XVI - Carcinoma of the Lung
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > Section XVI - Carcinoma of the Lung > Chapter 114 - Small Cell Lung Cancer
function show_scrollbar() {}
Chapter 114
Small Cell Lung Cancer
Frances A. Shepherd
Ronald Feld
David Payne
According to Ihde (1984), small cell lung cancer (SCLC) accounts for approximately 15% to 20% of all cases of lung cancer worldwide. Unlike the various subtypes of non small cell lung cancer (NSCLC), the relative proportion of SCLC with respect to lung cancer as a whole has not changed in recent decades. Minna and associates (1985) and Aisner and Matthews (1985) reported that the classic oat cell or lymphocyte tumor is composed of cells with small, round, or spindle-shaped, darkly staining nuclei and scant cytoplasm. Neurosecretory granules are often found in electron micrograph studies. The intermediate subtype of small cell carcinoma has cells with more fusiform or polygonal nuclei, and the cytoplasm is often more distinct (see Chapter 101). However, the differentiation of SCLC into these subtypes is not of great clinical importance because they are treated in similar fashion with similar outcomes.
Small cell lung cancer, as discussed by Bergsagel and one of us (RF) (1984) as well as Davis and colleagues (1985), differs in several biological and clinical respects from other types of lung cancer in that it has a large growth fraction, grows rapidly, and usually is widely disseminated at diagnosis. It is unlike NSCLC in that SCLC is very responsive to single-agent and combination chemotherapy, and more than three-fourths of patients, even those with advanced disease, achieve at least a partial response. Ihde (1984), as well as Shank (1985) and Stevens (1979) and their associates, reported that intensive early treatment can evoke complete responses in 25% to 60% of patients with limited disease and in 10% to 40% of those with extensive disease. Hyde and colleagues (1965, 1973) and Zelen (1973) noted that untreated patients have median survivals of only 6 to 17 weeks. As Davis (1985), Hansen (1980), Livingston (1984), and Sorensen (1986) and their colleagues point out, however, even with optimum treatment, fewer than 10% of patients are alive 5 years from the start of treatment. Improved integration of chemotherapy and radiation therapy has produced high survival rates in certain subsets of patients with this once rapidly fatal disease. This clinical experience served as a model for the combined-method approach to these tumors.
CELL BIOLOGY
Carney (1991, 1992) reported that it was possible to establish lung cancer cell lines from tumors obtained from both newly diagnosed and previously untreated patients as well as from patients who have relapsed after therapy. The cell lines could be established readily from various sites, including the primary tumor as well as specific metastatic sites. However, since SCLC is seldom resected surgically, most cell lines come from metastatic sites. In 68 patients with untreated SCLC, Stevenson and associates (1989) found no difference in the response rate or survival probabilities of patients in whom tumor cell lines were established compared with those in whom in vitro growth of tumor could not be accomplished.
The two types of lung cancer, SCLC and NSCLC, as reported by Carney (1991), are thought to come from a single stem cell. Carney (1986) suggests that a common stem cell may exist for all lung tumors, thus adding weight to the theory presented by Cuttitta (1981), Whang-Peng (1982), and Little (1983) and their colleagues that individual lung tumors may spontaneously change from one histologic type to another. This concept derives from clinical reports on mixed cell types as well as from autopsy series in which up to 40% of patients may have mixed histologic findings. In addition, the overlapping expression of endocrine biomarkers in small cell and non small cell tumors may reflect this fact biologically. Differences are noted in the markers produced by the two types of cancer cells, but, nonetheless, there is some overlap. Carney (1986), Bunn and Rosen (1985), and Carney (1985), Gazdar (1985), Cuttitta (1981), and Little (1983) and their co-workers looked at a number of cell lines from patients with SCLC and separated these lines into two major categories: classic and variant. Classic cell lines express elevated levels of biomarkers, including L-dopa decarboxylase, bombesin, neuron-specific enolase, and the brain isoenzyme of creatinine kinase. The variants express elevated levels of neuron-specific enolase and creatinine kinase only. Patients with the variant cell line have a
P.1701
less optimistic clinical prognosis. Amplification of C-myc has been associated with the variant class of SCLC, which may clarify the more malignant clinical behavior of variant tumors. Significantly, NSCLC lines only infrequently express any of the markers, allowing one to distinguish the tumor types using biological testing. This area has proved to be one of great interest that may lead to new approaches in treatment.
Also of interest is whether the neuroendocrine properties of lung cancer cells have any prognostic significance. These characteristics have been seen in NSCLC as well as SCLC tumors. It may be that NSCLC tumors with neuroendocrine differentiation may represent a distinct biological subset. Various studies reported by Skov (1991) and Sundaresan (1991) and their associates recounted data on the prognostic significance of neuroendocrine differentiation in clinical trials. Conflicting findings suggest that further studies are needed to establish conclusively the importance of this parameter.
Interest has been expressed in correlating the results of in vitro drug sensitivity testing with response to chemotherapy in patients with SCLC. Studies by Tsai (1990), Gazdar (1990a), and B. E. Johnson (1991a) and their colleagues confirmed that selection of individualized chemotherapy based on drug sensitivity testing is possible, but at the present time it is not considered useful in the management of SCLC patients. Although evaluations continue in the endeavor to recognize the mechanisms of resistance in patients with lung cancer, Carney (1992) notes that it is relatively clear that the multidrug-resistant phenotype is not a major determinant in this disease.
According to Brennan and associates (1991), C-myc, M-myc, and L-myc have been observed primarily in SCLC cell lines and fresh biopsy specimens. Carney (1991) found that amplification of C-myc has also been noted in variant SCLC cell lines, whereas both M-myc and L-myc have been demonstrated in classic cell types. Studies of large panels of cell lines reveal that amplification of oncogenes is more apt to be observed in cell lines established from heavily pretreated patients and is seen more frequently in established cell lines than in fresh biopsy specimens. Subsequently, Carney (1992) observed that myc amplification is seen more frequently in pretreated patients. The frequency of amplification was similar from fresh specimens and from cell lines in the same patient, suggesting that the myc family of oncogenes may accompany the more aggressive growth behavior observed at relapse. The clinical relevance of amplification of the myc oncogenes has not yet been demonstrated in prospective clinical trials.
Several studies undertaken by Weiner (1990) and Kern (1990) and their coauthors have provided evidence that cell lines from primary tumors that express c-erb-B2 genes in SCLC have shorter survival.
Cytogenetic abnormalities have been demonstrated in lung cancer cells. Carney (1991) found that lesions occur in the chromosome region 3p (14 23) in almost all cases of SCLC and have been shown in both primary and metastatic specimens, which suggests that it is a preliminary event in the biology of lung cancer. Of potential importance, as well, is the fact that the 3p deletion has not been demonstrated in extrapulmonary SCLC. Allele loss from chromosomes 13 and 17 has also been demonstrated. Some evidence also suggests that the expression of the p53 oncogene in lung cancer may be abnormal. Although other chromosomal abnormalities have been noted, Carney (1991) and Iggo and co-workers (1990) report that the mutation of this gene is the most commonly identified genetic change in human lung cancer. This area certainly demands more study.
Panels of monoclonal antibodies for identifying different types of lung cancer, including SCLC and NSCLC, may be feasible because many of the antibodies identified in lung cancer are under study by Boerman and associates (1991) for use in imaging, diagnosis, and target-directed therapy with toxins, as discussed by Carney (1992). Gazdar and colleagues (1990b) reported that monoclonal antibodies were used effectively for early detection and management of lung cancer using a sputum immunocytologic approach. This method was associated with 90% diagnostic accuracy 2 years before the ensuing diagnosis of cancer using orthodox techniques. Of significance, according to Carney (1991) and Woll and Rozengurt (1989), growth factors have been identified, at least in cell lines of lung cancer. These growth factors include bombesin (gastrin-releasing peptide), transferrin, and insulinlike growth factor. As reported by Macauley (1990) and Sausville (1990) and their colleagues, the latter may be an autocrine growth factor for SCLC. Interpreting how these factors function may be important in helping design a specific growth factor antagonist for therapeutic strategies, particularly in the treatment of SCLC. In studies of SCLC lines by Avalos and associates (1990), SCLC colony formation was enhanced by granulocyte colony-stimulating factor (G-CSF). Granulocyte colony-stimulating receptors were also shown on SCLC cells, which raises concern about the possible negative effects of using therapeutic G-CSF preparations in this patient population. This has not been a primary issue to date, however, and Crawford and colleagues (1991) have shown that G-CSF preparations have, in fact, been used successfully to lessen the myelosuppressive toxicity of chemotherapy in this disease with no apparent negative effect on survival. On the other hand, no survival advantage was observed in this landmark study and in a similar European study undertaken by Green and associates (1991), which creates uncertainty about whether the growth factors might be negatively affecting the outcome.
STAGING
Staging holds a key position in the choice of therapeutic treatment modalities for SCLC. Although chemotherapy is
P.1702
undoubtedly the main form of therapy used in SCLC, thoracic irradiation and, rarely, even surgery may also be helpful, depending on tumor stage before treatment. The most fundamental purpose for staging, however, is to determine prognosis. As one would expect, patients with less advanced SCLC have better long-term survival than do those with more advanced tumors.
Although the tumor, node, and metastasis (TNM) staging using the Union Internationale Contre le Cancer American Joint Committee on Cancer classification as defined by Mountain (1997) is now used routinely in NSCLC (see Chapter 105), this approach has not proved to be very useful for staging in SCLC. Most patients with this disease have stage III or IV disease at the time of diagnosis, thereby making the TNM staging system less likely to predict long-term survival. Most therapeutic trials in the treatment of SCLC have used the simple two-stage system originally suggested by the Veterans Administration Lung Cancer Study Group (VALG), which classifies patients into those with limited and those with extensive disease. Limited disease is described as a tumor confined to one hemithorax and its regional lymph nodes, including the ipsilateral mediastinal, ipsilateral supraclavicular, and contralateral hilar nodes. These sites should all be easily encompassed within a tolerable radiation therapy portal, as noted by Zelen (1973). Ipsilateral pleural effusions, left laryngeal nerve involvement, and superior vena cava (SVC) obstruction are judged limited, whereas pericardial involvement and bilateral pulmonary involvement are considered extensive because they would necessitate the use of too large a radiation therapy portal.
Some difficulty occurs when staging patients with contralateral mediastinal or supraclavicular lymph node metastases and patients with ipsilateral pleural effusions. These situations are often managed differently by different investigators. Some confusion exists about the lack of strict adherence to the VALG definition of limited disease. According to Ihde (1985), some investigators exclude ipsilateral pleural effusions and ipsilateral supraclavicular nodes, whereas others include contralateral supraclavicular nodes. For the most part, however, most groups adhere reasonably closely to the definition. In a consensus report prepared for the International Association for the Study of Lung Cancer Workshop on SCLC, Stahel and colleagues (1989) suggested that limited disease should include patients with disease restricted to one hemithorax with regional lymph node metastases (including hilar, ipsilateral, and contralateral mediastinal nodes and ipsilateral and contralateral supraclavicular nodes) and with ipsilateral pleural effusions, independent of whether cytology is positive or negative. The inclusion of contralateral mediastinal and supraclavicular metastases and ipsilateral pleural metastases in limited disease is recommended because the prognosis of patients with these sites of disease, including ipsilateral pleural effusions, is superior to that of patients with distant sites of metastases.
Stage may be affected by the number and type of staging procedures performed. If one investigator conducts more comprehensive staging than another does, a higher yield of patients with extensive disease results, but, surprisingly, the results in both groups of patients (limited and extensive disease) improve, although without influencing overall survival. As discussed by Pfister and colleagues (1990), this has usually been termed stage migration or the Will Rogers phenomenon. Although it is virtually impossible to correct for this effect, one must be aware of its possibility when unusually good results are reported. More sensitive diagnostic techniques will also detect a greater proportion of metastatic deposits, as was seen when staging of the brain evolved from radionuclide to computed tomographic (CT) and ultimately magnetic resonance imaging (MR) scanning.
The two-stage system generally separates patients with disparate outcomes well. Those with limited disease have a higher objective regression rate and a higher complete response rate, as well as notably longer disease-free and long-term survival, than do patients who have extensive disease. Patients who attain complete response in either stage do relatively well.
The University of Toronto group has identified a subgroup called very limited disease. This designation arose during a retrospective study of 180 limited-disease patients undertaken by one of us (FAS) and associates (1993). They found that the 33 patients without mediastinal involvement, supraclavicular node involvement, or pleural effusions had a projected 25% 5-year survival rate. It should be noted that this is the exact patient population that frequently is chosen for trials of intensive locoregional therapy, such as hyperfractionated radiation therapy or even surgery. Thus, favorable results in phase II trials may result in part from patient selection for study rather than from superior therapy.
Even within extensive disease, some subgroups of patients may have better prognosis. Ihde and co-workers (1971) report that patients with single sites of extensive disease have longer survival than do patients with multiple sites of metastases and, in fact, are not distinct from limited-disease patients. As well, Ihde (1985) found that patients with specific sites of involvement, including liver and brain, do particularly poorly. With current staging techniques approximately two-thirds of patients are found to have extensive disease.
Staging Procedures
The staging procedures that are most appropriate and essential for patients who are not participants in clinical trials are listed in Table 114-1. Extensive staging procedures in this setting may be difficult for some patients and can also needlessly escalate the cost of medical care. Extrathoracic staging is important, however, because the decision to incorporate radiation therapy into the overall treatment plan is based on confirmation of a limited-stage tumor. All patients
P.1703
must have a physical examination, chest radiograph, and simple blood tests before starting therapy. It has been suggested by the Memorial Sloan-Kettering Cancer Center that a certain order for the subsequent examinations be established and that the cost of staging could be reduced by canceling further investigation once a positive test was obtained. Although, in theory, this approach seems logical, it may not be practical because staging procedures, particularly CT scans, usually are done all at the same time, and sequential booking of tests and the waiting time for reporting may cause unnecessary delays in initiating treatment. For patients entering a clinical trial, pretherapy staging must be more extensive (Table 114-2); these procedures are comparable to those noted in a review by Stahel (1991) on the staging of patients with SCLC.
Table 114-1. Staging Procedures for Patients with Small Cell Lung Cancer Not Participating in Clinical Trials | |
|---|---|
|
Table 114-2. Possible Staging Procedures for Patients with Small Cell Lung Cancer Participating in Clinical Trials | |
|---|---|
|
During treatment, patients should undergo physical examination and have a chest radiograph and blood work before each treatment cycle to evaluate response. More extensive staging, as shown in Table 114-2, during therapy is probably indicated only in clinical trials. A study conducted by Richardson and colleagues (1991) proposes that a simpler approach to staging may be as good and economical.
At the completion of therapy, it is appropriate to repeat known positive studies at designated intervals to document the completeness of response. This is important because the decision to offer prophylactic cranial irradiation (PCI) is often based on confirmation of complete response. As reported by one of us (RF) and colleagues (1993), little evidence supports duplicating all pretherapy studies in patients with limited disease who seem to have attained a complete response based on the results of a radiograph of the chest. Although bronchoscopy may identify areas of occult residual disease, it is not necessary for the majority of patients or outside the clinical trial setting.
Areas of Controversy
Intrathoracic Tumor
Although chest radiographs are worthwhile for the evaluation of disease in the lungs, chest wall, and mediastinum, they may still underestimate the degree of disease in these sites. According to Hirsch (1989), as well as Lewis and colleagues (1990), CT scanning of the thorax is more precise in detecting tumors within the lung itself, and it is considered essential for radiation therapy planning. In up to 15% of patients, enlarged nodes in the mediastinum may not contain tumor and may misdirect the investigator into raising the stage of the patient being evaluated. The impact of incorrectly upstaging a patient with SCLC is minimal, however, because this tumor is seldom treated surgically and the mediastinum is always included in the radiation treatment field. Computed tomographic scans of the thorax should be extended to include the abdomen, which, of course, may assist in defining metastases in the liver or the adrenal glands. Abnormalities in the adrenal glands are fairly common, but available data have not clearly established that a patient with abnormalities (metastases or adenoma) at this site has a worse outcome than does a patient with limited disease.
Hirsch (1989) reports that MR imaging shows no benefit over CT in patients with SCLC. Although fluorodeoxyglucose positron emission tomography (FDG PET) has not often been used in patients with SCLC, it could prove to be useful in staging mediastinal and supraclavicular nodal involvement in patients with very limited intrathoracic disease on the chest radiograph. According to Erasmus and associates (1998), the accuracy of FDG PET in demonstrating intrathoracic metastatic nodal disease in NSCLC is greater than either that of CT or MR imaging, and this probably would be the same in SCLC patients. However, studies to confirm the accuracy of PET have not been undertaken in SCLC because patients with SCLC seldom undergo the surgical procedures that would be necessary to determine the accuracy of PET staging. Fiberoptic bronchoscopy is not
P.1704
necessarily useful unless surgery is contemplated, as discussed by Ginsberg (1989) and Ginsberg and Karrer (1989), although Stahel (1991) finds that a baseline may be useful if reevaluation is considered after possible response to therapy.
Liver Metastases
Liver metastases are extremely common in patients with SCLC. Hirsch (1989) notes that liver function tests alone are not useful for detection unless results are entirely normal, in which case tumor is rarely evident at this site. Sonography and CT of the liver are the favored approaches because both techniques may also detect adrenal metastases. Some investigators also suggest the addition of an ultrasound-guided needle biopsy or peritoneoscopy, particularly if the liver enzyme levels are elevated. However, these invasive tests are associated with potential morbidity and are probably superfluous because, as discussed by Ihde (1985), virtually all patients are treated with combination chemotherapy, which should treat any occult microscopic liver metastases that might be present. Again, whole-body FDG PET scanning might prove to be useful in demonstrating liver and adrenal gland involvement because FDG uptake is noted in metabolically active metastatic disease, as shown in the study of Rege and associates (1993) as well as others.
Bone Marrow
Bone marrow aspirates and biopsies are seldom believed to be necessary outside the clinical trial setting. Studies reported by Hirsch (1989) and by Campling and colleagues (1986) showed that few patients (less than 10%) have metastases at this site; even less frequently is the bone marrow the only site that classifies the patient as having extensive disease. For trial patients, single iliac crest aspirations and biopsies are still usually performed, and in some series, they have been done bilaterally. Stahel (1985) and Berendsen (1988) and their colleagues report that even more refined techniques increase the potential for finding bone marrow involvement by tumor (e.g., using specific monoclonal antibodies). The latter approach may be important for the evaluation of the small subgroup of patients who may be assessed for autologous bone marrow transplantation, but it is likely of far less importance for patients who are not undergoing this type of aggressive therapy. Carney and colleagues (1989) note that MR imaging of the marrow has been used, with early data suggesting that this procedure may be more sensitive. The data of Layer and Jarosch (1992), as well as that of Hochstenbag (1996), Trillet-Lenoir (1994), and Seto (1997) and their co-workers, demonstrate that MR imaging for detection of bone marrow metastasis is superior to that of either bone marrow biopsy or bone scintigraphy. However, whether identifying such metastasis in patients with limited disease influences the therapeutic approach is as yet unresolved.
Hirsch (1989) and Sagman and associates (1991a) found that lactate dehydrogenase (LDH) might provide comparable information without the need for this relatively uncomfortable invasive procedure. This theory is still controversial; therefore, at this time, bone marrow aspiration and biopsy should be undertaken only in patients who are potential candidates for clinical trials of limited disease.
Central Nervous System Metastases
Hirsch (1989) and Klastersky (1990) report that brain metastases are seen at presentation in approximately 10% of patients with SCLC, but they may be present at autopsy in up to 65%. The standard investigation for this site has been CT scanning, although MR imaging is probably superior to CT, as it is for most brain abnormalities. The role of FDG PET scans for identifying brain metastases is limited at best owing to the increased metabolism of normal brain tissue.
Carcinomatous meningitis is an infrequent presenting characteristic of this disease and may be confirmed by microscopic examination of the cerebrospinal fluid. According to Bunn (1978), Rosen (1982), and Aisner (1981) and their colleagues, however, several lumbar punctures may be required to demonstrate meningeal involvement. Nodular filling defects along the root sleeves may be seen at myelography or with MR imaging of the vertebral column when spinal cord compression is present.
Biomarkers
Many biomarkers have been studied in patients with this disease, and their expression may correlate with response to treatment. Adrenocorticotropic hormone, calcitonin, neuron-specific enolase, plasma neurophysin, and antidiuretic hormone have not been conclusively useful prognostic factors in these patients, as reported by one of us (RF) and co-workers (1988) and Hansen (1990). Pretreatment levels of carcinoembryonic antigen (CEA) correlate with the stage of the disease and, as proposed by Sculier and associates (1985a), may actually be an independent prognostic factor. However, levels are elevated only in approximately one-third of patients, thereby making CEA a less valuable indicator. According to Rawson and Peto (1990), Stahel (1991), and Albain and co-workers (1990), pretreatment LDH values may be a useful pretreatment prognostic factor. Values often return to normal if the patient responds to therapy. Recently Ahmed and colleagues (2000) have reported that they can detect the presence of circulating small cell lung cancer cells in the peripheral blood through real-time polymerase chain reaction for expressed neuropeptide. Their group did not correlate the finding of expression with outcome. As noted by one of us (RF) and co-workers (1988), as well as by Biran and colleagues (1991), several investigative groups have reported that rising levels of biomarkers sometimes precede clinical evidence of tumor relapse by weeks or months. Because of the lack of effective therapy
P.1705
at the time of relapse, as discussed by Boyer and associates (1992), the benefit of early knowledge of relapse of tumors is debatable. Combinations of markers may better predict early relapse. The consensus at the moment is that biomarkers other than perhaps LDH are of little value for pretreatment prognosis or as early evidence of relapse, although additional research continues on the subject.
A relatively new biomarker, noted by Holst and colleagues (1989), is the C-terminal flanking peptide of human gastrin-releasing peptide, which may indicate a worse prognosis. Giovanella and associates (1997) reported that the tumor marker neuron-specific enolase may be useful in monitoring therapy and in patient follow-up. However, with the exception of LDH, most of these markers have been used more as research tools than as important guides to treatments.
Restaging
Restaging is a distinct area of debate. In a retrospective study conducted by the National Cancer Institute of Canada (NCIC), one of us (RF) and colleagues (1992) found that routine restaging in patients with limited disease who had responded was probably of little value. Although a small survival benefit was demonstrated in a subgroup of patients who had negative posttreatment bronchoscopy compared with patients with positive bronchoscopic findings, the investigators suggested considering this approach only in a clinical trial. Economic analysis also supported the concept of not proceeding with restaging, although Stahel and co-workers (1989) and Stahel (1991) still encourage restaging, particularly repeat bronchoscopy. Some radiation oncologists require restaging before proceeding to prophylactic cranial irradiation.
Prognostic Factors
Prognostic factors may be useful for individual patient prognosis as well as for accurate stratification in clinical trials. The factors documented as important by Stahel (1991) and Rawson and Peto (1990), as well as by Ihde (1971), Albain (1990), and Stahel (1989) and their colleagues, encompass the following: stage of disease (limited versus extensive), performance status, and whether patients have received previous chemotherapy. Various investigators have found additional prognostic factors (Table 114-3). Female gender has been reported to be a favorable prognostic factor by several investigators, including Stahel (1992) and Ferguson and co-workers (1990). Consensus has not yet been reached as to which factors are most significant; the knowledge gleaned from staging and prognostic factors must be considered carefully when comparing results of therapy in reports of clinical trials in this disease. Newer statistical methods, such as recursive partitioning and amalgamation, may prove useful, as evidenced by two articles by Albain (1990) and Sagman (1991b) and their co-workers.
Table 114-3. Possible Prognostic Factors for Survival in Treated Patients with Small Cell Lung Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EVOLUTION OF THERAPY
Janne and colleagues (2001), in their review of cooperative group trials of various treatment strategies, have shown that survival for patients with SCLC, particularly those with limited disease, has improved significantly from 1980 to 1999. Although chemotherapy is the predominant form of treatment and is addressed in detail in this chapter, it is useful to review how treatment of SCLC developed to its present approach. Initially, surgery was the treatment of choice for patients with all types of lung cancer, but it was abandoned after the results of a randomized trial carried out in the United Kingdom by the British Medical Research Council comparing radiation therapy alone to surgery alone in patients with limited disease. The 5- and 10-year results of this study were reported by Miller and colleagues (1969) and Fox and Scadding (1973), respectively. Even though the mean survival time for all these patients was short (10 months, with only 5% of patients alive at 5 years), the fact that all surviving patients were in the radiation arm made radiation therapy the standard form of treatment from that point on.
R. A. Green and co-workers (1969) demonstrated the activity of cyclophosphamide against SCLC compared with placebo. They showed that the median survival times for patients with limited and extensive disease receiving placebo were approximately 12 weeks and 6 weeks, respectively. These data must be recognized when endeavoring to put into perspective the modest improvements observed in the treatment of this disease between the 1970s and 1990s.
P.1706
One of us (RF) and colleagues (1988) reported that many single agents in patients with SCLC yield response rates of 20% and higher. In a review of all studies from 1970 to 1990, Grant and co-workers (1992) identified 11 active drugs. Newer agents shown to be active have since been added to initial lists. Minna and colleagues (1989) reported that when active drugs were combined, an improved response rate was observed, with complete response rates ranging from 20% to 50% and rising even higher in patients with limited disease. Retrospective reviews of the median survival of patients treated with either single agents or combination chemotherapy showed that individuals receiving combinations survived longer. Randomized trials comparing single agents with combination chemotherapy with or without chest irradiation demonstrated benefit from the combinations, as emphasized by Ihde and associates (1991).
Bergsagel and colleagues (1972) showed that the addition of cyclophosphamide to conventional thoracic irradiation in patients with limited disease resulted in a survival benefit. This result was confirmed by Smyth (1984) and led to the use of combined-modality treatment in the early 1970s. Most frequently, thoracic irradiation is added to combination chemotherapy in patients with limited disease, and it is not routinely given to patients with extensive disease. Some groups add thoracic irradiation to the treatment plan for patients with extensive disease who achieve complete response or to those who present with very bulky thoracic tumors.
When analyses of relapse patterns showed that 50% of patients or more relapsed at the primary intrathoracic site, it became standard to administer thoracic irradiation during or after chemotherapy. Most of the studies showed that local relapse rates could be reduced by half, but significant prolongation of survival was not seen in any of the small trials. However, two meta-analyses of the randomized trials conducted by Arriagada and associates (1991a) and by Warde and one of us (DP) (1992) showed that thoracic irradiation adds significantly to survival for patients with limited disease. Accordingly, most physicians treat patients who achieve a complete response, and many treat patients who achieve at least a partial response, with this method of therapy. Controversy also surrounds how best to give thoracic irradiation. The issues include dose, fractionation, portal size, and at what point the radiation therapy should be given in reference to the beginning of combination chemotherapy. These questions are examined in more detail later in this chapter.
When it was observed that in many patients, relapse involved the central nervous system (CNS), Hansen and colleagues (1980) suggested that the brain was a potential sanctuary from chemotherapy. Subsequently, it became routine to administer PCI to all patients with SCLC, regardless of stage. In the 1980s, signs of neurologic toxicity were identified, and more rigorous criteria for the use of PCI have been advocated since then. In particular, it has been recommended that application of PCI be confined to patients who have shown a complete response because they are the most liable to benefit. According to Lishner and co-workers (1990), the possible disadvantage of PCI in this subpopulation (i.e., CNS toxicity, which was not observed in all studies) is probably worth risking (see Elective Brain Irradiation, later in this chapter).
In general, immunotherapy (biological responsive modifiers) has not proved to be of any superior benefit in this disease. One study by Cohen and colleagues (1979), who used thymosin fraction V, did show a survival benefit, but this effect was not validated in a study undertaken by Shank and co-workers (1985). In a study in Finland by Mattson and colleagues (1991), results suggested a benefit to maintenance therapy with interferon- in patients responding to standard methods of treatment. Cooperative groups in the United States have endeavored to corroborate this information, but conclusive analysis of these data is not yet available. A trial piloted by Jett and colleagues (1992) in the North Central Group using interferon maintenance therapy showed no benefit. At present, this form of therapy should not be considered standard.
CHEMOTHERAPY
Single Agents
Chemotherapy is currently the mainstay of treatment for all stages of SCLC. In the 1960s, R. A. Green and associates (1969) demonstrated improved survival in patients with extensive SCLC after three courses of cyclophosphamide compared with placebo. Since that time, many active drugs have been identified. A partial list of the most active single agents in SCLC is shown in Table 114-4. The agents used most frequently include etoposide (VP-16), cisplatin, cyclophosphamide, doxorubicin (Adriamycin), and vincristine. Promising new agents include gemcitabine, which has single-agent activity with a response rate of 27%, as reported by Cormier and colleagues (1994). Gemcitabine also has been the focus of a phase II study by Eisenhauer and colleagues (1992). Other agents are CPT-11, for which Masuda and colleagues (1992) have undertaken a study, and paclitaxel (Taxol) and its derivatives. Paclitaxel has shown single-agent activity with a 31% to 50% response, as pointed out by Ettinger (1993), Hainsworth and Greco (1995), and Bunn (1997). Other new agents include irinotecan, topotecan, and docetaxel, which are being studied in phase I and II trials. Topotecan, a new non cross-resistant chemotherapeutic agent, is a topoisomerase I inhibitor, and early studies by Schiller (1996), Perez-Soler (1996) and Ardizzoni (1997) and their associates have shown that it has significant activity in SCLC.
Table 114-4. Established Active Single Agents in the Treatment of Small Cell Lung Cancer | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Single-agent chemotherapy produces objective responses but seldom produces complete regression, even in previously untreated patients with SCLC. On the basis of studies
P.1707
carried out by Ettinger (1990) for the Eastern Cooperative Oncology Group (ECOG) and Evans and co-workers (1990) for NCIC, it seems both ethical and appropriate to treat previously untreated patients with extensive SCLC using an experimental agent. Evaluation should occur early in this case, and if no response is observed, the patient should be shifted to an active regimen before the disease is irretrievable. Expectation of response rates of 70% or greater should be used to estimate sample sizes in this population. Blackstein and co-workers (1990) note that less difficulty may be associated with the use of derivatives of known active agents, such as anthracyclines; this is also true of new platinum compounds. Both ECOG and NCIC have had experiences with active and inactive agents and have observed reasonable response rates and survival, regardless of the action of the new drug. Treating previously treated patients may result in artificially negative data, which may then not identify potentially useful drugs. Grant and colleagues (1992) suggested that using a lower response rate (10%) as an indication of activity in previously treated patients may be a useful approach. In addition to the active agents mentioned, Grant and colleagues (1992) note that phase II trials have found that many agents show little or no activity (Table 114-5) in patients with SCLC.
Table 114-5. Activity of Recently Tested Single Agents in Small Cell Lung Cancer | ||
|---|---|---|
|
Combination Chemotherapy
In spite of partial responses and occasional complete responses, the relatively poor results with single-agent chemotherapy led to efforts at combining these agents in patients with SCLC, as had been done with other malignancies. Less than 20% of 753 patients given single-agent chemotherapy had an objective response, and less than 3% obtained a complete response in a retrospective review carried out by Bunn and Ihde (1981). In contrast, among 1,236 patients receiving combination chemotherapy, a 70% objective response rate was seen, 31% being complete. Those receiving combination chemotherapy survived longer than did those receiving single agents. Authors of randomized trials have compared single agents and combination chemotherapy with or without chest irradiation and demonstrated a slight benefit from combination chemotherapy in objective tumor response and median survival. Bunn and Ihde (1981) and Minna and colleagues (1989) also reviewed the literature regarding the appropriate number of drugs to be included in combination for this disease. They found no significant difference in the complete response rate or long-term disease-free survival when more than three drugs were used in patients with limited disease.
Table 114-6 shows the most traditionally used and highly active combinations for treatment of this disease worldwide. Although these are among the most conventional regimens, virtually any combination of the most active agents has achieved reasonable results. Any of these regimens should result in response rates in excess of 80% (50% to 60% complete response) in patients with limited disease and of 65% to 70% in patients with extensive disease (10% to 20% complete response). If appropriate staging procedures
P.1708
are carried out, the median survival for patients with limited disease should be 12 to 15 months or more. In trials undertaken by Murray (1991b), D. H. Johnson (1987a, 1987b), and Tourani (1991) and their colleagues on combined-method treatment, median survivals of 18 to 20 months or more were observed for patients with limited disease. Ihde (1991) and Kristjansen (1991) and their associates found that the median survival for patients with extensive disease is still roughly 10 months or less, with a range of 8 to 12 months. Approximately 15% to 20% of patients with limited disease and less than 5% of those with extensive disease remain disease free for more than 2 years. Patients who achieve a complete response usually live longer than those who show only a partial response, the former being the only group with the potential for long-term disease-free survival. Patients with limited disease usually live significantly longer than do those with extensive disease, as do patients who have a superior performance status at presentation.
Table 114-6. Frequently Used Chemotherapy Combinations for Small Cell Lung Cancer | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Proper Dosing of Available Drugs
Diverse pharmacologic approaches with known active drugs may be illustrated by interest in the use of relatively low-dose oral etoposide (VP-16) on a continuous 14- or 21-day schedule, with approximately 1 week off, followed by restarting this therapy. This regimen was developed by B. E. Johnson and colleagues (1991b) from Vanderbilt University and Slevin and associates (1989), as well as by Einhorn and co-workers (1990) from Indiana University and Clark and co-workers (1990, 1991) from the United Kingdom. Carney and colleagues (1990) note that toxicity in formerly untreated patients seems tolerable and makes this a sound approach for elderly patients in whom an aggressive approach with a more conventional regimen is contraindicated or declined by the patient. Some patients also responded to injectable etoposide who had either responded in the past or did not respond at all. Oral etoposide has also been combined with cisplatin and carboplatin, but preliminary data presented by Murphy (1991) and Evans (1991) and their co-workers did not suggest a significant benefit over oral etoposide alone by continuous daily treatment. The favorable phase II results of single-agent etoposide led to two randomized trials of oral etoposide compared with intravenous chemotherapy. As reported by Harper for the London Lung Cancer Group (1996) and Girling for the Medical Research Council Lung Cancer Working Party (1996), both trials indicated significantly shorter survival for oral etoposide, and surprisingly, more toxicity. Similar results were reported by Souhami and associates (1997).
Dose Intensification
Dose Intensification by Increasing Chemotherapy Dose
Despite initial response rates of 80% to 90% to conventional chemotherapy, most patients experience relapse within 2 years and die with disseminated malignancy. Although improvement in local control is still required, it is likely that any substantial improvement or increases in cure rate will be secondary to the development of more effective systemic treatment. The concept of dose intensity in cancer chemotherapy has been reviewed by Dodwell and associates (1990). Although convincing evidence of a steep dose response curve for most chemotherapeutic agents when they are studied in vitro or in animal model systems exists, the clinical evidence is considerably less compelling when the randomized trials with SCLC are examined (Table 114-7).
Table 114-7. Prospective Randomized Trials of the Importance of Dose in Small Cell Lung Cancer | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In an older trial reported by Cohen and colleagues (1977), patients in the higher-dose arm demonstrated superior overall response rates and median survival. O'Donnell and co-workers (1985) undertook a similar study using higher doses of the same drugs and demonstrated a higher response rate but no significant prolongation of survival. This was a small trial of only 32 patients, however, and the cyclophosphamide dose of only 500 mg/m2 in the low-dose arm would be considered inadequate treatment today. Mehta and Vogl (1982) performed a larger trial using the same agents with the same results. Once again, though, the cyclophosphamide dose would be considered too low by today's standards. Figueredo and co-workers (1985) compared standard-dose cyclophosphamide, doxorubicin, and vincristine (CAV) to higher doses of the same drugs (i.e., doxorubicin, 20% increase; cyclophosphamide, 50% increase). No difference in complete response rate or duration of response could be identified. D. H. Johnson and colleagues (1987a, 1987b) also reported a higher response rate with higher doses of cyclophosphamide and doxorubicin but no improvement in survival. In a similar trial evaluating the usefulness of high-dose etoposide and cisplatin reported by Ihde and colleagues (1991), no difference in complete response rate or overall survival was seen for patients in the high-dose arm (i.e., 67% increase for both drugs). In the last study of dose, Arriagada and associates (1993) gave
P.1709
higher doses of cyclophosphamide and cisplatin only in cycle 1. Surprisingly, median and 2-year survival rates were both significantly higher in the high-dose arm.
Despite the relatively negative results of most of these initial trials, considerable interest in dose intensification for SCLC was generated in the 1990s. Even though the differences demonstrated in most of the trials did not reach statistical significance, each study did show a trend toward improved response and prolonged survival in the high-dose arm. In the assessment of dose intensity, the dosage of individual drugs as well as the duration of treatment and the interval between individual drug administrations should be taken into consideration, as discussed by Bonomi and co-workers (1985). Hryniuk and Levine (1986) and Hryniuk and associates (1987) define dose intensity as the amount of drug administered per unit of time, expressed for a single-drug regimen as milligrams per square meter per week. For a multiple-drug regimen, they recommend definition of an average relative dose intensity by comparison with a standard regimen and by giving a relative weight to each drug. In an analysis of 67 published studies, Klasa and co-workers (1991) attempted to correlate response and median survival time with dose intensity over the first 6 weeks of chemotherapy for SCLC. They identified a trend (P = 0.07) toward a positive correlation between dose intensity and median survival time for patients with extensive disease treated with CAV. When only randomized studies were considered, they noted a positive correlation (P = 0.001) for the relative dose intensity of doxorubicin with total response rate but not with overall survival. A similar correlation was also seen for etoposide-containing regimens for response rate and survival in patients with extensive disease.
On the basis of these observations, individual investigators and cooperative groups have continued to assess new chemotherapy strategies aimed at increasing the dose intensity of the regimen, either through alteration in the scheduling of drug delivery or by increasing the actual doses of chemotherapeutic agents.
Acceleration of Chemotherapy Delivery to Increase Dose Intensity
The mathematical model for the development of chemotherapy-resistant clones in malignancies proposed by Goldie and Coldman (1984) suggests that the number of drug-resistant clones of cells within the tumor is most likely at its lowest at the time of diagnosis. As tumor size increases, the number of drug-resistant clones also increases, either as a spontaneous event or in response to exposure to chemotherapeutic agents. This finding suggests that a potential therapeutic advantage may be gained by the early introduction of as many active agents (drugs or irradiation) as possible in the treatment protocol. Klimo and Connors (1985) first evaluated an intensive weekly chemotherapy protocol with the rapid alternation of myelosuppressive and nonmyelosuppressive agents over a short 9- to 12-week course for patients with diffuse large cell lymphoma. The favorable results achieved in lymphoma patients led Murray and co-workers (1991b) to develop a similar protocol for patients with extensive SCLC. Their CODE regimen [cisplatin, vincristine (Oncovin), doxorubicin, and etoposide], combined with a supportive care program of prednisone and cimetidine on alternate days and daily cotrimoxazole and ketoconazole, resulted in an overall response rate of 94%, a complete response rate of 40%, and a survival time of 61 weeks in 48 patients with extensive SCLC. The authors emphasized that the main toxicity for this regimen was constitutional, and they recommended administering 9 rather than 12 weeks of therapy, which resulted in a dose intensity that was almost twice as great as that achieved with standard 18-week protocols using the same drugs. Miles and colleagues (1991) reported another
P.1710
study using similar chemotherapeutic agents but substituted ifosfamide for vincristine. They also achieved a high overall response rate of 92%, a complete response rate of 48%, and median survival times of 58 weeks for limited disease and 42 weeks for extensive disease.
The regimens piloted by Murray (1991b) and Miles (1991) and their associates both contained four chemotherapeutic agents. In a Southwest Oncology Group (SWOG) pilot study reported by Taylor and co-workers (1990), six agents were alternated weekly for a total of 16 weeks. The overall response rate was 82%, and 38% of patients with extensive disease achieved complete remission. The median survival times for limited and extensive disease were 16.6 and 11.4 months, respectively. The Lung Group at the Institut Jules Bordet went one step further and tested a seven-drug combination. Sculier and colleagues (1988) reported an overall response rate of 78% for limited- and extensive-disease patient groups combined. Based on results of this pilot study, this regimen is to be compared to standard therapy in a European Organization for Research and Treatment of Cancer prospective randomized trial. However, the phase II study of Murray and associates (1991b) noted previously was followed by a randomized phase III trial undertaken by NCIC and the Southwest Oncology Group. Patients with extensive SCLC were randomized to receive either the CODE regimen or standard chemotherapy (alternation of CAV with etoposide and cisplatin). The study closed early when an excessive toxic death rate was observed in the CODE arm, with no evidence of a survival benefit from the dose-intensive treatment, as summarized by the report of Murray and coinvestigators (1999).
Colony-Stimulating Factors to Increase Dose Intensity
Myelosuppression is the dose-limiting toxicity for most chemotherapeutic agents that are active against SCLC. Several clinical studies have revealed that the recombinant CSFs, G-CSF, and granulocyte-macrophage CSF (GM-CSF) can accelerate the recovery of myelopoiesis after cytotoxic chemotherapy. In two similar randomized trials reported by Crawford (1991) and J. A. Green (1991) and their associates, patients were treated with cyclophosphamide, doxorubicin, and etoposide. They were randomized either to receive or not to receive G-CSF on the first cycle. Both trials showed that the incidence of febrile neutropenia and hospital admission was substantially reduced in the G-CSF arms. Although these trials were not designed to evaluate response and survival, no overall improvement in either outcome could be identified in the groups receiving G-CSF or GM-CSF.
The primary objective of these two trials was to ameliorate toxicity by reducing the period of absolute neutropenia and the incidence of neutropenia-associated sepsis. These efforts led to pilot studies to determine whether CSF would allow repeated administrations of higher doses of chemotherapy in an attempt to improve response rate and survival rate without an unacceptable increase in toxic effects. In a study undertaken by the Cancer and Acute Leukemia Group B, Mitchell and co-workers (1988) reported that the maximum tolerated doses of etoposide and cisplatin without CSF support were 200 mg/m2 etoposide and 35 mg/m2 cisplatin given intravenously daily for 3 days. In a dose-escalation study by the Cancer and Acute Leukemia Group B, three of six patients developed dose-limiting toxic effects with 200 mg/m2 per day etoposide for 3 days and 125 mg/m2 per day carboplatin with 10 g/kg of GM-CSF for 3 days. A greater degree of myeloprotection was achieved by increasing the dose of GM-CSF to 20 g/kg, but it was not possible to escalate chemotherapy doses further. Greater bone marrow protection was seen in a small cohort of patients treated with 5 g/kg of GM-CSF every 12 hours compared with either 10 or 20 g/kg once daily. In a similar trial reported by Mitchell and colleagues (1988), the addition of GM-CSF to etoposide and cisplatin at the maximum tolerated doses did not allow further dose escalation. Four of six patients developed febrile neutropenia or infections, and only one of six patients was able to tolerate six cycles of chemotherapy, and that patient required one dose reduction for hematologic toxicity. Furthermore, all patients who received more than one course of high-dose chemotherapy required blood product support (packed red blood cells and platelets).
Significant myelosuppression occurred in all the aforementioned weekly intensive chemotherapy protocols discussed. This result has led investigators to assess the role of CSF in accelerated chemotherapy programs. Ardizzoni and co-workers (1990) reported the results of a small nonrandomized pilot study of GM-CSF in which five patients received GM-CSF when grade IV leukopenia occurred and five patients received no growth factor support. The mean interval between chemotherapy courses and the mean duration of therapy were 10 and 57 days, respectively, in patients treated with GM-CSF, compared with 13 and 72 days in the control group. Overall, chemotherapy dose intensity was increased twofold in the patients given GM-CSF, compared with a 1.5-fold increase in the control patients. Other studies continue with GM-CSF, but at this time, as noted by Bishop (1991) and Anderson (1991a) and their associates, data are not as good as those observed with G-CSF.
The only reported prospective randomized trial of intensive weekly chemotherapy and G-CSF was undertaken by Fukuoka and colleagues (1991a). These investigators compared CODE alone to CODE with a small dose (50 g/m2) of G-CSF given daily on the nonchemotherapy days. The complete remission rate was similar in both arms, but the median survival in the G-CSF arm was 59 weeks, compared with 35 weeks in the control arm. The total dose delivered in the G-CSF arm was 85% of predicted, compared with 76% of predicted in the control arm. The median number of days of neutropenia was 1.33 in the G-CSF arm, compared with 3.31 in the control arm, and febrile episodes were seen in only 13 patients, compared with 36 patients in the group receiving no treatment. This degree of marrow protection was achieved despite a low dose of G-CSF. It is important
P.1711
to note, however, that these patients remained in the hospital throughout the 9-week course of therapy. It is also critical that the response rates and survival rates in the G-CSF arm are not superior to those reported by Murray and associates (1991b) for CODE given with alternate-day prednisone and prophylactic antibiotics and without CSF support.
In a randomized trial of dose intensity reported by Pujol and colleagues (1995), the addition of GM-CSF to the high-dose arm did not allow higher doses of drugs to be delivered. In fact, after the first dose, patients frequently required dose reduction for toxicity, and only 75% of intended doses could be administered despite the use of growth factors.
In a randomized trial of standard-dose vincristine, ifosfamide, carboplatin, and etoposide (V-ICE) versus intensified V-ICE, reported by Steward and associates (1995), there was a second randomization in each arm to either GM-CSF or placebo. No differences in dose delivery, dose intensity, remission rate, or survival were detected between the GM-CSF and placebo arms. Woll and colleagues (1995) reported the results of a similar trial that used the same drugs and G-CSF. They found no differences in the response rates or survival, and lethal toxicity was actually higher in the G-CSF arm.
Sculier and colleagues (2001) have recently reported the results of a European Lung Cancer Working Party trial of standard chemotherapy with ifosfamide, vindesine, and epirubicin given every 3 weeks or the same chemotherapy given with GM-CSF or prophylactic cotrimoxazole every 2 weeks in patients with extensive disease. There was no improvement in survival for the patients treated at 2-week intervals, and severe toxicity was significantly increased. A large European Organization for Research and Treatment of Cancer (EORTC) trial presented in abstract form by Tjan-Heijnen and associates (2001) showed that although dose sizes of cyclophosphamide, doxorubicin, and etoposide could be increased or the interval between doses could be decreased by the administration of G-CSF and prophylactic antibiotics, median and 1-year survival rates were not improved.
Clearly, CSFs can reduce the degree and duration of neutropenia after standard-dose chemotherapy, but they do not seem to allow repeated administration of higher doses of chemotherapy or dosing at significantly shorter intervals. If CSFs allow only modest dose escalation, their costs and toxicity likely are not justified. In summary, CSFs have very little role to play in the routine management of patients with SCLC who are receiving standard doses of chemotherapy either in classic 3- to 4-week intervals or in more dose-intense weekly schedules.
Bone Marrow Transplantation
High-dose chemotherapy followed by autologous bone marrow transplantation in patients with SCLC has been under investigation since the 1980s, and it is probably safe to say that this form of treatment is not appropriate for most patients with this tumor. Of necessity, almost all trials have been small phase I to II pilot studies, and diverse patient populations make comparisons between studies almost impossible. Some trials focused only on patients with limited disease, whereas others included those with extensive disease as well. Furthermore, high-dose therapy has been studied as initial induction treatment, as intensification after induction at standard doses, or as salvage at the time of relapse.
High-Dose Induction Chemotherapy
Several trials of high-dose induction chemotherapy are summarized in Table 114-8. The trial of D. H. Johnson and colleagues (1987a) is interesting because patients with extensive disease were treated with two courses of high-dose cyclophosphamide and etoposide plus high-dose cisplatin without bone marrow transplantation. Although myelosuppression was severe, 17 of 20 patients were able to receive
P.1712
their second course of high-dose chemotherapy. The complete response rate was 65%, but the median survival was only 41 weeks, and 2-year survival was approximately 20%.
Table 114-8. Trials of High-Dose Induction Chemotherapy for Small Cell Lung Cancer | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Several London hospitals evaluated intensive chemotherapy with autologous bone marrow transplantation, and Souhami and co-workers (1989) reviewed the results of four sequential trials undertaken by their group. Most patients in their studies had limited disease. In the first study, patients received cyclophosphamide alone, and in study II, etoposide was added. In studies III and IV, patients were treated with multidrug combinations consisting of cyclophosphamide, etoposide, doxorubicin, and vincristine or carboplatin, etoposide, and either melphalan or cyclophosphamide. Once again, high response rates were observed, but the 2-year survival was only 20% (data were available only for studies I, II, and III). Of interest, the highest response rate, median survival, and 2-year survival rate were seen in study I, in which only high-dose cyclophosphamide was used.
The Manchester group in the United Kingdom has performed a randomized phase II trial of six cycles of ifosfamide, carboplatin, and etoposide (ICE) at 4-week intervals compared with intensified ICE with G-CSF and autologous blood progenitor cells at 2-week intervals. Woll and colleagues (2001) reported on behalf of the group that intensified ICE could be given safely, but efficacy outcomes have not yet been published.
Late Intensification with High-Dose Chemotherapy and Autologous Bone Marrow Transplantation
The disappointing long-term results achieved with high-dose chemotherapy as initial induction treatment led several investigators to offer such treatment as late intensification only to patients who responded well to standard-dose induction therapy (Table 114-9).
Table 114-9. Late Intensification Therapy for Small Cell Lung Cancer | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cunningham and associates (1985) treated 22 patients (16 with limited and 6 with extensive disease) with high-dose chemotherapy consisting of 180 mg/kg cyclophosphamide and 1 g/m2 etoposide after induction with standard doses of cyclophosphamide, doxorubicin, methotrexate, etoposide, and vincristine. Autologous bone marrow was reinfused 36 hours after the beginning of high-dose chemotherapy. Eight patients with limited disease were in complete remission at the time of late intensification, and three (40%) achieved long-term survival. Ihde and colleagues (1986) reported the experience of the U.S. National Cancer Institute (NCI) in a small study of eight patients with extensive disease who also were treated with high-dose cyclophosphamide and etoposide and irradiation to the chest. The median survival was less than 1 year, and there were no 2-year survivors. Somewhat more encouraging results were reported by Goodman and coworkers (1991) for SWOG and by Elias and colleagues (1992) for the Dana Farber Cancer Institute. In the SWOG trial, 58 patients with limited disease were assessed for induction chemotherapy and late intensification. Only 21 patients received high-dose chemotherapy, which consisted of 150 mg/kg cyclophosphamide without autologous bone marrow transplantation. Of the 21 patients, 5 relapsed, 4 died as a result of toxicity from intensification, and 3 died of other causes but were in complete remission at the time of death. Nine of the 21 patients remain in complete remission with a median survival greater than 27 months. Elias and associates (1992) also studied 17 patients with limited disease who had responded to induction therapy (12 complete responses and 5 partial responses). Late intensification consisted of 5.6 g/m2 cyclophosphamide, 480 mg/m2 bischloroethylnitrosourea (carmustine),
P.1713
and 165 mg/m2 cisplatin followed by autologous bone marrow transplantation. The projected 2-year survival rate for that study is 75%.
Results from the two studies just mentioned are encouraging and suggest that further investigation should focus on eligible patients with limited disease of good performance status who have demonstrated an excellent response to standard-dose induction chemotherapy. It must be remembered, however, that this population represents less than one-half of patients with limited SCLC and a small percentage of the entire SCLC population. Certainly, randomized controlled trials testing this hypothesis are required. Does this review leave any room for optimism for high-dose therapy and autologous bone marrow transplantation? Although the long-term survival in most of the studies is disappointing, findings of three studies suggest that further investigation in this area is warranted. The early study of Cunningham and associates (1985) and the more recent study of Elias and co-workers (1992) both demonstrated that a significant proportion of patients with limited disease who underwent intense chemotherapy at the time of complete remission achieved long-term survival. Of even greater importance is a study of Humblet and colleagues (1987), which was part of a randomized trial in which patients underwent induction chemotherapy and were then randomized to late intensification with bone marrow transplantation or crossover to standard doses of the same drugs. The median relapse-free survival time after randomization for the intensified group was 28 weeks, compared with only 10 weeks for the standard-dose group (P = 0.002). The median overall survival was also longer for the intensified group despite four treatment-related deaths in that arm of the study. Relapse occurred frequently at the primary site, suggesting that local radiation therapy should be included in any further trials.
High-Dose Chemotherapy for Salvage
The very low response rate to standard-dose chemotherapy for patients with recurrent or refractory SCLC led some investigators to evaluate the role of very high-dose chemotherapy in this patient population. Lazarus (1990) and Postmus (1985) and their colleagues demonstrated that response could be achieved by dose intensification, but the response duration was short, toxicity was considerable, and few patients achieved long-term survival. For these reasons, high-dose chemotherapy is not appropriate for patients with resistant SCLC.
Alternating Non Cross-Resistant Chemotherapy
According to Goldie and Coldman (1984), tumor resistance to chemotherapy is likely a significant cause of treatment failure in SCLC. This resistance may exist at the start of therapy or it may be acquired during treatment. During the 1980s, the Goldie Coldman hypothesis, an approach to early resistance, had become popular. The authors proposed a mathematical model based on the hypothesis that tumor cell killing displays a logarithmic pattern and tumors continuously develop resistant mutations during treatment. The conjecture of Goldie and co-workers (1982) and Goldie and Coldman (1984) is that alternating two combinations of non cross-resistant drugs early in the course of treatment might lessen the development of drug-resistant clones and increase the chance of cure. In their model, it is essential that the combinations tested be truly clinically non cross-resistant and that both non cross-resistant combinations be active as initial treatment for the disease being evaluated. Bonadonna (1982) described the benefit of this approach, seen most often in the treatment of Hodgkin's disease, in which it appeared that treatment with MOPP [mechlorethamine (nitrogen mustard), vincristine (Oncovin), procarbazine, and prednisone] alternating with ABVD [doxorubicin (Adriamycin), bleomycin, vinblastine, and dactinomycin (DTIC)] was superior to MOPP alone. Studies by Santoro and colleagues (1987), however, show that ABVD may be superior to MOPP, which may mean that another explanation for the observation is still necessary. Preliminary data presented by Canellos and associates (1992) suggest that ABVD is equivalent to alternating MOPP and ABVD, again emphasizing the difference in the two regimens rather than the superiority of the alternating approach.
Because of the promising results in Hodgkin's disease and the availability of many active agents, resulting in many possible non cross-resistant combinations to test, this method has been used frequently in the treatment of patients with SCLC. A review by Elliott and colleagues (1984) pointed out shortfalls in some of the trial designs but also showed few clearly positive studies. In a Canadian study, CAV therapy alone was compared with alternating CAV with etoposide and cisplatin for a total of six courses in previously untreated patients with extensive SCLC. In this large trial, Evans and colleagues (1987) observed a 6-week difference in median survival, and, as noted by Goodwin and co-workers (1988), the treatment was cost-effective. A second study in Canadian patients with limited disease compared three courses of CAV followed by three courses of etoposide and cisplatin with six courses of the alternating regimen. As reported by one of us (RF) and associates (1987), no difference in outcome was found. It may be that etoposide and cisplatin is a superior combination, which could explain the positive result obtained in the Canadian limited-stage study but not that of the extensive-stage trial. Other studies, such as those carried out by Roth (1992) and Fukuoka (1991b) and their associates, do not totally advocate the concept that alternating combination chemotherapy is superior to standard regimens, although Fukuoka's group showed a significant survival advantage to alternation in patients with limited disease. In the Roth study, survival of patients treated with only four courses of etoposide and cisplatin was the same as that of patients treated with longer courses of alternating regimens. A review by Havemann (1990) upholds the opinion that results
P.1714
in the literature are conflicting, and no clear superiority of alternating chemotherapy can be proved.
Some authors believe that the results from the Canadian study undertaken by Evans and colleagues (1987) indicate that alternating chemotherapy should be standard treatment, at least in extensive disease, but with etoposide and cisplatin added to CAV rather than the alternation, as suggested by Ihde (1992). The consensus of investigators worldwide is that the alternating approach is sound but not necessarily superior to other approaches, such as four to six courses of etoposide and cisplatin alone.
Duration of Chemotherapy
Until the middle or late 1980s, it was not unusual to treat patients with chemotherapy for a minimum of 12 to 24 months. Results of retrospective studies, including a large one from the University of Toronto undertaken by one of us (RF) and colleagues (1984), suggested no benefit to prolonged therapy. A large randomized trial carried out by Splinter (1988, 1989) and colleagues (1986) in the EORTC Lung Group revealed no benefit in survival, at least in patients with limited disease, although there may have been the suggestion of benefit in patients with extensive disease. Ettinger (1990) from ECOG also showed no benefit to maintenance therapy. An update of a French trial by LeBeau and co-workers (1991a) that also tested this theory does show a small survival benefit in maintained patients with SCLC. Few studies promote the use of prolonged therapy in this disease. An update by Girling (1991) of the Medical Research Council trial shows no benefit of six courses of therapy over three courses.
Hanna and colleagues (2001) reported the results of a Hoosier Oncology Group trial that assessed the usefulness of 3-month maintenance therapy with oral etoposide after induction treatment with etoposide, ifosfamide, and cisplatin. Maintenance therapy was well tolerated and resulted in prolongation of relapse-free survival, but no significant improvement in overall survival.
One of us (FAS) (2001) reported the results of a large NCIC and EORTC trial that showed that the administration of the matrix metalloproteinase inhibitor Marimastat after chemotherapy to responding patients also failed to result in either disease-free survival or overall survival. Furthermore, Marimastat was associated with considerable toxicity and poorer quality-of-life scores.
At this point, it seems that four to six courses of chemotherapy for patients who show a response should be sufficient, and presently available maintenance chemotherapy is of no added benefit.
New Drug Development
Clearly, one of the most important approaches in the treatment of SCLC is the procurement of new active agents for managing the disease as well as defining better ways of using presently available therapy. One has to reemphasize the concept of using new agents in previously untreated patients. This practice seems to be safe as long as a crossover design is used, with early crossover to an established regimen to avoid patients being too ill to receive potentially valuable treatment after waiting too long. Grant and colleagues (1992) also looked for lower response rates in previously treated patients in studies conducted from 1970 to 1990.
Although a large number of new agents have become available for testing in this disease, few look promising, but as emphasized in a review by Ihde (1992), new drugs should be sought to try to improve the survival of these patients. High-dose epirubicin has been found by a number of groups, including D. H. Johnson (1989), as well as Blackstein (1990), Banham (1990), one of us (RF) (1992), Eckhardt (1990), Wils (1990) and their associates, to be active in both SCLC and NSCLC. Although not used routinely, as noted by D. H. Johnson (1989), Thatcher and Lind (1990), and Grant and co-workers (1992), carboplatin has been established as an active agent in this disease. It is unclear whether it is quite as active as cisplatin, but it is certainly a reasonable alternative to prevent or reduce neurotoxicity and nephrotoxicity in selected patients at high risk (e.g., patients with preexisting kidney or hearing problems). According to D. H. Johnson (1989, 1990), ifosfamide is also active in SCLC and is relatively nonmyelosuppressive compared with cyclophosphamide. Loehrer (1996) has shown that VIP (ifosfamide combined with etoposide and cisplatin) is superior to etoposide plus cisplatin in patients with extensive SCLC. Expense, the required use of mesna, and its usual requirement for in-hospital administration all make ifosfamide a somewhat more difficult agent to use in SCLC than most other available active agents.
The only other agents that look promising at this stage of development are gemcitabine, paclitaxel (Taxol), and the camptothecin analogues, topotecan and irinotecan (CPT-11). According to Anderson (1991b) and Lund (1991) and their colleagues, gemcitabine is also active in NSCLC. Earle and associates (1998), in a phase I study of gemcitabine, cisplatin, and etoposide in the treatment of SCLC, found that the response rate was 54% to 75%. The recommended dose of gemcitabine was 800 mg/m2 intravenously on days 1 and 8. However, gemcitabine has not been evaluated in randomized trials to compare it with standard regimens. Paclitaxel has undergone extensive phase I and II studies that have been summarized by Bunn (1996). The dose-limiting toxicity is leukopenia. Ettinger and colleagues (1995) noted a 58% development of grade 4 leukopenia; other toxic effects included involvement of the liver, lung, and heart. Perez and co-workers (1996) suggested a dose of 150 mg/m2 intravenously over 3 hours. Greco and Hainsworth (1996) conducted a phase II study of paclitaxel, carboplatin, and etoposide with an 83% response rate and complete response rate of 24%. Median survival was 7 months for patients with extensive disease and 17 months for those with limited disease. Neill and co-workers (1997) reported a response in seven of eight patients when paclitaxel was added to the carboplatin and etoposide regimen.
P.1715
Gatzemeier and associates (1997) reported a complete response rate of 37.1% and a partial response rate of 51.4%. In 117 patients, Hainsworth and colleagues (1998) used paclitaxel, carboplatin, and extended-schedule oral etoposide plus radiation therapy and found that median survival rates in patients with limited and extensive disease compared favorably with other accepted chemotherapy regimens.
Paclitaxel has now been evaluated in randomized studies by several groups. Mavroudis and co-workers (2000) compared etoposide and cisplatin to etoposide, cisplatin, and paclitaxel with G-CSF. The study was stopped prematurely due to the toxicity of the paclitaxel-containing arm without any evidence of superior efficacy. The CALGB also conducted a randomized trial of etoposide and cisplatin compared to etoposide, cisplatin, and paclitaxel with G-CSF. No survival benefit was seen with the addition of paclitaxel, and fatal toxicity was twice as high (Dr. Mark Green, personal communication).
A randomized trial in which paclitaxel has been combined with etoposide and carboplatin rather than cisplatin has also been undertaken in Germany, but final results are not yet available. However, an American group has presented preliminary results of a trial of etoposide and carboplatin with and without paclitaxel. With less than 90 patients in each arm, Birch and co-workers (1997, 2000) reported that response rates and survival were slightly, but not significantly, better for the paclitaxel arm.
Another drug that has demonstrated considerable activity against SCLC is topotecan, as reported by Schiller (1997). Topotecan is a topoisomerase I inhibitor that leads to a break in the DNA strand resulting in apoptosis and cell death. Lilenbaum (1998) and Kollmannsberger (1999) and their associates reported phase I studies evaluating dosage and toxicity of this potent antitumor agent. Topotecan has been assessed in both the first-line and the second-line setting for SCLC. D. Johnson and colleagues (2000) reported that the administration of topotecan following etoposide and cisplatin in a large ECOG trial resulted in a significant prolongation of progression-free survival time (3.4 months vs. 2.3 months, P = 0.0001) in patients with extensive SCLC. However, there was no improvement in overall survival. Quoix and co-workers (2001) reported preliminary results of a European phase II trial that compared topotecan and cisplatin to etoposide and cisplatin. The overall response rate was 57% in both arms, but final survival data were not presented. In the second-line setting, topotecan has been compared with the combination of cyclophosphamide, doxorubicin, and vincristine. Von Pawel and associates (1999) reported that topotecan resulted in a higher response rate (24.3% vs. 18.3%, P = 0.285), but median survival times and overall survival were the same for both treatments. Symptom improvement was slightly better for topotecan-treated patients. However, myelosuppression, in particular thrombocytopenia, was greater with topotecan.
Masuda and co-workers (1992) also reported activity in a small group of 16 refractory patients (47%) with another new agent, CPT-11. This led subsequently to a Japanese trial of irinotecan and cisplatin compared with etoposide and cisplatin in patients younger than 70 years with extensive SCLC. The results of this trial, which was stopped prematurely due to extreme results, have been reported by Noda and associates (2000) on behalf of the Japan Clinical Oncology Group. With only 77 patients in each arm, a significant difference in survival was seen in favor of the irinotecan arm (median 420 days and 1-year survival rate of 60% vs. median 300 days and 1-year survival of 40%, P = 0.0047 log rank). Several trials of similar design using different doses and schedules of irinotecan, etoposide, and cisplatin are about to open in North America and Europe in an attempt to confirm the Japanese results.
Other chemotherapeutic agents that have been evaluated for this disease include vinorelbine and docetaxel (Taxotere), as noted by Ariyoshi and Sugiura (1994). However, these drugs have not demonstrated superiority over other agents and so they are not used routinely in the first-line setting. In most parts of the globe, etoposide with either cisplatin or carboplatin remains the standard of care. Figueredo (1990) and Milroy (1991) and their colleagues have found that agents that attempt to bypass established drug resistance, such as verapamil, have to date not proved to be clearly helpful in patients with SCLC.
RADIATION THERAPY
Radiation therapy has been an important method of treatment of SCLC for many years, and it continues to play a vital role in the management of this disease. It has not been recommended as sole therapy since the British 5-year follow-up trial conducted by Miller and colleagues (1969) and the 10-year follow-up trial undertaken by Fox and Scadding (1973) and Fox and co-workers (1980, 1981) comparing it with surgery 30 years ago. It is, however, widely used in combination with chemotherapy for curative approaches and for palliation, when it is usually given alone. The last decade, in particular, has seen advances in the knowledge about radiation therapy in SCLC and its administration, both with and without chemotherapy. In this discussion, the role of radiation therapy in the routine management of the SCLC patient is considered and areas of recent progress are identified, emphasizing controversies in management and future directions of research in this field.
Radiation and Combined-Modality Therapy
The absorption of radiation by cells results in reproductive cell death in a proportion of the cell population. As discussed by Hall (1988), this proportion increases exponentially with dose, except for doses smaller than approximately 2 to 3 Gy, for which cell killing is somewhat less efficient. Thus, the cycling population tends to be progressively reduced as treatments are continued or the dose is increased. Malignant cells
P.1716
are distinguished by their ability to reproduce indefinitely and often rapidly; they therefore tend to be susceptible to this type of injury. When cell populations can be reduced to relatively low numbers by irradiation, host defenses may eliminate surviving cells, resulting in cure. However, normal tissues may also be injured through depletion of the stem cells of various tissue populations, resulting in chronic tissue damage. Ideally, one seeks to maximize tumor cell kill while minimizing normal tissue damage. In practice, cells may survive the irradiation for various reasons, including intrinsic radioresistance, technical error, or underdosage because of the need to protect critical structures.
The response of a tumor, in terms of volume change measured at some time after treatment, is not an important end point of treatment. It may be influenced by the intrinsic cell kinetics (rapidly cycling tumors may respond quickly, then regrow with similar speed), the proportion of cells actively cycling, and the response of associated tissues. More important end points of treatment after suitable periods of follow-up are locoregional control of tumor, survival without relapse, and overall survival.
In determining the radiation dose to be delivered to the intended volume, past experience of tissue tolerance to irradiation is of great value. Tolerance in general depends on the tissue type, notably its normal cell kinetics (rapidly proliferating epithelial tissues differ from slow or nonproliferating vascular, connective, or nervous tissues), the volume irradiated, and the presence of other conditions, such as diabetes. It was determined long ago that radiation therapy is more effective when administered over multiple treatment sessions, or fractions. According to Thames and Hendry (1987) and Arriagada and colleagues (1989), the radiobiological reasons relate to cellular repair, oxygenation of the cellular environment, and cell cycle responses, which are beyond the scope of this discussion. Many years of clinical-biological experience and insights have led to the development of dose fractionation schemes that tend to enhance antitumor effect while preserving, at least relatively, vital tissues. Important determinants of biological effect are the total dose, the dose per fraction (individual treatment session), the number of fractions into which the total dose is divided, the frequency of fractions, and the overall time required to administer all of the prescribed dose.
In particular, the understanding of the role of fraction size has advanced greatly since the late 1980s, as discussed by Fowler (1989) and Withers (1988). Many critical normal tissues, such as the nervous system and the microvasculature that supports function in all tissues, are characterized by cells that regenerate over long cycle times (months or years). Their response to radiation injury is related to this regeneration time. These tissues tend to manifest clinical damage after a latent period of months and slowly progress over a time scale of years. These changes are the so-called late effects seen in irradiation (e.g., myelopathy), in contrast to the acute effects of tissues that normally regenerate rapidly (e.g., mucosa) but are correspondingly rapidly depleted, hence manifesting injury early, such as with mucositis. Important investigations have shown that these late effects are sensitive to the size of the dose per fraction. The acutely responding tissues are less sensitive, and hence the severity of late injury cannot be predicted from the degree of early injury seen at or shortly after the time of treatment. Because the late effects on normal tissues are usually those that limit the ability to deliver radiation dose to the tumor, it should be possible to increase the biological dose to the tumor while continuing to respect the tolerance of critical normal tissues by designing regimens with smaller doses per fraction. A related observation suggests that small cell cancer cells may be particularly susceptible to small doses per fraction, which would also be tolerable by late-responding tissues. These insights from the 1980s are being subjected to clinical testing in a number of areas, usually with positive or promising results. These ideas may have profound effects on radiation therapy practice in the future.
In a discussion of the concepts of dose, time, and fractionation, it is useful to define certain terms that relate to various schedules of fractionation (i.e., the partitioning of the total radiation dose into individual treatment sessions or fractions) (Table 114-10). In a course of treatment, the fractions are usually, but not always, of equal dose. Fractionation is described as conventional when it is given in a single daily fraction of 1.8 to 2.0 Gy per day, 5 days per week. Typical courses given with curative intent administer a total of 60 to 65 Gy over 6.0 to 6.5 weeks using this fractionation. Treatment is termed hypofractionation when fraction sizes larger than 2 Gy (typically 3 to 8 Gy) are given, usually on a less frequent basis, such as once per week. An example might be 5-Gy fractions given once weekly for 12 weeks to a total of 60 Gy. Hyperfractionation, a more common unconventional program, refers to smaller fraction sizes, usually 1.0 to 1.5 Gy, which are typically given two or even three times per day, again normally 5 days per week. Small fraction sizes confer biological advantages, but for similar antitumor effect the total dose usually must be increased over a comparable conventional schedule, and the number of individual treatments then becomes larger. This means that multiple fractions per day must be given to maintain the overall time of treatment similar to that of a course of conventional fraction size. Administration of radiation therapy is termed accelerated when the total dose is delivered in a shorter overall time. Accelerated administration may be achieved by using hypofractionation techniques, by using multiple fractions per day and conventional fraction sizes, or by treating the patient 7 days per week. Some experimental regimens may incorporate more than one of these approaches.
Table 114-10. Typical Methods of Radiation Therapy Fractionation | |||
|---|---|---|---|
| |||
The manner in which chemotherapy and radiation therapy are combined temporally may have important effects on the outcome. Arriagada and associates (1988) recommend that the integration of the two methods be described as follows. When radiation therapy is given on days of the treatment cycle on which no chemotherapy is administered, without any delay in chemotherapy beyond that which would normally occur if chemotherapy were being given alone, is called alternating therapy. If chemotherapy and radiation therapy occur separately in time, therapy is sequential
P.1717
(e.g., chemotherapy followed by radiation therapy, or a delay in chemotherapy administration to permit delivery of radiation therapy). Finally, chemotherapy and irradiation may be administered concurrently, with both treatment methods given on the same day for some or even all of the cycle (Table 114-11).
Table 114-11. Combined-Method Radiation and Chemotherapy Regimens | ||
|---|---|---|
|
Clinical Considerations
Small cell lung cancer typically presents with locally advanced disease not suitable for surgery. The greatest burden of disease is found in the thorax, which requires a correspondingly intense locoregional therapy, but disease confined only to the thorax is considered potentially curable. Cancer in the lymph nodes of the mediastinum often indicates systemic disease, which is either occult or obviously evident by current clinical methods. The simple classification of limited disease (tumor confined to one hemithorax, mediastinum, or ipsilateral supraclavicular fossa, or both, but without malignant effusion) or extensive disease (metastatic disease, after standard staging procedures) has proved useful in identifying a group of potentially curable patients with SCLC. As already discussed, these definitions may vary in detail between different studies, which may influence results. Radiation with curative intent is usually given only to patients with limited disease.
The task of the radiation oncologist is to assess the patient and the tumor, determine appropriate therapy in light of available alternatives, and, if radiation therapy is indicated, prescribe and deliver an appropriate dose of irradiation to the tumor while minimizing the dose to normal tissues. This approach exploits the technical ability to focus radiation therapy beams on the tumor tissue and thus achieve a therapeutic advantage. For this reason, radiation oncologists make extensive use of advanced imaging techniques, including CT scanning, supplemented by computerized dose calculations and diagrams of distribution of radiation dose within the tissues.
The primary decision after assessment of the patient is whether to offer treatment with palliative or curative intent. For SCLC, radiation therapy is usually given in association with chemotherapy, although various temporal relations are possible. A target volume for treatment is determined from the diagnostic studies available, such as the chest radiograph and CT scans, and includes the known disease extent and the possible routes of spread, including the primary tumor and some or all of the mediastinal lymph nodes. The dose fractionation scheme reflects the goal of treatment and the tolerance of other organs. The target volume is identified radiologically at the time of treatment simulation, and skin reference points are established. A CT scan of the thorax in the treatment position permits visualization of the target volume in conjunction with treatment beams and computerized dose distributions. Shielding of nearby organs is implemented. The final step is verification by making radiographic portal images from each therapy beam, using the therapy treatment machine.
Thoracic Irradiation in Limited Disease
Early reports by Watson and Berg (1962) of radiation therapy for this disease demonstrated its tendency to respond dramatically ( melt away ), only to recur in a short period, often at a distant site. Subsequently, Bergsagel and co-workers (1972) noted the activity of chemotherapy in this disease, which led to two decades of investigations into combined-method treatments.
The best results for limited disease are obtained using a carefully integrated regimen of chemotherapy and thoracic irradiation. Ample evidence shows that radiation treatment produces objective responses, but its impact on survival has been difficult to demonstrate. The simple question of the benefit of thoracic irradiation combined with chemotherapy compared with administration of the same chemotherapy alone has been studied in many randomized trials. The drug regimens were different among the various trials, as were the radiation therapy details of dose, fractionation, and sequencing with respect to the chemotherapy. Most of the studies were considered to be negative in that they did
P.1718
not report a survival advantage for patients treated with thoracic radiation. However, trials may be falsely negative by chance, due to a low statistical power of finding a positive result when it truly exists; this is especially true of smaller trials. Some of the more recent larger trials showed a modest survival benefit, suggesting that sample size may have influenced the interpretation of the earlier studies.
A set of randomized trials may be analyzed collectively by the method of meta-analysis, as undertaken by Sacks and colleagues (1987), which analyzes the set of trial results together and effectively increases the chance of identifying a difference that exists but is modest in size. A meta-analysis by Warde and one of us (DP) (1992) of 11 SCLC trials (with adequate reporting, consisting of 1,911 randomized patients) was undertaken to determine the distribution of outcomes of these trials, such as improvements in survival rates and local control rates when thoracic radiation was added to systemic chemotherapy. The trials conducted by Bunn (1987), Osterlind (1986), Kies (1987), Ohnoshi (1986, 1987), Souhami (1984), Birch (1988), Perry (1987), Creech (1987), Nou (1988), Carlson (1991), and Rosenthal (1991) and their colleagues are summarized in Table 114-12. The additional study by Rosenthal and co-workers (1991) shows a trend to prolonged survival in the irradiated group, reporting 10-year follow-up of a study completed in 1979. The meta-analysis showed a modest but significant improvement in 2-year survival of approximately 5% to 6% (from approximately 17% to 23%) and a larger effect of approximately 25% (from approximately 23% to 48%) in local control at 2 years, associated with a small increase (1%) in treatment-related mortality. A more detailed meta-analysis by Pignon and colleagues (1992) as well as Joss (1985) and LeBeau (1991b) and their associates, with additional verification and follow-up, has been published and confirms these conclusions. These meta-analyses confirm, therefore, that the addition of thoracic irradiation contributes significantly to survival for patients with limited SCLC; it is now considered standard for all patients.
Table 114-12. Randomized Trials of Thoracic Irradiation with Chemotherapy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the meta-analysis of Pignon and colleagues (1992), it was noted that only younger patients achieved longer survival with radiation therapy. In fact, the addition of irradiation to chemotherapy in patients older than 70 years resulted in poorer survival, such that the overall risk of death actually increased. Quon and associates (1999), who reviewed the results of two NCIC trials of combined-modality therapy for limited SCLC, did not observe a negative impact of age. In their review, response rates, local relapse rates, and survival were the same for patients younger than and older than 70 years. Furthermore, the elderly patients did not experience greater early or late toxicity from radiation therapy, nor did they require more frequent treatment delays or longer time to complete treatment.
A large three-arm study of 399 patients reported by Perry and associates (1987) assessed the importance of adding radiation therapy to chemotherapy as well as the timing of radiation in relation to the chemotherapy treatment course. The chemotherapy was CAV, used every 21 days for up to 18 months. The thoracic radiation therapy was 50 Gy in 2-Gy fractions (40 Gy to the primary and mediastinum followed by 10 Gy to the primary, given after chemotherapy during either the first cycle, the fourth cycle, or not at all). All patients received elective brain irradiation to a dose of 30 Gy in 10 fractions. This study showed a doubling of the 2-year disease-free survival rate from 15% to 30%, and a doubling of local control from 26% to 58% (first failure in the chest) in favor of those patients receiving thoracic irradiation. In an update, Perry and associates (1998) reported that the advantage of the combined-modality regimens was maintained in 10 years of follow-up. A second study from the NCI by Bunn and co-workers (1987) randomized 74 patients to receive irradiation, 40 Gy in 15 fractions over 3 weeks given concurrently with the first cycle of CAV. This regimen resulted in an unacceptable mortality rate from pulmonary toxicity. In spite of this negative finding, a small but statistically significant survival benefit was seen for patients in the combined-method treatment arm. Lethal toxicity was
P.1719
not reported, however, for the combination of etoposide and cisplatin administered concurrently with thoracic irradiation even in elderly patients as reported by Quon and co-workers (1999) for the NCIC.
Why were the results of the early combined-modality trials so modest that meta-analysis techniques were required to demonstrate a survival advantage for the addition of thoracic irradiation? First, the chemotherapy used in many of these trials was not optimal by today's standards and did not always contain active agents, such as cisplatin, etoposide, or doxorubicin. Second, the radiation therapy may have been suboptimal with regard to dose, fractionation, or timing with respect to chemotherapy. Only one study of randomized doses of irradiation, undertaken by Coy and the NCIC (1988), has been attempted; the doses used in that trial designed almost 20 years ago are now considered to be low, and furthermore, they were not sufficiently different to determine an optimum. Optimal volume prescriptions are unknown; however, Liengswangwong and colleagues (1994), in a retrospective analysis from the Mayo Clinic, concluded that use of postchemotherapy volumes in treatment planning did not increase the risk of marginal failures. This issue becomes less relevant with the use of early concurrent therapy regimens. Further advances may be anticipated as these problems are addressed. In fact, as systemic therapy improves, the control of local disease to achieve overall cure becomes ever more important. It is accepted, however, that thoracic irradiation, carefully integrated with chemotherapy, has well-demonstrated benefits by the standards prevailing in lung cancer therapy and is well tolerated. It should therefore be adopted as proper routine management for the patient with limited SCLC.
Innovations in Combined-Modality Approaches
New insights from the clinic and from biology have suggested innovations, including timing of radiation therapy with respect to chemotherapy, the use of small doses per fraction, and the intercalation of chemotherapy and irradiation.
In Canada, Goldie and associates (1988) used drug-resistance data and mathematical modeling to develop the hypothesis that using multiple anticancer agents of different mechanisms of action so as to be non cross-resistant to each other was required to prevent the emergence of resistant cell clones during treatment. This suggested the clinical hypothesis that concurrent early administration of multiple agents might be advantageous. There have been several formal tests of this concept. Murray and colleagues (1991a, 1993) of the NCIC randomized 308 patients to receive thoracic irradiation (40 Gy in 15 fractions) administered concurrently with etoposide and cisplatin either early (with the second cycle of chemotherapy) or late (with the sixth cycle of chemotherapy). The early administration of radiation therapy significantly improved survival at 5 years from 13% to 22%, compared to giving it 12 weeks later. This finding suggests that the cancer may be developing drug-resistant cell clones within the chest, which can then metastasize to distant sites if radiation therapy is delayed or withheld. The tumor thus needs an aggressive early approach with all methods of treatment, including radiation therapy. On the other hand, the CALGB trial reported by Perry and associates (1987, 1998) included a randomized comparison of thoracic irradiation given with either the first or fourth cycle (a 9-week delay) and found no effect of the delay on survival. Delivery of chemotherapy was compromised by toxicity in the early-radiation-therapy arm of this study, which may account for the result. Another negative trial from Denmark by Work and colleagues (1997) randomized 199 patients to early radiation (40 to 45 Gy in split-course fashion, not concurrent with chemotherapy) or similar treatment 18 weeks later. The chemotherapy doses and nonoptimal radiation therapy administration raise questions about this trial. More recently, trials from Japan and Yugoslavia have both found 5-year survival rates in the range of 30% compared with approximately 15% in favor of early concomitant administration of radiation therapy and chemotherapy. The Japanese trial of Takada and associates (1996) used twice-daily fractions of 1.5 Gy, as did that of Jeremic and colleagues (1997). In the Jeremic study, the radiation therapy regimen was 54 Gy in 18 days over 3.6 weeks, commencing on day 1 or day 43 (a 6-week delay). All patients received elective brain irradiation. Both local control and survival rates were superior with the early combination. Finally, a small Greek trial reported by Samantas and colleagues (2000) compared early (with the first chemotherapy) versus late (with the fourth chemotherapy) twice-daily chest irradiation given concurrently with etoposide and carboplatin, and found no difference in response rates or survival. However, when reviewing all the trials, it appears that in a well-staged population of patients with limited disease and good prognostic factors, there may be an advantage to early administration of radiation therapy. This practice, although not universal, is becoming widespread.
The shape of the cell survival curve for irradiated SCLC suggests sensitivity to irradiation with small doses per fraction. Because a large number of small fractions is required to obtain adequate total dose, regimens of hyperfractionation (1.5 Gy given two fractions per day, to 45 Gy total) have been evaluated in the United States by Turrisi and Glover (1990). Administration of this form of chest radiation therapy was concurrent with the first cycles of chemotherapy (cisplatin and etoposide). Similar pilot studies recorded by Mornex and co-workers in France (1990) and B. E. Johnson and colleagues (1991a, 1991b) at the NCI were promising, with a 2-year survival rate of approximately 44% and a low rate of local failure (less than 16%). As a result, Turrisi and associates (1999) carried out a large randomized trial of the hyperfractionation approach. A total of 417 patients were treated with four cycles of chemotherapy (cisplatin and etoposide) together with several weeks of daily (five treatment days per month) chest irradiation, commencing on the first day of chemotherapy. They were randomized to receive 5 weeks of 1.8-Gy fractions given
P.1720
daily (total 45 Gy, 25 fractions) or 3 weeks of 1.5-Gy fractions given twice daily, 6 hours apart (total 45 Gy, 30 fractions). Treatment was safe and well tolerated, although esophagitis was more severe in the twice-daily group. The median survival times were similar in the two groups (19 and 23 months), but a significant advantage in survival emerged in favor of the twice-daily group (P = 0.04). With a median follow-up of 8 years, the survival advantage is 26% versus 16%, associated with significant reductions in both local and distant failure. This suggests that an important subgroup of potentially curable patients exists who may be more responsive to a strategy of hyperfractionation.
After a study by Seydel and associates (1983), the desire to integrate all methods early in the course of treatment while controlling toxicity prompted Arriagada and colleagues (1985) of the Institut Gustave Roussy group to develop the alternating method. This approach has experimental support in observations by Looney and co-workers (1985) that the radiation therapy, if added after chemotherapy at approximately the time of chemotherapy-induced tumor repopulation between cycles, could produce tumor control that was not possible with either method alone. The clinical trials followed the general chemotherapy (CT) and radiation therapy (RT) schema CT-CT-RT-CT-RT-CT-RT-CT-CT-CT in which - indicates a 1-week interval. In a series of trials with somewhat varying doses but using the same overall scheme of alternating therapies, Looney and colleagues also achieved 3-year disease-free survival rates in the range of 20% to 30%. Gregor and associates (1997a) tested this approach in a randomized clinical trial for the EORTC. They gave 50 Gy in 20 fractions at the end of a course of chemotherapy (cyclophosphamide, doxorubicin, and etoposide) as the sequential arm, compared with an alternating arm that integrated 10 Gy in four fractions over 1 week alternating with the same chemotherapy over four treatment cycles. This direct comparison of alternating and sequential approaches randomized 335 patients, but there was no evidence of superiority of either approach. Furthermore, the alternating regimen was characterized by severe hematologic toxicity, which compromised delivery of the treatment.
A logical extension of this approach combines the hyperfractionation procedure with the alternation of chemotherapy and radiation therapy. In the United States, the ECOG in a small trial conducted by D. H. Johnson and co-workers (1991) gave cisplatin and etoposide chemotherapy in four cycles of 3 weeks each. In each of the first three cycles, 1 week of hyperfractionated thoracic irradiation was administered (1.5 Gy twice daily to 15 Gy total in 1 week). In a similar fashion, Arriagada and colleagues (1991b) of the Institut Gustave Roussy group in Paris used doxorubicin, etoposide, cyclophosphamide, and cisplatin, together with three intercalated courses of radiation therapy. They gave the first irradiation doses as 1.4 Gy three times per day to a total of 21 Gy. Mornex and associates (1990) of the Lyon group reported on their experience with an alternating bifractionated regimen. No large randomized trial experience is available, and so definite conclusions about this approach cannot be made.
Because SCLC is very radiation sensitive, doses in the range of 45 Gy have traditionally been used for thoracic irradiation. However, at this dose, even when irradiation is given concurrently with chemotherapy, relapse at the primary site is seen in one-third or more of patients with limited-stage tumors. In an attempt to reduce this high failure rate, several investigators have evaluated higher doses of thoracic irradiation, increasing the dose to levels that are used for non SCLC. Choi and colleagues (1998) showed that the maximum tolerated dose in SCLC patients is 70 Gy given once daily with concurrent etoposide and cisplatin. Five of 19 patients suffered a local relapse. These investigators suggested that a trial to compare higher doses of standard daily radiotherapy to twice-daily radiotherapy would be of interest, but to date, such a trial has not been undertaken. However, despite the lack of randomized trial data, many centers in the United States now administer higher doses of thoracic irradiation, in the range of 60 to 65 Gy.
All regimens that combine radiation and chemotherapy, particularly when closely associated in time, are somewhat more toxic than single-modality or sequential protocols, and most pilot studies of these approaches revealed some severe morbidity and even mortality. In a meta-analysis of 11 randomized trials by Warde and one of us (DP) (1992), the addition of thoracic irradiation therapy produced a significant but small increase (1%) in treatment-related fatality. This change was substantially less than the overall survival gain produced by this treatment. However, it is important to note that a review of 88 elderly patients (age 70 years or older) in combined-modality trials of the NCIC reported by Quon and associates (1999) showed that their compliance with, tolerance of, and response to the thoracic irradiation was similar to that of the younger patients. Thus, potentially curative therapy should not be withheld from elderly patients purely on the basis of age.
In summary, combined-method therapies in patients with limited disease produce modestly superior rates of survival and tumor control in the chest, with some increase in toxicity associated with the concurrent administration of both methods. However, both theoretical considerations and clinical results support the use of early and concurrent combinations. Chest irradiation appears to be well tolerated when given concurrently with etoposide cisplatin regimens, but extensive concurrent experience with newer agents is not yet available. The use of a small dose per fraction seems to be beneficial, even if this means giving multiple fractions per day. In the only large randomized test so far, this method showed a significant improvement in survival at 5 years. The issue of total dose has not yet been addressed in large randomized studies.
No reason for complacency exists. Arriagada and colleagues (1991a) have shown that local failure is still frequent, with 33% of failures being local only, 25% distant
P.1721
only, and 9% local and distant simultaneously. The state of current practice with respect to radiation therapy and the directions of future research are evolving. Patients with limited SCLC should be referred to radiation oncology centers that conduct research, if possible. Alternatively, they should be referred to the local radiation oncologist for an opinion on thoracic irradiation, preferably at the outset of chemotherapy administration.
Elective Brain Irradiation
Elective brain irradiation is the practice of irradiating the entire brain electively on the principle that the patient is at risk for the development of brain metastases even if clinical tests do not demonstrate them. It is also known less accurately as prophylactic cranial irradiation, or PCI. Elective irradiation is recommended because chemotherapy alone often does not prevent the development of symptomatic brain metastases in approximately 25% of patients. Tumor cells in the brain sanctuary may be protected from chemotherapy by physiologic factors peculiar to the CNS. Kristjansen (1989) reported that this brain failure rate may be reduced to 5% if elective irradiation is given. This policy, however, results in approximately 75% of patients receiving a treatment that they may not need and that may be associated with toxicity. It is important to remember that the results from clinical trials depend on factors such as the duration of follow-up, the criteria required for diagnosing relapse, and whether isolated brain relapses are distinguished from those occurring in the setting of failure at multiple sites. Investigations tend to be performed reluctantly in terminally ill patients, so such data are not readily available.
The incidence of central nervous system metastases and the results of trials of elective whole brain irradiation (Table 114-13) are remarkably similar across studies, as reported by Jackson (1977), Cox (1978, 1980), Beiler (1979), Maurer (1980), Hansen (1980), Eagen (1981), Katsenis (1982), Seydel (1985), and Aroney (1983) and their colleagues. Furthermore, larger trials from groups in France, as reported by Arriagada (1995, 1998) and Laplanche (1998) and their colleagues, and elsewhere in Europe, as reported by Gregor and associates (1997b) (Table 114-13), have confirmed these earlier conclusions. The trials by Gregor (1997b) and Arriagada (1995) and their coauthors, however, included prospective neuropsychiatric evaluations and found no significant adverse effects in the treated group compared with the untreated patients. An interesting literature analysis by Suwinski and colleagues (1998) suggested that a threshold in dose response for prevention of brain relapse was evident if treatment was delayed. They suggested that optimum control is achieved with doses of 30 to 35 Gy at 2 Gy per fraction, administered early in the overall treatment regimen. The results of the trials of elective whole-brain irradiation for SCLC were very similar to those of thoracic irradiation in that local brain relapse was usually reduced by half by the administration of radiation. However, survival was never prolonged significantly. In an attempt to assess the impact of brain irradiation on survival, a large meta-analysis was undertaken and published by Auperin and colleagues (1999) on behalf of an international collaborative group. This analysis showed that elective brain irradiation was associated with an improvement in survival rate at 3 years from 15.3% to 20.7%.
Table 114-13. Randomized Trials of Elective Brain Irradiation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Advocates argue that the morbidity of a brain relapse compared with that of the treatment justifies the practice and improves overall quality of life in SCLC patients. The degree to which patients are actually harmed by the procedure remains controversial, however, as does the optimal dose. In an elderly, tobacco-abusing group, prior neurologic morbidity frequently exists, and, as suggested by Lishner and co-workers (1990), can cloud the interpretation of subsequent events. Whether long-term toxicities are a serious and typical problem is a controversial issue. The Toronto experience with PCI in SCLC, as reported by Lishner and colleagues (1990), has been relatively positive, with a low incidence of notable abnormalities not unlike those found in patients who do not receive PCI. The same issue of the Journal of Clinical Oncology in which the Lishner study
P.1722
appeared includes a study by Fleck and associates (1990) from Indiana University with extremely grave long-term complications. In a companion editorial, Turrisi (1990) endeavors to account for this result, and indicates that it may be associated with the fractionation schemes used.
Many questions in this area are unanswered. It is not known whether treatment failure occurs as a result of inadequate treatment of occult metastases or as a result of reseeding of the brain by cells from a persistent primary or other metastatic sites. Some clinicians believe that only patients exhibiting a complete response to treatment have the potential for cure and hence benefit from elective irradiation of a brain sanctuary site. One trial randomized only such patients, but other randomized trials have included patients with partial responses.
An alternative approach is to offer palliative therapeutic brain irradiation to patients only if and when they exhibit relapse in the brain. This treatment is generally thought to produce symptomatic improvement in up to 70% of patients. The available studies are retrospective, however, and have the methodologic difficulties of addressing this population, particularly in assessing their quality of life, so it must be stated that reliable data on valid end points of treatment are not available. Nevertheless, some clinicians continue to argue that a policy of therapeutic brain irradiation given only at the time of relapse would spare approximately 60% of responding patients from brain irradiation that was of no benefit to them because they were not destined to relapse in the brain in any case.
On the other hand, the elective administration of cranial irradiation may prolong the duration of life without brain metastases, and because these lesions usually cause significant morbidity, this may be a worthwhile goal. Judging whether it is worthwhile depends on the morbidity of the treatment itself. In dose ranges from 20 to 40 Gy, using fraction sizes from 2 to 4 Gy, patients may experience nausea, headaches, and, occasionally, vomiting along with alopecia and scalp erythema. The use of 20 Gy in five fractions produced nausea in up to 15% of patients, but this effect may be controlled by steroids and antinauseants. Of greater concern are the long-term effects on the neurologic status of survivors. Clinical and anatomic lesions evident by CT have been noted. In general, these patients are in the seventh decade and have had many years of tobacco use. Neurologic problems may thus be attributed to coexisting morbidity or to cytotoxic therapies, chemotherapy, or irradiation. A retrospective review of late survivors by Lishner and colleagues (1990) revealed that most observed neurologic problems had other plausible explanations besides the radiation therapy; indeed, some occurred in nonirradiated patients. This and other studies are not adequately controlled, however, and cannot be considered definitive. As noted, more recent prospective randomized trials with neurologic and neuropsychiatric evaluations by Arriagada and colleagues (1995) and the EORTC, as reported by Gregor and associates (1997b), strongly suggest that the concerns about adverse effects of elective brain irradiation have been overestimated. Furthermore, many of the cognitive defects identified in the study patients were present before the administration of brain irradiation.
At present, elective brain irradiation is standard at many centers, although certainly not all. Some centers offer treatment of complete responders only as an option and therapeutic brain irradiation for all others at the time of relapse; this policy is probably also widespread. On the other hand, the trials of the late 1990s have led to general acceptance that elective brain irradiation for complete responses with limited disease is appropriate treatment. This topic has been reviewed thoroughly by Kotalik and colleagues (2001) in a recently published practice guideline of Cancer Care Ontario on PCI.
Radiation Therapy for Patients with Extensive Small Cell Lung Cancer
When small cell cancer is metastatic at diagnosis, the prognosis is poor, with a median survival of 8 to 10 months. The amount of disease is usually great and difficult to control at multiple sites. The Medical Research Council (1991) reports that radiation therapy for extensive disease should, therefore, be directed only at symptomatic sites or those not controlled by chemotherapy. The palliative benefit is often good, especially for bone pain, venous obstruction, cerebral metastases, and so forth. Elective irradiation of chest or brain would normally be considered only for the rare complete responder to chemotherapy or for patients who responded completely at distant sites and who had only minimal residual thoracic disease. Because of the poor prognosis, no active investigation of the role of radiation therapy in extensive disease has been undertaken. The effort must await the development of more effective systemic chemotherapy, which, by prolonging survival, might demonstrate a more compelling need for aggressive local palliation.
Radiation for the Palliation of Small Cell Lung Cancer
The lung cancer patient has a wide variety of symptoms caused by the tumor or its metastases. Common symptoms of the primary tumor are hemoptysis, cough, dyspnea (especially if obstruction of significant airways exists), chest pain, hoarseness from vocal cord palsy, and sometimes SVC obstruction. Systemic effects are weight loss, anorexia, lethargy, and anemia. Localized metastases may produce bone pain, mediastinal compression with venous obstruction or esophageal compression, brain involvement with neurologic deficit, and spinal cord compression leading to paralysis. Radiation therapy often benefits patients because it can be directed to the site of the problem without causing side effects outside the field, and it is simple and painless to deliver, as described by Barkley (1986). Table 114-14
P.1723
illustrates the expected response rates for various symptoms based on a retrospective evaluation by Slawson and Scott (1979). Trials undertaken by Grafton (1991) and Simpson (1985) and their colleagues randomized patients to different dose levels in an attempt to assess the need for long treatment regimens in patients with a short expected survival. Results of these trials have shown little advantage to longer schedules, but the assessment of benefit in such trials poses many difficulties, and treatment decisions are usually individualized. The short overall survival of these patients means that duration of response is an important consideration, and modern trials now try to assess this interval. However, several randomized trials done by the Medical Research Council in the United Kingdom (1991, 1992, 1996) have consistently shown the usefulness of short, convenient fractionation schedules.
Table 114-14. Relief of Symptoms in 330 Patients with Metastatic Non Small Cell Lung Cancer Using Radiation Therapy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Major airway obstruction is an important problem, particularly for tumors involving the carina or trachea, especially after relapse. Whenever possible, endobronchial tumors should be treated by endoscopic d bridement by mechanical or laser methods. New therapy technology permits endoscopic placement of a catheter across the area of involvement. A wire provided with a 2.5-mm radioactive source at its tip may then be inserted. This source has high activity and delivers its dose rapidly to the region of the tumor. By retracting it in a series of 2.5-mm steps, under precise computer control of both position and duration, any desired portion of endobronchial wall can be irradiated selectively in a brief outpatient procedure using local anesthesia. Early experience with this device, as noted by Grafton and colleagues (1991), is promising, although the technique does not treat the deeper extent of the tumor. Endobronchial treatment modalities are discussed in Chapter 108.
Bone lesions from metastatic cancer are an important cause of morbidity in cancer patients and a frequent challenge to the radiation oncologist. Their frequency in lung cancer, their tendency to disable rather than kill, and their presence in patients refractory to therapy pose significant management problems. In addition, they are a common problem in patients who already have other lesions and a poor prognosis. The ability of irradiation to reach specific local sites with minimal adverse effects has made it an attractive mode of therapy in these situations. Although irradiation remains a mainstay of the palliative care of these patients, important questions remain about its optimal use. The major indications for irradiation of bone metastases are the need to: (a) alleviate or abolish pain, (b) prevent impending fracture or treat malignancy in conjunction with orthopedic fixation, (c) maintain activity and function, and (d) prevent or alleviate compression syndromes, especially of the spinal cord. Prolongation of survival is not usually a realistic goal of therapy.
Findings of prospective and retrospective studies suggest that common regimens ranging from 8 Gy in 1 fraction to 40 Gy in 20 fractions produce improvement in 70% to 80% of patients and result in sustained relief in 60% of patients surviving 1 year. A series of randomized trials undertaken by Price and colleagues (1986) showed that most bony sites can be treated effectively with a single dose of approximately 8 Gy. More fractionated courses do not appear to confer additional benefit. Many radiation oncologists prefer to administer larger doses to bones that bear weight or are close to the spinal cord, in the belief that patients should have the benefit of the small improvement in disease control that might arise from a higher dose.
Although maximal pain relief is usually achieved by 3 months, the time of onset of relief varies considerably, and the precise mechanisms involved in relief of pain are uncertain. Because late normal-tissue effects are not usually a limiting consideration, experience with small-dose-per-fraction, multiple-fraction-per-day regimens is minimal. Other experimental techniques include half-body radiation therapy in patients with widely metastatic disease. Studies by Salazar and associates (1986) have shown that prompt and meaningful responses are found in one-half or more of patients. This method may be associated with increased toxicity when combined with systemic agents because there may be shared adverse effects.
The syndrome of compression of the SVC is well known in lung cancer patients. Sculier and one of us (RF) (1985) have made recommendations for its management. Although SVC compression is sometimes considered an emergency, Ahmann (1984) suggests that a tissue diagnosis should always be obtained to guide treatment, particularly because in chemosensitive tumors such as small cell carcinoma, chemotherapy may be equally effective. Many series have shown the palliative benefit of radiation therapy. Patients often experience rapid subjective relief (within 1 to 2 days) long before radiologic improvement is seen. Nieto and Doty (1986) reported that when thrombotic occlusion is present, response is poor. On the other hand, a multivariate analysis of 408 cases by Wurschmidt and colleagues (1995) in Germany concluded that the syndrome might in fact be favorable prognostically, perhaps as an early symptom. They recommend a curative approach to such patients with otherwise limited disease.
Because SCLC is so sensitive to chemotherapy, it is frequently not necessary to administer radiation to palliate symptoms in newly diagnosed patients. The prompt administration of chemotherapy may result in symptom relief in a few days,
P.1724
with complete resolution of symptoms for many patients even before the second cycle. Thus, in hospitals or clinics where radiation therapy is not available, systemic treatment should not be delayed while attempting to transfer a patient to another treatment center for radiation. The exception to this would be patients with symptomatic central nervous system metastases, epidural metastases, or spinal cord compression.
Brain metastases are an important cause of morbidity, but they can be palliated successfully. As discussed by Kurtz (1981) and Mornex (1991) and their colleagues, typical regimens are well within brain tolerance and may make it possible for the patient to discontinue the use of steroids. The optimum regimen is unknown; doses usually range from 20 to 40 Gy over 1 to 4 weeks. A randomized trial of therapy, piloted by Patchell and colleagues (1990), for solitary brain metastases (by CT scan; mostly lung primaries) demonstrated better survival and function if the lesion was resected before irradiation; both groups received whole-brain irradiation (36 Gy in 12 fractions). Most patients with extensive SCLC, however, are not suitable for this combined approach because of multiple metastases or poor general condition.
Epidural metastasis producing compression of the spinal cord is an oncologic emergency. Rodichok and associates (1981) note that early diagnosis is essential because most patients have long-standing warning symptoms, and the onset of paresis may herald rapid development of paralysis. Paralysis may also occur, and some radiotherapists recommend the prophylactic use of steroids for these patients. According to Pedersen (1985) and Constans (1983) and their colleagues, as well as Byrne (1992), laminectomy or irradiation can produce relief of compression and symptoms; there are no standard indications for the use of both together.
Adverse Effects
Radiation effects on normal tissues are confined to the irradiated volume, especially the volume receiving doses intended for the tumor. The adverse effects depend on the nature of tissues within the irradiated volume, the intrinsic tolerance of the tissues to the dose fractionation scheme administered, and the degree to which the tissues have already been compromised by the malignancy, other treatment methods, or other nonmalignant disease processes. The clinical importance of radiation injury is influenced greatly by the proportion of the functional capacity of the organ that incurs the injury. Thus, severe fibrosis of a lobe of the lung caused by treatment may be acceptable, whereas the same therapy would be lethal if administered to a whole lung.
According to Maasilta (1991), McDonald and colleagues (1989), and Rubin and Casarett (1972), radiation injury to the lung is primarily mediated by damage to alveolar pneumocytes and vascular endothelial cells. The dose required to produce loss of function within the treated volume is equivalent to approximately 20 Gy in 10 fractions, an amount well below the doses usually required for treatment of cancer. Reactions considered early (within 2 to 3 months) are only rarely symptomatic, but a clinical syndrome is sometimes observed when large volumes of lung are treated. Symptoms include cough, dyspnea, and a low-grade fever. Hemoptysis and progressive pulmonary failure can develop. Characteristically, the involved portion of the lung corresponds to the treated volume. Infectious causes must be ruled out. Diffuse infiltrates develop over the next 2 weeks and then resolve. With or without clinical symptoms in the few months after treatment, progressive fibrotic change within the irradiated volume is invariable. This development constitutes the pattern of late pulmonary injury and is permanent. Although in general they correspond to the treatment changes in lung fields, secondary retraction of lung tissue may modify this pattern somewhat. Mah and Van Dyk (1988), as well as Brooks (1986) and Van Dyk (1989) and their colleagues, noted that density changes in lung display a definite dose response relation, as measured by CT methods. The combination of radiation therapy with chemotherapy, notably drugs such as doxorubicin and bleomycin, may result in more severe injury at the same level of radiation dose as the injury seen with radiation therapy alone.
The esophagus is usually included in the treatment volume for lung cancer. The early epithelial reaction results in dysphagia for solid food and sometimes liquids in many patients. This effect is transient and resolves shortly after the end of treatment. The later development of stricture is rare for doses up to 60 Gy, but more so when chemotherapy is also used, especially doxorubicin.
Cardiac toxicity may occur when large volumes of the heart are irradiated to 40 Gy or more. The usual manifestation is that of late pericardial problems, usually constriction. Patients with pacemakers may be treated safely. Lewin and colleagues (1984) recommend that the device not be directly irradiated, and cardiology consultation should be sought to assess pacemaker function after the use of linear accelerators because of their associated electromagnetic fields.
As noted by Dische and colleagues (1988), the most feared complication of thoracic radiation therapy is myelopathy. The doses required for lung cancer are well in excess of the tolerance of the spinal cord (45 to 50 Gy at 1.8 to 2.0 Gy per fraction). Much of the detailed treatment planning and shielding undertaken in a lung cancer patient is directed at limiting dose to this critical organ to less than 46 Gy. As a result, this complication is rare. When a previously treated patient develops signs of cord dysfunction at the upper thoracic level, it is imperative to investigate for spinal metastases, which are a more common cause of myelopathy.
Patients sometimes complain of fatigue or lethargy during thoracic irradiation, but this condition is rarely severe unless the liver is included in the irradiated volume. Greenberg and co-workers (1992) observed a similar syndrome in patients
P.1725
receiving localized breast irradiation, and it may be a systemic adaptation to the stress of being treated for cancer.
SURGERY
The initial optimism engendered by the success of combination chemotherapy in the 1970s was tempered by the observation that cure still remained an elusive goal for most patients with SCLC, even those with limited disease. The poor survival rate for patients in the surgical arm of the British Medical Research Council Study reported by Fox and Scadding (1973) led most investigators to abandon surgery as the initial treatment for bronchial neoplasms of this type. Nonetheless, almost all surgical series from the precombination chemotherapy era reported a small percentage of patients who achieved 5-year survival with surgical treatment alone. This observation led Shields and co-workers (1982) to review the VASOG results of surgical therapy for SCLC in an attempt to identify a subgroup of patients with limited disease who might benefit from surgical resection. They made the important observation that TNM staging, which has long been recognized to have prognostic importance for NSCLC, could also define prognostic subgroups for patients undergoing surgery for SCLC. Perhaps of even greater importance was Shields's observation of possible prolongation of survival in the patients who had received adjuvant chemotherapy in these early VASOG trials. The number of patients in these trials was insufficient for the results to achieve statistical significance, and the chemotherapy they received would certainly be considered inadequate by today's standards.
The poor survival of patients with SCLC who are treated by surgery alone is undoubtedly attributable to the presence of occult micrometastatic disease that cannot be detected even by current clinical staging techniques. For patients who have undergone surgical resection of the primary tumor, it should theoretically be possible to eradicate this small bulk of micrometastatic disease with chemotherapy. On the basis of Shields's observations from the VASOG studies, several groups initiated prospective trials of adjuvant chemotherapy after surgery for limited SCLC. One of us (FAS) and colleagues (1988) for the University of Toronto Lung Oncology Group reported a median survival of 83 weeks and projected 5-year survival of 31% for 63 patients who received adjuvant chemotherapy after surgical resection. Patients with pathologic stage I tumors had a projected 5-year survival of 48%. Karrer (1990) and Karrer and Shields (1991) reported similar results for the International Society of Chemotherapy Small Cell Program in Europe and in Asia. The projected 4-year survival rate for their patients with pathologic stage I tumors was 61%.
Elliott and colleagues (1987) have shown that the primary tumor bed and hilar or mediastinal lymph node areas are the most frequent single sites of failure after chemotherapy for limited disease. Despite the addition of thoracic irradiation, relapse occurs at the primary site in one-fourth to one-third of patients. In the early 1980s, investigators postulated that it might be possible to improve control at the primary site by the addition of surgery to standard combined-method therapy, which included chemotherapy and radiation therapy for patients with limited SCLC. Two small retrospective reviews suggested that indeed this might be possible, at least in patients whose condition permitted selection for surgery before the definitive histologic diagnosis was made. One of us (FAS) and co-workers (1983) noted relapse at the primary site in only 2 of 35 patients who had undergone surgical resection, and similar results were reported by Comis and colleagues (1982), who identified no local relapses after surgical treatment in their feasibility study of 22 patients.
During the 1980s, several groups initiated prospective feasibility studies of adjuvant surgical treatment after remission induced by chemotherapy for patients with limited SCLC. Surgery was usually offered only to responding patients, and on average, approximately 50% of the patients identified initially were considered candidates for surgery after induction chemotherapy. Surgical eligibility rates ranged from a low of 27% reported by Prager and colleagues (1984) to 79% reported by one of us (FAS) and associates (1989) for the University of Toronto Group. All groups have reported favorable median survival times of up to 2 years and projected 5-year survival rates of 60% to 80% for patients with pathologic stage I tumors.
These phase II trials led to observations that were important in the design of subsequent randomized trials of surgery for limited SCLC. They demonstrated that combined-modality treatment was feasible and that chemotherapy did not result in unacceptable postoperative morbidity or mortality. They emphasized the importance of TNM staging. Reviews by one of us (FAS) and associates (1991a, 1991b) and by Karrer (1990) suggested that combined-method treatment that included both systemic chemotherapy and surgery resulted in a 5-year survival rate for small cell patients that was almost indistinguishable from that of patients with NSCLC of similar stage (Fig. 114-1A). They also identified the difficulties associated with clinical staging for patients with SCLC. Clinical TNM stage correlated with final pathologic TNM stage in less than 50% of patients, and the survival differences identified by pathologic stage were not seen when the same patients were analyzed by clinical pretreatment stage (Fig. 114-1B). Most authors also reported a small number (approximately 10% to 15%) of patients with mixed histology tumors that included a non small cell component.
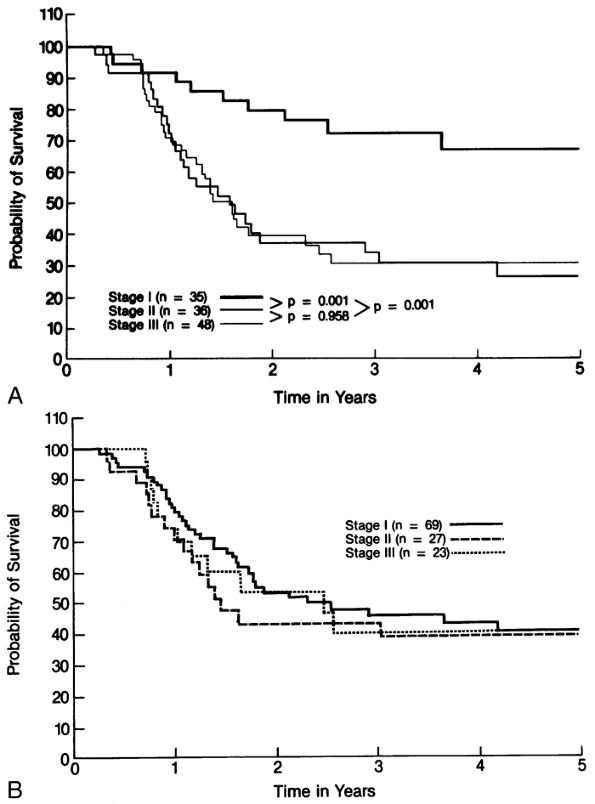 |
Fig. 114-1. A. Comparison of survival by pathologic stage for 119 patients who underwent surgery for limited small cell lung cancer. B. Comparison of survival by pretreatment clinical stage for 119 patients who underwent surgery for limited small cell lung cancer. From Shepherd FA, et al: Surgical treatment for limited small-cell lung cancer. J Thorac Cardiovasc Surg 101:385, 1991a. With permission. |
Because these feasibility studies were all nonrandomized trials, it was not possible to state that the favorable survival achieved was related to surgery rather than patient selection. In an attempt to answer this question, the Lung Cancer Study Group initiated a prospective randomized trial in 1983 in which all patients were treated initially with induction chemotherapy consisting of CAV and responding patients
P.1726
were randomized to surgical resection and radiation therapy or to radiation alone. Lad presented preliminary results of this trial on behalf of the Lung Cancer Study Group (1988). Three hundred forty patients were registered for this trial, but only 144 (42%) were randomized: 68 to surgery and 76 to no surgery. Attrition was related to progression in nonresponders or to reluctance of some patients to accept a randomization to receive or not receive a thoracotomy after several months of chemotherapy. Of the 68 patients randomized to surgery, 6 did not undergo operation, but a further 8 patients had off-study surgery, for a total of 70 thoracotomies. Fifty-eight patients (83%) underwent resection, of which 54 (77%) were thought to be complete. A complete pathologic response of 18% was seen, and non small cell pathologic change occurred in 11% of patients. The results of this trial were disappointing in that no difference in survival was seen between randomized patients in the surgical and nonsurgical arms (Fig. 114-2). Because only one-half of the randomized patients (one-fourth of all patients) in this study underwent surgical staging, it is not possible to compare survival based on stage or TNM subgroups. Within the surgical group, no difference in resectability could be identified for patients in any T or N subgroup, although there seemed to be a trend toward unresectability for patients with T3 tumors (P = 0.08).
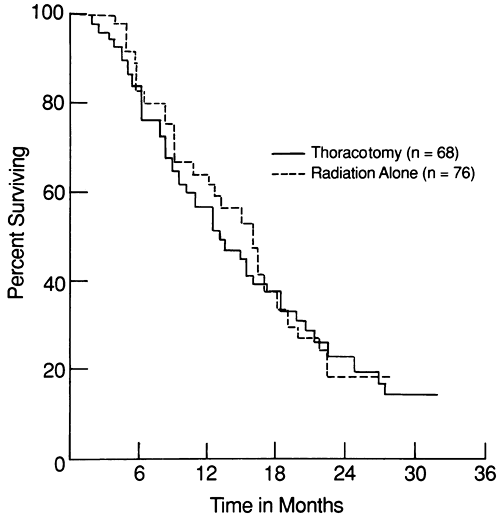 |
Fig. 114-2. Survival curves of the randomized patients with limited small cell lung cancer treated with initial chemotherapy and irradiation alone or with the addition of surgical resection after chemotherapy and irradiation in the North American Lung Cancer Study reported by Lad and coworkers (1991). |
How should the results of this Lung Cancer Study Group trial be interpreted? It is clear that surgical resection does not benefit the majority of patients with limited SCLC and that its future role will be minor in the management of this disease. Nonetheless, a small subgroup of patients (likely less than 10%) may benefit from combined-method therapy, with a significant chance of prolonged disease-free survival and even cure. It is essential, therefore, for medical oncologists and thoracic surgeons to identify such patients from the larger group of limited-disease SCLC overall. In general, the aforementioned phase II studies suggest that the criteria of operability applied to NSCLC are equally valid for SCLC. Surgery should be considered for all patients with T1 2N0 tumors. Whether surgery is offered as initial therapy or after induction chemotherapy is probably not important, as shown in Fig. 114-3, from the review by one of us (FAS) and co-workers (1991a). With respect to stage II disease, it is not possible to make firm generalizations about surgery, and treatment decisions should be individualized. Chemotherapy should be offered as initial treatment,
P.1727
and surgery may be considered for patients with an excellent performance status who have responded well to induction therapy. If a small cell tumor is identified unexpectedly at thoracotomy, complete resection and mediastinal node dissection should be undertaken whenever possible and chemotherapy should be administered postoperatively, even to patients with stage I tumors. Patients with N2 disease should not undergo a surgical resection; a standard chemotherapy regimen should be given postthoracotomy.
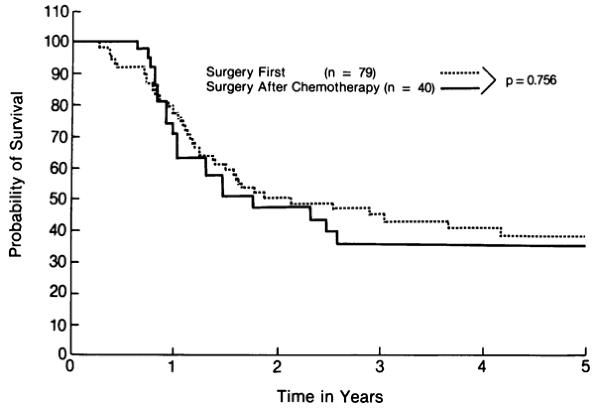 |
Fig. 114-3. Comparison of the survival of patients with small cell lung cancer who had surgery first or surgery after chemotherapy. From Shepherd FA, et al: Surgical treatment for limited small-cell lung cancer. J Thorac Cardiovasc Surg 101:390, 1991. With permission. |
Surgery plays a very limited role, if any, for patients with stage III tumors. Although most investigators have reported long-term survival for a small number of stage IIIA patients, most of these patients had only microscopic involvement of mediastinal lymph glands, or the disease was a T3N0 tumor, which is now considered IIB disease. Even though chemotherapy can result in dramatic resolution of bulky mediastinal disease, the addition of surgical resection does not contribute significantly to long-term survival for most of these patients.
A recent report by Inoue and associates (2000a) from Japan analyzed the results of resection in 95 SCLC patients, particularly in respect to the new TNM staging system proposed by Mountain (1997). Eighty-one patients had undergone a complete, curative resection with complete lymph node dissection, and 71 of these patients had undergone perioperative chemotherapy (two to four or more courses). Radiation therapy was infrequently utilized. The 5-year postoperative survival rate and median survival times were 56.1% and 67 months, respectively, for p-stage 1A, 30.0% and 23 months for p-stage 1B, 57.1% and 106 months for p-stage IIA, and 42.9% and 14 months for p-stage IIB. The median survival time was 9 months for p-stage IIIA and 4 months for p-stage IIIB patients. There was a significant survival advantage of p-T1 lesions versus p-T2 versus p-T3 lesions (p-T4 was similar to p-T3). No statistical survival benefit was noted between N0 and N1 disease, but both groups showed a significant survival benefit over p-N2 lesions. The authors concluded that surgical excision should be confined to appropriate surgical candidates with stages p1A through pIIB tumors, and at least four or more perioperative chemotherapy courses should be given. The presence of N2 disease contraindicates a surgical approach. Unfortunately, Inoue and colleagues (2000b) found that mediastinoscopy, although helpful in identifying the presence of N2 disease, was falsely negative in 15.6% of 32 patients in whom it was carried out. In a number of these patients, a video-assisted thoracoscopic exploration could have reduced the number of false-negative examinations. Whether or not the use of whole-body FDG PET scans or a technetium Tc 99m depreotide single-photon emission computed tomographic scan would further reduce the false-negative preoperative evaluation for N2 disease is yet to be determined.
Another group who may benefit from surgical resection is the 10% to 15% of patients with combined small cell and non small cell tumors. When such patients are identified at diagnosis, initial treatment should be chemotherapy to control the small cell component of the disease. Surgery should then be considered for the more chemotherapy-resistant non small cell component. For patients who demonstrate an unexpectedly poor response to chemotherapy or patients who experience localized late relapse after treatment for pure small cell tumors, a repeat biopsy should be performed to rule out non small cell pathology. In a small study of 28 patients who underwent salvage operations, one of us (FAS) and colleagues (1991b) identified 10 patients who had non small cell pathologic findings. Four of these patients achieved long-term disease-free survival, from 2 to 6 years after surgery. Although it is recognized that only a minority of patients fall into this favorable subgroup, it is important to take steps to identify them because curative therapy may be available. Shields and Karrer (1998) reviewed all the various aspects of the role of surgery in managing patients with SCLC. Their views are essentially the same as ours, although they prefer initial resection followed by chemotherapy in most of the highly selected patients who are surgical candidates.
BIOLOGICAL RESPONSE MODIFIERS AND OTHER NOVEL TREATMENTS
In general, Shank and colleagues (1985) report that numerous agents, including bacille Calmette-Gu rin, its methanol-extractive residue, and Corynebacterium parvum, have been investigated in all types of lung cancer with no definitive evidence that they are beneficial. The only encouraging study was conducted by Cohen and associates (1979) from the NCI, who initiated a randomized trial using thymosin fraction V. A notable increase in survival was seen by Shank and co-workers (1985) with the highest dose
P.1728
(60 mg/m2), but this has not been corroborated. Woll and Rozengurt (1989) reviewed the literature on this subject.
Antibodies directed against bombesin (a gastrinlike peptide hormone) have been evaluated in phase I studies. This approach was reviewed by Carney (1991, 1992), but these antibodies so far are not clinically usable.
Interferons and interleukins have not been found to be useful as therapy in lung cancer, as reviewed by one of us (FAS) (1997, 2001). In fact, maintenance therapy with interferon preparations was associated with shortened survival in some randomized trials, and with considerable toxicity leading to discontinuation of treatment in all studies. The only positive study using maintenance therapy is the one by Mattson and colleagues (1991) from Finland, who reported a survival advantage with long-term interferon- maintenance for patients with limited disease in complete remission. This study was repeated by SWOG, but the results were negative, and compliance with treatment was poor. The use of maintenance interferon was also tested by Jett and the North Central Oncology Group (1992) but was not found to be of benefit. Therefore, to date, it can be said that biological response modifiers alone are not part of the standard induction therapy of patients with SCLC.
The trials of anticoagulant therapy in SCLC using a variety of agents, including heparin, aspirin, and warfarin, have been reviewed by one of us (FAS) (2001). Aisner and coauthors (1992) reported on a pilot study showing an apparent improved response rate (survival compared with historic data) with warfarin added to standard therapy in patients with SCLC. This finding led to a randomized trial by the Cancer and Leukemia Group B that showed a nonsignificant trend in favor of warfarin treatment, as reported by Chahinian and associates for the group (1989). LeBeau and colleagues (1994) also showed improved survival for SCLC patients treated with heparin. Despite the somewhat promising results of the CALGB and French groups, there has been no further research into the addition of anticoagulant therapy in more than 10 years now.
The foremost example of using biological response modifiers in combination with other therapy is using growth factors such as G-CSF and GM-CSF (see the earlier section on colony-stimulating factors). In summary, G-CSF seems to decrease myelosuppression, febrile episodes, and days of hospitalization, but to date it has no proven survival benefit. It is possible that GM-CSF also has such an effect, but it has other inherent toxicity, including the possibility of enhanced thrombocytopenia. In fact, thrombocytopenia not ameliorated by either G-CSF or GM-CSF may present serious difficulties. When using myelosuppressive therapy, the dose-limiting hematologic toxicity is thrombocytopenia rather than granulocytopenia, as is the case when the presently available CSF preparations are used. New growth factors, such as interleukin-3, as reported by Postmus and co-workers (1992), alone or in combination, may be able to circumvent this problem in the future. It can be stated, however, that CSFs are not a required part of therapy in patients with SCLC treated with moderate-dose chemotherapy.
Other important new molecular biological therapeutic developments that include antigrowth factors (antagonists G and D), antimetastatic agents [matrix metalloproteinase (MMP) inhibitors], and other genetic products have been reviewed by Smyth (1996). The MMP inhibitors are believed to be associated with cellular metastatic potential and the development of tumor angiogenesis. Szabo (1998), Gonzalez-Avila (1998), and Okamoto (1999) and their colleagues, among others, have carried out many basic microbiological molecular investigations relating to the inhibition of the activity of MMP inhibitors. The activity of the MMP inhibitors is thought to have an anticancer effect in three ways: inhibition of local and regional tumor growth, inhibition of the formation of metastatic deposits, and restriction of tumor angiogenesis. Marimastat, an MMP inhibitor, has been studied in a phase I trial by Wojtowicz-Praga and associates (1998) to evaluate the safety and pharmacokinetics of various doses of the drug in humans. Marimastat is well absorbed from the gastrointestinal tract, with detectable drug levels in the plasma within hours after drug administration. Dose levels of 50 to 100 mg orally twice daily achieve plasma concentrations that are substantially higher than those required for MMP inhibition in vitro. The dose-limiting toxicity is severe inflammatory polyarthritis. Despite the promising preclinical evidence supporting a role for MMP inhibitors in the treatment of SCLC, one of us (FAS) and colleagues (2001) reported that the large NCIC and EORTC trial of adjuvant Marimastat for SCLC patients who had responded to induction chemotherapy was negative. Furthermore, Marimastat was associated with significant toxicity and deterioration in quality of life.
Other studies have examined monoclonal antibodies, which may inhibit tumor cell growth by binding to growth factors or may be conjugated to toxins or other chemotherapeutic agents, which results in tumor cell death. The success of our attempts to manipulate abnormal genes in malignant cells has yet to be resolved. However, our increasing knowledge and understanding of the regulation of normal and neoplastic cell growth at the molecular level may prove to be the key. Except for CSF preparations to attempt to reduce myelosuppression and its complications, however, biological response modifiers at present have no established role in patients with SCLC.
CARCINOMATOUS MENINGITIS
Several studies by Aisner (1979), Aroney (1981), Brereton (1978), and Wasserstrom (1982) and their colleagues, as well as by Greco and Fer (1978) and Oster and Fetell (1982), stressed that carcinomatous leptomeningitis may be more frequent in SCLC than was thought previously. Rosen and colleagues (1982) from the U.S. NCI observed that 60 (11%) of 526 of their patients who entered various protocols
P.1729
between August 1969 and June 1980 developed this complication; most cases arose after the time of presentation. Bunn and Rosen (1985) submit that the condition reaches an incidence of approximately 25% during the first 3 years and then plateaus. Only approximately 1% of patients relapse from a complete response in this way; the majority are discovered to have carcinomatous leptomeningitis concurrent with finding other progressive systemic disease.
The NCI review conducted by Rosen and associates (1982), using a multivariate analysis, indicated that liver metastasis was the element most strongly associated with the development of this complication, followed by bone and other CNS metastases. Because patients with CNS metastases (e.g., epidural., brain) are at high risk for developing carcinomatous leptomeningitis, they should probably have a lumbar puncture. Looking for tumor cells by means of standard methodology warrants cytocentrifuge examination. If the initial cerebrospinal fluid examination is abnormal but cytologically negative, repeat lumbar punctures should be carried out. A more detailed description of the clinical picture and of the evidence of this problem at autopsy is beyond the range of this chapter. These topics are well discussed by Bunn and Rosen (1985).
The fundamental issue is whether treatment is useful. The best treatment is clearly not established. Often, intrathecal drug therapy is administered, most often with methotrexate, combined with irradiation to the sites of bulk or systemic disease in the CNS. According to Rosen and colleagues (1982), combined treatment seemed to improve the symptomatology better than intrathecal treatment alone, at least in the NCI series. Because it is difficult to continue giving intrathecal medication without a catheter permanently in place, a subcutaneous (Ommaya) reservoir with a ventricular channel is placed. Use of this device ensures that all the injected drug enters the CNS, permits uniform dose distribution, and eliminates the requirement for repeated lumbar punctures. No comparative study in patients with solid tumors has been undertaken, but as discussed by Bleyer and Poplack (1979), this routine certainly seems preferable in patients with acute leukemia with leptomeningeal involvement.
Bunn and Rosen (1985) also noted that the only agents that seem active when given intrathecally or intraventricularly are methotrexate and thiotepa. Trump and colleagues (1981) propose that a combination of intrathecal methotrexate and thiotepa may be superior to methotrexate alone. In spite of all these treatments, the median survival appears to be only approximately 7 weeks. The patients typically deteriorate as a result of concurrent progressive systemic tumor in addition to the leptomeningeal involvement. Therefore, newer methods of treatment for this complication are unquestionably in demand. With the high drug levels observed in the brain with the use of high-dose etoposide, perhaps this regimen can be used as an adjunct in the future. Trials are essential, however, before this treatment is acceptable, and further research in this area is clearly in order.
COMPLICATIONS OF TREATMENT
Early Complications and Toxicities of Chemotherapy
All methods of therapy can produce significant individual toxicities. Those associated with individual types of therapy, as shown in Table 114-15, are classified into those that occur early and those that occur late after treatment. These toxicities must be considered when offering such treatment to patients who have SCLC and should be discussed with the patient in detail. One of us (RF) (1981, 1989a, 1994b, 1994) has provided a detailed description of these problems.
Table 114-15. Early and Possible Late Toxicities in Patients with Small Cell Lung Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
Of significance is that new approaches and supportive care may well counter many of these toxicities. For example, nausea and vomiting cause serious distress in patients with SCLC because cisplatin is frequently used along with other agents that cause moderate emesis. In an overview by Aapro (1991), the use of dexamethasone in combination with 5-hydroxytryptamine antagonists, as exemplified by ondansetron and granisetron, has improved the situation substantially. Nausea in the early posttreatment period is essentially eliminated, although delayed onset of nausea remains a possible issue for some patients.
Myelosuppression, with its capacity for infection, has been a potential serious problem in the treatment of SCLC. A common regimen used in the 1980s was CAE (cyclophosphamide, doxorubicin, and etoposide). This combination led to severe and prolonged myelosuppression, with a hospital admission rate as high as 40% to 50% for febrile neutropenia for these patients. Therapeutic results were reasonable, but the complication rate was likely unacceptable. This usage has been superseded by regimens such as etoposide and cisplatin, which is associated with less than 5% admission rates for this complication. Another approach to this problem has been the use of growth factors, particularly G-CSF, to lessen the extent and duration of neutropenia and thereby reduce the infection and hospitalization rate. Consequently, patients in the hospital are also discharged sooner. In a review by Crawford and associates (1991), the addition of G-CSF to CAE considerably reduced myelosuppressive and subsequent infectious complications. A similarly designed European study by Green and colleagues (1991) confirmed this result. This approach, however, is extremely costly. Alternate choices include less myelosuppressive but equally effective chemotherapy, as already proposed, or perhaps using oral prophylactic antibiotics, such as cotrimoxazole or various marketed fluoroquinolones.
Blackstein (1990) and Eckhardt (1990) and their colleagues observed that cardiotoxicity usually can be avoided by using less cardiotoxic anthracyclines, such as epirubicin, which is active in both SCLC and NSCLC, as reported by one of us (RF) and co-workers (1992). In addition, the use of only four to six courses of therapy, which is now standard throughout the world, decreases the occurrence of this problem and many of the other side effects, thereby enhancing the quality of life of these patients. D. H. Johnson (1990) found that
P.1730
mesna, especially when combined with ifosfamide, has essentially eradicated the problem of hemorrhagic cystitis with the latter agent. Mesna also can be used with high-dose cyclophosphamide to avoid hemorrhagic cystitis. If nephrotoxicity, ototoxicity, or emesis is a concern, one can substitute carboplatin for cisplatin, which usually either averts or stabilizes these side effects but also causes more myelosuppression. Carboplatin, as discussed by Green and associates (1992), is more expensive and possibly less active in this disease.
Late Complications of Chemotherapy
Peripheral neuropathy remains a frequent problem. Of specific concern is the late-onset neuropathy identified with cisplatin. This condition can begin even a few months after the final course of cisplatin and can be disabling. New approaches to this dilemma are needed.
Although many of the late complications of therapy are shown in Table 114-15, the one that is potentially of most concern is the formation of second malignancies in potentially cured patients. In a study by Sagman and colleagues (1992), second lung primaries in patients with SCLC are relatively frequent complications if patients survive long enough. A review by Heyne and associates (1992) from M. D. Anderson Hospital also revealed cases involving second primaries. Perhaps these complications can be prevented in future by the use of agents such as retinoids, as suggested by Ihde (1992). These lesions may be overlooked and presumed to be a relapse of the original tumor, thereby precluding possible surgical removal with the potential for cure. A second primary must always be contemplated when new lesions are observed in patients, undoubtedly beyond 2 years from diagnosis and even less in some cases. Other solid tumors also occur with reasonable frequency as second primaries, probably related in part to treatment but more likely associated with the patients' age (median 60+ years). Acute leukemia is an unusual occurrence and will become less typical with the discontinued use of nitrosourea and procarbazine for SCLC and the considerably shorter span of treatment involved. Second lung primaries and, indeed, acute leukemia are sometimes seen in patients with NSCLC, but they are less of a problem.
REFERENCES
Aapro MS: 5-HT3 receptor antagonists: an overview of their present status and future potential in cancer therapy-induced emesis. Drugs 42:551, 1991.
P.1731
Ahmann F: A reassessment of the clinical implications of the superior vena caval syndrome. J Clin Oncol 2:961, 1984.
Ahmed S, Coulson J, Woll P: Detection of small cell lung cancer cells in the peripheral blood by RT-PCR for expressed neuropeptides [Abstract]. Proc Am Soc Clin Oncol 19:486a, 2000.
Aisner J, et al: Meningeal carcinomatosis from small cell carcinoma of the lung. Consequence of improved survival. Acta Cytol 23:292, 1979.
Aisner J, et al: Leptomeningeal carcinomatosis in small cell carcinoma of the lung. Med Pediatr Oncol 9:47, 1981.
Aisner J, et al: Intensive combination chemotherapy, concurrent chest irradiation, and warfarin for the treatment of limited-disease small-cell lung cancer: a Cancer and Leukemia Group B pilot study. J Clin Oncol 10:1230, 1992.
Aisner SC, Matthews MJ: The pathology of lung cancer. In Aisner J (ed): Contemporary Issues in Clinical Oncology, Vol 3. Lung Cancer. New York: Churchill Livingstone, 1985.
Albain KS, et al: Determinants of improved outcome in small-cell lung cancer: an analysis of the 2,580-patient Southwest Oncology Group data base. J Clin Oncol 8:1563, 1990.
Anderson H, et al: Recombinant human GM-CSF in small cell lung cancer: a phase I/II study. Recent Results Cancer Res 121:155, 1991a.
Anderson H, et al: Phase II study of gemcitabine in non small cell lung cancer (NSCLC) [Abstract]. Proc Am Soc Clin Oncol 10:247, 1991b.
Ardizzoni A, et al: Accelerated chemotherapy with or without GM-CSF for small cell lung cancer: a non-randomised pilot study. Eur J Cancer 26:937, 1990.
Ardizzoni A, et al: Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 15:2090, 1997.
Ariyoshi Y, Sugiura T: New promising anticancer drugs for lung cancer [in Japanese]. Gan To Kagaku Ryoho 21:2578, 1994.
Aroney RS, et al: Meningeal carcinomatosis in small cell carcinoma of the lung. Am J Med 71:26, 1981.
Aroney RS, et al: Value of prophylactic cranial irradiation in prevention of central nervous system metastases in small cell lung cancer. Potential benefit restricted to patients with complete response. Cancer Treat Rep 67:675, 1983.
Arriagada R, et al: Alternating radiotherapy and chemotherapy schedules in small cell lung cancer, limited disease. Int J Radiat Oncol Biol Phys 11:1461, 1985.
Arriagada R, et al: Consensus report on combined radiotherapy and chemotherapy modalities in lung cancer. Antibiot Chemother 41:232, 1988.
Arriagada R, Pignon JP, Le Chevalier TL: Thoracic radiation therapy in small cell lung cancer: rationale for timing and fractionation. Lung Cancer 5:237, 1989.
Arriagada R, et al: Meta-analysis of randomized trials evaluating the role of thoracic radiation therapy in limited small cell lung carcinoma (SCLC) [Abstract]. Lung Cancer 7(suppl):98, 1991a.
Arriagada R, et al: Initial high dose chemotherapy and multifractionated radiation therapy in limited small cell lung cancer. Lung Cancer 7:159, 1991b.
Arriagada R, et al: Initial chemotherapeutic doses and survival with limited small cell lung cancer. N Engl J Med 329:1848, 1993.
Arriagada R, et al: Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 87:183, 1995.
Arriagada R, et al: Prophylactic cranial irradiation overview (PCIO) in patients with small cell lung cancer (SCLC) in complete remission [Abstract]. Proc Am Soc Clin Oncol 17:450, 1998.
Auperin A, et al: Prophylactic cranial irradiation for patients with small cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 341:476, 1999.
Avalos BR, et al: Human granulocyte colony-stimulating factor: biologic activity and receptor characterization of hematopoietic cells and small cell lung cancer cell lines. Blood 75:851, 1990.
Banham SW, et al: High dose epirubicin chemotherapy in untreated poorer prognosis small cell lung cancer. Respir Med 84:241, 1990.
Barkley H (ed): Radiation therapy for relief of intrathoracic symptoms arising from unresectable bronchial carcinoma. University of Texas System Cancer Center, Annual Clinical Conference on Cancer. Vol. 28. Houston: University of Texas Press, 1986, p. 191.
Beiler DD, et al: Low dose elective brain irradiation in small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 5:941, 1979.
Berendsen HH, et al: Detection of small cell lung cancer metastases in bone marrow aspirates using monoclonal antibody directed against neuroendocrine differentiation antigen. J Clin Pathol 41:273, 1988.
Bergsagel D, Feld R: Small cell lung cancer is still a problem [Editorial]. J Clin Oncol 2:1189, 1984.
Bergsagel DE, et al: Lung cancer: clinical trial of radiation therapy alone vs. radiation therapy plus cyclophosphamide. Cancer 30:621, 1972.
Biran H, Feld R, Malkin A: Circulating arginine-vasopressin, calcitonin, carcinoembryonic antigen, neuron-specific enolase, and beta-2 microglobulin fluctuations during combined modality induction therapy for small-cell bronchial carcinoma. Association of postchemotherapy AVP surge with high tumor response rate and durable remission. Tumour Biol 12:131, 1991.
Birch R, et al: Patterns of failure in combined chemotherapy and radiotherapy for limited small-cell lung cancer: Southeastern Chemotherapy Group experience. NCI Monogr 6:265, 1988.
Birch R, et al: A randomized study of etoposide and carboplatin with or without paclitaxel in the treatment of small cell lung cancer. Semin Oncol 24(4 suppl 12):S12 135, 1997.
Birch R, et al: Preliminary results of a randomized study comparing etoposide and carboplatin with and without paclitaxel in newly diagnosed small cell lung cancer [Abstract]. Proc Am Soc Clin Oncol 19:490a, 2000.
Bishop JF, et al: Dose and schedule of granulocyte macrophage colony stimulating factor (GM-CSF) carboplatin and etoposide in small cell lung cancer (SCLC) [Abstract]. Proc Am Soc Clin Oncol 10:240, 1991.
Blackstein M, et al: Epirubicin in extensive small-cell lung cancer: a phase II study in previously untreated patients: a National Cancer Institute of Canada Clinical Trials Group study. J Clin Oncol 8:385, 1990.
Bleyer WA, Poplack DG: Intraventricular versus intralumbar methotrexate for central nervous system leukemia: prolonged remission with the Ommaya reservoir. Med Pediatr Oncol 6:207, 1979.
Boerman OC, et al: Biodistribution of a monoclonal antibody (RNL-1) against the neural cell adhesion molecule (NCAM) in athymic mice bearing human small-cell lung-cancer xenografts. Int J Cancer 48:457, 1991.
Bonadonna G: Chemotherapy strategies to improve the control of Hodgkin's disease: the Richard and Linda Rosenthal Foundation Award Lecture. Cancer Res 42:4309, 1982.
Bonomi P, et al: Intensive induction treatment of small cell bronchogenic carcinoma with cyclophosphamide, methotrexate, and etoposide. Cancer Treat Rep 69:1007, 1985.
Boyer M, Feld R, Warr D: LDH alone does not predict relapse of small cell lung cancer (SCLC) [Abstract]. Proc Am Assoc Cancer Res 33:204, 1992.
Brennan J, et al: myc family DNA amplification in 107 tumors and tumor cell lines from patients with small cell lung cancer treated with different combination chemotherapy regimens. Cancer Res 51:1708, 1991.
Brereton HD, et al: Spinal meningeal carcinomatosis in small cell carcinoma of the lung. Ann Intern Med 88:517, 1978.
Brooks BJ Jr, et al: Pulmonary toxicity with combined modality therapy for limited stage small-cell lung cancer. J Clin Oncol 4:200, 1986.
Bunn PA Jr: The North American experience with paclitaxel combined with cisplatin or carboplatin in lung cancer. Semin Oncol 23:18, 1996.
Bunn PA Jr: Defining the role of paclitaxel in lung cancer: summary of recent studies and implications for future directions. Semin Oncol 24(4 suppl 12):S12 153, 1997.
Bunn PA Jr, Ihde DC: Small cell bronchogenic carcinoma: a review of therapeutic results. In Livingston RB (ed): Lung Cancer. Vol. 1. Boston: Martinus Nijhoff, 1981, p. 169.
Bunn PA Jr, Rosen ST: Central nervous system manifestation of small cell lung cancer. In Aisner J (ed): Contemporary Issues in Clinical Oncology. Vol. 3. Lung Cancer. New York: Churchill Livingstone, 1985, p. 287.
Bunn PA Jr, Nugent JL, Matthews MJ: Central nervous system metastases in small cell bronchogenic carcinoma. Semin Oncol 5:314, 1978.
Bunn PA Jr, et al: Chemotherapy alone or chemotherapy with chest radiation therapy in limited stage small-cell lung cancer. A prospective randomized trial. Ann Intern Med 106:655, 1987.
Byrne TN: Spinal cord compression from epidural metastases. N Engl J Med 327:614, 1992.
Campling B, et al: Is bone marrow examination in small-cell lung cancer really necessary? Ann Intern Med 105:508, 1986.
Canellos GP, et al: Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med 327:1478, 1992.
Carlson RW, et al: Late consolidative radiation therapy in the treatment of limited stage small cell lung cancer. Cancer 68:948, 1991.
P.1732
Carney DN: Recent advances in the biology of small cell lung cancer. Chest 89(suppl 4):253S, 1986.
Carney DN: Lung cancer biology. Curr Opin Oncol 3:288, 1991.
Carney DN: The biology of lung cancer. Curr Opin Oncol 4:292, 1992.
Carney DN, et al: Establishment and identification of small cell lung cancer cell lines have classic and variant features. Cancer Res 45:2913, 1985.
Carney DN, et al: Bone marrow involvement (BMI) by small cell lung cancer (SCLC) using magnetic resonance imaging (MRI) [Abstract]. Proc Am Soc Clin Oncol 9:228, 1989.
Carney DN, et al: Single-agent oral etoposide for elderly small cell lung cancer patients. Semin Oncol 17(1 suppl 2):49, 1990.
Chahinian P, et al: A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small cell lung cancer by the Cancer and Leukemia Group B. J Clin Oncol 7:993, 1989.
Choi N, et al: Phase I study to determine the maximum tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol 16:3528, 1998.
Clark PI, et al: Prolonged administration of single-agent oral etoposide in patients with untreated small cell lung cancer (SCLC) [Abstract]. Proc Am Soc Clin Oncol 9:226, 1990.
Clark P, et al: Two prolonged schedules of single-agent oral etoposide of differing duration and dose in patients with untreated small cell lung cancer (SCLC) [Abstract]. Proc Am Soc Clin Oncol 10:268, 1991.
Cohen MH, et al: Intensive chemotherapy of small cell bronchogenic carcinoma. Cancer Treat Rep 61:349, 1977.
Cohen MH, et al: Thymosin fraction V and intensive combination chemotherapy. Prolonging the survival of patients with small cell lung cancer. JAMA 241:1813, 1979.
Comis R, et al: The current results of chemotherapy (CTH) plus adjuvant surgery (AS) in limited small cell anaplastic lung cancer (SCALC) [Abstract]. Proc Am Soc Clin Oncol 1:147, 1982.
Constans JP, et al: Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg 59:111, 1983.
Cormier Y, et al: Gemcitabine is an active new agent in previously untreated extensive small cell lung cancer (SCLC). A study of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol 5:283, 1994.
Cox JD, et al: Prophylactic cranial irradiation in patients with inoperable carcinoma of the lung. Preliminary report of a cooperative trial. Cancer 42:1135, 1978.
Cox JD, et al: Results of whole brain irradiation for metastases from small cell carcinoma of the lung. Cancer Treat Rep 64:957, 1980.
Coy P, et al: The effect of dose of thoracic irradiation on recurrence in patients with limited stage small cell lung cancer. Initial results of a Canadian Multicenter Randomized Trial. Int J Radiat Oncol Bio Phys 14:219, 1988.
Crawford J, et al: Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325:164, 1991.
Creech R, Richter M, Finkelstein D: Combination chemotherapy with or without consolidation radiation therapy for regional small-cell carcinoma of the lung. Proc Am Soc Clin Oncol 6:66A, 1987.
Cunningham D, et al: High-dose cyclophosphamide and VP 16 as late dosage intensification therapy for small cell carcinoma of lung. Cancer Chemother Pharmacol 15:303, 1985.
Cuttitta F, et al: Monoclonal antibodies that demonstrate specificity for several types to human lung cancer. Proc Natl Acad Sci USA 78:4591, 1981.
Davis S, et al: Long-term survival in small cell carcinoma of the lung: a population experience. J Clin Oncol 3:80, 1985.
Dische S, Warburton M, Saunders M: Radiation myelitis and survival in the radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys 15:75, 1988.
Dodwell DJ, Gurney H, Thatcher N: Dose intensity in cancer chemotherapy. Br J Cancer 61:789, 1990.
Eagen RT, et al: A case for preplanned thoracic and prophylactic whole brain radiation therapy in limited small cell lung cancer. Cancer Clin Trials 4:261, 1981.
Earle CC, et al: A phase I study of gemcitabine/cisplatin/etoposide in the treatment of small cell lung cancer. Lung Cancer 22:235, 1998.
Eckhardt S, et al: Phase II study of 4'-epi-doxorubicin in patients with untreated, extensive small cell lung cancer. South-East European Oncology Group (SEEOG). Med Oncol Tumor Pharmacother 7:19, 1990.
Einhorn LH, Pennington K, McClean J: Phase II trial of daily oral VP-16 in refractory small cell lung cancer: a Hoosier Oncology Group study. Semin Oncol 17(1 suppl 2):32, 1990.
Eisenhauer E, et al: Gemcitabine is active in patients (pts) with previously untreated extensive small cell lung cancer (SCLC) a phase II study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) [Abstract]. Proc Am Soc Clin Oncol 11:309, 1992.
Elias AD, et al: High dose combination alkylating agents supported by autologous marrow (ABMT) with chest radiation therapy for responding limited stage (LD) small cell lung cancer (SCLC) [Abstract]. Proc Am Soc Clin Oncol 11:296, 1992.
Elliott JA, Osterlind K, Hansen HH: Cyclic alternating non-cross resistant chemotherapy in the management of small cell anaplastic carcinoma of the lung. Cancer Treat Rev 11:103, 1984.
Elliott JA, et al: Metastatic patterns in small-cell lung cancer: correlation of autopsy findings with clinical parameters in 537 patients. J Clin Oncol 5:246, 1987.
Erasmus JJ, et al: Thoracic FDG PET: state of the art. Radiographics 18:5, 1998.
Ettinger DS: Evaluation of new drugs in untreated patients with small-cell lung cancer: its time has come. J Clin Oncol 8:374, 1990.
Ettinger DS: Taxol in the treatment of lung cancer. J Natl Cancer Inst Monogr 15:177, 1993.
Ettinger DS, et al: Phase II study of paclitaxel in patients with extensive-disease small cell lung cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 13:1430, 1995.
Evans WK, et al: Superiority of alternating non-cross-resistant chemotherapy in extensive small cell lung cancer. A multicenter randomized clinical trial by the National Cancer Institute of Canada. Ann Intern Med 107:451, 1987.
Evans WK, et al: Phase II study of amonafide: results of treatment and lessons learned from the study of an investigational agent in previously untreated patients with extensive small-cell lung cancer. J Clin Oncol 8:390, 1990.
Evans WK, et al: Oral VP-16 and carboplatin for small cell lung cancer [Abstract]. Proc Am Soc Clin Oncol 10:247, 1991.
Feld R: Complications in the treatment of small cell carcinoma of the lung. Cancer Treat Rev 8:5, 1981.
Feld R: Late complications associated with the treatment of small-cell lung cancer. Cancer Treat Res 45:301, 1989a.
Feld R: Long term complications in small cell lung cancer. In Hansen HH (ed): Basic and Clinical Concepts of Lung Cancer. Boston: Kluwer, 1989b, p. 301.
Feld R: Complications associated with the treatment of small cell lung cancer. Lung Cancer 10:S307, 1994.
Feld R, et al: Combined modality induction therapy without maintenance chemotherapy for small cell carcinoma of the lung. J Clin Oncol 2:294, 1984.
Feld R, et al: Canadian multicenter randomized trial comparing sequential and alternating administration of two non-cross resistant chemotherapy combinations in patients with limited small cell carcinoma of the lung. J Clin Oncol 5:1401, 1987.
Feld R, Ginsberg R, Payne DG: Treatment of small cell lung cancer. In Roth JA, Ruckdeschel JC, Weisenburger TH, (eds): Thoracic Oncology. Philadelphia: WB Saunders, 1988, p. 229.
Feld R, et al: Phase I-II study of high dose epirubicin in advanced non-small cell lung cancer. J Clin Oncol 20:297, 1992.
Feld R, et al: The restaging of responding patients with limited small cell lung cancer. Is it really useful? Chest 103:1010, 1993.
Ferguson MK, et al: Sex-associated differences in presentation and survival in patients with lung cancer. J Clin Oncol 8:1402, 1990.
Figueredo A, et al: Addition of verapamil and tamoxifen to the initial chemotherapy of small cell lung cancer. A phase I/II study. Cancer 65:1895, 1990.
Figueredo AT, et al: Co-trimoxazole prophylaxis during high dose chemotherapy of small cell lung cancer. J Clin Oncol 3:54, 1985.
Fleck JF, et al: Is prophylactic cranial irradiation indicated in small-cell lung cancer? J Clin Oncol 8:209, 1990.
Fowler J: The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 62:679, 1989.
Fox RM, et al: A randomized study: small cell anaplastic lung cancer treated by combination chemotherapy and adjuvant radiotherapy. Int J Radiat Oncol Biol Phys 6:1083, 1980.
Fox RM, Tattersall MHN, Woods RL: Radiation therapy as an adjuvant in small cell lung cancer treated by combination chemotherapy: a randomized study [Abstract]. Proc Am Soc Clin Oncol 22:502, 1981.
Fox W, Scadding JG: Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small celled or oat celled carcinoma of the bronchus. Ten year follow-up. Lancet 2:63, 1973.
P.1733
Fukuoka M, et al: Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small cell lung cancer. J Natl Cancer Inst 83:855, 1991a.
Fukuoka M, et al: Dose intensive weekly CODE chemotherapy (CT) with or without recombinant human granulocyte colony-stimulating factor (rhG-CSF) in extensive-stage (ES) small cell lung cancer (SCLC). Lung Cancer 7:123, 1991b.
Gatzemeier U, et al: Paclitaxel, carboplatin, and oral etoposide: a phase II trial in limited-stage small cell lung cancer. Semin Oncol 24(4 suppl 12):S12 149, 1997.
Gazdar AF, et al: Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical., morphological and growth properties. Cancer Res 45:2924, 1985.
Gazdar AF, et al: Correlation of in vitro drug-sensitivity testing results with response to chemotherapy and survival in extensive-stage small cell lung cancer: a prospective clinical trial. J Natl Cancer Inst 82:117, 1990a.
Gazdar AF, Mulshine JL, Kramer BS: Biological, molecular, and clinical markers for the diagnosis and typing of lung cancer. Immunol Ser 53:453, 1990b.
Ginsberg RJ: Surgery and small cell lung cancer an overview. Lung Cancer 5:232, 1989.
Ginsberg RJ, Karrer K: Surgery in small cell lung cancer. Lung Cancer 5:139, 1989.
Giovanella L, et al: Immunoassay of neuron-specific enolase (NSE) and serum fragments of cytokeratin 19 (CYFRA 21.1) as tumor markers in small cell lung cancer: clinical evaluation and biological hypothesis. Int J Biol Markers 12:22, 1997.
Girling DJ: Prospective randomised trial of 3 or 6 courses of etoposide, cyclophosphamide, methotrexate and vincristine and of 6 courses of etoposide and ifosfamide in small cell lung cancer (SCLC). For the British Medical Research Council Lung Cancer Working Party, 6th World Conference on Lung Cancer, Melbourne. Lung Cancer 7(suppl): 103, 1991.
Girling DJ: Comparison of oral etoposide and standard intravenous multi-drug chemotherapy for small cell lung cancer: a stopped multi-centre randomised trial. Medical Research Council Lung Cancer Working Party. Lancet 348:563, 1996.
Goldie JH, Coldman AJ: The genetic origin of drug resistance in neoplasms: implications for systemic therapy. Cancer Res 44:3643, 1984.
Goldie JH, Coldman AJ, Gudauskas GA: Rationale for the use of alternating non-cross resistant chemotherapy. Cancer Treat Rep 66:439, 1982.
Goldie J, et al: A mathematical and computer-based model of alternating chemotherapy and radiation therapy in experimental neoplasms. Antibiot Chemother 41:11, 1988.
Gonzalez-Avila G, et al: 72-kD (MMP-2) and 92-kD (MMP-9) type IV collagenase production and activity in different histologic types of lung cancer cells. Pathobiology 66:5, 1998.
Goodman GE, et al: Treatment of limited small cell lung cancer with concurrent etoposide/cisplatin and radiotherapy followed by intensification with high-dose cyclophosphamide: a Southwest Oncology Group Study. J Clin Oncol 9:453, 1991.
Goodwin PJ, Feld R, Evans WK: Cost-effectiveness of cancer chemotherapy: an economic evaluation of a randomized trial in small cell lung cancer. J Clin Oncol 6:1537, 1988.
Grafton C, et al: High dose rate endobronchial brachytherapy using the MicroSelectron. Lung Cancer 7(suppl):97, 1991.
Grant SC, et al: Single-agent chemotherapy trials in small-cell lung cancer, 1970 to 1990: the case for studies in previously treated patients. J Clin Oncol 10:484, 1992.
Greco FA, Fer MF: Oat-cell carcinoma of the lung with carcinomatosis meningitis. Ann Intern Med 88:517, 1978.
Greco FA, Hainsworth JD: Paclitaxel, carboplatin, and oral etoposide in the treatment of small cell lung cancer. Semin Oncol 23:7, 1996.
Green JA, Trillet VN, Manegold C: 4-metHuG-Csf (G-CSF) with CDE chemotherapy (CT) in small cell lung cancer (SCLC): interim results from a randomized, placebo controlled trial [Abstract]. For the European G-CSF Lung Cancer Study Group. Proc Am Soc Clin Oncol 10: 243, 1991.
Green M, et al: Carboplatin in non-small cell lung cancer: an update on the Cancer and Leukemia Group B experience. Semin Oncol 19(1 suppl 2): 44, 1992.
Green RA, et al: Alkylating agents and bronchogenic carcinoma. Am J Med 46:516, 1969.
Greenberg DB, et al: Fatigue syndrome due to localized radiation. J Pain Symptom Manage 7:38, 1992.
Gregor A, et al: Randomized trial of alternating versus sequential radiotherapy/chemotherapy in limited-disease patients with small cell lung cancer: a European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group study. J Clin Oncol 15:2840, 1997a.
Gregor A, et al: Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer 33:1752, 1997b.
Hainsworth JD, Greco FA: Paclitaxel in lung cancer: 1-hour infusions given alone or in combination chemotherapy. Semin Oncol 22:45, 1995.
Hainsworth JD, et al: Paclitaxel, carboplatin, and extended-schedule oral etoposide for small cell lung cancer. Oncology (Huntingt) 12(1 suppl 2): 31, 1998.
Hall E: Radiobiology for the Radiologist. 3rd Ed. Philadelphia: Lippincott, 1988.
Hanna N, et al: Phase II trial of maintenance daily oral VP-16 versus no further therapy following induction chemotherapy with VP-16 plus ifosfamide plus cisplatin in extensive small cell lung cancer: a Hoosier Oncology Group Trial (LUN 93 2) [Abstract]. Proc Am Soc Clin Oncol 20:316a, 2001.
Hansen HH, et al: Prophylactic irradiation in bronchogenic small cell anaplastic carcinoma. A comparative trial of localized versus extensive radiotherapy including prophylactic brain irradiation in patients receiving combination chemotherapy. Cancer 46:279, 1980.
Hansen M: Paraneoplastic syndromes and tumor markers for small cell and non-small cell lung cancer. Curr Opin Oncol 2:345, 1990.
Harper P, et al: A randomized study of oral etoposide versus combination chemotherapy in poor prognosis small cell lung cancer [Abstract]. Proc Am Soc Clin Oncol 15:27, 1996.
Havemann MWK: Alternating chemotherapy in small cell lung cancer. Onkologie 13:157, 1990.
Heyne KH, et al: The incidence of second primary tumors in long-term survivors of small cell lung cancer. J Clin Oncol 10:1519, 1992.
Hirsch F: Staging and prognostic factors: 1. Staging procedures. Lung Cancer 5:152, 1989.
Hochstenbag MM, et al: Detection of bone marrow metastases in small cell lung cancer. Comparison of magnetic resonance imaging with standard methods. Eur J Cancer 32A:779, 1996.
Holst JJ, et al: Elevated plasma concentrations of C-flanking gastrin-releasing peptide in small cell lung cancer. J Clin Oncol 7:1831, 1989.
Hryniuk W, Levine MN: Analysis of dose intensity for adjuvant chemotherapy trials. J Clin Oncol 4:1161, 1986.
Hryniuk WM, Figueredo A, Goodyear M: Applications of dose intensity to problems of chemotherapy of breast and colorectal cancer. Semin Oncol 14:3, 1987.
Humblet Y, et al: Late intensification chemotherapy with autologous bone marrow transplantation in selected small cell carcinoma of the lung: a randomized study. J Clin Oncol 5:1864, 1987.
Hyde L, et al: Cell type and the natural history of lung cancer. JAMA 193:140, 1965.
Hyde L, et al: Natural course of inoperable lung cancer. Chest 64:309, 1973.
Iggo R, et al: Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet 335:675, 1990.
Ihde DC: Current status of therapy for small cell carcinoma of the lung. Cancer 54:2722, 1984.
Ihde DC: Staging evaluation and prognostic factors in small cell lung cancer. In Aisner J (ed): Contemporary Issues in Clinical Oncology. Vol 3. Lung Cancer. New York: Churchill Livingstone, 1985, p. 241.
Ihde DC: Chemotherapy of lung cancer. N Engl J Med 327:1434, 1992.
Ihde DC, et al: Prognostic implications of stage of disease and sites of metastases in patients with small cell carcinoma of the lung treated with intensive combination chemotherapy. Am Rev Respir Dis 123:500, 1971.
Ihde DC, et al: Late intensive combined modality therapy followed by autologous bone marrow infusion in extensive-stage small-cell lung cancer. J Clin Oncol 4:1433, 1986.
Ihde DC, et al: Randomized trial of high vs. standard dose etoposide (VP16) and cisplatin in extensive small cell lung cancer (SCLC) [Abstract]. For the 6th World Conference on Lung Cancer, Melbourne. Proc Am Soc Clin Oncol 10:240, 1991.
Inoue M, et al: Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg 70:1615, 2000a.
Inoue M, et al: Results of preoperative mediastinoscopy for small cell lung cancer. Ann Thorac Surg 70:1620, 2000b.
Jackson DV Jr, et al: Prophylactic cranial irradiation in small cell carcinoma of the lung. A randomized study. JAMA 237:2730, 1977.
P.1734
Janne P, et al: The survival of patients treated for limited stage small cell lung cancer in North America has increased during the past 20 years [Abstract]. Proc Am Soc Clin Oncol 20:317a, 2001.
Jeremic B, et al: Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small cell lung cancer: a randomized study. J Clin Oncol 15:893, 1997.
Jett JR, Su JQ, Maksymiuk AW: Phase III trial of recombinant interferon gamma (IFN- ) in complete responders (CR) with small cell lung cancer (SCLC) [Abstract]. Proc Am Soc Clin Oncol 11:287, 1992.
Johnson BE, et al: Limited (Ltd) stage small cell lung cancer (SCLC) treated with concurrent BID chest radiation therapy (RT) and etoposide cisplatin (VP/PT) followed by chemotherapy (CT) selected by in vitro drug sensitivity testing (DST). For the 6th World Conference on Lung Cancer, Melbourne. Lung Cancer 7(suppl):152, 1991a.
Johnson BE, et al: Limited (Ltd) stage small cell lung cancer treated with concurrent BID chest radiation therapy and etoposide/platinum followed by chemotherapy selected by in vitro drug sensitivity testing. Proc Am Soc Clin Oncol 10:240, 1991b.
Johnson D, et al: Topotecan vs. observation following cisplatin plus etoposide in extensive stage small cell lung cancer: a phase III trial of the Eastern Cooperative Oncology Group [Abstract]. Proc Am Soc Clin Oncol; 19:482a, 2000.
Johnson DH: New drugs in the management of SCLC. Lung Cancer 5:221, 1989.
Johnson DH: Overview of ifosfamide in small cell and non-small cell lung cancer. Semin Oncol 17(suppl 4):24, 1990.
Johnson DH, et al: High-dose induction chemotherapy with cyclophosphamide, etoposide, and cisplatin for extensive-stage small-cell lung cancer. J Clin Oncol 5:703, 1987a.
Johnson DH, et al: A randomized comparison of high-dose versus conventional-dose cyclophosphamide, doxorubicin and vincristine for extensive-stage small cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 5:1731, 1987b.
Johnson DH, et al: Alternating chemotherapy (CT) and thoracic radiation therapy (TRT) in limited small cell lung cancer (LSCLC): a test of the Looney hypothesis. For the Eastern Cooperative Oncology Group [Abstract]. Proc Am Soc Clin Oncol 10:243, 1991.
Joss R, et al: Combined modality treatment of small cell lung cancer (SCLC): randomized comparison of induction chemotherapy with or without radiation therapy to the chest (RT) [Abstract]. Proceedings of the Fourth World Conference on Lung Cancer. Toronto: International Association for the Study of Lung Cancer, 1985.
Karrer K: Is the progress in cancer treatment results adequate or are we confronted with a more or less world wide stagnation? J Cancer Res Clin Oncol 116:425, 1990.
Karrer K, Shields T: The importance of complete resection in the multimodality treatment of SCLC. For ISC-Lung Cancer Study Group, 6th World Conference on Lung Cancer, Melbourne. Lung Cancer 7(suppl): 71, 1991.
Katsenis AT, et al: Elective brain irradiation in patients with small-cell carcinoma of the lung: preliminary report. Tokyo: Lung Cancer International Congress Series, Excerpta Medica, 1982, p. 277.
Kern JA, et al: p185neu expression in human lung adenocarcinomas predicts shortened survival. Cancer Res 50:5184, 1990.
Kies MS, et al: Multimodal therapy for limited small cell lung cancer: a randomized study of induction combination chemotherapy with or without thoracic radiation in complete responders; and with wide-field versus reduced field radiation in partial responders: a Southwest Oncology Group study. J Clin Oncol 5:592, 1987.
Klasa RJ, Murray N, Colman AJ: Dose-intensity meta-analysis of chemotherapy regimens in small-cell carcinoma of the lung. J Clin Oncol 9: 499, 1991.
Klastersky J: Diagnosis and staging in small cell lung cancer. Curr Opin Oncol 2:331, 1990.
Klimo P, Connors JM: MACOP-B chemotherapy for the treatment of diffuse large-cell lymphoma. Ann Intern Med 102:596, 1985.
Kollmannsberger C, et al: Topotecan a novel topoisomerase I inhibitor: pharmacology and clinical experience. Oncology 56:1, 1999.
Kotalik J, et al: Practice guideline on prophylactic cranial irradiation in small-cell lung cancer. Int J Radiat Oncol Biol Phys 50:309, 2001.
Kristjansen PEG: The role of cranial irradiation in the management of patients with SCLC. Lung Cancer 5:264, 1989.
Kristjansen PEG, et al: A three-armed randomized trial in small cell lung cancer (SCLC) of two induction regimens with teniposide and cisplatin or carboplatin followed by alternating chemotherapy versus alternating chemotherapy. Lung Cancer 7(suppl):121, 1991.
Kurtz JM, et al: The palliation of brain metastases in a favorable patient population: a randomized clinical trial by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 7:891, 1981.
Lad T: The role of surgery in small cell lung cancer (SCLC): an intergroup prospective randomized trial. Lung Cancer 4:A83, 1988.
Lad T, et al: Thoracotomy staging of small cell lung cancer [Abstract]. For the 6th World Conference on Lung Cancer, Melbourne. Lung Cancer 7(suppl), 1991.
Laplanche A, et al: Controlled clinical trial of prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Lung Cancer 21:193, 1998.
Layer G, Jarosch K: Magnetic resonance tomography of the bone marrow for the detection of metastases of solid tumors [in German]. Radiologe 32:502, 1992.
Lazarus HM, Spitzer TR, Creger RJ: Phase I trial of high-dose etoposide, high-dose cisplatin, and reinfusion of autologous bone marrow for lung cancer. Am J Clin Oncol 13:110, 1990.
LeBeau B, Chastang CL, Brechot JM: Small cell lung cancer (SCLC): long term results of a randomized trial assessing chemotherapy continuation in patients reaching complete remission after six courses. For the Petites Cellules Group (02PC 83 Protocol), 6th World Conference on Lung Cancer, Melbourne. Lung Cancer 7(suppl):130, 1991a.
LeBeau B, et al: Small cell lung cancer (SCLC): negative results of a randomized trial on delayed thoracic radiation therapy administered to complete responders (CR) patients. Lung Cancer 7:94, 1991b.
LeBeau B, et al: Subcutaneous heparin treatment increases survival in patients with small cell lung cancer. Petites Cellules Group. Cancer 74: 38, 1994.
Lewin AA, et al: Radiation-induced failures of complementary metal oxide semiconductor containing pacemakers: a potentially lethal complication. Int J Radiat Oncol Biol Phys 10:1967, 1984.
Lewis JW Jr, et al: Can computed tomography of the chest stage lung cancer? Yes and no. Ann Thorac Surg 49:591, 1990.
Liengswangwong V, et al: Limited-stage small-cell lung cancer: patterns of intrathoracic recurrence and the implications for thoracic radiotherapy. J Clin Oncol 12:496, 1994.
Lilenbaum RC, et al: Phase I and pharmacologic study of continuous infusion topotecan in combination with cisplatin in patients with advanced cancer: a Cancer and Leukemia Group B study. J Clin Oncol 16:3302, 1998.
Lishner M, et al: Late neurological complications after prophylactic cranial irradiation in patients with small-cell lung cancer: the Toronto experience. J Clin Oncol 8:215, 1990.
Little CD, et al: Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature 306:194, 1983.
Littlewood TJ, Bentley DP, Smith AP: High-dose etoposide with autologous bone marrow transplantation as initial treatment of small cell lung cancer a negative report. Eur J Respir Dis 68:370, 1986.
Livingston RB, et al: Long-term survival and toxicity in small cell lung cancer. A Southwest Oncology Group study. Am J Med 77:415, 1984.
Loehrer PJ Sr: The role of ifosfamide in small cell lung cancer. Semin Oncol 23(suppl 7):40, 1996.
Looney WB, Hopkins HA, Carter WH Jr: Solid tumor models for the assessment of different treatment modalities. XXIII. A new approach to the more effective utilization of radiotherapy alternated with chemotherapy. Int J Radiat Oncol Biol Phys 11:2105, 1985.
Lund B, et al: Phase II study of gemcitabine in non-small cell lung cancer (NSCLC). For the 6th World Conference on Lung Cancer, Melbourne. Lung Cancer 7:121, 1991.
Maasilta P: Radiation-induced lung injury. From the chest physician's point of view. Lung Cancer 7:367, 1991.
Macaulay VM, et al: Autocrine insulin-like growth factor 1 in human small cell lung cancer cell lines and fresh tumor cells. Cancer Res 50: 2511, 1990.
Mah K, Van Dyk J: Quantitative measurement of change in human lung density following irradiation. Radiother Oncol 11:169, 1988.
Marangolo M, et al: High-dose etoposide and autologous bone marrow transplantation as intensification treatment in small cell lung cancer: a pilot study. Bone Marrow Transplant 4:405, 1989.
Masuda N, et al: CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 10:1225, 1992.
Mattson K, et al: Natural alpha interferon as maintenance therapy for small cell lung cancer. For the 6th World Conference on Lung Cancer, Melbourne. Lung Cancer 7(suppl):127, 1991.
P.1735
Maurer LH, et al: A randomized combined modality trial in small cell carcinoma of the lung: comparison of combination chemotherapy-radiation therapy versus cyclophosphamide-radiation therapy effects of maintenance chemotherapy and prophylactic whole brain irradiation. Cancer 45:30, 1980.
Mavroudis D, et al: A multicenter randomized phase III study comparing paclitaxel-cisplatin-etoposide versus cisplatin-etoposide as front line treatment of patients with small cell lung cancer [Abstract]. Proc Am Soc Clin Oncol 19:484a, 2000.
McDonald S, Rubin P, Maasilta P: Response of normal lung to irradiation. Tolerance doses/tolerance volumes in pulmonary radiation syndromes. Front Radiat Ther Oncol 23:255, 1989.
Medical Research Council Lung Cancer Working Party: Inoperable non-small-cell lung cancer (NSCLC): a Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Br J Cancer 63:265, 1991.
Medical Research Council Lung Cancer Working Party: A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Br J Cancer 65:934, 1992.
Medical Research Council Lung Cancer Working Party: Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Clin Oncol 8:167, 1996.
Mehta C, Vogl SE: High-dose cyclophosphamide in the induction therapy of small cell lung cancer. Minor improvements in rate of remission and survival. Proc Am Soc Cancer Res 23:155, 1982.
Miles DW, et al: Intensive weekly chemotherapy for the treatment of extensive-stage small-cell lung cancer. J Clin Oncol 9:1632, 1991.
Miller AB, Fox W, Tall R: Five-year follow-up of the Medical Research Council comparative trial of surgery and radiotherapy for the primary treatment of small-celled carcinoma or oat-celled carcinoma of the bronchus. Lancet 12:501, 1969.
Milroy R, et al: Randomised clinical study of Verapamil in addition to chemotherapy in small cell lung cancer (SCLC). For the 6th World Conference on Lung Cancer, Melbourne. Lung Cancer 7(suppl):114, 1991.
Minna JD, Higgins GA, Glatstein EJ: Cancer of the lung. In DeVita VT Jr, Hellman S, Rosenberg S (eds): Cancer Principles and Practice of Oncology. Philadelphia: JB Lippincott, 1985, p. 507.
Minna JD, et al: Cancer of the lung. In De Vita VT Sr, Hellman S, Rosenberg S (eds): Cancer Principles and Practice of Oncology. 3rd Ed. Philadelphia: JB Lippincott, 1989, p. 591.
Mitchell EP, et al: Etoposide (VP-16) and Cisplatin (DDP) in untreated extensive small cell lung cancer (SCLC): a dose-escalation study [Abstract]. Proc Am Soc Clin Oncol 7:206, 1988.
Mornex F, et al: Hyperfractionated radiotherapy alternating with multidrug chemotherapy in the treatment of limited small cell lung cancer (SCLC). Groupe Lyonnais d'Oncologie Thoracique. Int J Radiat Oncol Biol Phys 19:223, 1990.
Mornex F, Nayel H, Videira A: Efficacy of radiation therapy in 79 patients with brain metastases from primary lung cancer. Lung Cancer 7:98, 1991.
Mountain C: Revisions in the international system for staging lung cancer. Chest 111:1710, 1997.
Murphy PB, et al: Cisplatin (P) & prolonged administration of oral etoposide (E) in extensive small cell lung cancer (ESCLC) patients (PT): a phase II trial [Abstract]. Proc Am Soc Clin Oncol 10:257, 1991.
Murray N, et al: The importance of timing for thoracic irradiation (TI) in the combined modality treatment of limited stage small cell lung cancer (LSCLC) [Abstract]. Proc Am Soc Clin Oncol 10:243, 1991a.
Murray N, et al: Intensive weekly chemotherapy for the treatment of extensive-stage small-cell lung cancer. J Clin Oncol 9:1632, 1991b.
Murray N, et al: The importance of timing for thoracic irradiation in the combined modality treatment of limited stage small cell lung cancer. The National Cancer Institute of Cancer Clinical Trials Group. J Clin Oncol 11:336, 1993.
Murray N, et al: Randomized study of CODE versus CAV/EP for extensive-stage small-cell lung cancer: an intergroup study of the National Cancer Institute of Canada Clinical Trials Group and the Southwest Oncology Group. J Clin Oncol 17:2300, 1999.
Neill HB, et al: A phase II study evaluating the efficacy of carboplatin, etoposide, and paclitaxel with granulocyte colony-stimulating factor in patients with stage IIIB and IV non-small cell lung cancer and extensive small cell lung cancer. Semin Oncol 24(4 suppl 12):S12 130, 1997.
Nieto AF, Doty DB: Superior vena cava obstruction: clinical syndrome, etiology, and treatment. Curr Probl Cancer 10:441, 1986.
Noda K, et al: Randomized phase III study of irinotecan (CPT-11) and cisplatin versus etoposide and cisplatin in extensive disease small cell lung cancer: Japan Clinical Oncology Group Study [Abstract]. Proc Am Soc Clin Oncol 19:483a, 2000.
Nomura F, et al: High dose chemotherapy with autologous bone marrow transplantation for limited small cell lung cancer. Jpn J Clin Oncol 20: 94, 1990.
Nou E, Brodin O, Bergh J: A randomized study of radiation treatment in small cell bronchial carcinoma treated with two types of four drug chemotherapy regimens. Cancer 62:1079, 1988.
O'Donnell MR, et al: Intensive induction chemotherapy for small-cell anaplastic carcinoma of the lung. Cancer Treat Rep 69:571, 1985.
Ohnoshi T, et al: Randomized trial comparing chemotherapy alone and chemotherapy plus chest irradiation in limited stage small cell lung cancer: a preliminary report. Jpn J Clin Oncol 16:271, 1986.
Ohnoshi T, Hiraki S, Kimura I: Randomised trial of chemotherapy alone or with chest irradiation in limited-stage small-cell lung cancer. Lung Cancer International Congress Series, 1987, p. 186.
Okamoto I, et al: CD-44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene 18:1435, 1999.
Oster MW, Fetell M: Meningeal carcinomatosis in small cell carcinoma of the lung. Med Pediatr Oncol 10:157, 1982.
Osterlind K, et al: Chemotherapy versus chemotherapy plus irradiation in limited small cell lung cancer. Results of a controlled trial with 5 years follow-up. Br J Cancer 54:7, 1986.
Patchell RA, et al: A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494, 1990.
Pedersen AG, Bach F, Melgaard B: Frequency, diagnosis, and prognosis of spinal cord compression in small cell bronchogenic carcinoma. A review of 817 patients. Cancer 55:1818, 1985.
Perez EA, Buckwalter CA, Reid JP: Combinations of paclitaxel and etoposide in the treatment of lung cancer. Semin Oncol 223(suppl 15):21, 1996.
Perez-Soler R, et al: Treatment of patients with small cell lung cancer refractory to etoposide and cisplatin with the topoisomerase I poison topotecan. J Clin Oncol 14:2785, 1996.
Perry MC, et al: Chemotherapy with or without radiation therapy in limited small cell carcinoma of the lung. N Engl J Med 316:912, 1987.
Perry MC, et al: Thoracic radiation therapy added to chemotherapy for small cell lung cancer: an update of Cancer and Leukemia Group B Study 8083. J Clin Oncol 16:2466, 1998.
Pfister DG, et al: Classifying clinical severity to help solve problems of stage migration in nonconcurrent comparisons of lung cancer therapy. Cancer Res 50:4664, 1990.
Pignon J-P, et al: A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 327:1618, 1992.
Postmus PE, et al: High-dose cyclophosphamide and high-dose VP 16 213 for recurrent or refractory small cell lung cancer. A phase II study. Eur J Cancer Clin Oncol 21:1467, 1985.
Postmus PE, et al: Effects of recombinant human interleukin-3 in patients with relapsed small cell lung cancer treated with chemotherapy: a dose-finding study. J Clin Oncol 10:1131, 1992.
Prager RL, et al: The feasibility of adjuvant surgery in limited-stage small cell carcinoma: a prospective evaluation. Ann Thorac Surg 38:622, 1984.
Price P, et al: Prospective randomised trial of single and multifraction radiation therapy schedules in the treatment of painful bony metastases. Radiother Oncol 6:247, 1986.
Pujol JL, et al: Dose intensive chemotherapy in patients with advanced small cell lung cancer. Eur J Cancer 31:S21, 1995.
Quoix E, et al: Randomized phase II study of topotecan/cisplatin vs. topotecan/etoposide in patients with untreated extensive stage small cell lung cancer [Abstract]. Proc Am Soc Clin Oncol 20:318a, 2001.
Quon H, et al: The influence of age on the delivery, tolerance, and efficacy of thoracic irradiation in the combined modality treatment of limited stage small cell lung cancer. Int J Radiat Oncol Biol Phys 43:39, 1999.
Rawson NSB, Peto J: An overview of prognostic factors in small cell lung cancer: a report of prognostic factors in small cell lung cancer. A report from the Subcommittee for the Management of Lung Cancer in the United Kingdom, Coordinating Committee on Cancer Research. Br J Cancer 61:597, 1990.
Rege SD, et al: Imaging of pulmonary mass lesions with whole-body positron emission tomography and fluorodeoxyglucose. Cancer 72:82, 1993.
Richardson GE, et al: An algorithm for staging patients (PTS) with small cell lung cancer (SCLC) can save 40% of the initial evaluation costs [Abstract]. Proc Am Soc Clin Oncol 10:242, 1991.
P.1736
Rodichok LD, et al: Early diagnosis of spinal epidural metastases. Am J Med 70:1181, 1981.
Rosen ST, et al: Carcinomatous leptomeningitis in small cell lung cancer: a clinicopathologic review of the National Cancer Institute experience. Medicine (Baltimore) 61:45, 1982.
Rosenthal M, et al: Adjuvant thoracic radiation therapy in small cell lung cancer: ten year follow-up of a randomized study. Lung Cancer 7:235, 1991.
Roth BJ, et al: Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 10:282, 1992.
Rubin P, Casarett GI: A direction for clinical radiation pathology. Front Radiat Ther Oncol 6:1, 1972.
Sacks H, et al: Meta-analyses of randomized controlled trials. N Engl J Med 316:450, 1987.
Sagman U, et al: The prognostic significance of pretreatment serum lactate dehydrogenase in patients with small-cell lung cancer. J Clin Oncol 9:954, 1991a.
Sagman U, et al: Small cell carcinoma of the lung: derivation of a prognostic staging system. J Clin Oncol 9:1639, 1991b.
Sagman U, et al: Second primary malignancies following the diagnosis of small cell lung cancer. J Clin Oncol 10:1525, 1992.
Salazar OM, et al: Single-dose half-body irradiation for palliation of multiple bone metastases from solid tumours. Final Radiation Therapy Group report. Cancer 58:29, 1986.
Samantas E, et al: Randomized comparison of early versus late twice-daily chest irradiation concurrently with chemotherapy in limited disease small cell lung cancer: final results [Abstract]. Proc Am Soc Clin Oncol 19:491a, 2000.
Santoro A, et al: Long-term results of combination chemotherapy-radiation therapy approach in Hodgkin's disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol 5:27, 1987.
Sausville EA, Trepel JB, Moyer JD: Inhibitors of bombesin-stimulated intracellular signals: interruption of an autocrine pathway as a therapeutic strategy in small cell lung carcinoma. Clin Biol Res 354A:193, 1990.
Schiller JH: Topotecan in small cell lung cancer. Semin Oncol 24:S20 27, 1997.
Schiller JH, et al: Phase II study of topotecan in patients with extensive-stage small cell carcinoma of the lung: an Eastern Cooperative Oncology Group trial. J Clin Oncol 14:2345, 1996.
Sculier JP, Feld R: Superior vena cava obstruction syndrome: recommendations for management. Cancer Treat Rev 12:209, 1985.
Sculier JP, et al: Carcinoembryonic antigen: a useful prognostic marker in small cell lung cancer. J Clin Oncol 3:1349, 1985a.
Sculier JP, Klastersky J, Stritckmans P: Late intensification in small cell lung cancer: a phase I study of high doses of cyclophosphamide and etoposide with autologous bone marrow transplantation. J Clin Oncol 3:184, 1985b.
Sculier JP, et al: A 7-drug combination polychemotherapy for small cell lung cancer (SCLC): report of a phase-II study [Abstract]. Proc Am Soc Clin Oncol 7:215, 1988.
Sculier JP, et al: A three-arm phase III randomized trial testing accelerated chemotherapy with GM-CSF or cotrimoxazole in extensive small cell lung cancer [Abstract]. Proc Am Soc Clin Oncol 20:317a, 2001.
Seto T, et al: Effect on prognosis of bone marrow infiltration detected by magnetic resonance imaging in small cell lung cancer. Eur J Cancer 33:2333, 1997.
Seydel HG, et al: Combined modality treatment of regional small cell undifferentiated carcinoma of the lung. A cooperative study of the RTOG and ECOG. Int J Radiat Oncol Biol Phys 9:1135, 1983.
Seydel HG, et al: Prophylactic versus no brain irradiation in regional small cell lung carcinoma. Am J Clin Oncol 8:218, 1985.
Shank B, et al: Increased survival with high-dose multifield radiation therapy and intensive chemotherapy in limited small cell carcinoma of the lung. Cancer 56:2771, 1985.
Shepherd FA: Alternatives to chemotherapy and radiotherapy as adjuvant treatment for lung cancer. Lung Cancer 17(suppl 1):S121, 1997.
Shepherd FA: Alternatives to chemotherapy and radiotherapy in the treatment of small cell lung cancer. Semin Oncol 28(suppl 4):30, 2001.
Shepherd FA, et al: Reduction in local recurrence and improved survival in surgically treated patients with small cell carcinoma of the lung. J Thorac Cardiovasc Surg 86:498, 1983.
Shepherd FA, et al: Adjuvant chemotherapy following surgical resection for small cell carcinoma of the lung. J Clin Oncol 6:832, 1988.
Shepherd FA, et al: A prospective study of adjuvant surgical resection after chemotherapy for limited small cell lung cancer. A University of Toronto Lung Oncology Group study. J Thorac Cardiovasc Surg 97:177, 1989.
Shepherd FA, et al: Surgical treatment for limited small-cell lung cancer. The University of Toronto Lung Oncology Group experience. J Thorac Cardiovasc Surg 101:385, 1991a.
Shepherd FA, et al: Is there ever a role for salvage operations in limited small cell lung cancer? J Thorac Cardiovasc Surg 101:196, 1991b.
Shepherd FA, et al: Importance of clinical staging in limited small-cell lung cancer: a valuable system to separate prognostic subgroups. The University of Toronto Lung Oncology Group. J Clin Oncol 11:1592, 1993.
Shepherd F, et al: Randomized double-blind, placebo-controlled trial of Marimastat in patients with small cell lung cancer following response to first-line therapy: an NCIC-CTG and EORTC trial [Abstract]. Proc Am Soc Clin Oncol 20:4a, 2001.
Shields TW, Karrer K: Surgery for small cell lung cancer. In Roth JA, Cox JD, Hong WK (eds): Lung Cancer. 2nd Ed. Cambridge, MA: Blackwell Science, 1998, p. 115.
Shields TW, et al: Surgical resection in the management of small-cell carcinoma of the lung. J Thorac Cardiovasc Surg 84:481, 1982.
Simpson J, et al: Palliative radiotherapy for inoperable carcinoma of the lung: final report of a RTOG multi-institutional trial. Int J Radiat Oncol Biol Phys 11:751, 1985.
Skov BG, et al: Prognostic impact of histologic demonstration of chromogranin A and neuron-specific enolase in pulmonary adenocarcinoma. Ann Oncol 2:355, 1991.
Slawson RG, Scott RM: Radiation therapy in bronchogenic carcinoma. Radiology 132:175, 1979.
Slevin ML, et al: A randomized trial to evaluate the effect of schedule on the activity of etoposide in small cell lung cancer. J Clin Oncol 7:1333, 1989.
Smyth JF: The management of small cell anaplastic lung cancer. In Smyth JF (ed): The Management of Lung Cancer. London: Edward Arnold, 1984, p. 115.
Smyth JF: Cancer genetics and cell and molecular biology. Is this the way forward? Chest 109(suppl 5):125S, 1996.
Sorensen HR, Lund C, Alstrup P: Survival in small cell lung carcinoma after surgery. Thorax 41:479, 1986.
Souhami RL, et al: Radiotherapy in small cell cancer of the lung treated with combination chemotherapy: a controlled trial. Br Med J 288:1643, 1984.
Souhami RL, et al: Intensive chemotherapy with autologous bone marrow transplantation for small cell lung cancer. Cancer Chemother Pharmacol 24:321, 1989.
Souhami RL, et al: Five-day oral etoposide treatment for advanced small cell lung cancer: randomized comparison with intravenous chemotherapy. J Natl Cancer Inst 89:577, 1997.
Splinter TAW: EORTC 08825 induction versus induction plus maintenance chemotherapy in small cell lung cancer: definitive evaluation [Abstract]. For the EORTC Lung Co-operative Group. Proc Am Soc Clin Oncol 7:202, 1988.
Splinter TAW: Chemotherapy of SCLC: duration of treatment. Lung Cancer 5:186, 1989.
Splinter T, et al: EORTC 08825 induction versus induction plus maintenance chemotherapy (CT) in small cell lung cancer [Abstract]. Proc Am Soc Clin Oncol 5:188, 1986.
Stahel RA: Diagnosis, staging and prognostic factors of small cell lung cancer. Curr Opin Oncol 3:306, 1991.
Stahel RA: Morphology, surface antigens, staging and prognostic factors of small cell lung cancer. Curr Opin Oncol 4:308, 1992.
Stahel RA, et al: Detection of bone marrow metastasis in small cell lung cancer by monoclonal antibody. J Clin Oncol 3:455, 1985.
Stahel R, et al: Staging and prognostic factors in small cell lung cancer. Lung Cancer 5:119, 1989.
Stevens E, Einhorn L, Sohn R: Treatment of limited small cell cancer [Abstract]. Proc Am Assoc Cancer Res 20:435, 1979.
Stevenson HC, et al: Lack of relationship between in vitro tumour cell growth and prognosis in extensive-stage small-cell lung cancer. J Clin Oncol 113:923, 1989.
Steward W, et al: Dose intensification of V-ICE chemotherapy with GM-CSF in small cell lung cancer a prospective randomized study of 301 patients. Eur J Oncol 31:S18, 1995.
Sundaresan V, et al: Neuroendocrine differentiation and clinical behaviour in non-small cell lung tumours. Br J Cancer 64:333, 1991.
Suwinski R, Lee S, Withers HR: Dose-response relationship for prophylactic cranial irradiation in small cell lung cancer. Int J Radiat Oncol Biol Phys 40:797, 1998.
P.1737
Szabo E, et al: Overexpression of CC10 modifies neoplastic potential in lung cancer cells. Cell Growth Differ 9:475, 1998.
Takada M, et al: Phase III study of concurrent versus sequential thoracic radiation therapy in combination with cisplatin and etoposide for limited-stage small cell lung cancer: preliminary results of the Japan Clinical Oncology Group [Abstract]. Proc Am Soc Clin Oncol 15:1103, 1996.
Takimoto CH, Arbuck SG: Clinical status and optimal use of topotecan. Oncology (Huntingt) 11:1635, 1997.
Taylor CW, et al: Treatment of small-cell lung cancer with an alternating chemotherapy regimen given at weekly intervals: a Southwest Oncology Group pilot study. J Clin Oncol 8:1811, 1990.
Thames H, Hendry J: Fractionation in Radiation Therapy. Philadelphia: Taylor & Francis, 1987, p. 297.
Thatcher N, Lind M: Carboplatin in small cell lung cancer. Semin Oncol 17(suppl 2):40, 1990.
Tjan-Heijnen V, et al: Dose-intensification of cyclophosphamide, doxorubicin and etoposide chemotherapy does not improve survival in small cell lung cancer: final results of a randomized phase III EORTC trial [Abstract]. Proc Am Soc Clin Oncol 20:346a, 2001.
Tourani JM, et al: Short intensive five drug chemotherapy (CT) followed by intensive irradiation for limited small cell lung cancer (LSCLC). Improved response rate and survival. A pilot study [Abstract]. Proc Am Soc Clin Oncol 10:245, 1991.
Trillet-Lenoir VN, Arpin D, Brune J: Bone marrow metastases detection in small cell lung cancer: a review. Anticancer Res 14:2795, 1994.
Trump D, et al: Treatment of neoplastic meningitis with intrathecal methotrexate and thiotepa [Abstract]. Proc Am Soc Clin Oncol 22:160, 1981.
Tsai CM, et al: Correlation of in vitro sensitivity testing of long-term small cell lung cancer cell lines with response and survival. Eur J Cancer 26:1148, 1990.
Turrisi AT: Brain irradiation and systemic chemotherapy for small-cell lung cancer: dangerous liaisons? J Clin Oncol 8:196, 1990.
Turrisi AT III, Glover D: Thoracic radiotherapy variables: influence on local control in small cell lung cancer limited disease. Int J Radiat Oncol Biol Phys 19:1473, 1990.
Turrisi AT III, et al: Twice-daily compared with once-daily thoracic radiotherapy in limited small cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340:265, 1999.
Van Dyk J, Mah K, Keane T: Radiation-induced lung damage: dose-time-fractionation considerations. Radiother Oncol 14:55, 1989.
Von Pawel J, et al: Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small cell lung cancer. J Clin Oncol 17:658, 1999.
Warde P, Payne D: Does thoracic irradiation improve survival or local control in limited-stage small-cell carcinoma of the lung? A meta analysis. J Clin Oncol 10:890, 1992.
Wasserstrom WR, Glass JP, Posner JB: Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 49:759, 1982.
Watson W, Berg J: Oat cell lung cancer. Cancer 15:759, 1962.
Weiner DB, et al: Expression of the new gene-encoded protein (P185neu) in human non-small cell carcinoma of the lung. Cancer Res 50:421, 1990.
Whang-Peng J, et al: A specific chromosome defect associated with human small cell lung cancer detection; 3p (14 23). Science 215:181, 1982.
Wils J, et al: Phase II study of high dose epirubicin in non-small cell lung cancer. Eur J Cancer 26:1140, 1990.
Withers HR: Some changes in concepts of dose fractionation over 20 years. Front Radiat Ther Oncol 22:1, 1988.
Wojtowicz-Praga S, et al: Phase I trial of Marimastat, a novel matrix metalloproteinase inhibitor, administered orally to patients with advanced lung cancer. J Clin Oncol 16:2150, 1998.
Woll PJ, Rozengurt E: Therapeutic implications of growth factors in small cell lung cancer. Lung Cancer 5:287, 1989.
Woll PJ, et al: Can cytotoxic dose-intensity be increased by using granulocyte colony-stimulating factor? A randomized controlled trial of lenograstim in small cell lung cancer. J Clin Oncol 13:652, 1995.
Woll PJ, et al: Use of hematopoietic progenitors in whole blood to support dose-dense chemotherapy: a randomized phase II trial in small cell lung cancer patients. J Clin Oncol 19:712, 2001.
Work E, et al: Randomized study of initial versus late chest irradiation combined with chemotherapy in limited-stage small cell lung cancer. Aarhus Lung Cancer Group. J Clin Oncol 15:3030, 1997.
Wurschmidt F, Bunemann H, Heilmann HP: Small cell lung cancer with and without superior vena cava syndrome: a multivariate analysis of prognostic factors in 408 cases. Int J Radiat Oncol Biol Phys 33:77, 1995.
Zelen M: Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 4:31, 1973.
SUGGESTED READING
Arriagada R, Kramar A, Le Chevalier T: Competing events determining relapse-free survival in limited small cell lung carcinoma. The French Cancer Centers' Lung Group. J Clin Oncol 10:447, 1992.
EAN: 2147483647
Pages: 203