94 - Diffuse Lung Disease
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > Section XVI - Carcinoma of the Lung > Chapter 109 - Basic Principles of Radiation Therapy in Carcinoma of the Lung
Chapter 109
Basic Principles of Radiation Therapy in Carcinoma of the Lung
Bahman Emami
Nena Mirkovic
PHYSICAL ASPECTS OF RADIATION THERAPY
Ionizing radiation is part of the spectrum of electromagnetic energy that produces physical and biochemical events when they interact with the atoms of irradiated material. Gamma rays are nonparticle radiation beams emitted by naturally occurring radioactive isotopes. X-rays are nonparticle radiation beams produced by man-made sources, such as linear accelerators. Particle radiation beams most commonly used in radiation therapy include electron, proton, and neutron beams.
When a beam of ionizing radiation interacts with material, it ejects an electron from an atom. These high-speed electrons transfer their energy to the material by producing ionization and excitations of atoms along their pathways. The absorbed energy per mass unit of material is radiation-absorbed dose.
Units of Radiation
Radiation dose absorbed in tissue is measured in Gray (Gy), defined as energy deposited per kilogram of mass. The older unit of rad (radiation absorbed dose) equals 0.01 Gy. One cGy equals 1 rad. Roentgen (R) is a unit of radiation exposure in air. Activity of a radioactive isotope is measured in curies, which is a number of disintegrations per second undergone by 1 g of radium.
Nomenclature
The International Commission of Radiation Units and Measurements has recommended definitions of important concepts in treatment planning in radiation therapy [International Commission of Radiation Units and Measurements Report 50 (ICRU-50) (1993)] (Fig. 109-1):
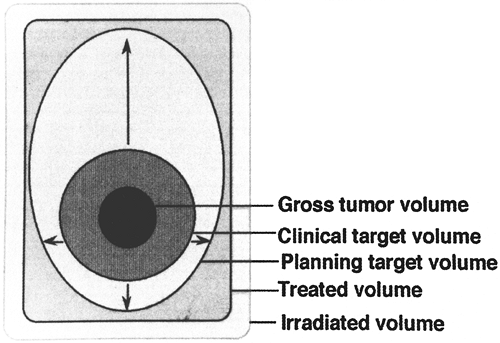 |
Fig. 109-1. Schematic illustration of the different volumes as defined by ICRU-50. From ICRU-50 Prescribing, Recording, and Reporting Photon Beam Therapy. Bethesda, MD: International Commission on Radiation Units and Measurements, 1993, p. 5. With permission. |
Gross tumor volume (GTV) is defined as all known gross tumor that is seen on imaging, palpable or determined in any other way. Gross tumor volume in lung cancer is determined primarily on lung windows of a computed tomographic (CT) scan. There may be several millimeters of difference between GTVs determined on lung windows and those determined on mediastinal windows. In addition, there is considerable interobserver and intraobserver variability.
Clinical target volume (CTV) is defined as areas suspected of harboring microscopic cancer cells. In lung cancer, CTV generally includes microscopic extension of the primary tumor into the surrounding apparently normal tissue or clinically normal lymph nodes suspected of bearing microscopic disease.
Planning target volume (PTV) is a margin of normal tissue surrounding the previously described volumes that is included in the radiation volume to account for organ movement and inaccuracies of daily setup. Inaccuracies in daily setup can be minimized by using proper immobilization technology. Organ movement is much more difficult to deal with. In lung cancer, respiratory and cardiac movements are considerable and need to be taken into account for planning (discussed subsequently).
EQUIPMENT USED IN CLINICALRADIATION THERAPY
High-energy x-rays produced by linear accelerators are by far the most commonly used beams for thoracic radiation therapy in the United States. Linear accelerators produce high-energy x-rays by bombarding a heavy-metal target with fast electrons. Low-energy x-rays such as those produced by orthovoltage or superficial machines have poor tissue penetration characteristics and deposit excessive dose in superficial tissues, thus making them unsuitable for treatment of deep-seated thoracic tumors.
P.1614
Cobalt teletherapy units house isotope cobalt 60 (60Co) that omits gamma rays. 60Co machines have been extensively used for thoracic radiation therapy in the past but now are largely replaced by linear accelerators. 60Co beams have relatively low energy and deposit excessive doses in normal lung before reaching the target, increasing the risk for radiation pneumonitis. Thus, the use of 60Co is no longer recommended for thoracic radiation therapy.
BIOLOGICAL EFFECTS OF IONIZING RADIATIONS AND BIOLOGICAL BASIS OF RADIATION THERAPY
The critical target in cell death is in the nucleus and is probably DNA. Ionizing radiation damages DNA in at least four different ways: (a) double-helix strand breaks, (b) single-strand breaks, (c) base damage, and (d) damage to cross-links in which both DNA DNA and DNA protein cross-links are involved.
These physical and biochemical phenomena take place in fractions of a second at the time of the radiation exposure. Various nuclear and cytoplasmic molecular changes then follow, leading to loss of reproductive ability, cell cycle delays, somatic transformation, and mutations, among others.
Chromosomes may be damaged by ionizing radiation. Classically, the damage is not detected in interphase and only appears as the cells go through cell division. Kaplan (1963) reported that the frequency of mutations produced by the single- or double-strand breaks on the DNA molecule depends on not only the number of initial breaks but also the adequacy of the repair mechanisms.
Carcinogenesis
Ionizing radiation has the potential to induce cancer in any organ. The adult thyroid gland may tolerate as much as 4,000 rad without significant changes, but in children who received between 200 and 800 rad to the neck for thymic enlargement, the development many years later of thyroid carcinoma, usually of the papillary type, has been reported.
Cell Kill by Ionizing Radiation
When a cell is hit by an ionizing particle, different kinds of damage may take place. Lethal damage occurs when the cell loses its ability for unlimited proliferation. After radiation exposure, the cell and its progeny die, although as many as five to six cell divisions may occur. Potentially lethal damage consists of slightly less severe impairment of the proliferative ability of the cell from which it may recover, but any modification in its environment interferes with repair and causes the cell to die.
Sublethal damage occurs when the injury induced by the ionizing radiation can be repaired by the cell. After exposure to ionizing radiation, the cells exhibit changes in their growth rate, including prolongation of the generation time and mitotic delay.
Factors Affecting the Biological Effects of Ionizing Radiation
Cell Sensitivity to Radiation
Terasima and Tolmach (1963) demonstrated in cell cultures and experimental animals that the sensitivity of cells to ionizing radiation and to most chemotherapeutic agents varies according to the phase of the cell cycle in which exposure of the cell to the physical or chemical event occurs.
In clinical practice, a distinction must be made between cell sensitivity to radiation on the one hand and tumor response and curability. Some tumors, such as seminomas, dysgerminomas, lymphomas, and small cell undifferentiated lung carcinoma, are sensitive to radiation and may disappear after low or moderated doses, but the patient may not necessarily be cured and eventually dies of disseminated disease. Epidermoid carcinoma [e.g., non small cell lung cancer (NSCLC)] requires high doses (6,500 to 7,500 rad or higher) for tumor control. In contrast to general concepts, adenocarcinomas are as curable with irradiation as squamous cell carcinomas.
Oxygen Enhancement Effect (Reoxygenation)
With sparsely ionizing radiation, such as x-rays or gamma rays, a given biological effect produced by a given dose is two to three times greater in the presence of oxygen
P.1615
than if it is absent. This augmentation is called the oxygen enhancement ratio. Oxygen must be present during the radiation exposure. The oxygen enhancement effect is lessened or absent in high linear energy transfer (LET) radiations, such as alpha particles, fast neutrons, and pi-mesons. Although increasing concentrations of oxygen result in more sensitization to radiation, Gray (1961) reported that no significant gain is observed when the oxygen pressure is higher than 30 mm Hg. Suit and Shalek (1963) suggested that the hypoxic cell population determines the response of a tumor to radiation and the probability of control of a tumor by radiation therapy. Fractionated radiation causes a decrease in the size of the tumor and the initial number of cells as well as new blood vessel proliferation and reoxygenation. Kallman (1972) noted that these changes result in a transfer of hypoxic tumor cells to a more oxygenated compartment, and this transfer in turn eventually leads to complete sterilization of the tumor without significant injury to the surrounding normal tissues. The lack of oxygen enhancement effect in the biological events induced by high LET particles has resulted in renewed interest in the clinical applications of these radiations.
Linear Energy Transfer
LET represents the energy transferred by an ionizing particle per unit length of pathway. Because most ionizing particles are not energetic, the LET that results from a beam of energy is an average of all the particles or photons in the beam. In addition, at the molecular level, the energy per unit length of track varies. Therefore, the LET of an ionizing particle depends in a complex way on the energy and charge of the particle: the greater the charge and the lesser the velocity, the higher the LET.
Because of these varying amounts of energy released in an absorber, equal doses of various types of radiation do not produce the same biological effects on the absorber, or patient. The term relative biological effect was established to compare the biological effectiveness of a given ionizing radiation with a certain standard, which are 250-kV x-rays. For instance, 60Co has a relative biological effect of about 0.95, and neutrons have a relative biological effect of between 2.0 and 2.5, depending on their energy.
Repair of Radiation Damage (Dose Fractionation)
Radiation therapy is given in daily fractions, four or five times per week, on the presumption that normal cells generally have a greater and faster capacity to repair sublethal damage and cell repopulation between fractions. This time-dose relation depends on individual sensitivity of the cells and their repair ability, size of the radiation fractions, total dose given, time between fractions and of overall treatment, initial hypoxic subpopulation and reoxygenation that takes place throughout the fractionated therapy, and the type of ionizing radiation used.
In analyzing the treatment of the patient, the specifications should include not only the volume of tissue treated and the total dose given but also the number of fractions and the overall period of time in which they are administered.
Dose Fractionation in Radiation Therapy of Lung Cancer
In the United States, typical dose fractionation used in lung cancer treatment is 1.8 to 2 Gy per fraction, delivered as one fraction per day, for overall treatment duration of about 6 weeks. In an effort to increase therapeutic efficacy, different fractionation schedules are explored, generally aimed to increase the number of daily fractions and decrease the overall treatment duration. The stated rationales for such an approach are several. If the cells are most sensitive in G2/M phase of the mitotic cycle, increasing the number of fractions should theoretically increase the probability of strike in the most sensitive phase. Furthermore, as the number of surviving cells is reduced by the initial part of therapy, the remaining cells proliferate more rapidly a phenomenon known as accelerated repopulation. More frequent radiation treatments should in theory counteract rapid proliferation. Finally, by splitting the daily radiation dose into two smaller fractions, the late effects are thought to be reduced, owing to difference in radiation sensitivity between rapidly cycling cells such as tumor and quiescent cells such as normal lung tissue. Due to a phenomenon known as shoulder on a cellular survival curve, rapidly proliferating cells sustain relatively more damage by smaller doses of radiation compared with noncycling cells. By repeating frequent administrations of smaller-than-usual fractions, the therapeutic window of radiation is increased, allowing for higher radiation dose. However, despite the attractive biological concept, the improved efficacy of such regimens has not been confirmed in extensive clinical research, such as the trial reported by Sause and associates (2000), with the possible exception of one trial reported by Turrisi and colleagues (1999) in small cell lung cancer.
PROCESS OF RADIATION THERAPY
The process of radiation therapy is initiated with evaluation and staging of the patient, both anatomically and pathologically. Comprehensive evaluation of the physiologic and functional status of the normal tissue organs such as lungs, heart, and spinal cord are an essential part of this evaluation. After the decision is made on the regimen to be used (e.g., conventional fractionation, hyperfractionation, radiation-chemotherapy), the technical process of administering radiation therapy begins with simulation. In the past,
P.1616
this planning process was with a single radiograph, called simulator film (Fig. 109-2). On obtaining the simulation film, which is usually in anteroposterior or lateral projection, a portal would be outlined by the radiation oncologist that presumably contained the area of gross tumor as well as areas of subclinical disease and outlined the area to be radiated. After appropriate measurements of the dimensions of this area of the film and the separation of the patient, calculations would have been carried out and the delivery of treatment would commence. This technique is currently labeled one-dimensional (1D) radiation therapy.
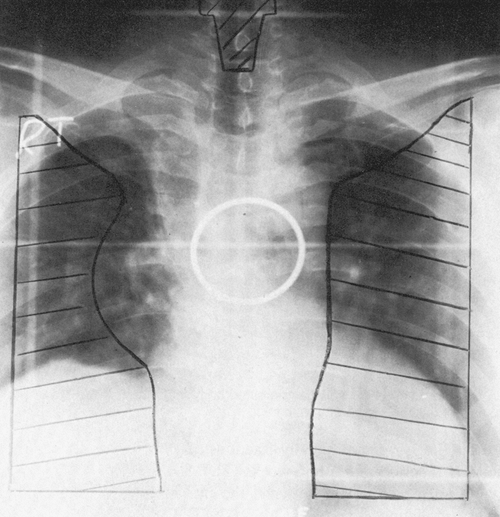 |
Fig. 109-2. An anteroposterior simulation film illustrating the target volume and Cerrobend blocks for protection of normal tissue. The ipsilateral hilum, entire mediastinum, and both supraclavicular areas are included in the target volume for positive regional lymph nodes. |
The introduction of CT scanning to the practice of radiation oncology in the 1970s was an important step, not only for evaluation of the patients but also for planning purposes, as reported by one of us (BE) and colleagues (1978). This technique of planning, which is currently used in many radiation therapy departments around the world, is labeled two-dimensional (2D) treatment planning, as subsequently described. Moving from the 1D to the 2D technique, as noted by one of us (BE) and colleagues (1978), was a major improvement. Nevertheless, even after two decades of use of using 2D technology, serious limitations exist with this technique (described subsequently).
Technologic Advances in Radiation Therapy
Two-dimensional planning has been practiced in radiation therapy for decades. The entrance path of the radiation beam is drawn on a plain chest radiograph (see Fig. 109-2), shaped around the tumor and subclinically involved areas. Dose distribution is then calculated on one or more slices of the CT scan. The plan is judged to be adequate if the desired isodose curve encompasses the desired target volume on the single CT slice (Fig. 109-3). There are a number of problems with that kind of planning. The gross tumor on a plain chest radiograph is frequently not well delineated, and mediastinal lymph nodes are obscured by mediastinal shadows. Soft tissue anatomy is poorly seen on plain radiographs. One CT slice is hardly representative of the entire thoracic volume. Even if isodose distribution is obtained on
P.1617
multiple CT slices, there is no algorithm to calculate dose distribution over the entire organ of interest. These shortcomings are thought to be at least partially responsible for poor results obtained with radiation therapy in the treatment of lung cancer.
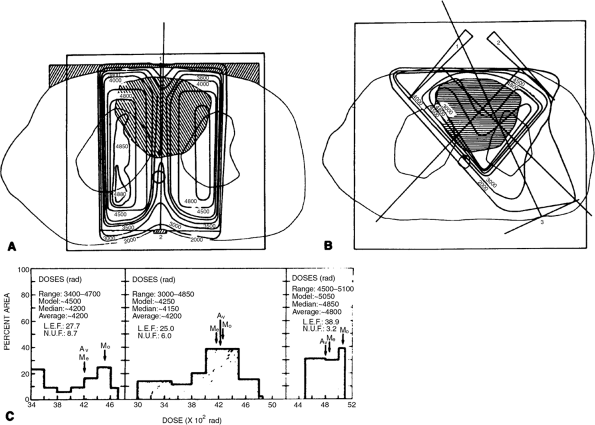 |
Fig. 109-3. A. Computed tomographic (CT) unoptimized treatment plan with lung transmission correction. Beam configuration and weights were identical to those in the conventional plan. Hatched area within patient's contour represents the target area as defined by a CT scan. Accurate lung transmission correction could be made because of the lung outline, and density could be obtained directly from CT scans. Hatched area outside the patient's contour represents the compensating filter and spinal cord block. B. CT optimized plan with lung transmission correction. Wedged beams improved the uniformity of dose and reduced the dose to normal structures. C. Doses and local efficiency and nonuniformity factors for each plan. Notice the decreased mediastinal dose resulting from the posterior spinal cord block. From Prasad S, Pilepich MV, Perez CA: Contribution of CT to quantitative radiation therapy planning. AJR Am J Roentgenol 136: 123, 1981. With permission. |
In the 1990s, the advent of computer technology led to development of three-dimensional conformal radiation therapy (3D-CRT) by one of us (BE) and associates (1991a) and by Purdy and one of us (BE) (1991), which has been replacing older, 2D technology, in many academic and nonacademic centers during the past decade (Fig. 109-4).
 |
Fig. 109-4. Radiation therapy treatment planning software three-dimensional rendering of a patient's skin (maximum gray), spinal cord (white), esophagus (very light gray), heart (medium gray), gross tumor volume (GTV) (gray), and planning target volume (PTV) (whitish gray). |
Three-dimensional conformal radiation therapy is not just an add-on to the 2D radiation oncology planning process. Rather, it represents a radical change in practice, particularly for radiation oncologists, as stressed by Purdy and collaborators (2000). The 2D treatment planning approach emphasizes the use of a conventional simulator for designing beam portals based on standardized beam arrangement techniques and bony landmarks visualized on planner radiographs. Three-dimensional treatment planning emphasizes a volumetric image-based visual simulation approach for defining tumor volume and organs at risk in the individual patient. A review of various stages and procedures involved in 3D-CRT is shown in Table 109-1.
Table 109-1. Three-dimensional Radiation Therapy Planning and Conformal Radiation Therapy | |
|---|---|
|
Clinical Experience with Three-dimensional Conformal Radiation Therapy
For most patients with unresectable NSCLC referred for radiation therapy, local control and survival rates remain poor. As reported by Arriagada and associates (1991) in a randomized study of more than 350 patients, the local control rate at 2 years with or without chemotherapy was 10%. Reports from the Radiation Therapy Oncology Group by Komaki (1998) and Sause (1995, 2000) and their co-workers, in which more than 452 patients were randomized to conventional radiation therapy, radiation therapy and chemotherapy, or hyperfractionated radiation therapy, the 5-year survival rates were 5%, 8%, and 6%, respectively. These extremely poor results may be attributed to poor local control. One of the possible reasons for the poor local control and poor survival was thought to be the use of traditional large volumes in combination with the use of 2D technology that precludes delivering a tumoricidal dose to the primary lung cancers commensurate with the tumor volume. It is generally accepted that for epithelial tumors, the dose for eradicating microscopic disease is 50 to 60 Gy, and for gross disease of 1- to 3-cm dimension, it is about 75 Gy. However, the significant majority of patients seen with inoperable lung cancer in radiation therapy clinics have an average tumor size of 4 to 6 cm. Even with knowledge of the aforementioned facts, in most centers, patients with inoperable lung cancer, irrespective of the size of the tumor, are treated with doses of 50 to 60 Gy. One of the possible reasons for using this low dose has been the limited tolerance of normal tissue to radiation and the inability of 2D technology to protect normal tissues adequately from high doses of radiation and thus to avoid unacceptable complications.
The initial clinical experience with 3D radiation therapy was promising (Table 109-2). However, more recently reported series show only modest progress. As reported by Sim and associates (2001), 152 patients with stage III non small cell lung cancer were treated with 3D-CRT. They reported median survival time and overall 2-year disease-free
P.1618
survival rate of 11.7 months and 13.5%, respectively. Adding chemotherapy improved these results to the median survival time of 18.1 months and 2-year disease-free survival rate of 20.5% (p = 0.001). Hayman and co-workers (2001) reported a dose escalation study in NSCLC using 3D-CRT. This study enrolled 104 patients; 81 patients completed protocol, and 63 were evaluable for escalation. Their report is shown in Table 109-3.
Table 109-2. Comparison of Survival Rates for Non Small Cell Lung Carcinoma | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
Table 109-3. Overall and Progression-Free Survival | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Despite these preliminary results from phase I trials, it is important to note that significant knowledge has been gained from these trials during the past decade that will have a major impact on radiation therapeutic management of lung cancer. These advancements have also uncovered many fundamental issues and questions, which undoubtedly will be the focus of research activities for the next several years.
As mentioned earlier, using ICRU-50 recommendations (GTV, CTV, and PTV) has become a routine practice in many institutions. However, several important issues still need to be answered. What window setting of the CT scan is a true representative of the actual pathology? The GTV is always different between the two window settings of soft tissues and lung windows. Interobserver and intraobserver variability in interpretation of radiologic findings remains a major problem for delineation of GTV and CTV. A significant problem in defining GTV is distinguishing between the actual tumor and postobstructive atelectasis or pneumonia. Inclusion of other imaging modalities, such as positron emission tomography (PET), may further improve tumor delineation. PET scanning has shown significant promise in both initial staging in lung cancer, as noted by Caldwell (2001) and Haberkorn (2001) and their associates, and target volume delineation for 3D-CRT, as reported by Erdi and colleagues (2002) (Fig. 109-5). Nodal involvement has been detected by PET scan in 80% to 100% of the studies in a meta-analysis of PET and CT in the detection of nodal metastases reported by Dwamena and co-workers (1999). In the Leuven Lung Cancer Group experience of the evaluation of 980 lymph node stations, the accuracy of PET was 85% (visually, 90%), as compared with 64% for the CT scan. The PET scan has been shown to be of significant help in distinguishing gross tumors from atelectasis or postobstructive phenomena. Figure 109-5 reveals that CT scan and corresponding PET scan can help in more accurate delineation
P.1619
of the gross tumor volume. Fusion of PET images into treatment-planning CT scans is becoming an increasingly routine practice.
 |
Fig. 109-5. Wire diagrams showing planning target volume (PTV) delineated from computed tomography (CT) (top) and from CT plus positron emission tomography (PET) (bottom) for patient 2. Involved paratracheal lymph nodes were detected on PET scans, which are included into the PTV once PET data are incorporated. From Erdi YE, et al: Radiation therapy treatment planning for patients with non-small cell lung cancer using positron emission tomography (PET). Radiother Oncol 62:51, 2002. With permission. |
Delineation of the CTV is also a difficult issue in cancer of the lung. Studies by Giraud (2000) and van Sornsen de Koste (2002) and their associates indicate that a margin of 4 to 6 mm would be adequate. The issue of margin around GTV to create CTV is far from clear and needs further investigation. If one decides to include hilar and ipsilateral mediastinum in their treatment of volume, it should be included in CTV. Regional nodes greater than 1.5 cm in short access are usually included in GTV. Nodes of smaller size, if proved by PET scan or by mediastinoscopy to harbor cancer, are included in CTV. Prophylactic radiation of uninvolved nodal regions is not indicated.
Designation of the PTV should account for day-to-day setup errors as well as organ motion. Movement of target volumes as well as critical organs during the breathing cycle has been well studied, as noted by Murphy (2002) and Nehmeh (2002) and their colleagues. The addition of PTV margins will invariably result in excessive radiation of large volumes of the lung. To solve this problem, gated radiation therapy with various techniques has been used, as reported by Ford and associates (2002). With this technique, the delivery of radiation has been correlated with specific breathing cycle. Various investigators have shown that, with this technique, the amount of normal lung irradiated is decreased, and therefore the possibility of complication has been significantly reduced.
Preliminary analysis of the results of 3D-CRT in bronchial carcinoma has uncovered other important issues and questions. Although the size of the gross tumor has been considered in the TNM staging of lung cancer, the true importance of this issue in radiotherapeutic management of patients with unresectable lung cancer has never been truly evaluated. It is a general belief that TNM staging is mostly based on surgical management of patients with lung cancer. Recent publications by Willner (2002) and Bradley (2002) and their co-workers have clearly shown the importance of tumor size in treatment of lung cancer. Table 109-4 reveals the correlation of some prognostic factors with therapeutic outcomes, such as survival, cause-specific survival, and local control. As can be seen, although tumor stage has no correlation, the tumor size (GTV) has statistically significant correlation with all of the outcomes. In a report by Bradley and associates (2002), the 5-year cause-specific survival for tumors with volumes equal to or less than 33 cm3 (cc) was 40%, compared with 5% to 20% for larger tumors. The 5-year cause-specific survival was 20% in patients receiving 70 Gy or higher doses and 10% in those treated with doses below 69 Gy. Patient with small tumors (less than 70 cc) treated with doses of 79 Gy or higher had a 70% 3-year survival rate, compared with 38% in those with lower doses.
Table 109-4. Tumor Size: Non Small Cell Lung Cancer | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
A recent publication by Ball and colleagues (2002) has revealed no correlation between median survival rate and 5-year survival rate with T stage in 243 patients treated with chemoradiation therapy (Table 109-5). Analysis of the recent literature on patients with lung cancer shows a high cure rate of small tumors with appropriate doses of radiation therapy. Sandler and colleagues (1990) in reporting on treatment of 77 patients with early-stage NSCLC have reported a 44% 5-year local control rate with doses of only 60 Gy. In this study, the 1-, 3-, and 5-year survival rates were
P.1620
65%, 17%, and 10%, respectively. Rosenthal and co-workers (1992), reporting the treatment of 62 patients with stage II NSCLC, have noted a 70% 1-year survival rate, a 20% 3-year survival rate, and a 12% 5-year survival rate, respectively.
Table 109-5. Non-Small Cell Lung Cancer | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Extension of this concept is the use of hypofractionated stereotactic radiation for small lung tumors, as noted by Nagata and associates (2002). This concept is not new; however, in the past, the lack of appropriate technique prevented its use. Currently, with technology such as stereotactic radiation therapy and intensity-modulated radiation therapy, clinical trials are on the way to test this concept. Table 109-6 reveals the high local control rate of small NSCLC with regimens of hypofractionated stereotactic irradiation. Considering the higher than 90% local control rate, subjecting these patients with small tumors to pneumonectomy and lobectomy will become a matter of judgment and debate.
Table 109-6. Several Schedules of Hypofractionated Stereotactic Irradiation for Extracranial Tumors | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Probably some of the most important knowledge gained from the analysis of the results of 3D-CRT in the treatment of NSCLC is the information on the dose volume relationship relative to the tolerance of normal tissue structures to therapeutic irradiation. Individual analysis by Graham and colleagues (1995) of 95 patients with medically inoperable NSCLC who were treated with 3D-CRT to doses ranging from 50 to 70 Gy revealed a correlation between the volume of lung irradiated to 20 Gy and the incidence of grade 2 or higher pneumonitis (Table 109-7). Based on these results, a phase I, II study was initiated in the early 1990s. In this protocol, patients were divided into three groups depending on the normal lung being irradiated to the dose of 2,000 cGy or higher. There was a dose escalation schema within each group. The most recent analysis of the results of this protocol are shown in Tables 109 8 and 109 9. From these and similar data, it becomes evident that mean lung dose is also an important prognostic factor. Based on these findings, there has been an attempt to develop a dose-volume
P.1621
histogram for pulmonary toxicity in the treatment of lung cancer (Fig. 109-6).
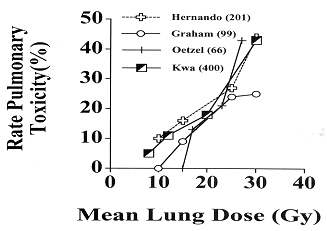 |
Fig. 109-6. Radiation-induced pulmonary toxicity. A dose-volume histogram analysis in 201 patients with lung cancer. From Hernando ML, et al: Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys 51:650, 2001. With permission. |
Table 109-7. Three-dimensional Conformal Radiation Therapy for Non Small Cell Lung Cancer: Incidence of Pneumonitis and Relationship to Volume of Lung Radiated to or Above 20 Gy | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Table 109-8. Lung Cancer: Three-dimensional Conformal Radiation Therapy Pulmonary Toxicity, Low-Risk Group | ||||||
|---|---|---|---|---|---|---|
|
Table 109-9. Lung Cancer: Three-dimensional Conformal Radiation Therapy Pulmonary Toxicity, Middle-Risk Group | ||||||
|---|---|---|---|---|---|---|
|
RADIOSENSITIZERS AND RADIOPROTECTIVE AGENTS
Radiation is a physical event that damages cells through biochemical mechanisms. The biochemical effects depend on cellular physiology (oxygen, cell cycle) and may be modifiable through biochemical additives (sensitizers, protectors). Berry (1965) and Doggett and colleagues (1967) noted that numerous drugs, including halogenated pyrimidines, dactinomycin, alkylating agents, and others, augment the effect of radiation on some tumors and normal tissues. The therapeutic aim of radiosensitizers may be additive or synergistic, depending on the degree of enhancement of effects.
Hypoxic sensitizers have been introduced in clinical trials. These drugs, which potentiate the effects of radiation on hypoxic cells, must be present at the time of irradiation. Phillips (1978) and Simpson and their colleagues (1987) reported on the results of clinical trials with radiosensitizers. In randomized trials, no significant improvement in tumor control or survival was noted when compared with radiation alone. Significant peripheral neuropathy was the main toxicity associated with these drugs. Even newer compounds, such as SR-2508, with less lipophilicity, allowing larger doses to be administered with the same peripheral neuropathy, have failed to show any improvement in randomized clinical trials reported by Brown (1984) and Chassagne and associates (1992).
Protection of uninvolved normal structures from detrimental effects of radiation therapy is one of the topics intensively investigated in various centers around the world. Although there are numerous laboratory and phase I studies being conducted, there is one product, Amifostine, which has gained popular use with some reasonable efficacy in reducing radiation-induced pneumonitis and esophagitis. This drug, which was created by the army in a nuclear warfare project under the name of WR-2721, is currently in extensive use in clinics. This drug is metabolized by membrane-bound alkaline phosphatase to the active form WR-1065 and presumably protects normal tissues from cytocide activity and free radicals produced by radiation and chemotherapy that alter structure and functional DNA. Several studies have shown that this product does not protect tumor cells and is essentially selective for normal cells. Some of the stated reasons are lower level of membrane-bound alkaline phosphatase, neutral pH in normal cells, and lower selective uptake by tumor tissues. An excellent review of this compound was recently published by Andreassen and associates (2003).
EFFECTS OF RADIATION ON NORMAL TISSUES
Esophagus
The effects of radiation on the esophagus are caused by damage to the germinal cell layers of the epithelium and the muscular layers and by vascular changes in the underlying tissues. Acute radiation esophagitis usually begins in the third week of radiation therapy, at the dose level of about 3,000 to 4,000 cGy. After 4,000 cGy, brisk mucositis and fibrinous exudate appear. These changes frequently are reversible. From the Radiation Therapy Oncology Group (RTOG) trials, the incidence of acute esophagitis was about 12% for grade II and 3% for grade III. With the current dose used, 6,000 to 6,500 cGy, the incidence of long-term sequelae such as esophageal stricture is rare. As one of us (BE) and colleagues (1991b) reported in the RTOG trials, this incidence was about 1%.
Lung
The most frequently reported sequela in the radiation therapy of thoracic neoplasms is pneumonitis. One of us (BE) and co-workers (1991b) noted that the incidence of grade II pneumonitis is about 10% and that of grade III pneumonitis is about 4.6%. Graham and associates (1994, 1999) reported the incidence and the grade of pneumonitis after radiation therapy in 99 patients treated with 3D-CRT. The incidence of grade II pneumonitis was 14%, that of grade III pneumonitis was 2%, and that of grade IV pneumonitis was 2%. The threshold dose for radiation pneumonitis is 2,000 cGy. The incidence and degree of radiation pneumonitis depend on the total dose, fractionation, and volume of lung irradiated.
P.1622
The maximum lung volume that can be included in a high-dose zone of irradiation without significant clinical symptoms is 25% of the total lung volume. Use of 3D-CRT in the management of lung cancer has given us significant insight into the volumetric radiation tolerance of lungs. Based on published information by Graham (1995) and Martel (1994) and their co-workers, it is generally agreed that if less than 25% of the total lung volume receives 2,000 cGy, that patient is considered at low risk for the development of pneumonitis. If a radiation therapy plan shows that 25% to 37% of the total lung volume would receive more than 2,000 cGy, then the patient is considered at medium risk for development of pneumonitis. An attempt should be made to develop alternative plans in order to reduce the risk for pneumonitis. If the treatment plan would result in more than 37% of total lung volume receiving more than 2,000 cGy, then we do not recommend that plan for treatment of the patient because such a patient is considered a high risk for pneumonitis. When more than 75% of the pulmonary tissue is radiated with doses of more than 2,000 cGy, patients may develop severe and sometimes fatal pneumonitis. The radiation-induced pneumonitis is usually manifested by respiratory distress, temperature peaks, and shortness of breath, and patients may even die from acute respiratory insufficiency and cor pulmonale. Sputum culture is negative unless the bacterial infection is superimposed, which is common and may be life threatening. Radiographs of the chest show diffuse pulmonary infiltrates that may coalesce and may be associated with pleural or interlobar effusions. The underlying pathologic damage consists of alveolar degeneration and hyaline membrane formation, followed by fibrosis of the alveolar wall and interlobar septa as well as capillary thrombosis and fibrinoid degeneration of the small arterioles. Many of these acute changes are reversible after 3 to 4 weeks with moderate doses of radiation therapy. After larger doses, however, the chronic changes become permanent and can be seen as early as 3 months after radiation treatment. Substantial functional impairment ensues. Decreased pulmonary blood flow can be demonstrated in nuclide ventilation-perfusion scanning. Pulmonary function studies in patients with lung cancer receiving radiation therapy demonstrated decreased compliance, diffusing capacity, and lung volumes. The decreased ventilatory and diffusing capacity results from alveolar cell degeneration and interstitial fibrosis.
Heart
The probability of radiation-induced cardiac disease following radiation therapy for lung cancer is relatively rare and depends on the volume irradiated, the size of the fraction, and the total dose of radiation. Transient, subacute, or chronic pericarditis is the most common form of clinical cardiac damage caused by radiation and may appear with doses of more than 5,000 cGy. Stewart and Fajardo (1972) reported an incidence of cardiac complications of 6.6% after a mean dose of 4,281 cGy. It is important to note that these patients were treated with the fractionation of 225 cGy per day, and in most of them, almost the entire volume of the heart was irradiated.
Spinal Cord
Acute radiation effects in the spinal cord are usually of minimal clinical significance and are caused mostly by transient edema. A transient radiation effect on the spinal cord is known as Lhermitte's syndrome. It occurs a few weeks after exposure and is characterized by some electric shocklike sensations that radiate along the spinal cord into the extremities. In most instances, the syndrome is reversible and does not correlate with subsequent permanent radiation myelopathy. A more severe type of brainstem or spinal cord injury may appear after 6 months or as late as several years after the irradiation. Radiation myelopathy causes motor and sensory changes referable to the injured segment. If the entire width of the cord is irradiated, complete paraplegia and anesthesia occur as a result of a total functional transection of the spinal cord. If only one half of the cord is irradiated, the neurologic picture is that of a Brown-S quard syndrome. These permanent changes are secondary to severe capillary degeneration, fibrinoid necrosis of the small arterioles, and necrosis of neurons and other oligodendrocytes, accompanied by demyelination of the white matter.
Small segments of brainstem or spinal cord may tolerate doses of more than 5,000 cGy, given in weekly increments of 1,000 cGy. With doses of 6,000 cGy in 6 weeks, the probability of radiation myelitis is 10% to 15%. A strong probability exists that higher doses will induce radiation myelopathy more frequently. Also, the greater the length of segment of the spinal cord being irradiated, the more likely is the possibility of developing permanent myelopathy. Table 109-10 presents the accepted tolerated doses for intrathoracic structures in the absence of chemotherapy.
Table 109-10. Normal Tissue Tolerance to Therapeutic Irradiation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Bone Marrow
The acute effects of radiation on the bone marrow, mostly caused by direct cell killing, are related to the volume irradiated and the dose and fractions given. Usually, a reduction in the number of lymphocytes occurs a reflection on the extreme sensitivity of circulating lymphocytes to interphase death. The lymphocyte count remains low for weeks or months; gradually, repopulation occurs. The granulocytes decrease 1 to 2 weeks after segmental irradiation, with recovery taking weeks or up to 18 months. Moderate thrombocytopenia is common; if the platelets decrease to less than 20,000, hemorrhages with ecchymosis, hematemesis, and melena, which complicate the fluid and electrolyte
P.1623
imbalance, may occur. The circulating red cells are extremely radioresistant, but chronic irritation may cause protracted anemia.
Knospe (1966) and Rubin (1973) and their colleagues noted that when the bone marrow is irradiated with more than 4,000 rad, more permanent chronic changes occur. Vascular degeneration, as pointed out by Rubin and Cassarett (1968), and fibrosis, as Lejar and associates (1966) noted, occur, preventing repopulation. These changes are more severe in patients who previously received bone marrow depressing chemotherapeutic agents and in patients who were treated with these drugs after radiation therapy. Special care should be exercised in patients receiving combination therapy or retreatment after radiation therapy or chemotherapy, and frequent leukocyte and platelet counts are necessary, particularly if the bone marrow is infiltrated with malignant cells.
EFFECT OF ADJUVANT CHEMOTHERAPY ON COMPLICATIONS
Chemotherapy significantly enhances the effect of irradiation. Phillips and Margolis (1972) reported a 50% incidence of radiation pneumonitis in patients treated with radiation alone about 2,650 rad in 20 fractions. When dactinomycin was added, the incidence of pneumonitis was 50% with delivery of 2,050 rad in 20 fractions. Patients receiving bleomycin may develop interstitial pulmonary fibrosis, which can accentuate the similar effects of irradiation.
Johnson and associates (1976) reported instances of severe esophageal fibrosis with stenosis following the administration of 3,000 rad in 2 weeks combined with intensive triple-agent chemotherapy (doxorubicin, vincristine, and cyclophosphamide) given the same day every 3 to 4 weeks. Also, the well-known cardiotoxicity of doxorubicin, coupled with the effects of radiation on the heart, makes this combination more toxic. When these two agents are combined, Chan and colleagues (1976) recommend that no more than a total of 4,000 rad be given to the entire heart, or that a maximum of 450 mg/m2 total dose of doxorubicin be administered. Also, the two agents should not be administered simultaneously.
The effects of combined irradiation and chemotherapy on the spinal cord have not been evaluated properly, but it is reasonable to believe that an additive or potentiating effect is present.
REFERENCES
Andreassen CN, Grau C, Lindegaard JC: Chemical radioprotection: a critical review of Amifostine as a cytoprotector in radiation therapy. Semin Radiat Oncol 13:62, 2003.
Arimoto T, et al: Small volume multiple non-coplanar arc radiotherapy for tumors of the lung, head and neck and the abdominopelvic region. In Lemke HU (ed): CAR'98 Computer Assisted Radiology and Surgery. Tokyo: Elsevier, 1998, pp. 257 261.
Arriagada R, et al: ASTRO (American Society for Therapeutic Radiology and Oncology) plenary. Effect of chemotherapy on locally advanced non-small cell lung carcinoma: a randomized study of 353 patients. GETCB (Groupe d'Etude et Traitement des Cancers Bronchiques), FNCLCC (Federation Nationale des Centres de Lutte contre le Cancer) and the CEBI trialists. Int J Radiat Oncol Biol Phys 20:183, 1991.
Ball D, et al: Failure of T stage to predict survival in patients with non-small-cell lung cancer treated by radiation therapy with or without concomitant chemotherapy. Int J Radiat Oncol Biol Phys 54:1007, 2002.
Berry RJ: Modification of radiation effects. Radiol Clin North Am 3:249, 1965.
Bradley JD, et al: Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 52:49, 2002.
Brown JM: Clinical trials of radiosensitizers. What should we expect? Int J Radiat Oncol Biol Phys 10:425, 1984.
Caldwell CB, et al: Observer variation in contouring gross tumor volume in patients with poorly defined non-small-cell lung tumors on CT: the impact of 18FDG-hybrid PET fusion. Int J Radiat Oncol Biol Phys 51: 923, 2001.
Chan YM, et al: Co-incident Adriamycin (A) and x-ray therapy (XRT) I bronchogenic carcinoma (BC): response and cardiotoxicity (CT). Proc AACR ASCO 17:276, 1976.
Chassagne D, et al: First analysis of tumor regression for the European randomized trial of etanidazole combined with radiation therapy in head and neck carcinomas. Int J Radiat Oncol Biol Phys 22:581, 1992.
Doggett R, et al: Combined therapy using chemotherapeutic agents and radiation therapy. In Wood C, Deeley TJ (eds): Modern Trends in Radiation Therapy. Vol. 1. New York: Appleton-Century-Crofts, 1967, p. 107.
Dwamena BA, et al: Metastases from non-small cell lung cancer: mediastinal staging in the 1990s-meta-analytic comparison of PET and CT. Radiology 213:530, 1999.
Emami B, et al: Value of computed tomography in radiation therapy of lung cancer. AJR Am J Roentgenol 131:63, 1978.
Emami B, et al: Three-dimensional treatment planning for lung cancer. Int J Radiat Oncol Biol Phys 21:217, 1991a.
Emami B, et al: Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21:109, 1991b.
Erdi YE, et al: Radiation therapy treatment planning for patients with non-small cell lung cancer using positron emission tomography (PET). Radiother Oncol 62:51, 2002.
Ford EC, et al: Evaluation of respiratory movement during gated radiation therapy using film and electronic portal imaging. Int J Radiat Oncol Biol Phys 52:522, 2002.
P.1624
Giraud P, et al: Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiation therapy planning. Int J Radiat Oncol Biol Phys 48:1015, 2000.
Graham MK, et al: Preliminary results of a radiation therapy oncology group trial (RTOG 9311), a dose escalation study using 3D conformal radiation therapy in patients with inoperable nonsmall cell lung cancer [Abstract]. ASTRO 2001. J Radiat Oncol Biol Phys 51:19, 2001.
Graham MV, et al: Three-dimensional radiation treatment planning study for patients with carcinoma of the lung. Int J Radiat Oncol Biol Phys 29:1105, 1994.
Graham MV. et al: Preliminary results of a prospective trial using three-dimensional radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 33:993, 1995.
Graham MV, et al: Clinical dose-volume histogram for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 45:323, 1999.
Gray LH: Radiobiologic basis of oxygen as modifying factor in radiation therapy. AJR Am J Roentgenol 85:803, 1961.
Haberkorn U., Schoenberg SO: Imagining of lung cancer with CT, MRI and PET. Lung Cancer 34:S13, 2001.
Hayman JA, et al: Dose escalation in non-small-cell lung cancer using three-dimensional conformal radiation therapy: update of a phase I trial. J Clin Oncol 19:127, 2001.
Herfath KK, et al: Stereotactic single dose radiation treatment of tumors in the lung. Radiology 217:148, 2000.
Hernando ML, et al: Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys 51:650, 2001.
ICRU-50 Prescribing, Recording, and Reporting Photon Beam Therapy. Bethesda, MD: International Commission on Radiation Units and Measurements, 1993, p. 5.
Johnson RE, Brereton HD, Kent CH: Small cell carcinoma of the lung: attempt to remedy causes of past therapeutic failure. Lancet 2:289, 1976.
Kallman RF: The phenomenon of reoxygenation and its implications for fractionated radiation therapy. Radiology 105:135, 1972.
Kaplan HS: Biochemical basis of reproductive death in irradiated cells. AJR Am J Roentgenol 909:907, 1963.
Knospe WH, Blom J, Crosby WH: Regeneration of locally irradiated bone marrow. I. Dose-dependent long-term changes in the rat, with particular emphasis upon vascular and stomal reaction. Blood 28:398, 1966.
Komaki R, et al: Failure patterns by prognostic group determined by recursive partitioning analysis (RPA) of 1547 patients on four radiation therapy oncology group (RTOG) studies in inoperable non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 42:263, 1998.
Leibel S: CD CRT for Lung Cancer. Proceedings San Francisco Cancer Symposium, February 26 27, 1994.
Lejar TJ, et al: Effects of focal irradiation on human bone marrow. AJR Am J Roentgenol 96:183, 1966.
Martel MK, et al: Dose-volume histogram and 3-D treatment planning evaluation of patients with pneumonitis. Int J Radiat Oncol Biol Phys 28:575, 1994.
Murphy MJ, et al: The effectiveness of breath-holding to stabilize lung and pancreas tumors during radiosurgery. Int J Radiat Oncol Biol Phys 53: 475, 2002.
Nagata Y, et al: Clinical outcomes of 3D conformal hypofractionated single high-dose radiation therapy for one or two lung tumors using a stereotactic body frame. Int J Radiat Oncol Biol Phys 52:1041, 2002.
Nehmeh SA, Erdi YE, Ling CC: Effect of respiratory gating on reducing lung motion artifacts in PET imaging of lung cancer. Med Phys 29:366, 2002.
Phillips T, Margolis L: Radiation pathology and the clinical response of lung and esophagus. In Vaeth JM (ed): Frontiers of Radiation Therapy and Oncology. Vol. 6. Baltimore: University Park Press, 1972, p. 254.
Phillips TL, et al: The hypoxic cell sensitizer program in the United States. Br J Cancer 37(Suppl. III) :276, 1978.
Prasad S, Pilepich MV, Perez CA: Contribution of CT to quantitative radiation therapy planning. AJR Am J Roentgenol 136:123, 1981.
Purdy JA, Emami B: Computed topography and three-dimensional approaches to radiation therapy I treatment planning. In Levitt S, Tapley N (eds): Technological Basis of Radiation Therapy: Practical Clinical Applications. Philadelphia: Lea & Febiger, 1991, pp. 56 66.
Purdy JA, et al: Three-dimensional conformal therapy and intensity modulated radiation therapy: practical potential benefits and pitfalls. Principles and Practice of Radiation Oncology Updates 1:2, 2000.
Rosenthal SA, et al: Clinical stage II non-small cell lung cancer treated with radiation therapy alone. The significance of clinically staged ipsilateral hilar adenopathy (N1 disease). Cancer 70:2410, 1992.
Rubin P, et al: Bone marrow regeneration and extension after extended field irradiation in Hodgkin's disease. Cancer 32:699, 1973.
Rubin P, Cassarett G: Clinical Radiation Pathology. Philadelphia: WB Saunders, 1968.
Sandler HM, Curran WJ Jr, Turrisi AT III. The influence of tumor size and pre-treatment staging on outcome following radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 19:9, 1990.
Sause W, et al: Final results of phase III trial in regionally advanced unresectable non-small-cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 117:358, 2000.
Sause WT, et al: Radiation Therapy Oncology Group (RTOG) 88 08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst 87:198, 1995.
Sim S, et al: Induction chemotherapy plus three-dimensional conformal radiation therapy in the definitive treatment of locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 51:660, 2001.
Simpson JR, et al: Large fraction irradiation with or without misonidazole in advanced non-oat cell carcinoma of the lung: a phase III randomized trial of the RTOG. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 13:861, 1987.
Stewart JR, Fajardo LF: Radiation-induces heart disease. In Vaeth JM (ed): Frontiers of Radiation Therapy and Oncology. Vol. 6. Baltimore: University Park Press, 1972, p. 274.
Suit HD, Shalek RJ: Response of anoxic C3H mouse mammary carcinoma isotransplants (1 25 MM3) to x-irradiation. J Natl Cancer Inst 31:479, 1963.
Terasima T, Tolmach LJ: Variations in several responses of hela cells to x-irradiation during division cycle. Biophys J 3:11, 1963.
Turrisi AT III, et al: Twice-daily compared with once-daily thoracic radiation therapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340:265, 1999.
van Sornsen de Koste JR, et al: What margins are necessary for incorporating mediastinal nodal mobility into involved-field radiation therapy for lung cancer? Int J Radiat Oncol Biol Phys 53:1211, 2002.
Uematsu M, et al: Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients. Cancer 82:1062, 1998.
Willner J, et al: Dose, volume, and tumor control predictions in primary radiation therapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 52: 382, 2002.
Wulf J, et al: Hypofractionated, high-dose radiation under stereotactic conditions in the stereotactic body frame: accuracy of re-positioning at 11 CT-simulations and 37 applications at the LINAC. Int J Radiat Oncol Biol Phys 42[Suppl 1]:215, 1998.
Reading References
Cox JD, et al: Dose-time relationships and the local control of small cell carcinoma of the lung. Radiology 128:205, 1078.
Eichorn HJ, Lessel A, Matschke S: Comparison between neutron therapy and 60Co gamma ray therapy of bronchial, gastric and esophagus carcinomata. Eur J Cancer 10:361, 1974.
Emami B, et al: Three-dimensional conformal radiation therapy: clinical aspects. In Principles and Practice of Radiation Oncology. Philadelphia: JB Lippincott, 1998, pp. 371 386.
Emami B, Graham MV: Lung. In Principles and Practice of Radiation Oncology. Philadelphia: JB Lippincott, 1998, pp. 1181 1220.
Fajardo LF: Pathology of Radiation Injury. Masson Publishing USA. 1982.
Hall EJ: Radiobiology for the Radiologist. 4th Ed. Philadelphia: Lippincott-Raven, 1993.
Prescribing, Recording, and Reporting Photon Beam Therapy (ICRU report, No 50). Intl Commission on Radiation, Nov. 1993.
EAN: 2147483647
Pages: 203