3 - Ultrastructure and Morphometry of the Human Lung
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section I - Anatomy of the Chest Wall and Lungs > Chapter 5 - Surgical Anatomy of the Lungs
Chapter 5
Surgical Anatomy of the Lungs
Thomas W. Shields
Until recent decades, the anatomy of the lungs was a little-understood and seemingly unimportant subject. With the development of radiographic and endoscopic techniques and the advancement of pulmonary surgery, detailed anatomic knowledge of the lungs became a necessity.
The essential anatomic unit of the lung, the bronchopulmonary segment, was established as that portion of the lung substance that represents the total branching of a major, segmental subdivision of a lobar bronchus. These units are named for their topographic position in the lung.
LOBES AND FISSURES
The right lung is composed of three lobes (the upper, middle, and lower) and is the larger of the two lungs. The left is made up of only two lobes, the upper and lower. Two fissures are usually present on the right. The oblique (major) fissure separates the lower lobe from the upper and middle lobes, and the horizontal (minor) fissure separates the other two (Fig. 5-1). In life, the oblique fissure on the right begins posteriorly at the level of the fifth rib or intercostal space, runs downward and forward approximating the course of the sixth rib, and ends at the diaphragm in the vicinity of the sixth costochondral junction. The horizontal fissure begins in the oblique fissure in the region of the midaxillary line at the level of the sixth rib and runs anteriorly to the costochondral junction of the fourth rib. On the left, the oblique (major) fissure is found (Fig. 5-2). This begins at a somewhat higher level posteriorly, between the third and fifth ribs, and runs downward and forward to end in the region of the sixth or seventh costochondral junction.
Variations in the fissures do occur, and often part or all of a fissure fails to develop. This is seen commonly as a more or less complete fusion of the middle lobe and the anterior portion of the upper lobe in more than 50% of lungs examined. Accessory fissures occur also, and certain portions of the lung may be demarcated into so-called accessory lobes. On occasion, such fissures are visible as linear shadows on the radiograph of the chest, and the accessory lobe may appear less radiolucent than the surrounding portions of the lung. The usual accessory lobes are the posterior accessory, inferior accessory, middle lobe of the left lung, and azygos lobe (Fig. 5-3). In contrast to the first three named, which are true accessory lobes made up of specific bronchopulmonary segments, the azygos lobe is not a true accessory lobe because it is formed of varying portions of one or two segments (apical and posterior) of the right upper lobe. The fissure is formed by an aberrant loop of the azygos vein and its mesentery of two layers of the parietal pleura and two of the visceral pleura (Fig. 5-4). On the radiograph, this fissure may appear as an inverted comma to the right of the mediastinum (Fig. 5-5). This anomaly is seen in 0.5% to 1.0% of the anatomic dissections and routine radiographs of the chest.
BRONCHOPULMONARY SEGMENTS
Each lobe of the right and left lungs is subdivided into several individual anatomic units, the bronchopulmonary segments. The general pattern is that of 18 segments, 10 in the right lung and 8 in the left. Initially, there were many differences in the nomenclature for the various segments as the result of the publications of the individual investigators working in North America and in Europe. Sealy and associates (1993) presented an excellent review of this topic. The terminology suggested by Brock (1946) and Jackson and Huber (1943) and that adopted by the Ad Hoch International Committee on Nomenclature that was published for the latter by Brock in 1950 are shown in Table 5-1. Subsequently, in 1989, the latest Nomina Anatomica nomenclature for the lung segments was published and is essentially that suggested originally by Jackson and Huber (1943) (Table 5-2). However, the numerical designations, especially those for the segments and structures of the right and left upper lobes, are dissimilar. Most surgeons in America
P.60
use the numbers suggested by Jackson and Huber (1993) and Boyden (1945, 1955) (see Table 5-2). However, Overholt and Langer (1947) and Beattie and colleagues (1992) have used the numerical designations adopted by the Nomina Anatomica (1989). Likewise, as noted by Wakabayashi (personal communication, 1996), the Japanese surgeons use the Nomina Anatomica numerical designations as well. Therefore, one should be aware of the discrepancies in the literature relative to the numerical designations for the various segments and the structure contained therein (bronchi, veins, and arteries). In this text, the terms and numbers used throughout are those suggested by Jackson and Huber (1943) and Boyden (1945, 1955). The topographic positions of the segments are shown in Fig. 5-6. Knowledge of the detailed anatomic features of the bronchial distribution and the vascular supply of each segment is essential for the surgeon. Although a general pattern exists in the anatomic features in each segment, variation is the rule. The usual pattern and the most common deviations from it are best portrayed by separate descriptions of the bronchial, arterial, and venous systems.*
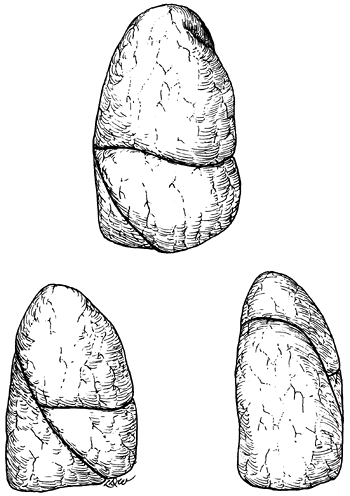 |
Fig. 5-1. Anterior, lateral, and posterior aspects of the right lung. Redrawn and modified from Anson BJ: Atlas of Human Anatomy. Philadelphia: WB Saunders, 1950, p. 199. With permission. |
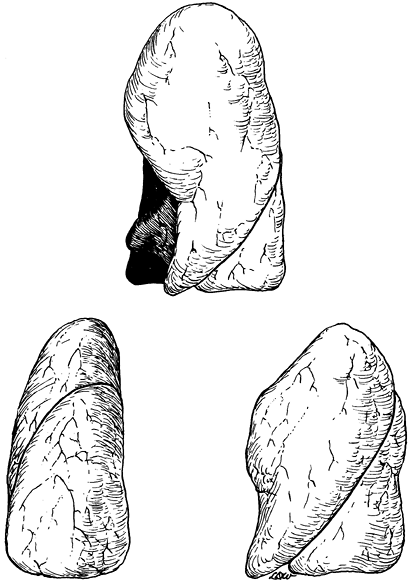 |
Fig. 5-2. Anterior, lateral, and posterior aspects of the left lung. Redrawn and modified from Anson BJ: Atlas of Human Anatomy. Philadelphia: WB Saunders, 1950, p. 199. With permission. |
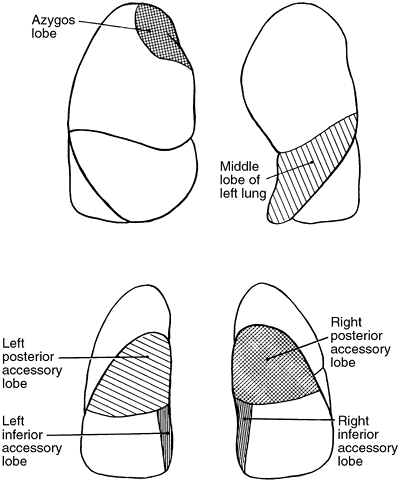 |
Fig. 5-3. Accessory lobes of the lungs. |
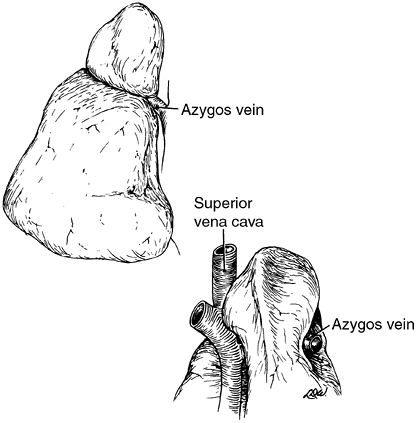 |
Fig. 5-4. Anterior and posterior views of the azygos lobe formed by an aberrant loop of the azygos vein. Redrawn and modified from Anson BJ: Atlas of Human Anatomy. Philadelphia: WB Saunders, 1950, pp. 203 204. With permission. |
Table 5-1. Nomenclature Adopted by the Ad Hoc International Committee Meeting at the Time of the International Congress of Otorhinolaryngology in 1949 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
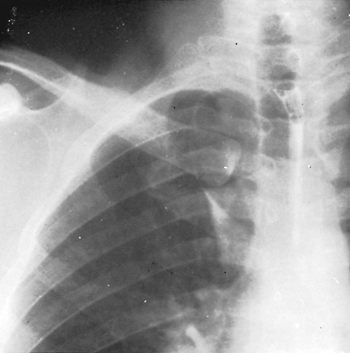 |
Fig. 5-5. Radiograph of the chest showing an azygos lobe. |
Table 5-2. Bronchopulmonary Segmental Nomenclature and Numerical Designations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
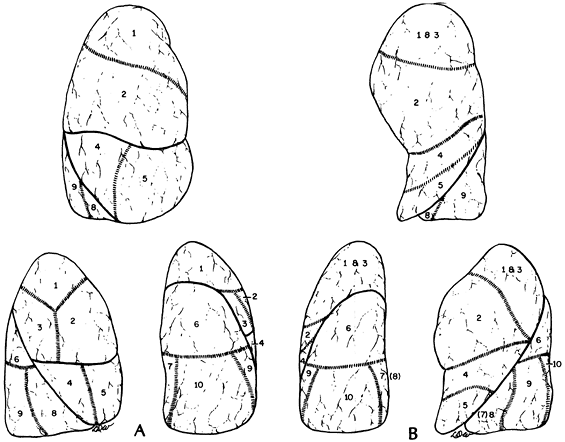 |
Fig. 5-6. A. Topographic positions of the bronchopulmonary segments of the right lung seen in anterior, lateral, and posterior views. B. Topographic positions of the bronchopulmonary segments of the left lung seen in anterior, lateral, and posterior views. |
P.61
P.62
P.63
BRONCHIAL TREE
The trachea bifurcates at about the level of the seventh thoracic vertebra into the right and left main-stem bronchi. Compared with the left bronchus, which arises at a sharper angle, the right bronchus arises in a more direct line with the trachea, an important factor in the localization of aspirated material.
Right Bronchial Tree
The length of the right main bronchus from the trachea to the point where the right upper lobe bronchus branches from its lateral wall is about 1.2 cm. The upper lobe bronchus, about 1 cm in length, in turn gives off three segmental bronchi one to the apical, one to the posterior, and one to the anterior segment. The branching may be a simple trifurcation or include varying combinations of the three major branches. The segmental bronchi further subdivide to supply the various portions of the segments.
Proceeding distally from the takeoff of the upper lobe bronchus, the primary bronchus is known as the bronchus intermedius, over which the main-stem pulmonary artery crosses, thus giving rise to the term eparterial bronchus to designate the right upper lobe bronchus. After a distance of about 1.7 to 2.0 cm, the middle lobe bronchus arises from the anterior surface of the bronchus intermedius. It varies in length between 1.2 and 2.2 cm before it bifurcates into lateral and medial branches. The superior segmental bronchus of the lower lobe arises from the posterior wall of the bronchus intermedius, slightly distal to the middle lobe bronchus. The superior segment is called the posterior accessory lobe when a fissure is present. This bronchus most often arises as a single branch and divides into three rami, usually by bifurcation or rarely by trifurcation. Distal to the superior bronchus, the basal stem bronchus sends off segmental bronchi to the medial (the inferior accessory lobe when a fissure is present), anterior, lateral, and posterior basal segments. The medial basal bronchus arises anteromedially and is distributed to the anterior and paravertebral surfaces of the lower lobe. The anterior basal branch arises on the anterolateral aspect of the basal trunk about 2 cm distal to the superior segmental bronchus and divides into two major rami. The lateral basal bronchus and the posterior basal bronchus most often arise as a common stem. Each of these bronchi, in turn, divides typically into two major subdivisions (Fig. 5-7).
Numerous variations occur, but the basic pattern encountered is as described. Infrequently, the upper lobe bronchus on the right undergoes two separate bifurcations to form the three bronchopulmonary segments. Of more interest is the rare occurrence of a tracheal bronchus that arises most commonly 2 cm above, or at the level of, the tracheal carina. A tracheal bronchus is estimated to be present in 0.1% to 2.0% of human tracheobronchial trees, depending on the manner of diagnosis and the population group studied, as noted by Barat and Konrad (1987). The tracheal bronchus occurs for all practical purposes exclusively on the right. Two major types exist: one consists of the right upper lobe bronchus and its divisions, and the other consists only of the apical segmental bronchus (ectopic or supernumerary) (Fig. 5-8). These, as well as other less common variations, have been described in the report by McLaughlin and colleagues (1985). Le Roux (1962) and
P.64
Atwell (1966, 1967) also discussed these anomalies. The diameter of the tracheal bronchus ranges between 0.5 and 1.0 cm and its length between 0.6 and 2.0 cm. Infrequently, an anomalous bronchus arises from the medial wall of the bronchus intermedius. It is found in 0.09% to 0.5% of the general population. It may occur at any level of the bronchus intermedius. Most often, however, it occurs at, or just distal to, the level and opposite to the orifice of the right upper lobe (Fig. 5-9). A few may arise more distally toward the level of the takeoff of the middle lobe bronchus. The anomalous bronchus, designated as the accessory cardiac bronchus by Brock (1946), may be only a short blind stump or diverticulum without any distal branches. About half the time it is present, according to McGuinness and associates (1993), the accessory cardiac bronchus may have a number of rudimentary or nearly normal branches associated with variable amounts of lung parenchyma. The length of this anomalous bronchus, according to Daskalakis (1983), may vary from 0.5 to 5.0 cm. On a rare occasion, two accessory cardiac bronchi may be present, as noted by Mangiulea and Stinghe (1968). These authors, as well as Jackson and Littleton (1988), also noted that an accessory cardiac bronchus may be found in association with a tracheal bronchus. The clinical findings and significance of a tracheal bronchus and an accessory cardiac bronchus are presented subsequently in this chapter. The variations in the middle lobe bronchus and its branchings, other than an occasional superoinferior spatial relationship of the segments rather than lateral and medial arrangements, are of little interest. In the division of the lower lobe bronchus, the presence of a subsuperior or an accessory subsuperior bronchus is a frequent finding. One to three such bronchi may be identified.
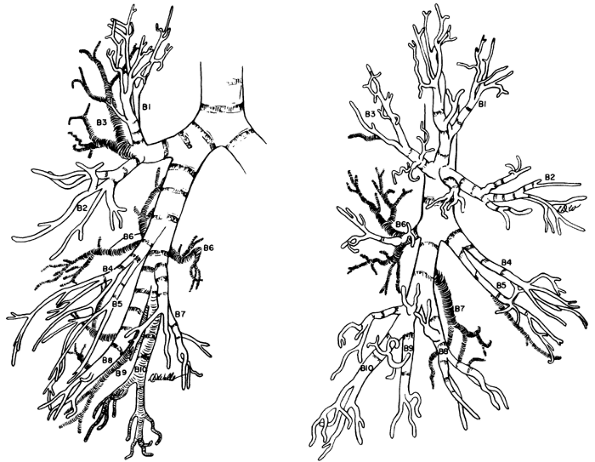 |
Fig. 5-7. Right bronchial tree, anterior and lateral views. Boyden's modification of numerical nomenclature used. Redrawn and modified from Brock RC: The Anatomy of the Bronchial Tree. 2nd Ed. London: Oxford University Press, 1954, pp. 190 191. With permission. |
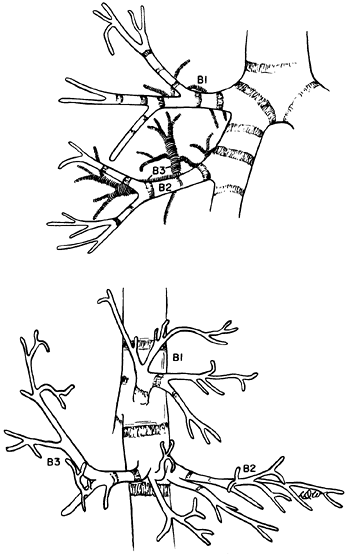 |
Fig. 5-8. Tracheal bronchus supplying the apical segment of the right upper lobe. Redrawn and modified from Bloomer WE, Liebow AA, Hales MR: Surgical Anatomy of the Bronchovascular Segments. Springfield, IL: Charles C. Thomas, 1960, p. 25. With permission. |
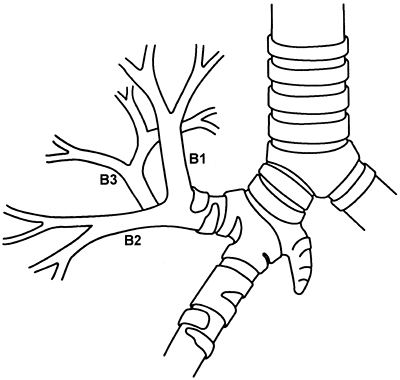 |
Fig. 5-9. Accessory cardiac bronchus arising from the medial wall of the bronchus intermedius at the level of and opposite to the orifice of the right upper lobe bronchus. Adapted from Keane MP, et al: Accessory cardiac bronchus presenting with haemoptysis. Thorax 52:490, 1997. With permission. |
Left Bronchial Tree
The left main bronchus is longer than the right, and its first branch arises anterolaterally as the left upper lobe bronchus about 4 to 6 cm distal to the tracheal carina. This bronchus is about 1.0 to 1.5 cm long and divides into superior and inferior (lingular) branches. The superior division ascends, and the inferior descends. The superior branch most often bifurcates into an apical posterior segmental bronchus and an anterior segmental bronchus. Occasionally, the anterior segment migrates inferiorly to create a trifurcate pattern. The inferior or lingular bronchus, the analog of the middle lobe, is variable in length (1 to 2 cm) and subsequently divides into superior and inferior divisions, the former of which, in turn, subdivides into posterior and inferior rami.
About 0.5 cm distal to the left upper lobe orifice, the lower lobe stem bronchus gives off its first branch, the superior segmental bronchus. This bronchus arises posteriorly and bifurcates in most instances, but trifurcation does occur. After giving off the superior branch, the basal trunk continues for an average distance of 1.5 cm as a single trunk. The bronchus then usually bifurcates into an anteromedial basal segmental
P.65
bronchus and a common stem bronchus for the lateral basal and posterior basal bronchi. These branches further subdivide into numerous rami for their respective segments (Fig. 5-10).
On the left side, the common variations are in the distribution of the segmental bronchi from the superior and inferior divisions of the left upper lobe bronchus and the presence of a subsuperior or accessory subsuperior bronchus arising from the lower lobe bronchus. Many of these deviations from normal have little clinical importance but are significant at the time of surgical resection of the various portions of the lungs.
A rare anomaly a so-called bridging bronchus has been described on three occasions. The bridging bronchus arises from the left main-stem bronchus and crosses the mediastinum to supply the right lower lobe and, at times, a portion of the middle lobe. This anomalous bronchus was first reported by Gonzalez-Crussi and associates in 1976. Subsequently, Starshak (1981) and Bertucci (1987) and their associates each reported a patient with this anomalous bridging bronchus (see Clinical Findings and Significance of Tracheobronchial Anomalies later in this chapter). This occurrence may be frequently associated with either a pulmonary venous or a pulmonary artery abnormality.
Absence of a Lobar Bronchus
The congenital absence of a lobar bronchus is rare. It appears that only 11 cases have been reported in the literature. The absence may be discovered in an infant but has been more commonly identified in adult patients. In the latter, the anomaly has been identified by bronchoscopy or bronchography, at the time of a pulmonary procedure, or infrequently at necroscopy. Ferguson and Neuhausen (1944) reported a patient with only a right upper lobe bronchus with the absence of a middle lobe and right lower lobe bronchus at bronchoscopy. Morton and associates (1950) reported the agenesis of the right upper and middle lobe bronchi in each of two patients. Storey and Marranooni (1954) reported the absence of a left lower lobe in a young adult man. Subsequently, Atwell (1967) at the Oserholt Thoracic Clinic described the absence of four lobar bronchi (one of the right upper lobe and three of the left upper lobe) in a series of 1,200 consecutive, complete, and bilateral bronchograms an overall incidence of 0.16%.
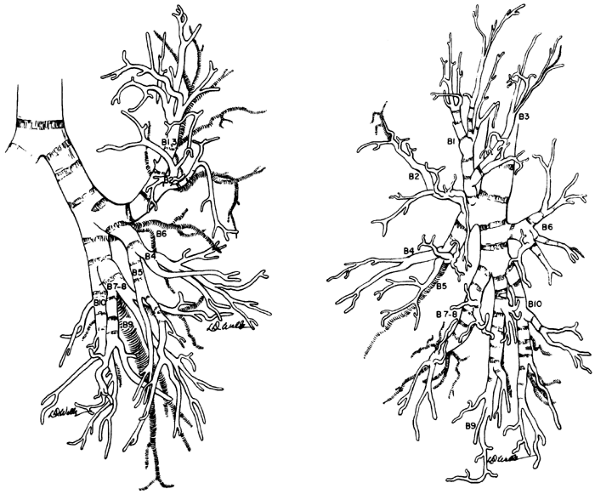 |
Fig. 5-10. Left bronchial tree, anterior and lateral views. Boyden's modification of numerical nomenclature is used. Redrawn and modified from Brock RC: The Anatomy of the Bronchial Tree. 2nd Ed. London: Oxford University Press, 1954, pp. 191 192. With permission. |
Landing and Wells (1973) presented a case in a 2-month-old infant with hypoplasia of the right lung as well as the absence of the middle lobe bronchus. These authors also reported a second infant with a scimitar syndrome associated with an absence of the right upper lobe bronchus. Both these infants had other multiple congenital defects, and the lobar bronchial agenesis was confirmed at the time of autopsy.
Undoubtedly, more examples of lobar agenesis have occurred throughout the intervening years, but the next report that was identified was not until that of Tsunezuka and colleagues in 2002. They reported the absence of a right upper lobe bronchus in an elderly woman.
Absence of a pulmonary artery to the missing lobe has been documented on several occasions. The symptomatology of the agenesis of a lobar bronchus is basically unknown, although the involved lung is usually smaller than the contralateral lung. No treatment per se is indicated unless the agenesis complicates a planned pulmonary resection.
P.66
PULMONARY ARTERIAL SYSTEM
The main pulmonary artery arises to the left of the aorta and passes superiorly and to the left. It occupies a position anterior to the left main bronchus and divides into the right and left main pulmonary arteries. These two vessels lie in an oblique line that is parallel and slightly superior to the pulmonary veins. The right main pulmonary artery is longer than the left, but its extrapericardial length up to its first branch is less than that of the left. The branching pattern of the pulmonary arteries is more variable than that of the bronchi, although the arteries tend to lie closely adjacent to the segmental bronchi and to follow their branching. No one pattern for either the right or the left pulmonary artery may be described as standard. A relatively typical distribution of the segmental arteries is often encountered, however, and from this, the multitude of variations may be readily understood (Figs. 5-11 and 5-12).
Right Pulmonary Artery
As it leaves the pericardial sac, the right pulmonary artery is anterior and inferior to the right main bronchus and posterior and superior to the superior pulmonary vein. The first branch is the truncus anterior, the major vessel carrying blood to the right upper lobe. It arises superolaterally and divides into two branches. The more superior branch of the truncus anterior again divides to form an apical branch that loops posteriorly over the upper lobe bronchus to supply a variable portion of the posterior segment. The latter vessel is known as the posterior recurrent artery. The more inferior branch of the truncus anterior goes to the anterior segment but also may give off a branch to the apical segment. The truncus anterior carries the entire blood supply to the right upper lobe in 1 of 10 individuals. In most people, one or more ascending vessels from the interlobar portion of the pulmonary artery are present also. The interlobar portion crosses over the bronchus intermedius. Generally, only one ascending vessel to the upper lobe is present. This branch, frequently small in caliber, supplies almost exclusively the posterior segment and is referred to as the posterior ascending artery. At the same level, or even either slightly proximal or distal to the posterior ascending artery, the middle lobe artery arises anteromedially from the interlobar portion of the pulmonary artery. The site of origin is usually at the level of the junction of the horizontal and oblique fissures. The artery is usually single, and bifurcation of the vessel is the rule, but the subdivisions are variable. The arterial branch to the superior segment of the lower lobe arises posteriorly and opposite to the middle lobe artery at the same level or slightly distal to it. The superior segmental artery is usually a single trunk that bifurcates. Distal to the aforementioned branchings of the interlobar portion of the artery, the vessel is considered the common basal trunk. The medial basal segmental artery may arise independently or may arise in common with the anterior basal branch. The remainder of the basal trunk then terminates with its division into the lateral and posterior segmental branches, the mode of actual branching being variable.
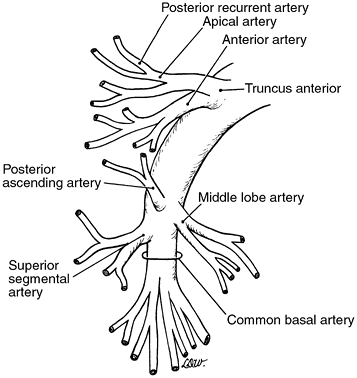 |
Fig. 5-11. Common pattern of branching of the right pulmonary artery. |
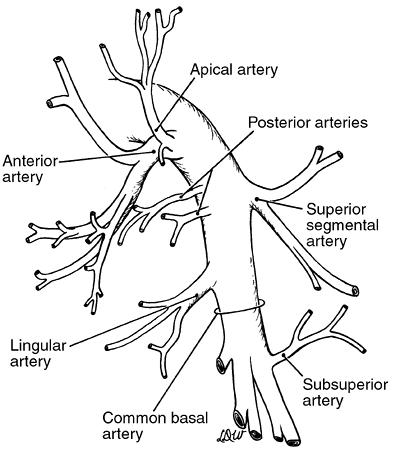 |
Fig. 5-12. Common pattern of branching of the left pulmonary artery. |
The major variations in the right arterial system occur with almost each of the aforementioned branchings. In as much as 20% of the population, two arteries arise from the anterior trunk. These vessels are designated the truncus anterior superior and the truncus anterior inferior. When they
P.67
are present, the recurrent posterior branch is almost always a branch of the truncus anterior superior. Infrequently, more than one ascending branch to the upper lobe arises from the interlobar portion of the artery; the more proximal branch supplies a portion of the anterior segment. On occasion, the posterior ascending artery arises from the superior segmental artery or, even more rarely, from the middle lobe artery. The middle lobe artery, as well as the superior segmental artery, although usually a single vessel, may be represented by two or, at times, even three vessels. Finally, in addition to the variable branchings of the common basal trunk, a subsuperior or accessory subsuperior artery may arise from either the common stem or the posterior basal branch.
Left Pulmonary Artery
The left pulmonary artery ascends to a higher level, passes more posteriorly, and has a greater extrapericardial length before giving off its first segmental branch to the lung than does the right pulmonary artery. The branches to the left upper lobe arise from the anterior, posterosuperior, and interlobar portions of the vessel. The number of branches may vary from two to seven, but four branches to the lobe form the most common pattern. Generally, the first branch arises from the anterior portion of the artery to supply the anterior segment, a part of the apical segment, and occasionally, the lingular division of the lobe. The first branch of this anterior segmental artery supplies the anterior segment and also may give off a lingular branch. Usually, it also branches to provide a vessel carrying blood to the apical segment. The second and, infrequently, a third branch from this first anterior trunk give rise to a vessel, or vessels, going to the anterior segment, to the apical segment, and, uncommonly, to the posterior segment. This anterior trunk is generally short, and often the branches may appear as separate vessels arising from a common opening from the main artery. A second branch from the main artery as it passes distally and posterosuperiorly over the left upper lobe bronchus and into the interlobar fissure is present in almost 80% of cases. This second arterial branch and, occasionally, a third is given off anterosuperiorly to the apical posterior segment. Posteriorly, as the artery passes into the interlobar fissure, it branches to form a vessel going to the superior segment of the lower lobe. This vessel usually is a single one that bifurcates or, infrequently, trifurcates at a variable distance from its takeoff from the main-stem arterial trunk. Most often, the lingular artery originates from the interlobar portion of the pulmonary artery distal to the superior segmental artery and constitutes the lingular arterial supply in toto in 80% of persons. At a variable distance from the origin of the lingular vessel, the pulmonary stem artery, now the common basal trunk, most commonly divides into two major branches. The more anterior branch supplies the anteromedial basal segment, and the posterior one supplies the lateral basal and posterior basal segments. The patterns of branching of the common basal trunk and its major divisions are variable.
Likewise, major variations may occur in all the segmental branches of the left pulmonary artery. As mentioned, the first anterior branch may supply the lingular division as well as other portions of the upper lobe, and although it occurs in fewer than 1 in 10 individuals, this branch may carry all the blood supplying the lingular division. Another variation is that the first anterior branch may carry only the blood supplying the apical segment; the anterior segment in this situation receives its arterial supply from the interlobar portion of the artery. As noted, the superior segmental artery usually arises proximal to the branch, or branches, going to the lingula, but in as many as one of three persons, the superior segmental branch may be distal to the lingular artery takeoff. Both these vessels may be multiple. Again, in one of three persons, a branch of one of the lingular vessels or even a direct branch from the interlobar portion of the artery may supply some blood to the anterior segment of the left upper lobe. Rarely, this vascular branch carries the entire arterial supply to this segment. As on the right, branches to the subsuperior segmental region are often found arising as single or multiple vessels from the common basal stem or, more frequently, from the posterior basal branch. Finally, a vessel may arise from the common basal stem or one of its branches to contribute to the lingular blood supply.
PULMONARY VENOUS SYSTEM
The venous drainage pattern of the lung reveals a greater number of variations than does the arterial pattern. The usual two major venous trunks from both lungs are the superior and inferior pulmonary veins. The tributaries of these veins are intersegmental and form various combinations to create the major trunks (Figs. 5-13 and 5-14).
Right Pulmonary Veins
The superior pulmonary vein lies anterior and somewhat inferior to the pulmonary artery. It usually is made up of four major branches, which drain the upper and middle lobes. The first three branches from above downward drain the upper lobe and are identified as the apical anterior, anterorinferior, and posterior branches. The posterior branch is composed of central and interlobar divisions. The fourth and most inferior trunk drains the middle lobe and generally is made up of two branches. Although the middle lobe vein most often joins the superior pulmonary vein, on occasion, it may enter the pericardium and drain into the atrium as a separate vessel. Rarely, it becomes a tributary of the inferior pulmonary vein. This variant on the right side has been described in two patients by Sujimoto and colleagues (1998). Yamashita (1978) has reported that the incidenceof
P.68
this anomaly was 4.8% in 120 specimens of the right lung and only 2.5% in the left lung.
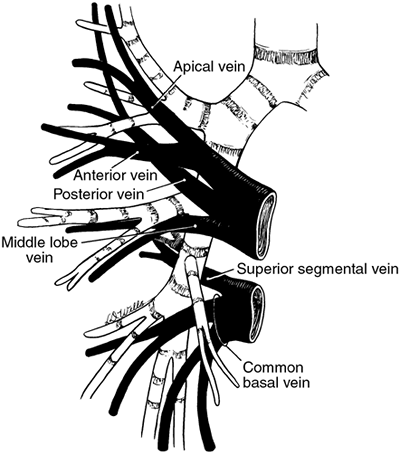 |
Fig. 5-13. Schematic representation of the tributaries of the right superior and inferior pulmonary veins. Adapted from Kubick S: Klinische Anatomie, Ein Farbfoto-Atlas der Topographie 2. Aufl (Band III-Thorax). Stuttgart: Georg Thieme, 1971, p. 97. With permission. |
The inferior pulmonary vein is inferior and posterior to the superior vein. It drains the lower lobe and as a rule is made up of two major trunks. The first is the superior segmental vein, which drains the superior segment. The other branch, known as the common basal vein, is made up of superior basal and inferior basal tributaries, and these vessels drain the various basal segments of the lower lobe.
Left Pulmonary Veins
On the left, the superior pulmonary vein is applied closely to the anteroinferior aspect of the pulmonary artery, and, as a result, obscures the anterior branches of the artery. This vein is made up of three to four tributaries that drain the entire upper lobe. The first division, the apical posterior vein, is made up of an apical ramus and a posterior ramus. The second division represents the anterior vein, which may have three rami: superior, inferior, and posterior. The third and fourth divisions represent the superior and inferior lingular veins. A single trunk may represent these veins in about 50% of persons. This trunk, as seen with the middle lobe vein on the right, may drain into the inferior pulmonary vein; this variant occurs more commonly on the left than on the right, although, as noted previously, the data of Yamashita (1978) do not support this statement.
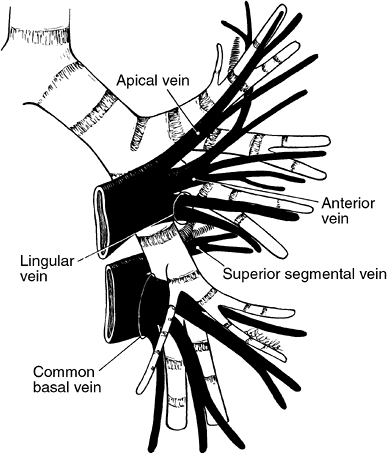 |
Fig. 5-14. Schematic representation of the tributaries of the left superior and inferior pulmonary veins. Adapted from Kubick S: Klinische Anatomie, Ein Farbfoto-Atlas der Topographie 2. Aufl (Band III-Thorax). Stuttgart: Georg Thieme, 1971, p. 97. With permission. |
The inferior pulmonary vein, as on the right, is located inferior and posterior to the superior vein and has two similar tributaries: the superior segmental and the common basal veins. The latter is made up of superior and inferior basal divisions, which drain the basal segments of the lobe.
Anomalous Unilateral Single Pulmonary Vein
On rare occasions, either in the right or left lung, the superior and inferior pulmonary veins may join within the lung substance or in the fissure to form a single trunk draining the entire lung before entering the pericardial sac. This occurrence is known as an anomalous unilateral single pulmonary vein (AUSPV). There is normal venous drainage into the left atrium; although, infrequently, a small secondary vein may drain into a systemic vein in the chest that flows into the right atrium. In some cases, the single vein from the right lung follows a circuitous route before entering the left atrium, resulting in a pseudo scimitar radiographic finding.
An anomalous unilateral single pulmonary vein has been identified infrequently. Twenty patients have been recorded in the literature. This anomaly may occur equally in either gender and is most commonly recognized in adults. An AUSPV has been observed more often in the right lung (65%) than in the left lung (35%). An AUSPV has been identified slightly more often as a single inferior pulmonary vein.
P.69
INTRAPERICARDIAL ANATOMY
The right pulmonary artery passes from the left to the right behind the ascending aorta and constitutes the superior border of the transverse sinus. It then lies behind the superior vena cava and forms the superior border of the postcaval recess of Allison (Fig. 5-15); the medial and inferior borders of this recess are the superior vena cava and right superior pulmonary vein. Although the right pulmonary artery is longer than the left pulmonary artery, it is not as accessible as the left. The left pulmonary artery passes inferior to the aortic arch and forms the superior border of the left pulmonary recess. The medial border of this recess is formed by the fold of Marshall (Fig. 5-16).
The superior and inferior pulmonary veins bulge into the pericardium and are invested to a greater or lesser extent by the pericardium's serous layer. On the right, these two vessels most often enter into the left atrium separately, although rarely they form one vessel. In contrast, on the left, the two veins form a common trunk in one of four persons.
The serous (parietal) pericardial investments of the vessels are important because these fibrous tissue layers must be divided to obtain free access to the entire circumference of the individual vessel. On the right, the serous layer leaves the lateral and posterior surfaces of the superior vena cava and comes to lie on the artery in the postcaval recess. At this point, only about one fifth of the circumference of the vessel is free. In contrast, three fourths of the circumference is free in the transverse sinus medial to the superior vena cava. From the artery, the serous layer passes inferiorly and reflects on the superior, anterior, and inferior surfaces of the superior vein; about one third of this vessel is not free posteriorly. The layer then descends to cover most of the inferior pulmonary vein and then passes down to envelop the inferior vena cava. On the left, the reflection of the serous pericardium passes over the anterior and inferior surfaces of the left pulmonary artery, and about one half of the vessel is free in the pericardial sac. The layer then descends inferiorly to the superior vein, so that only the posterior surface is not free in the sac. It then passes downward to envelop the inferior vein, which is subsequently almost totally free within the sac, except for a small surface located posteriorly.
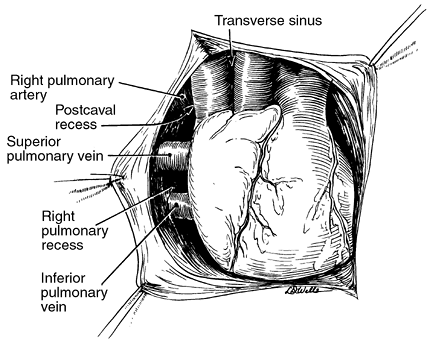 |
Fig. 5-15. Intrapericardial anatomy on the right. Redrawn from Healey JE Jr, Gibbon JH Jr: Intrapericardial anatomy in relation to pneumonectomy for pulmonary carcinoma. J Thorac Surg 19:864, 1950. With permission. |
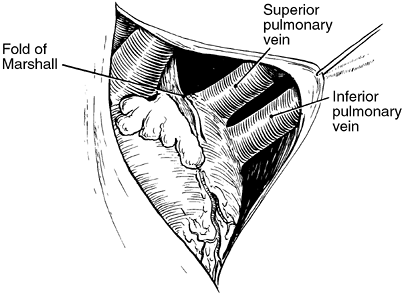 |
Fig. 5-16. Intrapericardial anatomy on the left. Redrawn from Healey JE Jr, Gibbon JH Jr. Intrapericardial anatomy in relation to pneumonectomy for pulmonary carcinoma. J Thorac Surg 19:864, 1950. With permission. |
BRONCHIAL ARTERIES AND VEINS
The bronchial arterial system arises from the systemic circulation and accounts for about 1% of the cardiac output. It empties mainly into the pulmonary veins and a lesser bronchial vein system that enters the azygos venous system on the right and the hemiazygos on the left. The origins of the arteries are variable from the aorta, intercostal arteries, and, occasionally, subclavian or innominate arteries. Rare origin from other systemic vessels of the chest (internal mammary artery) or even from a coronary artery has been recorded.
The most extensive anatomic study was reported by Caudwell and associates (1948). These investigators recorded nine patterns of origin. In 90% of the 150 cadaver specimens studied, the pattern was one of four types (Fig. 5-17), and the remaining 10% were distributed in five other, less common variations (Table 5-3). Liebow (1965) performed corrosion casts of the bronchial arterial system in 50 cadavers, and his findings were in essential agreement with those of the aforementioned authors.
Caudwell and colleagues (1948) reported that the level of origin of the bronchial arteries was from the third to eighth vertebral bodies, most commonly between the levels of the fifth and sixth thoracic vertebrae, and arose from the descending thoracic aorta and rarely from the arch. Most of the
P.70
bronchial vessels arose separately (74% of specimens), and only in 26% did two vessels have a common origin. The right bronchial arteries arose from the anterolateral or lateral surface of the aorta and rarely from its posterior aspect. In 88.7% of specimens, the right bronchial artery arose in common with an aortic intercostal vessel: 78% from the first, 7.3% from the second, and 1.3% from the third. Nathan and colleagues (1970) described the anatomy of this major right bronchial artery. They found that it arose anywhere from 0.5 to 5.0 cm from the origin of the intercostal artery from its origin from the aorta and courses upward and forward toward the right main-stem bronchus. In its courses on the right anterolateral aspect of the vertebral column, it passes to the right of the thoracic duct and crosses the esophagus to terminate at the lower level of the trachea near the origin of the right main-stem bronchus. At the level of the trachea, it crosses lateral to the vagus nerve. In its mediastinal course, the right bronchial artery generally runs parallel to the arch of the azygos vein by which it is overlapped.
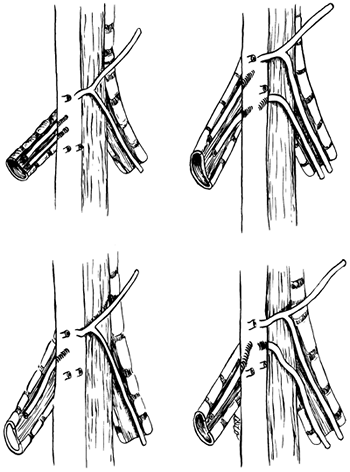 |
Fig. 5-17. The four most common sites of origin and numbers of bronchial arteries to the right and left lung (see Table 5-3 for percentage of occurrence of each). From Caudwell EW, et al: The bronchial arteries. An anatomic study of 150 human cadavers. Surg Gynecol Obstet 86:395, 1948. With permission. |
On the left, the bronchial arteries are more variable in their courses to the bronchus and, according to Caudwell and associates (1948), 94% arise directly from the aorta. Only 4% are associated with an intercostal vessel, which invariably is a right intercostal artery. In most instances, the bronchial vessels that arise from the aorta pass in back of the trachea, and in only a few cases does one pass in front of the trachea. Rarely, such a branch to the right may be in close proximity to the tracheal carina. It is possible a branch of such an anatomically situated vessel could be injured during a mediastinoscopy, as suggested by Miller and Nelems (1989).
Table 5-3. Origins and Number of Bronchial Arteries in 150 Dissected Autopsy Specimens | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
These anatomic studies by the aforementioned investigators have been confirmed by the angiographic observations of Olson and Athanasoulis (1982) and other interventional radiologists. Deffebach and associates (1987) reviewed the distribution of the bronchial arteries once they entered the hilus of the lung and course within the bronchial tree. Essentially, the arteries to either side form a communicating arc around the main bronchus. From here, the main arterial divisions radiate along the major bronchi. These vessels are closely applied to the bronchial wall, with generally two divisions, an anterior and posterior branch, along each bronchus. The vessels follow the course of the bronchus and divide, as do the bronchi. Networks of intercommunicating vessels are often present on the bronchial walls. It has been assumed that two thirds of this blood supply empties into the pulmonary veins and that the rest empties into the bronchial veins. The bronchial veins are present in the mucosa and also external to the bronchial cartilage. The direction of flow is to the venous plexus of the perihilar regions and then subsequently into either the azygos or hemiazygos systems.
CLINICAL FINDINGS AND SIGNIFICANCE OF TRACHEOBRONCHIAL ANOMALIES
Tracheal Bronchus
A tracheal bronchus may remain undetected throughout a person's life, but occasionally it is symptomatic in young
P.71
children and less commonly in adults. McLaughlin and colleagues (1985) identified 18 tracheal bronchi in children younger than the age of 5 years; eight of them were diagnosed by bronchoscopy in 412 children with respiratory symptoms. Five children underwent resection of the anomalous bronchus and its supplied lung tissue because of recurrent pneumonia. The tracheal bronchus often was associated with other congenital defects: tracheal stenosis, hypoplastic or fused first and second thoracic ribs, bilateral lumbar first ribs, and other minor vertebral anomalies. Down's syndrome often is present. In adults, complications are rare.
Epstein (1951) reported the occurrence of a bronchial adenoma (carcinoid tumor) that caused hemoptysis. Recently, Patrinou (2001) and Metin (2002) and their associates have recorded a second and a third patient with a typical carcinoid in this anomalous bronchus. In 1974, Uchikov and Nikolov published a case report of a patient with a non small cell carcinoma arising in a tracheal bronchus, as did Calvet and associates (1997), but whether or not resection was accomplished was not stated. Successful surgical resection of a carcinoma arising in a tracheal bronchus was carried out by Kim (1998), Kuo (1999), and Liu (2000) and their co-workers by means of a standard right upper lobectomy with division of the tracheal bronchus from the tracheal wall, whereas Sato and colleagues (2002) used a tracheobronchoplasty to effect a complete resection. Previously, Okubo and associates (2000) had carried out an upper sleeve lobectomy for a cancer of the right upper lobe; although, in this patient, the tumor did not originate in a supranumerary (ectopic) tracheal bronchus that was present. Finally, Moriya and colleagues (1985) reported the occurrence of a small cell cancer arising in this bronchus.
On rare occasions, bilateral tracheal bronchi may be present, the right and left tracheal bronchi supplying a portion or all of the respective upper lobe of the right and left lung. Holinger and colleagues (1952) were the first to describe this anomaly in a living infant, who subsequently died of respiratory complications. Cope and associates (1986) also identified a 14-month-old child with similar malformations who apparently had no difficulty because of their presence and was reported to be well at 2 years of age. Finally, Holinger and colleagues (1952) described an isolated left tracheal bronchus that curved posteriorly and medially to supply the right upper lobe.
Accessory Cardiac Bronchus
The presence of an accessory cardiac bronchus more often than not remains undetected until discovered on a diagnostic bronchoscopy or computed tomography (CT) of the lung; it is undetectable by standard chest radiography. Sotile and associates (1988) initially described the CT findings, and these were further defined by McGuinness and co-workers in 1993. Occasionally, most often in adults, this rare anomaly gives rise to symptoms as the result of infection within the anomalous bronchus. Hemoptysis from mucosal ulceration or persistent symptoms of unresolved pulmonary infection are the common clinical features, as noted by Keane (1997), Endo (2000), and Bentala (2002) and their colleagues, that lead to its identification. According to Daskalakis (1983), one patient developed a middle lobe syndrome because of the presence of this anomalous bronchus. In patients with a long accessory cardiac bronchus, pulmonary infection and subsequent pleural empyema may occur as one of the more serious complications. This has been reported by Suzuki and Hirata (1991) in the Japanese literature. Control of persistent symptoms is achieved by resection of the offending accessory cardiac bronchus. The rare occasion of the development of a squamous cell carcinoma in this bronchial anomaly was reported by Miyahara and associates (2002).
Bridging Bronchus
The three patients with a bridging bronchus were baby girls who demonstrated respiratory distress and pulmonary infection. All three had multiple congenital anomalies in addition to the bridging bronchus. Death occurred in each patient, in two after attempted surgical resection of the affected lobe.
CLINICAL AND RADIOGRAPHIC FEATURES OF AN ANOMALOUS UNILATERAL SINGLE PULMONARY VEIN
As a general rule, the presence of an AUSPV is asymptomatic but may have serious surgical implications when unrecognized, as noted by Meguro and co-workers (1998), who reported the necessity of a completion pneumonectomy following a left upper lobectomy when the entire venous drainage was through a single superior pulmonary vein.
A roentgenographic abnormality is present in most patients with an AUSPV. The roentgenographic abnormality may appear (a) as a varix, as reported by Ben-menachem (1975) and Hasuo (1981) and their colleagues; (b) as a possible pulmonary mass lesion, as noted by Benfield (1971) and Gilkeson (2000) and their co-workers; or (c) as an abnormal shadow along the right border simulating a scimitar syndrome, as described by Goodman (1972), Valdez-Davila (1978), and Cukier (1994) and their associates. Additional cases of AUSPV have been recorded by Kozuka and Nosaki (1968), Morgan and Forker (1971), Remy-Jardin and Remy (1999), and by Papamichael (1972), Tretheway (1974), Twersky (1976), Lutman (1986), Rey (1986), Belott (1997), and Moro (1999) and their associates. The essential findings in the 20 reported cases are listed in Table 5-4.
In the 1970s and 1980s, the diagnosis was established by pulmonary angiography, but presently, contrast-enhanced helical CT or magnetic resonance imaging is used. Three-dimensional reconstruction of the enhanced helical CT
P.72
scan is an excellent way to demonstrate the anomaly. Recognition of an AUSPV is, of course, mandatory when a pulmonary resection is required for an associated pathologic process.
Table 5-4. Anomalous Unilateral Single Pulmonary Vein | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
REFERENCES
Anson BJ: Atlas of Human Anatomy. Philadelphia: WB Saunders, 1950, p. 199.
Atwell SW: An aberrant bronchus. Ann Thorac Surg 2:438, 1966.
Atwell SW: Major anomalies of the tracheobronchial tree: with a list of the minor anomalies. Dis Chest 52:611, 1967.
Barat M, Konrad HR: Tracheal bronchus. Am J Otolaryngol 8:118, 1987.
Beattie EJ, Bloom ND, Harvey JC: Thoracic Oncology. New York: Churchill Livingstone, 1992.
Belott PH, et al: Single left pulmonary vein with normal pulmonary drainage: association with partial anomalous venous return. Cathet Cardiovasc Diagn 3:67, 1997.
Benfield JR, Gots RE, Mills D: Anomalous single left pulmonary vein mimicking a parenchymal nodule. Chest 59:101, 1971.
Ben-menachem Y, et al: The various forms of pulmonary varices. Report of three cases and review of the literature. AJR Am J Roentgenol 125:881, 1975.
Bentala M, et al: Cardiac bronchus: a rare cause of hemoptysis. Eur J Cardiothorac Surg 22:643, 2002.
Bertucci GM, et al: Bridging bronchus and posterior left pulmonary artery: a unique association. Pediatr Pathol 7:637, 1987.
Birnbaum GL: Anatomy of the Bronchovascular System. Its Application to Surgery. Chicago: Year Book Medical Publishers, 1954.
Bloomer WE, Liebow AA, Hales MR: Surgical Anatomy of the Bronchovascular Segments. Springfield, IL: Charles C. Thomas, 1960.
Boyden E: The intrahilar and related segmental anatomy of the lung. Surgery 18:706, 1945.
Boyden EA: Segmental Anatomy of the Lungs. New York: McGraw-Hill, 1955.
Brock RC: The Anatomy of the Bronchial Tree. London: Oxford University Press, 1946.
Brock RC (reporter): The nomenclature of broncho-pulmonary anatomy: an international nomenclature accepted by The Thoracic Society. Thorax 5:222, 1950.
Calvet P, et al: Right tracheal bronchus. J Radiol 78:135, 1997.
Caudwell EW, et al: The bronchial arteries. An anatomic study of 150 human cadavers. Surg Gynecol Obstet 86:395, 1948.
Cope R, Campbell JR, Wall M: Bilateral tracheal bronchi. J Pediatr Surg 21:443, 1986.
Cukier A, et al: Scimitar sign with normal pulmonary venous drainage and systemic arterial supply. Scimitar syndrome or bronchopulmonary sequestration? Chest 105:294, 1994.
Daskalakis MK: Middle lobe syndrome due to accessory cardiac bronchus. South Med J 76:941, 1983.
Deffebach ME, et al: The bronchial circulation. Small but a vital attribute of the lung. Am Rev Respir Dis 135:463, 1987.
Endo S, et al: Symptomatic accessory cardiac bronchus. Ann Thorac Surg 69:262, 2000.
Epstein I. Bronchial adenoma in supernumerary tracheal lobe; report of unusual case. J Thorac Surg 21:362, 1951.
Ferguson CF, Neuhausen EBD: Congenital absence of lung (agenesis) and other anomalies of the tracheo-bronchial tree. AJR Am J Roentgenol 52:459, 1944.
Gilkeson RC, Haaga JR, Ciancibello LM: Anomalous unilateral single pulmonary vein: multidetector CT findings. AJR Am J Roentgenol 175: 1464, 2000.
Gonzalez-Crussi F, et al: Bridging bronchus. A previously undescribed airway anomaly. Am J Dis Child 130:1015, 1976.
Goodman LR, Jamshidi A, Hipona FA: Meandering right pulmonary vein simulating the scimitar syndrome. Chest 62:510, 1972.
Hasuo K, et al: Anomalous unilateral single pulmonary vein mimicking pulmonary varices. Chest 79:602, 1981.
Holinger PH, et al: Congenital malformations of the trachea, bronchi, and lung. Ann Otol Rhinol Laryngol 61:1159, 1952.
Jackson CL, Huber JF: Correlated applied anatomy of bronchial tree and lungs with system nomenclature. Dis Chest 9:319, 1943.
Jackson GD, Littleton TJ: Simultaneous occurrence of anomalous cardiac and tracheal bronchi: a case study. J Thorac Imaging 3: 59, 1988.
Keane MP, et al: Accessory cardiac bronchus presenting with haemoptysis. Thorax 52:490, 1997.
Kim J, et al: Surgical resection of lung cancer originating in a tracheal bronchus. Ann Thorac Surg 66:944, 1998.
Kozuka T, Nosaki T: A pulmonary vein anomaly: unusual connection and tortuosity of the right lower lobe vein. Br J Radiol 41:232, 1968.
Kuo CW, Lee YC, Perng RP: Tracheal bronchus associated with lung cancer: a case report. Chest 116:1125, 1999.
Landing BH, Wells TR: Tracheobronchial anomalies in children. Perspect Pediatr Pathol 1:1, 1973.
Le Roux BT: Anatomical abnormalities of the right upper lobe bronchus. J Thorac Cardiovasc Surg 44:225, 1962.
Liebow AA: Patterns of origin and distribution of the major bronchial arteries in man. Am J Anat 117:19, 1965.
Liu HC, Hsu WH, Huang MH: Squamous cell carcinoma of the right upper lung congenial tracheal bronchus. Chung Hua i Hsueh Tsa Chih (Taipei) 63:424, 2000.
Lutman M, et al: Anomala unica vena polmonare unilateral (AUVPU). Importanza diagnostica della tomografia computerizzata. Radiol Med (Torino) 72:239, 1986.
Mangiulea VG, Stinghe RV: The accessory cardiac bronchus. Bronchologic aspect and review of the literature. Dis Chest 54:433, 1968.
McGuinness G, et al: Accessory cardiac bronchus: CT features and clinical significance. Radiology 189:563, 1993.
McLaughlin FJ, et al: Tracheal bronchus: association with respiratory morbidity in childhood. J Pediatr 106:751, 1985.
Meguro H, et al: A case of single left pulmonary vein with deficiency of left inferior pulmonary vein. J Jpn Assoc Chest Surg 12:539, 1998.
Metin M, et al: Tracheal bronchus obliterated with bronchial carcinoid. Eur J Cardiothorac Surg 21:155, 2002.
Miller RR, Nelems B: Mediastinal lymph node necrosis: a newly recognized complication of mediastinoscopy. Ann Thorac Surg 48:247, 1989.
Miyahara R, et al: A case of squamous cell carcinoma arising from accessory cardiac bronchus. Eur J Cardiothorac Surg 22:309, 2002.
Morgan JR, Forker AD: Syndrome of hypoplasia of the right lung and dextroposition of the heart: scimitar sign with normal pulmonary venous drainage. Circulation 43:27, 1971.
Moriya H, Kato H, Togawa T: Small cell lung cancer arising in an abnormal bronchus. Jpn J Chest Dis 441:1035, 1985.
Moro C, et al: Pulmonary varix: report of a case with additional anomalies of the vascular pulmonary tree. Am Heart J 95:243, 1999.
Morton DR, Klassen K, Baxter EH: Lobar agenesis of the lung. J Thorac Surg 20:665, 1950.
Nathan H, Orda R, Barkay M: The right bronchial artery. Anatomical considerations and surgical approach. Thorax 25:328, 1970.
Nomina Anatomica. 6th Ed. New York: Churchill Livingstone, 1989.
Okubo K, Ueno Y, Isobe J: Upper sleeve lobectomy for lung cancer with tracheal bronchus. J Thorac Cardiovasc Surg 120:1011, 2000.
P.73
Olson PR, Athanasoulis CA: Hemoptysis: treatment with transcatheter embolizations of the bronchial arteries. In: Athanasoulis CA, et al: Interventional Radiology. Philadelphia: WB Saunders, 1982, p. 196.
Overholt RH, Langer LA: New technique for pulmonary segmental resection. Its application in the treatment of bronchiectasis. Surg Gynecol Obstet 84:257, 1947.
Papamichael E, et al: Pulmonary varicosity associated with other congenital abnormalities. Chest 62:107, 1972.
Patrinou V, Kourea H, Dougenis D: Bronchial carcinoid of an accessory tracheal bronchus. Ann Thorac Surg 71:1034, 2001.
Remy-Jardin M, Remy J: Spiral CT angiography of the pulmonary circulation. Radiology 212:615, 1999.
Rey C, Vaksmann G, Francart C: Anomalous unilateral single pulmonary vein mimicking partial anomalous pulmonary venous return. Cathet Cardiovasc Diagn 12:330, 1986.
Sato M, et al: Tracheobronchoplasty for resection of lung cancer arising from a tracheal bronchus. Ann Thorac Surg 73:310, 2002.
Sealy WC, Connally SR, Dalton ML: Naming the bronchopulmonary segments and the development of pulmonary surgery. Ann Thorac Surg 55: 184, 1993.
Sotile SC, Brady MB, Brogdon BG: Accessory cardiac bronchus: demonstration by computed tomography. J Comput Tomogr 12:144, 1988.
Starshak RJ, et al: Bridging bronchus. A rare airway anomaly. Radiology 140:95, 1981.
Storey CF, Marranooni AG: Lobar agenesis of the lung. J Thorac Surg 28: 536, 1954.
Sujimoto S, et al: Anatomy of inferior pulmonary vein should be clarified in lower lobectomy. Ann Thorac Surg 66:1799, 1998.
Suzuki M, Hirata S: A case of accessory bronchus with acute empyema treated by open drainage. Nippon Kyobu Shikkan Gakkai Zasshi 29:600, 1991.
Tretheway DG, et al: Single left pulmonary vein with normal pulmonary venous drainage: a roentgenographic curiosity. Am J Cardiol 34:237, 1974.
Tsunezuka Y, et al: Congenital absence of the right upper lobe of the lung. Ann Thorac Surg 74:57, 2002.
Twersky J, et al: Further observation on pulmonary venous varix. AJR Am J Roentgenol 127:435, 1976.
Uchikov P, Nikolov P: A case of carcinoma developing from the base of an anomalous tracheal bronchus. Khirurgiia (Sofiia) 27:319, 1974.
Valdez-Davila O, et al: A variation of scimitar syndrome. ROFO Fortschr Geb Rontgenstr Unklearmed 128:271, 1978.
Yamashita H: Variations in the pulmonary segments and the bronchovascular tree. In: Yamashita H, ed. Roentgenographic Anatomy of the Lung. Tokyo: Igakushoin, 1978, p. 70.
READING REFERENCES
Allison PR: Intrapericardial approach to the lung root in the treatment of bronchial carcinoma by dissection pneumonectomy. J Thorac Surg 15:99, 1946.
Barrett RJ, Day JC, Tuttle WM: The arterial distribution to the left upper pulmonary lobe. J Thorac Surg 32:190, 1956.
Barrett RJ, O'Rourke PV, Tuttle WM: The arterial distribution to the right upper pulmonary lobe. J Thorac Surg 36:117, 1958.
Brock RC: The Anatomy of the Bronchial Tree. 2nd Ed. London: Oxford University Press, 1954.
Cory RAS, Valentine EJ: Varying patterns of the lobar branches of the pulmonary artery. Thorax 14:267, 1959.
Cudkowicz L: The Human Bronchial Circulation in Health and Disease. Baltimore: Williams&Wilkins, 1968.
Harris JH Jr: The clinical significance of the tracheal bronchus. AJR Am J Roentgenol 79:228, 1958.
Healey JE Jr, Gibbon JH Jr: Intrapericardial anatomy in relation to pneumonectomy for pulmonary carcinoma. J Thorac Surg 19:864, 1950.
Kent EM, Blades B: The surgical anatomy of the pulmonary lobes. J Thorac Surg 12:18, 1942.
Kubik S, Healey JE: Surgical Anatomy of the Thorax. Philadelphia: WB Saunders, 1970.
Milloy FJ, Wragg LE, Anson BJ: The pulmonary arterial supply to the right upper lobe of the lung based upon a study of 300 laboratory and surgical specimens. Surg Gynecol Obstet 116:35, 1963.
Milloy FJ, Wragg LE, Anson BJ: The pulmonary arterial supply to the upper lobe of the left lung. Surg Gynecol Obstet 126:811, 1968.
*The described anatomic patterns and variations of the bronchi and pulmonary arteries and veins have been selected by the author primarily from the studies of Birnbaum (1954); Bloomer, Liebow, and Hales (1960); and Boyden (1955).
EAN: 2147483647
Pages: 203