3 - Polycystic Ovary Syndrome
Authors: Unger, Jeff
Title: Diabetes Management in the Primary Care Setting, 1st Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > 3 - Polycystic Ovary Syndrome
function show_scrollbar() {}
3
Polycystic Ovary Syndrome
Take Home Points
Women with polycystic ovary syndrome (PCOS) experience menstrual irregularities, insulin resistance, and infertility
Early recognition of PCOS by the primary care physician (PCP) can reverse infertility, reduce the risk of cardiovascular disease, and slow the progression toward type 2 diabetes
The hallmark of PCOS is the excessive production of androgen hormones, which is driven by insulin resistance
Thirty percent to 40% of women with PCOS have impaired glucose tolerance and 10% develop type 2 diabetes by age 40
Treatment strategies for PCOS should be individualized and based on patient-driven outcomes. Insulin sensitizers are useful in inducing fertility and improving menstrual regularity. Oral contraceptives and spironolactone in combination can be useful to reverse the cosmetic effects of hyperandrogenemia. Treatment of metabolic abnormalities such as atherogenic dyslipidemia should be strongly encouraged. Lifestyle intervention is the cornerstone of therapy for all patients with PCOS.
Case 1
Rita is a 37-year-old Hispanic woman presenting for a routine physical examination. Although married for 15 years, she has failed to become pregnant despite numerous courses of clomiphene citrate. After achieving menarche at age 12, her periods have remained normal and consistent with intervals of 28 to 30 days. Over the past 10 years, Rita has noted an increase in both her appetite and weight. She has gained almost 20 pounds over the past 2 years. Following large meals, she tends to feel shaky, tired, and even confused. Rita is also concerned about excessive hair growth on her face and a long history of moderate cystic acne that is treatment resistant. The dark skin patches developing behind her neck are becoming thicker. In fact, she has tried unsuccessfully to scrub the areas off with bleach.
Rita's mother had angina and died at age 55 of a myocardial infarction. Her father is 62 years old and was recently diagnosed with type 2 diabetes complicated by chronic kidney disease. Two of her five siblings have hypertension and hyperlipidemia, but because they live outside of the United States, she knows little about their actual medical conditions.
Rita's physical exam and laboratory findings are as follows:
Body mass index (BMI) = 34 kg per m2
Blood pressure (BP) = 118/72 mm Hg
Waist circumference = 39 inches
Skin exam = Moderate facial acne associated with significant hirsutism involving the chin, side of the face, and chest. Midline alopecia is noted on the scalp. Prominent areas suggestive of acanthosis nigricans are noted around the neck and axilla.
Note: All lab studies were performed at 7 AM on day 3 of the patient's menstrual cycle.
Luteinizing hormone to follicle-stimulating hormone ratio (LH/FSH) = 3.50
Glucose, blood urea nitrogen (BUN), electrolytes, and liver panel all normal
Prolactin, 17-hydroxyprogesterone, and thyroid function tests all normal
Free testosterone = 68 ng per mL (normal 0 9.5 ng per mL)
Dehydroepiandrosterone sulphate (DHEAS) = 156 g per dL (normal 35 43 g per dL)
Sex-hormone binding globulin (SHBG) = 12 nmol per L (normal 18 114 nmol per L)
Lipid panel
Total cholesterol = 242 mg per dL
Triglycerides = 276 mg per dL
High-density lipoprotein cholesterol (HDL-C) = 35 mg per dL
Low-density lipoprotein cholesterol (LDL-C) = 120 mg per dL
Non-HDL-C = 207 mg per dL
P.89
Questions
What is the most likely reason for this patient's infertility?
Why are the patient's periods normal?
Is there a need to perform a pelvic ultrasound on this patient? Should a transvaginal or transabdominal ultrasound be ordered?
How should one manage her hirsutism and alopecia?
Previous attempts at treating her cystic acne have been unsuccessful. Would a course of isotretinoin be indicated, assuming the patient did not want to pursue pregnancy?
If the patient wanted to become pregnant, what pharmacologic interventions should be considered?
If pregnancy is achieved, should the medications be continued during pregnancy or discontinued during the first trimester?
How should the patient's metabolic risks be assessed and managed?
If the patient is successful at weight reduction, is there a chance that the acanthosis nigricans will improve or even resolve?
P.90
Polycystic ovary syndrome (PCOS) has a major effect on a woman's reproductive, metabolic, and cardiovascular well-being. Poetically described as the thief of womanhood, 1 PCOS can cause infertility, irregular menses, severe acne, and hirsutism. Women with PCOS constitute the largest group of women at risk for developing cardiovascular disease (CVD) and diabetes. PCOS has also been associated with an increased risk of endometrial carcinoma2 and obstructive sleep apnea.3 The cardiovascular and metabolic risk factors associated with PCOS are potentially reversible. When patients suspected of having PCOS are carefully evaluated and screened by their PCP, treatment can restore fertility, decrease the patient's cardiac risk, and slow the progression toward type 2 diabetes mellitus (T2DM).
Prevalence and Pathogenesis of Polycystic Ovary Syndrome
PCOS is one of the most common causes of menstrual irregularity and infertility in the United States. The prevalence of PCOS in adult women is estimated4 to be between 6% and 10%. The symptoms of PCOS usually begin around menarche, but onset after puberty may also occur because of environmental modifiers such as weight gain. Premature pubarche (appearance of pubic hair growth), the result of early secretion of adrenal steroids, may be a harbinger of the syndrome.5 Genetic studies support the increased frequency of PCOS in first-degree relatives of affected women.6
A normally functioning hypothalamic-pituitary-ovarian axis supports the maturation of ovarian follicles via the release of luteinizing hormone (LH) from the anterior pituitary gland. Follicle-stimulating hormone (FSH) stimulates the production of the ovarian follicles and is also secreted from the anterior pituitary gland. Within the ovarian follicle, theca cells synthesize androgen. Once formed, the androgens diffuse into the ovarian granulosa cells where they are converted into estrogen.
Patients with PCOS experience increased ovarian androgen biosynthesis as a result of abnormalities occurring at all levels of the hypothalamic-pituitary-ovarian axis:
Women with PCOS appear to have more frequent releases of LH, suggesting that the pulse frequency of gonadotropin-releasing hormone (GnRH) must be accelerated in PCOS.7
Increasing the GnRH pulse generator favors the synthesis and release of LH over FSH.
When the concentration of LH increases relative to FSH, the ovaries preferentially synthesize testosterone.
Insulin acts synergistically with LH to increase androgen production within the theca cell.
Insulin also inhibits the hepatic synthesis of SHBG, which normally binds testosterone. The higher levels of unbound or free testosterone increase the biologic activity of the circulating hormone.
Testosterone further inhibits (whereas estrogen stimulates) the hepatic synthesis of SHBG.8
P.91
An increase in free and total serum testosterone levels results in androgenization, the most obvious features of which are hirsutism, acne, and diffuse alopecia. Hyperandrogenemia interferes with the hypothalamic-pituitary axis, leading to anovulation (Fig. 3-1). The absence of a dominant follicle prevents development of nondominant follicles, resulting in the formation of multiple ovarian cysts.9 High androgen levels also affect metabolic parameters such as lipid concentrations.10
Women with PCOS typically have hyperinsulinemia. Thus, the concentration of free testosterone is often elevated, whereas the total testosterone level may be only slightly increased.11 Figures 3-1 and 3-2 summarize the pathogenesis and clinical outcomes associated with PCOS.
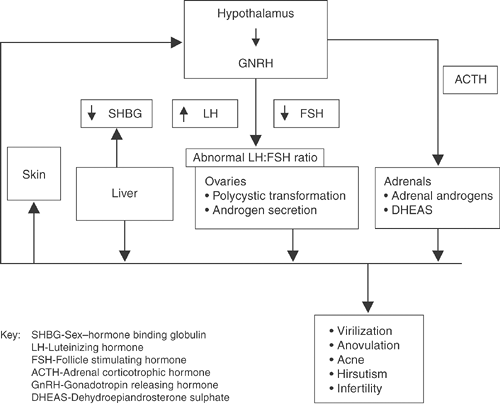 |
Figure 3-1 Polycystic Ovary Syndrome (PCOS) Neuroendocrine Dysfunction. |
P.92
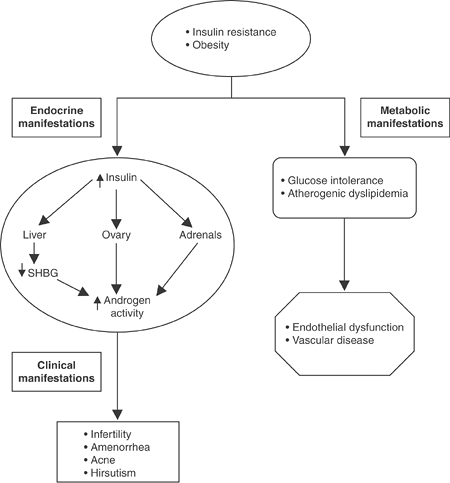 |
Figure 3-2 Metabolic Abnormalities Associated with Polycystic Ovary Syndrome (PCOS). |
Diagnostic Criteria for Polycystic Ovary Syndrome
The 2003 Rotterdam PCOS Consensus Conference, held by the European Society of Human Reproduction and Embryology in conjunction with the American Society for Reproductive Medicine, developed the following diagnostic criteria for PCOS12:
A history of irregular menstrual cycles and anovulation with onset at puberty. Twenty-five percent of women with PCOS have regular menstrual cycles.
Elevated total and free testosterone levels.
The presence of polycystic ovaries and the exclusion of other hormonal disorders with similar clinical features such as adult-onset congenital adrenal hyperplasia, hyperprolactinemia, adrenal or ovarian androgen-producing adenomas, hyperthecosis, and Cushing syndrome. The term hyperthecosis refers to the presence of nests of luteinized theca cells in the ovarian stroma secondary to differentiation of the ovarian interstitial cells into steroidogenically active luteinized stromal cells. These nests or islands of luteinized theca cells are scattered throughout the stroma of the ovary, rather than being confined to areas around cystic follicles as in PCOS. The result is greater production of androgens. The clinical features of hyperthecosis are similar to those of PCOS. However, women with hyperthecosis have more hirsutism and are much more likely to be virilized.
P.93
The Rotterdam PCOS Consensus Panel suggested that tests of insulin resistance are not necessary to make the diagnosis of PCOS or to select the type of treatment. Obese women with PCOS should be screened for metabolic syndrome (MS) and T2DM.
The Androgen Excess Society stresses the importance of hyperandrogenism as the cardinal feature of PCOS, placing less emphasis on ovarian morphology.
The clinical manifestations of PCOS are listed in Table 3-1.
TABLE 3-1 Clinical Manifestations of Polycystic Ovary Syndrome (PCOS) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||
P.94
Clinical Evaluation of Polycystic Ovary Syndrome
Figure 3-3 lists the historical and clinical features that should be evaluated in patients suspected of having PCOS. The typical history reveals a patient whose menarche begins as anticipated at the average age of 12 to 13 years. Shortly after menarche, the periods become irregular. After consulting a physician for irregular menses, patients may be prescribed oral contraceptives to help regulate the menstrual cycles. Oral contraceptives are beneficial for the treatment of PCOS, as they lower serum androgen levels, improve menstrual cycle regularity, and reduce hirsutism and acne. However, the use of oral contraceptive can also result in the delay of the PCOS diagnosis, thereby preventing teenagers from understanding the cardiovascular risks associated with this chronic disease.
Clinical Features Associated with Polycystic Ovary Syndrome
Hirsutism, Alopecia, and Acne
Male pattern hair and skin changes are common with PCOS. Hair growth occurs on the chin, cheek, sideburns, neck, and chest (Figs. 3-4 and 3-5) and between the breasts, on the periareolar area and upper arms, and below the umbilicus. The development of excessive body hair in a male-type distribution is a major clinical sign that is strongly associated with androgen excess. The onset of hirsutism in PCOS follows menarche, although some girls have earlier onset of pubic hair development and some degree of hirsutism. As patients gain more weight, the hirsutism intensifies.
Diffuse alopecia occurs in 40% to 70% of women with PCOS. Poor nutrition (particularly related to low protein intake), anemia, genetic predisposition, and zinc deficiencies in association with androgen excess increase the risk of developing alopecia14 (Fig. 3-6).
The PCOS-associated acne often precedes the development of hirsutism. Forty percent of girls with severe acne resistant to oral and topical agents including isotretinoin have PCOS.15 Severe or moderate acne persists and even worsens in women ages 20 to 40 (Fig. 3-5). The clinical finding of acne may be the sole manifestation of androgen excess in women with PCOS.15
Central Obesity
Women with PCOS may relate a sudden onset of weight gain over 6 to 18 months, further complicating symptoms associated with menstrual irregularities, infertility, acne, and hirsutism. The weight gain is often associated with a significant carbohydrate craving. Because of the exaggerated pancreatic beta-cell secretion of insulin following the consumption of carbohydrate-rich meals, patients may experience postprandial symptoms suggestive of hypoglycemia such as loss of concentration, hunger, sweating, tremor, and
P.95
P.96
P.97
insomnia. Weight gain is associated with an increase in visceral fat distribution, contributing to the development of impaired glucose tolerance (IGT), atherogenic dyslipidemia, and proinflammatory markers, which constitute components of MS16 (see Chapter 2). The central obesity favors progression
P.98
to T2DM and increases the risk of cardiovascular disease (CVD) in patients with PCOS (Fig. 3-7).
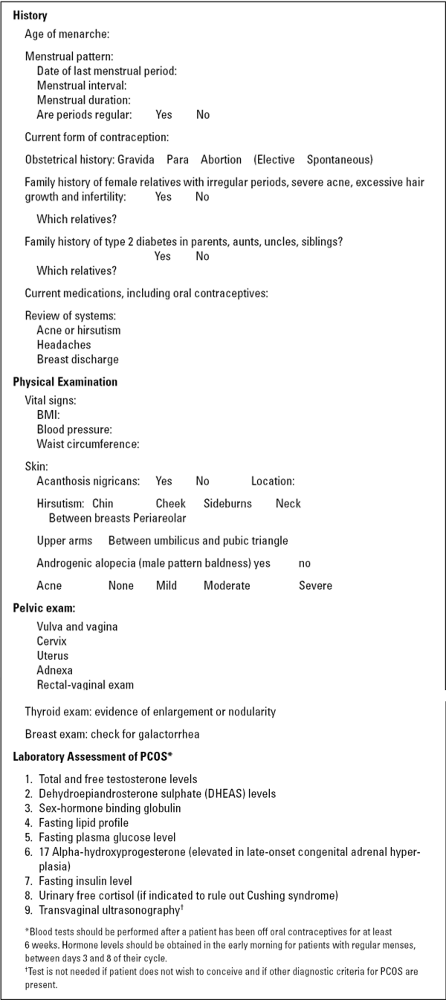 |
Figure 3-3 History, Physical, and Laboratory Workup of Patients with Polycystic Ovary Syndrome (PCOS) |
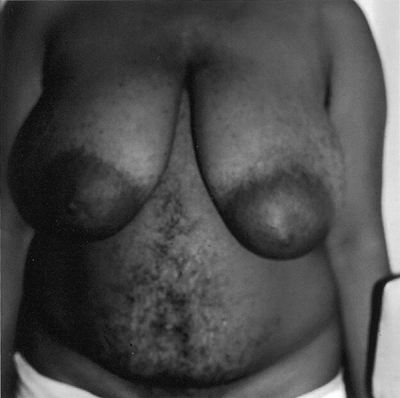 |
Figure 3-4 Patient with Polycystic Ovary Syndrome Displaying Diffuse Hirsutism on Abdomen and Chest. (Photo provided courtesy of Walter Futterweit, MD.) |
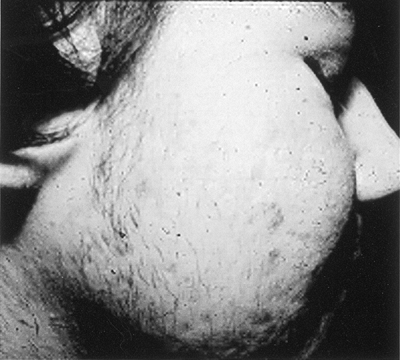 |
Figure 3-5 Severe Facial Acne and Hirsutism in Patient with Polycystic Ovary Syndrome. (Photo provided courtesy of Walter Futterweit, MD.) |
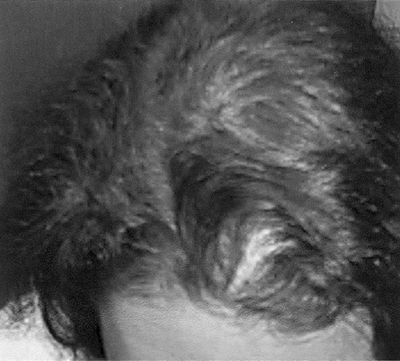 |
Figure 3-6 Alopecia Associated with Polycystic Ovary Syndrome. (Photo provided courtesy of Walter Futterweit, MD.) |
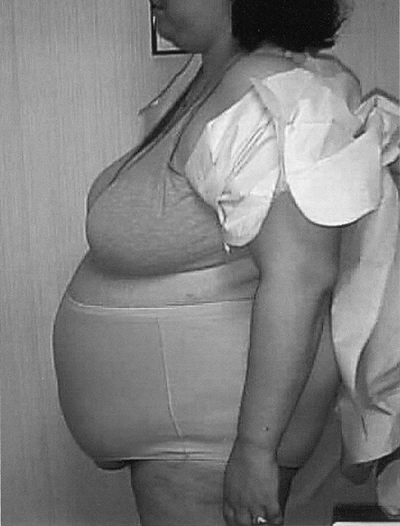 |
Figure 3-7 Central Obesity, a Hallmark of Polycystic Ovary Syndrome and Insulin Resistance, Is Strongly Associated with Cardiovascular Disease Risk. (Photo provided courtesy of Walter Futterweit, MD.) |
Sleep Apnea
In comparison with healthy subjects, patients with PCOS have a 4- to 30-fold increased prevalence of sleep apnea.17 Major symptoms of sleep apnea include snoring, daytime somnolence, and fatigue. Sleep apnea increases the risk of developing hypertension, myocardial infarctions, stroke, and diabetes.18 The Epworth scale may be used to screen patients for excessive sleepiness (Fig. 3-8).
P.99
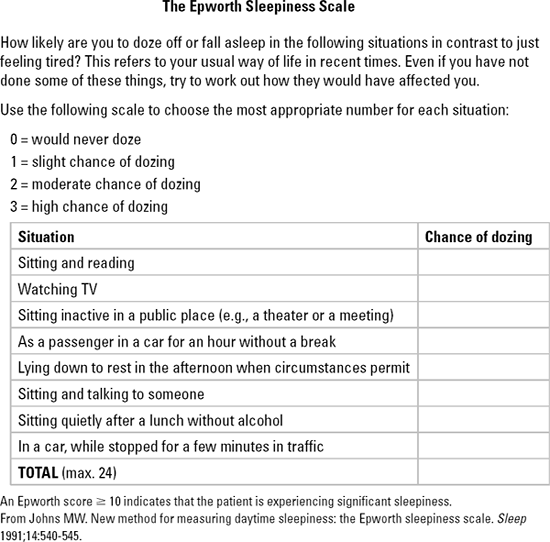 |
Figure 3-8 The Epworth Sleepiness Scale. |
Acanthosis Nigricans and Skin Tags
The findings of acanthosis nigricans (Fig. 3-9) with or without skin tags are clinical markers of insulin resistance.19 In the absence of a paraneoplastic syndrome, acanthosis nigricans is an epiphenomenon of dermal hyperplasia seen mostly in the nape of the neck, axillae, groin, inner lips of vulvae, periumbilical and inframammary areas, and the dorsum of the fingers and knuckles. They are characterized as brownish-grey, velvety or verrucous hyperparakeratotic pigmented areas, more commonly seen in obese women of Hispanic or African-American descent, although they may be also noted in lean and white populations. Parents of children with acanthosis nigricans often express their frustration in their inability to wash off the brown dirt with soap, water, and even bleach. Skin tags are not often seen in women before the age of 40 years, and if present earlier, the tags are an important clinical clue to the presence of hyperinsulinism or impaired insulin sensitivity.
P.100
Weight loss can improve both insulin resistance and the dark appearance of acanthosis nigricans.
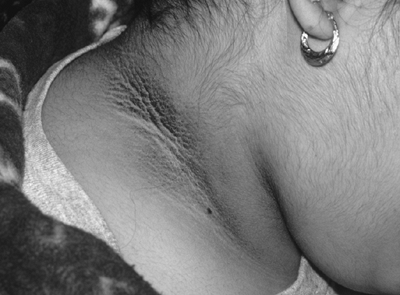 |
Figure 3-9 Acanthosis Nigricans in a Patient with Polycystic Ovary Syndrome Suggests the Presence of Insulin Resistance. (From Goodheart HP. Goodheart's Photoguide of Common Skin Disorders. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2003, with permission.) |
Laboratory Studies and Radiographic Evaluation of Polycystic Ovary Syndrome
Laboratory Studies
Hormonal studies are performed on patients with PCOS to determine the presence and severity of hyperandrogenism as well as whether the source is adrenal or ovarian. Although laboratory studies may be helpful in confirming the diagnosis of PCOS, no consensus statement has been published for the workup of these patients. Because of the circadian cyclicity of many of the hormones being evaluated, one should make note of the time the laboratory studies are obtained and when they are performed in relation to the patient's menstrual cycle. Note should also be made as to what medications the patient is taking at the time the tests are done, as some (such as oral contraceptives) may result in an inaccurate interpretation of the test results.
Because of the circadian cyclicity of many hormones, patients should submit to laboratory testing in the early morning. Those patients with regular menstrual cycles should have their blood samples obtained between days 3 and 8 of the menstrual cycle. All initial blood tests on patients suspected of having PCOS should be performed at least 6 weeks after the cessation of oral contraceptives. Blood values obtained while the patient uses oral contraceptives are not useful in diagnosing PCOS because oral contraceptives lower circulating androgen levels.20
P.101
Although elevations in androgen levels are frequently observed with PCOS, some patients have normal free testosterone and total testosterone values.21 Standardization of normal androgen levels in adolescents and older women is unavailable. Notwithstanding these limitations, measurement of the free testosterone is thought to be a sensitive method of assessing hyperandrogenemia.22 A total testosterone value greater than 50 ng per dL is considered elevated.23 The optimal value for serum testosterone in women is unknown. Most women with a level greater than 50 ng per dL will have irregular menses and clinical symptoms related to hyperandrogenism. The level of free (unbound) testosterone is usually elevated in patients with PCOS.
Dehydroepiandrosterone sulphate (DHEAS) is a major androgen precursor secreted from the adrenal gland. Patients having elevations of both DHEAS and total testosterone should be evaluated for an androgen-secreting tumor.24 Additional tests, such as 17-ketosteroids (measuring androgen metabolites in the urine) and 17-OH progesterone, are useful in determining if the patient has an adrenocortical tumor, adrenal cancer, or adrenal hyperplasia. These tests should be obtained in patients with a DHEAS level greater than 700 ng per dL. Normal DHEAS levels are 200 to 300 ng per dL (Table 3-2).
Patients with cushingoid features should have a urine cortisol determination. Levels greater than 100 g per 24 hours are strongly suggestive of Cushing syndrome.
If the 17-hydroxyprogesterone level at 8 AM (measured in the follicular phase of the menstrual cycle) is greater than 4 ng per mL, the patient probably has nonclassic adrenal hyperplasia resulting from a 21-hydroxylase deficiency. This diagnosis can be confirmed by a 60-minute ACTH stimulation test. Approximately 2% of women who present with hyperandrogenism and oligo-ovulation or anovulation have nonclassic adrenal hyperplasia resulting from a 21-hydroxylase deficiency. The prevalence of this congenital disorder varies markedly among different ethnic groups, from below 1% in Hispanic populations to as high as 5% to 8% in Ashkenazi Jewish populations.24a
A normally cycling premenopausal woman has an LH/FSH ratio of 1:1. Although a normal LH/FSH ratio does not exclude the diagnosis of PCOS, a ratio of 2:1 or 3:1 is found in 95% of patients with PCOS.9,25 An isolated rise in LH levels occurs in 60% of patients with PCOS.21 SHBG values are low in hyperandrogenemic and hyperinsulinemic states and in obesity.26
Patients suspected of having PCOS should have metabolic laboratory studies performed. A fasting lipid profile can identify patients with a typical atherogenic dyslipidemia. Most commonly, patients with PCOS will have a reduction in high-density lipoprotein cholesterol (HDL-C) and a rise in triglyceride levels (Table 3-3). This pattern is typically seen in patients with MS and T2DM.
IGT is present in 30% to 40% of women with PCOS, and in as many as 10% T2DM develops by age 40.27 These prevalence rates are among the highest known in women of similar age.28 Insulin resistance is more common in patients with PCOS than in unaffected counterparts matched for body mass index (BMI), fat-free mass, and body fat distribution.29 Insulin resistance alone
P.102
P.103
P.104
cannot fully account for the predisposition to and development of T2DM diabetes in PCOS patients. Most women with PCOS are able to fully compensate for their insulin resistance by increasing pancreatic secretion of insulin. However, those with a first-degree relative with T2DM have a disordered and insufficient beta-cell response to meals or a glucose challenge.30 Patients may develop clinical diabetes in response to pregnancy or glucocorticoid administration.31 A fasting plasma glucose level lower than 100 mg per dL is considered normal. Impaired fasting glucose (IFG) is diagnosed when the fasting glucose levels are 100 to 125 mg per dL. Fasting glucose levels greater than 126 mg per dL are diagnostic of diabetes. IGT is diagnosed if plasma glucose levels are greater than 140 mg per dL or less than 200 mg per dL at 2 hours after a 75-g glucose challenge is performed. A patient has diabetes if either her random or 2-hour postchallenge blood glucose level exceeds 200 mg per dL.
TABLE 3-2 Interpretation of Laboratory and Radiographic Studies in Patients Suspected of Having Polycystic Ovary Syndrome (PCOS) | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||
TABLE 3-3 Criteria for the Metabolic Syndrome in Women with Polycystic Ovary Syndromea | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Ultrasonographic Imaging
Transvaginal ultrasonography is necessary for infertility evaluation and management. If a patient does not wish to conceive and meets the Rotterdam criteria for PCOS (as listed on page 93), ultrasonography is not necessary. In these cases, confirmation of the morphologic characteristics of the patient's ovaries would not affect management. The specificity of morphologic evidence of polycystic ovaries on ultrasonography is limited by the fact that 23% of apparently normal women have characteristic findings of polycystic ovaries.32 Polycystic ovaries may also be observed in Cushing syndrome, idiopathic hirsutism, and hyperprolactinemia and with the use of medications such as valproic acid and ovulation-inducing agents.33 The best time to perform an ultrasound of the pelvis is in the early follicular phase (days 3 to 6 of the menstrual cycle) in women with regular menses. Anovulatory women with
P.105
oligomenorrhea may be scanned at random or 3 to 5 days after a progestin-induced withdrawal bleed.
TABLE 3-4 Technical Recommendations for Performing Ultrasonography on Patients Suspected of Having Polycystic Ovary Syndrome (PCOS) | ||
|---|---|---|
|
The criteria that suggest PCOS on ultrasound34 include the presence of 12 or more follicles measuring 2 to 9 mm in diameter in each ovary and/or increased ovarian volume of greater than 10 mL. Ultrasound may also be useful in identifying women who have endometrial hypertrophy, which may be diagnostic of endometrial carcinoma. Table 3-4 lists the technical recommendations for performing transvaginal ultrasonography in patients suspected of having PCOS.
Ultrasonography of the liver in patients with PCOS may identify patients with fatty liver infiltration.35 Despite the radiographic evidence, only 15% of patients with PCOS and nonalcoholic steatohepatitis (NASH) have abnormal liver chemistries.36 NASH is strongly associated with insulin resistance and is considered a precursor to clinical diabetes37 (see Chapter 4).
Polycystic Ovary Syndrome Associated with the Metabolic Syndrome, Insulin Resistance, Cardiovascular Disease, and Type 2 Diabetes
Linking Polycystic Ovary Syndrome with the Metabolic Syndrome
Women with PCOS are at high risk for developing T2DM and CVD related to MS. Therefore, PCPs must identify patients with PCOS not just as part of a routine workup for anovulation or infertility but to identify individuals with increased risk for cardiovascular morbidity and mortality. Of the 5 to 6 million women in the United States that have both PCOS and the MS, less than two thirds of these women are aware of either their diagnosis or their increased
P.106
cardiovascular risk.38 Approximately 50% of all women with PCOS have clinical characteristics that qualify them as having MS.39
MS is a constellation of interrelated risk factors that favor the development of atherosclerotic CVD and an increased risk for the development of T2DM. The National Cholesterol Education Program Adult Treatment Panel guidelines (NCEP ATP III)40 defining MS were based on data from the Third National Health and Nutrition Examination Survey (NHANES III).41 MS was defined as having three or more of the characteristics listed in Table 3-3. The 2003 American College of Endocrinology Position Statement also added IGT as a component of the syndrome, with a glucose level of 140 to 199 mg per dL obtained 120 minutes after a 75-g glucose challenge.42 The inclusion of other components such as inflammatory markers and obesity remains open to debate at this time, as discussed in Chapter 2.
Linking Polycystic Ovary Syndrome with Insulin Resistance
Insulin resistance is defined as a subnormal response to a given concentration of endogenous insulin. Insulin resistance is present in metabolic disorders such as obesity, physical stress (i.e., postsurgical, post-trauma), sepsis, acromegaly, pregnancy, hypertriglyceridemia, and Cushing syndrome. Patients with prediabetes eventually develop T2DM when the pancreatic beta cells become unable to produce enough insulin to keep fasting blood glucose levels less than 126 mg per dL and postprandial glucose levels less than 200 mg per dL. Whereas MS is present in 50% of patients with PCOS, insulin resistance is present in nearly all such women.43 Insulin resistance is associated with other metabolic abnormalities such as hypertension and dyslipidemia. The obvious need for evaluation and aggressive management of these comorbidities in patients with PCOS cannot be understated.
Excess adipose tissue in obese women with PCOS appears to be the primary source of insulin resistance, as discussed in Chapter 2. Obesity results in reduced levels of adiponectin that, in association with elevated circulating levels of free fatty acids, favor a state of insulin resistance. Insulin resistance in lean women with PCOS appears to be secondary to abnormalities in postreceptor insulin signaling.44 The compensatory hyperinsulinemia, which is associated with the insulin resistance state, favors the ovarian production of testosterone over estrogen, decreases production of SHBG from the liver, and affects gonadotropin release from the pituitary gland. Androgen levels rise, which impairs ovulation, reduces fertility, and further stimulates the intraovarian production of androgens.45
Accurate assessment of insulin resistance in the primary care setting is not possible. Testing fasting blood glucose and insulin levels is not standardized to reflect diagnostic cut-off points for insulin resistance.46 A simple and inexpensive way to assess the likelihood of a patient's being insulin resistant is to calculate the triglyceride-to-HDL-C ratio. A normal ratio is considered to be 3.5:1 or lower. A ratio of 3.5:1 or greater is suggestive of insulin resistance. For example,
P.107
a patient whose triglyceride level is 350 mg per dL and whose HDL-C is 35 would have a ratio of 10:1, favoring insulin resistance.46a In 2005, The American Association of Clinical Endocrinologists published a position statement suggesting that all patients diagnosed with PCOS should be considered as having insulin resistance and increased risks for developing T2DM and CVD.47
Patients with PCOS rapidly progress from normal glucose tolerance through prediabetes and into T2DM. Approximately 15% of PCOS patients convert from normal glucose tolerance to IGT yearly, and 50% of PCOS patients develop T2DM by age 30.48 The incidence of T2DM in patients with PCOS is 13%, with a mean age of 42 years in comparison with 1.4% of a control population.49 The more irregular the menses, the more likely women with PCOS are at risk for developing T2DM, particularly in obese patients.49
Cardiovascular risk is increased in women with PCOS who have additional metabolic abnormalities often seen in association with obesity. Seventy percent of obese women with PCOS have dyslipidemia.50 The pattern of dyslipidemia found in MS featuring elevated triglycerides and low HDL-C has been reported with obesity and PCOS but has not been found to be prevalent in weight-matched controls.51 Abnormalities in low-density lipoprotein cholesterol (LDL-C) have not been found consistently in association with PCOS.52
Although not clinically apparent initially, patients with PCOS are at risk for developing hypertension later in life.53 Inflammatory markers such as C-reactive protein and microalbuminuria are associated with endothelial cell dysfunction and increased CVD risk. Early screening for these atherogenic markers should be performed on patients suspected of having PCOS.
Polycystic Ovary Syndrome Increases Risk of Coronary Artery Disease
PCOS is associated with a high prevalence of coronary artery calcification as determined by electron-beam computed tomography54 and most likely due to an increased level of plasminogen activator inhibitor (PAI-1).55 Improvement in insulin sensitivity lowers PAI-1 levels. Metabolic abnormalities common to PCOS patients include hypertriglyceridemia, increased levels of very-low-density lipoproteins (VLDLs), decreased levels of HDL-C, and abnormal glucose levels. Insulin resistance coupled with hyperandrogenemia contributes to the atherogenic lipid state in PCOS. Testosterone decreases lipoprotein lipase activity in visceral fat cells, which favors an atherogenic dyslipidemic metabolic environment.
A risk factor model analysis has calculated that patients with PCOS have a four- to sevenfold higher risk of myocardial infarction in comparison with age-matched controls.56 This study also determined that women with PCOS had a sevenfold increased progression to T2DM (15% vs. 2.3% in control subjects).
A study evaluating 143 patients younger than age 60, whose complaint of chest pain required cardiac catheterization, determined that 42% had radiographic evidence of PCOS,57 double the frequency in the general population.58
P.108
Patients with PCOS exhibit more coronary artery segments with more than 50% stenosis and significantly greater clinical heart disease than do women with normal ovaries as detected by ultrasound.57
TABLE 3-5 Summary of Data Linking Polycystic Ovary Syndrome (PCOS) to Cardiovascular Disease | ||
|---|---|---|
|
Using oligomenorrhea as a surrogate marker for PCOS, a 2- to 2.5-fold increase in the incidence of T2DM was noted in the prospective Nurses' Health Study of more than 101,000 women. In a mean follow-up of 14 years, a history of oligomenorrhea was associated with a 100% increased prevalence of fatal and 50% increased incidence of nonfatal coronary artery disease after adjusting for age, smoking history, and BMI.49 Eighty percent of women with oligomenorrhea are thought to have PCOS.47,59 Table 3-5 summarizes the cardiovascular risks associated with PCOS.
The data relating to cardiovascular disease with PCOS can be summarized as follows:
The prevalence of T2DM (a myocardial infarction equivalent state) and IGT (a prediabetes condition associated with cardiovascular risk) are both increased in women with PCOS.
Many recognized cardiovascular risks are significantly increased in women with PCOS.
Women with PCOS have insulin resistance, which increases the risk for CVD.
Fifty percent of women with PCOS have diagnostic criteria that qualify them as having MS. This constellation of metabolic risk factors tends to increase the likelihood of developing CVD.
Imaging and cardiac studies in young women with PCOS have demonstrated a higher incidence of anatomic and functional abnormalities indicative of underlying CVD when compared with age-matched controls.
P.109
Although all studies evaluating the link between CVD, PCOS, and insulin resistance are not uniform in their conclusions, most consistently predict an increased risk for adverse cardiovascular events in patients with PCOS. Collectively, the data suggest that PCPs recognize these patients as being at high risk for developing T2DM and CVD. They should therefore undergo a comprehensive evaluation for recognized CVD and metabolic risk factors. Aggressive treatment to reduce cardiovascular risk and to slow the rate of progression to T2DM should be encouraged.
Cancer Risk Associated with Polycystic Ovary Syndrome
Endometrial carcinoma is more prevalent in patients with PCOS.60 This association has been attributed to the persistent stimulation of endometrial tissue by unopposed estrogen release. Ovarian progesterone secretion is minimized in PCOS, as patients do not ovulate. Endometrial carcinoma is also associated with T2DM and obesity.60 Breast and ovarian carcinomas may be more common in PCOS patients. However, the specific risk factors associated with breast and ovarian carcinomas are difficult to determine. Obesity, anovulation, infertility, and hormonal treatments for infertility may all have some influence on the development of breast and ovarian carcinomas.
Treatment of Polycystic Ovary Syndrome
Women with PCOS have a higher risk for developing T2DM and coronary artery disease. Owing to the lack of protective effect of female sex on coronary artery disease risk in patients with diabetes,61 heart disease prevalence is magnified in women with diabetes who have PCOS. An opportunity exists to reverse some of the associated risks that accompany PCOS, especially in younger women. Lifestyle modification with weight reduction, exercise, smoking cessation, and pharmacotherapy are all helpful in managing PCOS patients. Table 3-6 summarizes the treatment strategies for PCOS.
Pharmacologic therapy for PCOS should be individualized and based on the particular patient's metabolic, gynecologic, and cosmetic concerns. For example, a woman who desires to become pregnant might have the most success when treated with an insulin-sensitizing drug rather than oral contraceptives, which may reduce hirsutism and acne. Hirsutism might be addressed using spironolactone or oral contraceptives.
Restoring the Menstrual Cycle
Restoring natural menstrual cycling with oral contraceptives has both psychological and physiologic benefits. Oral contraceptives increase hepatic production of SHBG, which, in turn, binds free testosterone, making testosterone less likely to target androgen receptors. As a result, acne, hirsutism,
P.110
and alopecia may subside. The estrogenic component of the oral contraceptive suppresses LH, resulting in decreased ovarian androgen production. The choice of oral contraceptive is important because most progestins also possess androgenic effects. Contraceptives containing nonandrogenic progestins such as norgestimate and desogestrel are preferred.62 Drospirenone, an analog of spironolactone with unique antimineralocorticoid and antiandrogenic activities, is available for use in combination with ethinyl estradiol, thus potentially becoming the ideal oral contraceptive for women with PCOS.63 Oral contraceptives do not improve the metabolic abnormalities associated with PCOS and may increase insulin resistance.26 If insulin resistance deteriorates with the use of oral contraceptives, metformin may be added to the treatment regimen.
TABLE 3-6 Management Strategies for Patients with Polycystic Ovary Syndrome (PCOS) | ||
|---|---|---|
|
Antiandrogens
Spironolactone possesses moderate antiandrogenic effects when large doses (100 200 mg per day) are used.64 Patients who desire cosmetic improvements for their hirsutism, acne, and alopecia may be treated successfully with
P.111
a combination of oral contraceptives and spironolactone. Women desiring pregnancy should use both spironolactone and a combination of an estrogen and a progestin. The drug's effects on improving hirsutism may not become clinically apparent for up to 9 months. Cessation of spironolactone therapy often results in the recurrence of acne and hirsutism or worsening of alopecia within 3 months. Side effects of spironolactone include gastrointestinal discomfort, postural hypotension, increased urination, decreased libido, irritability, mastodynia, hyperkalemia, and muscle pain. Midcycle bleeding resulting from progestinlike properties at high doses of spironolactone may occur. Whether or not spironolactone is secreted in breast milk is uncertain.
Spironolactone and oral contraceptives are synergistic and can be used concomitantly for the following reasons: (a) spironolactone has a minimal effect on improving hirsutism, acne, and alopecia when compared with the combined effects of both drugs; (b) spironolactone cannot be used during pregnancy and has a Category D rating (risk to the fetus, but drug benefits may outweigh the risk); and (c) 50% to 70% of women who use spironolactone experience midcycle bleeding.
5-Alpha-reductase inhibitors suppress the conversion of testosterone to dihydrotestosterone (DHT) in the prostate. In the skin and other tissues, the effect of finasteride is mediated by inhibition of type 1 5- alpha-reductase. Dosages of 2.5 to 5.0 mg daily have been used to suppress hirsutism in women with PCOS65 with minimal side effects. A 32% to 78% reduction in hirsutism was associated with a decrease in plasma DHT.65 Finasteride is not approved by the U.S. Food and Drug Administration (FDA) for treating hirsutism in patients with PCOS.
Eflornithine hydrochloride, an inhibitor of the enzyme ornithine decarboxylase in human skin, has been approved for topical use in treating facial hirsutism. The inhibition of hair growth is its primary action, but clinical data are too limited to recommend its routine use.
Insulin Reducers
A reduction in insulin levels ameliorates sequelae associated with hyperinsulinemia and hyperandrogenemia. Therapies that normalize insulin levels can effectively treat the metabolic abnormalities associated with PCOS.
Metformin inhibits hepatic glucose production, thereby reducing the androgen production of theca cells.66 Metformin may also directly influence ovulation. A meta-analysis of 13 studies in which metformin was used by 543 patients reported that those taking metformin had an odds ratio for ovulation of 3.88 as compared with placebo and an odds ratio for ovulation of 4.41 for metformin plus clomiphene, when compared with clomiphene alone.67 Metformin also improved fasting insulin levels, blood pressure, and levels of LDL-C.
Women with PCOS who conceive while taking metformin have a lower likelihood of developing gestational diabetes or suffering a spontaneous abortion
P.112
when compared with PCOS women who are not prescribed metformin.68 A 30% to 40% reduction in the incidence of spontaneous abortions throughout pregnancy has been reported in patients using metformin.68 Metformin is a Category B drug whose use in pregnancy has not been shown to cause an increased rate of birth defects. Metformin should not be used while breast feeding.
Because metformin can cause previously anovulatory women to ovulate, unplanned pregnancies may occur. Caution should be used in prescribing metformin as sole therapy in adolescents and young adults without using some form of contraception and offering preconception planning (see Chapter 8). This is especially important when metformin is used in obese patients with T2DM. Most women with PCOS can have successful pregnancies without a dramatic increase in health risk.69 There are no guidelines for dosing metformin in patients with PCOS. However, a total daily dose of 2,000 mg is recommended. The drug should be taken with meals. Side effects include bloating, diarrhea, and nausea. When initiating metformin, one should begin with a low dosage of 500 mg daily taken with food for 7 to 10 days, then increased to twice daily with breakfast and dinner. Every 2 weeks the dose should be increased to a maximum of 2,000 mg as tolerated. After 6 to 8 weeks, the side effects of metformin become minimal with the exception of occasional diarrhea. Folic acid supplements should be prescribed, as metformin reduces the gastrointestinal absorption of this vitamin. Women taking metformin should discontinue use of the drug within 24 hours of undergoing any intravenous contrast study. Resumption of the drug is safe once renal function is retested and documented as normal. A serum creatinine level greater than 1.4 mg per dL might increase the risk of developing lactic acidosis when using metformin.
The chronic use of metformin in women with PCOS is an important area of debate. Whereas 10% of patients with PCOS have T2DM, nearly all women have insulin resistance and are at high risk for progressing rapidly toward clinically apparent T2DM. Nearly 30% to 50% of obese patients with both PCOS and IGT convert to T2DM by age 30 years.70 In these women, PCOS should be viewed less as an infertility or cosmetic disorder and more as a disorder that is likely to cause premature CVD and diabetes.70 In the obese cohort of the Diabetes Prevention Program (DPP), patients using metformin had a 31% reduction in progression to diabetes from IGT over 3 years when compared with patients taking placebo.71
The thiazolidinediones (TZDs) improve insulin action in the liver, skeletal muscle, and adipose tissue. TZDs, like metformin, affect ovarian steroid synthesis.72 TZDs improve the chances of ovulation, reduce free testosterone levels, increase levels of SHBG, reduce levels of PAI-1, and improve glycemia.73,74 TZDs have an FDA pregnancy Category C (a known teratogen in animal studies) rating. Oral contraceptives or barrier methods to prevent pregnancy should be prescribed when patients with PCOS use TZDs. Because of concern about using TZDs during pregnancy, metformin is more commonly used to treat PCOS.
P.113
TABLE 3-7 Pharmacotherapy for Patients with Polycystic Ovary Syndrome (PCOS) | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||
TZDs improve the frequency of menstrual cycles while reducing hyperinsulinemia and hyperandrogenism in PCOS. TZDs have proven beneficial in reducing the risk of progression to T2DM and in decreasing a number of inflammatory markers associated with the MS (see Table 2-7). In a head-to-head study, metformin tended to improve the frequency of menstrual cyclicity slightly more than rosiglitazone.75 TZDs may also be helpful in managing NAFLD, which may be present in 50% of women with PCOS.76
Patients who desire fertility should be placed on metformin rather than TZDs. Patients with respiratory failure, congestive heart failure, hypoxia, or liver transaminase levels exceeding three times the upper limit of normal at baseline should not be started on a TZD. Liver function testing should be monitored quarterly during the first year of TZD use and periodically thereafter. Fifteen percent of women who use a TZD will experience weight
P.114
gain and edema. Initial dosages are 15 mg daily of pioglitazone, increasing to 30 mg after 6 to 8 weeks. Rosiglitazone may be given initially at 2 mg daily and increased to 4 mg daily after 6 to 8 weeks.
Metabolic Risk Factors
Management of the cluster of metabolic risk factors associated with CVD is discussed elsewhere in this book (see Chapters 2 and 11).
The pharmacologic therapies used for PCOS are summarized in Table 3-7.
Summary
Patients with PCOS present with overt symptoms of infertility, menstrual irregularities, hirsutism, and acne. Many patients also possess metabolic and hormonal abnormalities that can significantly increase their risk for developing coronary artery disease, T2DM, and endometrial carcinoma. Many patients have obstructive sleep apnea. PCPs should screen patients suspected of having PCOS. Early recognition of this disorder may reverse the physical signs associated with the disease, while correcting the metabolic abnormalities that can pose a significant health risk for untreated individuals. Although lifestyle modification and pharmacotherapy are used to treat PCOS, there remains a paucity of long-term outcome data regarding the benefits of metabolic interventional strategies for patients with PCOS. Insulin sensitizers can improve ovulatory function, lower insulin resistance, lower androgen levels, and increase the likelihood of becoming pregnant.
References
1. Kitzinger C, Willmott J. The thief of womanhood : women's experience of polycystic ovarian syndrome. Soc Sci Med. 2002;54:349 361.
2. Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361:1810 1812.
3. Svatikova A, Wolk R, Gami AS, Pohanka M, Somers VK. Interactions between obstructive sleep apnea and the metabolic syndrome. Curr Diabetes Rep. 2005;5:53 58.
4. Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Prac Res Clin Obstet Gynaecol. 2004;18:671 683.
5. Ibanez L, Valls C, Potau N, Marcos MV, de Zegher F. Polycystic ovary syndrome after precocious pubarche: ontogeny of the low-birthweight effect. Clin Endocrinol (Oxf). 2001;55:667 672.
6. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774 800.
7. Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF Jr. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovary disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988;66:165 172.
8. Nelson VL, Qin KN, Rosenfield RL, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925 5933.
P.115
9. Sherif K. Polycystic ovary syndrome in primary care. Female Patient. 2005;30:24 28.
10. Sherif K, Kushner H, Falkner BE. Sex hormone-binding globulin and insulin resistance in African American women. Metabolism. 1998;47:70 74.
11. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223 1236.
12. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19 25.
13. Geist SH, Gains JA. Diffuse luteinization of the ovaries associated with masculinization syndrome. Am J Obstet Gynecol. 1942;43:975.
14. Carmina E. Prevalence of idiopathic hirsutism. Eur J Endocrinol. 1998;139:421 423.
15. Timpatanapong P, Rojanasakul A. Hormonal profiles and prevalence of polycystic ovary syndrome in women with acne. J Dermatol. 1997;24:223 229.
16. DeNino WF, Tchernof A, Dionne IJ, et al. Contribution of abdominal adiposity to age-related differences in insulin sensitivity and plasma lipids in healthy nonobese women. Diabetes Care. 2001;24:925 932.
17. Fogel RB, Malhotra A, Pillar G, et al. Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86: 1175 1180.
18. American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measured techniques in clinical research. The Report of an American Academy of Sleep Medicine Taskforce. Sleep. 1999;22:667 689.
19. Hermanns-Le T, Scheen A, Pierard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5:199 203.
20. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666 3672.
21. Laven JS, Imani B, Eijkermans MJ, Fauser BC. New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstet Gynecol Surv. 2002;57:755 767.
22. Cibula D, Hill M, Starka L. The best correlation of the new index of hyperandrogenism with the grade of increased hair. Eur J Endocrinol. 2000;143:405 408.
23. Luthold WW, Borges MF, Marcondes JA, Hakohyama M, Wajchenberg BL, Kirschner MA. Serum testosterone fractions in women: normal and abnormal clinical states. Metabolism. 1993;42:638 643.
24. Meldrum DR, Abraham GE. Peripheral and ovarian venous concentration of various steroid hormones in virilizing ovarian tumors. Obstet Gynecol. 1979;53:36 43.
24a. Redmond GP. Clinical evaluation of the woman with an androgenic disorder. In: Redmond GP, ed. Androgenic Disorders. New York: Raven Press; 1995:1 20.
25. Taylor AE, McCourt B, Martin K, Anderson EJ, Adams J, Schoebfeld D. Determinants of abnormal gonadotropin secretion in clinically defined women with PCOS. J Clin Endocrinol Metab. 1997;82:2248 2256.
26. Sherif K. Benefits and risks of oral contraceptives. Am J Obstet Gynecol. 1999;180(6 Pt 2): S343-S348.
27. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165 169.
28. Krosnick A. The diabetes and obesity epidemic among the Pima Indians. N Engl J Med. 2000;97:31 37.
29. Dunaif A. Insulin resistance and the polycystic ovary syndrome (PCOS): mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774 800.
30. Dunaif A. Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:942 947.
31. Kousta E, Cela E, Lawrence N. The prevalence of polycystic ovaries in women with a history of gestational diabetes. Clin Endocrinol (Oxf). 2000;53:501 507.
32. Polson DW, Adams J, Wadsworth J, et al. Polycystic ovaries: a common finding in normal women. Lancet. 1988;1(8590):870 872.
P.116
33. Yeh HC, Futterweit W, Thornton JC. Polycystic ovarian disease: US features in 104 patients. Radiology. 1987;163:111 116.
34. Balen A. Ovulation induction for polycystic ovary syndrome. Hum Fertil (Camb). 2003;3: 106 111.
35. Dewailly D, Ardaens Y, Robert Y. Ultrasound for the evaluation of PCOS. In: Azziz A, Nestler JE, Dewailly D, eds. Androgen Excess Disorders in Women. Philadelphia: Lippincott-Raven Publishers;1997:269 278.
36. Kinkhabwala S, Schiano TD, Futterweit W, et al. Prevalence of non-alcoholic fatty liver disease in polycystic ovary syndrome. Program of the 87th Annual Meeting of the Endocrine Society, San Diego, Calif, June 5, 2005;p 445 (Abstract P2-365).
37. Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450 455.
38. Peppard HR, Marfori J, Iuomo MJ, Nestler JE. Prevalence of polycystic ovary syndrome among premenopausal women with type 2 diabetes. Diabetes Care. 2001;24:1050 1052.
39. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287: 356 359.
40. Third Report of the National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143 3421.
41. US Department of Health and Human Services (DHHS). National Center for Health Statistics. Third National Health and Nutrition Examination Survey (NHANES III), 1988 1994. Hyattsville, Md: Center for Disease Control and Prevention;1996.
42. Insulin Resistance Syndrome Task Force. American College of Endocrinology Position Statement on the Insulin Resistance Syndrome. Endocr Pract. 2003;9;236 252.
43. Nestler JE. Insulin resistance syndrome and polycystic ovary syndrome. Endocr Pract. 2003;9(Suppl 2):86 89.
44. Dunaif A, Green G, Futterweit W, et al. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165 1174.
45. Futterweit W, Deligdisch L. Histopathological effects of exogenously administered testosterone in 19 female to male transsexuals. J Clin Endocrinol Metab. 1986;62:16 21.
46. Diamanti-Kandarakis E, Kouli C, Alexandraki K, et al. Failure of mathematical indices to accurately assess insulin resistance in lean, overweight, or obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:1273 1276.
46a. Laws A, Reaven GM. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J Intern Med. 1992;231:25 30.
47. American Association of Clinical Endocrinologists Polycystic Ovary Syndrome Writing Committee. American Association of Clinical Endocrinologists Clinical Position Statement on Metabolic and Cardiovascular Consequences of Polycystic Ovary Syndrome. Endocr Pract. 2005;11:126 134.
48. Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005; 90:3236 3242.
49. Solomon CG, Hu FB, Dunaif A, et al. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA. 2001;286:2421 2426.
50. Legro RS, Kunselman AR, Danaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607 613.
51. Holte J, Bergh T, Berne C, Tithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol (Oxf). 1994;41:463 471.
52. Robinson S. Henderson AD, Gelding SV, et al. Dyslipidaemia is associated with insulin resistance in women with polycystic ovaries. Clin Endocrinol (Oxf). 1996;44:277 284.
P.117
53. Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb). 2000;3:101 105.
54. Christian RC, Dumesic DA, Behrenbeck T, et al. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2562 2580.
55. Atiomo WU, Bates SA, Condon JE, Shaw S, West JH, Prentice AG. The plasminogen activator system in women with polycystic ovary syndrome. Fertil Steril. 1998;69:236 241.
56. Dahlgren E, Janson PO, Johansson S, Lapidus L, Oden A. Polycystic ovary syndrome and risk for myocardial infarction: evaluated from a risk factor model based on a prospective population study of women. Acta Obstet Gynecol Scand. 1992;71:599 604.
57. Birdsall MA, Farquhar CM, White HD. Association between polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med. 1997; 126:32 35.
58. Polson DW, Adams J, Wadsworth J, Franks S. Polycystic ovaries-a common finding in normal women. Lancet. 1988;1:870 872.
59. Solomon CG, Hu FB, Dunaif A. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87:2013 2017.
60. Balen A. Polycystic ovary syndrome and cancer. Hum Reprod Update. 2001;7:522 525.
61. Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in heart disease mortality in US adults. JAMA. 1999;281:129 1297.
62. Kuhl H. Comparitive pharmacology of newer progestogens. Drugs. 1996;51:188 215.
63. Guido M, Romualdi D, Giuliani M. Drospirenone for the treatment of hirsute women with polycystic ovary syndrome: a clinical, endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89:2817 2823.
64. Spritzer PM, Lisboa KO, Mattiello S, Lhullier F. Spironolactone as a single agent for long-term therapy of hirsute patients. Clin Endocrinol (Oxf). 2000;52:587 594.
65. Tartagni M, Schonauer MM, Cicinelli E, et al. Intermittent low-dose finasteride is as affective as daily administration for the treatment of hirsute women. Fertil Steril. 2004;82:752 756.
66. Mansfield R, Galea R, Brincat M, Hole D, Mason H. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril. 2003;79:956 962.
67. Lord JM, Flight IHK, Norman RJ. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev. 2003;3:CD003053.
68. Glueck CJ, Wang P, Goldenberg N, Sieve-Smith L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod. 2002;17:2858 2864.
69. Sharpless JL. Polycystic ovary syndrome and the metabolic syndrome. Clin Diabetes. 2003; 21:154 161.
70. Nestler JE. Should patients with polycystic ovary syndrome be treated with metformin?: an enthusiastic endorsement. Hum Reprod. 2002;17:1950 1953.
71. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393 403.
72. Mitwally MF, Witchel SF, Casper RF. Troglitazone: a possible modulator of ovarian steroidogenesis. J Soc Gynecol Investig. 2002;9:163 167.
73. Belli SH, Graffigna MN, Oneto A, Otero P, Schurman L, Levalle OA. Effect of rosiglitazone on insulin resistance, growth factors, and reproductive disturbances in women with polycystic ovary syndrome. Fertil Steril. 2004;81:624 629.
74. Romualdi D, Guido M, Ciampelli M. Selective effects of pioglitazone on insulin and androgen abnormalities in normo- and hyperinsuinaemic obese patients with polycystic ovary syndrome. Hum Reprod. 2003;18:1210 1218.
75. Baillargeon JP, Jakubowicz DJ, Iuorno MJ, et al. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. J Clin Endocrinol Metab. 2004;82:893 902.
76. Bloomgarden ZT. Thiazolidinediones. Diabetes Care. 2006;28:488 493.
EAN: 2147483647
Pages: 19