4 - Gastroenterology
Editors: Schrier, Robert W.
Title: Internal Medicine Casebook, The: Real Patients, Real Answers, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > Chapter 4 - Gastroenterology
function show_scrollbar() {}
Chapter 4
Gastroenterology
William R. Brown
Chronic Inflammatory Bowel Disease
What is the pathogenesis responsible for chronic ulcerative colitis (CUC) and Crohn's disease?
Compare and contrast the principal clinical features of CUC and Crohn's disease.
What are the respective risks of intestinal malignancy in CUC and Crohn's disease?
What are the principal medical therapeutic measures used for patients with CUC and Crohn's disease?
P.142
Discussion
What is the pathogenesis responsible for CUC and Crohn's disease?
The cause and pathogenesis of both these chronic inflammatory bowel diseases (CIBDs) are unknown. Both are characterized by a chronic inflammatory cell infiltrate of the bowel. However, whereas CUC is restricted to the colon, Crohn's disease can involve the entire alimentary tract from the mouth to the anus, although the distal ileum and colon are the portions most frequently affected. Another distinguishing feature of Crohn's disease is the involvement of all layers of the bowel, whereas the inflammation seen in CUC is mostly limited to the mucosa. In addition, focal granulomas are common in Crohn's disease but rare in CUC. However, neither disease has pathognomonic features, and Crohn's disease of the colon cannot be histologically distinguished from CUC in 15% to 25% of cases of chronic colitis.
Compare and contrast the principal clinical features of CUC and Crohn's disease.
The severity, clinical course, and prognosis of CUC and Crohn's disease are widely variable. Onset in both diseases occurs most often in early adulthood. The symptoms of CUC may range from slight rectal bleeding to fulminant diarrhea with colonic hemorrhage and hypotension. Most patients have intermittent attacks, although some can have continuous symptoms without remission. The clinical features of Crohn's disease depend on the severity and location of the bowel involvement; the principal features are diarrhea, abdominal pain, hematochezia, intestinal obstruction, fissures, and fistulas.
Extraintestinal manifestations are common in both Crohn's disease and CUC, but more common in CUC. The manifestations include arthritis, arthralgia, iritis, uveitis, liver disease, and skin lesions. The arthritis may present as a migratory arthritis, involving large joints, sacroiliitis, or ankylosing spondylitis. Primary sclerosing cholangitis, which is associated with an increased frequency of cholangiocarcinoma, and chronic hepatitis are common hepatobiliary abnormalities.
The principal features that differentiate Crohn's disease from CUC are listed in Table 4.1.
What are the respective risks of intestinal malignancy in CUC and Crohn's disease?
The frequency of intestinal cancer is increased in Crohn's disease, but not to the extent in CUC. According to some reports, the frequency of colon cancer in adults who have CUC involving the entire colon is approximately 25 times greater than that in the general population. The risk of colon cancer developing in patients with CUC is positively correlated with the extent and duration of the disease.
What are the principal medical therapeutic measures used for patients with CUC and Crohn's disease?
The general measures to control the symptoms of both diseases include correction of fluid electrolyte imbalances; iron, folate, or vitamin B12 supplementation as needed for the treatment of anemia; and dietary adjustments aimed at maintaining adequate nutrition. Total parenteral nutrition may be
P.143
required for the short-term treatment of severe acute disease, but bowel rest and hyperalimentation are of dubious value in the long term. Antidiarrheal agents such as loperamide are usually contraindicated in patients with CUC because they may contribute to the development of toxic megacolon, but they may help alleviate the diarrhea and abdominal cramps in the setting of stable Crohn's disease.Table 4-1 Features that Distinguish between Crohn's Disease and Ulcerative Colitis
Factors Crohn's Disease Ulcerative Colitis Pathologic features Transmural inflammation Mucosal inflammation Deep ulcers Superficial ulcers Granulomas common Granulomas absent Distribution Mouth to anus (ileum and proximal colon most common) Colon Clinical features Rectal bleeding 20% 40% 98% Fulminating episodes Uncommon Common Obstruction Common Rare Fistulas Common Rare Perianal disease Common Less common Sigmoidoscopic and radiographic findings Rectal involvement 50% 95% 100% Extent Patchy Continuous Ulcers Longitudinal, deep Shallow, collar button Pseudopolyps Uncommon Common Strictures Common Uncommon Ileal involvement Narrowed lumen with thickened wall Dilated lumen with diminished folds but histologically normal From Schaefer J, Mallory A. Gastrointestinal disease. In: Schrier RW, ed. Medicine: diagnosis and treatment. Boston: Little, Brown and Company, 1988. In CUC, corticosteroids are useful for inducing remissions or improvement in an acute attack, and they may be required for long-term management. However, the possible benefits of corticosteroids in the long term are offset by their many adverse side effects. The rectal administration of steroids or mesalamine can be beneficial, especially when rectal involvement (proctitis) is severe. However, significant absorption of rectal steroids can occur, so systemic effects of the agents (both beneficial and undesirable) may arise when they are given by this route. Sulfasalazine is metabolized by colonic bacteria, releasing sulfapyridine and 5-aminosalicylate (5-ASA); the latter is believed to be the
P.144
active compound. Sulfapyridine is absorbed systemically, which accounts for the side effects of sulfasalazine (e.g., headache, occasional megaloblastic anemia, skin rash). The greatest utility of sulfasalazine in patients with CUC is in long-term management, where it has been proved to reduce the frequency of relapses. 5-ASA, given rectally by enema or suppository, is well tolerated and effective. Given orally, 5-ASA is rapidly denatured by gastric acid, so alternatives to plain 5-ASA, such as microencapsulated (Pentasa; Hoechst Marion Roussel, Kansas City, MO) or acrylic-based resin-coated (Asacol; Procter & Gamble Pharmaceutical, Norwich, NY) forms of 5-ASA, may be used. Because the relative risk for development of CUC is greater in nonsmokers than in smokers (the opposite is true in Crohn's disease), nicotine is being tried in the treatment of CUC; some benefit has been reported, but additional research is needed.There is no uniformly effective treatment available for Crohn's disease. However, corticosteroids have documented efficacy in diminishing the activity of the disease process. Long-term use of corticosteroids is not recommended because of their many side effects, such as osteoporosis, diabetes, and cataracts. Sulfasalazine has some effectiveness, especially in colonic Crohn's disease, but is less effective than corticosteroids. Pentasa, in doses of more than 3 mg per day may be efficacious in mild to moderate Crohn's disease, particularly in ileal disease. Metronidazole may be at least as effective as sulfasalazine. When Crohn's disease cannot be controlled by these medications, the immunosuppressive agent azathioprine and its metabolite 6-mercaptopurine are often used. These drugs are effective in both inducing and maintaining remission in inflammatory-type and fistulizing-type Crohn's disease. Their use can result in a reduction in the corticosteroid dose needed, but this advantage may be offset by their toxic effects (e.g., pancreatitis, allergic reactions, and leucopenia). More recently, infliximab, a chimeric monoclonal antitumor necrosis factor antibody, has been shown to be effective in Crohn's disease, both in the inflammatory and the fistulizing types. The role of immunodulator drugs in CUC is less clear than in Crohn's disease.
Case
A 37-year-old man with documented CUC was first seen at 19 years because of severe bloody diarrhea and left lower quadrant abdominal pain that necessitated hospitalization. After 10 days of treatment with high-dose prednisone and sulfasalazine his symptoms were controlled, and he has since been managed with these medicines, with the dosages adjusted depending on his disease activity. He has not required corticosteroids except for flare-ups of disease. Subsequent to his initial presentation, after his disease activity had subsided, he underwent colonoscopy for histologic confirmation of the disease and to determine the extent of intestinal involvement; this examination revealed diffuse mucosal inflammation involving the entire colon (pancolitis). The terminal ileum appeared normal. Colonic biopsy specimens revealed a diffuse mucosal inflammatory infiltrate with little involvement of the submucosa, acute and chronic inflammatory cells, and frequent crypt abscesses but no granulomas.
P.145
The patient went on to graduate from college and was then hired as a sales representative for a pharmaceutical company. Because his disease has been quiescent and his schedule very busy he has not taken his medications regularly and has rarely seen his physician.
Approximately 2 months ago, he began to feel tired, and intermittent rectal bleeding developed. His physical examination findings are unremarkable, but the fecal occult blood test result is positive. The hemoglobin is 11 g/dL; hematocrit, 33%; and leukocyte count, 7,700 cells/mm3, with a normal differential count.
What is your differential diagnosis of his recent symptoms?
What tests are necessary to make the correct diagnosis?
How should this patient's CUC have been managed over the previous 18 years?
Case Discussion
What is your differential diagnosis of his recent symptoms?
The differential diagnosis in this patient includes three possibilities. First, this episode could be an acute flare-up or exacerbation of his ulcerative colitis. Second, he could have an acute, self-limited colitis superimposed on his ulcerative colitis; infection with Campylobacter, Salmonella, or Shigella species, or with parasites can cause such a colitis. Third, the rectal bleeding and anemia could be the result of adenocarcinoma.
What tests are necessary to make the correct diagnosis?
Stool cultures and the examination of stool for ova and parasites would be an important initial laboratory test in this patient. These proved to be negative.
Flexible sigmoidoscopy or colonoscopy with the acquisition of biopsy specimens is also an important diagnostic procedure. In contrast to CIBD, the histologic features of acute self-limited colitis consist of normal crypt architecture and an acute but not chronic inflammatory infiltrate in the lamina propria. Inflammation is more likely to be found in the upper mucosa in acute colitis, and in the crypt bases in CIBD. When an acute self-limited colitis, such as infection with Campylobacter jejuni, Salmonella, or Shigella, resolves, the mucosa is normal, whereas crypt distortion and atrophy are often seen in the setting of healed CIBD. In other acute colitides, the histologic features found in mucosal biopsy specimens may suggest a specific infection; these include viral inclusions, parasites, or pseudomembranes.
In this patient, flexible sigmoidoscopy was performed to a depth of 30 cm and revealed mild granularity of the mucosa without bleeding, although some blood was seen coming from above 30 cm. Active CUC almost always involves the rectum, so the finding of only mild changes in this patient's rectum suggests that the significant pathologic process was higher in the colon. A colonoscopic examination showed a sessile, fungating mass in the descending colon, which proved to be an adenocarcinoma.
How should this patient's CUC have been managed over the previous 18 years?
There is not yet agreement on the most cost-effective approach for the surveillance for colonic cancer in patients with CUC. However, after a patient has
P.146
had extensive disease for 8 to 10 years, it is probably wise to perform complete colonoscopy every 1 to 2 years, with multiple biopsy specimens obtained every 10 to 12 cm from normal-appearing mucosa and targeted specimens obtained from villous areas of mucosa, areas of ulceration with a raised edge, and strictures. Colectomy is recommended if multifocal or high-grade dysplasia is seen in the biopsy specimens and confirmed by an experienced pathologist. If a mass lesion associated with any degree of dysplasia is identified, this is also a generally accepted indication for colectomy. The management of persistent low-grade dysplasia without a mass is more controversial, but, increasingly, colectomy is being recommended for low-grade dysplasia (Fig. 4.1).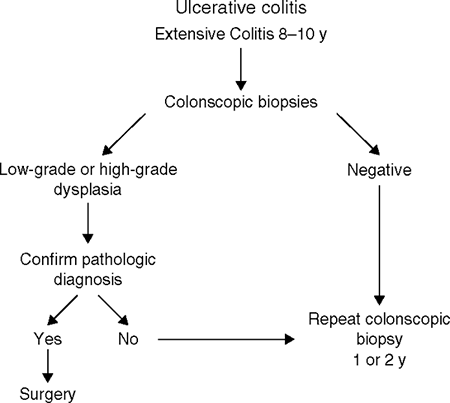
Figure 4-1 A proposed system of surveillance for cancer in ulcerative colitis using colonoscopy and biopsy. (From
Stenson WF and Korzenik J. Inflammatory bowel disease. In: Yamada T, Alpers, DH, Kaplowitz N, etal. eds. Textbook of gastroenterology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2003:1748, fig 83-29.
)Cancer prevention is an important topic to consider when advising young patients with extensive colitis about the possible need for surgical treatment. The decision to recommend prophylactic proctocolectomy after many years of colitis must be based on several considerations in the individual patient. These include the intractability of symptoms, age, psychological makeup, medical compliance, and the availability of newer surgical procedures. A prophylactic colectomy should be recommended to a noncompliant patient who acquires extensive ulcerative colitis at a young age. Patients who have CUC should be fully informed of their risk for development of cancer, as well as the limitations of endoscopic surveillance and the availability of surgical alternatives. If a patient is unwilling to assent to the surgical procedure, then he or she must be committed to undergoing regular surveillance.
P.147
Suggested Readings
Jewell DP. Ulcerative colitis. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran's gastrointestinal and liver disease. Pathophysiology, diagnosis, management, 7th ed. Philadelphia: WB Saunders, 2002: 2039 2067.
Sands BE. Crohn's disease. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran's gastrointestinal and liver disease. Pathophysiology, diagnosis, management, 7th ed. Philadelphia: WB Saunders, 2002: 2005 2038.
Stenson WF, Korzenik J. Inflammatory bowel disease. In: Yamada T, Alpers DH, Kaplowitz N, etal. eds. Textbook of gastroenterology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2003: 1699.
Chronic Liver Disease
What are some specific causes of chronic liver disease?
What are the principal laboratory abnormalities in the setting of chronic liver disease?
What are the two major histologic categories of chronic hepatitis due to viral infection?
Discussion
What are some specific causes of chronic liver disease?
Chronic liver disease may be the sequela of several kinds of toxic, metabolic, infectious, immunologic, or hereditary conditions. Table 4-2 contains a partial list.
What are the principal laboratory abnormalities in the setting of chronic liver disease?
The clinically available liver function tests include those that assess, at least in part, liver synthetic function (serum albumin and bilirubin concentrations, and prothrombin time) and those that mostly evaluate the hepatocellular release of enzymes (aminotransferases and alkaline phosphatase). Often, the levels of aminotransferase [alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase] are not markedly elevated in patients with chronic liver disease, and consequently do not accurately predict prognosis. The serum albumin and bilirubin concentrations and the prothrombin time are more likely to be distinctly abnormal, and more accurately reflect the true status of the liver's functional capacity.
What are the two major histologic categories of chronic hepatitis due to viral infection?
Categories of these diseases, constructed by international committees, consist of three components: the etiology of the diseases, grading of disease activity (i.e., the severity of the necroinflammatory process), and staging of the disease (i.e., the degree of fibrosis subsequent to necroinflammatory insults). The grading and staging are usually given a semiquantitative score (0 to 4) or a descriptive characterization (e.g., minimal to severe inflammation, or no fibrosis to cirrhosis).
P.148
Table 4-2 Specific Causes of Chronic Liver Disease | ||
|---|---|---|
|
Case
A 60-year-old man is brought to the hospital by his wife because he has not been acting his usual self. For the last 3 days, he has not been sleeping at night and has been napping during the day. There is no history of recent trauma, taking new medications, or suicidal ideation. He has been taking diazepam, 5 mg nightly, for insomnia. Risk factors for chronic liver disease, according to his wife, include the consumption of two beers nightly for 35 years and a blood transfusion for the treatment of a bleeding peptic ulcer 25 years ago, at which time he underwent an ulcer surgery.
On physical examination, he appears sleepy but arousable. The vital signs are normal. Several large spider angiomas are present on the torso. There is no scleral icterus. The parotid glands are enlarged bilaterally, and wasting of the temporal muscles is noted. The heart and lung examination findings are normal. His abdomen is slightly distended, and
P.149
shifting dullness and a midline scar are present. The liver is not palpable below the right costal margin but is palpable 10 cm below the xiphoid process; it is firm and percussed to a span of 8 cm in the right midclavicular line. The spleen is palpable. The abdomen is not tender to palpation or percussion. The testes are small. The rectum is found to contain hard, brown stool, which is positive for occult blood. There is mild edema of the legs and moderate muscle wasting. Asterixis is present. The cranial nerves and deep tendon reflexes are intact. The patient is somewhat uncooperative but his muscular strength is not focally diminished; his plantar reflexes (Babinski's sign) are normal.
Laboratory data are as follows: peripheral blood white cell count, 2,500 cells/mm3; hemoglobin, 10 g/dL; hematocrit, 33%; platelet count, 125,000/ mm3; serum AST, 100 IU/L (normal, <30 IU/L); ALT, 80 IU/L (normal, <45 IU/L); total bilirubin, 1.2 mg/dL; alkaline phosphatase, 150 IU/L (normal, <130 IU/L); total protein, 8.0 g/dL; albumin, 3.1 g/dL; and prothrombin time, 13 seconds (control, 11 seconds).
What features help you to diagnose chronic versus acute liver disease in this patient?
Does any particular factor help you determine the cause of this man's liver disease?
What reversible factors could be contributing to this man's presumed portosystemic encephalopathy (PSE)?
When, if ever, should this man's ascites be sampled? If it should, how and where should it be sampled?
What are three possible explanations for the occult blood in his stool?
What is the serum ascites albumin gradient, and of what value is it?
Would you start diuretic therapy now? Why or why not?
Why are his testes small?
Why are his parotid glands enlarged?
Is this man at increased risk for hepatocellular carcinoma?
How would you exclude hepatocellular carcinoma?
What is included in your differential diagnosis of this man's chronic liver disease?
Why is hepatitis A not in your differential diagnosis?
The results of additional tests are available within 4 hours of admission. The ascites is sampled from a left lower quadrant paracentesis, yielding a clear yellow fluid with a white blood cell count of 380 cells/mm3, 2% polymorphonuclear leukocytes, an albumin concentration of 0.5 g/dL, and a total protein level of 1 g/dL. No organisms are seen on Gram's-stained specimens.
Do the findings from the additional tests on the ascitic fluid support the diagnosis of portal hypertension-associated ascites? Why or why not?
With these data in mind, what treatment would you offer this patient now, and why?
What areas of the patient's history should you examine at greater length, and why?
Would you offer this patient a liver biopsy and, if so, when?
Case Discussion
What features help you to diagnose chronic versus acute liver disease in this patient?
In this patient, there are no pathognomonic features of chronic liver disease, but several that suggest this condition. Large spider angiomas are common in
P.150
the setting of chronic liver disease, but not acute liver disease, although small, nonpalpable spider angiomas may be present. Muscle wasting is common in moderately advanced chronic liver disease, but is not due to poor eating habits. Muscle wasting is not a feature of acute liver disease unless it is the result of a concomitant, unrelated problem. A palpable, firm left lobe of the liver (that portion palpable caudad to the xiphoid process) is usually a manifestation of chronic liver disease. It is always important to palpate and percuss for the liver in the midline, as well as in the midclavicular line. Ascites, due to portal hypertension, is much more a feature of chronic liver disease than of any other disorder. Ascites may occur in the setting of severe acute liver disease, but it is usually not of significant quantity to warrant treatment. One notable exception is the Budd-Chiari syndrome, in which there may be ascites, although the abdominal distention in this syndrome is partially due to a congested and enlarged liver stemming from the hepatic vein occlusion. Shifting dullness is indicative of a large amount (>1.0 to 1.5 L) of ascites.Pancytopenia is related to the splenic sequestration of blood cells and is not a prominent feature of liver disease unless the spleen is affected; when it is, it is usually enlarged. The degree of pancytopenia (or of individual cytopenias) may not correlate with spleen size. Hepatitis C may be associated with the development of aplastic anemia, but this is rare. Transient cytopenias may be seen in hepatitis, as in other viral infections. A low serum albumin level may be seen in any form of liver disease that has lasted for more than several weeks. A high serum globulin (total protein-albumin) level is a feature of chronic liver disease regardless of the cause. Extremely high serum globulin levels (i.e., 10 g/dL) should suggest the possibility of autoimmune or lupoid hepatitis; this disorder is usually seen in women and is frequently accompanied by other autoimmune features, such as thyroiditis. Autoimmune hepatitis is important to recognize because it can usually be treated with corticosteroids.
Does any particular factor help you determine the cause of this man's liver disease?
There are no particular factors that point to the cause of this patient's liver disease. The major differential diagnoses here are alcoholic liver disease and chronic active hepatitis (hepatitis C from his blood transfusion), probably in the cirrhotic stage. No feature of his history, physical examination, or routine laboratory tests helps distinguish between these two causes.
What reversible factors could be contributing to this man's presumed PSE?
Benzodiazepines, other sedative or hypnotic drugs, and opiates may precipitate PSE in a patient with severely impaired hepatic function. Constipation may also precipitate PSE in susceptible patients because of the colonic absorption of nitrogenous products. Both these reversible risk factors are present in this patient. Other reversible factors contributing to an episode of PSE include electrolyte disturbances, notably hypokalemia and metabolic alkalosis; increased intestinal absorption of nitrogenous products, resulting from relatively excessive dietary protein intake or an upper gastrointestinal (GI) hemorrhage; and a serious infection of any nature. In patients with chronic liver disease who have acute PSE, culture of the body fluids ascitic fluid, blood, urine, and sputum should be done. This patient's PSE indicates that he has severe liver disease.
When, if ever, should this man's ascites be sampled? If it should, how and where should it be sampled?
Diagnostic paracentesis should be performed as soon as possible to determine whether the patient has subacute bacterial peritonitis. This form of infectious peritonitis is a frequent cause of clinical deterioration in patients with chronic liver disease, and may be fatal if not recognized and treated early.
The three safest locations for paracentesis are the left lower quadrant, right lower quadrant, and the infraumbilical midline area. A supraumbilical approach should never be used because the umbilical or paraumbilical vessels, which course just under the parietal peritoneum, are frequently recanalized in patients with portal hypertension whose portal vein is patent. It is also important to always stay clear of (medial or lateral to) the rectus muscles because the superficial epigastric vessels course under them and may be punctured. Skin puncture through or near an abdominal scar in a patient with suspected or known portal hypertension should always be avoided.
What are three possible explanations for the occult blood in his stool?
Three possible explanations are (a) portal hypertensive gastropathy or enteropathy, (b) rectal varices, and (c) esophageal variceal hemorrhage due to portal hypertension. Variceal bleeding is usually a sudden event of large volume, although uncommonly varices ooze.
What is the serum ascites albumin gradient, and of what value is it?
The serum ascites albumin gradient is the numeric difference (not ratio) between the serum albumin concentration and the ascites albumin concentration. When the gradient is 1.1 or greater, portal hypertension is contributing to or entirely causing the ascites. When the gradient is less than 1.1, peritoneal carcinomatosis or inflammatory diseases are likely causes of the ascites. On the basis of this man's history, the two main causes to be considered are portal hypertension and peritoneal malignancy. Determination of the serum ascites albumin difference is a simple, minimally invasive, and fairly accurate way to diagnose portal hypertension.
Would you start diuretic therapy now? Why or why not?
No. Diuretics are not essential now, and they may only worsen the PSE and increase the risk of hepatorenal syndrome.
Why are his testes small?
In the setting of hepatic disease, the production of estrone from circulating androstenedione may be increased. The exact cause of this conversion is unknown but may be related to the decreased clearance of androstenedione by the liver. The consumption of excessive amounts of ethanol may also have contributed to the testicular atrophy in this patient.
Why are his parotid glands enlarged?
Parotid enlargement is seen in people who ingest excessive amounts of ethanol, and is associated with fatty infiltration of the glands. A similar situation may be seen in diabetic patients.
Is this man at increased risk for hepatocellular carcinoma?
Yes. There is a risk for the development of hepatocellular carcinoma in the setting of any form of cirrhotic liver, which this man most likely has. Certain conditions
P.152
are associated with higher risks than others. Those associated with highest risk are genetic hemochromatosis, chronic hepatitis B, chronic hepatitis C, and alcoholic liver disease.How would you exclude hepatocellular carcinoma?
Useful tests for identifying hepatocellular carcinoma are an imaging test [ultrasonography or computed tomography (CT) or magnetic resonance imaging] and a serum -fetoprotein level. The preferred imaging test (to exclude a focal lesion) depends on the expertise of the institution. Arterial-phase CT is regarded as most reliable. Hepatocellular carcinomas are especially difficult to detect in cirrhotic livers; therefore it is important that arterial-phase CT be used in this setting. The serum -fetoprotein level is very high in 60% of patients with alcoholic liver disease who have a superimposed hepatocellular carcinoma and in approximately 80% to 90% of patients with chronic hepatitis B who have this complication.
What is included in your differential diagnosis of this man's chronic liver disease?
The differential diagnosis in this patient includes alcoholic liver disease and chronic active hepatitis with cirrhosis, due to either hepatitis B or C, although hepatitis should be regarded as the more likely diagnosis. The hepatitis viruses may have been transmitted to him by the blood he received many years ago, or they may have been sporadically acquired.
Why is hepatitis A not in your differential diagnosis?
Hepatitis A has never been reported to cause chronic liver disease.
Do the findings from the additional tests on the ascitic fluid support the diagnosis of portal hypertension-associated ascites? Why or why not?
Yes, the findings from the tests on the ascitic fluid do support the diagnosis of portal hypertension-associated ascites because the serum ascites albumin gradient (2.6) exceeds 1.1. There are two caveats to remember when using the serum ascites albumin gradient in the diagnosis of ascites. First, if massive hepatic metastases cause enough liver disease to result in portal hypertension and ascites, the gradient resembles that seen in portal hypertension. Second, in ascites of mixed etiology (e.g., portal hypertension plus tuberculous peritonitis), the gradient usually resembles that seen in the setting of portal hypertension.
With these data in mind, what treatment would you offer this patient now, and why?
Hospital admission is required. Strict bed rest (for fear of self-harm) seems prudent. No benzodiazepines should be administered, although the patient should be monitored for the signs of ethanol withdrawal agitation, tachycardia, fever, and hallucinosis. The patient should receive an enema if he is constipated. Lactulose should also be administered (by mouth or nasogastric tube) if the patient becomes too disoriented and uncooperative. The oral or nasogastric lactulose dose is variable; the goal of therapy is to produce two to three soft stools per day. Alternatively, a nonabsorbable antibiotic could be used, such as neomycin at a dosage of 500 to 1,000 mg given orally or by nasogastric tube every 6 hours, or rifaximin. There is no evidence that giving lactulose and an antibiotic together is more effective than administering either alone. Lactulose is probably beneficial in the treatment of PSE by virtue of its ability to decrease the amount of nitrogen available for absorption (as urea) from the colon. Lactulose may accomplish this
P.153
by altering the colonic flora to more urease-negative forms and by inducing an osmotic diarrhea.What areas of the patient's history should you examine at greater length, and why?
One area of the patient's history that should be examined at greater length is his ethanol consumption history. This involves more interviewing of his family and friends. The alleged amount of ethanol ingested (per the patient's wife) is too low to cause liver disease in men because the alcohol content of two cans of beer is approximately 12 g. However, the parotid gland enlargement and testicular atrophy are findings that suggest his ethanol ingestion has been more than he has admitted. The amount and duration of alcohol ingestion necessary to cause chronic liver disease is highly variable among individuals, although the incidence of biopsy-proved cirrhosis, alcoholic hepatitis, or both, increases as consumption is increased. It is usually believed that the threshold amount of alcohol consumption that leads to these serious forms of chronic liver disease is in the order of 100 to 150 g per day for several years in men, but less in women. However, a large proportion of heavy drinkers do not contract serious liver diseases. It is advisable to record alcohol consumption in terms of grams per day times the number of years of consumption. A quart of 80 proof whiskey contains approximately 300 g of ethanol, a six-pack of 4% beer approximately 75 g, and 750 mL of wine approximately 90 g (150 g for fortified wine).
A second area of inquiry should be the patient's family history. In this patient, you should also ask whether anyone in the family has had liver disease, including genetic hemochromatosis. You might phrase the question in this way: Do you have any family members who have conditions that require blood to be removed as treatment? The manifestations of hemochromatosis may differ in various family members, and may consist of cardiomyopathy, diabetes, arthritis, or pituitary insufficiency. In this patient, the small liver is inconsistent with a diagnosis of hemochromatosis, although all else is. Moreover, he is an older man the typical age and sex of patients who have severe chronic liver disease caused by hemochromatosis.
Would you offer this patient a liver biopsy and, if so, when?
A liver biopsy would be of no help in the initial management of his decompensated liver disease. However, when conclusive documentation of the diagnosis would help determine management, liver biopsy might be important. This might be the case in a patient with suspected Budd-Chiari syndrome because it is often treatable by hepatic decompression (as, e.g., with a side-to-side portacaval anastomosis), or it might be the case in a patient with hemochromatosis. Once the patient's condition has stabilized, a liver biopsy might be offered, for three reasons. First, he may be a candidate for specific therapy. However, it is unlikely that there is any therapy for this patient. If, as seems likely, he has alcoholic cirrhosis there is no effective treatment other than abstinence; if he has hepatitis C related cirrhosis, interferon treatment may be dangerous because of the hepatocytolysis brought about by therapy. Second, some authorities believe that liver biopsy is indicated in patients with suspected alcoholic liver disease because confirmation of that diagnosis might help persuade the patient to abstain from further ethanol ingestion. Third, if the patient becomes a candidate for hepatic transplantation, most centers require a definitive preoperative diagnosis before going ahead with the procedure.
P.151
P.154
Suggested Readings
Batts KP, Ludwig J. Chronic hepatitis: an update on terminology and reporting. Am J Pathol 1995;19: 1409.
Dasarathy S, McCullough AJ. Alcoholic liver disease. In: Schiff ER, Sorrell MF, Maddrey WC, eds. Schiff's diseases of the liver, 9th ed. Philadelphia: Lippincott Williams & Wilkins, 2003: 1019 1057.
Davis GL. Hepatitis C. In: Schiff ER, Sorrell MF, Maddrey WC, eds. Schiff's diseases of the liver, 9th ed. Philadelphia: Lippincott Williams & Wilkins, 2003: 807 861.
Diarrhea
What is the diagnostic importance of nocturnal diarrhea in a patient with chronic diarrhea?
What is the difference between a secretory and an osmotic diarrhea?
What happens to diarrheal stool volume after fasting in the following settings: A vasoactive intestinal peptide (VIP) tumor, the abrupt onset of watery diarrhea after traveling outside of the United States, or diarrhea only when drinking large amounts of carbonated beverages?
What is the most likely cause of diarrhea in a patient who has recently taken ampicillin and then has low-grade fever and watery diarrhea? What is the most cost-effective way to diagnose this disease, and how would you treat this patient?
Why do patients with giardiasis often complain of increased stool volume and abdominal cramping when they consume milk products?
Which organisms are most commonly associated with diarrhea of less than 2 to 3 weeks' duration, and what are their clinical characteristics? How are such cases evaluated, and what are the various approaches to treatment?
What is the utility of staining stool specimens for leukocytes?
What would the clinician look for if surreptitious laxative abuse is suspected as a cause of chronic diarrhea?
A 24-year-old woman who has had a recurring rectovaginal fistula for 2 years complains of frequent small-volume stools, which occasionally contain blood and mucus. Stool cultures yield negative findings. What is the likely disease in this woman who has a rectovaginal fistula, and what would be the next step in evaluating her?
Discussion
What is the diagnostic importance of nocturnal diarrhea in a patient with chronic diarrhea?
Nocturnal diarrhea suggests an organic cause of the diarrhea. Patients with irritable bowel syndrome or other functional diarrheas rarely have diarrhea that awakens them from sleep.
What is the difference between a secretory and an osmotic diarrhea?
Secretory diarrhea is due to the active secretion of water and electrolytes into the intestinal lumen. The mechanism of action responsible for the release of the secretagogues is variable. For instance, the diarrhea of cholera, the classic example of a secretory diarrhea, is caused by the stimulation of adenylate cyclase activity by cholera toxin; this, in turn, causes an increase in the intracellular concentration of cyclic adenosine monophosphate, which stimulates electrogenic chloride secretion and inhibits electroneutral sodium chloride absorption. Increases in intracellular concentrations of Ca2+ as well as cyclic guanosine monophosphate have been proposed as the abnormalities at work in various other forms of secretory diarrhea.
In osmotic diarrhea, an unabsorbable solute (often a carbohydrate or divalent mineral) increases the osmolality of the intestinal contents. This increased osmolality passively drags water into the intestinal lumen. Patients with osmotic diarrhea usually have a stool osmolality measure that is much greater than that yielded by the formula: 2 serum Na+ + serum K+; this condition constitutes an osmotic gap. A common osmotic diarrhea is that which occurs after the ingestion of milk or milk products in people who are deficient in the intestinal enzyme lactase, or those who ingest magnesium-containing antacids or laxatives.
What happens to diarrheal stool volume after fasting in the following settings: a VIP tumor, the abrupt onset of watery diarrhea after traveling outside of the United States, or diarrhea only when drinking large amounts of carbonated beverages?
VIP is produced by the intestinal mucosa in increased amounts in the WDHA (watery diarrhea, hypokalemia, and achlorhydria) syndrome. VIP causes diarrhea by stimulating mucosal adenylate cyclase activity, and therefore would be expected to cause a secretory diarrhea. In such a condition, fasting would not decrease the stool volume until the patient becomes severely dehydrated.
Typically, travelers diarrhea is watery and occurs within 3 to 6 days of arriving in another country, or on return. Symptoms usually last for 2 to 3 days and resolve spontaneously. The most common pathogens responsible are the enterotoxigenic strains of Escherichia coli, which can elaborate heat-labile and heat-stable enterotoxins. The heat-labile toxin acts similarly to cholera toxin, whereas the heat-stable toxin stimulates mucosal guanylate cyclase activity. Other types of diarrhea-producing E. coli and their associated symptoms are the enteropathogenic type, which causes watery diarrhea, predominantly in children and newborns; the enteroinvasive type, which causes bloody diarrhea (dysentery) in children and adults, usually after the ingestion of contaminated food and water; and the enterohemorrhagic type, which causes bloody diarrhea in people of all ages and is transmitted through contaminated food (often poorly cooked hamburger). Serotype 0157:H7 of the enterohemorrhagic type has been identified in several outbreaks of infection characterized by particularly severe disease (hemolytic uremic syndrome).
P.156
Other pathogens associated with travelers diarrhea include Shigella and Salmonella species, C. jejuni, and Vibrio parahaemolyticus. Because of the numerous causes of traveler's diarrhea, the effect of fasting is usually unpredictable.
The osmotic diarrhea that occurs only after drinking large amounts of carbonated beverages is due to the ingestion of large amounts of fructose, which is the sugar used to sweeten these beverages (although not diet drinks) and comes in the form of corn syrup. Fructose is poorly absorbed by the proximal small intestinal mucosa. Cessation of fructose intake should stop the diarrhea.
What is the most likely cause of diarrhea in a patient who has recently taken ampicillin and then has low-grade fever and watery diarrhea? What is the most cost-effective way to diagnose this disease, and how would you treat this patient?
Pseudomembranous colitis (PMC) caused by Clostridium difficile is a likely diagnosis in this instance, given the patient's recent antibiotic use. The disease is usually self-limited, with the diarrhea dissipating 5 to 10 days after discontinuation of the offending antibiotic. Clindamycin was the first drug proved to cause PMC; later, ampicillin, because of its widespread use, was the drug most commonly implicated, but virtually any antibiotic can be responsible. In healthy adults, C. difficile colonization rates of 2% to 3% have been reported, whereas the rates in adults receiving antimicrobials but without diarrheal symptoms are as high as 10% to 15%.
The most commonly used method for diagnosing PMC is the cytotoxicity assay, which involves observation of the cytopathic effect produced by the toxin on a cell culture; the assay has a sensitivity of 95% to 97%. Although the latex agglutination test for the presence of toxin is both cheaper and faster to perform, it has a sensitivity of only approximately 85%. Gross colonic abnormalities in patients with PMC, which can be seen endoscopically, typically occur in the descending and sigmoid colon, making flexible sigmoidoscopy an adequate examination in most cases; however, cases with only right-sided involvement have been reported. The endoscopic findings in patients with PMC include erythematous, friable mucosa with characteristic pseudomembranes. Care must be taken to rule out bacterial or parasitic infections (especially C. jejuni and Entamoeba histolytica) and inflammatory bowel disease.
The recommended treatment for less severe cases of PMC consists of either oral metronidazole (250 mg four times a day) or vancomycin (125 to 500 mg four times a day). Parenteral doses of metronidazole [500 mg intravenously (IV) every 6 hours] should be given only when oral medication cannot be tolerated. The IV administration of vancomycin is not effective. The rates of relapse are similar for both metronidazole and vancomycin and range from 10% to 15%. Cholestyramine has been reported to be effective in the treatment of mild PMC or as an adjunctive measure, presumably by binding the toxin intraluminally. Cholestyramine may be used in conjunction with metronidazole but not with vancomycin, because it can bind and inactivate vancomycin. Recently, the oral administration of the nonabsorbed antibiotic rifaximin and probiotics (preparations of viable bacteria with therapeutic physiologic effects) has been reported effective in the treatment of recurrent PMC.
Why do patients with giardiasis often complain of increased stool volume and abdominal cramping when they consume milk products?
Giardia lamblia infection causes a deficiency in the intestinal disaccharidases, including lactase. The disaccharidase deficiency can cause cramping and flatulence after the ingestion of carbohydrates, especially milk products.
Which organisms are most commonly associated with diarrhea of less than 2 to 3 weeks' duration, and what are their clinical characteristics? How are such cases evaluated, and what are the various approaches to treatment?
The evaluation of a case of acute diarrhea involves routine culture of the stools, examination of the stools for the presence of ova and parasites, and, in some instances, flexible sigmoidoscopy.
One of the viral causes of acute diarrhea is the Norwalk agent that is seen in family and community epidemics, usually in older children and adults. It has an incubation period of 1 to 2 days. Vomiting and low-grade fever are common. Rotavirus infection is seen in infants and young children, primarily in winter; the incubation period is 1 to 3 days. Vomiting (occurring in 80%), upper respiratory symptoms, and fever (found in 30%) are common. Enteric adenovirus is a sporadic disease of infants and young children, and is often associated with fever and upper respiratory symptoms.
There are many bacterial causes of acute diarrhea. In Shigella infection, the major site of mucosal invasion is the colon. Penetration of the mucosa and invasion of the bloodstream are rare. Crampy abdominal pain and tenesmus are hallmarks of the disease. The organism produces an enterotoxin (Shiga toxin) that activates adenylate cyclase and causes a watery diarrhea in the early stages of the disease. Bloody diarrhea soon follows. The mainstay of therapy is supportive, with rehydration most important. Narcotics and anticholinergic medications should be avoided. Antibiotic treatment is reserved for those cases that do not resolve spontaneously in several days; ampicillin (500 mg four times a day, orally, for 5 days) is usually effective, but trimethoprim/sulfamethoxazole (one double-strength tablet twice daily) can be used for resistant strains. Chronic carriers, although uncommon, are prone to intermittent attacks of the disease.
The major site of Salmonella invasion is the ileal and, sometimes, the colonic mucosa. Bacteremia, with or without associated GI symptoms, occurs in approximately 10% of the cases. Carriers are usually asymptomatic, with the organism harbored in the gallbladder. Periumbilical pain and bloody diarrhea last approximately 5 days. Because antimicrobial treatment significantly increases the carrier rate, it is reserved for those cases that do not resolve spontaneously or for those patients who have an underlying predisposing condition.
C. jejuni is a common bacterial pathogen isolated from patients with acute bacillary diarrhea. Invasion of the mucosa occurs predominantly in the colon. Two features that may distinguish C. jejuni infection from other causes of bacterial diarrhea are (a) a prodrome of constitutional symptoms, and (b) a biphasic course, with initial improvement followed by worsening. No antibiotic regimen has been shown to lessen the symptoms or the time course of the disease.
P.158
Yersinia enterocolitica can cause enterocolitis, with a clinical picture consisting of fever, abdominal cramping, and bloody diarrhea lasting 1 to 3 weeks. Watery diarrhea is seen, possibly due to enterotoxin production. Invasive ileitis is also a feature of these infections.
Other diarrhea-producing enteric pathogens include E. histolytica, G. lamblia, and Strongyloides stercoralis.
What is the utility of staining stool specimens for leukocytes?
The presence of numerous leukocytes in stool specimens implies the existence of active inflammation of the intestinal mucosa. In cases of acute diarrhea, the presence of pus implies invasion (Shigella, Salmonella, C. jejuni, and E. histolytica). Although Shigella, E. histolytica, and C. jejuni infections are usually associated with most pus, patients with PMC also often have large numbers of fecal leukocytes. In cases of chronic diarrhea, the presence of pus most often implies tuberculosis, amebic colitis, ischemic colitis, or inflammatory bowel disease (ulcerative colitis more so than Crohn's disease, unless the latter involves the colon).
What would the clinician look for if surreptitious laxative abuse is suspected as a cause of chronic diarrhea?
There are several telltale clues to surreptitious laxative abuse. Melanosis coli, a dark pigmentation of the colorectal mucosa, may exist if the diarrhea is due to long-standing use of anthracene laxatives (aloe and cascara). The pigmentation usually disappears within 12 months of discontinuation of the laxative. If the ingestion of phenolphthalein-containing laxatives is the cause of the diarrhea, alkalization of a stool specimen by adding sodium hydroxide turns it pink. (However, raising the pH too high results in loss of the color and hence a false-negative result.) An osmotic gap of the stool may be present if the ingestion of magnesium sulfate is the cause of the diarrhea. Sodium sulfate and phosphate, however, which cause an osmotic diarrhea due to the formation of the anions sulfate and phosphate, do not cause an osmotic gap, and should be suspected in those thought to abuse laxatives but have an apparent secretory diarrhea.
What is the likely disease in this woman who has a rectovaginal fistula, and what would be the next step in evaluating her?
Crohn's disease is the most common cause of rectovaginal fistulas in young women and must be considered in the evaluation of such fistulas. Small-volume, bloody diarrhea is suggestive of anorectal involvement. After routine stool culture and examination for ova and parasites, flexible sigmoidoscopy or colonoscopy should be performed. In cases of Crohn's disease in which small bowel involvement is considered likely, a small bowel radiographic study might be next in order. In any event, cultures and tissue for histologic analysis should be obtained before empiric treatment with corticosteroids is instituted, to ensure that infectious colitis is not the cause.
P.155
P.157
Case
A 32-year-old woman with a history of Crohn's disease since childhood complains of having 10 to 12 loose, frothy, burning bowel movements. Blood is occasionally intermixed
P.159
in the stool. The increased stool frequency has been a problem ever since her most recent hospitalization for the treatment of small bowel obstruction, 5 weeks earlier. At that time, the remaining 120 cm of her ileum and 100 cm of her jejunum were resected because of fistulization and the formation of adhesions. She denies having fever, chills, and night sweats. Her appetite has been good, and she denies having abdominal pain associated with food ingestion; however, she has lost 15 lb (6.75 kg) since her last surgery. Her medications have not been changed since her discharge from the hospital, and consist of metronidazole (250 mg four times daily), prednisone (20 mg daily), calcium, and monthly vitamin B12 injections. She has had a long-standing history of watery diarrhea, which was controlled with the use of cholestyramine before the most recent surgery. Her weight had been stable for many years.
The patient is pale but in no acute distress and without fever. No orthostatic changes in her vital signs are noted. Her abdomen is soft with active bowel sounds. A healing midline incision and multiple scars from previous surgeries are present. The liver span is 7 cm in the midclavicular line. No abdominal or rectal masses are found. Her legs are slightly edematous. Her stool is dark brown and positive for occult blood.
The following laboratory results are obtained: hematocrit, 32%; mean corpuscular volume, 98 m3; serum sodium, 136 mEq/L; potassium, 3.0 mEq/L; chloride, 91 mEq/L; bicarbonate, 19 mEq/L; and creatinine, 0.6 mg/dL. The white blood cell count, platelet count, prothrombin time, and liver test results are all normal.
What is the first set of tests you would order in this patient to help explain the diarrhea?
Is this likely to be a flare-up of the patient's Crohn's disease?
If the cholestyramine treatment is resumed, how will this affect the volume of her diarrhea, and why?
What other treatment might be prescribed in an attempt to control her diarrhea and aid her nutrition?
Case Discussion
What is the first set of tests you would order in this patient to help explain the diarrhea?
The first step in evaluating the patient with postoperative diarrhea, whose condition is stable, is to rule out enteric infection, with cultures and examination of the stools for ova and parasites. Because this patient is taking metronidazole, the possibility of PMC should also be considered, and a stool cytotoxicity assay carried out. (Although metronidazole is commonly used to treat PMC, the drug has also been implicated in several cases as the causative antibiotic.) Flexible sigmoidoscopy could probably be safely performed soon after the resection, but as long as the patient's condition is stable, the procedure can be postponed pending the culture results.
Is this likely to be a flare-up of the patient's Crohn's disease?
No, because constitutional symptoms have not appeared or changed, and also because the character of the stool is more suggestive of a malabsorptive disorder than of an active flare-up of Crohn's disease.
If the cholestyramine treatment is resumed, how will this affect the volume of her diarrhea, and why?
Most likely the patient's diarrhea will worsen if she takes cholestyramine, whereas it was effective before her recent surgery. The explanation for this difference in effect is as follows: Because bile acids are normally absorbed actively in the distal ileum and resecreted by the liver into bile, when the distal ileum is either severely diseased or resected, unabsorbed bile acids enter the colon and stimulate water and electrolyte secretion, resulting in diarrhea. If the amount of ileum involved or resected is less than approximately 100 cm, the liver can compensate for the loss of bile acids by increasing bile acid synthesis, and fat malabsorption and weight loss are thereby largely prevented. Cholestyramine is effective in controlling diarrhea in this situation by binding bile acids in the small bowel and preventing their secretory effects in the colon. Such a set of circumstances apparently existed in this patient before her recent surgery. However, her last surgery resulted in the loss of her remaining ileum and part of the jejunum. Such a large loss of bowel would most likely result in depletion of her bile acid pool beyond the liver's ability to compensate for it by increasing bile acid synthesis, with consequent malabsorption of fat. The administration of cholestyramine would further deplete the bile acid pool and aggravate the fat malabsorption, which in turn would worsen the diarrhea. The mechanism responsible for this latter event is the stimulatory effect of unabsorbed fatty acids or their hydroxy derivatives on colonic water and electrolyte secretion.
What other treatment might be prescribed in an attempt to control her diarrhea and aid her nutrition?
Medium-chain triglycerides given orally should be tried to control her diarrhea and enhance nutrition. They do not require solubilization by bile acids for efficient absorption. At the same time, the patient should observe a low-fat diet.
P.160
Suggested Readings
Bartlett JG. Antibiotic-associated diarrhea. In: Blaser MJ, Smith PD, Ravdin JI, etal. eds. Infections of the gastrointestinal tract. New York: Raven Press, 1995:893.
DuPont HL. Traveler's diarrhea. In: Blaser MJ, Smith PD, Ravdin JI, etal. eds. Infections of the gastrointestinal tract. New York: Raven Press, 1995:299.
Powell DW. Approach to the patient with diarrhea. In: Yamada T, Alpers DH, Kaplowitz N, etal. eds. Textbook of gastroenterology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2003:844.
Schiller LR, Sellin JH. Diarrhea. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran's gastrointestinal and liver disease. Pathophysiology, diagnosis, management, 7th ed. Philadelphia: WB Saunders, 2002:131.
Malabsorption
What are the major steps in the digestion and absorption of dietary lipids, carbohydrates, and proteins?
What are the principal sites of intestinal absorption of various nutrients?
Of what does the enterohepatic circulation of bile acids consist?
What are some of the major disorders of maldigestion or malabsorption?
P.161
Discussion
What are the major steps in the digestion and absorption of dietary lipids, carbohydrates, and proteins?
The process of digestion can be divided into three major steps: (a) intraluminal digestion, including the action of bile acids and pancreatic enzymes; (b) digestion by the intestinal epithelium; and (c) the transport of nutrients across the epithelium to the circulation.
The major events in the digestion and absorption of dietary lipid include (a) the lipolysis of dietary triglycerides by pancreatic lipase; (b) micellar solubilization of the resulting long-chain fatty acids and -monoglycerides by bile acids; (c) the absorption of fatty acids and -monoglycerides into enterocytes; (d) the reesterification and incorporation (along with cholesterol, cholesterol esters, phospholipid, and -lipoproteins) into chylomicrons and very low density lipoproteins; and (e) the transport of chylomicrons from the mucosal cell into the intestinal lymphatics.
In the digestion and absorption of dietary carbohydrates, starch, which accounts for most of the carbohydrate intake, is initially hydrolyzed mostly by pancreatic amylase, yielding smaller sugars (maltose, maltotriose, and dextrins). These products, as well as ingested disaccharides such as lactose (milk sugar) and sucrose, are hydrolyzed further into their component monosaccharides by glucosidases (maltase, sucrase -dextrinase, and lactase), which are present in the brush border of epithelial cells in the proximal intestine. The monosaccharides are then absorbed by the epithelial cells and enter the portal circulation.
For the digestion and absorption of dietary protein to take place, proteins are first hydrolyzed by pancreatic enzymes in the intestinal lumen. These enzymes include endopeptidases (trypsin, chymotrypsin, and elastase) and exopeptidases (carboxypeptidases A and B). Oligopeptides produced by the pancreatic enzymes are further hydrolyzed by aminopeptidases located on the brush border as well as in the cytoplasm of intestinal epithelial cells. The resultant amino acids, and certain dipeptides and tripeptides, then enter the portal circulation.
What are the principal sites of intestinal absorption of various nutrients?
All dietary nutrients, with the exception of vitamin B12 (cobalamin), are absorbed preferentially in the proximal small intestine; most absorption of the components of a meal occurs within the first 150 cm, although absorption (especially of sugars and amino acids) can occur more distally (as in the event of disease or surgical bypass of the proximal intestine). Vitamin B12 is absorbed by the distal ileum, where there is a specific receptor for the cobalamin intrinsic factor complex.
Of what does the enterohepatic circulation of bile acids consist?
Bile acids are synthesized from cholesterol by the liver and are conjugated to either taurine or glycine before secretion into bile. During fasting, the bile acids are stored in the gallbladder. After a meal, they are secreted into the duodenum. The bile acids are very efficiently absorbed from the distal ileum, carried back to the liver by the portal vein, efficiently extracted and reconjugated by the liver, and then secreted again into bile. During each cycle, more than 95% of the bile acids are absorbed, but only small amounts are absorbed in the proximal small intestine.
What are some of the major disorders of maldigestion or malabsorption?
Table 4.3 lists the representative disorders.
P.162
Case
A 27-year-old woman complains of 11 months of diarrhea, gas, and abdominal cramps. She has five or six loose bowel movements a day, and diarrhea often awakens her from sleep. She also complains of abdominal cramps that are most severe just before a bowel movement, and are then temporarily relieved with the bowel movement. In addition,
P.163
she feels tired and has lost approximately 8 lb (3.6 kg) without dieting. She has noted a tendency to bruise easily. She drinks four glasses of milk a day.
Table 4-3 Major Disorders of Maldigestion or Malabsorption | |
|---|---|
|
Her past medical history is positive only for fatigue, for which she saw another physician 11 months ago, before the diarrhea developed. The physician told her that she had an iron-deficiency anemia. Since then, she has taken ferrous sulfate (300 mg four times daily), but still feels fatigued. She takes no other medication.
Physical examination reveals a young woman who appears mildly underweight but is otherwise normal.
Laboratory test results are as follows: white blood cell count, normal; hematocrit, 34%; mean corpuscular volume, 74 m3; serum iron, 50 mg/dL; total iron-binding capacity, 435 mg/dL; stool leukocyte test, negative; stool examination for ova and parasites, negative; serum albumin, 3.2 mg/dL; serum electrolytes, normal; and prothrombin time, 2 seconds greater than control.
While awaiting these laboratory results, you advise the patient to stop ingesting all milk products. The patient reports that this reduces but does not eliminate the diarrhea or gas.
What additional history should you obtain from the patient?
What might lead you to suspect that malabsorption is the cause of this patient's diarrhea, and why? What test should be performed to confirm this, and why?
This patient's fecal fat excretion is measured and found elevated, which proves she has maldigestion or malabsorption.
Considering that the patient has either maldigestion or malabsorption, what are the two disorders that may decrease the bile acid pool, two disorders that decrease pancreatic lipase activity, and two disorders that may decrease absorption by small bowel enterocytes?
How does the D-xylose test differentiate problems with digestion (e.g., bile salt depletion and pancreatic lipase deficiency) from problems with absorption? Name one disorder that may produce a false-positive result.
The D-xylose test in this patient reveals poor absorption of this sugar, which indicates that the small bowel absorption probably is abnormal.
On the basis of the results of the D-xylose test, what test should be performed now?
A small bowel biopsy specimen in this patient reveals mucosal villous atrophy and crypt hyperplasia, accompanied by an increased number of plasma cells and lymphocytes in the lamina propria and an increased number of lymphocytes in the epithelium.
Although the biopsy findings indicate celiac sprue, what other disorders could produce such a flat mucosa?
How can the diagnosis of celiac sprue be confirmed?
If the D-xylose test result was abnormal, but the small bowel biopsy findings were normal, a bacterial overgrowth in the proximal small intestine might be suspected. How should this possibility be evaluated?
If this patient's D-xylose absorption test result had been normal, what disorder might you suspect and how should you evaluate this possibility?
Why did the symptoms in this patient, who had celiac sprue, abate when she stopped drinking milk?
P.164
Case Discussion
What additional history should you obtain from the patient?
This patient has chronic diarrhea, which is arbitrarily defined as diarrhea that lasts longer than 3 weeks. Chronic diarrhea is a fairly common complaint, with a lengthy differential diagnosis. The clinical history remains the mainstay of the initial approach to diagnosis, and the history taking must include questions concerning the following factors:
Food: Milk consumption, sorbitol (added to diet foods and fruit), fructose (found in nondiet soft drinks, candy, and fruit), and unpasteurized milk (Yersinia infection).
Travel: To areas where giardiasis, amebiasis, or schistosomiasis might be contracted.
Iatrogenic factors: Surgeries in the GI tract. A partial gastrectomy can result in dumping (rapid emptying of the gastric contents into the small intestine) and, if a stagnant area of bowel is created, bacterial overgrowth can result (the blind loop syndrome). Medications are also a common cause of diarrhea. The administration of antibiotics can result in C. difficile colitis, and antacid use can produce an osmotic diarrhea. -Adrenergic antagonists, colchicine, laxatives, and innumerable other drugs can also cause diarrhea.
Risk factors for acquired immunodeficiency syndrome (AIDS).
Review of systems: This may reveal arthritis, which can accompany inflammatory bowel disease or Whipple's disease; peptic ulcer disease, which can be associated with the Zollinger-Ellison syndrome; symptoms or a history of diabetes; or hyperthyroidism.
Past medical problems, with an emphasis on childhood diarrhea or malnutrition and surgeries.
Further characterization of the diarrhea: Does it awaken the patient at night? Is it constant or does it alternate with constipation? The most common cause of chronic diarrhea in the U.S. population is the irritable bowel syndrome, which is a poorly understood motility disorder. It rarely results in diarrhea that awakens the patient at night, rarely produces weight loss, and may have diarrhea alternating with constipation.
What might lead you to suspect that malabsorption is the cause of this patient's diarrhea, and why? What test should be performed to confirm this, and why?
Malabsorption is suspected as the cause of the diarrhea because of the iron deficiency that does not respond to oral iron treatment and because the prothrombin time is elevated without signs of liver disease. A 2- or 3-day stool collection for quantitative fat analysis is the single most useful test to document malabsorption. Because fat absorption is a complex process (requiring the digestion of triglycerides by pancreatic lipase, solubilization of these products by bile salts, and absorption of the subsequent products by enterocytes of the small intestine), abnormalities in any of these steps result in fat malabsorption and an increase in fecal fat excretion. Therefore, measurement of the fecal fat content is a test for many steps in the
P.165
digestion and absorption pathways. One of the few kinds of malabsorption that does not cause increased fecal fat loss is that due to the lack of an intestinal enzyme needed in the digestion of a particular carbohydrate, despite a histologically normal intestine. The most common example of this is primary lactase deficiency, in which lactose is not absorbed normally but fat is.Considering that the patient has either maldigestion or malabsorption, what are the two disorders that may decrease the bile acid pool, two disorders that decrease pancreatic lipase activity, and two disorders that may decrease absorption by small bowel enterocytes?
Resection or disease of the distal small bowel can cause a decreased reabsorption of bile acids, resulting in insufficient bile salt concentrations in the proximal intestine to allow the normal solubilization and absorption of fat. Complete blockage of the common bile duct, as by pancreatic cancer or cancer of the duct, prevents bile acids from entering the duodenum.
Chronic pancreatitis or pancreatic cancer can block the pancreatic duct, resulting in decreased secretion of lipase. Increased acid content in the duodenum, such as occurs in the Zollinger-Ellison syndrome, can inactivate pancreatic lipase in the intestinal lumen.
Decreased absorption by small bowel enterocytes may be caused by celiac sprue, tropical sprue, Whipple's disease, small intestinal lymphoma, AIDS enteropathy, and several other diseases.
How does the D-xylose test differentiate problems with digestion (e.g., bile salt depletion and pancreatic lipase deficiency) from problems with absorption? Name one disorder that may produce a false-positive result.
D-xylose is a five-carbon sugar that can be absorbed without the aid of bile salts, pancreatic enzymes, or intestinal enzymes. It should be absorbed normally if the small bowel is intact. Therefore, the test is useful in distinguishing pancreatic enzyme insufficiency from enterocyte abnormalities. However, bacterial overgrowth in the proximal intestine is a condition that can cause malabsorption of D-xylose without affecting the enterocyte (the bacteria will consume the D-xylose before it can be absorbed), thereby producing a false-positive result.
On the basis of the results of the D-xylose test, what test should be performed now?
The small bowel should be examined, and there are two appropriate ways to do this: small bowel biopsy and a small bowel barium radiograph. A biopsy specimen gives more information about the mucosa, whereas the radiograph may permit better evaluation of diverticula, regional ileitis, or blind loops.
Although the biopsy findings indicate celiac sprue, what other disorders could produce such a flat mucosa?
Tropical sprue, soy and milk protein allergy (primarily in children), diffuse intestinal lymphoma, hypogammaglobulinemia, and the Zollinger-Ellison syndrome can produce a flat mucosal lesion that resembles that of celiac sprue.
How can the diagnosis of celiac sprue be confirmed?
The diagnosis of celiac sprue can be confirmed by observing the patient's response to a gluten-free diet. Adherence to a gluten-free diet should bring about a
P.166
cessation or marked reduction in the diarrhea and other intestinal symptoms, weight gain, and histologic improvement in the intestinal mucosa. Gluten is found in wheat, rye, barley, and oats, but not in rice and corn.If the D-xylose test result was abnormal, but the small bowel biopsy findings were normal, a bacterial overgrowth in the proximal small intestine might be suspected. How should this possibility be evaluated?
This would be more likely to occur in patients who have had a surgery that resulted in a blind loop of small intestine, or in elderly patients who are more likely to have multiple small bowel diverticula. A small bowel barium radiographic examination should reveal these abnormalities. The bile acid breath test could be used to document bacterial deconjugation of bile acids. In this test, a radiolabeled conjugated bile acid, such as [14C]-glycocholic acid, is given orally, and the amount and the time course of the [14C]-O2 exhaled is measured. Normally, most of the labeled bile acid is absorbed intact in the distal ileum; a minor amount reaches the colon, where anaerobic bacteria cleave the glycine moiety from the cholic acid moiety. The [14C]-O2 released in the colon is absorbed and exhaled. If the upper intestine is populated by excessive numbers of anaerobic bacteria, the deconjugation of [14C]-glycocholic acid occurs earlier and to a greater degree than normal, resulting in an early and high rise in the exhaled [14C]-O2 level.
If this patient's D-xylose absorption test result had been normal, what disorder might you suspect and how should you evaluate this possibility?
Pancreatic insufficiency should be suspected in patients who have a history of chronic pancreatitis or, less commonly, in middle-aged or elderly people who may present with a pancreatic cancer obstructing the pancreatic duct. A patient who has malabsorption and a history of pancreatitis should undergo a trial of pancreatic enzyme treatment. If this alleviates the diarrhea, the trial can be both diagnostic and therapeutic. The secretin test can be used to evaluate pancreatic function, but it is expensive and difficult to perform, so it is rarely used. If a pancreatic cancer is suspected, an imaging study such as CT scanning or endoscopic retrograde cholangiopancreatography (ERCP) should be performed.
Why did the symptoms in this patient, who had celiac sprue, abate when she stopped drinking milk?
Celiac sprue damages the intestinal epithelium, thereby decreasing the amounts of digestive enzymes, such as lactase, that are normally present in the villus cells.
Suggested Readings
Farrel RJ, Kelly CP. Celiac sprue and refractory sprue. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management, 7th ed. Philadelphia: WB Saunders, 2002:1817.
Hogenauer C, Hammer HF. Maldigestion and malabsorption. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management, 7th ed. Philadelphia: WB Saunders, 2002:1751.
P.167
Pancreatitis
What are the common and uncommon causes of acute pancreatitis?
What pathogenetic mechanism is hypothesized to be common to these causes of acute pancreatitis, and how does it explain the clinical features of the disease?
What symptoms and signs typify acute pancreatitis?
What difficulties may be encountered in confirming the diagnosis of acute pancreatitis through the measurement of amylase levels, and how might the diagnostic accuracy be improved?
What clinical and laboratory indices can be used to assess the prognosis in a case of acute pancreatitis?
What events signal the development of local complications of acute pancreatitis, and how are they best evaluated?
What are the mainstays of treatment of acute pancreatitis, and what is the rationale for their use?
What cardinal feature distinguishes chronic pancreatitis from acute pancreatitis?
How does the etiology of chronic pancreatitis differ from that of acute pancreatitis?
What are the mainstays of treatment of chronic pancreatitis?
Discussion
What are the common and uncommon causes of acute pancreatitis?
The common causes of acute pancreatitis are alcohol (60%), gallstones (25% to 30%), and idiopathic causes. Table 4.4 lists uncommon causes.
What pathogenetic mechanism is hypothesized to be common to these causes of acute pancreatitis, and how does it explain the clinical features of the disease?
Autodigestion is the pathogenetic mechanism common to all the causes of acute pancreatitis. Etiologic factors are believed to lead to the premature activation of pancreatic proenzymes within the gland. Destruction of the pancreas by the activated enzymes leads to local injury (edema, necrosis, and hemorrhage). In addition, the activation and release of proinflammatory cytokines, vasoactive peptides, and enzymes leads to the systemic effects that often accompany pancreatic injury (shock, disseminated intravascular coagulation, adult respiratory distress syndrome, renal failure, hyperglycemia, and hypocalcemia).
What symptoms and signs typify acute pancreatitis?
Pain is a characteristic symptom of acute pancreatitis and is located in the midepigastric and periumbilical regions. Commonly, it radiates to the back and is more constant and sustained than the pain associated with other abdominal processes. It is often more intense in the supine position and ameliorated by sitting forward. Patients may exhibit marked abdominal tenderness and guarding.
Nausea and vomiting are other symptoms. In this setting, the abdomen may be distended from the accumulation of intraabdominal and
P.168
fluid, paralytic ileus, and chemical peritonitis. The bowel sounds may be diminished.Table 4-4 Common and Uncommon Causes of Acute Pancreatitis
- Postoperative causes
- After endoscopic retrograde cholangiopancreatography
- Trauma
- Metabolic causes (hypertriglyceridemia, hyperparathyroidism, renal failure, and acute fatty liver of pregnancy)
- Hereditary causes
- Infections (mumps, Mycoplasma, coxsackie virus, and echovirus)
- Vasculitides (systemic lupus erythematosus, thrombotic thrombocytopenic purpura, Henoch-Schonlein purpura, necrotizing angiitis)
- Ampulla of Vater obstruction (Crohn'sease, duodenal diverticula, penetrating duodenal ulcer, pancreas divisum, and scorpion venom)
- Drugs
- Azathioprine/6-mercaptopurine
- Thiazide diuretics
- Estrogens
- Furosemide
- Sulfonamides (sulfasalazine, trimethoprim sulfamethoxazole)
- Tetracycline
- Methyldopa
- Sulindac
- Valproate
- Pentamidine
- Didanosine
- Oral 5-aminosalicylate (olsalzine and mesalamine)
- Octreotide
Hypotension may be present in as many as half of the patients; it results from vasodilation, myocardial depressant factor, and the loss of plasma and blood into the retroperitoneum.
Less common, but important, findings include periumbilical (Cullen's sign) or flank ecchymoses (Grey Turner's sign).
What difficulties may be encountered in confirming the diagnosis of acute pancreatitis through the measurement of amylase levels, and how might the diagnostic accuracy be improved?
Although the serum amylase level usually rises within 12 hours of the onset of pain and remains elevated for 3 to 5 days, a normal serum amylase value does not exclude pancreatitis. Spuriously normal serum amylase levels may result from the rapid clearance of amylase into the urine, and may be seen with hypertriglyceridemia and in late-stage ( burned out ) chronic pancreatitis. The magnitude of the amylase elevation in serum or urine does not correlate
P.169
with the severity of pancreatitis. In addition, hyperamylasemia is not a specific finding for pancreatitis because it may occur in a variety of pancreatic and nonpancreatic diseases. There are salivary as well as pancreatic-type isoamylases, and salivary amylase accounts for 60% to 65% of the total amylase content. Salivary hyperamylasemia can occur in the settings of diabetic ketoacidosis, alcoholism, and malignancy (especially with hepatic metastasis). Macroamylasemia occurs without any relationship to pancreatitis and results in elevated serum (but not urine) amylase levels.Attempts at improving the sensitivity, and especially the specificity, of the laboratory-based diagnosis of pancreatitis have included measurement of the renal amylase clearance and the ratio of renal amylase clearance to creatinine clearance (Cam/Ccr). However, the specificity of the Cam/Ccr is questionable because it may be elevated in the settings of diabetic ketoacidosis, burns, renal failure, chronic hemodialysis, pancreatic neoplasms, and alcoholic liver disease. Measurement of the pancreatic isoamylase levels has also been tried. This may provide information that changes the clinical diagnosis in 20% to 40% of patients with hyperamylasemia. Measurement of the serum lipase level is slightly less sensitive for the diagnosis of pancreatitis than that of the serum amylase level, but the lipase concentration remains elevated longer and is more specific than the amylase value.
What clinical and laboratory indices can be used to assess the prognosis in a case of acute pancreatitis?
A set of the early risk factors, known as Ranson's criteria, has been used to predict the potential complications and mortality in a patient with acute pancreatitis (see Tables 4.4 4.5).
The mortality rate associated with these signs has been determined as follows: two or fewer signs, 1%; three or four signs, 16%; five or six signs, 40%; and more than six signs, 100%.
Table 4-5 Ranson's
- At admission
- Age, older than 55 y
- White blood cell count, >16,000/ mm 3
- Blood glucose, > 200 mg/dL
- Serum lactate dehydrogenase, <350 IU/L
- Aspartate aminotransferase, > 250 IU/L
- During initial 48 hr
- Hematocrit decrease, > 10%
- Blood urea nitrogen rise, > 5 mg/dL
- Serum calcium, <8 mg/dL
- Arterial partial pressure of oxygen (Po2),<60 mm Hg
- Base deficit, >4 mEq/L
- Estimated fluid sequestration, > 6 L
P.170
Measurement of trypsinogen activation peptide in urine may distinguish mild from severe pancreatitis, but the test is not generally available.
- At admission
What events signal the development of local complications of acute pancreatitis, and how are they best evaluated?
Local and infectious complications of acute pancreatitis account for 80% of the mortality associated with the disease; therefore, detection of these complications is crucial in minimizing the likelihood of a fatal outcome. A pancreatic pseudocyst should be suspected in the setting of persistent pain and hyperamylasemia, and may be manifested as a palpable mass in the upper abdomen. Pancreatic necrosis or phlegmon, and pancreatic abscess are often difficult to distinguish because they both commonly cause prolonged abdominal pain and tenderness, fever, leukocytosis, and a palpable mass.
A CT scan with oral and IV contrast enhancement is the best method for imaging these complications. Extraluminal gas may be seen on the studies and can be used to distinguish pancreatic necrosis from pancreatic abscess. However, it is CT-guided percutaneous needle aspiration that usually allows for the early diagnosis of pancreatic infection and abscess, which require either percutaneous or surgical drainage.
What are the mainstays of treatment of acute pancreatitis, and what is the rationale for their use?
By eliminating oral intake (NPO), the neural and hormonal stimuli to pancreatic exocrine secretion may be minimized, thereby limiting the cycle of pancreatic autodigestion and inflammation. Eliminating food intake reduces the vagal stimulation of pancreatic secretion and reduces the delivery of acid, fatty acids, and amino acids to the duodenum, which would elicit release of secretin and cholecystokinin. Parenteral nutrition is often administered, but enteral nutrition through a tube placed in the jejunum is preferred because of its lower cost and fewer complications. Nasogastric suction is usually not advocated, but it may be useful in those patients experiencing nausea and vomiting resulting from paralytic ileus. Adequate replacement of fluid and electrolyte losses (especially calcium) stemming from the retroperitoneal inflammation and exudation is essential. Hypocalcemia is believed to result from a combination of factors: hypoalbuminemia, the sequestration of calcium in areas of fat necrosis, and an inadequate parathormone response. Analgesic administration is usually required to control the pain, which is often intense and prolonged.
What cardinal feature distinguishes chronic pancreatitis from acute pancreatitis?
The permanent destruction of the pancreatic gland is a cardinal feature of chronic pancreatitis. Pathologically, there is atrophy of the acini, a loss of islet cells, fibrosis, and plugging of irregular pancreatic ducts by protein. The protein plugs may be calcified and, on radiographic studies, 30% of patients exhibit pancreatic calcification. The clinical sequelae of glandular destruction include exocrine and endocrine insufficiency, manifested by steatorrhea and diabetes mellitus, respectively (the former occurring only when there is a more than 90% reduction in exocrine function). Abdominal pain is not
P.171
uniformly seen and may be intermittent, constant, or absent. Because of the acinar destruction, the serum amylase levels may be only mildly elevated or normal.How does the etiology of chronic pancreatitis differ from that of acute pancreatitis?
In western countries, most (approximately 90%) cases of chronic pancreatitis are attributable to alcoholism. Other possible causes include metabolic disorders such as hypercalcemia of any cause (perhaps hyperparathyroidism), hyperlipidemia, and congenital or hereditary conditions (pancreas divisum, cystic fibrosis, and hereditary pancreatitis).
What are the mainstays of treatment of chronic pancreatitis?
Acute relapses of chronic pancreatitis may require management identical to that for acute pancreatitis, and may be accompanied by pseudocyst formation and pancreatic ascites. Exocrine insufficiency resulting in steatorrhea and weight loss is treated with oral pancreatic enzyme replacement, whereas endocrine insufficiency (diabetes mellitus) requires insulin therapy. Management of the chronic pain has been problematic, and patients frequently become addicted to narcotic analgesics. The oral administration of high doses of pancreatic enzymes may reduce the pain. Surgical intervention (ganglionectomy, partial and total pancreatectomy, and pancreatic duct drainage operations) confers inconsistent benefits and is fraught with long-term morbidity.
Case 1
A 66-year-old man is admitted with complaints of progressively severe, constant upper abdominal pain, nausea, and vomiting of 48 hours duration. Recently, he has consumed large quantities of vodka, but has no history of biliary tract disease and is taking no medications.
He is a thin man, wincing and clutching his abdomen. His temperature is 38 C (100.4 F); blood pressure, 100/60 mm Hg; pulse, 90 beats per minute; and respirations, 18 per minute. His abdomen is flat and the bowel sounds are hypoactive. There is marked direct tenderness with guarding in the midepigastrium, but no peritoneal signs.
The following laboratory data are gathered: white blood cell count, 10,000 cells/mm3; hematocrit, 50%; serum creatinine, 1.3 mg/dL; total serum bilirubin, 3.4 mg/dL; alkaline phosphatase, 246 IU/L; AST, 209 IU/L; and serum amylase, 741 U/L.
Plain abdominal radiographs reveal the presence of scattered air-fluid levels, predominantly in the small bowel, but no calcification or subdiaphragmatic free air. An abdominal ultrasound examination reveals a dilated, fluid-filled gallbladder, a dilated common bile duct without definite calculi, and a poorly visualized pancreas because of overlying bowel gas.
A nasogastric tube is inserted and placed at low suction, and the patient remains NPO, receiving only IV fluids. Over the ensuing 48 hours, he requires regular doses of meperidine for the control of persistent, severe pain and is noted to have a rise in his bilirubin (8.0 mg/dL), alkaline phosphatase (450 IU/L), and AST (375 IU/L) levels. ERCP, performed on the third hospital day, demonstrates a dilated common bile duct that tapers smoothly in its intrapancreatic portion and contains no stones. The gallbladder is dilated and also contains no stones. No pancreatogram is obtained.
P.172
The aforementioned management is continued, and total parenteral nutrition is started. The patient's pain, abdominal tenderness, and liver test abnormalities gradually abate over the subsequent 10 days.
Why was an ERCP obtained?
What was the cause of the patient's biliary obstruction?
Case Discussion
Why was an ERCP obtained?
The patient's laboratory data included abnormal liver test results consistent with cholestasis, and common bile duct dilation was seen on the ultrasound examination. These findings and the failure of his symptoms to subside during the early hospital course raised concern about a gallstone at the ampulla of Vater and gallstone pancreatitis. Performing an emergency ERCP, with papillotomy when ampullary or common bile duct stones are found, has been advocated within 24 hours in patients who have acute biliary pancreatitis.
What was the cause of the patient's biliary obstruction?
Compression of the intrapancreatic common bile duct by an inflamed pancreas is the cause of the biliary obstruction in this patient. This is shown by the absence of gallstones on the ERCP study and the patient's gradual improvement with conservative management of acute pancreatitis.
Case 2
A 40-year-old, alcoholic man complains of chronic abdominal pain and weight loss. He had consumed two pints of bourbon daily for the last 10 years, until 4 years ago, when he had his first episode of abdominal pain, which was characterized as a sharp, continuous epigastric pain radiating to the back, and associated with nausea and vomiting. He was admitted to the hospital, where his symptoms gradually abated with treatment, consisting of bowel rest and IV fluids for 1 week. His abdominal radiographs at that time revealed calcification in the area of the pancreas. He subsequently reduced his alcohol intake, but required readmission to the hospital on several occasions after the consumption of relatively small quantities of alcohol.
In recent months, the patient has lost 25 lb (11.25 kg), coincident with the passing of persistently loose and occasionally greasy stools. His abdominal pain has become constant, and a macrocytic anemia has developed.
The patient is cachectic, weighing 125 lb (56.25 kg). He has a scaphoid abdomen with normal bowel sounds and mild direct tenderness in the midepigastrium in response to palpation. There is moderate pedal edema.
Relevant laboratory data are: white blood cell count, 4,900 cells/mm3, with 65% segmented cells, 20% lymphocytes, and 10% monocytes; hematocrit, 37%; mean corpuscular volume, 106 m3; prothrombin time, 14 seconds (control, 12 seconds); serum albumin, 2.7 g/dL; serum glucose, normal; serum and electrolytes and liver function tests are otherwise normal; serum vitamin B12, 96 pg/mL (normal, >200 pg/mL); serum folate, normal; and 72-hour fecal fat excretion, 42 g (normal, <15 g).
P.173
The patient is started on a regimen of monthly vitamin B12 injections and oral pancreatic enzymes, three capsules with each meal and one capsule with snacks. At first, he fails to gain weight and observes no reduction in the frequency of his bowel movements; his abdominal pain persists at a moderate severity. The dose of enzymes is increased to six capsules with each meal, and he also begins taking cimetidine (300 mg orally four times a day). Over a period of 1 month, his pain subsides considerably and he gains 15 lb (6.75 kg).
Is it unusual that the patient had his first attack of pancreatitis pain after 10 years of heavy alcohol consumption, and at that time he already had signs of chronic pancreatitis (pancreatic calcification)?
What is the pathophysiologic basis for vitamin B12 deficiency in the setting of chronic pancreatitis?
Why did the patient begin to gain weight only after his pancreatic enzyme dose was increased and cimetidine added?
Why might the patient's pain have subsided toward the end of the described course?
Case Discussion
Is it unusual that the patient had his first attack of pancreatitis pain after 10 years of heavy alcohol consumption, and at that time he already had signs of chronic pancreatitis (pancreatic calcification)?
No. It is believed that most people must consume at least 50 g of alcohol daily on a prolonged basis before chronic pancreatitis develops, and most have been drinking excessively for 5 to 20 years before their first attack. Alcohol-induced pancreatitis is probably chronic, even at the time of the first attack. Pancreatic calcifications are seen in 25% to 50% of the patients and are particularly common in alcoholics who have chronic pancreatitis.
What is the pathophysiologic basis for vitamin B12 deficiency in the setting of chronic pancreatitis?
The vitamin B12 deficiency stems from the exocrine insufficiency. Pancreatic proteases are necessary to cleave R protein from vitamin B12 in the proximal intestine, so that the latter may be absorbed as a complex with intrinsic factor (in the terminal ileum). Approximately 50% of patients with advanced pancreatitis have vitamin B12 deficiency due to exocrine insufficiency.
Why did the patient begin to gain weight only after his pancreatic enzyme dose was increased and cimetidine added?
Pancreatic enzymes can be inactivated by gastric acid, and this inactivation can be reduced by the administration of antacids or histamine 2 (H2) receptor antagonists. In addition, evidence suggests that certain enzyme capsules are more effective at delivering active enzyme to the small intestine than others.
Why might the patient's pain have subsided toward the end of the described course?
Sustained pain relief in patients with chronic pancreatitis often occurs after several years and only with marked progression of the pancreatic exocrine insufficiency, rather than being a result of therapeutic intervention. However, this
P.174
patient's pain seemed to subside rather quickly with the institution of high doses of pancreatic enzyme therapy. The suppression of pancreatic exocrine secretion has been accomplished in patients who received intraduodenal perfusions of pancreatic extract, and the pain of chronic pancreatitis has been shown to respond to treatment with orally administered pancreatic enzymes in some patients.
Suggested Readings
Owyang C. Chronic pancreatitis. In: Yamada T, Alpers DH, Kaplowitz N, etal. eds. Textbook of gastroenterology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2003: 2061.
Topazian M, Gorelick FS. Acute pancreatitis. In: Yamada T, Alpers DH, Kaplowitz N, etal. eds. Textbook of gastroenterology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2003:2026.
Acute Lower Gastrointestinal Hemorrhage
What is one of the most important diagnosis to rule out in a patient with large-volume hematochezia?
Which method of imaging the GI tract has no role in the evaluation of a patient with acute lower GI bleeding?
What are the two most common causes of acute major lower GI bleeding?
Discussion
What is one of the most important diagnosis to rule out in a patient with large-volume hematochezia?
An upper GI bleeding source must be ruled out in every patient with large-volume hematochezia. Lower GI bleeding is defined as bleeding from a source distal to the ligament of Treitz, the structure that divides the duodenum from the jejunum. Approximately 10% of patients with upper GI bleeding have hematochezia because of a rapid rate of blood loss and the subsequent rapid transit of blood through the GI tract. Because the strategy for treatment of an upper GI hemorrhage may differ drastically from that for a lower GI hemorrhage, first ruling out an upper GI bleeding source is mandatory in patients presenting with hematochezia.
The easiest way to exclude a significant upper GI hemorrhage with substantial reliability is through nasogastric lavage and aspiration. The aspiration of bilious contents from a nasogastric tube makes it very likely that the bleeding originates from a lower source, but this is not an infallible finding. If the source of bleeding remains in doubt after nasogastric lavage, upper GI endoscopy should be performed.
Which method of imaging the GI tract has no role in the evaluation of a patient with acute lower GI bleeding?
A barium enema examination should not be performed in the setting of acute lower GI bleeding because it has no therapeutic potential, and the barium interferes with the performance of more appropriate tests, namely technetium Tc 99m (99mTc)-labeled erythrocyte scanning, angiography, and colonoscopy.
At most hospitals, the 99mTc-labeled erythrocyte scan is the preferred nuclear medicine test for localizing the source of acute lower GI bleeding. To perform this, the patient's red blood cells are labeled with radioactive technetium. A scintillation camera then tracks where the labeled red blood cells collect in the patient. The 99mTc-labeled erythrocyte scan may help localize a bleeding source to the general region of the small bowel, right colon, or left colon, thereby directing the course of therapy. Under the correct circumstances, this scan may localize a source of bleeding at rates as low as 0.5 mL per minute.
If arterial bleeding is occurring at a rate of approximately 1 mL per minute, angiography is useful for both diagnosis and therapy. Once catheterized, the bleeding artery may then be selectively infused with vasopressin, or embolized with metal coils. Angiography is usually not useful if the bleeding has stopped.
Colonoscopy, because it may yield a diagnosis and provide a means for delivering therapy, regardless of whether the patient is actively bleeding, should be performed before nuclear medicine scans or angiography in most patients with acute lower GI bleeding. If possible, the lower bowel should be rapidly flushed with a polyethylene glycol electrolyte solution before colonoscopy is performed. With modern techniques, the diagnostic accuracy of colonoscopy is at least as good as that of angiography, unless the rate of bleeding is so brisk as to obscure colonoscopic visualization completely.
What are the two most common causes of acute major lower GI bleeding?
The most common cause of major lower GI bleeding is diverticulosis, accounting for approximately 40% of all cases. Diverticula are herniations in the colon wall that are believed to be acquired with age. Causal associations between low dietary fiber intake and diverticulosis have not been universally accepted, and the true etiology is probably multifactorial. In diverticulosis, as the colon wall herniates, the intramural arteries (vasa recta) may rupture, thereby producing a brisk but painless hemorrhage. Although the hemorrhage stops spontaneously in approximately 80% of patients, diverticular bleeding may lead to life-threatening blood loss, particularly in the elderly. Diverticula are more common in the left colon, yet diverticular bleeding most often originates from the right colon. The diagnosis is usually made on the basis of findings revealed by urgent colonoscopy or by angiography. Therapy with selective angiographic catheterization is successful in many cases.
Arteriovenous malformations (AVMs) or angiodysplasias in the colon are the second most common cause of major lower GI bleeding, accounting for approximately 20% of all cases. These vascular ectasias are located just beneath the columnar epithelium, and most are due to the degenerative changes of aging. A
P.176
causal association between aortic stenosis and colonic AVMs has been proposed but not definitely established. AVMs are usually located in the right colon and may present as either an acute lower GI hemorrhage or as chronic low-volume bleeding manifested by iron-deficiency anemia. If the bleeding is brisk and persistent, angiography is usually the preferred method for making the diagnosis and carrying out therapy. If the bleeding has slowed or stopped, urgent colonoscopic therapy with thermal cauterization or injection is often useful.Less common causes of acute lower GI bleeding are colonic neoplasms, inflammatory bowel disease, infectious colitis, and ischemic colitis. Ischemic colitis usually presents with acute, crampy lower abdominal pain, the urge to defecate, and passage of bloody diarrhea. Watershed areas of the colon, such as the splenic flexure and sigmoid colon, are most commonly involved because of their poor blood flow.
P.175
Case
A 70-year-old woman is seen in the emergency room complaining of rectal bleeding. Her first episode occurred approximately 6 hours ago, when she passed red blood and clots. At first she attributed the bleeding to her hemorrhoids, but she has had five more episodes since, the last of which was accompanied by a sensation of dizziness. She does not smoke or drink alcoholic beverages. She takes several aspirin a day for the treatment of arthritis. Physical examination reveals a woman who is pale and anxious. Her blood pressure and pulse in the supine position are 110/70 mm Hg and 100 beats per minute, respectively. When she stands, her blood pressure and pulse are 85/50 mm Hg and 130 beats per minute, respectively. The abdominal examination reveals no abnormal findings. Rectal examination reveals red blood in the vault and no masses.
What are the three most likely causes of this woman's hematochezia?
What diagnostic and therapeutic maneuvers must you do within the first hour?
What diagnostic and therapeutic tests should you consider doing over the next 24 to 48 hours?
Case Discussion
What are the three most likely causes of this woman's hematochezia?
Diverticulosis, colonic AVMs, and upper GI bleeding are the most likely causes of this woman's bleeding, which is associated with hemodynamic instability. Hemorrhoids, inflammatory bowel disease, and colonic neoplasms rarely cause bleeding of this degree.
What diagnostic and therapeutic maneuvers must you do within the first hour?
This woman exhibits significant hemodynamic instability, as demonstrated by the orthostatic changes in her blood pressure and pulse. You must place at least two large-bore (18-gauge) IV catheters and start IV volume expansion using crystalloid fluid (usually, 0.9% sodium chloride or an equivalent of lactated Ringer's solution). At the same time, you should draw blood for typing and cross-match studies, hemogram, coagulation studies, and serum chemistry profile. Next, you should place a nasogastric tube to obtain gastric contents and determine whether
P.177
there is an upper GI bleeding source. The aspiration of blood should prompt strong consideration for performing emergency upper GI endoscopy.What diagnostic and therapeutic tests should you consider doing over the next 24 to 48 hours?
If the bleeding slows or stops, rapid GI lavage with polyethylene glycol electrolyte solution followed by colonoscopy is usually the best test for diagnosis, with a yield of 40% to 50% in this setting. Colonoscopy can still be useful if the bleeding is persistently brisk; however, in this situation, angiography is the preferred test in many hospitals. If colonoscopy and angiography fail to identify the source of the bleeding, a 99mTc-labeled erythrocyte scan may help localize the source.
Suggested Readings
Elta GH. Approach to the patient with gross gastrointestinal bleeding. In: Yamada T, Alpers DH, Kaplowitz N, etal. eds. Textbook of gastroenterology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2003:698.
Zuccaro G Jr. American College of Gastroenterology. Practice Parameters Committee. Management of the adult patient with acute lower gastrointestinal bleeding. Am J Gastroenterol 1998;93:1202.
Acute Upper Gastrointestinal Hemorrhage
Is measurement of the hemoglobin concentration and hematocrit the best way to determine the severity of GI bleeding? Why or why not?
Is esophagitis a common cause of severe upper GI bleeding?
Is -adrenoreceptor blockade a treatment option for acute variceal bleeding? If so, why? If not, what are some treatment options?
Discussion
Is measurement of the hemoglobin concentration and hematocrit the best way to determine the severity of GI bleeding? Why or why not?
No. The best way to determine the severity of GI bleeding is to measure the vital signs with the patient in the supine and standing positions. If orthostatic changes in the vital signs (postural hypotension) occur in the setting of GI bleeding, they usually indicate at least a 20% loss in the total blood volume. This finding mandates immediate IV volume expansion and preparation for blood transfusion. Other physical findings associated with severe blood loss are resting tachycardia and hypotension, pallor, and agitation.
The initial blood count (hemogram) obtained from an acutely bleeding patient is a very poor reflection of the amount of blood lost. To be accurate, the blood count must be obtained when the patient's intravascular volume is normal. After an acute loss of blood, reequilibration may take up to 72 hours. The IV administration of crystalloid hastens this process (often known as hemodilution).
P.178
Placement of a nasogastric tube is very helpful in the initial assessment of a patient with GI bleeding, but the findings yielded can be misleading. The absence of bloody aspirate may suggest that bleeding has stopped for the moment, yet the amount of blood already lost may be life threatening. Conversely, up to 15% of patients with an actively bleeding upper GI source may have a nonbloody gastric aspirate. The presence of bile in the gastric aspirate offers some reassurance that an upper GI bleed has stopped; however, up to 50% of physicians misjudge the presence or absence of a bilious aspirate. The persistence of bloody gastric aspirate indicates significant ongoing bleeding.
Melena is produced when hemoglobin is degraded by bacteria in the GI tract, and may originate from either an upper or lower GI source. The ingestion of approximately 100 to 200 mL of blood is enough to cause melena; hence, the presence of melena alone does not necessarily mean the patient has had a substantial loss of blood. On the other hand, frequent episodes of melena indicate significant bleeding. Hematochezia resulting from an upper GI bleeding source indicates massive bleeding.
Is esophagitis a common cause of severe upper GI bleeding?
No. Esophagitis accounts only for approximately 8% of all cases of upper GI bleeding. It is usually caused by gastroesophageal reflux of acid, but may also be caused by infectious agents such as Candida albicans, herpes simplex virus, and cytomegalovirus. Severe bleeding resulting from esophagitis is rare and usually occurs in the setting of an already hospitalized and critically ill patient, especially during mechanical respiration.
Peptic ulcer disease, which accounts for 40% to 50% of all the cases of upper GI bleeding, is discussed in the next section.
Variceal bleeding accounts for 10% to 30% of all cases of upper GI bleeding. Varices are a complication of portal hypertension, the cause of which may be classified as prehepatic (portal vein obstruction), hepatic (cirrhosis), or posthepatic (thrombosis of the hepatic veins or inferior vena cava). The most common cause of portal hypertension in the U.S. population is ethanol-induced cirrhosis, although hepatitis C associated cirrhosis is an increasingly common cause. The 6-week mortality rate associated with bleeding varices is approximately 40%, and patients with variceal bleeding should be managed in an intensive care unit. These patients often have concurrent medical problems, such as a coagulopathy and hepatic encephalopathy.
Mallory-Weiss tears are partial-thickness mucosal lacerations near the gastroesophageal junction, usually caused by forceful retching, often in the setting of ethanol ingestion. These tears account for approximately 10% of upper GI hemorrhages. Most Mallory-Weiss tears stop bleeding spontaneously. Persistent bleeding can be treated with either endoscopic hemostasis or angiographic embolization.
Significant acute and chronic blood loss, sometimes leading to iron-deficiency anemia, can occur from gastric erosions or ulcers associated with large sliding hiatal hernias. These lesions, sometimes called Cameron lesions, which are usually located on the crests of mucosal folds at or near the level of
P.179
the diaphragm, seem to result from the riding motion of the herniated stomach in and out of the chest during respiration.Is -adrenoreceptor blockade a treatment option for acute variceal bleeding? If so, why? If not, what are some treatment options?
No. -Adrenoreceptor blockers, such as propranolol, produce a negative chronotropic and inotropic effect. The use of such drugs in an acutely bleeding patient, who is depending on an adrenergic response to maintain an adequate blood pressure, is therefore contraindicated. Some evidence supports the use of -adrenoreceptor blockers in selected patients to reduce the risk of recurrent variceal bleeding after the acute hemorrhage has been well controlled. Although the complete mechanism of action of these drugs is yet to be delineated, they are thought to exert their beneficial effect in part by lowering the portal pressure.
Other pharmacologic approaches to the control of acute variceal bleeding are the use of parenteral vasopressin or somatostatin. Although vasopressin has been commonly used, its efficacy has not been firmly established and it has associated cardiovascular side effects. The drug's mechanism of action is thought to be splanchnic arteriolar vasoconstriction, resulting in decreased portal pressure. Increasingly, somatostatin or its longer acting analog, octreotide, has become popular for the control of variceal bleeding, and also because of its purported lowering of portal pressure. Somatostatin has been shown to be as effective as, or more effective than, vasopressin in controlling acute variceal hemorrhage.
Endoscopic hemostasis can be achieved by endoscopic injection sclerotherapy or endoscopic variceal ligation. The latter is now the more popular technique because it is as effective as sclerotherapy and considerably safer.
If endoscopic techniques are unavailable or unsuccessful, acute hemostasis may be achieved with balloon tamponade devices. Although the tamponade accomplished with devices such as the Sengstaken-Blakemore tube or the Minnesota tube is effective in approximately 40% to 90% of patients, the rebleeding rate associated with their use is approximately 50%. There is also an approximately 30% rate of serious complications, such as aspiration pneumonia or esophageal rupture, resulting from the use of these tubes; therefore, their use is considered a temporary measure only.
Interventional radiologic techniques, such as the angiographic embolization of varices or the placement of a transjugular intrahepatic portosystemic stent shunt (TIPS), may also be useful if endoscopic hemostasis fails or is unavailable. The surgical creation of a portosystemic shunt in an acutely bleeding patient is associated with mortality rates of 50% to 80%, and has fallen out of favor. Furthermore, such shunts make liver transplantation technically more difficult.
Case
A 42-year-old man is brought to the emergency room by ambulance after an episode of hematemesis and syncope at a local bar. He has not had previous GI bleeding. He regularly takes aspirin for the relief of chronic back pain. During your interview, he passes several liquid, maroon stools. Physical examination reveals a supine blood pressure and pulse of 120/75 mm Hg and 110 beats per minute, respectively. When you make him
P.180
sit upright he complains of feeling faint and his systolic pressure drops to 90 mm Hg by palpation. His abdomen is nontender and distended. Shifting dullness is elicited and the spleen tip is palpable. The initial hemoglobin is 15 g/dL and the hematocrit is 45%.
How do you know this man has lost a significant amount of blood?
What are the most likely causes of this man's upper GI bleeding, and what should the next diagnostic step be?
Case Discussion
How do you know this man has lost a significant amount of blood?
He has significant orthostatic changes in his vital signs, indicating at least a 20% loss of total blood volume. The hemoglobin concentration or the hematocrit may be falsely reassuring in the setting of acute GI bleeding.
What are the most likely causes of this man's upper GI bleeding, and what should the next diagnostic step be?
The most likely causes of such severe upper GI bleeding are peptic ulcer disease and esophageal or gastric varices, the latter resulting from portal hypertension. This man has risk factors for both conditions. There is no way to distinguish one from the other based on the history and examination findings. After hemodynamic stabilization, emergency esophagogastroduodenoscopy (EGD) should be performed for diagnosis and, if indicated, to carry out acute hemostasis.
Suggested Readings
Barkun A, Bardou M, Marshall JK. Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003;139:843.
Elta GH. Approach to the patient with gross gastrointestinal bleeding. In: Yamada T, Alpers DH, Kaplowitz N, etal. eds. Textbook of gastroenterology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2003:698.
Peptic Ulcer Disease
What are the major risk factors for the development of peptic ulcers?
Is dietary adherence to bland meals and milk an accepted treatment of peptic ulcer disease? If not, what should the treatment be?
Discussion
What are the major risk factors for the development of peptic ulcers?
The major risk factors for the development of peptic ulcer disease are cigarette smoking, the ingestion of nonsteroidal antiinflammatory drugs (NSAIDs), and a family history of peptic ulcer. Peptic ulcers are thought to form
P.181
when the effects of gastric acid and pepsin overwhelm the protective mucosal barrier. Diseases such as the Zollinger-Ellison syndrome increase the secretion of gastric acid. Other factors promote the breakdown of the mucosal barrier.Ulcers are twice as likely to develop in cigarette smokers than in nonsmokers. In addition, ulcers heal more slowly and are more likely to recur in smokers. The mechanism responsible for cigarette smoke's ulcerogenic effect is not completely understood. NSAIDs disrupt the mucus bicarbonate barrier, allowing acid to damage the underlying mucosa. The GI complications of NSAIDs are a major cause of upper GI bleeding and perforation, particularly in elderly women, and are responsible for a two- to threefold increased mortality risk in long-term users of NSAIDs. The combined use of NSAIDs and corticosteroids appears to increase the risk even further.
People who have first-degree relatives with peptic ulcers have three times the risk of acquiring ulcers compared with the general population. The risk is even higher for the identical twin of a patient with ulcer disease.
Infection of the gastric mucosa by Helicobacter pylori is strongly associated with lower rates of duodenal ulcer healing and with higher rates of ulcer recurrence. The exact manner in which H. pylori infection promotes ulcers is not known.
No conclusive evidence links dietary substances, including ethanol, caffeine, and spicy foods, with the development of peptic ulcers. Similarly, although a critically ill hospitalized patient may have stress ulcers, environmental stressors at home or at work have not been conclusively linked with the development of peptic ulcers.
Is dietary adherence to bland meals and milk an accepted treatment of peptic ulcer disease? If not, what should the treatment be?
No. Before the advent of modern pharmacologic therapy, the treatment of ulcer disease with frequent bland meals and milk was widely accepted. Unfortunately, such treatment actually increases the production of gastric acid and does not accelerate ulcer healing.
H2 receptor antagonists, of which cimetidine was the first agent released for use, are widely accepted as safe and effective for the treatment of peptic ulcers. These agents directly inhibit histamine-stimulated gastric acid secretion and indirectly inhibit the histamine-potentiated, gastrin-stimulated acid secretion. When given in sufficient doses, the various H2 receptor antagonists act equally well, with duodenal ulcer healing rates of 75% after 4 weeks, and 85% to 95% after 8 weeks of therapy. The selection of a particular agent should be determined by the patient's ability to comply with the dosing regimen, as well as the cost per dose.
Proton pump inhibitors, such as omeprazole, and esomeprazole, are concentrated in the highly acidic environment of the parietal cell secretory canaliculi. When activated by protonation, these agents covalently bind to H+/K+ AT-Pase, thereby causing irreversible inhibition of the enzyme and a 90% to 99% suppression of gastric acid production within 24 hours. At doses of 20 to 40 mg per day, omeprazole achieves more rapid pain relief
P.182
and faster healing of peptic ulcers than do standard doses of H2 receptor antagonists. Proton pump inhibitors are the treatment of choice for patients with nonsurgically correctable Zollinger-Ellison syndrome. These agents have displayed an excellent short-term safety profile, and, with increasing use, their long-term risk seems less than initially feared.Sucralfate is an aluminum salt of sulfated sucrose. When placed in an acidic environment, it binds tenaciously to ulcers and promotes healing. It has no effect on acid secretion and has minimal acid-neutralizing effects. The entire mechanism of sucralfate's beneficial actions has not been determined. Sucralfate appears to be as effective as H2 receptor antagonists in promoting the healing of acute peptic ulcers. Its systemic absorption is minimal, although its long-term effects on aluminum deposition are unknown. Its primary side effect is dose-related constipation.
Antacids are also effective in promoting the healing of gastric and duodenal ulcers. Frequent dosing is usually required to achieve effectiveness equal to that of H2 receptor antagonists. Such a dosing schedule often results in poor patient compliance, not to mention the side effect of diarrhea associated with the use of magnesium-containing antacids.
There is no evidence to support the use of these agents in various combinations for the primary treatment of peptic ulcers. Combination therapy with antibiotics, acid-suppressive medications, and bismuth compounds is effective in healing duodenal ulcers associated with H. pylori infection, and in preventing the recurrence of such ulcers.
Case
A 50-year-old man has had recurrent and at times severe epigastric abdominal pain for the last several years. Antacids have given him symptomatic relief. The most recent episode began 1 week ago and has not responded completely to antacids. The pain now wakes him up at night. He smokes one pack of cigarettes per day, and he takes aspirin several times a week. His family history is unremarkable. Physical examination reveals moderate epigastric tenderness without evidence of a mass. The stool is brown and positive for occult blood.
What are this man's risk factors for peptic ulcer disease?
What diagnostic tests should you consider?
When would you consider treatment for H. pylori?
Case Discussion
What are this man's risk factors for peptic ulcer disease?
His smoking and NSAID ingestion are both risk factors for peptic ulcer disease.
What diagnostic tests should you consider?
If the patient were younger than 40 years, had only mild and intermittent symptoms, and had no evidence of systemic disease or risk factors for malignancy, a trial of empiric anti-ulcer therapy without prior diagnostic tests would be acceptable. Otherwise, either EGD or a double-contrast upper GI radiographic series is recommended. When there is a possibility of malignancy and if biopsy specimens
P.183
are needed, EGD is considered superior to radiography for the purpose of diagnosis. Because the man described is older than 40 years, smokes cigarettes, has occult blood in the stool, and is having increasingly severe pain, a diagnostic workup (preferably EGD) rather than empiric therapy is recommended.When would you consider treatment for H. pylori?
Eradication of H. pylori is usually advocated when associated with duodenal ulcer, and results in a dramatic reduction in ulcer recurrence. Infection can be demonstrated by endoscopic biopsy, serology, or radioisotope breath test findings. A multiple-drug regimen is required for reliable eradication of the organisms. A commonly used combination has been that of a bismuth-containing compound, tetracycline, metronidazole, and either a proton pump inhibitor or H2 receptor antagonist. Better patient compliance and equal efficacy have been reported with combinations of clarithromycin, amoxicillin, bismuth, and a proton pump inhibitor or H2 receptor antagonist.
Suggested Readings
Del Valle J, Chey WD, Scheiman JM. Acid peptic disorders. In: Yamada T, Alpers DH, Kaplowitz N, etal. eds. Textbook of gastroenterology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2003: 1321.
Spechler SJ. Peptic ulcer and its complications. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management, 7th ed. Philadelphia: WB Saunders, 2002:747.
Gallstone Disease
Which group of people has the highest known prevalence of gallstones?
What are the different types of gallstones, and how do they form?
Should all patients with gallstones undergo cholecystectomy?
What are the common symptoms of gallstone disease, and what percentage of patients with asymptomatic gallstones eventually exhibits symptoms?
What is the best imaging technique to demonstrate cholelithiasis?
What treatment of symptomatic cholelithiasis is the standard against which other treatments are compared?
Discussion
Which group of people has the highest known prevalence of gallstones?
Examination of autopsy findings have revealed that the highest known prevalence of gallstones is in the North American Pima Indians: approximately 60% for women and 25% for men. The population of Thailand has one of the lowest known prevalences: approximately 5% for women and 3% for men. The prevalence rate for whites in the United States and in north-central Europe is approximately 30% for women and 15% for men.
P.184
The composition of gallstones varies widely from population to population. The white population of the United States tends to have gallstones consisting largely of cholesterol, whereas the Asian population tends to have brown, calcium bilirubinate stones.
The widespread variation in gallstone prevalence rates, the variation in gallstone composition among ethnic populations, and the general female-to-male ratio of approximately 2:1 all strongly implicate both hereditary and environmental factors in the etiology of gallstone disease.
What are the different types of gallstones, and how do they form?
There are three types of gallstones: cholesterol, brown pigment, and black pigment. Cholesterol gallstones are composed primarily of cholesterol monohydrate crystals mixed with mucin glycoprotein. Brown pigment gallstones, which are associated with bacterial infection of the biliary tree and usually form in the bile ducts, are composed primarily of calcium bilirubinate. Black pigment gallstones form in the gall bladder and are associated with chronic hemolysis, advancing age, long-term parenteral nutrition and cirrhosis; these stones are composed primarily of an insoluble bilirubin pigment polymer.
The formation of gallstones depends on the interplay of three factors: the production of lithogenic bile, gallbladder motility, and the nucleation of gallstones. Conditions that foster increased biliary cholesterol secretion, such as obesity, reduced bile acid secretion (as in terminal ileal Crohn's disease), and increased bilirubin production (as in sickle cell hemoglobinopathy), may all cause the production of lithogenic bile. Biliary stasis, such as that associated with prolonged total parenteral nutrition, also promotes gallstone formation. Decreased biliary immunoglobulin A (IgA) secretion, such as that found in many Asians, may allow the growth of bacteria that produce -glucuronidase; the resulting hydrolysis of conjugated bilirubin promotes the precipitation of calcium bilirubinate. These calcium salts may then form the nuclei for gallstones.
Should all patients with gallstones undergo cholecystectomy?
No. Most patients with gallstones remain asymptomatic, and those who do become symptomatic are not at increased risk of death from either the disease or the surgery. This is also true for patients with concurrent diabetes mellitus. Therefore, prophylactic cholecystectomy is not recommended for most asymptomatic patients, including those with diabetes mellitus. However, prophylactic cholecystectomy for asymptomatic gallstones is recommended for certain groups who face a high risk of morbidity. The risk of gallbladder cancer is high in Native Americans with cholelithiasis. Symptoms develop in nearly all children who have cholelithiasis. The likelihood of complications after emergency cholecystectomy is increased in patients with sickle cell hemoglobinopathy.
Patients with asymptomatic stones in the common bile duct (choledocholithiasis) experience a more morbid course; in 50% of patients with choledocholithiasis found postmortem, these ductal stones contributed to their death. Therefore, such stones should be removed either surgically or by ERCP.
What are the common symptoms of gallstone disease, and what percentage of patients with asymptomatic gallstones eventually exhibit symptoms?
Only 10% to 20% of the people with asymptomatic gallstones eventually exhibit symptoms. The onset of symptoms most commonly consists of recurrent biliary pain due to a stone in the cystic duct. This pain starts usually in the right upper quadrant or epigastrium and may radiate to the back or right shoulder. Biliary pain typically is gradual in onset and lasts several hours. Contrary to common belief, there is no particular temporal relationship to food intake or diet.
Persistent blockage of the cystic duct results in acute inflammation of the gallbladder, or acute cholecystitis. Patients with acute cholecystitis usually experience nausea, vomiting, and fever, and they complain of severe right upper quadrant pain. Elicitation of right upper quadrant abdominal tenderness in combination with leukocytosis is also highly suggestive of acute cholecystitis. The definitive treatment is cholecystectomy.
Obstruction of the common bile duct by a gallstone may result in cholangitis. Charcot's triad of symptoms (fever, chills, and jaundice) is exhibited by only 50% to 75% of patients with acute cholangitis. Most patients respond rapidly to appropriate antibiotic therapy; however, definitive treatment consists of decompression of the bile duct by ERCP, percutaneous drainage, or biliary surgery.
Acute biliary pancreatitis may result from a common bile duct stone that is transiently blocking the pancreatic duct within the ampulla of Vater. Urgent ERCP with endoscopic sphincterotomy should be considered for these patients.
What is the best imaging technique to demonstrate cholelithiasis?
Ultrasonography is the best initial method for demonstrating cholelithiasis because it has 90% to 95% sensitivity and 95% specificity. Also, it is noninvasive and unencumbered by many technical limitations. Its main utility is in the demonstration of gallstones, although it is capable of detecting some additional findings, such as pericholecystic fluid, thickening of the gallbladder wall, and distention of the gallbladder, which are evidence of active inflammation. Ultrasonography can often reveal ductal dilatation or common bile duct stones, but failure to do so does not rule out choledocholithiasis.
Before ultrasonography became available, oral cholecystography was the test of choice for identifying gallstones. However, this procedure takes several hours to perform, is not effective when the bilirubin level exceeds 2.0 mg/dL, and can be made unreliable by vomiting and diarrhea. This technique may be useful if there is a strong clinical suspicion of cholelithiasis but the ultrasonographic findings are equivocal.
Hepatobiliary scintigraphy is primarily used to assist in establishing the diagnosis of acute cholecystitis. If the radioactive tracer does not image the gallbladder, obstruction of the cystic duct is highly likely; however, this technique cannot identify stones within the gallbladder.
P.186
ERCP is the best technique for diagnosing common bile duct stones, but it is far less sensitive in detecting stones in the gallbladder than ultrasonography or oral cholecystography. Magnetic resonance cholangiopancreatography (MRCP) is increasingly recognized as an accurate, noninvasive means of visualizing the bile ducts and pancreatic ducts.
In general, CT scanning visualizes gallstones poorly and adds little information in the overall effort to diagnose gallstone disease.
What treatment for symptomatic cholelithiasis is the standard against which other treatments are compared?
Open cholecystectomy has been the standard treatment of symptomatic gallstone disease. In a patient younger than 50 years who is free of complicating factors, the mortality rate associated with elective open cholecystectomy is less than 1%. The patient is usually hospitalized for approximately 5 days and remains on medical disability leave for an additional 4 to 6 weeks.
Increasingly, laparoscopic cholecystectomy has become the preferred treatment of symptomatic gallstone disease in most patients. Although this method has a slightly higher rate of common bile duct injury, patients who undergo this usually require a shorter hospital stay and less time off from work than those who undergo open cholecystectomy.
The dissolution of gallstones through the oral administration of bile acids, such as ursodeoxycholic acid (ursodiol), is reserved for those patients who are either unable or unwilling to undergo surgery. This therapy is most effective for those 15% of patients with cholelithiasis who have small cholesterol gallstones floating in a functional gallbladder. After the 6 to 12 months of therapy is completed, the recurrence rate of gallstones is approximately 50% at 5 years.
P.185
Case
A 54-year-old Hispanic woman presents to the emergency room complaining of constant, severe right upper quadrant pain radiating to her right scapula that has lasted for approximately 6 hours. She has vomited twice without relief of the pain. She experienced two similar, but less severe, episodes of such pain several weeks ago, for which she did not seek medical care. She does not have any chronic illness. Examination reveals a moderately obese woman with a temperature of 38 C (100.4 F). Her sclerae are slightly icteric. She exhibits abdominal guarding, with moderate right upper quadrant tenderness on palpation, halting of inspiration during palpation, and normal bowel sounds. The white blood cell count is 14,000 cells/mm3, and the alkaline phosphatase level is elevated at 200 IU/L. The total bilirubin level is 4 mg/dL. The serum aminotransferase values are normal.
What is the most likely diagnosis in this patient?
What imaging study should be performed?
How should you manage this patient's condition?
P.187
Case Discussion
What is the most likely diagnosis in this patient?
The most likely diagnosis is acute cholecystitis associated with choledocholithiasis and obstruction of the common bile duct by a gallstone. This woman's previous symptoms, which are consistent with recurrent biliary pain, suggest gallstone disease. The more severe symptoms she now has, which are associated with a leukocytosis, right upper quadrant abdominal tenderness and inspiratory arrest with palpation in the right upper quadrant (Murphy's sign), suggest acute cholecystitis. The elevated alkaline phosphatase and total bilirubin levels are evidence that the common bile duct is obstructed. (The total bilirubin level rarely rises above 3 mg/dL in cholecystitis alone.)
What imaging study should be performed?
Abdominal ultrasonography should be performed routinely in patients suspected of having gallstone disease. In this patient, the typical presentation, which points toward acute cholecystitis and cholelithiasis, makes additional imaging studies unnecessary. If the ultrasonogram fails to demonstrate stones, a hepatobiliary scintigram could assist in making the diagnosis.
How should you manage this patient's condition?
Initial management should consist of the IV administration of fluids and antibiotic coverage for gram-negative organisms, together with nasogastric suction. Cholecystectomy should be performed soon after the patient's condition has stabilized; a delay in surgery is associated with higher morbidity rates. If open cholecystectomy is performed, an exploration of the common bile duct should be strongly considered. If the laparoscopic method is chosen, preoperative ERCP should be performed to remove the stone in the common bile duct. If the patient's condition does not improve rapidly and she still has obstructive jaundice, an urgent ERCP should be performed to decompress the biliary system.
Suggested Readings
Lee SP, Ko CW. Gallstones. In: Yamada T, Alpers DH, Kaplowitz N, etal. eds. Textbook of gastroenterology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2003: 2177.
Pomposelli J, Jenkins RL. Surgical approaches to diseases of the biliary system. In: Schiff ER, Sorrell MF, Maddrey WC, eds. Schiff's diseases of the liver, 9th ed. Philadelphia: Lippincott Williams & Wilkins, 2003:713.
Acute Hepatocellular Disease
What are the major signs and symptoms of acute hepatocellular injury, and which are specific to a particular process?
At what serum bilirubin level is jaundice detectable, and what are the main determinants of the serum bilirubin concentration?
What are the four general causes of acute liver injury?
What are the features of viral hepatitis A, B, C, D, and E?
Match the following serologic results with the most likely clinical state.
a. HBsAg-, anti-HBs+, anti-HBc+ 1. Acute hepatitis B b. HBsAg-, anti-HBs+, anti-HBc- 2. Acute hepatitis A c. HBsAg+, IgM anti-HBc+ 3. Prior HBV infection, now immune d. HBsAg+, IgM anti-HBc- 4. Prior HAV infection, now immune e. Anti-HAV(total)+, IgM anti-HAV- 5. Hepatitis B chronic carrier f. Anti-HAV(total)+, IgM anti-HAV+ 6. Received hepatitis B vaccine HBsAg, hepatitis B surface antigen; anti-HBs, antibody to hepatitis B surface antigen; anti-HBc, antibody to hepatitis B core antigen; anti-HAV, antibodies to hepatitis A antigens [total and immunoglobulin M (IgM) class];-, negative; +, positive; HBV, hepatitis B virus; HAV, hepatitis A virus.
Which hepatic enzyme pattern suggests the presence of alcoholic liver disease?
What are the clinical and laboratory findings characteristic of ischemic liver injury?
What is fulminant hepatic failure?
P.188
Discussion
What are the major signs and symptoms of acute hepatocellular injury, and which are specific to a particular process?
The typical symptoms of acute hepatitis include malaise, fatigue, anorexia, nausea, dark urine, abdominal pain, headache, fever, myalgia, and arthralgia. Signs include jaundice, scleral icterus, hepatomegaly, tender liver, splenomegaly, and rash. In general, these features are nonspecific and do not help in identifying the cause of liver injury.
At what serum bilirubin level is jaundice detectable, and what are the main determinants of the serum bilirubin concentration?
A serum bilirubin level in the range of 2.5 to 3.0 mg/dL usually produces detectable scleral icterus. The serum bilirubin concentration is determined by the rates of bilirubin production (resulting from the catabolism of hemoglobin and other heme-containing enzymes) and elimination (including excretion into bile and the renal excretion of conjugated bilirubin). As a result, hemolysis and changes in renal function can considerably alter the serum bilirubin concentration.
What are the four general causes of acute liver injury?
Exposure to toxins is a common cause of acute liver injury. Such toxins include ethanol, acetaminophen, halogenated hydrocarbons, and the toxin from
P.189
the mushroom Amanita phalloides. Infections can also cause acute liver injury. The most common infections are those caused by hepatitis viruses A, B, C, D, and E, but parasites, bacteria, and fungi also can cause infectious hepatitis. Hepatic injury can also stem from ischemia; this is usually a result of severe systemic hypotension or congestive heart failure. Other sources of acute liver injury are the various metabolic disorders such as Wilson's disease and Reye's syndrome.What are the features of viral hepatitis A, B, C, D, and E?
Hepatitis A is caused by an RNA enterovirus that is usually transmitted by fecal oral contamination. The hepatitis A virus is present in the stool for approximately 2 weeks after infection, but symptoms do not appear until approximately 4 weeks after infection. This period of asymptomatic infectivity is partially responsible for the occasional outbreaks of hepatitis A spread by an unsuspecting food handler at a restaurant, or by children at a day-care center. Symptoms usually consist of nausea, vomiting, jaundice, and malaise, although the entire course of the disease may be subclinical, especially in children. Progression to fulminant hepatic failure is very rare, and full recovery is expected after 3 weeks of symptoms. The best serologic test for confirming acute viral hepatitis A is the IgM anti-HAV determination, which should be positive at the onset of symptoms. The presence of IgG anti-HAV implies that the person had hepatitis A in the past and is immune. Susceptible people should be passively immunized with human immune serum globulin within 2 weeks of exposure to the hepatitis A virus. Active prophylaxis against hepatitis A for certain high-risk populations and patients with chronic liver disease is available in the form of hepatitis A vaccine.
Hepatitis B is caused by a DNA virus that is transmitted by parenteral exposure to infected blood, usually through skin punctures by contaminated needles. Because this virus can also be transmitted through minute breaks in mucous membranes, risk factors for hepatitis B infection include sexual contact and the sharing of razors and toothbrushes with an infected person; transmission at birth from mother to child is also common. The hepatitis B virus is present in the blood approximately 2 months after infection, with symptoms appearing at approximately 3 months. IgM anti-HBc appears early in the disease and its measurement is the best single serologic test to confirm acute viral hepatitis B. The clinical course may progress to fulminant hepatic failure and death in up to 2% of the patients, or the infection may smolder in a chronic carrier state in up to 10% of the patients. Chronic carriers are at high risk for early death from cirrhosis or hepatocellular carcinoma and should be considered candidates for treatment with agents aimed at suppressing hepatitis B replication, for example, -interferon, lamivudine, or adefovir. Immunization with vaccine made from recombinant HBsAg is highly effective and confers long-lasting protection against infection.
Hepatitis C is caused by an RNA virus that, like the hepatitis B virus, is believed to be transmitted primarily by parenteral exposure to infected blood, although a substantial percentage of patients have no identifiable risk factors. Symptoms of acute infection are often mild. More than 50% of infected people
P.190
become chronic carriers. Such persons are at high risk for chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Patients with chronic hepatitis C should be considered candidates for treatment with -interferon or the combination of interferon and ribavirin. Serologic tests for hepatitis C continue to be improved as our knowledge of the virus increases. There is no known protective antibody and no known vaccine.Hepatitis D is caused by a defective RNA virus that requires the presence of HBsAg for expression. Hence, infection with the hepatitis D virus occurs only as a coinfection with hepatitis B virus, or as a superinfection in those who are chronic hepatitis B virus carriers. The symptoms of hepatitis D are usually more severe than those seen with acute hepatitis B, with progression to fulminant hepatic failure and death in up to 20% of the patients. The specific serologic test for hepatitis D, anti-HD, should be carried out only if the HBsAg serology is positive. There is no vaccine specific to the hepatitis D virus, although immunization against hepatitis B confers protection against hepatitis D.
Hepatitis E is caused by an RNA virus that, like the hepatitis A virus, is transmitted primarily by fecal oral contamination. Outbreaks of the disease can reach epidemic proportions in areas of the world where flooding and poor sanitation are prevalent. Although symptoms are mild in most patients, hepatitis E has a 20% mortality rate if it is acquired during pregnancy. Specific serologic tests are under development. There is no known vaccine for it.
Match the following serologic results with the most likely clinical state.
The correct pairings of the serologic results with the most likely clinical state are: a with 3, b with 6, c with 1, d with 5, e with 4, and f with 2. HBsAg is present in the settings of acute infection, chronic infection, and the carrier state. Anti-HBs and anti-HBc appear and the HBsAg level declines as the acute infection resolves. IgM anti-HBc or IgM anti-HAV is usually present only during acute infection, whereas the IgG classes of anti-HBc and anti-HAV persist, indicating a state of immunity after resolution of the acute infection. Anti-HBs appears alone, without anti-HBc, in response to hepatitis B vaccine.
Which hepatic enzyme pattern suggests the presence of alcoholic liver disease?
In the setting of alcoholic hepatitis, the serum AST level is usually higher than the serum ALT level. In addition, the serum level of -glutamyltranspeptidase is often elevated because of induction of this enzyme by chronic ethanol ingestion.
What are the clinical and laboratory findings characteristic of ischemic liver injury?
Ischemic liver injury, or shock liver, usually occurs in the setting of a recognized circulatory disturbance, such as hypotension or acute myocardial infarction. A rapid and dramatic rise in the AST and ALT levels is seen, with an equally rapid decline. The aminotransferase levels can rise into the thousands, approaching levels seen with acute viral hepatitis. A slow, steady increase in the serum bilirubin concentration subsequently occurs and peaks several days
P.191
later. A liver biopsy is not needed for diagnosis, but, when specimens are obtained, they show centrilobular necrosis.What is fulminant hepatic failure?
Fulminant hepatic failure is defined as progression to signs of liver failure, including hepatic encephalopathy, within 8 weeks of the onset of symptoms. Such a picture occurring 8 to 24 weeks from the onset of symptoms is considered subfulminant hepatic failure. Fulminant hepatic failure may be caused by viral, toxic, ischemic, or other causes of hepatocellular injury. The mortality rate for these entities is extremely high. Intensive support is indicated in affected patients, and liver transplantation should be considered if spontaneous recovery does not occur.
Case
A 37-year-old housewife reports 3 weeks of general fatigue, several days of dark urine, and 1 day of scleral icterus. She denies vomiting, but complains of mild, continuous pain in the right upper quadrant, and intermittent nausea.
Physical examination reveals the patient to be jaundiced but comfortable. She shows no signs of malnutrition and has no spider angiomas or palmar erythema. The liver is tender and measures 15 cm by percussion in the midclavicular line; it is palpable 4 cm below the costal margin on inspiration. The spleen is not palpable, and the examination findings are otherwise unremarkable.
What is your first diagnostic impression, and why? Match the laboratory findings with the various diagnostic possibilities.
AST (IU/L) ALT (IU/L) Total Bilirubin (mg/dL) Alkaline Phosphatase (IU/L) Diagnosis a. 235 90 5.5 190 1. Acute viral hepatitis b. 1,100 1,320 5.5 190 2. Chronic viral hepatitis c. 235 325 5.5 190 3. Alcoholic hepatitis d. 235 325 10.5 990 4. Bile duct obstruction AST, aspartate aminotransferase; ALT, alanine aminotransferase. What other historical information is needed pertaining to risk factors?
What tests would you order if you suspected acute viral hepatitis?
If the patient has acute hepatitis A or hepatitis B, what should you tell her about the risk to her family, and what is the appropriate follow-up after she recovers?
What if all initial viral hepatitis serology results are nonreactive?
If the patient has a strong family history of liver disease, what tests are available to screen for inherited disorders?
Is a liver biopsy indicated in this patient?
Should this patient be admitted to the hospital?
P.192
Case Discussion
What is your first diagnostic impression, and why? Match the laboratory findings with the various diagnostic possibilities.
The symptoms and examination findings are nonspecific, and the laboratory findings and the various diagnostic possibilities are paired, as follows: a with 3, b with 1, c with 2, and d with 4. Very high aminotransferase levels (>1,000 IU/L) usually indicate an acute hepatocellular injury. Moderately high levels (two to five times normal) can be seen in many situations, such as early or late in the course of an acute injury, or in chronic diseases such as chronic viral hepatitis or alcoholic liver disease. An AST/ALT ratio that exceeds 1 suggests the presence of alcoholic liver disease. Alkaline phosphatase and bilirubin levels that are elevated out of proportion to the aminotransferase concentrations suggest a biliary obstructive process, but these are not specific with regard to the level of obstruction (extrahepatic obstruction versus intrahepatic cholestasis).
What other historical information is needed pertaining to risk factors?
A patient presenting with liver disease should be asked about travel and hepatitis exposure (hepatitis A); parenteral risk factors, including transfusions, IV drug use, sexual contacts, and professional exposure (health care workers hepatitis B and C); medications; environmental exposure; alcohol intake; childhood liver disease; and family history. Although prolonged excessive alcohol intake is often easily recognized, sometimes it is covert.
What tests would you order if you suspected acute viral hepatitis?
The selection of tests should be guided by the nature of the clinical history. The following tests should be done in a person suspected of having acute viral hepatitis: (a) IgM anti-HAV to check for acute hepatitis A; (b) IgM anti-HBc to check for acute hepatitis B; and (c) antibodies to hepatitis C antigens (anti-HCV) to check for acute hepatitis C.
If the patient has acute hepatitis A or hepatitis B, what should you tell her about the risk to her family, and what is the appropriate follow-up after she recovers?
The household and sexual contacts of people with acute hepatitis A should be passively immunized with immune globulin, and they should exercise careful standard precautions to avoid fecal oral transmission. The household contacts of people with acute hepatitis B should avoid parenteral contact (the sharing of razors, toothbrushes, and the like). Sexual contact should be minimized during the acute stage of the illness. After clinical recovery, it is important to determine whether the HBsAg has disappeared and anti-HBs has appeared. Failure to clear HBsAg suggests development of a chronic hepatitis B carrier state. Sexual or household contacts of hepatitis B carriers should be immunized with hepatitis B vaccine.
What if all initial viral hepatitis serology results are nonreactive?
Repeat testing for anti-HCV in 6 months is appropriate because this test may not be positive in the acute setting.
If the patient has a strong family history of liver disease, what tests are available to screen for inherited disorders?
A low serum ceruloplasmin level and a high urinary copper excretion are highly suggestive of Wilson's disease. A high serum ferritin level and high transferrin saturation are highly suggestive of hemochromatosis. The definitive test for both of these disorders is a liver biopsy. Genetic testing for familial hemochromatosis is now available.
Is a liver biopsy indicated in this patient?
Liver biopsy is not usually needed for diagnosis or prognosis in patients with acute liver diseases. Exceptions might include establishing the diagnosis of drug-induced or toxic hepatitis, ischemic liver injury, granulomatous disease, and, rarely, alcoholic hepatitis.
Should this patient be admitted to the hospital?
Most patients with acute hepatitis do not require hospital admission. However, those who exhibit evidence of severe liver injury, such as hepatic encephalopathy, a bilirubin level above 15 mg/dL, or an increasing prothrombin time, and those with severe anorexia or nausea, should be hospitalized.
P.193
Suggested Readings
Berenguer M, Wright TL. Viral hepatitis. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management, 7th ed. Philadelphia: WB Saunders, 2002:1278.
Schiff ER. Viral hepatitis. In: Schiff ER, Sorrell MF, Maddrey WC, eds. Schiff's diseases of the liver, 9th ed. Philadelphia: Lippincott Williams & Wilkins, 2003:741.
EAN: 2147483647
Pages: 14
- Business Continuity Planning and Disaster Recovery Planning
- Initiation of the System Authorization Process
- Appendix B Glossary of Terms and Acronyms
- Appendix D The Information System Security Engineering Professional (ISSEP) Certification
- Appendix E The Information System Security Management Professional (ISSMP) Certification