V - Head and Neck
Editors: Mills, Stacey E.
Title: Histology for Pathologists, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > V - Head and Neck > 17 - Major Salivary Glands
function show_scrollbar() {}
17
Major Salivary Glands
Fernando Mart nez-Madrigal
Jaques Bosq
Odile Casiraghi
Introduction
The primary function of the salivary glands is to moisten the mucous membranes of the upper aerodigestive tract. In humans, this function is fulfilled by the continuous exocrine secretion of numerous minor salivary glands. These glands are located in the submucosa throughout the oral cavity, pharynx, and upper airways. In developed species, most of the saliva is elaborated by three pairs of major glands, or salivary glands, named by their location: the parotid, the submaxillary or submandibular, and the sublingual glands. They are connected symmetrically to the oral cavity, where they empty their secretion only under specific stimuli. The saliva produced by these glands (750 1000 mL/24 hours) plays an important role in preparing food for digestion, as well as in controlling the bacterial flora of the mouth. The quality of the saliva produced by the major glands is variable and depends on both the stimuli and the predominant participating gland.
Embryologic and Postnatal Developmental Changes
Parotid Gland
During embryologic life, the parotid is the first of the three major glands to appear and is seen by the sixth week. It derives from the ectoderm as an epithelial bud from the primitive oral epithelium, at the angle between the maxillary
P.446
process and the mandibular arch (1). As the primordia grow, they ramify into a bushlike system surrounded by mesenchymal tissue. This mesenchyma and, particularly, the basal lamina play an important role in the lobular organization of the gland (2,3,4,5), and in vascular and neural development. By the seventh week, the primitive gland moves in a dorsal and lateral direction and reaches the preauricular region. Development of the facial nerve divides the gland by approximately the tenth week into superficial and deep portions (6).
By the third month, the gland has attained its general pattern of organization. The epithelial structures are arranged in lobules, limited by a capsule of loose connective tissue (Figure 17.1A). The mesenchyma is then colonized by numerous lymphocytes that are later disposed in intraglandular and extraglandular lymph nodes. By the sixth month, the epithelial cordons are canalized and exhibit a double-cell ciliated cover. Cell differentiation begins in the excretory ducts with the progressive transformation of ciliated cells by columnar, squamous, and goblet cells (Figure 17.1B) (7). Intralobular duct and acinar differentiation, including myoepithelial cell formation, begins about the eighth month (8) and myoepithelial cell differentation by the nineteenth- to twenty-fourth week period. These cells, arranged in the basal portions of the acini and intercalated ducts, appear as clear cells with electron microscopy. Between 25 and 32 weeks, the myoepithelial cells become flattened and show cytoplasmal prolongations (9,10,11). Saliva production starts as a mucinous liquid at this time; but several studies in rodents suggest that full maturation is completed only after birth (12,13,14,15).
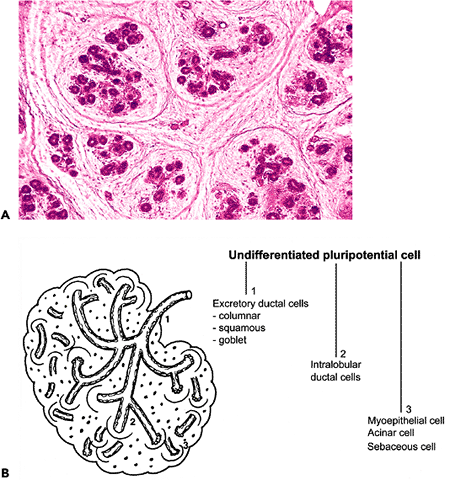 |
Figure 17.1 Development and histogenesis of the epithelial constituents of the salivary gland. A. Fetal parotid gland at 4 months; a lobular architecture is present. B. Drawing of (A), showing the cellular lines of differentiation for the excretory ducts, (1); excretory ducts, (2); intralobular ducts, (3); and acini. |
The definitive location of the parotid is behind the inferior facial nerve maxillary branch, below and in front of the external ear. It is enclosed within a fibroadipose capsule in a depression whose anterior limit is the masseter muscle. Its superior limit is the zygomatic arch; the posterior limit is the tragus, and the inferior limit is the anterior border of the sternocleidomastoid muscle (16). The adult parotid is the largest of the three major salivary glands and weighs between 14 and 28 g. The gland is surrounded by a fine capsule and is divided into two portions by the facial nerve. The main portion, or superficial lobe, is flattened and quadrilateral; it is here that the majority of salivary tumors develop. This observation has permitted the development of conservative surgical treatment of many parotid tumors. The rest of the gland, called the deep lobe, is irregularly wedge-shaped in anatomic relationship with the parapharyngeal space (17).
P.447
The surgical anatomic area where the parotid gland is located, is called the parotid region. In this region it is important to keep in mind the anatomical relations between the facial nerve, the gland, and the subcutaneous planes. The facial nerve has four parts, designated as retro-, inter-, intra-, and preglandular. The parotid gland is covered by a superficial musculoaponeurosis and the skin (18). Accessory parotid tissue is found in approximately 20% of cases. Nevertheless, in a recent study, the incidence was found to be 56% with no differences between right and left sides or between sexes (19). This accessory tissue may be found on the anterior surface of the gland, as well as along the parotid duct (20).
Like all the exocrine glands, the parotid is composed of numerous tubuloacinar units connected through the excretory ducts to a main duct (Stensen's duct) located in the anterior portion of the gland. The parotid duct follows a twisted course of 7 cm, crossing the masseter muscle, the corpus adiposum of the cheek, and the buccinator muscle before opening into the oral vestibule. The secretion of the accessory parotid is emptied by an independent duct reaching the parotid duct in the masseter portion.
Blood supply is by arterial branches from the external carotid. The veins are tributaries of the external jugular, and the lymphatics join the superficial and deep cervical lymph nodes. Innervation is derived from the sympathetic and auriculotemporal nerves.
Submaxillary Gland
In the submaxillary gland, the primordia appear at the end of the sixth week and, unlike the parotid, are probably of endodermal derivation (21,22). However, a recent study suggests that the sublingual process of the submandibular gland originates from a lateral ectodermal bud of the anlage of the submandibular gland (23). The epithelial bud appears in the groove between the lower jaw and tongue, at one side of the midplane. Extension of the glandular tissue in the mesenchyma goes backward beneath the lower jaw. Subsequent maturation and cell differentiation are similar to those of the parotid gland except for the lymphoid tissue, which is less obvious than in the parotid. In addition, there is no lymph node formation inside the gland. Recent studies have demonstrated that the extracellular matrix protein fibronectin is essential for cleft formation during the initiation of epithelial branching in submaxillary gland. It has also been demonstrated that inhibition of fibronectin blocks cleft formation and branching (24,25). This mechanism could be applied for other salivary glands and other exocrine glands (such as the mammary glands).
The submaxillary gland is finely encapsulated; it lies inside the submandibular triangle, an osteofibrous cavity from which it takes the form of a triangular prism. This gland weighs approximately 7 to 8 g and, like the other major salivary glands, is organized in lobules connected to a main excretory duct the submaxillary duct (Wharton's duct) which measures 5 cm in length and 2 to 3 mm in diameter. The duct originates near the surface; runs between the mylohyoideus, the hyoglossus, and genioglossus muscles; and finally opens through a narrow orifice in a small papilla called the caruncula sublingualis on each side of the frenulum linguae. A submandibular gland having three ducts that open separately into the oral cavity has also been reported (26). The blood supply is from branches of the facial and sublingual arteries. The secretomotor nerves are fibers from the cranial parasympathetic branch of the facial nerve; the vasomotor nerves are derived from the superior cervical ganglion. The lymph nodes are arranged in a row in the spaces between the mandible and the gland and are disposed in anterior, medial, and posterior groups (16,17).
Sublingual Gland
The sublingual glands are the last of the three major salivary glands to appear. Their primordia are located immediately lateral to the submaxillary glands for the greater sublingual glands and in the linguogingival sulcus for the lesser sublingual glands. The epithelial buds grow downward from the groove between the lower jaw and the tongue. Parenchymal organization and differentiation are similar to those of the submaxillary gland and are also probably of endodermal derivation.
The principal sublingual gland weighs 3 g. It lies in the sublingual fossa of the mandible and is surrounded by loose connective tissue. Its secretion is drained through a main duct called the Bartholin's duct, which opens into the submandibular duct, and various small ducts (Rivinus' ducts) that open separately into the mouth in the plica sublingualis or join the submandibular duct (16,17).
Vascular supplies come from the sublingual and submental arteries; the veins are tributaries of the external jugular. Innervation is similar to that of the submaxillary gland.
Apoptosis
In the literature, studies concerning apoptosis in salivary glands are not very frequent. Contrary to other glandular tissue, programmed cell death in the salivary glands has been sporadically studied in less-developed and developed species, using experimental animals such as rats or monkeys and, less frequently, humans (27,28,29).
In normal salivary glands, apoptosis was uncommon when observed in acinar cells and epithelial duct cells and never observed in myoepithelial cells. In specific pathologic contexts, such as duct obstruction or irradiation of the salivary glands, programmed cell death was observed with
P.448
many significant changes in the structure of the glands (30,31). In some salivary gland diseases, such as Sj gren's syndrome, accelerated apoptosis may explain acinar and ductal cell loss with the typical functional loss of the secretory activity seen in patients with this disease.
In experimental models, apoptosis is regulated by two kinds of protein families, bcl-2-related proteins and the inhibitor of apoptosis proteins (IAP). Activation of caspase-3 is inhibited by bcl-2 or Bcl-XL and accelerated by Bax. In addition, an X chromosome linked inhibitor of apoptosis called XIAP inhibits caspase-3 activation. Several gene products induce apoptosis in salivary gland diseases (32,33,34). A particular focus has been put on the expression of Fas (CD95) and its ligand FasL (CD95L), which are members of the TNF (tumor necrosis factor) receptors superfamily. In Sj gren syndrome, contradictory results have been found so the importance of apoptosis in epithelial cell loss in these patients remains speculative (35,36,37).
Duct obstruction due to calculi or strictures induces salivary gland atrophy. Histologically, the disappearance of acinar cells within the presence of groups of ductlike structures in a fibrous and inflammatory stroma was observed. The acinar cells had disappeared by apoptosis; this acinar cell death was observed a few hours after duct obstruction. In the first cell death, a few days after duct obstruction, the number of intraepithelial macrophages located in the acinar and duct epithelium of a normal salivary gland multiplied. These macrophages removed the dying cells and apoptotic cellular fragments by phagocytosis, and then they migrated to the interstitium. Simultaneous proliferation of duct epithelial cells by an increase in mitosis contributed to the groups of ductlike structures with some features of squamous metaplasia. Myoepithelial cells became more prominent at the periphery of residual ducts. According to many experimental studies, myoepithelial cells become more prominent as the epithelial cell loss increases, and no myoepithelial cell apoptosis has been observed (38,39,40,41,42,43,44,45).
Irradiation of the major salivary glands is unavoidable during radiotherapy for many head and neck cancers. In fact, radiotherapy induced dryness of the oral cavity, contributing to deterioration of oral mucosa and loss of teeth.
The death of salivary gland acinar cells by apoptosis was observed within 24 hours after irradiation. This mode of cell death occurs with relatively low doses of radiation. An acute inflammatory response located among the destroyed acini was observed.
In normal adult salivary glands, cell division is infrequent in the acini. Development and replenishment of acini destroyed by low doses of radiation comes from stem cells that are located in the terminal intralobular ducts. It is possible that radiation-induced apoptosis of acinar cells could be a stimulus for replication of duct stem cells. However, the death of the ductal stem cells by high-dose radiation did not induce complete regeneration of the destroyed acini and resulted in atrophy of the salivary glands (46).
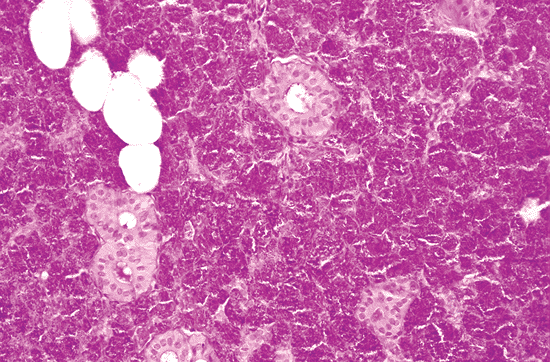 |
Figure 17.2 Serous-type acini of a parotid gland, with dense secretory granules. |
Light Microscopy
The salivary glands are compound exocrine tubuloacinar glands characterized by the aggregation of numerous secretory units. These units consist of acini, where secretion is produced, and a duct system that carries the secretion to the oral cavity and regulates the concentration of water and electrolytes. There are three types of salivary secretory units: the serous ones that contain amylase; the mucous ones that secrete sialomucins; and mixed units made up of mucous and serous cells. According to the predominance of these types of secretory units, the salivary glands may be classified into three categories: serous, mucous, and mixed glands.
With the exception of some mucous units, the parotid gland is of the serous type (Figure 17.2). The submaxillary and the sublingual glands are mixed, with a predominance of serous units in the submaxillary glands (Figure 17.3) and
P.449
a mucous predominance in the sublingual glands (Figure 17.4). Mixed secretory units can be found in accessory parotid glands (19).
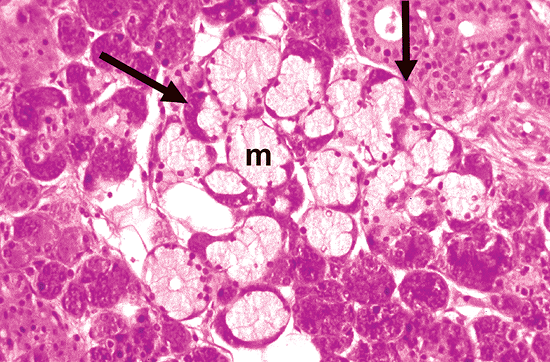 |
Figure 17.3 Histologic section of a submaxillary gland. In mixed units (arrows), serous cells are grouped in a crescent-shaped formation on the periphery of the acini, whereas the mucous cells (m) are in direct contact with the duct system. |
 |
Figure 17.4 Mucous-type acini of the sublingual gland; they are larger and more irregular than the serous and mixed types. Note an inconspicuous duct system. |
The lobular architecture of the glands is well defined by the anastomosed connective tissue trabeculae carrying the vascular and neural branches, as well as by the excretory ducts.
Secretory Units
Acini
Serous acini consist of pear-shaped groups of epithelial cells surrounded by a distinct basement membrane. The epithelial cells have a basal nucleus and dense cytoplasm packed with basophilic [periodic acid-Schiff (PAS)-positive] zymogen granules. They vary in number, depending on the different phases of the secretory cycle (Figure 17.2) (46). The primary enzyme present in the zymogen granules is amylase, or amylase, which splits starch into smaller water-soluble carbohydrates. However, there are other proteins in these granules, including agglutinin, proline-rich proteins, and histatins (47). Other enzymes, such as nonspecific antibacterial lysozyme, lactoferrin, trypsin- and chymotrypsin-like proteases, lysine endopeptidase, and histidine peptidase, also have been shown in the cytoplasm of acinar cells (47,48,49,50,51). Each acinus has a central lumen, rarely visible by light microscopy, through which the secretion drains into the intercalated ducts. The excretion seems to be promoted by the contraction of myoepithelial cells that lie between the outer surface of the acinus and the basement membrane. (Given the importance of myoepithelial cells in the pathology of salivary glands, a separate section, below, is devoted to their description.)
Mucous acini are larger than the serous type and have an irregular pattern (Figure 17.4). The secretory cells have abundant cytoplasm filled with clear mucous substance. They contain acid sialomucins (Alcian blue and mucicarmine-positive) and neutral sialmucins (PAS-positive) in different concentrations (52). The characteristics of these sialomucins also differ between submandibular and sublingual glands (53).
Mixed acini (Figure 17.3) are typically found in the submaxillary gland. These structures are characterized by the concentration of mucous cells near the intercalated duct and bordered by a crescent-shaped formation of serous cells. In mixed acini, the serous cells are more or less conspicuous, according to the amount of secretion accumulated in the mucous cells.
Ducts
A peculiar duct system transports the saliva from the gland to the oral cavity and modifies its water and electrolyte concentration. The first two segments, the intercalated and the striated ducts, are intralobular (Figure 17.5). They are also known as secretory ducts because of their metabolic activity (54). The other segments are interlobular and are called excretory ducts (54).
The intercalated duct lies directly in contact with the acinus. It is lined with a single layer of cuboidal epithelium and an irregular layer of myoepithelial cells. The epithelial cells show a progressive transformation between the secretory and ductal cells and a strong cytoplasmic activity of lactoferrin and lysozyme (55). The lengths of the intercalated ducts are variable in the three major glands (Figure 17.6). In the parotid gland, because they are relatively long, they are easy to recognize in the histologic sections (Figure 17.5A). In contrast, they are short in the submaxillary gland and hardly visible in the sublingual gland (Figure 17.4). Some authors have found undifferentiated cells on the basal side of the intercalated and striated ducts (56). These cells are positive for cytokeratins 13 and 16 on immunohistochemical staining (57,58).
The striated ducts are obvious in routine sections, particularly in the submaxillary gland, where they are relatively longer (Figures 17.3, 17.6). The epithelial lining is simple columnar. On the basal side, it has characteristic parallel striations caused by the deep cell membrane invaginations and mitochondria (Figure 17.5B). This structure represents a specialized surface on the epithelia involved in the transport of water and electrolytes. The numerous mitochondria are correlated with the strong eosinophilia of the duct. Various enzymes such as adenosine triphosphatase (ATPase), succinyl dehydrogenase, and carbonic anhydrase (59) are present in the cytoplasm of the striated ducts and provide them with a metabolic and energy system capable of concentrating some of the elements present in the saliva.
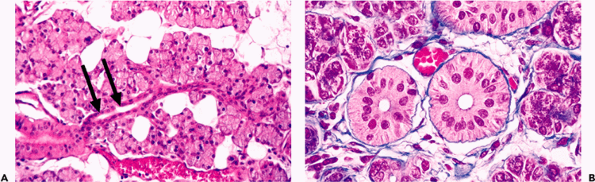 |
Figure 17.5 Parotid intralobular ducts. A. The intercalated ducts (arrows) (sectioned longitudinally) lie in contact with the acinus. B. The striated ducts (sectioned transversely) are lined with a columnar epithelium of basal-striated appearance. |
P.450
The striated ducts are connected with the interlobular ducts located in the septal connective tissue. These ducts are lined with a columnar pseudostratified epithelium with sparse goblet cells (Figure 17.7). They become progressively larger before joining the principal duct. The principal function of interlobular ducts is to transport saliva, but their role in regeneration is proposed by means of the hypothetical undifferentiated pluripotential cells. In theory, these cells may follow the same cellular lines as in embryonic development (Figure 17.1B) and are perhaps implicated in the metaplastic and neoplastic alterations of salivary glands (60), which are more frequent in these ducts.
The principal duct consists of a thick external fibrous coat of collagen (similar to dermal collagen) and elastic fiber bundles. The epithelium is pseudostratified columnar and becomes squamous and stratified near the opening in the mucous membrane (Figure 17.8).
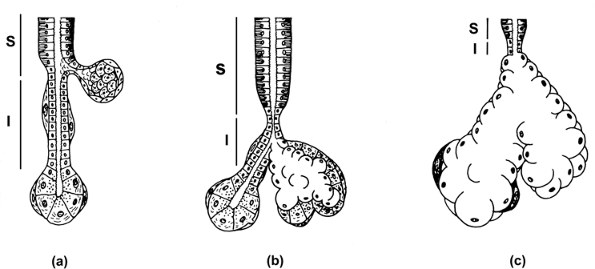 |
Figure 17.6 The morphology of the major salivary glands is characterized by three types of secretory unit. In the parotid (A) the intercalated duct (I) is longer than in the submaxillary (B) and sublingual glands (C). In contrast, the striated duct (S) is longer in the submaxillary gland. Sebaceous glands are more frequent in the parotid gland; in the sublingual gland. the intralobular ducts are inconspicuous. |
Ultrastructure
At the electron microscopy level, the serous cells show all the characteristics of a specialized cell for secretion and export of proteins. The cytoplasm possesses abundant endoplasmic reticulum, Golgi vesicles, mitochondria, lipid droplets, and secretory granules (Figure 17.9). The last are more common on the apical side of the cell. They consist of a membrane, which encloses the secretion, and a matrix of low-electron density and homogeneous aspect in immature granules and high-electron density in mature granules (61,62). The electron density of the granules differs according to the package of the different proteins within the same granule (46). All the cytoplasmic organelles increase during protein synthesis and decrease during discharge (59). The basal surface of the cell shows numerous folds of plasma and basal membranes. They cover the entire cell base and
P.451
extend beyond the lateral margins in the manner of foot processes. Therefore, the basal surface of the cell is greatly expanded, facilitating diffusion of materials into the cell (59). Most of the cytoplasm in mucous cells is occupied by mucous vacuoles and Golgi apparatus. Only a small basal and lateral portion of the cytoplasm contains endoplasmic reticulum and mitochondria (63).
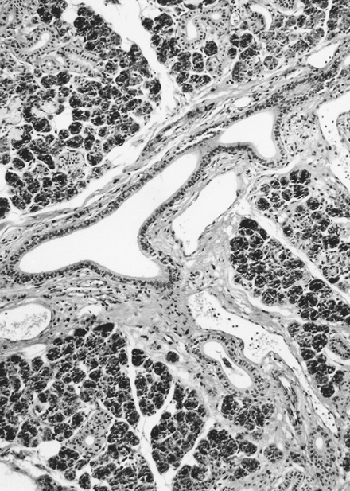 |
Figure 17.7 Interlobular duct adjacent to vessels in the septal connective tissue. |
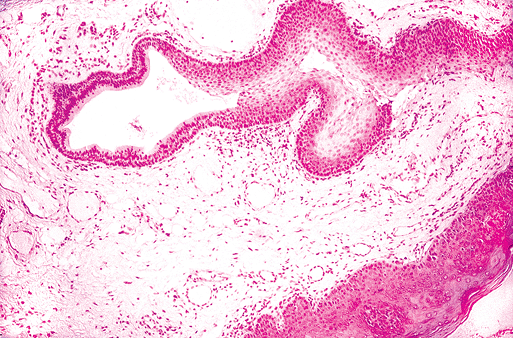 |
Figure 17.8 Main excretory duct in the caruncula sublingualis. Near the oral surface, the duct is lined with a squamous stratified epithelium. |
At the apical pole, the secretory cells are joined by an adhesive zone, whereas the basal side is adhered by desmosomes (62). Between the junctions, virtual spaces form the secretory capillaries, which are in continuation with the acinar lumen. Numerous microvilli protrude into the capillary lumen (Figure 17.9).
The cytoplasm of myoepithelial cells may be separated into two portions (61): one, in contact with the basal lamina, is occupied by myofilaments and pinocytotic vesicles; the other portion is in contact with the secretory cell and contains mitochondria, endoplasmic reticulum, lysosome-like complexes, and Golgi vesicles. The visualization of myofilaments may vary in different myoepithelial cells; some of them have only focal aggregates of myofilaments, while others show a typical basal distribution resembling
P.452
smooth muscle cells (62). The junction between myoepithelial and secretory cells is ensured by desmosomes.
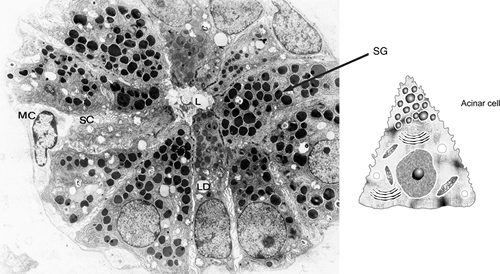 |
Figure 17.9 A. Ultrastructure of a parotid acinus: lumen (L), secretory granules (SG), lipid droplets (LD), secretory capillaries (SC), and myoepithelial cell (MC) ( 6000). B. Drawing of an acinar cell. |
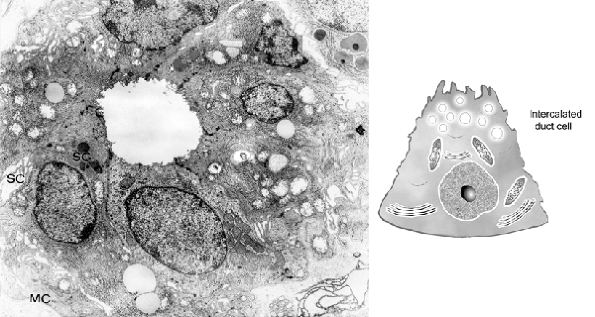 |
Figure 17.10 A. Intercalated duct of parotid gland. Some secretory granules (SG) and secretory capillaries (SC) are present on the apical side. On the external surface, myoepithelial cell prolongations (MC) are found ( 10,300). B. Drawing of an intercalated ductal cell. |
Transition from acinus to intercalated duct is gradual. In the latter, epithelial cells have relatively large nuclei and few cytoplasmic organelles, consisting principally of mitochondria, some cisternae of endoplasmic reticulum, lipid droplets, and a few apical secretory granules (54,58) (Figure 17.10). Scattered secretory capillaries may be found, as well. On the external surface, myoepithelial cell prolongations are usually present.
The striated ducts (Figure 17.11) are composed of tall cylindrical cells with central nuclei. On the basal side, the cell membrane is extensively folded into fingerlike structures. The space between these folds is occupied by vertically oriented mitochondria. This cytoplasmic organization is specialized in the active transport of water and electrolytes from the vascular system to the lumen of the duct; the rest of the cytoplasm contains mitochondria and scattered endoplasmic reticulum (63,64,65).
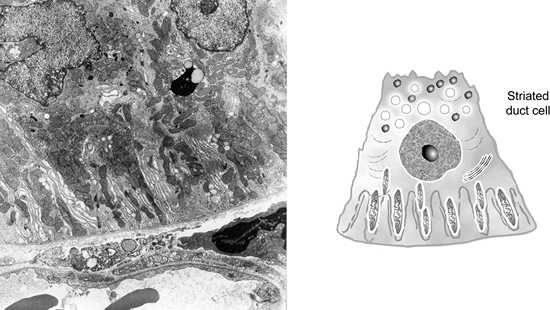 |
Figure 17.11 A. Striated duct cells possess vertically oriented mitochondria and extensively folded plasma membrane; the basal side is in close relationship to the blood capillary ( 7920). B. Drawing of a striated duct cell. |
Sebaceous Glands
In 1931, Hamperl (66) described the presence of sebaceous glands histologically similar to those of the cutaneous adnexa. Other authors have indeed recognized such structures, which appear as isolated cells in the wall of either an intercalated or a striated duct (Figure 17.12A) (67,68,69,70). Larger cell accumulations form a sebaceous gland limited by a well-defined basal membrane (Figure 17.12B C). They vary in size, have a diverticular aspect, and are permanently linked to an interlobular duct. At the periphery of the gland, the cells are flattened with round or oval nuclei. The central cells have an abundant vacuolated cytoplasm rich in lipids, which may be stained, in frozen sections, with fat colorations (Sudan III and IV, oil red O, osmic acid). The nuclei become irregular or pyknotic and finally disappear. When the gland reaches a certain size, the holocrine-type secretion is emptied into the ductal system and is mixed with the saliva (Figure 17.12D).
P.453
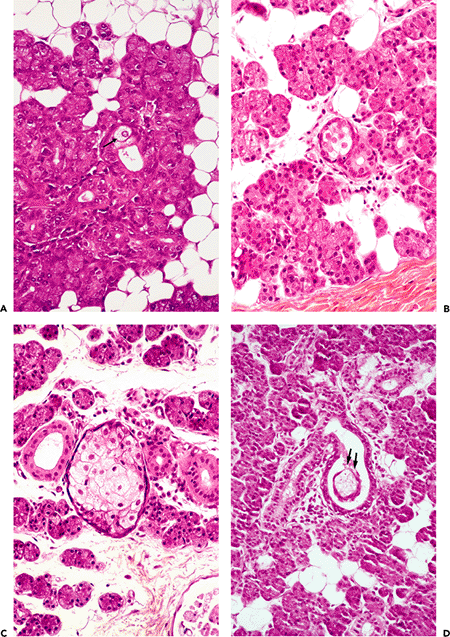 |
Figure 17.12 Sebaceous elements in a parotid gland. A. Isolated sebaceous cells can be seen in the duct wall (arrow). They proliferate to form a well-defined gland (B, C); secretion is present in the lumen of the duct (D) (arrows). E. Drawing illustrates the previous features. |
P.454
The number of sebaceous glands in major salivary glands varies; they are common in the parotid gland, rare in the submaxillary gland, and probably absent in the sublingual gland. They are diffusely scattered throughout the parenchyma, where their numbers also vary greatly. Their presence or absence in the different lobules is not related to either age or sex (70).
In a review of 100 parotid glands selected at random from our material, we found sebaceous glands in 42% of cases. They were also found in 5% of 100 submaxillary glands. These findings are in agreement with other authors. Thus, we conclude that their incidence in the parotid gland is more frequent than imagined. The more sections that are examined, the more sebaceous glands are found; it is, therefore, merely a question of looking for them. If the entire parotid gland were examined meticulously, it would be difficult not to find a sebaceous gland (69).
The presence of sebaceous glands in the salivary tissue has not been satisfactorily explained. A heterotopic phenomenon (67,68) similar to the occurrence of sebaceous glands in the oral mucosa (Fordyce's disease) seems unlikely. In the mouth, this condition may result from aberrant buds along the fetal line of closure (70,71,72); but in the parotid and submaxillary glands, there are no lines of closure. Metaplasia (66,70) beginning in the ducts does not explain the high frequency of sebaceous glands in parotid parenchyma. Therefore, it seems reasonable to consider the sebaceous glands as a normal holocrine differentiation. Their occurrence in the parotid gland appears to be related to a specific function that is not yet understood. Sebaceous glands analogous to those of the salivary glands have been found in the oral mucosa. In the oral mucosa, androgen receptors participate in the histogenesis of sebaceous glands (73,74). A similar mechanism may be proposed for the histogenesis of sebaceous glands in salivary glands.
The belief that a potential for sebaceous differentiation exists in the salivary parenchyma is further supported by the fact that salivary tumors or tumorlike conditions with a sebaceous character or sebaceous gland participation have been described. These rare lesions include sebaceous adenoma, sebaceous lymphadenoma, sebaceous carcinoma, and parotid cyst. They have also been noted in pleomorphic adenoma and mucoepidermoid carcinoma (69,74,75,76,77,78,79,80,81,82,83,84,85,86,87).
Myoepithelial Cells
Myoepithelial cells (basket cells) are derived from early modification and differentiation of primitive pluripotential salivary duct cells by the tenth week of gestation. It has also been suggested that the precursor of myoepithelial cells are the clear cells located in the terminal and striated ducts (63). In regeneration, these cells migrate from the acinar periphery to the duct-acinar region (32).
These cells lie between the epithelial cells and the basal lamina of acini, in the intercalated ducts, and probably also in the union of the striated and intercalated ducts (56,87,88). The cells are flat and have long cytoplasmic processes extending over the epithelial surface (Figure 17.13) in a network that makes it difficult to discern them in routine histologic sections. However, myoepithelial cells may assume morphologic modification at different anatomic locations within the ductal acinar structure (56,64). These cells are best studied by electron microscopy.
The most outstanding characteristic is the presence of cytoplasmic filaments on the basal side. Most of these consist of the myofilaments actin, tropomyosin, and myosin (63,88,89,90), which are arranged in a pattern similar to that of the smooth muscle. Tonofibril-like bundles of intermediate filaments are attached to the cellular junctions, particularly in desmosomes where the filaments are cytokeratin. Some forms of myoepithelial cells may also show scattered, rather than basal, cytoplasmic distribution of these filaments, which may reflect structural and functional
P.455
differences (64). The presence of vimentin is not constant, and the filament desmin is absent in normal myoepithelium (91). The cytoplasm shows a strong activity of ATPase and alkaline phosphatase (92,93).
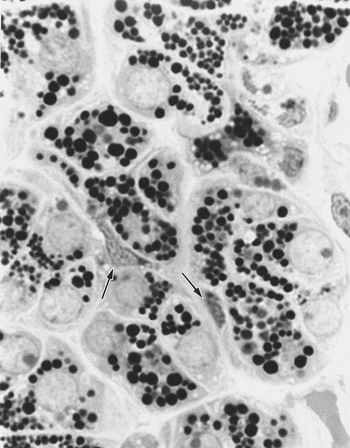 |
Figure 17.13 Myoepithelial cells (arrows) on the acinic surface (semithin section, toluidine blue). |
It is generally accepted that myoepithelial cells are contractile. This function speeds up the outflow of the saliva by increasing the pressure on the excretory unit (94). It has been extensively studied in the mammary gland of the rat, where these cells are more frequently found. Oxytocin-induced contraction in the myoepithelial cells is similar to that of true muscle cells (95). The presence of myofilaments in the cytoplasm of these cells correlates strongly with this function. In addition, myoepithelial cells support the underlying parenchyma and participate in the elaboration of the basal lamina. This last function is important in some hyperplastic and neoplastic alterations, where the myoepithelial cells produce fibronectin, laminin, and type III collagen (96,97,98,99,100,101,102,103). All these proteins are constituents of the basal lamina (104). In addition, myoepithelial cells are also involved in the production of tenascin, an extracellular matrix glycoprotein (105,106).
Regardless of endodermal or ectodermal origin, the myoepithelial cells share an epithelial and mesenchymal structure and function. Altered myoepithelial cells may manifest (in neoplastic proliferation) one or both characteristics. These cells are now considered the key factor in the morphology of many salivary neoplasms and in the morphologic variability of some tumors (106).
The Role of Myoepithelial Cells in Salivary Gland Tumors
The presence of myoepithelial cells in different histologic types of salivary gland tumors (Table 17.1) is well documented, as is the role of these cells in the pathogenesis and in its biologic behavior.
The role of myoepithelial cells in the histogenesis of the pleomorphic adenoma has been extensively studied. This tumor, sometimes called a mixed tumor because of the epithelial and mesenchymal mixture of tissues, is the most frequent neoplasm of the major salivary glands (107,108). It is now accepted that myoepithelial cells play a crucial role in the neoplastic process by expressing both epithelial and mesenchymal structures in the majority of pleomorphic adenomas (109,110,111,112,113,114,115,116,117). The participation of the contractile elements is also accepted in epithelial-myoepithelial carcinoma [clear cell carcinoma, a malignant tumor that mimics the normal structure of the intercalated duct (118,119,120,121,122,123)] and myoepithelioma [in which the myoepithelial cells are the only tumoral element showing different cellular forms (124,125,126,127,128,129)]. They also have been demonstrated in terminal duct adenocarcinoma (polymorphous low-grade adenocarcinoma) (130,131); monomorphic adenoma, which includes basaloid monomorphic adenoma (132,133,134); adenoid cystic carcinoma (96,100,113,135,136,137,138); and basaloid adenocarcinoma (139). Myoepithelial cell participation is also present in congenital tumors of salivary gland origin, such as sialoblastoma (140) and the salivary gland anlage tumor (141). Although there are rare reports of myoepithelial cells in mucoepidermoid carcinoma (135,142), it is difficult to fully accept these statements because mucoepidermoid carcinoma arises from a ductal segment that lacks myoepithelial cells.
Table 17.1 Salivary Gland Tumors with Myoepithelial Cell Participation | ||||||
|---|---|---|---|---|---|---|
|
P.456
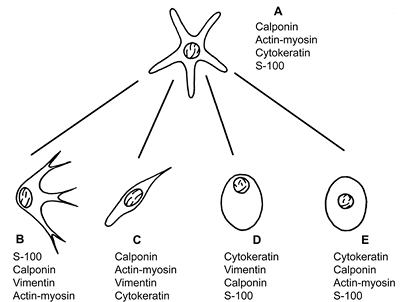 |
Figure 17.14 Morphology and histologic markers of the normal (A) and modified myoepithelial cells (B E). The most constant markers are present in order of frequency: chondromyxoid type (B), spindle-shaped type (C), hyalin or plasmocytoid type (D), and epithelial (clear) type (E). |
The principal morphologic types of altered myoepithelial cells are (Figure 17.14):
Stellate or myxoid cells, which are typically present in chondromyxoid areas of pleomorphic adenoma.
Spindle-shaped or myoid cells, which can be identified in pleomorphic adenoma and some types of myoepithelioma.
Hyalin or plasmocytoid cells (143), which can be seen in pleomorphic adenomas and can be present in myoepitheliomas. These cells show abundant cytoplasmic filaments that give a hyalin eosinophilic aspect.
Clear or epithelial cells, which are found in many salivary tumors on the external surface of ducts or ductlike structures. This is also characteristic in epithelial-myoepithelial carcinoma.
Bidirectional differentiation of the modified myoepithelium has been well documented. Myoepithelial cells with mesenchymal characteristics secrete mesenchymal mucins such as the acid glycosaminoglycans (hyaluronic acid, heparin sulfate, chondroitin-4-sulfate, and chondroitin-6-sulfate) (144,145), basement membrane constituents, elastin (111,112), and tenascin (105). In addition to contractile filaments (actin and myosin) and related proteins such as calponin, vimentin becomes strongly positive, particularly in spindle-shaped form (138,139,140,146,147,148). The S-100 protein, which is a common marker used for the myoepithelium, enhances its positivity in chondroid, myxoid, and stellate cells, particularly if they are associated with a myxoid stroma (104,149,150). Plasmocytoid myoepithelial cells are frequently negative for the muscular markers (151). However, these cells express several ultrastructural and immunomarkers characteristic of myoepithelial cells (127). On the other hand, epithelial differentiation may take the form of clear or squamous cells, which can contain both vimentin and keratin filaments in the same cell (110). Figure 17.14 illustrates the histologic and immunohistochemical patterns of modified myoepithelium.
Myoepithelial cells are a central element in the histologic formation and organization of different salivary gland tumors. The cytomorphologic features and the variability of the extracellular products account for the morphologic heterogeneity of these lesions. The presence of these cells varies widely, from minimal as in monomorphic adenomas (136), to marked, as in myoepitheliomas. Evidence of the protective effect of myoepithelial cells in malignant tumors is growing. Protease inhibitors like maspin, 1-antitrypsin, TIMP-1, and nexin II are anti-invasive factors produced by the myoepithelial cells in myoepithelial cells rich tumors (152,153). Now it is well accepted that malignant tumors with important myoepithelial cell components behave less aggressively. Myoepithelial carcinoma tends to be lobulated tumors rather than infiltrating, and patients with myoepithelial carcinoma show a better survival when they are compared to carcinomas that do not express myoepithelial cell differentiation. In addition, there is also evidence that myoepithelial cells may inhibit angiogenesis (154).
Lymphoid Tissue
The immune system of salivary glands comprises two elements of the mucosa-associated immune system. One is the secretory component, a glycoprotein receptor for dimeric IgA and pentameric IgM that is produced by epithelial cells in the acini, intercalated ducts, and striated ducts (155,156). The other element is lymphoid tissue, which is either distributed diffusely or organized in lymph nodes. Isolated
P.457
lymphoid cells are present in the connective tissue near the acini and ducts. They are variable in number in the different glands (157) and yield principally IgA (nearly 80% of the immunoglobulins in saliva), along with a lesser quantity of IgG and IgM (159). Small lymph nodes are usually present near the surface of the parotid gland but not in the other salivary glands. Nevertheless, lymphoid cell aggregates are present in fetal submandibular and sublingual glands (158). These nodes usually contain salivary ducts and acini in the medullary region (Figure 17.15A), a phenomenon probably due to the close relationship between developing gland and lymphoid tissue during embryonic life.
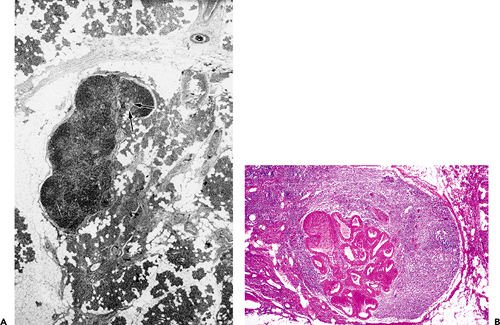 |
Figure 17.15 Lymph node of the parotid gland. A. Presence of glandular ducts in the medullary region (arrows). B. Duct dilatation and oncocytic transformation in another lymph node of the same patient. |
Lymph nodes are thought to participate in the histogenesis of Warthin's tumor. Although they have been pinpointed as the origin of the lymphoid tissue that characterizes this neoplasm, the hypothesis is still under debate. Different mechanisms have been proposed, but the most widely accepted one states that the lesion originates from the ducts inside lymph nodes within or adjacent to the parotid gland (85,160,161,162). According to this concept, the epithelial component of the tumor corresponds to altered ducts inside a lymph node, and the lymphatic component is, in fact, a lymph node. The following arguments support this hypothesis: salivary tissue is frequently present in intraparotid or periparotid lymph nodes (Figure 17.15A); with the exception of a few cases, this tumor is exclusive to the parotid region; it is common to find early stages of oncocytic and papillary transformation in lymph nodes of the parotid region (Figure 17.15B); and, finally, tumors identical to the epithelial structure of Warthin's tumor occur outside the lymph nodes but without lymphocytic components (160).
The diffuse lymphoid tissue may increase in chronic sialadenitis, particularly in immunologic reactions such as the benign lymphoepithelial lesion associated with Sj gren's syndrome. In these instances, lymphoid tissue may obscure the glandular parenchyma in a diffuse or nodular manner, and epimyoepithelial islands may be formed (91,160). The polyclonal character of the lymphoid cells and the presence of epimyoepithelial islands are useful criteria in the differential diagnosis of malignant lymphoma, which can occur in a preexisting benign lymphoepithelial lesion (162).
The Heterotopic Salivary Tissue and its Significance
The presence of salivary tissue outside the major salivary glands and the oral cavity, pharynx, and upper airways is considered heterotopia. This heterotopia may be classified as intranodal and extranodal in a number of locations in the head and neck.
P.458
Heterotopia is common in the lymph nodes near the parotid gland but is much less frequent in the submaxillary region and in other upper cervical nodes (163,164). The glandular elements are either normal or atrophic; they consist mainly of ducts, but acini are also found. They are localized in the medullary region and comprise a variable proportion of lymphoid and salivary tissues. Although all types of secretory units are found, serous ones are predominant. The histologic architecture is similar to a normal gland. The lymph nodes exhibit a normal structure or some degree of lymphoreticular hyperplasia.
The incidence of heterotopic salivary tissue in the lymph nodes has been well documented (165). It is typically found in the parotid region of fetuses during various stages of development, usually in more than one lymph node. In adults, although the incidence is not constant, it is nonetheless frequent (166). In most of the reports, the histogenetic mechanisms of this phenomenon have been related to embryonic development. From this point of view, the salivary tissue is trapped during embryologic development. In the fetus, the parotid gland is closely related to lymphoid tissue from the beginning of the second month. Moreover, Bairati (166) found that, at least in the first years of life, this salivary tissue is connected to the parotid gland. Although lymphatic dissemination has been proposed as a mechanism of salivary heterotopia in lymph nodes, in particular when they are located in the lower neck, this hypothesis is only speculative (167).
Extralymphatic heterotopias are rare and often latent but may be responsible for symptomatology. Heterotopic salivary tissue has been described in several sites, including head and neck, thorax, and abdomen (Table 17.2). In the upper neck, heterotopia is limited to the mandible, ear, palatine tonsil, mylohyoid muscle, pituitary gland, and cerebellopontine angle (164,168,169). All these sites, with the exception of the last, may be related to the embryonic migration of the salivary glands. In the lower neck, heterotopias are localized in the base of the neck, particularly around the sternoclavicular joint and in the thyroid gland and parathyroid glands (Figure 17.16) (167,171).
Table 17.2 Salivary Gland Heterotopias | |
|---|---|
|
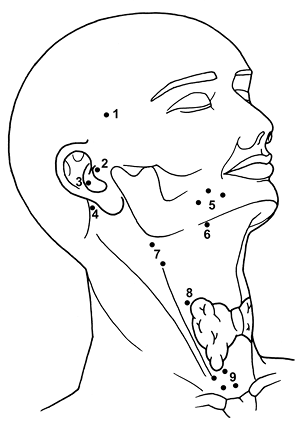 |
Figure 17.16 Extranodal salivary heterotopias. Pituitary gland (1), middle ear (2), external auditory canal (3), cerebellopontine angle (4), mandible (5), oropharynx (6), cervical superior (7), thyroid capsule (8), and lower anterolateral neck (9). |
Most examples of high salivary heterotopia are explained in relation to lines of migration of the parotid and submaxillary glands. In the mandible, the presence of salivary heterotopia may be related to a bone cavity and usually contains submandibular gland tissue. These inclusions are most common in the posterior lower jaw, near the angle. Originally described in 1942 by Stafne, they are often considered as heterotopias (172). This defect may be found in the anterior position (173). A recent study showed the heterotopic salivary tissue is present in 0.3% of maxillofacial marrow samples. The origin of this tissue is a matter of debate, but the most accepted hypothesis is the metaplasia of the odontogenic epithelial lesions (174). In the lower neck, an association with cysts and sinuses is frequent. This condition, along with the topographic presentation, has been related to the branchial apparatus (164,170) and, in particular, to a defective closure of the precervical His's sinus. The different abnormalities related to this defect (175) correspond embryologically to topographic distribution
P.459
through the neck from the ear to the clavicle. When this defect occurs, the salivary tissue is the result of abnormal tissue differentiation (heteroplasia). Willis (176) has argued that this is the mechanism of heterotopia. This mechanism may also account for the salivary tissue in remnants of Rathke's pouch (169) and the thyroglossal duct.
Neoplastic transformation in heterotopic salivary tissue may pose a problem in differential diagnosis of metastasis in a cervical lymph node (163). Pleomorphic adenoma, mucoepidermoid carcinoma, and adenoid cystic carcinoma are the most frequent tumors arising in heterotopic salivary tissue. In fact, heterotopia is an explanation for many aberrant salivary tumors (167,177) and some cervical lymph node metastases that are thought to be metastases of an unknown primary tumor (178). Primary salivary tumors of the mandible may originate in this aberrant tissue (179).
Aging Changes
Oncocytes
The oncocyte is an altered swollen epithelial cell characterized by an abundant eosinophilic granular cytoplasm that is rich in altered mitochondria and enzymes (Figure 17.17A) (180). This cell is frequently present in interlobular ducts and is less frequent in acinar cells. Oncocytes are more common in the parotid gland. They are rare before 50 years of age, increase in frequency with advancing years, and become constant after 70 years (66,181). Oncocytes may be detected by histochemical methods using phosphotungstic-acid hematoxylin or by more specific immunohistochemical methods with antibodies to human mitichondria (182).
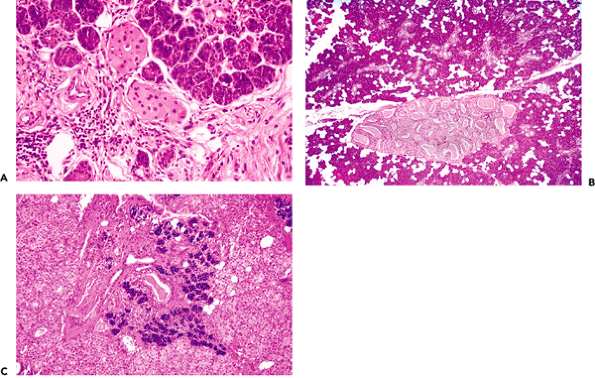 |
Figure 17.17 A. Oncocytes in intralobular ducts of a normal submaxillary gland. Note the abundant and granulated cytoplasm. B. Nodular oncocytic hyperplasia. C. Diffuse oncocytosis. |
The nature and function of oncocytes are unknown. Their proliferative character is a common finding in organs with endocrine function or endocrine dependence (183). Numerous researchers are presently interested in the production of various peptides with endocrine function in the intralobular ducts of rodent salivary glands (184,185,186). On the other hand, a number of workers are investigating the presence of neuroendocrine peptides such as the substance P like, -endorphin like (187,188), and a calcitonin-related peptide (189). In addition, neuroendocrine regulation of inflammation by the submandibular gland has been explained by an immunoneuroendocrine communication controlled by cervical sympathetic nerves (190).
The proliferation of oncocytes is called oncocytosis or oncocytic metaplasia when it is diffuse and generally without pathologic significance. In other cases, this proliferation presents a nodular pattern known as nodular hyperplasia (191). Histologically, it is easy to distinguish in the
P.460
salivary parenchyma as one or several small foci of oncocytes that are well circumscribed but not encapsulated. The cells may be arranged in solid cords or ductal structures and, in rare cases, may replace most of the gland (Figure 17.17B C). Oncocytes participate in various salivary gland tumors in approximately 10% of cases (192); the most common is Warthin's tumor, and they are also observed in basal cell adenoma, pleomorphic adenoma, myoepithelioma, polymorphous low-grade adenocarcinoma, mucoepidermoid carcinoma, and acinic cell carcinoma (193,194,195,196). The neoplastic proliferation called oncocytoma is rarely found in salivary glands (197).
Fatty Infiltration
Normally, some adipose cells are present in the areolar connective tissue of the salivary glands. This fatty tissue increases in adults; in the elderly, it forms an important proportion of salivary gland tissue. Fatty infiltration may reach huge proportions, especially in alcoholics and the malnourished (198).
Reactive Changes
Metaplasia
Squamous metaplasia may be present in larger salivary ducts in chronic inflammatory processes, particularly when associated with calculi (Figure 17.18A) (199). (Remember that when the major salivary ducts open into the oral cavity, they are lined with a stratified squamous epithelium). Metaplastic squamous transformation is also present in intralobular ducts and acini in ischemia and radiation injury (200,201).
Mucous metaplasia is found on interlobular ducts and, less frequently, on intralobular ducts in cases of obstructive and postradiotherapy forms of sialadenitis (202). An important proliferation of mucous goblet cells is accompanied by prominent ciliated cells mimicking the respiratory epithelium (Figure 17.18B). Necrotizing sialometaplasia is a type of metaplasia peculiar to salivary tissue, but it is exceptional in major salivary glands (Figure 17.19) (203). It consists of ischemic lobular infarction or necrosis of some acini, accompanied by extensive squamous metaplasia of salivary gland ducts and acini. Severe inflammation and granulation tissue are present. Necrosis of mucous acini is represented by small pools of mucin that, along with the squamous elements, may be mistaken for mucoepidermoid carcinoma (204). The preservation of general lobular morphology and prominent granulation tissue are criteria in favor of benignity. Subacute forms are also described (205).
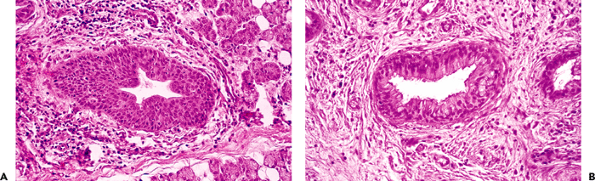 |
Figure 17.18 Metaplasia of excretory ducts. Squamous (A) and mucous metaplasia (B) showing cilliated cells simulating respiratory epithelium. |
Hyperplasia
Hyperplasia of mucous acini is an alteration exclusive to the minor salivary glands. In contrast, serous hyperplasia occurs in parotid and, rarely, in submaxillary and sublingual glands; it is called sialadenosis (206). This hyperplasia is associated with a number of metabolic, nutritional, and endocrine conditions or follows the ingestion of chemicals and drugs (207). In most cases, a bilateral swelling of the glands is caused by enlarged acini and an accumulation of secretory granules in the cytoplasm. In other cases, granulation is lost and the cytoplasm looks vacuolated (22). The myoepithelial cells may present nuclear pyknosis or cytoplasmic vacuolation. Adenomatoid salivary gland hyperplasia should be distinguished from true tumors. Recently, adenomatous ductal proliferation in salivary glands has been described; these cases may be misdiagnosed as true adenomas. Adenomatoid hyperplasia contains clusters or lobules of mucous or serous glands with normal or enlarged appearance, whereas adenomatous ductal proliferations show prominent ductal proliferation with some
P.461
acinar cells complexes, but the normal lobular pattern is preserved (208,209).
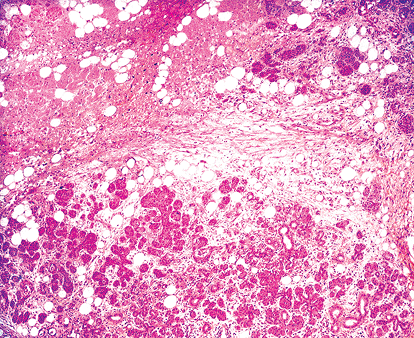 |
Figure 17.19 Necrotizing sialometaplasia of submaxillary gland. |
Atrophy
Atrophy of the salivary tissue is a common finding in surgical specimens of tumoral salivary glands that have one or more atrophied lobules. This atrophy is caused by partial or total obstruction of an excretory duct. Accordingly, secretory units distal to the obstructed duct are dilated, and the acinar lumina become visible. The secretory cells lose their granules and have a similar aspect to that of the intercalated duct. The atrophic lobule has an inflammatory component; the cells gradually disappear, and the parenchyma is replaced by adipose tissue and collagen fibers (Figure 17.20A). The extent of atrophy depends on the size of the affected duct. It is generally greater in lithiasic obstruction. The atrophy present in terminal stage chronic sialadenitis shows a diffuse pattern with prominent periductal sclerosis and dense inflammatory infiltration (Figure 17.20B) (202). In the submaxillary gland, diffuse atrophy is frequent after radiotherapy, and the gland has a firm consistency (Kuttner's tumor) that may be clinically mistaken for a submaxillary neoplasm. Atypical cells are often found in postradiotherapy atrophy. Dilated ducts lose cell polarity and exhibit hyperchromatic nuclei and prominent myoepithelium. In addition, interlobular ducts lose their continuity, and the interstitium is densely infiltrated with plasma cells (201). In experimental models, atrophy takes place after ligation of main salivary duct. Duct cells persist, in contrast to the disappearance of most acinar cells, which are deleted by apoptosis (210).
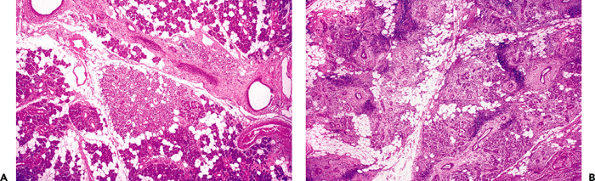 |
Figure 17.20 Atrophy of the salivary parenchyma. A. Focal atrophy in the vicinity of a parotid tumor; note the duct dilatation. B. Diffuse atrophy in chronic sialadenitis showing prominent periductal sclerosis. |
Regeneration
The parenchyma of salivary glands have a capacity for regeneration. It is particularly notable in the salivary gland a few weeks after partial resection. In general, regenerating tissue follows an embryonic pattern, showing solid buds and branching columns of undifferentiated cells that eventually form excretory units (22) (Figure 17.21A). In some cases, such regenerating tissue exhibits an atypical appearance. Proliferating solid buds of undifferentiated cells may simulate basal cell adenoma (211) or other undifferentiated cell neoplasms (Figure 17.21B). However, unlike neoplasms, the regenerating tissue preserves the lobular architecture characteristic of the normal salivary gland (Figure 17.21B C) (22,39). Regeneration after atrophy due to duct obstruction is completed from residual parotid ducts that differentiate into acinar cells (212); this is probably achieved by a stem cell that resides in one of the ducts. The pattern is similar to the high proliferative activity of the terminal tubule, proacinar, and acinar cells during normal embryonic development of the rat submandibular gland (213).
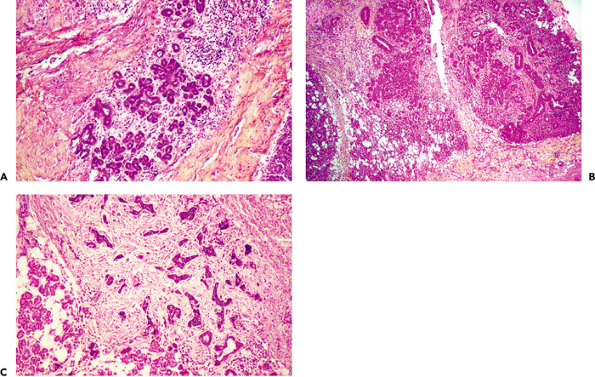 |
Figure 17.21 Regenerating salivary tissue of parotid gland. A. Formation of secretory units with an embryonic pattern. B. Atypical regenerating tissue; the lobular pattern is preserved. C. Infiltrating residual adenoid cystic carcinoma in a parotid gland. Solid buds consist of undifferentiated cells without lobular arrangement. |
P.462
Correlative Normal and Neoplastic Histology
A characteristic of salivary glands is the ability to give rise to a large number of histologically distinct tumors (214). The histogenetic origin of these neoplasms is an interesting topic, but the numerous mechanisms that have been proposed remain hypothetical. The most attractive hypotheses attempt to relate this phenomenon to embryonic development and, particularly, to the presence of ductal reserve cells (56,60,193,214,215,216,217). A conjectural role of these reserve cells is the regeneration of salivary parenchyma, as well as the development of metaplastic tissue in reactive conditions. Batsakis (216) hypothesized two stem cells progenitors located at proximal and distal regions of the duct system that are related to tumors mimicking the terminal ductoacinar complex and the excretory ducts system, respectively. Some researchers have demonstrated, by immunohistochemical methods, the presence of basal cells with an undifferentiated aspect in salivary ducts (57,58). However, this does not prove that they act as reserve cells. Conversely, experimental evidence exists of the proliferative capacity of differentiated acinar and myepithelial cells (218,219,220).
A correlation between the normal structure of the salivary gland and the histologic appearance of salivary tumors can help us to understand morphologic classifications. Nevertheless, we must realize that this histologic similarity does not necessarily imply that a particular tumor arises from the structure that it mimics (217). However, the morphogenetic approach proposed by Dardick relates morphology to cell differentiation derived from different genes' expression of a stem cell (221,222).
The intercalated duct represents the most important segment of the salivary gland in the morphologic organization of many salivary tumors (217). Many distinctive tumors have been related to it, including pleomorphic adenoma, adenoid cystic carcinoma, basal cell adenoma, epithelial-myoepithelial carcinoma, terminal duct carcinoma, basal cell carcinoma, and embryonic tumors (Figure 17.22). These tumors show both epithelial and myoepithelial cell differentiation (93,109,112,113,118,131,132,134,136,139,148,149,217,222,223), as does the normal intercalated duct. According to Batsakis (216), these tumors develop from intercalated duct reserve cells that follow the same direction as the embryonic terminal tubular cell. Statistically, approximately 80% of salivary tumors develop in the parotid gland, where the intercalated ducts are relatively long (Figure 17.6). In contrast, in the sublingual gland, which gives rise to less than 1% of salivary tumors (22), intercalated ducts are hardly visible.
P.463
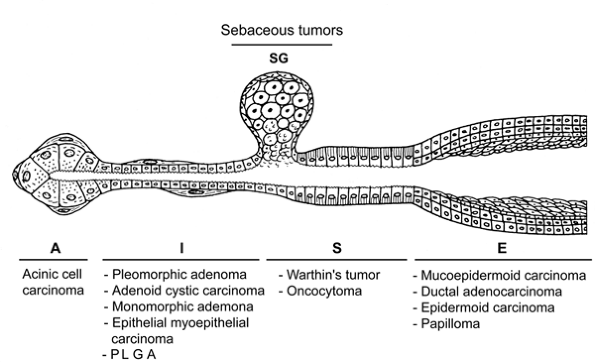 |
Figure 17.22 Morphologic similarity of some salivary tumors and the different epithelial structures of the salivary gland: acinus (A), intercalated duct (I), striated duct (S), sebaceous gland (SG), and excretory ducts (E). Not necessarily related to histogenesis. |
A similar morphologic link exists between the acini, the most differentiated structure of the gland, and the acinic cell tumor, a well-differentiated neoplasm (Figure 17.23A B) (224); between the mitochondria-rich cells of the striated duct, Warthin's tumor, and oncocytomas (180,181); and between the sebaceous glands and sebaceous tumors (69,76,86). A morphologic analogy also may be found between the larger excretory ducts and mucoepidermoid carcinoma, salivary duct adenocarcinoma, epidermoid carcinoma, adenosquamous carcinoma, and papillary tumors (Figure 17.22) (225,226,227,228,229,230,231,232,233).
Immunohistochemistry
An important aspect of cytodifferentiation in normal and neoplastic salivary tissue is the expression of different intermediate filaments, enzymes, immunologic components, and other proteins. These proteins may be stained by immunohistochemical methods.
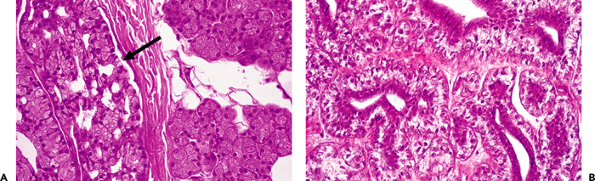 |
Figure 17.23 A. Acinic cell carcinoma of tha parotid gland (arrow), mimicking the normal gland. B. Epithelial-myoepithelial carcinoma of parotid gland, showing both epithelial and myoepithelial cells of the intercalated duct. |
In normal salivary glands, the immunohistochemistry may be summarized as follows (Table 17.3): the expression of cytokeratin varies according to individual cell types of the secretory unit. Acinar cells are stained by several types of lower weight cytokeratins such as CK7, CK8, and CK19. In addition, these cells may be marked by the enzymes usually present in the normal acinus, such as amylase and lysozyme (234). Cytokeratins 6 and 12 are weakly positive in acinar cells but strongly positive in ductal cells, especially in excretory ducts (Figure 17.24) (57). Basal cells are stained with anticytokeratin 19 and 18. Myoepithetial cells may coexpress cytokeratins 14, 17, 19, and vimentin (57,146,148). The most specific markers for myoepithelial cells are S-100 protein, -smooth muscle actin, calponin, caldesmon, and myosin heavy chain (149,235). In addition, normal myoepithelial cells are
P.464
sometimes stained with glial fibrillary protein (236). The secretory component, except for the mucous units, is present in acinar cells and intercalated and striated ducts (55,237). Carcinoembryonic antigen (CEA) is present in acinar and intercalated duct cells; it is strongly positive in inflamed glands (238). Basal cells are stained with CK14, CK17, and p63 (234). Ductal epithelial cells of intercalated, striated, and interlobular ducts are strongly positive for epithelial membrane antigen (EMA) (239). Recently, the presence of -fetoprotein in the human submandibular gland has been documented (240). Used as functional markers, the following enzymes may be stained: amylase and lactoferrin in serous acinar cells (241), lysozyme in intercalated ducts (49,50), and alkaline phosphatase and ATPase in myoepithelial cells (92). When used as histologic markers, all these proteins have proven useful in surgical pathology.
Table 17.3 Immunohistochemical Markers of Salivary Glands | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
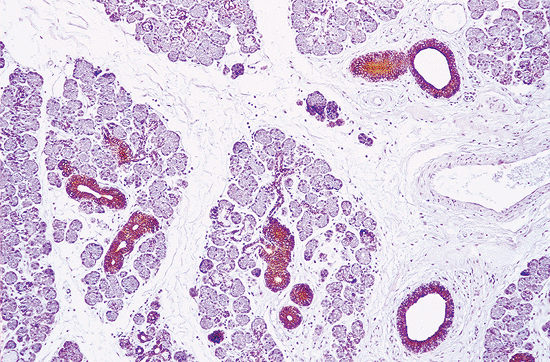 |
Figure 17.24 Immunostaining for high-molecular weight cytokeratin in a submaxillary gland. The acini are unstained, but intercalated, striated, and excretory ducts show a progressive immunoreaction (peroxidase-antiperoxidase method). |
Specimen Handling
The parotid gland is the most common location of major salivary gland tumors. Because most of them are benign and located on the superficial lobe, partial parotidectomy of the superficial lobe (lateral lobectomy) is the most common treatment. Superficial parotidectomy also suffices for small, well-differentiated, low-grade malignant neoplasms that have not compromised the facial nerve (242). The specimen should be sectioned horizontally, and the relationship between the tumor and the gland should be stated (Figure 17.25). The surgical limits should be described even in benign tumors (Figure 17.26). Total parotidectomy with preservation of the facial nerve is usually indicated in recurrent or multiple benign tumors and large benign tumors of the isthmus and deep lobe.
 |
Figure 17.25 Histologic section of a parotid gland showing a pleomorphic adenoma. The tumor is apparently well delimited; tumoral buds may be found separate from the principal tumor. |
P.465
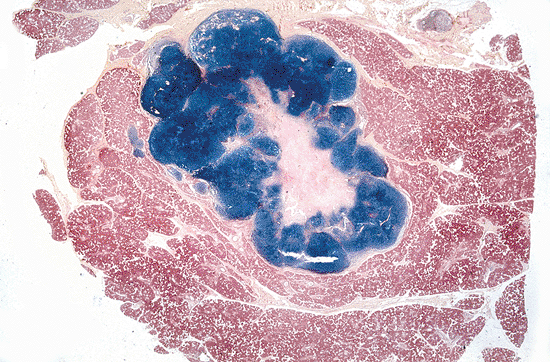 |
Figure 17.26 Superficial parotidectomy for a pleomorphic adenoma. Note the tumor is well delimited within the normal gland. |
Total parotidectomy, including facial nerve resection (radical parotidectomy), is indicated in aggressive malignant tumors, malignant tumors situated in the deep lobe, or those that have compromised the facial nerve. All these specimens should be sectioned on their major axis to evaluate periparotid tissue infiltration (Figure 17.27). Intraparotid and periparotid lymph nodes should be identified; and, when radical parotidectomy includes neck dissection, the lymph nodes should be separated according to the different anatomical regions.
Benign and malignant submandibular gland tumors are rare. Single gland resection is indicated for benign tumors, whereas malignant tumors are treated by a monobloc resection of the gland lesion along with associated muscles, nerves, and mucous membrane.
Sublingual tumors are rare, and most (80%) are malignant. They are also treated by local monobloc resection, including the sublingual compartment and surrounding tissues (243).
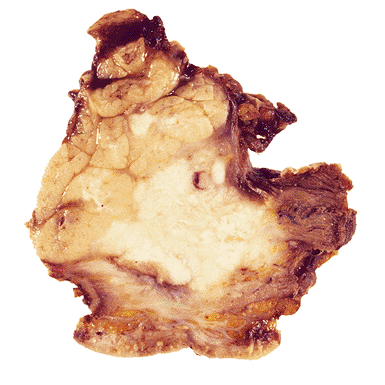 |
Figure 17.27 Radical parotidectomy for a high-grade mucoepidermoid carcinoma. Note the infiltration of the periparotid tissues. |
References
1. Arey LB. Developmental Anatomy. 7th ed. Philadelphia: WB Saunders; 1974.
2. Cohn RH, Banerjee SD, Bernfield MR. Basal lamina of embryonic salivary epithelia. Nature of glycosaminoglycan and organization of extracellular materials. J Cell Biol 1977;73:464 478.
3. Grobstein C. Epithelio-mesenchymal specificity in the morphogenesis of mouse sub-mandibular rudiments in vitro. J Exp Zool 1953;124:383 414.
4. Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr 1967;26:279 299.
5. Lawson KA. The role of mesenchyme in the morphogenesis and functional differentiation of rat salivary epithelium. J Embryol Exp Morphol 1972;27:497 513.
6. Gasser RF. The early development of the parotid gland around the facial nerve and its branches in man. Anat Rec 1970;167:63 77.
7. Azuma M, Sato M. Morphogenesis of normal human salivary gland cells in vitro. Histol Histopathol 1994;9:781 790.
8. Donath K, Dietrich H, Seifert G. Enwicklung und ultrastrukturelle cytodifferenzierung der parotis des menschen. Virchows Arch A Pathol Anat Histopathol 1978;378:297 314.
9. Lee SK, Hwang Jo, Chi JG, Yamada K, Mori M. Prenatal development of myoepithelial cell of human submandibular gland observed by immunohistochemistry of smooth muscle actin and rhodamine-phalloidin fluorescence. Pathol Res Pract 1993;189:332 341.
10. Adi MM, Chisholm DM, Waterhouse JP. Stereological and immunohistochemical study of development of human fetal labial salivary glands and their S-100 protein reactivity. J Oral Pathol Med 1994;23:36 40.
11. Lee SK, Kim EC, Chi JG, Hashimura K, Mori M. Immunohistochemical detection of S-100, S-100 alpha, S-100 beta proteins, glial fibrillary acidic protein, and neuron specific enolase in the prenatal and adult human salivary glands. Pathol Res Pract 1993;189:1036 1043.
12. Line SE, Archer FL. The postnatal development of myoepithelial cell in the rat submandibular gland. An immunohistochemical study. Virchows Arch B Cell Pathol 1972;10:253 262.
13. Gresik EW. Postnatal developmental changes in submandibular glands of rats and mice. J Histochem Cytochem 1980;28:860 870.
14. Strum JM. Unusual peroxidase-positive granules in the developing rat submaxillary gland. J Cell Biol 1971;51:575 579.
15. Yamashina S, Barka T. Localization of peroxidase activity in the developing submandibular gland of normal and isoproterenol-treated rats. J Histochem Cytochem 1972;20:855 872.
16. Testut L. Trait d'Anatomie Humaine. Paris: Octave Doin; 1931.
17. Gray H, Gross CM, eds. Gray's Anatomy of the Human Body. 29th ed. Philadelphia: Lea & Febiger; 1973.
18. Gola R, Chossegros C, Carreu P. Anatomie chirurgicale de la r gion parotidienne. Concepts actuels. Rev Stomatol Chir Maxillofac 1994;95:395 410.
19. Toh H, Kodama J, Fukuda J, Rittman B, Mackenzie I. Incidence and histology of human accessory parotid glands. Anat Rec 1993;236:586 590.
20. Frommer J. The human accessory parotid gland: its incidence, nature, and significance. Oral Surg Oral Med Oral Pathol 1977;43:671 676.
21. Hamilton WJ, Boyd JD, Mossman HW. Human Embryology, Prenatal Development of Form and Function. London: Williams & Wilkins; 1972.
22. Evans RW, Cruickshank AH. Epithelial Tumors of the Salivary Glands. Philadelphia: WB Saunders; 1970.
23. Merida-Velasco JA, Sanchez-Montesinos I, Espin-Ferra J, Garc a-Garc a ID, Garc a-Gomez S, Roldan-Schilling V. Development of the human submandibular salivary gland. J Dent Res 1993;73:1227 1232.
24. Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature 2003;423:876 881.
25. Hu MC, Rosenblum N. Genetic regulation of branching morphogenesis lessons learned from loss-of-function phenotypes. Pediatr Res 2003;54:433 438.
P.466
26. Gaur V, Choudhry R, Anand C, Choudhry S. Submandibular gland with multiple ducts. Surg Radiol Anat 1994;16:439 440.
27. Bowen ID, Morgan SM, Mullarkey K. Cell death in the salivary glands of metamorphosing Calliphora vomitoria. Cell Biol Int 1993;17:13 33.
28. Tomei LD, Cope FO, eds. Apoptosis: The Molecular Basis of Cell Death. Plainview, NY: Cold Spring Harbor Laboratory Press; 1991.
29. Lu QL, Poulsom R, Wong L, Hanby AM. Bcl-2 expression in adult and embryonic non-haematopoietic tissues. J Pathol 1993;169:431 437.
30. Walker NI, Gob GC. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J Pathol 1987;153:333 344.
31. Stephens LC, Schultheiss TE, Price RE, Ang KK, Peters LJ. Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer 1991;67:1539 1543.
32. Tamerin A. Submaxillary gland recovery from obstruction II. Electron microscopic alterations of acinar cells. J Ultrastruct Res 1971;34:288 302.
33. Burgess KL, Dardick I. Cell population changes during atrophy and regeneration of rat parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol and Endod 1998;85:699 706.
34. Takahashi S, Shinzato K, Domon T, Yamamoto T, Wakita M. Mitotic proliferation on myoepithelial cells during regeneration of atrophied rat submandibular glands after duct ligation. J Oral Pathol Med 2004;33:430 434.
35. Kong L, Ogawa N, Nakabayashi T, et al. Fas and Fas ligand expression in the salivary glands of patients with primary Sj gren's syndrome. Arthritis Rheum 1997;40:87 97.
36. Bolstand AI, Eiken HG, Rosenlund B, Alarcon-Riquelme M, Jonsson R. Increased salivary gland tissue expression of Fas, Fas ligand, cytotoxic T lymphocyte-associated antigen 4, and programmed cell death 1 in primary Sj gren's syndrome. Arthritis Rheum 2003;48;174 185.
37. Hand AR, Ho B. Liquid-diet-induced alterations of rat parotid acinar cells studied by electron microscopy and enzyme cytochemistry. Arch Oral Biol 1981;26:369 380.
38. Sharawy M, White SC. Morphometric and fine structural study of experimental autoallergic sialadenitis of rat submandibular glands. Virchows Arch B Cell Pathol 1978;28:255 273.
39. Chaudhry AP, Cutler LS, Yamane GM, Satchidanand S, Labay G, Sunderraj M. Light and ultrastructural features of lymphoepithelial lesions of the salivary glands in Mikulicz's disease. J Pathol 1986;148:239 250.
40. Fanidi A, Harrington EA, Evan GI. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature 1992;359:554 556.
41. Chen M, Quintans J, Fuks Z, Thompson C, Kufe DW, Weichselbaum RR. Suppression of Bcl-2 messenger RNA production may mediate apoptosis after ionizing radiation, tumor necrosis factor alpha, and ceramide. Cancer Res 1995;55:991 994.
42. Tamarin A. Submaxillary gland recovery from obstruction I. overall changes and electron microscopic alterations of glanular duct cells. J Ultrastruct Res 1971:34:276 287.
43. Matthews TW, Dardick I. Morphological alterations of salivary gland parenchyma in chronic sialadenitis. J Otolaryngol 1988;17:385 394.
44. Pammer J, Horvat R, Weninger W, Ulrich W. Expression of bcl-2 in salivary glands and salivary adenomas. A contribution to the reserve cell theory. Pathol Res Pract 1995;191:35 41.
45. Hockenbery DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A 1991;88:6961 6965.
46. Bloom W, Fawcett DW. A Textbook of Histology. 11th ed. Philadelphia: WB Saunders; 1986.
47. Donath K. Die Sialadenose der Parotis. Ultrastrukturelle, Klinische und Experimentelle Befunde zur Sekretionpathologie. Stuttgart: Gustav Fischer Verlag; 1976.
48. Takano K, Malamud D, Bennick A, Oppenheim F, Hand AR. Localization of salivary proteins in granules of human parotid and submandibular acinar cells. Crit Rev Oral Biol Med 1993;4:399 405.
49. Caselitz J, Jaup T, Seifert G. Lactoferrin and lysozyme in carcinomas of the parotid gland. A comparative immunocytochemical study with the occurrence in normal and inflamed tissue. Virchows Arch A Pathol Anat Histol 1981;394:61 73.
50. Reitamo S, Konttinen YT, Segerberg-Konttinen M. Distribution of lactoferrin in human salivary glands. Histochemistry 1980;66:285 291.
51. Xu L, Lal K, Santarpia RP III, Pollock JJ. Salivary proteolysis of histidine-rich polypeptides and the antifungal activity of peptide degradation products. Arch Oral Biol 1993;38:277 283.
52. Quintarelli G. Histochemical identification of salivary mucins. Ann N Y Acad Sci 1963;106:339 363.
53. Reddy MS, Bobek LA, Haraszthy GG, Biesbrock AR, Levine MJ. Structural features of the low-molecular-mass human salivary mucin. Biochem J 1992;287(pt 2):639 643.
54. Greep RO, Weiss L, eds. Histology. New York: McGraw-Hill; 1973.
55. Korsrud FR, Brandtzaeg P. Characterization of epithelial elements in human major salivary glands by functional markers: localization of amylase, lactoferrin, lysozyme, secretory component, and secretory immunoglobulins by paired immunofluorescence staining. J Histochem Cytochem 1982;30:657 666.
56. Riva A, Serra GP, Proto E, Faa G, Puxeddu R, Riva FT. The myoepithelial and basal cells of ducts of human major salivary glands: a SEM study. Arch Histol Cytol 1992;55(suppl):115 124.
57. Born IA, Schwechheimer K, Maier H, Otto HF. Cytokeratin expression in normal salivary glands and in cystadenolymphomas demonstrated by monoclonal antibodies against selective cytokeratin polypeptides. Virchows Arch A Pathol Anat Histopathol 1987;411:583 589.
58. Burns BF, Dardick I, Parks WR. Intermediate filament expression in normal parotid glands and in pleomorphic adenomas. Virchows Arch A Pathol Anat Histopathol 1988;413:103 112.
59. Seifert GA, Miehlke A, Haubrich J, Chilla R. Diseases of the Salivary Glands: Pathology, Diagnosis, Treatment, Facial Nerve Surgery. Stuttgart: Georg Thieme Verlag; 1986.
60. Regezi JA, Batsakis JG. Histogenesis of salivary gland neoplasms. Otolaryngol Clin North Am 1977;10:297 307.
61. Scott BL, Pease DC. Electron microscopy of the salivary and lacrimal glands of the rat. Am J Anat 1959;104:115 161.
62. Tandler B. Ultrastructure of the human submaxillary gland. I. Architecture and histological relationships of the secretory cells. Am J Anat 1962;111:287 307.
63. Tandler B, Denning CR, Mandel ID, Kutscher AH. Ultrastructure of human labial glands. III. Myoepithelium and ducts. J Morphol 1970;130:227 246.
64. Norberg L, Dardick I, Leung R, Burford-Mason AP, Rippstein P. Immunogold localization of actin and cytokeratin filaments in myoepithelium of human parotid salivary gland. Ultrastruct Pathol 1992;16:555 568.
65. Sj strand FS, Rhodin J. Ultrastructure of the proximal convoluted tubule of the mouse kidney as revealed by high resolution electron microscopy. Exp Cell Res 1953;4:426 465.
66. Hamperl H. Beitra ge zur normalen und pathologischen histologic menschlicher speicheldr sen. Z Mikorosk Anat Forsch 1931;27:1 55.
67. Harts PH. Development of sebaceous glands from intralobular ducts of the parotid gland. Arch Pathol 1946;41:651 654.
68. Marshall L Jr. Intraparotid sebaceous glands. Ann Surg 1949;129:152 155.
69. Meza-Chavez L. Sebaceous glands in normal and neoplastic parotid glands. Am J Pathol 1949;25:627 645.
70. Micheau C. Les glandes dites s bac es de la parotide et de la sous-maxillaires. Ann Anat Path 1969;14:119 126.
71. Patey DH, Thackray AC. The treatment of parotid tumours in the light of a pathological study of parotidectomy material. Br J Surg 1958;45:477 487.
72. Margolies A, Weidman F. Statistical and histologic studies of Fordyce's disease. Arch Derm Syph 1921;3:723 742.
73. Whitaker SB, Vigneswaran N, Singh BB. Androgen receptor status of the oral sebaceous glands. Am J Dermatopathol 1997;19:415 418.
74. Laine M, Blauer M, Ylikomi, et al. Immunohistochemical demonstration of androgen receptors in human salivary glands. Arch Oral Biol 1993;38:299 302.
P.467
75. Brocheriou C. Les tumeurs des glandes salivaires. In: Nezeloff C, ed. Nouvelles Acquisitions en Pathologie. Paris: Hermann; 1983:223 268.
76. Albores-Saavedra J, Morris AW. Sebaceous adenoma of the submaxillary salivary gland. Report of a case. Arch Otolaryngol 1963;77:500 503.
77. Assor D. Sebaceous lymphadenoma of the parotid gland: a case report. Am J Clin Pathol 1970;53:100 103.
78. Cheek R, Pitcock JA. Sebaceous lesions of the parotid. Report of two cases. Arch Pathol 1966;82:147 150.
79. Seifert G, Bull HG, Donath K. Histologic subclassification of the cystadenolymphoma of the parotid gland. Analysis of 275 cases. Virchows Arch A Pathol Anat Histol 1980;388:13 38.
80. Wasan SM. Sebaceous lymphadenoma of the parotid gland. Cancer 1971;28:1019 1022.
81. Kleinsasser O, H bner G, Klein HJ. Talgzellcarcinom des Parotis. Arch Klin Exp Ohren Nasen Kehlkopfheilk 1970;197:59 71.
82. Silver H, Goldstein MA. Sebaceous cell carcinoma of the parotid region. A review of the literature and a case report. Cancer 1966;19:1173 1179.
83. Mart nez-Madrigal F, Casiraghi O, Khattech A, Nasr-Khattech RB, Richard JM, Micheau C. Hypopharyngeal sebaceous carcinoma: a case report. Hum Pathol 1991;22:929 931.
84. Gnepp DR, Sporck FT. Benign lymphoepithelial parotid cyst with sebaceous differentiation. Cystic sebaceous lymphadenoma. Am J Clin Pathol 1980;74:683 687.
85. Peel RL, Gnepp DR. Diseases of the salivary glands. In: Barnes L, ed. Surgical Pathology of the Head and Neck. New York: Marcel Dekker; 1985:533 645
86. Rawson AJ, Horn RC Jr. Sebaceous glands and sebaceous gland-containing tumors of the parotid salivary gland; with a consideration of the histogenesis of papillary cystadenoma lymphomatosum. Surgery 1950;27:93 101.
87. Cutler LS, Chaudhry A, Innes DJ Jr. Ultrastructure of the parotid duct. Cytochemical studies of the striated duct and papillary cystadenoma lymphomatosum of the human parotid gland. Arch Pathol Lab Med 1977;101:420 424.
88. Archer FL, Kao VC. Immunohistochemical identification of actomyosin in myoepithelium of human tissues. Lab Invest 1968;18:669 674.
89. Drenckhahn D, Gr schel-Stewart U, Unsicker K. Immunofluorescence-microscopic demonstration of myosin and actin in salivary glands and exocrine pancreas of the rat. Cell Tissue Res 1977;183:273 279.
90. Tandler B. Ultrastructure of the human submaxillary gland. III. Myoepithelium. Z Zellforsch Mikroskop Anat 1965;68:852 863.
91. Franke WW, Schmid E, Freudenstein C, et al. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol 1980;84:633 654.
92. Hamperl H. The myoepithelia (myoepithelial cells). Curr Top Pathol 1970;53:161 220.
93. Shear M. The structure and function of myoepithelial cells in salivary glands. Arch Oral Biol 1966;11:769 780.
94. Garrett JR, Emmelin N. Activities of salivary myoepithelial cells: a review. Med Biol 1979;57:1 28.
95. Schroeder BT, Chakraborty J, Soloff MS. Binding of [3H] oxytocin to the cells isolated from the mammary gland of the lactating rat. J Cell Biol 1977;74:428 440.
96. Azumi N, Battifora H. The cellular composition of adenoid cystic carcinoma. An immunohistochemical study. Cancer 1987;60:1589 1598.
97. d'Ardenne AJ, Kirkpatrick P, Wells CA, Davies JD. Laminin and fibronectin in adenoid cystic carcinoma. J Clin Pathol 1986;39:138 144.
98. Donath K, Seifert G. Ultrastruktur und pathogenese der myoepithelialen sialadenitis. ber das vorkommen von myoepithelzellen bei der benignen lymphoepithelialen l sion. Virchows Arch A Pathol Anat Histopathol 1972;356:315 329.
99. Kallioinen M. Immunoelectron microscope demonstration of the basement membrane components laminin and type IV collagen in the dermal cylindroma. J Pathol 1985;147:97 102.
100. Orenstein JM, Dardick I, van Nostrand AW. Ultrastructural similarities of adenoid cystic carcinoma and pleomorphic adenoma. Histopathology 1985;9:623 638.
101. Seifert G, Donath K. Classification of the pathology of diseases of the salivary glands. Review of 2,600 cases in the salivary gland register. Beitr Pathol 1976;159:1 32.
102. Skalova A, Leivo I. Extracellular collagenous spherules in salivary gland tumors: immunohistochemical analysis of laminin and various types of callagen. Arch Pathol Lab Med 1992;116:649 653.
103. Skalova A, Leivo I. Basement membrane proteins in salivary gland tumors. Distribution of type IV collagen and laminin. Virchows Arch A Pathol Anat Histopathol 1992;420:425 431.
104. Laurie GW, Leblond CP, Martin GR. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol 1982;95:340 344.
105. Sunardhi-Widyaputra S, Van Damme B. Immunohistochemical expression of tenascin in normal human salivary glands and in pleomorphic adenomas. Pathol Res Pract 1993;189:138 143.
106. Savera AT, Zarbo RJ. Defining the role of myoepithelium in salivary gland neoplasia. Adv Anat Pathol 2004;11:69 85.
107. Eneroth CM. Salivary gland tumors in the parotid gland, submandibular gland, and the palate region. Cancer 1971;27:1415 1418.
108. Thackray AC, Sabin LH. Histological typing of salivary gland tumors. In: International Histological Classification of Tumors. No. 7. Geneva: World Health Organization; 1972.
109. Dardick I, van Nostrand AW, Jeans MT, Rippstein P, Edwards V. Pleomorphic adenoma. I. Ultrastructural organization of epithelial regions. Hum Pathol 1983;14:780 797.
110. Dardick I, van Nostrand AW, Phillips MJ. Histogenesis of salivary gland pleomorphic adenoma (mixed tumor) with an evaluation of the role of the myoepithelial cell. Hum Pathol 1982;13:62 75.
111. David R, Buchner A. Elastosis in benign and malignant salivary gland tumors. A histochemical and ultrastructural study. Cancer 1980;45:2301 2310.
112. Erlandson RA, Cardon-Cardo C, Higgins PJ. Histogenesis of benign pleomorphic adenoma (mixed tumor) of the major salivary glands. An ultrastructural and immunohistochemical study. Am J Surg Pathol 1984;8:803 820.
113. Hubner G, Klein HJ, Kleinsasser O, Schiefer HG. Role of myoepithelial cells in the development of salivary gland tumors. Cancer 1971;27:1255 1261.
114. Seifert G, Langrock I, Donath K. Pathomorphologische subklassifikation der pleomorphen speicheldr sen adenomen. Analyse von 310 pleomorphen parotisadenomen. HNO 1976;24:415 426.
115. Shirasuna K, Sato M, Miyazaki T. A myoepithelial cell line established from a human pleomorphic adenoma arising in minor salivary gland. Cancer 1980;45:297 305.
116. Yanagawa T, Hayashi Y, Nagamine S, Yoshida H, Yura Y, Sato M. Generation of cells with phenotypes of both intercalated duct-type and myoepithelial cells in human parotid gland adenocarcinoma clonal cells grown in athymic nude mice. Virchows Arch B Cell Pathol Incl Mol Pathol 1986;51:187 195.
117. Gallo O, Bani D, Toccafondi G, Almerigogna F, Storchi OF. Characterization of a novel cell line from pleomorphic adenoma of the parotid gland with myoepithelial phenotype and producing interleukin-6 as an autocrine growth factor. Cancer 1992;70:559 568.
118. Corio RL, Sciubba JJ, Brannon RB, Batsakis J. Epithelial-myoepithelial carcinoma of intercalated duct origin. A clinicopathological and ultrastructural assessment of sixteen cases. Oral Surg Oral Med Oral Pathol 1982;53:280 287.
119. Donath K, Seifert G, Schmitz R. Zur diagnose und ultrastruktur des tubul ren speichelgangkarzinoms. Epithelial-myoepitheliales schultst ckkarzinom. Virchows Arch A Pathol Anat Histopathol 1972;356:16 31.
120. Luna MA, Ordonez NG, Mackay B, Batsakis JG, Gillamandegui O. Salivary epithelial-myoepithelial carcinomas of intercalated ducts: a clinical, electron microscopic, and immunocytochemical study. Oral Surg Oral Med Oral Pathol 1985;59:482 490.
121. Batsakis JG, el-Naggar AK, Luna MA. Epithelial-myoepithelial carcinoma of salivary glands. Ann Otol Rhinol Laryngol 1992;101:540 542.
P.468
122. Nistal M, Garc a-Viera M, Mart nez-Garc a C, Paniagua R. Epithelial-myoepithelial tumor of the bronchus. Am J Surg Pathol 1994;18:421 425.
123. Palmer RM. Epithelial-myoepithelial carcinoma: an immunocytochemical study. Oral Surg Oral Med Oral Pathol 1985;59:511 515.
124. Crissman JD, Wirman JA, Harris A. Malignant myoepithelioma of the parotid gland. Cancer 1977;40:3042 3049.
125. Leifer C, Miller AS, Putong PB, Harwick RD. Myoepithelioma of the parotid gland. Arch Pathol 1974;98:312 319.
126. Luna MA, Mackay B, Gamez-Araujo J. Myoepithelioma of the palate. Report of a case with histochemical and electron microscopic observations. Cancer 1973;32:1429 1435.
127. Sciubba JJ, Brannon RB. Myoepithelioma of the salivary glands: report of 23 cases. Cancer 1982;49:562 572.
128. Dardick I, Cavell S, Boivin M, et al. Salivary gland myoepithelioma variants: histological, ultrastructural, and immunocytological features. Virchows Arch A Pathol Anat Histolpathol 1989;416:25 42.
129. Mart nez-Madrigal F, Santiago Payan H, Meneses A, Dom nguez Malag n H, Rojas ME. Plasmacytoid myoepithelioma of the laryngeal region: a case report. Hum Pathol 1995;26:802 804.
130. Frierson HR Jr, Mills SE, Garland TA. Terminal duct carcinoma of minor salivary glands. A nonpapillary subtype of polymorphous low-grade adenocarcinoma. Am J Clin Pathol 1985;84:8 14.
131. Gnepp DR, Chen JC, Warren C. Polymorphous low-grade adenocarcinoma of minor salivary gland. An immunohistochemical and clinicopathologic study. Am J Surg Pathol 1988;12:461 468.
132. Dardick I, Kahn HJ, van Nostrand AW, Baumal R. Salivary gland monomorphic adenoma. Ultrastructural, immunoperoxidase, and histogenetic aspects. Am J Pathol 1984;115:334 348.
133. Dardick I, van Nostrand AW. Myoepithelial cells in salivary gland tumors revisited. Head Neck Surg 1985;7:395 408.
134. Hoa W, Kech PC, Swerdlow MA. Ultrastructure of the basal cell adenoma of parotid. Cancer 1976;37:1322 1333.
135. Kahn HJ, Baumal R, Marks A, Dardick I, van Nostrand AW. Myoepithelial cells in salivary gland tumors: an immunohistochemical study. Arch Pathol Lab Med 1985;109:190 195.
136. Batsakis JG, Luna MA, el-Naggar AK. Basaloid monomorphic adenomas. Ann Otol Rhinol Laryngol 1991;100:687 690.
137. Chaudhry AP, Leifer C, Cutler LS, Satchidanand S, Labay GR, Yamane GM. Histogenesis of adenoid cystic carcinoma of the salivary glands. Light and electronmicroscopic study. Cancer 1986;58:72 82.
138. Chen JC, Gnepp DR, Bedrossian CW. Adenoid cystic carcinoma of the salivary glands: an immunohistochemical analysis. Oral Surg Oral Med Oral Pathol 1988;65:316 326.
139. Williams SB, Ellis GL, Auclair PL. Immunohistochemical analysis of basal cell adenocarcinoma. Oral Surg Oral Med Oral Pathol 1993;75:64 69.
140. Hsueh C, Gonzalez-Crussi F. Sialoblastoma: a case report and review of the literature on congenital epithelial tumors of salivary glands origin. Pediatr Pathol 1992;12:205 214.
141. Dehner LP, Valbuena L, Perez-Atayde A, Reddick RL, Askin FB, Rosai J. Salivary gland anlage tumor ( congenital pleomorphic adenoma ). A clinicopathologic, immunohistochemical and ultrastructural study of nine cases. Am J Surg Pathol 1994;18:25 36.
142. Dardick I, Daya D, Hardie J, van Nostrand AW. Mucoepidermoid carcinoma: ultrastructural and histogenetic aspects. J Oral Pathol 1984;13:342 358.
143. Lomax-Smith JD, Azzopardi JG. The hyaline cell: a distinctive feature of mixed salivary tumors. Histopathology 1978;2:77 92.
144. Quintarelli G, Robinson L. The glycosaminoglycans of salivary gland tumors. Am J Pathol 1967;51:19 37.
145. Takeuchi J, Sobue M, Yoshida M, Esaki T, Kato Y. Pleomorphic adenoma of the salivary gland. With special reference to histochemical and electron microscopic studies and biochemical analysis of glycosaminoglycans in vivo and in vitro. Cancer 1975;36:1771 1789.
146. Caselitz J, Osborn M, Seifert G, Weber K. Intermediate-sized filament proteins (prekeratin, vimentin, desmin) in the normal parotid-gland and parotid-gland tumors. Immunofluorescence study. Virchows Arch A Pathol Anat Histopathol 1981;393:273 286.
147. Savera AT, Gown AM, Zarbo RJ. Immunolocalization of three novel smooth muscle-specific proteins in salivary gland pleomorphic adenoma: assessment of the morphogenetic role of myoepithelium. Mod Pathol 1997;10:1093 1100.
148. Caselitz J, Osborn M, Wustrow J, Seifert G, Weber K. The expression of different intermediate-sized filaments in human salivary glands and their tumours. Pathol Res Pract 1982;175:266 278.
149. Morinaga S, Nakajima T, Shimosato Y. Normal and neoplastic myoepithelial cells in salivary glands. An immunohistochemical study. Hum Pathol 1987;18:1218 1226.
150. Markaki S, Bouropoulou V, Milas C. S-100 protein and neuron specific enolase (NSE) immunoreactivity in pleomorphic adenomas of the salivary glands and its relationship to the composition of their extracellular matrix. Arch Anat Cytol Pathol 1987;35:211 216.
151. Franquemont DW, Mills SE. Plasmacytoid monomorphic adenoma of salivary glands. Absence of myogenous differentiation and comparison to spindle cell myoepithelioma. Am J Surg Pathol 1993;17:146 153.
152. Sternlicht MD, Safarians S, Rivera SP, Barsky SH. Characterizations of the extracellular matrix and proteinase inhibitor content of human myoepithelial tumors. Lab Invest 1996;74:781 796.
153. Sternlicht MD, Barsky SH. The myoepitelial defense: a host defense against cancer. Med Hypotheses 1997;48:37 46.
154. Savera AT, Sloman A, Huvos AG, Klimtra DS. Myoepithelial carcinoma of the salivary glands. A clinicopathologic study of 25 patients. Am J Surg Pathol 2000;24:761 774.
155. Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components. Immunohistochemistry with a cold ethanol-fixation technique. Immunology 1974;26:1101 1114.
156. Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components: differential localization of free and bound SC in secretory epithelial cells. J Immunol 1974;112:1553 1559.
157. Korsrud FR, Brandtzaeg P. Quantitative immunohistochemistry of immunoglobulin- and J-chain-producing cells in human parotid and submandibular salivary glands. Immunology 1980;39:129 140.
158. Lee SK, Lim CY, Chi JG, et al. Immunohistochemical study of lymphoid tissue in human fetal salivary gland. J Oral Pathol Med 1993;22:23 29.
159. Bernier JL, Bhaskar SN. Lymphoepithelial lesions of salivary glands: histogenesis and classification based on 186 cases. Cancer 1958;11:1156 1179.
160. Hsu SM, Hsu PL, Nayak RN. Warthin's tumor: an immunohistochemical study of its lymphoid stroma. Hum Pathol 1981;12:251 257.
161. Thompson AS, Bryant HC Jr. Histogenesis of papillary cystadenoma lymphomatosum (Warthin's tumor) of the parotid salivary gland. Am J Pathol 1950;26:807 849.
162. Azzopardi JG, Evans DJ. Malignant lymphoma of parotid associated with Mikulicz disease (benign lymphoepithelial lesion). J Clin Pathol 1971;24:744 752.
163. Brown RB, Gaillard RA, Turner JA. The significance of aberrant or heterotopic parotid gland tissue in lymph nodes. Ann Surg 1953;138:850 856.
164. Micheau C. Les ectopies salivaires. Arch Anat Cytol Pathol 1969;17:179 186.
165. Neisse R. ber den einschluss von parotisl ppen in lymphknoten. Anat Hefte (Wisbaden) 1898;10:289 306.
166. Bairati A. Constate concrescenza fra noduli linfatici ed adenomeri delle ghiandole salivari nell omo durante lo sviluppo e nell adulto. Arch Biol (Paris) 1932;43:415 450.
167. Youngs LA, Scofield HH. Heterotopic salivary gland tissue in the lower neck. Arch Pathol 1967;83:550 556.
168. Curry B, Taylor CW, Fisher AW. Salivary gland heterotopia: a unique cerebellopontine angle tumor. Arch Pathol Lab Med 1982;106:35 38.
169. Schochet SS Jr, McCormick WF, Halmi NS. Salivary gland rests in the human pituitary: light and electron microscopical study. Arch Pathol 1974;98:193 200.
170. Jernstrom P, Prietto C. Accessory parotid gland tissue at base of neck. Arch Pathol 1962;73:473 480.
P.469
171. Bouquot JE, Gnepp DR, Dardick I, Hietanen JH. Intraosseous salivary tissue: jawbone examples of choristomas, hamartomas, embryonic rests, and inflamatory entrapment: another histogenetic source for intraosseous adenocarcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:205 217.
172. Stafne EC. Bone cavities situated near the angle of the mandible. J Am Dent Assoc 1942;29:1969 1972.
173. de Courten A, K ffer R, Samson J, Lombardi T. Anterior lingual mandibular salivary gland defect (Stafne defect) presenting as a residual cyst. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:460 464.
174. Carney JA. Salivary heterotopia, cysts, and the parathyroid gland: branchial pouch derivatives and remnants. Am J Surg Pathol 2000;24:837 845.
175. Willis RA. The Borderland of Embryology and Pathology. London: Butterworth; 1962.
176. Willis RA. Some unusual developmental heterotopias. Br Med J 1968;3:267 272.
177. Dhawan IK, Bhargava S, Nayak NC, Gupta RK. Central salivary gland tumors of jaws. Cancer 1970;26:211 217.
178. Singer MI, Appelbaum EL, Loy KD. Heterotopic salivary tissue in the neck. Laryngoscope 1979;89:1772 1778.
179. Mart nez-Madrigal F, Pineda-Daboin K, Casiraghi O, Luna MA. Salivary gland tumors of the mandible. Ann Diag Pathol 2002;347 353.
180. Micheau C, Riou G. Oncocytes et oncocytomes. Histoenzymologie, ultrastructure et description de l' ADN mitochondrial. Arch Anat Pathol 1975;23:123 132.
181. Meza-Chavez L. Oxyphilic granular cell adenoma of the parotid gland (oncocytoma). Report of five cases and study of oxyphilic granular cells (oncocytes) in normal parotid glands. Am J Pathol 1949;25:523 538.
182. Shintaku M, Honda T. Identification of oncocytic lesions of salivary glands by anti-mitochondrial immunohistochemistry. Histopathology 1997;31:408 411.
183. Hamperl H. Oncocyten und Oncocytome. Virchows Arch A Pathol Anat Histopathol 1962;335:452 483.
184. Barka T. Biologically active polypeptides in submandibular glands. J Histochem Cytochem 1980;28:836 859.
185. Bing J, Poulsen K, Hackenthal E, Rix E, Taugner R. Renin in the submaxillary gland: a review. J Histochem Cytochem 1980;28:874 880.
186. Murphy RA, Watson AY, Metz J, Forssmann WG. The mouse submandibular gland: an exocrine organ for growth factors. J Histochem Cytochem 1980;28:890 902.
187. Whitley BD, Ferguson JW, Harris AJ, Kardos TB. Immunohistochemical localization of substance P in human parotid gland. Int J Oral Maxillofac Surg 1992;21:54 58.
188. Pikula DL, Harris EF, Desiderio DM, Fridland GH, Lovelace JL. Methionine enkephalin-like, substance P-like, and beta-endorphin-like immunoreactivity in human parotid saliva. Arch Oral Biol 1992;37:705 709.
189. Salo A, Ylikoski J, Uusitalo H. Distribution of calcitonin gene-related peptide immunoreactive nerve fibers in the human submandibular gland. Neurosci Lett 1993;150:137 140.
190. Mathison R, Davison JS, Befus AD. Neuroendocrine regulation of inflammation and tissue repair by submandibular gland factors. Immunol Today 1994;15:527 532.
191. Blanck C, Eneroth CM, Jakobsson PA. Oncocytoma of the parotid gland: neoplasm or nodular hyperplasia? Cancer 1970;25:919 925.
192. Batsakis JG. Tumors of the Head and Neck. Baltimore: Williams & Wilkins; 1974.
193. Dardick I, Bireck C, Lingen M, Rowe PE. Differentiation and the cytomorphology of salivary gland tumors with specific reference to oncocytic metaplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:691 701.
194. Henley JD, Rehan J, Oda D, Gown AM, Gnepp DR. Oncocytic metaplastic tumors of salivary gland origin. Oral Surg Oral Med Oral Pathol 1977;84:187.
195. Chang A, Harawi SJ. Oncocytes, oncocytosis, and oncocytic tumors. Pathol Annu 1992;27(pt 1):263 304.
196. Palmer TJ, Gleeson MJ, Eveson JW, Cawson RA. Oncocytic adenomas and oncocytic hyperplasia of salivary glands: a clinicopathological study of 26 cases. Histopathology 1990;16:487 493.
197. Gray SR, Cornog JL Jr, Seo IS. Oncocytic neoplasms of salivary glands: a report of fifteen cases including two malignant oncocytomas. Cancer 1976;38:1306 1317.
198. Hemenway WG, Allen GW. Chronic enlargement of the parotid gland. Hypertrophy and fatty infiltration. Laryngoscope 1959;69:1508 1523.
199. Isacsson G, Lundquist PG. Salivary calculi as an aetiological factor in chronic sialadenitis of the submandibular gland. Clin Otolaryngol Allied Sci 1982;7:231 236.
200. Dardick I, Jeans MT, Sinnott NM, Wittkuhn JF, Kahn HJ, Baumal R. Salivary gland components involved in the formation of squamous metaplasia. Am J Pathol 1985;119:33 43.
201. Fajardo LF, Berthrong M. Radiation injury in surgical pathology. Part III. Salivary glands, pancreas and skin. Am J Surg Pathol 1981;5:279 296.
202. Seifert G, Donath K. Zur pathoegenese des K ttner-Tumors der submandibularis. HNO 1977;25:81 92.
203. Beer GM, Neuwirth A. Nekrotisierende sialometaplasie (speicheldr seninfarkt) der glandula submandibularis. Laryngol Rhinol Otol (Stuttg) 1983;62:468 470.
204. Abrams AM, Melrose RJ, Howell FV. Necrotizing sialometaplasia. A disease simulating malignancy. Cancer 1973;32:130 135.
205. Fowler B, Brannon RB. Subacute necrotizing sialadenitis. Report of 7 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;89:600 609.
206. Seifert G, Donath K. Die sialadenose der parotis. Dtsch Med Wochenschr 1975;100:1545 1548.
207. Mandel L, Baurmash H. Parotid enlargement due to alcoholism. J Am Dent Assoc 1971;82:369 373.
208. Campos LA. Hyperplasia of the sublingual glands in adult patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;81:584 585.
209. Yu GY, Donath K. Adenomatous ductal proliferation of the salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:215 221.
210. Walker NI, Gob GC. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J Pathol 1987;153:333 344.
211. Daley TD, Dardick I. An unusual parotid tumor with histogenetic implications for salivary gland neoplasms. Oral Surg Oral Med Oral Pathol 1983;55:374 381.
212. Takahashi S, Schoch E, Walker N. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int J Exp Pathol 1998;79:293 301.
213. Alavres EP, Sesso A. Cell proliferation, differentiation and transformation in the rat submandibular gland during early postnatal growth. A quantitative and morphological study. Arch Histol Jpn 1975;38:177 208.
214. Thackray AC, Lucas RB. Tumors of the major salivary glands. In: Atlas of Tumor Pathology, Series 2, Fascicle 10. Washington DC: Armed Forces Institute of Pathology; 1974:11 14
215. Eversole LR. Histogenetic classification of salivary tumors. Arch Pathol 1971;92:433 443.
216. Batsakis JG. Salivary gland neoplasia: an outcome of modified morphogenesis and cytodifferentiation. Oral Surg Oral Med Oral Pathol 1980;49:229 232.
217. Dardick I, van Nostrand AW. Morphogenesis of salivary gland tumors. A prerequisite to improving classification. Pathol Annu 1987;22(pt 1):1 53.
218. Barka T. Induced cell proliferation: the effect of isoproterenol. Exp Cell Res 1965;37:662 679.
219. Dardick I, Dardick AM, MacKay AJ, Pastolero GC, Guillane PJ, Burford-Mason AP. Pathobiology of salivary glands. IV. Histogenetic concepts and cycling cells in human parotid and submandibular glands cultured in floating collagen gels. Oral Surg Oral Med Oral Pathol 1993;76:307 318.
220. Burgess KL, Dardick I, Cummins MM, Burford-Mason AP, Bassett R, Brown DH. Myoepithelial cells actively proliferate during atrophy of the rat parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;82:674 680.
P.470
221. Zarbo RJ. Salivary gland neoplasia: a review for the practicing pathologist. Mod Pathol 2002;15:298 323.
222. Dardick I, Burford-Mason AP. Current status of the histogenetic and morphogenetic concepts of salivary gland tumorigenesis. Crit Rev Oral Biol Med 1993;4:639 677.
223. Dardick I, van Nostrand AW, Jeans MT, Rippstein P, Edwards V. Pleomorphic adenoma. II. Ultrastructural organization of stromal regions. Hum Pathol 1983;14:798 809.
224. Micheau C, Lacour J. Epithelioma acineux de la parotide. Ann Anat Pathol 1971;16:173 188.
225. Mills SE, Garland TA, Allen MS Jr. Low-grade papillary adenocarcinoma of palatal salivary gland origin. Am J Surg Pathol 1984;8:367 374.
226. Garland TA, Innes DJ Jr, Fechner RE. Salivary duct carcinoma: an analysis of four cases with review of literature. Am J Clin Pathol 1984;81:436 441.
227. Chen KT, Hafez GR. Infiltrating salivary duct carcinoma. A clinicopathologic study of five cases. Arch Otolaryngal 1981;107:37 39.
228. Allen MS Jr, Fitz-Hugh GS, Marsh WL Jr. Low-grade papillary adenocarcinoma of the palate. Cancer 1974;33:153 158.
229. Batsakis JG, McClatchey KD, Johns M, Regezi JA. Primary squamous cell carcinoma of the parotid gland. Arch Otolaryngol 1976;102:355 357.
230. Mart nez-Madrigal F, Baden E, Casiraghi O, Micheau C. Oral and pharyngeal adenosquamous carcinoma. A report of four cases with immunohistochemical studies. Eur Arch Otorhinolaryngol 1991;248:255 258.
231. Luna MA, Batsakis JG, Ordonez NG, Mackay B, Tortoledo ME. Salivary gland adenocarcinomas: a clinicopathologic analysis of three distinctive types. Semin Diagn Pathol 1987;4:117 135.
232. Micheau C, Lacour J, Genin J, Brug re J. Tumeurs muco pidermo des de la parotide et de la cavit buccale. Ann Anat Pathol 1972;17:59 71.
233. White DK, Miller AS, McDaniel RK, Rothman BN. Inverted ductal papilloma: a distinctive lesion of minor salivary gland. Cancer 1982;49:519 524.
234. Foschini MP, Eusebi V. Value of immunohistochemistry in the diagnosis of salivary gland tumors. Pathol Case Rev 2004;9:270 275.
235. Foschini MP, Scarpellini F, Gown AM, Eusebi V. Differential expression of myoepithelial markers in salivary, sweat and mammary glands. Int J Surg Pathol 2000;8:29 37.
236. Zarbo RJ, Hatfield JS, Trojanowski JQ, et al. Immunoreactive glial fibrillary acidic protein in normal and neoplastic salivary glands: a combined immunohistochemical and immunoblot study. Surg Pathol 1988;1:55 63.
237. Fantasia JE, Lally ET. Localization of free secretory component in pleomorphic adenomas of minor salivary gland origin. Cancer 1984;53:1786 1789.
238. Caselitz J, Jaup T, Seifert G. Immunohistochemical detection of carcinoembryonic antigen (CEA) in parotid gland carcinomas. Analysis of 52 cases. Virchows Arch A Pathol Anat Histol 1981;394:49 60.
239. Gusterson BA, Lucas RB, Ormerod MG. Distribution of epithelial membrane antigen in benign and malignant lesions of the salivary glands. Virchows Arch A Pathol Anat Histol 1982;397:227 233.
240. Tsuji T, Nagai N. Production of alpha-fetoprotein by human submandibular gland. Int J Dev Biol 1993;37:497 498.
241. Caselitz J, Seifert G, Grenner G, Schmidtberger R. Amylase as an additional marker of salivary gland neoplasms. An immunoperoxidase study. Pathol Res Pract 1983;176:276 283.
242. Cenley J, Baker DC. Cancer of the salivary glands. In: Suen JY, Myers EN, eds. Cancer of the Head and Neck. New York: Churchill Livingstone; 1981.
243. Rankow RM, Mignogna F. Cancer of the sublingual salivary gland. Am J Surg 1969;118:790 795.