8 - Skeletal Muscle
Editors: Mills, Stacey E.
Title: Histology for Pathologists, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > IV - Nervous System > 11 - Central Nervous System
function show_scrollbar() {}
11
Central Nervous System
Gregory N. Fuller
Peter C. Burger
Introduction
The central nervous system (CNS) is unparalleled among natural systems in terms of structural and functional complexity. As a consequence of its intricate regional architecture, heterogeneous cellular constituents, and an associated extensive and somewhat arcane lexicon, the nervous system is often viewed as a formidably Byzantine realm by many nonneuropathologists; and yet, a working familiarity with the normal morphology of this complex organ must precede optimal evaluation of the many disease states that afflict it. To this end, this chapter will present the salient features of regional neuroanatomy followed by a description of the essentials of microscopic anatomy of the CNS, with special emphasis on those aspects that constitute potential diagnostic pitfalls, including normal anatomic variations, alterations associated with advancing age, reactive changes, and common artifacts.
Regional Neuroanatomy
We have limited this discussion of regional neuroanatomy to those principles of structural organization that are of practical value to the diagnostician, emphasizing the rudiments of neuroembryology by which the basic organization
P.274
of the nervous system is best understood. Further details about topographical neuroanatomy can be found in the list of suggested readings provided at the end of the chapter.
Organization of the Spinal Cord and Brainstem
Embryologically, the nascent CNS is a hollow tube formed by the invagination of the neural plate ectoderm. This primitive cylinder is subdivided functionally into a dorsal sensory (alar) plate and a ventral motor (basal) plate. The two are separated by a lateral groove, termed the sulcus limitans, along which develops the efferent autonomic system (Figure 11.1). This primitive organizational pattern is retained, essentially unaltered, in the mature spinal cord. The central gray matter consists of: (a) dorsal horns that receive sensory input from the dorsal roots; (b) ventral horns that contain motor neurons whose axons are conducted to the somatic periphery by the ventral roots; and (c) the lateral autonomic gray matter. Spinal autonomic neurons are confined to thoracic (sympathetic) and sacral (parasympathetic) levels, forming the intermediolateral cell columns. The axons of these preganglionic neurons exit the spinal cord through the ventral roots, ultimately to synapse on postganglionic neurons in the peripheral autonomic ganglia. The sympathetic intermediolateral cell column produces a third horn of gray matter in the thoracic cord, termed the lateral horn (Figures 11.1,11.2). The parasympathetic intermediolateral cell column occupies a similar lateral position in the sacral cord (at the S2, S3, and S4 levels) but does not form a distinct horn.
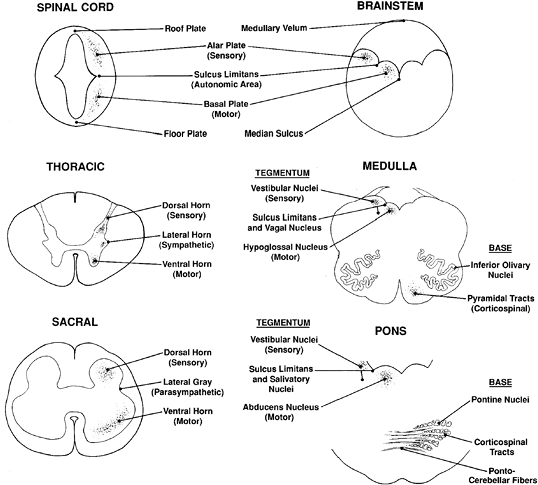 |
Figure 11.1 Structural organization of the spinal cord and brainstem. (See text for discussion.) |
Spinal Cord
The anatomy of the spinal cord varies according to the level (Figure 11.2). Two enlargements of the ventral horns, one in the cervical region (Figure 11.2A) and another in the lumbosacral region (Figure 11.2C), provide motor innervation for the upper extremities and lower extremities, respectively. In contrast, the ventral horns of the thoracic cord provide innervation for the more limited axial musculature of the trunk and are, accordingly, much smaller (Figure 11.2B). As mentioned earlier, the lateral horns of the gray matter (sympathetic neurons) are a unique feature of the thoracic cord. The thickness of the surrounding white matter fiber bundles (termed funiculi) also varies with cord level, being greatest in the cervical cord, where the thickness reflects the summated accrual of ascending fiber tracts that have successively entered at lower levels, as well as the maximum content of descending tracts that are en route to lower levels, and thinnest in the lumbosacral cord. The terminus of the spinal cord, the filum terminale, is composed primarily of meningeal connective tissue in the human and is discussed separately with the pia-arachnoid (Figure 11.56).
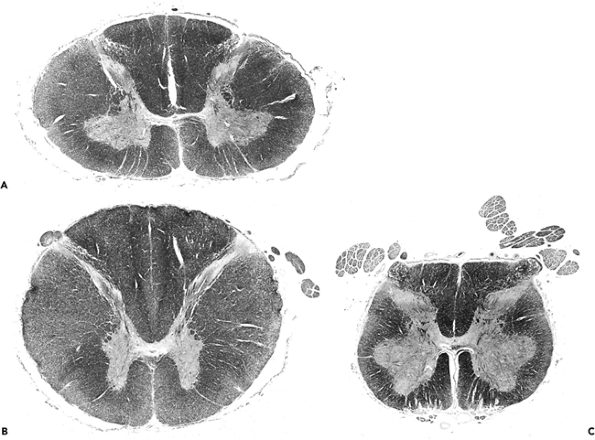 |
Figure 11.2 Spinal cord: regional variation of spinal cord structure is illustrated in these cross sections taken from the cervical enlargement (A), midthoracic cord (B), and lumbosacral enlargement (C). The cervical enlargement (A) is typified by an oval shape with large white matter funiculi and prominent, broad anterior gray horns that contain the motor neurons that innervate the upper extremities. In contrast, sections from thoracic cord (B) have a more rounded profile and exhibit small, slender, peglike anterior gray horns. In addition, lateral horns, which house the intermediolateral cell column neurons of the sympathetic nervous system, are unique to thoracic segments (see Figure 11.1). The lumbosacral cord (C) has very large anterior gray horns (motor supply to the lower extremities) like those of the cervical enlargement but only a very small surrounding mantle of white matter (see Figure 11.1). |
P.275
Brainstem
The brainstem is innately more complex than the spinal cord, but its basic organization is readily understood when viewed as a slightly modified version of the basic plan. Thus, the stem is also a neural tube but one that has been stretched dorsally and splayed out laterally so that the ventrally located embryonic motor plate is now medial and the dorsal sensory plate is lateral (Figure 11.1). Therefore, within the brainstem, the cranial nerve motor nuclei are located medially, the sensory nuclei laterally, and the autonomic nuclei are intermediate in position.
The brainstem can be further subcategorized in cross section into tectum, tegmentum, and base. The tectum is the roof of the ventricular system, as exemplified by the superior and inferior colliculi (corpora quadrigemina) of the midbrain and the superior medullary vela of the pons and medulla. The tegmentum forms the floor of the cerebral aqueduct and fourth ventricle and is divisible into the medial motor and lateral sensory areas discussed previously (Figure 11.1). The base is located subjacent to the tegmentum and is the most ventral portion of the stem. It is composed principally of the so-called long tracts that is, the descending motor pathways and ascending sensory pathways that link the spinal cord with higher neural centers. The combination of long tract signs with dysfunction of specific cranial nerves allows the precise anatomic localization of brainstem lesions by clinical examination.
Cerebellum
Embryologically, the cerebellum arises as a dorsal outgrowth of the fetal brainstem and remains connected to it in the adult by the three pairs of cerebellar peduncles: the superior (brachium conjunctivum), middle (brachium pontis), and inferior (restiform body). They join with the midbrain, pons, and medulla, respectively. The cerebellum is composed of three structural and functional compartments: cortex, medulla, and deep nuclei. The cortex
P.276
displays three distinct laminae: an outer hypocellular molecular layer, an intermediate single-cell thick Purkinje cell layer (described below), and a deep hypercellular granular cell layer (Figure 11.3). Before one year of age, the cerebellar cortex is conspicuous for remnants of a fourth layer of small neurons, the fetal external granular cell layer, which is located immediately subjacent to pia (Figure 11.3C). The external granular cells are gradually depleted during the first year of life as they descend the processes of Bergmann glia to reach their final position in the internal granular cell layer. Embedded within the white matter of the cerebellar medulla are four pairs of nuclei, from medial to lateral: fastigial, globosus, emboliform, and dentate. The dentate is by far the largest and is usually the only deep nucleus seen on routine sections. Its serpiginous profile is strikingly similar to that of the inferior olivary nucleus of the medulla oblongata (illustrated in Figure 11.1) which is a major source of afferent fibers to the cerebellum.
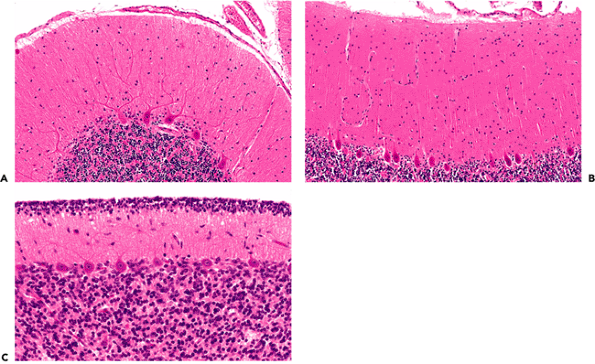 |
Figure 11.3 Cerebellar cortex. The adult cerebellar cortex is composed of three layers: an outer hypocellular molecular layer, middle Purkinje cell layer, and inner densely populated granular cell layer. Whereas the Purkinje cells are prototypically neuronal in appearance, the small cells of the granular layer are hardly recognizable as neurons by traditional histologic criteria (see Figure 11.7). A. A cross section of a cerebellar folium shows the typical broadly branching Purkinje cell dendritic arbor. B. However, sections taken parallel to the folia reveal the streamlined on edge appearance of the arbor, which should not be interpreted as pathological pruning. C. The fetal cerebellum has an additional cortical lamina, the external granular layer, applied to the surface of the cortex. This pool of cells populates the internal granular cell layer during development and is, thereby, depleted by the end of the first year of postnatal life. |
The Purkinje cell dendritic arbor extends into the molecular layer like a hand with outstretched fingers. Its broad, flat palm and radiating fingers are oriented perpendicular to the long axis of the cerebellar convolutions (folia). Thus, routine folia cross sections show the typical, elaborate dendritic branching pattern, whereas longitudinal sections present a dramatically different on edge view of the arbor (Figure 11.3). This should not be mistaken for pathologic dendritic tree pruning seen in some disease states.
Diencephalon
The diencephalon is interposed between the brainstem (midbrain, pons, and medulla) and the cerebrum. Four major divisions are recognized: epithalamus (pineal gland and habenula), thalamus, subthalamus, and hypothalamus. The medial and lateral geniculate nuclei of the thalamus are sometimes considered together as the metathalamus.
The strategic location of the thalamus is related to its major role in processing and relaying information passing between the cerebral cortex and brainstem and spinal cord. All sensory data (with the exception of olfaction) are processed by specific thalamic nuclei before distribution to the primary sensory cortices.
Of clinical significance to the pathologist, certain portions of the diencephalon immediately subjacent to the third ventricle (in particular, the large dorsomedial nuclei of the thalamus and the mammillary bodies of the hypothalamus) are often prominently involved in Wernicke's encephalopathy. The lesions at these sites are postulated to account for the memory disturbance that accompanies this disorder.
Cerebrum
Supratentorially, the CNS becomes so much more complicated that it is difficult to describe in terms of any general pattern of orientation. It remains a hollow structure, but
P.277
one that is no longer easy to consider as a tube with foldings and regional overgrowths. In light of this complexity, it is appropriate to review only those areas that are of particular diagnostic relevance.
Basal Ganglia
The term basal ganglia refers to the deep gray matter masses of the telencephalon and encompasses the caudate nucleus, putamen, globus pallidus, and amygdala (Figure 11.4). The term ganglion was formerly used interchangeably with nucleus, and ganglion cell was synonymous with neuron to earlier neuroanatomists. With the exception of the basal ganglia, the current definition of a ganglion is now generally restricted to mean a collection of neuronal cell bodies located outside the CNS, namely, the sensory and autonomic ganglia of the peripheral nervous system. Reference to CNS neurons as ganglion cells is still occasionally encountered, and this historical sense of the term is reflected in the names of such neoplastic entities as ganglioglioma, ganglioneuroma, and ganglion cell tumor.
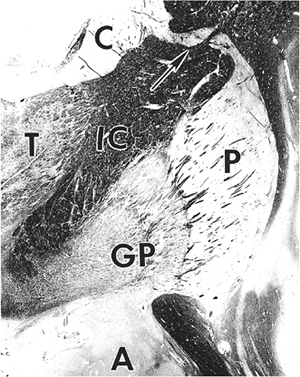 |
Figure 11.4 Basal ganglia. The gray matter of the telencephalon is broadly divisible into two components the superficial cortical gray matter mantle and the deep gray nuclei. The latter are known as the basal ganglia and consist of the caudate nucleus (C), putamen (P), globus pallidus (GP), and amygdala (A). The lenticular nucleus is composed of the medially situated, diffusely myelinated globus pallidus and the laterally placed putamen, whose myelinated fibers are grouped into slender fascicles known as the pencil bundles of Wilson. The internal capsule (IC) separates the lenticular nucleus from the caudate and the thalamus (T). Gray matter bridges (arrow) occasionally span the capsule to connect the caudate and putamen, a reflection of the close functional relationship between these two nuclei. The lenticular nucleus receives its blood supply from several lenticulostriate arteries, which are direct branches of the middle cerebral artery, and is the most common site of intracerebral hypertensive hemorrhage and lacunar infarction. The large lenticulostriate artery coursing through the lateral putamen was known in former times as Charcot's artery, or, more colorfully, as the artery of internal hemorrhage. The lenticulostriate vessels are often surrounded by dilated perivascular spaces that should not be mistaken for lacunar infarcts. |
The amygdala (archistriatum) is located in the mesial temporal lobe immediately rostral to the hippocampus and is functionally related to the limbic system. The remaining nuclei of the basal ganglia play an integral role in the modulation of motor function and probably participate in other higher neural systems as well. The caudate nucleus, as the name implies, has a long tapering tail that intimately follows the curvature of the lateral ventricle. The caudate is morphologically and functionally closely related to the putamen. These two nuclei are appropriately referred to collectively as the neostriatum, or simply the striatum. For descriptive purposes, the putamen and the medially situated globus pallidus (paleostriatum, or pallidum) are collectively referred to as the lenticular (lentiform) nucleus. The putamen and globus pallidus are separated from one another by the external medullary lamina of the globus pallidus, whereas the globus pallidus is itself divided into medial and lateral segments by the internal medullary lamina. The globus pallidus (pale globe) is so named because of its pallor in the fresh state compared to the putamen. This contrast is attributable histologically to the dense meshwork of myelinated fibers in the globus pallidus. In contrast, myelinated axons in the putamen are grouped into slender fascicles (pencil bundles of Wilson) that project medially to the globus pallidus and to the substantia nigra. The histologic appearance of the lentiform nucleus is distinctive and permits unambiguous identification of even very limited amounts of tissue from this site.
The basal ganglia are prominently involved in a variety of pathological processes, including kernicterus (literally, nuclear jaundice) in the neonate and lacunar infarction in adults. Carbon monoxide poisoning classically produces selective necrosis of the inner segment of the globus pallidus. A frequent incidental finding of no diagnostic significance on routine sections of the lentiform nucleus is micronodular mineralization of small blood vessels, which is typically most prominent in the globus pallidus. Histologically, similar micronodular mineralization is also commonly seen in the hippocampus (Figure 11.5C).
Hippocampal Formation
The hippocampal formation comprises the subiculum, Ammon's horn (hippocampus proper), and dentate gyrus (Figure 11.5). In coronal sections of the medial temporal lobe, the subiculum forms the inferior base of the hippocampal formation, joining the parahippocampal
P.278
P.279
gyrus with Ammon's horn. Ammon's horn, routinely abbreviated CA (for cornu Ammonis), is divided into four regions, CA1 through 4, based on cytological architecture and synaptic connectivity. (This nomenclature was introduced by Lorente de No in 1934.) The superiorly arching CA1 is joined by CA2 to form the medial floor of the temporal horn of the lateral ventricle. The dorsally situated CA2 is usually recognizable by the greater compactness of the pyramidal cell layer, as compared to CA1, and CA3 forms a descending medial arch that terminates in the hilus of the dentate gyrus. The final segment of Ammon's horn, CA4, lies within the hilus of the dentate gyrus and is often referred to as the end-plate. Essentially equivalent to Sommer's sector, CA1 is the zone that is most sensitive to various insults, including seizures, ischemia, and Alzheimer's disease changes. In contrast, the adjacent CA2 segment is known as the dorsal resistant zone, in recognition of its relative sparing compared to the other three sectors. The exquisite sensitivity of CA1 to injury, with sparing of the adjacent CA2, is routinely observed as mesial sclerosis of the hippocampus, which is seen in many temporal lobes resected for intractable epilepsy. The classic histologic description of the pattern of neuronal loss in Ammon's horn was based on observations made on the brains of such epileptic patients by Wilhelm Sommer in 1880; E. Brotz coined the term Sommer's sector in 1920.
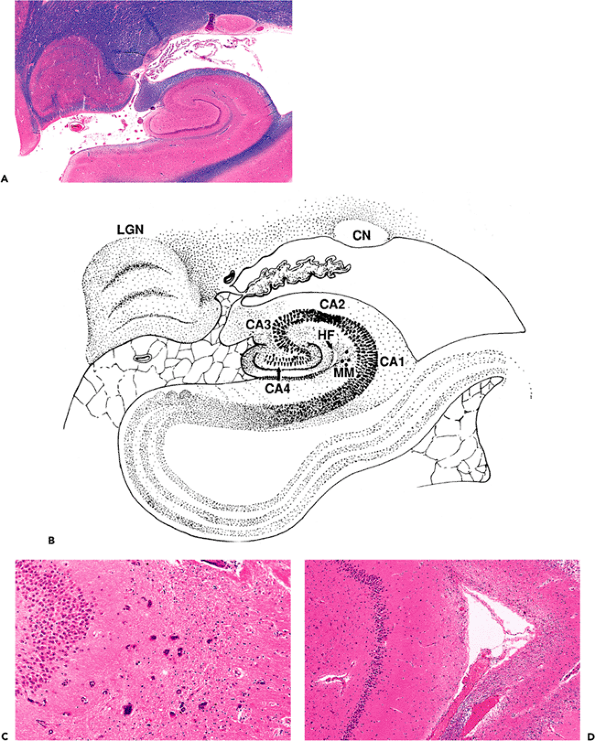 |
Figure 11.5 Hippocampal formation. The hippocampal formation is composed of the subiculum, Ammon's horn (cornu Ammonis, abbreviated CA; divided into regions CA1 CA4), and the dentate gyrus (A, B). CA1 is equivalent to Sommer's sector and is the region of the hippocampus that is most sensitive to a variety of insults. In contrast, the adjacent CA2 region is known as the dorsal resistant zone. Two common incidental findings are also illustrated micronodular mineralization (B, C), which is also seen in the globus pallidus, and a residual hippocampal fissure (B, D), which should not be misinterpreted as a healed infarct. [CN, tail of the caudate nucleus; HF, residual hippocampal fissure; LGN, lateral geniculate nucleus (note the Napoleon's hat profile and distinctive lamination); MM, micronodular mineralization. |
There are several notable features that are frequently encountered incidentally in the examination of routine hippocampal sections and can be mistakenly interpreted as evidence of disease. One is an asymptomatic micronodular mineralization comparable to that seen in the globus pallidus. In the hippocampal formation, it is most commonly seen just outside the apex of the dentate gyrus (Figure 11.5B, C). A second common finding is a residual hippocampal fissure, which produces a rarefied lamina or cystic cleft that can be mistaken for a healed infarct (Figure 11.5B, D). In addition, pyramidal neurons of the CA are often dark and shrunken in autopsy material, and care must be taken not to overinterpret such changes as evidence of ischemia (Figures 11.16, 11.17).
Cerebral Cortex
From antiquity, neuroscientists have sought to divide the cortical mantle into discrete, functionally significant units. Early efforts yielded fanciful maps akin to those of phrenology and physiognomy. More recently, the application of light microscopy and special staining techniques for cell bodies (Nissl stains), dendritic arbors and unmyelinated axons (Golgi's stains), and the myelin sheaths of myelinated axons (myelin stains such as the Weil's, Weigert's, and Luxol fast blue methods) have permitted a more scientific approach, although the details are beyond the scope of this chapter. In brief, the parcellation of the cortex is based on regional variation in the relative number, composition, and distribution of cortical neurons and their processes (cytoarchitectonics and myeloarchitechtonics). Various neuroanatomists have divided the cortex into a number of regions, varying from 20 to more than 200 and depending on the particular morphologic criteria and degree of subtlety employed. Currently, the most popular cortical map is that devised by Korbinian Brodmann in 1909. Brodmann's map and the classical nomenclature for the gross anatomy of the cerebral sulci and gyri are the two systems most commonly used at present for reference purposes in the neuroanatomic and clinical literature.
Within the context of even the simplest cortical map, it is generally not possible to assign a given histologic section of cortex to a precise anatomic locus without prior knowledge of the section's provenance. However, two cortical areas do exhibit distinctive features: primary motor cortex and primary visual cortex. The motor cortex, located on the precentral gyrus of the frontal lobe, is distinguished by the presence of the giant pyramidal cells of Betz. Pyramidal cells generally range from 10 to 50 mm in soma height from base to origin of the apical dendrite. By comparison, Betz cells may exceed 100 mm in soma height. The primary visual cortex, located on the banks of the calcarine fissure of the medial occipital lobe, is remarkable for the presence of a prominent external band of Baillarger, termed the line (or stria) of Gennari. This myelinated stratum located in lamina IV is usually visible to the naked eye and permits exact delineation of the primary visual cortex (Brodmann area 17) from the adjacent visual association cortex (Brodmann area 18).
Cellular Constituents of the Central Nervous System
Gray Matter and White Matter
By volume, most of the central nervous system is composed of gray matter and white matter. Specialized types of CNS tissues include the choroid plexus, pineal gland, circumventricular organs, and the infundibulum and neurohypophysis (discussed later). The hallmark of gray matter (Figure 11.6A) is the presence of neuronal cell bodies embedded within a finely textured eosinophilic background termed neuropil (Figure 11.6B, C). Neuropil (literally, nerve felt) is an interwoven meshwork of neuronal and glial cell processes. The individual neurites that compose the neuropil are not generally distinguishable in routine hematoxylin-eosin (H&E) stained sections (Figure 11.6B) but are resolvable at the ultrastructural level (Figure 11.6C). White matter, in contrast, is composed primarily of myelinated axons and the supporting cells, oligodendroglia, that produce and maintain the myelin sheaths (Figure 11.6D).
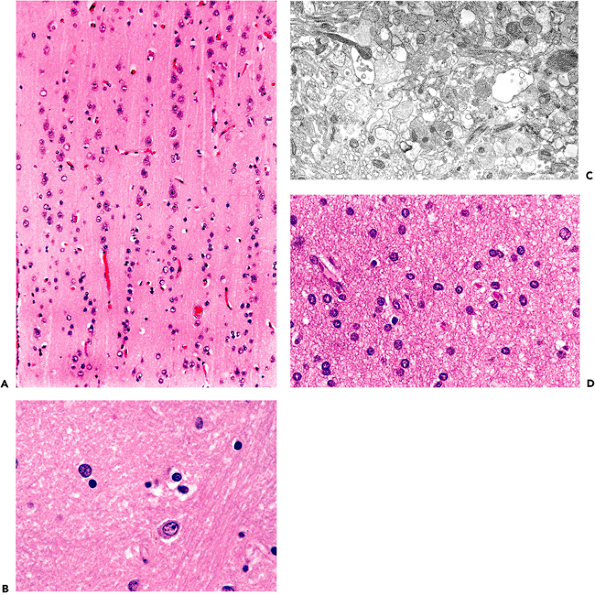 |
Figure 11.6 Gray matter and white matter. A. Gray matter contains abundant neuropil surrounding large neurons and smaller astrocytes and oligodendroglia. B. Neuropil is the term used for the fine amorphous eosinophilic background matrix of the central nervous system that fills the space between the cell bodies of the various cellular constituents as seen on H&E stains. C. Ultrastructural examination shows the neuropil to be composed of myriad intimately intermingling processes of the cellular constituents. D. White matter, in contrast, is composed primarily of oligodendroglia and the axons that they myelinate, and it displays a much more uniform appearance. |
P.280
Neurons
Normal Microscopic Anatomy
The prototypical neuron is exemplified by the large multipolar Betz cells of the motor cortex, the motor neurons of the ventral horn of the spinal cord, and the Purkinje cells of the cerebellum. These neurons are characterized by large perikarya (cell bodies or somas) with abundant Nissl substance (rough endoplasmic reticulum), robust dendritic arborizations, and large nuclei with prominent single nucleoli (Figure 11.7A). Such large multipolar forms, however, represent only one type of neuron; the diapason of neuronal morphologies is remarkable. This is readily apparent by comparison of a motor neurons with granular cell neurons (Figure 11.7A, D). These two neuronal populations typify the classical dichotomous subdivision of CNS neurons into large extroverted projection neurons with long axons (Golgi type I neurons) and small introverts that function regionally with restricted connections (Golgi type II
P.281
neurons). Between these two poles is a full spectrum of neuronal sizes and shapes, with an equally impressive variety of dendritic arbor configurations. The details of the latter are generally appreciable only with special stains for neuronal processes. The cell processes of neurons are separated into two categories: axons (Figure 11.7A C), of which each neuron only has one, and dendrites (Figure 11.7A), which are often multiple.
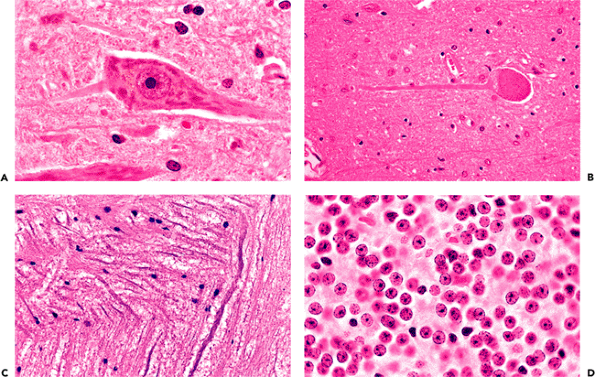 |
Figure 11.7 Neurons. A. Classical neuronal features, as illustrated by a motor neuron from the ventral horn of the spinal cord, include a large cell body (soma, perikaryon) with abundant cytoplasmic Nissl substance (rough endoplasmic reticulum, the tigroid substance of early microscopists), cytoplasmic processes, and a large nucleus with a single prominent nucleolus. The large process extending to the right is clearly recognizable as a dendrite by its content of Nissl substance, whereas, in this fortuitous section, the smaller process extending to the left is identified as the neuron's axon by its lack of Nissl substance. B. Axons are further distinguished from dendrites by their nontapering profile. C. The nontapering profile of axons is easily recognized in white matter. The extremes of neuron size and shape are readily apparent from a comparison of a large motor neuron (A) with the small granular cell neurons of the cerebellar cortex (D) that are approximately the same size as a motor neuron's nucleolus! |
With respect to morphologic variants, one unique population of CNS neurons merits brief mention. The mesencephalic nucleus of the trigeminal nerve, which is concerned chiefly with the mediation of jaw proprioception, is composed of true primary (first order) sensory neurons that possess only a single process emanating from the cell body (Figure 11.8A). This nucleus constitutes the only intraparenchymal example of this class of neurons; all other primary sensory neuronal perikarya are gathered outside the CNS in the spinal and cranial nerve ganglia (Figure 11.8B D).
Immunohistochemistry
Antibodies have been raised against a wide variety of the many unique neuronal proteins that are being isolated and characterized at an ever-increasing rate. A majority of these markers are confined to use for research purposes but several have found utility in the diagnostic laboratory. One of the earliest such markers, neuron specific enolase (NSE), has proven notoriously unreliable as a marker of neuronal differentiation. Its use for this purpose in evaluating neoplasms of the central nervous system is not recommended.
P.282
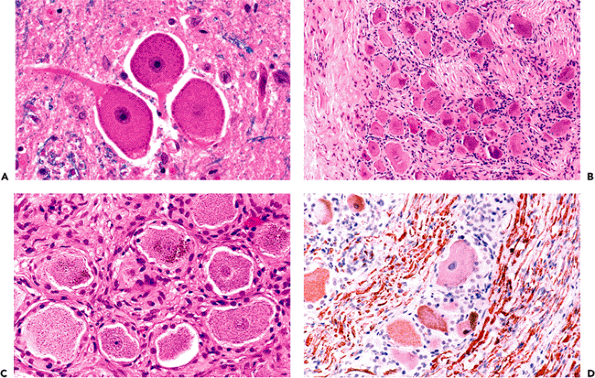 |
Figure 11.8 Unipolar neurons. Another neuronal morphologic variant is the unipolar (pseudounipolar) neuron. These large neurons possess only a single cell process, an axon, but no dendrites. A. Unipolar neurons are primary sensory neurons, and the only example of this class of neuron within the central nervous system is the mesencephalic nucleus of the trigeminal nerve in the upper pons and midbrain lateral to the periaqueductal gray matter. B. All other unipolar neurons are located in the peripheral nervous system ganglia. The dorsal root ganglia of the spinal cord provide a good example, with large unipolar neurons surrounded by satellite cells (B). C. Ganglionic neurons typically display cytoplasmic pigment. D. Their cell bodies and axons are strongly positive for phosphorylated neurofilament proteins. |
Antibodies directed against epitopes on the constituent proteins of neurofilaments, which are major cytoskeletal elements of the neuronal perikaryon and cytoplasmic processes, have been used extensively in both experimental and clinical studies (Figure 11.9).
One of the most useful and widely employed neuronal markers is synaptophysin. Synaptophysin is an integral membrane protein of synaptic vesicles. In the normal nervous system, antisynaptophysin antibodies yield a diffuse, finely granular pattern throughout the gray matter neuropil (Figure 11.10A). In addition, punctate granular decoration is seen along the cell bodies and proximal dendrites of several types of large, projection class neurons, including the Purkinje cells of the cerebellum, motor neurons of the spinal cord, extraocular motor neurons of the brainstem, and Betz cells of the precentral gyrus (Figure 11.10B).
Age-Related Neuronal Inclusions
A variety of inclusions, largely intracytoplasmic, appear with increasing frequency as we age. By far, the most common is lipofuscin (lipochrome, or aging pigment), whose yellow-to-pale-brown color is unaltered by most histologic procedures, including the H&E method (Figure 11.11). Its autofluorescence and partial avidity for the acid-fast stain can be used to visualize differentially this wear-and-tear pigment, although little functional significance is generally assigned to lipochrome accumulation in normal aging. In larger neurons, lipofuscin may accumulate to such an extent that it displaces organelles and creates an appearance similar to the cell swelling of central chromatolysis, described below (Figure 11.18). The lateral geniculate body provides an example of a densely populated nucleus whose constituent neuron's prominent accumulation of lipofuscin
P.283
is often discernible macroscopically as a distinctly mahogany hue compared to adjacent cortex. Interestingly, lipofuscin accumulation is not simply a function of cell size because some classes of large neurons appear comparatively immune to significant accrual (e.g., the cerebellar Purkinje cells).
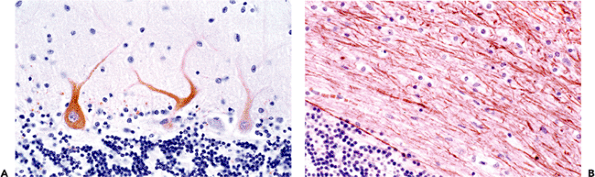 |
Figure 11.9 Neurofilament proteins (NFPs). Antibodies directed against neurofilament protein epitopes illuminate the cytoskeleton of neurons and their processes. As illustrated in the cerebellar cortex, specific antibodies directed against either nonphosphorylated (A) or phosphorylated (B) NFPs differentially identify cell bodies and dendrites or axons, respectively. |
Functionally more significant neuronal inclusions that may be seen in asymptomatic individuals are those associated with Alzheimer's disease. They are neurofibrillary tangles, neuritic plaques, granulovacuolar degeneration, and Hirano bodies. These illustrate the often ill-defined distinction between health and disease, because these changes can be seen in the elderly, albeit in limited numbers, in the absence of antemortem disturbances of mentation.
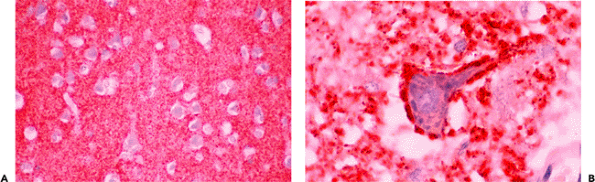 |
Figure 11.10 Synaptophysin is one of the most useful and widely employed markers of neuronal differentiation. A. The neuropil of gray matter, which is rich in synaptic contacts, shows a diffuse, finely granular pattern. B. Several specific types of large projection class neurons show prominent punctate decoration of the cell body and proximal dendrites, as illustrated here by a motor neuron in the hypoglossal nucleus of the medulla. Other groups of large neurons exhibiting this pattern of synaptophysin immunopositivity include Purkinje cells of the cerebellum, motor neurons of the ventral horn of the spinal cord, and Betz cells of the precentral gyrus in the cerebral cortex. |
In some cases, neurofibrillary tangles may be found in asymptomatic individuals in occasional neurons of the subiculum or CA. Although silver stains greatly aid visualization and quantitation, these structures may be identified on routinely stained H&E sections if the observer is familiar with their appearance. In pyramidal cells, they appear as a slightly basophilic wisp of faintly fibrillar material that extends out into cell processes, most notably the apical dendrite, and they are often more prominent on one side of the nucleus (flame-shaped tangle; Figure 11.12A). This morphology reflects the fact that tangles generally conform to the shape of the cell body. For example, in the pigmented neurons of the locus ceruleus, which are multipolar and lack the dominant apical dendrite of pyramidal neurons,
P.284
P.285
tangles that are globular in shape are occasionally encountered as an incidental finding.
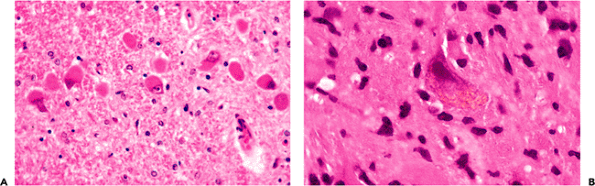 |
Figure 11.11 Lipofuscin. A. Prominent accumulation of lipofuscin pigment in large neurons with increasing age, as seen for example in the lateral geniculate nucleus, can result in peripheral displacement of the nucleus and Nissl substance, mimicking central chromatolysis. B. As a practical application of normal neurohistology, the presence of lipofuscin can sometimes aid in the identification of neurons in intraoperative frozen sections. As illustrated here, a neuron, identified by its lipofuscin content, is surrounded by tumor cells, thereby supporting a diagnosis of infiltrating glioma. |
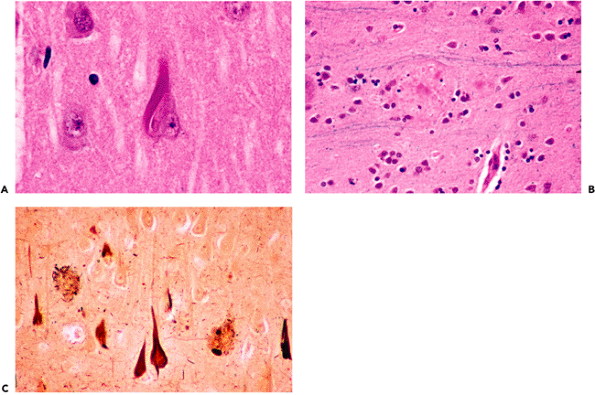 |
Figure 11.12 Neurofibrillary tangles and neuritic plaques. Neurofibrillary tangles (A) may be seen sporadically in the hippocampal formation of aging brains and have a fibrillary texture in H&E-stained sections. Mature senile (neuritic) plaques (B) deform the smooth texture of the neuropil and appear in H&E-stained sections as spherical, somewhat granular foci with a central eosinophilic core that is composed of amyloid. Note that adjacent myelinated axons are focally displaced as they pass by the plaque. In earlier stages, the plaques are less well defined and are not identifiable in H&E-stained sections. Although both neurofibrillary tangles and large mature neuritic plaques can be seen in H&E-stained sections, use of special techniques, such as silver stains (C), greatly facilitates visualization and quantitation. |
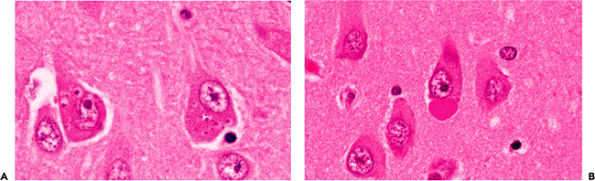 |
Figure 11.13 Granulovacuolar degeneration (of Simchowitz) (A) and Hirano bodies (B). Like neurofibrillary tangles, both of these intracytoplasmic inclusions can occasionally be seen in the hippocampal formation of normal older individuals. However, although these alterations are not pathognomonic for dementing illness, an appreciable number of affected cells should prompt a search for evidence of Alzheimer's disease in the form of a thorough examination for senile (neuritic) plaques and neurofibrillary tangles. |
The senile plaque is also a manifestation of cell injury, but one that, like slight atherosclerosis, is not an unexpected finding in the brains of asymptomatic adults. In such individuals, the plaque is usually seen in its primitive form as a somewhat ill-defined, roughly circular region of abnormal argyrophilic neurites that is not visualized in the H&E-stained section. As the plaques mature they become visible in the latter preparation, particularly when a central core of eosinophilic amyloid appears (Figure 11.12B). The latter can be more readily seen by Congo red or periodic acid-Schiff (PAS) staining. As noted earlier, both neurofibrillary tangles and neuritic plaques are more easily identified and quantitated with special techniques such as immunofluorescence or silver stains (Figure 11.12C).
Two additional intraneuronal inclusions that are seen in Alzheimer's disease, but only rarely in nondemented individuals, are granulovacuolar degeneration (GVD) and Hirano bodies (Figure 11.13). As the name implies, the inclusion of GVD consists of a dark, basophilic granule inside a small, clear vacuole. Clusters of these cytoplasmic inclusions may be present within a single neuron (Figure 11.13A). Depending on the plane of section, Hirano bodies appear in H&E-stained sections as brightly eosinophilic oval, elliptical, or elongated rodlike refractile inclusions that are located either in very close apposition to a neuronal perikaryon (Figure 11.13B) or within the neuropil. Ultrastructural examination supports a localization in neuronal cell bodies and processes, and immunohistochemical studies reveal the presence of actin and actin-associated proteins. Unlike neurofibrillary tangles and neuritic plaques, both GVD and Hirano bodies exhibit a very limited neuroanatomic distribution and are, in fact, virtually confined to the hippocampal formation. Encountering more than one or two cells with these alterations should raise the issue of Alzheimer's disease and prompt a search for other attendant histologic features.
A particularly striking cytoplasmic inclusion occasionally encountered in routine sections of the hypoglossal nuclei of the medulla (less often in the ventral horn motor nuclei of the spinal cord) is the hyalin (colloid) inclusion (Figure 11.14). These inclusions, which consist of ectatic cisternae of endoplasmic reticulum, are rarely seen in the first few decades of life but appear with increasing
P.286
frequency thereafter. They are occasionally mistaken by the uninformed for viral inclusions.
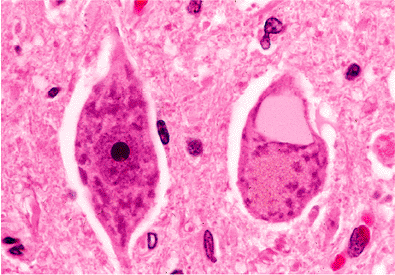 |
Figure 11.14 Hyalin (colloid) inclusion: these eye-catching inclusions, as seen in the neuron on the right, may be observed sporadically throughout the neuraxis but are most commonly encountered in the large motor neurons of the hypoglossal nuclei in the medulla (as in this micrograph). Less frequently, they may be seen in the motor neurons of the ventral horn of the spinal cord. Electron microscopic examination reveals ectatic cisternae of endoplasmic reticulum. |
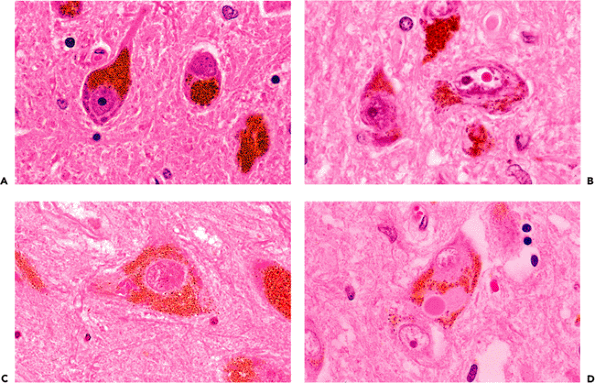 |
Figure 11.15 Pigmented neurons of the brainstem. A. Neuromelanin is a coarse, dark brown cytoplasmic pigment that is formed as a byproduct of catecholamine synthesis and is frequently encountered microscopically in scattered catecholaminergic neurons distributed widely throughout the brainstem. Two large populations are visible grossly: the substantia nigra (black substance) of the midbrain and the locus ceruleus (blue spot) of the pons. B. Marinesco bodies are eosinophilic, spheroidal, paranucleolar bodies that are often observed in the nuclei of pigmented neurons, especially those of the substantia nigra. The number of Marinesco bodies increases with advancing age and can be quite striking in some individuals. They should not be mistaken for intranuclear viral inclusions. C. Clusters of minute intracytoplasmic eosinophilic granules, seen in this micrograph to the left of the nucleus, may occasionally catch the eye of an obsessive observer. They have no known pathologic significance and are much smaller than Lewy bodies (D), which are the characteristic intracytoplasmic inclusions of Parkinson's disease. |
Catecholaminergic neurons throughout the brainstem gradually accumulate neuromelanin as a byproduct of neurotransmitter synthesis. The largest and most densely populated of these nuclei is the substantia nigra (black substance), which contains dopaminergic neurons. The locus ceruleus (blue spot), which is also seen by the unaided eye, is a collection of noradrenergic neurons in the rostral pontine tegmentum. It is of practical importance that the Lewy bodies of Parkinson's disease can be found in both of these neuroanatomic locales. Of the smaller and more diffusely distributed pigmented neurons, those in the vicinity of the dorsal motor nucleus of the vagus nerve in the medulla oblongata are most commonly encountered during routine histologic examination. Microscopically, neuromelanin appears as coarse, dark brown granules (Figure 11.15A) and should not be confused with melanocytic melanin. The latter is also present in the central nervous system but is confined to leptomeningeal melanocytes as discussed below (see Figure 11.57).
Several eosinophilic inclusions may be seen in pigmented brainstem neurons. The most striking of these are the commonly encountered Marinesco bodies (Figure 11.15B). These bright red, hyalin-appearing structures are located within the nucleus, often adjacent to and about the same size as a nucleolus (an alternative designation is paranucleolar body). Multiple Marinesco bodies may occur within a single nucleus, and, in some cases, a large percentage of pigmented neurons exhibit these eye-catching inclusions. In such cases, they may raise concern about a viral infection to the unaccustomed observer but are not
P.287
pathologic and have yet to be correlated with any significant process except advancing age. Two types of eosinophilic inclusions may be encountered in the cytoplasm of pigmented neurons. Clusters of diminutive acidophilic granules are occasionally noted (Figure 11.15C) but have no pathologic significance. Lewy bodies, in contrast, are much larger, notably displace the cytoplasmic neuromelanin from which they are separated by a small clear halo, and are associated with Parkinson's disease (Figure 11.15D).
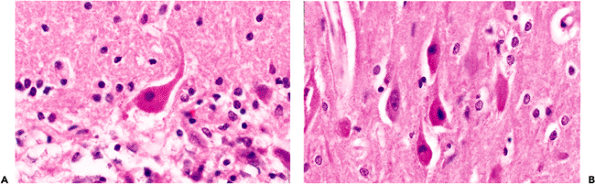 |
Figure 11.16 Ischemic injury: the sine qua non of ischemic damage to the nervous system is the so-called red neuron. As illustrated here by a Purkinje cell of the cerebellum (A) and pyramidal neurons of the hippocampal CA1 region (B), the soma (cell body) is shrunken, the cytoplasm is intensely eosinophilic, and the nucleus is pyknotic with no discernible nucleolus. It is largely the pronounced eosinophilia that distinguishes this cellular alteration from autolytic neuronal condensation, in which the cytoplasm is dark and basophilic (Figure 11.17). |
Autolysis and Basic Neuronal Reactions to Injury
As captured in their normal state by perfusion fixation or rapid immersion fixation, neurons are generally rotund with lightly eosinophilic cytoplasm that is stippled with basophilic Nissl substance in the case of the larger neurons. The surrounding glia are inconspicuous, and few clear vacuoles are seen. This perfection in fixation is rarely achieved in human material, however; and, in virtually all autopsy and surgical specimens, autolysis alters this ideal appearance to a greater or lesser extent. Neurons are, thereby, rendered somewhat contracted and basophilic. Nuclei are also somewhat condensed. Simultaneously, the processes of glia that surround neurons and blood vessels imbibe water to produce clear vacuoles (Figure 11.29). The neuronal response to injury overlaps in some cases with these autolytic changes, and it may not be possible to distinguish agonal hypotensive injury from autolysis in autopsy specimens. In the former setting, the neuronal contraction is pronounced, and the perineuronal and perivascular spaces are exceptionally prominent.
There are, however, three neuronal changes that provide unequivocal evidence of antemortem injury. One is the red neuron, which is the sine qua non of ischemic damage. The second is central chromatolysis, and the third is ferruginization. The red neuron is characterized by a shrunken cell body and intense cytoplasmic eosinophilia with complete loss of Nissl basophilia (Figure 11.16). The nucleus is dark and usually lacks a distinguishable nucleolus but may be pale and demonstrate early karyolysis. At times, it has a somewhat fragmented look suggesting karyorrhexis, although clearly defined karyorrhexis is rare. In surgical specimens, tissue-handling artifact ( crush artifact) also results in dark, shrunken neuronal perikarya; however, as with autolytic autopsy specimens, these cells lack the distinctive cytoplasmic eosinophilia of ischemia (Figure 11.17).
Central chromatolysis, the second unequivocally abnormal finding, consists of a loss of central basophilic staining of the cell body with peripheral margination of the Nissl substance (Figure 11.18). It is seen in a number of pathologic
P.288
states (the ballooned anterior horn cells of poliomyelitis being the classical example). There are numerous mimickers that lie in wait for the unwary. For example, some normal neuronal populations, such as the supraoptic and paraventricular nuclei of the hypothalamus and the dorsal nucleus of Clarke of the thoracic spinal cord, display Nissl substance that is preferentially distributed peripherally in the soma. Other neurons, such as those of the mesencephalic nucleus of the trigeminal nerve discussed previously (Figure 11.8), have large, exquisitely rounded somas and, hence, mimic that aspect of chromatolysis. The giant pyramidal cells of Betz in the motor cortex are so large in comparison to surrounding neurons, that at low magnification they may give an initial impression of chromatolytic swelling. Finally, as discussed earlier, one must be careful not to mistake the accumulation of various substances that displace the Nissl substance peripherally, such as lipofuscin, for central chromatolysis (Figure 11.11).
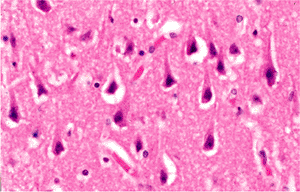 |
Figure 11.17 Neuronal contraction as a tissue-handling artifact. In contrast to ischemic insult (Figure 11.16), in crush artifacts the cytoplasm is dark and basophilic rather than brightly eosinophilic. |
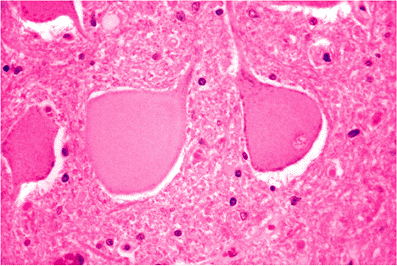 |
Figure 11.18 Central chromatolysis. Cytoplasmic hyalinization and swelling with peripheral displacement of the nucleus and lipofuscin can be a response to either intrinsic neuronal disease (such as in poliomyelitis or other viral infections) or to interruption of the axon in close proximity to the cell body. In the latter setting, the term axonal reaction is applied. |
A striking finding sometimes encountered near old infarcts is the presence of ferruginized or fossilized neurons, in which both perikarya and axons are encrusted by blue-staining minerals (Figure 11.19). This arresting phenomenon is not limited to the vicinal tissue of old infarcts, although this is the most common context, nor is it confined to the adult nervous system, because prenatal insults may result in similar findings. Clusters of axons thus affected can be mistaken for fungal hyphae (Figure 11.20).
A common reaction of axons to injury seen in a wide variety of pathologic states is the formation of localized dilatations known as axonal spheroids or axon retraction balls (Figure 11.21A). Ultrastructural examination shows greatly distended axis cylinders filled with bundles of neurofilaments and cellular organelles. A regional variant of this process may be observed in the granular cell layer of the cerebellum where focal dilatations of Purkinje cell axons are termed torpedoes. These structures are seen in a number of cerebellar degenerative diseases, as well as in normal aging. The most common site in the central nervous system where scattered axonal spheroids are routinely encountered as an incidental finding in aged individuals is in the rostral fasciculus gracilis of the medulla (Figure 11.21B). Spheroids in this location are often mineralized.
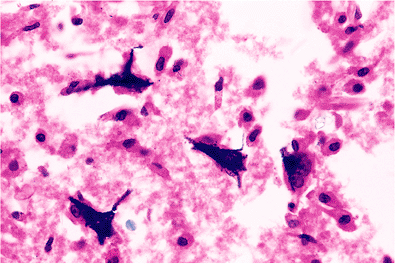 |
Figure 11.19 Mineralized (ferruginized) neurons. These encrusted relics resembling petrified tree trunks are most commonly encountered around the margins of old infarcts. |
Astrocytes
Normal Microscopic Anatomy
Like neurons, astrocytes are also heterogeneous. The cells of one class conform to the classic star shape and occur as either the fibrillary or protoplasmic form. Fibrillary astrocytes populate the white matter, whereas the latter inhabit the gray matter. Other important subtypes of astrocytes include the Bergmann astrocytes, which are distributed in a narrow lamina between the cell bodies of Purkinje neurons in the cerebellar cortex, and the pilocytic astrocytes of the periventricular region, cerebellum, and spinal cord (Figure 11.22).
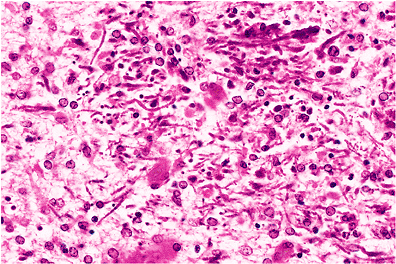 |
Figure 11.20 Mineralized axons. Clusters of mineralized axons have a superficial resemblance to fungal hyphae. |
P.289
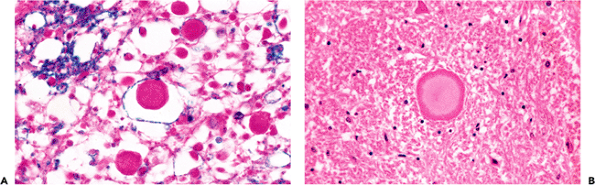 |
Figure 11.21 Axonal spheroids. A. Focal dilatations known as spheroids are a common axonal reaction to injury that are seen in a wide array of pathologic conditions, including radiation damage and posttraumatic diffuse axonal injury. B. Axonal spheroids are also frequently encountered incidentally in older individuals in the dorsal medulla oblongata (rostral fasciculus gracilis near the nucleus gracilis), where, as in this example, they are often mineralized. |
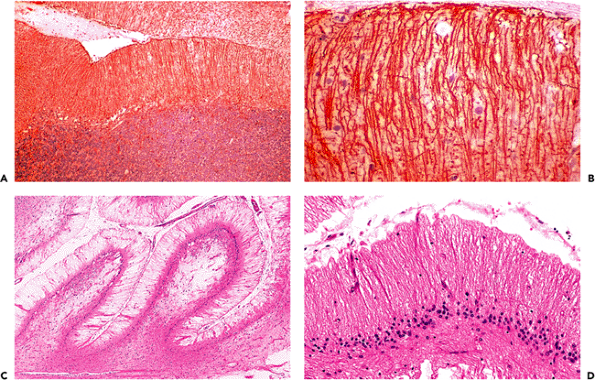 |
Figure 11.22 Bergmann glia. These astrocytes illustrate the fact that specialization is not confined to neurons. Bergmann astrocytes have cell bodies distributed in a narrow lamina of the cerebellar cortex coextensive with that of the Purkinje cells. Each cell sends an elongated process through the molecular layer to the subpial surface. A. and B. These processes are not usually well seen in healthy cerebellum with routine H&E staining (Figure 11.3A) but can be exquisitely visualized with immunohistochemistry for glial fibrillary acidic protein (GFAP). C. and D. An equally striking unmasking of this elegant architecture is often seen without the use of specialized staining techniques in areas of cerebellar cortex adjacent to healed infarcts, in which the degree of ischemia was sufficient to kill the indigenous neuronal populations but spared the more resistant Bergmann glia. Like other astrocytes throughout the central nervous system, Bergmann glia respond to ischemic insult by proliferating, resulting in an increased thickness of the cell body lamina referred to as Bergmann gliosis (D). |
P.290
In gray matter, nuclei of protoplasmic astrocytes cannot generally be distinguished from those of small neurons because the cytoplasm of both blends imperceptibly into the surrounding neuropil and is not normally discernible as a discrete entity. In white matter, it is usually difficult in H&E-stained sections to distinguish fibrillary astrocytes from the much more numerous oligodendroglia. The nuclei of oligodendrocytes are smaller and more hyperchromatic, but usually these two cell types do not fall into two clearly defined groups. In sections stained for myelin, a very small amount of eosinophilic cytoplasm may occasionally, but not invariably, be seen surrounding normal astrocytic nuclei. This helps distinguish this cell from the oligodendrocyte whose cytoplasm, other than the myelin sheath, is not usually apparent by conventional light microscopy (Figure 11.32). Astrocytic cytoplasm becomes much more prominent when astrocytes respond to CNS injury, culminating in the abundant glassy cytoplasm of the gemistocyte (Figure 11.24).
To appreciate the distinctive morphology of the star cell, one must visualize its radiating processes. These threadlike extensions reach out to define a sphere of influence that is many times greater in extent than one would have suspected by looking at an H&E-stained section alone. Historically, this tinctorial feat was achieved through the technically capricious metallic impregnations but now is accomplished with ease and predictability by the immunohistochemical localization of glial fibrillary acidic protein (GFAP) (Figure 11.24). In the case of the fibrillary astrocyte, processes branch infrequently, whereas those of the protoplasmic astrocyte are more numerous and divide more frequently. They are often less well stained with GFAP than the fibrillary types. Neither type of resting astrocyte is as apparent immunohistochemically as are reactive astrocytes.
The polar forms of astrocytes include the pilocytic and Bergmann types. The pilocytic astrocyte is not conspicuous in its native state but becomes so when responding as gliosis and forming Rosenthal fibers. The latter are hyaline, often corkscrew-shaped, eosinophilic structures that are wedged within one of the cell's bipolar processes (Figure 11.25). These structures are occasionally seen in normal brains in the hypothalamus or pineal gland but become much more prominent in gliosis about such lesions as craniopharyngiomas, pineal cysts, cerebellar hemangioblastomas, and chronic lesions of the spinal cord.
The Bergmann astrocytes are confined to a one-to-two-cell thick lamina. Their polar processes extend to the pial surface of the cerebellum and are only faintly seen with difficulty in standard sections. Yet, they are well visualized with immunohistochemistry for GFAP and at the margins of old cerebellar infarcts (Figure 11.22). The Bergmann glia provide an excellent illustration of astrocytic specialization. Their processes are a form of scaffold and serve as a reminder of the cooperative interplay between astrocytes and neurons during embryological development. At that time, the small neurons of the external granular cell layer spiral down the Bergmann processes to reach their final destination in the internal granular cell layer.
Age-Related Inclusions in Astrocytes: Corpora Amylacea
The ubiquitous corpora amylacea are, by far, the most salient astrocytic inclusions encountered in routine sections. These faintly laminated, slightly basophilic polyglucosan bodies accumulate with age and are observed in greatest numbers where astrocytic foot processes are most numerous, particularly around blood vessels and beneath the pia (Figure 11.23). The olfactory tracts of adults are also typically rich in corpora amylacea (Figure 11.45).
The similarity between corpora amylacea and fungal yeast forms such as cryptococcus is a source of potential diagnostic error since both are strongly positive for methenamine silver, Alcian blue, and PAS (Figure 11.23).
In some individuals, corpora amylacea are strikingly numerous although no pathologic significance has yet been attributed to this abundance.
Astrocytic Reactions to Injury
Although normally among the most morphologically demure of nervous system constituents (only naked nuclei are typically visible on routine H&E histology), astrocytes respond rapidly and dramatically to CNS injury. This response typically consists of two components: hypertrophy and hyperplasia. The initial hypertrophic response, an increase in cell size and cytoplasmic prominence, occurs rapidly following CNS insult. Conspicuous cytoplasm is generally indicative of reactive gliosis and constitutes prima facie evidence of CNS injury. Reactive astrocytes display a broad range of cytoplasmic quantity, from just barely perceptible to robustly embonpoint (Figure 11.24). The latter cells are known as gemistocytes (literally stuffed cells). Gliosis may, of course, also present as an increase in the number and density of astrocytic nuclei (hyperplasia) without attendant cytoplasmic prominence. This chronic type of gliosis is frequently subtle and often requires special stains for confirmation and quantitation.
The end result of acute reactive astrogliosis, such as that accompanying cerebral infarction, is frequently a dense fibrillary gliosis (Figure 11.24D). The often-invoked analogy of the astrocyte as the fibroblast of the CNS (i.e., a ubiquitously distributed cell with mitotic capability that responds with alacrity to a wide range of deleterious stimuli) is quite apt.
A distinctive cytoplasmic inclusion seen in fibrillary astrogliosis is the Rosenthal fiber (Figure 11.25). These strikingly eosinophilic, elongated, anfractuous structures are observed in a wide variety of reactive states that share in common significant chronicity. Rosenthal fibers are also characteristic of several specific nosologic entities, including
P.291
P.292
P.293
Alexander's disease and, perhaps most widely known, pilocytic astrocytoma. It should be stressed, however, that Rosenthal fibers may be strikingly abundant in the chronically compressed glial stroma surrounding a large number of nonneoplastic conditions (such as syringomyelia) and slowly expanding nonglial tumors (such as craniopharyngioma).
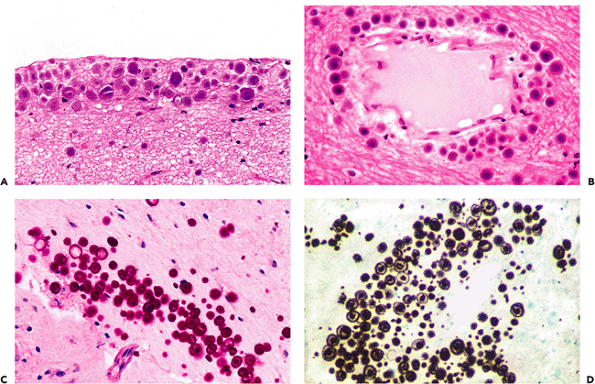 |
Figure 11.23 Corpora amylacea. These basophilic, lamellated polyglucosan bodies accumulate in astrocytic processes with age, most prominently in subpial (A) and perivascular (B) locations. Corpora amylacea can resemble fungal yeast forms and show strong positivity for fungal stains such as PAS-fungus (C) and Gomori's methenamine silver (GMS) (D). |
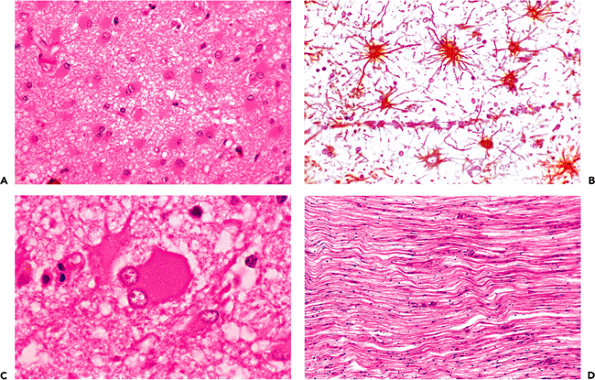 |
Figure 11.24 Reactive astrocytosis. The plainly visible cytoplasm and processes of the astrocytes (A) is proof of an insult to the nervous system. Under normal conditions, only bare nuclei are usually seen. The extensive, radiating cytoplasmic processes for which the astrocyte received its name are most readily appreciated when reactive astrocytes are immunostained for GFAP (B). Reactive astrocytes have been descriptively classified according to the amount and configuration of visible cytoplasm, and include the aptly named gemistocytic laden or stuffed cell (C), and pilocytic hair cell (D) types. Reactive gemistocytes are typical of the acute astrocytic reaction to CNS damage whereas dense fibrillary gliosis is commonly seen in longstanding lesions such as healed infarcts. |
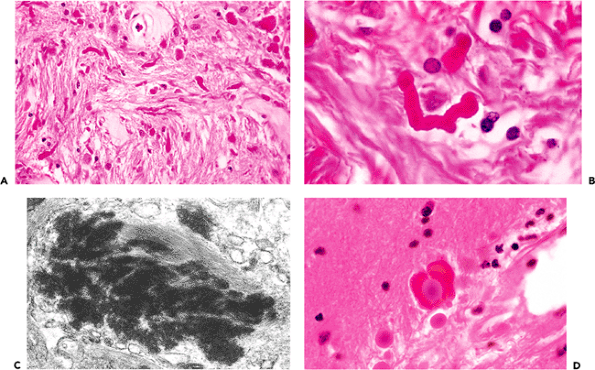 |
Figure 11.25 Rosenthal fibers. Chronic reactive fibrillary astrogliosis is often accompanied by Rosenthal fiber formation (A). Rosenthal fibers are brightly eosinophilic, lumpy, elongated structures (B) that, by ultrastructural examination, appear as electron-dense amorphous masses surrounded by and merging with dense bundles of glial filaments (C). Occasionally, the two most common intracytoplasmic inclusions of astrocytes (corpora amylacea and Rosenthal fibers) may coexist in the same astrocytic process (D). |
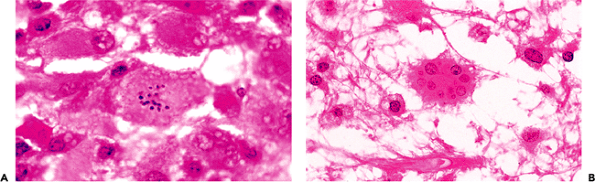 |
Figure 11.26 Granular mitoses (A) and Creutzfeldt astrocytes (B) in demyelinating diseases. These distinctive reactive astrocytes occur in a variety of pathologic conditions but are particularly characteristic of demyelinating diseases. |
There are several specialized forms of reactive astrogliosis that deserve brief description. Reactive astrocytes with multiple small, variably sized, nuclei (micronuclei), termed Creutzfeldt astrocytes, may be seen in a number of reactive states but are especially typical of demyelinative processes (Figure 11.26). A specific type of astrocytic reaction to injury is seen in a variety of hepatic diseases that produce hyperammonemia. The reaction consists of nuclear changes exclusively: swelling with contortion of the nuclear membrane, chromatin clearing, and development of one or two prominent nucleoli (Figure 11.27). In sharp contrast to all of the other types of reactive astrocytes, these Alzheimer type II astrocytes fail to exhibit prominent (or even subtle!) cytoplasm by routine H&E microscopy. Alzheimer type II astrocytes may be seen throughout the neuraxis but are particularly prominent in certain locations, most notably the globus pallidus. Alzheimer type I astrocytes differ from type II astrocytes in displaying abundant eosinophilic cytoplasm (Figure 11.27) and are only seen with frequency in
P.294
Wilson's disease (hepatolenticular degeneration). As the eponyms imply, both types of reactive astrocytic morphologies were described by Alois Alzheimer and have no relationship to the dementing disease of the same ilk.
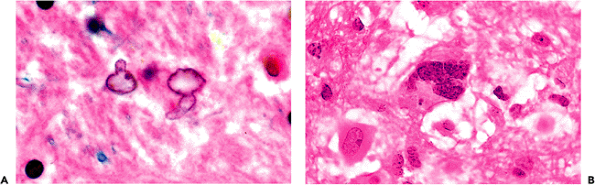 |
Figure 11.27 Alzheimer astrocytes in hyperammonemia. Two types of reactive astrocytic morphologies, termed Alzheimer type II and Alzheimer type I astrocytes, are associated with hyperammonemic conditions. They were described by Alois Alzheimer and bear his name but have nothing to do with the dementing disease that was also a subject of the famous neurologist's investigations. By far, the most frequently encountered are Alzheimer type II astrocytes (A). Typical features include an enlarged pale nucleus with an irregular contour and one or more small nucleoli. In marked contrast to other types of reactive astrocytes, visible cytoplasm is lacking. Alzheimer type II astrocytes are commonly seen in a wide variety of diseases that result in increased blood ammonia. In contrast, Alzheimer type I astrocytes (B) have large, irregularly lobulated or multiple nuclei and clearly discernible eosinophilic cytoplasm. These cells are not seen in most hyperammonemic diseases, with the exception of hepatolenticular degeneration (Wilson's disease). |
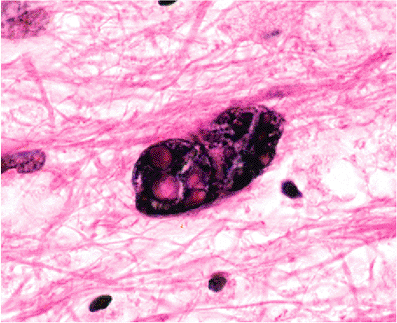 |
Figure 11.28 Bizarre reactive astrocytes of progressive multifocal leukoencephalopathy (PML). Atypical-appearing reactive astrocytes are sometimes the most striking finding in a PML biopsy and can be mistaken for neoplasia by the unprepared. |
Among astrocytic reactions to injury, none is more striking than that observed in some cases of progressive multifocal leukoencephalopathy (PML). Not infrequently, the most eye-catching aspect of a PML biopsy is an alarming nuclear hyperchromatism and pleomorphism exhibited by scattered astrocytes a vignette that has on more than one occasion elicited a mental frisson from even the most experienced observer (Figure 11.28).
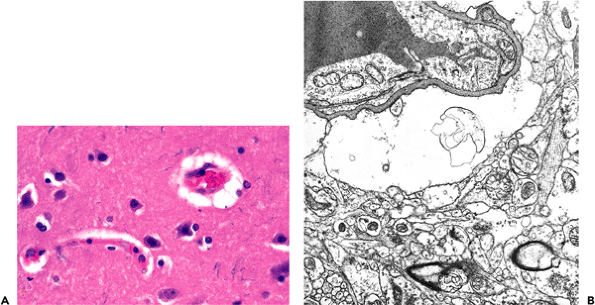 |
Figure 11.29 Perivascular astrocytic foot process swelling in autolysis. A. This common artifact of routine tissue processing is observed by light microscopy as apparent perivascular clearing of the neuropil. B. As seen by electron microscopy, the clearing is due to dilated astrocyte foot processes. |
Perivascular clearing is a routinely observed artifact of autolysis (Figure 11.29). By electron microscopy, these clear spaces are revealed to be greatly dilated astrocytic perivascular foot processes. This phenomenon of water imbibition by astrocytes is seen both as an autolytic change in virtually all autopsy specimens and, when extreme, as a marker of antemortem hypoxic/ischemic injury.
Oligodendroglia
The oligodendroglia ( few branch glia) are small cells that are active in the formation and maintenance of myelin and in the, as yet, poorly understood capacity of attending to neuronal cell bodies (satellitosis). In white matter, the oligodendrocytes' obligatory orientation to fiber pathways is occasionally made apparent by a fortuitous plane of section wherein the fascicular distribution of these cells is seen (Figure 11.30). In gray matter, oligodendrocytes are encountered as two to three small, dark nuclei that are pressed against the cell membrane of larger neurons (Figure 11.31). In surgical specimens obtained from infiltrating gliomas, these normal satellite oligodendroglia must be distinguished from infiltrating neoplastic cells that, like their nontransformed counterparts, are attracted to the immediate perineuronal region. Both astrocytomas and oligodendrogliomas
P.295
may exhibit such satellitosis, but it is most prominent in the latter neoplasm. Generally, the nuclei of the neoplastic satellites are larger, more pleomorphic, and more coarsely constructed than the normal orbiting cortical oligodendrocytes.
 |
Figure 11.30 Oligodendroglia. As seen here in a white matter tract (the corpus callosum) cut in longitudinal section, these glia may be identified, even at low power, as rows of nuclei that queue up between fascicles of myelinated axons. |
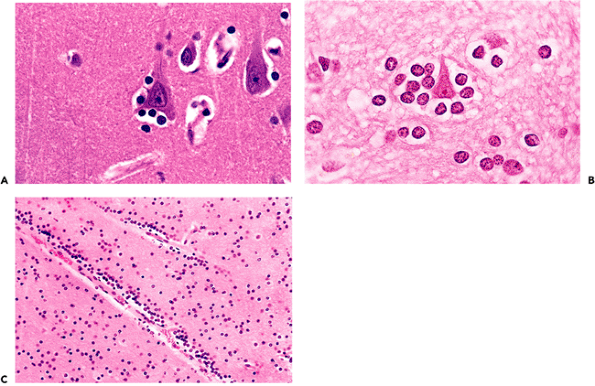 |
Figure 11.31 Perineuronal satellitosis. A. Normal perineuronal glia consist primarily of oligodendroglial satellite cells, together with occasional astrocytes and microglia. B. This affinity of normal oligodendroglia for neuronal perikarya is often retained by their neoplastic counterparts, oligodendrogliomas, in the form of neoplastic satellitosis. C. Nonneoplastic oligodendroglial hyperplasia can also be seen, as for example in some cases of longstanding epilepsy. |
Identification of normal oligodendroglia in both gray and white matter is greatly facilitated by these cells' perinuclear halo (the so-called fried egg appearance), which results from swelling and vacuolation of the cytoplasm (Figure 11.32A). This is analogous to the perivascular swelling and vacuolation of astrocytic foot processes. In oligodendroglial neoplasms (oligodendrogliomas), the perinuclear halo is a well-known, distinctive, and diagnostically useful feature (Figure 11.32B). Oligodendroglia and their neoplastic counterparts exhibit strong immunopositivity for S-100 protein (Figure 11.32C).
Ependyma
This cuboidal-to-columnar epithelium provides a lining for the CNS ventricular system (Figure 11.33) and specializes focally as a covering for the choroid plexus (Figure 11.47). The ciliated nature of the ependyma is readily appreciable in the child but is generally less so thereafter. Tanycytes (literally, stretched cells) are specialized constituents of the ependyma, the elongated abluminal processes of which reach the subependymal vasculature. Thus, these cells provide a physical link between the ventricular, vascular, and intraparenchymal compartments of the CNS. Tanycytes are
P.296
P.297
most numerous in the modified ependyma covering many of the circumventricular organs (discussed below). Visualization of these cells is best effected through use of the Golgi's stain.
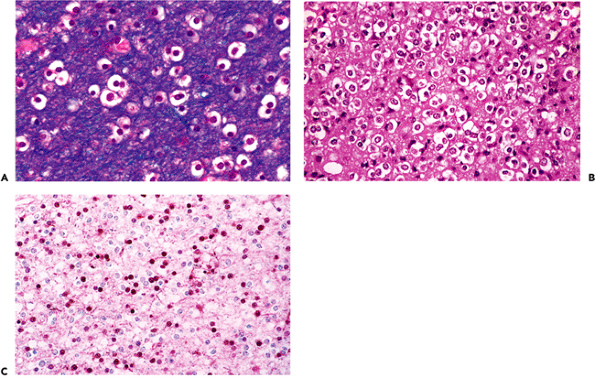 |
Figure 11.32 Oligodendroglia. In many specimens, oligodendroglia exhibit characteristic perinuclear halos (A). This fried egg appearance is an artifact of hypoxia/ischemia and delayed fixation and is a useful diagnostic feature that is also exhibited by oligodendrogliomas (B). Oligodendroglia, both normal and neoplastic, typically show strong nuclear immunopositivity for S-100 protein (C). |
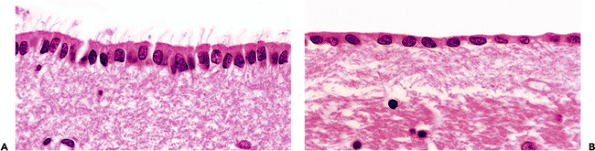 |
Figure 11.33 Ependyma and the subependymal plate. The lining of the ventricular system varies from a robust ciliated-columnar epithelium (A) to nearly squamous flattened cuboidal (B). The relative abundance of cilia and the height of the ependyma vary with anatomic location, and both decrease with age. The hypocellular fibrillary zone located immediately subjacent to the ependyma is known as the subependymal plate and contains scattered glia as single cells and in small clusters (Figure 11.36B). Glia of the subependymal plate respond to ependymal injury with a proliferative response termed granular ependymitis (Figure 11.36A). Subependymomas originate from the glia of the ependyma and subependymal plate. |
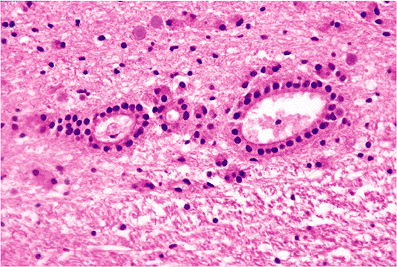 |
Figure 11.34 Ependymal rosettes. Clusters of ependymal rosettes may be found subjacent to the ependymal lining of the ventricular system throughout the neuraxis. They are particularly common in areas where opposed ventricular surfaces fuse during development, such as at the tips of the lateral ventriculur horns, especially the occipital horns (as illustrated here), and at the lateral angles of the fourth ventricle. These normal rosette clusters are occasionally sampled in surgical specimens and should not be misinterpreted as evidence of disease. |
The closely apposed ependymal surfaces of the tips of the lateral ventriculur horns frequently fuse during development, resulting in cords of ependymal cell nests and rosettes. This is especially typical of the distal portions of the posterior horns in the occipital lobes (Figure 11.34). The white matter in such areas appears pale and can simulate the rarefaction seen after ischemic insult. Detached ependymal rosettes may be encountered subjacent to the ventricular lining at any location throughout the neuraxis.
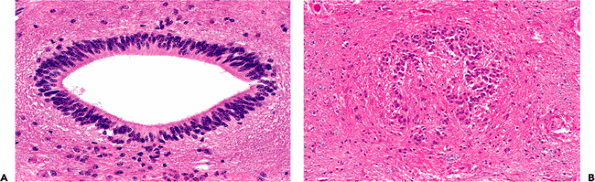 |
Figure 11.35 Central canal of the spinal cord. A. In the child, the central canal is widely patent and exhibits the ciliated columnar ependymal lining expected in a young individual. B. In contrast, the central canal of adults is typically obliterated over much of its length, with only residual small nests and occasional rosettes of ependymal cells. |
The ependyma-lined central canal of the spinal cord is patent in the child (Figure 11.35) but generally becomes obliterated about the time of puberty unless obstructive hydrocephalus is present. In the latter case, the canal may remain patent and even become dilated (hydromyelia). In the normal adult, however, the spinal ependymal cells have completed their role as a generative epithelium and remain as scattered clumps and rosettes (Figure 11.35). Occasional sections of adult spinal cord may exhibit a focally patent central canal.
The primary ependymal response to injury is loss. The resultant focal denudation of the ventricular wall is often accompanied by a proliferation of local cells, the subependymal glia. This nonspecific reaction, termed granular ependymitis (Figure 11.36A), is the potential product of a broad range of disparate etiologies, from viral infection to hydrocephalus. It is seen frequently at autopsy as a very focal, limited response and has, in this setting, little diagnostic significance. The normal ependyma is commonly thrown into folds, termed plicae, in many parts of the ventricular system (Figure 11.36B). These normal undulations should not be confused with granular ependymitis.
Microglia and the Monocyte Macrophage System
Normal Microscopic Anatomy
The small, dark, elongated nuclei of microglia are ubiquitous in the normal brain. They are so small and inconspicuous
P.298
in H&E-stained sections, however, that they are rarely noticed (Figure 11.37A). They must be distinguished from the commonly encountered tangential or en face sections of endothelial cells, which possess similarly elongated, albeit somewhat larger and plumper, nuclei. Special staining techniques such as the classic silver carbonate method, the more predictable lectin histochemistry (Figure 11.37B), and immunohistochemical markers such as HAM-56 uncloak the dendritic processes of microglia and permit unambiguous visualization. By these techniques, microglia are seen to be strikingly pervasive throughout the CNS parenchyma.
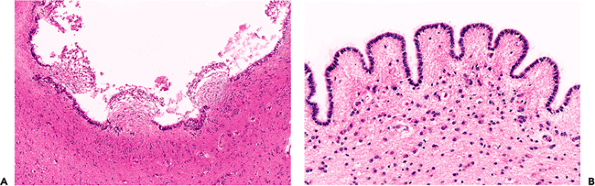 |
Figure 11.36 Granular ependymitis. A. The combination of focal denudation of the ependyma, coupled with an exophytic fusiform proliferation of the subependymal glia constitutes granular ependymitis. Despite the implication of an inflammatory etiology inherent in the name, this common alteration can result from many diverse insults, ranging from hydrocephalus to viral infections. B. Normal undulations of the ependyma, termed plicae, should not be confused with granular ependymitis. |
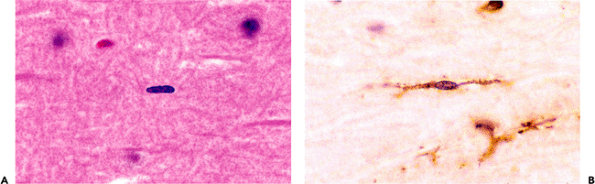 |
Figure 11.37 Microglia. A. These normally inconspicuous residents of the CNS parenchyma are identifiable by their classical rod-shaped nuclei on routine H&E staining. B. Dendritic processes, often bipolar, are vividly demonstrated by lectin staining. |
Response to Injury
In contrast to the relative passivity and anonymity of microglia in healthy nervous tissue, their activity is by no means subtle when called to action by parenchymal injury. Two variants are seen: microglial nodules and diffuse microgliosis. Microglial nodules (also called microglial stars) are frequent concomitants of viral or rickettsial infection, and they are generally acknowledged to consist of both astrocytes and microglia (Figure 11.38A). The microglia have an elongated shape and are known as rod cells. A form of glial nodule is also seen about degenerating neurons, as in amyotrophic
P.299
lateral sclerosis. Diffuse microgliosis is equally distinctive. In this context, the rod-shaped nuclei of microglia may be present in such numbers as to be easily identified (Figure 11.38B); however, the full extent of microgliosis is often best appreciated through immunohistochemistry for markers such as CD68 or HAM-56 (Figure 11.38C).
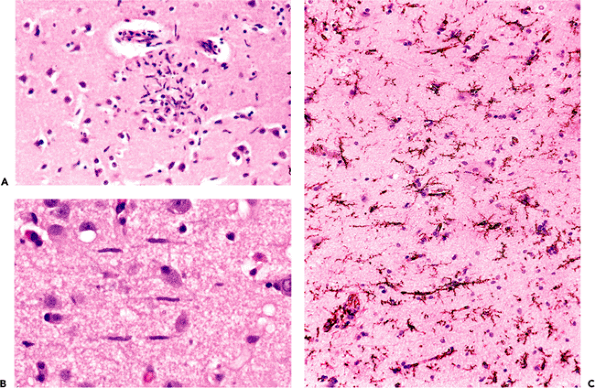 |
Figure 11.38 Microglia. Reactive microglia may assume several forms. Microglial nodules (A) are focal hypercellular collections of microglia together with reactive astrocytes that commonly form as a response to viral and rickettsial infections. In diffuse microgliosis (B), seen in a variety of conditions, including ischemia, the characteristic elongated rod-shaped nuclei can be identified on H&E-stained sections; however, the true extent of their presence is more accurately visualized through immunohistochemistry, as seen here with HAM-56 (C). |
Destruction of nervous tissue, by whatever mechanism, generally elicits a macrophage response that serves to clear nonviable debris (Figure 11.39A). Both the activation of autochthonous tissue microglia and the diapedesis of blood monocytes are sources for these scavengers. The weight of evidence suggests that the recruitment of blood monocytes plays a predominant role in large lesions such as infarcts but that the supply of indigenous cells is sufficient for lesser insults.
Macrophages are proliferative cells (Figure 11.39A, B). Mitotic figures will, therefore, usually be present in disease processes that elicit a macrophage response, such as infarction and demyelination. They should not be interpreted as suggestive of a neoplastic process. Macrophages often contribute substantially to the cellularity of tissue samples and, depending on preservation and fixation conditions, their identity may not always be obvious. For example, in some specimens, clearing of the macrophage cytoplasm lends an appearance similar to that of oligodendroglial cells, to the extent that, together with the attendant hypercellularity, an infiltrating glioma might be suspected. In such instances a number of antibodies, such as CD68 or HAM-56, can be used to identify the macrophage component (Figure 11.39C, D).
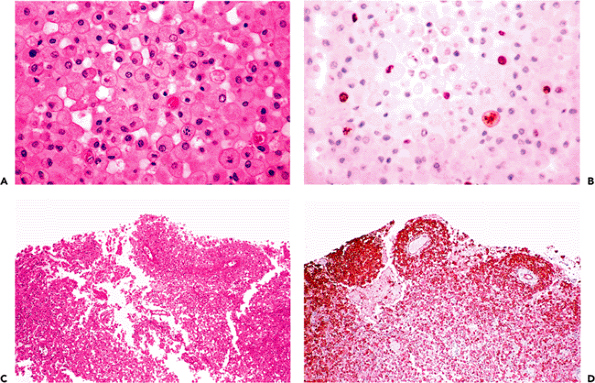 |
Figure 11.39 Macrophages. Discrete cell boundaries and vesicular cytoplasm serve to distinguish macrophages from other cellular constituents of the nervous system (A). Cognoscenti will be familiar with the colorful appellations given these cells in former times, including gitter cells (lattice cells) and compound granular corpuscles. Macrophages are mitotically active cells, and populations responding to CNS injury are readily labeled with proliferation markers such as the monoclonal antibody MIB-1 (B). Mitotic figures are, thus, to be expected in tissue samples from a wide range of nonneoplastic conditions that elicit a macrophage response, including infarcts and demyelinative diseases. In hypercellular biopsies (C), macrophages can be distinguished from other cellular constituents by a number of commercially available antibodies such as HAM-56 (D). |
P.300
Specialized Organs of the Central Nervous System
Pineal Gland
The pineal body (epiphysis, or conarium) presents a singular histologic appearance among CNS tissues with a prominently lobulated architecture (Figures 11.40,11.41). This glandular appearance might be mistaken for carcinoma by the unwary, and it can be difficult to distinguish the normal pineal gland from a well-differentiated pineocytoma in small surgical specimens.
Generally present in the pineal gland after puberty are corpora arenacea (acervuli cerebri, or brain sand). These mineralized concretions accrue with age and confer the radiologic hyperdensity that, before the era of computerized tomography and magnetic resonance imaging, made the normal midline position of the pineal gland a useful radiologic landmark (Figure 11.40).
The increase in corpora arenacea with senescence is accompanied by gradual gliosis and cystic change, with attendant effacement of the lush glandular appearance of the pineal gland seen in the earlier decades of life. The ubiquitous incidental pineal cysts typically have densely gliotic walls with scattered Rosenthal fiber formation (Figure 11.41C). The investing leptomeninges of the pineal contain arachnoid cell nests that occasionally give rise to meningiomas of the pineal region (Figure 11.41D).
Pineocytes express strong immunopositivity for the neuronal marker synaptophysin (Figure 11.42A). This useful phenotypic marker is retained by most pineal parenchymal neoplasms. In addition to pineocytes, the pineal gland also
P.301
contains an indigenous population of astrocytes whose distribution is revealed by immunostaining for GFAP (Figure 11.42B).
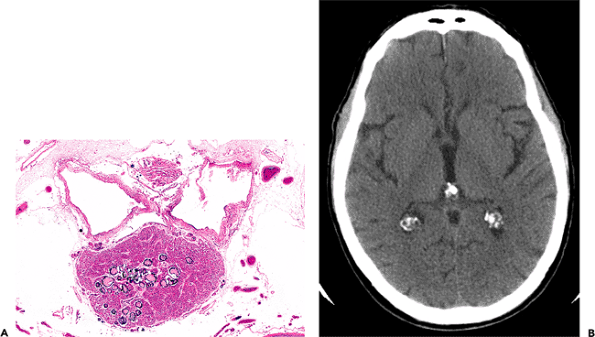 |
Figure 11.40 Pineal gland (epiphysis). A. Whole mount of a cross section of the pineal gland and its environs in situ reveals the typical mineralized concretions variously referred to as corpora arenacea (sand bodies), acervuli cerebri (little heaps), or simply brain sand. Superior to the pineal gland are the paired internal cerebral veins, and between them is the suprapineal recess of the third ventricle, which is lined with ependyma and often contains a tuft of choroid plexus. The loose connective tissue (redundant leptomeninges), in which all of these structures are located, is called the velum interpositum. B. The calcification of the pineal gland increases with age and was, thereby, quite useful as a radiographic midline marker prior to the advent of contemporary high resolution neuroimaging modalities. Also seen in this CT scan of a normal adult brain are prominently calcified tufts of choroid plexus (glomera choroidea; also Figure 11.47) in the atria of both lateral ventricles. |
Median Eminence and Infundibulum
The median eminence, infundibulum, and neurohypophysis display a unique constellation of morphologic features that reflect their specialized neuroendocrine functions. The background stroma is highly spindled (Figure 11.43A) and contains nodular microvascular tangles termed gomitoli (Figure 11.43B), spherical granular bodies called Herring bodies (Figure 11.43C; Table 11.1), which are storage sites for oxytocin and vasopressin, and scattered cells bearing lipofuscin-like brown pigment (Figure 11.43D). The constellation of features comprising a highly spindled background, vascular tangles, and granular bodies gives this region of the CNS more than a passing resemblance to pilocytic astrocytoma. An additional incidental finding, particularly in tissue sections of the infundibulum, is the presence of small clusters of granular cells, termed granular cell tumorlets (Figure 11.43E).
Olfactory Bulbs and Tracts
The intracranial components of the olfactory apparatus (the olfactory bulbs and tracts) have a very distinctive histologic appearance. Familiarity with these structures is useful, not only for the neuropathologist, but also for the general surgical pathologist who may encounter them in resections performed as part of the surgical treatment for regionally invasive entities of the nasal and paranasal sinuses. In such situations, the surgical pathologist may be called upon to render an intraoperative frozen-section assessment of tissue resected superior to the cribriform plate. The ability to recognize the normal histologic features of olfactory bulb tissue is, thus, of more than pedantic importance.
P.302
 |
Figure 11.41 Pineal histology. The pineal gland has a richly glandular architecture that is totally unlike any other region of the central nervous system. Salient features include a prominent lobular organization with connective tissue septa (A) and pineocytic rosettes (B). The latter impart a distinctly neuroendocrine character. Two additional histologic features of note are the ubiquitous incidental pineal cysts, whose walls (C) typically exhibit astrogliosis with scattered Rosenthal fibers, and arachnoid cell nests of the investing velum interpositum (D) that occasionally provide a source for meningiomas arising in this region. |
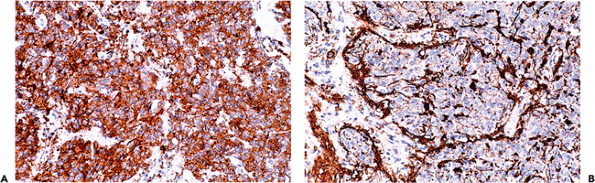 |
Figure 11.42 Pineal immunohistochemistry. A. Pineocytes (and their neoplastic progeny, the pineal parenchymal tumors) are strongly immunopositive for the neuronal marker synaptophysin.B. As expected, the indigenous population of pineal astrocytes are well visualized with antibodies directed against GFAP. |
P.303
 |
Figure 11.43 Median eminence, infundibulum, and neurohypophysis. This unique region of the CNS exhibits three distinguishing histologic features: A. a highly spindled stroma comprised of pituicytes; B. prominent capillary tangles of the hypothalamohypophyseal portal system called gomitoli; C. and spherical, eosinophilic axonal specializations for the storage of oxytocin and vasopressin called Herring bodies. This constellation of highly spindled stroma, vascular tangles, and granular bodies bears a resemblance to pilocytic astrocytoma. D. The presence of lipofuscin-like pigment is also very characteristic. E. Small clusters of granular cells (granular cell tumorlet) may be seen, particularly in the infundibulum, as an incidental finding but occasionally reach a sufficient size to produce compression of the infundibulum and subsequent clinical presentation with mildly elevated serum prolactin (stalk effect). |
P.304
Table 11.1 Granular Bodies in the CNS: Three Etiologic Classes | |
|---|---|
|
The olfactory bulb has a laminar organization (Figure 11.44). The outer layer is composed of spindled bundles of entering olfactory nerve fascicles intermixed with distinctive spherical, anuclear areas (termed glomeruli) that constitute specialized zones of synaptic contact between olfactory nerve collaterals and the dendrites of intrinsic olfactory bulb neurons. Mitral cells are large neurons, so-named for a resemblance of the perikaryon shape to a bishop's mitre. Their cell bodies are located in a lamina deep to that of the glomeruli (Figure 11.44). The deepest layer consists of a thick lamina of granular cell neurons that are comparable in size to those of the cerebellum and dentate gyrus.
The olfactory tracts (sometimes incorrectly referred to as olfactory nerves) extend posteriorly from the olfactory bulbs. They are triangular in cross section and, in adults, are notable for their profuse numbers of corpora amylacea (Figure 11.45).
Choroid Plexus
The choroid plexus is a specialized organ of the CNS that is responsible for the production of cerebrospinal fluid. It is found in the body, atrium, and temporal horns of the lateral ventricles, in the interventricular foramina of Monro, in the roof of the third ventricle, and in the roof and lateral recesses of the fourth ventricle. The frontal and occipital horns of the lateral ventricles and the aqueduct of Sylvius
P.305
are devoid of choroid plexus. The plexus is most obvious in the atria of the lateral ventricles (Figures 11.40B, 11.47), where prominent bilateral tufts (glomera choroidea) are formed. Cystic xanthomatous change is a common incidental finding in these botryoid structures. The plexus is also a normal resident in the subarachnoid space of the cerebellopontine angle (CPA) cisterns, which it reaches from the lateral recesses of the fourth ventricle by protruding through the foramina of Luschka (Figure 11.46). The paired foramina of Luschka (lateral), which open laterally into the ventral basilar CPA cisterns, are to be distinguished from the single foramen of Magendie (median), which opens in the dorsal midline into the cisterna magna.
 |
Figure 11.44 Olfactory bulb. The olfactory bulbs have a very distinctive laminar organization. The most superficial layer, termed the glomerular layer (1) is covered by the leptomeninges (pia-arachnoid) and the subarachnoid space (SAS). The glomerular layer displays a unique architecture, with spindled olfactory nerve fascicles intermixing with spherical hypocellular synaptic zones called glomeruli (1). Deeper layers include the external plexiform (2), mitral cell (3), internal plexiform (4), and granular cell layer (5). Deeper still is the anterior olfactory nucleus (6). A working familiarity with olfactory bulb histology is essential for the surgical pathologist because this structure is frequently seen in the frozen section laboratory during resection of superior nasal cavity tumors that may invade the cribriform plate and overlying olfactory bulbs. |
 |
Figure 11.45 Olfactory tracts. A. The olfactory tracts contain myelinated fiber bundles and are roughly triangular in cross section. B. A distinctive feature of the tracts frequently observed in adults is their remarkable content of corpora amylacea. |
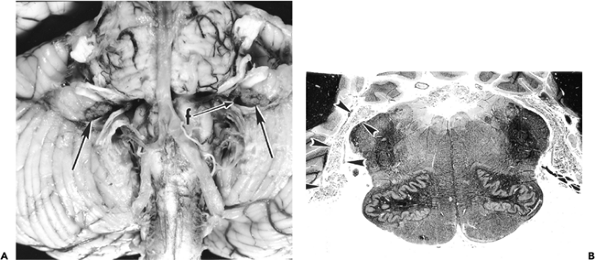 |
Figure 11.46 Choroid plexus. A. Small tufts of choroid plexus are normally visible on the basal surface of the brainstem in the cerebellopontine angle (arrows), and indicate the location of the lateral foramina of Luschka (f) from which they protrude. The dusty discoloration of the inferior medulla is due to the presence of leptomeningeal melanocytes (Figure 11.57). B: The ependyma-lined sleeve of the lateral recess of the fourth ventricle (arrowheads), together with the protruding tuft of choroid plexus, is referred to in the older literature as the flower basket of Bochdalek or cornucopia. Pieces of the ependymal cuff are often seen adherent to the lateral aspect of the medulla in autopsy brainstem sections and should be recognized as a normal finding. |
Microscopically, choroid plexus consists of invaginated fronds of vascular leptomeninges covered by an ependyma that is modified to become a highly secretory epithelium (Figure 11.47). The cells are larger and more cobblestoned than those of the adjacent ependyma (Figure 11.33). In addition to collagen and blood vessels, small nests of meningothelial (arachnoid) cells are common normal habitu s of the choroid plexus; whorls of these cells frequently give rise to psammoma bodies (Figure 11.47G). Nonspecific deposition of mineral salts also occurs commonly throughout the connective tissue core with increasing age and accounts for most of the plexuses' radiodensity. An additional aging change of no specific pathological significance is cytoplasmic vacuolization of the ependyma-derived lining cells (Figure 11.47E).
Circumventricular Organs
The circumventricular organs (CVOs) comprise a diverse group of specialized CNS centers that share two morphologic features: a periventricular location and vasculature that lacks the characteristic blood-brain barrier properties found throughout the rest of the brain and spinal cord. There are six CVOs: the pineal gland, subfornical organ, organum vasculosum of the lamina terminalis, area postrema, subcommissural organ, and the median eminence-infundibulum-neurohypophysis (Figures 11.48,11.49). Of these, all except the subcommissural organ are fully developed in the adult human (Figure 11.49). The subcommissural organ, which is located on the ventral surface of the posterior commissure just caudal to the pineal gland, is a very prominent CVO in most vertebrates (Figure 11.49B); although it generally regresses near the end of gestation in humans, vestigial remnants may be present (Figure 11.49C).
Intradural Elements of the Peripheral Nervous System
The major intradural representatives of the peripheral nervous system are the cranial and spinal nerves and small autonomic fibers in the adventitia of blood vessels. In all of the cranial nerves except cranial nerve VIII, the transition from central to peripheral nervous system occurs within 2
P.306
P.307
P.308
mm of the pial surface. In the eighth cranial nerve, the central nervous system extends out along the nerve for a centimeter or so to the level of the internal auditory meatus. At this point, the transition occurs between the medial central nervous system segment and the lateral peripheral segment that emerges from the apparatus for hearing and balance (Figure 11.50). The myelin of the central nervous system is formed by oligodendrocytes, whereas that of the peripheral nervous system is formed by Schwann cells. Peripheral nerve is noted for its content of interstitial collagen and the elongated nuclei of Schwann cells.
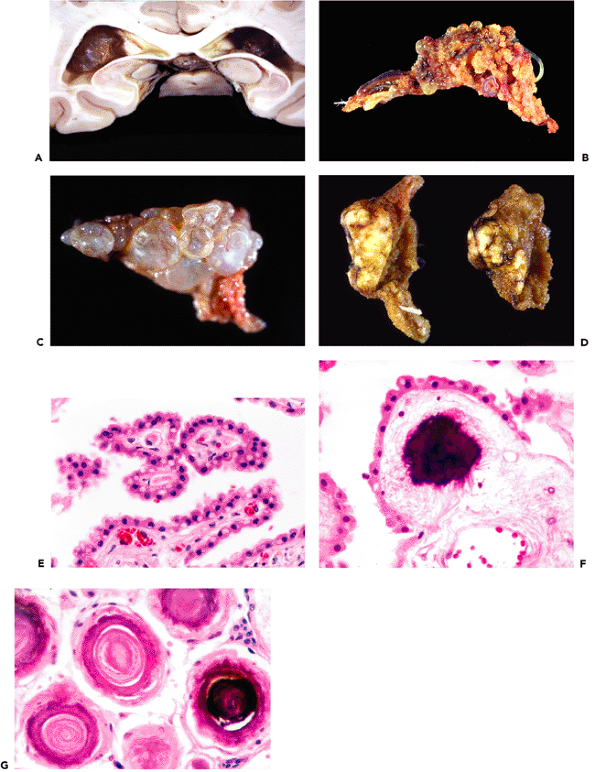 |
Figure 11.47 Choroid plexus. Choroid plexus produces the cerebrospinal fluid and is found in the lateral ventricles, foramen of Monro, roof of the third ventricle, and fourth ventricle. The largest tufts are called the glomera choroidea and are located in the atria of the lateral ventricles (A). Choroid plexus consists of a botryoid, finely tufted tangle of epithelium-covered fibrovascular tissue (B) that is formed during embryonic development through an invagination of the vascular pia-arachnoid into the ventricular system, whereby it acquires its covering of modified ependyma. Two common incidental findings on MRI scans and in autopsy specimens are cystic change (C) and xanthogranulomas (D), both of which may be bilateral. Histologically, choroid plexus is seen to be covered by simple cuboidal epithelium (modified ependyma); in adults, each choroid epithelial cell bears a single prominent paranuclear cytoplasmic vacuole (E). An additional aging change seen in normal choroid plexus is calcification, which occurs in two forms: nonspecific deposition of calcium salts in the collagenous stroma (F) and as psammoma bodies (G). The latter arise from meningothelial cell nests that normally are present in the choroid plexus as a result of the stroma's embryologic derivation from the leptomeninges. |
 |
Figure 11.48 Circumventricular organs. The circumventricular organs share a midline or paramidline position, proximity to the ventricular system, and lack of the usual blood-brain barrier. The subcommissural organ is present in the developing fetus but is vestigial in the adult. (AP, area postrema; ME, median eminence and infundibulum; OVLT, organum vasculosum of the lamina terminalis; PG, pineal gland; SCO, subcommissural organ; SFO, subfornical organ) |
 |
Figure 11.49 Circumventricular organs (CVOs). Histologically, all of the CVOs except for the subcommissural organ are very similar, with a loose neuropil that is highly vascular and lacks a blood-brain barrier as illustrated by the subfornical organ (A). The subcommissural organ is located in the region of the pineal gland just beneath the posterior commissure in the posterior dorsal third ventricle and is highly developed in most mammalian species, as illustrated by the mouse (B). In humans, the subcommissural organ is vestigial, but remnants are occasionally encountered (C). The organum vasculosum of the lamina terminalis (OVLT) and the median eminence-infundibulum-neurohypophysis are two additional CVOs that are in contact with the third ventricle (D). (I, infundibulum; LT, lamina terminalis; OC, optic chiasm) |
Two additional intrathecal components of the peripheral nervous system may pique interest on fortuitous encounter. The first is the so-called microneuroma, which is usually found in the parenchyma of the spinal cord or, more rarely, the medulla (Figure 11.51). These structures consist of a Gordian knot of unmyelinated axons that have been hypothesized to arise secondary to traumatic injury of peripheral nerve roots whose regenerating axons follow penetrating spinal or medullary arteries into the CNS parenchyma along the Virchow-Robin spaces. According to the hypothesis, the tapering perivascular spaces ultimately block further advance of the regenerating axons and, thereby, result in the observed neuroma.
An additional component of the peripheral nervous system that occasionally arouses interest is the unmyelinated terminal nerve (variously termed nervus terminalis, cranial nerve zero, and terminal nerve), which courses in the subarachnoid space covering the gyri recti of the orbital
P.309
surface of the frontal lobes (Figure 11.52). Although usually composed of multiple small anastomosing fascicles, it occurs as a single trunk in some specimens and can be quite striking. Rarely, intrafascicular ganglion cells may be observed.
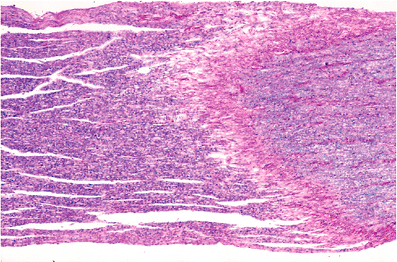 |
Figure 11.50 Transition zone from central to peripheral nervous system myelin. For cranial nerve VIII (vestibulocochlear), this transition occurs in the vicinity of the internal acoustic meatus. |
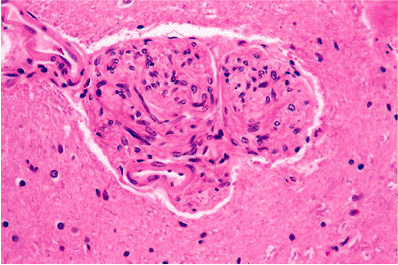 |
Figure 11.51 Microneuroma. These tangled balls of unmyelinated axons are most often encountered in the spinal cord, less often in the medulla, as an incidental finding in an otherwise unremarkable specimen. |
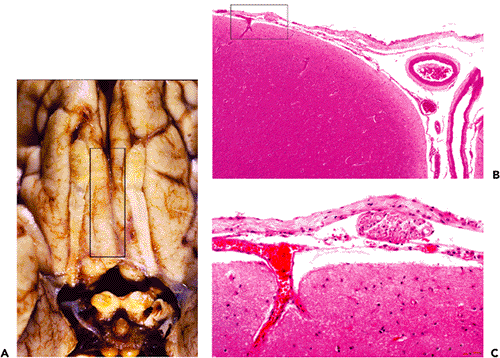 |
Figure 11.52 Cranial nerve zero. Cranial nerve zero (CN0), also known as the terminal nerve or nervus terminalis, is present in humans as a plexus of small peripheral nerve fascicles found in the subarachnoid space that covers the gyri recti that lie between the olfactory bulbs and tracts (A). Tissue sections taken through the gyri recti that include the overlying leptomeninges (B) will often include a terminal nerve fascicle cut in cross section (C). The small peripheral nerve fascicles of cranial nerve zero are one potential source of subfrontal schwannomas. |
Meninges
Dura Mater (Pachymeninx)
The dura mater is composed of two tightly annealed layers of fibrous connective tissue. The outer layer functions as the periosteum of the cranium, whereas the inner meningeal layer is joined to the arachnoid membrane by weak intercellular junctions and focally forms the four dural reduplications that compartmentalize the cranial cavity: the falx cerebri, falx cerebelli, tentorium cerebelli, and diaphragma sellae. The two layers of the dura separate to accommodate the dural venous sinuses; the inner meningeal layer is pierced by draining veins and by arachnoid villi. The latter conduct cerebrospinal fluid back into the venous circulation and are obvious over the superior parasagittal convexities of the cerebral hemispheres, where they project into the superior sagittal sinus (Figure 11.53). They are present in all other major venous sinuses, as well. They are often observed along the posterior margin of the cerebellar hemispheres in relation to the sinus confluens and the transverse venous sinuses. Small villi are also present intraspinally.
P.310
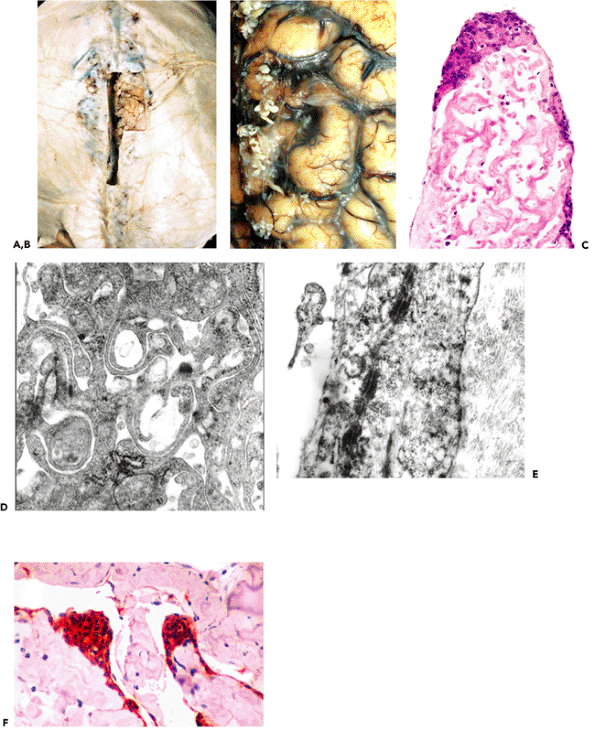 |
Figure 11.53 Arachnoid granulations (villi). Specialized structures of the arachnoid membrane serve to return cerebrospinal fluid from the subarachnoid space to the venous circulation and are accordingly found in relation to all major dural venous sinuses. The villi are most prominent in the superior sagittal (A, B) and transverse sinuses. With advancing age, they undergo collagenous hypertrophy, as seen in these micrographs, and may then be referred to as pacchionian bodies. The enlarged villi remodel the overlying bone of the inner table of the calvarium to produce small pits termed pacchionian foveolae, or foveolae granulares. Nests of meningothelial cells may be seen anywhere along the arachnoid membrane but are especially prominent in the apical regions of arachnoid granulations, where they are termed arachnoid cap cells (C), and in the arachnoid covering the orbitofrontal cortex. Normal meningothelial cells are innately inclined to form whorls and psammoma bodies, two features that are often retained by their neoplastic counterparts, meningiomas. The meningothelial cells of the arachnoid membrane, including the cap cells, serve an epithelial function. Accordingly, they possess elongated, intertwined cell processes (D) that are tightly spot welded together by numerous desmosomes (E) and exhibit strong immunopositivity for epithelial membrane antigen (EMA) (F). Like the tendency to form whorls and psammoma bodies, these epithelial phenotypic traits are retained by neoplastically transformed meningothelial cells and serve as useful diagnostic features of the vast majority of meningiomas, which otherwise exhibit a very broad range of light microscopic morphologies. |
P.311
The epithelial properties of arachnoid granulations are reflected ultrastructurally in elongated, interdigitating cell processes bonded together with desmosomes and immunohistochemically, by positivity for epithelial membrane antigen (Figure 11.53). These features are also characteristic of meningiomas.
With age, the deposition of collagen enlarges the arachnoid villi, which are then referred to as pacchionian bodies (Figure 11.53). Such large granulations frequently press through the overlying roof of the superior sagittal sinus and its lateral lacunae to produce small pits or depressions in the inner table of the calvarium. These are known as the foveolae granulares or pacchionian foveolae. Portions of the dura, particularly the falx cerebri and parasagittal dura associated with the superior sagittal sinus, often calcify nonspecifically with age. Calcification may also be seen in association with chronic renal failure. Focal ossification is sometimes encountered as an incidental finding.
Pia-Arachnoid (Leptomeninges)
The arachnoid forms a continuous sheet immediately subjacent to the dura. Based on descriptive and experimental ultrastructural observations, it is now generally accepted that the dura and arachnoid exist in vivo as a physically continuous tissue, with sparse but unequivocal intercellular junctions linking these two historically discrete membranes. The storied subdural space has, thus, taken its rightful place in the pantheon of neuromythology, alongside brain lymphatics and the syncytial theory of the neural net. It has been proposed that the term spatium subdurale be eliminated from the standardized nomenclature of Nomina Anatomica. Nevertheless, there is no disputing the fact that the interface between dura and arachnoid constitutes the weak link or path of least resistance for pathologic processes that tend to disrupt the meninges. It seems unlikely that such venerable terms as subdural hematoma will soon be cashiered.
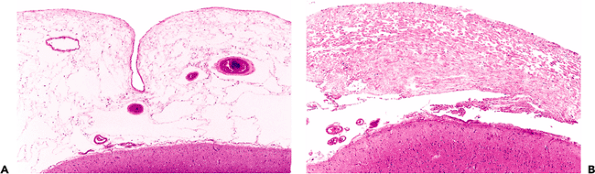 |
Figure 11.54 Subarachnoid space. A. The subarachnoid space is delimited by the arachnoid membrane externally and by the pia mater internally. Delicate arachnoid trabeculae course between these two membranes. B. In adults, gradual collagen deposition in the subarachnoid space results in grossly appreciable clouding of the leptomeninges. This aging fibrosis appears grossly as diffuse opacification with focal plaques and small punctate nodules. It is characteristically most prominent along the dorsal cerebral convexities adjacent to the superior sagittal sinus. |
The pia mater and arachnoid are often considered as a single delicate covering of the brain and spinal cord (the pia-arachnoid, or leptomeninges). The arachnoid is connected to the pia by delicate strands termed arachnoid trabeculae (Figure 11.54A). In the young, the arachnoid is crystal clear, but with age it becomes gradually thickened. The extent of this change varies considerably. In some cases, it is severe enough to raise concern about a pathologic process meningitis and meningeal carcinomatosis being the two usual suspects. This normal age-related arachnoid thickening is typically most pronounced over the dorsal parasagittal cerebral convexities. Microscopically, it results from the deposition of dense bundles of collagen (Figure 11.54B), analogous to the collagenous hypertrophy of arachnoid villi that occurs prominently in the same vicinity.
Focal nests of arachnoid cells (also called meningothelial cells) may be seen throughout the arachnoid membrane but are concentrated over the arachnoid villi (arachnoid cap cells) (Figure 11.53C). These distinctive elements become more obvious and more clustered with advancing age and, in the adult, often form whorls with centrally placed psammoma bodies. At this point, the resemblance of these nests to those of the meningioma is inescapable. As mentioned previously, small nests of arachnoid cells are also present intraventricularly in the vascular connective tissue core of the choroid plexus (Figure 11.47G). Both normal and neoplastic meningothelial cells are immunoreactive for epithelial membrane antigen an understandable property considering the epithelial phenotype of the desmosome-containing meningothelial cell (Figure 11.53).
The dorsal leptomeninges of the thoracic and lumbosacral spinal cord occasionally contain white waferlike
P.312
plaques (Figure 11.55), a finding that is often termed arachnoiditis ossificans. In fact, in the majority (but not all) of cases, these brittle lesions are roentgenographically and histologically devoid of bone or mineral. Rather, they most often consist of laminated, hyalinized fibrous tissue. True arachnoiditis ossificans generally occur in the context of prior symptomatic inflammation or trauma to the leptomeninges. Hyalin plaques, in contrast, are typically discovered as an incidental finding at autopsy in the absence of any relevant clinical history.
 |
Figure 11.55 Hyaline plaques of the spinal leptomeninges. These plaques are common incidental findings at autopsy and occur most frequently in the dorsal spinal arachnoid, although they may occasionally be seen in the cerebral leptomeninges as well. |
Like the dura, the pia is traditionally divided into two layers: the epipia, which covers the surface of the CNS parenchyma and surrounds the vasculature, and the intima pia, which extends into the CNS parenchyma as the posterior median and intermediate septa of the spinal cord. Classically, three specialized structures of the epipia are recognized: the denticulate ligaments on either side of the spinal cord, the linea splendens adjacent to the anterior spinal artery, and the filum terminale. All three structures are composed primarily of dense bundles of collagen.
 |
Figure 11.56 Filum terminale. A. The filum terminale is the terminus of the spinal cord and extends downward from the conus medullaris surrounded by the nerve roots of the cauda equina. B. As seen in cross section, the filum is composed primarily of dense collagenous tissue and contains blood vessels, small peripheral nerve fascicles, and, of significant clinical importance, a small, often eccentrically located, ependymal remnant of the central canal (upper right). The latter structure, shown at higher magnification in C, is the origin of myxopapillary ependymoma. D. A remnant of the embryonic terminal ventricle of Krause (ventriculus terminalis), which consists of a focal dilatation of the central canal located in the region of the junction of the conus medullaris with the filum, may be encountered in sections from this vicinity. |
The filum terminale, which forms the terminus of the spinal cord, warrants additional brief description. As noted earlier, it is composed largely of leptomeningeal collagen but also contains small blood vessels and occasional small nerve fascicles; it may harbor focal collections of adiposites in a minority of normal individuals. Most importantly, however, is an ependymal remnant of the central canal (Figure 11.56). This structure is the source of origin for a unique neoplasm of the conus medullaris and filum terminale: the myxopapillary ependymoma.
Leptomeningeal Melanocytes
True melanocytes like those found in the skin are normal cellular constituents of the meninges. They are typically most concentrated in the leptomeninges of the ventral aspect of the upper cervical spinal cord and medulla oblongata (Figure 11.57). In individuals with an abundant melanocytic presence, the distribution territory extends upward through the pontine cistern and mesencephalic interpeduncular fossa, lateral to the inferior cerebellar hemispheres and mesial aspects of the temporal lobes, and as far rostrally as the gyri recti of the orbitofrontal cortex. It is not unusual for melanocytes to follow the investing leptomeninges of the perivascular Virchow-Robin spaces around large penetrating arteries for short distances into the CNS parenchyma.
P.313
 |
Figure 11.57 Leptomeningeal melanocytes. True melanocytes (not to be confused with neuromelanin-containing catecholaminergic neurons) are normal constituents of the pia-arachnoid and are often grossly visible as a dusky brown discoloration of the leptomeninges overlying the ventral aspect of the brainstem (A) and cervical spinal cord (B). On cross section their rounded profiles might be confused with hemosiderin-laden macrophages; but on longitudinal section their elongated, dendritic quality is evident (C, D). |
P.314
Table 11.2 Brown Pigment in the Central Nervous System (CNS) | |
|---|---|
|
Intrinsic melanocytes of the leptomeninges may be involved in a spectrum of proliferative conditions ranging from benign melanocytoma to primary CNS melanoma, with all of these entities being exceptionally rare. In contrast, the normal presence of melanocytes in the leptomeninges must always be borne in mind when examining surgical biopsies from CNS sites known to harbor these distinctive elements; one must avoid misinterpreting them as evidence of a melanocytic neoplasm or as hemosiderin-laden macrophages (Table 11.2). With regard to the latter, the long dendritic processes of the melanocytes are generally quite distinctive (Figure 11.57).
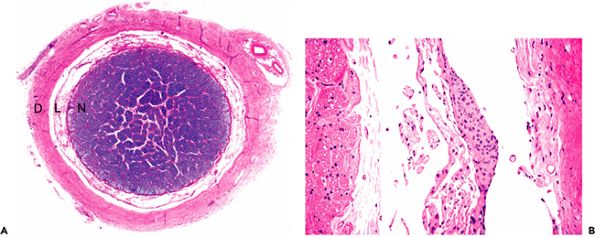 |
Figure 11.58 Optic nerve. A. A whole-mount cross section reveals that the optic nerve (N) is surrounded by leptomeninges (L), which include the pia mater and arachnoid together with the enclosed cerebrospinal fluid-containing subarachnoid space. The leptomeninges and subarachnoid space are in turn covered by the densely fibrous dura mater (D), which, in this location, is often referred to as the optic nerve sheath. B. At higher magnification, leptomeningeal arachnoid cell clusters are clearly seen. The presence of arachnoid cell nests around the optic nerve must be borne in mind when examining intraoperative frozen tissue sections from this neuroanatomic vicinity. |
Optic Nerve
The optic nerves (as well as the optic tracts and optic chiasm) are direct extensions of the CNS and not peripheral nerves. The significance of this fact is that the myelin of the optic nerves is of the central type and is produced by oligodendroglia, not Schwann cells. Thus, the optic nerves are susceptible to diseases of CNS white matter, such as multiple sclerosis. Being extensions of the CNS, the optic nerves are surrounded by the three meninges, pia mater, arachnoid, and dura mater, with the enclosed subarachnoid space (Figure 11.58). The presence of an arachnoid layer surrounding the optic nerve explains the occurrence of optic sheath meningiomas.
Fetal Brain
The two most distinctive histologic features of fetal brain compared to adult brain are active neurogenesis and paucity of myelin. The former is observed as a prominent, dense aggregation of neuroblasts and immature neurons in the periventricular and subpial zones. A similarly transient layer of migrating neurons in the fetal and infant
P.315
cerebellum (the external granular layer) has been discussed. These generative laminae begin involuting during the latter part of gestation; remnants are present during the first year of postnatal life (Figure 11.3C).
Table 11.3 Artifacts | |
|---|---|
|
Artifacts
A variety of gross and macroscopic artifacts may complicate evaluation of the CNS. Many of these are seen frequently in surgical neuropathology practice, while a few are limited primarily to autopsy neuropathology (Table 11.3). Artifacts can be broadly separated into those that hinder diagnostic evaluation versus those that mimic histopathologic lesions. Among the former are artifacts of the crush-burn-freeze-suck-soak group (Figure 11.59). The cavitron ultrasonic surgical aspirator (CUSA) is widely employed by neurosurgeons for the safe removal of diseased CNS tissue, particularly soft tumors, and a trap can be used to collect the aspirated tissue and saline irrigation solution for submission for histologic evaluation. Although microscopic examination of CUSA material can be very informative, the
P.316
pathologist must be aware of the artifacts that frequently accompany such specimens, including artificial distortion and smearing, and the introduction of extraneous material (bone dust, hemostatic agent); CUSA artifact is one cause of pseudonecrosis in CNS tissue samples (Table 11.4). Among artifacts that mimic lesions, the most common are perinuclear halo artifact, collapsed leptomeningeal vessels, and bone dust (Figure 11.60).
 |
Figure 11.59 Artifacts. Many artifacts encountered in surgical pathology of the central nervous system prevent or severely hinder interpretation of the specimen. Among these, some are unique to the consultation service, such as postal service crush artifact (A) and hot weather bubble-wrap paraffin pox (B), while others are secondary to surgical and laboratory tissue insults, such as severe freeze artifact (C), cautery artifact (D), and ultrasonic aspiration of brain tissue (E). |
Iatrogenically introduced foreign material is also encountered with regularity by pathologists who examine CNS specimens and warrants brief mention (Table 11.5; Figure 11.61). A variety of foreign agents are used to control bleeding during surgery and may be introduced preoperatively by the interventional radiologist for embolization of vascular lesions or intraoperatively by the surgeon to control hemorrhage during and after surgery. All of these agents periodically appear in tissue sections. Because they are designed to be resorbable and can therefore be left in place, the morphologic appearance will vary depending on the time interval from placement at the initial surgery and subsequent resection during a second surgery (as, for example, resection of recurrent tumor). Resorbable hemostatic agents elicit a chronic inflammatory reaction of variable intensity, which occasionally may be severe enough to create mass effect and clinical symptoms (textiloma, gossypiboma).
Table 11.4 Pseudonecrosis Etiologies | ||
|---|---|---|
|
There are a few artifacts with which the pathologist who examines postmortem CNS specimens should be familiar (Figure 11.62), the most common being autolysis of the cerebellar granular cell layer (cerebellar conglutination), mechanical distortion of the spinal cord produced by forceps pressure during removal ( toothpaste artifact), and the production of cystic cavities of varying size in the brain by the postmortem proliferation of gas-forming bacteria ( Swiss cheese brain ).
P.317
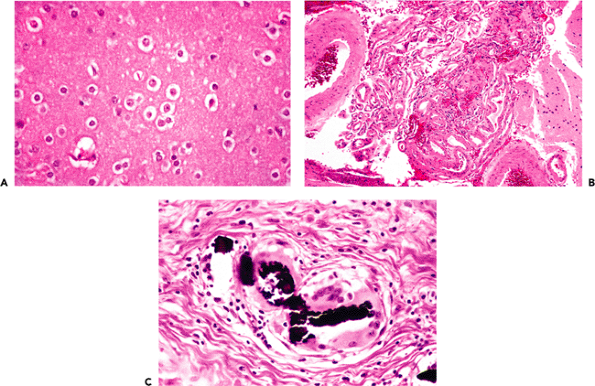 |
Figure 11.60 Artifacts. Several types of artifacts may not be recognized as artifactual in nature to the unaware and so may be particularly misleading. For example, one of the most characteristic morphologic features of normal oligodendrocytes and their derivative tumors, oligodendrogliomas, in formalin-fixed paraffin-embedded tissue sections is the presence of perinuclear halos. However, depending on fixation conditions and other factors, prominent halos may sometimes be seen around other cell types, including neurons (A); care must be exercised in such situations to avoid misdiagnosis. Another example of misleading artifact is the tangle of normal blood vessels that results from collapsed vascular leptomeninges (B). The result can mimic vascular malformation. Before rendering a diagnosis of vascular abnormality in such circumstances, the adjacent brain or spinal cord tissue should be examined for evidence of associated features, such as gliosis, hemosiderin deposition, and granular bodies. Finally, also under the category of misleading artifact is bone dust, which consists of microscopic fragments of cranial bone produced by the surgeon's drill that become intermixed with the tissue sample and can mimic calcification or ossification. In repeat operations, such bone dust fragments left in situ at the previous operation can be seen accompanied by a foreign body type giant cell reaction (C). |
Table 11.5 Iatrogenically-Introduced Foreign Material | |
|---|---|
|
P.318
 |
Figure 11.61 Artifacts. An additional category of artifacts seen in surgical neuropathology consists of foreign material placed by the interventional radiologist or neurosurgeon and subsequently encountered by the pathologist upon tissue resection. The most common examples of this are embolic and hemostatic agents. Embolic materials are introduced by catheter prior to surgery for highly vascular lesions to reduce intraoperative bleeding; the most common are gelatin foam (A), acrylic resin spheres (B), and polyvinyl alcohol particles (C). Hemostatic agents, in contrast, are placed in the surgical site to stop bleeding during the operation and often are left in place after closing to prevent postoperative bleeding. The most commonly employed agents are gelatin foam (D), oxidized cellulose (E), and microfibrillar bovine collagen (F). |
P.319
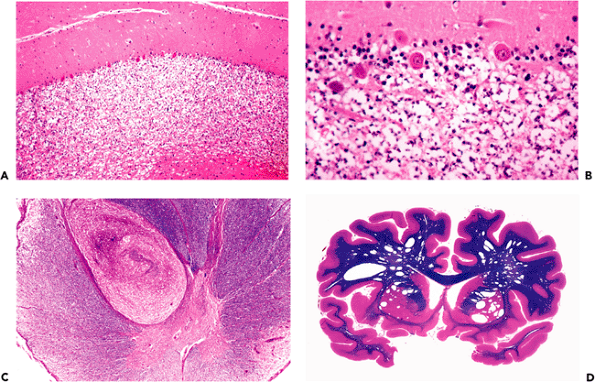 |
Figure 11.62 Artifacts. There are a number of gross and histologic artifacts that are usually encountered only in autopsy specimens of the central nervous system. The most common of these are cerebellar conglutination (A, B), also known as etat glace, which consists of autolytic dissolution of the granular cell layer of the cerebellum; toothpaste or squeeze artifact of the spinal cord (C), which results from focal crushing of the cord by forceps during removal at autopsy and resultant internal herniation of the central gray matter, mimicking malformation or heterotopia; and Swiss cheese brain (D), which is a striking macroscopic vacuolization of the brain resulting from postmortem proliferation of gas-forming bacteria. |
Suggested Readings
Neuropathology Textbooks
Burger PC, Scheithauer BW. Tumors of the central nervous system. In: Atlas of Tumor Pathology, series 4. Washington, DC: Armed Forces Institute of Pathology; 2006.
McLendon RE, Rosenblum M, Bigner DD. Russell & Rubinstein's Pathology of Tumors of the Nervous System. 7th ed. London: Arnold; 2006.
Prayson RA, ed. Neuropathology. Philadelphia: Elsevier; 2005.
Love S, Louis D, Ellison D, eds. Greenfield's Neuropathology. 8th ed. London: Arnold; 2006.
Burger PC, Scheithauer BW, Vogel FS. Surgical Pathology of the Nervous System and Its Coverings. 4th ed. New York: Churchill Livingstone; 2002.
Ironside JW, Moss TH, Louis DN, Lowe JS, Weller RO. Diagnostic Pathology of Nervous System Tumours. New York: Churchill Livingstone; 2002.
Neuropathology Review Books
Gray F, De Girolami U, Poirer J, eds. Escourolle and Poirier's Manual of Basic Neuropathology. 4th ed. Boston: Butterworth-Heinemann; 2004.
Nelson JS, Mena H, Parisi JE, Schochet SS, eds. Principles and Practice of Neuropathology, 2nd ed. New York: Oxford University Press; 2003.
Citow JS, Wollmann RL, Macdonald RL. Neuropathology and Neuroradiology: A Review. New York: Thieme; 2001.
Fuller GN, Goodman JC. Practical Review of Neuropathology. Philadelphia: Lippincott Williams & Wilkins; 2001.
Prayson RA. Neuropathology Review. Totowa, NJ: Humana Press; 2001.
Neuropathology Atlases
Ellison D, Love S, Chimelli L, Harding B, Lowe JS, Vinters H. Neuropathology: A Reference Text of CNS Pathology. 2nd ed. London: Mosby; 2004.
Hirano A. Color Atlas of Pathology of the Nervous System. 2nd ed. New York: Igaku-Shoin; 1988.
Okazaki H, Scheithauer BW. Atlas of Neuropathology. New York: Gower Medical; 1988.
Schochet SS, Nelson J. Atlas of Clinical Neuropathology. East Norwalk, CT: Appleton & Lange; 1989.
Weller RO. Color Atlas of Neuropathology. London: Oxford University Press; 1984.
Veterinary Neuropathology
Summers BA, Cummings JF, de Lahunta A, eds. Veterinary Neuropathology. St. Louis: Mosby; 1995.