7 - Adipose Tissue
Editors: Mills, Stacey E.
Title: Histology for Pathologists, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > IV - Nervous System > 10 - Peripheral Nervous System
function show_scrollbar() {}
10
Peripheral Nervous System
Carlos Ortiz-Hidalgo
Roy O. Weller
Introduction
From a practical point of view, the pathology of peripheral nerves falls into two main categories: (a) peripheral neuropathies, which are diagnosed and treated by physicians and for which an elective nerve or muscle biopsy may be performed as a diagnostic procedure rather than as a therapeutic exercise, and (b) tumors and traumatic lesions, which are removed surgically mainly as a therapeutic measure to alleviate symptoms.
For the diagnosis of peripheral neuropathies, a detailed knowledge of the structure, immunohistochemistry and ultrastructure of peripheral nerves, and clinicopathological correlations is essential. The diagnosis of tumors and traumatic lesions, conversely, relies more on identifying the cellular components within the lesion and their interrelationships. This chapter, therefore, concentrates first on how to identify different cellular components in normal peripheral nerves and, second, on how knowledge of the normal structure of peripheral nerves can be used to identify and assess pathological lesions.
Development of the Peripheral Nervous System
The first anatomical evidence of nervous system differentiation is the neural plate, which develops as a thickened
P.242
specialized area in the middorsal ectoderm of the late gastrula stage of the developing embryo. This zone later becomes depressed along the axial midline to form a neural groove that folds inward to form the neural tube (1). Before fusion is completed, groups of cells become detached from the lateral folds of the neural plate to form the neural crests. Anteriorly, neural crests are located at the level of the presumptive diencephalon and extend backward along the whole neural tube (2).
The neural crest yields pluripotent cells endowed with migratory properties (1). In the peripheral nervous system, the neural crest is the source of neurons and satellite cells in the autonomic and sensory ganglia; ectodermal placodes may also give rise to ganglion cells in the cranial region. Schwann cells are also derived from the neural crest. Migrating pluripotent neural crest cells and their subsequent development is determined and progressively limited, perhaps by the inductive effect of neuregulins and their receptors erbB2 and erbB3, by environmental factors, and by relations with other cell types (1,3). The transcription factor Sox-10, that is initially expressed in the earliest migrating neural crest cells, appears to be intimately involved in the development of Schwann cells from the neural crest. Interestingly, the major myelin protein, P0, is also a transcriptional target for Sox-10 (3).
Many of the events that occur during the later stages of development of peripheral nerves are recapitulated during the regeneration that follows nerve damage in postnatal life. Developing neuroblasts of the dorsal root ganglia (posterior sensory root ganglia) extend neurites both centrally into the neural tube and toward the periphery. Developing motor neurons in the anterior lateral parts of the neural tube extend their neurites toward the periphery. Schwann cells derived from the neural crest become associated with the developing peripheral nerves and eventually form myelin around many of the axons. The proximal portions of the anterior horn cell axons and the central axons of the sensory ganglion cells are myelinated within the neural tube by oligodendrocytes (Figure 10.1).
 |
Figure 10.1 Anatomy of spinal nerve roots. Motor axons arising from the anterior horn cell (A) are initially myelinated by oligodendrocytes (O) and then pass into the anterior root to be myelinated by Schwann cells (S). Sensory nerve axons pass into the dorsal root ganglion (DRG), and the central extension of the sensory neuron passes via the dorsal root into the spinal cord. Arachnoid (AR) appears to be continuous with the perineurium of the peripheral nerve (PN). Dura (D) extends from the spinal cord to coat the roots within the intervertebral foramen and is continuous with the epineurium (EN). |
Growth of Axons
One of the major questions that has been raised is how neuronal processes grow over long distances and arrive at specific terminal regions. Genetic determinants, growth factors, and the extracellular matrix appear to play important roles in the appropriate guidance of neuronal processes (4,5). In 1909, Santiago Ram n y Cajal proposed the concept of neurotrophic substances to explain the directionality and specificity of axonal growth in the developing nervous system, but it was not until the 1960s that nerve growth factor (NGF) was discovered by Rita Levi-Montalcini and Stanley Cohen, as a target-derived neurotrophic factor that supports the survival and differentiation of sensory and autonomic ganglia in the peripheral nervous system (6).
Nerve growth factor is a protein composed of three subunits alpha ( ), beta ( ), and gamma ( ) but only the -NGF has nerve growth promoting activity. Beta-NGF in humans is a 14.5 KDa polypeptide, -NGF is an arginyl esterase, whereas the function of the subunit is not known (6,7). Other substances that participate in axon growth are members of the NGF family [such as brain-derived neurotrophic factor (BDNF)]; neurotrophins 3 (NT-3), 4/5 (NT-4/5), and 6 (NT-6); semaphoring-3A, neuropilin-1, and ephrin (8). The tips of growing axons possess multiple surface receptors for soluble and bound molecules that provide information for the axons' growth course (8). Nerve growth factor interacts with the NGF receptor on the surface of the axon and promotes motility of the growing tip of the axon by interaction with the cytoskeleton of the cell. Mitochondria, neurotubules, neurofilaments, actin filaments, and some cisternae of smooth endoplasmic
P.243
reticulum are incorporated into the axonal growth cone by axoplasmic flow. In addition to its growth promoting properties, NGF also promotes the early synthesis of neurotransmitters.
Schwann cells in the developing nerve produce NGF and possess NGF receptors on their surface membranes, but expression of these receptors diminishes markedly as the peripheral nerve matures. As NGF binds to Schwann cell receptors and becomes concentrated on the surface of the primitive Schwann cell, it provides a chemotactic stimulus for growing axons (9). Failure of trophic interactions between the target organ and its innervation may result in nerve dysfunction. Indeed, cases of human neuropathies have been attributed to deficiency of neurotrophic factors; important data that provides a rational basis for the clinical use of neurotrophic agents in peripheral neuropathies (7).
The extracellular matrix also plays an important role in axonal growth and guidance. The tip of the growing axon has receptors for adhesion to extracellular substances such as collagen, fibronectin, laminin, and entactin; binding of extracellular components to these receptors promotes elongation of axons and stimulates cytoskeletal protein synthesis and therefore cell movement and axon growth. Some of these extracellular components are found within or near basement membranes surrounding Schwann cells (10,11).
Schwann Cells and Myelination
Schwann cells move freely between and around developing peripheral nerve axons, forming primitive sheaths around the neurites and growing in parallel with them. Contact with axons stimulates Schwann cell division in vitro (12). In vivo Schwann cell multiplication virtually ceases in the normal adult animal, but mitotic activity is induced by peripheral nerve damage. It is thought that exposure of the axon to the Schwann cell following loss of myelin sheaths (demyelination) or during axonal regeneration following axonal degeneration (wallerian degeneration) promotes Schwann cell division and that the relationship between Schwann cells and axons in the normal nerve induces some sort of contact inhibition in the Schwann cells. If axon regeneration does not occur following axon damage, Schwann cells gradually decrease in number, suggesting that Schwann cell growth and survival depend on contact with axons (12). Experimental evidence also suggests that continued axon regeneration depends on the presence of Schwann cells (13).
By the ninth week of gestation, fascicles of the human sural nerve are identifiable and contain large axon bundles surrounded by Schwann cell processes (14). Between weeks 10 and 15, Schwann cells extend several long flattened processes that wrap around large clusters of fine axons. At this stage, two to four Schwann cells are located within a common basement membrane and form Schwann families (15).
Myelination of peripheral nerves in humans commences between the twelfth and eighteenth week of gestation (16). Initiation of myelination depends on the diameter of the axon and its association with Schwann cells. By the time that axons have increased in diameter to between 1.0 and 3.2 m, they are in a 1:1 relationship with Schwann cells and have either formed mesaxons or membrane spirals with compact sheaths of 3 to 15 layers (12,15). The reason why some nerves become myelinated and others do not is not clear. Schwann cells around myelinated fibers and around unmyelinated fibers are both able to produce myelin, but the factors that determine whether myelination occurs are unknown. Certain transcription factors, such as Krox-20 and Oct-6, are known to be involved in the myelination program (3,4). In Oct-6 null mice, for instance, myelination is severely delayed, while in Krox-20 null mice myelination fails completely (3). Schwann cells in developing and regenerating peripheral nerves also express high levels of the neurotrophin receptor p75NTR. Neurotrophins are a family of proteins that play a variety of functions in the development and maintenance of the peripheral nervous system (17). Certain glycoproteins, such as myelin-associated glycoproteins, are believed to participate in establishing specific Schwann cell axon interactions in the developing peripheral nervous system (18).
Experimental studies have shown that axons may induce the formation of myelin if the unmyelinated sympathetic chain is grafted onto a myelinated nerve such as the saphenous nerve. Schwann cells that had not previously formed myelin will do so if they come into contact with large, regenerating axons that were previously myelinated (3). It appears also that Schwann cells may influence the caliber of axons since axonal diameter may be decreased markedly in some hereditary demyelinating neuropathies in which there is a genetic defect in Schwann cells and in myelination (12,13). It has been demonstrated that myelinating Schwann cells control the number and phosphorylation state of neurofilaments in the axon, leading to enlargement of the axon itself. Conversely, absence of myelin results in fewer neurofilaments, reduced phosphorylation levels, and therefore smaller axon diameters (18). Myelin-associated glycoprotein (MAG) acts as a myelin signal that modulates the caliber of myelinated axons (19). Maintenance of an axon therefore appears to depend not only on influences from the neuron cell body but also on interactions of the axon with the accompanying Schwann cells (12).
Some 70% of axons within a mixed sensory nerve, such as the sural nerve, are very small and will become segregated into groups of 8 to 15 axons lying in longitudinal grooves within one Schwann cell; these will form the unmyelinated fibers within the peripheral nerve. Thus, all axons in the peripheral nervous system are invaginated into the surfaces of Schwann cells, but myelin sheaths only form around the larger axons, which represent only a small proportion of peripheral nerve fibers.
P.244
Anatomy of Peripheral Nerves
An understanding of the anatomy of peripheral nerves is essential for the interpretation of clinical signs and symptoms and for planning an autopsy to investigate a patient with a peripheral neuropathy (14,15,19,20).
Major nerves, such as the sciatic and median nerves, contain motor, sensory, and autonomic nerve fibers; they are thus compound nerve trunks. It was Sir Charles Bell, the Scottish physician, who first demonstrated that motor function lay in the anterior roots; Fran ois Magendie, the French physiologist, showed that the sensory function lay in the posterior roots. This (anterior-motor; posterior-sensory) is known as the Bell-Magendie law. Motor nerves are derived from anterior horn cells in the spinal cord or from defined nuclei in the brainstem. The initial segment of the axon lies within the central nervous system and is ensheathed by myelin formed by oligodendrocytes (Figure 10.1). As the axons pass out of the brainstem or spinal cord they become myelinated by Schwann cells. Anterior spinal roots join the posterior roots as they pass through the intervertebral foramina to form peripheral nerve trunks. Cranial nerves leave the skull through a number of different foramina. The junction point between oligodendrocytes and the Schwann sheath of the cranial nerves, known as Obersteiner-Redlich zone (O-Rz), has some clinical significance. For example, the pulsatile compression of the O-Rz by a vessel in some exit foramina may be responsible for the clinical symptoms of trigeminal and glossopharyngeal neuralgia, hemifacial spasm, torticollis spasmodicus, or even symptoms of essential hypertension when a vascular cross-compression of the left vagus nerve occurs (21)
Motor nerves end peripherally at muscle endplates and many of the sensory nerves are associated with peripheral sensory endings. The cell bodies of sensory nerves lie outside the central nervous system in the dorsal root ganglia or in cranial nerve ganglia (15). Each ganglion contains numerous, almost spherical neurons (ganglion cells) with their surrounding satellite cells. Such satellite cells are derived from the neural crest and have an origin similar to that of Schwann cells (22). Satellite cells have been referred to in the past by a large variety of names such as amphicyte, capsular cells, perisomatic gliocyte, or perineuronal satellite Schwann cells.
Dorsal root ganglion cells were first described by the Swiss anatomist Albert von Kolliker in 1844. They are examples of pseudounipolar cells, which means that a single, highly coiled axon, or stem process, arises from each perikaryon; but, at varying distances from the neuron, there is a T- or Y-shaped bifurcation, always at a node of Ranvier, with the formation of central and peripheral axons. Thus, the initial segment of axon gives the impression that the cell is a unipolar neuron when it actually has two axons (Figure 10.1). The central axon passes into the spinal cord, either to synapse in the posterior sensory horn of gray matter or to pass directly into the dorsal columns. Peripheral axons pass into the peripheral nerves (15).
Autonomic nerves are either parasympathetic or sympathetic. Preganglionic parasympathetic fibers pass out of the brainstem in the cranial nerves III, VII, IX, and X and from the sacral cord in the second and third sacral nerves. Postganglionic neurons are situated near or within the structures being innervated. Sympathetic preganglionic fibers arise from neurons in the intermediolateral cell columns of gray matter in the thoracic spinal cord and pass out in thoracic anterior roots (15). These preganglionic fibers are myelinated and reach the sympathetic trunk through the corresponding anterior spinal roots; they synapse with the sympathetic ganglion cells in paravertebral or prevertebral locations. The autonomic nervous system innervates viscera, blood vessels, and smooth muscle of the eye and skin (15).
Histology, Immunocytochemistry, and Ultrastructure of Peripheral Nerves
Components of the Nerve Sheath
Macroscopic inspection of a normal peripheral nerve reveals glistening white bundles of fascicles bound together by connective tissue. The intraneural arrangement of fascicles is variable and changes continuously throughout the length of every nerve. Damaged peripheral nerves are often gray and shrunken due to the loss of myelin. Microscopically, transverse sections of a peripheral nerve (Figure 10.2) show how endoneurial compartments containing axons and Schwann cells are surrounded by perineurium to form individual fascicles embedded in epineurial fibrous tissue.
Epineurium
The epineurium consists of moderately dense connective tissue binding nerve fascicles together. It merges with the adipose tissue that surrounds peripheral nerves (Figure 10.2A), particularly in the subcutaneous tissue. In addition to fibroblasts, the epineurium contains mast cells. Although mostly composed of collagen, there are elastic fibers in the epineurium so that, when a specimen of unfixed nerve is removed from the body, there is some elastic recoil of the epineurium (20,23). The amount of epineurial tissue varies and is more abundant in nerves adjacent to joints. As nerve branches become smaller to consist of only one fascicle, epineurium is no longer present. In nerves that consist of several fascicles, one or more arteries, veins, and lymphatics run longitudinally in the epineurium parallel to the nerve fascicles (the vasa nervorum) (15,20,25) (Figure 10.2). Inflammation and occlusion of such arteries is an important cause of nerve damage in vasculitic diseases (25). The overgrowth of epineurial adipose tissue produces
P.245
the so-called lipofibromatous hamartoma, which classically affects the hands and is associated with enlargement of the affected digit (26).
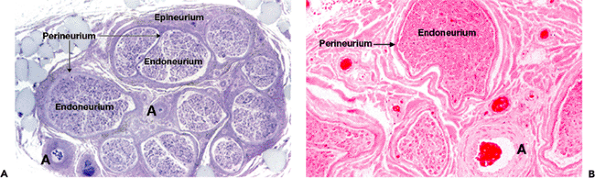 |
Figure 10.2 Peripheral nerve sheaths and compartments. A. A low-power view of a transverse section of a normal sural nerve. The nerve fascicles with roughly circular outlines are surrounded by perineurium and embedded in the connective tissue of the epineurium. Epineurial blood vessels (arrow) are also cut in cross section, and there is adherent adipose tissue (upper left) (1- m resin section, toluidine blue, 16). B. The endoneurial compartment containing myelinated and nonmyelinated nerve fibers and their accompanying Schwann cells is surrounded by perineurium. A large epineurial artery (arrow) is seen at the lower right (paraffin section, H&E, 45). |
Perineurium
Originally described by Friedrich G.J. Henle in the nineteenth century, the perineurium has, in the past, been known by a variety of different terms, such as mesothelium, perilemma, neurothelium, perineurothelium, and, more recently, perineurial epithelium (15,20).
Based on the pioneer work of the 1995 Nobel Prize winners Christiane N sslein-Volhard and Wieschaus, an intercellular signaling molecule secreted by Schwann cells known as Desert Hedgehog, was described, that functions as an important molecule in the formation of the perineurium. Apparently this molecule signals to the surrounding connective tissue cells to organize the perineurium (3).
The perineurium consists of concentric layers of flattened cells separated by layers of collagen (Figures 10.2,10.3,10.4). The number of cell layers varies from nerve to nerve and depends on the size of the nerve fascicle. In the sural nerve, for example, there are 8 to 12 layers of perineurial cells, but the number of layers decreases progressively so that a single layer of perineurial cells surrounds fine distal nerve branches (20). Perineurial cells eventually fuse to form the outer-core of the terminal sensory endings in pacinian corpuscles and muscle spindles (20,24,27). In motor nerves, the perineurial cells form an open funnel as the nerve ends at the motor endplate. Paraganglia of the vagus nerve may lie just underneath the perineurium (28).
By electron microscopy, perineurial cells are seen as thin sheets of cytoplasm containing small amounts of endoplasmic reticulum, filaments, and numerous pinocytotic vesicles that open on to the external and internal surfaces of the cell. Basement membrane is usually seen on both sides of each perineurial lamina (29,30). Numerous cell junctions, including well-formed tight junctions (zonulae occludentes), are present between adjacent perineurial cells and appear to be critical for the formation of
P.246
P.247
the blood-nerve barrier (15,30). Claudins are integral membrane proteins that play a major role in tight junctions and are present in normal and neoplastic perineurium. Claudins comprise a group of approximately 20 different proteins that are exclusively localized in tight junctions (31). In peripheral nerves, claudin-1 expression is largely limited to perineurial cells but is also present in paranodal regions and in the outer mesaxon along internodes (31,32). When tracer substances such as ferritin and horseradish peroxidase are injected into the blood, they do not enter peripheral nerves. Their entry is prevented by tight junctions in endoneurial capillaries and by the tight junctions in the inner layers of the perineurium. Thus, there is a blood-nerve barrier analogous to the blood-brain barrier (30). The blood-nerve barrier is present soon after birth and may prevent the entry of drugs and other substances into nerves that may otherwise interfere or block nerve conduction (30,33). No such blood-nerve barrier exists in the dorsal root ganglia or in autonomic ganglia; these sites in the peripheral nervous system are vulnerable to certain toxins, such as mercury (34).
 |
Figure 10.3 Diagram to show the major elements of peripheral nerve compartments. The epineurium (EP) contains collagen, blood vessels, and some adherent adipose tissue. The flattened cells of the perineurium (PN) are joined by tight junctions and form flattened layers separated by collagen fibers. Renaut bodies (R) project into the endoneurium (EN). Schwann cells forming lamellated myelin (M) (drawn uncompacted in this diagram) surround the larger axons. Multiple unmyelinated axons (UM) are invaginated into the surface of Schwann cells. Other elements include fibroblasts (Fb), mast cells (Mc), capillaries (cap), and collagen (col). |
 |
Figure 10.4 Immunocytochemistry of a normal peripheral nerve. A. Part of single nerve fascicle, cut in transverse section. Perineurium (top) surrounds the endoneurium containing myelinated nerve fibers (M). The nuclei are mainly those of Schwann cells (paraffin section, H&E, 160). B. Similar field to (A) stained for epithelial membrane antigen. The perineurium (top) is densely stained [immunoperoxidase technique (ABC) with antiepithelial membrane antigen (anti-EMA) antibody, 160]. C. Part of a nerve fascicle stained for neurofilament protein. Large myelinated axons are well stained, but unmyelinated axons are much smaller and more difficult to detect [immunoperoxidase technique (ABC) using an antibody against the 80 KDa neurofilament protein, 160]. D. Part of a nerve fascicle stained for S-100 protein showing densely stained Schwann cells [immunoperoxidase (ABC) using anti-S-100 protein antibody, 160]. E. Part of a nerve showing CD34+ endoneurial cells. These cells are clearly distinct from the Schwann cells that comprise the bulk of the cell in the nerve [immunoperoxidase technique (ABC) using anti-CD34 (QBend10) antibody, 160]. F. A traumatized nerve cut in longitudinal section showing regenerating axons (stained brown) (immunohistochemistry for GAP 43, 40). (Photograph provided by Professor James Nicoll.) |
If the perineurium is injured, there is breakdown of the blood-nerve barrier and perineurial cells migrate into the endoneurium to surround small fascicles of nerve fibres (35). This is classically seen in amputation neuromas but is also observed in focal compressive lesions of nerve (36). The swelling of the nerve and the concentric arrangement of the perineurial cells in the compressive lesions spawned the term localized hypertrophic neuropathy, but it is quite different from hypertrophic neuropathy (36), in which Schwann cells form whorls around individual axons in response to recurrent segmental demyelination (see below).
Whereas the epineurial sheath of the nerve is continuous with the dura mater at the junction of spinal nerves and spinal nerve roots (Figure 10.1), the perineurium blends with the pia-arachnoid. There are some morphological similarities between perineurium and arachnoid cells, although arachnoid cells are not usually coated by basement membrane. Immunocytochemically, perineurial cells and pia-arachnoid cells are positive for epithelial membrane antigen (EMA) (Figure 10.4) and vimentin but are negative for S-100 protein and CD57 (37,38). Perineurial cells also express insulin-dependent glucose transporter protein I (Glut-1) (32,39).
Epithelial membrane antigen belongs to a heterogenous family of highly glycosylated transmembrane proteins found originally on the surface of mammary epithelial cells (40) but which are also present in the cells of virtually all epithelial tumors (40). However, EMA is not restricted to epithelial structures and has been identified on plasma cells and on cells in certain lymphomas and soft tissue tumors (20,40). Perineurial cells, arachnoid, and pia share certain ultrastructural characteristics and express EMA and vimentin in their cytoplasm. Immunohistochemistry has demonstrated that perineurial cells proliferate in some conditions, such as traumatic neuroma, Morton's neuroma, neurofibroma, solitary circumscribed neuroma, neurothekeoma, pacinian neuroma, and in the mucosal neuromas associated with multiple endocrine neoplasia (vide infra) (33,41).
Some tumor cells break through the perineurial sheath to grow along the perineurial space; perineurial invasion has been correlated with decreased survival times in some cancers (42). The problem for the histopathologist, however, is that sometimes perineurial invasion cannot be unequivocally determined on hematoxylin and eosin (H&E) stained sections. Immunocytochemistry for Glut-1, EMA, and claudin-1 may be used to rapidly and accurately assess the presence of perineurial invasion (38,43). Care must be taken, however, when examining cases of vasitis nodosa, in which benign proliferating ductules may be found within the perineurium and endoneurium (44). Nerve involvement has also been reported in fibrocystic disease of the breast, normal and hyperplastic prostate, and normal pancreas (44).
Endoneurium
The endoneurium is the compartment that contains axons and their surrounding Schwann cells, collagen fibers, fibroblasts, capillaries, and a few mast cells (Figures 10.3,10.4,10.5).
In cross sections of peripheral nerves, some 90% of the nuclei belong to Schwann cells, 5% to fibroblasts, and 5% to other cells (such as mast cells and capillary endothelial cells). Within the endoneurium, CD34+ bipolar cells with delicate dendritic processes have been identified and are distinct from Schwann cells (45,46). Similar cells have been identified in peripheral nerve sheath tumors in various proportions (45).
Some investigators have observed endoneurial dendritic cells, distinct from Schwann cells and conventional fibroblasts, that may function as phagocytes under certain conditions (47). In this regard, it has been described within the human endoneurium, an intrinsic population of immunocompetent and potentially phagocytic cells (endoneurial macrophages), that share several lineage-related and functional markers with macrophages and may represent the peripheral counterpart of del-Rio-Hortega cells (microglia) of the CNS (48,49).
Nerve fibers may be myelinated or unmyelinated but not all nerves have the same nerve fiber composition. Most biopsies of peripheral nerves in humans are taken from the sural nerve at the ankle, and it is the composition of this nerve that has been most closely studied (50). Fibroblasts are ultrastructurally identical to fibroblasts elsewhere in the body. Mast cells are a normal constituent of the endoneurium and are also seen in sensory ganglia and in the epineurial sheath of peripheral nerves. There is an increase in the number of mast cells in some pathological conditions such as axonal (wallerian) degeneration
P.248
and in some neoplastic entities such as von Recklinghausen's disease (neurofibromatosis). A characteristically high number of mast cells is seen in neurofibromas, but they are only present in the Antoni B areas of schwannomas (24,51). Mast cells are thought to influence growth of neurofibromas because some of their mediators may also act as growth factors (52). Apparently the inciting factor for mast cell migration into nerve sheath tumors is Kit ligand that is hypersecreteted by NF / Schwann cell populations (52). Mast cell stabilizers are claimed to reduce proliferation and itching of neurofibromas (52). Following nerve injury, there is breakdown of the blood-nerve barrier as endoneurial vessels become permeable to fluid and protein; this increase in permeability may be related to the release of biogenic amines from mast cells within the endoneurium. Proteases released from mast cells have a high myelinolytic activity and may play a role in the breakdown of myelin in certain demyelinating diseases (52,53).
 |
Figure 10.5 High-power histology of human sural nerve in transverse section. A. Large- and smalldiameter myelinated fibers are seen. In the normal nerve, these fibers are separated from each other, but small numbers of clusters (see Figure 10.12B) are seen in this illustration (1- m resin section, toluidine blue, 160). B. Part of a sural nerve fascicle cut in transverse section. Perineurium is at the top right (PN). Both large and small myelinated fibers vary in cross-sectional outline. Splits within the sheath are Schmidt-Lanterman incisures. Also visible are an endoneurial blood vessel (BV) and a section through a fiber near the node of Ranvier (N). Unmyelinated axons are seen as unstained circles within Schwann cells (S). (1- m resin section, 310.) |
Collagen within the endoneurial compartment is highly organized and forms two distinct sheaths around myelinated and unmyelinated nerve fibers and their Schwann cells (see Figures 10.8, 10.10). The outer endoneurial sheath (of Key and Retzius) is composed of longitudinally oriented large diameter collagen fibers; the inner endoneurial sheath (of Plenk and Laidlaw) is composed of fine collagen fibers oriented obliquely or circumferentially to the nerve fibers. The term neurilemma has been applied to the combined sheath formed by the basement membrane of the Schwann cell and the adjacent inner endoneurial sheath (15,24). Thus the term neurilemmoma is inappropriate when used to describe tumors of Schwann cell origin (schwannomas). The longitudinal orientation of collagen fibers in the outer endoneurial sheath, together with the Schwann cell basement membrane tubes, may play an important role in guiding axons as they regenerate following peripheral nerve damage (3,24).
Renaut bodies (Figures 10.3, 10.6) are seen not infrequently in the endoneurium of human peripheral nerves. Described in the nineteenth century by the French physician Joseph Louis Renaut, they are cylindrical (circular in cross section), hyalin bodies attached to the inner aspect of the perineurium. Composed of randomly oriented collagen fibers, spidery fibroblasts, and perineurial cells, Renaut bodies stain positively with Alcian blue because of the presence of acid glycosaminoglycans. The rest of the endoneurium also contains Alcian blue positive mucoproteins (24,54). Renaut bodies express vimentin and EMA and produce extracellular matrix highly enriched in elastic fiber components (55). In longitudinal section, they may extend for some distance along the nerve and end in a blunt and abrupt fashion (55). These bodies are more prominent in horses and donkeys than in humans (2). Their precise function is not known, but Renaut himself thought that they may act as protective cushions within the nerve. They increase in number in compressive neuropathies and in a number of other neuropathies, including hypothyroid neuropathy, and may be a reaction to trauma (54,55).
Blood Supply of Peripheral Nerves
Vasa nervorum supplying peripheral nerves are derived from a series of branches from associated regional arteries. Branches from those arteries enter the epineurium (Figures 10.2, 10.3)
P.249
to form an intercommunicating or anastomosing plexus. From that plexus, vessels penetrate the perineurium obliquely and enter the endoneurium as capillaries often surrounded by pericytes (Figure 10.5). Tight junctions between the endothelial cells of the endoneurial capillaries constitute the blood-nerve barrier (30).
 |
Figure 10.6 Nerve fascicles showing Renaut bodies (arrows). A. Immunostaining for epithelial membrane antigen (EMA). B. Russell-Movat pentachrome, showing the Renaut bodies in blue. |
Complete infarction of peripheral nerves is very uncommon, probably due to the rich anastomotic connections of epineurial arteries. However, inflammation and thrombotic occlusion of epineurial arteries is seen in vasculitides (56), and occlusion by emboli occurs in patients with atherosclerotic peripheral vascular disease; both these disorders result in ischemic damage to peripheral nerves with axonal degeneration and consequent peripheral neuropathy (50).
Nerve Fibers
Most peripheral nerves contain a mixture of myelinated and unmyelinated nerve fibers. As the axons are oriented longitudinally along the nerve, quantitative estimates of the number of fibers in the nerve and their diameters are only adequately assessed in exact transverse sections. Staining techniques that can be used to identify nerve fibers and other components within peripheral nerves are summarized in Table 10.1. Longitudinal sections of peripheral nerve are less valuable than transverse sections, but teased nerve fibers (see Figure 10.15D) are very valuable for detecting segmental demyelination and remyelination and for assessing past axonal degeneration and regeneration (50).
In a transverse section of a human sural nerve, there are approximately 8,000 myelinated fibers/mm2, whereas the unmyelinated axons are more numerous at 30,000 myelinated fibers/mm2. Peripheral nerve fibers are classified as class A, class B, and class C fibers, according to their size, function, and the speed at which they conduct nerve impulses. Class A fibers are myelinated and are further subdivided into six groups covering three size ranges. The largest are 10 to 20 m diameter myelinated fibers that conduct at 50 to 100 m/sec; myelinated fibers 5 to 15 m in diameter conduct at 20 to 90 m/sec, and 1 to 7- m diameter myelinated fibers conduct at 12 to 30 m/sec. Class B fibers are myelinated preganglionic autonomic fibers some 3 m in diameter and conducting at 3 to 15 m/sec. Unmyelinated fibers are small (0.2 to 1.5 m in diameter), conduct impulses at 0.3 to 1.6 m/sec, and include postganglionic autonomic and afferent sensory fibers, including pain fibers (57).
Myelinated Axons
Ultrastructure
Although myelinated nerve fibers can be demonstrated in paraffin sections (Figure 10.4), they are best visualized by light microscopy in 0.5- to 1- m thick toluidine blue stained resin sections (Figure 10.5). They exhibit a bimodal
P.250
P.251
distribution of fiber diameter in the normal nerve, with peaks at 5 and 13 m and a range of 2 to 20 m. Most axons above 3 m in diameter are myelinated. Although along much of its length a myelinated nerve fiber has a circular outline in cross section, there is considerable variation in shape within the normal nerve, especially in the perinuclear regions and in the regions around the node of Ranvier (paranodal regions) (Figure 10.7).
Table 10.1 Histologic Techniques for Peripheral Nerves | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The axon itself is limited by a smooth plasma membrane (axolemma), that is separated from the encompassing Schwann cell by a 10 to 20 nm gap (periaxonal space of Klebs) (Figure 10.8). The axonal cytoplasm (axoplasm) contains mitochondria, cisternae of smooth endoplasmic reticulum, occasional ribosomes and glycogen granules, peroxisomes, and vesicles containing neurotransmitters. The most prominent components of the axoplasm, however, are the filamentous and tubular structures. Microfilaments, 5 to 7 m in diameter, are composed of chains of actin and comprise approximately 10% of the total axonal protein. They are virtually confined to the cortical zone of the axoplasm immediately beneath the axolemma (15). Neurofilaments (Figures 10.4C, 10.8) are 8- to 10- m diameter intermediate filaments of indeterminant length, and they constitute a major filamentous component in larger axons (58). They were described originally by Ram n y Cajal and Bielschowsky as argentophilic neurofibrillae. In the neuronal perikaryon, neurofilaments tend to appear in multiple whorled bundles with no clear orientation to elements of the cell. In the axons, however, neurofilaments appear in longitudinal, mostly parallel orientation (59). Small armlike filaments are seen by electron microscopy. They project from the surface of the neurofilaments to form an irregular polygonal lattice. Neurofilaments are composed of protein triplets that are chemically and immunochemically distinct (59). Three major subunits are recognized and are classified according to their molecular weights of 68, 150, and 200 KDa. Within axons, neurofilaments are phosphorylated and are immunocytochemically distinct from the nonphosphorylated filaments within neuron cell bodies. Immunocytochemistry for neurofilament protein (Figure 10.4C)
P.252
is often valuable for detecting large- or medium-sized axons in normal nerves, in traumatic lesions, in tumors involving peripheral nerves, and occasionally for detecting axonal processes in neuronal tumors (58,59).
 |
Figure 10.7 Diagram to show the relationships between (A) teased fibers, (B) nerve fibers in longitudinal section, and (C) nerve fibers in transverse section. A. In teased fibers, nodes of Ranvier (N) are separated by internodal portions of the Schwann cell and myelin sheath. The Schwann cell nucleus is roughly in the center of the internode. B. Longitudinal section through the node of Ranvier shows how the myelin sheath terminates as a series of end-loops. The axon narrows as it passes through the node of Ranvier. C. Transverse sections of peripheral nerve as seen in electron micrographs and 1 m resin sections are here related to the different portions of the internode and the node of Ranvier. From left to right, the paranodal region shows crenation of both axon and myelin sheath in larger fibers. At the node of Ranvier, the axon is small and coated by radially arranged Schwann cell processes and myelin end-loops. Throughout most of the internode, the myelinated fiber is circular. In the region of the nucleus, the axon and the myelin sheath may be ovoid rather than circular in outline. |
 |
Figure 10.8 Transverse section of a myelinated nerve fiber in the perinuclear region. The axon contains mitochondria, small vesicles, and numerous neurofilaments and neurotubules cut in cross section (inset,top left). A distinct periaxonal space separates the axon from its encompassing Schwann cell. Myelin is compacted except at the external mesaxon (EM) and internally around the internal mesaxon near the axon itself. Part of a Schmidt-Lanterman incisure is seen on the inside of the myelin sheath. Abundant rough and smooth endoplasmic reticulum is seen in the perinuclear cytoplasm of the Schwann cell. A basement membrane (BM) surrounds the Schwann cell plasma membrane, and endoneurial collagen fibers are seen cut in cross section (col). (Electron micrograph, 18,400); inset, 40,000.) |
The third filamentous component in the axoplasm is the microtubule (neurotubule). Microtubules are cylindrical (Figure 10.8), unbranched, longitudinally oriented, hollow tubules 24 nm in diameter and composed of globular subunits of tubulin 4 to 5 nm in diameter. Periodic radial projections of high-molecular weight proteins, which are part of the microtubule-associated proteins (MAPs), arise from the surface of the neurotubules. These armlike projections bind neurofilaments and actin filaments and together form neurotubule-neurofilament-actin filament lattices. The three-dimensional lattices form an ordered structure in the axoplasm that appears to play an important role in axonal transport and contributes directly to the axon's shape (60). Microtubules also direct the transport of vesicular organelles between the cell body and the axon and thereby determine, in part, the composition of the axon (60).
Axoplasmic Flow
In 1906, Scott proposed that neuron cell bodies secreted growing substances in order to maintain the function of the axon. He suggested that such substances pass down the axon cytoplasm to the axon terminals. This suggestion was endorsed by Ram n y Cajal when he observed how regeneration occurs from the proximal stump of a damaged axon as long as continuity with the cell body is maintained (15). More definitive evidence of axonal transport was provided later by experimental studies using autoradiography and other techniques. Not only can labeled substances such as tritiated leucine be traced by autoradiography as they are transported along axons from the cell body, but the transport of organelles within the axon can also be directly observed by the use of dark-field microscopy or Nomarski optics (60). The term axoplasmic flow was coined by Weiss to describe the movement of different materials along the axoplasm. Axoplasmic flow and transport occur in two directions, away from the cell body (anterograde) and toward the cell body (retrograde) (61).
Anterograde axoplasmic transport occurs at two velocities: fast and slow. Most organelles and large-molecular weight substances within the axon are conveyed by fast axoplasmic transport, up to 400 mm/day. If a ligature is placed around a nerve, transported material accumulates proximal to the ligature and to some degree distal to it, due to interference with anterograde and retrograde transport, which both occur at the same rate and by the same mechanisms. The filamentous lattice component of neurotubules, neurofilaments, and actin filaments is responsible for fast axoplasmic flow, and these three elements probably act as rails along which the various transported organelles and substances move. Fast axoplasmic transport is dependent on oxidative energy mechanisms and adenosine triphosphate (ATP); it also depends on calcium and magnesium ions and is blocked by calcium channel blocking agents. Some substances, such as trifluoperazine, that block calmodulin (calcium-activating protein) also block axoplasmic flow. Neurotubules, as an integral part of the axoplasmic transport mechanism, are depolymerized by cold and by colchicine; vincristine, and vinblastine are known to bind tubulin and prevent the normal assembly of neurotubules. Such substances block fast axoplasmic flow (60).
Retrograde axoplasmic transport may convey information and organelles back to the cell body. In immature nerves, nerve growth factor is taken up by nerve terminals and retrogradely transported to the cell body, where it may play a role in the maturation of neurons (62). It has been suggested that the transport of such growth factors may also influence the metabolism of mature neurons and that the absence of such signals from the distal part of the neuron when the axon is severed may trigger chromatolysis (61). Retrograde transport is also a pathway by which certain toxins (tetanus neurotoxin) and some metals (lead, cadmium, and mercury) may bypass the blood-brain barrier and accumulate in neurons (63). Neurotropic viruses such as herpes, rabies, and poliomyelitis may be transported to the central nervous system by retrograde transport (64,65). In addition to toxic neuropathies, axonal transport is defective in diabetes, peroneal muscular atrophy, and probably in amyotrophic lateral sclerosis. Axoplasmic transport is reduced with age (66).
Slow axoplasmic transport at 1 to 3 mm/day concerns the distal movement of cytoskeletal elements such as neurofilaments, microtubules, and actin. It is a one-way process, and neurofilaments are broken down by calcium-activated proteases at the distal end of the axon. Similarly, microtubules are depolymerized distally (67). Various toxins such as hexocarbons and their derivatives may interfere with slow axoplasmic transport so that neurofilaments accumulate and form large swellings within the axon (34,68). It is thought that neurofilaments within an axon may act primarily to maintain the bulk and the shape of large axons; neurofilaments are less numerous in small axons.
The Periaxonal Space of Klebs
As the Schwann cell enwraps the axon, it leaves a space, 20 nm wide, between the Schwann cell membrane and the axolemma (Figure 10.8); this is the periaxonal space of Klebs (69). This space is in continuity with the extracellular space at the node of Ranvier through a narrow helical channel at the site where the terminal cytoplasmic processes of the Schwann cell approach the axolemma (14) (Figure 10.7). The maintenance of the periaxonal space of Klebs appears to be mediated by an intrinsic 100 KDa myelin-associated glycoprotein (MAG) in the periaxonal membrane of the Schwann cell (70). This protein has a heavily glycosylated
P.253
domain, with sialic acid and sulfate residues on the external surface of the plasma membrane extending into the periaxonal space; in fact, about half of the peptide of MAG is in the periaxonal space (71). Mutant mice that do not express MAG do not form a periaxonal space, and the Schwann cell membrane fuses with the axolemma. Experimental studies with giant squid axons and mammalian nerve axons show that there is an increase in potassium concentration in the periaxonal space during repetitive conduction of nerve impulses. The full significance of the periaxonal space, however, is not clearly understood.
Schwann Cells
In his book on the microscopic structure of animals and plants published in Berlin in 1839, Theodore von Schwann identified a vague sheath of cells within nerve fibers; these cells have subsequently borne his name as Schwann cells. As described previously in the section on development of peripheral nerves, Schwann cells are derived from the neural crest and migrate with growing axons into the developing peripheral nerves (3,72). Schwann cells produce nerve growth factor both in development and during regeneration; and, as the nerves grow, Schwann cells divide axons into groups and eventually establish 1:1 relationships with the larger fibers that they will ultimately myelinate (15,25,72). Immature proliferating Schwann cells have a relatively large volume of cytoplasm compared with mature Schwann cells. The Schwann cytoplasm is rich in mitochondria, polyribosomes, Golgi cisternae, and rough endoplasmic reticulum (Figure 10.8). The cytoskeleton within the cells includes vimentin intermediate filaments and is particularly obvious during the active proliferative and migrating phases of development and regeneration.
Schwann cells in a normal adult peripheral nerve are associated with both myelinated fibers and unmyelinated fibers. In myelinated fibers the Schwann cytoplasm is divided into two compartments: (a) around the nucleus and on the outside of the myelin sheath, and (b) that thin rim of cytoplasm on the inside of the myelin sheath and around the internal mesaxon (Figure 10.8). Using electron microscopy, Schwann cells within a nerve can be identified by their relationship with myelinated or unmyelinated fibers. In damaged peripheral nerves, however, Schwann cells can be identified most easily by the presence of an investing basement membrane (Figure 10.8). Other cells within the endoneurium, such as fibroblasts, do not have a basement membrane; and, although macrophages may invade the basement membrane tubes, they have a distinct ruffled border that distinguishes them from Schwann cells. Perineurial cells may be found in the endoneurial compartment, particularly in damaged nerves; they possess a basement membrane, but they can be distinguished from Schwann cells by the presence of tight junctions that are not a feature of Schwann cells (3,54,72). With increasing age, normal Schwann cells accumulate lipofuscin and lamellated structures in the paranuclear cytoplasm in the form of pi ( ) granules of Reich. Such granules are composed of wide-spaced lamellated structures and amorphous osmiophilic material; they are rich in acid phosphatase and stain metachromatically with toluidine blue in frozen sections (73). Other inclusions such as the corpuscles of Erzholz are seen in Schwann cytoplasm; these bodies are spherical, 0.5 to 2.0 m in diameter, and stain intensely with the Marchi method. Few Pi granules remain in Schwann cells following nerve damage in which there has been extensive Schwann cell mitosis and proliferation (73).
In addition to an investing basement membrane, composed of laminin, fibronectin, and entactin/nidogen, Schwann cells also produce heparan sulfate, N-syndecan, glypican, collagens type I, III, IV, and V, 1 and 4 integrin, and the protein BM-40 (74). All these secreted products are incorporated into the basement membrane except type I and type II collagen (3,72). Schwann cells can be identified in paraffin sections by immunocytochemistry and by the presence of close investment by reticulin staining. There is a rich reticulin network investing each cell, not only in the normal peripheral nerve but also in Schwann cell tumors. The S-100 protein in the cytoplasm and nuclei of Schwann cells can be identified by immunocytochemistry (Figure 10.4D). This acidic protein, which is 100% soluble in ammonium sulfate at neutral pH, was described by Blake W. Moore in 1965, is a calcium-binding EF-hand type molecule, and has no known function, but it is present in Schwann cells and not in fibroblasts or perineurial cells (37,75) (Figure 10.4). In vitro studies have reported the presence of Schwann cells that are weakly reactive for S-100 protein and that may correspond to the non-myelinating (Remak) cell population (76). Schwann cells are also immunolabeled using CD57 and CD56 but perineurial cells are again negative (37,77). Calretinin, the 29-KDa, calcium-binding protein that also belongs to the family of EF-hand proteins, is expressed in Schwann cells but not consistantly in schwannomas (78). Normal and some neoplastic Schwann cells also express the cell adhesion molecule CD146 (79). Occasionally, Schwann cells are labeled by anti-GFAP antibodies, but this may depend on the antibody used (37,80). GFAP immunoreativity in the peripheral nervous system has been demonstrated in enteric ganglia, olfactory nerve cells, and in Schwann cells in the sciatic, splenic and vagus nerves (81). Schwann cells also participate in the formation, function, and maintenance of neuromuscular junctions and Meissner corpuscles (82,83). These terminal Schwann cells may be identified by their expression of Herp-protein, which is not present in nonterminal myelinating Schwann cells (84). An interesting and peculiar intermediate glial cell type known as the olfactory ensheathing cell (OEC) is associated with neuronal processes of the olfactory bulb; OECs share astrocytic and Schwann cell phenotypes,
P.254
promote axonal regeneration, and are potentially useful cells for xenotransplantation procedures (85).
Myelin
Myelin sheaths appear as slightly basophilic rings in H&E stained transverse paraffin sections of nerve (Figure 10.4). They can be more prominently stained by Luxol fast blue or by hematoxylin stains such as Loyez (Table 10.1). In frozen sections, myelin is well depicted by Sudan black staining; and, in unstained frozen sections, myelin can be identified due to its birefringence in polarized light, a technique that is particularly suitable for identifying myelin in enzyme histochemical preparations (57).
Myelin is formed by the fusion of Schwann cell membranes and, by electron microscopy, it is seen as a regularly repeating lamellated structure with a 12 to 18 nm periodicity (86). On the outer and inner aspects of the sheath, external and internal mesaxons can be traced from the cell surface (Figure 10.8). The myelin membrane is divided into two structurally and biochemically distinct domains: the compact and the noncompact myelin, each of which is characterized by a unique set of proteins. Compact myelin, for instance, contains P0, PMP22, and MBP, whereas noncompact myelin contains MAG, Cx32, 6 4 integrin and E-cadherin (3,72).
As the external aspects of the Schwann cell membranes fuse to produce compact myelin, an interrupted interperiod line forms in the myelin. The more densely stained period line is formed by fusion of the cytoplasmic aspects of the cell membrane. A narrow cleft can be resolved between the components of the interperiod line. In myelinating Schwann cells, noncompact myelin is present in paranodal loops, Schmidt-Lanterman incisures, nodal microvilli, and the inner and outer edges of the myelin (87). Several types of cell junction, including tight, gap and adherens junctions, are seen between the myelin lamellae (known as autotypic junctions) (87).
Biochemically, myelin is 75% lipid and 25% protein. The major lipids are cholesterol, sphingomyelin, and galactolipids, which are present in a rather higher proportion than they are in other cell membranes. It is the arrangement of the lipids that produces the liquid crystalline fluid birefringent myelin sheath, and it is esterification of the cholesterol in degenerating myelin that can be detected by Sudan dyes, by oil red O, and by the Marchi technique (Table 10.1). As myelin degenerates and the cholesterol becomes esterified, the ultrastructural lamellated pattern of myelin is lost and replaced by the amorphous osmiophilic globules seen in electron micrographs. More than half the protein in myelin is a transmembrane 28 to 30 KDa glycoprotein, P0 (88); other proteins are P1 and P2. The protein mediates homophilic adhesive interactions between Schwann cell plasma membranes, is a key structural constituent of both the major dense line and interperiod line of compact myelin, and is involved in myelin compaction (88). Numerous mutations in P0 have been described in a variety of demyelinating diseases (see below) (88).
Although the lipid composition of myelin in the peripheral nervous system (PNS) is very similar to that of the central nervous system (CNS), the protein components are markedly different. Central nervous system myelin has no P0 protein but has a proteolipid that is soluble in organic solvents; it also has an 18KDa basic protein that is probably homologous to the P1 protein of peripheral nerve myelin. These biochemical differences may account for differences in the structure between PNS and CNS myelin; for example, the spaces between the dense lines are less for CNS myelin (89). Biochemical differences in the proteins definitely account for the distinct antigenicities of peripheral and central nervous system myelin. Thus, injection of CNS myelin with Freund's adjuvant will produce allergic encephalomyelitis in experimental animals, with destruction of myelin in the brain and spinal cord (90), whereas injection of peripheral nervous system myelin with Freund's adjuvant will produce allergic neuritis with demyelination in the peripheral nervous system.
Myelin sheaths are essential for the normal functioning of the PNS; and, in those hereditary neuropathies in which myelination is defective, severe disability and retardation of development are seen (91). Acting as a biological electrical insulator, myelin allows discontinuous (saltatory) and very rapid conduction of a wave of depolarization along the nerve fiber. It appears that myelination is an evolutionary adaptation that allows increased conduction velocities without excessive increases in axon diameter (72).
Myelination in the PNS in humans occurs well in advance of that of the CNS (89). Although there is little myelin in human cerebral hemispheres at birth, myelin sheaths have already started to form around peripheral nerves at this time. Myelination is initiated by contact between Schwann cells and future myelinated axons. The Schwann cell rotates around the axon and may form 50 or more spirals, resulting in formation of the myelin sheath.
As the Schwann cell differentiates and produces a basement membrane, it acquires polarity via interaction of its cytoskeleton and some basement membrane components (mainly laminin and fibronectin) (3,92). The Schwann cell then begins to extend processes around individual axons. Once the lips of the Schwann cell start to wrap around the axon, they generate traction to pull the whole cell around, and a spiral wrapping made up of many lamellae is formed (18). The importance of basement membrane formation as a prerequisite for the formation of myelin is emphasized by the lack of myelination when the basement membrane is deficient (92,93).
Myelin-associated glycoprotein also plays an important role in myelination (88). It is present in the membranes of Schwann cells around myelinated fibers but not in those cells associated with unmyelinated fibers. Myelin-associated glycoprotein
P.255
probably functions through its interaction with the Schwann cell cytoskeleton, and this facilitates process lengthening and rotation during myelination (71). Periaxin, a 47 KDa protein constituent of the dystroglycan-dystrophin related protein-2 complex that links the Schwann cell cytoskeleton to the extracellular matrix, is located in the periaxonal region of Schwann cell plasma membrane that possibly interacts with myelin-associated glycoprotein during myelination (94). Mutations in the periaxin gene result in the autosomal recessive demyelinating Charcot-Marie-Tooth (CMT4F) and D j rine-Sottas diseases (94) (see below). As myelination proceeds, cytoplasm is expressed from the spiral of Schwann cell processes and membranes compact to form the 12 to 18 nm lamellated structure of myelin.
The length of an embryonic Schwann cell is 30 to 60 m, and it becomes associated with that length of axon in the developing nerve. As the nerve lengthens with growth of the body and limbs, so does the Schwann cell so that the length of the Schwann cell or internodal distance (Figure 10.7) in myelinated fibers reaches some 190 m at 18 weeks gestation and 475 m at birth. In the adult nerve, normal Schwann cells may extend for up to 1 mm in length along myelinated fibers. Schwann cells associated with unmyelinated fibers lengthen to reach approximately 250 m in the adult sural nerve. Following damage to a peripheral nerve, Schwann cell lengths revert to their embryonic length, and thus give short internodes in regenerating and remyelinating nerve fibers (see Figures 10.12, 10.14).
Schmidt-Lanterman Clefts or Incisures
Once viewed as artifacts, the clefts or incisures described by H.D. Schmidt and A.J. Lanterman (Figure 10.9) are now known to be fixed components of the myelin sheath (24). Each Schmidt-Lanterman incisure (S-L I) consists of a continuous spiral of Schwann cytoplasm that runs from the outer (nuclear) to the inner (paraxonal) Schwann cell compartment in an oblique fashion at about 9 degrees to the long axis of the sheath. The cleft splits the cytoplasmic membranes at the major dense line and forms a route for the passage of substances from the outer cytoplasmic layer through the myelin sheath to the inner cytoplasm. This function was suggested by Ranvier as early as 1897. Near the external surface of the cleft, stacks of desmosome-like structures and gap junctions rich in connexin 32, are sometimes seen, possibly maintaining the integrity of the spiral (95). The cell junction proteins claudin-5, MUPP1, E-cadherin, as well as a 155-KDa isoform of neurofascin, have been selectively detected at the S-L I (31). Cytoplasm in the clefts contains membrane-bound dense bodies, lysosomes, an occasional mitochondrion, and a single microtubule (Figure 10.9) that runs circumferentially around the fiber; this microtubule may be associated with transport and with stabilization of the cytoplasmic spiral (15). The number of S-L Is correlates with the diameter of the axon; the larger the fiber, the more clefts in the myelin sheath per Schwann cell. The presence of these clefts throughout myelogenesis suggests that they are an important functional part of the sheath (24). Balice-Gordon et al. (96) have suggested that the incisures may provide some degree of flexibility and may protect the peripheral nerve from mechanical stress during stretching and recoil. It also seems obvious that they are pathways of communication between the inner and outer Schwann cell cytoplasm, but their full significance remains to be elucidated.
 |
Figure 10.9 Longitudinal section of peripheral nerve: a Schmidt-Lanterman incisure. Blebs of cytoplasm are seen running through the myelin sheath. Densities in the cytoplasm (top left) suggest some form of junction between the spiral turns of the incisure. The axon is cut tangentially. (Electron micrograph, 30,000). (Reprinted from Weller RO, Cervos-Navarro J. Pathology of Peripheral Nerves: A Practical Approach. London: Butterworths; 1977 with permission.) |
Node of Ranvier
With the introduction of techniques whereby individually separated or teased myelinated nerve fibers could be stained black with osmium tetroxide, a new view of nerve fibers was obtained. In his publication of 1876, Louis-Antoine Ranvier, Professor of Histology in Paris, described and illustrated the constrictions or tranglements annulaires, that are now known as the nodes of Ranvier (20,24). The functions of the node at that time were not known, but Ranvier did suggest that the constrictions may prevent displacement or flow of the semiliquid myelin along the nerve fibers (20,24). He also suggested that the gap in the myelin sheath at the node of Ranvier might allow diffusion of nutrients into the axon (15).
P.256
In teased fibers stained with osmium tetroxide or viewed in polarized light, the nodal gap is readily visible, as is the bulbous swelling of the fiber on either side of the node of Ranvier (see Figure 10.15D). The distance between each node along a myelinated fiber (Figure 10.7) is approximately proportional to the thickness of the myelin sheath. In a normal adult mammalian nerve, internodal segments between the nodes of Ranvier vary from 200 to 1500 m in length; the Schwann cell nucleus is usually sited around the middle of the internode.
Histological study of 1- m transverse resin sections of nerve and electron microscopic observations reveal a complex structure at the node of Ranvier and in the paranodal regions. As the axon approaches a node of Ranvier, it may become cruciform in cross section, especially in large fibers (Figure 10.7). Deep furrows develop in the surrounding myelin sheath, and those furrows are filled with cytoplasm that is rich in mitochondria. As the axon passes through the node it is reduced to one-third or one-sixth of its internodal diameter. There may be a slight swelling at the midpoint of the node. Amorphous, osmiophilic material rich in ankyrin, Nr CRM, and neurofascin (97) may be deposited under the axolemma (98). Ankyrin-binding proteins are also localized in the initial segment of the axon, the voltage-dependent sodium channel, the sodium/potassium ATPase, and the sodium/calcium exchanger (97). These specialized areas of axon membrane may reflect the site of high ionic current density during transmission of a nerve impulse. Numerous ion channels are present in this region of the axolemma, and they are responsible for the changes in ionic milieu that occur during the conduction of nerve impulses (98).
There is considerable specialization of the Schwann cell and the myelin sheath at the node of Ranvier. The myelin sheath terminates by forming dilated looplike structures that are closely apposed to the axon surface (Figure 10.7). Occasionally, desmosome-like structures are formed between Schwann cell terminal loops. The tight junction protein claudin-2, and the ERM (ezrin, radizin, moesin) proteins have been identified as a ring that surrounds sodium channels at the node of Ranvier, possibly participating in the junctions formed at the outer collars of two adjacent Schwann cells at the node zone (31). The abundance of mitochondria in the paranodal cytoplasm is an indication of the high energy requirements of the node. Right in the center of the node, the myelin end-loops are replaced by multiple fingerlike Schwann cell processes (nodal villi) that contain f-actin and are 70 to 100 nm in diameter. The villi extend from the Schwann cells into the nodal gaps and interdigitate with processes of adjacent Schwann cells (98). This interlacing pattern of cell processes around the axon at the node of Ranvier is more prominent and complex in larger fibers.
Basement membrane from the two adjacent Schwann cells is continuous over the nodal gap. Around the villous Schwann cell processes, there is an electron-dense polyanionic-rich material that constitutes the extracellular matrix of the node. This gap substance creates a ringlike structure (ring of Nemiloff) and may provide an ion pool necessary for nodal function. It has been demonstrated that the gap substance contains glycosaminoglycans with cation binding substances (95).
The myelin sheath acts as a biological insulator for the internodal portion of the axons. Conduction of impulses along myelinated fibers proceeds in a discontinuous manner from node to node (saltatory conduction). Numerous sodium channels with a suggested density of approximately 100,000/ m2 are present on the axolemma at the node of Ranvier in contrast to the very low density of sodium channels (less than 25/ m2) in the internodal axon membrane; the internodal membrane may be regarded as inexcitable (3,98). Potassium channels show a complementary distribution to that of the sodium channels; they are less common than in the nodal membrane but are present in the paranodal and internodal axon membrane. Potassium channels contribute to the stabilization of the axon by preventing repetitive firing responses to a single stimulus and also help to maintain the resting potential of the myelinated fiber (98).
In demyelinating diseases, when the myelin sheath is stripped from the axon, there is gross slowing or cessation of nerve conduction along the affected fibers. Spread of a continuous wave of depolarization along the axon membrane is prevented due to the absence of an adequate density of sodium channels in the internodal axon membrane. Furthermore, the exposure of the internodal axon cell membrane, which is rich in potassium channels, will also interfere with induction of the impulse (15,98).
Unmyelinated Axons
Unmyelinated fibers can be detected as unstained structures by light microscopy in toluidine blue stained 0.5- m transverse resin sections of peripheral nerve (Figure 10.5) (99). However, at 1 to 3 m diameter, they are almost at the limit of resolution and are only seen in good quality sections. Such fibers can be stained by silver techniques, such as Palmgren's or Bodian's, but are poorly visualized in immunocytochemical preparations using antineurofilament antibodies (Figure 10.4), probably because unmyelinated fibers contain few neurofilaments and a high proportion of microtubules.
The structure of unmyelinated fibers and their quantitation are most adequately studied by transmission electron microscopy (Figure 10.10). They are more numerous than myelinated fibers in mixed peripheral nerves by a factor of 3 or 4:1 (25,54) and were first recognized in 1838 by the Polish physician Robert Remak as fibriae organicae ; the Schwann cells associated with unmyelinated axons are sometimes referred to as Remak cells (54). Schwann cells
P.257
have the potential to differentiate into either a myelinating or nonmyelinating ensheathing cell, depending upon the signals received from the axons that they contact. Schwann cells must form basal laminae in order to myelinate axons (24,54). Schwann cells around myelinated and unmyelinated axons may thus be regarded as originating from the same cell type but developing morphological, biochemical, and physiological differences (106).
 |
Figure 10.10 Unmyelinated axons (1.3 m in diameter) cut in transverse section. The axons (AX) are surrounded by Schwann cells, Mesaxons (MES). Stacks of Schwann cell processes (ST) are commonly seen in adult nerves. (Electron micrograph, 13,000.) |
The cytoplasm of Schwann cells associated with unmyelinated fibers contains a Golgi apparatus, rough endoplasmic reticulum, mitochondria, microtubules, and microfilaments and may exhibit centrioles near the nucleus. Pi ( ) granules, however, are not present, although there are lysosomes containing acid phosphatase present in the cytoplasm (73). The nuclei of these cells are ellipsoid with one or more prominent nucleoli. A continuous basement membrane surrounds each cell (98). Schwann cells associated with unmyelinated fibers express different phenotypic characteristics from Schwann cells around myelinated axons. Although both types of Schwann cell contain immunocytochemically detectable vimentin intermediate filaments and S-100 protein, and almost the same basement membrane components, Schwann cells associated with unmyelinated axons are more likely to express GFAP (101). Such cells also lack MAG, which is apparently necessary for segregation and myelination of axons. Mycobacterium leprae (Hansen's bacilli) colonize nonmyelinating Remak cells by attaching to laminin-2 and its receptor -dystroglycan. Myelin-forming Schwann cells seem to be relatively free from infection by M. leprae. There is often a strong cell-mediated immune response with extensive inflammation and peripheral nerve damage that causes paralysis and loss of sensation and frequently leads to unintentional mutilation of hands and feet (102).
Electron microscopy of transverse sections of normal peripheral nerve show how numerous unmyelinated axons 0.2 to 3.5 m in diameter are associated with a single Schwann cell. Short mesaxons extend from the surface of the cell (Figure 10.10), and the Schwann cell is separated from the axon plasma membrane by a space 10 to 15 nm wide that is analogous to the periaxonal space of Klebs seen around myelinated fibers. Although many axons may be gathered close to the cell body in the perinuclear region of the Schwann cell (24,54), away from the nuclear region, single axons become more widely separated and are enclosed by thin Schwann cell processes (Figures 10.3, 10.10). Each Schwann cell associated with unmyelinated axons in the sural nerve is between 200 and 500 m in length. As axons pass from one Schwann cell to another, they are surrounded by flattened irregular, fingerlike processes that interlock and become telescoped into the adjacent Schwann cell. The surface of the axon is therefore always in contact with the Schwann cell. In young children, only a single thin layer of Schwann cytoplasm surrounds each axon away from the nuclear region; but the picture is more complex in adult nerves, with several Schwann cell processes stacked together and associated with each unmyelinated axon.
Pockets of collagen bundles are frequently invaginated into the surface of Schwann cells associated with unmyelinated fibers (Figure 10.3), particularly in aging nerves and when there is loss of unmyelinated fibers. The pockets of collagen fibers are separated from the surface of the Schwann cell by a layer of basement membrane. The significance of this phenomenon is not fully known.
Endocrine cells have been identified within the perineurium in close contact with unmyelinated nerves in the lamina propria of the appendix (103). These cells were demonstrated in 1924 by Masson, and later Aub ck coined the term endocrine cell-nonmyelinated fiber complex, emphasizing the association between endocrine cells and unmyelinated fibers (104). These complexes are separated from the interstitial connective tissue by a common continuous basement membrane, leaving the cells in intimate contact with each other. It has been suggested that such endocrine cells could participate in the pathogenesis of the so-called neuromas of the appendix and appendiceal carcinoids (105,106). It is not known whether such endocrine cells exist in nerves other than those located in the wall of the appendix, but there are reports of extraepithelial carcinoid tumors in stomach, small intestine, and bronchus, which suggests that there may also be endocrine cells related to nerves in these regions (107).
Interesting immunological properties have been ascribed to Schwann cells. Numerous in vitro studies have shown that Schwann cells display a large repertoire of properties, ranging from the participation in antigen presentation, to secretion
P.258
of pro- and anti-inflammatory cytokines, chemokines, and neurotrophic factors (108). Schwann cells express Ia determinants on their membranes and are able to present foreign antigens to specific synergic T cells. When Schwann cells are exposed to inflammatory cytokines they have the capacity of inducing selective damage to T cells and have the potential of regulating the immune response in the peripheral nervous system (109). A role for Schwann cells has been suggested in myasthenia gravis (110).
Schwann cells also express complement receptor CR1 (CD35) and CD59, a 19 to 25 KDa glycoprotein that binds to complement proteins C8 and C9 in the assembling cytolytic membrane attack complex. This may indicate that regulation of complement activation by these proteins is important in neural host defense mechanisms and may be implicated in the complement-mediated damage occurring in inflammatory demyelinating diseases such as Guillain-Barr syndrome (111,112).
Correlation of Normal Histology with the Pathology of Peripheral Nerves
Handling and Preparation of Peripheral Nerve Biopsy and Autopsy Specimens
The sural nerve is the nerve that is most commonly biopsied in the investigation of peripheral neuropathies. It is a sensory nerve so that in some motor neuropathies it may be totally normal, in which case examination of small branches of motor nerves within a muscle biopsy may be more fruitful (48,54,54). At autopsy, a wider range of motor and sensory nerves may be sampled, depending on the clinical picture. Whether taken at biopsy or autopsy, peripheral nerves are very easily damaged. The myelin sheaths are semiliquid and may be crushed by indelicate handling (Figure 10.11). The specimen should be gripped at only one end and then gently dissected free before laying it, very gently stretched, on a piece of dry card and placing it in fixative or in liquid nitrogen for snap freezing. Fresh, frozen nerve should be used for enzyme and lipid histochemical studies whereas formalin-fixed nerve can be embedded in paraffin for the application of routine stains and immunocytochemistry (see Table 10.1). Although formalin-fixed material can be used for the preparation of 0.5- to 1- m resin-embedded sections and for electron microscopy, ideally the tissue should be fixed in glutaraldehyde and postfixed in osmium for ultrastructural studies. Teased fibers can be prepared from either glutaraldehyde- or formalin-fixed material (24,54).
The method of preparation really depends on the information sought. Frozen sections are ideal for detecting abnormal lipids, such as sulfatide in metachromatic leukodystrophy, and for detecting the cholesterol ester droplets of degenerating myelin by staining for Sudan red or oil red O. Increased lysosomal enzyme activity as in Krabbe's leukodystrophy or in human and experimental neuropathies in which axonal degeneration or segmental demyelination is suspected can be detected in frozen sections stained histochemically for acid phosphatase (54). Brief formalin or glutaraldehyde fixation can be used in some cases for electron microscopic enzyme histochemistry (54,113). Frozen sections can also be used for immunofluorescence for the detection of immunoglobulin binding to myelin sheaths in paraproteinemias. Transverse frozen sections of nerve are ideal for these purposes although they are often more difficult to prepare than longitudinal sections.
 |
Figure 10.11 Histologic artifact in a peripheral nerve. In this transverse 1- m resin section, the fascicle to the left of the picture is well-preserved. However, there is extensive recent hemorrhage (center) that occurred during the biopsy procedure; the myelinated axons in the nerve fascicle are squeezed and distorted (right) (toluidine blue, 40.) |
There is a variety of methods of preparing and examining fixed specimens of peripheral nerve, and each method reveals different information (24,54). Ideally, exact transverse sections should be cut from the peripheral nerve; occasionally, longitudinal sections are also useful, particularly for detacting regenerating axons by immunocytochemistry (Figure 10.4F). Paraffin-embedded sections can be stained for a variety of histological stains and for immunocytochemistry to reveal nerve components (Table 10.1). Blood vessels and inflammatory exudates are ideally studied in paraffin sections, but quantitation of nerve fibers, the detection of axon degeneration and regeneration, and the assessment of segmental demyelination and remyelination are more satisfactory in 0.5- to 1- m toluidine blue stained resin sections or by electron microscopy. The presence of amyloid in the endoneurium or giant axons in some hereditary neuropathies and in some toxic neuropathies can be detected both in paraffin- and in resin-embedded sections. Teased preparations are most useful for detecting segmental demyelination and remyelination and
P.259
for assessing whether axonal degeneration and regeneration have occurred within the nerve in the past (24,56).
Peripheral Neuropathies
The pathological diagnosis of a peripheral neuropathy usually requires close clinicopathologic correlation and knowledge of the electrophysiologic data, such as nerve conduction velocities and electromyography. Moderate slowing of nerve conduction velocities usually indicates loss of large myelinated fibers, whereas excessive slowing of conduction velocity suggests that segmental demyelination has occurred. Although there are a number of specific histopathological features that aid in the diagnosis of peripheral neuropathy [e.g., amyloid, the presence of M. leprae bacilli, abnormal lipids such as sulfatide within the nerve, giant axons, and vasculitis (15,56)], for the most part, assessment of peripheral nerve pathology depends on detection and quantitation of general pathological features and good clinicopathological correlation.
General Pathology of Peripheral Nerves
The general pathological reactions of peripheral nerves are, for most practical purposes, limited to (a) axonal degeneration and regeneration and (b) segmental demyelination and remyelination. Hypertrophic changes with onion-bulb formation occur most commonly as a result of recurrent segmental demyelination and are most often seen in hereditary neuropathies.
Axonal Degeneration and Regeneration
If a neuron in the anterior horn of the gray matter of the spinal cord or in a dorsal root ganglion dies, its axon degenerates and no regeneration occurs. Such neuronal destruction is seen in poliomyelitis, motor neuron disease (amyotrophic lateral sclerosis), spinal muscular atrophy, and infarction of the spinal cord. Dorsal root ganglion cells may be lost in viral infections such as varicella zoster or in a variety of hereditary sensory neuropathies. If an axon in a peripheral nerve is injured, for example, by trauma, entrapment, or ischemia, the distal end of the axon degenerates and subsequently regeneration occurs from the proximal stump of the damaged axon (Figure 10.12). The success of the regeneration depends on the distance of the site of damage from the nerve end organ (either motor endplate or sensory nerve ending) and the amount of scarring or other obstruction laid in the path of the regenerating axons.
Axonal degeneration was described by Waller in 1850 in London and the eponym wallerian degeneration is still used. Much of the fundamental work on nerve degeneration and regeneration, however, was performed by Ram n y Cajal in the early part of the twentieth century. Twenty-four hours after nerve injury, most myelinated and nonmyelinated axons start to show degenerative changes. There is retraction of myelin from the nodes of Ranvier and dilatation of Schmidt-Lanterman incisures in the proximal as well as in the distal stump. By 48 hours, myelin and axon changes become more obvious as the axon disintegrates and myelin sheaths become disrupted and form globules (Figure 10.13) in which axon fragments are enclosed. Disintegration of the myelin appears to start with dilatation of the Schmidt-Lanterman incisures during the first day or two after injury (24,54). Myelin debris is initially birefringent and has a lamellated ultrastructure, as does normal myelin. But, as proteins break down and lysosomal enzymes become active around the myelin debris (54,113,114), cholesterol within the myelin is esterified to cholesterol esters, and lipid debris loses its birefringence in polarized light and its lamellated ultrastructure to become amorphous globular lipid, which now stains strongly with Sudan dyes and oil red O. The Marchi technique also differentiates between normal myelin and degenerating myelin (56).
 |
Figure 10.12 Diagram summarizing the events occurring during axonal degeneration and regeneration. A. Normal nerve. B. By seven days after axonal damage, Schwann cells containing axon and myelin debris have divided to form bands of B ngner. C. Axon sprouts grow from the swollen end bulb of the proximal axon. D. An axon becomes myelinated. E. Connection with the end organ is reestablished; regenerated internodes are short. (Reprinted from: Weller RO, Cervos-Navarro J. Pathology of Peripheral Nerves: A Practical Approach. London: Butterworths; 1977 with permission.) |
During the second week after nerve injury, much of the myelin debris is removed from the distal part of the nerve, and regenerative features become more prominent. Axon fragments and myelin debris are broken down by both
P.260
Schwann cells and macrophages (116). Schwann cells directly attract macrophages by secretion of different proteins, probably regulated by autocrine circuits involving the neuropoietic cytokines, IL-6, and leukemia inhibitory factor (113). Macrophages, in addition to their phagocytic function may help to promote nerve repair through the elaboration of Schwann cell mitogens and may also affect neurons and axonal growth directly through the release of neurotrophins (115,117,118,119).
 |
Figure 10.13 Axonal degeneration and regeneration in transverse sections of peripheral nerve. A. Axonal degeneration four days after nerve section. Few axons are visible and myelin is forming globules in Schwann cells and macrophages (1- m section, toluidine blue, 310). B. Axonal regeneration in a human nerve biopsy. Normal myelinated fibers are interspersed with clusters of closely associated thinly myelinated regenerating fibers (top and bottom right) (1- m section, toluidine blue, 310). |
Although Schwann cell mitoses are seen as early as 24 hours after nerve injury, the peak of proliferation is between 3 and 15 days after nerve damage (120). As Schwann cells proliferate, they form columns (bands of B ngner) surrounded by basement membrane (Figure 10.12); often redundant, old Schwann cell basement membrane is associated with these bands. Regenerating axons grow along the bands of B ngner; and, if regeneration fails, the bands shrink and Schwann cells may disappear and become replaced by fibrous tissue (121).
Importins are proteins involved in the retrograde transport of substances collectively known as injury signals; such transport is through a complex of proteins acting in association with neurotubules and into the cell nucleus, where gene transcription subsequently occurs (122).
Several easily detectable histological changes occur during axonal regeneration The neuron cell bodies in the anterior horns of the spinal cord or in the dorsal root ganglia show changes of chromatolysis during the first three weeks after axonal injury (120). The nerve cell perikaryon swells by some 20%, and the nucleus becomes eccentric, as does the nucleolus. Nissl substance (a mixture of rough endoplasmic reticulum and polyribosomes) is dispersed so that the cytoplasm becomes pale when stained by H&E or by the Nissl stain (24,54). During this stage of chromatolysis, there is a marked increase in polyribosomal ribonucleic acid (RNA) with an upregulation of a number of regeneration-associated genes, including those encoding growth-associated protein-43 (GAP 43), cytoskeleton protein-23, and -tubulin, and in peptides such as galanin and vasoactive intestinal polipeptide (VIP), reflecting the metabolic events involved in axon regeneration (122,123,124). Regenerative changes in axons are seen within the first few hours after nerve damage but are most easily detected 5 to 20 days after injury. Using immunocytochemistry, GAP 43 can be identified in regenerating axons 4 to 21 days after injury (125) (Figure 10.4F). The proximal stump of the axon swells to create a balloonlike structure, often 50 m in diameter and 100 m in length. The balloons are filled with organelles and fibrils, which can be detected by electron microscopy; they can be visualized by light microscopy using immunocytochemical stains for neurofilament protein, GAP 43, or silver stains such as were used by Ram n y Cajal when he first described them. Myelin sheaths become stretched around the swollen axon balloons (114,126).
Starting around the fourth day after injury, multiple nerve sprouts, or neurites, extend from the axon balloon (growth cone) and grow distally at 1 to 2.5 mm/day. As the neurites enter the bands of B ngner, they become invaginated into the surface of the Schwann cell and, if growth continues, they become myelinated. Unmyelinated fibers regenerate in a similar way, but they are smaller and no myelin sheaths form around them. Regenerating neurites can be detected in the classical way by silver staining; but, in cross sections of peripheral nerve, they are best demonstrated in 0.5- to 1- m resin sections or by electron microscopy. Characteristically, regenerating axons form clusters encircled by a single basement membrane. In the light microscope, these clusters (Figure 10.13) are recognized by the close association of small, thinly myelinated axons within the nerve; myelinated nerve fibers in a normal
P.261
nerve are well-separated from each other by endoneurial collagen (Figure 10.5).
Axon growth and regeneration are stimulated by nerve growth factor that is synthesized by Schwann cells, fibroblasts, and macrophages and transported back along the axon by retrograde axoplasmic transport to stimulate nerve cell protein synthesis (60,126,127,128). In addition to growth factors, there appears to be topographical affinity between regenerating axons and certain pathways; for example, it appears that regenerating tibial nerve axons grow toward the distal tibial nerve rather than toward the distal peroneal nerves. Connective tissue elements may also play a role in guiding regenerating axons (10). Neurite outgrowth promoting factors on cell surfaces (cell adhesion molecules) or in the extracellular matrix promote extension of the axon by providing an appropriate adhesivness in the substrate (129). Both neurotrophic and neurite outgrowth promoting factors are essential for axonal growth after injury (129).
The success of regeneration, with axons reaching effective end organs, may be influenced by several factors. If the injury is far proximal from the end organ, few regenerating axons may make effective reconnections. But, regeneration over short distances may be very effective in the peripheral nervous system. The presence of scar tissue or discontinuity of anatomical pathways may also inhibit regeneration. A number of grafting techniques are employed to overcome this problem (13,120). If regeneration to the distal stump of a nerve is blocked by scar tissue, axons may grow outside the original course of the nerve and even back alongside the proximal stump (terminals of Perroncito); thus, small bundles of regenerating neurites, often surrounded by perineurial cells, form amputation neuromas. Microscopically, there are interlacing bundles containing axons surrounded by myelin sheaths and with fine perineurial coverings. Immunocytochemistry for neurofilament proteins (axons), EMA, and Glut-1 (perineurial cells), and S-100 (Schwann cells) may be very useful in establishing the structure and identity of the nerve bundles in an amputation neuroma. Immunohistochemical and radioimmunoassay data have shown a focal accumulation of sodium channels within the tips of injured axons that may be responsible, in part, for the ectopic axonal excitability and the resulting abnormal sensory phenomena (pain and paresthesia) which frequently complicate peripheral nerve injury (130). Macropaghes migrate into the neuroma within the first two weeks after the injury, and later they are seen with numerous large cytoplasmic vacuoles filled with myelin fragments. This suggests that macrophages may also participate in the genesis of chronic pain after the neuroma has formed possibly by: (a) creating demyelinating axonal regions susceptible to external stimuli; (b) by releasing substances that influence regeneration of axons; or (c) by direct action on the denuded remodelling membranes (131).
Axon degeneration, often with regeneration, is a feature of numerous peripheral neuropathies, including those associated with diabetes, amyloidosis, infections (such as leprosy), sarcoidosis, paraneoplastic syndromes, vascular disease, and metabolic diseases (24,54,132,133). Most toxic neuropathies (34,68) result in chronic axonal degeneration at the extreme distal ends of sensory and motor nerves (distal axonopathies). The distal ends of long tracts in the spinal cord (dorsal columns and corticospinal tracts) are often affected as well as the peripheral nerves. Timely withdrawal of the toxin may allow effective regeneration to occur, but only in the peripheral nerves, not in the spinal cord. Many peripheral neuropathies induced by the diseases itemized above are slowly progressive, so that nerve biopsies in these conditions do not usually reveal the early stages of axonal degeneration and regeneration. More frequently, the histological picture is characterized by loss of large myelinated axons and, to a lesser extent, loss of small myelinated and unmyelinated axons. Nerve root or peripheral nerve compression and trauma to peripheral nerve trunks result in axonal degeneration. Regeneration may be recognized in transverse sections of peripheral nerve by the presence of clusters (Figure 10.13). In teased fiber preparations, short internodes in the distal part of the nerve indicate that axonal degeneration and regeneration have occurred in the past (121).
Segmental Demyelination and Remyelination
When demyelination occurs in peripheral nerves, it has a segmental distribution with each segment representing the internodal portion of an axon myelinated by one Schwann cell (Figures 10.7, 10.14). Such segments can be contiguous, and thus demyelination may occur over long lengths of the nerve or in short sporadic segments (55,118). The axon remains intact except in severe demyelinating neuropathies in which secondary axonal degeneration occurs. Remyelination is often rapid and effective, with restoration of nerve function. Demyelination may occur as a result of direct interference with Schwann cell metabolism, as in diphtheria; myelin sheaths are broken down through the lysosomal action of Schwann cells, although macrophages are later involved in the destruction of myelin debris (116,120). Another mechanism that is seen in the commonest acute demyelinating neuropathy, Guillain-Barr syndrome, in which there is an immunological attack on peripheral nerve myelin by lymphocytes and macrophages; segmental demyelination occurs and is followed by remyelination (Figures 10.14, 10.15). Functional recovery occurs following both types of demyelination except in the most severe cases (134).
The first stages of segmental demyelination are seen at the node of Ranvier, where the nodal gap becomes widened; subsequently, the whole internode of myelin may be broken down (135). This destruction results in severe slowing of conduction of nerve impulses across the demyelinated segment and the onset of symptoms for the patient. Preserved axons remain invaginated within Schwann cells as the
P.262
myelin sheaths are broken down (Figures 10.15 and, 10.16). Schwann cells proliferate and within a few days start to remyelinate the demyelinated axons by a similar mechanism to that seen during myelination in the fetus. Remyelination may be well advanced by two weeks after demyelination as the thickness of myelin sheaths increases and conduction velocities return to normal.
 |
Figure 10.14 Summary of the events occurring in primary segmental demyelination and remyelination. A. Normal nerve. B. Early segmental demyelination; retraction of paranodal myelin with widening of the nodal gap. C. Destruction of myelin sheath and Schwann cell mitosis. D. and E. Remyelination; intercalated short segments. (Reprinted from: Weller RO, Cervos-Navarro J. Pathology of Peripheral Nerves: A Practical Approach. London: Butterworths; 1977 with permission.) |
Classically, segmental demyelination can be detected in teased nerve fibers, first by the presence of widening of the gap of the node of Ranvier and then by the presence of axons devoid of myelin sheaths. Intercalated thin myelin sheaths along the axon are seen as remyelination proceeds (Figure 10.15). In electron micrographs or in resin-embedded light microscope sections, naked axons can be recognized in transverse section and remyelinating fibers detected by the presence of inappropriately thin myelin sheaths (Figures 10.12,10.13,10.14). Segmental demyelination is a feature of a number of peripheral neuropathies (132,133), particularly mild vascular damage to peripheral nerves as in rheumatoid arthritis (136), diabetes (24), Guillain-Barr syndrome, occasional toxic neuropathies (131), and metabolic neuropathies such as metachromatic leukodystrophy (15,56) (Figure 10.15). Throughout the range of neuropathies, however, segmental demyelination is less common than axonal degeneration.
Hypertrophic Neuropathy
Recurrent segmental demyelination is a feature of a number of chronic hereditary neuropathies, particularly Charcot-Marie-Tooth (CMT) disease, D j rine-Sottas disease (hereditary motor and sensory neuropathy types I and III), and Refsum's disease (138,139). In such diseases, repeated segmental demyelination appears to be responsible for a florid proliferation of Schwann cells and the formation of onion-bulb whorls (139,140) (Figures 10.14, 10.15), giving a distinctive histological picture of hypertrophic neuropathy to these peripheral neuropathies. So far, at least 16 genetic loci and 9 caustive genes have been identified for primary heritable demyelinating neuropathies (HDN); therefore, although histopathological analysis is still important for the diagnosis of HDN, it is gradually being replaced by molecular diagnosis (139,141).
Traumatic Lesions of Peripheral Nerve
An understanding of the structure and staining reactions of normal peripheral nerve is essential for unraveling the complexities of traumatic lesions of nerve. Identification of cell types, recognition of patterns of organization, and the detection of normal elements within a traumatic lesion allow a more confident diagnosis and description to be formulated.
Amputation neuromas may develop as painful swellings at the distal ends of amputated limbs or at sites of nerve damage without amputation. They consist of disoriented bundles of axons surrounded by Schwann cells and divided into compartments by perineurial cells. In H&E stained paraffin sections, the tubular formation of the perineurial compartments can be recognized (56). By immunocytochemistry, axons can be stained with antibodies to neurofilament protein and GAP 43; the Schwann cells associated with them contain S-100 protein, and the perineurial cells are EMA-, Glut-1 , and claudin-1 positive but do not contain S-100 protein (142). Silver stains can also be used to identify the twisted and disoriented axons. The histological picture reflects the processes seen in the normal regeneration of peripheral nerve; but in amputation neuromas, appropriate regeneration along the distal part of the nerve is prevented.
In 1876, Thomas G. Morton, from Pennsylvania, described a neuroma that involves the plantar interdigital nerves, almost always between the third and the forth toes (Morton's metatarsalgia), and consists of small painful swellings on the nerves. Histologically, there is fibrosis and edema of the endoneurium and perineurium and the accumulation of mucosubstances similar to endoneurial glycosaminoglycans. The detection of axons and Schwann cells by immunohistochemistry is often a useful adjunct to the diagnosis of this lesion (142).
A pseudocyst or nerve sheath ganglion, containing mucinous material that stains with Alcian blue, may form on a
P.263
peripheral nerve, generally at a site of repeated trauma. Although the fibrous capsule of the cyst and its mucinous contents may dominate the picture, damaged nerve components can usually be detected adjacent to the cyst (142).
 |
Figure 10.15 Segmental demyelination and remyelination. A. Transverse section of peripheral nerve showing early remyelination in an experimental animal. There are normal, large myelinated fibers with axons 8 to 10 m in diameter and axons 3 to 5 m in diameter, which have thin myelin sheaths and are remyelinating. Myelin debris is seen in Schwann cells and macrophages (1- m resin section, toluidine blue, 310). B. Nerve biopsy from a child with metachromatic leukodystrophy (sulfatide lipidosis). Large axons with either thin myelin sheaths (remyelination) or with no myelin sheath at all (demyelinated) (right of center) are seen in the biopsy. Unmyelinated fibers (center) are unaffected (1- m resin section, toluidine blue, 310). (Reprinted from Ahmed AM, Weller RO. The blood-nerve barrier and reconstitution of the perineurium following nerve grafting. Neuropathol Appl Neurobiol 1979;5:469 483 with permission.) C. Hypertrophic neuropathy (Charcot-Marie-Tooth disease-HSMN type I). Demyelinated and remyelinated axons are seen at the centers of onion-bulb whorls formed by Schwann cell processes; there is abundant endoneurial collagen (pink) (1-um resin section, toluidine blue and carbol fuchsin, 240). D. Teased fibers. Normal fiber (lower) with a normal node of Ranvier (N). The fiber above has a thin, remyelinating segment (R) on one side of the node and a normal segment on the other side (osmium stain, 200). (Reprinted from:Weller RO. Colour Atlas of Neuropathology. Oxford: Oxford University Press and Harvey Miller; 1984 with permission.) |
Compressive lesions of peripheral nerves have resulted in some debate regarding their origin. Because of the resemblance of whorls within these lesions to those seen in hypertrophic neuropathies, they have been labeled localized hypertrophic neuropathy (36). Such lesions usually occur at sites of compression over the fibula or on the posterior interosseous nerve (137), although some lesions present with no obvious nerve compression. Although there is well-marked onion-bulb formation, ultrastructural studies have shown that the onion bulbs are formed not by Schwann cells, as in hypertrophic neuropathies, but by perineurial cells (141,143). Such compressive lesions should not be confused with perineuromas in which immunocytochemistry has confirmed that the cell whorls are formed by EMA-, Glut-1 , and claudin-1 positive perineurial cells (40,143), and there are abnormalities of chromosome 22. (143,144).
In 1972, Reed described a small (1 15 mm), typically solitary neuroma that presents in adults and affects the face,
P.264
with no clinical manifestations of neurofibromatosis or MEN-IIb (multiple endocrine neoplasia). This lesion is known as solitary circumscribed neuroma (SCN) (palisaded encapsulated neuroma) and is currently viewed as a form of true neuroma. Histologically, SCN appears as one or more circumscribed nodules in the dermis, sometimes partially encapsulated by a delicate compact EMA-positive perineurial layer. The process consists of a solid proliferation of tightly interwoven fascicles of Schwann cells, sometimes separated by narrow gaps that create a characteristic appearance of the lesion at low magnification. The Schwann cells are often arranged as palisades, and there may be numerous axons scattered troughout the lesion, which are best demonstrated with Bodian's silver stain or by immunohistochemistry for neurofilament proteins (145).
 |
Figure 10.16 Demyelination. A large diameter axon (top) is demyelinated and devoid of a myelin sheath. A small onion-bulb whorl has formed around this axon with the encirclement by Schwann cell processes (S), one of which contains an unmyelinated fiber (UM). Thickly myelinated fibers are seen at the bottom of the picture. (Electron micrograph, 6,600.) |
Tumors of the Peripheral Nervous System
A variety of cells and structures may be identified in tumors of the peripheral nervous system; they include perineurial cells, Schwann cells, axons, and neurons (142,146,147). The diagnosis frequently depends on the histologic analysis of the tumor and thus the detection of cellular components forming the tumor and their relationship with normal nerve structures (142).
Perineurioma is a rare true perineurial neoplasm (135). Perineuriomas can arise in a wide variety of sites and may exhibit different histological patterns with extra and intraneural forms (29,148). Histologically, intraneural perineuriomas affect individual fascicles with concentric proliferation of spindle cells around nerve fibers in the endoneurium; extraneural (soft tissue) perineuriomas show paucicellular to cellular forms (with some cases showing dense collagenization sclerosing perineurioma) with proliferation of spindle cells with extremely thin and elongated profile resembling normal perineurial cells (148). The key diagnostic finding is that the proliferation cells are labeled with EMA, Glut-1 and claudin1 but fail to stain for S-100 protein, neurofilaments, and CD34 (39,40,41,142). Perineuromas may be closely related to cutaneous meningioma since shared histologic features, and positive staining for vimentin and EMA in both conditions, suggest close similarities between these lesions (41).
Schwannomas are tumors of peripheral nerves composed almost entirely of Schwann cells (28,142,146). Neoplastic Schwann cells may produce and respond to trophic factors, particularly to the growth factor like polypeptides known as neuregulins, in an autocrine and/or paracrine fashion to promote proliferation. In fact the presence of neuregulins in certain schwannomas has been demonstrated by immunohistochemistry (149).
Histologically, schwannomas show the classical patterns of spindle cells in a compact (Antoni type A) or loose (Antoni type B) arrangement. Neoplastic Schwann cells may exhibit different morphologies (Figure 10.17) (142,146,150,151,152). Occasionally, the Schwann cells have an epithelioid appearance (epithelioid schwannomas) (152), are surrounded by an abundant myxoid stroma (nerve sheath myxoma) (153), have a high nuclear-cytoplasmic ratio (cellular schwannoma), exhibit a plexiform pattern (plexiform schwannoma), contain copious melanin (melanotic schwannoma) or exhibit xanthomatous change with many foamy cells containing oil red O positive neutral lipid (142,146,154). Schwannomas usually grow on the sides of
P.265
nerves and do not infiltrate nerve bundles (54). Thus, normal nerves may be seen within the fibrous capsule that is usually present around schwannomas.
 |
Figure 10.17 Panel showing the different morphology that the Schwann cells may present in schwannomas. A. Benign schwannoma, showing the typical biphasic appearance. B. Benign schwannoma with Verocay bodies. C. Reticulin (Gordon-Sweet) stain in a benign schwannoma, showing the positive basement membrane. D. Cellular schwannoma with uniform fascicular growth pattern. E. Granular cell schwannoma. Note the granular cells are originating from a nerve fascicle (N) (anti-S-100 protein). (Photograph supplied by Dr. Javier Baquera.) F. Melanotic schwannoma. G. Nerve sheath myxoma. H. Malignant peripheral nerve sheath tumor (malignant triton tumor) with rhabdomyoblastic differentiation (arrowheads). |
Histologically, the tumor cells have elongated nuclei and long eosinophilic processes, often forming palisades first described by the Uruguayan-born pathologist Jos Verocay and known today as Verocay bodies (155). The palisades are parallel arrays of tumor nuclei separated by the eosinophilic, periodic acid-Schiff (PAS) positive processes of Schwann cells. The basement membrane investing each Schwann cell in the tumor is well-demonstrated by electron microscopy but can also be demonstrated in reticulin stains by light microscopy (54,142). Collagen bundles within schwannomas may have a distinctive long spaced appearance (Luse bodies) (156). Immunocytochemistry shows the presence of S-100 protein, CD57 (Leu-7), CD56, and calretinin in schwannoma cells, but they are negative for EMA (37,38,157). Some schwannomas associated with the spinal cord and those deep in the body or close to major joints stain by immunocytochemistry for GFAP, while the superficial, subcutaneous schwannomas are negative (158). Schwann cells associated with unmyelinated fibers are more likely to express GFAP, and it has been proposed that the GFAP-positive schwannomas may arise from unmyelinated nerves (158).
Melanin may be seen in schwannomas; and, by electron microscopy, premelanosomes and melanosomes may be identified (142,145). Such structures emphasize the common origin of Schwann cells and melanocytes from the neural crest (159,160). There are two types of melanotic schwannomas.The conventional type is composed of plump spindle and epithelioid cells arranged in whorls and streaming fascicles containing melanin pigment. Psammomatous melanotic schwannomas are mainly in autonomic/visceral locations with the same cell arrangement as melanotic schwannomas but with the additional feature of PAS-positive, von Kossa-positive, mineralized, laminated calcospherites (161). Approximately 50% of patients with psammomatous melanotic schwannomas may have evidence of Carney's syndrome, which includes primary pigmented nodular adrenocortical disease; pituitary-independent, primary adrenal form of hypercortisolism; lentigines; ephelides and blue nevi of the skin and mucosae; and a variety of nonendocrine and endocrine tumors, such as myxomas, pituitary adenomas, testicular Sertoli cell tumor, and other benign and malignant neoplasms (including tumors of thyroid and ductal adenomas of the breast) (161).
Granular cell tumor (GCT, or Abrikosoff tumor) is not a specific entity. Granular cytoplasmic change is the expression of a metabolic alteration occurring often, but not exclusively, in Schwann cells (162). The tumor cells have abundant granular cytoplasm and a small eccentric nucleus; they invade small nerve branches in the skin. Immunocytochemical studies have shown that the tumor cells express S-100 protein, CD57, CD68, neurospecific enolase (NSE), Protein Gene Product PGP9.5, and also occasionally myelin (P0 and P2) and myelin-associated proteins (28,163). Expression of calretinin has been demonstrated in GCT. As this calcium-binding protein is typically expressed in neurons, ganglion cells, and Schwann cells, GTC expression may further support a neuronal origin or differentiation of these tumors (157). Interestingly, calretinin positivity may be increased in the pseudoepitheliomatous hyperplasia of the squamous epithelium overlying the tumor cells seen in some cases of GCT, which may indicate a role of calretinin in the interaction between GCT cells and hyperplastic epithelium (157). Granular cells have also been described in nerves undergoing wallerian degeneration (162).
Neurofibromas are complex benign lesions frequently associated with neurofibromatosis (147). As distinct from schwannomas, neurofibromas are diffuse lesions in the skin or around peripheral nerves that invade the peripheral nerve endoneurium and enlarge nerve branches (54,142,147). They also diffusely invade surrounding tissues and may cause bone destruction. Histologically, neurofibromas are as a rule not encapsulated, and they contain a variety of cells that are associated with peripheral nerves; unlike schwannomas, neurofibromas are not predominantly composed of Schwann cells. Mast cells are frequently seen in the stroma and can be identified using toluidine blue or Giemsa or by anti-triptase, CD117, and calretinin immunohistochemistry (51,52,152). Mast cells induce factor XIIIa-positive fibroblast-like cells to synthesize the large amount of extracellular matrix in neurofibromas, and mast cells are one of the inflammatory cell types involved in tumor promotion (52,164,165,166). It has been hypothesized that when mast cells are lost, as seen in cases of malignant peripheral nerve sheath tumors, there may be a diminution of the anti-cancer effect of mast cells in maintaining the benign nature of some of these proliferations (51,52). By immunocytochemistry, S-100- and CD57 (Leu-7)-positive Schwann cells can be detected within neurofibromas, in addition to EMA-, Glut-1 , and claudin-1 positive perineurial cells and CD34 endoneurial fibroblasts (37,38). Entrapped axons can be seen coursing through neurofibromas in immunocytochemical preparations for neurofilament protein or in silver-stained sections. All of these cellular components are set in a variable fibromyxoid stroma that in some cases may be so prominent (the myxoid part) that it may be mistaken for a myxoma or myxoid liposarcoma. Distorted structures resembling Wagner-Meissner or pacinian corpuscles are some times seen (pacinian neurofibromas), and melanin may be found in pigmented neurofibromas (167).
Neurofibromatosis is an autosomal dominant disease that is part of the group of neurocutaneous disorders collectively known as phacomatoses (137,142,168). It presents as two major diseases, peripheral (type 1, or von Recklinghausen's disease; NF1) and central (type 2; NF2) (163). Both type 1 and type 2 are inherited as autosomal dominant traits, but many cases are new mutations. Over 90% of cases
P.266
of neurofibromatosis are type 1 (peripheral); a gene defect has been located on the long arm of chromosome 17 (17q11.2; NF1 gene) near the centromere and has been linked to the locus encoding nerve growth factor (147,168). There is an increase in nerve growth factor in the serum of patients with this disorder (169). The NF1 suppresor gene, which spands over 350 kb of genomic DNA and contains 60 exons, encodes a ubiquitous protein known as neurofibromin. This 2818 amino acid protein has been shown to be associated with cytoplasmic microtubules and functions as a GTPase-activating protein for ras (170,171).
A wide variety of disorders occur in patients with NF1, including elephantiasis neuromatosa, in which there are redundant folds of skin associated with plexiform neurofibromas (24,162,168,170,171) and, more commonly, multiple cutaneous neurofibromata. Other tumors, such as gliomas, carcinoid tumors, pheochromocytomas, neuroblastomas, gastrointestinal stromal tumors, Wilms' tumors, and pigmented hamartomas of the iris (Lisch nodules), are also seen in patients with this disorder (142,167).
Central, or type 2, neurofibromatosis is much less common than NF1 and is characterized by bilateral vestibular schwannomas; skin lesions are uncommon. Approximately 40% of the vestibular schwannomas in NF2 tend to have a lobular grapelike pattern, while this arrangement is extremely uncommon in sporadic schwannomas (169,172). A proportion of these patients have multiple tumors, including meningiomas, intramedullary spinal ependymomas, and glial microhamartomas of the cerebral cortex (169,170). A gene deletion on chromosome 22q12 (NF2 gene) was identified in 1993 in patients with this disorder, and it is associated with abnormalities of glial growth factor and nerve growth factor activity (169). The NF2 gene spans 110 kb and encodes a membrane-cytoskeleton linking protein that is a member of the protein 4.1 family, known as merlin, (for moezin-ezrin-radixin-like protein). Merlin, also known as schwannomin, may be detected immunohistochemically in the cytoplasm of many cells, including Schwann cells, and it is believed to act as a tumor suppressor gene, the loss of function of which is a fundamental event in the genesis of schwannomas (172,173,174). Normal Schwann cells express merlin, which is known to play a crucial role in cytoskeleton-associated events. Since merlin is a tumor suppressor gene, and all schwannomas lack merlin, it is proposed that the mutation of NF2 may cause tumor formation through disruption of cell shape, cell matrix, and cell-cell communication or signaling functions, attributed to actin-cytoskeleton-plasma membrane interaction (173,174). Sporadic schwannomas from non-NF2 individuals also have NF2 mutations (175).
Neuronal tumors such as ganglioneuromas occur in association with autonomic ganglia in the peripheral nervous system. Histologically, neurons can be identified within these tumors; axons and Schwann cells may be identified by immunocytochemistry. Electron microscopy reveals the presence of 100-nm dense-core vesicles resembling catecholamine granules in the neurons. In addition to well-differentiated ganglioneuromas, primitive neuroectodermal tumors such as neuroblastoma and ganglioneuroblastomas arise within the abdomen and thorax (172). Cell types within the more primitive tumors may be difficult to identify by immunocytochemistry, but electron microscopy usually reveals the presence of 100-nm dense-core catecholamine vesicles within the tumor cell cytoplasm (142,176).
Malignant peripheral nerve sheath tumors (MPNST, or malignant schwannomas) (142) are derived from specialized cells of the endoneurium and perineurium and show great histological variation and many similarities to other soft tissue tumors (28,142). The term MPNST incorporates previously used terms such as neurofibrosarcoma and malignant schwannoma. Mutation in the NF1 tumor suppressor gene is the most important molecular genetic event in the development of MPNST (177). Malignant change in a benign scwhannoma is an extremely rare event (142); however, two forms of neurofibroma, plexiform and localized intraneural neurofibroma, are significant precursors of MPNST (178). Immunocytochemistry has demonstrated EMA-, S-100 , and CD57-positive cells in MPNST (154,177), and occasional tumors that are EMA-positive and S-100 negative have been described as MPNST with perineurial cell differentiation (179). This suggests that cells of MPNST may produce proteins characteristic of perineurial cells or of both perineurium and Schwann cells. Neurospecific enolase, neurofilament protein, and myelin-basic protein have also been occasionally identified in MPNST (17,180). Heterogeneity is common, and so histological sampling should include wide areas of tumor (142,181). Unusual elements (such as cartilage, bone, squamous elements, and muscle) may be encountered occasionally. Malignant peripheral nerve sheath tumors may also present with malignant glands (glandular malignant schwannoma) or rhabdomyosarcoma, features that may be highlighted by immunocytochemistry for cytokeratins and desmin, respectively. In an attempt to explain the occurrence of malignant muscle differentiation (rhabdomyosarcoma) in malignant schwannomas, Pierre Masson suggested that endoneurial cells might differentiate into muscle cells under the inductive influence of nerve cells, a situation that was thought to be operative in regenerating limbs in triton salamanders, hence the name triton tumor (malignant schwannoma with rhabdomyoblastic differentiation) (182).
Acknowledgments
We thank the staff of the Neuropathology and Cell Pathology laboratories and the Imaging Unit of Southampton University Hospitals NHS Trust, Southampton, England, and the Immunohistochemistry section of the ABC Medical
P.267
Center in Mexico City for their generous and expert cooperation. Special thanks are due to Margaret Harris, who typed the original manuscript, to Dr. Sergio Pi a-Oviedo, who drew the diagrams, and to Professor James Nicoll and Dr. Javier Baquera, who supplied Figures 10.4F and 10.17E respectively.
References
1. Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development 2004;131:4637 4650.
2. Gogly B. Embryology of neural crests and their classification [in French]. Rev Orthop Dento Faciale 1990;24:401 426.
3. Scherer SS. The biology and pathobiology of Schwann cells. Curr Opin Neurol 1997;10:386 397.
4. Jessen KR, Mirsky R. Signals that determine Schwann cell identity. J Anat 2002;200:367 376.
5. Young HM, Anderson RB, Anderson CR. Guidance cues involved in the development of the peripheral autonomic nervous system. Auton Neurosci 2004 31;112:1 14.
6. Levi-Montalcini R. The nerve growth factor and the neuroscience chess board. Prog Brain Res 2004;146:525 527.
7. Anand P. Neurotrophic factors and their receptors in human sensory neuropathies. Prog Brain Res 2004;146:477 492.
8. Petruska JC, Mendell LM. The many functions of nerve growth factor: multiple actions on nociceptors. Neurosci Lett 2004;361:168 171.
9. Markus A, Patel TD, Snider WD. Neurotrophic factors and axonal growth. Curr Opin Neurobiol 2002;12:523 531.
10. Chernousov MA, Carey DJ. Schwann cell extracellular matrix molecules and their receptors. Histol Histopathol 2000;15:593 601.
11. Letourneau PC, Condic ML, Snow DM. Extracellular matrix and neurite outgrowth. Curr Opin Genet Dev 1992;2:625 634.
12. Michailov GV, Sereda MW, Brinkmann BG, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science 2004;304:700 703.
13. Nadim W, Anderson PN, Turmaine M. The role of Schwann cells and basal lamina tubes in the regeneration of axons through long lengths of freeze-killed nerve grafts. Neuropathol Appl Neurobiol 1990;16:411 421.
14. Shield LK, King RHM, Thomas PK. A morphologic study of human fetal sural nerve. Acta Neuropathol (Berlin) 1986;70:60 70.
15. Thomas PK, Ochoa J. Microscopic anatomy of peripheral nerve fibers. In: Dyck PJ, Thomas PK, Lambert EH, Bunge R, eds. Peripheral Neuropathy. 2nd ed. Vol 1. Philadelphia: WB Saunders; 1984:39 96.
16. Moore JK, Linthicum FH Jr. Myelination of the human auditory nerve: different time courses for Schwann cell and glial myelin. Ann Otol Rhinol Laryngol 2001;110(pt 1):655 661.
17. Notterpek L. Neurotrophins in myelination: a new role for a puzzling receptor. Trends Neurosci 2003;26:232 234.
18. Martini R. The effect of myelinating Schwann cells on axons. Muscle Nerve 2001;24:456 466.
19. Yin X, Crawford TO, Griffin JW, et al. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci 1998;18:1953 1962.
20. Reina MA, L pez A, Villanueva MC, Andr s JA, Le n GI. Morfolog a de los nervios perif ricos, de sus cubiertas y de su vascularizaci n. Rev Esp Anestesiol Reanim 2000;47:464 475.
21. Sloniewski P, Korejwo G, Zielinski P, Morys J, Krzyzanowski M. Measurements of the Obersteiner-Redlich zone of the vagus nerve and their possible clinical applications. Folia Morphol (Warsz) 1999;58:37 41.
22. Lieberman AR. Sensory ganglia. In: Landon DN, ed. The Peripheral Nerve. London: Chapman and Hall; 1976:188 278.
23. Tassler PL, Dellon AL, Canoun C. Identification of elastic fibres in the peripheral nerve. J Hand Surg Br 1994;19:48 54.
24. Rushing EJ, Bouffard JP. Basic pathology of the peripheral nerve. Neuroimaging Clin N Am 2004;14:43 53, vii.
25. Cavanagh JB. Pathology of peripheral nerve diseases. In: Weller RO, ed. Systemic Pathology. 3rd ed. Vol. 4: Nervous System, Muscle and Eyes. Edinburgh: Churchill Livingstone; 1990:544 578.
26. Kameh DS, Perez-Berenguer JL, Pearl GS. Lipofibromatous hamartoma and related peripheral nerve lesions. South Med J 2000;93:800 802.
27. Vega JA, Del Valle ME, Haro JJ, Naves FJ, Calzada B, Uribelarrea R. The inner-core, outer-core and capsule cells of the human Pacinian corpuscles: an immunohistochemical study. Eur J Morphol 1994;32:11 18.
28. Enzinger FM, Weiss SW. Soft Tissue Tumors. 4th ed. St Louis: Mosby; 2001.
29. Tsang WYW. Perineuriomas: perineurial cell neoplasms with distinctive extra- and intraneural forms. Adv Anat Pathol 1996;212 222.
30. Reina MA, L pez A, Villanueva MC, De Andr s JA, Mach s F. La barrera hemato-nerviosa en los nervios. Rev Esp Anestesiol Reanim 2003;50:80 86.
31. Poliak S, Matlis S, Ullmer C, Scherer SS, Peles E. Distinct claudins and associated PZD proteins form different autotypic tight junctions in myelinating Schwann cells J Cell Biol 2002;159:361 372.
32. Folpe AL, Billings SD, McKenney JK, Walsh SV, Nusrat A, Weiss SW. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol 2002;26:1620 1626.
33. Erlandson RA. The enigmatic perineurial cell and its participation in tumors and in tumorlike entities. Ultrastruct Pathol 1991;15:335 351.
34. Cavanagh JB. Toxic and deficiency disorders. In: Weller RO, ed. Systemic Pathology. 3rd ed. Vol 4. Nervous System, Muscle and Eyes. Edinburgh: Churchill Livingstone; 1990:244 308.
35. Ahmed AM, Weller RO. The blood-nerve barrier and reconstitution of the perineurium following nerve grafting. Neuropathol Appl Neurobiol 1979;5:469 483.
36. Weller RO. Localized hypertrophic neuropathy and hypertrophic polyneuropathy. Lancet 1974;ii:529 593.
37. MacKeever PE. Immunohistochemistry of the nervous system. In: Daabs DJ, ed. Diagnostic Immunohistochemistry. Philadelphia: Chirchill Livingstone; 2002:559 624.
38. Hirose T, Tani T, Shimada T, Ishizawa K, Shimada S, Sano T. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol 2003;16:293 298.
39. Yamaguchi U, Hasegawa T, Hirose T, et al. Sclerosing perineurioma: a clinicopathological study of five cases and diagnostic utility of immunohistochemical staining for GLUT1. Virchows Arch 2003;443:159 163.
40. Wick MR, Cerilli LA. Applications of immunohistochemistry in the diagnosis of undifferentiated tumors. In: Lloyd RV, ed. Morphology Methods: Cell and Molecular Biology Techniques. Totowa, NJ: Human Press; 2001:323 360.
41. Zelger B, Weinlich G, Zelger B. Perineurioma. A frequently unrecognized entity with emphasis on a plexiform variant. Adv Clin Path 2000;4:25 33.
42. Hirai I, Kimura W, Ozawa K, et al. Perineural invasion in pancreatic cancer. Pancreas 2002;24:15 25.
43. Fogt F, Capodieci P, Loda M. Assessment of perineural invasion by GLUT-1 immunohistochemistry. Appl Immunohistochem 1995;3:194 197.
44. Zimmerman KG, Johnson PC, Paplanus SH. Nerve invasion by benign proliferating ductules in vasitis nodosa. Cancer 1983;51:2066 20610.
45. Khalifa MA, Montgomery EA, Ismiil N, Azumi N. What are the CD34+ cells in benign peripheral nerve sheath tumors? Double immunostaining study of CD34 and S-100 protein. Am J Clin Pathol 2000;114:123 126.
46. van de Rijn M, Rouse RV. CD34: a review. Appl Immunohistochem 1994;2:71 80.
47. Maurer M, Muller M, Kobsar I, Leonhard C, Martini R, Kiefer R. Origin of pathogenic macrophages and endoneurial fibroblast-like cells in an animal model of inherited neuropathy. Mol Cell Neurosci 2003;23:351 3510.
P.268
48. Bonetti B, Monaco S, Giannini C, Ferrari S, Zanusso G, Rizzuto N. Human peripheral nerve macrophages in normal and pathological conditions. J Neurol Sci 1993;118:158 168.
49. Griffin J, George R. The resident macrophages in the peripheral nervous system are renewed from the bone marrow: new variations on an old theme. Lab Invest 1993;3:257 260.
50. Gabriel CM, Howard R, Kinsella N, et al. Prospective study of the usefulness of sural nerve biopsy. J Neurol Neurosurg Psychiatry 2000;69:442 446.
51. Zochodne DW, Nguyen C, Sharkey KA. Accumulation and degranulation of mast cells in experimental neuromas. Neurosci Lett 1994;182:3 6.
52. Viskochil DH. It takes two to tango: mast cell and Schwann cell interactions in neurofibromas. J Clin Invest 2003;112:1791 1793.
53. Esposito B, De Santis A, Monteforte R, Baccari GC. Mast cells in Wallerian degeneration: morphologic and ultrastructural changes. J Comp Neurol 2002;445:199 210.
54. Weller RO, Cervos-Navarro J. Pathology of Peripheral Nerves: A Practical Approach. London: Butterworths; 1977.
55. Weis J, Alexianu ME, Heide G, Shroder JM. Renaut bodies contain elastic fiber components. J Neuropathol Exp Neurol 1993;52:444 451.
56. Weller RO. Colour Atlas of Neuropathology. Oxford: Oxford University Press and Harvey Miller; 1984.
57. Baker MD. Electrophysiology of mammalian Schwann cells. Prog Biophys Mol Biol 2002;78:83 103.
58. Lariviere RC, Julien JP. Functions of intermediate filaments in neuronal development and disease. J Neurobiol 2004;58:131 148.
59. Al-Chalabi A, Miller CC. Neurofilaments and neurological disease. Bioessays 2003;25:346 355.
60. Mukhopadhyay R, Kumar S, Hoh JH. Molecular mechanisms for organizing the neuronal cytoskeleton. Bioessays 2004;26:1017 1025.
61. von Bartheld CS. Axonal transport and neuronal transcytosis of trophic factors, tracers, and pathogens. J Neurobiol 2004;58:295 314.
62. Baas PW, Buster DW. Slow axonal transport and the genesis of neuronal morphology. J Neurobiol 2004;58:3 17.
63. Arvidson B. A review of axonal transport of metals. Toxicology 1994;88:1 14.
64. Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci 2002;25:532 537.
65. Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proc Natl Acad Sci U S A 2000;97:8146 8150.
66. Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol 2002;31:581 593.
67. Terada S. Where does slow axonal transport go? Neurosci Res 2003;47:367 372.
68. Spencer PS, Schaumburg HH, eds. Experimental and Clinical Neurotoxicology. 2nd ed. New York: Oxford University Press; 2000.
69. Katalymov LL, Glukhova NV. Some characteristics of periaxonal space in myelinated nerve fibers. Dokl Biol Sci 2003;388:9 11.
70. Trapp B, Quarles R, Suzuki K. Immunocytochemical studies of quaking mice support a role for myelin-associated glycoprotein in forming and maintaining the periaxonal space and periaxonal cytoplasmic collar of myelinating Schwann cells. J Cell Biol 1984;99:594 606.
71. Quarles RH. Myelin sheaths: glycoproteins involved in their formation, maintenance and degeneration. Cell Mol Life Sci 2002;59:1851 1871.
72. Mirsky R, Jessen KR. The neurobiology of Schwann cells. Brain Pathol 1999;293 311.
73. Weller RO, Herzog I. Schwann cell lysosomes in hypertrophic neuropathy and in normal human nerves. Brain 1970;93:347 356.
74. Tsiper MV, Yurchenco PD. Laminin assembles into separate basement membrane and fibrillar matrices in Schwann cells. J Cell Sci 2002;115(pt 5):1005 1015.
75. Gonzalez-Martinez T, Perez-Pinera P, Diaz-Esnal B, Vega JA. S-100 proteins in the human peripheral nervous system. Microsc Res Tech 2003;60:633 638.
76. Garavito ZV, Sutachan JJ, Muneton VC, Hurtado H. Is S-100 protein a suitable marker for adult Schwann cells? In Vitro Cell Dev Biol Anim 2000;36:281 283.
77. Le Forestier N, Lescs MC, Gherardi RK. Anti-NKH-1 antibody specifically stains unmyelinated fibres and non-myelinating Schwann cell columns in humans. Neuropathol Appl Neurobiol 1993;19:500 506.
78. Fine SW, Li M. Expression of calretinin and the alpha-subunit of inhibin in granular cell tumors. Am J Clin Pathol 2003;119:259 264.
79. Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol 1999;189:4 11.
80. Yasuda T, Sobue G, Ito T, et al. Human peripheral nerve sheath neoplasm: expression of Schwann cell-related markers and their relation to malignant transformation. Muscle Nerve 1991;14:812 8110.
81. Kawahara E, Oda Y, Ooi A, Katsuda S, Nakanishi Y, Umeda S. Expression of glial fibrillary acidic protein (GFAP) in peripheral nerve sheath tumors. A comparative study of immunoreactivity of GFAP, vimentin, S-100 protein, and neurofilament in 38 schwannomas and 18 neurofibromas. Am J Surg Pathol 1988;12:115 120.
82. Koirala S, Reddy LV, Ko CP. Roles of glial cells in the formation, function, and maintenance of the neuromuscular junction. J Neurocytol 2003;32:987 1002.
83. Par M, Elde R, Mazurkiewicz JE, Smith AM, Rice FL. The Meissner corpuscle revised: a multiafferented mechanoreceptor with nociceptor immunochemical properties. J Neurosci 2001;21:7236 7246.
84. Oda R, Yaoi T, Okajima S, Kobashi H, Kubo T, Fushiki S. A novel marker for terminal Schwann cells, homocysteine-responsive ER-resident protein, as isolated by a single cell PCR-differential display. Biochem Biophys Res Commun 2003;308:872 877.
85. Wewetzer K, Verd E, Angelov DN, Navarro X. Olfactory ensheathing glia and Schwann cells: two of a kind? Cell Tissue Res 2002;309:337 345.
86. Ffrench-Constant C, Colognato H, Franklin RJM. The mysteries of myelin unwrapped. Science 2004;304:688 6810.
87. Spiegel I, Peles E. Cellular junctions of myelinated nerves (Review). Mol Membr Biol 2002;19:95 101.
88. Eichberg J. Myelin PO. New knowledge and new roles. Neurochem Res 2002;27:1331 1340.
89. Garbay B, Heape AM, Sargueil F, Cassagne C. Myelin synthesis in the peripheral nervous system. Prog Neurobiol 2000;61:267 304.
90. Raine CS. Multiple sclerosis and chronic relapsing EAE: comparative ultrastructural neuropathology. In: Hallpike JF, Adams CWM, Tourtelotte WW, eds. Multiple Sclerosis. Pathology, Diagnostic and Management. London: Chapman and Hall; 1983:413 460.
91. Shy ME, Garbern JY, Kamholz J. Hereditary motor and sensory neuropathies: a biological perspective. Lancet Neurol 2002;1:110 118.
92. Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol 2003;163:889 8910.
93. Podratz JL, Rodriguez E, Windebank AJ. Role of the extracellular matrix in myelination of peripheral nerve. Glia 2001;35:35 40.
94. Takashima H, Boerkoel CF, De Jonghe P, et al. Periaxin mutations cause a broad spectrum of demyelinating neuropathies. Ann Neurol 2002;51:709 715.
95. Meier C, Dermietzel R, Davidson KG, Yasumura T, Rash JE. Connexin32-containing gap junctions in Schwann cells at the internode zone of partial myelin compaction and in Schmidt-Lanterman incisures. J Neurosci 2004;24:3186 3198.
96. Balice-Gordon RJ, Bone LJ, Scherer SS. Functional gap junctions in the Schwann cell myelin sheath. J Cell Biol 1998;142:1095 1104.
97. Custer AW, Kazarinova-Noyes K, Sakurai T, et al. The role of ankyrin-binding protein NrCAM in node of Ranvier formation. J Neurosci 2003;23:10032 10039.
P.269
98. Scherer SS. Nodes, paranodes, and incisures: from form to function. Ann N Y Acad Sci 1999;883:131 142.
99. Ochoa J. The unmyelinated fibre. In: Landon DN, ed. The Peripheral Nerve. London: Chapman and Hall; 1976:106 158.
100. Mirsky R, Jessen KR. The biology of non myelin-forming Schwann cells. Ann N Y Acad Sci 1986;486:132 146.
101. Gray MH, Rosenberg AE, Dickersin GR, Bhan AK. Glial fibrillary acidic protein and keratin expression by benign and malignant nerve sheath tumors. Hum Pathol 1989;20:1089 1096.
102. Britton WJ, Lockwood DNJ. Leprosy. Lancet 2004;363:1209 12110.
103. Schmidt HG, Schmid A, Domschke W. Nerve-neuroendocrine complexes in stomach mucosa in Zollinger-Ellison syndrome [in German]. Pathologe 1995;16:404 407.
104. Aub ck L, Hofler H. Extraepithelial intraneural endocrine cells as starting-points for gastrointestinal carcinoids. Virchows Arch A Pathol Anat Histopathol 1983;401:17 33.
105. Franke C, Gerharz CD, Bohner H, et al. Neurogenic appendicopathy in children. Eur J Pediatr Surg 2002;12:28 31.
106. Ortiz-Hidalgo C, de Le n-Bojorge B, Torres JE. Neuroma apendicular asociado a microcarcinoide solitario. Patolog a 1995;33:83 85.
107. Schmidt HG, Schmid A, Domschke W. Nerve-neuroendocrine complexes in stomach mucosa in Zollinger-Ellison syndrome [in German]. Pathologe 1995;16:404 407.
108. Colomar A, Marty V, Combe C, Medina C, Parnet P, Amedee T. The immune status of Schwann cells: what is the role of the P2X7 receptor? [in French] J Soc Biol 2003;197:113 122.
109. Bonetti B, Valdo P, Ossi G, et al. T-cell cytotoxicity of human Schwann cells: TNFalpha promotes fasL-mediated apoptosis and IFN gamma perforin-mediated lysis. Glia 2003;43:141 148.
110. Zhang YP, Porter S, Wekerle H. Schwann cells in myasthenia gravis. Preferential uptake of soluble and membrane-bound AChR by normal and immortalized Schwamm cells, and immunogenic presentation to SChR-specific T line lymphocytes. Am J Pathol 1990;136:111 112.
111. Vedeler C, Ulvestad E, Borge L, et al. The expression of CD-59 in normal human nervous tissue. Immunology 1994;82:542 547.
112. Gold R, Archelos JJ, Hartung HP. Mechanisms of immune regulation in the peripheral nervous system. Brain Pathol 1999;9:343 360.
113. Glass JD. Wallerian degeneration as a window to peripheral neuropathy. J Neurol Sci 2004;220:123 124.
114. Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol 1997;14:67 116.
115. Hirata K, Kawabuchi M. Myelin phagocytosis by macrophages and nonmacrophages during Wallerian degeneration. Microsc Res Tech 2002;57:541 547.
116. Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci 2002;22:6696 6703.
117. Shen ZL, Lassner F, Bader A, Becker M, Walter GF, Berger A. Cellular activity of resident macrophages during Wallerian degeneration. Microsurgery 2000;20:255 261.
118. Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus 2004;16:E1.
119. Horie H, Kadoya T, Hikawa N, et al. Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J Neurosci 2004;24:1873 1880.
120. Blackemore WK. Myelination, demyelination and remyelination. In: Thomas Smith W, Cavanagh JB, eds. Recent Advances in Neuropathology. Vol 2. Edinburgh: Churchill Livingstone; 1982:53 82.
121. Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg 2000;8:243 252.
122. Blesch A, Tuszynski MH. Nucleus hears axon's pain. Nat Med 2004;10:236 237.
123. Boeshore KL, Schreiber RC, Vaccariello SA, et al. Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J Neurobiol 2004;59:216 235.
124. Bergner AJ, Murphy SM, Anderson CR. After axotomy, substance P and vasoactive intestinal peptide expression occurs in pilomotor neurons in the rat superior cervical ganglion. Neuroscience 2000;96:611 618.
125. Khullar SM, Fristad I, Brodin P, Kvinnsland IH. Upregulation of growth associated protein 43 expression and neuronal co-expression with neuropeptide Y following inferior alveolar nerve axotomy in the rat. J Peripher Nerv Syst 1998;3:79 90.
126. Fenrich K, Gordon T. Canadian Association of Neuroscience review: axonal regeneration in the peripheral and central nervous systems: current issues and advances. Can J Neurol Sci 2004;31:142 156.
127. Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat 1999;194(pt 1):1 14.
128. Welcher AA, Suter U, De Leon M, Bitler CM, Shooter EM. Molecular approaches to nerve regeneration. Philos Trans R Soc Lond B Biol Sci 1991;331:295 301.
129. Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol 2003;27:277 324.
130. Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain 2004;127(pt 2):294 303.
131. Frisen J, Risling M, Fried K. Distribution and axonal relations of macrophages in neuroma. Neuroscience 1993;55:1003 1013.
132. King RM. Atlas of Peripheral Nerve Pathology. London: Edward Arnold; 19910.
133. Dyck PJ, Dyck PJB, Giannini C, Sahenk Z, Windebank AJ, Engelstad JN. Peripheral nerves. In: Graham DI, Lantos PL, eds. Greenfield's Neuropathology. 7th ed. Vol. 2. London: Arnold; 2002:551 676.
134. Kuwabara S. Guillain-Barre syndrome: epidemiology, pathophysiology and management. Drugs 2004;64:597 610.
135. Weller RO, Nester B. Early changes at the node of Ranvier in segmental demyelination: histochemical and electron microscopic observations. Brain 1972;95:665 674.
136. Weller RO, Bruckner FE, Chamberlain MA. Rheumatoid neuropathy: a histological and electrophysiological study. J Neurol Neurosurg Psychiatry 1970;33:592 604.
137. Weller RO, Mitchell J, Daves GD Jr. Buckthorn (Karwinskia humboldtiana) toxins. In: Spencer PS, Schaumburg HH, eds. Experimental and Clinical Neurology. Baltimore: Williams & Wilkins; 1980;336 347.
138. Shy ME, Garbern JY, Kamholz J. Hereditary motor and sensory neuropathies: a biological perspective. Lancet Neurol 2002;1:110 118.
139. Klein CJ. Pathology and molecular genetics of inherited neuropathy. J Neurol Sci 2004;220:141 143.
140. Bertorini T, Narayanaswami P, Rashed H. Charcot-Marie-Tooth disease (hereditary motor sensory neuropathies) and hereditary sensory and autonomic neuropathies. Neurologist 2004;10:327 337.
141. Zhou L, Griffin JW. Demyelinating neuropathies. Curr Opin Neurol 2003;16:307 313.
142. Ironside JW, Moss TH, Louis DN, Lowe JS, Weller RO. Tumours of peripheral nerves. In: Diagnostic Pathology of Nervous System Tumors. Churchill Livingstone; 2002:425 464.
143. Giannini C, Scheithauer BW, Jenkins RB, et al. Soft-tissue perineurioma. Evidence for an abnormality of chromosome 22, criteria for diagnosis, and review of the literature. Am J Surg Pathol 1997;21:164 173.
144. Rankine AJ, Filion PR, Platten MA, Spagnolo DV. Perineurioma: a clinicopathological study of eight cases. Pathology 2004;36:309 315.
145. Kossard S, Kumar A, Wilkinson B. Neural spectrum: palisaded encapsulated neuroma and verocay body poor dermal schwannoma. J Cutan Pathol 1999;26:31 36.
146. Kurtkaya-Yapicier O, Scheithauer B, Woodruff JM. The pathobiologic spectrum of Schwannomas. Histol Histopathol 2003;18:925 934.
147. Ferner RE, O'Doherty MJ. Neurofibroma and scwhannoma. Curr Opin Neurol 2002;15:679 684.
P.270
148. Canales-Ibarra C, Magari os G, Olsoff-Pagovich P, Ortiz-Hidalgo C. Cutaneous sclerosing perineurioma of the digits: an uncommon soft-tissue neoplasm. Report of two cases with immunohistochemical analysis. J Cutan Pathol 2003;30:577 581.
149. Hansen MR, Linthicum FH Jr. Expression of neuregulin and activation of erbB receptors in vestibular schwannomas: possible autocrine loop stimulation. Otol Neurotol 2004;25:155 1510.
150. de Saint Aubain Somerhausen N, Valaeys V, Geerts M, Andre J. Neuroblastoma-like schwannoma: a case report and review of the literature. Am J Dermatopathol 2003;25:32 34.
151. Kim YC, Park HJ, Cinn YW, Vandersteen DP. Benign glandular schwannoma. Br J Dermatol 2001;145:834 837.
152. Kindblom LG, Meis-Kindblom JM, Havel G, Busch C. Benign epithelioid schwannoma. Am J Surg Pathol 1998;22:762 770.
153. Laskin WB, Fetsch JF, Miettinen M. The neurothekeoma : immunohistochemical analysis distinguishes the true nerve sheath myxoma from its mimics. Hum Pathol 2000;31:1230 1241.
154. Scheithauer BW, Woodruff JM, Erlandson RA, eds. Neurofibroma. Tumors of the peripheral nervous system. Atlas of Tumor Pathology. Series 3, fascicle 24. Washington, DC: Armed Forces Institute of Pathology; 1999:177 218.
155. Ortiz-Hidalgo C, Verocay J. Verocay neurinomas and bodies and other contributions to medicine. Rev Neurol 2004;39:487 491.
156. Dingemans KP, Teeling P. Long-spacing collagen and proteoglycans in pathologic tissues. Ultrastruct Pathol 1994;18:539 547.
157. Fine SW, McClain SA, Li M. Immunohistochemical staining for calretinin is useful for differentiating schwannomas from neurofibromas. Am J Clin Pathol 2004;122:552 5510.
158. Yen SH, Fields KL. A protein related to glial filaments in Schwann cells. Ann N Y Acad Sci 1985;455:538 551.
159. Fullen DR, Reed JA, Finnerty B, McNutt NS. S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi. J Cutan Pathol 2001;28:393 3910.
160. Katati MJ, Martin JM, Massare E, Arjona V. Melanocytic schwannoma. A case report and review of the literature [in Spanish]. Rev Neurol 2000;31:427 430.
161. Stratakis CA, Matyakhina L, Courkoutsakis N, et al. Pathology and molecular genetics of the pituitary gland in patients with the complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas (Carney complex). Front Horm Res 2004;32:253 264.
162. Ordo ez NG. Granular cell tumor: a review and update. Adv Anat Pathol 1999;6:186 203.
163. Kurtin PJ, Bonin DM. Immunohistochemical demonstration of the lysosome-associated glycoprotein CD68 (KP-1) in granular cell tumors and schwannomas. Hum Pathol 1994;25:1172 1178.
164. Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin N Am 2004;15:157 166.
165. Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science 2002;296:920 922.
166. Takata M, Imai T, Hirone T. Factor-XIIIa-positive cells in normal peripheral nerves and cutaneous neurofibromas of type-1 neurofibromatosis. Am J Dermatopathol 1994;16:37 43.
167. Rosai J. Soft tissues. In: Rosai and Ackerman's Surgical Pathology. 9th ed. Vol 2. Edinburgh: Mosby; 2004:2266 22610.
168. Arun D, Gutmann DH. Recent advances in neurofibromatosis type 1. Curr Opin Neurol 2004;17:101 105.
169. Lim DJ, Rubenstein AE, Evans DG, et al. Advances in neurofibromatosis 2 (NF2): a workshop report. J Neurogenet 2000;14:63 106.
170. Yang FC, Ingram DA, Chen S, et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/- mast cells. J Clin Invest 2003;112:1851 1861.
171. Young H, Hyman S, North K. Neurofibromatosis 1: clinical review and exceptions to the rules. J Child Neurol 2002;17:613 621.
172. Baser ME, R Evans DG, Gutmann DH. Neurofibromatosis 2. Curr Opin Neurol 2003;16:27 33.
173. Xiao G-H, Chernoff J, Testa JR. NF2: the wizardry of Merlin. Genes Chromosomes Cancer 2003;38:389 3910.
174. Bashour AM, Meng JJ, Ip W, MacCollin M, Ratner N. The neurofibromatosis type 2 gene product, merlin, reverses the F-actin cytoskeletal defects in primary human schwannoma cells. Mol Cell Biol 2002;22:1150 1157.
175. Pelton PD, Sherman LS, Rizvi TA, et al. Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human Schwannoma cells. Oncogene 1998;17:2195 2201.
176. Mora J, Gerald WL. Origin of neuroblastic tumors: clues for future therapeutics. Expert Rev Mol Diagn 2004;4:293 302.
177. Bilgic B, Ates LE, Demiryont M, Ozger H, Dizdar Y. Malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1. Pathol Oncol Res 2003;9:201 205.
178. Woodruff JM. Pathology of tumors of the peripheral nerve sheath in type 1 neurofibromatosis. Am J Med Genet 1999;89:23 30.
179. Zamecnik M, Michal M. Malignant peripheral nerve sheath tumor with perineurial cell differentiation (malignant perineurioma). Pathol Int 1999;49:69 73.
180. Zamecnik M, Michal M. Perineurial cell differentiation in neurofibromas. Report of eight cases including a case with composite perineurioma-neurofibroma features. Pathol Res Pract 2001;197:537 544.
181. Yamaguchi U, Hasegawa T, Hirose T, et al. Low grade malignant peripheral nerve sheath tumour: varied cytological and histological patterns. J Clin Pathol 2003;56:826 830.
182. Malerba M, Garofalo A. A rare case of nerve-sheath sarcoma with rhabdomyoblastic differentiation (malignant triton tumor) [in Italian]. Tumori 2003;89:246 250.