3. The Hsp70 family is ubiquitous.
8.3 The Hsp70 family is ubiquitous. |
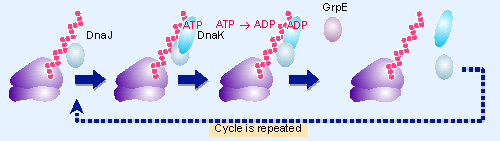 |
Figure 8.6 DnaJ assists the binding of DnaK (Hsp70), which assists the folding of nascent proteins. ATP hydrolysis drives conformational change. GrpE displaces the ADP; this causes the chaperones to be released. Multiple cycles of association and dissociation may occur during the folding of a substrate protein. |
The Hsp70 family is found in bacteria, eukaryotic cytosol, in the endoplasmic reticulum, and in chloroplasts and mitochondria. Hsp70 functions in conjunction with two further components, best characterized in bacteria as DnaJ and GrpE. Figure 8.6 shows that Hsp40 (DnaJ) binds first to a nascent protein as it emerges from the ribosome. Hsp40 contains a region called the J domain (named for DnaJ), which interacts with Hsp70. Hsp70 (DnaK) binds to both Hsp40 and to the unfolded protein. In effect, two interacting chaperones bind to the protein. The J domain accounts for the specificity of the pairwise interaction, and drives a particular Hsp40 to select the appropriate partner from the Hsp70 family.
The interaction of Hsp70 (DnaK) with Hsp40 (DnaJ) stimulates the ATPase activity of Hsp70. The ADP-bound form of the complex remains associated with the protein substrate until GrpE displaces the ADP. This causes dissociation of the complex, possibly by a series of events in which Hsp40 (DnaJ) dissociates, ATP binds to Hsp70 (DnaK), and then Hsp70 dissociates.
Protein folding is accomplished by multiple cycles of association and dissociation. As the protein chain lengthens, Hsp70 (DnaK) may dissociate from one binding site and then reassociate with another, thus releasing parts of the substrate protein to fold correctly in an ordered manner. Finally, the intact protein is released from the ribosome, folded into its mature conformation (for review see Georgopoulos and Welch, 1993; Hartl, 1966; Bukau and Horwich, 1998)
Different members of the Hsp70 class function on appropriate types of target proteins. Cytosolic proteins (the eponymous Hsp70 and a related protein called Hsc70) act on nascent proteins on ribosomes. Variants in the ER (called BiP or Grp78 in higher eukaryotes, called Kar2 in S. cerevisiae), or in mitochondria or chloroplasts, function in a rather similar manner on proteins as they emerge into the interior of the organelle on passing through the membrane.
| Reviews | |
| Bukau, B. and Horwich, A. L. (1998). The Hsp70 and Hsp60 chaperone machines. Cell 92, 351-366. | |
| Georgopoulos, C. and Welch, W. J. (1993). Role of the major heat shock proteins as molecular chaperones. Ann. Rev. Cell Biol. 9, 601-634. | |
| Hartl, F.-U. (1966). Molecular chaperones in cellular protein folding. Nature 381, 571-580. | |