9. TGFb signals through Smads
26.8 The JAK-STAT pathway |
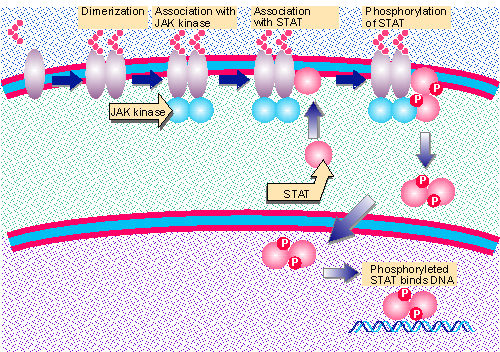 |
Figure 26.34 Cytokine receptors associate with and activate JAK kinases. STATs bind to the complex and are phosphorylated. They dimerize and translocate to the nucleus. The complex binds to DNA and activates transcription. Animated figure |
Some signal transduction pathways have large numbers of components (permitting a high degree of amplification) and many feedback circuits (permitting sensitive control of the duration and strength of the signal). The JAK-STAT pathway is much simpler, and consists of three components that function as illustrated in Figure 26.34.
JAK-STAT pathways are activated by several cytokine receptors. These receptors do not possess intrinsic kinase activities. However, binding of a cytokine causes its receptor to dimerize, which provides the signal to associate with and activate a JAK kinase. The JAK kinases take their name (originally Janus kinases) from the characteristic presence of two kinase domains in each molecule. Several members of the family are known (JAK1,2,3, etc.); each associates with a specific set of cytokine receptors. The interaction between the activated (dimeric) cytokine receptor and JAK kinase(s) in effect produces the same result as the ligand-induced dimerization of a tyrosine kinase receptor: the difference is that the receptor and kinase activities are held in different proteins instead of in the same protein.
The JAK kinases are tyrosine kinases whose major substrates are transcription factors called STATs. There are >7 STATs; each STAT is phosphorylated by a particular set of JAK kinases. The phosphorylation occurs while the JAK is associated with the receptor at the plasma membrane. A pair of JAK kinases associates with an activated receptor, and both may be necessary for the pathway to function. An example is that stimulation by the interferon IFNγ requires both JAK1 and JAK2 (Dale et al., 1989; Velazquez et al., 1992; for review see Darnell et al., 1994).
STAT phosphorylation leads to the formation of both homodimers and heterodimers. The basis for dimerization is a reciprocal interaction between an SH2 domain in one subunit and a phosphorylated Tyr in the other subunit (Shuai et al., 1994).
The STAT dimers translocate to the nucleus, and in some cases associate with other proteins. They bind to specific recognition elements in target genes, whose transcription is activated (for review see Schindler and Darnell, 1995).
Given a multiplicity of related cytokine receptors, JAK kinases, and STAT transcription factors, how is specificity achieved? The question is sharpened by the fact that many receptors can activate the same JAKs, but activate different STATs. Control of specificity lies with formation of a multipartite complex containing the receptor, JAKs, and STATs. The STATs interact directly with the receptor as well as with the JAKs, and an SH2 domain in a particular STAT recognizes a binding site in a particular receptor. So the major control of specificity lies with the STAT.
Stimulation of a JAK-STAT pathway is only transient. Its activation may be terminated by the action of a phosphatase. An example is the pathway activated by binding of erythropoietin (red blood cell hormone) to its receptor. This activates JAK2 kinase. Recruitment of another component terminates the reaction; the phosphatase SH-PTP1 binds via its SH2 domain to a phosphotyrosine site in the erythropoietin receptor. This site in the receptor is probably phosphorylated by JAK2. The phosphatase then dephosphorylates JAK2 and terminates the activation of the corresponding STATs. This creates a simple feedback circuit: erythropoietin receptor activates JAK2, JAK2 acts on a site in the receptor, and this site is recognized by the phosphatase that in turn acts on JAK2. This again emphasizes the way in which formation of a multicomponent complex may be used to ensure specificity in controlling the pathway (Klingmuller et al., 1995).
| Reviews | |
| Darnell, J. E., Kerr, I. M., and Stark, G. R. (1994). JAK-STAT pathways and transcriptional activation in response to IFNg and other extracellular signaling proteins. Science 264, 1415-1421. | |
| Schindler, C. and Darnell, J. E. (1995). Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Ann. Rev. Biochem 64, 621-651. | |
| Research | |
| Dale, T. C. et al. (1989). Rapid activation by interferon aof a latent DNA-binding protein present in the cytoplasm of untreated cells. Proc. Nat. Acad. Sci. USA 86, 1203-1207. | |
| Klingmuller, U. et al. (1995). Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 80, 729-738. | |
| Shuai, K. et al. (1994). Interferon activation of the transcription factor STAT91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell 76, 821-828. | |
| Velazquez, L. et al. (1992). A protein tyrosine kinase in the interferon a/bsignaling pathway. Cell 70, 313-322. | |