10. Specialized recombination involves breakage and reunion at specific sites
14.10 Specialized recombination involves breakage and reunion at specific sites |
| Key terms defined in this section |
| att sites are the loci on a phage and the bacterial chromosome at which recombination integrates the phage into, or excises it from, the bacterial chromosome. |
The conversion of lambda DNA between its different life forms involves two types of event. The pattern of gene expression is regulated as described in 11 Phage strategies. And the physical condition of the DNA is different in the lysogenic and lytic states:
- In the lytic lifestyle, lambda DNA exists as an independent, circular molecule in the infected bacterium.
- In the lysogenic state, the phage DNA is an integral part of the bacterial chromosome (called prophage).
Transition between these states involves site Vspecific recombination:
- To enter the lysogenic condition, free lambda DNA must be integrated into the host DNA.
- To be released from lysogeny into the lytic cycle, prophage DNA must be excised from the chromosome.
Integration and excision occur by recombination at specific loci on the bacterial and phage DNAs called attachment (att) sites. The attachment site on the bacterial chromosome is called attλ in bacterial genetics. The locus is defined by mutations that prevent integration of lambda; it is occupied by prophage λ in lysogenic strains. When the attλ site is deleted from the E. coli chromosome, an infecting lambda phage can establish lysogeny by integrating elsewhere, although the efficiency of the reaction is <0.1% of the frequency of integration at attλ. This inefficient integration occurs at secondary attachment sites, which resemble the authentic att sequences.
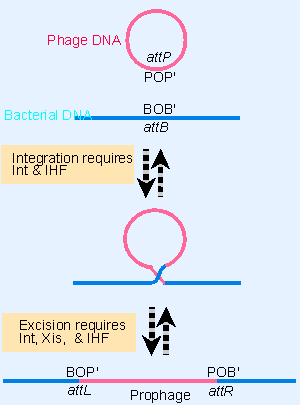 |
Figure 14.19 Circular phage DNA is converted to an integrated prophage by a reciprocal recombination between attP and attB; the prophage is excised by reciprocal recombination between attL and attR. |
For describing the integration/excision reactions, the bacterial attachment site (attλ) is called attB, consisting of the sequence components BOB′. The attachment site on the phage, attP, consists of the components POP′. Figure 14.19 outlines the recombination reaction between these sites. The sequence O is common to attB and attP. It is called the core sequence; and the recombination event occurs within it. The flanking regions B, B′ and P, P′ are referred to as the arms; each is distinct in sequence. Because the phage DNA is circular, the recombination event inserts it into the bacterial chromosome as a linear sequence. The prophage is bounded by two new att sites, the products of the recombination, called attL and attR.
An important consequence of the constitution of the att sites is that the integration and excision reactions do not involve the same pair of reacting sequences. Integration requires recognition between attP and attB; while excision requires recognition between attL and attR. The directional character of site-specific recombination is thus controlled by the identity of the recombining sites.
Although the recombination event is reversible, different conditions prevail for each direction of the reaction. This is an important feature in the life of the phage, since it offers a means to ensure that an integration event is not immediately reversed by an excision, and vice versa.
The difference in the pairs of sites reacting at integration and excision is reflected by a difference in the proteins that mediate the two reactions:
- Integration (attB attP) requires the product of the phage gene int and a bacterial protein called integration host factor (IHF).
- Excision (attL attR) requires the product of phage gene xis, in addition to Int and IHF.
So Int and IHF are required for both reactions. Xis plays an important role in controlling the direction; it is required for excision, but inhibits integration.
IHF is a 20 kD protein of two different subunits, coded by the genes himA and himD. IHF is not an essential protein in E. coli, and is not required for homologous bacterial recombination. It is one of several proteins with the ability to wrap DNA on a surface. Mutations in the him genes prevent lambda site-specific recombination, and can be suppressed by mutations in λ int, which suggests that IHF and Int interact.
Site-specific recombination can be performed in vitro by Int and IHF. Int is an integrase that catalyzes a precise breakage and reunion in the absence of any synthesis of DNA. The roles of the att sites can be investigated by making deletions on either side. It turns out that attP is much larger than attB. The function of attP requires a stretch of 240 bp, but the function of attB can be exercised by the 23 bp fragment extending from V 11 to +11, in which there are only 4 bp on either side of the core. The disparity in their sizes suggests that attP and attB play different roles in the recombination, with attP providing additional information necessary to distinguish it from attB.
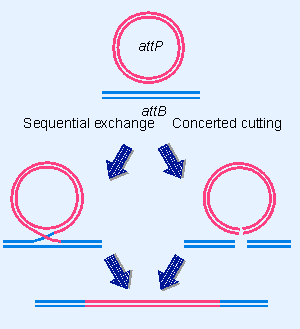 |
Figure 14.20 Does recombination between attP and attB proceed by sequential exchange or concerted cutting? |
Does the reaction proceed by a concerted mechanism in which the strands in attP and attB are cut simultaneously and exchanged? Or are the strands exchanged one pair at a time, the first exchange generating a Holliday junction, the second cycle of nicking and ligation occurring to release the structure? The alternatives are depicted in Figure 14.20.
The recombination reaction has been halted at intermediate stages by the use of "suicide substrates," in which the core sequence is nicked. The presence of the nick interferes with the recombination process. This makes it possible to identify molecules in which recombination has commenced but has not been completed. The structures of these intermediates suggest that exchanges of single strands take place sequentially. Int protein can resolve Holliday junctions, and is probably responsible for the cutting and ligation reactions.
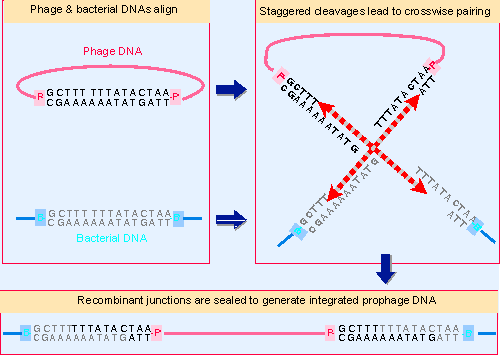 |
Figure 14.21 Staggered cleavages in the common core sequence of attP and attB allow crosswise reunion to generate reciprocal recombinant junctions. |
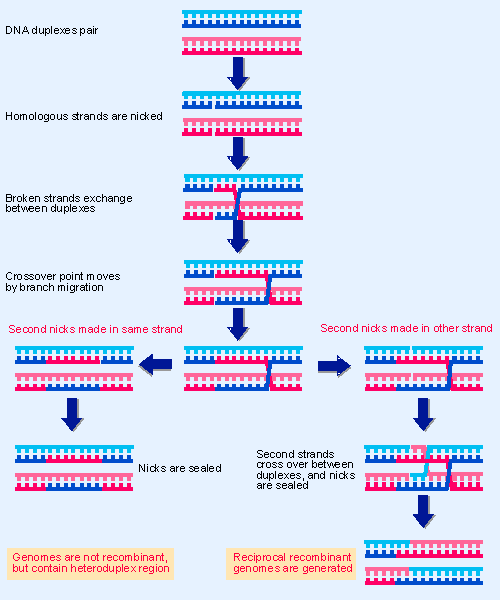 |
Figure 14.2 Recombination between two paired duplex DNAs could involve reciprocal single-strand exchange, branch migration, and nicking. |
The model illustrated in Figure 14.21 shows that if attP and attB sites each suffer the same staggered cleavage, complementary single-stranded ends could be available for crosswise hybridization. The distance between the lambda crossover points is 7 bp, and the reaction generates 3′ Vphosphate and 5′ VOH ends. The reaction is shown for simplicity as generating overlapping single-stranded ends that anneal, but actually occurs by a process akin to the recombination event of Figure 14.2. The corresponding strands on each duplex are cut at the same position, the free 3′ ends exchange between duplexes, the branch migrates for a distance of 7 bp along the region of homology, and then the structure is resolved by cutting the other pair of corresponding strands.
The in vitro reaction requires supercoiling in attP, but not in attB. When the reaction is performed in vitro between two supercoiled DNA molecules, almost all of the supercoiling is retained by the products. So there cannot be any free intermediates in which strand rotation could occur. This is consistent with the idea that the reaction proceeds through a Holliday junction. The breakage and reunion reaction resembles the activity of topoisomerase I, except that nicked strands from different duplexes are sealed together, instead of the ends of a broken strand from one duplex. (Int indeed has a [rather ineffectual] topoisomerase I ability to relax negatively supercoiled DNA.)
Large amounts of the Int and IHF proteins are needed for recombination in vitro. Int and IHF bind cooperatively to attP, and their affinity for the site is enhanced by supercoiling. The high stoichiometry suggests that the proteins do not function catalytically, but form a structure that supports only a single recombination event.
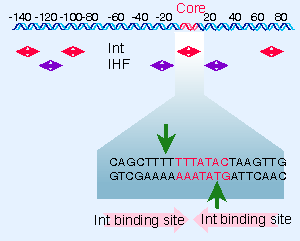 |
Figure 14.22 Int and IHF bind to different sites in attP. The Int recognition sequences in the core region include the sites of cutting. |
The proteins involved in site-specific recombination bind to specific sites in the att region. Int has two different modes of binding. It binds to inverted sites at the core sequence, positioning itself to make the cuts on each strand illustrated in Figure 14.22. These sites share a consensus sequence. It also binds to sites in the arms of attP that have a different consensus sequence. Different domains of Int recognize each type of sequence: an N-terminal domain recognizes the arms of attP, while a C-terminal domain recognizes the cores of attP and attB. The two domains probably bind DNA simultaneously, thus bringing the arms of attP close to the core.
IHF binds to sequences of ~20 bp in attP; the IHF binding sites are approximately adjacent to sites where Int binds. Xis binds to two sites located close to one another in attP, so that the protected region extends over 30 V40 bp. Together, Int, Xis, and IHF cover virtually all of attP. The binding of Xis changes the organization of the DNA so that it becomes inert as a substrate for the integration reaction.
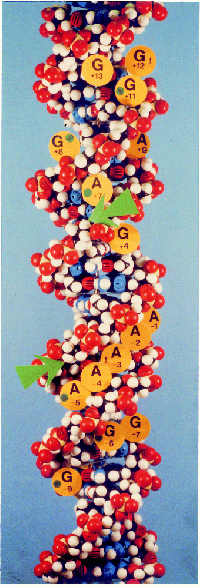 |
Figure 14.23 The Int binding sites in the core lie on one face of DNA. The large circles indicate positions at which methylation is influenced by Int binding; the large arrows indicate the sites of cutting. Photograph kindly provided by A. Landy. |
Figure 14.23 shows that when the core locations bound by Int are mapped on the double helix, virtually all of the contacts lie on one face of the DNA. The two sites of cutting are exposed in the major groove. IHF binding sites lie on the same face; and if the spacing between the Int-binding site and the flanking IHF-binding sites is altered so that it is no longer an integral number of helical turns, then integration is impeded.
When Int and IHF bind to attP, they generate a complex in which all the binding sites are pulled together on the surface of a protein. Supercoiling of attP is needed for the formation of this intasome.
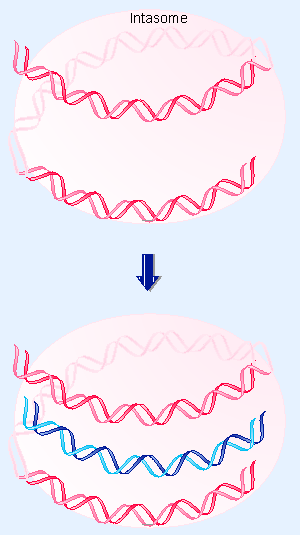 |
Figure 14.24 Multiple copies of Int protein may organize attP into an intasome, which initiates site-specific recombination by recognizing attB on free DNA. |
The only binding sites in attB are the two Int sites in the core. But Int does not bind directly to attB in the form of free DNA. The intasome is the intermediate that "captures" attB. Probably Int molecules that are part of the intasome bind to the sites in the core of attB, as indicated schematically in Figure 14.24.
According to this model, the initial recognition between attP and attB does not depend directly on DNA homology, but instead is determined by the ability of Int proteins to recognize both att sequences. The two att sites then are brought together in an orientation predetermined by the structure of the intasome. Sequence homology becomes important at this stage, when it is required for the strand exchange reaction.
The asymmetry of the integration and excision reactions is shown by the fact that Int can form a similar complex with attR only if Xis is added. This complex can pair with a condensed complex that Int forms at attL. IHF is not needed for this reaction.
Much of the complexity of site-specific recombination may be caused by the need to regulate the reaction so that integration occurs preferentially when the virus is entering the lysogenic state, while excision is preferred when the prophage is entering the lytic cycle. By controlling the amounts of Int and Xis, the appropriate reaction will occur.