8. Liver, Bile Ducts, Pancreas, and Spleen
Authors: Dahnert, Wolfgang
Title: Radiology Review Manual, 6th Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > Liver, Bile Ducts, Pancreas, and Spleen
function show_scrollbar() {}
Liver, Bile Ducts, Pancreas, and Spleen
Differential Diagnosis of Hepatic, Biliary, Pancreatic, and Splenic Disorders
Right Upper Quadrant Pain
BILE DUCTS
Biliary colic/bile duct obstruction
Acute cholecystitis/cholangitis
LIVER
Acute hepatitis: alcoholic, viral, drug-related, toxic
Hepatic abscess
Hepatic tumor: metastases, hepatocellular carcinoma, hemangioma, focal nodular hyperplasia, hepatic adenoma
Hemorrhagic cyst
Hepatic congestion: acute hepatic congestion, Budd-Chiari syndrome
Perihepatitis from gonococcal/chlamydial infection (Fitz-Hugh-Curtis syndrome)
PANCREAS
Acute pancreatitis
INTESTINES
Acute appendicitis
Peripyloric ulcer
Small bowel obstruction
Irritable bowel
Colitis/ileitis
Intestinal tumor
LUNG
Pneumonia
Pulmonary infarction
KIDNEY
Acute pyelonephritis
Ureteral calculus
Renal/perirenal abscess
Renal infarction
Renal tumor
OTHERS
Costochondritis
Herpes zoster
Liver
Diffuse Liver Disease
Fatty liver
Cirrhosis
Hepatitis
Hemochromatosis
Glycogen storage disease
Budd-Chiari syndrome
Diffuse Hepatic Enlargement = Hepatomegaly
METABOLIC
Fatty infiltration
Amyloid
Wilson disease
Gaucher disease
Von Gierke disease
Niemann-Pick disease
Weber-Christian disease
Galactosemia
MALIGNANCY
Lymphoma
Diffuse metastases
Diffuse HCC
Angiosarcoma
INFLAMMATION/INFECTION
Hepatitis
Mononucleosis
Miliary TB, histoplasmosis, sarcoid
Malaria
Syphilis
Leptospirosis
Chronic granulomatous disease of childhood
Sarcoidosis
VASCULAR
Passive congestion
OTHERS
Early cirrhosis
Polycystic liver disease
Hepatosplenomegaly
Disorders associated with extramedullary hematopoiesis + hemolytic anemia
Metabolic storage disease
Viral infection
Sarcoidosis
Leukemia, lymphoma, myeloproliferative disease
Increased Liver Attenuation
= abnormal deposits of substances with high atomic numbers
IRON
diffuse iron accumulation
Genetic/primary hemochromatosis
Erythropoietic hemochromatosis
Bantu siderosis
Transfusional iron overload
focal iron accumulation
Hemorrhagic metastases: choriocarcinoma, melanoma
Hepatic adenoma
Siderotic regenerative nodules of cirrhosis
 An iron-poor focus within a siderotic nodule on T2WI suggests HCC!
An iron-poor focus within a siderotic nodule on T2WI suggests HCC!
Focal hemochromatosis
COPPER
Wilson disease = hepatolenticular degeneration
= increased copper deposits in liver + basal ganglia
IODINE
Amiodarone (= antiarrhythmic drug with 37% iodine by weight)
 95 145 HU (range of normal for liver 30 70 HU)
95 145 HU (range of normal for liver 30 70 HU)
GOLD
Colloidal form of gold for therapy of rheumatoid arthritis
THOROTRAST
Alpha-emitter with atomic number of 90
THALLIUM
Accidental/suicidal ingestion of rodenticides (lethal dose is 0.2 1.0 gram)
ACUTE MASSIVE PROTEIN DEPOSITS
GLYCOGEN STORAGE DISEASE
P.668
| mnemonic: | GG CHAT |
Gold therapy
Glycogen storage disease
Cyclophosphamide
Hemochromatosis/hemosiderosis
Amiodarone
Thorotrast
Generalized Increase In Liver Echogenicity
Fatty liver
Steatohepatitis
Cirrhosis (fibrosis + fatty liver)
Chronic hepatitis
Vacuolar degeneration
Marked Decrease in Hepatic T2 Signal Intensity
= paramagnetic effect of intracellular iron deposition (ferritin, hemosiderin)
 signal intensity of pancreas does not help distinguish between primary + secondary hemochromatosis
signal intensity of pancreas does not help distinguish between primary + secondary hemochromatosis
Primary/hereditary hemochromatosis (dietary iron)
Secondary hemochromatosis
 bone marrow of low signal intensity (DDx: myelofibrosis)
bone marrow of low signal intensity (DDx: myelofibrosis)
Transfusional siderosis (RES)
 bone marrow of low signal intensity
bone marrow of low signal intensity decreased T2 signal in spleen
decreased T2 signal in spleen
Intravenous administration of ultrasmall superparamagnetic iron oxide
Liver Mass
 Hepatic masses account only for 5 6% of all intraabdominal masses in children!
Hepatic masses account only for 5 6% of all intraabdominal masses in children!
Primary Benign Liver Tumor
EPITHELIAL TUMORS
hepatocellular
Regenerative nodules
Adenomatous hyperplastic nodules
Focal nodular hyperplasia
Hepatic adenoma
cholangiocellular
Bile duct hamartoma/adenoma
Biliary cystadenoma
Papillary adenoma
MESENCHYMAL TUMORS
tumor of adipose tissue
Hepatic lipoma
Hepatic myelolipoma
Hepatic angiomyolipoma
tumor of muscle tissue
Leiomyoma
tumor of blood vessels
Infantile hemangioendothelioma
Hemangioma
Peliosis hepatis
mesothelial tumor
Benign mesothelioma
MIXED TISSUE TUMOR
Mesenchymal hamartoma
Hepatoblastoma
Benign teratoma
MISCELLANEOUS
Adrenal rest tumor
Pancreatic rest
Primary Malignant Liver Tumor
 Hepatic malignancies are the most common GI malignancy in children, but account for <2% of all pediatric malignancies!
Hepatic malignancies are the most common GI malignancy in children, but account for <2% of all pediatric malignancies!
EPITHELIAL TUMOR
hepatocellular
Hepatoblastoma (7%)
Hepatocellular carcinoma (75%)
cholangiocellular (6%)
Cholangiocarcinoma
Biliary cystadenocarcinoma
MESENCHYMAL TUMOR
tumor of blood vessels
Angiosarcoma
Epithelioid hemangioendothelioma
Kaposi sarcoma
other tumor
Embryonal sarcoma
Fibrosarcoma
TUMOR OF MUSCLE TISSUE
Leiomyosarcoma
Embryonal rhabdomyosarcoma of the biliary tree
MISCELLANEOUS
Carcinosarcoma
Teratoma
Yolk sac tumor
Carcinoid
Squamous carcinoma
Primary lymphoma
Solitary Liver Lesion
BENIGN TUMOR
Cavernous hemangioma
Adenoma
Focal nodular hyperplasia
Mesenchymal hamartoma
INFECTION
Pyogenic abscess
Echinococcal cyst
Inflammatory pseudotumor
TRAUMA
Hematoma
Traumatic cyst
MALIGNANT TUMOR
Primary tumor
Metastasis
OTHER
Fatty change
Simple cyst
P.669
SOLITARY ECHOGENIC LIVER MASS
| mnemonic: | Hyperechoic Focal Masses Affecting the Liver |
Hematoma, Hepatoma, Hemangioma, Hemochromatosis, Hepatoblastoma
Fatty infiltration, Focal nodular hyperplasia, Fibrosis
Metastasis
Adenoma
Lipoma
LIVER MASS SURROUNDED BY ECHOGENIC RIM
Metastasis: esp., cystic islet cell tumor
Adenoma
Hemangioma
Multiple Liver Lesions
BENIGN TUMOR
Cavernous hemangioma
Adenoma
Regenerating hepatic nodules
Multiple bile duct hamartoma
INFECTION
Multiple abscesses
Mycobacterial + fungal infection
Inflammatory pseudotumors
CONGENITAL
Polycystic disease
Caroli disease
MALIGNANCY
Metastases (most common malignant liver tumor)
Multifocal hepatoma
Lymphoma
OTHER
Sarcoidosis
Simple cysts
Langerhans cell histiocytosis (echogenic nodules)
BULL's-EYE LESIONS OF LIVER
Candidiasis (in immunocompromised)
Metastases
Lymphoma, leukemia
Sarcoidosis
Septic emboli
Other opportunistic infections
Kaposi sarcoma
MILIARY HEPATOSPLENIC LESIONS
Tuberculosis
Metastases
Fungal infections
Sarcoidosis
Lymphoma
Cystic Liver Lesion
NONNEOPLASTIC
Congenital hepatic cyst
Hematoma
Echinococcal cyst
Abscess
Fibropolycystic liver disease
NEOPLASTIC
Mesenchymal hamartoma
Undifferentiated sarcoma (embryonal sarcoma)
Malignant mesenchymoma
Biliary cystadenoma/cystadenocarcinoma
 <5% of intrahepatic cysts are of biliary origin!
<5% of intrahepatic cysts are of biliary origin!
Lymphangioma
Necrotic neoplasm
Cystic metastasis (ovarian/gastric carcinoma
FIBROPOLYCYSTIC LIVER DISEASE
= unique group of entities with derangement of embryonic biliary ductal plate development
 coexistence of hepatic + renal anomalies
coexistence of hepatic + renal anomalies
small interlobular bile ducts
Congenital hepatic fibrosis
Biliary hamartoma
Associated with: autosomal recessive (juvenile) polycystic kidney disease medium-sized bile ducts
Autosomal dominant polycystic disease
Associated with: autosomal dominant (adult) polycystic kidney disease large intrahepatic bile ducts
Caroli disease
large extrahepatic bile ducts
Choledochal cyst
Vascular Scar Tumor of Liver
Focal nodular hyperplasia
Giant cavernous hemangioma
Fibrolamellar carcinoma of liver
Well-differentiated hepatocellular carcinoma
Hypervascular metastasis
Intrahepatic cholangiocarcinoma
Liver Mass with Capsular Retraction
Cholangiocarcinoma
Fibrolamellar carcinoma or any hepatic malignancy
Low-density Mass in Porta Hepatis
Choledochal cyst
Hepatic cyst
Pancreatic pseudocyst
Enteric duplication
Hepatic artery aneurysm
Biloma
Embryonal rhabdomyosarcoma of biliary tree
Low-density Hepatic Mass with Enhancement
Hepatoma
Hypervascular metastases (lesions that may be obscured after contrast injection: pheochromocytoma, carcinoid, melanoma)
Cavernous hemangioma
Focal nodular hyperplasia with central fibrous scar
Hepatic adenoma
Fat-containing Liver Mass
Lipoma
Angiolipoma
Angiomyolipoma (eg, tuberous sclerosis)
Hepatocellular carcinoma
Hepatic adenoma
Liposarcoma metastasis
Malignant teratoma metastasis (+ calcifications)
Focal fatty change
P.670
Hyperintense Liver Mass on T1WI
Focal fat deposit
High protein content
Hemorrhage (methemoglobin)
Melanoma metastasis
Paramagnetic contrast agents + iodized oil
Hypervascular Liver Mass
 detected during hepatic arterial phase
detected during hepatic arterial phase
BENIGN
Focal nodular hyperplasia
Hepatocellular adenoma
Hemangioma
MALIGNANT
Primary malignant liver tumor
Hepatocellular carcinoma
Hemangioendothelioma
Angiosarcoma
Hypervascular liver metastases
Neuroendocrine tumors: islet cell, carcinoid
Renal cell carcinoma
Thyroid carcinoma
Choriocarcinoma
Melanoma
Breast carcinoma (some)
Hepatic Calcification
INFECTION (most common cause)
Granulomatous disease: tuberculosis (48%), histoplasmosis, brucellosis, coccidioidomycosis
 calcium involves entire lesion
calcium involves entire lesion
Echinococcal cyst (in 10 20%)
 curvilinear/ring calcification
curvilinear/ring calcification
CMV, toxoplasmosis, Pneumocystis carinii
Chronic granulomatous disease of childhood
Old pyogenic/amebic abscess
Schistosomiasis
 turtleback/tortoise shell calcifications
turtleback/tortoise shell calcifications
Cysticercosis, filariasis, paragonimiasis, Armillifer infection, dracunculiasis
Syphilitic gumma
VASCULAR
Hepatic artery aneurysm
Portal vein thrombosis
Hematoma
BILIARY
Intrahepatic calculi
Ascariasis, clonorchiasis
BENIGN TUMORS
Congenital cyst
Cavernous hemangioma
 large coarse centrally located calcification (in 10 20%)
large coarse centrally located calcification (in 10 20%)
Hepatocellular adenoma
Capsule of regenerating nodules
Infantile hemangioendothelioma
PRIMARY MALIGNANT TUMOR
Fibrolamellar carcinoma (calcified in 15 25%)
Hepatocellular carcinoma
Hepatoblastoma (10 20%)
Intrahepatic cholangiocarcinoma (in 18%)
 calcification accompanied by desmoplastic reaction
calcification accompanied by desmoplastic reaction
Epithelioid hemangioendothelioma
Cystadenocarcinoma
METASTATIC TUMOR
Mucin-producing neoplasm: carcinoma of colon, breast, stomach
Ovarian carcinoma (psammomatous bodies)
Melanoma, thyroid carcinoma, pleural mesothelioma, chondro- and osteosarcoma, carcinoid, leiomyosarcoma, neuroblastoma
| mnemonic: | 4H TAG MAP |
Hepatoma
Hemochromatosis
Hemangioma
Hydatid disease
Thorotrast
Abscess
Granulomas (healed)
Metastases
Absent mnemonic
Porcelain gallbladder
Spontaneous Hepatic Hemorrhage
Hepatocellular carcinoma
Hepatocellular adenoma
Focal nodular hyperplasia
Hepatic hemangioma
Hepatic metastases: lung, RCC, melanoma
HELLP syndrome
Amyloidosis
Peliosis hepatis
Angiomyolipoma
Liver Circulation
Transient Hepatic Parenchymal Enhancement
= Hyperperfusion Abnormalities Of Liver
= areas of early enhancement on arterial-dominant phase due to decreased portal blood flow/formation of intrahepatic arterioportal shunts/increased aberrant drainage through hepatic veins
LOBAR/SEGMENTAL
Portal vein obstruction:
portal vein thrombosis, tumor invasion, surgical ligation
Cirrhosis with arterioportal shunt
Hypervascular gallbladder disease
SUBSEGMENTAL
Obstruction of peripheral portal branches
Percutaneous needle biopsy + drainage procedure/ethanol ablation
Acute cholecystitis + cholangitis
SUBCAPSULAR
due to peripheral parenchymal compression
Rib compression
Perihepatic peritoneal implants
Pseudomyxoma peritonei
Perihepatic fluid collections
P.671
idiopathic/unexplained
PSEUDOLESIONS
= systemic venous blood flow draining into hepatic sinusoids
Accessory cystic vein of gallbladder fossa
Aberrant right gastric vein
Capsular veins
RETICULAR-MOSAIC PATTERN
Cirrhosis
Hereditary hemorrhagic telangiectasia
Hepatic vein obstruction
Arterioportal Shunt
= organic/functional communication between high-pressure hepatic arterial branch + low-pressure portal venous system
Cause:
Primary hepatic neoplasm
Hepatocellular carcinoma
Hemangioma
Cholangiocarcinoma
Metastatic tumor
Hepatic trauma
Blunt abdominal trauma
Iatrogenic: biopsy, percutaneous abscess drainage, percutaneous biliary drainage, ethanol injection
Cirrhosis
Rupture of hepatic artery pseudoaneurysm
Congenital malformation
Routes:
Macroscopic fistula
Transsinusoidal = between microscopic interlobular arteriole + portal venule
Transvasal = via tumor thrombus
Transtumoral = via draining vein from a hypervascular tumor
Transplexal/peribiliary = via capillary network surrounding bile ducts
Pathophysiology:
shunted contrast material enhances a focal area of liver parenchyma before adjacent parenchyma is enhanced via the usual splanchnic route
CECT (in hepatic arterial phase):
 pseudolesion = transient peripheral wedge-shaped hepatic parenchymal enhancement:
pseudolesion = transient peripheral wedge-shaped hepatic parenchymal enhancement: small shunt may resemble nodular lesion
small shunt may resemble nodular lesion lesion disappears in portal venous phase
lesion disappears in portal venous phase
 enhancement of portal vein branch main portal vein from periphery without enhancement of splenic vein/superior mesenteric vein
enhancement of portal vein branch main portal vein from periphery without enhancement of splenic vein/superior mesenteric vein
Hepatic Artery Enlargement
Cirrhosis (compensatory response to decreased portal venous flow)
Intrahepatic arteriovenous shunting
vascular neoplasm
hepatic artery-portal vein fistula
Cause: biopsy, trauma  turbulent high-velocity low-resistance flow
turbulent high-velocity low-resistance flow soft-tissue bruit (= random assignment of color in perivascular soft tissue due to tissue vibration)
soft-tissue bruit (= random assignment of color in perivascular soft tissue due to tissue vibration) arterialized frequently retrograde flow in portal vein
arterialized frequently retrograde flow in portal vein
Hereditary hemorrhagic telangiectasia
 large tortuous feeding arteries with high velocity + aliased flow
large tortuous feeding arteries with high velocity + aliased flow multiple dilated vessels (representing AVMs)
multiple dilated vessels (representing AVMs) large draining veins
large draining veins areas of fatty change + fibrosis
areas of fatty change + fibrosis
Chronic active hepatitis
Dampening of Hepatic Vein Doppler Waveform
= dampened oscillations of hepatic veins resembling portal vein flow due to shielding of hepatic veins from activity of right atrium
= portalization of hepatic vein flow pattern
Increased liver tissue stiffness
Liver cirrhosis
Various parenchymal abnormalities of liver
Intrinsic/extrinsic venous obstruction
Budd-Chiari syndrome
Inferior vena cava obstruction
Extrinsic compression of hepatic veins
Pulsatile Portal Vein
= waveform pulsatility with >2/3 change from peak to minimal velocity
Congestive heart failure
Hepatic artery-portal vein fistula
Arteriovenous shunt in cirrhosis
Portal-to-hepatic vein fistula
Portal Venous Gas
 Should be considered a life-threatening event and sign of bowel infarction + gangrene until proved otherwise!
Should be considered a life-threatening event and sign of bowel infarction + gangrene until proved otherwise!
Etiology:
INTESTINAL NECROSIS (in 74% of adults)
Bowel infarction secondary to arterial and venous occlusions (vascular accidents, superior mesenteric artery syndrome)
Ulcerative colitis
Necrotizing enterocolitis associated with mesenteric arterial thrombosis
Perforated gastric ulcer
GI OBSTRUCTION
Small bowel obstruction (duodenal atresia)
Imperforate anus
Esophageal atresia
MISCELLANEOUS
Hemorrhagic pancreatitis
Sigmoid diverticulitis
Intraabdominal abscess
Pneumonia
Iatrogenic injection of air during endoscopy
Dead fetus
Diabetes, diarrhea
| mnemonic: | BE NICE |
BE (air embolism during double contrast barium enema)
Necrotizing enterocolitis
Infarction (mesenteric)
Catheterization of umbilical vein
Erythroblastosis fetalis
P.672
Pathogenesis:
Intestinal wall alteration permitting passage of intraluminal air into intestinal venules:
ulceration of gastric, duodenal, bowel wall
sloughing of epithelial lining
enhanced mucosal permeability eg, intestinal ischemia with bowel necrosis (most common), perforated gastric carcinoma/ulcer, inflammatory bowel disease (Crohn disease, ulcerative colitis)
Prognosis: 75 90% mortality rate within 1 week of diagnosis Bowel distension with elevated intraluminal pressure causes minimal mucosal disruption + permits passage of intraluminal air into veins:
iatrogenic dilatation of hollow viscus (gastrostomy, sclerotherapy, ERCP, colonoscopy, barium enema)
spontaneous paralytic ileus, mechanical obstruction, acute gastric dilatation
blunt trauma (<1%) with acute pressure changes
barotrauma
Prognosis: surgery often not indicated Intraabdominal sepsis
? gas from septicemia in branches of mesenteric veins/portal vein (pylephlebitis)
? increased intraluminal fermentation of carbohydrates due to bacterial overgrowth
? mesocolic abscess causing inframesocolic perforation dissecting between peritoneal leaflets eg, diverticulitis, intra- or retroperitoneal abscess/gangrene, TB
Idiopathic (15%)
eg, organ transplantation (liver [18%], kidney, bone marrow), pulmonary disease (chronic obstructive pulmonary disease, bronchopneumonia, asthma), drugs (steroids, cytostatics), seizure
Composition of colonic gas:
methane, carbon dioxide, oxygen, nitrogen, hydrogen
Plain film:
 Substantial amount necessary for detection
Substantial amount necessary for detection branching linear gas densities:
branching linear gas densities: in periphery of liver extending to within 2 cm of liver capsule
in periphery of liver extending to within 2 cm of liver capsule predominantly within more anteriorly located left lobe of liver
predominantly within more anteriorly located left lobe of liver
 pneumatosis of intestinal wall
pneumatosis of intestinal wall
CT:
 Small amount of gas detectable
Small amount of gas detectable tubular areas of decreased attenuation in periphery of liver
tubular areas of decreased attenuation in periphery of liver gas in superior/inferior mesenteric veins
gas in superior/inferior mesenteric veins gas in small mesenteric veins at mesenteric border of bowel
gas in small mesenteric veins at mesenteric border of bowel
US:
 Small amount of gas detectable
Small amount of gas detectable intensely hyperechoic foci within lumen of portal vein + liver parenchyma
intensely hyperechoic foci within lumen of portal vein + liver parenchyma
Doppler:
 tall sharp bidirectional spikes (overloading of Doppler receiver from strong reflection of gas bubble in bloodstream) superimposed on normal portal vein spectrum
tall sharp bidirectional spikes (overloading of Doppler receiver from strong reflection of gas bubble in bloodstream) superimposed on normal portal vein spectrum
| DDx: | pneumobilia (located centrally within bile ducts close to liver hilum + within left lobe of liver) |
Gallbladder
Nonvisualization of Gallbladder on OCG
Peak opacification of gallbladder: 14 19 hours (13 35% of dose excreted in urine)
EXTRABILIARY CAUSES
Failure to ingest contrast
Fasting
Failure to reach absorptive surface of bowel
vomiting, nasogastric suction
esophageal/gastric obstruction
hiatal, umbilical, inguinal hernias
Zenker, epiphrenic, gastric, duodenal, jejunal diverticulum
gastric ulcer, gastrocolic fistula
malabsorption, diarrhea
postoperative ileus, severe trauma
inflammation: acute pancreatitis, acute peritonitis
Deficiency of bile salts
Crohn disease, surgical resection of terminal ileum, liver disease, cholestyramine therapy, abnormal communication between biliary system and gastrointestinal tract
INTRINSIC GALLBLADDER DISEASE
Cholecystectomy
Anomalous position
Obstruction of cystic duct
Chronic cholecystitis
Oral Cholecystogram (OCG)
| Dose: | 6 0.5 g tablets 2 hours after evening meal |
PATIENT SELECTION
bilirubin <5 mg% (not necessary if due to hemolysis)
 Contraindicated in serious liver disease!
Contraindicated in serious liver disease! Relative contraindications in peritonitis, postoperative ileus, acute pancreatitis!
Relative contraindications in peritonitis, postoperative ileus, acute pancreatitis!
TOXICITY
Nausea + vomiting (also noted in 29% on placebo)
Immediate anaphylactic response
Delayed hypotensive reaction (increased risk in cirrhosis)
Renal failure
Precipitation of hyperthyroidism
Nonvisualization of Gallbladder on US
Status post cholecystectomy
Obscured by costal margin
Anomalous position (intrahepatic, subphrenic)
Gallbladder carcinoma replacing gallbladder
Perforation of gallbladder
Congenital absence
Contracted gallbladder
nonfasting status without stones
in fasting status with stones
 wall-echo-shadow (WES triad) interfaces
wall-echo-shadow (WES triad) interfaces
P.673
Shadowing in Gallbladder Fossa
WES (wall-echo-shadow) triad
Gas in duodenum/colon obscuring gallbladder
Porcelain gallbladder
Emphysematous cholecystitis
Cholecystoenteric fistula
Status post ERCP with retrograde air injection
High-Density Bile
Hemorrhagic cholecystitis
Hemobilia
Prior contrast administration
vicarious excretion of urographic agent
cholecystopaque
Milk of calcium bile
Displaced Gallbladder
NORMAL IMPRESSION
by duodenum/colon (positional change)
HEPATIC MASS
hepatoma, hemangioma, regenerating nodule, metastases, intrahepatic cyst, polycystic liver, hydatid disease, hepar lobatum (tertiary syphilis), granuloma, abscess
EXTRAHEPATIC MASS
Retroperitoneal tumor (renal, adrenal)
Polycystic kidney
Lymphoma
Lymph node metastasis to porta hepatis
Pancreatic pseudocyst
Alteration in Gallbladder Size
Enlarged Gallbladder
= CHOLECYSTOMEGALY = HYDROPS OF GALLBLADDER
Size:
(a) infants <1 year: >3 cm in length (b) children: >7 cm in length (c) adults: >4 10 cm
OBSTRUCTION
Cystic duct obstruction (40%)
Hydrops: chronic cystic duct obstruction + distension with clear sterile mucus (white bile)
Empyema: acute/chronic obstruction with superinfection of bile
Cholelithiasis causing obstruction (37%)
Cholecystitis with cholelithiasis (11%)
Courvoisier phenomenon (10%)
secondary to neoplastic process in pancreas / duodenal papilla/ampulla of Vater/common bile duct
Pancreatitis
Infection: leptospirosis, ascariasis, typhoid fever, scarlet fever, familial Mediterranean fever
UNOBSTRUCTED (mostly neuropathic)
S/P vagotomy
Diabetes mellitus
Alcoholism
Appendicitis (in children)
Narcotic analgesia
WDHA syndrome
Hyperalimentation
Acromegaly
Kawasaki syndrome
Anticholinergics
Bedridden patient with prolonged illness
AIDS (in 18%)
Dehydration
Prolonged fasting
Total parenteral nutrition
Sepsis
NORMAL (2%)
Small Gallbladder
Chronic cholecystitis
Cystic fibrosis: in 25% of patients
Congenital hypoplasia/multiseptated gallbladder
Postprandial
Intrahepatic cholestasis (viral, drug-related)
Gallbladder Wall Thickening
Diffuse Gallbladder Wall Thickening
= anterior wall of gallbladder >3 mm
INTRINSIC
infection
Acute cholecystitis
Chronic cholecystitis (10 25%)
Xanthogranulomatous cholecystitis
Gallbladder perforation
Sepsis
Brucellosis
inflammation
AIDS cholangiopathy (average of 9 mm in up to 55%)
Sclerosing cholangitis
Eosinophilic cholecystitis
tumor infiltration
Gallbladder carcinoma (in 41% diffuse)
Leukemic infiltration (AML)
Multiple myeloma
others
Hyperplastic cholecystosis (in 91% diffuse)
Gallbladder varices
EXTRINSIC
liver disease
Hepatitis (in 80%)
Cirrhosis
Hepatic venous obstruction
fluid overload
Hypoalbuminemia
Renal failure
Right heart failure
Systemic venous hypertension
Ascites
Lymphatic obstruction (by portal nodes)
others
Graft-versus-host disease
Pancreatitis
drugs
Chemoinfusion of hepatic artery (ischemia)
Treatment with interleukin
P.674
PHYSIOLOGIC
= contracted gallbladder after eating
Focal Gallbladder Wall Thickening
METABOLIC
Metachromatic sulfatides
Hyperplastic cholecystoses
BENIGN TUMOR
Adenoma: glandular elements (0.2%)
Papilloma: fingerlike projections (0.2%)
Villous hyperplasia
Fibroadenoma
Cystadenoma:? premalignant
Neurinoma, hemangioma
Carcinoid tumor
MALIGNANT TUMOR
Carcinoma of gallbladder: adenocarcinoma/squamous cell carcinoma (in 59% focal)
Leiomyosarcoma
Metastases: from malignant melanoma (15%), lung, kidney, esophagus, breast, carcinoid, Kaposi sarcoma, lymphoma, leukemia
INFLAMMATION/INFECTION
Inflammatory polyp: in chronic cholecystitis
Parasitic granuloma: Ascaris lumbricoides, Paragonimus westermani, Clonorchis, filariasis, Schistosoma, Fasciola
Intramural epithelial cyst/mucinous retention cyst
Xanthogranulomatous cholecystitis (in 9% focal)
WALL-ADHERENT GALLSTONE = embedded stone
HETEROTOPIC MUCOSA
Ectopic pancreatic tissue
Ectopic gastric glands
Ectopic intestinal glands
Ectopic hepatic tissue
Ectopic prostatic tissue
Filling Defects of Gallbladder
Fixed Filling Defects of Gallbladder
| mnemonic: | PANTS |
Polyp
Adenomyomatosis
Neurinoma
Tumor, primary/secondary
Stone, wall-adherent
GALLBLADDER POLYPS
NONNEOPLASTIC (95%)
Cholesterol (60%): on average 8 polyps
Adenomyoma (25%): in gallbladder fundus
Inflammatory polyp (10%):
solitary (in 1/2), 2 5 (in 1/2)
Others: heterotopic gastric glands
NEOPLASTIC (5%)
Adenoma: solitary (in 66%); 2 5 (in 33%)
Metastasis
Fibroma, leiomyoma, lipoma, neurofibroma
Mobile Intraluminal Mass in Gallbladder
Tumefactive sludge
Blood clot
Nonshadowing stone
Comet-tail Artifact in Liver and Gallbladder
LIVER
Foreign metallic body (eg, surgical clip)
Intrahepatic calcification
Pneumobilia
Multiple bile duct hamartoma = von Meyenburg complex
GALLBLADDER
Rokitansky-Aschoff sinus
Intramural stone
Cholesterolosis of gallbladder
Echogenic Fat in Hepatoduodenal Ligament
= sign of pericholecystic inflammation
Cholecystitis
Perforated duodenal ulcer
Pancreatitis
Diverticulitis
Bile Ducts
Hemobilia
Iatrogenic trauma: percutaneous needle biopsy, transhepatic cholangiography/biliary drainage /portography
Blunt/penetrating trauma
Rupture of aneurysm/pseudoaneurysm
Gas in Biliary Tree = Pneumobilia
| mnemonic: | I GET UP |
Incompetent sphincter of Oddi (after sphincterotomy/passage of a gallstone)
Gallstone ileus
Emphysematous cholecystitis (actually in gallbladder)
Trauma
Ulcer (duodenal ulcer perforating into CBD)
Postoperative (eg, cholecystoenterostomy)
 gas outlines choledochus gallbladder
gas outlines choledochus gallbladder peripheral branches of bile ducts not filled
peripheral branches of bile ducts not filled
Obstructive Jaundice in Adult
Etiology:
BENIGN DISEASE (76%)
Traumatic/postoperative stricture (44%)
Calculi (21%)
Chronic pancreatitis (8%)
Sclerosing cholangitis (1%)
Recurrent pyogenic cholangitis
Parasitic disease (ascariasis)
Liver cysts
Aortic aneurysm
Papillary stenosis
MALIGNANCY (24%)
Pancreatic carcinoma (18%)
Ampullary/duodenal carcinoma (8%)
Cholangiocarcinoma (3%)
Metastatic disease (2%)
from stomach, pancreas, lung, breast, colon, lymphoma
P.675
Level and cause of obstruction:
INTRAPANCREATIC
Choledocholithiasis
 Most common cause of biliary obstruction (in 15% of patients with cholelithiasis)!
Most common cause of biliary obstruction (in 15% of patients with cholelithiasis)!
Chronic pancreatitis
Pancreatic carcinoma
SUPRAPANCREATIC (5%)
= between pancreas + porta hepatis
Cholangiocarcinoma
Metastatic adenopathy
PORTA HEPATIS (5%)
Klatskin tumor
Spread from adjacent tumor (GB, liver)
Surgical stricture
INTRAHEPATIC
Cystadenoma, cystadenocarcinoma
Mirizzi syndrome
Caroli disease
Cholangitis: recurrent pyogenic cholangitis, sclerosing cholangitis, AIDS cholangitis
Incidence of infected bile in bile duct obstruction:
incomplete/partial obstruction in 64%
complete obstruction in 10%
 Infection twice as high with biliary calculi than with malignant obstruction!
Infection twice as high with biliary calculi than with malignant obstruction!
| Organism: | E. coli (21%), Klebsiella (21%), enterococci (18%), Proteus (15%) |
Test Sensitivity for Common Bile Duct Obstruction:
Intravenous cholangiography
depends on level of bilirubin: <1 mg/dL in 92%; <2 mg/dL in 82%; <3 mg/dL in 40%; >4 mg/dL in <10%
False-negative rate: 45% Cx: adverse reactions in 4 10% US
88 90% sensitivity for dilatation of CBD
 In 27 95% correct level of obstruction determined by US
In 27 95% correct level of obstruction determined by US In 23 81% correct cause of obstruction determined by US
In 23 81% correct cause of obstruction determined by US CBD >4 6 mm/10% of patient's age in years
CBD >4 6 mm/10% of patient's age in years increase in CBD size after fatty meal
increase in CBD size after fatty meal Swiss cheese sign = abundance of fluid-filled structures on liver sections
Swiss cheese sign = abundance of fluid-filled structures on liver sections intrahepatic double channel / shotgun sign= two parallel tubular structures composed of portal vein + dilated intrahepatic bile ducts
intrahepatic double channel / shotgun sign= two parallel tubular structures composed of portal vein + dilated intrahepatic bile ducts intrahepatic bile duct >2 mm/>40% of adjacent portal vein branch
intrahepatic bile duct >2 mm/>40% of adjacent portal vein branch
False-negative: not dilated in acute obstruction (in 70%), sclerosing cholangitis, intermittent obstruction from choledocholithiasis False-positive: dilated hepatic artery in cirrhosis/portal hypertension/hepatic neoplasm, patients after cholecystectomy CT
100% visualization in tumorous obstruction, 60% in nontumorous obstruction
NUC
 delayed/nonvisualization of biliary system (93% specificity)
delayed/nonvisualization of biliary system (93% specificity) vicarious excretion of tracer through kidneys
vicarious excretion of tracer through kidneys
| DDx: | Hepatocellular dysfunction (delayed clearance of cardiac blood pool) |
Hyperbilirubinemia in Infants
= UNCONJUGATED HYPERBILIRUBINEMIA
PHYSIOLOGIC
Frequency: in 60% of full-term infants, in 80% of preterm infants Course: increase by day 2 3, peak by day 5 7 (up to 12 mg/dL in full-term babies, up to 14 mg/dL in premature infants)  Breast-fed babies may have an elevated bilirubin level until the end of 2nd week of life!
Breast-fed babies may have an elevated bilirubin level until the end of 2nd week of life!
NONPHYSIOLOGIC
onset of jaundice within first 24 hours
persistent/new-onset jaundice in infants 2 weeks of age
rise of serum bilirubin >5 mg/dL per 24 hours
direct bilirubin level >1 mg/dL
Neonatal Obstructive Jaundice
= severe persistent jaundice in a child beyond 3 4 weeks of age
Cause:
INFECTION
(a) bacterial: E. coli, Listeria monocytogenes (b) viral: TORCH, Coxsackie virus, echovirus, Adenovirus METABOLIC
(a) inherited: alpha-1 antitrypsin deficiency, cystic fibrosis, galactosemia, hereditary tyrosinemia (b) acquired: inspissated bile syndrome
= bile plug syndrome
(= cholestasis due to erythroblastosis); cholestasis due to total parenteral nutrition; choledocholithiasisBILIARY TRACT ABNORMALITIES
(a) extrahepatic: biliary obstruction/hypoplasia/atresia, choledochal cyst, spontaneous perforation of bile duct (b) intrahepatic: ductular hypoplasia/atresia IDIOPATHIC NEONATAL HEPATITIS
 The 3 most common causes of jaundice in neonates are hepatitis, biliary atresia, and choledochal cyst!
The 3 most common causes of jaundice in neonates are hepatitis, biliary atresia, and choledochal cyst!mnemonic: CAN Choledochal cyst
Atresia
Neonatal hepatitis
NUC imaging regimen:
Premedication with phenobarbital (5 mg/kg/day) over 5 days to induce hepatic microsomal enzymes, which enhance uptake and excretion of certain compounds and increase bile flow
IDA scintigraphy (50 Ci/kg; minimum of 1 mCi)
Imaging at 5-minute intervals for 1 hour + at 2, 4, 6, 8, 24 hours
P.676
Jaundice in Older Children
DISEASE OF HEPATOCYTES
hepatitis
Acute hepatitis: infection, toxic agents, drugs
Chronic hepatitis
metabolic
Wilson disease
Cystic fibrosis
Glycogen storage disease
Tyrosinemia
Alpha-1 antitrypsin deficiency
OBSTRUCTION
malignant neoplasm
Hepatoblastoma
Hepatocellular carcinoma
Sarcomas: angiosarcoma, lymphosarcoma, rhabdomyosarcoma of bile ducts, undifferentiated embryonal sarcoma
Metastatic disease: neuroblastoma, Wilms tumor, leukemia/lymphoma
benign neoplasm
Infantile hemangioendothelioma
Mesenchymal hamartoma
benign stricture
cholelithiasis/choledocholithiasis (uncommon)
Large Nonobstructed CBD
Passage of stone (return to normal after days to weeks)
Common bile duct surgery (return to normal in 30 50 days)
Postcholecystectomy dilatation (in up to 16%)
Intestinal hypomotility
Normal variant (aging)
Fatty-meal sonography (to differentiate from obstruction with 74% sensitivity, 100% specificity)
Method: peroral Lipomul (1.5 mL/kg) followed by 100 mL of water [cholecystokinin causes contraction of gallbladder, relaxation of sphincter of Oddi, increase in bile secretion], CBD measured before and 45/60 minutes after stimulation  little change/decrease in size = normal response
little change/decrease in size = normal response increase in size >2 mm = partial obstruction
increase in size >2 mm = partial obstruction
Filling Defect in Bile Ducts
ARTIFACT
Pseudocalculus
contracted sphincter of Boyden + Oddi with smooth arcuate contour
bridge of tissue between cystic duct + CHD
underfilling of cystic duct during ERCP
admixture defect at cystic duct junction
Air bubble: confirmed by positional changes
Blood clot: spheroid configuration, spontaneous resolution with time
BILIARY CALCULI
MIRIZZI SYNDROME
NEOPLASM
malignant
Cholangiocarcinoma: irregular stricture, intraluminal polypoid mass
Metastatic tumor (GI tract, pancreas, breast, melanoma, lymphoma)
Others: ampullary carcinoma, hepatoma, hamartoma, carcinoid, embryonal rhabdomyosarcoma of biliary tree
benign
Papilloma (most common benign neoplasm)
Histo: vascular connective tissue covered by single layer of columnar epithelium Adenoma
Histo: epithelial glandular tissue surrounded by fibrous tissue Fibroma, lipoma, neuroma
Granular cell myoblastoma (= Schwann-cell derived biliary tumor) in young black woman
PARASITES
Ascaris lumbricoides: long linear filling defect/discrete mass if coiled
Liver fluke (Clonorchis sinensis, Fasciola hepatica): intrahepatic epithelial hyperplasia, periductal fibrosis, cholangitis, liver abscess, hepatic duct stones, common duct obstruction
Schistosoma japonicum: portal vein infection
Hydatid cyst: after erosion into biliary tree
Echogenic Material in Bile Ducts
Calculi
Gas
Blood
Tumor
Parasites
Bile Duct Narrowing
BENIGN STRICTURE (44%)
trauma
Postoperative stricture (95 99%)
associated with cholecystectomy
Blunt/penetrating trauma
Hepatic artery embolization
Infusion of chemotherapeutic agents
inflammation
Sclerosing cholangitis
Recurrent pyogenic cholangitis
Eosinophilic cholangiopathy
Acute/chronic pancreatitis
Pancreatic pseudocyst
Perforated duodenal ulcer
Erosion by biliary calculus
Gallstones + cholecystitis
Abscess
Radiation therapy
Papillary stenosis
Acquired immunodeficiency syndrome
P.677
congenital
Choledochal cyst
MALIGNANT STRICTURE
Pancreatic carcinoma
Ampullary carcinoma
Cholangiocarcinoma
Compression by enlarged lymph node
Metastasis
Multifocal Intrahepatic Bile Duct Strictures
Primary sclerosing cholangitis
Ascending cholangitis due to stricture/stone/bile duct anomaly
Oriental cholangiohepatitis
AIDS-related cholangitis
Ischemia
floxuridine treatment
hepatic arterial thrombosis (in liver transplant)
Neoplasm
cholangiocarcinoma
metastases
Previous bile surgery
Congenital biliary anomalies
Papillary Stenosis
Etiology:
PRIMARY PAPILLARY STENOSIS (10%)
Congenital malformation of papilla
Sequelae of acute/chronic inflammation
Adenomyosis
SECONDARY PAPILLARY STENOSIS (90%)
Mechanical trauma of stone passage
(choledocholithiasis in 64%; cholecystolithiasis in 26%)
Functional stenosis: associated with pancreas divisum, history of pancreatitis
Reflex spasm = papillary dyskinesia
Scar from previous surgical manipulation
Periampullary neoplasm
 prestenotic dilatation of CBD
prestenotic dilatation of CBD increase in pancreatic duct diameter (83%)
increase in pancreatic duct diameter (83%) long smooth narrowing/beak (fibrotic stenosis)
long smooth narrowing/beak (fibrotic stenosis) prolonged bile-to-bowel transit time >45 minutes on Tc-IDA scintigraphy
prolonged bile-to-bowel transit time >45 minutes on Tc-IDA scintigraphy
Periampullary Tumor
Pancreatic carcinoma (85%)
Cholangiocarcinoma of distal common bile duct (6%)
Ampullary tumor (4%)
Duodenal wall tumor
adenocarcinoma, adenoma, carcinoid, smooth muscle tumor
Double-Duct Sign
= dilatation of common bile duct + pancreatic duct
Ampullary tumor (most common)
Pancreatic ductal adenocarcinoma
Stone impacted in ampulla of Vater
Papillary stenosis
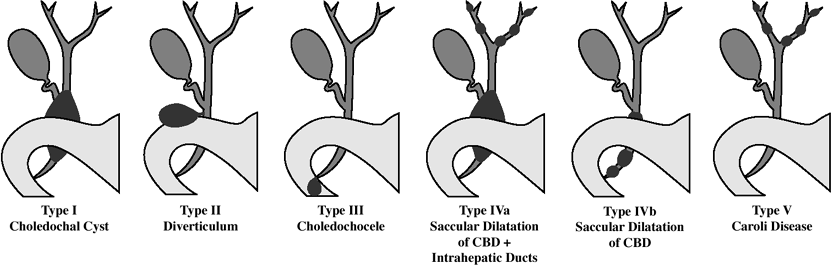
Congenital Biliary Cysts
(Todani classification)
Choledochal cyst (77 87%)
IA cystic dilatation of CBD IB focal segmental dilatation of CBD IC fusiform dilatation of CBD II. Diverticulum of extrahepatic ducts (1.2 3%)
originating from CBD/CHD
 neck of diverticulum open/closed
neck of diverticulum open/closed
III. Choledochocele (1.4 6%)
IV. Multiple segmental bile duct cysts
IVA multiple intra- and extrahepatic biliary cysts + saccular dilatation of CBD (19%) IVB multiple extrahepatic biliary cysts + normal intrahepatic bile ducts (rare) Caroli disease = intrahepatic biliary cysts
Pancreas
Congenital Pancreatic Anomalies
Pancreas divisum
Annular pancreas
Agenesis of dorsal pancreas
 |
| Classification of Congenital Biliary Cysts |
P.678
| May be associated with: | abnormal situs, polysplenia, intestinal malrotation |
Pancreatic Calcification
CHRONIC PANCREATITIS
Numerous irregular stippled calcifications of varying size; predominantly intraductal
Alcoholic pancreatitis (in 20 50%):
 calcifications limited to head/tail in 25%
calcifications limited to head/tail in 25%
Biliary pancreatitis (in 2%)
Hereditary pancreatitis (in 35 60%):
 round calcifications throughout gland
round calcifications throughout gland
Idiopathic pancreatitis
Pancreatic pseudocyst
NEOPLASM
Microcystic adenoma (in 33%):
 sunburst appearance of calcifications
sunburst appearance of calcifications
Macrocystic cystadenoma In 15%):
 amorphous peripheral calcifications
amorphous peripheral calcifications
Adenocarcinoma (in 2%): with sunburst pattern
Cavernous lymphangioma/hemangioma:
 multiple phleboliths
multiple phleboliths
Metastases from colon cancer
INTRAPARENCHYMAL HEMORRHAGE
Old hematoma/abscess/infarction
Rupture of intrapancreatic aneurysm
HYPERPARATHYROIDISM (in 20%):
 50% of patients develop chronic pancreatitis + concomitant nephrocalcinosis
50% of patients develop chronic pancreatitis + concomitant nephrocalcinosis Indistinguishable from alcoholic pancreatitis
Indistinguishable from alcoholic pancreatitis
CYSTIC FIBROSIS
Fine granular calcifications imply advanced pancreatic fibrosis
HEMOCHROMATOSIS
KWASHIORKOR = juvenile tropical pancreatitis
 Indistinguishable from alcoholic pancreatitis
Indistinguishable from alcoholic pancreatitis
Atrophy of Pancreas
Main pancreatic duct obstruction
Cystic fibrosis
 Most common cause in childhood!
Most common cause in childhood!
Schwachman-Diamond syndrome
Johanson-Blizzard syndrome (= pancreatic insufficiency, nasal alar hypoplasia, absence of permanent teeth, short stature, congenital deafness)
Hemochromatosis
Viral infection
Malnutrition
Cushing syndrome, steroid therapy, obesity
Pancreatic Mass
NEOPLASTIC
Adenocarcinoma
Islet cell tumor
Cystadenoma/-carcinoma
Solid and papillary neoplasm
Lymphoma
INFLAMMATORY
Acute pancreatitis
Pseudocyst
Pancreatic abscess
Pancreatic Neoplasm
| Origin: | in 99% exocrine ductal epithelium in 1% acinar portion of pancreatic glands in 0.1% malignant ampullary tumor with better prognosis |
EXOCRINE NEOPLASM
Ductal cell origin
Ductal adenocarcinoma (90%)
Ductectatic mucinous tumor
= mucin-hypersecreting carcinoma
Cystic neoplasm (10 15%)
serous microcystic neoplasm
mucinous macrocystic neoplasm
Solid and papillary epithelial neoplasm (rare)
Cystic changes of von Hippel-Lindau disease
Acinar cell origin
Acinar cell carcinoma (1%)
Adenoma
Indeterminate origin
Pancreatoblastoma = infantile pancreatic carcinoma
Dermoid cyst
Giant cell tumor
ENDOCRINE NEOPLASM
Nonfunctioning islet cell tumor
Functioning islet cell tumor
Insulinoma ( cells)
Glucagonoma
Gastrinoma ( cells)
Somatostatinoma
VIPoma (WDHA syndrome)
PP-oma = pancreatic polypeptide
Carcinoid
NONEPITHELIAL ORIGIN
Primary tumor
Primary lymphoma
<1% of pancreatic neoplasms
Primitive neuroectodermal tumor
Rhabdomyosarcoma
Mesenchymal tumor (1%)
Schwannoma
Neurofibroma
Lymphangioma
Teratoma
Lipoma
Metastases
Renal cell carcinoma
Lung cancer
Breast cancer
Colon cancer
Melanoma
Soft-tissue sarcoma, Kaposi sarcoma
Secondary lymphoma:
 large homogeneous solid mass, infrequently with central cystic area
large homogeneous solid mass, infrequently with central cystic area peripancreatic nodal masses
peripancreatic nodal masses peripancreatic vessels displaced + stretched
peripancreatic vessels displaced + stretched dilatation of pancreatic + bile duct uncommon
dilatation of pancreatic + bile duct uncommon
Ovarian cancer
Hepatocellular carcinoma
P.679
Hypervascular Pancreatic Tumors
PRIMARY
Islet cell tumor, microcystic adenoma, solid and papillary epithelial neoplasm
METASTASES from
angiosarcoma, leiomyosarcoma, melanoma, carcinoid, renal cell carcinoma, adrenal carcinoma, thyroid carcinoma
Pancreatic Cyst
INFLAMMATORY/INFECTIOUS
pseudocyst (85%): secondary to obstructive tumor/trauma/acute pancreatitis (in 2 4%), chronic pancreatitis (in 10 15%) [develop within 10 20 days, consolidated after 6 8 weeks]
acquired cyst:
Retention cyst (= exudate within bursa omentalis from acute pancreatitis)
Parasitic cyst: Echinococcus multilocularis, amebiasis
Pancreatic abscess
CONGENITAL (rare)
solitary true cyst
multiple true cysts (when associated with cystic disease of the liver/other organs):
Autosomal dominant polycystic kidney disease (hepatic cysts in 90% at autopsy)
 nearly always associated with renal cysts
nearly always associated with renal cysts
Von Hippel-Lindau disease (pancreatic cysts in 72% at autopsy; in only 25% on CT)
Cystic fibrosis
NEOPLASTIC
common cystic pancreatic neoplasms (5 15%):
Mucinous cystic neoplasm
 Most common cystic tumor of pancreas!
Most common cystic tumor of pancreas! Potentially malignant
Potentially malignant
Serous cystadenoma = microcystic adenoma
Intraductal papillary mucinous tumor (IPMT)
rare cystic pancreatic neoplasms:
Solid and papillary epithelioid neoplasm
Acinar cell cystadenocarcinoma
Retroperitoneal lymphangioma/hemangioma
Paraganglioma
solid pancreatic neoplasms with cystic degeneration:
Pancreatic adenocarcinoma
Cystic islet cell tumor (rare)
Cystic metastasis (3 12% at autopsy):
renal cell carcinoma, melanoma, lung tumors, breast carcinoma, hepatocellular carcinoma, ovarian carcinoma
Cystic teratoma
Pancreatic sarcoma (extremely rare)
Unilocular Pancreatic Cyst
 A cyst <3 cm is almost always benign + may be followed at 6-month intervals for 3 years!
A cyst <3 cm is almost always benign + may be followed at 6-month intervals for 3 years!
Pseudocyst
history of pancreatitis
Intraductal papillary mucinous neoplasm (IPMN)
 narrow neck at cyst-duct junction
narrow neck at cyst-duct junction
Unilocular serous cystadenoma
Lymphoepithelial cyst
Pancreatic Cyst with Solid Component
 All tumors are either malignant or have a high malignant potential!
All tumors are either malignant or have a high malignant potential!true cystic neoplasm
1. Mucinous cystic neoplasm
2. IPMN
cystically degenerated neoplasm
3. Islet cell tumor
4. Solid pseudopapillary tumor
5. Pancreatic adenocarcinoma
6. Metastasis
Macrocystic Lesion of Pancreas
= multilocular cyst, each compartment >2 cm in size
Mucinous cystic neoplasm
 in body + tail of pancreas
in body + tail of pancreas peripheral eggshell calcification
peripheral eggshell calcification
IPMN: side-branch/mixed
 septated cyst communicating with main duct
septated cyst communicating with main duct
Nonfunctioning neuroendocrine tumor
Congenital lymphangioma
Microcystic Lesion of Pancreas
= pancreatic lesion with >6 cysts each <2 cm in size
Serous cystadenoma
 fibrous central scar stellate pattern (30%)
fibrous central scar stellate pattern (30%) growth rate of 4 mm/year at follow-up
growth rate of 4 mm/year at follow-up
Hyperamylasemia
PANCREATIC
Acute/chronic pancreatitis
Pancreatic trauma
Pancreatic carcinoma
GASTROINTESTINAL
Perforated peptic ulcer
Intestinal obstruction
Peritonitis
Acute appendicitis
Afferent loop syndrome
Mesenteric ischemia/infarction
Portal vein thrombosis
TRAUMA
Burns
Cerebral trauma
Postoperative
OBSTETRICAL
Pregnancy
Ruptured ectopic pregnancy
RENAL
Transplantation
Renal insufficiency
METABOLIC
Diabetic ketoacidosis
Drugs
PNEUMONIA
SALIVARY GLAND LESION
P.680
Facial trauma
Mumps
Spleen
Nonvisualization of Spleen
Asplenia syndrome
Polysplenia syndrome
Traumatic fragmentation of spleen
Wandering spleen
Small Spleen
Infarction
Celiac disease
Congenital/hereditary hypoplasia
 Associated with recurrent bacterial infections!
Associated with recurrent bacterial infections!
Fanconi anemia
Irradiation
Partial splenectomy
Polysplenia syndrome
Atrophy
Splenomegaly
 inferior tip of spleen extends below tip of right lobe of liver
inferior tip of spleen extends below tip of right lobe of liver AP diameter of spleen >2/3 of abdominal diameter
AP diameter of spleen >2/3 of abdominal diameter
CONGESTIVE SPLENOMEGALY
heart failure, portal hypertension, cirrhosis, cystic fibrosis, portal/splenic vein thrombosis, acute splenic sequestration crisis of sickle cell anemia
NEOPLASM
leukemia, lymphoma, lymphoproliferative disease, Langerhans cell histiocytosis, metastases, primary neoplasm
STORAGE DISEASE
Gaucher disease, Niemann-Pick disease, mucopolysaccharidoses, gargoylism, amyloidosis, diabetes mellitus, hemochromatosis
INFECTION
bacterial: TB, subacute bacterial endocarditis, typhoid fever, syphilis, brucellosis
viral: hepatitis, infectious mononucleosis
protozoal: echinococcosis, malaria, kala azar, American leishmaniosis
fungal: histoplasmosis
HEMOLYTIC ANEMIA
hemoglobinopathy, hereditary spherocytosis, primary neutropenia, thrombotic thrombocytopenic purpura, extracorporeal membrane oxygenation (due to RBC damage)
EXTRAMEDULLARY HEMATOPOIESIS
osteopetrosis, myelofibrosis
COLLAGEN VASCULAR DISEASE
systemic lupus erythematosus, rheumatoid arthritis, Felty syndrome
SPLENIC TRAUMA
OTHERS
Sarcoidosis
 splenomegaly in up to 60%
splenomegaly in up to 60% inhomogeneous enhancement after bolus injection (multiple 2 3-cm hypodense nodular lesions)
inhomogeneous enhancement after bolus injection (multiple 2 3-cm hypodense nodular lesions) necrotic mass with focal calcifications
necrotic mass with focal calcifications
Hemodialysis
Autoimmune lymphoproliferative syndrome
Solid Splenic Lesion
MALIGNANT TUMOR
Lymphoma (Hodgkin disease, non-Hodgkin lymphoma, primary splenic lymphoma)
 Splenomegaly in non-Hodgkin lymphoma indicates involvement in most patients
Splenomegaly in non-Hodgkin lymphoma indicates involvement in most patients 30% of patients with splenomegaly have no involvement from non-Hodgkin lymphoma
30% of patients with splenomegaly have no involvement from non-Hodgkin lymphoma 30% of patients with lymphoma of any kind have splenic involvement without splenomegaly
30% of patients with lymphoma of any kind have splenic involvement without splenomegaly homogeneous splenomegaly (from diffuse infiltration)
homogeneous splenomegaly (from diffuse infiltration) miliary nodules
miliary nodules large 2 10-cm nodules (10 25%)
large 2 10-cm nodules (10 25%) nodes in splenic hilum (50%) in NHL; uncommon in Hodgkin disease
nodes in splenic hilum (50%) in NHL; uncommon in Hodgkin disease
Metastasis (7%)
melanoma (6 34%), breast carcinoma (12 21%), bronchogenic carcinoma (9 18%), colon carcinoma (4%), renal cell carcinoma (3%), ovary (8%), prostate (6%), stomach (7%), pancreas, endometrial cancer
Angiosarcoma
Malignant fibrous histiocytoma, leiomyosarcoma, fibrosarcoma
Langerhans cell histiocytosis
 splenomegaly
splenomegaly multiple hypoechoic nodules (less often)
multiple hypoechoic nodules (less often)
BENIGN TUMOR
Hamartoma = splenoma
Hemangioma
Hematopoietic
Sarcoidosis
 nodular lesions in liver and spleen in 5 c15%
nodular lesions in liver and spleen in 5 c15%(= coalescent granulomata) occurring within 5 years of diagnosis
 hepatosplenomegaly
hepatosplenomegaly abdominal adenopathy (mean size of 2.6 cm)
abdominal adenopathy (mean size of 2.6 cm)
Gaucher disease (islands of RES cells laden with glucosylceramide)
Inflammatory pseudotumor
Lymphangioma
SPLENIC INFARCTION
Cystic Splenic Lesion
CONGENITAL
Epidermoid cyst = true cyst = congenital cyst
VASCULAR
Splenic laceration/fracture
Hematoma
false cyst = posttraumatic cyst = nonpancreatic pseudocyst of the spleen
80% of all splenic cysts are pseudocysts
(= secondary cysts)
Cause: cystic end stage of trauma, infection, infarction  internal echoes from debris
internal echoes from debris calcifications within cyst wall may resemble eggshell
calcifications within cyst wall may resemble eggshell smaller size than true cyst
smaller size than true cyst
P.681
Cystic degeneration of infarct
occlusion of splenic a./branches (hemolytic anemia, endocarditis, SLE, arteritides, pancreatic cancer)
venous thrombosis of splenic sinusoids (massive splenomegaly)
Peliosis
INFECTION/INFLAMMATION
Pyogenic abscess
Prevalence: 0.1 0.7% Cause: hematogenous spread in sepsis (75%), penetrating trauma (15%), infarction (10%) Predisposed: endocarditis, drug abuse, penetrating trauma, neoplasm, sickle cell disease fever, chills, LUQ pain (in <50%)
 irregular borders without capsule
irregular borders without capsule gas bubbles within abscess
gas bubbles within abscess rim enhancement
rim enhancement
Rx: 76% success rate for percutaneous drain Microabscesses
Organism: fungus (especially Candida, Aspergillus, Cryptococcus) Prevalence: 26% of splenic abscesses Predisposed: immunocompromised patient  hepatosplenomegaly
hepatosplenomegaly multiple round hypoechoic/hypoattenuating target lesions of 5 10 mm often associated with hepatic + renal involvement
multiple round hypoechoic/hypoattenuating target lesions of 5 10 mm often associated with hepatic + renal involvement wheel-in-wheel appearance when central hyperechoic portion becomes necrotic + hypoechoic
wheel-in-wheel appearance when central hyperechoic portion becomes necrotic + hypoechoic
Granulomatous infection
Mycobacterium tuberculosis: miliary TB
 mild splenomegaly uncommon
mild splenomegaly uncommon
M. avium-intracellulare
 marked splenomegaly in 20%
marked splenomegaly in 20%
Pneumocystis carinii infection
 splenomegaly + multiple hypoattenuating foci
splenomegaly + multiple hypoattenuating foci
Parasitic cyst (Echinococcus)
Prevalence: in <2% of patients with hydatid disease Cause: systemic dissemination, intraperitoneal spread of ruptured liver cyst  solitary cyst subjacent daughter cysts
solitary cyst subjacent daughter cysts hydatid sand infolded membranes
hydatid sand infolded membranes linear calcification
linear calcification
Intrasplenic pancreatic pseudocyst
Prevalence: in 1 5% of patients with pancreatitis
CYSTIC NEOPLASM
Cavernous hemangioma
 Most common primary neoplasm of the spleen!
Most common primary neoplasm of the spleen! hyperdense lesion
hyperdense lesion
Lymphoma (most common malignant neoplasm!)
 splenomegaly
splenomegaly multiple small/large masses
multiple small/large masses
Lymphangioma/lymphangiomatosis
 multiple septated subcapsular cystic lesions
multiple septated subcapsular cystic lesions
Necrotic metastasis:
malignant melanoma (in 50%); breast, lung, ovarian, pancreatic, endometrial, colonic, prostatic, carcinoma; chondrosarcoma
 In 7% of patients with widespread metastasis!
In 7% of patients with widespread metastasis!
TRUE CYST (with epithelial lining)
Congenital cyst = epidermoid cyst
Parasitic cyst
FALSE CYST = PSEUDOCYST (lacking epithelial lining)
Traumatic cyst
Postinfarct cyst
Solitary Splenic Lesion
| mnemonic: | L'CHAIM |
Lymphoma
Cyst
Hematoma, Hemangioma, Hamartoma
Abscess
Infarct
Metastasis
Multiple Splenic Nodules and Masses
Lymphoma, leukemia
Metastases
Inflammatory lesions
Benign tumors
Splenic cysts
Splenic infarcts
Gaucher cells
Increased Splenic Density
Sickle cell anemia (in 5% of sicklers)
Hemochromatosis
Thorotrast exposure
Lymphangiography
Splenic Calcification
DISSEMINATED
Phlebolith: visceral angiomatosis
Granuloma (most common): histoplasmosis, TB, brucellosis
CAPSULAR & PARENCHYMAL
Pyogenic/tuberculous abscess
Pneumocystis carinii infection
Infarction (multiple)
Hematoma
VASCULAR
Splenic artery calcification
Splenic artery aneurysm
Splenic infarct
Autosplenectomy
CALCIFIED CYST WALL
Congenital cyst
Posttraumatic cyst
Echinococcal cyst
Cystic dermoid
Epidermoid
P.682
| mnemonic: | HITCH |
Histoplasmosis (most common)
Infarct (sickle cell disease)
Tuberculosis
Cyst (Echinococcus)
Hematoma
Iron Accumulation in Spleen
DIFFUSE
Multiple blood transfusions
Sickle cell anemia
FOCAL
Gamna Gandy bodies
Angiosarcoma
Hyperechoic Splenic Spots
Granulomas: miliary tuberculosis, histoplasmosis
Phleboliths
Lymphoma/leukemia
Myelofibrosis
Gamna-Gandy nodules (in portal hypertension)
Spontaneous Splenic Rupture
Posttraumatic delayed rupture
Splenomegaly
Hemangioma
Epidermoid cyst
Peliosis
Previous splenic infarction
P.683
Anatomy of Liver, Bile Ducts, Pancreas, and Spleen
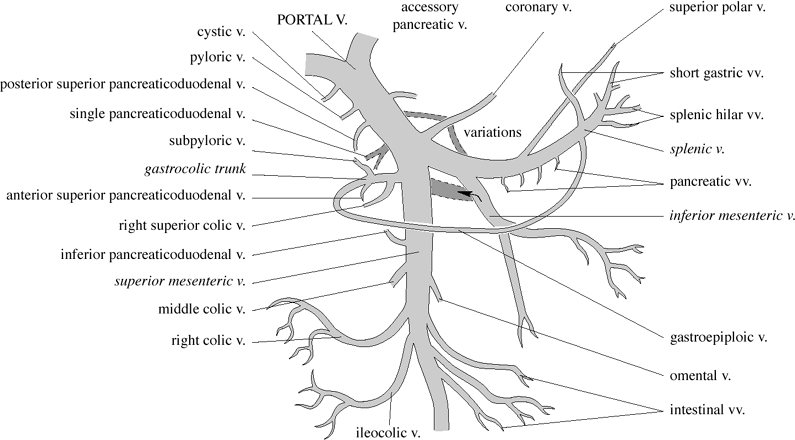 |
| Extrahepatic Portal Vein Tributaries |
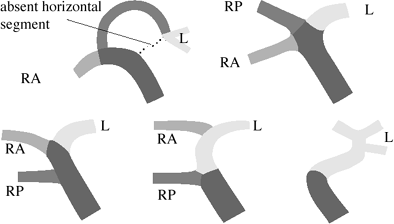 |
| Variations of Intrahepatic Portal Venous System |
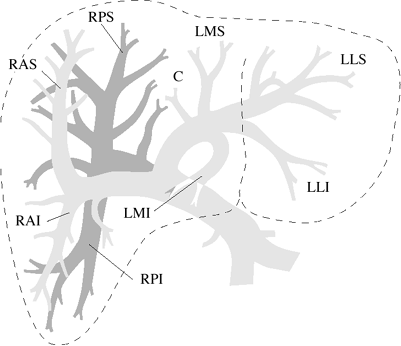 |
| Intrahepatic Portal Vein Branches |
P.684
 |
 |
| Level of Hepatic Vein Junction |
 |
| Level of Left Portal Vein |
 |
| Level of Right Portal Vein |
 |
| Level of Splenic Vein |
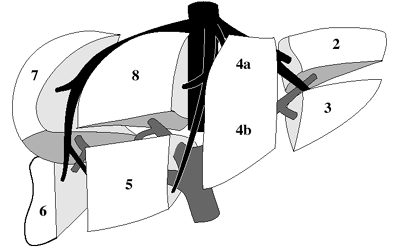
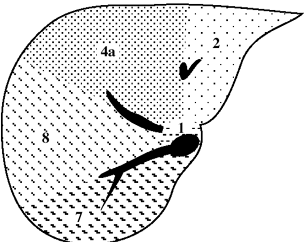
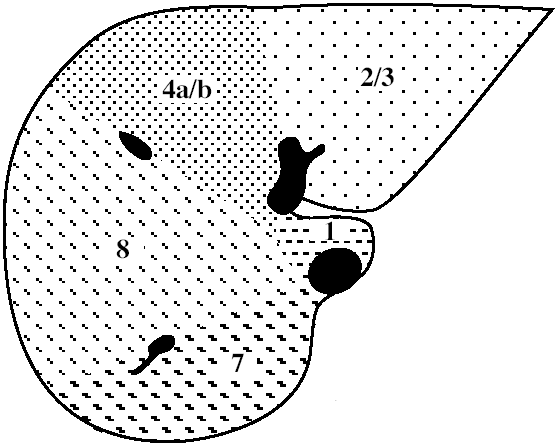
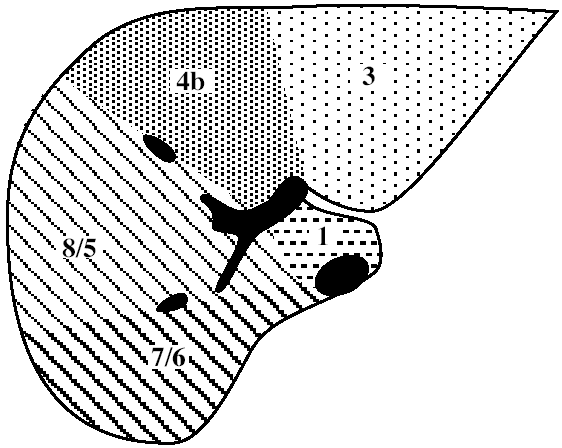
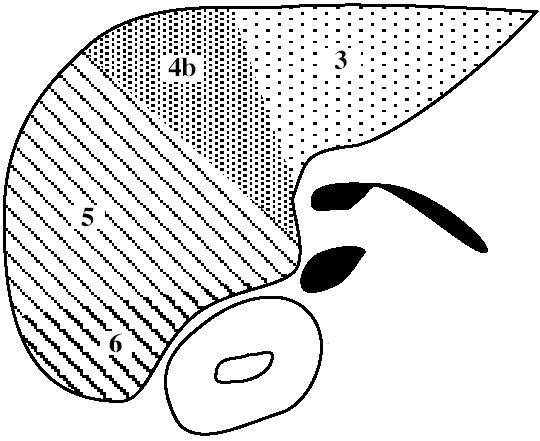
| Functional Segmental Live Anatomy | |||
|---|---|---|---|
| Goldsmith & Woodburne | Couinaud & Bismuth | ||
| CAUDATE LOBE | Caudate lobe | 1 | |
| LEFT LOBE | Left lateral segment | Left lateral superior subsegment | 2 |
| Left lateral inferior subsegment | 3 | ||
| Left medial segment | Left medial superior subsegment | 4a | |
| Left medial inferior subsegment | 4b | ||
| RIGHT LOBE | Right anterior segment | Right anterior inferior subsegment | 5 |
| Right anterior superior subsegment | 8 | ||
| Right posterior segment | Right posterior inferior subsegment | 6 | |
| Right posterior superior subsegment | 7 | ||
P.685
 |
| Michels Classification of Hepatic Arterial Anatomy |
Liver
Functional Segmental Liver Anatomy
based on distribution of 3 major hepatic veins:
middle hepatic vein
divides liver into right and left lobe. also separated by main portal vein scissura (Cantlie line) passing through IVC + long axis of gallbladder)
left hepatic vein
divides left lobe into medial + lateral sectors
right hepatic vein
divides right lobe into anterior + posterior sectors
Each of the four sections is further divided:
by an imaginary transverse line drawn through the right + left portal vein into anterior + posterior segments; the segments are numbered counterclockwise from IVC
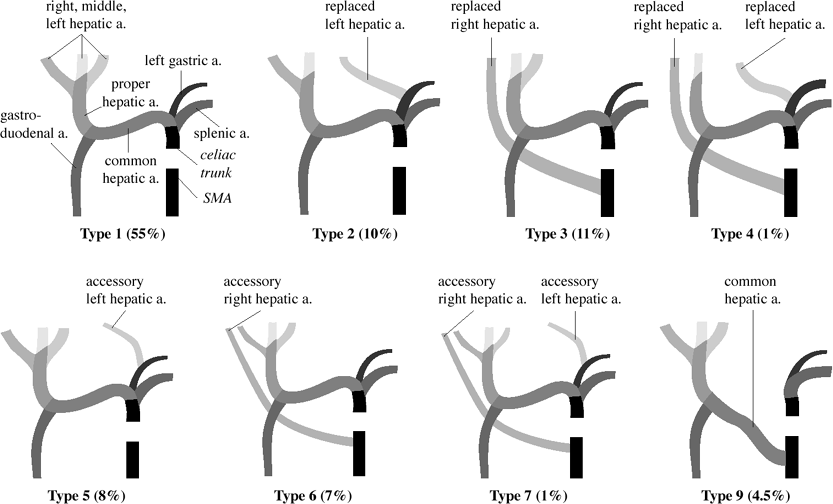
Hepatic Arterial Anatomy (Michels classification)
Type 1 (55%):
celiac trunk trifurcates into L gastric a. + splenic a. + common hepatic a.
common hepatic a. divides into gastroduodenal a. + proper hepatic a.
RT hepatic a. + LT hepatic a. arise from proper hepatic a.
middle hepatic a. (supplying caudate lobe) arises from:
L/R hepatic a.
proper hepatic a. (in 10%)
Type 2 (10%):
common hepatic a. divides into gastroduodenal + R hepatic a.
L hepatic a. replaced to L gastric a.
middle hepatic a. from R hepatic a.
Type 3 (11%):
common hepatic a. divides into gastroduodenal + L hepatic a.
R hepatic a. replaced to superior mesenteric a.
middle hepatic a. from L hepatic a.
Type 4 (1%):
common hepatic a. divides into middle hepatic a. + gastroduodenal a.
R hepatic a. + L hepatic a. are both replaced
Type 5 (8%):
accessory L hepatic a. arises from L gastric a.
Type 6 (7%):
accessory R hepatic a. arises from SMA
Type 7 (1%):
accessory R + L hepatic a.
Type 8 (2%):
combinations of accessory + replaced hepatic aa.
Type 9 (4.5%):
hepatic trunk replaced to superior mesenteric a.
Type 10 (0.5%):
hepatic trunk replaced to L gastric a.
Aberrant Hepatic Artery
= hepatic artery coursing between IVC + portal vein
P.686
Replaced right hepatic artery (50%)
Right hepatic artery with early bifurcation of common hepatic artery into right + left hepatic arteries (20%)
Accessory right hepatic artery (15%)
Replacement of entire hepatic trunk to SMA (15%)
Third Inflow to Liver
= aberrant veins supplying small areas of liver tissue + communicating with intrahepatic portal vein branches
| Effect: | focal decrease of portal vein perfusion resulting in areas of fat-sparing/fat accumulation |
Cholecystic veins
directly entering liver segments 4 + 5
veins joining the parabiliary veins via triangle of Calot
Parabiliary venous system
= venous network within hepatoduodenal ligament anterior to main portal vein
Tributaries:
cholecystic vein through triangle of Calot
pancreaticoduodenal vein
right gastric/pyloric vein
 pseudolesion at dorsal aspect of segment 4
pseudolesion at dorsal aspect of segment 4
Epigastric-paraumbilical venous system
= small veins around falciform ligament draining anterior part of abdominal wall directly into liver
Subgroups:
superior vein of Sappey
drains upper portion of falciform ligament + medial part of diaphragm
enters peripheral left portal vein branches
communicates with superior epigastric + internal thoracic veins
inferior vein of Sappey
drains lower portion of falciform ligament
enters peripheral left portal vein branches
communicates with branches of inferior epigastric vein around the umbilicus
vein of Burow
terminates in middle portion of collapsed umbilical vein
communicates with branches of inferior epigastric vein around the umbilicus
intercalary veins
interconnect vein of Burow + inferior vein of Sappey
Hepatic Fissures
Fissure for ligamentum teres = umbilical fissure
= invagination of ligamentum teres = embryologic remnant of obliterated umbilical vein connecting placental venous blood with left portal vein
located at dorsal free margin of falciform ligament
runs into liver with visceral peritoneum
divides left hepatic lobe into medial + lateral segments (divides subsegment 3 from 4)
Fissure for ligamentum venosum
= invagination of obliterated ductus venosus
= embryologic connection of left portal vein with left hepatic vein
separates caudate lobe from left lobe of liver
lesser omentum within fissure separates the greater sac anteriorly from lesser sac posteriorly
Fissure for gallbladder (GB)
= shallow peritoneal invagination containing the GB
divides right from left lobe of liver
Transverse fissure
= invagination of hepatic pedicle into liver
contains horizontal portion of left + right portal veins
Accessory fissures
Right inferior accessory fissure = from gallbladder fossa/just inferior to it to lateroinferior margin of liver
Others (rare)
Size of Liver
YOUNG INFANT
right hepatic lobe should not extend >1 cm below right costal margin
CHILD
right hepatic lobe should not extend below right costal margin
ADULT
midclavicular line (vertical/craniocaudad axis):
<13 cm = normal 13.0 15.5 cm = indeterminate (in 25%) >15.5 cm = hepatomegaly (87% accuracy) preaortic line <10 cm
prerenal line <14 cm
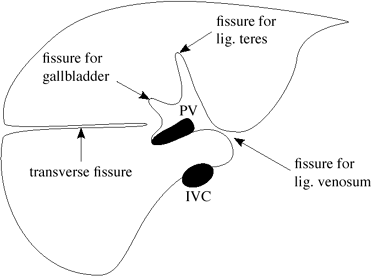 |
| Hepatic Fissures |
Liver Echogenicity & Attenuation
| US: | pancreatic > splenic hepatic > renal echogenicity |
| CT: | 40 70 HU (precontrast) |
| CECT: | early arterial phase (20 sec), late arterial phase (30 40 sec), portal venous phase (60 70 sec); maximal enhancement at 45 60 sec |
Maximum Cross-sectional Diameter of Portal Vein
| (a) child <10 years of age: | 8.5 mm |
| (b) 10 20 years of age: | 10.0 mm |
| (c) adult: | 13.0 mm |
P.687
Normal Hemodynamics Parameter of Liver
| Portal vein velocity: | >11 cm/sec |
| Congestion index (= cross-sectional area of portal vein divided by average velocity): | 0.070 0.09 |
| Hepatic artery resistive index: | 0.60 0.64 0.06 |
Liver Tests
Alkaline phosphatase (AP)
Formation: bone, liver, intestine, placenta High increase:
cholestasis with extrahepatic biliary obstruction (confirmed by rise in GT), drugs, granulomatous disease (sarcoidosis), primary biliary cirrhosis, primary + secondary malignancy of liver
Mild increase: all forms of liver disease, heart failure Gamma-glutamyl transpeptidase ( GT)
very sensitive in almost all forms of liver disease
Utility: confirms hepatic source of elevated AP, may indicate significant alcohol use Transaminases
high increase: viral/toxin-induced acute hepatitis aspartate aminotransferase (AST; formerly serum glutamic oxaloacetic transaminase [SGOT])
Formation: liver, muscle, kidney, pancreas, RBCs alanine aminotransferase (ALT; formerly serum glutamic pyruvic transaminase [SGPT])
Formation: primarily in liver
rather specific elevation in liver disease
Bilirubin
helps differentiate between various causes of jaundice
(a) unconjugated/indirect bilirubin = insoluble in water
Formation: breakdown of senescent RBCs Metabolism: tightly bound to albumin in vessels, actively taken up by liver, cannot be excreted by kidneys (b) conjugated/direct bilirubin = water-soluble
Formation: conjugation in liver cells Metabolism: excretion into bile; not reabsorbed by intestinal mucosa + excreted in feces Elevation:
overproduction: hemolytic anemia, resorption of hematoma, multiple transfusions
decreased hepatic uptake: drugs, sepsis
decreased conjugation: Gilbert syndrome, neonatal jaundice, hepatitis, cirrhosis, sepsis
decreased excretion into bile: hepatitis, cirrhosis, drug-induced cholestasis, sepsis, extrahepatic biliary obstruction
Lactic dehydrogenase (LDH)
nonspecific and therefore not helpful
high increase: primary or metastatic liver involvement Alpha fetoprotein (AFP)
>400 ng/mL strongly suggests that focal mass represents a hepatocellular carcinoma
Bile ducts
Normal Size of Bile Ducts
@ CBD at point of maximum diameter = free edge of gastrohepatic ligament (point of least constraint):
adolescents & adults
5 mm = normal; 6 7 mm = equivocal; 8 mm = dilated
 In patient >60 years of age add 1 mm/decade
In patient >60 years of age add 1 mm/decade Following cholecystectomy up to 8 mm
Following cholecystectomy up to 8 mm
neonates: <1 mm
infants up to 1 year of age: <2 mm
older children: <4 mm
@ CHD at porta hepatis + CBD in head of pancreas: 5 mm
@ right intrahepatic bile duct just proximal to CHD: 2 3 mm/<40% of diameter of accompanying portal vein
@ Cystic duct
Valves of Heister = normal mucosal folds
Diameter: 1.8 mm Average length: 1 2 cm distal cystic duct posterior to CBD (in 95%), anterior to CBD (in 5%)
Bile Duct Variants
| Prevalence: | 2.4% of autopsies; 13 18.5% of operative cholangiograms |
| Significance: | aberrant ducts near cystic duct /gallbladder have the greatest risk of iatrogenic injury at cholecystectomy |
| Cx: | (1) postoperative bile leak if severed (2) segmental biliary obstruction if ligated |
ABERRANT INTRAHEPATIC DUCT
may join CHD, CBD, cystic duct, right hepatic duct, gallbladder
major right segmental bile duct joins extrahepatic bile duct at/near cystic duct insertion (4 5%)
cysticohepatic duct (1 2%) = anomalous right hepatic duct inserts into cystic duct
anomalous left hepatic ducts: not susceptible to injury + therefore of no clinical significance
CYSTIC DUCT ENTERING RIGHT HEPATIC DUCT
DUCTS OF LUSCHKA
= small ducts from hepatic bed draining directly into gallbladder
DUPLICATION OF CYSTIC DUCT/CBD
duplication of gallbladder
CONGENITAL tracheobiliary FISTULA
= fistulous communication between carina and left hepatic duct
infants with respiratory distress
productive cough with bilious sputum
 pneumobilia
pneumobilia
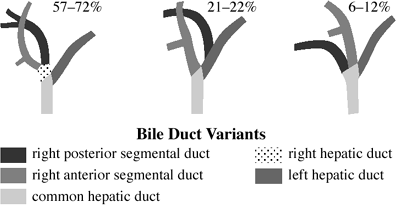 |
| Bile Duct Variants |
P.688
Variants of Cystic Duct Insertion
| Prevalence: | variations occur in 18 23% |
Craniocaudad direction:
proximal third = common hepatic duct high in porta hepatis
middle third of extrahepatic bile duct in 75%
distal third of extrahepatic bile duct in 10%
 cystic duct parallels extrahepatic bile duct (implies common fibrous sheath)
cystic duct parallels extrahepatic bile duct (implies common fibrous sheath)
Cx: during cholecystectomy
(1) common hepatic duct stricture
(2) inadvertent ligation/transection of extrahepatic bile duct
(3) long cystic duct remnant
Mediolateral direction:
right lateral
anterior spiral
posterior spiral
low lateral (with common sheath)
low medial (at/near ampulla of Vater)
Insertion into intrahepatic bile duct
right hepatic duct (0.3%)
left hepatic duct (rare)
absence of cystic duct
 gallbladder drains directly into common bile duct
gallbladder drains directly into common bile duct
Gallbladder
Size & Capacity & Wall Thickness
Length:
| (a) infant < 1 year old: | 1.5 3 cm in length |
| (b) older child: | 3 7 cm in length |
| (c) adult: | 7-10 cm in length; 2-3.5 cm in width |
| Capacity: | 30-50 mL |
| Wall thickness: | 2-3 mm |
| Bile volume: | 250-1,000 mL/day secreted by hepatocytes |
| GB function: | concentration of bile through absorption of 90% of water |
Congenital Gallbladder Anomalies
Agenesis of Gallbladder
| Incidence: | 0.04-0.07% (autopsy) |
Associated with:
common: rectovaginal fistula, imperforate anus, hypoplasia of scapula + radius, intracardiac shunt
rare: absence of corpus callosum, microcephaly, atresia of external auditory canal, tricuspid atresia, TE fistula, dextroposition of pancreas + esophagus, absent spleen, high position of cecum, polycystic kidney
Hypoplastic Gallbladder
congenital
associated with cystic fibrosis
Septations of Gallbladder
LONGITUDINAL SEPTA
Duplication of gallbladder
= two separate lumens + two cystic ducts
Incidence: 1:3,000-1:12,000 Bifid gallbladder = double gallbladder
= two separate lumens with one cystic duct
Triple gallbladder (extremely rare)
TRANSVERSE SEPTA
Isolated transverse septum
phrygian cap (2-6% of population)
= kinking/folding of fundus septum
Multiseptated gallbladder (rare)
= multiple cystlike compartments connected by small pores
Cx: stasis + stone formation
GALLBLADDER DIVERTICULUM
= persistence of cystohepatic duct
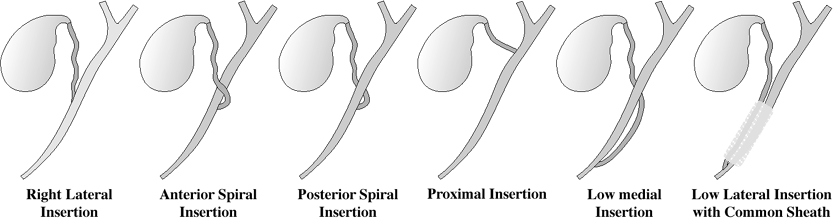 |
| Anatomic Variants of Cystic Duct Insertion |
Gallbladder Ectopia
Most frequent locations:
(1) beneath the left lobe of the liver > (2) intrahepatic > (3) retrohepatic
Rare locations:
(4) within falciform ligament, (5) within interlobar fissure, (6) suprahepatic (lodged between superior surface of right hepatic lobe + anterior chest wall), (7) within anterior abdominal wall, (8) transverse mesocolon, (9) retrorenal, (10) near posterior spine + IVC, (11) intrathoracic GB (inversion of liver)
Associated with: eventration of diaphragm P.689
Floating GB
= gallbladder with loose peritoneal reflections, may herniate through foramen of Winslow into lesser sac
Torqued GB
= results in hydrops
Pancreas
Size
| pancreatic head: | 1.0 2.2 cm |
| pancreatic body: 0.4 1.0 cm | |
| pancreatic tail: 0.8 1.8 cm |
Physiology of Pancreas
pancreatic islet cells = endocrine cells (1 2% of mass of pancreas) clustered in islets of Langerhans;
receive 10 15% of pancreatic blood flow
| Function: | secretion of |
insulin in b-cells; most abundant in center of islet
glucagon in a-cells
somatostatin in d-cells
VIP in d1-cells
serotonin in enterochromaffin cells
pancreatic polypeptide in PP cells (stimulate secretion of gastric and intestinal enzymes + inhibit intestinal motility)
Pancreatic Development & Anatomy
during the 4th week of gestation 2 endodermal diverti-cula form in the foregut near its junction with the yolk sac
dorsal diverticulum forms dorsal pancreas
ventral diverticulum forms liver, gallbladder, bile ducts, ventral pancreas
DORSAL ANLAGE (in mesoduodenum)
Origin: arises from dorsal wall of duodenum + is later displaced to the left  Forms cranial portion of head + isthmus + body + tail of pancreas
Forms cranial portion of head + isthmus + body + tail of pancreasprone to atrophy (poor in polypeptides)
 drains to the minor papilla through accessory duct of Santorini
drains to the minor papilla through accessory duct of Santorini
VENTRAL ANLAGE (below primordial liver bud)
Origin: ventral bud arises from ventral wall of duodenum and is composed of right + left lobes (the left ventral bud regresses completely), rotates dorsally and inferiorly + then to the left of the duodenum + fuses with dorsal anlage during 6 7th week GA  Forms caudal portion of the pancreatic head + uncinate process + CBD
Forms caudal portion of the pancreatic head + uncinate process + CBDnot prone to atrophy (rich in polypeptides)
 ventral duct drains with CBD through ampulla of Vater + becomes the major drainage pathway for the entire pancreas after fusion with dorsal duct
ventral duct drains with CBD through ampulla of Vater + becomes the major drainage pathway for the entire pancreas after fusion with dorsal duct
MAIN PANCREATIC DUCT OF WIRSUNG
[Johann Wirs ng (1589 1643), German physician in Padua, Italy]
distal portion of dorsal duct connects with ventral duct; proximal portion of dorsal duct may disappear
 measures 1 2 3 mm in diameter
measures 1 2 3 mm in diameter receives 20 35 tributaries/side branches that enter at right angles
receives 20 35 tributaries/side branches that enter at right angles usually drains through major papilla
usually drains through major papilla Major drainage route in 91% of individuals
Major drainage route in 91% of individuals
ACCESSORY PANCREATIC DUCT OF SANTORINI
[Giovanni Santorini (1681 1737), anatomist in Venice, Italy]
= proximal portion of dorsal duct, which has not atrophied
 Present in 44% of individuals
Present in 44% of individuals
AMPULLA OF VATER
= space within medial wall of second portion of duodenum below surface of papilla of Vater
MAJOR DUODENAL PAPILLA = papilla of Vater
 Drainage of common bile duct in 100%
Drainage of common bile duct in 100% Drainage of main pancreatic duct of Wirsung in 90%
Drainage of main pancreatic duct of Wirsung in 90%
MINOR DUODENAL PAPILLA (present in 60%)
 Drainage of accessory pancreatic duct of Santorini
Drainage of accessory pancreatic duct of Santorini Drainage of main pancreatic duct in 10%
Drainage of main pancreatic duct in 10% located a few cm orad to papilla of Vater
located a few cm orad to papilla of Vater
 |
| Pancreatic Duct Diameters |
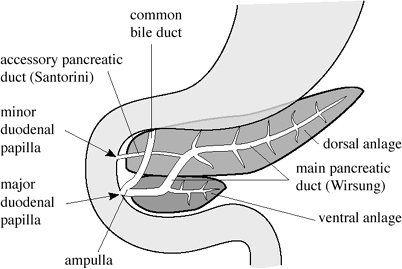 |
| Embryologic Development of Pancreas |
Pancreaticobiliary Junction Variants
Angle between CBD + pancreatic duct:
usually acute at 5 30
occasionally abnormal at up to 90
Sphincter of Oddi = sphincter of hepaticopancreatic ampulla
[Ruggero Oddi (1864 1913), physiologist in Genoa, Italy]
= muscle fibers encircling the CBD + pancreatic duct at choledochoduodenal junction
(a) choledochal sphincter (Boyden) = encircles distal CBD
(b) pancreatic duct sphincter (in 33% separate)
(c) ampullary sphincter
Types of union between CBD + pancreatic duct:
Normal junction = union inside duodenal wall
2 10 (mean 5) mm short common channel (55 85%) with a diameter of 3 5 mm
separate entrances into duodenum (42%)
8 15 mm long common channel
Anomalous junction = union outside duodenal wall beyond the influence of the sphincter of Boyden (1.5 3.2%)
pancreatic duct inserting into CBD >15 mm from entrance into duodenum
CBD inserting into pancreatic duct
P.690
 |
| Normal Union between CBD & Pancreatic Duct |
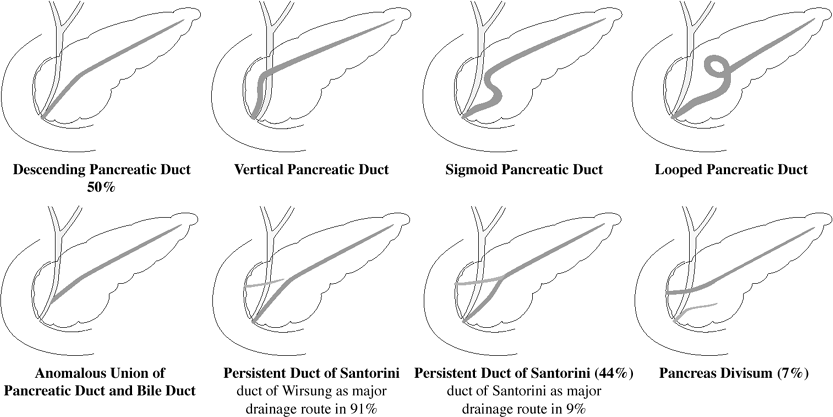 |
| Variations in Pancreatic Duct Anatomy |
Spleen
Size of Spleen
| in adults: | 12 cm length, 7 8 cm anteroposterior diameter, 3 4 cm thick; splenic index (L W H) of <480 |
| in children: | logarithmic increase in length with increasing age; formula for length = 5.7 + 0.31 age (in years) |
| in infants (0 3 months of age): | <6.0 cm in length |
Weight of Spleen
| at birth: | 15 g |
| in adults: | 150 (100 265) g |
estimated weight = splenic index 0.55
Embryology of Spleen
spleen arises from mesenchymal cells between layers of dorsal mesogastrium during 5th week GA
splenic primordium differentiates to form capsule, connective tissue framework, splenic parenchyma
major site of hematopoiesis until 28 weeks GA; retains capacity for extramedullary hematopoiesis well into adult life
 spleen recognizable by 12th week GA (as fusion of mesenchymal aggregates occurs)
spleen recognizable by 12th week GA (as fusion of mesenchymal aggregates occurs) splenic clefts/notches/lobules may persist
splenic clefts/notches/lobules may persist accessory spleen (in up to 30% by autopsy)
accessory spleen (in up to 30% by autopsy)
P.691
Histology of Spleen
RED PULP = numerous vascular sinuses
WHITE PULP = lymphoid follicles + cells of RES
| Development: | ratio of white to red pulp increases with age + progressive antigenic stimulation |
Imaging Characteristics of Spleen
CT ATTENUATION
without enhancement:
40 60 HU; 5 10 HU less than liver
with enhancement:
normal heterogeneous enhancement during first minute after bolus injection (due to different blood flow rates through the cords of the red + white pulp)
 arciform (alternating bands of high + low attenuation)/focal/diffuse heterogeneity
arciform (alternating bands of high + low attenuation)/focal/diffuse heterogeneity heterogeneity resolved in portal venous phase
heterogeneity resolved in portal venous phase
MR SIGNAL INTENSITY
directly related to ratio of white to red pulp
(a) neonate <8 months of age:
 T1WI- and T2WI-intensity: spleen < liver (due to predominance of red pulp)
T1WI- and T2WI-intensity: spleen < liver (due to predominance of red pulp)
DDx: hemochromatosis (b) adult + older child:
 T2WI-intensity: spleen > liver
T2WI-intensity: spleen > liver T1WI-intensity: liver > spleen > muscle
T1WI-intensity: liver > spleen > muscle
Iron metabolism
| Total body iron: | 5 g |
functional iron: 1g
Location: hemoglobin of RBCs, myoglobin of muscle, various enzymes stored iron: 1g
Location: hepatocytes, reticuloendothelial cells of liver (Kupffer cells) + spleen + bone marrow
| Absorption: | 1-2 mg/day through gut |
| Transport: | bound to transferrin intravascularly |
Deposition:
transferrin-transfer to:
hepatocytes, RBC precursors in erythron, parenchymal tissues (eg, muscle)
phagocytosis by:
reticuloendothelial cells phagocytize senescent erythrocytes (= extravascular hemolysis); RBC iron stored as ferritin/released and bound to transferrin
 |
| Extraperitoneal Spaces |
P.692
Disorders of Liver, Biliary Tract, Pancreas, and Spleen
Accessory Spleen
= failure of coalescence of several small mesodermal buds in the dorsal mesogastrium that comprise the spleen
| Incidence: | 10 30% of population; multiple (up to 6) in 10% |
 Undergoes hypertrophy after splenectomy and is responsible for recurrence of hematologic disorders (idiopathic thrombocytopenic purpura, hereditary spherocytosis, acquired autoimmune hemolytic anemia, hypersplenism)
Undergoes hypertrophy after splenectomy and is responsible for recurrence of hematologic disorders (idiopathic thrombocytopenic purpura, hereditary spherocytosis, acquired autoimmune hemolytic anemia, hypersplenism)Location:
near splenic hilum along the course of splenic vessels (most common)
within layers of omentum (gastrosplenic ligament, other suspensory ligaments of spleen)
anywhere in abdomen (eg, pancreas, pelvis)
attached to left ovary/testis = splenogonadal fusion (due to close relationship between developing spleen + mesonephros + left gonadal anlage)
NUC (Tc-99m sulfur colloid scan/spleen-specific Tc-99m denatured RBCs):
 usually <1 cm in diameter
usually <1 cm in diameter <10% identified when normal spleen present
<10% identified when normal spleen present
| Cx: | disease recurrence due to hypertrophy of accessory spleen after splenectomy for hypersplenism |
Ampullary Tumor
= benign/malignant tumors arising from glandular epithelium of ampulla of Vater
| Age: | 6th + 7th decade; M:F = 2:1 |
| Path: | average diameter of <3 cm |
| Histo: | (a) dysplastic epithelium in glandular/villous structures of tubular/villous adenoma (b) carcinoma in situ (c) invasive carcinoma often with desmoplastic reaction |
| Associated with: | familial adenomatous polyposis syndromes (eg, familial polyposis coli, Gardner syndrome) [100 200-fold risk], colon carcinoma |
malaise, epigastric pain, weight loss
intestinal bleeding (tumor ulceration)
intermittent jaundice (ductal obstruction)
gray aluminum/silver-colored stools (3%)
chills, fever, RUQ pain (ascending cholangitis) in up to 20%
endoscopy: tumor extending through orifice (63%), prominent papilla/submucosal mass (25%), not visualized (9%)
TNM staging:
| T1: | tumor confined to ampulla |
| T2: | tumor extending into duodenal wall |
| T3: | invasion of pancreas <2 cm deep |
| T4: | invasion of pancreas >2 cm deep |
International Union Against Cancer Staging:
| I = | tumor confined to ampulla |
| II = | tumor extension into duodenal wall/pancreas |
| III = | regional lymph node involvement (Lnn stations around head + body of pancreas, anterior + posterior pancreaticoduodenal, pyloric, common bile duct, proximal mesenteric) |
| IV = | invasion of pancreas >2 cm deep |
 tumor often inapparent due to small size
tumor often inapparent due to small sizeUGI:
 indentation of duodenal lumen at papilla of Vater with filling defect >1.5 cm
indentation of duodenal lumen at papilla of Vater with filling defect >1.5 cm surface irregularity + deep barium-filled crevices in villous tumor
surface irregularity + deep barium-filled crevices in villous tumor
Biliary imaging:
 dilatation of most distal segment of common bile duct
dilatation of most distal segment of common bile duct stenosis (circumferential tumor growth around ampulla/desmoplastic reaction)
stenosis (circumferential tumor growth around ampulla/desmoplastic reaction) irregular predominantly polypoid filling defect
irregular predominantly polypoid filling defect pancreatic dilatation = double-duct sign (may be absent if tumor small/accessory pancreatic duct decompresses pancreatic system/main pancreatic duct drains into minor papilla)
pancreatic dilatation = double-duct sign (may be absent if tumor small/accessory pancreatic duct decompresses pancreatic system/main pancreatic duct drains into minor papilla)
Endoscopic US (most sensitive technique):
87% staging accuracy
| Rx: | Whipple procedure (= pancreaticoduodenectomy) |
| Prognosis: | 28 70% 5-year survival for ampullary carcinomas (depending on stage) |
| DDx: |
|
Annular Pancreas
= second most common congenital anomaly wherein a ring of normal pancreatic tissue encircles the duodenum secondary to abnormal migration of ventral pancreas (head + uncinate)
| Incidence: | 1:20,000 autopsies |
| Age at discovery: | childhood (52%); adulthood (48%) |
| Associated with: | other congenital anomalies (in 75%): esophageal atresia, TE fistula, duodenal atresia/stenosis, duodenal diaphragm, imperforate anus, malrotation, Down syndrome |
| Location: | 2nd portion of duodenum (85%); 1st/3rd portion of duodenum (15%) |
mostly asymptomatic with incidental discovery
| neonate: | persistent vomiting (duodenal obstruction) |
| adult: | nausea, vomiting (60%), abdominal pain (70%), hematemesis (10%), jaundice (50%) |
 polyhydramnios (in utero)
polyhydramnios (in utero) double bubble = dilated duodenal bulb + stomach
double bubble = dilated duodenal bulb + stomach proximal duodenal dilatation
proximal duodenal dilatation enlargement of pancreatic head
enlargement of pancreatic headUGI:
 eccentric narrowing with lateral notching + medial retraction of 2nd part of duodenum
eccentric narrowing with lateral notching + medial retraction of 2nd part of duodenum concentric narrowing of mid-descending duodenum
concentric narrowing of mid-descending duodenum reverse peristalsis, pyloric incompetency
reverse peristalsis, pyloric incompetency
CT:
 pancreatic tissue surrounding descending duodenum
pancreatic tissue surrounding descending duodenum
ERCP (most specific)/MR pancreatography:
 normally located main duct in pancreatic body + tail
normally located main duct in pancreatic body + tail small duct originating on anterior left + passing posteriorly around duodenum communicates with main duct (in 85%)
small duct originating on anterior left + passing posteriorly around duodenum communicates with main duct (in 85%)
P.693
| Cx: | increased incidence of
|
| Rx: | gastrojejunostomy/duodenojejunostomy |
Ascariasis
Most frequent helminthic infection in humans
| Organism: | Ascaris lumbricoides, 25 35 cm long as adult worm; life span of 1 year |
| Country: | 644 million humans harbor the roundworm; 70 90% in America; in United States endemic in: Appalachian range, southern + Gulf coast states |
| Prevalence: | 25% of world population infected (a) in United States: 12% in blacks, 1% in whites (b) in parts of Africa, Asia, South America: 90% |
Cycle:
ingestion of contaminated water/soil/vegetable; larvae penetrate intestinal wall; migrate into mesenteric lymphatics + veins into liver; reach lung via right heart + pulmonary artery; mature in pulmonary capillary bed to 2 3 mm length; burrow into alveoli; ascend in respiratory tract; are swallowed and again reach small intestine, where they become adult worms whose eggs leave the body by the fecal route
abnormal liver function tests + biliary colic
hypereosinophilia only present during acute stage of larval migration
 barium study
barium study cholangiography (49%)
cholangiography (49%)US:
 tubular echogenic filling defect with 2 4-mm wide central sonolucent line (= worm with digestive tract) within dilated common bile duct
tubular echogenic filling defect with 2 4-mm wide central sonolucent line (= worm with digestive tract) within dilated common bile duct
| Cx: |
|
| Rx: | Mebendazole |
Autosomal Dominant Polycystic Disease
= POLYCYSTIC LIVER DISEASE
= biliary ductal plate malformation at the level of the small intrahepatic bile ducts with progressive dilation of the abnormal noncommunicating bile ducts of biliary hamartomas
M:F = 1:2
| Associated with: | polycystic kidney disease (in 50%) |
upper abdominal pain + distension from hepatomegaly
 enlarged diffusely cystic liver (cysts of 1 mm 12 cm in diameter)
enlarged diffusely cystic liver (cysts of 1 mm 12 cm in diameter) calcifications of cyst walls
calcifications of cyst walls diffuse dilatation of intra- and extrahepatic bile ducts
diffuse dilatation of intra- and extrahepatic bile ducts
| Cx: | infection, compression, bleeding, rupture of cysts |
Banti Syndrome
= NONCIRRHOTIC IDIOPATHIC PORTAL HYPERTENSION= NONCIRRHOTIC PORTAL FIBROSIS = HEPATOPORTAL SCLEROSIS
= syndrome characterized by
splenomegaly
hypersplenism
portal hypertension
| Etiology: | increased portal vascular resistance possibly due to portal fibrosis + obliterative venopathy of intrahepatic portal branches |
| Histo: | slight portal fibrosis, dilatation of sinusoids, intimal thickening with eccentric sclerosis of peripheral portal vein walls |
| Age: | middle-aged women; rare in America + Europe but common in India + Japan |
elevated portal vein pressure (without cirrhosis, parasites, venous occlusion)
normal liver function tests
cytopenia (due to hypersplenism)
normal/slightly elevated hepatic venous wedge pressure
 esophageal varices
esophageal varices patent hepatic veins
patent hepatic veins patent extrahepatic portal vein + multiple collaterals
patent extrahepatic portal vein + multiple collaterals
| Prognosis: | 90% 5-year survival; 55% 30-year survival |
Biliary Cystadenocarcinoma
= BILE DUCT CYSTADENOCARCINOMA
rare malignant multilocular cystic tumor originating from biliary cystadenoma
| Histo: | (a) with ovarian stroma (good prognosis), in females only (b) without ovarian stroma (bad prognosis) |
hemorrhagic internal fluid
 nodularity with septations are suggestive of malignancy
nodularity with septations are suggestive of malignancy coarse calcifications
coarse calcifications
| DDx: | no image differentiation from biliary cystadenoma |
Biliary Cystadenoma
= BILE DUCT CYSTADENOMA
= rare benign premalignant multilocular cystic tumor originating in bile ducts; probably deriving from ectopic nests of primitive biliary tissue
| Incidence: | 4.6% of all intrahepatic cysts of bile duct origin |
| Age: | >30 years (82%), peak incidence in 5th decade; M:F = 1:4; predominantly Caucasian |
| Path: | multilocular cystic tumor containing proteinaceous fluid with well-defined thick capsule |
| Histo: | single layer of cuboidal/tall columnar biliary-type epithelium with papillary projections, subepithelial stroma resembling that of the ovary Similar to mucinous cystic tumors of pancreas + ovary |
| Location: | intrahepatic:extrahepatic bile ducts = 85:15; right lobe (48%); left lobe (20 35%); both lobes (15 30%); gallbladder (rare) |
chronic abdominal pain
dyspepsia, anorexia, nausea + vomiting
jaundice
abdominal swelling with palpable mass (90%)
 mass of 1.5 35 cm in size
mass of 1.5 35 cm in size up to 11 liters of clear/cloudy, serous/mucinous/gelatinous, purulent/hemorrhagic/bilious fluid containing hemosiderin/cholesterol/necrosis
up to 11 liters of clear/cloudy, serous/mucinous/gelatinous, purulent/hemorrhagic/bilious fluid containing hemosiderin/cholesterol/necrosis papillary excrescences + mural nodules
papillary excrescences + mural nodules septations between cysts
septations between cystsUS:
 ovoid multiloculated anechoic mass with highly echogenic septations/papillary growths
ovoid multiloculated anechoic mass with highly echogenic septations/papillary growths may contain fluid-fluid levels
may contain fluid-fluid levels
CT:
 multiloculated mass of near water density
multiloculated mass of near water density contrast enhancement in wall + internal septa
contrast enhancement in wall + internal septa
MR:
 locules with variable signal intensity on T1WI + T2WI depending on their protein content
locules with variable signal intensity on T1WI + T2WI depending on their protein content
Angio:
 avascular mass with small clusters of peripheral abnormal vessels
avascular mass with small clusters of peripheral abnormal vessels stretching + displacement of vessels
stretching + displacement of vessels thin subtle blush of neovascularity in septa + wall
thin subtle blush of neovascularity in septa + wall
P.694
| Cx: | (1) malignant transformation into cystadenocarcinoma (indicated by invasion of capsule) (2) rupture into peritoneum/retroperitoneum |
| Rx: | surgical resection (recurrence common) |
| DDx: | liver abscess, echinococcal cyst, cystic mesenchymal hamartoma (children + young adults), undifferentiated sarcoma (children + young adults), necrotic hepatic metastasis, cystic primary hepatocellular carcinoma |
Biliary-Enteric Fistula
| Incidence: | 5% at cholecystectomy; 0.5% at autopsy |
| Etiology: | cholelithiasis (90%), acute/chronic cholecystitis, biliary tract carcinoma, regional invasive neoplasm, diverticulitis, inflammatory bowel disease, peptic ulcer disease, echinococcal cyst, trauma, congenital communication |
Communication with:
duodenum (70%), colon (26%), stomach (4%), jejunum, ileum, hepatic artery, portal vein (caused death of Ignatius Loyola), bronchial tree, pericardium, renal pelvis, ureter, urinary bladder, vagina, ovary
CHOLECYSTODUODENAL FISTULA (51 80%)
Perforated gallstone (90%):
associated with gallstone ileus in 20%
Perforated duodenal ulcer (10%)
Surgical anastomosis
Gallbladder carcinoma
CHOLECYSTOCOLIC FISTULA (13 21%)
CHOLEDOCHODUODENAL FISTULA (13 19%)
due to perforated duodenal ulcer disease
MULTIPLE FISTULAE (7%)
 pneumobilia = branching tubular radiolucencies, more prominent centrally within the liver
pneumobilia = branching tubular radiolucencies, more prominent centrally within the liver barium filling of biliary tree
barium filling of biliary tree shrunken gallbladder mimicking pseudodiverticulum of duodenal bulb
shrunken gallbladder mimicking pseudodiverticulum of duodenal bulb multiple hyperechoic foci with dirty shadowing
multiple hyperechoic foci with dirty shadowing
| DDx: | patulous sphincter of Oddi, ascending cholangitis, surgery (choledochoduodenostomy, cholecystojejunostomy, sphincterotomy) |
Budd-Chiari Syndrome
= syndrome of global/segmental hepatic venous outflow obstruction
Cause:
IDIOPATHIC (66%)
THROMBOSIS
hypercoagulable state:
polycythemia rubra vera (1/3), oral contraceptives, pregnancy + postpartum state, paroxysmal nocturnal hemoglobulinuria (12%), sickle cell disease
mnemonic: 5 P's Paroxysmal nocturnal hemoglobulinuria
Platelets (thrombocytosis)
Pill (birth control pills)
Pregnancy
Polycythemia rubra vera
injury to vessel wall:
phlebitis, trauma, hepatic radiation injury, chemotherapeutic + immunosuppressive drugs in patients with bone marrow transplants, venoocclusive disease from pyrrolizidine alkaloids (senecio) found in medicinal bush teas in Jamaica
NONTHROMBOTIC OBSTRUCTION
tumor growth into IVC/hepatic veins (renal cell carcinoma, hepatoma, adrenal carcinoma, metastasis, primary leiomyosarcoma of IVC)
membranous obstruction of suprahepatic IVC
= IVC diaphragm (believed to be a congenital web or an acquired lesion from long-standing IVC thrombosis); common in oriental + Indian population (South Africa, India, Japan, Korea); very rare in Western countries
right atrial tumor
constrictive pericarditis
right heart failure
| Pathophysiology: | hepatic venous thrombosis leads to elevation of sinusoidal pressure, which causes delayed/reversed portal venous inflow, ascites, alteration in hepatic morphology |
| Age: | all ages; M < F |
right upper quadrant pain
shortness of breath (due to decreased cardiac return)
nonspecific elevated transaminases, jaundice
lower-extremity edema
Location:
| Type I | : occlusion of IVC hepatic veins |
| Type II | : occlusion of major hepatic veins IVC |
| Type III | : occlusion of small centrilobar veins |
 hepatosplenomegaly (early sign)
hepatosplenomegaly (early sign) caudate lobe hypertrophy (88%) [DDx: cirrhosis]
caudate lobe hypertrophy (88%) [DDx: cirrhosis] ascites
ascites gallbladder wall thickening >6 mm
gallbladder wall thickening >6 mm nonvisualization of hepatic veins (75%)/vein diameter <3 mm (measured 2 cm from IVC)
nonvisualization of hepatic veins (75%)/vein diameter <3 mm (measured 2 cm from IVC) communications between right/middle hepatic vein and inferior right hepatic vein
communications between right/middle hepatic vein and inferior right hepatic vein enlarged inferior right hepatic vein (18%)
enlarged inferior right hepatic vein (18%) portal vein diameter >12 mm (in adults), >8 mm (in children)
portal vein diameter >12 mm (in adults), >8 mm (in children) visualization of collateral pathways:
visualization of collateral pathways:portosystemic: paraumbilical vein
bypassing IVC: azygos, hemiazygos
 narrowing/obstruction of intrahepatic IVC
narrowing/obstruction of intrahepatic IVCNECT:
 global liver enlargement + diffuse hypoattenuation
global liver enlargement + diffuse hypoattenuation
CT:
 flip-flop enhancement pattern:
flip-flop enhancement pattern: prominent enhancement of central liver + weak enhancement of peripheral liver on early images
prominent enhancement of central liver + weak enhancement of peripheral liver on early images enhancement of liver periphery + wash-out of contrast from central liver on delayed images
enhancement of liver periphery + wash-out of contrast from central liver on delayed images
 normal enhancement of enlarged caudate lobe (due to separate venous drainage directly into IVC)
normal enhancement of enlarged caudate lobe (due to separate venous drainage directly into IVC) mottled liver enhancement pattern (due to hepatic congestion):
mottled liver enhancement pattern (due to hepatic congestion): patchy liver enhancement (85%) with normal portal blood flow
patchy liver enhancement (85%) with normal portal blood flow hypodensity in atrophic areas/periphery (82%) with inversion of portal blood flow (= reversed portal venous blood flow due to increased postsinusoidal pressure produced by hepatic venous obstruction/rarely infarcts)
hypodensity in atrophic areas/periphery (82%) with inversion of portal blood flow (= reversed portal venous blood flow due to increased postsinusoidal pressure produced by hepatic venous obstruction/rarely infarcts)
 failure to identify hepatic veins
failure to identify hepatic veins hepatic vein thrombi (18 53%)
hepatic vein thrombi (18 53%)
MRI:
 reduction in caliber/complete absence of hepatic veins
reduction in caliber/complete absence of hepatic veins comma sign = multiple comma-shaped intrahepatic flow voids (due to intrahepatic collaterals)
comma sign = multiple comma-shaped intrahepatic flow voids (due to intrahepatic collaterals)
Doppler US (85 100% sensitive, 85% specific):
 one/more major hepatic veins reduced in size to <3 mm/filled with thrombus/not visualized
one/more major hepatic veins reduced in size to <3 mm/filled with thrombus/not visualized communicating intrahepatic venous collaterals
communicating intrahepatic venous collaterals decreased/absent/reversed blood flow in hepatic veins
decreased/absent/reversed blood flow in hepatic veins flat flow/loss of cardiac modulation in hepatic veins
flat flow/loss of cardiac modulation in hepatic veins demodulated portal venous flow = disappearance of portal vein velocity variations with breathing
demodulated portal venous flow = disappearance of portal vein velocity variations with breathing slow flow (<11 cm/sec)/hepatofugal flow in portal vein
slow flow (<11 cm/sec)/hepatofugal flow in portal vein portal vein congestion index >0.1
portal vein congestion index >0.1 portal vein thrombosis (20%)
portal vein thrombosis (20%) compression of IVC by enlarged liver/caudate lobe
compression of IVC by enlarged liver/caudate lobe sluggish/reversed/absent blood flow within IVC
sluggish/reversed/absent blood flow within IVC hepatic artery resistive index >0.75
hepatic artery resistive index >0.75
NUC (Tc-99m sulfur colloid):
 central region of normal activity (hot caudate lobe) surrounded by greatly diminished activity (venous drainage of hypertrophied caudate lobe into IVC by separate vein)
central region of normal activity (hot caudate lobe) surrounded by greatly diminished activity (venous drainage of hypertrophied caudate lobe into IVC by separate vein) colloid shift to spleen + bone marrow
colloid shift to spleen + bone marrow wedge-shaped focal peripheral defects
wedge-shaped focal peripheral defects
Angio (inferior venocavography, hepatic venography):
 absence of main hepatic veins
absence of main hepatic veins spider web pattern of collateral + recanalized veins
spider web pattern of collateral + recanalized veins high-pressure gradient between infra- and suprahepatic portion of IVC (due to enlarged liver)
high-pressure gradient between infra- and suprahepatic portion of IVC (due to enlarged liver) stretching + draping of intrahepatic arteries with hepatomegaly
stretching + draping of intrahepatic arteries with hepatomegaly inhomogeneous prolonged intense hepatogram with fine mottling
inhomogeneous prolonged intense hepatogram with fine mottling large lakes of sinusoidal contrast accumulation
large lakes of sinusoidal contrast accumulation
Portography:
 central hepatic enhancement (normal hepatopetal flow)
central hepatic enhancement (normal hepatopetal flow) reversed portal flow in liver periphery (supplied only by hepatic artery)
reversed portal flow in liver periphery (supplied only by hepatic artery) bidirectional/hepatofugal main portal vein flow
bidirectional/hepatofugal main portal vein flow
P.695
| Dx: | liver biopsy |
| Rx: | control of ascites with diuretics + sodium restriction; anticoagulation, thrombolytic therapy, surgery/balloon dilatation (depending on etiology); transjugular portosystemic shunt; orthotopic liver transplantation (for advanced cases) |
Acute Budd-Chiari Syndrome (1/3)
 Caudate lobe has not had time to hypertrophy!
Caudate lobe has not had time to hypertrophy!rapid onset of abdominal pain (liver congestion)
insidious onset of intractable ascites
 hepatomegaly without derangement of liver function
hepatomegaly without derangement of liver function ascites (97%)
ascites (97%)CT:
 diffuse hypodensity on NECT
diffuse hypodensity on NECT early enhancement of caudate lobe + central portion around IVC with decreased enhancement peripherally
early enhancement of caudate lobe + central portion around IVC with decreased enhancement peripherally hypodense lumina of hepatic veins on CECT
hypodense lumina of hepatic veins on CECT decreased attenuation of enhancing areas with patchy inhomogeneous enhancement in liver periphery on delayed scans
decreased attenuation of enhancing areas with patchy inhomogeneous enhancement in liver periphery on delayed scans
MR:
 peripheral liver parenchyma of moderately low signal intensity on T1WI + moderately high signal intensity on T2WI compared with central portion
peripheral liver parenchyma of moderately low signal intensity on T1WI + moderately high signal intensity on T2WI compared with central portion diminished + mottled peripheral enhancement
diminished + mottled peripheral enhancement
Chronic Budd-Chiari Syndrome (2/3)
insidious onset of jaundice, intractable ascites
portal hypertension, variceal bleeding
 enlargement of central region (= caudate lobe + adjacent central part of right lobe + medial segment of left lobe
enlargement of central region (= caudate lobe + adjacent central part of right lobe + medial segment of left lobe nonsegmental/lobar atrophy of affected liver (due to extensive fibrosis) with diminished attenuation before + after contrast administration
nonsegmental/lobar atrophy of affected liver (due to extensive fibrosis) with diminished attenuation before + after contrast administration progressive patchy enhancement radiating outward from major portal vessels (on dynamic bolus CT)
progressive patchy enhancement radiating outward from major portal vessels (on dynamic bolus CT) reticulated mosaic enhancement = diffuse patchy lobular enhancement separated by irregular linear areas of low density in central area
reticulated mosaic enhancement = diffuse patchy lobular enhancement separated by irregular linear areas of low density in central area delayed homogeneous enhancement of entire liver after several minutes
delayed homogeneous enhancement of entire liver after several minutes ascites
ascitesColor Doppler:
 bicolored hepatic veins (due to intrahepatic collateral pathways) are PATHOGNOMONIC
bicolored hepatic veins (due to intrahepatic collateral pathways) are PATHOGNOMONIC
MR:
 absence of flow within hepatic veins
absence of flow within hepatic veins minimal differences in signal intensity between central and peripheral portions of liver
minimal differences in signal intensity between central and peripheral portions of liver intrahepatic collateral vessels
intrahepatic collateral vessels
Candidiasis Of Liver
= almost exclusively seen in immunocompromised patients (acute leukemia, chronic granulomatous disease of childhood, renal transplant, chemotherapy for myeloproliferative disorders)
P.696
| Prevalence: | at time of autopsy in 50 70% of acute leukemia, in 50% of lymphoma patients Most common systemic fungal infection in immunocompromised patients! |
abdominal pain
persistent fever in neutropenic patient whose leukocyte count is returning to normal
elevated alkaline phosphatase
 hepatomegaly
hepatomegaly target / bull's-eye sign = multiple small hypoechoic/hypoattenuating masses with centers of increased echogenicity/attenuation distributed throughout liver
target / bull's-eye sign = multiple small hypoechoic/hypoattenuating masses with centers of increased echogenicity/attenuation distributed throughout liver Bull's-eye lesion becomes visible only when neutropenia resolves!
Bull's-eye lesion becomes visible only when neutropenia resolves!
 hyperintense lesions on T2WI
hyperintense lesions on T2WINUC:
 uniform uptake/focal photopenic areas
uniform uptake/focal photopenic areas diminished Ga-67 uptake
diminished Ga-67 uptake
| Dx: | biopsy evidence of yeast/pseudohyphae in central necrotic portion of lesion |
| DDx: | metastases, lymphoma, leukemia, sarcoidosis, septic emboli, other infections (MAI, CMV), Kaposi sarcoma |
Caroli Disease
[Jacques Caroli (1902 1979), surgeon in Paris, France]
COMMUNICATING CAVERNOUS ECTASIA OF INTRAHEPATIC DUCTS
rare congenital probably autosomal recessive disorder characterized by multifocal segmental saccular cystic dilatation of the large intrahepatic bile ducts, which retain their communication with the biliary tree
| Etiology: | (a)? perinatal hepatic artery occlusion (b)? hypoplasia/aplasia of fibromuscular wall components |
| Age: | childhood + 2nd 3rd decade, occasionally in infancy; M:F = 1:1 |
| Associated with: | benign renal tubular ectasia, medullary sponge kidney (in 80%), infantile polycystic kidney disease, choledochal cyst (rare), congenital hepatic fibrosis |
recurrent cramplike upper abdominal pain
fever, transient jaundice
cirrhosis/portal hypertension (very rare)
 multiple cystic structures converging toward porta hepatis as either localized/diffusely scattered cysts communicating with bile ducts (DDx: polycystic liver disease)
multiple cystic structures converging toward porta hepatis as either localized/diffusely scattered cysts communicating with bile ducts (DDx: polycystic liver disease) sludge/calculi in dilated ducts
sludge/calculi in dilated ductsCT/US/ MR:
 central dot sign = portal vein radicles completely surrounded by dilated bile ducts
central dot sign = portal vein radicles completely surrounded by dilated bile ducts
Cholangiography (diagnostic):
 segmental saccular/fusiform/beaded dilatation of intrahepatic bile ducts extending to periphery of liver up to 5 cm in diameter
segmental saccular/fusiform/beaded dilatation of intrahepatic bile ducts extending to periphery of liver up to 5 cm in diameter bridge formation across dilated lumina
bridge formation across dilated lumina intraluminal bulbar protrusions
intraluminal bulbar protrusions frequent ectasia of extrahepatic ducts + CBD
frequent ectasia of extrahepatic ducts + CBD
| Cx: | (1) Bile stasis with recurrent cholangitis (2) Biliary calculi (predominantly bilirubin) (3) Liver abscess (4) Septicemia (5) Increased risk for cholangiocarcinoma (7%) |
| DDx: | polycystic liver disease (noncommunicating), biliary hamartoma (noncommunicating); primary sclerosing cholangitis, recurrent pyogenic cholangitis |
Cholangiocarcinoma
| Incidence: | 0.5 1% of all cancers, 30% of hepatic primary malignancies Cholangiocarcinomas occur in 10 15% of patients with primary sclerosing cholangitis! |
Location:
INTRAHEPATIC
PERIPHERAL distal to 2nd-order branches
HILAR/CENTRAL at bifurcation/in 1st-order branches = Klatskin tumor
confluence of hepatic ducts in 10 26% left/right hepatic duct in 8 13%
EXTRAHEPATIC
common hepatic duct in 14 37% proximal CBD in 15 30% distal CBD in 30 50% cystic duct in 6%
Path:
exophytic (= mass-forming/nodular) type:
commonly in peripheral cholangiocarcinoma
 large irregular hypoattenuating mass
large irregular hypoattenuating mass stippled/punctate hyperattenuating foci
stippled/punctate hyperattenuating foci thin rimlike/thick bandlike enhancement around the tumor (early)
thin rimlike/thick bandlike enhancement around the tumor (early) progressive concentric filling in of contrast (late) due to slow diffusion into interstitial tumor spaces
progressive concentric filling in of contrast (late) due to slow diffusion into interstitial tumor spaces
diffuse = infiltrative (periductal) type:
commonly in hilar + extrahepatic cholangiocarcinoma
 mural thickening/encircling mass of bile duct wall
mural thickening/encircling mass of bile duct wall focal or diffuse stricture/complete obstruction of bile ducts
focal or diffuse stricture/complete obstruction of bile ducts
polypoid = papillary (intraductal) type: infrequent
 intraluminal polypoid mass
intraluminal polypoid mass
combination
| Histo: | well/moderately/poorly differentiated ductal (most common), papillary, mucinous, signet-ring cell, mucoepidermoid, adenosquamous, cystadenocarcinoma Unusual manifestation:
|
Predisposed:
Inflammatory bowel disease (10 increased risk); incidence of 0.4 1.4% in ulcerative colitis; latent period of 15 years; tumors usually multicentric + predominantly in extrahepatic sites; GB involved in 15% (simultaneous presence of gallstones is rare)
Biliary lithiasis: cholecystolithiasis (20 50%), intrahepatic lithiasis (5 10%)
Primary sclerosing cholangitis (10%)
Clonorchis sinensis infestation (Far East); most common cause worldwide
Choledochal cyst/congenital hepatic cyst/congenital biliary atresia
Ductal plate malformation:
Biliary hamartoma
Autosomal dominant polycystic disease
Congenital hepatic fibrosis
Caroli disease (due to chronic biliary stasis)
Papillomatosis of bile ducts
Recurrent pyogenic cholangitis
Choledochoenteric anastomosis
History of other malignancy (10%)
Thorotrast exposure
Alpha 1 antitrypsin deficiency
P.697
| Prognosis: | median survival of 7 months, 0 10% 5-year survival |
Intrahepatic Cholangiocarcinoma
= CHOLANGIOCELLULAR CARCINOMA
| Incidence: | 1/3 of all malignancies originating in the liver; 8 13% of all cholangiocarcinomas; 2nd most common primary hepatic tumor after hepatoma |
| Histo: | adenocarcinoma arising from the epithelium of a small intrahepatic bile duct with prominent desmoplastic reaction (fibrosis); mucin and calcifications |
| Average age: | 50 60 years; M > F |
abdominal pain (47%); painless jaundice (12%)
palpable mass (18%)
weight loss (18%)
| Spread: | (a) local extension along duct (b) local infiltration of liver substance (c) metastatic spread to regional lymph nodes (in 15%) |
 mass of 5 20 cm in diameter
mass of 5 20 cm in diameter satellite nodules in 65%
satellite nodules in 65% punctate/chunky calcifications in 18%
punctate/chunky calcifications in 18% calculi in biliary tree
calculi in biliary treeNUC:
 cold lesion on sulfur colloid/IDA scans
cold lesion on sulfur colloid/IDA scans segmental biliary obstruction
segmental biliary obstruction may show uptake on gallium scan
may show uptake on gallium scan
US:
 dilated biliary tree
dilated biliary tree predominantly homo-/heterogeneous mass
predominantly homo-/heterogeneous mass hyper- (75%)/iso-/hypoechoic (14%) mass
hyper- (75%)/iso-/hypoechoic (14%) mass mural thickening
mural thickening
CT:
 single predominantly homogeneous round/oval hypodense mass with irregular borders
single predominantly homogeneous round/oval hypodense mass with irregular borders peripheral washout sign = early minimal/moderate rim enhancement with progressive concentric filling and clearing of contrast material in rim of lesion on delayed images
peripheral washout sign = early minimal/moderate rim enhancement with progressive concentric filling and clearing of contrast material in rim of lesion on delayed images marked homogeneous delayed enhancement (74%)
marked homogeneous delayed enhancement (74%)
MR:
 large central heterogeneous hypointense mass on T1WI
large central heterogeneous hypointense mass on T1WI hyperintense periphery (viable tumor) + large central hypointensity (fibrosis) on T2WI
hyperintense periphery (viable tumor) + large central hypointensity (fibrosis) on T2WI gadolinium enhancement of lesion
gadolinium enhancement of lesion
Angiography:
 avascular/hypo-/hypervascular mass
avascular/hypo-/hypervascular mass stretched/encased arteries (frequent)
stretched/encased arteries (frequent) neovascularity in 50%
neovascularity in 50% lack of venous invasion
lack of venous invasion
| Prognosis: | <20% resectable; 30% 5-year survival |
Klatskin Tumor
[Gerald Klatskin (1910 1986), pathologist in yale, USA]
= INTRAhEPATIC CENTRAL ChoLANGIoCARCINoMA
= tumor at confluence of hepatic ducts (up to 70% of cholangiocarcinomas)
 direct signs of Klatskin tumor:
direct signs of Klatskin tumor: iso- to hyperechoic central porta hepatis mass/focal irregularity of ducts (for infiltrating cholangiocarcinoma = more common subtype)
iso- to hyperechoic central porta hepatis mass/focal irregularity of ducts (for infiltrating cholangiocarcinoma = more common subtype) polypoid/smooth nodular intraluminal mass (for papillary + nodular types of cholangiocarcinoma) with associated mural thickening
polypoid/smooth nodular intraluminal mass (for papillary + nodular types of cholangiocarcinoma) with associated mural thickening
 indirect signs of Klatskin tumor:
indirect signs of Klatskin tumor: segmental dilatation with nonunion of right and left ducts at porta hepatis + normal caliber of extrahepatic ducts
segmental dilatation with nonunion of right and left ducts at porta hepatis + normal caliber of extrahepatic ducts pressure effect/encasement/invasion/obliteration of portal vein and hepatic artery
pressure effect/encasement/invasion/obliteration of portal vein and hepatic artery lobar atrophy (14%) = dilated crowded ducts extending to liver surface geographic fatty change in one lobe
lobar atrophy (14%) = dilated crowded ducts extending to liver surface geographic fatty change in one lobe
Intrahepatic Peripheral Cholangiocarcinoma
no jaundice
| Location: | right lobe predilection |
 solitary mass (nodular form) without hypoechoic halo
solitary mass (nodular form) without hypoechoic halo diffusely abnormal liver texture (infiltrative form):
diffusely abnormal liver texture (infiltrative form): tumor more hypoechoic if <3 cm
tumor more hypoechoic if <3 cm tumor more hyperechoic if >3 cm
tumor more hyperechoic if >3 cm
 well-marginated cystic mass (papillary mucinproducing tumor) diffuse hyperechoic flecks of tumor calcification
well-marginated cystic mass (papillary mucinproducing tumor) diffuse hyperechoic flecks of tumor calcification dilatation of bile ducts peripheral to tumor (31%)
dilatation of bile ducts peripheral to tumor (31%)
| DDx: | metastatic adenocarcinoma/leiomyosarcoma; sclerosing hepatocellular carcinoma |
Extrahepatic Cholangiocarcinoma
= BILE DUCT CARCINoMA
| Age peak: | 6th 7th decade, M:F = 3:2 |
| Incidence: | <0.5% of autopsies; 90% of all cholangiocarcinomas; more frequent in Far East |
| Histo: | well-differentiated sclerosing adenocarcinoma (2/3), anaplastic carcinoma (11%), cystadenocarcinoma, adenoacanthoma, malignant adenoma, squamous cell = epidermoid carcinoma, leiomyosarcoma |
gradual onset of fluctuating painless jaundice
cholangitis (10%)
weight loss, fatigability
intermittent epigastric pain
elevated bilirubin + alkaline phosphatase
enlarged tender liver
Growth pattern:
Obstructive type (70 85%)
 U-/V-shaped obstruction with nipple, rattail, smooth/irregular termination
U-/V-shaped obstruction with nipple, rattail, smooth/irregular termination
Stenotic type (10 25%)
 strictured rigid lumen with irregular margins + prestenotic dilatation
strictured rigid lumen with irregular margins + prestenotic dilatation
Polypoid/papillary type (5 6%)
 intraluminal filling defect with irregular margins
intraluminal filling defect with irregular margins
P.698
Spread: (a) lymphatic spread: cystic + CBD nodes (>32%), celiac nodes (>16%), peripancreatic nodes, superior mesenteric nodes
(b) infiltration of liver (23%)
(c) peritoneal seeding (9%)
(d) hematogenous (extremely rare): liver, peritoneum, lung
UGI:
 infiltration/indentation of stomach/duodenum Cholangiography (PTC or ERC best modality to depict bile duct neoplasm):
infiltration/indentation of stomach/duodenum Cholangiography (PTC or ERC best modality to depict bile duct neoplasm): exophytic intraductal tumor mass (46%), 2 5 mm in diameter
exophytic intraductal tumor mass (46%), 2 5 mm in diameter frequently long/rarely short concentric focal stricture in infiltrating sclerosing cholangitic type with wall irregularities
frequently long/rarely short concentric focal stricture in infiltrating sclerosing cholangitic type with wall irregularities prestenotic diffuse/focal biliary dilatation (100%)
prestenotic diffuse/focal biliary dilatation (100%) progression of ductal strictures (100%)
progression of ductal strictures (100%)
US/CT:
 dilatation of intrahepatic ducts without extrahepatic duct dilatation
dilatation of intrahepatic ducts without extrahepatic duct dilatation failure to demonstrate the confluence of L + R hepatic ducts
failure to demonstrate the confluence of L + R hepatic ducts mass within/surrounding the ducts at point of obstruction (21% visible on US, 40% visible on CT)
mass within/surrounding the ducts at point of obstruction (21% visible on US, 40% visible on CT) infiltrating tumor visible as highly attenuating lesion in 22% on CT, in 13% on US
infiltrating tumor visible as highly attenuating lesion in 22% on CT, in 13% on US exophytic tumor visible in 100% on CT as low-attenuation mass, in 29% on US
exophytic tumor visible in 100% on CT as low-attenuation mass, in 29% on US polypoid intraluminal tumor visible as isoechoic mass within surrounding bile in 100% on US, in 25% on CT
polypoid intraluminal tumor visible as isoechoic mass within surrounding bile in 100% on US, in 25% on CT
CECT:
 hyperattenuating lesion at delayed imaging (due to delayed accumulation + washout of fibrous center)
hyperattenuating lesion at delayed imaging (due to delayed accumulation + washout of fibrous center)
Angiography:
 hypervascular tumor with neovascularity (50%)
hypervascular tumor with neovascularity (50%) arterioarterial collaterals along the course of bile ducts associated with arterial obstruction
arterioarterial collaterals along the course of bile ducts associated with arterial obstruction poor/absent tumor stain
poor/absent tumor stain displacement/encasement/occlusion of hepatic artery + portal vein
displacement/encasement/occlusion of hepatic artery + portal vein
| Cx: | (1) Obstruction leading to biliary cirrhosis (2) Hepatomegaly (3) Intrahepatic abscess (subdiaphragmatic, perihepatic, septicemia) (4) Biliary peritonitis (5) Portal vein invasion |
| Dx: | endoscopic brush biopsy (30 85% sensitive) |
| Prognosis: | median survival of 5 months; 1.6% 5-year survival; 39% 5-year survival for carcinoma of papilla of Vater |
| DDx: | sclerosing cholangitis, AIDS cholangitis, benign stricture, chronic pancreatitis, edematous papilla, idiopathic inflammation of CBD |
Cholangitis
Acute Obstructive/Ascending Cholangitis
= biliary duct obstruction associated with biliary infection
Cause:
benign disease:
Stricture from prior surgery (36%) after bile duct exploration/bilioenteric anastomosis
Calculi (30%)
Sclerosing cholangitis
Obstructed drainage catheter
Parasitic infestation
malignant disease:
Ampullary carcinoma
Types:
ACUTE NONSUPPURATIVE ASCENDING CHOLANGITIS
bile remains clear
patient nontoxic
ACUTE SUPPURATIVE ASCENDING CHOLANGITIS (14%)
Associated with: obstructing biliary stone or malignancy septicemia, CNS depression, lethargy, mental confusion, shock (50%)
purulent material fills biliary ducts
Prognosis: 100% mortality if not decompressed;40 60% mortality with treatment;13 16% overall mortality rate Organism: gram-negative enteric bacteria = E. coli > Klebsiella > Pseudomonas > Enterococci
recurrent episodes of sepsis + RUQ pain
Charcot triad (70%): fever + chills + jaundice
bile cultures in 90% positive for infection
 may have gas in biliary tree
may have gas in biliary treeCECT:
 transient hepatic parenchymal enhancement in periportal location on hepatic arterial phase
transient hepatic parenchymal enhancement in periportal location on hepatic arterial phase(= hyperemic changes around bile ducts)
| Cx: | miliary hepatic abscess formation; secondary sclerosing cholangitis |
AIDS-related Cholangitis
= AIDS CHOLANGIOPATHY
= infectious cholangitis characterized by opportunistic organisms
| Organism: | Cryptosporidium (protozoan parasite typically infecting GI tract epithelium), CMV |
| Histo: | marked periductal inflammatory response with interstitial edema + interstitial inflammatory cell infiltrates + necrotic biliary epithelium |
RUQ pain, fever, nausea, jaundice
elevated WBC count
abnormal LFTs (esp. serum alkaline phosphatase)
opportunistic organism isolated from bile (in 50%)
 irregular mild dilatation of intra- and extrahepatic bile ducts resembling sclerosing cholangitis
irregular mild dilatation of intra- and extrahepatic bile ducts resembling sclerosing cholangitisUS:
 stricture of distal CBD/papillary stenosis (due to papillitis)
stricture of distal CBD/papillary stenosis (due to papillitis) echogenic nodule at the distal end of the CBD
echogenic nodule at the distal end of the CBD mural thickening of gallbladder + bile ducts
mural thickening of gallbladder + bile ducts periductal echogenicity
periductal echogenicity pericholecystic fluid
pericholecystic fluid
P.699
CT:
 pseudogallstone appearance = marked circumferential edema of gallbladder wall + mucosal enhancement
pseudogallstone appearance = marked circumferential edema of gallbladder wall + mucosal enhancement periportal edema
periportal edema
Cholangiography:
 strictures + beading of central intrahepatic bile ducts
strictures + beading of central intrahepatic bile ducts pruning of peripheral bile ducts
pruning of peripheral bile ducts
DDx: acalculous cholecystitis, papillary stenosis, sclerosing cholangitis Chemotherapy-induced Cholangitis
= inflammatory fibrosing process about the portal triads simulating primary sclerosing cholangitis
Predisposed: patients with liver metastases from colon cancer Cause: direct effect of hepatic arterial infusion with chemotherapeutic agents (eg, floxuridine)/ischemia secondary to thrombosis of intrahepatic arterial branches  bile duct strictures as early as 2 months after therapy (in up to 15%)
bile duct strictures as early as 2 months after therapy (in up to 15%) stricture of common hepatic duct + sparing of distal CBD
stricture of common hepatic duct + sparing of distal CBD
Primary Sclerosing Cholangitis
= insidious progressive obliterative fibrosing inflammation of the biliary tree causing multifocal strictures, bile duct obliteration, cholestasis, and biliary cirrhosis
Etiology: idiopathic,? autoimmune process (speculative); altered bile acid metabolism with increase in lithocholic acid by bacterial overgrowth Prevalence: 1% as common as alcoholic liver disease Age: <45 years (2/3); mean 39 (range 21 67) years;M:F = 7:3 Histo:
Stage 1: degeneration of epithelial bile duct cells + infiltration with lymphocytes neutrophils; inflammation + scarring + enlargement of periportal triads (pericholangitis) Stage 2: fibrosis + inflammation infiltrating periportal parenchyma with piecemeal necrosis of hepatocytes; enlargement of portal triads; bile ductopenia Stage 3: portal-to-portal fibrous septa; severe degenerative changes + disappearance of bile ducts; cholestasis in periportal + paraseptal hepatocytes Stage 4: frank cirrhosis Associated with:
Inflammatory bowel disease (ulcerative colitis in 50 74%, Crohn disease in 13%)
 1 4% of patients with inflammatory bowel disease develop secondary sclerosing cholangitis!
1 4% of patients with inflammatory bowel disease develop secondary sclerosing cholangitis! 10% of patients with primary sclerosing cholangitis have Crohn disease
10% of patients with primary sclerosing cholangitis have Crohn disease
Cirrhosis, chronic active hepatitis, pericholangitis, fatty degeneration
Pancreatitis
Retroperitoneal/mediastinal fibrosis
Peyronie disease
Riedel thyroiditis, hypothyroidism
Retroorbital pseudotumor
Sj gren syndrome
abnormal liver function tests: serum bilirubin, serum alkaline phosphatase, -glutamyltransferase
progressive chronic/intermittent obstructive jaundice (75%)
history of previous biliary surgery (53%) + chronic/recurrent pancreatitis (14%)
fever, night sweats, chills, RUQ pain, pruritus (10 15%)
Location:
CBD almost always involved
Intra- and extrahepatic ducts (68 89%)
Cystic duct involved in 15 18%
Intrahepatic ducts only (1 11 25%)
Extrahepatic ducts only (2 3%)
 intrahepatic bile duct calculi (8 30%): soft black crushable stones/sandlike grit
intrahepatic bile duct calculi (8 30%): soft black crushable stones/sandlike gritUS:
 brightly echogenic portal triads
brightly echogenic portal triads echogenic biliary casts/punctate coarse calcifications along portal vein branches
echogenic biliary casts/punctate coarse calcifications along portal vein branches gallbladder wall thickening
gallbladder wall thickening
CT:
 dilatation, stenosis, pruning (decreased arborization), beading of tortuous intrahepatic bile ducts = tree-in-winter appearance (80%)
dilatation, stenosis, pruning (decreased arborization), beading of tortuous intrahepatic bile ducts = tree-in-winter appearance (80%) wall nodularity, duct wall thickening, mural contrast enhancement of extrahepatic bile ducts (100%)
wall nodularity, duct wall thickening, mural contrast enhancement of extrahepatic bile ducts (100%) hepatic metastases + lymph nodes in porta hepatis
hepatic metastases + lymph nodes in porta hepatis subtle foci of high attenuation in intrahepatic bile ducts
subtle foci of high attenuation in intrahepatic bile ducts lobar atrophy in preferentially affected portions
lobar atrophy in preferentially affected portions
Cholangiography:
 multifocal strictures with predilection for bifurcations + skip lesions (uninvolved duct segments of normal caliber) involving intra- and extrahepatic bile ducts:
multifocal strictures with predilection for bifurcations + skip lesions (uninvolved duct segments of normal caliber) involving intra- and extrahepatic bile ducts: CLASSIC string-of-beads appearance
CLASSIC string-of-beads appearance(= alternating segments of dilatation and focal annular stenoses)
 pruned tree appearance (= opacification of central ducts + nonvisualization of peripheral smaller radicles due to diffuse obstruction)
pruned tree appearance (= opacification of central ducts + nonvisualization of peripheral smaller radicles due to diffuse obstruction) cobblestone appearance (= coarse nodular mural irregularities) in 50%
cobblestone appearance (= coarse nodular mural irregularities) in 50% new strictures + lengthening of strictures between 6 months and 6 years (<20%)
new strictures + lengthening of strictures between 6 months and 6 years (<20%) minimal duct dilatation due to periductal inflammation + fibrosis
minimal duct dilatation due to periductal inflammation + fibrosis marked ductal dilatation (24%)
marked ductal dilatation (24%)DDx: ascending cholangitis, cholangiocarcinoma
 small eccentric saccular outpouchings (diverticula/pseudodiverticula) [up to 27%] = PATHOGNOMONIC
small eccentric saccular outpouchings (diverticula/pseudodiverticula) [up to 27%] = PATHOGNOMONIC webs = focal 1 2-mm-thick areas of incomplete circumferential narrowing
webs = focal 1 2-mm-thick areas of incomplete circumferential narrowing angles formed between central and peripheral ducts change from acute to obtuse
angles formed between central and peripheral ducts change from acute to obtuse polypoid mass (7%)
polypoid mass (7%) gallbladder irregularities uncommon
gallbladder irregularities uncommon
MR:
 periportal intermediate intensity on T1WI + hyperintense on T2WI (due to inflammation)
periportal intermediate intensity on T1WI + hyperintense on T2WI (due to inflammation)
NUC (Tc-99m-IDA scan):
 multiple persistent focal areas of retention in distribution of intrahepatic biliary tree
multiple persistent focal areas of retention in distribution of intrahepatic biliary tree marked prolongation of hepatic clearance
marked prolongation of hepatic clearance gallbladder visualized only in 70%
gallbladder visualized only in 70%
P.700
| Cx: | (1) Biliary cirrhosis (up to 49%) (2) Portal hypertension (3) Cholangiocarcinoma (clinically in 4 19%; in 7 36% at autopsy/liver transplantation) (4) Secondary cholangitis |
| Rx: | (1) Palliative: ursodeoxycholic acid, dilatation of dominant strictures (2) Curative: liver transplantation (4th leading indication) |
| DDx: | (1) Sclerosing cholangiocarcinoma (progressive cholangiographic changes within 0.5 1.5 years of initial diagnosis, marked ductal dilatation upstream from a dominant stricture, intraductal mass >1 cm in diameter) (2) Acute ascending cholangitis (history) (3) Primary biliary cirrhosis (disease limited to intrahepatic ducts, strictures less pronounced, pruning + crowding of bile ducts, normal AMA titer) (4) AIDS cholangiopathy (same on cholangiography) |
Recurrent Pyogenic Cholangitis
= PRIMARY CHOLANGITIS = RECURRENT PYOGENIC HEPATITIS/CHOLANGITIS = ORIENTAL CHOLANGIO-HEPATITIS = ORIENTAL CHOLANGITIS = HONG KONG DISEASE = INTRAHEPATIC PIGMENT STONE DISEASE
= chronic recurrent parasitic cholangitis resulting in progressive destructive cholangiopathy + liver failure
| Etiology: | ? Clonorchis sinensis infestation, coliform infection of bile, portal bacteremia, malnutrition |
| Incidence: | 3rd most common cause of an acute abdomen in Hong Kong after appendicitis and perforated ulcer; uncommon in United States |
| Epidemiology: | endemic to Southeast Asia (South China, Indochina, Taiwan, Japan, Korea); Asian immigrants in United States |
Associated intrabiliary infestation:
Clonorchis sinensis, Ascaris lumbricoides, E. coli
| Path: | pericholangitis, periductal abscesses, fibrosis of bile duct walls, heavy infiltration of portal tracts by PMNs, intraductal bile pigment calculi |
| Age: | 20 50 years; M:F = 1:1 |
recurrent attacks of fever, chills, abdominal pain, jaundice
| Location: | particularly in lateral segment of L lobe + posterior segment of R lobe |
 marked dilatation of proximal intrahepatic ducts (3 4 mm) in 100%
marked dilatation of proximal intrahepatic ducts (3 4 mm) in 100% decreased arborization of intrahepatic radicles
decreased arborization of intrahepatic radicles intra- and extrahepatic bile ducts filled with nonshadowing soft mudlike pigment (= calcium bilirubinate) stones (74%)
intra- and extrahepatic bile ducts filled with nonshadowing soft mudlike pigment (= calcium bilirubinate) stones (74%) dilatation of CBD (68%) + choledocholithiasis (30%)
dilatation of CBD (68%) + choledocholithiasis (30%) multifocal bile duct strictures (22%)
multifocal bile duct strictures (22%) pneumobilia (3 52%)
pneumobilia (3 52%) biloma
biloma segmental hepatic atrophy (36%)
segmental hepatic atrophy (36%) hepatic abscesses
hepatic abscessesCT:
 high-attenuation biliary calculi
high-attenuation biliary calculi enhancement of bile duct wall
enhancement of bile duct wall
Associated findings:
 gallstones
gallstones splenomegaly
splenomegaly varices
varices
ERCP:
 Worsening of cholangitis/sepsis if patients do not receive antibiotics!
Worsening of cholangitis/sepsis if patients do not receive antibiotics! acute tapering + straightening + rigidity of bile ducts
acute tapering + straightening + rigidity of bile ducts decreased arborization + increased branching angle of bile ducts
decreased arborization + increased branching angle of bile ducts
| Cx: | liver abscess (18%), splenomegaly (14%), biloma (4%), pancreatitis (4%), cholangiocarcinoma (2.5 6%) |
| Rx: | endoscopic sphincterotomy, choledochoduodenostomy |
| DDx: | (1) Caroli disease (saccular dilatation of intrahepatic bile ducts) (2) Primary sclerosing cholangitis (focal discontinuous bile duct dilatation) (3) Clonorchiasis (biliary ductal dilatation limited to intrahepatic bile ducts) |
Secondary Sclerosing Cholangitis
Cause:
Chronic bacterial cholangitis from bile duct stricture/choledocholithiasis
Ischemic bile duct damage from treatment with floxuridine
Infectious cholangiopathy in AIDS
Previous biliary tract surgery
Congenital biliary tree anomalies
Bile duct neoplasm
Cholecystitis
Acute Calculous Cholecystitis
| Etiology: | (a) in 80 95% cystic duct obstruction by impacted calculus; 85% disimpact spontaneously if stone <3 mm (b) in 10% acalculous cholecystitis |
| Pathogenesis: | chemical irritation from concentrated bile, bacterial infection, reflux of pancreatic secretions |
| Age peak: | 5th 6th decade; M:F = 1:3 |
| Associated with: | choledocholithiasis (15 25%) |
persisting (>6 hours) RUQ pain radiating to right shoulder/scapula/interscapular area (DDx: biliary colic usually <6 hours)
nausea, vomiting, chills, fever
RUQ tenderness + guarding
leukocytosis, elevated levels of alkaline phosphatase and transaminase and amylase
mild hyperbilirubinemia (20%)
Murphy sign = inspiratory arrest upon palpation of GB area (falsely positive in 6% of patients with cholelithiasis)
Oral cholecystography:
P.701
 nonvisualization/poor visualization of gallbladder
nonvisualization/poor visualization of gallbladder
US (81ndash; 100% sensitivity, 60 100% specificity, 92% PPV, 95% NPV):
 GB wall thickening >3 mm (45 72% sensitive, 76 88% specific):
GB wall thickening >3 mm (45 72% sensitive, 76 88% specific): hazy delineation of GB wall
hazy delineation of GB wall halo sign = GB wall lucency (in 8%) = 3-layered configuration with sonolucent middle layer (edema)
halo sign = GB wall lucency (in 8%) = 3-layered configuration with sonolucent middle layer (edema) striated wall thickening (62%) = several alternating irregular discontinuous lucent + echogenic bands within GB wall (100% PPV)
striated wall thickening (62%) = several alternating irregular discontinuous lucent + echogenic bands within GB wall (100% PPV)
 GB hydrops = distension with AP diameter >5 cm or enlargement of greater than 4 10 cm
GB hydrops = distension with AP diameter >5 cm or enlargement of greater than 4 10 cm positive sonographic Murphy sign (in 85 88%)
positive sonographic Murphy sign (in 85 88%)= maximum tenderness during compression with transducer directly over gallbladder (63 94% sensitive, 85 93% specific, 72% NPV)
False-negative sonographic Murphy sign:
lack of patient responsiveness, pain medication, inability to press directly on GB (position deep to liver/protected by ribs), GB wall necrosis
 crescent-shaped/loculated pericholecystic fluid (in 20%)
crescent-shaped/loculated pericholecystic fluid (in 20%)= inflammatory intraperitoneal exudate/abscess
 gallstones (83 98% sensitive, 52 77% specific):
gallstones (83 98% sensitive, 52 77% specific): impacted gallstone in GB neck/cystic duct
impacted gallstone in GB neck/cystic duct echogenic shadowing fat within hepatoduodenal ligament conspicuous color Doppler flow (due to inflammation)
echogenic shadowing fat within hepatoduodenal ligament conspicuous color Doppler flow (due to inflammation)DDx: bowel gas
 sludge
sludge
Color Doppler US:
 visualization of cystic artery >50% of the length of the gallbladder (30% sensitive, 98% specific)
visualization of cystic artery >50% of the length of the gallbladder (30% sensitive, 98% specific)
CECT:
 distended gallbladder
distended gallbladder gallbladder wall thickness >3 mm
gallbladder wall thickness >3 mm increased gallbladder wall attenuation
increased gallbladder wall attenuation transient focal increased attenuation around gallbladder fossa on hepatic arterial phase (due to hepatic arterial hyperemia + early venous drainage)
transient focal increased attenuation around gallbladder fossa on hepatic arterial phase (due to hepatic arterial hyperemia + early venous drainage) haziness of pericholecystic fat
haziness of pericholecystic fat pericholecystic fluid
pericholecystic fluid increased attenuation of bile
increased attenuation of bile
NUC (86 97% sensitivity, 73 100% specificity, 95 98% accuracy):
= functional information about gallbladder + cystic duct patency
 Tracer uptake hinges on adequate hepatic function+ fasting status
Tracer uptake hinges on adequate hepatic function+ fasting status nonvisualization of GB during 1st hour (in 83%)
nonvisualization of GB during 1st hour (in 83%)= evidence of cystic duct obstruction
 nonvisualization of GB by 4 hours (99% specific)
nonvisualization of GB by 4 hours (99% specific) nonvisualization of GB + CBD (in 13%)
nonvisualization of GB + CBD (in 13%) pericholecystic rim sign (34% sensitive) on initial images = increased hepatic activity adjacent to empty GB fossa (= local hepatocyte inflammation + hyperemia in transmural process); 57% PPV for gangrenous GB + 94% PPV for acute cholecystitis
pericholecystic rim sign (34% sensitive) on initial images = increased hepatic activity adjacent to empty GB fossa (= local hepatocyte inflammation + hyperemia in transmural process); 57% PPV for gangrenous GB + 94% PPV for acute cholecystitis increased perfusion to GB fossa during arterial phase (in up to 80%)
increased perfusion to GB fossa during arterial phase (in up to 80%)
Endpoint of imaging:
when tracer fills GB
4 hours of delayed imaging after tracer injection
45 minutes after morphine injection
False-positive scans (10 12%) = nonvisualization of GB without acute cholecystitis:
prolonged fasting, total parenteral nutrition, hyperalimentation, recent feeding <4 6 hours prior to study, severe intercurrent illness, CBD obstruction, congenital absence of GB, post-cholecystectomy, carcinoma of GB, chronic cholecystitis, acute pancreatitis, alcoholic liver disease, hepatocellular disease
Reduction to 2% false-positive scans through:
delayed images up to 4 hours
cholecystokinin (Sincalide ) injection 15 minutes prior to study
morphine IV (0.04 mg/kg) at 40 minutes + reimaging after 20 minutes (contraction of sphincter of Oddi + rise in intrabiliary pressure)
False-negative scans (4.8%) = visualization of GB despite acute cholecystitis:
rare calculous/acalculous cholecystitis without cystic duct obstruction
Cholangiography:
 sharply defined filling defect in contrast-material filled lumen of cystic duct
sharply defined filling defect in contrast-material filled lumen of cystic duct
MR cholangiopancreatogram (high sensitivity):
 low-signal intensity defect surrounded by high-signal intensity bile on T2WI
low-signal intensity defect surrounded by high-signal intensity bile on T2WI
Cx:
| mnemonic: | GAME BEG |
Gangrene
Abscess (pericholecystic)
Mirizzi syndrome
Emphysematous cholecystitis
Bouveret syndrome (= gallstone erodes into duodenum leading to duodenal obstruction)
Empyema
Gallstone ileus
GANGRENE OF GALLBLADDER
positive Murphy sign (33%)
 irregular/absent gallbladder wall
irregular/absent gallbladder wall shaggy, irregular, asymmetric wall (mucosal ulcers, intraluminal hemorrhage, necrosis)
shaggy, irregular, asymmetric wall (mucosal ulcers, intraluminal hemorrhage, necrosis) hyperechoic foci within GB wall (microabscesses in Rokitansky-Aschoff sinuses)
hyperechoic foci within GB wall (microabscesses in Rokitansky-Aschoff sinuses) intraluminal pseudomembranes (gangrene)
intraluminal pseudomembranes (gangrene) coarse nonshadowing nondependent echodensities (= sloughed necrotic mucosa/sludge/pus/clotted blood within gallbladder)
coarse nonshadowing nondependent echodensities (= sloughed necrotic mucosa/sludge/pus/clotted blood within gallbladder)
Pericholecystic Abscess
| Cause: | subacute perforation of gallbladder wall subsequent to gangrene + infarction due to acute cholecystitis |
| Prevalence: | 2 20% |
Location:
gallbladder bed (most common)
 area of low-level echoes in liver adjacent to GB
area of low-level echoes in liver adjacent to GB
intramural
 small area of low-level echoes within thickened gallbladder wall
small area of low-level echoes within thickened gallbladder wall
intraperitoneal
 area of low-level echoes within peritoneal cavity adjacent to gallbladder
area of low-level echoes within peritoneal cavity adjacent to gallbladder
P.702
Rx:
Emergency operation
Antibiotic treatment + elective operation
Percutaneous abscess drainage
Perforation of Gallbladder (in 2 20%)
Types:
Acute free perforation with peritonitis causing pericholecystic abscess in 33%
Subacute localized perforation causing pericholecystic abscess in 48%
Chronic perforation resulting in internal biliary fistula causing pericholecystic abscess in 18%
| Location: | most commonly at fundus |
 gallstone lying free in peritoneal cavity
gallstone lying free in peritoneal cavity sonolucent/complex collection surrounding GB
sonolucent/complex collection surrounding GB collection in liver adjacent to gallbladder
collection in liver adjacent to gallbladder
Empyema of Gallbladder
 multiple medium/coarse highly reflective intraluminal echoes without shadowing/layering/gravity dependence (purulent exudate/debris)
multiple medium/coarse highly reflective intraluminal echoes without shadowing/layering/gravity dependence (purulent exudate/debris)
Acute Acalculous Cholecystitis
| Frequency: | 5 15% of all acute cholecystitis cases |
| Associated with: | recent surgery in 50% |
| Etiology: | probably caused by decreased blood flow within cystic artery |
debilitated patients: depressed motility/starvation in trauma, burns, surgery, total parenteral nutrition, anesthesia, positive pressure ventilation, narcotics, shock, vasoactive amines, congestive heart failure, arteriosclerosis, polyarteritis nodosa, SLE, diabetes mellitus
 Diagnosis in the ICU patient sonographically difficult due to fasting state, medications, CHF, etc.
Diagnosis in the ICU patient sonographically difficult due to fasting state, medications, CHF, etc.
obstruction of cystic duct by extrinsic inflammation, lymphadenopathy, metastases
infection (only in 50%) from Salmonella, Helicobacter, cholera, Kawasaki syndrome, cytomegalovirus, cryptosporidiosis
 thickened gallbladder wall >4 5 mm
thickened gallbladder wall >4 5 mm echogenic bile/sludge
echogenic bile/sludge gallbladder distension
gallbladder distension pericholecystic fluid in absence of ascites
pericholecystic fluid in absence of ascites striated subserosal edema
striated subserosal edema sloughed mucosal membrane
sloughed mucosal membrane Murphy sign = pain + tenderness with transducer pressure over the gallbladder (difficult to assess in ICU patient with altered mental status)
Murphy sign = pain + tenderness with transducer pressure over the gallbladder (difficult to assess in ICU patient with altered mental status) decreased response to cholecystokinin
decreased response to cholecystokinin intramural gas
intramural gasCT:
 pericholecystic stranding (= edema)
pericholecystic stranding (= edema) decreased attenuation in adjacent liver (= perihepatitis)
decreased attenuation in adjacent liver (= perihepatitis)
| NUC: | same criteria as for calculous cholecystitis |
| Cx: | gallbladder perforation, gangrene, pericholecystic abscess |
| Rx: | percutaneous cholecystostomy trial (low threshold for ICU patients) |
| Prognosis: | 6.5% mortality rate |
Chronic Cholecystitis
 Most common form of gallbladder inflammation
Most common form of gallbladder inflammation gallstones
gallstones smooth/irregular GB wall thickening (mean of 5 mm)
smooth/irregular GB wall thickening (mean of 5 mm) mean volume of 42 mL
mean volume of 42 mLNUC:
 normal GB visualization in majority of patients
normal GB visualization in majority of patients delayed GB visualization (1 4 hours)
delayed GB visualization (1 4 hours) visualization of bowel prior to GB (sensitivity 45%, specificity 90%)
visualization of bowel prior to GB (sensitivity 45%, specificity 90%) noncontractility/decreased response after CCK injection (decreased GB ejection fraction)
noncontractility/decreased response after CCK injection (decreased GB ejection fraction)
Emphysematous Cholecystitis
= ischemia of gallbladder wall + infection with gas-producing organisms
| Etiology: | small-vessel disease with cystic artery occlusion, complication of acute cholecystitis |
| Organism: | Clostridium perfringens, Clostridium welchii, E. coli, staphylococcus, streptococcus |
| Age: | >50 years; M:F = 5:1 |
| Predisposed: | diabetics (20 50%), debilitating diseases; calculous (70 80%)/acalculous cystic duct obstruction |
WBC count may be normal (1/3)
point tenderness rare (diabetic neuropathy)
Plain film:
 gas appears 24 48 hours after onset of symptoms
gas appears 24 48 hours after onset of symptoms air-fluid level in GB lumen, air in GB wall within 24 48 hours after acute episode
air-fluid level in GB lumen, air in GB wall within 24 48 hours after acute episode pneumobilia (rare)
pneumobilia (rare)
US:
 arclike high-level echoes outlining GB wall
arclike high-level echoes outlining GB wall cholecystolithiasis (50%)
cholecystolithiasis (50%)
| Cx: | gangrene (75%); gallbladder perforation (20%) |
| Mortality: | 15% |
| DDx: | (1) Enteric fistula (2) Incompetent sphincter of Oddi (3) Air-containing periduodenal abscess (4) Periappendiceal abscess in malpositioned appendix (5) Lipomatosis of gallbladder |
Xanthogranulomatous Cholecystitis
= FIBROXANTHOGRANULOMATOUS INFLAMMATION= CEROID GRANULOMAS OF THE GALLBLADDER
= uncommon inflammatory disease of gallbladder characterized by presence of multiple intramural nodules
Etiology:
rupture of occluded Rokitansky-Aschoff sinuses with subsequent intramural extravasation of inspissated bile + mucin attracting histiocytes to phagocytize the insoluble cholesterol
| Incidence: | 1 2% |
| Age: | 7th + 8th decade |
| Histo: | mixture of ceroid (waxlike) xanthogranuloma with foamy histiocytes + multinucleated foreign body giant cells + lymphocytes + fibroblasts containing areas of necrosis (in newer lesions) |
| May be associated with: | gallbladder carcinoma (11%) |
P.703
 preservation of 2 3-mm thick mucosal lining (in 82%)
preservation of 2 3-mm thick mucosal lining (in 82%) thickened gallbladder wall: 91% diffuse, 9% focal
thickened gallbladder wall: 91% diffuse, 9% focal infiltration of pericholecystic fat: in 45% focal, in 54% diffuse
infiltration of pericholecystic fat: in 45% focal, in 54% diffuse hepatic extension (45%)
hepatic extension (45%) biliary obstruction (36%)
biliary obstruction (36%) lymphadenopathy (36%)
lymphadenopathy (36%)US:
 intramural hypoechoic nodules
intramural hypoechoic nodules
CT:
 5 20-mm small intramural hypoattenuating nodules
5 20-mm small intramural hypoattenuating nodules poor/heterogeneous contrast enhancement
poor/heterogeneous contrast enhancement
| DDx: | gallbladder carcinoma (in 59% focal, in 41% diffuse thickening of gallbladder wall, multiple masses within liver) |
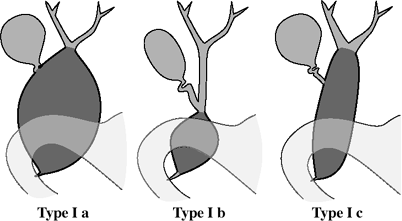 |
| Choledochal Cysts |
Choledochal Cyst
= CYSTIC DILATATION OF EXTRAHEPATIC BILE DUCT
= segmental aneurysmal dilatation of common bile duct without involvement of gallbladder/cystic duct; most common congenital lesion of bile ducts
| Etiology: | anomalous junction of pancreatic duct and CBD proximal to duodenal papilla; higher pressure in pancreatic duct and absent ductal sphincter allows free reflux of enzymes into CBD resulting in weakening of CBD wall |
Classification:
malunion of pancreaticobiliary duct
| Kimura type I | = pancreatic duct enters the proximal/mid CBD (10 58%) at right angle |
| Kimura type II | = CBD drains into pancreatic duct |
| Prevalence: | 1:13,000 admissions; high prevalence in Japanese/Asian infants |
| Age: | <10 years (60%) + young adulthood; 80% diagnosed in childhood; 7% during pregnancy; occasionally detected up to 8th decade; M:F = 1:4 |
| Histo: | fibrous cyst wall without epithelial lining |
Associated with:
dilatation, stenosis or atresia of other portions of the biliary tree (2%)
gallbladder anomaly (aplasia, double GB)
failure of union of left + right hepatic ducts
pancreatic duct + accessory hepatic bile ducts may drain into cyst
polycystic liver disease
Classic triad (20 30% of adult patients):
intermittent obstructive jaundice (33 50%)
 Uncommon cause of obstructive jaundice!
Uncommon cause of obstructive jaundice!
recurrent RUQ colicky pain (>75 90%), back pain
intermittent palpable RUQ abdominal mass (<25%)
recurrent fever, chills, weight loss, pruritus
Types:
marked cystic dilatation of CBD + CHD
focal segmental dilatation of CBD distally
cylindric dilatation of CBD + CHD
 size: diameter of 2 cm up to 15 cm (the largest choledochal cyst contained 13 liters)
size: diameter of 2 cm up to 15 cm (the largest choledochal cyst contained 13 liters) NO/mild peripheral intrahepatic bile duct dilatation
NO/mild peripheral intrahepatic bile duct dilatation may contain stones/sludge
may contain stones/sludgeUGI:
 soft-tissue mass in RUQ
soft-tissue mass in RUQ anterior displacement of 2nd portion of duodenum + distal portion of stomach (on LAT view)
anterior displacement of 2nd portion of duodenum + distal portion of stomach (on LAT view) widening of C-loop with inferior displacement of duodenum (on AP view)
widening of C-loop with inferior displacement of duodenum (on AP view)
US:
 ballooned/fusiform cyst beneath porta hepatis separate from gallbladder
ballooned/fusiform cyst beneath porta hepatis separate from gallbladder Communication with common hepatic/intrahepatic ducts needs to be demonstrated!
Communication with common hepatic/intrahepatic ducts needs to be demonstrated!
 abrupt change of caliber at junction of dilated segment to normal ducts
abrupt change of caliber at junction of dilated segment to normal ducts intrahepatic bile duct dilatation (16%) secondary to stenosis
intrahepatic bile duct dilatation (16%) secondary to stenosis
OB-US (earliest diagnosis at 25 weeks MA):
 right-sided cyst in fetal abdomen + adjacent dilated hepatic ducts
right-sided cyst in fetal abdomen + adjacent dilated hepatic ducts
DDx: duodenal atresia; cyst of ovary, mesentery, omentum, pancreas, liver NUC with HIDA:
 At times the choledochal cyst does not fill with radionuclide!
At times the choledochal cyst does not fill with radionuclide! photopenic area within liver that fills within 60 minutes + stasis of tracer within cyst
photopenic area within liver that fills within 60 minutes + stasis of tracer within cyst lack of tracer passage into small intestine
lack of tracer passage into small intestine prominent hepatic ductal activity (dilatation of ducts)
prominent hepatic ductal activity (dilatation of ducts)
DDx: often excludes hepatic cyst, pancreatic pseudocyst, enteric duplication, spontaneous loculated biloma Cholangiography/MR cholangiography (confirms diagnosis):
 anomalous junction of panreaticobiliary ductal system
anomalous junction of panreaticobiliary ductal system dilated intrahepatic bile ducts
dilated intrahepatic bile ducts intraductal calculi
intraductal calculi
| Cx: | (1) Stones in gallbladder, in CBD, within cyst, in intrahepatic biliary tree, in pancreatic duct (8 50%) (2) Malignant transformation into bile duct carcinoma + gallbladder carcinoma (increasing with age, <1% in 1st decade, 7 14% >age 20) (3) Recurrent pancreatitis (33%) (4) Cholangitis/cholecystitis (20%) (5) Cyst rupture with bile peritonitis (1.8 %) (6) Bleeding (7) Biliary cirrhosis + portal hypertension (8) Portal vein thrombosis (9) hepatic abscess |
| Rx: | excision of cyst + Roux-en-Y hepaticojejunostomy |
| DDx: | mesenteric, omental, ovarian, renal, adrenal, hepatic, enteric duplication cyst, pancreatic pseudocyst, hydronephrotic kidney, hepatic artery aneurysm, biloma (from spontaneous perforation of CBD) |
P.704
Choledochocele
= DUODENAL DUPLICATION CYST = ENTEROGENOUS CYST OF AMPULLA OF VATER/DUODENUM = INTRADUODENAL CHOLEDOCHAL CYST = DIVERTICULUM OF COMMON BILE DUCT
= cystic dilatation of the distal/intramural duodenal portion of the CBD with herniation of CBD into duodenum (similar to ureterocele)
Etiology:
congenital:
originates from tiny bud/diverticulum of distal CBD (found in 5.7% of normal population)
stenosis of ductal orifice/weakness of ductal wall
acquired:
stone passage followed by stenosis + inflammation
| Age: | 33 years (manifestation usually in adulthood) |
| Types: | (a) CBD terminates in cyst, cyst drains into duodenum (common) (b) cyst drains into adjacent intramural portion of CBD (less common) |
biliary colic, episodic jaundice, nausea, vomiting
| Associated with: | stones/sludge (frequent) |
UGI:
 smooth well-defined intraluminal duodenal filling defect in region of papilla
smooth well-defined intraluminal duodenal filling defect in region of papilla change in shape with compression/peristalsis
change in shape with compression/peristalsis
Cholangiography (diagnostic):
 opacified smooth clublike/saclike dilatation of intramural segment of CBD prolapsed into duodenum
opacified smooth clublike/saclike dilatation of intramural segment of CBD prolapsed into duodenum
| Cx: | pancreatitis, duodenal obstruction |
| Rx: | sphincterotomy/sphincteroplasty |
| DDx: | choledochal cyst (involves more than only terminal portion of CBD) |
Cholelithiasis
Prevalence:
25 million adults in United States; 10% of population + 2% of children;
increasing with age (40% of women in 9th decade);
in 3rd decade M:F = 2%:4%
in 7th decade M:F = 10%:25%
| Predisposing factors: | female, forty, fair, fat, fertile, flatulent |
Pathogenesis:
supersaturation of bile constituents, most notably cholesterol, related to defects in biliary lipid metabolism; biliary dysmotility; prolonged intestinal transit; aggravated by sedentary lifestyle + diet
Hemolytic disease:
sickle cell disease (7 37%), hereditary spherocytosis (43 85%), thalassemia, pernicious anemia (16 20%), prosthetic cardiac valves + mitral stenosis (hemolysis), cirrhosis (hemolysis secondary to hypersplenism), Rhesus/ABO blood group incompatibility (perinatal period)
Metabolic disorder = disruption of biliary lithogenic index:
diabetes mellitus, obesity, pancreatic disease, cystic fibrosis, hypercholesterolemia, type 4 hyperlipidemia, hemosiderosis (20%), hyperparathyroidism, hypothyroidism, prolonged use of estrogens/progesterone, pregnancy
Cholestasis
hepatic dysfunction: hepatitis, neonatal sepsis
biliary tree malformation: Caroli disease
biliary obstruction: parasitic infection, benign/malignant strictures, foreign bodies (sutures, ascariasis)
prolonged fasting (total parenteral nutrition)
methadone intake
Intestinal malabsorption
has a 10 increased risk of stone formation
Inflammatory bowel disease: Crohn disease (28 34%)
ileal resection
bypass surgery
Genetic predisposition = familial:
Navaho, Pima, Chippewa Indians
Others:
muscular dystrophy
Composition:
CHOLESTEROL STONE (70%)
= main component of most calculi
 lucent (93%), calcified (7%)
lucent (93%), calcified (7%) slightly hypodense compared with bile
slightly hypodense compared with bile
pure cholesterol stones (10%): yellowish, soft
 buoyancy in contrast-enhanced bile
buoyancy in contrast-enhanced bile density of <100 HU
density of <100 HU
mixture of cholesterol + calcium carbonate/bilirubinate (70%)
 laminated appearance
laminated appearance radiopaque on plain film (15 20%)
radiopaque on plain film (15 20%)
PIGMENT STONE (30%)
brown (common) = granular precipitate of calcium bilirubinate containing <25% cholesterol (by definition)
Cause: inflammation/infection of gallbladder, status post cholecystectomy black (less common) = compact lacquer of bilirubin derivatives with a high affinity for calcium carbonate
 multiple tiny faceted/spiculated homogeneously radiopaque stones
multiple tiny faceted/spiculated homogeneously radiopaque stonesCT:
 usually denser than bile
usually denser than bile
GAS-CONTAINING GALLSTONE
Mechanism: dehydration of older stones leads to internal shrinkage + dendritic cracks + subsequent nitrogen gas filling from negative internal pressure  crow-foot = Mercedes-Benz sign = radiating streaklike lucencies within stone, also responsible for buoyancy
crow-foot = Mercedes-Benz sign = radiating streaklike lucencies within stone, also responsible for buoyancy
FLOATING GALLSTONE (20 25%)
relatively pure cholesterol stones
gas-containing stones
rise in specific gravity of bile (1.03) from oral cholecystopaques (specific gravity of 1.06) causing stones (specific gravity of 1.05) to float
P.705
GALLBLADDER SLUDGE
= calcium-bilirubinate granules + cholesterol crystals associated with biliary stasis
Cause: prolonged fasting, parenteral nutrition, hyperalimentation, hemolysis, extrahepatic bile duct obstruction, cystic duct obstruction, acute + chronic cholecystitis  nonshadowing homogeneously echogenic material:
nonshadowing homogeneously echogenic material: fluid-sludge level
fluid-sludge level
 sludge ball = tumefactive sludge:
sludge ball = tumefactive sludge: slowly shifting with repositioning of patient
slowly shifting with repositioning of patient
DDx: gallbladder cancer Prognosis: may cause acute cholecystitis DDx: hemobilia with blood clot, parasitic infestation, mucus
Radiopacity:
 lucent stones (84%):
lucent stones (84%):cholesterol (85%), pigment (15%)
 calcified stones (15 20% on plain film, 60% on CT):
calcified stones (15 20% on plain film, 60% on CT):cholesterol (33%), pigment (67%)
Location of calcium:
 calcium phosphate deposited centrally within cholesterol stones
calcium phosphate deposited centrally within cholesterol stones calcium carbonate deposited radially within aging cholesterol/peripherally around cholesterol + pigmented stones
calcium carbonate deposited radially within aging cholesterol/peripherally around cholesterol + pigmented stones
Gallstones in Fetus
| EGA: | >28 weeks EGA |
| Cause: | hemolytic disease, cholestasis, maternal drug use |
| Prognosis: | usually resolve before/after delivery |
Gallstones in Neonate
 Rare without predisposing factors
Rare without predisposing factors
| Associated with: | obstructive congenital biliary anomaly, total parenteral nutrition, furosemide, GI dysfunction (short-gut syndrome), prolonged fasting, phototherapy, dehydration, infection, hemolytic anemia |
Gallstones in Older Children
| Associated with: | sickle cell disease, cystic fibrosis, malabsorption, total parenteral nutrition, Crohn disease, intestinal resection, hemolytic anemia, choledochal cyst |
Cholecystolithiasis
asymptomatic (60 65%); become symptomatic at a rate of 2% per year
biliary colic (misnomer) due to transient obstruction of cystic duct/common bile duct develops in 33% (18% overall risk in 20 years):
= acute RUQ/epigastric/LUQ/precordial/lower abdominal pain increasing over seconds/minutes + remaining fairly steady for 1 3( 6) hours associated with nausea + vomiting
no tenderness upon palpation
Abdominal plain film (10 16% sensitive):
 calcified gallstones
calcified gallstones
OCG (65 90% sensitive):
 filling defect in contrasted gallbladder lumen:
filling defect in contrasted gallbladder lumen: allows determination of size + number of gallstones
allows determination of size + number of gallstones demonstrates cystic duct patency
demonstrates cystic duct patency shows contractility after a fatty meal
shows contractility after a fatty meal
 nonvisualization of gallbladder (25%) = inconclusive
nonvisualization of gallbladder (25%) = inconclusive
CT (80% sensitive):
 hyperdense calcified gallstones in 60%
hyperdense calcified gallstones in 60% hypodense cholesterol stones 140 HU = pure cholesterol stone (= 80% cholesterol content):
hypodense cholesterol stones 140 HU = pure cholesterol stone (= 80% cholesterol content): Inverse relationship between CT attenuation number + cholesterol content
Inverse relationship between CT attenuation number + cholesterol content
 gallstones isointense to bile in 21 24% and thus undetectable by CT (<30 HU)
gallstones isointense to bile in 21 24% and thus undetectable by CT (<30 HU)
US (91 98% sensitive; in 5% falsely negative):
 bright (= highly reflective) echo from anterior surface of gallstone within gallbladder:
bright (= highly reflective) echo from anterior surface of gallstone within gallbladder: marked posterior acoustic shadowing
marked posterior acoustic shadowing mobile upon repositioning of patient (may infrequently be adherent to wall)
mobile upon repositioning of patient (may infrequently be adherent to wall) reverberation artifact
reverberation artifact Small calcifications <2 mm may not shadow
Small calcifications <2 mm may not shadow
 nonvisualization of GB + collection of echogenic echoes with acoustic shadowing (15 25%):
nonvisualization of GB + collection of echogenic echoes with acoustic shadowing (15 25%): wall-echo-shadow = double-arc shadow sign = 2 echogenic curvilinear parallel lines separated by sonolucent line (ie, anterior GB wall + bile + stone with acoustic shadowing)
wall-echo-shadow = double-arc shadow sign = 2 echogenic curvilinear parallel lines separated by sonolucent line (ie, anterior GB wall + bile + stone with acoustic shadowing)
 focal nonshadowing opacities <5 mm in diameter (in 70% gallstones)
focal nonshadowing opacities <5 mm in diameter (in 70% gallstones)False-negative US (5%):
contracted GB, GB in anomalous/unusual location, small gallstone, gallstone impacted in GB neck/cystic duct, immobile patient, obese patient, extensive RUQ bowel gas
| Prognosis: | stones <3 mm may pass through cystic duct |
| Cx: | acute cholecystitis (in 30%), choledocholithiasis, cholangitis, pancreatitis, duodenitis, biliary fistula, gallstone ileus, Mirizzi syndrome; cancer of GB + bile ducts (2 3 more frequent) |
Cholangiolithiasis
Choledocholithiasis
 Most common cause of bile duct obstruction!
Most common cause of bile duct obstruction!
| Etiology: | (a) passed stones originating in GB (b) primary development in intra-/extrahepatic ducts |
| Incidence: | in 12 15% of cholecystectomy patients; in 3 4% of postcholecystectomy patients; in 75% of patients with chronic bile duct obstruction |
Risk indicators for CBD stone:
recent history of jaundice
recent history of pancreatitis
elevated serum bilirubin >17 mol/L
elevated serum amylase >120 IU/L
dilated CBD >6 mm (16%)
obscured bile duct
P.706
asymptomatic: 10% of patients treated with cholecystectomy have unsuspected CBD calculi
recurrent episodes of right upper quadrant pain, jaundice, chills, fever (25 50%)
elevated serum bilirubin + alkaline phosphate levels
elevated transaminase (75%)
spontaneous passage with stones <6 mm size
Cholangiography (most specific technique):
 stone visualization in 92%
stone visualization in 92% dependent round filling defects
dependent round filling defects
DDx: air bubbles, neoplasm, concentrated bile Peroperative cholangiography:
prolongs operation by 30 minutes;
4% false-negatives; 4 10% false-positives
US (22 82% sensitive):
 stone visualization in 13 75% (more readily with CBD dilatation + good visibility of pancreatic head)
stone visualization in 13 75% (more readily with CBD dilatation + good visibility of pancreatic head) dilated ducts in 64 77%/duct <8 mm in diameter in 24 36%
dilated ducts in 64 77%/duct <8 mm in diameter in 24 36% increased dilatation of CBD with administration of fatty meal/cholecystokinin
increased dilatation of CBD with administration of fatty meal/cholecystokinin no stone in gallbladder (1.2 11%)
no stone in gallbladder (1.2 11%)
CT (88% sensitive, 97% specific, 94% accurate):
 stone visualization in 75 88% (isoattenuating to bile in 12 25%)
stone visualization in 75 88% (isoattenuating to bile in 12 25%) target sign = intraluminal mass with crescentic ring (= stone of soft-tissue density) in 85%
target sign = intraluminal mass with crescentic ring (= stone of soft-tissue density) in 85% subtle alternating low- and high-attenuation rings (= mixed cholesterol-calcium stones)
subtle alternating low- and high-attenuation rings (= mixed cholesterol-calcium stones)
MRCP (81 100% sensitive, 85 100% specific):
 dark filling defect within hyperintense fluid (stone must be >2 mm in diameter) (DDx: tumor, edematous papilla of Vater
dark filling defect within hyperintense fluid (stone must be >2 mm in diameter) (DDx: tumor, edematous papilla of Vater signal void on moderately T2WI (TE of 100 msec) BUT tumors have intermediate signal intensity
signal void on moderately T2WI (TE of 100 msec) BUT tumors have intermediate signal intensity no enhancement of calculus
no enhancement of calculus obscured/missed calculi:
obscured/missed calculi: visible signal intensity on T1WI due to sufficient water content
visible signal intensity on T1WI due to sufficient water content isointensity relative to bile
isointensity relative to bile
NUC:
 delayed bowel activity beyond 2 hours
delayed bowel activity beyond 2 hours persistent hepatic + common bile duct activity to 24 hours
persistent hepatic + common bile duct activity to 24 hours prominent ductal activity beyond 90 minutes with visualization of secondary ducts
prominent ductal activity beyond 90 minutes with visualization of secondary ducts
Stone in Cystic Duct Remnant
retained in 0.4% after surgery for choledocholithiasis
Chronic Granulomatous Disease Of Childhood
= recessive X-linked (60%)/autosomal (40%) immunodeficiency disorder resulting in purulent infections + granuloma formation primarily involving lymph nodes, skin, lungs
| Etiology: | polymorphonuclear leukocyte dysfunction characterized by inability to generate hydrogen peroxide causing prolonged intracellular survival of phagocytized catalase-positive bacteria with dissemination in reticuloendothelial system |
| Organism: | most commonly staphylococcus, Serratia marcescens, Nocardia species, mycobacteria, fungi |
| Path: | chronic infection with granuloma formation/caseation/suppuration |
| Age: | onset in childhood; M > F (more severe in boys) |
recurrent chronic infections
nitroblue tetrazolium test: low percentage of WBCs that reduce the dye after stimulation by phagocytosis/contact with endotoxin (normally >90%)
@ Bone
 osteomyelitis (commonly of spine, ribs, metatarsals)
osteomyelitis (commonly of spine, ribs, metatarsals)
@ Chest
 chronic pneumonia
chronic pneumonia hilar lymphadenopathy
hilar lymphadenopathy pleural + pericardial effusions
pleural + pericardial effusions
@ Liver
 hepatosplenomegaly
hepatosplenomegaly hepatic abscess (most common abdominal process)
hepatic abscess (most common abdominal process) liver calcifications
liver calcifications
@ GI tract
chronic diarrhea with malabsorption
vomiting, anorexia, heartburn, weight loss
 esophageal dysmotility, esophagitis, stricture
esophageal dysmotility, esophagitis, stricture gastric antral narrowing gastric outlet obstruction
gastric antral narrowing gastric outlet obstruction perianal fistula + abscess
perianal fistula + abscess
@ GU tract
dysuria
 cystitis
cystitis obstruction of urethra + ureters
obstruction of urethra + ureters
@ Lymph nodes
 suppurative lymphadenitis
suppurative lymphadenitis
@ Skin
pyoderma
| Rx: | prophylactic long-term trimethoprim-sulfamethoxazole + interferon gamma therapy |
Cirrhosis
= chronic liver disease characterized by diffuse parenchymal necrosis, regeneration and scarring with abnormal reconstruction of preexisting lobular architecture
Etiology:
TOXIC
Alcoholic liver disease in 75%
Drug-induced (prolonged methotrexate, oxyphenisatin, alpha-methyldopa, nitrofurantoin, isoniazid)
Iron overload (hemochromatosis, hemosiderosis)
INFLAMMATION
Viral hepatitis B/C
Schistosomiasis
BILIARY OBSTRUCTION
Cystic fibrosis
Inflammatory bowel disease
Primary biliary cirrhosis
obstructive infantile cholangiopathy
VASCULAR
P.707
Prolonged CHF = cardiac cirrhosis
hepatic venoocclusive disease (Budd-Chiari syndrome)
NUTRITIONAL
Intestinal bypass
Severe steatosis
Abetalipoproteinemia
HEREDITARY
Wilson disease
Alpha-1 antitrypsin deficiency
Juvenile polycystic kidney disease
Galactosemia
Type IV glycogen storage disease
hereditary fructose intolerance
Tyrosinemia
hereditary tetany
Osler-Weber-Rendu syndrome
Familial cirrhosis
IDIOPATHIC/CRYPTOGENIC (15%)
Cirrhosis in children:
chronic hepatitis, congenital hepatic fibrosis, cystic fibrosis, biliary atresia, alpha-1 antitrypsin deficiency, tyrosinemia, galactosemia, hemochromatosis, Wilson disease, schistosomiasis, total parenteral nutrition
Morphology:
micronodular cirrhosis (<3 mm): usually due to alcoholism, biliary obstruction, hemochromatosis, venous outflow obstruction, previous small-bowel bypass surgery, Indian childhood fibrosis
macronodular cirrhosis (3 15 mm, up to several cm): usually due to chronic viral hepatitis, Wilson disease, alpha-1 antitrypsin deficiency
mixed cirrhosis
Nodular lesions:
regenerative nodule = localized proliferation of hepatocytes + supporting stroma
dysplastic nodule
= cluster of hepatocytes >1 mm in diameter with evidence of dysplasia; common in hepatitis B and C, alpha-1 antitrypsin deficiency, tyrosinemia
low grade (macroregenerative nodule, type I/ordinary adenomatous hyperplasia)
high grade (macroregenerative nodule type II/adenomatous hyperplasia with atypia)
dysplastic nodule with subfocus of HCC (adenomatous hyperplasia with microscopic HCC)
hepatocellular carcinoma (adenomatous hyperplasia with macroscopic HCC)
| Associated with: | anemia, coagulopathy, hypoalbuminemia, cholelithiasis, pancreatitis, peptic ulcer disease, diarrhea, hypogonadism |
anorexia, weakness, fatigue, weight loss
jaundice, continuous low-grade fever
ascites, bleeding from esophageal varices, hepatic encephalopathy
 enlarged (early stage)/normal/shrunken liver
enlarged (early stage)/normal/shrunken liver shrinkage of right lobe (segments 5 8) and medial segment of left lobe (segments 4a + 4b) with concomitant hypertrophy of lateral segment of left lobe (segments 2 + 3) and caudate lobe (segment 1):
shrinkage of right lobe (segments 5 8) and medial segment of left lobe (segments 4a + 4b) with concomitant hypertrophy of lateral segment of left lobe (segments 2 + 3) and caudate lobe (segment 1): ratio of width of caudate to right lobe >0.65 on transverse images [sensitivity 43 84%, least sensitive in alcoholic cirrhosis, most sensitive in cirrhosis caused by hepatitis B; specificity 100%; 26% sensitivity; 84 96% accuracy] (DDx: Budd-Chiari syndrome)
ratio of width of caudate to right lobe >0.65 on transverse images [sensitivity 43 84%, least sensitive in alcoholic cirrhosis, most sensitive in cirrhosis caused by hepatitis B; specificity 100%; 26% sensitivity; 84 96% accuracy] (DDx: Budd-Chiari syndrome) diameter of quadrate lobe (segment 4) <30 mm (= distance between left wall of gallbladder and ascending portion of left portal vein) due to selective atrophy (95% specific)
diameter of quadrate lobe (segment 4) <30 mm (= distance between left wall of gallbladder and ascending portion of left portal vein) due to selective atrophy (95% specific)
 widened porta hepatis + interlobar fissure
widened porta hepatis + interlobar fissure surface nodularity + indentations (regenerating nodules)
surface nodularity + indentations (regenerating nodules) signs of portal hypertension
signs of portal hypertension splenomegaly
splenomegaly ascites (failure of albumin synthesis, overproduction of lymph due to increased hydrostatic pressure in sinusoids/decreased splanchnic output due to portal hypertension)
ascites (failure of albumin synthesis, overproduction of lymph due to increased hydrostatic pressure in sinusoids/decreased splanchnic output due to portal hypertension) associated with fatty infiltration (in early cirrhosis)
associated with fatty infiltration (in early cirrhosis)US (sensitivity 65 80%; DDx: chronic hepatitis, fatty infiltration):
Hepatic signs:
 surface nodularity (54% sensitive, 95% specific)
surface nodularity (54% sensitive, 95% specific) caudate lobe hypertrophy (41% sensitive, 91% specific)
caudate lobe hypertrophy (41% sensitive, 91% specific) portalization of hepatic vein waveform = dampened oscillations of hepatic veins resembling portal vein flow (57% sensitive, 76% specific)
portalization of hepatic vein waveform = dampened oscillations of hepatic veins resembling portal vein flow (57% sensitive, 76% specific) hepatomegaly (in 63%)
hepatomegaly (in 63%) increased hepatic parenchymal echogenicity in 66% (as a sign of superimposed fatty infiltration):
increased hepatic parenchymal echogenicity in 66% (as a sign of superimposed fatty infiltration): increased sound attenuation (9%)
increased sound attenuation (9%) decreased/normal definition of walls of portal venules (sign of associated fatty infiltration NOT of fibrosis)
decreased/normal definition of walls of portal venules (sign of associated fatty infiltration NOT of fibrosis)
 heterogeneous coarse (usually)/fine echotexture (in 7%)
heterogeneous coarse (usually)/fine echotexture (in 7%) occasional depiction of isoechoic regenerative nodules
occasional depiction of isoechoic regenerative nodules dilatation of hepatic arteries (increased arterial flow) with demonstration of intrahepatic arterial branches (DDx: dilated biliary radicals)
dilatation of hepatic arteries (increased arterial flow) with demonstration of intrahepatic arterial branches (DDx: dilated biliary radicals) increase in hepatic artery resistance (mean RI of 0.58 0.66 in normals to 0.63 0.85 in cirrhotics):
increase in hepatic artery resistance (mean RI of 0.58 0.66 in normals to 0.63 0.85 in cirrhotics): blunted increase in RI after meal ingestion (from 42% in normals to 7% in cirrhotics)
blunted increase in RI after meal ingestion (from 42% in normals to 7% in cirrhotics)
Extrahepatic signs:
 splenomegaly
splenomegaly ascites
ascites signs of portal hypertension
signs of portal hypertension
CT:
 native + enhanced parenchymal inhomogeneity
native + enhanced parenchymal inhomogeneity decreased attenuation (steatosis) in early cirrhosis
decreased attenuation (steatosis) in early cirrhosis isodense/hyperdense (siderotic) regenerative nodules
isodense/hyperdense (siderotic) regenerative nodules nodular/lobulated liver contour
nodular/lobulated liver contour predominantly portal venous supply to dysplastic nodules
predominantly portal venous supply to dysplastic nodules hypodense area adjacent to portal vein (= peribiliary cysts from obstructed extramural peribiliary glands)
hypodense area adjacent to portal vein (= peribiliary cysts from obstructed extramural peribiliary glands) rapid tapering of intrahepatic portal + hepatic venous branches
rapid tapering of intrahepatic portal + hepatic venous branches
CECT:
 enlarged tortuous hepatic artery (compensatory increase in arterial blood flow)
enlarged tortuous hepatic artery (compensatory increase in arterial blood flow) arterioportal shunts (through trans-sinusoidal shunts in liver periphery + transplexal shunts with hypertrophy of peribiliary plexus) in hepatic arterial phase:
arterioportal shunts (through trans-sinusoidal shunts in liver periphery + transplexal shunts with hypertrophy of peribiliary plexus) in hepatic arterial phase: poorly demarcated transient peripheral wedge-shaped hepatic parenchymal enhancement
poorly demarcated transient peripheral wedge-shaped hepatic parenchymal enhancementDDx: hepatocellular carcinoma (defect on portal venous phase)  early retrograde enhancement of portal vein branches
early retrograde enhancement of portal vein branches hepatofugal flow
hepatofugal flowCause: with occlusion of small hepatic venules the portal vein turns from a supplying vein into a draining vein
P.708
MR (problem-solving tool):
 no alteration of liver parenchyma
no alteration of liver parenchyma regenerative nodules ((due to iron deposits within nodules):
regenerative nodules ((due to iron deposits within nodules): hypo-/isointense relative to liver on T1WI
hypo-/isointense relative to liver on T1WI hypointense on T2WI
hypointense on T2WI no enhancement
no enhancement
 dysplastic nodule = iso-/hyperintense on T1WI + iso-/hypointense on T2WI
dysplastic nodule = iso-/hyperintense on T1WI + iso-/hypointense on T2WI HCC nodule = hypo-/iso-/hyperintense on T1WI + usually hyperintense on T2WI with marked enhancement during arterial phase
HCC nodule = hypo-/iso-/hyperintense on T1WI + usually hyperintense on T2WI with marked enhancement during arterial phase generalized decreased hepatic signal intensity on T2WI (mild iron deposition for unknown reasons)
generalized decreased hepatic signal intensity on T2WI (mild iron deposition for unknown reasons)
Angio:
 stretched hepatic artery branches (early finding)
stretched hepatic artery branches (early finding) enlarged tortuous hepatic arteries = corkscrewing (increase in hepatic arterial flow)
enlarged tortuous hepatic arteries = corkscrewing (increase in hepatic arterial flow) shunting between hepatic artery and portal vein
shunting between hepatic artery and portal vein mottled parenchymal phase
mottled parenchymal phase delayed emptying into venous phase
delayed emptying into venous phase pruning of hepatic vein branches (normally depiction of 5th order branches) = postsinusoidal compression by developing nodules
pruning of hepatic vein branches (normally depiction of 5th order branches) = postsinusoidal compression by developing nodules
NUC (Tc-99m labeled sulfur colloid):
 high blood pool activity secondary to slow clearance
high blood pool activity secondary to slow clearance colloid shift to bone marrow + spleen + lung
colloid shift to bone marrow + spleen + lung shrunken liver with little or no activity + splenomegaly
shrunken liver with little or no activity + splenomegaly mottled hepatic uptake (pseudotumors) on colloid scan (normal activity on IDA scans!)
mottled hepatic uptake (pseudotumors) on colloid scan (normal activity on IDA scans!) displacement of liver + spleen from abdominal wall by ascites
displacement of liver + spleen from abdominal wall by ascites
| Cx: | (1) Ascites: cause/contributor to death in 50% (2) Portal hypertension (3) Hepatocellular carcinoma (in 7 12%) (4) Cholangiocarcinoma |
Fatality from:
esophageal variceal bleeding (in 25%), hepatorenal syndrome (10%), spontaneous bacterial peritonitis (5 10%), complications from treatment of ascites (10%)
Primary Biliary Cirrhosis
= CHRONIC NONSUPPURATIVE DESTRUCTIVE CHOLANGITIS
| Histo: | idiopathic progressive destructive cholangitis of interlobar and septal bile ducts, portal fibrosis, nodular regeneration, shrinkage of hepatic parenchyma |
| Age: | 35 55 years; M:F = 1:9 |
Associated autoimmune disorders:
rheumatoid arthritis, Hashimoto thyroiditis, Sj gren syndrome, scleroderma, sarcoidosis
 66 100% of patients with primary biliary cirrhosis have sicca-complex signs of the Sj gren syndrome
66 100% of patients with primary biliary cirrhosis have sicca-complex signs of the Sj gren syndrome
fatigue, pruritus
xanthelasma/xanthoma (25%)
hyperpigmentation (50%)
insidious onset of pruritus (60%)
IgM increased (95%)
positive antimitochondrial antibodies (AMA) in 85 100%
 normal extrahepatic ducts
normal extrahepatic ducts cholelithiasis in 35 39%
cholelithiasis in 35 39%
CT:
 scattered dilated intrahepatic ducts with no apparent connection to main bile ducts
scattered dilated intrahepatic ducts with no apparent connection to main bile ducts caudate lobe hypertrophy (in 98%):
caudate lobe hypertrophy (in 98%): hypertrophied hyperattenuating caudate lobe surrounded by hypoattenuating rindlike right lobe (pseudotumor)
hypertrophied hyperattenuating caudate lobe surrounded by hypoattenuating rindlike right lobe (pseudotumor)
 atrophy of lateral segment of left hepatic lobe
atrophy of lateral segment of left hepatic lobe intrahepatic biliary calculi (20%)
intrahepatic biliary calculi (20%)
NUC:
 marked prolongation of hepatic Tc-99m IDA clearance
marked prolongation of hepatic Tc-99m IDA clearance uniform hepatic isotope retention
uniform hepatic isotope retention normal visualization of GB and major bile ducts in 100%
normal visualization of GB and major bile ducts in 100%
| DDx: | (1) Sclerosing cholangitis (young men) (2) CBD obstruction |
| Prognosis: | mean survival 6 (range 3 11) years after onset of cholestatic symptoms |
Complications of End-Stage Liver Disease
Hepatopulmonary Syndrome
| Dx: | (1) chronic liver disease (2) increased alveolar-arterial gradient (3) intrapulmonary vascular dilatation |
hypoxemia (in 1/3 of decompensated cirrhotic patients)
Pathomechanism:
elevation of unknown vasoactive substances in cirrhotic patient cause pulmonary vascular dilatation (from 8 15 m to 15 500 m) + result in diffusion-perfusion mismatch
 basilar nodular/reticulonodular areas of increased opacity (in 46 100%)
basilar nodular/reticulonodular areas of increased opacity (in 46 100%) dilated arterioles with an increased number of terminal branches extending to pleura
dilated arterioles with an increased number of terminal branches extending to pleura intrapulmonary arteriovenous shunt (demonstrated with Tc-99m macroaggregated albumin imaging, microbubble echocardiography)
intrapulmonary arteriovenous shunt (demonstrated with Tc-99m macroaggregated albumin imaging, microbubble echocardiography)
Hepatic Hydrothorax
= large pleural effusion in cirrhotic patient without primary pulmonary/cardiac disease
| Prevalence: | 10% |
| Mechanism: | pressure gradient favors fluid movement from peritoneal to pleural cavity through small diaphragmatic defects |
 pleural fluid: right in 67%, left in 17%, bilateral in 17%
pleural fluid: right in 67%, left in 17%, bilateral in 17%
Pulmonary Hypertension
| Prevalence: | 0.73% in patients with liver cirrhosis (versus 0.13% in all patients) |
P.709
Cause:
thromboembolic: portal venous thrombus reaches lung through spontaneous/surgically created portosystemic shunts
plexogenic: vasoactive substances (serotonin, thromboxane, neuropeptide Y, elastase) bypass the liver through portosystemic shunts
| Prognosis: | mean survival of 15 months |
Clonorchiasis
Rarely of clinical significance
| Country: | endemic to Southeast Asia: Japan, Korea, Central + South China, Taiwan, Indochina |
| Organism: | Chinese liver fluke = Clonorchis sinensis |
| Cycle: | parasite cysts digested by gastric juice, larvae migrate up the bile ducts, remain in small intrahepatic ducts until maturity (10 30 mm in length), travel to larger ducts to deposit eggs |
| Infection: | snail + freshwater fish serve as intermediate hosts; infection occurs by eating raw fish; hog, dog, cat, man are definite hosts |
| Path: | (a) desquamation of epithelial bile duct lining with adenomatous proliferation of ducts + thickening of duct walls (inflammation, necrosis, fibrosis) (b) bacterial superinfection with formation of liver abscess |
remittent incomplete obstruction + bacterial superinfection
 multiple crescent-/stiletto-shaped filling defects within bile ducts:
multiple crescent-/stiletto-shaped filling defects within bile ducts: echogenic focus/cast on US
echogenic focus/cast on US
 diffusely thickened bile ducts
diffusely thickened bile ducts
| Cx: | (1) Bile duct obstruction (conglomerate of worms/adenomatous proliferation) (2) Calculus formation (stasis/dead worms/epithelial debris) (3) Jaundice in 8% (stone/stricture/tumor) (4) Generalized dilatation of bile ducts (2%) |
Congenital Biliary Atresia
| Etiology: | ? variation of same infectious process as in neonatal hepatitis with additional component of sclerosing cholangitis or vascular injury |
| Prevalence: | <10 in 100,000 live births |
| Age: | neonate; M:F = 2:1 |
| Histo: | periportal fibrosis, proliferation of small intrahepatic bile ducts, mixed inflammatory infiltrates |
| In 15% associated with: | polysplenia, trisomy 18 |
Types:
| I | Focal = intrauterine vascular insult (extremely rare) |
| II | Intrahepatic biliary atresia = paucity of intrahepatic bile ducts (uncommon) |
| III | Extrahepatic biliary atresia = atresia of CBD + patent intrahepatic bile ducts |
Subtype 1 = perinatal type (66%)
jaundice develops after regression of physiologic jaundice
 bile duct remnant in porta hepatis
bile duct remnant in porta hepatis
Subtype 2 = embryonic/fetal type (34%)
normal decline in bilirubin does not occur
 NO bile duct remnant in porta hepatis
NO bile duct remnant in porta hepatis
Associated with: polysplenia (10 12%), intestinal malrotation, azygos continuation of IVC, symmetric bilobed liver, situs inversus, preduodenal portal vein, anomalous hepatic arteries, bilobed right lung, complex CHD
 |
| Biliary Atresia |
US:
 normal/increased size of liver
normal/increased size of liver normal/increased liver echogenicity
normal/increased liver echogenicity decreased visualization of peripheral portal veins (due to fibrosis)
decreased visualization of peripheral portal veins (due to fibrosis) triangular cord /tubular echogenic structure in porta hepatis (due to fibrous tissue) = PATHOGNOMONIC
triangular cord /tubular echogenic structure in porta hepatis (due to fibrous tissue) = PATHOGNOMONIC gallbladder findings:
gallbladder findings: nonvisualization of gallbladder
nonvisualization of gallbladder small gallbladder <1.5 cm in length + varying degrees of luminal compromise (DDx: hepatitis)
small gallbladder <1.5 cm in length + varying degrees of luminal compromise (DDx: hepatitis) normal gallbladder >1.5 cm in length (19%) when atresia of CBD distal to insertion of cystic duct
normal gallbladder >1.5 cm in length (19%) when atresia of CBD distal to insertion of cystic duct
 bile duct findings:
bile duct findings: no dilatation of intrahepatic bile ducts (due to panductal sclerosis)
no dilatation of intrahepatic bile ducts (due to panductal sclerosis) visualization of bile duct remnant in porta hepatis (depending on type of biliary atresia)
visualization of bile duct remnant in porta hepatis (depending on type of biliary atresia) small focal cystic dilatation of extrahepatic bile duct (= choledochal cyst) = patent segment of CBD with other parts being occluded due to fibrosis communication with gallbladder/intrahepatic bile ducts
small focal cystic dilatation of extrahepatic bile duct (= choledochal cyst) = patent segment of CBD with other parts being occluded due to fibrosis communication with gallbladder/intrahepatic bile ducts
NUC [phenobarbital-augmented cholescintigraphy] (90 97% sensitive, 60 94% specific, 75 90% accurate):
preparation of patient with 5 ng/kg/d phenobarbital twice a day for 3 7 days to stimulate biliary secretion (via induction of hepatic enzymes + increase in conjugation + excretion of bilirubin)
 good hepatic activity within 5 min (infants of <3 months of age have a normal hepatic extraction fraction)
good hepatic activity within 5 min (infants of <3 months of age have a normal hepatic extraction fraction) NO biliary excretion:
NO biliary excretion: NO visualization of bowel on delayed images at 6 and 24 hours
NO visualization of bowel on delayed images at 6 and 24 hours
 delayed clearance from cardiac blood pool
delayed clearance from cardiac blood pool increased renal excretion + bladder activity
increased renal excretion + bladder activity
| DDx: | severe hepatocellular dysfunction (DDx from neonatal hepatitis impossible in the absence of small bowel activity) requires liver biopsy |
P.710
MR cholangiography:
 nonvisualization of extrahepatic bile ducts
nonvisualization of extrahepatic bile ducts atrophic gallbladder
atrophic gallbladder periportal thickening
periportal thickening
Cholangiography (percutaneous/endoscopic/intraoperative) Liver Bx (60 97% accurate)
| Rx: |
|
DDx:
Neonatal hepatitis
Sclerosing cholangitis
Alagille syndrome = arteriohepatic dysplasia (abnormal facies, butterfly vertebra, pulmonic stenosis, complex CHD)
Congenital Hepatic Fibrosis
= congenital cirrhosis with rapid + fatal progression
| Histo: | fibrous tissue within hepatic parenchyma with excess numbers of distorted terminal interlobular bile ducts + cysts that rarely communicate with bile ducts |
| Age: | early childhood 6th decade; majority diagnosed in adolescence/early adulthood |
Associated with:
autosomal recessive polycystic kidney disease (invariably), Meckel-Gruber syndrome, vaginal atresia, tuberous sclerosis, nephronophthisis, medullary sponge kidney (80%), autosomal dominant polycystic kidney disease (rare)
liver function tests normal/mildly elevated
portal hypertension
predisposed to cholangitis + calculi
 lollipop-tree = ectasia of peripheral biliary radicles
lollipop-tree = ectasia of peripheral biliary radicles atrophy of right lobe + normal/enlarged medial segment of left lobe + hypertrophy of left lateral segment and caudate lobe
atrophy of right lobe + normal/enlarged medial segment of left lobe + hypertrophy of left lateral segment and caudate lobe splenomegaly
splenomegaly portosystemic varices
portosystemic varices enlarged hepatic artery associated with large multiacinar regenerative nodules
enlarged hepatic artery associated with large multiacinar regenerative nodules
| Cx: | cirrhosis, portal hypertension, hepatocellular carcinoma, cholangiocellular carcinoma |
Echinococcal Disease Of Liver
Echinococcus Granulosus
= HYDATID DISEASE = unilocular form
= E. cysticus (more common); man is accidental host
pastoral (European) form: dog is definite host; intermediate hosts are cattle, sheep, horses, hogs; endemic in sheep-raising countries: Australia, New Zealand, North + East Africa, USSR, Mediterranean countries, Near + Middle East, Japan, Argentina, Chile, Uruguay
sylvatic (northern) form: wolf is definite host; intermediate hosts are deer, moose; endemic in northwestern Canada, Alaska
| Cycle: | ingestion of contaminated material (eggs passed in feces of dog/other carnivore); eggs hatch in duodenum; larvae penetrate intestinal wall + mesenteric venules; larvae carried into portal circulation; larvae are filtered in capillaries of liver (first line of defense) > lung > other organs |
Histo:
CYST FLUID = antigenic clear/pale yellow fluid with neutral ph containing sodium chloride, proteins, glucose, ions, lipids, polysaccharides
ENDOCYST (parasitic component of capsule) = inner GERMINATIVE LAYER (resembling wet tissue paper) giving rise to brood capsules (daughter vesicles), which may
remain attached to cyst wall harboring up to 400,000 scolices
detach + form sediment in cyst fluid = hydatid sand
break up into numerous self-contained daughter cysts
ECTOCYST = CYST MEMBRANE = acellular laminated chitinlike substance secreted by parasite, allowing passage of nutrients
PERICYST = dense fibrous protective zone of host granulation tissue replacing tissue necrosis (from compression by the expanding cyst); marginal vascular rim of 0.5 4 mm
pain/asymptomatic
recurrent jaundice + biliary colic (transient obstruction by membrane fragments + daughter cysts expelled into biliary tree)
blood eosinophilia (20 50%)
urticaria + anaphylaxis (following rupture)
Tests:
Casoni intradermal test (60% sensitivity; may be falsely positive)
Complement fixation double diffusion (65% sensitivity)
Immunoelectrophoresis (most specific)
Indirect hemagglutination (85% sensitivity)
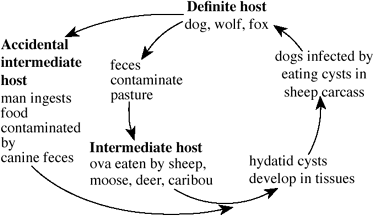 |
| Parasitic Cycle Of Echinococcus Granulosus |
| Time to diagnosis: | 11 81 (mean 51) years |
Affected organs:
liver (73%); lung (14%); peritoneum (12%); kidney (3 6%); spleen (0.9 8%); CNS (1%); orbit (1%); bone (0.5 4%); bladder; thyroid; prostate; heart
P.711
| Location: | right lobe > left lobe of liver; multiple cysts in 20% |
| Size: | up to 50 cm (average size of 5 cm), up to 16 liters of fluid; grows 2 3 cm annually |
Stages of cyst growth:
Unilocular cyst
Cyst with daughter vesicles/daughter cysts
Partially/completely calcified
Plain film:
 peripheral crescentic/curvilinear/polycyclic calcifications (10 20 33%), located in pericyst:
peripheral crescentic/curvilinear/polycyclic calcifications (10 20 33%), located in pericyst: Only complete calcification of all layers implies death of parasite!
Only complete calcification of all layers implies death of parasite!
 pneumohydrocyst (infection/communication with bronchial tree)
pneumohydrocyst (infection/communication with bronchial tree)
US:
 complex heterogeneous mass mimicking a solid mass (most common):
complex heterogeneous mass mimicking a solid mass (most common): Look for membranes/peripheral daughter vesicles
Look for membranes/peripheral daughter vesicles
 well-defined anechoic cyst (common):
well-defined anechoic cyst (common): cyst wall of double echogenic lines separated by hypoechoic layer
cyst wall of double echogenic lines separated by hypoechoic layer snowstorm sign = multiple internal echogenic foci settling to most dependent portion of cyst (= hydatid sand)
snowstorm sign = multiple internal echogenic foci settling to most dependent portion of cyst (= hydatid sand)
 multivesicular cyst of racemose /honeycomb appearance = multiple septa between daughter cysts inside mother cyst, CHARACTERISTIC but rare:
multivesicular cyst of racemose /honeycomb appearance = multiple septa between daughter cysts inside mother cyst, CHARACTERISTIC but rare: wheel spoke pattern = daughter cysts separated by echogenic material of hydatid matrix composed of broken daughter vesicles + scolices + hydatid sand
wheel spoke pattern = daughter cysts separated by echogenic material of hydatid matrix composed of broken daughter vesicles + scolices + hydatid sand HIGHLY SPECIFIC serpentine linear structures within hydatid matrix
HIGHLY SPECIFIC serpentine linear structures within hydatid matrix
 partial/complete detachment of endocyst from pericyst (due to decreasing intracystic pressure as a sign of degeneration/trauma/host response/response to therapy):
partial/complete detachment of endocyst from pericyst (due to decreasing intracystic pressure as a sign of degeneration/trauma/host response/response to therapy): localized split in wall with floating undulating membrane, CHARACTERISTIC but rare
localized split in wall with floating undulating membrane, CHARACTERISTIC but rare water lily sign = complete detachment of membrane
water lily sign = complete detachment of membrane Floating membrane does not indicate death of parasite!
Floating membrane does not indicate death of parasite!
 eggshell calcification in cyst wall (least common)
eggshell calcification in cyst wall (least common)
CT:
 well-demarcated round low-density mass of fluid attenuation (3 30 HU):
well-demarcated round low-density mass of fluid attenuation (3 30 HU): cyst wall of high attenuation on NECT
cyst wall of high attenuation on NECT linear areas of increased attenuation = detached laminated membrane
linear areas of increased attenuation = detached laminated membrane round peripheral fluid collection of lower attenuation (= daughter cysts)
round peripheral fluid collection of lower attenuation (= daughter cysts)
 enhancement of cyst wall + septations
enhancement of cyst wall + septations calcification of cyst wall/internal septa
calcification of cyst wall/internal septa
MR:
 cyst with hypointense rim (= collagenous pericyst) on T1WI + T2WI
cyst with hypointense rim (= collagenous pericyst) on T1WI + T2WI peripheral cysts within cyst hypointense on T1WI + hyperintense on T2WI (= daughter cysts)
peripheral cysts within cyst hypointense on T1WI + hyperintense on T2WI (= daughter cysts) twisted linear structures within cyst = collapsed parasitic membrane
twisted linear structures within cyst = collapsed parasitic membrane
Angio:
 avascular area with splaying of arteries
avascular area with splaying of arteries halo of increased density around cyst (inflammation/compressed liver)
halo of increased density around cyst (inflammation/compressed liver)
Cholangiography:
 cyst may communicate with bile ducts: right hepatic duct (55%), left hepatic duct (29%), CHD (9%), gallbladder (6%), CBD (1%)
cyst may communicate with bile ducts: right hepatic duct (55%), left hepatic duct (29%), CHD (9%), gallbladder (6%), CBD (1%)
Percutaneous aspiration:
fluid analysis positive for hydatid disease in 70% (fragments of laminated membrane in 54%; scolices in 15%; hooklets in 15%)
 Risk of anaphylactic shock (0.5%), asthma (3%), implantation of spilled protoscoleces
Risk of anaphylactic shock (0.5%), asthma (3%), implantation of spilled protoscoleces
Local Cx:
Rupture (50 90%)
contained = rupture of laminated membrane of endocyst, pericyst remains intact
 floating membranes
floating membranes
communicating = cyst contents escapes through biliary (5 15%)/bronchial tree
direct = tear of endocyst + ectocyst + pericyst with cyst contents spilling into pleural/peritoneal cavity (anaphylaxis, metastatic hydatidosis)
Infection (5 8%) following rupture
Transdiaphragmatic growth (0.6 16%) through bare area of liver
rupture into pleural cavity
seeding in pulmonary parenchyma
chronic bronchial fistula
Perforation into hollow viscus (0.5%)
Peritoneal seeding (13%) = encysted peritoneal hydatidosis
Compression of vital structures (bile ducts, portal vein)
| Rx: | (1) Surgery (in 10% recurrence) (2) Anthelmintics (albendazole, medendazole) (3) Injection of scolecidal agents (silver nitrate, 20/30% hypertonic saline solution,0.5% cetrimide solution, 95% ethanol) |
Echinococcus Multilocularis
= E. alveolaris = less common but more aggressive form of echinococcal disease
| Primary host: | fox, wolf |
| Secondary host: | rodents (moles, lemmings, wild mice); domestic cat; dog |
| Endemic to: | eastern France, southern Germany, western Austria, much of Soviet Union, Japan, Alaska, Canada, some areas in Turkey |
| Infection: | eating wild fruits contaminated with fox/wolf feces; direct contact with fox/wolf; contact with dogs/cats that have ingested infested rodents |
| Path: | larvae proliferate by exogenous extension + penetration of surrounding tissue (= diffuse + infiltrative process resembling malignancy); chronic granulomatous reaction with central necrosis, cavitation, calcification |
| Histo: | daughter cysts with thick lamellar wall arising on outer surface of original cyst, rarely containing scolices |
| Location: | liver (access via portal vein); widespread hematogenous dissemination not uncommon |
P.712
clinical manifestation 5 20 years after ingestion
abdominal discomfort, jaundice, hepatomegaly
eosinophilia
 aggressive growth pattern:
aggressive growth pattern: geographic infiltrating lesion with ill-defined margins
geographic infiltrating lesion with ill-defined margins invasion of IVC, diaphragm
invasion of IVC, diaphragm
 metastases to lung, heart, brain (in 10%)
metastases to lung, heart, brain (in 10%) faint/dense amorphous coalescent nodular/flame-shaped calcifications (dystrophic central calcifications scattered throughout necrotic + granulomatous tissue)
faint/dense amorphous coalescent nodular/flame-shaped calcifications (dystrophic central calcifications scattered throughout necrotic + granulomatous tissue)
US:
 echogenic geographic ill-defined single/multiple solid masses
echogenic geographic ill-defined single/multiple solid masses irregular cystic areas
irregular cystic areas propensity of spread to liver hilum
propensity of spread to liver hilum
CT:
 heterogeneous hypodense poorly marginated infiltrating masses
heterogeneous hypodense poorly marginated infiltrating masses pseudocystic necrotic regions of near water density surrounded by hyperdense solid component
pseudocystic necrotic regions of near water density surrounded by hyperdense solid component little/no enhancement
little/no enhancement
Angio:
 intrahepatic arterial tapering + obstruction
intrahepatic arterial tapering + obstruction
| Cx: | Budd-Chiari syndrome, IVC thrombosis, portal hypertension |
| Prognosis: | fatal within 10 15 years (if left untreated) |
| DDx: | hepatocellular carcinoma (biopsy!), large hemangioma (characteristic enhancement pattern), metastasis, epithelial hemangioendothelioma |
Embryonal Rhabdomyosarcoma Of Biliary Tree
rare tumor most commonly arising from CBD
| Median age: | 3 years; M > F |
| Path: | intraluminal biliary mass/cluster of grapelike masses (similar to rhabdomyosarcoma of bladder) |
| Histo: | same as sarcoma botryoides |
malaise, fever, jaundice
elevation of conjugated bilirubin
Metastases (in up to 30%) to:
retroperitoneal +mesenteric lymph nodes, lung
Location: common bile duct (most frequently)  8 20-cm bulky heterogeneous mass in porta hepatis
8 20-cm bulky heterogeneous mass in porta hepatis intrahepatic bile duct dilatation
intrahepatic bile duct dilatation displacement of duodenum, stomach, pancreas
displacement of duodenum, stomach, pancreasCholangiography:
 large bulky intraluminal mass/grapelike cluster of intraluminal masses focally distending common bile duct + obstructing proximal bile ducts
large bulky intraluminal mass/grapelike cluster of intraluminal masses focally distending common bile duct + obstructing proximal bile ducts
Epidermoid Cyst Of Spleen
EPITHELIAL CYST = PRIMARY CYST OF SPLEEN
| Incidence: | 10% of all benign nonparasitic cysts |
| Cause: | infolding of peritoneal mesothelium/collection of peritoneal mesothelial cells trapped within splenic sulci |
| Histo: | (1) mesothelial lining (2) squamous epithelial lining = epidermoid cyst = squamous metaplasia from embryonic inclusions within preexisting mesothelial surface epithelium |
| Age: | 2nd 3rd decade (average age of 18 years) |
| May be associated with: | polycystic kidney disease |
unilocular + solitary (80%)
multiple + multilocular (20%)
 well-defined thin-walled anechoic lesion of water density
well-defined thin-walled anechoic lesion of water density average size of 10 cm
average size of 10 cm peripheral septations/cyst wall trabeculations (in 86%)
peripheral septations/cyst wall trabeculations (in 86%) curvilinear calcification in wall (9 25%)
curvilinear calcification in wall (9 25%) may contain cholesterol crystals, fat, blood
may contain cholesterol crystals, fat, blood
| Cx: | trauma, rupture, infection |
Eosinophilic Cholangiopathy
rare benign cause of biliary obstruction
| Incidence: | 15 cases in literature |
| Cause: | unknown |
| Histo: | transmural eosinophilic infiltration of biliary tract |
Eosinophilic cholecystitis
Eosinophilic cholangitis
both
| May be associated with: | multiple organ involvement (in 50%) of GI tract, urinary tract, bone marrow, pancreas, lymph nodes |
jaundice
peripheral eosinophilia
 thickened bile duct wall biliary dilatation
thickened bile duct wall biliary dilatation wall irregularities in beaded pattern
wall irregularities in beaded pattern
| Rx: | steroids |
| DDx: | lymphoma, AIDS cholangiopathy, collagen vascular disease, cholangiocarcinoma, amyloidosis |
Epithelioid Hemangioendothelioma
rare primary malignant vascular tumor of liver (soft tissue, bone, lung)
| Age: | average age of 45 years; M:F = 1:2 |
| May be associated with: | oral contraceptives, exposure to vinyl chloride |
| Path: | multifocal nodules varying in size from a few mm to several cm involve both lobes of the liver (due to rapid perivascular extension); nodules may coalesce in liver periphery |
| Histo: | dendritic spindle-shaped cells + epithelioid round cells in a matrix of myxoid + fibrous stroma; neoplastic endothelial cells invade sinusoids + terminal hepatic + portal veins cutting off the tumor's blood supply |
in 80%: abdominal pain, weakness, anorexia, jaundice
| Metastases to: | spleen, mesentery, lymph nodes, lung, bone |
 multiple nodules (nodular form)
multiple nodules (nodular form) peripheral subcapsular growth (diffuse form) without deforming liver contour
peripheral subcapsular growth (diffuse form) without deforming liver contour increased tumor vascularity
increased tumor vascularity hypertrophy of uninvolved liver
hypertrophy of uninvolved liver
Plain film:
 hepatic calcifications within myxoid stroma (15%)
hepatic calcifications within myxoid stroma (15%)
US:
 typically hypoechoic lesions (due to central core of myxoid stroma)
typically hypoechoic lesions (due to central core of myxoid stroma)
P.713
CT:
 low-attenuation masses on NECT, may become isoattenuating with rest of liver on CECT (due to vasoformative growth + compensatory hepatic arterial flow with portal vein occlusion)
low-attenuation masses on NECT, may become isoattenuating with rest of liver on CECT (due to vasoformative growth + compensatory hepatic arterial flow with portal vein occlusion)
Angio:
 hyper- and hypovascularity (dependent upon degree of sclerosis + hyalinization)
hyper- and hypovascularity (dependent upon degree of sclerosis + hyalinization) invasion occlusion of portal + hepatic veins
invasion occlusion of portal + hepatic veins
NUC:
 decreased perfusion to central myxoid tumor portion + increased perfusion to cellular areas on sulfur colloid scan
decreased perfusion to central myxoid tumor portion + increased perfusion to cellular areas on sulfur colloid scan photopenic defect on static sulfur colloid scan
photopenic defect on static sulfur colloid scan NOT gallium avid
NOT gallium avid
| Prognosis: | 20% die within 2 years, 20% survive for 5 28 years treatment |
| DDx of multiple nodules: | metastatic disease |
| DDx of diffuse form: | sclerosing carcinoma, vasoocclusive disease |
Fatty Liver
FATTY INFILTRATION OF THE LIVER = HEPATIC STEATOSIS
Cause:
METABOLIC DERANGEMENT
poorly controlled diabetes mellitus (50%), obesity, hyperlipidemia, acute fatty liver of pregnancy, protein malnutrition, total parenteral hyperalimentation (TPN), malabsorption (jejunoileal bypass), glycogen storage disease, glycogen synthetase deficiency, cystic fibrosis, Reye syndrome, corticosteroids, severe hepatitis, trauma, chronic illness (TB, CHF)
HEPATOTOXINS
alcohol (>50%), carbon chlorides, phosphorus, amiodarone, chemotherapy
| Histo: | hepatocytes with large cytoplasmic fat vacuoles containing triglycerides; >5% fat of total liver weight |
No abnormal liver function tests
 rapid change with time (few days to >10 months) depending on clinical improvement (abstinence from alcohol, improved nutrition) + degree of severity
rapid change with time (few days to >10 months) depending on clinical improvement (abstinence from alcohol, improved nutrition) + degree of severity
Diffuse fatty infiltration
 hepatomegaly (75 80%)/normal sized liver
hepatomegaly (75 80%)/normal sized liverPlain film:
 radiolucent liver sign = enlarged radiolucent liver
radiolucent liver sign = enlarged radiolucent liver
US (sensitivity >90%, accuracy 85 97%):
 increased sound attenuation (scattering of sound beam) = poor definition of posterior aspect of liver
increased sound attenuation (scattering of sound beam) = poor definition of posterior aspect of liver fine (more typical)/coarsened hyperechogenicity (compared with kidney)
fine (more typical)/coarsened hyperechogenicity (compared with kidney) impaired visualization of borders of hepatic vessels
impaired visualization of borders of hepatic vessels attenuation of sound beam (feature of fat, NoT fibrosis)
attenuation of sound beam (feature of fat, NoT fibrosis)
CT:
 areas of lower attenuation than normal portal vein/IVC density
areas of lower attenuation than normal portal vein/IVC density reversal of liver-spleen density relationship (liver density is normally 6 12 hU greater than spleen)
reversal of liver-spleen density relationship (liver density is normally 6 12 hU greater than spleen) hyperdense intrahepatic vessels
hyperdense intrahepatic vessels
NUC:
Tc-99m sulfur colloid scan:
 diffuse heterogeneous uptake (68%)
diffuse heterogeneous uptake (68%) reversal of liver-spleen uptake (41%)
reversal of liver-spleen uptake (41%) increased bone marrow uptake (41%)
increased bone marrow uptake (41%)
Xe-133 ventilation scan:
 increased activity during washout phase (38%)
increased activity during washout phase (38%)
MR:
 slightly increased signal on T1WI + T2WI; relatively insensitive (10% fat by weight will alter SE signal intensities only by 5 15%)
slightly increased signal on T1WI + T2WI; relatively insensitive (10% fat by weight will alter SE signal intensities only by 5 15%) fat turns black with Dixon technique
fat turns black with Dixon technique
Fat-Spared Area in diffuse fatty infiltration
| Cause: | direct drainage of systemic blood into liver |
Location:
posterior edge of segment 4 = anterior to portal vein bifurcation (drainage of aberrant gastric vein)
next to gallbladder bed (drainage of cystic vein)
subcapsular skip areas
 hypoechoic ovoid/spherical/sheetlike mass
hypoechoic ovoid/spherical/sheetlike mass No mass effect (undisplaced course of vessels)
No mass effect (undisplaced course of vessels)
| DDx: | tumor mass |
Focal Fatty Infiltration
| Etiology: | ? vascular origin, focal tissue hypoxia |
| Distribution: | (a) lobar/segmental uniform lesions (b) lobar/segmental nodular lesions (c) perihilar lesions (d) diffuse nodular lesions (e) diffuse patchy lesions predominantly in centrilobar + periportal regions, subcapsular distribution may be due to variants of blood supply (due to third inflow from connection between peripheral portal radicles + perforating capsular/accessory cystic veins) |
| Location: | right lobe, caudate lobe, perihilar region |
 fan-shaped lobar/segmental distribution with angulated/interdigitating geographic margins
fan-shaped lobar/segmental distribution with angulated/interdigitating geographic margins lesions extend to periphery of liver
lesions extend to periphery of liver NO mass effect (undisplaced course of vessels, no bulging of liver contour)
NO mass effect (undisplaced course of vessels, no bulging of liver contour)US:
 hyperechoic area with poorly defined/sharp margins
hyperechoic area with poorly defined/sharp margins multiple/rarely single echogenic nodules simulating metastases (rare)
multiple/rarely single echogenic nodules simulating metastases (rare)
CT:
 patchy areas of decreased attenuation ranging from 40 to +10 HU (DDx: liver tumor)
patchy areas of decreased attenuation ranging from 40 to +10 HU (DDx: liver tumor) NO contrast enhancement
NO contrast enhancement
MR (not sensitive for fat):
 high signal on T1WI + low/isointense signal on T2WI
high signal on T1WI + low/isointense signal on T2WI
NUC with colloid:
 no significant changes on sulfur colloid images (SPECT imaging may detect focal fatty infiltration)
no significant changes on sulfur colloid images (SPECT imaging may detect focal fatty infiltration)
| DDx: | primary/secondary hepatic tumor |
Focal Nodular Hyperplasia
FNH = rare benign congenital hamartomatous malformation or reparative process in areas of focal injury; SPECIFIC DIAGNOSIS RARELY POSSIBLE
P.714
| Prevalence: | 0.9%; 2nd most common benign tumor of liver after hemangioma; 3 8% of all primary hepatic tumors in adult population, 4% in pediatric population; twice as common as hepatocellular adenoma |
| Cause: | (?) congenital arteriovenous malformation triggers focal hepatocellular hyperplasia owing to a regional increase in blood flow Oral contraceptives DO NOT cause FNH, but exert a trophic effect on its growth! |
| Path: | localized, well-delineated, usually solitary (80 95%), subcapsular mass composed of numerous small nodules within an otherwise normal liver; no true capsule; frequently central fibrous scar in area of interconnection of fibrous bands (HALLMARK); angioarchitecture typically has one/more thick-walled arteries within fibrous septa dividing into numerous capillaries connected to sinusoids, which are drained by large hepatic veins (no portal veins!); FNH hepatocytes contain steatosis in 50% |
| Histo: | composed of multiple spherical aggregates of hepatocytes often containing increased amounts of fat (in 50%) + triglycerides + glycogen; Kupffer cells line sinusoids; bile duct proliferation within fibrous septa without connection to biliary tree; thick-walled arteries within fibrous septa radiating from the center toward the periphery; absent portal triads + central veins; difficult differentiation from regenerative nodules of cirrhosis + hepatocellular adenoma |
Pathologic classification:
Classic FNH (80%):
abnormal nodular architecture, malformed vessels, cholangiolar proliferation
Nonclassic FNH (20%) without septa/central scar:
 globally resembling hepatic adenoma
globally resembling hepatic adenoma no prominent septa
no prominent septa vaguely lobulated contours
vaguely lobulated contours central scar (in only 4%)
central scar (in only 4%)telangiectatic FNH (15%)
FNH with cytologic atypia (3%)
mixed hyperplastic + adenomatous FNH (2%)
 Difficult to differentiate from other liver masses with imaging
Difficult to differentiate from other liver masses with imaging
| Age peak: | 3rd 4th decade (range: 7 months to 75 years); M:F = 1:8 |
| Associated with: | hepatic hemangioma (in 23%), meningioma, astrocytoma, arterial dysplasia of other organs in case of multiple FNH |
initially often asymptomatic (in 50 90% incidental discovery)
vague abdominal pain (10 15%) due to mass effect
normal liver function
hepatomegaly/abdominal mass
| Location: | right lobe:left lobe = 2:1; multiple in 20% |
| Size: | <5 cm (in 85%) |
 well-circumscribed nonencapsulated nodular cirrhotic-like mass in an otherwise normal liver:
well-circumscribed nonencapsulated nodular cirrhotic-like mass in an otherwise normal liver: often near liver surface
often near liver surface pedunculated mass (in 5 20%)
pedunculated mass (in 5 20%) multiple masses (in 20%)
multiple masses (in 20%)
 central stellate scar = central fibrous core with radiating fibrous septa containing arteriovenous malformation= spoke-wheel pattern (in 50%)
central stellate scar = central fibrous core with radiating fibrous septa containing arteriovenous malformation= spoke-wheel pattern (in 50%) highly vascular tumor:
highly vascular tumor: supplied by enlarged anomalous hepatic artery
supplied by enlarged anomalous hepatic artery venous drainage always into hepatic veins(DDx: HCC drains into portal vein system in 98%)
venous drainage always into hepatic veins(DDx: HCC drains into portal vein system in 98%) hemorrhage is unlikely
hemorrhage is unlikely
 pseudocapsule of a few mm in thickness (due to surrounding compressed hepatic parenchyma, perilesional vessels, inflammatory reaction)
pseudocapsule of a few mm in thickness (due to surrounding compressed hepatic parenchyma, perilesional vessels, inflammatory reaction) calcifications are EXTREMELY rare
calcifications are EXTREMELY rare
NECT:
 iso-/slightly hypoattenuating homogeneous mass
iso-/slightly hypoattenuating homogeneous mass
CECT (3 phases necessary!):
 transient intense hyperdensity of most of the lesion during arterial phase (30 60 sec after bolus injection) except for central scar
transient intense hyperdensity of most of the lesion during arterial phase (30 60 sec after bolus injection) except for central scar hypodense central scar during arterial phase (15 33%) (DDx: fibrolamellar HCC)
hypodense central scar during arterial phase (15 33%) (DDx: fibrolamellar HCC) lesion becomes isodense during portal venous + equilibrium phase
lesion becomes isodense during portal venous + equilibrium phase hyperdense central scar on delayed images (delayed washout of contrast from myxomatous scar tissue)
hyperdense central scar on delayed images (delayed washout of contrast from myxomatous scar tissue)
US:
 iso-/mildly hypo-/mildly hyperechoic (33%) homogeneous mass
iso-/mildly hypo-/mildly hyperechoic (33%) homogeneous mass hypoechoic halo (of compressed liver parenchyma/displaced hepatic vessels)
hypoechoic halo (of compressed liver parenchyma/displaced hepatic vessels) hyperechoic central scar in 18%
hyperechoic central scar in 18%
Doppler:
 enlarged afferent blood vessel with central arterial hypervascularity + centrifugal filling to the periphery in a spoke-wheel pattern
enlarged afferent blood vessel with central arterial hypervascularity + centrifugal filling to the periphery in a spoke-wheel pattern large draining veins at tumor margins
large draining veins at tumor margins may show high-velocity Doppler signals with arterial pulsatility from arteriovenous shunts
may show high-velocity Doppler signals with arterial pulsatility from arteriovenous shunts
MR (70% sensitive, 98% specific, requires 3 phases):
 usually homogeneous signal intensity of lesion
usually homogeneous signal intensity of lesion T1WI:
T1WI: iso- to hypointense (94 100%)
iso- to hypointense (94 100%) atypically hyperintense lesion in 6%
atypically hyperintense lesion in 6%
 T2WI: slightly hyper- to isointense (94 100%)
T2WI: slightly hyper- to isointense (94 100%) central scar (more often detected than on US/CT)
central scar (more often detected than on US/CT) hypointense on T1WI
hypointense on T1WI hyperintense on T2WI in 75 84% (due to vascular channels + edema)
hyperintense on T2WI in 75 84% (due to vascular channels + edema) hypointense on T2WI in 25% (absent or minimal edema)
hypointense on T2WI in 25% (absent or minimal edema)
CEMR (2-D/3-D GRE):
 intense enhancement in arterial phase
intense enhancement in arterial phase isointense during portal venous phase
isointense during portal venous phase late + prolonged enhancement of central scar (due to increased interstitial space + fluid content gradually taking up contrast material)
late + prolonged enhancement of central scar (due to increased interstitial space + fluid content gradually taking up contrast material) occasionally prolonged enhancement (due to entrapment of Gd-DTPA by functioning hepatocytes inside tumor followed by 1% excretion into biliary tree)
occasionally prolonged enhancement (due to entrapment of Gd-DTPA by functioning hepatocytes inside tumor followed by 1% excretion into biliary tree) enhancement of pseudocapsule on delayed images
enhancement of pseudocapsule on delayed images less uptake of IV superparamagnetic iron oxide (ferucarbotran, mangafodipir trisodium) than surrounding
less uptake of IV superparamagnetic iron oxide (ferucarbotran, mangafodipir trisodium) than surrounding P.715
liver (uptake mechanism similar to that of sulfur colloid) Use iron oxide in lesions with atypical features!
Use iron oxide in lesions with atypical features!
NUC:
Sulfur colloid scan:
 Only FNH contains sufficient Kupffer cells to cause normal/increased uptake
Only FNH contains sufficient Kupffer cells to cause normal/increased uptake normal uptake (33%)
normal uptake (33%) increased uptake (33%) = virtually DIAGNOSTIC
increased uptake (33%) = virtually DIAGNOSTIC cold spot (33%) due to less Kupffer cells (DDx: hepatic adenoma, hemangioma, hepatoblastoma, liver herniation, hepatocellular carcinoma)
cold spot (33%) due to less Kupffer cells (DDx: hepatic adenoma, hemangioma, hepatoblastoma, liver herniation, hepatocellular carcinoma)
Tc-HIDA:
 normal/increased uptake (40 70%), cold spot (60%)
normal/increased uptake (40 70%), cold spot (60%)
Tc-99m tagged RBCs:
 increased uptake during early phase
increased uptake during early phase defect relative to liver on delayed images
defect relative to liver on delayed images
Angio:
 discretely marginated hypervascular mass (90%) with intense capillary blush/hypovascular (10%)
discretely marginated hypervascular mass (90%) with intense capillary blush/hypovascular (10%) enlargement of main feeding artery with central blood supply (= spoke-wheel pattern in 33%)
enlargement of main feeding artery with central blood supply (= spoke-wheel pattern in 33%) homogeneous parenchymal stain
homogeneous parenchymal stain decreased vascularity in central stellate fibrous scar
decreased vascularity in central stellate fibrous scar
| Rx: | (1) Discontinuation of oral contraceptives (2) Resection of pedunculated mass (3) Diagnostic excisional biopsy for extensive tumor (FNH seldom requires surgery) |
| Cx: | rarely rupture with hemoperitoneum (increased incidence in patients on oral contraceptives 14%) |
DDx:
Fibrolamellar carcinoma (scar calcified, metastases, retroperitoneal adenopathy, tumor hemorrhage + necrosis causing pain, hypointense scar on T2WI)
Hepatic adenoma (10 cm large tumor, symptomatic due to propensity for hemorrhage in 50%, central scar atypical)
Well-differentiated hepatocellular carcinoma (internal necrosis + hemorrhage, vascular invasion, metastases, persistent rim-enhancement of tumor capsule)
Giant cavernous hemangioma (larger tumor, may calcify, globular peripheral enhancement followed by centripetal filling, retention of contrast on delayed images, central scar with CSF-like behavior on MRI)
Hypervascular metastasis (hypovascular during portal venous phase, older patient)
Intrahepatic cholangiocarcinoma (less vascular, dominant large central scar, metastases)
Telangiectatic Focal Nodular Hyperplasia
| Frequency: | 10% of all FNH |
| Age: | mean age 38 years; women |
| Associated with: | oral contraceptives (mean time, 15 years) |
| Mean size: | 7 cm |
 multiple lesions in 20 50%
multiple lesions in 20 50% strong arterial enhancement
strong arterial enhancement persistent lesion enhancement (61%) due to sinusoidal dilatation
persistent lesion enhancement (61%) due to sinusoidal dilatation absence of a central scar (92%)
absence of a central scar (92%) heterogeneous pattern (43%) due to necrosis, sinusoidal dilatation, hemorrhagic foci
heterogeneous pattern (43%) due to necrosis, sinusoidal dilatation, hemorrhagic foci
MR:
 hyperintensity on T1WI (53%) due to intrasinusoidal dilatation
hyperintensity on T1WI (53%) due to intrasinusoidal dilatation strong hyperintensity on T2WI (44%)
strong hyperintensity on T2WI (44%)
| DDx: | hepatic adenoma |
Gallbladder Carcinoma
| Incidence: | 0.4 4.6% of biliary tract operations; most common biliary cancer (9 more common than extrahepatic bile duct cancer); 6th most common gastrointestinal malignancy (after colon, pancreas, stomach, liver, esophagus); 3% of all intestinal neoplasms; 7,000 new cases/year in United States |
| Demographics: | most common in Israel, Bolivia, Chile, northern Japan, New Mexico |
| Ethnicity: | Native Americans + Hispanic Americans (associated with increased prevalence of gallstones) |
| Median age: | 72 years; M:F = 1:3 1:4; whites > blacks 85% occur in 6th decade or later! |
| Risk factors: | increased body mass, female gender, postmenopausal status, cigarette smoking, chronic Salmonella typhi infection, exposure to chemicals (rubber, automobile, wood finishing, metal fabricating industries) |
Associated with:
Disorder of gallbladder:
Cholelithiasis in 74 92%
 Gallbladder carcinoma occurs in only 1% of all patients with gallstones!
Gallbladder carcinoma occurs in only 1% of all patients with gallstones!
Porcelain gallbladder (in 4 60%): prevalence of gallbladder carcinoma in 10 25% of autopsies
Chronic cholecystitis
Gallbladder polyp: a polyp >2 cm is likely malignant!
Disorder of bile ducts:
Primary sclerosing cholangitis
Congenital biliary anomalies: cystic dilatation of biliary tree, choledochal cyst, anomalous junction of pancreaticobiliary ducts, low insertion of cystic duct
Inflammatory bowel disease (predominantly ulcerative colitis, less common in Crohn disease)
Familial polyposis coli
| Path: | diffusely infiltrating lesion (68%), intraluminal polypoid growth (32%) |
| Histo: |
|
P.716
Modified Nevin Stage:
| I | mucosa only (in situ carcinoma) |
| II | mucosal + muscular invasion |
| III | mucosa + muscularis + serosa |
| IV | gallbladder wall + lymph nodes |
| V | hepatic/distant metastases |
Early diagnosis usually unsuspected due to lack of specific signs + symptoms:
history of past GB disease (50%)
malaise, vomiting, weight loss
chronic RUQ pain (54 76%)
obstructive jaundice (35 74%)
abnormal liver function tests (20 75%)
elevated -fetoprotein and CEA
| Location: | fundus (60%), body (30%), neck (10%) |
Growth types:
 mass replacing the gallbladder (40 65%)
mass replacing the gallbladder (40 65%) thickening of GB wall (20 30%) due to submucosal spread:
thickening of GB wall (20 30%) due to submucosal spread: focal (59%)/diffuse (41%) wall thickening
focal (59%)/diffuse (41%) wall thickening
DDx: acute/chronic inflammation (usually <10 mm)  intraluminal polypoid/fungating cauliflower-like mass with wide base (15 25%)
intraluminal polypoid/fungating cauliflower-like mass with wide base (15 25%)
 replacement of gallbladder by mass (37 70%)
replacement of gallbladder by mass (37 70%) pericholecystic infiltration: in 76% focal, in 24% diffuse
pericholecystic infiltration: in 76% focal, in 24% diffuse dilatation of biliary tree (38 70%):
dilatation of biliary tree (38 70%): infiltrative tumor growth along cystic duct
infiltrative tumor growth along cystic duct lymph node enlargement causing biliary obstruction
lymph node enlargement causing biliary obstruction intraductal tumor spread
intraductal tumor spread
 fine granular/punctate flecks of calcification (mucinous adenocarcinoma)
fine granular/punctate flecks of calcification (mucinous adenocarcinoma) lymph node enlargement in porta hepatis
lymph node enlargement in porta hepatis
| N.B.: | misdiagnosis by US/CT in 50%, especially in the presence of gallstones |
Abdominal radiograph:
 calcified gallstones
calcified gallstones porcelain gallbladder
porcelain gallbladder RUQ gas collection (after invasion of adjacent bowel)
RUQ gas collection (after invasion of adjacent bowel)
Cholangiography:
 malignant stricture/obstruction of extrahepatic bile ducts/right and left bile duct confluence, intrahepatic duct of right lobe
malignant stricture/obstruction of extrahepatic bile ducts/right and left bile duct confluence, intrahepatic duct of right lobe intraluminal GB filling defect (= tumor/stones)
intraluminal GB filling defect (= tumor/stones) mass displacing/invading gallbladder
mass displacing/invading gallbladder intraductal filling defects (= tumor/stones)
intraductal filling defects (= tumor/stones)
US:
 gallbladder replaced by mass with irregular margins + heterogeneous echotexture (= tumor necrosis)
gallbladder replaced by mass with irregular margins + heterogeneous echotexture (= tumor necrosis) immobile intraluminal well-defined round/oval mass
immobile intraluminal well-defined round/oval massDDx: tumefactive sludge  echogenic foci = coexisting gallstones/wall calcifications/tumoral calcification
echogenic foci = coexisting gallstones/wall calcifications/tumoral calcification tumor inseparable from liver
tumor inseparable from liver
CT:
 hypo-/isoattenuating mass in gallbladder fossa:
hypo-/isoattenuating mass in gallbladder fossa: low-attenuation areas of necrosis
low-attenuation areas of necrosis areas of enhancement (= viable tumor)
areas of enhancement (= viable tumor)
 subtle extension beyond wall of GB
subtle extension beyond wall of GB invasion of liver with protrusion of anterior surface of medial segment of left lobe
invasion of liver with protrusion of anterior surface of medial segment of left lobe
MR:
 hypointense mass on T1WI + ill-defined early contrast enhancement
hypointense mass on T1WI + ill-defined early contrast enhancement
| Metastases: | in 75 77% at time of diagnosis |
direct extension (most common mode): invasion of liver (34 65 89%), duodenum (12 15%), colon (9 15%), pancreas (6%), stomach, bile duct, right kidney, abdominal wall
Cause: thin GB wall with only a single muscle layer + no substantial lamina propria + perimuscular connective tissue continuous with interlobular connective tissue of liver lymphatic spread (26 41 75%): cystic, pericholedochal, celiac, superior mesenteric, foramen of Winslow, paraaortic nodes, superior + posterior pancreaticoduodenal
intraperitoneal seeding (common)
hematogenous spread (less common): liver, lung, bones, heart, pancreas, kidney, adrenal, brain
neural spread (frequent): associated with more aggressive tumors
intraductal spread (least common): particularly in papillary adenocarcinoma
| Cx: | perforation of gallbladder + abscess formation gallstones located within abscess |
| Prognosis: | 75% unresectable at presentation; average survival is 6 months; 5% 1-year survival rate; 6% 5-year survival rate |
| DDx: | (1) Xanthogranulomatous cholecystitis (lobulated mass filling gallbladder + stones) (2) Acute/chronic cholecystitis (generalized gallbladder wall thickening <10 mm) (3) Liver tumor invading gallbladder fossa (4) Tumors from adjacent organs (pancreas, duodenum) (5) Metastases (melanoma, leukemia, lymphoma) (6) Polyps: cholesterol polyp, hyperplastic polyp, granulation polyp (7) Adenomyomatosis |
Glycogen Storage Disease
autosomal recessive diseases with varying severity and clinical syndromes
Von Gierke Disease (Type I)
| Etiology: | defect in glucose-6-phosphatase with excess deposition of glycogen in liver, kidney, intestines |
| Dx: | failure of rise in blood glucose after glucagon administration |
| Age at presentation: | infancy |
 hepatomegaly
hepatomegalyUS:
 increased echogenicity (glycogen/fat)
increased echogenicity (glycogen/fat)
CT:
 increased (glycogen)/normal/decreased (fat) parenchymal attenuation
increased (glycogen)/normal/decreased (fat) parenchymal attenuation
| Prognosis: | death in infancy, may survive into adulthood with early therapy |
| Cx: | (1) Hepatic adenoma (2) Hepatocellular carcinoma |
P.717
Pompe Disease (Type II)
= abnormal metabolism with enlargement of myocardial cells due to glycogen deposition; similar to endocardial fibroelastosis
| Etiology: | defect in lysosomal glucosidase |
 massive cardiomegaly with CHF
massive cardiomegaly with CHF hepatomegaly
hepatomegaly
| Prognosis: | sudden death in 1st year of life (due to conduction abnormalities); survival rarely beyond infancy |
Cori Disease (Type III)
Andersen Disease (Type IV)
McArdle Disease (Type V)
Hers Disease (Type VI)
Hemochromatosis
excess iron deposition in various parenchymal organs (liver, pancreas, spleen, kidneys, heart) leading to cirrhosis with portal hypertension
| Cause: | excess iron deposition from
|
CT (60% sensitivity for iron):
 diffuse/rarely focal increase in liver density (up to 75 130 HU)
diffuse/rarely focal increase in liver density (up to 75 130 HU) depiction of portal + hepatic veins against background of hyperattenuating liver on NECT
depiction of portal + hepatic veins against background of hyperattenuating liver on NECT dual energy CT (at 80 + 120 kVp) can quantitate amount of iron deposition
dual energy CT (at 80 + 120 kVp) can quantitate amount of iron deposition
Genetic Hemochromatosis
= IDIOPATHIC/PRIMARY/HEREDITARY HEMOCHROMATOSIS
= excessive duodenal absorption + parenchymal retention of dietary iron that favors accumulation within non-RES organs (liver, pancreas, heart, pituitary gland)
| Cause: | autosomal recessive disorder (abnormal HFE gene located near human-leukocyte antigen [HLA] on short arm of chromosome 6) with increased absorption of intestinal iron |
| Prevalence: | 1:220 whites of northern European ancestry; homozygote frequency up to 0.25 0.50%; heterozygote carriers >10% |
Pathophysiology:
absorbed iron is selectively bound to transferrin; increased transferrin saturation in portal circulation favors selective iron uptake by periportal hepatocytes as initial site of iron accumulation; RES cells are incapable of storing excess iron
| Path: | excess iron stored as crystalline iron oxide (ferric oxyhydroxide) within cytoplasmic ferritin + lysosomal hemosiderin; iron overload affects parenchymal cells (liver, pancreas, heart) NOT Kupffer cells/RE cells of bone marrow + spleen (abnormal function of RES) |
| Age: | after middle age; female iron loss during menses and pregnancy provides some protection |
asymptomatic during 1st decade of disease
symptomatic in 80 90% if iron deposits >10 g
hyperpigmentation (90%)
hepatomegaly (90%)
arthralgias (50%)
diabetes mellitus (30%) secondary to insulin resistance by hepatocytes + pancreatic -cell damage from iron deposition
CHF + arrhythmia (15%)
loss of libido, impotence, amenorrhea, testicular atrophy, loss of body hair
liver iron index > 2 (= liver iron concentration [ mol per gram of dry weight] per patient's age)
serum Fe >300 mg/dL
serum transferrin saturation > 50%
MR:(skeletal muscle = good signal intensity reference)
 significant signal loss in liver on T2WI with signal intensity equal to background noise (paramagnetic susceptibility of ferritin + ferric ions leads to profound shortening of T1 + T2 relaxation times of adjacent protons)
significant signal loss in liver on T2WI with signal intensity equal to background noise (paramagnetic susceptibility of ferritin + ferric ions leads to profound shortening of T1 + T2 relaxation times of adjacent protons) normal pancreatic signal intensity in noncirrhotics
normal pancreatic signal intensity in noncirrhotics pancreatic signal intensity equal to/less than muscle (in 90% of cirrhotic patients)
pancreatic signal intensity equal to/less than muscle (in 90% of cirrhotic patients) normal signal intensity of spleen (in 86%) due to abnormal RES function
normal signal intensity of spleen (in 86%) due to abnormal RES function normal bone marrow signal
normal bone marrow signal
| Dx: | liver biopsy |
| Cx: | (1) Periportal fibrosis resulting in cirrhosis (if iron concentration >22,000 g/g of liver tissue) (2) Hepatocellular carcinoma (14 30%) (3) Insulin-dependent diabetes mellitus (30 60%) (4) Congestive cardiomyopathy (15%) |
| Rx: | phlebotomies in precirrhotic stage |
| Prognosis: | normal life expectancy with early diagnosis and treatment |
Secondary Hemochromatosis
[= HEMOSIDEROSIS = increased iron deposition without organ damage]
Cause:
Erythrogenic hemochromatosis = increased duodenal absorption of iron secondary to erythroid hyperplasia in ineffective erythropoiesis (eg, thalassemia, congenital dyserythropoietic anemia, sideroblastic anemia [= impaired protoporphyrin production], myelodysplasia, NOT in sickle cell anemia)
Path: no excess Kupffer cell iron Bantu siderosis = excessive dietary iron from food preparation in iron containers (Kaffir beer)
Iron overload siderosis
| Path: | iron deposition initially in RES (phagocytosis of intact RBC) with sparing of parenchymal cells of pancreas; after saturation of RES storage capacity parenchymal cells of other organs accumulate iron (liver, pancreas, myocardium) |
| Age: | 4th 5th decade; M:F = 10:1 |
P.718
MR:
 signal loss in liver on T2WI with signal intensity greater than background noise (iron in Kupffer cells with sparing of parenchymal liver cells)
signal loss in liver on T2WI with signal intensity greater than background noise (iron in Kupffer cells with sparing of parenchymal liver cells) splenic signal intensity less than muscle
splenic signal intensity less than muscle low signal intensity of bone marrow (= siderotic marrow)
low signal intensity of bone marrow (= siderotic marrow)
Transfusional Siderosis
= iron deposited in liver, spleen, and bone marrow in patients receiving >40 units of blood (iron storage capacity of RES = 10 g of iron)
abnormal iron deposition in RES is clinically of little significance (no damage of affected organs)
 parenchymal iron deposition can cause organ dysfunction
parenchymal iron deposition can cause organ dysfunction low signal intensity of bone marrow (= siderotic marrow)
low signal intensity of bone marrow (= siderotic marrow) strong signal decrease in spleen
strong signal decrease in spleen low signal intensity of liver + pancreas
low signal intensity of liver + pancreas
| Rx: | iron chelation therapy to remove excess iron |
Hepatic Abscess
LIVER ABSCESS
localized collection of pus in the liver resulting from any infectious process with destruction of the hepatic parenchyma + stroma
| Types: | pyogenic (85%), fungal (9%), amebic (6%) |
| Location: | multiple in 50% A pyogenic abscess tends to be centrally located, an amebic abscess peripherally! |
 hepatomegaly
hepatomegaly elevation of right hemidiaphragm
elevation of right hemidiaphragm pleural effusion
pleural effusion right lower lobe atelectasis/infiltration
right lower lobe atelectasis/infiltration gas within abscess (esp. Klebsiella)
gas within abscess (esp. Klebsiella)
MR:
 hypointense on T1WI + hyperintense on T2WI (72%)
hypointense on T1WI + hyperintense on T2WI (72%) perilesional edema (35%)
perilesional edema (35%) double target sign on T2WI = hyperintense center (fluid) + hypointense sharply marginated inner ring (abscess wall) + hyperintense poorly marginated ring (perilesional edema)
double target sign on T2WI = hyperintense center (fluid) + hypointense sharply marginated inner ring (abscess wall) + hyperintense poorly marginated ring (perilesional edema) rim enhancement (86%)
rim enhancement (86%)
Amebic Abscess
| Organism: | Entamoeba histolytica |
| Etiology: | spread of viable amebae from colon to liver via portal system |
| Incidence: | in 1 25% of intestinal amebiasis |
| Age: | 3rd 5th decade; M:F = 4:1 |
amebic dysentery
amebic hepatitis (15%)
| Location: | liver abscess (right lobe) in 2 25%; systemic dissemination by invasion of lymphatics/portal system (rare); liver:lung:brain = 100:10:1 |
| Size: | 2 12 cm; multiple liver abscesses in 25% |
 nonspecific variable appearance
nonspecific variable appearance nodularity of abscess wall (60%)
nodularity of abscess wall (60%) internal septations (30%)
internal septations (30%) not gas-containing (unless hepatobronchial/hepatoenteric fistula present)
not gas-containing (unless hepatobronchial/hepatoenteric fistula present) disruption of diaphragm
disruption of diaphragm
CT:
 nonspecific hypoattenuating area
nonspecific hypoattenuating area enhancing wall
enhancing wall
US:
 homogeneous hypoechoic area
homogeneous hypoechoic area posterior acoustic enhancement
posterior acoustic enhancement well-defined smooth thin wall
well-defined smooth thin wall
NUC:
 sensitivity of sulfur colloid scan is 98%
sensitivity of sulfur colloid scan is 98% photon-deficient area surrounded by rim of uptake on Ga-67 scan
photon-deficient area surrounded by rim of uptake on Ga-67 scan
Aspiration:
typically opaque reddish/dirty brown/pink material ( anchovy paste / chocolate sauce ), usually sterile, parasite confined to margin of abscess
| Cx: | (1) Diaphragmatic disruption (rare) is strongly suggestive of amebic abscess (2) Fistulization into colon, right adrenal gland, bile ducts, pericardium |
| Rx: | conservative treatment with chloroquine/metronidazole (Flagyl ); percutaneous drainage for left hepatic abscess (spontaneous rupture into pericardium + tamponade possible) |
| Prognosis: | resolution under therapy may take from 1 month to 2 years; permanent cysts may remain behind |
Pyogenic Liver Abscess
 Most common type of liver abscess
Most common type of liver abscess
| Organism: | E. coli, aerobic streptococci, St. aureus, anaerobic bacteria (45%); polymicrobial (>50%) |
| Incidence: | 0.016% |
| Predisposed: | steroids, immunosuppressed state, excessive antibiotics usage |
Etiology:
Biliary disease (60%):
ascending cholangitis from obstructive biliary tract disease (malignant/benign stricture), Crohn disease (sclerosing cholangitis, gallstones), cholecystitis
Portal phlebitis:
suppurative appendicitis, colitis, diverticular disease
Disseminated sepsis via hepatic artery:
infarction from sickle cell/embolism/postembolization/septicemia; indwelling arterial catheters
Direct contiguous spread from a local infection:
cholecystitis, peptic ulcer, subphrenic sepsis
Trauma:
rupture, penetrating wounds, biopsy, surgery or liver transplantation
Cryptogenic in 45%:
invasion of cysts; superinfection of dead tissue (eg, primary/secondary hepatic tumor) by pyogenic intestinal flora
| Age: | 6th 7th decade; M > F |
pyrexia (79%)
abdominal pain (68%)
nocturnal sweating (43%)
vomiting/malaise (39%)
jaundice (0 20%)
elevated WBCs
elevated alkaline phosphatase
positive blood culture (50%)
P.719
| Location: | solitary abscess in right lobe (40 75%), in left lobe (2 10%); multiple abscesses in 10 34 73% (more often of biliary than hematogenous origin) |
US:
 hypoechoic round lesion with well-defined mildly echogenic rim
hypoechoic round lesion with well-defined mildly echogenic rim posterior acoustic enhancement
posterior acoustic enhancement coarse clumpy debris/low-level echoes/fluid-debris level with internal movement
coarse clumpy debris/low-level echoes/fluid-debris level with internal movement intensely echogenic reflections with reverberations (from gas) in 20 30%
intensely echogenic reflections with reverberations (from gas) in 20 30%
CT:
 inhomogeneous hypoattenuating (0 45 HU) single/multiloculated cavity
inhomogeneous hypoattenuating (0 45 HU) single/multiloculated cavity double target sign = wall-enhancement + surrounding hypodense zone (6 30%)
double target sign = wall-enhancement + surrounding hypodense zone (6 30%) cluster sign = several microabscesses each <2 cm within the same anatomic area; suggestive of biliary origin
cluster sign = several microabscesses each <2 cm within the same anatomic area; suggestive of biliary origin air density
air density
MR:
 decreased T1 signal + increased T2 signal (varies with protein content)
decreased T1 signal + increased T2 signal (varies with protein content) enhancement of peripheral rim
enhancement of peripheral rim
NUC:
 photon-deficient area on sulfur colloid + IDA scan
photon-deficient area on sulfur colloid + IDA scan Ga-67 citrate uptake in 80%
Ga-67 citrate uptake in 80% In-111 tagged WBC uptake is highly specific (since WBCs normally go to liver, may need sulfur colloid test for correlation)
In-111 tagged WBC uptake is highly specific (since WBCs normally go to liver, may need sulfur colloid test for correlation)
| Cx: | (1) Septicemia (2) Rupture into right subphrenic space (3) Rupture into abdominal cavity (4) Rupture into pericardium (5) Empyema (6) Common hepatic duct obstruction |
| Mortality: | 20 80%; 100% if unrecognized/untreated |
Hepatic Adenoma
= HEPATOCELLULAR ADENOMA = LIVER CELL ADENOMA
 The most frequent hepatic tumor in young women after use of contraceptive steroids!
The most frequent hepatic tumor in young women after use of contraceptive steroids!
| Prevalence: | half as common as FNH |
| Path: | pseudocapsule due to compression of liver tissue containing multiple large vessels; high incidence of hemorrhage + necrosis + fatty change; no scar |
| Histo: | solitary spherical benign growth of hepatocytes; sheets of hepatocytes; sheets of hepatocytes without portal veins or central veins; scattered thin-walled vascular channels + bile canaliculi; decrease in number of abnormally functioning Kupffer cells; hepatocytes contain increased amounts of glycogen fat |
| Age: | young women in childbearing age; not seen in males unless on anabolic steroids; rare in children |
Associated with:
oral contraceptives (2.5 risk after 5-year use, 7.5 risk after 9-year use, 25 risk >9-year use)
anabolic steroids
pregnancy
diabetes mellitus
type Ia glycogen storage disease (von Gierke) in 60%
Fanconi anemia
 Pregnancy may increase tumor growth rate + lead to tumor rupture!
Pregnancy may increase tumor growth rate + lead to tumor rupture! Tumor regression may occur with dietary therapy leading to normal insulin, glucagon, and serum glucose levels
Tumor regression may occur with dietary therapy leading to normal insulin, glucagon, and serum glucose levelsasymptomatic (20%)
RUQ pain as sign of mass effect (40%)/intratumoral or intraperitoneal hemorrhage (40%)
hepatomegaly
| Location: | right lobe of liver in subcapsular location (75%); multiple in 20 30% (eg, hepatic adenomatosis without risk factors) |
| Size: | between 6 and 30 cm in size (average size of 8 10 cm) |
 round well-circumscribed pseudo-encapsulated mass
round well-circumscribed pseudo-encapsulated mass intraparenchymal/pedunculated (in 10%)
intraparenchymal/pedunculated (in 10%) unusual nodule-in-nodule appearance in large tumors (DDx: hepatocellular carcinoma)
unusual nodule-in-nodule appearance in large tumors (DDx: hepatocellular carcinoma) occasional eccentric dystrophic calcifications
occasional eccentric dystrophic calcifications
CT:
 round isoattenuating mass
round isoattenuating mass mass of decreased density due to fat + areas of necrosis (30 40%)
mass of decreased density due to fat + areas of necrosis (30 40%) hyperdense areas of fresh intratumoral hemorrhage (22 50%)
hyperdense areas of fresh intratumoral hemorrhage (22 50%)
CECT:
 transient avid enhancement on arterial-phase images in small adenomas/initial peripheral enhancement with centripetal filling in larger adenomas (due to supply by hepatic artery)
transient avid enhancement on arterial-phase images in small adenomas/initial peripheral enhancement with centripetal filling in larger adenomas (due to supply by hepatic artery) iso-/hypoattenuating on delayed-phase images
iso-/hypoattenuating on delayed-phase images
US:
 usually small well-demarcated solid heterogeneous mass of variable echogenicity (echogenic for areas of fat or hemorrhage/complex hyper- and hypoechoic):
usually small well-demarcated solid heterogeneous mass of variable echogenicity (echogenic for areas of fat or hemorrhage/complex hyper- and hypoechoic): hyperechoic lesion with well-defined hypoechoic rim
hyperechoic lesion with well-defined hypoechoic rim anechoic cystic areas if large
anechoic cystic areas if large color flow in peripheral peritumoral sinusoids
color flow in peripheral peritumoral sinusoids
MR:
 inhomogeneous on all pulse sequences (indistinguishable from HCC)
inhomogeneous on all pulse sequences (indistinguishable from HCC) often hyperintense areas on T1WI (due to presence of fatladen hepatocytes/hemorrhage) in 35 77%
often hyperintense areas on T1WI (due to presence of fatladen hepatocytes/hemorrhage) in 35 77% isointense sheets of hepatocytes on T2WI
isointense sheets of hepatocytes on T2WI hyperintense areas of necrosis/hemorrhage on T2WI in 47 74%
hyperintense areas of necrosis/hemorrhage on T2WI in 47 74% usually signal loss with out-of-phase imaging
usually signal loss with out-of-phase imaging
NUC:
 focal photopenic lesion on sulfur colloid scan (because lesion composed of hepatocytes + nonfunctioning Kupffer cells) surrounded by rim of increased uptake (due to compression of adjacent normal liver containing Kupffer cells); may show uptake equal to/slightly less than liver (23%)
focal photopenic lesion on sulfur colloid scan (because lesion composed of hepatocytes + nonfunctioning Kupffer cells) surrounded by rim of increased uptake (due to compression of adjacent normal liver containing Kupffer cells); may show uptake equal to/slightly less than liver (23%) usually increased activity on hIDA scan
usually increased activity on hIDA scan No gallium uptake
No gallium uptake
P.720
Angio:
 usually hypervascular mass
usually hypervascular mass homogeneous but not intense stain in capillary phase
homogeneous but not intense stain in capillary phase enlarged hepatic artery with feeders at tumor periphery (50%)
enlarged hepatic artery with feeders at tumor periphery (50%) hypo-/avascular regions (secondary to hemorrhage/necrosis)
hypo-/avascular regions (secondary to hemorrhage/necrosis) neovascularity
neovascularity
| CAVE: | percutaneous biopsy carries high risk of bleeding! |
| Cx: | (1) Spontaneous hemorrhage with subcapsular hematoma/hemoperitoneum (41%) (2) Malignant transformation (? contiguous development of hepatocellular carcinoma) (3) Recurrence after resection |
| Rx: | hormone therapy stopped; screening for malignant degeneration with -fetoprotein; surgical resection (to prevent rupture) |
| DDx: | FNH, hemangioma, fibrolamellar hepatocellular carcinoma; metastasis |
Hepatic Angiomyolipoma
rare benign mesenchymal tumor
| Associated with: | tuberous sclerosis |
| Histo: | smooth muscle cells, fat, proliferating blood vessels |
asymptomatic
 intratumoral fat is DIAGNOSTIC
intratumoral fat is DIAGNOSTIC soft-tissue component may enhance
soft-tissue component may enhance
| Cx: | intratumoral hemorrhage |
Hepatic Angiosarcoma
HEMANGIOENDOTHELIAL SARCOMA = KUPFFER CELL SARCOMA = HEMANGIOSARCOMA
| Prevalence: | 0.14 0.25 per million; <2% of all primary liver neoplasms; most common sarcoma of liver (followed by fibrosarcoma > malignant fibrohistiocytoma > leiomyosarcoma) |
Etiology:
thorotrast = thorium dioxide (7 10%) with latent period of 15 24 years
arsenic
polyvinyl chloride (latent period of 4 28 years)
| Associated with: | hemochromatosis, anabolic steroids, cirrhosis, von Recklinghausen disease |
Path:
multifocal/multinodular lesions (71%) of up to >5 cm in size
large solitary mass with hemorrhage + necrosis
Histo:
vessels lined with malignant endothelial cells (eg, sinusoids) causing atrophy of surrounding liver
vasoformative = forming poorly organized vessels (responsible for RBC trauma + platelet trapping)
forming solid nodules of malignant spindle cells
| Age: | 6th 7th decade; M:F = 4:1 |
abdominal pain, weakness, fatigue, weight loss
spontaneous hemoperitoneum (27%)
jaundice
microangiopathic hemolytic anemia (23%), thrombocytopenia (54%), DIC (31%)
NO elevation of -fetoprotein
Early metastases to:
lung (23%), spleen (16 46%), porta hepatis nodes, portal vein, thyroid, peritoneal cavity, bone marrow (rapid metastatic spread)
 portal vein invasion
portal vein invasion hemorrhagic ascites
hemorrhagic ascites
Plain film:
 circumferential displacement of residual thorotrast
circumferential displacement of residual thorotrast
NUC:
 single/multiple photopenic areas on sulfur colloid scan
single/multiple photopenic areas on sulfur colloid scan increased gallium uptake
increased gallium uptake perfusion blood pool mismatch (initial decrease followed by slow increase in RBC concentration) as in hemangioma on 3-phase red blood cell scan
perfusion blood pool mismatch (initial decrease followed by slow increase in RBC concentration) as in hemangioma on 3-phase red blood cell scan
US:
 solid/mixed mass with anechoic areas (hemorrhage/necrosis)
solid/mixed mass with anechoic areas (hemorrhage/necrosis) multiple nodules
multiple nodules
CT:
 hypodense masses with high-density regions (hemorrhage)/low-attenuation regions (old hemorrhage/necrosis)
hypodense masses with high-density regions (hemorrhage)/low-attenuation regions (old hemorrhage/necrosis) focal areas of peripheral enhancement on dynamic CT as in large hemangioma (60%)
focal areas of peripheral enhancement on dynamic CT as in large hemangioma (60%)
MR:
 hypointense on T1WI with irregular areas of high signal (hemorrhage)
hypointense on T1WI with irregular areas of high signal (hemorrhage) hyperintense on T2WI + fluid-fluid levels
hyperintense on T2WI + fluid-fluid levels peripheral Gd-pentetate enhancement on T1WI
peripheral Gd-pentetate enhancement on T1WI
Angio:
 hypervascular stain around tumor periphery in late arterial phase with puddling; NO arterial encasement
hypervascular stain around tumor periphery in late arterial phase with puddling; NO arterial encasement
| CAVE: | Biopsy may lead to massive bleeding in 16%! Have surgical backup available! |
| Prognosis: | rapid deterioration with median survival of 6 months (13 months under chemotherapy) |
| DDx for multiple lesions: | hypervascular metastases |
| DDx for single lesion: | cavernous hemangioma, HCC (no splenic metastases) |
Hepatic Cyst
 Second most common benign hepatic lesion after hemangioma
Second most common benign hepatic lesion after hemangioma
| Prevalence: | 2 7%; increasing with age; M < F |
ACQUIRED HEPATIC CYST
secondary to trauma, inflammation, parasitic infestation, neoplasia
CONGENITAL HEPATIC CYST
= defective development of aberrant/obstructed intrahepatic bile ducts; derived from bile duct hamartoma
Incidence: liver cysts detected at autopsy in 50%;
in 22% detected during lifeAge of detection: 5th 8th decade Histo: cyst surrounded by fibrous capsule + lined by columnar epithelium, related to bile ducts within portal triads; no communication with bile duct Associated with:
Tuberous sclerosis
Polycystic kidney disease (25 33% have liver cysts)
Polycystic liver disease: autosomal dominant
hepatomegaly (40%); pain (33%); jaundice (9%)
P.721
| Size of cyst: | range from microscopic to huge (average 1.2 cm; in 25% largest cyst <1 cm; in 40% largest cyst >4 cm; maximal size of 20 cm) |
| Number of cysts: | multiple cysts spread throughout liver (in 60%)/solitary cyst |
 unilocular simple cyst:
unilocular simple cyst: imperceptible wall
imperceptible wall may show fluid-fluid interface
may show fluid-fluid interface
 water attenuation (0 10 HU)
water attenuation (0 10 HU) no enhancement
no enhancement cold spot on IDA, Ga-68, Tc-99m sulfur colloid scans
cold spot on IDA, Ga-68, Tc-99m sulfur colloid scans
| Rx: | sclerosing therapy with minocycline hydrochloride (Dose: 1 mg per 1-mL cyst content up to 500 mg in 10 mL of 0.9% saline + 10 mL 1% lidocaine) following contrast opacification of cyst to confirm absence of communication with biliary tree/leakage into peritoneal cavity |
Hepatic Hemangioma
Cavernous Hemangioma of Liver
= most common benign liver tumor (78%); second most common liver tumor after metastases
| Incidence: | 1 4%; autopsy incidence 0.4 7.3%; increased with multiparity |
| Cause: | ? enlarging hamartoma present since birth,? true vascular neoplasm |
| Age: | rarely seen in young children; M:F = 1:5 |
| Path: | large vascular channels filled with slowly circulating blood; lined by single layer of mature flattened endothelial cells separated by thin fibrous septa; no bile ducts; thrombosis of vascular channels common resulting in fibrosis + hemorrhage + myxomatous degeneration + calcifications |
| Pathophysiology: | large blood volume with low blood flow |
| Associated with: | (1) Hemangiomas in other organs (2) Focal nodular hyperplasia (3) Rendu-Osler-Weber disease |
asymptomatic if tumor small (50 70%)
may present with spontaneous life-threatening hemorrhage if large (5%)
hepatomegaly
may enlarge during pregnancy
abdominal discomfort + pain (from thrombosis in large hemangioma)
Kasabach-Merritt syndrome (= hemangioma + thrombocytopenia) rare
| Location: | frequently peripheral/subcapsular in posterior right lobe of liver; 20% are pedunculated; multiple in 10 20% |
| Size: | <4 cm (90%); >4 6 12 cm = giant cavernous hemangioma |
 well-circumscribed lobulated mass
well-circumscribed lobulated mass blood supply from hepatic artery
blood supply from hepatic artery may have central area of fibrosis = areas of nonenhancement/nonfilling/cystic space (occurrence increases with age)
may have central area of fibrosis = areas of nonenhancement/nonfilling/cystic space (occurrence increases with age) central septal calcifications within areas of fibrosis/phleboliths (5 20%)
central septal calcifications within areas of fibrosis/phleboliths (5 20%)
US:
 uniformly hyperechoic (60 70%) mass due to multiple interfaces created by blood-filled spaces separated by fibrous septa
uniformly hyperechoic (60 70%) mass due to multiple interfaces created by blood-filled spaces separated by fibrous septa inhomogeneous hypoechoic mass (up to 40%) in larger hemangiomas with well-defined thick/thin echogenic lobulated border due to hemorrhagic necrosis, scarring, myxomatous change centrally
inhomogeneous hypoechoic mass (up to 40%) in larger hemangiomas with well-defined thick/thin echogenic lobulated border due to hemorrhagic necrosis, scarring, myxomatous change centrally homogenous (58 73%)/heterogeneous (fibrosis, thrombosis, hemorrhagic necrosis)
homogenous (58 73%)/heterogeneous (fibrosis, thrombosis, hemorrhagic necrosis) hypoechoic center possible
hypoechoic center possible may show acoustic enhancement (37 77%)
may show acoustic enhancement (37 77%) unchanged in size/appearance (82%) on 1 6-year follow-up
unchanged in size/appearance (82%) on 1 6-year follow-up no Doppler signals/signals with peak velocity of <50 cm/sec
no Doppler signals/signals with peak velocity of <50 cm/sec
CT (combination of precontrast images, good bolus, dynamic scanning):
 well-circumscribed spherical/ovoid low-density mass:
well-circumscribed spherical/ovoid low-density mass: may have areas of higher/lower density within mass
may have areas of higher/lower density within mass
 typical pattern of low density on NECT + peripheral enhancement + complete fill-in on delayed images 3 30 minutes post IV bolus (55 89%):
typical pattern of low density on NECT + peripheral enhancement + complete fill-in on delayed images 3 30 minutes post IV bolus (55 89%): peripheral (72%)/central (in 8%)/diffuse dense (in 8%) enhancement
peripheral (72%)/central (in 8%)/diffuse dense (in 8%) enhancement complete (75%)/partial (24%)/no (2%) fill-in to isodensity in delayed phase
complete (75%)/partial (24%)/no (2%) fill-in to isodensity in delayed phase
 rapid contrast filling (16%), more often in small lesions (in 42% of hemangiomas <1 cm)
rapid contrast filling (16%), more often in small lesions (in 42% of hemangiomas <1 cm)DDx: hypervascular tumor (do not remain hyperattenuating on delayed-phase images)  central scar may not enhance at any time
central scar may not enhance at any time
MR (90 95% accuracy):
 spheroid/ovoid (87%) mass with smooth well-defined lobulated margins (87%); no capsule
spheroid/ovoid (87%) mass with smooth well-defined lobulated margins (87%); no capsule homogeneous internal architecture if <4 cm, hypointense internal inhomogeneities if >4 cm (due to fibrosis)
homogeneous internal architecture if <4 cm, hypointense internal inhomogeneities if >4 cm (due to fibrosis) hypo-/isointense mass on T1WI
hypo-/isointense mass on T1WI markedly hyperintense light bulb appearance (due to slow flowing blood) increasing with echo time) o T2WI (DDx: hepatic cyst, hypervascular tumor, necrotic tumor, cystic neoplasm)
markedly hyperintense light bulb appearance (due to slow flowing blood) increasing with echo time) o T2WI (DDx: hepatic cyst, hypervascular tumor, necrotic tumor, cystic neoplasm) same enhancement pattern as CT:
same enhancement pattern as CT: uniform enhancement at 1 second in 40% of small hemangiomas <1.5 cm after gadolinium-DTPA
uniform enhancement at 1 second in 40% of small hemangiomas <1.5 cm after gadolinium-DTPA peripheral nodular enhancement progressing centripetally with centrally uniform enhancement (50%)/persistent hypointensity (30%)
peripheral nodular enhancement progressing centripetally with centrally uniform enhancement (50%)/persistent hypointensity (30%) mildly hyperintense on T2WI for hyalinized hemangioma + lack of enhancement in early phase + slight peripheral enhancement in late phase (DDx: malignant hepatic tumor)
mildly hyperintense on T2WI for hyalinized hemangioma + lack of enhancement in early phase + slight peripheral enhancement in late phase (DDx: malignant hepatic tumor)
Angio (historical gold standard):
 dense opacification of well-circumscribed, dilated, irregular, punctate vascular lakes/puddles in late arterial + capillary phase starting at periphery in ring-/C-shaped configuration
dense opacification of well-circumscribed, dilated, irregular, punctate vascular lakes/puddles in late arterial + capillary phase starting at periphery in ring-/C-shaped configuration normal-sized feeders; AV shunting (very rare)
normal-sized feeders; AV shunting (very rare) contrast persistence late into venous phase
contrast persistence late into venous phase
NUC (95% accuracy with SPECT):
| Indication: | lesions >2 cm (detectable in 70 90%) |
 initially cold lesion on Tc-99m labeled RBC scans (dose of 15 20 mCi) with increased activity on delayed images at 1 2 hours
initially cold lesion on Tc-99m labeled RBC scans (dose of 15 20 mCi) with increased activity on delayed images at 1 2 hours cold defect on sulfur colloid scans
cold defect on sulfur colloid scans
| Bx: | may be biopsied safely provided normal liver is present between tumor + liver capsule nonpulsatile blood (73%) endothelial cells without malignancy (27%) |
| Prognosis: | no growth when <4 cm in diameter; giant cavernous hemangiomas may enlarge |
| Cx (rare): | (1) Spontaneous rupture (4.5%) (2) Abscess formation (3) Kasabach-Merritt syndrome (platelet sequestration) |
| DDx: | hypervascular malignant neoplasm/metastasis (quick homogeneous filling during arterial phase of small hemangiomas) |
P.722
Giant Hepatic Cavernous Hemangioma
= at least one dimension exceeding 8 10 cm (in literature no agreement on size)
| Associated with: | coexistent smaller <5 cm hemangioma in 13% |
| Histo: | hemorrhage, thrombosis, extensive hyalinization, liquefaction, fibrosis; central cleft due to cystic degeneration/liquefaction |
RUQ pain/fullness; abdominal mass
US:
 heterogeneous mass
heterogeneous mass
NECT:
 heterogeneous hypoattenuating mass with marked central areas of low attenuation
heterogeneous hypoattenuating mass with marked central areas of low attenuation
CECT:
 early peripheral globular enhancement
early peripheral globular enhancement incomplete filling of central portions
incomplete filling of central portions
MR:
 sharply marginated hypointense mass with cleftlike area of lower intensity on T1WI
sharply marginated hypointense mass with cleftlike area of lower intensity on T1WI large markedly hyperintense cleftlike area with some hypointense internal septa inside a hyperintense mass on T2WI
large markedly hyperintense cleftlike area with some hypointense internal septa inside a hyperintense mass on T2WI
CEMR:
 peripheral nodular enhancement
peripheral nodular enhancement central cleftlike area remains hypointense
central cleftlike area remains hypointense
| DDx: | metastasis, hepatocellular carcinoma, cholangiocarcinoma, hepatic adenoma, FNH (smaller and less hyperintense central scar on T2WI), focal fatty infiltration |
Infantile Hemangioendothelioma of Liver
= INFANTILE HEPATIC HEMANGIOMA = CAPILLARY/CAVERNOUS HEMANGIOMA
 Most common benign hepatic tumor during first 6 months of life!
Most common benign hepatic tumor during first 6 months of life!
| Histo: | multiple anastomosing thick-walled vascular spaces similar to cavernous hemangioma lined by plump immature endothelial cells in single or (less often) multiple cell layers; areas of extramedullary hematopoiesis/thrombi; scattered bile ducts; involutional changes (infarction, hemorrhage, necrosis, scarring) |
Classification:
hemangioendothelioma type 1 (more common): orderly proliferation of small blood vessels
hemangioendothelioma type 2: more aggressive histologic pattern
DDx: angiosarcoma Cavernous hemangioma:
dilated vascular spaces lined by flat endothelial cells
 Relationship to adult cavernous hemangioma unknown!
Relationship to adult cavernous hemangioma unknown!
| Age at presentation: | <6 months in 85%, during 1st month in 33%, >1 year in 5%; M: F = 1:1.4-1:2 |
abdominal mass secondary to hepatomegaly
cutaneous hemangiomas (9-45-87%) occur with multinodular form
may present with high-output ChF secondary to AV shunts within tumor (8 -15-25%)
Kasabach-Merritt syndrome (in 11%)
= hemorrhagic diathesis due to platelet sequestration by tumor/disseminated intravascular coagulation; characterized by an association of hemangioma, or hemangioendothelioma, or angiosarcoma with thrombocytopenia and purpura (secondary to increased systemic fibrinolysis)
Prognosis: fatal outcome in 20-30% hemolytic anemia
| Size: | several mm up to 20 cm (average size of 3 cm) |
 diffuse involvement of entire liver, rarely focal
diffuse involvement of entire liver, rarely focal single mass (50%)/multiple masses (50%)
single mass (50%)/multiple masses (50%) enlargement of celiac + hepatic arteries + proximal aorta
enlargement of celiac + hepatic arteries + proximal aorta rapid decrease in aortic caliber below celiac trunk
rapid decrease in aortic caliber below celiac trunk enlarged hepatic veins (increased venous flow)
enlarged hepatic veins (increased venous flow)
Plain film:
 fine speckled/fibrillary calcifications in 16-25% (DDx: hepatoblastoma, hamartoma, metastatic neuroblastoma)
fine speckled/fibrillary calcifications in 16-25% (DDx: hepatoblastoma, hamartoma, metastatic neuroblastoma)
US:
 heterogeneous predominantly hypoechoic/complex/hyperechoic lesion
heterogeneous predominantly hypoechoic/complex/hyperechoic lesion multiple sonolucent areas (= enlarging vascular channels secondary to initial rapid growth) (DDx: mesenchymal hamartoma):
multiple sonolucent areas (= enlarging vascular channels secondary to initial rapid growth) (DDx: mesenchymal hamartoma): vascular components demonstrated by color Duplex
vascular components demonstrated by color Duplex
 calcifications (in up to 50%)
calcifications (in up to 50%)
OB-US:
 polyhydramnios + fetal hydrops
polyhydramnios + fetal hydrops
NECT:
 large well-defined hypoattenuating mass
large well-defined hypoattenuating mass hemorrhage (not uncommon)
hemorrhage (not uncommon) calcifications (in up to 16%)
calcifications (in up to 16%)
CECT (similar to cavernous hemangioma):
 early peripheral enhancement (72%)
early peripheral enhancement (72%) variable delayed central enhancement
variable delayed central enhancement
MR:
 heterogeneous hypointense multinodular lesion on T1WI hyperintense areas of hemorrhage
heterogeneous hypointense multinodular lesion on T1WI hyperintense areas of hemorrhage varying degrees of hyperintensity on T2WI (resembling adult hemangioma)
varying degrees of hyperintensity on T2WI (resembling adult hemangioma) decreasing signal intensity with fibrotic replacement on T2WI
decreasing signal intensity with fibrotic replacement on T2WI
P.723
NUC (sulfur colloid, tagged RBC):
 increased flow in viable portions of lesion during angiographic phase
increased flow in viable portions of lesion during angiographic phase increased activity mixed with central photopenic areas (hemorrhage, necrosis, fibrosis) on delayed tagged RBC images
increased activity mixed with central photopenic areas (hemorrhage, necrosis, fibrosis) on delayed tagged RBC images photopenic defect on delayed sulfur colloid images
photopenic defect on delayed sulfur colloid images
Angio:
 enlarged, tortuous feeding arteries and stretched intrahepatic vessels
enlarged, tortuous feeding arteries and stretched intrahepatic vessels hypervascular tumor with inhomogeneous stain; clusters of small abnormal vessels
hypervascular tumor with inhomogeneous stain; clusters of small abnormal vessels pooling of contrast material in sinusoidal lakes with rapid clearing through early draining veins (AV shunting)
pooling of contrast material in sinusoidal lakes with rapid clearing through early draining veins (AV shunting)
| Prognosis: | rapid growth in first 6 months followed by tendency to involute within 6 8 months; 32 75% survival rate in complicated cases |
| Cx: | (1) Congestive heart failure (2) Hemorrhagic diathesis (3) Obstructive jaundice (4) Hemoperitoneum (rupture of tumor) (5) Malignant transformation into angiosarcoma (rare) |
| Rx: | (1) No treatment if asymptomatic (2) Reduction in size with steroids/radiotherapy/chemotherapy (3) Embolization(4) Surgical resection/liver transplantation |
| DDx: | (1) Hepatoblastoma (>1 year of age, elevated -fetoprotein, more heterogeneous) (2) Mesenchymal hamartoma (usually multilocular cystic mass) (3) Metastatic neuroblastoma (elevated catecholamines in urine, adrenal mass, nonenhancing multiple liver masses) |
Hepatic Venoocclusive Disease
= occlusion of small centrilobular veins without involvement of major hepatic veins
| Etiology: | radiation and chemotherapy in bone-marrow transplant patients; bush tea (alkaloid) consumption in Jamaica |
 main hepatic veins + IVC normal
main hepatic veins + IVC normal bidirectional/reversed portal venous flow
bidirectional/reversed portal venous flow gallbladder wall thickening
gallbladder wall thickening
Hepatitis
| Cause: | alcohol, medication, viral infection, NASH (nonalcoholic steatohepatitis) |
Acute Hepatitis
markedly elevated AST + ALT
increase in serum-conjugated bilirubin
 hepatomegaly/normal size of liver
hepatomegaly/normal size of liver gallbladder wall thickening
gallbladder wall thickening lymphadenopathy
lymphadenopathy
CT:
 periportal low attenuation (lymph edema)
periportal low attenuation (lymph edema)
US:
 diffuse decrease in liver echogenicity
diffuse decrease in liver echogenicity increased brightness of portal triads ( starry sky pattern) = centrilobular pattern due to edema in hepatocytes (DDx: leukemic infiltrate, diffuse lymphomatous involvement, toxic shock syndrome)
increased brightness of portal triads ( starry sky pattern) = centrilobular pattern due to edema in hepatocytes (DDx: leukemic infiltrate, diffuse lymphomatous involvement, toxic shock syndrome) edema of gallbladder fossa + gallbladder wall thickening
edema of gallbladder fossa + gallbladder wall thickening thickening + increase in echogenicity of fat within falciform ligament, ligamentum venosum, porta hepatis, periportal connective tissue
thickening + increase in echogenicity of fat within falciform ligament, ligamentum venosum, porta hepatis, periportal connective tissue
Chronic Hepatitis
= process present for at least 6 months
| Cause: | autoimmune hepatitis; hepatitis B, C, D; cryptic hepatitis; chronic drug hepatitis; primary biliary cirrhosis; primary sclerosing cholangitis; Wilson disease; alpha-1 antitrypsin deficiency |
US:
 increased liver echogenicity
increased liver echogenicity coarsening of hepatic echotexture
coarsening of hepatic echotexture silhouetting/loss of definition of portal venules = decreased visualization of the walls of the peripheral portal veins
silhouetting/loss of definition of portal venules = decreased visualization of the walls of the peripheral portal veins NO sound attenuation
NO sound attenuation
| Cx: | cirrhosis (10% for hepatitis B; 20 50% for hepatitis C) |
Neonatal Hepatitis
Cause:
INFECTION: virus, protozoa, spirochete, toxoplasmosis, rubella, CMV, herpes, hepatitis A/B, syphilis
METABOLIC: alpha-1 antitrypsin deficiency, familial recurrent cholestasis, errors of metabolism (nesidioblastosis = idiopathic hyperinsulin hypoglycemia of infancy)
IDIOPATHIC
| Age: | 1 4 weeks of age; M > F |
| Histo: | multinucleated giant cells with hepatic parenchymal disruption, relatively little bile within bile duct canaliculi |
US:
 normal-sized/enlarged liver
normal-sized/enlarged liver increase in parenchymal echogenicity
increase in parenchymal echogenicity decreased visualization of peripheral portal veins
decreased visualization of peripheral portal veins normal bile duct system
normal bile duct system gallbladder of normal size/small (with decrease in bile volume in severe hepatocellular dysfunction)
gallbladder of normal size/small (with decrease in bile volume in severe hepatocellular dysfunction) decrease in gallbladder size after milk feeding (DDx: congenital biliary atresia)
decrease in gallbladder size after milk feeding (DDx: congenital biliary atresia)
NUC:
| Technique: | often performed after pretreatment with phenobarbital (5 mg/kg 5 days) to maximize hepatic function |
 normal/decreased hepatic tracer accumulation
normal/decreased hepatic tracer accumulation prolonged clearance of tracer from blood pool
prolonged clearance of tracer from blood pool bowel activity faint/delayed usually by 24 hours (best seen on lateral view; covering liver activity with lead shielding is helpful)
bowel activity faint/delayed usually by 24 hours (best seen on lateral view; covering liver activity with lead shielding is helpful) gallbladder may not be visualized
gallbladder may not be visualized
P.724
| Prognosis: | spontaneous remission |
| DDx: | biliary atresia (NO small bowel activity) |
Radiation Hepatitis
Acute Radiation-induced Hepatitis
| Time of onset: | 2 6 weeks after completion of radiation therapy with dose >3,500 rad (35 Gy) |
abnormal liver function tests
right upper quadrant discomfort
 hepatomegaly
hepatomegaly ascites
ascites
| Prognosis: | complete recovery in majority |
Chronic Radiation-induced Hepatitis
 increased attenuation in irradiated parenchyma (no fatty infiltration)
increased attenuation in irradiated parenchyma (no fatty infiltration) geographic areas of hypointensity on T1WI + hyperintensity on T2WI (due to increased water content)
geographic areas of hypointensity on T1WI + hyperintensity on T2WI (due to increased water content)
| Viral Markers of Hepatitis | ||
|---|---|---|
| Virus | Tests | Interpretation |
| HAV | Anti-HAV IgM | acute hepatitis (can remain positive for >1 year) |
| Anti-HAV IgG | past hepatitis, lifelong immunity | |
| HBV | HBsAg | acute/chronic disease |
| Anti-HBc IgM | acute infection (if titer high); chronic infection (if titer low) | |
| Anti-HBc IgG | past/recent HBV contact (may be only serum indicator of past infection) | |
| HBe | active viral replication | |
| Anti-HBe | low/absent replicative state (typically present in long-standing HBV carriers) | |
| Anti-HBs | immunity after vaccination | |
| HBV-DNA | active viral replication | |
| HCV | Anti-HCV | past/current infection |
| RIBA | test for various viral components | |
| HCV-RNA | active viral replication | |
| HDV | Anti-HDV IgM | acute/chronic infection |
| Anti-HDV IgG | chronic infection (if titer high + IgM positive); past infection if titer low + IgM negative) | |
| HDV-RNA | active viral replication | |
| HEV | Anti-HEV IgM | acute hepatitis |
| Anti-HEV IgG | past hepatitis | |
| HEV-RNA | viral replication | |
Hepatoblastoma
| Incidence: | 3rd most common abdominal tumor in children; most frequent malignant hepatic tumor in infants + children <3 years of age |
| Incidence increased with: | hemihypertrophy, Beckwith syndrome |
Histo:
epithelial type = small cells resembling embryonal/fetal liver
mixed type = epithelial cells + mesenchymal cells (osteoid, cartilaginous, fibrous tissue)
| Age: | <3 years; <18 months (in 50%); peak age between 18 and 24 months; range from newborn to 15 years; M:F = 2:1 |
upper abdominal mass, weight loss, nausea, vomiting
jaundice, pain
precocious puberty (production of endocrine substances)
persistently + markedly elevated -fetoprotein (66%)
| Metastases to: | lung (frequent) |
| Location: | right lobe of the liver |
 usually solitary mass with an average size of 10 12 cm
usually solitary mass with an average size of 10 12 cm multifocal (20%)
multifocal (20%) coarse calcifications/osseous matrix (12 30%)
coarse calcifications/osseous matrix (12 30%)
US:
 large heterogeneous echogenic mass, often with calcifications, occasionally cystic areas (necrosis/extramedullary hematopoiesis)
large heterogeneous echogenic mass, often with calcifications, occasionally cystic areas (necrosis/extramedullary hematopoiesis)
CT:
 hypointense tumor with peripheral rim enhancement
hypointense tumor with peripheral rim enhancement
MR:
 inhomogeneously hypointense on T1WI with hyperintense foci (hemorrhage)
inhomogeneously hypointense on T1WI with hyperintense foci (hemorrhage) inhomogeneously hyperintense with hypointense bands (fibrous septa) on T2WI
inhomogeneously hyperintense with hypointense bands (fibrous septa) on T2WI
NUC:
 photopenic defect
photopenic defect
Angio:
 hypervascular mass with dense stain
hypervascular mass with dense stain marked neovascularity; NO AV-shunting
marked neovascularity; NO AV-shunting vascular lakes may be present
vascular lakes may be present avascular areas (secondary to tumor necrosis)
avascular areas (secondary to tumor necrosis) may show caval involvement (= unresectable)
may show caval involvement (= unresectable)
| Prognosis: | 60% resectable; 75% mortality; better prognosis than hepatoma; better prognosis for epithelial type than mixed type |
| DDx: | hemangioendothelioma (fine granular calcifications), metastatic neuroblastoma, mesenchymal hamartoma, hepatocellular carcinoma (>5 years of age, no calcifications) |
Hepatocellular Carcinoma
= HEPATOMA
= most frequent primary visceral malignancy in the world; 80 90% of all primary liver malignancies; 2nd most frequent malignant hepatic tumor in children (39%) after hepatoblastoma
| Incidence: | (a) in industrialized world: 0.2 0.8% (b) in sub-Saharan Africa, Southeast Asia, Japan, Greece, Italy: 5.5 20% |
| Peak age: | (a) industrialized world: 6th 7th decade; M:F = 2.5:1; fibrolamellar subtype (in 3 10%) below age 40 years (b) high incidence areas: 30 40 years; M:F = 5:1 (c) in children: >5 years of age (peak at 12 14 years); M:F = 4:3 |
P.725
Etiology:
Cirrhosis (60 90%)
alcohol
hemochromatosis
cardiac
biliary atresia
Latent period: 8 months to 14 years from onset of cirrhosis Incidence of HCC:
44% in macronodular (= postnecrotic) cirrhosis due to hepatitis B virus, alcoholism, hemochromatosis
6% in micronodular cirrhosis due to alcoholism
 5% of alcoholic cirrhotics develop HCC!
5% of alcoholic cirrhotics develop HCC!
Chronic hepatitis B/C: 12% develop HCC
Carcinogens
aflatoxin
siderosis
thorotrast
oral contraceptives/anabolic androgens
Inborn errors of metabolism
alpha-1 antitrypsin deficiency
galactosemia
type I glycogen storage disease (von Gierke)
Wilson disease
tyrosinosis
| mnemonic: | WHAT causes HCC? |
Wilson disease
Hemochromatosis
Alpha-1-antitrypsin deficiency
Tyrosinosis
Hepatitis
Cirrhosis (alcoholic, biliary, cardiac)
Carcinogens (aflatoxin, sex hormones, thorotrast)
| Path: | soft tumor due to lack of stroma, often hemorrhagic + necrotic |
| Histo: | HCC cells resemble hepatocytes in appearance + structural pattern (trabecular, pseudoglandular = acinar, compact, scirrhous); (a) expansive encapsulated HCC: collapsed portal vein branches at capsule (b) infiltrative nonencapsulated HCC: portal venules communicate with tumoral sinusoids = often invasion of portal hepatic veins |
Growth pattern:
solitary massive (27 50 59%):
bulk in one (most often right) lobe with satellite nodules
multifocal small nodular (15 25%):
small foci of usually <2 cm (up to 5 cm) in both hepatic lobes
diffuse microscopic infiltrating form (10 15 26%):
tiny indistinct nodules closely resembling cirrhosis
| Vascular supply: | hepatic artery, portal vein in 6% |
-fetoprotein elevated in 75 90% (DDx: negative -fetoprotein in cholangiocarcinoma)
elevated liver function tests
persistent RUQ pain, hepatomegaly, ascites
fever, weight loss, malaise
Paraneoplastic syndromes:
sexual precocity/gynecomastia
hypercholesterolemia
erythrocytosis (tumor produces erythropoietin)
hypoglycemia
hypercalcemia
carcinoid syndrome
| Metastases to: | lung (most common = 8%), adrenal, lymph nodes, bone |
 portal vein invasion (25 33 48%)
portal vein invasion (25 33 48%) arterioportal shunting (4 63%)
arterioportal shunting (4 63%) invasion of hepatic vein (16%)/IVC (= Budd-Chiari syndrome)
invasion of hepatic vein (16%)/IVC (= Budd-Chiari syndrome) occasionally invasion of bile ducts
occasionally invasion of bile ducts calcifications in ordinary HCC (2 9 25%); however, common in fibrolamellar (30 40%) and sclerosing HCC
calcifications in ordinary HCC (2 9 25%); however, common in fibrolamellar (30 40%) and sclerosing HCC hepatomegaly and ascites
hepatomegaly and ascites tumor fatty metamorphosis (2 17%)
tumor fatty metamorphosis (2 17%)
CT (sensitivity of 63% in cirrhosis, 80% without cirrhosis):
 hypodense mass/rarely isodense/hyperdense in fatty liver:
hypodense mass/rarely isodense/hyperdense in fatty liver: dominant mass with satellite nodules
dominant mass with satellite nodules mosaic pattern = multiple nodular areas with differing attenuation on CECT (up to 63%)
mosaic pattern = multiple nodular areas with differing attenuation on CECT (up to 63%) diffusely infiltrating neoplasm
diffusely infiltrating neoplasm
 encapsulated HCC = circular zone of radiolucency surrounding the mass (12 32 67%)
encapsulated HCC = circular zone of radiolucency surrounding the mass (12 32 67%)
| False-positive: | confluent fibrosis, regenerative nodule |
Biphasic CECT:
 enhancement during hepatic arterial phase (80%)
enhancement during hepatic arterial phase (80%) decreased attenuation during portal venous phase with inhomogeneous areas of contrast accumulation
decreased attenuation during portal venous phase with inhomogeneous areas of contrast accumulation isodensity on delayed scans (10%)
isodensity on delayed scans (10%) thin contrast-enhancing capsule (50%) due to rapid washout
thin contrast-enhancing capsule (50%) due to rapid washout wedge-shaped areas of decreased attenuation (segmental/lobar perfusion defects due portal vein occlusion by tumor thrombus)
wedge-shaped areas of decreased attenuation (segmental/lobar perfusion defects due portal vein occlusion by tumor thrombus)
CT with intraarterial ethiodol injection:
 hyperdense mass detectable as small as 0.5 cm US (86 99% sensitivity, 90 93% specificity, 50 94% accuracy)
hyperdense mass detectable as small as 0.5 cm US (86 99% sensitivity, 90 93% specificity, 50 94% accuracy)
US:
 variable echogenicity:
variable echogenicity: hyperechoic HCC (13%) due to fatty metamorphosis or marked dilatation of sinusoids
hyperechoic HCC (13%) due to fatty metamorphosis or marked dilatation of sinusoids hypoechoic HCC (26%) due to solid tumor
hypoechoic HCC (26%) due to solid tumor HCC of mixed echogenicity (61%) due to nonliquefactive tumor necrosis
HCC of mixed echogenicity (61%) due to nonliquefactive tumor necrosis
 Doppler peak velocity signals >250 cm/sec
Doppler peak velocity signals >250 cm/sec calcifications (rare)
calcifications (rare)
MR:
 hypointense (50%)/iso- to hyperintense (with fatty metamorphosis) on T1WI
hypointense (50%)/iso- to hyperintense (with fatty metamorphosis) on T1WI ring sign = well-defined hypointense capsule on T1WI (24 44%), double layer of inner hypointensity (fibrous tissue) + outer hyperintensity (compressed blood vessels + bile ducts) on T2WI in expansive type of HCC
ring sign = well-defined hypointense capsule on T1WI (24 44%), double layer of inner hypointensity (fibrous tissue) + outer hyperintensity (compressed blood vessels + bile ducts) on T2WI in expansive type of HCC moderately hyperintense on T2WI
moderately hyperintense on T2WI may contain central scar of fibrosis/calcifications/necrosis hypointense on T1WI + T2WI
may contain central scar of fibrosis/calcifications/necrosis hypointense on T1WI + T2WI
CEMR:
 Gd-DTPA enhancement peripherally (21%)/centrally (7%)/mixed (10%)/no enhancement (21%)
Gd-DTPA enhancement peripherally (21%)/centrally (7%)/mixed (10%)/no enhancement (21%) central scar without much enhancement
central scar without much enhancement improved lesion detectability after intravenous administration of superparamagnetic iron oxide
improved lesion detectability after intravenous administration of superparamagnetic iron oxide
P.726
NUC:
 Sulfur colloid scan: single cold spot (70%), multiple defects (15-20%), heterogeneous distribution (10%)
Sulfur colloid scan: single cold spot (70%), multiple defects (15-20%), heterogeneous distribution (10%) Tc-HIDA scan: cold spot/atypical uptake in 4% (delayed images)
Tc-HIDA scan: cold spot/atypical uptake in 4% (delayed images) Gallium-scan: avid accumulation in 70-90% (in 63% greater, in 25% equal, in 12% less uptake than liver)
Gallium-scan: avid accumulation in 70-90% (in 63% greater, in 25% equal, in 12% less uptake than liver)
Angio:
 thread and streaks = linear parallel vascular channels coursing along portal venous radicles seen with portal venous involvement
thread and streaks = linear parallel vascular channels coursing along portal venous radicles seen with portal venous involvement in differentiated HCC: enlarged arterial feeders, coarse neovascularity, vascular lakes, dense tumor stain, arterioportal shunts
in differentiated HCC: enlarged arterial feeders, coarse neovascularity, vascular lakes, dense tumor stain, arterioportal shunts in anaplastic HCC: vascular encasement, fine neovascularity, displacement of vessels + corkscrew-like vessels of cirrhosis
in anaplastic HCC: vascular encasement, fine neovascularity, displacement of vessels + corkscrew-like vessels of cirrhosis
| Prognosis: | >90% overall mortality; 17% resectability rate; 6 months average survival time; 30% 5-year survival time |
| Cx: | spontaneous rupture (in 8%) |
| Rx: | (1) Resection (2) I-131 antiferritin IgG (remission rate >40% up to 3 years) |
| DDx: | hepatocarcinoma, cholangiocarcinoma, focal nodular hyperplasia, hemangioma, hepatic adenoma |
Fibrolamellar Carcinoma of Liver
= uncommon variant of hepatocellular carcinoma
| Prevalence: | 1-9% of all HCCs; up to 35% of hCCs in patients <50 years of age |
| Age: | 5-69 (mean 23) years; mostly 2nd-3rd decade; M:F = 1:1 |
| Path: | large well-circumscribed lobulated nonencapsulated strikingly desmoplastic tumor with calcifications + fibrous central scar |
| Histo: | large hepatocyte-like cells with granular eosinophilic cytoplasm growing in sheets/cords/trabeculae separated by broad bands of fibrous stroma arranged in parallel lamellae resulting in compartmentalized appearance |
| Risk factors: | NONE known; underlying cirrhosis or hepatitis in <5% |
| Demographics: | less common in Europe; rare in Japan + China |
pain, cachexia; palpable RUQ mass, hepatomegaly
gynecomastia (rare) from conversion of androgens to estrogens by tumor-elaborated enzyme aromatase
jaundice (5%) from biliary compression
alpha;-fetoprotein usually negative/mildly elevated to <200 ng/ L (in up to 10%)
transaminase levels <100 IU/L
 partially/completely encapsulated solitary mass (in 80-90%):
partially/completely encapsulated solitary mass (in 80-90%): intrahepatic (80%)/pedunculated (20%)
intrahepatic (80%)/pedunculated (20%) 5-20 (mean 13) cm in diameter
5-20 (mean 13) cm in diameter prominent central fibrous scar (45-60%)
prominent central fibrous scar (45-60%) capsular retraction (10%)
capsular retraction (10%) punctate/nodular/stellate calcifications located within scar (33-55%)
punctate/nodular/stellate calcifications located within scar (33-55%) intratumoral hemorrhage + necrosis (10%)
intratumoral hemorrhage + necrosis (10%) vascular invasion (<5%)
vascular invasion (<5%)
 mass + small peripheral satellite lesions (10-15%)
mass + small peripheral satellite lesions (10-15%) diffuse multifocal masses (<1%)
diffuse multifocal masses (<1%) regional adenopathy (50 70%): porta hepatis
regional adenopathy (50 70%): porta hepatis distant metastases (20%): lung, peritoneal implants
distant metastases (20%): lung, peritoneal implants
US:
 mixed echogenicity (60%)
mixed echogenicity (60%) central hyperechoic scar (33 60%)
central hyperechoic scar (33 60%)
CT:
 mass of low attenuation
mass of low attenuation enhancement of non-scar portion:
enhancement of non-scar portion: prominent heterogeneous enhancement in arterial + portal venous phase
prominent heterogeneous enhancement in arterial + portal venous phase less pronounced enhancement during equilibrium phase
less pronounced enhancement during equilibrium phase
 delayed enhancement of scar (25%) + pseudocapsule of compressed liver tissue (15%)
delayed enhancement of scar (25%) + pseudocapsule of compressed liver tissue (15%)
MRI:
 large lobulated mass
large lobulated mass T1WI:
T1WI: hypointense (86%)/isointense (14%)
hypointense (86%)/isointense (14%) homogeneous (80%)/heterogeneous (20%)
homogeneous (80%)/heterogeneous (20%)
 T2WI:
T2WI: hyperintense/heterogeneous (85%)
hyperintense/heterogeneous (85%) isointense/homogeneous (15%)
isointense/homogeneous (15%)
 hypointense central scar on T1WI + T2WI
hypointense central scar on T1WI + T2WI
Angio:
 dense tumor stain
dense tumor stain enlarged feeding arteries
enlarged feeding arteries NO arteriovenous/arterioportal shunting
NO arteriovenous/arterioportal shunting avascular central scar
avascular central scar
NUC:
 photopenic defect on sulfur colloid scan
photopenic defect on sulfur colloid scan increased activity during arterial phase + photo-penic during delayed imaging on labeled RBC scan
increased activity during arterial phase + photo-penic during delayed imaging on labeled RBC scan
| Prognosis: | 48% resectability rate; 32 months average survival time; 67% 5-year survival time |
| DDx: | focal nodular hyperplasia (young + middle-aged women, <5 cm in size, calcifications uncommon, isointense to liver on all CT + MR images with pronounced homogeneous enhancement during arterial phase, hyperintense central scar on T2WI, uptake of sulfur colloid/super paramagnetic iron oxide) |
Hyperplastic Cholecystosis
= variety of degenerative + proliferative changes of gallbladder wall characterized by hyperconcentration, hyperexcitability, and hyperexcretion
| Incidence: | 30 50% of all cholecystectomy specimens; M:F = 1:6 |
Adenomyomatosis of Gallbladder
= increase in number + height of mucosal folds
| Histo: | hyperplasia of epithelial + muscular elements with mucosal outpouching of epithelium-lined cystic spaces into (46%) or all the way through (30%) a thickened muscular layer as tubules/crypts/2 8 mm saccules (= intramural diverticula = Rokitansky-Aschoff sinus); develop with increasing age [Karl Rokitansky (1804 1878), pathologist in Vienna, Austria] [Carl Aschoff (1866 1942), pathologist in Bonn, Germany] |
| Incidence: | 2 5% of all cholecystectomy specimens |
| Age: | >35 years; M:F = 1:3 |
| Associated with: | (1) Gallstones in 25 75% (2) Cholesterolosis in 33% |
P.727
Types:
generalized form = ADENOMYOMATOSIS
 pearl necklace gallbladder = tiny extraluminal extensions of contrast on OCG (enhanced after contraction with fatty meal)/MRCP
pearl necklace gallbladder = tiny extraluminal extensions of contrast on OCG (enhanced after contraction with fatty meal)/MRCP comet-tail = sound reverberation artifact between cholesterol crystals in Rokitansky-Aschoff sinuses (PATHOGNOMONIC)
comet-tail = sound reverberation artifact between cholesterol crystals in Rokitansky-Aschoff sinuses (PATHOGNOMONIC)
N.B.: Rokitansky-Aschoff sinuses of <5 mm cannot be identified segmental form
compartmentalization most often in neck/distal 1/3
localized form in fundus = ADENOMYOMA
 smooth sessile mass in GB fundus
smooth sessile mass in GB fundus= solitary adenomyoma + extraluminal diverticula-like formation
annular form
 hourglass configuration of GB with transverse congenital septum
hourglass configuration of GB with transverse congenital septum
| DDx: | gallbladder carcinoma |
Cholesterolosis
= abnormal deposits of cholesterol esters in macrophages within lamina propria (foam cells) + in mucosal epithelium
Strawberry Gallbladder
= LIPID CHOLECYSTITIS = cholesterosis
= planar form = seedlike patchy/diffuse thickening of the villous surface pattern (disseminated micronodules)
| Associated with: | cholesterol stones in 50 70% |
not related to serum cholesterol level
 radiologically not demonstrable
radiologically not demonstrable
Cholesterol Polyp (90%)
= polypoid form
= abnormal deposit of cholesterol esters and triglycerides producing a villouslike structure covered with a single layer of epithelium and attached via a delicate stalk
| Prevalence: | 4%; most common (50%) fixed filling defect of gallbladder |
| Age: | 40 50 years; M:F=1:3 |
| Location: | commonly in middle 1/3 of gallbladder |
 multiple (on average 8) small filling defects <10 mm (rarely up to 20 mm) in diameter
multiple (on average 8) small filling defects <10 mm (rarely up to 20 mm) in diameter
| DDx: | papilloma, adenoma, inflammatory granuloma |
Inspissated Bile Syndrome
= uncommon cause of jaundice in neonate
| Associated with: | massive hemolysis (Rh incompatibility), hemorrhage (intraabdominal, intracranial, retroperitoneal), increased enterohepatic circulation (Hirschsprung disease, intestinal atresia, stenosis) |
US:
 sludge in gallbladder
sludge in gallbladder sludge within bile ducts + partial/complete obstruction (affected ducts may blend with surrounding hepatic parenchyma)
sludge within bile ducts + partial/complete obstruction (affected ducts may blend with surrounding hepatic parenchyma)
Intraductal Papillary Mucinous Tumor Of Pancreas
= IPMT = MUCINOUS DUCTAL ECTASIA = DUCTECTATIC MUCINOUS CYSTIC TUMOR OF PANCREAS = INTRADUCTAL MUCIN-HYPERSECRETING NEOPLASM = MUCIN-PRODUCING PANCREATIC TUMOR = MUCINOUS VILLOUS ADENOMATOSIS
= rare intraductal tumor originating from papillary epithelial lining typified by voluminous mucin secretions
| Path: | conglomeration of communicating cysts covered by a rim of normal pancreatic parenchyma + thin fibrous capsule |
| Histo: | cysts represent a dilated duct lined with innumerable papillae coated with hyperplastic/atypical/malignant epithelium (adenoma-carcinoma sequence) |
| Age: | elderly patients; M>F |
recurrent episodes of dull pain/acute pancreatitis (due to impaired outflow of pancreatic secretions):
hyperamylasemia (occasionally)
viscosity of fluid greater than normal serum (89% sensitive, 100% specific)
| Prognosis: | low-grade malignancy with better prognosis than pancreatic adenocarcinoma |
| Dx: | ERCP (bulging ampulla, mucin pouring from papilla, communication between pancreatic duct + cystic cavity) |
| Rx: | Whipple operation (main duct IPMT/partial pancreatectomy (branch duct IPMT) |
| DDx: | chronic obstructive pancreatitis, serous/mucinous cystic tumors, pseudocyst |
Main Duct IPMT
| Age: | 57 (range, 34 75) years; M:F = 1:1 |
 hyperechoic, hyperdense, T2-hypointense filling defect within dilated duct (= enhancing papillary mural nodule/gravity-dependent mucin glob)
hyperechoic, hyperdense, T2-hypointense filling defect within dilated duct (= enhancing papillary mural nodule/gravity-dependent mucin glob) dilatation of main pancreatic duct:
dilatation of main pancreatic duct:dilatation of entire main pancreatic duct
 homogeneous hypoechoic, hypodense, T1-hypointense and T2-hyperintense main duct
homogeneous hypoechoic, hypodense, T1-hypointense and T2-hyperintense main duct pancreatic parenchymal atrophy
pancreatic parenchymal atrophy dilatation of branch ducts (usually in pancreatic tail + uncinate process)
dilatation of branch ducts (usually in pancreatic tail + uncinate process) dilatation of major minor papilla bulging into duodenal lumen
dilatation of major minor papilla bulging into duodenal lumen obstruction of CBD (due to tumor/impacted mucin)
obstruction of CBD (due to tumor/impacted mucin)
Cx: pancreaticobiliary/-duodenal fistula, pseudomyxoma peritonei DDx: chronic obstructive pancreatitis segmental dilatation of main pancreatic duct
 cyst in pancreatic body/tail + normal remaining pancreatic parenchyma
cyst in pancreatic body/tail + normal remaining pancreatic parenchyma cyst in pancreatic head + upstream dilatation of main pancreatic duct
cyst in pancreatic head + upstream dilatation of main pancreatic duct
| DDx: | peripheral mucinous cystic tumor (main duct almost always normal) |
P.728
ERCP:
thick jellylike mucus protruding from a bulging patulous duodenal papilla
 plugging of the papilla of Vater
plugging of the papilla of Vater amorphous intraluminal filling defects in main pancreatic duct
amorphous intraluminal filling defects in main pancreatic duct usually small mural polypoid/flat tumor
usually small mural polypoid/flat tumor dilated main + branch pancreatic ducts without obstructive ductal stricture
dilated main + branch pancreatic ducts without obstructive ductal stricture
| N.B.: | reflux of contrast material due to excess of mucin/patent papillary orifice hinders filling of ductal tree |
Branch Duct IPMT
| Age: | 63 (range, 37 76) years; M:F=1:1 |
usually incidental finding when tumor small
symptoms mimicking acute/chronic pancreatitis
| Location: | mainly in uncinate process >> pancreatic tail > pancreatic body |
| Path: | macrocystic/microcystic pattern; malignancy suggested by irregular thick wall + septa and solid nodules |
 round/ovoid small lobulated intraductal mass (frequently not visualized):
round/ovoid small lobulated intraductal mass (frequently not visualized): dilated main pancreatic duct
dilated main pancreatic duct normal main pancreatic duct (almost always normal in small tumor)
normal main pancreatic duct (almost always normal in small tumor) Secretin administration distends ducts and enhances detection of communication with main pancreatic duct!
Secretin administration distends ducts and enhances detection of communication with main pancreatic duct!
 uni-/multilocular cyst 10-20 mm large with sparse septa
uni-/multilocular cyst 10-20 mm large with sparse septa
| DDx: | mucinous cystadenoma (no communication with main pancreatic duct); pseudocyst (no intraluminal filling defects) |
 multiple thin septa separating fluid-filled lacunae
multiple thin septa separating fluid-filled lacunae
| DDx: | serous cystadenoma (no communication with main pancreatic duct) |
 severe pancreatic atrophy
severe pancreatic atrophy protrusion of papilla into duodenum
protrusion of papilla into duodenum
ERCP:
 contrast spills from main duct into cystically dilated branch ducts
contrast spills from main duct into cystically dilated branch ducts elongated band-/threadlike or nodular filling defects in dilated ducts (= depiction of mucin)
elongated band-/threadlike or nodular filling defects in dilated ducts (= depiction of mucin)
| Cx: | seeding to main pancreatic duct resulting in main duct IPMT |
Lipoma of Liver
Extremely rare
asymptomatic
| May be associated with: | tuberous sclerosis |
| Size: | few mm 13 cm |
US:
 echogenic mass
echogenic mass striking acoustic refraction (sound velocity in soft tissue 1,540 m/sec, in fat 1,450 m/sec)
striking acoustic refraction (sound velocity in soft tissue 1,540 m/sec, in fat 1,450 m/sec)
| Prognosis: | no malignant potential |
Liver Transplant
Indication:
chronic viral hepatitis: chronic active hepatitis (4% in childhood)
metabolic disease: alpha-1 antitrypsin deficiency (9% in childhood), hemochromatosis, Wilson disease
cholestatic liver disease: primary biliary cirrhosis, primary sclerosing cholangitis, biliary atresia (52% in childhood)
autoimmune hepatitis
cryptogenic cirrhosis (6% in childhood)
alcoholic liver disease
acute fulminant hepatic failure (11% in childhood): viral hepatitis, drug-induced hepatitis (eg, by acetaminophen, isoniazid), hepatotoxins (eg, mushrooms)
| Contraindications: | AIDS, extrahepatic malignant tumors, active IVDA/alcohol abuse |
Normal posttransplant findings
Periportal edema (21%)
Cause: lymphedema in early posttransplantation period (= dilatation of lymphatic channels due to lack of normal lymphatic drainage)  periportal collar of low attenuation on CT + hyperechogenicity on US
periportal collar of low attenuation on CT + hyperechogenicity on US resolution within weeks to months
resolution within weeks to months
Fluid collection around falciform ligament (11%), at vascular anastomoses (liver hilum, IVC), biliary anastomosis, lesser sac
Small right pleural effusion
Peri-/subhepatic hematoma/free intraabdominal fluid
Vascular Complications in Liver Transplant (9%)
 Most frequent cause of graft loss
Most frequent cause of graft lossliver failure, bile leak, abdominal bleeding, septicemia
1. Anastomotic narrowing of IVC/portal vein
 Discrepancies in caliber between donor + recipient vessel have no pathologic significance!
Discrepancies in caliber between donor + recipient vessel have no pathologic significance!venous hypertension of lower part of body
portal hypertension
 narrowing of portal vein + poststenotic dilatation
narrowing of portal vein + poststenotic dilatation 3 4-fold velocity increase compared with prestenotic segment
3 4-fold velocity increase compared with prestenotic segment
2. Thrombosis/stenosis of portal vein (1 3%)
Cause: faulty surgical technique, vessel misalignment, differences in vessel caliber creating turbulent flow, hypercoagulable state, prior portal vein surgery, prior thrombosis in recipient portal vein portal hypertension, liver failure, massive ascites, edema
 filling defect/focal narrowing at anastomosis
filling defect/focal narrowing at anastomosis
Rx: percutaneous transluminal angioplasty stent placement, surgical thrombectomy, venous jump graft, creation of portosystemic shunt, retransplantation 3. Thrombosis/stenosis of IVC (<1%)
pleural effusions, hepatomegaly, ascites, extremity edema
 compression of IVC (due to swelling of graft)
compression of IVC (due to swelling of graft) size discrepancy between donor + recipient IVC
size discrepancy between donor + recipient IVC
4. Hepatic artery stenosis (5 13%)
Location: at/near anastomotic site Time of onset: within 3 months  marked focal increase in velocity >200 300 cm/sec + poststenotic turbulence (in >50% stenosis)
marked focal increase in velocity >200 300 cm/sec + poststenotic turbulence (in >50% stenosis) intrahepatic tardus et parvus waveform = slowed systolic acceleration time (SAT >0.08 sec) distal to stenosis (73% sensitive)
intrahepatic tardus et parvus waveform = slowed systolic acceleration time (SAT >0.08 sec) distal to stenosis (73% sensitive) diminished pulsatility (RI <0.5) due to ischemia
diminished pulsatility (RI <0.5) due to ischemiaDDx: normal in early post-transplantation period  biliary dilatation (due to stricture), infarction, biloma
biliary dilatation (due to stricture), infarction, biloma
P.729
Rx: revascularization surgery, balloon angioplasty 5. hepatic artery thrombosis (3 9 16% in adults, 9 19 42% in children)
Risk factors: significant caliber difference between donor + recipient artery, preexisting celiac artery stenosis, prolonged cold ischemia of donor liver, ABo blood type incompatibility, rejection Time of onset: usually within first 2 months Three types of clinical presentation:
fulminant hepatic necrosis + rapid deterioration
bile leak, bile peritonitis, bacteremia, sepsis
relapsing bacteremia
 absence of hepatic artery flow
absence of hepatic artery flowFalse-positive Doppler (10%):
low flow state, small vessel size, severe liver edema (in first 72 hours after transplantation, viral hepatitis, rejection)
False-negative Doppler: arterial collaterals  multiple hypoechoic lesions in liver periphery (= infarcts)
multiple hypoechoic lesions in liver periphery (= infarcts)
Mortality: 27-58% 6. hepatic artery pseudoaneurysm (uncommon)
Location: at vascular anastomosis Cx: massive intraperitoneal hemorrhage, portal vein fistula, biliary fistula Rx: surgical resection, embolization, exclusion by stent placement
Parenchymal Complications in Liver Transplant
Rejection
 Can ONLY be diagnosed with liver biopsy!
Can ONLY be diagnosed with liver biopsy!
Infarction (10%)
 may calcify
may calcify may liquefy developing into intrahepatic biloma
may liquefy developing into intrahepatic biloma
Graft infection
Biliary Complications in Liver Transplant (6-34%)
 Second most common cause of liver dysfunction after rejection
Second most common cause of liver dysfunction after rejection
| Time of onset: | within first 3 months |
Biliary obstruction
anastomotic stricture (extrahepatic)
Cause: iatrogenic trauma resulting in ischemia + scar formation nonanastomotic (intrahepatic) stricture
Cause: hepatic arterial thrombosis/stenosis (in 50%), prolonged preservation time, bacterial/viral cholangitis, rejection, recurrent primary sclerosing cholangitis, cholangiocarcinoma, kinking of redundant CBD, sphincter of oddi dysfunction tension mucocele of allograft cystic duct remnant
Cause: ligation of cystic duct proximally + distally
 extrinsic mass compressing ChD
extrinsic mass compressing ChD fluid collection adjacent to ChD
fluid collection adjacent to ChD
Cx: ascending cholangitis Bile leak
T-tube exit site: 50% within 10 days
anastomosis of choledochocholedochostomy: 70% within 1st month
bile duct necrosis (hepatic artery occlusion)
 The intrahepatic biliary epithelium is perfused solely by the hepatic artery!
The intrahepatic biliary epithelium is perfused solely by the hepatic artery!
after liver biopsy
common hepatic duct leak
Incidence: 4.3-23% Stone/sludge formation
Cause: alteration in bile composition
Lymphoma of liver
PRIMARY LYMPHOMA (rare)
 solid solitary mass
solid solitary mass
SECONDARY LYMPHOMA (common)
autoptic incidence of liver involvement:
60% in hodgkin disease
50% in non-hodgkin lymphoma
Pattern:
infiltrative diffuse (most common): no alteration in hepatic architecture
focal nodular: detectable by cross-sectional imaging
combination of diffuse + nodular (3%)
| Detection rate (for CT, MRI): | <10% |
Mesenchymal Hamartoma Of Liver
= rare developmental cystic liver tumor
| Histo: | disordered arrangement of primitive fluid-filled mesenchyme, bile ducts, hepatic parenchyma; stromal/cystic predominance with cysts of a few mm up to 14 cm in size; no capsule |
| Age peak: | 15 24 months (range from newborn to 19 years); M:F = 2:1 |
slow progressive abdominal enlargement
respiratory distress and lower extremity edema
| Location: | right lobe:left lobe = 6:1; 20% pedunculated |
| Size: | 5 29 (mean 16) cm |
 grossly discernible cysts in 80%
grossly discernible cysts in 80%
US:
 multiple rounded cystic areas on an echogenic background
multiple rounded cystic areas on an echogenic background may appear solidly echogenic in fetus/younger infant (with microcysts creating innumerable tissue-fluid interfaces)
may appear solidly echogenic in fetus/younger infant (with microcysts creating innumerable tissue-fluid interfaces)
CT:
 multiple lucencies of variable size + attenuation (depending on composition of stromal versus cystic elements)
multiple lucencies of variable size + attenuation (depending on composition of stromal versus cystic elements) hemorrhage (rare)
hemorrhage (rare) enhancement of stromal component
enhancement of stromal component
MR:
 varying signal intensity (varying concentrations of protein in cystic predominance type)/hypointense on T1WI (mesenchymal predominance type)
varying signal intensity (varying concentrations of protein in cystic predominance type)/hypointense on T1WI (mesenchymal predominance type) marked hyperintensity of cystic locules/hypointense fibrosis on T2WI
marked hyperintensity of cystic locules/hypointense fibrosis on T2WI
NUC:
 one/more areas of diminished uptake on sulfur colloid scan
one/more areas of diminished uptake on sulfur colloid scan
P.730
Angio:
 hypovascular mass
hypovascular mass may show patchy areas of neovascularity
may show patchy areas of neovascularity enlarged irregular tortuous feeding vessels
enlarged irregular tortuous feeding vessels
Metastases To Gallbladder
| Organ of origin: | melanoma, renal cell carcinoma (late in course of disease), lymphoma (in AIDS), malignant fibrous histiocytoma |
| in children: | embryonal cell sarcoma, rhabdomyosarcoma |
Metastases To Liver
 Most common malignant lesion of the liver
Most common malignant lesion of the liver
| Incidence: | the liver is the most common metastatic site after regional lymph nodes; incidence of metastatic carcinoma is 20 greater than primary carcinoma; metastases represent 22% of all liver tumors in patients with known malignancy |
| Organ of origin: | colon (42%), stomach (23%), pancreas (21%), breast (14%), lung (13%) |
 involvement of liver + spleen typical in leukemia/lymphoma + melanoma
involvement of liver + spleen typical in leukemia/lymphoma + melanoma
| in children: | neuroblastoma, Wilms tumor |
hepatomegaly (70%)
abnormal liver enzymes (50 75%)
| Location: | both lobes (77%), right lobe (20%), left lobe (3%) |
| Number: | multiple (50 98%), solitary (2%) |
| Size: | >33% smaller than 2 cm |
Enhancement characteristics compared with normal liver:
 lesion enhancement during arterial phase (metastases are supplied by hepatic artery)
lesion enhancement during arterial phase (metastases are supplied by hepatic artery) less enhancement during portal venous phase (metastases have a negligible portal venous supply)
less enhancement during portal venous phase (metastases have a negligible portal venous supply) extracellular space agents accumulate more in tumor tissue (metastases have a larger interstitial space)
extracellular space agents accumulate more in tumor tissue (metastases have a larger interstitial space)
| NUC: | 80 95% sensitivity in lesions >1.5 cm; lesions <1.5 cm are frequently missed; sensitivity increases with metastatic deposit size, peripheral location, and use of SPECT |
| NECT: | important for hypervascular tumors (eg, renal cell carcinoma, carcinoid, islet cell tumors), which may be obscured by CECT |
CECT:
Technique:
optimal is bolus technique with dynamic incremental scanning; sensitivity is decreased relative to NCCT if scans are obtained during equilibrium phase of contrast administration
 circumferential bead- or bandlike enhancement during arterial phase + peripheral washout on delayed images
circumferential bead- or bandlike enhancement during arterial phase + peripheral washout on delayed images no (35%), peripheral (37%), mixed (20%), central (8%) enhancement
no (35%), peripheral (37%), mixed (20%), central (8%) enhancement complete isodense fill-in on delayed scans in 5% (DDx: hemangioma)
complete isodense fill-in on delayed scans in 5% (DDx: hemangioma) CT-sensitivity 88 90%; specificity 99%; lesions of approx. 1 cm can usually be detected!
CT-sensitivity 88 90%; specificity 99%; lesions of approx. 1 cm can usually be detected!
CT-Angiography (most sensitive imaging modality):
| Indication: | patients with potentially resectable isolated liver metastases/preoperative to partial hepatectomy for detection of additional metastases (additional lesions detected in 40 55%) |
CT arteriography = angiography catheter in hepatic artery, detects lesions by virtue of increased enhancement
CT arterial portography = angiography catheter in SMA, detects hypodense lesions on a background of increased enhancement of normal surroundings in portal venous phase
CT-delayed iodine scanning:
= CT performed 4 6 hours following administration of 60 mg iodine results in detection of additional lesions in 27%
| Rx: | Exclusion criteria for metastasectomy: (1) advanced stage of primary tumor (2) >4 metastases (3) extrahepatic disease (4) <30% normal liver tissue/function available after resection |
Calcified Liver Metastases
| Incidence: | 2 3% |
Mucinous carcinoma of GI tract (colon, rectum, stomach)
Endocrine pancreatic carcinoma
Leiomyosarcoma, osteosarcoma
Malignant melanoma
Papillary serous ovarian cystadenocarcinoma
Lymphoma
Pleural mesothelioma
Neuroblastoma
Breast cancer
Medullary carcinoma of the thyroid
Renal cell carcinoma
Lung carcinoma
Testicular carcinoma
| mnemonic for mucinous adenocarcinoma: | COBS |
Colon carcinoma
Ovarian carcinoma
Breast carcinoma
Stomach carcinoma
Hypervascular Liver Metastases
Renal cell carcinoma
Carcinoid tumor
Pancreatic islet cell tumor
Melanoma
Thyroid cancer
Choriocarcinoma
Ovarian cystadenocarcinoma
Sarcomas
Pheochromocytoma
| mnemonic: | CHIMP |
Carcinoid
Hypernephroma
Islet cell carcinoma
Melanoma
Pheochromocytoma
Hypovascular Liver Metastases
Stomach
Colon
Pancreas
Lung
Breast
P.731
Hemorrhagic Liver Metastases
| mnemonic: | CT BeComes MR |
Colon carcinoma
Thyroid carcinoma
Breast carcinoma
Choriocarcinoma
Melanoma
Renal cell carcinoma
Echogenic Liver Metastases
| Incidence: | 25% |
Colonic carcinoma (mucinous adenocarcinoma) 54%
Hepatoma 25%
Treated breast carcinoma 21%
Liver Metastases of Mixed Echogenicity
| Incidence: | 37.5% |
Breast cancer 31%
Rectal cancer 20%
Lung cancer 17%
Stomach cancer 14%
Anaplastic cancer 11%
Cervical cancer 5%
Carcinoid 1%
Cystic Liver Metastases
Mucinous ovarian carcinoma
Colonic carcinoma
Sarcoma
Melanoma
Lung carcinoma
Carcinoid tumor
| mnemonic: | LC GOES |
Leiomyosarcoma (and other sarcomas)
Choriocarcinoma
Gastric carcinoma
Ovarian carcinoma
Endometrial carcinoma
Small cell carcinoma
Echopenic Liver Metastases
| Incidence: | 37.5% |
Lymphoma 44%
Pancreas 36%
Cervical cancer 20%
Lung (adenocarcinoma)
Nasopharyngeal cancer
Metastases To Pancreas
| Frequency: | 3 10% (autopsy) |
| Organ of origin: | renal cell carcinoma (30%), bronchogenic carcinoma (23%), breast carcinoma (12%), soft-tissue sarcoma (8%), colonic carcinoma (6%), melanoma (6%) |
 solitary (78%)/multiple (17%) ovoid masses with discrete smooth margins
solitary (78%)/multiple (17%) ovoid masses with discrete smooth margins diffuse pancreatic enlargement (5%)
diffuse pancreatic enlargement (5%)
CECT:
 heterogeneously (60%)/homogeneously (17%) hyperattenuating relative to pancreas
heterogeneously (60%)/homogeneously (17%) hyperattenuating relative to pancreas hypoattenuating relative to pancreas (20%)
hypoattenuating relative to pancreas (20%) isoattenuating relative to pancreas (5%)
isoattenuating relative to pancreas (5%)
Concomitant intraabdominal metastases to:
liver (36%), lymph nodes (30%), adrenal glands (30%)
| DDx: | ductal pancreatic adenocarcinoma (uniformly nonenhancing mass, encasement of vessels) |
Milk Of Calcium Bile
= LIMY BILE = CALCIUM SOAP
= precipitation of particulate material with high concentration of calcium carbonate, calcium phosphate, calcium bilirubinate
| Associated with: | chronic cholecystitis + gallstone obstruction of cystic duct |
 diffuse opacification of GB lumen with dependent layering
diffuse opacification of GB lumen with dependent layering usually functionless GB on oral cholecystogram
usually functionless GB on oral cholecystogram
US:
 intermediate features between sludge + gallstones
intermediate features between sludge + gallstones
Mirizzi Syndrome
= extrinsic right-sided compression of common hepatic duct by large gallstone impacted in cystic duct/gallbladder neck/cystic duct remnant; accompanied by chronic inflammatory reaction
| Frequently associated with: | formation of fistula between gallbladder and common hepatic duct |
jaundice
 cystic duct course usually parallel to CHD
cystic duct course usually parallel to CHD normal CBD below level of impacted stone
normal CBD below level of impacted stone TRIAD:
TRIAD:gallstone impacted in GB neck
dilatation of bile ducts above level of cystic duct
smooth curved segmental stenosis of CHD
Cholangiography:
 partial obstruction of CHD due to external compression on lateral side of duct/eroding stone
partial obstruction of CHD due to external compression on lateral side of duct/eroding stone
| DDx: | lymphadenopathy, neoplasm of GB/CHD |
Mucinous cystic neoplasm
= MACROCYSTIC ADENOMA OF PANCREAS
= thick-walled uni-/multilocular low-grade malignant tumor composed of large mucin-containing cystic spaces
| Frequency: | 10% of pancreatic cysts; 1% of pancreatic neoplasms |
| Mean age: | 50 years (range of 20 95 years); in 50% between 40 60 years; M:F = 1:9 |
| Path: | large smooth round/lobulated multiloculated cystic mass encapsulated by a layer of fibrous connective tissue |
| Histo: | similar to biliary and ovarian mucinous tumors; cysts lined by tall columnar, mucin-producing cells subtended by a densely cellular mesenchymal stroma (reminiscent of ovarian stroma), often in papillary arrangement, lack of cellular glycogen (a) mucinous cystadenoma (b) mucinous cystadenocarcinoma = stratified papillary epithelium |
P.732
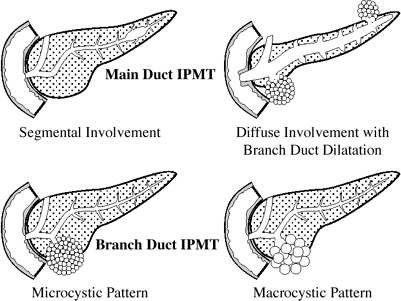 |
| Intraductal Papillary Mucinous Tumor (IPMT) |
 All mucinous cystic neoplasms should be considered as low-grade malignant neoplasms
All mucinous cystic neoplasms should be considered as low-grade malignant neoplasms
asymptomatic
abdominal pain, anorexia
| Location: | often in pancreatic tail (90%)/body, infrequently in head |
 well-demarcated thick-walled mass of 2 36 (mean, 10 12) cm in diameter
well-demarcated thick-walled mass of 2 36 (mean, 10 12) cm in diameter multi-/unilocular large cysts >2 cm with thin septa <2 mm:
multi-/unilocular large cysts >2 cm with thin septa <2 mm: A tumor with <6 cysts of >2 cm in diameter is in 93 95% a mucinous cystic neoplasm!
A tumor with <6 cysts of >2 cm in diameter is in 93 95% a mucinous cystic neoplasm!
 solid papillary excrescences protrude into the interior of tumor (sign of malignancy)
solid papillary excrescences protrude into the interior of tumor (sign of malignancy) amorphous discontinuous peripheral mural calcifications (10 15%)
amorphous discontinuous peripheral mural calcifications (10 15%) hypovascular mass with sparse neovascularity
hypovascular mass with sparse neovascularity vascular encasement and splenic vein occlusion may be present
vascular encasement and splenic vein occlusion may be present great propensity for invasion of adjacent organs
great propensity for invasion of adjacent organs
US:
 cysts may contain low-level echoes
cysts may contain low-level echoes
CT:
 internal septations may not be visualized without contrast enhancement
internal septations may not be visualized without contrast enhancement cysts with attenuation values of water; may have different levels of attenuation within different cystic cavities
cysts with attenuation values of water; may have different levels of attenuation within different cystic cavities enhancement of cyst walls
enhancement of cyst walls
Angio:
 predominantly avascular mass
predominantly avascular mass cyst wall + solid components may demonstrate small areas of vascular blush + neovascularity
cyst wall + solid components may demonstrate small areas of vascular blush + neovascularity displacement of surrounding arteries + veins by cysts
displacement of surrounding arteries + veins by cysts
Metastases:
 round thick-walled cystic lesions in liver
round thick-walled cystic lesions in liver
| Prognosis: | invariable transformation into cystadenocarcinoma; 17-63% 5-year survival rate |
| Rx: | complete surgical excision (5-year survival rate of 74-90%) |
DDx:
Pseudocyst (most common pancreatic cyst): inflammatory changes in peripancreatic fat, pancreatic calcifications, temporal evolution, history of alcoholism, elevated levels of amylase
Lymphangioma/hemangioma
Variants of ductal adenocarcinoma:
mucinous colloid adenocarcinoma/ductectatic mucinous tumor of pancreas = mucin-hypersecreting carcinoma
papillary intraductal adenocarcinoma
adenosquamous carcinoma: squamous component predisposes to necrosis + cystic degeneration
anaplastic adenocarcinoma: lymphadenopathy + metastases at time of presentation
Solid and cystic papillary epithelioid neoplasm: hemorrhagic cystic changes in 20%
Cystic islet cell tumor: hypervascular component
Cystic metastases: history of malignant disease
Atypical serous cystadenoma: smaller tumor with greater number of smaller cysts
Sarcoma
Infection: amebiasis, Echinococcus multilocularis
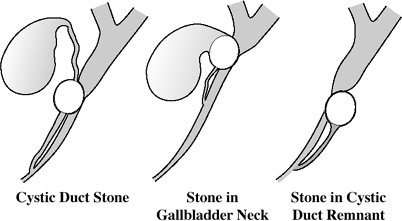 | Mirizzi Syndrome |
Multiple Bile Duct Hamartomas
= BILIARY MICROHAMARTOMAS = VON MEYENBURG COMPLEX
| Incidence: | 0.15-2.8% of autopsies |
| Etiology: | failure of involution of embryonic bile ducts |
| Histo: | cluster of proliferated bile ducts lined by single layer of cuboidal cells embedded in fibrocollagenous tissue with single ramified lumen, communication with biliary system usually obliterated |
| Associated with: | fibropolycystic liver disease |
| Size: | 0.1-10 mm |
asymptomatic
 nonspecific imaging appearance
nonspecific imaging appearance
CT:
 multiple scattered round/irregular hypodense lesions of up to 15 mm in diameter
multiple scattered round/irregular hypodense lesions of up to 15 mm in diameter rim of little peripheral/no enhancement
rim of little peripheral/no enhancement
US:
 multiple small cysts/echogenic areas (if size not resolved) up to 10 mm comet-tail artifact
multiple small cysts/echogenic areas (if size not resolved) up to 10 mm comet-tail artifact
MR:
 hypointense on T1WI
hypointense on T1WI iso-/slightly hyperintense on T2WI
iso-/slightly hyperintense on T2WI
P.733
 hypointense after gadopentetate dimeglumine
hypointense after gadopentetate dimeglumine
Angio:
 multiple areas of abnormal vascularity in form of small grapelike clusters persisting into venous phase
multiple areas of abnormal vascularity in form of small grapelike clusters persisting into venous phase
DDx:
Metastatic liver disease (more variable in size and attenuation/signal intensity)
Simple hepatic cysts (not as numerous or uniformly small)
Autosomal dominant polycystic disease (cysts usually larger and more numerous)
Multiple Endocrine Neoplasia
= MEN = MULTIPLE ENDOCRINE ADENOMAS (MEA)
= familial autosomal dominant adenomatous hyperplasia characterized by neoplasia of more than one endocrine organ
| Theory: | cells of involved principal organs originate from neural crest and produce polypeptide hormones in cytoplasmic granules, which allow amine precursor uptake and d ecarboxylation = APUD cells |
| reminder: | ||
| Type 1 | = Wermer syndrome | PPP |
| Type 2 | = Sipple syndrome (type 2A) | PMP |
| Type 3 | = Mucosal neuroma syndrome (type 2B) | MPM |
MEN 1 Syndrome
= WERMER SYNDROME
= autosomal dominant trait with high penetrance; M:F = 1:1
| Cause: | genetic defect on chromosome 11 |
Organ involvement (PPP):
Parathyroid hyperplasia (97%): multiglandular
primary HPT (in 95%) is usually presenting feature
Pancreatic islet cell tumor (30 80%):
 Likely multiple + behaving malignant!
Likely multiple + behaving malignant! Primary cause of morbidity + mortality!
Primary cause of morbidity + mortality!
gastrinoma (most common type, in 50 60%)
Zollinger-Ellison syndrome
 multiple masses associated with diffuse gastric wall thickening
multiple masses associated with diffuse gastric wall thickening multiple duodenal microgastrinomas (<5 mm) account for >50% of gastrinomas
multiple duodenal microgastrinomas (<5 mm) account for >50% of gastrinomas
insulinoma (in 30%)
 coexistence with gastrinomas in 10%
coexistence with gastrinomas in 10%
VIPoma = WDHH-syndrome (watery diarrhea, hypokalemia, hypochlorhydria)
Anterior pituitary gland tumor (15 30 50%):
nonfunctioning
prolactin (60%), growth hormone (<25%), adrenocorticotropic hormone (5%), TSH
presenting feature of the syndrome in 10%
Combination of parathyroid + pancreas + pituitary involvement (40%)
Adrenocortical hyperplasia/adenomas (up to 33 40%)
rarely functional
Carcinoid (2 5%)
Location: thymus, bronchus, stomach (30-fold increased incidence), duodenum Lipoma
usually asymptomatic
multiple facial angiofibromas (in 85 90% of MEN 1 patients)
May be associated with:
thyroid tumor (20%), thymoma, buccal mucosal tumor, colonic polyposis, M n trier disease
| Screening population: | <35-year old patient with HPT, 2 endocrine organ tumors, 1st-degree relative of MEN 1 patient |
Imaging surveillance:
renal US + abdominal radiograph for abdominal calculi; abdominal MR for islet cell + adrenal tumors + liver mets; pituitary MR for adenoma (every 3 years)
| Types of Multiple Endocrine Neoplasia | |||
|---|---|---|---|
| MEA | Type 1 | Type 2 | Type 3 |
| Pituitary adenoma | + | ||
| Parathyroid adenoma | + | + | |
| Medullary thyroid carcinoma | + | + | |
| Pancreatic island cell tumor | + | ||
| Pheochromocytoma | + | + | |
| Ganglioneuromatosis | + | ||
MEN 2 Syndrome
= SIPPLE DISEASE = MEN Type 2A
= autosomal dominant cancer syndrome
| Cause: | genetic defect on chromosome 10 |
Organ involvement (PMP):
Parathyroid hyperplasia/neoplasia in multiple glands
hyperparathyroidism (later onset than in MEN 1)
Medullary carcinoma of thyroid (almost 100%)
serum calcitonin commonly elevated
Pheochromocytoma (50%): bilateral in 50%; malignant in 3% diagnosed before (in 10%)/after detection (in 17%) of medullary thyroid carcinoma
| May be associated with: | carcinoid tumors, Cushing disease |
| Screening population: | all patients with medullary thyroid cancer/pheochromocytoma, 1st-degree relative of MEN 2 patient |
Imaging surveillance:
abdominal MR for pheochromocytoma (every 3 years); MIBG scintigraphy (optional)
MEN 3 Syndrome
= MUCOSAL NEUROMA SYNDROME = MEN Type 2B
| Cause: | new mutation |
Organ involvement (MPM):
Medullary carcinoma of thyroid
Pheochromocytoma
Mucosal neuroma = oral + intestinal neuroganglioneuromatosis
 Mucosal neuromas are PATHOGNOMONIC
Mucosal neuromas are PATHOGNOMONIC Usually precede the appearance of thyroid carcinoma + pheochromocytoma!
Usually precede the appearance of thyroid carcinoma + pheochromocytoma!
long slender extremities (marfanoid appearance)
thickened lips (due to submucosal nodules)
nodular deformity of tongue (mucosal neuromas of tongue often initially diagnosed by dentists)
prognathism
corneal limbus thickening
constipation alternating with diarrhea
P.734
@ GI tract
 thickened/plaquelike colonic wall
thickened/plaquelike colonic wall chronic megacolon = dilated colon with abnormal haustral markings
chronic megacolon = dilated colon with abnormal haustral markings alternating areas of colonic spasm + dilatation (rarely associated with Hirschsprung disease)
alternating areas of colonic spasm + dilatation (rarely associated with Hirschsprung disease) multiple submucosal neuromas throughout small bowel, may act as lead point for intussusception
multiple submucosal neuromas throughout small bowel, may act as lead point for intussusception
Pancreas Divisum
= most common anatomic variant of pancreas due to failure of fusion of the ventral and dorsal anlage at 8th week of fetal life with main dorsal pancreatic duct (Santorini) draining through minor (accessory) papilla + ventral pancreatic duct (Wirsung) with CBD draining through major papilla
| Prevalence: | 4 9 14% in autopsy series; 2 8% in ERCP series; 3 7% in normal population; 12 26% in patients with idiopathic recurrent pancreatitis |
| Hypothesis: | relative/actual functional stenosis of minor papilla predisposes to nonalcoholic recurrent pancreatitis in dorsal segment |
| Age: | young/middle-aged adult |
chronic relapsing pancreatitis (clinical relevance continues to be debated)
Pancreatography:
 The ONLY reliable means for diagnosis
The ONLY reliable means for diagnosis contrast injection into major papilla demonstrates CBD + only short ventral pancreatic duct with early arborization
contrast injection into major papilla demonstrates CBD + only short ventral pancreatic duct with early arborization contrast injection into minor papilla fills dorsal pancreatic duct
contrast injection into minor papilla fills dorsal pancreatic duct no communication between ventral + dorsal ducts
no communication between ventral + dorsal ducts
CT:
 oblique fat cleft between ventral + dorsal pancreas (25%)
oblique fat cleft between ventral + dorsal pancreas (25%) failure to see union of dorsal + ventral pancreatic ducts (rare)
failure to see union of dorsal + ventral pancreatic ducts (rare)
Pancreatic Acinar Cell Carcinoma
= rare neoplasm of exocrine origin
| Age: | 40 81 (mean 62) years; M:F = 86:14; 87% Caucasian |
increased serum lipase amylase
syndrome of elevated lipase:
disseminated subcutaneous + intraosseous fat necrosis (usually distal to knees/elbows)
polyarthropathy
skin lesions resembling erythema nodosum
biliary obstruction distinctly uncommon
 lobulated well-defined mass of 2 15 cm in diameter
lobulated well-defined mass of 2 15 cm in diameter thin enhancing capsule
thin enhancing capsule tumor necrosis usually present
tumor necrosis usually present moderately vascular tumor + neovascularity + arterial and venous encasement
moderately vascular tumor + neovascularity + arterial and venous encasement
| Prognosis: | median survival of 7 9 months |
| DDx: | (1) pancreatic adenocarcinoma (small, irregular, locally invasive, without capsule, biliary obstruction if located in head of pancreas) (2) Nonfunctioning islet-cell tumor (3) Microcystic cystadenoma (4) Solid and papillary epithelial neoplasm (5) Oncocytic tumor of pancreas |
Pancreatic Ductal Adenocarcinoma
= DUCT CELL ADENOCARCINOMA = PANCREATIC ADENOCARCINOMA
 Duct cells comprise only 4% of pancreatic tissue!
Duct cells comprise only 4% of pancreatic tissue!
| Incidence: | 95% of malignant tumors of pancreas; 5th leading cause of cancer death in the United States (27,000 per year) |
| Etiology: | alcohol abuse (4%), diabetes (2 more frequent than in general population, particularly in females), hereditary pancreatitis (in 40%); cigarette smoking (risk factor 2 x) |
| Path: | scirrhous infiltrative adenocarcinoma with a dense cellularity + sparse vascularity |
| Peak age: | 7th (range, 4th 8th) decade; M:F = 2:1 |
| Stage | I = confined to pancreas II = + regional lymph node metastases III = + distant spread |
 At presentation:
At presentation:65% of patients have advanced local disease/distant metastases
21% of patients have localized disease with spread to regional lymph nodes
14% of patients have tumor confined to pancreas
Extension:
local extension beyond margins of organ (68%): posteriorly (96%), anteriorly (30%), into porta hepatis (15%), into splenic hilum (13%)
invasion of adjacent organs (42%):
duodenum > stomach > left adrenal gland > spleen > root of small bowel mesentery
local lymph node spread:
pancreaticosplenic nodes accompanying splenic a., pancreaticoduodenal nodes, superior mesenteric preaortic nodes
| Metastases: | liver (30 36%), regional lymph nodes >2 cm (15 28%), ascites from peritoneal carcinomatosis (7 10%), lungs (pulmonary nodules/lymphangitic), pleura, bone |
weight loss, anorexia, fatigue
pain in hypochondrium radiating to back
obstructive jaundice (75%):
 Most frequent cause of malignant biliary obstruction
Most frequent cause of malignant biliary obstructionpainless jaundice (25%)
acute onset diabetes (25 50%), steatorrhea
hyperamylasemia
spontaneous vein thrombosis (Trousseau syndrome)
| Location: | pancreatic head (60%); body (15%); tail (5%); diffuse involvement (20%) |
| Size: | 2 10 cm (in 60% between 4 6 cm) |
UGI:
 antral pad sign = extrinsic indentation of the posteroinferior margin of antrum
antral pad sign = extrinsic indentation of the posteroinferior margin of antrum Frostberg inverted-3 sign = inverted 3 contour to the medial portion of the duodenal sweep
Frostberg inverted-3 sign = inverted 3 contour to the medial portion of the duodenal sweep spiculated duodenal wall + traction + fixation (neoplastic infiltration of duodenal mucosa/desmoplastic response)
spiculated duodenal wall + traction + fixation (neoplastic infiltration of duodenal mucosa/desmoplastic response) irregular/smooth nodular mass with ampullary carcinoma
irregular/smooth nodular mass with ampullary carcinoma
BE:
 diffuse tethering throughout peritoneal cavity (from intraperitoneal seeding)
diffuse tethering throughout peritoneal cavity (from intraperitoneal seeding) localized haustral padding/flattening/narrowing with serrated contour at inferior aspect of transverse colon/splenic flexure
localized haustral padding/flattening/narrowing with serrated contour at inferior aspect of transverse colon/splenic flexure
P.735
CT (75 96% detection rate for dynamic CT scan):
 hypovascular lesion best depicted during parenchymal/portal venous phase (11% not visualized)
hypovascular lesion best depicted during parenchymal/portal venous phase (11% not visualized) pancreatic mass (95%)/diffuse enlargement (4%)/normal scan (1%)
pancreatic mass (95%)/diffuse enlargement (4%)/normal scan (1%) mass with central zone of diminished attenuation (75 83%)
mass with central zone of diminished attenuation (75 83%) indirect signs:
indirect signs: convex deformity of pancreatic contour
convex deformity of pancreatic contour double-duct sign = pancreatic + bile duct obstruction without detectable mass (4%)
double-duct sign = pancreatic + bile duct obstruction without detectable mass (4%) duct dilatation (58%): 3/4 biductal, 1/10 isolated to one duct; dilated pancreatic duct (67%); dilated bile ducts (38%)
duct dilatation (58%): 3/4 biductal, 1/10 isolated to one duct; dilated pancreatic duct (67%); dilated bile ducts (38%) atrophy of pancreatic body + tail (20%)
atrophy of pancreatic body + tail (20%)
 calcifications (2%)
calcifications (2%) postobstructive pseudocyst (11%)
postobstructive pseudocyst (11%) obliteration of peripancreatic fat (50%) = pancreas lacks a capsule
obliteration of peripancreatic fat (50%) = pancreas lacks a capsule vascular invasion:
vascular invasion: thickening of celiac axis/SMA (invasion of perivascular lymphatics) in 60%
thickening of celiac axis/SMA (invasion of perivascular lymphatics) in 60% dilated collateral veins (12%)
dilated collateral veins (12%) high probability of unresectability if circumferential contiguity of tumor to vessel >50% (84% sensitive, 98% specific)
high probability of unresectability if circumferential contiguity of tumor to vessel >50% (84% sensitive, 98% specific)
 thickening of Gerota fascia (5%)
thickening of Gerota fascia (5%) local tumor extension posteriorly, into splenic hilum, into porta hepatis (68%)
local tumor extension posteriorly, into splenic hilum, into porta hepatis (68%) contiguous organ invasion (duodenum, stomach, mesenteric root) in 42%
contiguous organ invasion (duodenum, stomach, mesenteric root) in 42% hepatic metastases (75% sensitive)
hepatic metastases (75% sensitive)
US:
 hypoechoic pancreatic mass
hypoechoic pancreatic mass focal/diffuse (10%) enlargement of pancreas
focal/diffuse (10%) enlargement of pancreas contour deformity of gland; rounding of uncinate process
contour deformity of gland; rounding of uncinate process dilatation of pancreatic biliary duct
dilatation of pancreatic biliary duct
MR (no diagnostic improvement over CT; valuable in evaluation of an enlarged pancreatic head):
 hypointense lesion on T1WI (accentuated on fat-suppressed images)
hypointense lesion on T1WI (accentuated on fat-suppressed images) hypovascular lesion during capillary phase compared with surrounding pancreas (due to desmoplastic fibrotic component)
hypovascular lesion during capillary phase compared with surrounding pancreas (due to desmoplastic fibrotic component) lesion enhancement >1 minute after contrast injection (due to desmoplasia with large interstitial spaces)
lesion enhancement >1 minute after contrast injection (due to desmoplasia with large interstitial spaces) abnormally low signal intensity of pancreatic tail + body (due to atrophy/secondary chronic pancreatitis) on T1WI reducing the contrast relative to focal cancer
abnormally low signal intensity of pancreatic tail + body (due to atrophy/secondary chronic pancreatitis) on T1WI reducing the contrast relative to focal cancer
Angiography (70% accuracy):
 hypovascular tumor/neovascularity (50%)
hypovascular tumor/neovascularity (50%) arterial encasement: SMA (33%), splenic artery (14%), celiac trunk (11%), hepatic artery (11%), gastroduodenal artery (3%), left renal artery (0.6%)
arterial encasement: SMA (33%), splenic artery (14%), celiac trunk (11%), hepatic artery (11%), gastroduodenal artery (3%), left renal artery (0.6%) venous obstruction: splenic vein (34%), SMV (10%)
venous obstruction: splenic vein (34%), SMV (10%) venous encasement: SMV (23%), splenic vein (15%), portal vein (4%)
venous encasement: SMV (23%), splenic vein (15%), portal vein (4%)
Cholangiography:
 rat tail/nipplelike occlusion of CBD
rat tail/nipplelike occlusion of CBD nodular mass/meniscuslike occlusion in ampullary tumors
nodular mass/meniscuslike occlusion in ampullary tumors double duct sign = abrupt obstruction of common bile + pancreatic duct
double duct sign = abrupt obstruction of common bile + pancreatic duct
Pancreatography (abnormal in 97%):
 irregular, nodular, rat-tailed, eccentric obstruction
irregular, nodular, rat-tailed, eccentric obstruction localized encasement with prestenotic dilatation
localized encasement with prestenotic dilatation acinar defect
acinar defect
Prognosis:
10% 1-year survival, 2% 3-year survival, <1% 5-year survival; 14 months medial survival after curative resection, 8 months after palliative resection, 5 months without treatment; tumors resectable in only 8-15% at presentation, 5% 5-year survival rate after surgery
Survival rate & tumor size:
100% 5-year survival rate for tumors <1 cm without parenchymal/vascular/lymphatic invasion; 30% for tumors <2 cm
| DDx: | focal pancreatitis, islet cell carcinoma, metastasis, lymphoma, normal variant |
Pancreatic Islet Cell TUmors
| Origin: | embryonic neuroectoderm, derivatives of APUD (amine precursor uptake and decarboxylation) cell line arising from islet of Langerhans (APUDoma) |
| Prevalence: | 1-5:1,000,000 population/year; isolated or part of MEN I syndrome (= Wermer syndrome) |
| Path: | (a) small tumor: solid well-demarcated (b) large tumor: cystic changes + necrosis + calcifications |
| In order of frequency: | insulinoma > gastrinoma > glucagonoma > VIPoma > somatostatinoma |
| Histo: | sheets of small round cells + numerous stromal vessels |
Average time from onset of symptoms to diagnosis is 2.7 years
Classification:
functional (85%)
 tumors small at presentation as a result of symptoms
tumors small at presentation as a result of symptoms
nonfunctional (15%) below threshold of detectability/hypofunctional
 tumors large at presentation
tumors large at presentation
| Metastases: | in 60-90% to liver regional lymph nodes |
 Liver metastasis often hypervascular with enhancement during arterial phase
Liver metastasis often hypervascular with enhancement during arterial phase hyperechoic liver metastasis is suggestive of islet cell tumor rather than pancreatic adenocarcinoma!
hyperechoic liver metastasis is suggestive of islet cell tumor rather than pancreatic adenocarcinoma!
 marked enhancement on immediate images (hypervascularity)
marked enhancement on immediate images (hypervascularity) marked enhancement on delayed images (large interstitial space with loose edematous stroma + abundant blood vessels)
marked enhancement on delayed images (large interstitial space with loose edematous stroma + abundant blood vessels) No vascular encasement/duct obstruction
No vascular encasement/duct obstruction calcifications + large size highly suggestive of malignancy
calcifications + large size highly suggestive of malignancy
US:
transabdominal US (70% sensitive)
endoscopic US (nearly 95% sensitive, 93% specific), less sensitive for distal pancreatic body + tail
intraoperative US (combined with palpation by surgeon)
 homogeneous hypoechoic mass
homogeneous hypoechoic mass
CT (71-82% sensitive)
 Tumors often small (<20 mm) and multiple + difficult to detect
Tumors often small (<20 mm) and multiple + difficult to detect isoattenuating tumor at NECT
isoattenuating tumor at NECT avid enhancement in arterial phase
avid enhancement in arterial phase tumor may be cystic/hypoattenuating (rare)
tumor may be cystic/hypoattenuating (rare)
P.736
MR:
 low signal intensity on T1WI
low signal intensity on T1WI high signal intensity on moderately T2WI
high signal intensity on moderately T2WI intense enhancement on arterial-phase postcontrast images
intense enhancement on arterial-phase postcontrast images hypervascular liver metastases
hypervascular liver metastases
NUC (70 90% sensitive, only 50% sensitive for gastrinomas + insulinomas)
Somatostatin Receptor Scintigraphy with Indium-111 octreotide
 whole-body scintigraphy detects tumors >10 mm in size
whole-body scintigraphy detects tumors >10 mm in size predicts which patients will respond to radionuclide therapy
predicts which patients will respond to radionuclide therapy monitors response to therapy
monitors response to therapy
Hepatic venous sampling (88% sensitive):
 performed together with pancreatic angiography
performed together with pancreatic angiography rise in venous hormone concentration after selective injection of secretagogue (eg, calcium) into arteries supplying the pancreas
rise in venous hormone concentration after selective injection of secretagogue (eg, calcium) into arteries supplying the pancreas
| Prognosis: | 50% 5-year survival rate |
| DDx: | (1) Pancreatic ductal adenocarcinoma (hypovascular, smaller, encasement of SMA + celiac trunk) (2) Microcystic adenoma (benign tumor, small cysts, older women) (3) Metastatic tumor: renal cell carcinoma (clinical Hx) (4) Solid and papillary epithelial neoplasm (young female, hemorrhagic areas) (5) Paraganglioma (6) Sarcoma (rare) |
ACTH-producing Tumor
= Corticotrophinoma
rare cause of Cushing syndrome
increased level of serum cortisol
impaired glucose tolerance > central obesity > hypertension, oligomenorrhea > osteoporosis > purpura > striae > muscle atrophy
| Prognosis: | almost all malignant with metastases at time of diagnosis |
Gastrinoma
 2nd most common islet cell tumor
2nd most common islet cell tumor
| Age: | 8% in patients <20 years; M > F |
| Histo: | in cells/ cells |
| Path: | (a) islet cell hyperplasia (10%) (b) benign adenoma (30%): in 50% solitary, in 50% multiple (especially in MEN 1) (c) malignant (50 60%) with metastases to liver, spleen, lymph nodes, bone |
| Associated with: | MEN 1 (in 50 60%); the most common islet cell tumor in MEN 1 |
Zollinger-Ellison syndrome: severe recurrent peptic ulcer disease (>90%), malabsorption, hypokalemia, gastric hypersecretion, hyperacidity/occasionally hypoacidity, diarrhea (from gastric hypersecretion)
 Only 1:1,000 patients with peptic ulcer disease has a gastrinoma!
Only 1:1,000 patients with peptic ulcer disease has a gastrinoma!
epigastric pain (recurrent/intractable peptic ulcer disease)
GI bleeding
elevated serum levels of gastrin (DIAGNOSTIC)
Location: usually multiple 87% in pancreas (50% solitary in head/tail)
ectopic (7 33%):
duodenal wall (13% in medial wall of duodenum = gastrinoma triangle)
peripancreatic nodes/spleen
stomach, jejunum
omentum, retroperitoneum
ovary
frequently in gastrinoma triangle (= triangle defined by porta hepatis as apex of triangle + 2nd and 3rd parts of duodenum as the base)
 ulcer in unusual location, eg, postbulbar
ulcer in unusual location, eg, postbulbar average tumor size 3.4 cm (up to 15 cm)
average tumor size 3.4 cm (up to 15 cm) occasionally calcifications
occasionally calcifications homogeneous hypoechoic mass
homogeneous hypoechoic mass
Angio:
 hypervascular lesion (70%)
hypervascular lesion (70%) hepatic venous sampling after intraarterial stimulation with secretin
hepatic venous sampling after intraarterial stimulation with secretin
CT:
 transiently hyperdense on dynamic CT (majority)
transiently hyperdense on dynamic CT (majority) thickening of gastric rugal folds
thickening of gastric rugal folds
MR:
 low-intensity mass on fat-suppressed T1WI
low-intensity mass on fat-suppressed T1WI diminished central + peripheral ring enhancement
diminished central + peripheral ring enhancement high-intensity mass on fat-suppressed T2WI
high-intensity mass on fat-suppressed T2WI
Sensitivity of preoperative localization:
25% for US, 35% for CT, 20% for MRI, 42 63% for transhepatic portal venous sampling for gastrin, 68 70% for selective angiography, 77% for arteriography combined with intra-arterial injection of secretin
| Rx: | surgery curative in 30% |
| Cx: | frequently malignant degeneration (liver metastases at time of diagnosis in 30%) |
Glucagonoma
| Incidence: | uncommon tumor |
| Age: | middle age; M < F |
| Histo: | derived from cells |
| Associated with: | MEN |
necrolytic erythema migrans (erythematous macules/papules on genitals, lower extremity, groin, buttocks, face) in >70% of patients
4D syndrome = dermatosis, diarrhea, depression, deep vein thrombosis
painful glossitis/stomatitis, weight loss, anemia
plasma glucagon level > 1,000 ng/L (DIAGNOSTIC);
diabetes mellitus due to elevated glucagon
elevation of insulin, serotonin, gastrin
Location: predominantly in pancreatic body/tail  tumor size 2.5 25 cm (mean 6.4 cm) with solid + necrotic components (in 70% >5 cm in size)
tumor size 2.5 25 cm (mean 6.4 cm) with solid + necrotic components (in 70% >5 cm in size) hypervascular in 90%; successful angiographic localization in 15%
hypervascular in 90%; successful angiographic localization in 15%
| Cx: | deep vein thrombosis + pulmonary embolism |
| Prognosis: | in 60 80% malignant transformation (liver metastases at time of diagnosis in 50 60%); 55% 5-year survival rate |
P.737
Insulinoma
 Most common syndromic islet cell tumor!
Most common syndromic islet cell tumor!
| Age: | 4th 6th decade; M:F = 2:3 |
| Associated with: | MEN type I (in 10%) |
| Path: | (a) single benign adenoma (80 90%) (b) multiple adenomas/microadenomatosis (5 10%) (c) islet cell hyperplasia (5 10%) (d) malignant adenoma (5 10%) |
Whipple triad: starvation attack + hypoglycemia (fasting glucose <50 mg/dL) + relief by IV dextrose
elevated levels of plasma insulin
neuroglycopenic symptoms: headaches, confusion, coma
hypoglycemia exacerbated by fasting results in frequent meals to avoid symptoms
sweating, palpitations, tremor (secondary to catecholamine release in response to hypoglycemia)
obesity
firm rubbery palpable mass at surgery (in >90%)
Location: no predilection for any part of pancreas,
2 5% in ectopic location; 10% multiplememonic: 10% are associated with MEN 1;
10% are multiple (especially in MEN 1)
10% have islet cell hyperplasia;
10% are malignant average tumor size 1 2 cm; <1.5 cm in 70%
average tumor size 1 2 cm; <1.5 cm in 70%
US (20 75% preoperative and 75 100% endoscopic + intraoperative sensitivity):
 round/oval smoothly marginated solid homogeneously hypoechoic mass
round/oval smoothly marginated solid homogeneously hypoechoic mass
Angio:
 hypervascular tumor (66%): accurate angiographic localization in 50 90%
hypervascular tumor (66%): accurate angiographic localization in 50 90% transhepatic portal venous sampling (correct localization in 95%)
transhepatic portal venous sampling (correct localization in 95%) hepatic venous sampling after intraarterial stimulation with calcium gluconate
hepatic venous sampling after intraarterial stimulation with calcium gluconate
CECT (30 75% sensitivity):
 hypo-/iso-/hyperattenuating lesion
hypo-/iso-/hyperattenuating lesion
MR:
 low signal intensity on fat-suppressed T1WI
low signal intensity on fat-suppressed T1WI hyperintense on T2WI + dynamic contrast-enhanced + suppressed inversion recovery images
hyperintense on T2WI + dynamic contrast-enhanced + suppressed inversion recovery images tumors >2 cm show ring enhancement
tumors >2 cm show ring enhancement
| Prognosis: | malignant transformation in 5 10% |
| Rx: | surgery curative |
Nonfunctioning Islet Cell Tumor
= Nonsyndromic ICT
 3rd most common islet cell tumor!
3rd most common islet cell tumor!
| Incidence: | 15 25 50% of all islet cell tumors |
| Histo: | derived from either or cells |
| Age: | 24 74 (mean 57) years |
mostly asymptomatic (= tumors may be hormonally active but without clinical evidence of hormone production)
abdominal pain, jaundice, gastric variceal bleeding
palpable mass, gastric outlet obstruction
| Location: | predominantly in pancreatic head |
 tumor size 6 20 cm (>5 cm in 72%) with solid + necrotic components
tumor size 6 20 cm (>5 cm in 72%) with solid + necrotic components coarse nodular calcifications (20 25%)
coarse nodular calcifications (20 25%) CT contrast enhancement in 83%
CT contrast enhancement in 83% hypoechoic mass
hypoechoic mass late dense capillary stain
late dense capillary stain large irregular pathologic vessels with early venous filling
large irregular pathologic vessels with early venous filling
| Prognosis: | in 80 100% malignant transformation with metastases to liver + regional nodes; 60% 3-year survival; 44% 5-year survival |
| Rx: | may respond to systemic chemotherapy |
Somatostatinoma
| Origin: | derived from cells |
| Incidence: | fewer than 200 cases reported in literature |
| May be associated with: | NF 1 |
inhibitory syndrome = inhibitory action of somatostatin on other pancreatic + bowel peptides (growth hormone, TSH, insulin, glucagon, gastric acid, pepsin, secretin)
diabetes, cholelithiasis, steatorrhea, hypochlorhydria
elevated plasma level of somatostatin (DIAGNOSTIC)
Location: predominantly in pancreatic head/duodenum at ampulla of Vater  tumor size 0.6 20 cm (average >4 cm)
tumor size 0.6 20 cm (average >4 cm) hypervascular
hypervascular obstruction of duodenum
obstruction of duodenum
| Prognosis: | 50 90% malignant transformation; metastatic disease to liver/lymph nodes in 50 70% at time of initial diagnosis |
VIPoma
= solitary tumor liberating Vasoactive Intestinal Peptide acting directly on cyclic adenosine monophosphate (AMP) within epithelial cells of bowel relaxing vascular smooth muscle + causing electrolyte secretion; sporadic occurrence
| Histo: | adenoma/hyperplasia; M:F = 1:2 |
WDHA syndrome = watery diarrhea + hypokalemia + achlorhydria (VIP inhibits gastrin poroduction);
more recently + more accurately described as:
WDHH syndrome = watery diarrhea + hypokalemia + hypochlorhydria = pancreatic cholera = Verner-Morrison syndrome
dehydration due to massive diarrhea (>1 L/day)
Location:
pancreas: from cells predominantly in pancreatic body/tail (75%)
extrapancreatic: retroperitoneal ganglioblastoma, pheochromocytoma, lung, neuroblastoma (in children)
 average size 5 10 cm with solid + necrotic tissue
average size 5 10 cm with solid + necrotic tissue mostly hypervascular tumor
mostly hypervascular tumor dilatation of gallbladder
dilatation of gallbladder
| Prognosis: | in 50 80% malignant transformation |
| DDx: | small cell carcinoma of lung/neuroblastoma may also cause WDHH syndrome |
Pancreatic Lipomatosis
= FATTY REPLACEMENT = FATTY INFILTRATION
= deposition of fat cells in pancreatic parenchyma
Predisposing factors:
Atherosclerosis of elderly
Obesity
Steroid therapy
Diabetes mellitus
Cushing syndrome
Chronic pancreatitis
Main pancreatic duct obstruction
Cystic fibrosis (most common cause in childhood)
Malnutrition/dietary deficiency
Hepatic disease
Hemochromatosis
Viral infection
Schwachman-Diamond syndrome
Johanson-Blizzard syndrome
P.738
 fatty replacement often uneven:
fatty replacement often uneven: increase in AP diameter of pancreatic head with focal fatty replacement = lipomatous pseudohypertrophy
increase in AP diameter of pancreatic head with focal fatty replacement = lipomatous pseudohypertrophy
 prominently lobulated external contour
prominently lobulated external contour
US:
 increased pancreatic echogenicity
increased pancreatic echogenicity
CT:
 marbling of pancreatic parenchyma/total fatty replacement/lipomatous pseudohypertrophy
marbling of pancreatic parenchyma/total fatty replacement/lipomatous pseudohypertrophy
Pancreatic Fatty Sparing
= sparing of fatty change in pancreatic head + uncinate process (ventral pancreatic anlage) as initial stage in pancreatic lipomatosis
| Histo: | ventral pancreatic anlage has smaller + more densely packed acini with scanty/absent interacinar fat |
US:
 rounded/triangular hypoechoic area within pancreatic head/uncinate process + diffusely increased echogenicity in remainder of gland
rounded/triangular hypoechoic area within pancreatic head/uncinate process + diffusely increased echogenicity in remainder of gland
CT:
 higher-density region of pancreatic head + uncinate process with diffusely decreased attenuation of pancreatic body + tail
higher-density region of pancreatic head + uncinate process with diffusely decreased attenuation of pancreatic body + tail
Pancreatic Pseudocyst
= collection of pancreatic fluid encapsulated by fibrous tissue
| Etiology: | (1) Acute pancreatitis; requires >4 weeks to form; pseudocysts mature in 6 8 weeks (2) Chronic pancreatitis (3) Posttraumatic (4) Pancreatic cancer |
| Incidence: | 2 4% in acute pancreatitis; 10 15% in chronic pancreatitis |
| Location: | 2/3 within pancreas |
Atypical location (may dissect along tissue planes in 1/3):
intraperitoneal: mesentery of small bowel/transverse colon/sigmoid colon
retroperitoneal: along psoas muscle; may present as groin mass/in scrotum
intraparenchymal: liver, spleen, kidney
mediastinal (through esophageal hiatus > aortic hiatus > foramen of Morgagni > erosion through diaphragm): may present as neck mass
| May communicate with: | duodenum, stomach, spleen |
Plain film/contrast radiograph:
 smooth extrinsic indentation of posterior wall of stomach/inner duodenal sweep (80%)
smooth extrinsic indentation of posterior wall of stomach/inner duodenal sweep (80%) indentation/displacement of splenic flexure/transverse colon (40%)
indentation/displacement of splenic flexure/transverse colon (40%) downward displacement of duodenojejunal junction
downward displacement of duodenojejunal junction gastric outlet obstruction
gastric outlet obstruction splaying of renal collecting system/ureteral obstruction
splaying of renal collecting system/ureteral obstruction
US (pseudocyst detectable in 50-92%; 92-96% accuracy):
 usually single + unilocular cyst
usually single + unilocular cyst multilocular in 6%
multilocular in 6% fluid-debris level/internal echoes (may contain sequester, blood clot, cellular debris from autolysis)
fluid-debris level/internal echoes (may contain sequester, blood clot, cellular debris from autolysis) septations (rare; sign of infection/hemorrhage)
septations (rare; sign of infection/hemorrhage) may increase in size (secondary to hypertonicity of fluid, communication with pancreatic duct, hemorrhage, erosion of vessel)
may increase in size (secondary to hypertonicity of fluid, communication with pancreatic duct, hemorrhage, erosion of vessel) obstruction of pancreatic duct/CBD
obstruction of pancreatic duct/CBD
CT:
 fluid in pseudocyst (0-30 HU)
fluid in pseudocyst (0-30 HU) cyst wall calcification (extremely rare)
cyst wall calcification (extremely rare)
Pancreatography:
 communication with pancreatic duct in up to 50-70%
communication with pancreatic duct in up to 50-70%
Indications for pseudocyst drainage:
pain, suspected infection, persistence of pseudocyst >5 cm, increasing size, biliary/gastrointestinal obstruction
Cx (in 40%):
Rupture into abdominal cavity, stomach, colon, duodenum
hemorrhage/formation of pseudoaneurysm
Infection = pancreatic abscess
usually occurs >4 weeks after acute pancreatitis
symptomatology of infection
 gas bubbles (DDx: fistulous communication to GI tract)
gas bubbles (DDx: fistulous communication to GI tract) increase in attenuation of fluid contents
increase in attenuation of fluid contents
Dx: transcutaneous needle aspiration Intestinal obstruction
| Prognosis: | spontaneous resolution (in 20 50%) secondary to rupture into GI tract/pancreatic/bile duct |
| DDx: | pancreatic cystadenoma, cystadenocarcinoma, necrotic pancreatic carcinoma, fluid-filled bowel loop, fluid-filled stomach, duodenal diverticulum, aneurysm |
Pancreatic Transplantation
| Complications: | sepsis, rejection, pancreatitis, pseudocyst, pancreatic abscess (22%), anastomotic leak |
| Prognosis: | 40% survival rate >1 year |
Graft-vessel thrombosis in pancreatic transplant (2-19%)
Early thrombosis (<1 month after transplantation)
Cause: technical error in fashioning anastomosis, microvascular damage due to preservation injury Late thrombosis (>1 month after transplantation)
Cause: alloimmune arteritis with gradual occlusion of small blood vessels
Acute Rejection of Pancreatic Transplant
focal tenderness over transplant
measurement of urinary + serum amylase, blood glucose (nonspecific for diagnosis of rejection)
US:
 poor margination of transplant
poor margination of transplant acoustic inhomogeneity
acoustic inhomogeneity dilated pancreatic duct
dilated pancreatic duct
P.739
 |
| Pancreatic Transplantation |
Pancreatitis
Cause:
IDIOPATHIC (20%)
ALCOHOLISM (25%): acute pancreatitis (15%); chronic pancreatitis (70%)
CHOLELITHIASIS (50 70%): acute pancreatitis (75%); chronic pancreatitis (20%)
METABOLIC DISORDERS
Hypercalcemia in hyperparathyroidism (10%), multiple myeloma, amyloidosis, sarcoidosis
Hereditary pancreatitis: autosomal dominant, only Caucasians affected, most common cause of large spherical pancreatic calcifications in childhood (in 50%), recurrent episodes of pancreatitis, development into pancreatic carcinoma in 20 40%; pronounced dilatation of pancreatic duct; pseudocyst formation (50%); associated with type I hypercholesterolemia
Hyperlipidemia types I and V
Cystic fibrosis
INFECTION/INFESTATION
Viral infection (mumps, hepatitis, Coxsackie virus, mononucleosis)
Parasites (ascariasis, clonorchis)
TRAUMA
 One of the most common causes of pancreatitis in childhood!
One of the most common causes of pancreatitis in childhood!Penetrating ulcer
Blunt/penetrating trauma; nonaccidental trauma
Surgery (in 0.8% of Billroth-II resections, 0.8% of splenectomies, 0.7% of choledochal surgery, 0.4% of aortic graft surgery)
STRUCTURAL ABNORMALITIES
Pancreas divisum
 In 12 50% of cases with no other underlying abnormalities
In 12 50% of cases with no other underlying abnormalities
Choledochocele
DRUGS
Azathioprine, thiazide, furosemide, ethacrynic acid, sulfonamides, tetracycline, phenformin, steroids (eg, renal transplant), l-asparaginase, acetaminophen, procainamide
MALIGNANCY
Pancreatic carcinoma (in 1%), metastases, lymphoma
MULTISYSTEM CONDITIONS
Sepsis and shock
Hemolytic-uremic syndrome
Reye syndrome
Systemic lupus erythematosus
Theories of pathogenesis:
reflux of bile/pancreatic enzymes/duodenal succus
(a) terminal duct segment shared by common bile duct + pancreatic duct
(b) obstruction at papilla of Vater from inflammatory stenosis, edema/spasm of sphincter of Oddi, tumor, periduodenal diverticulum
(c) incompetent sphincter of Oddi
Acute Pancreatitis
= inflammatory disease of pancreas producing temporary changes with potential for restoration of normal anatomy + function following resolution
Path:
INTERSTITIAL EDEMATOUS PANCREATITIS (75 95%): edema, congestion, leukocytic infiltrates; mortality rate of 4%
NECROTIZING PANCREATITIS (5 25%):
proteolytic destruction of pancreatic parenchyma; mortality rate of 80 90%
(a) HEMORRHAGIC PANCREATITIS:
+ fat necrosis and hemorrhage (due to erosion of small vessels)
falling hematocrit
(b) SUPPURATIVE PANCREATITIS:+ bacterial infection
Clinical stages:
I = EDEMATOUS PANCREATITIS (75%)
rapid improvement following conservative Rx
gradual decrease of elevated enzymes
Mortality: 1 5% II = PARTIALLY NECROTIZING PANCREATITIS
delayed/no response to conservative Rx
delayed/no normalization of enzymes:
hyperglycemia of <200 mg/100 mL
hypocalcemia of >4 mval/L
base deficit of <4 mval/L
leukocytosis of <16,000
Mortality: 30 75% III = TOTALLY NECROTIZING PANCREATITIS
deterioration under conservative therapy:
hyperglycemia of >200 mg/100 mL
hypocalcemia of <4 mval/L
base deficit of >4 mval/L
leukocytosis of >16,000
P.740
| Mortality: | 100% (40% by 2nd day, 75% by 5th day, 100% by 10th day) |
acute epigastric pain radiating to back/chest (peaking after a few hours, resolving in 2 3 days)
nausea, vomiting
raised pancreatic amylase + lipase in blood + urine
increased amylase-creatinine clearance ratio
signs of hemorrhagic pancreatitis:
Cullen sign = periumbilical ecchymosis
Grey-Turner sign = flank ecchymosis
Fox sign = infrainguinal ecchymosis
subcutaneous nodules + fat necrosis + polyarthritis
Distribution:
Diffuse pancreatitis (52%)
Focal pancreatitis (48%):
location of head:tail = 3:2
 NO findings on US/CT in 29%
NO findings on US/CT in 29%
Abdominal film:
 colon cutoff sign (2 52%) = dilated transverse colon with abrupt change to a gasless descending colon (inflammation via phrenicocolic ligament causes spasm + obstruction at the splenic flexure impinging on a paralytic transverse colon)
colon cutoff sign (2 52%) = dilated transverse colon with abrupt change to a gasless descending colon (inflammation via phrenicocolic ligament causes spasm + obstruction at the splenic flexure impinging on a paralytic transverse colon) sentinel loop (10 55%) = localized segment of gas-containing bowel in duodenum (in 20 45%)/terminal ileum/cecum
sentinel loop (10 55%) = localized segment of gas-containing bowel in duodenum (in 20 45%)/terminal ileum/cecum renal halo sign = water-density of inflammation in anterior pararenal space contrasts with perirenal fat; more common on left side
renal halo sign = water-density of inflammation in anterior pararenal space contrasts with perirenal fat; more common on left side mottled appearance of peripancreatic area (secondary to fat necrosis in pancreatic bed, mesentery, omentum)
mottled appearance of peripancreatic area (secondary to fat necrosis in pancreatic bed, mesentery, omentum) intrapancreatic gas bubbles (from acute gangrene/suppurative pancreatitis)
intrapancreatic gas bubbles (from acute gangrene/suppurative pancreatitis) gasless abdomen = fluid-filled bowel associated with vomiting
gasless abdomen = fluid-filled bowel associated with vomiting ascites
ascites
CXR (findings in 14 71%):
 pleural effusion (in 10 20%), usually left-sided, with elevated amylase levels (in 85%)
pleural effusion (in 10 20%), usually left-sided, with elevated amylase levels (in 85%) left-sided diaphragmatic elevation
left-sided diaphragmatic elevation left-sided subsegmental atelectasis (20%)
left-sided subsegmental atelectasis (20%) parenchymal infiltrates, pulmonary infarction
parenchymal infiltrates, pulmonary infarction pulmonary edema, ARDS
pulmonary edema, ARDS pleural empyema, pericardial effusion
pleural empyema, pericardial effusion mediastinal abscess, mediastinal pseudocyst
mediastinal abscess, mediastinal pseudocyst pancreatico-bronchial/-pleural/-pulmonary fistula
pancreatico-bronchial/-pleural/-pulmonary fistula
UGI:
 esophagogastric varices (from splenic vein obstruction)
esophagogastric varices (from splenic vein obstruction) enlarged tortuous edematous rugal folds along antrum + greater curvature (20%)
enlarged tortuous edematous rugal folds along antrum + greater curvature (20%) widening of retrogastric space (from pancreatic enlargement/inflammation in lesser sac)
widening of retrogastric space (from pancreatic enlargement/inflammation in lesser sac) diminished duodenal peristalsis + edematous folds
diminished duodenal peristalsis + edematous folds widening of duodenal sweep + downward displacement of ligament of Treitz
widening of duodenal sweep + downward displacement of ligament of Treitz Poppel sign = edematous swelling of papilla
Poppel sign = edematous swelling of papilla Frostberg inverted-3 sign = segmental narrowing with fold thickening of duodenum
Frostberg inverted-3 sign = segmental narrowing with fold thickening of duodenum jejunal + ileal fold thickening (proteolytic spread along mesentery)
jejunal + ileal fold thickening (proteolytic spread along mesentery)
BE:
 narrowing, nodularity, fold distortion along inferior haustral row of transverse colon descending colon
narrowing, nodularity, fold distortion along inferior haustral row of transverse colon descending colon
Cholangiography:
 long gently tapered narrowing of CBD
long gently tapered narrowing of CBD prestenotic biliary dilatation
prestenotic biliary dilatation smooth/irregular mucosal surface
smooth/irregular mucosal surface
Bone films (findings in 6%):
| Cause: | metastatic intramedullary lipolysis + fat necrosis + trabecular bone destruction |
| Time of onset: | usually 3 6 weeks after peak of clinical pancreatitis |
 punched out/permeative mottled destruction of cancellous bone + endosteal erosion
punched out/permeative mottled destruction of cancellous bone + endosteal erosion aseptic necrosis of femoral/humeral heads
aseptic necrosis of femoral/humeral heads metaphyseal infarcts, predominantly in distal femur + proximal tibia
metaphyseal infarcts, predominantly in distal femur + proximal tibia
US (pancreatic visualization in 62 78%):
 hypoechoic diffuse/focal enlargement of pancreas
hypoechoic diffuse/focal enlargement of pancreas dilatation of pancreatic duct (if head focally involved)
dilatation of pancreatic duct (if head focally involved) perivascular cloaking = spread of inflammatory exudate along perivascular spaces
perivascular cloaking = spread of inflammatory exudate along perivascular spaces extrapancreatic hypoechoic mass with good acoustic transmission (= phlegmonous pancreatitis)
extrapancreatic hypoechoic mass with good acoustic transmission (= phlegmonous pancreatitis) fluid collection: lesser sac (60%), L > R anterior pararenal space (54%), posterior pararenal space (18%), around left lobe of liver (16%), in spleen (9%), mediastinum (3%), iliac fossa, along transverse mesocolon/mesenteric leaves of small intestine
fluid collection: lesser sac (60%), L > R anterior pararenal space (54%), posterior pararenal space (18%), around left lobe of liver (16%), in spleen (9%), mediastinum (3%), iliac fossa, along transverse mesocolon/mesenteric leaves of small intestineFate of fluid collection:
complete resolution
pseudocyst formation
bacterial infection = abscess
 pseudocyst formation (52%): extension into lesser sac, transverse mesocolon, around kidney, mediastinum, lower quadrants of abdomen
pseudocyst formation (52%): extension into lesser sac, transverse mesocolon, around kidney, mediastinum, lower quadrants of abdomen
CT (pancreatic visualization in 98%):
 no detectable change in size/appearance (29%)
no detectable change in size/appearance (29%) enlargement of pancreas with convex margins + indistinctness of gland + parenchymal heterogeneity:
enlargement of pancreas with convex margins + indistinctness of gland + parenchymal heterogeneity: hypodense (5 20 HU) mass in phlegmonous pancreatitis; may persist long after complete recovery
hypodense (5 20 HU) mass in phlegmonous pancreatitis; may persist long after complete recovery hyperdense areas (50 70 HU) in hemorrhagic pancreatitis for 24 48 hours
hyperdense areas (50 70 HU) in hemorrhagic pancreatitis for 24 48 hours
 thickening of anterior pararenal fascia
thickening of anterior pararenal fascia halo sign = sparing of perirenal space
halo sign = sparing of perirenal space non contrast-enhancing parenchyma during bolus injection (= pancreatic necrosis)
non contrast-enhancing parenchyma during bolus injection (= pancreatic necrosis) fluid collection
fluid collection
Angiography:
 may be normal
may be normal hypovascular areas (15 56%)
hypovascular areas (15 56%) hypervascularity + increased parenchymal stain (12 45%)
hypervascularity + increased parenchymal stain (12 45%) venous compression secondary to edema
venous compression secondary to edema formation of pseudoaneurysms (in 10% with chronic pancreatitis): splenic artery (50%), pancreatic arcades, gastroduodenal artery
formation of pseudoaneurysms (in 10% with chronic pancreatitis): splenic artery (50%), pancreatic arcades, gastroduodenal artery
P.741
Cx:
Phlegmon (18%)
Pseudocyst formation (10%)
Hemorrhagic pancreatitis (2 5%)
Abscess (2 10%)
Pancreatic ascites
Biliary duct obstruction
Thrombosis of splenic vein/SMV
Pseudoaneurysm
rupture into preexisting pseudocyst
digestion of arterial wall by enzymes
Incidence: in up to 10% of severe pancreatitis Location: splenic artery (most common), gastroduodenal, pancreatico-duodenal, hepatic, left gastric artery Mortality: 37% for rupture, 16 50% for surgery Thoracopancreatic fistula
pancreaticopleural fistula
pancreaticopericardial fistula
pancreaticoesophageal fistula
pancreaticobronchial fistula
mediastinal pseudocyst
Rx:
Conservative (NPO, gastric tube, atropine, analge-sics, sedation, prophylactic antibiotics) for stage I
Early surgery in stages II and III
Mild Acute Pancreatitis (75%)
minimal organ dysfunction
| Path: | interstitial edema |
| Prognosis: | improvement within 48 72 hours following conservative therapy with gradual decrease of elevated enzymes |
| Mortality: | 1 5% |
Severe Acute Pancreatitis
 Develops shortly after onset of untreated mild acute pancreatitis
Develops shortly after onset of untreated mild acute pancreatitisincreased abdominal tenderness, rebound distension, hypoactive bowel sounds
| Associated with: | organ failure/local complications |
| Path: | pancreatic cell breakdown + necrosis |
| Cx: | acute fluid collection, pancreatic necrosis, pseudocyst, abscess |
Acute Fluid Collections (30 50%)
= early form of acute pseudocyst/pancreatic abscess
| Path: | lack of a defined wall of fibrous/granulation tissue; pancreatic phlegmon [misnomer, no infection] = solid boggy inflammatory mass characterized by edema, infiltration of inflammatory cells + necrosis of retroperitoneal fat |
| Location: | extension into lesser sac, anterior pararenal space, transverse mesocolon, small bowel mesentery, retroperitoneum, pelvis |
 near 0 HU on CT
near 0 HU on CT
| Prognosis: | spontaneous regression (in 40 50%) |
Pancreatic Necrosis
= focal/diffuse area of nonviable pancreatic parenchyma
| Path: | clumps of devitalized pancreatic parenchyma + hemorrhage in pancreatic and peripancreatic tissues |
| Histo: | extensive interstitial fat necrosis with vessel damage + necrosis of acinar cells, islet cells, ductal system |
| Associated with: | peripancreatic fat necrosis |
 focal/diffuse well-marginated zones of unenhanced pancreatic parenchyma > 3 cm/involving >30% of the pancreatic gland
focal/diffuse well-marginated zones of unenhanced pancreatic parenchyma > 3 cm/involving >30% of the pancreatic gland
Acute Pseudocyst
= collection of pancreatic fluid enclosed by a wall of fibrous/granulation tissue
| Path: | absence of epithelium-lined wall |
| Cause: | acute pancreatitis, pancreatic trauma, chronic pancreatitis |
| Time of onset: | >4 weeks after acute pancreatitis |
amylase-rich fluid
| Prognosis: | persistent pseudocyst usually communicates with pancreatic duct; spontaneous resolution in 44%; <4 cm: resolution anticipated; >7 cm: treatment recommended |
| Cx: | hemorrhage, infection; spontaneous rupture into hollow viscera |
PANCREATIC ABSCESS
= well-demarcated fluid collection of pus usually close to the pancreas
| Time of onset: | 2-4 weeks after severe acute pancreatitis |
| Organism: | most commonly due to E. coli |
liquefied tissue with little/no necrosis
 may contain gas within pancreatic bed in 30-50% (DDx of gas: cutaneous/enteric fistula, ruptured duodenum, iatrogenic gas collection)
may contain gas within pancreatic bed in 30-50% (DDx of gas: cutaneous/enteric fistula, ruptured duodenum, iatrogenic gas collection)
| DDx: | infected necrosis |
Chronic Pancreatitis
= continued inflammatory disease of pancreas characterized by scarring with irreversible permanent damage to anatomy
+ function primarily of the exocrine pancreas
| Incidence: | 4:100,000 (in Western countries) |
Etiology:
UNKNOWN CAUSE (20%)
CHRONIC CALCIFYING PANCREATITIS
Alcoholism (70%)
Juvenile tropical pancreatitis = Kwashiorkor: in equatorial third-world countries, associated with pure protein malnutrition, patients present with diabetes + chronic abdominal pain
hereditary pancreatitis
Inborn errors of metabolism
hyperlipidemia
hypercalcemia
CHRONIC OBSTRUCTIVE PANCREATITIS
P.742
acute exacerbation of epigastric pain (93%): decreasing with time due to progressive destruction of gland, usually painless after 7 years
jaundice (42%) from common bile duct obstruction
steatorrhea (80%)
diabetes mellitus (58%)
secretin test with decreased amylase + bicarbonate in duodenal fluid
Plain film:
 numerous irregular calcifications (in 20 50% of alcoholic pancreatitis) PAThoGNoMoNIC
numerous irregular calcifications (in 20 50% of alcoholic pancreatitis) PAThoGNoMoNIC
UGI:
 displacement of stomach/duodenum by pseudocyst
displacement of stomach/duodenum by pseudocyst shrinkage/fold induration of stomach (DDx: linitis plastica)
shrinkage/fold induration of stomach (DDx: linitis plastica) stricture of duodenum
stricture of duodenum
Cholangiopancreatography (most sensitive imaging modality):
 side-branch ectasia = slight ductal ectasia/clubbing of side branches (minimal disease)
side-branch ectasia = slight ductal ectasia/clubbing of side branches (minimal disease) nipping = narrowing of the origins of side branches
nipping = narrowing of the origins of side branches dilatation >2 mm, tortuosity, wall rigidity, main ductal stenosis (moderate disease)
dilatation >2 mm, tortuosity, wall rigidity, main ductal stenosis (moderate disease) beading, chain of lakes, string of pearls = multifocal dilatation, stenosis, obstruction of main pancreatic duct + side branches (severe disease)
beading, chain of lakes, string of pearls = multifocal dilatation, stenosis, obstruction of main pancreatic duct + side branches (severe disease) intraductal filling defects due to mucinous protein plugs/calculi/debris
intraductal filling defects due to mucinous protein plugs/calculi/debris prolonged emptying of contrast material
prolonged emptying of contrast material may have stenosis/obstruction + prestenotic dilatation of CBD
may have stenosis/obstruction + prestenotic dilatation of CBD filling of pseudocysts (<50%)
filling of pseudocysts (<50%)
US/CT:
 irregular (73%)/smooth (15%)/beaded (12%) pancreatic ductal dilatation (in 41-68%)
irregular (73%)/smooth (15%)/beaded (12%) pancreatic ductal dilatation (in 41-68%) small atrophic pancreas (in 10-54%)
small atrophic pancreas (in 10-54%) pancreatic calcifications (4 50 68%)
pancreatic calcifications (4 50 68%) inhomogeneous gland with increased echogenicity (62%)
inhomogeneous gland with increased echogenicity (62%) irregular pancreatic contour (45 60%)
irregular pancreatic contour (45 60%) focal (12 32%)/diffuse (27 45%) pancreatic enlargement during flare up (DDx: pancreatic carcinoma)
focal (12 32%)/diffuse (27 45%) pancreatic enlargement during flare up (DDx: pancreatic carcinoma) mostly mild biliary ductal dilatation (29%)
mostly mild biliary ductal dilatation (29%) intra-/peripancreatic pseudocysts (20 34%)
intra-/peripancreatic pseudocysts (20 34%) segmental portal hypertension (= splenic vein thrombosis + splenomegaly) in 11%
segmental portal hypertension (= splenic vein thrombosis + splenomegaly) in 11% arterial pseudoaneurysm formation
arterial pseudoaneurysm formation peripancreatic fascial thickening + blurring of organ margins (16%)
peripancreatic fascial thickening + blurring of organ margins (16%) ascites/pleural effusion (9%)
ascites/pleural effusion (9%) no abnormalities (7%)
no abnormalities (7%)
MR:
 loss of signal intensity on fat-suppressed T1WI (from loss of aqueous protein in pancreatic acini secondary to fibrosis)
loss of signal intensity on fat-suppressed T1WI (from loss of aqueous protein in pancreatic acini secondary to fibrosis) diminished heterogeneous contrast enhancement (from loss of normal capillary network replaced by fibrous tissue)
diminished heterogeneous contrast enhancement (from loss of normal capillary network replaced by fibrous tissue)
Angiography:
 increased tortuosity + angulation of pancreatic arcades + intrahepatic arteries (88%)
increased tortuosity + angulation of pancreatic arcades + intrahepatic arteries (88%) luminal irregularities/focal fibrotic arterial stenoses (25 75%)/smooth beaded appearance
luminal irregularities/focal fibrotic arterial stenoses (25 75%)/smooth beaded appearance irregular parenchymal stain
irregular parenchymal stain venous compression/occlusion (20 50%)
venous compression/occlusion (20 50%) portoportal shunting + gastric varices without esophageal varices
portoportal shunting + gastric varices without esophageal varices
| Cx: | pancreatic carcinoma (2 4%), jaundice, pseudocyst formation, pancreatic ascites, thrombosis of splenic/mesenteric/portal vein |
| Rx: | surgery for infected pseudocyst, GI bleeding from portal hypertension, common bile duct obstruction, gastrointestinal obstruction |
| DDx: | pancreatic carcinoma (extrapancreatic spread) |
Chronic Alcoholic Pancreatitis
= characterized by heterogeneous lobular distribution; typically >8 years of heavy ethanol abuse
Pathophysiology:
thick pancreatic secretions with increased protein concentration precipitate in pancreatic ductules causing obstruction; protein plugs may calcify
 protein plugs/calculi within ductal system
protein plugs/calculi within ductal system ductal abnormalities more severe in smaller branches
ductal abnormalities more severe in smaller branches
Chronic Obstructive Pancreatitis
Etiology:
Congenital/acquired lesions of pancreatic duct
Trauma/surgical duct ligation
Sphincter of Oddi dysfunction, ampullary stenosis
Primary sclerosing cholangitis
Idiopathic fibrosing pancreatitis
Renal failure
Slow growing ampullary tumor
 dilatation of main pancreatic duct
dilatation of main pancreatic duct normal sized/focally or diffusely enlarged/small atrophic gland
normal sized/focally or diffusely enlarged/small atrophic gland calcifications uncommon
calcifications uncommon
Nonalcoholic Duct-Destructive Chronic Pancreatitis
= characterized by periductal inflammation + duct destruction
| Location: | body + tail of pancreas |
 focal/diffuse enlargement with decreased attenuation
focal/diffuse enlargement with decreased attenuation narrow main pancreatic duct without irregularities
narrow main pancreatic duct without irregularities no calcifications
no calcifications
MR:
 homogeneously decreased signal intensity on T1WI isointense relative to spleen
homogeneously decreased signal intensity on T1WI isointense relative to spleen variable intensity on T2WI
variable intensity on T2WI
Pancreatoblastoma
= rare childhood tumor often misdiagnosed as neuroblastoma/hepatoblastoma
| Age: | <7 years |
palpable mass, anorexia, vomiting
 up to 10 cm large well-defined lobulated solid/multiloculated mass in region of lesser sac
up to 10 cm large well-defined lobulated solid/multiloculated mass in region of lesser sac
Papillary Adenoma Of Bile Ducts
= very rare benign tumor of biliary tract
| Path: | usually solitary tumor/papillomatosis with papillary fronds extending into lumen |
P.743
| Histo: | columnar epithelium supported by connective tissue from lamina propria |
biliary obstruction
| Location: | common bile duct > right/left hepatic duct |
 usually small intraductal mass
usually small intraductal mass visualized at cross-sectional imaging only if large enough
visualized at cross-sectional imaging only if large enough
| Prognosis: | high rate of recurrence after surgical resection |
| Cx: | malignant transformation (rare) |
Passive Hepatic Congestion
| Cause: | CHF, constrictive pericarditis |
| Pathophysiology: | chronic central venous hypertension transmitted to hepatic sinusoids results in centrilobular congestion + eventually hepatic atrophy, necrosis, fibrosis |
abnormal liver function tests
CT:
 globally delayed enhancement (36%)
globally delayed enhancement (36%) enhancement of portal veins + hepatic arteries + immediately adjacent parenchyma (56%)
enhancement of portal veins + hepatic arteries + immediately adjacent parenchyma (56%) reticulated mosaic pattern = lobular patchy areas of enhancement separated by coarse linear regions of diminished attenuation (100%)
reticulated mosaic pattern = lobular patchy areas of enhancement separated by coarse linear regions of diminished attenuation (100%) diminished periportal attenuation (24%)
diminished periportal attenuation (24%) diminished attenuation around intrahepatic IVC (8%)
diminished attenuation around intrahepatic IVC (8%) prominent IVC + hepatic vein enhancement (due to contrast reflux from right atrium into dilated IVC)
prominent IVC + hepatic vein enhancement (due to contrast reflux from right atrium into dilated IVC)
| DDx: | Budd-Chiari syndrome (regional/lobular distribution of reticulated mosaic pattern, caudate lobe hypertrophy) |
Peliosis
[pelios, Greek = purple]
= rare benign disorder characterized by multiple blood-filled cavities within organs of the RES
| Cause: | (a)? acquired: chronic infection (disseminated TB), hepatotoxic drugs (androgen-anabolic steroids, corticosteroids, tamoxifen citrate, chemotherapeutic agents, azathioprine, oral contraceptives, thorium dioxide injection), diabetes mellitus, chronic renal failure, advanced malignancy (Hodgkin disease, myeloma, disseminated cancer) (b) bacillary peliosis hepatis in AIDS (lesions contain bacilli of Rochalimaea species) responsive to antibiotics (c)? congenital: angiomatous malformation |
| Histo: | (1) Phlebectatic peliosis hepatis (early stage) = endothelial-lined cysts (=? dilatation of central veins) communicating with dilated hepatic sinusoids + compression of surrounding liver (2) Parenchymal peliosis hepatis (late stage) = irregularly shaped cysts without lining communicating with dilated hepatic sinusoids + areas of liver cell necrosis |
| Associated with: | hormonally induced benign/malignant tumors |
| Location: | liver (most common), spleen, bone marrow, lymph nodes, lungs) |
| Age: | fetal life (rare) to adult life |
incidental discovery
 hepatomegaly, splenomegaly
hepatomegaly, splenomegaly
US:
 multiple indistinct areas of hypo-/hyperechogenicity
multiple indistinct areas of hypo-/hyperechogenicity
CECT:
 initially hypoattenuating, with passage of time isoattenuating/enhancing round lesions
initially hypoattenuating, with passage of time isoattenuating/enhancing round lesions
MR:
 mixed signal intensity due to repeated hemorrhage (deoxyhemoglobin + methemoglobin + siderotic nodules)
mixed signal intensity due to repeated hemorrhage (deoxyhemoglobin + methemoglobin + siderotic nodules)
Angio:
 multiple small (several mm to 1.5 cm) round collections of contrast medium scattered throughout liver in late arterial phase of hepatic arteriogram
multiple small (several mm to 1.5 cm) round collections of contrast medium scattered throughout liver in late arterial phase of hepatic arteriogram simultaneous opacification of hepatic veins
simultaneous opacification of hepatic veins
| Prognosis: | reversible after drug withdrawal/progression to hepatic failure/intraperitoneal hemorrhage leading to death |
Porcelain Gallbladder
=calcium carbonate incrustation of gallbladder wall
| Incidence: | 0.6 0.8% of cholecystectomy patients; M:F = 1:5 |
| Histo: | (a) flakes of dystrophic calcium within chronically inflamed + fibrotic muscular wall (b) microliths scattered diffusely throughout mucosa, submucosa, glandular spaces, Rokitansky-Aschoff sinuses |
| Associated with: | gallstones in 90% |
minimal symptoms
 curvilinear (muscularis)/granular (mucosal) calcifications in segment of wall/entire wall
curvilinear (muscularis)/granular (mucosal) calcifications in segment of wall/entire wall nonfunctioning GB on oral cholecystogram
nonfunctioning GB on oral cholecystogram highly echogenic shadowing curvilinear structure in GB fossa (DDx: stone-filled contracted GB)
highly echogenic shadowing curvilinear structure in GB fossa (DDx: stone-filled contracted GB) echogenic GB wall with little acoustic shadowing (DDx: emphysematous cholecystitis)
echogenic GB wall with little acoustic shadowing (DDx: emphysematous cholecystitis) scattered irregular clumps of echoes with posterior acoustic shadowing
scattered irregular clumps of echoes with posterior acoustic shadowing
| Cx: | 10 20% develop carcinoma of gallbladder |
Portal Hypertension
= portal venous pressure >10 mm Hg
normal hepatic blood flow of 550 900 mL/min (= 25% of cardiac output) passes through portal system (2/3) + through hepatic artery (1/3)
Classification:
DYNAMIC/HYPERKINETIC PORTAL HYPERTENSION
congenital/traumatic/neoplastic arterioportal fistula
INCREASED PORTAL RESISTANCE
@ Prehepatic
portal vein thrombosis (portal phlebitis, oral contraceptives, coagulopathy, neoplastic invasion, pancreatitis, neonatal omphalitis)
portal vein compression (tumor, trauma, lymphadenopathy, portal phlebosclerosis, pancreatic pseudocyst)
@ Intrahepatic (= obstruction of portal venules)
presinusoidal
Congenital hepatic fibrosis
Idiopathic noncirrhotic fibrosis
Primary biliary cirrhosis
alpha-1 antitrypsin deficiency
Wilson disease
Sarcoid liver disease
Toxic fibrosis (arsenic, copper, PVC)
Reticuloendotheliosis
Myelofibrosis
Felty syndrome
Schistosomiasis
Cystic fibrosis
Chronic malaria
P.744
sinusoidal
Hepatitis
Sickle cell disease
postsinusoidal
Cirrhosis (most frequent): Laennec cirrhosis, postnecrotic cirrhosis from hepatitis
Venoocclusive disease of liver
@ Posthepatic
Budd-Chiari syndrome
Constrictive pericarditis
CHF (tricuspid incompetence)
Pathophysiology:
continued elevated pressure despite formation of portal venous collateral vessels may be explained by
backward flow theory = hypodynamic flow theory
= increase in sinusoidal pressure due to deposition of collagen in the spaces of Disse + hepatocyte swelling
low/stagnant portal venous flow rates
forward flow theory = hyperdynamic flow theory
= splanchnic flow increases secondary to mesenteric vasodilators + increase in cardiac output to preserve hepatic perfusion + intrahepatic endogenous vasoconstrictors
increased portal venous flow rates >15 mL/min/kg
Flow direction:
hepatopetal (petere, Latin = to seek)
hepatofugal (fugere, Latin = to flee) = flow reversal
Cause: intrahepatic arterioportal communications (inside portal triads vasa vasorum of portal veins + hepatic arteries connect via bile duct capillaries to portal vein)
| Spontaneous Portosystemic Shunts | |
|---|---|
| Type of Varices | Frequency (%) |
| Coronary venous | 80 86 |
| Esophageal | 45 65 |
| Paraumbilical | 10 43 |
| Abdominal wall | 30 |
| Perisplenic | 30 |
| Retrogastric/gastric | 2 27 |
| Paraesophageal | 22 |
| Omental | 20 |
| Retroperitoneal paravertebral | 18 |
| Mesenteric | 10 |
| Splenorenal | 10 |
| Gastrorenal | 7 |
elevated hepatic wedge pressure (HWP) = portal venous pressure; normal values seen in presinusoidal portal hypertension
caput medusae = drainage from paraumbilical + omental veins through superficial veins of chest (lateral thoracic vein to axillary vein; superficial epigastric vein to internal mammary vein and subclavian vein) + abdominal wall (circumflex iliac vein and superficial epigastric vein to femoral vein; inferior epigastric vein to external iliac vein)
hemorrhaging esophageal varices (50%)
@ Splanchnic system:
 portal vein >13 mm (57% sensitivity, 100% specificity)
portal vein >13 mm (57% sensitivity, 100% specificity) SMV + splenic vein >10 mm; coronary vein >4 mm; recanalized umbilical vein >3 mm (size of vessels not related to degree of portal hypertension or presence of collaterals)
SMV + splenic vein >10 mm; coronary vein >4 mm; recanalized umbilical vein >3 mm (size of vessels not related to degree of portal hypertension or presence of collaterals) loss of respiratory increase of splanchnic vein diameters of <20% (81% sensitive, 100% specific)
loss of respiratory increase of splanchnic vein diameters of <20% (81% sensitive, 100% specific) portal vein aneurysm
portal vein aneurysm portal vein thrombosis
portal vein thrombosis cavernous transformation of portal vein
cavernous transformation of portal vein increased echogenicity + thickening of portal vein walls
increased echogenicity + thickening of portal vein wallsDoppler US:
 continuous monophasic portal venous flow pattern without respiratory fluctuations
continuous monophasic portal venous flow pattern without respiratory fluctuations reduction of mean portal vein velocities to 7 12 cm/sec (normally 12 30 cm/sec)
reduction of mean portal vein velocities to 7 12 cm/sec (normally 12 30 cm/sec) loss of flow increase in portal venous system during expiration
loss of flow increase in portal venous system during expiration congestive index >0.13 cm/sec (= ratio of area of portal vein divided by flow velocity; 67% sensitive)
congestive index >0.13 cm/sec (= ratio of area of portal vein divided by flow velocity; 67% sensitive) may have bidirectional/hepatofugal (<10%) flow within spontaneous splenorenal shunts (indicates high incidence of hepatic encephalopathy)
may have bidirectional/hepatofugal (<10%) flow within spontaneous splenorenal shunts (indicates high incidence of hepatic encephalopathy) dilated hepatic artery may demonstrate elevated resistive index >0.78
dilated hepatic artery may demonstrate elevated resistive index >0.78
@ Spontaneous portosystemic shunts:
high frequency of hepatic encephalopathy
 varices = serpentine tubular rounded structures
varices = serpentine tubular rounded structures coronary (left gastric) vein >5 6 mm (in 26%)
coronary (left gastric) vein >5 6 mm (in 26%) gallbladder wall varices in thickened gallbladder wall (in 80% associated with portal vein thrombosis)
gallbladder wall varices in thickened gallbladder wall (in 80% associated with portal vein thrombosis)
connection to SVC
Esophageal varices (= subepithelial + submucosal veins) supplied by anterior branch of left gastric vein
Paraesophageal varices (endoscopically not visible) supplied by posterior branch of coronary (= left gastric) vein draining into azygos + hemiazygos vv. + vertebral plexus
 NOT connected to esophageal varices!
NOT connected to esophageal varices! mediastinal/lung mass on CXR in 5 8%
mediastinal/lung mass on CXR in 5 8%
connection to pulmonary circulation
Gastropulmonary shunt (between gastric/esophageal vv. and left pericardiophrenic/inferior pulmonary vv.)
 nodularity along left cardiac border on CXR
nodularity along left cardiac border on CXR
retrograde mesenteric flow
Veins of Retzius (= anastomoses between portal vein and IVC)
ileocolic veins right gonadal vein IVC
pancreaticoduodenal vein IVC
proximal small left branches of SMV left gonadal vein left renal vein
ileocolic veins directly into IVC
retroperitoneal collaterals
Splenorenal/splenoadrenorenal shunt
Gastrorenal shunt
Mesenterorenal shunt (between SMV + right renal v.)
Mesenterogonadal shunt (between ileocolic v. + right testicular v.)
Splenocaval shunt (between splenic v. + left hypogastric v.)
intrahepatic shunt (portal v. to hepatic v.)
P.745
@ Cruveilhier-von Baumgarten syndrome (20 35%)
= recanalized paraumbilical veins (NoT recanalized umbilical veins)
 hypoechoic channel in ligamentum teres
hypoechoic channel in ligamentum teressize <2 mm (in 97% of normal subjects; in 14% of patients with portal hypertension)
size mm (86% sensitivity for portal hypertension)
 arterial signal on Doppler US in 38%
arterial signal on Doppler US in 38% hepatofugal venous flow (82% sensitivity, 100% specificity for portal hypertension)
hepatofugal venous flow (82% sensitivity, 100% specificity for portal hypertension)
@ Spleen
 splenomegaly (absence does not rule out portal hypertension)
splenomegaly (absence does not rule out portal hypertension) siderotic Gamna-Gandy nodules in 13% (= small foci of perifollicular + trabecular hemorrhage):
siderotic Gamna-Gandy nodules in 13% (= small foci of perifollicular + trabecular hemorrhage): multiple 3 8-mm low-intensity spots on FLASh/GRASS images
multiple 3 8-mm low-intensity spots on FLASh/GRASS images multiple hyperechoic spots on US
multiple hyperechoic spots on US
 multiple faint calcifications on CT
multiple faint calcifications on CT
 ascites
ascites
| Cx: | Acute gastrointestinal bleeding (mortality of 30 50% during 1st bleeding) |
Segmental Portal Hypertension
= splenic vein occlusion/superior mesenteric vein occlusion
Portosystemic Surgical Connections
NONSELECTIVE SHUNT
= decompression of the entire portal system with increased risk of hepatic encephalopathy
Portocaval shunt
= portal vein to IVC end-to-side/side-to-side
Mesocaval shunt
= synthetic graft between SMV and IVC
short H-graft to posterior wall of SMV
long C-graft to anterior wall of SMV
direct mesocaval shunt dividing IVC (rare)
Mesorenal shunt
Mesoatrial shunt
= polytetrafluoroethylene (PTFE) graft between anterior wall of SMV superior to pancreas and right atrium coursing through abdomen + diaphragm into right thoracic cavity
SELECTIVE SHUNT
= decompression of parts of the portal system with preservation of blood flow to the liver
 Contraindicated in patients with ascites
Contraindicated in patients with ascitesDistal splenorenal shunt = Warren shunt (popular)
= splenic vein to left renal vein
Doppler criteria for shunt patency:
 increased local velocities
increased local velocities turbulence + severe spectral broadening
turbulence + severe spectral broadening dilatation of recipient vein at shunt site
dilatation of recipient vein at shunt site phasic flow pattern in portal tributaries
phasic flow pattern in portal tributaries hepatofugal flow in intrahepatic portal vein branches
hepatofugal flow in intrahepatic portal vein branches reduction in size + number of portosystemic collaterals
reduction in size + number of portosystemic collaterals reduction/absence of ascites or splenomegaly
reduction/absence of ascites or splenomegaly
Transjugular Intrahepatic Portosystemic Shunt (TIPS)
= portal decompression through percutaneously established shunt with expandable metallic stent between hepatic + portal veins within the liver
| Indication: | patients with esophageal + gastric variceal hemorrhage/refractory ascites due to advanced liver disease with portal hypertension, hepatorenal syndrome |
| Type of stent: | 10-mm Wall stent (curved), Palmaz stent (straight), Strecker stent, spiral Z stent |
| Shunt surveillance: | at regular 3 6-month intervals for |
Assessment:
MORPHOLOGY
Ascites
Portosystemic collaterals
Size of spleen
Diameter of stent (usually 8 10 mm)
Configuration of stent: areas of narrowing
Extension of stent into portal + hepatic veins
HEMODYNAMICS
Direction of flow in: extrahepatic portal vein, R + L portal vein, SMV, splenic vein, all 3 hepatic veins, intrahepatic IVC, paraumbilical vein, coronary vein
Peak blood flow velocity within main portal vein
Peak blood flow velocity within proximal + mid + distal aspects of stent
Hepatic artery: PSV, EDV, RI
 high-velocity turbulent flow (50 270 cm/s) at least double that of pre-TIPS values
high-velocity turbulent flow (50 270 cm/s) at least double that of pre-TIPS values superimposed cardiac + respiratory variations
superimposed cardiac + respiratory variations increase in hepatic artery velocities from 77 cm/s (pre-TIPS) to 119 cm/s (post-TIPS)
increase in hepatic artery velocities from 77 cm/s (pre-TIPS) to 119 cm/s (post-TIPS) reversed flow direction within portal vein branches
reversed flow direction within portal vein branches
| Cx: |
|
| Mortality: | <2% (intraperitoneal hemorrhage) |
P.746
TIPS failure
| Cause: | acute thrombosis, improper stent placement, intimal hyperplasia, hepatic vein stenosis, change in stent configuration, bulging of liver parenchyma into shunt |
| Prevalence: | 31% at 1 year, 42% at 2 years |
recurrent bleeding = shunt abnormality in 100%
>50% stenosis (in 30 80% within 12 months)
 irregular filling defects along wall of shunt on color Doppler
irregular filling defects along wall of shunt on color Doppler Pseudointimal hyperplasia is isoechoic to blood!
Pseudointimal hyperplasia is isoechoic to blood!
 generalized decrease in shunt velocity:
generalized decrease in shunt velocity: maximal shunt velocity of <60 cm/sec (>95% sensitive + specific)
maximal shunt velocity of <60 cm/sec (>95% sensitive + specific) gradual decrease in shunt velocity over 1 6 months (due to intimal hyperplasia)
gradual decrease in shunt velocity over 1 6 months (due to intimal hyperplasia) decrease in peak flow velocity in similar location within stent >50 cm/sec relative to initial baseline study
decrease in peak flow velocity in similar location within stent >50 cm/sec relative to initial baseline study decrease in maximal portal vein velocity >33% from baseline
decrease in maximal portal vein velocity >33% from baseline
 local increase in shunt velocity:
local increase in shunt velocity: velocity transition zone within stent with flow acceleration by a factor of 2
velocity transition zone within stent with flow acceleration by a factor of 2 increase in peak flow velocity in similar location within stent >50 cm/sec relative to initial baseline study
increase in peak flow velocity in similar location within stent >50 cm/sec relative to initial baseline study
 change in flow direction:
change in flow direction: reversal of portal venous flow direction (100% sensitive, 92% specific, 71% PPV, 100% NPV)
reversal of portal venous flow direction (100% sensitive, 92% specific, 71% PPV, 100% NPV) change in flow direction in collateral veins from baseline
change in flow direction in collateral veins from baseline retrograde flow in RHV (developing stenosis of right hepatic venous outflow tract)
retrograde flow in RHV (developing stenosis of right hepatic venous outflow tract)
 loss of pulsatility of portal/shunt flow:
loss of pulsatility of portal/shunt flow: venous pulsatility index (Vmax Vmin)/Vmax <0.16 (94% sensitive, 87% specific)
venous pulsatility index (Vmax Vmin)/Vmax <0.16 (94% sensitive, 87% specific)
 developing/worsening ascites/splenomegaly
developing/worsening ascites/splenomegaly
Occlusion
 absent flow within shunt
absent flow within shunt echogenic material within stent
echogenic material within stentacute cause: prolonged procedural catheterization, leakage of bile into/around stent
delayed cause: pseudointimal hyperplasia, stent shortening with delayed stent expansion
| Pre- and Post-TIPS Baseline Study (under stable fasting conditions) | ||
|---|---|---|
| Pre-TIPS | Post-TIPS | |
| Portal vein velocity (cm/s) | 10 30 | 40 60 |
| Mean portal vein velocity (cm/s) | 18 6 | 55 7 |
| Portal pressure (mm hg) | 37 8 | 22 6 |
| Shunt peak velocity (cm/s) | 0 | 95 58 |
Portal Vein Thrombosis
Etiology:
IDIOPATHIC (mostly):? neonatal sepsis
SECONDARY:
Cirrhosis + portal hypertension (5%)
Malignancy: tumor invasion by HCC, cholangiocarcinoma, pancreatic carcinoma, gastric carcinoma, metastasis/extrinsic compression by tumor
Trauma: umbilical venous catheterization; surgery; Cx of splenectomy (7%, higher in patients with myeloproliferative disorders)
Hypercoagulable state: blood dyscrasia; clotting disorder; estrogen therapy; severe dehydration
Intraperitoneal inflammatory process (portal vein phlebitis): perinatal omphalitis; pancreatitis; appendicitis; ascending cholangitis
| Age: | predominantly children, young persons |
abdominal pain
portal systemic encephalopathy
hematemesis (esophageal varices)
Acute Portal Vein Thrombosis
Plain film:
 hepatosplenomegaly
hepatosplenomegaly enlarged azygos vein
enlarged azygos vein paraspinal varices
paraspinal varices
UGI:
 esophageal varices
esophageal varices thickening of bowel wall
thickening of bowel wall
US:
 echogenic material within vessel lumen (67%)
echogenic material within vessel lumen (67%) increase in portal vein diameter (57%)
increase in portal vein diameter (57%) portosystemic collateral circulation (48%)
portosystemic collateral circulation (48%) enlargement of thrombosed segment >15 mm (38%)
enlargement of thrombosed segment >15 mm (38%) thickening of lesser omentum
thickening of lesser omentum
Doppler-US:
 no flow on postprandial Doppler color scans:
no flow on postprandial Doppler color scans: Malignant thrombus tends to distend vein + exhibit pulsatile flow, a bland thrombus does not!
Malignant thrombus tends to distend vein + exhibit pulsatile flow, a bland thrombus does not!
 decrease in hepatic artery resistive index:
decrease in hepatic artery resistive index: RI <0.50 (in acute occlusive portal vein thrombosis)
RI <0.50 (in acute occlusive portal vein thrombosis) minimal decrease/normal RI (in chronic portal vein thrombosis/nonocclusive thrombosis)
minimal decrease/normal RI (in chronic portal vein thrombosis/nonocclusive thrombosis)
NECT:
 decreased attenuation of affected hepatic parenchyma (due to edema, depletion of hepatocytes, fibrosis)
decreased attenuation of affected hepatic parenchyma (due to edema, depletion of hepatocytes, fibrosis)
CECT:
 transient high attenuation during hepatic arterial phase (due to increased arterial flow)
transient high attenuation during hepatic arterial phase (due to increased arterial flow) low-density center of portal vein thrombus surrounded by peripheral enhancement:
low-density center of portal vein thrombus surrounded by peripheral enhancement: portal vein density 20 30 HU less than aortic density
portal vein density 20 30 HU less than aortic density
MR:
 absent flow void in portal area + abnormal signal intensity in main portal vein
absent flow void in portal area + abnormal signal intensity in main portal vein hyperintense thrombus on T1WI + T2WI (if <5 weeks old)
hyperintense thrombus on T1WI + T2WI (if <5 weeks old) filling defect on MRA
filling defect on MRA
P.747
Angio:
 thread and streaks sign of tumor thrombus (streaky contrast opacification of tumor vessels)
thread and streaks sign of tumor thrombus (streaky contrast opacification of tumor vessels)
| Cx: | (1) Cavernous transformation (19%) (2) Hepatic infarction (3) Bowel infarction |
Chronic Portal Vein Thrombosis
Pathophysiology:
central part (caudate lobe + lateral segment) is well supplied by collateral venous vessels; the peripheral zone (mainly right lobe) receives less portal venous blood resulting in increased arterial flow
 nonvisualization of extrahepatic portal vein (= fibrotic portal vein)
nonvisualization of extrahepatic portal vein (= fibrotic portal vein) calcification within clot/wall of portal vein
calcification within clot/wall of portal vein cavernous transformation (= cavernoma) of portal vein:
cavernous transformation (= cavernoma) of portal vein: presence of a racemose conglomerate of collateral veins with portal venous flow linking pancreas + duodenum + gallbladder fossa
presence of a racemose conglomerate of collateral veins with portal venous flow linking pancreas + duodenum + gallbladder fossa
 splenomegaly
splenomegaly ascites
ascites
CECT:
 peripheral scattered areas of high attenuation in liver during hepatic arterial phase
peripheral scattered areas of high attenuation in liver during hepatic arterial phase
US:
 echogenic/nonvisualized portal vein
echogenic/nonvisualized portal vein
MR:
 hypointense portal vein on T1WI + hyperintense on T2WI (2 18 months old)
hypointense portal vein on T1WI + hyperintense on T2WI (2 18 months old) numerous abnormal flow voids in porta hepatis
numerous abnormal flow voids in porta hepatis
Postcholecystectomy Syndrome
= symptoms recurring/persisting after cholecystectomy
Incidence:
mild recurrent symptoms in 9 25%; severe symptoms in 2.6 32% (result of 1,930 cholecystectomies):
completely cured (61%)
satisfactory improvement with
persistent mild dyspepsia (11%)
mild attacks of pain (24%)
failure with
occasional attacks of severe pain (3%)
continuous severe distress (1.7%)
recurrent cholangitis (0.7%)
Cause:
BILIARY CAUSES
Incomplete surgery
Gallbladder/cystic duct remnant
Retained stone in cystic duct remnant (1%)
Overlooked CBD stone (5%)
Operative trauma
Bile duct stricture
Bile peritonitis
Suture granuloma of cystic duct remnant
Bile duct pathology
Fibrosis of sphincter of Oddi
Biliary dyskinesia
Biliary fistula
Cystic duct mucocele
Residual disease in neighboring structures
Pancreatitis
Hepatitis
Cholangitis
Overlooked bile duct neoplasia
EXTRABILIARY CAUSES (erroneous preoperative diagnosis)
Other GI tract disease:
Inadequate dentition
Hiatus hernia
Peptic ulcer
Spastic colon
Anxiety state, air swallowing
Abdominal angina
Carcinoma outside gallbladder
Coronary artery disease
Richter Syndrome
= development of large cell/diffuse histiocytic lymphoma in patients with CLL
| Etiology: | transformation/dedifferentiation of CLL lymphocytes |
| Incidence in CLL patients: | 3 10% |
| Median age: | 59 years |
| Medium time interval after diagnosis of CLL: | 24 months |
fever (65%) without evidence of infection
increasing lymphadenopathy + hepatosplenomegaly (46%)
weight loss (26%), abdominal pain (26%)
| Location: | bone marrow, lymph nodes, liver, spleen, bowel, lung, pleura, kidney, dura |
| Prognosis: | median survival time: 4 months from diagnosis of lymphoma; 14% rate of remission rate |
Schistosomiasis
 Major cause of portal hypertension worldwide: 200 million people affected
Major cause of portal hypertension worldwide: 200 million people affected
Types:
SCHISTOSOMA MANSONI
occurs in >70 million inhabitants of parts of Africa, Caribbean, Arabic peninsula, West Indies, northern part of South America
SCHISTOSOMA JAPONICUM
coastal areas of China, Japan, Formosa, Philippines, Celebes
SCHISTOSOMA HAEMATOBIUM
in Africa, Mediterranean, Southwest Asia typically affects urinary tract
Cycle:
cercariae enter lymphatics + blood system via thoracic duct; larvae are transported into mesenteric capillaries; mature in portal system + liver into worms; worms live in pairs in copula within portal vein + tributaries for 10 15 years; female swims against blood flow to reach venules of urinary bladder (S. haematobium) or intestine + rectum (S. mansoni, S. japonicum); deposits eggs in wall of urinary bladder or intestines, eggs pass with urine + feces; hatch within water to release miracidia which infect snail hosts; cercariae emerge after maturation from snails
| Infection: | cercariae penetrate human skin/buccal mucosa from contaminated water (slow-moving streams, irrigation canals, paddy fields, lakes) |
| Histo: | granulomatous reaction + fibrosis along portal vein branches |
clinically mild infection with chronic course
@ Liver & spleen (10%)
 hepatosplenomegaly
hepatosplenomegaly portal vein dilatation in 73% (= presinusoidal portal hypertension)
portal vein dilatation in 73% (= presinusoidal portal hypertension) marked diffuse thickening of echogenic walls of portal venules = periportal fibrosis
marked diffuse thickening of echogenic walls of portal venules = periportal fibrosis Schistosoma infection is the most frequent cause of liver fibrosis worldwide!
Schistosoma infection is the most frequent cause of liver fibrosis worldwide!
 normal parenchymal echogenicity + small peripheral hyperechoic foci in 50%
normal parenchymal echogenicity + small peripheral hyperechoic foci in 50% hyperechoic gallbladder bed
hyperechoic gallbladder bed thickened gallbladder wall
thickened gallbladder wall capsular turtleback/tortoise shell calcifications extending perpendicularly from the surface toward the center (Schistosomiasis japonicum)
capsular turtleback/tortoise shell calcifications extending perpendicularly from the surface toward the center (Schistosomiasis japonicum)
@ GI tract
 gastric + esophageal varices
gastric + esophageal varices polypoid bowel wall masses (esp. in sigmoid)
polypoid bowel wall masses (esp. in sigmoid) granulomatous colitis
granulomatous colitis strictures with extensive pericolic inflammation
strictures with extensive pericolic inflammation
| Cx: | ileus |
P.748
Schwachman-Diamond Syndrome
= rare probably autosomal recessive condition characterized by congenital absence of pancreatic exocrine tissue
 2nd most frequent cause of exocrine pancreatic insufficiency in childhood after cystic fibrosis!
2nd most frequent cause of exocrine pancreatic insufficiency in childhood after cystic fibrosis!pancreatic insufficiency, steatorrhea
recurrent respiratory and skin infections (secondary to bone marrow hypoplasia)
normal electrolytes in sweat
failure to thrive
tends to improve with time
 total fatty replacement of pancreas
total fatty replacement of pancreas metaphyseal chondrodysplasia resulting in dwarfism
metaphyseal chondrodysplasia resulting in dwarfism
| DDx: | cystic fibrosis (pancreatic calcifications, cyst formation, abnormal sweat test) |
Serous Cystadenoma Of Pancreas
= MICROCYSTIC CYSTADENOMA = GLYCOGEN-RICH CYSTADENOMA
= benign lobulated neoplasm composed of innumerable small cysts (1 20 mm) containing proteinaceous fluid separated by thin connective tissue septa
| Incidence: | approximately 50% of all cystic pancreatic neoplasms |
| Histo: | cyst walls lined by cuboidal/flat glycogen-rich epithelial cells derived from centroacinar cells of pancreas (DDx: lymphangioma), thin fibrous pseudocapsule |
| Age: | 34 88 years; mean age 65 years; 82% over 60 years of age; M:F = 1:2 4 |
| Associated with: | von Hippel-Lindau syndrome |
pain, weight loss, malaise, anorexia, fatigue, jaundice
palpable mass
| Location: | any part of pancreas affected, slight predominance for head + neck |
 well-demarcated lobulated mass 1 25 (mean 5) cm in diameter with smooth/nodular contour
well-demarcated lobulated mass 1 25 (mean 5) cm in diameter with smooth/nodular contour innumerable small <2 cm cysts of honeycomb/bunch of grapes appearance; uncommonly few large cysts (in <5%)/cyst up to 8 cm in diameter
innumerable small <2 cm cysts of honeycomb/bunch of grapes appearance; uncommonly few large cysts (in <5%)/cyst up to 8 cm in diameter prominent central stellate scar (ChARACTERISTIC)
prominent central stellate scar (ChARACTERISTIC) amorphous central calcifications (in 18%) in dystrophic area of stellate central scar ( sunburst )
amorphous central calcifications (in 18%) in dystrophic area of stellate central scar ( sunburst ) pancreatic duct + CBD may be displaced, encased, or obstructed
pancreatic duct + CBD may be displaced, encased, or obstructedUS:
 solid predominantly echogenic mass with mixed hypoechoic + echogenic areas
solid predominantly echogenic mass with mixed hypoechoic + echogenic areas
CT:
 attenuation values close to water
attenuation values close to water contrast enhancement
contrast enhancement
Angio:
 hypervascular mass with dilated feeding arteries, dense tumor blush, prominent draining veins, neovascularity, occasional AV shunting, No vascular encasement
hypervascular mass with dilated feeding arteries, dense tumor blush, prominent draining veins, neovascularity, occasional AV shunting, No vascular encasement
MR:
 delayed enhancement of scar on contrast-enhanced FLASh images
delayed enhancement of scar on contrast-enhanced FLASh images
| Prognosis: | no malignant potential |
| Rx: | surgical excision/follow-up examinations |
| DDx: | malignant mucinous cystic neoplasm (younger age, body + tail of pancreas, >10 cm large at presentation) |
Solid And Papillary Neoplasm Of Pancreas
= SOLID AND CYSTIC TUMOR = PAPILLARY-CYSTIC NEOPLASM = SOLID AND PAPILLARY EPITHELIAL NEOPLASM = HAMOUDI TUMOR
= rare, low-grade malignant tumor; often misclassified as nonfunctioning islet cell tumor, cystadenoma, or cystadenocarcinoma of pancreas
| Prevalence: | 0.17 2.7% of all nonendocrine pancreatic tumors |
| Mean age: | 25 (range 10 74) years; M:F = 1:9; especially in black and East Asian patients |
| Path: | large well-encapsulated mass with considerable hemorrhagic necrosis + cystic degeneration |
| Histo: | sheets + cords of cells arranged around a fibrovascular stroma |
vague upper abdominal discomfort and pain
gradually enlarging abdominal mass
| Location: | tail of pancreas (most frequently) |
 well-encapsulated inhomogeneous round/lobulated pancreatic mass with solid + cystic portions
well-encapsulated inhomogeneous round/lobulated pancreatic mass with solid + cystic portions may be completely cystic (when complicated by extensive necrosis + internal hemorrhage)
may be completely cystic (when complicated by extensive necrosis + internal hemorrhage) fluid-debris level (20%)
fluid-debris level (20%) mean diameter of 9 cm (range 3 15 cm)
mean diameter of 9 cm (range 3 15 cm) stippled/punctate/amorphous dystrophic calcification (33%)
stippled/punctate/amorphous dystrophic calcification (33%) hypovascular with no contrast enhancement/enhancement of solid tissue projecting toward center of mass
hypovascular with no contrast enhancement/enhancement of solid tissue projecting toward center of massUS:
 echogenic mass with necrotic center
echogenic mass with necrotic center
MR:
 high signal intensity on T1WI (consistent with hemorrhagic necrosis)
high signal intensity on T1WI (consistent with hemorrhagic necrosis)
P.749
| Prognosis: | (1) excellent after excision (2) metastases (in 4%): omentum, lymph nodes, liver |
| DDx: | (1) Microcystic adenoma (innumerable tiny cysts, older age group) (2) Mucinous cystic neoplasm (large uni-/multilocular cysts, older age group) (3) Nonfunctioning islet cell tumor (hypervascular) (4) Pleomorphic carcinoma of pancreas (smaller tumor in older patient) (5) Pancreatoblastoma (childhood tumor) (6) Calcified hemorrhagic pseudocyst |
Splenic Angiosarcoma
| Incidence: | rare, <100 cases in literature |
| Cause: | usually not due to thorotrast or toxic exposure to vinyl chloride/arsenic as in liver angiosarcoma |
| Age: | 50 60 years |
splenomegaly, abdominal pain
 multiple nodules of varying size usually enlarging the spleen
multiple nodules of varying size usually enlarging the spleen solitary complex mass with variable contrast enhancement
solitary complex mass with variable contrast enhancement metastasizes to liver (70%)
metastasizes to liver (70%) spontaneous rupture (33%)
spontaneous rupture (33%)
MR:
 focal/diffuse hypointense foci on T1WI + T2WI (iron deposition from hemorrhage)
focal/diffuse hypointense foci on T1WI + T2WI (iron deposition from hemorrhage)
| Prognosis: | 20% survival rate after 6 months |
Splenic Hamartoma
SPLENOMA
rare typically single nonneoplastic lesion composed of a mixture of normal splenic elements
| Etiology: | congenital |
| May be associated with: | hamartomas elsewhere as in tuberous sclerosis |
| Histo: |
|
asymptomatic
CT:
 attenuation equal to/hypodense to splenic tissue
attenuation equal to/hypodense to splenic tissue prolonged heterogeneous enhancement
prolonged heterogeneous enhancement
MR:
 heterogeneously hyperintense on T2WI
heterogeneously hyperintense on T2WI diffuse heterogeneous enhancement, more homogeneous on delayed images
diffuse heterogeneous enhancement, more homogeneous on delayed images
Splenic Hemangioma
| Cause: | congenital, arising from sinusoidal epithelium |
| Prevalence: | 0.03 14% (autopsy); M > F Most common primary splenic tumor! |
| Age: | 20 50 years |
| Histo: | proliferation of vascular channels lined by single layer of endothelium; mostly of cavernous type; may contain areas of infarction, hemorrhage, thrombosis, fibrosis |
| Associated with: | generalized angiomatosis Klippel-Tr naunay-Weber syndrome, Beckwith-Wiedemann syndrome, Turner syndrome |
asymptomatic/pain + fullness in LUQ
 usually small single lesion <4 cm, up to 17 cm in size
usually small single lesion <4 cm, up to 17 cm in size foci of speckled/snowflakelike calcifications
foci of speckled/snowflakelike calcificationsUS:
 well-marginated predominantly hyperechoic lesion
well-marginated predominantly hyperechoic lesion
CT:
 predominantly cystic lesion is avascular
predominantly cystic lesion is avascular solid areas hypo-/isodense to normal spleen and enhancing
solid areas hypo-/isodense to normal spleen and enhancing
MR:
 hypo-/isointense on T1WI + hyperintense on T2WI
hypo-/isointense on T1WI + hyperintense on T2WI hypointense areas due to hemosiderin deposits
hypointense areas due to hemosiderin deposits progressive centripetal enhancement with persistent uniform enhancement on delayed images
progressive centripetal enhancement with persistent uniform enhancement on delayed images
NUC:
 no uptake of Tc-99m sulfur colloid
no uptake of Tc-99m sulfur colloid
| Prognosis: | slow growth, thus becoming symptomatic in adulthood |
| Cx: | (1) Spontaneous splenic rupture (in up to 25%) (2) Kasabach-Merritt syndrome (= anemia, thrombocytopenia, coagulopathy) with large hemangioma (3) Portal hypertension (4) Malignant degeneration |
Splenic Infarction
 Most common cause of focal defects!
Most common cause of focal defects!Cause:
Embolic: bacterial endocarditis (responsible in 50%), atherosclerosis with plaque emboli, cardiac thrombus (atrial fibrillation, left ventricular thrombus), metastatic carcinoma
Local thrombosis: sickle cell disease (leading to functional asplenia), myelo-/lymphoproliferative disorders (CML most common), polycythemia vera, myelofibrosis with myeloid metaplasia + splenomegaly, Gaucher disease, collagen vascular disease, portal hypertension
Vasculitis: periarteritis nodosa
Vascular compromise of splenic artery: focal inflammatory process (eg, pancreatitis), thrombus from splenic artery aneurysm, splenic torsion
Therapeutic complication: transcatheter hepatic arterial embolization
| mnemonic: | PSALMS |
Pancreatic carcinoma, Pancreatitis
Sickle cell disease/trait
Adenocarcinoma of stomach
Leukemia
Mitral stenosis with emboli
Subacute bacterial endocarditis
| Anatomy: | branches of the splenic artery are noncommunicating end arteries |
LUQ pain, fever
elevated erythrocyte sedimentation rate, leukocytosis
abnormal lactate dehydrogenase levels
 single/multiple focal wedge-shaped peripheral defects
single/multiple focal wedge-shaped peripheral defects global infarction
global infarctionUS:
 initially ill-defined hypoechoic lesion (due to inflammation, edema, necrosis)
initially ill-defined hypoechoic lesion (due to inflammation, edema, necrosis) later increasingly well-defined echogenic lesion (due to organization of infarct with fibrosis)
later increasingly well-defined echogenic lesion (due to organization of infarct with fibrosis)
CT phases:
hyperacute phase (day 1)
 mottled area of increased attenuation on NECT (hemorrhage)
mottled area of increased attenuation on NECT (hemorrhage) large focal hyperattenuating lesion on CECT
large focal hyperattenuating lesion on CECT mottled pattern of contrast enhancement
mottled pattern of contrast enhancement
acute (days 2 4) + subacute phase (days 4 8)
 focal progressively more well-demarcated areas of decreased attenuation without enhancement
focal progressively more well-demarcated areas of decreased attenuation without enhancement
chronic phase (2 4 weeks)
 size decreases + attenuation returns to normal
size decreases + attenuation returns to normal complete resolution/residual contour defect
complete resolution/residual contour defect areas of calcification
areas of calcification
P.750
| Cx: | acute febrile illness, abscess formation, pseudocyst formation, splenic rupture, hemorrhage |
Splenosis
= posttraumatic autotransplantation of splenic tissue to other sites (heterotopic splenic tissue)
| Age: | young men with history of trauma/splenectomy |
| Time of detection: | mean of 10 years (range of 6 months to 32 years) after trauma |
| Location: | diaphragmatic surface, liver, greater omentum, small bowel serosa, parietal peritoneum, pleura after diaphragmatic rupture (attaches to peritoneal/pleural surface) |
 multiple small encapsulated sessile implants
multiple small encapsulated sessile implants
| Size: | few mm to 3 cm (due to limited blood supply from local neovascularization) |
CT:
 isodense to normal spleen with homogeneous enhancement
isodense to normal spleen with homogeneous enhancement
MR:
 hypointense on T1WI
hypointense on T1WI heterogeneous enhancement (red + white pulp differences)
heterogeneous enhancement (red + white pulp differences) hyperintense/rarely hypointense (due to iron deposition) on T2WI
hyperintense/rarely hypointense (due to iron deposition) on T2WI
NUC:
 uptake by Tc-99m sulfur colloid; In-111 labeled platelets; Tc-99m heat-damaged RBC (best detection rate without uptake by liver)
uptake by Tc-99m sulfur colloid; In-111 labeled platelets; Tc-99m heat-damaged RBC (best detection rate without uptake by liver)
Significance:
protects against infection in pediatric patients
may be confused with metastases/lymphoma
responsible for disease recurrence after splenectomy (eg, idiopathic thrombocytopenic purpura)
| DDx: | accessory spleen |
Spontaneous Perforation Of Common Bile Duct
| Pathogenesis: | unknown (? CBD obstruction, localized mural malformation, ischemia, trauma) |
| Age: | 5 weeks to 3 years of age |
vague abdominal distension
mild persistent hyperbilirubinemia
varying acholic stools
US:
 biliary ascites/loculated subhepatic fluid
biliary ascites/loculated subhepatic fluid localized pseudocholedochal cyst in porta hepatis
localized pseudocholedochal cyst in porta hepatis
Hepatobiliary scintigraphy:
 radioisotope diffusely throughout peritoneal cavity
radioisotope diffusely throughout peritoneal cavity
Thorotrastosis
Thorotrast = 25% colloidal suspension of thorium dioxide; used as contrast agent between late 1920s and mid 1950s, in particular for cerebral angiography and liver spleen imaging; chemically inert with high atomic number of 90; >100,000 people injected
Thorium dioxide = consists of 11 radioactive isotopes (thorium-232 is major isotope); decay by means of alpha, beta, and gamma emission; biologic half-life of 1.34 1010 years; hepatic dose of 1,000 3,000 rads in 20 years
| Distribution: | phagocytized by RES + deposited in liver (70%), spleen (30%), bone marrow, abdominal lymph nodes (20%) |
 linear network of metallic density contrast material in spleen, lymph nodes, liver
linear network of metallic density contrast material in spleen, lymph nodes, liver spleen may be shrunken/nonfunctional
spleen may be shrunken/nonfunctional
| Cx: | hepatic fibrosis, angiosarcoma (50%), cholangiocarcinoma, hepatocellular carcinoma (latency period of 3 40 years; mean 26 years) |
Tyrosinemia
= rare autosomal recessive metabolic disorder
| Country: | increased prevalence in Canadian province of Quebec and parts of Scandinavia |
| Biochemistry: | deficiency of enzyme fumarylacetoacetase (last step in catabolic pathway of tyrosine, serum methionine, urinary succinylacetone); elevated levels of serum tyrosine as a precursor of dopamine, norepinephrine, epinephrine, melanin, thyroxin |
ACUTE FORM
fulminant liver failure, often by 1 year of age
CHRONIC FORM
= Fanconi syndrome with renal tubular dysfunction
vitamin D-resistant rickets
intermittent porphyria-like symptoms
progressive liver failure in early childhood
anemia, abnormal liver function tests
elevated levels of -fetoprotein
 hepatosplenomegaly
hepatosplenomegaly micro- and macronodular cirrhosis (early childhood):
micro- and macronodular cirrhosis (early childhood): regenerating nodules of 2 20 mm: hyper- (mostly) /iso-/hypoattenuating; hypo-/occasionally hyperechoic
regenerating nodules of 2 20 mm: hyper- (mostly) /iso-/hypoattenuating; hypo-/occasionally hyperechoic portal hypertension
portal hypertension increased echogenicity (fibrosis + fatty infiltration)
increased echogenicity (fibrosis + fatty infiltration)
 nephromegaly with uniformly thickened renal cortices
nephromegaly with uniformly thickened renal cortices nephrocalcinosis
nephrocalcinosis
| Prenatal Dx: | enzyme deficiency demonstrable in hepatocytes, skin fibroblasts, lymphocytes, amniocytes |
| Cx: | hepatocellular carcinoma (in 37% beyond 2 years of age) |
| Rx: | (1) Diet restricted in phenylalanine + tyrosine (alleviates kidney damage but does not prevent fatal outcome) (2) 2-2-nitro-4-trifluoro-methylbenzoyl-1,3-cyclohexanedione (NTBC) inhibits 4-hydroxyphenylpyruvate dioxygenase + prevents formation of maleylacetoacetate and fumarylacetoacetate (3) Liver transplantation (before HCC develops) |
P.751
Undifferentiated Sarcoma Of Liver
= EMBRYONAL SARCOMA
| Incidence: | 4th/5th most common liver tumor in pediatric population |
| Age: | <2 months (in 5%); 6 10 years (in 52%); by 15 years (in 90%); up to 49 years; M:F = 1:1 |
| Histo: | primitive undifferentiated stellate/spindle-shaped sarcomatous cells closely packed in whorls + sheets/scattered loosely in a myxoid ground substance with foci of hematopoiesis (50%) |
painful RUQ mass and fever
mild anemia + leukocytosis (50%)
elevated liver enzymes (33%)
fever (5%)
| Location: | right lobe (75%); left lobe (10%);both lobes (15%) |
 7 14 21 cm in size
7 14 21 cm in size well-defined margins (fibrous pseudocapsule)
well-defined margins (fibrous pseudocapsule)NUC:
 photodefect on sulfur colloid scan
photodefect on sulfur colloid scan
US/CT:
 large intrahepatic mass with cystic areas up to 4 cm in diameter (myxoid stroma + necrosis + hemorrhage)
large intrahepatic mass with cystic areas up to 4 cm in diameter (myxoid stroma + necrosis + hemorrhage) discordant finding between US (solid) + CT (cystlike)
discordant finding between US (solid) + CT (cystlike)
Angio:
 hypo-/hypervascular with stretching of vessels
hypo-/hypervascular with stretching of vessels scattered foci of neovascularity
scattered foci of neovascularity
| Prognosis: | mostly results in death within 12 months |
| DDx: | mesenchymal hamartoma (a) solid lesion + cystic degeneration: hepatocellular carcinoma, fibrolamellar carcinoma, intrahepatic cholangiocarcinoma, angiosarcoma, epithelioid hemangio-endothelioma, other sarcomas, lymphoma, metastatic disease, hepatocellular adenoma (b) solitary cystic lesion: biliary cystadenoma/biliary carcinoma, cystic degeneration of hepatocellular carcinoma, bacterial/parasitic abscess, metastatic disease, posttraumatic resolving hematoma |
Wandering Spleen
= ABERRANT/FLOATING/PTOTIC/DRIFTING/DYSTOPIC/DISPLACED/PROLAPSED SPLEEN
= excessively mobile spleen on an elongated pedicle displaced from its usual position in LUQ
| Cause: | embryologically absent/malformed gastrosplenic + splenorenal ligaments; deficient/lax abdominal musculature (prune-belly syndrome, pregnancy) |
| Age: | any (higher frequency in women of childbearing age) |
asymptomatic mobile abdominal/pelvic mass
chronic vague lower abdominal/back pain
nausea, vomiting, eructation, flatulence
acute abdomen (with splenic infarction from torsion)
 empty splenic fossa + associated soft-tissue mass in center of abdomen/pelvis
empty splenic fossa + associated soft-tissue mass in center of abdomen/pelvis inverted malpositioned stomach
inverted malpositioned stomach splenic hilum often located anteriorly
splenic hilum often located anteriorly displaced large spleen (congestion during torsion)
displaced large spleen (congestion during torsion)Cx:
Torsion with prolonged venous occlusion: perisplenitis, localized peritonitis, adhesions, venous thrombosis, hypersplenism
 no flow within spleen on Doppler US
no flow within spleen on Doppler US elevated resistive index in proximal splenic artery
elevated resistive index in proximal splenic artery low attenuation with heterogeneous enhancement on CT
low attenuation with heterogeneous enhancement on CT whorled appearance of twisted splenic pedicle
whorled appearance of twisted splenic pedicle
Torsion with arterial occlusion: hemorrhagic infarction, subcapsular/intrasplenic hemorrhage, gangrene, degenerative cysts, functional asplenism
GI complications:
@ Stomach: compression, distension, volvulus, traction diverticulum, varices
@ Small bowel: dilatation, obstruction
@ Colon: compression, volvulus, laxity, ptosis
| Rx: | 1. Splenectomy (4% postsplenectomy sepsis) 2. Splenopexy 3. Conservative treatment (if asymptomatic) |
EAN: 2147483647
Pages: 24
- Chapter I e-Search: A Conceptual Framework of Online Consumer Behavior
- Chapter VII Objective and Perceived Complexity and Their Impacts on Internet Communication
- Chapter VIII Personalization Systems and Their Deployment as Web Site Interface Design Decisions
- Chapter XV Customer Trust in Online Commerce
- Chapter XVIII Web Systems Design, Litigation, and Online Consumer Behavior