6 - Observation Unit - Treatment Protocols
Editors: Peacock, W. Frank
Title: Short Stay Management of Heart Failure, 1st Edition
Copyright 2006 Lippincott Williams & Wilkins
> Table of Contents > 6 - Observation Unit Treatment Protocols
6
Observation Unit Treatment Protocols
J. Douglas Kirk
Introduction
Although there are guidelines from various sources about the management of patients with heart failure, most pertain to chronic management.1 Limited data are available from randomized controlled trials of acute decompensated heart failure (ADHF) patients in the emergency department (ED), much less the observation unit (OU). As a result, little consensus exists regarding their management, adding to the inconsistent care. This chapter focuses on therapeutic management, with respect to general supportive measures, pharmacologic therapy, and, most important, specific treatment protocols or algorithms that can be implemented in your institution.
General Support
The majority of patients admitted to the OU with ADHF have a chief complaint of dyspnea, and supplemental oxygen should be administered initially in essentially all patients. Pulse oximetry should be used to measure the effectiveness, with a target of maintaining an oxygen saturation of 95% or greater. This may require high-flow oxygen by facemask in some patients, whereas others may need oxygen only by nasal cannula.
Patients with severe dyspnea or hypoxia, typically seen in cases of flash pulmonary edema from severe hypertension and diastolic dysfunction (a unique syndrome more related to hemodynamic mismatch 2 than true cardiac failure ), may require more aggressive airway maneuvers. In such cases, endotracheal intubation may be warranted or inevitable, but every attempt should be made to avoid intubation because of its associated morbidity in these patients. Obviously, patients this ill are not good candidates for OU care. However, the use of aggressive airway adjuncts such as noninvasive ventilation (NIV) may assist in avoiding the need for intubation while maintaining adequate oxygenation and ventilation. NIV should not be considered a substitute for intubation or, more importantly, other pharmacologic management but rather as a bridge to therapies
P.55
directed at reducing filling pressures and pulmonary congestion. Further, brief periods of NIV should not exclude patients from the OU by definition, especially in patients with acute pulmonary edema from hemodynamic mismatch.
Initial Management of Acute Pulmonary Edema
Although many patients with acute pulmonary edema are too sick for subsequent OU management, a number will turn around quickly with aggressive ED treatment, particularly those with hemodynamic mismatch. Concurrent with the previously mentioned airway maneuvers, all efforts should be directed at reducing pulmonary congestion. The most rapid improvement will be achieved with potent vasodilators such as nitroglycerin, nesiritide, or nitroprusside. Although each is quite effective, their immediate intravenous use often requires too much time to set up, a luxury these patients may not have. Initiation of sublingual nitroglycerin therapy, in doses larger than those typically used for chest pain (two to six 0.4-mg tablets or sprays) can be quite effective.3 One can achieve significant reductions in pulmonary capillary wedge pressure and blood pressure (afterload) with an improvement in respiratory symptoms, often within minutes. Patients can then be transitioned to one of the aforementioned intravenous vasodilators and typically are reasonable candidates for the OU.
The addition of an intravenous diuretic to this strategy is common and makes some practical sense because it will result in significant diuresis and hence a drop in preload, although probably not immediately. However, a number of these patients do not suffer necessarily as much from total fluid overload as from maldistribution of fluid into the pulmonary bed. By limiting diuretic use in these patients, important deleterious effects may be avoided, confirming the primary role of vasodilators in this population (Table 6-1). Although the data are limited in the use of any of these agents in the setting of acute pulmonary edema and respiratory distress, there appears to be an immediate benefit from rapid administration of sublingual nitroglycerin with or without an intravenous loop diuretic. Further recommendations on the use of these agents cannot be made until further research elucidates the utility and safety of such an approach.
Pharmacologic Therapy
Although general supportive measures such as maintaining adequate oxygenation are critical, the mainstay of therapy is pharmacologic. The primary goal is to decrease filling pressures. An additional important goal is improving cardiac output through a reduction in afterload or improvement in contractility. In patients with diastolic dysfunction, improving the ventricle's ability to fill with blood is key, through efforts to improve myocardial relaxation.
P.56
TABLE 6-1 Untoward Effects of Therapeutic Agents for ADHF | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Diuretics
Diuretics are often first-line therapy in the OU management of patients with ADHF and as such have become a mainstay of many treatment protocols in the OU. The rationale is that patients are volume overloaded, and, although this may be true, more important than a total increase in volume is the acute elevation in filling pressures. Nonetheless, diuretics are effective in reducing preload and removing excess fluid. The loop diuretic furosemide is most commonly used, although other loop diuretics are equally effective. Suggested starting doses are 40 mg of intravenous furosemide in diuretic naive patients or an amount equivalent to the patient's total usual daily dose given intravenously. Peak diuresis should occur within 30 to 60 minutes. Repeated doses, in some instances double the initial dose, are often used in patients who fail to respond. Doses greater than 160 mg of furosemide are likely to produce as many side effects as results and should be discouraged. In patients with diuretic resistance, use of an additional diuretic that works on the proximal tubule (e.g., metolazone) may produce an effective diuresis. Caution should be exercised with excessive diuretic use. In addition to the well-described electrolyte depletion (K+, Mg+2), recent literature demonstrates that diuretics result in decreased renal perfusion and neurohormonal activation by increasing renin and norepinepherine.4,5 The short-term gains with diuretic therapy may be offset by the decrease in renal perfusion and resultant deleterious long-term effects (Table 6-1).
Vasodilators
A minority of patients have mild exacerbations of ADHF and therapy with oxygen and loop diuretics may be sufficient, especially if their visit is due to brief periods of medical or dietary noncompliance. However, this frequently
P.57
is not adequate and the addition of vasodilators becomes necessary, particularly in patients with severe hypertension and diastolic dysfunction. Most are well perfused and hence are best treated with vasodilators such as nitroglycerin, nesiritide, or nitroprusside. Some patients with mild ADHF may respond to sublingual, oral, topical, or intravenous nitrates, and several OU treatment protocols advocate this approach.3,6 Others have promoted the use of sublingual angiotensin-converting enzyme inhibitors (ACEIs) in this setting, based largely on a small trial of 22 patients who showed symptomatic improvement after treatment with sublingual captopril.7
Data from ADHERE, a multicenter heart failure registry, suggest that patients treated with an intravenous vasodilator initiated in the ED versus later in the hospital or not at all had lower mortality (4.3% vs. 10.9%, unadjusted, p <0.0001) and shorter hospital lengths of stay (3 vs. 7 days, p <0.001).8 These data generate some enthusiasm that early goal-directed therapy initiated in the ED or OU may hold promise and further study is warranted.
Despite their widespread acceptance as standard therapy, surprisingly little clinical outcome data exist for the vasodilators nitroglycerin and nitroprusside to support their use in ADHF. Physician familiarity with nitroglycerin use in patients with chest pain makes the combination of nitroglycerin and diuretics frequent first-line therapy. Nitroprusside can also be particularly useful in patients with acute pulmonary edema associated with severe hypertension but its use is uncommon. However, there are several limitations to these therapies, including the deleterious effects of neurohormonal activation and the need for titration and hemodynamic monitoring (Table 6-1). The latter two characteristics make these agents ill-suited for use in the OU. This has led to a search for better therapeutic agents, ideally ones that improve acute symptoms and hemodynamics as well as mortality. Further, ease of use is an important consideration in choosing an agent to be used in the OU.
Natriuretic Peptides
Nesiritide is the only intravenous vasodilator studied to date in the OU environment. Approved by the Food and Drug Administration in 2001, it became the first commercially available natriuretic peptide used for the treatment of ADHF. It is identical to human endogenous B-type natriuretic peptide (BNP) and serves as an antagonist to pathologic neurohormonal activation that occurs in heart failure. This feature is common among heart failure pharmacologic agents with proven mortality benefit, including ACEIs and beta-blockers. Its most important effect is the general counterbalancing of vasoconstrictive neurohormones in patients with poor cardiac output.
Nesiritide produces significant reductions in pulmonary capillary wedge pressure, right atrial pressure, and systemic venous resistance within minutes and concomitant increases in stroke volume and cardiac output.9 In addition, it does not possess many of the untoward properties associated with diuretics, inotropes, or other vasodilators (Table 6-1). In the PRECEDENT
P.58
trial, a comparison of nesiritide with dobutamine, the investigators found fewer arrhythmias and no increase in heart rate with nesiritide.10 Data from the VMAC trial demonstrated that nesiritide decreased pulmonary capillary wedge pressure more than either nitroglycerin or standard therapy at 3 hours and more than nitroglycerin at 24 hours.11 Dyspnea and global clinical status were improved compared with standard therapy and similar to that of nitroglycerin. In addition, nesiritide's hemodynamic effects were longer lasting, without a need for up-titration, which was frequently necessary in the nitroglycerin group to maintain adequate reduction in wedge pressure.12 To date nesiritide is the only therapy that has been shown in randomized controlled trials of ADHF to provide significant symptomatic and hemodynamic improvement compared with placebo plus standard care.11 However, it has not been studied in a trial prospectively designed or adequately powered to evaluate its effect on mortality, and some have questioned its safety.13
Nesiritide possesses several characteristics that provide convenience and ease of use: (a) no proarrhythmic effect, (b) no tachyphylaxis, and (c) no need for titration [hence not mandated for intensive care unit (ICU) use], making it quite suitable for the ED or OU population.14
In 237 OU patients randomized to either standard care or at least 12 hours of nesiritide therapy in the PROACTION trial,15 the investigators report nesiritide use was associated with a substantial decrease in the sum length of stay over the ensuing month after the index OU visit (2.5 vs. 6.5 days, p <0.032). Mortality and complications were uncommon and not statistically different between the two groups.
Nesiritide is typically administered as a bolus of 2 g per kilogram followed by an infusion of 0.01 mg/kg/minute. In patients with relatively low systolic blood pressure (SBP) (90 110 mm Hg), the bolus can be reduced or eliminated altogether. If hypotension occurs, the infusion should be discontinued. It may be restarted at a lower dose when the blood pressure has stabilized, but this decision will vary on a case-by-case basis. Diuretics may be continued but typically at lower doses due to the potentiation of effects from nesiritide. ACEIs may also be used but not at the expense of curtailing the dose of nesiritide. The use of beta-blockers is not contraindicated, although their use in patients with ADHF is typically reduced or discontinued until patients are more hemodynamically stable. After therapeutic targets have been achieved, typically within 12 to 24 hours, nesiritide may be discontinued and patients should be started on proven mortality-reducing outpatient regimens (ACEI, beta-blocker, aldosterone antagonist) with appropriate adjustments in dosing based on clinical status.
Inotropes
The use of inotropes has essentially no role in the OU management of patients with ADHF. Patients exhibiting clear signs of decreased perfusion or overt cardiogenic shock should be managed in an ICU with appropriate hemodynamic monitoring, and thus further discussion here is not warranted.
P.59
Management Algorithms in the Observation Unit
Appropriate management of ADHF in the ED and/or OU is challenging. Conclusive evidence identifying suitable patients who clearly benefit from a particular therapy is lacking, as are specific guidelines to drive management. It does appear, however, that patient risk stratification16 and initiation of aggressive treatment in the ED8 may limit potentially irreversible myocardial toxicity, especially in those with moderate to severe ADHF. The algorithm depicted in Figure 6-1 attempts to provide some guidance for the diagnostic and prognostic evaluation of the suspected ADHF patient, in addition to recommendations for therapeutic strategies and disposition decisions.17 For the purposes of this chapter's focus on OU care, sections pertaining to the potentially life-threatening complications of respiratory failure or cardiogenic shock are not discussed in detail.
Using typical historical, physical examination, and diagnostic test features, a clinical profile is defined, identifying patients in whom pulmonary congestion predominates the clinical presentation versus those with more of an element of hypoperfusion. A minority of patients will have mild exacerbations of ADHF, and the mainstay of therapy for them may be intravenous diuretics, particularly if they have been noncompliant with diet or medications. Topical or sublingual nitrates may be warranted if moderate hypertension (SBP 140 160 mm Hg) is present or a history of diastolic dysfunction exists.
The majority of ADHF visits are of moderate severity and often characterized by significant hypertension. An abrupt increase in blood pressure may precipitate acute pulmonary edema, especially in patients with diastolic dysfunction. This presentation is more related to hemodynamic mismatch. Accordingly, the clinical target is blood pressure control with early, aggressive vasodilation, more so than diuresis. This is particularly true when pulmonary congestion is related to fluid maldistribution more than an increase in total fluid volume. Preliminary data suggest these patients should be aggressively treated with intravenous vasodilators early in the ED course,8 and a substantial number may be appropriate for OU management.15 The ideal choice of a specific vasodilator in these cases can be difficult. Nitroglycerin and loop diuretics are effective in symptomatically improving these patients but are associated with neurohormonal activation and several limitations to their ED or OU use (Table 6-1). From a practical standpoint, the most important limitation is the need for titration and admission to an ICU. In many institutions, this renders these agents ineffectual because their use would not be permitted in the OU setting. If for no other reason, the use of nesiritide and its characteristic ease of use may play a significant role here. Using the guidelines to estimate severity from Figure 6-1, the majority of patients classified as moderate risk and those at low risk can all be managed in an OU.
P.60
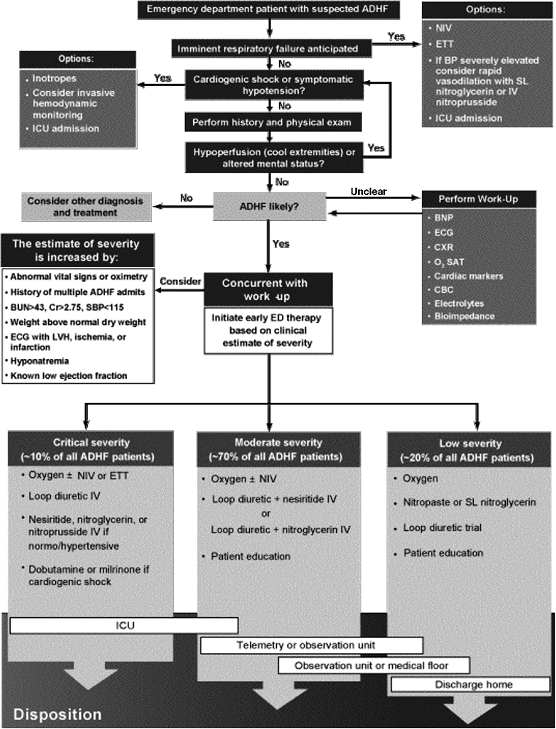 |
FIGURE 6-1 Algorithm for the early stabilization of acute decompensated heart failure in the emergency department. ADHF, acute decompensated heart failure; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CBC, complete blood count; Cr, creatinine; CXR, chest radiograph; ECG, electrocardiogram; ETT, endotracheal tube; ICU, intensive care unit; LVH, left ventricular hypertrophy; NIV, noninvasive ventilation; O2SAT, oxygen saturation; prn, as needed; SBP, systolic blood pressure; SL, sublingual. (Adapted from Peacock WF, Allegra J, Ander D, et al. Management of acutely decompensated heart failure in the emergency department. CHF 2003;9[Suppl 1]:3 18.) |
P.61
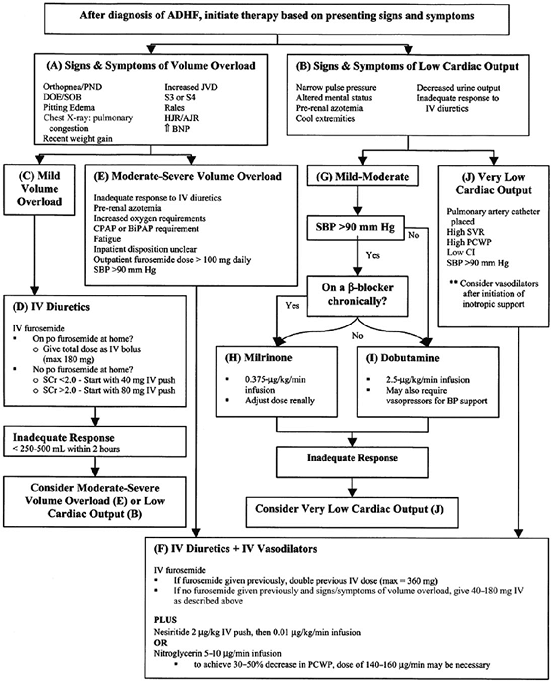 |
FIGURE 6-2 Acute decompensated heart failure (ADHF) treatment algorithm. AJR, abdominal jugular reflex; BiPAP, bilevel positive airway pressure; BNP, B-natriuretic peptide; CI, cardiac index; CPAP, continuous positive airway pressure; DOE, dyspnea on exertion; HJR, hepatojugular reflex; JVD, jugular venous distention; PCWP, pulmonary capillary wedge pressure; PND, paroxysmal nocturnal dyspnea; SBP, systolic blood pressure; SCr, serum creatinine; SOB, shortness of breath; SVR, systemic vascular resistance. (Adapted from DiDomenico RJ, Park HY, Southworth MR, et al. Guidelines for acute decompensated heart failure treatment. Ann Pharmacother 2004;38:649 660, with permission.) |
Another algorithm with more patient-specific treatment recommendations for management of ADHF in the OU was reported by DiDomenico et al.18 and is described in Figure 6-2. The timeline for key elements of these guidelines is depicted in Figure 6-3 and provides
P.62
clinicians with specific clinical targets that should be achieved during the OU stay. The authors felt strongly that the diagnosis of ADHF should be established within 2 hours after presentation to the ED. Intravenous therapy should be initiated within 2 hours of establishing the diagnosis, and within 2 hours of initiating intravenous therapy the patient's response should be assessed and additional therapy added as necessary. Over the following 6 to 8 hours, reassessment of the patient's response should continue, and after 12 hours of observation, disposition should be determined (i.e., hospital admission or discharge home). Transfer out of the OU should then proceed within 24 hours of the initial OU contact.
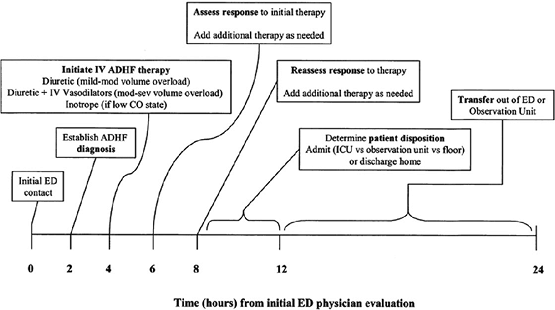 |
FIGURE 6-3 Timeline for the management of acute decompensated heart failure (ADHF) in the emergency department/observation unit. CO, cardiac output; ED, emergency department; ICU, intensive care unit; mod-sev, moderate to severe. (Adapted from DiDomenico RJ, Park HY, Southworth MR, et al. Guidelines for acute decompensated heart failure treatment. Ann Pharmacother 2004;38:649 660.) |
In this strategy, treatment of ADHF is generally based on the presence or absence of volume overload and an assessment of the patient's cardiac output. On the left side of Figure 6-2 (boxes A, C, D, E, and F) treatment recommendations are given for patients with ADHF experiencing signs and symptoms of volume overload, manifested by pulmonary congestion. One of the limitations of this algorithm is grouping all patients with pulmonary congestion together, regardless of the etiology. There is no consideration of the patient with hemodynamic mismatch and acute pulmonary edema, whose primary therapy should be control of blood pressure with intravenous vasodilators. Nonetheless, it is quite helpful with general management principles. The right side of the algorithm provides treatment recommendations for patients with low cardiac output.
P.63
In most OUs these patients are excluded, and therefore little discussion of this component of the algorithm is warranted.
The major emphasis of this protocol is managing volume overload, which is further divided into mild and moderate-severe groups. Patients with mild volume overload (Figure 6-2, box C) are treated with intravenous diuretic therapy, typically loop diuretics (Figure 6-2, box D). Dosages in patients previously taking diuretics are guided by the total home daily dose, given as an intravenous bolus. Therapy for patients not taking oral diuretics at home is based on renal function, although clinicians should exercise caution with diuretic therapy in such patients to avoid further worsening of renal function. Success of diuretic therapy is driven by urine output goals and recommendations for repeat diuretic dosing are described in the algorithm. Again, caution should be exercised with extremely high doses of loop diuretics. In addition to the prerenal azotemia, electrolyte abnormalities, particularly hypokalemia and hypomagnesemia, are common and should be recognized and treated quickly. A management strategy for electrolyte disturbances in this setting is included in the accompanying standing orders (Figure 6-4).
The authors recognize that patients with more severe volume overload, which typically includes those with hemodynamic mismatch and resultant acute pulmonary edema, are likely to have an inadequate response to intravenous diuretic therapy alone. In these patients, the initial pharmacologic regimen should be more aggressive and include both an intravenous diuretic and a parenteral vasodilator (Figure 6-2, box F). Intravenous nitroglycerin or nesiritide should be used to produce a more rapid response and more effectively relieve the signs and symptoms of congestion in these patients. No specific recommendations are provided as to which vasodilator should be used. However, untoward effects of conventional vasodilators (Table 6-1) and ease of use characteristics associated with nesiritide therapy should be considered when choosing the optimal agent to be used in the OU. Further, the suggested starting dose of nitroglycerin (5 10 g per minute) noted in Figure 6-2, box F should be considerably higher.
Corresponding physician order sets for ADHF management in the OU have been developed and are presented in Figure 6-4. These are a vital part of any OU management algorithm and are typically necessary to standardize the evaluation and treatment of ADHF patients. These orders are for sample purposes only and should be modified accordingly to accommodate institutional variations in practice. Again, the inclusion of orders for inotropic therapy in this example is typically not permitted in the majority of observation units.
Similar algorithms have also been published by other groups and warrant mention here to provide alternatives and to highlight important differences in OU management strategies. The Midwest Heart Specialists Heart Failure Program produced an algorithm for the early goal-directed therapy of ADHF patients (Figure 6-5).19 Several key decision points are
P.64
emphasized: (a) early and accurate diagnosis of ADHF with clinical variables and BNP testing; (b) identification of those ADHF patients with shock, followed by prompt admission; (c) treatment of the ADHF patient with renal insufficiency with vasoactive medication; and (d) frequent
P.65
re-evaluation of the ED/OU treated patient to identify early treatment failures, followed by prompt institution of vasoactive therapy in these patients. In many instances, treatment recommendations are similar to those proposed by DiDomenico et al.18 However, important differences include distinguishing patients with renal insufficiency and instituting early intravenous nesiritide
P.66
P.67
plus continuous infusion of a loop diuretic or bolus loop diuretic. Nitroglycerin therapy is not included in this approach. Also, patients without renal insufficiency are treated with bolus loop diuretic monotherapy and reassessed every 2 to 4 hours. Those who fail to improve and/or do not achieve urine output targets are started on continuous infusion loop diuretics. Failure to improve by 6 to 8 hours prompts additional therapy with intravenous nesiritide. Similar to the aforementioned algorithm,18 patients with acute pulmonary edema due to hemodynamic mismatch are not distinguished with respect to initial therapy with intravenous vasodilators.
 |
FIGURE 6-4 Physician order set for the initial management of acute decompensated heart failure in the emergency department/observation unit. AP, anterior/posterior; BNP, B-natriuretic peptide; BUN, blood urea nitrogen; CBC, complete blood cell count; CK, creatine kinase; CK-MB, creatine kinase MB isoenzyme; ECG, electrocardiogram; INR, international normalized ratio; IVP, intravenous push; PT, prothrombin time; PTT, partial thromboplastin time; SBP, systolic blood pressure; SCr, serum creatinine; Clcr, creatinine clearance. (Adapted from DiDomenico RJ, Park HY, Southworth MR, et al. Guidelines for acute decompensated heart failure treatment. Ann Pharmacother 2004;38: 649 660.) |
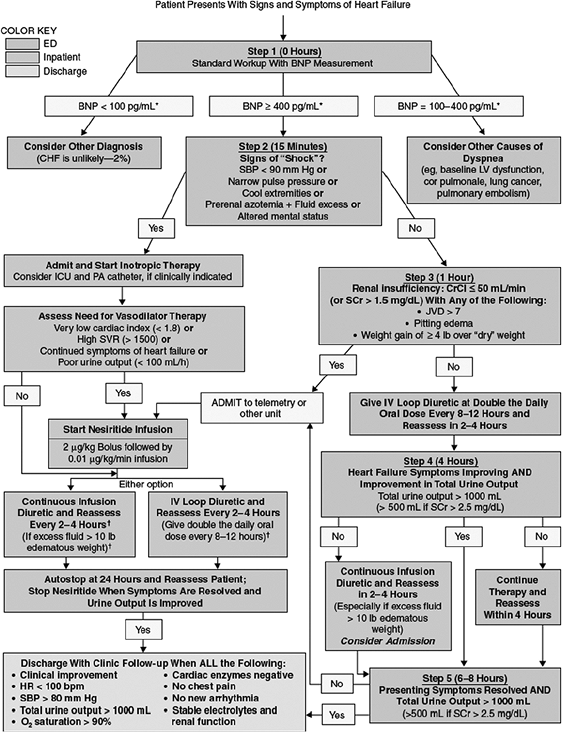 |
FIGURE 6-5 Algorithm for early goal-directed therapy for acute decompensated heart failure. ED, emergency department; BNP, B-type natriuretic peptide; CHF, congestive heart failure; ICU, intensive care unit; PA, pulmonary artery; SVR, systemic vascular resistance; HR, heart rate; SBP, systolic blood pressure; LV, left ventricular; CrCl, creatinine clearance; SCr, serum creatinine; JVD, jugular venous distention. *Clinical decisions should not be based solely on BNP level. BNP levels shown are for the Triage (Biosite) assay. Consider decreasing dose of diuretic by 50% if receiving nesiritide. (Adapted from Saltzberg MT. Beneficial effects of early initiation of vasoactive agents in patients with acute decompensated heart failure. Rev Car diovasc Med 2004;5[Suppl 4]:S17 S27.) |
Using the Midwest heart algorithm, an analysis of data before and after implementation provided insight into the potential impact of such an approach.19 Hospital and telemetry unit length of stay was significantly reduced among patients with concomitant renal insufficiency and those requiring intravenous vasoactive therapy. Vasoactive use increased after algorithm utilization but still was seen in only one third of all hospitalized ADHF patients. In patients with renal insufficiency, the treatment algorithm resulted in a doubling of nesiritide use when compared with similar patients for whom the algorithm was not used. Nesiritide use as an overall percentage of vasoactive therapy (50%) was unchanged, and only 15% of all ADHF patients who were subsequently admitted had been treated with nesiritide. Despite the increased use of nesiritide, overall drug costs and costs associated with vasoactive therapy were actually reduced, suggesting that an early goal-directed approach can be implemented in a cost-effective manner while simultaneously improving outcomes.
Conclusions
Evidence-based guidelines for the management of ADHF patients in the ED and OU are lacking. Although treatment protocols and management algorithms appear vital to the success of any OU strategy, they are currently based largely on anecdotal experience or, at best, data from small trials. Cornerstones of these algorithms are appropriate patient risk stratification and recognition of those primarily with pulmonary congestion versus those with cardiogenic shock. Further delineation based on (a) severity of volume overload, (b) associated renal insufficiency, or (c) hemodynamic mismatch as an etiology appears to aid with management decisions. Unfortunately, there is no consensus among authors regarding an overall approach. Systematic use of therapeutic agents (intravenous diuretics and vasodilators) with a priori defined clinical targets is a must. Further, data seem to support the early use of vasodilators in the ED or OU,8 and nesiritide in particular appears well suited for the OU setting.15 Additional recommendations await publication of institutional experience with algorithms such as those presented here.
P.68
References
1. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2001;38:2101 2113.
2. Gheorghiade M, Zannad F, Sopko G, et al. for The International Working Group on Acute Heart Failure Syndrome. Acute heart failure syndrome: current state and framework for future research. Circulation 2005;112(25):3958 3968.
3. Bussmann W, Schupp D. Effect of sublingual nitroglycerin in emergency treatment of severe pulmonary edema. Am J Cardiol 1978;41:931 936.
4. Gottlieb SS, Brater DC, Thomas I, et al. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation 2002;105:1348 1353.
5. Brewster UC, Setaro JF, Perazella MA. The renin-angiotensin-aldosterone system: cardiorenal effects and implications for renal and cardiovascular disease states. Am J Med Sci 2003;326:15 24.
6. Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet 1998;351:389 393.
7. Hamilton RJ, Carter WA, Gallagher EJ. Rapid improvement of acute pulmonary edema with sublingual captopril. Acad Emerg Med 1996;3:205 212.
8. Peacock WF, Emerman CL, Costanzo MR, et al. Early initiation of intravenous vasoactive therapy improves heart failure outcomes: an analysis from the ADHERE registry database. Ann Emerg Med 2003;42:S26.
9. Mills RM, LeJemtel TH, Horton DP, et al. On behalf of the Natrecor Study Group. Sustained hemodynamic effects of an infusion of nesiritide (human B-type natriuretic peptide) in heart failure: a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol 1999;34:155.
10. Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J 2002;144: 1102 1108.
11. Publication Committee for the VMAC Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA 2002;287:1531 1540.
12. Elkayam U, Akhter MW, Singh H, et al. Comparison of effects on left ventricular filling pressure of intravenous nesiritide and high-dose nitroglycerin in patients with decompensated heart failure. Am J Cardiol 2004;93:237 240.
13. Sackner-Bernstein JD, Kowalski M, Fox M. Short-term risk of death after treatment with nesiritide for decompensated heart failure. A pooled analysis of randomized controlled trials. JAMA 2005;293:1900 1905.
14. Fonarow GC, ADHERE Scientific Advisory Committee. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med 2003;4[Suppl 7]: S21 S30.
15. Peacock WF, Holland R, Gyarmathy R, et al. Observation unit treatment of heart failure with nesiritide: results from the PROACTION trial. J Emerg Med 2005;29(3):243 252.
16. Fonarow GC, Adams KF Jr, Abraham WT, et al, for the ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572 580.
P.69
17. Peacock WF, Allegra J, Ander D, et al. Management of acutely decompensated heart failure in the emergency department. CHF 2003;9[Suppl 1]:3 18.
18. DiDomenico RJ, Park HY, Southworth MR, et al. Guidelines for acute decompensated heart failure treatment. Ann Pharmacother 2004;38:649 660.
19. Saltzberg MT. Beneficial effects of early initiation of vasoactive agents in patients with acute decompensated heart failure. Rev Cardiovasc Med 2004;5[Suppl 4]:S17 S27.
EAN: 2147483647
Pages: 18