79 - Compression of the Trachea by Vascular Rings
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XIV - Congenital, Structural, and Inflammatory Diseases of the Lung > Chapter 93 - Solitary Pulmonary Nodule
function show_scrollbar() {}
Chapter 93
Solitary Pulmonary Nodule
Ronald B. Ponn
In contrast to diffuse lung disease, discussed in Chapter 94, a single focal pulmonary density, usually termed a solitary pulmonary nodule (SPN), is frequently asymptomatic and often due to neoplasm. Most lung neoplasms are malignant. In this category, the incidence of lung cancer greatly exceeds that of all other primary and metastatic tumors. Many lung cancers that present as an SPN are early-stage lesions and potentially curable by resection. Likewise, isolated uncommon primary parenchymal malignant lung tumors, such as carcinoids and salivary gland-type tumors (see Chapters 116 and 117), are associated with a high surgical cure rate. Numerous reports also suggest that there is benefit from resection of tumors metastatic to the lung from a variety of primary sites. Although not universally confirmed, patients with a single metastasis may have a longer disease-free survival following resection than do those with multiple foci (see Chapter 120). In contrast to the benefit of resection for localized malignant lung neoplasms, resection of benign tumors and nonneoplastic nodules is almost always purely diagnostic, hence unnecessary, at least from a therapeutic standpoint. When a lung density is detected by imaging, therefore, the clinical imperative is either to establish a specific diagnosis or, in the absence of certainty, to reach a high level of probability that the lesion is benign and that a strategy of periodic follow-up is appropriate. This goal should be accomplished in a timely fashion with the least discomfort, morbidity, and cost. The general thoracic surgeon should be sufficiently familiar with the spectrum of focal lung lesions and their features in order to participate knowledgeably in the decision pathway with respect to whether, when, and how to pursue invasive diagnostic procedures.
Although approaches to the SPN vary, it is clear that the rate of unnecessary thoracotomy (i.e., open resection for benign disease) has decreased markedly over time. In the era before computed tomography (CT), Steele (1963) reported a cooperative Veterans Administration Armed Forces study in which only 36% of 941 solitary lung nodules resected by full posterolateral thoracotomy between 1958 and 1963 were malignant. In contemporaneous series, malignancy rates for surgically removed lesions varied from as low as 10% to as high as 68%. In a later study, Toomes and associates (1983) found malignancy in just less than one half of 955 nodules resected between 1970 and 1980. Using the same inclusion criteria as the earlier review applied to a similar population of male veterans, Rubins and Rubins (1996) analyzed all solitary nodules removed without a preoperative diagnosis at their institution between 1981 and 1994. In contrast to the earlier experience, 79% of the masses were malignant, and 94% were primary lung cancers. This dramatic improvement is due to advances in thoracic imaging.
The judicious application of modern and continuously improving imaging technology and newer alternative biopsy modalities should continue to reduce the necessity for major interventions in patients with SPN, without an increase in missed or delayed detection of cancer arising in or metastatic to the lung. In the past, the options for diagnosing an SPN were a simple dichotomy: watch it or wedge it. In practice, the choices were either a period of observation by serial plain films or linear tomograms versus early thoracotomy for diagnosis and possibly treatment or later thoracotomy prompted by radiographic change. Many thoracic physicians and surgeons justifiably advised resection of all SPNs that did not have definitive radiographic signs of benignancy or documented stability. Currently, our options have expanded greatly. We can watch it over time by plain film or vastly more sensitive imaging techniques. Similarly, tissue diagnosis no longer always requires us to wedge it by posterolateral thoracotomy because flexible bronchoscopy, percutaneous transthoracic biopsy, limited incisions, and video-assisted thoracic surgery are often reliable alternatives.
DEFINITION
There is no universally accepted definition of what constitutes an SPN. Variations in nomenclature are based on the size and characteristics of the density and the presence or absence of other radiographic abnormalities. Some authors
P.1318
include lesions as large as 6 cm, whereas others limit an SPN to 3 or 4 cm in maximum diameter, a larger opacity being a mass. A few series even apply a lower size limit of 1 cm. Some use SPN to denote a small spherical, smoothly marginated density entirely surrounded by lung parenchyma the classic coin lesion. Nodules with air bronchograms or cavitation are variously included or excluded. The absence of other radiographic abnormalities, such as lymphadenopathy or satellite parenchymal lesions, is variably required for defining an SPN. Some require that an SPN be discovered on a plain chest film rather than other imaging studies such as CT. Another confounding factor is that sometimes patients with symptoms are included, whereas other reports discuss only the asymptomatic SPN. Clearly, the incidence of malignancy and the conclusions of any series will vary depending on the definition. For the most part, these specific definitions are arbitrary and clinically unhelpful because the approach to the patient is rarely changed significantly by their application. Absent clear signs of benignancy or malignancy, the size, contour, and associated findings relate far more to how best to make a reliable diagnosis than to debating what constitutes a true SPN. This is especially true currently, when nodules smaller than those included in the older SPN literature are detected by more sensitive modalities and are presented daily to medical, surgical, and radiology chest physicians. The question is not one of definitional minutiae, but rather by what means we ensure that cancers are not being missed, while simultaneously not overusing resources or harming patients.
The emphasis of this chapter is on the approach to the patient with a radiographic lung density that may represent cancer and may benefit from thoracic surgical evaluation. Based on these considerations, a narrow definition of SPN would be insufficiently inclusive. SPN will be used to encompass lesions that are, or at the time of discovery by any imaging modality appear to be, located in the lung, are predominantly solid (i.e., nodular or masslike rather than infiltrative and diffuse), and do not have clearcut signs of malignancy such as invasion or metastasis to lymph nodes, bone, or other areas on the basis of the presentation radiographs. Two major changes since the prior edition of this text have greatly affected discussion of the SPN. First, screening CT scan as a means of identifying an SPN that is missed by plain films is receiving intense interest and increasing the number of patients who present with an SPN. Second, there is more experience with and greater availability of positron emission tomography (PET) as a means of differentiating benign from malignant nodules. The impetus for the proliferation of PET is based both on science and on the willingness of many payers to approve this study in cases of proven or suspected lung cancer a vignette of modern medicine that is beyond the scope of this chapter and is not at all unique to lung cancer.
An SPN that cannot be classified radiographically with certainty as benign or malignant is an indeterminate nodule. A nodule that is indeterminate at presentation may be diagnosed as benign after additional radiographic studies or review of prior films. If a benign etiology is not proven by imaging criteria or biopsy, the lesion remains indeterminate, even when the suspicion of malignancy is low.
PREVALENCE OF SOLITARY PULMONARY NODULE AND INCIDENCE OF MALIGNANCY
The prevalence of SPNs in the general population is unknown. The reported detection rate varies depending on the definition of SPN, the study population, and increasingly, the imaging modalities employed. A higher rate of detection is expected in older people, smokers, people with a current or prior extrapulmonary neoplasm, and those who live in areas where fungal or mycobacterial lung infections are common (see Chapter 89). Good and Wilson (1958) reported finding two SPNs per 1,000 chest radiographs. Because of superior resolution, CT is a more sensitive technique than conventional radiography for detecting parenchymal nodules. Edwards and Fry (1982) reviewed 100 consecutive CT scans obtained to evaluate nonpulmonary thoracic abnormalities in patients without known malignant disease. They detected a solitary nodule in two of the 100 studies, twice the rate of plain films. In a group of 25 patients with known malignancy, Schaner and associates (1978) found 15 nodules by CT that were not suspected by chest films or conventional tomograms. Because 9 of the 15 lesions turned out to be benign, the authors concluded that there is an appreciable prevalence of asymptomatic benign nodules in the general population. Keogan and colleagues (1993) reached a similar conclusion after finding that 16% of 551 lung cancer patients undergoing CT were found to have separate nodules, at least 70% of which were benign.
Although the true prevalence of SPN is uncertain, it is clear that such densities are encountered frequently in clinical practice. Stoller and associates (1988) estimated that in 1987 there were 133,000 newly discovered solitary lung nodules in the United States and emphasized that this figure rivals the annual incidence of other major clinical pulmonary problems, such as lung cancer and the adult respiratory distress syndrome. Lillington (1991) estimated that there are 150,000 new SPN cases per year. The recent interest in screening low-dose helical CT scan in patients who are at higher than normal risk for developing lung cancer was generated by the report by Henschke and colleagues (1999). Screening for lung cancer is discussed in Chapter 99. Although there is a vigorous ongoing debate regarding the risk-to-benefit ratio and the cost effectiveness of this approach, screening CT clearly sheds new light on the prevalence of SPN. In the aforementioned study, noncalcified nodules were detected in 233 of 1,000 screened patients, who were older than 60 years, had at least a 10-pack-year smoking history, had no known cancer, and were fit for resection. It is to the credit of the multidisciplinary group involved
P.1319
in this study that 27 of the 28 patients (among 233 with nodules) who underwent biopsy turned out to have lung cancer, 23 cases of which were stage I lesions. In the series of 1,520 people older than 50 years of age and with a 20-pack-year or more smoking history reported by Swenson and associates (2002), one or more SPNs were identified on CT 1 year after baseline study in an astounding 66% of cases that is, 1,000 patients. Twenty-five cases were lung cancer, and 22 of these underwent potentially curative resection. However, seven patients underwent resection of benign nodules. Although the methodology in these and other studies differs, it is clear that a high false-positive rate exists. For the purposes of this chapter, there are two lessons. First, screening CT has already greatly increased the number of patients with SPN who require attention. Second, even in the high-risk groups, most lesions detected in this manner are benign. Mahadevia and associates (2003) presented a comprehensive analysis of the potential costs and clinical ramifications of screening CT. With respect to incidence, they calculated that if half of Americans aged 45 to 75 years who ever smoked underwent a single scan, 5 million SPNs would be detected. The cost for this program would be at least $115 billion annually. Although, to date, no major thoracic specialty organization has recommended screening CT, direct patient advertising is rampant, and centers for the early detection of lung cancer by CT continue to sprout in institutions with no present or past multidisciplinary thoracic program. It is clearer than ever that the SPN constitutes a major clinical issue that requires knowledgeable evaluation in order to achieve the goals of avoiding unnecessary morbidity, anxiety, and expense, while simultaneously achieving timely detection and treatment of malignancies.
Once an SPN has been identified, what is the likelihood that it is malignant? The surgical literature traditionally cites malignancy rates of 30% to 50%. These figures are based on older analyses of resected lesions, such as the reports of Steele (1963) and Toomes and colleagues (1983) noted earlier. There is a selection bias in favor of finding more cancers in patients who have been directed toward resection, even during an era when early resection was often the most common approach to SPN. During a similar time period, in contrast, the incidence of malignancy in SPN in radiographic general population surveys was much lower. Holin and associates (1959) found an incidence of only 3%. Similarly, only 6% of nodules were ultimately determined to be malignant in the series collected by McClure and colleagues (1961). The importance of geographic location is emphasized by the experience reported by Trunk and colleagues (1974), who reviewed 137 consecutive nodules resected at a U.S. Air Force hospital in Illinois between 1963 and 1971. Benign lesions were identified in more than 84%, most of which (103) were granulomas. In this category, about one half were proved to be due to histoplasmosis, a fungus endemic to the region. As noted earlier, when CT scan is used as the baseline study, even in high-risk cohorts, most SPNs are benign.
None of these figures represents the current odds of malignancy in the global spectrum of SPNs. Although one might speculate that the lung cancer epidemic in recent decades should increase the likelihood of cancer in solitary lesions, the actual proportion is unknown. The more clinically relevant figure, however, is the incidence of malignancy in a lesion that remains indeterminate after modern imaging, nonsurgical biopsy, or both. In a large cooperative study examining the role of CT, Zerhouni and associates (1986) found malignancy in 56% of nodules ultimately diagnosed by biopsy. The rate of malignancy, however, rose to 77% for nodules that were not called benign by imaging criteria. In the 14-year experience reported by Rubins and Rubins (1996), 90% to 100% of resected nodules were malignant in each of the later years of the study, as opposed to less than 60% in the earlier time period. The lowest current malignancy rates occur in series of thoracoscopic wedge excisions of SPNs. In the reports of Mack (1993), Bernard (1996), and DeCamp (1995) and their colleagues, malignancy was found in 48%, 56%, and 60% of cases, respectively. Lower rates in thoracoscopic series are likely related to the emergence of this technique as an effective, low-risk, and definitive method for diagnosing peripheral SPNs and to the resultant inclusion in these series of patients with nodules of lower clinical suspicion, as an alternative to long-term surveillance.
The odds of malignancy in a pulmonary nodule that remains indeterminate after an evaluation that may include combinations of imaging modalities and nonsurgical biopsy is of paramount clinical relevance to thoracic surgeons. This figure is the one that should be used to formulate further recommendations and the one quoted to the patient who must ultimately decide how to proceed.
ETIOLOGY
The differential diagnostic possibilities for an SPN encompass an extensive list of diverse pathologic processes (Table 93-1). Although the reported frequency of benign versus malignant etiologies is subject to case selection bias, it is clear that lung cancer makes up 85% to 90% of malignant lesions, whereas granulomas make up a similar proportion of the benign group. All of the cell types of lung cancer can present as a solitary pulmonary nodule. Although metastatic spread to the lung from extrapulmonary tumors most often manifests as multiple nodules, an isolated deposit is sufficiently common that metastases make up the next most frequent source of malignant SPNs, usually reported in the range of 5% to 10% of resected nodules. Carcinoid tumors account for 1% to 3% of malignant lesions. All other types of malignant primary lung neoplasms are extremely rare (see Chapters 117 and 119).
Benign causes of SPN include neoplastic and nonneoplastic processes. Benign lung tumors are uncommon (see Chapter 118). Despite the long list of histologies in this category,
P.1320
hamartomas account for a higher proportion of benign tumors than do lung cancers among malignant tumors. Fein and co-workers (1998) noted that hamartoma accounted for 192 of 3,802 resected nodules (5%) collated from six large series. Nonneoplastic benign nodules are overwhelmingly more common than benign tumors. In the United States, most are due to granulomas from prior infection with the fungal organisms Histoplasma capsulatum or Coccidioides immitis. In many other parts of the world, granulomatous lesions more commonly represent the residua of pulmonary infection with Mycobacterium tuberculosis. Noninfectious granulomas, such as macronodular sarcoidosis and Wegener's granulomatosis, classically occur as multiple lesions, but may be confined to a single density or a dominant mass. Youssem and Hochholzer (1987) noted that 40% of pulmonary hyalinizing granulomas were solitary. Fichtenbaum and associates (1990) described a case of eosinophilic granuloma, also typically a diffuse reticulonodular process, presenting as an SPN.
Table 93-1. Causes of Solitary Pulmonary Nodules | |
|---|---|
|
Some apparent SPNs are factitious. Kundel and colleagues (1978) reported that up to 20% of subtle opacities considered to be lung nodules on chest film turned out to be due to other causes. Extrapulmonary densities that may simulate nodules include overlapping normal structures, nipple shadows, and soft tissue or osseous lesions of the chest wall. Differentiation from pulmonary nodules is accomplished by the use of markers, repeat radiographs, oblique views, fluoroscopy, spot films, and CT scans. In a series of 502 solitary lung nodules suspected by plain radiography, Huston and Muhm (1987) were able to determine by radiographic means other than CT that 62 opacities (12%) were either nonpulmonary or nonexistent. In current practice, there is a low threshold to proceed to CT scan when any doubt remains.
CLINICAL EVALUATION
A patient's personal profile and medical history may be helpful in the differential diagnosis of an SPN. Older age, male gender, and smoking increase the likelihood of lung cancer. In contrast, lung cancer is uncommon in people younger than 35 years of age. Cummings and associates (1986a) have shown that the probability of malignancy increases in direct proportion to the number of cigarettes consumed per day. Conversely, the likelihood of lung cancer diminishes in ex-smokers concordant with an increasing time interval since smoking cessation. Although the changing epidemiology of lung cancer, notably the increasing incidence in women and possibly more cases presenting at an earlier age and in nonsmokers (see Chapter 98), may have lessened the importance of age, gender, and smoking habits, these factors remain important in assigning probabilities for clinical decision making. Residence and travel history may raise the possibility of a granulomatous nodule. People who currently reside or have previously lived in the midwestern and southwestern United States are more likely to have granulomas due, respectively, to histoplasmosis and coccidioidomycosis. The symptoms that may have occurred during the primary infection are usually mild and nonspecific, temporally remote, and rarely recalled by the patient. A history of exposure to tuberculosis or emigration from countries where mycobacterial infection is endemic may aid in differential diagnosis.
The presence of pulmonary symptoms has been variously reported to correlate with an increased or a decreased chance of malignancy. Although some authors believe that a nodule must be unassociated with any symptoms to be classified as an SPN, this limitation seems artificial because the diagnostic issues are similar in cases with or without symptoms. The presence of symptoms should form part of the clinical synthesis for approaching these lesions. Central masses are more often associated with dyspnea, wheezing,
P.1321
hemoptysis, pneumonia, and sputum production. Peripheral lesions are more often asymptomatic. The presence of a dry, nonspecific cough, however, and even vague chest pain in cases of peripheral subpleural masses, is not uncommon in patients who are ultimately found to harbor malignancy. Acute pulmonary symptoms such as cough productive of purulent sputum, limited hemoptysis, and dyspnea, especially when coupled with systemic complaints such as malaise, fatigue, anorexia, and fever, are more often associated with an acute or subacute infectious or inflammatory process. In this setting, it is reasonable in some cases to follow the patient by plain film for a short period before embarking on more advanced imaging or invasive studies. Although the empiric use of antibiotics is controversial and should be individualized, cultures should be sent and treated if positive.
A history of prior or synchronous extrapulmonary malignancy is an important consideration (see Chapter 120). Multiple new nodules most often indicate metastatic disease. Occasionally, however, multiple densities result from a reaction to chemotherapeutic agents or from infectious processes secondary to immunosuppression from the malignancy or its treatment. As noted earlier, multiple nodules detected synchronously, especially when found by CT rather than plain films, usually represent metastases, but may be benign, especially if they are small. Johnson and associates (1982) noted that less than one third of nodules smaller than 0.5 cm were metastases. Carucci and co-workers (2001) reported that in 31 patients with clustered nodules on CT scan, defined as geographically localized multiple nodules within 1 cm of each other and without a dominant nodule, none proved to be malignant, even though 68% of the images were performed for screening in the setting of known malignancy.
The situation is more complex when a solitary nodule is detected in a patient previously treated for a nonpulmonary cancer. Although a history of malignancy increases the chance that the nodule is a metastasis, a large proportion of SPNs in this setting are due to benign causes or to primary lung cancer. Overall, as emphasized by Coppage and colleagues (1987) and by Davis (1991), single lung nodules in patients with known extrapulmonary cancers more often represent primary lung carcinoma than metastatic foci. The probability that an SPN in a patient with a cancer history is metastatic depends on the type and initial stage of the primary neoplasm as well as the individual's risk for primary lung cancer or a benign process. The more advanced the primary, the more likely that an SPN is a metastasis.
The site of origin of the primary neoplasm also influences the probability of metastasis. At one extreme, newly detected lung opacities in people with germ cell tumors or sarcomas, as reported by Pass and co-workers (1985), prove to be metastatic in most cases. This high probability results from the biologic propensity of these tumors to spread preferentially to the lung, combined with a lower risk for lung cancer due to a younger mean age in this population. Likewise, most new SPNs in patients treated for melanoma are metastatic. Although Pogrebniak and associates (1988) found that many SPNs in melanoma cases were benign, the benign etiologies in their series were rare, including hematoma, Pneumocystis, histiocytosis, and nonspecific pneumonitis. In contrast to primary sites strongly associated with metastasis in an SPN, squamous cancers of the head and neck have a propensity to precede or appear synchronously with primary malignant lung tumors. Smoking is the common factor that puts these individuals at risk for multiple aerodigestive malignancies. About one fourth of head and neck cancer patients develop second primary tumors within 8 years following initial treatment, most occurring in the lung. According to Cahan and co-workers (1977), a new SPN in patients treated for early-stage squamous cancer of the head and neck is a primary lung cancer in more than 90% of instances. Malefatto and associates (1984) found a lower rate of lung cancer (53%) and a higher proportion of benign lesions (28%). The two series are similar, however, in that a minority of the nodules were due to metastasis. Most of the common extrapulmonary adenocarcinomas, such as those of the breast and colon, are associated with an intermediate likelihood of metastasis in an SPN. Cahan and colleagues (1974) found that slightly less than half of synchronous or metachronous SPNs in patients with colon cancer were metastatic deposits, the remainder being primary lung cancers. In a series of patients with breast cancer, the same authors (1975) noted that 32% of SPNs were metastases, 60% lung cancer and 8% benign. Casey and associates (1984) reported a similar experience in breast cancer 43% metastatic, 52% lung cancer, and 5% benign. Although renal cell carcinoma metastasizes to the lung in 30% to 50% of cases, Libby and colleagues (1990) pointed out that synchronous primary lung and kidney cancers are common. In a serious of resected SPNs in patients with prior nonpulmonary cancers, Andrea and associates (2001) found that 42% were metastatic, 32% primary lung cancers, and 27% benign. Metastasis was found in 43% of colorectal cases, 60% of breast cancers, and 42% of melanomas. Although there were only two sarcoma cases, both nodules proved to be metastatic.
In patients with a history of lung cancer, a new nodule is most often also due to lung cancer. When the histology of the two cancers is the same and carcinoma in situ is not seen, the new lesion is considered either a metastasis or a metachronous primary cancer, depending on the definitions applied (see Chapter 106). In fact, it is often not possible to determine with certainty whether the new lesion is a second primary or a metastasis, and definitions are often arbitrary, as discussed by the author (2000). Although not an SPN, a synchronous nodule separate from the presenting site and usually detected by CT in a patient with a current lung cancer raises similar diagnostic issues. Staging requires determination of whether the lesion is benign or malignant and, in the latter case, assessment of the often complex question of synchronous primary lung cancer versus metastasis.
P.1322
In patients without a history of cancer and without symptoms or findings suggestive of an extrapulmonary neoplasm, the chance that an SPN is a metastasis from an occult primary tumor is small. Extensive evaluation to assess this possibility is not warranted. In addition to a complete history, physical examination, and basic blood tests, however, optimal care of patients older than 50 years of age with suspicious SPNs should include recent mammography and gynecologic examination in women, serum prostatic-specific antigen in men, and stool testing for blood and serum carcinoembryonic antigen in both men and women. These tests are probably more useful to identify coincident common cancers, rather than to discover an occult primary origin of an SPN.
Other elements of the medical history may be helpful. Ongoing or recently resolved systemic, respiratory, cutaneous, or musculoskeletal symptoms may suggest diagnoses such as bronchiolitis obliterans organizing pneumonia, sarcoidosis, unresolved pneumonitis, or pulmonary infarction. These are rare causes of SPN, and in most cases tissue confirmation is required. The experience reported by Jolles and co-workers (1989) is instructive. Of seven patients with severe rheumatoid arthritis who underwent bronchoscopy for new SPNs consistent with necrobiotic ( rheumatoid ) nodules, all were found to have carcinoma. Another important factor is immunosuppression from the human immunodeficiency virus (HIV) or other cause. Although most patients in this category present with symptoms and with more than one nodule, SPNs do occur. The differential diagnosis in this setting is different from that of the general population (see below and Chapter 121).
An important, and often neglected, aspect of the history is the determination of whether there exist prior chest radiographs. Although patients are often unsure, a call to their primary physicians and to hospitals to which they have been admitted often uncovers these studies. Ideally, any extant films should be examined in direct comparison with the current studies. If the radiographs are no longer available, the radiologist's report is helpful only if it specifically indicates the size, exact location, and description of the opacity in question.
Physical findings that may provide a clue to the etiology of an SPN are uncommon. Examples include telangiectasias in hereditary cases of arteriovenous malformation, signs of an asymptomatic extrapulmonary primary cancer such as a cutaneous melanoma or breast or abdominal mass, or signs suggesting lung cancer such as clubbing and ipsilateral scalene lymphadenopathy.
RADIOGRAPHIC ASSESSMENT
Although the clinical evaluation may be helpful on occasion, it is the radiographic assessment of an SPN that is most often determinative with respect to plans for resection, biopsy, observation, or foregoing further evaluation. Advances in thoracic imaging are responsible for the decline in resection of benign SPNs by thoracotomy. Most solitary nodules are discovered on chest film, although, as alluded to earlier, screening CT scan is already a common modality for the initial identification of SPNs. In either case, however, in the absence of documented chronicity, further evaluation is required. The high kilovoltage employed for standard chest films limits contrast resolution and the ability to detect subtle calcification. Low-kilovoltage plain tomography and fluoroscopy were for many years the standard techniques for assessing lung nodules. Huston and Muhm (1987) showed that conventional tomography combined with fluoroscopy can be employed with good results. Classifying 502 nodules on the basis of calcification and shape, they were able to reach a correct benign or malignant diagnosis in 67% of cases. Modifications of the chest radiograph to improve the assessment of SPNs have been described, but are not widely applied at present. Kelcz and associates (1994) showed improved observer detection and characterization of nodules by employing dual-energy radiographs to eliminate rib shadows with tissue-selective images and to enhance calcified areas with bone-selective images. Chiles and Sherrier (1990) reported histogram analysis of digitized plain chest films. Newer imaging methods designed to assess tissue and cellular features have been applied to the evaluation of SPNs. Although magnetic resonance (MR) imaging has not generally proved valuable in initial assessment, experience with PET is promising. In most centers, however, CT is currently the pivotal imaging modality for lung nodules that require further evaluation after review of chest films. CT has been refined by the development of high-resolution computed tomography (HRCT) and offers far superior capability over conventional radiography and tomography to delineate morphologic characteristics as well as the detection of unsuspected coexistent nodules or extrapulmonary thoracic abnormalities that may aid in diagnosis. CT also allows precise localization of nodules with respect to the pleura, fissures, blood vessels, and airways, information that is often helpful for planning biopsy approaches. In addition, quantitative CT measurements of the baseline attenuation or contrast-enhanced density of a nodule may suggest a benign or malignant etiology. CT scanning is now universally available in the United States, and rapid spiral HRCT is the standard. During the course of a few years, CT has evolved from a time-consuming, patient-dependent major undertaking to a test that can be accomplished in a single breath-hold and that can be reconstructed by a variety of computer algorithms to provide a vast amount of information (see Chapter 10).
Two radiographic features of an SPN are diagnostic of a benign etiology, namely certain patterns of calcification and stability over time. In some cases, in addition, baseline density by CT or enhancement by CT or PET can be strongly indicative of a benign or malignant process. The CT features of an SPN, alone or in combination with clinical history or other imaging studies, are often of great value
P.1323
in generating a high, intermediate, or low level of suspicion of a malignant etiology and thereby in planning the optimal diagnostic pathway.
Calcification and Density
Although calcification occurs more commonly in benign nodules than in malignant lesions, the presence of calcium per se is not definitive. Calcification in malignant tumors can result from neoplastic incorporation of an adjacent granuloma or parenchymal scar, dystrophic calcium deposition in areas of ischemia, and necrosis or calcium production by the cancer. Four patterns of calcium distribution, however, correlate so closely with benignity that their identification is considered diagnostic. A laminated pattern results from calcium deposition in concentric complete or partial rings and is also referred to as laminar, lamellated, target, or bull's eye calcification. A benign central pattern requires identification of a dense nidus of calcium in the center of a nodule (Fig. 93-1). By plain film, the central location of the nidus must be confirmed in two planes. The diffuse pattern is a dense homogeneous distribution of calcium throughout the entire nodule. The fourth benign pattern is popcorn calcification, made up of larger chunks of calcium noted throughout the mass, and is diagnostic of pulmonary hamartoma, although only a minority of hamartomas display this feature. Calcification that is eccentric or consists of fine scattered flecks (stippled) can be found in both benign and malignant nodules and cannot be relied on as a sole indicator of a benign process (Fig. 93-2). Stippled calcification may be central, but is differentiated from the benign central type by its fine pattern.
As a general rule, benign lesions are associated with more obvious, easily detectable macrocalcifications than are malignant nodules. Malignant lesions rarely contain calcification that is sufficiently extensive or dense to be appreciated on plain chest films. Although calcium was found on radiographs of the resected specimens in 10 of 72 patients (14%) with adenomas, metastatic tumors, or lung cancers studied by O'Keefe and associates (1957), in only one case (1.4%) was calcification noted on the preoperative chest film. In the same study, in contrast, calcium was detected by specimen radiography in one half of 135 benign lesions and in one third of the corresponding chest films. The chest film showed calcification in 35 of 90 granulomas (39%) and in 11 of 32 hamartomas (34%). Theros (1977) reported calcification on chest radiographs in only 7 of 1,267 (0.5%) cases of primary lung tumors from the Armed Forces Institute of Pathology.
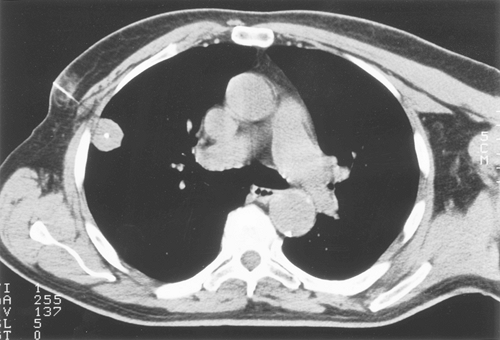 |
Fig. 93-1. Computed tomography scan showing a dense central nidus of calcification. Core biopsy documented amyloid. |
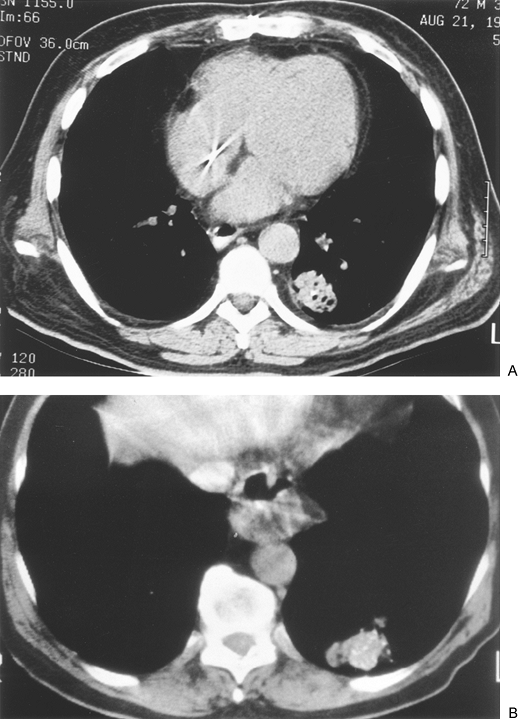 |
Fig. 93-2. Computed tomography scans of adenocarcinomas with calcification. A. A single eccentric focus. B. Scattered or stippled calcification. |
Siegelman and associates (1986a), Proto and Thomas (1985), and others have shown that CT is capable of detecting calcification in one fourth to one third of nodules that appear uncalcified on plain radiography. In addition, HRCT can detect calcium that is missed by standard CT collimation. In cases in which calcification is identified directly by examining thin sections through the nodule, the benign patterns of calcium deposition noted earlier are relevant to the evaluation. In other nodules, calcium is not appreciated
P.1324
grossly, but the presence of microcalcification is inferred from the high density of the entire lesion or of its central area. CT densitometry is the measurement of attenuation within a nodule using a computer printout of a matrix of CT numbers. Application of this approach for identifying benign nodules was first reported by Siegelman and associates (1980). In this initial series, all nodules with a representative CT number greater than 164 Hounsfield units (HU) were reliably confirmed to be benign. After these initial promising results could not be universally verified by other investigators, the problem was traced in large measure to variability among CT scanners. Zerhouni and associates (1986) devised and tested a high-density calcium carbonate standard reference nodule simulator or phantom. In this method, after the clinical nodule is scanned, the phantom model is constructed to simulate the size and location of the nodule and the chest wall thickness of the patient and is then scanned using the same settings. If the nodule is denser than the phantom, it is likely benign. If the phantom is denser, the nodule is indeterminate. Although ingenious, because of less than optimal results in subsequent series and the development of superior approaches, this modality is no longer used for the evaluation of SPNs. In addition, modern CT scanners, as noted by Webb (1997), provide reproducible and accurate attenuation numbers in standard testing.
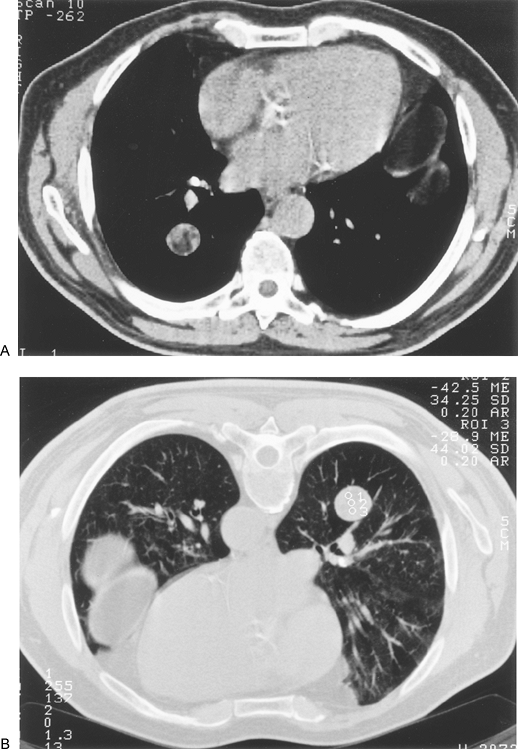 |
Fig. 93-3. Computed tomography scan showing smoothly marginated right lower lobe mass. A. Inhomogeneous density. B. Computed tomography numbers obtained at time of planned biopsy (prone position) were negative in all regions of interest, confirming a hamartoma. Biopsy was not performed. |
Experience with CT has emphasized that cancers contain calcium more often than appreciated by other techniques 13.4% of malignant lesions in the series of Siegelman and associates (1986a), a proportion similar to that noted by O'Keefe and co-workers (1957) on specimen radiographs and to the histologic incidence of calcification in resected cancers. Therefore, radiographic diagnosis must be both quantitative and qualitative. It is suggested that benign calcification not only must be diffuse or central but also must involve more than 10% of the nodule. Exceptions will still be found. Maile and associates (1982) noted that metastatic lesions may contain significant amounts of calcium, including chondrosarcoma, osteogenic sarcoma, thyroid cancer, and mucinous adenocarcinoma of the colon. Zweibel and colleagues (1991) found calcification by CT in 39% of central carcinoids but only 8% (one case) of peripheral carcinoids. Rarely, a primary lung cancer will demonstrate calcification involving more than 10% of the lesion. These are generally adenocarcinomas that are 3 cm or larger.
At the opposite end of the attenuation spectrum, very low density indicates the presence of fat. Fat within a pulmonary nodule is diagnostic of a benign process and usually indicates a hamartoma (Fig. 93-3). Lipoid pneumonia and pulmonary lipoma are rare causes of fat density. Using HRCT, Siegelman and colleagues (1986b) were able to make a specific diagnosis of hamartoma in 28 of 47 cases (60%). Fat alone was found in 38%, and fat combined with calcification in 21%. The ability of HRCT to diagnose at least half of pulmonary hamartomas, and thereby avoid resection or biopsy, is a definite advantage.
In summary, if a nodule exhibits a benign pattern of calcification and has no other features worrisome for malignancy, no further follow-up is indicated. If a benign diagnosis is based on densitometry alone, or if there are any other characteristics of the nodule that arouse suspicion, close observation is mandatory, and early biopsy must be considered. The evaluation of enhancement as a sign of malignancy or benignancy is different from baseline density and is discussed later.
Stability and Growth Rate
In addition to fat density or benign calcification, lack of growth over time is a reliable indication of benignity. The requisite period of stability for a confident benign diagnosis is often cited as 2 years. Yankelevitz and Henschke (1997) reviewed this concept. They confirmed that 2-year stability
P.1325
is accepted as indicative of a benign SPN in textbooks of pulmonary medicine, thoracic surgery, and chest radiology as well as in journal articles. They trace the origin of this belief to a report by Good and Wilson (1958), but point out that examination of the original data yields a predictive value for benignancy based on stability of only 65%. They caution that our current ability to detect smaller nodules casts further doubt on the usefulness of 2-year stability. Numerous exceptions have been reported. Davis and associates (1956) reported an adenocarcinoma that was unchanged by chest film for 8 years and suggested that stability for 5 years be required to consider a nodule benign. In a study of 105 surgically treated cases of bronchoalveolar carcinoma, Dumont and colleagues (1998) found that in 12% of the 85 cases presenting as an SPN, the lesion had been radiographically stable for 2 to 7 years. Several of these tumors did not have associated fibrosis, indicating that not all were scar carcinomas.
The concept that lack of growth over time suggests a benign etiology is based on the fact that malignant neoplasms exhibit cell division and growth. Because some benign lesions also increase in size, the rate of growth of an SPN has been suggested as a means of differentiating benign from malignant processes. Analysis of growth rates is also relevant to the question of how much weight to assign to temporal stability of a nodule. Collins and Loeffler (1956) established the concept of tumor doubling time, the time it takes for a nodule to increase twofold in volume. Use of doubling time to determine growth rates is based on three assumptions. First, nodules are assumed to be spherical. The relationship of volume to radius, therefore, is volume = 4/3 r3. Second, it is assumed that growth rate is either uniformly constant or randomly steady over time. Third, it is assumed that the entire area or volume of opacity seen radiographically is due to neoplastic cells. Based on this model, a single cancer cell that measures 10 m in diameter requires 30 doublings of volume to reach a diameter of 1 cm. Only five more doublings are needed to achieve a diameter of 3 cm. By 40 doublings, the tumor would be 10 cm in diameter. Five more doublings would yield a theoretical tumor of 32 kg. It is assumed that, in most cases, death from local invasion or distant metastasis occurs at or before the 10-cm stage. Thus, by the time a tumor is visible by plain film (1 cm), at least three fourths of its natural history has elapsed. For lung nodules, doubling time can be determined if two radiographs obtained at different times show an increase in diameter. Garland (1966) calculated that the duration of growth from one cell to a 2-cm tumor averaged 8 years for primary squamous and undifferentiated cancers and 15 years for adenocarcinomas. Masses with very short doubling times are generally infectious or inflammatory, whereas those with long doubling times are likely granulomas or benign neoplasms. Unfortunately, there is much overlap. Nathan (1974) and Nathan and associates (1962) reported that all but three cases in a group of 177 malignant lung nodules had doubling times of 280 days or less. The three slowest-growing lesions had doubling times of 375, 395, and 465 days. All nodules with doubling times of more than 465 days were benign. The lower limit of doubling time consistent with a malignant etiology in these series of primary and metastatic nodules was 7 days. If unusually rapidly progressive (and rare) lesions such as choriocarcinoma and embryonal cell carcinoma are excluded, however, a reasonable lower limit of doubling time for malignant processes is between 30 and 40 days. Mizuno and associates (1984) reported that the mean doubling time for adenocarcinoma was 177 days, that for large cell carcinoma was 111 days, and that for squamous cancer was 102 days. Small cell carcinomas grew considerably faster than non small cell neoplasms, with a mean doubling time of only 62 days. There was, however, a wide range of doubling times within cell types. Doubling times for squamous cancers, for example, ranged from less than 30 to just more than 350 days. The fastest growing adenocarcinomas had doubling times similar to those of rapidly doubling squamous cell tumors, but three adenocarcinomas displayed doubling times of more than 450 days, with two cases of more than 500 days. With the mean doubling times in this series, the time from the 1-cm stage to the predicted death of the patient (10-cm size) was 4.9 years for adenocarcinoma, 3 years for large cell cancer, 2.8 years for squamous carcinoma, and 1.6 years for small cell tumors. Although for the group as a whole, the actual survival time correlated well with predicted survival based on doubling time, the authors noted that for many resected patients, actual survival far exceeded the interval calculated by doubling time. Hayabuchi and associates (1983) also emphasized that prolonged doubling time for lung cancer is not uncommon, especially for adenocarcinomas. They followed people exposed to radiation from the atomic blasts in Japan with biennial chest films. Slow-growing tumors were defined by a doubling time of more than 5 months. By this definition, 17% of peripheral squamous cancers were slow growing, with a mean doubling time of 160 days. Forty-two percent of peripheral adenocarcinomas were slow growing, with a mean doubling time of 367 days. Large cell and small cell cancers, in contrast, were all associated with a doubling time of less than 5 months. Among metastases, thyroid cancer and adenoid cystic carcinoma of the salivary glands are often very indolent.
Although growth is universal in malignant pulmonary nodules, benign lesions may increase in size as well. Good and Wilson (1958) found that 4% of hamartomas and 6% of granulomas enlarged over time. Goodwin and Snell (1969) noted that histoplasmomas could increase in diameter by as much as 1.7 mm per year, a rate that overlaps many cancers. They reported that granulomas due to tuberculosis and coccidioidomycosis could also enlarge. Opacities due to rounded atelectasis can also progress, as noted by Silverman and Marino (1987) and by Hillerdal (1989). Eschelman and colleagues (1991) reported growth in a case of pulmonary hyalinizing granuloma. Gotway and colleagues (2001) described sarcoid presenting as an enlarging SPN.
P.1326
Arteriovenous malformations may enlarge over time and, without true progression, may appear radiographically larger or smaller depending on the respiratory cycle. Conversely, malignant densities sometimes appear to shrink. Garland (1966) reported that 5% of malignant nodules appeared to decrease in diameter temporarily during observation. This phenomenon is probably related to radiographic technique, observer variation, or changes in areas of opacity due to nonneoplastic reaction or obstruction rather than to an actual decline in the volume of cancer cells.
The use of temporal stability and doubling time for decision making should generally be limited to retrospective applications based on comparison of prior and current radiographs. Except in rare cases, watchful waiting in order to calculate doubling time prospectively is not prudent. Despite exceptions to the 2-year rule, a lesion that is stable for 2 years by high-quality radiographs (doubling time more than 730 days) is very likely benign. In contrast, most nodules that have demonstrated any growth should be resected. If, however, review of prior films clearly documents minimal change over a prolonged period, especially if other signs of benignity are present, it may be reasonable to continue radiographic surveillance. Another problem with using doubling time for prospectively following SPNs is that small lesions can increase significantly in volume with minimal change in diameter. Recalling the formula, volume = 4/3 r3, a 0.5-cm nodule that doubles in volume increases in diameter by 1.4 mm, and a 1-cm mass by only 2.4 mm. Such small increments can be missed by plain radiography and even by CT. This observation indicates that stability over time and prolonged doubling time carry more clinical weight in the case of larger lesions. It also suggests that CT is generally the imaging modality of choice if one elects to follow a small nodule. Cases in which the concept of doubling time might have more prospective usefulness are those characterized by very rapid progression, that is, doubling time of less than 30 days. In most instances, these will be infectious or inflammatory lesions.
Other Radiographic Features
In current practice, the location, size, and morphologic features of an indeterminate SPN are most often defined by CT. The conventional teaching is that certain internal and edge findings are characteristic of malignant lesions, whereas their opposites are associated with benign nodules. For example, edges with spiculation, notching (Rigler's sign), irregularity, lobulation, or indistinctness are thought of as denoting invasive growth into the adjacent lung parenchyma, as opposed to the smooth or angular margination of a benign nodule. The classic metastatic deposit is spherical and smoothly marginated. Although these generalizations are basically valid, analysis of large numbers of cases shows that most are insufficiently specific to be of value as univariate predictors. Malignant nodules can be associated with a pushing edge as they grow; that is, the interface between the lesion and the surrounding lung remains smooth because the tumor is not infiltrating. Conversely, benign processes may insinuate locally or create a reaction that simulates cancerous infiltration. Although the radiographic features of an SPN, especially in combination, may suggest an etiology, they are only occasionally definitive. Processes that often can be diagnosed radiographically include mucus plugging, arteriovenous malformation, rounded atelectasis, and fungal nodule.
Location of the Solitary Pulmonary Nodule
Nodule location is of little diagnostic value. Primary lung cancers and tuberculous granulomas occur more often in the upper and middle lobes, whereas hamartomas show an equal lobar distribution. Metastases most often are located peripherally. In a radiographic-pathologic study by Scholten and Kreel (1977), 92% of metastases were located in the peripheral third of the lung fields, and 67% were directly subpleural. Crow and associates (1981) noted that 82% of metastatic deposits were peripheral. Banko and associates (1996) and Yokomise and colleagues (1998) found that intrapulmonary lymph nodes resected for the diagnosis of indeterminate SPNs were located in the lower lobes in 70% and 72% of the cases.
Size of the Solitary Pulmonary Nodule
Larger nodule size correlates with an increased probability of cancer. The converse, that small lesions are likely benign, is reported in older series. Although more recent studies confirm that larger nodules are overwhelmingly malignant 93% to 99% of resected nodules larger than 3 cm in the reports of Zerhouni and colleagues (1986) and of Siegelman and associates (1986a) current experience indicates that small size does not always carry a high probability of a benign process. Steele (1963) found that 80% of nodules larger than 3 cm were malignant, whereas 80% of those under 2 cm and 93% of SPNs 1 cm or smaller were benign. More recently, almost half of the reported lesions smaller than 2 to 3 cm are malignant. In the 1986 studies noted earlier, 42% of 177 malignant lesions were smaller than 2 cm, and 15% were smaller than 1 cm. In a group of 40 consecutive nodules, all smaller than 3 cm, evaluated between 1990 and 1993 by Libby and co-workers (1995), 53% overall were cancers, as were 80% of those larger than 2 cm and 43% of those 2 cm or smaller. In a group of 65 nodules 1 cm or smaller resected by video-assisted thoracic surgery (VATS), Munden and colleagues (1997) found an incidence of malignancy of 58%. The increasing incidence of cancer in smaller nodules may be the result of improved radiographic detection coupled with a greater willingness to apply a strategy of watchful waiting in low-suspicion cases. The lesson, however, is that small nodules detected by modern imaging are often malignant.
P.1327
Shape and Margins of the Solitary Pulmonary Nodule
Huston and Muhm (1987) used fluoroscopy and conventional tomography to classify nodules by shape and edge features. Lesions with totally circumferential spiculation, known as a corona radiata or corona maligna, were considered malignant; nodules were called benign if they had a linear or angular shape or were composed of multiple tiny densities. Although all 38 nodules diagnosed as benign remained stable for 2 years, and 31 of 33 lesions classified as malignant proved to be so on biopsy, the limitation of pure morphologic analysis by plain tomography is highlighted by the fact that 70% of all noncalcified lesions in this large series were radiographically indeterminate. Among the indeterminate lesions, 77% were found to be benign and 23% malignant. In the cooperative CT study of Zerhouni and associates (1986), 100 of 178 nodules with smooth or lobulated borders proved to be benign (56%), as contrasted with 91 lesions with irregular, spiculated margins, of which only 11 were benign (12%). Unfortunately, many of these nodules were incorrectly diagnosed by CT criteria. Siegelman and colleagues (1986a) used a scale of 1 to 4 for edge analysis by CT, in which 1 was sharp and smooth, 2 moderately smooth, 3 showing some undulations or spiculations, and 4 grossly irregular with circumferential spiculations. Although most nodules with smooth margination (type 1) were benign, 20% were malignant. A type 2 edge was common in both categories: 58% malignant, 42% benign. Eighty-eight percent of types 3 and 4 lesions were malignant (Fig. 93-4). Zwirewich and associates (1991) correlated edge and internal characteristics determined by HRCT with histology in 93 patients. Although certain features were statistically more common in malignancy, there was much overlap. Spiculation correlated with lymphatic spread and infiltrative tumor growth in some cases but was most often due to irregular peripheral fibrosis and was present in 87% of malignant nodules and 55% of benign lesions. Similarly, a pleural tag or pleural tail, due to a desmoplastic reaction extending from the lesion to the visceral pleura and variously cited as indicative of cancer or of a benign process, was present in 58% of malignancies and also in 27% of benign nodules (Fig. 93-5). Lobulation was typical of malignancies and represented nodular excrescences of neoplastic growth, but was seen in 27% of benign nodules as well. In the benign cases, lobulation on HRCT was most often due to coalescent granulomas. Sone and associates (1997) also emphasized that irregularity, including spiculation,
P.1328
indistinctness, lobulation, and pleural tails, is usually due to nonmalignant factors, namely, a desmoplastic reaction or scar formation with retraction. They reemphasized that a tumor with uniform, solid growth often displays a well-defined, smooth margin.
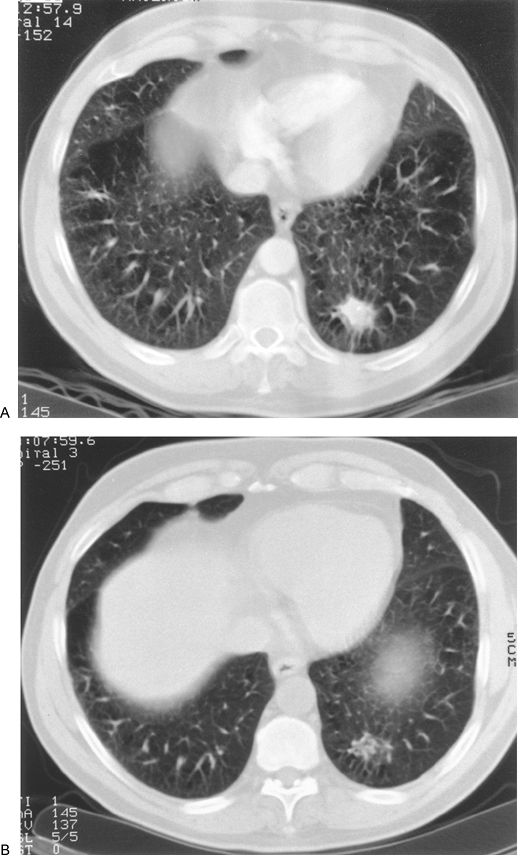 |
Fig. 93-4. A. Computed tomography scan showing circumferential spiculation (corona radiata, corona maligna) in a left lower lobe nodule. B. Although circumferential spiculation is considered a sign of malignancy, this lesion had regressed 2 weeks later at the time of planned biopsy and ultimately resolved entirely. |
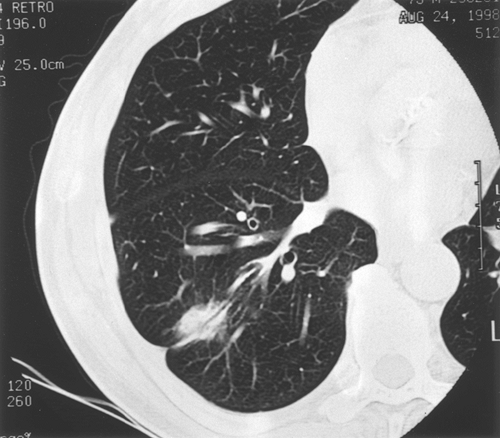 |
Fig. 93-5. Computed tomography scan showing a pleural tag in a right lower lobe carcinoma. Benign lesions may also have this feature. |
Internal Composition of the Solitary Pulmonary Nodule
With respect to internal composition, Zwirewich and associates (1991) found that homogeneous attenuation correlated more often with benign processes (55%), but was also present in 20% of primary and metastatic SPN. The 80% of malignant opacities with inhomogeneous internal findings displayed cavitation, necrosis without cavitation, and air bronchograms or areas of bubblelike low attenuation due to small, patent, air-containing bronchi within the mass. Although considered typical of squamous cell cancer, cavitation can occur in all cell types.
The extremes of wall thickness of a cavitary lesion may be helpful. Malignant lesions tend to have thicker, more irregular walls. In the series of Woodring and Fried (1983), all cavitary masses with a wall thickness of less than 1 mm were benign; 95% of those with wall thickness of less than 4 mm were also benign; of those between 5 and 15 mm, 27% were malignant; and 84% of those thicker than 15 mm were malignant (Fig. 93-6). Air bronchograms and bubblelike areas, sometimes termed pseudocavitation, are typical of bronchoalveolar carcinoma. Kuriyama and co-workers (1991) used HRCT to determine the frequency of air bronchograms or bronchiolograms and the histologic correlate of a patent bronchus or bronchiole in 20 lung lesions smaller than 2 cm as compared with 20 benign nodules. The authors concluded that an air bronchogram should raise a strong suspicion of malignancy because 72% of adenocarcinomas had an air bronchogram, but only 5% of benign masses demonstrated this feature. Air bronchograms may also be seen in pulmonary lymphoma. Although ground-glass opacity (GGO), defined as a hazy increased density that does not obscure normal vessels, is usually associated with diffuse lung diseases, recent reports have noted focal GGO in SPNs as well. Nakata and colleagues (2002) resected 43 such lesions that had persisted a mean of 3.7 months and found bronchoalveolar carcinoma in 23, mixed adenocarcinoma in 11, and atypical hyperplasia in 9. All lesions larger than 1 cm were malignant. In addition, a combination of solid attenuation with GGO correlated with malignancy in 93% of cases. Although recent reports from Japan, such as that of Suzuki and colleagues (2002), suggested that GGO indicates low-grade, node-negative cancers that may be treated by limited resection without surgical lymph node staging (see Chapter 106), this assertion remains unproven (Fig. 93-7).
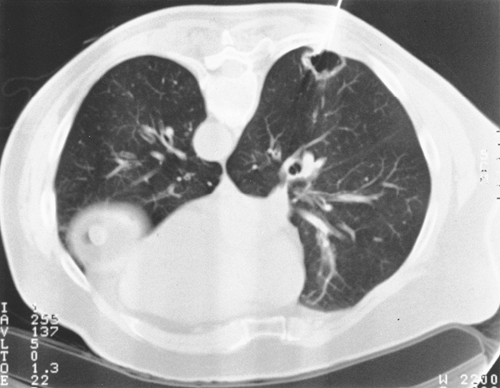 |
Fig. 93-6. Computed tomography scan during needle biopsy of wall of right lower lobe cavitary lesion. Biopsy and subsequent resection showed bronchoalveolar carcinoma, T2, N0. Any cell type may be cavitary; thin-walled cavities may be malignant. |
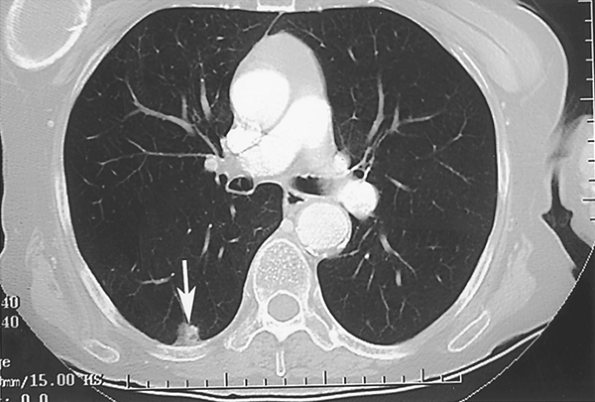 |
Fig. 93-7. Right lower lobe nodule with at least 50% ground-glass opacity. Although most such lesions represent bronchoalveolar cancer or well-differentiated adenocarcinoma, this was a poorly diffentiated squamous cancer throughout, T1, N0. |
Associated Radiographic Findings
The presence of certain associated radiographic abnormalities may be helpful. Satellite lesions, an undefined term but generally thought of as nodular densities smaller than and in close proximity to the primary nodule, usually indicate malignancy. The coexistence of radiographic hilar or mediastinal lymphadenopathy along with an SPN usually indicates primary lung cancer, but may be seen with other processes, such as metastasis and sarcoidosis. An adrenal mass without characteristics of a benign adenoma on CT or magnetic resonance imaging also increases the odds of malignancy. In both these scenarios, that is, an SPN with signs of regional nodal or distant spread, small cell cancer must be considered a prime possibility. Calcification in the thoracic
P.1329
lymph nodes, liver or spleen increase the likelihood that an SPN is a granuloma. It must never be assumed a priori, however, that the pulmonary and nodal or extrathoracic findings are necessarily related.
Relationship to Vascular and Airway Structures
The relationship of an SPN to blood vessels or airways may be suggestive of a diagnosis and, in some cases, pathognomonic. Naidich and Garay (1991) reviewed angiocentric and bronchocentric focal lung lesions. CT can be helpful in identifying metastases, septic emboli, and bland or noninfectious infarcts and can be diagnostic for invasive aspergillosis and for arteriovenous malformations (AVM). Metastatic deposits, because of their hematogenous mode of dissemination, may be shown on CT to be supplied by a feeding pulmonary artery. Septic pulmonary emboli and noninfected areas of infarction are usually multiple, but occasionally solitary. Infarcts are typically wedge shaped and pleural based. The perimeter of an infarct may enhance with intravenous contrast owing to collateral bronchial arterial blood supply. A pulmonary artery may be identified at the apex of the triangular infarct. In the case of septic emboli, Huang and colleagues (1989) and Kuhlman and associates (1990) found feeding pulmonary vessels by CT in about two thirds of peripheral lesions. Invasive pulmonary aspergillosis, also hematogenously disseminated and rarely solitary, may be associated with a feeding vessel, as well as with an air-crescent sign a rim of air surrounding an intracavitary fungus ball. The fungal conglomerate may move with changes in patient position. This constellation of findings in an immunocompromised host is diagnostic, but is not typical of early lesions, before tissue necrosis has occurred. Similarly, in the case of AVMs, plain film or CT identification of a round or serpiginous density with a feeding and draining vessel is pathognomonic. Although AVMs enhance markedly after administration of intravenous contrast, this finding is often superfluous in the presence of clearcut vessels and is also nonspecific because most tumors enhance as well. AVMs can be demonstrated to change size with changes in cardiac output induced by the Valsalva maneuver. Rounded atelectasis characteristically appears as a peripheral round or oval mass abutting a thickened pleural surface. Bronchi and blood vessels entering the mass in a curvilinear fashion form a comet tail and blur the central margin. Rounded atelectasis is also often associated with volume loss in the affected lobe and with signs of asbestosis or other chronic pleural fibrotic process, including postcardiac surgery left pleural effusion.
HRCT is particularly suited to demonstrating the relation of a focal lesion to the airways. SPNs due to lung cancers or carcinoid tumors may have an endobronchial component, whereas granulomas usually do not. In some cases, the opacity seen on a plain chest film represents an area of consolidation or atelectasis distal to an obstructing endobronchial mass. In these instances, CT may show a small intraluminal mass, raising the possibility of carcinoid, squamous papilloma, and rarer endobronchial lesions. Mucus plugging, usually seen as multiple lesions, may appear as a solitary branching linear opacity with bulges in the expected distribution of the airways. In rare instances, a carcinoid or metastasis may simulate this picture.
Enhancement by Computed Tomography, Positron Emission Tomography, and Depreotide
There has been considerable interest in studying and refining methods of separating benign from malignant SPNs based on their differential uptake of systemically injected substances. Because of quantitative and qualitative differences in vascularity as well as cellular metabolism, malignancies generally enhance more than benign processes. Although linear tomography, angiography, Doppler ultrasound, and gadolinium-enhanced MR imaging, as reported by Kono (1993) and Guckel (1996) and their colleagues, have been used in this manner, most current clinical focus is on enhancement by CT and PET. A smaller literature exists on labeled somatostatin analogs.
Computed Tomography Enhancement
Swenson and colleagues (1996) corroborated their prior extensive work on CT contrast enhancement with a prospective study of 107 indeterminate SPNs 7 mm to 3 cm in diameter. Malignant nodules enhanced a median of 46 HU from baseline precontrast levels (range, 11 to 110 HU), statistically significantly more than benign neoplasms and granulomas (median, 8 HU; range, 10 to 94 HU). Using a cutoff of 20 HU and above to define malignancy, the sensitivity was 98%, specificity 73%, and accuracy 85% (Fig. 93-8). Only 1 of 52 primary and metastatic malignant lung neoplasms enhanced less than 20 HU. Combining this series with their prior reports yielded 270 cases with a sensitivity of 99%, specificity of 75%, accuracy of 90% and, importantly, a negative predictive value of 99%. The authors also showed that enhancement correlated directly with increased central nodule vascularity as determined by tissue staining with antibody to factor VIII associated antigen, an endothelial cell marker. Similar results were achieved by Yamashita and associates (1995), who found that all 18 cases of lung cancer studied enhanced 25 or more HU as compared with less than 15 HU for 13 of 14 benign lesions. In a series of 65 indeterminate SPNs, Zhang and Kono (1997) noted significantly more enhancement in malignant nodules (41.9 HU) than in benign, noninflammatory lesions (13.4 HU). They cautioned, however, that active inflammatory processes, such as organizing pneumonia or tuberculosis, can enhance within the range of malignant neoplasms and thereby yield false-positive tests (Fig. 93-9). Swenson and co-workers (1996) noted similar caveats. The negative predictive value of the test, however, remained
P.1330
acceptable, with only 2 of 42 malignancies enhancing with less than 20 HU. False-negative readings were due to areas of tumor necrosis. The authors point out that avoidance of necrotic areas when selecting the region of interest for measurement of baseline and contrast-enhanced attenuation can minimize this problem. Yamashita and colleagues (1997) emphasized that larger tumors are associated with a higher incidence of necrosis and may be less suitable for this technique. Specifically, lesions of 3 cm or less had a 16% incidence of radiographically evident necrosis, whereas 80% of tumors larger than 3 cm exhibited necrotic regions. Miyake and colleagues (1995) reported that mucin-producing adenocarcinomas could also contain areas of diminished vascularity that may produce false-negative enhancement.
P.1331
Zhang and Kono (1997) noted two other methods for improving discrimination. First, despite equal peak enhancement for active inflammatory and malignant nodules, time-attenuation curves showed faster washout for the inflammatory processes. Second, the nodule-to-aorta contrast ratio was more than 6% for all malignant lesions. By combining this latter parameter with the 20-HU absolute threshold, there would have been no false-negative results in their series. In addition to quantitative measures, qualitative patterns are also being investigated. Benign lesions tend to enhance more peripherally, whereas malignant nodules enhance centrally. Central tumor necrosis, however, can sometimes reverse this pattern and must be taken into account. Swenson and associates (2000) have recently updated the use of contrast enhancement in a seven-center report, including 356 relatively spherical, homogeneous, 4- to 40-mm nodules. The prevalence of malignancy was 48%. Malignant lesions enhanced significantly more than benign nodules, with a median above baseline of 38.1 HU versus 10 HU, respectively. They concluded that enhancement equal to or less than 15 HU is strongly predictive of benignity. Miles and colleagues (2001) noted that the standardized perfusion value based on CT enhancement correlated with the standard uptake value obtained by PET. CT enhancement remains a valuable modality in centers with expertise in this area. Advantages of this modality include the universal availability in the United States of modern CT scanners, rapid scanning times, and the ability to use the required contrast injections to perform optimal chest and abdominal scans.
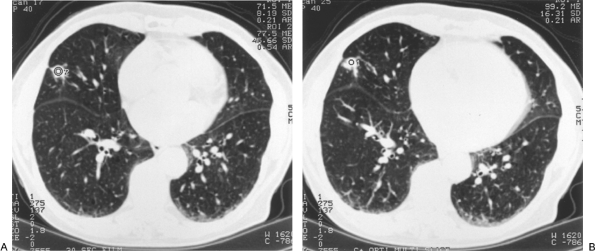 |
Fig. 93-8. A. Suspicious irregular right middle lobe nodule with pleural tail. B. Post contrast enhancement less than 15 HU. Video-assisted thoracic surgical resection confirmed a granuloma. |
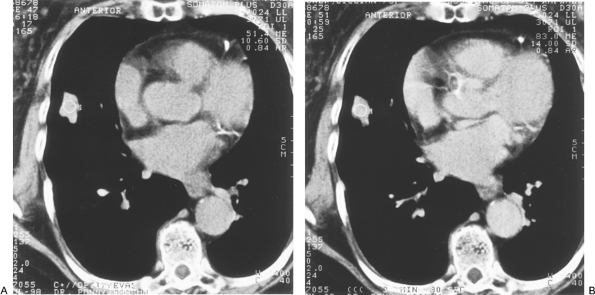 |
Fig. 93-9. False-positive enhancement study. A. Baseline B. Post contrast enhancement by 30 HU. Resection documented active tuberculosis. |
Positron Emission Tomography
Since the prior edition of this text, PET scanners have become increasingly available and are gaining a greater role in the assessment of the SPN and the staging of lung and other cancers. PET scan in thoracic disease is discussed in Chapter 12. PET scanning with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose (FDG) relies on increased metabolic activity measured as augmented glycolysis in malignant as compared with normal cells. FDG is a D-glucose analog that enters cells and is phosphorylated by hexokinase. Thereafter, most of the FDG undergoes no further metabolism and is retained in the intracellular space. PET detects the radioactive fluorine-18 positron-emitting label attached to the analog. Scans are interpreted qualitatively as positive (any activity greater than surrounding areas) or quantitatively by measurement of the standardized uptake value (SUV) or ratio (SUR). PET has been studied as a diagnostic modality for lung nodules. Although early studies reported a higher accuracy than recent series, ongoing technical refinements and improved appreciation with experience of the role of PET in the overall clinical picture maintain PET as a valuable tool in many cases of SPN. Gupta and associates (1992) found that FDG-PET was completely accurate in 20 patients with indeterminate SPN. Hypermetabolism by qualitative analysis was detected in all 13 biopsy-proven malignant lesions, as compared with none of seven benign nodules, and SUV was significantly higher for malignant processes. In a series of 51 nodules, Patz and co-workers (1993) found increased uptake in all malignant lesions, but two false-positive studies occurred in cases of active tuberculosis, yielding a sensitivity of 100% and a specificity of 89%. Gupta and colleagues (1996) later reported an expanded series of 61 patients showing a positive predictive value of 95%, but, because of three malignant lesions misinterpreted as benign, a negative predictive value of 82%. Similarly, Scott and associates (1994) noted positive and negative predictive values of 94% and 80%, respectively, in 61 cases. Higashi and co-workers (1997) found that four of seven bronchoalveolar tumors failed to demonstrate increased uptake of FDG. In this regard, it is interesting to note that Duhaylongsod and associates (1995) correlated increased FDG activity with more rapid tumor doubling times. Knight and colleagues (1996) reported a 100% negative predictive value for lesions larger than 1 cm, but there were six false-positive studies in a series of 48 patients. Lowe and Naunheim (1998) also pointed out that small cancers may be missed by current PET technology. In a group of 107 patients with suspected lung cancer, Sazon and associates (1997) found a high sensitivity but a low specificity (52%) because 12 of 25 patients with benign lesions showed FDG uptake. The scans in this study, however, were interpreted qualitatively only. Hagberg and colleagues (1997) in a series of 49 cases achieved a sensitivity of 93% but a specificity of only 70%. Using likelihood ratios, Dewan and associates (1997) found that PET was superior to standard radiographic assessment and to formal probabilistic systems as well. In two recent series, the first retrospective and the second prospective, Lowe and associates (1997, 1998) found sensitivities of 96% and 92% and improved specificities of 77% and 90%, respectively. Imdahl and colleagues (2001) reported an overall sensitivity of 86% in 107 cases, a sensitivity of only 43% for inflammatory lesions, and a correlation of sensitivity with nodule size. PET was not accurate in 27% of lesions 1 cm or smaller, 10% of those from 1.1 to 2 cm, and 12% of those larger than 2 cm. Marom and associates (2002) showed that there was a statistically greater likelihood for PET to miss small and low-grade cancers. Kim and colleagues (1998) and Higashi and co-workers (1998) also noted that bronchoalveolar cancers were often negative on PET (Fig. 93-10). False-positive studies are most often due to infectious or inflammatory processes, including mycobacterial nodules, other infectious granulomas, sarcoidosis, necrotizing granulomas, and many less common SPNs. Nine consecutive patients having PET scans and ultimately shown to have tuberculomas had positive studies in a series from South Korea described by Goo and associates (2000). Croft and colleagues (2002) similarly pointed out the limitations of PET in an area with a high prevalence of histoplasmosis. In their experience, PET demonstrated a sensitivity of 93%, but a specificity of only 40%, and positive and
P.1332
negative predictive values of 88% and 55%, respectively.
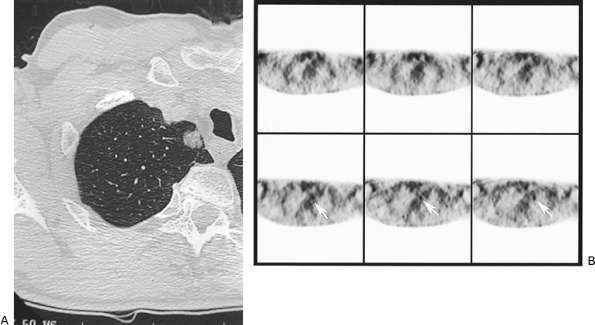 |
Fig. 93-10. Computed tomography scan (A) and positron emission tomography scan (B) of small right upper lobe solitary pulmonary nodule. Video-assisted thoracic surgical resection showed bronchoalveolar carcinaoma. Note ground-glass opacity by computed tomography. |
The impact on decision making of false-positive and false-negative PET studies can be modulated by assessing this study in the entire context of the clinical and imaging profile of each case. The problem of false-negative studies in small and low-grade cancers will likely be lessened over time by improved technology, greater availability of dedicated scanners, and alteration of study parameters, such as waiting a longer period from injection to scanning following an initial negative study. Mathies and colleagues (2002) found that sensitivity increased from 80% to 100% when delayed scans were performed in cases in which the initial 70-minute scan had an SUV of less than 2.5. In this small study, benign lesions did not increase in SUV over time, whereas certain malignant nodules did. If confirmed in larger series, this dual time point scanning may be helpful when there is suspicion of bronchoalveolar cancer or other low-grade tumor. In addition, there is current interest in optimal ways of fusing CT and PET images, as described by Goerres (2002) and Skalski (2002) and their associates, a technology that will likely progress rapidly.
Depreotide Scanning
Lung nodule enhancement by a technetium-labeled synthetic analog of somatostatin, 99mTc depreotide, has been studied carefully but less extensively than positron-emission tomography (PET) and appears promising (see Chapter 13). Blum and associates (2000) reported a multicenter phase III trial using this agent for assessing 114 indeterminate SPNs, with single-photon emission computed tomography (SPECT) as the major imaging modality. Scans were interpreted qualitatively as positive or negative. Tissue confirmation was obtained in all cases. Of 88 malignant nodules, 85 (96%) were correctly identified correctly. Among the 26 benign SPNs, there were seven false positive studies (27%), six granulomas, and one hamartoma. The sensitivity was 96%, accuracy 91%, and specificity 73%. Aside from a lower specificity, these results are similar to PET. More experience is needed to assess the role of 99mTc depreotide in evaluating SPNs as well as its role in mediastinal and extrathoracic staging, an area that has been much more extensively studied with FDG PET (see Chapters 12 and 105). Although both tests are time consuming, SPECT uses less expensive imaging hardware and radionuclide agent.
TISSUE SAMPLING OPTIONS
When the clinical and radiographic assessment are neither confirmatory nor sufficiently suggestive of a benign etiology to forego further evaluation or to choose a course of periodic surveillance, the next step is either resection or a nonsurgical means to establish a tissue diagnosis.
Sputum Cytology
The simplest approach is sputum cytology. In most classic asymptomatic SPNs, sputum cytology is negative. Indeed, a positive cytology, especially if squamous carcinoma,
P.1333
in the presence of a small peripheral nodule should prompt a search for a large airway source of the malignant cells. Sputum cytology is of value in the workup of central endobronchial lesions, but its role in the evaluation of larger SPNs remains a subject of controversy. In a decision analysis study of cost-effectiveness, Raab and associates (1997) found that sputum cytology was a worthwhile first test, not only for central lesions, but also for peripheral masses 3 cm or larger. In contrast, Goldberg-Kahn and colleagues (1997), also using a decision analysis model comparing sputum cytology, transthoracic needle aspiration, bronchoscopy, and open biopsy as the initial approach to central and peripheral lesions, determined that sputum cytology is not useful. The authors relate this finding to low sensitivity and the frequent use of other tests following negative sputa. They point out that the increased prevalence of adenocarcinomas as compared with squamous cancers, which more often shed cells into the airways, has reduced the sensitivity of sputum cytology. The Early Lung Cancer Detection Program reported by Frost and co-workers (1984) noted that sputum analysis is of little value in nonsquamous tumors. In practical terms, sputum cytology in patients with asymptomatic, small, peripheral SPNs is not helpful. For larger lesions, especially if associated with pulmonary symptoms, cytology may be diagnostic and is risk free and inexpensive. A negative study, however, should not delay the further expeditious evaluation of an SPN.
Bronchoscopy
Bronchoscopy, like sputum cytology, is limited by the fact that most SPNs do not communicate with an airway visually accessible by fiberoptic endoscopy. Absence of airway communication is especially typical of metastatic malignancies and benign lesions. In practice, however, the flexible bronchoscope in many cases can be directed close to the lesion, and the brush and biopsy forceps often can reach the SPN. Fluoroscopic guidance increases the diagnostic yield, as shown by Fletcher and Levin (1982). The use of a transbronchial aspirating needle catheter may aid in obtaining diagnostic material, both for cytology as well as a core of tissue for histology. Gasparini and colleagues (1995) made a diagnosis of malignancy in 54% of cases with bronchoscopic biopsy forceps and 69% with transbronchial needle aspiration. The two techniques in combination resulted in a diagnosis in 75%. Shure and Fedullo (1983) similarly reported that adding aspiration to biopsy, brushings and washings increased the yield from 48% to 69%. Overall, however, the reported efficacy of bronchoscopy for SPNs varies widely. Bronchoscopy more often confirms malignancy (20% to 80%) than establishes a specific benign diagnosis (0% to 20%). Higher accuracy is achieved with larger lesions, more central location, and when CT demonstrates a bronchus leading to or passing through a nodule a positive bronchus sign (Fig. 93-11). Radke and colleagues (1979) showed that diagnostic material was obtained more than twice as often from nodules larger than 2 cm in diameter (64%) as compared with smaller SPNs (28%) and noted a low yield for lesions in the outer third of the lung fields. Mehta and associates (1995) found an average yield of 55% for SPNs larger than 2 cm, but only 11% for smaller lesions. Naidich and co-workers (1988) established a diagnosis by bronchoscopy in 60% of cases with a positive bronchus sign, but in only 30% of cases without bronchial communication by standard CT. Further, when high-resolution computed tomography (HRCT) failed to show a tumor-bronchial association, endoscopy was diagnostic in only 14% of cases. Gaeta and associates (1993) reported that transbronchial biopsy and brushings were diagnostic in 81% of lesions with a positive bronchus sign, as contrasted to 45% of those without this feature. Biaceroglu and colleagues (1998) established a diagnosis in 82% of cases with this feature versus 44% of those without it. Using biplane fluoroscopy, however, Chechani (1996) reported a high yield for SPNs without a positive bronchus sign. In this study, nodules located in the basilar segments of the lower lobes or apical segments of the upper lobes were less often diagnosed (58%) than were lesions in other areas (83%), likely because of suboptimal radiographic localization. Baalini and colleagues (2000) reviewed the results of bronchoscopy in 177 cases, using a C-arm fluoroscope and multiplane imaging for localization, and washings, brushings, and transbronchial biopsy for sampling. Inclusion criteria required a classic SPN, without associated atelectasis, cavitation, pneumonitis, or satellite lesions and without radiographic or bronchoscopic evidence of endobronchial extension or extrinsic bronchial compression. When grouped by distance from the hilum, diagnostic yields for central, intermediate, and peripheral lesions were 82%, 61%, and 53%, respectively. Although these differences in location were statistically significant, nodule size proved more predictive. Peripheral lesions 2 cm or smaller were diagnosed in only 14% of cases, versus 31% if they were central or intermediate. Of note is that
P.1334
only two small peripheral lesions were diagnosed by bronchoscopy, and both were due to tuberculosis. There was a trend toward better results for middle lobe and lingular SPNs.
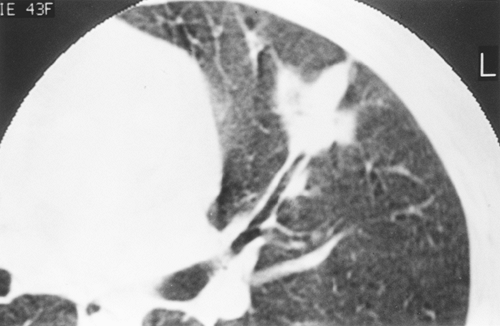 |
Fig. 93-11. Left upper lobe mass with positive bronchus sign. |
In summary, the results of standard flexible bronchoscopy for SPNs vary. HRCT can aid in deciding whether the lesion is likely to be accessible. Fluoroscopic guidance is helpful, and transbronchial aspiration should be part of the bronchoscopist's expertise. In general, peripheral nodules smaller than 2 cm are unlikely to be diagnosed. With modern imaging, this group makes up a significant percentage of SPNs. Diagnostic yield can be improved by techniques such as rapid on-site cytopathology, ultrasonographic guidance, and CT-guided endoscopy, as discussed by Diette and associates (2000), Prakash (1999), and Wagner and colleagues (1996), respectively. Availability and experience in these areas, however, is limited. Finally, as shown by Goldberg and associates (1993) and by Torrington and Kern (1993), routine staging bronchoscopy to assess the airways as a separate procedure before resection of an SPN is unproductive and not warranted.
Transthoracic Needle Biopsy
Transthoracic needle biopsy (TNB) or fine-needle aspiration (FNA) under CT or fluoroscopic guidance is an established means of assessing SPNs. TNB is used here to denote both techniques. Westcott (1988) and Klein and Zarka (1997) emphasize that improvements in imaging, biopsy needles, and cytopathology have decreased the risk for TNB while increasing its reliability. Regardless of the imaging modality chosen for the procedure, a CT scan is essential for determining the feasibility of TNB, planning the approach and assessing the risk. Specially designed needles are currently used that are finer and more versatile than those employed in the past. Coaxial needles can be employed to obtain multiple samples without reinsertion. In addition, finer-gauge devices increase the safety of multiple passes. In most cases, it is possible to obtain cores or fragments of tissue for histologic study in addition to aspirates for cytology. Advances in cytopathology have improved the ability to interpret tiny samples. Techniques such as immunohistochemical staining, flow cytometry, DNA probes, electron microscopy, and hormone receptor assays can often be applied to minute amounts of material. The importance of close communication between the radiologist and pathologist involved in TNB is highlighted by a prospective, randomized study reported by Santambrogio and associates (1997), who achieved an accuracy for malignant and benign SPNs of 99% in 110 cases when immediate cytologic evaluation was performed. If the specimen was inadequate, more material was obtained. In contrast, accuracy was 81% in the control group of 110 patients when the adequacy of the sample was assessed only by its gross characteristics.
As for bronchoscopy, the reported results of TNB for SPNs vary. Although Calhoun and co-workers (1986) found that about one third of resected lesions with a preoperative nondiagnostic aspirate turned out to be cancer, current sensitivity for malignancy should be 90% to 97%, as reported by Khouri and associates (1985) and Caskey and colleagues (1990). Although virtually any area of the thorax can be sampled by TNB, the procedure is especially suited for lesions not amenable to bronchoscopic diagnosis, that is, smaller, peripheral nodules. Although Berquist and associates (1980) reported a yield of only 65% for nodules smaller than 1 cm, more recently Westcott and colleagues (1997) and Li and co-workers (1996) obtained diagnostic samples from 95% to 100% of small nodules. Wallace and associates (2002) reported a diagnostic accuracy of 88% in nodules 1 cm or smaller. Although there remains debate about whether core biopsy in addition to FNA adds to the diagnostic yield, Kim and associates (2002) found that the combination of the two methods resulted in a sensitivity of 97% versus 94% for aspirate alone in malignant lesions, and 34% versus 20%, respectively, for benign lesions. Gasparini and colleagues (1995) reported a sensitivity of 95% for malignancy and 59% for benign nodules by integrating as one procedure bronchoscopy, immediate cytology and TNB if the bronchoscopic sample was nondiagnostic. Although appealing, the logistics of this approach would be daunting in most institutions.
The most common complications of TNB are pneumothorax and bleeding. Systemic air embolism is exceedingly rare, and malignant seeding of the needle tract appears to have been eliminated since the introduction of finer needles. The incidence of pneumothorax is 15% to 30%, but half or fewer of the patients require chest tubes. Severe emphysema, small SPNs, deeper location, and multiple needle passes are among the risk factors. The radiologist performing the biopsy usually treats and follows the pneumothorax. My colleagues and I (1997) have shown that most patients with chest tubes after TNB can be managed with Heimlich valves on an ambulatory basis. Hemoptysis, the second most common complication, occurs in about 5% of cases and is usually self-limited. Massive intrathoracic bleeding from biopsy of mediastinal masses or vascular pulmonary lesions such as renal cell carcinoma, inadvertent puncture of an intercostal or internal mammary artery, or torn apical adhesions from TNB-induced pneumothorax has been reported, but is rare. Overall, the mortality rate of TNB for all thoracic processes is about 0.02%.
It is clear that in experienced hands, TNB is effective and safe for establishing malignant diagnoses. The false-negative rate is 5% or less. Two areas of controversy, however, arise from the limitations of TNB. First, the cell type identified by TNB sometimes does not match the histology of the resected lesion. Most often, this discrepancy occurs within the subtypes of non small cell carcinoma, small cell cancers being rarely misinterpreted. DiBonito and associates (1991), comparing cytologic typing of lung cancers with
P.1335
autopsy histology, found that aspiration cytology was accurate in 90% of squamous cancers, 70% of adenocarcinomas, 50% of large cell cancers, and 100% of small cell tumors. Because the treatment for all non small cell cases is the same, this limitation is of no practical importance at present. The second and more important issue is a low yield of specific diagnoses; stated otherwise, a high incidence of nonspecific findings, when a benign lesion is present. To paraphrase Klein and Zarka (1997), a specific benign diagnosis requires the confident identification of a named benign process that renders further evaluation for malignancy unnecessary. Benign lesions that can often be diagnosed with specificity by TNB include hamartoma, chondroma, granuloma, lipoid pneumonia, pulmonary infarct, Wegener's granulomatosis, abscess, and other infectious processes. The yield may be increased by obtaining multiple samples from representative parts of the nodule (e.g., both the wall and center of a cavitary SPN), core biopsies, expert cytologic input and liberal use of microbiologic cultures and stains, and rapid DNA techniques when fungal or mycobacterial infection is suspected, as reported by Shim and colleagues (1998). The reported rate for clinically identifying benign SPNs varies markedly, from 5% to 89%, but the rate for a specific diagnosis is in the range of 10% to 20%.
The approach to cases in which TNB is negative (cancer cells are not identified) but nonspecific (no specific benign diagnosis) is controversial. In the past, most authors correctly regarded a nondiagnostic TNB to be of insufficient reliability to obviate thoracotomy. The clinical corollary is that TNB is not helpful in decision making in operable patients because the ultimate outcome will be changed only in the minority of cases with a specific benign result. This philosophy was recently emphasized in guidelines suggested by Rivera and colleagues (2003), who recommended against TNB in operative candidates with SPNs that are even moderately suspicious for lung cancer, unless preoperative therapy was planned.
Many clinicians, however, are willing to follow certain indeterminate SPNs based on a nonspecific TNB. The specifics of the individual TNB are important. A biopsy in which the radiologist and pathologist are confident that adequate material has been obtained carries more weight than a suboptimal biopsy. It must be clear that all relevant portions of the lesion have been sampled and that areas of postobstructive pneumonia, associated reaction, or necrosis have not been mistaken for active tumor. As emphasized by Westcott (1988), a second TNB is often indicated following a nonspecific initial result and can establish a malignant diagnosis in some cases. On the other hand, two or more TNBs yielding sufficient amounts of negative material, especially if core biopsies are obtained, make a malignant process very unlikely. Reliability for a benign process is increased by the demonstration of a cellular specimen that contains inflammatory cells, histiocytes, or multinucleated giant cells. A factor of great importance in judging the validity of a nonspecific biopsy is the track record of the specialists involved the local expertise. When the clinical and radiographic pictures suggest cancer, however, resection is indicated regardless of the results of TNB. Bronchoscopy and TNB in the diagnosis of lung lesions are discussed in depth in recent reviews by Mazzone (2002) and associates and by Schreiber and McCrory (2003).
Advantages of having a preoperative diagnosis by bronchoscopy or TNB include the ability to discuss with the patient exactly what to expect at the time of operation and postoperatively and the elimination of the logistic problems with respect to operating times and uncertain allocation of other hospital resources, as noted earlier. Clearly, in the absence of a preoperative diagnosis, all of this can and must be dealt with by a more complex discussion and more variable planning, based on intraoperative findings. The author finds that the availability of reliable TNB allows a more focused preoperative patient discussion, with less confusion and anxiety during and immediately after the operation for family members and the patient. As an analogy, aside from the increase in options for treating many breast cancers, there is a human aspect to the change in this area from frozen section to mastectomy in a single procedure with the patient unaware of the diagnosis and outcome until after the operation. Other reasons for establishing a preoperative diagnosis include avoidance of tumor manipulation, especially stapling close to larger and deeper lesions to provide tissue for frozen section (see Chapter 26), and elimination of the period of waiting for a frozen section diagnosis, a factor that varies by institution, during what should be a relatively short operation. In some cases, operation without a diagnosis is unavoidable due to nondiagnostic biopsy, nonfeasibility of TNB, or lack of local expertise. In cases of high probability of cancer, the argument to bypass biopsy is stronger than in those with a moderate probability, a term that is not universally defined but that clearly indicates that a significant proportion of nodules so classified will be benign. It must also be emphasized that some highly suspicious SPNs turn out to be benign, hence the term probability.
Video-Assisted Thoracic Surgery
In recent years, video-assisted thoracoscopy, globally termed video-assisted thoracic surgery (VATS), has emerged as an option for diagnosing SPNs and in some instances for treating malignant nodules (see Chapters 18, 32, 33). VATS is increasingly being employed as an alternative to bronchoscopy, TNB, and direct thoracotomy for definitively diagnosing SPNs. VATS is best suited for peripheral nodules 2 cm or smaller. In such cases, the procedure is expeditious, and an acceptable stapled margin can be achieved. Techniques have been developed for locating deeper or very small peripheral lesions, including intrapleural ultrasound and preoperative percutaneous CT-guided marking using mammographic localizing wires, subpleural injection of
P.1336
methylene-blue or colored collagen, preoperative radionuclide injection and intraoperative probes, and percutaneous intraparenchymal placement of angiographic coils or contrast injection with operative identification by fluoroscopy. Yttrium-aluminum-garnet (YAG) laser alone or in combination with endostaplers has been used for resecting deep SPNs. In all cases, the specimen should be retrieved in a plastic bag before extraction through the chest wall incision in order to prevent spillage of neoplastic cells or infected material. As an alternative to wedge excision, Bousamra and Clowry (1997) reported success with VATS needle aspiration and immediate cytology in a small series, emphasizing the rapidity of diagnosis (10 to 20 minutes) and the cost savings realized because staplers are not used for the diagnostic portion of the procedure.
The next step in a VATS procedure for an SPN depends on the frozen section or cytologic diagnosis and the preoperative clinical evaluation. If the SPN is benign, the procedure is terminated. If the lesion is a solitary metastasis or low-grade primary lung tumor, in many cases, the diagnostic wedge resection will suffice for therapy. The decision process is more complex in the case of bronchogenic carcinoma. Although some would not consider a pulmonary density associated with hilar or mediastinal lymph node enlargement an SPN, in practice VATS may be employed in such instances. If the nodes have not been sampled by mediastinoscopy or other means before VATS, ipsilateral nodes may be assessed at the time of VATS. If there is no involvement that would preclude curative resection, the nature of and approach to resection depend on the surgeon's judgment and preference. Most patients are best treated by lobectomy, which can be accomplished either by converting to thoracotomy or by a VATS lobectomy. Alternatively, in certain high-risk cases, the surgeon may feel that wedge resection constitutes optimal treatment. The issue of whether local excision of lung cancer is appropriate for the patient able to tolerate anatomic resection remains a subject of controversy (see Chapter 106). Because of the need for general anesthesia for both procedures, the potential for tumor dissemination by VATS, as reported by Downey and associates (1996), and the current absence of proven equivalent options for treatment, definitive resection should be considered at the time of VATS diagnosis of lung cancer. The various possibilities should all be discussed with the patient preoperatively. Another problem that may arise is the finding in a patient with a prior or synchronous extrapulmonary cancer that the SPN is also of the same cell type, usually an adenocarcinoma. The pathologist reading the frozen section should be made aware of the history and ideally should examine the two specimens. The decision to stop or to proceed with lobectomy will depend on the pathologic diagnosis of primary versus metastatic tumor. Often, a firm differentiation cannot be made, and the surgeon must decide on the basis of the entire clinical synthesis. This dilemma, of course, is not specific to VATS, but also occurs with thoracotomy and with nonsurgical biopsy.
The role of VATS in lung cancer treatment remains a subject of controversy (see Chapters 33 and 106). In addition, VATS for therapy in patients with solitary metastases has been criticized for missing radiographically occult deposits that would be identified by open operation with bimanual palpation (see Chapter 120). Despite such therapeutic concerns, VATS is versatile, definitive, safe, and cost effective in many cases. The sensitivity and specificity of VATS approaches 100% for both benign and malignant SPNs. VATS is associated with lower risk and less pain than thoracotomy. Several groups, including Mack (1993), Hazelrigg (1993), DeCamp (1995), and Bernard (1996) and their colleagues, have reported favorable results with VATS in series devoted to or including large numbers of SPNs. The series of Mack (1993) describes 242 VATS resections for nodules smaller than 3 cm located in the outer third of the lung. Fifty-two percent of the lesions were benign, and 48% malignant. Only two patients (0.8%) required thoracotomy to locate the SPN. In patients with good pulmonary function and a frozen section diagnosis of lung cancer, open or VATS lobectomy was carried out. There was no mortality. The morbidity rate was 3.6%, mean hospital stay was 2.4 days for patients having VATS only, and diagnostic accuracy was complete. Although a significantly shorter hospital stay following VATS versus open anatomic resection has not uniformly been reported, wedge resection by VATS should be associated with fewer hospital days. We were the first group to report VATS performed on an outpatient basis (author and associates, 1997). The feasibility of ambulatory VATS for SPNs has been confirmed by Chang and colleagues (2002) and by others for diffuse lung disease. The cost of this approach is clearly less than that of thoracotomy or inpatient VATS and may be less than the cumulative expense of one or more nonspecific TNBs or bronchoscopic examinations followed by periodic imaging studies for 2 years or more. In addition, VATS may make a rapid definitive benign diagnosis and thus spare the patient the anxiety associated with ongoing imaging. My current approach to healthy patients with indeterminate SPNs with low suspicion for cancer that are easily amenable to VATS is to discuss this approach, versus TNB and versus follow-up, and to allow the patient to decide. If the suspicion is very low, follow-up is encouraged over biopsy. Patients with high-suspicion, VATS-accessible SPNs are also offered preoperative TNB versus VATS biopsy and muscle-sparing thoracotomy for anatomic resection during the same anesthetic, based on the frozen section. In most cases of metastasis and in some cases of lung cancer, VATS excision suffices, with lymph node sampling preferred in the latter. If a patient prefers a separate therapeutic procedure after final histologic evaluation of cancer by VATS or if the frozen section is equivocal about primary versus metastatic tumor, the definitive resection is performed as soon as possible. For the large group of SPNs of moderate suspicion, an individualized approach is formulated. All such decisions are based on the liberal use of PET scan and consideration
P.1337
of TNB. The frequent use of the phrase immediate thoracotomy in the SPN literature appears to me to be extreme. Nonetheless, it is clear that numerous valid algorithms exist and are cited in many of the references of this chapter.
Thoracotomy
Thoracotomy remains a reasonable initial approach in operative candidates with lesions that are highly suspicious for malignancy, especially if not easily amenable to TNB or VATS or if the patient prefers this approach. Open resection is also indicated for suspicious lesions even after a nondiagnostic bronchoscopy or TNB, especially if these studies were suboptimal. Thoracotomy is also indicated for SPNs with a moderate likelihood of cancer in the increasingly uncommon circumstance of a lesion not accessible by bronchoscopy, TNB or VATS. It is instructive to note that in a modern series (1990 1993) of SPNs 3 cm or smaller, reported by Libby and colleagues (1995), thoracotomy was the initial method of diagnosis in only 20% of the cases, despite a 53% incidence of malignancy.
DECISION MAKING
The clinician has four options from which to choose as the initial approach to an indeterminate SPN: no further follow-up, periodic radiographic surveillance ( watchful waiting or wait and watch strategy), nonsurgical biopsy (bronchoscopy, TNB, or both), or resection. Open or VATS resection is a form of biopsy but is considered a separate clinical pathway because of its greater magnitude as compared with nonsurgical tissue acquisition methods as well as its potential role as therapy if the nodule is malignant.
In some cases, a decision for no intervention is straightforward based on clinical grounds, radiography, or patient preference. Severe comorbidity or advanced age may preclude consideration of any treatment modality for malignancy. After discussion of the possibilities and the risks of testing for, treatment of, and failure to diagnose cancer, a patient may refuse intervention. No special follow-up is needed in cases with unequivocal signs of benignancy, such as classic benign calcification, fat density, or prolonged radiographic stability. Because of rare exceptions to the 2-year rule, however, most noncalcified lesions should be kept under surveillance for a longer period. Observation without intervention is reasonable in patients whose clinical and radiographic synthesis is highly suggestive of a benign SPN (e.g., young age, nonsmoker, endemic fungal area, radiographic small size, benign morphology, high CT numbers, stability under 2 years, prolonged doubling time if known, negative PET or other enhancement study). It must be emphasized that watchful waiting is an active process that requires periodic reassessment with appropriate imaging at intervals suited to the individual case and early intervention when warranted. If further experience with CT enhancement, PET, and CT-PET fusion confirms their reliability, a larger proportion of SPNs may fall into this wait and watch category in the future, especially because smaller lesions are being detected with increasing frequency.
In most cases at present, however, the major problem is choosing the initial approach among the invasive options. Sometimes, this decision is straightforward. Some patients, for example, may be deemed too tenuous to tolerate general anesthesia and any form of resection but might benefit from nonsurgical therapy if cancer is found. In these instances, diagnostic bronchoscopy or TNB is indicated. The choice of biopsy approach depends on the size and location of the SPN, as discussed earlier. The decision to employ nonsurgical versus surgical biopsy in patients who are operative candidates and whose lesions have a moderate to high suspicion of malignancy should be based on the factors noted in the previous section, the surgeon's preference, and input from the patient. If the SPN appears likely to be accessible by bronchoscopy, a preoperative endoscopy may achieve the same goal. In addition, some patients are reluctant to proceed directly to resection without a diagnosis. Alternatively, especially for suspicious lesions, the surgeon and patient may prefer to avoid a separate biopsy procedure with its low but finite risks. VATS is an especially useful initial approach in cases suspicious for lung cancer for surgeons who perform lobectomy by this route or when the patient's cardiopulmonary status precludes anatomic resection. Finally, in cases of lower suspicion but without strong benign features, the expert application of TNB or VATS can avoid many thoracotomies for benign SPNs.
As emphasized by Lillington (1991) and Lillington and Caskey (1993), the decision to follow or intervene depends on the probability of cancer (pCA). There are no infallible systems or clinicians for making this determination. In daily practice, the pCA is arrived at nonquantitatively, in large part intuitively, based partly on patient-specific data, but more often on the radiographic features of the lesion. This task can be accomplished equally well by an experienced pulmonologist or thoracic surgeon. The participation of an expert thoracic radiologist is essential. CT scan should be part of the initial evaluation for all SPNs that are not definitively benign or factitious on the basis of plain films. PET is becoming more important in initial decisions. If an expectant approach is selected, the imaging modality or combinations used for surveillance will vary according to individual circumstances. Unfortunately, there exists no universally accepted surveillance program for following SPNs. The general rule is obvious: more frequent assessment early after identification, with increasing intervals between imaging based on stability over time. As noted, the length of time a lesion must be followed is unclear, but the 2-year rule has been called into question. Tan and associates (2003) recommend CT scan at 3, 6, 9, 12, and 24 months for patients deemed suitable for follow-up. Although
P.1338
many of us follow a similar plan, the authors point out that there is very little objective evidence for the frequency of monitoring, rating the level of evidence as poor, and the grade of recommendation as C, indicating that the panel agreed on the recommendation but had little solid grounds for it. If confirmed, the use of volumetrically determined growth rates of small nodules based on three-dimensional HRCT, as described by Yankelevitz and colleagues (1999), may help resolve this issue. Their initial in vitro and clinical work indicates that growth may be detected in as little as 30 days even if the lesion changes shape. The possibility for early detection of cancers, as well as shorter follow-up times for benign lesions, is exciting.
Mathematical models for determining pCA have been reported. Cummings and associates (1986a) first described the use of Bayes' theorem for generating an odds ratio of the pCA by assessing the predictive value of individual clinical and radiographic variables. The prevalence of cancer in the general population may also be considered. Various combinations of patient history (age, smoking, prior malignancy), nodule size, edge features, calcification, growth rate, and location are included. Using decision analysis to compare the life expectancy of patients managed by observation, biopsy, or resection based on the pCA, the same authors (1986b), found that for a pCA of 5% or less, observation equalled or was superior to intervention, whereas with increasing pCA, intervention became superior to surveillance, and at pCA levels of more than 60%, resection or nonsurgical biopsy was clearly indicated. Edwards and colleagues (1989) validated this approach, although their series was not limited to the classic SPN. Gurney and associates (1993) found that radiologists using bayesian analysis were able to estimate the pCA better than expert radiologists judging the same cases who did not employ this methodology. Gurney and Swenson (1995) compared neural network analysis, a nonlinear mathematical model capable of learning from example and identifying relationships among data that are not apparent to human analysis, with the Bayesian method and found the latter superior. Mathematical models for determining the approach to an SPN, however, cannot be accepted in the absence of large prospective studies that include modern imaging. In most reports, the input data are limited, and the variables are weighted on the basis of older published series. The conundrum
P.1339
of predicting malignancy is highlighted by the misclassification of 12 of 66 (18%) cases, of which 8 of 44 (18%) were false-negative diagnoses, in the series of Gurney and colleagues (1993). As noted earlier, in one study, PET alone was more accurate than Bayesian analysis.
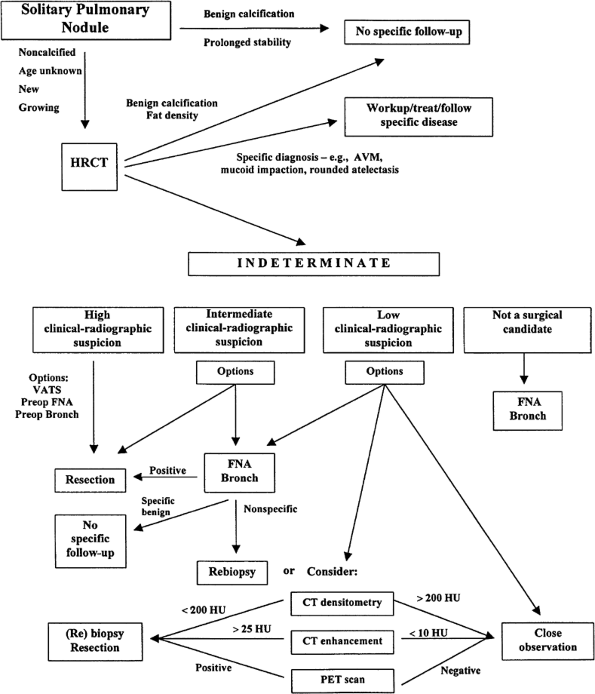 |
Fig. 93-12. A sample algorithm for management of a solitary pulmonary nodule. AVM, arteriovenous malformation; bronch, bronchoscopy; TNB, transthoracic needle biopsy or aspiration; HRCT, high-resolution computed tomography; PET, positron emission tomography; SPECT, single-photon emission computed tomography; VATS, video-assisted thoracic surgery. |
CONCLUSION
The SPN remains a common and challenging problem in thoracic medicine and surgery. Advances in imaging and in surgical and nonsurgical biopsy techniques have improved our ability to diagnose these lesions without missing opportunities for treatment of early cancers, while minimizing major interventions. New promising modalities for the diagnosis of the SPN are undergoing active investigation. At present, the optimal approach to an SPN remains an individualized clinical decision best made by a general thoracic surgeon or pulmonologist in close collaboration with a thoracic radiologist. A sample algorithm is presented in Figure 93-12.
ACKNOWLEDGMENTS
The author gratefully acknowledges the assistance of Jack L. Westcott, M.D., Frank Mele, M.D., and Vicente Caride, M.D. for providing illustrative radiographs.
REFERENCES
Andrea S, et al: Significance of a single pulmonary nodule in patients with previous history of malignancy. Eur J Cardiothorac Surg 20:1101 1105, 2001.
Baalini WA, et al: Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 117:1049 1054, 2000.
Banko MS, et al: Prevalence of pathologically proven intrapulmonary lymph nodes and their appearance on CT. AJR Am J Roentgenol 167: 629 630, 1996.
Bernard A, et al: Resection of pulmonary nodules using video-assisted thoracic surgery. Ann Thorac Surg 61:202 205, 1996.
Berquist TH, et al: Transthoracic needle biopsy: accuracy and complications in relation to location and type of lesion. Mayo Clin Proc 55: 475 481, 1980.
Biaceroglu S, et al: CT bronchus sign-guided bronchoscopic multiple diagnostic procedures in carcinomatous solitary pulmonary nodules and masses. Respiration 65:49 55, 1998.
Blum J, et al: A multicenter trial with a somatostatin analog 99mTcdepreotide in the evaluation of solitary pulmonary nodules. Chest 117: 1232 1238, 2000.
Bousamra M, Clowry L: Thoracoscopic fine-needle aspiration of solitary pulmonary nodules. Ann Thorac Surg 64:1191 1193, 1997.
Cahan WG, Castro EB, Hajdu SI: The significance of a solitary lung shadow in patients with colon carcinoma. Cancer 33:414 421, 1974.
Cahan WG, Castro EB: Significance of a solitary lung shadow in patients with breast cancer. Ann Surg 181:137 143, 1975.
Cahan WG: Multiple primary cancers of the lung, esophagus and other sites. Cancer 40:[Suppl]:1954 1960, 1977.
Calhoun P, et al: The clinical outcome of needle aspirations of the lung when cancer is not diagnosed. Ann Thorac Surg 41:592 596, 1986.
Carucci LR, Maki DD, Miller WT: Clustered pulmonary nodules: highly suggestive of benign disease. J Thorac Imaging 16:103 105, 2001.
Casey JJ, et al: The solitary pulmonary nodule in the patient with breast cancer. Surgery 96:801 805, 1984.
Caskey CI, Templeton PA, Zerhouni EA: Current evaluation of the solitary pulmonary nodule. Radiol Clin North Am 28:511 520, 1990.
Chang AC, Yee J, Orringer MB, Iannettoni MD: Diagnostic thoracoscopic lung biopsy: an outpatient experience. Ann Thorac Surg 74: 1942, 2002.
Chechani V: Bronchoscopic diagnosis of solitary pulmonary nodules and lung masses in the absence of endobronchial abnormality. Chest 109: 620 625, 1996.
Chiles C, Sherrier RH: Analysis of the solitary pulmonary nodule by means of digital techniques. J Thorac Imaging 5:55 60, 1990.
Collins VP, Loeffler RK: Observations on growth rates of human tumors. Am J Roentgenol Rad Ther 76:988 1000, 1956.
Coppage L, Shaw C, Curtis AM: Metastatic disease to the chest in patients with extrathoracic malignancy. J Thorac Imaging 2:24 37, 1987.
Croft DR, et al: FDG-PET imaging and the diagnosis of non-small cell lung cancer in a region of high histoplasmosis prevalence. Lung Cancer 36:297 301, 2002.
Crow J, Slavin G, Kreel L: Pulmonary metastasis: a pathologic and radiologic study. Cancer 47:2595 2602, 1981.
Cummings SR, Lillington GA, Richard RJ: Estimating the probability of malignancy in solitary pulmonary nodules. A Bayesian approach. Am Rev Respir Dis 134:449 452, 1986a.
Cummings SR, Lillington GA, Richard RJ: Managing pulmonary nodules. The choice of strategy is a close call. Am Rev Respir Dis 134:453 460, 1986b.
Davis EW, Peabody JW, Katz S: The solitary pulmonary nodule: a ten-year study based on 215 cases. J Thorac Cardiovasc Surg 32:728 771,1956.
Davis S: CT evaluation for pulmonary metastasis in patients with extrathoracic malignancy. Radiology 180:1 12, 1991.
DeCamp MM, et al: The safety and versatility of video-thoracoscopy: a prospective analysis of 895 consecutive cases. J Am Coll Surg 181: 113 120, 1995.
Dewan NA, et al: Likelihood of malignancy in a solitary pulmonary nodule: comparison of Bayesian analysis and results of FDG-PET scan. Chest 112:416 422, 1997.
DiBonito L, et al: Cytologic typing of primary lung cancer: study of 100 cases with autopsy confirmation. Diagn Cytopathol 7:7 10, 1991.
Diette GB, et al: Utility of on-site cytopathology assessment for bronchoscopic evaluation of lung masses and adenopathy. Chest117:1186 1190, 2000.
Downey RJ, et al: Dissemination of malignant tumors after video-assisted thoracic surgery: a report of twenty-one cases. J Thorac Cardiovasc Surg 111:954 960, 1996.
Duhaylongsod FG, et al: Lung tumor growth correlates with glucose metabolism measured by fluoride 18 fluorodeoxyglucose positron emission tomography. Ann Thorac Surg 60:1348 1352, 1995.
Dumont P, et al: Bronchoalveolar carcinoma. Histopathological study of evolution in a series of 105 surgically treated patients. Chest 113:391 395, 1998.
Edwards FH, et al: Use of artificial intelligence for the preoperative diagnosis of pulmonary lesions. Ann Thorac Surg 48:556 559, 1989.
Edwards SE, Fry IK: Prevalence of lung nodules on computed tomography of patients without known malignant disease. Br J Radiol 55: 715 716, 1982.
Eschelman DJ, et al: Pulmonary hyalinizing granuloma: a rare cause of a solitary pulmonary nodule. J Thorac Imaging 6:54 56, 1991.
Fein AM, Feinsilver SH, Ares CA: The solitary pulmonary nodule: a systemic approach. In Fishman AP (ed): Pulmonary Diseases and Disorders. 3rd Ed. New York: McGraw-Hill, 1998.
Fichtenbaum CJ, Kleinman GM, Haddad RG: Eosinophilic granuloma of the lung presenting as a solitary pulmonary nodule. Thorax 45:905 906, 1990.
Fletcher EC, Levin DC: Flexible fiberoptic bronchoscopy and fluoroscopically-guided transbronchial biopsy of solitary pulmonary nodules. West J Med 136:477 483, 1982.
Frost JK, et al: Results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis 130:549 554, 1984.
Gaeta M, et al: Carcinomatous solitary pulmonary nodules: evaluation of the tumor-bronchi relationship with thin-section CT. Radiology 187:535 539, 1993.
Garland LH: The rate of growth and natural duration of primary bronchial cancer. AJR Am J Roentgenol 96:604 611, 1966.
P.1340
Gasparini S, et al: Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses: experience with 1027 consecutive cases. Chest 108:131 137, 1995.
Goerres GW, et al: PET-CT image coregistration in the thorax: influence of respiration. Eur J Nucl Med Mol Imaging 29:351 360, 2002.
Goldberg-Kahn B, Healy JC, Bishop JW: The cost of diagnosis: a comparison of four different strategies in the workup of solitary radiographic lung lesions. Chest 111:870 876, 1997.
Goldberg SK, Walkenstein MD, Steinbach A, et al: The role of staging bronchoscopy in the preoperative assessment of a solitary pulmonary nodule. Chest 104:94 97, 1993.
Goo JM, et al: Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology 216:117 121, 2000.
Good CA, Wilson TW: The solitary circumscribed pulmonary nodule: study of seven hundred five cases encountered roentgenologically in a period of three and one-half years. JAMA 166:210 215, 1958.
Goodwin RA, Snell JD: The enlarging histoplasmoma: concept of a tumor-like phenomenon encompassing the tuberculoma and coccidioidoma. Am Rev Respir Dis 100:1 12, 1969.
Gotway MB, et al: Sarcoidosis presenting as an enlarging solitary pulmonary nodule. J Thorac Imaging 16:117 122, 2001.
Guckel C, Schnabel K, Deimling M, et al: Solitary pulmonary nodules: MR evaluation of enhancement patterns with contrast-enhanced dynamic snapshot gradient-echo imaging. Radiology 200:681 686, 1996.
Gupta NC, et al: Solitary pulmonary nodules: detection of malignancy with PET with 2-(F-18)-fluoro-2-deoxy-D-glucose. Radiology 184: 441 444, 1992.
Gupta NC, Maloof, J, Gunel E: Probability of malignancy in solitary pulmonary nodules using fluorine-18-FDG and PET. J Nucl Med 37:943 948, 1996.
Gurney JW, Lyddon DM, McKay JA: Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Radiology 186:415 422, 1993.
Gurney JW, Swenson SJ: Solitary pulmonary nodules: determining the likelihood of malignancy with neural network analysis. Radiology 196:823 829, 1995.
Hagberg RC et al: Characterization of pulmonary nodules and mediastinal staging of bronchogenic carcinoma with F-18 fluorodeoxyglucose positron emission tomography. Eur J Cardiothor Surg 12:92 97, 1997.
Hayabuchi N, Russell WJ, Murakami J: Slow-growing lung cancer in a fixed population sample: radiologic assessments, Cancer 52:1098 1104, 1983.
Hazelrigg SR, Nunchuck SK, LoCicero J: Video-assisted Thoracic Surgical Study Group data. Ann Thorac Surg 56:1039 1044, 1993.
Henschke CI, et al: Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 354:99, 1999.
Higashi K, et al: Bronchioloalveolar carcinoma: false-negative results on FDG-PET. J Nucl Med 38:79P, 1997(abst).
Higashi K, et al: Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med 39:1016 1020, 1998.
Hillerdal G: Rounded atelectasis: clinical experience with 74 patients. Chest 95:836 841, 1989.
Holin SM, et al: Solitary pulmonary nodules found in a community-wide chest roentgenographic survey: a five-year followup study. Am Rev Tuberc 79:427 439, 1959.
Huang RM, et al: Septic pulmonary emboli: CT-radiographic correlation. AJR Am J Roentgenol 153:41 45, 1989.
Huston J, Muhm JR: Solitary pulmonary opacities: plain tomography. Radiology 163:481 485, 1987.
Imdahl A, et al: Validation of FDG positron emission tomography for differentiation of unknown pulmonary lesions. Eur J Cardiothorac Surg 20:324 329, 2001.
Johnson H, Fatone J, Flye MW: Histologic evaluation of the nodules resected in the treatment of pulmonary metastatic disease. J Surg Oncol 21:1 4, 1982.
Jolles H, Moseley, PL, Peterson MW: Nodular pulmonary opacities in patients with rheumatoid arthritis. Chest 96:1022 1025, 1989.
Kelcz F, et al: Conventional chest radiography vs dual-energy computed radiography in the detection and characterization of pulmonary nodules. AJR Am J Roentgenol 162:271 278, 1994.
Keogan MT, et al: The significance of pulmonary nodules detected on CT staging for lung cancer. Clin Radiol 48:94 96, 1993.
Khouri NF, et al: Transthoracic needle aspiration biopsy of benign and malignant lung lesions. AJR Am J Roentgenol 144:281 288, 1985.
Kim B, et al: Localized form of bronchioloalveolar carcinoma: FDG-PET findings. AJR Am J Radiol 170:935 939, 1998.
Kim HK, et al: Transthoracic fine needle aspiration and core biopsy of pulmonary lesions. A study of 296 patients. Acta Cytol 46:1061 1068, 2002.
Klein JS, Zarka MA: Transthoracic needle biopsy: an overview. J Thorac Imaging 12:232 249, 1997.
Knight SB, Delbeke D, Stewart JR, Sandler MP: Evaluation of pulmonary lesions with FDG-PET: comparison of findings in patients with and without a history of prior malignancy. Chest 109:982 988, 1996.
Kono M, Adachi S, Kusumoto M, et al: Clinical utility of Gd-DTPA-enhanced magnetic resonance imaging in lung cancer. J Thorac Imaging 8:18 26, 1993.
Kuhlman JE, Fishman EK, Teiger C: Pulmonary septic emboli: diagnosis with CT. Radiology 174:211 213, 1990.
Kundel HH, Nodine CF, Carmody D: Visual scanning, pattern recognition and decision-making in pulmonary nodule detection. Invest Radiol 13:175 181, 1978.
Kuriyama K, et al: Prevalence of air bronchograms in small peripheral carcinomas of the lung on thin-section CT: comparison with benign tumors. AJR Am J Roentgenol 156:921 924, 1991.
Li H, et al: Diagnostic accuracy and safety of CT-guided percutaneous aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol 167:105 109, 1996.
Libby DM, et al: Simultaneous pulmonary and renal malignancy. Chest 98:153 156, 1990.
Libby DM, Henschke CI, Yankelevitz DF: The solitary pulmonary nodule: update 1995. Am J Med 99:491 496, 1995.
Lillington GA: Management of solitary pulmonary nodules. Dis Mon 37:271 318, 1991.
Lillington GA, Caskey CI: Evaluation and management of solitary and multiple pulmonary nodules. Clin Chest Med 14:111 119, 1993.
Lowe VJ, et al: Pulmonary abnormalities and PET data analysis: a retrospective study. Radiology 202:435 439, 1997.
Lowe VJ, et al: Prospective investigation of positron emission tomography in lung nodules. J Clin Oncol 16:1075 1084, 1998.
Lowe VJ, Naunheim KS: Positron emission tomography in lung cancer. Ann Thorac Surg 65:1821 1829, 1998.
Mack MJ, et al: Thoracoscopy for the diagnosis of the indeterminate solitary pulmonary nodule. Ann Thorac Surg 56:825 832, 1993.
Mahadevia PJ, et al: Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effective analysis. JAMA 289:313, 2003.
Maile CW, et al: Calcification in pulmonary metastases. Br J Radiol 55: 108 113, 1982.
Malefatto JP, et al: The clinical significance of radiographically detected pulmonary neoplastic lesions in patients with head and neck cancer. J Clin Oncol 2:625 630, 1984.
Marom EM, et al: T1 lung cancers: sensitivity of diagnosis with fluorodeoxyglucose. PET Radiology 223:453 459, 2002.
Mathies A, et al: Dual time point 18-FDG PET for the evaluation of pulmonary nodules. J Nucl Med43:871 875, 2002.
Mazzone P, et al: Bronchoscopy and needle biopsy techniques for diagnosis and staging of lung cancer. Clinics in Chest Medicine 23:137 158, 2002.
McClure CD, et al: The solitary pulmonary nodule and primary lung malignancy. Arch Environ Health 3:127 139, 1961.
Mehta AC, et al: Role of bronchoscopy in the evaluation of solitary pulmonary nodules. J Bronchol 2:315 322, 1995.
Miles KA, Griffiths MR, Fuentes MA: Standardized perfusion value: universal CT contrast enhancement scale that correlates with FDG PET in lung nodules. Radiology 220:548 553, 2001.
Miyake H, et al: Mucin-producing tumor of the lung: CT findings. J Thorac Imaging 10:96 98, 1995.
Mizuno T, et al: Comparison of actual survivorship after treatment with survivorship predicted by actual tumor-volume doubling time from tumor diameter at first observation. Cancer 53:2716 2720, 1984.
Munden RF, et al: Small pulmonary lesions detected at CT: clinical importance. Radiology 202:105 110, 1997.
Naidich DP, et al: Solitary pulmonary nodules: CT-bronchoscopic correlation. Chest 93:595 598, 1988.
Naidich DP, Garay SM: Radiographic evaluation of focal lung disease. Clin Chest Med 12:77 95, 1991.
Nakata M, et al: Focal ground-glass opacity detected by low-dose helical CT. Chest 121:1464 1467, 2002.
P.1341
Nathan MH, Collins VP, Adams RA: Differentiation of benign and malignant pulmonary nodules by growth rate. Radiology 79:221 232, 1962.
Nathan MH. Management of solitary pulmonary nodules. An organized approach based on growth rate and statistics. JAMA 227:1141 1144, 1974.
O'Keefe ME, Good CA, McDonald JR: Calcification in solitary nodules of the lung. AJR Am J Roentgenol 77:1023 1033, 1957.
Pass HJ, et al: Detection of pulmonary metastases in patients with osteogenic and soft tissue sarcomas: the superiority of CT scans compared with conventional linear tomograms using dynamic analyses. J Clin Oncol 3:1261 1265, 1985.
Patz EF et al: Focal pulmonary abnormalities: evaluation with F 18 fluorodeoxyglucose PET scanning. Radiology 188:487 490, 1993.
Pogrebniak HW, et al: Resection of pulmonary metastases from malignant melanoma: results of a 16-year experience. Ann Thorac Surg 46:20 23, 1988.
Ponn RB, Silverman H, Federico JA: Outpatient chest tube management. Ann Thorac Surg 64:1437 1440, 1997.
Ponn RB: Lightening can strike twice: second primary lung cancers. [Editorial.] Chest 118:1526 1529, 2000.
Prakash UBS: Advances in bronchoscopic procedures. Chest 116: 1403 1408, 1999.
Proto AV, Thomas SR: Pulmonary nodules studied by computed tomography. Radiology 153:149 153, 1985.
Raab SS, Hornberger J, Raffin T: The importance of sputum cytology in the diagnosis of lung cancer. Chest 112:937 945, 1997.
Radke JR, et al: Diagnostic accuracy in peripheral lung lesions: factors predicting success with flexible fiberoptic bronchoscopy. Chest 76:176 179, 1979.
Rivera MP, et al: Diagnosis of lung cancer: the guidelines Chest 123: 129S 136S, 2003.
Rubins J, Rubins HB: Temporal trends in the prevalence of malignancy in resected solitary pulmonary lesions. Chest 109:100 103, 1996.
Santambrogio L, et al: CT-guided fine-needle aspiration cytology of solitary pulmonary nodules: a prospective randomized study of immediate cytologic evaluation. Chest 112:423 425, 1997.
Sazon DAD, et al: Fluorodeoxyglucose-positron emission tomography in the detection and staging of lung cancer. Am J Respir Crit Care Med 153:417 421, 1997.
Schaner EG, et al: Comparison of conventional and computed tomography and conventional whole lung tomography in detecting pulmonary nodules: a prospective radiologic-pathologic study. AJR Am J Roentgenol 131:51 54, 1978.
Scholten ET, Kreel L: Distribution of lung metastases in the axial plane. Radiol Clin 46: 248 265, 1977.
Schreiber G, McCrory DC: Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published experience. Chest 123:115S 128S, 2003.
Scott WJ, et al: Positron emission tomography of lung tumors and mediastinal lymph nodes using F-18 fluorodeoxyglucose. Ann Thorac Surg 58:698 703, 1994.
Shim JJ, et al: Nested polymerase chain reaction for detection of mycobacterium tuberculosis in solitary pulmonary nodules. Chest 113:20 24, 1998.
Shure D, Fedullo PF: Transbronchial needle aspiration of peripheral masses. Am Rev Respir Dis 128:1090 1092, 1983.
Siegelman SS, et al: CT of the solitary pulmonary nodule. AJR Am J Roentgenol 135:1 13, 1980.
Siegelman SS, et al: Solitary pulmonary nodules: CT assessment. Radiology 160:307 312, 1986a.
Siegelman SS, et al: Pulmonary hamartoma: CT findings. Radiology 160: 313 317, 1986b.
Silverman SP, Marino L: Unusual cases of enlarging pulmonary mass. Chest 91:457 458, 1987.
Skalski J, Wahl RL, Meyer CR: Comparison of mutual information-based warping accuracy for fusing body CT and PET by 2 methods: CT mapped on PET emission scan versus CT mapped onto PET transmission scan. J Nucl Med 43:1184 1187, 2002.
Sone S, et al: Factors affecting the radiologic appearance of peripheral bronchogenic carcinomas. J Thorac Imaging 12:159 172, 1997.
Steele JD: The solitary pulmonary nodule: report of a cooperative study of resected asymptomatic pulmonary nodules in males. J Thorac Cardiovasc Surg 46:12 39, 1963.
Stoller JK, Ahmad M, Rice TW: The solitary pulmonary nodule. Cleve Clin J Med 55:68 74, 1988.
Suzuki K, et al: Early peripheral lung cancer: prognostic significance of ground glass opacity on thin-section tomographic scan. Ann Thorac Surg 74:1635 1639, 2002.
Swenson SJ, et al: Lung nodule enhancement at CT: prospective findings. Radiology 201:447 455, 1996.
Swenson SJ, et al: Lung nodule enhancement at CT: multicenter study. Radiology 214:73 80, 2000.
Swenson SJ, et al: Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 165:508, 2002.
Tan BB, et al: The solitary pulmonary nodule. Chest 123:89S 96S, 2003.
Theros EG: Varying manifestations of peripheral pulmonary neoplasms: a radiographic-pathologic correlative study. AJR Am J Roentgenol 128: 893 914, 1977.
Toomes H, et al: The coin lesion of the lung: a review of 955 resected coin lesions. Cancer 51:534 537, 1983.
Torrington KG, Kern JD: The utility of fiberoptic bronchoscopy in the evaluation of the solitary pulmonary nodule. Chest 104:1021 1024, 1993.
Trunk G, Gracey DR, Byrd RB: The management and evaluation of the solitary pulmonary nodule. Chest 66:236 239, 1974.
Wagner U, et al: Computer tomographically guided fiberbronchoscopic transbronchial biopsy of small pulmonary lesions: a feasibility study. Respiration 63:181 186, 1996.
Wallace MJ, et al: CT-guided percutaneous fine-needle aspiration biopsy of small ( 1 cm) pulmonary lesions. Radiology 225:823, 2002.
Webb WR: The solitary pulmonary nodule. In Freundlich IM, Bragg DG (eds): A radiologic approach to diseases of the chest. Baltimore: Williams & Wilkins, 1997.
Westcott JL: Percutaneous transthoracic needle biopsy. Radiology 169: 593 601, 1988.
Westcott JL, Rao N, Colley DP: Transthoracic needle biopsy of small pulmonary nodules. Radiology 202:97 103, 1997.
Woodring JH, Fried AM: Significance of wall thickness in solitary cavities of the lung: a follow-up study. AJR Am J Roentgenol 140:473 474, 1983.
Yamashita K, et al: Solitary pulmonary nodule: preliminary study of evaluation with incremental dynamic CT. Radiology 194:399 405, 1995.
Yamashita K, et al: Intratumoral necrosis of lung carcinoma: a potential diagnostic pitfall in incremental dynamic computed tomography analysis of solitary pulmonary nodules. J Thorac Imaging 12:181 187, 1997.
Yankelevitz DF, Henschke CI: Does 2-year stability imply that pulmonary nodules are benign? AJR Am J Roentgenol 168:325 328, 1997.
Yankelevitz DF, et al: Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 217:251 256, 1999.
Yokomise H, et al: Importance of intrapulmonary lymph nodes in the differential diagnosis of small pulmonary nodular shadows. Chest 113: 703 706, 1998.
Youssem S, Hochholzer L: Pulmonary hyalinizing granuloma. Am J Clin Pathol 87:1 6, 1987.
Zerhouni EA, et al: CT of the pulmonary nodule: a cooperative study. Radiology 160:319 327, 1986.
Zhang M, Kono M: Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology 205:471 478, 1997.
Zweibel BR, Austin JHM, Grines MM: Bronchial carcinoid tumors: assessment with CT f location and intratumoral calcification in 31 patients. Radiology 179:483 486, 1991
Zwirewich CV, et al: Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology 179:469 476, 1991.
EAN: 2147483647
Pages: 203