77 - Management of Nonneoplastic Diseases of the Trachea
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XIV - Congenital, Structural, and Inflammatory Diseases of the Lung > Chapter 91 - Hydatid Disease of the Lung
Chapter 91
Hydatid Disease of the Lung
Nickolaos N. Harlaftis
Homeros A. Aletras
Panagiotis N. Symbas
Hydatid disease, which is caused by the Echinococcus granulosus tapeworm and is known as echinococcosis or hydatidosis, has been acknowledged as a clinical entity since ancient times. Organs of scarified animals were described in the Talmud as bladders full of water, and Hippocrates referred to hydatid disease in the aphorism, When the liver is filled with water and bursts into the epiploon, the belly is filled with water and the patient dies. Rudolphi (1928) first used the term hydatid cyst for the description of echinococcosis in humans.
Echinococcosis remains a significant health problem in endemic areas where sheep and cattle are raised, including the Middle East, the Mediterranean countries, South America, Australia, New Zealand, Central Asia, China, Alaska, East Africa, and among Indian tribes of Canada. Humans become infected from food or water contaminated with canine feces or by direct contact with dogs. Although the disease has been rare in North America and Western Europe, an increase in the frequency of this clinical entity can be expected with the increase in travel and migration of people. Therefore, the presence of hydatid disease should be considered in a patient who presents with a well-defined spherical density of the lung, particularly a patient who has lived or traveled in an endemic area. Ramos and associates (2001) contend that the disease is more frequent at the early ages and there is predominance in males over females.
PATHOPHYSIOLOGY
The primary hosts of the infecting organism are the members of the Canidae family, usually dogs, wolves, and coyotes. Feline species are seldom naturally infected but the parasite has been reported in the cat, wildcat, jaguar, and panther. The primary host contracts echinococcosis by ingesting mature and productive echinococcal cysts in the viscera of an intermediate host (e.g., sheep, goats, cattle, hogs, moose, reindeer, deer, elk, and other herbivorous animals). In the intestines of the primary host the scolices of the hydatid cyst develop into a parasitic worm composed of a scolex, neck, and three proglottids. (i.e., segments). The last proglottid, which is approximately one half the length of the entire parasite, contains 400 to 800 ova. The proglottid matures and breaks off from the scolex. The ova are released in the feces of the primary host and are then introduced into intermediate hosts by ingestion of contaminated grass, water, vegetables, and such. The larval stage, which cannot occur in the main host, begins in the intermediate host and leads to the development of hepatic and pulmonary hydatid cysts. These organs are then ingested by the primary hosts and thus the cycle continues (Fig. 91-1).
In the gastrointestinal tract of the intermediate host, including humans, the chitinous embryophore that surrounds the hexacanth embryo is lysed and the embryo is released. As Smyth (1968) described, the embryo with the aid of its hooklets attaches to and penetrates the mucosa of the duodenum and jejunum, enters the mesenteric venules, and proceeds to the portal vein. From the portal vein the embryo enters the liver where it becomes embedded, and if it is not destroyed by phagocytosis develops into a cyst. In most series of patients, such as that of Toole and associates (1960), the incidence of hepatic involvement in echinococcosis is 50% to 60%. Saidi (1976) reported an incidence of 70% to 80%. In the portal circulation, some embryos whose diameters do not exceed 0.3 mm may pass through the sinus capillaries of the liver and by way of the hepatic veins and vena cava proceed to the right side of the heart and the pulmonary capillaries, where there may become embedded. Here, as in the liver, the embryos that survive phagocytosis hypertrophy, the hooklets disappear, and the embryos enter the larval stage.
The lungs are the second most common sites of lodgment of the parasite, as Pieters and colleagues (1976) and Peleg and associates (1985) noted, with an incidence varying between 10% and 40%. One alternative pathway of the parasites' entrance into the lung is the lymphatic circulation. The embryo enters the lymphatics of the small intestine, proceeds to the thoracic duct, to the central venous system, to
P.1299
the right side of the heart, and then to the lungs. Another possible route is a venovenous anastomosis in the liver and the space of Retzius. Burgos and associates (1999) contend that the disease can be contracted through the inhalation of air contaminated with echinococcus. Secondary pulmonary cysts may develop when ova enter the venous circulation because of rupture of extrapulmonary cysts. Ivanissevich and Rivas (1962) reported that the site of the primary hydatid cyst, producing secondary metastatic pulmonary echinococcus cysts in 31 patients, was the heart in 64%, the liver in 26% and the iliac bone in 10%. In such instances, it is difficult to distinguish between primary and secondary cysts.
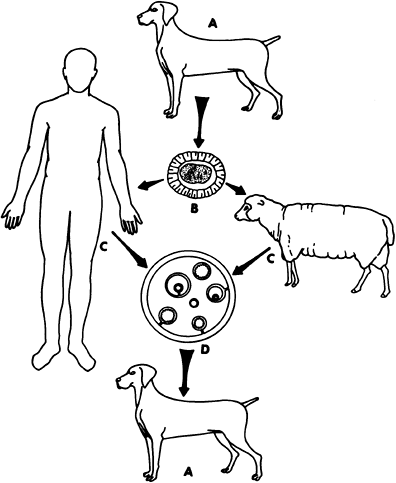 |
Fig. 91-1. Life cycle of Echinococcus granulosus. Primary host A ingests viscera of intermediate host C containing hydatid cyst development of ova producing parasitic worm in intestine of primary host A B ova shed with feces from primary host A, contaminating vegetables, grass, etc. ingestion of contaminated vegetable or grass by intermediate host C development of hydatid cyst D. |
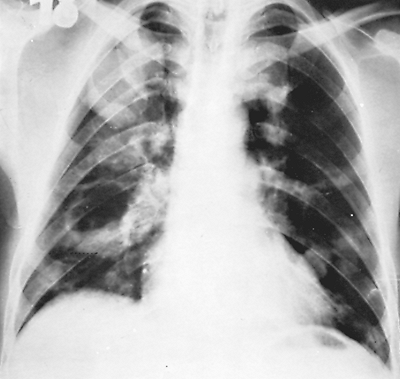 |
Fig. 91-2. Frontal chest radiograph showing multiple hydatid cysts of the lung. |
Peschiera (1964) pointed out that the most common areas of involvement of pulmonary echinococcosis are the right lung and both of the lower lobes. One of us (HA) (1968) and Barret and Thomas (1952) noted that many cysts have a simultaneous development in either one or both lungs, with a reported incidence of 14% to 24%. Aytac and colleagues (1977) reported that 15% of their patients had multiple unilateral cysts and 13% had multiple bilateral cysts. Aubert and Viard (1983) found that 10% of their patients presented with multiple unilateral cysts and 7.4% with multiple bilateral cysts. Each cyst grows and matures independently of any coexisting cyst (Fig. 91-2). Todorov and Boeva (2000) emphasized that children are more likely to develop pulmonary rather than hepatic echinococcus cysts. Some evidence suggests that echinococcal cysts develop more rapidly in the lungs of children than of adults, which may explain the more common appearance of pulmonary cysts in children. Sometimes the entire hemithorax of young children is occupied by parasitic cysts (Fig. 91-3). In these children, either the contamination must have occurred early after birth or the cysts grew rapidly. Hepatic hydatid disease in patients with pulmonary echinococcosis was found in 8.8% and 36.5% of the patients in the series of Dogan (1989), Athanassiadi (1998), Burgos (1999), and Petrov (2001) and their associates.
Two types of hydatid cysts occur in humans: the unilocular and the alveolar. This discussion is limited to the unilocular variety, because these are the cysts of clinical importance in the lungs. A hydatid cyst is composed of the wall and the hydatid fluid. There are three layers in the wall of the cyst. Two of these layers, the outer laminated membrane and the inner germinal layer or germinative membrane, derive from the parasite, and one layer, the pericyst or adventitia, is produced by the host. The adventitia, also known as the ectocyst, is rarely thicker than a few millimeters and is composed entirely of the host's cells. It is the result of the inflammatory response of the organ. When the hexacanth embryo embeds in the host's tissue it causes a local reaction and migration of mononuclear leukocytes, lymphocytes, and eosinophils to the area. Polymorphonuclear leukocytes are not present since they prevent the parasite from implanting at sites where they occur. The original cells are gradually replaced by fibroblasts so that this layer eventually is transformed into a thin and easily discernible capsule of fibrous matter, connective tissue, and compressed parenchymal cells. Functionally this layer provides mechanical protection
P.1300
and nutrition to the parasite. Therefore, whenever degenerative changes of the membrane develop such as calcification, an amorphous degeneration or automatic absorption of the hydatid cyst occurs. The laminated membrane is 1 to 3 mm thick and is surrounded by a pericystic layer. The membrane is white gelatinous, rich in polysaccharides, and characteristically laminated, which is often obvious to the naked eye. It is composed of hyaline and elastic tissue with no host blood vessels entering into it, and it is easily discernible from the pericystic layer, which is vitally important to the surgeons. The laminated membrane as described by Morseth (1967) is composed of a plexus of fine fibers with a dispersed, thick, reticular substance, which as Schwabe (1959) and Larbaoui (1989) noted is permeable to calcium, potassium, chlorides, water, and colloids. Larbaoui (1989) emphasized that when this layer is intact, it constitutes a perfect filter for microorganisms while allowing the entrance of colloids and crystalloids. Nutritional and other substances useful to the parasite traverse the membrane by diffusion, but active transport may also play a role.
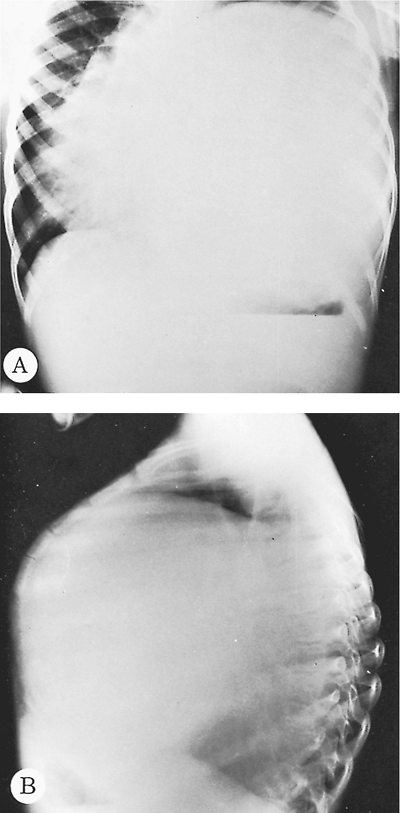 |
Fig. 91-3. Frontal (A) and lateral (B) preoperative and postoperative chest radiographs of a 5-year-old boy with a giant left lower lobe hydatid cyst producing chest deformity. |
The germinal layer (germinative membrane or endocyst) is the inner layer of the cyst wall. It is a thin, transparent, and granular membrane that is lined with small papillae, which are brood capsules at different stages of development. These capsules, which are formed by the proliferation of the cells of the germinal layer, develop buds of scolices. The scolices have suckers and hooks and represent the mature parasite larvae. The germinative membrane is the living part of the parasite and produces the laminated membrane and reproduces the parasite. Some cysts cannot regenerate, but every undamaged cyst regardless of size must be considered capable of reproduction.
The daughter cysts, a rare finding in pulmonary echinococcosis, are produced from the germinal membrane, from the brood capsule, or from the scolices. Daughter cysts vary in size from a few millimeters to a few centimeters; their number ranges from a few to thousands, and they contain viable scolices. Some of the daughter cysts may be degenerated, whereas others may contain their own daughter cysts.
The hydatid vesicle is filled with hydatid fluid, which is colorless, odorless, and sterile and resembles crystal-clear water. The specific gravity of the fluid is 1.008 to 1.015, the pH is 6.7 to 7.2, and the concentration of sodium, potassium, chloride, and carbon dioxide is approximately that of the host's blood. Yuksel and colleagues (1997) contend that the pressure of the fluid ranges from 21 to 61 cm H2O. It contains antigenic elements that may cause anaphylactic phenomena when the cyst ruptures. The function of the hydatid fluid is similar to that of amniotic fluid, because it suspends the daughter cysts. A large production of hydatid fluid can disrupt the nutrition from the host to the interior of the cyst and cause the death of the parasite.
The rate of growth of any particular pulmonary cyst varies and may be faster in children than in adults. Their diameter can increase, as Sarsam (1971) and others noted, from a few millimeters up to approximately 5 cm a year. A cyst with a diameter of 10 cm contains approximately 400 mL of hydatid fluid, and any cyst that grows to a diameter of 6 to 7 cm must be removed. Usually the rate of growth of echinococcus in the lungs is constant, but precipitous growth spurts can occur. The growth of pulmonary cysts is more rapid than in other organs, mainly because of the negative pressure and the great elasticity of the pulmonary tissue. In the course of their natural evolution, many cysts gradually cease growing and degenerate. During the growth period of the cyst, it may rupture spontaneously or during coughing, sneezing, or any other cause of increased intrathoracic pressure or after injury during diagnostic thoracentesis. Large cysts are especially vulnerable to rupture because of the increased pressure exerted by the fluid. The
P.1301
rupture may occur within the boundaries of the pericystic layer, into the pleural space, or into a neighboring organ, bronchus, or blood vessel. Rupture of the germinal membrane toward the interior of the cyst may result in the formation of daughter cysts. Such formation after a rupture of the laminated membrane is rare. Rupture of the cyst toward the surrounding tissues may be followed by secondary echinococcosis, and rupture into a blood vessel may lead to embolization of a portion of the cyst. Rupture of the cyst and evacuation of the cystic content into a main bronchus rarely results in spontaneous cure. As Ramos and colleagues (2001) noted, this complication exposes the patient to asphyxia, anaphylactic shock, secondary hydatid spread, and hemoptysis. Suppuration of the cyst can occur after rupture and secondary infection. Intrapleural rupture is limited by the development of pleural adhesions. The consequences of intrapleural rupture are anaphylactic reaction of the patient, infection of the cyst, or secondary pleural hydatidosis. However, the frequency of secondary pleural hydatidosis is low. Ramos and colleagues (2001) found 5 such cases among 20 pulmonary cysts that broke spontaneously into the pleural cavity. Although hydatid cysts of the liver commonly calcify, calcification of such a cyst in the lung is rare. The calcification, which resembles an eggshell, takes place in the adventitia of complicated cysts and does not always indicate that the hydatid is dead. A calcified lung cyst is almost always in communication with the bronchial tree and is probably infected. Some investigators, including Bakir and Al-Omeri (1967), Saidi (1976), and Smyth (1968), contend that the remaining pericystic cavity becomes obliterated after the cystic contents are evacuated through the bronchus or after excision of the cyst. Others, such as Kourias and Tobler (1957), P rez-Fontana(1948) and Peschiera (1964), believe that the residual cavity persists because of epithelialization of the adventitial sacs.
CLINICAL MANIFESTATIONS
Intact or simple hydatid cysts of the lung produce no characteristic symptoms. Their clinical manifestations depend on the site and the size of the cyst. Small peripherally located cysts are usually asymptomatic, whereas large central cysts may manifest with symptoms of compression of adjacent organs. If the patient is symptomatic, the first complaint is often a nonproductive cough; some patients, particularly those with centrally located cysts, may have blood-streaked sputum, although massive hemoptysis does not occur. Some patients complain of a dull or acute chest pain or they present with a sensation of pressure in the chest with no aggravating or relieving features. During infancy the hydatid cyst may disturb the growth of the child. In children who have a supple chest wall, a bulge in the ipsilateral chest may also be observed (see Fig. 91-3).
Rupture of the cyst into an adjacent bronchus may be manifested by vigorous coughing and expectoration of salty sputum consisting of mucous hydatid fluid and occasionally fragments of the laminated membrane, generally described as grape skin or frothy blood. In addition, the patient may develop a severe hypersensitivity reaction manifested by generalized rash, high fever, pulmonary congestion, and severe bronchospasm. Occasionally the intrabronchial rupture of the cyst manifests with sudden and severe dyspnea, which may lead to suffocation and death from complete tracheal obstruction by fragments from the hydatid membrane. Arce (1941) pointed out that the diagnosis of rupture of the hydatid cyst is unequivocally made when the hooklets of the parasite are found during microscopic examination of the sputum. Complete intrabronchial evacuation of the hydatid contents may result in cure of the disease in few patients. More often the parasitic membrane and a significant quantity of parasitic fluid remains in the pericystic cavity and the patient experiences complications from local infection. In this case the most usual manifestations are fever, chronic cough, mucopurulent or dark bloody sputum, anorexia, and weight loss. Rupture of the cyst into the pleural cavity is a severe but infrequent complication. It occurred in 5% of the patients of Ramos and colleagues (2001) and in 3.5% of the patients of Kilani and colleagues (1988). The symptoms are usually insidious and moderate; they consist of dry cough, chest pain, moderate dyspnea, generalized malaise, and fever. These relatively mild clinical manifestations result from preexisting pleural adhesions, which prevent the dissemination of the cyst contents into the whole pleural space. In some patients, particularly those without preexisting pleural adhesions, intrapleural rupture of the cyst produces an acute and dramatic clinical picture consisting of intense chest pain, persistent cough, severe dyspnea and even cyanosis, shock, and suffocation. Frequently symptoms of generalized urticaria, intense pruritus, severe anaphylactic shock, and even death can occur. The symptoms of intrapleural rupture of a hydatid cyst are accompanied by the physical findings of localized or generalized hydropneumothorax. Intrapleural rupture of an infected cyst causes hydatid empyema, which has the clinical signs of a pleural empyema but lacks the allergic manifestations of a simple uninfected cyst.
DIAGNOSIS
Diagnosis of an intact echinococcal cyst is usually based on a suspicion resulting from an unexpected finding on routine chest radiographs. Radiographically the cyst appears as a homogeneous spherical opacity with definite edges (Fig. 91-4). Sharma and Eggleston (1969) emphasized that a change from a spherical to an oval shape may be observed only during deep inhalation (Escudero-Nemerow sign). The radiologic picture depends mainly on the size and location of the cyst. A small cyst may appear as a small vesicle and is difficult to recognize until it grows large enough to
P.1302
present a clear image on the chest radiograph. A cyst may cause distal bronchial obstruction, manifested as atelectasis and pneumonitis beyond the cyst. Centrally located cysts may compress the bronchovascular structures, presenting radiographically as a depression or indentation at the site of pressure, the so-called notch sign. The cyst may also have a clear crescentic shadow on the top or on one side, referred to as a pneumocyst by D v (1935), perivesicular pneuma by Arce (1941), perivesicular meniscus by Peschiera (1972), moon sign or crescent sign by Barret and Thomas (1952), and pulmonary meniscus sign by Saidi (1976). This sign has been attributed to the air that enters the perivesicular space, becoming trapped between the adventitia and the unruptured vesicle after vigorous coughing, straining, or direct trauma to the cyst, and is the first radiographic sign of impending rupture of the cyst. A double-dome arch sign, as Arce (1941) noted, may appear when a small additional amount of air enters the hydatid vesicle. The entrance of free air into the cyst and the perivesicular space after the complete rupture of the laminated membrane displaces the fluid. This finding has been termed a camalote sign by Arce (1941) or a water lily sign by Lagos-Garcia and Segers (1924). It is produced by the floating membrane of the cyst (Fig. 91-5). The three diagnostic signs described, however, are radiographic rarities. Beggs (1985) reviewed the radiographic findings. Most cysts present as a solid mass in the right lower lobe. They were multiple in 29% of the patients of Burgos and colleagues (1999) and in 28% of the patients of Aytac and colleagues (1977). The incidence of bilateral pulmonary hydatidosis varies from 2% to 30%, according to Solak (1988), Dogan (1989), Mutaf (1994), and Karaoglanoglou (2001) and their colleagues. Therefore, every discrete radiologic lesion observed in any patient over 3 years of age in an endemic area should be considered a hydatid cyst. The radiographic pleural manifestations in the acute stage of rupture of a cyst vary from loculated hydropneumothorax to nonloculated partial, complete, or tension hydropneumothorax. The water lily sign can also be observed in instances of rupture of the cyst into the pleura (Fig. 91-6). A nodular extrapulmonary appearance suggests secondary hydatidosis of the pleura. As Dogan and colleagues (1989) noted, the usual radiographic signs after rupture of a cyst into the bronchi are an air meniscus, water lily sign, and incarcerated membrane.
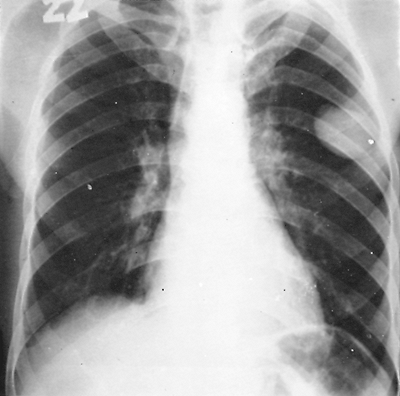 |
Fig. 91-4. Routine frontal chest radiograph showing a small peripheral hydatid cyst in the left upper lung field. |
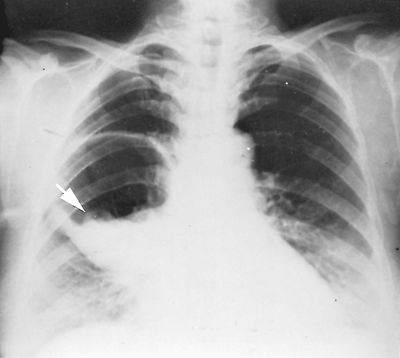 |
Fig. 91-5. Frontal chest radiograph of a ruptured echinococcal cyst in the right lower lung field, with the cystic membrane floating on the hydatid fluid (arrow), the water lily sign. |
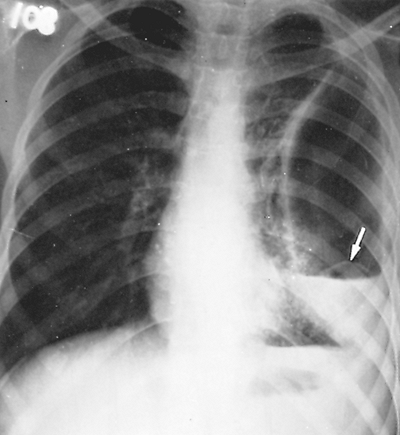 |
Fig. 91-6. Frontal chest radiograph showing a collapsed echinococcal membrane lying above the air fluid level (arrow), the water lily sign, in a 15-year-old boy admitted with a pyothorax. |
P.1303
CT scanning, as Saksouk (1986) and Lewall (1986) and their associates reported, has added to the diagnosis of hydatid disease of the lung, particularly to the early discovery of coexistent small cysts in the lung and of pending or existing rupture of the cyst. CT scanning also elucidates the cystic nature of a lung mass, particularly one located in the mediastinum. von Sinner (1991) reported two diagnostic signs indicating a collapsed parasitic membrane, the serpent sign and the whirl sign. Also, as Kalovidouris and colleagues (1984) noted, CT scanning appears to be valuable in the follow-up of patients who have had resection or evacuation of hydatid disease of the lung.
Magnetic resonance imaging may show detached membranes, local host reactions, or communications between the cyst and the bronchial tree in ruptured cysts. It may also show the regression of the cyst during chemotherapy with albendazole, which destroys the germinal layer and accelerates the degeneration of the parasite or increases the local host reaction. von Sinner (1991) and von Sinner and colleagues (1991) reported that the local host reaction is shown as a ring enhancement sign (hypervascularization) or as halo sign (allergic atelectatic or inflammatory response).
The diagnosis of a hydatid cyst of the lung necessitates investigation for other possible locations of the disease. Ultrasonography and echocardiography are two methods for evaluation of hepatic or pericardial-cardiac cysts.
The laboratory diagnosis of hydatid disease is complementary to the clinical and radiologic methods. As Faust and Russel (1964) and one of us (HA) (1968) reported, eosinophilia occurs in 20% to 34% of patients with echinococcosis. Because an increase in eosinophils is also observed in many other diseases, the test has limited diagnostic value. Casoni's intradermal reaction and Weinberg's complement fixation test were widely used in the past. These tests are no longer recommended because of their variable sensitivity and limited specificity. As Biava and colleagues (2001) note, many serologic tests are being proposed today. They include the indirect immunofluorescence assay; the indirect hemagglutination test, where the soluble antigen is coated with latex particles or sheep red blood cells treated with formaldehyde or glutaraldehyde; immunoelectrophoresis or counterimmunoelectrophoresis with antigen 5; and the enzyme-linked immunosorbent assay, especially with an enzyme-conjugated immunoglobulin G. Biava and colleagues (2001) emphasize that a judicious application of these techniques confirms the diagnosis in 80% to 94% of hepatic hydatidosis cases and in the 65% of pulmonary cases. However, seronegativity cannot exclude hydatidosis. False-negative results are obtained when the cysts are calcified, even if they are fertile. Serologic tests do not replace imaging methods. They can, however, confirm the hydatid origin of a cyst. A serologic survey is necessary for the follow-up of patients after surgery. The specific antibodies increase 4 to 6 weeks after surgery, followed by a slow decrease for the next 12 to 18 months. Biava and colleagues (2001) report that persistently high antibody titers or a secondary increase in the antibody titers 6 to 12 months postoperatively indicate a recurrence.
TREATMENT
The treatment of pulmonary hydatid cyst is essentially surgical. The benzimidazole compounds albendazole (ABZ) and mebendazole (MBZ) exert a direct effect on cumulus oophorus and perhaps also on the wall of the cyst. Saimot (2001) contends that factors influencing the efficacy of benzimidazoles correlate inversely with the size and age of the parasite, calcification of the cyst wall, and pericystic fibrosis. For these reasons lung cysts are more accessible to the chemotherapeutic agents than are cysts in other organs. Morris and colleagues (1985) used ABZ (10 mg/kg/day) and noticed some remission in 15 of 22 patients. Aggarwal and Wali(1991), however, used the same agent in 10 patients with pulmonary hydatid cyst for 8 weeks with little response. Because the individual studies produced inconsistent results, the World Health Organization (WHO) conducted multicenter trials of the efficacy of chemotherapy on human hydatidosis. Davis and colleagues (1986) studied 121 patients, and the success rate (partial or total) was 14% in the MBZ group and 30% in the ABZ group. A second trial by Davis and colleagues (1989) analyzed the results from 112 patients and suggested that ABZ was more effective than MBZ (39.1% vs. 16.7% success ). Saimot (2001) reported that the available data until 1997 gave an overall cyst response rate (cure or improvement) of 72.6%. The most common adverse events of patients treated with ABZ according to WHO guidelines (1996) are changes in liver enzymes and alopecia. The WHO guidelines recommend chemotherapy for inoperable primary liver or lung echinococcosis and for patients with multiple cysts in two or more organs. Morris (1987) reported that preoperative use of ABZ or MBZ may reduce the risk for recurrence of echinococcosis and facilitate the operation. Dogan and colleagues (1989) and Topcu and colleagues (2000) noted successful results with postoperative chemotherapy in the prevention of postoperative local recurrence. Dhaliwal and Kalkat (1997) advocate ABZ postoperatively for complicated hydatidosis and for the treatment of patients with inoperable cysts (10 14 mg/kg/day in cycles of 1 month with 2-week interval between cycles).
The surgical treatment of hydatid cyst of the lung has undergone considerable change since the 1950s. Numerous surgical procedures based on the evacuation of the cyst through the chest wall in one or two stages have been used. These methods are not applicable when the cysts are near the hilum of the lung and are inadequate because secondary echinococcal cysts may develop in the surgical wound and a chronic bronchocutaneous fistula may be
P.1304
created. The objective of the surgical treatment of pulmonary hydatidosis is to eradicate the parasite, to prevent intraoperative rupture of the cyst and subsequent dissemination, and to eliminate the residual cavity, with the maximum preservation of lung tissue. Because of the great variability in the pathology of pulmonary hydatid disease, surgical treatment must be individualized for each case. Numerous researchers, including Cetin (1988), Halezeroglou (1997), Burgos (1999), Topcu (2000), Karaoglanoglou (2001), and Aribas (2002) and their associates, prefer as a first choice of treatment lung-sparing operations such as enucleation of the cyst (Barrett's technique) or pericystectomy (P rez-Fontana technique) with closing of the bronchial openings with or without capitonnage of the pericystic space. Unnecessary resection of the lung must be avoided because the compressed lung is usually healthy and reexpands after excision of the cyst. Segmental resection is indicated for the treatment of large simple cysts that almost completely occupy the involved segment. It can also be used for complicated cysts of moderate size, if infection does not extent beyond the segmental plane. Lobectomy should be performed only when the size and number of cysts and the degree of infection exclude lesser procedures. The principal indications for lobectomy are large cysts involving more than 50% of the lobe, cysts with severe pulmonary suppuration not responding to preoperative treatment, multiple unilocular cysts, and sequelae of hydatid disease, such as pulmonary fibrosis, bronchiectasis, or severe hemorrhage. Pneumonectomy is rarely indicated for the treatment of hydatid disease of the lung and should be used only when the whole lung is involved in the disease process, leaving no salvageable pulmonary parenchyma. Although the reasons for conservative surgery are well known, different rates of resection are reported. The anatomic resection rate ranges from 4.3% in the series of Ayuso and colleagues (1981) to 48.3% in the cases of Burgos and colleagues (1999).
The preoperative preparation of the patient with hydatid disease is similar to the preparation of the patient undergoing thoracotomy for other comparable pulmonary lesions. Patients with small peripheral lesions require limited preparation, but patients with suppurative cysts should be treated with postural drainage, antibiotics, and other supportive measures until the suppurative process is as minimal as possible. Because echinococcal cysts not detected by the preoperative evaluation may exist in the liver or elsewhere, the possibility of future surgical intervention should be discussed with the patient preoperatively.
Bilateral lung cysts should be resected in two stages (Fig. 91-7). In a patient with uncomplicated cysts, the lung with the larger cyst or more numerous cysts should be approached first. In a patient with a lung cyst larger than 4 or 5 cm in one lung and a ruptured cyst in the other lung, the intact cyst should be removed first in order to prevent its rupture. The contralateral lesions are then resected 2 to 4 weeks after the first operation (Fig. 91-8).
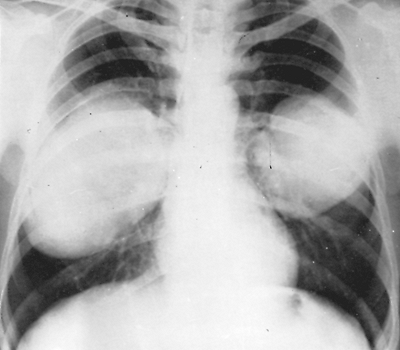 |
Fig. 91-7. Frontal chest radiograph shows two intact echinococcal cysts, one in each lung. Both were enucleated by separate thoracotomies at a 2-month interval. |
A double-lumen endotracheal tube is used to prevent blockage of the tracheobronchial tree by the cyst contents during the operation. A posterolateral thoracotomy through the fifth, sixth, or seventh intercostal space or rarely through the bed of the fifth, sixth, or seventh rib is performed while taking extreme care not to rupture the cyst. The most superficial portion of the intact cyst is devoid of pulmonary parenchyma, is a round, grayish white area, and is devoid of blood vessels (Fig. 91-9A). The perimeter of this area is dark, ill defined, and blends into the normal lung
P.1305
tissue. This appearance and particularly the elastic feel on palpation of the mass differentiate the hydatid cyst from a neoplastic lesion. The management of bilateral pulmonary cysts as suggested in earlier publications by one of us (HA) (1968), as well as Xanthakis (1972), Aytac (1977), and Dogan (1989) and their co-workers was resection in two stages with an interval of 2 to 3 weeks between interventions. According to Petrov and colleagues (2001), the indications for a two-stage operation are: (a) a larger number of pulmonary cysts, especially in complicated echinococcosis; (b) when lobectomy is required in the presence of pleural adhesions; (c) poor cardiopulmonary reserve; and (d) uncompensated chronic conditions.
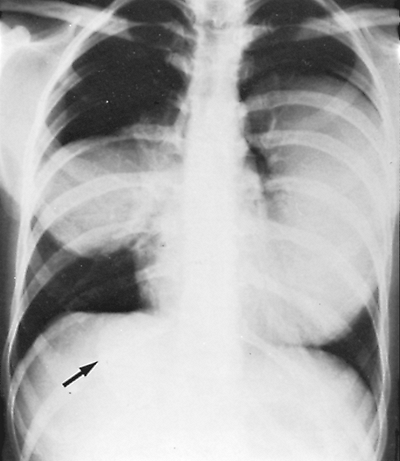 |
Fig. 91-8. Frontal chest radiograph shows three echinococcal cysts, one in each lung and one in the liver. Left hydatid cyst was removed first. Six weeks later, the cysts (arrow) from the right lung and liver were treated simultaneously. |
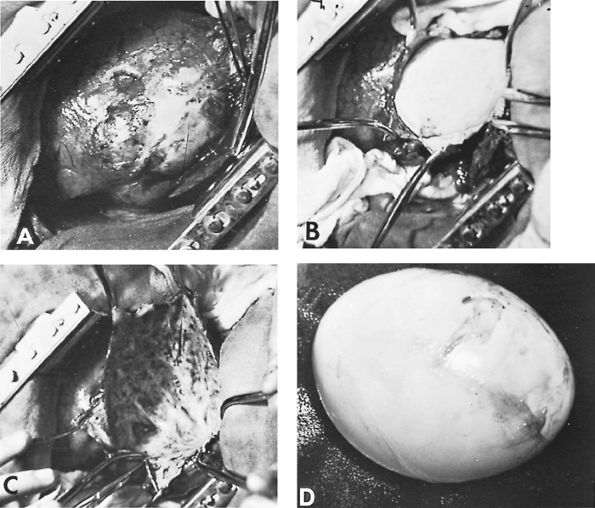 |
Fig. 91-9. Intraoperative photographs. A. The lung containing the hydatid cyst. Note the grayish white surface of the lung where the cyst is located. B. The hydatid cyst being dissected. C. Residual pericystic cavity after cyst removal. D. Hydatid cyst after excision. |
Many researchers advocate various operative strategies for the one-stage management of bilateral lung echinococcosis.Kariev (1986), Cetin (1988), Burgos (1999), and Petrov (2001) and their colleagues prefer median sternotomy. Denis and associates (1969) and Galindo and co-workers (1981) have reported one-stage bilateral thoracotomies. Burgos and associates (1999) and Narbonaand Elarre (1975) have used the transsternal submammarian thoracotomy (Clamshell incision) for bilateral pulmonary cysts. Minithoracotomy with video-assisted thoracotomy has been used for small solitary cysts in both lungs by Petrov (2001). Although Karapetyan (1997) and Burgos (1999) and their colleagues recommend median sternotomy only for bilateral cysts located in an anterior position, Cetin (1988) and Petrov (2001) and their co-workers suggest that median sternotomy can be used for any size, number, or location of cysts in patients in good general condition. However, median sternotomy is not recommended when extensive resection is required (particularly left lower lobectomy) or infection of the cyst or pleural complications exist.
P.1306
Intact Cysts
Resection by Enucleation without Needle Aspiration
Excision of an intact hydatid cyst without needle aspiration is accomplished by careful separation of the laminated membrane from the pericystic zone. The separation of these two components of the parasitic cyst is feasible even though they adhere intimately to one another. Barret (1947) reported that the enucleation of small cysts can usually be accomplished without difficulty. Large cysts, however, present a greater challenge because of the increased possibility of rupture during the separation of the pericystic zone from the laminated membrane. Because this complication is occasionally unavoidable, the surgical field must be protected from the possibility of spillage of the parasitic material with resultant contamination of the pleural space and surgical wound. Therefore, after the hydatid cyst is identified, the surgical wound and the adjacent lung tissue are covered with packed gauze steeped in normal saline solution, so that only the area of the lung that contains the cyst is exposed. Because the gauze material filters only macroscopic material, the hydatid fluid may still enter the pleural space and precipitate an allergic reaction. For this reason, before the dissection is begun, two suctions must always be present. After the part of the lung containing the cyst is isolated, the tissue overlying the cyst is incised and the cyst is exposed. A cruciate or stellate incision is then made on the pericystic zone. With blunt dissection, a small space is created between this zone and the laminated membrane. The separation of the two zones is facilitated by traction on the edges of the pericystic zone (Fig. 91-9B). During the dissection, the airway pressure is lowered to avoid the protrusion of the laminated membrane through the opening of the pericystic zone. After the two zones are completely separated, the airway pressure is increased in order to facilitate removal of the cyst. Before and during delivery of the cyst, the laminated membrane should never be grasped with an instrument, and significant manual pressure on the pericystic zone should not be used to avoid its rupture.
After removal of the cyst, the residual cavity, which is devoid of epithelium and always has some bronchial openings, must be managed appropriately (Fig. 91-9C). The management of the residual cavity has historically passed through different stages. Posadas (1899) advised only suturing of the bronchial openings. This practice, however, did not prevent air leak sufficiently. Therefore, fixation of the edges of the sutured pericystic zone to the thoracotomy incision was later added to this method. D lb t (1899) advocated the folding of the pericystic zone by sutures, a method named capitonnage. Crausaz (1967) used purse-string sutures from the base of the pericystic cavity upward to obliterate the cavity. Allende and Langer (1947) supplemented this method with suturing of the individual bronchial openings within the cavity. Chrysospathis (1966) closed the bronchial communications at a more proximal point. Each of these methods, in the hands of their proponents, yielded good results. One of us (HA) favors, especially for a large pericystic cavity, closure of the bronchial openings, partial pericystectomy (i.e., resection of the free portion of the pericystic zone), elimination of the residual cavity by capitonnage, and closure of its edges with continuous sutures. It is generally agreed, however, that the most important point of the management of the residual cavity is secure closure of patent bronchial openings. After the grossly evident bronchial openings are closed, smaller sites can easily be detected by filling the residual cavity with normal saline solution. With the application of positive pulmonary pressure, air escaping through any bronchial openings is visualized by the formation of bubbles. This maneuver must be repeated until sealing of all air leaks is achieved.
Removal of Intact Cyst After Needle Aspiration
The danger of development of secondary hydatid cysts because of spillage of hydatid fluid as a result of violation of the cyst's integrity by needle aspiration or rupture has led to the development of various chemical substances capable of rendering the hydatid fluid sterile. Formalin and formaldehyde solutions have mainly been used in the past as scolecoidal agents during operation. The escape of these substances into the pericystic space causes irritation of the tissue which results in impairment of healing and, as Saidi (1976) noted, in the formation of bronchial fistulae. Silver nitrate solution, 0.5%, has scolecoidal properties and is used accordingly. Hypertonic saline solution, which is considered to have scolecoidal properties, does not affect tissue healing. Accordingly, we prefer this agent.
Before the needle aspiration is begun, two suctions should be available. The surgical wound and the lung except for the segment containing the cyst are covered with packed gauze, as described previously. The lobe that contains the cyst is immobilized, the lung is maintained inflated, and a 20-or 21-gauge needle, which is connected to a 20- or 50-mL syringe, is inserted into the most prominent portion of the cyst. The hydatid fluid is incompletely aspirated while the needle is maintained immobile.
The remaining fluid is then removed in one of two ways. First, after withdrawal of the aspirating needle, the wall of the parasitic cyst is incised, a suction tip is immediately introduced into the cyst, and the remaining contents of the cyst are removed. The suction tip should be of the sump type, to avoid blocking of the suction channel by portions of membrane or, rarely, by daughter cysts. While the preceding evacuation is being executed, a second suction is used to remove any hydatid fluid that may overflow from the cystic cavity. Second, after most of the hydatid cyst is aspirated, instead of opening the pericystic and cystic walls, a trocar with a side arm connected to a suction apparatus is inserted in the cyst through the same orifice immediately after the removal of the aspiration needle. The remaining
P.1307
hydatid contents are evacuated, the trocar is removed, the opening of the cyst is enlarged, and a sump suction tip is introduced to withdraw the remaining contents of the cyst. Throughout the entire period of evacuation of the parasitic contents of the cyst, the lungs must be kept inflated. With the maintenance of constant positive endotracheal pressure, none of the escaped parasitic fluid in the pericystic cavity can advance through the bronchial openings into the bronchial tree. Finally, the remainder of the laminated membrane is removed, the residual cavity is irrigated with hypertonic saline solution, and the bronchial openings are closed in the manner described previously.
Many surgeons, including Burgos (1999), Ramos (2001), Petrov (2001), Karaoglanoglou (2001), Aribas (2002) and their associates, prefer Barret's method for small cysts without tension. In most cases they remove the cyst after preliminary hydatid cyst fluid aspiration. Qian (1988) reported that in 106 patients operated with Barret's method, operative rupture occurred in 35 (33%), with recurrence in 2 (1.9%). In the same series in 316 patients treated with aspiration of the cyst recurrence was found in 5 cases (3.7%).
Pericystectomy
P rez-Fontana (1951) described removal of the pericystic zone with the intact cyst. The technical difficulty with this method is the creation of the appropriate plane through the pulmonary tissue, near and around the parasitic cyst, with resulting bleeding and air leak. This method can be easily applied in superficially located small cysts.
Ruptured Cysts
The management of a ruptured cyst during the acute stages is mainly directed toward the prevention of major complications resulting from the evacuation of the cystic contents into the tracheobronchial tree or the pleural space. Preventive precautions include the maintenance of the airway free of secretions and cystic tissue by appropriate orotracheal suction or bronchoscopy, evacuation of the hydropneumothorax, and treatment of anaphylactic reaction. After the acute period, the most conservative treatment should be used to save as much lung tissue as possible. Ramos (2001) and Kilani (1988) and their associates advocate that the rupture of a cyst into the pleura must be treated urgently using an operation that eliminates the hydatid elements that are found in the pleural cavity. An infected cyst is opened with minimal damage to the adjacent lung parenchyma, its contents are evacuated, and the cavity is thoroughly irrigated. The bronchial openings with or without capitonnage of the residual cavity are then closed and the pleural space is drained. When the infection is significant and there is advanced pericystic pneumonitis, lobectomy is the operation of choice.
PROGNOSIS
Postoperative complications are influenced by the size and number of cysts and the type of operation. In the series of Ayuso (1981), Halezeroglou (1997), Karaoglanoglou (2001), and Aribas (2002) and their colleagues, the complication rate ranged between 12.9% and 19%. Statistical analysis of 8,384 cases of pulmonary hydatidosis by Aubert and Viard (1983) revealed that the most common complications were pleural infection in 2.54% of the patients and prolonged air leakage in 2.19%.
The operative mortality in large series doesn't exceed 2%. Dogan and colleagues (1989) reported an operative mortality rate of 1.25%, and Petrov and colleagues (2001) reported a rate of 0.78%. Athanassiadi (1998) and Aribas (2002) and their colleagues (2002) do not mention any hospital mortality. The recurrence rate is also very low. Burgos and colleagues (1999) reported that 98.3% of the surviving patients were free of hydatid disease 18 years after the operation. Ayuso and colleagues (1981) found on overall recurrence rate of 2.7%, while in the series of Petrov and colleagues (2001) and Karaoglanoglou and co-workers (2001) no recurrence was noted.
In a series of 115 patients treated by one of us (HA), 78 underwent enucleation of the cyst and capitonnage of the remaining cavity, with individual closure of the bronchial openings. Two patients had cystectomy and wedge resection of the surrounding parenchyma; 3 patients underwent cystectomy according to the P rez-Fontana method; 14 had enucleation without capitonnage; and 1 patient had enucleation with pleurectomy. Twelve patients underwent lobectomy, and two had segmentectomy. No deaths were encountered. In a follow-up period of 2 to 25 years, no recurrence has been noted. Therefore with appropriate treatment, the prognosis is excellent.
REFERENCES
Aggarwal P, Wali J Jr: Albendazole in the treatment of pulmonary echinococcosis. Thorax 46:599, 1991.
Aletras HA: Hydatid cyst of the lung. Scand J Thorac Cardiovasc Surg 2:918, 1968.
Allende JM, Langer L: Tratamiento de los quistes hydatidicos del pulmon. Boletin y Trabajos. Acad Argentina Chir 31:537, 1947.
Arce J: Hydatid cyst of the lung. Arch Surg 43:789, 1941.
Aribas OK, et al: Comparison between pulmonary and hepatopulmonary hydatidosis. Eur J Cardiothorac Surg 21:489, 2002.
Athanassiadi K, et al: Surgical treatment of echinococcosis by a transthoracic approach: a review of 85 cases. Eur J Cardiothorac Surg 14:134, 1998.
Aubert M, Viard P: Etude statistique sur l'hydatidose pleuro-pulmonaire dans le basin M diterran en en 1982: a propos de 8384 cas. Ann Chir 37:74, 1983.
Aytac A, et al: Pulmonary hydatid disease: report of 100 patients. Ann Thorac Surg 23:145, 1977.
Ayuso LA, et al: Surgical treatment of pulmonary hydatidosis. J Thorac Cardiovasc Surg 82:569, 1981.
Bakir F, Al-Omeri MM: Echinococcal tension pneumothorax. Thorax 24:547, 1969.
Barret NR: Surgical treatment of the hydatid cyst of the lung. Thorax 2:21, 1947.
P.1308
Barret NR, Thomas D: Pulmonary hydatid disease. Br J Surg 40:222, 1952.
Beggs I: The radiology of the hydatid disease. AJR 145:639, 1985.
Biava MF, Dao A, Fortier B: Laboratory diagnosis of cystic hydatid disease. World J Surg 25:10, 2001.
Burgos R, et al: Pulmonary hydatidosis: surgical treatment and follow-up of 240 cases. Eur J Cardiothorac Surg 16:628, 1999.
Cetin G, et al: Surgical treatment of bilateral hydatid disease of the lung via median sternotomy: experience in 60 consecutive patients. J Thorac Cardiovasc Surg 36:114, 1988.
Chrysospathis P: Echinococcus cysts of the lung. Dis Chest 49:278, 1966.
Crausaz PH: Surgical treatment of the hydatid cyst of the lung and hydatid cyst of the liver with intrathoracic evolution. J Thorac Cardiovasc Surg 53:116, 1967.
Davis A, Dixon H, Pawlowski ZS: Multicentre clinical trials of benzimidazole-carbamates in human echinococcosis (phase 2). Bull WHO 67: 503,1989.
Davis A, Pawlowski ZS, Dixon H: Multicentre clinical trials of benzimidazole carbamates in human echinococcosis. Bull WHO 64:383, 1986.
D lb t P: Kystes hydatiques du foie trait s par le capitonnage et al suture sans drainage. Bull Mem D Soc Chir Paris 25:30, 1899.
Denis B, et al: Traitement chirurgical du kyste hydatique du poumon. A propos de 573 cas. Ann Chir Thorac Cardiovasc 8:189, 1969.
D v F: Sur la st rilisation du samble hydatique par les solutions formol s et les solutions iod es. C R Soc Biol 119:352, 1935.
Dhaliwal RS, Kalkat MS: One-stage surgical procedure for bilateral lung and liver hydatid cysts. Ann Thorac Surg 64:338, 1997.
Dogan A, et al: Surgical treatment of hydatid cysts of the lung: report on 1055 patients. Thorax 44:192, 1989.
Faust EC, Russel PF: Craig and Faust's Clinical Parasitology. London: Kimpton, 1964, p:678.
Galindo R, et al: Le traitement du kyste hydatique pulmonaire ches l'enfant. Ann Chir 35:213, 1981.
Halezeroglou S, et al: Giant hydatid cysts of the lung. J Thorac Cardiovasc Surg 113:712, 1997.
Ivanissevich O, Rivas CI: Equinococcosis Hidatidica. Buenos Aires: Talleres Gr ficos Ministerio Educacion y Justicia, 1962, pp. 160 172.
Kalovidouris A, et al: Postsurgical evaluation of hydatid disease with CT: diagnostic pitfalls. J Comput Assist Tomogr 8:1114, 1984.
Karaoglanoglou N, et al: Giant hydatid lung cysts. Eur J Cardiothorac Surg 19:914, 2001.
Karapetyan E, Hovhannisyan A, Galstryan K: Simultaneous surgical operations in the presence of bilateral lung echinococcosis. In International Congress of Thorax Surgery. Athens, Greece, July 1 8, 1997. Bologna: Monduzzi Editore, 1997, pp. 83 86.
Kariev T, Ingamberrdiev I, Tuhastinov I: Ednoetapnaia dvustoronnaia ehinokokektomia lehkih iz cherezgrydinnogo dostupa. Khirurgia 5:23, 1986.
Kilani T, et al: Les complication pleurales du kyste hydatique du pnoumon: a propos de seize cas. Ann Chir 42:145, 1988.
Kourias B, Tobler AL: L'avenir loigen des op res pour kyste hydatique du poumon. Etude de 265 cas sur 305 op res. Lyon Chir 53:209, 1957.
Lagos-Garcia C, Segers A: Consideraciones sobre un caso de quiste hidatico pulmonar abierto in bronquios. Semin Med Bs As 31:271, 1924.
Larbaoui D: Le kyste hydatique de poumon. Rev Pneumol Clin 45:49, 1989.
Lewall DB, Bailey TM, McCorkell SJ: Echinococcal matrix: computed tomographic, sonographic and pathologic correlation. J Ultrasound Med 5:33, 1986.
Morris DL: Preoperative albendazole therapy for hydatid cyst. Br J Surg 74:805, 1987.
Morris DL, et al: Albendazole: objective evidence of response in human hydatid disease. JAMA 253:2053, 1985.
Morseth DJ: Fine structure of the hydatid cyst and protoscolex of echinococcus granulosus. J Parasitol 53:312, 1967.
Mutaf O, et al: Pulmonary hydatidosis in children. Eur J Pediatr Surg 40:70, 1994.
Narbona B, Elarre A: Traitement des kystes hydatiques pulmonaires. A propos de 455 kystes operes. Helth Hir Acta 42:303, 1975.
Peleg H, Best LA, Gaitini D: Simultaneous operation for hydatid cysts of right lung and liver. J Thorac Cardiovasc Surg 90:783, 1985.
P rez-Fontana V: La patologia del guiste hydatico del pulmon. Arch Int Hidatid 8:47, 1948.
P rez-Fontana V: Traitement chirurgical de kyste hydatique dus poumon. La m thode ruguayenne ou extirpation du perikyste. Arch Int Hydatid 72:469, 1951.
Peschiera CA: Hydatid cyst of the lung. In Steele JD (ed): The Treatment of Mycotic and Parasitic Diseases of the Chest. Springfield. IL: Charles C Thomas, 1964, p. 201.
Peschiera CA: Hydatid disease of the lung. In Shields TW (ed): General Thoracic Surgery. Philadelphia: Lea & Febiger, 1972.
Petrov DB, et al: Surgical treatment of bilateral hydatid disease of the lung. Eur J Cardiothorac Surg 19:918, 2001.
Pieters G, Van den Spiefel A, Demeester P: Le kyste hydatique pulmonaire (257 cas). Acta Chir Belg 75:563, 1976.
Posadas A: Traitement des kystes hydatiques. Rev Chir 19:374, 1899.
Qian Z: Thoracic hydatid cysts: a report of 842 cases treated over a thirty- year period. Ann Thorac Surg 46:342, 1988.
Ramos G, Ordu a A, Garcia-Yuste MG: Hydatid cyst of the lung: diagnosis and treatment. World J Surg 25:46, 2001.
Rudolphi KA: Entozoorum Sive Verminum Intestinalium. Historia Naturalis. Vol. 2 in Taberna, Libraria and Artinum, Amsterdam 1808, p. 247. Saidi F: Surgery of the Hydatid Disease. Philadelphia: WB Saunders, 1976.
Saimot AG: Medical treatment of liver hydatidosis. World J Surg 25:15, 2001.
Saksouk FA, Fahl MH, Rizk GH: Computed tomography of pulmonary hydatid disease. J Comput Assist Tomogr 10:226, 1986.
Sarsam A: Surgery of the pulmonary hydatid cysts: review of 155 cases. J Thorac Cardiovasc Surg 62:663, 1971.
Schwabe CW: Host-parasite relationship in echinococcosis: observations on the permeability of the hydatid cyst wall. Am J Trop Hyg 8:20, 1959.
Sharma SK, Eggleston FC: Management of hydatid disease. Arch Surg 99:59, 1969.
Smyth JD: In vitro studies and host-specificity in echinococcus. Bull WHO 39:5, 1968.
Solak H, et al: Surgery in hydatid cyst of the lung. A report of 460 cases. Scand J Thorac Cardiovasc Surg 22:101, 1988.
Todorov T, Boeva V: Echinococcosis in children and adolescents in Bulgaria: a comparative study. Ann Trop Med Parasitol 94:135, 2000.
Toole H, et al: Consid ration sur la therapie actuelle des kystes hydatiques de poumon. Appr cition de proc des op ratoires. Rev Med Moyen Orient 17:758, 1960.
Topcu S, et al: Surgical treatment of pulmonary hydatid cysts in children. J Thorac Cardiovasc Surg 120:1097, 2000.
von Sinner WW: New diagnostic signs in hydatid disease: radiography, ultrasound, CT and MRI correlated to pathology. Eur J Radiol 12:150, 1991.
von Sinner WW, et al: MR imaging in hydatid disease. AJR 157:741, 1991.
WHO Informal Working Group on Echinococcosis. Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull WHO 74:231, 1996.
Xanthakis D, et al: Hydatid disease of the chest. Report of 91 patients surgically treated. Thorax 27:517, 1972.
Yuksel M, et al: Correlation between size and intracystic pressures of hydatid cysts. Eur J Cardiothorac Surg 12:903, 1997.
EAN: 2147483647
Pages: 203