22 - Conduct of Anesthesia
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section VII - Pulmonary Resections > Chapter 29 - Pneumonectomy and Its Modifications
Chapter 29
Pneumonectomy and Its Modifications
Thomas J. Kirby
Stanley C. Fell
Evarts Graham performed the first successful one-stage pneumonectomy in April 1933 on James Gilmore, a 48-year-old physician with a biopsy-proven carcinoma of the left upper lobe, as outlined by one of us (SCF) (2002). Graham had planned a lobectomy, but the operative findings were that the carcinoma extended into the left lower bronchus. Graham decided that pneumonectomy was the only acceptable procedure.
He initially occluded the pulmonary hilum with a catheter to assess the effects of sudden obstruction of the pulmonary artery, fearing that the symptoms of pulmonary embolism might ensue. The patient remained stable, however, and Graham proceeded with pneumonectomy by mass ligature of the hilum using chromic catgut. He also inserted 7 radon seeds of 1.5 millicurie each into the hilar stump. Horrified by the size of the empty pleural space, he performed a seven-rib thoracoplasty and closed the chest with catheter drainage. Gilmore's pathology specimen was subsequently reviewed and noted to be T2, N1, stage IIB.
The following month in 1933, Graham presented the case at the sixteenth Annual Meeting of the American Association for Thoracic Surgery in Washington, D.C. The case report was published by Graham and Singer in 1933 and also subsequently by Graham in 1949. Dr. Gilmore actually resumed practice and lived for 30 more years, ultimately dying of cardiorenal disease. Ironically, he outlived Graham, who succumbed to widely metastatic lung cancer in 1957.
Graham's presentation stimulated Archibald in Montreal, who performed a left pneumonectomy by hilar dissection in July 1933. Archibald closed the bronchus with a twisted silver wire. Two weeks after Archibald's successful case, Rienhoff (1936) at Johns Hopkins performed the first pneumonectomy by hilar dissection and closure of the bronchus with interrupted sutures, a technique employed to this day.
INDICATIONS FOR PNEUMONECTOMY
Currently, pneumonectomy is performed for carcinoma of the lung, usually bulky and centrally located. Occasionally, pneumonectomy is indicated for a destroyed lung secondary to chronic infections such as tuberculosis, bronchiectasis, and fungal disease.
All patients require a thorough evaluation to rule out metastatic disease and to ascertain the patient's physiologic capacity to withstand the resection (see Chapters 19 and 20). Pneumonectomy should never be performed in the absence of a definite histologic diagnosis.
OVERVIEW
The key tenet for a safe pneumonectomy is proper exposure. The customary approach is a posterolateral thoracotomy that enters the chest through the fifth interspace or the bed of the subperiosteally resected fifth rib. The operation can also be carried out using a muscle-sparing incision provided exposure is adequate (see Chapter 25). Video-assisted pneumonectomy has been reported, but should be employed only by surgeons with significant experience with this technique (see Chapter 33). Single-lung ventilation using either a double-lumen tube or a bronchial blocker has made pneumonectomy a technically easier procedure and should be used if possible.
Upon entering the chest, resectability is first assessed as described by Fry (1984). Inspection of the pleural space to rule out intrapleural metastases should be done and suspicious areas resected for biopsy. Locoregional spread, including invasion of major vascular and mediastinal structures such as the aorta, heart, vena cava, esophagus, spine, and trachea, precludes complete resection in virtually all instances and negates any benefit from proceeding with pneumonectomy (see Chapter 106). Mediastinal lymph nodes should be fully staged whether or not mediastinoscopy has been performed. This includes biopsy of paratracheal (level 2), tracheobronchial (level 4), subaortic (level 5), subcarinal (level 7), and pulmonary ligament (level 9) lymph nodes as described by Naruke and associates (1978). Any other suspect nodes should also be sampled. We do not routinely perform
P.471
mediastinal lymph node dissection, although many surgeons believe otherwise. In patients who have undergone induction therapy for clinical N1 or N2 disease, mediastinal lymphadenectomy is warranted. The main point is that any approach to the lymph nodes short of systematic sampling is unacceptable. Multistation involvement and extracapsular spread indicate that complete and therefore curative resection is not possible, again, a contraindication to pneumonectomy.
Finally, the surgeon must carefully evaluate the need for pneumonectomy. Occasionally, intraoperative findings allow lesser resections such as sleeve resection of the bronchus or pulmonary artery to be performed, allowing either the upper or lower lobe to be spared, as reported by Jensik and associates (1972) and subsequently confirmed by others (see Chapter 28).
The usual sequence of management of the hilar structures is artery, vein, and bronchus. However, circumstances may dictate that a different sequence be employed. For example, initial division of the bronchus facilitates exposure of the pulmonary artery. In instances in which identification of the hilar structures is obscured because of inflammation, transsection of the inferior pulmonary vein first will often facilitate identification and dissection of the remaining hilar structures. Tumor encroachment or invasion of a pulmonary vein should lead the surgeon to divide the veins first, often intrapericardially, to prevent tumor embolization. Some surgeons prefer to control the veins first based on the theory that this might lessen hematogenous spread of malignant cells. Contrary to older beliefs, the lung does not become engorged if the venous outflow is occluded before the artery (see Chapter 26).
Left Pneumonectomy
Hilar dissection is best performed using scissors. The mediastinal pleura is circumferentially incised around the hilus, and the lymph nodes are evaluated as previously described. The phrenic nerve is preserved and not sacrificed unless it is directly invaded by tumor in an otherwise resectable situation. Dissection of the mediastinal fat and lymph node bearing tissue around the main pulmonary artery is performed. The artery is then freed in the subadventitial
P.472
plane (Fig. 29-1). Division of the vagal branches to the posterior wall of the bronchus allows exposure of this structure. The areolar tissue between the anteromedial wall of the bronchus and pulmonary artery is now divided (Fig. 29-2). Pledget and finger dissection using the thumb and forefinger is used to encircle the artery, allowing a vessel loop to be placed around it (Fig. 29-3).
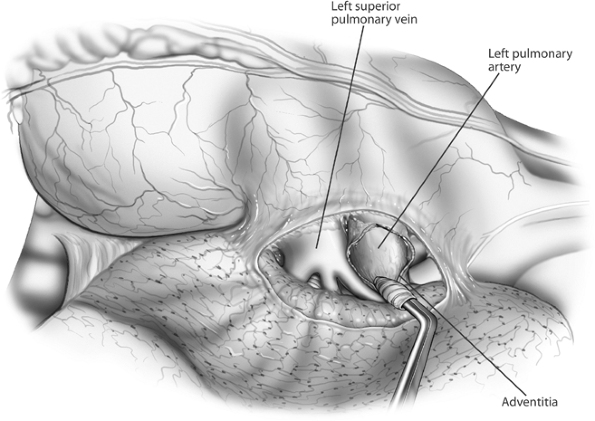 |
Fig. 29-1. Anterior aspect of the left hilum. The mediastinal pleura has been incised and the pulmonary artery rolled out of its adventitia, separating it from the superior pulmonary vein. |
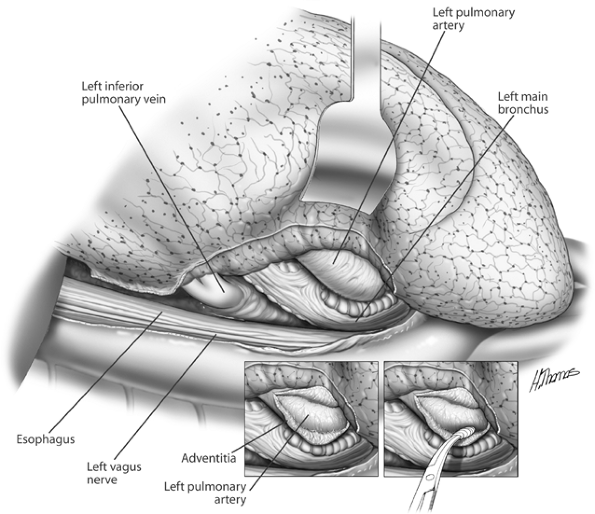 |
Fig. 29-2. Posterior aspect of the left hilum. The pulmonary artery is being separated from the left bronchus. |
The artery should now be occluded with a vascular clamp, tourniquet, or manually for at least 1 minute and up to 5 minutes to assess the patient's hemodynamic stability. Hypotension, tachycardia, and right ventricular dysfunction or dilation are indications that the patient will not physiologically tolerate a pneumonectomy. The procedure should be abandoned, or, if feasible, a Swan-Ganz catheter should be placed to assess hemodynamic parameters accurately and determine whether either fluid, inotropic support, or pulmonary vascular vasodilators (such as milrinone or nitric oxide) will improve cardiopulmonary function sufficiently to allow resection to proceed safely.
If further length of the artery is required, dissection may be carried proximally, transecting the ligamentum arteriosum and avoiding injury to the left recurrent laryngeal nerve. Additional length can be obtained by carrying out intrapericardial dissection of the artery, as described by Allison in 1946. The pericardium anterior to the phrenic nerve and just superior to the entrance of the superior pulmonary vein is opened and extended superiorly to the inferoanterior edge of the pulmonary artery. By sharp dissection, the artery can be freed from its pericardial investments. If the artery is to be divided at its origin, care should be taken not to compromise the lumen of the main pulmonary artery, an
P.473
event that would lead to obstruction of the right ventricular outflow tract.
The artery can now be safely transected using a variety of techniques. The proximal pulmonary artery can be secured with a vascular staple line, the distal artery taken with a Sarot clamp, and the artery slowly divided just proximal to the clamp to assess the integrity of the vascular staple line. The distal end can then be suture ligated (Fig. 29-4). Alternatively, the artery can be divided between two firings of a vascular stapler. The artery can also be divided between vascular clamps, and the ends oversewn with a vascular suture such as No. 5-0 Prolene. Adequate length of pulmonary artery is mandatory to perform the latter two techniques safely.
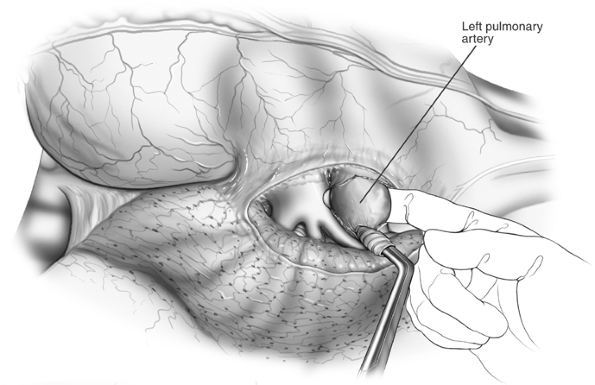 |
Fig. 29-3. Finger being passed around the artery. |
Attention is now directed to the bronchus, which must not be stripped of its adventitia. A denuded bronchus is subject to impaired healing with resultant bronchopleural fistula. A bronchial clamp is applied to the distal aspect of the left main-stem bronchus. Traction on the bronchus facilitates dissection of the proximal bronchus from the surrounding mediastinal fibroadipose and nodal tissue to the level of the carina. If a left-sided double-lumen tube or bronchial blocker is in place, it should be withdrawn into the distal trachea. Even if a right-sided double-lumen tube is in place, it should be withdrawn into the distal trachea so as not to interfere with a tension-free closure of the bronchus. If a stapler is used, it should be applied to the bronchus just distal to the carina, preventing a long stump, which may increase the risk for bronchopleural fistula. Before firing the stapler, airway pressures should be checked. A sudden and dramatic increase in airway pressure
P.474
with closure of the stapler indicates that the remaining airway may be compromised. The stapler should be reapplied correctly. The stapler can now be safely fired and the bronchus transected distal to the staple line with a scalpel using the anvil of the stapler as a guide. A suitable bronchus clamp is applied to the specimen end of the bronchus to avoid contamination of the operative field with airway secretions and to provide traction (see Fig. 29-5).
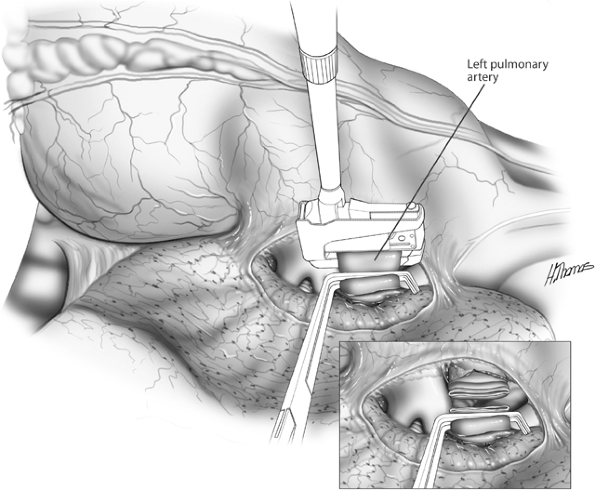 |
Fig. 29-4. Vascular stapler applied to proximal artery, which is clamped distally. Inset: The artery is divided and the distal stump suture ligated. |
If the bronchus is to be closed by manual suturing, the following technique is employed: Stay sutures are inserted at the upper and lower angles of the bronchus just proximal to the anticipated line of transsection. A bronchial clamp is applied to the specimen side of the bronchus, and the bronchus is divided using a scalpel and the lung removed. The bronchus is closed with interrupted No. 4-0 Vicryl sutures starting at the midpoint. The stay sutures are tied in situ. No sutures are cut until they are all in place.
The bronchial stump is checked for air leaks by flooding the chest with saline and having the anesthesiologist apply at least 20 cm of airway pressure. Any bubbling from the stump indicates an air leak that requires repair by additional suturing.
One of us (SCF) prefers to divide the bronchus before the veins. This maneuver provides better access and greater length to the veins as they are mobilized. The bronchus is dissected and divided as previously described. A bronchial clamp is applied to the specimen end of the bronchus, which when retracted, allows for exposure of the posterior aspects of the veins (see Fig. 29-5). The veins are now managed as previously described. Intrapericardial exposure of the left hilum is shown in Fig. 29-6.
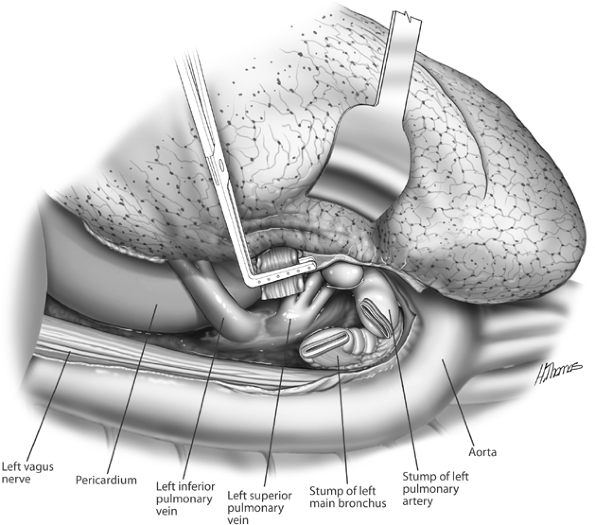 |
Fig. 29-5. The left main bronchus stapled and divided. Retracting the clamp on the distal bronchus facilitates exposure of the superior pulmonary vein. The inferior pulmonary ligament has been divided and the inferior vein exposed. The veins are then stapled. |
P.475
The hemithorax is irrigated and hemostasis secured. One us of (TJK) dissolves 160 mg of gentamycin along with 2 g of vancomycin in 500 mL of saline, which is left behind in the pneumonectomy space. One of us (SCF) does not balance the mediastinum, nor is the chest routinely drained except in infected cases or if the procedure has been bloody (i.e., pleuropneumonectomy, completion pneumonectomy). In infected cases, the pneumonectomy space is handled as a postpneumonectomy empyema, as described in Chapter 37, and balanced drainage is instituted. Otherwise, the chest tube is placed to straight drainage, clamped, and unclamped the next morning to drain any blood that may have accumulated overnight. If the patient is hemodynamically stable, the tube is removed.
One of us (SCF) routinely places a No. 28F catheter just above the diaphragm, which is connected to underwater-seal drainage without suction. When the patient is placed supine and the mediastinum reverts to its normal position, air will bubble through the underwater seal. Within a few minutes, 4 to 6 cm of negative intrathoracic pressure will be evident. The position of the trachea is checked. If it is found to be deviated from the midline, further drainage of air is indicated. The chest tube is then
P.476
clamped and the tube removed the following day if the chest radiograph is satisfactory. Alternatively, the drain can be left on underwater-seal drainage overnight. In infected or wet cases, the tube is allowed to drain for 24 hours, when the drainage becomes serous and is then removed. Commercially available balanced drainage systems can be used. Short-term drainage with a standard chest tube can be helpful in the postoperative period. If the patient becomes unstable, mediastinal shift is not a diagnostic issue with a properly functioning tube. Likewise, bleeding can be assessed rapidly and serially without the need for frequent radiographs or blood tests to confirm or rule out this possibility.
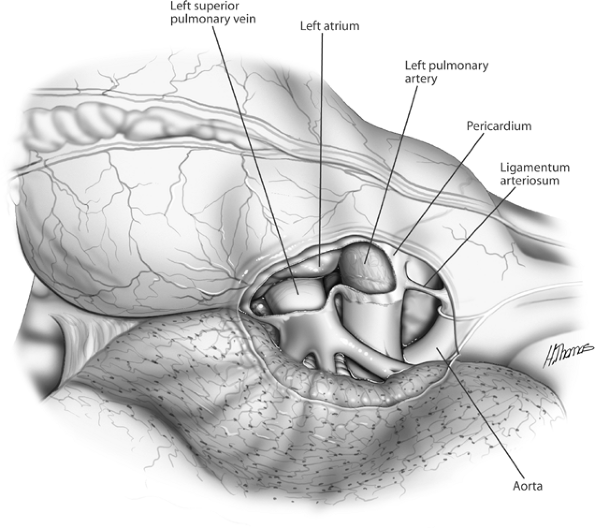 |
Fig. 29-6. Intrapericardial exposure of the left hilum. |
Right Pneumonectomy
Because the right lung compromises 60% of the total lung volume, right pneumonectomy is more physiologically taxing than a left pneumonectomy and is associated with a higher morbidity and mortality.
After the thoracotomy, the mediastinal pleura is sharply incised circumferentially, and complete lymph node staging is performed as previously described (Fig. 29-7). The inferior border of the azygos vein is dissected, and the vein is reflected superiorly off the bronchus. Division of this vein is rarely required. With the lung retracted posteriorly and inferiorly, the pulmonary artery is identified and dissected
P.477
P.478
in the subadventitial plane. Dissection is carried posterior to the superior vena cava, which is reflected medially using a pledget (Fig. 29-8). The superior margin of the right main-stem bronchus is now freed, allowing the main pulmonary artery to be safely encircled using a thumb and forefinger or a Semb dissector.
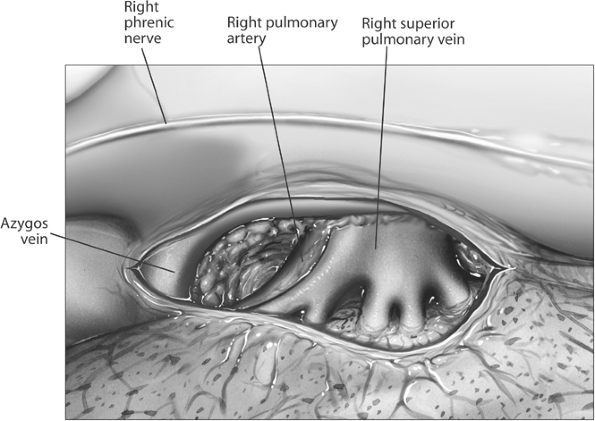 |
Fig. 29-7. Right hilum, mediastinal pleura incised. Note that only the truncus anterior of the pulmonary artery is visible at this point. |
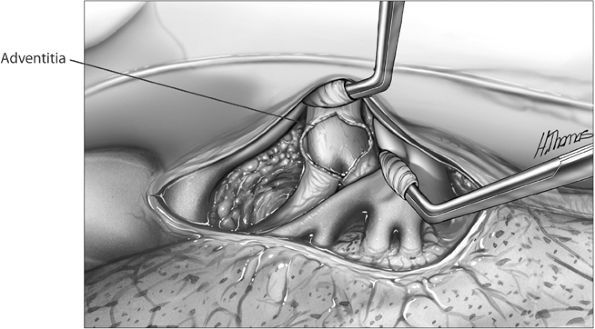 |
Fig. 29-8. Superior vena cava retracted and pulmonary artery dissected in subadventitial plane. |
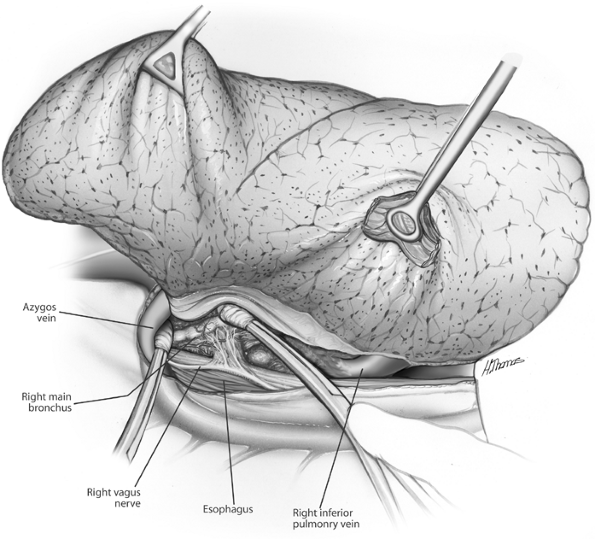 |
Fig. 29-9. Dissection of posterior hilum Azygos vein retracted, bronchus exposed, and inferior pulmonary ligament divided, exposing inferior pulmonary vein. |
The lung is retracted medially. The vagus nerve is identified, and its branches to the bronchus are sharply divided (Fig. 29-9). The bronchial arterial branches require clips or ligatures. The posteroinferior border of the bronchus is freed, retracting the esophagus posteriorly. Finger dissection frees the circumference of the bronchus (Fig. 29-10).
Attention is now directed toward the veins. The inferior pulmonary ligament is sharply divided to the lower margin of the inferior vein (Fig. 29-11). The anterior and posterior aspects of the vein are cleared of surrounding mediastinal tissue, and a Semb clamp is passed around the vein at its superior margin. The superior vein is dissected free using similar technique. During the entire dissection, the surgeon continually assesses technical resectability.
The artery, vein, and bronchus are now divided using the techniques previously described. Caveats include the following. First, adequate length of pulmonary artery must be obtained and may require an intrapericardial dissection to achieve (Figs. 29-12 and 29-13), and traction on the artery must be avoided because injury to the vessel at this level is often irretrievable. Second, after removal of the lung, the bronchial stump is inspected to ascertain that it is sufficiently short. The stump should always be buttressed. Options include parietal pleura, azygos vein, pericardial fat
P.479
P.480
P.481
pad, and intercostal muscle bundle (Fig. 29-14). The latter is our preference because it provides the best blood supply and strongest tissue. We do not recommend the use of the esophagus for this purpose. The remainder of the operation is completed as described for left pneumonectomy.
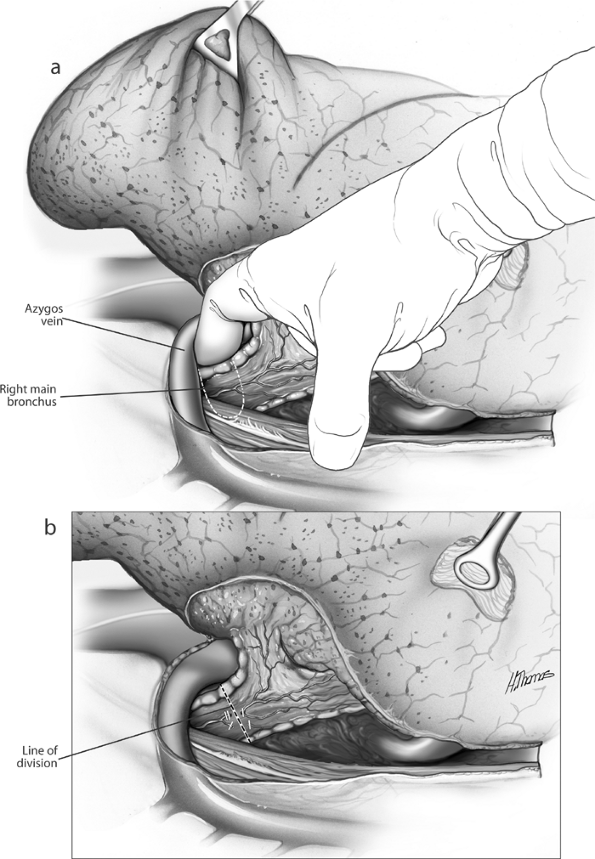 |
Fig. 29-10. A. Fingers freeing the bronchus. B. Site of bronchial division following application of stapler. |
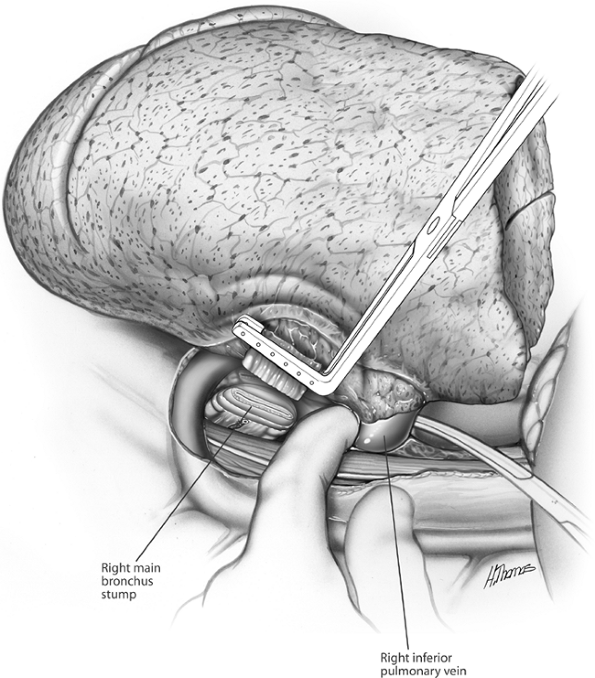 |
Fig. 29-11. The inferior pulmonary vein is isolated, stapled, and divided. The superior pulmonary vein is similarly managed. |
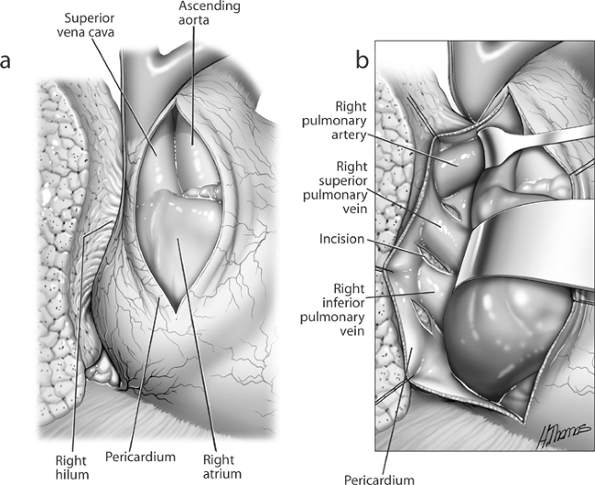 |
Fig. 29-12. Intrapericardial dissection of right hilum. A. Pericardial incision. B. Exposure of pulmonary vessels lateral to superior vena cava. |
Technical Modifications of Pneumonectomy
Preoperatively, a decision can be made that a large central tumor makes control of the pulmonary hilum difficult or unsafe through a posterolateral thoracotomy. In these instances, a transsternal approach is indicated, as described by Cooper and associates (1978) and in Chapter 34. Using this approach, dissection between the superior vena cava and aorta exposes the proximal right main pulmonary artery and bronchus (see Fig. 29-13). The vascular supply to the lung is now easily controlled. Occasionally, the sternotomy is closed, and the resection is completed by a thoracotomy. Pneumonectomy for an infected or destroyed lung requires careful preoperative planning with the anesthesiologist to prevent contamination of the opposite airway and the pneumonectomy space.
Operative findings often lead to modifications in the extent of resection required. Chest wall involvement should be resected en bloc with the lung, achieving acceptable surgical margins. Invasion of the pericardium, adventitia of the aorta, or superficial muscle of the esophagus and limited involvement of the superior vena cava
P.482
are not absolute contraindications to resection. If the surgeon judges that a complete resection with negative margins can be accomplished, the procedure should be carried out. Any areas where resection margins are close should be marked with vascular clips to facilitate postoperative radiation therapy to the area. Major defects in the pericardium should be repaired primarily or with a synthetic mesh such as Dacron. Involvement of the vagus or phrenic nerve is also not necessarily a contraindication to resection, but if the vagus needs to be sacrificed proximal to the takeoff of the left recurrent laryngeal nerve, this can present problems with postoperative cough because of a paralyzed left vocal cord. Sleeve pneumonectomies are formidable procedures that should be undertaken only by surgeons with experience with this type of resection (see Chapter 30).
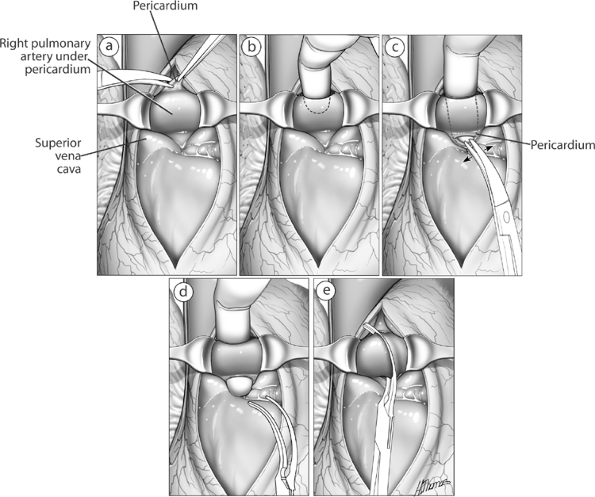 |
Fig. 29-13. A. The superior vena cava is retracted laterally and the aorta medially, and the adventitia over the pulmonary artery is incised. B E. Further steps in the isolation of the proximal pulmonary artery. |
POSTOPERATIVE MANAGEMENT
Patients are treated perioperatively with the accepted antibiotic coverage in place at the surgeon's hospital for clean surgical cases. After pneumonectomy, patients need to be monitored in an intensive care unit with strict bed rest for the first 24 hours. Fluid restriction is mandatory, such that measured input equals output. The surgeon should also assess the amount of intraoperative fluid given by the anesthesiologists because patients often require
P.483
P.484
diuretics to remove excess fluid administered intraoperatively. Fluid management must be discussed with the anesthesiologist preoperatively. Whether the patient should receive routine prophylaxis for atrial arrhythmias is up to the surgeon, although the literature is supportive of such management, as reported by Wijeysundera and Beattie (2003) as well as by Amar (2000) and Lanza (2003) and their colleagues. Patients may be nursed semirecumbent or with the nonoperative side elevated. Incentive spirometry and chest physiotherapy should be routinely performed. Excess tracheobronchial secretions that the patient cannot clear should lead the surgeon to consider bronchoscopy with minimal sedation. Chest films are done daily to ensure that the postpneumonectomy space gradually fills. Any sudden drop in the air fluid level is suspicious for a bronchopleural fistula, and the bronchial stump should be assessed by flexible bronchoscopy. Multiple air-fluid levels in the postpneumonectomy space are an indication for thoracentesis of the pneumonectomy space to rule out empyema even in the absence of other signs and symptoms of infection.
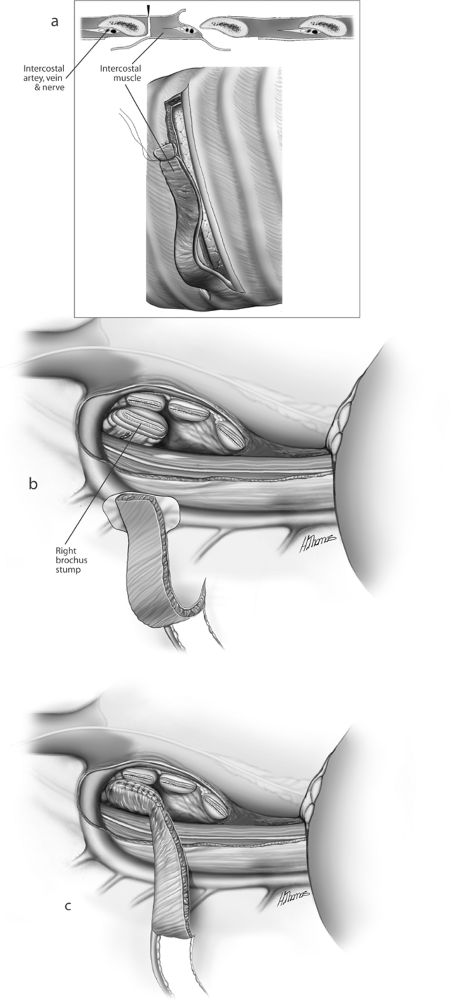 |
Fig. 29-14. A. Construction of intercostal pedicle flap. B. Right hilum before application of intercostal pedicle flap of bronchus. C. Flap applied to bronchus and suture applied to peribronchial tissues. |
REFERENCES
Allison PR: Intrapericardial approach to the lung root in the treatment of bronchial carcinoma by dissection pneumonectomy. J Thorac Cardiovasc Surg 15:99, 1946.
Amar D, et al: Effects of diltiazem prophylaxis on the incidence and clinical outcome of atrial arrhythmias after thoracic surgery. J Thorac Cardiovasc Surg 120:790, 2000.
Cooper JD, Nelems JM, Pearson FG: Extended indications for median sternotomy in patients requiring pulmonary resection. Ann Thorac Surg 26:413, 1978.
Fell SC: Special article: a brief history of pneumonectomy. Chest Surg Clin N Am 12:10, 2002.
Fry WA: Decision making at the time of exploratory thoracotomy. Ann Thorac Surg 38:310, 1984.
Graham EA: The first pneumonectomy. Cancer Bulletin 2:2, 1949.
Graham EA, Singer JJ: Successful removal of an entire lung for carcinoma of the bronchus. JAMA 101:1371, 1933.
Jensik RJ, et al: Sleeve lobectomy for carcinoma. A ten-year experience. J Thorac Cardiovasc Surg 64:400, 1972.
Lanza LA, et al: Low-dose amiodarone prophylaxis reduces atrial fibrillation after pulmonary resection. Ann Thorac Surg 75:223, 2003.
Naruke T, Suemasu K, Ishikawa S: Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg 76:832, 1978.
Rienhoff WF: The surgical technique of total pneumonectomy. Arch Surg 32:218, 1936.
P.485
Wijeysundera D, Beattie WS: Calcium channel blockers for reducing cardiac morbidity after noncardiac surgery: a meta-analysis. Anesth Analg 97:634, 2003.
Reading References
Bignall JR, Martini M, Smither DW: Survival of 6,086 cases of bronchial carcinoma, Lancet 1:1067, 1967.
Brewer LA III: The first pneumonectomy. Historical notes. J Thorac Cardiovasc Surg 88:810, 1984.
Churchill ED, et al: The surgical management of carcinoma of the lung. J Thorac Surg 20:349, 1950.
Deslauriers J, Faber LP: Pneumonectomy, part I. Chest Surg Clin N Am 9:2, 1999.
Deslauriers J, Faber LP: Pneumonectomy, part II. Chest Surg Clin N Am 9:3, 1999.
Ginsberg RJ et al: Modern thirty-day operative mortality for surgical resections in the lung. J Thorac Cardiovasc Surg 86:654, 1983.
Ochsner A: The development of pulmonary surgery with special emphasis on carcinoma and bronchiectasis. Am J Surg 135:732, 1978.
Van Mieghem W, Demedts M: Cardiopulmonary function after lobectomy or pneumonectomy for pulmonary neoplasm. Respir Med 83:199, 1989.
Wilkins EW, Scannell JG, Carver JG: Four decades of experiences with resections for bronchogenic carcinoma at the Massachusetts General hospital. J Thorac Cardiovasc Surg 76:364, 1978.
EAN: 2147483647
Pages: 203