17 - Invasive Diagnostic Procedures
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section VI - Anesthetic Management of the General Thoracic Surgical Patient > Chapter 22 - Conduct of Anesthesia
function show_scrollbar() {}
Chapter 22
Conduct of Anesthesia
Andranik Ovassapian
Technological advances have increased the application of video-assisted thoracic surgery (VATS). The need for one-lung ventilation (OLV) is absolute during VATS because retraction of the operative lung by the surgeon is not an option as it is during thoracotomy. The new generations of thoracic surgeons favor isolation and OLV during all intrathoracic operations, therefore increasing the demand for expert use of various devices and techniques applied for separation of the lungs. This demand has also increased the interest in the development of a new bronchial blocker that has proven to be extremely useful in patients with a difficult airway or when nasotracheal intubation is the only option.
Pulmonary function tests and ventilation-perfusion studies are critical for selection of patients for lung resection. In addition to routine anesthetic problems, anesthesia for thoracic surgery is complicated by several factors: Opening the chest produces a pneumothorax; manipulation of the lung, heart, and major vessels by the surgeon may interfere with ventilatory exchange and cardiovascular stability; and the lateral decubitus position changes the distribution of blood flow and pattern of ventilation and exposes the lower lung to the danger of contamination by secretions from the operative lung. Thus, for safe conduct of anesthesia for thoracic surgery, the anesthesiologist should be knowledgeable about the physiology of OLV and be skillful in techniques for isolation of the lungs.
The introduction of double-lumen endobronchial tubes for one-lung anesthesia by Bjork and Carlens in 1950 represented a major advance in thoracic anesthesia. In 1958, Jenkins and Clark advocated the routine use of double-lumen tubes for all intrathoracic operations. Yet the difficulty in blindly positioning these tubes and the resulting possibility of life-threatening complications discouraged their use by many anesthesiologists. Several advances have made one-lung anesthesia safer and the double-lumen tube more popular. These include the availability of disposable double-lumen tubes and of new bronchial blockers; the introduction of the flexible bronchoscope (FB) by Shinnick and Freedman (1982) and by me and my colleagues (1983) for precise positioning of endobronchial tubes; and the use of different treatment methods for management of hypoxemia during OLV and application of arterial blood gas or pulse oximetry for monitoring of blood oxygenation.
CHOICE OF ANESTHESIA
After a thorough preoperative assessment and preparation of the patient, the anesthesiologist should choose an anesthetic plan that is both safe for the patient and suitable for the needs of the surgeon. An appropriate preoperative medication should be prescribed to relax and free the patient of apprehension. Narcotics minimize patient's discomfort during placement of arterial cannulae and large intravenous lines. The respiratory depression caused by narcotics in patients with advanced pulmonary disease, however, should be kept in mind. Judicious use of intravenous narcotics such as fentanyl while the patient is in the operating room and before any procedures provides the necessary analgesia. Oxygen (2 to 3 L) through a nasal cannula should be instituted if conscious sedation is provided for placement of various lines and an epidural catheter. Respiratory depression and hypoxemia are common with intravenous sedation. Anticholinergic agents such as atropine or glycopyrrolate are prescribed with the premedication to decrease secretions during airway instrumentation and to facilitate visualization of the airways when a fiberscope is used. Thorburn and colleagues (1986) showed that anticholinergic agents also improve pulmonary mechanics before general anesthesia. Because of the lower incidence of undesirable side effects, glycopyrrolate is the anticholinergic agent of choice.
General endotracheal and endobronchial anesthesia with controlled ventilation is an ideal anesthetic technique for most intrathoracic surgical procedures. A variety of high-frequency ventilation (HFV) techniques have been developed and recommended for operations performed on the airway and for intrathoracic procedures. During HFV, ventilatory excursions of the lungs are of low amplitude, which may facilitate surgical exposure and resection during intrathoracic
P.368
operations. HFV has been used successfully in situations in which access to the airway is impaired. The success or failure of HFV and its advantages and disadvantages compared with conventional mechanical ventilation depend on the following: the type of HFV used, whether one-lung or two-lung ventilation is applied, and the type of surgical procedure.
The three basic forms of HFV are high-frequency positive-pressure ventilation (HFPPV), high-frequency jet ventilation (HFJV), and high-frequency oscillation (HFO). El-Baz and associates (1981, 1982) used one-lung HFPPV successfully for sleeve pneumonectomy and surgical procedures on large airways. Smith and colleagues (1981) reported successful use of HFPPV for pulmonary lobectomy. Hildebrand and co-workers (1984) applied HFJV for intrathoracic surgery, providing satisfactory operating conditions and ventilatory exchange. They indicated, however, that OLV using a double-lumen tube provides the optimal conditions if the difficulties associated with placement of double-lumen tubes and related complications can be avoided. Glenski and co-workers (1986) demonstrated that HFO resulted in adequate pulmonary gas exchange and excellent surgical conditions for peripheral lung procedures; however, for procedures on the major airways or mediastinal structures, surgical conditions were unsatisfactory during HFO. They believe that the disadvantages of HFO outweigh the advantages during intrathoracic surgery.
A unique feature of thoracic anesthesia is the use of OLV. The selection of an anesthetic technique and agent is influenced by whether OLV is used and whether a high concentration of inspired oxygen can be delivered to the patient. The volatile halogenated anesthetic agents, such as isoflurane, sevoflurane, and desflurane, permit the administration of a high inspired concentration of oxygen. Sevoflurane offers smooth induction of and rapid recovery from anesthesia, a combination that is desirable for patients with lung disease. Abe and associates (1998) demonstrated that arterial oxygen tension during OLV with sevoflurane anesthesia was similar to that with isoflurane. Halogenated agents depress the airway reflexes, cause bronchodilation, and can be eliminated through the lungs. The high concentration of inhalation anesthetic agents may interfere with hypoxic vasoconstriction, however, diverting blood flow from the ventilated to the nonventilated lung, increasing intrapulmonary shunting, and decreasing Pao2. Another disadvantage of inhalation agents is the ease with which they may depress the myocardium and decrease the cardiac output. When narcotic analgesics are added to the anesthetic regimen, the concentration of inhalation agent is lowered. This adjustment helps alleviate the aforementioned problems associated with inhalation agents.
Induction of anesthesia is achieved by the intravenous administration of 5 g/kg fentanyl and 3 to 4 mg/kg sodium thiopental or by 1 to 3 mg/kg propofol. Anesthesia is maintained with 50% nitrous oxide and 0.5% to 0.8% isoflurane, 1% to 2% sevoflurane, or 6% to 8% desflurane. An additional 10 to 15 g/kg of fentanyl is given before and during OLV when nitrous oxide is discontinued and anesthesia is maintained with a low concentration of inhalation agent and oxygen. At the conclusion of OLV, nitrous oxide is started again; no additional narcotic is given thereafter. If necessary, higher concentrations of an inhalation agent are used to maintain adequate depth of anesthesia. Muscle relaxants are used for intubation of the trachea and to prevent diaphragmatic movement during the operation. Weinrich and associates (1980) recommended a combination of ketamine, nitrous oxide, and muscle relaxant as an anesthetic for thoracic surgery. Ketamine has sympathomimetic properties, and, therefore, supports the cardiovascular system and causes bronchodilation. It is a useful drug for the induction of general anesthesia in hypovolemic patients with an unstable cardiovascular system.
The use of epidural anesthesia to supplement a light general anesthesia is another approach that warrants consideration. It offers the advantage of decreasing the use of neuromuscular blocking drugs or narcotic analgesics intraoperatively. This technique may receive more attention as continuous epidural infusions of analgesics are used increasingly for the management of postsurgical pain. Training and experience in this technique, especially when a high or midthoracic approach is used, are needed. The technique should be used regularly to help sustain the expertise needed for its safe conduct. During intrathoracic operations, it may be necessary to give various additional drugs for the control of cardiac dysrhythmias, systemic blood pressure, and cardiac output changes or acid-base balance. Their dosage is similar to that used in other types of anesthesia. Caution should be exercised, however, in the use of vasodilators and vasopressors during OLV. Vasodilators such as sodium nitroprusside and nitroglycerin interfere with hypoxic pulmonary vasoconstriction. The vasopressors exert more vasoconstriction in oxygenated vessels than in hypoxic vessels. Consequently, both groups of drugs may divert blood flow from the ventilated to the nonventilated hypoxic lung, which may then increase shunt and hypoxemia.
MONITORING
Monitoring requirements differ among individual patients because of their general physical conditions, the presence or absence of cardiopulmonary disease, and the nature of their operative procedures. Monitoring of patients who are healthy and undergoing minor intrathoracic operations may be limited to the routine monitors used in all patients undergoing general anesthesia. The pulse oximeter provides a noninvasive, continuous monitoring of the hemoglobin saturation and the heart rate. Improvement in the design of pulse oximeters, especially of the sensors, and introduction of an earlobe oximeter probe have made the pulse oximeter reliable and easy to use. The pulse oximeter displays the percentage of oxyhemoglobin. Over a wide
P.369
range of arterial blood oxygen tension, the percentage of oxyhemoglobin does not change. Direct arterial monitoring of blood pressure not only provides beat-to-beat measurement of pressure but also permits analysis of arterial blood gases and acid-base status during the operation. Continuous monitoring of arterial blood gases and pH, which is now available, provides a better continuous monitoring method during OLV.
The measurement of central venous pressure (CVP) is indicated in hypovolemic patients, when large volume shifts are anticipated, in patients with trauma and multiple injuries, and in patients with right ventricular dysfunction. Continuous or serial measurements are useful in the management of fluid and blood replacement when venous tone and myocardial function remain stable. The response of the CVP to a rapid volume infusion is a useful test of right ventricular function. The CVP is subject to mechanical interference, however, especially during thoracic surgery. The internal jugular and subclavian veins are common sites for central venous cannulation. It is wise to use the vein ipsilateral to the thoracotomy, because pneumothorax is a known complication of deep vein cannulation. In patients with left ventricular dysfunction, the CVP may not provide accurate information about left ventricular filling pressure. Under these circumstances, a pulmonary capillary wedge pressure should be measured by using a flow-directed pulmonary artery catheter. In most patients, the pulmonary capillary wedge pressure corresponds well with the left atrial pressure. The pulmonary artery catheter allows measurements of pulmonary artery systolic, diastolic, and mean pressures, along with the pulmonary capillary wedge pressure, CVP, and cardiac output. In addition, mixed venous blood can be sampled and the shunt can be calculated. Peripheral and pulmonary vascular resistance can be calculated. Serial measurements of cardiac output can help to assess the status of the circulation and guide any necessary supportive therapy. Information derived from centrally placed catheters indicates how the myocardium manages the fluid load; however, the CVP and pulmonary wedge pressure reflect changes in blood volume when depth of anesthesia and myocardial performance are unchanged. The combination of a decrease in CVP or pulmonary wedge pressure and systemic arterial pressure suggests hypovolemia, whereas a high CVP and pulmonary wedge pressure with a low arterial pressure may indicate poor myocardial performance.
New anesthesia machines are equipped with monitors and alarm systems. These include an inspired oxygen concentration monitor and a spirometer to measure expired tidal volume, a low and high airway pressure, and an apnea alarm system. The use of muscle relaxants is monitored by a peripheral nerve stimulator, and the body temperature is measured by an esophageal or a tympanic membrane probe. The availability of mass spectrometry or end-tidal CO2 analysis provides additional monitoring of the patient's ventilation and confirms the proper placement of an endotracheal tube. The addition of pulse oximetry, used to monitor arterial oxygen saturation noninvasively, permits the early detection of impaired oxygenation, especially during OLV.
ONE-LUNG ANESTHESIA
One-lung anesthesia is absolutely required under certain circumstances and provides safety for the patient and better operative conditions for the surgeon. Because of its complexity, however, it adds to anesthetic difficulties. The most common indication for one-lung anesthesia is to provide the surgeon with a quiet operating field. A bronchopleurocutaneous fistula, communicating empyema, bronchial hemorrhage, lung abscess, giant air cyst, or operations performed through VATS are absolute indications for isolating the individual lungs. This measure prevents the spread of secretions to the healthy lung and helps to ensure adequate ventilation. Thoracoscopy, although not a new surgical technique, has gained popularity in recent years with the development of appropriate video equipment and is applied for many diagnostic and therapeutic intrathoracic operations, such as drainage of empyema, evacuation of hemothorax, bleb resection, and mediastinal lymph node and lung biopsy.
Methods of Obtaining One-Lung Ventilation
Three categories of bronchial tubes or blockers are used to achieve separation of the lungs. These are double-lumen tubes of different sizes and types, bronchial blockers of different kinds, and single-lumen tubes with one or two cuffs.
Double-Lumen Endobronchial Tubes
Double-lumen endobronchial tubes consist of two lumens of unequal length that are separated proximally into two tubes; a tracheal cuff is located proximal to the opening of the tracheal lumen, and a bronchial cuff is located at the tip of the bronchial tube. Tubes designed for intubation of the right main-stem bronchus have an opening in the bronchial cuff (bronchial cuff slit) to permit ventilation of the right upper lobe. When a double-lumen tube is positioned properly and the bronchial and tracheal cuffs are inflated, a separate airway is formed for each lung. The main advantage of double-lumen bronchial tubes is that, although isolation of the lungs is preserved, one or both lungs can be ventilated. Hammer (2001) and colleagues (1999) report that disposable double-lumen tubes are now available in 35F, 37F, 39F, and 41F for adult patients, as well as in 26F, 28F, and 32F sizes for pediatric use. The Carlens double-lumen tube described in 1949, which has a carinal hook, and the Robertshaw double-lumen tube described in 1962, which has no carinal hook, are used most commonly. Edwards and Hatch (1965) reviewed their clinical experience with them. Burton and associates (1983) demonstrated the
P.370
advantages of the disposable polyvinyl double-lumen tubes. The advantages of these tubes include a softer and more flexible structure, thin-walled tracheal and bronchial cuffs, a gentler distal curve, and a greater inside-to-outside diameter ratio.
After the patient is anesthetized and paralyzed, the double-lumen tube is inserted into the trachea using a rigid laryngoscope. The left-sided tube is held 90 degrees rotated clockwise (to the right), and the right-sided tube is held 90 degrees rotated counterclockwise (to the left) so the tip of the bronchial lumen faces anteriorly. After the tip is advanced beyond the vocal cords, the double-lumen tube is rotated 90 degrees to the appropriate side and then advanced into the intended bronchus. The Carlens (left-sided) and White (right-sided) double-lumen tubes with carinal hooks are rotated 180 degrees as the tip of the bronchial lumen passes the vocal cords to bring the carinal hook anteriorly to negotiate the vocal cords. After the carinal hook passes beyond the vocal cords, the Carlens tube is rotated 90 degrees clockwise and the White tube is rotated 90 degrees counterclockwise to position bronchial tubes toward the intended bronchus.
If rigid laryngoscopy proves difficult for tracheal intubation with a double-lumen tube, a flexible bronchoscope (FB) is used for this purpose, as described by the author (1996). The FB is passed through the bronchial lumen of the tube. After exposure of the vocal cords, the bronchoscope is advanced into the lower trachea. The double-lumen tube is maneuvered such that the tip of the tube faces posteriorly to pass beneath the epiglottis and then is rotated 180 degrees to bring the tip anteriorly to pass through the vocal cords. After entering the trachea, the tube is rotated 90 degrees right or left to position the bronchial lumen toward the intended bronchus.
Once the double-lumen tube is placed in the trachea, the tracheal cuff is inflated and mechanical ventilation is started with a tidal volume of 10 mL/kg and a rate of 8 breaths per minute. The rate of ventilation is adjusted to maintain a Paco2 between 35 and 40 mm Hg.
Bronchoscopic Positioning of Left-Sided Double-Lumen Tubes
The FB is introduced into the bronchial lumen rotated 90 degrees to the left and is advanced into the left main-stem bronchus to evaluate its patency and length. Two techniques are used for positioning the left endobronchial tube:
After inspection of the left main-stem bronchus, the FB is pulled back to position, its tip 2 mm beyond the distal end of the endobronchial tube lumen. The bronchoscope is rotated 90 degrees to the left and the tip is angulated to view the lateral wall of the trachea. The tracheal cuff is deflated, and the FB and the tube are held together and advanced into the left main-stem bronchus. The orifice of the left upper lobe bronchus comes into view when the bronchoscope reaches 10 to 15 mm above this opening. The tube and FB are advanced until the distal end of the tube is 5 mm above the opening of the left upper lobe bronchus (Fig. 22-1).
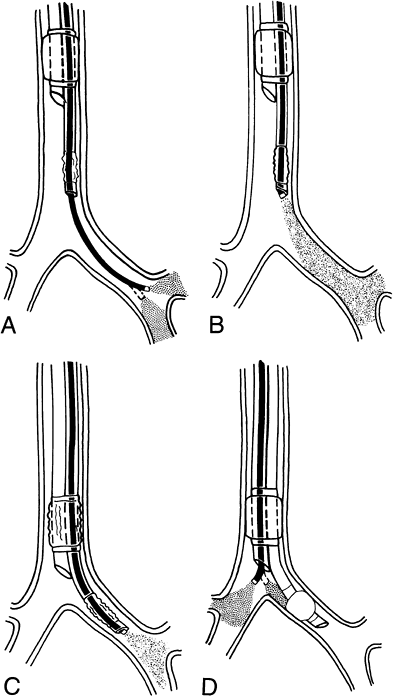
Fig. 22-1. Flexible bronchoscopic placement and positioning of left-sided, double-lumen tube. A. The FB is passed through the bronchial lumen into the left main-stem bronchus. The patency, length, and anatomy of the left main-stem bronchus and the position of the orifice of the left upper lobe bronchus are evaluated. B. The FB is pulled back to position its tip 2 mm beyond the distal end of the bronchial lumen. The FB is rotated 90 degrees to the left with the tip angulated anteriorly toward the lateral wall of the trachea. C. The tracheal cuff is deflated, and the tube and FB are advanced together inside the left main-stem bronchus until the orifice of the left upper lobe comes into view. At this point, the tip of the FB is 10 to 15 mm above the left upper lobe orifice. The tube is advanced further to position the distal end of the tube approximately 5 mm above the orifice of the left upper lobe bronchus. D. The FB is passed through the tracheal lumen to check the position of the bronchial cuff and right main-stem bronchus.
After evaluating the anatomy of the left main-stem bronchus, the tip of the FB is positioned 10 mm above the orifice of the left upper lobe bronchus. The tracheal
P.371
cuff is deflated, and the tube is advanced over the bronchoscope into the left bronchus until it passes approximately 5 mm beyond the tip of the bronchoscope. The bronchoscope is then advanced beyond the endobronchial tube lumen to visualize both the upper and lower lobe bronchi.
After positioning the left endobronchial tube, the FB is withdrawn from the bronchial lumen and inserted into the tracheal lumen. The relation of the tracheal lumen distal opening to the tracheal wall and the position of the bronchial cuff and the opening of the right main-stem bronchus are evaluated. If the endobronchial tube cuff is seen outside the left main-stem bronchus, the tracheal cuff is deflated and the tube is advanced further until the proximal edge of the bronchial cuff lies 3 to 5 mm inside the left main-stem bronchus. This placement ensures separation of the lungs and stability of the tube, and minimizes the possibility of herniation of the bronchial cuff into the trachea. The bronchial and tracheal cuffs are then inflated using the minimal leak technique (Fig. 22-2).
Bronchoscopic Positioning of Right-Sided Double-Lumen Tubes
Proper positioning of right-sided double-lumen tubes is technically more difficult because of the short, variable length of the right main-stem bronchus. Rigg (1980) reported a high incidence of failure when these tubes were positioned blindly, but Campos and associates (2000) reported no upper lobe atelectasis with right-side double-lumen tubes when positioning was accomplished with a flexible bronchoscope. I (1987) applied the following techniques successfully. The FB is passed through the bronchial lumen of the tube into the right main-stem bronchus. The patency, length, and anatomy of the right main-stem bronchus, as well as the bronchus intermedius, are evaluated. If the right main-stem bronchus is 15 mm or longer, placement of a right-sided double-lumen tube is possible. Either of the following two techniques can then be applied:
After inspection of the right main-stem bronchus, the FB is withdrawn inside the lumen of the endobronchial tube, the bronchoscope is rotated 90 degrees to the right, and the tip is flexed anteriorly to position the tip of the FB at the proximal end of the slit (Murphy eye) in the endobronchial tube. The tracheal cuff is deflated, and the tube and bronchoscope, while being held together, are advanced toward the right main-stem bronchus. The view through the endobronchial tube slit is the red mucosa of the tracheal and right main-stem bronchial walls. The first indication of approaching the opening of the right upper lobe bronchus is a dark area at the distal end of the endobronchial tube slit the termination of the bronchial wall and the beginning of the orifice to the right upper lobe bronchus. The tube and FB are advanced further until the orifice of the right upper lobe bronchus comes into full view through the endobronchial tube slit (Fig. 22-3). If necessary, the FB can be advanced through the endobronchial tube slit into the right upper lobe bronchus to visualize the three segments of the right upper lobe. The FB is then rotated to its neutral position and is advanced beyond the tip of the endobronchial tube
P.372
to check the opening of the right middle and lower lobe bronchi. The FB is then withdrawn from the endobronchial tube lumen and is passed through the tracheal lumen to check the position of the bronchial cuff and the opening of the left main-stem bronchus (see Fig. 22-3).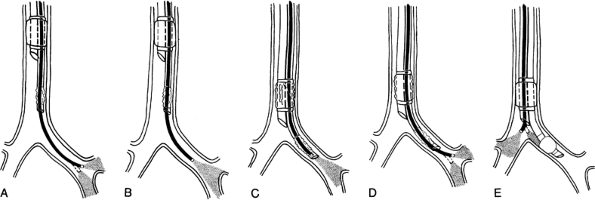
Fig. 22-2. Alternate approach to flexible bronchoscopic placement and positioning of left-sided, double-lumen tube. A. The FB is passed through the bronchial lumen into the left main-stem bronchus. The patency, length, and anatomy of the left main-stem bronchus and the position of the orifice of the left upper lobe bronchus are evaluated. B. The FB is withdrawn, and its tip is positioned 10 mm above the origin of the left upper lobe bronchus. C. The tracheal cuff is deflated, and the tube is advanced over the FB into the left main-stem bronchus until it comes into view beyond the tip of the FB. D. The FB is advanced beyond the bronchial lumen to visualize the left upper lobe bronchus. E. The FB is passed through the tracheal lumen to check the position of the bronchial cuff and the opening of the right main-stem bronchus.
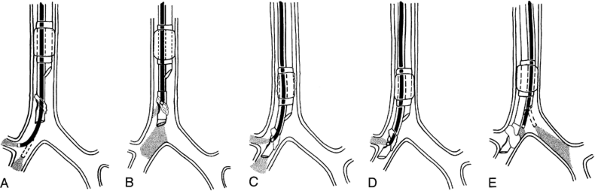
Fig. 22-3. Alternate approach to flexible bronchoscopic placement and positioning of right-sided, double-lumen tube. A. The FB is passed through the bronchial lumen into the right main-stem bronchus. The patency, length, and anatomy of the right bronchial tree are evaluated. The FB is then rotated 90 degrees to the right, and the tip of the FB is flexed anteriorly to visualize the right upper lobe bronchus. While the FB is held stationary, its tip is returned to the neutral position. B. The tracheal cuff is deflated, and the tube is advanced over the FB into the right main-stem bronchus until it comes into view beyond the tip of the FB. C. The FB is withdrawn inside the bronchial lumen to visualize the orifice of the right upper lobe bronchus through the slit of the bronchial cuff. D. The FB is passed through the tracheal lumen to check the position of the bronchial cuff and the opening of the left main-stem bronchus.
After inspection of the right main-stem bronchus and bronchus intermedius, the FB is pulled back and rotated 90 degrees clockwise, and its tip is flexed anteriorly to visualize the orifice of the right upper lobe bronchus. While the FB is held stationary, the tip of the FB is returned to the neutral position. The tracheal cuff is deflated, and the double-lumen tube is advanced over the FB into the right main-stem bronchus. When the distal end of the tube comes into view through the FB, advancement of the tube stops (Fig. 22-4). At this position, the bronchial tube slit would be at the level of the orifice for the right upper lobe bronchus. It is critical that during advancement of the tube, the FB is stabilized. The FB is then withdrawn a few millimeters inside the bronchial lumen, and its tip is flexed anteriorly to visualize the orifice of the right upper lobe bronchus through the slit in the endobronchial tube. Minor adjustments of the tube often are necessary to have a clear view of the right upper lobe orifice. The asymmetric design of the bronchial cuff in right-sided tubes makes it possible to position the tube correctly when the right main-stem bronchus is as short as 15 mm. The upper border of the bronchial cuff often is just at the level of the carina. As a result, air leak around the bronchial cuff is encountered more often with a right-sided tube than with a left-sided double-lumen tube after the patient is placed in the lateral position. Advancing the tube and repositioning the FB are achieved easily while the patient is in the lateral position.
Blind Positioning of Left-Sided Double-Lumen Tube
If the FB is not available, a left-sided double-lumen tube can be positioned blindly with a high degree of success. After the tube is placed in the trachea, it is advanced to the depth of 28 to 31 cm at teeth level, depending on the size of the patient. Both the tracheal and bronchial cuffs are inflated to separate the lungs. Upon clamping the tracheal lumen, the breath sounds should now only be present on the left lung. If the breath sound is present on the right lung but is absent on the left lung, the bronchial tube has entered the wrong bronchus. After ensuring the proper bronchial intubation, the tracheal and bronchial tubes are clamped sequentially to confirm the proper depth position of the tube. Placing the bronchial tube too deep blocks the left upper lobe bronchus, causing atelectasis, and may prevent air entry into the right lung. If the bronchial tube is not advanced far enough, the bronchial cuff herniates from the left main-stem bronchus, causing partial or complete occlusion of the right main-stem bronchus and thus preventing ventilation of the right lung. Separation of the lungs will not be achieved if the bronchial tube remains above the carina.
Bahk and Oh (1998) have described another approach for blind positioning of the left-sided double-lumen tube using bronchial cuff pressure as a guide. The double-lumen tube is inserted deeply until resistance is felt. The pilot of the bronchial cuff is connected to a pressure gauge, and the cuff is inflated with 1 to 2 mL of air until a pressure close to 30 cm H2O is obtained. The double-lumen tube is slowly withdrawn until the intracuff pressure decreases to 15 cm. The bronchial cuff is deflated, and the tube is advanced 1 cm. This technique allowed Bahk and Oh to position the tube correctly 97.5% of the time.
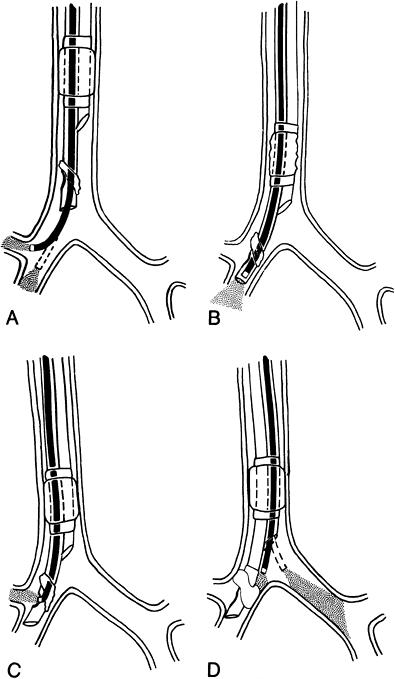 |
Fig. 22-4. Alternate approach to flexible bronchoscopic placement and positioning of right-sided, double-lumen tube. A. The FB is passed through the bronchial lumen into the right main-stem bronchus. The patency, length, and anatomy of the right bronchial tree are evaluated. The FB is then rotated 90 degrees to the right, and the tip of the FB is flexed anteriorly to visualize the right upper lobe bronchus. While the FB is held stationary, its tip is returned to the neutral position. B. The tracheal cuff is deflated, and the tube is advanced over the FB into the right main-stem bronchus until it comes into view beyond the tip of the FB. C. The FB is withdrawn inside the bronchial lumen to visualize the orifice of the right upper lobe bronchus through the slit of the bronchial cuff. D. The FB is passed through the tracheal lumen to check the position of the bronchial cuff and the opening of the left main-stem bronchus. |
P.373
Verifying the Functional Status of Double-Lumen Tubes
The following procedures help to determine whether the separation of the lungs has been achieved and adequate ventilation can be applied through each lung. With a mechanical ventilator set at a tidal volume of 10 mL/kg of ideal body weight and a rate of 8 breaths per minute, the exhaled tidal volume and the peak and plateau airway pressures are measured, and the expiratory flow rate on the respirometer and the humidification of both lumens by exhaled air are observed. After the tracheal connector tube is clamped, the breath sounds should only be present on the bronchial side, and the respirometer may show a 10% to 15% decrease in the exhaled tidal volume, whereas the expiratory flow rate should remain the same. The peak airway pressure should increase by no more than 50% when compared with two-lung ventilation. The clamp is then moved to the bronchial connector tube. Breath sounds should be present on the tracheal side. Tidal volume, expiratory flow rate, and peak airway pressure should change little from one lung to another. The clamp is then removed and the tube is secured. After the patient assumes the lateral position, the measurements of tidal volume and airway pressures are repeated with each lumen sequentially occluded.
Placement is considered unsatisfactory if separation of the lungs is incomplete; if, when changing from two-lung ventilation to OLV, the tidal volume decreases by more than 15%; if the expiratory flow rate of either lung slows dramatically; or if the peak airway pressure increases by more than 50%.
If tube placement is unsatisfactory, the FB is used to check the position of the bronchial cuff and to ensure that the tip of the bronchial or tracheal lumen is neither pressed against the bronchial or tracheal walls nor blocking the orifice to the left upper lobe bronchus. For right-sided tubes, the position of the slit in the bronchial cuff with respect to the orifice of the right upper lobe must be rechecked, as well as the patency of the right middle and lower lobes. In the presence of advanced lung disease with loss of lung tissue, empyema, or atelectasis, more exaggerated changes in the preceding variables are expected when switching from two-lung ventilation to diseased lung ventilation. Bronchospasm may occur after bronchial intubation in lightly anesthetized patients or in patients with a reactive airway. In the presence of bronchospasm, more exaggerated changes in the variables are expected. These changes can be lessened by deepening anesthesia. The peak airway pressures and tidal volume measurements during OLV are presented in Table 22-1.
Table 22-1. Tidal Volumes and Peak Airway Pressures During One-Lung Ventilation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
P.374
Bronchial Blockers
Bronchial blockers are introduced into the bronchus of the diseased lung while the healthy nonoperative lung is ventilated through a tracheal or contralateral bronchial tube. Three different kinds of bronchial blockers are in use today.
Wire-guided endobronchial blocker. Arndt and associates (1999a, 1999b) have introduced an Arndt or wire-guided endobronchial blocker (WGB) designed for separation of the lungs (Fig. 22-5). The WGB is placed coaxially through a conventional single-lumen endotracheal tube. The WGB is placed through a special endotracheal tube adapter that has three ports: one for introduction of the FB, one for passage of the blocker, and one to continue ventilation during positioning of the blocker. The passing of the FB through the guidewire loop of the blocker enables the endoscopist to direct the blocker in the desired location in the bronchial tree.
The adult WGB is 9F and is placed with a 3- to 4-mm FB through an 8-mm or larger-size single-lumen endotracheal tube. The size 5F pediatric blocker is placed with a 2-mm FB through a 4.5- or 5-mm endotracheal tube. The high-volume, low-pressure, elliptical-shaped balloon accommodates up to 15 mL of air. Typically, 5 to 8 mL is required to block the main bronchus in adult patients. The blocker's lumen can be used to insufflate or suction gas from the occluded lung. The balloon of the blocker may be less likely to dislodge because of its elliptical shape and the high-volume, low-pressure cuff. This new bronchial blocker represents a significant advancement in the management of the patient who needs separation of lungs and who may also present with a difficult airway. This system minimizes some of the traditional difficulties associated with the use of independent bronchial blockers and the Univent tube.
The WGB has special advantages when postoperative mechanical ventilation of a patient is planned or expected, the patient has a difficult airway, and oral access is denied. Flexible bronchoscopy is essential for effective use of the WGB. Endobronchial blockers are not suitable when bilateral thoracic operations are planned requiring sequential one-lung ventilation.
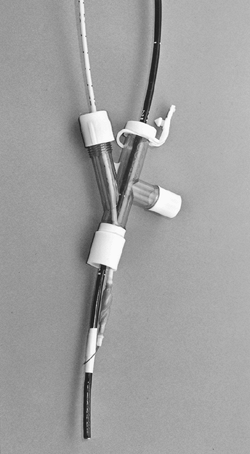
Fig. 22-5. Arndt or wire-guided endobronchial blocker.
The Univent tube. The Univent tube (Fuji Systems Corporation, Tokyo, Japan) is an endotracheal tube with two lumens: a larger lumen for ventilation and a smaller lumen containing a hollow-core bronchial blocker (Fig. 22-6). Univent tubes are now available in small sizes (3.5- to 4.5-mm inner diameter) for use in children older than 6 years.
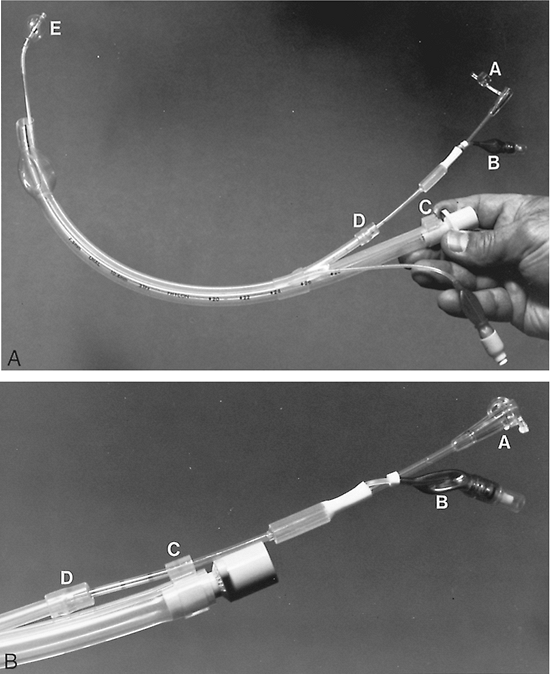
Fig. 22-6. Univent tube. A. The Univent tube with bronchial blocker is advanced outside the lumen of the tube and the balloon is inflated. (A) Attached plug to close the internal lumen of the blocker when one-lung ventilation is not applied. This cap should not be engaged (bronchial blocker lumen open) during one-lung ventilation. (B) Bronchial blocker inflation balloon. (C) Hand stopper that secures the bronchial blocker and prevents its movement. (D) Movable cap on the shaft of the bronchial blocker to seal off the leak between the blocker and its housing lumen. It also keeps the blocker from moving. (E) Inflated bronchial blocker cuff. B. Close-up of the proximal end of the Univent tube. (A) Plug that closes the lumen of the blocker. (B) Bronchial blocker balloon. (C) Hand stopper that secures the bronchial blocker. (D) Movable cap on the shaft of bronchial blocker.
P.375
A movable cap is incorporated on the shaft of the bronchial blocker to seal the gap between the blocker and its housing lumen and to keep the blocker from moving. After tracheal intubation, the bronchial blocker is placed in the intended bronchus. Inflating the tracheal and bronchial cuffs provides separation of the lungs and allows OLV.
The bronchial blocker is lubricated and is retracted inside the housing lumen before the Univent tube is placed inside the trachea using a rigid laryngoscope. The Univent tube is rotated 90 degrees toward the operative lung the side to be blocked and the bronchial blocker is advanced into the intended main-stem bronchus. Karwande (1987) reported successful blind placement of the blocker in 45 of 50 cases (90%). For five patients, blocker placement had to be done under flexible bronchoscopic guidance. In two cases, the blocker was dislodged after it was positioned. Hultgren and co-workers (1986) reported successful use of Univent tubes in 30 consecutive patients, but in one patient, the blocker entered the wrong side. MacGillivray (1988) reported successful seal of the intended bronchus only in one of eight patients. Bronchoscopic manipulation resulted in successful placement in another four patients, but a good seal could not be obtained in three patients and the Univent tube was replaced by a double-lumen tube.
With the help of a flexible bronchoscope passed through the Univent tube, positioning of the bronchial blocker is achieved faster and with a higher success rate. The blocker is left in the desired position with the cuff deflated. Before going to OLV, the tube is disconnected from the anesthesia machine to facilitate lung collapse. After the lung is partially collapsed, the blocker balloon is inflated. The advantages of the Univent tube include easier placement than occurs with double-lumen tubes and no need to change it for fiberoptic bronchoscopy or for postoperative ventilatory support.
The tip of the blocker is hard, and repeated attempts during blind placement may traumatize the tracheobronchial tree. Arai and Hatano (1987) reported dislodgment of the tip of the bronchial blocker cap. If a Univent tube is left in place for postoperative ventilation, precaution should be taken to avoid movement of the blocker into the trachea and inadvertent inflation of its cuff, causing acute airway obstruction. One such incident was reported by Dougherty and Hannallah in 1992. MacGillivray (1988) reported that herniation of the bronchial cuff is common with the Univent tube.
Fogarty embolectomy catheter. Fogarty arterial embolectomy catheters have been used in children by Vale (1969) and Hogg and Lorhan (1970), as well as by Cay and associates (1975). They may be positioned with the help of a rigid or flexible bronchoscope. Ginsberg (1981) advocated the use of Fogarty catheters in adults, whereas Dalens and colleagues (1982) used Swan-Ganz pulmonary artery catheters in children. Aspiration of secretions or temporary ventilation of the diseased lung is not possible without losing the separation of the lungs. Dislodgment of blockers is common with coughing, changing from the supine to the lateral position, or during surgical manipulation.
After the patient is anesthetized and paralyzed, rigid laryngoscopy is performed, and first the Fogarty catheter and then the tracheal tube are placed inside the trachea. As suggested by Ginsberg (1981), the distal end of the Fogarty catheter is angled to 30 degrees to facilitate advancement into the desired main-stem bronchus. A bronchoscopy adapter is placed on the tracheal tube connector, and the FB is passed through the adapter into the trachea to observe and, if needed, to assist passage of the Fogarty catheter into the desired bronchus. Lee and colleagues (2002) reported successful use of a Fogarty catheter to block an aberrant right upper lobe tracheal bronchus, while a Univent tube was used to block the rest of the right lung.
For left-lung blockade, the tip of the Fogarty catheter is positioned above the orifice of the left upper lobe bronchus. For blockade of the right lung, the balloon is placed against the orifice of the right upper lobe bronchus. Rao and associates (1981) placed a Fogarty catheter in the diseased lung and a tracheal tube in the main-stem bronchus of the healthy lung in children to achieve the same objective as the double-lumen tube achieves in adults. If the Fogarty catheter fails to block the diseased bronchus, the dependent lung may still be protected by the presence of the bronchial tube. Baraka and colleagues (1982) reported a high incidence of right upper lobe collapse with blind intubation of the right main-stem bronchus. This complication can be avoided by accurately positioning the tracheal tube with a flexible bronchoscope. The balloons of most catheters used for bronchial blockade have low-volume, high-pressure properties, and overdistention can rupture the bronchus.
Single-Lumen Endotracheal Tubes
Single-lumen endotracheal tubes with one or two inflatable cuffs were the first tubes used for one-lung anesthesia. Single-lumen endotracheal tubes with two cuffs are rarely used today; however, Hammer (2001) has stated that the single-lumen endotracheal tube with or without an inflatable cuff is the simplest way of providing OLV in infants and children. They are introduced into the bronchus of the nonoperative lung. The distance from the tip of the tube to the proximal cuff must be shorter than the length of the main-stem bronchus so that the upper lobe bronchus is not obstructed. Their major disadvantage is that the operative lung cannot be ventilated or suctioned without losing the separation of the lungs.
One-Lung Ventilation
Larsson and associates (1987) demonstrated that functional residual capacity (FRC), compliance, and fraction of
P.376
total ventilation decrease slightly in the dependent lung when the anesthetized patient is placed in the lateral position. The FRC decreases further when the pleural cavity is opened, presumably because of further downward shift of the mediastinum. These findings are similar to those described by Froese and Bryan (1974), who attributed these changes in the lower dependent lung to compression of the lung by the weight of the mediastinum and to the elevation of the diaphragm. General anesthesia and muscle relaxation further decrease the FRC and cause atelectasis in the dependent lung. This decrease in FRC with maldistribution of ventilation in relation to perfusion results in further decrease in Pao2. To avoid atelectasis of the dependent lung, a tidal volume of 10 mL/kg is used. Kerr and associates (1973) showed that if minute ventilation is not decreased during OLV, the arterial carbon dioxide tension is maintained at a similar level to that of two-lung ventilation. The use of a large tidal volume to ventilate one lung results in increasing peak airway pressure by approximately 50%. When airway pressure increases, Cote and colleagues (1983) showed that a larger proportion of the delivered total volume may be wasted because of the compression effect on gases, distention of the anesthesia machine breathing circuit, or both. The result may be a slight decrease in alveolar ventilation and increase in Paco2.
The factors contributing to hypoxemia during OLV are shunting in the nonventilated lung, demonstrated by Kerr and associates (1973), ventilation-perfusion abnormalities in the ventilated lung, and reduction in the cardiac output. To counteract hypoxemia, the nonoperative lung should be ventilated with 100% oxygen with a large tidal volume. In a small percentage of patients, however, the Pao2 may still remain suboptimal. Various techniques have been applied to improve the Pao2 under these circumstances. One such technique is insufflation of oxygen into the nonventilated lung. Results are inconclusive: Rees and Wansbrough (1982) showed improvement, whereas Capan and associates (1980) showed that it is ineffective without application of continuous positive airway pressure (CPAP). The use of CPAP to the nonventilated lung improves arterial oxygenation, but leads to overdistention of the operative lung and suboptimal surgical conditions. Applying HFJV with low driving pressure to the operative lung, Wilks and co-workers (1985) demonstrated improved oxygenation during OLV while maintaining a good surgical field. Nakatuska and associates (1988) compared the effect of CPAP and HFJV to the nondependent lung on the cardiac output and Pao2 during OLV. The application of HFJV to the nondependent lung caused a significant increase in Pao2 compared with deflation of the lung to atmosphere pressure and also maintained better cardiac output compared with CPAP. In addition, HFJV provides a quiet surgical field and satisfactory surgical exposure by delivering small tidal volumes with a low airway pressure in spite of vibratory movement of the surgical field. Malmkvist (1989) reported that intermittent reinflation of the upper lung with 2 L of oxygen every 5 minutes improved Pao2 during OLV. It seems logical that gentle independent ventilation of the operative lung with small tidal volumes coordinated with the surgeon's movements would be a simple, inexpensive approach to improving Pao2.
In the dependent lung, compression by the mediastinum and cephalad movement of the paralyzed diaphragm result in a decrease in FRC. This results in underventilation of well-perfused alveoli and an increase in airway closure. Trapped gas comes into equilibrium with mixed venous blood, contributing to arterial desaturation. Application of positive end-expiratory pressure to the dependent, ventilated lung may improve the situation. Khanam and Branthwaite (1973), however, observed that application of positive end-expiratory pressure to the ventilated lung may increase not only FRC, but also intraalveolar pressure, shifting a higher proportion of pulmonary blood flow to the nonventilated lung and contributing to a reduction in cardiac output.
Ashton and Cassidy (1985) showed that decreased cardiac output and systemic vascular resistance are induced by cardiac depressor reflexes as a result of stimulation of pulmonary stretch receptors. The magnitude of this cardiac depressor reflex is proportional to the magnitude of lung inflation. The effect of the reflex is antagonized by arterial baroreceptors, and a large tidal volume can reduce baroreceptor activity. This decrease in cardiac output in the face of systemic hypoxemia may lead to a significant decrease in oxygen transport. Depending on the degree of increased intraalveolar pressure during application of positive end-expiratory pressure and its effect on the pulmonary circulation and cardiac output, the PaH02 may increase, decrease, or remain the same, according to Katz and associates (1982). Pulmonary vascular congestion, interstitial edema of lower lung, hypovolemia, dysrhythmia, myocardial depression, and surgical manipulation can all decrease the cardiac output and contribute to arterial desaturation. Ligations of the branches of the pulmonary artery to the collapsed section of lung reduce the shunt and improve the arterial blood oxygenation.
Kerr (1973) and Flacke (1976) and their associates have shown that the shunt through the nonventilated lung is approximately 20% to 25% of the cardiac output. This degree of shunt is less than one would expect from complete collapse of the entire lung. Several factors are responsible. First, the effect of gravity and hydrostatic pressure in the lateral decubitus position increases the blood flow to the dependent lung. Second, the operative lung may have decreased pulmonary blood flow because of underlying pathologic conditions. Hurford and associates (1987) showed that in many patients the degree of preoperative perfusion and ventilation of the operative lung correlated inversely with intraoperative oxygenation during OLV. Many patients with normal perfusion of the operative lung, however, did have an adequate level of oxygenation during OLV. This diminishes the value of operative lung ventilation perfusion as a predictor of hypoxemia during OLV. Third, Benumof (1979) showed that hypoxic pulmonary vasoconstriction increases pulmonary
P.377
vascular resistance in the operative lung, which diverts blood flow away from the operative lung and toward the nonoperative, dependent lung.
Anesthetic concentrations of inhalation agents may abolish hypoxic pulmonary vasoconstriction, thereby increasing the blood flow to the nonventilated lung and consequently decreasing the Pao2. Rogers and Benumof (1983) showed that halothane and isoflurane cause an insignificant change in the amount of shunted blood when used in a concentration of 1 minimum alveolar concentration or less. To avoid the possible inhibition of hypoxic pulmonary vasoconstriction from higher concentrations of inhalation anesthetics and to minimize the respiratory depressant effect of high doses of narcotics, a combination of a narcotic and inhalation anesthetics may be particularly useful for intrathoracic operations.
Complications
Complications related to the isolation of lungs and OLV fall into two categories: technical and physiologic (Table 22-2). Because of the shape and large size of double-lumen tubes, the incidence of difficult tracheal intubation is higher than with those with the use of single-lumen tubes. The practical difficulties encountered with Robertshaw tubes were reviewed by Black and Harrison (1975). Complications include laceration of the tracheobronchial tree and malposition of the tube. The site of laceration is usually the posterior membranous wall of the trachea or a main-stem bronchus. The diagnosis may be difficult to make, but it can be confirmed by fiberoptic bronchoscopy. Improper positioning of double-lumen endobronchial tubes, as reported by Read and associates (1977), includes failure to advance the tube far enough down the intended bronchus. Difficulties resulting from improperly positioned endobronchial tubes include incomplete isolation of the lungs, failure to collapse the operative lung, difficulty in ventilating one or both lungs, air entry into the wrong lung, and air trapping and unsatisfactory deflation of the lung. If not recognized, air trapping can eventually cause rupture of the lung and tension pneumothorax. If any of these circumstances arises, two-lung ventilation should be resumed, and the cause of the problem should be identified and corrected before OLV is attempted once again. The physiologic complication of OLV is hypoxemia.
Table 22-2. Complications of Bronchial Intubation and One-Lung Ventilation | |
|---|---|
|
BLOOD AND FLUID REPLACEMENT
Blood and fluid replacement during thoracic surgery is a delicate task and an extremely important part of anesthetic management. Great care must be taken not to overload the circulation, especially in patients undergoing lobectomy or pneumonectomy, because the pulmonary venous capacitance is greatly reduced. Blood loss during most intrathoracic operations does not necessitate a transfusion, but major bleeding can occur at any time. A large intravenous cannula that allows blood and fluid to be administered rapidly is essential, as is the ready availability of blood. All fluids, especially blood, should be warmed during administration.
The perioperative fluid regimen recommended by Giesecke and Egbert (1986) is to replace insensible loss occurring while the patient receives nothing by mouth with a maintenance-type solution of lactated Ringer's or 5% dextrose in 0.45% saline at a rate of 2 mL/kg per hour. During surgery, in addition to 2 mL/kg per hour for insensible loss, 6 mL/kg per hour of replacement-type solution of lactated Ringer's or saline is recommended.
This regimen is followed only for the first 1 or 2 hours of the operation to avoid overhydration. Hutchin and associates (1969) discussed the danger of overhydration of patients during pneumonectomy. Infusion of large volumes of fluids may improve the urine output and circulatory dynamics, but at the risk of developing pulmonary edema and impaired lung mechanics. Twigley and Hillman (1985) stated that crystalloid solutions given intraoperatively go to the interstitial space. Baek and associates (1975) noted that excessive amounts of crystalloid solutions increase interstitial fluid, which causes peripheral and pulmonary edema without correcting the plasma volume deficit. To maintain an adequate blood volume and urine output and to avoid overhydration and congestion of the tissues, including the lungs, Twigley and Hillman (1985) suggested using colloid solutions perioperatively. This suggestion is based on the fact that most intraoperative cardiovascular changes are secondary to an absolute or relative change in intravascular circulating volume, caused by bleeding and the vasodilatory effects of anesthetic drugs. These changes are ideally corrected with a colloid. Colloid solutions are useful to expand plasma volume and should be considered for replacing blood and fluid loss when the patient's blood pressure and pulse rate reflect signs of hypovolemia. The controversy regarding crystalloid or colloid use for blood and fluid replacement continues unresolved.
P.378
In addition to monitoring blood pressure, pulse rate, and urine output, monitoring of CVP and pulmonary artery occlusive pressure is helpful in guiding appropriate fluid therapy especially in patients with poor general health and during operations with major blood and fluid losses. As indicated by Wittnich and colleagues (1986), however, care is needed in techniques of measurement and interpretation of pulmonary artery occlusive pressure. These authors have shown that after pneumonectomy, inflation of the balloon of the pulmonary artery catheter can result in considerable occlusion of the remaining cross-sectioned area of pulmonary circulation. This increase in right ventricular afterload results in reduced cardiac output and reduced left atrial pressure. Therefore, pulmonary artery occlusive pressure may reflect the correct pressure of left atrial pressure, although this is a result of an acute change in cardiac output. Measuring pulmonary wedge pressure by advancing the catheter to peripheral vessels without inflation of the balloon provides a more accurate reading of existing pressures.
Continuous measurement of CVP and pulmonary artery wedge pressures is helpful in patients with myocardial disease or advanced pulmonary disease. In a patient with a healthy heart, however, a serious overload of crystalloid solutions is possible without significant elevation of the CVP or pulmonary artery wedge pressure, particularly if infusion of fluid is constant over several hours. Soft tissue edema and increased urine output are the best signs of overload with intravenous fluids. Edema is seen most easily in the scleral conjunctiva. Congestion and edema of tissues caused by overhydration are also position dependent. In the lateral decubitus position, the nonoperative, healthy lung is dependent and accumulates more fluids. A moderate fluid overload can result in decreased Pao2 intraoperatively and moderate to severe hypoxemia in the immediate postoperative period.
The shortage of blood, together with transfusion hazards, has stimulated a search for alternatives to the use of homologous blood since the 1970s. Transmission of acquired immunodeficiency syndrome (AIDS) through blood transfusions has further increased the public's fear of accepting blood transfusion. Autologous transfusion by aspiration from the surgical field was reported by Bregman and co-workers (1974). Brewster and associates (1979) showed that intraoperative autotransfusion significantly reduced the use of homologous blood transfusion. Normovolemic hemodilution is possible on the day of the operation if the physical condition of the patient permits.
SPECIFIC PROCEDURES AND SUGGESTED MANAGEMENT
Bronchoscopy
A new airway device, the laryngeal mask airway (LMA), has become the technique of choice for flexible bronchoscopy performed under general anesthesia or under topical anesthesia in critically ill patients. The LMA has made sharing of the airway by the surgeon and anesthesiologist an easy task, without compromising the patient's ventilation. The LMA was developed by Brain (1983) of England and consists of a silicone rubber tube with a distal end opening at a 30-degree angle into an elliptical-shaped mask (Fig. 22-7). Two bars are incorporated into the junction of the tube and mask, preventing the epiglottis from herniating into the tube. Before use, the cuff is deflated so that the edge of the cuff appears smooth and faces away from the mask inlet.
The tube of the LMA is held between the index finger and thumb, with the tip of the index finger placed at the anterior aspect of the tube, at the junction of the tube and mask. The patient's head is extended in sniffing position, and the LMA is placed into the mouth (Fig. 22-8). The tip of the LMA is pressed upward against the hard palate and advanced as deep as possible toward the hypopharynx using the index finger. As the index finger is withdrawn, the LMA is advanced further until resistance is felt. The cuff is inflated with the recommended volume of air (20, 30, and 40 mL for sizes 3, 4, and 5, respectively), and the LMA is secured with tape. With perfect positioning, the laryngeal inlet is in full view through the LMA (Fig. 22-9). Ferson and colleagues (1997) have reported that the LMA, in contrast to the endotracheal tube, allows a complete flexible bronchoscopic survey of the larynx and trachea.
Flexible Bronchoscopy Under Topical Anesthesia
Brimacombe and colleagues (1992, 1997) reported the use of an LMA for flexible bronchoscopy under topical anesthesia. Administration of glycopyrrolate, 0.3 to 0.4 mg intramuscularly 30 minutes before bronchoscopy or 0.2 to 0.3 mg intravenously 10 minutes before bronchoscopy, is essential for the topical anesthesia to be effective. After sedation, 4% aerosolized lidocaine is sprayed on the base of the tongue and tonsillar fossae; after 1 minute, lidocaine jelly is spread on the base of the tongue with a tongue blade. Lidocaine, 3 mL of 4%, is injected through the
P.379
cricothyroid membrane to provide anesthesia of the larynx and trachea. If injection of local anesthetic through the cricothyroid membrane is undesirable, laryngotracheal anesthesia is achieved by spraying local anesthetic through the FB working channel (the spray-as-you-go technique). Graham and associates (1992) have shown that injection of lidocaine through the cricothyroid membrane provides a better topical anesthesia of the larynx and trachea and is as acceptable to the patient as the spray-as-you-go technique.
 |
Fig. 22-7. The laryngeal mask airway with cuff inflated. |
 |
Fig. 22-8. A. Placement of the LMA. The LMA is placed inside the mouth with its tip against the hard palate. With the index finger, the LMA is advanced into the hypopharynx while pressing against the palate. B. The LMA is pushed as far down as possible with the index finger. C. The other hand holds the LMA in position while the index finger is withdrawn from the pharynx. The LMA is pushed further downward with the hand to ensure the mask is inserted as deep as possible. Courtesy of LMA North America, Inc., San Diego, CA. |
A bronchoscopy swivel adapter is mounted on the LMA to allow passage of the FB. The LMA is connected to the anesthesia breathing system, which allows assistance and monitoring of the patient's ventilation. If an LMA is not available, an Ovassapian (1987) fiberoptic intubating airway (Fig. 22-10) is used, which keeps the tongue in an anterior position, facilitates exposure of the larynx, and protects the FB from being bitten by the patient. The FB is advanced through the intubating airway to expose the larynx.
Flexible Bronchoscopy Under General Anesthesia
When general anesthesia is required for flexible bronchoscopy, traditionally the anesthesia is provided by an endotracheal tube or a face mask. The ratio of the external diameter of the FB to that of the internal diameter of the endotracheal tube is critical because the instrument reduces the effective ventilatory area of the endotracheal tube lumen. A 5-mm FB reduces the internal diameter of an 8-mm endotracheal tube to such a degree that effective positive-pressure
P.380
ventilation is impossible, which limits the time for bronchoscopy.
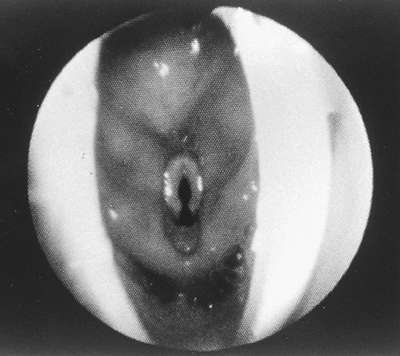 |
Fig. 22-9. Flexible bronchoscopic view of the larynx through a perfectly positioned LMA. The epiglottis is pushed upward and outside of the LMA, allowing complete exposure of the laryngeal inlet. In most cases, the tip of the epiglottis remains inside the LMA, blocking 25% to 50% of the laryngeal view. Courtesy of LMA North America, Inc., San Diego, CA. |
Using a face mask for administration of anesthesia also limits the time of bronchoscopy because ventilation is interrupted during bronchoscopy. Modification of the face mask to provide an entry port for the FB allows bronchoscopy without interruption of ventilation, but the maintenance of the airway and ventilation of the patient could be difficult or impossible in some patients. The LMA not only provides an excellent airway for the patient but also allows unhurried examination of the larynx, vocal cord, upper trachea, and bronchial tree. The LMA permits the use of a larger FB without compromising airway resistance or effective ventilation.
 |
Fig. 22-10. The Ovassapian fiberoptic intubating airway. |
Rigid Bronchoscopy
For rigid bronchoscopy in an anesthetized and paralyzed patient, ventilation can be carried out by intermittent positive-pressure ventilation (IPPV) using a ventilating bronchoscope; by manual jet ventilation using a Venturi injector device described by Sanders (1967); by HFJV, as introduced by Erickson and Sjostrand (1977); or by positive-negative external compression or HFPPV, as reported by Hayek and associates (1985). Manual jet ventilation can be achieved through a rigid bronchoscope, as described by Satyanarayana and colleagues (1980). Vourc'h and co-workers (1983) compared manual jet ventilation with HFJV during bronchoscopy in patients with tracheobronchial stenosis. Arterial blood gas tensions were identical during both manual jet ventilation and HFJV at a rate of 150 per minute. From the endoscopist's point of view, HFJV is preferable to manual jet ventilation because the tracheobronchial wall remains immobile. During HFPPV, no air entrainment occurs, so that anesthetic gases can be delivered at known concentrations. With Hayek external positive-negative internal compression, the airway is not intubated and the surgeon has access to the airway without interference.
Mediastinoscopy
Mediastinoscopy can be performed using local anesthesia, but endotracheal general anesthesia is more pleasant for the patient, provides more flexibility for the surgeon, and facilitates management of a major complication that may occur during this procedure. Compression of the innominate artery by the mediastinoscope can diminish or block blood flow to the right carotid and subclavian arteries. Lee and Salvatore (1976) reported a sudden loss of pulse and blood pressure in the right arm during mediastinoscopy, which was misdiagnosed as a cardiac arrest. The right radial artery pulse should therefore be monitored by palpation or finger plethysmography to detect compression of the innominate artery. Sudden hypotension, bradycardia, or dysrhythmia may occur as a result of mechanical stimulation or compression of the trachea, vagus nerve, or great vessels. Repositioning of the mediastinoscope and intravenous administration of atropine or ephedrine may be necessary to restore the pulse rate and blood pressure. Massive bleeding caused by accidental injury to a major vessel is a distinct but rare possibility. The management of such bleeding necessitates thoracotomy and major surgical intervention. The anesthesiologist should be ready for massive replacement of fluids and blood. An intraoperative tension pneumothorax manifested by increased peak airway pressure, hypotension, and cyanosis is uncommon but requires immediate diagnosis and treatment, as stated by Furgang and Saidman (1972). Other reported complications associated with mediastinoscopy, reported by Ashbaugh (1970), include injury
P.381
to the recurrent laryngeal nerve, phrenic nerve, or esophagus; transient hemiparesis; and air embolism.
Cysts of the Lung
IPPV or vigorous coughing might result in a dangerous increase in alveolar pressure and rupture of a cyst. Ting and associates (1963) reported that the size of a cyst increases if it is in communication with the bronchus and has a valve-type action so that air may enter but not leave the cyst during IPPV. In a closed cyst, administration of nitrous oxide should be avoided, as was suggested by Isenhower and Cucchiara (1976), because it could rapidly increase the volume of and pressure within the cyst. As the size of the cyst increases, it may cause compression atelectasis and mediastinal shift and interfere with adequate gas exchange. Ventilation also may become inadequate if a significant portion of the tidal volume enters and leaves the communicating cyst without participating in gas exchange. Overinflation and rupture of the cyst may cause tension pneumothorax and cardiopulmonary insufficiency.
If the disease is confined to one lung, Isenhower and Cucchiara (1976) suggested that isolation of the lungs with a double-lumen endobronchial tube avoids IPPV to the diseased side. Bilateral air cysts represent a difficult problem because of the possible increase in their size and their interference with gas exchange in the dependent lung while the surgeon operates on the upper lung. Bilateral thoracotomy may be necessary. Normandale and Feneck (1985) successfully applied HFJV for the anesthetic management of patients with bullous cystic lung disease.
Bronchopleural Fistula
The complications associated with a large bronchopleural fistula are loss of ventilation, contamination of the contralateral lung, and development of pneumothorax when IPPV is applied. These complications are best avoided by the passage of an endobronchial tube before induction of general anesthesia. Securing the airway with a double-lumen endobronchial tube in a conscious patient is a safe, but not always easy, approach. Placing patients in a head-up lateral decubitus position with the affected side down minimizes the chance of secretions moving into the tracheobronchial tree during intubation of the trachea. Francis and Smith (1962) indicated that the double-lumen endobronchial tube permits IPPV of the healthy lung without the loss of minute ventilation through the fistula, and prevents soiling of the healthy lung. If tracheal intubation is difficult or not desirable in the conscious patient, general anesthesia with spontaneous ventilation can be used until the airway is secured with a double-lumen endobronchial tube.
If a double-lumen tube cannot be applied and a single-lumen tube is placed, Barker and co-workers (1971) suggested maintaining spontaneous ventilation until the chest is opened. During surgery, the air leak can be minimized by manually packing the lung. Carlon and co-workers (1980) successfully applied high-frequency positive pressure and carbon dioxide removal in a patient with a large bronchopleural fistula. Hildebrand and associates (1984) indicated that HFJV at 100 cycles per minute was unsuitable during lobectomy with an open bronchus and resulted in a rapid deterioration in Pao2 and an increase in Paco2. Their results conflict with those of Moulaert and Rolly (1983), who claimed that ventilation with an open bronchus was possible with HFJV at a rate of 250 cycles per minute.
Pneumothorax and Hemothorax
An important feature in the anesthetic management of pneumothorax and hemothorax is to drain them under local anesthesia before inducing general anesthesia or administering IPPV.
Tracheal Resection
A thorough preoperative evaluation and understanding of the airway problem is essential. Good rapport must be established for the patient, and heavy premedication should be avoided. Close communication between the surgeon and anesthesiologist is essential during tracheal reconstruction, and each one should be fully aware of the other's plan, approach, and readiness before induction of anesthesia.
Various methods of maintaining adequate ventilation have been applied during operations on the trachea or bronchi. Use of a single-lumen endotracheal tube placed above the tracheal lesion preoperatively and advanced inside the trachea or bronchi below the tracheal lesion during surgery has been described by Belsey (1950) and Geffin and associates (1969). It is safer to secure the airway while the patient is awake.
If awake intubation is not possible, a slow inhalation induction with a halogenated anesthetic agent with oxygen should be performed, with spontaneous ventilation maintained until tracheal intubation is achieved. The FB can be used to apply topical anesthesia to the larynx and trachea, to evaluate the site and degree of tracheal stenosis, and to intubate the trachea. The instrument enables the anesthesiologist to place the tip of the tube above the stenotic area and to avoid any trauma. After exposure of the trachea, as the surgeon starts resecting the lesion, the orotracheal tube is advanced beyond the lesion into the lower section of the trachea. The surgeon completes the resection and anastomosis in the presence of the endotracheal tube. To avoid the presence of an endotracheal tube in the surgical field, Akdikmen and Landmesser (1965) and Geffin and associates (1969) described the use of two separate endotracheal tubes. The first endotracheal tube is placed orally above the tracheal lesion. The second sterile, armored endotracheal
P.382
tube is inserted by the surgeon into the distal trachea or one of the main-stem bronchi after cutting the trachea distal to the lesion. The second endotracheal tube is then connected to the anesthesia machine using sterile corrugated tubing and a Y piece, and anesthesia is continued. After resection of the lesion and placement of sutures in the posterior tracheal wall, the surgeon removes the endotracheal tube placed in the distal trachea. The original orotracheal tube is advanced beyond the suture line into the lower trachea or one of the main-stem bronchi until the surgeon completes the repair of the trachea.
A third approach is the use of HFJV through a small catheter, as described by Erickson (1975) and Rogers (1985) and their co-workers; manual jet ventilation, as reported by Lee and English (1974); or HFPPV, as applied by El-Baz and colleagues (1982). Scamman and Choi (1986) used a sterile nasogastric tube for application of low-frequency jet ventilation and measurement of end-tidal CO2 during tracheal resection. The distal end of the nasogastric tube was cut off above the highest side hole and placed 2 cm into the distal stump of the trachea. The larger lumen was connected to a Sander's jet apparatus, and the smaller to a CO2 analyzer. Normal arterial and end-tidal gas tensions were maintained while the surgeon completed the posterior and lateral wall anastomosis. Neuman and associates (1984) described successful use of HFJV for tracheal resection in a 7-year-old child.
To avoid contamination of the operating room from inhalation agents, intravenous anesthetics are used during HFPPV or jet ventilation. Early extubation is highly desirable to minimize the compromise of blood flow to the trachea. Woods (1961) and Coles (1976) and their associates applied extracorporeal circulation for management of tracheobronchial resection.
Laser Surgery
Laser surgery of the airway presents several potential anesthetic problems. These include ventilation and oxygenation through a compromised and shared airway and hazards introduced by the laser beam. The major hazards from laser surgery are fires and destruction of normal tissues. Fire can occur when the laser strikes a rubber or plastic endotracheal tube in an oxygen-rich anesthetic mixture. Nitrous oxide, like oxygen, supports combustion, whereas halogenated anesthetic agents are not flammable and do not support combustion. To minimize the danger of fire, Brutinel and associates (1983) recommended using 50% oxygen or less in nitrogen, whereas Eisenman and Ossoff (1986) favor a mixture of oxygen and helium during general anesthesia. The use of metallic or noncombustible disposable endotracheal tubes and protection of standard rubber or plastic endotracheal tubes by wrapping them with aluminum or copper tape have been thoroughly reviewed by Hermens and co-workers (1983). Endotracheal tubes wrapped with metallic tape may cause pharyngeal and laryngeal injury because of rough edges, and pieces of tape can loosen, break off, and be aspirated. All oil-based ointments and lubricants should be avoided because they are combustible and can be ignited. In case of fire, the procedure should be stopped and the endotracheal tube should be removed immediately. The lungs as well as the trachea may be injured by either smoke inhalation or a direct thermal burn.
Using a Hayek (1985) positive and negative external compressor allows air exchange without intubation. This device is in its early stages of experimental use. If proved safe and effective, it will eliminate the need for intubation, and therefore the surgeon will have sole access to the airway during total intravenous anesthesia.
The surgical field should be immobile to minimize the chance of laser damage of normal tissue. The choice of anesthesia for bronchoscopic laser surgery depends on the surgical technique and the age and condition of the patient. Rontal and associates (1986) favor topical anesthesia with sedation whenever possible, but particularly in patients with higher-grade airway obstruction. General anesthesia is the method of choice for most children and for adults who cannot tolerate local anesthesia. Dumon and associates (1984) favor spontaneous ventilation, whereas Brutinel (1983) and Vourc'h (1983) and their co-workers recommend controlled ventilation. If jet ventilation is chosen, scavenging of inhalation anesthetic agents is difficult, and total intravenous anesthesia must be provided. Prolonged respiratory depression, causing the need for postoperative ventilatory support, is a potential complication of total intravenous anesthesia.
Whatever the anesthetic technique, the basic principles of anesthetic management of patients with a compromised airway should be followed. Communication between the anesthesiologist and the surgical team is essential, and a plan for management of total airway obstruction must be decided upon before induction of anesthesia. All routine safety precautions during laser surgery, both for the patient and the operating room personnel, should be observed. A sign noting that a laser is in use should be placed on the outside of the door. Finally, postoperative care is extremely important, because respiratory depression, laryngospasm, bronchospasm, airway obstruction, and hemorrhage can all occur and require immediate treatment.
REFERENCES
Abe K, Mashimo T, Yoshiya I: Arterial oxygenation and shunt fraction during one-lung ventilation: a comparison of isoflurane and sevoflurane. Anesth Analg 86:1266, 1998.
Akdikmen S, Landmesser CM: Anesthesia for surgery of the intrathoracic portion of the trachea. Anesthesiology 26:117, 1965.
Arai T, Hatano Y: Yet another reason to use a fiberoptic bronchoscope to properly site a double lumen tube. Anesthesiology 66:581, 1987.
Arndt GA, et al: Single-lung ventilation in a critically ill patient using a fiberoptically directed wire-guided endobronchial blocker. Anesthesiology 90:1484, 1999a.
Arndt GA, et al: Wire-guided endobronchial blockade in a patient with a limited mouth opening. Can J Anaesth 46:87, 1999b.
Ashbaugh DG: Mediastinoscopy. Arch Surg 100:568, 1970.
P.383
Ashton JH, Cassidy SS: Reflex depression of cardiovascular function during lung inflation. J Appl Physiol 58:137, 1985.
Baek SM, et al: Plasma expansion in surgical patients with high central venous pressure: the relationship of blood volume to hematocrit, CVP, pulmonary wedge pressure and cardiorespiratory changes. Surgery 78:304, 1975.
Bahk JH, Oh YS: A new and simple maneuver to position the left-sided double-lumen tube without the aid of fiberoptic bronchoscopy. Anesth Analg 86:1271, 1998.
Baraka A, et al: One lung ventilation of children during surgical excision of hydatid cysts. Br J Anaesth 54:523, 1982.
Barker WL, et al: Management of bronchopleural fistulas. J Thorac Cardiovasc Surg 62:393, 1971.
Belsey R: Resection and reconstruction of the intrathoracic trachea. Br J Surg 38:200, 1950.
Benumof JL: Mechanism of decreased blood flow to atelectatic lung. J Appl Physiol 46:1047, 1979.
Bjork VO, Carlens E: The prevention of spread during pulmonary resection by the use of a double-lumen catheter. J Thorac Cardiovasc Surg 20:151, 1950.
Black AMS, Harrison GA: Difficulties with positioning Robertshaw double-lumen tubes. Anaesth Intensive Care 3:299, 1975.
Brain AIJ: The laryngeal mask a new concept in airway management. Br J Anaesth 55:801, 1983.
Bregman D, et al: Intraoperative autotransfusion during emergency thoracic and elective open heart surgery. Ann Thorac Surg 18:590, 1974.
Brewster DC, et al: Intraoperative autotransfusion in major vascular surgery. Am J Surg 137:507, 1979.
Brimacombe J, et al: A potential new technique for awake fiberoptic bronchoscopy use of the laryngeal mask airway. Med J Aust 156:876, 1992.
Brimacombe JR, Brain AIJ, Berry AM: The Laryngeal Mask Airway: A Review and Practical Guide. London: WB Saunders, 1997.
Brutinel WM, McDougall JC, Cortese DA: Bronchoscopic therapy with neodymium-yttrium-aluminum-garnet laser during intravenous anesthesia. Effect on arterial blood gas levels, pH, hemoglobin saturation, and production of abnormal hemoglobin. Chest 84:518, 1983.
Burton NA, et al: Advantages of a new polyvinyl chloride double lumen tube in thoracic surgery. Ann Thorac Surg 36:78, 1983.
Campos JH, Massa FC, Kernstine KH: The incidence of right upper-lobe collapse when comparing a right-sided double-lumen tube versus a modified left-sided double-lumen tube for left sided thoracic surgery. Anesth Analg 90:535, 2000.
Capan LM, et al: Optimization of arterial oxygenation during one-lung anesthesia. Anesth Analg 59:847, 1980.
Carlens E: A new flexible double-lumen catheter for bronchospirometry. J Thorac Cardiovasc Surg 18:742, 1949.
Carlon GC, et al: High frequency positive pressure ventilation in management of a patient with bronchopleural fistula. Anesthesiology 52:60, 1980.
Cay DL, et al: Selective bronchial blocking in children. Anaesth Intensive Care 3:127, 1975.
Coles JC, et al: A method of anesthesia for imminent tracheal obstruction. Surgery 80:379, 1976.
Cote CJ, et al: Wasted ventilation measured in vitro with eight anesthetic circuits with and without inline humidification. Anesthesiology 59:442, 1983.
Dalens B, Labbe A, Haberer JP: Selective endobronchial blocking vs. selective intubation. Crit Care Med 10:643, 1982.
Dougherty P, Hannallah M: A potentially serious complication that resulted from improper use of the Univent tube. Anesthesiology 77:835, 1992.
Dumon JF, et al: Principles for safety in application of neodymium-YAG laser in bronchology. Chest 86:163, 1984.
Edwards EM, Hatch DJ: Experiences with double-lumen tubes. Anaesthesia 20:461, 1965.
Eisenman TS, Ossoff RH: Anesthesia for bronchoscopic laser surgery. Otolaryngol Head Neck Surg 94:45, 1986.
El-Baz N, et al: One-lung high-frequency pressure ventilation for sleeve pneumonectomy: an alternative technique. Anesth Analg 60:683, 1981.
El-Baz N, et al: One-lung frequency ventilation for tracheoplasty and bronchoplasty: a new technique. Ann Thorac Surg 34:564, 1982.
Erickson I, Sjostrand I: Experimental and clinical evaluation of high-frequency positive-pressure ventilation (HFPPV) and the pneumatic valve principle in bronchoscopy under general anesthesia. Acta Anesthesiol Scand Suppl 64:83, 1977.
Erickson I, et al: High-frequency positive-pressure ventilation (HFPPV) during resection of tracheal stenosis and during preoperative bronchoscopic examination. Acta Anaesthesiol Scand 19:113, 1975.
Ferson DZ, et al: The laryngeal mask airway: a new standard for airway evaluation in thoracic surgery. Ann Thorac Surg 63:768, 1997.
Flacke JW, Thompson DS, Read RC: Influence of tidal volume and pulmonary artery occlusion on arterial oxygenation during endobronchial anesthesia. South Med J 69:619, 1976.
Francis JG, Smith KG: An anesthetic technique for the repair of bronchopleural fistula. Br J Anaesth 34:817, 1962.
Froese AB, Bryan AC: Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 41:242, 1974.
Furgang FA, Saidman LJ: Bilateral tension pneumothorax associated with mediastinoscopy. J Thorac Cardiovasc Surg 63:329, 1972.
Geffin B, Bland J, Grillo HC: Anesthetic management of tracheal resection and reconstruction. Anesth Analg 48:884, 1969.
Giesecke AH, Egbert LD: Perioperative fluid therapy; crystalloids. In Miller RD (ed): Anesthesia. 2nd Ed. New York: Churchill Livingstone, 1986, p. 1313.
Ginsberg RJ: New technique for one-lung anesthesia using an endobronchial blocker. J Thorac Cardiovasc Surg 82:542, 1981.
Glenski JA, Crawford M, Rehder K: High-frequency, small-volume ventilation during thoracic surgery. Anesthesiology 64:211, 1986.
Graham DR, et al: Comparison of three different methods used to achieve local anesthesia for fiberoptic bronchoscopy. Chest 102:704, 1992.
Hammer GB: Pediatric thoracic anesthesia. Anesth Analg 92:1449, 2001.
Hammer GB, Fitzmaurice BG, Brodsky JB: Methods for single-lung ventilation in pediatric patients. Anesth Analg 89:1426, 1999.
Hayek Z, et al: External high frequency ventilation without intubation. Crit Care Med 19:406A, 1985.
Hermens JM, et al: Anesthesia for laser surgery. Anesth Analg 62:218, 1983.
Hildebrand PJ, et al: High frequency jet ventilation. A method for thoracic surgery. Anaesthesia 39:1091, 1984.
Hogg CE, Lorhan PH: Pediatric bronchial blocking. Anesthesiology 33:560, 1970.
Hultgren BL, et al: A new tube for one lung ventilation: experience with Univent tube [Abstract]. Anesthesiology 65:A481, 1986.
Hurford WE, Kolker AC, Strauss HW: The use of ventilation/perfusion lung scans to predict oxygenation during one-lung anesthesia. Anesthesiology 67:841, 1987.
Hutchin P, et al: Pulmonary congestion following infusion of large fluid loads in thoracic surgical patients. Ann Thorac Surg 8:339, 1969.
Isenhower N, Cucchiara RF: Anesthesia for vanishing lung syndrome: report of a case. Anesth Analg 55:750, 1976.
Jenkins VA, Clark G: Endobronchial anesthesia with the Carlens catheter. Br J Anaesth 30:12, 1958.
Karwande S: A new tube for single lung ventilation. Chest 92:761, 1987.
Katz JA, et al: Pulmonary oxygen exchange during endobronchial anesthesia: effects of tidal volume and PEEP. Anesthesiology 56:164, 1982.
Kerr JH, et al: Observations during endobronchial anesthesia. I. Ventilation and carbon dioxide clearance. Br J Anaesth 45:159, 1973.
Khanam T, Branthwaite MA: Arterial oxygenation during one-lung anesthesia. Anaesthesia 28:280, 1973.
Larsson A, Malmkvist G, Werner O: Variation in lung volume and compliance during pulmonary surgery. Br J Anesth 59:585, 1987.
Lee HL, et al: Successful one-lung ventilation in a patient with aberrant tracheal bronchus. Anesth Analg 95:492, 2002.
Lee JH, Salvatore A: Innominate artery compression simulating cardiac arrest during mediastinoscopy: a case report. Anesth Analg 55:748, 1976.
Lee P, English IC: Management of anesthesia during tracheal resection. Anaesthesia 29:305, 1974.
MacGillivray RG: Evaluation of a new tracheal tube with a movable bronchial blocker. Anaesthesia 43:687, 1988.
Malmkvist G: Maintenance of oxygenation during one-lung ventilation. Effect of intermittent reinflation of the collapsed lung with oxygen. Anesth Analg 68:763, 1989.
Moulaert P, Rolly G: High frequency of jet ventilation for pulmonary resection. In Sheck PA, Sjostrand NH, Smith RB (eds): Perspectives in High Frequency Ventilation. Boston: Martinus-Nijhoff, 1983, p. 227.
Nakatuska M, Wetstein L, Keenan RL: Unilateral high-frequency jet ventilation during one-lung ventilation for thoracotomy. Ann Thorac Surg 44:654, 1988.
Neuman CG, et al: High-frequency jet ventilation for tracheal resection in a child. Anesth Analg 63:1039, 1984.
Normandale JP, Feneck RO: Bullous cystic lung disease: its anaesthetic management using high frequency jet ventilation. Anaesthesia 40:1182, 1985.
Ovassapian A: A new fiberoptic intubating airway. Anesth Analg 66:S132, 1987.
P.384
Ovassapian A: Fiberoptic aided bronchial intubation. In Ovassapian A (ed): Fiberoptic Endoscopy and the Difficult Airway. Philadelphia: Lippincott-Raven, 1996, p. 117.
Ovassapian A, Schrader S: Fiberoptic-aided bronchial intubation. Semin Anesth 6:133, 1987.
Ovassapian A, et al: Endobronchial intubation using flexible fiberoptic bronchoscope. Anesthesiology 59:A501, 1983.
Rao CC, et al: One-lung pediatric anesthesia. Anesth Analg 60:450, 1981.
Read RC, Friday CD, Eason CN: Prospective study of the Robertshaw endobronchial catheter in thoracic surgery. Ann Thorac Surg 24:156, 1977.
Rees D, Wansbrough SR: One-lung anesthesia: percent shunt and arterial oxygen tension during continuous insufflation of oxygen to the nonventilated lung. Anesth Analg 61:507, 1982.
Rigg D: A comparison of the Robertshaw and Carlens-type double-lumen tubes for thoracic anesthesia. Anaesth Intensive Care 8:460, 1980.
Robertshaw FL: Low resistance double-lumen endobronchial tubes. Br J Anaesth 34:576, 1962.
Rogers RC, et al: High frequency jet ventilation for tracheal surgery. Anaesthesia 40:32, 1985.
Rogers SN, Benumof JL: Halothane and isoflurane do not impair arterial oxygenation during one-lung ventilation to patients undergoing thoracotomy. Anesthesiology 59:A532, 1983.
Rontal M, et al: Anesthetic management for tracheobronchial laser surgery. Ann Otol Rhinol Laryngol 95:556, 1986.
Sanders RD: Two ventilating attachments for bronchoscopes. Del Med J 39:170, 1967.
Satyanarayana T, et al: Bronchofiberscope jet ventilation. Anesth Analg 59:350, 1980.
Scamman FL, Choi WW: Low frequency jet ventilation for tracheal resection. Laryngoscope 96:678, 1986.
Shinnick JP, Freedman AP: Bronchofiberscopic placement of a double-lumen endotracheal tube. Crit Care Med 10:544, 1982.
Smith RB, et al: High frequency ventilation during pulmonary lobectomy: three cases. Respir Care 26:437, 1981.
Thorburn JR, et al: Comparison of the effects of atropine and glycopyrrolate on pulmonary mechanics in patients undergoing fiberoptic bronchoscopy. Anesth Analg 65:1285, 1986.
Ting EY, et al: Mechanical properties of pulmonary cysts and bullae. Am Rev Respir Dis 87:538, 1963.
Twigley AJ, Hillman KM: The end of the crystalloid era? Anaesthesia 40:860, 1985.
Vale R: Selective bronchial blocking in a small child. Case report. Br J Anaesth 41:453, 1969.
Vourc'h G, et al: High frequency jet ventilation versus manual jet ventilation during bronchoscopy in patients with tracheo-bronchial stenosis. Br J Anaesth 55:969, 1983.
Weinrich A, et al: Continuous ketamine infusion for one-lung anesthesia. Can J Anaesth 27:485, 1980.
Wilks D, et al: Selective high frequency jet ventilation of the operative lung improves oxygenation during thoracic surgery. Anesthesiology 63:A586, 1985.
Wittnich C, et al: Misleading pulmonary wedge pressure after pneumonectomy: its importance in postoperative fluid therapy. Ann Thorac Surg 42:192, 1986.
Woods F, et al: Resection of the carina and mainstem bronchi with extracorporeal circulation. N Engl J Med 254:492, 1961.
EAN: 2147483647
Pages: 203