15 - Molecular Diagnostic Studies in Pulmonary Disease
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section V - Assessment of the Thoracic Surgical Patient > Chapter 20 - Preoperative Cardiac Evaluation of the Thoracic Surgical Patient
Chapter 20
Preoperative Cardiac Evaluation of the Thoracic Surgical Patient
Andrew S. Rudin
Dan J. Fintel
It is neither possible nor appropriate to clear a patient for surgery during a preoperative evaluation. The principal goals of the preoperative evaluation are to estimate the risk for and to minimize the incidence of postoperative cardiovascular complications. Additionally, it is valuable to recommend therapies that may reduce the long-term risk for cardiac events. The aim of this chapter is to outline a rational preoperative evaluation strategy and to discuss four principal postoperative cardiovascular complications: myocardial infarction (MI), congestive heart failure, arrhythmias, and hypertension. Thoracic surgery creates special challenges for the cardiac patient. In one recent study by Melendez and Carlon in 1998 of 188 patients aged 15 to 87 years undergoing thoracic surgery, 14% had cardiovascular complications. Mangano and colleagues, in 1996, found that patients who have episodes of ischemia or who experience nonfatal MIs in the first week after surgery have a 2- to 20-fold risk for serious cardiovascular outcome in the 2 years after surgery. There are numerous physiologic reasons why the thoracic surgery patient is at especially high risk for cardiac complications. Significant atelectasis, decreased lung compliance, and decreased diffusing capacity after thoracic surgery may lead to hypoxia, hypercarbia, or increased work of breathing, which all decrease myocardial oxygen supply and increase myocardial oxygen demand. This mismatch may precipitate ischemia, which in turn can lead to arrhythmias, congestive heart failure, or even MI. Second, postoperative patients develop a hypercoagulable state that may exacerbate fixed coronary stenoses, contribute to new coronary plaque rupture, or place strain on the heart through the development of pulmonary emboli. Third, after major lung resections, the decrease in the pulmonary vascular bed results in increased preload, which can worsen congestive heart failure. Complicating matters further are the high catecholamine levels and enormous fluid shifts found in many patients after thoracic surgery.
PREOPERATIVE EVALUATION OF RISK
The guiding principle in preoperative consultation is the same as in the everyday practice of medicine: Order tests only if the results have a reasonable likelihood of changing management. To appreciate fully whether a test result may change management, an understanding of Bayes' theorem is required. Bayes' theorem states that the post-test probability of a person having a disease is related to the sensitivity, specificity, and the prevalence of that disease in the population being studied. For instance, if the clinical suspicion for a disease is high (pretest probability), a negative noninvasive test never rules out that disease. Here is a real-life example: In January 1993, a 37-year-old man, long-time smoker, with elevated cholesterol and a family history of coronary artery disease presented to an emergency department in Sedgwick County, Kansas. He had complaints consistent with classic unstable angina. He began experiencing chest discomfort 2 days earlier while washing his car. He subsequently developed substernal chest tightness while loading boxes at work, radiating to his arm and associated with diaphoresis and shortness of breath. The symptoms resolved with 5 to 10 minutes of rest. On the surface, his physician performed all of the appropriate tests, admitting him overnight for observation, ruling out myocardial infarction (MI), and performing a treadmill stress test the next day. He exercised for 12 minutes without electrocardiographic changes and was discharged. Four days later, he died. His autopsy disclosed extensive three-vessel coronary stenoses. His widow sued, citing Bayes' theorem, and was awarded a large sum of money. The jury went so far as to state that Bayes' theorem is part of the medical standard of care.
Mangano and colleagues in 1996 reported that morbidity and mortality due to cardiovascular disease are prevalent and costly for the 30 million patients who undergo noncardiac surgery annually, affecting more than 1 million of them. Ten percent of these patients have known coronary
P.346
artery disease or are at significant risk. Several small clinical trials have investigated the effect of preoperative nitrates, calcium channel blockers, and 2-agonists with somewhat encouraging but not conclusive results. Because postoperative ischemic events are at least partially related to the persistently exaggerated sympathetic response commonly seen in these patients, -blocker therapy merits special examination. In fact, two studies have demonstrated significant morbidity and mortality benefits with the perioperative use of -blockers. Mangano and associates (1996) randomized 200 patients to receive atenolol (a 2-selective -blocker) or placebo and followed the patients for 2 years. Atenolol was given intravenously (10 mg) immediately before and after surgery and then orally (100 mg daily) until hospital discharge. Thirty patients (15.6%) died during the 2-year follow up period. Twenty-one of these deaths (12 of which were from cardiac causes) occurred in the placebo group, compared with 9 (4 of which were from cardiac causes) in the atenolol group, resulting in a 55% reduction in overall mortality and a 65% reduction in cardiovascular mortality. Predictably, most of the -blocker benefit was seen during the first 6 to 8 months, when no cardiac deaths occurred in the atenolol group, compared with seven cardiac deaths in the placebo group (p <0.001). Poldermans and colleagues (2001) subsequently described a randomized trial of bisoprolol, another 2-selective -blocker, in patients undergoing major subdiaphragmatic vascular surgery who had evidence of dobutamine-induced hypokinesis (ischemia) by stress echocardiography. One hundred and twelve patients were followed for 30 days postoperatively after receiving perioperative bisoprolol plus standard care versus standard care alone. A dose of 5 mg was started at least 1 week before surgery and was increased as tolerated to 10 mg with a goal heart rate of 60 beats/min. Intravenous metoprolol was given postoperatively if patients were not able to take the oral medication. There were two (3.4%) cardiac deaths in the bisoprolol group versus nine (17%) in the standard care group (p = 0.02); nonfatal infarcts were zero versus nine (17%) (p = 0.001); and the combined end point of death plus nonfatal infarct was 3.4% versus 34% (p = 0.001), with a relative risk of 0.09 [95% confidence interval (CI) of 0.02 to 0.37]. Note that chronic obstructive pulmonary disease (COPD) is no longer regarded as a contraindication to -blocker use. Gottlieb and co-workers (1998) demonstrated in a recent analysis of more than 40,000 patients with COPD after MI that these medications are both safe and effective. The 9,228 patients who received -blockers had a relative risk for death of 0.60 (CI, 0.57 to 0.63) compared with the 32,586 patients with COPD who did not receive -blockers after infarction. Another medication that may become important in preventing postoperative cardiovascular complications is aspirin. This may be especially true postoperatively in vascular and cardiothoracic surgery. Mangano and associates (1996) prospectively studied 5,065 patients who survived at least 48 hours after coronary artery bypass surgery. Among the patients who received aspirin within the first 48 hours after surgery, the mortality rate was 1.3%, compared with 4.0% among patients who did not receive aspirin. This finding certainly will require further study before routinely recommending early postoperative aspirin therapy to patients undergoing noncardiac thoracic surgery.
PREOPERATIVE EVALUATION OF CARDIOVASCULAR RISK
Preoperative cardiovascular evaluation does not routinely require a stress test before clearance for surgery. Which patients warrant noninvasive cardiac stress testing (treadmill test, stress echocardiography, or a nuclear stress test)? Which patients should proceed directly to coronary angiography? Who should have no testing at all? To answer these questions, it is important to understand that many patients will remain at high risk despite demonstrating no ischemia on noninvasive testing, and others will remain at low risk despite an abnormal study. For example, an 80-year-old patient with diabetes who suffered a recent MI and has decompensated heart failure and high-grade atrioventricular block is still at high risk even with a stress test that is without evidence of ischemia. The purpose of noninvasive stress testing is to help stratify patients into low, intermediate, or high risk. The American College of Cardiology/American Heart Association (ACC/AHA) published guidelines on preoperative testing in December 2001 that are drawn from the aforementioned principles of bayesian analysis and of only ordering tests that have a reasonable likelihood of changing management. They recommend a stepwise approach to preoperative cardiac assessment that is logical and evidence based (Fig. 20-1; Table 20-1).
Step 1. Is the surgery emergent? If so, the task of the consultant may be to recommend perioperative -blockade if ischemic heart disease is present or suspected, or preoperative diuresis and a consideration for a pulmonary artery catheter if left ventricular dysfunction is present. In appropriate cases, a postoperative ischemia evaluation is indicated in patients whose need for surgery is urgent.
Step 2. Has the patient undergone a bypass within the last 5 years or percutaneous coronary intervention (PCI) from 6 months to 5 years previously? According to Mahar and co-workers (1978), if the clinical symptoms have remained stable and there are no recurrent signs or symptoms of ischemia, then the likelihood of a perioperative cardiac death or MI is extremely low. Further cardiac testing is generally not necessary.
Step 3. Has the patient undergone a stress test or an angiogram in the past 2 years? If the patient has not experienced a change in symptoms since that examination, then a repeat evaluation is not necessary.
Step 4. Does the patient have a major clinical predictor of risk? Examples of such clinical conditions include acute
P.347
P.348
coronary syndromes, decompensated congestive heart failure, significant arrhythmias, or severe valvular disease. Usually, elective surgery is delayed until the acute cardiac problem is adequately treated.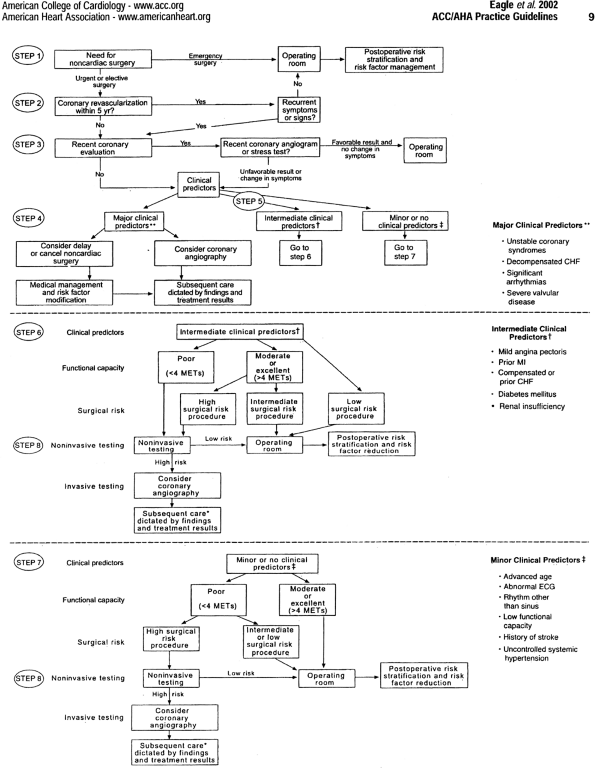
Fig. 20-1. Stepwise approach to preoperative cardiac assessment. Steps are discussed in the text. *Subsequent care may include cancellation or delay of surgery, coronary revascularization followed by noncardiac surgery, or intensified care. CHF, congestive heart failure; ECG, electrocardiogram; MET, metabolic equivalent; MI, myocardial infarction.
Table 20-1. Clinical Predictors of Increased Perioperative Cardiovascular Risk (Myocardial Infarction, Heart Failure, Death)
Major
Unstable coronary syndromes
Acute or recent MIa with evidence of important ischemic risk by clinical symptoms or noninvasive study
Unstable or severeb angina (Canadian class III or IV)c
Decompensated heart failure
Significant arrhythmias
High-grade atrioventricular block
Symptomatic ventricular arrhythmias in the presence of underlying heart disease
Supraventricular arrhythmias with uncontrolled ventricular rate
Severe valvular disease
Intermediate
Mild angina pectoris (Canadian class I or II)
Previous MI by history or pathologic Q waves
Compensated or prior heart failure
Diabetes mellitus (particularly insulin-dependent)
Renal insufficiency
Minor
Advanced age
Abnormal ECGT (left ventricular hypertrophy, left bundle-branch block, ST-T abnormalities
Rhythm other than sinus (e.g., atrial fibrillation)
Low functional capacity (e.g., inability to climb one flight of stairs with a bag of groceries)
History of stroke
Uncontrolled systemic hypertensionECG, electrocardiogram; MI, myocardial infarction
a The American College of Cardiology National Database Library defines recent MI as 7 days but 1 month (30 days); acute MI is within 7 days.
b May include stable angina in patients who are unusually sedentary.
c Campeau L. Grading of angina pectoris. Circulation 54:522, 1976.Step 5. Does the patient have intermediate clinical predictors of risk? Examples include mild angina pectoris, prior MI, compensated or prior congestive heart failure, diabetes mellitus, or renal insufficiency. These patients can be stratified by their functional capacity (less than 4 metabolic equivalents (METS) by history) and the surgery-specific risk. Activities that require more than 4 METS include moderate cycling, climbing hills, ice-skating, roller-blading, singles tennis, and jogging.
Most general thoracic surgeries are considered intermediate surgical risk procedures by the ACC/AHA task force, as reported by Eagle and associates (2002), unless the surgical procedure is anticipated to be prolonged with extensive blood loss and large fluid shifts. Intermediate surgical risk procedures have a combined end point of the risk for MI or death between 1% and 5%. Low-risk procedures such as breast surgery and endoscopic procedures have a combined end point of less than 1%. High-risk procedures such as aortic and vascular surgery have a combined end point of greater than 5%. These include aortic and vascular surgeries. Noninvasive testing is only indicated in most thoracic surgery patients who have intermediate clinical predictors and poor functional capacity.
Step 6. Patients with intermediate clinical risk factors and moderate or excellent functional capacity (greater than 4 METS) normally require no further testing before undergoing most general thoracic surgeries. However, if the surgery is complicated and the patient has two or more intermediate clinical risk factors, then noninvasive testing should be considered.
Step 7. General thoracic operations are generally safe for patients with moderate or excellent functional capacity and no or only minor clinical risk factors. These patients warrant no further workup. If the functional capacity is poor and the surgical procedure high risk, then a stress test should be considered.
Step 8. The results of noninvasive testing can be used to assist the team in deciding on the appropriate perioperative management such as intensified medical therapy or coronary revascularization. It is almost never appropriate to recommend coronary bypass surgery or percutaneous cardiac intervention in an effort to reduce the risk for surgery unless they would be otherwise indicated. Thus, if an asymptomatic patient has a small amount of ischemia on a myocardial perfusion stress test and is found to have single-vessel disease at angiography, then the recommendation is not to perform PCI.
PERIOPERATIVE MANAGEMENT
There are times when patients with considerable risk will need an urgent operation and situations when a patient will develop a cardiac complication after operation. The management of cardiac conditions both before operation and during the perioperative period is similar.
Implantable Defibrillators and Pacemakers
No formal guidelines have been developed for the perioperative management of these devices. Adverse interactions may occur primarily due to the interactions between the electrical currents of electrocautery, anesthetic agents, and the metabolic derangements so often present during thoracic surgery. Electrocautery is generally applied in a unipolar fashion between the cautery device and a plane perpendicular to the patient's skin. The possibility of interference with an implanted device is related to the amount of current in the adjacent area. The interference can lead to a variety of responses by the device: (a) temporary or permanent
P.349
resetting to a backup mode such as VOO or VVI pacing, (b) temporary or permanent inhibition of pacemaker output, (c) an increase in pacing rate due to the activation of a rate response sensor, (d) ICD firing, and (e) myocardial injury that may cause failure to sense or capture. The probability of these events occurring has fallen considerably with the use of bipolar leads and improved pacemaker design. However, the following recommendations can still be made. Pacemakers and implantable defibrillators should be interrogated before and after surgery. The defibrillator should be programmed off before the operation and then on immediately afterward. Pacemakers that are in a rate-responsive mode should be reprogrammed. All attempts should be made to avoid excessive electrocautery near the device whenever possible. If emergent cardioversion is required, then the paddles should be placed as far as possible from the device, preferably in an anterioposterior position.
Myocardial Infarction
Perhaps the most feared postoperative complication is MI. The World Health Organization definition of MI requires that two of the following three conditions be met: (a) symptoms, (b) myocardial enzyme release, and (c) compatible electrocardiogram changes. However, this definition poses a dilemma to categorize the many postoperative patients who exhibit myocardial enzyme release but are devoid of symptoms or electrocardiographic changes. Most cardiologists consider a significant enzyme release as an MI despite the failure to meet strict criteria. It is worth noting that several conditions may result in the release of troponin I and T. These include blunt myocardial trauma, aortic dissection, pulmonary embolism, esophageal rupture, peptic ulcer disease, pancreatitis, and even cholecystitis.
After exclusion of these impostors, it is important to understand the various mechanisms that may result in myocardial injury in order to assist with treatment strategies. Myocardial injury is generally caused by significant mismatches in myocardial supply and demand (two notable exceptions are trauma and myocarditis). The usual mechanism for an MI in the non-postoperative setting is coronary artery plaque rupture resulting in obstruction of a vessel causing myocardial damage due to this abrupt change in supply. The process, which leads to rupture, is atherosclerosis. This atherosclerosis is characterized primarily by intimal thickening due to cellular and lipid accumulation. This accumulation starts out as a fatty streak and progresses to form fibroatheromas by developing a cap containing smooth muscle cells and collagen. Fatty streaks have been found in the intima of infants. These early lesions progress without compromising lumen diameter because of a compensatory vascular enlargement termed remodeling. It is important to realize that the culprit lesions in acute coronary syndromes are usually mildly stenotic and often not even detectable by angiography. In 1995, Falk and associates performed a meta-analysis of four studies of serial angiography before and after MI and demonstrated that fully 68% of culprit lesions were less than 50% stenotic, 18% were between 50 to 70%, and only 14% were greater than 70% before plaque rupture. These studies were conducted in nonpostoperative settings. The typical lesion (plaque) that ruptures has a large lipid core, a thin fibrous cap, and a high content of inflammatory cells (mostly macrophages). These rupture-prone lesions are often called soft or vulnerable plaques. As the lesions mature, macrophages digest most of the lipid material, resulting in a diminished role of inflammatory cells and less release of cytokines. Additionally, the fibrous cap strengthens and thickens, which makes plaque rupture much less likely. Treatments for acute coronary syndromes (plaque ruptures) include antithrombotic regimens, therapies designed to decrease myocardial oxygen demand, and when appropriate, myocardial revascularization. This is in contradistinction to demand MIs, in which no plaque rupture has occurred and hence there is a diminished role for antithrombotic agents. Most postoperative MIs are not the result of vulnerable plaque rupture but rather are due to increased myocardial oxygen demand or decreased supply secondary to blood loss. However, this distinction must be made on a case-by-case basis. Evidence for plaque rupture as the culprit must be sought after adequate control of blood pressure, heart rate, volume status, and anemia has been achieved. This evidence may include recurrent chest pain, persistent electrocardiographic changes, ventricular arrhythmias, or refractory heart failure.
If in fact the MI is deemed the result of plaque rupture, then the various choices of antithrombotic agents or early cardiac catheterization should be considered with regard to their risk-to-benefit ratio. Unstable angina, non-ST elevation MIs, and ST elevation MIs represent a continuum of degree of coronary artery occlusion and amount of myocardial necrosis, with ST-elevation infarctions representing 100% occlusions and significant necrosis. ST elevation MIs are usually treated aggressively with aspirin, oxygen, -blockers, morphine, intravenous nitroglycerin, and early cardiac catheterization with percutaneous intervention. These are exceedingly rare in the postoperative patient.
For acute non-ST elevation MIs and unstable angina, the treatment is largely medical, only rarely requiring urgent percutaneous intervention. The risks for hemorrhage in the postoperative patient must be weighed against the potential benefits of each therapy to make an informed decision as to which treatments to use.
Several randomized trials have shown the benefits of aspirin in unstable coronary syndromes with a relative risk reduction for death or MI of more than 50% by the RISC study group in 1990. A meta-analysis of six randomized trials by Oler and co-workers in 1996 demonstrated that patients treated with a combination of aspirin and heparin had a 33% reduction in death or MI compared with patients treated with aspirin alone. A meta-analysis by Yusuf and associates (1988)
P.350
demonstrated a 13% reduction in the progression to acute MI with the use of these agents in unstable angina.
Low-molecular-weight heparins have several advantages over conventional unfractionated heparin. These include ease of subcutaneous administration, reliable bioavailability and anticoagulant effect, lower rates of heparin-induced thrombocytopenia, and decreased sensitivity to platelet factor 4 inhibition. These agents inhibit thrombin and possess potent anti-Xa activity. The largest studies to date in patients with unstable angina or non-ST elevation MI, ESSENCE with 3,171 patients and TIMI 11B with 4,020 patients, found that the primary end points of death, MI, or recurrent ischemia are significantly reduced with enoxaparin compared with unfractionated heparin. In the ESSENCE trial, the 30-day end point was reached in 19.8% in the enoxaparin group compared with 23.3% in the unfractionated heparin group (p = 0.016). In TIMI 11B, there was a 15% relative risk reduction in the primary end point at 14 days (p = 0.03).
Glycoprotein 2B3A inhibitors have been shown to be useful in acute coronary syndromes as well. These agents inhibit platelet aggregation, reducing distal embolization and preventing subsequent MIs. They also display late benefit (up to 3 years), which may be due to antiinflammatory properties caused by inhibition of Mac-1 receptors on white blood cells and by plaque stabilization, both of which reduce long-term target vessel revascularization. These agents reduce adverse cardiac events by 22% to 56% at 30 days in high- and low-risk patients undergoing percutaneous coronary interventions. In patients with acute coronary syndromes not undergoing percutaneous interventions, there is more modest evidence of benefit with these agents.
-blockers also have been shown to improve mortality in acute coronary syndrome patients. In the ISIS-1 trial, 16,027 patients were randomized to receive intravenous atenolol, 5 to 10 mg, followed by oral atenolol, 100 mg daily. The atenolol patients had a 7-day reduction in mortality from 4.3% to 3.7%. Another study demonstrating the benefits of -blockers in acute MI was the MIAMI trial, in which more than 5,700 patients were randomized to receive intravenous metoprolol up to 15 mg, followed by oral metoprolol or placebo. The -blocker patients had a 13% reduction in mortality at 15 days. As opposed to aspirin, heparin, and -blockers, there is no clear mortality benefit associated with use of nitroglycerin. The GISSI-3 trial randomized 19,394 patients to a 24-hour infusion of intravenous nitroglycerin followed by topical nitrates or placebo. At 6 weeks, there was an insignificant reduction in total mortality in the nitrate group (6.52% versus 6.92%). In the ISIS-4 trial (1995), 58,050 patients were randomized to receive isosorbide mononitrate versus placebo and again demonstrated a small, nonsignificant benefit. However, most postoperative MIs are of the demand variety; hence, the above-mentioned data have limited relevance.
The goals of managing patients who have suffered demand MIs either before or after operation are simple: decrease myocardial oxygen demand and increase myocardial oxygen supply. The simplest and most effective way to decrease demand is to decrease heart rate and blood pressure using -blockers. The mortality benefits of this agent were previously discussed. It is also important to pay attention to the intravascular volume status of the postoperative patient because increased filling pressures can cause increased oxygen demand and lead to increased catecholamine levels, creating a viscous cycle. Another factor that significantly increases myocardial oxygen demand is fever. Elevation of the body temperature by 2 C can result in a rise of energy consumption of more than 20%, as reported by Kluger and colleagues (1996). Therefore, standing doses of antipyretics should be considered in a patient with a recent MI (or stroke) in whom fever is present. When there is myocardial (or cerebral) necrosis, a surrounding area is stunned and still vulnerable to death from further ischemic insult.
Congestive Heart Failure
Congestive heart failure is a syndrome in which the cardiac output is insufficient to meet the body's needs. Congestive heart failure is the only cardiovascular disorder that is increasing in incidence, prevalence, and overall mortality. According to Weitz (2001), it currently affects 2% of the U.S. population with a prevalence of 6% to 10% in people older than 65 years. The causes of congestive heart failure are not limited to cardiomyopathy but include ischemia, hypertension, valvular, pericardial disease, and arrhythmic as well as diastolic dysfunction. Goldman and associates (1977) found that a history of congestive heart failure was absent in most patients who developed perioperative heart failure. Signs and symptoms of congestive heart failure were the best predictors. Data on perioperative congestive heart failure in the 1950s and 1960s show that most cases developed within 1 hour of cessation of anesthesia. This was due to a combination of hypertension or hypotension, ischemia, intraoperative fluid administration, sympathetic stimulation, cessation of positive-pressure ventilation, and hypoxia. According to Weitz (2001), a second peak occurred between 24 and 48 hours and may be related to the reabsorption of interstitial fluid, myocardial ischemia, and possibly withdrawal of long-term oral medications. Insertion of a pulmonary artery catheter perioperatively remains controversial. In January of 2003, Sandham and colleagues reported a randomized trial of 1,994 high-risk elderly patients undergoing major surgery. In-hospital mortality at 6 months and at 12 months did not differ appreciably between the two groups. The only significant difference obtained was the development of pulmonary embolism in 8 patients with a pulmonary artery catheter versus none in the standard care group (p = 0.004). Another interesting finding was a trend toward improved survival in New York Heart Association Functional Class (NYHAFC) 3 or 4 patients, although the number of patients in this category was small.
P.351
The appropriate course of action obviously depends on the cause, with a critical distinction made between acute management and chronic therapy. To understand this distinction better, one needs to understand the current paradigm for treating chronic heart failure patients. This paradigm is referred to as the neurohumoral paradigm. When the heart is failing, a number of neurohormones circulate at very high levels, helping the body to maintain perfusion of the brain and organs. In the short term, this conveys an obvious survival benefit by maintaining an adequate cardiac output and blood pressure. Unfortunately, these neurohormones are toxic to the heart over the long run, resulting in fibrosis, apoptosis, and remodeling while increasing the myocardial propensity to fibrillate. Examples of these potentially toxic substances include angiotensin 2, aldosterone, epinephrine, and possibly endothelin. Most of the medications used in chronic heart failure are antagonists of the above-mentioned toxins. Angiotensin-converting enzyme inhibitors are used not only for their benefits in afterload reduction but also as general inhibitors of the generation of angiotensin 2. Hydralazine is a much more potent afterload reducer but lacks the antitoxin effects of the aforementioned agents and hence has little, if any mortality benefits.
This is in contradistinction to acute heart failure management theory. Here, the paradigm is a simple pump theory. The pump theory treats the heart as a pump that is affected by preload, inotropism, and afterload. Diastolic heart failure is discussed in a separate section.
The first step in managing heart failure in the perioperative period is to identify destabilizing factors such as fluid overload, anemia, or fever and remedy these if possible. The next step is simple pump theory with various medical and mechanical tools at your disposal.
Nitroglycerin is a potent preload reducer that works by venodilation. Other agents that reduce preload include diuretics (including hemodialysis) and positive-pressure ventilation, including bilevel positive airway pressure (BiPAP).
Digoxin is too weak to be considered an inotrope in the acute management phase. On the other hand, dobutamine, a nonselective -agonist, is a potent inotrope as well as an afterload reducer. Dopamine is also a nonselective -agonist in doses of 5 to 10 g/kg/min. However, dopamine tends to increase afterload owing to its 1-agonist activity, making it a less desirable agent in acute heart failure. Milrinone, a phosphodiesterase inhibitor, is a potent inotrope and an afterload reducer. Diuretics also decrease afterload.
Nitroprusside is the most potent afterload reducer. This agent must be used with caution and usually with an arterial line owing to its ability to cause severe hypotension. Additionally, thiocyanate levels should be checked in any patient taking this drug for more than 24 hours, especially in patients with renal failure. Hydralazine is another suitable pure afterload-reducing agent that can be given intravenously or orally. Nesiritide is recombinant human brain type natriuretic peptide that acts as both a venous and arterial dilator as well as a diuretic, as noted by Colluci and colleagues (2000). Additionally, this agent antagonizes both the renin-angiotensin-aldosterone system and the sympathetic nervous system.
Arrhythmias
Cardiac arrhythmias are common in the perioperative period, but fortunately, most are clinically benign. Transient arrhythmias occur in 64% to 84% of patients in the perioperative period. However, according to Kuner (1967) and Mahla and associates (1998), only about 5% of the arrhythmias are clinically important. The arrhythmias are primarily supraventricular and consist predominantly of wandering atrial pacemaker, isorhythmic atrioventricular dissociation, nodal rhythms, and sinus bradycardia. A study by Polanczyk and co-workers (1998) of 4,181 patients undergoing major noncardiac surgery found a 6.1% incidence of postoperative sustained supraventricular arrhythmias; 87% of these patients required specific therapy. The development of supraventricular arrhythmias (in this study predominantly atrial fibrillation or flutter) was associated with an increased risk for an acute cardiac event (congestive heart failure, MI, or unstable angina), infection, or cerebrovascular accident. Additionally, these arrhythmias were associated with a 33% increase in the length of hospital stay. To put these numbers in perspective, in a study by Schein and colleagues (2000) of patients undergoing cataract surgery, the incidence of postoperative arrhythmias requiring treatment was only 0.12%. The causes of perioperative arrhythmias are multifactorial and include electrolyte disturbances, acid base abnormalities, anesthesia, hypoxia or hypercarbia, anemia, volume status, changes in autonomic tone, hypertension, and myocardial ischemia.
Cardiac arrhythmias occur frequently in thoracic surgery patients as well. The primary arrhythmia seen in this patient population, according to Sloan and Weitz (2001), is atrial fibrillation, with a peak incidence between postoperative days 2 and 4. In various series by Amar (1997, 1998) and by Amar (1996) and Cardinale (1999) and their colleagues, the incidence of supraventricular arrhythmias following lung resection varies from 10 to 33%. In a study by Amar and associates (1996) of patients undergoing resection for non small cell lung cancer (NSCLC), the development of atrial fibrillation was associated with a poorer 2-year survival and longer length of stay. However, another study by Sloan and Weitz (2001) showed no differences in these two end points. According to Ritchie and co-workers (1993), the incidence of supraventricular arrhythmias is between 13% and 60% in patients undergoing esophagectomy. The mechanism of atrial fibrillation in thoracic surgery patients is uncertain but may be related to stimulation of the pulmonary veins, according to Haissaguerre and colleagues (1998), which seems to be the culprit in most patients suffering from this arrhythmia. Ischemia is rarely the sole cause of atrial fibrillation in any setting.
P.352
Despite the widespread use of digoxin to decrease atrial fibrillation, Ritchie (1993) and Amar (1997) and their associates found that it is not effective for one simple reason: It shortens the atrial refractory period and actually promotes atrial fibrillation. A study by Amar and co-workers (2000) of 330 patients undergoing major noncardiac thoracic surgery found that intravenous diltiazem reduces the incidence of postoperative supraventricular arrhythmias from 25% to 15% compared with digoxin. Diltiazem is not classically considered an antiarrhythmic agent, but recent data in both animals and humans from Amar (1997), Daoud (1997), and De Simone (1999) and their colleagues suggest that blocking L-type calcium channels may have some efficacy against atrial fibrillation. Early studies with esmolol and propranolol by Cruickshank and Prichard (1994) have demonstrated a reduction of perioperative supraventricular and ventricular arrhythmias. The principal mechanism may be catecholamine blockade. However, these studies should be viewed with some caution because the percentage of patients who were suffering from -blocker withdrawal in the control groups was unknown.
Amar and associates (2002) examined 581 patients undergoing pneumonectomy or lobectomy to determine the incidence and outcome of ventricular arrhythmias after these operations. They reported a ventricular tachycardia incidence of 15% over the first 72 to 96 hours postoperatively but found that none of those patients had sustained ventricular tachycardia (<30 seconds) or suffered any hemodynamic consequences. Additionally, ventricular tachycardia was not associated with poor outcome. The development of ventricular tachycardia was strongly associated with postoperative atrial fibrillation and atrial couplets, prompting the authors to speculate that vagal withdrawal, adrenergic hyperactivity, or both may have a role in precipitating ventricular tachycardia in the early postoperative period.
The treatment of postoperative VT is principally aimed at identifying the underlying precipitators. The list of causes ranges from ischemia and heart failure to electrolyte disturbances. The treatment inevitably includes -blockade. However, it is important to recognize the frequency with which this rhythm occurs and how benign it can be in the perioperative thoracic surgery patient. If the VT is occurring very frequently, is associated with hemodynamic compromise, or is sustained, then consideration should be given to intravenous amiodarone or lidocaine. Lidocaine is a sodium channel blocker that has a rapid onset and is primarily effective against VT due to metabolic causes or in patients who are acutely ischemic. Lidocaine is not very effective in patients with a normal metabolic state who have VT due to a reentrant pathway from the old scar of an MI. Lidocaine is 90% metabolized by the liver; thus, in severe liver dysfunction or cardiogenic shock (resulting in decreased hepatic blood flow), this medication should generally not be used. Toxicities include hemodynamic collapse and central nervous system depression and seizures. Lidocaine should always be administered in 2 to 3 separate boluses to prevent subtherapeutic levels, which usually occur 20 to 60 minutes after the initial bolus. A recommended regimen is to administer 1.5 mg/kg initially followed by 0.8 mg/kg boluses at 8-minute intervals for two additional doses. Doses should be reduced by about 50% for patients in heart failure. Amiodarone is effective for all types of ventricular tachycardia. It is a sodium, potassium, and calcium blocker as well as a -blocker. Intravenous amiodarone may cause some hypotension but is generally well tolerated. It can be used safely in renal failure and even in severe liver dysfunction in the acute setting. Amiodarone is usually given as a 150-mg intravenous bolus over 10 minutes followed by another bolus 10 to 30 minutes later, followed by an intravenous drip at 1 mg/min for 6 hours, followed by 0.5 mg/min until stable. If long-term amiodarone usage is desired, then 8 to 10 g of loading over several days is usually required before maintenance dosing. The load can either given orally or intravenously, with bioavailability ranging from 20% to 80% (average, 50%). Requiring special mention is the entity of amiodarone lung disease. Chronic lung fibrosis was first described in the early 1980s and manifests insidiously as a cough and minor dyspnea, which is confirmed by a downward trend in diffusion capacity on pulmonary function testing or fibrosis on chest imaging. This syndrome is up to nine times more frequent in patients with preexisting lung disease and is dose related, occurring most frequently when the cumulative dose is greater than 140 to 230 g. Much more underappreciated is amiodarone's role in acute pulmonary toxicity. The principal mechanism of acute pulmonary toxicity, according to Ashrafian and Davey (2001), is a hypersensitivity, immune complex, oxidation reaction with the clinical manifestation being adult respiratory distress syndrome or bronchiolitis obliterans organizing pneumonia. Lung biopsy may show a characteristic lipoid pneumonitis. Unfortunately, there is no clear treatment of this condition. Of course, the amiodarone should be held, and pulsed steroids are often used, but their role is unclear.
Hypertension
Weitz (2001) noted that perioperative hypertension or hypotension occurs in 25% of hypertensive patients who undergo surgery. Hypertensive events occur most frequently in patients with a resting diastolic blood pressure greater than 110 mm Hg undergoing carotid, abdominal aortic, peripheral vascular, intraperitoneal, or intrathoracic surgeries. A multivariate analysis performed by Browner and colleagues (1992) in male veterans after noncardiac surgery found a 3.8 odds ratio for postoperative death in hypertensive patients compared with normotensive patients. However, other data are conflicting. A prospective randomized multicenter study by Forrest and associates (1992) of more than 17,000 patients found that preoperative hypertension was associated with bradycardia, tachycardia, and hypertension but had no impact on mortality.
P.353
Weitz (2001) noted that perioperative hypertension tends to occur at four different times: (a) during intubation and induction of anesthesia (due to sympathetic stimulation with adrenergic mediated vasoconstriction), (b) intraoperatively secondary to pain, (c) early postoperatively secondary to pain, hypothermia, hypoxia, and volume overload or hypovolemia, and (d) 24 to 48 hours postoperatively as fluid is immobilized and following withdrawal from preoperative antihypertensive agents and sedatives. However, -blocker withdrawal sometimes occurs up to 4 days postoperatively.
The treatment of perioperative hypertension depends on the cause. If hypertension occurs during intubation, surgical incision, or emergence from anesthesia, then it is often due to increased sympathetic tone, and the treatment is generally short-acting -blockers or narcotics. If the hypertension occurs 24 to 96 hours postoperatively, then the preoperative antihypertensives should be restarted as a first line of therapy. Prevention here is key. Often, long-acting preparations can be given before surgery, or if an extended period of bowel rest is expected postoperatively, then standing intravenous antihypertensive therapy should be considered. It is generally indicated to continue the outpatient antihypertensive regimen throughout the perioperative period, especially with regard to -blockers and clonidine because these medications tend to cause the most severe withdrawal reactions. Finally, congestive heart failure is a frequent cause of postoperative hypertension. A combination of nitrates and diuretics is effective first-line therapy.
REFERENCES
Amar D: Prevention and management of dysrhythmias following thoracic surgery. Chest Surg Clin N Am 7:817, 1997.
Amar D: Cardiac arrhythmias. Chest Surg Clin N Am 8:479, 1998.
Amar D, Zhang H, Roistacher N: The incidence and outcome of ventricular arrhythmias after noncardiac thoracic surgery. Anesth Analg 95:537, 2002.
Amar D, et al: Relationship of early postoperative dysrhythmias and long-term outcome after resection of non-small lung cancer. Chest 110:437, 1996.
Amar D, et al: Effects of diltiazem versus digoxin on dysrhythmias and cardiac function after pneumonectomy. Ann Thorac Surg 63:1374, 1997.
Amar D, et al: Effects of diltiazem prophylaxis on the incidence and clinical outcome of atrial arrhythmias after thoracic surgery. J Thorac Cardiovasc Surg 120:790, 2000.
Ashrafian H, Davey P: Is amiodarone an under recognized cause of acute respiratory failure in the ICU? Chest 120:275, 2001.
Browner WS, Li J, Mangano D: In-hospital and long-term mortality in male veterans following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 268:228, 1992.
Cardinale D, et al: Atrial fibrillation after operation for lung cancer: clinical and prognostic significance. Ann Thorac Surg 68:1827, 1999.
Colluci WS, et al: Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med 343:246, 2000.
Campeau L: Grading of angina pectoris. Circulation 54:522, 1976.
Corti R, Farkouh ME, Badimon JJ: The vulnerable plaque and acute coronary syndromes. Am J Med 113:668, 2002.
Cruickshank JM, Prichard BNC: Beta-Blockers in Clinical Practice. Edinburgh: Churchill Livingstone 1994, pp. 1 1204.
Daoud EG, et al: Effect on verapamil and procainamide in atrial fibrillation induced electrical remodeling in humans. Circulation 96:1542, 1997.
De Simone A, et al: Pretreatment with verapamil in patients with persistent or chronic atrial fibrillation who underwent electrical cardioversion. J Am Coll Cardiol 34:810, 1999.
Eagle KA, et al: ACC/AHA guideline update on perioperative cardiovascular evaluation for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. [On-line.] Accessed 2002: http://www.acc.org/clinical/guidelines/perio/clean/perio_index.htm.
Falk, E, Shah PK, Fuster V: Coronary plaque disruption. Circulation 92:657, 1995.
Forrest JB, et al: Multicenter study of general anesthesia. III. Predictors of severe perioperative adverse outcomes {published erratum appears in Anesthesiology 77:222, 1992}. Anesthesiology 76:3, 1992.
Goldman L, et al: Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med 297:845, 1977.
Gottlieb SS, McCarter RJ, Vogel RA: Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 339:489, 1998.
Haissaguerre M, et al: Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339:659, 1998.
ISIS-4: A randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulfate in 58,050 patients with suspected acute myocardial infarction. (Fourth International Study of Infarct Survival) Collaborative Group. Lancet 345:669, 1995.
Kluger MJ, et al: The adaptive value of fever. Infect Dis Clin North Am 10:1, 1996.
Kuner J, et al: Cardiac arrhythmias during anesthesia. Dis Chest 52: 580, 1967.
Mahar LJ, et al: Perioperative myocardial infarction in patients with coronary artery disease with and without aorta/-coronary artery bypass grafts. J Thorac Cardiovasc Surg 76:533, 1978.
Mahla E, et al: Perioperative ventricular dysrhythmias in patients with structural heart disease undergoing noncardiac surgery. Anesth Analg 86:16, 1998.
Mangano DT, et al: Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 335:1713, 1996.
Melendez JA, Carlon VA: Cardiopulmonary Risk Index does not predict complications after thoracic surgery. Chest 114: 69, 1998.
Oler A, et al: Adding heparin to aspirin reduces the incidence of myocardial infarction and death in patients with unstable angina. A meta-analysis. JAMA 276:811, 1996.
Polanczyk CA, et al: Supraventricular arrhythmia in patients having noncardiac surgery: clinical correlates and effect on length of stay. Ann Intern Med 129:279, 1998.
Poldermans D, et al: Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients for as long as 2 years after successful major vascular surgery. Eur Heart J 22:1353, 2001.
RISC Group: Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. Lancet 336:827, 1990.
Ritchie AJ, et al: Cardiac dysrhythmia in total thoracic oesophagectomy: a prospective study. Eur J Cardiothoracic Surg 7:420, 1993.
Sandham JD, et al, for the Canadian Critical Care Clinical Trials Group: A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 348:5, 2003.
Schein OD, et al: The value of routine preoperative medical testing before cataract surgery. Study of Medical Testing for Cataract Surgery. N Engl J Med 342:168, 2000.
Sloan SB, Weitz HH: Postoperative arrhythmias and conduction disorders. Med Clin North Am 85:1171, 2001.
Weitz HH: Perioperative cardiac complications. Med Clin North Am 85:1151, 2001.
Yusuf S, Wittes J, Friedman L: Overview of results of randomized clinical trials in heart disease. 2. Unstable angina, heart failure, primary prevention with aspirin, and risk factor modification. JAMA 260:2259, 1988.
EAN: 2147483647
Pages: 203