155 -The Thymus
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Mediastinum > Section XXIX - Primary Mediastinal Tumors and Syndromes Associated with Mediastinal Lesions > Chapter 183 - Biological Markers and Pathology of Mediastinal Lymphomas
Chapter 183
Biological Markers and Pathology of Mediastinal Lymphomas
Michael J. Kornstein
Lymphomas (Hodgkin's disease and non-Hodgkin's lymphomas) account for 15% to 19% of all primary mediastinal lesions, as reported by Davis and colleagues (1987) and Whooley and associates (1999). Among the lymphomas, about one third are Hodgkin's disease and two thirds are non-Hodgkin's lymphomas. As to mediastinal location, lymphomas account for approximately 20% of lesions in the anterior and middle compartments but are uncommon in the posterior area. Cohen and colleagues (1991) have reported similar findings. In pediatric patients, up to 45% of anterior mediastinal tumors are lymphomas, as reported by Mullen and Richardson (1986) and reviewed by myself (1995). Mediastinal lymphomas most likely arise from the thymus or lymph nodes; hence, the greater likelihood for origin in the anterior or middle mediastinum.
As reported by Kaplan (1972), Maity (1992), and Johnson (1983) and their colleagues, 60% of Hodgkin's lymphoma patients have mediastinal disease at presentation; only 3% have tumor limited to intrathoracic sites. As to non-Hodgkin's lymphomas, 20% involve the mediastinum, according to Patchefsky (1974) and Levitt (1982) and their colleagues as well as Strickler and Kurtin (1991), whereas less than 10% are limited to this site. The non-Hodgkin's lymphomas make up a heterogeneous group of disorders that are characterized by the clonal proliferation of lymphoid cells.
CLASSIFICATIONS OF MEDIASTINAL LYMPHOMAS
Over the past 30 years, numerous lymphoma classifications have been proposed, as Frizzera (1997) and Berard and Hutchison (1997) have reviewed. The earlier systems, including the Rappaport Classification and the National Cancer Institute Working Formulation (Table 183-1), were based only on tumor histopathology (e.g., nodular vs. diffuse growth pattern, cell size, and appearance of the nuclei). Subsequently, studies divided lymphomas into B-cell versus T-cell phenotype on the basis of cell surface antigens. The Revised European-American Lymphoma (REAL) classification was proposed by Harris and colleagues (1994). It was revised and published as the World Health Organization (WHO) lymphoma classification by Jaffe and associates (2001), as discussed by Chan (2001) (Table 183-2). This system divides lymphomas into B-cell neoplasms, T-cell (and putative natural killer cell) neoplasms, and Hodgkin's disease. Mathe (1996) is among those who have criticized the REAL classification for inaccuracies and inconsistencies.
At present, the WHO classification has been advocated for use. Nevertheless, knowledge of the previous classifications will continue to be useful, at least for communicating with those not acquainted with the more recent proposals.
Like the lymphocytes from which they arise, lymphomas express either B-cell or T-cell markers, as Braylan and colleagues (1997) and myself (1995) have reviewed (Fig. 183-1). The B lymphocytes are immunoglobulin- and antibody-producing cells; therefore, most B-cell lymphomas have surface, cytoplasmic, or both kinds of immunoglobulin. The immunoglobulin molecule is composed of two heavy chains and two light chains. The heavy chains are alpha (IgA), gamma (IgG), mu (IgM), delta (IgD), or epsilon (IgE). Light chains are either kappa or lambda. Thus, B cells express immunoglobulin having either kappa or lambda light chains. In a reactive process, approximately twice as many B cells have immunoglobulin with kappa light chains compared with lambda. In contrast, in a B-cell lymphoma, the B cells are clonal and have either kappa or lambda light chains. Hence, the study of kappa and lambda light chains may be helpful in discriminating between a reactive (benign) lymphocyte population and a B-cell lymphoma.
Figure 183-2 diagrams T-cell development in the thymus. As reviewed by LeClercq and Plum (1996) and by myself (1995), the earliest T cells express CD2 and 7 and are located in the subcapsular areas of the thymus. With increasing development, the T cells also express CD1, 3, 4, 5, 7, and 8, and move to the thymic cortex. As the cells move to
P.2683
the medulla, they acquire a mature phenotype with loss of CD1 and retention of either CD4 or CD8. The cells then migrate from the thymus as helper/inducer T cells (CD4+) or suppressor/cytotoxic T cells (CD8+).
Table 183-1. Working Formulation for Clinical Usage | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
A wide range of antibodies is available to characterize cell antigen expression (see Appendix). These studies can be performed on tissue sections or cytospin preparations by immunocytochemistry or in cell suspensions by flow cytometry. Initially, all studies for lymphocyte surface antigens required fresh or frozen tissue. Currently, techniques have advanced, and many of the antigens can be studied by immunocytochemistry using routinely processed, formalin-fixed specimens, as reviewed by Linder (1991) and Knowles (2001).
Studies of DNA also may help in characterizing lymphomas, as I have noted (1995). As lymphocytes mature, they undergo rearrangement of their DNA to produce immunoglobulin (B cells) or antigen receptors (T cells). On this basis, molecular biology techniques, including Southern blots and the polymerase chain reaction, can determine whether a population of lymphocytes includes a clone of cells. Finding a clonal population of lymphoid cells in a tissue specimen usually indicates a lymphoma, as noted by Williams and colleagues (1987).
LYMPHOBLASTIC LYMPHOMA
As discussed by myself (1995) and by Thomas and Kantarjian (2001), lymphoblastic lymphoma has distinct clinical and pathologic features. This neoplasm is most common in children, among whom it accounts for 30% to 50% of all non-Hodgkin's lymphomas, according to Murphy (1980) and Griffith and associates (1987). In adults, this tumor is uncommon and comprises only 5% of non-Hodgkin's lymphomas, according to Simon and colleagues (1988). In children, the median age is 9 years, and it is seen twice as often in boys as in girls. Up to 75% of pediatric patients have a mediastinal mass. Most patients present with disseminated disease. Other than the mediastinum, sites of involvement may include extrathoracic lymph nodes, bone marrow, central nervous system, head and neck, lung, pleura, liver, pericardium, peritoneum, skin, and gonads.
Lymphoblastic lymphoma is closely related to acute lymphoblastic leukemia, which has been reviewed by Uckun and associates (1998). Both disorders generally involve lymph nodes, thymus, or both. However, leukemia is diagnosed if the patient also has significant bone marrow involvement. Lymphoblastic lymphoma is diagnosed when the patient has fewer than 25% lymphoblasts in the bone marrow.
Histologically, lymphoblastic lymphoma is a diffuse infiltrate of immature lymphoid cells (Fig. 183-3). The tumor cells are 12 m in diameter (i.e., intermediate in size between small lymphocytic lymphoma and large cell lymphoma). The nuclei have finely dispersed chromatin without prominent nucleoli. In contrast to most other mediastinal lymphomas, fibrosis is scant. Tissue sections of lymphoblastic lymphoma show extensive effacement by round, blue cells. The tumor cells have a high rate of proliferation, as evidenced by frequent mitotic figures and numerous tingible-body macrophages. Necrosis is often widespread. Biopsy samples may consist entirely of necrotic tumor. Another problem is that the cells are damaged easily. Crush artifact in a tissue biopsy may be so extensive as to obscure the diagnosis. Wright-stained cytologic preparations (e.g., touch imprint, fine-needle aspiration) are helpful in that the morphology of individual cells is better appreciated. For a
P.2684
touch imprint, the tumor is gently touched to the slide. These preparations demonstrate the diffuse chromatin pattern, basophilic cytoplasm, and occasional vacuoles.
Table 183-2. World Health Organization Classification of Neoplastic Diseases of the Hematopoietic and Lymphoid Tissues | ||
|---|---|---|
|
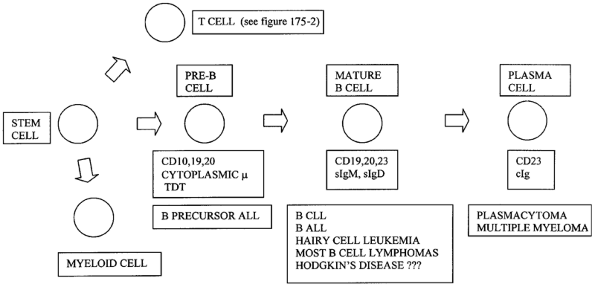 |
Fig. 183-1. B-lymphocyte surface markers: correlation with differentiation and neoplasia. ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; sIgd, surface immunoglobulin D; sIgM, surface immunoglobulin M; TDT, terminal deoxynucleotidyl transferase. |
Immunologically, mediastinal lymphoblastic lymphoma almost always has an immature T-cell phenotype. Rarely, precursor B-cell lymphoblastic lymphoma presents with mediastinal disease, as described by Lin and colleagues (2000). Lymphoblastic lymphoma of either T-cell or B-cell origin is positive for terminal deoxynucleotidyl transferase (TDT), a marker of immature lymphoid cells. As described by Soslow and associates (1998), TDT can be demonstrated in routinely processed tissue, as can other markers commonly expressed by lymphoblastic lymphoma, including CD99 (MIC2) and CD34. However, no single marker should be relied on for diagnosis, because none is entirely specific. For example, CD99 is expressed in other small round cell tumors of childhood. Mathewson and colleagues (1997) have reported that even TDT may be expressed in nonlymphoid small round cell tumors. The finding of immature lymphoid cells is not specific for lymphoblastic lymphoma. Onciu and associates (2002) reported TDT-positive
P.2685
lymphoid cells in benign reactive lymph nodes from children. Immature T cells are also present in normal thymus, thymic hyperplasia, and lymphocytic thymoma.
 |
Fig. 183-2. T-cell surface markers: correlation with differentiation in thymus and neoplasia. TDT, terminal deoxynucleotidyl transferase. |
 |
Fig. 183-3. A. Biopsy of precursor T-lymphoblastic leukemia/lymphoma showing diffuse effacement by lymphoid cells. Tingible-body macrophages are interspersed (hematoxylin and eosin stain, original magnification 200). B. FNA biopsy of precursor T-lymphoblastic leukemia/lymphoma showing immature lymphoid cells (Diff-Quik, original magnification 400). |
Most commonly, the tumor phenotype in mediastinal lymphoblastic lymphoma corresponds to the stage of cortical thymic lymphocyte differentiation in which the cells express the T-cell antigens CD1, 2, 4, 7, and 8, as described by Crist (1988) and Sheibani (1987) and their associates. Alternatively, the phenotype may correspond to an earlier stage of differentiation in which the cells express only CD2, 5, 7, or all three, or a later stage with additional expression of either CD4 or CD8. The level of differentiation does not consistently influence prognosis, although relatively few cases have been studied in this regard.
Lymphoblastic lymphomas expressing the natural killer cell phenotype (CD56+) have been described. Natural killer cells are granular lymphocytes that are positive for CD16 and CD56 but lack surface CD3. These cells have major histocompatibility antigen-unrestricted cytotoxicity. Lymphomas expressing the natural killer cell phenotype have a predilection for the nasopharyngeal region and skin. Koita and associates (1998) report a case with nasal and mediastinal involvement that histologically appeared lymphoblastic and expressed TDT. Uncommonly, lymphoblastic lymphoma with a precursor B-cell phenotype involves the mediastinum, as described by Lin and colleagues (2000).
Lymphoblastic lymphoma should be included in the differential diagnosis of a mediastinal tumor, particularly in pediatric patients. These patients typically have peripheral lymphadenopathy. As described by myself together with Wakely (1996), pathologic diagnosis usually can be made on a fine-needle aspiration (FNA) biopsy of a peripheral lymph node. The FNA biopsy sample can be studied morphologically on Wright-stained smears. The diagnosis can be confirmed by immunologic studies for cell surface markers performed by flow cytometry on cell suspensions, or by immunocytochemistry on cytospin preparations. In a mediastinal biopsy, lymphoblastic lymphoma must be distinguished from a lymphocytic thymoma and thymic hyperplasia. A thymoma is a tumor of thymic epithelial cells which should be identifiable by immunohistochemistry for cytokeratin. Thymic tissue has a lobular structure with cortex and medulla. This differential diagnosis can be difficult on a fine-needle aspirate of a mediastinal mass. In particular, lymphoblastic lymphoma involving the thymus can be impossible to distinguish from a lymphocytic thymoma. Nevertheless, FNA biopsy samples have been used successfully to diagnosis mediastinal lymphoma, as reviewed by Hughes and colleagues (1998). Another caution is that steroid treatment prior to biopsy may interfere with the histopathologic diagnosis, as noted by Borenstein and associates (2000). In most cases, whether by FNA or core-needle biopsy, as discussed by Sklair-Levy and associates (2000), the combination of the appropriate clinical setting, the characteristic morphology, and the early T-cell phenotype should allow for the proper diagnosis.
MEDIASTINAL LARGE B-CELL LYMPHOMA
Numerous series and reviews of mediastinal large cell lymphomas have been published, including those by Aisenberg (1999) and by Lazzarino (1997), Abou-Elella (1999), al-Sharabati, (1991), Davis (1990), Ergul (2002), Lamarre (1989), Jacobson (1988), Lavabre-Bertrand (1992), Kirn (1993), Todeschini (1990), Nakagawa (1993), and Bertini (1991), Barth (2001, 2002), and van Besien (2001) and their colleagues.
Evidence favors the concept that mediastinal large cell lymphoma has clinical and pathologic features distinct from large cell lymphomas of other sites. As reviewed by myself (1995) and those cited in the previous paragraph,
P.2686
mediastinal large cell lymphoma usually occurs in young adults (median age 30 years; range 10 71). Women predominate over men in a 2:1 ratio. Most presenting symptoms relate to the local effects of the anterior mediastinal mass and include cough, dyspnea, chest pain, dysphagia, hemoptysis, and superior vena cava syndrome. Fever, night sweats, and weight loss (B symptoms) are present in one third of patients. Depending on the study, 13% to 54% of patients have extrathoracic disease, most commonly of the supraclavicular, axillary, or intraabdominal lymph nodes, at presentation. Bone marrow involvement is uncommon. A case report by Stein and associates (1997) describes a mediastinal large cell lymphoma presenting with a pneumothorax. Piira (1995) and Lones (2000) and colleagues reported that mediastinal large cell lymphoma in children (ages 10 to 17 years) has features similar to those in adults.
The histopathology of mediastinal large cell lymphoma consists of intermediate to large lymphoid cells amid prominent sclerosis (Fig. 183-4). The sclerosis varies from broad bands to fine strands. The latter may encircle nests of tumor cells in the pattern of compartmentalizing fibrosis. Fine interstitial fibrosis surrounding individual tumor cells also may be seen. Nuclei are often multilobulated. Cytoplasm ranges from clear to eosinophilic. Cell size may appear smaller than that usually encountered in most large cell lymphomas. Because of this variability in cell size, Stein and Dallenbach (2000) and Paulli and colleagues (1999) advocate the designation primary mediastinal B-cell lymphoma. Nevertheless, the WHO classification uses the term mediastinal large B-cell lymphoma. The older terminology, histiocytic lymphoma, had been replaced by large cell lymphoma, because lymphomas derived from histiocytes (true histiocytic lymphomas) are rare. Kamel and colleagues (1995) described a series of 12 true histiocytic lymphomas, one of which presented with mediastinal disease. Occasionally, biopsies of mediastinal lymphoma demonstrate residual thymic tissue, as shown by Davis and associates (1990).
 |
Fig. 183-4. This section of a mediastinal large B-cell lymphoma illustrates the atypical lymphoid cells. Thin fibrous strands create a compartmentalizing pattern (hematoxylin and eosin stain, original magnification 400). |
Although the thymus is the site for T-cell differentiation, nearly all mediastinal large cell lymphomas express B-cell antigens, including CD19 and CD20, as noted by Addis and Isaacson (1986) and Menestrina (1986), Lamarre (1989), Moller (1989), and Kirn (1993) and their associates. Most cases are negative for CD10 (cALLA), CD21, CD30, and for T-cell markers. Higgins and Warnke (1999) described CD30 expression in 69% of mediastinal large B-cell lymphomas. De Leval and associates (2001) described bcl-2 expression in 19 of 19 cases and CD10 positivity in 32%. Yang and associates (2002) reported an unusual example of a large T-cell mediastinal lymphoma. As discussed by Moller (1987, 1989) and Barth (2001) and their colleagues, the phenotype of most mediastinal lymphomas corresponds to a terminal stage of B-cell differentiation. Yet, the tumor cells show some anomalous features, including lack of immunoglobulin and downregulation of major histocompatibility complex class II antigens. The thymic medulla does contain occasional B cells. It is these medullary B cells that are thought to give rise to mediastinal large cell lymphoma.
The mediastinal large cell lymphomas have genotypic features of B cells. Southern blot analyses reported by Scarpa and colleagues (1987) have demonstrated heavy- and light-chain immunoglobulin gene rearrangements. This further supports the B-cell nature of these tumors. Scarpa and colleagues (1999a) reported C-myc oncogene abnormalities in two of six mediastinal large cell lymphomas. Bcl-2 and Epstein-Barr virus (EBV) did not appear to be involved. Reports by Bentz (2001), Copie-Bergman (1999), Scarpa (1999a, 1999b), Palanisamy (2002), and Rigaud (2001) and colleagues indicated that mediastinal large B-cell lymphomas have genetic features different from lymphomas of other sites. However, the genetic differences are not consistent among the various studies.
Treatment consists of chemotherapy or radiation therapy or both (reviewed by van Besien and colleagues, 2001; also see Chapter 184). Residual fibrosis in the area of the tumor mimics persistent disease on radiographic studies. Thus, a persistent, yet nonprogressive, small, residual shadow on computed tomographic scan is more likely to be fibrosis than neoplasm.
ANAPLASTIC LARGE CELL LYMPHOMA
As reviewed by Jaffe (2001) and by Fiorani (2001) and Stein (2000) and their colleagues, anaplastic large cell lymphoma has clinicopathologic features distinct from other large cell lymphomas. Clinically, most patients are in the pediatric to young adult age range, with a predominance in males of up to 6:1. Although most patients present with lymphadenopathy, extranodal sites of involvement (such as the bone, soft tissue, and skin) are common. Mediastinal disease is not unusual. Anaplastic large cell lymphoma limited to the skin (primary cutaneous disease) appears to be a separate entity with different clinical course and pathogenesis,
P.2687
as described by Jaffe (2001) as well as by Bekkenk and colleagues (2000).
Histologic features include a sinusoidal pattern of infiltration by large cells with basophilic cytoplasm, prominent Golgi zone, and lobulated (horseshoe-shaped) nucleus. Nucleoli are usually multiple, small, and basophilic. Small cell and lymphohistiocytic variants have been described (Fig. 183-5). The tumor cells express CD30 or Ki-1, and hence, the tumor has been called Ki-1 lymphoma. Greer and colleagues (1991) reported 77% of large cell anaplastic lymphomas with a T-cell phenotype, whereas 13% were B cell and 10% indeterminate. In describing the WHO classification, Jaffe and colleagues (2001) included among the anaplastic large cell lymphomas only those with a T-cell or null cell phenotype. Those with a B-cell phenotype are believed to be less distinctive and are included within the diffuse large B-cell lymphoma category.
Falini and associates (1990) reported that most anaplastic large cell lymphomas are negative for CD45, or leukocyte common antigen. Also, many are positive for epithelial membrane antigen. The immunohistochemistry (CD45 , epithelial membrane antigen positive) may suggest metastatic carcinoma. Based on the pleomorphic histologic appearance, the lesion also may get misdiagnosed as metastatic melanoma. Hodgkin's lymphoma is a diagnostic consideration in some cases. Additional marker studies (e.g., CD30, cytokeratin, other lymphocyte antigens) are often necessary for the proper diagnosis.
Among others, Bitter and associates (1990) described anaplastic large cell lymphomas as having a characteristic cytogenetic abnormality: a reciprocal translocation between chromosomes 2 and 5 (t2;5)(p23;q35). Subsequently, Morris and colleagues (1994) reported that the translocation results in fusion of the nucleolar phosphoprotein gene to the ALK receptor tyrosine kinase gene. An antibody to the resulting unique protein (p80), or ALK, is useful for immunohistochemistry. Indeed, Benharroch and colleagues (1998) reported ALK protein expression defined by immunohistochemistry to be specific for the 2:5 translocation. Nakamura and colleagues (1997) described the use of this antibody in distinguishing anaplastic large cell lymphoma from Hodgkin's disease. Tilly and colleagues (1997) have reported patients with anaplastic large cell lymphoma to have a significantly more favorable outcome than other large cell lymphomas when treated with aggressive chemotherapy. Others, such as Williams and colleagues (2002) and as reviewed by Harris and associates (1994), have described the prognosis as similar to other large cell lymphomas. In general, anaplastic large cell lymphoma is aggressive clinically, but responds well to combination chemotherapy, according to a review by Jaffe (2001).
 |
Fig. 183-5. Anaplastic large cell lymphoma is characterized by large, lymphoid cells with prominent nucleoli (hematoxylin and eosin stain, original magnification 200). |
MARGINAL ZONE LYMPHOMA
Extranodal marginal zone lymphoma of MALT type (malignant lymphoma of mucosa-associated lymphoid tissue, MALT lymphoma, or maltoma) involving the thymus (Fig. 183-6) has been the subject of case reports by Isaacson (1990), Takagi (1992), and Yokose (1998) and their associates. In general, MALT lymphomas are usually low grade and most commonly affect the gastrointestinal tract and salivary glands, as reviewed by Cavalli and associates (2001). Thymic MALT lymphomas are rare. As in other sites, the thymic MALT lymphomas are low grade and may be associated with autoimmune vascular disease, such as Sj gren's syndrome. Patients have an indolent course and usually have prolonged survival without antineoplastic therapy.
OTHER NON-HODGKIN'S LYMPHOMAS
Virtually all types of lymphoma may involve the mediastinum, particularly when the disease is disseminated. Jenkins and colleagues (1981) reported that 21% of all patients with non-Hodgkin's lymphoma had mediastinal
P.2688
lymphadenopathy. Mantle cell lymphoma is one of the more recently recognized types of lymphomas; therefore, this entity is discussed specifically.
 |
Fig. 183-6. Thymic marginal zone B-cell lymphoma contains monotonous, small lymphoid cells. A Hassall's corpuscle (center) is infiltrated (hematoxylin and eosin stain, original magnification 400). |
According to Harris and Jaffe (2001) and their associates, as well as Swerdlow and Williams (2002) and Densmore and Williams (2000), mantle cell lymphoma is a designation for a low- to intermediate-grade lymphoma that has been described under numerous terms, including lymphocytic lymphoma of intermediate differentiation and mantle zone lymphoma. In the Working Formulation, most of these cases were classified as diffuse small cleaved cell lymphoma. This lesion is composed of small to intermediate-sized lymphoid cells with slightly irregular nuclei. The pattern is diffuse or vaguely nodular. In many cases, the tumor cells occupy the mantle zone area that surrounds reactive germinal centers. Mantle cell lymphoma has a B-cell phenotype that corresponds to the mantle zone. Like small lymphocytic lymphoma, mantle cell lymphoma cells coexpress CD5 with B-cell markers, such as CD20. Absence of CD23 distinguishes mantle cell lymphoma from small lymphocytic lymphoma. Genetic studies have identified a characteristic t(11:14) chromosomal translocation involving the bcl-1 oncogene. This translocation results in overexpression of the PRAD1 gene, which encodes for the protein cyclin D1. Immunohistologic demonstration of cyclin D1 is helpful in the diagnosis of mantle cell lymphoma, as noted by Yatabe and colleagues (2000).
Bosch and co-workers (1998) have reviewed 59 patients with mantle cell lymphoma. They ranged in age from 39 to 83 years, with a median age of 63. Seventy-four percent of patients were men. Ninety-five percent of patients presented with widespread disease (stage III or IV), and 92% had extranodal disease. Most common extranodal sites included bone marrow, spleen, liver, lung, pleura, and gastrointestinal tract. Fifty-eight percent had peripheral blood involvement. Median survival was 49 months. Mantle cell lymphomas have no predilection for the mediastinum, but may occasional be diagnosed on a mediastinal biopsy.
The differential diagnosis between mantle cell lymphoma and lymphoblastic lymphoma may be difficult by morphology alone. As reported by Soslow and associates (1997), immunoperoxidase stains are helpful in that blastic transformation of mantle cell lymphoma expresses BCL-1 (PRAD-1/Cyclin D-1), whereas lymphoblastic lymphoma is positive for TDT. Similarly, Dunphy and colleagues (1997) reported that CD23 is helpful in distinguishing small lymphocytic lymphomas (CD23+) from mantle cell lymphoma (CD23 ).
HODGKIN'S LYMPHOMA
As reviewed by Hudson and Donaldson (1997) and by myself (1995), Hodgkin's lymphoma (Hodgkin's disease) has characteristic clinicopathologic features. Its incidence shows a bimodal age distribution with one peak in the third decade and the second in individuals over 45. Differences are noted between patients in the two age groups. Those in the younger group are more likely to have the nodular sclerosing histopathology, whereas those in the older group are more likely to have the mixed cellularity type. Seventy percent of younger patients have mediastinal disease, compared with 44% of older ones. In the United States and Europe, the risk in young adults for Hodgkin's lymphoma increases with increasing social class. Geographic differences are also evident, in that underdeveloped countries have a greater percentage of children among patients with Hodgkin's lymphoma. Also, in these areas of the world, the mixed cellularity histopathology is more frequent, and patients tend to present at a more advanced stage. These clinicopathologic findings have led to speculation that Hodgkin's lymphoma results from infection. In particular, EBV has been implicated. One EBV-related disorder, infectious mononucleosis, is more likely to have occurred in individuals subsequently diagnosed with Hodgkin's lymphoma. Also, Herbst and colleagues (1992) noted that EBV genomes and gene products can be demonstrated in biopsy samples from approximately 50% of patients with Hodgkin's lymphoma. In a Chinese study, Zhou and associates (2001) found evidence of EBV in tumor cells in 89% of pediatric patients with Hodgkin's lymphoma, versus 38% of adults.
Hodgkin's lymphoma in patients with acquired immunodeficiency syndrome (AIDS) has certain characteristics, including the presence of EBV in nearly all cases, as reported by Herndier (1993) and Bellas (1996) and their colleagues. The latter group of investigators compared clinical and pathologic features of Hodgkin's lymphoma in 24 patients with positive results for human immunodeficiency virus (HIV) with those in 56 HIV-negative patients. Significant differences included a higher frequency of the mixed cellularity type and a higher frequency of EBV latent membrane protein expression. Mediastinal disease with nodular sclerosis is less common among those with HIV infection. Certain countries, such as Italy and Spain, have a higher incidence of Hodgkin's lymphoma in AIDS patients than others, including the United States and France. Roithmann and co-workers (1990) reported data indicating that among AIDS patients, Hodgkin's lymphoma occurs preferentially in intravenous drug abusers. It is believed that those countries with a predominance of intravenous drug use associated AIDS have a greater incidence of AIDS-associated Hodgkin's lymphoma than those areas where the prevailing HIV risk group is homosexual men.
As noted, although 60% of all Hodgkin's lymphoma patients have mediastinal involvement at presentation, only 3% have disease limited to intrathoracic sites, usually the anterior mediastinum.
Histopathology is categorized based on the Rye Classification, which lists four types of Hodgkin's lymphoma: (a) nodular sclerosis, (b) lymphocyte predominant, (c) mixed cellularity, and (d) lymphocyte depleted. As reported by Pileri and associates (2002), WHO modified the classification published by Jaffe and collaborators (2001) with division first into lymphocyte predominance and classic types. The classic types are then divided into (a) lymphocyte rich,
P.2689
(b) nodular sclerosing, (c) mixed cellularity, and (d) lymphocyte depleted. As noted by several studies, including those of Urba and Longo (1992), Kennedy and associates (1992), and Sloane (1987), the histopathologic classification is not an independent prognostic factor. All types of Hodgkin's lymphoma are characterized by the presence of Reed-Sternberg cells (Fig. 183-7). These are large, bilobed cells with prominent eosinophilic nucleoli. Large cells without the characteristic bilobed nucleus are Reed-Sternberg cell variants.
Nodular sclerosis is the most common type of Hodgkin's lymphoma in the United States and accounts for over 50% of cases. Adolescents and young adults are usually affected. It has a high incidence of mediastinal involvement. The lymph node is effaced by broad collagenous bands dividing the tumor into nodules (see Fig. 183-7). Reed-Sternberg cells and variants are present along with lymphocytes, eosinophils, plasma cells, histiocytes, and neutrophils. Also typical of nodular sclerosis are the lacunar cells, Reed-Sternberg cell variants that appear to be situated within empty spaces called lacunae. The empty space represents artifact from formalin fixation.
Nodular sclerosis classical Hodgkin's lymphoma varies in cellularity. Tumors that are mostly fibrotic may be termed lymphocyte depleted, whereas lesions that have little fibrosis and more atypical cells are described as the cellular phase. Some studies, including that by MacLennan and colleagues (1989), report that lymphocyte-depleted tumors have a worse prognosis. As studied by Ben-Yehuda-Salz and colleagues (1990), the syncytial type of nodular sclerosing Hodgkin's lymphoma is characterized by sheets of large, malignant-appearing cells. Focal areas typical of nodular sclerosing type need to be identified. In addition, the immunophenotype should be characteristic. Nevertheless, distinction of syncytial Hodgkin's lymphoma from CD30+ (Ki-1) large cell anaplastic lymphoma is difficult.
Lymphocyte-predominant Hodgkin's lymphoma accounts for approximately 10% of cases, according to Glaser and Swartz (1990). Most patients with this tumor are asymptomatic and present with localized disease. Although generally indolent, relapses do occur, especially with the nodular type. Mediastinal involvement is uncommon. Histologically, this lesion is characterized by popcorn cells that have pale cytoplasm, lobulated nuclei, and small nuclei. These cells are present amid a background of small lymphocytes. Epithelioid histiocytes are often prominent. Reed-Sternberg cells are infrequently present. The growth pattern may be either nodular or diffuse. The lymphocyte rich type of classical Hodgkin's lymphoma is distinguished most clearly by the phenotype that is discussed subsequently. Mixed cellularity classical Hodgkin's lymphoma lacks the fibrosis. Lymph nodes are diffusely effaced by a polymorphous cell population, including lymphocytes, plasma cells, neutrophils, histiocytes, and eosinophils. Reed-Sternberg cells and variants are frequent. Compared with the nodular sclerosis type, the mixed cellularity type more often is associated with advanced stage and systemic symptoms.
As summarized by Kant and associates (1986), the lymphocyte depleted type closely resembles large cell non-Hodgkin's lymphoma, and in fact, the distinction is often not clear. Many cases originally classified as lymphocyte-depleted Hodgkin's disease are diagnosed as large cell lymphoma on review.
The diagnosis is often based not only on histopathology but also on immunophenotyping studies. Immunoperoxidase
P.2690
stains using routinely processed tissue specimens usually, but not always, demonstrate a characteristic phenotype for classic Hodgkin's lymphoma: the Reed-Sternberg cells and variants are positive for CD15 (Leu-M1) and CD30 (Ki-1) but negative for CD45 (leukocyte common antigen). Of note, CD30 is also expressed by many mediastinal large B-cell lymphomas and by anaplastic large cell lymphomas, as described by Higgins and Warnke (1999). Expression of other lymphoid markers, both B cell and T cell, is variable. In lymphocyte-predominant Hodgkin's disease the malignant cells typically express CD45 and B-cell marker CD20 but are negative for CD15 and CD30. Also characteristic of lymphocyte-predominant Hodgkin's disease are the presence of numerous CD57+ cells among the benign lymphocytes and the absence of EBV latent membrane protein, according to von Wasielewski and associates (1997). EBV latent membrane protein is present frequently in Hodgkin's lymphoma of the mixed cellularity type.
 |
Fig. 183-7. Hodgkin's lymphoma of anterior mediastinum. A. Gross photograph of tumor adherent to pericardium. Nodularity is a consequence of fibrous bands characteristic of nodular sclerosing classical Hodgkin's lymphoma. B. A Reed-Sternberg cell (binucleated cell with prominent nucleoli) is in the center and is surrounded by small lymphocytes (hematoxylin and eosin stain, 400). A from Kornstein MJ: Pathology of the Thymus and Mediastinum. Philadelphia: WB Saunders, 1995. With permission. |
The cell of origin for the Reed-Sternberg cells and variants of Hodgkin's disease has long been controversial. As reviewed by myself (1995) and Urba and Longo (1992), most evidence favors a cell of lymphocyte lineage. Many cases share antigenic similarities with activated lymphocytes. According to Weiss and associates (1997), lymphocyte-predominant Hodgkin's disease is different in that the neoplastic cells are clonal and of B-cell origin. The finding that the malignant cells are of B-cell origin leads to a change in terminology: WHO advocates the term Hodgkin's lymphoma rather than Hodgkin's disease.
MYELOID LEUKEMIA
Rarely, myeloid leukemias may present with a mediastinal mass. Myeloid cell origin should be considered, when lymphoid markers are negative. Nounou and coinvestigators (2002) describe a patient with an extramedullary myeloid cell tumor localized to the mediastinum. When a bone marrow examination demonstrated acute myeloid leukemia, the previously obtained mediastinal biopsy (initially diagnosed as lymphoma) was found to be positive for myeloperoxidase. Ye and co-workers (2002) described a patient with T-cell blast crisis of chronic myelogenous leukemia presenting as a mediastinal mass.
Appendix: Nomenclature for Lymphocyte Surface Antigens (CD Margers) Useful in Hematopathology | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
P.2691
REFERENCES
Abou-Elella AA, et al: Primary mediastinal large B cell lymphoma: a clinicopathologic study of 43 patients from the Nebraska Lymphoma Study Group. J Clin Oncol 17:784, 1999.
Addis BJ, Isaacson PG: Large cell lymphoma of the mediastinum: a B cell tumour of probable thymic origin. Histopathology 10:379, 1986.
Aisenberg AC: Primary large cell lymphoma of the mediastinum. Semin Oncol 26:251, 1999.
al-Sharabati M, et al: Primary anterior mediastinal B-cell lymphoma. A clinicopathologic and immunohistochemical study of 16 cases. Cancer 67:2579, 1991.
Barth TF, Leithauser F, Moller P: Mediastinal B-cell lymphoma, a lymphoma type with several characteristics unique among diffuse large B-cell lymphomas. Ann Hematol 80(suppl 3):B49, 2001.
Barth TF, et al: Mediastinal (thymic) large B-cell lymphoma: where do we stand. Lancet Oncol 3:229, 2002.
Bekkenk MW, et al: Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood 95:3653, 2000.
Bellas C, et al: Pathological, immunological, and molecular features of Hodgkin's disease associated with HIV infection. Comparison with ordinary Hodgkin's disease. Am J Surg Pathol 20:1520, 1996.
Benharroch D, et al: ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 91:2076, 1998.
Bentz M, et al: Gain of chromosome arm 9p is characteristic of primary mediastinal B-cell lymphoma (MBL): comprehensive molecular cytogenetic analysis and presentation of a novel MBL cell line. Genes Chromosomes Cancer 30:393, 2001.
Ben-Yehuda-Salz D, et al: Syncytial variant of nodular sclerosing Hodgkin's disease. A new clinicopathologic entity. Cancer 65:1167, 1990.
Berard CW, Hutchison RE. The problem of classifying lymphomas: an orderly prescription for progress. Ann Oncol 8:S3, 1997.
Bertini M, et al: Stage II large B cell lymphoma with sclerosis treated with MACOP-B. Ann Oncol 2:733, 1991.
Bitter MA, et al: Morphology in Ki-1(CD30)-positive non-Hodgkin's lymphoma is correlated with clinical features and the presence of a unique chromosomal abnormality, t(2;5)(p23;q35). Am J Surg Pathol 14:305, 1990.
Borenstein SH, et al: The effects of prebiopsy corticosteroid treatment on the diagnosis of mediastinal lymphoma. J Pediatr Surg 35:973, 2000.
Bosch F, et al: Mantle cell lymphoma: presenting features, response to therapy, and prognostic factors. Cancer 82:567, 1998.
Braylan RC, et al: US-Canadian Consensus recommendations on the immunophenotypic analysis of hematologic neoplasia by flow cytometry: data reporting. Cytometry 30:213, 1997.
Cavalli F, et al: MALT Lymphomas. Hematology (Am Soc Hematol Educ Program) 241, 2001.
Chan JK: The new World Health Organization classification of lymphomas: the past, the present, and the future. Hematol Oncol 19:129, 2001.
Cohen AJ, et al: Primary cysts and tumors of the mediastinum. Ann Thorac Surg 51:378, 1991.
Copie-Bergman C, et al: The MAL gene is expressed in primary mediastinal large B cell lymphoma. Blood 94:3567, 1999.
Crist WM, et al: Clinical features and outcome in childhood T-cell leukemia-lymphoma according to stage of thymocyte differentiation: a Pediatric Oncology Group study. Blood 72:1891, 1988.
Davis RD Jr, Oldham HN Jr, Sabiston DC Jr: Primary cysts and neoplasms of the mediastinum: recent changes in clinical presentation, methods of diagnosis, management, and results. Ann Thorac Surg 44:229, 1987.
Davis RE, Dorfman RF, Warnke RA: Primary large cell lymphoma of the thymus: a diffuse B-cell neoplasm presenting as primary mediastinal lymphoma. Hum Pathol 21:1262, 1990.
de Leval L, et al: Expression of bcl-6 and CD10 in primary mediastinal large B cell lymphoma: evidence for derivation from germinal center B cells? Am J Surg Pathol 25:1277, 2001.
Densmore JJ, Williams ME: Mantle cell lymphoma. Curr Treat Options Oncol 1:281, 2000.
Dunphy CH, Wheaton SE, Perkins SL: CD23 expression in transformed small lymphocytic lymphomas/chronic lymphocytic leukemias and blastic transformations of mantle cell lymphoma. Mod Pathol 10:818, 1997.
Ergul SM, et al: Primary mediastinal large B cell lymphoma. South Med J 95:1005, 2002.
Falini B, et al: Variable expression of leucocyte-common (CD45) antigen in CD30 (Ki1)-positive anaplastic large-cell lymphoma: implications for the differential diagnosis between lymphoid and nonlymphoid malignancies. Hum Pathol 21:624, 1990.
Fiorani C, et al: Primary systemic anaplastic large cell lymphoma (CD30+): advances in biology and current therapeutic approaches. Clin Lymphoma 2:29, 2001.
Frizzera G: Recent progress in lymphoma classification. Curr Opin Oncol 9:392, 1997.
Glaser SL, Swartz WG: Time trends in Hodgkin's disease incidence: the role of diagnostic accuracy. Cancer 66:2196, 1990.
Greer JP, et al: Clinical features of 31 patients with Ki-1 anaplastic large cell lymphoma. J Clin Oncol 9:539, 1991.
Griffith RC, et al: A morphologic study of childhood lymphoma of the lymphoblastic type: the Pediatric Oncology Group experience. Cancer 59:1126, 1987.
Harris NL, et al: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 84:1361, 1994.
Herbst H, et al: Distribution and phenotype of Epstein-Barr virus harboring cells in Hodgkin's disease. Blood 80:484, 1992.
Herndier BG, et al: High prevalence of Epstein-Barr virus in the Reed-Sternberg cells of HIV-associated Hodgkin's disease. Am J Pathol 142: 1073, 1993.
Higgins JP, Warnke RA: CD30 expression is common in mediastinal large B cell lymphoma. Am J Clin Pathol 112:241, 1999.
Hudson MM, Donaldson SS: Hodgkin's disease. Pediatr Clin North Am 44:891, 1997.
Hughes JH, et al: Fine-needle aspiration cytology of mediastinal non-Hodgkin's nonlymphoblastic lymphoma. Cancer 84:26, 1998.
Isaacson PG, et al: Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue arising in the thymus. A thymic lymphoma mimicking myoepithelial sialadenitis. Am J Surg Pathol 14:342, 1990.
Jacobson JO, et al: Mediastinal large cell lymphoma: an uncommon subset of adult lymphoma curable with combined modality therapy. Cancer 62:1893, 1988.
Jaffe ES: Anaplastic large cell lymphoma: the shifting sands of diagnostic hematopathology. Mod Pathol 14:219, 2001.
Jaffe ES, et al: World Health Organization Classification of Tumours: Hematopoietic Neoplasms. Lyon: IARC Press, 2001.
Jenkins PF, et al: Non-Hodgkin's lymphoma, chronic lymphatic leukaemia and the lung. Br J Dis Chest 75:22, 1981.
Johnson DW, et al: Hodgkin's disease limited to intrathoracic sites. Cancer 52:8, 1983.
Kamel OW, et al: True histiocytic lymphoma: a study of 12 cases based on current definition. Leuk Lymphoma 18:81, 1995.
Kant JA, et al: The pathologic and clinical heterogeneity of lymphocyte-depleted Hodgkin's disease. J Clin Oncol 4:284, 1986.
Kaplan HS: Hodgkin's Disease. Cambridge, MA: Harvard University Press, 1972.
Kennedy BJ, et al: Survival in Hodgkin's disease by stage and age. Med Pediatr Oncol 20:100, 1992.
Kirn D, et al: Large-cell and immunoblastic lymphoma of the mediastinum: prognostic features and treatment outcome in 57 patients. J Clin Oncol 11:1136, 1993.
Knowles DM: Immunophenotypic markers useful in the diagnosis and classification of hematopoietic neoplasms. In Knowles DM (ed): Neoplastic Hematopathology. Baltimore: Williams & Wilkins, 2001, p. 93.
Koita H, et al: Lymphoblastic lymphoma expressing natural killer cell phenotype with involvement of the mediastinum and nasal cavity. Am J Surg Pathol 21:242, 1998.
Kornstein MJ: Pathology of the Thymus and Mediastinum. Philadelphia: WB Saunders, 1995.
Lamarre L, et al: Primary large cell lymphoma of the mediastinum: a histologic and immunophenotypic study of 29 cases. Am J Surg Pathol 13:730, 1989.
Lavabre-Bertrand T, et al: A study of 15 cases of primary mediastinal lymphoma of B-cell type. Cancer 69:2561, 1992.
Lazzarino M, et al: Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol 15:1646, 1997.
LeClercq G, Plum J: Thymic and extrathymic T cell development. Leukemia 10:1853, 1996.
P.2692
Levitt LJ, et al: Primary non-Hodgkin's lymphoma of the mediastinum. Cancer 50:2486, 1982.
Lin P, et al: Precursor B-cell lymphoblastic lymphoma: a predominantly extranodal tumor with low propensity for leukemic involvement. Am J Surg Pathol 24:1480, 2000.
Linder J: Antibodies marking paraffin-embedded leukocytes. Status report 1991. Am J Clin Pathol 95:607, 1991.
Lones MA, et al: Large cell lymphoma arising in the mediastinum in children and adolescents is associated with an excellent outcome: a Children's Cancer Group report. J Clin Oncol 18:3845, 2000.
MacLennan KA, et al: Relationship of histopathologic features to survival and relapse in nodular sclerosing Hodgkin's disease. A study of 1659 patients. Cancer 64:1686, 1989.
Maity A, et al: Mediastinal masses in children with Hodgkin's disease: an analysis of the Children's Hospital of Philadelphia and the Hospital of the University of Pennsylvania experience. Cancer 69:2755, 1992.
Mathe G: The last revised Euro-American classification of lymphoid leukemias and non-Hodgkin's lymphomas: the same inaccuracies and inconsistencies in a chaotic complexity. Biomed Pharmacother 50:97, 1996.
Mathewson RC, Kjeldsberg CR, Perkins SL: Detection of terminal deoxynucleotidyl transferase (TdT) in nonhematopoietic small round cell tumors of children. Pediatr Pathol Lab Med 17:835, 1997.
Menestrina F, et al: Mediastinal large-cell lymphoma of B-type, with sclerosis: histopathological and immunohistochemical study of eight cases. Histopathology 10:589, 1986.
Moller P, et al: Mediastinal lymphoma of clear cell type is a tumor corresponding to terminal steps of B cell differentiation. Blood 69:1087, 1987.
Moller P, et al: Immunophenotypic similarities of mediastinal clear cell lymphoma and sinusoidal (monocytoid) B cells. Int J Cancer 43:10, 1989.
Morris SW, et al: Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 263:1281, 1994.
Mullen B, Richardson JD: Primary anterior mediastinal tumors in children and adults. Ann Thorac Surg 42:338, 1986.
Murphy SB: Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: dissimilarities from lymphomas in adults. Semin Oncol 7:332, 1980.
Nakagawa A, et al: Clinicopathologic study of primary mediastinal non-lymphoblastic non-Hodgkin's lymphomas among the Japanese. Acta Pathol Jpn 43:44, 1993.
Nakamura S, et al: Anaplastic large cell lymphoma: a distinct molecular pathologic entity: a reappraisal with special reference to p80 (NPM/ ALK) expression. Am J Surg Pathol 21:1420, 1997.
Nounou R, et al: Extramedullary myeloid cell tumours localised to the mediastinum: a rare clinicopathological entity with unique karyotypic features. J Clin Pathol 55:221, 2002.
Onciu M, et al: Terminal deoxynucleotidyl transferase-positive lymphoid cells in reactive lymph nodes from children with malignant tumors: incidence, distribution pattern, and immunophenotype in 26 patients. Am J Clin Pathol 118:248, 2002.
Palanisamy N, et al: Similar patterns of genomic alterations characterize primary mediastinal large B-cell lymphoma and diffuse large B-cell lymphoma. Genes Chromosomes Cancer 33:114, 2002.
Patchefsky AS, et al: Non-Hodgkin's lymphomas: a clinicopathologic study of 293 cases. Cancer 34:1173, 1974.
Paulli M, et al: Mediastinal B cell lymphoma: a study of its histomorphologic spectrum based on 109 cases. Hum Pathol 30:178, 1999.
Piira T, et al: Primary mediastinal large cell lymphoma in children: a report from the Children's Cancer Group. Pediatr Pathol Lab Med 15:561, 1995.
Pileri SA, et al: Hodgkin's lymphoma: the pathologist's viewpoint. J Clin Pathol 55:162, 2002.
Rigaud G, et al: Alteration of chromosome arm 6p is characteristic of primary medastinal B cell lymphoma, as identified by genome-wide allelotyping. Genes Chromosomes Cancer 31:191, 2001.
Roithmann S, Tourani JM, Andrieu JM: Hodgkin's disease in HIV-infected intravenous drug abusers. N Engl J Med 323:275, 1990.
Scarpa A, et al: Mediastinal large-cell lymphoma with sclerosis. Genotypic analysis establishes its B nature. Virchows Arch A Pathol Anat Histopathol 412:17, 1987.
Scarpa A, et al: Molecular features of primary mediastinal B cell lymphoma: involvement of p16INK4A, p53, and c-myc. Br J Haematol 107:106, 1999a.
Scarpa A, et al: Nonrandom chromosomal imbalances in primary mediastinal B cell lymphoma detected by arbitrarily primed PCR fingerprinting. Genes Chromosomes Cancer 26:203, 1999b.
Sheibani K, et al: Antigenically defined subgroups of lymphoblastic lymphoma: relationship to clinical presentation and biologic behavior. Cancer 60:183, 1987.
Simon R, et al: The non-Hodgkin's lymphoma pathologic classification project: long term follow-up of 1153 patients with non-Hodgkin's lymphomas. Ann Intern Med 109:939, 1988.
Sklair-Levy M, et al: CT-guided core needle biopsy in the diagnosis of medastinal lymphoma. Eur Radiol 10:714, 2000.
Sloane JP: Histopathology of Hodgkin's disease. In Selby P, McElwain TJ (eds): Hodgkin's Disease. Oxford: Blackwell Scientific Publications, 1987, p. 4.
Soslow RA, Bhargava V, Warnke RA: MIC2, TdT, bcl-2, and CD34 expression in paraffin-embedded high-grade lymphoma/acute lymphoblastic leukemia distinguishes between distinct clinicopathologic entities. Hum Pathol 28:1158, 1998.
Soslow RA, et al: BCL-1 (PRAD-1/Cyclin D-1) overexpression distinguishes the blastoid variant of mantle cell lymphoma from B-lineage lymphoblastic lymphoma. Mod Pathol 10:810, 1997.
Stein H, Dallenbach F: Diffuse large cell lymphomas of B and T cell type. In Knowles DM (ed): Neoplastic Hematopathology. 2nd Ed. Baltimore: Lippincott Williams & Wilkins, 2000, p. 675.
Stein H, et al: CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood 96:3681, 2000.
Stein ME, et al: Chemotherapy-induced spontaneous pneumothorax in a patient with bulky mediastinal lymphoma: a rare oncologic emergency. Oncology 54:15, 1997.
Strickler JG, Kurtin PJ: Mediastinal lymphoma. Semin Diagn Pathol 8:2, 1991.
Swerdlow SH, Williams ME: From centrocytic to mantle cell lymphoma: a clinicopathologic and molecular review of 3 decades. Hum Pathol 33:7, 2002.
Takagi N, et al: Malignant lymphoma of mucosa-associated lymphoid tissue arising in the thymus of a patient with Sjogren's syndrome. A morphologic, phenotypic, and genotypic study. Cancer 69:1347,1992.
Thomas DA, Kantarjian HM: Lymphoblastic lymphoma. Hematol Oncol Clin North Am 15:51, 2001.
Tilly H, et al: Primary anaplastic large-cell lymphoma in adults: clinical presentation, immuno-phenotype, and outcome. Blood 90:3727, 1997.
Todeschini G, et al: Mediastinal large-B-cell lymphoma with sclerosis: a clinical study of 21 patients. J Clin Oncol 8:804, 1990.
Uckun FM, et al: Biology and treatment of childhood T-lineage acute lymphoblastic leukemia. Blood 91:735, 1998.
Urba WJ, Longo DL: Hodgkin's disease. N Engl J Med 326:678, 1992.
van Besien K, et al: Primary mediastinal B cell lymphoma: a review of pathology and management, J Clin Oncol 19:1855, 2001.
von Wasielewski R, et al: Lymphocyte-predominant Hodgkin's disease: an immunohistochemical-analysis of 208 reviewed Hodgkin's disease cases from the German Hodgkin Study Group. Am J Pathol 150:793, 1997.
Wakely PE Jr, Kornstein MJ: Aspiration cytology of lymphoblastic lymphoma and leukemia: the MCV experience. Pediatr Pathol Lab Med 16:243, 1996.
Weiss LM, Arber DA, Chang, KL: Clonality in lymphocyte predominant Hodgkin's disease. Cancer Surv 30:125, 1997.
Whooley BP, et al: Primary tumors of the mediastinum. J Surg Oncol 70: 95, 1999.
Williams DM, et al: Anaplastic large cell lymphoma in childhood: analysis of 72 patients treated on The United Kingdom Children's Cancer Study Group chemotherapy regimens. Br J Haematol 117:812, 2002.
Williams ME, et al: Immunoglobulin and T cell receptor gene rearrangements in human lymphoma and leukemia. Blood 69:79, 1987.
Yang GC, et al: TIA-1+ cytotoxic large T-cell lymphoma of the mediastinum: case report. Diagn Cytopathol 26:154, 2002.
Yatabe Y, et al: Significance of cyclin D1 overexpression for the diagnosis of mantle cell lymphoma: a clinicopathologic comparison of cyclin D1-positive MCL and cyclin D1-negative MCL-like B-cell lymphoma. Blood 95:2253, 2000.
P.2693
Ye CC, et al: T-cell blast crisis of chronic myelogenous leukemia manifesting as a large mediastinal tumor. Hum Pathol 33:770, 2002.
Yokose T, et al: Low-grade B cell lymphoma of mucosa-associated lymphoid tissue in the thymus of a patient with rheumatoid arthritis. Pathol Int 48:74, 1998.
Zhou XG, et al: Epstein-Barr virus (EBV) in Chinese pediatric Hodgkin disease: Hodgkin disease in young children is an EBV-related lymphoma. Cancer 92:1621, 2001.
Reading References
Chim CS, et al: Primary B-cell lymphoma of the mediastinum. Hematol Oncol 14:173, 1996.
Zinzani PL, et al: Primary mediastinal large B cell lymphoma with sclerosis: a clinical study of 89 patients treated with MACOP-B chemotherapy and radiation therapy. Haematologica 86:187, 2001.
EAN: 2147483647
Pages: 203