140 - Inflammatory Diseases of the Esophagus
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Mediastinum > Section XXVIII - Mediastinal Infections, Overview of Mass Lesions in the Mediastinum, and Control of Vascular Obstructing Symptomatology > Chapter 165 - Acute and Chronic Mediastinal Infections
Chapter 165
Acute and Chronic Mediastinal Infections
Michael J. Liptay
Sameer Kanaan
The vast majority of acute mediastinal infections are the result of esophageal perforations or an infection of the mediastinum after a transsternal cardiac procedure. A small number of acute infections are the result of the spread of an infection arising from the oropharynx, with descending necrotizing mediastinitis being the most severe.
Chronic infections are uncommon. Most are the result of fungal disease originating in the various mediastinal node groups, while a few are secondary to mycobacterial organisms. Chronic fungal or tubercular infections may be self-limiting but may progress into the clinical entity of chronic fibrosing mediastinitis.
PERFORATION OF THE ESOPHAGUS
Perforation of the esophagus can be caused by either spontaneous or iatrogenic trauma. Disruption of the esophagus in the thoracic cavity permits the egress of oropharyngeal bacteria and gastric contents into the visceral compartment of the mediastinum. Perforation of the cervical esophagus, on the other hand, results in leakage of oropharyngeal secretions and infection of the fascial spaces within the neck, which communicate with the anterior and visceral compartments of the mediastinum. The causes, clinical manifestations, diagnostic interventions, treatment, and outcome of esophageal perforations have been reviewed by Jones and Ginsberg (1992), as well as by Whyte (1995), Wright (1995), Engum (1996), Bufkin (1996), and Iannettoni (1997) and their colleagues, and are beyond the scope of this chapter (see Chapter 138). Management strategies of esophageal perforation including the accompanying mediastinitis is based on four principles:
Eliminate source of soilage by primary repair of or diversion away from the esophageal perforation.
Provide thorough and wide mediastinal drainage to control ongoing mediastinal suppuration that occurs after primary repair or diversion. In addition, gastrostomy tube decompression should be performed to diminish gastric reflux and decrease mediastinal soilage.
Appropriate antibiotics should be administered to augment host defenses that must be effective against both gram-positive and gram-negative bacteria and against both aerobic and anaerobic bacteria.
Maintain adequate nutrition. The ultimate goal is to restore alimentary tract continuity as emphasized by Burnett and associates (1990).
The details of the techniques of management of esophageal perforations are discussed in Chapter 138.
POSTOPERATIVE STERNAL INFECTION AND MEDIASTINITIS
The incidence of mediastinitis after cardiac surgical intervention is between 0.15% and 5%, as reported in a 10-year review by Baskett and colleagues (1999). The causes and risk factors include diabetes, chronic obstructive pulmonary disease, congestive heart failure, use of bilateral mammary grafts, current smoker, reoperation, lower ejection fraction, prolonged ventilation, obesity, age, use of bone wax, preoperative renal failure, duration of operation, off-center sternotomy, improper stabilization of the sternum, need for repeated blood transfusions in the early postoperative period, and use of electrocautery (see Reading References for more information).
The bacterial pathogens found are usually Staphylococcus aureus and Staphylococcus epidermidis, which account for 50% to 80% of isolates. This supports the idea that skin flora at the time of operation are responsible for the infection. Also, the leg incision used for saphenous vein graft harvest could be a source of pathogens and may be responsible for gastrointestinal flora found in some cases. Postoperative contamination is also a possible source of infection. Mixed infections account for up to 40% of cases. Gram-negative and fungal infections are infrequent causes of mediastinitis.
The associated mortality rate with mediastinitis after coronary bypass surgery is 10% to 50%. Braxton and associates (2000) reported that the first-year survival rate after
P.2478
coronary artery bypass graft was 78% with mediastinitis and 95% without, with a threefold increase in mortality rate at 4 years' follow-up.
Treatment of mediastinitis uses aggressive surgical d bridement, open drainage, and delayed closure with or without muscle or omental transposition. The reported mortality rate with this technique is now quoted to be 1% 10% compared to historical treatment with open drainage and packing, which was associated with a 50% mortality rate.
DESCENDING NECROTIZING MEDIASTINITIS
Acute purulent mediastinitis caused by oropharyngeal infection was termed by Estrera and associates (1983) as descending necrotizing mediastinitis. Such infections typically develop fulminantly, and lead to sepsis and death.
Etiology
Descending necrotizing mediastinitis is an uncommon but still lethal form of mediastinitis. Of the reported cases, 60% 70% are secondary to odontogenic infections, usually arising from the second or third molar as recorded by Estrera (1983), Mathieu (1995), and Wheatley (1990) and their associates. Odontogenic and peritonsillar abscesses may extend to involve the submandibular space and the parapharyngeal space, which readily communicates with all major cervical fascial planes as pointed out by McCurdy and coinvestigators (1977). Parapharyngeal abscesses may extend into the retrovisceral space, which provides a ready path into the posterior visceral compartment of the mediastinum (Fig. 165-1).
Chow (1990) and Brook and Frazier (1996) have recorded that the microbiological features of descending necrotizing mediastinitis are polymicrobial with aerobes and anaerobes, reflecting the indigenous microflora of the oral cavity. The most common organisms isolated include Prevotella, Peptostreptococcus, Fusobacterium, Veillonella, Actinomyces, oral Streptococcus, Bacteroides, Staphylococcus aureus, Hemophilus species, and Bacteroides melaninogenicus. Symbiosis between one or more species of gram-negative aerobic bacteria and an anaerobe can result in synergistic necrotizing cellulitis.
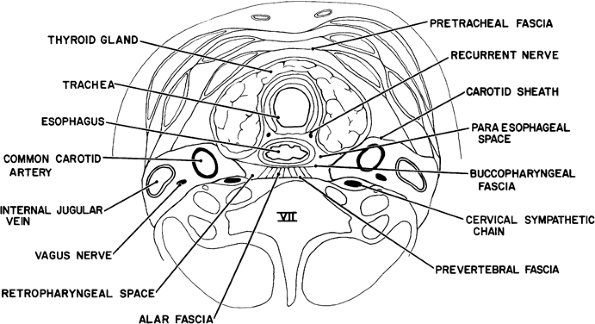 |
Fig. 165-1. Cross-section of the neck at the level of the seventh cervical vertebra. The pretracheal, paraesophageal, and retropharyngeal-retrovisceral spaces extend directly into the mediastinum. Infection can extend also along the carotid sheaths. |
Other causes of descending necrotizing mediastinitis include trauma to the neck, including neck or mediastinal surgery; cervical lymphadenitis; and endotracheal intubation as reported by Guardia and associates (1991), Uram and Hauser (1988), and Gould and colleagues (1974). Alsoub and Chacko (1994) listed the many causes of this lethal infection.
Mathieu and associates (1995) described predisposing conditions that may favor this infection process, which include diabetes (13.3%), alcoholism (17.7%), neoplasm (4.4%), and radionecrosis (3.3%). In particular, they found age greater than 70 years and underlying diabetes were fatal risk factors.
Diagnosis
The criteria used for diagnosis of descending necrotizing mediastinitis are clearly defined by Estrera and colleagues (1983) and include: (a) clinical evidence of severe oropharyngeal infection, (b) characteristic roentgenographic features of mediastinitis, (c) documentation of necrotizing mediastinal infection at the operation or postmortem or both, and (d) establishment of the relationship between descending necrotizing mediastinitis and the oropharyngeal process.
Because this infection progresses rapidly, early diagnosis is essential. Computed tomographic (CT) scanning is more reliable than chest radiography and can provide precise information on the extent of the infection that will guide the optimal approach used for surgical drainage.
Clinical Manifestations
Descending necrotizing mediastinitis is seen most often in a patient who is under treatment for a deep cervical infection
P.2479
resulting from one of the aforementioned causes. Despite antibiotics and even drainage of the deep cervical space, the infection progresses to involve the mediastinum. Early diagnosis is often difficult because of the vagueness of early symptoms that would indicate mediastinal involvement. Descending necrotizing mediastinitis may occur anytime after the occurrence of cervical infection, manifested by signs and symptoms of sepsis with stiffness, swelling, and pain in the neck. Dysphagia may or may not be present. Mediastinal involvement may occur as soon as 12 hours to as late as 2 weeks, but most commonly is seen within 48 hours after the onset of deep cervical infection. Continuing sepsis is evident. Diffuse brawny induration of the neck and upper anterior chest wall is seen. Pitting edema and crepitance may be present in the area. Substernal pain, increased dysphagia, cough, and dyspnea may also develop. Pleural and pericardial involvement may also occur as the necrotizing process involves these adjacent spaces. Pleural effusion, nonspecific electrocardiographic changes, and even infection of the retroperitoneal space of the abdomen may develop as the inflammatory process ensues.
Radiographic Features
Estrera and associates (1983) reported four radiographic features of the neck and chest present in descending necrotizing mediastinitis: (a) widening of the retrocervical space with or without an air fluid level, (b) anterior displacement of the tracheal air column, (c) mediastinal emphysema, and (d) loss of the normal cervical spine lordosis. Also, the superior mediastinal shadow can be widened, and findings of pleural or pericardial involvement can be evident (Fig. 165-2).
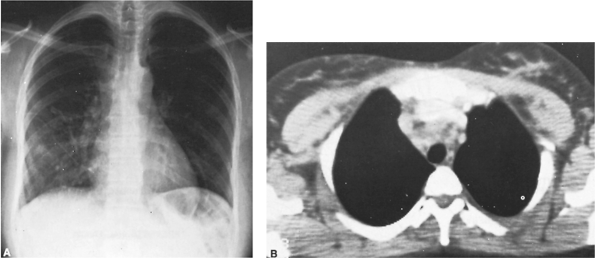 |
Fig. 165-2. Patient with an acute febrile illness with cough and substernal chest pain. A. Posteroanterior radiograph of the chest reveals a nodular infiltrate in the right lower lung field and an ill-defined enlargement of the superior mediastinal shadow. B. Computed tomographic scan reveals an inflammatory process in the superior portion of the visceral compartment of the mediastinum with associated enlargement of the adjacent lymph node. |
CT scans of the chest are better than chest radiographs in delineating the infectious process. Carrol (1987) and Breatnach (1986) and their associates outlined several CT findings in descending necrotizing mediastinitis: (a) abscess formation, (b) soft tissue infiltration with loss of the normal fat planes, (c) absence of prominent lymphadenopathy, and (d) presence of gas bubbles. Air and fluid can be seen in the visceral or anterior compartments, as can pleural or pericardial effusions.
Treatment
Knowledge of the anatomy is essential not only in the diagnosis, but also in the treatment of descending necrotizing mediastinitis. Three potential pathways for spread exist along the fascial planes: (a) the pretracheal route to the anterior mediastinum, (b) the lateral pharyngeal route to the middle mediastinum, and (c) the retropharyngeal/retrovisceral route to the posterior mediastinum. Moncada and associates (1978) reported that 70% of cases of descending necrotizing mediastinitis spread along the retropharyngeal/retrovisceral route to the posterior mediastinum. Odontogenic and peritonsillar abscesses may extend to involve the submandibular space and the parapharyngeal space, which, as McCurdy and colleagues (1977) noted, readily communicates with all major cervical fascial spaces.
Management of descending necrotizing mediastinitis includes surgical drainage, antimicrobial therapy, and airway management. The surgical approach depends on the location of the abscess. Estrera and co-workers (1983) stated that if the infection is in the space below the level of the tracheal bifurcation anteriorly or the fourth thoracic vertebra posteriorly,
P.2480
mediastinal drainage should be performed via a transthoracic approach. If only the superior mediastinum is involved and the infection is contained above the level of the carina or the fourth thoracic vertebra, standard transcervical mediastinal drainage may be adequate, as suggested by Wheatley and colleagues (1990). Marty-Ane and colleagues (1994) proposed a more aggressive approach regardless of the level of infection to include the transthoracic approach through a standard thoracotomy in addition to the cervical drainage. The transthoracic drainage has been demonstrated to result in better d bridement and improved survival, as reported by Temes and co-workers (1998). Further evidence for the inclusion of routine transthoracic drainage is provided by Corsten and associates' (1997) meta-analysis comparing neck and thoracic drainage (19% mortality) with transcervical drainage alone (41% mortality, p < 0.05). Recent reports describe the use of a clamshell incision as suggested by Ris and colleagues (1996), as well as the use of thoracoscopic drainage described by Roberts and associates (1997) in descending necrotizing mediastinitis. The thoracoscopic approach has decreased morbidity versus a thoracotomy and has improved drainage of the mediastinum compared with cervical drainage.
Antimicrobial therapy should be given promptly and cover both aerobes and anaerobes. At the present time, a single agent such as carbapenem, as noted by Sakamoto and co-workers (2000), effectively covers both. Initial antibiotic choice should offer coverage as broad as possible, with combinations used as necessary. Later on, when culture results are available, the antibiotics can be tailored accordingly.
Airway management by using a tracheostomy has been advised by many researchers, including Alexander (1968), Estrera (1983), Allen (1985), Wheatley (1990), and Cordero (1996) and their associates. The rationale is that with severe esophageal and cervical edema often present, a dislodgement of an oral endotracheal tube may prove fatal. However, many others, such as Brunelli (1996) and Sakamoto (2000) and their colleagues, believe that a tracheostomy is not always necessary. The reasoning is that during the tracheostomy, fascial planes are further opened, risking contamination and further spread of infection into the pretracheal space into the anterior mediastinum. Other options include intubation using inhalation anesthesia and endoscopic tracheal intubation by experienced anesthesiologists.
Prognosis
The mortality rate for patients with descending necrotizing mediastinitis before the antibiotic era was approximately 50%, yet the rate has only decreased to 40% despite the introduction of antibiotics, surgical techniques, and intensive care monitoring, as noted by Guardia (1991), Estrera (1983), and Levine (1986) and their co-workers. The reasons for this are that the infection rapidly spreads, leads to fulminant sepsis, and, as a rule, there is a significant delay in diagnosis and initiation of the appropriate therapy.
Since 1990, there has been a decrease in the mortality rate to 15.4%, largely because of the more aggressive approach taken to treat these infections as discussed by Brunelli and associates (1996). Death may result from fulminant sepsis, blood vessel erosion with exsanguination, aspiration, metastatic intracranial infection, empyema, and purulent pericarditis with tamponade.
SUBACUTE MEDIASTINITIS
The definition of subacute mediastinitis is unclear, but this term should embrace those inflammatory processes involving the mediastinum that produce minimal to mild and evanescent symptomatology (substernal pain, fever, night sweats) and an identifiable anterior or visceral mediastinal mass by radiographic or CT examination. These infections most often are the result of fungal, mycobacterial, or, rarely, actinomycotic organisms. These subacute infections are observed only infrequently in previously normal healthy persons but are becoming more common in immunocompromised patients, particularly those with acquired immunodeficiency syndrome (AIDS).
In nonimmunocompromised patients, subacute infections attributed to histoplasmosis and to primary progressive Mycobacterium tuberculosis infections are encountered most often. Actinomycotic infections are rare. Morgan and associates (1990) described two cases of mediastinal actinomycosis. In immunocompromised patients, Mycobacterium avium complex infections as well as those involving M. tuberculosis (Fig. 165-3) are prone to involve the mediastinal lymph nodes. Pitchenik and Robinson (1985) reported that mediastinal or hilar adenopathy was one of the dominant findings in 59% of AIDS patients with M. tuberculosis infections.
The inflammatory nature of the mediastinal mass may be suggested by the CT features of the lesions, but it is best confirmed by gallium scintigraphy, especially in AIDS patients, as suggested by Bitran (1987) and Mehta (1987) and their co-workers (Fig. 165-4). Indium leukocyte scintigraphy also may be useful in identifying these subacute infections, although as Spies (1991) noted, its effectiveness decreases as the inflammatory process becomes more chronic.
The final diagnosis is made by identification of the specific organism by tissue stains or culture. Adequate samples usually are obtained by needle aspiration, although more invasive mediastinal interventions may be necessary. Treatment consists of administering the appropriate drug or combination of drugs for the specific organism identified.
FIBROSING MEDIASTINITIS
Fibrosing mediastinitis is an uncommon benign process resulting in the deposition and proliferation of dense fibrous tissue throughout the visceral compartment of the mediastinum. This chronic inflammatory or inflammatorylike
P.2481
process can lead to entrapment and compression of vital mediastinal structures such as central systemic veins like the vena cava, the esophagus, the trachea or airways, and the pulmonary arteries or veins. The deposition of thick encasing fibrous tissue typically involves the superior mediastinum in the region of the vena cava. When using a more restrictive definition as used by Loyd and associates (1988), the process should involve and obstruct the major airways (tracheal carina or main-stem bronchi) or the pulmonary arteries and veins, or both. With this definition, the superior vena cava is less commonly involved, but this subset of patients is more seriously affected and has a poorer prognosis.
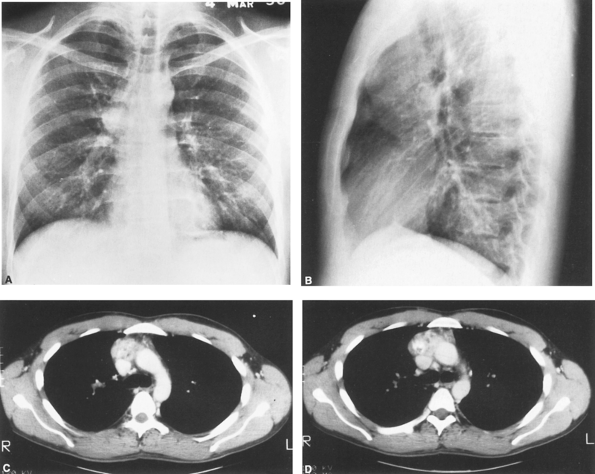 |
Fig. 165-3. Posteroanterior (A) and lateral (B) radiographs of a young adult man with AIDS who developed the anterior mediastinal mass in a 6-month period. C, D. Computed tomographic scans reveal a nonhomogeneous anterior mediastinal mass with areas of calcification. Biopsy revealed a granulomatous process containing many acid-fast organisms typical of Mycobacterium tuberculosis. |
This entity also has been termed sclerosing mediastinitis, fibrous mediastinitis, and granulomatous mediastinitis. Recently, the term idiopathic fibroinflammatory lesion of the mediastinum has been proposed by Flieder and colleagues (1999). The exact cause in most cases is unknown, but recently, many cases in the United States are thought to be secondary to an abnormal response to Histoplasma capsulatum infection by many investigators, including Goodwin (1972), Wieder (1982), Loyd (1988), and Urschel (1990) and their associates, as well as by Garrett and Roper (1986), Wieder and Rabinowitz (1977), and Mathisen and Grillo (1992).
Etiology
Fibrosing mediastinitis may result from a number of causes (Table 165-1). Most cases in the United States are thought to be caused by fungal infections, primarily H. capsulatum. Mycobacterial infections are a less common cause. Goodwin and associates (1972) identified histoplasmosis to
P.2482
be the offending organism in 26 of 38 cases, with the remainder caused by tuberculosis. Eggleston (1980) found that most cases are caused by histoplasmosis, and Urschel and colleagues (1990) reported that 12 of 22 cases of fibrosing mediastinitis were secondary to histoplasmosis, with one case attributed to tuberculosis. The exact mechanism and pathogenesis of fibrosing mediastinitis is not clear though, yet the link between fibrosing mediastinitis and H. capsulatum is based on several observations. First, most cases occur in the United States in areas where histoplasmosis is endemic. Second, many affected patients test positive for H. capsulatum antigens. Third, H. capsulatum organisms are occasionally identified in histopathologic specimens as reported by Rossi and associates (2001). However, because definitive histopathologic proof is absent in many cases, the current thinking is that fibrosing mediastinitis results not from direct H. capsulatum infection, but rather from an abnormal immunologic reaction to its antigens. Goodwin (1972) and Baum (1960) and their associates believe that the cause is an exaggerated delayed hypersensitivity reacting to antigens from infected lymph nodes. Marchevsky and Kaneko (1992) support these findings as well with the presence of strongly positive skin and serum reactivity to H. capsulatum in many patients and hypergammaglobulinemia and hypocomplementemia in others. Sherrick and co-workers (1994) believe that as the acute histoplasmosis infection heals, caseation develops in the mediastinal and hilar lymph nodes. Dunn and colleagues (1990) suggest that these nodes then proceed to rupture, spreading necrotic antigenic material throughout the mediastinum that results in either localized or diffuse fibrosis.
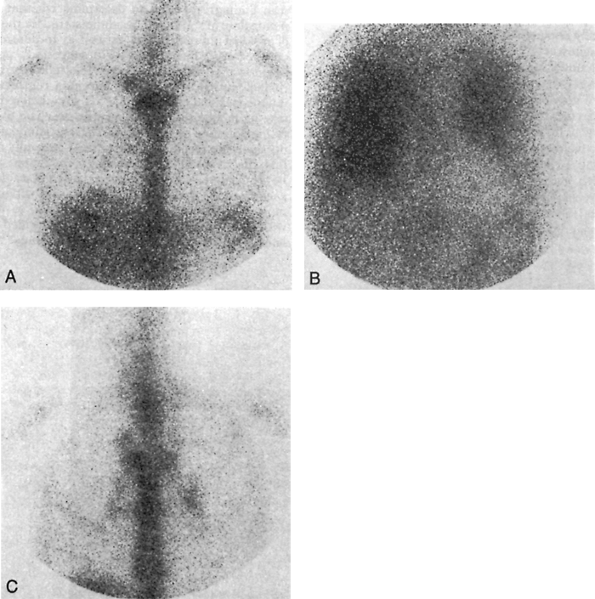 |
Fig. 165-4. Gallium imaging in immunocompromised patients. A. Normal 48-hour anterior gallium image of the chest. B. A 72-hour anterior chest image demonstrates diffuse bilateral increased pulmonary uptake in a 61-year-old man with AIDS and a fever. Note the negative cardiac silhouette. The abnormality was also evident on 24-hour images. Initial chest radiograph results were negative, but later studies showed perihilar infiltrates. Bronchial aspirates were positive for Pneumocystis carinii pneumonia. C. A 72-hour anterior chest image shows bilateral hilar and right paratracheal lymphadenopathy in a 36-year-old man with AIDS who presented with nonproductive cough and fever. The patient proved to have Mycobacterium avium intracellulare infection, which also involved the liver and bone marrow. |
Controversy does exist with regard to fibrosing mediastinitis and the mediastinal granuloma. Some researchers believe that mediastinal granuloma is a precursor to fibrosing mediastinitis. For example, Dines and colleagues (1979) concluded that 34% of 31 patients with mediastinal granulomas eventually developed fibrosing mediastinitis and that
P.2483
the granulomas should be resected to prevent this from occurring. However, Loyd and associates (1988) concluded the opposite. They found no evidence of mediastinal granuloma progression into fibrosing mediastinitis and determined that resection should be performed only for diagnosis or relief of symptoms.
Table 165-1. Etiologic Factors in Granulomatous Mediastinitis with Fibrosis | ||
|---|---|---|
|
Other less common causes of fibrosing mediastinitis are bacterial infections, tuberculosis [as reported by Lee and co-workers (1996)], aspergillosis, mucormycosis, blastomycosis [as described by Lagerstrom and associates (1992)], cryptococcosis, autoimmune diseases in association with Beh et's disease [as reported by Othmani and associates (2000)], rheumatic fever, radiation therapy [as implicated by Dechambre and co-workers (1998)], trauma, Hodgkin's disease, and drug therapy with methysergide maleate. Many of these unusual causes were discussed by Mole and associates (1995) in their report of 18 cases. Fibrosing mediastinitis can also be associated with a number of disease syndromes such as retroperitoneal fibrosis as reported by Fenner (1987) and Morgan (1966) and their colleagues. Sclerosing cholangitis, Riedel's thyroiditis, and pseudotumor of the orbit have also been associated with fibrosing mediastinitis. In a number of cases, as noted in Urschel and colleagues' (1990) series, the cause remains unknown and the term idiopathic is used.
Pathology
Fibrosing mediastinitis is characterized grossly by a diffuse, ill-defined fibrotic infiltration of mediastinal structures. The mass of tissue is dense, white fibrous tissue in cut sections and is described as woody hard. Urschel and associates (1990) likened it to cement poured in the chest. Tissue planes are obscured, and in rare cases the fibrotic infiltration extends into the soft tissues of the neck as noted by Meredith and co-workers (1993), the posterior mediastinum observed by Kountz and colleagues (1989), and the lung as reported by Rossi and associates (2001).
Histologically, bands of hyalinized fibrous connective tissue entrap adjacent structures, and infiltrate and obscure adipose tissue. The fibrous tissue can contain mononuclear cells. The bands are arranged haphazardly, but Razzuk and colleagues (1973) state that the bands can be arranged concentrically around granulomas. The fibrous bands blend with the adjacent nerves, veins, and lymphatics. Zones of new collagen production and scattered aggregates of lymphocytes and plasma cells are present throughout.
Clinical Features
Fibrosing mediastinitis may be self-limiting, but serious persistent complications can incapacitate the patient and even be fatal. Patients are typically young at presentation, but a number of cases may be seen in the fourth to fifth decades of life. It affects men and women equally. A recent study by Flieder and coinvestigators (1999) has suggested an increased predilection in African-Americans, but this has not been seen in other studies.
Approximately 40% of patients are asymptomatic, and the disease is discovered as an incidental radiographic finding. In the other 60% of patients, the clinical features vary with the visceral mediastinal structures involved. Most patients present with signs and symptoms related to compression or obstruction of the central airways, superior vena cava, pulmonary veins, pulmonary arteries, and esophagus. Feigin (1979), Kalweit (1996), and Cochrane (1991), and their colleagues have recorded that the heart, pericardium, aorta, aortic branches, and coronary arteries are less frequently involved. The most common presenting complaints include cough, dyspnea, pleuritic chest pain, fever, wheezing, recurrent pulmonary infection, hemoptysis, and dysphagia. Patients can present with systemic signs such as fever or weight loss. Compression and occlusion of the superior vena results in the superior vena cava syndrome (see Chapter 170). Fibrosing mediastinitis has been reported by Dines and colleagues (1979) as well as by Wieder and Rabinowitz (1977) to be the most common benign cause of superior vena cava syndrome (SVCS). Obstruction of the central airways is common and manifests with cough, dyspnea, and a history of recurrent or persistent pneumonia. Patients with venous occlusion present with progressive or exertional dyspnea as well as hemoptysis; this symptomatology has been termed the pseudo mitral stenosis syndrome by Rossi (2001). Espinosa (1993) and Berry (1986) and their colleagues have noted that chronic pulmonary venous occlusion can result in cor pulmonale and secondary pulmonary arterial hypertension, which is an important
P.2484
cause of morbidity and mortality in patients with fibrosing mediastinitis. Pulmonary venous occlusion can also lead to pulmonary infarction as recorded by Chazova (2000) and Williamson (1992) and their associates. Hoarseness caused by compression of the left recurrent laryngeal nerve is infrequent.
Radiographic Findings
Chest Radiography
Chest radiographs of patients with fibrosing mediastinitis usually appear abnormal, with the extent of disease frequently underestimated, as pointed out by McAdams (1995). The most common finding is widening of the mediastinum (50% 90%) (Fig. 165-5A); other features are a hilar mass (23% 39%), calcification (10% 32%), superior vena cava obstruction (33% 39%), parenchymal opacities (17% 33%), pleural effusion (9%), airway narrowing (36%), and septal thickening (4%), as described by Feigin (1979), Loyd (1988), Sherrick (1994), and Mole (1995) and their co-workers, among others. Rarely, as noted by Katzenstein and Mazur (1980), wedge-shaped areas of consolidation may be apparent. The right side of the mediastinum is more commonly affected than the left, as reported by Feigin and associates (1979), as well as by Williams and Jones (1997).
Computed Tomographic Scans
A CT scan with intravenous contrast is suggested as the preferred method for evaluation of fibrosing mediastinitis by Rodriguez and co-workers (1998). It can often delineate the areas of involvement and the degree of compression of the great vessels, trachea, and esophagus (Fig. 165B-D). Major bronchi can also be involved (Fig. 165-6). Weinstein and colleagues (1983) documented with CT scans, the presence of: (a) hilar mass (100%), (b) mediastinal mass (100%), (c) calcification (86%), (d) airway stenosis (71%), and (e) parenchymal opacities (57%) in patients with fibrosing mediastinitis. Sherrick and associates (1994) noted two distinct CT scan patterns of fibrosing mediastinitis. They found 82% of patients to have relatively localized mediastinal disease affecting the right paratracheal and subcarinal regions. Sixty-three percent of these patients had evidence of prior histoplasmosis or tuberculosis infection. The other 18% of patients were observed to have a diffusely infiltrating disease process affecting multiple structures in the mediastinum. These patients had no evidence of prior granulomatous disease, and almost 50% had associated conditions such as retroperitoneal fibrosis. CT accurately depicts the extent, level, and length of venous stenosis and shows collateral vessels as well. It is also useful for assessing the site, length, and severity of airway stenosis.
Magnetic Resonance Imaging
Rossi and colleagues (2001) reported that fibrosing mediastinitis manifests on T1-weighted images as a heterogeneous, infiltrative mass of intermediate signal intensity. T2-weighted images show both increased and decreased signal intensity. The increased signal is thought to represent active inflammation while the decreased signal is thought to be calcifications or fibrous tissue. Magnetic resonance (MR) imaging is useful when the use of contrast material is contraindicated.
Other Radiographic Investigations
Esophageal involvement is best demonstrated by contrast esophagography, as noted by Ramakantan and Shah (1990). Goenka and co-workers (1995) stated that the typical findings include both circumferential narrowing and long strictures in the junction of the upper and middle two thirds. Standard contrast venography can be performed to demonstrate the anatomy and location of obstruction of the superior vena cava as well as collateral circulation (Fig. 165-7). Pulmonary arteriography is performed in patients with suspected pulmonary vessel involvement, as suggested by Moreno (1983) and Sherrick (1994) and their associates. Typical findings include long-segment, smooth, or funnel-like stenoses of affected vessels.
Radionuclide Studies
Positron emission tomographic findings of fibrosing mediastinitis have been reported in a single case by Imran and co-workers (1999) demonstrating the majority of the lesion to be hypometabolic with focal areas of increased metabolic activity that when subjected to biopsy, revealed typical findings of fibrosing mediastinitis. Lastly, according to McAdams (1995), radionuclide scans with xenon 133 or technetium 99m diethylenetriamine pentaacetic acid may be used and can show ventilation defects in patients with lobar or segmental bronchial occlusion.
Diagnosis
The major aims of diagnostic interventions are to establish that the process is benign and determine the cause if possible. Because chest radiographs of fibrosing mediastinitis are nonspecific and MR imaging poorly depicts calcifications, a CT scan is considered the mainstay in diagnosis by Rossi and associates (2001). Bronchoscopy and mediastinoscopy are also sufficient, but thoracotomy and video-assisted thoracic surgery may be necessary to establish the benign nature of the fibrosis. Cervical mediastinoscopy in the setting of superior vena cava obstruction may carry a higher morbidity, but Jahangiri and associates (1995) reviewed 34 patients and concluded that mediastinoscopy
P.2485
can be performed safely in the setting of superior vena cava obstruction. Esophagoscopy with contrast is indicated when dysphagia is a major complaint. Once the diagnosis has been established, CT or MR imaging plays an important role to define the extent of disease, particularly if surgical resection is being considered.
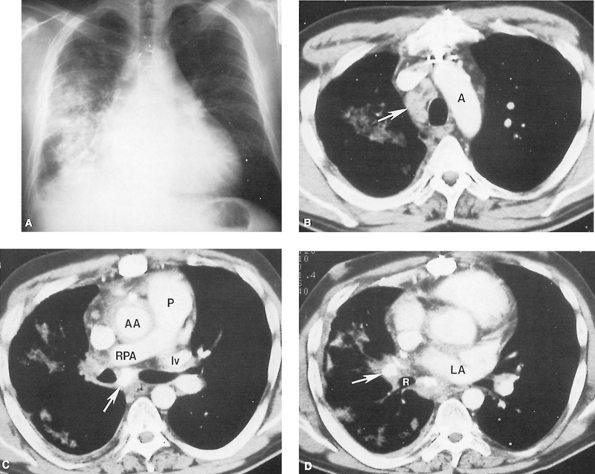 |
Fig. 165-5. Fibrosing mediastinitis. A. Posteroanterior chest radiograph demonstrates diffuse right lung consolidation and some volume loss, plus a small right pleural effusion. Mild right paratracheal widening is present. Cardiomegaly and surgical changes from a previous coronary bypass procedure are seen. B. Post contrast-enhanced computed tomographic study at the level of the aortic arch (A) demonstrates right paratracheal lymphadenopathy (arrow). C. More caudad at the level of the pulmonary artery (P), complete obstruction of the right pulmonary artery (RPA) has occurred. No opacification of the right superior pulmonary vein is seen, although the left superior pulmonary vein (lv) is well enhanced. Calcified lymph nodes (arrow) are present. Abnormal soft tissue encircles the ascending aorta (AA) and infiltrates into the right hilum; on MR imaging this was shown to be of low signal intensity on T2-weighted images, compatible with fibrosis. D. More caudad at the level of the left atrium (LA), calcified lymph nodes (arrow) also are seen occluding the right middle lobe bronchus. R, right lower lobe bronchus. A through D, courtesy of Stuart S. Sagel, MD, and Paul L. Molina, MD, Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, MO. |
Skin testing for mycobacterial and fungal diseases is indicated, as are complement fixation studies for histoplasmosis, coccidioidomycosis, and blastomycosis. Elevated titers are suggestive of disease but are not diagnostic. Increasing serial titers are suggestive of a continuing subacute process. Cultures and histologic examination of any biopsy material for fungal and acid-fast organisms are essential but are often unrewarding.
Treatment
Fibrosing mediastinitis usually takes an unpredictable course, with either spontaneous remission or exacerbation
P.2486
of symptoms reported. Most patients, particularly those with SVCS, improve with time as collateral venous circulation develops. Three general approaches to treatment are possible: (a) medical management with systemic antifungals or corticosteroids, (b) surgical resection, and (c) local therapy for complications.
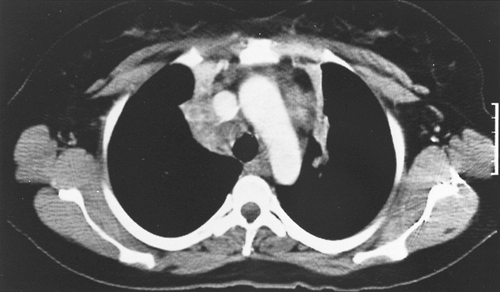 |
Fig. 165-6. Computed tomographic scan of a young woman with fibrosing mediastinitis with compression of the upper lobe bronchi bilaterally with resultant atelectasis of upper lobe segments in both right and left lungs. |
Patients treated with systemic antifungal agents or corticosteroids have supporting data from case reports or small series, with no prospective, randomized controlled trials performed to date as noted by Mathisen and Grillo (1992), as well as by Dunn (1990) and Urschel (1990) and their coinvestigators. The limited data suggest that ketoconazole may stabilize the disease process or improve symptoms minimally, but Loyd and associates (1988) believe that is of no benefit. Goodwin and Des Prez (1978) do not believe that amphotericin B is of value. As noted by Dunn and colleagues (1990), most studies have shown little or no benefit with corticosteroids. Savelli and associates (1997) documented a case of fibrosing mediastinitis in a young woman with dysphagia treated successfully with tamoxifen and prednisone. Radiographic and symptomatic resolution of the disease was noted, although symptoms returned after cessation of tamoxifen. After its reinstitution, the fibrosis regressed and the woman is currently asymptomatic. The mechanism of tamoxifen in this case is unclear, but the researchers proposed the inhibition of epidermal growth factors by tamoxifen. Tamoxifen's utility in treating fibrosing mediastinitis awaits further study.
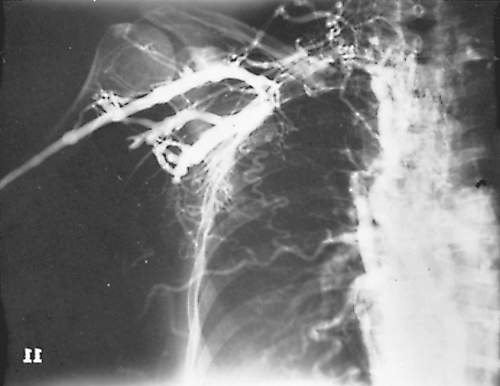 |
Fig. 165-7. Venogram in a young adult woman with a SVCS caused by fibrosing mediastinitis but with a normal standard chest radiograph. Right subclavian and right innominate veins and superior vena cava are obstructed. Extensive collateral channels include the intercostal veins, internal mammary veins, and lateral thoracic branches. Drainage to the inferior vena cava is both by the azygos and hemiazygos veins. |
Surgical resection of localized disease can be curative or result in amelioration of signs and symptoms as reported by Garrett and Roper (1986) and Mathisen and Grillo (1992), as well as by Dunn and associates (1990), but on the whole, results have been disappointing, with an associated high morbidity and as high as 50% mortality in cases requiring pneumectomy. With SVCS, when no improvement occurs with time, Doty and associates (1990) have recommended superior vena cava bypass to relieve the distressing symptoms. The use of spiral saphenous vein grafts, as suggested by Doty and colleagues (1990) or azygos vein transposition when feasible, have been successful in this regard. Thoracotomy may be used in progressive, resistant cases to relieve tracheal or esophageal compression. Lung resection may be required to eradicate persistent infections or pulmonary obstruction.
Symptomatic patients can also be treated with local therapies directed toward reopening occluded or stenotic airways, pulmonary arteries, or the vena cava. Laser therapy, balloon dilation, and stents in the vasculature or endobronchially have all been used with some success by Kandzari (2000) and Dodds (1994) and their colleagues, as well as by Sheski and Mathur (1998) and Watkinson and Hansell (1993).
Prognosis
Loyd and associates (1988) reported a mortality rate of greater than 30%, which is higher than in other studies. Causes of death are usually recurrent infections, hemoptysis, or cor pulmonale. The interval between onset of symptoms and death was approximately 6 years. The mortality rates in patients with subcarinal or bilateral mediastinal involvement are higher than in those with localized disease. Moreover, the health of many surviving patients was severely compromised as a result of their disease.
REFERENCES
Alexander DW, Leonard JR, Trail ML: Vascular complications of deep neck abscesses. A report of four cases. Laryngoscope 78:361, 1968.
Allen D, Loughnan TE, Ord RA: A re-evaluation of the role of tracheostomy in Ludwig's angina. J Oral Maxillofac Surg 43:436, 1985.
Alsoub H, Chacko KC: Descending necrotising mediastinitis. Postgrad Med J 71:98, 1995.
P.2487
Baskett RJF, MacDougall CE, Ross DB: Is mediastinitis a preventable complication? A 10-year review. Ann Thorac Surg 67:462, 1999.
Baum GL, Green RA, Schwartz J: Enlarging pulmonary histoplasmoma. Am Rev Respir Dis 82:721, 1960.
Berry DF, et al: Pulmonary vascular occlusion and fibrosing mediastinitis. Chest 89:296, 1986.
Bitran J, et al: Patterns of gallium-67 scintigraphy in patients with acquired immunodeficiency syndrome and the AIDS related complex. J Nucl Med 28:1103, 1987.
Braxton JH, et al: Mediastinitis and long-term survival after coronary artery bypass graft surgery. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg 70:2004, 2000.
Breatnach E, Nath PH, Delaney DJ: The role of computed tomography in acute and subacute mediastinitis. Clin Radiol 37:139, 1986.
Brook I, Frazier EH: Microbiology of mediastinitis. Arch Intern Med 156: 333, 1996.
Brunelli A, et al: Descending necrotizing mediastinitis. Surgical drainage and tracheostomy. Arch Otolaryngol Head Neck Surg 122:1326, 1996.
Bufkin BL, Miller JI Jr, Mansour KA: Esophageal perforation: emphasis on management. Ann Thorac Surg 61:1447, 1996.
Burnett CM, Rosemurgy AS, Pfeiffer EA: Life-threatening acute posterior mediastinitis due to esophageal perforation. Ann Thorac Surg 49:979, 1990.
Carrol CL, et al: CT evaluation of mediastinal infections. J Comput Assist Tomogr 11:449, 1987.
Chazova I, et al: Venous and arterial changes in pulmonary veno-occlusive disease, mitral stenosis and fibrosing mediastinitis. Eur Respir J 15:116, 2000.
Chow AW: Infections of the oral cavity, neck, and head. In Mandell GL, Douglas RG Jr, Benett JE (eds): Principles and Practice of Infectious Diseases. 3rd Ed. New York: Churchill Livingstone, 1990, p. 516.
Cochrane A, et al: Fibrosing mediastinitis with coronary artery involvement. Ann Thorac Surg 51:652, 1991.
Cordero L, Torre W, Freire D: Descending necrotizing mediastinitis and respiratory distress syndrome treated by aggressive surgical treatment. J Cardiovasc Surg (Torino) 37:87, 1996.
Corsten MJ, et al: Optimal treatment of descending necrotising mediastinitis. Thorax 52:702, 1997.
Dechambre S, et al: Bronchial stenosis and sclerosing mediastinitis: an uncommon complication of external thoracic radiotherapy. Eur Respir J 11:1188, 1998.
Dines DE, et al: Mediastinal granuloma and fibrosing mediastinitis. Chest 73:320, 1979.
Dodds GA III, et al: Relief of superior vena cava syndrome due to fibrosing mediastinitis using the Palmaz stent. Chest 106:315, 1994.
Doty BD, Doty JR, Jones KW: Bypass of superior vena cava: fifteen years' experience with spiral vein graft for obstruction of superior vena cava due to benign disease. J Thorac Cardiovasc Surg 99:889, 1990.
Dunn EJ, et al: Surgical implications of sclerosing mediastinitis: a report of six cases and review of the literature. Chest 97:338, 1990.
Eggleston JC: Sclerosing mediastinitis. In Fenoglio CM, Wolff M (eds): Progress in Surgical Pathology. Vol 2. New York: Masson, 1980.
Engum SA, et al: Improved survival in children with esophageal perforation. Arch Surg 131:604, 1996.
Espinosa RE, et al: Idiopathic pulmonary hilar fibrosis: an unusual cause of pulmonary hypertension. Mayo Clin Proc 68:778, 1993.
Estrera AS, et al: Descending necrotizing mediastinitis. Surg Gynecol Obstet 157:545, 1983.
Feigin DS, Eggleston JC, Siegelman SS. The multiple roentgen manifestations of sclerosing mediastinitis. Johns Hopkins Med J 144:1, 1979.
Fenner MN, et al: Retroperitoneal fibrosis and sclerosing mediastinitis. Indiana Med 80:334, 1987.
Flieder DB, Suster S, Moran CA: Idiopathic fibroinflammatory (fibrosing/ sclerosing) lesions of the mediastinum: a study of 30 cases with emphasis on morphologic heterogeneity. Mod Pathol 12:257, 1999.
Garrett HE Jr, Roper CL. Surgical interventions in histoplasmosis. Ann Thorac Surg 42:711, 1986.
Goenka MK, et al: Mediastinal fibrosis: an unusual cause of esophageal stricture. J Clin Gastroenterol 20:331, 1995.
Goodwin RA Jr, Des Prez RM: State of the art: histoplasmosis. Am Rev Respir Dis 117:929, 1978.
Goodwin RA, Nickell JA, Des Prez R: Mediastinal fibrosis complicating healed primary histoplasmosis and tuberculosis. Medicine (Baltimore) 51:227, 1972.
Gould K, Barnett JA, Stanford JP: Purulent pericarditis in the antibiotic era. Arch Intern Med 134:923, 1974.
Guardia SN, Cameron R, Phillips A: Fatal necrotizing mediastinitis secondary to acute suppurative parotitis. J Otolaryngol 20:54, 1991.
Iannettoni MD, et al: Functional outcome after surgical treatment of esophageal perforation. Ann Thorac Surg 64:1606, 1997.
Imran MB, et al: Sclerosing mediastinitis: findings on fluorine-18 fluorodeoxyglucose positron emission tomography. Clin Nucl Med 24:305, 1999.
Jahangiri M, et al: The role of mediastinoscopy in superior vena caval obstruction. Ann Thorac Surg 59:453, 1995.
Jones WG II, Ginsberg RJ: Esophageal perforation: a continuing challenge. Ann Thorac Surg 53:534, 1992.
Kalweit G, et al: Mediastinal compression syndromes due to idiopathic fibrosing mediastinitis: report of three cases and review of the literature. Thorac Cardiovasc Surg 44:105, 1996.
Kandzari DE, et al: Percutaneous stenting of right pulmonary artery stenosis in fibrosing mediastinitis. Catheter Cardiovasc Interv 49:321, 2000.
Katzenstein AL, Mazur MT: Pulmonary infarct: an unusual manifestation of fibrosing mediastinitis. Chest 77:521, 1980.
Kountz PD, Molina PL, Sagel SS: Fibrosing mediastinitis in the posterior thorax. AJR 153:489, 1989.
Lagerstrom CF, et al: Chronic fibrosing mediastinitis and superior vena caval obstruction from blastomycosis. Ann Thorac Surg 54:764, 1992.
Lee JY, et al: Tuberculous fibrosing mediastinitis: radiologic findings. AJR 167:1598, 1996.
Levine TM, Wurster CF, Krespi YP: Mediastinitis occurring as a complication of odontogenic infections. Laryngoscope 96:747, 1986.
Loyd JE, et al: Mediastinal fibrosis complicating histoplasmosis. Medicine (Baltimore) 67:295, 1988.
Marchevsky AM, Kaneko M: Surgical Pathology of the Mediastinum. 2nd Ed. New York: Raven, 1992.
Marty-Ane C, et al: Descending necrotizing mediastinitis: advantage of mediastinal drainage with thoracotomy. J Thorac Cardiovasc Surg 107:55, 1994.
Mathieu D, et al: Cervical necrotizing fascitis: clinical manifestations and management. Clin Infect Dis 21:51, 1995.
Mathisen DJ, Grillo HC: Clinical manifestations of mediastinal fibrosis and histoplasmosis. Ann Thorac Surg 54:1053, 1992.
McAdams HP: Chest case of the day: fibrosing mediastinitis. AJR 165: 189, 1995.
McCurdy JA Jr, MacInnis EL, Hayes LL: Fatal mediastinitis after a dental infection. J Oral Surg 35:726, 1977.
Mehta AC, Spies WG, Spies SM: Utility of gallium scintigraphy in AIDS. Radiology 165:72, 1987.
Meredith SD, et al: Cervical manifestations of fibrosing mediastinitis: a diagnostic and therapeutic dilemma. Head Neck 15:561, 1993.
Mole TM, Glover J, Sheppard MN: Sclerosing mediastinitis: a report on 18 cases. Thorax 50:280, 1995.
Moncada R, et al: Mediastinitis from odontogenic and deep cervical infection: anatomical pathways of propagation. Chest 73:497, 1978.
Moreno AJ, et al: Angiographic and scintigraphic findings in fibrosing mediastinitis. Clin Nucl Med 8:167, 1983.
Morgan AD, Loughridge LW, Calne RY: Combined mediastinal and retroperitoneal fibrosis. Lancet 1:67, 1966.
Morgan DE, et al: Mediastinal actinomycosis. AJR 155:735, 1990.
Othmani S, et al: Mediastinal fibrosis combined with Beh et's disease: three case reports. Rev Med Interne 21:330, 2000.
Pitchenik AE, Robinson HA: The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis 131:393, 1985.
Ramakantan R, Shah P: Dysphagia due to mediastinal fibrosis in advanced pulmonary tuberculosis. AJR 154:61, 1990.
Razzuk MA, Urschel HC Jr, Paulson DL: Systemic mycoses primary pathogenic fungi. Ann Thorac Surg 15:644, 1973.
Ris HB, et al: Descending necrotizing mediastinitis: surgical treatment via clamshell approach. Ann Thorac Surg 62:1650, 1996.
Roberts JR, et al: Thoracoscopic management of descending necrotizing mediastinitis. Chest 112:850, 1997.
Rodriguez E, et al: Fibrosing mediastinitis: CT and MR findings. Clin Radiol 53:907, 1998.
Rossi SE, et al: Fibrosing mediastinitis. Radiographics 21:737, 2001.
Sakamoto H, et al: Descending necrotizing mediastinitis due to odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89:412, 2000.
P.2488
Savelli BA, Parshley M, Morganroth ML: Successful treatment of sclerosing cervicitis and fibrosing mediastinitis with tamoxifen. Chest 111: 1137, 1997.
Sherrick AD, et al: The radiographic findings of fibrosing mediastinitis. Chest 106:484, 1994.
Sheski FD, Mathur PN: Long-term results of fiberoptic bronchoscopic balloon dilation in the management of benign tracheobronchial stenosis. Chest 114:796, 1998.
Spies WG: Radionuclide studies of the mediastinum. In Shields TW (ed): Mediastinal Surgery. Philadelphia: Lea & Febiger, 1991, p. 50.
Temes RT, et al: Mediastinitis without antecedent surgery. Thorac Cardiovasc Surgeon 46:84, 1998.
Uram J, Hauser MS: Deep neck and mediastinal necrotizing infection secondary to a traumatic intubation: report of a case. J Oral Maxillofac Surg 46:788, 1988.
Urschel HC Jr, et al: Sclerosing mediastinitis: improved management of histoplasmosis titre and ketoconazole. Ann Thorac Surg 50:215, 1990.
Watkinson AF, Hansell DM: Expandable Wallstent for the treatment of obstruction of the superior vena cava. Thorax 48:915, 1993.
Weinstein JB, Aronberg DJ, Sagel SS: CT of fibrosing mediastinitis: findings and their utility. AJR 141:247, 1983.
Wheatley MJ, et al: Descending necrotizing mediastinitis transcervical drainage is not enough. Ann Thorac Surg 49:780, 1990.
Whyte RI, Iannettoni MD, Orringer MB: Intrathoracic esophageal perforation: the merit of primary repair. J Thorac Cardiovasc Surg 109:140, 1995.
Wieder S, Rabinowitz JG: Fibrous mediastinitis: a late manifestation of mediastinal histoplasmosis. Radiology 125:305, 1977.
Wieder S, et al: Pulmonary artery occlusion due to histoplasmosis. AJR 138:243, 1982.
Williams SM, Jones ET: General case of the day. Allergic (or hypersensitivity) bronchopulmonary aspergillosis (ABPA). Radiographics 17:1597, 1997.
Williamson WA, et al: Pulmonary venous infarction secondary to squamous cell carcinoma. Chest 102:950, 1992.
Wright CD, et al: Reinforced primary repair of thoracic esophageal perforation. Ann Thorac Surg 60:245, 1995.
READING REFERENCES
Blomquist IK, Bayer AS: Life-threatening deep fascial space infections of the head and neck. Infect Dis Clin North Am 2:237, 1988.
Demmy TL, et al: Recent experience with major sternal wound complications. Ann Thorac Surg 49:458, 1990.
Ehrenkranz NJ, Pfaff SJ: Mediastinitis complicating cardiac operations: evidence of postoperative causation. Rev Infect Dis 13:803, 1991.
El Oakley RM, Wright JE: Postoperative mediastinitis: classification and management. Ann Thorac Surg 61:1030, 1996.
Farrington M, et al: Study of cardiothoracic wound infection at St. Thomas Hospital. Br J Surg 72:759, 1985.
Gottlieb LJ, et al: Approaches to sternal wound infections. Adv Card Surg 7:147, 1996.
Grossi EA, et al: A survey of 77 major infectious complications of median sternotomy: a review of 7,949 consecutive operative procedures. Ann Thorac Surg 40:214, 1985.
Jones G, et al: Management of the infected median sternotomy wound with muscle flaps. The Emory 20-year experience. Ann Surg 225:766, 1997.
Kohman LJ, Coleman MJ, Parker FB: Bacteremia and sternal infection after coronary artery bypass grafting. Ann Thorac Surg 49:454, 1990.
Loop FD, et al: J. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity and cost of care. Ann Thorac Surg 49:179, 1990.
Nagachinta T, et al: Risk factors for surgical wound infection following cardiac surgery. J Infect Dis 156:967, 1987.
Ottino G, et al: Major sternal wound infection after open-heart surgery: a multivariate analysis of risk factors in 2,579 consecutive operative procedures. Ann Thorac Surg 44:173, 1987.
Santos GH, Shapiro BM, Komisar A: Role of transoral irrigation in mediastinitis due to hypopharyngeal perforation. Head Neck Surg 9:116, 1986.
Sarr MG, Gott VL, Townsend TR: Mediastinal infection after cardiac surgery. Ann Thorac Surg 38:415, 1984.
Smith JM, et al: Sternal wound complications after open heart surgery: results from 3524 consecutive operative procedures. Contemp Surg 43:197, 1993.
EAN: 2147483647
Pages: 203