6 - Insulin Pump Therapy
Authors: Unger, Jeff
Title: Diabetes Management in the Primary Care Setting, 1st Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > 6 - Insulin Pump Therapy
function show_scrollbar() {}
6
Insulin Pump Therapy
Take Home Points
Insulin pump therapy allows patients to manage their diabetes intensively by using a method that is pharmacologically superior to multiple daily injections (MDIs)
Pumped insulin is delivered via basal, bolus, and supplemental insulin
Patients who use insulin pumps must be trained in using MDIs before beginning pump therapy. If the pump malfunctions, the patient will need to revert to using MDIs on a temporary basis
Although providing superior physiologic insulin replacement therapy when compared with MDIs and consistently being favored in quality-of-life assessment studies over other forms of insulin replacement therapy, pumping insulin is much more costly than traditional therapies.
Any patient who uses insulin is a potential candidate for an insulin pump
The newest technology combines insulin pumping with continuous glucose sensing. This allows patients to receive an alarm that is transmitted from the sensor site to the insulin pump, warning them if their interstitial glucose levels are too high or too low. An immediate therapeutic adjustment can be made. Patients who use the pump-augmented sensor are able to efficiently modify their continuous subcutaneous insulin infusion (CSII) prescribed parameters
Prescribers and patients desiring CSII should carefully consider the advantages and disadvantages of insulin pump therapy over syringes, vials, and pen-injector devices.
P.266
Case 1
Leonardo, age 15, was diagnosed as having type 1 diabetes (T1DM) 6 months ago after a 3-day viral upper respiratory infection. Seven days after the upper respiratory infection resolved, he began experiencing weakness, frequent urination, mild abdominal pain, and weight loss, despite having a voracious appetite. His family physician, noting that Leonardo appeared dehydrated, performed a random fingerstick blood glucose level measurement, which was 338 mg per dL. After the patient was rehydrated in the office, insulin therapy was initiated by using a BID 70/30 mixed analogue insulin-pen injector. Within 4 days, Leonardo immediately began to gain weight and feel more energetic, although his blood glucose readings remained far above target. Four months later, Leonardo's A1C was 7.8%. However, he was experiencing wide glycemic swings, with glucose levels ranging from 50 to 275 on a near-daily basis. The family doctor suggested that Leonardo be started with basal-bolus insulin therapy consisting of bedtime glargine and premeal glulisine with pen injectors. With the help of a registered dietician, Leonardo was able to learn carbohydrate (carb) counting. He began checking his blood glucose levels six to eight times daily, and his A1C levels decreased 1.5% within 3 months of intensifying his insulin regimen.
Over a 30-day recording period, 10% of the patient's presupper readings were less than 60 mg per dL on basal-bolus insulin. His fasting glucose levels were averaging 145 mg per dL. Leonardo was a cross-country runner, and his low blood glucose levels correlated with the days on which he intensively trained for more than 60 minutes. Despite reducing the dose of his mealtime bolus by 80% 4 hours before beginning his training session and targeting a pre-exercise blood glucose level of 180 to 240 mg per dL, Leonardo would still become hypoglycemic either during the run or within 4 hours of completing the training session. Although he had no evidence of severe hypoglycemia, the patient and his mother were both concerned about how else they should modify his insulin regimen to minimize his significant glycemic excursions, especially with his active lifestyle.
Leonardo's family physician suggested that using an insulin pump might help maintain a more predictable level of glycemic control. The physician explained that some of the newest-generation pumps combined glucose-sensing technology with insulin pumping. Real-time interstitial glucose readings are displayed on the pump screen, and an alarm would warn Leonardo when the glucose levels appeared to be decreasing too quickly or becoming elevated above the 240-mg per dL target. On hearing the alarm, Leonardo should check his blood glucose level with his standard home blood glucose meter and take the appropriate action to correct the hyperglycemia or hypoglycemic event. Although the pumps are more expensive than insulin pens, Leonardo's long-term diabetes management would certainly be simplified. Pumping insulin should reduce not only his risk of short-term complications [diabetic ketoacidosis (DKA) and hypoglycemia] but also long-term complications such as macrovascular and microvascular disease.
Introduction
The use of continuous subcutaneous insulin infusion (CSII), also known as insulin pump therapy, allows patients with diabetes to achieve improved glycemic control (lower A1Cs) while using less daily insulin, reducing the likelihood of weight gain, and limiting diurnal glycemic variability compared
P.267
with syringes, vials, and pen-injected insulin.1 Because of the lifestyle flexibility that insulin pumpers enjoy, quality-of-life scores consistently favor CSII over MDIs.2 Switching from MDIs to CSII will limit the number of short-term complications such as hypoglycemia and DKA, as well as long-term microvascular disease.1,3,4 Although CSII is certainly the most sophisticated and precise insulin-delivery method currently available, patients who initiate pump therapy must become solidly committed to diabetes self-management. Blood glucose levels must be checked 6 to 8 times daily. Patients must understand insulin pharmacokinetics, carbohydrate counting, and some exercise physiology. Because a pump is a mechanical device that may, on occasion, malfunction, patients must be adept at troubleshooting and correcting unexplained hyperglycemia by using the survival skills they learned from using MDIs.
Pumpers are the most knowledgeable, dedicated, and determined individuals that primary care physicians (PCPs) will come to know within their diabetes patient population. Many of these patients are self-sufficient, confident, and totally committed to improving their own outcomes. PCPs who feel comfortable managing patients with diabetes should also commit themselves to learning the dynamics and benefits of insulin pump therapy.
Insulin pump therapy is designed to simulate normal pancreatic beta-cell function by physiologically delivering both basal and bolus insulin to patients with type 1 and type 2 diabetes. The basal insulin limits the hepatic glucose production that occurs in the fasting state. Prandial (bolus insulin) is normally secreted from the pancreatic beta cells in a first- and second-phase response to meals. The first-phase insulin response occurs as one prepares to eat, whereas the second-phase insulin response continues as long as necessary to prevent postprandial hyperglycemia occurring as nutrients are absorbed from the gut. The tightly controlled glycemic range (70 to 140 mg per dL) is reflective of the normal relation between basal insulin and glucose, as well as the response of the beta cells to a mealtime carbohydrate challenge, as shown in Figure 6-1.
Ideally, exogenous insulin replacement should mimic the normal glucose and insulin response to the fasting and prandial states. However, prandial injection therapy, whether given by a syringe or a pen device, cannot provide both a first- and second-phase physiologic insulin dosage. Exogenous insulin is provided in hopes that the rate of the drug's absorption will coincide with the increase in prandial glucose. Basal insulin, provided as either glargine or detemir, once given, assumes that one's basal insulin requirements do not change during a 24-hour period. However, if one exercises, basal insulin requirements are reduced. Before getting up in the morning, basal insulin requirements increase in response to physiologic insulin resistance caused by increased production of cortisol and growth hormone. In the afternoon hours, insulin requirements are typically lower than during the morning and evening hours. Unfortunately, once the injection of basal insulin is given, the drug's influence on basal insulin levels cannot be altered. That is, one cannot turn up or down the level of glargine or detemir injected the evening before in response to exercise or varying degrees of insulin resistance.
P.268
 |
Figure 6-1 Normal Physiology of Insulin and Glucose Response to Meals. In a nondiabetic individual, basal glucose levels range between 70 and 100 mg per dL while fasting. Basal glucose supplies the heart and central nervous system with an immediate and constant source of energy. Basal insulin levels prevent the liver from accelerating its release of glucose into the plasma via glycogenolysis and gluconeogenesis. If the patient is in the fed state, glucose levels will increase, triggering the pancreatic beta cells to release enough insulin to maintain postprandial glucose levels less than 140 mg per dL. Note that the basal and prandial glucose levels vary according to the time of day as well as the quantity and quality of consumed nutrients at any given meal. In the early morning hours, levels of circulating counterregulatory hormones (growth hormone and cortisol) are increased, resulting in a state of insulin resistance. A normally functioning pancreas overcomes this state of insulin resistance by secreting more insulin in response to these counterregulatory hormones. Therefore, insulin requirements are normally elevated in the morning. In the afternoon hours, insulin resistance is minimized, and insulin requirements decrease. Suppers tend to be the largest meal of the day, during which time one consumes the most calories, carbohydrates, and fats. The higher fat content in food will delay gastric emptying as well as the absorption of carbohydrates from the gut. Patients who must use exogenous insulin should attempt to replicate this complex interactive scheme to maintain blood glucose levels as near to normal as possible. Different basal and bolus-delivery patterns must be used to match this physiologic endogenous insulin milieu. One can see why even seasoned insulin pump patients have difficulty achieving normalcy by using exogenous insulin. |
By having the ability to program changes in basal and bolus insulin-delivery rates, pump users can simulate normal beta-cell insulin secretion. One can program higher basal rates in anticipation of periods of heightened insulin resistance (ie, dawn phenomenon) and lower basal rates in the afternoon hours when insulin resistance is minimal. Different mealtime bolus patterns may be used to control postprandial glucose excursions better.
As of 2006, an estimated 280,000 insulin pump users were registered in the United States.5 As pumps become more user friendly and technologically
P.269
advanced, the number of patients inquiring about insulin pumps will undoubtedly increase. PCPs who are familiar with intensive insulin replacement therapy can successfully manage pump patients. Determinations of the basal rates, mealtime boluses, and supplemental insulin requirements are similar to the formulas used to calculate the different components of MDIs. Certified pump trainers, provided by the pump-manufacturing companies, can be invaluable assistants when pump therapy is initiated. Nearly all pump trainers are certified diabetic educators (CDEs) and are qualified to teach patients every aspect of insulin pump use, including safety issues, proper pump-insertion techniques, infusion-site monitoring, basal and bolus delivery patterns, sick-day management, as well as troubleshoot acute hyperglycemia and pump malfunctions (Table 6-1). Sales personnel can help direct interested personnel toward insulin pump education programs while serving as liaisons between the insurance companies, pump manufacturer, physician office staff, and the patient. Once the physician and the patient agree to consider pump therapy, a letter of medical necessity (similar to the template letter shown in Appendix 1) should be written to the third-party payor. The physician must then calculate and prescribe the pump parameters (basal rates, anticipated mealtime boluses, insulin-sensitivity factor, insulin-to-carbohydrate ratio, personal lag time, and type of insulin to be pumped), which can be written on a form like that in Appendix 2. The patient and the pump trainer can meet in the physician's office to initiate the pump therapy by using the prescribed written parameters.
Patients who are initially informed about insulin pumps may be afraid of wearing a highly technical device on their belts 24 hours a day. However, patients who are new to pump therapy need only understand how to self-insert the pump's infusion set properly and how to dose a proper and physiologic mealtime bolus. The other bells and whistles can be worked out over time as the patients become more confident with their ability to handle the pump.
Patients must understand that the pump does not cure diabetes, but offers them an opportunity to manage their diabetes physiologically. By improving one's overall control, the risk of diabetes-related complications both short and long term will be minimized. Pumpers will need to become experts in diabetes self-management. For many patients committed to insulin pump therapy, managing diabetes becomes both challenging and exciting!
Evolution of Modern Pump Technology
In the 1960s, a pediatrician named Arnold Kadesh developed the first insulin pump. Worn on a backpack (Fig. 6-2A), the pump delivered insulin and glucagon intravenously. Although initially intended as a research instrument in the 1970s, pumps were found to be effective in improving glycemic control in patients with brittle type 1 diabetes 6 (Fig. 6-2B). After the MiniMed 502 pump was introduced and marketed in 1983, pump use began to grow in popularity. By 1990, the United States was home to 6,600 registered pump users7 (Fig. 6-2C).
P.270
P.271
P.272
P.273
TABLE 6-1 Responsibilities of the Physician, Sales Personnel, Pump Trainer, and Patients Who Are Involved in Pump Therapy | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
 |
Figure 6-2 Insulin Pumps: Past, Present, and Future. A: In 1963, Arnold Kadesh, MD, developed the first insulin pump prototype designed to provide his daughter, who had type 1 diabetes, with improved glycemic control. This backpack pump delivered both intravenous insulin and glucagons. B: From 1978 to 1987, pumps became miniaturized. C: Since the mid-1980s, pumps have become more technologically advanced and are designed to deliver insulin in a manner that simulates normal beta-cell function. *Not commercially available |
P.274
The Diabetes Control and Complications Trial (DCCT) was published in 1993.8 At the conclusion of the DCCT, 42% of the intensively managed patients were using insulin pump therapy, and 56% were on MDIs. Patients in the DCCT who were on insulin pump therapy had a 0.3% lower A1C compared with the intensively managed individuals who used MDIs. As CSII gained global popularity, the size, quality, reliability, mechanics, comfort, efficiency, and software of the pumps continued to improve. Insulin pumping now provides patients with diabetes their best hope of achieving physiologic glycemic control while reducing their incidence of short- and long-term complications.
From a primary care prospective, more patients are becoming proactive and inquiring about the benefits of using CSII to treat their own diabetes. These concerns should be addressed in an intelligent and supportive manner, remembering that any patient who requires insulin may be considered a candidate for an insulin pump. Patients are also appearing in emergency departments or operating room suites wearing their insulin pumps. Not infrequently, pumpers have been asked to remove their cell phones while in the emergency department, or you won't be needing that machine in the hospital. In reality, patients who use insulin pumps are extremely motivated, knowledgeable, and well versed in diabetes self-management skills. Physicians who choose to care for pump patients will need to advance their own insights into and knowledge regarding diabetes management. In so doing, these physicians will be able to provide a higher level of care to all of their diabetes patients whether or not they are using pump therapy. PCPs should sympathize with patients who might benefit from pump therapy, yet may need to wait months before seeing a diabetes specialist and even longer before actually receiving their insulin pumps. By becoming pump prescribers, PCPs will allow patients to benefit more rapidly from intensification of their diabetes regimen.
The antique pump infusion sets were anchored into the abdominal skin by a metal needle. These infusion sets tended to be uncomfortable, became easily dislodged from the skin, and had to be manually and painfully inserted by the patient every 2 days. The newer pumps use a spring-loaded self-inserter device (Fig. 6-3), which implants the Silastic infusion catheter painlessly into the skin. Infusion sets have an easy point of disconnect from the site of insertion, allowing patients to be apart from their pump at any time for showering, swimming, exercising, or engaging in intimate activities. Because the infusion sets are either 23 or 43 inches in length, the pump can easily be placed under a pillow during sleep or hidden in clothing.
Some pumps allow discrete bolusing by using a remote control. A woman who wears the pump under a dress could use the remote control device to signal a mealtime bolus rather than struggle with trying to remove the pump from her clothing during a board-room meeting. An insulin reservoir within the insulin pump provides a preprogrammed individualized basal rate to each patient. Before mealtimes, the patient simply determines the blood glucose level and provides a physiologic bolus over a specified time through the infusion set.
P.275
 |
Figure 6-3 One of Several Available Spring-loaded Insulin Pump Infusion Set Insertion Devices. After the skin is cleaned with an alcohol-based adhesive pad, the Silastic infusion-set tip is placed in the cocked inserter. As the inserter is positioned in parallel with the skin surface, light pressure is applied to the distal end of the device, which quickly and painlessly projects the catheter tip through the skin. Removing the white adhesive strips from around the catheter tips will prevent the catheter from dislodging from the infusion site for up to 72 hours. |
The ultimate challenge in treating insulin-requiring diabetes is to design a reliable closed-loop artificial pancreas system. Such a futuristic model would include a self-contained insulin-delivery device, such as an insulin pump, and a continuous glucose sensor. The sensor would transmit the patient's ambient glucose readings to the pump, which would, in turn, respond by infusing the exact amount of basal and bolus insulin required to reach the patient's targeted blood glucose level. Such technology would certainly lead to a reduction in diurnal glycemic variations, a process that is believed to be the cornerstone of microvascular and macrovascular diabetes-related complications. The two anchoring components of the closed-loop artificial pancreas (pumps and sensors) are already commercially available. In 2006, the first U.S. Food and Drug Administration (FDA) approved pump-sensor device was marketed: the Medtronic Real-Time Paradigm 722 pump and sensor (Fig. 6-4).
Users of the Paradigm 722 self-insert both the infusion set and a sensor-transmitting device into two separate areas in the abdomen (Fig. 6-4). The sensor reads interstitial glucose levels every 5 minutes and transmits the data to the insulin pump surface panel for viewing and interpretation. Although the transmitting sensor data do not drive pump insulin delivery, the pump can be programmed to alarm for both high and low glucose values. On hearing the alarm, the patient acquires a confirmatory fingerstick glucose level and corrects the abnormality. Real-time trend graphs may be downloaded to a PC or displayed on the pump screen, which can be useful in identifying glycemic trends in response to physical activity, meals, insulin, menstruation, illness, and so on. Pump sensors would be useful for patients age 18 and older who
Have hypoglycemic unawareness
Have wide glycemic variations with or without CSII therapy
Are pregnant or contemplating pregnancy
Have rigid or intense athletic-training schedules
Are members of certain professions (shift workers, physicians/surgeons, firefighters, police officers)
Have autonomic neuropathy (cardiac, gastroparesis)
Have had a recent myocardial infarction, angioplasty, or coronary artery bypass grafting
Have chronic kidney disease (to improve glycemic control and delay disease progression)
P.276
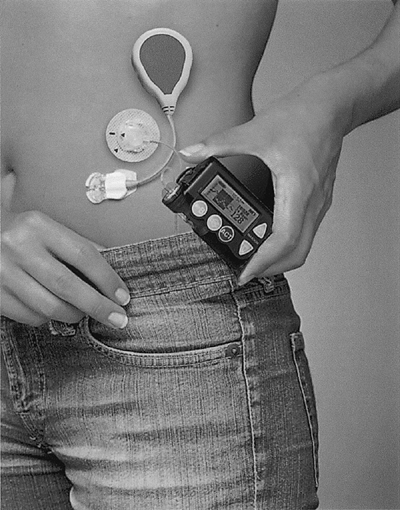 |
Figure 6-4 Medtronic 722 Sensor-augmented Pump System. |
To Pump or Not to Pump: That Is the Question!
Anyone considering pump therapy should consider the advantages and disadvantages associated with CSII, as discussed later and summarized in Table 6-2.
P.277
P.278
TABLE 6-2 Advantages and Disadvantages of Insulin Pump Therapy | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Improved Overall Glycemic Control and Glycemic Variability
The improved A1C values associated with CSII reflect the beneficial pharmacokinetics associated with pump therapy. Insulin pumps use only a single short-acting insulin analogue for both basal and bolus insulin delivery. Multiple basal insulin rates can be programmed into the pump to match the metabolic requirements of individual patients. Neither CSII or MDIs result in complete normalization of glucose concentrations throughout the day. However, if the A1C is reduced safely to as near normal as possible, long- and short-term diabetic complication rates will be reduced. The DCCT has demonstrated superiority of CSII versus MDIs to reduce A1C levels. CSII is superior to MDIs using NPH in reducing A1C levels9 and has been found to be superior to MDIs when fast-acting bolus insulin is used for meals.10 A1C levels are lower in pump patients than in those using MDIs with glargine insulin.11
In addition to improvement in A1C levels, glycemic variability can be reduced in patients using pumps. As discussed in Chapter 7, a strong correlation exists between glycemic variability and the likelihood of developing long-term complications. Glycemic variability is minimized in pump patients. Because a single rapid-acting analogue is absorbed from a single insulin depot, day-to-day
P.279
glycemic variability averages 3% versus 52% with NPH.12 Improved glycemic variability is accompanied by a 15% to 20% reduction in total daily insulin requirements when compared with that in patients using MDI.12
Insulin pumps provide patients with basal, bolus, and supplemental insulin in a physiologic, programmable format, providing patients with more predictable and reproducible glycemic control (Fig. 6-5). This physiologic insulin delivery ensures conscientious pump patients of achieving near-normal basal and postprandial glucose control while minimizing glycemic variability. Most patients require one or two different basal rates, including one that reduces hyperglycemia in the early morning hours before a patient awakens. When
P.280
this dawn phenomenon is better controlled, patients will awaken with near-normal blood glucose levels. However, if fasting blood glucose levels are consistently elevated, patients will be working to correct hyperglycemia throughout the day.
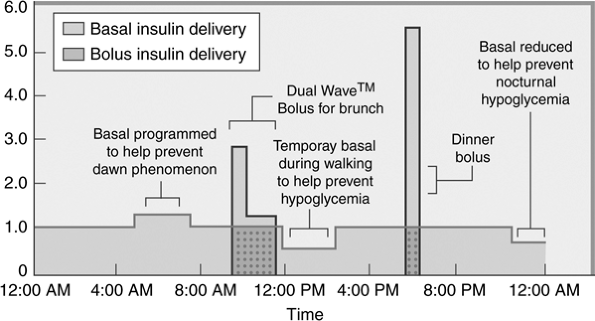 |
Figure 6-5 Programmable Insulin Delivery with Pump Therapy. Patients with diabetes can receive individualized, programmable insulin delivery via pump therapy. By using only a single type of rapid-acting insulin analogue (lispro, aspart, or glulisine), the day-to-day absorption of insulin varies by less than 3%, meaning that the patient's glycemic control will be more predictable and reliable than by using other forms of basal/bolus insulin therapy. Basal rates can be programmed to match the patient's individual basal glucose requirements. For example, a higher basal rate can be programmed for the early morning hours to limit the hyperglycemia caused by the dawn phenomenon. As insulin resistance begins to decrease in the early afternoon hours, the basal rates can be reduced. If a patient exercises consistently during a certain time of day, the basal rate can be programmed at a lower rate, beginning at the anticipated onset of activity, and increased 1 to 2 hours after the exercise session ends. Patients can set a temporary basal rate in anticipation of activities that may induce hypoglycemia (such as intense exercise) or in response to mild hypoglycemia, so that the blood glucose level will increase by hepatic glucose production rather than by the patients having to eat their way out of hypoglycemia. Bolus insulin may be provided for meals in several forms. An immediate bolus may be given all at one time to correct hyperglycemia or to prevent an increase in blood glucose levels, which will occur in response to a between-meal snack. The square-wave (extended-wave) bolus provides the patient with mealtime insulin delivery over several hours and mimics the physiologic second-phase insulin response of euglycemic individuals. A dual-wave bolus combines an immediate and extended-wave bolus, so that a percentage of the total mealtime dose of insulin is provided at the onset of the meal, and the remainder is given over a 2- to 5-hour period. |
The pump's basal insulin delivery maintains ambient plasma glucose readings in the fasting state by limiting hepatic glucose production. The programmable basal rates are calculated based on the patient's size, age, gender, activity level, degree of insulin resistance, and other outliers, as shown in Table 6-3.
Patients may choose to bypass the prescribed basal delivery rate by placing themselves on a temporary basal rate to either slow or increase the rate of insulin delivery. For example, mild hypoglycemia may be treated by setting a temporary basal rate and slowing insulin delivery. By allowing the plasma glucose level to increase spontaneously, patients will not gain weight by eating their way out of hypoglycemia.
Bolus insulin delivered by the pump simulates physiologic first- and second-phase insulin response (Fig. 6-5). The amount of insulin to be delivered as a bolus as well as the time over which the bolus is administered is based on preprandial glucose levels, the amount of carbohydrates and fats that will be consumed during a meal, and the anticipated activity level after a meal. The three types of pumped boluses include the following:
Immediate (now) bolus: If one miscalculates the mealtime bolus and records an elevated glucose level 2 to 4 hours after eating, a supplemental (correction bolus) may be administered through the pump to correct the hyperglycemia. An immediate bolus may also be given for small snacks,
P.281
before exercise in patients who are hyperglycemic, and after the insertion of a new infusion set.TABLE 6-3 Initial Total Daily Dose Requirements for a Patient with Type 1 Diabetes Mellitus
Dose (U/kg/day) Patient's Condition 0.5 Conditioned or trained athlete 0.6 Motivated exerciser, woman in first phase (follicular) of menstrual cycle 0.7 Woman in last week (luteal phase) of menstrual cycle or in first trimester of pregnancy, adult mildly ill with a virus, child starting puberty 0.8 Woman in second trimester of pregnancy, child in midpuberty, adult with a severe or localized viral infection 0.9 Woman in third trimester of pregnancy, child at the peak of puberty, adult ill with bacterial infection 1.0 Woman at term of pregnancy, pubescent child who is ill, adult with a severe bacterial infection or illness 1.5 2.0 Severely ill man or woman, child at peak pubescence who is ill Extended (square-wave) bolus: Insulin is infused over time, typically 30 minutes to 4 hours. The extended bolus is useful for managing patients with gastroparesis and may also be administered for snacks with high fat content, such as ice cream.
Combination (dual-wave) bolus: By combining the immediate and the extended-wave bolus, this becomes the most physiologic means by which prandial insulin can be delivered. After determining the premeal glucose, the patient calculates how much additional insulin should be added or subtracted to the prescribed dose of insulin in the immediate portion of the bolus that will allow the patient to attain the targeted glucose level. Carbohydrate counting or a predetermined dose of insulin may then be administered by using the combination bolus. The extended bolus continues to deliver insulin for 2 to 4 hours, depending on the quantity and fat content of the meal. Fat tends to delay carbohydrate absorption, requiring longer extended-bolus times.
Reduction in Frequency and Severity of Hypoglycemia
The incidence of severe hypoglycemia is less frequent with CSII than with MDIs, yet the ability to perceive low blood glucose levels is less apparent to pump patients. Teaching patients to administer a premeal bolus properly and avoid insulin stacking will significantly reduce the frequency and severity of hypoglycemia (see Chapter 5). Pump patients often monitor their glucose levels before meals, 2 hours after eating, at bedtime, before and after exercise, before driving, and any time they suspect hypoglycemia.
The most serious adverse effect of intensive insulin therapy is severe hypoglycemia, which requires the assistance of another person for reversal. In the DCCT, the incidence of severe hypoglycemia was three times greater in the group receiving MDIs than in conventional therapy (twice-daily injection) patients. In a study by Bode et al,13 patients switched from MDIs to CSII had a reduction in severe hypoglycemia events from 138 episodes per 100 patient-years, with MDIs, to 22 per 100 patient-years in the first year of CSII use. Hypoglycemic events remained lower in pump patients through years 2 to 4, with no increase in A1C levels. Multiple studies have confirmed a reduction in frequency of hypoglycemia associated with CSII.14
Hypoglycemia may occur whenever any form of insulin therapy becomes intensified. If the pump basal insulin rate is programmed inappropriately high, hypoglycemia will follow. Patients must check blood glucose levels frequently and be prepared to treat hypoglycemia in a rapid, efficient, and safe manner.
Patients who are extremely sensitive to small changes in circulating exogenous insulin levels are less likely to become hypoglycemic by using pump therapy.15 The basal and bolus delivery rates of insulin pumps can be adjusted
P.282
by tenths of units, allowing precision dosing of the insulin during both the fasting and prandial states.
In patients who have had T1DM for more than 5 years, autonomic dysfunction develops, which precludes them from recognizing the symptoms of hypoglycemia (fatigue, sweating, blurred vision, dizziness, palpitations, and impaired cognition). Patients with hypoglycemic unawareness also lose their ability to produce enough counterregulatory hormones (cortisol, growth hormone, epinephrine, and glucagon) to protect them from and reverse the hypoglycemia. Activities such as driving, exercising, and caring for children may be challenging and dangerous for patients with hypoglycemic unawareness. Insulin pump therapy can restore one's ability to perceive hypoglycemia. Simply reducing the pump's basal rates and mealtime boluses will allow patients to maintain a higher target blood glucose level while minimizing the frequency and severity of hypoglycemia. For example, a target of 150 to 220 mg per dL in a patient with frequent hypoglycemia might be preferable to the standard glycemic target of 80 to 120 mg per dL. After 6 to 8 weeks of hypoglycemic avoidance, the basal rate of the insulin pump can be increased, allowing the physician to reduce the target glucose range safely toward normal.12 As glucose levels improve, hypoglycemic awareness returns.
P.283
Case 2
This 36-year-old computer design engineer was diagnosed with T1DM at age 6. At the time he was started on CSII, he had nonproliferative retinopathy; chronic kidney disease, stage III; diabetic peripheral neuropathic pain; and orthostatic hypotension. His A1C was 5.9%. Based on his body weight and prior insulin dose requirements, he was placed on a basal rate of 1.0 unit per hour and used carbohydrate counting (1 unit covers 10 g of carbohydrates) to determine his combination wave prandial insulin dose. His home blood glucose meter download (Fig. 6-6A) shows that the patient has significant frequent hypoglycemia. Another indication of frequent hypoglycemia can be determined by evaluating the mathematical relation between the standard deviation (SD) and the mean blood glucose (MBG) average. Doubling the SD value of 75 raises the level to greater than the MBG average of 122, implying a tendency toward hypoglycemia. Of this patient's 85 total blood glucose values (N) during this 28-day period, 35.3% are less than 70 mg per dL, and he recorded 21 events less than 60 mg per dL. At no time did the patient report any symptoms of hypoglycemia. After reviewing the download data, the pump's basal rate was reduced to 0.8 units per hour, and he was provided with a new insulin-to-carbohydrate ratio (1 unit covers 15 g of carbohydrates). The patient was advised to give 25% of his mealtime bolus to coincide with the start of the meal and 75% over a 3-hour period to lessen the likelihood of immediate postprandial hypoglycemia.
After adjusting the pump parameters, the patient was able to avoid hypoglycemia (Fig. 6-6B). The SD of 65 when doubled now is less than the MBG level of 184, suggesting that the patient is experiencing less glycemic variation with no trend toward hypoglycemia. Only once during this 28-day period did the patient have a blood glucose value less than 60 mg per dL, which he was able to detect and correct without assistance. The basal rates can be adjusted in the future if his blood glucose levels remain elevated and his A1C increases to greater than 6.5%.
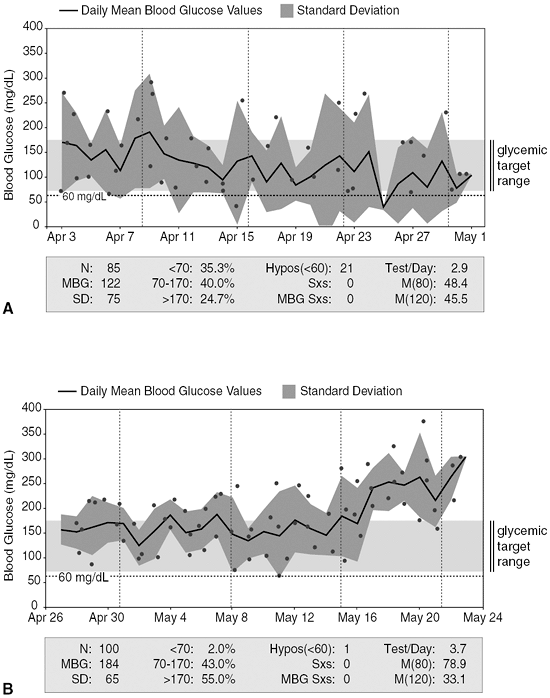 |
Figure 6-6 Home Blood Glucose Meter Download. A: Readings show that patient has significant frequent hypoglycemia. B: Downloads after adjustments to his regimen, which improved his hypoglycemia. Hypos, events of hypoglycemia; MBG, mean blood glucose; N, number of readings; SD, standard deviation. |
P.284
Pump Therapy Reduces Total Daily Insulin Requirements and Limits Weight Gain
Reduced insulin requirements and greater flexibility in food intake may result in minimal weight gain for pump users when compared with MDI patients.8,13 Patients intensively managed in the DCCT gained on average 10 pounds more over a 6-year period than did those using conventional therapy. Because pumps infuse insulin efficiently, using only a single subcutaneous depot and one rapid-acting insulin analogue, one's total daily dose of insulin can be reduced in many cases by 10% to 15% when compared with MDIs. Less insulin use limits weight gain.
Equally important is how pumpers can physiologically manage episodes of mild hypoglycemia. Instead of eating their way out of hypoglycemia, pump patients may either suspend the pump or place the pump on a temporary basal rate until the blood glucose levels increase in response to increased hepatic glucose production. When one eats to correct hypoglycemia, extra calories are consumed, and the corrected blood glucose levels tend to surpass the intended target of 70 to 100 mg per dL. Faced with a postcorrection glucose of 280 after the consumption of a bowl of ice cream to correct a glucose level of 54 mg per dL, the patient may decide to give a supplemental bolus to correct the hyperglycemia. This may result in insulin stacking and a return to hypoglycemia, followed by even greater food consumption. Because pump patients tend to have less frequent episodes of hypoglycemia that they can manage with glucose tablets rather than with candy bars and ice cream, weight gain is minimized.
Pumps Reduce and Reverse Diabetic Retinopathy
Insulin pumps have been shown to reduce and even reverse diabetic retinopathy. An Italian study16 of 20 patients with T1DM with an average age of 37 years and average duration of diabetes of 18 years who were followed up for 2 years showed an overall pump-driven reduction in A1C from 9.1% to 7.5%. Of 10 patients with nonproliferative diabetic retinopathy (4 mild, 5 moderate, and 1 severe), 6 showed regression to a lower grade of diabetic retinopathy. Two of 4 patients with proliferative retinopathy showed a reduction in retinal lesions. The enhancement in glycemic control achieved via insulin pump therapy resulted in significant improvement in diabetic retinopathy.
Lifestyle Flexibility
CSII allows patients to have a more flexible lifestyle. Pumping simplifies irregular meal schedules, exercise, traveling, and other unplanned activities. These advantages may explain why 50% of healthcare professionals with T1DM who are members of the American Diabetes Association (ADA) and American Association of Diabetes Educators use insulin pumps. Patient acceptance of pump therapy is extremely high. Of patients using pumps, 97% show long-term continuation rates.17
P.285
Continuous Subcutaneous Insulin Infusion is more Expensive than Multiple daily Injections
Insulin pump therapy is more expensive than traditional insulin delivery devices such as vials, syringes, and pen injectors (Table 6-2). Initial start-up costs for pump therapy average $5,500. Infusion sets, reservoirs, and catheters cost $1,500 to $1,800 per year. Pump patients are usually more willing to perform home blood glucose monitoring, adding to the expense of this necessary procedure. Pregnant patients with T1DM who are intensively managed on pump therapy will spend on average $140 per month more on all diabetes-related supplies than will patients using MDIs.18
Kanakis et al.19 reviewed the relative costs of insulin pumping in 2002. Excluding the initial start-up costs of pump therapy, CSII costs approximately $2,000 more per year than syringes and vials, and $1,500 more than pen injectors.
Medicare and Medicaid in most states and insurance companies now cover insulin pumps and supplies with proper documentation and prior approval (Appendix 3). Patients with Medicare and third-party payors must pay 10% to 20% of the initial cost (durable equipment coverage) and a proportion of continuing supplies.
Skin Infections and Irritations
Subcutaneous skin infections at the infusion site are likely to occur if the patient does not change the infusion site at least every 72 hours. Patients must be trained to recognized signs of early abscesses, which include persistent itching, pain, or redness around the infusion site, or a gradual increase in blood glucose levels as insulin absorption is reduced from an infected site. Most subcutaneous abscesses can be treated empirically with antibiotics directed at Staphylococcus aureus infections. Larger abscesses will require surgical drainage. Proper insertion technique and infusion-site monitoring will reduce the risk of an abscess developing.
On rare occasions, the author has noted recurring localized skin irritation occurring at the insulin infusion site within 24 to 48 hours after initiating the new infusion set. Initially patients experience mild itching around the site, which prompts them to change their infusion sets and reinsert them in a different area. However, the irritation recurs within 48 hours in association with an increase in blood glucose levels. Some patients may attempt to use supplemental insulin boluses to normalize their glucose levels, which only worsens the localized skin reaction. Being forced to change the infusion sets every 2 days increases the cost of pump therapy. Patients become frustrated by the fact that they are forced to make frequent site changes, while their glucose levels fail to normalize. Some may decide to stop pumping altogether and return to MDIs. However, by switching to a different insulin formulation (i.e., from lispro to glulisine or aspart), the patient will notice significant and rapid improvement in the suspected localized insulin allergy.20,21
P.286
Some Physical Activities May Damage the Pump
Pumps cannot be worn during certain types of sporting activities, such as playing football or rugby, or SCUBA diving. Although some pumps may be detailed as being waterproof, patients should be aware that pumps exposed to water pressures below 10 feet may not maintain their protective status. Patients are therefore strongly encouraged to disconnect their pumps while participating in water sports.
Diabetic Ketoacidosis May Occur Rapidly and Without Warning in Pump Patients
DKA may occur rapidly in pump patients under the following situations: (a) an underlying infection develops, such as a viral illness or appendicitis; (b) the infusion set becomes disconnected or is improperly inserted; (c) the infusion set becomes obstructed, clogged, or filled with a large air bubble (in which case, the patient's insulin dose is substituted with air); (d) the patient simply miscalculates the proper dose of insulin to administer to cover the amount of carbohydrates consumed during a meal; (e) the patient omits a mealtime dose of insulin; (f) the patient runs out of insulin in the pump reservoir; or (g) the pump has a major malfunction and fails to deliver insulin. DKA may occur in even the most compliant of patients, which emphasizes the importance of careful pump education and follow-up.
Frequent blood glucose self-monitoring should alert patients to increasing blood glucose levels, which would prompt them to institute their emergency protocol. Patients must be informed that when faced with increasing blood glucose levels, they must always fix the diabetes first before troubleshooting the insulin pump to determine the cause of the interruption in insulin delivery. Subcutaneous insulin injections may be administered until the pump malfunctions are determined and corrected.
Indications for Insulin Pump Therapy
Although anyone with diabetes may be a potential pump candidate, some patients deserve special consideration for pump initiation.
Failure to Achieve Targeted A1C with Multiple Daily Injections
Failure to achieve treatment targets with elevated plasma glucose levels and A1C levels is an indication for a more aggressive yet physiologic treatment approach. One of the most common reasons certain patients do not achieve glucose treatment targets is the fear of hypoglycemia. Many patients consciously overeat to prevent hypoglycemia. By selecting insulin pump therapy, the patient can better match medical nutritional therapy and exercise with insulin administration. Precise and predictable insulin dosing is provided through the insulin pump. For example, 0.9 units might be excessive, but
P.287
0.4 units would be appropriate. A dose of 0.5 units would be difficult to administer via a syringe or even a pen injector. The basal insulin infusion via CSII is more predictable and reliable, yet adjustable. Because the basal insulin infusion is peakless, the risk for hypoglycemia is decreased.
Hypoglycemia and Hypoglycemic Unawareness
The fear of becoming hypoglycemic is the rate-limiting factor in successfully achieving glucose-treatment targets. With other forms of insulin delivery, the patient with diabetes may strive for prescribed treatment targets, have hypoglycemia, and then overeat to compensate with resultant hyperglycemia. The patient is then on a roller-coaster glycemic curve. With CSII, a closer carbohydrate-to-insulin ratio is maintained by administering minute amounts of insulin when necessary. If the plasma glucose should decrease below the treatment target range, the patient may suspend or cease insulin infusion for a time. Weight gain is avoided through immediate insulin suspension rather than supplemental carbohydrates. In this fashion, the roller-coaster effect is minimized, hypoglycemia is less common, and the glucose level approximates treatment targets. In addition, excessive weight gain is avoided in those patients who eat their way out of hypoglycemia.
Athletes and Patients Who Incorporate Exercise into Their Daily Routines
Exercise poses a special problem for patients receiving insulin. Ideally, patients have been instructed to exercise at those times when injected subcutaneous insulin is not peaking. Each subcutaneous injection of insulin establishes a different subcutaneous depot from which insulin may be absorbed over time. As one exercises, insulin absorbed from multiple depots is more likely to result in hypoglycemia than is insulin absorbed from a single infusion site. With CSII, the patient can safely either suspend insulin infusion for a short time (<1 hour) or modify the basal infusion to a very low rate. If suspending infusion, the patient can determine whether to cease infusion abruptly or to administer a bolus (usually no more than 50% of the planned infusion during the exercise period) before ceasing infusion.
Although the pump should not be worn by patients who participate in contact or water sports, glycemic control is much more predictable while exercising with pumps than when using MDIs of insulin.
Persistent Fasting Hyperglycemia on Rising (Dawn Phenomenon)
In some patients, a dawn phenomenon corresponds to an increase in plasma glucose levels during the early morning hours (4 to 9 AM).22 Insulin resistance intensifies in direct proportion to the physiologic increase in cortisol and growth hormone levels. A patient who does not have diabetes simply
P.288
produces more endogenous insulin in response to these counterregulatory hormones. Patients with diabetes who inject insulin at bedtime cannot automatically increase the circulating dose of that insulin in response to an increase in glucose levels. However, programming a 100% to 150% increase in basal insulin delivery on an insulin pump for 4 to 5 hours, beginning 1 hour before the anticipated onset of the dawn phenomenon, allows the patient to waken with near-normal glycemic levels.
Pregnancy
CSII is an excellent method to achieve and maintain the meticulous glucose control required during pregnancy for the prevention of fetal malformations and obstetric complications. Frequent basal rates can be set during the 24-hour period, and one can administer a bolus immediately with food intake to control postprandial plasma glucose. Programmed basal-rate changes are needed, as insulin requirements are different during each of the pregnancy trimesters (see Chapter 8). Patients contemplating pregnancy will also find that using CSII simplifies their management of hyperglycemia.23
Shift Workers
Shift workers tend to have a very difficult time with glycemic control.7 The physiologic stress caused by chronic sleep deprivation and altered work schedules results in increasing serum cortisol levels. Insulin resistance is exacerbated in response to the higher cortisol levels.24 Patients who work altered shifts can adjust their basal rate profiles to correspond to their schedules. For example, a registered nurse who works the graveyard shift Monday through Friday can use a designated pump pattern on those days that would provide less basal insulin from midnight through 7 AM. On her days off, the pump pattern may be altered automatically by the patient so that additional insulin is provided as she sleeps from midnight through 7 AM. Many women also find this pattern tool useful, as their insulin requirements may jump 10% to 15% during the 3 to 5 days prior to the onset of menstruation.25 When the patient recognizes an increase in her insulin requirements, she simply switches her pump setting from pattern A to pattern B. Pattern A can be resumed once again after 5 to 7 days as progesterone levels fall and insulin resistance wanes.
Pediatric Patients, Highly Insulin-sensitive Individuals, and Poorly Compliant Adolescents
Pediatric patients may require minute amounts of insulin, which can be delivered via CSII in variations of 0.1 units of insulin for bolus and 0.05 units for basal alterations. CSII allows minute variations in the amounts of insulin infused to accommodate these specific requirements. This accuracy surpasses the dilution of U-100 insulin then injected subcutaneously.
P.289
In a review of 80 adolescent patients transferred from MDIs to either CSII or MDIs using glargine and fast-acting insulin, those switched to pumps had a significant A1C decrease from 8.4% to 7.8%, whereas MDI patients did not have a significant decrease in A1C (8.5% to 8.2%). The risk of both moderate and severe hypoglycemia declined in both groups.26
In 65 very young children with a mean age of 4.5 years at pump initiation (range, 1.4 to 6.9 years), mean A1C decreased significantly from 7.4% to 6.9% at 12 months, with further reductions by 2 years.4 Severe hypoglycemia was reduced by 53%. In a randomized trial of CSII versus MDIs27 in a group of young children with a mean age of 3.6 years, no difference in A1C or hypoglycemia was defined, although pump therapy was found to be safe and effective in young children.
Teenagers who require frequent hospitalization for DKA or find little pleasure in following an insulin regimen by using traditional pen-injector devices have been shown to reduce their incidence of hospitalizations and DKA by using CSII.22
Technical expertise is needed for patients using insulin pumps. To be successful pumpers, patients should become proficient at the skills listed in Table 6-1. Children and adolescents who use insulin pumps must have the assistance and encouragement of their parent(s) to manage pump therapy effectively and safely. Parents must supervise adolescents to make certain that they are not taking dangerous short cuts. For example, some adolescents may forget to give a mealtime bolus when they are among friends because they do not want to attract attention to their disease state. Others may simply administer a bolus of insulin without first checking a blood glucose level, preferring to go by how they feel rather than by what they know is best for them. Occasionally, teenagers may delay changing their infusion set on schedule, because they have more pressing issues to which they must attend, or they may inadvertently allow their reservoirs to run out of insulin.
Disturbed eating behavior is very common in young women with T1DM. Between 45% and 80% of teenage girls are binge eaters, and up to 40% will eat while omitting insulin in an attempt to control their weight.28 Eating disorders are associated with many negative medical outcomes, including poor metabolic control, increased frequency of diabetes-related hospitalizations, and higher rates of diabetes-related complications, particularly retinopathy and perhaps neuropathy.28 Patients with clinically significant eating disorders should not be placed on insulin pump therapy.
Special Situations That May Affect Glycemic Control: Menstruation and Traveling
Shortly before and during menstruation, some women face higher insulin requirements. Increasing the basal infusion rate in anticipation of and during menses often results in improved glucose control. A separate basal-rate profile may be initiated as glycemic control begins to worsen before the onset of
P.290
menstruation. Resumption of the original basal rates can begin on day 5 to 7 of the menstrual cycle.
Traveling between time zones is simplified with the use of an insulin pump. If one normally injects the prescribed basal insulin at 10 PM while at home in Los Angeles, and then flies to New York, the basal insulin should be injected at 1 AM. This may require the patient to wake up from sleep to administer the injection. An insulin pump provides basal insulin 24 hours a day, so one does not have to be concerned about consistency. The flexibility provided by the insulin pump allows patients to travel safely and confidently (see Chapter 9 for more details).
Discussing Pump Therapy with Prospective Patients
P.291
Case 3
Jerry, 37 years old, is seen for his initial office visit. After being diagnosed with T1DM at age 24, Jerry feels as though he is ready for the next step in managing his diabetes. With NPH and regular insulin twice daily, Jerry's A1C is 5.2%. He has been checking his blood glucose levels intermittently over the past month. His electronic logs indicate that 94% of his glucose levels lie between 70 and 170 mg per dL, and on average, he is monitoring blood glucose levels 1.6 times a day. He has experienced no hypoglycemic events in the past month. Jerry's laboratory studies and physical examination are normal, and he has no diabetes-related symptoms or complications. As a buyer for a large clothing chain, Jerry's job requires him to board a plane and travel on 10 of 30 days each month. He flies internationally 6 times a year. Making no secret as to his agenda, Jerry announces that he wants to purchase an insulin pump. He is a very busy man and cannot afford to take time off work to start the pump. He wants to pay cash for the pump and supplies, saying Don't worry about my insurance coverage. I'll deal with them later.
The following questions regarding Jerry should be addressed:
Is Jerry an insulin pump candidate?
Anyone who requires insulin therapy is certainly an insulin pump candidate. Assuming that he is willing to learn intensive diabetes management, carbohydrate counting, sick-day management, diabetes traveling strategies, emergency pump protocols, insulin pharmacokinetics, agree to participate in follow-up appointments, and perform frequent home blood glucose monitoring, Jerry would be an excellent pump candidate.
How should one address the appropriateness of pump therapy with this patient?
Insulin pumping does not cure diabetes. Rather, the pump simply provides a more physiologic means of delivering insulin than do subcutaneous injections. To achieve the most benefit from CSII, patients must become dedicated diabetes self-managers. Simply replacing the infusion set every 3 days and administering bolus insulin before meals will not allow patients to become successful pumpers. Pumping requires dedication, education, and a long-term commitment to improving one's diabetes short- and long-term outcomes.
What steps can the physician take on this visit that might encourage Jerry to raise the bar on his commitment to diabetes self-management in preparation for initiating pump therapy?
Jerry should be placed on a physiologic basal-bolus therapy by using a rapid-acting insulin analogue and a once-daily basal insulin analogue. Preferably, he should be instructed on how to use an insulin-pen injector, as these devices may be useful as a backup should a mechanical dysfunction occur during travel with the pump. Jerry must agree to perform home monitoring before each meal to determine the proper amount of insulin he will need to inject before eating. Bedtime glucose levels will also be recorded to lessen the likelihood of nocturnal hypoglycemia developing. Recognizing and treating hypoglycemia properly by using glucose tabs or gels is encouraged. Because Jerry does participate in cardiovascular conditioning 4 times weekly, the management of exercise for patients with T1DM should also be addressed. Jerry should join the ADA and participate in the monthly group diabetes education programs at the local hospital, which are taught by the CDE. Finally, Jerry may choose to attend a local pump club meeting so that he can interact with other pumpers and prospective pump patients.
When evaluating a patient as a potential candidate for an insulin pump, the pros and cons of pump therapy must be discussed. Blood glucose levels will have to be checked more frequently than for patients using MDIs, and pumpers must be compliant with treatment suggestions and follow-up office visits. Although most patients placed on insulin pumps are avid participants in diabetes self-management, some individuals who poorly manage diabetes with MDIs might become successful pumpers. Many inadequately controlled patients have become frustrated and discouraged because MDIs have not helped them achieve their targeted glycemic goals. As patients begin to use insulin pumps, their enthusiasm for developing a partnership with the healthcare team grows. Patients often feel more energetic and in control of their diabetes with the use of the pump.
Physicians should be familiar with the different types of insulin pumps that are commercially available (Table 6-4). On occasion, patients will request a prescription for a certain type of insulin pump. A specialist in diabetes care is likely to have ancillary medical personnel in the office who can assist patients on the working mechanics of individual insulin pumps. PCPs, in contrast, may wish to become as familiar as possible with a single type of insulin pump to improve their comfort level with managing pump patients. Learning everything about one pump is easier than learning small tidbits about multiple pumps. Inquiries regarding educational programs for PCPs interested in managing pump patients should be directed to the pump manufacturers' Web sites.
Many patients with diabetes are not considered candidates for pumps because of underlying comorbidities. A poster presentation29 examined
P.292
P.293
P.294
P.295
13 pump-challenged patients, whose comorbidities included severe hypoglycemic unawareness, movement disorders, attention deficit disorder, mental retardation, schizophrenia, drug or alcohol abuse, personality disorder, depression or severe panic attacks, a history of falsifying glucose records, hypoglycemic encephalopathy, and extreme insulin resistance that required U-500 insulin. Pre-CSII education took 5 months on average, and A1C levels decreased from 9.4% to 8.4% over a 6- to 12-month period. Severe hypoglycemia was reduced by 76%. The authors concluded that success is relative, and pointed out that when dealing with this group of patients, the skill and patience of the prescribing physician and healthcare team are crucial.
TABLE 6-4 Insulin Pump Comparison | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Placing individuals on a pump if they are unable to master MDIs first is a prescription for failure and danger. Patients must be familiar with using MDIs of insulin before beginning pump therapy. All medical devices, including insulin pumps, may malfunction. Patients must be able to troubleshoot the possible mechanisms for the pump malfunction and know how to manage any resultant hyperglycemia. The rule for managing any insulin pump malfunction is to always treat the diabetes first. This may require reverting back to using MDIs temporarily until a new pump becomes available. Although insulin dosing is similar with MDIs and CSII, their primary difference is in the physiologic means of insulin delivery and the flexibility they afford. Adult patients who have no knowledge of MDIs cannot safely be placed on pump therapy.
Initiating Pump Therapy in the Primary Care Setting
Pumps may be initiated in the practitioner's office by a certified pump trainer in about 1 hour. A patient who is new to pump therapy basically must understand how to insert the infusion set and provide a mealtime bolus. Pump students should not be initially overchallenged to learn every feature that is incorporated into their pump. The basic skills any first-time pumper must master prior to leaving the office include (a) how and when to change the insulin reservoir and insert the infusion set, (b) how and when to properly administer a prandial bolus of insulin, (c) how often to check blood glucose levels, and (d) how to respond appropriately to hyperglycemia and hypoglycemia. Patients should be provided with open access to the pump trainer and to the physician should any issues or questions arise regarding their insulin pump. Follow-up visits should be scheduled for 2 weeks after pump initiation, at which time parameters may be fine tuned and additional skills may be addressed. Over time, patients can be instructed on carbohydrate counting, emergency management of pump malfunction, bolus wizard features, pump data management, sick-day regimens, travel procedures, pump-augmented sensor systems, use of supplemental insulin, and initiation of temporary basal rates. Patients do not need to be given saline to practice their button pushing before being given insulin. To those pump trainers who advocate using saline initially, I ask, Would you live in a cave for a week before you move into your brand-new mansion? As
P.296
a PCP managing more than 280 pump patients, not once have I recommended that anyone be placed on saline prior to using insulin
TABLE 6-5 Online Pump Training | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Various training opportunities are available to patients, including pump videos, online virtual pump programs (Table 6-5), and pump manuals. Acceptance of CSII is quite high once the pump is initiated. Physicians who prescribe pump therapy must be able to determine the safe and effective initial pump parameters that will allow patients to achieve adequate glycemic control without becoming hypoglycemic. During the subsequent follow-up appointments, physicians may choose to add additional basal rates, change the nature of the mealtime bolus, and monitor the patient for acute and chronic complications related to CSII.
Setting the Pump Parameters (Don't Worry This Is the Easy Part!)
The following case will illustrate the steps required to determine the initial pump parameters for a patient who is beginning CSII.
P.297
P.298
P.299
P.300
P.301
P.302
P.303
P.304
P.305
Case 4
Jen, age 20, is preparing to start insulin pump therapy today. She weighs 70 kg, and she has a habit of skipping breakfast but eating a modest lunch and large dinner. She has consistent hyperglycemia on rising each morning (6 AM), with her fasting blood glucose values averaging 40 mg per dL greater than her prelunch values. Jen exercises for 60 minutes, 5 days a week, between 4 and 8 PM, and eats dinner around 9 PM. Her blood glucose levels at bedtime before initiating pump therapy have been averaging 200 mg per dL.
The following steps will guide the physician in the initial steps required for pump programming:
What is Jen's anticipated total daily dose of insulin requirement? (Fig. 6-7, item 1)
Determine the patient's total daily dose (TDD) of insulin.
The first order of business to consider when prescribing pump parameters is to determine how much insulin a patient requires over a 24-hour period. By taking the patient's current weight in kilograms and multiplying that weight by 0.7 units, one can determine the TDD of insulin required for a 24-hour period as follows:
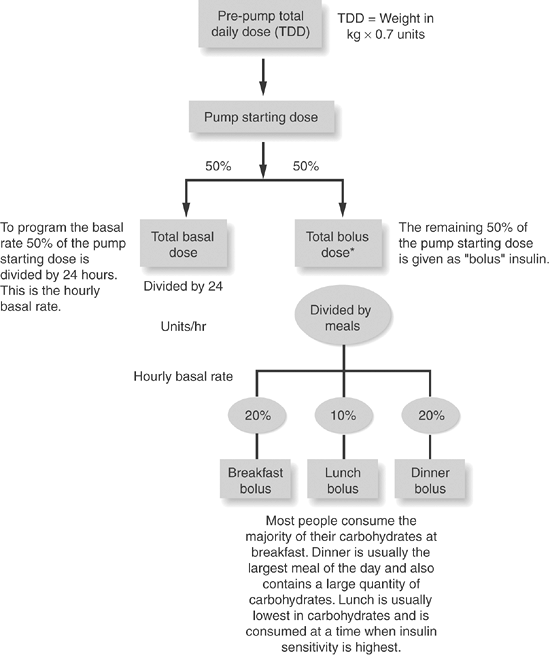
Figure 6-7 Formulas for Programming Pump Parameters. These include total daily dose (TDD) of insulin, insulin-sensitivity factor (ISF), insulin/carbohydrate ratio (I/C), personal lag time, and rule for managing hypoglycemia. *Although many people have a 50/50 ratio of basal/bolus insulin, others may have a ratio of 40/60 ratio, meaning that more insulin is required to maintain PPG than FPG levels.
TDD = Weight in kg 0.7 units
In Jen's case, 70 kg 0.7 = 49 units (OK to round this off to 50 U per day)
The TDD of insulin also varies depending on the patient's level of physical conditioning, age, gender, degree of the patient's physical stress, and body weight. A well-conditioned athlete, for example, would have a TDD of weight in kg 0.5 units. Table 6-3 suggests dosing strategies for calculating the TDD based on these multiple factors. Keep in mind that this allows the physician to establish the initial dose of insulin.
Assign how much of the TDD is applied to basal and how much to bolus insulin per day.
Once the TDD of insulin is calculated, of that total daily dose is assigned to basal insulin delivery, and , to bolus insulin delivery:
50% of the TDD = basal insulin
50% of the TDD = bolus insulin
In Jen's case, 25 units is applied to the basal insulin and 25 units to the bolus insulin.
Determine the patient's hourly basal insulin requirements.
The initial hourly basal insulin requirements are determined by dividing the total basal insulin by 24 hours.
For Jen, 25 U per 24 hours = 1.0 U per hour. Thus the initial basal rate of insulin is 1.0 U per hour.
Additional basal rates may be programmed at future visits based on the presence of the dawn phenomenon; timing, frequency, and severity of recorded episodes of hypoglycemia; and the patient's glycemic response to exercise. Most patients require 1 to 2 basal rates. Generally, the more basal rates programmed, the more complicated insulin pumping becomes for both the patient and the physician. Patients who have multiple basal rates (more than 4) programmed into their pumps, yet are unable to maintain adequate A1C levels (6.5% to 7.5%) while showing signs of wide glycemic variation, may improve by simply limiting them to 1 to 2 basal rates.
What is Jen's insulin-sensitivity factor? (Fig. 6-7, item 2)
The insulin sensitivity factor (ISF) allows patients to calculate the anticipated reduction in glucose level after a 1-unit insulin bolus.
Patients can use the ISF to correct hyperglycemia resulting from underdosing of prandial insulin, the insulin resistance that occurs on sick days, or pre-exercise glycemic elevations. Glycemic targets may vary throughout the day. Patients may be advised to maintain a daytime target of 80 to 140 mg per dL, but a night-time target of 150 to 180 mg per dL to prevent nocturnal hypoglycemia. The default safe target for correcting hyperglycemia is 150 mg per dL. This allows patients to overshoot their mark slightly without becoming hypoglycemic. If the target is set too low (100 mg per dL), patients will commonly overcorrect into a state of hypoglycemia.
The ISF is calculated by dividing 1,700/total daily dose of insulin. Jen's ISF would be 1,700/50 = 34. Thus, if the glycemic target is 150 mg per dL, and Jen's current blood glucose level is 250 mg per dL, Jen would do the following calculation to reach the target:
Step 1: 250 - 150 = 100 mg per dL
Step 2: 100/34 = 2.9 units
Step 3: Target goal =150 during the day
Giving a 2.9-U dose at noon will allow Jen to achieve a normal glucose level within 1 to 2 hours after administering the bolus. (Note that the pumps allow patients to administer insulin by tenths of units. This cannot be done with syringes and vials or insulin pens.)
When correcting hyperglycemia, patients should be encouraged to recheck their blood glucose levels within 1 to 2 hours of the correction dose to make certain that they are approaching, yet have not exceeded, the target.
Patients who must frequently correct postprandial hyperglycemia should be provided with a revised insulin-to-carbohydrate ratio. Extending the square-wave portion of the bolus for a longer time may obviate the necessity for frequent corrections. Hyperglycemia that frequently occurs in the preprandial or fasting state requires adjustments in basal-rate insulin delivery.
What is Jen's insulin/carbohydrate ratio (I/C)? (Fig. 6-7, item 3)
The I/C ratio provides patients with an estimation of how much 1 unit of insulin will cover the glycemic excursion anticipated with carbohydrate ingestion.
Divide 450/TDD insulin to get the I/C.
In Jen's case, 450/50 = 9. Thus, if Jen consumes 100 g of carbohydrates, she will need to administer a bolus of 100/9 = 11.1 U of insulin.
I/Cs may vary throughout the day, often being lower in the morning and higher in the evening. Jen may actually have an I/C of 1:8 for breakfast and 1:11 for dinner. Patients who are educated on their I/C values will realize that consuming carbohydrates will necessitate a dose of insulin. For example, if Jen goes to a movie and eats popcorn, she will need to provide insulin for that carbohydrate snack. Otherwise, her blood glucose level will be very high by the time she returns home after the show.
What is Jen's personal lag time ?
Absorption rates differ for each patient. By calculating a personal lag time, postprandial glycemic excursions might be minimized.30 The methods required to calculate the personal lag time appropriately are detailed in Figure 6-7, item 4.
A patient with blood glucose levels greater than 180 mg per dL should increase the lag time, allowing the insulin to improve glycemic control before eating. Patients who are hypoglycemic before meals should delay the mealtime bolus until after the meal is completed to prevent a worsening of the hypoglycemic state.
How should Jen's boluses be monitored and adjusted?
A percentage of the total amount of insulin assigned for mealtime boluses during a 24-hour period should be assigned to breakfast, lunch, and dinner. As shown in Figure 6-1, glycemic requirements are highest during the early morning hours and in the evening hours. Therefore, prandial insulin requirements are highest for breakfast and supper and lowest for lunch.
In Jen's case a total of 25 units is assigned to prevent postprandial hyperglycemia. Jen can apply these 25 units to meals based on the amount of carbohydrates she will be consuming at each meal. However, if Jen has had trouble with carbohydrate counting, a prescribed (suggested) baseline dosage of insulin may be delivered at each meal. From this baseline dosage, the patient can increase or decrease the dose of prandial insulin based on several factors:
Increase 1 to 2 units for large meals
Decrease 1 to 2 units for smaller meals
Calculate the ISF to determine how much insulin would be required to adjust the preprandial glucose level to 150 mg per dL and then add this total to the prescribed dose. For example, if the initial blood glucose level is 250 mg per dL, preprandial glucose target is 150 mg per dL, ISF is 1:50 mg per dL, and the suggested lunch-time dose of insulin is 4 units, the patient would take a total of 6 units for lunch (250 mg per dL - 150 mg per dL = 100/50 = 2 units. These 2 units plus the suggested lunch dose of 4 units = 6 units).
Therefore Jen is advised to make the following decisions:
If the preprandial glucose level is between 70 to 170 mg per dL, she may either count carbohydrates or plan to administer a bolus of approximately 10 units of insulin for breakfast, 4 units for lunch, and 11 units for dinner.
Whenever possible, use a dual-wave bolus to control the anticipated hyperglycemia associated with mealtime. The dual-wave bolus consists of a rapid immediate bolus simulating normal first-phase insulin response and a square-wave bolus similar to a normal second-phase insulin release, which is administered over a 2- to 3-hour period (Fig. 6-5).
The dual-wave bolus can be administered in many different ways. Providing 50% of the total bolus immediately and 50% over a 2- to 3-hour period will work well for many patients. If the premeal glucose levels are greater than 150 mg per dL, supplemental insulin can be calculated into the initial bolus to target a 150 mg per dL glucose level. For example, if the prelunch glucose level is 250 mg per dL and the ISF is 1:25, Jen should give 4 + 2 or 6 units as an immediate bolus and 2 units as square-wave delivery over a 2-hour period. If the prelunch glucose level is less than 70 mg per dL, Jen can either reduce the immediate bolus to 1 unit and give the usual square-wave bolus, or begin the dual-wave bolus after eating to avoid hypoglycemia. Utilization of the bolus wizard available on Medtronic pumps may also be beneficial for determining the amount and timing of an individual meal bolus. With practice, patients will learn how to deliver the most physiologic dual-wave bolus for each meal consumed. Patients who use pramlintide with their insulin pumps may experience less hypoglycemia if 25% of their mealtime bolus plus their correction bolus is given with the meal and 75% of the calculated bolus is provided over a 3-hour period. Pramlintide patients who are prone to postprandial hypoglycemia may even begin their mealtime boluses after they complete their meal rather than just before the onset of the carbohydrate intake.
How can Jen's bolus wizard feature be used to her advantage?
The bolus wizard feature allows patients to receive assistance from the actual insulin pump software to dose an insulin bolus properly. The bolus wizard, which is unique to Medtronic insulin pumps, reduces math errors, decreases the number of correction boluses required, and helps prevent insulin stacking. This advanced feature can be linked directly to a BD Logic blood glucose meter or uploaded manually from a different blood glucose meter.
A most useful tool in the bolus wizard system is the ability to recognize active insulin levels given in a previous bolus. Active insulin depots remain to be absorbed from a prior bolus. This will reduce the chance of stacking insulin before a prior bolus is completely absorbed. For example, if Jen attempts to use a correction dose of 6 units 2 hours after eating a meal, the pump will advise her that the safest way to infuse insulin might be to reduce the correction bolus by 60%, as that quantity of insulin remains to be absorbed after the initial bolus. When Jen attempts to give this supplemental insulin, the pump will ask her if she would prefer reducing the dose by 40% to prevent insulin stacking. These calculations are preprogrammed into the pump. The bolus wizard also may be helpful when providing supplemental insulin for high blood glucose levels.
What type of insulin should Jen use while pumping?
The majority of patients use a fast-acting insulin analogue (lispro, aspart, or glulisine) as their pumped insulin.
All fast-acting analogues are FDA approved for use in insulin pumps. Patients with severe insulin resistance who require high-volume basal and bolus flow rates, resulting in more than 150 units of daily insulin, might be candidates for concentrated U-500 regular insulin.31 Pump reservoirs are designed to carry between 200 and 300 units of insulin. If a patient uses 200 units of insulin per day, the reservoir and infusion sets would have to be changed every 24 to 36 hours rather than every 3 days, adding to the overall expense for pump supplies. U-500 insulin can be beneficial for insulin-resistant patients. Because U-500 has a much slower onset of action than a rapid-acting insulin analogue, patients using concentrated insulin should delay eating for between 45 and 60 minutes after starting their dual-wave bolus. Because U-500 insulin is 5 times as potent as standard U-100 insulin, patients can reduce their basal and bolus rates by 80%. For example, a basal rate of 3.0 U per hour can be reduced to 0.6 U per hour on U-500. Meal boluses totaling 50 units can be changed to a dual-wave bolus of 5 units immediately and 5 units over a 3-hour period. Families of patients using U-500 insulin should be trained in the administration of emergency glucagon for rare cases of insulin-induced hypoglycemia.
What instructions should be given regarding pump emergency protocols ?
Patients using insulin pumps must be able to anticipate, screen for, and manage short-term complications such as hyperglycemia, DKA, and hypoglycemia. Although these tasks are often taught by certified pump educators and CDEs, physicians should periodically assess patients' understanding of emergency pump protocols.
If Jen awakens with a fasting blood glucose level of 280 mg per dL, she may administer a correction bolus to target the glucose level to 150 mg per dL. In Jen's case, her target blood glucose of 150 is 130 mg per dL below her current level. Knowing that her ISF is 1:30, Jen determines that she must provide an immediate bolus of 4.3 units for the correction. One hour after administering the bolus, another blood glucose level should be determined. If the blood glucose level is decreasing, she can assume that the infusion set line is patent and the pump mechanics are in order. However, if the 1-hour postcorrection blood glucose level is going higher despite the use of the supplemental bolus, she must proceed to troubleshoot the pump, infusion set, and the infusion site.
Hyperglycemia may result from a mismatch between the dose of insulin provided and the quantity of food consumed, a loss of insulin potency (incorrect shipping and packaging of mail-ordered insulin may result in reduced potency), an underlying infection resulting in an acute insulin-resistant state, an insulin-flow obstruction through the infusion set, or a pump mechanical malfunction. If a pump is subject to a static discharge, similar to a finger-tip shock one experiences in winter while walking on carpet, the pump parameters may be erased from the pump's internal memory. Most pumps will deliver an auditory or vibrating alarm if insulin delivery is interrupted. Insulin cannot be delivered if the infusion catheter is kinked within the insertion site. The pump will sense when abnormal pressure is required to pump insulin against an obstruction and subsequently emit an alarm.
When patients experience unexpected hyperglycemia, they should always fix the diabetes first by giving a subcutaneous correction dose of insulin via a syringe or pen device, targeting a 150 mg per dL blood glucose level. The infusion set and reservoir should be changed and an alternative infusion site chosen. Once the new infusion set has been inserted, the patient should carefully monitor blood glucose levels over the next 2- to 3-hour period, making certain that the hyperglycemia is not worsening (which could be indicative of a systemic infection) or that hypoglycemia has developed from using an excessive correction bolus.
TABLE 6-6 Managing Diabetic Ketoacidosis
If nausea or vomiting is present, immediately check your blood glucose and ketones. If your blood glucose is above 250 mg/dL and/or ketones are present: - Call your healthcare provider.
- Take an injection of a rapid-acting insulin analogue with a syringe or pen device (not through the pump). The amount should be the same as if you were taking a correction bolus.
- Change entire infusion set system (new reservoir, infusion set, and cannula). Troubleshoot the pump. If help is needed, call the 24-h Product Help Line (the phone number is on the back of your pump).
- Drink liquids with no calories every 30 min (for example, 8 oz. diet ginger ale, broth, water).
- Check your blood glucose and urine ketones in 1 h. Blood ketones can be detected via the Precision QID home blood glucose meter (Abbott) (http://www. diabeteshealthconnection.com/products/monitors/precision/precisionxtra.aspx).
- Continue to take insulin as discussed with healthcare professional.
- Call your healthcare professional immediately if your blood glucose and ketones are not decreasing or you are unable to drink fluids.
- If your blood glucose is less than 200 mg/dL and ketones are present, drink liquids containing calories (for example, juice or caffeine-free, non-diet soda). Also, additional insulin is usually required. Contact your healthcare professional for specific guidelines for insulin doses when ketones are present.
Patients should always be provided with a copy of their prescribed parameters in case the pump needs emergency reprogramming. A delay in reprogramming the pump may result in hyperglycemia and DKA. Patients can get assistance on reprogramming their pumps directly from the pump manufacturers' telephone help lines (Table 6-4).
Can Jen safely manage DKA while wearing an insulin pump?
Because insulin pumps use rapid-acting insulin analogues, an interruption in drug delivery will quickly result in hyperglycemia. Any patient in whom hyperglycemia develops (blood glucose >240 mg per dL) in association with nausea, vomiting, abdominal pain, and an altered level of consciousness should be evaluated for ketosis. Urine ketone test kits are readily available, and some home blood glucose monitors (Precision Xtra, Abbott) can be used to screen for the presence of ketone bodies. Table 6-6 lists the management strategies for DKA in pump patients.
Owing to the high rate of fetal mortality associated with DKA in pregnancy, pump users should consider injecting 0.2 U per kg of NPH at bedtime to prevent DKA if the infusion set becomes dislodged for any reason.22 This small amount of insulin will not affect nocturnal glycemia. Pregnant patients may choose to use alternative infusion sites such as arms or legs, as their abdomens expand in the third trimester.
How often should Jen change the infusion set and pump reservoir?
To prevent irritation and infection at the infusion-set insertion site while maintaining adequate insulin flow into the subcutaneous insulin depot site, the infusion sets and reservoirs should be changed every 2 to 3 days. Sites may need to be changed sooner if discomfort or irritability develops at the insertion site. A steady increase in glucose levels may be indicative of impaired insulin absorption from the infusion-site subcutaneous depot. After inserting a new infusion set into the skin, the patient should provide a immediate 1- to 2-unit bolus of insulin to prime the site and establish a depot from which the insulin may be continuously absorbed. The priming bolus will not reduce the patient's blood glucose level. Infusion sets should be changed only during the waking hours, because an improperly inserted set can result in significant hyperglycemia, which may go undetected while the patient sleeps. Two hours after the new infusion set is inserted, a blood glucose level should be determined to make certain that the patient's glycemic control is within the prescribed target range. Otherwise, a correction bolus may be administered.
Pump batteries should be replaced every 30 days. Pumps will also emit a warning alarm advising the patient to change the batteries. Once the alarm sounds, the patient should change the batteries within 24 hours.
What precautions should Jen take when she travels with the pump?
Patients on insulin pumps who travel should always have the following emergency supplies in their possession, not in their checked baggage:
Backup blood glucose meter, lancets, lancet device, and strips
Glucose tablets to manage hypoglycemia
A list of the pump parameters
Extra pump and blood glucose meter batteries
Extra infusion sets and reservoirs (if necessary, the infusion sets can be placed through the skin manually, without the use of a self-insertion device)
Extra vial of insulin
Pen injector of rapid-acting insulin analogue plus extra needles
Food and water, in case access is limited
If going to a foreign country, be sure to carry information on who to contact in case of a pump malfunction. The U.S. pump manufacturers can assist patients with this information.
Remember that insulin preparations available outside of the United States are not identical to what is marketed in this country. Plan accordingly when preparing to travel with any insulin-delivery devices.
TABLE 6-7 Sick-day Protocol for Pump Users
- Monitor your blood glucose every 2 h while you are awake and at least once overnight.
- Depending on the results of blood glucose and ketone testing, you may need to make periodic insulin adjustments while you are sick.
- You will still require insulin, even when you are unable to eat.
- If your blood glucose is above 250 mg/dL, follow the hyperglycemia protocol to avoid the potential of diabetic ketoacidosis from illness or infection.
- Remember to call your healthcare provider if your blood glucose is above 250 mg/dL and ketones are present.
How should Jen manage sick days while on the pump?
Sick days are easily managed with pump therapy (Table 6-7).
Temporary basal rates may need to be set for mild persistent hyperglycemia that may occur in response to a viral infection or the temporary use of corticosteroids (see Fig. 7-12, showing a patient with Bell palsy who is taking prednisone). Patients should be instructed that mealtime boluses are not mandatory and should be programmed when a meal is consumed. If a patient is unable to eat, the basal insulin flow can be sufficient to control glycemia. Supplemental insulin may be used every 4 to 6 hours to control acute hyperglycemia.
What is the proper protocol for treating hypoglycemia?
Mild symptomatic hypoglycemia is managed by patients using the rule of 15 (Fig. 6-8). Pump patients and their families should always be trained in using a glucagon emergency kit for reversing severe hypoglycemia.
Fine-tuning Pump Therapy
Patients should be re-evaluated within 1 to 2 weeks of pump initiation to begin their parameter adjustments. Basal and bolus doses are adjusted according to the patient's blood glucose measurements taken before meals, 2 hours after meals, at bedtime, at midnight, and at 3 AM (Table 6-8).
The basal rate should be increased or decreased by 0.1 units per hour to keep the preprandial and overnight blood glucose levels within a 30-mg glucose excursion from baseline. If the glucose level increases more than 30 mg per dL from the 3 AM measurement to the prebreakfast measurement, a second basal rate (approximately 1.5 times that of the initial basal rate) is added for 4 to 6 hours, beginning 2 to 3 hours before the usual breakfast time. Daytime basal rates are adjusted only if significant glucose excursions occur more than 4 hours after the mealtime bolus. To guide daytime basal-rate adjustments, the
P.306
patient should be instructed to delay or skip a meal while monitoring blood glucose levels every 2 hours in the fasting state. This should be done periodically to ascertain that basal rates are not set too high or too low.
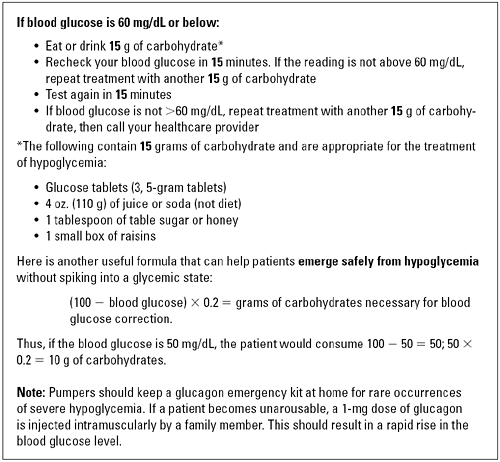 |
Figure 6-8 Managing Hypoglycemia. The Rule of 15 and emerging safely from hypoglycemia. |
Bolus doses are adjusted according to blood glucose measurements taken 2 hours after meals. Patients are provided specific guidelines for adjusting insulin boluses or the I/C to maintain reasonable glucose excursions. If the blood glucose level 2 hours after eating is 40 mg or less higher than the premeal glucose level, the I/C was correctly determined. However, if the 2-hour postprandial level is greater than 40 mg per dL, a higher I/C for a given meal should be used the next time the patient eats that same meal. One common source of frustration among patients who attempt, yet fail to count carbohydrates correctly, is that this method of premeal insulin determination is best used when blood glucose levels are within the targeted range of 70 to 170 mg per dL. Carbohydrate counting when a patient's blood glucose values
P.307
are consistently more than 300 mg per dL, for example, will only result in persistent hyperglycemia. Patients can use both carbohydrate counting and their ISFs to determine their premeal insulin dose; a calculation that is performed automatically by the Bolus Wizard feature on Medtronic pumps.
TABLE 6-8 Adjusting Pump Parameters | ||
|---|---|---|
|
Using electronic home blood glucose download data is very helpful in determining the efficacy of the patient's bolus regimen. If at least 50% of the patient's postmeal glucose levels (taken 2 hours after eating) for each meal are within the 70 to 170 mg per dL range, the patient boluses are adequate. If more than 3% of the patient's postprandial levels are less than 70 mg per dL, the patient should be advised to reduce the total bolus for that particular meal by 10% to 20% to avoid hypoglycemia. Patients who show persistent elevations in postprandial glucose levels should increase their premeal insulin bolus or consider working harder on correctly counting carbohydrates.
Patients with an A1C level greater than 8.5% should focus their pump adjustments on improving their fasting blood glucose levels. As A1C levels
P.308
become normalized (A1C less than 8.5%), adjustments in boluses will further improve the A1C levels:
Change I/C for certain meals
Change ISF
Determine and use one's personal lag time
Carry extended bolus over a 2- to 3-hour period
Provide 25% of total bolus immediately with the onset of the meal and 75% over the following 2- to 3-hour period
Consider using pramlintide to improve glycemic variation
Case 5
This patient received her insulin pump on July 20 (Fig. 6-9). Because she traveled a long distance to the office, the patient was advised to call in her blood glucose levels on July 24. The basal rates were subsequently adjusted over the phone. On August 9, the patient's home blood glucose meter was downloaded at the office. The adjustments made on the pump parameters resulted in significant improvement in glycemic control, with 80% of her blood glucose readings in the range of 70 to 170 mg per dL. Her A1C decreased from 9% to 6.8% with the use of the insulin pump.
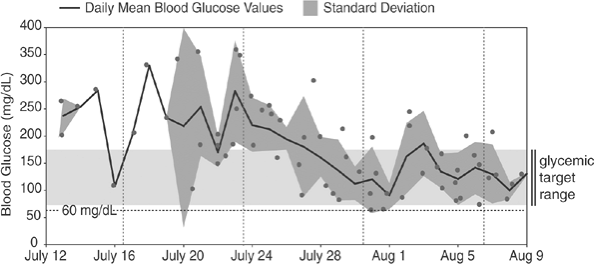 |
Figure 6-9 Blood Glucose Levels from a New Pump Patient. The levels indicate a decrease in AIC. |
Patients who exhibit frequent episodes of hypoglycemia (>3% of the meter downloads are <60 mg per dL or doubling the meter SD for any mealtime readings is greater than their average blood glucose levels at that time) reduce their insulin doses. Many of these patients may be stacking insulin by administering boluses of additional drug before the absorption of the prior dose has been completed. Others may simply be overcalculating the amount
P.309
of insulin they actually need for any given meal. Some patients are just as fearful of being hyperglycemic as others are of being hypoglycemic. No matter what the cause of the hypoglycemia, the incidence of low blood glucose levels must be reduced. Patients should be treated to the lowest yet safest level of A1C that they can achieve.
Long-term Follow-up of Insulin Pump Patients
Once the patient is stabilized on the pump parameters, patients may be re-evaluated every 3 to 5 months, with attention paid to the following issues:
Home blood glucose monitoring electronic logs should be carefully inspected to determine if fine-tuning of the basal and boluses is necessary.
Review the protocol for managing hypoglycemia (Fig. 6-8), hyperglycemia (using supplemental insulin), prevention, and initial treatment of DKA (Fig. 6-5), sick-day management (Table 6-7), and the emergency protocol for pump malfunction (Table 6-8).
Review the pump parameters (Table 6-8) to make certain that the physician-directed basal rates have not been changed by the patient. Patients should understand that they are in charge of their mealtime and supplemental boluses. However, the medical team should direct any alterations in basal rates.
Evaluate the areas surrounding the infusion sites to make certain that abscesses and scar tissue are not forming. Areas of inflammation may be indicative of a localized insulin allergy, which may require switching to a different short-acting insulin analogue.
Insulin stacking should be discussed, especially for patients experiencing more than 5% hypoglycemia on their electronic logs.
A1C levels should be monitored 3 to 4 times a year.
Remember that approximately 50% of the patient's total daily insulin dose should be provided as bolus insulin, and 50%, as basal insulin. If the patient is providing multiple correction and supplemental boluses, the ratio of basal to bolus insulin will be far less than 1:1. The basal rates will have to be analyzed and readjusted.
Table 6-9 discusses questions that should be explored for pump patients having difficulty achieving a targeted A1C less than 7%.
Exercising with an Insulin Pump
Exercise is beneficial for patients with diabetes. The use of an insulin pump can facilitate the administration of insulin to maintain normoglycemia and avoid dangerous hypoglycemia after exercise. Exercise can result in initial hyperglycemia, followed by postexercise hypoglycemia. As exercise begins, free fatty acids are released from the adipose tissue as an initial source of
P.310
energy. Within 10 minutes of beginning exercise, the liver accelerates the production and release of glucose, which provides skeletal muscles with a sustainable source of energy. If glucose levels increase in an insulinopenic patient who is already hyperglycemic (blood glucose level >240 mg per dL), exercise-induced ketosis may occur. Some individuals may need to give a small insulin bolus at the onset of exercise if their blood glucose level is more than 240 mg per dL.
TABLE 6-9 Questions to Ask Pump Patients When Their Follow-up A1C Levels Are >7% | |
|---|---|
|
An insulin pump can be worn during most exercise and sports activities. Precautions must be addressed in patients involved in contact sports. Patients need to be taught to make safe basal-rate alterations in preparation for exercise and how to prevent inadvertent disruption/dislocation of catheter or pump during intense exercise or sports activities.
Adjustments in basal insulin delivery while exercising should be individualized. The basic rule of thumb is that a well-conditioned athlete who is participating in the same activity for which he has been training rarely needs to alter the rate of insulin delivery or suspend the pump while exercising. However, a poorly conditioned individual who is embarking on a moderate or
P.311
more intensive exercise regimen will need to reduce or suspend the insulin delivery while active. Patients should always check the blood glucose levels before beginning exercise and every 30 minutes during exercise to determine how a given activity might affect their glycemic control. The blood glucose should also be monitored immediately after exercising as well as 4 hours after completing the activity in case a delayed hypoglycemic response to the exercise develops. Blood glucose levels should be maintained in the target range of 120 to 180 mg per dL during the exercise program.
When exercising in the basal state (before breakfast or more than 4 hours after the previous meal), the basal infusion rate is initially reduced by 50%. Subsequently, the adjusted temporary basal infusion rate is adjusted according to self-monitoring blood glucose (SMBG). For example, if a patient with a usual basal rate of 1.6 units per hour jogs or cycles for 1 hour in the early morning before breakfast, this rate is reduced to 0.8 units per hour. After individual SMBG measurements, this temporary basal infusion rate can be further adjusted. Some athletes choose to decrease the infusion rate for 30 to 60 minutes during the postexercise period because of increased insulin sensitivity. In addition, the subsequent mealtime bolus is often reduced by 30% to 50%. When the insulin pump is continued during any exercise, the patient should monitor for hyperglycemia due to malfunction or dislodging of the infusion catheter. To limit weight gain, many exercising patients choose insulin adjustment rather than supplemental food intake.
With prolonged endurance exercise (eg, hiking, walking, running, cycling), a steady state develops, and patients maintain plasma glucose levels within a predictable range while balancing carbohydrate intake and exercise. They may balance a low-dose temporary basal infusion rate with intermittent food to offset caloric expenditure. In this situation, the temporary basal rate is set at 0.1 to 0.2 units per hour. With experience, many patients maintain glucose levels between 100 mg per dL and 140 mg per dL during several hours of activity.
A general guideline for carbohydrate consumption with exercise is 15 to 30 g every 30 to 60 minutes. Less insulin and fewer calories are required with improved fitness level and favorable environmental conditions. When the individual is exercising during weather extremes, adjustments in both basal insulin and caloric intake are often necessary. Exercising at a lower fitness level in cold weather may require more calories, whereas exercising in a hot environment may lead to hypoglycemia due to poor appetite and reduced caloric intake, suggesting a lower insulin requirement.
Suggestions for Exercising While on an Insulin Pump
Check blood glucose at the time of exercise. The target blood glucose level for beginning exercise is 120 to 180 mg per dL. If the blood glucose is greater than 240 mg per dL, check for urine ketones. If positive (+), delay or postpone exercise until ketones clear and blood glucose levels return to
P.312
the target range. If ketones are negative (-), consider giving a small bolus of insulin before beginning exercise.If blood glucose levels are less than 120 mg per dL before exercise, consume 15 g of carbohydrates and begin a temporary basal rate (TBR), as described later.
Check blood glucose 30 to 45 minutes into exercise to evaluate for hyperglycemia.
Check blood glucose 2 to 4 hours after completion of exercise to monitor for hypoglycemia.
In general, physical conditioning over time reduces glycemic variability associated with exercise. Untrained athletes may experience more glucose fluctuations and become hypoglycemic after concluding exercise.
If exercise times are consistent, basal rates may be lowered beginning at the onset of anticipated exercise and continued for 2 to 4 hours after exercise is completed.
Patients may choose to set a TBR while exercising. Reduce the prescribed basal rate by 50% to 75% while exercising, and continue this TBR for 1 to 2 hours after finishing the training.
Use of Insulin Pump Therapy for Patients with Type 2 Diabetes
Patients with T2DM can successfully be trained in an outpatient setting to manage their diabetes by using insulin pump therapy. A study by Raskin et al.32 demonstrated that pumping insulin was as safe and effective at improving glycemic control in insulin-naive patients as was MDIs. This 24-week open-label study assigned 136 patients to initiate insulin therapy by using either CSII with insulin aspart or basal-bolus MDIs by using NPH plus aspart with pen injectors. Hypoglycemic events were mild, and the recorded events equally distributed between the two groups. The treatment-satisfaction surveys favored pumping over MDIs at the end of the study. Ninety-three percent of the CSII-treated patients believed that the pump was convenient, simple to use, and offered lifestyle flexibility. The significantly greater satisfaction scores reported by CSII-treated subjects suggest that CSII may be the preferred method of intensive insulin therapy for capable patients with T2DM who desire optimal glycemic control.
Summary
Intensive diabetes management can be achieved with the use of insulin pump therapy. Compared with MDIs, CSII has better insulin pharmacokinetics, less variability in insulin absorption, and decreased risk of hypoglycemia. Patients using insulin pumps enjoy greater lifestyle flexibility and often become more proactive in their approach to diabetes self-management. Although more
P.313
expensive than MDIs, pump therapy offers patients a much more physiologic approach to controlling their diabetes. Careful evaluation of pump candidates, patient education, and timely follow-up visits are vital to the success of pump therapy.
As we enter the dawn of a new age of diabetes management with mechanical sensors and pumps working in unison, improvement in glycemic control for patients with diabetes seems to be heading in a positive direction. Until a closed-loop feedback system between the pump and sensor is developed and marketed, basal bolus insulin therapy using an insulin pump remains state of the art. PCPs should take a proactive role in promoting and managing patients using insulin pump therapy.
P.314
Appendix 6-1
Sample Letter of Medical Necessity, Which Can Be Sent to a Third-party Payor for a Patient in Need of Insulin Pump Therapy
Dear Sir or Madam:
__________is a ___-year-old patient who was diagnosed as having diabetes at age__. The patient has the following diabetes-related complications: (hypoglycemic unawareness; wide glycemic variations; nocturnal hypoglycemia; coronary artery disease; peripheral vascular disease; chronic kidney disease, stage___; autonomic neuropathy; peripheral neuropathy; pregnancy). The patient has been using physiologic insulin-replacement therapy since_____. Despite practicing carbohydrate counting, medical nutritional therapy, participating in a healthy-lifestyle program, and performing frequent blood glucose monitoring, his or her glycemic control could be optimized with the use of continuous subcutaneous insulin infusion (pump) therapy. I believe that the patient's short- and long-term glycemic control, as well as his or her quality of life will be enhanced with the use of an insulin pump. I am therefore recommending that a (specific pump name here) pump be approved for his or her immediate use.
It has become apparent in recent years that the present conventional treatment of the type I diabetes patient who requires insulin is often unable to control the blood glucose levels adequately. Now general agreement exists among diabetes specialists that the lifespan of patients with diabetes mellitus can be increased and the incidence and severity of diabetic complications, such as blindness and kidney disease, can be reduced and/or prevented by attention to maintaining the blood glucose level at as nearly normal levels as possible throughout the patient's lifetime.
Further, recent studies indicate that the onset of diabetic complications, such as nephropathy and retinopathy, may be delayed by using intensive insulin therapeutic regimens to maintain near-normal blood sugar levels.
Beginning in the late 1970s, multiple investigators have reported that near-normal blood glucose control could be achieved with an external insulin pump.
Portable insulin-pump therapy is cost effective because it can reduce the number of hospitalizations necessary for uncontrolled brittle diabetes mellitus and/or hypoglycemia.
Theoretically, it can also decrease the number of hospitalizations necessary for the treatment of the complications of diabetes mellitus. Diabetes mellitus is the leading cause of blindness in the United States, accounts for 75% of all nontraumatic amputations, and will account for 50% of all patients who reach end-stage renal disease and will require either renal dialysis or renal transplantation or both in the 1990s.
The insulin pump is an open-loop device that instills subcutaneously a predetermined amount of insulin (the basal rate). The basal rate can be programmed into the pump to reflect changes in basal insulin requirements as they are affected by daily activity, stress, infection, and other parameters that change the blood sugar level.
Additionally, the pump has a bolus feature that allows the patient to instill varying amounts of insulin to stimulate the physiologic response of the pancreas to meals or other hyperglycemic events.
Pump therapy thus differs from conventional therapy in that it is a much more physiologic form of treatment for patients with diabetes mellitus. It has further advantages in that because only regular- or short-acting insulin is infused, the patient has
P.315
much more liberty to go about his or her daily activities without fear of absorption peaks of insulin causing hypoglycemia.
The device in (this patient's) case is a necessity and not merely a convenience. He or she has been unable to bring the diabetes under good metabolic control despite strict adherence to a complex regimen of diet, exercise, self-monitoring of blood glucose, and multiple daily injections of three different types of insulin.
Use of this insulin pump will allow me to assist this patient in achieving the lowest and safest possible A1C levels that have been recommended by numerous professional consensus guidelines, including the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE).
If you require any further information, please do not hesitate to contact me.
Sincerely,
P.316
P.317
Appendix 6-2
 |
P.318
Appendix 6-3
 |
Commonly seen Beta Cell Tests:
ICA (Islet Cell Autoantibody)
GAD (Glutamic acid decarboxylase)
IAA (Insulin Auto Antibody)
IA-2 (RIA)
All test results should be documented by obtaining copies of actual lab reports.
Patient must also meet all other Medicare criteria.
These criteria are also required for ongoing pump supplies.
P.319
References
1. Weissbert-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care. 2003;26:1079 1087.
2. Graff MR, Rubin RR, Walker EA. How diabetes specialists treat their own diabetes: findings from a study of AADE and ADA membership. Diabetes Educ. 2000;26:460 467.
3. Boland EA, Greg M, Oesterle A, et al. Continuous subcutaneous insulin infusion: a new way to lower risk of severe hypoglycemia, improve metabolic control, and enhance coping in adolescents with type 1 diabetes. Diabetes Care. 1999;22:1779 1784.
4. Weinzimer S, Ahern J, Boland E, Steffen A, Tamborlane W. Continuous subcutaneous insulin infusion is safe and effective in very young children with type 1 diabetes: program and abstracts of the 63rd Scientific Sessions of the American Diabetes Association; June 13 17, 2003. New Orleans, Louisiana: Abstract 1744-P.
5. Millennium Research Group. US Markets for Drug Delivery 2005. Toronto: Millenium Research Group, 2004.
6. Pickup JC, Keen H, Parsons JA, Alberti KGMM. The use of continuous subcutaneous insulin infusion to achieve normoglycaemia in diabetic patients [abstract]. Diabetologia. 1977;13:425A.
7. Bode BW, Tamborlane WV, Davidson PC. Insulin pump therapy in the 21st century: strategies for successful use in adults, adolescents, and children with diabetes. Postgrad Med. 2002;111. Online: URL: http://www.postgradmed.com/issues/2002/05_02/bode3.htm. Accessed 11/13/05.
8. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977 986.
9. Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomized controlled trials. Br Med J. 2002;324:705 710.
10. Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1C. Diabetes Care. 1994;27:2590 2596.
11. Doyle EA, Weinzimer SA, Steffen AT, et al. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care. 2004;27:1554 1558.
12. Unger J. A primary care approach to continuous subcutaneous insulin infusion. Clin Diabetes. 1999;7:113 120.
13. Bode BW, Steed RD, Davidson PC. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type 1 diabetes. Diabetes Care. 1996;19:324 327.
14. Chantelau E, Spraul M, Muhlhauser I, et al. Long-term safety, efficacy and side effects of continuous subcutaneous insulin infusion treatment for type 1 (insulin-dependent) diabetes mellitus: a one centre experience. Diabetologia. 1989;32:421 426.
15. Shade DS, Valentine V. To pump or not to pump. Diabetes Care. 2002;25:2100 2102.
16. Aragona M, Giannarelli R, Coppelli A. Improvement in retinopathy in type 1 diabetic patients treated with continuous subcutaneous insulin infusion: program and abstracts of the 63rd Scientific Sessions of the American Diabetes Association; June 13 17, 2003, New Orleans, Louisiana, Abstract 435-P.
17. Bode BW, Gross T, Steed D, et al. A recent assessment of continuation rates for continuous subcutaneous insulin infusion (CSII) [abstract 393]. Diabetes. 1998;21(suppl 1):393.
18. Gabbe SG, Holing E, Temple P, Brown ZA. Benefits, risks, costs, and patient satisfaction associated with insulin pump therapy for the pregnancy complicated by type 1 diabetes mellitus. Am J Obstet Gynecol. 2000;182:1283 1291.
19. Kanakis SJ, Watts C, Leichter SB. The business of insulin pumps in diabetes care: clinical and economic considerations. Clin Diabetes. 2002;20:214 216.
20. Abraham MR, al-Sharafi BA, Saavedra GA, Khardori R. Lispo in the treatment of insulin allergy. Diabetes Care. 1999;22:1916 1917.
P.320
21. Ottensen JL, Nilsson P, Jami J, et al. The potential immunogenicity of human insulin and insulin analogues evaluated in transgenic mouse model. Diabetologia. 1994;37:1178 1185.
22. Unger J, Marcus A. Insulin pump therapy: what you need to know. Emerg Med. 2002;34: P24-P33.
23. Unger J. Preconception management of women with type 1 diabetes. The Female Patient (OB/GYN edition). 2001;26:45 51.
24. Lac G, Chamoux A. Elevated salivary cortisol levels as a result of sleep deprivation in a shift worker. Occup Med. 2003;53:143 145.
25. Unger J. Intensive management of type 1 diabetes. Emerg Med. 2001;33:30 42.
26. Alemzadeh R, Ellis JN, Holzum MK, Parton EA. Comparison of continuous (subcutaneous insulin infusion (CSII) with flexible multiple daily insulin (FMDI) regimen using insulin glargine in pediatric type 1 diabetes mellitus: program and abstracts of the 63rd Scientific Sessions of the American Diabetes Association, June 13 17, 2003. New Orleans, Louisiana. Abstract 434-P.
27. Wilson D, Buckingham B, Kunselman E, et al. A two center randomized controlled trial of insulin pump therapy in young children with diabetes: program and abstracts of the 63rd Scientific Sessions of the American Diabetes Association, June 13 17, 2003. New Orleans, Louisiana: Abstract 1745-P.
28. Bryden KS, Neil A, Mayou RA, et al. Eating habits, body weight, and insulin misuse: a longitudinal study of teenagers and young adults with type 1 diabetes. Diabetes Care. 1999;22: 1956 1960.
29. Lenhard M, Maser R. Success is relative: CSII in challenged patients: program and abstracts of the 63rd Scientific Sessions of the American Diabetes Association; June 13 17, 2003. New Orleans, Louisiana: Abstract 2129-PO.
30. Hirsch IB. Intensive treatment of type 1 diabetes. Med Clin North Am. 1998;82:689 719.
31. Knee TS, Seidensticker DF, Walton JL, Solberg LM, Lasseter DH. A novel use of U-500 insulin for continuous subcutaneous insulin infusion in patients with insulin resistance: a case series. Endocr Pract. 2003;9:181 186.
32. Raskin P, Bode BW, Marks JB, et al. Continuous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes. Diabetes Care. 2003;26:2598 2603.
EAN: 2147483647
Pages: 19