3 - Glaucoma
Editors: Tasman, William; Jaeger, Edward A.
Title: Wills Eye Hospital Atlas of Clinical Ophthalmology , The, 2nd Edition
Copyright 2001 Lippincott Williams & Wilkins
> Table of Contents > Chapter 3 - Glaucoma
function show_scrollbar() {}
Chapter 3
Glaucoma
George L. Spaeth
Augusto Azuara-Blanco
Jeffrey D. Henderer
L. Jay Katz
Marlene R. Moster
Jonathan S. Myers
Douglas J. Rhee
Annette K. Terebuh
Richard P. Wilson
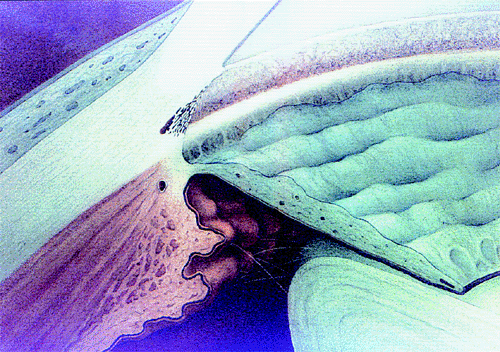 |
| A normal anterior chamber angle of a blue-eyed human eye. |
P.92
What is Glaucoma?
We define glaucoma in this chapter as the process of ocular tissue damage caused at least partially by intraocular pressure (IOP). Note that elevated IOP by itself is not a criterion for diagnosing glaucoma. Note also that, while IOP plays a pathogenetic role in every glaucoma, IOP need not be elevated in the sense of being above average. Glaucoma can be associated with IOP as low as 10 mm Hg or perhaps even lower than that. (See sections on IOP, pages 97,125 126).
A second consideration is whether glaucoma can occur without tissue damage. Tissue damage can occur without being detected by current methods. For example, many optic neurons must become nonfunctional before visual field loss is detectable. Also, certain levels of IOP are so certain to cause damage that it would seem sensible to use the label glaucoma. However, these arguments are not sufficient: first, if damage cannot be detected it is not certain that it will occur; and second, there is great variability in the level of IOP that can be tolerated. We limit the definition of glaucoma, then, to situations in which tissue damage is detectable. If it seems virtually certain that the person will develop glaucoma damage, we use the term preglaucoma. If there is a question about whether tissue damage has occurred, the phrase glaucoma suspect is employed.
Glaucoma is a group of conditions. Even entities characteristically considered as specific diagnostic groups, such as primary open-angle glaucoma (POAG), are usually composites of different conditions.
To repeat, then, glaucoma is the process of ocular tissue damage caused at least in part by IOP.
Classification: Terminology and Risk Factors
The broad classification used in this chapter includes glaucoma, which indicates detectable tissue damage (Fig. 3.1); preglaucoma, which indicates some finding (e.g., IOP of 80 mm Hg) that makes it virtually certain that the patient will develop tissue damage; and glaucoma suspect, which means that there is something about the patient that raises the suspicion of glaucoma, but does not denote with certainty whether tissue damage has occurred. Glaucoma suspects are individuals in whom the development of glaucoma is considered to be more likely than in individuals who possesses none of the risk factors for glaucoma. The glaucoma suspect is further categorized as an individual who has a suspicious finding, such as an optic disc asymmetry, or an individual with a presumed predisposition to glaucoma because of some risk factor, such as a positive family history or pigment dispersion syndrome.
Stage, Stability, and Treatable Factors
This chapter is employing a different classification scheme than has been traditional (Table 3.1). Glaucoma can be classified
P.93
by the stage of the condition, the stability of the condition, and the treatable factors of the condition, including whatever is affecting the control of IOP. This newer classification recognizes that the treatment of glaucoma is based on attempts to avoid or minimize tissue damage. However, the mechanism for damage of the optic nerve in conditions as apparently similar as focal and POAG or primary angle-closure glaucoma varies. In POAG, the damage may be solely mechanical or almost solely vascular. In primary angle-closure glaucoma, in which the patient has low-grade recurrent attacks of angle-closure, the mechanism for optic nerve damage may be a mechanical change similar to that in POAG, but in the cases in which the elevation of IOP is extremely marked, the mechanism of damage is an acute ischemic optic neuropathy. Because treatment of the many conditions called glaucoma depends on the stage of the condition and the stability of the condition, those elements are stressed.
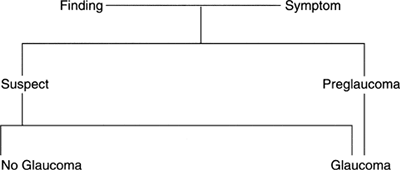 |
Figure 3.1. Classification of glaucoma. |
Table 3-1. Management Considerations: Factors that Influence the Type and Vigor of Therapy | |
|---|---|
|
Stage
The stage of glaucoma refers to amount of damage. Although this refers typically to the optic nerve, it also applies to the health of other tissues, such as the cornea.
In the earliest stage, the diagnosis is suspected but not definite. There is only a suspicious finding, such as an IOP above 30 mm Hg or moderate disc asymmetry. The patient has no ocular symptoms and no evidence of definite damage. The physician may be suspicious that damage has occurred or may occur but cannot ascertain this at the time of the evaluation. The second stage is early glaucoma. In early glaucoma, the patient is still asymptomatic, but there is definite damage, such as an unmistakable nasal step in the visual field, a notch in the optic nerve, a hemorrhage on the optic nerve, or a minimal but definite afferent pupillary defect. The third stage is glaucomatous disease. The patient has some symptoms. He or she notices the field loss, is aware of halos or pain, or notices something else that interferes with visual function. The fourth stage is worsening disease. The patient notices functional impairment and is aware that the functional impairment is worsening.
Stability
The stability of the glaucoma must also be assessed (Fig. 3.2). The patient's condition may be improving such that the symptoms or even the visual field or optic nerve head is improving. The patient's condition may be stable, with no detectable change on repeated evaluations of the objective or the subjective aspects of the patient's condition. The patient does not think that he or she is changing or worse, and the physician notices no change in the results of the physical evaluation. If the stability of the patient's condition is worsening, the symptoms are getting worse, or the optic disc or visual field is worsening. Sometimes, the stability of the condition is uncertain. When a patient is first evaluated, it may not be possible at that point to establish the stability of the patient's condition. Repeated evaluations of the history or the findings are usually necessary to determine the stability of the condition. If it cannot be determined, physicians must be honest about this difficulty and not mislead themselves or the patient.
The determination of stability is influenced by the patient's life expectancy. Many physicians cringe at this idea and feel that they are playing God. However, a fairly accurate estimate of life expectancy is not difficult to determine. Life insurance companies do a good job of anticipating life expectancy, and physicians can as well. Life expectancy can be determined by obtaining a thorough family history, including the ages at death of the parents and the health of siblings. Added to this is information about the patient's lifestyle. Does the patient smoke cigarettes, drink excessively, use drugs, or in some other way abuse his or her body? Is the patient overweight? Does the patient eat well? Does the patient exercise regularly? An estimate of the patient's general health is essential. This information can be obtained with relative ease; it must be known to make sense of the information related to the stability of the condition and the rapidity with which that stability is changing.
On the vertical axis of the graph in Figure 3.3 is the visual function, from normal to totally nonfunctional. On the horizontal axis is the duration of the patient's life, from birth to death; because this varies among individuals, it is not given in terms of years. The stability of the patient's condition and the rapidity with which that stability is changing are reflected by the slope of the line that describes
P.94
the course of the patient's glaucoma. For example, Patient 1 (Fig. 3.2A) has early glaucoma and a long life expectancy. This early-stage glaucoma would need minimal treatment if its stage were changing slowly (top line), but because of the patient's long life expectancy, it requires vigorous treatment if the stability is changing rapidly. In contrast, Patient 2 (Fig. 3.3) has a short life expectancy. If such a patient had early-stage glaucoma, treatment would be justified only if the rate of change were fairly rapid. If the patient had an advanced stage of glaucoma, as is shown on the graph, treatment must be vigorous, even if the rate of change is relatively slow.
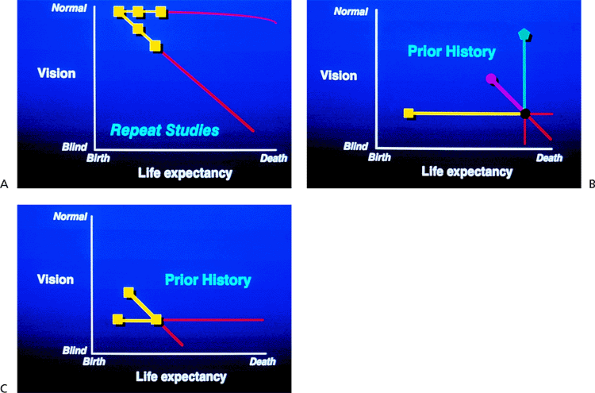 |
Figure 3.2. A: Stability of the glaucomatous disease is a factor in management. B: A careful history may be the only clue to the rate of change when the diagnosis is made late in the glaucomatous disease process. C: A patient diagnosed with advanced glaucoma but with a long life expectancy may demand vigorous treatment. |
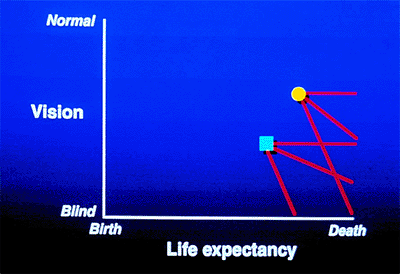 |
Figure 3.3. Two patients demonstrate the different rates of change in glaucoma. One patient has more advanced disease and a longer life expectancy. Even if the rate of deterioration is slow, this patient will become blind before death. The other patient, who has less severe disease and a shorter life expectancy, will retain vision until death, although the rate of deterioration is the same as that in the other patient. If the rate of deterioration is rapid, both patients would become blind before death. |
The stability of the patient's condition is affected by how rapidly the stage is changing. The development of a graph indicating this phenomenon can help the physician and the patient (Fig. 3.2A). In the first or second stages of glaucoma, the most important way of determining the rapidity of change is by repeating meticulous evaluations of the optic nerve. The optic nerve should be drawn carefully at every visit to determine whether change is occurring. Photographs or image analysis methods may be helpful. The visual field needs to be repeated sufficiently frequently to notice any early defect that may develop or any change that may occur. If it becomes apparent that the optic disc is worsening within a period of 3 months and still worse 3 months later, the condition is rapidly worsening. However, if a patient has an early visual field defect that shows only a tiny deterioration over 15 years, the rate of worsening is extremely slow.
It is essential to determine the rate of change in the patient's stability, because it is the rate of change that determines the vigor of therapy that is appropriate. When the glaucoma is advanced (Fig. 3.2B), a careful history is the
P.95
best method for determining the rate of change. Consider the patient whose condition is graphed in Figure 3.2C. The person has a long life expectancy and has already lost much vision (i.e., stage 3 or 4). If the condition is unchanging, the patient can expect to continue to see until he dies; if the condition is changing rapidly or even relatively slowly, vision will be lost before death. The importance of stability for prognosis and for determining the needed therapy is demonstrated in Figure 3.3, in which the different slopes depict different clinical outcomes. However, the clinical course is rarely linear, and there may be sudden downturns or plateaus in an extended graph of the disease.
Intraocular Pressure
Traditional classifications of glaucoma have been based on the mechanism for pressure elevation, but the pressure need not be elevated for glaucoma to develop. However, pressure is a factor, and the mechanism for pressure regulation is essential to understand, because alteration of the IOP is the major way in which the course of glaucoma can be altered.
Treatable Factors
Determining which factors are treatable is the next step in the management of the patient considered to have glaucoma or to be at significant risk for developing glaucoma (Table 3.2). An anterior chamber angle that is narrow enough to be occluded, or one in which occlusion has been proven because of the presence of peripheral anterior synechiae of the type that are seen in primary angle-closure glaucoma, is usually a sufficient justification for a peripheral iridotomy, assuming that damage to the optic nerve or other ocular disease is not present.
The method of lowering IOP is directed at the mechanism responsible for the control of IOP. In virtually all patients, this may involve medical or surgical therapy. In those with idiopathic POAG, the pigment dispersion syndrome, and the exfoliation syndrome, argon laser trabeculoplasty (ALT) is appropriate to consider as adjunctive or primary treatment. Surgical correction of an interference with aqueous flow is often appropriate if such a blockage is the primary problem.
Table 3-2. Risk Factors for Glaucoma | |
|---|---|
|
Factors other than IOP can be treated. Methods of evaluating the adequacy of blood flow to the optic nerve are still rudimentary, but it seems likely that treatments that affect blood flow, especially those that limit vasospasm, will become a standard part of the treatment of patients with glaucoma. Certain aspects of a patient's lifestyle affect the patient's glaucoma, and it is appropriate to direct attention toward some of these. Patients with low-tension glaucoma who have a sedentary lifestyle have a more rapid progression of their glaucoma than patients who exercise. Exercise can lower IOP by about 4 mm Hg. In Japan, obesity was found to correlate strongly with progressive glaucomatous nerve damage. Malnutrition sensitizes the nerve to further damage. Systemic hypertension should be avoided, as should sudden decreases in blood pressure.
Risk Factors
Glaucoma is a progressive disease. However, the rate at which it progresses varies markedly from person to person. It was once believed that the only important factor to be controlled was the IOP, but it has become clear that many other factors affect the course of the disease (Table 3.2). Because the major damage that occurs in most persons with glaucoma is deterioration of the optic nerve, the most important risk factor is the ability of the optic nerve to resist damage. Because most glaucomas are chronic conditions and because they are usually responsive to treatment, the ability of the patient to manage his or her own life is a critical factor in determining whether the patient's glaucoma deteriorates. The patients with the best prognoses are those who know how to develop a relationship with the physician in which the physician acts as a junior partner, who take primary responsibility for their health, and who do those things most likely to keep them healthy.
Intraocular Pressure and Glaucoma
For many years, IOP was considered the definition of glaucoma. Persons with pressures two standard deviations above the population norm (usually 21 mm Hg) had the disease, and those that fell within the normal range did not. In more recent years, this view has steadily lost favor as several lines of evidence indicate that glaucoma can exist at normal levels of IOP and persons with elevated levels of pressure may not have the disease. Yet there is compelling evidence
P.96
to indicate that IOP is somehow related to this disease, even for persons who have glaucomatous damage with a normal IOP. In fact current thinking views glaucoma as ocular tissue damage related in some way to IOP, and IOP is a risk factor, perhaps even the most important risk factor, for developing glaucoma.
Evidence for IOP as a component of the pathogenesis of glaucoma comes from several directions. First, population-based glaucoma surveys indicate that the prevalence of glaucoma and the probability of glaucoma increase as the level of IOP increases. Second, in population studies of ocular hypertension, the incidence of conversion to glaucoma rises with increased IOP. This has been demonstrated in different racial groups and in different locations around the world (Table 3.3). Third, persons who acquire a pressure asymmetry in both eyes, if they develop glaucoma, tend to develop it in the eye with the higher pressure. Fourth, investigators have repeatedly demonstrated in controlled randomized clinical trials of glaucoma therapy that persons being treated for glaucoma seemed to do worse if their pressures were elevated compared to those who pressures were reduced. Persons who had their pressures reduced had lower rates of progression, and if the IOP could be lowered to the low-normal range (usually considered less than 15 mm Hg), the rate of progression was slowed markedly. Finally, the Normal Tension Glaucoma, a randomized, prospective, clinical trial, more conclusively demonstrated the role of IOP in glaucoma. This landmark clinical trial indicated that an IOP reduction of 30% could significantly reduce the rate of progression of so-called normal-tension glaucoma. This
P.97
finding has provided the most convincing clinical evidence to indicate that IOP is a component of glaucoma.
Table 3-3. Natural History of Ocular Hypertension | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
However, despite the importance of IOP, there is likely more to the pathogenesis than pressure. Evidence for this comes from the fact that IOP above the population norm is not uncommon, and yet the vast majority of those with elevated IOP do not have, and will never develop, glaucoma (Table 3.3). There is still no consensus on how to approach patients with elevated IOP and no evidence of glaucoma. Evidence both for withholding and starting treatment has been published. A large multicenter, prospective, randomized, controlled trial is currently underway to investigate whether there is any benefit to IOP reduction in this class of patient. Further evidence comes from clinical experience that indicates that despite pressure reduction, even very significant pressure reduction, some patients continue to worsen. Additionally, persons with a history of migraine headache and Raynaud's syndrome who have presumed vasospastic disease may be at increased risk for open-angle glaucoma. Certainly genetics is related to glaucoma and may or may not be related to IOP. The optic nerve itself may be susceptible to injury secondary to problems with glial cells and the extracellular matrix. Myopia may be a risk factor as well, and is unlikely to be related to IOP.
Our current understanding of the relationship between IOP and glaucoma has evolved considerably and is still evolving. Convincing evidence has finally arrived to implicate IOP in the pathogenesis of the disease, yet clinical experience indicates that IOP is not the whole story. Genetic defects, blood flow defects, optic nerve structural defects or problems related to ocular neurotoxins and neurotrophic growth factors may yet be found to be important in the pathogenesis of glaucoma. Important animal research and human clinical trials investigating novel non pressure-related treatments of glaucoma are currently underway. For now, however, despite this shift in thinking, the fact that IOP remains an important component of glaucoma means that, from a treatment standpoint, the goal is still a reduction of IOP.
Evaluation of the Anterior Chamber Angle
Evaluation of the anterior chamber angle is essential for the diagnosis and management of glaucoma. It is important for the practitioner to recognize the normal anterior chamber angle (Fig. 3.4A-D) and to use a consistent method for grading the anterior chamber angle (Figs. 3.5, 3.6, 3.7). The angle shown in the photograph in Figure 3.4D is wide open, with no pigmentation of the posterior trabecular meshwork. It is graded E40c. This includes an estimate of the place where the iris inserts onto the inner wall of the eye, of the depth of the peripheral anterior chamber angle, and of the peripheral curvature of the iris. All three of these factors must be evaluated if a meaningful description of the angle configuration is to be obtained. These measurements can be easily and rapidly recorded using the system described.
Narrow Angles and Optically Closed Angles
Figure 3.8A shows an optically closed angle (i.e., Goldmann lens). Figure 3.8B shows the angle opened by indentation gonioscopy. A narrow angle viewed through the Zeiss lens is seen in Figure 3.9A. The posterior trabecular meshwork is just barely visible. With Zeiss lens indentation gonioscopy, the angle can be deepened; extensive adhesions are present and can now be seen (Fig. 3.9B).
Angle Grading System
The exact nature of the angle configuration is easily described using the grading system shown in Figures 3.4, 3.5, 3.6, 3.7, 3.8, 3.9. The angle shown in Figure 3.10 is partially optically closed. The parenthetic designation describes the deepest structure visible before indentation. The capital D refers to the site of adhesions between the iris and the inner wall of the eye.
Angle Pigmentation
The amount and character of pigment in the anterior chamber angle must also be determined, because it helps to provide an accurate diagnosis and is critical in deciding on appropriate treatment (Fig. 3.11). The examiner should determine whether the pigment is brownish, which is characteristic of the pigment in the normal angle (Fig. 3.11A) or that seen in the pigment dispersion syndrome, or black, which is more characteristic of the pigment in the exfoliation syndrome. The pigment in the pigment dispersion syndrome is phagocytized and is more characteristically limited to the trabecular meshwork (Fig. 3.11B), but in the exfoliation syndrome, the pigment is more prominent inferiorly than superiorly and tends to spread over the angle recess and up onto Schwalbe's line, where inferiorly it is seen as the highly characteristic Sampaolesi's line (Fig. 3.11C). Sampaolesi's line is a scalloped line of pigment on or anterior to Schwalbe's line and is diagnostic of the exfoliation syndrome.
Patients with minimal pigmentation of the posterior trabecular meshwork do not respond well to ALT, but patients with 2+ (on a scale of 0 to 4+) pigmentation or more in the trabecular meshwork are more likely to have a beneficial effect from ALT. Figure 3.11D shows an angle with 3+ pigmentation after ALT, with disappearance of the pigment in treated areas.
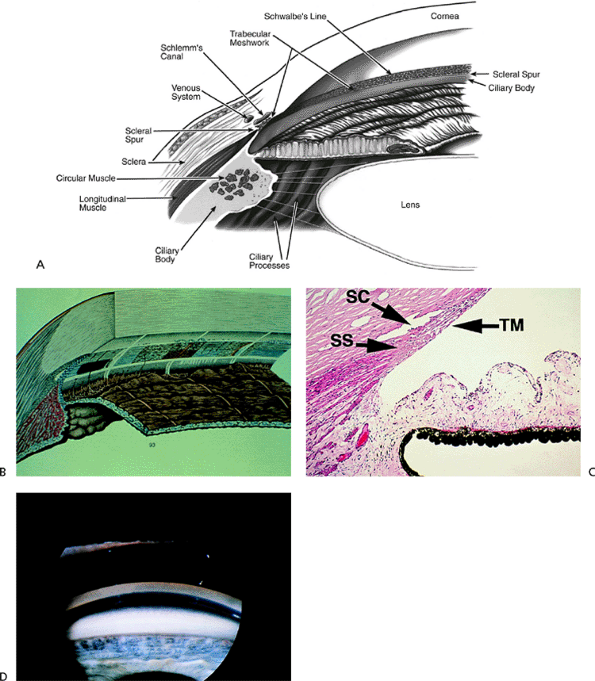 |
Figure 3.4. A: Aqueous pathway and anatomy of the angle. The production of aqueous humor is the result of a complex sequence of events that occurs in the ciliary processes. Secretion involves active transport and hydrostatic pressure that forces fluid, electrolytes, and small molecules through the fine capillaries and loosely connected cells of the double-layered epithelium (i.e., pigmented and nonpigmented) of the ciliary body and into the posterior chamber. Aqueous passes between the iris and the lens and into the anterior chamber and is responsible for supplying nutrients and removing metabolic waste from the nonvascularized structures of the anterior segment of the eye. The angle refers to the junction of the cornea and the iris, and contains the drainage pathway for 90% of the aqueous. This area cannot be seen directly; a contact lens or mirror must be used for visualization (i.e., gonioscopy). Schwalbe's line is considered the posterior limit of the cornea. The trabecular meshwork consists of a network of beams and interconnecting spaces and fibers that extends from Schwalbe's line to the iris insertion or scleral spur. The trabecular meshwork is actually a double layer of interlacing fibers; the innermost layer is the uveal trabecula, and the outermost layer is the corneoscleral trabecula. The trabecular meshwork is normally translucent, although it frequently contains pigment in its posterior portion. The scleral spur is a short extension of the sclera anteriorly and appears shiny white on gonioscopy. It serves as a point of insertion for the corneoscleral trabecular fibers and separates the meshwork from the anterior portion of the ciliary body, which is seen as a darker band in the posterior angle on gonioscopy. The trabecular meshwork is the most important structure to identify properly at the time of gonioscopy, because its nature (e.g., amount of pigment, presence of a neovascular membrane, closed angle, iris adhesions) determines the level of intraocular pressure (IOP) and the direction of therapy. The trabecular meshwork can best be recognized by noticing that it has depth, unlike any of the other angle structures. The examiner can see into the trabecular meshwork. Schlemm's canal lies outward from the trabecular meshwork and is not normally visible. However, sometimes the canal fills with blood during gonioscopy, and this can serve as a valuable landmark in identifying angle anatomy. Aqueous passes through the trabecular meshwork into Schlemm's canal and then to the episcleral venous circulation. B: An open angle showing normal variations in the pigmentation of the trabecular meshwork, iris processes, and configuration of the last roll of the iris. The dark, rectangular window (left) is the ideal position for a trabeculectomy excision for uncontrolled IOP. C: Histologic section of the normal angle. (SC, Schlemm's canal; SS, scleral spur; TM, trabecular meshwork.) (Courtesy of Ralph C. Eagle Jr., M.D., Philadelphia, PA.) D: Gonioscopic view of an open angle. The brown line just anterior to the iris is the anterior portion of the ciliary body. The remainder of the trabecular meshwork is devoid of pigmentation and barely visible. |
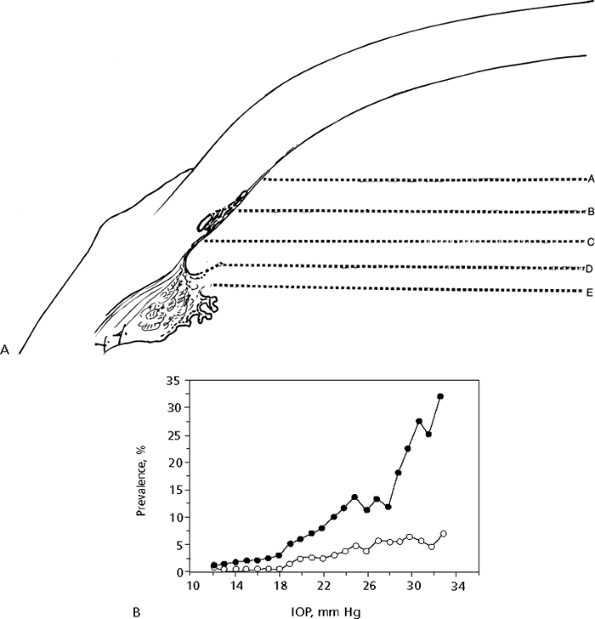 |
Figure 3.5. A: Angle grading system. Each capital letter (A through E) refers to the place where the iris attaches to the wall of the eye. B: Prevalence of primary open-angle glaucoma (POAG) in relation to screening intraocular pressure (IOP). The curve is smoothed using a running mean with window width of 7 mm Hg. (Caucasian American subjects, n=5,700 eyes [open circles]; African American subjects, n=4,674 eyes [closed circles].) (Reproduced from Alfred E. Sommer, M.D. Relationship between intraocular pressure and POAG among white and black Americans. Arch Ophthalmol 1991;109:1090, with permission. Copyright 1991, American Medical Association.) |
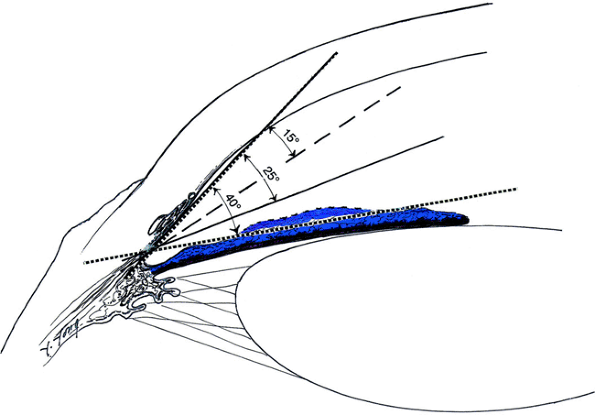 |
Figure 3.6. Angle grading system. The number of degrees refers to the depth of the peripheral anterior chamber. |
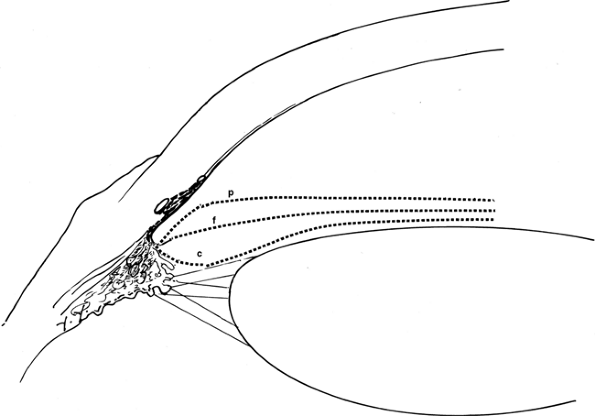 |
Figure 3.7. Angle grading system. Each lower-case letter refers to an estimate of the peripheral curvature of the iris. |
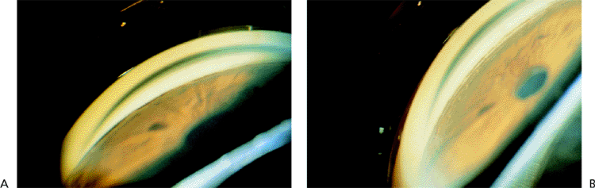 |
Figure 3.8. A: An optically closed angle as seen through a Goldmann three-mirror lens during gonioscopy. B: The same angle partially opened by the indentation during gonioscopy. |
 |
Figure 3.9. A: A narrow angle viewed through the Zeiss lens. The posterior trabecular meshwork is barely visible. B: During indentation, the angle is deepened, and adhesions can be seen. |
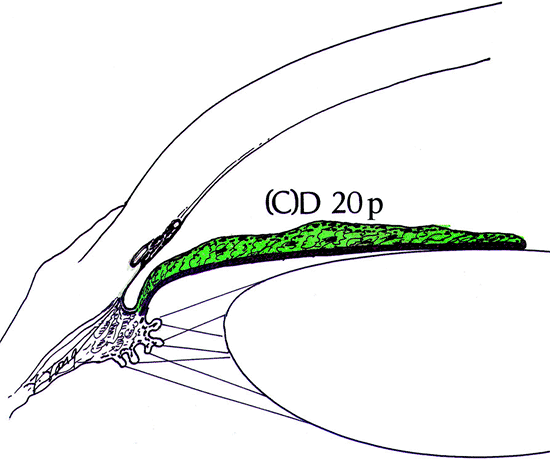 |
Figure 3.10. A partially closed angle that would be graded (C)D 20p. The (C) refers to the deepest structure visible before indentation, and the D refers to the actual site of adhesions between the iris and the inner wall of the eye. |
 |
Figure 3.11. A: Brownish pigmentation of the normal angle. B: Heavier but still brownish pigmentation in a patient with pigment dispersion syndrome. C: Pigmentation of the inferior angle in a patient with pseudoexfoliation syndrome. The pigment tends to be darker and is spread over the angle recess. A distinct black line is seen inferiorly at the level of Schwalbe's line and is known as Sampaolesi's line. D: An angle with 3+ pigmentation after argon laser trabeculoplasty and disappearance of pigment in treated areas. |
P.98
P.99
P.100
P.101
P.102
Evaluation of the Optic Disc and Nerve Fiber Layer
Introduction
Optic nerve head (ONH) evaluation is integral to the diagnosis and management of glaucoma for several reasons. Glaucoma causes characteristic, although not pathognomonic, defects in the ONH, which aids in the diagnosis. Glaucoma also causes progressive changes in the ONH that occur slowly, typically over months to years. Therefore, serial observation and recording of appearance are good indicators for overall disease management. Because documenting the ONH does not require the patient to perform the test, it is more objective than perimetry. However, ascertaining progression of the ONH by clinical examination and photography involves subjective interpretation by the clinician, which is susceptible to intra- and interobserver variability. Newer imaging techniques attempt to limit this variability to increase the sensitivity of detecting subtle change.
Defects in the retinal nerve fiber layer (RNFL) occur in patients with glaucoma. Recent evidence suggests that RNFL defects may predate visual field or ONH changes. Changes as small as 10 to 20 m may indicate disease progression before visual field changes are noted. RNFL imaging technology's potential for earlier diagnosis and finer sensitivity to disease progression is actively being evaluated. Its exact clinical role has not yet been established.
Optic Nerve Head
Optic Nerve Head Changes Occurring in Glaucoma
Progressive Thinning of the Rim
The only change that is completely diagnostic of glaucomatous damage is a progressive change in the appearance of the optic nerve: thinning of the neural rim (Fig. 3.12). This may occur concentrically (Figure 3.12A, B) or focally (Figure 3.12C). A large cup by itself, however, is not diagnostic of glaucoma; Figures 3.12A and B, for example, could be photographs
P.103
of normal optic nerves. Only by documenting that the cup was once smaller can these discs be considered glaucomatous. Figures 3.12D and E show worsening of the disc before surgery, and Figure 3.12F shows improvement after surgery.
 |
Figure 3.12. A: Slight concentric enlargement in the cup is present in a patient with early glaucoma. No visual field loss would be present here. B: More extensive concentric cup enlargement is seen in a patient with more advanced glaucoma. No visual field loss would be present here. C: Inferior, focal extension of the cup. D: Concentric enlargement of the cup. E: Further enlargement of the cup in the same patient with inadequate control of the intraocular pressure. F: Improvement in disc appearance in the same patient after trabeculectomy. |
Cupping With No Rim Remaining
Though large cups are less frequent in normal persons than are small cups, the size of the cup does not rule out or confirm glaucoma. If there is no rim, the nerve is abnormal (Fig. 3.13), but cup-to-disc ratios of 0.3 or less do not rule out the presence of glaucoma (Fig. 3.12F), and cup-to-disc ratios of 0.7 or larger are not always indicative of glaucoma (Fig. 3.12B).
Focal Glaucoma
The appearance of the nerve is often helpful in determining whether it is glaucomatous. An acquired pit of the optic nerve is virtually diagnostic of glaucoma damage (Fig. 3.14).
P.104
These pits are usually at the 5:30 or 6:30 position and less frequently found at the 1:30 or 11:30 position. They are found at the outer edge of the optic nerve.
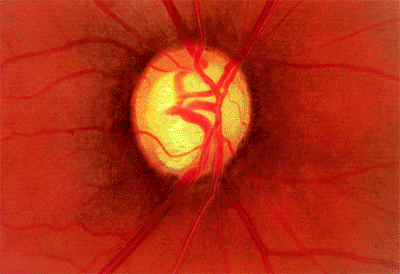 |
Figure 3.13. Enlarged cup with a thin temporal rim but normal intraocular pressure and marked visual field loss. |
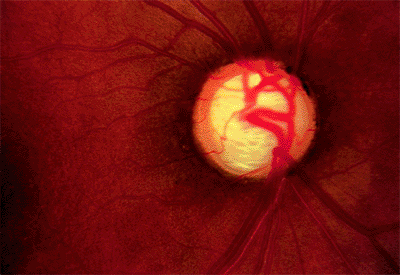 |
Figure 3.14. Acquired pit of the inferior portion of the optic nerve rim (at 6:30). This is diagnostic of glaucoma. |
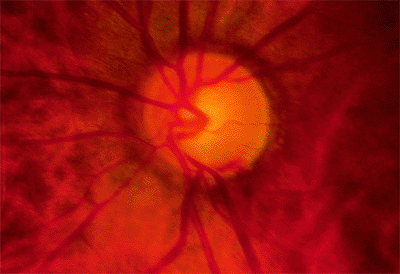 |
Figure 3.15. Striate hemorrhage at the inferior disc margin. This finding frequently indicates that the glaucoma is not well controlled. |
Hemorrhage
A superficial hemorrhage crossing the optic nerve edge is highly characteristic of glaucoma, and this should be specifically looked for by the examiner (Fig. 3.15). These hemorrhages are uncommon, occurring in a small percentage of patients. However, they are almost always a sign that the glaucoma is uncontrolled.
Disc Asymmetry
Asymmetry of the two optic nerves is highly suggestive of an acquired change, alerting the examiner to the possible presence of glaucoma nerve damage. In the patient illustrated, neither eye shows much damage, but tissue loss is more apparent in the left eye (Fig. 3.16B) than in the right eye (Fig. 3.16A). Myopic eyes tend to have larger scleral rims than hyperopic eyes, and cup asymmetry may in some cases be accounted for by anisometropia. (Also see page 144, Fig. 3.88).
Clinical Evaluation of the Optic Nerve Head
Historical Perspective
The optic nerve was first observed in vivo in 1851 when Hermann von Helmholtz (1821 to 1894) invented the direct ophthalmoscope. Edward von Jaeger (1818 to 1884) published the first ONH drawing from a patient with glaucoma in 1854. He used Helmholtz's direct ophthalmoscope with a candle as the illumination source; a single disc drawing required 80 to 120 hours. Both he and Albrecht von Graefe (1828 to 1870), confused by the two-dimensional view of the direct ophthalmoscope, believed that the optic nerve in glaucoma was elevated. In 1855, Adolf Weber (1829 to 1915), a student of von Graefe, suggested that the optic nerve was instead depressed, which was later confirmed by the German anatomist Heinrich Muller (1820 to 1864) in 1858. The first photographs of the human fundus were published by Howe in 1886. Nordenson modified a Zeiss camera to create the first stereoscopic camera in 1925.
Clinical examination remains the mainstay for evaluation of the ONH. It is inexpensive, quick to perform, and highly sensitive and specific. The direct ophthalmoscope gives a very magnified, direct view of the ONH, but it is a two-dimensional image. The slip lamp biomicroscope using a Hruby, 60 diopter (D), 78 D, or 90 D lens offers a three-dimensional view. The indirect ophthalmoscope gives a very minified image of the ONH, which makes a detailed evaluation of the ONH difficult.
Photographic Techniques
The most basic ONH imaging technique is a simple two-dimensional photograph. However, cupping can only be inferred
P.105
indirectly by observations of color and vessel course. Stereoscopic photography allows a three-dimensional perception of the ONH. There are two methods of obtaining a stereoscopic pair of ONH photos sequential, which captures two separate images photographed consecutively, and simultaneous, which creates the stereoscopic pair from a single image reflection. Simultaneous methods provide greater reproducibility of disc assessments. Figure 3.17 shows images obtained simultaneously.
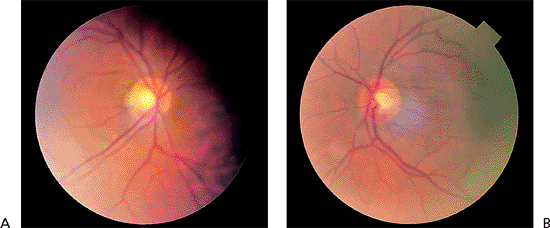 |
Figure 3.16. Disc asymmetry is present in a 55-year-old patient with an elevated pressure in the right eye associated with the exfoliation syndrome. The cup in the right eye (A) is larger than that in the left eye (B). (Also see page 144, Fig. 3.88). |
The next advancement involved digitization of the photographic image and using a computer program to create a topographic map of the ONH. Examples of this include the Nidek 3Dx (Nidek Technologies, Inc., Pasadena, CA) and the Topcon TRC-SS2 (Topcon Instrument Corp. of America, Paramus, NJ). The computer relies on a ratio of distances between the same reference point that appears in each stereo photo to generate this map. In one comparison, the Topcon system was twice as sensitive at detecting vessel shift as conventional stereo disc photos. This system is no longer commercially available. In other studies, the Nidek system was more reproducible than images from a Zeiss camera using a sequential photo technique, and less variable in disc assessments than conventional simultaneous photos using a Donaldson camera. The Nidek 3Dx generally estimates a smaller horizontal cup-to-disc (c/d) ratio than the Topcon.
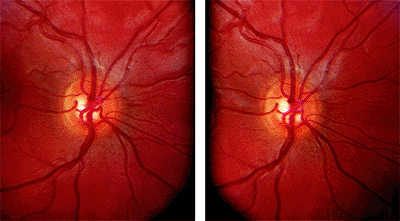 |
Figure 3.17. Optic nerve head simultaneous stereoscopic pair using a Topcon. |
The Discam (Marcher Enterprises Ltd., Hereford, England) utilizes a digital camera and stereochronoscopy with alternation flicker to generate a stereopair of images (Fig 3.18A). In addition to measuring horizontal and vertical c/d ratios, this system looks for neuroretinal rim loss along a chevron, which uses the supero- and inferotemporal aspects of the cup as the axes (Fig. 3.18B). However, the placement of the chevron and determination of the location of the neuroretinal rim rely on human interpretation. This method provides reproducible inter- and intraobserver estimates of optic disc measurements.
In summary, the conventional photographic cameras have the same degree of image resolution. The Zeiss camera using a sequential technique is still probably the most common method for obtaining stereo disc photos despite evidence in the literature that simultaneously acquired photos may have an advantage.
Confocal Scanning Laser Ophthalmoscopy (CSLO)
CSLO is another imaging technique that also generates topographic maps of the ONH. Conventional photography uses broad spectrum white light and captures the reflected light from the entire area of interest. CSLO uses a diode laser, focused on a very small point, and captures light in a collector focused only on that very spot. The amount of time it takes to receive the reflected light is used to calculate the distance to the surface. This information is then used to create the topography map. The cup is defined as the area below a reference plane set 50 m below the surface of the optic nerve head. There are several manufacturers of confocal scanning laser ophthalmoscopes Heidelberg Retina Tomograph (HRT, Heidelberg Engineering, Heidelberg, Germany), Topographic Scanning System (TopSS, Laser Diagnostic Technologies, San Diego, CA), and the Zeiss Laser Topographic Scanner (LTS, Zeiss Instruments, Thornwood, NJ no longer commercially available). When the HRT and Topcon TRC were compared, the Topcon measured the rim
P.106
P.107
area and cup volume to be larger, but the c/d ratio was the same. Additionally, the HRT was more reproducible regarding disc parameters than the Topcon System. Serial HRT analysis may be able to detect conversion from ocular hypertension to glaucoma before automated achromatic perimetry. One study comparing the ability of HRT, optical coherence tomography (OCT), and scanning laser polarimetry (SLP) (note: OCT and SLP are ways of measuring RNFL thickness see below) to detect patients with glaucoma found HRT to be the most sensitive and specific method. Figure 3.19A, B
P.108
shows an example of CSLO of the same patient using two different machines. Figure 3.20 is an example of CSLO in a patient with glaucoma using the HRT.
 |
Figure 3.18. A: Two-dimensional photo and stereoscopic pair with (B) chevron drawn. (Courtesy of Donald L. Budenz, M.D., Miami, FL.) |
Figure 3.19. Confocal scanning laser ophthalmoscopy of the same patient using the (A) Heidelberg Retina Tomograph and (B) Topographic Scanning System machines. |
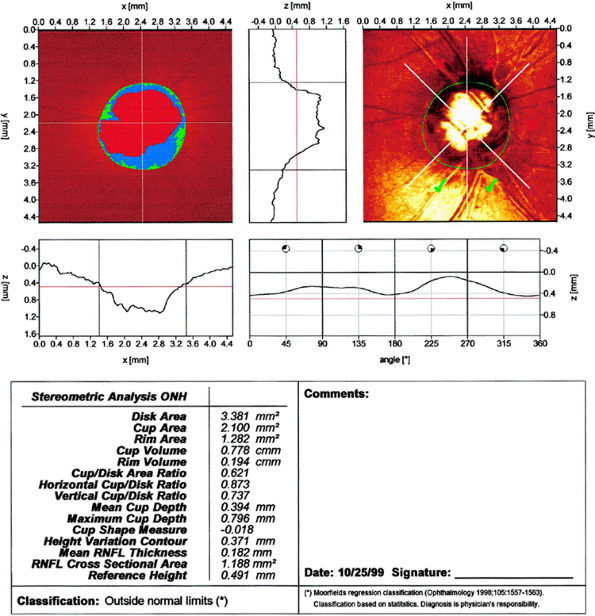 |
Figure 3.20. Confocal scanning laser ophthalmoscopy using a Heidelberg Retina Tomograph from a patient with glaucoma. |
Retinal Nerve Fiber Layer
Introduction
The RNFL is composed of ganglion cell axons, neuroglia, and astrocytes. The RNFL becomes thicker as one approaches the optic nerve head. The superior and inferior poles are thicker than the nasal and temporal poles; this gives the classic double hump seen in graphic representations of RNFL thickness (Fig 3.21). (See also double hump in Figure 3.15.) The presence of RNFL defects may aid in the diagnosis of glaucoma, but RNFL photography lacks the reproducibility to be more useful than ONH evaluation or perimetry for monitoring progression of disease. Newer technologies indirectly measure the actual thickness of the RNFL.
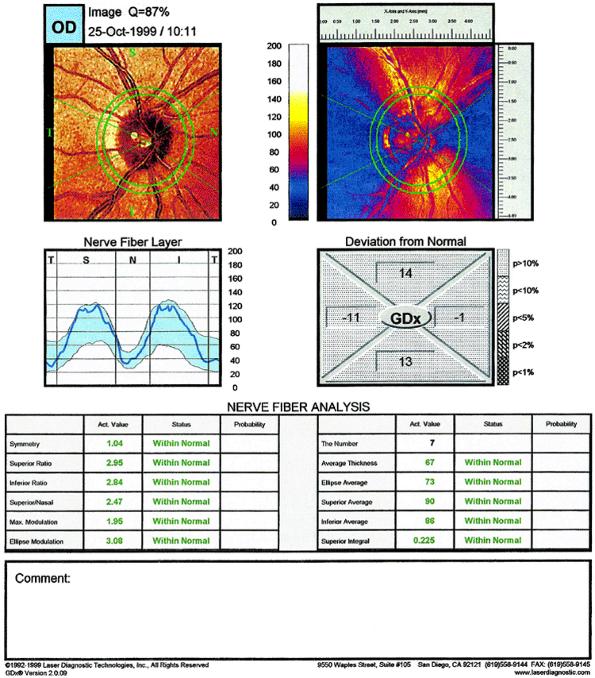 |
Figure 3.21. Graphic representation of retinal nerve fiber layer thickness results from scanning laser polarimetry GDx of the same normal patient seen in Figure 3.20. One sees the double-hump appearance. (T, temporal; S, superior; N, nasal; I, inferior.) |
P.109
P.110
Clinical Evaluation of the Retinal Nerve Fiber Layer
Historical Perspective
Clinical examination of the nerve fiber layer was first described by Vogt in 1917. The first published photograph of the nerve fiber layer was taken by Behrendt and Wilson in 1965. Examination of the RNFL was repopularized by Hoyt and Newman in the early 1970s.
Scanning Laser Polarimetry (SLP)
This technology exploits the birefringent nature of the RNFL to change the polarization of reflected light from the retina and RNFL. Assuming that the rotation of the polarized light is proportional to the RNFL thickness, the amount of rotation observed can be used to quantitatively measure RNFL thickness termed retardance (Fig. 3.22). The only SLP machine currently available is the GDx (Laser Diagnostic Technologies, Inc., San Diego, CA). Patients with glaucoma have a thinner RNFL (Fig. 3.23), which is reflected in a flattening of the double hump.
Optical Coherence Tomography
Optical coherence tomography (OCT; Zeiss-Humphrey Inst., San Leandro, CA) uses low coherence interferometry and measures the time it takes for light to be reflected from the different structures to generate its images. This is analogous to A-mode ultrasound, which instead uses sound waves. OCT can differentiate between patients clinically diagnosed as normal, ocular hypertensive, or glaucomatous, and glaucomatous versus nonglaucomatous eyes. In a direct comparison, OCT had a higher correlation between RNFL structure and visual function than SLP; however, further study is needed. Like SLP, OCT shows RNFL thinning in glaucomatous eyes (Fig. 3.24) compared to normals (Fig. 3.25).
Visual Field Loss
Glaucoma causes the loss of visual function. The evaluation of the effects of the glaucomatous process on visual function is essential in the diagnosis and management of the condition. In some patients, interference with other functions may be more marked; contrast sensitivity, dark adaptation, color vision, and the ability to discriminate flicker are all affected to some extent by the glaucomatous process. However, for tests to be clinically useful, they must be sensitive and specific. A clinically useful test must be able to find the condition (i.e., have few false-negative results) and must not be positive for other conditions (i.e., have few false-positive results). Of all the tests of visual function investigated, evaluation of the visual field combines the highest level of specificity and sensitivity. It is the best standardized of all the tests and continues to be clinically the most useful method of evaluating visual function in patients with glaucoma. And, perhaps most importantly, it is the only test currently available that can somewhat objectively assess the subjective experience of the patient's visual function.
Confrontation Fields
Determining the visual field by confrontation methods can often provide extremely useful information, and the value of the test should not be forgotten. Confrontation fields are underused as methods of evaluating visual function in patients with glaucoma. For patients with very poor vision from cataract, for example, it may be the only practical test possible. For patients with extremely poor vision, the unshielded light of a penlight is a useful method, and for patients with slightly less impaired vision, covering the penlight with a finger so that a red glow is produced is a useful method of evaluating the field. For patients with good vision, a small, colored object, such as the red top on a bottle of a cycloplegic agent, can be used to determine a color field by confrontation. To evaluate the field in the setting of large central nervous system (CNS) disease such as stroke, finger counting may be used as well.
With any of these methods, the patient is first asked to fixate upon the examiner's nose, eye, or face. For patients with very poor vision, fixation can be maintained upon the source of the examiner's voice. The examiner monitors fixation during the subsequent testing phase. Next, the object is moved in from the periphery, and the patient is requested to indicate when he or she first sees the object or, in the case of the red top bottle, can see the red as a clear red color. The four quadrants corresponding to superonasal, superotemporal, inferonasal, and inferotemporal are tested. A nasal step is specifically sought by moving the object vertically on the nasal side of the patient's visual field, asking the patient to state whether the object changes in brightness or color as it crosses the horizontal line through the visual axis. Scotomas can be mapped in a similar fashion. The red object can be moved from a scotoma into a visible location and vise versa to define the extent of the lesion.
One very useful confrontation technique is a modification of the Tangent screen to test for functional visual field loss. Tunnel vision is a typical pattern of functional field loss and can be readily demonstrated by mapping the field while sitting a few feet from the patient. Typically, there will be an extremely constricted field. The examiner then increases the distance between himself or herself and the patient. The field is retested, and failure to find an overall expanded field is indicative of tunnel vision.
The results of the confrontation field test should be charted as exactly as possible, providing a baseline for future reference to determine whether the field is changing.
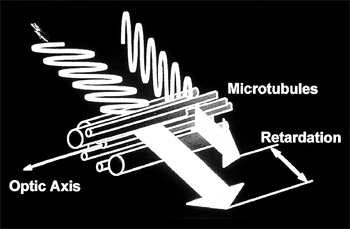 |
Figure 3.22. Graphic representation of retardance. (Reprinted from Schuman JS, Noecker RJ. Imaging of the optic nerve and nerve fiber layer in glaucoma. Ophthalmol Clin North Am 1995;8:259, with permission.) |
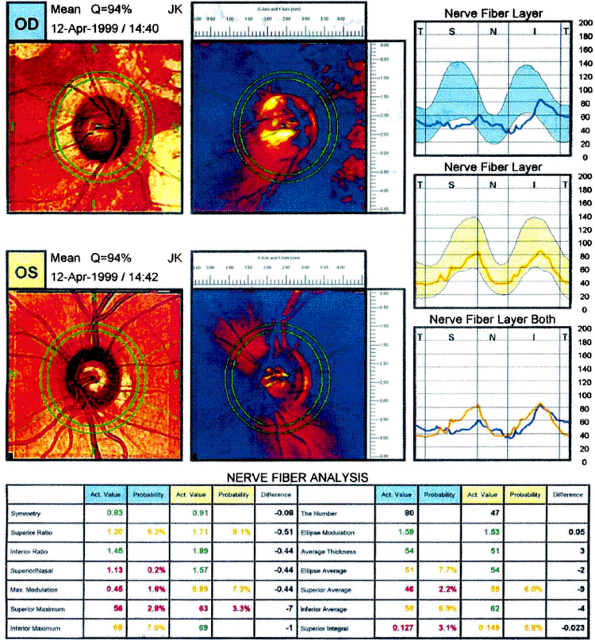 |
Figure 3.23. Scanning laser polarimetry from a patient with glaucoma. Note the flattening of the double hump on the graphic representation of the retinal nerve fiber layer. (Courtesy of Laser Diagnostics.) |
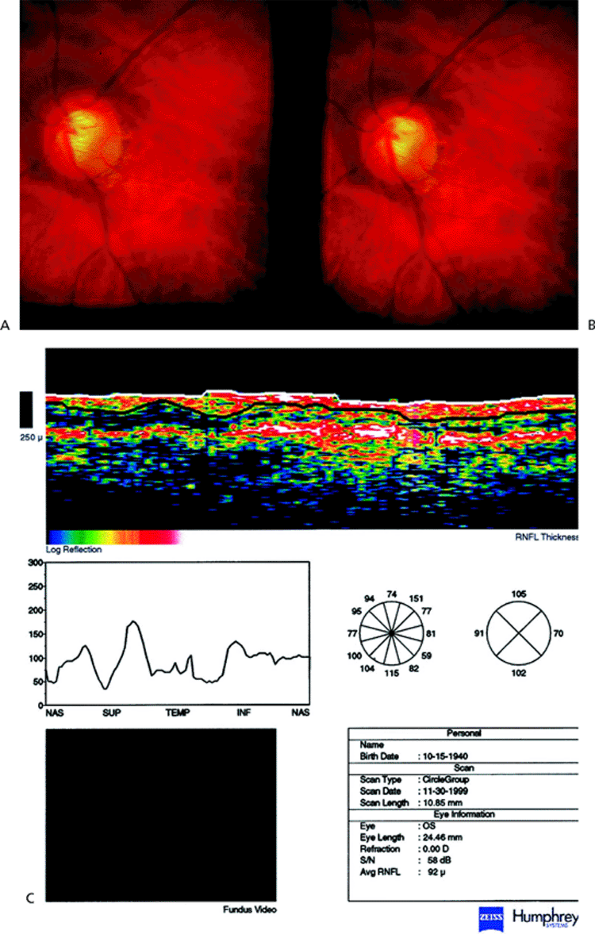 |
Figure 3.24. A: Optic nerve head with Nidek 3Dx from a patient with glaucoma. B: Corresponding achromatic visual field. C: Optical coherence tomography image from the same glaucomatous patient demonstrating retinal nerve fiber layer thinning in a glaucomatous eye. (Courtesy of Joel S. Schuman, M.D., Boston, MA.) |
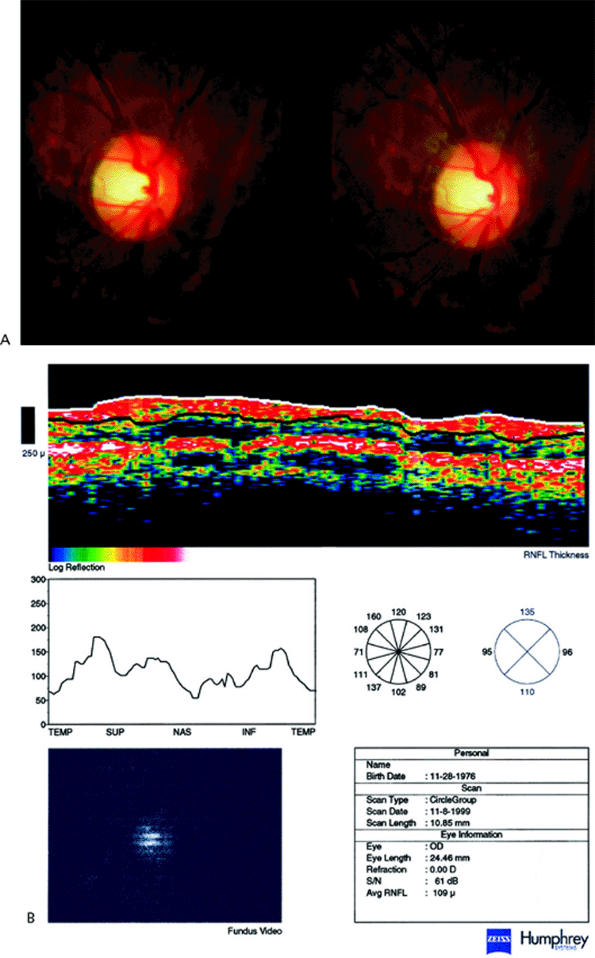 |
Figure 3.25. A: Optic nerve head image with Nidek 3Dx from a normal patient. B: Optical coherence tomography image from the same patient of a normal retinal nerve fiber layer. (Courtesy of Joel S. Schuman, M.D., Boston, MA.) |
P.111
P.112
P.113
P.114
Kinetic Perimetry
The visual field has been described by Traquair as an island of vision in a sea of blindness. The central portion of the island is peaked, and this corresponds to the most sensitive portion of the field to light. The peripheral portion is less sensitive to light and is therefore lower in elevation, like the shores of the island. In order to create a two-dimensional map of this three-dimensional island, a topographic map must be made. The basis of kinetic perimetry is to take a stimulus of fixed intensity and size and move it across the visual field to create a ring that, identically to a contour interval on a topographic map, defines where that stimulus is seen. The stimulus intensity is then reduced to test the more sensitive parts of the field (the peak of the island) or increased to test the less sensitive parts of the field. Eventually, a map of the island of vision is created.
The Goldmann kinetic perimeter is the most well known example of kinetic perimetry. Until automated static perimetry was developed, it was the best way to test the visual field. Although static perimetry has now replaced it as the test of choice for glaucoma, the Goldmann kinetic perimeter remains a viable option for certain patients who cannot perform static perimetry.
Computerized Static Perimetry
The most widely accepted method of evaluating the visual field is computerized perimetry, using a technique known as static perimetry. In contrast to kinetic perimetry where a stimulus of fixed intensity is moved in the visual field, static perimetry maps the island of vision by varying the intensity of the stimulus in a fixed location. This is akin to a mountain climber taking altitude measurements at various fixed locations all over the island of vision. The advantage is that not only can computers perform the testing, thereby permitting standardization, reproducibility, and statistical analysis, but the very fine changes in stimulus intensity that can be performed also permit very sensitive maps of the island of vision, and glaucomatous defects can be detected earlier than they become apparent using kinetic perimetry.
The most commonly used automated perimeters are Humphrey or Octopus instruments. Full threshold tests, such as the Humphrey 24-2 examination, provide a high level of sensitivity and specificity. However, these levels are not absolute. A normal visual field examination, which is a test in which no defect is found, does not mean that the patient does not have glaucoma. Repeated visual field tests that show no apparent deterioration do not prove that the patient's glaucoma is not deteriorating. Many neurons must be lost before a field defect is evident, and many more neurons must be lost before a visual field defect progresses.
Additionally, the visual field may demonstrate a visual field defect that is not due to glaucoma. Droopy lids, small pupils, refractive error, opacities in the media, retinal disease, and CNS disease all can cause visual field defects. Even age, with its accompanying smaller pupil and hazy lens, typically causes loss of sensitivity in the peripheral field. These changes may mimic the changes that occur as glaucoma gets better or worse. Additionally, changes in testing strategy, such as switching from one machine to another, or even different testing strategies on the same machine, can demonstrate changes in the field that may, or may not, be real. The astute examiner must be aware of the changes that can cause an apparent improvement or, more commonly, a deterioration of the field, and must consider those possibilities when interpreting the visual field examination.
Most of the new instruments provide software analyses of the hardware results. These analyses provide much useful information for the practitioner trying to decide whether the field is normal or abnormal, stable or changing. The individual points on the patient's visual field are compared with the age-matched controls to determine what is abnormal about the field, and then a subtraction technique is used to attempt to eliminate the factors that may decrease the overall sensitivity of the field as a result of opacities in the media. These statistical calculations attempt to highlight localized abnormal areas of the field that are in excess of any generalized depression and so may be buried in overall darkness of the field. Such localized defects are more typical of glaucoma. Additional tests are built into the automated examination to investigate the intratest reliability. Fixation losses, false-positives, and false-negatives are routinely calculated. On some testing protocols, certain defined points in the field are retested in an attempt to measure the patient's internal consistency. This measure of short-term fluctuation provides an estimate of the reproducibility of the data and assists the interpreter by providing a useful method of estimating the reliability of the test.
It is essential for those interpreting visual field examinations to understand the difference between reliability and validity. A visual field evaluation may be highly reproducible and, therefore, may be reliable. However, that reliable visual field may not be a valid indicator of the patient's visual field. For example, when tests are repeatedly done with the correcting lens too far from the eye, there is a reproducible peripheral contraction, but the peripheral contraction is not a valid indicator of the patient's field.
When interpreting the visual field, we recommend a systematic approach. We believe this will provide that examiner the best opportunity to interpret the field correctly. We recommend that a seven-step strategy be followed:
Which test was performed?
Is the patient's demographic and clinical information correct?
Is the field reliable?
Is the field abnormal?
What is the pattern of the abnormality?
Is the current examination different (worse) than previous examinations?
Is the abnormality or worsening due to disease or artifact?
P.115
Each strategy will be examined in some detail using the Humphrey perimeter as the model. This is not the only perimeter that can be used, but is the most widely used and is the one used at the Wills Eye Hospital. See Figure 3.28 for a typical Swedish interactive threshold algorithm (SITA) single-field printout.
The test that was performed is indicated at the top of the printout. This is important, not only because it will determine how to interpret that field, but also because for patients who have undergone multiple field examinations, a change in testing protocol can alter the appearance of the field. We believe that a consistent approach of using the same testing protocol over time is often best. Patients who are switched to one of the newer, faster test protocols may require two or more fields to be performed to establish a new baseline.
The patient demographic information is critical. Care must be taken with persons with the same name, and extreme care must be taken to ensure that the age of the patient and the refraction used for the examination are correct. Problems in either of these areas will often greatly affect the results of the test.
Reliability of the field is best assessed by examining the fixation losses (or the gaze tracker on the newer Humphrey Field Analyzer [HFA] II), false-positives, false-negatives, and short-term fluctuation. Fixation losses are a measure of how attentive the patient was in looking at the fixation target during the test. Generally speaking, a high number indicates unreliability, but in certain cases where the remaining reliability parameters are normal, this can be acceptable. False-positives measure times that the patient indicated a stimulus was seen even when no stimulus was presented. Such errors tend to make the field look better than it actually is and can seriously confound interpretation. We consider more than two false-positives (or 10% to 15% on a SITA exam) to be concerning. False-negatives measure the patient's lack of response to a retest with a stimulus that was previously seen in a certain location. High false-negatives generally result in making the field appear worse than it is, but can be understood and even expected in patients with pathologic defects in their fields due to fluctuation inherent in a scotoma. Therefore, a high number may not be terribly concerning if the overall field is poor. Short-term fluctuation is calculated by retesting certain defined points in the field and determining the difference in sensitivity for the two tests. This is a useful measure of reliability and can even represent the first change in glaucoma or glaucomatous progression. However, it takes time and has been abandoned on the newer SITA testing algorithms.
Abnormality of the visual field is best demonstrated on the total deviation plot. This plot is a comparison of each point in the field with a database of normal age-matched controls and represents the deviation from the normal expected value for each point in the field. A statistical calculation is then performed to indicate the level of abnormality and is indicated by various shaded boxes.
The real interpretation work begins with deciding if there is artifact in the field and what pathology the pattern of field loss indicates. Extensive atlases of visual field artifacts are available, and a thorough discussion of these is beyond the scope of this text. Suffice it to say that knowledge of nonphysiologic (such as lens-rim artifact) and nonglaucomatous (such as ptosis) patterns of field loss is essential to proper interpretation. To aid the interpreter who is interested in testing for glaucoma, the Humphrey printout contains a plot called the pattern deviation plot. This plot is a subtraction plot based on the total deviation plot and is designed to normalize an island of vision that has been diffusely depressed, typically by media opacity or small pupil size. It should reveal the focal areas of depression in excess of the generalized background depression that may indicate glaucoma is present. Statistical measures of the amount of deviation from normal are also presented. Note that this plot cannot adjust for other causes of field loss, such as retinal or CNS, that may simulate the focal losses of glaucoma. A correlation of the visual field and the clinical examination is required to confirm which disease process is present.
There is no universally accepted definition of what constitutes a glaucomatous defect; however, there are three major patterns of visual field loss that are characteristic of glaucoma: a diffuse decrease in sensitivity, primarily peripherally; an ocular scotoma involving the area about 15 degrees from fixation (i.e., arcuate); and a dense paracentral scotoma that starts nasal to fixation. There are probably other patterns that have been less well established. These three separate patterns can occur individually or in various combinations. The diffuse type of decreased sensitivity tends to be typical of ischemic, high-pressure glaucomas, while the arcuate type of defect is characteristic of chronic, moderate pressure elevation glaucomas, and the paracentral type of defect is more typical of the glaucomas that occur with normal levels of IOP.
Arbitrary classification schemes have been developed to attempt to classify glaucomatous visual field defects according to their level of severity. For the most part, these are complicated research tools and are not useful in clinical practice. One scale, however, is relatively simple and can be used to classify defects for use in deciding treatment strategies. The Hodapp-Parrish-Anderson Scale (Hodapp, 1993) requires that certain minimal abnormalities be present before a field is called glaucomatous, and then classifies defects as early, moderate, or severe. The definitions (and our own modifications for SITA testing) are based on a Humphrey perimeter equipped with STATPAC 2 and are found in
P.116
Table 3.4. See Figures 3.26, 3.27, and 3.28 for examples of the Hodapp Interpretation Scale applied to a visual field. At Wills, we have devised our own arbitrary grading scale that divides field abnormalities into seven stages of progressively more and more damage. It is based upon the Humphrey visual field pattern deviation plot, but can be relatively easily adapted to other perimeters. This method simply counts the number of depressed points on the plot and assigns a point value. Although there is no allowance made for a glaucoma hemifield test or clusters of points in typical glaucomatous regions, there is some adjustment made for location as the central four points, if depressed, count for triple value. This scale is designed for use in conjunction with a new optic nerve staging scale that we have also developed in order to arrive at a Glaucoma Likelihood Score (GLS) that seeks to estimate the chance that a patient has glaucoma. The visual field scale is seen in Table 3.5, and examples of visual fields are seen in Figures 3.26, 3.27, and 3.28.
Determining if the current examination differs from previous examinations may be even harder than deciding if the field is abnormal in the first place. In glaucoma, typically the field is followed over time to assess if it is stable or worsening. A series of visual fields in which there is no apparent change does not necessarily indicate stability of the patient's condition, and a series of visual fields indicating progressive change does not always indicate instability of the patient's glaucoma. Confounding factors such as pupil size, developing cataract, fatigue, improperly performed tests, and long-term fluctuation must always be considered and ruled out before concluding that a change in a visual field is a valid indicator of a change in the patient's status. Once again, there are no universally accepted criteria for what determines if one field is different from another, but again, there are three patterns of glaucomatous progression: a new scotoma developing in a previously normal area; deepening of an existing scotoma; and enlargement of a preexisting scotoma. Scales to determine progression, much like scales to determine abnormality, are arbitrary and generally very complex and used only for research. The Hodapp-Parrish-Anderson criteria are relatively easy and readily applicable. The criteria for a new scotoma are identical to those discussed above. For deepening of an existing scotoma, two points must be depressed by 10 dB from baseline, and for enlargement of an existing scotoma, three points must be depressed by 10 dB from baseline. An additional criterion is if the mean deviation declines at a rate of p<5% (about 1 dB per year) on the Humphrey glaucoma change analysis plot. This criterion is less specific for glaucoma, but may serve to identify the less common group of patients that demonstrate progression not by focal change, but by diffuse change in the visual field. Whichever criteria are used, the most important factor to remember is the effect of long-term fluctuation. Recent data indicate that the vast majority of visual fields that appear to have worsened will normalize (or return to baseline) upon repeat testing. Treatment decisions based upon the worsening of a single visual field, especially in the setting of other subjective and objective signs of stability, may be unnecessarily hasty.
Table 3-4. Hodapp-Parrish-Anderson Visual Field Grading Scale (Hodapp, 1993) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
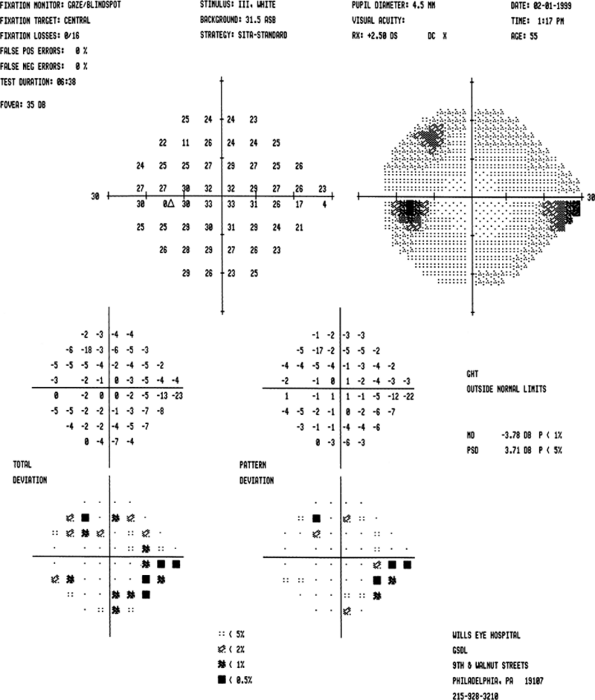 |
Figure 3.26. Early visual field loss. This field meets each of the three criteria that constitute the Minimal category of the Hodapp-Parrish-Anderson classification system and should probably be considered in the Early category. According to the Spaeth classification system, this is a stage 2 field (mild) as there are 6 points depressed at the 1% or worse level. |
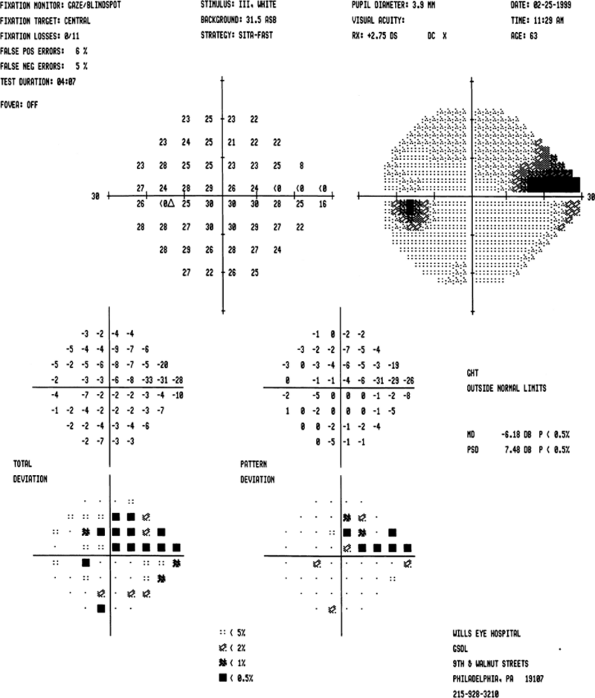 |
Figure 3.27. Moderate visual field loss. This field barely meets the mean deviation criterion that constitutes the Moderate category of the Hodapp-Parrish-Anderson classification system. If the visual field contained more evidence of cataract on the total deviation plot or the foveal threshold, one might consider the mean deviation to be a reflection of more than just glaucoma, and thereby downgrade the field to the Early category. According to the Spaeth classification system, this is a stage 3 (mild-to-moderate) field as there are 8 points depressed at the 1% or worse level. Note that there is a point depressed at the 2% level within 5 degrees of fixation. If this were depressed at the 1% level or worse, then there would be 11 abnormal points (points depressed at this level in this location are counted three times) and the field would still be stage 3. |
 |
Figure 3.28. Moderate visual field loss. This field contains a point of 0 dB within 5 degrees of fixation, has a mean deviation of more than 12 dB, and the total number of points depressed at the 1% level or greater on the pattern deviation plot exceeds one quadrant. According to the Spaeth classification system, this is a stage 4 field (moderate loss) as there are 20 points depressed at the 1% or worse level (remember to triple the point depressed at the 5 degrees location). |
Table 3-5. Spaeth Visual Field Grading Scale | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
P.117
P.118
P.119
P.120
Newer Visual Field Tests
So far, the discussion has centered on the traditional methods of automated perimetry. These employ a white stimulus displayed upon a white background and have the ability to detect scotomas that are completely asymptomatic to the patient. Yet, as more information about the nature of the cellular injuries of glaucoma has become available, tests have been devised to attempt to preferentially target the function of those cells thought to be damaged first in glaucoma. Such special methods of perimetry, including the presentation of a blue stimulus against a yellow background (short wavelength automated perimetry, or SWAP) and frequency-doubling technology, may offer earlier detection of glaucoma than standard white-on-white perimetry. However these methods have not been proven superior and probably are best used only in centers that are investigating their value.
Specific Entities
Angle-Closure Glaucomas
Angle-closure glaucomas are characterized by temporary or permanent contact of peripheral iris against the trabecular meshwork, resulting in obstruction of aqueous outflow. The clinical presentation can be acute or chronic. It is much less common than POAG.
The most common mechanism of angle-closure glaucoma is relative pupillary block (e.g., primary angle-closure glaucoma). The aqueous cannot flow from the posterior to the anterior chamber because of a functional obstruction between the lens and the iris. The peripheral angle structures are not visible or are partially obscured, and the iris is bowed forward, making contact with the anterior chamber angle. Other possible causes for pupillary block are discussed below.
Angle-closure glaucoma may be related to ocular structures that push the iris forward (e.g., plateau iris syndrome, aqueous misdirection, tumors, cysts) and tissues or membranes that obstruct the outflow directly (e.g., neovascular glaucoma, iridocorneal endothelial [ICE] syndrome, inflammatory debris).
Acute Primary Angle-Closure Glaucoma
Patients with acute angle-closure glaucoma present most often with pain, elevated IOP (often greater than 50 mm Hg), a shallow or flat peripheral anterior chamber, corneal edema, and visual loss (Fig. 3.29). Nausea and vomiting may occur. Predisposed patients are usually older than 50 years, hyperopes, with a shallow anterior chamber, a short axial length of the globe, and a thicker, more anteriorly displaced lens. It is more common in females and there is a familial tendency. Factors than can precipitate an attack include emotional stress, mydriasis, or anticholinergic drugs. Pupillary block is the most common mechanism (Figs. 3.30, 3.31).
Management
Acute angle-closure glaucoma is a medical emergency. The first goals are to break the acute attack, usually with medical therapy, to relieve permanently the
P.121
pupillary block with neodymium:yttrium-argon-garnet (Nd:YAG) laser iridotomy, and to treat the fellow eye. The urgency of treatment is a factor of the amount of pain present and the likelihood of permanent optic nerve damage. When the optic nerve is already damaged, it is more susceptible to further damage. In such cases, lowering the IOP is urgent. Examination of the optic nerve is an essential part of the evaluation of a patient with an angle-closure glaucoma!
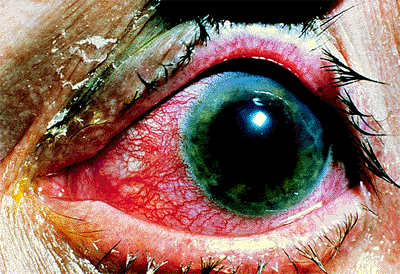 |
Figure 3.29. Typical external appearance of acute angle closure caused by pupillary block, with diffuse hyperemia of the conjunctiva, mid-dilated pupil, and steamy cornea. The intraocular pressure is 64 mm Hg. |
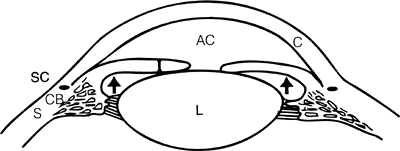 |
Figure 3.30. In pupillary block glaucoma, a physiologic block is created between the iris and the lens, preventing the normal flow of aqueous from the posterior chamber to the anterior chamber. Pressure (arrows) within the posterior chamber forces the peripheral iris forward, closing the angle and causing a sudden increase in intraocular pressure. (AC, anterior chamber; C, cornea; CB, ciliary body; I, iris; L, lens; S, sclera; SC, Schlemm's canal.) |
Initial medical therapy includes administration of intravenous acetazolamide (500 mg), topical aqueous suppressants ( blocker, carbonic anhydrase inhibitor, 2 agonist), and topical pilocarpine (1% to 2%, twice over 30 minutes). Frequent topical corticosteroids are started. If the attack is not broken, an osmotic agent is given (oral 50% glycerin, oral 45% isosorbide, or intravenous 20% mannitol, 1 to 2 g/kg). In recalcitrant cases, argon laser peripheral iridoplasty (or gonioplasty) or Nd:YAG iridotomy can be tried to break the acute attack. The view is usually suboptimal from corneal edema, and topical glycerin can be helpful.
Permanent relief of pupillary block is achieved with Nd:YAG laser peripheral iridotomy (see below). If laser iridotomy is not possible, argon laser peripheral iridoplasty (see below) or surgical peripheral iridectomy is necessary. The anterior chamber angle may appear open or show peripheral anterior synechiae of variable extension after the attack. Goniosynechialysis can be effective to open the angle if closure occurred within 12 months of surgical intervention. The fellow eye should undergo prompt prophylactic laser iridotomy to prevent an acute attack.
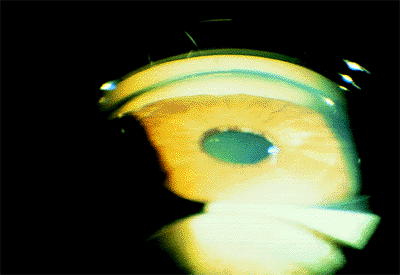 |
Figure 3.31. Gonioscopic view of a closed angle during an acute angle-closure attack. |
Chronic Angle-Closure Glaucoma
Some patients describe episodes of ocular discomfort, blurred vision or colored halos around lights that, in the presence of a narrow angle, are suggestive of subacute angle-closure glaucoma. These symptoms typically occur at night. Repeated subacute attacks may result in the development of permanent peripheral anterior synechiae and chronic angle-closure glaucoma. The synechiae tend to be broad based, and are most commonly seen in the superior quadrant.
Creeping angle-closure glaucoma is another form of chronic angle-closure glaucoma in which the anterior chamber angle narrows progressively over time. This form of glaucoma is the most common form of angle-closure glaucoma in black patients, especially in those on miotic therapy.
Diagnosis is based on gonioscopy (Fig. 3.32). Indentation gonioscopy (see page 99) is important to differentiate parts of the angle with appositional or permanent/synechial closure.
Management
Laser peripheral iridectomy is the initial treatment of choice. However, if most of the anterior chamber angle is closed, laser iridotomy will not be helpful in controlling the IOP. Long-term aqueous suppressants and, sometimes, filtering surgery, may be required. If more than
P.122
one quadrant of the anterior chamber angle has appositional closure and opens with indentation gonioscopy, laser iridotomy may be enough to arrest the progression of the disease (Fig. 3.33).
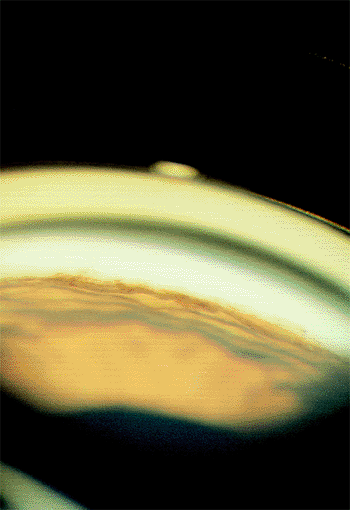 |
Figure 3.32. Gonioscopic view of peripheral anterior synechiae commonly seen in chronic angle closure. |
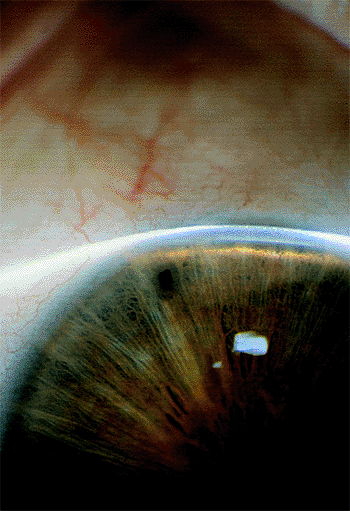 |
Figure 3.33. Laser peripheral iridectomy successfully reversed the appositional closure. |
Plateau Iris Syndrome
Plateau iris configuration refers to a flat iris configuration with an abrupt posterior turn near the iris insertion and, therefore, with a narrow anterior chamber angle. This is graded as a P angle (see page 100). Most eyes with plateau iris configuration have a component of relative pupillary block, and laser iridotomy can widen the anterior chamber angle. Occasionally, however, laser iridotomy does not affect the positioning and shape of the iris and the anterior chamber angle. The term used to describe this condition is plateau iris syndrome, which is due to an anterior position of ciliary processes. Patients with plateau iris may develop acute or chronic angle closure.
Management
All patients with plateau iris should undergo laser peripheral iridectomy. If peripheral iridectomy does not deepen the angle, chronic miotic therapy can be effective to prevent or treat angle closure, although the treatment of choice is laser peripheral iridoplasty (see below).
Neovascular Glaucoma
Neovascular glaucoma occurs when a fibrovascular membrane proliferates from the iris onto the angle structures (Fig. 3.34, Fig. 3.35). With progression of the disease, the fibrovascular membrane contracts, resulting in secondary angle-closure glaucoma. Fibrovascular membrane growth is related to ocular ischemic microvascular disease such as diabetes and central retinal vein obstruction. Other conditions can lead to neovascularization, such as uveitis, central retinal artery obstruction, branch vein obstruction, and tumors.
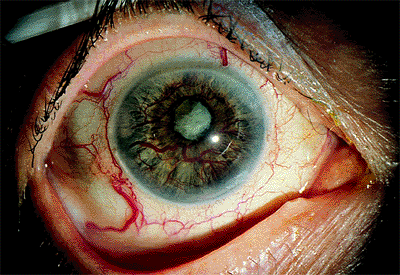 |
Figure 3.34. Neovascularization of the iris with large-caliber vessels on the surface in an 80-year-old diabetic woman. The angle is closed, and the intraocular pressure is 66 mm Hg. Notice the absence of corneal edema as a result of the longstanding duration of the disease. |
Management
Prompt laser panretinal photocoagulation is employed to cause regression of the neovascularization. If the angle is still open, or partially open, effective laser treatment may revert the condition. If the angle is closed, medical treatment with atropine, steroids, and aqueous suppressants can lower the IOP. If this fails, a tube-shunt procedure is usually the procedure of choice, providing there is useful vision. If the eye is blind, then surgery usually should not be considered, and treatment with atropine, steroids, and topical glaucoma drops is appropriate.
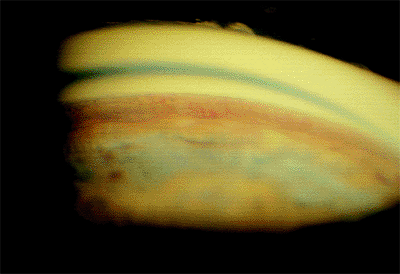 |
Figure 3.35. Blood vessels within the angle in a 64-year-old man 3 months after a central vein occlusion. |
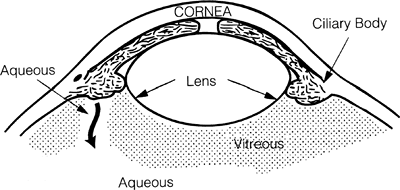 |
Figure 3.36. In aqueous misdirection, aqueous is directed posteriorly into the vitreous cavity, pushing the vitreous, lens, and iris forward; closing the angle; and increasing the intraocular pressure. |
P.123
Aqueous Misdirection Syndrome
Previously called malignant glaucoma because of its inexorably worsening course and frequently grave outcome, aqueous misdirection syndrome is seen in eyes with small anterior segments. A change in aqueous dynamics brought on by the addition of miotics, surgery, or injury can direct aqueous production posteriorly into the vitreous cavity (Figs. 3.36, 3.37). The increasing volume posteriorly pushes the lens, iris, and hyaloid face forward, progressively making the anterior chamber shallower, closing the angle, and increasing the IOP. Studies with the high-frequency biomicroscope have revealed small, anterior, suprachoroidal effusions in many of the cases examined. The forward rotation of the iris, lens, and ciliary body around scleral spur caused by the effusion could well be the inciting feature in some of the cases with this syndrome. Characteristically, the space between the central cornea and lens is much less than would be expected if the IOP rise were due to angle-closure glaucoma.
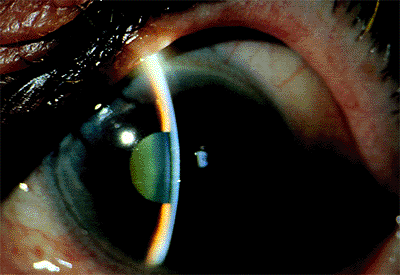 |
Figure 3.37. This posttrabeculectomy patient shows the characteristic features of aqueous misdirection syndrome: low bleb because little aqueous is entering the anterior chamber, shallow to flat central anterior chamber, no choroidal detachments, and elevated intraocular pressure. |
Differential Diagnosis
Pupillary block (deeper central anterior chamber, peripheral iridectomy curative)
Suprachoroidal hemorrhage (history of pain, visible dark choroidal detachment)
Suprachoroidal effusion (visible choroidal detachment, low IOP unless anterior rotation of lens and hyaloid face causes relative aqueous misdirection and pressures in the teens)
Management
The crescendo of flattening anterior chamber and increasing IOP makes medical and, frequently, surgical intervention imperative. Medical treatment consists of maximum aqueous suppression with topical blockers, 2 agonists, topical and/or systemic carbonic anhydrase inhibitors, maximum cycloplegia with atropine to pull the lens-iris diaphragm posteriorly, and intravenous mannitol every 12 hours to shrink the vitreous maximally and deepen the anterior chamber. Steroids are added to minimize inflammation. If there is access to the anterior hyaloid face, it can be ruptured with the Nd:YAG laser to resolve the misdirection. A capsulotomy often is required in pseudophakes. If no access to the hyaloid face is available, a pars plana anterior vitrectomy is required. Infusion through a corneal paracentesis with pars plana vitrectomy facilitates removal of the anterior vitreous and hyaloid face.
Other Pupillary Block Glaucomas
Conditions than may lead to secondary pupillary block include subluxed or dislocated crystalline lens (Fig. 3.38), iris contact with the anterior vitreous face, a secluded pupil with 360 degrees of posterior synechiae from inflammation (Fig. 3.39, 3.40), and an anterior chamber intraocular lens if there is no patent peripheral iridectomy (Fig. 3.41). There is often a prominent iris bomb , with a billowing of the iris,
P.124
P.125
especially at the periphery, and coming into direct apposition with the cornea (Fig. 3.40).
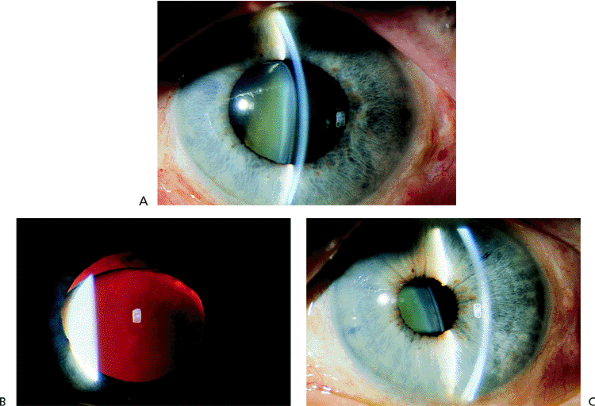 |
Figure 3.38. A: Patient with a traumatic subluxation of the crystalline lens that was displaced forward at the superior pole. The zonules were disrupted superiorly, with vitreous presenting through the pupil. B: With retroillumination, the lens can be seen below the superior edge of the pupil. The pupil could not be constricted, and the intraocular pressure was markedly elevated. C: After laser iridectomy, the anterior chamber deepened, and the pupil constricted promptly with miotics. |
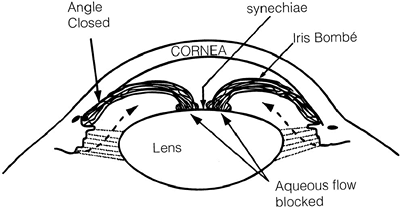 |
Figure 3.39. Posterior synechiae may form between the iris and lens in patients with chronic anterior uveitis, preventing the flow of aqueous into the anterior chamber. An iris bomb is created, closing the angle and increasing the intraocular pressure. |
 |
Figure 3.40. A: Patient with sarcoid uveitis, with 360 degrees of posterior synechiae leading to a secluded pupil. B: A patient with chronic uveitis developed posterior synechiae through 360 degrees of the pupil margin. C: An iris bomb developed that closed the angle and resulted in an increase in the intraocular pressure. |
 |
Figure 3.41. A: A pseudophakic patient with a posterior chamber intraocular lens developed a marked iris bomb . An imperforate peripheral iridectomy is evident inferiorly. B: A laser iridectomy was performed in several locations, including the imperforate area, and the anterior chamber deepened dramatically. The iris bomb disappeared, and the intraocular pressure was reduced. |
Management
Medical treatment is undertaken only as a temporizing measure. By vigorous dilation and the use of aqueous suppressants, the pupillary block may be broken and the IOP lowered. The most effective treatment is an Nd:YAG laser iridectomy or, if not possible, surgical peripheral iridectomy or trabeculectomy.
Tumor-Related Glaucoma
Unilateral secondary glaucoma may result from the presence of a primary or metastatic ocular tumor (Figs. 3.42, 3.43). Several mechanisms may be involved. A ciliary body mass may result in forward displacement of the lens-iris diaphragm or a chronic angle-closure type related to neovascularization and synechial closure of the angle. An iris or ciliary body melanoma may directly infiltrate the trabecular meshwork or liberate cells into the anterior chamber that impair the outflow facility of the trabecular meshwork in an otherwise open angle (Fig. 3.44). Gonioscopy is essential to establish the mechanism of elevated IOP.
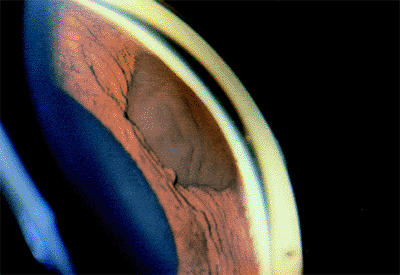 |
Figure 3.42. Melanotic lesion of the iris extending into the angle as seen on gonioscopy. |
Management
Initial management is medical, with aqueous suppressants. If medical treatment is unable to control the IOP, cyclodestructive procedures or enucleation should be considered. Filtration surgery is usually not recommended because it might facilitate the spread of tumor cells outside the eye.
Open-Angle Glaucomas
The open-angle glaucomas are a heterogeneous group of glaucomas sharing in common the presence of an open anterior chamber angle and pressure-related damage, or the potential for such, to the optic nerve.
Primary Open-Angle Glaucomas
POAG is a condition in which the nerve becomes damaged in association with relatively elevated IOP, but there are no
P.126
other evident abnormalities on ophthalmic examination. This glaucoma is the most common glaucoma in the United States, affecting over half of all patients with glaucoma. There are probably many entities that lead to POAG, but they are indistinguishable on clinical examination. Current research on the genetic basis of glaucoma holds the promise to identify the underlying pathophysiology of POAG, and reveal the underlying subtypes.
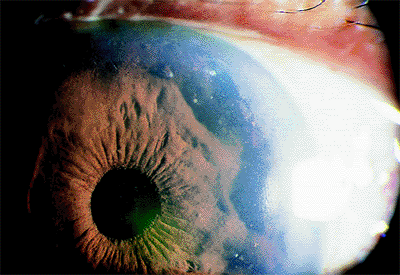 |
Figure 3.43. Diffuse metastatic lesions of the iris resulting in angle closure. |
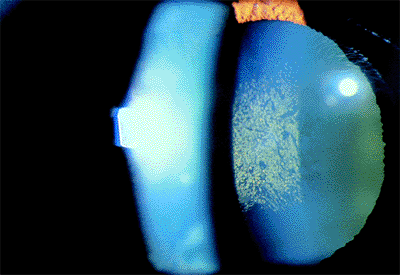 |
Figure 3.44. Deposition of pigmented tumor cells on the anterior lens capsule. |
Low-pressure glaucoma, or normal-tension glaucoma, is essentially POAG without elevated IOP. Some clinicians object to the term normal-tension glaucoma because of the arbitrary and judgmental nature of the term normal, as well as the unnecessarily confusing term tension for patients. Others clinicians object to the term low-pressure glaucoma, as the pressure is not lower than that found in the general population. Historically, this entity was thought not to exist, and then later a pressure of 22 mm Hg was chosen to distinguish this from POAG. This distinction is now recognized to be completely arbitrary, and most clinicians view primary and low-tension glaucoma as part of an overlapping spectrum of optic nerve damage associated with greater or lesser degrees of pressure-dependent mechanisms of damage, as opposed to other proposed mechanisms, such as vascular, excitotoxic, and growth factor deficiency related. Certain clinical features are more common at the extremes. For example, reports have linked a greater incidence of paracentral field defects, disc hemorrhages, and vasospastic phenomena to low-pressure glaucoma than to POAG.
Both POAG and low-pressure glaucoma are diagnoses of exclusion. All secondary causes of glaucoma must be excluded before those diagnoses can be made. This requires a thorough ophthalmic examination, including a detailed history, slit lamp examination, gonioscopy, and funduscopic examination. Rarely, additional testing, such as neurologic imaging studies, is required. For low-pressure glaucoma, the history is especially important with regard to possible prior elevated IOP now resolved (e.g., due to oral steroid use in the past), episodes of ischemia (e.g., shock-related optic neuropathy), related vasospastic phenomena (e.g., migraines, Raynaud's phenomenon), or other neurologic signs and symptoms (e.g., headaches related to an intracranial mass).
POAG and low-pressure glaucoma are treated by reduction of IOP. The Normal Tension Glaucoma Study demonstrated that a 30% reduction from maximal IOP effectively reduces the rate of glaucomatous progression. Some clinicians favor nonvasoactive agents in the medical treatment of low-pressure glaucoma, because of concern regarding already compromised optic nerve blood flow, but increased efficacy of these agents has not been demonstrated. Calcium channel blockers are also employed by some clinicians, but conflicting reports regarding their efficacy have prevented the widespread acceptance of their use in glaucoma.
Developmental Glaucomas
Infantile glaucomas are relatively uncommon, affecting approximately 1 in 10,000 live births. The classic triad of signs is epiphora, photophobia, and blepharospasm. Additional important signs are enlargement of the globe and corneal clouding. Isolated congenital glaucoma is generally sporadic, is bilateral in 75% of the cases, and affects males slightly more than females. Typically, the iris inserts anteriorly and flatly at the trabecular meshwork.
Other conditions presenting with infantile glaucoma include aniridia, Sturge-Weber syndrome, neurofibromatosis, homocystinuria, Lowe syndrome, rubella, retinoblastoma, juvenile xanthogranuloma, Axenfeld's syndrome, Rieger's syndrome, Peters' syndrome, microcornea, microspherophakia, and persistent hyperplastic primary vitreous, among others.
An examination under anesthesia is usually necessary to allow detailed, accurate measurements including IOP (measured immediately after induction of anesthesia, given the pressure-lowering effects of most general anesthetics), corneal diameter, retinoscopy, axial length, and detailed anterior and posterior segment evaluation for associated anomalies, as allowed by the clarity of the media. For the most part, the developmental glaucomas are treated surgically, given the limited success and high potential for side effects of current medications. Most clinicians begin with goniotomy or trabeculotomy for isolated trabeculodysgenesis, which often responds well. It is crucial that refractive and amblyopic issues are addressed promptly.
Secondary Open-Angle Glaucomas
The secondary open-angle glaucomas involve elevated IOPs that are related to pathologic changes. These may be broken down into pretrabecular, trabecular, and posttrabecular mechanisms of outflow obstruction.
Pretrabecular Outflow Obstruction in Secondary Open-Angle Glaucomas
The pretrabecular secondary open-angle glaucomas include neovascular, ICE, epithelial downgrowth, fibrous ingrowth,
P.127
and angle recession glaucomas. The first four of these glaucomas may also produce angle-closure glaucoma with proliferation and contraction of the pretrabecular membranes. Neovascular and ICE syndrome related glaucomas are discussed later in this chapter.
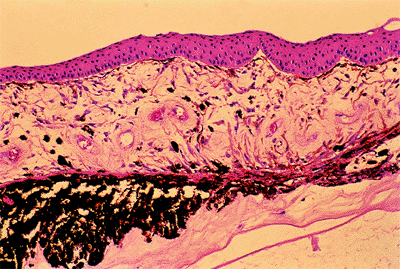 |
Figure 3.45. Epithelial ingrowth. An epithelial membrane covers the anterior iris and the anterior chamber angle recess. (Courtesy of Ralph C. Eagle, Jr., M.D.) |
Epithelial Downgrowth and Stromal Ingrowth
Perforating injuries create a pathway for epithelial and fibrous connective tissue to grow into the eye. Membranes, often with scalloped edges, may proliferate across anterior chamber structures, including the iris and corneal endothelium (Fig. 3.45). These membranes cover the trabecular meshwork and lead to reduced outflow. The membranes may be identified by argon laser treatment over affected portions of the iris. The laser will create white, rather than brown, spots.
Treatment of these conditions is extremely difficult. Filtering surgeries often fail quickly, even with antimetabolite use, as the internally proliferating membranes close the sclerostomy. Tube shunts have been advocated, but may also fail. Cryotherapy to the cornea and iris has been advocated to destroy the advancing membranes, but may lead to damage to other ocular structures. In selected cases, meticulous surgical removal of the involved areas of iris with membrane peeling from the cornea may be successful.
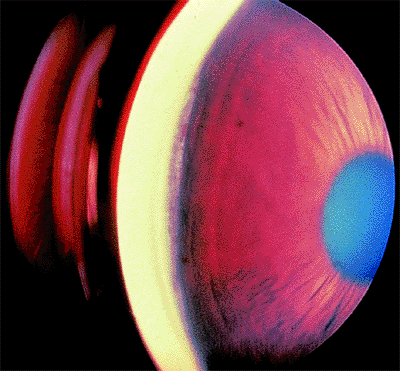 |
Figure 3.46. Traumatic angle recession. Temporally, for 2 clock hours, a widened band of the ciliary body has been exposed, in contrast to the remainder of the angle, where only the anterior portion of the ciliary body is visible. |
Angle Recession
Angle recession glaucoma is an important late complication of ocular trauma (Figs. 3.46, 3.47A,B). Angle recession, with or without elevated pressure, is seen in the majority of patients in whom trauma has caused a hyphema. IOPs may remain normal for years to decades before becoming severely elevated. Patients must be strongly advised of the need for lifelong, regular follow-up after significant ocular
P.128
trauma. Elevated IOP is more likely to occur in eyes with 180 degrees or more of angle recession, and may affect 6% to 20% of eyes with angle recession over a 10-year period. Angle recession must be considered in chronic unilateral glaucomas. Since patients often do not recall the long-past trauma, clinicians must search for clues to the prior trauma, such as facial and corneal scars, pupillary sphincter tears, iris transillumination, iridodonesis, phacodonesis, Vossius' ring, dark brown or black deposits in the inferior angle recess, and localized anterior synechiae as a sign of prior intraocular inflammation, especially at the 6-o'clock position. With time, angle recession may scar, becoming less evident by gonioscopy in apparent severity and extent. The affected areas still have nonfunctioning meshwork, secondary to scarring or their closure by a Descemet's-like membrane. Interestingly, it has been noted that the fellow eye is at significantly increased risk of developing open-angle glaucoma.
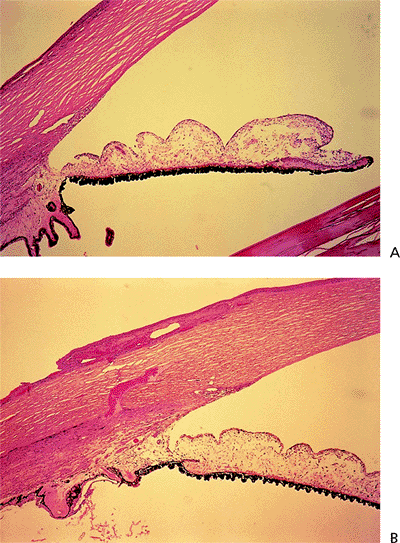 |
Figure 3.47. Traumatic angle recession. A: Normal anterior chamber angle. A line parallel to the visual axis through the trabecular meshwork crosses the tip of the first ciliary process. B: Traumatic angle recession. The iris insertion is moved posteriorly with rotation of the ciliary body. A line parallel to the visual axis through the trabecular meshwork passes internal to the retracted ciliary body processes. (Courtesy of Ralph C. Eagle, Jr., M.D.) |
Angle recession glaucoma may respond to aqueous suppressants. Miotics and argon laser trabeculoplasty are rarely effective. Long-term success rates for trabeculectomy are worse than for POAG; some surgeons use antimetabolites on primary procedures for this reason.
Glaucoma Associated With the Exfoliation Syndrome
Exfoliative glaucoma was previously called pseudoexfoliative glaucoma to differentiate the syndrome from the splitting off of a layer of the anterior lens capsule exposed to intense heat as seen with glass blowers and blast furnace workers. However, studies have shown the unknown substance found on the lens, zonules, and other intraocular tissues is exfoliated. It is thought to be related to elastic fibers and basement membrane components. Also discovered was the fact that exfoliation in the eye is a local manifestation of a systemic disorder. Transmission electron microscopy has revealed exfoliative fibrils in the connective tissue components of skin, heart, lungs, liver, kidney, and cerebral meninges, suggesting an aberrant connective-tissue metabolism throughout the body. These systemic changes have not been shown to cause an increase in cardiovascular or cerebrovascular mortality.
While exfoliative glaucoma occurs worldwide, its prevalence varies from region to region. Scandinavians and South African blacks are disparate populations who are especially at risk. There is a clear association with age, but males and females are affected equally. An increased prevalence of occludable angles and angle-closure glaucoma has been demonstrated.
The classic appearance of exfoliation is a whitish, dandruff-like material coating the anterior surface of the crystalline lens. The excursions of the pupil over the lens surface have worn off the exfoliative material in a band around a central untouched disc, also leaving the more peripheral material untouched (Fig. 3.48). Constant movement over a rough surface leaves the underside of the pupillary margin worn away in a saw tooth pattern, with transillumination of the peripupillary iris and an impaired blood-aqueous barrier. Black pigment exfoliated from the neuroepithelium of the uveal tract and later pigment chafed off the posterior pigment epithelium of the iris is deposited on the anterior iris surface and in the inferior angle. Unlike pigmentary dispersion syndrome, where the pigment is evenly distributed for 360 degrees around the angle, in the exfoliation syndrome the pigment is mostly deposited inferiorly. Gonioscopy in exfoliation patients reveals three distinct lines: pigment in the trabecular meshwork, pigment layered out on Schwalbe's line, and pigment deposited in a serpentine line well anterior to Schwalbe's line (Fig. 3.49). The latter is called Sampaolesi's line and can be especially important when making the diagnosis of exfoliative glaucoma in the presence of a small pupil that cannot be dilated. Other clues are exfoliative material on the pupillary margin, anterior iris surface, corneal
P.129
endothelium, and anterior vitreous face. When discovered, the exfoliation syndrome is usually unilateral or asymmetric (Table 3.6). Even in fellow eyes with no visible exfoliation, exfoliation material can be detected ultrastructurally and immunohistochemically around iris blood vessels.
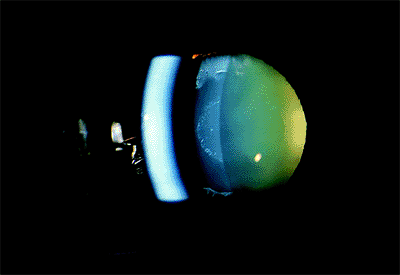 |
Figure 3.48. The anterior lens capsule of a patient with the exfoliation syndrome demonstrating the typical central disc of exfoliative material, clear capsule under the edge of the pupil, and peripheral exfoliative material. |
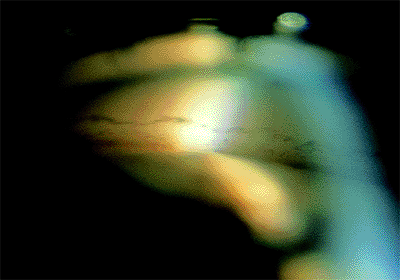 |
Figure 3.49. Inferior angle showing three distinct pigment lines: in trabecular meshwork, on Schwalbe's line, and a serpentine line well anterior to Schwalbe's line. |
Table 3-6. Exfoliation Syndrome Characteristics | |
|---|---|
|
Early recognition of the patient with exfoliation syndrome is important in order to foster close follow-up and allow early detection and treatment of glaucoma. At the time of diagnosis, IOP and its diurnal variation are usually higher and the visual field defects greater in exfoliative glaucoma compared to POAG. Exfoliative glaucoma has also been shown to have a poorer prognosis than POAG. Because lowering IOP has been shown to improve visual field prognosis more in exfoliative glaucoma than in POAG, glaucoma progression is felt to be more pressure related in exfoliative glaucoma.
Recognition of the exfoliation syndrome is also crucial in the patient seeking cataract extraction. Because zonules attach to the surface of the lens capsule, exfoliation material forming under the insertion of the zonules into the lens capsule undermines the strength of the zonules and can result in phakodonesis, lens subluxation, and vitreous loss at the time of surgery (Fig. 3.50).
 |
Figure 3.50. This peripheral iridectomy allows a look at how exfoliative material forming under the zonular attachment to the lens allows the zonules to pull free of the lens, causing marked phakodonesis in this patient. |
Management
Treatment of exfoliative glaucoma varies little from that of POAG, with the following exceptions. Of all the sundry glaucomas, argon laser trabeculoplasty works best in exfoliative glaucoma, enhancing its position in the therapeutic armamentarium. Trabecular aspiration, a surgical procedure in which exfoliative material, pigment, and debris are vacuumed from the trabecular meshwork, has been used in Germany with lesser effectiveness in controlling IOP but greater safety than trabeculectomy.
Pigment Dispersion Syndrome and Pigmentary Glaucoma
The pigment dispersion syndrome is characterized by deposition of pigment in the posterior trabecular meshwork (Fig. 3.51A,B), on the endothelial surface of the cornea (Krukenberg's spindle), and on the equatorial surface of the
P.130
lens (Zentmeyer line) (Fig. 3.52). There is also a characteristic spoke-like loss of pigment from the posterior pigment epithelium in the mid to far periphery of the iris (Fig. 3.53). It is the pigment from the iris that causes the deposits of pigment on the central corneal endothelium in the posterior trabecular meshwork (Fig. 3.51) and on the lens. The anterior surface of the iris may also be involved.
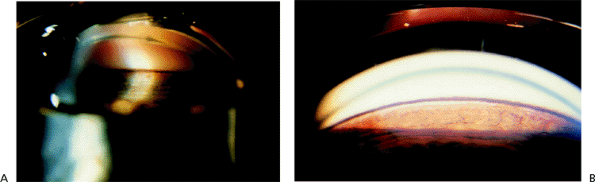 |
Figure 3.51. A, B: Dense, even pigmentation of the trabecular meshwork over 360 degrees is also a requisite for the diagnosis of pigmentary glaucoma and differentiates this disorder from most of the diseases in the differential diagnosis. The peripheral iris often slopes posteriorly from its insertion, draping over the zonules and, more centrally, over the lens. |
 |
Figure 3.52. Pigmentary deposition on the equatorial surface of the lens (Zentmeyer line). |
 |
Figure 3.53. Midperipheral iris transillumination defects are pathognomonic of pigmentary glaucoma and reveal the source of the malady. |
If the pigment deposition and secondary changes to the outflow pathways are sufficient to cause relative blockage of aqueous outflow, the IOP may increase and play a role in the development of optic nerve damage. The combination of the pigment dispersion syndrome with glaucoma is called pigmentary glaucoma. This type of glaucoma occurs most commonly in myopic individuals between the ages of 20 and 45 years and affects men approximately twice as often as women.
Not all the findings described for the pigment dispersion syndrome are present in every individual. In fact, it is not unusual for some of the findings to be absent. Iris transillumination defects are rarely seen in patients with dark brown irides. In addition, an extraordinarily important aspect of the pigment dispersion syndrome is lattice degeneration of the retina. Patients with the pigment dispersion syndrome are predisposed to retinal detachment, not simply because they are myopic, but also, apparently, as a result of the pigment degeneration itself.
The cause of the pigment dispersion is partly related to reverse pupillary block. This reverse pupillary block is a phenomenon in which aqueous is pumped anteriorly from the posterior chamber into the anterior chamber, with accommodation or blinking, and the aqueous in the anterior chamber then forces the peripheral, unsupported portion of the iris posteriorly back towards the zonules. The posterior pigment epithelium can then rub against the zonules, which predisposes the pigment epithelium to be released. This reverse pupillary block is illustrated in Figure 3.54. If blinking is prevented, the anterior chamber configuration can spontaneously return to normal (Fig. 3.55). This posterior bowing of the iris is called a q iris configuration. The pigment that is lost from the posterior pigment epithelium floats to the trabecular meshwork, where it is phagocytized by the endothelial cells lining the trabecular beams. If overloaded, these cells die, leaving denuded trabecular beams that can no longer rid themselves of further deposition of pigment, glycosaminoglycans, and other debris. Conse-quently, there is an elevation of IOP.
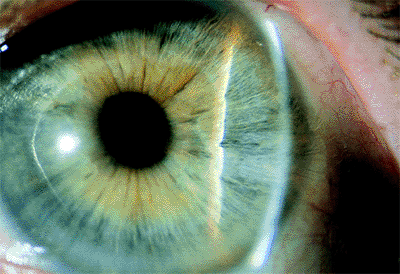 |
Figure 3.54. Reverse pupillary block. The iris is pushed back against the lens and zonules during normal blinking in this eye with loose zonules. |
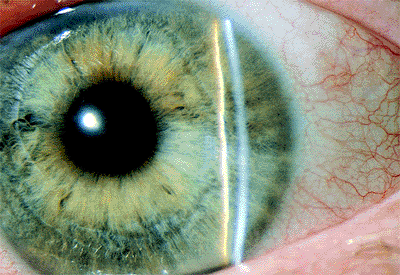 |
Figure 3.55. The same eye as in Figure 3.54 after 1 minute without blinking. |
P.131
Pigmentary glaucoma may be especially serious in some individuals, at least partially because of the tendency of myopes to develop optic nerve damage more easily than emmetropes or hyperopes.
Some authors comment on the tendency of the pigment dispersion syndrome and pigmentary glaucoma to become less severe with increasing age. They point out that as the individual ages the pupil becomes smaller and the lens enlarges, as a result of which there is an increase in the degree of relative pupillary block. This results in elimination of the q configuration and development of an actual anterior convexity, decreasing the contact between the posterior pigment epithelium and the zonules, and therefore decreasing the amount of pigment release. While this phenomenon is probably real, it is probably also of little clinical significance. It is unwise to think that a patient who has pigmentary glaucoma will have a disappearance of the glaucoma with increasing age.
The differential diagnosis of pigmentary glaucoma is shown in Table 3.7.
Management
Two methods have been recommended by some authors to stop or lessen the pigment dispersion syndrome in patients with pigmentary glaucoma who have elevated IOP characterized by posterior bowing of the peripheral iris. Miotics can cause increased lens-iris apposition and therefore increase the amount of relative anterior pupillary block. This can, as mentioned above, decrease the contact between the posterior pigment epithelium and the zonules, decreasing the amount of pigment release. However, miotics also predispose to retinal detachment, a condition to which patients with pigment dispersion syndrome and pigmentary glaucoma are strongly predisposed. Consequently, if miotics are to be considered, they should only be used after a meticulous examination of the peripheral retina. Additionally, because miotics cause a marked myopic shift in individuals below the age of 40, they are usually not well tolerated. Administering pilocarpine through a sustained-release system such as the pilocarpine Ocusert has the advantage that the constant release of a small amount of pilocarpine causes less accommodative spasm, allows for a constant refraction, and therefore may allow the pilocarpine to be tolerated, even by younger individuals. However, the pilocarpine Ocusert can sometimes malfunction, allowing a sudden release of pilocarpine, predisposing the patient to retinal detachment. Additionally, the long-term use of miotics results in small, rigid pupils in a condition that makes the use of miotics undesirable in most patients. A different method of reducing the pigment release has been suggested by authors who recommend peripheral iridotomy. The peripheral iridotomy can decrease the reverse pupillary block. However, the long-term benefit of peripheral iridotomy has not been demonstrated. Additionally, patients who develop marked pigment release with exercise do not appear to be significantly helped with peripheral iridotomy. The benefit, then, is not proven, but there is a risk, specifically, a pressure elevation that may push the marginally compensated individual into a need for a glaucoma filtering procedure.
Table 3-7. Differential Diagnosis of Pigmentary Glaucoma | |
|---|---|
|
Argon laser trabeculoplasty is usually highly effective in patients with the pigment dispersion syndrome, even relatively young individuals. The amount of energy needs to be reduced, in view of the intense pigmentation of the posterior trabecular meshwork, and powers of 400 mW or sometimes even less are usually adequate.
Aphakic and Pseudophakic Glaucomas
Increased IOP is a potential complication of cataract extraction. Many etiologies lead to postoperative pressure elevations and are best considered by time of onset.
Within the first week, many of the materials dispersed during surgery can lead to elevated pressures. -Chymotrypsin frequently resulted in pressure spikes in the first 2 days following intracapsular cataract extraction. Retained viscoelastic material is a source of trabecular obstruction manifesting in the first postoperative day. Increased IOPs are seen with all current viscoelastics; no definitive difference in incidence has been shown among them. Meticulous removal at time of surgery may reduce the frequency of this complication. Hyphema following surgery also blocks outflow and can lead to elevated IOP. Debris, inflammation, and trabecular edema may also play roles in acutely
P.132
elevated pressures postoperatively. Preexisting glaucoma increases the frequency and severity of acute postoperative pressure elevations. These complications are best treated medically with aqueous suppressants and topical steroids.
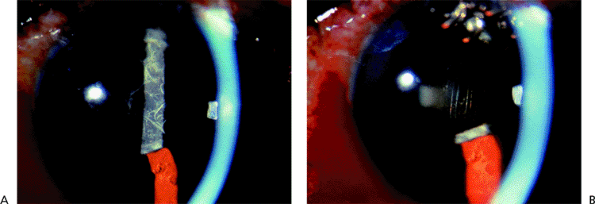 |
Figure 3.56. Fibrin clot over posterior chamber intraocular lens with elevated intraocular pressure (IOP) following combined guarded filtration procedure and cataract extraction (A). Same eye (B) following intracameral injection of tissue plasminogen activator. Visual acuity improved dramatically and IOP dropped from 38 to 10 mm Hg, and remained well controlled. |
Fibrin clots may be treated with intracameral injection of tissue plasminogen activator (Fig. 3.56A, B). Surgical evacuation of viscoelastic or blood is reserved for pressures that do not respond to medical therapy and threaten the optic nerve.
After the first week, other factors may lead to glaucoma. Vitreous in the anterior chamber may reduce aqueous outflow. Hyphema related to wound construction or intraocular lens position may occur. Ghost cell glaucoma results from longstanding hyphema or vitreous hemorrhage leading to degenerated erythrocytes that are less flexible and block trabecular channels. Retained lens particles become hydrated and more prominent, blocking the trabecular meshwork. Postoperative inflammation related to retained lens particles, intraocular lens position, vitreous traction, and surgical trauma may become more evident. Corticosteroid induced glaucoma must be considered as a common source of increased IOP in postoperative patients on topical steroids. Discontinuation of steroids or a therapeutic challenge of the fellow eye with topical steroids often will aid in the diagnosis.
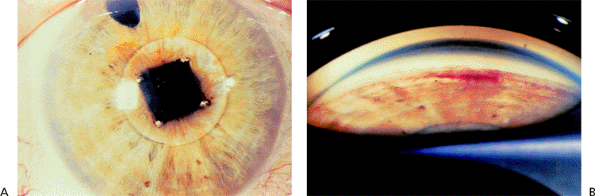 |
Figure 3.57. Right eye of a patient with intraocular lenses and the uveitis, glaucoma, hyphema syndrome in both eyes. This patient had multiple hyphemas over a period of years. In some instances, the inflammation was readily apparent, but the hyphema was only appreciated on gonioscopy. A: Iris-supported intraocular lens. B: Gonioscopy reveals small hyphema. |
Late pressure elevations, occurring months to years following surgery, may also be secondary to inflammation and hemorrhage. Propionibacterium acnes endophthalmitis may lead to chronic inflammation with increased pressures. Intraocular lenses may be a source of inflammation and hyphema in the UGH (uveitis, glaucoma, hyphema) syndrome (Figs. 3.57A,B; 3.58A,B). Anterior chamber lenses may directly damage the trabecular meshwork. Vitreous in the anterior chamber may still block outflow. Following YAG laser capsulotomy, inflammation may lead to quite significant pressure elevations whose frequency is greatly reduced by pretreatment with apraclonidine.
 |
Figure 3.58. Left eye of the same patient seen in Figure 3.57. A: Solid one-piece anterior chamber intraocular lens. B: Gonioscopy shows haptic through iridectomy abutting ciliary body. |
P.133
Angle-closure glaucoma may be seen throughout the postoperative course, related to inflammation, pupil block, or shallow chambers. Malignant glaucoma is a relatively uncommon but important cause of postoperative glaucoma. Epithelial downgrowth is another rare cause of late postoperative glaucoma that represents a severe threat to the eye.
Cyclodialysis Cleft Closure
Traumatic cyclodialysis clefts divert aqueous away from the trabecular meshwork, and may lead to hypotony (Fig. 3.59). These clefts may be caused by trauma or may occur during intraocular surgery, such as cataract extraction. The unused trabecular meshwork becomes less permeable to aqueous. Months or years later, spontaneous closure of the cyclodialysis cleft may lead to dramatic elevations of IOP. Patients present with sudden onset of ocular pain, headaches, and reduced vision. Slit lamp and gonioscopic evaluation differentiates this entity from the similar presentation of acute angle-closure glaucoma. Aqueous suppressants are often effective in reducing IOP chronically, or until the trabecular meshwork recovers and better outflow facility resumes. Miotics are avoided as they may reopen cyclodialysis clefts. An exception to this is the sudden closure of a longstanding cleft with prior good IOP control. In this situation, immediate use of a strong miotic, such as pilocarpine 4%, may open and reestablish the cleft. Occasionally, filtering surgery may be necessary in cases of persistently elevated pressures.
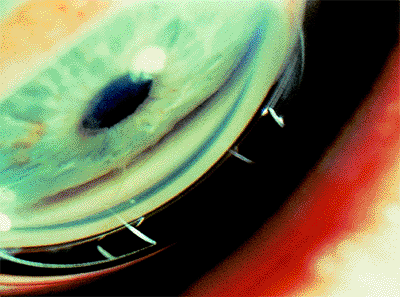 |
Figure 3.59. Two small areas of cyclodialysis just able to be visualized in hypotonus eye. Intracameral injection of viscoelastic may aid in the gonioscopic examination. |
Corticosteroid-Induced Glaucoma
The use of corticosteroids in any form may lead to the development of a secondary open-angle glaucoma. Increased IOP has been demonstrated with topical, periocular, inhalational, and systemic steroids, as well as with increased endogenous steroids in adrenal hyperplasia and Cushing's syndrome (Figs. 3.60, 3.61). Past steroid use and glaucoma may simulate the presentation of normal-tension glaucoma.
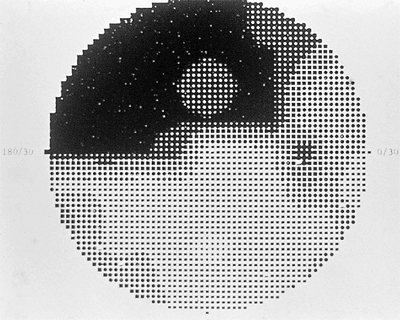 |
Figure 3.60. Advanced visual field loss in a doctor who self-prescribed topical steroids for vernal conjunctivitis for many years without eye examinations. |
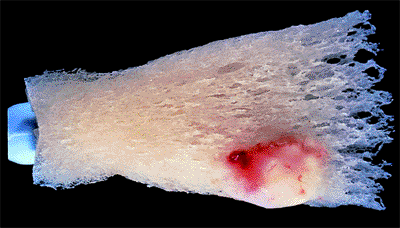 |
Figure 3.61. For 5 months following vitrectomy, intraocular pressures were elevated to as high as 44 mm Hg, despite medications in a patient without previous glaucoma. The subconjunctival steroid depot, shown here, was excised and the glaucoma resolved completely in 1 month. |
P.134
Topical steroids are more likely to elevate IOP than systemic steroids. IOPs rise more frequently and more severely with the more potent steroids and with greater dosing. Pressure elevation typically manifests 2 to 6 weeks after the initiation of steroids. Patients with POAG respond much more frequently and severely to steroids with elevated IOP. Depending on steroid dose, duration, and diagnostic criteria, over 90% of patients with POAG may have pressure elevations with steroids compared to 5% to 10% of normals. Other risk factors for steroid responsiveness include ocular hypertension, angle recession glaucoma (in either eye), primary relative of patient with POAG, pigmentary glaucoma, myopia, diabetes, and history of connective tissue disease, but not exfoliative glaucoma.
Corticosteroids result in the accumulation of glycosaminoglycans in the trabecular meshwork, reducing outflow facility. Theories for the mechanism of this accumulation of glycosaminoglycans include increased production and reduced clearance, mediated either by nuclear steroid receptors or by membrane stabilization.
The diagnosis may be made either by withdrawing steroid therapy and observing reduced pressures or by challenging the fellow eye in topical steroid cases. IOPs usually may begin to drop in days and return to baseline within 1 to 2 months, but sometimes remain high in patients who have been on steroids for long periods of time. Elevated pressures following periocular injection of steroids may require the excision of depot steroids. Aqueous suppressants and miotics can be effective in treating the elevated pressure. Argon laser trabeculoplasty may be useful, although less effective than in POAG, pigmentary glaucoma, and exfoliative glaucoma.
Posttraumatic Glaucomas
Nonpenetrating injuries of the eye secondary to blunt trauma are usually related to the anterior-to-posterior compression of the eye with secondary equatorial stretching. This stretching may result in pupil sphincter tears, iridodialysis, angle recession, cyclodialysis, trabecular dialysis, disruption of the lens zonule, and retinal dialysis or detachment.
Tears in the face of the ciliary body, usually between the circular and longitudinal muscles, may disrupt the major arterial circle of the iris and the arterial and venous connections of the ciliary body, resulting in hyphema. Severity may range from a microhyphema, in which the slit-lamp microscope is necessary to appreciate the presence of blood in the anterior chamber, to total hyphema, in which the anterior and posterior chambers are entirely filled with blood (Figs. 3.62; 3.63A,B).
IOP may be elevated or reduced, depending on the balance of several factors. Aqueous secretion may be acutely reduced; uveoscleral outflow may be increased by an accompanying cyclodialysis; blood may obstruct an injured and inflamed trabecular meshwork. Although red blood cells normally pass through the pores in the meshwork, acute inflammation and swelling of the meshwork combined with excessive amounts of red blood cells and debris may overwhelm the meshwork's capacity. Aqueous suppressants are effective in many cases. Steroids help to reduce inflammation. Miotics are generally avoided as they may predispose to posterior synechiae, further compromise the blood aqueous barrier, and reduce uveoscleral outflow. Patients with hyphema are generally kept at bedrest and treated with mydriatics and topical steroids. Aminocaproic acid, an antifibrinolytic agent, is also used in some centers to reduce the incidents of rebleeds, which may be severe.
Prolonged elevation of IOP may require surgical evacuation of the blood. Timing is dependent on the ability of the nerve to withstand elevated pressures; otherwise, healthy nerves may tolerate pressures of up to 50 mm Hg for several days. Evacuation may also be necessary because of the development of blood staining, especially in those at risk for amblyopia (Fig. 3.64). Surgical evacuation is ideally performed near the fourth day posttrauma. This is past the peak incidence of rebleeding and allows time for the anterior
P.135
chamber blood to clot and retract somewhat from ocular structures, facilitating removal.
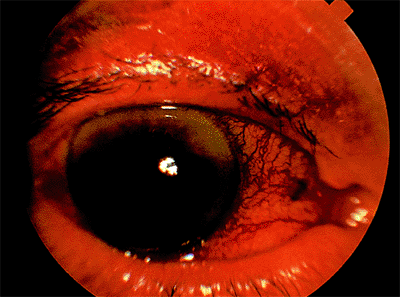 |
Figure 3.62. Traumatic hyphema. Two fluid levels exist as the red blood cells settle and clot. |
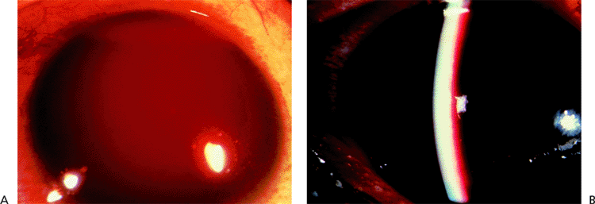 |
Figure 3.63. Total hyphema (A). The incidence of elevated intraocular pressure increases with the amount of blood in the anterior chamber. More than half of those with total hyphemas have significant pressure elevations; with blackball or eight-ball hyphemas (B) this approaches 100%. Patients with sickle cell disease may suffer severe pressure elevations with relatively minor hyphemas. |
Patients with sickle cell disease are at greater risk for complications from hyphemas. Significant IOP elevations may occur with even small hyphemas. Sickling has been demonstrated in the anterior chamber blood; this may reduce clearance and increase IOP. Central retinal arterial obstruction may occur at lower pressures following hyphemas in these patients. Carbonic anhydrase inhibitors are avoided in sickle cell disease as they may worsen sickling and reduce clearance of blood by increasing aqueous ascorbic acid and by promoting systemic acidosis. Black patients with hyphemas should be screened for sickle cell hemoglobin; positive patients should undergo hemoglobin electrophoresis. All available therapies should be used to keep IOPs below 25 mm Hg.
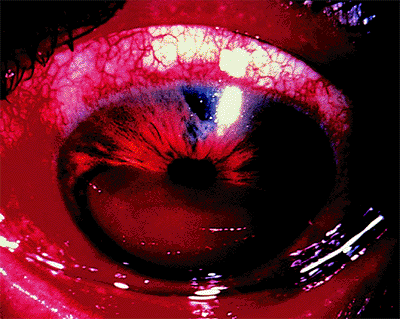 |
Figure 3.64. Corneal blood staining following a traumatic hyphema. These generally clear over weeks to months, starting at the periphery, following hyphema resolution. In patients in the amblyopic age group, incipient blood staining may require surgical evacuation of the blood. |
Trauma may also lead to angle-closure glaucoma through a variety of mechanisms. Clots may cover the pupil, leading to pupillary block and angle closure. Dislocated lenses may cause pupillary block. Posttraumatic inflammation may lead to extensive peripheral anterior and posterior synechiae. Shallow anterior chambers with penetrating trauma also may create extensive anterior synechiae.
Trauma may also lead to retinal detachments. A rare cause of open-angle glaucoma in the setting of a retinal detachment is Schwartz's syndrome.
Schwartz's Syndrome
Schwartz's syndrome is elevated IOP secondary to obstruction of the trabecular meshwork by photoreceptor outer segments. These photoreceptor outer segments are released from a chronic retinal detachment through a tear in the retina (Fig. 3.65). The retinal detachments are often low lying and peripheral and may be easily overlooked. The photoreceptor outer segments may be mistaken for white blood cells and the glaucoma erroneously diagnosed as uveitic. Pressures may fluctuate widely depending on the balance of trabecular meshwork outflow obstruction and increased outflow through the retinal detachment. The retinal pigment epithelium has a pumping function that normally evacuates subretinal fluid, keeping the retina firmly attached to the pigment epithelium. In the case of a retinal detachment, this pumping function helps to lower IOP. This can very rarely lead to the iris retraction syndrome, in which patients with Schwartz's syndrome and extensive posterior synechiae show iris retraction when placed on aqueous suppressants. The theory is that the reduced inflow from aqueous suppression, coupled with the reduced flow into the anterior chamber and increased outflow through the retinal detachment, leads to hypotony and an exceptionally deep anterior chamber.
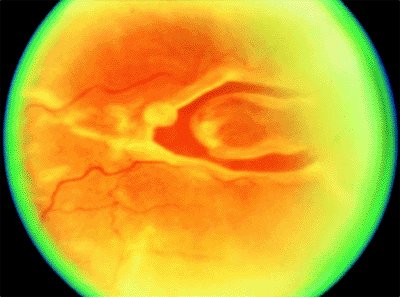 |
Figure 3.65. Peripheral horseshoe tear of the retina. Retinal tears with detachments can release photoreceptor outer segments. In Schwartz's syndrome these segments may clog the trabecular meshwork and increase intraocular pressure. |
P.136
Ghost Cell Glaucoma
Ghost cells are degenerated red blood cells whose leaky membranes have allowed much of the cell's hemoglobin to escape. Residual, degenerated hemoglobin precipitates on inner cell walls in the form of Heinz bodies. These spherical cells are much less pliable than red blood cells and cannot pass through the intertrabecular spaces, leading to pressure elevations at relatively lower concentrations than expected with normal red blood cells.
The formation of ghost cells requires the sequestration of red blood cells in the vitreous for several weeks. The source of vitreous hemorrhage does not matter a bleed from diabetic neovascularization of the retina, hemorrhage secondary to trauma, or a postsurgical hyphema with spill-over into the vitreous through a compromised vitreous face may evolve into a secondary glaucoma. After several weeks, the degenerated cells migrate back into the anterior chamber through a defect in the vitreous face, surgical or traumatic, and obstruct the trabecular meshwork. Tan or khaki-colored cells are seen in the anterior chamber, the anterior vitreous, and the trabecular meshwork, and may also form a pseudohypopyon (Fig. 3.66A,B). If the vitreous face is intact, the ghost cells do not reach the anterior chamber and no glaucoma results.
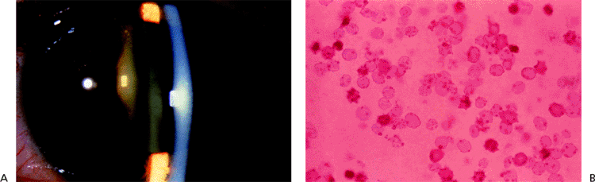 |
Figure 3.66. This patient with ghost cell glaucoma had 2+ anterior chamber khaki-colored cells and marked khaki-colored cells in the anterior vitreous (A). Vitrectomy specimen showed ghost cells (B). |
Several other blood-related elevations of IOP may be differentiated from ghost cell glaucoma. Hyphemas may result in acutely elevated pressure, especially in patients with sickle cell disease. This is secondary to obstruction of the meshwork by red blood cells in the first days following the hyphema. Rarely, an eight-ball, total hyphema may persist long enough to contain ghost cells that add to the trabecular obstruction. Hemolytic glaucoma occurs when the contents of ruptured red blood cells are ingested by macrophages that then obstruct the meshwork. Hemolytic glaucoma is also typically seen in the first days to weeks following the initial hemorrhage. Hemosiderotic glaucoma, in which iron-containing blood breakdown products stain and poison the cells of the trabecular meshwork, occurs much later, often years after the hemorrhage.
Aqueous suppressants often control the IOP. Miotics are rarely helpful as the meshwork is obstructed; similarly, argon laser trabeculoplasty is not indicated. Anterior chamber lavage with balanced salt solution may resolve the glaucoma. If unsuccessful, or too great a reservoir of ghost cells remains in the vitreous, total vitrectomy, removing as much hemorrhagic material as possible, is usually effective in resolving the glaucoma.
Inflammatory Glaucomas
Inflammation within the eye alters aqueous humor dynamics and can lead to increased IOP. Acute inflammation may reduce aqueous secretion and increase uveoscleral outflow, reducing IOP. However, inflammatory material, consisting
P.137
of white blood cells, macrophages, and proteins, may obstruct the trabecular meshwork. Chemical mediators of inflammation may further compromise trabecular function, as may trabeculitis, inflammation of the trabecular meshwork itself. Trabecular dysfunction may be transient, clearing with resolution of the inflammation, or may persist, with permanent structural changes reducing trabecular outflow facility.
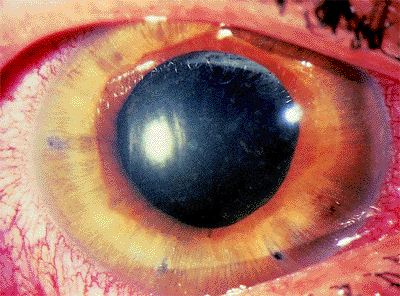 |
Figure 3.67. Idiopathic uveitis resulting in secondary glaucoma with closed- and open-angle components. Note the multiple laser peripheral iridotomies for previous acute iris bomb . Following resolution of acute attacks, patient had persistent elevation of intraocular pressure in spite of large of areas of anterior chamber angle on gonioscopy. |
Additionally, inflammatory scarring may lead to peripheral anterior synechiae and posterior synechiae, resulting in angle-closure glaucoma. Chronic uveitis may lead to neovascularization and neovascular glaucoma. Patients may manifest some or all of these findings, the balance dictating the nature of the glaucoma. For example, a patient may present acutely during a uveitic episode with uveitic hypotony, progress to a secondary open-angle glaucoma as white blood cells block the meshwork, develop an angle-closure component as synechiae form, and then be left with a chronic mixed mechanism secondary glaucoma with residual synechiae and scarring of the meshwork.
Virtually all sources of ocular inflammation can lead to glaucoma. Idiopathic uveitis (Fig. 3.67), glaucomatocyclitic crisis (Fig. 3.68), Fuch's heterochromic cyclitis (Fig. 3.69), lens-related uveitis (Fig. 3.70), herpes (Fig. 3.71), and sarcoidosis (Fig. 3.72) may all result in increased IOP. Other uveitic glaucomas include HLA-B27 related uveitides, pars planitis, sarcoidosis, juvenile rheumatoid arthritis, Beh et's disease, Crohn's disease, syphilis, toxoplasmosis, coccidioidomycosis, mumps, rubella, leprosy, sympathetic ophthalmia, Vogt-Koyanagi-Harada syndrome, and other less common entities.
Treatment strategies are similar for these entities. Resolution of inflammation is the first goal, which may require topical or systemic steroids, antibiotics for infectious diseases, and lens extraction in lens-related conditions. Increased IOP secondary to steroid therapy or to increased aqueous production with resolving inflammation may confuse the clinical picture. Cycloplegics are effective through many mechanisms. Dilation prevents the formation of a small secluded pupil and relieves the discomfort of ciliary spasm. Cycloplegics also improve uveoscleral outflow and stabilize the blood-aqueous barrier. Topical and systemic aqueous suppressants are effective. Miotics are avoided as these agents further reduce the blood-aqueous barrier and reduce uveoscleral outflow. Similar concerns argue against the use of prostaglandin analogs, although limited data are available. Argon laser trabeculoplasty is generally ineffective
P.138
P.139
and increases inflammation. Filtering surgery is less successful in these patients, especially during acute attacks. Adjunctive use of antimetabolites such as 5-fluorouracil (5-FU) and mitomycin-C may improve results. Drainage tube implants may also be effective. Cyclodestructive procedures can be used in refractory cases, but contribute to intraocular inflammation and pose significant risks to vision.
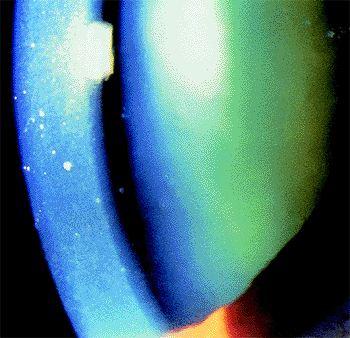 |
Figure 3.68. Glaucomatocyclitic crisis (Posner-Schlossman syndrome). Unilateral involvement consisting of fine keratic precipitates in a patient with minimal anterior chamber reaction and markedly elevated intraocular pressure (IOP). Following multiple similar attacks, patient developed chronically elevated IOP and optic nerve damage requiring trabeculectomy. |
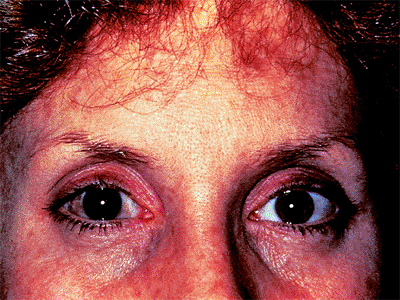 |
Figure 3.69. Fuch's heterochromic cyclitis. Note the much lighter iris color in the involved eye. The triad of heterochromia, uveitis, and cataract typically appears in the third to fourth decade. The uveitis is asymptomatic. Open-angle glaucoma is common, increasing with duration of the disease. Friable angle vessels may bleed easily with surgery; however, frank neovascularization of the iris and neovascular glaucoma are uncommon. |
 |
Figure 3.70. Lens-related glaucoma. In phacolytic glaucoma, a hypermature lens leaks proteins into the anterior chamber through a grossly intact capsule (A); these proteins and ingesting macrophages block the trabecular meshwork. A swollen, mature cataract may also lead to pupillary block in phacomorphic glaucoma; note the narrow angle present in a patient with phacolytic glaucoma in (B). A hypermature cataract with liquefied cortex and dense nucleus may also lead to phacolytic glaucoma (C). Retained lens cortex (D) following cataract extraction may increase in size with hydration and lead to glaucoma through trabecular obstruction by lens material. Phacoanaphylaxis exists when lens material is present outside the capsule following surgery or trauma and leads to granulomatous inflammation. A previous history of trauma to the lens or cataract surgery in the fellow eye is typical. Phacoanaphylaxis often leads to significant pressure elevation. |
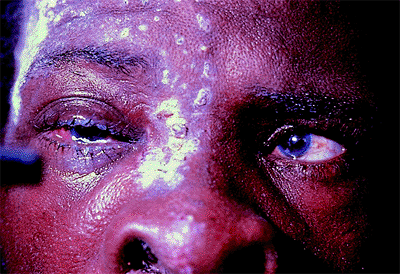 |
Figure 3.71. Herpes zoster ophthalmicus with characteristic skin lesions affecting the dermatome of the first branch of the facial nerve. The tip of the nose is involved, a harbinger of ocular involvement (Hutchinson's sign). Marked conjunctival injection and a granulomatous uveitis are present. Glaucoma is a frequent complication in herpes zoster and herpes simplex uveitis. |
 |
Figure 3.72. Sarcoid uveitis with posterior synechiae and cataract. Up to half of the patients with sarcoid suffer from uveitis at some point during the disease process. Granulomatous anterior uveitis is a common manifestation, frequently becoming bilateral, recurrent, and chronic. Sarcoid nodules on the iris (Busacca nodules in the crypts, Koeppe nodules at the pupillary margin) may be seen. Secondary open- and closed-angle glaucoma is common. As the disease is more common in blacks, clinicians must be wary of steroid-induced glaucoma as a complication of treatment. |
Glaucoma Associated With Ocular Tumors
Benign and malignant intraocular tumors may cause glaucoma by a variety of mechanisms. Direct infiltration of the trabecular meshwork can block aqueous outflow. Free-floating tumor cells may obstruct the meshwork. Hemorrhage may produce a secondary open-angle glaucoma. Tumor-related neovascularization of the iris and angle produces glaucoma in some cases. Large posterior masses sometimes may press the iris and lens anteriorly, resulting in angle closure. Tumor-related glaucomas are typically unilateral. Treatment of the glaucoma without diagnosis of the tumor may have serious consequences for the patient. A thorough examination will reveal most intraocular malignancies, especially for clinicians alert to such tip-offs as a sentinel vessel, a prominent episcleral vessel overlying a choroidal tumor.
Uveal melanomas are the most common intraocular malignancy in adults, and are the most common cause of tumor-related glaucoma. Iris melanomas may obstruct the trabecular meshwork by direct invasion or by hemorrhage (Fig. 3.73). Ciliary body melanomas may be more difficult to detect, remaining hidden until relatively large. Glaucoma most often results from the tumor pushing the iris anteriorly to close the anterior chamber angle. Glaucoma may also result due to direct extension of the tumor into the trabecular meshwork, iris neovascularization, hyphema, or tumor necrosis causing obstruction of the meshwork by cells and debris (Fig. 3.74). Choroidal melanomas less commonly cause glaucoma. Mechanisms of glaucoma in choroidal melanomas include iris and angle neovascularization most commonly, as well as angle closure, hemorrhage, and necrosis.
 |
Figure 3.73. Iris melanoma with involvement of the trabecular meshwork. |
 |
Figure 3.74. Hyphema causing secondary open-angle glaucoma in a patient with uveal melanoma. |
Histologically benign tumors of the iris may also cause glaucoma. Diffuse uveal melanomas may block the meshwork with melanoma cells or pigment. Melanocytomas, most commonly found on the optic disc but also seen in the iris or ciliary body, can produce glaucoma. These tumors have a propensity to undergo necrosis and fragmentation, blocking the trabecular meshwork with debris, producing the so-called melanomalytic glaucoma. Differentiation of these tumors from their malignant counterparts is a crucial step in treatment, often requiring histopathologic study.
Intraocular metastases are typically to the uveal tissues. Glaucoma is found more frequently in metastases to the iris and ciliary body (64% and 67%, respectively) than in metastases to the choroid (2%). Common primary tumors include those of the breast and lung, as well as tumors of the gastrointestinal tract, kidney, thyroid, and skin. Efforts to identify the primary neoplasm are not always successful. Friable metastases often seed the anterior chamber angle, obstructing the trabecular meshwork (Figs. 3.75, 3.76). Pseudohypopyons and ring infiltrates are sometimes seen.
Benign reactive lymphoid hyperplasia can directly invade the trabecular meshwork. Large cell lymphoma (reticulum cell sarcoma, histiocytic lymphoma) and leukemia may also infiltrate the meshwork and lead to glaucoma.
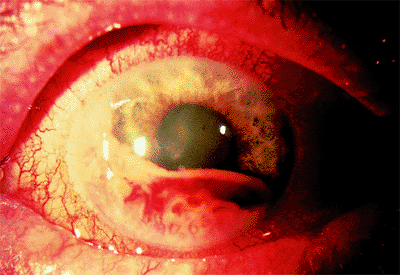 |
Figure 3.75. Metastatic tumor from primary tumor in lung to iris. |
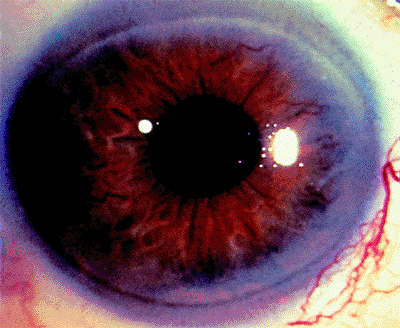 |
Figure 3.76. Multiple myeloma invading iris. |
P.140
Retinoblastoma is the most common intraocular malignancy of childhood. Glaucoma is not uncommon, occurring in 17% of cases in one study. In that study of 303 eyes, the glaucoma was thought to be secondary to iris neovascularization with or without hemorrhage in 72%, angle closure in 26%, and tumor seeding in 2% (Fig. 3.77A, B). The less common but benign medulloepithelioma may also cause glaucoma secondary to iris neovascularization, as well as direct invasion of angle structures or obstruction of the meshwork by hyphema and tumor-related debris.
Various ocular tumors are manifested by the phakomatoses. Unilateral glaucoma, congenital or later onset, may be seen in neurofibromatosis of both types (Fig 3.78). Half of all eyes with plexiform neuromas of the upper lid develop glaucoma. The glaucoma associated with encephalotrigeminal angiomatosis (Sturge-Weber syndrome) is discussed along with other causes of increased episcleral venous pressure. Congenital glaucoma may also be seen. Oculodermal melanocytosis (nevus of Ota) has been reported to have an incidence of glaucoma of 10%, including angle-closure, open-angle, and congenital varieties.
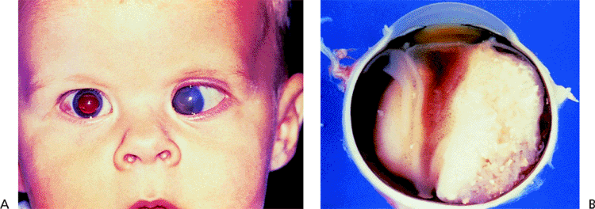 |
Figure 3.77. Retinoblastoma causing glaucoma. A: External photo. B: Gross pathologic specimen. |
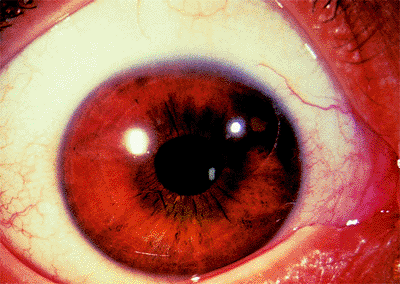 |
Figure 3.78. Neurofibromatosis type 1 with Lisch nodules, most prominently superonasal. Lisch nodules, variably pigmented collections of melanocytic spindle cells, are seen bilaterally in all patients over age 16 with neurofibromatosis type 1. Nodules are rare and unilateral in neurofibromatosis type 2. |
Posttrabecular Outflow Obstruction in Secondary Open-Angle Glaucomas
Increased Episcleral Venous Pressure
The Goldmann equation,
Po = F/C + Pev
where Po is IOP, F is aqueous production, C is outflow facility, and Pev is episcleral venous pressure, describes a direct relation of IOP to episcleral venous pressure. Normal episcleral venous pressure is between 9 and 10 mm Hg. Increases in episcleral venous pressure lead to increases in IOP. Experimentally, this relationship is slightly less than 1:1, possibly due to decreased aqueous inflow or increased uveoscleral outflow with increased IOP. However, any cause of increased episcleral venous pressure may increase IOP.
The aqueous outflow pathway may be summarized as follows:
Anterior chamber; Schlemm's canal; aqueous veins; episcleral
P.141
veins; anterior ciliary veins; superior ophthalmic vein; cavernous sinus; internal jugular vein.
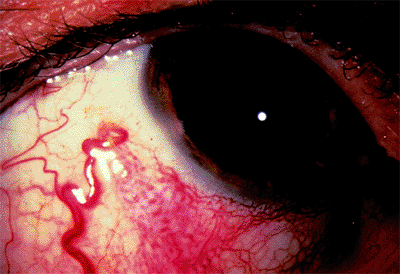 |
Figure 3.79. Sturge-Weber syndrome with glaucoma. Note dilated conjunctival vessel and episcleral hemangioma. |
Some conjunctival veins also carry aqueous via the facial vein to the external jugular vein.
Obstruction of venous drainage along any of these pathways may lead to increased episcleral venous pressure and increased IOP. Dilated vessels are seen beneath the conjunctiva that do not blanch with epinephrine. Blood may be seen in Schlemm's canal on gonioscopy. Orbital congestion in thyroid ophthalmopathy may increase episcleral pressure and IOP; this may be responsive to steroids, radiation, and decompression. Thrombosis of an orbital vein or the cavernous sinus can produce similar findings. Retrobulbar tumors, jugular vein obstruction, and superior vena cava syndrome also lead to increased episcleral pressure.
Arteriovenous anomalies, by exposing the venous system to the greater pressures of the arterial system, result in increased episcleral venous pressure. Sturge-Weber syndrome (Figs. 3.79, 3.80, 3.81), carotid cavernous fistulas (Fig. 3.82A,B), and orbital varices can in this manner produce
P.142
glaucoma. Idiopathic cases of increased episcleral venous pressure exist as well (Fig. 3.83A,B).
 |
Figure 3.80. Infant with Sturge-Weber syndrome undergoing examination under anesthesia. Intraocular pressure was normal. Glaucoma is more common with upper lid involvement by the hemangioma. |
 |
Figure 3.81. Resolving suprachoroidal hemorrhage following trabeculectomy in a patient with Sturge-Weber syndrome. Although prophylactic partial-thickness scleral windows with full-thickness sclerostomies were performed, a moderately large hemorrhage still occurred. |
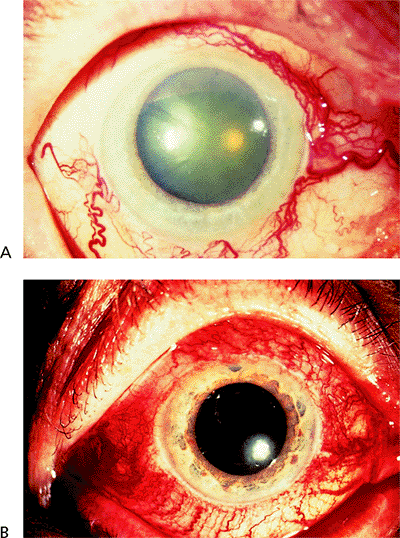 |
Figure 3.82. Dilated and tortuous episcleral vessels in patients with increased intraocular pressure secondary to A: carotid cavernous fistula and B: dural cavernous fistula. Although typically dural cavernous fistulas have a less dramatic appearance, this is not the case in these two patients. These patients are also at risk for ocular ischemia leading to neovascular glaucoma, uveal effusions with angle closure, and central retinal arterial and venous obstruction. |
 |
Figure 3.83. Patient with sporadic, idiopathic elevated episcleral venous pressure shows mildly dilated episcleral vessels (A) and blood in Schlemm's canal on gonioscopy (B). A familial form of idiopathic elevated episcleral venous pressure exists as well. |
Treatment of the underlying cause of increased venous pressure is ideal but not always possible or worthwhile. Embolization of carotid cavernous fistulas carries significant risk of CNS vascular accident; many close spontaneously. Aqueous suppressants often are helpful. Miotics and argon laser trabeculoplasty are not often useful, given the typically normal facility of outflow. Trabeculectomy is effective as it bypasses the elevated episcleral venous pressure, but has a higher incidence in these patients of serous and hemorrhagic choroidal detachments. Prophylactic partial thickness scleral flaps with full thickness sclerostomies have been advocated.
Medical Therapy for Glaucoma
The goal of treatment of a patient with glaucoma or suspected of having glaucoma is enhanced health. That goal is achieved by preserving vision, helping the individual develop a better understanding of what health is and how to achieve it, and, importantly, avoiding doing those things that are likely to make the person feel unhealthy. These three issues will all be briefly discussed in reverse order.
Avoidance of Actions That Make the Person Unhealthy
Merely telling a person he or she has glaucoma decreases the person's quality of life. Consequently, physicians need to be circumspect in this regard. To label a person as having glaucoma requires that the person have definite signs of damage. Even though certain genes have now been found to be associated with the diagnosis of glaucoma, it is still impossible on the basis of a laboratory test to determine with certainty whether or not a person has this entity. However, there are relative degrees of certainty, and when the findings are sufficiently intense or characteristic, it is possible to say with relative certainty that the person does, indeed, have glaucoma.
At the present time, the two most important indicators of the presence or absence of glaucoma are the nature of the optic nerve and the nature of the visual field. Clues suggesting the presence of glaucoma are indicated in Table 3.8 (Figs. 3.84; 3.85; 3.86; 3.87A, B; 3.88A-F; 3.89A, B).
The likelihood of an optic nerve being glaucomatous can be estimated based on a scale of disc damage (Fig 3.90). Note that what is determined here is the width of the neuroretinal rim. This is at least a partially subjective determination. However, by the width of the rim, we mean that portion of the optic nerve that appears to be on about the same plane as the retina. The internal edge of the rim is defined as that point where there is a sudden change in the curvature of the disc. Some discs have rims that slope so gradually toward the center that it is virtually impossible to determine with certainty the point at which the neuroretinal rim starts and stops. When considering these optic nerves, it is probably best to admit defeat rather than make a spurious estimate as to the width of the alleged neuroretinal rim.
The rim width is utilized rather than the cup/disc ratio. A cup/disc ratio may be small, such as perhaps .2, but may be highly pathologic if eccentrically placed. This problem also relates to rim width but is of less concern.
In staging the optic nerve, the area of thinnest rim width is chosen. When the examiner cannot determine with reasonable certainty whether the rim is at a particular figure or slightly smaller, the number chosen is always the larger. For example, if a rim width is considered to be about .2, but the
P.143
P.144
examiner thinks it may be slightly smaller than that, the figure chosen is .2.
Table 3-8.* Features of the Optic Nerve Head in Glaucoma | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
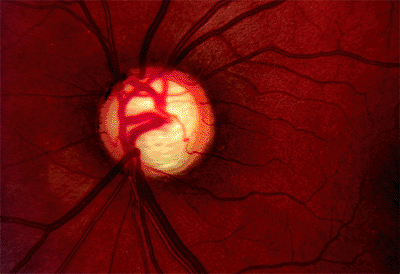 |
Figure 3.84. This optic nerve shows an acquired pit of the optic nerve, pathognomonic of glaucoma. |
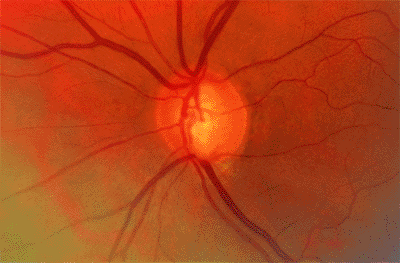 |
Figure 3.85. A notch consists of an irregularity in the optic nerve in which there is a change in the curvature of the rim localized to an area of less than 2 clock hours. The notch may extend all the way to the edge of the rim, or may be only partial. Both are highly indicative of the presence of a glaucomatous process. |
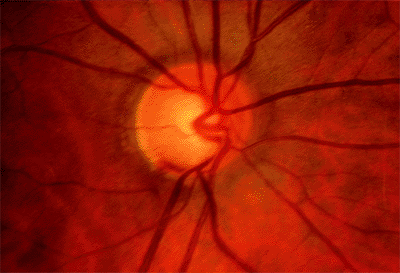 |
Figure 3.86. A flame-shaped hemorrhage crossing the rim of the optic nerve is highly suggestive of glaucoma. A similar finding may be seen rarely in patients with systemic hypertension or following a posterior vitreous detachment. |
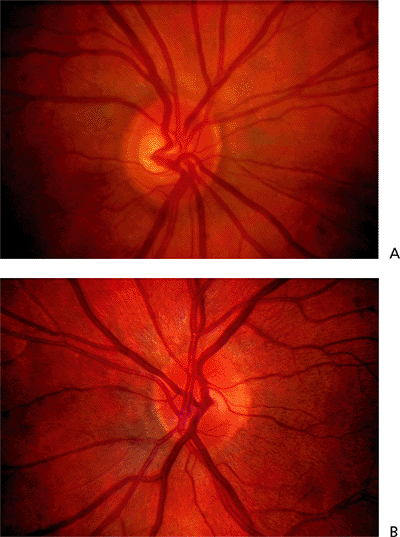 |
Figure 3.87. A, B: This patient with an essential iris atrophy has a significantly thinner rim in the left eye than in the right, without any other apparent cause for the asymmetry except asymmetry of intraocular pressure. The discs are the same size. The asymmetry is highly suggestive of glaucoma damage. |
It is essential to determine the disc size when using the Disc Damage Score. Indeed, it is essential to examine the disc size whenever evaluating the optic disc in a meaningful way. This can be readily done with a slit lamp using a lens with a strong dioptric power such as a 60, 66, 78, or 90 D lens. With good illumination and excellent fixation, and with the oculars properly adjusted so as to be parfocal for the examiner, the vertical height of the optic disc is measured from the pole at 12 o'clock to the pole at 6 o'clock. It is important to make sure that one does not measure peripapillary atrophy or the scleral ring, and that one measures from the outer edge of the rim to the outer edge of the rim. It is helpful if the illumination is reduced slightly, and if the width of the beam is such that it extends very slightly beyond the nasal and the temporal edges of the optic disc. Thus, the examiner uses a rectangle to isolate the optic nerve and estimate the vertical disc diameter. This diameter is then read directly from the graticule of the slit lamp and the appropriate correction factors made. These correction factors are shown in Table 3.9. There are advantages to using a Volk 66 D lens as no correction needs to be made. The measured size on the slit lamp is multiplied by the appropriate correction factor. This applies only to eyes with refractive errors less than +5 D. The optic nerve will appear spuriously small in eyes with more than 5 D of hyperopia and spuriously large in those with more than 5 D of myopia. The exact correction factors for these amounts of refractive error have not been determined, but it has been suggested that a correction can be made by multiplying the disc size by 1.1 for each diopter of myopia or dividing by 1.1 for each diopter of hyperopia. This is not an established method and is mentioned only as a rough method of estimating disc size.
For purposes of estimating the amount of disc damage with our staging system, discs are divided into small, average, and large discs. Small discs are those with vertical disc diameters less than 1.50 mm; large discs, those with vertical disc diameters greater than 2.0 mm; and average, in between (1.50 to 2.00 mm).
The stage of disc damage is determined by estimating the thinnest rim width, measuring the vertical disc diameter, and then using the nomogram to assign a disc stage. From a practical point of view, this becomes quite easy for discs with an average size if one remembers that discs with a thinnest-rim-width greater than .2 are grade 1, those with thinnest-rim-width slightly smaller than .2 are grade 2, and those with extremely thin rims are grade 3. The final four stages, 4, 5, 6, and 7, are utilized when there is an area of the disc in which no rim is present. Stage 4 indicates that rim is absent for less than 45 degrees; stage 5, absent between 45 degrees and 90 degrees; stage 6, absent between 90 degrees and 180 degrees; and stage 7, absent over more than 180 degrees.
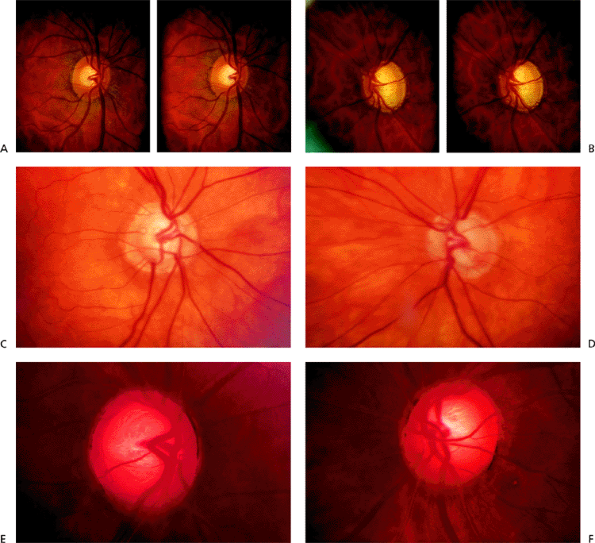 |
Figure 3.88. A-F: In this patient, the optic nerve with the narrower rim is considerably larger than the optic nerve with the wider rim. The difference in the width of the rim is due to the size of the optic disc, not to the damaging effect of glaucoma. |
P.145
The likelihood that glaucoma damage is present is small for discs of grade 0 or 1, intermediate for discs of grade 2, and virtually certain for discs of grades 3 and higher (Table 3.10).
The other primary estimate of glaucoma damage is visual field loss. This is discussed elsewhere in this section. A simple grading system intended to be used in conjunction with the Disc Damage Score is shown on page 148. This ranges from 0, indicating no apparent visual field loss, to 7, denoting advanced field loss. Staging visual fields is even more problematic than staging optic disc damage, because the field test is more subjective, more variable in the way it is administered, and more dependent upon the methodology of testing and the response of the patient. These grades, then, are highly approximate.
A relatively complete estimate of the likelihood of glaucoma damage can be obtained by adding the Disc Damage Score and the Visual Field Damage Score. A total score of 2 or less would strongly suggest that the patient did not have significant glaucoma damage, whereas a total score of 5 or more would be highly indicative of the presence of actual
P.146
P.147
glaucoma damage. The Glaucoma Damage Likelihood Score (GDLS) is indicated in Table 3.11. This refers, of course, only to the eye under consideration, not to the person.
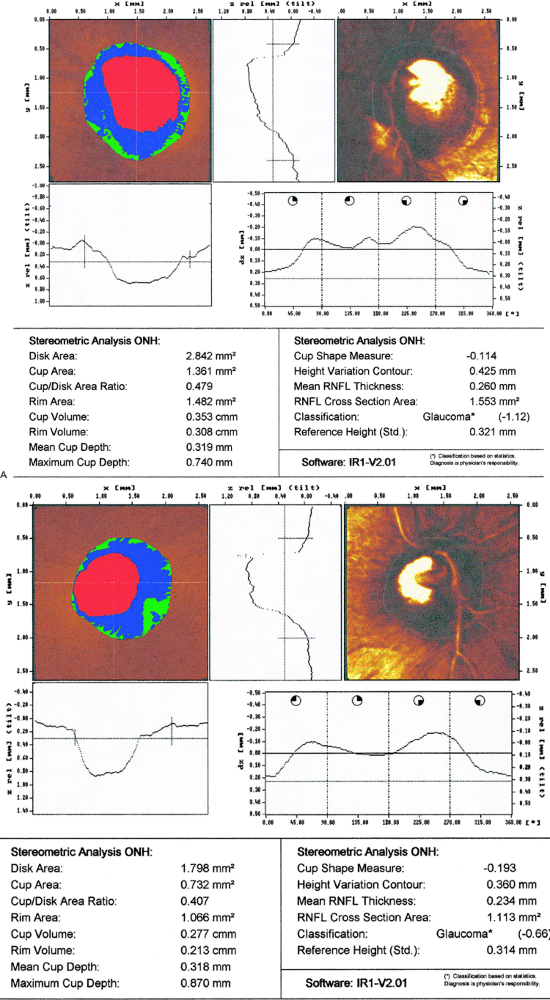 |
Figure 3.89. A, B: These plots of the Heidelberg Retinal Tomograph show a large disc and a small disc. It is likely that the disc with the narrower rim has a narrower rim simply because the disc is larger, and not because of glaucoma. |
The reader will note that these scales deal with glaucoma damage. There is a period of time when the glaucomatous process is advancing, but before glaucoma damage can be detected. The most important factor known to influence the likelihood that glaucoma damage will occur is IOP. The nature of the person's constitution, that is the genetic structure and family history, also plays a role. Consequently, in estimating the likelihood that a person will develop damage in the future, it is useful to include a measure of IOP and a consideration of the family history. However, the most important consideration is the actual presence of damage. Where damage is already present, it is likely to progress. Consequently, in estimating the likelihood that a person has the glaucomatous process, that is, a condition in which the optic nerve deteriorates over time, it is helpful to include an estimate of IOP, family history, and the amount of damage believed already present. These three criteria make up the Glaucoma Probability Score, as indicated in Table 3.12. The Glaucoma Probability Score, then, provides an estimate of the likelihood that the individual is affected with the glaucomatous process, whereas the Glaucoma Damage P.148
P.149
Score indicates the likelihood that the person actually has glaucoma damage already present.
 |
Figure 3.90. The Disc Damage Scale. |
Table 3-9. Approximate Correction Factors of Estimating Disc Size With a Slit Lamp | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 3-10. Disc Damage Score: Likelihood that One Has Optic Nerve Damage Due to Glaucoma | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
 |
Table 3-11. Glaucoma Damage Score: Likelihood that an Eye Has Glaucoma Based on the Sum of the Scales Indicating Disc and Field Damage |
Table 3-12. Glaucoma Probability Score: Likelihood of the Presence of the Glaucomatous Process | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Examples:
IOP=37 mm Hg with no visual field loss and with slightly suspicious optic discs, which are symmetrical.
GDLS=2
GDLS asymmetry=0
IOP=3
IOP asymmetry=0
Ethnic origin=0
Genetic score=0
Refraction=0
GPS=5
Interpretation: Glaucomatous process suspect
Presence of a probable early notch in both eyes, making the discs highly suspicious, with IOP of 22 mm Hg in one eye and 16 mm Hg in the other in a black patient with a family history of glaucomatous visual loss.
GDLS=3
GDLS asymmetry=0
IOP=2
IOP asymmetry=1
Ethnic origin=
Genetic score=
Refraction=
Total=GPS=7
Interpretation: Glaucomatous process likely
Patient with healthy-appearing optic discs and visual fields, with IOP of 58 mm Hg in one eye and 49 mm Hg in the other, and 10 D of myopia in both eyes.
GDLS=1
GDLS asymmetry=0
IOP=7
IOP asymmetry=1
Ethnic origin=0
Genetic score=0
Refraction=
Total=GPS=9
Interpretation: Glaucomatous process certain
Definite visual field loss with probable optic nerve damage, IOP of 17 mm Hg in a Caucasian patient.
GDLS=5
GDLS asymmetry=0
IOP=1
IOP asymmetry=1
Ethnic origin=0
Genetic score=0
Refraction=0
Total=GPS=7
Interpretation: Glaucomatous process likely
These two scores are discussed in detail because one wishes to avoid the damaging effects of treatments where possible. Every treatment for glaucoma has some damaging
P.150
effect. This may simply be cost or inconvenience. It is more often a topical or systemic side effect. Consequently, one needs to have a relative degree of certainty that the person in question will actually become damaged by the glaucomatous process before there is justification for initiating any type of treatment.
 |
Table 3-13. Likelihood that a Person Will Have Symptoms as a Result of Glaucomatous Optic Nerve Damage* |
To repeat: One needs to have a relative degree of certainty that the person in question will actually become damaged by the glaucomatous process before there is justification for initiating any type of treatment.
The likelihood that an eye will become damaged can be estimated by using a graph that considers three aspects of the eye's condition: first, the stage of glaucoma; second, the duration that it is anticipated the glaucoma will exist; and third, the rate of change of this stage of glaucoma. The stage of glaucoma can be estimated by using either the Disc Damage Scale or preferably the Glaucoma Damage Likelihood Scale, which includes both the Disc Damage Scale, and the Visual Field Damage Scale.
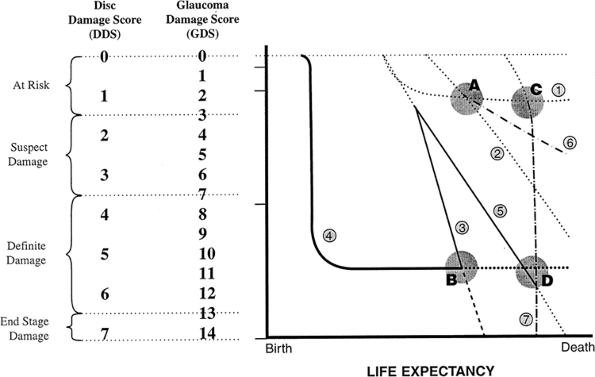 |
Figure 3.91. The Glaucoma Graph. |
This graph refers to one eye only. The likelihood that a person will have symptoms as a result of glaucoma damage is highly complex. It depends upon the type and intensity of field loss, the visual awareness and needs of the person, and the person's psychological make-up. A rough indication of the likelihood that a person will have symptoms from glaucoma, however, can be gathered by combining the Glaucoma Damage Likelihood Score for the two eyes, as shown in Table 3.13.
The Glaucoma Graph
The Glaucoma Graph (Fig. 3.91) is a way of determining and understanding the clinical course of glaucoma in an individual patient.
On the y axis of the graph is the stage of the glaucoma, and on the x axis is the life expectancy. The slope and the curve of each of the individual lines are determined and graphed in different ways:
Dotted lines indicate that the slope and the curve have
P.151
been determined by plotting the results of serial studies, such as repeated disc photographs taken yearly or repeated visual field examinations.
Solid lines depict the clinical course as described in the patient's history.
Dashed lines are extrapolations that are presumed to represent what will happen in the future. These hypothetical, extrapolated future courses are based on the nature of the previous courses and on knowledge of what has happened since a known point in time.
This illustration shows the courses of seven different patients with different manifestations of glaucoma:
A patient at point A has minimal glaucoma, and about one third of his or her life still to live.
A patient at point B has advanced glaucoma and has about one third of his or her life still to live.
A patient at point C has very early glaucoma and only a few years to live.
A patient at point D has advanced glaucoma and only a few years to live.
Patient 1, considered at point A, has one third of his or her life to live and is in an early stage of glaucoma. About one third of his or her life earlier, this patient was noted to have elevated pressure and followed without treatment. The patient continued to be followed without treatment and no damage to the optic nerve or visual field was ever noted. It is reasonable to assume that, if the patient continues to have IOPs around the same level as those noted initially, he or she will probably follow the course described by line 1, and will die without any evidence of glaucoma damage.
Patient 2 is also considered at point A, ie, having minimal damage with one third of his or her life left to live. In this case, however, the patient's IOP rose continuously, and the patient was noted to develop early disc and field damage, which then continued at the rate depicted by the dotted line 2. This patient, if untreated, would develop definite asymptomatic damage. However, the patient would have no functional loss at the time of his or her death.
Patients 3 and 4 are at point B. Both have advanced glaucoma and one third of their lives left to live. However, Patient 3 is deteriorating rapidly and will be blind long before he or she dies, whereas Patient 4, who had a blow to the eye as a child and lost vision to a steroid-induced glaucoma at that time, has had stable vision for most of his or her life, and it is reasonable to expect that it will continue to be stable.
Patients at points C and D both have only a few years to live, but those at point C (like Patients 1 and 2 at point A ) have minimal damage, and those at point D (like Patient 4 at point B ) have marked damage.
Patient 5 started with a clinical course similar to that of Patient 3 (advanced glaucoma and deteriorating rapidly), but around the midpoint of his or her life, the glaucoma became less severe. Nevertheless, this patient will be blind at the time of his or her death unless there is effective intervention. Compare Patient 4, who at point D has the same life expectancy and the same amount of damage as Patient 5 (only a few years to live and advanced glaucoma). Patient 4, however, has a stable clinical course and does not appear to need a change in therapy. In contrast, Patient 5 needs lowering of IOP urgently.
Patient 6, at around point C, also has only a few years of life remaining, but has a glaucoma that is getting worse a little bit more slowly than that affecting Patients 2 and 5. However, since Patient 6 has so little damage to start with, no treatment is necessary, even though he or she is getting worse. Even without treatment, he or she will not have enough damage or visual loss due to glaucoma at the time of death that he or she will have any awareness of being ill, and will have no limitation in function.
Patient 7, at point C, has only a few years left to live, but has a type of glaucoma that is deteriorating so rapidly that even though he or she has only a short period of time to live, he or she will be blind well before the time of death.
Using the Glaucoma Graph to define and characterize the nature of the clinical course helps the physician and patient to understand that:
Patients 1, 4, and 6 do not need any treatment at all; Patient 1 will never develop damage, Patient 4 has marked damage but it is not getting worse, and Patient 6 is getting worse so slowly that it will not interfere with his or her life.
Patients 3, 5, and 7 can be seen to need treatment urgently in order to prevent them from becoming totally blind prior to the time of their deaths.
In Patient 2, the need for treatment is controversial. Since this patient would never develop glaucoma, perhaps he or she should not be treated at all. But since he or she would develop some damage, those who want to prevent any damage at all would advise therapy.
The Glaucoma Graph is used by locating the individual on the vertical axis based on the Disc Damage Score or the Glaucoma Damage Score, and on the horizontal axis based on estimated life expectancy. Life expectancy cannot be estimated with certainty of course, but meaningful estimates can be made. Without an estimate, one cannot rationally decide upon treatment. Factors that help arrive at a meaningful estimate of life expectancy are indicated in Table 3.14.
The evaluation of the optic disc and the visual field are repeated at appropriate intervals between 3 and 6 months, depending upon the suspicion that the individual is getting worse. The new scores are plotted on the graph and given an estimate of the stability or rate of change (improvement or deterioration) of the patient's condition. Once enough points have been established to develop a meaningful line, then the line can be extrapolated so that one gets an estimate of the likelihood that the patient will pass into the field of symptomatic damage prior to his or her death.
The less the amount of damage, the less the justification for starting treatment. The greater the damage, the greater the need to start treatment, as even slow amounts of deterioration
P.152
will result in the patient becoming symptomatically worse.
Table 3-14. Factors Affecting Life Expentancy | |
|---|---|
|
The likelihood of the patient developing an attack of angle-closure glaucoma or developing some other type of glaucoma is not included in the system just described. However, it is important when counseling a person who has an occludable anterior chamber angle not to say that the person has glaucoma. Indeed, the person does not yet have glaucoma, but merely has the predisposition to glaucoma. That is an important predisposition that usually indicates the need for therapy. The treatment, then, is preventive. It is important for patients to understand the distinction between the prevention of glaucoma and the actual treatment of glaucoma. There are justifiable reasons for trying to prevent glaucoma from occurring, either the angle-closure type of glaucoma or the glaucoma that occurs as progressive optic nerve damage. Thus, simply because a person does not yet have glaucoma does not mean that therapy is not indicated. Therapy is indicated when the likelihood is sufficiently great that the individual's life will be affected in a harmful way by the glaucomatous process.
Enhancing the Patient's Health
The major goal of any treatment is to preserve or enhance the patient's health. This can be done in glaucoma by helping an individual understand what it means to have a chronic disease or the likelihood of the development of an acute disease. It is not known exactly how much a person's glaucoma benefits from improving the general state of health. There is fairly good evidence that losing weight and exercising can lower IOP in some individuals, perhaps by about as much as 4 mm Hg. There is also reason to believe that avoiding systemic hypertension may prevent a microvascular disease that could accelerate the progress of glaucoma. Some studies have suggested that cigarette smoking and obesity are risk factors for glaucomatous deterioration. Telling patients that their glaucoma will be benefited by measures that enhance their general health is probably too strong a statement. On the other hand, there are sufficient indicators that abnormalities of blood flow play a role in the development of glaucoma that it seems prudent to try to maintain cardiovascular fitness. If maintaining one's general health is beneficial from the point of view of glaucoma, that is certainly a plus. If it is not beneficial to the glaucoma, it is at any rate beneficial to the person who adopts a lifestyle that is conducive to maintaining a good state of health. This will clearly vary from person to person and includes the full spectrum of factors that affect health: diet, exercise, lifestyle, attitudes, and emotional characteristics. The goal is physical, social, emotional, and spiritual health. All of those interrelate, and complete health requires wholeness in all areas. Assisting patients to develop healthier lifestyles, more positive attitudes, better socialization, and more meaningful spiritual lives is one of the responsibilities and the joys of every physician, including those involved in caring for patients with glaucoma or suspected of having glaucoma.
Alternative therapies for glaucoma have been suggested. Some ophthalmologists use agents such as ginkgo to try to improve blood flow or some of the calcium channel blockers. Antioxidants have been suggested by others. When considering any of these agents, the potential risks need to be measured against the potential benefits. Because at this time there is little convincing demonstration of any real benefits to the glaucomatous eye of alternative therapies, and because all agents have some risk, the risk/benefit ratio of all therapies must usually be unfavorable. There is some evidence that a subgroup of patients with progressive glaucoma and low IOPs who have abnormal regulation of the vasculature as demonstrated by provocation with perturbation of blood gases may benefit from calcium channel blockers. This is not established, and unless one is willing to utilize the appropriate studies, the use of the calcium channel blockers should be considered experimental.
P.153
Lowering Intraocular Pressure
The only treatment that to date has been proven to prevent development of progressive optic nerve damage in patients with glaucoma is lowering IOP. It is not known whether this effect is due to absolute lowering of IOP, or to stabilization of IOP. Either or both may be true. It appears that, for a considerable number of patients with glaucoma, it is necessary to lower the IOP by approximately 30% if one is to obtain a meaningful alteration in the clinical course of the disease. However, it has not been established that it is necessary to lower the pressure by 30% in every individual, nor has it been established that lowering IOP by 30% will be beneficial in every individual. Nevertheless, the 30% figure is a guideline that has some evidence to support it. Having said that, however, it seems clear that IOP needs to be lowered by a greater percentage in people whose IOPs are initially low in order to have a beneficial effect than in people whose IOPs are initially high. Zeyen has suggested a method of arriving at a target pressure that takes into account this variability. He suggests that a reasonable approach is to try to lower the IOP by a percentage that is equal to the IOP at which the optic nerve is being damaged. Thus, if an individual has an IOP of 40 mm Hg, at which pressure the optic nerve is being damaged, then it would be reasonable to lower the pressure 40%, that is, by 16 mm Hg, and set a target pressure of around 24 mm Hg. In contrast, in the person who is getting worse with a pressure of 15 mm Hg, it may be appropriate to lower the IOP 15% of 15 mm Hg, or slightly more than 2 mm Hg. An estimate of a suitable target pressure in such a patient may be around 12 mm Hg.
Some ophthalmologists believe that when the optic nerve is already damaged it is more prone to subsequent damage. This has not been established, but has a ring of logic to it. It may simply be that those individuals who develop marked damage are those individuals whose optic discs are very sensitive to damage, and that the likelihood that patients with advanced damage will progress is not related to the advanced damage but rather to the nature of the underlying disease. Nevertheless, it seems prudent to be slightly more vigorous in those individuals in whom disc damage is advanced. As such, the ophthalmologist may wish to add or subtract 1 or 2 mm Hg from the target pressure described by Zeyen in those individuals in whom there is far advanced damage.
Target Pressure Range
There are benefits to both the physician and the patient to set a target pressure. However, for both the physician and the patient, it should be clear that the target pressure represents merely a range. If the target pressure can be easily reached without inducing troublesome symptomatology, then it seems prudent to continue to try to keep the IOP around the same level as the selected target pressure. However, where the target pressure is not able to be reached with ease, then the ophthalmologist must consider carefully the risks of pushing therapy more vigorously. By and large, it is helpful to think of a target range. Thus, the ophthalmologist may indicate on the chart and tell the patient that the goal is to get the IOP down to a range of between x and y mm Hg (Table 3.15). For example, x could be 10 and y 15, or, perhaps, x 25 and y 30 mm Hg.
Initiation of Therapy on the Basis of Risk/Benefit Ratio
Risks of Therapy
Whether to start the patient on medicinal, laser, or surgical therapy depends upon the diagnosis and the risk/benefit ratio of the intended therapy. The Glaucoma Graph is of great assistance in this regard. Risks of therapy include topical and systemic considerations. They exist for medicinal, laser, or surgical treatments. It is extraordinarily difficult to try to balance the relative risks of these modes of therapy because the onset and permanence of the risks is so different. For example, the risk to ocular and systemic health in association with the use of a topical carbonic anhydrase inhibitor is initially so small as to be barely measurable. However, the risks are cumulative, and with many years of use the risk becomes a real factor. In contrast, the risk to sight or general health in association with a surgical procedure is largely related to the time immediately around the procedure itself. Thus, considering the relative importance of immediate and long-term risks (and benefits) is essential in trying to establish a meaningful risk/benefit ratio. It is, of course, not sufficient merely to consider the risks of treatment; one must also consider the risks of not utilizing treatment, that is, what is the expected clinical course in the absence of therapy. This has been discussed in detail on pages 149 152. Table 3.16 provides rough guidelines about the relative effect of various types of therapeutic approaches to glaucoma.
Factors that influence whether to start with medicinal therapy or an argon laser trabeculoplasty are suggested in Table 3.16. An argon laser trabeculoplasty is especially appropriate in elderly individuals with a clear-cut diagnosis of a primary type of open-angle glaucoma and marked pigmentation of the posterior trabecular meshwork, and is probably the treatment of choice in such individuals. Additionally, argon laser trabeculoplasty has a role in those individuals in whom problems of systemic health introduce concerns with regard to the use of the medicinal agents.
Table 3-15. Estimating Target Pressure Range | ||
|---|---|---|
|
P.154
Choice of Medications Used to Treat Glaucoma
Two different considerations are of major help in deciding upon which agent is most appropriate for a particular individual. The first has to do with the characteristics of the drug, and the second, the characteristics of the patient. The challenge is to match the two as perfectly as possible. Table 3.17 categorizes patients into groups. Agents are categorized as either usually inappropriate, occasionally appropriate, or usually a good choice for each of the patient groups. The way to use the table is to consider all those characteristics that describe the patient under consideration, and find which agent or agents are most likely to fit that particular
P.155
P.156
patient with those particular characteristics. Clearly, these are merely guidelines. Finally, Table 3.18 lists classes of side effects and agents most likely to cause them.
Table 3-16. Relative Effects of Various Therapeutic Methods for Treating Glaucoma | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
Table 3-17. Factors that Suggest a Topical Drug is Particularly Inappropriate or Appropriate for Use in a Patient with Glaucoma |
Table 3-18. Classes of Side Effects and Agents Most Likely to Cause Them* | ||
|---|---|---|
|
Surgical Therapy for Glaucoma
Principles of Glaucoma Surgery
The objective of lowering or stabilizing IOP with surgery for glaucoma involves either improving aqueous outflow or reducing aqueous inflow. Lasers have proven to be invaluable for glaucoma therapy. They have not replaced but rather only complemented incisional operations. Individualized assessment for each eye of each patient will help direct the proper sequence of surgical intervention if medical therapy proves insufficient or is deemed inappropriate. The goal must be clear. In other words, is the treatment for pain relief from high IOP in a blind eye, or is it for maintaining vision in the patient's only seeing eye? With informed consent, the patient will also have the opportunity to voice his or her preference.
Lasers
Laser Peripheral Iridectomy
Goal
A peripheral iridectomy can prevent or alleviate a lens pupillary block leading to angle-closure glaucoma.
Indications
narrow angle capable of angle closure
primary angle closure leading to chronic or acute angle closure
iris bomb (posterior iris-lens synechia)
dramatic/spontaneous lens subluxation
microspherophakia
phacomorphic glaucoma
Technique
Nd:YAG lasers are typically preferred over the argon or diode laser because of the relative ease with which the iridotomy can be performed.
Preoperative Care
Pilocarpine to constrict the pupil and an agonist (aproclonidine or brimonidine) to minimize bleeding and blunt any IOP spike.
Contact Lens
Abraham off-center button lens or similar magnifying lens (CC-I 1.4).
Location of Treatment
11 o'clock or 1 o'clock (12 o'clock may lead to bubbles that obscure viewing). A lower location may increase the likelihood of ghost images. The far periphery is ideal, as the iris is thinnest in those regions.
 |
Figure 3.92. Biomicroscopic confirmation of iridotomy. |
P.157
Power
6.0 mJ
Bursts
1 to 3
Total Number of Shots
1 to 6 on average (brown greater than blue)
End Point
Posterior pigment blows into the anterior chamber. Special situation with a bleeding diathesis (aspirin or Coumadin), for example: Pretreat with the argon laser to coagulate and protect against potential bleeding. The argon settings are: power, 400 to 800 mW; size, 50 to 100 m; number, 20 to 40; duration, 1.0 seconds.
Patient must look to side to avoid macular burn.
Potential Complications
corneal abrasion
hyphema
uveitis
IOP spike
ghost image
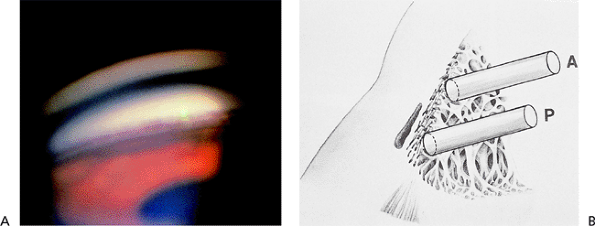 |
Figure 3.93. A: Spots at the junction of pigmented trabecular meshwork with 180 degrees or 360 degrees of angle being treated. B: Location of anterior (A) and posterior (B) beam placement. |
Postoperative Care
Agonist pre- and posttreatment. Topical steroid or nonsteroidal antiinflammatory agent four times a day for 4 days.
Measurement of Success
Gonioscopic confirmation that angle is deeper. Transillumination is an unreliable means of confirming iridotomy patency. Must be able to see the iridotomy is full thickness (Fig. 3.92).
Laser Trabeculoplasty
Goal
Reduction of intraocular pressure by enhancing outflow through the trabecular meshwork.
Indications
Open angle with clear visualization of angle structures, good prognosis. Diagnosis of POAG, low-tension glaucoma, pigmentary glaucoma, or pseudoexfoliation glaucoma. Age: the older the better, the exception being with pigmentary glaucoma. Trabecular meshwork pigmentation, heavy, greater than light.
Technique
Argon laser predominates, although diode- and frequency-doubled Nd:YAG laser also have been found to be equally effective.
Preoperative Treatment
Agonists.
Lens
Single or three-mirrored Goldmann lens or a Ritch lens.
Location
Spots at the junction of pigmented trabecular meshwork with 180 degrees or 360 degrees of angle being treated at one sitting 360 degrees in most cases, 180 degrees in high-risk cases (Fig. 3.93A, B).
End Point
Mild blanching of trabecular meshwork.
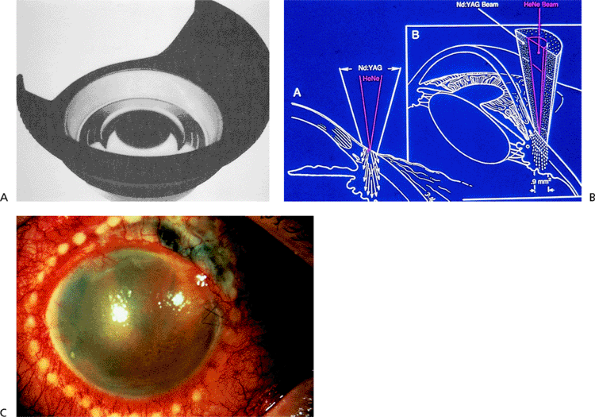 |
Figure 3.94. A: Shields lens. B: Focus of beam on ciliary body. C: External appearance of eye after cyclophotocoagulation. |
P.158
Power
200 to 800 mW.
Size
50 m.
Number of Shots
Total of 70 to 100 over 360 degrees.
Potential Complications
corneal abrasion
IOP spike
hyphema
uveitis
discomfort (inadvertent ciliary body shots)
Postoperative Care
Agonists posttreatment, topical steroid or nonsteroidal agent four times a day for 4 days. Examine at 1 week and 4 weeks.
Measure of Success
Determined at 4 to 6 weeks after the laser.
Cyclophotocoagulation
Goal
IOP reduction by partial ciliary body ablation, which slows aqueous production.
Indications
Eyes with limited visual potential, or those that are not candidates for laser trabeculoplasty or filtering surgery, such as those with neovascular glaucoma or multiple intraocular surgeries.
Technique
Three commonly used delivery systems:
Slit-lamp delivery (YAG laser on thermal mode)
Lens: Shields lens (Fig. 3.94A-C)
Power: 4 to 6 J
No. shots: 30 to 40 over 360 degrees
Contact probe (diode G probe) (Fig. 3.95A-C)
Power: 5 to 7 W, 1 to 2 seconds
No. shots: 18 over 360 degrees
Endoscopic (diode or argon) limbal incision with intraocular probe (Fig. 3.96)
Power: 400 to 800 mW, 1 second
No. shots: 30 to 40 over 360 degrees
Potential Complications
uveitis
phthisis
sympathetic ophthalmia
vitreous hemorrhage
globe perforation, especially in thin scleral regions that appear blue
endophthalmitis (for the endoscopic delivery system)
cataract
P.159
 |
Figure 3.95. A: Diode G probe. B: Placement of G probe. C: Artist's side view of G probe application. |
Measure of Success
It usually takes 4 to 6 weeks to evaluate the full effect of treatment. If it is inadequate, additional applications of cyclophotocoagulation can be tried.
Postoperative Care
Cycloplegic and topical steroids given over 1 to 3 months.
Incisional Surgery
Guarded Filtering Surgery
Goal
Create an alternative pathway for aqueous into the subconjunctival space, which leads to a filtering bleb.
Indications
Whenever medication and/or laser treatment has proven ineffective or is not indicated. Adequate conjunctival tissue at the limbus is required for procedure. Table 3.19 lists the risk factors for failure of guarded filtering surgery.
Technique
Guarded filtering surgery (trabeculectomy) has replaced full-thickness operations such as thermosclerostomy or posterior lip sclerectomy because of the lower complication rate, such as flat anterior chambers and suprachoroidal hemorrhages.
Conjunctival flap (Fig. 3.97A, B). A limbal- (A) or fornix-based (B) conjunctival flap may be used with comparable success rates, although with different bleb morphologies, in that a limbal flap has more localized bleb, and a fornix flap has a more diffuse, flatter bleb.
Antimetabolite use. Risk factor assessment helps determine the need and extent of antimetabolite use to minimize conjunctival scarring (see Table 3.20).
Scleral flap (Fig. 3.98). A one-half-thickness flap is raised with the hinge at the limbus. The size, shape, and thickness of the scleral flap have not proven to be critical determinants of success.
Paracentesis. Using a 25-gauge needle, a clear corneal paracentesis tract is created.
Figure 3.96. Endoscopic limbal incision with intraocular probe.
Table 3-19. Risk Factors for Failure of Guarded Filtering Surgery
- African-American
- Pseudophakic or aphakic
- Uveitis
- Previous filtering surgery
- Previous eye surgery such as retinal detachment or pars plana vitrectomy
- Regression of neovascular glaucoma
- Noncompliance
- Multiple eyedrop regimens; long-term eyedrop use
- Severe glaucomatous injury requiring a very low intraocular pressure
- Age less than 20 years
Sclerectomy. The sclerectomy is placed eccentrically, close to the edge of the scleral flap (Fig. 3.99). This ensures a good amount of flow into the subconjunctival space of the region. The corneoscleral bloc can be excised free hand, or it can be punched out with a scleral punch on the inferior wound edge.
Peripheral iridectomy. A generous iridectomy prevents iris plugging up the sclerostomy site.
Scleral flap sutures. Sutures are placed into the scleral flap with the intent of cutting or releasing the sutures in the postoperative period to encourage further subconjunctival filtration. There are two ways suture may be cut preoperatively:
P.161
Laser suture lysis using argon or diode laser. Lens: Hoskins (Fig. 3.100) or a Ritch compression lens. Setting: .05 seconds. Power: 200 to 800 mW. Spot size: 75 m. Special situation: If the conjunctiva is hemorrhagic, then a diode is preferred because of less heat absorption into the subconjunctival tissue, which may lead to a hole and possible leak.
Releasable externalized sutures (Fig. 3.101A-D). Sutures may be preplaced so that they can be easily retrieved and removed to release the scleral flap at that position. A commonly used releasable suture is one in which a slipknot is placed prior to the suture being exteriorized into the cornea and passed in such a fashion that only the elbow of the suture is exposed. In this way, that elbow can be grasped in the postoperative period and pulled, releasing and opening up the scleral flap in that region.
Table 3-20. Suggested Antimetabolite Use Depending on Degree of Risk of Failure
Risk for Failure Antimetabolite Use 1. Low None 2. Some 5-fluorouracil soak for 2 minutes 3. Moderate 5-fluorouracil soak for 5 minutes 4. Considerable Mitomycin soak for 1 minute 5. High Mitomycin soak for 3 minutes 
Figure 3.97. A: Limbal-based conjunctival flap in guarded filtration procedure. B: Fornix-based conjunctival flap.
Figure 3.98. Scleral flap in guarded filtration procedure.
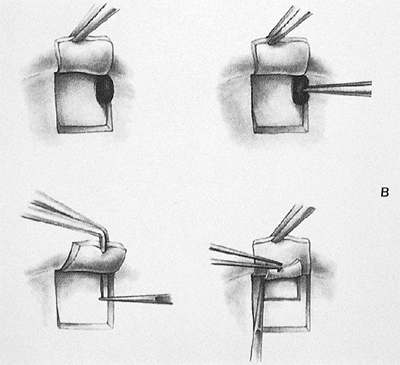
Figure 3.99. The sclerectomy is placed eccentrically, close to the edge of the scleral flap.
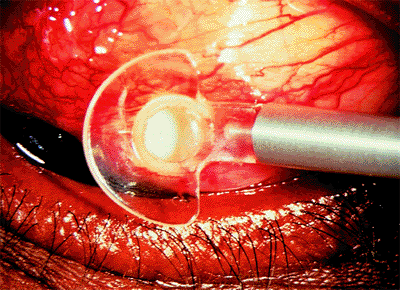
Figure 3.100. Laser suture lysis with a Hoskins lens.
Assessment of outflow irrigating through the paracentesis tract. The anterior chamber is reformed and a slow leakage should be evident at the trabeculectomy site.
Conjunctiva closure (Fig. 3.102A, B). Using an absorbable suture, such as 8-0 Vicryl, the conjunctiva is closed in a locking, running fashion for Tenon's layers and in a running, nonlocking, manner for the conjunctiva. A vascular needle tip is preferred when available, so as to minimize the chance of creating a hole in the conjunctiva that may lead to a bleb leak.
Evaluating bleb integrity. Upon irrigating through the paracentesis tract through the anterior chamber, three events ideally should occur: (1) the eye should firm up slightly before softening spontaneously; (2) the anterior chamber should deepen and not shallow after reformation; and (3) the bleb should form and remain elevated.
P.160
Potential Complications
Intraoperatively: suprachoroidal hemorrhage, subconjunctival buttonhole. Postoperatively: suprachoroidal hemorrhage, bleb infection endophthalmitis, flat anterior chamber with choroidal attachments, bleb failure, hypotony maculopathy (Table 3.21).
Postoperative Care
Laser suture lysis or release. Timing: with no antimetabolite may be up to 2 weeks; with 5-FU, up to 1 month; with mitomycin C, 2 months or longer, will still often give an adequate response.
Measure of Success
IOP at desired target level with a functioning limbal filtering bleb without visual loss or discomfort.
Postoperative Care
Cycloplegia and topical antibiotic. Topical steroids are given on a tapering schedule over a 4- to 8-week period.
Tube Shunts (See Table 3.22)
Goal
Lowering IOP by shunting aqueous from the anterior chamber into the subconjunctival space.
Indications
refractory glaucoma not amenable to trabeculectomy
neovascular glaucoma
previously failed guarded filtering procedure
uveitis
contact-lens wearer with a fear of limbal bleb infection
extensive limbal scarring from previous ocular surgery
Technique (using the Baerveldt shunt as an example)
Conjunctival incision (either a fornix-based or conjunctival flap can be fashioned) (Fig. 3.103)
Preparation of the tube ligature. A 4-0 nylon suture is placed inside the lumen of the tube (Fig. 3.104). An external 6-0 Vicryl suture is tied externally around the tube. If the tube is totally occluded, a 1- to 2-mm venting
P.162
slit may be put in the proximal portion of the tube with a Sharp blade. These vents act as pseudovalves. Alternatively, the external ligature tension can be adjusted to titrate outflow rather than totally obstructing it.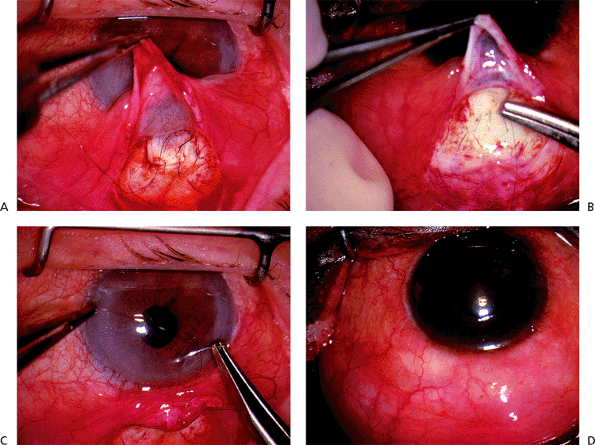
Figure 3.101. A-D: Four steps in placement of releasable externalized sutures.
Superotemporal quadrant optimal location. Superior and lateral recti are identified with a muscle hook. The reservoir is placed, with the wings of the reservoir underneath the recti. The reservoir is secured to the globe with two 6-0 Mersilene sutures.

Figure 3.102. A, B: Two steps in conjunctiva closure in guarded filtration procedure.
The tube is trimmed to desired length and placed in the anterior chamber through the paracentesis tract previously made by a 23-gauge needle. The placement of the tube should leave 1 to 2 mm of the distal end in the anterior chamber, with no iris or corneal touch (Fig. 3.105).
Table 3-21. Troubleshooting List Postoperatively
Intraocular Pressure Bleb Anterior Chamber Depth Problem Elevated Flat Deep Failure of trabeculectomy flap Shallow Aqueous misdirection or suprachoroidal hemorrhage Low High Deep or shallow Overfiltration Low Flat Shallow or deep Bleb leak Low Low Shallow Choroidal detachment Table 3-22. Tube Shunts Currently Available
Type Size (mm2) Valve Molteno Single plate 135 No Double plate 270 No Baerveldt 250, 350, 425 No Krupin 185 Yes Ahmed 185 Yes 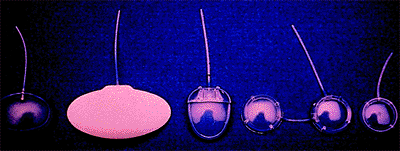
Figure 3.103. Conjunctival incision for placing the Baerveldt shunt.

Figure 3.104. A 4-0 nylon suture is placed inside the lumen of the Baerveldt shunt tube.
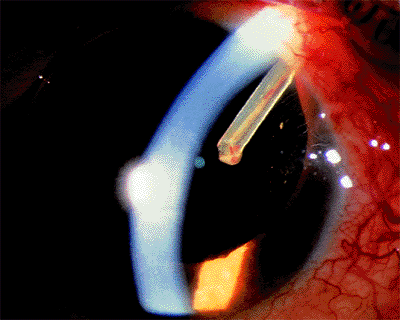
Figure 3.105. Placement of the tube leaves 1 to 2 mm of the distal end in the anterior chamber, with no iris or corneal touch.
The donor patch graft (either cadaver or autologous sclera, pericardium, or fascia lata) is placed over the tube to prevent later tube erosion through the conjunctiva (Fig. 3.106).
The conjunctiva is closed with an absorbable suture such as Vicryl.
P.163
The Ahmed tube shunt placement is similar to that of the Baerveldt with two exceptions: (1) the valve is primed by injecting fluid through the tube, which opens up the valve, composed of two adjacent membranes; and (2) the reservoir is placed between the two recti muscles rather than underneath them.
Potential Complications
suprachoroidal hemorrhage
aqueous misdirection
flat anterior chamber
choroidal detachments
choroidal edema
tube erosion extrusions
tube retraction from the anterior chamber
Postoperative Care
Cycloplegics and antibiotics for 1 to 2 weeks. A topical steroid may be used for 2 months. It is prudent to defer
P.164
pulling the intraluminal suture until conditions are ideal, usually 1 month postoperatively. It is removed by making a small cut down in the conjunctiva to externalize the distal end of the suture, grabbing the suture with a forceps and gently pulling it out.
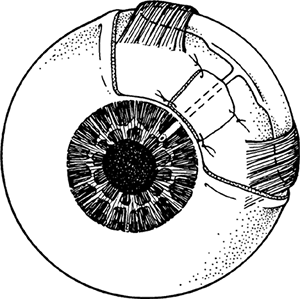 |
Figure 3.106. Donor patch graft is placed over the tube. |
Bibliography
Anderson DR, Cynader MS. Glaucomatous optic nerve cupping as an optic neuropathy. Clin Neurosci 1997;4:274.
Anderson DR, Patella VM. Automated static perimetry. 2nd ed. St. Louis: Mosby; 1999:278.
Armaly M. Ocular pressure and visual fields. Arch Ophthalmol 1969;81:25.
Armaly M, Krueger D, Maunder L, et al. Biostatistical analysis of the Collaborative Glaucoma Study. Arch Ophthalmol 1980;98:2163.
Azuara-Blanco A, Spaeth GL, Nicholl J, Lanzl M, Augsburger JJ. Comparison between laser scanning tomography and computerized image analysis of the optic disc. Br J Ophthalmol 1999;83:295.
Azuara-Blanco A, Harris A, Cantor LB. Reproducibility of optic disk topographic measurements with the Topcon Imagenet and the Heidelberg Retina Tomograph. Ophthalmologica 1998;212:95.
Becker B, Morton W. Topical epinephrine in glaucoma suspects. Am J Ophthalmol 1966;62:272.
Behrendt T, Wilson LA. Spectral reflectance photography of the retina. Am J Ophthalmol 1965:59;1079.
Boes DA, Spaeth GL, Mills RP, Smith M, Nicholl JE, Clifton BC. Relative optic cup depth assessments using three stereo photograph viewing methods. J Glaucoma 1996;5:9.
Bowd C, Weinreb RN, Williams JM, Zangwill LM. The retinal nerve fiber layer thickness in ocular hypertensive, normal, and glaucomatous eyes with optical coherence tomography. Arch Ophthalmol 2000;188:22.
Brusini P, Busatto P. Frequency doubling perimetry in glaucoma early diagnosis. Acta Ophthalmol Scand Suppl 1998;227:23.
Campbell DG. Pigmentary dispersion and glaucoma. A new theory. Arch Ophthalmol 1979;79:1667.
Cartwright MJ, Anderson DR. Correlation of asymmetric damage with asymmetric intraocular pressure in normal-tension glaucoma (low-tension glaucoma). Arch Ophthalmol 1988;106:898.
Chauhan B, Drance S, Douglas G. The effect of long-term intraocular pressure reduction on the differential light sensitivity in glaucoma suspects. Invest Ophthalmol Vis Sci 1988;29:1478.
Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 1998;126:487.
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol 1998;126:498.
Daubs J, Crick R. Effect of refractive error on the risk of ocular hypertension and open-angle glaucoma. Trans Ophthalmol Soc UK 1981;101:121.
Davanger M, Ringvold A, Blika S. The probability of having glaucoma at different IOP levels. Acta Ophthalmol (Copenh) 1991;69:565.
David R, Livingston D, Luntz MH. Ocular hypertension: a comparative follow-up of black and white patients. Br J Ophthalmol 1978;62:676.
Epstein D, Krug J, Hertzmark E, Remis L, Edelstein D. A long-term clinical trial of timolol therapy versus no treatment in the management of glaucoma suspects. Ophthalmology 1989;96:1460.
Glaucoma Laser Trial Research Group. The glaucoma laser trial (GLT) and glaucoma laser trial follow-up study: 7. Results. Am J Ophthalmol 1995;120:718.
Graham PA. The definition of pre-glaucoma. A prospective study. Trans Ophthalmol Soc UK 1969;88:153.
Greenfield DS, Zacharia P, Schuman JS. Comparison of Nidek 3Dx and Donaldson simultaneous stereoscopic disk photography. Am J Ophthalmol 1993;116:741.
Haynes WL, Alward WLM, Tello C, Liebmann JM, Ritch R. Incomplete elimination of exercise-induced pigment dispersion by laser iridotomy in pigment dispersion syndrome. Ophthalmic Surg Lasers 1995;26:484.
Hernandez MR, Pena JDO. The optic nerve head in glaucomatous optic neuropathy. Arch Ophthalmol 1997;115:389.
Hodapp E, Parrish R, Anderson D. Clinical decisions in glaucoma. St. Louis: Mosby-Year Book Inc; 1993.
Hoh ST, Greenfield DS, Mistlberger A, Liebmann JM, Ishikawa H, Ritch R. Optical coherence tomography and scanning laser polarimetry in normal, ocular hypertensive, and glaucomatous eyes. Am J Ophthalmol 2000;129:129.
Howe L. Photography of the interior of the eye. Trans Am Ophthalmol Soc 1885 7;4:568.
Hoyt WF, Newman NM. The earliest defect in glaucoma? Lancet 1972;1:692.
Jacobi PC, Krieglstein GK. Trabecular aspiration, a new mode to treat pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci 1995;36:2270.
Jaeger E. Ueber Staar and Sarroperationen 1854. Verlag von L.W. Seidel.
Jaeger E. Preface to 1869 edition. In: Albert DM. Jaeger's atlas of diseases of the ocular fundus Philadelphia: WB Saunders; 1972:vii.
Jay J, Allan D. The benefit of early trabeculectomy versus conventional management in primary open-angle glaucoma relative to severity of disease. Eye 1989;3:528.
Jay JL, Murdoch JR. The rate of visual field loss in untreated primary open angle glaucoma. Br J Ophthalmol 1993;77:176.
Johnson CA, Brandt JD, Khong AM, Adams AJ. Short-wavelength automated perimetry in low-, medium-, and high-risk ocular hypertensive eyes. Initial baseline results. Arch Ophthalmol 1995;113:70.
Johnson DH. Options in the management of malignant glaucoma. Arch Ophthalmol 1998;116:799.
Kamal DS, Vismanathan AC, Garway-Heath DF, Hitchings RA, Poinoosawmy D, Bunce C. Detection of optic disc change with the Heidelberg retina tomograph before confirmed visual field change intraocular hypertensives converting to early glaucoma. Br J Ophthalmol 1999;83:290.
Karickhoff JR. Pigmentary dispersion syndrome and pigmentary glaucoma: A new mechanism concept, a new treatment, and a new technique. Ophthalmic Surg 1992;23:169.
Kass M, Gordon M, Hoff M, et al. Topical timolol administration reduces the incidence of glaucomatous damage in ocular hypertensive individuals. A randomized, double-masked, long-term clinical trial. Arch Ophthalmol 1989;107:1590.
Kass M, Kolker A, Becker B. Prognostic factors in glaucomatous visual field loss. Arch Ophthalmol 1976;94:1274.
Katz J, Tielsch JM, Quigley HA, Sommer A. Automated perimetry detects visual field loss before manual Goldmann perimetry. Ophthalmology 1995;102:21.
Katz LJ, Spaeth GL, Cantor LB, et al. Reversible optic disc cupping and visual field improvement in adults with glaucoma. Am J Ophthalmol 1989;107:485.
Kitazawa Y. Prophylactic therapy of ocular hypertension: a prospective study. Trans Ophthalmol Soc NZ 1981;33:30.
Kitazawa Y, Horie T, Aoki S, Suzuki M, Nishioka K. Untreated ocular hypertension. A long term prospective study. Arch Ophthalmol 1977;95:1180.
Krohn MA, Keltner JL, Johnson CA. Comparison of photographic techniques and films used in stereophotogrammetry of the optic disc. Am J Ophthalmol 1979;88:859.
Kronfeld PC. Glaucoma. In: Albert DM, Edwards DD. The history of ophthalmology. Cambridge, MA: Blackwell Science; 1996:203.
P.165
K chle M, Nguyen NX, Horn F, Naumann GO. Quantitative assessment of aqueous flare and aqueous cells in pseudoexfoliation syndrome. Acta Ophthalmol 1992;70:201.
Leske M, Connell A, Wu S-Y, et al. Four-year incidence and progression of open-angle glaucoma preliminary data from the Barbados Incidence Study of Eye Diseases. Invest Ophthalmol Vis Sci 1997;38:S728.
Lichter PR. Variability of expert observers in evaluating the optic disc. Trans Am Ophthalmol Soc 1977;74:532.
Liebmann JM, Weinreb RN, Ritch R. Angle-closure glaucoma associated with occult annular ciliary body detachment. Arch Ophthalmol 1998;116:731.
Linn r E. Ocular hypertension. I. The clinical course during ten years without therapy. Aqueous humour dynamics. Acta Ophthalmol (Copenh) 1976;54:707.
Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Discussion. Ophthalmology 1994;101:1651.
Muller H. Ueber niveau-Veranderngen an der eintrittsstelle des sehnerven. Archiv fur Ophthalmologie 1858;4:1.
Nakla M, Nduaguba C, Rozier M, Hoffman D, Caprioli J. Comparison of imaging techniques to detect glaucomatous optic nerve damage. Invest Ophthalmol Vis Sci 1999;40:S397.
Nicholl JE, Lesk MR, Katz LJ, Spaeth GL, Araujo SV. Comparison of Topcon Imagenet stereometric disc analysis of the Topcon TRC-SS and Nidek 3Dx simultaneous stereoscopic fundus cameras. Invest Ophthalmol Vis Sci 1995;36:970.
Nordenson JW. Augenkamera zum stationaren Ophthalmoskop von Gullstrand. Ber Dtsch Ophthalmol Ges 1925;45:278.
Norskov K. Glaucoma screening. II. A five-year follow-up carried through in relation to a glaucoma screening among members of the Volunteer Donor Corps of the island of Falster (Denmark). Acta Ophthalmologica Kbh 1970;48:418.
Perkins E. The Bedford glaucoma survey. I. Long-term follow up of borderline cases. Br J Ophthalmol 1973;57:179.
Perkins E, Phelps C. Open angle glaucoma, ocular hypertension, low-tension glaucoma, and refraction. Arch Ophthalmol 1982;100:1464.
Pieroth L, Schuman JS, Hertzmark E, et al. Evaluation of focal defects of the nerve fiber layer using optical coherence tomography. Ophthalmology 1999;106:570.
Pohjanpelto P, Palva J. Ocular hypertension and glaucomatous optic nerve damage. Acta Ophthalmol 1974;52:194.
Ponte F, Giuffre G, Giammanco R, Dardanoni G. Risk factors of ocular hypertension and glaucoma. The Casteldaccia Eye Study. Doc Ophthalmol 1994;85:203.
Quigley HA. Better methods in glaucoma diagnosis. Arch Ophthalmol 1985;103:186.
Quigley HA, Duneklberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of larger optic nerve fibers. Ophthalmology 1988;95:357.
Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. Arch Ophthalmol 1982;100:135.
Quigley HA, Duneklberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol 1989;107:453.
Quigley HA, Enger C, Katz J, Sommer A, Scott R, Gilbert D. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol 1994;112:644.
Ritch R, Liebmann J, Robin A, et al. Argon laser trabeculoplasty in pigmentary glaucoma. Ophthalmology 1993;100:909.
Ritch R. Pigmentary dispersion syndrome. Am J Ophthalmol 1998;126:442.
Rodrigues MM, Spaeth GL, Sivalingam E, et al. Value of trabeculectomy specimens in glaucoma. Ophthalmic Surg 1978;9(2):29.
Rosenthal AR, Kottler MS, Donaldson DD, Falconer DG. Comparative reproducibility of the digital photogrammetric procedure utilizing three methods of stereoscopic photography. Invest Ophthalmol Vis Sci 1977;15:54.
Schl tzer-Schrehardt UM, Koca MR, Naumann GOH, Volkholz H. Pseudoexfoliation syndrome, ocular manifestation of a systemic disorder? Arch Ophthalmol 1992;110:1752.
Shin D, Kolker A, Kass M, Kaback M, Becker B. Long-term epinephrine therapy of ocular hypertension. Arch Ophthalmol 1976;94:2059.
Shrum KR, Hattenhauer MG, Hodge D. Cardiovascular and cerebrovascular mortality associated with ocular pseudoexfoliation. Am J Ophthalmol 2000;129:83.
Shuttleworth GN, Khong CH, Diamond JP. A new digital optic disc stereo camera: intraobserver and interobserver repeatability of optic disc measurements. Br J Ophthalmol 2000;84:403.
Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber layer atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol 1991;109:77.
Sommer A, Quigley HA, Robin AL, Miller NR, Katz J, Arkell S. Evaluation of nerve fiber layer assessment. Arch Ophthalmol 1984;102:1766.
Sommer A. Doyne lecture. Glaucoma: facts and fancies. Eye 1996;10:295.
Sommer A. Intraocular pressure and glaucoma. Am J Ophthalmol 1989;107:186.
Sommer A, Miller NR, Pollack I, Maumenee AE, George T. The nerve fiber layer in the diagnosis of glaucoma. Arch Ophthalmol 1977;95:2149.
Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol 1991;109:1090.
S rensen P, Nielsen N, N rskov K. Ocular hypertension. A 15-year follow-up. Acta Ophthalmol 1978;56:363.
Tello C, Chi T, Shepps G, Liebmann J, Ritch R. Ultrasound biomicroscopy in pseudophakic malignant glaucoma. Ophthalmology 1993;100:1330.
Tielsch JM, Katz J, Quigley HA, Miller NR, Sommer A. Intraobserver and interobserver agreement in measurement of optic disc characteristics. Ophthalmology 1988;95:350.
Trope GE, Pavlin CJ, Bau A, Baumal CR, Foster FS. Malignant glaucoma: clinical and ultrasound biomicroscopic characteristics. Ophthalmology 1994;101:1030.
Tsai JC, Barton KA, Mioller MH, Khaw PT, Hitchings RA. Surgical results in malignant glaucoma refractory to medical or laser therapy. Eye 1997;11:677.
Varma R, Spaeth GL, Hanau C, Steinmann WC, Feldman RM. Positional changes in the vasculature of the optic disc in glaucoma. Am J Ophthalmol 1987;104:45.
Vesti E, Kivel AT. Exfoliation syndrome and exfoliation glaucoma. Prog Retin Eye Res 2000;19:345.
Vogt A. Die Nervenfaserstreifung der menschlichen netzhaut mit besonderer berucksichtigung der differentialdiagnose gegenuber pathologischen streifenformigen reflexen (praretinalen faltelungen). Klin Monatsbl Augenhheilkd 1917:58;399.
Walker W. Ocular hypertension. Follow-up of 109 cases from 1963 to 1974. Trans Ophthalmol Soc UK 1974;94:525.
Wang JJ, Mitchell P, Smith W. Is there an association between migraine headache and open-angle glaucoma? Findings from the Blue Mountains Eye Study. Ophthalmology 1997;104:1714.
Weber A. Ein fall von partieller hyperamie der choroidea beieinem kanninchen. Archiv fur Ophthalmologie 1855;2:133.
Wilensky J, Podos S, Becker B. Prognostic indicators in ocular hypertension. Arch Ophthalmol 1974;91:200.
Wilson MR, Hertzmark E, Walker AM, Childs-Shaw K, Epstein DL. A case-control study of risk factors in open angle glaucoma. Arch Ophthalmol 1987;105:1066.
Zeyen T. Target pressures in glaucoma. In: Gramer E, Grehn F. Pathogenesis and risk factors of glaucoma. Berlin: Springer; 1999:209.
EAN: 2147483647
Pages: 17