22.4 Quality Management System (QMS)
|
22.4 Quality Management System (QMS)
4.1: General Requirements
QMS Responsibility The Growth Corporation (Growth) quality management system (GCQMS) is defined in its quality manual (manual). The Growth executive management team (GEMT), which consists of the president and direct reporting staff, is responsible for the extent and content of the manual. The manual is maintained and kept current by the director of quality assurance, who also serves as the ISO 9000 management representative.
It is the direct responsibility of the GEMT to ensure that there is a continual improvement in the effectiveness of the GCQMS through constant oversight of its processes. The requirements of the ISO 9001:2000 Standard (Standard) are used to establish the framework within which these processes interact.
Eight Quality Management Principles The GEMT applies the eight quality management principles defined by the Standard as a means to ensure continual performance improvement in the GCQMS. Specific activities that relate to each principle include the following:
-
Customer focus—an extensive customer service organization to complement and supplement marketing and sales continuous analysis of customer response and complaints based on periodic customer satisfaction surveys.
-
Leadership—an annual business plan prepared by GEMT that includes quantitative quality objectives, metrics, and targets for all managers and supervisors.
-
Involvement of people—the use of cross-functional teams in the setting of quality objectives and the solution of process and product nonconformances.
-
Process approach—the establishment and analysis of core competencies for each company function.
-
System approach to management—the integration of business objectives with quality objectives so that the company processes represent a total quality approach to continual improvement.
-
Continual improvement—the goal of all employees based on quality objectives and intense training in quality management system concepts and implementation.
-
Factual approach to decision making—decisions primarily based on the analysis of quality objective progress against targets. The GEMT reserves the right to make decisions based on less statistical information when appropriate. Decision making at Growth is a holistic process that incorporates all available internal and external data.
-
Mutually beneficial supplier relationships—a vigorous supplier partnership program that provides suppliers with periodic evaluations of their performance and the necessary support to aid in nonconformance resolution.
Exclusion Statement The GCQMS is fully responsive to all requirements of the Standard. There are no exclusions.
Growth's Core Competencies Growth has structured its company organizational processes in terms of core competencies. The core competencies are as follows (with main process champions noted):
-
Executive (GEMT)—president and CEO;
-
Finance and administration—controller;
-
MIS management—MIS manager;
-
Sales and marketing—vice president of sales and marketing;
-
Design engineering—vice president of design engineering;
-
Quality assurance—vice president of quality assurance;
-
Manufacturing—vice president of manufacturing.
Processes Defined The full set of Growth business processes consists of the core competencies (main processes) and their associated subprocesses. The structure of the full set of processes is illustrated below in Figure 22.1. The set of process documents shown in this figure are available online in a secure intranet network. The document control subprocess provides protocols for hard copy, as necessary.
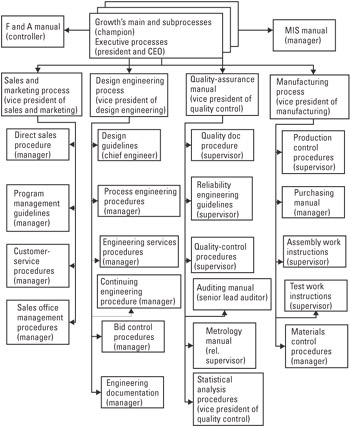
Figure 22.1: Growth's process management and quality management system.
Process Sequence and Interaction Defined Figure 22.2 describes graphically how the main processes are designed to produce customer-desired products and how customer feedback is obtained and analyzed within the GCQMS structure.
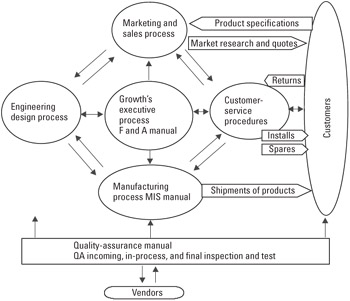
Figure 22.2: Growth's sequence and interaction of its main processes (refer to Figure 22.1 for subprocess interaction).
Figure 22.2 illustrates the following processes:
-
The development of product specifications is a joint effort of marketing and sales with design engineering. Design engineering interacts with the customer only when engineering is part of a design engineer and product manager team.
-
Design engineering hands off production packages to manufacturing by means of cross-functional team relationships and the engineering change order process. The packages contain the required test fixtures and instrumentation needed to produce customer-specified product characteristics. The MIS department manages the intranet system that integrates all of the QMS operational functions, including those required by engineering design, quality assurance, manufacturing, and customer service.
-
The team of design engineering, purchasing, and quality assurance evaluates and selects vendors. Subcontractors are chosen on an as-needed basis by the department heads.
-
Manufacturing works with continuing engineering to optimize its processes as a means of shipping on time to desired performance levels. Quality assurance supports the calibration of measuring and monitoring devices in both manufacturing and engineering.
-
After-sales activities and installations are managed by customer service under marketing and sales oversight. Customer service has a direct contact with the customer via returned goods and also orders spare parts from manufacturing and sells them directly to the customer. Customer service data is provided on a daily basis to quality assurance.
-
Quality assurance works across the board to ensure the operational integrity of the processes and performs reliability studies, manages metrological activities, performs audits of the company and of vendors, and provides the cost-of-quality analysis for the company in concert with finance and administration. The department serves as part of the design engineering cross-functional team and performs incoming, in-process, and final inspection and testing.
-
Finance and administration and MIS manage the online computer systems that are used by all departments.
-
The GEMT uses a series of reviews to ensure the integrity and efficiency of the total management process. The reviews include the quarterly management review supplemented by monthly department reviews and weekly operational reviews. All of the reviews are documented and kept as records by either the ISO 9000 management representative or the appropriate local area manager.
Criteria and Methods The GEMT formulates quality objectives, their metrics, and targets as part of the annual growth business plan. This is a way to establish the set of criteria and methods to be used to effectively manage Growth's main processes and subprocesses. ![]() GBP.Doc
GBP.Doc
Resource and Information Availability The Growth business plan also establishes the capital and personnel resources required to maintain an effective QMS and creates the framework for companywide information sources that include management reviews at all company levels; a monthly company newsletter; and quarterly company meetings by the GEMT for all employees.
Process Monitoring, Measuring, and Analysis
An intensive computer-aided program is used to analyze data obtained through the monitoring and measurement of key process parameters. The manager of statistical analysis is responsible for this function, which includes the corrective and preventive action process located in the quality-assurance manual. ![]() QAManual.Doc
QAManual.Doc
Continual Process Improvement The GEMT uses a cross-functional action team structure to analyze and resolve process improvement issues. Oversight is accomplished both through specific action team reviews and quarterly GEMT reviews. The action teams complement the corrective and preventive action program. All action team activities are documented. ![]() TEAMS.Doc
TEAMS.Doc
Outsource Management Printed circuit board fabrication and painting are typical processes that Growth regularly outsources. In addition, circuit board layout and mechanical design are typically outsourced by engineering on an as-needed basis. Control of such outsourced processes is coordinated by the purchasing supervisor as part of Growth's supplier partnership program. All outsourced engineering projects are managed by the pertinent project engineer and regularly reviewed by the chief engineer. ![]() SPP.Doc
SPP.Doc
4.2: Documentation Requirements
4.2.1: General Requirements
The GCQMS includes the following:
-
A controlled quality policy document that is posted as both an electronic file and on hard copy about the facility. Refer to Section 5.3 of this manual.
-
A controlled set of quality objectives with metrics and targets based on the Growth Business Plan. Refer to Section 5.4.1 of this manual.
-
The six procedures required by the Standard:
-
Control of documents in two procedures (i.e., QA document control and engineering document control).
 QADC.Doc and
QADC.Doc and  ENGDC.Doc
ENGDC.Doc -
Control of records in the procedure (i.e., records control).
 Records.Doc
Records.Doc -
Internal audit in the procedure (i.e., auditing manual).
 Audits.Doc
Audits.Doc -
Control of nonconformity in the quality-assurance manual.
 QAManual.Doc
QAManual.Doc -
Corrective action in the quality-assurance manual.
 QAManual.Doc
QAManual.Doc -
Preventive action in the quality-assurance manual.
 QAManual.Doc
QAManual.Doc
Additional Documentation The GCQMS also includes a large number of other documents whose purpose is to ensure the overall effective planning, operation, and control of the QMS. The extent of the mandatory documentation and the supplemental documentation is explained next.
Life Cycle The QMS documentation is designed to impact the entire life cycle of Growth's hardware and software products. As a result, the product plans are based on the effective inclusion of all aspects of the product's life (i.e., from market share to after-sales service).
Four-Tier Structure The documentation is primarily online at the tier I and tier II level, and is a hybrid system otherwise (i.e., a mixture of electronic and hard-copy files). The system is illustrated in Figure 22.3. The online system includes an MRP system used by manufacturing and engineering.
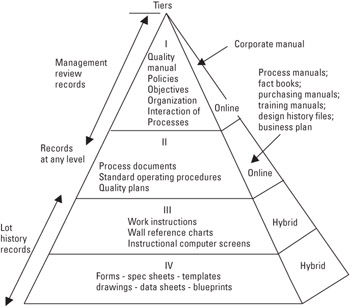
Figure 22.3: Growth's four-tier operational pyramid.
Records As indicated in the pyramid, records can occur at any level and form their own particular documentation hierarchy. (Refer to Section 4.2.4 of this manual.)
4.2.2: Quality Manual
As illustrated in Figure 22.3, Growth's quality manual (manual) is the highest level document in the QMS. It defines Growth's quality policy statements for all five sections of the Standard and those portions of ANSI/ISO/ASQ Q9000-3-1997 that are applicable.
The president is responsible for the review and approval of the manual. As with all Growth documents, the manual is reviewed and updated either upon revision or during the quality audit process.
Scope The manual covers the design, manufacture, marketing, selling, and servicing of modular hardware and software products as a means to display and process commercial and industrial images on personal computers and workstations. As previously noted, there are no exclusions to the Standard's requirements.
Process interaction: Defined in the previous chart entitled, Growth's sequence and interaction of its main processes.
Linkage: Linkage from document to document within the QMS is by means of hyperlinks for the electronic files and references to hard-copy documents. Only the document title is used (i.e., electronic files are not numbered). In this manner, the reader is directed from the manual to the process documents and then to procedural and format documents, as appropriate. Hard-copy documents such as drawings and schematics are controlled numerically under engineering change order document control.
To expedite navigation, it is always advisable to begin with the manual and then go directly to the Appendix, entitled "Growth's Master List of Hub Documents." The hub document can be likened to an airport hub. Once you reach the hub document, it leads the reader to the next levels of system information. As an aid to navigation, the manual's cover page contains key hyperlinks.
Procedures defined: It is important to note that Growth's "procedures" are in the form of either very high-level process documents or lower level procedures or work instructions. The high-level process documents are equivalent to standard operating procedures and are in the form of flow charts with a supplemental text document attached. The two documents form a single process document.
Implementation: The effective implementation of the QMS is ensured through a comprehensive management review defined in Section 5.6 of this manual. Most importantly, great care is taken to track all preventive actions achieved by Growth employees and to reward such activities commensurate with their contribution to the overall company's productivity.
Skill levels: All of Growth's documents are created to serve highly skilled and extensively trained employees. Moreover, Growth's employees are required to work for lengths of time without close supervision and to carry out multitasking work. As a result, the level of detail in the documents varies from engineering guidelines to detailed test protocols based on the specific operational and administrational tasks. Most importantly, the documentation is designed to support minimal supervision by being readily available yet unobtrusive.
4.2.3: Control of Documents
Procedure The description of Growth's approach to the control of all hardware and software documentation and data is contained in two documents (i.e., QA document control and engineering document control). ![]() QADC.Doc and
QADC.Doc and ![]() ENGDC.Doc
ENGDC.Doc
Responsibility Control of documents is shared by the quality documentation supervisor and the engineering documentation manager. Both functions maintain master lists of documents. The master lists of documents are maintained to ensure the correct distribution of documents and that users have the most recently revised documents at their work sites. ![]() QCMLDOC.Doc; and
QCMLDOC.Doc; and ![]() ENGMLDOC.Doc
ENGMLDOC.Doc
Control of Externally Received Documents Documents such as the ISO 9000 Standard, vendor documents, and customer specifications are controlled locally by the appropriate user. Such documents are used either in the design, manufacture, or preventive maintenance of product and associated instrumentation.
Document and Data Approval and Issue Control of the documents is maintained in the following ways:
-
All released engineering documents are under engineering change order (ECO) control whether on hard copy or online. Program managers and project engineers have ready access to such documents via the engineering computer system (ECS). All policy, process, and procedural documents are controlled by means of either the document's name or by the code numbers and "red" control numerals, as necessary., Each process document is assigned a "champion" who is responsible for the review, approval (sign off), and release of the documents.
-
Tier IV documents (e.g., forms contained in a forms master manual) are controlled by the quality documentation supervisor and distributed to the local managers upon request.
-
Memos, reports, and similar documentation are controlled at the localmanager level and do not require a master list.
-
Obsolete documents are removed from use at the local-manager level upon receipt of revised documents.
-
Documents that are maintained for legal and informational purposes are marked accordingly on their containers and archived and maintained by the accounting department, or by the chief engineer, as appropriate. Department managers are authorized to determine that obsolete documents are to be retained.
Document and Data Changes Revision control is handled in the following manner:
-
Changes at the policy, process, procedural, and forms level are made via the department change order (DCO).
-
Changes to released hardware and software engineering documents are incorporated via the ECO.
-
Document and data change orders for hardware and software products are reviewed and approved by the designees defined in the process and are usually the document owners or their designees.
-
Previous revisions and pertinent background information are directly available as part of the DCO and ECO formats. Such formats also contain descriptive material for the nature of the change.
-
Revisions to documents occur as part of the corrective and preventive action program, [e.g., audits, nonconformance material reports (NCMRs), corrective action reports (CARs), and preventive action reports (PARs)]. However, all documents that have not been revised for over two years are reviewed for currency by the document's champion.
-
Online document legibility is inherent. Hard-copy documents are kept in steel case files and/or banker's boxes when stored. Internal audits ensure that document deterioration is minimized.
Online control is handled differently. The online documentation systems are secured via passwords and periodically backed up and stored electronically by the MIS manager. ![]() MISMANUAL.Doc
MISMANUAL.Doc
4.2.4: Control of Records
Procedure The records control procedure and its associated records master list detail the procedures for identifying, collecting, indexing, accessing, filing, storing, maintaining, and disposing of quality records. ![]() RECORDS.Doc and
RECORDS.Doc and ![]() RECORDSML.Doc
RECORDSML.Doc
Responsibility Maintenance of records is the responsibility of each local area manager. However, for the sake of continuity, the ISO 9000 management representative maintains the records master list that is an online summary of all core competency master lists.
Control Quality records are identified by either title or number. The records master list indicates the location of the records. They may be online or hard copy. The master list includes who is responsible for the record, its retention time, and its status (online, hard copy, obsolete, or obsolete but retained).
Subcontractor data, in the form of certificates of compliance or analysis, is included in the master list.
Filing The hard-copy records are filed in either commercial-grade steel case files or in corrugated containers such as banker's boxes. Online records are managed by the MIS manager. All files are kept on site. Records are kept within the easy reach of the users to facilitate usage.
Legibility The hard-copy quality records are typed for legibility whenever possible. Handwritten records are written with permanent black ink pens. Online records are inherently legible.
Conformance Growth uses its quality records as a key source of information for presenting the status of its internal audit and preventive and corrective action program to management for review. Most importantly, the records are a source of quantitative information used in trend analysis.
Process Control Many records are kept by the local area manager (e.g., process control records are kept in the individual customer job folders in production and include, when available, assembly drawings, bills of materials, fabrication drawings, visual aids, process control documents, and measurement documents).
Contractual Agreements In those cases when Growth enters into a contractual agreement, records are made available to the customer or its representative for evaluation for a limited period.
Disposition Because Growth's records consist of records from all of the company's core competencies, the disposal of records requires controller approval, and, in the case of company proprietary information, the approval of the president.
|
EAN: 2147483647
Pages: 155