2.6 Molecular Recognition in Chemical Systems
|
2.6 Molecular Recognition in Chemical Systems
Before turning to futuristic systems, it is useful to describe how molecular recognition takes place in the following areas (among others): catalysis, membrane transport, and photochemistry.
Supramolecular Reactivity and Catalysis
A catalyst is a substance that accelerates a chemical reaction but is not consumed in the reaction and does not affect its equilibrium. This occurs because a catalyst provides a new and easier pathway for reactant molecules to be converted into product molecules. The effects of molecular recognition are made manifest in systems involving enzymes and other molecules of similarly high molecular weight. Enzymes demonstrate in their high selectivity the full power of molecular recognition.
Supramolecular reactivity involves two main steps: binding, which selects the substrate, and transformation of the bound species into products within the supramolecule formed. Both steps take part in the molecular recognition of the productive substrate and require the correct molecular information in the reactive receptor. Compared to molecular reactivity, a binding step is involved that precedes the reaction itself. Catalysis additionally comprises a third step, the release of the substrate. The catalyst is then free to participate in a new cycle.
The selection of the substrate is not the only function of the binding step. In order to promote a given reaction, the binding should strain the substrate (or pair of substrates) so as to bring it toward the transition state of the reaction; thus efficient catalysis should bind the transition state more strongly than the free state of the substrate in order to lower the free energy of activation.
A major role is played by the existence of strong interactions between the substrate and the receptor site of the catalyst. They may be used to facilitate the reaction in several ways: a thermodynamic effect (strong binding forcing the substrate into contact with the reactive groups); a steric effect (i.e., fixation of the substrate being able to distort it toward its transition state geometry); and an electrostatic (electronic, protonic, ionic) effect, consisting of a possible activation of the functional groups of the catalyst (or substrate) by modification of their physicochemical properties as a consequence of substrate fixation (e.g., perturbation of the charge distribution in both the substrate and catalyst with respect to their free, unbound state).
Examples of catalytic behavior are processes such as ester cleavage and phosphoryl transfer in ATP hydrolysis. A case of a catalyst for the latter is as follows: a multifunctional anion receptor containing a macrocyclic polyamine as an anion binding site, an acridine group as a stacking site, and a catalytic site for hydrolysis; it acts as a catalyst in ATP hydrolysis with increased ATP/ADP selectivity. One possible pathway is shown in figure 2.9 (Hosseini, Blacker, and Lehn 1990). Another area of major interest, as mentioned above, is molecular recognition of the structural and base sequence features in DNA together with the selective cleavage of nucleic acids. Being able to design selective DNA and RNA cleavage reagents is a very active area of research at present, particularly in light of their potential uses in biotechnology.
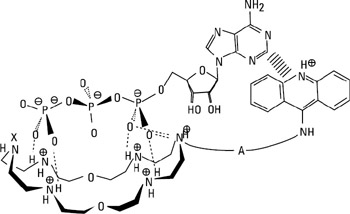
Figure 2.9: Hypothetical structure of the ATP complex in the catalysis of ATP hydrolysis. (Reprinted with permission from Hosseini, Blacker, and Lehn, 1990. 1990 American Chemical Society.)
A further step beyond bond cleavage is the design of systems capable of inducing bond formation. To this end, the presence of several binding and reactive groups is essential. Such is the case for coreceptor molecules in which subunits may cooperate for substrate binding and transformation (figure 2.10) (Lehn 1995).
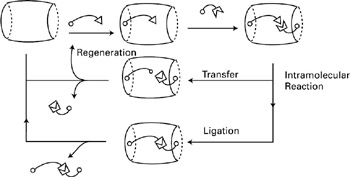
Figure 2.10: Cocatalysis cycle, with final products either being the transfer of a subunit or the ligation of two subunits. (With permission from Supramolecular Chemistry, J.-M. Lehn. 1995 VCH Verlagsgesellschaft.)
This ability of bringing together substrates and cofactors, mediating reactions between them within the supramolecular architecture, is called cocatalysis. As an example, the same macrocycle as was used in the studies of ATP hydrolysis was also found to mediate the synthesis of pyrophosphate from acetylphosphate (Lehn 1979, 1986).
Another example of interest is the enhanced ligation of DNA, effected by an imidazole-functionalized spermine binding in the minor groove of the double helix (figure 2.11) (Zuber, Sirlin, and Behr 1993).
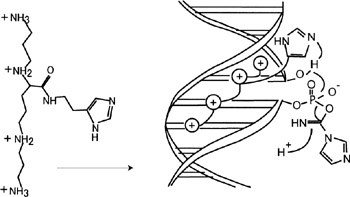
Figure 2.11: Enhancement of ligation of DNA as effected by an imidazole-functionalized spermine binding in the minor groove of the double helix. (Reprinted with permission from Zuber, Sirlin, and Behr. 1993 American Chemical Society.)
Carrier-Mediated Transport
The assisted transport of molecules across membranes is extremely important for processes in biology. This is of particular significance when the flow of molecules occurs against a gradient or occurs faster than free diffusion would allow. Biological systems accomplish this feat through the judicious use of molecular recognition and transport processes. Carrier-mediated transport consists of the transfer of a substrate across a membrane, facilitated by a carrier molecular located in the membrane. It is a cyclic process comprising four steps, as shown in figure 2.12: (1) formation of the carrier-substrate complex at one interface; (2) diffusion of the complex through the membrane phase; (3) release of the substrate at the other interface; and (4) back diffusion of the free carrier.
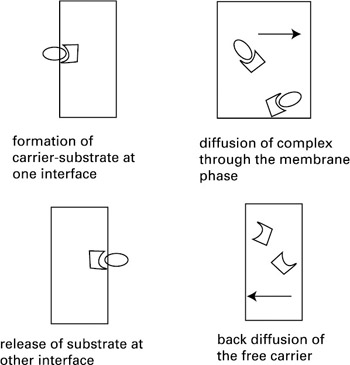
Figure 2.12: Schematic showing the four steps of a transport cycle.
Because of its cyclic nature, this process presents analogies with molecular catalysis; it may be considered as physical catalysis producing a change in location. In transport, the substrate is translocated, just as chemical catalysis produces a transformation into products. The carrier is the transport catalyst that strongly increases the rate of passage of the substrate with respect to free diffusion and shows enzymelike features.
Carrier design requires taking into account factors specific for transport processes. Whereas a molecular receptor should display high stability, high selectivity, and a slow exchange rate with respect to its substrate, a carrier molecule should be highly selective but should not bind its substrate too tightly. It must be flexible enough to allow sufficiently fast exchange rates for loading and unloading and to avoid carrier saturation. In addition, the carrier must have a suitable lipophilic-hydrophilic balance so as to be readily soluble in the membrane phase, while retaining the ability to reach the interface and enter into contact with the aqueous phase. Finally, it should not be too bulky to diffuse rapidly.
A major goal in transport chemistry is to design carriers and processes that involve the coupled flow of two (or more) species in either the same (symport) or in opposite (antiport) directions. Such parallel or antiparallel vectorial processes make it possible to set up a pumped system in which a species is carried in the potential created by physicochemical gradients of electrons (redox gradient), protons (pH gradient), or other species (concentration gradient). Gradients may be generated by chemical reactions, as occurs in vectorial bioenergetics. For transport, either two or more individual carriers for different substrates may be used simultaneously or the appropriate subunits may be introduced into a single species, a so-called cocarrier. In such coupled systems, carrier design allows transport processes to be endowed with regulation of rates and selectivity, as well as with coupling to energy sources for the transport of a species against its own concentration gradient.
Photonic Molecular Devices
One reason for the emphasis on photochemistry is due to its potential for devising (a) parallel input, (b) processing, and (c) output functions. How to read information in and out of molecular systems should be considered as one of the defining problems of the field. This is particularly true in light of the fact that much of the information stored may not even be localizable. Even if it is, the value may make no sense unless it is compared to the measured values of all other incident values in the same system. An example is the location of the "information" encoded in a neural network. Many of the systems under discussion will require some form of parallel output or value definition arising from collective behavior.
The formation of supramolecular entities from photoactive components may be expected to perturb the ground-state and excited-state properties of the individual species, giving rise to novel properties that define a supramolecular photochemistry. This could include such features as excitation energy migration, photoinduced charge separation by electron or proton transfer, perturbation of optical transitions and polarizabilities, modification of redox potential or excited states, photoregulation of binding properties, and selective photochemical reactions, among other possibilities.
Supramolecular photochemistry, like catalysis, may involve three steps: binding of substrate and receptor, mediating a photochemical process (such as energy, electron, or proton transfer), followed by either restoration of the initial state for a new cycle or a chemical reaction.
In principle, supramolecular photonic devices require a complex organization and adaptation of the components in space, energy, and time, leading to the generation of photosignals by energy transfer (ET) or electron transfer (eT), substrate binding, chemical reactions, and so on. Numerous types of devices may thus be imagined, involving oriented energy migration, antenna effects, vectorial transfer of charge, conversion of light into chemical energy, optical signal generation, and photoswitching, to name a few. Being able to carry out separately the various steps of an overall photochemical process (such as in conversion of absorbed light into emitted light of another wavelength, for example) would allow the customization and optimization of each part. Light conversion requires a device involving three basic operations and consisting of two discrete components: (a) a light collector (antenna) using strongly absorbing units and (b) an emitter, thus allowing the separate optimization of absorption and emission. For maximum effectiveness, intercomponent energy transfer between the antenna and emitter must occur as efficiently as possible. Hence the three-step mode of absorption, energy transfer, and emission.
As an example of such a process, luminescent cryptates of europium(III) and terbium(II) have been formed with macrobicyclic structures including various groups that can serve as light-collecting antenna components. These complexes present a unique combination of features arising from both their cryptate structure and the nature of the emitting species: protection of the included ion from deactivation due to interaction with water molecules; very high thermodynamic stability and kinetic inertness; multiple photosensitizing groups suitable for energy transfer and displaying strong UV light absorption; and characteristic long wavelength and long lifetime of the emission. They display a bright luminescence in an aqueous solution, whereas the free ions do not emit under the same conditions. Absorption of UV light by the organic antenna groups is followed by energy transfer to the included lanthanoid cation, which then emits its characteristic visible radiation (figure 2.13).
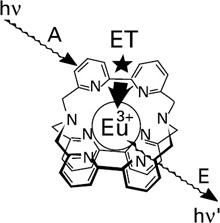
Figure 2.13: Eu(III) cryptate showing the three steps (absorption, energy transfer, and emission) in light conversion. (Reprinted with permission from Supramolecular Chemistry, J.-M. Lehn. 1995 VCH Verlagsgesellschaft.)
Such photoactive cryptates are of interest as novel luminescent materials and as labels for biological applications such as labeling of monoclonal antibodies, oligonucleotides, and membrane components (Prat, Lopez, and Mathis 1991; Lopez et al. 1993). Linked, cascade systems performing very long range energy transfer (VLRET) may be imagined as well. Such systems could be considered as examples of supramolecular wires in the field of molecular and supramolecular electronics.
Another area of interest is the use of receptor molecules bearing photosensitive groups. Such molecules may display marked modifications in their photophysical properties on the binding of substrate species, leading to changes in their light absorption (color generation) or emission features, allowing their detection by spectroscopic measurements.
Chromoor lumino-ionophores of macrocyclic or macropolycyclic type respond to the binding of metal ions and may be of much interest as analytical tools for environmental applications or for the study of ionic changes in biological processes such as cell signaling. These types of receptors combine the strong and selective complexing ability of cryptands with the intense absorption or emission properties of photosensitive groups (azophenol, spirobenzopyran, anthracene, or coumarin). They display marked changes in absorption on complexation of alkali-metal cations or protonation, thus acting as fluorescent signaling systems.
Yet another example is the strong enhancement of the acridine fluorescence observed when ATP binds to a receptor molecule formed by a (24)-N6O2 macrocycle bearing a lateral acridine group (Hosseini, Blacker, and Lehn 1990). This receptor is a sensitive and selective ATP probe that generates a fluorescence signal on ATP binding.
One present, very active area of research is the search for substances effecting singleor double-strand nucleic acid recognition and fitted with a photoreacting group to perform sequence-specific photocleavage on DNA or RNA.
The field of supramolecular photochemistry is a rapidly expanding field and promises much activity in the years to come.
|