36 - Prostate
Editors: Mills, Stacey E.
Title: Histology for Pathologists, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > XI - Endocrine > 45 - Parathyroid
function show_scrollbar() {}
45
Parathyroid
Sanford I. Roth
Nicole A. Belsley
Graziella M. Abu-Jawdeh
Introduction
The surgical pathology of the parathyroid glands largely involves interpretation of their histology in patients with hyperfunction due to neoplastic or primary hyperplastic processes;1 that is, primary hyperparathyroidism (1,2,3,4,5,6,7). Secondary hyperparathyroidism, in contrast, is a consequence of stimulation of parathyroid gland growth (secondary hyperplasia) and hormone secretion by hypocalcemia due to diseases of other organs, such as renal insufficiency, malabsorption (8,9,10), or genetic defects in the parathyroid or other organs (11). Secondary hyperplasia is histologically indistinguishable, but clinically easily distinguishable from primary hyperplasia. Since other disorders, such as hypoparathyroidism, pseudohypoparathyroidism, and familial hypocalciuric hypercalcemia (12), are not treated surgically and neonatal severe hyperparathyroidism (12) is rare, the parathyroid glands are rarely available for examination in these diseases. In order to adequately interpret the pathology of the parathyroid glands, the surgical pathologist must have a thorough knowledge and understanding of calcium metabolism, the biochemistry of the parathyroid glands, the differential diagnosis and pathobiology of parathyroid disease, and the normal and pathologic anatomy of the glands (13). This chapter presents the normal histology, the embryology and its molecular biology, the alterations of the parathyroid glands that occur with age, and a brief discussion of normal calcium metabolism.
Historical Review
In 1850 Professor Richard Owen (14), before the Royal College in London, first described the parathyroid glands in the Indian rhinoceros as a small, compact yellow glandular body attached to the thyroid at the point where the veins emerge. Interestingly this paper (14) was not published for twelve years after its initial presentation. Remak (15) in 1855 and Virchow (16) in 1863 described similar glands in cats and humans, respectively. However, it was not until 1880 that the anatomy and histology of the parathyroid glands of several species, including humans, were clearly established by a medical student, Ivor Sandstr m (17,18). He demonstrated that the glands, which he named glandulae parathyroidae, were structures separate from the thyroid. He used this terminology to suggest the physical, as well as the
P.1150
possible embryologic, relationship to the thyroid. Kohn (19,20) proposed the term Epithelk perchen after he demonstrated by animal experiments that the glands originated independently from the thyroid. However, this terminology has survived only in the German literature.
Embryology
Although an ectodermal origin of the glands has been suggested (21,22), the consensus is that the glands arise from the endodermal region of the branchial pouch (23). Neural crest cells participate in the formation of the mesenchyme of the glands (24). Embryonic parathyroid hormone production has been demonstrated by immunohistochemical studies (Roth, unpublished data). At 8 3/7; weeks gestational age (3.1 cm crown-rump length) a few positive cells are seen in the glands. By 17 to 20 weeks (Figure 45.1) (25,26,27,28,29,30) abundant parathyroid hormone is present in the glands.
The parathyroid glands arise as diverticula of the endoderm of the third and fourth branchial pouches (31,32,33,34). They make their first appearance as bilateral localized proliferations along the anterodorsal surface of pouch III and the lateral portion of the dorsal extremity of pouch IV during the 5th week of gestation (9-mm embryo). The glands are thus referred to as parathyroid III or parathyroid IV, depending on their pouch of origin (the singular is used because of the symmetrical growth). Parathyroid III, along with the thymus, forms as the third pouch separates from the pharynx. At this stage parathyroid III lies cephalad and lateral to parathyroid IV, and the two are separated by the medial thyroid. Differential growth rates of the thymus and the adjacent medial structures determine the final position the glands occupy after birth. The thymus, the most lateral of these structures, attaches to the pericardium and comes to lie largely in the thorax. The attached parathyroid III also develops a more caudad position as the more medial structures grow cephalad. In the 18 mm embryo, when parathyroid III is at the level of the lower pole of the thyroid, it usually separates from the thymus, forming the lower parathyroid gland. Variations in the level at which this separation takes place are frequent and account for the marked anatomic variations in the final position of the adult glands. Parathyroid IV and the lateral thyroid (the ultimobranchial or postbranchial body) derive from the caudad part of the fourth branchial pouch (Figure 45.2). Together they form a bilobate complex and, as they separate, parathyroid IV acquires its adult position as the upper gland, near the intersection of the recurrent laryngeal nerve and the medial thyroid artery. Due to its medial position, closer association with midline structures, and shorter embryonic migrations, parathyroid IV has a more constant location and is usually cephalad to parathyroid III. During development, small portions of the gland may become separated from the main gland or a gland may separate into two or more parts (see below).
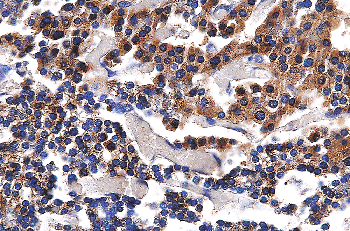 |
Figure 45.1 Immunohistochemical stain demonstrating PTH in the gland of a 20-week fetus. |
 |
Figure 45.2 An embryonic parathyroid gland still attached to the remnant of the branchial pouch. The chief cells are closely packed and uniform. No stroma is visible. |
Molecular Biology
The successful differentiation, development, and migration of the parathyroid glands require multiple genetic events. These events, though not fully understood, are largely known by identification of the genetic defects in various forms of human hypoparathyroidism, particularly DiGeorge Syndrome/velocardiofacial syndrome2, and by studies on mouse animal models (35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55). Though early studies (53) proposed an origin of the parathyroid glands from neural crest cells migrating into the pharyngeal pouches, it is now recognized that parathyroid epithelial
P.1151
cells develop from the third pharyngeal pouch endoderm. Gene products from neural crest cells in the parathyroid mesenchyme are required for modulation of the branchial cleft endoderm into the parathyroid cells (53,54). The development and migration of the parathyroid glands requires a proper balance between epithelial cell death (apoptosis) and proliferation, as well as the appropriate interaction of endoderm with the associated mesenchyme.
Several genes and their products have been identified which are required for normal differentiation, development, and migration of the parathyroid glands (37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,55), though undoubtedly there are many other not yet identified genes and signaling factors vital to these events. The known factors include genes encoding transcription factors, Hoxa3, Pax9, Tbx1, GATA3, Glial cell missing (Gcm) 2, and Eyes absent (Eya)1, as well as genes encoding secreted signaling molecules, fibroblast growth factor (Fgf) 8, Chordin (Chrd), bone morphogenetic proteins (BMPs), TGF (A) and Sonic hedgehog (Shh). The signaling molecules act by modulating the expression of the respective transcription factors. In homozygous mutant mouse models for many of these genes, amongst multiple pharyngeal and cardiac developmental anomalies, the parathyroids are often aplastic or hypoplastic (37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53).
Hoxa3, a member of the Hox family, is a gene that encodes a transcription factor present in the third pharyngeal pouch endoderm as well as the neural crest cells. Mice knockout studies for Hoxa3 highlight its function in the development of the organs arising from the third and fourth pharyngeal pouches and their dependence on mesenchymal neural crest cells for differentiation (38,39,40). Hoxa3 mutants develop multiple anomalies involving the throat, heart, and thyroid and fail to develop the thymus and parathyroids. The failure to develop parathyroids is thought to stem from a defect in the neural crest cells' ability to induce proper differentiation of the pharyngeal pouch endoderm (38,39).
Mammalian gcm2 (GCMB in humans) encodes a transcription factor also found in the third pharyngeal pouch endoderm, but is specifically expressed in a domain restricted to the parathyroid. Gcm2 deficient mice lack parathyroid glands, demonstrating the crucial role of gcm2 in the differentiation and function of the parathyroid (41). Interestingly, although gcm2 expression is restricted to the parathyroid, gcm2 knockout mice showed parathyroid hormone production originating in the thymus driven by gcm1, another homolog of the Drosophila gcm family that functions in placental development. Expression of gcm is absent in Hoxa3 mutant mice, suggesting it acts downstream of Hoxa3 (41,42).
GATA-3 and Pax9, additional transcription factors expressed in the third pharyngeal pouch, also result in hypoparathyroidism when absent. In humans with mutations in GATA-3, the resulting disease includes hypoparathyroidism manifest by hypocalcemia and low parathyroid hormone (43). Mice deficient for Pax-9 show initial formation of the pharyngeal pouches, but have arrested formation of the epithelial buds of the third pharyngeal pouch (44).
Eya1 is expressed in the third pharyngeal endoderm, mesenchyme and ectoderm as well as the organs derived from these structures. In order to investigate the role of Eya1 in animals, mice knockout experiments were carried out and studied at gestational days 11.5 12.5 (45). In contrast to the wild-type embryos that formed epithelial buds of the thymus and parathyroid primordia at gestational day 12, the Eya1-/- mice failed to form these epithelial buds. In addition, gcm2 expression was down-regulated, suggesting an upstream control by Eya1. HoxA3, Pax1 and Pax9 expression were not affected. Since HoxA3 and Pax1 have been shown to regulate Gcm2 expression (45), the control of Gcm2 by Eya1 suggests Eya1 may lie in a pathway downstream from HoxA3 and Pax1 (46).
The close relationships of the endoderm, ectoderm and neural crest cells are demonstrated by the signaling protein FGF8. The protein is expressed in the pharyngeal endoderm and ectoderm; however, the severe developmental anomalies caused by its deficiency are a result of its effect on the neural crest cells (47,48). Decreased FGF8 causes increased apoptosis of the cranial and cardiac neural crest cells, resulting in improper development of the heart and pharynx, mimicking DiGeorge syndrome (48). Studies showed dependence of this secreted molecule on the transcription factor Tbx-1, a gene mapped to the microdeletion of chromosome 22 associated with DiGeorge syndrome (49,50).
Antagonistic secreted signaling molecules, Chrd and the BMPs, regulate the dorsal and ventral developmental organization respectively. Chrd is present in the pharyngeal endoderm and acts antagonistically by binding the BMPs. Loss of Chrd results in an expansion of the allantois (ventral) and reduction in the embryonic mesoderm (dorsal). In addition, the mutant mice show features similar to those found in DiGeorge syndrome, including parathyroid hypoplasia, indicating a role of Chrd in not only positioning information but differentiation as well (51). Its role in parathyroid development may be due to decreased expression of Tbx1 and Fgf8 caused by loss of Chrd (49). An additional gene, Sonic hedgehog (Shh), also contributes to the balance between Chrd and BMPs. Shh encodes a secreted protein expressed in the pharyngeal endoderm that acts as a negative regulator. Shh -/- mutant mice showed abnormal third pouch development thought to be due to an overexpression of Bmp4 (52). The normal repression of Bmp by Shh in the third pharyngeal pouch is lost in the mutant mice, upsetting the dorsal/ventral balance in favor of ventral expansion of the thymus and absence of the dorsal parathyroid. Although the gland primordium initially formed appropriately, Gcm expression was later absent in these mice, correlating with the lack of parathyroid glands (52).
Aside from organogenesis, numerous genes have been implicated in parathyroid disease (55,56,57,58,59,60,61). Most neoplastic
P.1152
diseases of the parathyroid, primary chief cell hyperplasia, adenomas and carcinomas, result from alterations in tumor suppressor genes, including Rb, p53, and HRPT2, or cell cycle regulators such as cyclin D1 (55,56,57,58,59). In addition, genetic mutations involving proteins specific to the parathyroid gland's role in calcium homeostasis have been identified (60), specifically the calcium receptor gene which encodes a gene located in the parathyroid as well as the kidney (61). Although not located in the parathyroid, mutations in vitamin D receptors and PTH receptors indirectly affect the proper functioning of the parathyroid, leading to alterations in calcium homeostasis (62).
Calcium and Parathyroid Metabolism
Calcium is one of the most closely controlled ions in mammals (63). Ninety-nine percent (99%) of the calcium is in the hydroxyapatite of the bones and 1% is in the extracellular fluids and soft tissues (64). The serum level of ionized calcium (Ca2+) is maintained within the limits of laboratory error in most individuals. The serum calcium concentration ([Ca2+]) is commonly measured as the total calcium, although it is the ionized calcium, approximately 50% of the total serum calcium (64), that is meaningful. In normal humans, calcium ions are bound, primarily to proteins (~40%) such as albumin, but also to chelating agents such as citrate. The serum [Ca2+] is regulated through five organs: the parathyroid glands, the C cells of the thyroid (probably of minimal importance in humans), the bones, the kidneys, and the gastrointestinal tract (65). In humans the two primary hormones responsible for control of [Ca2+] are the parathyroid hormone (PTH) and the metabolites of vitamin D, primarily 1 ,25 (OH)2 vitamin D (66).
Acute control of the serum [Ca2+] is the primary responsibility of PTH (31). PTH operates through a cell surface receptor, primarily found in the proximal convoluted tubule of the kidney and the osteoblasts of the bone. PTH acts to increase the serum calcium. It promotes tubular reabsorption of calcium in the kidney, increases the activity of the 1 vitamin D hydroxylase [thus increasing the synthesis of the active form of vitamin D (1 ,25 (OH)2 vitamin D) and increasing bone resorption and gastrointestinal calcium transport]. The effect on bone requires the osteoblasts and the osteoclast precursors (see Chapter 4), which have PTH receptors. These cells produce paracrine factors that stimulate osteoclastic bone resorption since the osteoclasts lack PTH receptors. PTH also operates through the osteocytes, resulting is osteocytic osteolysis of the perilacunar bone.
The most active metabolite of vitamin D, 1 ,25 (OH)2 vitamin D, increases calcium resorption from the gastrointestinal tract and acts in synergy with PTH to increase bone resorption (67). 1 ,25 (OH)2 vitamin D also acts as a negative feedback on PTH synthesis and secretion. This action is mediated by inhibiting translation of prepro-PTH messenger through a vitamin D responsive element related to an upstream promotor of the PTH gene (68).
PTH synthesis and secretion are controlled by the ambient [Ca2+]. Increased ambient [Ca2+] decreases PTH synthesis and secretion and intracellular [Ca2+], whereas decreased serum [Ca2+] increases PTH synthesis and secretion. This is in contrast to other organs, where increased intracellular [Ca2+] increases hormone synthesis and secretion, whereas decreased [Ca2+] decreases synthesis and secretion.
A calcium ion sensing cell surface receptor has been identified (69,70,71). This receptor is a member of the superfamily of guanine-nucleotide-regulatory G proteins. The gene for this receptor is located on the long arm of chromosome 3 (69,70,71). The receptor has been identified on the cell membrane of the parathyroid glands and is thought to be responsible for mediating the effect of calcium on the synthesis and secretion of PTH, much as vitamin D acts through its receptor.
Mutations of the calcium receptor gene have been identified in familial hypocalciuric hypercalcemia and severe neonatal hyperparathyroidism (69,72,73,74). These heterozygous and homozygous mutations result in inactivation of the receptor. The chief cell is thus stimulated to hypersecrete. In some cases of hypoparathyroidism, hyperactivity mutations have been identified in this gene (75), resulting in decreased synthesis and secretion of PTH.
Paracrine control of PTH secretion has been demonstrated in tissue culture, with decreasing cell density resulting in decreasing hormone secretion (76). Chromogranin A, which is cosecreted with PTH and its proteolytic products pancreastatin and parastatin, also inhibit PTH secretion (77,78,79).
Number and Location
Ninety percent (90%) to 97% of patients have four parathyroid glands (80,81,82,83,84,85,86). However, the number has been reported to vary between two and 12 glands (80,81). The incidence of supernumerary glands in adults varies between 2 and 6.5% (86). Supernumerary glands are most commonly intrathymic and are thought to result from embryonic division of one or more glands (Figure 45.3). Small accumulations of parathyroid tissue as a result of parathyroid cell dispersion during embryonic migration may result in the presence of numerous small nests of cells. Proliferation of these nests, especially in the presence of secondary hyperparathyroidism, [i.e., parathyromatosis (88)], results in multiple accumulations of parathyroid implants that
P.1153
must be distinguished from metastatic implants from a carcinoma. Ectopic glands are one of the factors responsible for persistent hyperparathyroidism after surgical therapy of primary hyperplasia of the parathyroids (86,87) and must be distinguished from other causes of recurrent or persistent hyperparathyroidism such as parathyroid carcinoma and parathyromatosis (88). It is probably rare for there to be less than four glands in the absence of other abnormalities such as thymic aplasia. Because studies of the number of glands are based on autopsy material, we feel that most of the cases with fewer than four glands are due to failure to locate aberrant glands in unusual locations. Despite the known variation in the adult position of the parathyroid glands (20,28,29,30,69,70), there is a definite pattern in their anatomic distribution, which is related to their embryonic derivation (Figure 45.4). The most common location of the upper or superior (parathyroid IV) glands (77%) is at the cricothyroid junction posteriorly or just above the intersection of the recurrent laryngeal nerve and inferior thyroid artery (70%) (70,82). The second most common location is behind the upper pole of the thyroid (22%) (82), in which case the glands are often within the surgical capsule of the thyroid (Figure 45.5). Other uncommon locations include a more caudad position near the inferior thyroid artery, within the thyroid capsule or parenchyma (Figure 45.5), within or near the pharyngeal wall, a retropharyngeal or retroesophageal position, or within or near the carotid bifurcation. Abnormal upper glands can descend into ectopic locations as low as the posterior mediastinum (70).
 |
Figure 45.3 Parathyroid gland from a 60-year-old female, divided into several portions by the vascular bundle and recurrent laryngeal nerve. |
The lower or inferior parathyroid glands (parathyroid III) have a more diverse distribution but are most commonly located between the lower pole of the thyroid and the thymus (Figure 45.6). They can occur as high in the neck as the hyoid bone, the pharynx, or the pyriform sinus, or as low as the pericardium (89,90). In 42 to 61% of the cases they are located on either side of the lower thyroid or in a juxtathyroidal location. Another common location is the thymic tongue or cervical extension of the thymus. Uncommon locations are the mediastinal thymus and the anterior mediastinum (82,83,84,85,86). Ectopic intravagal parathyroid tissue has been reported (91). The gland locations are symmetrical in 80% of patients.
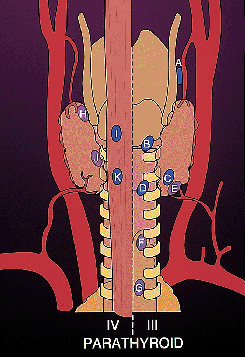 |
Figure 45.4 Diagram demonstrating the location of the parathyroid glands. (Modified with permission from: Gilmour JR. The gross anatomy of the parathyroid glands. J Pathol Bacteriol 1938;46:133 149 and reprinted with permission from:Roth SI. Parathyroid glands. In: Damjanov I, Linder J, eds. Anderson's Pathology. 10th ed. St. Louis: Mosby-Year Book; 1966:1980 2007. ) |
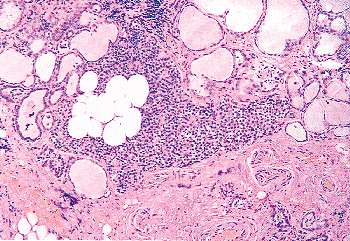 |
Figure 45.5 A parathyroid gland from a 21-year-old male just below the thyroid gland capsule in the thyroid parenchyma. A large accumulation of adipocytes is present within the parathyroid. Only chief cells are seen within the parathyroid. |
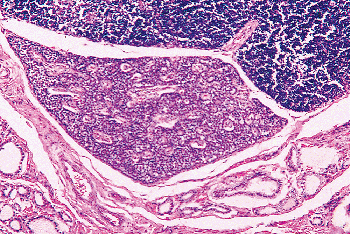 |
Figure 45.6 A fetal parathyroid (33 weeks' gestation) is seen between the thymus (top) and lower edge of the thyroid (bottom). A small amount of stroma surrounds the parathyroid vessels. The gland is composed of pure tightly packed chief cells. |
P.1154
Gross Appearance, Size, and Shape
Each adult gland measures 3 to 6 mm in length, 2 to 4 mm in width, and 0.5 to 2.0 mm in thickness (Figure 45.7). The shape of the gland varies because it is molded by the adjacent structures. The glands are a flattened, ovoid pancake with sharp edges. The capsule is grey and almost transparent often with a fine network of small vessels. The underlying parenchyma is yellow to orange tan, depending on the amount of stromal fat, the number of oxyphil cells, and degree of vascularity (5). The glands are soft and malleable, although they may show a marked increase in firmness, swelling, and a dark red color if surgical manipulation causes intraglandular hemorrhage. Abnormal glands are usually more bulbous, with rounded edges, and have a firmer consistency and a darker red tan color (5).
 |
Figure 45.7 Four normal parathyroid glands removed at autopsy from a 53-year-old man. |
Gland weight and parenchymal cell content are important parameters used in the histopathologic assessment of the parathyroid gland (92,93,94,95,96,97,98,99,100,101,102), and all parathyroid glands or part of glands removed at surgery must be carefully dissected free of capsular fat or thymic tissue, measured and weighed to the nearest milligram. The total parathyroid weight gradually increases throughout embryonic life, reaching a mean total of 5 to 9 mg at the postpartum age of 3 months (97,98,99). This is followed by a steep linear increase in total parathyroid weight until the third or fourth decade of life, when it levels off at a mean of 120 3.5 mg in men and 142 5.2 mg in women (5). The mean weight per gland is 31.1 mg in men and 29.8 mg in women (100). The lower parathyroid glands are larger then the upper glands (97,101). The parenchymal cell content of the glands is extremely variable and difficult to evaluate. It is reported to average 74% of the weight of the gland in adults (85). It is a somewhat better indicator of gland function than gland weight alone but requires careful morphometric analysis in conjunction with careful evaluation of the total gland weight. The average parenchymal weight per gland is 21.6 mg for men and 18.2 mg for women (100), whereas the mean total parenchymal weight for four glands is 82.0 2.6 mg for men and 88.9 3.9 mg for women (96,97,98). The total gland weight has been reported to be higher in blacks than in whites (102). In a selected series of patients with primary hyperparathyroidism due to a single adenoma, who had a second gland totally removed because the surgeon felt the gland was enlarged, 15 histologically normal glands weighed over 60 mg (103). The largest gland weighed slightly over 160 mg. The total (10) and parenchymal (101) gland weight are inversely related to serum calcium concentration in patients with secondary hyperparathyroidism. In these patients a direct relationship has been found between the total gland weight and the serum phosphorus and renal function as expressed by serum urea nitrogen (10).
Histology
The normal parathyroid gland has a thin fibrous capsule that separates it from the adjacent thyroid thymus or adipose tissue (Figures 45.8 and 45.9), which, except for those glands embedded in the thyroid or its capsule, is adipose tissue or thymus. At the vascular pole, an artery and vein are present surrounded by fibrous tissue. These branch into smaller arteries and veins, which form a complex readily visible in the capsule. The capsular arteries and veins are connected by arterioles, capillaries, and venules located in
P.1155
the fibrous septa between the parenchymal cells (102). This capillary network abuts every chief cell. Due to the rich capillary network, the cut surface bleeds readily, providing an easy way for the surgeon to distinguish the parathyroid from lymph nodes, adipose tissue, thymus, and thyroid, which do not show such prominent bleeding from their cut surfaces. The capillary endothelial lining cells have pores or fenestrations resembling those seen in other endocrine glands (104,105). Dense bodies, Weibel-Palade bodies, pinocytotic vesicles, tight junctions, or zonulae occludens are constituents of the parathyroid capillary endothelium. Two interconnecting plexuses of lymphatic capillaries in the capsule surround the parathyroid glands. From the inner plexus, loops of lymphatics dip into the gland parenchyma, whereas the efferent lymphatics arise from the outer plexus via special lymphatics or those of the thyroid (106). The interstitial space is limited by the basement membranes of the chief cells and capillaries and contains collagen bundles and elastic fibers (104). Nerve bundles in close proximity to chief cells suggest autonomic innervation (107,108,109,110). In the rabbit the nerves have been shown to originate in the medulla oblongata, the dorsal nucleus of the vagus, and the vagus nerve (107,110).
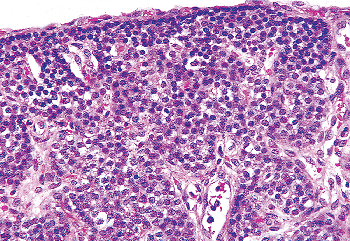 |
Figure 45.8 A parathyroid gland from a 7.5-month-old girl. The chief cells are the only cell type seen and are arranged in sheets, between the large vascular channels. The capillaries are present between individual chief cells. The chief cells show central regular nuclei and a clear amphophilic cytoplasm. A thin fibrous capsule separates the gland from the stroma. |
 |
Figure 45.9 A parathyroid from a 27-year-old woman showing only sheets of chief cells and no stromal fat. The vascular pole can be seen at the right margin of the micrograph. A thin fibrous capsule is seen. |
In infants and children the interstitium consists only of the capillary network and the extracellular space. Little or no collagen or stroma is present (Figure 45.8). With age, there is focally increasing collagenization of the perivascular stroma. This forms a delicate fibrous septa in adult glands, imparting to them a somewhat lobulated appearance. Surrounding the capillaries and lymphatics are interstitial cells that consist of fibroblasts, pericytes, mast cells, and a few lymphocytes. Adipocytes are sparse in the stroma of infants and children. Stromal fat cells begin to appear late in the first decade of life and increase throughout life. At puberty, especially in women, there is an increased rate of accumulation of adipocytes. Stromal fat cells increase in number with increasing age, reaching a maximum in the third to fifth decades of life. There is a marked variation in the amount and distribution of stromal fat within a single gland, between glands in the same individual, and among individuals of the same age (Figures 45.8,45.9,45.10,45.11,45.12,45.13,45.14,45.15). Recent studies (96,111,112) confirmed the initial reports of Gilmour (32) that, in adults, adipocytes occupy an average of 50% of the stromal volume rather than of the total parathyroid volume. Women have a higher percentage of stromal fat than do men. The amount of stromal fat is affected by the same factors that affect total body fat; for example, diet, nutrition, chronic illness (such as malignancy), and genetics. These variations make interpretation of the level of parathyroid function difficult to assess on the
P.1156
basis of the stromal fat. In normal glands, the stromal fat appears to compress the surrounding parenchyma; whereas, in hyperplastic or adenomatous glands, the fat cells appear scattered among the parenchymal cells. In the fourth decade there is a relative decrease in parenchymal adipocytes.
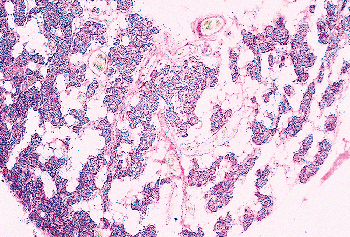 |
Figure 45.10 Photomicrograph of a parathyroid from a 39-year-old man. There is abundant stromal fat separating the cords of chief cells. |
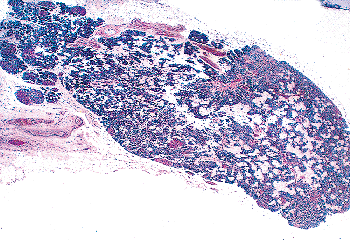 |
Figure 45.11 A parathyroid from a 69-year-old woman. There is a moderate amount of stromal fat, largely concentrated in the center of the gland. A few small oxyphil nodules can be seen in the parenchyma. |
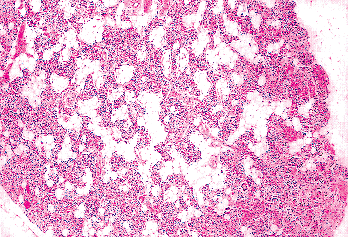 |
Figure 45.12 A parathyroid from a 76-year-old man. There is a moderate amount of stromal fat, largely in the center of the gland. Small oxyphil nodules are present. |
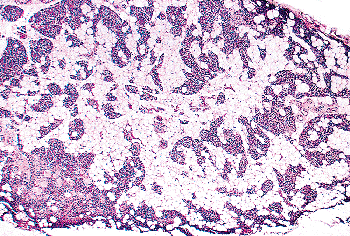 |
Figure 45.13 A parathyroid from a 77-year-old man with abundant stromal fat. The parenchyma is composed entirely of chief cells. |
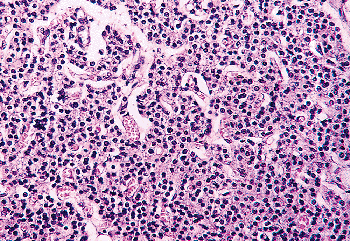 |
Figure 45.14 A normal parathyroid from a 70-year-old woman showing almost no stromal fat or oxyphil cells. |
Each parenchymal cell is separated from the stroma by a prominent basement membrane. The parenchymal cells are arranged in irregular sheets without prominent stroma in infants and prepubertal children. In infants and young children only one type of cell is present, the chief cell. The chief cells of the newborn and infantile parathyroid gland are small and regular, measuring 6 to 8 m in diameter (Figure 45.16). The chief cell membranes are poorly demarcated, and the cytoplasm is amphophilic, relatively lucent, and occasionally vacuolated. Intracellular fat in the chief cells of children is low compared with the adult gland, with only 30 to 40% of the chief cells of children containing large intracellular fat droplets on fat stains (113). The
P.1157
nuclei are often molded and they overlap. The nuclei are centrally located, with uniform chromatin and small, inconspicuous nucleoli.
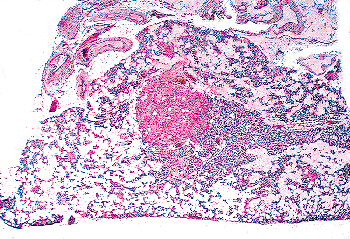 |
Figure 45.15 A parathyroid gland of a 77-year-old man. The stroma is almost completely replaced by adipocytes. There is a large oxyphil cell nodule in the center of the gland. |
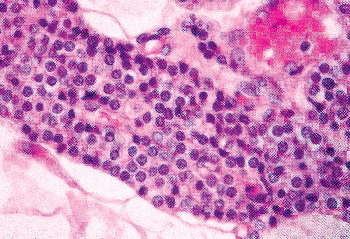 |
Figure 45.16 Sheets of chief cells in a newborn. The stroma has little collagen and is outside the large sheet. The cell membranes are poorly demarcated. The cytoplasm is eosinophilic. |
In the adult, the parenchymal cells are arranged in solid nests, rounded or lobulated masses and trabeculae, or a combination of these (Figures 45.8,45.9,45.10,45.11,45.12,45.13,45.14,45.15,45.16,45.17). A pseudofollicular pattern also has been described in normal glands (Figure 45.18) (114,115,116). Ultrastructural evidence indicates that follicle formation in the parathyroid is the result of a proliferation of parenchymal cells, with ischemic necrosis and degeneration of those cells separated from their blood supply by other parenchymal cells.
These pseudofollicles are usually filled with cellular debris and a pink eosinophilic homogeneous material resembling the colloid seen in thyroid follicles (Figure 45.18) (114). This material contains glycoproteins, as evidenced by the positive periodic acid-Schiff (PAS) reaction, and PTH as evidenced by immunostaining. Several investigators have further reported that it stains with Congo red and shows the apple-green birefringence characteristic of amyloid (115). A similar finding has been reported in the follicles of pathologic parathyroid glands (116,117). Cinti et al. (114) were unable to confirm either the presence of amyloid or glycoproteins in the follicles of a series of normal parathyroids removed at the time of thyroidectomy. However, electron microscopy of follicles in a pathologic parathyroid gland did demonstrate fibrils closely resembling those of amyloid in pseudofollicles (113).
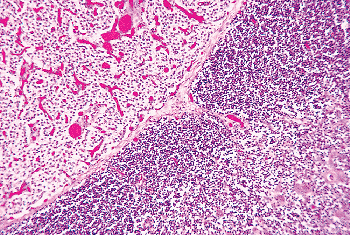 |
Figure 45.17 Sheets of parathyroid chief cells showing amphophilic clear cytoplasm, adjacent to the thymus. The prominent vascularity of the gland is visible. |
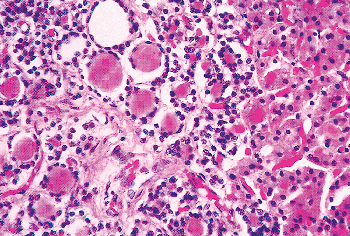 |
Figure 45.18 Numerous pseudofollicles in a normal parathyroid of a 58-year-old white man. Lining the pseudofollicles are chief cells. At the right of the micrograph is the edge of an oxyphil cell nodule. |
There are two types of parenchymal cells recognized by light microscopy in the adult normal parathyroid gland. These are the chief cells (Figures 45.15, 45.18,45.19,45.20), in their active and inactive forms, and the oxyphil cell (Figures 45.15, 45.18,45.19,45.20) (19,118,119). In adults the chief cells are arranged in sheets, cords, trabeculae, and small
P.1158
nodules. They are spherical and measure 8 to 12 m in diameter. The cell borders are poorly defined. The cytoplasm is amphophilic or faintly eosinophilic, with 70 to 80% of the chief cells containing large prominent fat droplets, (Figure 45.21) (5,17), corresponding to the lipid bodies seen in the resting chief cells by electron microscopy (120). It is of interest that these lipid droplets were first recognized in human chief cells by Sandstr m (17,18), although he did not appreciate their significance. By ordinary light microscopy, the active chief cell may be difficult to identify. The inactive chief cell may be recognized by the vacuolated clear appearance of the cytoplasm that is filled with lipid, glycogen, and lysosomes (120,121,122,123). Deposition of silver particles on the secretory granules is responsible for the argyrophil reaction seen in normal parathyroid glands using the Grimelius silver nitrate stain (120,124,125,126). The granules correspond to the location of PTH (Figure 45.22) (127,128,129,130,131) and chromogranin (Figure 45.23) (128). Differences in the parathyroid and chromogranin content of the chief cells indicate a variation in the secretory granule content and supports the presence of a secretory cycle in the parathyroid chief cell. The nuclei are round, centrally located, have a sharp nuclear outline, even chromatin, and small rare nucleoli (5,113).
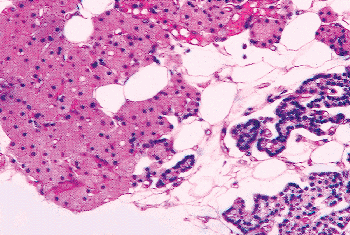 |
Figure 45.19 Nests and cords of oxyphil cells and chief cells among the adipocytes of the stroma. |
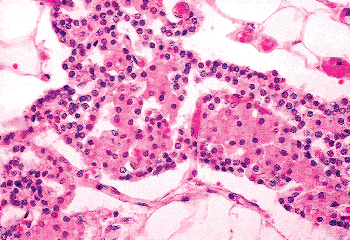 |
Figure 45.20 Trabecula of parathyroid showing mixture of oxyphil and chief cells. |
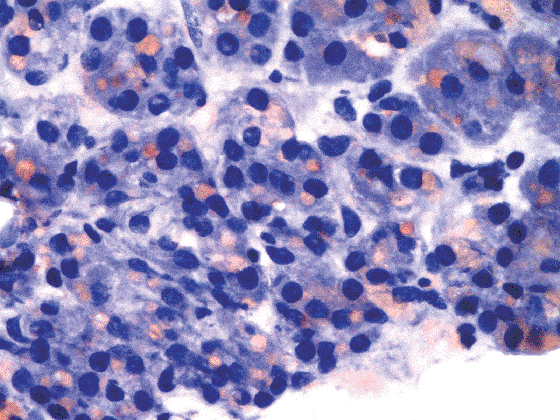 |
Figure 45.21 Chief cells with intracytoplasmic lipid droplets (Oil-Red-O stain, hematoxylin counterstain). |
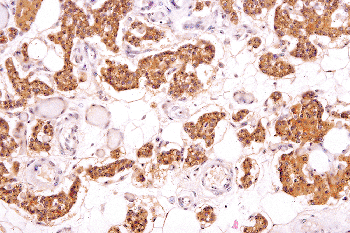 |
Figure 45.22 Chief cells showing abundant PTH in the parathyroid gland. Note the variation in the amount of hormone in various chief cells. (Immunoperoxidase stain for PTH, hematoxylin counterstain.) |
Ultrastructural features of the chief cell correlate with its functional activity (120,121,122,129,130,131,132,133,134,135). The chief cell, considered the basic functional unit of the parathyroid gland, is responsible for the production and secretion of PTH and, in turn, in the maintenance of the homeostasis of ionized calcium (136,137,138,139). To achieve this, each individual chief cell undergoes a secretory cycle (120,121,122,129,130,131,132,133) (Figure 45.24) similar to that of the follicle of the thyroid. Each chief cell is polarized with the Golgi apparatus and granular endoplasmic reticulum farthest from the basement membrane lined perivascular surface (140).
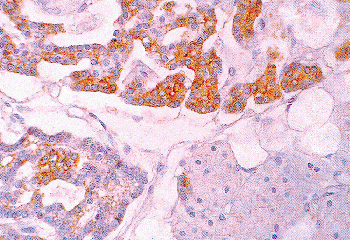 |
Figure 45.23 Parathyroid gland showing abundant chromogranin in the chief cells. The chief cells have different amounts of chromogranin. The oxyphil cells are free of chromogranin. |
P.1159
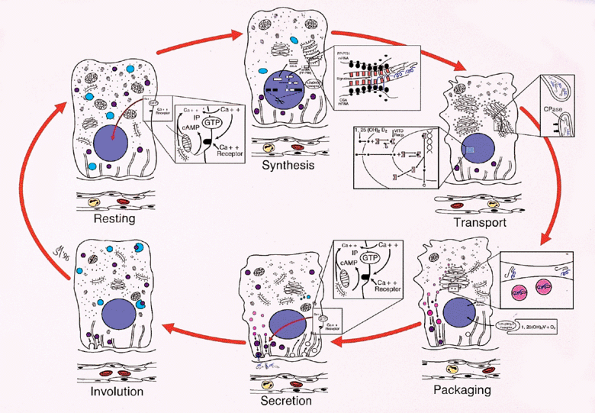 |
Figure 45.24 Diagram of the secretory cycle of the parathyroid chief cells (derived from electronmicroscopic studies (9). Although these comprise a continuum, typical stages have been recognized ultrastructurally. During the resting phase, the cells are rich in glycogen and lipid bodies with dispersion of the granular endoplasmic reticulum (GER) and free ribosomes. Dense-core secretory granules are relatively uncommon and scattered throughout the cytoplasm. Rare lysosomes are seen. The Golgi apparatus is small and inconspicuous, with few vesicles and vacuoles. The cell membranes are relatively straight with few interdigitations. At the end of the resting phase, presumably due to decreased cytoplasmic [Ca2+] and release of vitamin D, metabolite from the upstream promotor site, the cell begins the process of PTH synthesis. As the synthetic phase begins, transcription of the gene for PTH begins with production of the complimentary RNA for prepro-PTH and presumably accompanied by a similar transcription of chromogranin. The mRNA is produced by removal of the introns and splicing of the exons. The mRNA moves to the granular endoplasmic reticulum, which aggregates [the perinuclear body of Pappenheimer and Wilens (48)]. This is accompanied by aggregation of the free ribosomes to polysomes. The preprohormone crosses into the lumen of the GER, where the preportion of the molecule is removed by a ligase. Other alterations include depletion of the dense-core granules, lysosomes, glycogen, and lipid bodies. The cell membrane increases in tortuosity, probably due to loss of cytoplasmic volume. The synthesis and secretion of hormone is halted, presumably by the action of 1 ,25 dihydroxyvitamin D on the upstream promotor region of PTH and the cytosolic [Ca2+]. In the transport phase, the hormone, supposedly accompanied by chromogranin, is transported through the cisternae of the GER to the Golgi apparatus as the pro segment is removed by a clipase. The hormone is conveyed to the Golgi vesicles. Further depletion of lipid bodies, glycogen, and secretory granules occurs, and the cisternae of the GER disperse. In the packaging phase, the hormone is bundled along with chromogranin into dense-core granules and the Golgi apparatus begins its involution. Secretion occurs as the dense-core granules containing PTH and chromogranin move along the microtubules, fuse with the cell membrane, and release the hormone into the pericapillary extracellular space. Depending on the ambient [Ca2+], the granules are rapidly passed along the microtubules and secreted (low [Ca2+]) or left free in the cytoplasm (high [Ca2+]), to be destroyed by lysosomes or to be secreted at a later time. The Golgi continues to decrease in size and complexity. The free ribosomes disaggregate. The cells begin to involute toward the resting phase with gradual loss of secretory granules and further involution of the Golgi apparatus. The cells begin to accumulate glycogen and lipid bodies and approach the resting phase. Lysosomes containing acid phosphatase accumulate in the cells. These presumably serve to destroy excess hormone and unsecreted secretory granules. (Modified with permission from: Roth SI. Parathyroid glands. In: Damjanov I, Linder J, eds. Anderson's Patology. 10th ed. St. Louis: Mosby-Year Book; 1966:1980 2007. ) |
P.1160
The resting phase, which corresponds to the inactive chief cell, is characterized by accumulation of glycogen and large lipid bodies that correspond to the lipid seen by light microscopy (see Figure 45.21). The rest of the cell organelles, the Golgi apparatus, the granular endoplasmic reticulum, and secretory granules are small and inconspicuous. This is best seen in the chronically suppressed cell in atrophic parathyroid glands (120,121,122,129,132,134,135). During this stage, lysosomes (141) as well as variable numbers of small dense-core secretory granules are present, the latter being peripherally located. Sacs of granular endoplasmic reticulum are dispersed throughout the cytoplasm, whereas free ribosomes are only partially aggregated into polysomes. The Golgi apparatus is small and inconspicuous, with few vacuoles and prosecretory granules. The cell membranes are straight with few interdigitations (104).
The synthetic phase is marked by the parallel aggregation of the cisternae of rough granular endoplasmic reticulum and is noted at the light microscopic level by the presence of the body of Pappenheimer and Wilens (120,142). Free ribosomes aggregate into polysomes. It is during this phase that prepro-PTH and chromogranin (120,134) are synthesized within the granular endoplasmic reticulum. During the next phase, the prohormone is split and transferred to the Golgi apparatus which begins to enlarge with increases in smooth membranes, vesicles and vacuoles, and prosecretory granules of different electron densities. As the packaging phase emerges, the granular endoplasmic reticulum disperses throughout the cytoplasm, and larger dense-core secretory granules appear in the Golgi region, gradually moving to the periphery. PTH and chromogranin A are present in the aggregated granular endoplasmic reticulum and dense core granules (128,129). As the secretory granules progress along the microtubules toward the cell surface, there is an involution of the Golgi apparatus; acid phosphatase appears at the secretory face and eventually is transferred into large lysosomes (141). Separation of the secretory granules from the microtubules into the cytoplasm could account for the second compartment of PTH storage postulated by Cohn and MacGregor (136). The cell cytoplasm in the synthetic and secretory phases becomes depleted of glycogen and lipid bodies. During the last phase, there is margination of the lysosomes and secretory granules, fusion of the plasma membrane with that of the secretory granules, and emptying of the products into the extracellular space. The membranes of the secretory granules and lysosomes are probably recycled (143). Shannon and Roth (141) and Hashizume et al. (144) demonstrated that the stored parathyroid secretory granules fuse with lysosomes which provide a mechanism for intracellular degradation of PTH. The chief cell returns to the resting phase with a resultant accumulation of glycogen and complex lipid bodies, which are the best indicators of the resting or functionally suppressed cell. In chronically suppressed normal glands adjacent to a hyperfunctioning adenoma, 90 to 95% of the chief cells are inactive. In comparison, the normal adult gland has 70 to 80% of the chief cells in the resting phase, and the normal prepubertal gland has 30 to 40% in the resting phase (113,121).
Correlative morphologic studies in normal, adenomatous, and hyperplastic glands have shown that the intracellular content of fat in the chief cells is inversely related to its endocrine activity and is a better indicator of hormonal function than is the stromal fat. An increased cytoplasmic lipid content is a feature of a functionally suppressed chief cell (120,121,145,146,147,148,149,150), whereas hormonally active cells of adenoma and hyperplasia are largely in the active stages of hormone synthesis and secretion and thus are fat depleted. Based on this fact, evaluation of intracellular fat content by Oil-Red-O stain (Figure 45.21) has been demonstrated to be useful in differentiating between normal and adenomatous or hyperplastic parathyroid glands (141,142,143,144,145,146,147,148,149,150). Care must be taken in interpreting these fat stains because some areas of adenomas and hyperplasias may contain intracellular fat (151,152,153,154).
Cell culture studies (155,156) using a sequential hemolytic plaque assay confirm the cyclical secretion of both PTH and chromogranin A by individual parathyroid cells. Roth and Raisz (134) demonstrated that the ambient ionized [Ca2+] controls the length of the resting phase of the chief cell cycle. Proliferation of parathyroid chief cells is also controlled by the ambient ionized [Ca2+] (157). Molecular studies (158) have shown that the mechanism of this control may be via depression of cyclins D1 and D2.
The second cell type in the adult gland is the oxyphil cell (see Figures 45.9, 45.12, 45.15, 45.17, 45.19, and 45.25). These are felt to be derived from the chief cells (99,159,160,161,162), although the stimulus for the development of oxyphil cells has not yet been identified. Before puberty, only extremely rare oxyphil cells are present in the glands. Beginning at puberty and increasing throughout life, increasing numbers of these cells appear (159). They are distributed among the chief cells as individual cells, sheets, and small or large nodules (5,113), indicating that there is both a continual transformation (see Figures 45.19, 45.20, and 45.24) from the chief cells and clonal proliferation of the oxyphil cells (160). Rarely, these nodules enlarge enough to be visible grossly. When this happens, it is not possible to differentiate these presumably nonfunctioning oxyphil nodules from functioning oxyphil adenomas (5). The normal oxyphil cells contain only minimal PTH or chromogranin (see Figure 45.23).
Oxyphil cells in multiple organs, including abnormal parathyroid glands, have been demonstrated to contain decreased cytochrome oxidase (163). Studies on oncocytic salivary gland tumors (164) manifested decreased concentrations of mitochondrial enzymes when measured against the amount of mitochondrial protein. This is in contrast to histochemical studies showing marked increases in mitochondrial
P.1161
enzymes (165,166,167). These studies, along with the fact that oxyphil cells increase with age, suggest that there is a mitochondropathy, which may lead to the proliferation of the mitochondria within the cells. PTH-related peptide has been described in oxyphil cells (168), although its function is not clear.
The oxyphil cell measures 12 to 20 m in diameter and has a clearly demarcated cell membrane, a pyknotic nucleus, and abundant eosinophilic granular cytoplasm (Figure 45.25), rich in mitochondria and resembling the H rthle cells of the thyroid or the oncocytes of other endocrine organs (159,160,161,162). Ultrastructurally, their cytoplasm is completely filled with mitochondria, often with a bizarre shape and size, as well as occasional lysosomes and lipofuscin granules (120). Their function is unknown; however, the oxyphil cells in normal glands do not seem to contain the organelles responsible for PTH synthesis and secretion [i.e., granular endoplasmic reticulum, Golgi apparatuses, and secretory granules (119,120)], as indicated by electron microscopy and the absence of PTH and chromogranin (see Figure 45.23). However, cases of functioning oxyphil cell adenomas (5,6,169) and chief cell hyperplasias (121) have been reported. Protein secretory and synthetic organelles and dense core granules have been demonstrated in these tumor cells.
Transitional oxyphil cells may be recognized by light microscopy by the decreased density of their cytoplasmic eosinophilia. By electron microscopy, these cells have lesser numbers of mitochondria and some of the organelles associated with PTH synthesis and secretion, such as granular endoplasmic reticulum, Golgi apparatuses, and dense-core secretory granules. Both forms of oxyphil cells contain sparse amounts of lipid droplets (150,151).
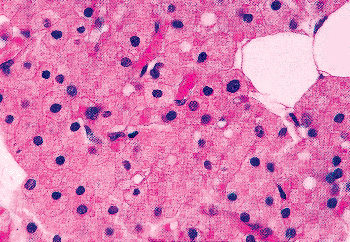 |
Figure 45.25 Oxyphil cells of the parathyroid in an 80-year-old woman. The centrally located, variably sized, pyknotic nuclei are seen within the granular eosinophilic cytoplasm. The cell membranes and the vascular channels are easily visible. |
Cytology of previously localized tumors has been proposed as useful in the preoperative identification of parathyroid neoplasms (170,171,172,173). Intraoperative imprints of tumors and normal glands may allow differentiation of adenomas and hyperplasia (174,175,176). Touch preps or smears from normal glands (Figure 45.26) show small nests or flat sheets of chief cells, with attached stromal fat, as well as naked nuclei in the background. Microfollicles may be present. The nuclei are round to oval and slightly pleomorphic with granular chromatin. The chief cells have pale vacuolated cytoplasm, some with intracytoplasmic lipid droplets (Figure 45.26). Oxyphil cells, with abundant cytoplasm and small, centrally located nuclei, may be
P.1162
identified as well. In contrast, the cytology of parathyroid adenomas shows large dense clusters of crowded cells with increased pleomorphism and decreased intracytoplasmic lipid. (177).
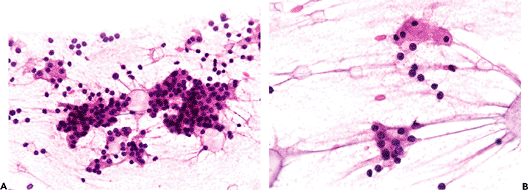 |
Figure 45.26 A. Parathyroid gland smear preparation obtained at autopsy, showing sheets and nests of parenchymal chief cell with attached stromal fat and naked nuclei in the background. B. Intracytoplasmic lipid droplets can be seen as well (Hematoxylin and eosin). |
The use of pure histologic criteria in the estimation of the functional or hormonal activity of the gland and in the differentiation between normal, hyperplastic, and adenomatous glands is difficult. The gland size and weight, the shape, and the relative proportion of stromal adipocytes and chief cells are all important criteria in making these distinctions. Account must be taken of the number of oxyphil cells because in normal glands, but not adenomas or primary hyperplastic glands, oxyphil cells are nonfunctional. We have found that the appraisal of these elements, along with the patient's clinical history, and evaluation of cellular cytology and intracellular fat content of the chief cells, all must be carefully considered in arriving at a proper pathologic diagnosis.
Footnotes
1 Though hyperplasia is commonly used to describe the pathology of primary hyperplasia involving all four glands, studies of patients with multiple endocrine neoplasia and familial disease (2) demonstrate that these are in fact neoplastic diseases.
2 Result of a hetologous deletion of a portion of the long arm of chromosome 22(del22q11.2), containing Tbx1.
References
1. Mallette LE. Review. Primary hyperparathyroidism, an update: incidence, etiology, diagnosis, and treatment. Am J Med Sci 1987;293:239 249.
2. Cope O, Keynes WM, Roth SI, Castleman B. Primary chief-cell hyperplasia of the parathyroid glands: a new entity in the surgery of hyperparathyroidism. Ann Surg 1958;148:375 388.
3. Cope O. Hyperparathyroidism: diagnosis and management. Am J Surg 1960;99:394 403.
4. Aurbach GD, Potts JT Jr. The parathyroids. Adv Metab Dis 1964;1:45 93.
5. Castleman B, Roth SI. Tumors of the parathyroid glands. In: Atlas of Tumor Pathology. 2nd series, fascicle 2. Washington, DC: Armed Forces Institute of Pathology; 1978:1 94.
6. DeLellis RA. Tumors of the parathyroid gland. In: Atlas of Tumor Pathology. 3rd series, fascicle 6. Washington, DC: Armed Forces Institute of Pathology; 1993:1 102.
7. O'Malley BW, Kohler PO. Hypoparathyroidism. Postgrad Med 1968;44:71 75, 77 81, 182 186.
8. Hanley DA, Sherwood LM. Secondary hyperparathyroidism in chronic renal failure. Pathophysiology and treatment. Med Clin North Am 1978;62:1319 1339.
9. Katz AI, Hampers CL, Merrill JP. Secondary hyperparathyroidism and renal osteodystrophy in chronic renal failure. Analysis of 195 patients, with observations on the effects of chronic dialysis, kidney transplantation and subtotal parathyroidectomy. Medicine (Baltimore) 1969;48:333 374.
10. Roth SI, Marshall RB. Pathology and ultrastructure of the human parathyroid glands in chronic renal failure. Arch Intern Med 1969;124:397 407.
11. Yagi H, Ozono K, Miyake H, Nagashima K, Kuroume T, Pike JW. A new point mutation in the deoxyribonucleic acid-binding domain of the vitamin D receptor in a kindred with hereditary 1,25-dihydroxyvitamin D-resistant rickets. J Clin Endocrinol Metab 1993;76:509 512.
12. Roth SI. Parathyroid glands. In: Damjanov I, Linder J, eds. Anderson's Pathology. 10th ed. St. Louis: Mosby-Year Book; 1966:1980 2007.
13. Roth SI, Wang CA, Potts JT Jr. The team approach to primary hyperparathyroidism. Hum Pathol 1975;6:645 648.
14. Owen R. On the anatomy of the Indian rhinoceros (Rh. unicornis, L). Trans Zool Soc (Lond) 1862;4:31 58.
15. Remak R. Untersuchungen ber die Entwickelung der Wirbelthiere. Berlin: G. Reimer; 1855:3 40, 122 124.
16. Virchow R. Die krankhaften Geschw lste. Vol 3. Berlin: Hirschwald; 1863:13.
17. Sandstr m I. Om en ny K rtel hos menniskan och tskilliga d ggdjur. Upsala Lakaref Forh 1880;15:441 471.
18. Seipel CM. An English translation of Sandstr m's Glandulae Parathyroideae with biographical notes by Professor J August Hammar. Bull Inst Hist Med 1938;6:179 222.
19. Kohn A. Studien ber die Schilddr se. Arch f Mikr Anat 1895;44:366 422.
20. Kohn A. Die Epithelk rperchen. Ergeb Anat Entwicklungsgeshch 1899;9:194 252.
21. Pearse AG, Takor TT. Neuroendocrine embryology and the APUD concept. Clin Endocrinol (Oxf) 1976;5(suppl):S229 S244.
22. Merida-Velasco JA. Experimental study of the origin of the parathyroid glands. Acta Anat (Basel) 1991;141:163 169.
23. Graham A. Development of the pharyngeal arches. Am J Med Genet A 2003;119:251 256.
24. Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol 1975;34:125 154.
25. Norris EH. Anatomical evidence of prenatal function of the human parathyroid glands. Anat Rec 1946;96:129 142.
26. Nakagami K, Yamazaki Y, Tsunoda Y. An electron microscopic study of the human fetal parathyroid gland. Z Zellforsch Mikrosk Anat 1968;85:89 95.
27. Ishizaki N, Shoumura S, Emura S, et al. Ultrastructure of the parathyroid gland of the mouse fetus after calcium chloride or ethylenediaminetetraacetic acid administration. Acta Anat 1989;135:164 170.
28. Leroyer-Alizon E, David L, Anast CS, Dubois PM. Immunocytological evidence for parathyroid hormone in human fetal parathyroid glands. J Clin Endocrinol Metab 1981;52:513 516.
29. Scothorne RJ. Functional capacity of fetal parathyroid glands with reference to their clinical use as homografts. Ann N Y Acad Sci 1964;120:669 676.
30. MacIsaac RJ, Heath JA, Rodda CP, et al. Role of the fetal parathyroid glands and parathyroid hormone-related protein in the regulation of placental transport of calcium, magnesium and inorganic phosphate. Reprod Fertil Dev 1991;3:447 457.
31. Welsh DA. Concerning the parathyroid glands: a critical anatomical and experimental study. J Anat Physiol 1898;32:292 307.
32. Gilmour JR. The embryology of the parathyroid glands, the thymus and certain associated rudiments. J Pathol Bacteriol 1937;45:507 522.
33. Weller GL Jr. Development of the thyroid, parathyroid and thymus glands in man. Contr Embryol Carnegie Inst 1933;24 (141):95 138.
34. Norris EH. The parathyroid glands and the lateral thyroid in man: their morphogenesis, histogenesis, topographic anatomy and prenatal growth. Contrib Embryol Carnegie Inst 1937;26:247 294.
35. Trump D, Dixon PH, Mumm S, et al. Localization of X linked recessive idiopathic hypoparathyroidism to a 1.5 Mb region on Xq26-q27. J Med Genet 1998;35:905 909.
36. Kelly T, Blanton S, Saif R, Sanjad S, Sakati N. Confirmation of the assignment of the Sanjad-Sakati (congenital hypoparathyroidism) syndrome (OMIM 241410) locus to chromosome 1q42 43. J Med Genet 2000;37:63 64.
37. Wurdak H, Ittner L, Lang K, et al. Inactivation of TGFbeta signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev 2005;19:530 535.
38. Kameda Y, Arai Y, Nishimaki T, Chisaka O. The role of Hoxa3 gene in parathyroid gland organogenesis of the mouse. J Histochem Cytochem 2004;52:641 651.
39. Manley N, Capecchi M. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol 1998;195:1 15.
40. Su D, Ellis S, Napier A, Lee K, Manley NR. Hoxa3 and pax1 regulate epithelial cell death and proliferation during thymus and parathyroid organogenesis. Dev Biol 2001;236:316 329.
41. Gunther T, Chen Z, Kim J, et al. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature 2000;406:199 203.
P.1163
42. Hogan B, Hunter M, Oates A, et al. Zebrafish gcm2 is required for gill filament budding from pharyngeal ectoderm. Dev Biol 2004;276:508 522.
43. Nesbit M, Bowl M, Harding B, et al. Characterization of GATA3 mutations in the hypoparathyroidism, deafness, and renal dysplasia (HDR) syndrome. J Biol Chem 2004;279:22624 22634.
44. Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev 1998;12:2735 2747.
45. Manley N, Capecchi M. The role of Hoxa-3 in mouse thymus and thyroid development. Development 1995;121:1989 2003.
46. Xu P, Zheng W, Laclef C, et al. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development 2002;129:3033 3044.
47. Frank D, Fotheringham L, Brewer J, et al. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development 2002;129:4591 4603.
48. Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 2002;129:4613 4625.
49. Vitelli F, Taddei I, Morishima M, Meyers EN, Lindsay EA, Baldini A. A genetic link between Tbx1 and fibroblast growth factor signaling. Development 2002;129:4605 4611.
50. Packham EA, Brook JD. T-box genes in human disorders. Hum Mol Genet 2003;12:R37 R44.
51. Bachiller D, Klingensmith J, Shneyder N, et al. The role of chordin/Bmp signals in mammalian pharyngeal development and DiGeorge syndrome. Development 2003;130:3567 3578.
52. Moore-Scott B, Manley N. Differential expression of Sonic hedgehog along the anterior-posterior axis regulates patterning of pharyngeal pouch endoderm and pharyngeal endoderm-derived organs. Dev Biol 2005;278:323 335.
53. Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol 1975;34:125 154.
54. Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opinion Genet Dev 2003;13:529 536.
55. Nos V, Khan A. Recent developments in the molecular biology of the parathyroid. In: Lloyd RV, ed. Endocrine Pathology: Differential Diagnosis and Molecular Advances. Totowa, NJ: Humana Press; 2004:131 151.
56. Cryns VL, Thor A, Xu HJ, et al. Loss of the retinoblastoma tumor suppressor gene in parathyroid carcinoma. N Eng J Med 1994;330:757 761.
57. Cryns VL, Rubio MP, Thor AD, Louis DN, Arnold A. p53 abnormalities in human parathyroid carcinoma. J Clin Endocrinol Metab 1994;78:1320 1324.
58. Hsi E, Zuckerberg LR, Yang WI, Arnold A. Cyclin D1/PRAD1 expression in parathyroid adenomas: an immunohistochemical study. J Clin Endocrinol Metab 1996;81:1736 1739.
59. Vasef MA, Byrnes R, Sturm M, Bromley C, Robinson RA. Expression of cyclin D1 in parathyroid carcinomas, adenomas, and hyperplasias: a paraffin immunohistochemical study. Mod Pathol 1999;12:412 416.
60. Pollack MR, Brown EM, Chou YH, et al. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 1993;75:1297 1303.
61. Janicic N, Soliman E, Pausova Z, et al. Mapping of the calcium-sensing receptor gene (CASR) to human chromosome 3q13.3 21 by fluorescence in situ hybridization, and localization to rat chromosome 11 and mouse chromosome 16. Mamm Genome 1995;6:798 801.
62. Thakker RV. The molecular genetics of hypoparathyroidism. In: Bilezikian JP, Marcus R, Levine MA, eds. The Parathyroids: Basic and Clinical Concepts. 2nd ed. San Diego: Academic Press; 2001:779 790.
63. Brown EM. Physiology of calcium homeostasis. In: Bilezikian JP, Maarcus R, Levine MA, eds. The Parathyroids: Basic and Clinical Concepts. 2nd ed. San Diego: Academic Press; 2001:167 182.
64. Broadus AE. Mineral balance and homeostasis. In: Favus MJ, ed. Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism. 5th ed. Washington, DC: American Society for Bone and Mineral Research Press; 2003:105 11.
65. Brown EM. Homeostatic mechanisms regulating extracellular and intracellular calcium metabolism. In: Bilezikian JP, Levine MA, Marcus R, eds. The Parathyroids: Basic and Clinical Concepts. New York: Raven Press; 1994:15 54.
66. Bringhurst RF, Demay MB, Krane SM, Kronenberg HM. Bone and mineral metabolism in health and disease, Part 14, Endocrinology and metabolism, Sect 2, Disorders of bone and mineral metabolism. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL. Jameson L, Isselbacher KJ, eds. Harrison's Principles of Internal Medicine. 16th ed. 2005 [cited 1 June 2006]; New York: The McGraw-Hill Companies [117 lines, 2 figures]. Available at: http://www.accessmedicine.com.ezp1.harvard.edu. Also available in paper copy from the publisher.
67. Adams JS, Hollis BW. Vitamin D: Synthesis, metabolism, and clinical measurement. In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. 2nd ed. Philadelphia: Williams & Wilkins; 2002:157 174.
68. Silver J. Naveh-Many T. Parathyroid hormone-synthesis and secretion. In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. 2nd ed. Philadelphia: Williams & Wilkins; 2002:74 101.
69. Brown EM, Pollack M, Seidman CE, et al. Calcium-ion sensing cell-surface receptors. N Engl J Med 1995;333:234 240.
70. Thompson MW, Gauger PG. Ectopic locations of parathyroid glands. In: Bilezikian JP, Maarcus R, Levine MA, eds. The Parathyroids: Basic and Clinical Concepts. 2nd ed. San Diego: Academic Press; 2001:499 514.
71. Brown EM, Pollak M, Chou YH, et al. The cloning of extracellular Ca++-sensing receptors from parathyroid and kidney: molecular mechanisms of extracellular Ca++-sensing. J Nutr 1995;125 (suppl 7):1955S 1970S.
72. Pollak MR, Chou YH, Marx SJ, et al. Familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Effects of mutant gene dosage on phenotype. J Clin Invest 1994;93:1108 1112.
73. Chou YH, Pollak MR, Brandi ML, et al. Mutations in the human Ca2+-sensing-receptor gene that cause familial hypocalciuric hyperpercalcemia. Am J Hum Genet 1995;56:1075 1079.
74. Pollak MR, Brown EM, Chou YH, et al. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 1992; 75:1297 1303.
75. Pollak MR, Brown EM, Estep HL, et al. Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation. Nat Genet 1994;8:303 307.
76. Sun F, Maercklein P, Fitzpatrick LA. Paracrine interactions among parathyroid cells: effect of cell density on cell secretion. J Bone Miner Res 1994;9:971 976.
77. Cohn DV, Fasciotto BH, Reese BK, Zhang JX. Chromogranin A: a novel regulator of parathyroid gland secretion. J Nutr 1995; 125(suppl 7):S2015 S2019.
78. Zhang JX, Fasciotto BH, Darling DS, Cohn DV. Pancreastatin, a chromogranin A-derived peptide, inhibits transcription of the parathyroid hormone and chromogranin A genes and decreases the stability of the respective messenger ribonucleic acids in parathyroid cells in culture. Endocrinology 1994;134:1310 1316.
79. Fasciotto BH, Trauss CA, Greeley GH, Cohn DV. Parastatin (porcine chromogranin A347 419), a novel chromogranin A-derived peptide, inhibits parathyroid cell secretion. Endocrinology 1994;133:461 466.
80. Gilmour JR. The gross anatomy of the parathyroid glands. J Pathol Bacteriol 1938;46:133 149.
81. Vail AD, Coller FC. The number and location of parathyroid glands recovered from 202 routine autopsies. Mo Med 1966;63:347 350.
82. Wang CA. The anatomic basis of parathyroid surgery. Ann Surg 1976;183:271 275.
83. Alveryd A. Parathyroid glands in thyroid surgery. I. Anatomy of parathyroid glands. II. Postoperative hypoparathyroidism identification and autotransplantation of parathyroid glands. Acta Chir Scand 1968;389:1 120.
P.1164
84. kerstr m G, Malmaeus J, Bergstr m R. Surgical anatomy of human parathyroid glands. Surgery 1984;95:14 21.
85. Grimelius L, kerstr m G, Johansson H, Bergstr m R. Anatomy and histopathology of human parathyroid glands. Pathol Annu 1981;16(pt 2):1 24.
86. Hooghe L, Kinnaert P, Van Geertruyden J. Surgical anatomy of hyperparathyroidism. Acta Chir Belg 1992;92:1 9.
87. Edis AJ, Purnell DC, van Heerden JA. The undescended parathymus. An occasional cause of failed neck exploration for hyperparathyroidism. Ann Surg 1979;190:64 68.
88. Fitko R, Roth SI, Hines JR, Roxe DM, Cahill E. Parathyromatosis in hyperparathyroidism. Hum Pathol 1990;21:234 237.
89. Halstead W, Evans H. The parathyroid glandules. Their blood supply and their preservation in operation upon the thyroid gland. Ann Surg 1907;46 489 506.
90. Brewer LA. The occurrence of parathyroid tissue within the thymus: report of four cases. Endrocrinol 1934;18:393 408.
91. Lack EE, Delay S, Linnoila RI. Ectopic parathyroid tissue within the vagus nerve. Incidence and possible clinical significance. Arch Pathol Lab Med 1988;112:304 306.
92. kerstr m G, Grimelius L, Johansson H, Lundqvist H. Estimation of the parenchymal-cell content of the parathyroid gland, using density-gradient columns. Preliminary report. Acta Pathol Microbiol Scand A 1977;85:555 557.
93. kerstr m G, Grimelius L, Johansson H, Pertoft H, Lunqvist H. Estimation of the parathyroid parenchymal cell mass by density gradients. Am J Pathol 1980;99:685 694.
94. kerstr m G, Grimelius L, Johansson H, Lunqvist H, Pertoft H, Bergstrom R. The parenchymal cell mass in normal human parathyroid glands. Acta Pathol Microbiol Scand A 1981;89:367 375.
95. Grimelius L, kerstr m G, Johansson H, Lundqvist H. Estimation of parenchymal cell content of human parathyroid glands using the image analyzing computer technique. Am J Pathol 1978;93:793 800.
96. Dufour DR, Wilkerson SY. The normal parathyroid revisited: percentage of stromal fat. Hum Pathol 1982;13:717 721.
97. Gilmour JR, Martin WJ. The weight of the parathyroid glands. J Pathol Bacteriol 1937;44:431 462.
98. Parfitt AM. Parathyroid growth: normal and abnormal. In: Bilezikian JP, Maarcus R, Levine MA, eds. The Parathyroids: Basic and Clinical Concepts. 2nd ed. San Diego: Academic Press; 2001:293 330.
99. Roth SI. Recent advances in parathyroid gland pathology. Am J Med 1971;50:612 622.
100. Dufour DR, Wilkerson SY. Factors related to parathyroid weight in normal persons. Arch Pathol Lab Med 1983;107:167 172.
101. Matsushita H, Hara M, Shishiba Y, Nakazawa H. An evaluation of the size of the parathyroid glands. Endocrinol Jpn 1984;31:127 131.
102. Ghandur-Mnaymneh L, Cassady J, Hajianpour MA, Paz J, Reiss E. The parathyroid gland in health and disease. Am J Pathol 1986;125:292 299.
103. Yao K, Singer FR, Roth SI, Sassoon A, Ye C, Giuliano AE. Weight of normal parathyroid glands in patients with parathyroid adenomas. J Clin Endocrinol Metab 2004;89:3208 3213.
104. Thiele J. Human parathyroid gland: a freeze fracture and thin section study. Curr Top in Pathol 1977:31 80.
105. Mazzocchi G, Meneghelli V, Frasson F. The human parathyroid glands: an optical and electron microscopic study. Lo Sperimentale 1967;117:383 447.
106. Balashev VN, Ignashkina MS. Lymphatic system of parathyroid glands in man. Fed Proc Transl Suppl 1965;24:603 604.
107. Alten hr E. Electron microscopical evidence for innervation of chief cells in human parathyroid gland. Experientia 1971;27:1077.
108. Yeghiayan E, Rojo-Ortega JM, Genest J. Parathyroid vessel innervation: an ultrastructural study. J Anat 1972;112(pt 1):137 142.
109. Isono H, Shoumura S. Effects of vagotomy on the ultrastructure of the parathyroid gland of the rabbit. Acta Anat (Basel) 1980;108:273 280.
110. Shoumura S, Iwasaki Y, Ishizaki N, et al. Origin of autonomic nerve fibers innervating the parathyroid gland in the rabbit. Acta Anat (Basel) 1983;115:289 295.
111. Saffos RO, Rhatigan RM, Urgulu S. The normal parathyroid and the borderline with early hyperplasia: a light microscopic study. Histopathology 1984;8:407 422.
112. Dekker A, Dunsford HA, Geyer SJ. The normal parathyroid gland at autopsy: the significance of stromal fat in adult patients. J Pathol 1979;128:127 132.
113. Roth SI. The parathyroid gland. In: Silverberg SG, ed. Principles and Practice of Surgical Pathology. 2nd ed. Vol 2. New York: Churchill Livingstone; 1989:1923 1955.
114. Cinti S, Balercia G, Zingaretti MC, Amati S, Osculati F. The normal human parathyroid gland. A histochemical and ultrastructural study with particular reference to follicular structures. J Submicrosc Cytol 1983;15:661 679.
115. Anderson TJ, Ewen SWB. Amyloid in normal and pathological parathyroid glands. J Clin Pathol 1974;27:656 663.
116. Lieberman A, DeLellis RA. Intrafollicular amyloid in normal parathyroid glands. Arch Pathol 1973;95:422 423.
117. Leedham PW, Pollock DJ. Intrafollicular amyloid in primary hyperparathyroidism. J Clin Pathol 1970;23:811 817.
118. Gilmour JR. The normal histology of the parathyroid glands. J Pathol Bacteriol 1939;48:187 222.
119. Roth SI, Olen E, Hansen L. The eosinophilic cells of the parathyroid (oxyphil cells), salivary (oncocytes), and thyroid (H rthle cells) glands. Lab Invest 1962;11:933 941.
120. Munger BL, Roth SI. The cytology of the normal parathyroid glands of man and Virginia deer: a light and electron microscopic study with morphologic evidence of secretory activity. J Cell Biol 1963;16:379 400.
121. Roth SI, Munger BL. The cytology of the adenomatous, atrophic, and hyperplastic parathyroid glands of man. A light- and electron-microscopic study. Virchows Arch Pathol Anat Physiol Klin Med 1962;335:389 410.
122. Roth SI, Capen CC. Ultrastructural and functional correlations of the parathyroid gland. Int Rev of Exp Pathol 1974;13:161 221.
123. Capen CC, Roth SI. Ultrastructural and functional relationships of normal and pathologic parathyroid cells. Pathobiol Annul. 1973;3:129 175.
124. Frigerio B, Capella C, Wilander E, Grimelius L. Argyrophil reaction in parathyroid glands: a light and electron microscopic study. Acta Pathol Microbiol Immunol Scand A 1982;90:323 326.
125. Weymouth RJ, Baker BL. The presence of argyrophilic granules in the parenchymal cells of the parathyroid glands. Anat Rec 1954;119:519 527.
126. Weymouth RJ. The cytology of the parathyroid glands of the rat after bilateral nephrectomy, administration of parathyroid hormone and hypophysectomy. Anat Rec 1957;127:509 525.
127. Futrell JM, Roth SI, Su SP, Habener JF, Segre GV, Potts JT Jr. Immunocytochemical localization of parathyroid hormone in bovine parathyroid glands and human parathyroid adenomas. Am J Pathol 1979;94:615 622.
128. Ravazzola M, Orci L, Habener JF, Potts JT Jr. Parathyroid secretory protein: immunocytochemical localization within cells that contain parathyroid hormone. Lancet 1978;2:371 372.
129. Stork PJ, Herteaux C, Frazier R, Kronenburg H, Wolfe HJ. Expression and distribution of parathyroid hormone and parathyroid hormone messenger RNA in pathological conditions of the parathyroid [abstract]. Lab Invest 1989;60:A92.
130. Personal communication, Komminoth P, Wolfe H (13).
131. Kendall CH, Potter L, Brown R, Jasani B, Pringle JH, Lauder I. In situ correlation of synthesis and storage of parathormone in parathyroid gland disease. J Pathol 1993;169:61 66.
132. Thiele J. The human parathyroid chief cell a model for a polypeptide hormone producing endocrine unit as revealed by various functional and pathological conditions. A thin section and freeze-fracture study. J Submicrosc Cytol 1986;18:205 220.
133. Thiele J, K rner J, Fischer R. Ultrastructural morphometry on human parathyroid tissue. Morphological and functional implications. J Submicrosc Cytol Pathol 1988;20:491 500.
134. Roth SI, Raisz LG. The course and reversibility of the calcium effect on the ultrastructure of the rat parathyroid gland in organ culture. Lab Invest 1966;15:1187 1211.
135. Alten hr E. Ultrastructural pathology of parathyroid glands. Curr Top Pathol 1972;56:2 54.
P.1165
136. Cohn DV, MacGregor RR. The biosynthesis, intracellular processing, and secretion of parathormone. Endocr Rev 1981;2:1 26.
137. Habener JF, Rosenblatt M, Potts JT Jr. Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev 1984;64:958 1053.
138. MacCallum WG, Voegtlin C. On the relation of tetany to the parathyroid glands and to calcium metabolism. J Exp Med 1909;11:118 151.
139. Patt HM, Luckhardt AB. Relationship of low blood calcium to parathyroid secretion. Endocrinology 1942;31:384 392.
140. Svensson O, Wernerson A, Reinholt FP. The parathyroid glands in the rat as seen by ultrathin step and serial sectioning. Bone Miner 1989;6:237 248.
141. Shannon WA Jr, Roth SI. An ultrastructural study of acid phosphatase activity in normal, adenomatous and hyperplastic (chief cell type) human parathyroid glands. Am J Pathol 1974;77:493 506.
142. Pappenheimer AM, Wilens SL. Enlargement of the parathyroid glands in renal disease. Am J Pathol 1935;11:73 91.
143. Wild P, Schraner EM, Eggenberger E. Quantitative aspects of membrane shifts in rat parathyroid cells initiated by decrease in serum calcium. Biol Cell 1984;50:263 272.
144. Hashizume Y, Waguri S, Watanabe T, Kominami E, Uchiyama Y. Cysteine proteinases in rat parathyroid cells with special reference to their correlation with parathyroid hormone (PTH) in storage granules. J Histochem Cytochem 1993;41:273 282.
145. Roth SI, Gallagher MJ. The rapid identification of normal parathyroid glands by the presence of intracellular fat. Am J Pathol 1976;84:521 528.
146. Sasano H, Geelhoed GW, Silverberg SG. Intraoperative cytologic evaluation of lipid in the diagnosis of parathyroid adenoma. Am J Surg Pathol 1988;12:282 286.
147. Ljungberg O, Tibblin S. Preoperative fat staining of frozen sections in primary hyperparathyroidism. Am J Pathol 1979;95:633 642.
148. King DT, Hirose FM. Chief cell intracytoplasmic fat used to evaluate parathyroid disease by frozen section. Arch Pathol Lab Med 1979;103:609 612.
149. Bondeson AG, Bondeson L, Ljungberg O, Tibblin S. Fat staining in parathyroid disease diagnostic value and impact on surgical strategy: clinicopathologic analysis of 191 cases. Hum Pathol 1985;16:1255 1263.
150. Monchik JM, Farrugia R, Teplitz C, Teplitz J, Brown S. Parathyroid surgery: the role of chief cell intracellular fat staining with osmium carmine in the intraoperative management of patients with primary hyperparathyroidism. Surgery 1983;94:877 886.
151. Alpern HD, Roth SI, Olson JE. Intracellular lipid droplets in functioning transitional parathyroid oxyphil adenomas. A caveat. Arch Surg 1990;125:410 411.
152. Chen KTK. Fat stain in hyperparathyroidism. Am J Surg Pathol 1982;6:191 192.
153. Kasdon EJ, Rosen S, Cohen RB, Silen W. Surgical pathology of hyperparathyroidism. Usefulness of fat stain and problems in interpretation. Am J Surg Pathol 1981;5:381 384.
154. Dekker A, Watson CG, Barnes EL Jr. The pathologic assessment of primary hyperparathyroidism and its impact on therapy. A prospective evaluation of 50 cases with oil-red-O stain. Ann Surg 1979;190:671 675.
155. Ritchie CK, Cohn DV, Maercklein PB, Fitzpatrick LA. Individual parathyroid cells exhibit cyclic secretion of parathyroid hormone and chromogranin-A (as measured by a novel sequential hemolytic plaque assay). Endocrinology 1992;131:2638 2642.
156. Fitzpatrick LA. Heterogeneous secretory response of parathyroid cells. Recent Prog Horm Res 1993;48:471 475.
157. Lee MJ, Roth SI. Effect of calcium and magnesium on deoxyribonucleic acid synthesis in rat parathyroid glands in vitro. Lab Invest 1975;33:72 79.
158. Bianchi S, Fabiani S, Muratori M, et al. Calcium modulates the cyclin D1 expression in a rat parathyroid cell line. Biochem Biophys Res Commun 1994;204:691 700.
159. Hamperl H. ber das Vorkommen von Onkocyten in verschiedenen Organen und ihren Geschw lsten (Mundspeicheldr sen, Bauschpeicheldr se, Epithelk rperchen, Hypophyse, Schilddr se, Eileiter). Virchows Arch f Path Anat 1936;25:327 375.
160. Hamperl H. Onkocyten and Onkocytome. Virchows Arch Pathol Anat Physiol Klin Med 1962;335:452 483.
161. Tremblay G. The oncocytes. Methods Achiev Exp Pathol 1969; 4:121 140.
162. Christie AC. The parathyroid oxyphil cells. J Clin Pathol 1967;20:591 602.
163. M ller-H cker J. Random cytochrome-C-oxidase deficiency of oxyphil cell nodules in the parathyroid gland. A mitochondrial cytopathy related to cell ageing? Pathol Res Pract 1992;188:701 706.
164. Tandler B, Hoppel CL. Mitochondria. New York: Academic Press; 1972:1 59.
165. Balogh K Jr, Cohen RB. Oxidative enzymes in the epithelial cells of normal and pathological human parathyroid glands: a histochemical study. Lab Invest 1961;10:354 360.
166. Fischer R. ber den histochemischen Nachwies oxidativer Enzyme in Onkozyten verschiedener Organe. Virchows Arch A 1961;334:445 452.
167. Tremblay G, Cartier GE. Histochemical study of oxidative enzymes in the human parathyroid. Endocrinology 1961;69:658 661.
168. Kitazawa R, Kitazawa S, Fukase M, et al. The expression of parathyroid hormone-related protein (PTHrP) in parathyroid: histochemistry and in situ hybridization. Histochemistry 1992;98:211 215.
169. Wolpert HR, Vickery AL Jr, Wang CA. Functioning oxyphil cell adenomas of the parathyroid gland. A study of 15 cases. Am J Surg Pathol 1989;13:500 504.
170. Shapiro MJ, Batang ES. Needle aspiration biopsy of the thyroid and parathyroid. Otolaryngol Clin North Am. 1990;23:217 229.
171. Bergenfelz A, Forsberg L, Henderstr m E, Ahren B. Preoperative localization of enlarged parathyroid glands with ultrasonically guided fine needle aspiration for parathyroid hormone assay. Acta Radiol 1991;32:403 405.
172. Halbauer M, Crepinko I, Tomc Brzac H, Simonovic I. Fine needle aspiration cytology in the preoperative diagnosis of ultrasonically enlarged parathyroid glands. Acta Cytol 1991;35:728 735.
173. Tikkakoski T, Stenfors LE, Typpo T, Lohela P, Apaja-Sarkkinen M. Parathyroid adenomas: pre-operative localization with ultrasound combined with fine-needle biopsy. J Laryngol Otol 1993; 107:543 545.
174. Silverberg SG. Imprints in the intraoperative evaluation of parathyroid disease. Arch Pathol 1975;99:375 378.
175. Geelhoed GW, Silverberg SG. Intraoperative imprints for the identification of parathyroid tissue. Surgery 1984;96:1124 1131.
176. Roth SI. The parathyroid gland In: Silverberg SG, DeLellis RA, Frable WJ, eds. Principles and Practice of Surgical Pathology and Cytopathology. 3rd ed. New York: Churchill Livingstone; 1997:2709 2750.
177. DeMay RM. Parathyroid. In: The Art and Science of Cytopathology. Volume II: Aspirate Cytology. Chicago: ASCP Press; 1996:648 649.