35 - Urinary Bladder, Ureter, and Renal Pelvis
Editors: Mills, Stacey E.
Title: Histology for Pathologists, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > XI - Endocrine > 44 - Thyroid
function show_scrollbar() {}
44
Thyroid
Maria Luisa Carcangiu
Embryology
The human thyroid first appears as a median anlage and two lateral anlagen. The median anlage develops in the floor of the primitive pharynx at the foramen cecum (a dimplelike depression at the base of the tongue) from a median ductlike invagination that grows caudally. This formation, known as the thyroglossal duct, contains at its base the developing thyroid gland, which is at first spherical but later, when approaching its final site in front of the trachea at about 7 weeks of gestation, becomes bilobed (1). During this downward migration, the thyroglossal duct undergoes atrophy, leaving as a vestige the pyramidal lobe in about 40% of individuals. Faulty downward migration of the medial anlage or persistence of parts of the thyroglossal duct gives rise to ectopic thyroid tissue, thyroglossal duct cysts, and cervical fistulae.
The initially solid thyroid anlage begins to form cords and plates of follicular cells during the 9th week of gestation. Small follicles appear by the 10th week. Inside these primitive follicles, a finely granular material begins to collect, which by the 20th week acquires the morphologic features of colloid. By week 14 there are well-developed follicles with a central lumen containing colloid. Both the cytoplasm of the follicular cells and the intralumional colloid are thyroglobulin (TGB)-positive (Figure 44.1A, B). Labeled amino acid studies have shown that TGB synthesis actually begins at a much earlier stage, when the thyroid is still a solid mass at the base of the tongue and long before follicle formation and colloid secretion can be identified morphologically (2,3).
PAX8, a transcription factor expressed in the adult mouse thyroid where it is involved in the maintenance of functional differentiation in follicular cells, has been demonstrated in the human median thyroid anlage, in the tyroglossal duct, and in the ultimobranchial bodies.
Thyroid transcription factor-1 (TTF1), also is expressed in the median thyroid anlage (4,5).
P.1130
 |
Figure 44.1 Developing thyroid gland in a 14-week fetus. A. Rare primitive follicles are seen within a mostly solid proliferation. B. The cytoplasm of the follicular cells and the material contained in the lumen of the primitive follicles are immunoreactive for TGB. C. C cells, as seen in a CT immunostain preparation, are scattered within follicles. |
Immunohistochemically, ghrelin, a growth hormone releasing hormone, has been shown in the follicular cells of fetal thyroid (from 8 to 38 weeks of gestational age) (6). Fetal thyroid stromal tissue is immunoreactive for galectin 1 but not for galectin 3 (7).
The two lateral anlagen of the thyroid derive from the ultimobranchial bodies (UBBs), which in turn originate from the IVth Vth branchial pouch complex. UBBs, while still connected to the pharynx, start their migration downward on each side of the neck together with the parathyroid IV anlage. At 7 to 8 weeks they separate from the pharynx and the parathyroid. Their lumens become obliterated by proliferating cells, so that at 8 to 9 weeks they appear as solid masses that fuse with the dorsolateral aspects of the median thyroid anlage and become incorporated into the developing lateral lobes (8).
After its fusion with the medial thyroid (at 9 weeks to term) the UBB enters in the dissolution phase and divides into a central thick-walled stratified epithelial cyst and a peripheral component composed of cell groups dispersed among the follicles: the C cells (9,10) (Figure 44.1C). In postnatal life, the central epithelial cyst largely disappears, its occasional remnants corresponding to the so-called solid cell nests (SCNs).
C cells are thought to derive from the neural crest and to migrate to the UBBs before the incorporation of the latter in the thyroid (11,12,13,14). Evidence for a relationship between UBBs and C cells comes from several sources:
Patients with Di George syndrome, characterized by complete or partial absence of derivatives of the IIIrd and IVth Vth pouch complexes, have C cells in their thyroid in only 25% of cases (15,16).
C cells are completely absent in thyroglossal duct remnants and cysts, as well as in lingual thyroid (17).
In the adult thyroid gland, C cells and follicles carrying such cells in their walls are especially numerous in the vicinity of the UBB remnants (17).
There is some controversy as to whether the role of UBBs in thyroid development is limited to the production of C cells as described above or whether they also contribute to the follicular cell population. Williams et al. (18) described five cases of maldescent of the medial thyroid anlage in which cystic structures were present in the lateral neck in the region of the upper parathyroids. Four of these cystic structures contained intercystic glandular nodules composed of solid areas of irregularly distributed cells that stained positively for calcitonin (CT) and CT gene related peptide; these cells were intermixed with follicular structures that were immunoreactive for thyroglobin. On the basis of these observations, the investigators concluded that the UBB contributes both C cells and follicular cells to the
P.1131
thyroid gland, and they speculated on the possible role of UBB-derived cells in the genesis of so-called intermediate or mixed medullary and follicular carcinomas. This hypothesis, which was independently postulated by Ljungberg (17), is however contradicted by the observation that in humans with thyroid nondescent (or unilateral aplasia) there are no recognizable thyroid follicles in the usual locations (19).
The fetal thyroid gland develops rapidly until the 4th month of intrauterine growth (crown rump length 18 mm). After birth the thyroid growth rate parallels that of the body, reaching the normal adult weight at around 15 years of age.
Gross Anatomy
The normal adult thyroid has a shape reminiscent of a butterfly, with two bulky lateral lobes connected by a thin isthmus. Each lateral lobe is 2 to 2.5 cm wide, 5 to 6 cm long, and 2 cm deep. Their upper and lower extremities (one having a pointed shape and the other featuring blunt contours) are referred to as upper and lower thyroid poles, respectively. One lobe may be larger than the other, and the isthmus may be exceptionally wide. The pyramidal lobe, a vestige of the thyroglossal duct, is found in about 40% of thyroids; it appears as a narrow projection of thyroid tissue that extends upward from the isthmus to lie on the surface of the thyroid cartilage.
The thyroid gland is located in the midportion of the neck, where it is attached to the anterior trachea by loose connective tissue. The two lateral lobes surround the ventral and lateral aspects of the larynx and trachea, reaching the lower halves of the thyroid cartilage and covering the second, third, and fourth tracheal rings.
The normal weight of the adult thyroid is 15 to 25 g in nongoitrous areas. However, there are significant individual variations, most of them related to gender, age, corporal weight, hormonal status, functional status of the gland, and iodine intake (20). In women, the thyroid volume is known to increase during the secretory phase of the menstrual cycle (21).
A thin fibrous capsule invests the thyroid. Connected to this capsule are numerous fibrous septa that penetrate the thyroid parenchyma and divide it into lobules (so-called thyromeres). The microscopic integrity of the capsule was assessed in a study on 138 thyroid glands from autopsies of adults (age 20 40 years) by Komorowski and Hanson (22). Although grossly all of these capsules seemed complete, microscopically they were focally incomplete in 62% of the cases. Furthermore, thyroid follicles were found within the thyroid capsule in 14% of cases and in the pericapsular connective tissue in 88%. In the latter location, they were mostly seen as nodular aggregates.
The color of the normal thyroid is red brown. A phenomenon exceptionally seen in normal thyroid glands of elderly individuals is the accumulation in the follicular cells of a melanin-like pigment that imparts to the gland a characteristic coal black stain, easily apparent on gross examination. The terms melanosis thyroid and black thyroid are used to refer to this phenomenon. These changes are qualitatively identical to those seen in more florid form in thyroids of patients on chronic minocycline therapy (23,24).
Nodularity of thyroid parenchyma is identified grossly in about 10% of the glands of endocrinologically normal individuals (25).
The blood supply of the thyroid gland derives primarily from the inferior thyroid artery (which originates from the thyrocervical trunk of the subclavian artery) and the superior thyroid artery (which arises from the external carotid). A thyroidea ima artery also may be present, which varies widely in size from a small vessel to one the size of the inferior thyroid artery. The superior and medial thyroid veins and the inferior vein drain (via a venous plexus in the thyroid capsule) into the internal jugular and the brachiocephalic vein, respectively (26,27).
An intricate lymphatic network permeates the thyroid gland, encircling the follicles and connecting the two lateral lobes through the isthmus. It empties into subcapsular channels, which in turn give rise to collecting trunks within the thyroid capsule in close proximity to the veins. The lymph vessels draining the superior portion of the thyroid lobes and isthmus collect into the internal jugular lymph nodes, whereas those draining the inferior portion of the gland collect into the pre- and paratracheal and prelaryngeal lymph nodes. The pretracheal lymph node situated close to the isthmus is also known as the Delphian node (28). Other lymph node stations are the recurrent laryngeal nerve chain and the retropharyngeal and retroesophageal groups. The anterosuperior mediastinal nodes are secondary to the recurrent laryngeal nerve chain and pretracheal groups; however, injection studies have shown that dye injected into the thyroid isthmus can drain directly into the mediastinal nodes (29).
Some correlations exist between the site of a thyroid tumor within a given lobe and the location of the initial lymph node metastasis. However, the degree of anastomosing between these various nodal groups is such that any of them can be found to be the site of disease regardless of the precise location of the primary tumor.
Vasomotor nonmedullated postganglionic neural fibers originating from the superior and midline cervical sympathetic ganglia influence indirectly the secretory activity of the thyroid gland through their action on the blood vessels. In addition, adrenergic receptors in follicular cells and a network of adrenergic fibers ending near the follicular basement membrane have been demonstrated (30). It has been hypothesized, therefore, that thyroid secretion is regulated
P.1132
both by direct neural signals and by indirect vascular nerve signals (31,32,33). A role for direct neural influences in the secretion of calcitonin (CT) and other C cell derived hormones is supported by the demonstration in chickens of a rich cholinergic network encircling the C cells (34).
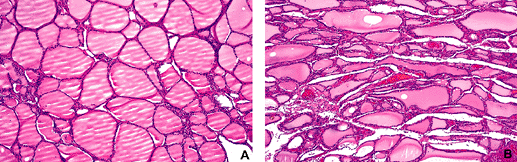 |
Figure 44.2 A. Low-power view of normal adult thyroid gland. The follicles have a round-to-oval shape. B. Elongated follicles as a result of compression are seen in the vicinity of an adenoma (not shown in this picture). |
Small paraganglia are normally present close to the thyroid and are occasionally found beneath the thyroid capsule; their presence explains the rare occurrence of peri- and intrathyroidal paragangliomas.
Microscopic Anatomy
The fundamental unit of the thyroid is the follicle, a round to slightly oval structure lined by a single layer of epithelial cells resting on a basement membrane (Figure 44.2A). The lumen of the follicle contains colloid, a viscous material that is mostly composed by proteins secreted by the follicular cells, including TGB. The follicles, which are separated from each other by a loose fibroconnective tissue, have an average diameter of 200 m. Their size may vary even within the same gland depending on the functional status of the thyroid and the age of the individual. Variations in the shape of follicles exist, but elongated follicles are a feature of hyperplastic or neoplastic conditions or are the result of compression adjacent to an expansile mass (Figure 44.2B). A characteristic structure present in the normal thyroid but more often seen in hyperplastic conditions, is the Sanderson's polster. (See page 1136.)
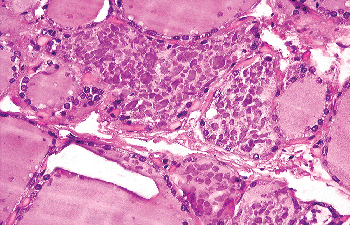 |
Figure 44.3 Clumps of condensed colloid within follicular lumina. |
The colloid, which is pale eosinophilic in the actively secreting gland, acquires a deeply eosinophilic staining quality in resting follicles. Often, numerous clumps with various shapes appear within the colloid of resting follicles, suggesting an artifactual coagulation-type phenomenon (Figure 44.3). In some follicles the colloid may have an amphophilic or basophilic staining quality, probably the result of an increase in the amount of acidic groups in the TGB molecule (Figure 44.4). In the most advanced expression
P.1133
of this phenomenon, the intraluminal material acquires a distinct mucinlike appearance (Figure. 44.5).
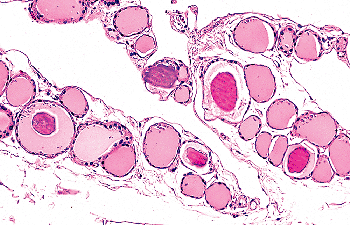 |
Figure 44.4 The colloid accumulated in these follicles exhibits different densities and tintorial qualities, the latter ranging from acidophilic to basophilic. |
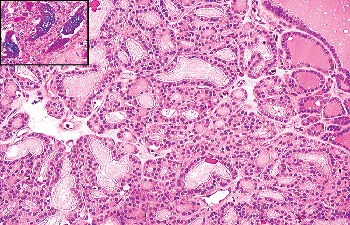 |
Figure 44.5 The basophilic colloid present in most of these follicles contrasts with the more typical red colloid present in the follicles on the right upper corner. Inset: Alcian blue-PAS highlights the mucinous character of the basophilic colloid. |
The glycoproteic material present within the follicles stains for periodic acid-Schiff (PAS) and alcian blue and is immunoreactive for TGB. A row of small vacuoles is seen at the interface between follicular epithelium and the colloid in actively functioning glands; these are referred to as resorption vacuoles. In addition, it is not unusual to find a large round or oval clear space within the colloid; this often appears empty but it may contain birefringent calcium oxalate crystals (See page 1137.)
Another morphologic variation of the colloid is represented by collections of round basophilic corpuscles clustering at one pole of the follicle.
The epithelial glandular cells lining the follicle are known as follicular cells or thyrocytes; among them, there is a second cellular component known as C cells.
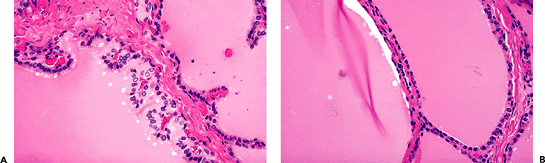 |
Figure 44.6 A. The epithelium of one follicle is low cuboidal and relatively inactive. The adjacent follicle shows a taller epithelium and reabsorption vacuoles. B. The epithelium of the same follicle is flattened on one side and cuboidal on the other, as an expression of functional polarization. |
Follicular Cells
The cells lining the follicles follicular cells or thyrocytes show variations in their shape and size according to the functional status of the gland. Three major types, expressions of a morphologic continuum, are described: flattened (endothelioid), cuboidal, and columnar (cylindrical) (Figure 44.6A). Flattened cells are relatively inactive. Cuboidal cells (their height equaling their width) are the most numerous and their major function is to secrete colloid. The rarer columnar cells resorb the TGB-containing colloid, liberate the active hormones, and excrete these hormones into blood vessels; they may feature an apical cuticle, apical lipid droplets, and one or more basilar vacuoles (vacuoles of Bensley).
Functional polarity is apparent at the level of the follicle and the follicular cell. A single follicle may have flattened cells on one side and cuboidal or low columnar cells on the other (Figure 44.6B), the best expression of this phenomenon being the already mentioned Sanderson's polster.
At the cellular level, all follicular cells manifest a definite polarity, resting with their bases on the basement membrane and having the apexes directed toward the lumen of the follicle. Size and position of the nucleus and some components of the cytoplasm may vary considerably. In the resting thyroid, the nucleus is round or oval, is located toward the center of the cell, and usually contains one nucleolus that is eccentrically located. Its chromatin may be finely granular or clumped. In actively secreting cells, the nucleus is enlarged; because of the mostly apical enlargement of the cytoplasm, it acquires a basal position. The cytoplasm is usually weakly eosinophilic; only exceptionally in an otherwise normal thyroid, does it appear granular and intensely eosinophilic; that is, oncocytic (so-called Hurthle cells). In contrast to parathyroid cells, little or no intracytoplasmic glycogen is present. Occasionally, the
P.1134
follicular cell cytoplasm contains a golden brown pigment of lipofuscin type (Figure 44.7A), which should be distinguished from the melanin-like pigment already mentioned (Figure 44.7B).
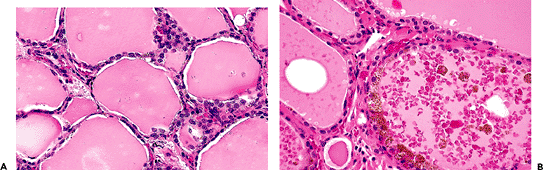 |
Figure 44.7 A. Lipofuscin in the cytoplasm of the follicular cells. B. Granular black pigment in the follicular epithelium and colloid of the thyroid of a 73-year-old patient who was not on minocycline therapy. |
Ultrastructurally, the follicular cells are arranged in a single layer around the colloid and rest on a basement membrane, approximately 35 to 40 nm in thickness, that separate them from the interstitial stroma. Microvilli emanate from the surface of the cells, their number being increased and their length greater in actively functioning cells. Cell membranes of adjacent cells interdigitate in a complex fashion and are joined by junctional complexes toward the apex (35,36). The cytoplasm contains variable amounts of endoplasmic reticulum, mitochondria of usually small size, and lysosomes. When the number of mitochondria is highly increased, the cell acquires at the light microscopic level an intensely eosinophilic granular cytoplasmic appearance (corresponding to the above mentioned Hurthle cells).
Immunohistochemistry
A wide variety of markers with various degrees of specificity and diagnostic significance are expressed by the normal adult follicular cells.
Thyroglobulin (TGB)
This is the most specific immunohistochemical marker for normal follicular cells and the tumors composed of them. It can be demostrated with either monoclonal or polyclonal antibodies and the reactivity is both in the cytoplasm and in the colloid (37,38). Oncocytes show a much lesser degree of positivity. Despite its high specificity, TBG can give rise to a common pitfall. Because of its tendency to leak out of the cytoplasm of the follicular cells and to diffuse into the adjacent tissues, where it can then be incorporated into the cytoplasm of other types of cells (e.g., metastatic carcinoma), it can cause a false positivity (39).
Thyroid Transcription Factor-1 (TTF-1)
This is another very useful marker for thyroid follicular cells and tumors composed of them. This nuclear transcription factor, first identified in thyroid follicular cells and later in pneumocytes, is necessary for the development of thyroid and pulmonary tissues. In the normal adult thyroid its distribution is related to that of thyroglobulin and thyroperoxidase (40,41).
Keratins
Normal follicular cells are immunoreactive only for low-molecular-weight keratin, whereas high-molecular-weight types have been found to be expressed in inflammatory and neoplastic conditions (42,43,44).
Vimentin
Some normal follicular cells occasionally express this intermediate filament in conjunction with keratin, although less commonly than in neoplastic conditions (45).
Epithelial Membrane Antigen (EMA)
Follicular cells are variably positive, with accentuation of the cell membranes.
Triiodothyronine (T3) and Thyroxine (T4)
These hormones can be detected immunohistochemically both in the cytoplasm of the follicular cells and in the intraluminal colloid but their use for diagnostic purposes is negligible.
Estrogen and Progesterone Receptors
Estrogen and progesterone receptors positivity, restricted to the follicular cell nuclei, shows some correlation with the age and sex of the individual (46,47).
P.1135
S-100 protein
This marker, which is mainly detected in inflammatory/hyperplastic and neoplastic thyroid conditions, is only focally and weakly expressed by normal follicular cells (48,49).
Epidermal Growth Factor Receptor (EGFR)
In normal follicular cells, and because of their functional polarization, the location of this receptor is mainly basal or basolateral (50).
Thyroid Peroxidase
This enzyme is responsible for the oxidation of iodide to iodine. At the immunohistochemical level it shows a pattern of staining correlated with the age of the individual. A cytoplasmic pattern of staining with apical membrane accentuation is seen in children and young adults, and a perinuclear ring distribution is seen in older individuals (51).
Sodium Iodide Symporter
At the immunohistochemical level this molecule, responsible for the active iodide intake into the follicular epithelium, is localized mainly to the lateral basal portion of the cells (52,53).
Physiology
The main function of the thyroid gland is the production of thyroid hormones, the most important being thyroxin (T4) and triiodothyronine (T3). These hormones regulate metabolism, increase protein synthesis in every tissue of the body, and increase O2 consumption. Thyroid hormones are particularly important for body development and for the normal maturation of the central and peripheral nervous system.
Steps in thyroid hormone biosynthesis include ingestion of iodine ions from water and food, their absorption and transport as iodide into the extracellular fluid and their concentration within the thyroid where their intracellular levels are 30 times higher than in peripheral blood. The active iodide uptake across the basement membrane is mediated by human sodium iodide symporter (hNIS) in a process coupled with the flow of sodium (52). The intrathyroidal iodide is then oxidized to iodine. This last step is dependent on the action of iodide peroxidase, which oxidates the iodine ion to a highly reactive form of iodine, which in turn binds to tyrosine. The results are monoiodotyrosine (MIT) when one iodine molecule is attached, or diiodotyrosine (DIT) when two iodine molecules are attached. The iodotyrosine residues are condensed to form the biologically active thyroid hormones thyroxin (T4) and triiodotyronine (T3). Thyroxin results from the coupling of two molecules of DIT, and triiodotyronine from the coupling of one molecule of MIT with a molecule of DIT (33).
Thyroid hormones are stored in TGB, with numerous iodinated tyrosine residues, including biologically active T4 and T3. TGB, a large protein with a 19 S sedimentation coefficient and a molecular weight of 670,000, is formed by two identical subunits with a 12 S sedimentation coefficient to which many oligosaccharides are linked. Variations in the sugar chains of the thyroglobulin molecule have been evaluated by analysis of reactivity to various lectins and found to differ between the normal gland and various pathologic states, including neoplasms (54).
Thyroglobulin is encoded by a gene spreading over more than 200 kilobases in the bovine genome (55). The molecular mechanisms involved in the tissue-specific and hormone-dependent expression of the thyroglobulin gene have been studied in follicular cells in primary cultures and cell lines (56,57). TGB is collected at the center of the thyroid follicles and is the main constituent of colloid.
Ultrastructural studies have correlated the morphologic changes that accompany thyroid hormone production and secretion. The synthesis of TGB begins in the endoplasmic reticulum and continues in the Golgi apparatus, where the end sugars of the carbohydrate site are incorporated; it is then packaged in small apical microvesicles, the contents of which are discharged into the follicular lumen after fusion of the vesicle membranes with the luminal side of the plasma membrane.
Resorption of TGB takes place through cytoplasmic pseudopodia (streamers) that engulf minute portions of colloid, which are then drawn into the cell in the form of membrane-bound colloid droplets. These subsequently fuse with lysosomes, and their content is digested by the lysosomal enzymes (58,59,60,61). The breakdown products, including T3 and T4, diffuse in the blood stream, where they are transported primarily by the specific carrier protein, thyroxin-binding globulin (TBG). TBG normally transports more than 70% of thyroid hormones. Approximately 20% of circulating thyroid hormones is carried by transthyretin (prealbumin) and albumin (62). Only a small portion of circulating thyroid hormones (approximately 0.05% of T3 and 0.015% of T4) is unbound and, therefore, biologically active. Free, circulating, biologically active T3 and T4 are in equilibrium with the hormones bound to the carrier proteins. The amount of circulating T4 is much larger than that of T3; however, T3 is about four times more active biologically; as a result, the final contribution of T3 to the biologic activity of thyroid hormones equals that of T4 (63).
Thyroid hormones stimulate metabolism, increase oxygen consumption, and cause a rise in heat production, cardiac output, and heart rate. They are essential for normal development, growth, and maturation. The acceleration of growth may result from a direct action on the cells to increase their rate of division, by acting permissively for other hormones, or by inducing the synthesis of a variety of growth-promoting hormones (58,62,64,65,66,67,68).
P.1136
Thyroid biosynthetic and secretory activities are controlled by the blood level of thyroid stimulating hormone (TSH), a glycoprotein synthesized and secreted by the anterior pituitary gland (69). TSH binds to a specific receptor located on the basolateral surface of the follicular cell membrane, and by activating the adenylate-cyclase pathway regulates the complex mechanism responsible for T3 and T4 synthesis (70,71).
Stimulation of the thyroid gland by thyrotropin increases its secretory activity and vascularity and results in both hypertrophy and hyperplasia of follicular cells, accompanied by reduction of colloid storage. At the functional level, this is reflected by an increase in iodide concentration and organic binding, hormone synthesis, and hormone secretion (63,72). TSH release is in turn regulated by a tripeptide secreted by the hypothalamus, thyrotropin releasing hormone (TRH). TSH and TRH release are regulated by the circulating levels of free T3 and T4, via a negative feedback on the pituitary and hypothalamus (low levels of free T3 and T4 stimulate the release of TSH and TRH). In contrast, TSH and TRH releases are inhibited by high levels of circulating free T3 and T4 (69,72,73).
Microscopic Variations
Sanderson's Polster
A characteristic structure, present in the normal thyroid but accentuated in hyperplastic conditions, is the so-called Sanderson's polster. This refers to an aggregate of small follicles lined by flattened epithelium and covered by an undulating layer of columnar epithelium that is seen bulging into the lumina of larger follicles (Figure 44.8). This perfectly benign and to some extent physiologic change, most likely the morphologic expression of the functional polarization of the thyroid follicle, needs to be distinguished from papillary microcarcinoma.
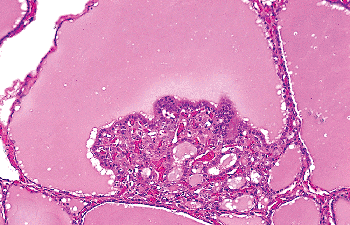 |
Figure 44.8 Sanderson's polster protruding into the lumen of a follicle. |
Granulomas
Granulomas are a relatively common finding in otherwise normal surgically resected thyroids and in autoptic specimens. Both foreign material and colloid may elicit this process. Suture material is the most frequent cause of formation of granulomas in completion thyroidectomy specimens. Larger foreign body granulomas sometimes clinically simulating a thyroid nodule have been reported in thyroids of patients who underwent laryngeal injection of Polytef (polytetrafluoroethylene) (74,75). This material may migrate through the lymphatics into adjacent tissues, where it may start the inflammatory process.
Rarely, interstitial granulomas are seen as a reaction to oxalate crystals that have been released by broken follicles (76).
Granulomatous lesions originated by the rupture of follicles and their invasion by macrophages and leukocytes as a reaction to the extruded colloid are a common incidental finding in surgically resected thyroids. Carney et al. (77) referred to this process as palpation thyroiditis (and also as multifocal granulomatous folliculitis) and attributed it to the minor trauma resulting from physical examination. Support for this interpretation comes from the observation that the number and size of the granulomas is related to the intensity of the palpation and the fact that similar changes have been described in individuals engaged in martial arts (so-called martial-arts thyroiditis) (77,78).
Grossly, a gland affected by palpation thyroiditis appears normal or shows tiny foci of hemorrhage. Histologically, multiple small granulomas centered in a disrupted follicle and composed of histiocytes, lymphocytes, and plasma cells are seen scattered in the thyroid gland (Figure 44.9A). Some of the histiocytes are foamy, while others have the appearance of multinucleated giant cells. The appearance depends on the stage of the process, a common picture being a cluster of foamy macrophages hanging from the follicular epithelium into the lumen (Figure 44.9B). Necrosis, hemosiderin, and iron deposition are seen only rarely. Sometimes up to four or five follicles are involved in a single granuloma.
Immunohistochemically, most of the lymphocytes are T cells; among the plasma cells, K-positive cells predominate (79).
Palpation thyroiditis seems to represent a variation in the theme of colloidophagy, a process described many years ago and characterized by a granulomatous reaction to colloid in follicles allegedly undergoing spontaneous rupture in thyroids affected by goiter or thyroiditis (80).
Palpation thyroiditis needs to be distinguished from interstitial giant cell thyroiditis (in which the granulomas are centered not in the follicles but in the interstitium), necrotizing granulomas following surgical procedures (similar to those more commonly seen in the bladder and the prostate and characterized by a central area of necrosis surrounded by a palisading of epithelioid cells), and aggregates of C cells (which are immunoreactive for CT) (81,82).
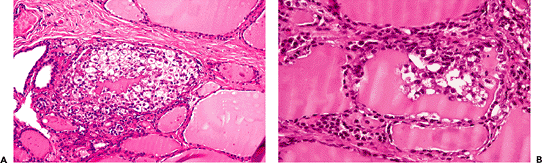 |
Figure 44.9 Palpation thyroiditis. A. The thyroid follicle in the center is packed with histiocytes and other inflammatory cells, with a clump of residual colloid in the center. The follicular epithelium is barely discernible. B. In this case the follicle is only partially involved. Inflammatory cells and desquamated follicular cells protrude into the lumen. |
P.1137
Crystals
Anisotropic crystals of calcium oxalate may be present within the colloid in normal adult thyroid glands. They may be seen in ordinary light, but are more easily identified under polarized light (Figure 44.10). Their shape varies from rhomboid to irregular plaques and their size shows wide variations (83).
In autoptic studies they have been found with a frequency of up to 85% of thyroids examined (84). Their number appears to increase with age; this, together with the observation that the crystals have been found more frequently in colloid with low positivity for TGB, has prompted the suggestion that they result from variations in colloid and calcium concentration in the gland secondary to a low functional state of the thyroid (83,84,85).
In one study the number of crystals was markedly elevated in glands with subacute thyroiditis, where they were found in the giant cells, in remnants of colloid, and in the thyroid stroma. In the same study crystals were identified only rarely in thyroids with chronic thyroiditis or glandular hyperplasia (86).
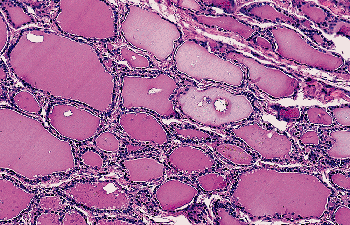 |
Figure 44.10 Multiple birefringent calcium oxalate crystals are seen in the lumina of normal thyroid follicles (polarized light). |
Oxalate crystals in the thyroid are also seen in large number in patients undergoing dialysis for renal failure. In this setting, the thyroid is just another site of oxalate deposition, together with the kidney, myocardium, and other sites (87). Rarely, crystals released by follicular breakdown may elicit a granulomatous reaction in the nearby thyroid stroma (76).
At the time of frozen section, their identification within a follicular structure can be useful in distinguishing thyroid from parathyroid gland tissue (88).
Squamous Metaplasia
Benign squamous cells occur as an expression of squamous metaplasia of follicular cells in various benign and malignant thyroid lesions and, under exceptional circumstances, in an otherwise normal thyroid (89,90,91). They need to be distinguished from transversally cut follicles and from the SCNs of UBB derivation. It also should be mentioned that squamous epithelium is regularly observed as a component of the epithelium of thyroglossal duct cysts.
C CELLS
C cells (parafollicular cells) represent a minor component of the thyroid gland. It has been estimated that they comprise not more that 0.1% of the glandular mass. They have a neuroendocrine function, being responsible for the production of the peptide hormone CT. The term C cells was introduced by Pearse (92) to underline their role in secreting and storing this hormone. More recently, other hormones have been found to be produced by C cells, but only in small quantity and not in every cell.
C cells are identified only with difficulty in sections stained with hematoxylin and eosin, where they appear polygonal and with a granular weakly eosinophilic
P.1138
cytoplasm that is larger and paler than that of follicular cells. The nucleus is round to oval, pale, with a centrally located nucleolus.
C cells are located, individually or in small groups, within thyroid follicles. Specifically, most are found at the periphery of the follicular wall (hence the qualifier parafollicular), within its basement membrane and without contact with the follicular lumen. Electron microscopy has shown that C cells occupy an intrafollicular (rather than interfollicular) position, and that they are separated from the thyroid interstitium by the follicular basal lamina. The presence of C cells in the interfollicular stroma has never been convincingly demonstrated ultrastructurally (93).
Occasional C cells have prominent cytoplasmic processes that extend beyond the adjacent follicular cells. In normal adults and neonates, C cells are restricted to the midupper and upper thirds of the lateral lobes of the thyroid, in the area where UBBs (from which they derive) fuse with the thyroid median anlage. The number of C cells varies with the development of the gland, being more numerous in early age. In one study, up to 100 C cells per low-power field were demonstrated in neonates and children; whereas in adults only a maximum of 10 cells per low-power field were counted (94). In another study, no difference in the number of C cells was found between young and middle-aged groups, but in the elderly the number of such cells was variable, with groups of up to 20 or more cells sometimes being observed (95). However, no statistically significant differences among the various age groups in adults were demonstrated. Other studies have since confirmed that normal adult thyroid glands may contain numerous C cells, sometimes in the form of small nodules (22,96) (Figure 44.11). Gibson et al. (96) suggested that such clusters of C cells, in the absence of disturbances in calcium metabolism and of a family history of medullary carcinoma, do not constitute a precursor of medullary carcinoma but may be instead the expression of either a partial failure of embryonic C-cell migration and dispersion within the gland or of age-related hyperplasia. It needs to be mentioned that C-cell hyperplasia of presumed reactive nature has been observed in the immediate periphery of nonmedullary thyroid neoplasms (97,98), in association with lymphocytic thyroiditis (99,100,101), and in secondary hyperparathyroidism (102). As already mentioned, C cells tend to aggregate in the vicinity of SCNs.
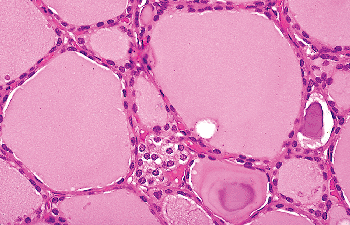 |
Figure 44.11 Clusters of C cells in the thyroid of an elderly individual with no known clinical or laboratory evidence of calcium disturbance. |
The main ultrastructural characteristic of C cells is the presence of secretory granules, which range in diameter from 60 to 550 nm (103). Two main types of granules have been identified. Type I granules have an average diameter of 280 nm and a moderately electron-dense, finely granular content which is closely applied to the limiting membranes of the granules. Type II granules are smaller (average diameter of 130 nm) with a more electron-dense content, which are separated from the limiting membranes by a small but distinct electron-lucent space. Most normal C cells are filled with type I secretory granules, with no or few type II granules. Immunocytochemical studies performed at the ultrastructural level have shown that both type I and II secretory granules contain immunoreactive CT (104).
Histochemistry and Immunohistochemistry
Histochemically, normal C cells are characterized by the following:
Argyrophilia
In sections stained with argyrophil techniques such as the Grimelius reaction or analogous stains, the cytoplasm of the C cell is characterized by the deposition of fine silver-positive granules (103).
Lead hematoxylin
The cytoplasm of C cells is selectively stained by this type of hematoxylin (105).
Toluidine blue and coriophosphine 0
These dyes confer marked metachromasia to C cells after acid hydrolysis of tissue sections (105).
Lectin Ulex europaeus agglutinin I
Selective reactivity to this marker has been demonstrated (106).
These methods, widely used in the past for the identification of C cells, have been largely replaced by the use of immunohistochemical techniques.
Immunohistochemically C cells have been found to be reactive to:
Calcitonin (Figure 44.12) (103,104,107,108).
Calcitonin gene related peptide (CGRP) (109)
Katacalcin (110)
Somatostatin (111,112,113), substance P (114), helodermin (115) and gastrin-releasing peptide (116,117). A small proportion of CT-positive cells are also positive for these neuropeptides.
Thyrotropin-releasing hormone. In some species, C cells have been found to contain this hormone as detected immunohistochemically (118).
Serotonin and other biologically active amines (119).
Cytokeratins. C cells are immunoreactive only for low-molecular-weight keratin.
Pan-endocrine markers, such as neuron-specific enolase, chromogranin A, and synaptophysin (120).
Carcinoembryonic antigen (CEA) (103).
P.1139
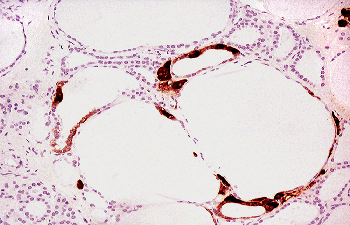 |
Figure 44.12 Immunoperoxidase stain for CT demonstrates C cells within follicles, arranged either individually or in small groups. |
It is possible that neuroendocrine cells other than C cells exist in the thyroid and that they represent the cells of origin of the rare thyroid neuroendocrine carcinomas having histologic and immunohistochemical features different from those of medullary carcinoma.
Physiology
CT is a 32 amino acid peptide whose main function is the regulation of the level of calcium in the plasma by a feedback mechanism. This is brought about by the inhibition of osteoclastic activity. When calcium plasma levels are increased, CT is released from the thyroid. CT also acts in the kidney to enhance the production of vitamin D.
The major physiological role of CT is most likely the protection of the skeleton during periods of calcium stress such as growth, pregnancy and lactation (121). However, absence of CT is not associated with hypercalcemia, nor does a marked excess of the hormone (as seen in patients with medullary thyroid carcinoma) produce hypocalcemia. In addition to calcium, both gastrin and cholecystokinin induce the secretion of CT, as does the chronic administration of estrogenic hormones.
The CT gene is located on the short arm of chromosome 11 and consists of six axons that encode katacalcin (C-terminal flanking peptide) and CGRP (110,121,122). The primary transcript of the CT gene gives rise to two different mRNAs by tissue-specific alternative splicing events, leading to the production of CT and CGRP mRNAs. The CT-CGRP gene is expressed both in thyroid and nervous tissues, but CT is produced in large quantities only in the thyroid.
In normal male adults, basal CT levels range from 3 to 36 pg/ml (0.9 to 10.5 pmol/L). Plasma levels in females range from 3 to 17 pg/ml (0.9 to 5.0 pmol/L). Normal values after pentagastrin stimulation are less than 106 pg/ml (30.9 pmol/L) for males and less than 29 pg/ml (8.5 pmol/L) for females.
Katacalcin, the C-terminal flanking peptide of CT, is a 21 amino acid peptide that is cosecreted with CT in equimolar amounts (110). Its function, however, is unknown. CGRP is a 37 amino acid peptide that is an extremely potent vasodilator and also serves a neuromodulator or neurotransmitter function (121).
Stroma
Lymphocytes
In thyroids surgically resected because of a mass, it is not uncommon to observe in the interstitium of the normal portion of the gland a few collections of lymphocytes, sometimes admixed with rare plasma cells. Simple chronic thyroiditis and focal lymphocytic thyroiditis are the names given to this process that most likely does not represent a nosologic entity but rather the epiphenomenon of etiologically different conditions. Similar changes may in fact be seen in the proximity of neoplasms, in thyroids of patients taking lithium, or in individuals who have received low-dose external radiotherapy (123).
Fibrous Tissue
The usually thin fibrous septa that separate the thyroid lobules may exhibit microscopic variations. In a study on normal thyroids collected at autopsy from young adults, Komorowski and Hanson (22) found that 8% of the thyroid glands showed extensive fibrosis. According to their description, dense and largely acellular collagen fibers divided the thyroid into small nodules, giving it an appearance akin to micronodular cirrhosis of liver.
Another change that may occur in the interstitium, albeit rarely, is so-called multifocal sclerosing thyroiditis. It is characterized histologically by numerous microscopic foci of stellate-shaped fibrosis composed of cellular fibroblastic tissue frequently entrapping few thyroid follicles in the center. Even if at low power the individual lesions appear similar to those of papillary microcarcinoma, the epithelial component of such lesions lack the cytoarchitectural features of a papillary neoplasm (Figure 44.13A, B). Furthermore, the number of lesions in multifocal sclerosing thyroiditis greatly exceeds that seen in the usual case of papillary microcarcinoma. The etiology and pathogenesis of this process are not known.
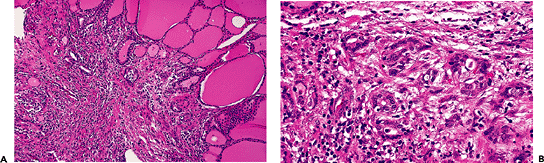 |
Figure 44.13 A. Multifocal sclerosing thyroiditis. On low power, the appearance resembles that of a papillary microcarcinoma. B. At higher power, the follicles entrapped in the fibrosis are irregularly shaped but do not show any of the cytologic features of papillary carcinoma. |
P.1140
Adipose Tissue and Skeletal Muscle
Thyroid stroma may undergo adipose metaplasia, resulting in the presence of islands of mature adipose tissue between follicles (Figure 44.14). Mature fat also occasionally may be seen in proximity to the thyroid gland capsule, its presence in this location most likely resulting from the close relationship of fat and thyroid tissue during fetal life (124).
Other tissues that grow in close proximity to the thyroid gland during their development and that can be found within the capsule of adults are cartilage and muscle.
In one study, striated muscle was found within the thyroid parenchyma of 19 glands, usually in the region of the isthmus or in the pyramidal lobe of the gland (22). Conversely, in 10 specimens thyroid follicles were found within fascicles of strap muscle from the same areas (Figure 44.15). Follicles entrapped in perithyroidal skeletal muscle are more easily identifiable when they undergo hyperplastic changes (125).
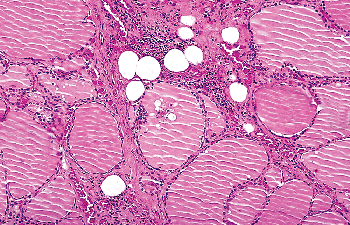 |
Figure 44.14 Adipose metaplasia of thyroid stroma. Mature adipocytes are seen between follicles. |
Calcification
Dystrophic calcifications may be seen in normal thyroid of old age, particularly in relation to vessels. They can easily be distinguished from psammoma bodies because of the lack of laminations and the irregularity of their contours.
Psammoma bodies rarely have been described in benign thyroid lesions but not in normal thyroids (126,127,128). Finding psammoma bodies in an otherwise normal thyroid or in a cervical lymph node should always prompt a careful search for an occult papillary carcinoma (Figure 44.16).
Branchial Pouch Derived and Other Ectopic Tissues
Branchial pouch-related structures are found within the thyroid in various forms: solid cell rests (a remnant of the
P.1141
ultimobranchial body or branchial pouch complex IV V), epithelium-lined cysts, parathyroid glands, thymic tissue, salivary gland type tissue, and heterotopic cartilage.
 |
Figure 44.15 Clusters of thyroid tissue intimately admixed with bundles of skeletal muscle adjacent to the thyroid gland. |
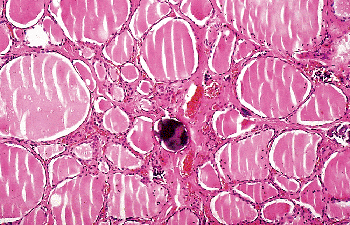 |
Figure 44.16 Psammoma body in nonneoplastic thyroid tissue adjacent to a papillary carcinoma (not shown in the picture). |
Solid Ultimobranchial Body Remnants (Solid Cell Nests)
So-called solid cell nests are clusters of epithelial cells interspersed among the follicles. Because they may exhibit squamous differentiation, they have sometimes been misinterpreted in the past as foci of squamous metaplasia in follicles (91). However, a UBB origin, for what in retrospect are clearly the same formations, had already been suggested by Erdheim in 1904 (129) and Getzowa in 1907 (130), following their demonstration of clusters of epithelial cells with solid or rarely cystic appearance in individuals with thyroid aplasia. Additional evidence along these lines was provided by the demonstration of marked similarities of human SCNs with the normal UBB of the rat and the hyperplastic or neoplastic UBB remnants in bulls (131,132,133).
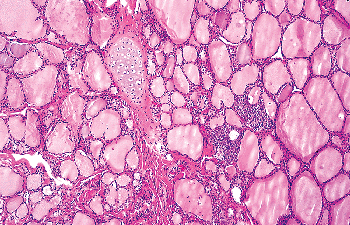 |
Figure 44.17 Cartilage island is seen in the proximity of SCNs. |
SCNs are relatively common in normal thyroid, the probability of finding them increasing with the number of sections examined. In one study, SCNs were found in only 3% of routinely examined thyroids but in as many as 61% of specimens when the gland was blocked serially at 2- to 3-mm intervals (134). For unknown reasons SCNs are more common in males than in females. Most SCNs measure an average 0.1 mm in diameter, but occasionally they can reach 2 mm or more. They may be single or multiple. They are usually surrounded by stroma and more or less demarcated by the adjacent thyroid follicles. Adipose tissue and cartilage may be present in their vicinity (Figure 44.17). Most SCNs are found along the central axis of the middle and upper third of the lateral lobes (i.e., in the same area where C cells usually occur); this constitutes additional proof for their origin from the UBB, as it does the fact that the number of C cells is increased in the vicinity of SCNs (135,136).
SCNs are often grouped in clusters featuring a multilobed shape on low-power examination (Figure 44.18A). They have a dual cell population. The main component is made
P.1142
up of cells of polygonal-to-oval shape, elongated nuclei with finely granular chromatin, and acidophilic cytoplasm. Some of these cells show clear-cut squamous differentiation. Immunohistochemically, they are reactive for high- and low-molecular-weight keratins, carcinoembryonic antigen (CEA) and p63 (Figure 44.18B). Ultrastructurally they feature bundles of tonofilaments, desmosomes, and intraluminal cytoplasmic projections (137,138,139). The positivity for p63 (a p53 homolog that is expressed in basal/stem cells of stratified epithelia), together with the expression of basal cell-type keratins (such as 34betaE12), telomerase and bcl-2, is compatible with a basal/stem cell phenotype for this cellular component (140,141). The second cell population, numerically less conspicuous, is characterized at the light microscopic level by clear cytoplasm and round nuclei, at the ultrastructural level by dense-core secretory granules, and at the immunohistochemical level by immunoreactivity to CT, CGRF, and chromogranin (135,137,138,142). All of these features are indicative of a C-cell nature for this population and constitute a further link between SCNs and the UBBs. Cystic cavities containing acid mucin are frequently observed in association with SCNs (see next section). A variation in the theme is represented by the admixture of SCNs (pure or admixed with a cystic component) with groups of small follicles lined by low cuboidal TGB-immunoreactive epithelium, forming the so-called mixed follicles (Figure 44.19). The fact that a similar admixture is seen in mixed medullary-follicular carcinomas has led some investigators to suggest that these rare tumors may arise from uncommitted stem cells of the UBBs that have the potential to differentiate into C cells, follicular cells, or both (143).
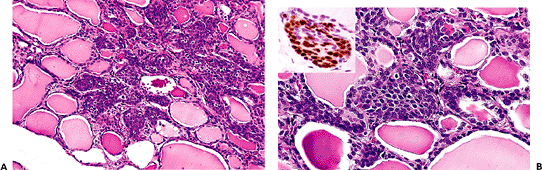 |
Figure 44.18 A. Low-power view shows the multilobed shape often exhibited by groups of SCNs. B. SCN in normal thyroid. Note the uniform appearance of the epithelial cells. Inset: Strong nuclear immunoreactivity for p63. |
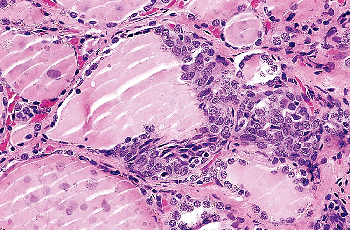 |
Figure 44.19 So-called mixed follicle. An SCN merges with a follicle lined by a flattened epithelium with colloid in the lumen. |
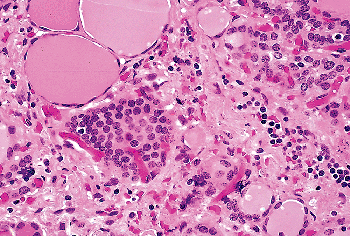 |
Figure 44.20 Tangential cut of a follicle. This should not be misinterpreted as an SCN. |
SCNs need to be distinguished from collections of C cells, follicles with squamous metaplasia, and tangential sections of normal follicles (Figure 44.20).
Cystic Ultimobranchial Body Remnants
UBB remnants also may take the form of cysts. These occur most commonly in the soft tissues of the neck adjacent to the thyroid. Indeed, it is possible that some of the clinically evident branchial pouch cysts located in close proximity to the thyroid gland and sometimes confused clinically with thyroid lesions or lymph nodes are of UBB origin.
Cystic UBB remnants may also develop within the thyroid itself (144). In the latter instance, they may occur by themselves, may be adjacent to SCNs, or may be intimately admixed with them (Figure 44.21). The cysts are lined most frequently by a flattened multilayered epithelium of squamous type, and less commonly by a ciliated columnar epithelium (Figure 44.22) and often contain clumps of eosinophilic material in their lumen. They are especially common in neonates. Cystic UBB remants may have an associated lymphoid component (lymphoepithelial cysts) and are more commonly seen in glands with Hashimoto's thyroiditis (145,146,147,148). Pancreatic tissue, a representative of foregut remnants, has been described in the wall of perithyroidal epithelial cysts by Langlois et al. (149).
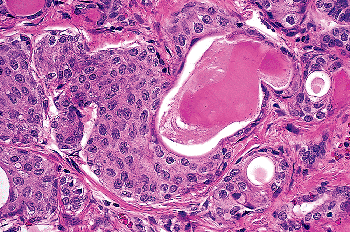 |
Figure 44.21 SCN with associated cystic formation. A dense eosinophilic material fills the lumen of the cyst. |
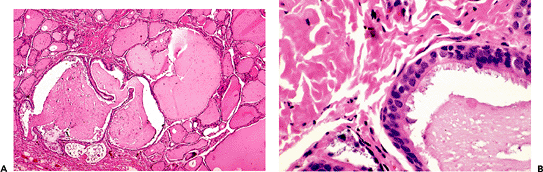 |
Figure 44.22 A. Intrathyroidal cyst of probable branchial pouch derivation. B. Higher-power view showing ciliated epithelium. |
P.1143
Parathyroid Tissue
The development of the parathyroid glands and the thymus from the branchial pouches in close proximity to the thyroid gland explains why these organs occasionally may be found adjacent to the thyroid capsule or even within the thyroid itself (Figure 44.23).
True intrathyroidal parathyroid glands in adults are rare. However, in a study where 58 human fetal thyroid glands obtained at autopsy were systematically studied for the presence of intrathyroidal parathyroid tissue, the latter was found in 13 thyroid lobes from 12 fetuses (22.4%). It was located subcapsularly in nine of 58 cases (15.5%), and it was lying deep in thyroid tissue in four (68%) (150). These intra- and perithyroidal parathyroid structures can be affected by primary or secondary chief cell hyperplasia, adenoma, or carcinoma and represent an often overlooked cause of surgical failure in primary hyperparathyroidism (151).
Thymic Tissue
Most of the thymus derives embryologically from the third branchial pouch, together with the lower pair of parathyroid glands. There is also a small and inconstant portion that derives from the fourth branchial pouch together with the upper pair of parathyroid glands and the UBB, which form the lateral thyroid anlage. It is from the latter source that the islands of thymic tissue occasionally found in or around the thyroid are thought to derive (Figure 44.24) (152). The fact that ectopic thymic tissue is observed more frequently in neonates and infants supports this hypothesis. Harach and Vujanic (153) searched systematically for the presence of intrathymic tissue in 58 thyroid glands obtained at autopsy from fetuses with proven retrosternal thymus. Subcapsular thymic tissue was found in two cases (3.4%) and intrathyroid thymic tissue in one (1.7%). An entire thymic gland within the thyroid of an infant has been described by Neill (154). Mizukami et al. (155) reported thymic tissue in the interlobular septum of the thyroid of a patient with Graves disease. Damiani et al. (156) found thymic rests in 1.4% of 2,575 adult thyroid glands that they examined.
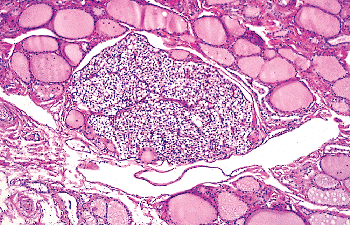 |
Figure 44.23 Parathyroid gland entirely located within thyroid. |
Ectopic thymic tissue may show cystic changes and present clinically as a cystic neck mass. It also may be the source of peri- and intrathyroidal thymomas (157).
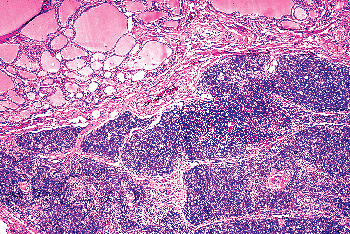 |
Figure 44.24 Intrathyroidal thymic tissue. |
P.1144
Salivary Gland Type Tissue
Rarely salivary gland type tissue has been found within the thyroid. Most of the reported cases have been seen in association with a benign thyroid condition, such as multinodular goiter (158).
Heterotopic Cartilage
Most intrathyroidal islands of mature cartilage probably represent remnants of the branchial pouch apparatus (18,159).
Thyroid Tissue in Abnormal Locations
The presence of nonneoplastic thyroid tissue outside the normal anatomical confines of the gland may be caused by a variety of mechanisms, ranging from congenital abnormalities to acquired processes. Their main practical interest resides in the fact that lack of knowledge of their occurrence may lead to a mistaken diagnosis of metastatic thyroid carcinoma.
In Midline Structures
Ectopic thyroid is derived from abnormalities in migration patterns of the medial anlage and is therefore more commonly found in the neck in a midline position, at any point in the normal pathway of descent of the thyroglossal duct from the foramen cecum to the lower neck (160,161,162). In most cases the ectopy is partial, clinically insignificant, and discovered accidentally. The base of the tongue (lingual thyroid) and the hyoid bone and its surroundings (as a component of a thyroglossal cyst) are the most common sites. The opposite phenomenon is represented by exaggerated descent of the median anlage into the mediastinum, which may lead to location of thyroid tissue substernally in the preaortic area, in the pericardial cavity, and in the substance of the heart (163,164,165). However, the majority of mediastinal goiters represent a dislocation downward of normal glands that have been pulled down by the hyperplastic changes that occurred in them.
Lingual thyroid is a relatively common incidental microscopic finding. The follicles appear normal but because of their intimate relationship with the surrounding skeletal muscle they may raise the differential diagnosis with carcinoma (166). Sauk (167) and Baughman (168) found thyroid tissue in the tongue in 10% of individuals examined at autopsy, with the sex distribution being equal. The tongue is the most common location of ectopic thyroid tissue in the rare cases of total ectopy (169). In this condition ectopic glands are prone to functional insufficiency, frequently followed by compensatory hyperplasia, which may be the cause of dyspnea or dysphagia. Acute hypothyroidism may follow the removal of this ectopic tissue.
The other site where ectopic thyroid tissue is found more commonly is the wall of thyroglossal duct cysts. It appears in the form of small groups of follicles and is present in 25% to 65% of cysts examined histologically, its frequency being related to the number of sections submitted for histologic examination (170). The medial location and the presence of thyroid tissue in the wall distinguish thyroglossal duct cysts from the rarer branchial pouch cysts. Ectopic thyroid derived from abnormalities in migration of the medial anlage typically does not contain C cells. In one study of median anlage anomalies including 23 cases of thyroglossal cysts with adjacent thyroid tissue and one case of lingual thyroid, not a single C cell was found in either the thyroid tissue or the epithelium lining the cysts (17).
In Pericapsular Soft Tissues and Skeletal Muscles
As already discussed, the presence of thyroid tissue in these locations is not a rare event. It most likely results from the intimate relationship of the thyroid gland with the mesodermal structures of the neck during development.
In the Lateral Neck
This phenomenon, frequently referred to as lateral aberrant thyroid, has different pathogeneses. It has been suggested that surgery and trauma may cause implantation of thyroid tissue in the lateral neck. Typically when this is the case, a few nodules of normal-appearing thyroid tissue, always of microscopic size and frequently surrounded by a fibrous capsule, are seen in the lateral neck close to the cervical lymph nodes (171,172,173). History of previous trauma or surgery on the neck, the presence of suture material (in cases of previous surgery), and the benign appearance of the dislocated thyroid tissue are useful in distinguishing them from metastatic carcinoma. It has to be kept in mind that the latter may appear deceptively benign on microscopic examination. Spontaneous separation of thyroid tissue with subsequent implant in the lateral neck may occur in nodular goiter or Hashimoto's thyroiditis (174,175). In both of these conditions, nodules of thyroid tissue extrude and separate from the surface of the gland and deposit in the extrathyroidal soft tissue, where they may acquire an autonomous blood supply (so-called parasitic nodules). The differential diagnosis with metastatic lymph nodes may be very problematic, especially in the presence of Hashimoto's thyroiditis.
In Cervical Lymph Nodes
Normal-appearing thyroid tissue in medially located cervical lymph nodes is rarely the result of a developmental
P.1145
anomaly (176). When this is the case, a few microscopic nests of benign-looking follicles are seen in the marginal sinus of the lymph node (Figure 44.25). The follicular cells that compose them should lack all of the cytologic features typical of papillary carcinoma (177). Psammoma bodies and papillae should also be absent. Numerous sections are sometimes needed to rule out a metastasis from a papillary microcarcinoma, which is by far the most frequent cause of thyroid tissue in cervical nodes.
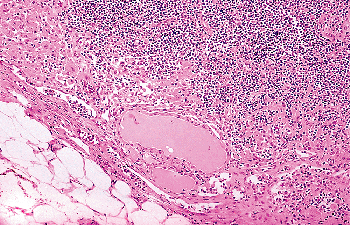 |
Figure 44.25 A group of benign-appearing thyroid follicles is seen close to the marginal sinus of a cervical lymph node. This patient did not have a carcinoma in the thyroid gland. |
In Other Sites
Rarely, one can find thyroid tissue in locations outside its place of embryonic development and occasionally quite distantly from it. These locations include the sella turcica, larynx, trachea, esophagus, heart, diaphragm, gallbladder, common bile duct, region of the porta hepatis, retroperitoneum, inguinal region, adrenal gland, uterus, vagina, and last but not least the ovary. In the latter site, the thyroid tissue represents a component of a teratoma and sometimes is the only evidence for it (struma ovarii) (178,179,180,181,182,183,184,185).
References
1. Hoyes AD, Kershaw DR. Anatomy and development of the thyroid gland. Ear Nose Throat J 1985;64:318 333.
2. Shepard TH. Onset of function in the human fetal thyroid: biochemical and radioautographic studies from organ culture. J Clin Endocrinol Metab 1967;27:945 958.
3. Gitlin D, Biasucci A. Ontogenesis of immunoreactive thyroglobulin in the human conceptus. J Clin Endocrinol Metab 1969;29:849 853.
4. Pasca di Magliano M, Di Lauro R, Zannini M. Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci U S A 2000;97:13144 13149.
5. Trueba SS, Auge J, Mattei G, et al. PAX8, TITF1, and FOXE1 gene expression patterns during human development: new insights into human thyroid development and thyroid dysgenesis-associated malformations. J Clin Endocrinol Metab 2005;90:455 462.
6. Volante M, Allia E, Fulcheri E, et al. Ghrelin in fetal thyroid and follicular tumors and cell lines: expression and effects on tumor growth. Am J Pathol 2003;162:645 654.
7. Savin SB, Cvejic DS, Jankovic MM. Expression of galectin-1 and galectin-3 in human fetal thyroid gland. J Histochem Cytochem 2003;51:479 483.
8. Norris EH. The parathyroid glands and the lateral thyroid in man: their morphogenesis, histogenesis, topographic anatomy and prenatal growth. Contrib Embryol Carnegie Inst 1937;159:249 294.
9. Chan AS, Conen PE. Ultrastructural observations on cytodifferentiation of parafollicular cells in the human fetal thyroid. Lab Invest 1971;25:249 259.
10. Sugiyama S. The embryology of the human thyroid gland including ultimobranchial body and others related. Ergeb Anat Entwicklungsgesch 1971;44:3 111.
11. Le Dourain NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol 1973;30:31 48.
12. Le Douarin N, Fontaine J, Le Lievre C. New studies on the neural crest origin of the avian ultimobranchial glandular cells interspecific combinations and cytochemical characterization of C cells based on the uptake of biogenic amine precursors. Histochemistry 1974;38:297 305.
13. Nadiz J, Weber E, Hedinger C. C-cell in vestiges of the ultimobranchial body in human thyroid glands. Virchows Arch B Cell Pathol 1978;27:189 191.
14. Ito M, Kameda Y, Tagawa T. An ultrastructural study of the cysts in chicken ultimobranchial glands, with special reference to C-cells. Cell Tissue Res 1986;246:39 44.
15. Conley ME, Beckwith JB, Mancer JF, Tenckhoff L. The spectrum of the DiGeorge syndrome. J Pediatr 1979;94:883 890.
16. Burke BA, Johnson D, Gilbert EF, Drut RM, Ludwig J, Wick MR. Thyrocalcitonin-containing cells in the DiGeorge anomaly. Hum Pathol 1987;18:355 360.
17. Ljungberg O. Biopsy Pathology of the Thyroid and Parathyroid. London: Chapman & Hall; 1992.
18. Williams ED, Toyn CE, Harach HR. The ultimobranchial gland and congenital thyroid abnormalities in man. J Pathol 1989;159:135 141.
19. Harada T, Nishikawa Y, Ito K. Aplasia of one thyroid lobe. Am J Surg 1972;124:617 619.
20. Hegedus L, Perrild H, Poulsen LR, et al. The determination of thyroid volume by ultrasound and its relationship to body weight, age, and sex in normal subjects. J Clin Endocrinol Metab 1983;56:260 263.
21. Hegedus L, Karstrup S, Rasmussen N. Evidence of cyclic alterations of thyroid size during the menstrual cycle in healthy women. Am J Obstet Gynecol 1986;155:142 145.
22. Komorowski RA, Hanson GA. Occult thyroid pathology in the young adult: an autopsy study of 138 patients without clinical thyroid disease. Hum Pathol 1988;19:689 696.
23. Bell CD, Kovacs K, Horvath E, Rotondo F. Histologic, immunohistochemical, and ultrastructural findings in a case of minocycline-associated black thyroid. Endocr Pathol 2001;12:443 451.
24. Landas SK, Schelper RL, Tio FO, Turner JW, Moore KC, Bennett-Gray J. Black thyroid syndrome: exaggeration of a normal process? Am J Clin Pathol 1986;85:411 418.
25. Brown RA, Al-Moussa M, Beck J. Histometry of normal thyroid in man. J Clin Pathol 1986;39:475 482.
26. Imada M, Kurosumi M, Fujita H. Three-dimensional imaging of blood vessels in thyroids from normal and levothyroxine sodium-treated rats. Arch Histol Jpn 1986;49:359 367.
27. Imada M, Kurosumi M, Fujita H. Three-dimensional aspects of blood vessels in thyroids from normal, low iodine diet-treated, TSH-treated and PTU-treated rats. Cell Tissue Res 1986;245:291 296.
28. Feind C. The head and neck. In: Haagensen CD, Feind C, Herter FP, Slanetz CA Jr, Weinberg JA, eds. The Lymphatics in Cancer. Philadelphia: WB Saunders; 1972:59 222.
29. Crile G Jr. The fallacy of the conventional radical neck dissection for papillary carcinoma of the thyroid. Ann Surg 1957;145:317 320.
P.1146
30. Uchiyama Y, Murakami G, Ohno Y. The fine structure of nerve endings on rat thyroid follicular cells. Cell Tissue Res 1985;242:457 460.
31. Melander A, Ericson LD, Sundler F, Ingbar SH. Sympathetic innervation of the mouse thyroid and its significance in thyroid hormone secretion. Endocrinology 1974;94:959 966.
32. Tice LW, Creveling CR. Electron microscopic identification of adrenergic nerve endings on thyroid epithelial cells. Endocrinology 1975;97:1123 1129.
33. Ingbar SH. The thyroid gland. In: Wilson JD, Foster DW, eds. Williams Textbook of Endocrinology. 7th ed. Philadelphia: WB Saunders; 1985:682 815.
34. Kameda Y, Okamoto K, Ito M, Tagawa T. Innervation of the C cells of chicken ultimobranchial glands studied by immunohistochemistry, fluorescence microscopy, and electron microscopy. Am J Anat 1988;182:353 368.
35. Heimann P. Ultrastructure of human thyroid. A study of normal thyroid, untreated and treated diffuse goiter. Acta Endocrinol (Copenh) 1966;53(suppl 110):1 102.
36. Klinck GH, Oertel JE, Winship T. Ultrastructure of normal human thyroid. Lab Invest 1970;22:2 22.
37. Kurata A, Ohta K, Mine M, et al. Monoclonal antihuman thyroglobulin antibodies. J Clin Endocrinol Metab 1984;59:573 579.
38. Stanta G, Carcangiu ML, Rosai J. The biochemical and immunohistochemical profile of thyroid neoplasia. Pathol Annu 1988; 23(pt 1):129 157.
39. Rosai J, Carcangiu ML. Pitfalls in the diagnosis of thyroid neoplasms. Pathol Res Pract 1987;182:169 179.
40. Katoh R, Kawaoi A, Miyagi E, et al. Thyroid transcription factor-1 in normal, hyperplastic, and neoplastic follicular thyroid cells examined by immunohistochemistry and nonradioactive in situ hybridization. Mod Pathol 2000;13:570 576.
41. Lau SK, Luthringer DJ, Eisen RN. Thyroid transcription factor-1: a review. Appl Immunohistochem Mol Morphol 2002;10:97 102.
42. Miettinen M, Franssila K, Lehto VP, Paasivuo R, Virtanen I. Expression of intermediate filament proteins in thyroid gland and thyroid tumors. Lab Invest 1984;50:262 270.
43. Miettinen M, Kovatich AJ, Karkkainen P. Keratin subsets in papillary and follicular thyroid lesions. A paraffin section analysis with diagnostic implications. Virchows Arch 1997;431:407 413.
44. Fonseca E, Nesland JM, Hoie J, Sobrinho-Simoes M. Pattern of expression of intermediate cytokeratin filaments in the thyroid gland: an immunohistochemical study of simple and stratified epithelial-type cytokeratins. Virchows Arch 1997;430:239 245.
45. Viale G, Dell'Orto P, Coggi G, Gambacorta M. Coexpression of cytokeratins and vimentin in normal and diseased thyroid glands. Lack of diagnostic utility of vimentin immunostaining. Am J Surg Pathol 1989;13:1034 1040.
46. Bur M, Shiraki W, Masood S. Estrogen and progesterone receptor detection in neoplastic and non-neoplastic thyroid tissues. Mod Pathol 1993;6:469 472.
47. Kawabata W, Suzuki T, Moriya T, et al. Estrogen receptors (alpha and beta) and 17beta-hydroxysteroid dehydrogenase type 1 and 2 in thyroid disorders: possible in situ estrogen synthesis and actions. Mod Pathol 2003;16:437 444.
48. McLaren KM, Cossar DW. The immunohistochemical localization of S100 in the diagnosis of papillary carcinoma of the thyroid. Hum Pathol 1996;27 633 636.
49. Nishimura R, Yokose T, Mukai K. S-100 protein is a differentiation marker in thyroid carcinoma of follicular cell origin: an immunohistochemical study. Pathol Int 1997;47:673 679.
50. Westermark K, Lundqvist M, Wallin G. EGF-receptors in human normal and pathological thyroid tissue. Histopathology 1996;28:221 227.
51. Lima MA, Gontijo VA, Schmitt FC. Thyroid peroxidase and thyroglobulin expression in normal human thyroid glands. Endocr Pathol 1998;9:333 338.
52. Lin JD, Hsueh C, Chao TC, Weng HF. Expression of sodium iodide symporter in benign and malignant human thyroid tissues. Endocr Pathol 2001;12:15 21.
53. Ringel MD, Anderson J, Souza SL, et al. Expression of the sodium iodide symporter and thyroglobulin genes are reduced in papillary thyroid cancer. Mod Pathol 2001;14:289 296.
54. Maruyama M, Kato R, Kobayashi S, Kasuga Y. A method to differentiate between thyroglobulin derived from normal thyroid tissue and from thyroid carcinoma based on analysis of reactivity to lectins. Arch Pathol Lab Med 1998;122:715 720.
55. de Martynoff G, Pohl V, Mercken L, van Ommen GJ, Vassart G. Structural organization of the bovine thyroglobulin gene and of its 5 -flanking region. Eur J Biochem 1987;164:591 599.
56. Christophe D, Gerard C, Juvenal G, et al. Identification of a cAMP-responsive region in thyroglobulin gene promoter. Mol Cell Endocrinol 1989;64:5 18.
57. Lee NT, Nayfeh SN, Chae CB. Induction of nuclear protein factors specific for hormone-responsive region during activation of thyroglobulin gene by thyrotropin in rat thyroid FRTL-5 cells. J Biol Chem 1989;264:7523 7530.
58. Green WL. Physiology of the thyroid gland and its hormones. In: Green WL, ed. The Thyroid. New York: Elsevier; 1987:1 46.
59. Bjorkman U, Ekholm R, Elmqvist LG, Ericson LE, Melander A, Smeds S. Induced unidirectional transport of protein into the thyroid follicular lumen. Endocrinology 1974;95:1506 1517.
60. Ericson LE, Engstrom G. Quantitative electron microscopic studies on exocytosis and endocytosis in the thyroid follicle cell. Endocrinology 1978;103:883 892.
61. Ide M. Immunoelectron microscopic localization of thyroglobulin in the human thyoid gland. Acta Pathol Jpn 1984;34:575 584.
62. Sterling K. Thyroid hormone action at the cell level (first of two parts). N Engl J Med 1979;300:117 123.
63. Liddle GW, Liddle RA. Endocrinology. In: Smith LH, Thier SO, eds. Pathophysiology: The Biological Prinicples of Disease. Philadelphia: WB Saunders; 1981.
64. M ller MJ, Seitz HJ. Thyroid hormone action on intermediary metabolism. Part I. Respiration, thermogenesis and carbohydrate metabolism. Klin Wochenschr 1984;62:11 18.
65. M ller MJ, Seitz HJ. Thyroid hormone action on intermediary metabolism. II. Lipid metabolism in hyper- and hypothyroidism. Klin Wochenschr 1984;62:49 55.
66. M ller MJ, Seitz HJ. Thyroid hormone action on intermediary metabolism. Part III. Protein metabolism in hyper- and hypothyroidism. Klin Wochenschr 1984;62:97 102.
67. Oppenheimer JH. Thyroid hormone action at the nuclear level. Ann Intern Med 1985;102:374 384.
68. Oppenheimer JH, Samuels HH, eds. Molecular Basis of Thyroid Hormone Action. New York: Academic Press; 1983.
69. Larsen PR. Thyroid pituitary interaction: feedback regulation of thyrotropin secretion by thyroid hormones. N Engl J Med 1982;306:23 32.
70. Davies T, Marians R, Latif R. The TSH receptor reveals itself. J Clin Invest 2002;110:161 164.
71. Farid NR, Szkudlinski MW. Minireview: structural and functional evolution of the thyrotropin receptor. Endocrinology 2004;145:4048 4057.
72. Pittman JA Jr. Thyrotropin-releasing hormone. Adv Intern Med 1974;19:303 325.
73. Wilber JF. Thyrotropin releasing hormone: secretion and actions. Annu Rev Med 1973;24:353 364.
74. Walsh FM, Castelli JB. Polytef granuloma clinically simulating carcinoma of the thyroid. Arch Otolaryngol 1975;101:262 263.
75. Sanfilippo F, Shelburne J, Ingram P. Analysis of a polytef granuloma mimicking a cold thyroid nodule 17 months after laryngeal injection. Ultrastruct Pathol 1980;1:471 475.
76. Chaplin AJ. Histopathological occurrence and characterization of calcium oxalate: a review. J Clin Pathol 1977;30:800 811.
77. Carney JA, Moore SB, Northcutt RC, Woolner LB, Stillwell GK. Palpation thyroiditis (multifocal granulomatous folliculitis). Am J Clin Pathol 1975;64:639 647.
78. Blum M, Schloss MF. Martial-arts thyroiditis. N Engl J Med 1984;311:199 200.
79. Harach R, Jasani B. Thyroid multifocal granulomatous folliculitis (palpation thyroiditis): an immunocytochemical study. Endocr Pathol 1993;4:105 109.
80. Hellwig CA. Colloidophagy in the human thyroid gland. Science 1951;113:725 726.
81. Manson C, Cross P, De Sousa B. Post-operative necrotizing granulomas of the thyroid. Histopathology 1992;21:392 393.
P.1147
82. Harach HR. Palpation thyroiditis resembling C cell hyperplasia. Usefulness of immunohistochemistry in their differential diagnosis. Pathol Res Pract 1993;189:488 490.
83. Richter MN, McCarty KS. Anisotropic crystals in the human thyroid gland. Am J Pathol 1954;30:545 553.
84. Katoh R, Suzuki K, Hemmi A, Kawaoi A. Nature and significance of calcium oxalate crystals in normal human thyroid gland. A clinicopathological and immunohistochemical study. Virchows Arch A Pathol Anat Histopathol 1993;422:301 306.
85. Reid JD, Choi CH, Oldroyd NO. Calcium oxalate crystals in the thyroid. Their identification, prevalence, origin, and possible significance. Am J Clin Pathol 1987;87:443 454.
86. Gross S. Granulomatous thyroiditis with anisotropic crystalline material. AMA Arch Pathol 1955;59:412 418.
87. Fayemi AO, Ali M, Braun EV. Oxalosis in hemodialysis patients: a pathologic study of 80 cases. Arch Pathol Lab Med 1979;103:58 62.
88. Isotalo PA, Lloyd RV. Presence of birefringent crystals is useful in distinguishing thyroid from parathyroid gland tissues. Am J Surg Pathol 2002;26:813 814.
89. Klinck G, Menk K. Squamous cells in the human thyroid. Mil Surgeon 1951;109:406 414.
90. Harcourt-Webster JN. Squamous epithelium in the human thyroid gland. J Clin Pathol 1966;19:384 388.
91. LiVolsi VA, Merino MJ. Squamous cells in the human thyroid gland. Am J Surg Pathol 1978;2:133 140.
92. Pearse AG. The cytochemistry of the thyroid C cells and their relationship to calcitonin. Proc R Soc Lond B Biol Sci 1966;164:478 487.
93. Teitlebaum SL, Moore KE, Shieber W. Parafollicular cells in the normal human thyroid. Nature 1971;230:334 335.
94. Wolfe HJ, DeLellis RA, Voelkel EF, Tashjian AH Jr. Distribution of calcitonin-containing cells in the normal neonatal human thyroid gland: a correlation of morphology with peptide content. J Clin Endocrinol Metab 1975;41:1076 1081.
95. O'Toole K, Fenoglio-Preiser C, Pushparaj N. Endocrine changes associated with the human aging process. III. Effect of age on the number of calcitonin immunoreactive cells in the thyroid gland. Hum Pathol 1985;16:991 1000.
96. Gibson WCH, Peng TC, Croker BP. Age-associated C-cell hyperplasia in the human thyroid. Am J Pathol 1982;106:388 393.
97. Albores-Saavedra J, Gorraez de la Mora T, de la Torre-Rendon F, Gould E. Mixed medullary-papillary carcinoma of the thyroid: a previously unrecognized variant of thyroid carcinoma. Hum Pathol 1990;21:1151 1155.
98. Scopsi L, Di Palma S, Ferrari C, Holst JJ, Rehfeld JF, Rilke F. C-cell hyperplasia accompanying thyroid diseases other than medullary carcinoma: an immunocytochemical study by means of antibodies to calcitonin and somatostatin. Mod Pathol 1991;4:297 304.
99. Libbey NP, Nowakowski KJ, Tucci JR. C-cell hyperplasia of the thyroid in a patient with goitrous hypothyroidism and Hashimoto's thyroiditis. Am J Surg Pathol 1989;13:71 77.
100. Biddinger PW, Brennan MF, Rosen PP. Symptomatic C-cell hyperplasia associated with chronic lymphocytic thyroiditis. Am J Surg Pathol 1991;15:599 604.
101. Guyetant S, Wion-Barbot N, Rousselet MC, Franc B, Bigorgne JC, Saint-Andre JP. C-cell hyperplasia associated with chronic lymphocytic thyroiditis: a retrospective quantitative study of 112 cases. Hum Pathol 1994;25:514 521.
102. Tomita T, Millard DM. C-cell hyperplasia in secondary hyperparathyroidism. Histopathology 1992;21:469 474.
103. DeLellis RA, Wolfe HJ. The pathobiology of the human calcitonin (C)-cell: a review. Pathol Annu 1981;16(pt 2):25 52.
104. DeLellis RA, Nunnemacher G, Wolfe HJ. C-cell hyperplasia. An ultrastructural analysis. Lab Invest 1977;36:237 248.
105. Pearse AG. Common cytochemical and ultrastructural characteristics of cells producing polypeptide hormones (the APUD series) and their relevance to thyroid and ultimobranchial C cells and calcitonin. Proc R Soc Lond B Biol Sci 1968;170:71 80.
106. Gonzalez-Campora R, Sanchez Gallego F, Martin Lacave I, Mora Marin J, Montero Linares C, Galera-Davidson H. Lectin histochemistry of the thyroid gland. Cancer 1988;62:2354 2362.
107. Bussolati G, Pearse AG. Immunofluorescent localization of calcitonin in the C cells of pig and dog thyroid. J Endocrinol 1967;37:205 209.
108. McMillan PJ, Hooker WM, Deptos LJ. Distribution of calcitonin-containing cells in the human thyroid. Am J Anat 1974;140:73 79.
109. Schmid KW, Kirchmair R, Ladurner D, Fischer-Colbrie R, Bocker W. Immunohistochemical comparison of chromogranins A and B and secretogranin II with calcitonin and calcitonin gene-related peptide expression in normal, hyperplastic and neoplastic C-cells of the human thyroid. Histopathology 1992;21:225 232.
110. Ali-Rachedi A, Varndell IM, Facer P, et al. Immunocytochemical localization of katacalcin, a calcium-lowering hormone cleaved from the human calcitonin precursor. J Clin Endocrinol Metab 1983;57:680 682.
111. Van Noorden S, Polak JM, Pearse AG. Single cellular origin of somatostatin and calcitonin in the rat thyroid gland. Histochemistry 1977;53:243 247.
112. Yamada Y, Ito S, Matsubara Y, Kobayashi S. Immunohistochemical demonstration of somatostatin-containing cells in the human, dog and rat thyroids. Tohoku J Exp Med 1977;122:87 92.
113. Kusumoto Y. Calcitonin and somatostatin are localized in different cells in the canine thyroid gland. Biomed Res 1980;1:237 241.
114. Kakudo K, Vacca LL. Immunohistochemical study of substance P-like immunoreactivity in human thyroid and medullary carcinoma of the thyroid. J Submicrosc Cytol 1983;15:563 568.
115. Sundler F, Christophe J, Robberecht P, et al. Is helodermin produced by medullary thyroid carcinoma cells and normal C-cells? Immunocytochemical evidence. Regul Pept 1988;20:83 89.
116. Kameya T, Bessho T, Tsumuraya M, et al. Production of gastrin releasing peptide by medullary carcinoma of the thyroid. An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol 1983;401:99 107.
117. Sunday ME, Wolfe HJ, Roos BA, Chin WW, Spindel ER. Gastrin-releasing peptide gene expression in developing hyperplastic, and neoplastic human thyroid C-cells. Endocrinology 1988;122:1551 1558.
118. Gkonos PJ, Tavianini MA, Liu CC, Roos BA. Thyrotropin-releasing hormone gene expression in normal thyroid parafollicular cells. Mol Endocrinol 1989;3:2101 2109.
119. Nunez EA, Gershon MD. Thyrotropin-induced thyroidal release of 5-hydroxytryptamine and accompanying ultrastructural changes in parafollicular cells. Endocrinology 1983;113:309 317.
120. DeLellis RA. Endocrine tumors. In: Colvin RB, Bhan AK, McCluskey RT, eds. Diagnostic Immunopathology. New York: Raven Press; 1988.
121. MacIntyre I. Calcitonin: physiology, biosynthesis, secretion, metabolism and mode of action. In: DeGroot LJ, ed. Endocrinology. 2nd ed. Vol 2. Philadelphia: WB Saunders; 1989:892 901.
122. Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 1982;298:240 244.
123. Kontozoglou T, Mambo N. The histopathologic features of lithium-associated thyroiditis. Hum Pathol 1983;14:737 739.
124. Carpenter GR, Emery JL. Inclusions in the human thyroid. J Anat 1976;122(pt 1):77 89.
125. Gardiner WR. Unusual relationships between thyroid gland and skeletal muscle in infants; a review of the literature and four case reports. Cancer 1956;9:681 691.
126. Klinck GH, Winship T. Psammoma bodies and thyroid cancer. Cancer 1959;12:656 662.
127. Batsakis JG, Nishiyama RH, Rich CR. Microlithiasis (calcospherites) and carcinoma of the thyroid gland. Arch Pathol 1960;69:493 498.
128. Dugan JM, Atkinson BF, Avitabile A, Schimmel M, LiVolsi VA. Psammoma bodies in fine needle aspirate of the thyroid in lymphocytic thyroiditis. Acta Cytol 1987;31:330 334.
129. Erdheim J. I. Uber Schilddrusenaplasie. II. Geschwulste des Ductus Thyreoglossus. III. Uber einige menschliche Kiemenderivate. Beitr Pathol Anat 1904;35:366 433.
P.1148
130. Getzowa S. Zur Kenntnis des postbranchialen Korpers und der branchialen Kanalchen des Menschen. Virchows Arch 1907;88:181 235.
131. Calvert R, Isler H. Fine structure of a third epithelial component of the thyroid gland of the rat. Anat Rec 1970;168:23 41.
132. Black HE, Capen CC, Young DM. Ultimobranchial thyroid neoplasms in bulls. A syndrome resembling medullary thyroid carcinoma in man. Cancer 1973;32:865 878.
133. Ljungberg O, Nilsson PO. Hyperplastic and neoplastic changes in ultimobranchial remnants and in parafollicular (C) cells in bulls: a histologic and immunohistochemical study. Vet Pathol 1985;22:95 103.
134. Harach HR. Solid cell nests of the human thyroid in early stages of postnatal life. Systematic autopsy study. Acta Anat (Basel) 1986;127:262 264.
135. Janzer RC, Weber E, Hedinger C. The relation between solid cell nests and C cells of the thyroid gland: an immunohistochemical and morphometric investigation. Cell Tissue Res 1979;197:295 312.
136. Chan JK, Tse CC. Solid cell nest associated C-cells: another possible explanation for C-cell hyperplasia adjacent to follicular cell tumors. Hum Pathol 1989;20:498 499.
137. Cameselle-Teijeiro J, Varela-Duran J, Sambade C, Villanueva JP, Varela-Nunez R, Sobrinho-Simoes M. Solid cell nests of the thyroid: light microscopy and immunohistochemical profile. Hum Pathol 1994;25:684 693.
138. Mizukami Y, Nonomura A, Michigishi T, et al. Solid cell nests of the thyroid. A histologic and immunohistochemical study. Am J Clin Pathol 1994;101:186 191.
139. Yamaoka Y. Solid cell nest (SCN) of the human thyroid gland. Acta Pathol Jpn 1973;23:493 506.
140. Reis-Filho JS, Preto A, Soares P, Ricardo S, Cameselle-Teijeiro J, Sobrinho-Simoes M. p63 expression in solid cell nests of the thyroid: further evidence for a stem cell origin. Mod Pathol 2003;16:43 48.
141. Preto A, Cameselle-Teijeiro J, Moldes-Boullosa J, et al. Telomerase expression and proliferative activity suggest a stem cell role for thyroid solid cell nests. Mod Pathol 2004;17:819 826.
142. Martin V, Martin L, Viennet G, Hergel M, Carbillet JP, Fellmann D. Ultrastructural features of solid cell nest of the human thyroid gland: a study of 8 cases. Ultrastruct Pathol 2000;24:1 8.
143. Ljungberg O, Nilsson PO. Intermediate thyroid carcinoma in humans and ultimobranchial tumors in bulls: a comparative morphological and immunohistochemical study. Endocr Pathol 1991;2:24 39.
144. Beckner ME, Shultz JJ, Richardson T. Solid and cystic ultimobranchial body remnants in the thyroid. Arch Pathol Lab Med 1990;114:1049 1052.
145. Louis DN, Vickery AL Jr, Rosai J, Wang CA. Multiple branchial cleft like cysts in Hashimoto's thyroiditis. Am J Surg Pathol 1989;13:45 49.
146. Apel RL, Asa SL, Chalvardjian A, LiVolsi VA. Intrathyroidal lymphoepithelial cysts of probable branchial origin. Hum Pathol 1994;25:1238 1242.
147. Streutker CJ, Murray D, Kovacs K, Higgins HP. Epithelial cyst of thyroid. Endocr Pathol 1997;8:75 80.
148. Ryska A, Vokurka J, Michal M, Ludvikova M. Intrathyroidal lymphoepithelial cyst. A report of two cases not associated with Hashimoto's thyroiditis. Pathol Res Pract 1997;193:777 781.
149. Langlois NE, Krukowski ZH, Miller ID. Pancreatic tissue in a lateral cervical cyst attached to the thyroid gland a presumed foregut remnant. Histopathology 1997;31:378 380.
150. Harach HR, Vujanic GM. Intrathyroidal parathyroid. Pediatr Pathol 1993;13:71 74.
151. Spiegel AM, Marx SJ, Doppman JL, et al. Intrathyroidal parathyroid adenoma or hyperplasia. An occasionally overlooked cause of surgical failure in primary hyperparathyroidism. JAMA 1975;234:1029 1033.
152. LiVolsi V. Branchial and thymic remnants in the thyroid and cervical region: an explanation for unusual tumors and microscopic curiosities. Endocr Pathol 1993;4:115 119.
153. Harach HR, Vujanic GM. Intrathyroidal thymic tissue: an autopsy study in fetuses with some emphasis on pathological implications. Pediatr Pathol 1993;13:431 434.
154. Neill J. Intrathyroid thymoma. Am J Surg Pathol 1986;10:660 661.
155. Mizukami Y, Nonomura A, Michigishi T, et al. Ectopic thymic tissue in the thyroid gland. Endocr Pathol 1993;4:162 164.
156. Damiani S, Filotico M, Eusebi V. Carcinoma of the thyroid showing thymoma-like features. Virchows Arch A Pathol Anat Histopathol 1991;418:463 466.
157. Miyauchi A, Kuma K, Matsuzuka F, et al. Intrathyroidal epithelial thymoma: an entity distinct from squamous cell carcinoma of the thyroid. World J Surg 1985;9:128 135.
158. Cameselle-Teijeiro J, Varela-Duran J. Intrathyroid salivary gland-type tissue in multinodular goiter. Virchows Arch 1994;425:331 334.
159. Finkle HI, Goldman RL. Heterotopic cartilage in the thyroid. Arch Pathol 1973;95:48 49.
160. Guimaraes SB, Uceda JE, Lynn HB. Thyroglossal duct remnants in infants and children. Mayo Clin Proc 1972;47:117 120.
161. Ellis PD, Van Nostrand AW. The applied anatomy of thyroglossal tract remnants. Laryngoscope 1977;87(pt 1):765 770.
162. Larochelle D, Arcand P, Belzile M, Gagnon NB. Ectopic thyroid tissue a review of the literature. J Otolaryngol 1979;8:523 530.
163. De Andrade MA. A review of 128 cases of posterior mediastinal goiter. World J Surg 1977;1:789 797.
164. de Souza FM, Smith PE. Retrosternal goiter. J Otolaryngol 1983;12:393 396.
165. Kantelip B, Lusson JR, De Riberolles C, Lamaison D, Bailly P. Intracardiac ectopic thyroid. Hum Pathol 1986;17:1293 1296.
166. Wapshaw H. Lingual thyroid. A report of a case with unusual histology. Br J Surg 1974;30:160 165.
167. Sauk JJ Jr. Ectopic lingual thyroid. J Pathol 1970;102:239 243.
168. Baughman RA. Lingual thyroid and lingual thyroglossal tract remnants. A clinical and histopathologic study with review of the literature. Oral Surg Oral Med Oral Pathol 1972;34:781 799.
169. Ulrich HF. Lingual thyroid. Ann Surg 1932;95:503 507.
170. LiVolsi VA, Perzin KH, Savetsy L. Carcinoma arising in median ectopic thyroid (including thyroglossal duct tissue). Cancer 1974;34:1303 1315.
171. Block MA, Wylie JH, Patton RB, Miller JM. Does benign thyroid tissue occur in the lateral part of the neck? Am J Surg 1966;112:476 481.
172. Klopp CT, Kirson SM. Therapeutic problems with ectopic non-cancerous follicular thyroid tissue in the neck: 18 case reports according to etiological factors. Ann Surg 1966;163:653 664.
173. Moses DC, Thompson NW, Nishiyama RH, Sisson JC. Ectopic thyroid tissue in the neck. Benign or malignant. Cancer 1976;38:361 365.
174. Hathaway BM. Innocuous accessory thyroid nodules. Arch Surg 1965;90:222 227.
175. Sisson JC, Schmidt RW, Beierwaltes WH. Sequestered nodular goiter. N Engl J Med 1964;270 927 932.
176. Meyer JS, Steinberg LS. Microscopically benign thyroid follicles in cervical lymph nodes. Serial section study of lymph node inclusions and entire thyroid gland in 5 cases. Cancer 1969;24:302 211.
177. Frantz VK, Forsythe R, Hanford JM, Rogers WM. Lateral aberrant thyroids. Ann Surg 1942;115:161 183.
178. Donegan JO, Wood MD. Intratracheal thyroid familial occurrence. Laryngoscope 1985;95:6 8.
179. Kaplan M, Kauli R, Lubin E, Grunebaum M, Laron Z. Ectopic thyroid gland. A clinical study of 30 children and review. J Pediatr 1978;92:205 209.
180. Kurman RJ, Prabha AC. Thyroid and parathyroid glands in the vaginal wall: report of a case. Am J Clin Pathol 1973;59:503 507.
181. Rahn J. ber eine eigenartige. Heterotopie von Schildr sengewebe. Zentralbl Allg Pathol 1958;99:80 86.
182. Ruchti C, Balli-Antunes H, Gerber HA. Follicular tumor in the sellar region without primary cancer of the thyroid. Heterotopic carcinoma? Am J Clin Pathol 1987;87:776 780.
183. Sekine S, Nagata M, Hamada H, Watanabe T. Heterotopic thyroid tissue at the porta hepatis in a fetus with trisomy 18. Virchows Arch 2000;436:498 501.
184. Shiraishi T, Imai H, Fukutome K, Watanabe M, Yatani R. Ectopic thyroid in the adrenal gland. Hum Pathol 1999;30:105 108.
185. Yilmaz F, Uzunlar AK, Sogutcu N. Ectopic thyroid tissue in the uterus. Acta Obstet Gynecol Scand 2005;84:201 202.