27 - Anal Canal
Editors: Mills, Stacey E.
Title: Histology for Pathologists, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > IX - Genitourinary Tract > 34 - Kidney
function show_scrollbar() {}
34
Kidney
William L. Clapp
Byron P. Croker
Introduction
The kidney has an intricate structure that underlies its diverse roles of excreting waste products, regulating body fluid and solute balance, regulating blood pressure, and secreting hormones. A familiarity with the basic structure of the kidney facilitates the evaluation and comprehension of diseases and functional disorders that can affect the kidney. The structure of the normal human kidney is considered in this chapter. Although the focus is on the human kidney, analogous renal structures in other mammalian species are discussed or illustrated when pertinent.
Pediatric Kidney
Renal enthusiasts, especially developmental biologists and pathologists, have long been fascinated with how a kidney develops from primitive mesoderm into such a wondrously complex organ. A basic understanding of nephrogenesis provides a framework to enhance our knowledge of congenital kidney disease. The human kidney is structurally immature at the time of birth, and important morphologic changes occur during infancy and childhood. Pathologists not familiar with the histologic peculiarities of the pediatric kidney may mistake normal findings for abnormalities or fail to observe significant abnormalities of renal maturation. The following section covers the pediatric kidney, focusing first on kidney development prior to birth, and second, on the kidney after birth.
Kidney Development
During development, cells proliferate, migrate, differentiate, die, and interact with other cells to form tissues and organs. These different aspects of cell behavior are controlled by genes in a temporal and spatial manner. The kidney has long been considered an excellent model system for the study of organogenesis. However, when one considers the elaborate architecture and heterogenous cellular elements of the organ, it is not surprising that understanding the mechanisms of kidney development remains a considerable challenge. Detailed reviews of kidney development are available for more information (1,2,3,4,5,6,7,8,9,10,11,12).
Embryonic Kidneys
Organogenesis begins during the third week of human embryogenesis with the initial formation of the central nervous and cardiovascular systems. The urogenital system represents the last organ system to develop. Kidney development goes through three successive stages: pronephros, mesonephros and metanephros. All three systems develop from the intermediate mesoderm, located between the dorsal somites and lateral plate mesoderm and extending from the cervical to the caudal regions of the embryo. The
P.840
pronephros and mesonephros are transient structures in mammals. However, all three systems are essential for the formation of each subsequent organ and are dependent on the presence of the preceeding structure. The mesonephros forms before the pronephros regresses, and the metanephros develops before the mesonephros disappears (Figure 34.1). This developmental scheme may be likened to a wave of nephrogenesis moving in a cervical to caudal direction through the intermediate mesoderm. Some genes that regulate metanephric kidney development are also believed to be involved in forming the earlier embryonic kidneys.
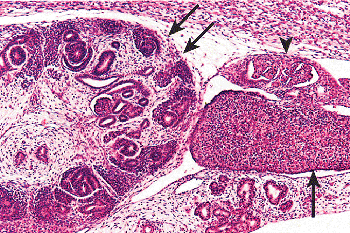 |
Figure 34.1 Mesonephros amd metanephros. The mesonephros (arrowhead) contributes somatic cell lineages to the gonadal ridge (single arrow), which will develop into the gonad. Early nephron formation is present in the metanephros (double arrows), whose development is dependent on the presence of the mesonephros. Reprinted with permission from: Murphy WM, Grignon DJ, Perlman EJ. Tumors of the kidney, bladder, and related urinary structures. In: Atlas of Tumor Pathology. 4th series, fascicle 1. Washington, DC: Armed Forces Institute of Pathology; 2004. |
Pronephros
The pronephros develops in the cervical region at the end of the third week of human gestation. However, most of our knowledge of the pronephros has come from the study of lower vertebrates (13,14). The pronephros consists of a glomus (glomerulus-like structure), tubules, and a duct. The glomus, not physically connected to the tubules, projects into the coelomic cavity and filters blood.
Ciliated tubules, called nephrostomes, open into the coelom and collect the filtrate. The nephrostomes connect to proximal tubules, which empty into a distal tubule that joins the pronephric duct. In humans, the pronephros is a rudimentary organ and does not function. As the pronephric duct extends caudally, the glomus and tubules regress. However, the pronephric duct persists and becomes the mesonephric duct.
Mesonephros
The human mesonephros develops in the middle of the fourth week of gestation as a thoracic organ. Considerable variation in structure and function of the mesonephros exists, even among mammalian species (15). The human mesonephros contains 20 to 40 nephrons, consisting of glomeruli directly connected to tubules, with proximal and distal segments, some of which directly connect to the mesonephric duct (wolffian duct). The distal mesonephric duct fuses with the cloaca, a precursor of the urinary bladder. In some mammals, two sets of mesonephric tubules exist. The caudal tubules, representing the majority of the mesonephric nephrons, never fuse with the mesonephric duct, whereas the more cephalad tubules are connected to the duct. Moreover, mice deficient for the Wilms' tumor suppressor gene, WT1, lack the caudal set of mesonephric tubules but develop the cephalad ones (16). Thus, WT1 appears to regulate only caudal mesonephric development, which may have some molecular events similar to metanephric development since both require WT1 for formation. The excretory function of the human mesonephros is believed to be limited. As observed with the pronephros, the mesonephros undergoes apoptosis and degenerates (17).
In the male, some mesonephric tubules form the efferent ducts of the epididymis, whereas the mesonephric duct gives rise to the duct of the epididymis, the seminal vesicle, and the ejaculatory duct. In females, the mesonephros undergoes dissolution, with the epo phoron, paro phoron, and Gartner's duct remaining as vestigial structures. Evidence has emerged indicating the mesonephros contributes cell lineages for other organ systems. A region including the dorsal aorta, gonad, and mesonephros (AGM) is the first site in which adult-type hematopoietic stem cells are generated (18,19).
Metanephros
Overview
The metanephros, the definitive and permanent kidney, develops from an inductive interaction between the ureteric bud and the mesenchyme of the caudal intermediate mesoderm, called the metanephric mesenchyme, or blastema. During the fifth week of gestation, the first step in metanephric development occurs. Factors expressed by the metanephric mesenchyme induce the ureteric bud, a branch of the caudal mesonephric duct, to grow dorsally until it encounters the mesenchyme. The ureteric bud undergoes iterative branching to form the renal pelvis, calyces, and collecting ducts. Induced by the ureteric bud, the metanephric mesenchyme differentiates into the glomeruli, proximal and distal tubules, and Henle's loops. Thus, cells of the metanephric kidney originate from two different lineages to form the collecting ducts and nephrons. The reciprocal inductive interaction between the ureteric bud and the metanephric mesenchyme is the central process of metanephrogenesis. For detailed information, the reader is directed to the classic light
P.841
microscopic (1,2,3), microdissection (4,5,6), and experimental (7,8) studies.
Formation of the Renal Pelvis and Calyces
The complex three-dimensional branching pattern of the ureteric bud and its derivatives creates an elaborate renal architecture. The ureteric bud and its branches consist of a tubule portion, which elongates, and an actively growing ampullary tip. Various types of branching have been observed, including bifid and trifid branching from the ampullary tip and different modes of lateral branching (Oliver's closed and open divided models) from the tubule portion of the ureteric bud. The complexity of branching morphogenesis varies according to the period of nephrogenesis and also among mammalian species (4,5,6,20,21,22).
The first three to five generations of ureteric bud branches form the renal pelvis, with more divisions occuring in the poles than in the midpolar region (Figure 34.2). Urine production is accompanied by progressive dilatation and coalescence of the earlier branches to form the early pelvic-calyceal system by 11 to 12 weeks. Subsequent generations of branches form the calyces. Extensive tissue remodeling of the calyceal system occurs. By 11 to 14 weeks, the calyces become compressed between the expanding renal pelvis and the aggregation of nephrons induced by collecting ducts in the developing papillae. The minor calyces convert from a bulbous configuration to their definitive cuplike shape, and the papillae become conical (Figure 34.3). The fate of the very first nephrons formed, presumably induced by and attached to the first generations of the ureteric bud that form the pelvis and calyces, remains a question. They are believed to either degenerate or attach to a later generation branch that elongates eventually to reach the juxtamedullary cortex.
Formation of the Collecting System
At eight weeks, the first nephrons can be observed attached to ureteric bud branches. The organogenetic processes of collecting duct branching/elongation and nephron differentiation occur simultaneously. Collecting duct morphogenesis has been divided into four periods (5). In the first period, from the fifth to the fourteenth week of gestation, branching occurs from the ampullary tips, and individual nephrons remain attached to their ampullae. In an iterative bifurcation model of branching, one of the two new ampullae retains the old nephron whereas the other induces the formation of a new one. The second period, weeks 14 through 22, is characterized by the formation of arcades. Ampullae rarely branch, but single elongating tips repeatedly induce new nephrons while carrying attached older nephrons. As new nephrons are formed, the connecting tubule of the older nephron merges its point of attachment away from the ampulla to the connecting tubule of the newer nephron. Repetition of this process results in three to seven nephrons forming around a single ampulla, joined to one another in an arcade by their connecting tubules. Arcades are associated with juxtamedullary nephrons in the inner cortex of the fully developed kidney.
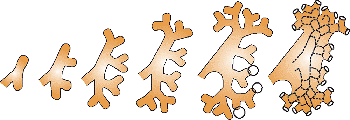 |
Figure 34.2 Diagram depicting early branches of ureteric bud that dilate and coalesce to form the renal pelvis. Examples of third, fourth, and fifth generation branches are circled. Modified with permission from: Potter EL. Normal and Abnormal Development of the Kidney. Chicago: Year Book; 1972. |
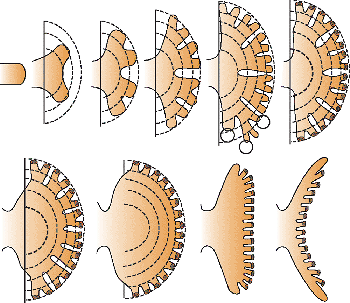 |
Figure 34.3 Diagram illustrating later branches of ureteric bud forming a minor calyx and papilla. Circles indicate generation branches that may expand to form part of the calyx or, if not expanding, form papillary ducts. The expanding pelvis and the peripheral zone of differentiating nephrons compress the original saccular cavity, producing the cuplike shape of the calyx and the conical configuration of the papilla. Modified with permission from: Potter EL. Normal and Abnormal Development of the Kidney. Chicago: Year Book; 1972. |
In the third period, weeks 20 through 36, the ampullae advance beyond the attachment point of the arcade, toward the outer surface. The ampullae do not branch but induce five to seven nephrons, each of which will have a direct connection to the developing collecting tubule. This type of nephron attachment predominates in the outer cortex of the mature kidney (Figure 34.4). Since nephrons retain contact with their ampullae of origin, either through arcades or directly, the longitudinal growth of the collecting tubules positions the attached glomeruli in the cortex. In the fourth period, beginning at 32 to 36 weeks, the ampullae disappear, and no new nephrons form. Normally, nephrogenesis does not occur beyond 36 weeks of gestation. The last
P.842
nephrons formed are in the outer cortex with their glomeruli near the renal capsule.
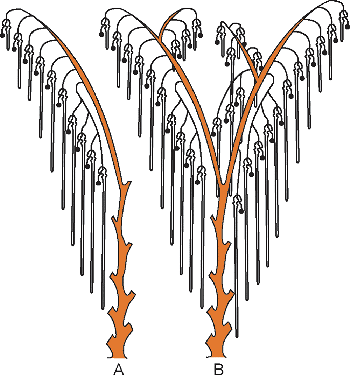 |
Figure 34.4 Diagram demonstrating the pattern of nephrons and collecting tubules at birth. A. The most common arrangement is for each collecting tubule to have a single arcade composed of three to five nephrons and five to seven nephrons individually attached. B. Depending on the division of the ampullary tips, other variations are possible. Modified with permission from: Potter EL. Normal and Abnormal Development of the Kidney. Chicago: Year Book; 1972. |
Nephron Formation
Over 100 years ago, investigations by Herring and Huber provided a fairly accurate morphologic view of human nephron development (Figure 34.5) (1,2). They were also prescient in regard to some mechanisms of nephrogenesis; for example, the development of glomerular capillaries. From eight weeks of gestation, the nephrons and collecting duct system develop together. The stages in individual nephron development do not vary and occur continously throughout the periods of collecting duct formation. The formation of nephrons can be divided into two phases: the induction stage and the morphogenetic stage (7,8,23). In the induction stage, the mesenchyme condenses around the ampullary tips in response to inducing signals from the ureteric bud. Two types of mesenchymal condensates form in this induction stage prior to epithelial differentiation of the mesenchyme (24). The first condensate, called the cap, closely surrounds each ampullary tip. A short time later, another condensate, termed the pretubular aggregate, forms at the lateral edges of the ampullary tip, below the cap. At the stage of ureteric bud division, forming a T-shaped structure, two pretubular aggregates may be observed, one on each side of the T bud. The caps and pretubular aggregates can be distinguished by histology. The cap is believed to regulate ureteric bud branching, whereas the pretubular aggregate is destined to form the nephron.
The morphogenetic stage of nephron formation involves several complex phases (Figure 34.5). First, the cells of the pretubular aggregate undergo a mesenchyme-to-epithelium transition, characterized by expression of epithelial markers and synthesis of basement membrane matrix glycoproteins. The cells develop intercellular junctions and become polarized and surrounded by a basal lamina, forming a structure termed the vesicle. A central cavity may be observed in the vesicle. Soon after formation, the vesicle fuses to the ureteric duct epithelium, and a continuous basal lamina surrounds both the vesicle and the duct. Opposite the area of fusion between the vesicle and the ureteric duct, a vascular cleft develops representing the site where the glomerular capillaries will emerge. The vesicle becomes a comma-shaped tubular structure. Another crevice forms near the fusion between the comma structure and the ureteric duct. After elongation and folding, an S-shaped figure (representing an early nephron) forms (Figure 34.6). At this stage, the S-shaped body is already compartmentalized into distinct cell types that are arranged into three areas. The vascular cleft lies below the upper and middle limbs of the S-shaped body and above the lower limb. The upper limb (connected to the ureteric duct) and the middle limb (also known as Stoerk's complex) generate the proximal and distal convoluted tubules and the loops of Henle. The lower limb, most distant from the ureteric bud, differentiates into the parietal and visceral epithelium of the glomerulus.
Active nephron formation occurs across the developing renal cortex in a band, known as the nephrogenic zone (termed the neogenic zone in early studies) (Figures 34.7,34.8,34.9). After nephron formation ceases, generally by 36 weeks of gestation, the nephrogenic zone disappears (Figure 34.10). The growth of the collecting ducts and the incremental formation of nephrons result in a centrifugal developmental pattern extending through the renal cortex. The earliest nephrons to form are found in the juxtamedullary zone of cortex, whereas the last nephrons to develop are in the outer cortex. This principle is fundamental to understanding postnatal structural changes in the kidney and is sometimes useful in the timing of developmental disturbances in the cortex. For example, a disturbance during the early months of development may result in an abnormality of the entire cortical thickness, whereas one that occurs in the last half of gestation may involve only the outermost layers of cortical nephrons. In summary, the coincident processes of ureteral-derived epithelial branching and nephron formation largely establish the basic architectural organization of the kidney.
P.843
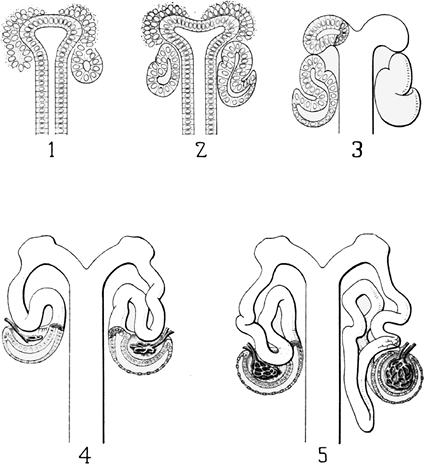 |
Figure 34.5 Huber's schematic drawings of nephron development. 1. Condensation stage with the cap and the pretubular aggregate. A renal vesicle (right) is present. 2. Comma-shaped body. 3. S-shaped body. 4. Early glomerular capillary development, Bowman's capsule formation and tubule elongation. 5. Glomerular and tubule maturation (right). Reprinted from: Huber GC. On the development and shape of uriniferous tubules of certain of the higher mammals. Am J Anat 1905;4(suppl):1 98. |
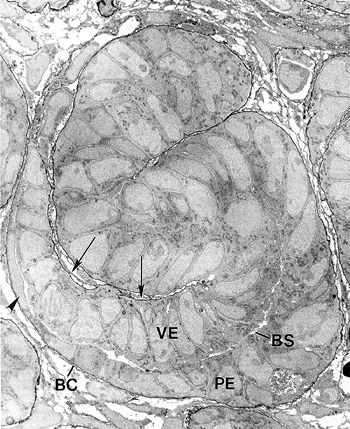 |
Figure 34.6 Electron micrograph of S-shaped figure from newborn mouse kidney. The basement membranes are rendered black by labeling with anti-laminin IgG conjugated to horseradish peroxidase. The vascular cleft (arrows), visceral epithelial cells (VE), Bowman's space (BS), parietal epithelial cells (PE), and Bowman's capsule (BC) can be seen. The visceral epithelial cells will differentiate into podocytes. Some parietal epithelial cells are becoming squamous (arrowhead) and will line Bowman's capsule. The epithelial cells above the vascular cleft will give rise to the proximal tubules, loops of Henle, and distal convoluted tubules. (Magnification 5000.) Modified with permission from: Clapp WL, Abrahamson DR. Development and gross anatomy of the kidney. In: Tisher CC, Brenner BM, eds. Renal Pathology. 2nd ed. Philadelphia: JB Lippincott; 1994:3 59. |
P.844
 |
Figure 34.7 Developing kidney at 21 weeks of gestation showing two medullary pyramids with surrounding cortex. The nephrogenic zone represents a thin layer outlining the peripheral aspects of the lobes, both at the surface and in the midplane of the septa (column) of Bertin, between the two renal lobes. |
Glomerulogenesis
To appreciate how some glomerular diseases arise or how the glomerulus responds to injury, an understanding of glomerular development is indispensable. Glomerular development proceeds through a sequence of structures described as vesicle, comma-shaped, S-shaped, capillary loop, and maturing glomerulus stages (9,25,26). The vesicle and comma-shaped stages were discussed previously. At the S-shaped stage, the lower limb beneath the vascular cleft separates into two layers (lips) divided by a narrow developing Bowman's space (Figure 34.6). Lining the upper, internal lip are the visceral epithelial cells, which will differentiate into podocytes. On the opposite side of Bowman's space, the cells of the lower, outer lip will become the parietal epithelial cells lining Bowman's capsule.
 |
Figure 34.8 Nephrogenic zone from developing kidney at 26 weeks of gestation illustrating several stages of nephron formation. |
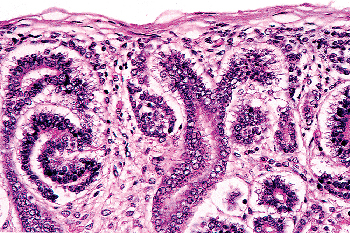 |
Figure 34.9 Higher magnification of same field as in Figure 34.7. In the center, pretubular aggregates (early renal vesicles) are present on either side of the ureteric duct. An early S-shaped body is present (left). |
During the S-shaped stage, microvessels can often be identified within the vascular cleft of the S-figure. Because this vascular cleft is the site where the glomerular capillaries emerge, the origin of the microvessels has generated considerable study. A long-standing question has been whether the glomerular endothelial cells have an angiogenic or a vasculogenic origin. Earlier evidence favored the process of angiogenesis, whereby endothelial cells sprout from external vessels that grow into the kidney. More recent studies
P.845
provide compelling evidence for a vasculogenic mechanism, whereby endothelial cells of the early glomerular capillaries originate from intrinsic angioblasts, likely derived from the metanephric mesenchyme (27,28). Release of growth factors, such as vascular endothelial growth factor (VEGF), from the immature podocytes may attract the angioblasts, expressing VEGF receptors (as Flk1) into the vascular clefts. Other signaling systems such as the angiopoietin (ligand)-Tie (receptor) axis also play a role in endothelial cell and vascular development (29). At this stage, the endothelial cells contain few fenestrae. The early podocytes are cuboidal or columnar, whereas the parietal epithelial cells are already flattening. Situated between the endothelial and podocyte layers are two basement membranes. The basement membrane beneath the podocytes is usually thicker and more continuous than the one underneath the endothelial cells.
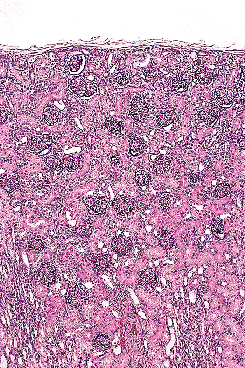 |
Figure 34.10 Newborn kidney (40 weeks of gestation). Note the absence of a nephrogenic zone. Some glomeruli are near the renal capsule. |
During the capillary loop stage, the capillaries start to fill out into an expanding Bowman's space. The endothelial cells flatten and develop numerous fenestrae. The podocytes develop a complex cellular architecture as they become terminally differentiated and cease to undergo mitosis. They flatten and form cytoplasmic primary processes, which in turn, extend foot processes that interdigitate with those from adjacent podocytes and adhere to the developing glomerular basement membrane (GBM). Intercellular junctional complexes are present at the apical membranes between podocytes. With foot process development, these junctions migrate down the lateral surfaces of the emerging foot processes and disappear, when they are either replaced by or converted into the slit diaphragms (26). Slit diaphragms, specialized intercellular junctions, bridge the space between adjacent foot processes. The slit diaphragm is connected to the podocyte cytoskeleton as part of a multifunctional protein complex that includes nephrin, CD2-associated protein (CD2AP), and podocin (30,31). Nephrin is the protein encoded by the NPHS1 gene that is mutated in congenital nephrotic syndrome of the Finnish type, which is associated with loss of the slit diaphragm, abnormal foot processes, and massive proteinuria (32). Thus, the slit diaphragm is critical for maintaining podocyte architecture and the glomerular filtration barrier. The dual GBM, synthesized by both the endothelium and podocytes, is still present, but areas of fusion between the two membranes are found. Beginning during the capillary loop stage, a complex series of transitions in the GBM protein composition occurs. There is developmental switching of both type IV collagen and laminin isoforms in the GBM, events which are essential for forming normal glomerular capillaries (26,33).
Glomeruli in the maturing stage resemble adult glomeruli by histology but are smaller in diameter. The podocytes of the maturing glomeruli may have a cuboidal appearance. A single fused GBM predominates, and areas of dual unfused basement membranes are rarely seen. At this time, the synthesis of components for the GBM is largely by the podocytes. In areas where foot process interdigitation is continuing, irregular outpocketings of basement membrane are found beneath the podocytes. These outpocketings, or loops, reflect newly synthesized GBM that will be deposited into the existing GBM.
The development of the mesangium occurs relatively later in glomerulogenesis. Although the mesangial cells likely derive from the metanephric mesenchyme, their origin is not entirely clear. The mesangial cell precursors are distinct from the VEGF receptor expressing angioblasts that differentiate into the glomerular endothelial cells. However, the emergence of mesangial cells in the glomerulus is dependent on platelet-derived growth factor-B (PDGF-B), produced by podocytes and endothelial cells, and its receptor, PDGF receptor-B (PDGF-RB), expressed on mesangial cells (34).
Development of the Juxtaglomerular Apparatus
In the human mesonephros, a complete juxtaglomerular apparatus has not been observed, although renin-expressing cells have been noted (35). Renin expression in the metanephric kidney has been detected as early as eight weeks of gestation, and renin mRNA levels are significantly higher in the developing kidney than in the adult organ (35,36). In the developing kidney, renin-expressing cells are found in intrarenal arteries, including the arcuate and interlobular arteries. As development progresses, the distribution of renin-expressing cells shifts from the larger vessels to the juxtaglomerular apparatus primarily to the terminal afferent arteriole in the mature kidney (37,8). However, within the afferent arterioles themselves, heterogeneous patterns of renin expression exist (39). The juxtaglomerular (JG) cell, as a cellular component of the mature juxtaglomerular apparatus, is located in the wall of the terminal afferent arteriole close to the glomerulus. Studies of the embryonic origin and lineage of JG cells have demonstrated that JG cells originate from renin-expressing precursor cells of the metanephric blastema rather than an extrarenal source (40). Furthermore, studies have provided in vivo genetic evidence that renin precursor cells, in addition to differentiation into JG cells, can also differentiate into non renin-expressing cells, such as vascular smooth muscle cells and glomerular mesangial cells (41).
Development of the Interstitium
Compared to other parenchymal components, there is far less known about the interstitium (stroma) in kidney development. In addition to providing a structural framework around the other components, an emerging view is that the developing interstitium plays an essential role in nephron and collecting duct differentiation (42,43,44). A traditional view is that cells of the metanephric mesenchyme not induced by the ureteric bud will become interstitial (stroma) cells. However, it is now believed that stroma cells arise
P.846
from different cell lineages within the metanephric mesenchyme and also separate from the mesenchyme. A loose stroma containing spindle-shaped cells surrounds the early ureteric bud branches and early nephrons and is known as the primary interstitium (or clear-cell type stroma). As nephrogenesis proceeds, a cortical interstitium and a medullary interstitium, each with distinct cellular phenotypes, forms. Although the interstitial cells resemble fibroblasts, dendritic cells, macrophages, or lymphocytes according to morphologic and immunophenotypical findings, their origins and functions are mysterious (45,46,47). There is accumulated evidence to indicate that signals emanating from cells in the interstitium as well as the renal capsule are essential for normal nephron and collecting duct development (42,43,44). These important stromal cell expressed molecules include Foxd1, RAR , RAR 2, FGF-7, BMP4, Pod1 and Pbx1 (Table 34.1).
Structural fibers extending between the ureteric bud ampulla and the renal capsule may represent a morphologic correlate that in part mediates the signals between the developing collecting duct and the capsule (48).
Apoptosis
In addition to prominent cell proliferation in the nephrogenic zone of the cortex, cell proliferation also contributes to the differentiation of the medullary tubules (49,50). Considering the widespread nature of apoptosis during embryologic development, it is not surprising that it occurs in kidney organogenesis. However, unlike the well-known function of apoptosis in the formation of nonwebbed digits, the biologic role of apoptosis in kidney development is not as apparent. During normal nephrogenesis, apoptosis has been observed within uninduced metanephric mesenchyme (51,52), comma-shaped bodies (53), developing tubules (52,54,55), stromal cells surrounding the tubules (51), and immature glomeruli (56). As shown by several genetic defects in signaling between the mesenchyme and ureteric bud (Table 34.1), the metanephric mesenchyme is programmed to undergo massive apoptosis if it fails to be induced by the ureteric bud. At later stages of kidney development, apoptosis plays a role in remodeling the cell composition of medullary collecting ducts (54) and in the differentiation of the loops of Henle (55). Intercalated cells, involved in urine acidification, are removed from the developing medullary collecting duct by apoptosis or simple extrusion from the epithelium (Figures 34.11,34.12) (54). Also, in developing glomeruli, endothelial cells are removed by apoptosis during capillary lumen formation (55), and apoptosis can be observed in the parietal epithelium during glomerular development (Figure 34.13).
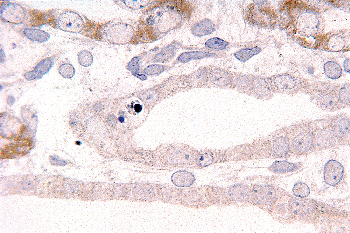 |
Figure 34.11 Developing medullary collecting duct in a postnatal kidney. After etching with sodium methoxide, toluidine blue is removed from normal nuclei but remains in the nuclear fragments of apoptotic bodies (magnification 300; courtesy of Dr. Jin Kim). |
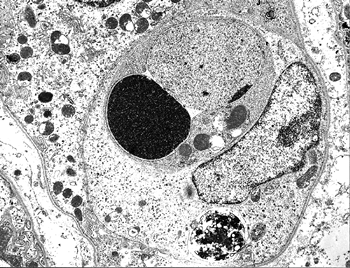 |
Figure 34.12 Electron micrograph of a postnatal medullary collecting duct illustrating a phagocytosed apoptotic body composed of a nucleus with condensed chromatin and organelle remnants (magnification 1200; courtesy of Dr. Jin Kim). |
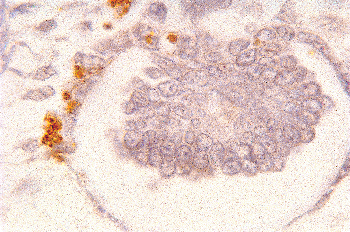 |
Figure 34.13 Micrograph from cortical nephrogenic zone of a fetal kidney demonstrating labeling for apoptosis in parietal epithelium of a developing glomerulus. Cells with fragmented DNA are identified by a Tdt-mediated dUTP-biotin nick end-labeling (TUNEL) immunoperoxidase method. (Courtesy of Dr. Jin Kim.) |
P.847
There are disparate findings considering the quantitative scale of apoptosis during nephrogenesis (52,57). However, a role for apoptosis during nephrogenesis is further evidenced by the fact that mice deficient for Bcl-2, the major antiapoptotic regulator, have fulminant apoptosis of the metanephric mesenchyme, develop multiple cysts, and die of renal failure (58). Considering the above findings and the complex morphogenetic processes occurring during nephrogenesis, it seems likely that apoptosis plays a role in sculpting the kidney.
Molecular Regulation of Kidney Development
To make a kidney requires an orchestration of numerous complex cellular and molecular events. Several experimental approaches and model systems have enhanced our understanding of kidney development. A variety of in vitro studies have been valuable, including organic culture of metanephric rudiments, pioneered by Grobstein (7), and cell cultures of individual nephrogenic lineages. Gene targeting studies such as gene ablation in mice ( knockout mice) have provided powerful in vivo evidence for the role of certain genes in nephrogenesis. A detailed review of the molecular aspects of nephrogenesis is beyond the scope of this chapter, but several reviews are available (9,10,11,12,59,60,61). Table 34.1 outlines some of the genes involved in mammalian kidney development, their encoded protein functions, their normal expression in the developing kidney, and the mutant kidney phenotypes that result from their ablation in mice. In addition, the known human syndromes caused by naturally occurring mutations in these genes are noted. The genes are listed under the cellular process (specification of nephrogenic mesenchyme, cell survival, cell proliferation, ureteric bud branching, mesenchymal-to-epithelial transition, glomerulogenesis and nephron differentiation) in which they are believed to have an important function (60). Where some of the genes are temporally positioned (upstream or downstream to one another) in the functional cascade of kidney organogenesis remains to be determined.
Compelling evidence exists to indicate that some genes, for example WT1, Pax 2, and Pod1, play multiple roles during nephrogenesis. Note that these particular genes are placed under more than one nephrogenic cellular process in Table 34.1. The listing of the genes in Table 34.1 is based predominantly on in vivo genetic evidence. The generation of knockout mice using homologous recombination in embryonic stem cells has been very informative. However, embryonic lethality and pleiotropic phenotypes may occur, preventing an analysis of the significance of the targeted gene in nephrogenesis. Creating conditional knockout mice by disrupting genes only in specific renal cell types using site-specific DNA recombinase systems (e.g., Cre-lox P system) will provide important insights into kidney development (62). Moreover, some of the mutant kidney phenotypes will serve as valuable models for human disease.
In the near future, a list similar to Table 34.1 will be significantly lengthened. There will be a greater appreciation for the multiple roles that some genes have during nephrogenesis. However, our understanding of the cellular and molecular events that underlie the dynamics of kidney formation will be enhanced. In addition to a morphogenetic role during kidney organogenesis, some genes have an important physiologic function in the adult kidney. Also, the tabulated genes and their encoded proteins will no doubt be useful as lineage- or cell-specific markers to study renal diseases, including tumors.
Gross Anatomy
Kidney Position and Blood Supply
Upon formation, the metanephric kidneys are situated close to each other in the pelvis at the level of the upper sacrum. Between the sixth and ninth weeks of gestation, the kidneys are found further apart and at higher levels in the abdomen until they reach their final upper lumbar position (178). This ascent of the kidneys is believed to result largely from differential growth of the caudal part of the embryo away from the kidneys (179,180). However, others have argued that the cephalad movement of the kidneys is active and not caused by differential growth of the vertebral column (181). With this migration, the renal hilum, where the main vessels enter and exit, rotates from a ventral orientation to face anteriomedially. Initially, the kidneys receive their blood supply from branches of the common iliac arteries. With their ascension, the kidneys are supplied by arteries originating from progressively higher levels of the distal aorta (178). The question of whether some of these vessels anastomose in a periaortic plexus is not well studied (182). As the ascending kidneys receive new branches from the aorta, the older, caudal branches undergo involution. Persistence of these inferior vessels may result in accessory renal arteries. The most cephalad branches arising from the abdominal aorta become the permanent main renal arteries.
Kidney Weight and Configuration
The various reference values reported for fetal and neonatal kidney weights correspond relatively closely, despite potential variability due to factors such as the social and economic status and the level of health care in a given population (183,184,185,186,187,188). Separate values from nonmacerated and macerated cases are available (188), as well as values using prefixation (187,188) and postfixation weighing (183,186) of the kidneys. Data for the combined weight (right and left) of the kidneys during the second
P.848
P.849
P.850
P.851
P.852
P.853
P.854
and third trimesters are shown in Figure 34.14. Different reference values published for combined kidney weights during infancy and childhood also favorably compare (183,189). The data of Emery and Mithal (183) are illustrated (Figure 34.15).
Table 34.1 Genes Involved in Kidney Development | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
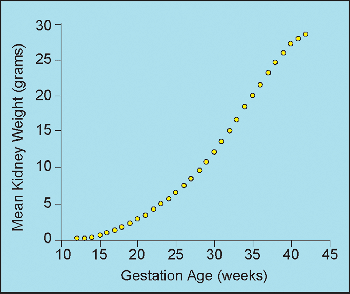 |
Figure 34.14 Mean combined (right and left) weight of kidneys from second and third trimester fetuses and neonates. Modified with permission from: Hansen K, Sung CJ, Huang C, Pinar H, Singer DB, Oyer CE. Reference values for second trimester fetal and neonatal organ weights and measurements. Pediatr Dev Pathol 2003;6:160 167. |
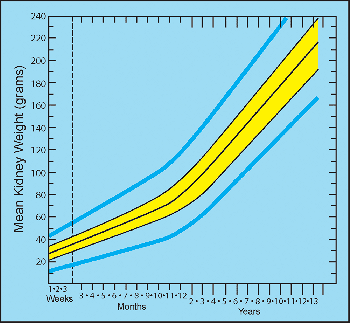 |
Figure 34.15 Mean combined (right and left) weight of kidneys at various postnatal ages. The middle black line represents the means. The 50th percentile (yellow band) and 95th percentile (blue lines) ranges are shown. Modified with permission from: Emery JL, Mithal A. The weights of kidneys in late intra-uterine life and childhood. J Clin Pathol 1960;13:490 493. |
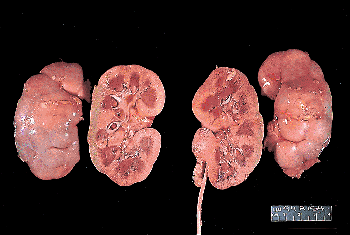 |
Figure 34.16 Gross appearance of newborn kidneys. The rounded configuration with a relatively deeper sinus, characteristic of the infantile kidney, is seen on the sectioned surface. Fetal lobations are prominent on the external surface. |
The newborn kidney has a shorter, more rounded configuration than that of the adult. The upper and lower poles project further medially, so the renal sinus is relatively deeper in the infant (Figures 34.16,34.17) (190). The renal sinus of infants contains much less fat and connective tissue than in the adult, and the cortical septa (columns) of Bertin approach much closer to the pelvic-calyceal system. Figure 34.17 illustrates the process of unrolling of the renal poles during childhood, as the kidney assumes the more elongated configuration observed in adults. This change in configuration produces a shallower renal sinus with partial exteriorization of the pelvis. As the pelvic-calyceal system assumes a more exterior position, it also becomes displaced further from the parenchyma lining the sinus. The additional space created between the pelvic-calyceal system and the cortical columns of Bertin is normally filled with fat, which increases in quantity as the kidney approaches maturity.
Fetal Lobations
A renal lobe consists of a medullary pyramid and its surrounding cortical parenchyma (Figures 34.7, 34.16). The centrilobar cortex covers the base of a pyramid, whereas the septal cortex surrounds the sides of a pyramid. Thus, a septum (column) of Bertin represents the confluence of two layers of septal cortex from two adjacent lobes. Although a lobe does not represent a functional renal unit, it may be viewed as an anatomic organizational unit (191). Lobation begins in the human kidney at six to seven weeks and proceeds to maximun development with an average of 14 lobes at 28 weeks of gestation (192,193). At this stage, generally 14 papillae and calyces are present, corresponding with the same number of lobes. Deep clefts on the surface separate the lobes. After the twenty-eighth week, a process of variable lobar fusion decreases the number of surface fissures, papillae, and calyces. The degree of calyceal fusion is greater than papillae fusion. In full-term infant kidneys, the mean
P.855
number reported for calyces is 9 and for papillae, it is 11 (193). Considerably more lobar fusion, creating compound papillae, occurs in the polar regions than in the midpolar region, where simple papillae are more likely to be retained.
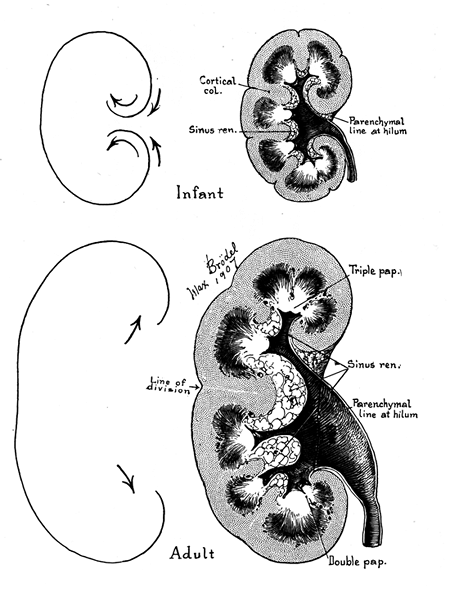 |
Figure 34.17 Max Br del's classic illustration of the process of unrolling of the kidney in postnatal life (from Kelley HA, Burnam CF. Diseases of the Kidneys, Ureters, and Bladder. Vol.1. New York: Appleton; 1925 ). The rounded configuration of the infantile organ becomes elongated as the upper and lower poles diverge and pelvic-calyceal structures are partially everted from their original position within the renal sinus. The space thus created within the renal sinus is filled by fat, which is far more abundant in the adult kidney than in the infant kidney. |
The surface of the neonatal kidney is divided into polygons by prominent fissures that correspond roughly, although not precisely, to the lobar outlines (Figure 34.16). These fetal lobations usually decrease in prominence with advancing age, persisting longer on the ventral surface than the dorsal surface of the organ. Although there is considerable individual variation in the chronology of their disappearance, they are usually inconspicuous by 4 to 5 years of age (194). The only valid generalization is that fetal lobations usually diminish in number and prominence in the first few years of life, but they remain apparent, especially on the ventral surfaces, in a significant proportion of adult kidneys. In one study, one or more interlobar fissures were detected in up to 50% of adult kidneys (193). In older children and adults, it is important to distinguish persistent fetal lobations from cortical scars. Fetal lobation is a more accurate term than fetal lobulation because a lobule is an architectural feature of the cortex, primarily observed on the histologic level.
Histology
Cortical Architecture
The developing renal cortex has unique temporal and spatial features of organization. Each generation of nephrons, most easily observed histologically by the glomeruli, forms a layer over the preceeding generation. The earliest formed nephrons are situated in the inner cortex (juxtamedullary) near the future medulla, whereas the nephrogenic zone in the outer cortex (superficial) contains the last nephrons formed. Between the thirty-second and thirty-sixth weeks of gestation, nephron formation ceases and the nephrogenic zone disappears. Although the nephrogenic zone persists in kidneys of children born prematurely (195,196), evidence suggests that postnatal nephrogenesis is suboptimal and does not occur after 40 days (197).
The histologic features of the developing renal cortex have been used as an index of fetal maturation (196). The ratio of the width of the nephrogenic zone to the width of the remaining cortex decreases in a linear fashion as the birth weight increases (185,198). This approach has been used to detect infants with reduced intrauterine growth in whom this ratio is less than expected for the birth weight. Using glomeruli as representative of nephrons, studies have employed counting the number of layers (or rows) of glomeruli from inner to outer cortex as a method to evaluate nephrogenesis (199,200,201). Each successive layer is assumed to represent a new glomerular generation. These estimates are performed on well-oriented sections that are orthogonal to the cortex and display a well-defined corticomedullary junction.
The studies are in fair agreement between gestations of 24 to 36 weeks, in which the average number of rows (nephron generations) of glomeruli are as follows: 5 to 7 (24 weeks), 8 to 9 (28 weeks), 9 to 10 (32 weeks), and 10 to 14 (36 weeks). After the full complement of nephrons is attained by the normal fetus, subsequent renal growth reflects hypertrophy and maturation of the nephrons. As tubules elongate and increase in diameter, they become interposed between glomeruli. The glomeruli in the outer cortex of newborns are crowded together (Figure 34.10), whereas the older, more mature glomeruli deeper in the cortex are more widely separated. This process of tubular growth not only separates glomeruli from one another, but it also tends to separate them from the cortical surface near where they originally developed. In a normal term newborn, one observes many glomeruli very close to the renal capsule (Figure 34.10). By about 2 months of age, the process of nephron growth has begun to separate the outermost glomeruli, and a narrow zone largely devoid of glomeruli develops beneath the renal capsule (Figure 34.18). This latter zone has been termed the cortex corticis (202). The cortex corticis becomes progressively wider during childhood (Figure 34.19). Although it is normal to find an occasional glomerulus adjacent to the
P.856
capsular surface in normal infants and children, the presence of numerous very superficial glomeruli suggests defective renal growth during late fetal or early postnatal life. Abnormally crowded glomeruli also can be an important clue to defects in nephron growth and differentiation, which may involve only the outer cortex or the entire cortical mantle. Thus, the cortical architecture may be viewed as a record of the developmental history of the kidney.
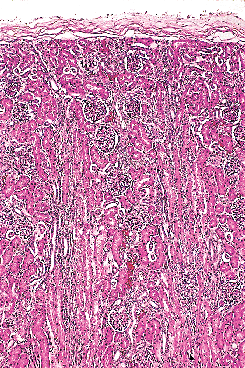 |
Figure 34.18 Renal cortex at 2 months of age. Glomeruli in the outer cortex are becoming more widely spaced due to tubular elongation. Superficial glomeruli are beginning to separate from the renal capsule, the first indication of the cortex corticis. |
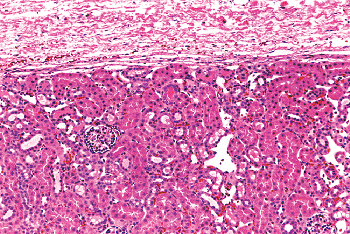 |
Figure 34.19 Micrograph of well-developed renal cortex corticis. The zone without glomeruli beneath the distinct renal capsule is evident. Reprinted with permission from: Murphy WM, Grignon DJ, Perlman EJ. Tumors of the kidney, bladder, and related urinary structures. In: Atlas of Tumor Pathology. 4th series, fascicle 1. Washington, DC: Armed Forces Institute of Pathology; 2004. |
The cortex is subdivided into distinctly demarcated lobules by radially oriented groups of tubules termed medullary rays that extend from the base of the pyramids upward into the cortex (Figure 34.20). A lobule is defined as the cortical domain surrounding a medullary ray. Despite their name, the medullary rays (of Ferrein) actually are part of the cortex and contain the straight segments of the proximal tubule, the thick ascending limbs, and the collecting ducts. Medullary rays usually extend to near the cortical surface in infants but not in older children. The presence of complete medullary rays is a good indicator that the plane of a given section is perpendicular and reflective of the true thickness of the cortex. Medullary ray nodules are complex tangled tubular configurations commonly seen in the medullary rays of infants during the early months of life, being most prominent between 1 and 6 months of age (Figure 34.21) (203). These structures apparently represent a normal transitory developmental phenomenon of variable prominence, which is pathologic only when extreme.
Nephron Number
The number of nephrons in a kidney is determined in utero. The final number is dependent on gestational age and a favorable intrauterine environment. Unbiased, precise stereological methods have been used to count glomeruli, which serve as a surrogate for nephron number. One study of human intrauterine renal growth revealed the glomerular number increased from 15,000 at 15 weeks of gestation to 740,000 by 40 weeks (204). The greatest rate of nephron induction has been observed between 15 to 17 weeks of
P.857
gestation; however, approximately 60% of the total nephrons are believed to form during the third trimester (204). Nephron number, inferred from glomerular number estimates in children and adults without renal disease, is strongly correlated with birth weight (205). Intrauterine growth retardation has been shown to impair nephron formation, as measured in fetuses and in infants dying within a year of birth (206,207).
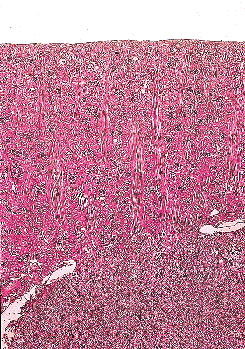 |
Figure 34.20 Kidney at 21 months of age. This perpendicularly oriented section illustrates the full length of several medullary rays that extend from the corticomedullary junction to a level near the cortex corticis. Each medullary ray marks the center of a cortical lobule. |
 |
Figure 34.21 Medullary ray nodule. In the center of the micrograph, a tangled cluster of collecting ducts forms a nodule near the midportion of a medullary ray. This structure is usually transitory, being uncommon in the first month of life and extremely rare after 1 year of age. |
It is apparent that a kidney having a quantitative abnormality, such as low nephron number, may not demonstrate an obvious defect of nephron spatial topography on histologic examination. However, studies have demonstrated an inverse correlation between the number of glomeruli and mean glomerular volume, suggesting glomeruli increase in size to compensate for an innate low nephron number (205,207). Large glomeruli may be susceptible to scarring. Glomerulomegaly has been used as an adverse factor to assess the risk of disease progression in childhood nephrotic syndrome (208). Thus, increased glomerular size (volume or area) may be useful as an indicator for nephron deficiency in individuals susceptible to renal disease.
Until a few years ago, each human kidney was believed to contain one million nephrons. It is now appreciated that there is a remarkably wide variation in total nephrons per kidney among normal adults, ranging from as low as 227,000 to over 2,000,000 (209,210,211). Nephron endowment is programmed in the perinatal period, capping the nephron number in an individual's lifetime. Some time ago, Brenner et al. (212) postulated that an inborn deficit of nephrons predisposes to acquired renal disease, including hypertension, in adults. A study showing that hypertensive individuals had fewer nephrons but a larger glomerular volume than age-matched normotensive controls supports this hypothesis (213). The endowment of nephrons from nephrogenesis and the developmental origins of renal disease are fertile areas for investigation.
Glomerular Maturation and Growth
Newly formed glomeruli are structurally distinctive, and their evolution toward a mature form is a gradual process. Because the period of glomerular development spans a six- to seven-month period of fetal life, a spectrum of maturational stages is normally present in infant kidneys. As mentioned before, this spectrum is organized in a temporal-spatial manner in the cortex. Familiarity with normal glomerular maturation can facilitate an assessment of the renal developmental status in infants. Dramatic changes in glomerular structure and size occur through the early months and years of life. For convenience of study, several stages of glomerular development have been defined in studies (214,215,216,217). Figure 34.22 shows representative glomeruli from the midcortical region of infants and children from birth to 9 years of age to illustrate the maturational changes. Figure 34.22A shows the characteristic appearance of recently formed glomeruli from a term newborn infant. In addition to their small size, the most obvious distinctions from mature glomeruli are the simple character of the tufts with relatively few capillary loops and the layer of cuboidal cells surfacing the visceral layer of the tuft. This cuboidal layer, representing developing podocytes, is often continuous and covers most of the circumference of the tufts. Ultrastructurally, these primitive podocytes are closely approximated to one another and often lack foot processes (Figure 34.23A). The glomerular basement membrane (GBM) is thin and two lamina densa structures, representing basal laminae produced by endothelial cells and podocytes prior to their fusion into a single lamina, may be focally observed. The thickness of the GBM increases progressively with age (Figure 34.23). The approximate values for GBM thickness during childhood range as follows: 100 to 130 nm in fetal kidneys; 170 nm ( 30) at birth; 208 nm ( 24) at 1 year; 245 nm ( 49) at 2 years; 268 nm ( 43) at 6 years; and 300 nm ( 42) by 10 years (216,218,219). The growth rate is greatest prior to 2 years of age. In contrast to adults, the GBM thickness in children appears less sex dependent (206). The data of Volger and colleagues, illustrating the increase in GBM (Figure 34.24A) and lamina densa widths (Figure 34.24B) with age are shown. Further GBM growth in adolescence, which is less documented, must occur to reach the adult GBM thickness in men (373 nm 42) and women (326 nm 45) (220).
P.858
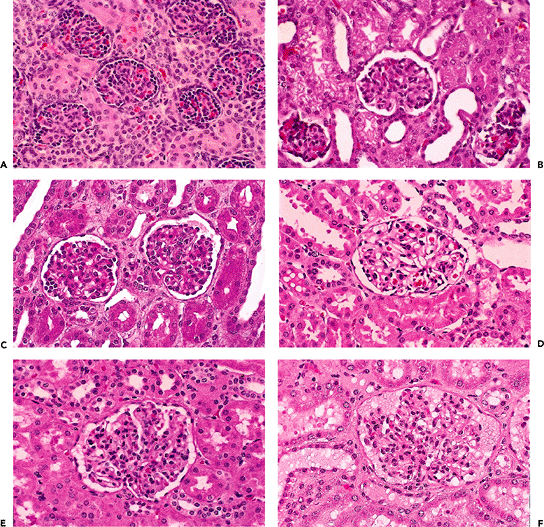 |
Figure 34.22 Normal midcortical glomeruli of six infants and children from birth to 9 years of age. All the micrographs were taken at the same magnification. A. Newborn. B. 6 months. C. 11 months. D. 21 months. E. 5 years. F. 9 years. |
P.859
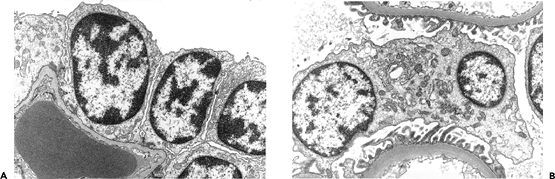 |
Figure 34.23 A. Electron micrograph of a normal glomerulus from an infant 2 months of age. Note the continuous layer of primitive podocytes lacking foot processes and the thin glomerular basement membrane. B. Mature glomerulus from a patient 16 years of age, photographed at the same magnification as in A. There is prominent foot process development, and the glomerular basement membrane is distinctly thicker than in the infant. (Courtesy of Dr. Gary W. Mierau.) |
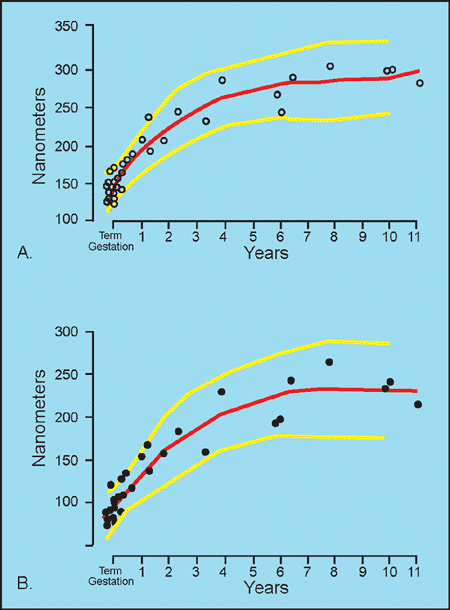 |
Figure 34.24 Thickness of glomerular basement membrane (GBM) in children. A. Total thickness of GBM. B. Thickness of lamina densa (LD) portion of GBM. The thickness of both the total GBM and the LD increases rapidly in early childhood and slows in growth after 3 years of age. The mean GBM and LD thicknesses (red lines) and the 95th percentile ranges (yellow lines) are shown. Modified with permission from: Volger C, McAdams J, Homan SM. Glomerular basement membrane and lamina densa in infants and children: an ultrastructural evaluation. Pediatr Pathol 1987;7:527 534. |
The continuous cuboidal layer of cells is transient as the maturing podocytes flatten over the surfaces of the developing capillaries. A few small clusters of cuboidal podocytes remain in infant glomeruli (Figure 34.22 B C). After a glomerulus has been in existence for more than 12 months, remnants of the cuboidal layer are usually not seen in normally developed glomeruli. In 1940, Gruenwald and Popper suggested that some podocytes may be sloughed into Bowman's space as part of the maturation process (214). In fact, several studies have demonstrated that podocytes are shed from the glomerulus and excreted in the urine in various glomerular diseases, as well as in healthy individuals (221,222). Moreover, most of the urinary podocytes are viable as evidenced by their ability to grow in culture under in vitro conditions. Whether podocyturia occurs in the normal neonate and infant remains to be established. The above podocyte alterations are coincident with the emergence of more conspicuous capillary loops (Figure 34.22 C D). Eventually, the capillary loops are arranged into lobules (Figure 34.22 E F). However, an occasional glomerulus with the small size and immature appearance of a neonatal glomerulus may be observed in older infants, especially in the outer one-third of the cortex (Figure 34.25).
Glomerular growth during childhood has been evaluated in several investigations (223,224,225,226,227). In these studies, the source of renal tissue (postmortem or biopsy specimen), the observational technique (histology or microdissection), and the morphometric method varied. However, it is well established that glomerular size increases from birth into adolescence. Spouster and Emery (225) reported that the midcortical and juxtamedullary mean glomerular area in fetuses actually decreased between 12 and 20 weeks gestation. After this initial decrease, the
P.860
glomeruli remained at the same size until birth, after which they steadily grew. Moore et al. (226) observed that the mean glomerular diameter in normal children increased from 112 to 167 m between birth and 15 years of age, averaging 3.6 m per year during this period. Akaoka et al. (227) found that the mean glomerular tuft area in children with minimal change nephrotic syndrome and recurrent hematuria, increased from 6,600 m2 to 11,000 m2 between 2 and 15 years of age. In the latter study, the glomerular capillary lumina area did not correlate with the glomerular tuft area, whereas the number of capillaries per glomerulus showed a positive correlation with the glomerular tuft area. Although some of the glomeruli were not normal in this study, the findings support the concept that glomerular growth occurs by an increase in the number or length of capillaries rather than by hemodynamic capillary dilatation.
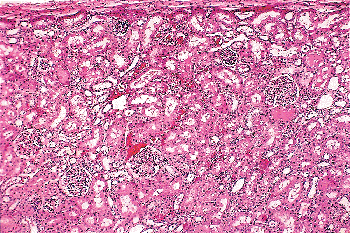 |
Figure 34.25 Persistent immature glomeruli at 12 months of age. Two miniature glomeruli with cuboidal cells at the periphery of the tuft are present near the renal capsule. Small numbers of defective glomeruli can be found in infant kidneys, but they are destined to undergo sclerosis and involution. |
Juxtamedullary glomeruli are larger than superficial glomeruli at birth and during infancy. However, some uncertainty exists regarding these regional differences in glomerular size in later childhood and young adults. Some authors have observed no size difference between juxtamedullary and superficial glomeruli by the fourteenth to thirty-sixth postnatal month (223,225), whereas others have found a size difference persists until at least 15 years of age (224,226). Methodological differences in the studies or the wide variation in glomerular size existing among individuals (228) may account for these disparate findings.
Investigations using stereological methods have provided estimates for the number of cells in glomeruli. Steffes et al. (229) observed that the total number of cells per glomerulus increased along with the mean glomerular volume in comparing normal individuals younger than 20 years of age to those greater than 20 years. The number of endothelial cells and mesangial cells increased with age, whereas the number of podocytes remained unchanged with age. These results are consistent with the large amount of animal data indicating that mature podocytes are largely terminally differentiated and do not replicate. This concept is also supported by the expression pattern of cell cycle regulatory proteins during glomerulogenesis (230,231,232). The proliferation marker Ki-67 is expressed in podocyte precursors in comma- and S-shaped bodies, but its expression is markedly reduced in podocytes of the glomerular capillary loop stage. The cyclin-dependent kinase (CDK) inhibitors p27 and p57 are absent in the comma- and S-shaped bodies but expressed in maturing podocytes of the capillary loop stage, as well as in mature podocytes in adult kidneys. These findings suggest the CDK inhibitors are involved with arresting the cell cycle of podocytes at the capillary loop stage and maintaining the fully mature podocytes in a quiescent differentiated state.
Early Juxtamedullary Glomeruli
The maturational process in the very early generations of glomeruli may be accelerated because, even in very young fetuses, these juxtamedullary glomeruli rarely possess a cuboidal layer and are considerably larger than their immediate superficial neighbors. Attention was drawn to these large juxtamedullary glomeruli in humans by Kampmeier, who noted that they subsequently disappeared, suggesting that they were transient structures (233). Tsuda observed in human fetuses that the diameter of juxtamedullary glomeruli were nearly twice that of superficial glomeruli as early as three months of gestation (199). Emery and Macdonald noted that the disappearance of these large glomeruli, in the early months after birth, was associated with the presence of scarred glomeruli in the same region (234). These findings support Kampmeier's suggestion that they represent a transient population of nephrons. It is presumed that these precociously formed glomeruli are functionally important during fetal life and likely undergo involution early in the postnatal period. Relatively little study has been made of these interesting structures.
Glomerulosclerosis in Infants
Glomerulosclerosis in infantile kidneys is commonly observed. In most cases, it is a presumably normal phenomenon that must be distinguished from the pathologic changes of glomerular disease. In 1909, Herxheimer concluded that sclerotic glomeruli usually represented defective development of glomeruli in otherwise normal kidneys and were not manifestations of a disease process (235). Other investigators have suggested this context of glomerulosclerosis might result from a vascular origin, excretion of toxic substances, or renal infection (236,237).
Emery and Macdonald conducted thorough studies of glomerulosclerosis in children's kidneys that were
P.861
considered morphologically within normal limits (234). Their series of 475 cases included kidneys from fetuses at 24 weeks of gestation to children 15 years of age. The percentage of sclerotic glomeruli in each kidney was most often in the range of 1 to 2% (65% of cases), although higher percentages from 3 to 10% affected glomeruli (30% of cases) were observed. The proportion of infants having sclerotic glomeruli was age dependent. Scarred glomeruli occurred in 25 to 40% of kidneys from late fetuses and newborns. They were detected in 70% of kidneys by 2 months of age, remaining near this level throughout the first year. Afterwards, their incidence steadily declined and was about 10% of children at 6 years of age.
Emery and Macdonald found that sclerotic glomeruli localized to two areas; the deep inner cortex (juxtamedullary zone near the arcuate vessels) and the superficial outer cortex (near the capsule). Sclerotic glomeruli in the juxtamedullary zone were more common in the first six months of life than in older children. Affected glomeruli in the outer cortex were most prominent in the first two years after birth. The presence of scarred glomeruli in the juxtamedullary zone coincided with the disappearance of the large glomeruli seen in this region during the months after birth, as discussed previously. Thus, it appears the sclerosing glomeruli in the juxtamedullary zone represent the involution of the large glomeruli that localize to this zone during nephrogenesis. The glomerular scarring in the outer cortex near the capsule likely reflects a different etiologic process that, if linked to nephrogenesis, is probably occurring relatively late. In a study of 800 infant kidneys, Thomas reported a similar distribution pattern of sclerotic glomeruli in the cortex (238). The general consensus is that these lesions are defects of development but without functional or clinical significance. Their major significance for pathologists is that they not be interpreted as evidence of glomerular disease, unless their number is considerably above the usual range (greater than 20%) mentioned above.
A typical example of infantile glomerulosclerosis is illustrated in Figure 34.26. The scarred glomeruli may occur singly or in small groups. They are usually smaller than normal, immature in appearance, and variably hyalinized. The afferent arteriole is often thickened, and periglomerular fibrosis may be present as well as some chronic inflammatory cells in the interstitium. In later stages, only a small globule of hyalin material in a small focus of sclerosis without capillary lumens may be seen. The tubules associated with the sclerotic glomeruli often contain proteinaceous material and apparently disappear along with the glomeruli.
Ectopic Glomeruli
In the kidneys from fetuses and infants, glomeruli are often found outside the confines of the renal parenchyma, either in the renal sinus (Figure 34.27) or in the connective tissue around interlobar vessels. These ectopic glomeruli occur in several mammalian species as well as in young humans (239). They appear to degenerate during postnatal life and are not found in adult human kidneys. It has been suggested that some vessels supplying the pelvic mucosa and medulla may be derived from degenerated ectopic glomeruli (239,240). The ectopic glomeruli may represent the early large juxtamedullary glomeruli, described by Kampmeier, that have persisted at least until infancy rather than degenerate.
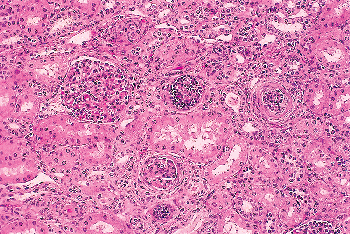 |
Figure 34.26 Infantile glomerulosclerosis at 9 months of age. One small, developmentally immature glomerulus is seen near the center, and two adjacent glomeruli are undergoing involutional sclerosis. |
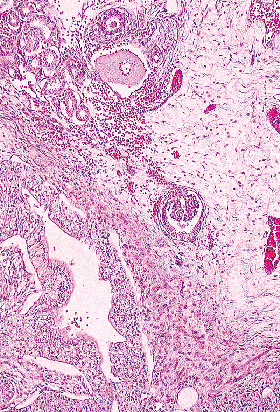 |
Figure 34.27 Ectopic glomerulus in the renal sinus. |
P.862
Tubular Maturation and Growth
There is less known about the maturation and growth of tubules between birth and adulthood compared to glomeruli. Although all tubular segments increase in size during postnatal maturation, the proximal convoluted tubules undergo very prominent elongation and increased tortuosity (5). The results of microdissection studies reported from two laboratories were fairly similar (223,241). The mean lengths of proximal tubules observed were: about 2 mm at birth, 3.5 mm at 3 months, 6.5 mm at 1 year of age, 7.7 mm at 2 years, and 12.0 mm at 12 years. The mean proximal tubular length of 20 mm in adult kidney indicates that proximal tubule elongation continues through adolescence and young adulthood. Proximal tubules at birth are less uniform in size than glomeruli. Proximal tubules in the outer cortex of the newborn kidney were observed to be the shortest, those in the midcortex intermediate in length, and those from the juxtamedullary cortex the longest, consistent with a centrifugal pattern of development (223). These regional differences in proximal tubules decreased significantly after 1 month of age and disappeared by 14 months. Whether tubular function, such as solute and volume reabsorption, of the maturing proximal tubules from different cortical regions also follows a centrifugal pattern of maturation remains uncertain (242). It is interesting that in the adult kidney, the proximal tubules from the outer cortex have been observed to be longer than those from the mid- and juxtamedullary cortex (223). The ratio of glomerular surface area to proximal tubular volume was proposed as a theoretical anatomic correlate of functional glomerulotubular balance, that is, the balance between the capacity of the glomerulus to filter and the tubule to reabsorb the filtrate (223). Values for this ratio (28 in the term newborn kidney, 13 in the 3-month kidney, 6 in the 6-month kidney, and 3 in the adult kidney) suggested morphologic dominance of glomeruli over tubules early in life until the tubules, in effect, grew up to their glomeruli (223). However, it is difficult to correlate these morphologic ratios with experimental functional data indicating proportionate increases in glomerular filtration rate (GFR) and proximal tubule reaborption after birth, consistent with maintenance of glomerulotubular balance during postnatal maturation (243).
During postnatal maturation, the loops of Henle undergo striking elongation (5). The newborn kidney lacks a well-formed inner medulla and contains loops of Henle that are relatively short (2,4,5). At birth, the loops of Henle are shortest in the younger nephrons, whereas longer loops belong to the older nephrons. As the kidney increases in size after birth, the loops of Henle elongate, the medullary interstitium increases, and the medulla becomes separated into outer and inner zones. From birth until full maturation, loops of Henle may increase in length as much as three-fold. In the mature kidney, the location of the tips of the loops of Henle relates to the age of their associated nephrons. The loops of the last-formed nephrons (outer cortex) reach the junction between the outer and inner medulla and are known as short loops of Henle. In contrast, the loops of the earliest formed nephrons (juxtamedullary cortex) extend deep into the inner medulla near the tip of the papilla and are referred to as long loops of Henle. Prior to birth, thin portions of loops of Henle are only observed in the long loops of Henle. These thin portions are present in the descending limbs of the long loops of Henle and continue to lengthen after birth. Descending thin limbs are not seen in short loops of Henle until after birth. Only long loops of Henle develop ascending thin limbs, which are derived from the thick ascending limbs, likely by an ascending process of apoptotic remodeling (55,244).
Adult Kidney
Gross Anatomy
The kidneys lie within the retroperitoneum and extend from the twelfth thoracic (T12) to the third lumbar (L3) vertebrae, with the right kidney usually slightly more caudad. They are situated within the perirenal space, which contains abundant fat and is traversed by fine fibrous septae (245,246,247). Visualization of the renal fascia with radiologic procedures has been reported in normal individuals (248,249). Each kidney weighs 125 to 170 grams in men and 115 to 155 grams in women (250). If differences in body build are considered, kidney weight correlates best with body surface area, whereas age, sex, and race have less influence (251). Each kidney is 11 to 12 cm in length, 5 to 7.5 cm in width, and 2.5 to 3 cm in thickness. The left kidney tends to be slightly larger and may demonstrate irregularities of the lateral contour from compression by the spleen in up to 10% of normal individuals (252). A glistening tough fibroelastic capsule surrounds the kidney.
In the hilar region, the main renal artery branches to form anterior and posterior divisions (Figure 34.28), which in turn divide into segmental arteries that supply the apical, upper, middle, lower, and posterior segmental regions of the parenchyma (253,254). No collateral circulation has been demonstrated between the segmental arteries. Some of the so-called accessory arteries actually represent normal segmental arteries with an early origin from the main-stem renal artery or aorta (254). Therefore, ligation of such a segmental artery in the belief that it is an accessory vessel results in necrosis of the corresponding parenchymal segment. The intrarenal veins do not follow a segmental distribution, and there are numerous anastomoses of the veins throughout the kidney. There are variable drainage patterns of the large extra-renal veins that join to form the main renal vein (255). A relatively common occurrence is a posterior primary venous tributary, whose retropelvic
P.863
position should be remembered during renal surgical intervention. An outer pale region (the cortex) and an inner darker region (the medulla) can be distinguished on the cut surface of a bisected kidney. The presence of glomeruli and convoluted tubules results in the cortex having a more granular appearance.
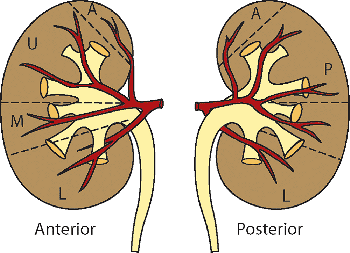 |
Figure 34.28 Diagram of the vascular supply of the human kidney. The anterior division of the renal artery divides into three segmental branches that supply the upper (U) and middle (M) segments of the anterior surface and most of the lower (L) segment. The small apical (A) segment is usually supplied by a branch from the anterior division. The posterior division of the renal artery supplies the posterior (P) segment, which represents more than half of the posterior surface of the kidney. Modified with permission from: Graves FT. The anatomy of the intrarenal arteries and its application to segmental resection of the kidney. Br J Surg 1954;42:132 139. |
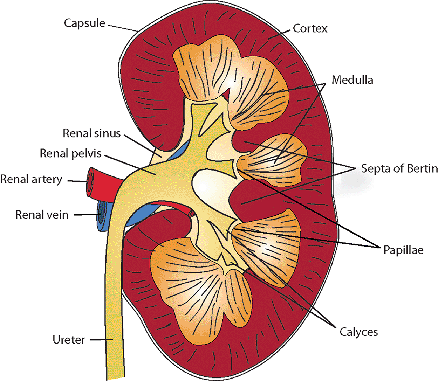 |
Figure 34.29 Diagram of a bisected kidney illustrating major anatomic structures. |
The human kidney is a multipapillary type of mammalian kidney (256), with the medulla divided into 8 to 18 striated conical masses called pyramids (Figure 34.29). The striated appearance reflects the parallel linear orientation of the loops of Henle and collecting ducts. The base of each pyramid is located at the corticomedullary junction, whereas the apex extends toward the renal pelvis, forming a papilla. The tip of each papilla is perforated by 20 to 70 small openings (6) that represent the distal ends of the collecting ducts (of Bellini). The cortex is about 1 cm in thickness, encircles the base of each pyramid, and extends downward between pyramids to form the septa (columns) of Bertin. Despite well-described radiologic features (257,258), an enlarged septum of Bertin on occasion has been clinically mistaken for a renal tumor. Longitudinal striations extending from the base of the pyramids out into the cortex are termed the medullary rays (of Ferrein). Regardless of their name, they are actually part of the cortex and are formed by the straight segments of the proximal tubules (PSTs), the thick ascending limbs (TALs), and the collecting ducts. The medullary rays may be visualized during excretory urography in conditions with tubular fluid stasis (259).
A single pyramid with its surrounding cortical parenchyma constitutes a renal lobe (191,260) (Figure 34.30). The human kidney has an average of 14 lobes (102). During development, variable lobar fusion leads to coalescence of some papillae and remodeling of the corresponding calyces, gradually reducing the number of papillae and calyces. The mean number of calyces and papillae
P.864
reported is 9 and 11, respectively (193). There is a greater degree of lobar fusion in the polar regions than in the midpolar region of the kidney. A degree of persistent fetal lobation may be observed in some adult kidneys.
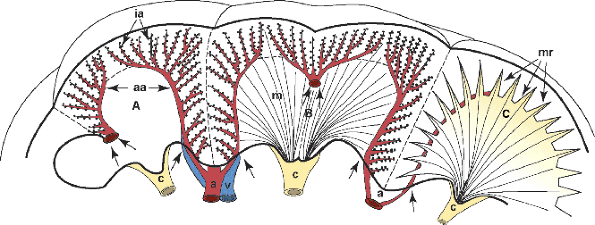 |
Figure 34.30 Diagram of three renal lobes. A. Arcuate (aa) and interlobular (ia) arteries. B. Cortex and medulla (m) are illustrated in a double lobe with fused double papillae. C. Lobe showing medullary rays (mr). A septum of Bertin represents the approximation of two layers of septal cortex from two adjacent lobes. (Small double arrows in A and B, subsidiary septal arteries; single arrows, location where arcuate vessels enter the renal parenchyma; a, interlobar arteries; v, interlobar vein; c, calyces). Modified with permission from: Hodson CJ. The renal parenchyma and its blood supply. Curr Probl Diagn Radiol 1978; 7:1 32. |
There are two main types of renal papillae (261). Simple papillae drain only one lobe and have convex tips containing small, often slitlike orifices. Compound papillae drain two or more adjacent fused lobes and have flattened, ridged, or concave tips with round, often gaping orifices. The distribution of papillae types within the kidney is related to the embryologic pattern of fusion involving the lobes, papillae, and calyces (Figure 34.31). It is believed that the more open orifices of compound papillae are less capable of preventing intrarenal reflux (262), which may be associated with an increase in intrapelvic pressure. This concept is supported by the observation that pyelonephritic scars associated with intrarenal reflux are present more commonly in the renal poles, where the compound papillae predominantly occur.
The renal pelvis is the saclike expansion of the upper ureter. Two or three outpouchings or major calyces (infundibula) extend from the pelvis and divide into the minor calyces, into which the papillae protrude. In addition, elaborate leaflike extensions, termed fornices, extend from the minor calyces into the medulla, and secondary pouches increase the pelvic surface area (263).
The renal sinus is located on the medial or concave aspect of each kidney (Figure 34.32) (264,265). It contains the renal pelvis, the major renal arteries and veins, the lymphatics, and neural structures that supply the kidney. The renal hilus is the entry into the sinus. Fat fills the renal sinus
P.865
and is contiguous with the perirenal fat. Within the renal sinus, the renal capsule does not enclose the cortical parenchymal surface. Beckwith called attention to the importance of the renal sinus as a pathway for tumor dissemination in Wilms' tumor (266), and this has also been shown in renal cell carcinomas (267,268). A detailed description of the gross anatomy of the kidney is provided elsewhere (9).
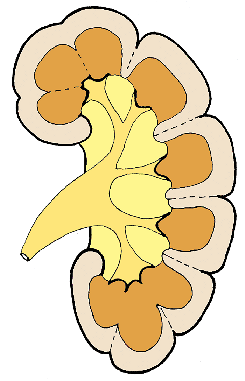 |
Figure 34.31 Schematic representation of the lobar architecture. In the polar regions, there is a greater degree of lobe fusion, resulting in the formation of compound papillae and calyces and the loss of septal cortex. The individual lobes tend to be retained in the midpolar region, and the septal cortex extends between renal pyramids, as septa of Bertin, to the renal sinus. Modified with permission from: Hodson CJ. The renal parenchyma and its blood supply. Curr Probl Diagn Radiol 1978; 7:1 32. |
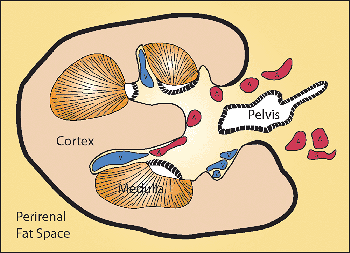 |
Figure 34.32 Diagram of kidney cross section, showing the renal sinus which is filled with fat (yellow). The renal sinus contains the pelvic-calyceal system and the major renal vessels. The renal capsule (thick black line) surrounds the convex surface of the kidney but disappears from the cortical surface as the latter enters the renal sinus. The cortical surfaces, including the septa of Bertin, facing the renal sinus lack a capsule. The capsule surrounding the pelvic-calyceal system is indicated by a cross-hatched thick black line. The capsule covering the calyces, appears to extend over the medullary pyramids on cross section, and it envelopes the renal pelvis as well. Modified with permission from: Murphy WM, Grignon DJ, Perlman EJ. Tumors of the kidney, bladder, and related urinary structures. In: Atlas of Tumor Pathology. 4th series, fascicle 1. Washington, DC: Armed Forces Institute of Pathology; 2004. |
Nephron
The structural and functional unit of the kidney is the nephron, which consists of the renal corpuscle (glomerulus and Bowman's capsule), proximal tubule, thin limbs, and distal tubule, all of which originate from the metanephric blastema. The total number of nephrons in a human kidney varies markedly among normal individuals (209,210,211). Although a tenfold variation in nephron number has been reported, the usual range is about 600,000 to 1,200,000 nephrons per kidney. Nephrons can be classified according to the position of their glomeruli in the cortex or the length of their loop of Henle (Figure 34.33). In the former scheme, superficial, midcortical, and juxtamedullary nephrons are distinguished. Superficial nephrons have glomeruli located in the outer cortex, and their efferent arterioles usually ascend to the cortical surface. The glomeruli of juxtamedullary nephrons are located immediately above the corticomedullary junction in the inner cortex, and their efferent arterioles form the descending vasa recta. The glomeruli of midcortical nephrons are situated in the midcortex above the juxtamedullary region but below the superficial nephrons.
In the more commonly used classification, there are two main populations of nephrons: those with a short loop of Henle and those with a long loop. The length of the loop of
P.866
Henle is generally related to the location of its parent glomerulus in the cortex. The short loops form their bend at various levels within the inner stripe of the outer medulla, whereas the long loops of Henle enter and turn back within the inner medulla. Although there are numerous gradations between these two main types of nephrons, there are seven times more short than long loop nephrons in human kidneys (269). A correlation between the urinary concentrating ability and the relative length of the medulla has been established in several mammalian kidneys (270).
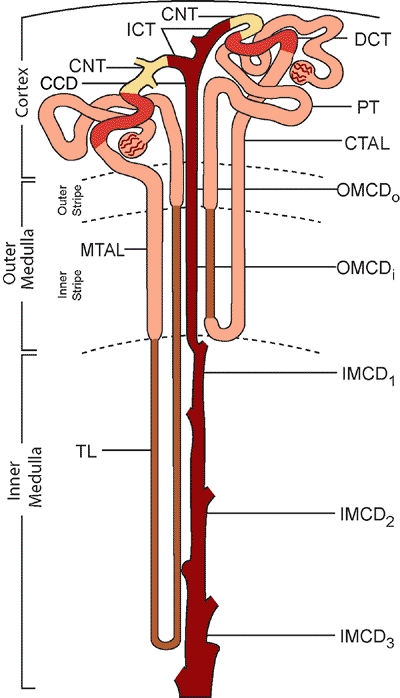 |
Figure 34.33 Diagram illustrating the segments of the nephron and the zones of the kidney. (PT, proximal tubule; TL, thin limb of Henle's loop; MTAL, medullary thick ascending limb; CTAL, cortical thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; ICT, initial collecting tubule; CCD, cortical collecting duct; OMCDo, collecting duct in outer stripe of outer medulla; OMCDi, collecting duct in inner stripe of outer medulla; IMCD1, outer third of inner medullary collecting duct; IMCD2, middle third of inner medullary collecting duct; IMCD3, inner third of inner medullary collecting duct. Modified with permission from: Madsen KM, Tisher CC. Structural-functional relationships along the distal nephron. Am J Physiol 1986;250(pt 2):F1 F15. |
The connecting tubule, a transitional segment, joins the nephron to the collecting duct system, which originates from the ureteric bud. Although not correct in a strict anatomic sense, for practical considerations, the term nephron is commonly used to include the connecting segment and entire collecting duct. The collecting duct system can be divided into the cortical, outer medullary, and inner medullary collecting ducts (271,272). Structural and functional heterogeneity exist along the nephron (273). Internephron heterogeneity refers to the differences between analogous segments in superficial and juxtamedullary nephrons. Intranephron or axial heterogeneity may be defined as the differences between early and successive later portions of an individual nephron segment.
Architecture
The renal cortex can be divided into lobules. A renal lobule consists of a centrally positioned medullary ray and its surrounding cortical parenchyma, containing all nephrons draining into the collecting ducts of the medullary ray. In contrast to lobules of other organs, renal lobules are not distinctly separated by fine connective tissue septa; therefore, they are difficult to distinguish histologically. Furthermore, because it has been difficult to establish any structural-functional significance, the concept of the renal lobule is not commonly used.
The nephron segments and blood vessels in the cortex and medulla have a specific geometric arrangement (274). This intricate architecture allows integration (axial) of complex transport functions along the length of a specific nephron segment, as well as integration (regional) between different nephron segments in a specific region or zone (275).
Two architectural regions of the renal cortex can be distinguished: the cortical labyrinth and the medullary rays (Figure 34.34). The cortical labyrinth represents a continuous parenchymal zone that surrounds the regularly distributed medullary rays. Glomeruli, proximal and distal convoluted tubules, interlobular vessels (also termed cortical radial vessels), and a rich capillary network are situated in the cortical labyrinth. The large majority of convoluted tubular profiles are proximal tubules. Connecting tubules of juxtamedullary nephrons fuse and form so-called arcades, which are adjacent to the interlobular vessels within the cortical labyrinth. Individual nephrons with their interlobular vessels, glomeruli, and attached tubular segments, are difficult to distinguish in this complex topography by histology. The medullary rays (Figures 34.35,34.36) contain the proximal and distal straight tubules and collecting ducts, all of which enter into the medulla. The distal straight tubules are the thick ascending limbs. Within an individual medullary ray, the straight tubules of superficial nephrons are situated centrally, the straight tubules of midcortical nephrons are localized peripherally, and the collecting ducts occupy a position between the two groups. The straight tubules of juxtamedullary nephrons decend directly into the medulla without ever entering the medullary rays.
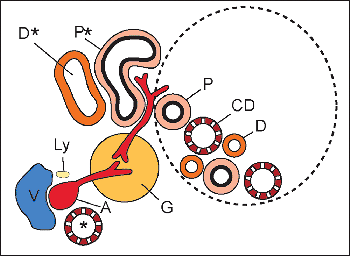 |
Figure 34.34 Diagram of architectural regions of the renal cortex. A medullary ray is encircled by the dotted line, and the cortical labyrinth is outside the dotted line. The proximal straight (P) and distal straight ascending limb (D) tubules and the collecting ducts (CD) are located in the medullary ray. The adjacent cortical labyrinth contains the interlobular vessels: arteries (A), veins (V), and lymphatics (Ly); arcades (*) of connecting tubules; glomeruli (G); and the proximal (P*) and distal (D*) convoluted tubules. Modified with permission from: Kriz W, Kaissling B. Structural organization of the mammalian kidney. In: Seldin DW, Giebisch D, eds. The Kidney: Physiology and Pathophysiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:587 654. |
The localization of specific segments of the nephrons at various levels in the medulla account for the division of the medulla into an outer and inner zone, with the former subdivided into an inner and outer stripe (Figure 34.33). The relative tissue volumes for the cortex and the outer and inner medulla are 70, 27, and 3% respectively (272).
The outer stripe of the outer medulla is relatively thin. It contains the terminal portions of the proximal straight tubules, the thick ascending limbs, and the collecting ducts. The outer stripe is also distinguished by the absence of thin limbs of Henle. Glomeruli are not present in the medulla. In contrast to the outer stripe, the inner stripe of the outer medulla is thicker. It contains thin descending limbs, thick ascending limbs, and collecting ducts. It is further characterized by the absence of the proximal straight tubules. Aggregations of descending and ascending vasa recta, known as vascular bundles, develop in the
P.867
outer stripe but are located predominantly in the inner stripe. Compared with the kidneys of some mammals with very high urine concentrating ability, the human kidney has a simple medulla (274). In contrast to the complex medulla, the vascular bundles of the simple medulla do not fuse to form larger vascular structures, and they do not incorporate the descending thin limbs of short loops (Figure 34.37). The inner medulla contains the thin descending and thin ascending limbs of long loops, as well as the collecting ducts. Thick ascending limbs are absent in the inner medulla.
 |
Figure 34.35 Longitudinal section of cortex demonstrating two linear aggregates of tubules representing medullary rays (original magnification 50). |
 |
Figure 34.36 Cross section of cortex illustrating medullary rays that are regularly distributed within the cortical labyrinth (original magnification 50). |
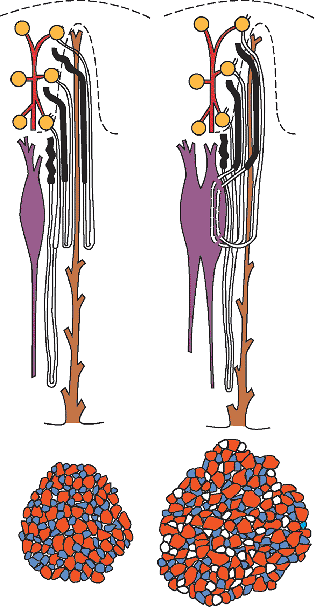 |
Figure 34.37 Schematic diagram demonstrating the simple and complex types of medulla. Upper left: In the simple medulla, the loops of Henle remain separate from the vascular bundle (purple). The vascular bundle itself (lower left cross section) contains only descending (red) and ascending (blue) vasa recta. Upper right: In the complex medulla, the descending thin limbs (DTLs) of short loops of Henle descend within the vascular bundles (purple), which tend to fuse. Therefore, the complex bundles (lower right cross section) contain the DTLs of short loops (white) in addition to descending (red) and ascending (blue) vasa recta. Modified with permission from: Jamison RL, Kriz W. Urinary Concentrating Mechanism: Structure and Function. New York: Oxford University Press;1982. |
Parenchyma
An accurate morphologic evaluation of the kidney requires a detailed systematic examination of the glomeruli, tubules, interstitium, and blood vessels of the renal
P.868
parenchyma. A standard nomenclature for structures of the kidney exists (276). A detailed approach to the histopathologic evaluation of the kidney has been described (277), and technical guidelines for handling the renal biopsy are available (278,279). The following discussion emphasizes normal morphologic aspects and structural-functional relationships in the kidney. The reader is directed to detailed discussions for more information (274,280).
Glomerulus
Overview
In 1666, Malpighi first described the glomeruli and demonstrated their continuity with the renal vasculature (281,282). About 175 years later, Bowman elucidated in detail the capillary architecture of the glomerulus and the continuity between its surrounding capsule and the proximal tubule (283,284). The renal corpuscle consists of a tuft of interconnected capillaries and an enclosing capsule named after Bowman. The term glomerulus is commonly used to refer to the glomerular capillary tuft and Bowman's capsule, although the term renal corpuscle is more accurate in a strict anatomic sense. The glomerulus does not simply represent a ball of capillaries. Providing structural support for the capillary tuft is a central region termed the mesangium, which contains cells and their surrounding matrix material. The capillaries are lined by a thin layer of endothelial cells, contain a basement membrane, and are covered by epithelial cells (also called podocytes) that form the visceral layer of Bowman's capsule. The parietal epithelium is continuous with the visceral epithelium at the vascular pole where the afferent arteriole enters the glomerulus and the efferent arteriole exits. The glomerulus somewhat resembles a blind-pouched extension (Bowman's capsule) of the proximal tubule invaginated by a tuft of capillaries (Figure 34.38) (285). The cavity situated between the two epithelial layers of Bowman's capsule is called Bowman's space, or the urinary space. At the urinary pole, this space and the parietal layer of Bowman's capsule continue into the lumen and epithelium of the proximal tubule. The glomerular tuft originates from the afferent arteriole, which enters the glomerulus at the vascular pole and divides into several lobules. Anastomoses are believed to exist between individual capillaries within a lobule, as well as between lobules (274,286,287).
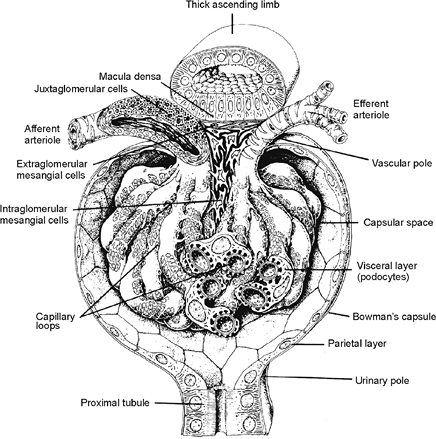 |
Figure 34.38 Schematic three-dimensional representation of the glomerulus. Modified with permission from: Geneser F. Textbook of Histology. Philadelphia: Lea & Febiger; 1986. |
The efferent arteriole is formed by rejoined capillaries and leaves the glomerulus at the vascular pole. In contrast to the afferent arteriole, the efferent arteriole has a more continuous intraglomerular segment. The glomerulus is responsible for the ultrafiltration of plasma. The glomerular filtration barrier consists of the fenestrated endothelium, the peripheral glomerular basement membrane (GBM), and the slit diaphragms between the podocyte foot processes.
P.869
The glomerulus has a round configuration and an average diameter of about 200 m (274,280). Although the diameter of juxtamedullary glomeruli has been reported up to 20 to 50% greater than that of superficial glomeruli (274), other researchers have found no significant size difference between these glomerular populations in the normal adult kidney (288). It has been reported that the glomeruli in solitary functioning kidneys are significantly larger than those in control patients with two kidneys (289). Although the glomerulus has a lobular architecture, the lobulation is often inconspicuous in light microscopic sections. An accentuated degree of lobulation may be more prominent in autopsy kidneys than in biopsy specimens (277). An apparent increase in the number of glomerular cells can be observed with an increased section thickness, therefore an accurate assessment of glomerular cellularity requires histologic sections 2 to 4 m thick (Figure 34.39). Generally, the presence of more than three cells in a mesangial area away from the vascular pole constitutes hypercellularity. The delicate character of the glomerular capillary walls can be observed on thin histologic and frozen sections (Figure 34.40) (290).
Global glomerulosclerosis may occur as part of aging, without renal disease. The mean percentage of global glomerulosclerosis in normal kidneys was reported as follows: less than 1% between 1 and 20 years of age; 2% between 20 and 40 years; 7% between 40 and 60 years; and 11% between 60 and 80 years (291,292,293). There are no differences between the sexes in the development of glomerulosclerosis in aging humans (294). The 90th percentile for global glomerulosclerosis may be generally estimated for any age by subtracting 10 from half the patient's age (293).
Endothelial Cells
A thin fenestrated endothelium lines the glomerular capillaries. By light microscopy, the endothelial cells have light eosinophilic cytoplasm and slightly oval nuclei. Their nuclei are present within the capillary lumina. The endothelial cells are extremely attenuated around the capillary lumen, and the thicker portions of the cells containing the nuclei lie adjacent to the mesangium away from the urinary space. The cytoplasm contains microtubules, microfilaments, and intermediate filaments (295). The attenuated portion of endothelial cytoplasm is perforated by fenestrae 70 to 100 nm in diameter (286). It has been argued that endothelial fenestrae do not simply arise from fusion of plasma membrane invaginations, called caveolae (296). It has been widely stated that glomerular endothelial fenestrae lack diaphragms, but thin bridging diaphragms have been observed when certain methods of fixation are used (297). The endothelial cell surface carries a negative charge because of the presence of polyanionic glycoproteins, including podocalyxin, a major sialoprotein of glomerular endothelial cells and podocytes (298,299). Results from recent studies have suggested that glomerular endothelial cells have a glycocalyx surface layer about 60 nm thick, and this surface coat fills the fenestrae forming sieve plugs (300,301). There is emerging evidence that the glomerular endothelial glycocalyx may be an important component of the glomerular filtration barrier (302,303). Vascular endothelial growth factor (VEGF), produced by podocytes, is an important regulator of glomerular endothelial cell function; VEGF induces fenestrae and increases permeability of endothelial cells, both in vivo and in vitro (304,305).
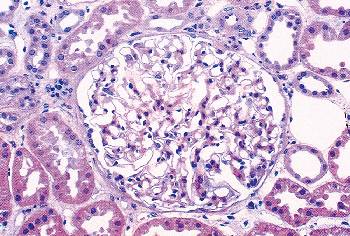 |
Figure 34.39 Glomerulus exhibiting round configuration and normal cellularity (original magnification 250). |
 |
Figure 34.40 Fluorescent micrograph of an H&E-stained frozen section. Note the delicate character of the glomerular capillaries. (Courtesy of Dr. Stephen M. Bonsib.) |
Current evidence indicates that maintenance of glomerular endothelial differentiation appears dependent on podocyte-derived VEGF (306). Glomerular endothelial cells synthesize nitric oxide (NO) and endothelin-1, a vasoconstrictor (307). There is strong expression of CD34 in glomerular endothelial cells (Figure 34.41).
Mesangial Cells
The mesangium, composed of mesangial cells and their surrounding matrix, is observed as a periodic acid-Schiff
P.870
(PAS)- and methenamine silver-positive structural support for the glomerular capillary loops (Figure 34.42). By light microscopy, the mesangial cells usually can be distinguished by their mesangial location and dark-staining nuclei. Ultrastructurally, they are irregular in shape and have elongated cytoplasmic processes that may extend between the endothelium and the glomerular basement membrane. The mesangial cell processes have microfilaments that contain actin, myosin, and -actinin (308,309). With smooth muscle contractile properties, the mesangial cell has been proposed to be a specialized pericyte that likely modulates glomerular filtration (310). Whereas the endothelium forms a continuous layer around the inner circumference of the glomerular capillary, the basement membrane and the visceral epithelial cell layer do not completely encircle the capillary but enclose the mesangial matrix and cells between the capillaries (Figure 34.43). The mesangial matrix is similar but not identical to the GBM and contains several types of collagen, as well as fibronectin and small proteoglycans. The matrix is especially rich in microfibrils, unbranched, noncollagenous structures that contain fibrillins and microfibril-associated glycoproteins (MAGPs) (311,312). The presence of microfibril-mediated attachments between mesangial cell processes and the GBM suggests that the mesangial cell and the GBM represent a biomechanical functional unit (313,314,315). It has been proposed that the contractile apparatus of the mesangium appears to maintain the structure of the capillary walls by counteracting the distention caused by the intracapillary hydraulic pressure (316). The mesangial cell also has phagocytic capability and plays a role in the clearance of macromolecules and debris from the mesangium (317). Mesangial cells can respond to, as well as generate, a variety of molecules, including interleukin-1, platelet-derived growth factor, and arachidonic acid metabolites, which may play a central role in the response to glomerular injury (318). It is recognized that a small subpopulation of cells in the mesangium are bone marrow-derived macrophages and play a role in immune responsiveness (Figure 34.44) (319,320,321). Occasional leukocytes are observed in the
P.871
normal glomerulus and label with leukocyte common antigen (CD45 and CD45RB) staining (322,323,324).
 |
Figure 34.41 Glomerular endothelium showing immunoreactivity for CD34. Some peritubular capillaries show staining along the upper border. (CD34 immunoperoxidase, original magnification 300.) |
 |
Figure 34.42 PAS-stained normal glomerulus illustrating the PAS-positive mesangium within the central regions of the glomerular capillaries (original magnification 250). |
 |
Figure 34.43 Schematic diagram illustrating the relationship between the mesangium and the glomerular capillaries. The visceral epithelial cell (podocyte) cytoplasm (red) and endothelial cell cytoplasm (yellow) are depicted. Note that the glomerular basement membrane (green) encloses the mesangium and its attached capillaries. The central mesangial cell is represented by dark brown cytoplasm and a black nucleus, and the mesangial matrix is represented by the tan fibrillar texture. The cytoplasmic processes of mesangial cells are connected to the glomerular basement membrane directly or indirectly by microfibrils in the mesangial matrix. Modified with permission from: Kriz W, Elger M, Lemley K, Sakai T. Structure of the glomerular mesangium: a biomechanical interpretation. Kidney Int Suppl 1990;30:S2 S9 . |
 |
Figure 34.44 KP-1 immunohistochemical stain illustrating that a small percentage of cells in the mesangium label as tissue monocytes. (KP-1 immunoperoxidase, original magnification 210.) |
Interestingly, it has been demonstrated that mesangial cells residing in the extraglomerular mesangial region of the juxtaglomerular apparatus (JGA) may migrate and repopulate the intraglomerular mesangium upon glomerular injury (325).
Glomerular Basement Membrane
The glomerular basement membrane (GBM) can be demonstrated on light microscopy by PAS and methenamine silver stains (Figure 34.45). The silver preparation is more specific. Examination of a normal peripheral capillary loop away from the vascular pole and the mesangium shows a delicate basement membrane (Figure 34.46). Hematoxylin and eosin (H&E) and PAS preparations may stain capillary luminal contents and the cytoplasm of the endothelial and podocyte cell layers, resulting in an apparent thickening of the GBM. The basement membrane is situated between the endothelium and the podocytes in the glomerular capillary wall. On ultrastructural examination, the GBM consists of a central dense layer (the lamina densa) and two surrounding thinner electron-lucent layers (the lamina rara interna and the lamina rara externa). In comparison with laboratory animals, the electron-lucent layers appear less prominent in the human glomerulus (Figure 34.47). This may be due in part to different fixation conditions.
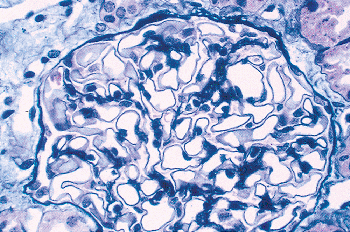 |
Figure 34.45 Silver methenamine-stained normal glomerulus illustrating the silver-positive glomerular basement membrane and positive basement membrane of Bowman's capsule (original magnification 500). |
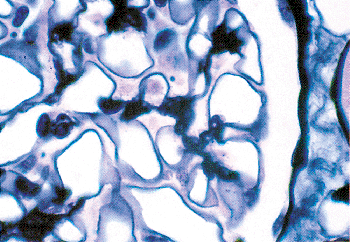 |
Figure 34.46 Higher magnification micrograph demonstrating thin regular glomerular basement membranes of peripheral capillary loops. (Silver methenamine stain, original magnification 1250.) |
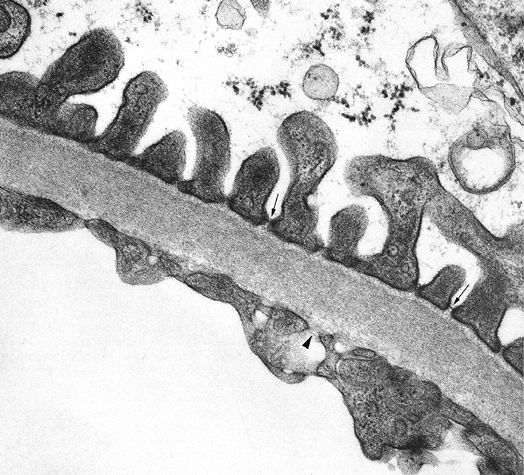 |
Figure 34.47 Transmission electron micrograph of a glomerulus. Bowman's space is above and the capillary lumen is below the glomerular capillary wall. A fenestra or pore (arrowhead) in the endothelium is evident. Note the regular alignment of the foot processes of the podocytes. Filtration slit diaphragms (arrows) are present between individual foot processes. The glomerular basement membrane consists primarily of the lamina densa. The electron-lucent lamina rara interna and lamina rara externa are not prominent (original magnification 48,000). |
P.872
The adult GBM ranges between 310 and 380 nm in mean thickness (326,327,328). It is significantly thicker in men (mean 373 nm) than in women (mean 326 nm) and increases in width until the fourth decade of life (220). Quantitative data on the normal adult glomerular capillary structure include the following values: mean glomerular volume of 1.38 106 m3, average capillary diameter of 6.75 m, and capillary filtration surface/glomerulus of 200 103 m2 (329).
The major components of the GBM include type IV collagen, laminin, nidogen (also called entactin), and proteoglycans, including perlecan and agrin (26). Collagen IV is the major constituent of the GBM. Six chains, 1(IV) through 6(IV), make up the collagen IV protein family (330,331). Three chains of collagen IV self-associate to form triple-helical molecules called protomers. Despite many possible combinations, the six chains of collagen IV form only three types of protomers, which are designated as 1. 1. 2(IV), 3. 4. 5(IV), and 5. 5. 6(IV). The triple helical protomers unite at the noncollagenous domain (NC1) at the carboxy terminus to form hexamers, which in turn, assemble to form a polymerized network that serves as a scaffold for integration of other GBM components (330,331). Three canonical sets of hexamers form three distinct networks in basement membranes: the 1. 1. 2(IV) 1. 1. 2(IV), the 3. 4. 5(IV) 3. 4. 5(IV), and the 1. 1. 2(IV) 5. 5. 6(IV) networks (332). The 3. 4. 5(IV) 3. 4. 5(IV) network predominates in the adult GBM, and mutations of the genes encoding the 3, 4, and 5(IV) chains cause Alport syndrome (333). In Goodpasture syndrome, autoantibodies are targeted to the 3(IV) chain (333).
Laminins are heterotrimers composed of three chains; , and . Laminin-11, containing the 5, 2, and 1 chains, is the major laminin isoform in the adult GBM (33). Nidogen/entactin binds to laminin and is present in the GBM (334). Proteoglycans consist of glycosaminoglycan (GAG) chains bound to a core protein (335). They are primarily responsible for the negative charge of the GBM (336,337). Of three heparan sulfate proteoglycans, agrin (338) and collagen type XVIII (339) are more abundant in the GBM than is perlecan (340). Bamacan, a chondroitin sulfate proteoglycan, is present in the immature GBM but disappears from the adult GBM, remaining in the mesangial matrix (341).
Podocytes
The podocytes (visceral epithelial cells) are the largest cells in the glomerulus. By light microscopy, they are positioned on the outside of the glomerular capillary wall, often bulge into the urinary space, and have prominent nuclei and abundant light eosinophilic cytoplasm. Scanning electron microscopy shows that the podocytes have long cytoplasmic ramifications, the primary processes that surround the glomerular capillaries and divide into individual foot processes (Figure 34.48) (342). The foot processes cover the capillary wall, contact the lamina rara externa of the GBM, and interdigitate with foot processes from different podocytes. It is generally believed that the cell body and primary processes of the podocyte are not directly attached to the GBM and are suspended within Bowman's space. There is morphologic evidence for an even more complex cytoarchitecture of the podocyte. Neal et al. (343) demonstrated foot processes (termed anchoring processes) directly between the podocyte cell body and the GBM. Moreover, their study characterized three distinct compartments of Bowman's space. The subpodocyte space (SPS), first described by Gautier et al. in 1950 (344), exists as a restricted area under the podocyte cell body and is associated with a reported 60% of the glomerular filtration surface (343). The interpodocyte space (IPS) was characterized as a narrow anastomosing region interconnecting the subpodocyte space with the main peripheral Bowman's space (343). The physiologic role of these podocyte partitions of Bowman's space remains to be clarified. By transmission electron microscopy, the cells have abundant rough endoplasmic reticulum, a well-developed Golgi apparatus, and prominent lyosomes. There are numerous intermediate filaments, microtubules, and microfilaments in the cytoplasm (295). The intermediate filaments (vimentin, desmin) and microtubules predominate in the cell body and primary processes, whereas the foot processes contain a dense microfilament contractile apparatus (345,346,347). The latter, containing actin, myosin, -actinin, talin, and vinculin, connects to the intermediate filaments and microtubules of primary processes (348).
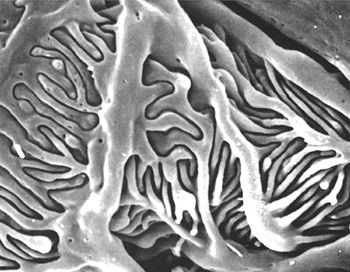 |
Figure 34.48 Scanning electron micrograph of glomerulus illustrating the primary processes of podocytes wrapping around the capillary loops. Note the interdigitation of the foot processes (original magnification 13,000; courtesy of Dr. Jill W. Verlander). |
This ultrastructural arrangement of contractile proteins may allow the podocytes to play an active role in modifying
P.873
the glomerular filtration surface area. Although the GBM is synthesized by both endothelial cells and podocytes, the latter play a greater role in the adult (349).
The adjacent foot processes near the GBM are separated by a 30- to 40-nm space termed the filtration pore (or slit), which is bridged by a thin extracellular structure called the filtration slit diaphragm. On ultrastructural examination, the slit diaphragm has always attracted the attention of pathologists. Over 30 years ago, based on electron microscopy, Karnovsky and co-workers proposed an isoporous zipperlike structural model for the slit diaphragm (350,351,352). In this model, a central filament, corresponding to the central dot of the diaphragm on cross section, is connected to the adjacent foot processes by spaced crossbridges, between which are rectangular pores. Recent three-dimensional reconstruction of the slit diaphragm by electron tomography has provided results that generally agree with the Karnovsky model (353). The electron tomographic study showed that the slit diaphragm consists of a network of winding strands, about 30 to 35 nm long, which merge centrally into a longitudinal density. The strands, which create a slit diaphragm thickness between 5 and 10 nm, surround pores that are the same size or smaller than albumin molecules. The pores appear more irregular than previously supposed.
The slit diaphragm appears unique but shares similarities with tight and adherens junctions (354,355). It separates the different membrane surfaces of the podocyte. Podocytes, similar to other epithelial cells, are polarized with distinct membrane domains (356) (Figure 34.49). The basal membrane domain and the apical membrane domain are located below and above the slit diaphragm, respectively. The slit diaphragm area (including the bridging diaphragm, adjacent podocyte foot process membrane, and cytoplasm) may also be considered a surface domain, a very specialized one.
A major advance in our understanding of the podocyte was the identification of the protein nephrin, encoded by NPHS1, the gene mutated in congenital nephrotic syndrome of the Finnish type (30,32). Nephrin, a transmembrane adhesion protein of the immunoglobulin superfamily, localizes to strands of the slit diaphragm (353,357). Lack of nephrin in humans or animals leads to the loss of the slit diaphragm, foot process effacement, and massive proteinuria (32,148). An increasing number of other proteins localize to the slit diaphragm domain, where they interact with nephrin and other partners, forming a multifunctional complex (Figure 34.50) (348,356,358,359,360). Some proteins are present in the actual slit diaphragm itself. For example, there is evidence nephrin and Neph1, a protein similar to nephrin, each form homodimers and form heterodimeric interactions with each other in the slit diaphragm (361,362,363,364).
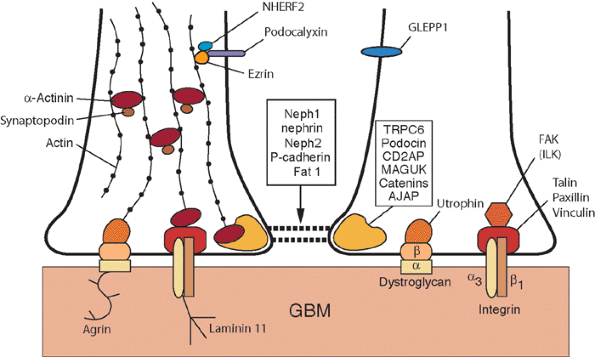 |
Figure 34.49 Schematic drawing of the membrane domains of the podocyte: the filtration slit diaphragm, basal membrane, and apical membrane domains. Molecular interactions within these domains of the podocyte foot processes are represented. See the text for further explanations. Modified from: Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 2003;83:253 307 ;Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 2001;108:1583 1587. |
Another member in the Neph protein family, Neph2, localizes to the slit diaphragm, homodimerizes, and forms heterodimers with nephrin but not with Neph1 (365). Other proteins are present in the podocyte cytoplasm or plasma membrane adjacent to the slit diaphragm. For example, members of the membrane-associated guanylate kinase (MAGUK) family of scaffolding proteins (including MAGI-1, MAGI-2, CASK, and ZO-1) localize adjacent to the slit diaphragm (358,359,360). These scaffolding proteins connect junctional membrane proteins to the actin cytoskeleton and signaling cascades. In addition, multiple adherens junction associated proteins (AJAP) (including -actinin, IQGAP1, II spectrin, and II spectrin) are also components of this expanding nephrin-associated multiprotein complex (358,359,360).
Mutations or deficiencies of genes encoding some of the proteins that comprise the slit diaphragm domain complex, including nephrin (32,148), Neph1 (155), Fat1 (156), podocin (150), CD2AP (149), -actinin (151,152), and TRPC6 (153,154), cause glomerular diseases in humans and animals as characterized by absent slit diaphragms, foot process effacement, and proteinuria (Table 34.1). In addition to functioning as a critical barrier to
P.874
filtration, the slit diaphragm appears to regulate actin cytoskeletal dynamics in the foot processes (366). For example, -actinin, an important protein in the slit diaphragm domain, interacts with synaptopodin (another actin-associated protein) to facilitate the formation of long unbranched parallel actin filaments in differentiated podocytes (367). The slit diaphragm also functions as a signaling center (368). For example, nephrin and CD2AP interact to stimulate antiapoptotic signaling in podocytes by activating the serine/threonine kinase AKT (369).
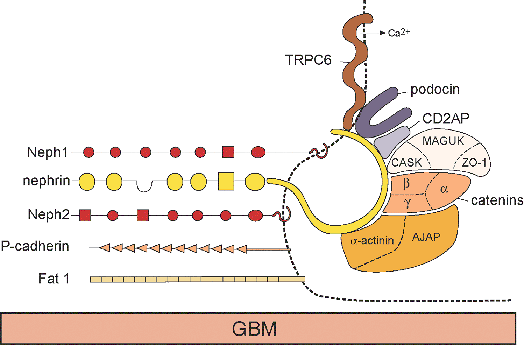 |
Figure 34.50 Schematic drawing of a working model of the podocyte filtration slit diaphragm domain. The left side portrays the molecular composition of the slit diaphragm itself, which spans between adjacent foot processes. The right side illustrates the molecular interactions of the nephrin-associated multiprotein complex within the podocyte foot process membrane and cytoplasm. See the text for further explanations. Modified from: Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 2003;83:253 307 ;Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 2001;108:1583 1587 ;Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 2005;14:211 216 ;Lehtonen S, Ryan JJ, Kudlicka K, Iino N, Zhou H, Farquhar MG. Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci U S A 2005;102:9814 9819. |
The basal membrane domain, the sole of the podocyte foot process, is embedded in the underlying GBM (Figure 34.49). Two types of surface receptors in the basal membrane, integrins and dystroglycan, anchor the foot processes by binding to their ligands in the GBM (370,371). The 3 1 integrin binds to collage type IV, laminin, and nidogen/entactin, whereas dystroglycan binds to laminin, agrin, and perlecan (348). Both integrins and dystroglycan are coupled by adapter molecules to the actin cytoskeleton.The integrins bind the talin, paxillin, vinculin complex (346), and dystroglycan binds utrophin (371). The induction of cellular responses from integrin-ligand interactions, known as outside-in signaling, is believed to be mediated by FAK (focal adhesion kinase) and ILK (integrin-linked kinase) (348,372). Similar to the filtration slit domain, an intact basal membrane domain is required to maintain foot process integrity.
The apical membrane domain, above the slit diaphragm, has a prominent glycocalyx surface of negatively charged glycoproteins (Figure 34.49) (373). These include podocalyxin (374) and GLEPP1 (375). Podocalyxin interacts with a complex composed of ezrin and NHERF2 (Na+/H+ exchanger-regulatory factor 2), which in turn, associates with the actin cytoskeleton (376,377). Genetic evidence exists indicating podocalyxin is important for foot process stability (159). The development of the glomerulus as a vascular structure distinct from the remainder of the nephron is reflected in the intrarenal distribution of intermediate filaments. Vimentin is present in glomerular endothelial and mesangial cells and podocytes (Figures 34.51,34.52) (347,378,379,380,381).
Human podocytes often do not stain for desmin, however rat podocytes may show desmin expression, especially in response to injury (381,382). The glomerular tuft does not stain for keratins.
The Wilms' tumor suppressor gene, WT1, plays an indispensable role in the regulation of cell growth and differentiation during early nephrogenesis. Embyronic mice homozygous for a targeted mutation of WT1 fail to develop kidneys (77). Striking evidence also exists for the importance of WT1 in glomerular podocyte differentiation. During kidney development, WT1 expression is detected in the metanephric mesenchyme and becomes stronger in the
P.875
renal vesicle, but highest levels occur during glomerulus formation within the podocyte cell layer (383,384). Expression of the WT1 protein, a transcription factor, in the nuclei of podocytes does not disappear with glomerular maturation but persists in the adult kidney (Figure 34.53). Greater than 95% of patients with the Denys-Drash syndrome (nephrotic syndrome and genital anomalies and/or Wilms' tumor), characterized by shrunken glomeruli with hypertrophied podocytes, have point mutations affecting the zinc finger DNA-binding domain of WT1 (385). These findings provide a functional link between a molecular defect of WT1 and podocyte pathology and suggest that WT1 has a role in maintenance of podocyte structure and function in the mature kidney.
 |
Figure 34.51 Vascular structures including the glomerulus are heavily labeled with antibody to vimentin. Tubular and interstitial components show weaker and sparse labeling. (Vimentin immunoperoxidase, original magnification 62.) |
 |
Figure 34.52 Prominent expression of vimentin is seen in glomerular endothelium, podocytes, and parietal epithelium. (Vimentin immunoperoxidase, original magnification 300.) |
Glomerular Filtration Barrier
The glomerular capillary wall functions as a filter that is selective for the size and charge of molecules. To pass through the capillary wall, a molecule must journey along an extracellular pathway through the fenestrated endothelium, the GBM, and the filtration slit diaphragm. Historically, the GBM has been considered the main barrier to filtration. More recently, the role of the podocyte slit diaphragm has emerged. The permeability of the glomerular capillary wall to water and small molecules is very high, whereas its permeability to molecules the size of albumin and larger is very low. Studies employing mathematical modeling have suggested that the GBM and filtration slits contribute equally to the total resistance to water filtration (386). A model incorporating ultrastructural data has shown that the slit diaphragm is the most restrictive part of the barrier to the filtration of macromolecules (387).
 |
Figure 34.53 Light micrograph demonstrating expression of the WT1 protein in podocyte nuclei of an adult glomerulus. (WT1 immunoperoxidase, original magnification 210.) |
Mutations or deficiencies of genes encoding proteins of the filtration slit diaphragm domain, including nephrin and several of its interacting protein partners, result in massive proteinuria, providing genetic evidence for the crucial role of the slit diaphragm in glomerular permselectivity (348,358). Although the protein networks within the GBM contribute to the size selectivity of the filtration barrier, the slit diaphragm appears to be the most important size-selective filter.
The GBM has been favored as the principal structure responsible for the charge-selective permeability of the glomerular capillary wall (388). This charge selectivity in the GBM has been associated with the presence of polyanionic molecules, such as heparan sulfate proteoglycans. Therefore, it was somewhat surprising that mice deficient for the heparan sulfate side chains of Perlecan were found to have no morphologic GBM defects and exhibited no proteinuria (389).
However, since agrin appears to be the major heparan sulfate proteoglycan in the GBM (338), it will be important to evaluate its contribution to the charge barrier in the GBM. The distribution of negatively charged molecules in the cellular layers of the glomerular capillary wall are also likely important. Experimental removal of polyanionic sialic acids from the plasma membrane of podocytes leads to proteinuria (390). Although relatively neglected, there is emerging evidence that negative charges within the glomerular endothelial glycocalyx may play a role in charge selectivity (302,303).
The integrated view of the glomerular filtration barrier is that the endothelium, the GBM, and the podoctyes and their slit diaphragms do not act independently but are linked to one another in a functional unit. Each of these components is important for normal glomerular filtration. Detailed reviews of the the filtration barrier are available (391,392,393).
Parietal Epithelial Cells
The parietal layer of Bowman's capsule consists of relatively flat squamous epithelial cells. They have prominent proliferative potential (394). Keratins, cadherens, and the transcription factor Pax-2 are expressed in parietal epithelial
P.876
cells (Figure 34.54) (347,379,380,381,395). Cytokeratin 8 has been reported to be a marker for activated parietal epithelial cells (394). The cells are 0.1 to 0.3 m in height but may increase to 2 to 3.5 m at the nucleus (284). The epithelium rests on the basement membrane of Bowman's capsule, which has been reported to range from 1,200 to 1,500 nm in thickness and may have a lamellated appearance (284). In contrast to the GBM, the basement membrane of Bowman's capsule expresses the 6 chain of type IV collagen, which is part of the 1. 1. 2(IV) 5. 5. 6(IV) network (396), and bamacan, a chondroitin sulfate proteoglycan (397).
 |
Figure 34.54 Keratin expression is not observed in the glomerular tuft, but immunoreactivity is seen in the parietal epithelial cells. The vascular pole is on the right. The macula densa (far right center) and other tubule segments are immunoreactive for keratin. (CAM 5.2 immunoperoxidase, original magnification 120.) |
Peripolar Cells
A peripolar cell, situated between the visceral and parietal epithelial cell layers at the origin of the glomerular tuft in Bowman's space, has been most commonly observed in sheep but also identified in humans (398,399,400,401). However, these cells have been detected only in approximately 1% of human glomeruli by light microscopy (399). Ultrastructurally, the peripolar cell contains multiple membrane-bound electron-dense secretory type granules. It has been proposed that this cell represents a component of the juxtaglomerular apparatus (398). The exact function of the peripolar cells is unknown, but they may have a secretory function and discharge their contents into Bowman's space.
Juxtaglomerular Apparatus
The juxtaglomerular apparatus (JGA), discovered by Golgi (402), is situated at the vascular pole of the glomerulus and includes the afferent and efferent arterioles, extraglomerular mesangial region, and macula densa (MD) (Figure 34.55). The JGA is the major structural unit of the renin-angiotensin system. Although the general outline of this anatomical unit usually can be observed in light microscopic sections (Figure 34.56), histochemical or immunocytochemical methods are usually required to demonstrate the distinctive juxtaglomerular granular cells. These cells tend to occur in clusters and are most
P.877
abundant in the wall of the afferent arteriole, but they are also found in the wall of the efferent arteriole and the extraglomerular mesangial region (403,404,405). Ultrastructural analysis shows the presence of myofilaments, attachment bodies, a well-developed endoplasmic reticulum and Golgi apparatus, and numerous membrane-bound granules (Figure 34.57). The granules are variable in shape and size. It is believed that the smaller, often rhomboid-shaped granules with a crystalline substructure (called protogranules) observed in the Golgi region represent mature granules. Renin and angiotensin II have been immunolocalized to the granules of these cells (406,407). Renin release occurs by exocytosis. It is believed to be modulated by adrenergic nerve activity (408,409).The extraglomerular mesangium, also called the lacis or the cells of Goormaghtigh, is located between the afferent and efferent arterioles and has extensive contact with the basal surface of the MD. This extraglomerular region is continuous with the intraglomerular mesangium, and the Goormaghtigh's cells are similar in ultrastructure to the mesangial cells. There are numerous gap junctions between the extraglomerular mesangial cells and the cells of the intraglomerular mesangium and glomerular arterioles (410,411). These morphologic features and the central position within the JGA suggest that the extraglomerular mesangium may represent the structural-functional link between the MD and the glomerular arterioles and mesangium. The MD represents a plaque of specialized tubular cells within the cortical thick ascending limb of Henle adjacent to the hilus of the glomerulus. The cells are low columnar and may protrude into the tubular lumen (Figure 34.58). By electron microscopy, they have apical nuclei, cellular organelles largely lateral to and beneath the nuclei, and basal cellular processes that interdigitate with the extraglomerular mesangial cells. The lateral intercellular spaces between the MD cells vary in width but usually are more dilated compared with the lateral intercellular spaces of other nephron segments (412). In contrast with contiguous portions of the thick ascending limb, there is evidence that the MD lacks epidermal growth factor and Tamm-Horsfall protein but may be water permeable (Figure 34.59) (413,414,415).
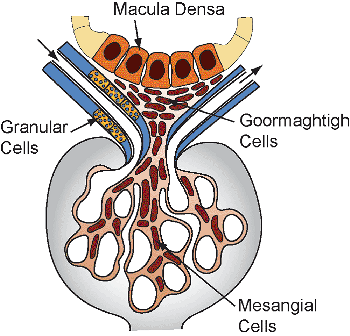 |
Figure 34.55 The basic components of the juxtaglomerular apparatus. Modified with permission from: Kriz W, Kaissling B. Structural organization of the mammalian kidney. In: Seldin DW, Giebisch D, eds. The Kidney: Physiology and Pathophysiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:587 654. |
 |
Figure 34.56 Light micrograph depicting the juxtaglomerular apparatus. From the top to the bottom of the micrograph are the macula densa, extraglomerular mesangium, afferent arteriole, and glomerulus (original magnification 750; courtesy of Dr. Luciano Barajas). Reprinted with permission from: Barajas L, Salido EC, Smolens P, Hart D, Stein JH. Pathology of the juxtaglomerular apparatus including Bartter's syndrome. In: Tisher CC, Brenner BM, eds. Renal Pathology with Clinical and Functional Correlations. 2nd ed. Philadelphia: JB Lippincott; 1994:948 978. |
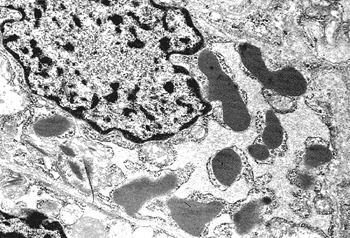 |
Figure 34.57 Electron micrograph of a juxtaglomerular granular cell. Note the prominent cytoplasmic membrane-bound granules (original magnification 19,000; courtesy of Dr. Luciano Barajas). Reprinted with permission from: Barajas L, Bloodworth JMB Jr, Hartroft PM. Endocrine pathology of the kidney. In: Bloodworth JMB Jr, ed. Endocrine Pathology: General and Surgical. 2nd ed. Baltimore: Williams & Wilkins; 1982:723 766. |
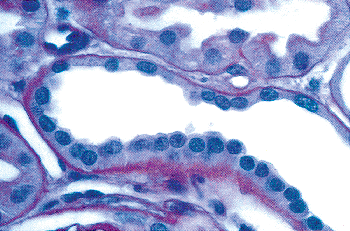 |
Figure 34.58 Macula densa characterized as a morphologically distinct plaque of low columnar cells with apically situated nuclei (PAS stain, original magnification 750). |
The anatomic arrangement of the juxtaglomerular apparatus is suited for functional regulation of the adjacent structures. The MD plays a role in tubuloglomerular feedback, a mechanism whereby luminal concentrations of
P.878
sodium and/or chloride are sensed by the MD, leading to the transfer of a signal to the glomerular arterioles to regulate the glomerular filtration rate (GFR) (416). For example, increased luminal salt concentration at the MD results in a decreased GFR. The neuronal isoform of nitric oxide synthase (nNOS) and the cyclooxygenase enzyme COX-2 immunolocalize to the MD (417,418,419). There is evidence that both nitric oxide (NO)- and COX-2 generated prostaglandins play a role in the signaling between the MD and the renin-secreting cells in the afferent arteriole (420,421).
 |
Figure 34.59 Differential interference contrast image of an isolated thick ascending limb segment perfused in vitro. The tubular lumen (L) and macula densa (dashed lines enclose the ends of the macula densa plaque) are observed. In response to a reduction in tubular luminal osmolality, there is dilatation of the lateral intercellular spaces (arrow) in the macula densa, suggesting increased water flow (original magnification 1250) Modified with permission from: Kirk KL, Bell PD, Barfuss DW, Ribadeneira M. Direct visualization of the isolated and perfused macula densa. Am J Physiol 1985;248(pt 2):F890 F894. |
Proximal Tubule
The proximal tubule is divided into an initial convoluted portion (PCT), the pars convoluta, and a straight portion (PST), the pars recta. The convoluted portion forms several coils around its parent glomerulus in the cortex and continues into the straight portion, which is located in the medullary ray.
The human proximal tubule is approximately 14 mm in length (422). In histologic sections of the cortex, sectioned profiles of proximal convoluted tubules represent the major parenchymal component. The appearance of the cortex and especially the proximal tubules varies, according to the method of fixation. A decrease in blood pressure results in decreased filtration and renal volume (423,424). After immersion fixation of excised pieces of renal tissue, the cortex has a more homogeneous compact appearance, and there is collapse of the proximal tubular lumens (Figure 34.60) (425). Free nuclei and vesicular membranous material may be observed in the proximal tubular lumens. Fixation of an experimental functioning kidney in situ by rapid freezing, dripping of fixative on the renal surface, or vascular perfusion results in more conspicuous intertubular interstitial spaces and widely open lumens of the proximal tubules. The cells of the proximal tubule are cuboidal to low columnar with eosinophilic, often granular cytoplasm and round nuclei situated in the center or near the base of the cells (Figure 34.61). The lateral cell borders are indistinct because of extensive interdigitations of lateral cellular processes from adjacent cells (Figure 34.62). In the basal part of the cells there are vertical striations that represent numerous elongated mitochondria. Cytoplasmic apical vacuoles and granules correspond to a well-developed endocytic-lysosomal apparatus. There is a prominent PAS-positive luminal brush border composed of the numerous densely packed long microvilli (Figure 34.63). The brush border, apical cytoplasmic vacuoles, and basal striations are less prominent in the pars recta. Lectins have been used as selective probes to delineate renal tubular segments (426,427,428).
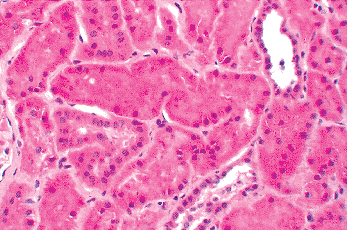 |
Figure 34.60 Immersion-fixed renal biopsy specimen demonstrating diffuse collapse of the proximal tubular lumens. Note the patent lumens of the distal nephron segments. (Original magnification 250.) |
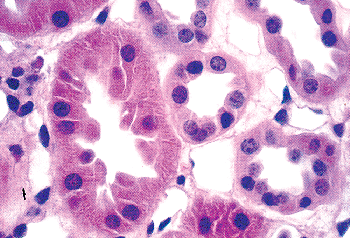 |
Figure 34.61 Cross section of a proximal tubule (left of center). The proximal tubular cells are taller and more eosinophilic than the cells of the distal nephron segments (right). (Original magnification 750.) |
Although a certain degree of nonspecificity has been reported, the lectin Lotus tetragonolobus has been used as a marker of proximal tubular epithelium (Figure 34.64).
P.879
Keratins 8 and 18 are expressed in both the convoluted and straight portions of the proximal tubule, whereas keratin 19 is focally expressed in the straight portion (379,380,429). The specific expression of the cell adhesion protein cadherin-6 in the proximal tubule has been reported (430).
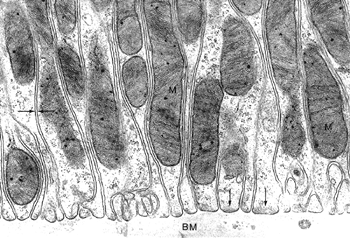 |
Figure 34.62 Electron micrograph illustrating extensive interdigitation of cellular processes in the basal region of proximal tubular cells. The mitochondria (M) are elongated. The width of the extracellular space (opposing arrows) is constant, and there are bundles of cytoplasmic filaments (single arrows) adjacent to the basement membrane (BM) (original magnification 40,000). Reprinted with permission from: Maunsbach AB, Christensen EI. Functional ultrastructure of the proximal tubule. In: Windhager EE, ed. Handbook of Physiology. Renal Physiology. New York: Oxford University Press; 1992:41 107. |
In most mammals, distinct segments of the tubule portion of the nephron can be distinguished by structural and functional differences. The structural differences have been characterized mainly on the ultrastructural level (431). However, these tubule segments often can be detected on light microscopy because of their known distribution within specific zones of the kidney (Figure 34.33). In general, the degree of tubule segmentation has not been characterized in detail in the human kidney. In several mammals, the proximal tubule can be divided into three morphologically distinct segments (Figure 34.65) (431). The S1 segment originates at the glomerulus and constitutes one-half to two-thirds of the pars convoluta. The S2 segment represents the remainder of the pars convoluta and the initial part of the pars recta. The S3 corresponds to the remainder of the pars recta and is located in the inner cortex and outer stripe of the outer medulla. Although a pars convoluta and a pars recta have been described in the human kidney (432), the segmentation of the proximal tubule into three divisions has not been closely examined.
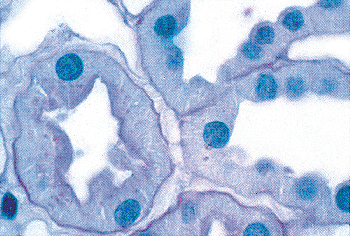 |
Figure 34.63 PAS-stained cross section of proximal tubule to the left of the center of the micrograph. Note the prominent PAS-positive brush border (original magnification 1250). |
 |
Figure 34.64 Staining of the brush border of the proximal tubules with lectin Lotus tetragonolobus. The distal nephron segments and glomeruli are negative. (Courtesy of Dr. Randolf A. Hennigar.) |
The cells in the S1 segment have a tall brush border, a well-developed endocytic lysosomal apparatus, numerous elongated mitochondria, and extensive basolateral invaginations and interdigitations.
The cells in the S2 segment are similar to those in the S1 segment; however, the brush border is shorter, and the endocytic organelles, mitochondria, and basolateral invaginations and interdigitations are less prominent (Figure 34.66). The cells in the S3 segment are more cuboidal and have relatively fewer endocytic organelles, small mitochondria, and inconspicuous membrane invaginations and interdigitations. The length of the brush border in the S3 segment varies among species.
The proximal tubule is responsible for the reabsorption of about 60% of the glomerular ultrafiltrate. The reabsorption of chloride, bicarbonate, glucose, amino acids, and fluid is coupled to the active transport of sodium (433). An excellent correlation exists along the length of the proximal tubule between the elaborately developed basolateral membrane expressed as surface area (Figure 34.67), the high sodium potassium (Na+/K+)-ATPase activities that are localized to the basolateral membrane, and the capacity
P.880
to transport sodium and ions (434,435). The 1 1 heterodimer is the main Na+/K+-ATPase isozyme of the kidney, but the 2- and 3-isoforms have been detected (436,437). This sodium pump mediated active transport of Na+ out of the cell across the basolateral membrane establishes a lumen-to-cell concentration gradient for Na+.
P.881
The transport of Na+ from the lumen into the proximal tubule cell, down its concentration gradient, is mediated by the Na+/H+ exchanger, NHE3, expressed in the brush border (438,439). The numerous mitochondria located in close proximity to the plasma membrane provide a source for the cellular energy required for active transport. In general, the intrinsic rates at which fluid and solutes are transported decrease along the proximal tubule from S1 to S3. The discovery of the aquaporins, a family of water channel proteins, has enhanced our understanding of the kidney's role as the primary organ that regulates water balance (440,441). Aquaporin-1 (AQP1), abundant in both the apical and basolateral membranes of the proximal tubule, is believed to mediate osmotic water permeability in this segment (442,443).
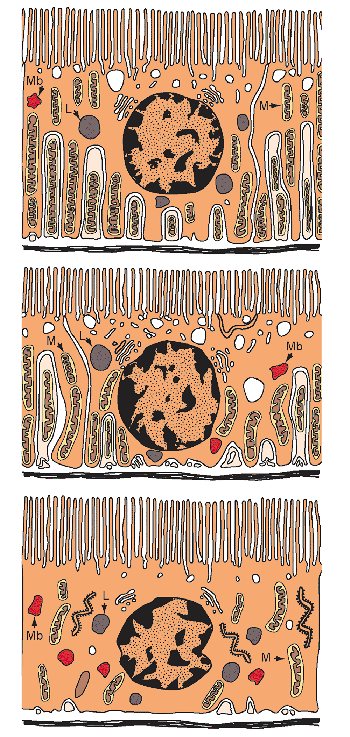 |
Figure 34.65 Schematic diagram of the three segments of the proximal tubule: upper, S1; middle, S2; lower, S3. The prominent basolateral processes are lined with mitochondria. The interdigitating cellular processes that come from adjacent cells are shaded lighter. (Mb, microbody; M, mitochondrion; L, lysosome) Modified with permission from: Maunsbach AB, Christensen EI. Functional ultrastructure of the proximal tubule. In: Windhager EE, ed. Handbook of Physiology. Renal Physiology. New York: Oxford University Press; 1992:41 107. |
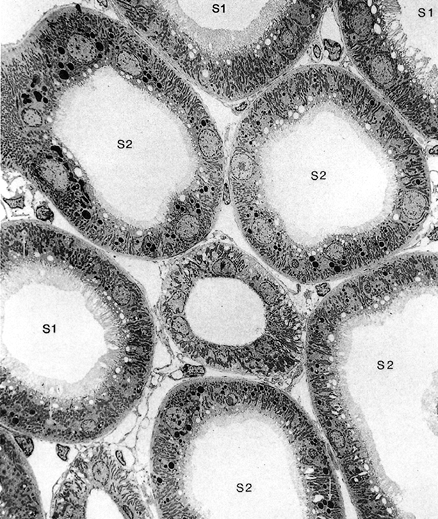 |
Figure 34.66 Electron micrograph of rat renal cortex. The cells in the S1 segment are taller and have a more prominent brush border than the cells in the S2 segment (original magnification 1870). Reprinted with permission from: Maunsbach AB, Christensen EI. Functional ultrastructure of the proximal tubule. In: Windhager EE, ed. Handbook of Physiology. Renal Physiology. New York: Oxford University Press; 1992:41 107. |
 |
Figure 34.67 Three-dimensional schematic drawing of the proximal convoluted tubule illustrating the complex basal and lateral cellular processes that interdigitate with those from adjacent cells. Modified with permission from: Welling LW, Welling DJ. Shape of epithelial cells and intercellular channels in the rabbit proximal nephron. Kidney Int 1976;9:385 394. |
The well-developed endocytic-lysosomal apparatus in the proximal tubule (Figure 34.68) plays an important role in reabsorption and degradation of albumin and low-molecular weight proteins filtered by the glomerulus (444). Proteins are absorbed by endocytosis and transferred through the endosomal compartment to the lysosomes, where they are degraded. This is a selective process dependent upon the size and charge of the protein molecule (445,446,447). The capacity for protein degradation decreases from the S1 to the S3 segment (448). Upon exposure to an acidic environment in the endosomes (449), the internalized ligand-receptor complexes are segregated, and the receptors are recycled back to the luminal membrane via small vacuolar structures, termed apical dense tubules (450). Megalin and cubilin are multiligand endocytic receptors expressed throughout the endocytic apparatus of the proximal tubule (451). The receptors function independently but also interact as a dual complex to facilitate the uptake of albumin (452), as well as numerous ligands, including low-molecular weight proteins, vitamin-binding proteins, hormones, lipoproteins, and drugs (453).
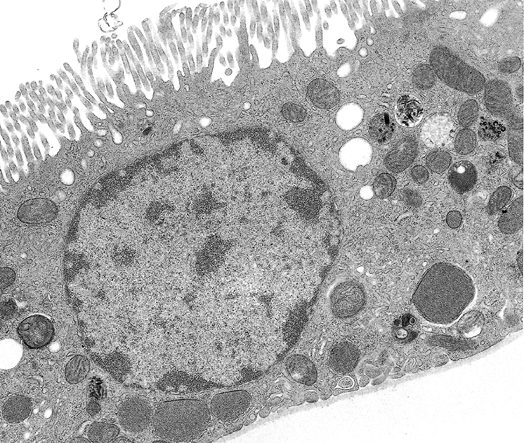 |
Figure 34.68 Electron micrograph illustrating an isolated perfused S2 segment of the rabbit proximal tubule. Note the endocytic compartment consisting of coated pits and vesicles, apical tubules, small endocytic vesicles, and larger endocytic vacuoles. The lysosomes are heterogeneous and contain electron-dense material. The mitochondria are numerous (original magnification 15, 000). Reprinted with permission from: Clapp WL, Park CH, Madsen KM, Tisher CC. Axial heterogeneity in the handling of albumin by the rabbit proximal tubule. Lab Invest 1988;58:549 558. |
The normal adult kidney has a relatively low rate of cell turnover with little proliferation (454,455,456). However, renal cell proliferation is accelerated during hypertrophy and following injury. Histologic assessment of cell proliferation can be made by determining a mitotic index or immunostaining with an antibody that detects proteins present during the cell cycle (457). During recovery after tubular injury, such as ischemia or toxin exposure, cell proliferation in tubular cells, especially in the proximal tubule, increases markedly (Figure 34.69) (458,459). Apoptosis has been documented in the adult kidney during the repair response to various forms of tubular injury, including ischemia, toxic insults, and hydronephrosis (Figure 34.70) (460,461,462,463).
Thin Limbs of Henle's Loop
The transition of the thin limbs of Henle's loop with other nephron segments marks the borders between certain zones of the kidney (Figure 34.33). Between the outer and inner stripes of the outer medulla, there is an abrupt transition from the proximal tubule to the descending thin limb (DTL) of Henle's loop. Short-looped nephrons have only a short DTL located in the inner stripe of the outer medulla. Near the hairpin turn of the short loop, the DTL continues into the thick ascending limb. Long-looped
P.882
nephrons have both a long DTL and a long ascending thin limb (ATL). The long DTL traverses the inner stripe of the outer medulla and enters the inner medulla, whereas the long ATL resides entirely within the inner medulla. At the border between the outer and inner medulla, the long ATL continues into the thick ascending limb. By light microscopy, the thin limb is lined with a flat, simple epithelium about 1 to 2 m thick (Figure 34.71). The lenticularly shaped nucleus bulges slightly into the lumen. Four types of epithelium have been described in the thin limb in several mammals (Figure 34.72) (272,464). It is not known if four types exist in humans, but at least two different types of epithelium have been demonstrated (465). Type I is present in the DTL of short-looped nephrons. It is an extremely thin, simple epithelium with few cellular interdigitations and cell organelles. Type II epithelium lines the initial part of the DTL of long-looped nephrons located in the outer medulla. This epithelium exhibits species variation and is characterized by taller cells, short microvilli, and more prominent cell organelles than in the other epithelial types. In the rat and mouse, type II epithelium is complex and characterized by extensive lateral interdigitations; whereas in the rabbit and human kidney, the interdigitations are less prominent (272). Type III epithelium, found in the DTL of long-looped nephrons in the inner medulla, is composed of simple cells with few organelles and without lateral interdigitations. Type IV epithelium forms the bends of the long loops and lines the entire ATL in the inner medulla. It is characterized by low, flattened cells with few organelles and no microvilli but abundant lateral interdigitations. Thin limb epithelium has been reported to be immunoreactive for keratins 7, 8, 18, and 19 (379,380).
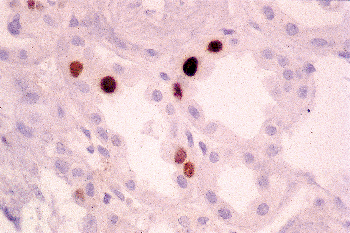 |
Figure 34.69 Biopsy of kidney allograft with cyclosporin A toxicity. Normally there is sparse labeling for Ki-67, a nuclear protein expressed by proliferating cells. In this example, there is a prominent increase in labeling of the tubular nuclei. (Ki-67 immunoperoxidase, original magnification 210.) |
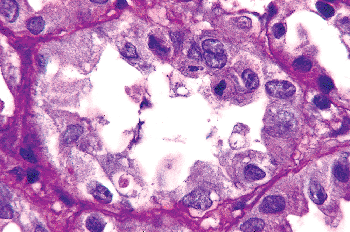 |
Figure 34.70 Micrograph from same case as in Figure 34.69. Several apoptotic nuclei are present (1 to 2 o'clock positions) in the tubular epithelium. (PAS stain, original magnification 300). |
 |
Figure 34.71 Several thin limbs of Henle are depicted in the center of the light micrograph. The lining epithelium is extremely attenuated, and the nuclei protrude into the lumens (original magnification 500). |
 |
Figure 34.72 Schematic drawing of the four types of epithelium in the thin limbs of Henle's loop. (The interdigitating cellular processes that come from adjacent cells are shaded lighter.) Modified with permission from: Madsen KM, Tisher CC. Anatomy of the kidney. In: Brenner BM, ed. Brenner and Rector's The Kidney. 7th ed. Philadelphia: WB Saunders; 2004:3 72. |
The thin limb of Henle's loop plays an important role in the countercurrent multiplication component of urinary concentration. Physiologic studies have demonstrated that the DTL is permeable to water but has low permeability to sodium chloride, whereas the thin ascending limb is largely impermeable to water but has a high permeability to sodium chloride (272,466). These physiologic investigations are supported by immunohistochemical studies. The
P.883
aquaporin water channel protein, AQP1, is prominently expressed in the DTL but not in the ATL (442,467). The kidney-specific chloride channel C1C-K1 is expressed exclusively in the ATL (468,469). Mice and humans lacking AQP1 (470,471) and mice deficient for C1C-K1 (472) have impaired urine concentrating ability. In the passive model proposed by Kokko and Rector (473) and Stephenson (474), a hypertonic medullary interstitium concentrates sodium chloride in the DTL by extraction of water. The fluid that then enters the ATL has a higher sodium chloride concentration, resulting in passive salt absorption and dilution of the fluid of the ATL. Thus, the thin limb contributes to the maintenance of a hypertonic medullary interstitium and delivers a dilute fluid to more distal segments. The morphologic features of a simple epithelium with few organelles in the ATL are consistent with the lack of demonstrable active transport in this segment.
Distal Tubule
The distal tubule consists of three distinct segments: the thick ascending limb of Henle's loop (TAL), the macula densa (MD), and the distal convoluted tubule (DCT) (Figure 34.33). The MD, as previously discussed (in the section entitled Juxtaglomerular Apparatus), is a specialized plaque of cells within the TAL. In short-looped nephrons, the transition from the descending thin limb to the TAL occurs before the hairpin turn. In long-looped nephrons, the transition from the thin ascending limb to the TAL marks the border between the inner medulla and the inner stripe of the outer medulla. The TAL can be divided into a medullary (MTAL) and a cortical segment (CTAL). The cells are eosinophilic and cuboidal, and the round nucleus tends to be located in the apical region and causes a bulge of the cell into the lumen (Figure 34.73). Similar to the proximal tubule cells, the cells of the TAL have indistinct lateral cell borders because of elaborate basolateral membrane invaginations and interdigitations. They also have cytoplasmic basal striations because of elongated mitochondria. These morphologic features are characteristic for epithelial cells involved in active transport. However, in contrast to the proximal tubule, the cells are lower and less eosinophilic, and there is no brush border in the TAL. As the TAL ascends into the cortex, there is a gradual decrease in cell height, basolateral membrane area, and size of the mitochondria (475). Scanning electron microscopy has shown two luminal surface configurations of cells in the TAL (476). Cells with a relatively smooth surface are most commonly found in the medullary segment, whereas cells with a rough surface due to luminal microprojections and apical lateral membrane invaginations predominate in the cortical segment. The functional significance of these structural findings remains unexplained. The TAL continues into the distal convoluted tubule just beyond the MD. The cells of the TAL synthesize Tamm-Horsfall protein and secrete it into the tubular lumen (414). This segment expresses keratins 8 and 18 (378,380,429), and also kidney-specific (Ksp) cadherin (477).
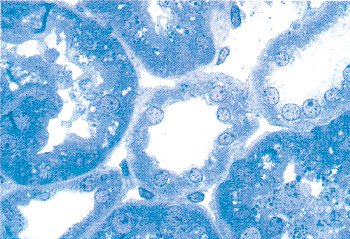 |
Figure 34.73 Light micrograph demonstrating a cross section of a thick ascending limb in the center. There is no brush border, and the cells are lower than the adjacent proximal tubular cells. (Toluidine blue-stained, 1- m Epon section, original magnification 750.) |
An important function of the TAL is the active reabsorption of sodium chloride. There is a correlation between structure and function in the ascending limb. The basolateral membrane surface area, the Na+/K+-ATPase activity, and the reabsorptive capacity for sodium chloride are all greater in the medullary segment than in the cortical segment of the TAL (433,475,478). The reabsorption of sodium chloride in both the medullary and cortical segments of the TAL is mediated by a bumetanide-sensitive Na+/K+/2Cl- cotransporter (BSC-1), which localizes to the TAL apical plasma membrane (479). This reabsorption of salt coupled with the water impermeability of the TAL results in a hypertonic interstitium and delivery of a hypotonic fluid to more distal tubular segments.
The DCT begins just beyond the MD in the cortex and represents the terminal part of the distal tubule. The cells of the DCT are similar to those of the TAL and contain numerous mitochondria. However, the DCT cells are taller, characteristically have nuclei closer to the lumen, and lack lateral interdigitations in the apical region between adjacent cells (Figure 34.74). In comparison with the proximal tubule, the cells of the DCT are lower and less eosinophilic, have a less prominent apical endocytic apparatus, and lack a brush border. More nuclei are observed in a cross section than in the proximal tubule, and the lumen is normally open. The epithelium of the DCT shows immunoreactivity for keratins 8, 18, and 19 (379,429) and for Ksp-cadherin (477). In the DCT, reabsorption of sodium chloride is mediated by the Na+/Cl- cotransporter, TSC, which localizes to the apical plasma membrane and apical cytoplasmic vesicles (480,481). The cotransporter TSC, the target of thiazide diuretics, is distinct from the cotransporter BSC-1,
P.884
present in the thick ascending limb. Biochemical studies have demonstrated that the DCT has a higher level of Na+/K+-ATPase activity than any other tubular segment (116). Morphologic and physiologic studies have provided evidence that the DCT, similar to the TAL, is relatively impermeable to water but responsible for the reabsorption of sodium chloride (482,483,484).
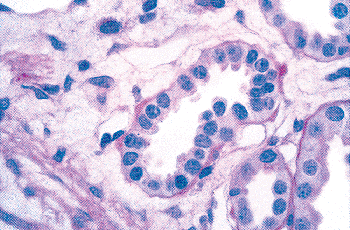 |
Figure 34.74 Light micrograph showing a distal convoluted tubule. Note the absence of a brush border and the nuclei situated close to the lumen. (PAS stain, original magnification 750.) |
Connecting Segment
The connecting segment (or tubule) is a transitional segment that joins the DCT with the collecting duct system. In superficial nephrons, the connecting segment (CS) continues directly into an initial collecting tubule (Figure 34.75). In contrast, the connecting segments of juxtamedullary nephrons and of many midcortical nephrons join to form an arcade that ascends in the cortex before draining into an initial collecting tubule. In humans, most nephrons empty individually into initial collecting tubules (272).
Fourteen percent of the nephrons are connected to arcades, and each arcade consists of about three nephron attachments (6). Each cortical collecting tubule receives an average of 11 nephrons (6). In most species, including humans, the CS contains different cell types as a result of an intermixing of cells from the adjacent DCT and cortical collecting duct (485). The connecting tubule cell is the most characteristic cell type of this transitional segment. They display ultrastructural features intermediate between the DCT cells and the principal cells of the cortical collecting duct and contain deep true infoldings of the basal cell membrane (486). The connecting tubule cell appears to be the only cell type in the kidney (of those thus far identified) that shows immunoreactivity for the proteolytic enzyme kallikrein (487,488). The physiologic relevance of this finding remains uncertain. Various types of intercalated cells, similar to those in the cortical collecting duct, are also present in the CS and are likely involved in tubular acid-base regulation. The CS is an important site of potassium secretion regulated by mineralocorticoids (484). An ATP-sensitive K+ channel, ROMK, localizing to the apical membrane of connecting tubule cells, likely mediates the potassium secretion (489,490). The expression of a Na+/Ca2+ exchanger and a Ca2+-ATPase in the basolateral membrane of the connecting tubule cells suggests the CS has a significant role in calcium reaborption (491,492).
Collecting Duct
The collecting duct begins in the cortex and descends to the tip of the papilla, also called the area cribrosa, where the inner medullary segments terminate as the ducts of Bellini. These terminal collecting duct segments were apparently described by Eustachio nearly 100 years before the observation of Bellini (493). During its course, there is an increase in diameter from the cortical portion to the terminal segments at the area cribrosa. The collecting duct can be divided into the cortical (CCD), outer medullary (OMCD), and inner medullary collecting ducts (IMCD). Significant cellular heterogeneity exists along the collecting duct (271). Although there is a degree of nonspecificity, the lectins Dolichos biflorus and Arachis hypogaea have been used as markers for collecting duct epithelium (Figure 34.76) (427). The distal tubules and collecting ducts show variable but generally more intense staining for keratins than do the proximal tubules (Figures 34.77,34.78). Keratins 8, 18, and 19 are prominently expressed throughout the cortical and medullary collecting ducts (379,380,429). There is less
P.885
staining for keratin 7. Scattered keratin 7- and 19-negative cells have been observed to be intercalated cells (379). Keratins 5/6, 17, and 20, as well as vimentin, are restricted primarily to medullary collecting ducts (429). There is less immunoreactivity for Ksp-cadherin in collecting ducts compared to the thick ascending limbs and distal convoluted tubules (477).
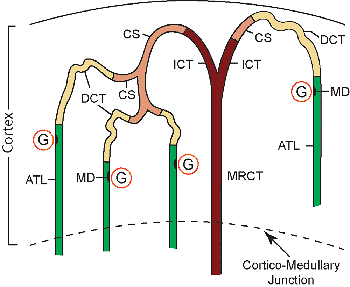 |
Figure 34.75 Diagram of the various anatomic arrangements of the distal tubule connecting to the cortical collecting duct in superficial, midcortical and juxtamedullary nephrons. (G, glomerulus; ATL, ascending thick limb (of Henle); MD, macula densa; DCT, distal convoluted tubule; CS, connecting segment; ICT, initial collecting tubule; MRCT, medullary ray collecting tubule) |
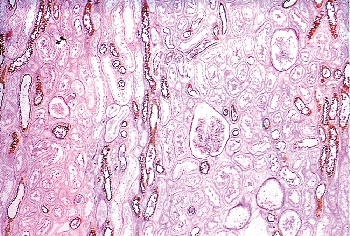 |
Figure 34.76 Staining of the collecting ducts and thick ascending limbs with lectin Arachis hypogaea. The proximal tubules and glomeruli are negative. (Courtesy of Dr. Randolf A. Hennigar.) |
Cortical Collecting Duct
The cortical collecting duct can be subdivided further into the initial collecting tubule and the medullary ray portion. By light microscopy, the epithelium of the cortical collecting duct consists of cuboidal cells with distinct lateral cell borders and central round nuclei (Figure 34.79). The lumen is prominently open, and there is no brush border. The cortical collecting duct is composed of principal cells and intercalated cells. Principal cells are mainly responsible for salt and water transport, and the intercalated cells are involved in acid-base regulation. It is difficult to distinguish principal cells from intercalated cells on H&E paraffin sections. The principal cells on light microscopy have an extremely light or clear cytoplasm. By electron microscopy, the principal cells have relatively few cell organelles and no interdigitations of lateral cellular processes from adjacent cells, which accounts for the distinct cell borders observed on light microscopy (Figure 34.80). However, there are prominent infoldings of the basal plasma membrane, which gives the basal region an accentuated clear appearance on light microscopy (494). The principal cell has a fairly smooth luminal surface with short microvilli and a single cilium by scanning electron microscopy (Figure 34.88).
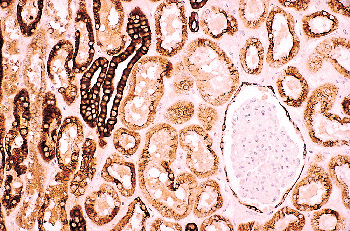 |
Figure 34.77 Distal tubules, collecting ducts, proximal tubules, and parietal epithelium lining Bowman's capsule showing expression of keratins. The distal tubules and collecting ducts label more intensely than do the proximal tubules. (CAM 5.2 immunoperoxidase, original magnification 62.) |
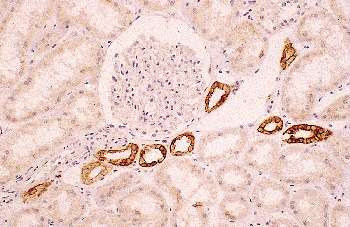 |
Figure 34.78 Detection of keratin expression varies depending on the specificity and dilution of the antibody. In this micrograph, there is prominent immunoreactivity of the distal tubules and collecting ducts. (35 H11 immunoperoxidase, original magnification 62.) |
Principal cells are involved in sodium reaborption and potassium secretion. Sodium reaborption is mediated by
P.886
an amiloride-sensitive sodium channel, ENaC, located in the apical membrane of principal cells throughout the entire collecting duct (495,496). Experimental conditions of dietary potassium loading or mineralocorticoid stimulation have shown increases in potassium secretion and Na+/K+-ATPase activity in the cortical collecting duct, along with an increase in the surface area of the basolateral membrane of the principal cells (497,498,499,500,501). These findings indicate that the principal cells are involved in potassium secretion in the cortical collecting duct. The entire collecting duct becomes permeable to water in the presence of the antidiuretic hormone vasopressin. After vasopressin binds to its receptor on the basolateral membrane of principal cells (502), small apical cytoplasmic tubulovesicles (called aggrephores) containing the aquaporin water channel AQP2 are shuttled to the apical membrane, which markedly increases water permeability (503,504). The presence of the aquaporin water channels AQP3 and AQP4 in the basolateral membranes of principal cells facilitate the final exit of water into the interstitium (505,506).
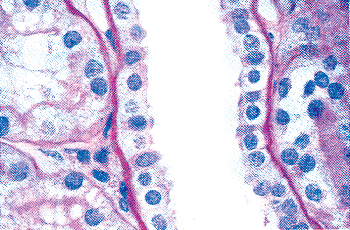 |
Figure 34.79 Micrograph illustrating a cortical collecting duct. Note the distinct lateral cell orders (original magnification 500). |
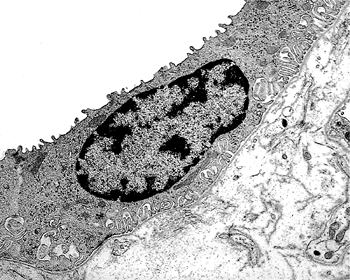 |
Figure 34.80 Electron micrograph of principal cell from the collecting duct. Note the relatively prominent infoldings of the basal plasma membrane (original magnification 12,500). |
The intercalated or dark cells are interspersed in the lining epithelia of the collecting duct. Although intercalated cells usually represent the minority cell type in epithelia where they are found, they constitute 30 to 40% of the cells in the cortical collecting duct in some mammals (271,280). They are also present in the connecting segment, the outer medullary collecting duct, and the initial portion of the inner medullary collecting duct. Intercalated cells may be identified on 1- m thick toluidine blue-stained Epon sections by their densely staining cytoplasm and their often convex luminal surface covered with numerous microprojections (Figure 34.81). The darkly staining cytoplasm is due in part to the presence of relatively more organelles, especially mitochondria. Two distinct populations of intercalated cells, types A and B, have been described in the cortical collecting duct of mammals (507,508). On ultrastructural examination, the type A intercalated cells have prominent microprojections of the apical membrane and extensive tubulovesicular structures in the apical cytoplasm (Figure 34.82). In comparison with the type A cells, the type B intercalated cells have a denser cytoplasm, more mitochondria, a smaller apical membrane area, a small number of microprojections on the apical surface, more spherical vesicular structures throughout the cytoplasm (but fewer vesicles beneath the apical membrane), and a larger basolateral membrane surface area (Figure 34.83). By scanning electron microscopy, the type A cells have a large convex luminal surface covered with numerous complex microprojections called microplicae (Figure 34.84), whereas the type B cells display a small angular luminal surface with relatively small microvilli (Figure 34.85) (508). The type B intercalated cells may be inconspicuous on scanning electron microscopy. More recently, a third type of intercalated cell has been characterized in the cortical
P.887
collecting duct of some mammals (509,510). They account for 40 to 50% of intercalated cells in the mouse connecting segment and initial collecting duct. The non-A non-B intercalated cells are larger than type A and type B intercalated cells, have abundant mitochondria, and have prominent apical microprojections similar to those of type A cells. Compared to the type A and B intercalated cells, the non-A non-B intercalated cells have been studied in fewer species, mainly the rat and mouse. There are significant differences in the prevalence and distribution of the different types of intercalated cells throughout the connecting segment and cortical collecting duct among mammalian species (280).
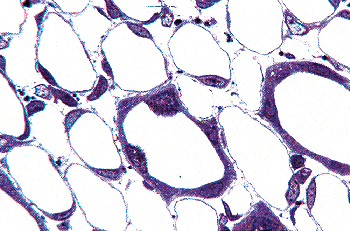 |
Figure 34.81 Light micrograph of the outer medulla showing intercalated cells in the collecting ducts. The intercalated cells exhibit a bulging apical surface covered with microprojections and dark-staining cytoplasm. (One-micron toluidine blue-stained Epon section, original magnification 160.) |
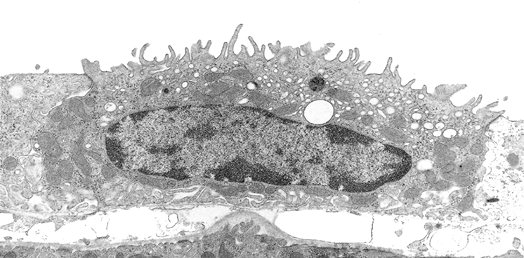 |
Figure 34.82 Electron micrograph of a type A intercalated cell in the cortical collecting duct. Note the prominent tubulovesicular membrane compartment in the apical cytoplasm and the numerous microprojections on the luminal surface. (Original magnification 11, 800; courtesy of Dr. Jill W. Verlander.) |
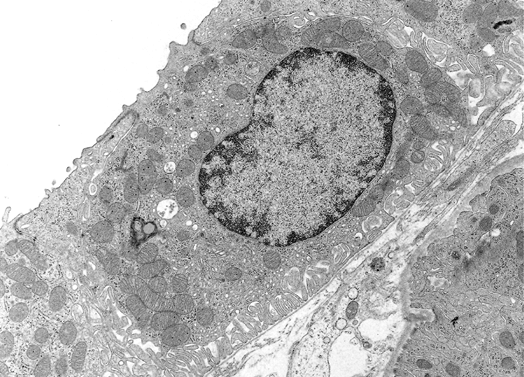 |
Figure 34.83 Electron micrograph of a type B intercalated cell in the cortical collecting duct. There are numerous vesicles throughout the cytoplasm, and the basolateral membrane is prominent. Note the paucity of microprojections on the luminal surface. Compared to the type A intercalated cell, there are fewer vesicles beneath the apical membrane. (Original magnification 11,800; courtesy of Dr. Jill W. Verlander.) |
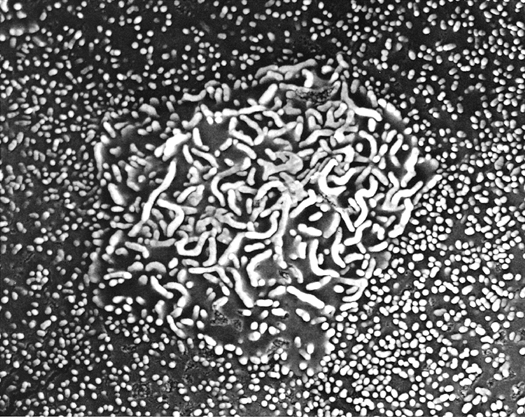 |
Figure 34.84 Scanning electron micrograph of the luminal surface of a type A intercalated cell in the cortical collecting duct. The type A cell is well demarcated and has a large luminal surface covered primarily with microplicae but also microvilli. (Original magnification 15,000; courtesy of Dr. Jill W. Verlander.). |
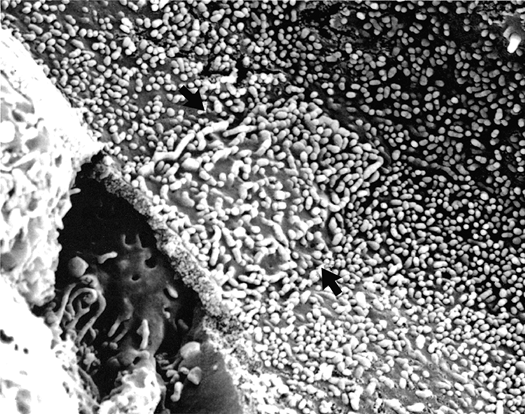 |
Figure 34.85 Scanning electron micrograph of the luminal surface of a cortical collecting duct. A type B intercalated cell (arrows) displays a small angular luminal surface covered with short microprojections, mainly microvilli. (Original magnification 15,000; courtesy of Dr. Jill W. Verlander.) |
Intercalated cells are typified by their high levels of carbonic anhydrase, the enzyme that catalyzes the interconversion of carbon dioxide (CO2) to bicarbonate (HCO3-), suggesting they are involved in urine acidification (511). Physiologic studies have demonstrated that the cortical collecting duct reabsorbs bicarbonate in acid-loaded animals (512) and secretes bicarbonate in alkali-loaded animals (513). In a study of experimental acute respiratory acidosis, there was a striking increase in the apical membrane surface area of the type A intercalated cells, whereas no morphologic changes were observed in the type B cells (508).
Studies have immunolocalized the vacuolar-type proton pump H+-ATPase in the apical membrane (514,515,516) and the Cl-/HCO3- exchanger, AE1 (Band 3), in the basolateral membrane (507,516,517,518) of type A intercalated cells. These findings indicate that type A cells are responsible for H+ secretion in the cortical collecting duct. The immunolocalization of the H+-ATPase to the basolateral membrane of type B intercalated cells (515,516) and the physiologic evidence for an apical Cl-/HCO3- exchange in these cells (519) indicate that type B cells are involved in bicarbonate secretion. Evidence has accumulated to indicate that the apical Cl-/HCO3- exchange in the type B cell is mediated by the protein pendrin, which immunolocalizes to the apical membrane and apical cytoplasmic vesicles of the B cell (520,521,522). Furthermore, the renal cortical expression of pendrin is increased in alkali-loaded animals and decreased in acid-loaded animals (523). Type B intercalated cells are most numerous in the cortical collecting duct. Pendrin is not in the same protein family as AE1 (524). Mutations of the gene encoding pendrin result in
P.888
Pendred's syndrome, a disorder mainly associated with a thyroid goiter and deafness (525). The non-A non-B intercalated cells express pendrin in the apical membrane and cytoplasmic vesicles like type B intercalated cells but also express the H+-ATPase in the apical membrane-like type A intercalated cells (522,526). It is unclear whether the non-A non-B cells function as H+- or HCO3--secreting cells or possibly switch between these two functions. Thus, the types of intercalated cells may be defined by their cellular distribution of the H+-ATPase and the presence or absence of the anion exchangers, AE1 and pendrin (527). Alternatively, it has been proposed that intercalated cells exhibit a high degree of plasticity and may reverse their apical-basolateral polarity in response to changes in the acid-base status (528). For example, it has been reported that type B intercalated cells can convert to type A cells in response to metabolic acidosis (529).
Outer Medullary Collecting Duct
The collecting duct traverses the outer medulla without receiving tributaries. The outer medullary collecting duct is lined by principal cells and intercalated cells (Figure 34.86). The principal cells in this segment are similar to those in the cortical collecting duct but are taller and have fewer organelles and basal membrane infoldings. The intercalated cells constitute 18 to 40% of the cells in the outer medullary collecting duct in some species and gradually decrease along this segment (530,531). The intercalated cells in the outer medullary collecting duct resemble the type A intercalated cells in the cortical collecting duct but are taller and have a less dense cytoplasm.
 |
Figure 34.86 Light micrograph illustrating longitudinal section of an outer medullary collecting duct (original magnification 250). |
The outer medullary collecting duct plays a major role in urine acidification. An increase in the surface area of the apical plasma membrane of the intercalated cells in this segment has been demonstrated after hydrogen ion stimulation (532,533). The apical and basolateral membranes of these cells label with antibodies against the H+-ATPase and the chloride/bicarbonate exchanger, respectively (514,518). These findings suggest that the intercalated cells in the outer medullary collecting duct are responsible for hydrogen ion secretion. In addition, this segment may be an important site of potassium reabsorption. The functional presence of H+/K+-ATPase activity in the outer medullary collecting duct (534,535) and the immunohistochemical (536) and in situ hybridization (537,538) localization of H+/K+-ATPase in the intercalated cells of this segment indicate that these cells are involved in potassium reabsorption in exchange for hydrogen ion secretion.
Inner Medullary Collecting Duct
The inner medullary collecting duct (IMCD) represents the terminal portion of the collecting duct. Although the IMCD is often called the papillary collecting duct, only the inner two-thirds of the IMCD are located in the papilla.
Descending through the inner medulla, the collecting ducts join in successive fusions, which result in an arborescent architectural arrangement. There is a significant increase in diameter and height of the epithelium as the ducts descend (539). The height of the cells increases gradually from cuboidal to columnar (Figure 34.87). However, in the terminal portion of the human inner medulla there is often an abrupt transition between collecting ducts lined with cuboidal cells and the ducts of Bellini, which are composed of tall columnar cells.
Structural and functional heterogeneity exist along the IMCD (539). It can be subdivided arbitrarily into three portions:
P.889
the outer third (IMCD1), middle third (IMCD2), and inner third (IMCD3). However, there is physiologic evidence for the division of the IMCD into two functionally distinct segments, which are termed the initial IMCD and the terminal IMCD (540,541).
 |
Figure 34.87 Micrograph illustrating columnar cells of the collecting duct in the inner medulla (original magnification 500). |
The initial IMCD is the outer segment and mainly corresponds to the IMCD1, whereas the terminal IMCD includes most of the IMCD2 and the IMCD3. The initial IMCD consists mainly of principal cells that are similar in structure to the principal cells in the OMCD. In the rat, intercalated cells similar to the type A intercalated cells in the OMCD comprise approximately 10% of the cells in the initial IMCD (Figure 34.88) (542). Intercalated cells are rare to absent in the initial IMCD of the human (485) and rabbit (530). The terminal IMCD is composed of mainly one cell type, the IMCD cell. Compared with principal cells, the IMCD cells are taller and have lighter staining cytoplasm containing numerous ribosomes, small lysosomes in the basal cytoplasm, and fewer infoldings of the basal plasma membrane (Figure 34.89) (543). By scanning electron microscopy, the IMCD cells display more numerous small microvilli and lack the central cilium characteristic of principal cells (Figure 34.90).
The inner medullary collecting duct has an important role in urinary concentration. The reabsorption of urea and water in this segment causes the formation of a concentrated urine. Physiologic studies demonstrated that urea and water permeabilities are low in the initial IMCD and relatively high in the terminal IMCD (540,541). Water permeability is increased by vasopressin in both subsegments and is mediated by the aquaporin water channel AQP2 present in the apical membrane of the IMCD cells (503). Vasopressin increases urea permeability only in the terminal IMCD. The urea transporters UT-A1 and UT-A3 immunolocalize to the terminal IMCD and mediate urea transport in this segment (544,545,546). There is evidence that the IMCD is also involved in urine acidification. Acid secretion mediated by an H+/K+-ATPase has been demonstrated in isolated perfused segments from this region, although no immunoreactivity for this transport protein has been demonstrated in the IMCD (547).
 |
Figure 34.88 Scanning electron micrograph of the inner medullary collecting duct. The intercalated cell is round and exhibits a convex luminal surface covered with microplicae without cilia. The adjacent principal cells are characterized by short microvilli and a single central cilium on their luminal surface. (Original magnification 12,000.) Reprinted with permission from: Clapp WL, Madsen KM, Verlander JW, Tisher CC. Morphologic heterogeneity along the rat inner medullary collecting duct. Lab Invest 1989;60:219 230. |
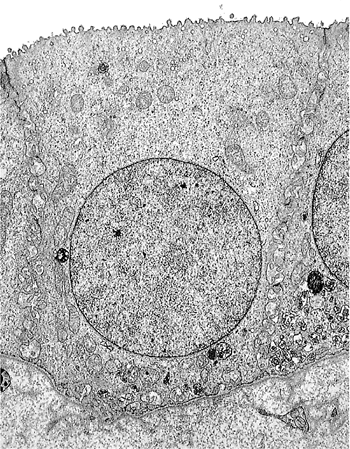 |
Figure 34.89 Electron micrograph of an IMCD cell. The cell is tall, has extensive lateral membranes, and exhibits small stubby microvilli. Infoldings of the basal plasma membrane are not prominent. (Original magnification 12,500.) Reprinted with permission from: Clapp WL, Madsen KM, Verlander JW, Tisher CC. Morphologic heterogeneity along the rat inner medullary collecting duct. Lab Invest 1989;60:219 230. |
Interstitium
The renal interstitium consists of an extracellular matrix containing sulfated and nonsulfated glucosaminoglycans and interstitial cells (548). In humans, estimates of the relative
P.890
cortical interstitial volume range from 5 to 20%, with a mean of 12% (549,550,551). A significant increase with age has been reported (551). The peritubular interstitial tissue in the normal cortex is inconspicuous on light microscopy, and the tubules and capillaries often have a back-to-back architectural appearance (Figure 34.91). Types I and III collagen and fibronectin are present (552). The periarterial connective tissue constitutes a loose sheath around the intrarenal arteries and contains the lymphatic vessels (553). The periarterial connective tissue should not be overinterpreted as representing focal interstitial fibrosis in the cortex. Two types of cortical interstitial cells have been described: a fibroblast-like cell and a lymphocyte-like cell (548). The fibroblast-like cells show immunoreactivity for ecto-5-nucleotidase (5-NT) (554). These 5-NT-postive interstitial fibroblasts are the erythropoietin-producing cells in the kidney (555,556).
 |
Figure 34.90 Scanning electron micrograph of the terminal IMCD. The entire luminal surface of the IMCD cells is covered with abundant short microvilli. There is an absence of cilia. (Original magnification 12,500). Reprinted with permission from: Clapp WL, Madsen KM, Verlander JW, Tisher CC. Morphologic heterogeneity along the rat inner medullary collecting duct. Lab Invest 1989;60:219 230. |
The relative volume of the renal interstitium increases from the cortex to the tip of the papilla. The interstitial volume has been reported from 10 to 20% in the outer medulla to approximately 30 to 40% at the papillary tip in some species (557). This appreciable amount of interstitium in the medulla should not be mistaken for interstitial fibrosis by the pathologist. The medullary interstitium has a gelatinous appearance on light microscopy (Figure 34.92). The interstitial cells in the medulla include lymphocyte-like cells virtually identical to the ones in the cortex, pericytes situated near the descending vasa recta, and prominent lipid-containing cells mainly localized to the inner medulla (548). The latter, the renomedullary interstitial cells, are often arranged in rows between the loop of Henle and the vasa recta, have irregular, long cytoplasmic processes, and contain lipid inclusions. These cells can be observed on 1- m thick toluidine blue-stained sections of the inner medulla. The lipid droplets contain mainly triglycerides that are rich in unsaturated fatty acids, including arachidonic acid, phospholipids, and cholesterol (548). In addition to the synthesis of the extracellular matrix of the interstitium, the renomedullary interstitial cells are believed to contribute to the endocrine-like antihypertensive function of the renal medulla (558,559).
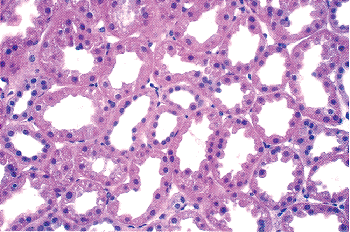 |
Figure 34.91 Biopsy specimen of the cortex of a kidney donated for transplantation. Note the compact arrangement of the tubules and the limited amount of interstitial tissue. (Original magnification 250.) |
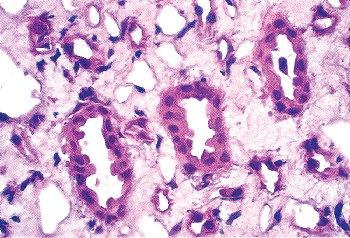 |
Figure 34.92 Renal biopsy specimen illustrating the inner medulla. Note the prominent amount of interstitium surrounding the tubules. (Original magnification 500.) |
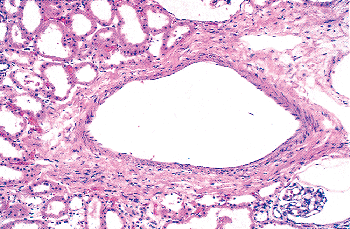 |
Figure 34.93 Micrograph of the corticomedullary junction illustrating an arcuate artery (original magnification 125). |
P.891
Vasculature
A detailed description of the renal vasculature is available (560). The segmental arteries, originating from the anterior and posterior divisions of the main renal artery, divide to form the interlobar arteries, which course toward the cortex along the septa of Berlin between adjacent renal pyramids. At the corticomedullary junction, the interlobar arteries give rise to the arcuate arteries, which follow a gently curved course along the base of the pyramids parallel to the kidney surface (Figure 34.93). The interlobular arteries branch sharply from the arcuate arteries and ascend in the cortex in a radial fashion toward the renal surface. Because the renal lobules cannot be clearly distinguished, it has been recommended that the interlobular arteries be called cortical radial arteries (276,560).
Most afferent arterioles originate from the interlobular arteries, and each supplies a single glomerulus. The angle of origin of the afferent arterioles becomes less recurrent and more open as the interlobular arteries extend to the outer cortex (Figure 34.94) (561). The length of the afferent arterioles is variable; average values of 170 to 280 m have been reported (Figure 34.95) (562,563). Some rare branches of the intrarenal arteries that do not terminate in glomeruli, the so-called aglomerular vessels, may result from degeneration of the connected glomeruli (561). Aglomerular arterioles near the corticomedullary junction have been observed to enter the medulla, and shunt arterioles between afferent and efferent arterioles have been reported (564,565,566).
 |
Figure 34.94 Juxtamedullary glomerulus with a connected hilar arteriole. Note the recurrent angle of the arteriole. (Silver methenamine stain, original magnification 250.) |
 |
Figure 34.95 Micrograph depicting the transverse course of an afferent arteriole supplying a glomerulus. (PAS stain, original magnification 250.) |
The wall structure of the intrarenal arteries and the proximal portion of the afferent arterioles resembles that of blood vessels of the same size elsewhere in the body. The endothelium stains for factor VIII related antigen (Figure 34.96) (567,568) and CD34 (Figure 34.97) (569,570), whereas the muscularis stains for smooth muscle actin (Figure 34.98) (571) and vimentin.
 |
Figure 34.96 Factor VIII is produced by endothelium. The micrograph shows factor VIII immunoperoxidase staining of a medium-sized artery (center), vein (right), and glomerulus (left) (original magnification 62). |
P.892
 |
Figure 34.97 CD34 immunoperoxidase staining demonstrates a greater variety of vascular structures that label more intensely than with factor VIII. In the micrograph, arteries, veins, glomeruli, and peritubular capillaries are immunoreactive (original magnification 62). |
The efferent arterioles from the glomeruli branch to form a complex postglomerular microcirculation (Figure 34.99). Although gradations exist, three basic types of efferent arterioles may be distinguished (572,573). The superficial or outer cortical efferent arterioles are fairly long and divide into extensive capillary networks that supply the convoluted tubules of the cortical labyrinth. These capillaries are readily identified by CD34 and smooth muscle actin staining (Figures 34.98, 34.100,34.101). The midcortical efferent arterioles are variable in length and supply the cortical labyrinth and the straight tubules of the medullary rays. With the exception of the outer cortex, there is dissociation between the tubule segments and the efferent arterioles of their parent glomeruli. In the midcortex and inner cortex, tubule segments are supplied by capillaries of efferent arterioles from other glomeruli (574,575).
P.893
The efferent arterioles from juxtamedullary nephrons descend and supply the entire medulla. In contrast to the efferent arterioles of superficial and midcortical glomeruli (Figure 34.102), those from juxtamedullary glomeruli are larger in diameter, display more layers of smooth muscle cells, and have more endothelial cells on cross sections (274,560).
 |
Figure 34.98 Smooth muscle actin immunoperoxidase stain illustrating a labeled large artery (upper left) and arterioles at the corticomedullary junction and two positive-stained columns of vasa recta (midright) penetrating the medulla. Two venous profiles (upper center) have minimal muscularis. (Original magnification 11.) |
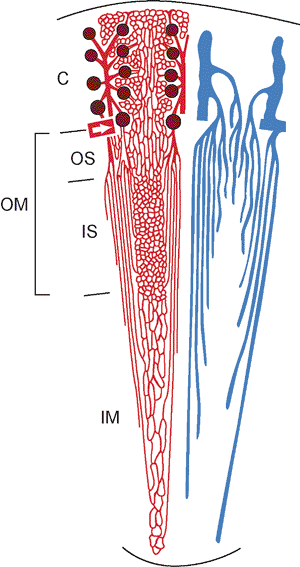 |
Figure 34.99 The renal microvasculature. The left side (red) illustrates the arterial vessels, glomeruli, and capillaries. An interlobular artery originates from an arcuate artery (white arrow) and gives rise to the afferent arterioles, which supply the glomeruli (dark brown). The efferent arterioles of the superficial and midcortical glomeruli supply the capillary plexuses of the cortical labyrinth and the medullary rays. The efferent arterioles of the juxtamedullary glomeruli descend into the medulla and form the descending vasa recta, which supply the adjacent capillary plexuses. Note the prominence of the capillary plexus in the inner stripe of the outer medulla. The right side (blue), which may be superimposed on the left side, displays the venous system. The ascending vasa recta drain the medulla and empty into the arcuate and interlobular veins, which drain the cortex. The vasa recta from the inner medulla ascend within the vascular bundles, whereas most vasa recta from the inner stripe ascend between the bundles. (C, cortex; OM, outer medulla; OS, outer stripe; IS, inner stripe; IM, inner medulla) Modified with permission from: Kriz W, Kaissling B. Structural organization of the mammalian kidney. In: Seldin DW, Giebisch D, eds. The Kidney: Physiology and Pathophysiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:587 654. |
 |
Figure 34.100 The extensive cortical peritubular capillary network is shown by endothelial labeling with CD34 antibody. (CD34 immunoperoxidase, original magnification 120.) |
In the outer stripe of the outer medulla, the efferent arterioles of juxtamedullary nephrons divide to form the descending vasa recta that descend in the vascular bundles but at intervals leave the bundles to form capillary plexuses. The ascending (or venous) vasa recta drain the renal medulla. The ascending vasa recta from the inner medulla join the vascular bundles, whereas most from the inner stripe of the outer medulla ascend between the bundles (560). This architectural arrangement creates a functional separation of the blood flow to the outer and inner medulla. The close proximity of the arterial descending and venous ascending vasa recta within the vascular bundles permits effective countercurrent exchange (274,560). The ascending vasa recta at the corticomedullary junction empty into the arcuate and interlobular veins (Figure 34.98), which do form extensive anastomoses, in contrast to the arcuate arteries.
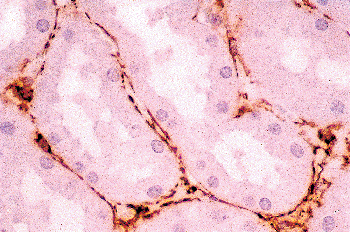 |
Figure 34.101 Smooth muscle actin expression complements and parallels the CD34 expression in documenting the cortical peritubular capillaries. (Smooth muscle actin immunoperoxidase, original magnification 120.) |
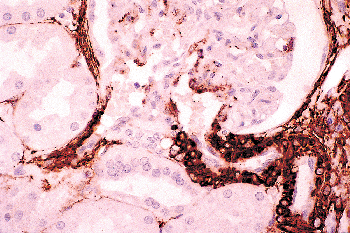 |
Figure 34.102 Smooth muscle actin immunoperoxidase of a superficial glomerulus delineating the more prominent smooth muscle investment of the afferent arteriole (right) compared with the efferent arteriole (left) (original magnification 120). |
The interlobular veins, which accompany the interlobular arteries, drain the cortex and empty into the arcuate veins. In sections the intrarenal veins have less musculature than comparably sized veins in other organs (Figure 34.98). The arcuate veins empty into the interlobar veins, which converge to form a single renal vein that exits at the hilus of the kidney.
Lymphatics
The lymphatic vessels of the kidney are embedded in the loose periarterial connective tissue in the cortex (Figure 34.103) (576,577,578). They are not prominent on routine histologic sections. The lymphatics originate as small vessels around the interlobular arteries and empty into arcuate and interlobar lymphatics, which finally drain into larger lymph vessels at the renal hilus. The interlobar and hilar lymphatics possess valves. Lymphatics are not believed to exist in the renal medulla (560,578). There is a less prominent subcapsular network of lymphatic vessels that appears to communicate with the major intrarenal lymphatics within the cortex (578). It has been proposed that the periarterial spaces and the lymphatics may function as a unit to allow exchange with the venous system and serve as a route for the intrarenal distribution of hormones and inflammatory cells (579). Several markers of lymphatic endothelium are now available including: VEGFR-3 (receptor for VEGF-C, VEGF-D, lymphatic angiogenic factor), LYVE-1 (a CD44 homologous hyaluronate receptor), Prox-1 (lymphatic
P.894
P.895
vessel transcription factor), and podoplanin (a membrane mucoprotein) (580).
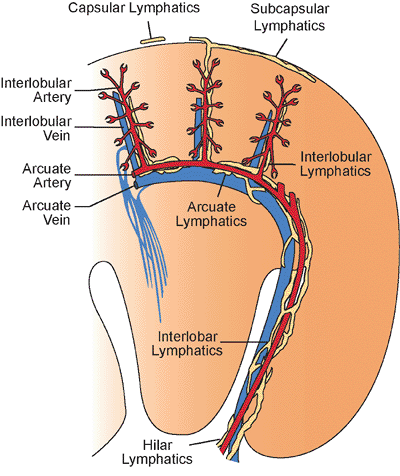 |
Figure 34.103 The lymphatic vessels of the kidney. The arteries (red), veins (blue), and lymphatics (yellow) are illustrated. The lymphatics are primarily distributed in the cortex, although a subcapsular network is also present. Note the absence of the lymphatics in the medulla. Modified with permission from: Madsen KM, Tisher CC. Anatomy of the kidney. In: Brenner BM, ed. Brenner and Rector's The Kidney. 7th ed. Philadelphia: WB Saunders; 2004:3 72. |
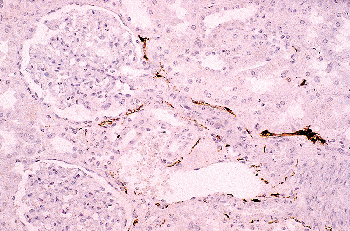 |
Figure 34.104 S-100 immunoperoxidase stain demonstrating nerves extending along the afferent arteriole to the vascular pole of the glomerulus (original magnification 62). |
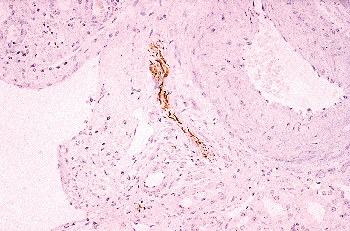 |
Figure 34.105 Phosphoneurofilament (pNF) immunoperoxidase stain showing a nerve between an artery (right) and a vein (left) (original magnification 62). |
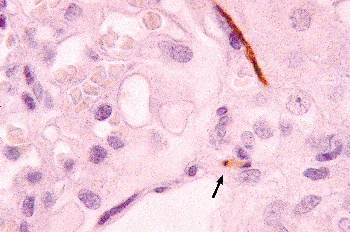 |
Figure 34.106 Two axons are demonstrated in this view of the vascular pole of a glomerulus (center). One axon has a longitudinal profile (upper right), and the other is observed in cross-section as a dot (arrow). (pNF immunoperoxidase, original magnification 300.) |
Nerves
The kidney is innervated by adrenergic fibers mainly derived from the celiac plexus (581). The nerve fibers generally accompany the arteries and arterioles in the cortex and outer medulla (582). Staining for myelin by S-100 (Figure 34.104) or for peripheral axons with antibodies against phosphoneurofilament (pNF) (Figure 34.105) demonstrates the nerve fibers (583,584,585,586). There is prominent innervation of the juxtaglomerular apparatus (Figure 34.106) (587). The efferent arterioles and the descending vasa recta are accompanied by nerve fibers as long as they contain a surrounding smooth muscle layer (588). Although the direct relationship of nerve terminals to tubules is somewhat controversial, autoradiographic studies have provided evidence for monoaminergic innervation of cortical tubules (589,590).
Acknowledgments
We are most grateful to Dr. Bruce Beckwith, whose figures and text from the previous edition of this book were in considerable measure retained. Dr. Beckwith has not only advanced our understanding of pediatric tumors, especially Wilms' tumor, but also provided insights into normal and abnormal kidney development. We also thank Roger Hoover of the University of Florida Biomedical Media Services and Ron Irby of the North Florida South Georgia Veterans Health System for their expert and dedicated assistance with the figures.
References
1. Herring PT. The development of the Malpighian bodies of the kidney, and its relation to pathological changes which occur in them. J Pathol Bacteriol 1900;6:459 496.
2. Huber GC. On the development and shape of uriniferous tubules of certain of the higher mammals. Am J Anat 1905;4(suppl):1 98.
3. Felix W. The development of the urogenital organs. In: Kiebel F, Mall FP, eds. Manual of Human Embryology. Vol. 2. Philadelphia: JB Lippincott; 1912:752 979.
4. Peter K. Untersuchungen ber Bau und Entwicklung der Niere. 2nd ed. Jena, Germany: Verlag Gustav Fischer; 1927.
5. Potter EL. Normal and Abnormal Development of the Kidney. Chicago: Year Book; 1972.
6. Oliver J. Nephrons and Kidneys: A Quantitative Study of Developmental and Evolutionary Mammalian Renal Architectonics. New York: Harper & Row; 1968.
7. Grobstein C. Inductive interaction in the development of the mouse metanephros. J Exp Zool 1955;130:319 340.
8. Saxen L. Organogenesis of the Kidney. Cambridge: Cambridge University Press; 1987.
9. Clapp WL, Abrahamson DR. Development and gross anatomy of the kidney. In: Tisher CC, Brenner BM, eds. Renal Pathology. 2nd ed. Philadelphia: JB Lippincott; 1994:3 59.
10. Risdon RA, Woolf AS. Development of the kidney. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, eds. Heptinstall's Pathology of the Kidney. 5th ed. Philadelphia: Lippincott-Raven; 1998:67 84.
11. Vize PD, Woolf AS, Bard JB. The Kidney: From Normal Development to Congenital Disease. San Diego: Academic Press; 2003.
12. Bush KT, Stuart RO, Nigam SK. Developmental biology of the kidney. In: Brenner BM, ed. Renner & Rector's the Kidney. 7th ed. Philadelphia: WB Saunders; 2004:73 103.
13. Jones EA. Xenopus: a prince among models for pronephric kidney development. J Am Soc Nephrol 2005;16:313 321.
14. Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol 2005;16:299 304.
15. Sainio K. Development of the mesonephric kidney. In: Vize PD, Woolf AS, Bard JB, eds. The Kidney. From Normal Development to Congenital Disease. San Diego: Academic Press; 2003:75 86.
16. Sainio K, Hellstedt P, Kreidberg JA, Saxen L, Sariola H. Differential regulation of two sets of mesonephric tubules by WT-1. Development 1997;124:1293 1299.
17. Pole RJ, Qi BQ, Beasley SW. Patterns of apoptosis during degeneration of the pronephros and mesonephros. J Urol 2002;167:269 271.
18. Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 1996;86:897 906.
19. Kumaravelu P, Hook L, Morrison AM, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development 2002;129:4891 4899.
20. Davies J. Development of the ureteric bud. In: Vize PD, Woolf AS, Bard JB, eds. The Kidney: From Normal Development to Congenital Disease. San Diego: Academic Press; 2003:165 179.
21. al-Awqati Q, Goldberg MR. Architectural patterns in branching morphogenesis in the kidney. Kidney Int 1998;54:1832 1842.
22. Watanabe T, Costantini F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol 2004;271:98 108.
23. Bard J. The metanephros. In: Vize PD, Woolf AS, Bard JB, eds. The Kidney: From Normal Development to Congenital Disease. San Diego: Academic Press; 2003:139 148.
24. Sariola H, Sainio K, Bard J. Fates of the metanephric mesenchyme. In: Vize PD, Woolf AS, Bard JB, eds. The Kidney: From Normal Development to Congenital Disease. San Diego: Academic Press; 2003:181 193.
25. Abrahamson DR. Glomerulogenesis in the developing kidney. Semin Nephrol 1991;11:375 389.
26. Abrahamson DR, Wang R. Development of the glomerular capillary and its basement membrane. In: Vize PD, Woolf AS, Bard JBL, eds. The Kidney: From Normal Development to Congenital Disease. San Diego: Academic Press; 2003:221 249.
P.896
27. Robert B, St John PL, Hyink DP, Abrahamson DR. Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vasculogenic angioblasts. Am J Physiol 1996;271(pt 2):F744 F753.
28. Robert B, St John PL, Abrahamson DR. Direct visualization of renal vascular morphogenesis in Flk1 heterozygous mutant mice. Am J Physiol 1998;275(pt 2):F164 F172.
29. Woolf AS, Yuan HT. The development of kidney blood vessels. In: Vize PD, Woolf AS, Bard JB, eds. The Kidney: From Normal Development to Congenital Disease. San Diego: Academic Press; 2003:251 266.
30. Tryggvason K. Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 1999;10:2440 2445.
31. Ly J, Alexander M, Quaggin SE. A podocentric view of nephrology. Curr Opin Nephrol Hypertens 2004;13:299 305.
32. Kestila M, Lenkkeri U, Mannikko M, et al. Positionally cloned gene for a novel glomerular protein nephrin is mutated in congenital nephrotic syndrome. Mol Cell 1998;1:575 582.
33. Miner JH. Building the glomerulus: a matricentric view. J Am Soc Nephrol 2005;16:857 861.
34. Betsholtz C, Lindblom P, Bjarnegard M, Enge M, Gerhardt H, Lindahl P. Role of platelet-derived growth factor in mesangium development and vasculopathies: lessons learned from platelet-derived growth factor and platelet-derived growth factor receptor mutations in mice. Curr Opin Nephrol Hypertens 2004;13:45 52.
35. Celio MR, Groscurth P, Inagami T. Ontogeny of renin immunoreactive cells in the human kidney. Anat Embryol (Berl) 1985;173:149 155.
36. Gomez RA, Lynch KR, Chevalier RL et al. Renin and angiotensinogen gene expression in maturing rat kidney. Am J Physiol 1988;254(pt 2):F582 F587.
37. Minuth M, Hackenthal E, Poulsen K, Rix, Taugner R. Renin immunocytochemistry of the differentiating juxtaglomerular apparatus. Anat Embryol (Berl) 1981;162:173 181.
38. Gomez RA, Lynch KR, Sturgill BC, et al. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol 1989;257(pt 2):F850 F858.
39. Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol 1998;9:63 71.
40. Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 2001;281:F345 F356.
41. Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 2004;6:719 728.
42. Alcorn D, Maric C, McCausland J. Development of the renal interstitium. Pediatr Nephrol 1999;13:347 354.
43. Levinson R, Mendelsohn C. Stromal progenitors are important for patterning epithelial and mesenchymal cell types in the embryonic kidney. Semin Cell Dev Biol 2003;14:225 231.
44. Cullen-McEwen LA, Caruana G, Bertram JF. The where, what and why of the developing renal stroma. Nephron Exp Nephrol 2005;99:e1 e8.
45. Sundelin B, Bohman SO. Postnatal development of the interstitial tissue of the rat kidney. Anat Embryol (Berl) 1990;182:307 317.
46. Maric C, Ryan GB, Alcorn D. Embryonic and postnatal development of the rat renal interstitium. Anat Embryol (Berl) 1997;195:503 514.
47. Marxer-Meier A, Hegyi I, Loffing J, Kaissling B. Postnatal maturation of renal cortical peritubular fibroblasts in the rat. Anat Embryol (Berl) 1998;197:143 153.
48. Schumacher K, Strehl R, De Vries U, Groene HJ, Minuth WW. SBA-positive fibers between the CD ampulla, mesenchyme, and renal capsule. J Am Soc Nephrol 2002;13:2446 2453.
49. Nadasdy T, Lajoie G, Laszik Z, Blick KE, Molnar-Nadasdy G, Silva FG. Cell proliferation in the developing human kidney. Pediatr Dev Pathol 1998;1:49 55.
50. Cha JH, Kim YH, Jung JY, Han KH, Madson KM, Kim J. Cell proliferation in the loop of Henle in the developing rat kidney. J Am Soc Nephrol 2001;12:1410 1421.
51. Koseki C, Herzlinger D, al-Awqati Q. Apoptosis in metanephric development. J Cell Biol 1992;119:1327 1333.
52. Coles HS, Burne JF, Raff MC. Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development 1993;118:777 784.
53. Winyard PJ, Nauta J, Lirenman DS, et al. Deregulation of cell survival in cyctic and dysplastic renal development. Kidney Int 1996;49:135 146.
54. Kim J, Cha JH, Tisher CC, Madsen KM. Role of apoptotic and nonapoptotic cell death in removal of intercalated cells from developing rat kidney. Am J Physiol 1996;270(pt 2):F575 F592.
55. Kim J, Lee GS, Tisher CC, Madsen KM. Role of apoptosis in development of the ascending thin limb of the loop of Henle in rat kidney. Am J Physiol 1996;271(pt 2):F831 F845.
56. Fierlbeck W, Liu A, Coyle R, Ballermann BJ. Endothelial cell apoptosis during glomerular capillary lumen formation in vivo. J Am Soc Nephrol 2003;14:1349 1354.
57. Foley GD, Bard JB. Apoptosis in the cortex of the developing mouse kidney. J Anat 2002;210:477 484.
58. Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 1993;75:229 240.
59. Cho EA, Dressler GR. The formation and development of nephrons. In: Vize PD, Woolf AS, Bard JB, eds. The Kidney: From Normal Development to Congenital Disease. San Diego: Academic Press; 2003:195 210.
60. Carroll TJ, McMahon AP. Overview: the molecular basis of kidney development. In: Vize PD, Woolf AS, Bard JB, eds. The Kidney: From Normal Development to Congenital Disease. San Diego: Academic Press; 2003:343 376.
61. Yu J, McMahon AP, Valerius MT. Recent genetic studies of mouse kidney development. Curr Opin Genet Dev 2004;14:550 557.
62. Gawlik A, Quaggin SE. Conditional gene targeting in the kidney. Curr Mol Med 2005;5:527 536.
63. Karavanov AA, Karavanova I, Perantoni A, Dawid IB. Expression pattern of the rat Lim-1 homeobox gene suggests a dual role during kidney development. Int J Dev Biol 1998;42:61 66.
64. Tsang TE, Shawlot W, Kinder SJ, et al. Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev Biol 2000;223:77 90.
65. Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 2005;132:2809 2823.
66. Xu PX, Adams, J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet 1999;23:113 117.
67. Sajithlal G, Zou D, Silvius D, Xu PX. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol 2005;284:323 336.
68. Li X, Oghi KA, Zhang J, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature 2003;426:247 254.
69. Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development 2003;130:3085 3094.
70. Ruf RG, Xu PX, Silvius D, et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A 2004;101:8080 8095.
71. Nishinakamura R, Matsumoto Y, Nakao K, et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 2001;128:3105 3115.
72. Patterson LT, Pembaur M, Potter SS. Hoxa11 and Hoxd11 regulate branching morphogenesis of the ureteric bud in the developing kidney. Development 2001;128:2153 2161.
73. Wellik DM, Hawkes PJ, Capecchi MR. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev 2002;16:1423 1432.
74. Dressler GR, Deutsch U, Chowdhury C, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 1990;109:787 795.
P.897
75. Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development 1995;121:4057 4065.
76. Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 2001;128:4747 4756.
77. Kreidberg JA, Sariola H, Loring J, et al. WT-1 is required for early kidney development. Cell 1993;74:679 691.
78. Donovan MJ, Natoli TA, Sainio K, et al. Initial differentiation of the metanephric mesenchyme is independent of WT1 and the ureteric bud. Dev Genet 1999;24:252 262.
79. Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 2000;127:1387 1395.
80. Kume T, Deng K, Hogan BL. Minimal phenotype of mice homozygous for a null mutation in the forkhead/winged helix gene, Mf2. Mol Cell Biol 2000;20:1419 1425.
81. Grieshammer U, Ma L, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell 2004;6:709 717.
82. Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 1995;9:2795 2807.
83. Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 1995;9:2808 2820.
84. Godin RE, Takaesu NT, Robertson EJ, Dudley AT. Regulation of BMP7 expression during kidney development. Development 1998;125:3473 3482.
85. Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR. FGF8 is required for cell survival at distinct stages of nephrogenesis and for the regulation of gene expression in nascent nephrons. Development 2005;132:3847 3857.
86. Perantoni AO, Timofeeva O, Naillat F, et al. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 2005;132:3859 3871.
87. Sorenson CM, Rogers SA, Korsmeyer SJ, Hammerman MR. Fulminant metanephric apoptosis and abnormal kidney development in bcl-2-deficient mice. Am J Physiol 1995;268(pt 2):F73 F81.
88. Sorenson CM, Padanilam B, Hammerman MR. Abnormal postpartum renal development and cytogenesis in the bcl-2 (-/-) mouse. Am J Physiol 1996;271:F184 F193.
89. Moser M, Pscherer A, Roth C, et al. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2 beta. Genes Dev 1997;11:1938 1948.
90. Moser M, Dahmen S, Kluge R, et al. Terminal renal failure in mice lacking transcription factor AP-2 beta. Lab Invest 2003;83:571 578.
91. Favor J, Sandulache R, Neuhauser-Klaus A, et al. The mouse Pax21Neu mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc Natl Acad Sci U S A 1996;93:13870 13875.
92. Porteous S, Torban E, Cho N-P, et al. Primary renal hypoplasia in humans and mice with PAX2 mutations: evidence of increased apoptosis in fetal kidneys of Pax21Neu +/- mutant mice. Hum Mol Genet 2000;9:1 11.
93. Torban E, Eccles MR, Favor J, Goodyer PRl. PAX2 suppresses apoptosis in renal collecting duct cells. Am J Pathol 2000;157:833 842.
94. Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 1999;126:547 554.
95. Bates CM, Kharzai S, Erwin T, Rossant J, Parada LF. Role of N-myc in the developing mouse kidney. Dev Biol 2000;222:317 325.
96. Cano-Gauci DF, Song HH, Yang H, et al. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol 1999;146:255 264.
97. Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol 2001;231:31 46.
98. Sanchez MP, Silos-Santiago L, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 1996;382:70 73.
99. Pichel JG, Shen L, Sheng HZ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 1996;382:73 76.
100. Moore MW, Klein RD, Farinas I, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature 1996;382:76 79.
101. Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 1994;367:380 383.
102. Schuchardt A, D'Agati V, Pachnis V, Costantini F. Renal agenesis and hypodysplasia in ret-k-mutant mice result from defects in ureteric bud development. Development 1996;122:1919 1929.
103. Enomoto H, Araki T, Jackman A, et al. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron 1998;21:317 324.
104. Esquela AF, Lee SJ. Regulation of metanephric kidney development by growth/differentiation factor 11. Dev Biol 2003;257:356 370.
105. Basson MA, Akbulut S, Watson-Johnson J, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 2005;8:229 239.
106. Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development 1997;124:1653 1664.
107. Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development 1999;126:1139 1148.
108. Batourina E, Gim S, Bello N, et al. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet 2001;27:74 78.
109. Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 2000;105:863 873.
110. Quaggin SE, Schwartz L, Cui S, et al. The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis. Development 1999;126:5771 5783.
111. Bullock SL, Fletcher JM, Beddington RS, Wilson VA. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev 1998;12:1894 1906.
112. Muller U, Wang D, Denda S, Meseses JJ, Pederson RA, Reichardt LF. Integrin alpha-8/beta-1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell 1997;88:603 613.
113. Kreidberg JA, Donovan MJ, Goldstein SL, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 1996;122:3537 3547.
114. Schnabel CA, Godin RE, Cleary ML. Pbx1 regulates nephrogenesis and ureteric branching in the developing kidney. Dev Biol 2003;254:262 276.
115. Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 2004;131:3401 3410.
116. Majumdar A, Vainio S, Kispert A, McMahaon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 2003;130:3175 3185.
117. Maas R, Elfering S, Glaser T, Jepeal L. Deficient outgrowth of the ureteric bud underlies the renal agenesis phenotype in mice manifesting the limb deformity (ld) mutation. Dev Dyn 1994;199:214 228.
118. Niimura F, Labosky PA, Kakuchi J, et al. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest 1995;96:2947 2954.
119. Nagata M, Tanimoto K, Fukamizu A, et al. Nephrogenesis and renovascular development in angiotensinogen-deficient mice. Lab Invest 1996;75:745 753.
P.898
120. Yanai K, Saito T, Kakinuma Y, et al. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem 2000;275:5 8.
121. Takahashi N, Lopez ML, Cowhig JE Jr, et al. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 2005;16:125 132.
122. Esther CR Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest 1996;7:953 965.
123. Tsuchida S, Matsusaka T, Chen X, et al. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest 1998;101:755 760.
124. Oliverio MI, Kim HS, Ito M, et al. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci U S A 1998;95:15496 15501.
125. Nishimura H, Yerkes E, Hohenfellner K, et al. Role of the angiotensin type 2 receptor gene and congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell 1999;3:1 10.
126. Oshima K, Miyazaki Y, Brock JW III, Adams MC, Ichikawa I, Pop JC IV. Angiotensin type II receptor expression and ureteral budding. J Urol 2001;166:1848 1852.
127. Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 1994;372:679 683.
128. Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 2005;9:283 292.
129. Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev 1996;10:1467 1478.
130. Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 2005;132:529 539.
131. Cui S, Schwartz, Quaggin SE. Pod1 is required in stromal cells for glomerulogenesis. Dev Dyn 2003;226:512 522.
132. McGregor L, Makela V, Darling SM, et al. Fraser syndrome and mouse blebbed phenotype caused by mutations in FRAS1/Fras1 encoding a putative extracellular matrix protein. Nat Genet 2003;34:203 208.
133. Vrontou S, Petrou P, Meyer BI, et al. Fras1 deficiency results in cryptophthalmos, renal agenesis and blebbed phenotype in mice. Nat Genet 2003;34:209 214.
134. Takamiya K, Kostourou V, Adams S, et al. A direct functional link between the multi-PDZ domain protein GRIP1 and the Fraser syndrome protein Fras1. Nat Genet 2004;36:172 177.
135. Mah SP, Saueressig H, Goulding M, Kintner C, Dressler GR. Kidney development in cadherin-6 mutants: delayed mesenchyme-to-epithelial conversion and loss of nephrons. Dev Biol 2000;223:38 53.
136. Patek CE, Fleming S, Miles CG, et al. Murine Denys-Drash syndrome: evidence of podocyte de-differentiation and systemic mediation of glomerulosclerosis. Hum Mol Genet 2003;12:2379 2394.
137. Gao F, Maiti S, Sun G, et al. The Wt1+R394W mouse displays glomerulosclerosis and early-onset renal failure characteristic of human Denys-Drash syndrome. Mol Cell Biol 2004;24:9899 9910.
138. Hammes A, Guo JK, Lutsch G, et al. Two splice variants of the Wilms' tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 2001;106:319 329.
139. Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 1994;8:1875 1887.
140. Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 1994;8:1888 1896.
141. McCright B, Gao X, Shen L, et al. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development 2001;128:491 502.
142. Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 2003;111:707 716.
143. Miner JH, Li C. Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol 2000;217:278 289.
144. Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet 1995;10:400 406.
145. Cosgrove D, Meehan DT, Grunkemeyer JA, et al. Collagen COL4A3 knockout: a mouse model for autosomal Alport syndrome. Genes Dev 1996;10:2981 2992.
146. Miner JH, Sanes JR. Molecular and functional defects in kidneys of mice lacking collagen alpha 3(IV): implications for Alport syndrome. J Cell Biol 1996;135:1403 1413.
147. Lu W, Phillips CL, Killen PD. Insertional mutation of the collagen genes Col4a3 and Col4a4 in a mouse model of Alport syndrome. Genomics 1999;61:113 124.
148. Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 2001; 10:1 8.
149. Shih NY, Li J, Karpitskii V, et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 1999;286:312 315.
150. Roselli S, Heidet L, Sich M, et al. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol 2004;24:550 560.
151. Kaplan JM, Kim SH, North KN, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 2000;24:251 256.
152. Kos CH, Le TC, Sinha S, et al. Mice deficient in alpha-actinin-4 have severe glomerular disease. J Clin Invest 2003;111:1683 1690.
153. Winn MP, Conlon PJ, Lynn KL, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 2005;308:1801 1804.
154. Reiser J, Polu KR, Moller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 2005;37:739 744.
155. Donoviel DB, Freed DD, Vogel H, et al. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 2001;21:4829 4836.
156. Ciana L, Patel A, Allen ND, et al. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalites and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol 2003;23:3575 3582.
157. Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in Lmxb1 mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet 1998;19:51 55.
158. Miner JH, Morello R, Andrews KL, et al. Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J Clin Invest 2002;109:1065 1072.
159. Doyonnas R, Kershaw DB, Duhme C, et al. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med 2001;194:13 27.
160. Wharram BL, Goyal M, Gillespie PJ, et al. Altered podocyte structure in GLEPP1(Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate. J Clin Invest 2000;106:1281 1290.
161. Sadl VS, Jin F, Yu J, et al. The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev Biol 2002;249:16 29.
162. Lu W, Peissel B, Babakhanlou H, et al. Perinatal lethality with kidney and pancreas defects in mice with a targeted Pkd1 mutation. Nat Genet 1997;17:179 181.
P.899
163. Lu W, Fan X, Basora N, et al. Late onset of renal and hepatic cysts in Pkd1-targeted heterozygotes. Nat Genet 1999;21:160 161.
164. Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sanford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci U S A 2001;98:12174 12179.
165. Chauvet V, Qian F, Boute N, et al. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am J Pathol 2002; 160:973 983.
166. Wu G, Markowitz GS, Li L, et al. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 2000;24:75 78.
167. Wu G, D'Agati V, Cai Y, et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 1998;93:177 188.
168. Jadeja S, Smyth I, Pitera JE, et al. Identification of a new gene mutated in Fraser syndrome and mouse myelencephalic blebs. Nat Genet 2005;37:520 525.
169. Morham SG, Langenbach R, Loftin CD, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 1995;83:473 482.
170. Norwood VF, Morham SG, Smithies O. Postnatal development and progression of renal dysplasia in cyclooxygenase-2 null mice. Kidney Int 2000;58:2291 2300.
171. Threadgill DW, Dlugosz AA, Hansen LA, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotypes. Science 1995;269:230 234.
172. Bernardini N, Bianchi F, Lupetti M, Dolfi A. Immunohistochemical localization of the epidermal growth factor, transforming growth factor alpha, and their receptor in the human mesonephros and metanephros. Dev Dyn 1996;206:231 238.
173. Nakai S, Sugitani Y, Sato H, et al. Crucial roles of Brn1 in distal tubule formation and function in mouse kidney. Development 2003;130:4751 4759.
174. Wang P, Pereira FA, Beasley D, Zheng H. Presenilins are required for the formation of comma- and S-shaped bodies during nephrogenesis. Development 2003;130:5019 5029.
175. Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 2003;130:5031 5042.
176. Lo SH, Yu QC, Degenstein L, Chen LB, Fuchs E. Progressive kidney degeneration in mice lacking tensin. J Cell Biol 1997;136:1349 1361.
177. Dahl U, Sjodin A, Larue L, et al. Genetic dissection of cadherin function during nephrogenesis. Mol Cell Biol 2002;22:1474 1487.
178. Moore KL, Persaud TVN. The Developing Human: Clinically Oriented Embryology. 7th ed. Philadelphia: WB Saunders; 2003.
179. Gruenwald P. The normal changes in the position of the embryonic kidney. Anat Rec 1943;85:163 176.
180. Friedland GW, de Vries P. Renal ectopia and fusion. Embryologic basis. Urology 1975;5:698 706.
181. Muller F, O'Rahilly R. Somitic-vertebral correlation and vertebral levels in the human embryo. Am J Anat 1986;177:3 19.
182. Bremer JL. The origin of the renal artery in mammals and its anomalies. Am J Anat 1915;18:179 200.
183. Emery JL, Mithal A. The weights of kidneys in late intra-uterine life and childhood. J Clin Pathol 1960;13:490 493.
184. Gruenwald P, Minh HN. Evaluation of body and organ weights in perinatal pathology. I. Normal standards derived from autopsies. Am J Clin Pathol 1960;34:247 253.
185. Singer DB, Sung CJ, Wigglesworth JS. Fetal growth and maturation: with standards for body and organ development. In: Wigglesworth JS, Singer DB, eds. Textbook of Fetal and Perinatal Pathology. 2nd ed. Oxford: Blackwell; 1998:8 40.
186. Guihard-Costa AM, Menez F, Delezoide AL. Organ weights in human fetuses after formalin fixation: standards by gestational age and body weight. Pediatr Dev Pathol 2002;5:559 578.
187. Hansen K, Sung CJ, Huang C, Pinar H, Singer DB, Oyer CE. Reference values for second trimester fetal and neonatal organ weights and measurements. Pediatr Dev Pathol 2003;6:160 167.
188. Maroun LL, Graem N. Autopsy standards of body parameters and fresh organ weights in nonmacerated and macerated human fetuses. Pediatr Dev Pathol 2005;8:204 217.
189. Coppoletta JM, Wolbach SB. Body length and organ weights of infants and children. Am J Pathol 1933;9:55 70.
190. Kelley HA, Burnam CF. Diseases of the Kidneys, Ureters, and Bladder. Vol.1. New York: Appleton; 1925.
191. Hodson J. The lobar structure of the kidney. Br J Urol 1972;44:246 261.
192. L fgren F. Das topographische System der Malpighischen Pyramiden der Menschenniere. Uppsala, Sweden: Lund Hakan Ohlssons Boktryckeri; 1949.
193. Sykes D. The morphology of renal lobulations and calices, and their relationship to partial nephrectomy. Br J Surg 1964;51:294 304.
194. Crelin ES. Functional Anatomy of the Newborn. New Haven, CT: Yale University Press; 1976.
195. Campos ES. Pathological changes in the kidney in congenital syphilis. Johns Hopkins Hosp Bull 1923;34:253 263.
196. Potter EL, Thierstein ST. Glomerular development in the kidney as an index of fetal maturity. J Pediatr 1943;22:695 706.
197. Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo G. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 2004;7:17 25.
198. Singer DB, Klish W. Morphometric studies of the renal glomerulogenic zone. Am J Pathol 1970;59:32a.
199. Tsuda S. Histologic investigation of the foetal kidney. Jap J Obstet Gynecol 1934;17:337 341.
200. Dorovini-Zis K, Dolman CL. Gestational development of brain. Arch Pathol Lab Med 1977;101:192 195.
201. Hinchliffe SA, Sargent PH, Chan YF, et al. Medullary ray glomerular counting as a method of assessment of human nephrogenesis. Pathol Res Pract 1992;188:775 782.
202. Peter K. Harnorgane, Organe Uropoietica. In: Peter K, Wetzel G, Heiderich F, eds. Handbuch der Anatomie des Kindes. Vol. 2. Munich: JF Bergmann; 1938:1 41.
203. Benjamin DR, Beckwith JB. Medullary ray nodules in infancy and childhood. Arch Pathol 1973;96:33 35.
204. Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 1991;64:777 784.
205. Hughson M, Farris AB III, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 2003;63:2113 2122.
206. Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol 1992;99:296 301.
207. Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int 2000;58:770 773.
208. Fogo A, Hawkins EP, Berry PL, et al. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int 1990;38:115 123.
209. Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 1992;232:194 201.
210. Merlet-Benichou C, Gilbert T, Vilar J, Moreau E, Freund N, Lelievre-Pegorier M. Nephron number: variability is the rule. Causes and consequences. Lab Invest 1999;79:515 527.
211. Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K. Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol 2005;16:2557 2564.
212. Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more of the other? Am J Hypertens 1988;1(pt 1):335 347.
213. Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Eng J Med 2003;348:101 108.
214. Gruenwald P, Popper H. The histogenesis and physiology of the renal glomerulus in early postnatal life: histological examination. J Urol 1940;43:452 459.
P.900
215. MacDonald MS, Emery JL. The late intrauterine and postnatal development of human renal glomeruli. J Anat 1959;93:331 340.
216. Vernier RL, Birch-Andersen A. Studies of the human fetal kidney. I. Development of the glomerulus. J Pediatr 1962;60:754 768.
217. Thony HC, Luethy CM, Zimmermann A, et al. Histological features of glomerular immaturity in infants and small children with normal or altered tubular function. Eur J Pediatr 1995;154(suppl 4):S65 S68.
218. Volger C, McAdams J, Homan SM. Glomerular basement membrane and lamina densa in infants and children: an ultrastructural evaluation. Pediatr Pathol 1987;7:527 534.
219. Ramage IJ, Howatson AG, McColl JH, Maxwell H, Murphy AV, Beattie TJ. Glomerular basement membrane thickness in children: a stereologic assessment. Kidney Int 2002;62:895 900.
220. Steffes MW, Barbosa J, Basgen JM, Sutherland DE, Najarian JS, Mauer SM. Quantitative glomerular morphology of the normal human kidney. Lab Invest 1983;49:82 86.
221. Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol 2003;285:F40 F48.
222. Petermann AT, Krofft R, Blonski M, et al. Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int 2003;64:1222 1231.
223. Fetterman GH, Shuplock NA, Philipp FJ, Gregg HS. The growth and maturation of human glomeruli and proximal convolutions from term to adulthood: studies by microdissection. Pediatrics 1965;35:601 619.
224. Zolnai B, Palkovits M. Glomerulometrics III. Data referring to the growth of the glomeruli in man. Acta Biol Sci Hung 1965;15:409 423.
225. Souster LP, Emery JL. The sizes of renal glomeruli in fetuses and infants. J Anat 1980;130(pt 3):595 602.
226. Moore L, Williams R, Staples A. Glomerular dimensions in children under 16 years of age. J Pathol 1993;171:145 150.
227. Akaoka K, White RH, Raafat F. Human glomerular growth during childhood: a morphometric study. J Pathol 1994;173:261 268.
228. Samuel T, Hoy WE, Douglas-Denton R, Hughson MD, Bertram JF. Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol 2005;16:3102 3109.
229. Steffes MW, Schmidt D, McCrery R, Basgen JM; International Diabetic Nephropathy Study Group. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 2001;59:2104 2113.
230. Combs HL, Shankland SJ, Setzer SV, Hudkins KL, Alpers CE. Expression of the cyclin kinase inhibitor, p27kip1, in developing and mature human kidney. Kidney Int 1998;53:892 896.
231. Nagata M, Nakayama K, Terada Y, Hoshi S, Watanabe T. Cell cycle regulation and differentiation in the human podocyte lineage. Am J Pathol 1998;153:1511 1520.
232. Hiromura K, Haseley LA, Zhang P, et al. Podocyte expression of the CDK-inhibitor p57 during development and disease. Kidney Int 2001;60:2235 2246.
233. Kampmeier OF. The metanephros or so-called permanent kidney in part provisional and vestigial. Anat Rec 1926;33:115 120.
234. Emery JL, Macdonald MS. Involuting and scarred glomeruli in the kidneys of infants. Am J Pathol 1960;36:713 723.
235. Herxheimer G. Uber hyaline Glomeruli der Neugeborenen und Sauglinge. Frankfurt Ztschr Path 1909;2:138 152.
236. Schwarz L. Weitere Beitrage zur Kenntnis der anatomischen Nierenveranderungen der Neugeborenen und Sauglinge. Virchows Arch Path Anat 1928;267:654 689.
237. Friedman HH, Grayzel DM, Lederer M. Kidney lesions in stillborn and newborn infants. Congenital glomerulosclerosis. Am J Pathol 1942;18:699 713.
238. Thomas MA. Congenital glomerulosclerosis. Pathology 1969;1:105 112.
239. Moffat DB, Fourman J. Ectopic glomeruli in the human and animal kidney. Anat Rec 1964;149:1 7.
240. MacCallum DB. The bearing of degenerating glomeruli on the problem of the vascular supply of the mammalian kidney. Am J Anat 1939;65:69 103.
241. Darmady EM, Prince J, Stranack F, Offer J. The proximal convoluted tubule in the renal handling of water. Lancet 1964;2:1254 1257.
242. Evan AP, Larsson L. Morphologic development of the nephron. In: Edelmann CM Jr, Bernstein J, Meadow SR, Spitzer A, Travis LB, eds. Pediatric Kidney Disease. 2nd ed. Boston: Little Brown & Co; 1992:19 48.
243. Satlin LM, Woda CB, Schwartz GJ. The development of function in the metanephric kidney. In: Vize PD, Woolf AS, Bard JB, eds. The Kidney: From Normal Development to Congenital Disease. San Diego: Academic Press; 2003:267 325.
244. Neiss WF. Histogenesis of the loop of Henle in the rat kidney. Anat Embryol (Berl) 1982;164:315 330.
245. Raptopoulos V, Kleinman PK, Mark S Jr, Synder M, Silverman PM. Renal fascial pathway: posterior extension of pancreatic effusions within the anterior pararenal space. Radiology 1986;158:367 374.
246. Tobin CE. The renal fascia and its relation to the transversalis fascia. Anat Rec 1944;89:295 311.
247. Kunin M. Bridging septa of the perinephric space: anatomic, pathologic, and diagnostic considerations. Radiology 1986;158:361 365.
248. Kochkodan EJ, Hagger AM. Visualization of the renal fascia: a normal finding in urography. AJR Am J Roentgenol 1983;140:1243 1244.
249. Parienty RA, Pradel J, Picard JD, Ducellier R, Lubrano JM, Smolarski N. Visibility and thickening of the renal fascia on computed tomograms. Radiology 1981;139:119 124.
250. Wald H. The weight of normal adult human kidneys and its variability. Arch Pathol Lab Med 1937;23:493 500.
251. Kasiske BL, Umen AJ. The influence of age, sex, race, and body habitus on kidney weight in humans. Arch Pathol Lab Med 1986;110:55 60.
252. Frimann-Dahl J. Normal variations of the left kidney. An anatomical and radiologic study. Acta Radiol 1961;55:207 216.
253. Graves FT. The anatomy of the intrarenal arteries and its application to segmental resection of the kidney. Br J Surg 1954;42:132 139.
254. Graves FT, Graves D. Anatomical Studies for Renal and Intrarenal Surgery. Bristol, England: John Wright; 1986.
255. Satyapal KS. Classification of the drainage patterns of the renal veins. J Anat 1995;186(pt 2):329 333.
256. Sperber I. Studies on the mammalian kidney. Zool Bidrag Uppsala 1944;22:249 432.
257. Hodson CJ, Mariani S. Large cloisons. AJR Am J Roentgenol 1982;139:327 332.
258. Lafortune M, Constantin A, Breton G, Vallee C. Sonography of the hypertrophied column of Bertin. AJR Am J Roentgenol 1986;146:53 56.
259. Bigongiari LR, Patel SK, Appelman H, Thombury JR. Medullary rays. Visualization during excretory urography. Am J Roentgenol Ther Nucl Med 1975;125:795 803.
260. Hodson CJ. The renal parenchyma and its blood supply. Curr Probl Diagn Radiol 1978;7:1 32.
261. Ransley PG, Risdon RA. Renal papillary morphology in infants and young children. Urol Res 1975;3:111 113.
262. Ransley PG. Intrarenal reflux: anatomical, dynamic and radiologic studies part I. Urol Res 1977;5:61 69.
263. Schmidt-Nielsen B. The renal pelvis. Kidney Int 1987;31:621 628.
264. Murphy WM, Grignon DJ, Perlman EJ. Tumors of the kidney, bladder, and related urinary structures. In: Atlas of Tumor Pathology. 4th series, fascicle 1. Washington, DC: Armed Forces Institute of Pathology; 2004.
265. Amis ES Jr, Cronan JJ. The renal sinus: an imaging review and proposed nomenclature for sinus cysts. J Urol 1988;139:1151 1159.
266. Beckwith JB. National Wilms Tumor Study: an update for pathologists. Pediatr Dev Pathol 1998;1:79 84.
267. Bonsib SM, Gibson D, Mhoon M, Greene GF. Renal sinus involvement in renal cell carcinomas. Am J Surg Pathol 2000;24:451 458.
268. Bonsib SM. The renal sinus is the principal invasive pathway: a prospective study of 100 renal cell carcinomas. Am J Surg Pathol 2004;28:1594 1600.
P.901
269. Oliver J. Architecture of the Kidney in Chronic Bright's Disease. New York: Hoeber; 1939.
270. Schmidt-Nielsen B, O'Dell R. Structure and concentrating mechanism in the mammalian kidney. Am J Physiol 1961;200:1119 1124.
271. Madsen KM, Tisher CC. Structural-functional relationships along the distal nephron. Am J Physiol 1986;250(pt 2):F1 F15.
272. Jamison RL, Kriz W. Urinary Concentrating Mechanism: Structure and Function. New York: Oxford University Press;1982.
273. Sands JM, Kokko JP, Jacobson HR. Intrarenal heterogeneity: vascular and tubular. In: Seldin DW, Giebisch D, eds. The Kidney: Physiology and Pathophysiology. 2nd ed. New York: Raven Press; 1992:1087 1155.
274. Kriz W, Kaissling B. Structural organization of the mammalian kidney. In: Seldin DW, Giebisch D, eds. The Kidney: Physiology and Pathophysiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:587 654.
275. Knepper M, Burg M. Organization of nephron function. Am J Physiol 1983;244:F579 F589.
276. Kriz W, Bankir L. A standard nomenclature for structures of the kidney. The Renal Commission of the International Union of Physiological Sciences (IUPS). Kidney Int 1988;33:1 7.
277. Croker BP, Tisher CC. Indications for and interpretation of the renal biopsy: evaluation by light, electron and immunofluorescence microscopy. In: Schrier RW, ed. Diseases of the Kidney. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
278. Pirani CL, Croker BP. Handling and processing of renal biopsy and nephrectomy specimens. In: Tisher CC, Brenner BM, eds. Renal Pathology. With Clinical and Functional Correlations. 2nd ed. Philadelphia: JB Lippincott; 1994:1683 1694.
279. Walker PD, Cavallo T, Bonsib SM; Ad Hoc Committee on Renal Biopsy Guidelines of the Renal Pathology Society. Practice guidelines for the renal biopsy. Mod Pathol 2004;17:1555 1563.
280. Madsen KM, Tisher CC. Anatomy of the kidney. In: Brenner BM, ed. Brenner and Rector's The Kidney. 7th ed. Philadelphia: WB Saunders; 2004:3 72.
281. Malpighi M. De Viscerum Structura Exercitatio Anatomica. Bonn, Germany: De Liene; 1666.
282. Hayman JM Jr. Malpighi's Concerning the structure of the kidneys. Ann Med Hist 1925;7:242 263.
283. Bowman W. On the structure and use of the Malpighian bodies of the kidney, with observations on the circulation through that gland. Philos Trans R Soc Lond 1842;132:57 80.
284. Fine LG. William Bowman's description of the glomerulus. Am J Nephrol 1985;5:437 440.
285. Geneser F. Textbook of Histology. Philadelphia: Lea & Febiger; 1986.
286. Jorgensen F. The Ultrastructure of the Normal Human Glomerulus. Copenhagen: Munksgaard; 1966.
287. Tisher CC, Brenner BM. Structure and function of the glomerulus. In: Tisher CC, Brenner BM, eds. Renal Pathology. With Clinical and Functional Correlations. 2nd ed. Philadelphia: JB Lippincott; 1994:143 161.
288. Newbold KM, Sandison A, Howie AJ. Comparison of size of juxtamedullary and outer cortical glomeruli in normal adult kidney. Virchows Arch A Pathol Anat Histopathol 1992;420:127 129.
289. Newbold KM, Howie AJ, Koram A, ADu D, Michael J. Assessment of glomerular size in renal biopsies including minimal change nephropathy and single kidneys. J Pathol 1990;160:255 258.
290. Bonsib SM, Reznicek MJ. Renal biopsy frozen section: a fluorescent study of hematoxylin and eosin-stained sections. Mod Pathol 1990;3:204 210.
291. Kaplan C, Pasternak B, Shah H, Gallo G. Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol 1975;80:227 234.
292. Kappel B, Olsen S. Cortical interstitial tissue and sclerosed glomeruli in the normal human kidney, related to age and sex. A quantitative study. Virchows Arch A Pathol Anat Histol 1980;387:271 277.
293. Smith SM, Hoy WE, Cobb L. Low incidence of glomerulosclerosis in normal kidneys. Arch Pathol Lab Med 1989;113:1253 1255.
294. Neugarten J, Gallo G, Silbiger S, Kasiske B. Glomerulosclerosis in aging humans is not influenced by gender. Am J Kidney Dis 1999;34:884 888.
295. Vasmant D, Maurice M, Feldmann G. Cytoskeleton ultrastructure of podocytes and glomerular endothelial cells in man and in the rat. Anat Rec 1984;210:17 24.
296. Sorensson J, Fierlbeck W, Heider T, et al. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol 2002;13:2639 2647.
297. Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous sieve plugs in capillary fenestrae. Microvasc Res 1997;53:1 13.
298. Horvat R, Hovoka A, Dekan G, Poczewski H, Kerjaschki D. Endothelial cell membranes contain podocalyxin the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol 1986;102:484 491.
299. Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 1984;98:1591 1596.
300. Rostgaard J, Qvortrup K. Sieve plugs in fenestrae of glomerular capillaries site of the filtration barrier? Cells Tissue Organs 2002;170:132 138.
301. Hjalmarsson C, Johansson BR, Haraldsson B. Electron microscopic evaluation of the endothelial surface layer of glomerular capillaries. Microvas Res 2004;67:9 17.
302. Ciarimboli G, Hjalmarsson C, Bokenkamp A, Schurek HJ, Haraldsson B. Dynamic alterations of glomerular charge density in fixed rat kidneys suggest involvement of endothelial cell coat. Am J Physiol Renal Physiol 2003;285:F722 F730.
303. Jeansson M, Haraldsson B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am J Physiol Renal Physiol 2006;290:F111 F116.
304. Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci 1995;108(pt 6):2369 2379.
305. Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol 1998;140:947 959.
306. Ballermann BJ. Glomerular endothelial cell differentiation. Kidney Int 2005;67:1668 1671.
307. Ballermann BJ, Marsden PA. Endothelium-derived vasoactive mediators and renal glomerular function. Clin Invest Med 1991;14:508 517.
308. Becker CG. Demonstration of actomyosin in mesangial cells of the renal glomerulus. Am J Pathol 1972;66:97 110.
309. Drenckhahn D, Schnittler H, Nobiling R, Kriz W. Ultrastructural organization of contractile proteins in rat glomerular mesangial cells. Am J Pathol 1990;137:1343 1351.
310. Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J 1987;1:272 281.
311. Sterzel RB, Hartner A, Schlotzer-Schrehardt U, et al. Elastic fiber proteins in the glomerular mesangium in vivo and in cell culture. Kidney Int 2000;58:1588 1602.
312. Schaefer L, Mihalik D, Babelova A, et al. Regulation of fibrillin-1 by biglycan and decorin is important for tissue preservation in the kidney during pressure-induced injury. Am J Pathol 2004;165:383 396.
313. Mundel P, Elger M, Sakai T, Kriz W. Microfibrils are a major component of the mesangial matrix in the glomerulus of the rat kidney. Cell Tissue Res 1988;254:183 187.
314. Sakai T, Kriz W. The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat Embryol (Berl) 1987;176:373 386.
315. Kriz W, Elger M, Lemley K, Sakai T. Structure of the glomerular mesangium: a biomechanical interpretation. Kidney Int Suppl 1990;30:S2 S9.
316. Kriz W, Elger M, Mundel P, Lemley KV. Structure-stabilizing forces in the glomerular tuft. J Am Soc Nephol 1995;5:1731 1739.
317. Michael AF, Keane WF, Raij L, Vernier RL, Mauer SM. The glomerular mesangium. Kidney Int 1980;17:141 154.
318. Sterzel RB, Lovett DH. Interactions of inflammatory and glomerular cells in the response to glomerular injury. In: Wilson CB, Brenner BM, Stein JH, eds. Immunopathology of Renal Disease. New York: Churchill Livingstone; 1988:137 173.
P.902
319. Schreiner GF, Kiely JM, Cotran RS, Unanue FR. Characterization of resident glomerular cells in the rat expressing Ia determinants and manifesting genetically restricted interactions with lymphocytes. J Clin Invest 1981;68:920 931.
320. Falini B, Flenghi L, Pileri S, et al. PG-M1: a new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am J Pathol 1993;142:1359 1372.
321. Imasawa T, Utsunomiya Y, Kawamura T, et al. The potential of bone marrow-derived cells to differentiate to glomerular mesangial cells. J Am Soc Nephrol 2001;12:1401 1409.
322. Hall PA, d'Ardenne AJ, Stansfeld AG. Paraffin section immunohistochemistry. I. Non-Hodgkin's lymphoma. Histopathology 1988;13:149 160.
323. Streuli M, Morimoto C, Schrieber M, Schlossman SF, Saito H. Characterization of CD45 and CD45R monoclonal antibodies using transfected mouse cell lines that express individual human leukocyte common antigens. J Immunol 1988;141:3910 3914.
324. Pulido R, Cebrian M, Acevedo A, de Landazuri MO, Sanchez-Madrid F. Comparative biochemical and tissue distribution study of four distinct CD45 antigen specificities. J Immunol 1988;140:3851 3857.
325. Hugo C, Shankland SJ, Bowen-Pope DF, Couser WG, Johnson RJ. Extraglomerular origin of the mesangial cell after injury. A new role of the juxtaglomerular apparatus. J Clin Invest 1997;100:786 794.
326. Jorgensen F, Weis Bentzon M. The ultrastructure of the normal human glomerulus; thickness of glomerular basement membranes. Lab Invest 1968;18:42 48.
327. Osawa G, Kimmelstiel P, Seiling V. Thickness of glomerular basement membranes. Am J Clin Pathol 1966;45:7 20.
328. Osterby R. Morphometric studies of the peripheral glomerular basement membrane in early juvenile diabetes. I. Development of initial basement membrane thickening. Diabetologia 1972;8:84 92.
329. Ellis EN, Mauer SM, Sutherland DE, Steffes MW. Glomerular capillary morphology in normal humans. Lab Invest 1989;60:231 236.
330. Hudson BG, Reeders ST, Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 1993;268:26033 26036.
331. Abrahamson DR, Vanden Heuvel GB, Clapp WL. Nephritogenetic antigens in the glomerular basement membrane. In: Neilson EG, Couser WG, eds. Immunologic Renal Diseases. Philadelphia: Lippincott-Raven; 1997:217 234.
332. Hudson BG. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J Am Soc Nephrol 2004;15:2514 2527.
333. Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Eng J Med 2003;348:2543 2556.
334. Katz A, Fish AJ, Kleppel MM, Hagen SG, Michael AF, Butkowski RJ. Renal entactin (nidogen): isolation, characterization and tissue distribution. Kidney Int 1991;40:643 652.
335. Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol 2005;6:646 656.
336. Farquhar MG. The glomerular basement membrane. A selective macromolecular filter. In: Hay ED, ed. Cell Biology of Extracellular Matrix. 2nd ed. New York: Plenum; 1991:365 418.
337. Mahan JD, Sisson-Ross SS, Vernier RL. Anionic sites in the human kidney: ex vivo perfusion studies. Mod Pathol 1989;2:117 124.
338. Groffen AJ, Ruegg MA, Dijkman H, et al. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J Histochem Cytochem 1998;46:19 27.
339. Halfter W, Dong S, Schurer B, Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J Biol Chem 1998;273:25404 25412.
340. Groffen AJ, Hop FW, Tryggvason K, et al. Evidence for the existence of multiple heparan sulfate proteoglycans in the human glomerular basement membrane and mesangial matrix. Eur J Biochem 1997;247:175 182.
341. McCarthy KJ, Bynum K, St John PL, Abrahamson DR, Couchman JR. Basement membrane proteoglycans in glomerular morphogenesis: chondroitin sulfate proteoglycan is temporally and spatially restricted during development. J Histochem Cytochem 1993;41:401 414.
342. Arakawa M. A scanning electron microscope of the human glomerulus. Am J Pathol 1971;64:457 466.
343. Neal CR, Crook H, Bell E, Harper SJ, Bates DO. Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J Am Soc Nephrol 2005;16:1223 1235.
344. Gautier A, Bernhard W, Oberling C. [The existence of a pericapillary lacunar apparatus in the malpighian glomeruli revealed by electronic microscopy]. CR Seances Soc Biol Fil 1950;144:1605 1607.
345. Andrews PM, Bates SB. Filamentous actin bundles in the kidney. Anat Rec 1984;210:1 9.
346. Drenckhahn D, Franke R. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 1988;59:673 682.
347. Holthofer H, Miettinen A, Lehto V, Lehtonen E, Virtanen I. Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult human kidneys. Lab Invest 1984;50:552 529.
348. Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 2003;83:253 307.
349. Abrahamson D. Structure and development of the glomerular capillary wall and basement membrane. Am J Physiol 1987;253(pt 2):F783 F794.
350. Rodewald R, Karnovsky MJ. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 1974;60:423 433.
351. Karnovsky MJ, Ryan GB. Substructure of the glomerular slit diaphragm in freeze-fractured normal rat kidney. J Cell Biol 1975;65:233 236.
352. Schneeberger EE, Levey RH, McCluskey RT, Karnovsky MJ. The isoporous substructure of the human glomerular slit diaphragm. Kidney Int 1975;8:48 52.
353. Wartiovaara J, Ofverstedt L-G, Khoshnoodi J, et al. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest 2004; 114:1475 1483.
354. Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 1990;111:1255 1263.
355. Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 2000;11:1 8.
356. Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 2001;108:1583 1587.
357. Ruotsalainen V, Ljungberg P, Wartiovaara J, et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A 1999;96:7962 7967.
358. Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 2005;14:211 216.
359. Lehtonen S, Ryan JJ, Kudlicka K, Iino N, Zhou H, Farquhar MG. Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci U S A 2005;102:9814 9819.
360. Hirabayashi S, Mori H, Kansaku A, et al. MAGI-1 is a component of the glomerular slit diaphragm that is tightly associated with nephrin. Lab Invest 2005;85:1528 1543.
361. Gerke P, Huber TB, Sellin L, Benzing T, Walz G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol 2003;14:918 926.
362. Khoshnoodi J, Sigmundsson K, Ofverstedt L-G, et al. Nephrin promotes cell-cell adhesion through homophilic interactions. Am J Pathol 2003;163:2337 2346.
363. Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem 2003;278:19266 19271.
P.903
364. Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest 2003;112:209 221.
365. Gerke P, Sellin L, Kretz O, et al. NEPH2 is located at the glomerular slit diaphragm, interacts with nephrin and is cleaved from podocytes by metalloproteinases. J Am Soc Nephrol 2005;16:1693 1702.
366. Oh J, Reiser J, Mundel P. Dynamic (re)organization of the podocyte actin cytoskeleton in the nephrotic syndrome. Pediatr Nephrol 2004;19:130 137.
367. Asanuma K, Kim K, Oh J, et al. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest 2005;115:1188 1198.
368. Benzing T. Signaling at the slit diaphragm. J Am Soc Nephrol 2004;15:1382 1391.
369. Huber TB, Hartleben B, Kim J, et al. Nephrin and CD2AP associate with phosphoinositide-3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 2003;23:4917 4928.
370. Kerjaschki D, Ojha PP, Susani M, et al. A beta1-integrin receptor for fibronectin in human kidney glomeruli. Am J Pathol 1989;134:481 489.
371. Regele HM, Fillipovic E, Langer B, et al. Glomerular expression of dystroglycans is reduced in minimal change nephrosis but not in focal segmental glomerulosclerosis. J Am Soc Nephrol 2000;11:403 412.
372. Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta1-integrin-linked protein kinase. Nature 1996; 379:91 96.
373. Barisoni L, Mundel P. Podocyte biology and the emerging understanding of podocyte diseases. Am J Nephrol 2003;23:353 360.
374. Sawada H, Stukenbrok H, Kerjaschki D, Farquhar MG. Epithelial polyanion (podocalyxin) is found on the sides but not the soles of the foot processes of the glomerular epithelium. Am J Pathol 1986;125:309 318.
375. Wiggins RC, Wiggins JE, Goyal M, Wharram BL, Thomas PE. Molecular cloning of cDNAs encoding human GLEPP1, a membrane protein tyrosine phosphatase: characterization of the GLEPP1 protein distribution in human kidney and assignment of the GLEPP1 gene to human chromosome 12p 12-p13. Genomics 1995;27:174 181.
376. Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 2001;108:289 301.
377. Orlando RA, Takeda T, Zak B, et al. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol 2001;12:1589 1598.
378. Stamenkovic I, Skalli O, Gabbiani G. Distribution of intermediate filament proteins in normal and diseased human glomeruli. Am J Pathol 1986;125:465 475.
379. Moll R, Hage C, Thoenes W. Expression of intermediate filament proteins in fetal and adult human kidney. Modulations of intermediate filament patterns during development and in damaged tissue. Lab Invest 1991;65:74 86.
380. Oosterwijk E, van Muijen GNP, Oosterwijk-Wakka JC, Warnaar SO. Expression of intermediate-sized filaments in developing and adult human kidney and in renal cell carcinoma. J Histochem Cytochem 1990;38:385 392.
381. Yaoita E, Franke WW, Yamamoto T, Kawasaki K, Kihara I. Identification of renal podocytes in multiple species: higher vertebrates are vimentin positive/lower vertebrates are desmin positive. Histochem Cell Biol 1999;111:107 115.
382. Floege J, Alpers CE, Sage EH, et al. Markers of complement-dependent and complement-independent glomerular visceral epithelial injury in vivo. Expression of antiadhesive proteins and cytoskeletal changes. Lab Invest 1992;67:486 497.
383. Pritchard-Jones K, Fleming S, Davidson D, et al. The candidate Wilms' tumor gene is involved in genitourinary development. Nature 1990;346:194 197.
384. Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B. Nuclear localization of the protein encoded by the Wilms' tumor gene WT1 in embryonic and adult tissues. Development 1993;119:1329 1341.
385. Pelletier J, Bruening W, Kashtan CE. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell 1991;67:437 447.
386. Drummond MC, Deen WM. Structural determinants of glomerular hydraulic permeability. Am J Physiol 1994;266(pt 2):F1 F12.
387. Edwards A, Daniels BS, Deen WM. Ultrastructural model for size selectivity in glomerular filtration. Am J Physiol 1999;276(pt 2): F892 F902.
388. Kanwar YS, Liu ZZ, Kashihara N, Wallner EI. Current status of the structural and functional basis of glomerular filtration and proteinuria. Semin Nephrol 1991;11:390 413.
389. Rossi M, Morita H, Sormunen R, et al. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J 2003;22:236 245.
390. Gelberg H, Healy L, Whiteley H, Miller LA, Vimr E. In vivo enzymatic removal of alpha 2 6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab Invest 1996;74:907 920.
391. Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol 2001;281: F579 F596.
392. Haraldsson B, Sorensson J. Why do we not all have proteinuria? An update of our current understanding of the glomerular barrier. News Physiol Sci 2004;19:7 10.
393. Tryggvason K, Wartiovaara J. How does the kidney filter plasma? Physiology (Bethesda) 2005;20:96 101.
394. Dijkman H, Smeets B, van der Laak J, Steenbergen E, Wetzels J. The parietal epithelial cell is crucially involved in human idiopathic focal segmental glomerulosclerosis. Kidney Int 2005;68:1562 1572.
395. Ohtaka A, Ootaka T, Sato H, Ito S. Phenotypic change of glomerular podocytes in primary focal segmental glomerulosclerosis: developmental paradigm? Nephrol Dial Transplant 2002;17(suppl 9):11 15.
396. Peissel B, Geng L, Kalluri R, et al. Comparative distribution of the alpha 1(IV), alpha 5(IV), and alpha 6(IV) collagen chains in normal human adult and fetal tissues and in kidneys from X-linked Alport syndrome patients. J Clin Invest 1995;96:1948 1957.
397. Couchman JR, Kapoor R, Sthanam M, Wu RR. Perlecan and basement membrane-chondroitin sulfate proteoglycan (bamacan) are two basement membrane chondroitin/dermatan sulfate proteoglycans in the Engelbreth-Holm-Swarm tumor matrix. J Biol Chem 1996;271:9595 9602.
398. Ryan GB, Coghlan JP, Scoggins BA. The granulated peripolar epithelial cell: a potential secretory component of the renal juxtaglomerular complex. Nature 1979;277:655 656.
399. Gall JA, Alcorn D, Butkus A, Coghlan JP, Ryan GB. Distribution of glomerular peripolar cells in different mammalian species. Cell Tissue Res 1986;244:203 208.
400. Gardiner DS, Lindop GB. The granular peripolar cell of the human glomerulus: a new component of the juxtaglomerular apparatus? Histopathology 1985;9:675 685.
401. Ryan GB, Alcorn D, Coghlan JP, Hill PA, Jacobs R. Ultrastructural morphology of granule release from juxtaglomerular myoepithelioid and peripolar cells. Kidney Int Suppl 1982;12:S3 S8.
402. Golgi C. Annotazioni intorno all'istologia dei reni dell'uomo e di altri mammiferi e sull'istogenesi: dei canalicoli oriniferi. Atti R Accad Naz Lincei Rendiconti 1889;5:337 342.
403. Barajas L. Anatomy of the juxtaglomerular apparatus. Am J Physiol 1979;237:F333 F343.
404. Barajas L, Bloodworth JMB Jr, Hartroft PM. Endocrine pathology of the kidney. In: Bloodworth JMB Jr, ed. Endocrine Pathology: General and Surgical. 2nd ed. Baltimore: Williams & Wilkins; 1982:723 766.
405. Barajas L, Salido EC, Smolens P, Hart D, Stein JH. Pathology of the juxtaglomerular apparatus including Bartter's syndrome. In: Tisher CC, Brenner BM, eds. Renal Pathology with Clinical and Functional Correlations. 2nd ed. Philadelphia: JB Lippincott; 1994:948 978.
P.904
406. Cantin M, Gutkowska J, Lacasse J, et al. Ultrastructural immunocytochemical localization of renin and angiotensin II in the juxtaglomerular cells of the ischemic kidney in experimental renal hypertension. Am J Pathol 1984;115:212 224.
407. Taugner R, Mannek E, Nobiling R, et al. Coexistence of renin and angiotensin II in epithelioid cell secretory granules of rat kidney. Histochemistry 1984;81:39 45.
408. Barajas L, Wang P. Localization of tritiated norepinephrine in the renal arteriolar nerves. Anat Rec 1979;195:525 534.
409. Kopp UC, DiBona GF. Neural regulation of renin secretion. Semin Nephrol 1993;13:543 551.
410. Pricam C, Humbert F, Perrelet A, Orci L. Gap junctions in mesangial and lacis cells. J Cell Biol 1974;63:349 354.
411. Taugner R, Schiller A, Kaissling B, KRiz W. Gap junctional coupling between the JGA and the glomerular tuft. Cell Tissue Res 1978;186:279 285.
412. Kaissling B, Kriz W. Variability of intercellular spaces between macula densa cells: a transmission electron microscopic study in rabbits and rats. Kidney Int Suppl 1982;12:S9 S17.
413. Salido EC, Barajas L, Lechago J, Laborde NP, Fisher DA. Immunocytochemical localization of epidermal growth factor in mouse kidney. J Histochem Cytochem 1986;34:1155 1160.
414. Sikri KL, Foster CL, MacHugh N, Marshall RD. Localization of Tamm-Horsfall glycoprotein in the human kidney using immunofluorescence and immunoelectron microscopical techniques. J Anat 1981;132(pt 4):597 605.
415. Kirk KL, Bell PD, Barfuss DW, Ribadeneira M. Direct visualization of the isolated and perfused macula densa. Am J Physiol 1985;248(pt 2):F890 F894.
416. Schnermann J. Homer W. Smith Award lecture. The juxtaglomerular apparatus: from anatomical peculiarity to physiological relevance. J Am Soc Nephrol 2003;14:1681 1694.
417. Wilcox CS, Welch WJ, Murad F, et al. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci U S A 1992;89:11993 11997.
418. Mundel P, Bachmann S, Bader M, et al. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int 1992;42:1017 1019.
419. Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 1994;94:2504 2510.
420. Welch WJ, Wilcox CS, Thomson SC. Nitric oxide and tubuloglomerular feedback. Semin Nephrol 1999;19:251 262.
421. Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol 2001;281:F1 F11.
422. Rouiller C. General anatomy and histology of the kidney. In: Rouiller C, Muller AF, eds. The Kidney: Morphology, Biochemistry, Physiology. New York: Academic Press; 1969:61 156.
423. Swann HG. The functional distention of the kidney: a review. Tex Rep Biol Med 1960;18:566 595.
424. Hodson CJ. Physiological changes in size of the human kidney. Clin Radiol 1961;12:91 94.
425. Parker MV, Swann HG, Sinclair JG. The functional morphology of the kidney. Tex Rep Biol Med 1962;20:425 445.
426. Faraggiana T, Malchiodi F, Prado A, Churg J. Lectin-peroxidase conjugate reactivity in normal human kidney. J Histochem Cytochem 1982;30:451 458.
427. Hennigar RA, Schulte BA, Spicer SS. Heterogeneous distribution of glycoconjugates in human kidney tubules. Anat Rec 1985;211:376 390.
428. Silva FG, Nadasdy T, Laszik Z. Immunohistochemical and lectin dissection of the human nephron in health and disease. Arch Pathol Lab Med 1993;117:1233 1239.
429. Skinnider BF, Folpe AL, Hennigar RA, et al. Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol 2005;29:747 754.
430. Paul R, Ewing CM, Robinson JC, et al. Cadherin-6, a cell adhesion molecule specifically expressed in the proximal renal tubule and renal cell carcinoma. Cancer Res 1997;57:2741 2748.
431. Maunsbach AB, Christensen EI. Functional ultrastructure of the proximal tubule. In: Windhager EE, ed. Handbook of Physiology. Renal Physiology. New York: Oxford University Press; 1992:41 107.
432. Tisher CC, Bulger RE, Trump BF. Human renal ultrastructure. I. Proximal tubule of healthy individuals. Lab Invest 1966;15:1357 1394.
433. Moe OW, Baum M, Berry CA, et al. Renal transport of glucose, amino acids, sodium, chloride and water. In: Brenner BM, ed. Brenner & Rector's The Kidney. 7th ed. Philadelphia: WB Saunders; 2004:413 452.
434. Welling LW, Welling DJ. Shape of epithelial cells and intercellular channels in the rabbit proximal nephron. Kidney Int 1976;9:385 394.
435. Welling LW, Welling DJ. Relationship between structure and function in renal proximal tubule. J Electron Microsc Tech 1988;9:171 185.
436. Ahn KY, Madsen KM, Tisher CC, Kone BC. Differential expression and cellular distribution of mRNAs encoding alpha- and beta-isoforms of Na+-K+-ATPase in rat kidney. Am J Physiol 1993;265(pt 2):F792 F801.
437. Clapp WL, Bowman P, Shaw GS, Patel P, Kone BC. Segmental localization of mRNAs encoding Na+-K+-ATPase alpha- and beta-subunit isoforms in rat kidney using RT-PCR. Kidney Int 1994;46:627 638.
438. Biemesderfer D, Pizzonia J, Abu-Alfa A, et al. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol 1993;265(pt 2):F736 F742.
439. Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 1995;48:1206 1215.
440. Agre P, King LS, Yasui M, et al. Aquaporin water channels from atomic structure to clinical medicine. J Physiol 2002;542(pt 1):3 16.
441. King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol 2004;5:687 698.
442. Nielsen S, Smith BL, Christensen EI, Knepper MA, Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol 1993;120:371 383.
443. Maunsbach AB, Marples D, Chin E, et al. Aquaporin-1 water channel expression in human kidney. J Am Soc Nephrol 1997;8:1 14.
444. Maack T. Renal filtration, transport, and metabolism of proteins. In: Seldin D, Giebisch G, eds. The Kidney: Physiology and Pathophysiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:2235 2267.
445. Christensen EI, Rennke HG, Carone FA. Renal tubular uptake of protein: effect of molecular charge. Am J Physiol 1983;244:F436 F441.
446. Park CH, Maack T. Albumin absorption and catabolism by isolated perfused proximal convoluted tubules of the rabbit. J Clin Invest 1984;73:767 777.
447. Park CH. Time course and vectorial nature of albumin metabolism in isolated perfused rabbit PCT. Am J Physiol 1988;255(pt 2):F520 F528.
448. Clapp WL, Park CH, Madsen KM, Tisher CC. Axial heterogeneity in the handling of albumin by the rabbit proximal tubule. Lab Invest 1988;58:549 558.
449. Larsson L, Clapp WL III, Park CH, Cannon JK, Tisher CC. Ultrastructural localization of acidic compartments in cells of isolated rabbit PCT. Am J Physiol 1987;253(pt 2):F95 F103.
450. Christensen EI. Rapid membrane recycling in renal proximal tubule cells. Eur J Cell Biol 1982;29:43 49.
451. Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol 2001;280:F562 F573.
452. Birn H, Fyfe JC, Jacobsen C, et al. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Invest 2000;105:1353 1361.
453. Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 2002;3:256 266.
P.905
454. Olsen S, Solez K. Acute tubular necrosis and toxic renal injury. In: Tisher CC, Brenner BM, eds. Renal Pathology with Clinical and Functional Correlations. 2nd ed. Philadelphia: JB Lippincott; 1994:769 809.
455. Nadasdy NT, Laszik Z, Blick KE, Johnson LD, Silva FG. Proliferative activity of intrinsic cell populations in the normal human kidney. J Am Soc Nephrol 1994;4:2032 2039.
456. Droz D, Zachar D, Charbit L, Gogusev J, Chretein Y, Iris L. Expression of the human nephron differentiation molecules in renal cell carcinomas. Am J Pathol 1990;137:895 905.
457. Gerdes J, Becker MHG, Key G, Cattoretti G. Immunohistochemical detection of tumor growth fraction (Ki-67 antigen) in formalin-fixed and routinely processed tissues. J Pathol 1992; 168:85 86.
458. Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 1994;93:2175 2188.
459. Kliem V, Johnson RJ, Alpers CE, et al. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int 1996;49:666 678.
460. Gobe GC, Axelsen RA. Genesis of renal tubular atrophy in experimental hydronephrosis in the rat. Role of apoptosis. Lab Invest 1987;56:273 281.
461. Gobe GC, Axelsen RA, Searle JW. Cellular events in experimental unilateral ischemic renal atrophy and in regeneration after contralateral nephrectomy. Lab Invest 1990; 63:770 779.
462. Schumer M, Colombel MC, Sawczuk IS, et al. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol 1992;140:831 838.
463. Shimizu A, Yamanaka N. Apoptosis and cell desquamation in repair process of ischemic tubular necrosis. Virchows Arch B Cell Pathol Incl Mol Pathol 1993;64:171 180.
464. Dieterich HJ, Barrett JM, Kriz W, Bulhoff JP. The ultrastructure of the thin loop limbs of the mouse kidney. Anat Embryol (Berl) 1975;147:1 18.
465. Bulger RE, Tisher CC, Myers CH, Trump BF. Human renal ultrastructure. II. The thin limb of Henle's loop and the interstitium in healthy individuals. Lab Invest 1967;16:124 141.
466. Knepper MA, Gamba G. Urine concentration and dilution. In: Brenner BM, ed. Brenner & Rector's The Kidney. 7th ed. Philadelphia: WB Saunders; 2004:599 636.
467. Nielsen S, Pallone T, Snith BL, Christensen EI, Agre P, Maunsbach AB. Aquaporin-1 water channels in short and long loop descending thin limbs and in descending vasa recta in rat kidney. Am J Physiol 1995;268(pt 2):F1023 F1037.
468. Uchida S, Sasaki S, Nitta K, et al. Localization and functional characterization of rat kidney-specific chloride channel, C1C-K1. J Clin Invest 1995;95:104 113.
469. Takeuchi Y, Uchida S, Marumo F, Sasaki S. Cloning, tissue distribution, and intrarenal localization of C1C chloride channels in human kidney. Kidney Int 1995;48:1497 1503.
470. Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem 1998;273:4296 4299.
471. King LS, Choi M, Fernandez PC, Cartron JP, Agre P. Defective urinary-concentrating ability due to a complete deficiency of aquaporin-1. N Eng J Med 2001;345:175 179.
472. Matsumura Y, Uchida S, Kondo Y, et al. Overt nephrogenic diabetes insipidus in mice lacking the CLC-K1 chloride channel. Nat Genet 1999;21:95 98.
473. Kokko JP, Rector FC Jr. Countercurrent multiplication system without active transport in inner medulla. Kidney Int 1972;2:214 223.
474. Stephenson JL. Concentration of urine in a central core model of the renal counterflow system. Kidney Int 1972;2:85 94.
475. Kone BC, Madsen KM, Tisher CC. Ultrastructure of the thick ascending limb of Henle in the rat kidney. Am J Anat 1984;171:217 226.
476. Allen F, Tisher CC. Morphology of the ascending thick limb of Henle. Kidney Int 1976;9:8 22.
477. Shen SS, Krishna B, Chirala R, Amato RJ, Truong LD. Kidney-specific cadherin, a specific marker for the distal portion of the nephron and related renal neoplasms. Mod Pathol 2005;18:933 940.
478. Garg LC, Knepper MA, Burg MB. Mineralocorticoid effects on Na-K-ATPase in individual nephron segments. Am J Physiol 1981;240:F536 F544.
479. Nielsen S, Maunsbach AB, Ecelbarger CA, Knepper MA. Ultrastructural localization of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am J Physiol 1998;275(pt 2):F885 F893.
480. Bachmann S, Velazquez H, Obermuller N, Reilly RF, Moser D, Ellison DH. Expression of the thiazide-sensitive Na-Cl cotransporter by rabbit distal convoluted tubule cells. J Clin Invest 1995;96:2510 2514.
481. Plotkin MD, Kaplan MR, Verlander JW, et al. Localization of the thiazide-sensitive Na-Cl cotransporter, rTSC1, in the rat kidney. Kidney Int 1996;50:174 183.
482. Woodhall PB, Tisher CC. Response of the distal tubule and cortical collecting duct to vasopressin in the rat. J Clin Invest 1973;52:3095 3108.
483. Gross JB, Imai M, Kokko JP. A functional comparison of the cortical collecting tubule and the distal convoluted tubule. J Clin Invest 1975;55:1284 1294.
484. Kaissling B. Structural aspects of adaptive changes in renal electrolyte excretion. Am J Physiol 1982;243:F211 F226.
485. Myers CE, Bulger RE, Tisher CC, Trump BF. Human ultrastructure. IV. Collecting duct of healthy individuals. Lab Invest 1966;15:1921 1950.
486. Kaissling B, Kriz W. Structural analysis of the rabbit kidney. Adv Anat Embryol Cell Biol 1979;56:1 123.
487. Vio CP, Figueroa CD. Subcellular localization of renal kallikrein by ultrastructural immunocytochemistry. Kidney Int 1985;28:36 42.
488. Barajas L, Powers K, Carretero O, Scicli AG, Inagami T. Immunocytochemical localization of renin and kallikrein in the rat renal cortex. Kidney Int 1986;29:965 970.
489. Mennitt PA, Wade JB, Ecelbarger CA, Palmer LG, Frindt G. Localization of ROMK channels in the rat kidney. J Am Soc Nephrol 1997;8:1823 1830.
490. Xu JZ, Hall AF, Peterson LN, Bienkowski MJ, Eessalu TE, Hebert SC. Localization of the ROMK protein on apical membranes of rat kidney nephron segments. Am J Physiol 1997;273(pt 2): F739 F748.
491. Reilly RF, Shugrue CA, Lattanzi D, Biemesderfer D. Immunolocalization of the Na+/Ca2+ exchanger in rabbit kidney. Am J Physiol Renal Physiol 1993;265:F327 F332.
492. Borke JL, Caride A, Verma AK, Penniston JT, Kumar R. Plasma membrane calcium pump and 28-kDa calcium binding protein in cells of rat kidney distal tubules. Am J Physiol 1989;257(pt 2): F842 F849.
493. Fine LG. Eustachio's discovery of the renal tubule. Am J Nephrol 1986;6:47 50.
494. Welling LW, Evan AP, Welling DJ. Shape of cells and extracellular channels in rabbit cortical collecting ducts. Kidney Int 1981;20:211 222.
495. Duc C, Farman N, Canessa CM, Bonvalet JP, Rossier BC. Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: localization by in situ hybridization and immunocytochemistry. J Cell Biol 1994;127(pt 2):1907 1921.
496. Hager H, Kwon TH, Vinnikova AK, et al. Immunocytochemical and immunoelectron microscopic localization of alpha-, beta-, and gamma-ENaC in rat kidney. Am J Physiol Renal Physiol 2001;280:F1093 F1096.
497. Stanton BA, Biemesderfer D, Wade JB, Giebisch G. Structural and functional study of the rat nephron: effects of potassium adaptation and depletion. Kidney Int 1981;19:36 48.
498. Petty KJ, Kokko JP, Marver D. Secondary effect of aldosterone on Na-KATPase activity in the rabbit cortical collecting tubule. J Clin Invest 1981;68:1514 1521.
P.906
499. Mujais SK, Chekal MA, Jones WJ, Hayslett JP, Katz AI. Regulation of renal Na-K-ATPase in the rat: Role of the natural mineralo- and glucocorticoid hormones. J Clin Invest 1984;73:13 19.
500. Kaissling B, Le Hir M. Distal tubular segments of the rabbit kidney after adaptation to altered Na- and K-intake. I. Structural changes. Cell Tissue Res 1982;224:469 492.
501. Wade JB, O'Neil RG, Pryor JL, Boulpaep EL. Modulation of cell membrane area in renal collecting tubules by corticosteroid hormones. J Cell Biol 1979;81:439 445.
502. Kirk KL, Buku A, Eggena P. Cell specificity of vasopressin binding in renal collecting duct: computer-enhanced imaging of a fluorescent hormone analog. Proc Natl Acad Sci U S A 1987;84:6000 6004.
503. Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A 1993;90:11663 11667.
504. Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A 1995;92:1013 1017.
505. Ecelbarger CA, Terris J, Frindt G, et al. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol 1995;269(pt 2):F663 F672.
506. Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem 1994;269:5497 5500.
507. Schuster VL, Bonsib SM, Jennings ML. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol 1986;251(pt 1):C347 C355.
508. Verlander JW, Madsen KM, Tisher CC. Effect of acute respiratory acidosis on two populations of intercalated cells in the rat cortical collecting duct. Am J Physiol 1987;253(pt 2):F1142 F1156.
509. Teng-umnuay P, Verlander JW, Yuan W, Tisher CC, Madsen KM. Identification of distinct subpopulations of intercalated cells in the mouse collecting duct. J Am Soc Nephrol 1996;7:260 274.
510. Kim J, Kim YH, Cha JH, Tisher CC, Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. J Am Soc Nephrol 1999;10:1 12.
511. Lonnerholm G. Histochemical demonstration of carbonic anhydrase activity in the human kidney. Acta Physiol Scand 1973;88:455 468.
512. McKinney TD, Burg MB. Bicarbonate absorption by rabbit cortical collecting tubules in vitro. Am J Physiol 1978;234:F141 F145.
513. McKinney TD, Burg MB. Bicarbonate secretion by rabbit cortical collecting tubules in vitro. J Clin Invest 1978;61:1421 1427.
514. Brown D, Gluck S, Hartwig J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J Cell Biol 1987;105:1637 1648.
515. Brown D, Hirsh S, Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature 1988;331:622 624.
516. Alper SL, Natale J, Gluck S, Lodfish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci U S A 1989;86:5429 5433.
517. Drenckhahn D, Schluter K, Allen DP, Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science 1985;230:1287 1289.
518. Verlander JW, Madsen KM, Low PS, Allen DP, Tisher CC. Immunocytochemical localization of band 3 protein in the rat collecting duct. Am J Physiol 1988;255(pt 2):F115 F125.
519. Weiner ID, Hamm LL. Regulation of intracellular pH in the rabbit cortical collecting tubule. J Clin Invest 1990;85:274 281.
520. Royaux IE, Wall SM, Karniski LP, et al. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 2001;98:4221 4226.
521. Soleimani M, Greeley T, Petrovic S, et al. Pendrin: an apical Cl-/OH-/HCO3- exchanger in the kidney cortex. Am J Physiol Renal Physiol 2001;280:F356 F364.
522. Kim YH, Kwon TH, Frische S, et al. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 2002;283:F744 F754.
523. Frische S, Kwon TH, Frokiaer J, Madsen KM, Nielsen S. Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol 2003;284:F584 F593.
524. Romero MF. Molecular pathophysiology of SLC4 bicarbonate transporters. Curr Opin Nephrol Hypertens 2005;14:495 501.
525. Everett LA, Glaser B, Beck JC, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 1997;17:411 422.
526. Wall SM, Hassell KA, Royaux IE, et al. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 2003;284:F229 F241.
527. Wall SM. Recent advances in our understanding of intercalated cells. Curr Opin Nephrol Hypertens 2005;14:480 484.
528. Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature 1985;318:368 371.
529. Schwartz GJ, Tsuruoka S, Vijayakumar S, Petrovic S, Mian A, Al-Awqati Q. Acid incubation reverses the polarity of intercalated cell transporters, an effect mediated by hensin. J Clin Invest 2002;109:89 99.
530. LeFurgey A, Tisher CC. Morphology of rabbit collecting duct. Am J Anat 1979;115:111 124.
531. Hansen GP, Tisher CC, Robinson RR. Response of the collecting duct to disturbances of acid-base and potassium balance. Kidney Int 1980;17:326 337.
532. Madsen KM, Tisher CC. Cellular response to acute respiratory acidosis in rat medullary collecting duct. Am J Physiol 1983;245: F670 F679.
533. Madsen KM, Tisher CC. Response of intercalated cells of rat outer medullary collecting duct to chronic metabolic acidosis. Lab Invest 1984;51:268 276.
534. Garg LC, Narang N. Ouabain-insensitive K-adenosine triphosphatase in distal nephron segments of the rabbit. J Clin Invest 1988;81:1204 1208.
535. Wingo CS. Active proton secretion and potassium absorption in the rabbit outer medullary collecting duct. Functional evidence for proton-potassium-activated adenosine triphosphatase. J Clin Invest 1989;84:361 365.
536. Wingo CS, Madsen KM, Smolka A, Tisher CC. H-K-ATPase immunoreactivity in cortical and outer medullary collecting duct. Kidney Int 1990;38:985 990.
537. Ahn KY, Kone BC. Expression and cellular localization of mRNA encoding the gastric isoform of H+-K+-ATPase -subunit in rat kidney. Am J Physiol 1995;268(pt 2):F99 F109.
538. Campbell-Thompson ML, Verlander JW, Curran KA, et al. In situ hybridization of H-K-ATPase B-subunit mRNA in rat and rabbit kidney. Am J Physiol 1995;269(pt 2):F345 F354.
539. Madsen KM, Clapp WL, Verlander JW. Structure and function of the inner medullary collecting duct. Kidney Int 1988;34:441 454.
540. Sands JM, Knepper MA. Urea permeability of mammalian inner medullary collecting duct system and papillary surface epithelium. J Clin Invest 1987;79:138 147.
541. Sands JM, Nonoguchi H, Knepper MA. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol 1987;253(pt 2):F823 F832.
542. Clapp WL, Madsen KM, Verlander JM, Tisher CC. Intercalated cells of the rat inner medullary collecting duct. Kidney Int 1987;31:1080 1087.
543. Clapp WL, Madsen KM, Verlander JW, Tisher CC. Morphologic heterogeneity along the rat inner medullary collecting duct. Lab Invest 1989;60:219 230.
544. Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Cellular and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc Natl Acad Sci U S A 1996;93:5495 5500.
545. Shayakul C, Knepper MA, Smith CP, DiGiovanni SR, Hediger MA. Segmental localization of urea transporter mRNAs in rat kidney. Am J Physiol 1997;272(pt 2):F654 F660.
546. Terris JM, Knepper MA, Wade JB. UT-A3: localization and characterization of an additional urea transporter isoform in the IMCD. Am J Physiol Renal Physiol 2001;280:F325 F332.
P.907
547. Wall SM, Truong AV, DuBose TD Jr. H+-K+-ATPase mediates net acid secretion in rat terminal inner medullary collecting duct. Am J Physiol 1996;271(pt 2):F1037 F1044.
548. Bohman SO. The ultrastructure of the renal medulla and the interstitial cells. In: Cotran RS, ed. Tubulo-Interstitial Nephropathies. New York: Churchill Livingstone; 1983:1 34.
549. Hestbech J, Hansen HE, Amdisen A, Olsen S. Chronic renal lesions following long-term treatment with lithium. Kidney Int 1977;12:205 213.
550. Bohle A, Grund KE, Mackensen S, Tolon M. Correlations between renal interstitium and level of serum creatinine. Morphometric investigations of biopsies in perimembranous glomerulonephritis. Virchows Arch A Pathol Anat Histol 1977;373:15 22.
551. Kappel B, Olsen S. Cortical interstitial tissue and sclerosed glomeruli in the normal human kidney, related to age and sex. A quantitative study. Virchows Arch A Pathol Anat Histol 1980;387:271 277.
552. Mounier F, Foidart JM, Gubler MC. Distribution of extracellular matrix glycoproteins during normal development of human kidney. An immunohistochemical study. Lab Invest 1986;54:394 401.
553. Lemley KV, Kriz W. Anatomy of the renal interstitium. Kidney Int 1991;39:370 381.
554. Kaissling B, Hegyi I, Loffing J, Le Hir M. Morphology of interstitial cells in the healthy kidney. Anat Embryol (Berl) 1996;193:303 318.
555. Bachmann S, Le Hir M, Eckardt KU. Colocalization of erythropoietin mRNA and ecto-5-nucleotidase immunoreactivity in peritubular cells of the rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem 1993;41:335 341.
556. Maxwell PH, Osmond MK, Pugh CW, et al. Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int 1993;44:1149 1162.
557. Pfaller W. Structure function correlation in rat kidney. Quantitative correlation of structure and function in normal and injured rat kidney. Adv Anat Embryol Cell Biol 1982;70:1 106.
558. Muirhead EE. Antihypertensive functions of the kidney. Arthur C. Corcoran memorial lecture. Hypertension 1980;2:444 464.
559. Muirhead EE. Discovery of the renomedullary system of blood pressure control and its hormones. Hypertension 1990;15:114 116.
560. Lemley KV, Kriz W. Structure and function of the renal vasculature. In: Tisher CC, Brenner BM, eds. Renal Pathology with Clinical and Functional Correlations.. 2nd ed. Philadelphia: JB Lippincott; 1994:981 1026.
561. Fourman J, Moffat DB. The Blood Vessels of the Kidney. Oxford, England:Blackwell Scientific; 1971.
562. More RH, Duff GL. The renal arterial vasculature in man. Am J Pathol 1951;27:95 117.
563. Edwards JG. Efferent arterioles of glomeruli in the juxtamedullary zone of the human kidney. Anat Rec 1956;125:521 529.
564. Casellas D, Mimran A. Shunting in renal microvasculature of the rat. A scanning electron microscopic study of corrosion casts. Anat Rec 1981;201:237 248.
565. Ljungqvist A. Ultrastructural demonstration of a connection between afferent and efferent juxtamedullary glomerular arterioles. Kidney Int 1975;8:239 244.
566. Ljungqvist A. Fetal and postnatal development of the intrarenal arterial pattern in man. A microangiographic and histologic study. Acta Paediatr 1963;52:443 464.
567. Mukai K, Rosai J, Burgdorf WH. Localization of factor VIII-related antigen in vascular endothelial cells using an immunoperoxidase method. Am J Surg Pathol 1980;4:273 276.
568. Sanfilippo F, Pizzo SV, Croker BP. Immunohistochemical studies of cell differentiation in a juxtaglomerular tumor. Arch Pathol Lab Med 1982;106:604 607.
569. Fina L, Molgaard HV, Robertson D, et al. Expression of the CD34 gene in vascular endothelial cells. Blood 1990;75:2417 2426.
570. Civin CL, Trischmann TM, Fackler MJ, et al. Summary of CD34 cluster workshop section. In: Knapp W, ed. Leucocyte Typing IV. London: Academic Press; 1989:818 825.
571. Gabbiani G, Schmid E, Winter S, et al. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A 1981;78:298 302.
572. Rollh user H, Kriz W, Heinke W. Das Gef ss System der Rattenniere. Z Zellforsch Mikrosk Anat 1964;64:381 403.
573. Kriz W, Barrett JM, Peter S. The renal vasculature: anatomical functional aspects. In: Thurau K, ed. Kidney and Urinary Tract Physiology II. Baltimore: University Park Press; 1976:1 21.
574. Beeuwkes R III, Bonventre JV. Tubular organization and vascular-tubular relations in the dog kidney. Am J Physiol 1975;229:695 713.
575. Beeuwkes R. Vascular-tubular relationship in the human kidney. In: Leaf A, Giebisch G, Bolis L, Gorini S, eds. Renal Pathophysiology: Recent Advances. New York: Raven Press; 1980:155 163.
576. Pierce EC. Renal lymphatics. Anat Rec 1944;90:315 335.
577. Bell RD, Keyl MJ, Shrader FR, Jones EW, Henry LP. Renal lymphatics: the internal distribution. Nephron 1968;5:454 463.
578. Kriz W, Dieterich HJ. Das lymphagefass system der niere bei einigen saugetieren: licht-und elektronenmikroskopische untersuchungen. Z Anat Entwickl Gesch 1970;131:111 147.
579. Kriz W. A periarterial pathway for intrarenal distribution of renin. Kidney Int Suppl 1987;20:S51 S56.
580. Colvin RB. Emphatically lymphatic. J Am Soc Nephrol 2004;15:827 829.
581. Mitchell GA. The nerve supply of the kidneys. Acta Anat (Basel) 1950;10:1 37.
582. Gosling JA. Observations on the distribution of intrarenal nervous tissue. Anat Rec 1969;163:81 88.
583. Stefansson K, Wollmann RL, Jerkovic M. S-100 protein in soft-tissue tumors derived from Schwann cells and melanocytes. Am J Pathol 1982;106:261 268.
584. Nakajima T, Watanabe S, Sato Y, Kameya T, Hirota T, Shimosato Y. An immunoperoxidase study of S-100 protein distribution in normal and neoplastic tissues. Am J Surg Pathol 1982;6:715 727.
585. Trojanowski JQ, Lee VM, Schlaepfer WW. An immunohistochemical study of human central and peripheral nervous system tumors, using monoclonal antibodies against neurofilaments and glial filaments. Hum Pathol 1984;15:248 257.
586. Lee VM, Carden MJ, Schlaepfer WW. Structural similarities and differences between neurofilament proteins from five different species as revealed using monoclonal antibodies. J Neurosci 1986;6:2179 2186.
587. Barajas L. Innervation of the renal cortex. Fed Proc 1978;37:1192 1201.
588. Fourman J. The adrenergic innervation of the efferent arterioles and the vasa recta in the mammalian kidney. Experientia 1970;26:293 294.
589. Barajas L, Powers K, Wang P. Innervation of the renal cortical tubules: a quantitative study. Am J Physiol 1984;247(pt 2): F50 F60.
590. Barajas L, Powers K. Innervation of the thick ascending limb of Henle. Am J Physiol 1988;255(pt 2):F340 F348.