4 - Bone
Editors: Mills, Stacey E.
Title: Histology for Pathologists, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > III - Musculoskeletal System > 7 - Adipose Tissue
function show_scrollbar() {}
7
Adipose Tissue
John S.J. Brooks
Patricia M. Perosio
Introduction
In compiling this chapter, our intention was to provide practicing surgical pathologists with both a description of normal and abnormal adipose tissue and a reference source. We were inclusive in our approach and considered all bodily lesions containing mature fat appropriate for discussion regardless of site. The section on development should provide a deeper understanding for the diagnostician and a starting point for the researcher. Collected and detailed as a group are the fatty infiltrations of organs, the inflammations affecting fat, the hamartomas and mesenchymomas, and the lipomas and variants thereof. Up-to-date definitions are provided where necessary. Importantly, we have also summarized clinical and genetic syndromes in which fat cells may participate. Unusual but distinctive histologies are enumerated, such as may occur in starvation, pancreatic fat necrosis, and true lipodystrophy. All topics are well-referenced, hopefully providing the reader with a valuable resource. In short, we have attempted to describe
P.166
as many lesions as possible, not just primary fatty entities, but also anything extraneous within adipose tissue or confused with it.
White Fat
Prenatal Development
The morphology of developing adipose tissue has been studied in detail. By examining serial sections obtained from 805 human fetuses of various ages, Poissonnet et al. (1) have determined that prior to the second trimester of pregnancy, adipose tissue primordia cannot be identified by light microscopy. After 14 weeks' gestation, aggregates of mesenchymal cells are seen condensed around proliferating primitive blood vessels. They refer to these findings as stage II in the development of adipose tissue (Figure 7.1). Prior to this time, future adipose tissue is characterized by loose spindle cells and ground substance (stage I). Later on, capillaries continue to proliferate into a rich network, around which preadipocytes become stellate and organized into a mesenchymal lobule (stage III). These preadipocytes do not contain lipid. With further development, fine lipid vacuoles characteristic of stage IV accumulate within cytoplasm (Figure 7.2). Continued proliferation of the components of the lobule results in the formation of densely packed aggregates of vacuolated fat cells with a rich capillary vascular network. Finally, condensation of perilobular mesenchyme at the periphery of the lobule results in formation of fibrous interlobular septa in stage V. This process occurs over the 10-week period between the 14- and 24-week gestation period. From approximately 24 to 29 weeks, the number of fat lobules is relatively constant. Continued growth occurs mainly due to proliferation of capillaries and adipocytes, causing an increase in the size of the fat lobules (Figure 7.2).
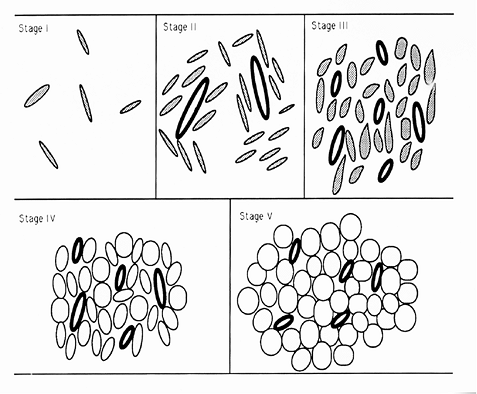 |
Figure 7.1 Developmental stages of adipose tissue. Stage I: Stellate cells (stippled) embedded in amorphous ground substance. Stage II: As angiogenesis begins, mesenchymal cells (stippled) condense around the blood vessels (bold ovals). Stage III: A rich capillary network develops from each vessel, forming a glomerulus-like network around which each lobule forms. The preadipocytes become more stellate. Stage IV: With accumulation of lipid, these adipocytes, with multiple small lipid droplets, become closely packed around the capillaries. Stage V: Further accumulation of lipid with many unilocular cells (clear circles) is evident. The perilobular mesenchyme condenses into interlobular septa at this stage. |
The same sequence of development of adipose tissue occurs at all sites throughout the body (2). The earliest white fat lobules appear first in the face, neck, breast, and abdominal wall at 14 weeks' gestation. By 15 weeks, they are also evident over the back and shoulders. Development in the upper and lower extremities and anterior chest begins around the sixteenth week. By the end of the twenty-third week, a layer of subcutaneous fat completely covers the extremities.
There is a very close association of adipocyte development and angiogenesis. Fat appears first in well-vascularized regions, such as the shoulder joint, before differentiation can be identified in the less well-supplied adjacent subcutaneous tissue. There is also an important physiologic significance to this close anatomic relationship. Lipoprotein lipase, the hormone responsible for transfer of triglyceride from circulating lipoproteins to adipose tissue, is synthesized by adipocytes and transferred to the luminal surface of the capillary endothelium (3). Thus, this close spatial relationship provides efficient transfer of enzyme and lipid.
Because of this close developmental association of capillaries and adipocytes, some have proposed that the adipocyte precursor, or preadipocyte, actually is derived from endothelial cells (4). Others have felt the preadipocyte may be a perivascular reticulum cell, perivascular fibroblast-like cell, or undifferentiated mesenchymal cell. The presumptive adipocyte precursor has been characterized ultrastructurally in the newborn rat (5). The preadipocyte is
P.167
a spindle cell with four to five cytoplasmic extensions along its long axis and abundant rough endoplasmic reticulum (ER). Lipid accumulates first as small droplets adjacent to the nucleus. As more lipid appears, it coalesces into a single large vacuole, and the cell takes on an oval then, finally, a round shape. The amount of rough ER decreases as the cell matures. Although cell shape and abundance of rough ER were taken as supportive evidence for the common origin of the preadipocyte and the fibroblast, which has a similar ultrastructural appearance, these similarities may have been a coincidence. The immature adipocyte needs to synthesize and excrete lipoprotein lipase thus the abundant rough ER. Fibroblasts synthesizing and secreting procollagen would be expected to have a similar array of organelles.
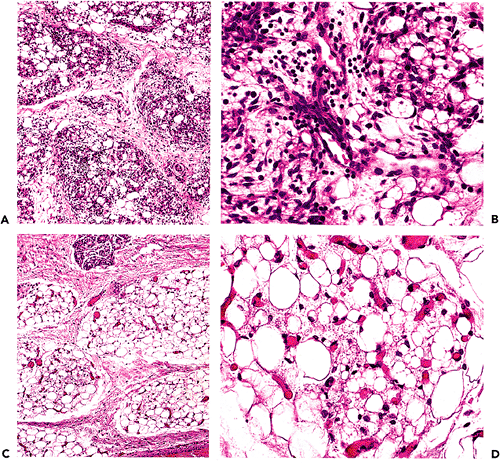 |
Figure 7.2 Fetal fat. A. Fat lobules from a 25-week fetus with a myxoid quality and prominent vasculature. B. At high power, both univacuolated and multivacuolated cells are noted together with small capillaries. C. and D. By 37 weeks, the lobules are more developed (C) and many of the cells are univacuolated (D). |
As the preadipocyte accumulates lipid to become an adipocyte, both multilocular and unilocular adipocytes can be seen. Multilocular adipocytes predominate at first. With further lipid accumulation, more cells assume the unilocular appearance characteristic of mature adipocytes. Thus, attempts to differentiate brown from white adipocyte tissue at the light microscopic level, which rely on the presence of multivacuolated cells to characterize and identify brown fat, are not reliable. Reliance on ultrastructural and biochemical differences helps to distinguish between these different forms of adipose tissue.
Molecular Biology
Through work on the adipocytic neoplasm known as myxoid liposarcoma (MLS), at least one gene involved in adipocytic differentiation has been identified. The translocation t(12;16)(q13;p11) of that tumor disrupts the normal function of the CHOP gene found at 12q13. First, the CHOP gene was shown to be rearranged in nearly all MLS (6), and, subsequently, the actual breakpoint was cloned (7,8). The CHOP gene, also known as GADD153, encodes a member of the CCAAT/enhancer binding protein (C/EBP) family and has a DNA binding domain. It appears to be involved in normal adipocyte differentiation because the protein it produces may be a dominant inhibitor of other C/EBP transcription factors known to be important in cell proliferation (9). Members of this C/EBP group are highly expressed in fat and are involved in the differentiation of fibroblasts into adipocytes and in the growth arrest of terminally differentiated adipocytes (6). CHOP itself is
P.168
induced in the differentiation of 3T3-L1 cells to adipocytes. In the neoplasm, the translocation results in a fusion gene involving CHOP and TLS (translocated in liposarcoma), an RNA binding gene with much similarity structurally and functionally to the EWS gene of Ewing's sarcoma. Presumably, the lack of the normal inhibitory function of an intact CHOP gene allows the fatty tumor to proliferate unchecked. The use of both Southern blots and fluorescent in situ hybridation (FISH) techniques in detecting the rearranged gene will have usefulness in the diagnosis of fatty tumors. Likewise, when it becomes commercially available, antibody to the CHOP protein might be used immunohistochemically to detect such tumors.
Apoptosis, or programmed cell death, probably occurs in adipocytic tissues, but studies localizing the bcl-2 protein in human fetal tissues fail to mention its detection in fat (10).
Postnatal Development
At birth, the average-size infant has approximately 5 billion adipocytes (11). This represents only 16% of the total number of adipocytes in adults. Adipose tissue continues to grow in parallel with general growth throughout the first 10 years of life. Fat cells enlarge significantly during the first six months of life without much increase in cell number (12). Until puberty, the cell size remains fairly constant while the number of adipocytes progressively increases. At puberty, there is a substantial increase in adipocyte size and number (12). Although at the end of puberty, the total number of adipocytes is similar to the adult, new adipocytes may continue to form throughout life (13). Studies on adult rats have shown that overfeeding results in proliferation of adipocyte precursors and development of new fat cells (14). De novo adipocyte formation can be triggered by overdistension of existing fat cells and the mass of stored triglycerides (15). Loss of fat cells may also occur and has been shown in overweight women following several years of strict dietary restriction (16).
Gender Differences
The differences in body fat content noted between men and women begins in early childhood. Young girls are fatter than boys. Studies on fetuses, however, have not noted differences in the pattern of distribution or quantity of fat in prenatal life (1). The distribution of adipose tissue, however, even in prenatal life is not homogeneous throughout the body. Gender differences in the distribution of adipose tissue following puberty are well-known and thought to be related to steroid hormone secretion (12). In humans, estrogens and progesterone induce an increase in trochanteric fat. The localization of more fat in the lower body in women results in the so-called gynecoid habitus. These same deposits are reduced by androgens in men, resulting in an android distribution of fat. The percentage of body fat also differs in men and women. Males reach a peak in body fat content during early adolescence, whereas women continue to accumulate fat relative to body weight throughout the teen years.
Functions
White adipose tissue is the body's largest energy store, and it possesses the enzymes necessary for the uptake and release of triglycerides. Briefly, triglycerides circulate in the blood in the form of chylomicrons from the intestine and very low density lipoproteins from the liver (17). Lipoprotein lipase present on the luminal surface of endothelial cells hydrolyzes the triglyceride to release free fatty acids. This enzyme is synthesized by adipocytes and transferred to the endothelial cells. Most of the free fatty acids are taken up by the fat cells and reesterified to glycerol phosphate within the adipocyte to form triacylglycerol, which is then stored within the cell's lipid droplet. The fat is mobilized through the action of hormone-sensitive lipase, which hydrolyzes stored triglycerides. The released free fatty acids may be reesterified or released to the circulation and bound to albumin for transfer to other cells.
Until recently the main endocrine function of adipose tissue was thought to be the conversion of androstenedione to estrone, the major source of estrogen in men and postmenopausal women. The aromatase action, however, has been localized to the stromal cell fraction of adipose tissue and not the adipocyte (18). More recently a dynamic role of adipose tissue has emerged with expression of several hormones, growth factors, and cytokines identified in adipocytes, stromal cells, and macrophages that are localized to adipose tissue. These include leptin, a regulator of energy expenditure and appetite; interleukin-6, which may play a role in the metabolic syndrome; and several important regulators of glucose and lipid metabolism, the complement cascade, and the fibrinolytic system.
Leptin, the protein product of the ob gene, is synthesized exclusively by adipocytes and acts on the hypothalamus to increase energy expenditure and decrease appetite (19). This pathway is well-established in rats. In humans, fasting lowers serum leptin levels and increases appetite (20). Unfortunately elevated or rising levels of leptin do not show the reverse effect, and leptin has not been shown to have an anti-obesity action in humans. The majority of obese individuals have elevated serum leptin levels, proportional to the amount of adipose tissue, and it is postulated that humans are leptin resistant (21). Leptin receptors are present on most tissues, and leptin may play a role outside the adipose tissue to accelerate wound healing, increase vascular tone, and inhibit bone formation (22).
Cytokines are secreted by adipocytes, stromal cells, and resident macrophages. Interleukin-6 (IL-6) is made by adipocytes and macrophages, and adipose tissue accounts for approximately 30% of circulating IL-6 in humans (23).
P.169
Like leptin, serum IL-6 levels are highly correlated with percent body fat. The IL-6 released from intra-abdominal stores enters the portal circulation. Hepatic triglyceride secretion is stimulated by IL-6, and this may contribute to the hypertriglyceridemia seen with visceral obesity (22). Interleukin-6 also stimulates hepatic secretion of acute phase reactants, increases platelet number and activity, and increases expression of endothelial adhesion molecules. There are ongoing investigations of the role this cytokine (which is derived in large part from adipose tissue) plays in the metabolic syndrome and the risk of cardiovascular disease in obesity.
Adipocytes also secrete C3 and adipsin, the proteins of the alternate complement pathway (24). Plasminogen activator inhibitor-1 (PAI-1) is a potent inhibitor of the fibrinolytic system and favors the development of thromboemboli. Insulin induces expression of PAI-1 by adipocytes, and elevated levels are seen with obesity (25).
Regulation
White adipose tissue contains numerous receptors for hormones, cytokines, catecholamines, and lipoproteins. Catecholamines acting through -2 receptors inhibit lipolysis, and a predominance of -2 receptors in gluteal fat of women is thought to impact maintenance of these fat stores despite weight loss (17). Regional differences in lipoprotein lipase levels (LPL) also occur in women. Gluteal fat in premenopausal women tends to have high LPL levels, and these regions contain larger fat cells. Such regional differences disappear after menopause and are not present in obese men (26,27). This suggests that the sex steroids also play a role in adipose tissue distribution and activity. Both androgens and estrogen modulate ob gene expression and control adipose tissue development (28,29). Androgens are antiadipogenic and estrogens proadipogenic. These may play a role in the regional differences in fat distribution and the development of the android and gynecoid patterns of obesity.
Insulin stimulates lipogenesis and glucose uptake while inhibiting fat breakdown. Insulin and glucocorticoids stimulate DNA synthesis in cultured human adipocytes and conversion of preadipocytes to mature adipocytes. These effects are enhanced on cells obtained from obese, as compared to lean, people. Estradiol-17 has also been shown to stimulate division of cultured preadipocytes obtained from both men and women. Progesterone acts in vitro to stimulate both preadipocyte division and LPL activity (30). This dual role facilitates triglyceride accumulation in women. Fibroblast growth factor 1, secreted by adipose-derived microvascular endothelial cells, stimulates preadipocyte differentiation and acculumlation of triglycerides (31).
Tumor necrosis factor alpha (TNF- ) and IL-6 have the opposite effect and are implicated along with leptin in the weight loss and anorexia of chronic wasting illnesses and cancer (32,33). TNF- is expressed in preadipocytes and acts to block differentiation to mature adipocytes through CCAAT/enhancer binding protein alpha (C/EBP- ) (34). It also suppresses lipoprotein lipase and stimulates the mobilization of fatty acids. The TNF- induces the release of IL-6 and leptin from adipose tissue, and the action of these cytokines is closely interrelated.
In addition to adipocyte function, fat cell size and number are also regulated. Numerous studies using tritiated thymidine incorporation as a marker for cell division in adipose tissue have been done in rats to identify mitotically active cells within fat. Mature lipid-laden adipocytes are generally considered to be incapable of cell differentiation because of the absence of mitotic figures seen histologically in normal adipose tissue. Sampling fat from rats injected with tritiated thymidine at 1 day and 3 days of age, which are then sacrificed at various times up to 5 months of age, has shown that the number of labeled cells in subcutaneous fat initially rises due to cell proliferation. The concentration of radioactivity then falls, probably as a result of a dilutional effect resulting from continued cell division (35). This study, however, failed to distinguish adipocyte from stromal labeling. Similar studies had been performed on rats in which the subcutaneous tissue is separated into stromal and adipose components. In one study, the specific radioactivity of the adipocyte fraction did not increase until two to five days after injection (36). Thus, they concluded that DNA synthesis occurs in nonlipid-laden cells or preadipocytes. As these cells accumulate lipid, labeled cells are detected within the adipocyte fraction.
Gross Aspects
Fatty tissue is typically a homogeneous, bright cadmium-like yellow, with a glistening and greasy surface texture, and finely divided by faint septa. Any variation in color indicates a pathologic process: white to white/yellow in fat necrosis, paler yellows in many lipomas, reddish tinge to orange/yellow in angiolipoma, definite gray/white to whitish streaks in spindle cell lipoma, and white/yellow to white nodules in liposarcoma.
Histology
Microscopically, a mature white fat cell is spherical and measures up to 120 m in diameter (37). The cytoplasm is compressed at the perimeter of the cell, and only a thin rim of cell membrane is evident on hematoxylin-eosin (H&E) stained sections. Reticulin and periodic acid-Schiff (PAS) stains highlight the adipocyte basement membrane (Figure 7.3). The cytoplasm is displaced by a single lipid vacuole, and the cells are fairly uniform in size (Figure 7.4). The nucleus, although oval, is thin and small with finely distributed chromatin; when seen in profile, a central minute clear vacuole may be seen within the nucleus (Figure 7.4). Normal subcutaneous fat is finely divided into ill-defined lobules by thin bands of collagen (Figure 7.5).
P.170
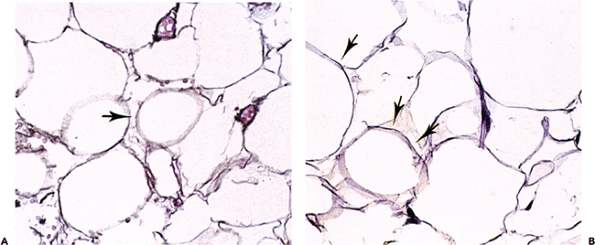 |
Figure 7.3 Normal adult adipocyte. A. On a reticulin stain, each adipocyte is outlined by reticulin (arrow), which is present outside the cytoplasm. B. The same is true on PAS stain, where the basement membrane is highlighted (arrows) and encompasses the pale residue of cytoplasm remaining after fixation and embedding. |
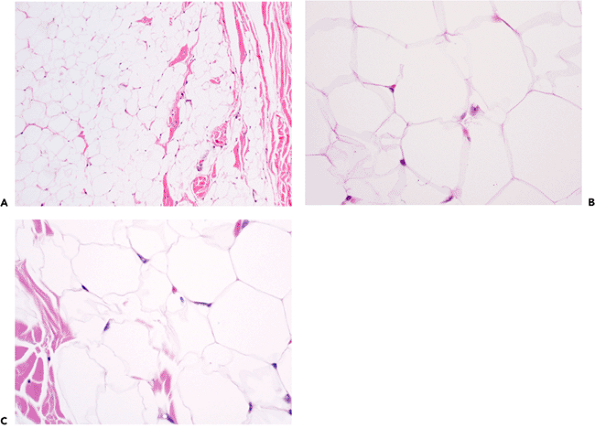 |
Figure 7.4 A. At medium power the size of subcutaneous adipocytes appears relatively uniform. B. At high power, pale areas represent portions of basement membrane and cytoplasm cut on the bias. Nuclei of capillary endothelial cells are present at intersections between multiple cells. C. In contrast to other nuclei, an ideal section of an adipocyte nucleus shows a pale character due to its thin nature and the common central vacuole, or Locherne. The wrinkled cell outlines are an artifact occasionally seen, the result of improper fixation. |
P.171
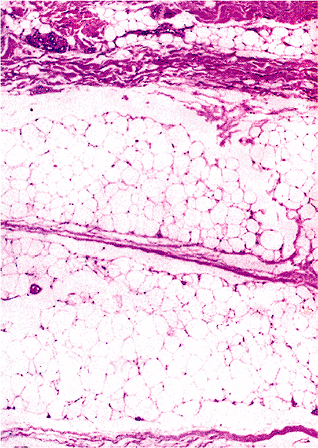 |
Figure 7.5 Adult subcutaneous fat lobule with associated microvasculature; note the thin and delicate fibrous tissue septa. |
Ultrastructure
The ultrastructure of developing adipocytes has previously been discussed. In brief, a spindle shape with abundant endoplasmic reticulum and small spherical mitochondria characterizes preadipocytes (37). Lipid accumulates as small perinuclear inclusions that coalesce to form larger lipid droplets. The mitochondria become filamentous and the endoplasmic reticulum less prominent. In a mature adipocyte, the nucleus is flattened against the cytoplasmic membrane by a large lipid droplet. There is only a thin, tenuous rim of cytoplasm that surrounds it. Pinocytotic vesicles are seen in variable numbers but are very numerous following periods of starvation. Adjacent to the cell membrane are deposits of basement membrane. Capillaries are closely opposed to the adipocyte basement membrane. Only rarely have nerves been identified adjacent to white fat cells, although they may be seen in intercellular collagenous septa.
Brown Fat
Prenatal Development
The development of brown adipose tissue has been studied in animal models. The brown adipocyte precursors are spindle cells closely related to a network of capillaries (38). As the cells and vessels proliferate, they are organized into lobules by connective tissue septa. As the cells accumulate lipid, they initially are unilocular. However, with further lipid accumulation, multiple cytoplasmic lipid vacuoles appear. As in white fat, the close association of developing adipocytes and blood vessels has led some to speculate that adipocytes actually develop from endothelial cells. Although similar ultrastructural features are cited as supportive evidence of theory, more recent investigations have attributed these similarities to a common origin from undifferentiated mesenchyme. In fact, ultrastructural and biochemical studies that have examined developing brown adipose tissue have shown that unique features such as large mitochondria and a unique mitochondrial protein are found early in development and distinguish brown from white fat.
Fetal necropsy studies have identified lobules of developing brown fat in the human fetus (39). The largest of these are from the posterior cervical, axillary, suprailiac, and perirenal regions. Those in the neck and axillae are closely associated with the major blood vessels of these regions in such a way that they extend along the course of the cervical blood vessels into the root of the neck. The suprailiac collections lie deep to the abdominal muscles, yet superficial to the peritoneum, and invest the anterior abdominal wall to the diaphragm. Intermediate-sized brown fat pads are seen in the interscapular paralateral trapezius and deltoid regions. Small collections are evident in the intercostal area. In this study, no difference was noted in distribution between the sexes or among the races. The amount of brown fat increases in proportion to growth throughout life. Deposits are well-established by the fifth month of gestation.
Postnatal Development
The presence of brown fat beyond the neonatal period in humans has been debated. An autopsy study by Heaton (40), however, has identified lobules of brown fat throughout life to the eighth decade. Brown fat is most widely distributed in young children and, over the next several decades, gradually disappears from most sites. In children under age 10, identifiable deposits of brown fat were identified in the interscapular region, around the neck vessels and muscles, around the structures of the mediastinum, and adjacent to the lung hila. Intra-abdominal and retroperitoneal deposits were noted around the kidneys, pancreas, spleen, mesocolon, and omentum, as well as in the anterior abdominal wall. The extremities were not sampled. Although brown fat disappeared from most areas, it was found to persist around the kidneys, adrenals, and aorta and within the mediastinum and neck throughout adult life. As in fetal life, no difference in distribution based on gender was noted.
P.172
Function
The main function of brown adipose tissue is heat production. It has been estimated that the maximal aerobic capacity per gram of tissue is almost 10 times that of skeletal muscle (41). It has been estimated that even in humans the small quantities of brown fat present are capable of raising heat production by over 20% (42). The production of heat is closely related to the active sympathetic innervation of brown fat and stimulation by norepinephrine. Release of norepinephrine results in the production of cyclic adenosine monophosphate (AMP) and lipolysis to release free fatty acids (43). These undergo oxidation within the mitochondria to produce adenosine triphosphate (ATP). Brown fat mitochondria contain a unique uncoupling protein, also known as thermogenin, which uncouples the oxidation of fatty acids from generation of ATP (44,45). The resultant energy is dissipated as heat. In small rodents and hibernating animals, brown fat is activated by cold temperatures to produce heat, resulting in what is known as nonshivering thermogenesis. Teleologically, this would be useful in those at risk for hypothermia. Thus, neonates, unable to alter the external environment in order to maintain body temperature, would be expected to have relatively more active brown fat than adults. In addition, brown fat accumulation and activation may play a role in weight regulation. Experimentally overfed rats show a compensatory increase in brown fat activation in metabolic rate, minimizing weight gain (46). Many types of obesity in laboratory mice and rats are related to defective regulation of brown adipose tissue, including that seen in ob/ob mice (47). In contrast, exaggerated leanness may be associated with excessive brown adipose tissue responsiveness to external factors, such as sympathetic stimulation. Although brown adipose tissue is present in humans, its role in weight regulation, obesity, and thermal regulation in adults remains controversial (48). Increased amounts of periadrenal brown fat in malnourished people at autopsy suggest a compensatory increase in nonshivering thermogenesis to maintain body temperature in those with diminished subcutaneous fat and cachexia (49).
Regulation
Unlike white fat, brown fat is highly innervated and regulated by sympathetic stimulation. Nerves enter each lobe and branch within the interlobular septa, running along the vessels to terminate on the fat cells (50). Brown fat cells have numerous 1- and 2-adrenoreceptors that regulate lipolysis and thermogenesis (43). The -adrenoreceptors, although present, probably do not act directly in heat production. Norepinephrine also may act to increase the number and character of brown fat cells. Using continuous infusions of norepinephrine, Mory et al. (51) have shown that such chronic sympathetic stimulation results in increased cellularity, increased protein content, and increased mitochondrial density in brown fat. Because of this close association of sympathetic activity and brown fat activity, several investigators have used pheochromocytoma as a model to study brown fat activities in humans. These studies have provided evidence supportive of early autopsy studies. Functional brown adipose tissue was identified in adults with pheochromocytomas that had similar biochemical features to the better-characterized brown adipose tissue of rodents (52).
Hormones also play a role in brown fat regulation, but it is minor in comparison to the sympathetic system. Thyroid hormone, although active in regulating metabolic rate, has little importance in diet-induced or nonshivering thermogenesis (43). Insulin stimulates glucose intake into brown adipose tissue. Both cortisol and gonadal steroid hormones inhibit thermogenesis, thus promoting energy conservation.
Histology
The term brown fat was applied to this tissue because of its characteristic gross appearance. It is incorrect to refer to it as fetal fat because it is present throughout life. The abundant vascularity and numerous mitochondria within the cells impart a characteristic reddish-brown color to the tissue. Brown fat has a glandular lobulated appearance. This is in contrast to the more diffuse growth pattern of white fat. Histologically, brown fat is organized into lobules of cells that are made up of adipocytes, capillaries, nerves, and connective tissue. These are surrounded by a thin, fibrous capsule containing blood vessels, nerves, and scattered white adipose cells (53). The cells are polygonal in shape, with a mixture of multivacuolated and univacuolated cells (Figure 7.6). The occurrence of both cell types is emphasized, and
P.173
their presence in developing white fat initially confused studies on its origin. The multivacuolated cell, characteristic of brown fat, has a highly granular cytoplasm with numerous lipid inclusions. Its granular appearance is due to the numerous mitochondria necessary for thermogenesis. The nucleus is spherical and often centrally located, although a large lipid inclusion may displace it toward the periphery of the cell or, rarely, to the extreme perimeter (as in white fat). Small nucleoli are common. The unilobular cells are indistinguishable histologically from the mature signet-ring cell type white adipocytes but are different ultrastructurally. On average, the size of the brown fat cells is smaller than white adipocytes, approximately 25 to 40 m. In animals that hibernate, marked seasonal variation in cell size has been noted. Both exposure to cold and starvation result in lipid depletion, causing reduction in cell size and wrinkling of the cell membrane.
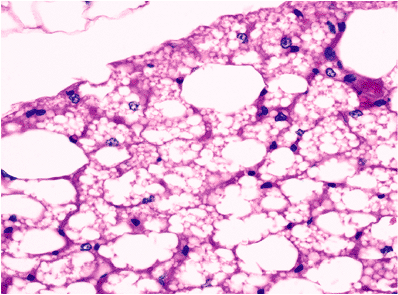 |
Figure 7.6 Normal adult brown fat. Nearly all cells have centrally placed nuclei and multivacuolated cytoplasm. Rare cells (top left) are nonvacuolated. An arborizing thin capillary network is noted. |
Brown adipose cells are surrounded by a network of collagen fibers that contain numerous minute nerve axons and blood vessels. Nonmyelinated axons terminate on the fat cells, providing an avenue for direct sympathetic regulation. The vascularity is quite prominent with numerous capillaries coursing between the adipocytes. It is estimated in rats that the vascularity of brown fat is four to six times greater than that of white fat (53).
Histochemistry
Enzyme Histochemistry
In development, enzyme histochemistry within developing adipocytes is related to the stage of adipocyte differentiation. In fact, in some systems, such as the rat, it is clear that enzymatic differentiation of adipocytes precedes morphologic differentiation (54,55). In regions destined to become adipose tissue, undifferentiated morphology is initially present without a capillary bed and without any enzymatic capacity. Subsequently, immature cells or what can be termed preadipocytes exist in the form of spindle cells within an area containing a capillary bed. These cells lack any lipid or a basal lamina and have a large complement of enzymatic activity; but they lack the capability to release fat as a result of the absence of esterase (lipase). In mature lobules, adipocytes in the form of rounded cells now contain lipid, a basal lamina, and a well-developed capillary bed; the entire complement of enzymatic activity is present, including NADH-tetrazolium reductase, ADPH-tetrazolium reductase, and glucose 6-phosphate dehydrogenase (G6PDH). Malate dehydrogenase (NADP) activity is acquired only by late-stage adipocytes (54). Hausman and Thomas (54) demonstrated the presence of such enzymatic differentiation before the assumption of an obviously rounded cell shape consistent with an adipocyte.
Lipoprotein lipase is an enzyme found at high concentration in fatty tissues. It is involved in the transport of serum triglycerides into adipocytes in the form of fatty acids. However, it can be found in other tissues such as skeletal muscles (56,57) and cardiac muscle (58), where it may be localized to endothelial cells. Concentration in fat is directly related to the serum insulin concentration.
Lipid Histochemistry
Lipids in adipose tissue are generally identified using various stains, such as oil red O and Sudan IV (59,60,61,62,63,64,65). It should be noted that lipids are lost in formaldehyde after prolonged fixation, and, thus, cases to be tested using frozen cryostat sections of fixed material should be obtained as soon as possible. Of the two time-honored lipid stains mentioned, oil red O gives the more intense stain and is more rapid to perform. Sudan black B may stain nonlipid substances (such as coagulated proteins) nonspecifically. As a rule, neutral fats are detected using these fat stains. However, a differential staining pattern between neutral fats and fatty acid components and phospholipids can be obtained with the Nile blue sulfate stain (66); with this stain, neutral fat stains pink to red, and fatty acids and phospholipids stain bluish. The lipid composition of fatty tissues may also be investigated using new techniques such as the hot-stage polarizing-light microscopic method (67).
The normal composition of lipid in white adipose tissue consists of 99% triglyceride in the form of neutral fat and less than 1% in the form of phospholipid, cholesterol, and fatty acids (53). In less-differentiated adipocytes, such as those found in liposarcomas, there is a shift away from neutral fat to phospholipids and cholesterol (66). Unfortunately, lipid stains appear to have little use in the everyday examination of adipocyte lesions. The droplets seen on the stains may represent nonspecific staining, and a variety of other mesenchymal lesions may contain lipid (64). An exception is the distinction between lesions with artificial vacuoles, such as epithelioid smooth muscle lesions, which are negative with fat stains.
Intracellular Lipid in Nonadipocytes
Lipid may accumulate in a variety of other cell types and in nonadipocytic tumors.
Steatosis
According to Stedman's Medical Dictionary (68), steatosis has two main meanings: adiposis and fatty degeneration (e.g., steatosis cordis = fatty degeneration of heart). These terms (and the terms used in a variety of pathology texts) are unclear, and the distinction between intracellular lipid accumulation and adipocyte infiltration of organs is not
P.174
made. When nonadipocytes store lipid intracellularly, the phrase lipid accumulation is accurate; in the liver, the term steatosis is used; and, in major pathology texts (69,70), the term appears to be applied solely to the hepatocyte. However, intracytoplasmic lipid can be found within other solid organs, such as the heart [in the myocardial fibers in hypoxia (69)] and the kidney [in the renal tubule in diabetes, poisonings, Reye's syndrome (71)]. Theoretically, there is no reason why these processes cannot be referred to as myocardial or renal tubular steatosis. Regardless, in referring to intracellular lipid accumulation, terms such as lipid accumulation or steatosis are preferable to unclear and archaic designations, such as adiposis or fatty degeneration. Discussion of adipocyte infiltration of organs is found later (see the section entitled Syndromes Associated With Fatty Lesions (including Lipomatosis).
Lipid accumulation may occur in the placenta after prolonged parenteral nutrition; there, it takes the form of foamy vacuoles within the syncytial and Hofbauer cells of the chorionic villi (72).
Aside from adipocytes, lipid in the form of cholesterol and cholesterol esters may be identified in cells with a steroid-producing function in organs, such as the adrenal, ovary, and testis (and tumors thereof). In addition, other types of lipid are found within a variety of tumors. In practice, it is generally thought that a lipid stain (e.g., oil red O) can aid in the differential diagnosis of certain tumors. For example, it is well-known that renal cell carcinomas typically contain lipid (73), and most pathologists have the impression that many other tumors do not. However, it is clear from studies four decades ago (74) that fat stains are positive in the majority of cancers (Table 7.1). Thus, there are problems with the use of the fat stain in the diagnosis of carcinoma, and caution should be exercised in interpretation. Furthermore, although some clear cell lesions in the differential diagnosis of renal cancer contain glycogen (benign sugar tumor of lung) (75), others such as xanthoma of bone (76) contain lipid and thus a fat stain is of no assistance.
Table 7.1 Oil Red O Positive Carcinomasa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Immunohistochemistry
Currently, there is no commercially available specific immunohistochemical marker for adipose tissue. However, an adipocyte lipid-binding protein, p422 or aP2, is a protein expressed exclusively in preadipocytes late in adipogenesis. Preliminary studies with an antibody to aP2 demonstrate that it stains only lipoblasts and brown fat cells and is capable of identifying liposarcomas selectively (77). This may be quite useful diagnostically in the future.
Adipocytes and tumors thereof stain positively for vimentin; and, in our experience, adipocytic tumors have been negative for cytokeratin, desmin, and muscle-specific actin. In 1983, Michetti et al. (78) were the first to describe S-100 immunoreactivity in adipocytes, specifically of rat origin. The S-100 protein was extracted and shown to be identical to that found in the rat brain. Ultrastructurally, S-100 reactivity was widely dispersed within adipocyte cytoplasm but was not found within mitochondria, lipid droplets, or most of the endoplasmic reticulum. In a similar ultrastructural study, Haimoto et al. (79) identified S-100 protein in the plasma membranes, in membranes of microvesicles, and within polysomes. The Golgi apparatus was negative for this marker, although some reactivity was found within the rough endoplasmic reticulum. During the process of lipolysis in fat cells, Haimoto et al. (79) noted a change in the distribution of S-100 antigen and suggested that S-100 protein molecules interact with free fatty acids, indicating that this protein may act as a carrier protein for free fatty acids.
The S-100 protein is a highly acidic calcium-binding protein of molecular weight 21,000. It consists of two polypeptide chains ( and ) and may occur as dimers in three ways: S-100a ( , ), S-100b ( , ), or S-100ao ( , ) (80). When fat cells have been analyzed, they have been shown to contain only S-100b ( form), like Schwann cells (80,81). In the routine practice of immunohistochemistry, adipose tissue reacts in a variable fashion (Figure 7.7), accounting for some negative reactions observed by Kahn et al. (82). Although lipomas and liposarcomas are reported to be frequently positive with S-100 (83,84,85,86), in our experience, this has not been true. Regardless of fixation with formalin or Bouin's solution, very few liposarcomas have exhibited S-100 immunoreactivity.
Adipocytic lesions do not stain with antibodies to neuron-specific enolase (87).
Obesity
Human obesity is thought to be approximately 60% genetic in origin, with multiple genetic and environmental factors involved (88). The role of brown adipose tissue (BAT) was briefly alluded to earlier, and new research continues to underscore its importance. For example, in transgenic mice engineered to lack BAT, obesity develops routinely (89). In
P.175
mice, the genetics of obesity are more clear than in humans. A team at the Rockefeller University led by Dr. Friedman first reported the identification of the ob mouse gene and showed that mutations of it are associated with the development of obesity (90). These same researchers have located the human counterpart (OB gene) (90) and mapped its location to chromosome 7 (91). The human protein produced by this gene has 84% homology with the mouse protein, appears to be a hormone secreted by adipose tissue, and likely functions as part of a pathway to regulate body fat. If, indeed, a defective hormone is responsible for some forms of obesity, then there is an immediate therapy available in the form of fully intact native hormone. In 1995, several research groups (92,93,94) have shown that injection of the ob protein into mice causes the animals to lose weight and maintain their weight loss. Even obesity due to a nongenetic defect like excess diet fat is corrected by the ob protein, now called leptin.
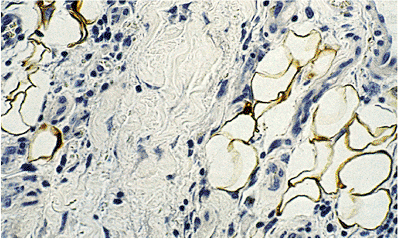 |
Figure 7.7 S-100 immunohistochemistry. Reactivity is seen both in the nuclei and in the cytoplasm surrounding the lipid droplets. Such S-100 positive results appear to vary considerably from case to case, probably reflecting fixation differences. |
Finally, the receptor for this protein has been identified recently and shown to be nonfunctional in obese animals (95). Clearly, there have been major advances constituting a breakthrough in obesity research, and the fruits of this research should affect human therapy soon.
Adipocyte Lesions
Terminology
In contrast to other human tissue cell types, the terms hypertrophy and hyperplasia are not usually applied to the adipocyte. It is stressed here, however, that adipocyte hypertrophy (or increased fat cell size) is a recognized phenomenon and is found, for example, in obesity. Enlarged, or hypertrophic, fat cells (>120 m or so) can also be identified in neoplasia (lipoma and liposarcoma) where cells appear to have three or four times the normal diameter (e.g., >300 m). Hyperplasia (or an increased number of adipocytes), in contrast to widespread belief, is a definite occurrence. Again, it is common in obese patients, but it may also be seen in organ-based infiltrations; these are a type of site-specific adipocyte hyperplastic processes. Mature adipocytes are incapable of regeneration, and new fat cells are added through in situ mesenchymal cell differentiation recruited from primitive perivascular cells. No disease or change involving adipocytes can appropriately be termed a degeneration (as mentioned earlier), other than liquefaction with necrosis. Atrophy of adipocytes may be seen in malnutrition, starvation, or as the effect of chemotherapy (see the section entitled Atrophy). The appearance of mature fat cells as small foci in unusual places is termed metaplasia and is discussed in a later section. Localized new growths of either pure adipocytes or mixtures of adipocytes in other tissue constitute neoplasia and are presumably clonal entities.
Degeneration
In the condition sclerema adiposum neonatorum, the subcutaneous fat is grossly and microscopically abnormal. Rubbery plaques are due to fat necrosis and degenerative individual fat cells with intracellular needle-shaped crystals (96). This fat crystallization is brown and can be highlighted by polarization. Such crystals apparently may also be identified in up to 30% of stillbirths as a general degeneration following intrauterine demise (96). In another disease, Neu Laxova syndrome, a defect in lipid metabolism causes a lardlike appearance to the adipose tissue and is lethal.
Atrophy
The changes in fat lobules during starvation or malnutrition are particularly noticeable in the subcutaneous region or the omentum. Individual fat cells are reduced in size and fat content, and those without much lipid take on a rounded or epithelioid appearance (97). In the extreme, lobules of these epithelioid cells can simulate tumor nodules histologically (Figure 7.8). The cytoplasm is variable in amount and is eosinophilic or granular with or without small lipid vacuoles of differing size, depending on the severity of the malnutrition. Some cells have a multivacuolated appearance. The intervening region between cells is constituted by homogeneous eosinophilic or amphophilic myxoid ground substance (Figure 7.8) that is probably an extract of serum, although stimulation of proteoglycan matrix by the process of starvation (98) is possible. As part of this involution process, lipofuscin is deposited within the shrinking cells (Figure 7.8). Importantly, each lobule retains its overall oval shape, although markedly reduced in size and considerably separated from other lobules (Figure 7.8). In extreme cachexia, only streaks of tissue remain.
P.176
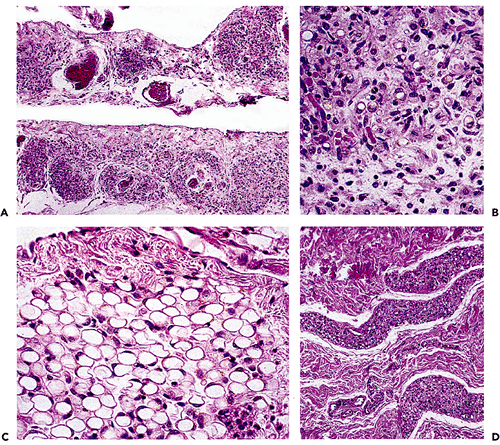 |
Figure 7.8 A. The extreme atrophy seen here in the omentum of a patient with anorexia nervosa may mimic tumor deposits. B. At high power, shrunken eosinophilic cells are seen with occasional vacuoles and lipofuscin pigment. C. In less severe starvation, these omental adipocytes are well-recognized, although much smaller than normal size; again, note the presence of pigment. D. In the skin, severe cachexia secondary to a cancer resulted in marked involution of the cutaneous fat lobules, which appear only as elongated streaks. |
Nearly identical changes can also be seen in the white adipose tissue of fasted animals. As the cells gradually lose their lipid, the single lipid droplet breaks up into multiple vacuoles. Gradually, all lipid disappears. These cells become small and ovoid in shape, sometimes measuring only 15 m in diameter (99). There is an apparent expansion of pericellular collagen in such a way that these cells appear as clusters of mesenchymal cells in fibrous stroma. Ultrastructurally, multiple pinocytotic vesicles are seen clustered along the entire cell membrane (53). Lipid is not seen within these vesicles, and their significance is unknown.
In the bone marrow, chemotherapy causes changes referred to as serous atrophy or gelatinous transformation (100,101). The majority of the fat cells have been destroyed, leaving scattered adipocytes of varying size remaining. No lobular appearance is present in the marrow, but the interstitial compartment is composed of the same eosinophilic myxoid substance described previously, again probably consisting of serum fluid and proteins. Droplets of lipid scattered about are also found and, upon regeneration, may appear as foci of lipogranulomas.
Although the microscopic features of the starvation effect on human brown fat have not been described, animals maintained on a dextrose-thiamine diet are known to show distinct morphologic changes in brown fat (37). The mitochondria are disrupted and large, irregular electron-dense inclusions are seen within the mitochondrial matrix. The cristae may assume a mosaic pattern with compartmentalization of the material. These cells revert to normal after 24 hours of a normal diet. Similar changes in white fat mitochondria have not been seen with starvation, suggesting that the active mitochondria of brown fat are particularly labile and sensitive to dietary changes.
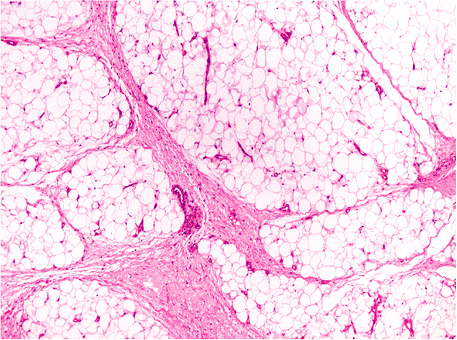 |
Figure 7.9 Accentuated fat lobules in ischemia of the lower extremity. Loose myxoid connective tissue widens the septa between lobules; edema and a mild inflammatory infiltrate are present. |
P.177
Cellulite
The term cellulite is applied to the external skin when it exhibits linear depressed streaks (mattress phenomenon) or frank dimpling. Cellulite is typically found on the thigh and buttocks and is more common in females; it can be divided into incipient cellulite and full-blown cellulite. The former results from an uneven undersurface of the dermal-hypodermal interface, with fibrous tissue surrounding the protruding papillae adiposa; vertical fibrous strands of uneven thickness divide the hypodermal fat (102). In contrast, full-blown cellulite consists of a delicate meshwork of collagen fibers produced by increased hypodermal pressure of fat accumulation and increasing fat volume. Scattered CD34+ fibroblasts are seen in both forms of cellulite. Unlike women, men have a more smooth, strand-free dermal interface in the thigh and buttock areas (102).
Ischemia
Little is written about the effect of ischemia on the adipocyte. We have observed changes in the subcutaneous fat of legs removed for atherosclerotic vascular disease. They consist of accentuation of the lobular architecture by thickening of the fibrous septa; wider and more myxoid in quality, the septa are edematous and also contain scattered inflammatory cells (Figure 7.9). Actual necrosis was not observed.
Metaplasia
As surgical pathologists, we most frequently encounter adipocytic metaplasia, usually calcified, in cardiac valves (Figure 7.10). There is little in the literature or textbooks on this phenomenon. The emergence of mature adipose tissue seems to parallel the appearance of osteoblasts forming bone within the calcific deposits. Once adipose tissue is present, bone marrow precursors may become resident, presumably from circulating cells, and cause hematopoiesis. Metaplasia is not limited to this site and may be encountered in calcified large vessels or elsewhere, such as in laryngeal cartilage undergoing ossification. We have even seen it in small ossified bronchioles. A similar phenomenon of hematopoiesis without adipose tissue and bone has been reported within acoustic neuromas (103).
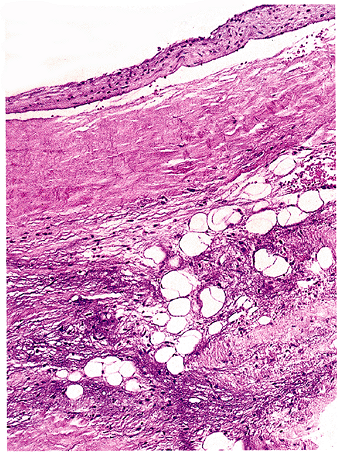 |
Figure 7.10 Fatty metaplasia of cardiac valve. Mature adipocytes are found in a myxoid background but are more commonly seen in association with calcification or ossification. |
P.178
Lipodystrophy
Although there are several different entities referred to in the past under this name, some (idiopathic intestinal lipodystrophy, or Whipple's disease) are infectious and others (mesenteric lipodystrophy; see section entitled Mesenteritis) are inflammatory disorders without fundamental changes in the fat cells themselves. The one example of a true lipodystrophy is called membranous lipodystrophy, a relatively new clinical entity characterized by abnormal fat cells, bone cysts with pathologic fractures, and leukodystrophy of the brain (104,105,106). The marrow fat is particularly affected (104), but the membranocystic lesions are also present to a lesser degree in the subcutaneous adipose tissue (106). The characteristic and pathognomonic finding is the highly shrivelled, undulating outline of individual fat cell membranes, giving them hyalin eosinophilic convolutions or arabesque profiles. Multiple small cysts are found, apparently formed by fusion of ruptured adipocytes. Young adults are affected in Japan and Finland primarily, but five cases have been seen in the United States (105). Its etiology and pathogenesis are unknown; it is probably related to an enzyme deficiency (104). A secondary form of membranous lipodystrophy has been described in association with lupus erythematosus and morphea profunda (107). Interestingly, the membranous changes in fat characteristic of lipodystrophy can also be seen in normal fat affected by radiation therapy (108).
Adipocytes in Organs
Fatty Infiltration
As distinct from lipid accumulation or steatosis (see section entitled Steatosis), fatty infiltration is defined as the presence of mature adipose tissue in sites not normally containing fat. This is a disorder or condition relating to adipocyte cell growth and, therefore, the term fatty degeneration is a misnomer and incorrect. In some situations, such as within extremity muscle groups, the process of fatty infiltration is often related to atrophy of the involved site (109). This association between fatty infiltration and atrophy or involution is also noted in other organs [thymus (110), bone marrow (111), and kidney (112)], and apparently signifies the propensity for adipocytes to fill a vacuum, in a sense, left by atrophic processes (97). Whatever the stimulus may be, the adipocytes probably arise from pleuripotent mesenchymal cells adjacent to blood vessels (97). The reversal of this relationship is found in the parathyroid gland, where there is an inverse relationship between parenchymal cells and adipocytes, to the point where no adipocytes are present in complete parathyroid hyperplasia.
Nonatrophic organs can also accumulate fat cells (lipomatosis), and the classic examples are the heart and pancreas (69). In these locations, no parenchymal damage is discerned, and the process is a type of accidental lipogenesis (97). In the case of the pancreas, normal parenchymal histology and function are present even though the pancreas may be nearly invisible grossly (69,113). This type of pancreatic lipomatosis is correlated with age and obesity and also occurs in diabetics (113). The amount of pancreatic tissue is thought to be either completely normal (69) or partially depleted (113). However, true pancreatic atrophy with resultant lipomatosis also exists as a rare condition known as Schwachman syndrome (113) [see Table 7.2 in section entitled Syndromes Associated with Fatty Lesions (Including Lipomatosis)]. Fatty infiltration of the heart is most often an innocuous condition with no effect on the myocardial fiber or cardiac function (69). However, there are rare exceptions in which severe adipocity has resulted in cardiac rupture (97). Another clinically important lesion is termed lipomatous hypertrophy of the interatrial septum (114), a focal enlargement that may cause sudden death, arrhythmias, or congestive failure (115,116). Be mindful that the occasional appearance of fat in endocardial biopsies in no way indicates cardiac perforation (117).
Isolated fat cells can be found within lymph nodes in childhood, but enlarged nodes with prominent fatty infiltration mainly occur in adults, particularly in obesity (118). Common in the abdomen and retroperitoneum, such lipolymph nodes can be mistaken for lipomas (118) or be interpreted as positive in a lymphangiogram for lymphoma or Hodgkin's disease (personal observation), mimicking lymphoma relapse (119). Rarely, a lipoma or angiomyolipoma occurs in the liver (120), but those lesions should not be confused with the hepatic pseudolipoma (121). This pseudolipoma is often found as a bulge on the surface of the liver and probably represents capture of previously detached appendices epiploicae. In the mouth, fat is one of the components contributing to macroglossia in certain conditions (122).
The Ito cells of the liver are fat-containing cells along the sinuses and are a variation on normal histology (123); they may become prominent in the condition known as lipopeliosis (124) and may be involved in the benign neoplasm called spongiotic pericytoma (125).
Fat Biopsy for Amyloid
It is becoming increasingly popular to perform a subcutaneous fat biopsy for the diagnosis of amyloidosis. In such instances, the Congo red stain may reveal amyloid around blood vessels and, occasionally, between adipocytes (126,127,128). This procedure is at least as sensitive as the rectal biopsy (128), can identify up to 84% of cases (127), can be combined with other studies to determine amyloid type (126), and is a safe and innocuous way to make the diagnosis (127).
P.179
Biopsy analysis of adipose tissue may become important in the future, to assess a given individual's storage content of toxic chemicals. A variety of industrial and environmental hydrocarbons are stored predominantly in fat, and subcutaneous adipose tissue deposits may be analyzed and results correlated with the development of diseases such as neoplasia.
Inflammations
Fat Necrosis
Three histologically distinct types of fat necrosis exist: the ordinary variety secondary to trauma and other inflammation, that associated with pancreatitis, and infarction of fat. Histologically, ordinary fat necrosis is typified by the presence of epithelioid histiocytes, foamy macrophages, and giant cells in adipose tissue, often surrounding and isolating individual adipocytes (Figure 7.11). Lymphocytes and plasma cells are also found in small numbers. Occasionally, unusual crystalloids may be seen (129). Fat cells become destroyed, and the released lipid may fuse to result in a single droplet larger than the average cell or in minute droplets. This process may resolve with mild fibrosis or, if extensive, may cause cyst formation with eventual dense fibrosis and even calcification at the periphery. Such cysts with central liquefaction may be located on the buttocks and be the final result of trauma, secondary to an injection. Just beneath the cyst wall, necrotic outlines of adipocytes are usually present, signifying the origin of the end-stage cyst in fat necrosis.
An unusual type of fat necrosis forming cystic spaces has been designated membranous fat necrosis by Poppiti et al. (130). In this example, actual cysts are formed that contain pseudopapillary structures and central debris. Although the fat cell outlines are normal in appearance, the formation of these cysts resembles that seen in membranous lipodystrophy. Membranous fat necrosis can also occur secondary to radiation therapy (108).
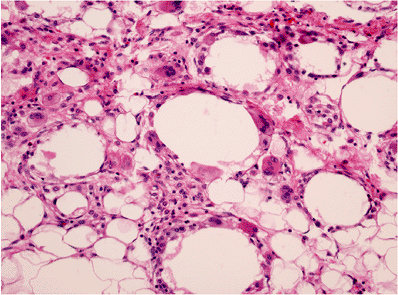 |
Figure 7.11 Fat necrosis, ordinary type. Multinucleated histiocytic giant cells surround a large lipid vacuole formed by fusion of destroyed adipocytes. Scattered lymphocytes and monocytes occupy expanded spaces between cells at top. |
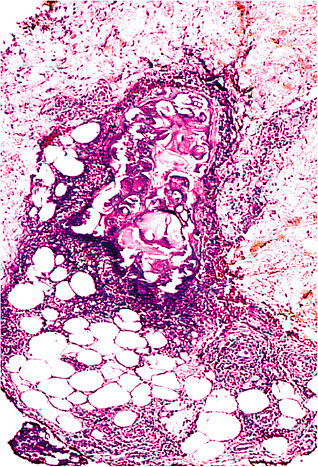 |
Figure 7.12 Fat necrosis, pancreatic type. In contrast to regular fat necrosis, numerous neutrophils are found, together with central liquefaction. The central material may give either an eosinophilic or basophilic appearance, and disrupted cell membranes can be appreciated. |
Fat necrosis secondary to acute pancreatitis is histologically distinctive (Figure 7.12). Rather than consisting of a histiocytic infiltrate, the pancreatic fat necrosis is accompanied by an infiltrate of neutrophils predominantly, and liquefaction of fat is apparent (131,132). In the center of the lesion, the infarctlike outlines of fat cells can be seen, and fat cell membranes are ruptured, releasing their contents into a central eosinophilic or basophilic material. The entire region is bordered by an acute inflammatory infiltrate. The process is thought to be secondary to the action of pancreatic lipolytic enzymes in the serum acting on susceptible foci.
The infarction type of fat necrosis, in which eosinophilic outlines of fat cells without nuclei or inflammation are present histologically, may be seen in lipomas and in detached peritoneal tissue originating from appendices epiploicae. The lipomas containing infarction may be pedunculated with twisting, causing compromise of blood flow.
P.180
Calciphylaxis
Another disorder that often manifests itself as skin and subcutaneous fat necrosis is called calciphylaxis; here, the characteristic vascular necrosis with calcium precipitation will aid in the diagnosis (133). It is a painful and often lethal complication of dialysis and renal failure (134). Small vessels (including arterioles in the fat) show mural calcification and necrosis, along with thrombosis and necrosis of surrounding tissues. In some cases, an association with primary hyperparathyroidism has been reported (135).
Panniculitis
Numerous diseases and conditions may cause an inflammatory infiltrate of the subcutaneous adipose tissue, namely, a panniculitis; readers are referred to a variety of textbooks on skin pathology for an in-depth enumeration of these. Only a few relevant points are made here. First, the condition called Weber-Christian disease, or febrile nodular nonsuppurative panniculitis of the subcutaneous fat, was described early in this century and is consistently referred to in discussions of this topic. However, it became clear in the 1960s and 1970s that this disease was not a clinically distinct entity, but rather had many separate etiologies, including steroid withdrawal, diabetes mellitus, tuberculosis, pancreatic disease, and systemic lupus erythematosus (136). Thus, it is generally agreed today that Weber-Christian disease was a clinical description of a presentation for numerous diseases and is a term to be avoided (137).
Panniculitis, as a rule, can be divided into those that are septal and those that involve the lobules of adipose tissue (137). The character of the infiltrate is important, and note should be made of the presence of eosinophils (138), neutrophils and granulomas (139), histiocytes with lymphophagocytosis (140), or other specific changes (141). Autoimmune diseases such as scleroderma (142) and lupus (143) may be causative, indicating the importance of historical detail. Unusual causes, such as 1-antitrypsin deficiency (137), have a characteristic histology, as does pancreatic fat necrosis (described in other texts). Even withdrawal from steroids may cause a panniculitis (144).
Mesenteritis
Inflammation of the mesenteric fat is a recognizable clinical entity that has more recently been termed mesenteric panniculitis to signify the active inflammatory stage and retractile mesenteritis to signify the fibrotic stage (145). Other terms complicate the literature, but it is generally held that they all refer to the same disease process and spectrum: liposclerotic mesenteritis, sclerosing mesenteritis, mesenteric lipodystrophy (ML), and Weber-Christian disease of the mesentery [see recent review by Kelly and Hwang (146)].
The process consists of a chronic inflammatory infiltrate of lymphocytes, plasma cells, foamy histiocytes, and giant cells, along with recognizable fat necrosis, edema, and a variable amount of fibrosis and calcification. Myofibroblasts proliferate and are directly involved in the pathogenesis of the retractile disease (146). While it most often thickens the mesentery (type 1 ML), it can appear as a single tumefaction at the mesenteric base (type 2 ML) or as multiple discrete nodules (type 3 ML) (147). Other space-occupying lesions, such as inflammatory pseudotumors, xanthogranulomatosis (see below), and fibromatosis, are in the differential diagnosis (119). Affected patients are usually middle-aged and predominantly male, and they complain of vague abdominal discomfort and weight loss, with over one-half presenting with fever. Nearly one-half of them are, oddly enough, asymptomatic (146). Rare cases have been fatal, but the prognosis is generally excellent. Mass lesions regress within 2 years in about two-thirds of the patients, and any pain disappears in three-quarters of them (145). Steroids are commonly given to treat the disease, but it is unclear whether the course of the disease or the progression to fibrosis is changed (145).
Retroperitoneal xanthogranulomatosis can be due to a primary inflammatory process of the kidney, or it can represent involvement of the retroperitoneum by the mesenteritis. Many foamy histiocytes and lymphocytes are seen. Rarely, it can be associated with Erdheim-Chester disease (multisystem fibroxanthomas with bone pain and sclerotic bone lesions) (148).
Lipogranuloma
Small collections of epithelioid histiocytes with lipid droplets are commonly encountered in lymph nodes, draining the gastrointestinal tract (mesenteric, porta hepatis, retroperitoneal), and in the liver, spleen, and bone marrow. They do not imply a pancreatitis (in which necrosis should be present) or other pathologic process and are completely incidental.
Tumors and Tumorlike Lesions
Brown Fat Lesions
Hibernoma
The only pathologic lesion of brown fat known to date is the hibernoma, the neoplastic counterpart given its name by Gery (149). Although many of the cells in the hibernoma are multivacuolated, some cells lack vacuoles completely and are eosinophilic and granular in appearance. Both of these cell types have a centrally placed nucleus. Importantly, univacuolated cells with peripherally placed nuclei
P.181
resembling white adipocytes can be identified, as they can in normal brown fat (149,150). The red-brown color of a hibernoma is the result of the increased vascularity in numerous mitochondria. The ultrastructure of hibernoma is similar to brown fat (151); and, indeed, when cellular organelles are compared, the ultrastructure suggested to a number of authors (149,150) is that brown fat and white fat are two distinct tissues, with different ultrastructural features.
Concerning location, many hibernomas arise in sites corresponding to the distribution of normal brown fat interscapular area, neck, mediastinum, and axilla (149); other cases have been reported in the abdominal wall, thigh, and popliteal space (149), all sites considered devoid of brown fat (152). Generally medium-sized tumors (5 to 10 cm), hibernomas may obtain a huge dimension [23 cm (153)] and are often present for years prior to excision. The tumors typically occur in young adults with a median age of 26 years, much younger than patients with ordinary lipoma (149). Interestingly, endocrine activity has been noted within these tumors, with steroid hormones (including cortisol and testosterone) detected (154). Hibernomas do not recur, but whether malignant hibernomas exist has been a controversial topic. A case having atypical mitoses and bizarre nuclei was reported by Enterline et al. (155), and a similar case with ultrastructural features was documented by Teplitz et al. (156).
White Fat Lesions
Adipose Tissue within Nonfatty Lesions
Almost any malignant tumor may invade and incorporate mature fat cells. Occasionally, however, the presence of fat cells within mesenchymal proliferation can be confusing. For example, nodular fasciitis may incorporate individual fat cells that can appear smaller than normal, mimicking lipoblasts (136). Likewise, a very prominent component of adipose tissue accompanies intramuscular angiomatosis and lymphangiomatosis of the extremities (152). Benign teratomas of the ovary (157) and lung (158,159) occasionally contain mature adipose tissue as an incidental finding. So-called fibrous polyps of the esophagus (160) also contain adipose tissue. Other nonlipomatous tumors that may contain fat include the pleomorphic adenoma of the salivary gland and the benign spindle cell breast tumor described by Toker et al. (161). This lesion may be what has been described recently as a myofibroblastoma (162) with the incorporation of adipose tissue.
Perhaps by a process of cellular metaplasia, fat may also be found occasionally in the endometrium (163) or in epithelial tumors of various types (see below).
Ectopic Adipose Tissue
Ectopic fat either in cardiac valves or within organs was discussed earlier in the Metaplasia and Fatty Infiltration sections. Oddly enough, ectopic fat may occur in the dermis, where it causes a pedunculated appearance; this has been termed nevus lipomatosis superficialis or, more recently, pedunculated lipofibroma (164).
Hamartomas Containing Fat Cells
Many of us are aware that the benign pulmonary chondroma, or hamartoma, may contain fat (158). In fact, approximately 75% of these lesions do (165), and the presence of such a tissue foreign to the lung parenchyma supports the concept that these lesions are benign mesenchymomas (158,165). Occasionally, the lipomatous component may be so dominant as to suggest a lipoma (165,166).
Amazingly, adipose tissue can be a component of many other unusual lesions. It may be coupled with vascular, fibrous, and myofibroblastic components in multiple congenital mesenchymal hamartomas [multiple sites (167)]; with undifferentiated spindle cells and fibroblasts in the fibrous hamartoma of infancy [mainly in shoulder and axillary regions (168,169,170)]; with fibrous tissue and mature nerve in the sometimes congenital fibrolipomatous hamartoma of nerve with or without macrodactyly [palm, wrist, or fingers (171,172,173)]; or with smooth muscle and vessels in the angiomyolipoma (174,175). These hamartomatous lesions of tuberous sclerosis will be discussed further. In another oddity, adipose tissue is one component of human tails and pseudotails (176), along with skin and other tissues.
Massive Localized Lymphedema
In morbidly obese patients, huge subcutaneous masses as large as 50 cm may form, clinically mimicking liposarcoma (177,178). Pedunculated masses of adipose tissue show dilated lymphatics and edema and thus this condition is known as massive localized lymphedema (MLL). Grossly, the fat is marbled in appearance secondary to coarse bands of fibrous tissue intersecting fat lobules. Microscopically, the adipose tissue is dissected by fibrosis simulating sclerosing liposarcoma; however, the lesion is superficial, and there are no atypical stromal cells nor lipoblasts. In the edematous septa, scattered myofibroblasts are noted. Aside from the often postsurgical abdominal sites reported initially, MLL may also occur in the thigh, scrotum, and inguinal regions and be associated with hypothyroidism (178).
Mesenchymomas
Adipose tissue is a nearly constant component of benign mesenchymomas growth that should be redefined as having more than two mesenchymal elements. LeBer and Stout (179) required the presence of at least two different mesenchymal elements to make a diagnosis of mesenchymoma. However, we believe the trend has evolved in favor of more than two elements, and those lesions with only two
P.182
elements currently appear to be designated separately as chondrolipoma, fibrolipoma, and so on (152,180). This seems appropriate since the secondary element, usually in a lipoma, is frequently a very focal finding (as it may be in a liposarcoma). Thus, aside from lesions with focal metaplasia, lesions with three or more elements can be designated true mesenchymomas. For instance, a description of a trigeminal neurilemmoma (181) was really a mesenchymoma with cartilage, bone, hemangioma, schwannoma, and adipose tissue. Also, a thoracic tumor with smooth muscle, angiomatoid spaces, fibrous tissue, and adipose tissue is another mesenchymoma, reported in association with hemihypertrophy (182). Angiomyolipoma is another example of a benign mesenchymoma and is frequently found in the kidney, where approximately 40% are associated with tuberous sclerosis (175). Although the fat seen here is practically always mature, rarely lipoblast-like cells may be seen in these (152,183). Angiomyolipomas have also been reported in other sites, such as lymph nodes (184).
Lipomas
The distinction between adipose tissue lobules and true lipoma occasionally arises in the practice of surgical pathology, necessitating a strict definition of lipoma. Although lipoma is well described in two major texts (152,180), definitions are concise without detail. Lipoma is herein defined as a superficial or deep-circumscribed and expansile benign neoplasm composed of mature adipose tissue, which is commonly (but need not be) encapsulated. Such a definition emphasizes its well-differentiated and clonal nature (see following) and serves to distinguish most lipomas from normal fat and prominent posttraumatic skin folds, or fat fractures (185). As Allen (180) emphasizes, the capsule may be quite thin and poorly defined. Nonetheless, it is a crucial requirement for superficial tumors; deep lesions, on the contrary, are often nonencapsulated. When a subcutaneous lipoma is excised in a piecemeal fashion, the lesion may be diagnosed by noting the presence of portions of capsular fibrous tissue in the form of a circular arc of collagen of varying width at the edge of tissue fragments. In the absence of a clear-cut capsule or fragments thereof, a diagnosis of a superficial lipoma cannot be made.
Clinically, the majority of lipomas seen in surgical pathology are subcutaneous tumors typically in the middle-aged to elderly patient. Males and females are probably equally affected, and there are no racial differences. Most tumors are located on the trunk or upper extremities; if other sites are encountered, consideration should be given to one of the lipoma subtypes (e.g., forearm for angiolipoma, neck for spindle and pleomorphic types). Lipomas probably outnumber all other soft tissue tumors combined (152). Interesting facts about lipomas include (a) a nearly static size after the initial growth period (152); (b) the relative rarity of lesions on the hands, feet, face, and lower leg despite the presence of fat (152); (c) hardness after the application of ice, a diagnostic sign (152); (d) the lack of size reduction in starvation (152,180); (e) a definite, but low, recurrence rate [1 to 4% (152,180)]; (f) an unknown etiology; (g) a possible relation to potassium intake (186); and (h) a possible association with an increased incidence of cancer [46% (187)].
Many lesions of the subcutaneous region come to surgical pathology labeled as lipomas; and, not uncommonly, a portion of these actually turn out to be something else that is frequently more interesting.
When one views normal fat histologically, the size of fat cells appears to vary somewhat due to the sectioning plane; however, the variation is relatively small [80 to 120 m (personal observation); Figure 7.4]. In lipomas [including atypical lipoma (188)], there is a tendency for cell size to vary more widely, with larger cells (e.g., >300 m) being apparent. Practically, this means that a medium-power view will often disclose a two- to fivefold size range (Figure 7.13). Normal fat has a netlike structure of fibrous tissue, wherein such dispersed fibrous bands or septa dissect the adipose tissue randomly. The fibrous tissue is thicker in quality in bodily regions exposed to pressure, such as the hands, feet, and buttocks (97). This netlike fibrous tissue arrangement is recapitulated within lipomas (Figure 7.14), particularly at the periphery where small lobules are often found. A high degree of vascularity is a feature associated with lipogenic malignancy, but we should be aware that this refers to a visible network of capillaries, often in strings and branching arrays. However, normal adipose tissue and lipomas are likewise highly vascular, except the capillary vascular bed is more difficult to visualize. A PAS stain of a lipoma, for example, can highlight the minute but diffuse capillaries, particularly at the junctions between cells, where they are made more difficult to see due to compression. A delicate reticulin
P.183
network is also present in lipomas, contributed to by the basement membranes of both lipocytes and capillaries; each lipocyte is completely encircled by reticulin in a manner similar to normal fat cells (Figure 7.3). Normally, lipomas have a low degree of cellularity and no nuclear atypia; the presence of either is cause for concern. Sometimes increased cellularity is due to a diffuse low-grade form of fat necrosis (Figure 7.15). The ultrastructure of lipoma recapitulates that of its normal counterpart (189).
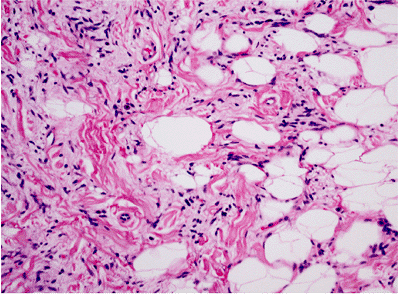 |
Figure 7.13 Variation in fat cell size in spindle cell lipoma. Some adipocytes are three to five times the size of a normal adipocyte; compare with Figure 7.4. Increased numbers of spindle cells together with collagen bands characterize this lipoma subtype, although the size variation seems to be present in all unusual types of lipoma. |
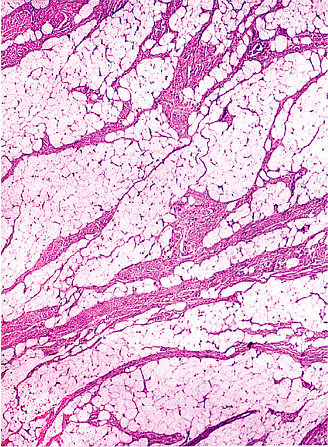 |
Figure 7.14 Lipoma with accentuated lobulation. In certain sites such as the buttock, foot, and hand (depicted here), thick fibrous septa are noted throughout; these correspond to the thicker septa within the normal adipose tissue in these regions. |
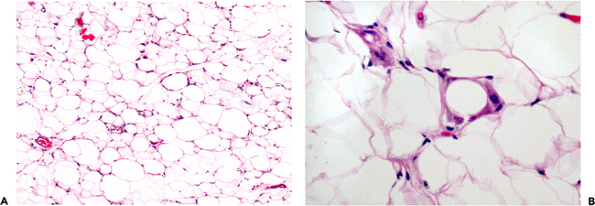 |
Figure 7.15 Lipoma. A. In some tumors, an increased cellularity at medium power may cause concern, but it is frequently due to a mild but diffuse fat necrosis. B. The lipocytes are falsely enlarged by the histiocytes without much other inflammation. |
Myxoid Change
In rare lipomas, the mature fat cells are separated by varying amounts of a loose basophilic ground substance, probably proteoglycan (Figure 7.16). When prominent, the lesion may be designated a myxolipoma or myxoid lipoma (152,180). The myxoid quality often raises the possibility of a myxoid liposarcoma. However, these areas contain only widely scattered bland cells and are never hypercellular. Furthermore, the plexiform capillary network so typical of the malignant tumor is absent, as are lipoblasts. As Enzinger and Weiss (152) observed, rare cells may be vacuolated but contain bluish mucoid material.
Intramuscular Lipoma
Deep lipomas may be either intermuscular or intramuscular, with the latter unencapsulated tumors being the more common. Intramuscular lipomas (190), also known as infiltrating lipomas, involve the large muscles of the extremities (particularly the thigh, shoulder, and upper arm) or the paraspinal muscles. For extremity lesions, an inapparent mass may become visible upon voluntary contraction. Microscopically, the lipocytes are typically mature, and mitoses or atypical nuclei are not found. Muscle fibers are widely dispersed throughout the lesion (Figure 7.17). Any unusual features should raise the suspicion of a well-differentiated liposarcoma (191). Often, intramuscular lipomas extend beyond the muscle fascia to involve the intervening connective tissue space. Therefore, it is often difficult to completely excise such lesions, and the recurrence rate is higher than that for ordinary subcutaneous lipoma. This has been particularly true for paraspinal intramuscular lipomas.
Intramuscular angiolipomas are lesions considered to be intramuscular hemangiomas with a variable fat content (152).
Lipoma arborescens is a special type of lipoma occurring in a joint: it has a characteristic villiform gross appearance,
P.184
and the patients typically have a highly painful knee (180). The mere presence of adipose tissue on a synovial biopsy is not synonymous with this entity.
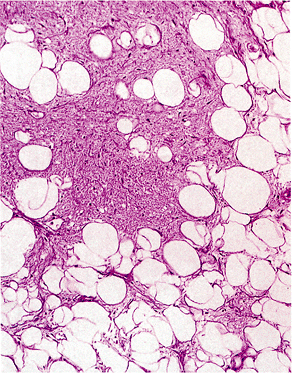 |
Figure 7.16 Lipoma with myxoid change. Features that differentiate this from myxoid liposarcoma are the lack of branching capillary vessels and significant cellularity in the myxoid component. |
Other Elements in Lipomas
Aside from the ordinary lipoma, extraneous elements of various types can be associated with an adipose tissue benign proliferation, including combinations with epithelial or other mesenchymal components.
Mesenchymal Components
Perhaps the most common mesenchymal component associated with the lipoma is, as surgical pathologists are aware, benign cartilaginous metaplasia (Figure 7.18). So-called chondrolipomas may occur in almost any site of the body, including the breast (192) and mediastinum (193). Although the term benign mesenchymoma has been applied to such lesions, the chondroid metaplasia is practically always an extremely minor component in the form of very small isolated islands of cartilage; therefore, the designation of mesenchymoma appears to be an exaggeration (as it is when cartilaginous metaplasia occurs in liposarcoma). Allen (194) also prefers to avoid the term mesenchymoma.
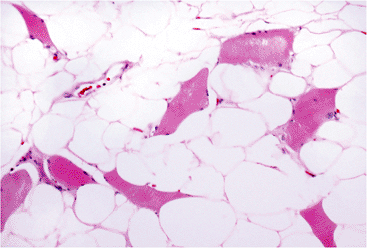 |
Figure 7.17 Lipoma, intramuscular type. The light fat cells proliferate between dark individual skeletal muscle fibers in this commonly unencapsulated tumor (trichrome stain). |
Lipochondromatosis is a recently reported entity that involves the tendons and synovium of the ankle region as a mass lesion (195). Rarely benign osteoid is also found in lipomas, either solely or coupled with cartilage (196). Some of these osteolipomas are in contact with periosteum and may be termed periosteal lipoma (196). Smooth muscle lesions, particularly of the uterus, may be combined with adipose tissue to produce lipoleiomyomas (197) and lipoleiomyomatosis (198). Prominent blood vessels are a frequent component of superficial small subcutaneous tumors called angiolipomas (152). These lesions are interesting, as they may be multiple, cause pain due to frequent microthrombi, and give rise to the differential diagnosis of Kaposi's sarcoma when the angiomatoid component completely overcomes the lipocytic component (Figure 7.19). These fat-poor variants are designated cellular angiolipomas (199). In such instances, the diagnosis is made by finding rare-to-scattered mature fat cells, usually at the periphery of the lesion.
Some lipomas contain an increased content of fibrous tissue. These usually superficial tumors have been called fibrolipomas. However, it is likely that the amount of fibrous
P.185
tissue in a lipoma is directly related to its anatomic site of origin (Figure 7.14). Dense thicker fibrous tissue is typically found in lipomas of the pressure-bearing regions of the body such as the hands, feet, and buttocks; the lobular architecture accentuated by such fibrous bands may be apparent grossly.
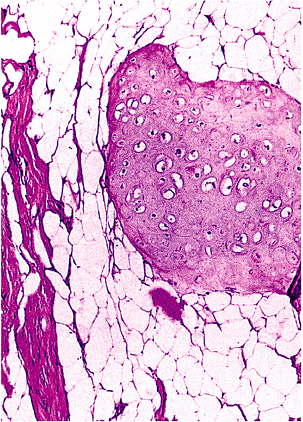 |
Figure 7.18 Chondrolipoma. Small nodules of mature cartilage are present, often very focally; this combination alone should not be labeled a mesenchymoma. |
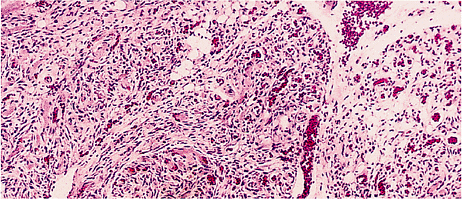 |
Figure 7.19 Angiolipoma. In this unusual example, the rarity of adipocytes (top middle) makes the tumor resemble a deep Kaposi's-like lesion; the location, circumscription, frequent microthrombi (center), and isolated islands of fat cells at the periphery aid in the diagnosis. |
Epithelial Components
In some superficial lipomas, eccrine glands may be incorporated into the lesion. Eccrine glands may be found at the junction of dermal collagen; the subcutaneous fat and lipomas arising in this region can cause displacement of these glands, well within the substance of the lipoma. This phenomenon has been noted in locations such as the hand and buttock (personal observation).
Adipose tissue may accompany adenomas (i.e., lipoadenomas) of the thyroid (200) and parathyroid (201). Aside from lipoadenomas, other lesions of the thyroid gland may contain fat including colloid nodules, lymphocytic thyroiditis, and papillary carcinomas (202,203). Another unusual phenomenon is the formation of the thymolipoma (204). As listed in Table 7.2 [see section entitled Syndromes Associated with Fatty Lesions (Including Lipomatosis)], an unusual lipomatous syndrome is described that consists of thyrolipoma, thymolipoma, and pharyngeal lipoma (205).
Lymphocytes in Lipomas
Occasionally, one may observe a dense perivascular lymphocytic infiltrate in scattered vessels within and outside ordinary lipomas. Although not generally described, the authors have observed this phenomenon several times and investigated the patients; they have not exhibited evidence of chronic lymphocytic leukemia or autoimmune disease. Perhaps this may represent a localized host reaction to the proliferation.
Special Lipoma Types
In the spindle cell (206,207,208) and pleomorphic (209,210) lipomas, the fat cells appear variable in size at low power. In spindle cell lipoma (Figure 7.13), the spindle cell content may vary from scanty to abundant, and the nuclei of the spindle cells are wavy, resembling nerve sheath lesions. Dense fibrous tissue is also found sometimes with a keloidal quality. Similar cells may be seen in pleomorphic lipoma, which has, in addition, characteristic floret tumor giant cells (Figure 7.20). Both of these lesions are encapsulated and have characteristic locations commonly limited to the head and neck of elderly males. They may be related entities (211). Interestingly, immunoreactivity for androgen receptor has recently been demonstrated in the fibroblast-like spindle cells of spindle cell lipoma (Brooks, personal data).
The chondroid lipoma is a well-circumscribed lesion with two elements: mature adipose tissue and focal or prominent areas containing strands and nests of eosinophilic vacuolated cells resembling chondroblasts or lipoblasts. A hyalinized myxoid matrix is also seen. This tumor is S-100 protein, vimentin, and CD68-positive, and may be cytokeratin-positive. It occurs mainly in women in the superficial soft tissues or skeletal muscle of extremities, head, and neck. While worrisome in appearance, the lesion does not recur or metastasize (212,213).
Finally, an unusual fatty tumor of the mediastinum with elastic tissue has been described as elastofibrolipoma (214).
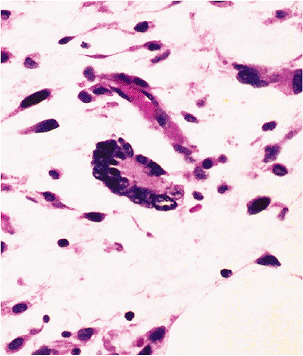 |
Figure 7.20 Floret cell. The wreath of nuclei at the periphery characterizes this cell, which is classically present in pleomorphic lipoma but may occur in some liposarcomas. |
P.186
Lipoblastoma
Frequently a congenital lesion, the lipoblastoma (215,216,217,218,219,220,221) is a benign solitary proliferation of fat, retaining the lobular architecture of developing fetal white adipose tissue. Nearly 90% of these superficial lesions occur before the age of 3 (152). Interestingly, lesions tend to mature with the age of the patient. Tumors may be predominantly myxoid with spindle cells, predominantly lipocytic, or mixed; all types have a prominent capillary bed and are often encapsulated. When mature fat cells are present, they are typically in the central portion of the lobules, in turn surrounded by collagen. In contrast, the presence of maturing adipocytes in myxoid liposarcoma is frequently found at the periphery of the lobule (220). Thus, while these tumors bear a resemblance to myxoid liposarcoma, there are clear differences, and the lobular accentuation with collagen is quite typical (Figure 7.21), as is the age at presentation. Rare cells resembling brown fat or hibernoma cells have been identified in lipoblastoma (219). If these lesions are single, they should be termed lipoblastoma (216) and not lipoblastomatosis (218,221); that was the original designation given appropriately by Vellios et al. in 1958 for a diffuse form (215).
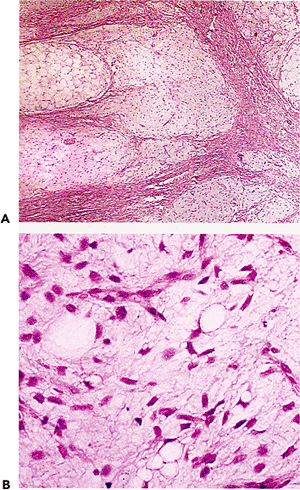 |
Figure 7.21 Lipoblastoma. A. At low power, a distinctly lobulated appearance can be observed. In some lobules, differentiation has started in the center. B. Within the myxoid lobules, small lipoblasts and spindle cells are found. The spindle cells are similar to those in developing fat (see Figure 7.2B). |
Lipoblastomatosis
Lipoblastomatosis is the proper designation for the less common diffuse form of lipoblastoma. About one-third of the patients have diffuse tumors, which (in contrast to the solitary form) are usually deeply situated, more poorly circumscribed, infiltrating muscle, and with a higher tendency to recur (216).
Cytogenetics of Lipomas
Chromosomal karyotypes of lipomas have been studied (222,223,224,225,226,227,228,229) and reveal nonrandom changes involving chromosomes 3 and 12, indicative of clonality. The balanced translocation t(3;12) is a common finding (224,225), with breakpoints described at probably identical locations q27;q13 (223) and q28;q14 (222). The breakpoint on chromosome 12 is very close to the one described in the t(12;16) translocation in myxoid liposarcomas (224). This balanced translocation involving chromosome 12 is seen in roughly 50% of lipomas (225) and may involve other chromosomes such as 21 and 7 (225). Another one-third of lipomas show a ring chromosome (225) originally described by Heim et al. (226) as a possible rearrangement of chromosome 3; this may be a marker for lipogenic tumors. Rarely, chromosome 6 has shown an abnormality (229). Interestingly, subgroups of lipomas may show different cytogenic changes (227).
Likewise, clonal chromosomal changes are noted in lipoblastoma with the abnormality at 8q11-q13 (230).
Syndromes Associated with Fatty Lesions (Including Lipomatosis)
The word lipomatosis may appropriately refer to two separate conditions: the presence of multiple subcutaneous lipomas and the infiltration of organs or sites such as the pelvis (231,232) by adipose tissue. The bilateral multiple symmetrical lipomatosis (MSL) syndrome [Madelung's disease (233,234,235,236,237)] is said to be frequently accompanied by a high intake of alcohol (180). However, there is increasing evidence that there is no association of MSL with alcohol abuse (238), that there may be a constitutional mitochondrial dysfunction (239), that mitochondral DNA may be abnormal (240), that patients have plasma lipid anomalies (241), and that the cells involved may be distorted brown fat cells supportive of a neoplastic nature to MSL (242).
Lipomatosis may involve a single portion of the body, such as the face (243), the spinal epidural area (244,245,246,247), the mesentery (248), the mediastinum and abdomen (249), the mediastinum alone (250), the brain (251,252), and the
P.187
kidney (253), as well as subcutaneous tissue (254). Syndromes relating to many of these are delineated in Table 7.2.
Lipomas, either as single or multiple tumors, may be part of a variety of syndromes (Table 7.2), some of which are autosomal dominant [Gardner's syndrome (255,256), MEA type 1 (257,258), Bannayan's syndrome (259), or tuberous sclerosis (260)]. Pathologists may find it interesting to note that lipomatous lesions may also occur in Cowden's disease (261), Beckwith's hemihypertrophy (180), and as fat within a pulmonary hamartoma in Carney's syndrome (262,263).
Furthermore, adipose tissue lesions may be found in association with other clinical syndromes as well (264,265,269). The listing in Table 7.2 is meant to be as complete as possible for informational purposes. The lipodystrophies (membranous and intestinal or mesenteric) were discussed earlier.
Table 7.2 Syndromes Associated with Fatty Processes | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Mimics of Fat Cells
Mature Fat Cells
Pathologists visualize adipocytes as clear cells or white holes on routine sections. Therefore, other cells or processes with this white hole appearance may be confused with them. Some lesions are fairly obvious like the vacuolated lymphadenopathy of lymphangiogram effect.
P.188
Dilated superficial lymphatics if closely clustered, as they may be in a nasal polyp, remind one of adipocytes at medium power. The submucosal cystic spaces of pneumatosis cystoides intestinalis (270) are composed of gas with a lining of inflammatory cells, histiocytes, and giant cells. Cysts very similar in histology are occasionally noted within ovarian teratomas; here, it is probably a reaction to internal rupture. Likewise, small gaseous cysts without any lining in the intestinal mucosa truly mimic lipocytes in an entity termed pseudolipomatosis (270). Similar clear but artifactual vacuoles in the skin have been called pseudolipomatosis cutis (271). Termed villous edema in placental texts, this artifact of chorionic villi gives them a pseudolipomatous appearance. Lipid-filled sinusoidal Ito cells in the liver simulate small adipocytes in vitamin A toxicity (272).
Lipoblasts
The response to the lipidlike substance silicone after the rupture of a breast implant can cause concern: when the response to the silicone is marked with sheets of histiocytes containing a single dominant vacuole, the cells resemble lipoblasts and the lesion may be mistaken for liposarcoma.
Tumors with vacuoles also cause the pathologist to consider a lipocytic origin. Metastases to the skin or subcutaneous region of signet-ring carcinoma or signet-ring melanoma (273) (Figure 7.22) may resemble lipoblasts, and other helpful features such as nesting or spindling are not always present. Lymphomas of both B- and T-cell origin exhibiting a vacuolated or signet-ring appearance have recently been described (274,275,276,277,278), may mimic liposarcoma (278), and should be in the differential diagnosis of cutaneous, nodal, or retroperitoneal tumors.
Mesenchymal tumors such as epithelioid smooth muscle lesions and fibrohistiocytic neoplasms (Figure 7.23) can be vacuolated as well, due to an artifact and proteoglycan material, respectively. These two tumor groups, particularly in the form of gastrointestinal (GI) stromal malignancies (leiomyosarcomas) and myxoid malignant fibrous histiocytoma, probably account for the largest number of lesions mistaken for liposarcoma. In the GI tumors, the perinuclear vacuole coupled with a cellular epithelioid morphology can closely mimic the round cell or cellular myxoid liposarcoma. In myxoid fibrohistiocytic tumors of various types, vacuolated cells superficially simulate the lipoblast, but closer inspection reveals a delicate basophilic substance in the cytoplasm, apparently due to matrix production by the tumor cells (Figure 7.23). Unusual paragangliomas with vacuoles (279,280) may also be puzzling. Other lesions most often simulating lipocytes are those of endothelial origin because a true and often large vacuole is produced. Such cells may be identified in the histiocytoid hemangioma (281), in other epithelioid angiomas (282,283), in the spindle cell hemangioendothelioma (Figure 7.24) (284), in epithelioid hemangioendothelioma (285), and in some poorly differentiated angiosarcomas (Figure 7.25). In contrast to most large lipoblasts, the large vacuoles in endothelial tumors show a central septation. Chordomas, particularly with a sacral presentation, may be confused with a lipocytic tumor due to the prominent
P.189
vacuolization of the physaliphorous cells. Mesotheliomas may also be vacuoled mimicking liposarcoma (286).
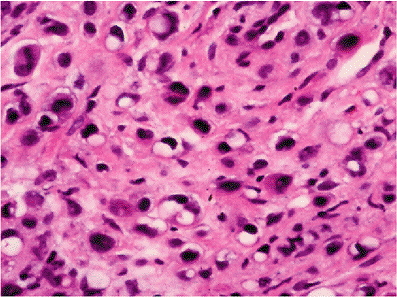 |
Figure 7.22 Adipocyte mimic. Subcutaneous metastases from either signet-ring carcinoma or melanoma (seen here) may rarely imitate a lipocytic tumor. |
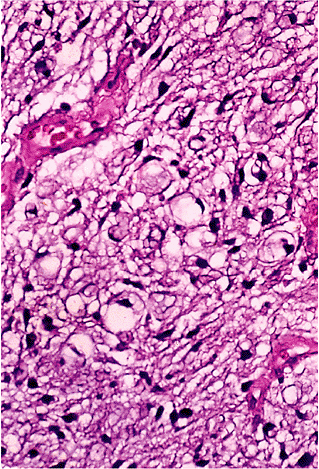 |
Figure 7.23 Lipoblast mimic. In fibrohistiocytic tumors like myxoid dermatofibrosarcoma and myxoid malignant fibrous histiocytoma, cells with a vacuolated appearance may be confused with lipoblasts; however, the vacuole contains a wispy bluish coloration due to the presence of proteoglycan matrix. |
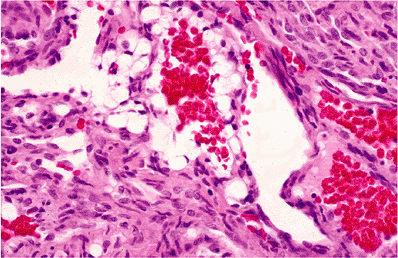 |
Figure 7.24 Adipocyte mimic. Large vacuolated cells can be found in the spindle cell hemangioma, but they are endothelial in nature and often line vascular spaces as seen here. |
The best defense against a misdiagnosis of another tumor as a lipocytic one is strict adherence to the definition of a lipoblast: a cell, occasionally large but usually small, with a vacuole or vacuoles indenting the nucleus. The requirement for nuclear indentation assures an intracellular/cytoplasmic location for the vacuole and also excludes the semicircular nuclei around small vascular channels. Extracellular vacuoles are a common phenomenon, particularly in lesions with areas of mucoid matrix, and are often mistaken for a true intracellular finding; however, the nucleus is never affected since the substance is noncytoplasmic.
True liposarcomatous differentiation may be rarely identified in nonfatty malignancies such as medulloblastoma (287), cystosarcoma phyllodes (288), and even mesothelioma (289).
 |
Figure 7.25 Adipocyte mimic. In some poorly differentiated angiosarcomas, vacuolated endothelial cells also resemble fat cells; however, note the presence of occasional septated vacuoles (center), a feature typical for proliferating endothelial cells and unlike adipocytes. |
References
1. Poissonnet CM, Burdi AR, Bookstein FL. Growth and development of human adipose tissue during early gestation. Early Hum Dev 1983;8:1 11.
2. Poissonnet CM, Burdi AR, Garn JM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev 1984;10:1 11.
3. Robinson DS. In: Florkin M, Stotz EH, eds. Comparative Biochemistry. Vol. 18 Amsterdam: Elsevier; 1970:51 116.
4. Hausman GJ, Champion DR, Martin RJ. Search for the adipocyte precursor cell and factors that promote its differentiation. J Lipid Res 1980;21:657 670.
5. Napolitano L. The differentiation of white adipose cells: an electron microscope study. J Cell Biol 1963;18:663 679.
6. Aman P, Ron D, Mandahl N, et al. Rearrangement of the transcription factor gene CHOP in myxoid liposarcomas with t(12; 16)(q13;p11). Genes Chromosomes Cancer 1992;5:278 285.
7. Crozat A, Aman P, Mandahl F, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature 1993;363:640 644.
8. Rabitts TH, Forster A, Larson R, Nathan P. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nature Genet 1993;4:175 180.
9. Ladanyi M. The emerging molecular genetics of sarcoma translocations. Diagn Mol Pathol 1995;4:162 173.
10. LeBrun DP, Warnke RA, Cleary ML. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol 1993;142:743 753.
11. Martin RJ, Ramsay T, Hausman GJ. Adipocyte development. Pediatr Ann 1984;13:448 453.
12. Poissonnet CM, LaVelle M, Burdi AR. Growth and development of adipose tissue. J Pediatr 1988;113(pt 1):1 9.
13. Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab 1976 5:299 311.
14. Faust IM. Factors which affect adipocyte formation in the rat. In: Bjorntorp P, Cairella M, Howard AN, eds. Recent Advances in Obesity Research III. Proceedings of the 3rd International Congress on Obesity. London: John Libbey; 1981:52 57.
15. Bjorntorp P. Adipocyte precursor cells. In: Bjorntorp P, Cairella M, Howard AN, eds. Recent Advances in Obesity Research III. Proceedings of the 3rd International Congress on Obesity. London: John Libbey;1981:58 69.
16. Sjostrom L, William-Olsson T. Prospective studies on adipose tissue development in man. Int J Obes 1981;5:597 604.
17. Kolata G. Why do people get fat? Science 1985;227:1327 1328.
18. Hirsch J, Fried SK, Edens NK, Leibel RL. The fat cell. Med Clin North Am 1989;73:83 96.
19. Cinti S, Frederich RC, Zingaretti C, De Matteis R, Flier JS, Lowell BB. Immunohistochemical localization of leptin and uncoupling protein in white and brown adipose tissue. Endocrinology 1997;138:797 804.
20. Hukshorn CJ, Saris WH. Leptin and energy expenditure. Curr Opin Clin Nutr Metab Care 2004;7:629 633.
21. Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci 2002;967:379 388.
22. Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab 2001;280:E827 E847.
23. Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 2004;15:2792 2800.
24. Choy LN, Rosen BS, Spiegelman BM. Adipsin and an endogenous pathway of complement from adipose cells. J Biol Chem 1992;267:12736 12741.
25. Birgel M, Gottschling-Zeller H, Rohrig K, Hauner H. Role of cytokines in the regulation of plasminogen activator inhibitor-1 expression and secretion in newly differentiated subcutaneous human adipocytes. Arterioscler Thromb Vasc Biol 2000;20:1682 1687.
P.190
26. Rebuffe-Scrive M, Enk L, Crona N, et al. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J Clin Invest 1985;75:1973 1976.
27. Fried SK, Kral JB. Sex differences in regional distribution of fat cell size and lipoprotein lipase activity in morbidly obese patients. Int J Obes 1987;11:129 140.
28. Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord 1996;20:291 302.
29. Kopelman PG. Effects of obesity on fat topography: metabolic and endocrine determinants. In: Kopelman PG, Stock MJ eds. Clinical Obesity. Oxford, UK: Blackwell Science; 1998:158 175.
30. Bjorntorp P. Fat cell distribution and metabolism. Ann NY Acad Sci 1987;499:66 72.
31. Hutley L, Shurety W, Newell F, et al. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes 2004;53:3097 3106.
32. Hube F, Hauner H. The role of TNF-a in human adipose tissue: prevention of weight gain at the expense of insulin resistance? Horm Metab Res 1999;31:626 631.
33. Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 1992;89:1681 1684.
34. Stephens JM, Pekala PH. Transcriptional repression of GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. J Biol Chem 1991;266:21839 21845.
35. Hellman B, Hellerstrom C. Cell renewal in the white and brown fat of the rat. Acta Pathol Microbiol Scand 1961;51:347 353.
36. Hollenberg CH, Vost A. Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J Clin Invest 1969;47:2485 2498.
37. Napolitano L. The fine structure of adipose tissues. In: Reynold AE, Cahill GF, eds. Handbook of Physiology. Section 5: Adipose Tissue. Washington, DC: American Physical Society; 1965:109 123.
38. Nnodim JO. Development of adipose tissue. Anat Rec 1987;219:331 337.
39. Merklin RJ. Growth and distribution of human fetal brown fat. Anat Rec 1974;178:637 646.
40. Heaton JM. The distribution of brown adipose tissue in the human. J Anat 1972;112(pt 1):35 39.
41. Girardier L. Brown fat: an energy dissipating tissue. In: Girardier L, Stock MJ, eds. Mammalian Thermogenesis. London: Chapman and Hall, 1983:50 98.
42. Rothwell NJ, Stock MJ. Brown adipose tissue. In: Baker PF, ed. Recent Advances in Physiology. Volume 10. Edinburgh: Churchill Livingstone; 1984:349 384.
43. Rothwell NJ, Stock MJ. Whither brown fat? Biosci Rep 1986;6:3 18.
44. Bouillaud F, Combes-George M, Ricquier D. Mitochondria of adult human brown adipose tissue contain a 32 000-Mr uncoupling protein. Biosci Rep 1983;3:775 780.
45. Cunningham S, Leslie P, Hopwood D, et al. The characterization and energetic potential of brown adipose tissue in man. Clin Sci (Lond) 1985;69:343 348.
46. Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 1979;281:31 35.
47. Himms-Hagen J. Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J 1990;4:2890 2898.
48. Blaza S. Brown adipose tissue in man: a review. J R Soc Med 1983;76:213 216.
49. Santos GC, Araujo MR, Silveira TC, Soares FA. Accumulation of brown adipose tissue and nutritional status: a prospective study of 366 consecutive autopsies. Arch Pathol Lab Med 1992;116:1152 1154.
50. Cottle WH. The innervation of brown adipose tissue. In: Lindberg O, ed. Brown Adipose Tissue. New York: Elsevier; 1970:155 178.
51. Mory G, Bouillaud F, Combes-George M, Ricquier D. Noradrenaline controls the concentration of the uncoupling protein in brown adipose tissue. FEBS Lett 1984;166:393 396.
52. Ricquier D, Nechad M, Mory G. Ultrastructural and biochemical characterization of human brown adipose tissue in pheochromocytoma. J Clin Endocrinol Metab 1982;54:803 807.
53. Afzelius BA. Brown adipose tissue: its gross anatomy, histology, and cytology. In: Lindberg O, ed. Brown Adipose Tissue. New York: Elsevier; 1970:1 31.
54. Pearse AG. Histochemistry: Theoretical and Applied. Vol 2. 3rd ed. Baltimore: Williams & Wilkins; 1972.
55. Hausman GJ. Anatomical and enzyme histochemical differentiation of adipose tissue. Int J Obes 1985;9(suppl 1):1 6.
56. Lithell J, Boberg J, Hellsing K, Lundqvist G, Vessby B. Lipoprotein-lipase activity in human skeletal muscle and adipose tissue in the fasting and the fed states. Atherosclerosis 1978;30:89 94.
57. Lithell H, Hellsing K, Lundqvist G, Malmberg P. Lipoprotein-lipase activity of human skeletal-muscle and adipose tissue after intensive physical exercise. Acta Physiol Scand 1979;105:312 315.
58. Fielding CJ, Havel RJ. Lipoprotein lipase. Arch Pathol Lab Med 1977;101:225 229.
59. Zugibe FT. Diagnostic Histochemistry. St. Louis: CV Mosby; 1970.
60. Pearse AG. Histochemistry: Theoretical and Applied. Vol. 2. 3rd ed. Baltimore: Williams & Wilkins; 1972.
61. Pearse AG. Histochemistry: Theoretical and Applied. Vol. 2. 3rd ed. Baltimore: Williams & Wilkins; 1972.
62. Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. St. Louis: CV Mosby; 1973.
63. Filipe MI, Lake BD, eds. Histochemistry in Pathology. Edinburgh: Churchill Livingstone; 1983.
64. Spicer SS, ed. Histochemistry in Pathologic Diagnosis. New York: Marcel Dekker; 1987.
65. Hausman GJ. Techniques for studying adipocytes. Stain Technol 1981;56:149 154.
66. Popper H, Knipping G. A histochemical and biochemical study of a liposarcoma with several aspects on the development of fat synthesis. Pathol Res Pract 1981;171:373 380.
67. Waugh DA, Small DM. Methods in laboratory investigation: identification and detection of in situ cellular and regional differences of lipid composition and class in lipid-rich tissue using hot stage polarizing light microscopy. Lab Invest 1984;51:702 714.
68. Stedman TL. Stedman's Medical Dictionary. 21st ed. Baltimore: Williams & Wilkins; 1966.
69. Robbins SL, Cotran RS, Kumar V. The Pathologic Basis of Disease. 3rd ed. Philadelphia: WB Saunders; 1984.
70. Rubin E, Farber JL. eds. Pathology. Philadelphia: Lippincott; 1988.
71. Heptinstall RH. Pathology of the Kidney. 2nd ed. Boston: Little, Brown & Co; 1974.
72. Jasnosz KM, Pickeral JJ, Graner S. Fat deposits in the placenta following maternal total parenteral nutrition with intravenous lipid emulsion. Arch Pathol Lab Med 1995;119:555 557.
73. Bennington JL. Proceedings: Cancer of the kidney: etiology, epidemiology, and pathology. Cancer 1973;32:1017 1029.
74. Elizalde N, Korman S. Cytochemical studies of glycogen, neutral mucopolysaccharides and fat in malignant tissues. Cancer 1968;21:1061 1068.
75. Andrion A, Mazzucco G, Gugliotta P, Monga G. Benign clear cell (sugar) tumor of the lung: a light microscopic, histochemical, and ultrastructural study with a review of the literature. Cancer 1985;56:2657 2663.
76. Bertoni F, Unni KK, McLeod RA, Sim FH. Xanthoma of bone. Am J Clin Pathol 1988;90:377 384.
77. Bennett JH, Shousha S, Puddle B, Athanasou NA. Immunohistochemical identification of tumours of adipocytic differentiation using an antibody to aP2 protein. J Clin Pathol 1995;48:950 954.
78. Michetti F, Dell'Anna E, Tiberio G, Cocchia D. Immunochemical and immunocytochemical study of S-100 protein in rat adipocytes. Brain Res 1983;262:352 356.
79. Haimoto H, Kato K, Suzuki F, Nagura H. The ultrastructural changes of S-100 protein localization during lipolysis in adipocytes. An immunoelectron-microscopic study. Am J Pathol 1985;121:185 191.
80. Takahashi K, Isobe T, Ohtsuki Y, Akagi T, Sonobe H, Okuyama T. Immunochemical study of the distribution of alpha and beta subunits of S-100 protein in human neoplasms and normal tissues. Virchows Arch Cell Pathol 1984;45:385 396.
81. Nakazato Y, Ishida Y, Takahashi K, Suzuki K. Immunohistochemical distribution of S-100 protein and glial fibrillary acidic protein in normal and neoplastic salivary glands. Virchows Arch A Pathol Anat Histopathol 1985;405:299 310.
82. Kahn HJ, Marks A, Thom H, Baumal R. Role of antibody to S100 protein in diagnostic pathology. Am J Clin Pathol 1983;79:341 347.
P.191
83. Nakajima T, Watanabe S, Sato Y, Kameya T, Hirota T, Shimosato Y. An immunoperoxidase study of S-100 protein distribution in normal and neoplastic tissues. Am J Surg Pathol 1982;6:715 727.
84. Cocchia D, Lauriola L, Stolfi V, Tallini G, Michetti F. S-100 antigen labels neoplastic cells in liposarcoma and cartilaginous tumours. Virchows Arch A Pathol Anat Histopathol 1983;402:139 145.
85. Weiss SW, Langloss JM, Enzinger FM. Value of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest 1983;49:299 308.
86. Hashimoto H, Daimaru Y, Enjoji M. S-100 protein distribution in liposarcoma. An immunoperoxidase study with special reference to the distinction of liposarcoma from myxoid malignant fibrous histiocytoma. Virchows Arch A Pathol Anat Histopathol 1984;405:1 10.
87. Haimoto H, Takahashi Y, Koshikawa T, Nagura H, Kato K. Immunohistochemical localization of gamma-enolase in normal human tissues other than nervous and neuroendocrine tissues. Lab Invest 1985;52:257 263.
88. Stunkard AJ, Wadden TA, eds. Obesity: Theory and Therapy. 2nd ed. New York: Raven Press; 1993.
89. Lowell BB, Susulic VS, Hamann A, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993;366:740 742.
90. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425 432.
91. Green ED, Maffei M, Braden VV, et al. The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7. Genome Res 1995;5:5 12.
92. Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995;269:540 543.
93. Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995;269:543 546.
94. Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 1995;269:546 549.
95. Chua SC Jr, Chung WK, Wu-Peng XS, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 1996;271:994 996.
96. Raife T, Landas SK. Intracellular crystalline material in visceral adipose tissue: a common autopsy finding [abstract]. Am J Clin Pathol 1990;94:511.
97. Tedeschi CG. Pathologic anatomy of adipose tissue. In: Renold AE, Cahill GF, eds. Handbook of Physiology. Section 5: Adipose Tissue. Baltimore: Waverly Press; 1965.
98. Manthorpe R, Helin G, Kofod B, Lorenzen I. Effect of glucocorticoid on connective tissue of aorta and skin in rabbits. Biochemical studies on collagen, glycosaminoglycans, DNA and RNA. Acta Endocrinol (Copenh) 1974;77:310 324.
99. Napolitano LM. Observations on the fine structure of adipose cells. Ann NY Acad Sci 1965;131:34 42.
100. Seaman JP, Kjeldsberg CR, Linker A. Gelatinous transformation of the bone marrow. Hum Pathol 1978;9:685 692.
101. Wittels B. Bone marrow biopsy changes following chemotherapy for acute leukemia. Am J Surg Pathol 1980;4:135 142.
102. Pierard GE, Nizet JL, Pierard-Franchimont C. Cellulite: from standing fat herniation to hypodermal stretch marks. Am J Dermatopath 2000;22:34 37.
103. Gruskin P, Canberry JN. Pathology of acoustic neuromas. In: House WF, Leutje CM, eds. Acoustic Tumors. Baltimore: University Park Press; 1979:85 148.
104. Wood C. Membranous lipodystrophy of bone. Arch Pathol Lab Med 1978;102:22 27.
105. Bird TD, Koerker RM, Leaird BJ, Vlcek BW, Thorning DR. Lipomembranous polycystic osteodysplasia (brain, bone and fat disease): a genetic cause of presenile dementia. Neurology 1983;33:81 86.
106. Kitajima I, Suganuma T, Murata F, Nagamatsu K. Ultrastructural demonstration of Maclura pomifera agglutinin binding sites in the membranocystic lesions of membranous lipodystrophy (Nasu Hakola disease). Virchows Arch A Pathol Anat Histopathol 1988;413:475 483.
107. Chun SI, Chung KY. Membranous lipodystrophy: secondary type. J Am Acad Dermatol 1994;31:601 605.
108. Coyne JD, Parkinson D, Baildam AD. Membranous fat necrosis of the breast. Histopathology 1996;28:61 64.
109. Adams RD. Diseases of Muscle: A Study in Pathology. 3rd ed. New York: Harper & Row; 1975.
110. Rosai J, Levine GD. Tumors of the thymus. In: of Atlas of Tumor Pathology. 2nd series, fascicle 13. Washington, DC: Armed Forces Institute of Pathology; 1976.
111. Rywlin AM. Histopathology of the Bone Marrow. Boston: Little, Brown & Co; 1976:19.
112. Ackerman LV, Rosai J. Surgical Pathology. 5th ed. St Louis: CVMosby; 1974:649.
113. Seifert G. Lipomatous atrophy and other forms. In: Kloppel G, Heitz PU, eds. Pancreatic Pathology. New York: Churchill Livingstone; 1984.
114. Heggtveit HA, Fenoglio JJ, McAllister HA. Lipomatous hypertrophy of the interatrial septum: An assessment of 41 cases. Lab Invest 1976;34:318.
115. McAllister HA, Fenoglio JJ. Tumors of the Cardiovascular System. Washington, DC: Armed Forces Institute of Pathology; 1978:44 46.
116. Rokey R, Mulvagh SL, Cheirif J, Mattox KL, Johnston DL. Lipomatous encasement and compression of the heart: antemortem diagnosis by cardiac nuclear magnetic resonance imaging and catheterization. Am Heart J 1989;117 952 953.
117. Waller BF, ed. Pathology of the Heart and Great Vessels. New York: Churchill Livingstone; 1988.
118. Symmers WSC. The lymphoreticular system. In: Symmers WSC, ed. Systemic Pathology. Vol. 2. Edinburgh: Churchill Livingstone, 1978:647 651.
119. Smith T. Fatty replacement of lymph nodes mimicking lymphoma relapse. Cancer 1986; 58:2686 2688.
120. Takayasu K, Shima Y, Muramatsu Y, et al. Imaging characteristics of large lipoma and angiomyolipoma of the liver. Case reports. Cancer 1987;59:916 921.
121. Pounder DJ. Hepatic pseudolipoma. Pathology 1983;15:83 84.
122. Shafer WG, Hine MK, Levy BM. Development disterbances of oral and paraoral structures. A Textbook of Oral Pathology. 4th ed. Philadelphia: WB Saunders; 1983:24 25.
123. Ramadori G. The stellate cell (Ito-cell, fat-storing cell, lipocyte, perisinusoidal cell) of the liver. New insights into pathophysiology of an intriguing cell. Virchows Arch B Cell Pathol Incl Mol Pathol 1991;61:147 158.
124. Cha I, Bass N, Ferrell LD. Lipopeliosis: an immunohistochemical and clinicopathologic study of five cases. Am J Surg Pathol 1994;18:789 795.
125. Stroebel P, Mayer F, Zerban H, Bannasch P. Spongiotic pericytoma: a benign neoplasm deriving from the perisinusoidal (Ito) cells in rat liver. Am J Pathol 1995;146:903 913.
126. Orfila C, Giraud P, Modesto A, Suc JM. Abdominal fat tissue aspirate in human amyloidosis: light, electron, and immunofluorescence microscopic studies. Hum Pathol 1986;17:366 369.
127. Duston MA, Skinner M, Shirahama T, Cohen AS. Diagnosis of amyloidosis by abdominal fat aspiration: analysis of four years' experience. Am J Med 1987;82:412 414.
128. Gertz MA, Li CY, Shirahama T, Kyle RA. Utility of subcutaneous fat aspiration for the diagnosis of systemic amyloidosis (immunoglobulin light chain). Arch Intern Med 1988;48:929 933.
129. Keen CE, Buk SJ, Brady K, Levison DA. Fat necrosis presenting as obscure abdominal mass: birefringent saponified fatty acid crystalloids as a clue to diagnosis. J Clin Pathol 1994;47:1028 1031.
130. Poppiti RJ Jr, Margulies M, Cabello B, Rywlin AM. Membranous fat necrosis. Am J Surg Pathol 1986;10:62 69.
131. Bennett RG, Petrozzi JW. Nodular subcutaneous fat necrosis: a manifestation of silent pancreatitis. Arch Dermatol 1975;111:896 898.
132. Hughes PS, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol 1975;111:506 510.
133. Fischer AH, Morris DJ. Pathogenesis of calciphylaxis: study of three cases and literature review. Hum Pathol 1995;26:1055 1064.
P.192
134. Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial 2002;15:172 86.
135. Mirza I, Chaubay D, Gunderia H, Shih W, El-Fanek H. An unusual presentation of calciphylaxis due to primary hyperparathyroidism. Arch Pathol Lab Med 2001;125:1351 1353.
136. Macdonald A, Feiwel M. A review of the concept of Weber Christian panniculitis with a report of five cases. Br J Dermatol 1968;80:355 361.
137. Sweatt HL, Hardman WJ, Solomon AR. Non-neoplastic diseases of the skin. In: Mills SE, ed. Sternberg's Diagnostic Surgical Pathology. 4th ed. New York: Lippincott Williams Wilkins; 2004:40 43.
138. Winkelmann RK, Frigas E. Eosinophilic panniculitis: a clinicopathologic study. J Cutan Pathol 1986;13:1 12.
139. Blaustein A, Moreno A, Noguera J, de Moragas JM. Septal granulomatous panniculitis in Sweet's syndrome: report of two cases. Arch Dermatol 1985;121:785 788.
140. Suster S, Cartagena N, Cabello-Inchausti B, Robinson MJ. Histiocytic lymphophagocytic panniculitis: an unusual extranodal presentation of sinus histiocytosis with massive lymphadenopathy (Rosai Dorfman disease). Arch Dermatol 1988;124:1246 1249.
141. Alegre VA, Winkelmann RK, Aliaga A. Lipomembranous changes in chronic panniculitis. J Am Acad Dermatol 1988;19(pt 1):39 46.
142. Vincent F, Prokopetz R, Miller RA. Plasma cell panniculitis: a unique clinical and pathologic presentation of linear scleroderma. J Am Acad Dermatol 1989;21(pt 2):357 360.
143. Izumi AK, Takiguchi P. Lupus erythematosus panniculitis. Arch Dermatol 1983;119:61 64.
144. Silverman RA, Newman AJ, LeVine MJ, Kaplan B. Poststeroid panniculitis: a case report. Pediatr Dermatol 1988;5:92 93.
145. Sleisenger MH, Fordtran JS, eds. Gastrointestinal Disease: Pathophysiology, Diagnosis, Management. 3rd ed. Philadelphia: WB Saunders; 1983.
146. Kelly JK, Hwang WS. Idiopathic retractile (sclerosing) mesenteritis and its differential diagnosis. Am J Surg Pathol 1989;13:513 521.
147. Scully RE, Galdabini JJ, McNeely BU. Lipodystrophy of mesentery (Case 30-1976). N Engl J Med 1976;295:214 218.
148. Eble JN, Rosenberg AE, Young RH. Retroperitoneal xanthogranulomatosis in a patient with Erdheim Chester disease. Am J Surg Pathol 1994;18:843 848.
149. Seemayer TA, Knaack J, Wang NS, Ahmed MN. On the ultrastructure of hibernoma. Cancer 1975;36:1785 1793.
150. Dardick I. Hibernoma: a possible model of brown fat histogenesis. Hum Pathol 1978;9:321 329.
151. Gaffney EF, Hargreaves HK, Semple E, Vellios F. Hibernoma: distinctive light and electron microscopic features and relationship to brown adipose tissue. Hum Pathol 1983;14:677 687.
152. Enzinger FM, Weiss SW. Soft Tissue Tumors. 2nd ed. St. Louis: CV Mosby; 1988.
153. Rigor VU, Goldstone SE, Jones J, Bernstein R, Gold MS, Weiner S. Hibernoma. A case report and discussion of a rare tumor. Cancer 1986;57:2207 2211.
154. Allegra SR, Gmuer C, O'Leary GP Jr. Endocrine activity in a large hibernoma. Hum Pathol 1983;14:1044 1052.
155. Enterline HT, Lowry LD, Richman AV. Does malignant hibernoma exist? Am J Surg Pathol 1979;3:265 271.
156. Teplitz C, Farrugia R, Glicksman AS. Malignant hibernoma does exist. Lab Invest 1980;42:59A.
157. Talerman A. Germ cell tumors of the ovary. In: Kurman R, ed. Blaustein's Pathology of the Female Genital Tract. 3rd ed. New York: Springer-Verlag; 1987:689.
158. Dail DH. Uncommon tumors. In: Dale DH, Hammar SP, eds. Pulmonary Pathology. New York: Springer-Verlag; 1988:847 972.
159. Ali MY, Wong PK. Intrapulmonary teratoma. Thorax 1964;19:228 235.
160. Lee RG. Esophagus. In: Sternberg SS, ed. Diagnostic Surgical Pathology. New York: Raven Press; 1989:928.
161. Toker C, Tang CK, Whitely JF, Berkheiser SW, Rachman R. Benign spindle cell breast tumor. Cancer 1981;48:1615 1622.
162. Wargotz ES, Weiss SW, Norris HJ. Myofibroblastoma of the breast: sixteen cases of a distinctive benign mesenchymal tumor. Am J Surg Pathol 1987;11:493 502.
163. Nogales FF, Pavcovich M, Medina MT, Palomino M. Fatty change in the endometrium. Histopathology 1992;20:362 363.
164. Nogita T, Wong TY, Hidano A, Mihm MC Jr, Kawashima M. Pedunculated lipofibroma: a clinicopathologic study of thirty-two cases supporting a simplified nomenclature. J Am Acad Dermatol 1994;31(pt 1):235 240.
165. Tomashefski JF Jr. Benign endobronchial mesenchymal tumors: their relationship to parenchymal pulmonary hamartomas. Am J Surg Pathol 1982;6:531 540.
166. Palvio D, Egeblad K, Paulsen SM. Atypical lipomatous hamartoma of the lung. Virchows Arch A Pathol Anat Histopathol 1985;405:253 261.
167. Benjamin SP, Mercer RD, Hawk WA. Myofibroblastic contraction in spontaneous regression of multiple congenital mesenchymal hamartomas. Cancer 1977;40:2343 2352.
168. Enzinger FM. Fibrous hamartoma of infancy. Cancer 1965;18:241 248.
169. Reye RD. A consideration of certain subdermal fibromatous tumours of infancy. J Pathol Bacteriol 1956;72:149 154.
170. Fletcher CD, Powell G, van Noorden S, McKee PH. Fibrous hamartoma of infancy: a histochemical and immunohistochemical study. Histopathology 1988;12:65 74.
171. Silverman TA, Enzinger FM. Fibrolipomatous hamartoma of nerve: a clinocopatholigic analysis of 26 cases. Am J Surg Pathol 1985;9:7 14.
172. Silverman TA, Enzinger FM. Fibrolipomatous hamartoma of nerve: a clinicopathologic analysis of 26 cases. Am J Surg Pathol 1985;9:7 14.
173. Aymard B, Bowman-Ferrand F, Vernhes L, Floquet A, Floquet J, Morel O, Merle M, Delagoutte JP. Hamartome lipofibromateux des nerfs p riph riques. Etude anatomo-clinique de 5 cas dont 2 avec tude ultrastructurale. Ann Pathol 1987;7:320 324.
174. Price EB Jr, Mostofi FK. Symptomatic angiomyolipoma of the kidney. Cancer 1965;18:761 774.
175. McCullough DL, Scott R, Seybold HM. Renal angiomyolipoma (hamartoma): review of the literature and report on 7 cases. J Urol 1971;105:32 44.
176. Dao AH, Netsky NG. Human tails and pseudotails. Hum Pathol 1984;15:449 453.
177. Farshid G, Weiss SW. Massive localized lymphedema in the morbidly obese: a histologically distinct reactive lesion simulating liposarcoma. Am J Surg Pathol 1998;22:1277 1283.
178. Wu D, Gibbs J, Corral D, Intengan M, Brooks JJ. Massive localized lymphedema: additional locations and association with hypothyroidism. Hum Pathol 2000:31:1162 1168.
179. LeBer MS, Stout AP. Benign mesenchymomas in children. Cancer 1962;15:595 605.
180. Allen P. Tumors and Proliferations of Adipose Tissue. New York; Masson, 1981.
181. Kasantikul V, Brown WJ, Netsky MG. Mesenchymal differentiation in trigeminal neurilemmoma. Cancer 1982;50:1568 1571.
182. Majeski JA, Paxton ES, Wirman JA, Schrieber JT. A thoracic benign mesenchymoma in association with hemihypertrophy. Am J Clin Pathol 1981;76:827 832.
183. Rosai J. Case presentation at the European Society of Pathology meeting in Porto, Portugal; September 1989.
184. Brecher ME, Gill WB, Straus FH. Angiomyolipoma with regional lymph node involvement and long-term follow-up study. Hum Pathol 1986;17:962 963.
185. Meggitt BF, Wilson JN. The battered buttock syndrome fat fractures: a report on a group of traumatic lipomata. Br J Surg 1972;59:165 169.
186. Wilson JE. Lipomas and potassium intake. Ann Intern Med 1989;110:750 751.
187. Solvonuk PF, Taylor GP, Hancock R, Wood WS, Frohlich J. Correlation of morphologic and biochemical observations in human lipomas. Lab Invest 1984;51:469 474.
188. Azumi N, Curtis J, Kempson R, Hendrickson M. Atypical and malignant neoplasms showing lipomatous differentiation: a study of 111 cases. Am J Surg Pathol 1987;11:161 183.
189. Fu YS, Parker FG, Kaye GI, Lattes R. Ultrastructure of benign and malignant adipose tissue tumors. Pathol Annu 1980;15(pt 1):67 69.
P.193
190. Kindblom L, Angervall L, Stener B, Wickbom I. Intermuscular and intramuscular lipomas and hibernomas. A clinical, roentgenologic, histologic and prognostic study of 46 cases. Cancer 1974;33:754 762.
191. Evans HL, Soule EH, Winkelmann RK. Atypical lipoma, atypical intramuscular lipoma, and well differentiated retroperitoneal liposarcoma: a reappraisal of 30 cases. Cancer 1979;43:574 584.
192. Marsh WL Jr, Lucas JG, Olsen J. Chondrolipoma of the breast. Arch Pathol Lab Med 1989;113:369 371.
193. Lim YC. Mediastinal chondrolipoma. Am J Surg Pathol 1980;4:407 409.
194. Allen P. Letter to the case. Pathol Res Pract 1989;184:444 445.
195. Hayden JW, Abellera RM. Tenosynovial lipochondromatosis of the flexor hallucis, common toe flexor, and posterior tibial tendons. Clin Orthop Relat Res 1989;245:220 222.
196. Katzer B. Histopathology of rare chondroosteoblastic metaplasia in benign lipomas. Pathol Res Pract 1989;184:437 445.
197. Honore LH. Uterine fibrolipoleiomyoma: report of a case with discussion of histogenesis. Am J Obstet Gynecol 1978;132:635 636.
198. Brescia RJ, Tazelaar HD, Hobbs J, Miller AW. Intravascular lipoleiomyomatosis: a report of two cases. Hum Pathol 1989;20:252 256.
199. Hunt SJ, Santa Cruz DJ, Barr RJ. Cellular angiolipoma. Am J Surg Pathol 1990;14:75 81.
200. DeRienzo D, Truong L. Thyroid neoplasms containing mature fat: a report of two cases and review of the literature. Mod Pathol 1989;2:506 510.
201. Perosio P, Brooks JJ, LiVolsi VA. Orbital brown tumor as the initial manifestation of a parathyroid lipoadenoma. Surg Pathol 1988;1:77 82.
202. Gnepp DR, Ogorzalek JM, Heffess CA. Fat-containing lesions of the thyroid gland. Am J Surg Pathol 1989;13:605 612.
203. Bruno J, Ciancia EM, Pingitore R. Thyroid papillary adenocarcinoma; lipomatous-type. Virchows Arch A Pathol Anat Histopathol 1989;414:371 373.
204. Otto HF, Loning T, Lachenmayer L, Janzen RW, Gurtler KF, Fischer K. Thymolipoma in association with myasthenia gravis. Cancer 1982;50:1623 1628.
205. Trites AE. Thyrolipoma, thymolipoma and pharyngeal lipoma: a syndrome. Can Med Assoc J 1966;95:1254 1259.
206. Enzinger FM, Harvey DA. Spindle cell lipoma. Cancer 1975;36:1852 1859.
207. Angervall L, Dahl I, Kindblom LG, Save-Soderbergh J. Spindle cell lipoma. Acta Pathol Microbiol Scand A 1976;84:477 487.
208. Fletcher CD, Martin-Bates E. Spindle cell lipoma: a clinicopathological study with some original observations. Histopathology 1987;11:803 817.
209. Shmookler BM, Enzinger FM. Pleomorphic lipoma: a benign tumor simulating liposarcoma: a clinicopathologic analysis of 48 cases. Cancer 1981;47:126 133.
210. Azzopardi J, Iocco J, Salm R. Pleomorphic lipoma: a tumour simulating liposarcoma. Histopathology 1983;7:511 523.
211. Beham A, Schmid C, H dl S, Fletcher CD. Spindle cell and pleomorphic lipoma: an immunohistochemical study and histogenetic analysis. J Pathol 1989;158:219 222.
212. Meis JM, Enzinger FM. Chondroid lipoma. A unique tumor simulating liposarcoma and myxoid chondrosarcoma. Am J Surg Pathol 1993;17:1103 1112.
213. Kindblom LG, Meis-Kindblom JM. Chondroid lipoma: an ultrastructural and immunohistochemical analysis with further observations regarding its differentiation. Hum Pathol 1995;26:706 715.
214. De Nictolis M, Goteri G, Campanati G, Prat J. Elastofibrolipoma of the mediastinum: a previously undescribed benign tumor containing abnormal elastic fibers. Am J Surg Pathol 1995;19:364 367.
215. Vellios F, Baez J, Schumacker HB. Lipoblastomatosis: a tumor of fetal fat different from hibernoma; report of a case, with observations on the embryogenesis of human adipose tissue. Am J Pathol 1958;34:1149 1159.
216. Chung EB, Enzinger FM. Benign lipoblastomatosis: an analysis of 35 cases. Cancer 1973;32:482 492.
217. Bolen JW, Thorning D. Benign lipoblastoma and myxoid liposarcoma: a comparative light- and electron-microscopic study. Am J Surg Pathol 1980;4:163 174.
218. Alba Greco M, Garcia RL, Vuletin JC. Benign lipoblastomatosis: ultrastructure and histogenesis. Cancer 1980;45:511 515.
219. Chaudhuri B, Ronan SG, Ghosh L. Benign lipoblastoma: report of a case. Cancer 1980;46:611 614.
220. Hanada M, Tokuda R, Ohnishi Y, Takami M, Takahashi T, Kimura M. Benign lipoblastoma and liposarcoma in children. Acta Pathol Jpn 1986;36:605 612.
221. Dudgeon DL, Haller JA Jr. Pediatric lipoblastomatosis: two unusual cases. Surgery 1984;95:371 373.
222. Turc-Carel C, Dal Cin P, Rao U, Karakousis C, Sandberg AA. Cytogenetic studies of adipose tissue tumors: I. A benign lipoma with reciprocal translocation t(3;12)(q28;q14). Cancer Genet Cytogenet 1986;23:283 289.
223. Heim S, Mandahl N, Kristoffersson U, et al. Reciprocal translocation t(3;12)(q27;q13) in lipoma. Cancer Genet Cytogenet 1986;23:301 304.
224. Sandberg AA, Turc-Carel C. The cytogenetics of solid tumors. Relation to diagnosis, classification and pathology. Cancer 1987;59:387 395.
225. Heim S, Mitelman F. Cancer Cytogenetics. New York: Alan R. Liss; 1987:240 241.
226. Heim S, Mandahl N, Kristoffersson U, et al. Marker ring chromosome a new cytogenetic abnormality characterizing lipogenic tumors? Cancer Genet Cytogenet 1987;24:319 326.
227. Heim S, Mandahl N, Rydholm A, Willen H, Mitelman F. Different karyotypic features characterize different clinicopathologic subgroups of benign lipogenic tumors. Int J Cancer 1988;42:863 867.
228. Turc-Carel C, Dal Cin P, Boghosian L, Leong SP, Sandberg AA. Breakpoints in benign lipoma may be at 12q13 or 12q14. Cancer Genet Cytogenet 1988;36:131 135.
229. Sait SN, Dal Cin P, Sandberg AA, et al. Involvement of 6p in benign lipomas. A new cytogenetic entity? Cancer Genet Cytogenet 1989;37:281 283.
230. Dal Cin P, Sciot R, De Wever I, Van Damme B, Van den Berghe H. New discriminative chromosomal marker in adipose tissue tumors. The chromosome 8q11 q13 region in lipoblastoma. Cancer Genet Cytogenet 1994;78:232 235.
231. Bechtold R, Shaff MI. Pelvic lipomatosis with ureteral encasement and recurrent thrombophlebitis. South Med J 1983;76:1030 1032.
232. Henriksson L, Liljeholm H, Lonnerholm T. Pelvic lipomatosis causing constriction of the lower urinary tract and the rectum. Case report. Scand J Urol Nephrol 1984;18:249 252.
233. Shugar MA, Gavron JP. Benign symmetrical lipomatosis (Madelung's disease). Otolaryngol Head Neck Surg 1985;93:109 112.
234. Keller SM, Waxman JS, Kim US. Benign symmetrical lipomatosis. South Med J 1986;79:1428 1429.
235. Cinti S, Enzi G, Cigolini M, Bosello O. Ultrastructural features of cultured mature adipocyte precursors from adipose tissue in multiple symmetric lipomatosis. Ultrastruct Pathol 1983;5:145 152.
236. Enzi G. Multiple symmetric lipomatosis: an updated clinical report. Medicine (Baltimore) 1984;63:56 64.
237. Pollock M, Nicholson GI, Nukada H, Cameron S, Frankish P. Neuropathy in multiple symmetric lipomatosis. Madelung's disease. Brain 1988;111(pt 5):1157 1171.
238. Boozan JA, Maves MD, Schuller DE. Surgical management of massive benign symmetric lipomatosis. Laryngoscope 1992;102:94 99.
239. Berkovic SF, Andermann F, Shoubridge EA, et al. Mitochondrial dysfunction in multiple symmetrical lipomatosis. Ann Neurol 1991;29:566 569.
240. Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology 1994;44:862 866.
241. Deiana L, Pes GM, Carru C, Campus GV, Tidore MG, Cherchi GM. Extremely high HDL levels in a patient with multiple symmetric lipomatosis. Clin Chim Acta 1993;223:143 147.
P.194
242. Zancanaro C, Sbarbati A, Morroni M, et al. Multiple symmetric lipomatosis. Ultrastructural investigation of the tissue and preadipocytes in primary culture. Lab Invest 1990;63:253 258.
243. DeRosa G, Cozzolino A, Guarino M, Giardino C. Congenital infiltrating lipomatosis of the face: report of cases and review of the literature. J Oral Maxillofac Surg 1987;45:879 883.
244. Quint DJ, Boulos RS, Sanders WP, Mehta BA, Patel SC, Tiel RL. Epidural lipomatosis. Radiology 1988;169:485 490.
245. Vazquez L, Ellis A, Saint-Genez D, Patino J, Nogues M. Epidural lipomatosis after renal transplantation complete recovery without surgery. Transplantation 1988;46:773 774.
246. Doppman JL. Epidural lipomatosis. Radiology 1989;171:581 582.
247. Kaplan JG, Barasch E, Hirschfeld A, Ross L, Einberg K, Gordon M. Spinal epidural lipomatosis: a serious complication of iatrogenic Cushing's syndrome. Neurology 1989;39:1031 1034.
248. Siskind BN, Weiner FR, Frank M, Weiner SN, Bernstein RG, Luftschein S. Steroid-induced mesenteric lipomatosis. Comput Radiol 1984;8:175 177.
249. Enzi G, Digito M, Marin R, Carraro R, Baritussio A, Manzato E. Mediastino-abdominal lipomatosis: deep accumulation of fat mimicking a respiratory disease and ascites. Clinical aspects and metabolic studies in vitro. Q J Med 1984;53:453 463.
250. Shukla LW, Katz JA, Wagner ML. Mediastinal lipomatosis: a complication of high dose steroid therapy in children. Pediatr Radiol 1988;19:57 58.
251. Al-Mefty O, Fox JL, Sakati N, Bashir R, Probst F. The multiple manifestations of the encephalocraniocutaneous lipomatosis syndrome. Childs Nerv Syst 1987;3:132 134.
252. Brumback RA, Leech RW. Fishman's syndrome (encephalocraniocutaneous lipomatosis): a field defect of ectomesoderm. J Child Neurol 1987;2:168 169.
253. Arora PK. Re: Non-operative diagnosis of renal sinus lipomatosis simulating tumour of the renal pelvis [letter]. Br J Urol 1989;63:445.
254. Rubinstein A, Goor Y, Gazit E, Cabili S. Non-symmetric subcutaneous lipomatosis associated with familial combined hyperlipidaemia. Br J Dermatol 1989;120:689 694.
255. Scully RE, Galdabini JJ, McNeely BU. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercise. Case 53-1976 (Gardner's syndrome). N Engl J Med 1976;295:1526 1532.
256. Scully RE, Galdabini JJ, McNeely BU. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 47-1978 (Gardner's syndrome). N Engl J Med 1978;299:1237 1244.
257. Snyder N III, Scurry MT, Diess WP. Five families with multiple endocrine adenomatosis. Ann Intern Med 1972;76:53 58.
258. Johnson GJ, Summerskill WH, Anderson VE, Keating FR Jr. Clinical and genetic investigation of a large kindred with multiple endocrine adenomatosis. N Engl J Med 1967;277:1379 1385.
259. Higginbottom MC, Schultz P. The Bannayan syndrome: an autosomal dominant disorder consisting of macrocephaly, lipomas, hemangiomas, and a risk for intracranial tumors. Pediatrics 1982;69:632 634.
260. Klein JA, Barr RJ. Diffuse lipomatosis and tuberous sclerosis. Arch Dermatol 1986;122:1298 1302.
261. Weinstock JV, Kawanishi H. Gastrointestinal polyposis with orocutaneous hamartomas (Cowden's disease). Gastroenterology 1978;74(pt 1):890 895.
262. Carney JA. The triad of gastric epithelioid leiomyosarcoma, functioning extra-adrenal paraganglioma, and pulmonary chondroma. Cancer 1979;43:374 382.
263. Carney JA. The triad of gastric epithelioid leiomyosarcoma, pulmonary chondroma, and functioning extra-adrenal paraganglioma: a five-year review. Medicine (Baltimore) 1983;62:159 169.
264. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev 1988;17:1083 1085.
265. Juhlin L, Strand A, Johnsen B. A syndrome with painful lipomas, familial dysarthria, abnormal eye-movements and clumsiness. Acta Med Scand 1987;221:215 218.
266. Aleksic S, Budzilovich G, Greco MA, et al. Intracranial lipomas, hydrocephalus and other CNS anomalies in oculoauriculo-vertebral dysplasia (Goldenhar Gorlin syndrome). Childs Brain 1984;11:285 297.
267. Oochi N, Rikitake O, Maeda T, Yamaguchi M. A case of Laurence Moon Biedl syndrome associated with bilateral adrenal lipomas and renal abnormalities. Nippon Naika Gakkai Zasshi 1984;73:89 93.
268. Humphrey AA, Kinsley PC. Familial multiple lipomas: report of a family. Arch Derm Syph 1938;37:30 34.
269. Temtamy SA, Rogers JG. Macrodactyly, hemihypertrophy, and connective tissue nevi: report of a new syndrome and review of the literature. J Pediatr 1976;89:924 927.
270. Petras RE. Nonneoplastic intestinal diseases. In: Mills SE, ed. Sternberg's Diagnostic Surgical Pathology. 4th ed. New York: Lippincott Wilkins; 2004:1519 1520.
271. Trotter MJ, Crawford RI. Pseudolipomatosis cutis: superficial dermal vacuoles resembling fatty infiltration of the skin. Am J Dermatopathol 1998:20:443 447.
272. Russell RM, Boyer JL, Bagheri SA, Hruban Z. Hepatic injury from chronic hypervitaminosis a resulting in portal hypertension and ascites. N Engl J Med 1974;291:435 440.
273. Sheibani K, Battifora H. Signet-ring cell melanoma. A rare morphologic variant of malignant melanoma. Am J Surg Pathol 1988;12:28 34.
274. Iossifides I, Mackay B, Butler JJ. Signet-ring cell lymphoma. Ultrastruct Pathol 1980;1:511 517.
275. Hanna W, Kahn HJ, From L. Signet ring lymphoma of the skin: ultrastructural and immunohistochemical features. J Am Acad Dermatol 1986;14(pt 2):344 350.
276. Cross PA, Eyden BP, Harris M. Signet ring cell lymphoma of T cell type. J Clin Pathol 1989;42:239 245.
277. Uccini S, Pescarmona E, Ruco LP, Baroni CD, Monarca B, Modesti A. Immunohistochemical characterization of a B-cell signet ring cell lymphoma. Report of a case. Pathol Res Pract 1988;183:497 504.
278. Mathur DR, Ramdeo IN, Sharma SP, Singh H. Signet ring cell lymphoma simulating liposarcoma a case report with brief review of literature. Indian J Cancer 1988;25:52 55.
279. Jacobs DM, Waisman J. Cervical paraganglioma with intranuclear vacuoles in a fine needle aspirate. Acta Cytol 1987;31:29 32.
280. Spagnolo DV, Paradinas FJ. Laryngeal neuroendocrine tumour with features of a paraganglioma, intracytoplasmic lumina and acinar formation. Histopathology 1985;9:117 131.
281. Rosai J, Gold J, Landy R. The histiocytoid hemangiomas. A unifying concept embracing several previously described entities of skin, soft tissue, large vessels, bone, and heart. Hum Pathol 1979;10:707 730.
282. Barnes L, Koss W, Nieland M. Angiolymphoid hyperplasia with eosinophilia: a disease that may be confused with malignancy. Head Neck Surg 1980;2:425 434.
283. Kung IT, Gibson JB, Bannatyne PM. Kimura's disease: a clinico-pathological study of 21 cases and its distinction from angiolymphoid hyperplasia with eosinophilia. Pathology 1984;16:39 44.
284. Weiss SW, Enzinger FM. Spindle cell hemangioendothelioma. A low-grade angiosarcoma resembling a cavernous hemangioma and Kaposi's sarcoma. Am J Surg Pathol 1986;10:521 530.
285. Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer 1982;50:970 981.
286. Shimazaki H, Aida S, Iizuka Y, Yoshizu H, Tamai S. Vacuolated cell mesothelioma of the pericardium resembling liposarcoma: a case report. Hum Pathol 2000;31:767 770.
287. Chimelli L, Hahn MD, Budka H. Lipomatous differentiation in a medulloblastoma. Acta Neuropathol (Berl) 1991;81:471 473.
288. Powell CM, Rosen PP. Adipose differentiation in cystosarcoma phyllodes. A study of 14 cases. Am J Surg Pathol 1994;18:720 727.
289. Krishna J, Haqqani MT. Liposarcomatous differentiation in diffuse pleural mesothelioma. Thorax 1993;48:409 410.