36 - Infectious Diseases: Mycotic
Editors: McPhee, Stephen J.; Papadakis, Maxine A.; Tierney, Lawrence M.
Title: Current Medical Diagnosis & Treatment, 46th Edition
Copyright 2007 McGraw-Hill
> Table of Contents > 39 - Poisoning
function show_scrollbar() {}
39
Poisoning
Kent R. Olson MD
Initial Evaluation: Poisoning or Overdose
Patients with drug overdoses or poisoning may initially have no symptoms or they may have varying degrees of overt intoxication. The asymptomatic patient may have been exposed to or may have ingested a lethal dose of a poison but not yet exhibit any manifestations of toxicity. It is important to (1) quickly assess the potential danger, (2) consider gut decontamination to prevent absorption, and (3) observe the patient for an appropriate interval.
Assess the Danger
If the drug or poison is known, its danger can be assessed by consulting a text or computerized information resource (eg, Poisindex) or by calling a regional poison control center. (Dialing 800 222-1222 will direct the call to the appropriate United States regional poison control center.) Assessment will usually take into account the dose ingested (in milligrams per kilogram of body weight); the time interval since ingestion; the presence of any symptoms or clinical signs; preexisting cardiac, respiratory, renal, or liver disease; and, occasionally, specific serum drug or toxin levels. Be aware that the history given by the patient or family may be incomplete or unreliable.
The manufacturer or its local representative may be able to provide information over the phone concerning the toxic ingredients in question and can be contacted directly or via the regional poison control center (800 222-1222).
Gut Decontamination
The choice of gut decontamination procedure depends on the toxin and the circumstances. (See below for a more detailed discussion of methods.)
Observation of the Patient
Asymptomatic or mildly symptomatic patients should be observed for at least 4 6 hours. Longer observation is indicated if the ingested substance is a sustained-release preparation or is known to slow gastrointestinal motility or if there may have been exposure to a poison with delayed onset of symptoms (such as acetaminophen, colchicine, or hepatotoxic mushrooms). After that time, the patient may be discharged if no symptoms have developed and adequate gastric decontamination has been provided. Before discharge, psychiatric evaluation should be performed to assess suicidal risk. Intentional ingestions in adolescents should raise the possibility of unwanted pregnancy or sexual abuse.
The Symptomatic Patient
In symptomatic patients, treatment of life-threatening complications takes precedence over in-depth diagnostic evaluation. Patients with mild symptoms may deteriorate rapidly, which is why all potentially significant exposures should be observed in an acute care facility. The following complications may occur, depending on the type of poisoning.
Coma
Assessment & Complications
Coma is commonly associated with ingestion of large doses of antihistamines, barbiturates, benzodiazepines and other sedative-hypnotic drugs, -hydroxybutyrate (GHB), ethanol, opioids, antipsychotic drugs, or antidepressants. The most common cause of death in comatose patients is respiratory failure, which may occur abruptly. Pulmonary aspiration of gastric contents may also occur, especially in victims who are deeply obtunded or convulsing. Hypoxia and hypoventilation may cause or aggravate hypotension, arrhythmias, and seizures. Thus, protection of the airway and assisted ventilation are the most important treatment measures for any poisoned patient.
Table 39-1. Initial management of coma. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Treatment
A. Emergency Management
The initial emergency management of coma can be remembered by the mnemonic ABCD, for Airway, Breathing, Circulation, and Drugs (dextrose, thiamine, and naloxone or flumazenil), respectively (Table 39-1).
P.1640
1. Airway
Establish a patent airway by positioning, suction, or insertion of an artificial nasal or oropharyngeal airway. If the patient is deeply comatose or if there is no gag or cough reflex, perform endotracheal intubation. These airway interventions may not be necessary if the patient is intoxicated by an opioid or a benzodiazepine and responds rapidly to intravenous naloxone or flumazenil (see below).
2. Breathing
Clinically assess the quality and depth of respiration, and provide assistance if necessary with a bag-valve-mask device or mechanical ventilator. Provide supplemental oxygen. The arterial blood CO2 tension is useful in determining the adequacy of ventilation. The arterial blood PO2 determination may reveal hypoxemia, which may be caused by respiratory arrest, bronchospasm, pulmonary aspiration, or noncardiogenic pulmonary edema. Pulse oximetry provides an assessment of oxygenation but is not reliable in patients with methemoglobinemia or carbon monoxide poisoning.
3. Circulation
Measure the pulse and blood pressure and estimate tissue perfusion (eg, by measurement of urinary output, skin signs, arterial blood pH). Place the patient on continuous electrocardiographic monitoring. Insert an intravenous line, and draw blood for complete blood count, glucose, electrolytes, serum creatinine and liver tests, and possible quantitative toxicologic testing.
4. Drugs
a. Dextrose and thiamine
Unless promptly treated, severe hypoglycemia can cause irreversible brain damage. Therefore, in all comatose or convulsing patients, give 50% dextrose, 50 100 mL by intravenous bolus, unless a rapid bedside blood sugar test is available and rules out hypoglycemia. In alcoholic or very malnourished patients who may have marginal thiamine stores, give thiamine, 100 mg intramuscularly or over 2 3 minutes intravenously.
b. Narcotic antagonists
Naloxone, 0.4 2 mg intravenously, may reverse opioid-induced respiratory depression and coma. If opioid overdose is strongly suspected, give additional doses of naloxone (up to 5 10 mg may be required to reverse the effects of potent opioids or propoxyphene). Caution: Naloxone has a much shorter duration of action (2 3 hours) than most common opioids; repeated doses may be required, and continuous observation for at least 3 4 hours after the last dose is mandatory. Nalmefene, a newer opioid antagonist, has a duration of effect longer than that of naloxone but still shorter than that of the opioid methadone.
c. Flumazenil
Flumazenil, 0.2 0.5 mg intravenously, repeated every 30 seconds as needed up to a maximum of 3 mg, may reverse benzodiazepine-induced coma. Caution: Flumazenil has a short duration of effect (2 3 hours), and resedation requiring additional doses is common. Furthermore, flumazenil should not be given if the patient has coingested a tricyclic antidepressant, is a user of high-dose benzodiazepines, or has a seizure disorder because its use in these circumstances may precipitate seizures. In most circumstances, use of flumazenil is not advised as the potential risks outweigh its benefits.
Hypothermia
Assessment & Complications
Hypothermia commonly accompanies coma due to opioids, ethanol, hypoglycemic agents, phenothiazines, barbiturates, benzodiazepines, and other sedative-hypnotics and depressants. Hypothermic patients may have a barely perceptible pulse and blood pressure and often appear to be dead. Hypothermia may cause or aggravate hypotension, which will not reverse until the temperature is normalized.
Treatment
Treatment of hypothermia is discussed in Chapter 38. Gradual rewarming is preferred unless the patient is in cardiac arrest.
Hypotension
Assessment & Complications
Hypotension may be due to poisoning by many different drugs and poisons, including antihypertensive drugs, -blockers, calcium channel blockers, disulfiram (ethanol interaction), iron, theophylline, phenothiazines and other antipsychotic agents, and antidepressants. Poisons causing hypotension include cyanide, carbon monoxide, hydrogen sulfide, arsenic, and certain mushrooms.
Hypotension in the poisoned or drug-overdosed patient may be caused by venous or arteriolar vasodilation, hypovolemia, depressed cardiac contractility, or a combination of these effects. The only certain way to determine the cause of hypotension in any individual patient is to insert a pulmonary artery catheter and calculate the cardiac output and peripheral vascular resistance. Alternatively, a central venous pressure (CVP) monitor may indicate a need for further fluid therapy.
P.1641
Treatment
Most patients respond to empiric treatment with 200 mL intravenous boluses of 0.9% saline or other isotonic crystalloid up to a total of 1 2 L. If fluid therapy is not successful, give dopamine, 5 15 mcg/kg/min by intravenous infusion. Consider pulmonary artery catheterization if hypotension persists.
Hypotension caused by certain toxins may respond to specific treatment. For hypotension caused by overdoses of tricyclic antidepressants or related drugs, administer sodium bicarbonate, 50 100 mEq by intravenous bolus injection. Norepinephrine 4 8 mcg/min by intravenous infusion is more effective than dopamine in some patients with overdoses of tricyclic antidepressants or of drugs with predominantly vasodilating effects. For -blocker overdose, glucagon (5 10 mg intravenously) may be of value. For calcium channel blocker overdose, administer calcium chloride, 1 2 g intravenously (repeated doses may be necessary; doses of 5 10 g and more have been given in some cases).
Hypertension
Assessment & Complications
Hypertension may be due to poisoning with amphetamines, anticholinergics, cocaine, ephedrine-containing performance-enhancing products, monoamine oxidase (MAO) inhibitors, and other drugs.
Severe hypertension (eg, diastolic blood pressure > 105 110 mm Hg in a person who does not have chronic hypertension) can result in acute intracranial hemorrhage, myocardial infarction, or aortic dissection. Patients often present with headache, chest pain, or encephalopathy.
Treatment
Treat hypertension if the patient is symptomatic or if the diastolic pressure is greater than 105 110 mm Hg especially if there is no prior history of hypertension.
Hypertensive patients who are agitated or anxious may benefit from a sedative such as lorazepam, 2 3 mg intravenously. For persistent hypertension, administer phentolamine, 2 5 mg intravenously, or nitroprusside sodium, 0.25 8 mcg/kg/min intravenously. If excessive tachycardia is present, add propranolol, 1 5 mg intravenously, or esmolol, 25 100 mcg/kg/min intravenously. Caution: Do not give -blockers alone, since doing so may paradoxically worsen hypertension as a result of unopposed -adrenergic stimulation.
Arrhythmias
Assessment & Complications
Arrhythmias may occur with a variety of drugs or toxins (Table 39-2). They may also occur as a result of hypoxia, metabolic acidosis, or electrolyte imbalance (eg, hyperkalemia or hypokalemia, hypocalcemia), or following exposure to chlorinated solvents or chloral hydrate overdose. Atypical ventricular tachycardia (torsade de pointes) is often associated with drugs that prolong the QT interval.
Table 39-2. Common toxins or drugs causing arrhythmias. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Treatment
Arrhythmias are often caused by hypoxia or electrolyte imbalance, and these conditions should be sought and treated. If ventricular arrhythmias persist, administer lidocaine at usual antiarrhythmic doses. Caution: Avoid class Ia agents (quinidine, procainamide, disopyramide), which may aggravate arrhythmias caused by tricyclic antidepressants, calcium channel blockers, or -blockers. Wide QRS complex tachycardia in the setting of tricyclic antidepressant overdose (or quinidine and other class Ia drugs) should be treated with sodium bicarbonate, 50 100 mEq intravenously by bolus injection. (See discussion of tricyclic antidepressant poisoning.) Torsade de pointes associated with prolonged QT interval may respond to intravenous magnesium (2 g intravenously over 2 minutes) or overdrive pacing.
For tachyarrhythmias induced by chlorinated solvents, chloral hydrate, Freons, or sympathomimetic agents, use propranolol or esmolol (see doses given above in hypertension section).
Seizures
Assessment & Complications
Seizures may be due to poisoning with many drugs and poisons, including amphetamines, antidepressants (especially
P.1642
tricyclic antidepressants and bupropion), antihistamines, antipsychotics, cocaine, isoniazid, phencyclidine (PCP), and theophylline.
Seizures may also be caused by hypoxia, hypoglycemia, hypocalcemia, hyponatremia, withdrawal from alcohol or sedative-hypnotics, head trauma, central nervous system infection, or idiopathic epilepsy.
Prolonged or repeated seizures commonly lead to hypoxia, metabolic acidosis, hyperthermia, and rhabdomyolysis.
Treatment
Administer lorazepam, 2 3 mg, or diazepam, 5 10 mg, intravenously over 1 2 minutes, or if intravenous access is not immediately available midazolam, 5 10 mg intramuscularly. If convulsions continue, administer phenobarbital, 15 20 mg/kg slowly intravenously over no less than 30 minutes; or phenytoin, 15 mg/kg intravenously over no less than 30 minutes (maximum infusion rate, 50 mg/min). For drug-induced seizures, phenobarbital is preferred over phenytoin. The drugs may be used together if necessary. Maintenance doses may be required if drug toxicity is expected to last more than 18 24 hours.
Seizures due to a few drugs and toxins may require antidotes or other specific therapies (as listed in Table 39-3).
Hyperthermia
Assessment & Complications
Hyperthermia may be associated with poisoning by amphetamines (especially ecstasy), atropine and other anticholinergic drugs, cocaine, dinitrophenol and pentachlorophenol, PCP, salicylates, strychnine, tricyclic antidepressants, and various other medications. Overdoses of serotonin reuptake inhibitors (eg, fluoxetine, paroxetine, sertraline) or use in a patient taking an MAO inhibitor may cause agitation, hyperactivity, and hyperthermia ( serotonin syndrome ). Haloperidol and other antipsychotic agents can cause rigidity and hyperthermia (neuroleptic malignant syndrome [NMS]). (See section on schizophrenia and other psychotic disorders in Chapter 25.) Malignant hyperthermia is a rare disorder associated with general anesthetic agents.
Table 39-3. Seizures related to toxins or drugs requiring special consideration.1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Hyperthermia is a rapidly life-threatening complication. Severe hyperthermia (temperature > 40 41 C) may rapidly cause brain damage and multiorgan failure, including rhabdomyolysis, renal failure, and coagulopathy (see Chapter 38).
Treatment
Treat hyperthermia aggressively by removing all clothing, spraying the patient with tepid water, and fanning the patient. If this is not rapidly effective, as shown by a normal rectal temperature within 30 60 minutes, or if there is significant muscle rigidity or hyperactivity, induce neuromuscular paralysis with a nondepolarizing neuromuscular blocker (eg, pancuronium, vecuronium). Once paralyzed, the patient must be intubated and mechanically ventilated. In patients with seizures, absence of visible muscular convulsive movements may give the false impression that brain seizure activity has ceased; however, this must be confirmed by electroencephalography.
Dantrolene (2 5 mg/kg intravenously) may be effective for hyperthermia associated with muscle rigidity that does not respond to neuromuscular blockade (ie, malignant hyperthermia). Bromocriptine, 2.5 7.5 mg orally daily, has been recommended for neuroleptic malignant syndrome. Cyproheptadine, 4 mg orally every hour for three or four doses, has been used to treat serotonin syndrome.
Antidotes & other Treatment
Antidotes
Give an antidote (if available) when there is reasonable certainty of a specific diagnosis (Table 39-4). Antidotes themselves may have serious side effects. The indications and dosages for specific antidotes are discussed in the respective sections for specific toxins.
Table 39-4. Some toxic agents for which there are specific antidotes.1 | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Decontamination of the Skin
Corrosive agents rapidly injure the skin and eyes and must be removed immediately. In addition, many toxins are readily absorbed through the skin, and systemic absorption can be prevented only by rapid action.
P.1643
Wash the affected areas with copious quantities of lukewarm water or saline. Wash carefully behind the ears, under the nails, and in skin folds. For oily substances (eg, pesticides), wash the skin at least twice with plain soap and shampoo the hair. Specific decontaminating solutions or solvents (eg, alcohol) are rarely indicated and in some cases may paradoxically enhance absorption. For exposure to chemical warfare poisons such as nerve agents or vesicants, some authorities recommend use of a dilute hypochlorite solution (household bleach diluted 1:10 with water).
Decontamination of the Eyes
Act quickly to prevent serious damage. Flush the eyes with copious amounts of saline (preferred) or water. (If available, instill local anesthetic drops in the eye before beginning irrigation.) Remove contact lenses if present. Direct the irrigating stream so that it will flow across both eyes after running off the nasal bridge. Lift the tarsal conjunctiva to look for undissolved particles and to facilitate irrigation. Continue irrigation for 15 minutes or until each eye has been irrigated with at least 1 L of solution. If the toxin is an acid or a base, check the pH of the tears after irrigation, and continue irrigation until the pH is between 6.5 and 7.5.
After irrigation is complete, perform a careful examination of the eye, using fluorescein and a slit lamp or Wood's lamp to identify areas of corneal injury. Patients with serious conjunctival or corneal injury should be immediately referred to an ophthalmologist.
Gastrointestinal Decontamination
Removal of ingested poisons was a routine part of emergency treatment for decades. However, studies in volunteers indicate that if more than 60 minutes has passed, induced emesis and gastric lavage are relatively ineffective, and prospective clinical studies have failed to demonstrate improved outcome after gastric emptying. For small or moderate ingestions of most substances, toxicologists generally recommend oral activated charcoal alone without prior gastric emptying. Exceptions are large ingestions of anticholinergic compounds and salicylates, which often delay gastric emptying, and ingestion of sustained-release or enteric-coated tablets, which may remain intact for several hours.
Gastric emptying is not generally used for ingestion of corrosive agents or petroleum distillates, because further esophageal injury or pulmonary aspiration may result. However, in certain cases, removal of the toxin may be more important than concern over possible complications. Consult a medical toxicologist or regional poison control center (800 222-1222) for advice.
Emesis
Emesis using syrup of ipecac can partially evacuate gastric contents if given very soon after ingestion (eg, at work or at home). However, it may increase the risk of pulmonary aspiration and delay or prevent the use of oral activated charcoal. Therefore, it is no longer used in the routine management of ingestions.
Gastric Lavage
Gastric lavage is more effective for liquid poisons or small pill fragments than for intact tablets or pieces of mushroom. It is most useful when started within 60 minutes after ingestion. However, the lavage procedure may delay administration of activated charcoal and may stimulate vomiting and pulmonary aspiration in an obtunded patient. It is no longer used in the routine management of overdose.
A. Indications
Gastric lavage is sometimes used after very large ingestions (eg, massive aspirin overdose), for collection and examination of gastric contents for identification of poison, and for convenient administration of charcoal and antidotes.
B. Contraindications
Do not use lavage for stuporous or comatose patients with absent gag reflexes unless they are endotracheally intubated
P.1644
beforehand. Some authorities advise against lavage when caustic material has been ingested; others regard it as essential to remove liquid corrosives from the stomach.
C. Technique
In obtunded or comatose patients, the danger of aspiration pneumonia is reduced by performing endotracheal intubation with a cuffed tube before the procedure. Gently insert a lubricated, soft but noncollapsible stomach tube (at least 37 40 F) through the mouth or nose into the stomach. Aspirate and save the contents, and then lavage repeatedly with 50- to 100-mL aliquots of fluid until the return fluid is clear. Use lukewarm tap water or saline.
Activated Charcoal
Activated charcoal effectively adsorbs almost all drugs and poisons. Poorly adsorbed substances include iron, lithium, potassium, sodium, cyanide, mineral acids, and alcohols.
A. Indications
Activated charcoal should be used for prompt adsorption of drugs or toxins in the stomach and intestine. Studies in volunteers show that activated charcoal given alone may be as effective as or more effective than ipecac-induced emesis or gastric lavage. However, evidence of benefit in clinical studies is lacking. Administration of charcoal, especially if mixed with sorbitol, can provoke vomiting, which could lead to pulmonary aspiration in an obtunded patient.
B. Contraindications
Activated charcoal should not be used for comatose or convulsing patients unless it can be given by gastric tube and the airway is first protected by a cuffed endotracheal tube. It is also contraindicated for patients with ileus or intestinal obstruction or those who have ingested corrosives for whom endoscopy is planned.
C. Technique
Administer activated charcoal, 60 100 g orally or via gastric tube, mixed in aqueous slurry. Repeated doses may be given to ensure gastrointestinal adsorption or to enhance elimination of some drugs (see below).
Catharsis
A. Indications
Cathartics are used by some toxicologists for stimulation of peristalsis to hasten the elimination of unabsorbed drugs and poisons and the activated charcoal slurry. There is no clinical evidence to support their use, and some agents (eg, sorbitol) can provoke vomiting, increasing the risk of pulmonary aspiration.
B. Contraindications and Cautions
Do not use mineral oil or other oil-based cathartics. Do not give a cathartic to patients with suspected intestinal obstruction. Avoid sodium-based cathartics in patients with hypertension, renal failure, and congestive heart failure and magnesium-based cathartics in patients with renal failure. Sorbitol (an osmotic cathartic found in some prepackaged activated charcoal slurry products) can cause hypotension and dehydration due to third-spacing and also causes intestinal cramping and vomiting.
C. Technique
Magnesium sulfate 10%, 2 3 mL/kg, or other agents given orally or via gastric tube.
Whole Bowel Irrigation
Whole bowel irrigation uses large volumes of balanced polyethylene glycol-electrolyte solution to mechanically cleanse the entire intestinal tract. Because of the composition of the irrigating solution, there is no significant gain or loss of systemic fluids or electrolytes.
A. Indications
Whole bowel irrigation is particularly effective for massive iron ingestion in which intact tablets are visible on abdominal x-ray. It has also been used for ingestions of sustained-release and enteric-coated tablets as well as swallowed drug-filled packets.
B. Contraindications
Do not use in patients with suspected intestinal obstruction. Use with caution in patients who are obtunded or have depressed airway protective reflexes.
C. Technique
Administer a balanced polyethylene glycol-electrolyte solution (CoLyte, GoLYTELY) into the stomach via gastric tube at a rate of 1 2 L/h until the rectal effluent is clear. This may take several hours. It is most effective when patients are able to sit on a commode to pass the intestinal contents.
Increased Drug Removal
A. Urinary Manipulation
Forced diuresis is hazardous; the risk of complications (pulmonary edema, electrolyte imbalance) usually outweighs its benefits. Acidic drugs (eg, salicylates, phenobarbital) are more rapidly excreted with an alkaline urine. Acidification (sometimes promoted for amphetamines, phencyclidine) is not very effective and is contraindicated in the presence of rhabdomyolysis or myoglobinuria.
B. Hemodialysis
The indications for dialysis are as follows: (1) Known or suspected potentially lethal amounts of a dialyzable drug (Table 39-5). (2) Poisoning with deep coma, apnea, severe hypotension, fluid and electrolyte
P.1645
or acid-base disturbance, or extreme body temperature changes that cannot be corrected by conventional measures. (3) Poisoning in patients with severe renal, cardiac, pulmonary, or hepatic disease who will not be able to eliminate toxin by the usual mechanisms.
Table 39-5. Recommended use of hemodialysis in poisoning.1 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Peritoneal dialysis may rarely be used for acute poisonings when hemodialysis is not available, but it is very inefficient. Continuous renal replacement therapy (also known as continuous venovenous hemodiafiltration) is of uncertain benefit for elimination of most poisons but has been used successfully in the management of lithium intoxication.
C. Repeat-Dose Charcoal
Repeated doses of activated charcoal, 20 30 g orally or via gastric tube every 3 4 hours, may hasten elimination of some drugs (eg, theophylline, phenobarbital) by absorbing drugs excreted into the gut lumen ( gut dialysis ). However, clinical studies have failed to prove better outcome using multiple-dose charcoal. Sorbitol or other cathartics should not be used with each dose, or resulting large stool volumes may lead to dehydration or hypernatremia.
Bond GR: The role of activated charcoal and gastric emptying in gastrointestinal decontamination: a state-of-the-art review. Ann Emerg Med 2002;39:273.
Heard K: Gastrointestinal decontamination. Med Clin North Am 2005;89(6):1067.
Proudfoot AT et al: Position paper on urine alkalinization. J Toxicol Clin Toxicol 2004;42:1.
Diagnosis of Poisoning
The identity of the ingested substance or substances is usually known, but occasionally a comatose patient is found with an unlabeled container or refuses or otherwise fails to give a coherent history. By performing a directed physical examination and ordering common clinical laboratory tests, the clinician can often make a tentative diagnosis that may allow empiric interventions or may suggest specific toxicologic tests.
Physical Examination
Important diagnostic variables in the physical examination include blood pressure, pulse rate, temperature, pupil size, sweating, and the presence or absence of peristaltic activity. Poisonings with many drugs fit into one of four common syndromes.
Sympathomimetic Syndrome
The blood pressure and pulse rate are elevated, though with severe hypertension reflex bradycardia may occur. The temperature is often elevated, pupils are dilated, and the skin is sweaty, though mucous membranes are dry. Patients are usually agitated, anxious, or frankly psychotic.
Examples: Amphetamines, cocaine, ephedrine and pseudoephedrine.
Sympatholytic Syndrome
The blood pressure and pulse rate are decreased and body temperature is low. The pupils are small or even pinpoint. Peristalsis is usually decreased. Patients are usually obtunded or comatose.
Examples: Barbiturates, benzodiazepines and other sedative hypnotics, GHB, clonidine and related antihypertensives, ethanol, opioids.
Cholinergic Syndrome
Stimulation of muscarinic receptors causes bradycardia, miosis, sweating, and hyperperistalsis as well as bronchorrhea, wheezing, excessive salivation, and urinary incontinence. Nicotinic receptor stimulation may produce initial hypertension and tachycardia as well as fasciculations and muscle weakness. Patients are usually agitated and anxious.
Examples: Carbamates, nicotine, organophosphates (including nerve agents), physostigmine.
Anticholinergic Syndrome
Tachycardia with mild hypertension is common, and the body temperature is often elevated. Pupils are widely dilated. The skin is flushed, hot, and dry. Peristalsis is decreased, and urinary retention is common. Patients may have myoclonic jerking or choreoathetoid
P.1646
movements. Agitated delirium is frequently seen, and severe hyperthermia may occur.
Examples: Atropine, scopolamine, other naturally occurring and pharmaceutical anticholinergics, antihistamines, tricyclic antidepressants.
Laboratory Tests
The following clinical laboratory tests are recommended for screening of the overdosed patient: measured serum osmolality and osmolar gap, electrolytes, glucose, creatinine, blood urea nitrogen (BUN), urinalysis (eg, oxalate crystals with ethylene glycol poisoning, myoglobinuria with rhabdomyolysis), and electrocardiography. Serum acetaminophen and ethanol quantitative levels should be determined in all patients with drug overdoses.
Osmolar Gap
The osmolar gap is defined and calculation of the gap is described in Table 39-6. It is increased in the presence of large quantities of low-molecular-weight substances, most commonly ethanol. Common poisons associated with increased osmolar gap are acetone, ethanol, ethylene glycol, isopropyl alcohol, methanol, and propylene glycol. Note: Severe alcoholic ketoacidosis and diabetic ketoacidosis can also cause an elevated osmolar gap resulting from the production of ketones and other low-molecular-weight substances.
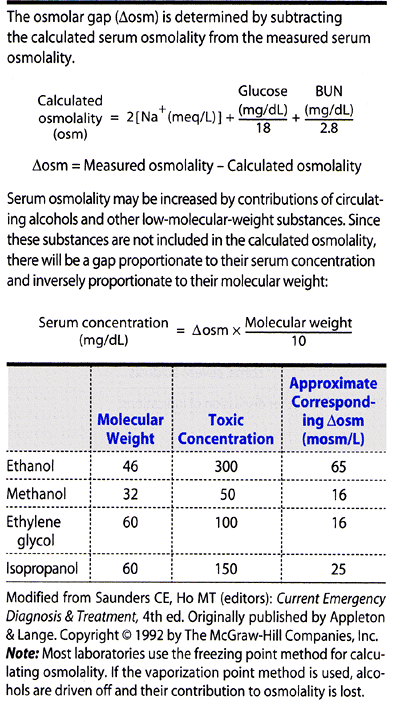 |
Table 39-6. Use of the osmolar gap in toxicology. |
Anion Gap
Metabolic acidosis associated with an elevated anion gap is usually due to an accumulation of lactic acid or other acids (see Chapter 21). Common causes of elevated anion gap in poisoning include carbon monoxide, cyanide, ethylene glycol, medicinal iron, isoniazid, methanol, metformin, ibuprofen and salicylates.
The osmolar gap should also be checked; combined elevated anion and osmolar gap suggests poisoning by methanol or ethylene glycol, though this may also occur in patients with diabetic ketoacidosis and alcoholic ketoacidosis.
Toxicology Laboratory Examination
A comprehensive toxicology screen is of little value in the initial care of the poisoned patient on the contrary, it is time-consuming and expensive. Specific quantitative levels of certain drugs may be extremely helpful (Table 39-7), however, especially if specific antidotes or interventions (eg, dialysis) would be indicated based on the results.
If a toxicology screen is required, urine is the best specimen. Many hospitals can perform a quick but limited screen for drugs of abuse (typically these screens include only opioids, amphetamines, and cocaine, and some add benzodiazepines, barbiturates, and tetrahydrocannabinol [marijuana]). There are numerous false-positive and false-negative results. Blood samples may be saved for possible quantitative testing, but blood is not generally used for screening purposes since it is relatively insensitive for many common drugs, including psychotropic agents, opioids, and stimulants.
Table 39-7. Specific quantitative levels and potential therapeutic interventions.1 | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Abdominal X-Rays
A plain film of the abdomen may reveal radiopaque iron tablets, drug-filled condoms, or other toxic material. Studies suggest that few tablets are predictably visible (eg, ferrous sulfate, sodium chloride, calcium carbonate, and potassium chloride). Thus, the x-ray is useful only if positive.
P.1647
Bartlett D: Understanding the anion and osmolal gaps laboratory values: what they are and how to use them. J Emerg Nurs 2005;31:109.
Goldfrank LR (editor): Goldfrank's Toxicologic Emergencies, 8th ed. McGraw-Hill, 2004.
Hovda KE et al: Anion and osmolal gaps in the diagnosis of methanol poisoning: clinical study in 28 patients. Intensive Care Med 2004;30:1842.
Olson KR (editor): Poisoning and Drug Overdose, 4th ed. McGraw-Hill, 2004.
Selected Poisonings
Acetaminophen
Acetaminophen (paracetamol in the UK, Europe) is a common analgesic found in many nonprescription and prescription products. After absorption, it is metabolized mainly by glucuronidation and sulfation, with a small fraction metabolized via the P450 mixed-function oxidase system (2E1) to a highly toxic reactive intermediate. This toxic intermediate is normally detoxified by cellular glutathione. With acute acetaminophen overdose (> 140 mg/kg, or 7 g in an average adult), hepatocellular glutathione is rapidly depleted and the reactive intermediate attacks other cell proteins, causing necrosis. Patients with enhanced P450 2E1 activity, such as chronic alcoholics and patients taking isoniazid, are at increased risk of developing hepatotoxicity. Hepatic toxicity may also occur after chronic accidental overuse of acetaminophen eg, as a result of taking two or three acetaminophen-containing products concurrently or intentionally exceeding the recommended maximum dose of 4 g/d.
Clinical Findings
Shortly after ingestion, patients may have nausea or vomiting, but there are usually no other signs of toxicity until 24 48 hours after ingestion, when hepatic aminotransferase levels begin to increase. With severe poisoning, fulminant hepatic necrosis may occur, resulting in jaundice, hepatic encephalopathy, renal failure, and death. Rarely, massive ingestion (eg, serum levels over 500 1000 mg/L) can cause acute coma, hypotension, and metabolic acidosis unrelated to hepatic injury.
The diagnosis after acute overdose is based on measurement of the serum acetaminophen level. Plot the serum level versus the time since ingestion on the acetaminophen nomogram shown in Figure 39-1. Ingestion of sustained-release products or coingestion of an anticholinergic agent, salicylate, or opioid drug may cause delayed elevation of serum levels and may render the nomogram useless. The nomogram is not useful after chronic overdose.
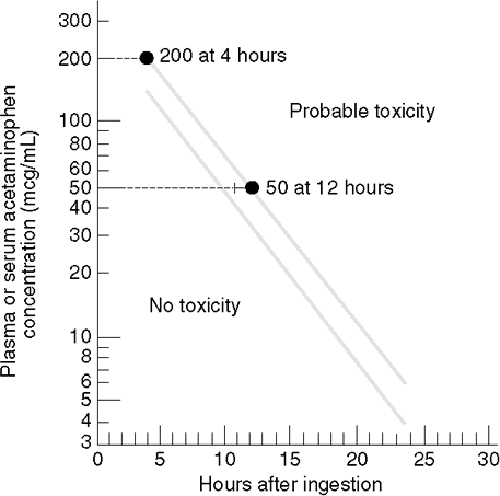 |
Figure 39-1. Nomogram for prediction of acetaminophen hepatotoxicity following acute overdosage. The upper line defines serum acetaminophen concentrations known to be associated with hepatotoxicity; the lower line defines serum levels 25% below those expected to cause hepatotoxicity. To give a margin for error in the estimation of the time of ingestion and for patients at higher risk for hepatotoxicity, the lower line is often used as a guide to treatment. (Modified and reproduced, with permission, from Rumack BH, Matthew H: Acetaminophen poisoning and toxicity. Pediatrics 1975;55:871. ) |
Treatment
A. Emergency and Supportive Measures
Administer activated charcoal (see p 1644) within 1 2 hours of the ingestion. Although charcoal may interfere with absorption of the oral antidote acetylcysteine, this is not considered clinically significant.
B. Specific Treatment
Although the general recommendation is to treat if the serum acetaminophen level is above the toxic line on the nomogram (Figure 39-1), many clinicians prefer to use the lower line as a guide to treatment, as it provides
P.1648
a 25% safety margin. Begin treatment with a loading dose of N-acetylcysteine, 140 mg/kg orally, followed by 70 mg/kg every 4 hours. Dilute the solution to 5% with water, juice, or soda. If vomiting interferes with oral N-acetylcysteine administration, consider giving the antidote intravenously (see below). The most widely used oral N-acetylcysteine protocol in the United States calls for 72 hours of treatment. However, other regimens have demonstrated equivalent success with 20 48 hours of treatment. A 20-hour intravenous regimen was recently approved by the FDA (Acetadote). Treatment with N-acetylcysteine is most effective if started within 8 10 hours after ingestion. If the precise time of ingestion is unknown or if the patient is at higher risk of hepatotoxicity (eg, alcoholic, liver disease, chronic use of P450-inducing drugs), then use a lower threshold for initiation of N-acetylcysteine (in some case reports, a level of 100 mg/L at 4 hours was suggested in very high-risk patients).
The conventional oral formulation may also be given intravenously using a micropore filter and a slow rate of infusion. Call a regional poison control center or medical toxicologist for assistance.
Lavonas EJ et al: Intravenous administration of N-acetylcysteine: oral and parenteral formulations are both acceptable. Ann Emerg Med 2005;45:223.
Sivilotti ML et al: A new predictor of toxicity following acetaminophen overdose based on pretreatment exposure. Clin Toxicol (Phila) 2005;43:229.
Acids, Corrosive (Table 39-8)
The strong mineral acids exert primarily a local corrosive effect on the skin and mucous membranes. Symptoms include severe pain in the throat and upper gastrointestinal tract; bloody vomitus; difficulty in swallowing, breathing, and speaking; discoloration and destruction of skin and mucous membranes in and around the mouth; and shock. Severe systemic metabolic acidosis may occur both as a result of cellular injury and from systemic absorption of the acid.
Severe deep destructive tissue damage may occur after exposure to hydrofluoric acid because of the penetrating and highly toxic fluoride ion. Systemic hypocalcemia and hyperkalemia may also occur after fluoride absorption, even following skin exposure.
Inhalation of volatile acids, fumes, or gases such as chlorine, fluorine, bromine, or iodine causes severe irritation of the throat and larynx and may cause upper airway obstruction and noncardiogenic pulmonary edema.
Treatment
A. Ingestion
Dilute immediately by giving a glass (4 8 oz) of milk or water to drink. Do not give bicarbonate or other neutralizing agents, and do not induce vomiting. Some experts recommend immediate placement of a small
P.1649
flexible gastric tube and removal of stomach contents followed by lavage, particularly if the corrosive is a liquid or has important systemic toxicity.
Table 39-8. Common corrosive agents. | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Perform flexible endoscopic esophagoscopy promptly to determine the presence and extent of injury. X-rays of the chest and abdomen may reveal the presence of free air in patients with esophageal or gastric perforation. Perforation, peritonitis, and major bleeding are indications for surgery.
B. Skin Contact
Flood with water for 15 minutes. Use no chemical antidotes; the heat of the reaction may cause additional injury.
For hydrofluoric acid burns, soak the affected area in benzalkonium chloride solution or apply 2.5% calcium gluconate gel (prepared by adding 3.5 g calcium gluconate to 5 oz of water-soluble surgical lubricant, eg, K-Y Jelly); then arrange immediate consultation with a plastic surgeon or other specialist. Binding of the fluoride ion may be achieved by injecting 0.5 mL of 5% calcium gluconate per square centimeter under the burned area. (Caution: Do not use calcium chloride.) Intra-arterial infusion of calcium is sometimes required for extensive burns or those involving the nail bed; consult with a hand surgeon.
C. Eye Contact
Anesthetize the conjunctiva and corneal surfaces with topical local anesthetic drops (eg, proparacaine). Flood with water for 15 minutes, holding the eyelids open. Check pH with pH 6.0 8.0 test paper, and repeat irrigation, using 0.9% saline, until pH is near 7.0. Check for corneal damage with fluorescein and slit lamp examination; consult an ophthalmologist about further treatment.
D. Inhalation
Remove from further exposure to fumes or gas. Check skin and clothing. Treat pulmonary edema.
Dunser MW et al: Critical care management of major hydrofluoric acid burns: a case report, review of the literature, and recommendations for therapy. Burns 2004;30;391.
Alkalies (Table 39-8)
The strong alkalies are common ingredients of some household cleaning compounds and may be suspected by their soapy texture. Those with alkalinity above pH 12.0 are particularly corrosive. Clinitest tablets and disk batteries are also a source. Alkalies cause liquefactive necrosis, which is deeply penetrating. Symptoms include burning pain in the upper gastrointestinal tract, nausea, vomiting, and difficulty in swallowing and breathing. Examination reveals destruction and edema of the affected skin and mucous membranes and bloody vomitus and stools. X-ray may reveal the presence of disk batteries in the esophagus or lower gastrointestinal tract.
Treatment
A. Ingestion
Dilute immediately with a glass of water. Do not induce emesis. Some gastroenterologists recommend immediate placement of a small flexible gastric tube and removal of stomach contents followed by gastric lavage after ingestion of liquid caustic substances to remove residual material.
Immediate endoscopy is recommended to evaluate the extent of damage. If x-ray reveals the location of ingested disk batteries in the esophagus, immediate endoscopic removal is mandatory.
The use of corticosteroids to prevent stricture formation is of no proved benefit and is definitely contraindicated if there is evidence of esophageal perforation.
B. Skin Contact
Wash with running water until the skin no longer feels soapy. Relieve pain and treat shock.
C. Eye Contact
Anesthetize the conjunctival and corneal surfaces with topical anesthetic (eg, proparacaine). Irrigate with water or saline continuously for 20 30 minutes, holding the lids open. Check pH with pH test paper, and repeat irrigation, using 0.9% saline, for additional 30-minute periods until the pH is near 7.0. Check for corneal damage with fluorescein and slit lamp examination; consult an ophthalmologist for further treatment.
Ramasamy K et al: Corrosive ingestion in adults. J Clin Gastroenterol 2003;37:119.
Amphetamines & Cocaine
Amphetamines and cocaine are widely abused for their euphorigenic and stimulant properties. Both drugs may be smoked, snorted, ingested, or injected. Amphetamines and cocaine produce central nervous system stimulation and a generalized increase in central and peripheral sympathetic activity. The toxic dose of each drug is highly variable and depends on the route of administration and individual tolerance. The onset of effects is most rapid after intravenous injection or smoking. Amphetamine derivatives and related drugs include methamphetamine ( crystal meth, crank ), methylenedioxymethamphetamine (MDMA, ecstasy ), ephedrine ( herbal ecstasy ), and methcathinone ( cat ). Nonprescription medications and nutritional supplements may contain stimulant or sympathomimetic drugs such as ephedrine or caffeine (see Theophylline, below): Phenylpropanolamine was withdrawn from the market because of an increased incidence of hypertensive intracerebral hemorrhage in young women.
Clinical Findings
Presenting symptoms may include anxiety, tremulousness, tachycardia, hypertension, diaphoresis, dilated
P.1650
pupils, agitation, muscular hyperactivity, and psychosis. Metabolic acidosis may occur. In severe intoxication, seizures and hyperthermia may occur. Sustained or severe hypertension may result in intracranial hemorrhage, aortic dissection, or myocardial infarction. Hyponatremia has been reported after MDMA use; the mechanism is not known but may involve excessive water intake, syndrome of inappropriate antidiuretic hormone (SIADH), or both.
The diagnosis is supported by finding amphetamines, cocaine, or the cocaine metabolite benzoylecgonine in the urine. Blood screening is generally not sensitive enough to detect these drugs.
Treatment
A. Emergency and Supportive Measures
Maintain a patent airway and assist ventilation, if necessary. Treat coma or seizures as described at the beginning of this chapter. Rapidly lower the body temperature (see hyperthermia, above) in patients who are hyperthermic (40 C).
For poisoning by ingestion, administer activated charcoal (p 1644). Do not induce emesis because of the risk of seizures.
B. Specific Treatment
Treat agitation, psychosis, or seizures with a benzodiazepine such as lorazepam, 2 3 mg intravenously. Add phenobarbital 15 mg/kg intravenously for persistent seizures. Treat hypertension with a vasodilator drug such as phentolamine (1 5 mg intravenously) or a combined - and -adrenergic blocker such as labetalol (10 20 mg intravenously). Do not administer a pure -blocker such as propranolol alone, as this may result in paradoxic worsening of the hypertension as a result of unopposed -adrenergic effects.
Treat tachycardia or tachyarrhythmias with a short-acting -blocker such as esmolol (25 100 mcg/kg/min by intravenous infusion). Treat hyponatremia as outlined in Chapter 21.
Greene SL et al: Multiple toxicity from 3,4-methylenedioxymethamphetamine ( ecstasy ). Am J Emerg Med 2003;21:121.
Kashani J et al: Methamphetamine toxicity secondary to intravaginal body stuffing. J Toxicol Clin Toxicol 2004;42:987.
Anticoagulants
Warfarin and related compounds (including ingredients of many commercial rodenticides) inhibit the clotting mechanism by blocking hepatic synthesis of vitamin K-dependent clotting factors.
Anticoagulants may cause hemoptysis, gross hematuria, bloody stools, hemorrhages into organs, widespread bruising, and bleeding into joint spaces. The prothrombin time is increased within 12 24 hours (peak 36 48 hours) after a single overdose. After ingestion of brodifacoum and indanedione rodenticides (so-called superwarfarins ), inhibition of clotting factor synthesis may persist for several weeks or even months after a single dose.
Treatment
A. Emergency and Supportive Measures
Discontinue the drug at the first sign of gross bleeding, and determine the prothrombin time (international normalized ratio, INR). If the patient has ingested an acute overdose, administer activated charcoal (see p 1644).
B. Specific Treatment
Do not treat prophylactically with vitamin K wait for evidence of anticoagulation (elevated prothrombin time). If the INR is elevated, give phytonadione (vitamin K1), 10 25 mg orally, and additional doses as needed to restore the prothrombin time to normal. Doses as high as 200 mg/d have been required after ingestion of superwarfarins. Give fresh-frozen plasma or activated Factor VII as needed to rapidly correct the coagulation factor deficit if there is serious bleeding. If the patient is chronically anticoagulated and has strong medical indications for being maintained in that status (eg, prosthetic heart valve), give much smaller doses of vitamin K (1 mg orally) and fresh-frozen plasma (or both) to titrate to the desired prothrombin time.
If the patient has ingested brodifacoum or a related superwarfarin, prolonged observation (over weeks) and repeated administration of large doses of vitamin K may be required.
Ingels M et al: A prospective study of acute, unintentional, pediatric superwarfarin ingestions managed without decontamination. Ann Emerg Med 2002;40:73.
Zupancic-Salek S et al: Successful reversal of anticoagulant effect of superwarfarin poisoning with recombinant activated factor VII. Blood Coagul Fibrinolysis 2005;16:239.
Anticonvulsants
Anticonvulsants (carbamazepine, phenytoin, valproic acid) are widely used in the management of seizure disorders. In addition, carbamazepine and valproic acid are increasingly used for treatment of mood disorders.
Phenytoin can be given orally or intravenously. Rapid intravenous injection of phenytoin can cause acute myocardial depression and cardiac arrest owing to the solvent propylene glycol; a newer form of phenytoin (fosphenytoin) is available that does not contain this diluent. Chronic phenytoin intoxication can occur following only slightly increased doses because of zero-order kinetics and a small toxic-therapeutic window. Phenytoin intoxication can also occur following acute intentional or accidental overdose. The overdose syndrome is usually mild even with high
P.1651
serum levels. The most common manifestations are ataxia, nystagmus, and drowsiness. Choreoathetoid movements have been described.
Carbamazepine was first used for the treatment of trigeminal neuralgia. It has since become a first-line agent for temporal lobe epilepsy and other seizure disorders. Intoxication causes drowsiness, stupor, and, with high levels, coma and seizures. Dilated pupils and tachycardia are common. Toxicity may be seen with serum levels greater than 20 mg/L, though severe poisoning is usually associated with concentrations greater than 30 40 mg/L. Because of erratic and slow absorption, intoxication may progress over several hours to days.
Valproic acid intoxication produces a unique syndrome consisting of hypernatremia (from the sodium component of the salt), metabolic acidosis, hypocalcemia, elevated serum ammonia, and mild liver aminotransferase elevation. Hypoglycemia may occur as a result of hepatic metabolic dysfunction. Coma with small pupils may be seen and can mimic opioid poisoning. Encephalopathy and cerebral edema can occur.
The newer anticonvulsants lamotrigine and tiagabine have also been reported to cause seizures after overdose. Topiramate intoxication has caused acute agitation and confusion.
Treatment
A. Emergency and Supportive Measures
For recent ingestions, give activated charcoal orally or by gastric tube. For large ingestions of carbamazepine or valproic acid especially of sustained-release formulations consider whole bowel irrigation (see p 1644). Multiple-dose activated charcoal may be beneficial in ensuring gut decontamination for large ingestions and might enhance elimination of absorbed drugs.
B. Specific Treatment
There are no antidotes. Naloxone was reported to have reversed valproic acid overdose in one anecdotal case. Consider hemodialysis for massive intoxication with valproic acid or carbamazepine (eg, carbamazepine levels > 100 mg/L or valproic acid levels > 1000 mg/L).
Lofton AL, Klien-Schwartz W: Evaluation of lamotrigine toxicity reported to poison centers. Ann Pharmacother 2004;38(11):1811.
Singh SM et al: Extracorporeal management of valproic acid overdose: a large regional experience. J Nephrol 2004;17:43.
Arsenic
Arsenic is found in some pesticides and industrial chemicals, and arsenic trioxide has recently been reintroduced as a chemotherapeutic agent. A massive epidemic of chronic arsenic poisoning has occurred in Bangladesh due to naturally occurring arsenic in deep aquifers. Symptoms of acute poisoning usually appear within 1 hour after ingestion but may be delayed as long as 12 hours. They include abdominal pain, vomiting, watery diarrhea, and skeletal muscle cramps. Profound dehydration and shock may occur. In chronic poisoning, symptoms can be vague but often include pancytopenia, painful peripheral sensory neuropathy, and skin changes including melanosis, keratosis, and desquamating rash. Urinary arsenic levels may be falsely elevated after certain meals (eg, seafood) that contain large quantities of a nontoxic form of organic arsenic.
Treatment
A. Emergency Measures
After recent ingestion (within 1 2 hours), perform gastric lavage and administer 60 100 g of activated charcoal (see p 1643 1644). Administer intravenous fluids to replace losses due to vomiting and diarrhea.
B. Antidote
For patients with severe acute intoxication, give dimercaprol injection (bronchoalveolar lavage, BAL), 10% solution in oil, 3 5 mg/kg intramuscularly every 4 6 hours for 2 days. The side effects include nausea, vomiting, headache, and hypertension. Follow dimercaprol with oral succimer (dimercaptosuccinic acid, DMSA), 10 mg/kg every 8 hours, for 1 week. Consult a medical toxicologist or regional poison control center (800 222-1222) for advice regarding chelation.
Kalia K et al: Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health 2005;47:1.
Yoshida T et al: Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicol Appl Pharmacol 2004;198:243.
Atropine & Anticholinergics
Atropine, scopolamine, belladonna, diphenoxylate with atropine, Datura stramonium, Hyoscyamus niger, some mushrooms, tricyclic antidepressants, and antihistamines are antimuscarinic agents with variable central nervous system effects. The patient complains of dryness of the mouth, thirst, difficulty in swallowing, and blurring of vision. The physical signs include dilated pupils, flushed skin, tachycardia, fever, delirium, myoclonus, ileus, and flushed appearance. Antidepressants and antihistamines may induce convulsions.
Antihistamines are commonly available with or without prescription. Diphenhydramine commonly causes delirium, tachycardia, and seizures. Massive overdose may mimic tricyclic antidepressant poisoning. The first-generation nonsedating agents terfenadine and astemizole caused QT interval prolongation and torsade de pointes (atypical ventricular tachycardia) and were removed from the United States market. Loratadine and fexofenadine have not caused this problem.
P.1652
Treatment
A. Emergency and Supportive Measures
Administer activated charcoal (see p 1644). Tepid sponge baths and sedation, or neuromuscular paralysis in rare cases, are indicated to control high temperatures (see p 1642).
B. Specific Treatment
For pure atropine or related anticholinergic syndrome, if symptoms are severe (eg, agitated delirium or excessively rapid tachycardia), give physostigmine salicylate, 0.5 1 mg slowly intravenously over 5 minutes, with electrocardiographic monitoring, until symptoms are controlled. Bradyarrhythmias and convulsions are a hazard with physostigmine administration, and it should be avoided in patients with tricyclic antidepressant overdose.
DeFrates LJ et al: Antimuscarinic intoxication resulting from the ingestion of moonflower seeds. Ann Pharmacother 2005;39:173. Epub 2004 Nov 30.
Sharma AN et al: Diphenhydramine-induced wide complex dysrhythmia responds to treatment with sodium bicarbonate. Am J Emerg Med 2003;21:212.
-Adrenergic Blockers
There are a wide variety of -adrenergic blocking drugs, with varying pharmacologic and pharmacokinetic properties (see Table 11-7). The most toxic -blocker is propranolol. Propranolol competitively blocks 1 and 2 adrenoceptors and also has direct membrane-depressant and central nervous system effects.
Clinical Findings
The most common findings with mild or moderate intoxication are hypotension and bradycardia. Cardiac depression from more severe poisoning is often unresponsive to conventional therapy with -adrenergic stimulants such as dopamine and norepinephrine. In addition, with propranolol and other lipid-soluble drugs, seizures and coma may occur.
The diagnosis is based on typical clinical findings. Routine toxicology screening does not usually include -blockers.
Treatment
A. Emergency and Supportive Measures
Initially, treat bradycardia or heart block with atropine (0.5 2 mg intravenously), isoproterenol (2 20 mcg/min by intravenous infusion, titrated to the desired heart rate), or an external transcutaneous cardiac pacemaker. However, these measures are often ineffective, and specific antidotal treatment may be necessary (see below).
For ingested drugs, administer activated charcoal (see p 1644).
B. Specific Treatment
If the above measures are not successful in reversing bradycardia and hypotension, give glucagon, 5 10 mg intravenously, followed by an infusion of 1 5 mg/h. Glucagon is an inotropic agent that acts at a different receptor site and is therefore not affected by -blockade.
Bailey B: Glucagon in beta-blocker and calcium channel blocker overdoses: a systematic review. J Toxicol Clin Toxicol 2003;41:595.
Wax PM et al: Beta-blocker ingestion: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2005;43:131.
Calcium Channel Blockers
Calcium channel blockers used in the United States include verapamil, diltiazem, nifedipine, nicardipine, amlodipine, felodipine, isradipine, nisoldipine, and nimodipine. These drugs share the ability to cause arteriolar vasodilation and depression of cardiac contractility, especially after acute overdose. Patients may present with bradycardia, atrioventricular (AV) nodal block, hypotension, or a combination of these effects. With severe poisoning, cardiac arrest may occur.
Treatment
A. Emergency and Supportive Measures
Maintain a patent airway and assist ventilation, if necessary. Treat coma, hypotension, and seizures as described at the beginning of this chapter. Treat bradycardia with atropine (0.5 2 mg intravenously), isoproterenol (2 20 mcg/min by intravenous infusion), or a transcutaneous or internal cardiac pacemaker.
For ingested drugs, administer activated charcoal (see p 1644). In addition, whole bowel irrigation should be initiated as soon as possible if the patient has ingested a sustained-release product.
B. Specific Treatment
If bradycardia and hypotension are not reversed with these measures, administer calcium chloride intravenously. Start with calcium chloride 10%, 10 mL, or calcium gluconate 10%, 20 mL. Repeat the dose every 3 5 minutes. The optimum (or maximum) dose has not been established, but there are reports of success after as much as 10 12 g of calcium chloride. Calcium is most useful in reversing negative inotropic effects and is less effective for AV nodal blockade and bradycardia. Epinephrine infusion (1 4 mcg/min initially) and glucagon (5 10 mg intravenously) have also been recommended. In addition, high doses of insulin (0.5 1 U/kg intravenous bolus followed by 0.5 1 U/kg/h infusion) along with sufficient dextrose to maintain euglycemia have been reported to be beneficial but there are no controlled studies.
DeWitt CR et al: Pharmacology, pathophysiology and management of calcium channel blocker and beta-blocker toxicity. Toxicol Rev 2004;23:223.
P.1653
Marques M et al: Treatment of calcium channel blocker intoxication with insulin infusion: case report and literature review. Resuscitation 2003;57:211.
Shepherd G et al: High-dose insulin therapy for calcium-channel blocker overdose. Ann Pharmacother 2005;39:923. Epub 2005 Apr 5.
Carbon Monoxide
Carbon monoxide is a colorless, odorless gas produced by the combustion of carbon-containing materials. Poisoning may occur as a result of suicidal or accidental exposure to automobile exhaust, smoke inhalation in a fire, or accidental exposure to an improperly vented gas heater or other appliance. Carbon monoxide avidly binds to hemoglobin, with an affinity approximately 250 times that of oxygen. This results in reduced oxygen-carrying capacity and altered delivery of oxygen to cells (see also Smoke Inhalation in Chapter 9).
Clinical Findings
At low carbon monoxide levels (carboxyhemoglobin saturation 10 20%), victims may have headache, dizziness, abdominal pain, and nausea. With higher levels, confusion, dyspnea, and syncope may occur. Hypotension, coma, and seizures are common with levels greater than 50 60%. Survivors of acute severe poisoning may develop permanent obvious or subtle neurologic and neuropsychiatric deficits. The fetus and newborn may be more susceptible because of high carbon monoxide affinity for fetal hemoglobin.
Carbon monoxide poisoning should be suspected in any person with severe headache or acutely altered mental status, especially during cold weather, when improperly vented heating systems may have been used. Diagnosis depends on specific measurement of the arterial or venous carboxyhemoglobin saturation, although the level may have declined if high-flow oxygen therapy has already been administered, and levels do not always correlate with clinical symptoms. Routine arterial blood gas testing and pulse oximetry are not useful because they give falsely normal Po2 and oxyhemoglobin saturation determinations, respectively.
Treatment
A. Emergency and Supportive Measures
Maintain a patent airway and assist ventilation, if necessary. Remove the victim from exposure. Treat patients with coma, hypotension, or seizures as described at the beginning of this chapter.
B. Specific Treatment
The half-life of the carboxyhemoglobin (CoHb) complex is about 4 5 hours in room air but is reduced dramatically by high concentrations of oxygen. Administer 100% oxygen by tight-fitting high-flow reservoir face mask or endotracheal tube. Hyperbaric oxygen (HBO) can provide 100% oxygen under higher than atmospheric pressures, further shortening the half-life; it may also reduce the incidence of subtle neuropsychiatric sequelae. Recent studies disagree about the benefit of HBO, but recommended indications for HBO in patients with carbon monoxide poisoning include a history of loss of consciousness, CoHb greater than 25%, metabolic acidosis, age over 50 years, and cerebellar findings on neurologic examination.
Henry CR et al: Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA 2006;295:398.
Juurlink DN et al: Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev 2005;CD002041.
Weaver LK et al: Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med 2002;347:1057.
Chemical Warfare: Nerve Agents
Nerve agents used in chemical warfare work by cholinesterase inhibition and are most commonly organophosphorus compounds. Agents such as tabun (GA), sarin (GB), soman (GD), and VX are similar to insecticides such as malathion but are vastly more potent. They may be inhaled or absorbed through the skin. Systemic effects due to unopposed action of acetylcholine include miosis, salivation, abdominal cramps, diarrhea, and muscle paralysis producing respiratory arrest. Inhalation also produces severe bronchoconstriction and copious nasal and tracheobronchial secretions.
Treatment
A. Emergency and Supportive Measures
Perform thorough decontamination of exposed areas with repeated soap and shampoo washing. Personnel caring for such patients must wear protective clothing and gloves, since cutaneous absorption may occur through normal skin.
B. Specific Treatment
Give atropine in an initial dose of 2 mg intravenously, and repeat as needed to reverse signs of acetylcholine excess. (Some victims have required several hundred milligrams.) Treat also with the cholinesterase-reactivating agent pralidoxime, 1 2 g intravenously initially followed by an infusion at a rate of 200 400 mg/h. United States military personnel in the Iraq invasion were equipped with autoinjectable units containing 2 mg of atropine plus 600 mg of the cholinesterase-reactivating agent pralidoxime.
Barthold CL et al: Organic phosphorus compounds nerve agents. Crit Care Clin 2005;21:673.
Leikin JB et al: A review of nerve agent exposure for the critical care physician. Crit Care Med 2002;30:2346.
P.1654
Chemical Warfare: Ricin
Ricin is a naturally occurring toxin found in minute quantities in the castor bean (Ricinus communis). It can cause toxicity if castor beans are thoroughly chewed or blenderized, although the quantity of ricin is small and it is poorly absorbed from the gastrointestinal tract, so symptoms following castor bean ingestion are usually limited to diarrhea and abdominal pain. Less commonly, severe gastroenteritis can lead to volume depletion and renal failure. On the other hand, purified ricin is extremely toxic if administered parenterally: the LD50 for injected ricin in animals is as low as 0.1 mcg/kg. A fatal case of suspected ricin poisoning by homicidal injection of an estimated 0.28 mg of ricin was associated with diffuse organ damage and death from cardiac failure after 2 days. Inhalation of ricin powder has not been reported in humans, but animal studies suggest it could cause hemorrhagic tracheobronchitis and pneumonia.
Treatment
A. Emergency and Supportive Measures
After suspected ricin inhalation or exposure to powdered ricin, remove clothing and wash skin with water. Personnel caring for such patients should wear protective respiratory gear, clothing, and gloves.
B. Specific Treatment
There is no known antidote or other specific treatment. Provide supportive care for volume loss due to gastroenteritis and cardiac and respiratory support as needed.
Audi J et al: Ricin poisoning: a comprehensive review. JAMA 2005;294:2342.
Doan LG: Ricin: mechanism of toxicity, clinical manifestations, and vaccine development. A review. J Toxicol Clin Toxicol 2004;42:201.
Chlorinated Insecticides
Lindane (Kwell) and other chlorinated insecticides (chlorophenothane [DDT], lindane, toxaphene, chlordane, aldrin, endrin) are central nervous system stimulants that can cause poisoning by ingestion, inhalation, or direct contact. Most of these agents have been removed from the United States market because of their acute toxicity and their potential to accumulate in the food chain. The estimated lethal dose is about 20 g for DDT, 3 g for lindane, 2 g for toxaphene, 1 g for chlordane, and less than 1 g for endrin and aldrin. The manifestations of poisoning are nervous irritability, muscle twitching, seizures, and coma. Arrhythmias may occur. Hepatic and renal damage are reported.
Treatment
Give activated charcoal (see p 1644) and consider gastric lavage for large recent ingestions (see p 1643). Repeat-dose activated charcoal may be effective for large ingestions. For seizures, give diazepam, 5 10 mg slowly intravenously, or other anticonvulsants as described on p 1642.
Perform thorough decontamination of exposed areas with repeated soap and shampoo washing. Personnel caring for such patients must wear protective clothing and gloves, since cutaneous absorption may occur through normal skin.
Forrester MB et al: Epidemiology of lindane exposures for pediculosis reported to Poison Centers in Texas, 1998 2002. J Toxicol Clin Toxicol 2004;42:55.
Clonidine & other Sympatholytic Antihypertensives
Overdosage with these agents (clonidine, guanabenz, guanfacine, methyldopa) causes bradycardia, hypotension, miosis, respiratory depression, and coma. (Transient hypertension occasionally occurs after clonidine overdosage, a result of peripheral -adrenergic effects of this drug in high doses.) Symptoms are usually resolved in less than 24 hours, and deaths are rare. Similar symptoms may occur after ingestion of topical nasal decongestants chemically similar to clonidine (oxymetazoline, tetrahydrozoline, naphazoline). Brimonidine is used as an ophthalmic preparation for glaucoma. Tizanidine is a centrally acting muscle relaxant structurally related to clonidine; it produces similar toxicity in overdose.
Treatment
A. Emergency and Supportive Measures
Give activated charcoal (see p 1644). Maintain the airway and support respiration if necessary. Symptomatic treatment is usually sufficient even in massive overdose. Maintain blood pressure with intravenous fluids. Dopamine can also be used. Atropine is usually effective for bradycardia.
B. Specific Treatment
There is no specific antidote. Although tolazoline has been recommended for clonidine overdose, its effects are unpredictable and it should not be used. Naloxone has been reported to be successful in a few anecdotal and poorly substantiated cases.
Spiller HA et al: Retrospective review of tizanidine (Zanaflex) overdose. J Toxicol Clin Toxicol 2004;42:593.
Spiller HA et al: Toxic clonidine ingestion in children. J Pediatr 2005;146:263.
Cocaine
See Amphetamines & Cocaine, above.
Cyanide
Cyanide is a highly toxic chemical used widely in research and commercial laboratories and many industries.
P.1655
Its gaseous form, hydrogen cyanide, is an important component of smoke in fires. Cyanide-generating glycosides are also found in the pits of apricots and other related plants. Cyanide is generated by the breakdown of nitroprusside, and poisoning can result from rapid high-dose infusions. Cyanide is also formed by metabolism of acetonitrile, a solvent found in some over-the-counter fingernail glue removers. Cyanide is rapidly absorbed by inhalation, skin absorption, or ingestion. It disrupts cellular function by inhibiting cytochrome oxidase and preventing cellular oxygen utilization.
Clinical Findings
The onset of toxicity is nearly instantaneous after inhalation of hydrogen cyanide gas but may be delayed for minutes to hours after ingestion of cyanide salts or cyanogenic plants or chemicals. Effects include headache, dizziness, nausea, abdominal pain, and anxiety, followed by confusion, syncope, shock, seizures, coma, and death. The odor of bitter almonds may be detected on the victim's breath or in vomitus, though this is not a reliable finding. The venous oxygen saturation may be elevated (> 90%) in severe poisonings because tissues have failed to take up arterial oxygen.
Treatment
A. Emergency and Supportive Measures
Remove the victim from exposure, taking care to avoid exposure to rescuers. For suspected cyanide poisoning due to nitroprusside infusion, stop or slow the rate of infusion. (Metabolic acidosis and other signs of cyanide poisoning usually clear rapidly.)
For cyanide ingestion, administer activated charcoal (see p 1644). Although charcoal has a low affinity for cyanide, the usual doses of 60 100 g are adequate to bind typically ingested lethal doses (100 200 mg).
B. Specific Treatment
In the United States, the cyanide antidote package (Taylor Pharmaceuticals) (Table 39-9) contains nitrites (to induce methemoglobinemia, which binds free cyanide) and thiosulfate (to promote conversion of cyanide to the less toxic thiocyanate). Administer amyl nitrite by crushing an ampule under the victim's nose or at the end of the endotracheal tube, and administer 3% sodium nitrite solution, 10 mL intravenously. Caution: Nitrites may induce hypotension and dangerous levels of methemoglobin. Also administer 25% sodium thiosulfate solution, 50 mL intravenously (12.5 g).
Table 39-9. Currently available (prepackaged) cyanide antidotes. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
Gracia R et al: Cyanide poisoning and its treatment. Pharmacotherapy 2004;24:1358.
Mannaioni G et al: Acute cyanide intoxication treated with a combination of hydroxycobalamin, sodium nitrite, and sodium thiosulfate. J Toxicol Clin Toxicol 2002;40:181.
Digitalis & other Cardiac Glycosides
Cardiac glycosides are derived from a variety of plants and are widely used to treat heart failure and supraventricular arrhythmias. These drugs paralyze the Na+-K+-ATPase pump and have potent vagotonic effects. Intracellular effects include enhancement of calcium-dependent contractility and shortening of the action potential duration. Digoxin and ouabain are highly tissue-bound, but digitoxin has a volume of distribution of just 0.6 L/kg, making it the only cardiac glycoside accessible to enhanced removal procedures such as hemoperfusion or repeated doses of activated charcoal. There are a number of plants (eg, oleander, foxglove, lily-of-the-valley) that contain cardiac glycosides. Bufotenin, a cardiotoxic steroid found in certain toad secretions and used as an herbal medicine and a purported aphrodisiac, has pharmacologic properties similar to cardiac glycosides.
Clinical Findings
Intoxication may result from acute single exposure or chronic accidental overmedication. After acute overdosage, nausea and vomiting, bradycardia, hyperkalemia, and AV block frequently occur. Patients in whom toxicity develops gradually during long-term therapy are often hypokalemic and hypomagnesemic owing to concurrent diuretic treatment and more commonly present with ventricular arrhythmias (eg, ectopy, bidirectional ventricular tachycardia, or ventricular fibrillation). Digoxin levels may be only slightly elevated in patients with intoxication from cardiac glycosides other than digoxin because of limited cross-reactivity of immunologic tests.
Treatment
A. Emergency and Supportive Measures
Maintain a patent airway and assist ventilation, if necessary. Monitor potassium levels and cardiac rhythm closely. Treat ventricular arrhythmias initially with lidocaine (2 3 mg/kg intravenously) or phenytoin (10 15
P.1656
mg/kg intravenously slowly over 30 minutes) and treat bradycardia initially with atropine (0.5 2 mg intravenously) or a transcutaneous external cardiac pacemaker.
After acute ingestion, administer activated charcoal (see p 1644).
B. Specific Treatment
For patients with significant intoxication, administer digoxin-specific antibodies (digoxin immune Fab [ovine]; Digibind or DigiFab). Estimation of the Digibind dose is based on the body burden of digoxin calculated from the ingested dose or the steady-state serum digoxin concentration:
1. From the ingested dose
Number of vials = approximately 1.5 % ingested dose (mg).
2. From the serum concentration
Number of vials = serum digoxin (ng/mL) % body weight (kg) % 10-2. Note: This is based on the equilibrium digoxin level; after acute overdose, serum levels are falsely high before tissue distribution is complete, and overestimation of the Digibind or DigiFab dose is likely.
3. Empiric dosing
Empiric dosing of Digibind or DigiFab may be used if the patient's condition is relatively stable and an underlying condition (eg, atrial fibrillation) suggests a residual level of digitalis activity. Start with one or two vials and reassess the clinical condition after 20 30 minutes. For cardiac glycosides other than digoxin or digitoxin, there is no formula for estimation of vials needed and treatment is empiric.
Note: After administration of digoxin-specific Fab antibody fragment, serum digoxin levels may be falsely elevated depending on the assay technique.
Barrueto F Jr et al: Cardioactive steroid poisoning from an herbal cleansing preparation. Ann Emerg Med 2003;41:396.
Bateman DN: Digoxin-specific antibody fragments: how much and when? Toxicol Rev 2004;23:135.
Husby P et al: Immediate control of life-threatening digoxin intoxication in a child by use of digoxin-specific antibody fragments (Fab). Paediatr Anaesth 2003;13:541.
Ethanol, Barbiturates, Benzodiazepines, & other Sedative-Hypnotic Agents
The group of agents known as sedative-hypnotic drugs includes a variety of products used for the treatment of anxiety, depression, insomnia, and epilepsy. Ethanol and other selected agents are also popular recreational drugs. All of these drugs depress the central nervous system reticular activating system, cerebral cortex, and cerebellum.
Clinical Findings
Mild intoxication produces euphoria, slurred speech, and ataxia. Ethanol intoxication may produce hypoglycemia, even at relatively low concentrations. With more severe intoxication, stupor, coma, and respiratory arrest may occur. Carisoprodol commonly causes muscle jerking or myoclonus. Death or serious morbidity is usually the result of pulmonary aspiration of gastric contents. Bradycardia, hypotension, and hypothermia are common. Patients with massive intoxication may appear to be dead, with no reflex responses and even absent electroencephalographic activity. Diagnosis and assessment of severity of intoxication are usually based on clinical findings. Ethanol serum levels greater than 300 mg/dL (0.3 g/dL; 65 mmol/L) usually produce coma in persons who are not chronically abusing the drug, but regular users may remain awake at much higher levels. Phenobarbital levels greater than 100 mg/L usually cause coma.
Treatment
A. Emergency and Supportive Measures
Administer activated charcoal (see p 1644). Repeat-dose charcoal may enhance elimination of phenobarbital, but it has not been proved to improve clinical outcome. Hemodialysis may be necessary for patients with severe phenobarbital intoxication.
B. Specific Treatment
Flumazenil is a benzodiazepine receptor-specific antagonist; it has no effect on ethanol, barbiturates, or other sedative-hypnotic agents. If used, flumazenil is given slowly intravenously, 0.2 mg over 30 60 seconds, repeated in 0.5 mg increments as needed up to a total dose of 3 5 mg. Caution: Flumazenil may induce seizures in patients with preexisting seizure disorder, benzodiazepine addiction, or concomitant tricyclic antidepressant overdose. If seizures occur, diazepam and other benzodiazepine anticonvulsants will not be effective. As with naloxone, the duration of action of flumazenil is short (2 3 hours) and resedation may occur, requiring repeated doses.
Isbister GK et al: Alprazolam is relatively more toxic than other benzodiazepines in overdose. Br J Clin Pharmacol 2004;58:88.
Olshaker JS et al: Flumazenil reversal of lorazepam-induced acute delirium. J Emerg Med 2003;24:181.
Seger DL: Flumazenil treatment or toxin. J Toxicol Clin Toxicol 2004;42:209.
-Hydroxybutyrate
GHB has become a popular drug of abuse. It originated as a short-acting general anesthetic and is occasionally used in the treatment of narcolepsy. It gained popularity among bodybuilders for its alleged growth hormone stimulation and found its way into social settings, where it is consumed as a liquid. It has been used to facilitate sexual assault ( date-rape drug). Symptoms after ingestion include drowsiness and lethargy followed by coma with respiratory depression. Muscle twitching and seizures are sometimes observed. Recovery is usually rapid, with patients awakening within a few hours. Other related chemicals with similar
P.1657
effects include butanediol and -butyrolactone (GBL). A prolonged withdrawal syndrome has been described in some heavy users.
Treatment
For recent ingestions, give activated charcoal orally or by gastric tube (see p 1644). There is no specific treatment. Most patients recover rapidly with supportive care. GHB withdrawal syndrome may require very large doses of benzodiazepines.
Anderson IB et al: Trends in gamma-hydroxybutyrate (GHB) intoxication: 1999 2003. Ann Emerg Med 2006;47:177.
Lora-Tamayo C et al: Intoxication due to 1,4-butanediol. Forensic Sci Int 2003;133:256.
Mason PE et al: Gamma hydroxybutyric acid (GHB) intoxication. Acad Emerg Med 2002;9:730.
Tarabar AF et al: The gamma-hydroxybutyrate withdrawal syndrome. Toxicol Rev 2004;23:45.
Iron
Iron is widely used therapeutically for the treatment of anemia and as a daily supplement in multiple vitamin preparations. Most children's preparations contain about 12 15 mg of elemental iron (as sulfate, gluconate, or fumarate salt) per dose, compared with 60 90 mg in most adult-strength preparations. Iron is corrosive to the gastrointestinal tract and, once absorbed, has depressant effects on the myocardium and on peripheral vascular resistance. Intracellular toxic effects of iron include disruption of Krebs cycle enzymes.
Clinical Findings
Ingestion of less than 30 mg/kg of elemental iron usually produces only mild gastrointestinal upset. Ingestion of more than 40 60 mg/kg may cause vomiting (sometimes with hematemesis), diarrhea, hypotension, and acidosis. Death may occur as a result of profound hypotension due to massive fluid losses and bleeding, metabolic acidosis, peritonitis from intestinal perforation, or sepsis. Fulminant hepatic failure may occur. Survivors of the acute ingestion may suffer permanent gastrointestinal scarring.
Serum iron levels greater than 350 500 mcg/dL are considered potentially toxic, and levels over 1000 mcg/dL are usually associated with severe poisoning. A plain abdominal x-ray may reveal radiopaque tablets.
Treatment
A. Emergency and Supportive Measures
Maintain a patent airway and assist ventilation if necessary. Treat hypotension aggressively with intravenous crystalloid solutions (0.9% saline or lactated Ringer's solution). Fluid losses may be massive owing to vomiting and diarrhea as well as third-spacing into injured intestine.
Perform whole bowel irrigation to remove unabsorbed pills from the intestinal tract (see p 1644). Activated charcoal is not effective but may be appropriate if other ingestants are suspected.
B. Specific Treatment
Deferoxamine is a selective iron chelator. It is not useful as an oral binding agent. For patients with established manifestations of toxicity and particularly those with markedly elevated serum iron levels (eg, greater than 800 1000 mcg/dL) administer 10 15 mg/kg/h by constant intravenous infusion; higher doses (up to 40 50 mg/kg/h) have been used in massive poisonings. Hypotension may occur. The presence of an iron-deferoxamine complex in the urine may give it a vin ros appearance. Deferoxamine is safe for use in pregnant women with acute iron overdose. Caution: Prolonged infusion of deferoxamine (> 36 48 hours) has been associated with development of acute respiratory distress syndrome (ARDS) the mechanism is not known.
Bar-Oz B et al: Medications that can be fatal for a toddler with one tablet or teaspoonful: a 2004 update. Paediatr Drugs 2004;6:123.
Manoguerra AS et al: Iron ingestion: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2005;43:553.
Isoniazid
Isoniazid (INH) is an antibacterial drug used mainly in the treatment and prevention of tuberculosis. It may cause hepatitis with long-term use, especially in alcoholic patients and elderly persons. It produces acute toxic effects by competing with pyridoxal 5-phosphate, resulting in lowered brain -aminobutyric acid (GABA) levels. Acute ingestion of as little as 1.5 2 g of INH can cause toxicity, and severe poisoning is likely to occur after ingestion of more than 80 100 mg/kg.
Clinical Findings
Confusion, slurred speech, and seizures may occur abruptly after acute overdose. Severe lactic acidosis out of proportion to the severity of seizures is probably due to inhibited metabolism of lactate. Peripheral neuropathy and acute hepatitis may occur with long-term use.
Diagnosis is based on a history of ingestion and the presence of severe acidosis associated with seizures. Isoniazid is not usually included in routine toxicologic screening, and serum levels are not readily available.
Treatment
A. Emergency and Supportive Measures
Seizures may require higher than usual doses of benzodiazepines (eg, lorazepam, 3 5 mg intravenously) or administration of pyridoxine as an antidote (see below).
Administer activated charcoal (see p 1644). Do not induce emesis, because of the risk of abrupt onset of seizures.
P.1658
B. Specific Treatment
Pyridoxine (vitamin B6) is a specific antagonist of the acute toxic effects of INH and is usually successful in controlling convulsions that do not respond to benzodiazepines. Give 5 g intravenously over 1 2 minutes or, if the amount ingested is known, give a gram-for-gram equivalent amount of pyridoxine. Patients taking INH are usually given 10 20 mg of pyridoxine orally daily to help prevent neuropathy.
Huang YS et al: Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 2003;37:924.
Topcu I et al: Seizures, metabolic acidosis and coma resulting from acute isoniazid intoxication. Anaesth Intensive Care 2005;33:518.
Lead
Lead is used in a variety of industrial and commercial products, such as storage batteries, solders, paints, pottery, plumbing, and gasoline and is found in some traditional Hispanic and Ayurvedic ethnic medicines. Lead toxicity usually results from chronic repeated exposure and is rare after a single ingestion. Lead produces a variety of adverse effects on cellular function and primarily affects the nervous system, gastrointestinal tract, and hematopoietic system.
Clinical Findings
Lead poisoning often goes undiagnosed initially because presenting symptoms and signs are nonspecific and exposure is not suspected. Common symptoms include colicky abdominal pain, constipation, headache, and irritability. Severe poisoning may cause coma and convulsions. Chronic intoxication can cause learning disorders (in children) and motor neuropathy (eg, wrist drop). Lead-containing bullet fragments in or near joint spaces can result in chronic lead toxicity.
Diagnosis is based on measurement of the blood lead level. Whole blood lead levels less than 10 mcg/dL are usually considered nontoxic. Levels between 10 and 25 mcg/dL have been associated with impaired neurobehavioral development in children. Levels of 25 50 mcg/dL may be associated with headache, irritability, and subclinical neuropathy. Levels of 50 70 mcg/dL are associated with moderate toxicity, and levels greater than 70 100 mcg/dL are often associated with severe poisoning. Other laboratory findings of lead poisoning include microcytic anemia with basophilic stippling and elevated free erythrocyte protoporphyrin.
Treatment
A. Emergency and Supportive Measures
For patients with encephalopathy, maintain a patent airway and treat coma and convulsions as described at the beginning of this chapter.
For recent acute ingestion, if a large lead-containing object (eg, fishing weight) is still visible in the stomach on abdominal x-ray, repeated cathartics (see p 1644), whole bowel irrigation (see p 1644), endoscopy, or even surgical removal may be necessary to prevent subacute lead poisoning. (The acidic gastric contents may corrode the metal surface, enhancing lead absorption. Once the object passes into the small intestine, the risk of toxicity declines.)
Conduct an investigation into the source of the lead exposure.
Workers with a single lead level greater than 60 mcg/dL (or three successive monthly levels greater than 50 mcg/dL) or construction workers with any single blood lead level greater than 50 mcg/dL must by federal law be removed from the site of exposure. Contact the regional office of the United States Occupational Safety and Health Administration (OSHA) for more information. Several states mandate reporting of cases of confirmed lead poisoning.
B. Specific Treatment
The indications for chelation depend on the blood lead level and the patient's clinical state. A medical toxicologist or regional poison control center (800 222-1222) should be consulted for advice about selection and use of these antidotes.
Note: It is impermissible under the law to treat asymptomatic workers with elevated blood lead levels in order to keep their levels under 50 mcg/dL rather than remove them from the exposure.
1. Severe toxicity
Patients with severe intoxication (encephalopathy or levels greater than 70 100 mcg/dL) should receive edetate calcium disodium (ethylenediaminetetraacetic acid, EDTA), 1500 mg/m2/kg/d (approximately 50 mg/kg/d) in four to six divided doses or as a continuous intravenous infusion. Some clinicians also add dimercaprol (BAL), 4 5 mg/kg intramuscularly every 4 hours for 5 days.
2. Less severe toxicity
Patients with less severe symptoms and asymptomatic patients with blood lead levels between 55 and 69 mcg/dL may be treated with edetate calcium disodium alone in dosages as above. An oral chelator, succimer (DMSA), is available for use in patients with mild to moderate intoxication. The usual dose is 10 mg/kg orally every 8 hours for 5 days, then every 12 hours for 2 weeks.
Brewster UC et al: A review of chronic lead intoxication: an unrecognized cause of chronic kidney disease. Am J Med Sci 2004;327:341.
Needleman H: Lead poisoning. Annu Rev Med 2004;55:209.
Weide R et al: Severe lead poisoning due to Ayurvedic Indian plant medicine. Dtsch Med Wochenschr 2003;128:2418.
Lsd & other Hallucinogens
A variety of substances ranging from naturally occurring plants and mushrooms to synthetic substances
P.1659
such as PCP, toluene and other solvents, and lysergic acid diethylamide (LSD) are abused for their hallucinogenic properties. The mechanism of toxicity and the clinical effects vary for each substance.
Many hallucinogenic plants and mushrooms produce anticholinergic delirium, characterized by flushed skin, dry mucous membranes, dilated pupils, tachycardia, and urinary retention. Other plants and mushrooms may contain hallucinogenic indoles such as mescaline and LSD, which typically cause marked visual hallucinations and perceptual distortion, widely dilated pupils, and mild tachycardia. PCP, a dissociative anesthetic agent similar to ketamine, can produce fluctuating delirium and coma, often associated with vertical and horizontal nystagmus. Toluene and other hydrocarbon solvents (butane, trichloroethylene, chemo, etc) cause euphoria and delirium and may sensitize the myocardium to the effects of catecholamines, leading to fatal dysrhythmias.
Treatment
A. Emergency and Supportive Measures
Maintain a patent airway and assist respirations if necessary. Treat coma, hyperthermia, and seizures as outlined at the beginning of this chapter. For recent large ingestions, consider giving activated charcoal orally or by gastric tube.
B. Specific Treatment
Patients with anticholinergic delirium may benefit from a dose of physostigmine, 0.5 1 mg intravenously, not to exceed 1 mg/min. Dysphoria, agitation, and psychosis associated with LSD or mescaline intoxication may respond to benzodiazepines (eg, lorazepam, 1 2 mg orally or intravenously) or haloperidol (2 5 mg intramuscularly or intravenously). Monitor patients who have sniffed solvents for cardiac dysrhythmias (most commonly premature ventricular contractions, ventricular tachycardia, ventricular fibrillation); treatment with -blockers such as propranolol (1 5 mg intravenously) or esmolol (250 500 mcg/kg intravenously, then 50 mcg/kg/min by infusion) may be more effective than lidocaine.
Gertsch JH et al: Case report: an ingestion of Hawaiian Baby Woodrose seeds associated with acute psychosis. Hawaii Med J 2003;62:127.
Tang HL: Renal tubular acidosis and severe hypophosphataemia due to toluene inhalation. Hong Kong Med J 2005;11:1:50.
Mercury
Acute mercury poisoning usually occurs by ingestion of inorganic mercuric salts or inhalation of metallic mercury vapor. Ingestion of the mercuric salts causes a burning sensation in the throat, discoloration and edema of oral mucous membranes, abdominal pain, vomiting, bloody diarrhea, and shock. Direct nephrotoxicity causes acute renal failure. Inhalation of high concentrations of metallic mercury vapor may cause acute fulminant chemical pneumonia. Chronic mercury poisoning causes weakness, ataxia, intention tremors, irritability, and depression. Exposure to alkyl (organic) mercury derivatives from contaminated fish or fungicides used on seeds has caused ataxia, tremors, convulsions, and catastrophic birth defects.
Treatment
A. Acute Poisoning
There is no effective specific treatment for mercury vapor pneumonitis. Remove ingested mercuric salts by lavage, and administer activated charcoal (see p 1644). For acute ingestion of mercuric salts, give dimercaprol (BAL) at once, as for arsenic poisoning. Unless the patient has severe gastroenteritis, consider succimer (DMSA), 10 mg/kg orally every 8 hours for 5 days and then every 12 hours for 2 weeks. Unithiol is a chelator that can be given orally or parenterally, but is not commonly available In the United States. Maintain urinary output. Treat oliguria and anuria if they occur.
B. Chronic Poisoning
Remove from exposure. Neurologic toxicity is not considered reversible with chelation, although some authors recommend a trial of succimer or uniothiol (contact a regional poison center or medical toxicologist for advice).
Johnson CL: Mercury in the environment: sources, toxicities, and prevention of exposure. Pediatr Ann 2004;33:437.
Saper RB et al: Heavy metal content of ayurvedic herbal medicine products. JAMA 2004;292:2868.
Weil M et al: Blood mercury levels and neurobehavioral function. JAMA 2005;293:1875.
Wilson JF: Balancing the risks and benefits of fish consumption. Ann Intern Med 2004;141:977.
Methanol & Ethylene Glycol
Methanol (wood alcohol) is commonly found in a variety of products, including solvents, duplicating fluids, record cleaning solutions, and paint removers. It is sometimes ingested intentionally by alcoholic patients as a substitute for ethanol and may also be found as a contaminant in bootleg whiskey. Ethylene glycol is the major constituent in most antifreeze compounds. The toxicity of both agents is caused by metabolism to highly toxic organic acids methanol to formic acid; ethylene glycol to glycolic and oxalic acids.
Clinical Findings
Shortly after ingestion of either of these agents, patients usually appear drunk. The serum osmolality (measured with the freezing point device) is usually increased, but acidosis is often absent early. After several
P.1660
hours, metabolism to toxic organic acids leads to a severe anion gap metabolic acidosis, tachypnea, confusion, convulsions, and coma. Methanol intoxication frequently causes visual disturbances, while ethylene glycol often produces oxalate crystalluria and renal failure.
Treatment
A. Emergency and Supportive Measures
For patients presenting within 30 60 minutes after ingestion, empty the stomach by gastric lavage (see p 1643). Charcoal is not very effective but should be administered if other poisons or drugs have also been ingested.
B. Specific Treatment
Patients with significant toxicity (manifested by severe metabolic acidosis, altered mental status, serum methanol or ethylene glycol level > 50 mg/dL, or osmolar gap > 10 mosm/L) should undergo hemodialysis as soon as possible to remove the parent compound and the toxic metabolites. Treatment with folic acid, thiamine, and pyridoxine may enhance the breakdown of toxic metabolites.
Ethanol blocks metabolism of the parent compounds by competing for the enzyme alcohol dehydrogenase. The desired serum ethanol concentration is 100 mg/dL. To achieve this, administer a loading dose of approximately 750 mg/kg orally or in a dilute intravenous solution (available from the pharmacy in 5% and 10% solution), and then provide a maintenance infusion of 100 150 mg/kg/h. The infusion will have to be increased to about 175 250 mg/kg/h during hemodialysis to replace dialysis elimination of ethanol. Fomepizole (4-methylpyrazole; Antizol) blocks alcohol dehydrogenase and can be used instead of ethanol. A regional poison control center (800 222-1222) should be contacted for indications and dosing.
Megarbane B et al: Current recommendations for treatment of severe toxic alcohol poisonings. Intensive Care Med 2005;31:189. Epub 2004 Dec 31.
Mycyk MB et al: Antidote review: fomepizole for methanol poisoning. Am J Ther 2003;10:68.
Methemoglobinemia-Inducing Agents
A large number of chemical agents are capable of oxidizing ferrous hemoglobin to its ferric state (methemoglobin), a form that cannot carry oxygen. Drugs and chemicals known to cause methemoglobinemia include benzocaine (a local anesthetic found in some topical anesthetic sprays and a variety of nonprescription products), aniline, nitrites, nitrogen oxide gases, nitrobenzene, dapsone, phenazopyridine (Pyridium), and many others. Dapsone has a long elimination half-life and may produce prolonged or recurrent methemoglobinemia.
Clinical Findings
Methemoglobinemia reduces oxygen-carrying capacity and may cause dizziness, nausea, headache, dyspnea, confusion, seizures, and coma. The severity of symptoms depends on the percentage of hemoglobin oxidized to methemoglobin; severe poisoning is usually present when methemoglobin fractions are greater than 40 50%. Even at low levels (15 20%), victims appear cyanotic because of the chocolate brown color of methemoglobin, but they have normal PO2 results on arterial blood gas determinations. Pulse oximetry gives inaccurate oxygen saturation measurements; the reading is often between 85% and 90%. Severe metabolic acidosis may be present. Hemolysis may occur, especially in patients susceptible to oxidant stress (ie, those with glucose-6-phosphate dehydrogenase deficiency).
Treatment
A. Emergency and Supportive Measures
Administer high-flow oxygen. If the causative agent was recently ingested, administer activated charcoal (see p 1644). Repeat-dose activated charcoal may enhance dapsone elimination (see p 1645).
B. Specific Treatment
Methylene blue enhances the conversion of methemoglobin to hemoglobin by increasing the activity of the enzyme methemoglobin reductase. For symptomatic patients, administer 1 2 mg/kg (0.1 0.2 mL/kg of 1% solution) intravenously. The dose may be repeated once in 15 20 minutes if necessary. Patients with hereditary methemoglobin reductase deficiency or glucose-6-phosphate dehydrogenase deficiency may not respond to methylene blue treatment.
Armstrong C et al: Benzocaine-induced methemoglobinemia: a condition of which all endoscopists should be aware. Can J Gastroenterol 2004;18:625.
Bradberry SM: Occupational methaemoglobinaemia. Mechanisms of production, features, diagnosis and management including the use of methylene blue. Toxicol Rev 2003;22:13.
Monoamine Oxidase Inhibitors
Overdoses of MAO inhibitors (isocarboxazid, phenelzine, selegiline, moclobemide) cause ataxia, excitement, hypertension, and tachycardia, followed several hours later by hypotension, convulsions, and hyperthermia.
Table 39-10. Poisonous mushrooms. | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ingestion of tyramine-containing foods may cause a severe hypertensive reaction in patients taking MAO inhibitors. Foods containing tyramine include aged cheese and red wines. Hypertensive reactions may also occur with any sympathomimetic drug. Severe or fatal hyperthermia (serotonin syndrome) may occur if patients receiving MAO inhibitors are given meperidine, fluoxetine, paroxetine, fluvoxamine, venlafaxine, tryptophan, dextromethorphan,
P.1661
tramadol, or other serotonin-enhancing drugs. This reaction can also occur with the newer selective MAO inhibitor moclobemide, and the antibiotic linezolid, which has MAO-inhibiting properties. The serotonin syndrome has also been reported in patients taking selective serotonin reuptake inhibitors (SSRIs) in large doses or in combination with other SSRIs, even in the absence of an MAO inhibitor or meperidine.
Treatment
Administer activated charcoal (see p 1644). Treat severe hypertension with nitroprusside, phentolamine, or other rapid-acting vasodilators (see p 1641). Treat hypotension with fluids and positioning, but avoid use of pressor agents if possible. Observe patients for at least 24 hours, since hyperthermic reactions may be delayed. Treat hyperthermia with aggressive cooling; neuromuscular paralysis may be required (see p 1642). Cyproheptadine, 4 mg orally (or by gastric tube) every hour for three or four doses, has been reported to be effective against serotonin syndrome.
Boyer EW et al: The serotonin syndrome. N Engl J Med 2005;352:1112.
Gillman PK: Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth 2005;95:434. Epub 2005 Jul 28.
Mushrooms
There are thousands of mushroom species that cause a variety of toxic effects. The most dangerous species of mushrooms are Amanita phalloides, Amanita verna, Amanita virosa, Gyromitra esculenta, and the Galerina species, all of which contain amatoxin, a potent cytotoxin. Ingestion of even a portion of one mushroom of a dangerous species may be sufficient to cause death.
The characteristic pathologic finding in fatalities from amatoxin-containing mushroom poisoning is acute massive necrosis of the liver.
Clinical Findings (Table 39-10)
A. Symptoms and Signs
1. Amatoxin-type cyclopeptides
(A phalloides, A verna, A virosa, and Galerina species.) After a latent interval of 8 12 hours, severe abdominal cramps and vomiting begin and progress to profuse diarrhea, followed in 1 2 days by hepatic necrosis, hepatic encephalopathy, and frequently renal failure. The fatality rate is about 20%. Cooking the mushrooms does not prevent poisoning.
2. Gyromitrin type
(Gyromitra and Helvella species.) Toxicity is more common following ingestion of uncooked mushrooms. Vomiting, diarrhea, hepatic necrosis, convulsions, coma, and hemolysis may occur
P.1662
after a latent period of 8 12 hours. The fatality rate is probably less than 10%.
3. Muscarinic type
(Inocybe and Clitocybe species.) Vomiting, diarrhea, bradycardia, hypotension, salivation, miosis, bronchospasm, and lacrimation occur shortly after ingestion. Cardiac arrhythmias may occur. Fatalities are rare.
4. Anticholinergic type
(Eg, Amanita muscaria, Amanita pantherina.) This type causes a variety of symptoms that may be atropine-like, including excitement, delirium, flushed skin, dilated pupils, and muscular jerking tremors, beginning 1 2 hours after ingestion. Fatalities are rare.
5. Gastrointestinal irritant type
(Eg, Boletus, Cantharellus.) Nausea, vomiting, and diarrhea occur shortly after ingestion. Fatalities are rare.
6. Disulfiram type
(Coprinus species.) Disulfiram-like sensitivity to alcohol may persist for several days. Toxicity is characterized by flushing, hypotension, and vomiting after coingestion of alcohol.
7. Hallucinogenic
(Psilocybe and Panaeolus species.) Mydriasis, nausea and vomiting, and intense visual hallucinations occur 1 2 hours after ingestion. Fatalities are rare.
8. Cortinarius orellanus
This mushroom may cause acute renal failure due to tubulointerstitial nephritis. Amanita smithiana has also been reported to cause acute renal failure.
Treatment
A. Emergency Measures
After the onset of symptoms, efforts to remove the toxic agent are probably useless, especially in cases of amatoxin or gyromitrin poisoning, where there is usually a delay of 12 hours or more before symptoms occur and patients seek medical attention. However, induction of vomiting or administration of activated charcoal is recommended for any recent ingestion of an unidentified or potentially toxic mushroom (see p 1644).
B. General Measures
1. Amatoxin-type cyclopeptides
A variety of antidotes (eg, thioctic acid, penicillin, corticosteroids) have been suggested for amatoxin-type mushroom poisoning, but controlled studies are lacking and experimental data in animals are equivocal. Aggressive fluid replacement for diarrhea and intensive supportive care for hepatic failure are the mainstays of treatment. Silymarin (a derivative of milk thistle) is commonly used in Europe (20 mg/kg over 24 hours given in four 2-hour infusions) and has recently become available in the United States (Apothecare, 1 800-969 6601).
Interruption of enterohepatic circulation of the amatoxin by the administration of activated charcoal and laxatives may be of value. However, by the time this method is used, most of the amatoxin has already caused cellular damage and has already been excreted. Charcoal hemoperfusion has been recommended but is of unproved value.
Liver transplant may be the only hope for survival in gravely ill patients contact a liver transplant center early.
2. Gyromitrin type
For gyromitrin poisoning, give pyridoxine, 25 mg/kg intravenously.
3. Muscarinic type
For mushrooms producing predominantly muscarinic-cholinergic symptoms, give atropine, 0.005 0.01 mg/kg intravenously, and repeat as needed.
4. Anticholinergic type
For anticholinergic type, physostigmine, 0.5 1 mg intravenously, may calm extremely agitated patients and reverse peripheral anticholinergic manifestations, but it may also cause bradycardia, asystole, and seizures. Alternately, use a benzodiazepine such as lorazepam, 1 2 mg intravenously.
5. Gastrointestinal irritant type
Treat with antiemetics and intravenous or oral fluids.
6. Disulfiram type
For Coprinus ingestion, avoid alcohol. Treat alcohol reaction with fluids and supine position.
7. Hallucinogenic type
Provide a quiet, supportive atmosphere. Diazepam or haloperidol may be used for sedation.
8. Cortinarius
Provide supportive care and hemodialysis as needed for renal failure.
Diaz JH: Syndromic diagnosis and management of confirmed mushroom poisonings. Crit Care Med 2005;33:427.
Enjalbert F et al: Treatment of amatoxin poisoning: 20-year retrospective analysis. J Toxicol Clin Toxicol 2002;40:715.
Ganzert M et al: Indication of liver transplantation following amatoxin intoxication. J Hepatol 2005;42:202.
Opioids
Prescription and illicit opioids (morphine, heroin, codeine, oxycodone, propoxyphene, etc) are popular drugs of abuse and the cause of frequent hospitalizations for overdose. These drugs have widely varying potencies and durations of action; for example, some of the illicit fentanyl derivatives are up to 2000 times more potent than morphine. All of these agents decrease central nervous system activity and sympathetic outflow by acting on opiate receptors in the brain. Tramadol is a newer analgesic that is unrelated chemically to the opioids but acts on opioid receptors. Buprenorphine is a partial agonist-antagonist opioid recently introduced for the outpatient treatment of opioid addiction.
Clinical Findings
Mild intoxication is characterized by euphoria, drowsiness, and constricted pupils. More severe intoxication
P.1663
may cause hypotension, bradycardia, hypothermia, coma, and respiratory arrest. Pulmonary edema may occur. Death is usually due to apnea or pulmonary aspiration of gastric contents. Propoxyphene may cause seizures and prolongation of the QRS interval. Methadone has been associated with QT interval prolongation and torsade de pointes. Tramadol, dextromethorphan, and meperidine also occasionally cause seizures. With meperidine, the metabolite normeperidine is probably the cause of seizures and is most likely to accumulate with repeated dosing in patients with renal insufficiency. While the duration of effect for heroin is usually 3 5 hours, methadone intoxication may last for 48 72 hours or longer. Most opioids, with the exception of illicit newer fentanyl derivatives, tramadol, oxycodone, and methadone, are usually detectable on routine urine toxicology screening. Wound botulism has been associated with skin-popping, especially involving black tar heroin. Buprenorphine added to an opioid regimen may produce acute narcotic withdrawal symptoms.
Treatment
A. Emergency and Supportive Measures
Protect the airway and assist ventilation. Administer activated charcoal (see p 1644).
B. Specific Treatment
Naloxone is a specific opioid antagonist that can rapidly reverse signs of narcotic intoxication. Although it is structurally related to the opioids, it has no agonist effects of its own. Administer 0.4 2 mg intravenously, and repeat as needed to awaken the patient and maintain airway protective reflexes and spontaneous breathing. Very large doses (10 20 mg) may be required for patients intoxicated by some opioids (eg, propoxyphene, codeine, fentanyl derivatives). Caution: The duration of effect of naloxone is only about 2 3 hours; repeated doses may be necessary for patients intoxicated by long-acting drugs such as methadone. Continuous observation for at least 3 hours after the last naloxone dose is mandatory.
Buajordet I et al: Adverse events after naloxone treatment of episodes of suspected acute opioid overdose. Eur J Emerg Med 2004;11:19.
Clarke SF et al: Naloxone in opioid poisoning: walking the tightrope. Emerg Med J 2005;22:612.
Sporer KA: Buprenorphine: a primer for emergency physicians. Ann Emerg Med 2004;43:580.
Tharp AM et al: Fatal intravenous fentanyl abuse: four cases involving extraction of fentanyl from transdermal patches. Am J Forensic Med Pathol 2004;25:178.
Paraquat
Paraquat is used as a herbicide. Concentrated solutions of paraquat are highly corrosive to the oropharynx, esophagus, and stomach. The fatal dose after absorption may be as small as 4 mg/kg. If ingestion of paraquat is not rapidly fatal because of its corrosive effects, the herbicide may cause progressive pulmonary fibrosis, with death ensuing after 2 3 weeks. Patients with plasma paraquat levels above 2 mg/L at 6 hours or 0.2 mg/L at 24 hours are likely to die.
Treatment
Remove ingested paraquat by immediate induced emesis, or by gastric lavage if the patient is already in a health care facility (see p 1643). Clay (bentonite or fuller's earth) and activated charcoal are effective adsorbents. Administer repeated doses of 60 g of activated charcoal by gastric tube every 2 hours for at least three or four doses. Charcoal hemoperfusion, 8 hours per day for 2 3 weeks, has been anecdotally reported to be lifesaving, but clinical and animal studies are equivocal. Supplemental oxygen should be withheld unless the PO2 is less than 70 mm Hg because oxygen may contribute to the pulmonary damage, which is mediated through lipid peroxidation.
Eddleston M et al: Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: a systematic review. QJM 2003;96:809.
Sittipunt C: Paraquat poisoning. Respir Care 2005;50:383.
Pesticides: Cholinesterase Inhibitors
Organophosphorus and carbamate insecticides (organophosphates: parathion, malathion, etc; carbamates: carbaryl, aldicarb, etc) are widely used in commercial agriculture and home gardening and have largely replaced older, more environmentally persistent organochlorine compounds such as DDT and chlordane. The organophosphates and carbamates also called anticholinesterases because they inhibit the enzyme acetylcholinesterase cause an increase in acetylcholine activity at nicotinic and muscarinic receptors and in the central nervous system. There are a variety of chemical agents in this group, with widely varying potencies. Most of them are poorly water-soluble, are formulated with an aromatic hydrocarbon solvent such as xylene, and are well absorbed through intact skin. Most chemical warfare nerve agents (see above) are organophosphates.
Clinical Findings
Inhibition of cholinesterase results in abdominal cramps, diarrhea, vomiting, excessive salivation, sweating, lacrimation, miosis (constricted pupils), wheezing and bronchorrhea, seizures, and skeletal muscle weakness. Initial tachycardia is usually followed by bradycardia. Profound skeletal muscle weakness, aggravated by excessive bronchial secretions and wheezing, may result in respiratory arrest and death. Symptoms and
P.1664
signs of poisoning may persist or recur over several days, especially with highly lipid-soluble agents such as fenthion or dimethoate.
The diagnosis should be suspected in patients who present with miosis, sweating, and hyperperistalsis. Serum and red blood cell cholinesterase activity can be measured in the laboratory and is usually depressed at least 50% below baseline in those victims who have severe intoxication.
Treatment
A. Emergency and Supportive Measures
If the agent was recently ingested, empty the stomach by gastric lavage and administer activated charcoal (see p 1643). If the agent is on the victim's skin or hair, wash repeatedly with soap or shampoo and water. Providers must take care to avoid skin exposure by wearing gloves and waterproof aprons. Dilute hypochlorite solution (eg, household bleach diluted 1:10) is reported to help break down organophosphate pesticides and nerve agents.
B. Specific Treatment
Atropine reverses excessive muscarinic stimulation and is effective for treatment of salivation, wheezing, abdominal cramping, and sweating. However, it does not interact with nicotinic receptors at autonomic ganglia and at the neuromuscular junction and has no effect on muscle weakness. Administer 2 mg intravenously, and give repeated doses as needed to dry bronchial secretions and decrease wheezing; as much as several hundred milligrams of atropine has been given to treat severe poisoning.
Pralidoxime (2-PAM, Protopam) is a specific antidote that reverses organophosphate binding to the cholinesterase enzyme; therefore, it is effective at the neuromuscular junction as well as other nicotinic and muscarinic sites. It should be started as soon as possible, to prevent permanent binding of the organophosphate to cholinesterase. Administer 1 2 g intravenously as a loading dose, and begin a continuous infusion (200 500 mg/h, titrated to clinical response). Constant infusion is more effective because of the short duration of action of single doses. Continue to give pralidoxime as long as there is any evidence of acetylcholine excess. Pralidoxime is of questionable benefit for carbamate poisoning, because carbamates have only a transitory effect on the cholinesterase enzyme. High-dose sodium bicarbonate (5 mEq/kg intravenously over 5 minutes) has been reported effective although the mechanism is unclear and the treatment has not been widely adopted.
Balali-Mood M et al: Effect of high doses of sodium bicarbonate in acute organophosphorous pesticide poisoning. Clin Toxicol (Phila) 2005;43:571.
Buckley NA et al: Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev 2005;CD005085.
Petroleum Distillates & Solvents
Petroleum distillate toxicity may occur from inhalation of the vapor or as a result of pulmonary aspiration of the liquid during or after ingestion. Acute manifestations of aspiration pneumonitis are vomiting, coughing, and bronchopneumonia. Some hydrocarbons ie, those with aromatic or halogenated subunits can also cause severe systemic poisoning after oral ingestion (Table 39-11). Hydrocarbons can also cause systemic intoxication by inhalation. Vertigo, muscular incoordination, irregular pulse, myoclonus, and seizures occur with serious poisoning and may be due to hypoxemia or the systemic effects of the agents. Chlorinated and fluorinated hydrocarbons (trichloroethylene, freons, etc) and many other hydrocarbons can cause ventricular arrhythmias due to increased sensitivity of the myocardium to the effects of endogenous catecholamines.
Treatment (Table 39-11)
Remove the patient to fresh air. Since aspiration is the primary danger after ingestion of many common products, use of lavage or emesis is not recommended; administration of activated charcoal may be helpful if the preparation contains toxic solutes (eg, an insecticide) or is an aromatic or halogenated product. Observe the victim for 6 8 hours for signs of aspiration pneumonitis (cough, localized rales or rhonchi, tachypnea, and infiltrates on chest radiograph). Corticosteroids are not recommended. If fever occurs, give a specific antibiotic only after identification of bacterial pathogens by laboratory studies. Because of the risk of arrhythmias, use bronchodilators with caution in patients with chlorinated or fluorinated solvent intoxication.
Finch CK et al: Acute inhalant-induced neurotoxicity with delayed recovery. Ann Pharmacother 2005;39:169. Epub 2004 Dec 8.
Harris D et al: Butane encephalopathy. Emerg Med J 2005;22:676.
Phenothiazines & other Antipsychotic Agents
Promethazine, prochlorperazine, chlorpromazine, haloperidol, droperidol, risperidone, olanzapine, ziprasidone, quetiapine, and aripiprazole are used as antiemetics and antipsychotic agents and as potentiators of analgesic and hypnotic drugs.
Table 39-11. Clinical features of hydrocarbon poisoning. | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Phenothiazines (particularly chlorpromazine) induce drowsiness and mild orthostatic hypotension in as many as 50% of patients. Larger doses can cause obtundation, miosis, severe hypotension, tachycardia, convulsions, and coma. Abnormal cardiac conduction may occur, resulting in prolongation of QRS or QT intervals (or both) and ventricular arrhythmias. Droperidol now has a black box warning about prolonged QT interval and the risk of torsade de pointes.
With therapeutic or toxic doses, an acute extrapyramidal dystonic reaction similar to Parkinson's
P.1665
disease may develop in some patients, with spasmodic contractions of the face and neck muscles, extensor rigidity of the back muscles, carpopedal spasm, and motor restlessness. This reaction is more common with haloperidol and the butyrophenones and less common with newer atypical antipsychotics such as ziprasidone, olanzapine, and quetiapine. Severe rigidity accompanied by hyperthermia and metabolic acidosis ( neuroleptic malignant syndrome ) may occasionally occur and is life-threatening (see Chapter 25).
Treatment
A. Emergency and Supportive Measures
Administer activated charcoal. For severe hypotension, treatment with fluids and pressor agents may be necessary. Treat hyperthermia as outlined on p 1642. Maintain cardiac monitoring.
P.1666
B. Specific Treatment
Hypotension and cardiac arrhythmias associated with widened QRS intervals on the ECG in a patient with thioridazine poisoning may respond to intravenous sodium bicarbonate as used for tricyclic antidepressants. Prolongation of the QT interval and torsade de pointes is usually treated with intravenous magnesium or overdrive pacing.
For extrapyramidal signs, give diphenhydramine, 0.5 1 mg/kg intravenously, or benztropine mesylate, 0.01 0.02 mg/kg intramuscularly. Treatment with oral doses of these agents should be continued for 24 48 hours.
Bromocriptine (2.5 7.5 mg orally daily) may be effective for mild or moderate neuroleptic malignant syndrome. Dantrolene (2 5 mg/kg intravenously) has also been used for muscle contractions but is not a true antidote.
Carstairs SD et al: Overdose of aripiprazole, a new type of antipsychotic. J Emerg Med 2005;28:311.
Palenzona S et al: The clinical picture of olanzapine poisoning with special reference to fluctuating mental status. J Toxicol Clin Toxicol 2004;42:27.
Strachan EM et al: Electrocardiogram and cardiovascular changes in thioridazine and chlorpromazine poisoning. Eur J Clin Pharmacol 2004;60:541. Epub 2004 Sep 15.
Quinidine & Related Antiarrhythmics
Quinidine, procainamide, and disopyramide are class Ia antiarrhythmic agents, and flecainide and propafenone are class Ic agents. These drugs have membrane-depressant effects on the sodium-dependent channel responsible for cardiac cell depolarization. Manifestations of cardiotoxicity include arrhythmias, syncope, hypotension, and widening of the QRS complex on the ECG (> 100 120 ms). With type Ia drugs, a lengthened QT interval and atypical or polymorphous ventricular tachycardia (torsade de pointes) may occur. The antimalarials chloroquine and hydroxychloroquine have similar effects in overdose.
Treatment
A. Emergency and Supportive Measures
Administer activated charcoal (see p 1644); consider gastric lavage after large recent overdose. Assist ventilation if needed. Perform continuous cardiac monitoring.
B. Specific Treatment
Treat cardiotoxicity (hypotension, QRS interval widening) with intravenous boluses of sodium bicarbonate, 50 100 mEq. Ventricular tachycardia of the torsade de pointes variety may be treated with intravenous magnesium or overdrive pacing.
Clarot F et al: Fatal propafenone overdoses: case reports and a review of the literature. J Anal Toxicol 2003;27:595.
Messant I: Massive chloroquine intoxication: importance of early treatment and pre-hospital treatment. Resuscitation 2004;60:343.
Salicylates
Salicylates (aspirin, methyl salicylate, etc) are found in a variety of over-the-counter and prescription medications. Salicylates uncouple cellular oxidative phosphorylation, resulting in anaerobic metabolism and excessive production of lactic acid and heat, and they also interfere with several Krebs cycle enzymes. A single ingestion of more than 200 mg/kg of salicylate is likely to produce significant acute intoxication. Poisoning may also occur as a result of chronic excessive dosing over several days. Although the half-life of salicylate is 2 3 hours after small doses, it may increase to 20 hours or more in patients with intoxication.
Clinical Findings
Acute ingestion often causes nausea and vomiting, occasionally with gastritis. Moderate intoxication is characterized by hyperpnea (deep and rapid breathing), tachycardia, tinnitus, and elevated anion gap metabolic acidosis. Serious intoxication may result in agitation, confusion, coma, seizures, cardiovascular collapse, pulmonary edema, hyperthermia, and death. The prothrombin time is often elevated owing to salicylate-induced hypoprothrombinemia.
Diagnosis is suspected in any patient with metabolic acidosis and is confirmed by measuring the serum salicylate level. Patients with levels greater than 100 mg/dL (1000 mg/L) after an acute overdose are more likely to have severe poisoning. On the other hand, patients with subacute or chronic intoxication may suffer severe symptoms with levels of only 60 70 mg/dL. The arterial blood gas typically reveals a respiratory alkalosis with an underlying metabolic acidosis.
Treatment
A. Emergency and Supportive Measures
Administer activated charcoal (see p 1644). Gastric lavage followed by administration of extra doses of activated charcoal may be needed in patients who ingest more than 10 g of aspirin (see p 1643). The desired ratio of charcoal to aspirin is about 10:1 by weight; while this cannot always be given as a single dose, it may be administered over the first 24 hours in divided doses every 2 4 hours. Treat metabolic acidosis with intravenous sodium bicarbonate. This is critical because acidosis (especially acidemia, pH < 7.40) promotes greater entry of salicylate into cells, worsening toxicity. Brief hypoventilation during rapid sequence intubation may cause sudden and severe deterioration if the pH is allowed to fall.
B. Specific Treatment
Alkalinization of the urine enhances renal salicylate excretion by trapping the salicylate anion in the urine. Add 100 mEq (two ampules) of sodium bicarbonate to 1 L of 5% dextrose in 0.2% saline, and infuse this solution intravenously at a rate of about 150 200 mL/h. Unless the patient is oliguric, add 20 30 mEq of potassium chloride to each liter of intravenous fluid. Patients who are volume-depleted often fail to produce an alkaline urine (paradoxical aciduria) unless potassium is given.
Hemodialysis may be lifesaving and is indicated for patients with severe metabolic acidosis, markedly altered mental status, or significantly elevated salicylate levels (eg, > 100 120 mg/dL [1000 1200 mg/L] after acute overdose or > 60 70 mg/dL [600 700 mg/L] with subacute or chronic intoxication).
Parker D et al: The analysis of methyl salicylate and salicylic acid from Chinese herbal medicine ingestion. J Anal Toxicol 2004;28:214.
Rivera W et al: Delayed salicylate toxicity at 35 hours without early manifestations following a single salicylate ingestion. Ann Pharmacother 2004;38:1186.
Seafood Poisonings
A variety of intoxications may occur after eating certain types of fish or other seafood. These include scombroid, ciguatera, paralytic shellfish, and puffer fish poisoning. The mechanisms of toxicity and clinical presentations are described in Table 39-12. In the majority of cases, the seafood has a normal appearance and taste (scombroid may have a peppery taste).
Table 39-12. Common seafood poisonings. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Treatment
A. Emergency and Supportive Measures
Caution: Abrupt respiratory arrest may occur in patients with acute paralytic shellfish and puffer fish poisoning. Observe patients for at least 4 6 hours. Replace fluid and electrolyte losses from gastroenteritis with intravenous saline or other crystalloid solution.
For recent ingestions, it may be possible to adsorb residual toxin in the gut with activated charcoal, 50 60 g orally (see p 1644).
B. Specific Treatment
There is no specific antidote for paralytic shellfish or puffer fish poisoning.
1. Ciguatera
There are anecdotal reports of successful treatment of acute neurologic symptoms with mannitol, 1 g/kg intravenously.
P.1667
2. Scombroid
Antihistamines such as diphenhydramine, 25 50 mg intravenously, and the H2 blocker cimetidine, 300 mg intravenously, are usually effective. For severe reactions, give also epinephrine, 0.3 0.5 mL of a 1:1000 solution subcutaneously.
Isbister GK et al: Neurotoxic marine poisoning. Lancet Neurol 2005;4:219.
Kiernan MC et al: Acute tetrodotoxin-induced neurotoxicity after ingestion of puffer fish. Ann Neurol 2005;57:339.
Snake Bites
The venom of poisonous snakes and lizards may be predominantly neurotoxic (coral snake) or predominantly cytolytic (rattlesnakes, other pit vipers). Neurotoxins cause respiratory paralysis; cytolytic venoms cause tissue destruction by digestion and hemorrhage due to hemolysis and destruction of the endothelial lining of the blood vessels. The manifestations of rattlesnake envenomation are mostly local pain, redness, swelling, and extravasation of blood. Perioral tingling, metallic taste, nausea and vomiting, hypotension, and coagulopathy may also occur. Neurotoxic envenomation may cause ptosis, dysphagia, diplopia, and respiratory arrest.
Treatment
A. Emergency Measures
Immobilize the patient and the bitten part in a neutral position. Avoid manipulation of the bitten area. Transport the patient to a medical facility for definitive treatment. Do not give alcoholic beverages or stimulants; do not apply ice; do not apply a tourniquet. The trauma to underlying structures resulting from incision and suction performed by unskilled people is probably not justified in view of the small amount of venom that can be recovered.
B. Specific Antidote and General Measures
1. Pit viper (eg, rattlesnake) envenomation
A new antivenin (CroFab) has replaced the Wyeth horse serum-based product. With local signs such as swelling, pain, and ecchymosis but no systemic symptoms, give 4 6 vials of crotalid antivenin (CroFab) by slow intravenous drip in 250 500 mL saline. Repeated doses of 2 vials every 6 hours for up to 18 hours has been recommended. For more serious envenomation with marked local effects and systemic toxicity (eg, hypotension, coagulopathy), higher doses and additional vials may be required. Monitor vital signs and the blood coagulation profile. Type and cross-match blood. The adequacy of venom neutralization is indicated by improvement in symptoms and signs, and the rate of swelling slows. Prophylactic antibiotics are not indicated after a rattlesnake bite.
2. Elapid (coral snake) envenomation
Give 1 2 vials of specific antivenom as soon as possible. To locate antisera for exotic snakes, call a regional poison control center (800 222-1222).
Camilleri C et al: Conservative management of delayed, multicomponent coagulopathy following rattlesnake envenomation. Clin Toxicol (Phila) 2005;43:201.
P.1668
Gold BS et al: North American snake envenomation: diagnosis, treatment, and management. Emerg Med Clin North Am 2004;22:423.
Spider Bites & Scorpion Stings
The toxin of most species of spiders in the United States causes only local pain, redness, and swelling. That of the more venomous black widow spiders (Latrodectus mactans) causes generalized muscular pains, muscle spasms, and rigidity. The brown recluse spider (Loxosceles reclusa) causes progressive local necrosis as well as hemolytic reactions (rare). Stings by most scorpions in the United States cause only local pain. Stings by the more toxic Centruroides species (found in the southwestern United States) may cause muscle cramps, twitching and jerking, and occasionally hypertension, convulsions, and pulmonary edema. Stings by scorpions from other parts of the world are not discussed here.
Treatment
A. Black Widow Spider Bites
Pain may be relieved with parenteral narcotics or muscle relaxants (eg, methocarbamol, 15 mg/kg). Calcium gluconate 10%, 0.1 0.2 mL/kg intravenously, may relieve muscle rigidity, though its effectiveness is questionable. Antivenin is available, but because of concerns about acute hypersensitivity reactions it is often reserved for very young or elderly patients or those who do not respond to the above measures. Horse serum sensitivity testing is required. (Instruction and testing materials are included in the antivenin kit.)
B. Brown Recluse Spider Bites
Because bites occasionally progress to extensive local necrosis, some authorities recommend early excision of the bite site, whereas others use oral corticosteroids. Anecdotal reports have claimed success with dapsone and colchicine. All of these treatments remain of unproved value.
C. Scorpion Stings
No specific treatment is available for envenomations by scorpions found in the United States. For Centruroides stings, some toxicologists use a specific antivenom developed in Arizona, but this is neither FDA-approved nor widely available.
Foex B et al: Best evidence topic report. Scorpion envenomation: does antivenom reduce serum venom concentrations? Emerg Med J 2005;22:195.
Isbister GK et al: Antivenom treatment in arachnidism. J Toxicol Clin Toxicol 2003;41:291.
LoVecchio F et al: Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol 2003;41:937.
Saucier JR: Arachnid envenomation. Emerg Med Clin North Am 2004;22:405.
Theophylline & Caffeine
Theophylline toxicity may be caused by several of its pharmacologic effects, including inhibition of phosphodiesterase and adenosine and release of catecholamines. Theophylline may cause intoxication after an acute single overdose, or intoxication may occur as a result of chronic accidental repeated overmedication or reduced elimination resulting from hepatic dysfunction or interacting drug (eg, cimetidine, erythromycin). The usual serum half-life of theophylline is 4 6 hours, but this may increase to more than 20 hours after overdose. Caffeine and caffeine-containing herbal products can produce similar toxicity.
Clinical Findings
Mild intoxication causes nausea, vomiting, tachycardia, and tremulousness. Severe intoxication is characterized by ventricular and supraventricular tachyarrhythmias, hypotension, and seizures. Status epilepticus is common and often intractable to the usual anticonvulsants. After acute overdose (but not chronic intoxication), hypokalemia, hyperglycemia, and metabolic acidosis are common. Seizures and other manifestations of toxicity may be delayed for several hours after acute ingestion, especially if a sustained-release preparation such as Theo-Dur was taken.
Diagnosis is based on measurement of the serum theophylline concentration. Seizures and hypotension are likely to develop in acute overdose patients with serum levels greater than 100 mg/L. Serious toxicity may develop at lower levels (ie, 40 60 mg/L) in patients with chronic intoxication.
Treatment
A. Emergency and Supportive Measures
After acute ingestion, administer activated charcoal (see p 1644). Repeated doses of activated charcoal may enhance theophylline elimination by gut dialysis. Addition of whole bowel irrigation should be considered for large ingestions involving sustained-release preparations.
Hemodialysis is effective in removing theophylline and is indicated for patients with status epilepticus or markedly elevated serum theophylline levels (eg, > 100 mg/L after acute overdose or > 60 mg/L with chronic intoxication).
B. Specific Treatment
Treat seizures with benzodiazepines (lorazepam, 2 3 mg intravenously, or diazepam, 5 10 mg intravenously) or phenobarbital (10 15 mg/kg intravenously). Phenytoin is not effective. Hypotension and tachycardia which are mediated through excessive -adrenergic stimulation may respond to -blocker therapy even in low doses: Administer esmolol, 25 50 mcg/kg/min by intravenous infusion, or propranolol, 0.5 1 mg intravenously.
P.1669
Barnes PJ: Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med 2003;167:813.
Kerrigan S et al: Fatal caffeine overdose: two case reports. Forensic Sci Int 2005;153:67.
Tricyclic & other Antidepressants
Tricyclic and related cyclic antidepressants are among the most dangerous drugs involved in suicidal overdose. These drugs have anticholinergic and cardiac depressant properties ( quinidine-like sodium channel blockade). Tricyclic antidepressants produce more marked membrane-depressant cardiotoxic effects than the phenothiazines.
Newer antidepressants such as trazodone, fluoxetine, citalopram, paroxetine, sertraline, bupropion, venlafaxine, and fluvoxamine are not chemically related to the tricyclic antidepressant agents and do not generally produce quinidine-like cardiotoxic effects. However, they may cause seizures in overdoses and they may cause serotonin syndrome (see Monoamine Oxidase Inhibitors, above). Seizures are also reported rarely after therapeutic doses of bupropion.
Clinical Findings
Signs of severe intoxication may occur abruptly and without warning within 30 60 minutes after acute tricyclic overdose. Anticholinergic effects include dilated pupils, tachycardia, dry mouth, flushed skin, muscle twitching, and decreased peristalsis. Quinidine-like cardiotoxic effects include QRS interval widening (> 0.12 s; see Figure 39-2), ventricular arrhythmias, AV block, and hypotension. Rightward-axis deviation of the terminal 40 ms of the QRS has also been described. Prolongation of the QT interval has been reported with citalopram and venlafaxine. Seizures and coma are common with severe intoxication. Life-threatening hyperthermia may result from status epilepticus and anticholinergic-induced impairment of sweating. Among newer agents, bupropion and venlafaxine have been associated with a greater risk of seizures.
 |
Figure 39-2. Cardiac arrhythmias resulting from tricyclic antidepressant overdose. A: Delayed intraventricular conduction results in prolonged QRS interval (0.18 s). B and C: Supraventricular tachycardia with progressive widening of QRS complexes mimics ventricular tachycardia. (Reproduced, with permission, from Benowitz NL, Goldschlager N: Cardiac disturbances in the toxicologic patient. In: Clinical Management of Poisoning and Drug Overdose, 3rd ed. Haddad LM, Winchester JF [editors]. Saunders, 1998. ) |
The diagnosis should be suspected in any overdose patient with anticholinergic side effects, especially if there is widening of the QRS interval or seizures. For intoxication by most tricyclics, the QRS interval correlates with the severity of intoxication more reliably than the serum drug level.
Serotonin syndrome should be suspected if a patient taking serotonin reuptake inhibitors develops agitation, delirium, muscular hyperactivity, and fever.
Treatment
A. Emergency and Supportive Measures
Observe patients for at least 6 hours, and admit all patients with evidence of anticholinergic effects (eg, delirium, dilated pupils, tachycardia) or signs of cardiotoxicity (see above).
Administer activated charcoal, and consider gastric lavage after recent large ingestions (see p 1644). All of these drugs are highly tissue-bound and are not effectively removed by hemodialysis procedures.
B. Specific Treatment
Cardiotoxic sodium channel-depressant effects may respond to boluses of sodium bicarbonate (50 100 mEq intravenously). Sodium bicarbonate provides a large sodium load that alleviates depression of the sodium-dependent channel. Reversal of acidosis may also have beneficial effects at this site. Maintain the pH between 7.45 and 7.50. Alkalinization does not promote excretion of tricyclics. Prolongation of the QT interval or torsade de pointes is usually treated with intravenous magnesium or overdrive pacing.
Mild serotonin syndrome may be treated with benzodiazepines and withdrawal of the antidepressant. Moderate cases may respond to cyproheptadine (4 mg orally or via gastric tube hourly for three or four doses). Severe hyperthermia should be treated with neuromuscular paralysis and endotracheal intubation in addition to external cooling measures.
Bailey B et al: A meta-analysis of prognostic indicators to predict seizures, arrhythmias or death after tricyclic antidepressant overdose. J Toxicol Clin Toxicol 2004;42:877.
Isbister GK et al: Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol 2004;42:277.
Kelly CA et al: Toxicity of citalopram and the newer antidepressants after overdose. J Toxicol Clin Toxicol 2004;42:67.
EAN: 2147483647
Pages: 49