13 - Blood
Editors: McPhee, Stephen J.; Papadakis, Maxine A.; Tierney, Lawrence M.
Title: Current Medical Diagnosis & Treatment, 46th Edition
Copyright 2007 McGraw-Hill
> Table of Contents > 16 - Breast
function show_scrollbar() {}
16
Breast
Armando E. Giuliano MD
Benign Breast Disorders
Fibrocystic Condition
![]() Essentials of Diagnosis
Essentials of Diagnosis
Painful, often multiple, usually bilateral masses in the breast.
Rapid fluctuation in the size of the masses is common.
Frequently, pain occurs or worsens and size increases during premenstrual phase of cycle.
Most common age is 30 50. Rare in postmenopausal women not receiving hormonal replacement.
General Considerations
Fibrocystic condition is the most frequent lesion of the breast. Although commonly referred to as fibrocystic disease, it does not, in fact, represent a pathologic or anatomic disorder. It is common in women 30 50 years of age but rare in postmenopausal women who are not taking hormonal replacement medications. Estrogen is considered a causative factor. There may be an increased risk in women who drink alcohol, especially women between 18 and 22 years of age. Fibrocystic condition encompasses a wide variety of histologic changes. These lesions are always associated with benign changes in the breast epithelium, some of which are found so commonly in normal breasts that they are probably variants of normal breast histology but have nonetheless been termed a condition or disease.
The microscopic findings of fibrocystic condition include cysts (gross and microscopic), papillomatosis, adenosis, fibrosis, and ductal epithelial hyperplasia. Although fibrocystic condition has generally been considered to increase the risk of subsequent breast cancer, only the variants in which proliferation (especially with atypia) of epithelial components is demonstrated represent true risk factors.
Clinical Findings
A. Symptoms and Signs
Fibrocystic condition may produce an asymptomatic lump in the breast that is discovered by accident, but pain or tenderness often calls attention to the mass. There may be discharge from the nipple. In many cases, discomfort occurs or worsens during the premenstrual phase of the cycle, at which time the cysts tend to enlarge. Fluctuation in size and rapid appearance or disappearance of a breast mass are common with this condition. Multiple or bilateral masses are common, and many patients will give a history of a transient lump in the breast or cyclic breast pain.
B. Diagnostic Tests
Because a mass due to fibrocystic condition is frequently indistinguishable from carcinoma on the basis of clinical findings, suspicious lesions should be biopsied. Fine-needle aspiration cytology may be used, but if a suspicious mass that is nonmalignant on cytologic examination does not resolve over several months, it should be excised. Surgery should be conservative, since the primary objective is to exclude cancer. Occasionally, core needle biopsy will suffice. Simple mastectomy or extensive removal of breast tissue is rarely, if ever, indicated for fibrocystic condition.
Differential Diagnosis
Pain, fluctuation in size, and multiplicity of lesions are the features most helpful in differentiating fibrocystic condition from carcinoma. If a dominant mass is present, the diagnosis of cancer should be assumed until disproved by biopsy. Final diagnosis depends on pathologic analysis of the excisional biopsy specimen. Mammography may be helpful, but the breast tissue in these young women is usually too radiodense to permit a worthwhile study. Sonography is useful in differentiating a cystic mass from a solid mass.
Treatment
When the diagnosis of fibrocystic condition has been established by previous biopsy or is likely because the history is classic, aspiration of a discrete mass suggestive of a cyst is indicated to alleviate pain and, more importantly, to confirm the cystic nature of the mass. The patient is reexamined at intervals thereafter. If no fluid is obtained by
P.720
aspiration, if fluid is bloody, if a mass persists after aspiration, or if at any time during follow-up a persistent or recurrent lump is noted, biopsy is performed.
Breast pain associated with generalized fibrocystic condition is best treated by avoiding trauma and by wearing a good supportive brassiere during the night and day. Hormone therapy is not advisable, because it does not cure the condition and has undesirable side effects. Danazol (100 200 mg orally twice daily), a synthetic androgen, has been used for patients with severe pain. This treatment suppresses pituitary gonadotropins, but androgenic effects (acne, edema, hirsutism) usually make this treatment intolerable; in practice, it is rarely used. Similarly, tamoxifen reduces some symptoms of fibrocystic condition, but because of its side effects it is not useful for young women unless it is given to reduce the risk of cancer. Postmenopausal women receiving hormone replacement therapy may stop hormones to reduce pain. The use of evening primrose oil (a natural form of gamolenic acid) has been shown in studies to decrease pain in 44 58% of users and should be considered for treatment. The dose of gamolenic acid is six capsules of 500 mg orally twice daily. Studies have also demonstrated a low-fat diet or decreasing dietary fat intake may reduce the painful symptoms associated with fibrocystic condition.
The role of caffeine consumption in the development and treatment of fibrocystic condition is controversial. Some studies suggest that eliminating caffeine from the diet is associated with improvement while other studies refute the benefit entirely. Many patients are aware of these studies and report relief of symptoms after giving up coffee, tea, and chocolate. Similarly, many women find vitamin E (400 IU daily) helpful. However, these observations remain anecdotal.
Prognosis
Exacerbations of pain, tenderness, and cyst formation may occur at any time until the menopause, when symptoms usually subside, except in patients receiving hormonal replacement therapy. The patient should be advised to examine her own breasts each month just after menstruation and to inform her practitioner if a mass appears. The risk of breast cancer developing in women with fibrocystic condition showing proliferative or atypical changes in the epithelium is higher than that of the general population. These women should be monitored carefully with physical examinations and imaging studies, such as mammography.
Lucas JH et al: Breast cyst aspiration. Am Fam Physician 2003; 68:1983.
Marchant DJ: Benign breast disease. Obstet Gynecol Clin North Am 2002;29:1.
Morrow M: The evaluation of common breast problems. Am Fam Physician 2000;61:2371.
Norlock FE: Benign breast pain in women: a practical approach to evaluation and treatment. J Am Med Womens Assoc 2002;57:85.
Terry MB et al: Lifetime alcohol intake and breast cancer risk. Ann Epidemiol 2006;16:230.
Fibroadenoma of the Breast
This common benign neoplasm occurs most frequently in young women, usually within 20 years after puberty. It is somewhat more frequent and tends to occur at an earlier age in black women. Multiple tumors are found in 10 15% of patients.
The typical fibroadenoma is a round or ovoid, rubbery, discrete, relatively movable, nontender mass 1 5 cm in diameter. It is usually discovered accidentally. Clinical diagnosis in young patients is generally not difficult. In women over 30 years, fibrocystic condition of the breast and carcinoma of the breast must be considered. Cysts can be identified by aspiration or ultrasonography. Fibroadenoma does not normally occur after the menopause but may occasionally develop after administration of hormones.
No treatment is usually necessary if the diagnosis can be made by needle biopsy or cytologic examination. Excision or vacuum-assisted core needle removal with pathologic examination of the specimen is performed if the diagnosis is uncertain. In a 2005 study, cryoablation, or freezing of the fibroadenoma, appears to be a safe procedure if the lesion is consistent with fibroadenoma on histology prior to ablation. Cryoablation is not appropriate for all fibroadenomas because some are too large to freeze. The advantages of cryoablation over observation are not clear. It is usually not possible to distinguish a large fibroadenoma from a phyllodes tumor on the basis of needle biopsy results.
Phyllodes tumor is a fibroadenoma-like tumor with cellular stroma that grows rapidly. It may reach a large size and, if inadequately excised, will recur locally. The lesion can be benign or malignant. If benign, phyllodes tumor is treated by local excision with a margin of surrounding breast tissue. The treatment of malignant phyllodes tumor is more controversial, but complete removal of the tumor with a rim of normal tissue avoids recurrence. Because these tumors may be large, simple mastectomy is sometimes necessary. Lymph node dissection is not performed, since the sarcomatous portion of the tumor metastasizes to the lungs and not the lymph nodes.
Grady I et al: Ultrasound-guided, vacuum-assisted, percutaneous excision of breast lesions: an accurate technique in the diagnosis of atypical ductal hyperplasia. J Am Coll Surg 2005;201: 14.
Hartmann LC et al: Benign breast disease and the risk of breast cancer. N Engl J Med 2005;353:229.
Jacklin RK et al: Optimising preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol 2006 [Epub ahead of print].
Kaufman CS et al: Office based cryoablation of breast fibroadenomas with long-term follow-up. Breast J 2005;11:344.
Nipple Discharge
In order of decreasing frequency, the following are the most common causes of nipple discharge in the nonlactating breast: duct ectasia, intraductal papilloma, and carcinoma.
P.721
The important characteristics of the discharge and some other factors to be evaluated by history and physical examination are as follows:
Nature of the discharge (serous, bloody, or other).
Association with a mass.
Unilateral or bilateral.
Single or multiple duct discharge.
Discharge is spontaneous (persistent or intermittent) or must be expressed.
Discharge is produced by pressure at a single site or by general pressure on the breast.
Relation to menses.
Premenopausal or postmenopausal.
Patient is taking contraceptive pills or estrogen.
Spontaneous, unilateral, serous or serosanguineous discharge from a single duct is usually caused by an intraductal papilloma or, rarely, by an intraductal cancer. A mass may not be palpable. The involved duct may be identified by pressure at different sites around the nipple at the margin of the areola. Bloody discharge is suggestive of cancer but is more often caused by a benign papilloma in the duct. Cytologic examination may identify malignant cells, but negative findings do not rule out cancer, which is more likely in women over age 50 years. In any case, the involved duct and a mass if present should be excised. A ductogram (a mammogram of a duct after radiopaque dye has been injected) is of limited value since excision of the suspicious ductal system is indicated regardless of findings. Ductoscopy, evaluation of the ductal system with a small scope inserted through the nipple is being studied as a means of identifying intraductal lesions but is not yet practical in the clinical setting.
In premenopausal women, spontaneous multiple duct discharge, unilateral or bilateral, most noticeable just before menstruation, is often due to fibrocystic condition. Discharge may be green or brownish. Papillomatosis and ductal ectasia are usually detected only by biopsy. If a mass is present, it should be removed.
A milky discharge from multiple ducts in the nonlactating breast occurs from hyperprolactinemia. Serum prolactin levels should be obtained to search for a pituitary tumor. Thyroid-stimulating hormone (TSH) helps exclude causative hypothyroidism. Numerous antipsychotic drugs and other drugs may also cause a milky discharge that ceases on discontinuance of the medication.
Oral contraceptive agents or estrogen replacement therapy may cause clear, serous, or milky discharge from a single duct, but multiple duct discharge is more common. In the premenopausal woman, the discharge is more evident just before menstruation and disappears on stopping the medication. If it does not stop and is from a single duct, exploration may be considered.
A purulent discharge may originate in a subareolar abscess and require removal of the abscess and the related lactiferous sinus.
When localization is not possible, no mass is palpable, and the discharge is nonbloody, the patient should be reexamined every 3 or 4 months for a year, and mammography should be done. Although most discharge is from a benign process, patients may find it annoying or disconcerting. To eliminate the discharge, proximal duct excision can be considered both for treatment and diagnosis. Cytologic examination of the nipple discharge for exfoliated cancer cells may rarely be helpful in determining a diagnosis. In addition, the duct may be catheterized and washed out with an isotonic solution (ductal lavage) to evaluate cells for atypia. Regardless of the method of analysis, ductal excision is both therapeutic as well as diagnostic.
Dietz JR et al: Directed duct excision by using mammary ductoscopy in patients with pathologic nipple discharge. Surgery 2002;132:582.
Dooley WC et al: Office-based breast ductoscopy for diagnosis. Am J Surg 2004;188:415.
Escobar PF et al: The clinical applications of mammary ductoscopy. Am J Surg 2006;191:211.
Pritt B et al: Diagnostic value of nipple cytology: study of 466 cases. Cancer 2004;102:233.
Sauter ER et al: Fiberoptic ductoscopy findings in women with and without spontaneous nipple discharge. Cancer 2005; 103:914.
Sauter ER et al: The association of bloody nipple discharge with breast pathology. Surgery 2004;136:780.
Simmons R et al: Nonsurgical evaluation of pathologic nipple discharge. Ann Surg Oncol 2003;10:113.
Fat Necrosis
Fat necrosis is a rare lesion of the breast but is of clinical importance because it produces a mass (often accompanied by skin or nipple retraction) that is indistinguishable from carcinoma. Trauma is presumed to be the cause, though only about 50% of patients give a history of injury. Ecchymosis is occasionally present. If untreated, the mass effect gradually disappears. The safest course is to obtain a biopsy. Needle biopsy is often adequate, but frequently the entire mass must be excised, primarily to exclude carcinoma. Fat necrosis is common after segmental resection, radiation therapy, or flap reconstruction after mastectomy.
Tan PH et al: Fat necrosis of the breast A review. Breast 2006;15:313.
Breast Abscess
During nursing, an area of redness, tenderness, and induration may develop in the breast. The organism most commonly found in these abscesses is Staphylococcus aureus. In the early stages, the infection can often be treated while nursing is continued from that breast by administering an antibiotic such as dicloxacillin or oxacillin, 250 mg orally four times daily for 7 10 days (see Puerperal Mastitis, Chapter 18). If the lesion progresses to form a localized mass with local and systemic signs of infection, surgical drainage is performed and nursing is discontinued. Often needle
P.722
or catheter drainage is adequate, but surgical incision and drainage may be necessary.
A subareolar abscess may develop (rarely) in young or middle-aged women who are not lactating. These infections tend to recur after incision and drainage unless the area is explored during a quiescent interval, with excision of the involved lactiferous duct or ducts at the base of the nipple. Otherwise, infection in the nonlactating breast is very rare. In the nonlactating breast, inflammatory carcinoma must always be considered. Thus, findings suggestive of abscess or cellulitis in the nonlactating breast are an indication for incision and biopsy of any indurated tissue that does not resolve promptly with antibiotics. If the abscess can be percutaneously drained and completely resolves, the patient may be monitored conservatively.
Berna-Serna JD et al: Percutaneous management of breast abscesses. An experience of 39 cases. Ultrasound Med Biol 2004:30:1.
Dener C et al: Breast abscesses in lactating women. World J Surg 2003;27:130.
Disorders of the Augmented Breast
At least 4 million American women have had breast implants. Breast augmentation is performed by placing implants under the pectoralis muscle or, less desirably, in the subcutaneous tissue of the breast. Most implants are made of an outer silicone shell filled with a silicone gel, saline, or some combination of the two. Capsule contraction or scarring around the implant develops in about 15 25% of patients, leading to a firmness and distortion of the breast that can be painful. Some require removal of the implant and capsule.
Implant rupture may occur in as many as 5 10% of women, and bleeding of gel through the capsule is noted even more commonly. Although silicone gel may be an immunologic stimulant, there is no increase in autoimmune disorders in patients with such implants. The Food and Drug Administration (FDA) has advised symptomatic women with ruptured implants to discuss possible surgical removal with their physicians. However, women who are asymptomatic and have no evidence of rupture of a silicone gel prosthesis should probably not undergo removal of the implant. Women with symptoms of autoimmune illnesses should address the possibility of removal with their practitioner.
Studies have failed to show any association between implants and an increased incidence of breast cancer. However, breast cancer may develop in a patient with a silicone gel prosthesis, as it does in women without them. Detection in patients with implants is more difficult because mammography is less able to detect early lesions. However, after a woman who had mastectomy undergoes breast reconstruction with implants, local recurrence of cancer is usually cutaneous or subcutaneous and is easily detected by palpation.
If a cancer develops in a patient with implants, it should be treated in the same manner as in women without implants. Such women should be offered the option of mastectomy or breast-conserving therapy, which may require removal or replacement of the implant. Radiotherapy of the augmented breast often results in marked capsular contracture. Adjuvant treatments should be given for the same indications as for women who have no implants.
Adams WP et al: Decision and management algorithms to address patient and food and drug administration concerns regarding breast augmentation and implants. Plast Reconstr Surg 2004;114:1252.
Brinton LA et al: Risk of connective tissue disorders among breast implant patients. Am J Epidemiol 2004;160:619.
Englert H et al: Augmentation mammoplasty and silicone-osis. Intern Med J 2004;34:668.
Fryzek JP et al: Silicone breast implants. J Rheumatol 2005;32:201.
Carcinoma of the Female Breast
![]() Essentials of Diagnosis
Essentials of Diagnosis
Risk factors include delayed childbearing, positive family history of breast cancer or genetic mutations (BRCA1, BRCA2), and personal history of breast cancer or some types of fibrocystic condition.
Most women with breast cancer do not have identifiable risk factors.
Early findings: Single, nontender, firm to hard mass with ill-defined margins; mammographic abnormalities and no palpable mass.
Later findings: Skin or nipple retraction; axillary lymphadenopathy; breast enlargement, erythema, edema, pain; fixation of mass to skin or chest wall.
Incidence & Risk Factors
Next to skin cancer, breast cancer is the most common type of cancer in women, second only to lung cancer as a cause of death. The probability of developing breast cancer increases throughout life. The mean and the median age of women with breast cancer is between 60 and 61 years.
There will be about 214,640 new cases of breast cancer and about 41,430 deaths from this disease in women in the United States in 2006. An additional
P.723
61,980 cases of ductal carcinoma in situ will be detected, principally by screening mammography. Breast cancer will develop in one of every eight or nine American women during her lifetime. The incidence of breast cancer continues to increase, but recently mortality has appeared to decrease slightly. This reflects both early detection and increased use of systemic therapy. Breast cancer is three to four times more likely to develop in women whose mothers or sisters had breast cancer than in those without this family history. Risk is further increased in patients whose mothers' or sisters' breast cancers occurred before menopause or were bilateral and in those with a family history of breast cancer in two or more first-degree relatives as well as in women of Ashkenazi Jewish descent. However, there is no history of breast cancer among female relatives in over 75% of patients. Nulliparous women and women whose first full-term pregnancy was after age 35 have a 1.5 times higher incidence of breast cancer than multiparous women. Late menarche and artificial menopause are associated with a lower incidence, whereas early menarche (under age 12) and late natural menopause (after age 50) are associated with a slight increase in risk. Fibrocystic condition, when accompanied by proliferative changes, papillomatosis, or atypical epithelial hyperplasia, is associated with an increased incidence. A woman who had cancer in one breast is at increased risk for cancer developing in the other breast. In these women, a contralateral cancer develops at the rate of 1% or 2% per year. Women with cancer of the uterine corpus have a risk of breast cancer significantly higher than that of the general population, and women with breast cancer have a comparably increased risk for endometrial cancer. In the United States, breast cancer is more common in whites. The incidence of the disease among nonwhites (mostly blacks) is increasing, especially in younger women. In general, rates reported from developing countries are low, whereas rates are high in developed countries, with the notable exception of Japan. Some of the variability may be due to underreporting in the developing countries, but a real difference probably exists. Dietary factors, particularly increased fat consumption, may account for some differences in incidence. Oral contraceptives do not appear to increase the risk of breast cancer. There is evidence that administration of estrogens to postmenopausal women may result in a slightly increased risk of breast cancer, but only with higher, long-term doses of estrogens. Concomitant administration of progesterone and estrogen may markedly increase the incidence of breast cancer compared with the use of estrogen alone. The Women's Health Initiative prospective randomized study of hormone replacement therapy stopped treatment with estrogen and progesterone early because of an increased risk of breast cancer compared with untreated controls or women treated with estrogen alone. Alcohol consumption increases the risk slightly. Some inherited breast cancers have been found to be associated with a gene on chromosome 17. This gene, BRCA1, is mutated in families with early-onset breast cancer and ovarian cancer. Breast cancer will develop in as many as 85% of women with BRCA1 gene mutations during their lifetime. Other genes are associated with increased risk of breast and other cancers, such as BRCA2 (associated with a gene on chromosome 13); ataxia-telangiectasia mutation; and mutation of p53, the tumor suppressor gene. Mutations to p53 have been found in approximately 1% of breast cancers in women under 40 years of age. Genetic testing is commercially available for women at high risk for breast cancer. Women with genetic mutations in whom breast cancer develops may be treated in the same way as women who do not have mutations (ie, lumpectomy), though data are emerging to suggest an increased recurrence rate for these women. Such women with mutations often elect bilateral mastectomy as treatment. Some states have enacted legislation to prevent insurance companies from considering mutations as preexisting conditions, preventing insurability.
Women at greater than normal risk for developing breast cancer (Table 16-1) should be identified by their practitioners, taught the techniques of breast self-examination (BSE), and followed carefully. Those with an exceptional family history should be counseled and given the option of genetic testing. Some of these high-risk women may consider prophylactic mastectomy or tamoxifen.
The National Surgical Adjuvant Breast Project (NSABP) conducted the Breast Cancer Prevention Trial (BCPT), which studied the efficacy of tamoxifen as a preventive agent in women who never had breast cancer but were at high risk for developing the disease. Women who received tamoxifen for 5 years had about a 50% reduction in noninvasive and invasive cancers compared with women taking placebo. However, women above the age of 50 who received the drug had an increased incidence of endometrial cancer and deep venous
P.724
thrombosis. Unfortunately, no survival data will be produced from this trial because it was stopped.
Table 16-1. Factors associated with increased risk of breast cancer.1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
The selective estrogen receptor modulator (SERM) raloxifene, effective in preventing osteoporosis, has also shown some promise in preventing breast cancer. The Multiple Outcomes of Raloxifene Evaluations (MORE) trial demonstrated that raloxifene reduced breast cancer risk in women being treated with the drug for osteoporosis. The MORE trial, whose principal aim was to determine the effect of raloxifene on bone, was extended by 4 years (Continuing Outcomes Relevant to Evista (CORE) trial) to better evaluate the effect of raloxifene on breast cancer risk. After 8 years of treatment, raloxifene demonstrated an overall reduction of invasive breast cancer of 66%. Although, it appears that raloxifene is more effective than tamoxifen in reducing the risk of breast cancer, the studies are not comparable since the tamoxifen trial was observing women at increased risk for breast cancer while the MORE/CORE trial was observing women with low bone density and with a lower risk of breast cancer. While it does appear that older women with osteopenia will benefit from the bone effects and breast cancer risk reduction of raloxifene, its efficacy compared with tamoxifen still requires study.
The Study of Tamoxifen and Raloxifene (STAR) trial is ongoing with early results expected in 2007. Similar to tamoxifen, aromatase inhibitors (AI) have shown great success in treating breast cancer with fewer side effects, although bone loss is a significant side effect of this long-term treatment.
Several large multicenter studies (eg, International Breast Cancer Intervention Study II [IBIS-II] and National Cancer Institute of Canada Clinical Trials Group [NCIC CTG]) are underway to determine whether AIs have a role in preventing breast cancer.
In addition to pharmaceutical therapy, patients continue to seek a way to prevent breast cancer. There has been considerable research on incorporating diet and exercise into the lifestyle of women who may be at risk for cancer. The Women's Health Initiative Randomized Controlled Dietary Modification Trial was conducted to determine whether decreasing dietary fat intake would reduce the incidence of breast cancer recurrence after initial treatment. Although the trial demonstrated a decrease in recurrence in the follow-up period, it did not reach statistical significance.
Andrews L et al: Psychological impact of genetic testing for breast cancer susceptibility in women of Ashkenazi Jewish background: a prospective study. Genet Test 2004;8:240.
Cauley JA et al: Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat 2001;65:125.
Colditz GA: Estrogen, estrogen plus progestin therapy, and risk of breast cancer. Clin Cancer Res 2005;11(2 Pt 2):909s.
Cuzick J: Aromatase inhibitors for breast cancer prevention. J Clin Oncol 2005;23:1636.
Ettinger B et al: Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999;282:637.
Fabian CJ et al: Selective estrogen-receptor modulators for primary prevention of breast cancer. J Clin Oncol 2005;23: 1644.
Fisher B et al: Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 2005;97:1652.
Jemal A et al: Cancer Statistics, 2006. CA Cancer J Clin 2006; 56:106.
Kalidas M et al: Aromatase inhibitors for the treatment and prevention of breast cancer. Clin Breast Cancer 2005;6:27.
Martino S et al; CORE Investigators: Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004;96:1751.
Miller WR: Aromatase inhibitors and breast cancer. Minerva Endocrinol 2006;31:27.
Narod SA et al: Prevention and management of hereditary breast cancer. J Clin Oncol 2005;23:1656.
Palma M et al: BRCA1 and BRCA2: the genetic testing and the current management options for mutation carriers. Crit Rev Oncol Hematol 2006;57:1.
Prentice RL et al: Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295: 629.
Rebbeck TR et al: Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004;22:1055.
Rossouw JE et al: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321.
U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Ann Intern Med 2005;143:355.
Vogel VG et al: The study of tamoxifen and raloxifene: preliminary enrollment data from a randomized breast cancer risk reduction trial. Clin Breast Cancer 2002;3:153.
Wrensch MR et al: Breast cancer risk in women with abnormal cytology in nipple aspirates of breast fluid. J Natl Cancer Inst 2001;93:1791.
Early Detection of Breast Cancer
Screening Programs
A number of mass screening programs consisting of physical and mammographic examination of the breasts of asymptomatic women have been conducted. Such programs frequently identify about 10 cancers per 1000 women older than age 50 years and about two cancers per 1000 women younger than age 50 years. About 80% of these women have negative axillary lymph nodes at the time of surgery, whereas only 50% of nonscreened women found in the course of usual medical practice
P.725
have uninvolved axillary nodes. Detecting breast cancer before it has spread to the axillary nodes greatly increases the chance of survival, and about 85% of such women will survive at least 5 years.
Both physical examination and mammography are necessary for maximum yield in screening programs, since about 35 50% of early breast cancers can be discovered only by mammography and another 40% can be detected only by palpation by clinician. About one-third of the abnormalities detected on screening mammograms will be found to be malignant when biopsy is performed. The probability of cancer on a screening mammogram is directly related to the Breast Imaging and Reporting Data System (BIRADS) assessment, and work-up should be performed based on this classification. Women 20 40 years of age should have a breast examination as part of routine medical care every 2 3 years. Women over age 40 years should have annual breast examinations. The sensitivity of mammography varies from approximately 60% to 90%. This sensitivity depends on several factors, including patient age (breast density) and tumor size, location, and mammographic appearance. In young women with dense breasts, mammography is less sensitive than in older women with fatty breasts, in whom mammography can detect at least 90% of malignancies. Smaller tumors, particularly those without calcifications, are more difficult to detect, especially in dense breasts. The lack of sensitivity and the low incidence of breast cancer in young women have led to questions concerning the value of mammography for screening in women 40 50 years of age. The specificity of mammography in women under 50 years varies from about 30% to 40% for nonpalpable mammographic abnormalities to 85% to 90% for clinically evident malignancies.
Screening recommendations for women in their 40s are based, in part, on trials from Sweden. Two trials showed a statistical advantage for screening women in their 40s, and a meta-analysis similarly revealed a statistical survival advantage for screened women with longer follow-up. In March 1997, the National Cancer Advisory Board recommended that women in their 40s with average risk factors should have screening mammography every 1 2 years and that women at higher risk should seek medical advice on when to begin screening. Studies continue to support the value of screening mammography in women over 40 years. Such women should have annual mammography and physical examination.
The beneficial effect of screening in women aged 50 69 years is undisputed and has been confirmed by all clinical trials. The efficacy of screening in older women those older than 70 years is inconclusive and is difficult to determine because few women were screened.
Self-Examination
BSE has not been shown to improve survival. Despite this and despite possible increased biopsy rates, it is a useful technique since many patients do detect their own cancer, and women often feel more in control and proactive by performing BSE. Because of the absence of strong evidence supporting the value of BSE, the American Cancer Society no longer recommends monthly BSE beginning at age 20 years. The recommendation is that patients be made aware of the potential benefits, limitations, and harms (increased biopsies or false-positive results) associated with BSE. Women who chose to perform BSE should be advised regarding the proper technique. Premenopausal women should perform the examination 7 8 days after the menstrual period. The breasts should be inspected initially while standing before a mirror with the hands at the sides, overhead, and pressed firmly on the hips to contract the pectoralis muscles. Masses, asymmetry of breasts, and slight dimpling of the skin may become apparent as a result of these maneuvers. Next, in a supine position, each breast should be carefully palpated with the fingers of the opposite hand. Some women discover small breast lumps more readily when their skin is moist while bathing or showering. Physicians should instruct women in the technique of self-examination and advise them to report a mass or other abnormality. While BSE is not a recommended practice, patients should recognize and report any breast changes to their practitioners as it remains an important facet of proactive care.
Imaging
Mammography is the most reliable means of detecting breast cancer before a mass can be palpated. Slowly growing cancers can be identified by mammography at least 2 years before reaching a size detectable by palpation. Film screen mammography delivers less than 0.4 cGy to the mid breast per view and has largely replaced the older xeromammographic technique, which delivers more radiation. Although full-field digital mammography provides an easier method to maintain and review mammograms, it has not been proven that it provides better images or increases detection rates more than film mammography. A large study of 50,000 women comparing film screen to digital mammography showed no difference in overall cancer detection. However, in subset analysis, digital mammography seems slightly superior in young women with dense breasts. Computer-assisted detection (CAD) has not shown any increase in detection of cancers and is not routinely performed at centers with experienced mammographers.
Calcifications are the most easily recognized mammographic abnormality. The most common findings associated with carcinoma of the breast are clustered polymorphic microcalcifications. Such calcifications are usually at least five to eight in number, aggregated in one part of the breast and differing from each other in size and shape, often including branched or V- or Y-shaped configurations. There may be an associated mammographic mass density or, at times, only a mass density with no calcifications. Such a density usually
P.726
has irregular or ill-defined borders and may lead to architectural distortion within the breast. A small mass or architectural distortion, particularly in a dense breast, may be subtle and difficult to detect.
Indications for mammography are as follows: (1) to screen at regular intervals women at high risk for developing breast cancer (see above); (2) to evaluate each breast when a diagnosis of potentially curable breast cancer has been made, and at yearly intervals thereafter; (3) to evaluate a questionable or ill-defined breast mass or other suspicious change in the breast; (4) to search for an occult breast cancer in a woman with metastatic disease in axillary nodes or elsewhere from an unknown primary; (5) to screen women prior to cosmetic operations or prior to biopsy of a mass, to examine for an unsuspected cancer; (6) to monitor those women with breast cancer who have been treated with breast-conserving surgery and radiation; and (7) to monitor the contralateral breast in those women with breast cancer treated with mastectomy.
Patients with a dominant or suspicious mass must undergo biopsy despite mammographic findings. The mammogram should be obtained prior to biopsy so that other suspicious areas can be noted and the contralateral breast can be checked. Mammography is never a substitute for biopsy because it may not reveal clinical cancer in a very dense breast, as may be seen in young women with fibrocystic changes, and may not reveal medullary cancers.
Communication and documentation among the patient, the referring practitioner, and the interpreting physician are critical for high-quality screening and diagnostic mammography. The patient should be told about how she will receive timely results of her mammogram; that mammography does not rule out cancer; and that she may receive a correlative examination such as ultrasound at the mammography facility if referred for a suspicious lesion. She should also be aware of the technique and need for breast compression and that this may be uncomfortable. The mammography facility should be informed in writing of abnormal physical examination findings. It is strongly recommended in the Agency for Health Care Policy and Research (AHCPR) Clinical Practice Guidelines that all mammography reports be communicated with the patient as well as the health care provider in writing. Additional phone communication about any abnormal findings should take place between the interpreting and referring practitioners. MRI and ultrasound may be useful screening modalities in women who are at high risk for breast cancer, but not for the general population. The sensitivity of MRI is much higher than mammography; however, the specificity is significantly lower and this results in multiple unnecessary biopsies. The increased sensitivity despite decreased specificity may be considered a reasonable trade-off for those at increased risk for developing breast cancer, but not for normal-risk population. MRI is useful in women with breast implants to determine the character of a lesion present in the breast and to search for implant rupture. In addition, positron emission tomography (PET) may play a role in imaging atypical lesions but only after diagnostic mammography has been performed. PET has demonstrated the ability to improve breast cancer diagnosis in small pilot studies, but the primary role remains evaluation of metastatic deposits.
Baxter N: Canadian Task Force on Preventive Health Care: Preventive health care, 2001 update: should women be routinely taught breast self-examination to screen for breast cancer? CMAJ 2001;164:1837.
Byrne AM et al: Positron emission tomography in the staging and management of breast cancer. Br J Surg 2004;91:1398.
Elmore JG et al: Screening for breast cancer. JAMA 2005; 293:1245.
Humphrey LL et al: Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137(5 Part 1):347.
Kosters JP et al: Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev 2003;(2):CD003373.
Kriege M et al: The Magnetic Resonance Imaging Screening Study Group: Efficacy of magnetic resonance imaging and mammography for breast cancer screening in women with a familial or genetic predisposition. Obstet Gynecol Surv 2005;60:107.
Kumar R et al: Potential of dual-time-point imaging to improve breast cancer diagnosis with (18)F-FDG PET. J Nucl Med 2005;46:1819.
Nystrom L et al: Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet 2001;359:909.
Pisano ED et al; Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group: Diagnostic performance of digital versus film mammography for breast cancer screening. N Engl J Med 2005;353:1773.
Reddy DH et al: Incorporating new imaging models in breast cancer management. Curr Treat Options Oncol 2005;6: 135.
Smith RA et al: American Cancer Society guidelines for the early detection of cancer, 2005. CA Cancer J Clin 2005; 55:31.
Taylor P et al: Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography. Health Technol Assess 2005;9:1.
Weaver DL et al: Pathologic findings from the Breast Cancer Surveillance Consortium: population-based outcomes in women undergoing biopsy after screening mammography. Cancer 2006;106:732.
Clinical Clues to Early Detection of Breast Cancer
A. Symptoms and Signs
The presenting complaint in about 70% of patients with breast cancer is a lump (usually painless) in the breast. About 90% of breast masses are discovered by the patient herself. Less frequent symptoms are breast pain; nipple discharge; erosion, retraction, enlargement, or itching of the nipple; and redness, generalized hardness, enlargement, or shrinking of the breast. Rarely, an axillary
P.727
mass or swelling of the arm may be the first symptom. Back or bone pain, jaundice, or weight loss may be the result of systemic metastases, but these symptoms are rarely seen on initial presentation.
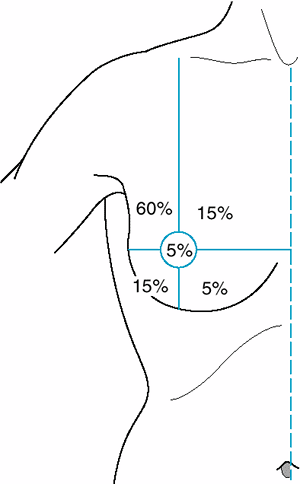 |
Figure 16-1. Frequency of breast carcinoma at various anatomic sites. |
The relative frequency of carcinoma in various anatomic sites in the breast is shown in Figure 16-1.
Inspection of the breast is the first step in physical examination and should be carried out with the patient sitting, arms at her sides and then overhead. Abnormal variations in breast size and contour, minimal nipple retraction, and slight edema, redness, or retraction of the skin can be identified. Asymmetry of the breasts and retraction or dimpling of the skin can often be accentuated by having the patient raise her arms overhead or press her hands on her hips to contract the pectoralis muscles. Axillary and supraclavicular areas should be thoroughly palpated for enlarged nodes with the patient sitting (Figure 16-2). Palpation of the breast for masses or other changes should be performed with the patient both seated and supine with the arm abducted (Figure 16-3). Palpation with a rotary motion of the examiner's fingers as well as a horizontal stripping motion has been recommended.
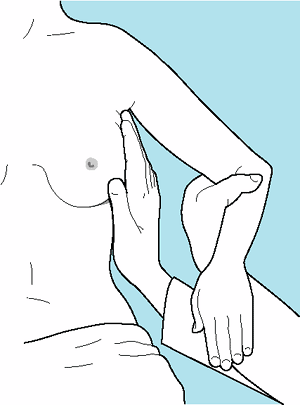 |
Figure 16-2. Palpation of axillary region for enlarged lymph nodes. |
Breast cancer usually consists of a nontender, firm or hard mass with poorly delineated margins (caused by local infiltration). Slight skin or nipple retraction is an important sign. Minimal asymmetry of the breast may be noted. Very small (1 2 mm) erosions of the nipple epithelium may be the only manifestation of Paget's carcinoma. Watery, serous, or bloody discharge from the nipple is an occasional early sign but is more often associated with benign disease.
A lesion smaller than 1 cm in diameter may be difficult or impossible for the examiner to feel and yet may be discovered by the patient. She should always be asked to demonstrate the location of the mass; if the practitioner fails to confirm the patient's suspicions, the examination should be repeated in 2 3 months, preferably 1 2 weeks after the onset of menses. During the premenstrual phase of the cycle, increased innocuous nodularity may suggest neoplasm or may obscure an underlying lesion. If there is any question regarding the nature of an abnormality under these circumstances, the patient should be asked to return after her period. Ultrasound is often valuable and mammography essential when an area is felt by the patient to be abnormal but the physician feels no mass. MRI may be considered, but the lack of specificity should be discussed by the practitioner and the patient.
Metastases tend to involve regional lymph nodes, which may be palpable. One or two movable, nontender, not particularly firm axillary lymph nodes 5 mm or less in diameter are frequently present and are
P.728
generally of no significance. Firm or hard nodes larger than 1 cm are typical of metastases. Axillary nodes that are matted or fixed to skin or deep structures indicate advanced disease (at least stage III). Microscopic metastases are present in about 30% of patients with clinically negative nodes. On the other hand, if the examiner thinks that the axillary nodes are involved, that impression will be borne out by histologic section in about 85% of cases. The incidence of positive axillary nodes increases with the size of the primary tumor. Noninvasive cancers (in situ) do not metastasize.
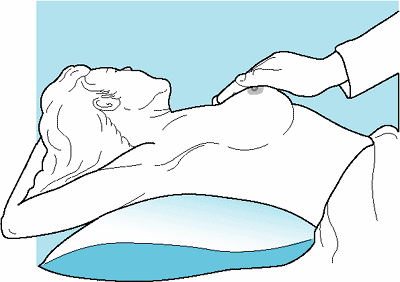 |
Figure 16-3. Palpation of breasts. Palpation is performed with the patient supine and arm abducted. |
In most cases, no nodes are palpable in the supraclavicular fossa. Firm or hard nodes of any size in this location or just beneath the clavicle are suggestive of metastatic cancer and should be biopsied. Ipsilateral supraclavicular or infraclavicular nodes containing cancer indicate that the tumor is in an advanced stage (stage III or IV). Edema of the ipsilateral arm, commonly caused by metastatic infiltration of regional lymphatics, is also a sign of advanced cancer.
B. Laboratory Findings
A consistently elevated sedimentation rate may be the result of disseminated cancer. Liver or bone metastases may be associated with elevation of serum alkaline phosphatase. Hypercalcemia is an occasional important finding in advanced cancer of the breast. Carcinoembryonic antigen (CEA) and CA 15 3 or CA 27 29 may be used as markers for recurrent breast cancer but are not helpful in diagnosing early lesions. Many scientists are further investigating breast cancer markers through proteomics and hormone assays. These studies are ongoing and may prove to be helpful in early detection or evaluation of prognosis.
C. Imaging for Metastases
Chest radiographs may show pulmonary metastases. CT scanning of the liver and brain is of value only when metastases are suspected in these areas. Bone scans utilizing 99mTc-labeled phosphates or phosphonates are more sensitive than skeletal radiographs in detecting metastatic breast cancer. Bone scanning has not proved to be of clinical value as a routine preoperative test in the absence of symptoms, physical findings, or abnormal alkaline phosphatase or calcium levels. The frequency of abnormal findings on bone scan parallels the status of the axillary lymph nodes on pathologic examination. PET has been shown to be less useful than a bone scan to identify metastatic bone lesions. It is effective in soft tissue or visceral metastases in patients with signs or symptoms of metastatic disease. PET scanning combined with CT (PET-CT) is an effective screening method for detecting soft tissue metastases and is replacing CT scans.
D. Diagnostic Tests
1. Biopsy
The diagnosis of breast cancer depends ultimately on examination of tissue or cells removed by biopsy. Treatment should never be undertaken without an unequivocal histologic or cytologic diagnosis of cancer. The safest course is biopsy examination of all suspicious masses found on physical examination and of suspicious lesions demonstrated by mammography. About 60% of lesions clinically thought to be cancer prove on biopsy to be benign, and about 30% of lesions believed to be benign are found to be malignant. These findings demonstrate the fallibility of clinical judgment and the necessity for biopsy.
All breast masses require a histologic diagnosis with one probable exception, that beinga nonsuspicious, presumably fibrocystic mass, in a premenopausal woman. Rather, these masses can be observed through one or two menstrual cycles. However, if the mass does not completely resolve during this time, it must be biopsied. Figures 16-4 and 16-5 present algorithms for management of breast masses in premenopausal and postmenopausal patients.
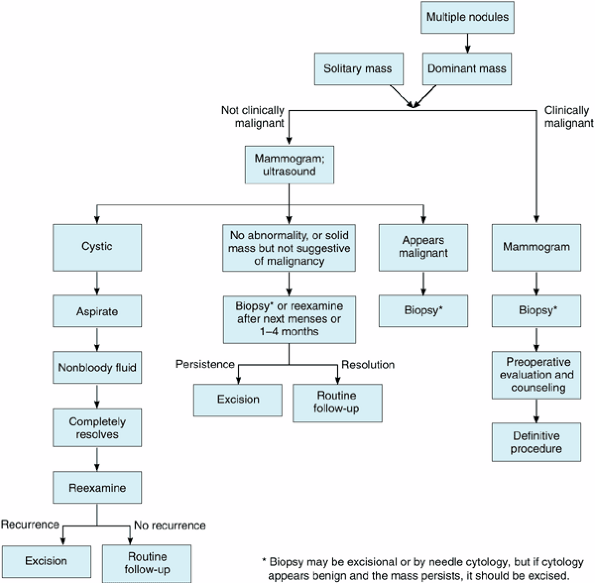 |
Figure 16-4. Evaluation of breast masses in premenopausal women. (Modified from Giuliano AE: Breast disease. In: Practical Gynecologic Oncology, 3rd ed. Berek JS, Hacker NF [editors]. Williams & Wilkins, 2000. ) |
The simplest biopsy method is needle biopsy, either by aspiration of tumor cells (fine-needle aspiration cytology) or by obtaining a small core of tissue with a hollow needle (core biopsy).
Fine-needle aspiration cytology is a useful technique whereby cells are aspirated with a small needle and examined cytologically. This technique can be performed easily with no morbidity and is much less expensive than excisional or open biopsy. The main disadvantages are that it requires a pathologist skilled in the cytologic diagnosis of breast cancer and that it is subject to sampling problems, particularly because deep lesions may be missed. Furthermore, noninvasive cancers usually cannot be distinguished from invasive cancers. The incidence of false-positive diagnoses is extremely low, perhaps 1 2%. The false-negative rate is as high as 10%. Most experienced clinicians would not leave a suspicious dominant mass in the breast even when fine-needle aspiration cytology is negative unless the clinical diagnosis, breast imaging studies, and cytologic studies were all in agreement, such as a fibrocystic lesion or fibroadenoma.
Large-needle (core needle) biopsy removes a core of tissue with a large cutting needle. Hand-held biopsy devices make large-core needle biopsy of a palpable mass easy and cost effective in the office with local anesthesia. As in the case of any needle biopsy, the main problem is sampling error due to improper positioning of the needle, giving rise to a false-negative test result.
Open biopsy under local anesthesia as a separate procedure prior to deciding upon definitive treatment is the most reliable means of diagnosis. Needle biopsy or aspiration, when positive, offers a more rapid approach with less expense and morbidity, but when nondiagnostic it must be followed by open biopsy. Open biopsy consists of either an incisional biopsy or an excisional biopsy. An incisional biopsy is one in which an incision is made and only a portion of the breast abnormality is removed for histologic evaluation. An excisional biopsy is also done through an incision in the skin, but with the intent to remove the entire abnormality, not simply a sample. Incisional biopsies are rarely performed.
P.729
Additional evaluation for metastatic disease and therapeutic options can be discussed with the patient after the histologic or cytologic diagnosis of cancer has been established. This approach has the advantage of avoiding unnecessary procedures, since cancer is found in the minority of patients biopsied for a breast lump. In situ cancers are not easily diagnosed cytologically and usually require excisional biopsy.
As an alternative in highly suspicious circumstances, the patient may be admitted to the hospital, where the diagnosis is made on frozen section of tissue obtained by open biopsy under general anesthesia. If the frozen section is positive, the surgeon can proceed immediately with operation. This one-step method is rarely used today except when a cytologic study has suggested cancer but is not diagnostic and there is a high clinical suspicion of malignancy in a patient well prepared for the diagnosis of cancer and its treatment options.
In general, the two-step approach outpatient biopsy followed by definitive operation at a later date is preferred in the diagnosis and treatment of breast cancer, because patients can be given time to adjust to the diagnosis of cancer, can consider alternative forms of therapy, and can seek a second opinion if they wish. There is no adverse effect from the short delay of the two-step procedure, and this is the recommendation of the NCI.
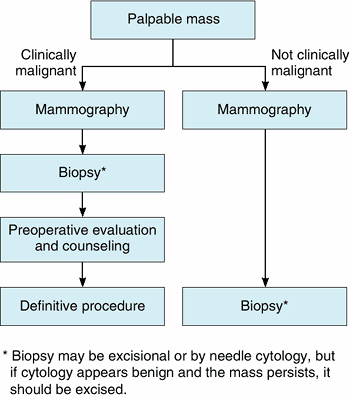 |
Figure 16-5. Evaluation of breast masses in postmenopausal women. (Modified from Giuliano AE: Breast disease. In: Practical Gynecologic Oncology, 3rd ed. Berek JS, Hacker NF [editors]. Williams & Wilkins, 2000. ) |
2. Ultrasonography
Ultrasonography is performed primarily to differentiate cystic from solid lesions. Though not diagnostic, ultrasound may reveal features highly suggestive of malignancy such as irregular margins
P.730
on a new solid mass. Ultrasonography may show an irregular mass within a cyst in the rare case of intracystic carcinoma. If a tumor is palpable and feels like a cyst, an 18-gauge needle can be used to aspirate the fluid and make the diagnosis of cyst. If a cyst is aspirated and the fluid is nonbloody, it does not have to be examined cytologically. If the mass does not recur, no further diagnostic test is necessary. Nonpalpable mammographic densities that appear benign should be investigated with ultrasound to determine whether the lesion is cystic or solid. These may even be needle biopsied with ultrasound guidance.
3. Mammography
When a suspicious abnormality is identified by mammography alone and cannot be palpated by the clinician, the lesion should be biopsied by a computerized stereotactic guided core needle technique. Under mammographic guidance, a biopsy needle can be inserted into the lesion by the mammographer, and a core of tissue for histologic examination or cells for cytology can then be examined. Vacuum assistance increases the amount of tissue obtained and improves diagnosis.
Mammographic localization biopsy is performed by obtaining a mammogram in two perpendicular views and placing a needle or hook-wire near the abnormality so that the surgeon can use the metal needle or wire as a guide during operation to locate the lesion. After mammography confirms the position of the needle in relation to the lesion, an incision is made and the subcutaneous tissue is dissected until the needle is identified. Using the films as a guide, the abnormality can then be localized and excised. It often happens that the abnormality cannot even be palpated through the incision this is the case with microcalcifications and thus it is essential to obtain a mammogram of the specimen to document that the lesion was excised. At that time, a second marker needle can further localize the lesion for the pathologist. Stereotactic core needle biopsies have proved equivalent to mammographic localization biopsies. Core biopsy is preferable to mammographic localization for accessible lesions since an operation can be avoided by the use of stereotactic biopsy techniques.
4. Other imaging modalities
Other modalities of breast imaging have been investigated. Automated breast ultrasonography is useful in distinguishing cystic from solid lesions but should be used only as a supplement to physical examination and mammography. Ductography may be useful to define the site of a lesion causing a bloody discharge, but since biopsy is almost always indicated, ductography may be omitted and the blood-filled nipple system excised. Ductoscopy has shown some promise in identifying intraductal lesions, especially in the case of pathologic nipple discharge, but the utility of this procedure is still being studied. MRI is highly sensitive but not specific and should not be used for screening, but it may be of value in highly selective cases. It is useful, for example, in differentiating scar from recurrence postlumpectomy and may be valuable to screen high-risk women (eg, women with BRCA mutations). It may also be of value to examine for multicentricity when there is a known primary cancer; to examine the contralateral breast in women with cancer; to examine the extent of cancer, especially lobular carcinomas; or to determine the response to neoadjuvant chemotherapy. PET scanning does not appear useful in evaluating the breast itself but is valuable to examine regional lymphatics and distant metastases.
5. Cytology
Cytologic examination of nipple discharge or cyst fluid may be helpful on rare occasions. As a rule, mammography (or ductography) and breast biopsy are required when nipple discharge or cyst fluid is bloody or cytologically questionable. Ductal lavage, a technique that washes individual duct systems with saline and loosens epithelial cells for cytologic evaluation, is being evaluated as a risk assessment tool but appears to be of little value.
Baker JA et al: Breast US: assessment of technical quality and image interpretation. Radiology 2002;223:229.
Dooley WC: Routine operative breast endoscopy for bloody nipple discharge. Ann Surg Oncol 2002;9:920.
Eubank WB et al: Evolving role of positron emission tomography in breast cancer imaging. Semin Nucl Med 2005;35:84.
Hollingsworth AB: Perspectives on preoperative staging with breast MRI. J Am Coll Surg 2004;199:173.
Lenahan C et al: The role of tumor markers in breast cancer management. Curr Surg 2004;61:532.
Ljung BM et al: Cytology of ductal lavage fluid of the breast. Diagn Cytopathol 2004;30:143.
P.731
Differential Diagnosis
The lesions to be considered most often in the differential diagnosis of breast cancer are the following, in descending order of frequency: fibrocystic condition of the breast, fibroadenoma, intraductal papilloma, lipoma, and fat necrosis.
Staging
Currently, the American Joint Committee on Cancer and the International Union Against Cancer have agreed on a TNM (tumor, regional lymph nodes, distant metastases) staging system for breast cancer. The use of this uniform TNM staging system enhances communication between investigators and clinicians. Table 16-2 sets forth the TNM classification.
Pathologic Types
Numerous pathologic subtypes of breast cancer can be identified histologically (Table 16-3). These types are distinguished by the histologic appearance and growth pattern of the tumor. In general, breast cancer arises either from the epithelial lining of the large or intermediate-sized ducts (ductal) or from the epithelium of the terminal ducts of the lobules (lobular). The cancer may be invasive or in situ. Most breast cancers arise from the intermediate ducts and are invasive (invasive ductal, infiltrating ductal), and most histologic types are merely subtypes of invasive ductal cancer with unusual growth patterns (colloid, medullary, scirrhous, mucinous, etc). Ductal carcinoma that has not invaded the extraductal tissue is intraductal or in situ ductal. Lobular carcinoma may be either invasive or in situ. In situ lobular carcinoma is primarily a risk factor for the development of invasive ductal cancer.
Except for the in situ cancers, the histologic subtypes have only a slight bearing on prognosis when outcomes are compared after accurate staging. Various histologic parameters, such as invasion of blood vessels, tumor differentiation, invasion of breast lymphatics, and tumor necrosis have been examined, but they too seem to have little prognostic value.
The noninvasive cancers by definition are confined by the basement membrane of the ducts and lack the ability to spread. However, in patients whose biopsies show noninvasive intraductal cancer, associated invasive ductal cancers metastasize to lymph nodes in about 1 3% of cases.
Special Clinical Forms of Breast Cancer
Paget's Carcinoma
The basic lesion is usually an infiltrating ductal carcinoma, usually well differentiated, or a ductal carcinoma in situ (DCIS). The ducts of the nipple epithelium are infiltrated, but gross nipple changes are often minimal, and a tumor mass may not be palpable. The first symptom is often itching or burning of the nipple, with superficial erosion or ulceration. The diagnosis is established by biopsy of the erosion.
Paget's carcinoma is not common (about 1% of all breast cancers), but it is important because the nipple changes appear innocuous and the diagnosis frequently is missed. The nipple changes are often diagnosed and treated as dermatitis or bacterial infection, leading to delay in detection. When the lesion consists of nipple changes only, the incidence of axillary metastases is less than 5%, and the prognosis is excellent. When a breast mass is also present, the incidence of axillary metastases rises, with an associated marked decrease in prospects for cure by surgical or other treatment.
Inflammatory Carcinoma
This is the most malignant form of breast cancer and constitutes less than 3% of all cases. The clinical findings consist of a rapidly growing, sometimes painful mass that enlarges the breast. The overlying skin becomes erythematous, edematous, and warm. Often there is no distinct mass, since the tumor infiltrates the involved breast diffusely. The diagnosis should be made when the redness involves more than one-third of the skin over the breast and biopsy shows infiltrating carcinoma with invasion of the subdermal lymphatics. The inflammatory changes, often mistaken for an infection, are caused by carcinomatous invasion of the subdermal lymphatics, with resulting edema and hyperemia. If the practitioner suspects infection but the lesion does not respond rapidly (1 2 weeks) to antibiotics, biopsy should be performed. Metastases tend to occur early and widely, and for this reason inflammatory carcinoma is rarely curable. Mastectomy is seldom indicated unless chemotherapy and radiation have resulted in clinical remission with no evidence of distant metastases. In these cases, residual disease in the breast may be eradicated. Radiation, hormone therapy, and chemotherapy are the measures most likely to be of value rather than operation.
Breast Cancer Occurring during Pregnancy or Lactation
Breast cancer complicates approximately one in 3000 pregnancies. The diagnosis is frequently delayed, because physiologic changes in the breast may obscure the lesion. This results in a tendency of both patients and practitioners to misinterpret findings and to delay biopsy. When the cancer is confined to the breast, the 5-year survival rate after mastectomy is about 70%. Axillary metastases are already present in 60 70% of patients, and for them the 5-year survival rate after mastectomy is only 30 40%. Pregnancy (or lactation) is not a contraindication to operation, and treatment should be based on the stage of the disease as in the nonpregnant (or nonlactating) woman. Overall survival rates have improved, since cancers are now diagnosed in pregnant women earlier than in the past. Breast-conserving surgery may be performed and radiation and chemotherapy given even during the pregnancy.
Table 16-2. TNM staging for breast cancer. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
P.732
P.733
Bilateral Breast Cancer
Clinically evident simultaneous bilateral breast cancer occurs in less than 5% of cases, but there is as high as a 20 25% incidence of later occurrence of cancer in the second breast. Bilaterality occurs more often in familial breast cancer, in women under age 50 years, and when the tumor in the primary breast is lobular. The incidence of second breast cancers increases directly with the length of time the patient is alive after her first cancer about 1 2% per year.
In patients with breast cancer, mammography should be performed before primary treatment and at regular intervals thereafter, to search for occult cancer in the opposite breast or conserved ipsilateral breast. Routine biopsy of the opposite breast is usually not warranted even for lobular cancer.
Table 16-3. Histologic types of breast cancer. | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Noninvasive Cancer
Noninvasive cancer can occur within the ducts (ductal carcinoma in situ, DCIS) or lobules (lobular carcinoma in situ, LCIS). LCIS, although thought to be a premalignant lesion or a risk factor for breast cancer, in fact may behave like DCIS. In a 2004 analysis of multiple NSABP studies, invasive lobular breast cancer not only developed in patients with LCIS but it developed in the same breast and indexed location as the original LCIS. Although more research needs to be done in this area, the invasive potential of LCIS is
P.734
being reconsidered. DCIS tends to be unilateral and most often progresses to invasive cancer if untreated. In approximately 40 60% of women who have DCIS treated with biopsy alone, invasive cancer develops within the same breast.
The treatment of intraductal lesions is controversial. DCIS can be treated by wide excision with or without radiation therapy or with total mastectomy. Conservative management is advised in this patient population with small lesions amenable to lumpectomy until further data are developed. Although research is defining the malignant potential of LCIS, it may be well managed with observation, but patients unwilling to accept the increased risk of breast cancer may be offered surgical excision of the area in question or even bilateral total mastectomy. Currently, accepted standards of care offer the alternative of chemoprevention, using agents such as tamoxifen, which is effective in preventing invasive breast cancer from developing in both LCIS and intraductal carcinoma in situ that has been completely excised. Axillary metastases from in situ cancers should not occur unless there is an occult invasive cancer. Sentinel node biopsy may be indicated in large DCIS treated with mastectomy.
Barni S et al: Locally advanced breast cancer. Curr Opin Obstet Gynecol 2006;18:47.
Fisher ER et al: Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: twelve-year observations concerning lobular carcinoma in situ. Cancer 2004; 100:238.
Kawase K et al: Paget's disease of the breast: there is a role for breast-conserving therapy. Ann Surg Oncol 2005;12:391.
Khan A et al: Diagnosis and management of ductal carcinoma in situ. Curr Treat Options Oncol 2004;5:131.
Lerebours F et al: Update on inflammatory breast cancer. Breast Cancer Res 2005;7:52.
Ring AE et al: Breast cancer and pregnancy. Ann Oncol 2005; 16:1855.
Tai P et al: Short- and long-term cause-specific survival of patients with inflammatory breast cancer. BMC Cancer 2005; 5:137.
Biomarkers
The presence or absence of estrogen receptors (ER) and progesterone receptors (PR) in the cytoplasm of tumor cells is of paramount importance in managing patients with breast cancer. Patients whose primary tumors are receptor-positive have a more favorable course than those whose tumors are receptor-negative. Receptors are of value in determining adjuvant therapy and for treatment of advanced disease. Up to 60% of patients with metastatic breast cancer will respond to hormonal manipulation if their tumors contain estrogen receptors. Fewer than 5% of patients with metastatic, ER-negative tumors can be treated successfully in this fashion.
Receptor status is valuable not only in managing metastatic disease but also in helping select patients for adjuvant therapy. Adjuvant hormonal therapy (tamoxifen) or AIs with receptor-positive tumors and adjuvant chemotherapy with receptor-negative tumors improve survival rates even in the absence of lymph node metastases (see Adjuvant Therapy, below).
PR status may be a more sensitive indicator than ER status of patients who may respond to hormonal manipulation. Up to 80% of patients with metastatic PR-positive tumors improve with hormonal manipulation. Receptors have no relationship to response to chemotherapy.
In addition to ER status and PR status, the rate at which tumor divides and the differentiation of the cells (proliferative indices) are important. In order to establish the rate of growth and differentiation, the amount and type of DNA is measured with flow cytometry.
The ER status, PR status, proliferative indices, and HER-2/neu status of the tumor should be determined at the time of initial biopsy. This is performed on paraffin-fixed tissue by immunohistochemistry. HER-2/neu overexpression is scored using a numerical system: 1+ is not an overexpressor, 2+ is borderline, and 3+ is an overexpressor. In the case of 2+ expression, fluorescence in situ hybridization (FISH) is recommended to more accurately assess HER-2/neu amplification and provide better prognostic information. It is critical to understand the receptor status prior to initiating any adjuvant therapy because it may change after hormonal therapy or chemotherapy as well as aid in the assessment of prognosis. While individually these biomarkers provide insight to appropriate adjuvant therapy, when combined they provide a great deal of information regarding risk of recurrence. A new test, Oncotype DX, combines 21 genetic markers, including estrogen receptor, progesterone receptor, and HER-2/neu expression in a tumor specimen. The researchers were able to categorize risk of recurrence into
P.735
three groups: high risk, intermediate risk, and low risk. In addition, the test is able to identify that the high-risk group was more likely to benefit from chemotherapy in addition to tamoxifen while the low risk group did not. This type of test is quite helpful when the survival advantage of therapy is difficult to determine but is only appropriate for ER-positive node-negative tumors. Its applicability is limited by its experience and lack of prospective corroboration.
Another promising biomarker being studied is vascular endothelial growth factor (VEGF), a protein that stimulates the growth of blood vessels. Elevated levels of VEGF may be a marker for a tumor that is more aggressive since it has the ability to develop blood vessels and grow. While researchers look for more specific markers to determine the presence of breast cancer, these markers also provide insight to targeted methods of treatment. Other markers being evaluated are p53, nm23, DNA 5c exceeding rate (DNA 5cER), G-actin, urokinase-type plasminogen activator (u-PA), and its type-1 inhibitor (PAI-1).
Konecny G et al: Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 2003;95:142.
Paik S et al: A multigene assay to predict recurrence of tamoxifen treated, node-negative breast cancer. N Eng J Med 2004; 351:2817.
Winston JS et al: HER-2/neu evaluation in breast cancer are we there yet? Am J Clin Pathol 2004;121:S33.
Zhang W et al: Biomarker analysis on breast ductal lavage cells in women with and without breast cancer. Int J Cancer 2006 [Epub ahead of print].
Curative Treatment
Treatment may be curative or palliative. Curative treatment is advised for clinical stage I, II, and III disease (Table 16-2). Patients with locally advanced (T3, T4) and even inflammatory tumors may be cured with multimodality therapy, but in most palliation is all that can be expected. Palliative treatment is appropriate for all patients with stage IV disease and for previously treated patients in whom distant metastases develop or who have unresectable local cancers.
The growth potential of tumors and host resistance factors vary widely from patient to patient and may be altered during the course of the disease. The doubling time of breast cancer cells ranges from several weeks in a rapidly growing lesion to years in slowly growing ones. Assuming that the rate of doubling is constant and that the neoplasm originates in one cell, a carcinoma with a doubling time of 100 days may not reach clinically detectable size (1 cm) for about 8 years. Rapidly growing cancers have a much shorter preclinical course and a greater tendency to metastasize by the time a breast mass is discovered.
The long preclinical growth phase and the tendency of breast cancers to metastasize have led clinicians to believe that most breast cancer is a systemic disease at the time of diagnosis. Although it may be true that breast cancer cells are released from the tumor prior to diagnosis, variations in the host-tumor relationship prohibit the growth of disseminated disease in many patients. Clearly, not all breast cancer is systemic at the time of diagnosis. For this reason, a pessimistic attitude concerning the management of breast cancer is unwarranted. Most patients can be cured.
Controversy surrounds the timing of surgery with respect to the menstrual cycle. Some suggest that operation during the time of unopposed estrogen adversely affects survival, but most studies support no such effect. Several randomized trials are currently examining this question.
Choice of Primary Therapy
The extent of disease and its biologic aggressiveness are the principal determinants of the outcome of primary therapy. Clinical and pathologic staging help in assessing extent of disease (Table 16-2), but each is to some extent imprecise. Other factors such as DNA flow cytometry, tumor grade, hormone receptor assays, and oncogene amplification may be of prognostic value but are not important in determining the type of local therapy.
Controversy surrounds the choice of primary therapy of stage I, II, and III breast carcinoma. A number of states require physicians to inform patients of alternative treatment methods in the management of breast cancer. Currently, the standard of care for stage I, stage II, and most stage III cancer is surgical resection followed by adjuvant radiation or systemic therapy when indicated.
Breast-Conserving Therapy
Many nonrandomized trials, the randomized Milan trial, and a large randomized trial conducted by the NSABP in the United States show that disease-free survival rates are similar for patients treated by partial mastectomy plus axillary dissection followed by radiation therapy and for those treated by modified radical mastectomy (total mastectomy plus axillary dissection). All patients whose axillary nodes contained tumor received adjuvant chemotherapy.
In the NSABP trial, patients were randomized to three treatment types: (1) lumpectomy (removal of the tumor with confirmed tumor-free margins) plus whole breast irradiation, (2) lumpectomy alone, and (3) total mastectomy. All patients underwent axillary lymph node dissection, and some had tumors as large as 4 cm with (or without) palpable axillary lymph nodes. With 20 years of follow-up, the lowest local recurrence rate was among patients treated with lumpectomy and postoperative irradiation, approximately 14%; the highest nearly 40% was among patients treated with lumpectomy alone. However, the overall survival as well as the distant disease-free survival were
P.736
similar among the three treatment groups. This study shows that lumpectomy and axillary dissection with postoperative radiation therapy are as effective as modified radical mastectomy for the management of patients with stage I and stage II breast cancer.
The results of these and other trials have demonstrated that much less aggressive surgical treatment of the primary lesion than has previously been thought necessary gives equivalent therapeutic results and may preserve an acceptable cosmetic appearance.
Tumor size is a major consideration in determining the feasibility of breast conservation. The lumpectomy trial of the NSABP randomized patients with tumors as large as 4 cm. To achieve an acceptable cosmetic result, the patient must have a breast of sufficient size to enable excision of a 4-cm tumor without considerable deformity. Therefore, large size is only a relative contraindication. Subareolar tumors, also difficult to excise without deformity, are not contraindications to breast conservation. Clinically detectable multifocality is a relative contraindication to breast-conserving surgery, as is fixation to the chest wall or skin or involvement of the nipple or overlying skin. The patient not the surgeon should be the judge of what is cosmetically acceptable.
Axillary dissection is valuable in preventing axillary recurrences, in staging cancer, and in planning therapy. Intraoperative lymphatic mapping and sentinel node dissection identify lymph nodes most likely to harbor metastases if present in the axillary nodes. A trial from Milan with very short follow-up showed no survival difference between axillary dissection and sentinel node biopsy in node-negative women. Results suggest that sentinel node biopsy can safely replace axillary dissection for staging and treatment in histopathologically node-negative women at experienced centers. At an international consensus conference in Philadelphia in 2001, participants recommended sentinel node biopsy as an alternative to axillary dissection in selected patients with invasive cancer. Bone marrow biopsy with examination by immunocytochemistry to detect early metastases may be as sensitive a staging procedure as axillary dissection and may identify patients at high risk for disseminating disease.
Recommendations
Earlier consensus held that breast-conserving surgery with radiation was the preferred form of treatment for patients with early-stage breast cancer. Despite the numerous randomized trials showing no survival benefit of mastectomy over breast-conserving partial mastectomy and irradiation, breast-conserving surgery appears underutilized and mastectomy remains the more common treatment. About 25% of patients in the United States with stage I or stage II breast cancer are treated with breast-conserving surgery and radiation therapy, compared with 75% treated with mastectomy. Use of breast-conserving surgery and radiation therapy varies by region of the country, ranging from 15% in the South Central United States to 30% in the Pacific Region.
Modified radical mastectomy (total mastectomy plus axillary lymph node dissection) has been the standard therapy for most patients with breast cancer. This operation removes the entire breast, overlying skin, nipple, and areolar complex as well as the underlying pectoralis fascia with the axillary lymph nodes in continuity. The major advantage of modified radical mastectomy is that radiation therapy may not be necessary. The disadvantage, of course, is the psychological impact associated with breast loss. Radical mastectomy, which removes the underlying pectoralis muscle, should be performed rarely, if at all. Axillary node dissection is not indicated for noninfiltrating cancers, because nodal metastases are rarely present. Skin-sparing mastectomy is currently gaining favor but is appropriate in only a small subgroup of patients.
Radiotherapy after partial mastectomy consists of 5 6 weeks of five daily fractions to a total dose of 5000 6000 cGy. Most radiation oncologists use a boost dose. Currently, several studies are underway examining the utility and recurrence rates after intraoperative radiation or dose dense radiation in which the time course of radiation is shortened. Current studies suggest that radiotherapy after mastectomy may improve survival in a subset of patients and meta-analyses suggest radiation after lumpectomy may improve survival. The use of radiation in mastectomy patients is being further researched in a large cooperative trial to better identify which subgroups will benefit. Researchers are also examining the utility of axillary irradiation as an alternative to axillary dissection in the clinically node-negative patient with sentinel node metastases.
Preoperatively, full discussion with the patient regarding the rationale for operation and various alternative forms of treatment is essential. Breast-conserving surgery and radiation should be offered whenever possible, since most patients would prefer to save the breast. Breast reconstruction, immediate or delayed, should be discussed with patients who choose or require mastectomy. Patients should have an interview with a reconstructive plastic surgeon to discuss options prior to making a decision regarding reconstruction. Time is well spent preoperatively in educating the patient and family about these matters.
Adjuvant Systemic Therapy
Following surgery and radiation therapy, chemotherapy or hormonal therapy is advocated for most patients with curable breast cancer. The objective of adjuvant systemic therapy is to eliminate the occult metastases responsible for late recurrences while they are microscopic and most vulnerable to anticancer agents. In addition, adjuvant chemotherapy may decrease local recurrence in patients treated with breast conservation, whereas adjuvant hormonal manipulation decreases contralateral breast cancer occurrence.
P.737
Even the earliest studies comparing placebo with chemotherapy drugs having minimal activity such as L-phenylalanine mustard showed an improvement in both disease-free and overall survival for women disease free postoperatively. The landmark study from Milan, Italy evaluating the effect of 1 year of adjuvant cyclophosphamide, methotrexate, and fluorouracil (CMF) given on days 1 and 8 of each month for 12 months, showed a significant improvement in survival for premenopausal women with node-positive disease. After 20 years of follow-up, significant improvement in survival persisted among those receiving chemotherapy. CMF rapidly became the standard management for premenopausal women with node-positive breast cancer. Subsequently, the use of chemotherapy for postmenopausal women and those at less risk than node-positive women was evaluated. Systemic chemotherapy improves survival in all groups of women treated. The improvement in survival appears to be about 30% of the patients' risk of death; that is, a woman with a 30% chance of recurrence and death derives about a 10% overall improvement in survival. This risk reduction analysis has been confirmed in numerous studies and meta-analyses.
On the basis of the superiority of anthracycline-containing regimens in metastatic breast cancer, both doxorubicin and epirubicin have been studied extensively in the adjuvant setting and have been compared to CMF regimens. Studies comparing Adriamycin (doxorubicin) and cyclophosphamide (AC) or epirubicin and cyclophosphamide (EC) with CMF have shown that treatment with anthracycline-containing regimens are at least as effective, and perhaps more effective, as treatment with CMF. The NSABP B-23 compared four cycles of AC with six cycles of CMF and demonstrated the equivalence of these two regimens in node-negative, ER-negative disease. Whereas four cycles of AC or EC have not demonstrated improved survival compared with CMF, the use of six cycles of fluorouracil plus AC (FAC) or fluorouracil plus EC (FEC) has shown improved survival compared with CMF alone. For node-negative patients, most oncologists offer four cycles of AC or six cycles of CMF in the adjuvant setting.
For node-positive patients, taxanes are now frequently combined with anthracycline-based regimens. The Cancer and Leukemia Group B (CALGB) study comparing four cycles of AC to four cycles of AC followed by four cycles of paclitaxel showed about a 20% proportional reduction in recurrence and a 4% absolute improvement in disease-free survival with the use of paclitaxel. Paclitaxel is FDA-approved for and increasingly used as adjuvant therapy in node-positive breast cancer. Unfortunately, a subsequent study by the NSABP failed to show any benefits of the use of paclitaxel except in ER-negative patients with positive nodes as did later results of the CALGB study. A 2002 National Institutes of Health (NIH) consensus panel felt that firm conclusions about the use of taxanes could not be drawn and recommended that patients receive adjuvant taxanes only in the context of a clinical trial. However, based on trends in improved survival, most oncologists add a taxane to AC for node-positive women. A trial comparing six cycles of FAC to six cycles of docetaxel, doxorubicin, and cyclophosphamide (TAC) showed an improvement in disease-free survival for patients receiving the addition of paclitaxel. This benefit was most marked for patients with positive nodes and was seen in both ER-negative and ER-positive tumors. Until more information is obtained, the role of taxanes in the adjuvant setting remains unclear.
Controversy exists as to whether patients whose tumors overexpress the HER-2/neu oncogene benefit more from anthracycline regimens than from CMF regimens. Retrospective analysis of randomized trials suggests that patients with HER-2/neu overexpression may benefit more from doxorubicin than patients with HER-2/neu-negative disease. These retrospective studies have numerous problems including the analysis of HER-2/neu on paraffin tissue blocks. Trastuzumab (Herceptin), when studied in the metastatic setting, has proved effective in combination with chemotherapy in patients with HER-2/neu overexpression. Published in early 2006, a multicenter trial from Finland by the Fin-Her collaborative group, studied the use of trastuzumab in combination with docetaxel or vinorelbine for patients with early breast cancer that demonstrated HER-2/neu overexpression. The 3-year recurrence-free survival was better in those who took trastuzumab than in those who did not receive the antibody, 89% versus 78%, respectively. In another study from Brussels, the HERA trial, a similar disease-free survival at interim analysis was demonstrated when giving trastuzumab subsequent to adjuvant chemotherapy in early breast cancer. While both these studies require additional follow-up, the ability to reduce recurrence in the adjuvant setting is promising and many medical oncologists are adding trastuzumab to their adjuvant chemotherapy regimens in early breast cancer with HER-2/neu overexpression.
The overall duration of adjuvant chemotherapy still remains uncertain. However, based on the meta-analysis performed in the Oxford Overview (Early Breast Cancer Trialists' Collaborative Group), the current recommendation is for 3 6 months of the commonly used regimens. The addition of taxanes required an additional duration of therapy of up to 6 months. Increasing the frequency of chemotherapy administration (dose dense chemotherapy) has been shown to be superior to standard dosing. It is often used when there is a greater risk of recurrence or in the younger patient, since it is a difficult regimen to tolerate physically.
Adjuvant hormonal therapy is also highly effective in decreasing recurrence and mortality in women with ER-positive tumors. The standard regimen has been tamoxifen for 5 years. Hormonal therapy decreases the risk of breast cancer mortality by approximately 25%. This appears to be effective regardless of age and may
P.738
be used in both premenopausal and postmenopausal women. More recently, the AIs have been shown to be effective in the adjuvant setting. The large Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial in postmenopausal women with ER-positive disease showed improved disease-free survival in patients treated with anastrozole compared with those treated with tamoxifen alone or even with the combination of tamoxifen and anastrozole. In addition, anastrozole showed a decrease of over 50% in the recurrence of contralateral breast tumors and fewer side effects such as endometrial cancers, hot flushes, and thromboembolic events. However, anastrozole did have an increase in fractures related to bone loss and the use of the drug. Anastrozole is increasingly being used in the adjuvant setting in postmenopausal women. Because of the extensive long-term data supporting the use of tamoxifen, the American Society of Clinical Oncology, continues to recommend the use of tamoxifen for adjuvant hormonal therapy in the absence of significant contraindications; anastrozole is recommended when there are contraindications to tamoxifen. In addition, anastrozole is being used after patients complete tamoxifen therapy or prior to completing therapy (year 2 or 3) to further decrease recurrences.
Use of high-dose chemotherapy with stem cell support has not demonstrated a consistent, favorable impact on survival and should not be used outside of clinical trials. Although it is clear that dose intensity to a specific threshold is essential, there is no clear benefit to high-dose therapy with stem cell support.
An NIH consensus conference has reexamined the standards for adjuvant therapy of breast cancer. Since the last conference on this topic in 2000, the long-term advantage of systemic therapy has been further established. No new prognostic factors have been validated to aid in the selection of patients for adjuvant treatment. Its use should be based on the patient's age; on the size, histopathologic grade, and hormone receptor status of the breast tumor; and on the status of the regional lymph nodes. The value of HER-2/neu, p53, angiogenesis factors, and vascular invasion is being investigated, but they remain to be proven prognostic factors. Studies are being conducted evaluating trastuzumab in the adjuvant and neoadjuvant setting in a group of patients with newly diagnosed disease in which the tumors overexpress HER-2/neu. The NIH panel concluded that regardless of other factors, adjuvant systemic chemotherapy with drug combinations improves survival and should be used for most women who have potentially curable breast cancer. The use of anthracyclines is superior to combinations without anthracyclines. Tamoxifen should be used as a systemic agent in all women whose tumors are hormone receptor positive regardless of age, menopausal status, or other prognostic factors. HER-2/neu status should not affect the choice of agents or the use of hormone therapy. Ovarian ablation in premenopausal patients with ER-positive tumors may produce a benefit similar to that of adjuvant systemic chemotherapy. Taxanes have demonstrated benefit in patients with metastatic cancer and are being used in node-negative patients as is trastuzumab. Adjuvant systemic therapy should not be given to women who have small node-negative breast cancers with favorable histologic findings and tumor markers such as mucinous or tubular carcinoma, ER-positive, low grade.
In practice, most medical oncologists are currently using systemic adjuvant therapy for patients with either node-negative or node-positive breast cancer. Prognostic factors other than nodal status being used to determine the patient's risks are tumor size, ER and PR status, nuclear grade, histologic type, proliferative rate, and oncogene expression (Table 16-4). The assumption is made that all patients with node-negative aggressive tumors should receive adjuvant therapy except those who have serious coexistent medical problems. In general, systemic chemotherapy decreases the chance of recurrence by about 30%. Most patients tolerate at least tamoxifen. The use of chemotherapy or hormonal therapy prior to resection of the primary tumor (neoadjuvant) is gaining popularity. This enables the assessment of in vivo chemosensitivity. A complete tumor response in vivo prior to operation appears to be associated with improvement in survival. Neoadjuvant chemotherapy also permits breast conservation by shrinking the primary tumor in women who would otherwise need mastectomy for local control. There was considerable concern as to the timing of sentinel lymph node biopsy (SLNB), since the chemotherapy may affect any cancer present in the lymph nodes. After several studies, it is still unclear as to the appropriate timing of the procedure. A large multicenter study, NSABP B-27, studied this question as an adjunct to a neoadjuvant efficacy trial. The false-negative
P.739
rate was as high as 10.7%, which is well above the false-negative rate outside the neoadjuvant setting (< 1 5%). Many physicians recommend performing SLNB prior to administering the chemotherapy in order to avoid a false-negative result. If a complete dissection is necessary, this can be completed at the time of the definitive surgery. While SLNB is critical to staging, the timing remains physician dependent and its role with neoadjuvant therapy is unclear.
Table 16-4. Prognostic factors in node-negative breast cancer. | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Important questions remaining to be answered are the timing and duration of adjuvant and neoadjuvant chemotherapy, which chemotherapeutic agents should be applied for which subgroups of patients, the use of combinations of hormonal therapy and chemotherapy, and the value of prognostic factors other than hormone receptors in predicting response to therapy. Adjuvant systemic therapy is not generally used in patients with small tumors and those with negative lymph nodes who have favorable tumor markers. However, a small disease-free survival benefit, even in patients with small favorable tumors, has been suggested. It appears that adjuvant systemic therapy benefits all breast cancer patients, but the clinician and patient must decide if the benefits outweigh the risks, complications, and expense.
Delaney G: Recent advances in the use of radiotherapy to treat early breast cancer. Curr Opin Obstet Gynecol 2005;17:27.
Early Breast Cancer Trialists' Collaborative Group: Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet 2000;355:1757.
Fisher B et al: Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233.
Fisher B et al: Treatment of axillary lymph node-negative, estrogen receptor-negative breast cancer: updated findings from National Surgical Adjuvant Breast and Bowel Project clinical trials. J Natl Cancer Inst 2004;96:1823.
Fisher B et al: Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2005;23:2694.
Howell A et al: ATAC Trialists' Group: Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 2005;365:60.
Joensuu H et al; FinHer Study Investigators: Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006;354:809.
Keshtgar MR et al: New approaches in breast cancer management: sentinel node biopsy and intraoperative radiotherapy. Int J Fertil Womens Med 2005;50(5 Pt 1):218.
Lemanski C et al: Intraoperative radiotherapy given as a boost for early breast cancer: Long-term clinical and cosmetic results. Int J Radiat Oncol Biol Phys 2006;64:1410.
Love RR: Meeting highlights: international consensus panel on the treatment of primary breast cancer. J Clin Oncol 2002;20:1955.
Mamounas EP et al: Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 2005;23: 3686.
Muller V et al: Bone marrow micrometastases and circulating tumor cells: current aspects and future perspectives. Breast Cancer Res 2004;6:258.
Piccart-Gebhart MJ et al; Herceptin Adjuvant (HERA) Trial Study Team: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659.
Posther KE et al: Sentinel node skills verification and surgeon performance: data from a multicenter clinical trial for early-stage breast cancer. Ann Surg 2005;242:593.
Sokolowicz LE et al: Hormonal therapy for primary breast cancer: scientific rationale and status of clinical research. Curr Oncol Rep 2005;7:31.
Stolier AJ et al: Postlumpectomy insertion of the MammoSite brachytherapy device using the scar entry technique: initial experience and technical considerations. Breast J 2005;11: 199.
The National Institutes of Health Consensus Development Conference: Adjuvant Therapy for Breast Cancer. Bethesda, Maryland, USA. November 1 3, 2000. Proceedings. J Natl Cancer Inst Monogr 2001;(30):1.
Veronesi U et al: Twenty-year follow-up of randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227.
Vinh-Hung V et al: Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst 2004;96: 115.
Vogel C et al: Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002;3:719.
Palliative Treatment
This section covers palliative therapy of disseminated disease incurable by surgery (stage IV).
Radiotherapy
Palliative radiotherapy may be advised for primary treatment of locally advanced cancers with distant metastases to control ulceration, pain, and other manifestations in the breast and regional nodes. Irradiation of the breast and chest wall and the axillary, internal mammary, and supraclavicular nodes should be undertaken in an attempt to cure locally advanced and inoperable lesions when there is no evidence of distant metastases. A small number of patients in this group are cured in spite of extensive breast and regional node involvement.
Palliative irradiation is of value also in the treatment of certain bone or soft tissue metastases to control pain or avoid fracture. Radiotherapy is especially useful in the treatment of isolated bony metastasis, chest wall recurrences, brain metastases, and acute spinal cord compression.
Table 16-5. Agents commonly used for hormonal management of metastatic breast cancer. | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hormone & Targeted Therapy
Disseminated disease may shrink or grow less rapidly after endocrine therapy such as administration of
P.740
hormones (eg, estrogens, androgens, progestins; see Table 16-5); ablation of the ovaries, adrenals, or pituitary; or administration of drugs that block hormone receptor sites (eg, antiestrogens) or drugs that block the synthesis of hormones (eg, AIs). Hormonal manipulation is usually more successful in postmenopausal women even if they have received estrogen replacement therapy. Treatment should be based on the presence of ER protein in the primary tumor or metastases. The rate of response is nearly equal in premenopausal and postmenopausal women with ER-positive tumors. A favorable response to hormonal manipulation occurs in about one-third of patients with metastatic breast cancer. Of those whose tumors contain ERs, the response is about 60% and perhaps as high as 80% for patients whose tumors contain PRs as well. Because only 5 10% of women whose tumors do not contain ERs respond, they should not receive hormonal therapy except in unusual circumstances, eg, in an older patient who cannot tolerate chemotherapy. Because the quality of life during a remission induced by endocrine manipulation is often superior to a remission following cytotoxic chemotherapy, it is usually best to try endocrine manipulation first in cases in which the ER status is unknown. In addition, women with ER-positive tumors who do not respond to hormone therapy or experience progression should be placed on a different form of hormonal manipulation. Women whose tumor has failed to respond to tamoxifen and gone on to a third-generation AI have shown equal if not better response than those who respond to tamoxifen. However, when receptor status is unknown and the disease is progressing rapidly or involves visceral organs, endocrine therapy is rarely successful, and introducing it may waste valuable time.
In addition to radiotherapy, bisphosphonate therapy has shown excellent results in delaying and reducing skeletal events in women with bony metastases. Bisphosphonates are also sometimes used in conjunction with AIs to decrease the potential bony events associated with those drugs. Further research examining the utility of bisphosphonates in conjunction with other therapies and in early breast cancer treatment is being conducted.
In general, only one type of therapy should be given at a time unless it is necessary to irradiate a destructive lesion of weight-bearing bone while the patient is receiving another regimen. The regimen should be changed only if the disease is clearly progressing. This is especially important for patients with destructive bone metastases, since changes in the status of these lesions are difficult to determine radiographically. A plan of therapy that would simultaneously minimize toxicity and maximize benefits is often best achieved by hormonal manipulation.
The choice of endocrine therapy depends on the menopausal status of the patient. Women within 1 year of their last menstrual period are arbitrarily considered to be premenopausal, whereas women whose menstruation ceased more than a year ago are postmenopausal. If endocrine therapy is the initial choice, it is referred to as primary hormonal manipulation; subsequent endocrine treatment is called secondary or tertiary hormonal manipulation.
Trastuzumab is a monoclonal antibody that binds to HER-2/neu receptors on the cancer cell and has been
P.741
shown to be highly effective in HER-2/neu-expressive cancers. In metastatic disease, for patients with HER-2/neu oncogene overexpression, trastuzumab has been shown to increase survival when combined with AC or paclitaxel. Ongoing studies are evaluating trastuzumab in combination with other agents for adjuvant chemotherapy regimens in newly diagnosed cases.
In addition, compelling data have been reported regarding the use of an antiangiogenesis drug bevacizumab (Avastin) in the treatment of metastatic disease. Based on the amount of VEGF detected in the primary tumor, bevacizumab can increase overall survival and disease-free survival when used in combination with chemotherapy when metastases are present. Studies are ongoing and hold a great deal of promise.
A. The Premenopausal Patient
1. Primary hormonal therapy
The potent antiestrogen tamoxifen is by far the most common and preferred method of hormonal manipulation in the premenopausal patient. Tamoxifen is usually given orally in a dose of 20 mg daily. There is no significant difference in survival or response between tamoxifen therapy and bilateral oophorectomy. The average remission is about 12 months. Tamoxifen can be given with little morbidity and few side effects. Toremifene, a tamoxifen analog, has similar side effects but is less likely to cause uterine cancer. Controversy continues about whether a response to tamoxifen is predictive of probable success with other forms of endocrine manipulation.
Bilateral oophorectomy is less desirable than primary hormonal manipulation in premenopausal women because tamoxifen is so well tolerated. However, oophorectomy can be achieved rapidly and safely by surgery, or if the patient is a poor operative risk, by irradiation of the ovaries. Chemical ovarian ablation using a gonadotropin-releasing hormone (GnRH) analog can also be utilized. Oophorectomy presumably works by eliminating estrogens, progestins, and androgens, which stimulate growth of the tumor. AIs should not be used in a patient with functioning ovaries.
2. Secondary or tertiary hormonal therapy
Although patients who do not respond to tamoxifen or oophorectomy should be treated with cytotoxic drugs, those who respond and then relapse may subsequently respond to another form of endocrine treatment (Table 16-5). The initial choice for secondary endocrine manipulation has not been clearly defined.
Patients who improve after oophorectomy but subsequently relapse should receive tamoxifen or an AI. If one fails, the other may be tried but is not likely to succeed. Megestrol acetate, a progesterone agent, may be considered. Both drugs cause less morbidity and mortality than surgical adrenalectomy, can be discontinued once the patient improves, and are not associated with the many problems of postsurgical hypoadrenalism, so that patients who require chemotherapy are more easily managed. Adrenalectomy or hypophysectomy induced regression in 30 50% of patients who previously responded to oophorectomy, but these procedures are rarely done today. Pharmacologic hormonal manipulation has replaced these invasive procedures. Toremifene has shown no added value when tumors no longer respond to tamoxifen. AIs are of value when a tumor had responded to tamoxifen or oophorectomy but then progresses.
B. The Postmenopausal Patient
1. Primary hormonal therapy
Tamoxifen, 20 mg orally daily, or anastrozole, 1 mg orally daily, is the initial therapy of choice for postmenopausal women with metastatic breast cancer amenable to endocrine manipulation. Anastrozole (an AI) has fewer side effects than tamoxifen, the former therapy of choice, and is at least equally as effective. The main side effects of tamoxifen are nausea, vomiting, skin rash, and hot flushes. Rarely, it may induce hypercalcemia in patients with bony metastases. The main side effects of anastrozole are similar but lower in incidence; however, osteoporosis and bone fractures are significant. Other AIs are letrozole or exemestane. They have similar efficacy and side effect profiles.
2. Secondary or tertiary hormonal therapy
AIs have achieved the status of primary hormonal therapy in postmenopausal women since trials comparing an AI, anastrozole, with tamoxifen suggest that the former is just as effective and has fewer side effects. AIs are also available for the treatment of advanced breast cancer in postmenopausal women after tamoxifen treatment. In the event that the patient responds to AI, but then has progression of disease, an antiestrogen, fulvestrant (Faslodex) has shown a great deal of promise with about 20 30% of women benefiting from use. Postmenopausal patients who do not respond to SERM or AI should be given cytotoxic drugs such as CMF or AC. Postmenopausal women who respond initially to a SERM or AI but later manifest progressive disease may be crossed over to cytotoxic drugs. Androgens have many toxicities and should rarely be used. As in premenopausal patients, neither hypophysectomy nor adrenalectomy is being performed.
Chemotherapy
Cytotoxic drugs should be considered for the treatment of metastatic breast cancer (1) if visceral metastases are present (especially brain or lymphangitic pulmonary), (2) if hormonal treatment is unsuccessful or the disease has progressed after an initial response to hormonal manipulation, or (3) if the tumor is ER-negative. The most useful single chemotherapeutic agent to date is doxorubicin (Adriamycin), with a response rate of 40 50%. Single agents are rarely used but rather are given in combination with other cytotoxic drugs.
Combination chemotherapy using multiple agents has proved to be more effective, with objectively observed favorable responses achieved in 60 80% of patients with stage IV disease. Various combinations of drugs have been used, and clinical trials are always ongoing to identify a combination to increase survival and reduce undesirable
P.742
side effects. Doxorubicin (40 mg/m2 intravenously on day 1) and cyclophosphamide (200 mg/m2 orally on days 3 6) produce an objective response in about 85% of patients so treated. Other chemotherapeutic regimens have consisted of various combinations of drugs, including cyclophosphamide, vincristine, methotrexate, fluorouracil, and taxanes with response rates ranging up to 60 70%. Prior adjuvant chemotherapy does not seem to alter response rates in patients who relapse. Researchers continue to study new drugs and combinations of chemotherapy agents such as capecitabine, mitoxantrone, vinorelbine, gemcitabine, irinotecan, cisplatin, and carboplatin. Many of these agents or combinations are available to patients in a clinical trial setting or by physician's choice. For patients whose tumors have progressed after many therapies and who are considering additional therapy, clinical trial participation with experimental drugs in phase II or III testing should be encouraged.
Nausea and vomiting are well controlled with drugs that directly affect the central nervous system, such as ondansetron and granisetron. These drugs are selective antagonists of serotonin receptors in the central nervous system and block nausea caused by cytotoxic chemotherapy. Growth factors such as erythropoietin (epoetin alfa), which stimulates red blood cell production and mimics the effect of erythropoietin, and filgrastim (granulocyte colony-stimulating factor; G-CSF), which stimulates proliferation and differentiation of hematopoietic cells, prevent life-threatening anemia and neutropenia seen commonly with high doses of chemotherapy. These agents greatly diminish the incidence of infections that may complicate the use of myelosuppressive chemotherapy.
The taxanes (paclitaxel and docetaxel) have been shown to be very effective for patients with metastatic breast cancer. They have usually been given after failure of combination chemotherapy for metastatic disease or relapse shortly after completion of adjuvant chemotherapy. However, they are becoming more important in both the management of metastatic disease and even adjuvant therapy. These drugs have response rates of 30 40% in patients with metastatic disease. They may be especially valuable in treating anthracycline-resistant tumors. Both agents are being used after treatment with anthracyclines in patients with advanced disease as well as in adjuvant and neoadjuvant settings. High-dose chemotherapy and autologous bone marrow or stem cell transplantation aroused widespread interest for the treatment of metastatic breast cancer. With this technique, the patient receives high doses of cytotoxic agents, eradicating the marrow, for which the patient subsequently undergoes autologous bone marrow or stem cell transplantation. Complete response rates are as high as 30 35% considerably better than what can be achieved with conventional chemotherapy. Most randomized trials, however, comparing high-dose chemotherapy with stem cell support show no improvement in survival over conventional chemotherapy. A study purporting to show a survival advantage to high-dose chemotherapy in South Africa was found to be falsified and discredited. Enthusiasm for high-dose chemotherapy with stem cell support has waned, but additional studies continue and recently showed a beneficial effect in some high-risk women. The technique is extremely costly, and the treatment itself is associated with a mortality rate of about 3 7%.
Bernard-Marty C et al: Facts and controversies in systemic treatment of metastatic breast cancer. Oncologist 2004;9:617.
Bhatnagar AS: Review of the development of letrozole and its use in advanced breast cancer and in the neoadjuvant setting. Breast 2006;15(1 Suppl):3.
Fricker J: Letrozole better than tamoxifen in postmenopausal women. Lancet Oncol 2005;6:137.
Harvey HA: Optimizing bisphosphonate therapy in patients with breast cancer on endocrine therapy. Semin Oncol 2004;31 (6 Suppl 12):23.
Gould RE et al: Update on aromatase inhibitors in breast cancer. Curr Opin Obstet Gynecol 2006;18:41.
Hussain SA et al: Endocrine therapy and other targeted therapies for metastatic breast cancer. Expert Rev Anticancer Ther 2004;4:1179.
Ingle JN et al; North Central Cancer Treatment Group Trial N0032: Fulvestrant in women with advanced breast cancer after progression on prior aromatase inhibitor therapy: North Central Cancer Treatment Group Trial N0032. J Clin Oncol 2006;24:1052.
Mouridsen HT: Aromatase inhibitors in advanced breast cancer. Semin Oncol 2004;31(6 Suppl 12):3.
Pandit-Taskar N et al: Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med 2004; 45:1358.
Slamon DJ et al: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783.
Smith I: Goals of treatment for patients with metastatic breast cancer. Semin Oncol 2006;33(1 Suppl 2):2.
Stadtmauer EA et al: Conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer. N Engl J Med 2000;342:1069.
Prognosis
Stage of breast cancer is the most reliable indicator of prognosis (Table 16-6). Patients with disease localized to the breast and no evidence of regional spread after microscopic examination of the lymph nodes have by far the most favorable prognosis. Axillary lymph node status is the best-analyzed prognostic factor and correlates with survival at all tumor sizes. In addition, increased number of axillary nodes involved correlates directly with lower survival rates. Biologic markers of tumor aggressiveness such as estrogen, PRs, grade, and others are important prognostic variables because patients with aggressive tumors and no evidence of metastases to the axillary lymph nodes have a much higher recurrence rate than do patients with hormone receptor-positive tumors and no regional metastases. The histologic subtype of breast cancer (eg, medullary, lobular, colloid) seems to have little significance in prognosis once these tumors are truly invasive. Flow cytometry
P.743
of tumor cells to analyze DNA index and S-phase frequency aid in prognosis. Tumors with marked aneuploidy have a poor prognosis (see Table 16-4). HER-2/neu oncogene amplification, epidermal growth factor receptors, and cathepsin D may have some prognostic value, but no markers are as significant as lymph node metastases in predicting outcome.
Table 16-6. Approximate survival (%) of patients with breast cancer by TNM stage. | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The mortality rate of breast cancer patients exceeds that of age-matched normal controls for nearly 20 years. Thereafter, the mortality rates are equal, though deaths that occur among breast cancer patients are often directly the result of tumor. Five-year statistics do not accurately reflect the final outcome of therapy.
When cancer is localized to the breast, with no evidence of regional spread after pathologic examination, the clinical cure rate with most accepted methods of therapy is 75% to greater than 90%. Variations to this generalization may be related to the hormonal receptor content of the tumor, tumor size, host resistance, or associated illness. Patients with small mammographically detected biologically favorable tumors and no evidence of axillary spread have a 5-year survival rate greater than 95%. When the axillary lymph nodes are involved with tumor, the survival rate drops to 50 70% at 5 years and probably around 25 40% at 10 years. In general, breast cancer appears to be somewhat more malignant in younger than in older women, and this may be related to the fact that fewer younger women have ER-positive tumors.
For those patients whose disease progresses despite treatment, studies suggest supportive group therapy may improve survival. As they approach the end of life, such patients will require meticulous efforts at palliative care (see Chapter 5).
Hayes DF: Prognostic and predictive factors for breast cancer: translating technology to oncology. J Clin Oncol 2005;23: 1596.
Follow-Up Care
After primary therapy, patients with breast cancer should be monitored for life for at least two reasons: to detect recurrences and to observe the opposite breast for a second primary carcinoma. Local and distant recurrences occur most frequently within the first 2 5 years. During this period, the patient should be examined more frequently. Thereafter, examination is done annually. Special attention is paid to the contralateral breast because a new primary breast malignancy will develop in 20 25% of patients. The patient should examine her own breast monthly, and a mammogram should be obtained annually. In some cases, metastases are dormant for long periods and may appear 10 15 years or longer after removal of the primary tumor. Estrogen and progestational agents are rarely used for a patient free of disease after treatment of primary breast cancer, particularly if the tumor was hormone receptor positive. Studies nevertheless have failed to show an adverse effect of hormonal agents in patients who are free of disease. Even pregnancy has not been clearly associated with shortened survival of patients rendered disease free yet most oncologists are reluctant to advise a young patient with breast cancer that she may become pregnant, and most are less than enthusiastic about prescribing hormone replacement therapy for the postmenopausal breast cancer patient. The use of estrogen replacement therapy may be considered for a woman with a history of breast cancer after discussion of the benefits and risks of such therapy for conditions such as osteoporosis and hot flushes, but it is not recommended.
Local Recurrence
The incidence of local recurrence correlates with tumor size, the presence and number of involved axillary nodes, the histologic type of tumor, the presence of skin edema or skin and fascia fixation with the primary tumor, and the type of initial local (breast) therapy. Local recurrence on the chest wall after total mastectomy and axillary dissection develops in as many as 8% of patients. When the axillary nodes are not involved, the local recurrence rate is less than 5%, but the rate is as high as 25% when they are heavily involved. A similar difference in local recurrence rate was noted between small and large tumors. Factors such as multifocal cancer, in situ tumors, positive resection margins, chemotherapy, and radiotherapy have an effect on local recurrence in patients treated with breast-conserving surgery.
Chest wall recurrences usually appear within the first several years but may occur as late as 15 or more years after mastectomy. All suspicious nodules and skin lesions should be biopsied. Local excision or localized radiotherapy may be feasible if an isolated nodule is present. If lesions are multiple or accompanied by evidence of regional involvement in the internal mammary or supraclavicular nodes, the disease is best managed by radiation treatment of the entire chest wall including the parasternal, supraclavicular, and axillary areas and usually by systemic therapy.
Local recurrence after mastectomy usually signals the presence of widespread disease and is an indication for studies to search for evidence of metastases. Distant metastases
P.744
will develop within 2 years in most patients with locally recurrent tumor after mastectomy. When there is no evidence of metastases beyond the chest wall and regional nodes, irradiation for cure or complete local excision should be attempted. Patients with local recurrence may be cured with local resection and radiation. After partial mastectomy, local recurrence may not have as serious a prognostic significance as after mastectomy. However, those patients in whom a recurrence develops have a worse prognosis than those who do not. It is speculated that the ability of a cancer to recur locally after radiotherapy is a sign of aggressiveness and resistance to therapy. Completion of the mastectomy should be done for local recurrence after partial mastectomy; some of these patients will survive for prolonged periods, especially if the breast recurrence is DCIS or more than 5 years after initial treatment. Systemic chemotherapy or hormonal treatment should be used for women in whom disseminated disease develops or those in whom local recurrence occurs.
Edema of the Arm
Significant edema of the arm occurs in about 10 30% of patients after axillary dissection with or without mastectomy. It occurs more commonly if radiotherapy has been given or if there was postoperative infection. Partial mastectomy with radiation to the axillary lymph nodes is followed by chronic edema of the arm in 10 20% of patients. In the past, it was routine to perform an axillary dissection as it is more accurate for staging than axillary sampling. In an axillary dissection, it is recommended that at least level I and II lymph nodes (approximately 15 20 lymph nodes) be removed, in combination with partial mastectomy or as part of the mastectomy. After considerable research in the last decade, sentinel lymph node dissection has proved to be a more accurate form of axillary staging with less morbidity. Because a sentinel lymph node dissection usually only removes the first one to three lymph nodes that would be affected by carcinoma if it had spread to the axilla, it can provide accurate staging without the side effects of edema or infection. It does not replace axillary dissection if the sentinel lymph nodes are involved with metastases, although this is still being studied. Judicious use of radiotherapy, with treatment fields carefully planned to spare the axilla as much as possible, can greatly diminish the incidence of edema, which will occur in only 5% of patients if no radiotherapy is given to the axilla after a partial mastectomy and lymph node dissection.
Late or secondary edema of the arm may develop years after treatment, as a result of axillary recurrence or of infection in the hand or arm, with obliteration of lymphatic channels. Infection in the arm or hand on the dissected side should be treated with antibiotics, rest, and elevation. When edema develops, careful examination of the axilla for recurrence should be done. If there is no sign of recurrence, the swollen extremity should be treated with rest and elevation. A mild diuretic may be helpful. If there is no improvement, a compressor pump or manual decompression decreases the swelling, and the patient is then fitted with an elastic glove or sleeve. Most patients are not bothered enough by mild edema to wear an uncomfortable glove or sleeve and will treat themselves with elevation or manual decompression alone. Benzopyrones have been reported to decrease lymphedema but are not approved for this use in the United States. Rarely, edema may be severe enough to interfere with use of the limb.
Breast Reconstruction
Breast reconstruction is usually feasible after standard or modified radical mastectomy. Reconstruction should be discussed with patients prior to mastectomy, because it offers an important psychological focal point for recovery. Reconstruction is not an obstacle to the diagnosis of recurrent cancer. The most common breast reconstruction has been implantation of a silicone gel prosthesis in the subpectoral plane between the pectoralis minor and pectoralis major muscles. Although the FDA has placed a moratorium on the purely cosmetic use of silicone gel implants because of possible leakage of silicone and possible associated autoimmune phenomena, they can be used in breast reconstruction after mastectomy with appropriate prior patient consent. Most plastic surgeons currently would place a saline-filled prosthesis rather than a silicone gel implant. Alternatively, autologous tissue can be used for reconstruction.
Autologous tissue flaps are aesthetically superior to implant reconstruction in most patients. They also have the advantage of not feeling like a foreign body to the patient. The most popular autologous technique currently is the trans-rectus abdominis muscle flap (TRAM flap), which is done by rotating the rectus abdominis muscle with attached fat and skin cephalad to make a breast mound. The free TRAM flap is done by completely removing the rectus with overlying fat and skin and using microvascular surgical techniques to reconstruct the vascular supply on the chest wall. A latissimus dorsi flap can be swung from the back but offers less fullness than the TRAM flap and is therefore less acceptable cosmetically. Reconstruction may be performed immediately (at the time of initial mastectomy) or may be delayed until later, usually when the patient has completed adjuvant therapy. When considering reconstructive options, concomitant illnesses should be considered, since the ability of an autologous flap to survive depends on medical comorbidities. In addition, the need for radiotherapy may affect the choice of reconstruction as radiation may increase fibrosis around an implant or decrease the volume of a flap.
Risks of Pregnancy
Data are insufficient to determine whether interruption of pregnancy improves the prognosis of patients who are identified to have potentially curable breast cancer and who receive definitive treatment during pregnancy. Theoretically, the increasingly high levels of estrogen produced by the placenta as the pregnancy progresses could
P.745
be detrimental to the patient with occult metastases of hormone-sensitive breast cancer. Moreover, occult metastases are present in most patients with positive axillary nodes, and treatment by adjuvant chemotherapy could be potentially harmful to the fetus early in gestation, although chemotherapy may be given to pregnant women later. Under these circumstances, interruption of early pregnancy seems reasonable, with progressively less rationale for the procedure as term approaches. The decision is affected by many factors, including the patient's desire to have the baby and the prognosis especially when axillary nodes are involved.
Equally important is the advice regarding future pregnancy (or abortion in case of pregnancy) to be given to women of child-bearing age who have had definitive treatment for breast cancer. It is assumed that pregnancy will be harmful if occult metastases are present, though this has not been demonstrated. Patients whose tumors are ER negative (most younger women) may not be affected by pregnancy. To date, no adverse effect of pregnancy on survival of pregnant women who have had breast cancer has been demonstrated, though most oncologists advise against it.
In patients with inoperable or metastatic cancer (stage IV disease), induced abortion is usually advisable because of the possible adverse effects of hormonal treatment, radiotherapy, or chemotherapy upon the fetus.
Ascherman JA et al: Implant reconstruction in breast cancer patients treated with radiation therapy. Plast Reconstr Surg 2006;117:359.
Cocquyt VF et al: Better cosmetic results and comparable quality of life after skin-sparing mastectomy and immediate autologous breast reconstruction compared to breast conservative treatment. Br J Plast Surg 2003;56:462.
Ducic I et al: Safety and risk factors for breast reconstruction with pedicled transverse rectus abdominis musculocutaneous flaps: a 10-year analysis. Ann Plast Surg 2005;55:559.
Langer S et al: Lymphatic mapping improves staging and reduces morbidity in women undergoing total mastectomy for breast carcinoma. Am Surg 2004;70:881.
Salhab M et al: Skin-sparing mastectomy and immediate breast reconstruction: patient satisfaction and clinical outcome. Int J Clin Oncol 2006;11:51.
van der Veen P et al: Lymphedema development following breast cancer surgery with full axillary resection. Lymphology 2004; 37:206.
Carcinoma of the Male Breast
![]() Essentials of Diagnosis
Essentials of Diagnosis
A painless lump beneath the areola in a man usually over 50 years of age.
Nipple discharge, retraction, or ulceration may be present.
General Considerations
Breast cancer in men is a rare disease; the incidence is only about 1% of that in women. The average age at occurrence is about 60 somewhat older than the most common presenting age in women. There may be an increased incidence of breast cancer in men with prostate cancer. The prognosis, even in stage I cases, is worse in men than in women. Blood-borne metastases are commonly present when the male patient appears for initial treatment. These metastases may be latent and may not become manifest for many years. As in women, hormonal influences are probably related to the development of male breast cancer. There is a high incidence of both breast cancer and gynecomastia in Bantu men, theoretically owing to failure of estrogen inactivation by a liver damaged by associated liver disease. It is important to note that first-degree relatives of men with breast cancer are considered to be at high risk. This risk should be taken into account when discussing options with the patient and family. In addition, BRCA2 mutations are common in men with breast cancer. Men with breast cancer, especially with a history of prostate cancer, should receive genetic counseling.
Clinical Findings
A painless lump, occasionally associated with nipple discharge, retraction, erosion, or ulceration, is the primary complaint. Examination usually shows a hard, ill-defined, nontender mass beneath the nipple or areola. Gynecomastia not uncommonly precedes or accompanies breast cancer in men. Nipple discharge is an uncommon presentation for breast cancer in men but is an ominous finding associated with carcinoma in nearly 75% of cases.
Breast cancer staging is the same in men as in women. Gynecomastia and metastatic cancer from another site (eg, prostate) must be considered in the differential diagnosis. Benign tumors are rare, and biopsy should be performed on all males with a defined breast mass.
Treatment
Treatment consists of modified radical mastectomy in operable patients, who should be chosen by the same criteria as women with the disease. Breast conserving therapy is rarely performed. Irradiation is the first step in treating localized metastases in the skin, lymph nodes, or skeleton that are causing symptoms. Examination of the cancer for hormone receptor proteins is of value in predicting response to endocrine ablation. Men commonly have ER-positive tumors. Adjuvant chemotherapy is used for the same indications as in breast cancer in women.
P.746
Because breast cancer in men is frequently a disseminated disease, endocrine therapy is of considerable importance in its management. Tamoxifen is the main drug for management of advanced breast cancer in men. Tamoxifen (20 mg orally daily) should be the initial treatment. There is little experience with AIs though they should be effective. Castration in advanced breast cancer is a successful measure and more beneficial than the same procedure in women but is rarely used. Objective evidence of regression may be seen in 60 70% of men with hormonal therapy for metastatic disease approximately twice the proportion in women. The average duration of tumor growth remission is about 30 months, and life is prolonged. Bone is the most frequent site of metastases from breast cancer in men (as in women), and hormonal therapy relieves bone pain in most patients so treated. The longer the interval between mastectomy and recurrence, the longer the remission following treatment. As in women, there is correlation between ERs of the tumor and the likelihood of remission following hormonal therapy.
AIs should replace adrenalectomy in men as it has in women. Corticosteroid therapy alone has been considered to be efficacious but probably has no value when compared with major endocrine ablation. Either tamoxifen or AIs may be primary or secondary hormonal manipulation.
Estrogen therapy 5 mg of diethylstilbestrol three times daily orally may be effective hormonal manipulation after others have been successful and failed, just as in women. Androgen therapy may exacerbate bone pain. Chemotherapy should be administered for the same indications and using the same dosage schedules as for women with metastatic disease or for adjuvant treatment.
Prognosis
The prognosis of breast cancer is poorer in men than in women. The crude 5- and 10-year survival rates for clinical stage I breast cancer in men are about 58% and 38%, respectively. For clinical stage II disease, the 5- and 10-year survival rates are approximately 38% and 10%. The survival rates for all stages at 5 and 10 years are 36% and 17%. For those patients whose disease progresses despite treatment, meticulous efforts at palliative care are essential (see Chapter 5).
Fentiman IS et al: Male breast cancer. Lancet 2006;367:595.
Kwiatkowska E et al: Somatic mutations in the BRCA2 gene and high frequency of allelic loss of BRCA2 in sporadic male breast cancer. Int J Cancer 2002;98:943.
Loerzel VW et al: Male breast cancer. Clin J Oncol Nurs 2004; 8:191.
Weiss JR et al: Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev 2005;14:20.
EAN: 2147483647
Pages: 49