9. TGFbBMPs are diffusible morphogens
29.8 Dorsal-ventral development uses localized receptor-ligand interactions |
Dorsal-ventral development displays a complex interplay between the oocyte and follicle cells. The formation of pattern starts with the expression of genes in the oocyte that are needed for proper development of the follicle cells. And then expression of genes in the follicle cells transmits a signal to the oocyte that results in development of ventral structures. Another pathway is responsible for development of dorsal structures in the developing egg. Each of these systems functions by activating a localized ligand-receptor interaction that triggers a signal transduction pathway (Schupbach, 1987).
The process starts with the localization of gurken mRNA. This passes through two stages, the first playing a role in anterior-posterior patterning, the second in dorsal-ventral patterning. These are key events that define the spatial asymmetry of the egg chamber. (The requirement of gurken for both pathways is the only feature that breaches their independence.)
First gurken mRNA is localized on the posterior side of the oocyte. This results in a signal that causes adjacent follicle cells to become posterior. The follicle cells signal back to the oocyte in a process that results in the establishment of a polarized network of microtubules. This is necessary for the localization of the maternal transcripts of bicoid and oskar to opposite poles, as discussed earlier.
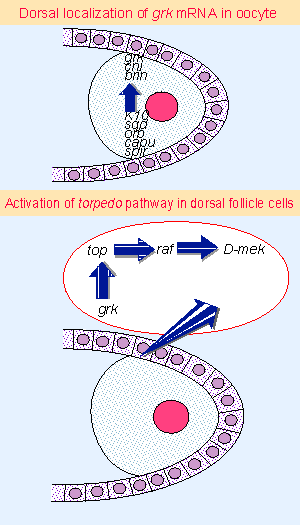 |
Figure 29.11 Dorsal and ventral identities are first distinguished when grk mRNA is localized on the dorsal side of the oocyte. Synthesis of Grk activates the receptor coded by torpedo, which triggers a MAPK pathway in the follicle cells. |
Dorsal-ventral polarity is established later when gurken mRNA becomes localized on the dorsal side of the oocyte. Figure 29.11 illustrates the pathway and its consequences. The products of cornichon and brainiac are needed for proper localization of the gurken mRNA or for activation of the protein. Of the group of loci that act earlier, the products of K10 and squid are needed to localize the RNA; and cappuccino and spire mutants have an array of defects that suggest their products have a general role in organizing the cytoskeleton of the oocyte. Accordingly, cappuccino and spire are required also for the earlier localization of gurken mRNA involved in anterior-posterior patterning.
gurken codes for a protein that resembles the growth factor TGFα. The next locus in the pathway is torpedo, which codes for the Drosophila EGF receptor. It is expressed in the follicle cells. So the pathway moves from oocyte to follicle cells when the ligand (Gurken), possibly in a transmembrane form that exposes the extracellular domain on the oocyte, interacts with the receptor (Torpedo) on the plasma membrane of a follicle cell.
An interesting and general principle emerges from the activation of Torpedo, which is a typical receptor tyrosine kinase. Activation of Torpedo leads to the activation of a Ras signaling pathway, which proceeds through Raf and D-mek (the equivalent of MAPKK), to activate a classic MAP kinase pathway. The ultimate readout of this pathway is not known, but its effect is to prevent activation on the dorsal side of the embryo of the ventral-determining pathway that we discuss next.
The utilization of this pathway shows that similar pathways may be employed in different circumstances to produce highly specific effects. The trigger to activate the pathway in the oocyte-follicle cell interaction is the specific localization of Gurken. The consequence is a change in the properties of follicle cells that prevents them from acquiring ventral fates. The basic components of the pathway, however, are the same as those employed in signal transduction of proliferation signals in vertebrate systems. The same pathway is employed again in the specific development of retinal cells in Drosophila itself, where another receptor-counter receptor interaction activates the Ras pathway, with specific, but very different effects on cell differentiation. So essentially the same pathway can be employed to interpret an initial signal and produce a response that is predetermined by the cell phenotype.
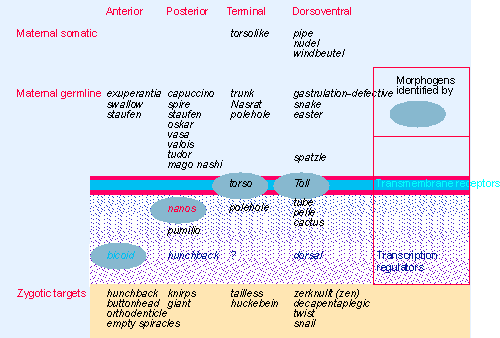 |
Figure 29.4 Each of the four maternal systems that functions in the egg is initiated outside the egg. The pathway is carried into the egg, where each pathway has a localized product that is the morphogen. This may be a receptor or a regulator of gene expression. The final component is a transcription factor, which acts on zygotic targets that are responsible for the next stage of development. |
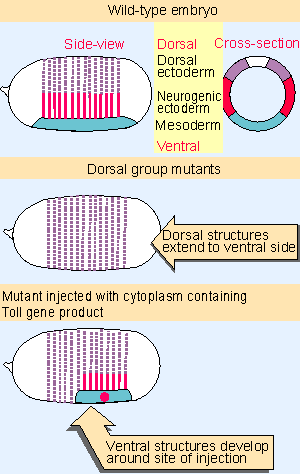 |
Figure 29.12 Wild-type Drosophila embryos have distinct dorsal and ventral structures. Mutations in genes of the dorsal group prevent the appearance of ventral structures, and the ventral side of the embryo is dorsalized. Ventral structures can be restored by injecting cytoplasm containing the Toll gene product. |
Development of ventral structures requires a group of 11 maternal genes whose products establish the dorsal-ventral axis between the time of fertilization and cellular blastoderm (see Figure 29.4). Figure 29.12 shows that the dorsal-ventral pattern can be viewed from the side by the phenotype of the cuticle, and can be seen in cross-section to represent the formation of different types of tissues. The dorsal system is necessary for the development of ventral structures including the mesoderm and neurogenic ectoderm. (The system was named for the effects of mutations [to dorsalize], rather than for the role of the gene products [to ventralize].) Mutants in any genes of the dorsal group lack ventral structures, and have dorsal structures on the ventral side, as indicated in the figure. But injecting wild-type cytoplasm into mutant embryos rescues the defect and allows ventral structures to develop (for review see Morisato and Anderson, 1995).
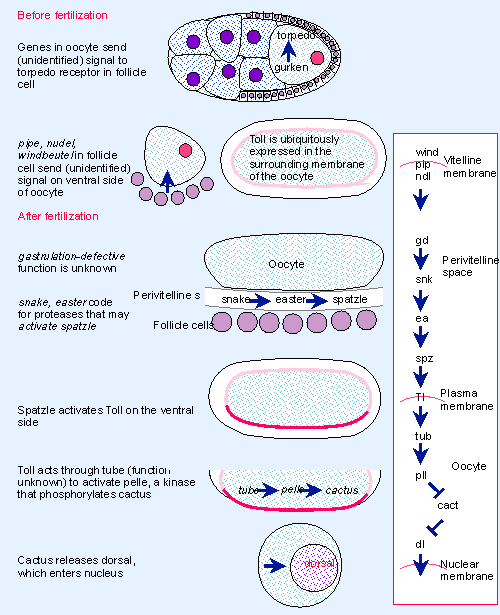 |
Figure 29.13 The dorsal-ventral pathway is summarized on the right and shown in detail on the left. It involves interactions between follicle cells and the oocyte. The pathway moves into the oocyte when spatzle binds to Toll and activates the morphogen. The pathway is completed by transporting the transcription factor dorsal into the nucleus. |
The ventral-determining pathway also begins in the follicle cell and ends in the oocyte. The pathway is summarized in Figure 29.13. The initial steps are not well defined, and require the expression of three loci in the follicle cells on the ventral side. These loci function before fertilization, but the egg does not receive the signal until after fertilization. The nature of the signal is not known, although we know most of the events that ensue in the oocyte.
The signal leads to a series of proteolytic cleavages that occur in the perivitelline space (the outermost layer of the oocyte). The product of snake probably cleaves the product of easter, which in turn is activated to cleave the spatzle product. Cleavage activates spatzle, so that it can provide a ligand for a receptor coded by the Toll gene. Toll is the first component of the pathway that functions in the oocyte.
Rescue experiments identify Toll as the crucial gene that conveys the signal into the oocyte. Toll V mutants lack any dorsal-ventral gradient, and injection of Toll induces the formation of dorsal-ventral structures. The other genes of the dorsal group code for products that either regulate or are required for the action of Toll, but they do not establish the primary polarity (Anderson et al., 1985).
There is a paradox in the distribution of Toll protein. Toll gene product activity is found in all parts of a donor embryo when cytoplasm is extracted and tested by injection. Yet it induces ventral structures only in the appropriate location in normal development. An initial general distribution of Toll gene product must therefore in some way be converted into a concentration of active product by local events.
Toll is a transmembrane protein homologous to the vertebrate interleukin-1 (IL1) receptor. Binding of ligand is sufficient to activate the ventral-determining pathway. The reaction occurs on the ventral side of the perivitelline space. The spatzle ligand either cannot diffuse far from the site of cleavage, or perhaps it binds to Toll very rapidly, with the result that Toll is activated only on the ventral side of the embryo. Loss-of-function mutations in Toll are dorsalized, because the receptor cannot be activated. There are also dominant (TollD) mutations, which confer ventral properties on dorsal regions; these are gain-of-function mutations, which are ventralized because the receptor is constitutively active. Genetic analysis shows that toll acts via tube and pelle. Tube is probably an adaptor protein that recruits the kinase pelle to the activated receptor. The target for the pelle kinase is not proven, but its activation leads to phosphorylation of the product of cactus, which is the final regulator of the transcription factor coded by dorsal.
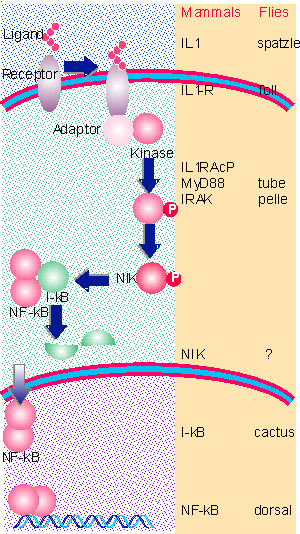 |
Figure 29.14 Activation of IL1 receptor triggers formation of a complex containing adaptor(s) and a kinase. The IRAK kinase activates NIK, which phosphorylates I-kB. This triggers degradation of I-kB, releasing NF-kB, which translocates to the nucleus to activate transcription. |
Figure 29.14 shows the parallels between the toll signaling pathway in flies and the IL1 vertebrate pathway (where the biochemistry is well characterized). Activation of the receptor causes a complex to assemble that includes adaptor proteins (several in vertebrates), which bind a kinase. Activation of the vertebrate kinase (IRAK) in turn activates the kinase NIK, which phosphorylates I-κB. It is not clear whether the fly kinase (pelle) acts directly on cactus (the equivalent of Iκ-B) or through an intermediate.
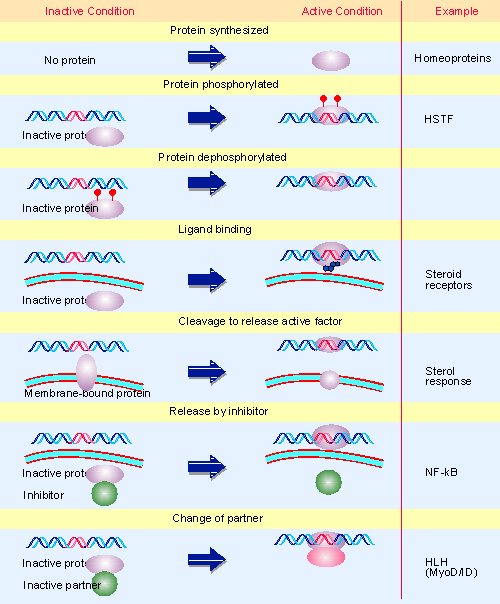 |
Figure 21.2 The activity of a regulatory transcription factor may be controlled by synthesis of protein, covalent modification of protein, ligand binding, or binding of inhibitors that sequester the protein or affect its ability to bind to DNA. Multiple figure |
At all events, dorsal and cactus form an interacting pair of proteins that are related to the transcription factor NF-κB and its regulator IκB. NF-κB consists of two subunits (related in sequence) which are bound by IκB in the cytoplasm. When IκB is phosphorylated, it releases NF-κB, which then moves into the nucleus, where it functions as a transcription factor of genes whose promoters have the κB sequence motif. (An example of the pathway is illustrated in Figure 21.2.) It seems likely that cactus regulates dorsal in the same way that IκB regulates NF-κB. A cactus-dorsal complex is inert in the cytoplasm, but when cactus is phosphorylated, it releases dorsal protein, which enters the nucleus. The pathway is therefore conserved from receptor to effector, since activation of interleukin-1 receptor has as a principal effect the activation of NF-κ B, and activation of Toll leads to activation of dorsal.
 |
Figure 29.15 Dorsal protein forms a gradient of nuclear localization from ventral to dorsal side of the embryo. On the ventral side (lower) the protein identifies bright nuclei; on the dorsal side (upper) the nuclei lack proteDorsal protein forms a gradient of nuclear localization from ventral to dorsal side of the embryo. On the ventral side (lower) the protein identifies bright nuclei; on the dorsal side (upper) the nuclei lack protein and show as dark holes in the bright cytoplasm. Photograph kindly provided by Michael Levine. |
As a result of the activation of Toll, a gradient of dorsal protein in the nucleus is established, from ventral to dorsal side of the embryo. On the ventral side, dorsal protein is released to the nucleus, but on the dorsal side of the embryo it remains in the cytoplasm. A steep gradient is established at the stage of syncytial blastoderm, and becomes sharper during the transition to cellular blastoderm. The proportion of dorsal protein that is in the nucleus correlates with the ventral phenotype that will be displayed by this region. An example of a gradient visualized by staining with antibody against dorsal protein is shown in Figure 29.15. The total amount of dorsal protein in the embryo does not change: the gradient is established solely by a redistribution of the protein between nucleus and cytoplasm (Roth et al., 1989; for review see Belvin and Anderson, 1996).
Dorsal both activates and represses gene expression. It activates the genes twist and snail, which are required for the development of ventral structures. And it represses the genes dpp and zen, which are required for the development of dorsal structures; as a result, these genes are expressed only in the 40% most dorsal of the embryo. We discuss the determination of Dorsal-ventral structures by the Dpp pathway in the next section.
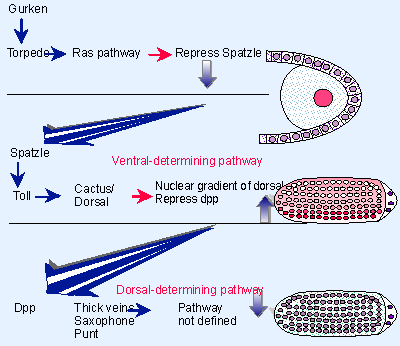 |
Figure 29.16 Dorsal-ventral patterning requires the successive actions of three localized systems. |
One of the crucial aspects of dorsal-ventral development is the relationship between the systems. This is summarized in Figure 29.16. The ability of one system to repress the next is responsible for restricting the localized activities to the appropriate part of the embryo. The initial interaction between gurken and torpedo leads to the repression of spatzle activity on the dorsal side of the embryo. This restricts the activation of dorsal protein to the ventral side of the embryo. Nuclear localization of dorsal protein in turn represses the expression of dpp, so that it forms a gradient diffusing from the dorsal side. In this way, ventral structures are formed in the nuclear gradient of dorsal protein, and dorsal structures are formed in the gradient of dpp protein.
The terminal system is initiated in a way that is similar to the dorsal-ventral system. A transmembrane receptor, coded by the torso gene, is produced by translation of a maternal RNA after fertilization. The receptor is localized throughout the embryo. It is activated at the poles by local production of an extracellular ligand. Torso protein has a kinase activity, which initiates a cascade that leads to local expression of the tailless and huckebein RNAs, which code for factors that regulate transcription.
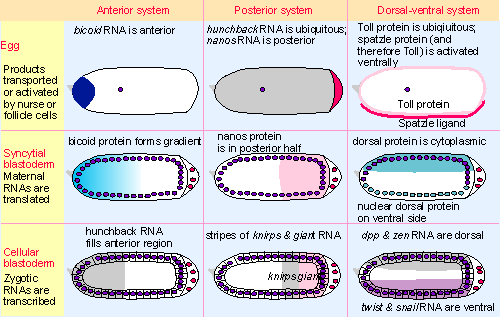 |
Figure 29.17 In each axis-determining system, localized products in the egg cause other maternal RNAs or proteins to be broadly localized at syncytial blastoderm, and zygotic RNAs are transcribed in bands at cellular blastoderm. |
The pattern of regulators at each stage of development for each of the systems is summarized in Figure 29.17. Two types of mechanism are used to create the initial asymmetry. For the anterior-posterior axis, an RNA is localized at one end of the egg (bicoid for the anterior system, nanos for the posterior system); localization depends upon the interaction of sequences in the 3′ end of the RNA with maternal proteins. In the case of the dorsal-ventral and terminal systems, a receptor protein is specifically activated in a localized manner, as the result of the limited availability of its ligand. All of these interactions depend on RNAs and/or proteins expressed from maternal genes.
The local event leads to the production of a morphogen, which forms a gradient, either quantitatively (bicoid) or by nucleocytoplasmic distribution (dorsal), or is localized in a broad restricted region (nanos). The extent of the region in which the morphogen is active is ~50% across the egg for each of these systems. The morphogens are translated from maternal RNAs, and development is therefore still dependent on maternal genes up to this stage.
Establishing anterior-posterior and dorsal-ventral gradients is the first step in determining orientation and spatial organization of the embryo. Under the direction of maternal genes, gradients form across the common cytoplasm and influence the behavior of the nuclei located in it. The next step is the development of discrete regions that will give rise to different body parts. This requires the expression of the zygotic genome, and the loci that now become active are called zygotic genes. Genes involved at this stage are identified by segmentation mutants.
The products of the segmentation genes form bands that distinguish individual regions on the anterior-posterior axis. When we consider the results of the anterior and posterior systems together, we see that there are several broad regions (the two regions generated by the anterior system are adjacent to the two regions defined by the posterior system; see below). On the dorsal-ventral axis, there are three rather broad bands that define the regions in which the mesoderm, neuroectoderm, and dorsal ectoderm form (proceeding from the ventral to the dorsal side).
| Reviews | |
| Belvin, M. P. and Anderson, K. V. (1996). A conserved signaling pathway: the Drosophila Toll-Dorsal pathway. Ann. Rev. Cell Dev. Biol. 12, 393-416. | |
| Morisato, D. and Anderson, K. V. (1995). Signaling pathways that establish the dorsal-ventral pattern of the Drosophila embryo. Ann. Rev. Genet. 29, 371-399. | |
| Research | |
| Anderson, K. V., Bokla, L., and Nusslein-Volhard, C. (1985). Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell 42, 791-798. | |
| Roth, S., Stein, D., and Nusslein-Volhard, C. (1989). A gradient of nuclear localization of the dorsal protein determines Dorsal-ventral pattern in the Drosophila embryo. Cell 59, 1189-202. | |
| Schupbach, T. (1987). Germ line and soma cooperate during oogenesis to establish the Dorsal-ventral pattern of egg shell and embryo in Drosophila melanogaster. Cell 49, 699-707. | |