9. RB is a tumor suppressor that controls the cell cycle
28.8 Oncoproteins may regulate gene expression |
It goes almost without saying that it is necessary to make changes in gene expression in order to convert a cell to the transformed phenotype. Many oncogenes act at early stages in pathways that lead ultimately to changes in gene expression. Some act directly at the level of transcription. Retroviral oncogenes include examples derived from the major classes of cellular transcription factors. Several prominent gene families coding for transcription factors are identified by v-onc genes: rel, jun, fos, erbA, myc, and myb. In the cases of Rel, Jun, and ErbA proteins, there are differences in transcriptional activity between the c-Onc and v-Onc proteins that may be related to transforming capacity.
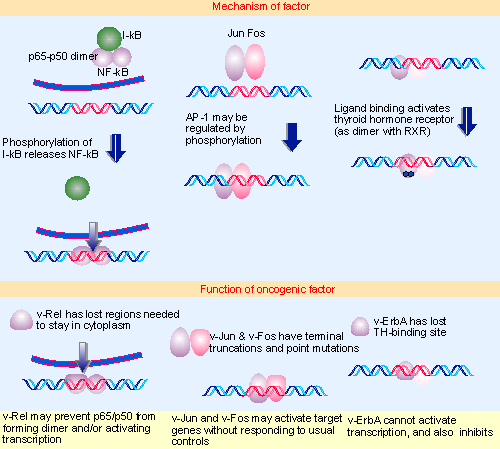 |
Figure 28.19 Oncogenes that code for transcription factors have mutations that inactivate transcription (v-erbA and possibly v-rel) or that activate transcription (v-jun and v-fos). Multiple figure |
The actions of v-onc genes may in principle be quantitative or qualitative; and those that affect transcription might either increase or decrease expression of particular genes. By virtue of increased expression or activity they could turn up transcription of genes whose products can be tolerated only in small amounts. Failure to respond to normal regulation of activity by other cellular factors also might lead to increased gene expression. A less likely possibility is the acquisition of specificity for new target promoters. Alternatively, if the oncoproteins are defective in the ability to activate transcription, they might function as dominant negative suppressors of the cellular transcription factors. The first steps towards distinguishing these possibilities lie with determining which functions are altered in v-Onc compared with c-Onc proteins: is DNA-binding altered either quantitatively or quantitatively; is the ability to activate transcription altered? Figure 28.19 summarizes the properties of some of these oncoproteins.
The oncogene v-rel was identified as the transforming function of the avian (turkey) reticuloendotheliosis virus. The retrovirus is highly oncogenic in chickens, where it causes B-cell lymphomas. v-rel is a truncated version of c-rel, lacking the ~100 C-terminal amino acids, and has a small number of point mutations in the remaining sequence.
The rel gene defines a family with several members, which form various pairwise combinations that regulate transcription. The best characterized family member is the transcription factor NF-κB. This is a dimer of two subunits, p65 and p50, which is held in the cytoplasm by a regulator, I-κB (which masks the nuclear localization sequence). When I-κB is phosphorylated, it is degraded and therefore releases NF-κB, which enters the nucleus and activates transcription of target genes whose promoters or enhancers have the κB motif (see 21 Regulation of transcription). This regulatory story is essentially recapitulated in Drosophila development, where dorsal codes for an NF-κB homologue that is held in the cytoplasm by the cactus product, an IκB homologue (see 29 Gradients, cascades, and signaling pathways). There is sequence relationship between the two subunits of NF-κB, and c-rel has 60% similarity with p50.
NF-κB is one of the most pleiotropic transcription factors; indeed, it has been suggested that it may constitute a general second messenger. Many types of stimulus to the cell result in activation of NF-κb and a broad range of genes is activated via the presence of κB binding sites. It remains to be seen what changes in v-rel make it oncogenic, and how they affect the transcriptional regulation exercised by the Rel-related proteins. One possibility is that the v-rel product forms dimers with cellular family members, and that these dimers are inactive, so that v-Rel prevents NF-κB or related factors from functioning. v-Rel is exclusively nuclear, because it has lost the sequences required for export to the cytoplasm.
The transcription factor AP1 is the nuclear factor required to mediate transcription induced by phorbol ester tumor promoters (such as TPA). An AP1 binding site confers TPA-inducibility upon a target gene. The canonical AP1 factor consists of a dimer of two subunits, coded by the genes c-jun and c-fos, which activates genes whose promoters or enhancers have an AP1 binding site.
Jun and Fos are transcription factors of the leucine zipper class. Each protein is a member of a family, and a series of pairwise interactions between Jun family members and Fos family members may generate a series of transcription factors related to AP1. Mutations of v-jun or v-fos that abolish the ability to bind DNA also render the product non-transforming, providing a direct proof that ability to bind to DNA is required for transforming activity. Beyond this, however, it is not clear what changes in transcription are required for transformation: they could involve quantitative changes (over-expression or under-expression of particular target genes) or qualitative changes (alteration in the pattern of genes that responds to the factor).
The cellular gene c-erbA codes for a thyroid hormone receptor, a member of the general class of steroid hormone receptors (see 21 Regulation of transcription). Upon binding its ligand, a typical steroid receptor activates expression of particular target genes by binding to its specific response element in a promoter or enhancer. The mode of action for thyroid hormone is distinct: it is located permanently in the nucleus, and, indeed, may bind its response element whether or not ligand is present. The effect of hormone binding may therefore be to activate transcription by previously bound receptor.
Ability to bind DNA is required for transforming capacity. v-erbA is truncated at both ends and has a small number of substitutions relative to c-erbA. Hormone binding is altered; the c-erbA product binds triiodothyronine (T3) with high affinity, but the v-erbA product has little or no affinity for the ligand in mammalian cells. This suggests that loss of the ligand-binding capacity (perhaps together with other changes) may create a protein whose function has become independent of the hormone. The consequence of losing the response to ligand is that the factor can no longer be stimulated to activate transcription.
These results place v-erbA as a dominant negative oncogene, one that functions by overcoming the action of its normal cellular counterpart. The implication is that genes usually activated by c-ErbA act to suppress transformation. In this particular case, it seems likely that these genes usually promote differentiation; blocking this action allows the cells to proliferate.
c-jun, c-fos, c-rel, and also c-myc are "immediate early" genes, members of a class of genes that are rapidly induced when resting cells are treated with mitogens, which suggests that they may be involved in a cascade that initiates cycling. So their targets are likely to be concerned with initiating or promoting growth. We should therefore expect an increase in their activities to be associated with oncogenesis, an expectation that may be fulfilled for v-fos and v-myc, but does not explain the behavior of v-rel.
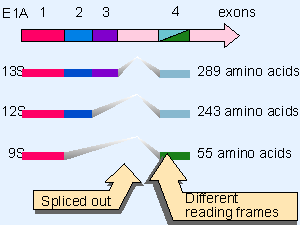 |
Figure 28.20 The adenovirus E1A region is spliced to form three transcripts that code for overlapping proteins. Domain 1 is present in all proteins, domain 2 in the 289 and 243 residue proteins, and domain 3 is unique to the 2The adenovirus E1A region is spliced to form three transcripts that code for overlapping proteins. Domain 1 is present in all proteins, domain 2 in the 289 and 243 residue proteins, and domain 3 is unique to the 289 residue protein. The C-terminal domain of the 55 residue protein is translated in a different reading frame from the common C-terminal domains of the other two proteins. |
The adenovirus oncogene E1A provides an example of a protein that regulates gene expression indirectly, that is, without itself binding to DNA. The E1A region is expressed as three transcripts, derived by alternative splicing, as indicated in Figure 28.20. The 13S and 12S mRNAs code for closely related proteins and are produced early in infection. They possess the ability to immortalize cells, and can cooperate with other oncoproteins (notably Ras) to transform primary cells (see later). No other viral function is needed for this activity (for review see Nevins, 1987).
The E1A proteins exercise a variety of effects on gene expression. They activate the transcription of some genes, but repress others. Loci that are activated include genes transcribed by RNA polymerase III as well as RNA polymerase II.
Mutation of the E1A proteins suggests that transcriptional activation requires only the short region of domain 3, found only in the 289 amino acid protein coded by 13S mRNA. Repression of transcription, induction of DNA synthesis, and morphological transformation all require domains 1 and 2, common to both the 289 and 243 amino acid proteins. This suggests that repression of target genes is required to cause transformation. E1A proteins act by binding to several cellular proteins that in turn repress or activate transcription of appropriate target genes. Among these targets are the CBP and p300 coactivators, the TBP basal transcription factor, and the cell cycle regulators RB and p27.
A powerful new approach to analyzing the roles of individual genes in cancer has been made possible by the development of techniques to allow simultaneous screening for the expression of many genes. High density DNA microarrays contain probes to the mRNAs of up to 10,000 genes (typically immobilized on a glass slide). The technique is at its most effective for comparing the genes expressed in two related cell types. The technique can be applied to a tumor cell when it is possible to compare it with the original cell type from which it arose, or can be used to compare related tumor cells with different proeprties. Tumor cell lines can be obtained that vary in their ability to metastasize (to spread from the site of origin to colonize new sites in the body). A highly metastatic cell line can be selected from a line that is poorly metastatic without apparently changing the properties of the tumor as such Xonly the ability to spread appears to be affected. A comparison in two such cases showed that only a small number (<20) genes have a significant change in transcription. This suggests the possibility that metastasis involves relatively minor changes in the pattern of gene expression. Among the affected genes are several whose products act on the actin cytoskeleton, suggesting that changes in cellular motility and/or adhesion to other cells or substratum could be the basis for metastasis (Clark et al., 2000).
This section updated 8-21-2000
| Reviews | |
| Nevins, J. R. (1987). Regulation of early adenovirus gene expression. Microbiol. Rev. 51, 419-430. | |
| Research | |
| Clark, E. A. , Golub, T. R. , Lander, E. S. , and Hynes, R. O. (2000). Genomic analysis of metastasis reveals an essential role for RhoC . Nature 406, 532-535. | |
- ERP Systems Impact on Organizations
- Challenging the Unpredictable: Changeable Order Management Systems
- ERP System Acquisition: A Process Model and Results From an Austrian Survey
- Context Management of ERP Processes in Virtual Communities
- Development of Interactive Web Sites to Enhance Police/Community Relations