3. Retroviral oncogenes have cellular counterparts
28.2 Transforming viruses carry oncogenes |
Transformation may occur spontaneously, may be caused by certain chemical agents, and, most notably, may result from infection with tumor viruses. There are many classes of tumor viruses, including both DNA and RNA viruses, and they occur widely in the avian and animal kingdoms.
 |
Figure 28.3 Transforming viruses may carry oncogenes. |
The transforming activity of a tumor virus resides in a particular gene or genes carried in the viral genome. Oncogenes were given their name by virtue of their ability to convert cells to a tumorigenic (or oncogenic) state. An oncogene initiates a series of events that is executed by cellular proteins. In effect, the virus throws a regulatory switch that changes the growth properties of its target cell. Figure 28.3 summarizes the general properties of the major classes of transforming viruses. The oncogenes carried by the DNA viruses specify proteins that inactivate tumor suppressors, so their action in part mimics loss-of-function of the tumor suppressors. The oncogenes carried by retroviruses are derived from cellular genes and therefore may mimic the behavior of gain-of-function mutations in animal proto-oncogenes.
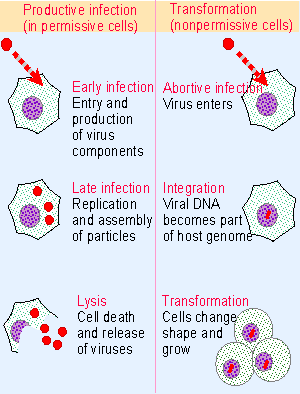 |
Figure 28.4 Permissive cells are productively infected by a DNA tumor virus that enters the lytic cycle, while nonpermissive cells are transformed to change their phenotype. |
Polyomaviruses and adenoviruses have been isolated from a variety of mammals. Although perpetuated in the wild in a single host species, a virus may be able to grow in culture on a variety of cells from different species. The response of a cell to infection depends on its species and phenotype and falls into one of two classes, as illustrated in Figure 28.4:
- Permissive cells are productively infected. The virus proceeds through a lytic cycle that is divided into the usual early and late stages. The cycle ends with release of progeny viruses and (ultimately) cell death.
- Nonpermissive cells cannot be productively infected, and viral replication is abortive. Some of the infected cells are transformed; in this case, the phenotype of the individual cell changes and the culture is perpetuated in an unrestrained manner.
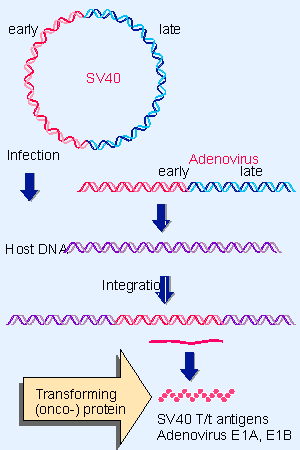 |
Figure 28.5 Cells transformed by polyomaviruses or adenoviruses have viral sequences that include the early region integrated into the cellular genome. Sites of integration are random. |
A common mechanism underlies transformation by DNA tumor viruses. Oncogenic potential resides in a single function or group of related functions that are active early in the viral lytic cycle. When transformation occurs, the relevant gene(s) are integrated into the genomes of transformed cells and expressed constitutively. This suggests the general model for transformation by these viruses illustrated in Figure 28.5, in which the constitutive expression of the oncogene generates transforming protein(s) (oncoproteins).
Polyomaviruses are small. Polyomavirus itself is common in mice, the analogous virus SV40 (simian virus 40) was isolated from rhesus monkey cells, and more recently the human viruses BK and JC have been characterized. All of the polyomaviruses can cause tumors when injected into newborn rodents.
During a productive infection, the early region of each virus uses alternative splicing to synthesize overlapping proteins called T antigens. (The name reflects their isolation originally as the proteins found in tumor cells.) The various T antigens have a variety of functions in the lytic cycle. They are required for expression of the late region and for DNA replication of the virus.
Cells transformed by polyomaviruses contain integrated copies of part or all of the viral genome. The integrated sequences always include the early region. The T antigens have transforming activity, which rests upon their ability to interact with cellular proteins. This is independent of their ability to interact directly with the viral genome. SV40 requires "big T" and "little t" antigens, and polyoma requires "T" and "middle T" antigens for transformation.
Papillomaviruses are small DNA viruses that cause epithelial tumors; there are ~75 human papillomaviruses (HPVs); most are associated with benign growths (such as warts), but some are associated with cancers, in particular cervical cancers. Two virus-associated products are expressed in cervical cancers; these are the E6 and E7 proteins, which can immortalize target cells.
Adenoviruses were originally isolated from human adenoids; similar viruses have since been isolated from other mammals. They comprise a large group of related viruses, with >80 individual members. Human adenoviruses remain the best characterized, and are associated with respiratory diseases. They can infect a range of cells from different species.
Human cells are permissive and are therefore productively infected by adenoviruses, which replicate within the infected cell. But cells of some rodents are nonpermissive. All adenoviruses can transform nonpermissive cultured cells, but the oncogenic potential of the viruses varies; the most effective can cause tumors when they are injected into newborn rodents. The genomes of cells transformed by adenoviruses have gained a part of the early viral region that contains the E1A and E1B genes, which code for several nuclear proteins.
Epstein-Barr is a human herpes virus associated with a variety of diseases, including infectious mononucleosis, nasopharyngeal carcinoma, African Burkitt lymphoma, and other lymphoproliferative disorders. EBV has a limited host range for both species and cell phenotype. Human B lymphocytes that are infected in vitro become immortalized, and some rodent cell lines can be transformed. Viral DNA is found in transformed cells, although it has been controversial whether it is integrated. It remains unclear exactly which viral genes are required for transformation.
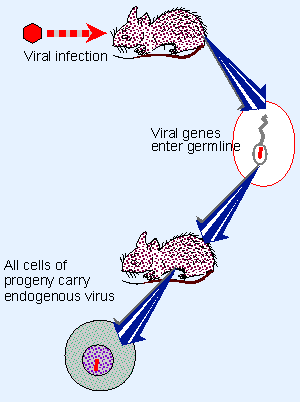 |
Figure 28.6 Retroviruses transfer genetic information horizontally by infecting new hosts; information is inherited vertically if a virus integrates in the genome of the germline. |
Retroviruses present a different situation from the DNA tumor viruses. They can transfer genetic information both horizontally and vertically, as illustrated in Figure 28.6. Horizontal transfer is accomplished by the normal process of viral infection, in which increasing numbers of cells become infected in the same host. Vertical transfer results whenever a virus becomes integrated in the germline of an organism as an endogenous provirus; like a lysogenic bacteriophage, it is inherited as a Mendelian locus by the progeny (see 11 Phage strategies).
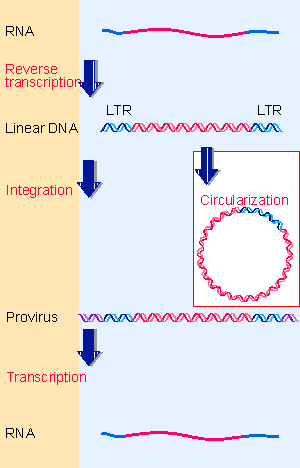 |
Figure 16.2 The retroviral life cycle proceeds by reverse transcribing the RNA genome into duplex DNA, which is inserted into the host genome, in order to be transcribed into RNA. |
The retroviral life cycle propagates genetic information through both RNA and DNA templates. A retroviral infection proceeds through the stages illustrated previously in Figure 16.2, in which the RNA is reverse-transcribed into single-stranded DNA, then converted into double-stranded DNA, and finally integrated into the genome, where it may be transcribed again into infectious RNA. Integration into the genome leads to vertical transmission of the provirus. Expression of the provirus may generate retroviral particles that are horizontally transmitted. Integration is a normal part of the life cycle of every retrovirus, whether it is nontransforming or transforming.
The tumor retroviruses fall into two general groups with regard to the origin of their tumorigenicity:
- Nondefective viruses follow the usual retroviral life cycle. They provide infectious agents that have a long latent period, and often are associated with the induction of leukemias. Two classic models are FeLV (feline leukemia virus) and MMTV (mouse mammary tumor virus). Tumorigenicity does not rely upon an individual viral oncogene, but upon the ability of the virus to activate a cellular proto-oncogene(s).
- Acute transforming viruses have gained new genetic information in the form of an oncogene. This gene is not present in the ancestral (nontransforming virus); it originated as a cellular gene that was captured by the virus by means of a transduction event during an infective cycle. These viruses usually induce tumor formation in vivo rather rapidly, and they can transform cultured cells in vitro. Reflecting the fact that each acute transforming virus has specificity toward a particular type of target cell, these viruses are divided into classes according to the type of tumor that is caused in the animal: leukemia, sarcoma, carcinoma, etc.
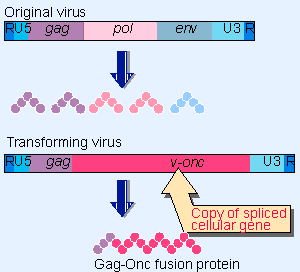 |
Figure 28.7 A transforming retrovirus carries a copy of a cellular sequence in place of some of its own gene(s). |
When a retrovirus captures a cellular gene by exchanging part of its own sequence for a cellular sequence (see 16 Retroviruses and retroposons), it generates the structure summarized in Figure 28.7. This type of event is rare, but creates a transducing virus that has two important properties:
- Usually it cannot replicate by itself, because viral genes needed for reproduction have been lost by the exchange with cellular sequences. So almost all of these viruses are replication-defective. But they can propagate in a simultaneous infection with a wild-type "helper" virus that provides the functions that were lost in the recombination event. (RSV is an exceptional transducing virus that retains the ability to replicate.)
- During an infection, the transducing virus carries with it the cellular gene(s) that were obtained in the recombination event, and their expression may alter the phenotype of the infected cell. Any transducing virus whose cellular genetic information assists the growth of its target cells could have an advantage in future infective cycles. If a virus gains a gene whose product stimulates cell growth, the acquisition may enable the virus to spread by stimulating the growth of the particular cells that it infects. (This is important also because a retrovirus can replicate only in a proliferating cell; see 11 Phage strategies.) After a virus has collected a cellular gene, the gene may gain mutations that enhance its ability to influence cell phenotype.
Of course, transformation is not the only mechanism by which retroviruses affect their hosts. A notable example is the HIV-1 retrovirus, which belongs to the retroviral group of lentiviruses. The virus infects and kills T lymphocytes carrying the CD4 receptor, devastating the immune system of the host, and inducing the disease of AIDS. The virus carries the usual gag-pol-env regions, and also has an additional series of reading frames, which overlap with one another, to which its lethal actions are attributed.