3. Coated vesicles transport both exported and imported proteins
25.2 Oligosaccharides are added to proteins in the ER and Golgi |
Virtually all proteins that pass through the secretory apparatus are glycosylated. Glycoproteins are generated by the addition of oligosaccharide groups either to the NH2 group of asparagine (N-linked glycosylation) or to the OH group of serine, threonine, or hydroxylysine (O-linked glycosylation). N-linked glycosylation is initiated in the endoplasmic reticulum and completed in the Golgi; O-linked glycosylation occurs in the Golgi alone. The stages of N-glycosylation are illustrated in the next three figures.
 |
Figure 25.3 An oligosaccharide is formed on dolichol and transferred by glycosyl transferase to asparagine of a target protein. |
The addition of all N-linked oligosaccharides starts in the ER by a common route, as illustrated in Figure 25.3. An oligosaccharide containing 2 N-acetyl glucosamine, 9 mannose, and 3 glucose residues is formed on a special lipid, dolichol. Dolichol is a highly hydrophobic lipid that resides within the ER membrane, with its active group facing the lumen. The oligosaccharide is constructed by adding sugar residues individually; it is linked to dolichol by a pyrophosphate group, and is transferred as a unit to a target protein by a membrane-bound glycosyl transferase enzyme whose active site is exposed in the lumen of the endoplasmic reticulum.
The acceptor group is an asparagine residue, located within the sequence Asn-X-Ser or Asn-X-Thr (where X is any amino acid except proline). It is recognized as soon as the target sequence is exposed in the lumen, when the nascent protein crosses the ER membrane.
Some trimming of the oligosaccharide occurs in the ER, after which a nascent glycoprotein is handed over to the Golgi. The oligosaccharide structures generated during transport through the ER and Golgi fall into two classes, determined by the fate of the mannose residues. Mannose residues are added only in the ER, although they can be trimmed subsequently.
- High mannose oligosaccharides are generated by trimming the sugar residues in the ER. Figure 25.4 shows that almost immediately following addition of the oligosaccharide, the 3 glucose residues are removed by the enzymes glucosidases I and II. For proteins that reside in the ER, a mannosidase removes some of the mannose residues to generate the final structure of the oligosaccharide. The ER mannosidase attacks the first mannose quickly, and the next 3 more slowly; the total number of mannose residues that is removed varies with the individual substrate protein.
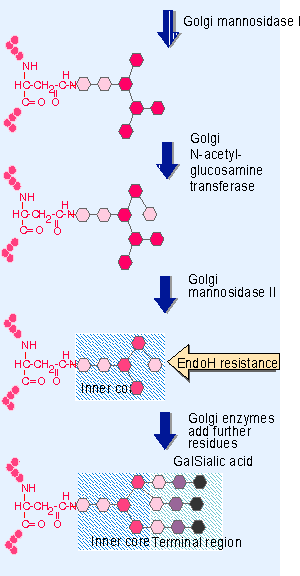
Figure 25.5 Processing for a complex oligosaccharide occurs in the Golgi and trims the original preformed unit to the inner core consisting of 2 N-acetyl-glucosamine and 3 mannose residues. Then further sugars can be added, in the order in which the transfer enzymes are encountered, to generate a terminal region containing N-acetyl-glucosamine, galactose, and sialic acid. - Complex oligosaccharides result from additional trimming and further additions carried out in the Golgi. Golgi modifications occur in the fixed order illustrated in Figure 25.5. The first step is further trimming of mannose residues by Golgi mannosidase I. Then a single sugar residue is added by the enzyme N-acetyl-glucosamine transferase. Then Golgi mannosidase II removes further mannose residues. This generates a structure called the inner core, consisting of the sequence NAc-Glc PNAc-Glc PMan3. At this point, the oligosaccharide becomes resistant to degradation by the enzyme endoglycosidase H (Endo H). Susceptibility to Endo H is therefore used as an operational test to determine when a glycoprotein has left the ER.
 |
Figure 25.4 Sugars are removed in the ER in a fixed order, initially comprising 3 glucose and 1-4 mannose residues. This trimming generates a high mannose oligosaccharide. |
Additions to the inner core generate the terminal region. The residues that can be added to a complex oligosaccharide include N-acetyl-glucosamine, galactose, and sialic acid (N-acetyl-neuraminic acid). The pathway for processing and glycosylation is highly ordered, and the two types of reaction are interspersed in it. Addition of one sugar residue may be needed for removal of another, as in the example of the addition of N-acetyl-glucosamine before the final mannose residues are removed.
We do not know what determines how each protein undergoes its particular characteristic pattern of processing and glycosylation. We assume that the necessary information resides in the structure of the polypeptide chain; it cannot lie in the oligosaccharide, since all proteins subject to N-linked glycosylation start the pathway by addition of the same (preformed) oligosaccharide.
 |
Figure 25.6 The Golgi apparatus consists of a series of individual membrane stacks. Photograph kindly provided by Alain Rambourg. |
The individual cisternae of the Golgi are organized into a series of stacks, somewhat resembling a pile of plates. A typical stack consists of 4 V8 flattened cisternae. Figure 25.6 shows an example. A major feature of the Golgi apparatus is its polarity. The cis side faces the endoplasmic reticulum; the trans side in a secretory cell faces the plasma membrane. The Golgi consists of compartments, which are named cis, medial, trans, and TGN (trans-Golgi network), proceeding from the cis to the trans face. Proteins enter a Golgi stack at the cis face and are modified during their transport through the successive cisternae of the stack. When they reach the trans face, they are directed to their destination.
Membrane structure changes across the Golgi stack. The main difference is an increase in the content of cholesterol proceeding from cis to trans. As a result, fractionation of Golgi preparations generates a gradient in which the densest fractions represent the cis cisternae, and the lightest fractions represent the trans cisternae. The positions of enzymes on the gradient, and in situ immunochemistry with antibodies against individual enzymes, suggest that certain enzymes are differentially distributed proceeding from cis to trans. The difference between the cis and trans faces of the Golgi is clear, but it is not clear how the concept of compartments relates to individual cisternae; there may rather be a continuous series of changes proceeding through the cisternae.
 |
Figure 25.7 A Golgi stack consists of a series of cisternae, organized with cis to trans polarity. Protein modifications occur in order as a protein moves from the cis face to the trans face. |
Nascent proteins encounter the modifying enzymes as they are transported through the Golgi stack. Figure 25.7 illustrates the order in which the enzymes function. This may be partly determined by the fact that the modification introduced by one enzyme is needed to provide the substrate for the next, and partly by the availability of the enzymes proceeding through the cisternae.
The addition of a complex oligosaccharide can change the properties of a protein significantly. Glycoproteins often have a mass with a significant proportion of oligosaccharide. What is the significance of these extensive glycosylations? In some cases, the saccharide moieties play a structural role, for example, in the behavior of surface proteins that are involved in cell adhesion. Another possible role could be in promoting folding into a particular conformation. One modification Xthe addition of mannose-6-phosphate Xconfers a targeting signal.
- Chapter II Information Search on the Internet: A Causal Model
- Chapter XIII Shopping Agent Web Sites: A Comparative Shopping Environment
- Chapter XIV Product Catalog and Shopping Cart Effective Design
- Chapter XVI Turning Web Surfers into Loyal Customers: Cognitive Lock-In Through Interface Design and Web Site Usability
- Chapter XVII Internet Markets and E-Loyalty