7. Single-stranded DNA is needed for replication
13.7 Single-stranded DNA is needed for replication |
| Key terms defined in this section |
| SSB is the single-strand protein of E. coli, a protein that binds to single-stranded DNA. |
As the replication fork advances, it unwinds the duplex DNA. One of the template strands is rapidly converted to duplex DNA as the leading daughter strand is synthesized. The other remains single-stranded until a sufficient length has been exposed to initiate synthesis of an Okazaki fragment of the lagging strand in the backward direction. The generation and maintenance of single-stranded DNA is therefore a crucial aspect of replication. Two types of function are needed to convert double-stranded DNA to the single-stranded state:
- A helicase is an enzyme that separates the strands of DNA, usually using the hydrolysis of ATP to provide the necessary energy. A helicase is generally multimeric, often a hexamer, and typically translocates along DNA by using its multimeric structure to provide multiple DNA-binding sites. It is likely to have one conformation that binds to duplex DNA and another that binds to single-stranded DNA. Alternation between them drives the motor that melts the duplex, and requires ATP hydrolysis Xtypically 1 V2 ATPs are hydrolyzed for each base pair that is unwound. A helicase usually initiates unwinding at a single-stranded region adjacent to a duplex, and may function with a particular polarity, preferring single-stranded DNA with a 3′ end (3′-5′ helicase) or with a 5′ end (5′-3′ helicase).
- A single-strand binding protein (SSB) binds to the single-stranded DNA, preventing it from reforming the duplex state. The SSB binds as a monomer, but typically in a cooperative manner in which the binding of additional monomers to the existing complex is enhanced.
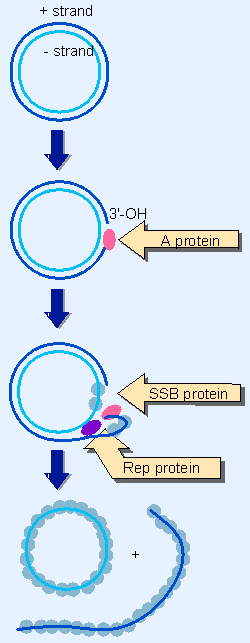 |
Figure 13.10 fX174 DNA can be separated into single strands by the combined effects of 3 functions: nicking with A protein, unwinding by Rep, and single-strand stabilization by SSB. |
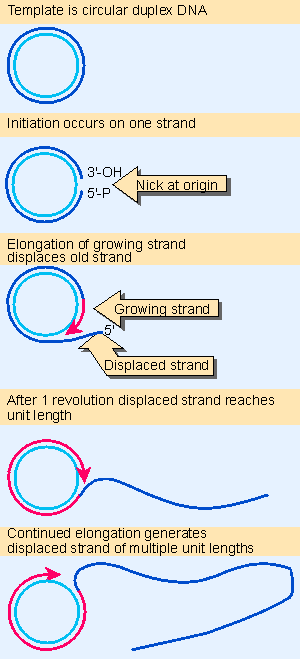 |
Figure 12.16 The rolling circle generates a multimeric single-stranded tail. Animated figure |
The conversion of φX174 double-stranded DNA into individual single strands illustrates the features of the process. Figure 13.10 shows that a single strand is peeled off the circular strand, resembling the rolling circle described previously in Figure 12.16. The reaction can occur in the absence of DNA synthesis when the appropriate 3 proteins are provided in vitro.
The phage A protein nicks the viral (+) strand at the origin of replication. In the presence of 2 host proteins, Rep and SSB, and ATP, the nicked DNA unwinds. The Rep protein provides a helicase that separates the strands; the SSB traps them in single-stranded form. The E. coli SSB is a tetramer of 74 kD that binds cooperatively to single-stranded DNA.
The significance of the cooperative mode of binding is that the binding of one protein molecule makes it much easier for another to bind. So once the binding reaction has started on a particular DNA molecule, it is rapidly extended until all of the single-stranded DNA is covered with the SSB protein. Note that this protein is not a DNA-unwinding protein; its function is to stabilize DNA that is already in the single-stranded condition.
Under normal circumstances in vivo, the unwinding, coating, and replication reactions proceed in tandem. The SSB binds to DNA as the replication fork advances, keeping the two parental strands separate so that they are in the appropriate condition to act as templates. SSB is needed in stoichiometric amounts at the replication fork. It is required for more than one stage of replication; ssb mutants have a quick-stop phenotype, and are defective in repair and recombination as well as in replication. (Some phages use different SSB proteins, notably T4; this shows that there may be specific interactions between components of the replication apparatus and the SSB; we discuss this later.)