5 Some energy sources
5 Some energy sources
5.1 Primary and rechargeable batteries
The more frequent sources in the form of primary and rechargeable batteries exist in a wide variety of technologies and types. For choice, it is absolutely necessary to have a method of selection related to criteria linked to technical characteristics and uses. Many parameters must be taken into account: energy density (Figure 24.2), instant and mean power, use temperature, self discharge , lifetime, shape and flexibility, recycling and cost.
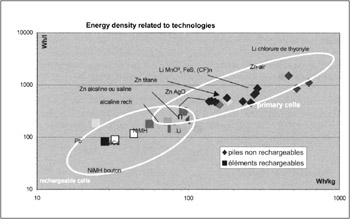
Figure 24.2: Characteristics of primary and rechargeable batteries
Primary cells are of interest only for weak energy need, ie for objects that consume little or that are occasionally used unless one prefers to recharge the battery. One sees that, sometimes, the object (camera or walkman) must accept both options, with the choice of standard compatible formats. But it may be emphasised that there is a risk with down market primary cells of leaks and that they cannot be stored for a long time.
Some examples of ordinary current choices illustrate the selection criteria that are prevalent :
The very small objects with high consumption and frequent use operate with economical and high density energy primary batteries, for example hearing aids that use Zinc-air cells. A clock uses a zinc-silver cell of which the voltage is ultra -stable. Alkaline cells are generally used for high currents but are of relatively short lifetime. Small lithium cells are soldered (memory back-up: clock calendar, camera, TV channel, measurement parameters). High density energy on a very wide range of temperature is required in the cameras (lithium battery LiFeSO 2 or Li/MnO 2 ). An original solution would be that society Powerpaper develops a solution to print flexible batteries on the surface of packing cardboard. This would complete the offer of printable electronics.
For intense usage, rechargeable batteries, though expensive, are unbeatable for annual cost because they can perform many cycles .
The following table (Table 24.1) sums up the main features of rechargeable batteries and shows in what the choice may be different depending on use.
| Technology | Energy density - Wh/kg - Wh/1 | -Life time - Cycles 100% | Complexity of charge Charge maxi | Self charge | Cost c euro/Wh/cycle | Use |
|---|---|---|---|---|---|---|
| Lead 2 V | 20 “40 <80 | 5 years 200 | Easy 5 to 15 min | < 10%/month | 0,1 to 0,3 | Robot, low cost, high power |
| NiCd 1,2 V | 40 “60 <120 | 10 years 1500 | Easy 5 to 15 min | 10% in some days 100% in 5 months | 0,5 | Wireless, tools, wide range of temperature, solid, high power |
| NiMH 1,2 V | 60 “120 <350 | 5 years 500 | Mean 1h | 100% in 3 months | 0,4 to 0,6 | DECT, GSM, PC, picture, games image recoder |
| Li-ion 3,6 V | 110 “160 250 | 2 years 400 | Sophisticated 1h | < 10%/month | 1,5 to 5 | GSM, PenPC, PDA, camcorder |
| Li-ion polymer 3,6 V | 130 “170 400 | 2 years 500 | Sophisticated 1h | < 10%/month | ? | Replacement of flexible Li-ion and safer |
It must be emphasised that nickel batteries need, due to their high self discharge, frequent recharging (weekly to monthly).
For the objects that use rechargeable batteries, the issue of the charger is crucial. One sees that the charger is often much heavier and bigger than the battery. It may weigh 10 times the battery weight (200 g for 20 g in a GSM for a charge of 3 hours). Faster chargers exist (for example 600 mAh lithium-ion charged in 1 h) that are a little lighter at the price of sophistication.
This type of analysis about energy related to use, choice of the source and functional evolution will have to be made for every object. For example, the arrival of 3G mobiles (high bandwidth, sound and image remote download) that consume more energy, leads main manufacturers of lithium batteries for mobiles (Sanyo, Panasonic etc) to search for battery packs that have a capacity of 1000 mAh weighing less than 30 g. Projections are 400 Wh/1 and 250 Wh/kg in 2005/7. As the autonomy of objects is a essential need difficult to obtain due to increase of power consumption, much research is focused on an external autonomous source complementary or competitive to the classical charger [2 MIT]. The solutions range from single sources (primary battery, solar cell, inductive coil, piezzo-electric, dynamo etc) to hybrid sources that associate several sources and energy storage (demonstration of a pen-computer with solar panel: Figure 24.3).

Figure 24.3: Solar pen-computer (France T l com)
Ubiquitous and opportunist battery charge is also possible using ultrafast charge or connection to a hybrid telecom+energy network.
5.2 Photovoltaic cells
Small size cells (Figure 24.4), especially adapted to indoor light, can supply some ¼ W under natural light. For example, a panasonic cell (16 — 58 mm) gives 24 ¼ A under 1,3 V ie 30 ¼ W under a 200 lux fluorescent light or under a 40 lux incandescent bulb. An outdoor cell close to a window of some mm 2 will supply some mA.
Figure 24.4: Thin film solar cells
The combination of sources is necessary to overcome the problem in micropower (memory backup), for example, a photovolta c connected to a rechargeable battery or a capacitor or an ultra- capacitor :
-
the solar source charges the energy store that powers the object;
-
in calculators , a solar cell saves the primary battery that may therefore be installed for the lifetime. When the battery is exhausted, the objects partially operate ie only in light.
5.3 Ultra-capacitors
The ultra-capacitors are very large capacitors (100 times more than ordinary capacitors) based on a molecular double layer capacity effect. The energy density reaches almost the capacity of a rechargeable battery (up to 10 Wh/kg).
An ultracapacitor can operate associated with a solar cell in replacement of a rechargeable battery, or associated with a high capacity primary or a rechargeable battery but of low power, to provide peak power. It can serve as an ultrafast charge storage (some seconds in a charging zone) for a small objects that consume little. The advantages are a very long lifetime (> 10 years), several hundred of thousand charge/discharge cycles, of a low impedance of some m . So, combined with a primary or rechargeable battery, it helps provide high power pulses .
5.4 Fuel cells
These generators operate with a gaseous, liquid or solid fuel and oxygen of the air. The chemical reaction is a slow oxidation freeing electrons in an external circuit. Depending on the technology, the products of reactions are water, carbon dioxide or ionic metal salts . The efficiency is 30 to 50%. The operating temperature is around 60 to 80 °C.
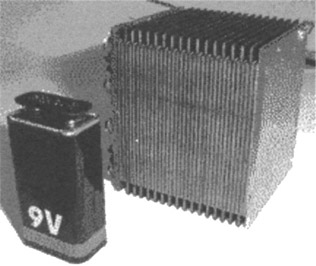
Figure 24.5: Fuel cell
The fuel is contained in micro-storage: methanol-ethanol-water cartridges (Motorola, Sanyo, Medys), pressurised hydrogene or metal hydride (Los Alamos, CEA) or borohydride bulb (Manhattan scientific, CEA). The energy density target is 3000 Wh/kg. The challenge is very strong because such a battery, weighing as much as a battery charger, should give 20 to 50 equivalent charges with the advantages of suppressing the dependency on the electric plug and offering ultrafast energy recovery by simply replacing a cartridge. First industrial products will not be available before 2005/7 with a competitive price compared to lithium batteries. Reducing the use of platinum catalyst and the price of proton exchange membrane are the mains issues. But there also exists a promising approach with the metal-air battery (zinc-air AER or aluminium-air TRIMOLE reaching 400 to 800 Wh/l), perhaps easier to put into practice.
Still more surprising research is taking place about a biobattery that, with the help of conventional batteries, would digest organic material (sugar, alcohol) producing usable hydrogen in the battery core . This approach is very interesting because one could take care of communicating objects like plants or animals!
5.5 UPTI wiring (Universal Power & Telecom Interface)
A possible solution to power ever-more numerous communicating objects in the coming year, is to imagine a safety ultra low voltage bipolar cable that necessitates only partial insulation. It may be 12 V complying with the safety standard CEI 950 and complying with car voltage. The circuit can be very easy to install like a decorative piece and allows one to clip small objects almost everywhere to power and charge them.
A hybrid function of information transport on the energy cable and a leakage antenna option to send the information at very short distance may be envisaged. This UPTI cable could suit in buildings but also in cars and public transportation. A PC, a mobile or a PDA could be almost charged on UPTI. As well as a communicating device, the holder may have electrical charging area. It only remains to define a standard!
EAN: 2147483647
Pages: 191