14 - Drug Therapy
Editors: Kane, Robert L.; Ouslander, Joseph G.; Abrass, Itamar B.
Title: Essentials of Clinical Geriatrics, 5th Edition
Copyright 2004 McGraw-Hill
> Table of Contents > Part III - General Management Strategies > Chapter 13 - Sensory Impairment
Chapter 13
Sensory Impairment
Because as many as 75 percent of older adults have significant visual and auditory dysfunction not reported to their physicians, adequate screening for these problems is important. These disorders may limit functional activity and lead to social isolation and depression. Correction of remediable conditions may improve the ability to perform daily activities.
VISION
![]() Physiologic and Functional Changes
Physiologic and Functional Changes
The visual system undergoes many changes with age (Table 13-1). Decreases in visual acuity in old age may be caused by morphological changes in the choroid, pigment epithelium, and retina, or by decreased function of the rods, cones, and other neural elements. Older patients frequently have difficulties turning their eyes upward or sustaining convergence. Intraocular pressure slowly increases with age.
TABLE 13-1 PHYSIOLOGICAL AND FUNCTIONAL CHANGES OF THE EYE | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The refractive error may become either more hyperopic or more myopic. In the young, hyperopia may be overcome by the accommodative power of the ciliary muscle on the young lens. However, with age, this latent hyperopia becomes manifest because of loss of accommodative reserve.
Other older patients may show an increase in myopia with age, caused by changes within the lens. The crystalline lens increases in size with age as old lens fibers accumulate in the lens nucleus. The nucleus becomes more compact and harder (nuclear sclerosis), increasing the refractive power of the lens and worsening the myopia.
Another definitive refractive change of aging is the development of presbyopia from nuclear sclerosis of the lens and atrophy of the ciliary muscle. As a result, the closest distance at which one can see clearly slowly recedes with
P.336
age. At approximately age 45, the near point of accommodation is so far that comfortable reading and near work become cumbersome and difficult. Corrective lenses are then needed to enable the patient to move that point closer to the eyes.
Diminished tear secretion in many older patients, especially postmenopausal women, may lead to dryness of the eyes, which can cause irritation and discomfort. This condition may endanger the intactness of the corneal surface. The treatment consists mainly in substitution therapy, with artificial tears instilled at frequent intervals.
The corneal endothelium often undergoes degenerative changes with aging. Because these cells seldom proliferate during adult life, the cell population is decreased. This may leave an irregular surface on the anterior chamber side, where pigments may accumulate. This type of endothelial dystrophy is frequently seen in older patients, and dense pigment accumulation may slightly decrease visual acuity. In some patients, the endothelial dystrophy will spontaneously progress and lead to corneal edema. Such cases require corneal transplants.
P.337
![]() Blindness
Blindness
The prevalence of visual problems and blindness increases with age (Fig. 13-1). The most common causes of blindness are cataracts, glaucoma, macular degeneration, and diabetic retinopathy. Screening for these disorders should include testing visual acuity, performing an ophthalmoscopic evaluation, and checking intraocular pressure (Table 13-2).
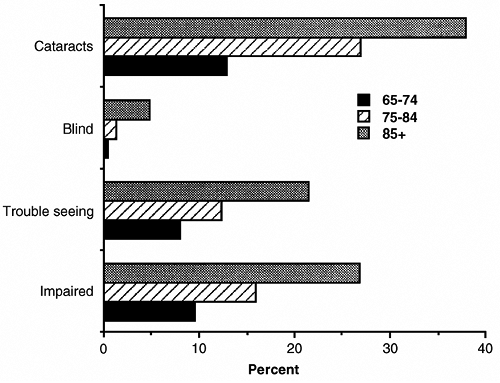 |
FIGURE 13-1 Prevalence of vision problems in older persons, 1984. (From Havlik, 1986.) |
TABLE 13-2 OPHTHALMOLOGICAL SCREENING | |||||||
|---|---|---|---|---|---|---|---|
|
Senile Cataract
Opacification of the crystalline lens is a frequent complication of aging. In the Framingham Eye Study, the prevalence of cataracts was associated with age and reached 46 percent at ages 75 to 85 (Kini et al., 1978).
The cause of age-related cataracts is unknown, but the opacifications in the lens are associated with the breakdown of the g-crystalline proteins. Epidemiological data and basic research suggest that ultraviolet light may be a contributing factor in cataract development. The pathological process may occur in either the cortex or the nucleus of the lens. Cortical cataracts have various stages of development. Early in the process, opacities are in the periphery and do not decrease visual acuity. At the mature stage, opacifications are more widespread and involve the pupillary area, leading to a slow decrease in visual acuity. In the mature stage, the entire lens becomes opaque. The nuclear cataract does not have these stages of development but is a slowly progressing central opacity, which frequently shows a yellowish discoloration, therefore preventing certain colors from reaching the retina.
Cataracts of mild degree may be managed by periodic examination and optimum eyeglasses for an extended period. Ultraviolet lenses may be of benefit. When a cataract progresses to the point where it interferes with activities, cataract surgery is generally indicated. The surgeon may use several methods to remove it, and the decision regarding the best method for each patient should be made by the ophthalmologist.
In intracapsular cataract extractions, the entire cataract and surrounding capsule are removed in a single piece. This removes the entire opacity. In extracapsular cataract extractions, the cataractous lens material and a portion of the capsule are removed. The posterior capsule is left in place to hold an intraocular lens implant.
P.338
After cataract removal, the eye has decreased refractive power. Three methods of restoring useful vision are available: eyeglasses, contact lenses, and intraocular lenses (Table 13-3). Approximately 95 percent of those who undergo cataract surgery now receive intraocular lens implants.
TABLE 13-3 METHODS OF RESTORING VISION AFTER CATARACT SURGERY | ||||||
|---|---|---|---|---|---|---|
|
Eyeglasses required after surgery are usually thick and heavy. These correct the focus of the eye and permit excellent vision through the central portion. However, they increase the apparent size of the object by approximately 25 percent, introduce optical distortion, and interfere with peripheral vision. Patients must learn to turn the head instead of the eyes to see clearly to the side. Eyeglasses can be used for patients who have had surgery on both eyes or surgery on one eye and decreased vision in the other. However, eyeglasses cannot usually be used for patients who have had surgery in one eye and have normal vision in the other eye because of the difference in image size.
Contact lenses correct the focus of the eye, permit both central and peripheral vision, and increase apparent object size by 6 percent. However, handling contact lenses is difficult for some individuals, and most lenses must be removed and inserted daily. Extended-wear contact lenses are available, and approximately 50 to 70 percent of elderly patients are able to wear them after surgery. Contacts are useful in patients who have had cataract surgery in one
P.339
or both eyes. The lenses correct for distant vision, but eyeglasses are required for reading.
The intraocular lens is surgically placed inside the iris and is expected to remain permanently in place. This lens corrects the focus of the eyes and permits central and peripheral vision; object size is increased by only 1 percent. It is appropriate for patients with cataracts in one or both eyes and is particularly useful for patients unable to wear a contact lens. Bifocal eyeglasses are usually required to aid distant or near vision.
Glaucoma
The glaucomas are a group of eye disorders characterized by increased intraocular pressure, progressive excavation of the optic nerve head
P.340
with damage to the nerve fibers, and a specific loss in the visual field. Most cases of primary glaucoma occur in older patients. In the Framingham Eye Study, prevalence of open-angle glaucoma increased with age to 7.2 percent at ages 75 to 85, with men having much higher rates than women (Kini et al., 1978).
Angle-closure glaucoma is an acute and relatively infrequent type of glaucoma, characterized by a sudden painful attack of increased intraocular pressure accompanied by a marked loss in vision. The treatment consists of normalizing the intraocular pressure by the application of miotic eye drops or other medication (such as carbonic anhydrase inhibitors or osmotic agents). The definitive treatment, however, is surgical excision of a peripheral piece of iris or, more frequently now, by laser iridectomy, ensuring free flow of aqueous humor. Because the disease is usually bilateral, some physicians propose prophylactic iridectomy on the second eye.
Chronic open-angle glaucoma is the more frequent variety of primary glaucoma. It is characterized by an insidious onset, slow progression, and the appearance of typical defects of the visual fields. Early in the disease, intraocular pressure is only moderately elevated, and optic nerve head excavation progresses slowly and sometimes asymmetrically. While central visual acuity may remain normal for a long time, the defects in the peripheral visual field are characteristic and gradually progressive. Initially, there is a paracentral scotoma, which may coalesce. A nasal step of the visual field is another important sign. Finally, the entire field will constrict and eventually involve the visual centers.
The treatment is usually medical, with miotics of various kinds used first. Beta-blocking agents may also be used and have the advantage of not changing the diameter of the pupil. However, care should be taken because these agents may be systemically absorbed. In severe cases, combination drops may be used with systemic medications such as carbonic anhydrase inhibitors. Surgery or laser therapy is indicated only if disease progresses on maximal medical therapy.
Age-Related Macular Degeneration
The macular area of the retina lying at the posterior pole of the globe is the site of highest visual acuity. This area depends entirely on choriocapillaries for nutrition.
Any disturbance in the vessel wall of the choroidal capillaries, in the permeability or thickness of the Bruch's membrane, or in the retinal pigment epithelium may interfere with the exchange of nutrients and oxygen from the choroidal blood to the central retina. Such disturbances occur frequently in older patients. Senile degeneration of the macula is one of the most frequent causes of visual loss in older adults and is the commonest cause of legal blindness (20/200 or worse). In the Framingham Eye Study, the prevalence was 28 percent at ages 75 to 85 years, with a higher rate in women than in men (Kini et al., 1978). In addition to older age, risk factors include family history of the disorder, cigarette smoking, low dietary intake or plasma concentrations of antioxidant vitamins and zinc, and white race for wet lesions (Fine et al., 2000).
P.341
Ophthalmoscopic findings vary and do not always parallel loss of vision. In the dry form of degeneration, there are areas of depigmentation alternating with zones of hyperpigmentation caused mainly by changes in the retinal pigment epithelium. In another form, the degeneration involves the Bruch's membrane, leading to the pigmentation of well-circumscribed, roundish yellow areas.
The second type of degeneration is an exudative or wet type. Here there is an elevated focus in the macular area, which at first contains serous fluid but later contains blood derived from blood vessels sprouting from the choroid to the subretinal space. The blood may become organized and form a plaque.
In all these cases, central visual acuity will be markedly affected. These patients will gradually lose the ability to read or see any other details. Most macular degeneration is not treatable; however, laser treatment applied at a specific stage of exudative macular degeneration has been effective in preventing central visual loss in some patients (Macular Photocoagulation Study Group, 1993, 1994). Total blindness does not occur, as patients retain peripheral vision and therefore are able to perform activities that do not necessitate acute central vision.
Diabetic Retinopathy
In the geriatric population, a significant amount of visual loss is attributed to diabetic retinopathy. The Framingham Eye Study showed an age-associated increase in prevalence up to 7 percent at ages 75 to 85 years (Kini et al., 1978). In the adult-onset diabetic with background changes, the visual loss is usually related to vascular changes in and around the macula. Leakage of serous fluid from vessels surrounding the macula leads to macular edema and deterioration of visual acuity. This may respond to laser photocoagulation.
Hemorrhages within the macula may lead to more permanent visual loss. A loss of retinal capillaries may lead to macular ischemia and poor prognosis of visual recovery.
Recent clinical trials have demonstrated that intensive blood-glucose control and tight blood pressure control reduce the risk of microvascular disease, including retinopathy in type 2 diabetes (see Chap. 12).
![]() General Factors
General Factors
Table 13-4 summarizes the general patterns of signs and symptoms associated with common visual problems of older adults. In addition to the specific treatment discussed above, some simple techniques, such as use of a magnifying device, large-print reading material, lighting intensifiers, and reduction of glare, can help maximize visual function (see Table 13-5).
TABLE 13-4 SIGNS AND SYMPTOMS ASSOCIATED WITH COMMON VISUAL PROBLEMS IN OLDER ADULTS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 13-5 AIDS TO MAXIMIZE VISUAL FUNCTION | |
|---|---|
|
Health care providers should also be aware of the significant systemic absorption of ophthalmic medications. These agents may lead to other organ systems' dysfunction and interact with other medications (Table 13-6). The patient's other medical problems and medications should be assessed and the minimum dose to
P.342
P.343
P.344
achieve the desired effect should be used. Patients should also be monitored for systemic toxicity.
TABLE 13-6 POTENTIAL ADVERSE EFFECTS OF OPHTHALMIC SOLUTIONS | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
HEARING
This section covers four areas related to hearing problems in older adults: a review of the major parts of the auditory system, tests used to evaluate the hearing system, effects of aging on hearing performance, and specific pathologic disorders affecting the auditory system.
Hearing problems are common in the elderly, especially in a highly industrialized society where noise and age interact to cause hearing loss (Fig. 13-2). In the National Health and Nutrition Examination Survey, hearing loss was present in 35.1 percent of those surveyed aged 55 to 74 years (Reuben et al., 1998). Hearing loss in the elderly is usually of the sensorineural type, caused by damage of the hearing organ, the peripheral nervous system, and/or the central nervous system. These hearing problems are not usually amenable to medical or surgical
P.345
intervention, and thus require hearing aids, aural rehabilitation, and understanding as the major avenues of remediation.
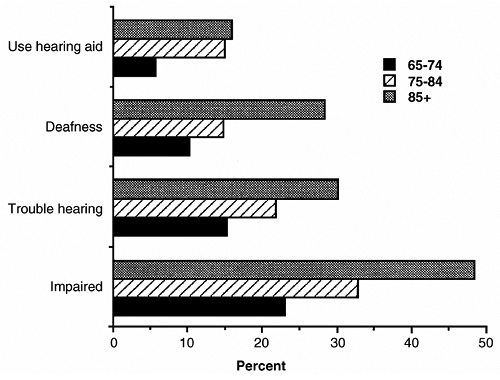 |
FIGURE 13-2 Prevalence of hearing problems in older persons, 1984. (From Havlik, 1986.) |
![]() The Auditory System
The Auditory System
On a functional basis, the auditory system can be divided into three major parts: peripheral, brainstem, and cortical areas (Table 13-7). Each part of the hearing system has unique functions, which combine to allow hearing and understanding of speech. Table 13-8 lists these functions.
TABLE 13-7 PERIPHERAL AND CENTRAL AUDITORY NERVOUS SYSTEM | |
|---|---|
|
TABLE 13-8 FUNCTIONAL COMPONENTS OF THE AUDITORY SYSTEM | |
|---|---|
|
The main functions of the peripheral auditory system are to change sound into a series of electrical impulses and to transmit those to the brainstem. The major brainstem function is binaural interaction. Binaural interaction allows localization of sound and extraction of a signal from a noisy environment. The cortex brings sound to consciousness and allows interpretation of speech and initiation of appropriate reactions to sound signals.
P.346
![]() Assessment
Assessment
Assessment of hearing function can be divided into three kinds of hearing tests: standard, binaural, and difficult speech. The standard tests are useful for evaluating the peripheral system, binaural tests for evaluating the brainstem, and difficult speech tests for evaluating cortical problems (Table 13-9). Standard tests are performed by presenting pure tones or single words at varying intensity. An audiometer (AudioScope, Welch-Allyn, Inc.) that will deliver pure tones is available for the office screening of hearing deficits.
TABLE 13-9 ASSESSMENT OF HEARING FUNCTION | |
|---|---|
|
Tympanic membrane movement is assessed with a probe. Loudness comparison assesses the individual's ability to balance intensity of sound coming from both ears; lateralization tests the individual's ability to fuse sounds from both ears; and masking level differences assesses the ability to pick out specific sounds from a background of noise. Monotic degraded tasks present difficult sounds such as noise background, filtered sound, and time-compressed speech; dichotic tasks simultaneously present sense and nonsense speech, which the individual is asked to repeat.
![]() Aging Changes
Aging Changes
Many changes in the peripheral and central auditory system during aging have effects on the hearing mechanism (Table 13-10). These changes lead to diminished performance by older subjects (Table 13-11), including the loss of sensitivity and distortion of signals that succeed in passing to higher levels, difficulty in localizing signals and in taking advantage of two-ear listening, difficulty understanding
P.347
speech under unfavorable listening conditions, and problems with language, especially when aging is compounded by stroke.
TABLE 13-10 EFFECTS OF AGING ON THE HEARING MECHANISM | |
|---|---|
|
TABLE 13-11 HEARING PERFORMANCE IN OLDER ADULTS | |
|---|---|
|
Three major factors enhance the progression of hearing loss with advancing age: previous middle-ear disease, vascular disease, and exposure to noise. These factors alone, however, do not account for the hearing loss of old age, called presbycusis. Although clinically and pathologically complex, this is a distinct progressive sensorineural hearing loss associated with aging. The deterioration is not limited to the peripheral sensory receptor. Presbycusis affects 60 percent of individuals older than age 65 in the United States. However, only a fraction of these have a functional deficit necessitating aural rehabilitation.
P.348
![]() Sensitivity
Sensitivity
Beginning with the third decade of life, there is a deterioration in the hearing threshold. At first, sensitivity at the high frequencies declines gradually. This age-associated loss has been confirmed in populations not exposed to high levels of noise. This gradual impairment is sensorineural and can be tested by pure-tone audiometry, which reveals useful information about the physiological condition of hearing, but does not disclose some important aspects of deterioration.
![]() Speech
Speech
Although there is a close relationship between pure tone loss and the ability to hear speech, the audiogram does not precisely measure hearing for speech. To assess this auditory function, speech audiometry can be performed by presenting the undistorted test words above threshold intensities in the absence of background noise.
Older people with hearing impairment may have difficulty understanding speech under less-favorable conditions, as with background noise, under poor acoustic conditions, or when speech is rapid. This difficulty may be caused in part by the longer time required by higher auditory centers to identify the message. Such hearing loss may necessitate testing of desired signals with the presentation of a competing signal. This will more accurately reflect hearing of speech in social circumstances.
Speech occurring in rooms that cause long reverberations is also much less intelligible to the elderly. Auditory temporal discrimination and auditory reaction time and frequency discrimination also decline with age. Because consonant
P.349
sounds are of higher frequency and shorter duration, the loss of high-frequency hearing in the elderly may affect these sounds, which encode much of speech information. Lipreading may compensate to some extent for this effect on understanding speech, but other factors of processing information still remain.
![]() Loudness
Loudness
A common auditory problem of the elderly is abnormal loudness perception. This can occur as hypersensitivity to sounds of high intensity and appears as increased loudness recruitment, in which gradually increasing loudness, such as amplified sound, is unpleasantly harsh and difficult to tolerate. In older adults with hearing impairment, this abnormality is manifest when a speaker is asked to speak louder or the output of a hearing aid is increased. It may result from a sensorineural loss attributable to changes in the hair cells of the inner ear.
![]() Localization
Localization
Sound localization contributes to effectiveness of signal detection and helps with discrimination. Loss of directional hearing results in greater hearing difficulty in a noisy environment. Localization is disturbed in older adults with hearing loss and may be partly caused by the aging brain's deranged processing of interaural intensity differences and time delays. A strongly asymmetrical hearing loss also disturbs localization.
![]() Tinnitus
Tinnitus
Tinnitus, an internal noise generated within the hearing system, occurs in many types of hearing disorders at all ages but is much more frequent in older adults. Tinnitus, however, is not necessarily associated with hearing loss and may occur in older adults without hearing impairment. Estimates of prevalence of tinnitus in the United States are about 10 percent, with the majority of persons with tinnitus being 40 to 80 years of age (Peifer et al., 1999). Treatment is generally unsatisfactory.
![]() Other Hearing Disorders
Other Hearing Disorders
One of the most easily treatable but too easily overlooked causes of hearing loss is cerumen that occludes the external auditory canal (Table 13-12).
P.350
Cerumen usually affects low-frequency sounds and complicates existing hearing impairments.
TABLE 13-12 DISORDERS OF HEARING IN OLDER ADULTS | |
|---|---|
|
Hearing loss in the geriatric patient may be caused by scarring of the tympanic membrane. In tympanosclerosis, there is calcification of the tympanic membrane that results in stiffening of the drumhead.
Otosclerosis may cause fixation of the ossicular chain and lead to a conduction hearing loss. The bony capsule may also be affected, leading to sensorineural loss. Paget's disease may also lead to both kinds of hearing loss and should be evaluated radiologically and by an alkaline phosphatase determination.
Ototoxic medication is an acquired cause of hearing loss producing cochlear damage. The aminoglycoside antibiotics require special caution. At high doses, ethacrynic acid and furosemide may be ototoxic.
High doses of aspirin may cause a reversible hearing impairment. Unfortunately, except for aspirin, removal of the offending drug usually does not reverse the sensorineural loss.
Sound trauma is an environmental factor with neurosensory consequences. Superimposed on the changes of aging, sound trauma can have a severe impact on a patient's communicative ability.
Vascular or mass lesions may affect hearing at one of several levels, including the middle and inner ear, auditory nerve, brainstem, and cortex.
![]() Aural Rehabilitation
Aural Rehabilitation
Every individual who has communication difficulties caused by a permanent hearing loss should have an ear, nose, and throat evaluation to rule out remediable disease and then an audiological evaluation to assess the roles of amplification
P.351
and aural rehabilitation. Table 13-13 lists the factors that should be considered during the evaluation for a hearing aid. In the severely impaired, in addition to a hearing aid, aural rehabilitation with speech reading may be necessary.
TABLE 13-13 FACTORS IN EVALUATION FOR A HEARING AID | |
|---|---|
|
Patient and family counseling may improve use of and satisfaction with a hearing aid. Realistic expectations should be explained to the patient. Hearing aids are most useful in one-on-one conversations and are less effective in noisy, group settings. They are also less useful in improving understanding of less-familiar accents and languages, for example, a British accent in a movie or television production. Such understanding can be improved by use of the closed caption feature on television. Facing the speaker and lipreading also improves understanding. Improvements and modifications in design and construction of hearing aids have enabled a greater proportion of the hearing-impaired population to profit from amplification. The old adage that hearing aids will not help people with sensorineural loss is simply not true. The aid can be adjusted to a specific frequency rather than all frequencies, thus decreasing loudness problems, improving discrimination, and making the aid more acceptable. Binaural aids improve sound localization and discrimination.
The hearing aid that is worn on the body provides the greatest amplification but is necessary only for patients with the most severe hearing loss. The controls are large and therefore more easily managed by some elderly persons. However, many elderly people prefer behind-the-ear or in-the-ear devices. The in-the-ear devices are small, cosmetically more acceptable, but more difficult to manipulate.
P.352
Although expensive, cochlear implants can restore hearing in individuals with severe hearing loss not corrected by hearing aids.
TASTE
During aging there is a significant loss of lingual papillae and an associated diminution of ability to taste. Salivary secretion also diminishes, thus decreasing solubilization of flavoring agents. Upper dentures may cover secondary taste sites and decrease taste acuity.
Olfactory bulbs also show significant atrophy with old age. In a population-based, cross-sectional study of adults aged 53 to 97, the prevalence of impaired olfaction by olfaction testing was 24.5 percent and increased with age to 62.5 percent of 80- to 97-year-olds (Murphy et al., 2002). Taste and olfactory changes together may account for the lessened interest in food shown by older adults.
POLYNEUROPATHY
Patients with polyneuropathy have impairments in balance and an increased risk for falls and falls causing injury (Richardson, 2002). Epidemiological data on polyneuropathy are relatively limited. In a study from Italy of subjects ages 55 and older, the prevalence of polyneuropathy was 11 percent. Diabetes mellitus was the most common risk factor (44 percent of patients with polyneuropathy). The next most common risk factors were alcoholism, nonalcoholic liver disease, and malignancy (Beghi and Monticelli, 1998). In a natural history study of type 2 diabetes mellitus, 42 percent of the diabetic population had nerve conduction abnormalities consistent with polyneuropathy after 10 years (Partanen et al., 1995). The prevalence of diabetes mellitus in older persons is increasing, and, therefore, the prevalence of polyneuropathy is likely to increase as well.
In chronic polyneuropathies, such as diabetes mellitus, symptoms usually begin in the lower extremities and sensory symptoms usually precede motor symptoms. In demyelinating polyneuropathies, such as Guillain-Barr syndrome, weakness rather than sensory loss is more typical. The physical examination should focus on the sensory examination including pin prick, light touch, vibration, cold, and proprioception, and on muscle strength testing and appearance of muscle wasting. Extensive diagnostic testing is usually not necessary in a patient with mild symptoms and a known underlying diagnosis such as diabetes mellitus or alcohol abuse. In patients with no clear etiology, electrodiagnostic testing should be the initial diagnostic study (Dyck et al., 1996). Rutkove has described an algorithm for a diagnostic approach to polyneuropathy
P.353
(Rutkove, 2002; see Fig. 13-3). Laboratory tests, which might include a complete blood count, erythrocyte sedimentation rate, thyroid stimulating hormone, serum and urine protein electrophoresis, blood glucose, vitamin B12 level, antinuclear antibody, and urinalysis, should be directed by the electrodiagnostic testing results.
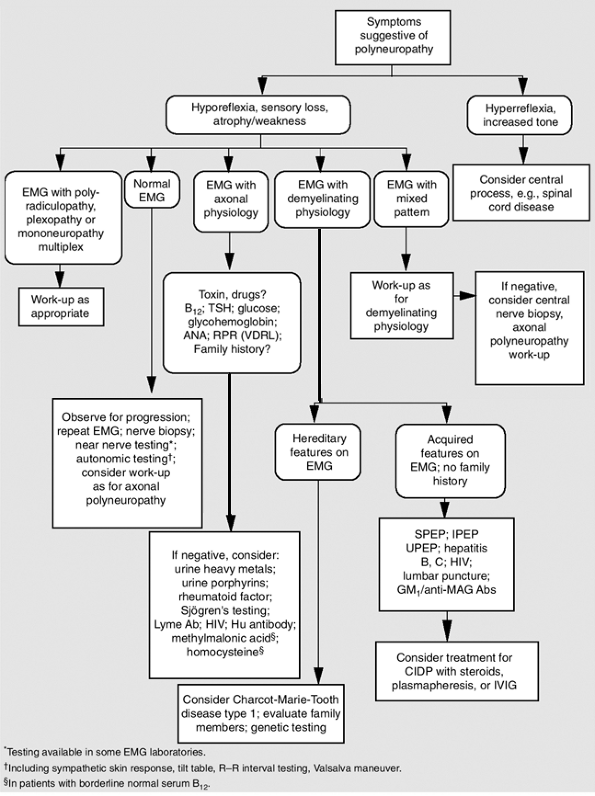 |
FIGURE 13-3 Diagnostic approach to polyneuropathy. Ab = antibody; CIDP = chronic inflammatory demyelinating polyneuropathy; EMG = electromyography; GM1 = ganglioside antibodies; Hu = human; IPEP = immunoprotein electrophoesis; IVIG = intravenous immunoglobulin; MAG = myelin-associated glycoprotein; SPEP = serum protein electrophoresis; UPEP = urine protein electrophoresis. (From Rutkove, 2002.) |
Treatment should address the underlying disease process and alleviation of symptoms. Avoidance of toxins, such as alcohol or drugs, is the most important step. In patients with diabetes, tight control may help maintain nerve function (see Chap. 12). In painful neuropathies, tricyclic antidepressants are effective, as is gabapentin. In patients with weakness, physical therapy evaluation is important and use of ankle foot orthosis, splints, and walking-assistance devices can improve function. Proper foot and nail care is important in reducing risk for foot ulcers.
ACKNOWLEDGMENT
The authors wish to thank Dr. Douglas Noffsinger for his assistance in preparing material for an earlier version of the section on hearing in this chapter.
References
Anand KB, Eschmann E: Systemic effects of ophthalmic medication in the elderly. NY State J Med 88:134 136, 1988.
Beghi E, Monticelli ML: Chronic symmetric symptomatic polyneuropathy in the elderly: a field screening investigation of risk factors for polyneuropathy in two Italian communities. J Clin Epidemiol 51:697 702, 1998.
Dyck PJ, Dyck PJB, Grant IA, et al: Ten steps in characterizing and diagnosing patients with peripheral neuropathy. Neurology 47:10 17, 1996.
Fine SL, Berger JW, Maguire MG, et al: Age-related macular degeneration. N Engl J Med 342:483 492, 2000.
Havlik RJ: Aging in the eighties, impaired senses for sound and light in persons age 65 years and over. NCHS Advance Data, No. 125, 1986.
Kini MM, Liebowitz HM, Colton T, et al: Prevalence of senile cataract, diabetic retinopathy, senile macular degeneration, and open-angle glaucoma in the Framingham Eye Study. Am J Ophthalmol 85:28 34, 1978.
Macular Photocoagulation Study Group: Laser photocoagulation for subfoveal neovascular lesions of age-related macular degeneration: updated findings from two clinical trials. Arch Ophthalmol 111:200 209, 1993.
Macular Photocoagulation Study Group: Laser photocoagulation for juxtafoveal choroidal neovascularization: five-year results from randomized clinical trials. Arch Ophthalmol 112:500 509, 1994.
P.354
P.355
P.356
Murphy C, Schubert CR, Cruickshanks KJ, et al: Prevalence of olfactory impairment in older adults. JAMA 288:2307 2312, 2002.
Partanen J, Niskonen L, Lehtinen J, et al: Natural history of peripheral neuropathy in patients with non-insulin dependent diabetes mellitus. N Engl J Med 89:333:89 94, 1995.
Peifer KJ, Rosen GP, Rubin AM: Tinnitus: etiology and management. Clin Geriatr Med 15:193 204, 1999.
Reuben DB, Walshk K, Moore AA, et al: Hearing loss in community-dwelling older persons: national prevalence data and identification using simple questions. J Am Geriatr Soc 46:1008 1011, 1998.
Richardson JK: Factors associated with falls in older patients with diffuse polyneuropathy. J Am Geriatr Soc 50:1767 1773, 2002.
Rutkove SB: Overview of polyneuropathy. UpToDate 2002, see http://www.uptodate.com.
Suggested Readings
Gottlieb JL: Age-related macular degeneration. JAMA 288:2233 2236, 2002.
Kollarits CR, Rubin AM, Goebel JA (eds): Visual and auditory challenges. Clin Geriatr Med 15:1 204, 1999.
Lavizzo-Mourey RJ, Siegler EL: Hearing impairment in the elderly. J Gen Intern Med 7:191 198, 1992.
Mulrow CD, Lichtenstein MJ: Screening for hearing impairment in the elderly: rationale and strategy. J Gen Intern Med 6:249 258, 1991.
Uhlmann RF, Rees TS, Psatz BM, et al: Validity and reliability of auditory screening tests in demented and non-demented older adults. J Gen Intern Med 4:90 96, 1989.
EAN: 2147483647
Pages: 23