10 - Acetylsalicylic Acid, Aspirin
Editors: Norris, John W.; Hachinski, Vladimir
Title: Stroke Prevention, 1st Edition
Copyright 2001 Oxford University Press
> Table of Contents > II - Secondary Prevention > 10 - Acetylsalicyuc Acid (Aspirin)
10
Acetylsalicyuc Acid (Aspirin)
Natan M. Bornstein
Aspirin is an old and widely-used drug. Its antithrombotic role the base of its clinical use for prevention of stroke. The development ASA as an active drug for stroke prevention underwent some important milestones over time. The salutary effects of willow bark (saltix alba) have been known to several cultures for centuries. Salicin, its active ingredient, is a bitter glycoside from which sodium salicylate was isolated in 1829 by Henri Leroux, who demonstrated its antipyretic effects. The pharmaceutical chemist Felix Hoffman found a way of acetylating the hydroxyl group on the benzene ring of salicylic acid to form acetylsalicylic acid, which was shown to have anti-inflammatory and analgesic effects.
Acetylsalicylic acid was introduced into clinical medicine at the turn of nineteenth century under the name aspirin, which was given to the new drug by Bayer's chief pharmacologist, Heinrich Dreser.1 In 1953, Craven2 suggested that aspirin may act as an anticoagulant and, in 1956, he suggested that it might prevent ischemic vascular disease.3 In 1963, Blatrix4 noted that aspirin increases bleeding time. The inhibitory effect of this compound on the action blood platelets was not discovered until the late 1960s.5 In 1968 O'Brien6 described a specific inhibitory effect of aspirin on the aggregation response blood. This property was then linked to the irreversible inhibition of cyclooxygenase enzyme responsible for the synthesis of eicosanoids (prostacyclin and thromboxane) and involved in arachidonic acid metabolism,7 which is responsible for the
P.178
conversion of arachidonic acid to TxA2 in platelets and for the conversion arachidonic acid to prostacycline in the vascular wall.8
Mechanism of Action
Aspirin competes with arachidonic acid for binding to the hydroxyl group of single amino acid residue (serine 529) in the polypeptide chain of platelet prostaglandin G/H synthase 1. As a result, aspirin completely and irreversibly inhibits the action of the enzyme cyclooxygenase, thereby suppressing the production thromboxane A2 (TxA2) in platelets, an affect that induces platelet aggregation and vasoconstriction. This irreversible effect persists for the life span of the platelet. On the other hand, aspirin reduces the production of prostacyclin (PGI2) on the vessel wall, an effect that inhibits platelet aggregation and induces vasodilation and, therefore, might have some anti-thrombogenic effect. However, endothelial cells can rapidly recover the inhibitory effect of aspirin cyclooxygenase synthesis, in contrast to platelets.9,10
In addition to its anti-aggregate effect, aspirin has other actions that may potentially play a role in stroke prevention, namely; antiinflammatory and antioxidant activities. Aspirin is rapidly absorbed in the stomach and upper intestine: the peak plasma concentration occurs 15 20 minutes after ingestion and the antiaggregate activity is evident within 1 hour after administration.10 Thus, the inhibitory effect is rapid and lasts for the life-span of the platelet. Some mechanisms of aspirin on hemostasis are cyclooxygenase independent and should be taken into account. Aspirin may increase fibrinolytic activity for up to 4 hours after its administration,11 and may lower vitamin K-dependent clotting factors II, VII, IX, and X.12 However, the dose-response relationship, duration, occurrence in the clinical setting, and relevance to the antithrombotic effect of aspirin have not been established.
In summary, the two opposing actions of aspirin on blood platelets are blocking of the pro-aggregatory and vasoconstrictive effects TxA2 on the one hand and diminishing vasodilatation and anti-aggregate activity of prostacyclin on the other hand, which led to the coining of the term aspirin dilemma .13 The aspirin dilemma refers mainly to the debate concerning the use of higher vs. lower doses of aspirin in patients who are at high risk of cerebrovascular thrombosis.
Aspirin and Stroke Prevention
Primary Prevention
Several trials aimed at investigating the use of aspirin for primary prevention of stroke yielded inconclusive results. The U.S. Physicians' Health Study14 was a double-blind, placebo-controlled trial of 325 mg of aspirin taken every other day
P.179
with or without beta carotene, conducted among 22,071 U.S. male physicians initially aged 40 to 84 years, with an average follow-up of 5 years. The study revealed a 44% reduction in the incidence of myocardial infarction, and that motivated the early termination of the study. Over 5-year period, incidence of cardiovascular death was similar in the aspirin and placebo groups, a nonsignificantly increased risk of stroke all types, particularly for the small subgroup of hemorrhagic strokes (23 vs. 12), was shown in the aspirin-treated group compared with placebo group. However, the number of events were too small to draw any firm conclusions.
The British Doctors' Trial15 randomized 5139 male physicians initially aged 50 to 78 years in an open design between one group taking 500 mg of aspirin daily (two-thirds of the patients) and those advised to avoid aspirin (one-third the patients). This trial was much smaller, unblinded, unbalanced, and less rigid than the U.S. Physicians' Health Study. After 6 years, no statistically significant difference was detected between the two groups, either for the combined outcome event vascular death, stroke, or myocardial infarction, for any of these events alone.16 However, a slight increase in disabling strokes and a decrease transient ischemic attacks (TIAs) among those allocated to aspirin. Only limited data were available on which of the strokes were hemorrhagic and were thrombotic, but no excess of any particular type stroke was shown with aspirin. Barnett17 suggested that any future primary prevention studies designed to evaluate possible stroke reduction should be required to continue for 10 years longer or to involve a population of subjects 10 years older than the ones in these two studies, because stroke incidence peaks 10 years later in life than myocardial infarction.
An overview of these two trials primary prevention16,18 showed a 32% (SD 8%) reduction in the odds of suffering a nonfatal myocardial infarction, and a 13% (SD 6%) reduction of combined vascular events, but a nonsignificant increase for nonfatal stroke (18% SD 13%). The results of these two large studies led to the conclusion that the routine use of aspirin by healthy men should not be universally recommended when the side effects of aspirin are compared with the reduction in risk of nonfatal myocardial infarction.16,19
The Nurses' Health Study20 included females taking from one to six aspirin per week or placebo was conducted in the United States. The analysis based on 87,678 registered nurses aged 34 to 65 years and free of diagnosed coronary heart disease, stroke, and cancer at baseline. The study demonstrated that women who had taken aspirin a reduced risk of a first myocardial infarction, but no alteration in the risk of stroke was observed. Cardiovascular death slightly but nor significantly reduced.
Several points should be emphasized regarding these studies. They included individuals who were engaged in health services and could be considered as comprising an especially health-minded group. This also may explain the rate of vascular
P.180
events being lower than expected in the general population. Kronmal et al.21 have since reported the intriguing results that aspirin use was associated with increased risk for ischemic stroke in women and hemorrhagic both men and women in a cohort of elderly people. The authors mentioned the possibility of there having been some confounding of results stemming from aspirin use per se as opposed to cause and effect.
In another randomized study, the Hypertension Optimal Treatment (HOT) study,22 in which 9391 hypertensive patients were assigned to receive 75 mg of daily aspirin and 9391 patients received placebo, there was a significant beneficial effect of aspirin on myocardial infarction reduced by 36% but there was no effect on stroke. In these studies, a small but definite chance of adverse events existed, with about 1 per 1000 cases of excessive bleeding due to aspirin.
A recent data analysis conducted by Hart et al.23 concluded that aspirin is ineffective in primary prevention of stroke for individuals without clinically identified vascular disease. This is in contrast to its benefit in decreasing myocardial infarction and to its protective effect against stroke in patients with manifest vascular disease.
Efficacy of aspirin for stroke reduction in patients with asymptomatic carotid stenosis is doubtful. In a double-blind, placebo-controlled trial, Cote et al.24 demonstrated that aspirin had no significant long-term protective effect in asymptomatic patients with high-grade (>50%) carotid stenosis. The median duration of follow-up was 2.3 years. The annual rate of all ischemic events and death from any cause was 12.3% for the placebo group and 11.0% aspirin group (p = 0.61). The annual rates for vascular events only were 11% the placebo group and 10.7% for the aspirin group (p = 0.99).
The role of aspirin in preventing initial stroke patients with nonvalvular atrial fibrillation remains unclear. Only two placebo-controlled, randomized, primary prevention trials in patients with atrial fibrillation have been performed, using warfarin and various doses of aspirin. They were terminated early as monitoring of the results showed significant differences.25
The Atrial Fibrillation, Aspirin, Anticoagulation (AFAS AK) study from Copenhagen, Denmark,26 and the Stroke Prevention in Atrial Fibrillation (SPAF) study from the United States27 formally evaluated use of aspirin as an alternative treatment. The AFASAK study used 75 mg of aspirin per day and the SPAF trial used 325 mg per day. Another study, Boston Area Anticoagulation Trial for Atrial Fibrillation (BAATAF), 28 which was not specifically designed to evaluate the role of aspirin, allowed patients in the placebo group to receive aspirin at a dose of 325 mg per day. The BAATAF and AFASAK trials were not blinded, and the SPAF study was blinded for ASA but not for warfarin therapy.
In the AFASAK,26 1007 patients were randomly allocated to receive warfarin, aspirin (75 mg/day), or placebo. At the end of 2 years, the incidence of stroke, TIA, and systemic embolism was significantly lower in the warfarin group (1.5%) than in the aspirin and placebo group (6% each). A reduction of about 20% in
P.181
the risk of important vascular events occurred in the aspirin group, but because of very small numbers of events the results were inconclusive. The trial was reported as negative with respect to aspirin. However, its unblinded design and the fact that 38% of the patients assigned to the warfarin group withdrew from this study, together with the fact that analysis was an efficacy analysis, necessitated confirmation of these findings by other trials.25
In the SPAF Study,27 588 patients with nonvalvular atrial fibrillation were randomly selected to receive warfarin, aspirin (325 mg/day), or placebo. In addition, 656 patients not eligible for treatment with warfarin received aspirin or placebo in a double-blind fashion. At the end of 1 year, the placebo arm of the study was terminated because active treatment (either warfarin or aspirin) reduced the risk of stroke and systemic embolism by an impressive 81%. The study also revealed that aspirin reduced the risk of stroke and embolism by 50% but was not effective in patients older than 75 years.
Various mechanisms of stroke may explain the lack effectiveness aspirin in the AFASAK study compared with that in the SPAF study. The entered older patients, who were probably at higher risk of stroke than those in the SPAF study. This difference may be related to a higher prevalence of left atrial or ventricular stasis-related thrombi, which are anticoagulant sensitive, in the older patients in the AFASAK study, as indicated by the threefold to fourfold higher incidence of heart failure and the twofold higher incidence previous myocardial infarction in patients that study. Aspirin reduced the occurrence of stroke categorized as noncardioembolic significantly (p = 0.01) more than it did those categorized as cardioembolic, an important finding of the SPAF investigation.29 Another difference between the two studies was a lower (75 mg) dose of aspirin that was used in the AFASAK compared to 325 mg of SPAF. In addition, the Danish study included more females, and this also may be a confounder. Thus, ASA may have some stroke prevention benefit in patients with nonvalvular atrial fibrillation who tend to develop platelet rich in situ thrombi or emboli. In these patients, atrial fibrillation is probably only a marker of vascular disease rather than a cause of left arterial thromboembolism.30
In light of these findings, it emerges that there are presently no approved prescription indications for aspirin in the primary prevention of cerebrovascular and cerebrovascular disease, and that formal policy recommendations need to await the results of the randomized trials. In meantime, the American Heart Association suggests that aspirin may outweigh the harm in men at high risk for coronary disease, but no guidelines have been issued for women.
Secondary Prevention
Since the late 1970s, many clinical trials have been conducted to determine value of aspirin in the prevention ischemic stroke. The first placebo-controlled randomized trial, which was done in Canada,31 involved almost 600 patients (290
P.182
patients assigned to the two groups that included aspirin treatment were compared with 295 patients assigned to one of the two groups that did not take the drug) and showed that 1300 mg of aspirin per day reduced the incidence stroke and death by 31%.
Another randomized controlled study conducted in France on 604 patients with previous TIA (16%) or completed stroke (84%) showed that 1000 mg of aspirin daily significantly reduced the risk of stroke (40%) in both sexes, but that mortality rate was not reduced.32 In this study, cotreatment with dipyridamole did not confer additional benefits. Since then, several other trials have been conducted with different doses of aspirin, ranging from 30 to 1000 mg per day. The results of three trials were published in 1991. One was the final report the U.K. TIA aspirin trial,33 in which 2435 patients with or minor stroke were randomly allocated to receive blind treatment with aspirin at 1200 mg daily, aspirin at 300 mg daily, or placebo. Patients were followed for a mean of about 4 years. The outcomes (i.e., death, myocardial infarction, and stroke) were similar in the two groups that had been allocated aspirin neither dose of aspirin was significantly better than placebo. It is noteworthy that the number of patients in each aspirin group was 815 and 806, which might be too small to rule out a type II error. In the final analysis, when both aspirin dose groups were combined, the investigators found a significant (15%) reduction in the risk of death, myocardial infarction, and stroke, but only a 7% reduction in disabling stroke death, and 3% reduction in disabling stroke vascular death. It is important to mention that the population of the U.K. TIA study was somewhat different from other stroke studies in that there was a relative low annual rate of stroke (3.2% in the placebo group, compared to 5.9% 7.3% reported other studies.31,34
A randomized double-blind Dutch TIA trial34 compared the effectiveness of 30 mg aspirin daily to 283 mg on the occurrence of nonfatal stroke, myocardial infarction, and vascular death in patients after TIA or minor stroke (there was no placebo group). A total of 3131 patients were included and followed for an average of 2.6 years. No significant difference was found in vascular events between the 30 mg daily group (14.7%) compared with 283 mg daily group (15.2%).
The Swedish Aspirin Low-Dose Trial (SALT)35 was a double-blind randomized trial that compared 75 mg of aspirin daily with placebo for the prevention of stroke and death following TIA or minor stroke. The 1360 study patients were followed for a mean of 32 months. A significant 18% reduction was found in the primary out come events (stroke or death).
The recently completed second European Stroke Prevention Study (ESPS-2) was a randomized, placebo-controlled, double-blind trial comparing the effect of low dose aspirin (50 mg daily), modified-release dipyridamole (DP, 400 daily), to the combination of both drugs with the effects placebo in 6602 patients with a prior ischemic stroke or TIA.36 The investigators reported that the
P.183
combined therapy was more effective in preventing stroke (37% reduction) than aspirin alone (18.1% reduction) or DP alone (16.3% reduction). For the combination end-point of stroke death, the combination regimen was associated with a 24.4% risk reduction. The combination of aspirin and DP compared with aspirin alone was associated with a 12.9% (95% CI0 25%; p = 0.056) relative risk reduction in primary outcome event of stroke and death, 22% (95% CI:9 33%) relative event vascular death, nonfatal stroke, or myocardial infarction.37,38 None of the treatments significantly reduced the risk of death alone or of fatal stroke. Before the ESPS-2, four studies that had compared combination of aspirin and DP with alone in TIA/stroke patients had collectively shown that the combination of the two drugs was associated with only a 3% (95% CI: from 22 to 22%) reduction in the vascular events compared with aspirin alone.10,31 These results are somewhat different from the result obtained by EPSP-2. A meta-analysis of all trials, including ESPS-2, indicates that among the 2473 patients with prior stroke or TIA who were assigned to the combination of aspirin and DP, 356 (14.6%) experienced vascular event compared with 419 (17.2%) of 2436 patients assigned to receive aspirin a relative risk reduction of 15% (p = 0.012).38 If the results this meta-analysis are correct, the combination of aspirin and DP prevents twice as many vascular events does aspirin alone. However, it is known that large randomized trials may contradict previous meta-analyses. In a recent review of all the DP, Wilterdink and Easton39 concluded that . another randomized clinical trial showing a significant benefit of the combination dipyridamole plus aspirin over aspirin alone may be needed before the addition of dipyridamole to aspirin is widely accepted for prevention of stroke.
The results of ESPS-2 were criticized on several issues:
There were ethical concerns regarding the use of a placebo arm when efficacy of aspirin was proven.
There were concerns that emerged from one of the participating centers having been excluded from analysis after 438 fictitious patients were enrolled.
Among the 25% of patients who withdrew from study, most were in the P and the combination groups and compliance was higher among DP patients (97%) than in the aspirin groups (84%).
The low dose of aspirin (50 mg daily) was regarded by many as a placebo.
The predominant effect of the combination regimen was in reducing nonfatal stroke, with little effect on myocardial infarction and fatal which is different from the effect of other antiplatelet agents.
The Ticlopidine Aspirin Stroke Study (TASS)40 was a triple -blind study comparing the effect of aspirin 1300 mg daily vs. ticlopidine 250 mg twice daily in
P.184
3069 patients with TIA (1300) and minor stroke who were followed for up to 5.8 years. The primary analysis was an intention-to-treat assessment of death from all causes or nonfatal stroke. Ticlopidine effected a 13% greater reduction than aspirin in the primary end-points and a reduction of 21% 3-year event rate for fatal or non-fatal stroke (Fig. 10.1). It was interesting to note that there was 42% relative risk reduction (RRR) for stroke and death in the first year a 47% RRR for stroke and death. This was largely maintained over the next 2 years, but the RRR declined to 21% after 3 years. The superiority of ticlopidine over aspirin in the reduction of stroke was seen both males and females.
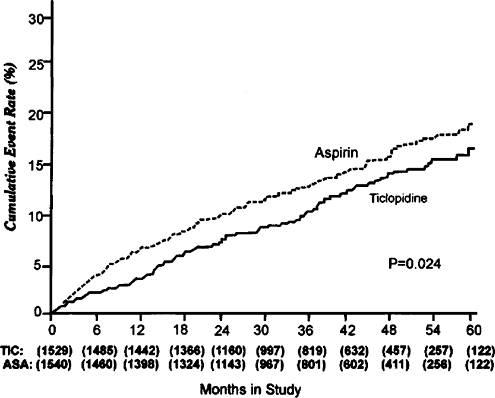 |
FIGURE 10.1. Cumulative event-rate curves for fatal and nonfatal stroke. Values in parentheses indicate the number of patients in the ticlopidine (TIC) and aspirin (ASA) groups. |
A large (19,185 patients) randomized blinded, international trial of clopidogrel vs. aspirin in patients at risk of ischemic events (CAPRIE) was conducted and reported in 1964.41 CAPRIE was the largest clinical trial of a secondary prevention strategy to prevent various vascular end-points in a high-risk population. The trial was designed to assess the relative efficacy of clopidogrel 75 mg once daily and aspirin 325 mg daily in reducing the risk of a composite outcome cluster of ischemic stroke, myocardial infarction, or vascular death. Three groups of patients at significant risk of vascular events, those with recent ischemic stroke (6431), recent myocardial infarction (6302); or symptomatic peripheral arterial disease (6452), were followed for 1 3 years. The result of the outcome cluster
P.185
showed a significant RRR of 8.7% in favor clopidogrel (95% CI:0.3 16.5%; p = 0.043) and an absolute risk reduction of 0.51% (Fig. 10.2). There were no significant differences in adverse events between the two regimens: specifically, there was no increased risk of neutropenia in the clopidogrel group. In a posthoc analysis, there were significant differences in the RRR for each of three entry groups (stroke, myocardial infarction, and peripheral arterial disease), with the most striking effect appearing to be in patients with peripheral arterial disease (RRR 23.8%; CI 8.9 36.2). For stroke patients, there was nonsignificant benefit for clopidogrel over aspirin (RRR 7.3%; 95% CI 5.7 18.7). However, the trial was not powered to detect a realistic treatment effect in each of the three clinical subgroups. Moreover, an additional analysis of these patients in the ischemic stroke and peripheral arterial disease subgroups with a previous history of myocardial infarction demonstrated clear benefit for clopidogrel over aspirin. Hence, the conclusion of this study appears to strongly confirm and be consistent with the previous ticlopidine studies. When absolute risk reduction of 0.5% is taken in consideration, it is calculated that 200 patients per year would need to be treated with clopidogrel rather than aspirin save one end-point.
Thus, it seems probable that clopidogrel will replace ticlopidine but that is less likely to replace aspirin as the first-line therapy for secondary stroke prevention, given its only modest superiority and presumed higher cost.
In 1994, the antiplatelet Trialists' Collaboration published results of a metaanalysis18 in which they analyzed 18 placebo-controlled clinical trials on 10,000
P.186
patients with TIAs or minor stroke: allocation to a mean duration of 33 months of antiplatelet therapy produced a highly significant (2p < 0.00001) reduction of 37 per 1000 in risk of suffering another vascular events (i.e., myocardial infarction, stroke or vascular death) with a standard deviation (SD) of 8. The proportional reduction in important vascular events these trial was 22% (SD 5%).
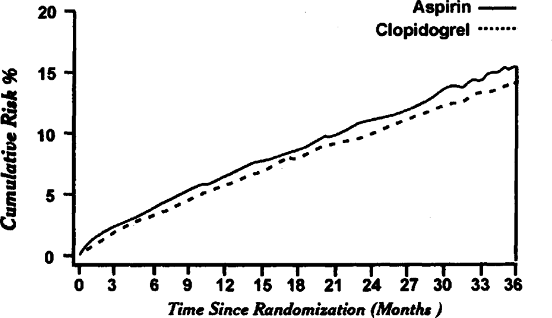 |
FIGURE 10.2. Cumulative risk of composite endpoint (ischemic stroke, myocardial infarction, and vascular death) in CAPRIE patients treated with aspirin or clopidogrel. Adapted from CAPRIE with permission. |
Algra and Van Gijn conducted a mini-meta analysis on data from 10 randomized trials of aspirin only vs. control treatment in 6171 patients after TIA or minor stroke.42 They concluded that aspirin at any dose above 30 mg daily prevents 13% (95% CI; 4 12) of vascular event (i.e., vascular death, stroke, or myocardial infarction).
The data of meta-analyses are not free criticism. If one wishes to compare the results of studies already published, one immediately is confronted with difficulties because of the strikingly different methodologies that were applied and diagnosis that was not always determined under the same conditions. One has to ask if the same therapy may be applied after TIA and stroke. The criteria used to evaluate the efficiency of the therapy varied and very little information concerning the compliance of the patients to criteria was given.
Comparisons can be direct or indirect, and the latter is not always acceptable. Dyken43 warned that direct comparisons between clinical trials are difficult because of the types patients entered, duration treatment, quality followups, and because endpoint definitions often differ. He also warned that several variables other than the study drug could influence sizes of risk reduction between trials. There are other problems with making comparisons between studies that test high doses of aspirin to those examining low doses. Many the former studies were performed up to 15 years before the latter were carried out. The changes in medical treatment of hypertension heart disease may have altered the impact of aspirin.
Adverse effects
Regular use of aspirin increases the risk of gastrointestinal (GI) side effects, such as epigastric pain, peptic ulcer, gastritis, and GI bleeding. Administration of enteric-coated aspirin, but not the buffered type, may lessen the damage to the gastric mucosa.44,45,46 It is difficult to compare the incidence of these side effects among studies because the criteria and the definitions are not the same, nor are the auditing procedures. Therefore, comparison of bleeding complications between different groups within one study gives more valuable information.
In both the U.K. TIA33 and the Dutch TIA trials, bleeding complications were more frequent in the higher aspirin dose group. In U.K. TIA trial, GI bleeding occurred in 1.6% of patients on placebo, 2.6% on 300 mg aspirin and per day, 4.7% on 1200 mg per and approximately half of the patients in each group required hospitalization. It is a widely held view that all-site bleeding with
P.187
aspirin is not dose-related.47 In the Dutch TIA, although major bleeding occurred only slightly less in the 30 mg group, minor bleeds were significantly less that group.
Aspirin and risk of hemorrhagic stroke was recently evaluated using a metaanalysis of the data from 16 randomized controlled trials48 involving 55,462 participants and 108 hemorrhagic strokes. The mean dosage of aspirin was 273 mg per day, and the mean duration of treatment was 37 months. Aspirin treatment was associated with an absolute risk increase in hemorrhagic stroke of 12 events per 10,000 persons (95% CI, 5 20, p < 0.001). However, the overall benefit of aspirin on myocardial infarction and ischemic stroke may outweigh its slight increase in the risk of hemorrhagic stroke.
Dose of Aspirin
The inhibition of platelet thromboxane (TxA2) synthesis is presumed the major mediator of the clinical antithrombotic effect aspirin. Therefore, an optimal aspirin dosage regimen must maximally suppress (TxA2) production. It should be taken in consideration that the synthesis of the beneficial prostacyclin is also suppressed by aspirin. These opposite actions of aspirin were termed the aspirin dilemma .13
Experimental Data
It was initially suggested that the higher doses of aspirin have a greater chance of platelet inhibition.49 Weksler et al.8 suggested that relatively large doses of aspirin (1000 mg-3000 per day) were necessary to inhibit the cyclooxygenase enzyme in both platelets and endothelial cells. The dose-dependent effect of aspirin on reduction of the stable metabolite TxA2, thromboxane 62 (TxB2), was demonstrated by Thogi et al.50 The serum (TxB2)-generated ex-vivo incubation was reduced after 40 mg per day of aspirin (by 85%) and decreased further with increasing aspirin doses to 320 mg per day (by 96%) and 1280 mg per day (by 99%) aspirin doses. In another study,51 serum TxB2 synthesis was inhibited by more than 99% with 300 mg and 500 mg of aspirin 2 hours after the first administration.
Helgason et al.52 had demonstrated that partial inhibition of platelet aggregation, named aspirin resistance, occurred in the patients with or without acute stroke even when increasing doses of aspirin (325, 650, 975, and 1300 mg per day) were administered. Ackerman and Newman53 noted that there was a progressive increase in the number of nonresponders as dose aspirin decreased below 975 mg per day. About 16% of those who received less than 65 per day were nonresponders, but all who were taking over 975 mg per day experienced a full effect.
P.188
Hormes et al.54 reported that aspirin doses of 325 mg and 650 mg per day clearly more effectively altered aggregation of platelets than did 41 mg per day. A daily dose of oral aspirin improved aggregation more than a dose every other day or every 3 days. The antiaggregation response occurred with greater doses of aspirin in the patients who were hyperaggregable at baseline. Advances development of analytical tools to quantitate the dose dependence and time dependence of the effect aspirin on platelet biochemistry and function led to progressive reduction in the dose clinical trials. A level of aspirin dose exists which no effect is seen. It was reported that 10 mg of aspirin does not alter platelet function.55 However, a single administration of a small dose, from 12 mg of aspirin, causes an incomplete inhibition of platelets.56 The cumulative effects doses as low as 20 mg per day are sufficient for attaining complete inhibition of TxT2 in all platelets, and it takes between 3 and 12 days to reach a steady state of complete TxT2 suppression by use of doses between 20 and 40 mg.56,57,58 In other words, 20 mg of aspirin is the minimum daily dose (in terms of TxA2 suppression) needed to acetylate the fraction of new platelets formed each day (10% 15%).56
The studies that have demonstrated complete suppression of TxA2 by daily doses of aspirin between 20 mg and 50 mg have included not only volunteers55,59,60,61,62 but also patients with TIA56 or myocardial ischemia.63 It was shown64 that aspirin at doses of 25 75 mg per day during long-term treatment is sufficient to inhibit platelet aggregation in patients with cerebrovascular disease. Aggregation of platelet-rich plasma induced by a stroke was significantly reduced after 40 mg per day of aspirin (p < 0.005).50 This dose did not decrease the urinary concentration of prostaglandin-containing products, in contrast to higher aspirin doses. In another study,51 it was shown that a loading dose of 40 mg of aspirin in combination with 40 mg as a maintenance dose was less effective the inhibition of platelet aggregation and of thromboxane synthesis than a loading dose of 300 mg combined with 40 mg of aspirin. Also, a low loading dose of aspirin (40 mg) in combination with 40 mg of as a maintenance dose reached its maximal effect very late at day 7 of the observation period compared with other combinations.
In healthy persons, in vivo and ex studies showed that a single dose of at least 160 mg had a profound antiplatelet effect within 1 hour of administration.59,65 A loading dose of at least 100 mg of aspirin may have a complete antiplatelet effect that can be reached within 1 hour.62,65 This degree of inhibition then be sustained by daily administration of doses between 20 mg and 40 mg. If entericcoated aspirin is used instead of the regular form, the required minimum dosage for complete inhibition of TxA2 may increase because prolonged contact with the intestinal juice enhances hydrrolysis of the drug. For a slow release preparation, the necessary dose was 50 mg66 and 80 mg for a granular form.67
P.189
Clinical Experience
Although the experimental data concerning antithrombotic effect of low-dose aspirin on platelet activity may be convincing, the correlation between platelet aggregation with in vivo efficacy for patients with cerebrovascular disease is unclear. In reviewing the randomized clinical trials that were conducted since late 1970s, it becomes clear that several issues were critical elements in the aspirin-dose controversy: (1) The aspirin-dilemma, which led to the hypothesis that arterial thrombosis might occur more frequently with the higher dose of aspirin, and (2) the link between higher dose absolute rate of serious sideeffects (i.e., major hemorrhage). The main problem with a meta-analysis conducted in order to evaluate the usefulness of aspirin stroke prevention18,42 is the lack of direct comparison between a low (100 mg) and a high (1000 dose of aspirin in a large randomized controlled trial. It is likely that the relative efficacy of aspirin for stroke prevention varies between patient populations with different spectra of stoke mechanisms regardless aspirin dose, thereby confounding indirect comparisons.
Nevertheless, the relative risk reduction for stroke and death was 25% to 42% in the higher dose trials compared to only 7% to 18% for the lower dose trial.68 There are also several clinical observations suggesting that higher doses of aspirin may confer a greater benefit in patients with high risk for stroke.22,32 On the other hand, an assessment of benefits and risks should be taken in consideration as well. A direct comparison of GI bleeding was conduction in the U.K. TIA study33: it occurred in 1.6% of the patients on placebo, 2.6% 300 mg of aspirin, and 4.7% on 1200 mg of aspirin. Minor side effects were reported by 24% of the placebo patients, 29% of those on 300 mg of aspirin, and 39% of those on 1200 mg of aspirin. In the Dutch TIA Trial Study,34 a dose of 30 mg/day was compared to 283 mg/day. There were small absolute increases in major hemorrhage (0.3% per year) and fatal bleeding (0.15% that were not statistically significant. In absolute terms, the incremental increase in major hemorrhage with higher doses of aspirin is small, although minor side effects are more common in higher doses of aspirin. Therefore, if a 30% in risk reduction exists with higher doses of aspirin vs. approximately 20% by lower doses69 in a high risk group (10% of stroke or death in 1 year), 100 patients would need to be treated per year with higher doses to prevent one additional event effect by about one additional hemorrhage. One issue in the aspirin dilemma was recently solved (i.e., the optimal dose of aspirin to reduce risk of stroke, myocardial infarction and death after carotid endarterectomy).70 In a randomized double-blind, controlled trial, 2849 patients scheduled for carotid endarterectomy were randomly assigned to received 81 mg, 325 mg, 650 mg, or 1300 mg of aspirin prior to and for 3 months after this procedure. The risk of stroke, myocardial
P.190
infarction, and death was lower in patients taking 81mg or 325 mg of aspirin, therefore, the recommended dose of aspirin after carotid endarterectomy is 325 mg for the prevention of a perisurgical complication.
Conclusion
In conclusion, assuming that our clinical practice should be conducted on the basis of evidence, no scientific data are available to recommend with confidence either high or low dose aspirin for the prevention of stroke. The definitive way of solving this problem is to have a head-to-head comparison of the two doses in a randomized clinical trial. In practical terms, surveys around the world and among experts show that 300 to 325 mg is the most widely used dose of aspirin.71-72 According to interviews, 45% of participants at the European Stroke Conference recommended 100 mg, 45% favored 300 mg, but only 5% > 1000 mg of aspirin.73 The Ad Hoc Committee on Guidelines for Management of Transient Ischemic Attacks of the Stroke Council American Heart Association74 favors the dosage of 325 mg per day since this promotes compliance and minimized gastrointestinal side effects, although they accept a dose range as wide as 30 mg to 1300 mg per day as recommendable treatment.
Acknowledgments
The author thanks Madeleine Bianco and Esther Eshkol for secretarial editorial assistance.
References
1. Flower RJ, Moncada S, Vane JR. Analgetic-antipyretics and anti-inflammatory agents: Drugs employed in the treatment of gout. In: Oilman AG, Goodman IS, Rail TW, Murad F, eds. Goodman and Oilman's The Pharmacological Basis of Therapeutics, 7th ed. New York: Macmillan, 1985, 674 675.
2. Craven LL. Experience with aspirin (acetylsalicylic acid) in the non-specific prophylaxis of coronary thrombosis. Mississippi Valley Med J 1953;75:38 44.
3. Craven LL. Prevention of coronary and cerebral thrombosis. Mississippi Valley Med J 1956;78:213.
4. Blatrix C. Allongement du temps de saignement sous l'influence certains medicaments. Nouv Rev F Hematol 1963;3:346.
5. Weiss HJ, Aledort LM. Impaired platelet-connective-tissue reaction in man after aspirin ingestion. Lancet 1967;2:495 497.
6. O'Brien JR. Effects of salicylates on human platelets. Lancet 1968;l:779 783.
7. Majerus PW. Arachidonate metabolism in vascular disorders. J Clin Invest 1983;72: 1521 1525.
8. Weksler BB, Pett SB, Alonso D, et al. Differential inhibition by aspirin of vascular and platelet prostaglandin synthesis in atherosclerotic patients. TV Engl J Med 1983; 308:800 805.
9. Jaffe E, Weksler B. Recovery of endothelial cell prostacyclin production after inhibition by low doses of aspirin. Clin Invest 1979;63:532 535.
P.191
10. Patrono C. Aspirin as an antiplatelet drug: Review article. N Engl J Med 1994;330: 1287 1294.
11. Bjornsson TD, Scheider DE, Berger H. Aspirin acetylates fibrinogen and enhances fibrinolysis. J Pharmacol Exp Ther 1989;250:154 161.
12. Lekstram JA, Bell WR. Aspirin in the prevention of thrombosis. Medicine 1991;70: 161 178.
13. van Gijn J. Aspirin: Dose and indications in modem stroke prevention. In: Barnett HJM, Hachinski VC, eds. Cerebral Ischemia: Treatment and Prevention. Philadelphia: W.B. Saunders, 1992;10:1:193 207.
14. The Steering Committee of the Physicians' Health Study Research Group: Final report on the aspirin component of ongoing Physicians' Health Study. N Engl J Med 1989;321:129 135.
15. Peto R, Gray Collins et al. Randomised trial of prophylactic daily aspirin in British male doctors. BMJ (Clin Res Ed) 1988;296:313 316.
16. Hennekens CH, Buring JE, Sandercock P, et al. Aspirin and other antiplatelet agents in the secondary and primary prevention of cardiovascular disease. Circulation 1989; 80:749 756.
17. Barnett HJM. 35 years of stroke prevention: challenges, disappointments and successes. Cerebrovasc Dis 1991;1:61 70.
18. The Aspirin Papers. benefits patients with vascular disease and those undergoing revascularisation. Collaborative overview of randomised trials antiplatelet therapy. I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ 1994;308:71 106.
19. Herbert P, Fuster V, Hennekens CH. Antiplatelet and anticoagulant therapy in evolving myocardial infarction and primary prevention. In: Fuster V, Verstraete M eds. Thrombosis in Cardiovascular Disorders. Philadelphia: W.B. Saunders, 1992:261 273.
20. Manson JE, Stampfer MJ, Colditz GA, et al. A prospective study of aspirin use and primary prevention of cardiovascular disease in women. JAMA 1991;266:521 527.
21. Kronmal RA, Hart RG, Manolio TA, et al. Aspirin use and incident stroke in the cardiovascular health study. Stroke 1998;29:887 894.
22. Hansson L, Zanchetti A, George S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension. Optimal Treatment (HOT) randomised trial. Lancet 1998;351:1755 1762.
23. Hart RG, Halperin JL, McBride R, et al. Aspirin for the primary prevention of stroke and other major vascular events. Meta-analysis and Hypotheses. Arch Neural 2000; 57:326 332.
24. Cote R, Battista RN, Abrahamowicz M, et al: Lack of effect of aspirin in asymptomatic patients with carotid bruits and substantial carotid narrowing. Ann Intern Med 1995;123:649 655.
25. Ezekowitz MD, Cohen IS, Gornick CC, et al. Atrial fibrillation. In: Daniel WG, Kronson I, Mugge A, eds. Cardiogenic Embolism. Baltimore: Williams & Wilkins, 1996: 27 44.
26. Petersen P, Boysen G, Godtfredsen J, et al. Placebo-controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: The Copenhagen AFaspirinK Study. Lancet 1989; 1:175 179.
27. Stroke Prevention in Atrial Fibrillation Investigators. Stroke Prevention Fibrillation Study: Final results. Circulation 1991;84:527 539.
P.192
28. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. The effect of low-dose warfarin on the risk of stroke in nonrheumatic atrial fibrillation. TV Engl J Med 1990;323:1505 15ll.
29. Miller VT, Rothrock JF, Pearce LA, et al., on behalf of the Stroke Prevention in Atrial Fibrillation investigators. Ischemic stroke in patients with atrial fibrillation: effect of aspirin according to stroke mechanism. Neurology 1993;43:32 36.
30. Chesebro JH, Fuster V, Halperin JL: Atrial fibrillation risk marker for stroke. N Engl J Med 1990;323:392 394.
31. Canadian Cooperative Study Group. A randomized trial of aspirin and sulfinpyrazone in threatened stroke. N Engl J Med 1978;299:53 59.
32. Bousser MG, Eschwege E, Haguemau M, et al. AICLA Controlled trial of aspirin and dipyridamole in the secondary prevention of atherothrombotic cerebral ischemia. Stroke 1983; 15:5 14.
33. The United Kingdom Transient Ischemic Attack (UK-TIA) Aspirin Trial: Final results, UK-TIA study group. J Neurol Neurosurg Psychiatry 1991;54:1044 1054.
34. The Dutch TIA Study Group. A comparison of two doses aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack of minor ischemic stroke. NEnglJMed 1991;325:1261 1266.
35. Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischemic events. The SALT Collaborative Group. Lancet 1991; 338:1345 1349.
36. Diener H, Cunha L, Forbes C, et al. European Stroke Prevention Study: Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996;43: 1 13.
37. Van Gijn J, Algra A. Secondary stroke prevention with antithrombotic drugs: What to do next? Cerebrovasc Dis 1997; 7(suppl 6):30 32.
38. Hankey GJ. One year after CAPRIE, 1ST and ESPS 2. Any changes in concepts? Cerebrovasc Dis 1998; 8(suppl 5): 1 7.
39. Wilterding JL, Easton JD. Dipyridamole plus aspirin in cerebrovascular disease. Arch Neurol 1999;56:1087 1092.
40. Hass WK, Easton JD, Adams HP, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. N Engl J Med 1989;321:501 507.
41. CAPRIE Steering Committee. A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE). Lancet 1996;348:1329 1339.
42. Algra A, Van Gijn J. Aspirin at any dose above 30 mg offers only modest protection after cerebral ischaemia. J Neurol Neurosurg Psychiatry 1996;60:197 199.
43. Dyken ML. Meta-analysis in the assessment of therapy for stroke prevention. Cerebrovasc Dis 1992;2(suppl):35 40.
44. Robbins DC, Schwartz RS, Kutny K, et al. Comparative effects of aspirin and enteric-coated aspirin on loss of chromium. Clin Ther 1984;6:461 66.
45. Lanza FL, Rover GL, Nelson RS. Endoscopic evaluation of the effects of aspirin, buffered aspirin, and enteric-coated aspirin on gastric duodenal mucosa. N Engl J Med 1990; 303: 136 138.
46. Kelly JP, Kaufman DW, Jurgelson JM, et al. Risk of aspirin-associated major uppergastrointestinal bleeding with enteric-coated or buffered product. Lancet 1996;348: 1413 1416.
47. Adams HP, Bendixen BH. Lowversus high-dose aspirin in prevention of ischemic stroke. Clin Neuropharmacol 1993;16:485 500.
48. Jian H, Whelton PK, Ba BV, et al. Aspirin and risk of hemorrhagic stroke. A metaanalysis of randomized controlled trials. JAMA 1998;280:1930 1935.
P.193
49. O'Brien JR, Etherington MD. How much aspirin? (letter). Thromb Haemost 1990; 64:486.
50. Tohgi H, Konno S, Tamura K, Kimura B, Kawano K. Effects of low-to-high doses aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin. Stroke 1992;23:1400 1403.
51. Buerke M, Pittroff W, Meyer J, et al. Aspirin therapy: Optimized platelet inhibition with different loading and maintenance doses. Am Heart J 1995;130:465 472.
52. Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke 1993;24:345 350.
53. Ackerman RH, Newman KL: Incomplete antiplatelet effects in patients on aspirin compounds (abstract). Ann Neurol 1990;28:224.
54. Hormes JT, Austin JH, James G, et al. Toward an optimal antiplatelet' dose of aspirin: Preliminary observations. J Stroke Cerebrovasc Dis 991; 1:27 35.
55. Kallmann R, Nieuwenhuis HK, deGroot PG, et al. Effects of low doses aspirin, lOmg and 30mg daily, on bleeding time, thromboxane reduction, 6-keto-PGl alpha excretion in healthy subjects. Thromb Res 1989;45:355 361.
56. Patrono C, Ciabattoni G, Patrignani P, et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation 1985;72:1177 1184.
57. Weksler BB, Kent JL, Rudolph D, et al. Effect of low dose aspirin on platelet function in patients with recent cerebral ischemia. Stroke 1985;16:5 9.
58. Toghi H, Tamura K, Kimura A, M, Suzuki H. Individual variation of platelet aggregability and serum thromboxane B2 concentrations after low-dose aspirin. Stroke 988;19:700 703.
59. Patrignani P, Filabozzi Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest 1982;69: 1366 1372.
60. FitzGerald GA, Gates JA, Hawiger J, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest 1983;71:678 688.
61. Preston FE, Greaves M, Jackson CA, et al. Low-dose aspirin inhibits platelet and venous cyclo-oxygenase in man. Thromb Res 1982;27:447 456.
62. de Caterina R, Giannessi D, Bernini W, et al. Selective inhibition of thromboxanerelated platelet function by low-dose aspirin in patients after myocardial infarction. Am J Cardiol 1985;55:589 590.
63. de Caterina R, Giannessi D, Boem A, et al. Equal antiplatelet effects of aspirin 50 or 324 mg/day in patients after acute myocardial infarction. Thromb Haemost 1985;54: 528 532.
64. Boysen G, Bottcher J, Olsen JS. Platelet cyclo-oxygenase inhibition by minimal doses of acetylsalicylic acid in patients with cerebrovascular disease. Acta Neurol Scand 1982:65(suppl. 90): 178 179.
65. Patrono C, Ciabattoni G, Pinca E, et al. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb Res 1980; 17:317 327.
66. Roberts MS, Joyce RM, McLeod LJ, et al. Slow-release aspirin and prostaglandin inhibition. Lancet 1986:1:1153 1158.
67. Jakubowski JA, Stampfer MJ, Vaillancourt R, et al. Cumulative antiplatelet effect of ow-dose enteric coated aspirin. Br J Haematol 1985;60:635 642.
68. Barnett HJM, Meldrum EM. Drugs and surgery in the prevention of ischemic stroke. N Engl J Med 1995;332:238 248.
69. Matchar DB, McCrory DC, Barnett HJM, et al. Treatment for stroke prevention. Ann Intern Med 1994;121:41 53.
P.194
70. Taylor DW, Barnett HIM, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: randomised controlled trial. Lancet 1999;353:2179 2184.
71. Dyken ML, Barnet HJM, Easton DJ, et al. Low-dose aspirin and stroke It Ain't Necessarily So. Stroke 1992;23:1395 1399.
72. Hart RG, Harrison MJG. Aspirin Wars. The optimal dose of aspirin to prevent stroke. Stroke 1996;27:585 587.
73. Bogousslavsky J, Easton JD. Round table discussion: Assessment of benefit/risk therapy in stroke prevention. Cerebrovasc Dis 1992;2(suppl l):41 47.
74. Ad Hoc Committee on Guidelines for management of transient ischemic attacks the stroke council of the American Heart Association. Guidelines for management of transient ischemic attack. Stroke 1994;25:1320 1335.
EAN: 2147483647
Pages: 23