31 - Segmentectomy and Lesser Pulmonary Resections
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section VIII - Postoperative Management of the General Thoracic Surgical Patient > Chapter 39 - General Principles of Postoperative Care
Chapter 39
General Principles of Postoperative Care
Joseph LoCicero III
Major pulmonary and esophageal procedures exert tremendous physiologic stress on patients. To obtain the best results, perioperative care must be optimal. This care begins long before the procedure starts and does not end until long after the patient leaves the hospital.
PREOPERATIVE PREPARATION
Patients' Perceptions
As Cykert and colleagues (2000) point out, patients are most concerned about their functional outcome following a major operation such as pulmonary resection, esophagectomy, or chest wall resection. They fear being limited in physical activity, having a bed-to-chair existence, and requiring complete assistance with activities of daily living. They do not seem to fear postoperative atelectasis, pneumonia, 3 days of mechanical ventilation, or even death. However, nonparticipation by the patient in the postoperative period may lead to postoperative atelectasis and pneumonia, which might lead to significant respiratory compromise and to some of the dreaded outcomes patients are trying to avoid. According to Wright and colleagues (1997), the most common problems delaying discharge from the hospital include inadequate pain control, prolonged air leak, severe nausea, fever, debility, and arrhythmias (Table 39-1). Efforts to improve the patient's preparation prior to the day of operation should be directed at preventing complications that would lead to limited physical function.
Preoperative Teaching
To achieve the best patient participation in his or her own postoperative care, the patient and family should be as fully informed as possible. When the patient and the family know what to expect, they are better prepared to deal with the problems as they arise. The surgeon should have frank and open discussions with the patient and family concerning the anticipated outcome and the expected postoperative problems, along with the usual measures to combat those problems. Such discussions help the patient and his or her family understand that the postoperative course may not be smooth and that aggressive measures may be required in order to achieve ultimate recovery.
Studies such as those by Turner and Williams (2002) and Hekkenberg and colleagues (1997) show that patients retain only about half of what is discussed, so repeated sessions may be necessary to ensure that the patient and family anticipate the perioperative journey. Included in these discussions should be planning for what happens after hospitalization. Involvement of social services at the time of scheduling the operation will facilitate a smooth transition from the acute care setting.
Preoperative Pulmonary Exercise and Training
If surgical intervention is elective, a short period of preparation (preferably 3 weeks) may be beneficial if directed at improving physical status and specifically at pulmonary preparation, conditioning exercises, and nutrition. Gracey and colleagues (1979) studied 157 patients about to undergo a major operation. They administered a standard pulmonary preparation program used at that time. They found that complications were significantly reduced, but that postoperative pulmonary complications were related to the extent of the operation. They made no specific conclusions.
However, in 1999, Debigare and associates studied preparation for lung volume reduction procedures. Because many patients traveled a great distance, they devised a home exercise training program. The program included incentive spirometry, muscle exercises, and aerobic training. The program began with detailed teaching and follow-up and was ensured through weekly phone calls and a diary filled out by each patient. They discovered significant increases in the 6-minute walk test, quality of life perception, peak work rate, peak oxygen consumption, endurance time, and muscle strength and concluded that such training
P.602
was beneficial when time permits a delay in the timing of the operation.
Table 39-1. Common Reasons for Delay in Discharge | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Smoking
Smoking cessation has always been considered an important issue in preparation for an operation. However, the evidence shows that the effects of cigarette smoking linger long after cessation and that inordinately long preoperative delays would be necessary to achieve any significant improvement. Chodoff and colleagues (1975) studied five heavy smokers who quit for 1 week. They could not identify any significant changes in any pulmonary measurements. The Lung Health Study Research Group has published many reports concerning the effects of smoking cessation. Anthonisen and colleagues (1994) reported the results of one of the aforementioned group's studies, namely, that individuals with documented early chronic obstructive pulmonary disease (COPD) who stop smoking experienced improvement of lung function, with the greatest benefit being noted in the first year. No conclusions can be made concerning the early effects of smoking cessation, because their first observation point was 3 months after intervention.
In a study of rats exposed to smoking followed by cessation, Hannan and associates (1989) found that smoking significantly decreased alveolar macrophage fluidity, which persisted up to 18 weeks after cessation. Jeffery and colleagues (1988) studied tracheal surface epithelium and ciliary function in rats exposed to smoke. They found an increase in the frequency of cilia in intraluminal mucus and an increase in the presence of secretory cells of types IV (i.e., merocrine) and V (i.e., apocrine). They also found that the area of trachea covered by cilia, as determined by point counting, increased significantly.
Verra and associates (1995) studied human cilia of individuals who were smokers and ex-smokers or nonsmokers. They noted that the percentage of axonemal ultrastructural abnormalities was higher in smokers and ex-smokers than in nonsmokers or control subjects, a condition that seemed to persist long after smoking cessation. The axonemal ultrastructural abnormalities were polymorphic, characteristic of acquired ultrastructural changes. These results suggest that chronic smoking may induce an increased number of abnormal cilia, which may lead to impaired clearance of mucus.
Bertram and Rogers (1981) noted that epithelial recovery can occur for smokers who have quit for 2 years. Andersson and associates (2000) studied bronchial lavage fluid from former smokers, finding that Clara cell secretory protein was increased in smokers and remained elevated for up to 12 months after smoking cessation. Despite the lack of firm evidence, it is still recommended that patients quit smoking for as long as possible prior to operation.
Nutrition
Preoperative nutritional repletion remains controversial. Fogliani and co-workers declared in 1977 that they could give patients with esophageal cancer sufficient nutrition and return them to positive nitrogen balance using a diet consisting of an average of 2,000 to 2,600 calories as carbohydrate and lipids and 12 to 14 g of nitrogen. Lim and colleagues (1981) used total parenteral nutrition to achieve positive nitrogen balance but noted that this took a minimum of 4 weeks. Despite this difference, the goal for preoperative preparation of the patient is to maintain nutrition at all possible costs to prevent additional weight loss before operation and to schedule the procedure as soon as the patient is prepared.
Medications
Preoperative medications should be continued up to the time of operation. The only exceptions are anticoagulant medications. Patients on warfarin (Coumadin), low-molecular-weight heparin, unfractionated heparin, or clopidogrel (Plavix) should stop their medications long enough prior to the procedure so that the effects of these drugs are minimal. Cessation of aspirin is an individual preference. For pulmonary and esophageal surgery, there is no evidence that aspirin increases bleeding. There is also no evidence that addition of preoperative short-term bronchodilators changes operative outcomes.
POSTOPERATIVE MANAGEMENT
Prevention of Readmission
The goal of postoperative management is to prevent or minimize the effects of the most common problems that lead to a poor outcome. One surrogate measure of a poor outcome other than death is the rate of readmission. As Handy and colleagues (2001) point out, the rate is much higher than most surgeons care to admit. The aforementioned authors operated on 374 patients and had a 2.1% mortality rate. Of the 366 patients discharged, 69 (18.9%) were readmitted a
P.603
total of 113 times. Table 39-2 lists the reasons for readmission. By far the most common reason for readmission was pulmonary complications (27%). Fifty-one percent of the total number of patients were readmitted as inpatients, with the remainder being emergency department evaluations.
Table 39-2. Most Common Reasons for Readmission After Thoracotomy | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
No significant differences in demographics, diagnosis, extent of procedure, postoperative complications, length of stay, or reason for readmission were identified between inpatient and emergency department readmissions. Patients undergoing larger resections, such as pneumonectomy and lobectomy, were admitted more frequently. Moreover, the study found that the patients undergoing pulmonary resections who were readmitted had a difference in subsequent mortality over the 5 years of study. Twelve (4.0%) of the 297 patients not requiring readmission died, whereas 8 (11.6%) of the 69 readmitted patients died. Based on this and personal experience, a number of institutions have developed patient care pathways to address the major causes of morbidity.
Conduct of the Operation
Perioperative management and the conduct of anesthesia are discussed in Chapters 21 and 22. However, several points are worth emphasizing. Anesthetic management begins with proper preparation of patients so that they will be able to emerge smoothly and promptly after the end of the operation. Avoidance of long-acting narcotics and paralytics is essential to this philosophy. The anesthesiologist should be judicious with fluids, keeping the additional crystalloid above fluid losses to 500 to 750 mL. Pain control is extremely important in early mobilization of patients. Regardless of approach (epidural, continuous bupivacaine infusion, patient-controlled analgesia, or a combination), the goal is continuous pain relief sufficient to allow the patient to participate in pulmonary rehabilitation and have early mobilization without significant restrictions placed by the method of analgesia.
Postoperative Hospital Disposition
Patients should go to a unit familiar with the management of these complex thoracic surgical patients. The setting should be monitored, and the nurse-to-patient ratio in the early postoperative period should be sufficiently high to permit assessment and intervention as needed and staff for early mobilization of the patient. Each institution defines the staffing ratio differently, but a 1:2 nurse-to-patient ratio is ideal.
Chest Drainage Systems
Surgeons now have several different options for draining the chest. Although actual tube choice is beyond the scope of this chapter, most surgeons still place one tube anteriorly and one tube posteriorly in the chest. The tubes are attached to a drainage system that permits one-way drainage only, with a portion of the device set up to collect fluid. These devices use a variety of valves and liquid to establish a one-way system. All of the collection systems are designed to provide suction on the tubes if the surgeon desires. In the past, the manufacture of these devices was governed by a single American standard published by the American Society for Testing and Materials (2000) that specified the minimum safety and performance standards for pleural and mediastinal drainage. In 2003, this standard was withdrawn by the society and replaced by the three standards on suction devices written by the International Standards Organization (1999a, 1999b, 1999c). These new standards specify looser criteria but apply the standards worldwide.
In the past, all chest tubes were placed on suction at -20 cm H2O. In recent years, the advisability of the ubiquitous use of suction has been questioned. Several authors, including the author (1985), Cerfolio (2001a), and Wain (2001) and their respective co-workers, contend that if the lung is fully expanded with the tube not on suction, then the patient will do well. Now, there is more individual preference concerning chest tube suction.
Regardless of types of chest tubes and the use of suction, the drainage tubes must be assessed at least daily for patency, function, air leakage, and drainage. Inspection of the tube and drainage system for clots or blockages assures patency. Obstructions are removed by stripping the tubing. This is accomplished by occluding the tubing and pulling the tubing away from the patient to produce a local suction effect. If this does not work, a balloon-tipped catheter may be passed up the tubing to remove the clot, or a suction catheter may be used for the same purpose.
A functioning tube is one that shows variation in the fluid within the tubing when the patient breathes quietly. This may be observed while talking with the patient at the bedside. Good respiratory variation indicates proper functioning of the tube. Limited changes in the level of the fluid may indicate partial blockage, and the tubing may require further stripping. The tubing should be placed so that it does not coil, leaving low points to collect fluid. Such collections impede fluid flow and may cause positive pressure to build up in the tubing and back up into the patient.
P.604
Air leakage is assessed by observing the water-seal chamber on the drainage device. Air leakage should first be assessed off suction at quiet respiration. Next the patient is asked to cough, and the chamber observed. Finally, the patient may be placed back on suction if suction is being employed, and the chamber again observed. Several grading systems have been devised. In general, air leaks should be characterized by the force necessary to produce the air leak and the amount of the air leak. The smallest leak is an intermittent one produced on suction only, and the largest one is a continuous air leak.
Drainage should be measured daily and during the preceding 8 hours so that an estimate can be made concerning whether the rate of fluid drainage is increasing or decreasing. Nurses usually record the drainage in 8-hour shifts and provide a total daily drainage for the last three shifts. Also, the character of the drainage should be noted. Change in the character of the fluid from sanguinous to serous is usually a good sign. Change from serous to purulent connotes potential empyema. In planning chest tube removal, the drainage must significantly decrease to levels acceptable to the surgeon. Although exact numbers are not scientifically verified for the amount of pleural fluid produced per day while a chest tube is in place, a convenient number is 3 mL/kg per day, or 1 mL/kg per 8 hours. For the average patient, this would amount to 240 to 300 mL per day.
Chest tubes and drainage systems are intended to keep the lung expanded and prevent the development of a space infection. Once air leakage ceases and drainage has decreased to acceptable levels, the system has performed its function and should be removed. In an age of cost containment, this could be anytime after the operation. Wain and colleagues (2001) noted that for major pulmonary resections, the average length of time to chest tube removal was 4.5 days. This remains an operator-dependent variable and still can be decreased significantly. There is no scientific proof that a chest tube must be on suction for 2 days after operation followed by 2 days of water seal before removing the tube.
Medications
Patients are encouraged to take their medicines up to the time of surgery, with the exception of warfarin. However, most medications can be withheld safely for a few days postoperatively until normal metabolism and bowel function have returned. There is no indication for the use of prophylactic medications. The use of perioperative antibiotics for pulmonary or esophageal resection should be individualized, as Mangram and colleagues (1999) reported in a guideline for prevention of surgical site infection sponsored by the Centers for Disease Control and Prevention. They noted that prophylactic antibiotics should be used for operations with an anticipated high infection rate or for those with severe or life-threatening consequences if infection occurs, and that administration of an antimicrobial agent should be selected based on published recommendations for a specific operation and efficacy against most common pathogens. In fact, Luchette and associates (2000) found in a meta-analysis that data supporting the use of prophylactic antibiotics for emergency thoracotomies and chest tubes were minimal; they recommended only a 24-hour duration of antibiotics. A survey conducted by the author (1990) showed that few surgeons used prophylactic antibiotics for thoracotomies.
Pain relief is an important part of the postoperative care of the patient undergoing a thoracotomy. Patients deserve to be as pain free as possible. In addition to patient comfort, one of the important reasons for good pain management is that the patient needs to participate fully in postoperative rehabilitation. Deep breathing, coughing, and ambulation from the time of transfer from the recovery room help to prevent unwanted complications. Methods of pain relief are not covered in detail here. As Savage and colleagues (2002) point out, when choosing an approach to postthoracotomy pain management, the thoracic surgeon and anesthesiologist must consider the following: the physician's experience, familiarity, and personal complication rate with specific techniques; the desired extent of local and systemic pain control; the presence of contraindications to specific analgesic techniques and medications; and the availability of appropriate facilities for patient assessment and monitoring postthoracotomy. Whatever method of pain relief is employed, it should facilitate early participation in rehabilitation. If the pain control approach prevents this by limiting mobility or altering mental capacity, another approach should be considered.
Use of prophylactic anticoagulation or methods of venous thrombosis prevention should be considered. Although the operation itself is not considered a high-risk procedure for the development of deep venous thrombosis, the patients fit many of the categories outlined by Wain and associates (2001) and listed in Table 39-3. The majority of patients having a thoracotomy fit the high-risk category as
P.605
defined in Table 39-4. These patients have a calf thrombosis rate of 20% to 40%, a pulmonary embolus rate of 2% to 4%, and a fatality rate of 0.4% to 1.0%. The methods of choice for prevention of deep venous thrombosis in this category include intermittent pneumatic calf compression, low-dose heparin, and low-molecular-weight heparin.
Table 39-3. Common Risk Factors for the Development of Postoperative Deep Venous Thrombosis | |
|---|---|
|
Table 39-4. Classification of Risk Levels for a Postoperative Thromboembolic Event | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||
Postoperative Nutrition
During the operation, fluid management is designed to limit free water that might produce interstitial edema and limit oxygen diffusion capacity. Following operation, this philosophy continues. Patients should have minimum maintenance fluids at 0.5 mL/kg per hour or less. During the first 24 to 48 hours, urine output will be lower than most nursing staff are used to seeing. One must resist the urge to increase the fluid or give boluses of crystalloid. On the first postoperative day, the patient may begin a regular diet without fluid restrictions, and the maintenance fluids may be stopped. For patients having esophageal resection, jejunal feeding should be initiated in the postanesthesia care unit with saline at 10 mL per hour. If tolerated overnight, full-strength feedings can be substituted and increased every 8 hours until the patient's feedings reach a calculated goal.
Cardiac Issues
Postoperative cardiac arrhythmias and ischemia remain common problems after thoracotomy. Groves and colleagues (1999) monitored the heart rates of 82 patients having a major thoracotomy. They discovered that the number of patients suffering from silent myocardial ischemia doubled following thoracotomy. Patients who had ischemia had an average postoperative resting heart rate of 93 beats per minute, as opposed to those without ischemia, who had an average postoperative resting heart rate of 82 beats per minute. They noted that 12% of patients developed atrial arrhythmias, none of which were associated with myocardial ischemia.
The treatment of postoperative arrhythmias is controversial. A brief review of the most commonly used medications is presented in Table 39-5, and a suggested treatment algorithm is presented in Fig. 39-1. Because atrial arrhythmias are far more common, their management is discussed in some detail subsequently. In contrast, because ventricular arrhythmias are rare after thoracotomy and their treatment relies primarily upon resuscitation of the hemodynamically unstable patient, little else will be said about them. The principles of correcting electrolyte abnormalities and ruling out myocardial ischemia should be paramount for patients demonstrating ventricular ectopy or tachycardia or both.
Once the diagnosis of an atrial tachyarrhythmia has been established, the first priority is to assess hemodynamic stability. If the patient experiences syncope, or if the blood pressure is less than 80 mm Hg systolic, then synchronous electrical cardioversion should be performed. The first shock is typically delivered at 200 joules (J), with subsequent shocks at 300 J and 360 J, respectively. For the syncopal patient, no premedication is required. For the patient who is mentating, however, some sedation should be
P.606
administered prior to cardioversion. Sometimes this will depress the blood pressure further, requiring intravenous fluid administration.
Table 39-5. Commonly Used Antiarrhythmic Agents | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
If the patient is hemodynamically stable and mentating, then the next priority should be to achieve control over the ventricular rate, because it is usually rapid (140 to 200 beats per minute). Drugs such as digoxin, verapamil, diltiazem, or metoprolol all depress atrioventricular nodal conduction and are most useful here. Many thoracic surgical patients exhibit bronchospasm, however, and therefore -adrenergic blocking drugs are relatively contraindicated. Furthermore, -blockers are frequently longer-acting than calcium channel blocking drugs and are not as easy to reverse should an adverse effect appear. For these reasons, digoxin (0.5 mg i.v. bolus, followed by two 0.25 mg i.v. boluses spaced 4 hours apart) combined with verapamil (5 to 10 mg i.v. bolus every 5 to 10 minutes) or diltiazem (0.25 to 0.35 mg/kg i.v. bolus every 5 to 10 minutes) to control ventricular rate is a reasonable choice. If these drugs are not successful, or if the patient's hemodynamics deteriorate, then synchronous cardioversion should be undertaken immediately. Once rate control has been achieved, the patient may be transitioned to equivalent doses of oral digoxin and verapamil or diltiazem over the next 24 hours.
During this period of time, electrolytes such as potassium and magnesium should be assayed and replaced as needed. Myocardial ischemia should be ruled out by electrocardiography. In the absence of these factors, the natural history of postoperative atrial tachyarrhythmias is self-termination. Therefore, usually nothing more than a day or two of rate control is required. If the patient does spontaneously convert to normal sinus rhythm over the next 24 hours, the medication can be discontinued and no further treatment is required. If, however, the patient remains in a rate-controlled fibrillation or flutter beyond 24 hours, then cardioversion should be attempted. Usually this is begun as a trial of chemical cardioversion with antiarrhythmic medication. Unfortunately, there is no single drug that demonstrates high efficacy at converting postoperative atrial fibrillation or atrial flutter to sinus rhythm. The class I-A agents, such as procainamide and quinidine, exhibit an approximately 30% conversion rate, similar to placebo. The class I-C agents, such as flecainide and propafenone, claim a somewhat higher conversion rate (about 40% to 60%), but their use is contraindicated in patients following a recent myocardial infarction or in patients with a known depressed ejection fraction. The new class III agent ibutilide claims a very high conversion rate of approximately 60% but is associated with both a high relapse rate and the appearance of malignant ventricular arrhythmias such as torsades de pointes. One of the older class III agents, d-sotalol, has been shown to be effective at converting atrial fibrillation to sinus rhythm, but the racemic mixture of d- and l-sotalol has significant beta-blocking activity and is relatively contraindicated in thoracic surgical patients.
Despite its relative lack of efficacy, the most often used and recommended drug for chemical cardioversion is procainamide. It is easy to give a loading dose (17 mg/kg i.v. over 20 minutes), has relatively few acute limiting side effects (hypotension, nausea), and is quickly metabolized. Unlike quinidine, which has anticholinergic side effects, procainamide has some antiadrenergic effects and can therefore double as an atrioventricular nodal blocking drug. Once the patient has been given a loading dose of procainamide, he or she is started on a continuous infusion of 2 to 4 mg/min. If the patient is taking oral medications, then Procanbid can be started by mouth once the loading dose is completed. The infusion is stopped after the second oral dose.
If the patient converts to sinus rhythm, then the oral antiarrhythmic drug should be continued for at least 30 days after surgery. It may be stopped in the outpatient setting, because the risk of relapsing into atrial fibrillation or atrial flutter is extremely unlikely so long after the operation. If the arrhythmia persists, however, two options are available. First, a semielective electrical cardioversion may be undertaken at this time, with adequate serum levels of antiarrhythmic drug present. Second, the patient may be given anticoagulation with heparin and then maintained on warfarin.
Typically, if patients are discharged from the hospital in rate-controlled atrial fibrillation with adequate anticoagulation, they will spontaneously convert to sinus rhythm as
P.607
P.608
outpatients. If, however, they remain in atrial fibrillation beyond 30 postoperative days, then they should be at low risk for an outpatient electrical cardioversion, providing they have remained therapeutically anticoagulated.
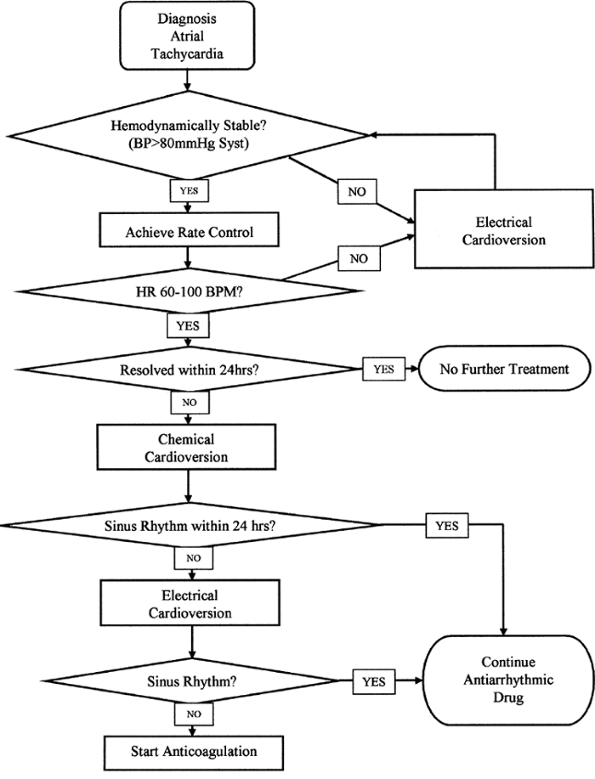 |
Fig. 39-1. Suggested treatment algorithm for the management of atrial arrhythmias. |
Laboratory Testing
Routine laboratory testing is unnecessary except for specific patient conditions such as blood glucose level for diabetes mellitus. Other tests for specific indications might include a blood count for significant hemorrhage or serum potassium level for chronic diuretic usage. Chest radiographs likewise should not be performed on a routine basis. It is not necessary to obtain a radiograph every day and for each manipulation of the chest drainage system, such as the routine cessation of the use of suction. If the immediate postoperative radiograph shows good lung expansion, the air leakage is minimal or nonexistent, and the physical exam does not change, there is no need to repeat the radiograph. In general, patients can average three or fewer postoperative radiographs.
In-Hospital Rehabilitation
In the early postoperative course, the most significant potential complication following thoracotomy is pneumonia. A significant risk factor for the development of pneumonia is atelectasis. Atelectasis is a common problem following thoracotomy and can be minimized only with the patient's help. Several techniques for prevention of atelectasis have been tried and investigated over the years. Stock and associates (1985) performed a trial of continuous positive airway pressure (CPAP), incentive spirometry, and coughing and deep breathing in patients following upper abdominal operations. They found that CPAP increased functional residual capacity faster than the other methods but was not tolerated as well as the other techniques. They also found that coughing and deep breathing and incentive spirometry were equivalent techniques.
In 1991 Hall and colleagues reported on a trial of incentive spirometry or chest physiotherapy in patients following upper abdominal operations. They found that there was little difference between the techniques. They preferred chest physiotherapy. However, most hospitals have eliminated the respiratory therapist from routine postoperative care, and the nurse is too busy to perform effective chest physiotherapy. In 1998 Chumillas and colleagues instructed patients with significant emphysema to perform home exercises consisting of incentive spirometry and coughing and deep breathing and found improved outcomes and fewer complications following operation.
Thus, the most effective approach to the prevention of atelectasis is preoperative home exercises with coughing and deep breathing and possibly incentive spirometry, and continuation of the same exercises with adequate pain control following operation. Because the nursing and other professional staff are stretched thin and can provide only sporadic attention, the families are encouraged to support the patient's efforts on a regular basis during waking hours.
Clinical Pathways
Several institutions have initiated guidelines for all professional staff to follow in the management of postoperative patients. Such protocols have been used successfully for the cardiac surgical patient. Knott-Craig and colleagues (1997) reported that the standard use of preoperative digitalis, aggressive perioperative pulmonary therapy, subcutaneous heparin, and venoocclusive stockings led to a decrease in postoperative complications in thoracotomy patients. That same year, Wright and associates (1997) used data collected from the previous year to make changes in the pathway management of patients. The pathway detailed daily goals for the patient and care team to achieve. Items detailed on a daily basis included assessments, test ordering, physical therapy, medications, diet, oxygen therapy, patient education, social service and case management, pain management, chest tube management, and wound care. The pathway represented the idealized patient and allowed the clinically naive to rapidly gain an insight into the total care of the patient. They noted significant decreases in length of stay and hospital costs. Zehr and colleagues (1998) were able to show similar results at Johns Hopkins Hospital. Cerfolio and co-workers (2001b) studied 500 patients using a clinical pathway and found increased patient satisfaction.
Preparation for Discharge
If postoperative care is well planned and executed, the patient should be able to go home. However, unforeseen events may lead to having to plan for the patient to go to a rehabilitation facility prior to going home. The exact placement of patients depends on how sick they are and how much rehabilitation they can perform. In a short-term acute care rehabilitation facility, the patient must be able to do at least 6 hours per day of rehabilitation, and the length of stay is limited to 5 days. A skilled nursing facility may take patients who can do only a few hours of rehabilitation daily, but with an average length of stay of 21 days. Long-term acute care facilities may take very ill patients with rehabilitation potential to return to full activity, including those on ventilators, and are permitted an average length of stay of 25 days.
After discharge from the acute care facility or one of the rehabilitation facilities, home care is often needed. This care provides checks on the general well-being of the patient, nutritional care, respiratory care, and wound care.
P.609
Planning far in advance of discharge will ensure a smooth and rapid transition back to home.
REFERENCES
American Society for Testing and Materials: Standard specification for medical and surgical suction and drainage systems. F960 86(2000). Withdrawn 2003.
Andersson O, et al: Clara cell secretory protein. Levels in BAL fluid after smoking cessation. Chest 118:180, 2000.
Anthonisen NR, et al: Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA 272:1497, 1994.
Bertram JF, Rogers AW: Recovery of bronchial epithelium on stopping smoking. Br Med J (Clin Res Ed) 283:1567, 1981.
Cerfolio RJ, Bass C, Katholi CR: Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg 71:1613, 2001a.
Cerfolio RJ, et al: Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 122:318, 2001b.
Chodoff P, Margand PM, Knowles CL: Short term abstinence from smoking: its place in preoperative preparation. Crit Care Med 3:131, 1975.
Chumillas S, et al: Prevention of postoperative pulmonary complications through respiratory rehabilitation: a controlled clinical study. Arch Phys Med Rehabil79:5, 1998.
Cykert S, Kissling G, Hansen CJ: Patient preferences regarding possible outcomes of lung resection: what outcomes should preoperative evaluations target? Chest 117:1551, 2000.
Debigare R, et al: Feasibility and efficacy of home exercise training before lung volume reduction. J Cardiopulm Rehabil 19:235, 1999.
Fogliani J, et al: Preoperative assessment: value and limitations in the preparation for surgery of esophageal or cardia carcinoma. Ann Anesthesiol Fr 18:377, 1977.
Gracey DR, Divertie MB, Didier EP: Preoperative pulmonary preparation of patients with chronic obstructive pulmonary disease: a prospective study. Chest 76:123, 1979.
Groves J, et al: Perioperative myocardial ischaemia, heart rate and arrhythmia in patients undergoing thoracotomy: an observational study. Br J Anesth 83:850, 1999.
Hall JC, et al: Incentive spirometry versus routine chest physiotherapy for prevention of pulmonary complications after abdominal surgery. Lancet 337:953, 1991.
Handy JR Jr, et al: Hospital readmission after pulmonary resection: prevalence, patterns, and predisposing characteristics. Ann Thorac Surg 72: 1855, 2001.
Hannan SE, et al: Cigarette smoke alters plasma membrane fluidity of rat alveolar macrophages. Am Rev Respir Dis 140:1668, 1989.
Hekkenberg RJ, et al: Informed consent in head and neck surgery: how much do patients actually remember? J Otolaryngol 26:155, 1997.
Hirsh J, Dalen JE, Guyatt G: The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis. Chest 119:1S, 2000.
International Standards Organization: Medical suction equipment. Part 1: Electrically powered suction equipment safety requirements. ISO 10079 1, 1999a.
International Standards Organization: Medical suction equipment. Part 2: Manually powered suction equipment. ISO 10079 2, 1999b.
International Standards Organization: Medical suction equipment. Part 3: Suction equipment powered from a vacuum or pressure source. ISO 10079 3, 1999c.
Jeffery PK, et al: Response of laryngeal and tracheo-bronchial surface lining to inhaled cigarette smoke in normal and vitamin A-deficient rats: a scanning electron microscopic study. Scanning Microsc 2:545, 1988.
Knott-Craig CJ, et al: Improved results in the management of surgical candidates with lung cancer. Ann Thorac Surg 63:1405, 1997.
Lim ST, et al: Total parenteral nutrition versus gastrostomy in the preoperative preparation of patients with carcinoma of the oesophagus. Br J Surg 68:69, 1981.
LoCicero J: Prophylactic antibiotic usage in cardiothoracic surgery. Chest 98:719, 1990.
LoCicero J, et al: New applications of the laser in pulmonary surgery: hemostasis and sealing of air leaks. Ann Thorac Surg 40:546, 1985.
Luchette FA, et al: Practice management guidelines for prophylactic antibiotic use in tube thoracostomy for traumatic hemopneumothorax: the EAST practice management guidelines work group. Eastern Association for Trauma. J Trauma 48:753, 2000.
Mangram AJ, et al: Guideline for prevention of surgical site infection 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20:250, 1999.
Savage C, et al: Postthoracotomy pain management. Chest Surg Clin N Am 12:251, 2002.
Stock MC, et al: Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest 87: 151, 1985.
Turner P, Williams C: Informed consent: patients listen and read, but what information do they retain? N Z Med J 115:U217, 2002.
Verra F, et al: Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med 151:630, 1995.
Wain JC, et al: Trial of a novel synthetic sealant in preventing air leaks after lung resection. Ann Thorac Surg 71:1623, 2001.
Wright CD, et al: Pulmonary lobectomy patient care pathway: a model to control cost and maintain quality. Ann Thorac Surg 64:299, 1997.
Zehr KJ, et al: Standardized clinical care pathways for major thoracic cases reduce hospital costs. Ann Thorac Surg 66:914, 1998.
EAN: 2147483647
Pages: 203