152 - Surgical Palliation of Inoperable Carcinoma of the Esophagus
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Mediastinum > Section XXIX - Primary Mediastinal Tumors and Syndromes Associated with Mediastinal Lesions > Chapter 178 - Video-Assisted Thymectomy
Chapter 178
Video-Assisted Thymectomy
Michael J. Mack
Thymectomy is an effective and accepted treatment for myasthenia gravis (MG). Many surgical approaches for the performance of thymectomy in patients with MG have been described. It is generally accepted that whatever approach is used, completeness of thymectomy is mandatory in order to obtain optimal clinical results. Standard accepted approaches have been primarily transsternal. In an attempt to make the surgical procedure less invasive to the patient, Cooper and associates (1988) described the transcervical approach for thymectomy. Although comparable results were obtained by this approach, it was not widely accepted by most surgeons, mainly due to the added technical challenges posed by this limited access approach.
In the early 1990s, following the introduction of video-assisted techniques into general and orthopedic surgery, thoracoscopy or video-assisted thoracic surgery (VATS) began to be used for a number of thoracic applications. Now, 10 years later, the VATS approach is generally accepted for a wide variety of intrathoracic surgical procedures, including sympathectomy, management of spontaneous pneumothorax, drainage of empyema, lung biopsy, and resection of pulmonary nodules. Although significant experience has been gained in a number of surgical centers with more complex procedures, including esophagectomy and lobectomy, the technical challenges associated with these procedures as well as the lack of compelling clinical outcome data demonstrating clear-cut clinical benefit has limited widespread acceptance.
Experience has been gained with minimally invasive approaches for performance of thymectomy in patients with MG in the decade since the early 1990s. I and my colleagues (1996) reported our initial experience as part of a multicenter experience, and I and Scruggs updated that experience 2 years later. In this chapter I will review our standard surgical technique, describe other approaches that have been used, and review the published medical literature regarding these approaches.
OPERATIVE TECHNIQUE OF VIDEO-ASSISTED THYMECTOMY
Preoperative Management
Because of the less invasive approach used, our group has not found the surgical procedure to cause significant exacerbation of myasthenic symptoms perioperatively. Therefore, we have not used specific preoperative management. If the patient has recently been in crisis, we wait until the disease course has been clearly stabilized. If the patient is being treated with steroids, we administer additional intravenous steroids perioperatively as we would for any other surgical procedure in a patient on chronic steroid therapy. Specifically, plasmapheresis has not been found to be necessary in any of our patients.
Anesthesia Management
All procedures are performed under general anesthesia with a double-lumen endotracheal tube to administer selective unilateral pulmonary ventilation. A multiagent technique is used with short-acting narcotics and avoidance of neuromuscular blockage acting agents. Extubation is immediate in the operating room upon completion of the procedure.
Operative Positioning
Although initially our group used a left-sided approach, our most recent experience has been totally through the right hemithorax. Therefore, the patient is placed in a supine position with the right side slightly elevated to approximately 10 to 20 degrees. The right arm is placed on an arm holder across the upper portion of the patient and draped out of the operative field. These maneuvers afford
P.2639
wide exposure of the right hemithorax and maximal room for instrument and thoracoscope excursion.
Operative Approach
It is my current technique to perform thymectomy from a right video-assisted approach. We prefer approach from the right side for a number of reasons. First, there is more room for maneuverability from within the right thoracic cavity because of the absence of the pericardial sac. Second, the superior vena cava serves as an excellent landmark for initiation of the surgical dissection and for identification of the innominate vein. Third, for right-handed surgeons, approaching from the right side facilitates access to the cervical area.
Surgical Technique
The approach has changed minimally over the past 5 years. I use four trocars for access. The first three trocars are placed in an inverted triangle, with the apex of the triangle being in the fifth intercostal space in the midaxillary line. The second and third trocars (the base of the inverted triangle) are placed in approximately the third and the sixth intercostal spaces in the anterior axillary line. The fourth trocar, which is used for retraction of thymic tissues mobilized during the course of the dissection, is usually placed further inferiorly later in the procedure. Ascertainment of the correct placement of this trocar is usually performed after the dissection has begun, when retraction of thymic tissues is necessary and is usually in approximately the seventh intercostal space in the midaxillary line. I use 5-mm sealed trocars so that carbon dioxide insufflation can be used. Advancements in videoscopic technology have allowed adequate lighting and resolution to be obtained through a 5-mm scope. The use of a 30-degree angled scope facilitates exposure during this dissection, especially in the left inferolateral portion of the dissection to identify the left phrenic nerve as it courses over the pericardium and to adequately visualize the exposure of the cervical area above the innominate vein without sword fighting with surgical instruments.
Carbon dioxide insufflation to 8 to 10 cm H20 is routinely used. Its benefits are multiple. First, insufflation causes retraction of the collapsed lung out of the operative field. Second, the positive pressure opens up the mediastinum and allows more room for both visualization and dissection. Third, the positive pressure within the mediastinum minimizes minor bleeding that may occur from dissection. Fourth, and most important, as dissection is carried into the cervical area, the tissue planes are opened up and visualization is enhanced. The routine use of both a 30-degree scope and CO2 insufflation has significantly facilitated the technical aspects of the procedure. Surgical instruments used during the procedure include a 5-mm endoscopic grasping instrument, a 5-mm hook cautery, and a fan retractor for retraction of tissue in the latter stages of dissection. The only other instruments routinely used include a 5-mm endoscopic clip applier for ligation before division of the inferior thymic vein, and an endoscopic bag in which the completely dissected specimen is placed prior to extraction through an anterior trocar sight. Occasionally an endoscopic Kittner is helpful for blunt dissection. A complete list of all instruments routinely used is listed in Table 178-1.
Table 178-1. Instruments for Video-Assisted Thymectomy | |
|---|---|
|
Thymic Dissection
Once ventilation has been stopped to the ipsilateral lung, a sealed 5-mm trocar is placed. The thoracic cavity safely accessed and two additional 5-mm sealed ports are placed as previously described. After general examination of the thoracic cavity, all appropriate structures and landmarks, including the thymus gland, phrenic nerve, superior vena cava, and internal mammary vessels, are identified (Fig. 178-1).
All anterior mediastinal tissue, whether clearly thymic tissue or mediastinal fat, is routinely removed. The dissection is begun inferior and posterior just anterior to the
P.2640
phrenic nerve and is carried cephalad to the junction of the superior vena cava (SVC) and innominate vein (Fig. 178-2). This dissection is performed with a hook electrocautery at 25 watts. The dissection is then carried anteriorly just behind the internal mammary vessels along the posterior portion of the sternum. This circumferential line of dissection is then carried inferiorly down to the pericardial reflection and connected with the beginning dissection line. Once the limits of the resection have been defined, dissection is commenced. I usually perform the more posterior portion of the dissection first, sweeping the tissue from caudad to cephalad. Dissection in the more posterior portion first is helpful in case any bleeding is encountered when dissection is performed in the more anterior portion. An avascular plain along the pericardium is usually encountered, and with retraction and careful dissection with the cautery device, complete dissection of all tissue off of the pericardium and superior vena cava can be achieved. When the tissue has been sufficiently mobilized to the level of the innominate vein, I then begin dissection of tissue off of the posterior sternal area. Again, an avascular plain is easily achieved and by general retraction downward and use of the hook cautery all tissue can be resected off of the retrosternal area.
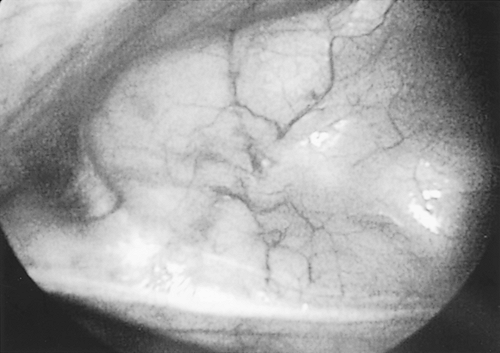 |
Fig. 178-1. View of thymus gland in anterior mediastinum from the right hemithorax. Patient's head is to the left and phrenic nerve is across the bottom. |
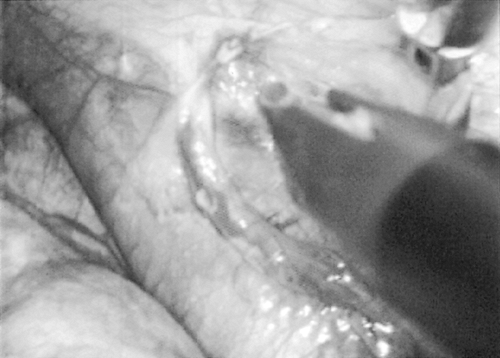 |
Fig. 178-2. Dissection has begun along the superior vena cava (SVC) toward the cephalad portion. Phrenic nerve is clearly seen on the SVC. |
Next the junction of the superior vena cava and innominate vein is addressed. Quite typically the vascular supply emanating from near the origin of the internal mammary vessels is encountered here. Ligation of these vessels with an endoscopic clip applier followed by division opens up the dissection planes along the innominate vein. At this time, it is usually helpful to place the fourth port for placement of the fan retractor for additional exposure. As the thymic tissue is retracted to the left and downward, exposure along the innominate vein is usually quite adequate (Fig. 178-3). Identification of the thymic vein is readily possible by careful blunt dissection along the innominate vein. Again, double ligation of this vessel with a 5-mm endoclip followed by division significantly opens up the area posterior to the innominate vein for dissection of tissue. If there is significant thymic tissue within the mediastinum, it is usually readily apparent at this time. Devascularization of the thymus gland causes a deeper yellow coloration to the tissue so that discernment from adjacent mediastinal fat is more readily possible than at the initial stages of the procedure.
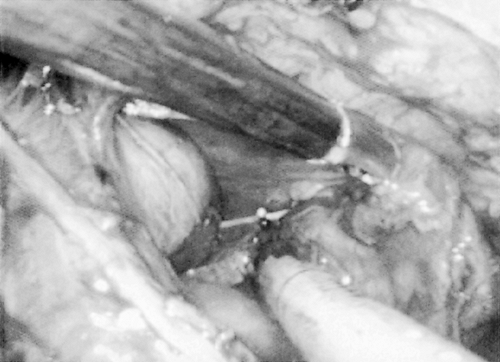 |
Fig. 178-3. Thymic tissue is swept to the right away from the innominate vein in the center. Phrenic nerve is seen on the superior vena cava in the lower left corner. |
I have changed the technique to a minor degree in that I usually perform the cervical dissection next. By proper angulation of the scope, good visualization of the superior horns of the thymic gland coursing into the neck is possible. Gentle downward traction by the endoscopic forceps with careful sharp dissection with the hook electrocautery allows the ligamentous attachments at the superior poles to be exposed and divided. First the right-sided pole is mobilized and dissected free, followed by the left-sided pole.
Routinely the most technical challenging portion of the procedure is the dissection along the left pleural envelope. It is helpful, if possible, but not mandatory, to maintain the left pleura intact. Although no special management is necessary in case the pleura is inadvertently entered, dissection is facilitated if the pleura remains intact. The dissection is usually carried from the cephalad portion of the left pleural envelope toward the inferior aspect along the pericardial phrenic groove. Although the left phrenic nerve can be identified the majority of the time, this is not always the case. Therefore, I frequently perform this portion of the procedure with blunt dissection with either a retractor or endoscopic Kittner, sweeping all tissue off the left pleural envelope. By using this technique, I have been able to completely remove all mediastinal tissue and have encountered no phrenic nerve injuries. The last portion of the dissection is along the left pericardial phrenic space. Again, with the judicious use of retraction with a fan retractor upward and to the right, exposure of the tissue extending between the
P.2641
phrenic nerve and the pericardial sack can be completely removed.
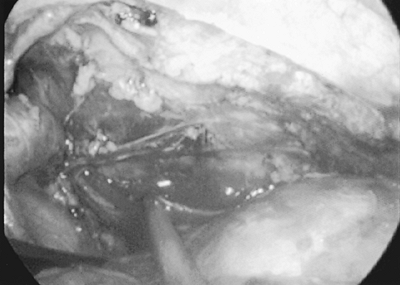 |
Fig. 178-4. View of the anterior mediastinum after all tissue has been removed. Innominate vein is at left, pericardium to the bottom right, and left pleura is in the background. |
Once all anterior mediastinal tissue has been mobilized, the tissue is placed into an endoscopic bag that has been placed through an anterior trocar site. Occasionally, slight enlargement of the 10-mm trocar site through which the fan retractor was placed is necessary to remove large specimens. The anterior mediastinal space is then reinspected for both any residual tissue that may remain and for hemostasis (Fig. 178-4). Once removed from the thoracic cavity, the specimen is examined for completeness of dissection (Fig. 178-5). Once the procedure is completed, we evacuate the air from the chest cavity by placement of a rubber catheter through an anterior trocar site and expansion of the lung under water seal. Unless inadvertent lung injury has occurred, I do not routinely use a chest tube.
Postoperative Management
The patient is extubated immediately in the operating room and then taken to the recovery room. A chest radiograph is obtained in the recovery room to confirm full expansion of both lungs. Care in an intensive care unit has not been necessary, and patients are routinely returned to a standard ward room. Preoperative medications are resumed at the same doses. Oral narcotics are used for pain relief, and respiratory therapy with incentive spirometry is administered. Discharge is routinely the morning following the operative procedure. Preoperative medications are maintained at the same dosage for the first 2 to 3 months after surgery, whereupon, depending on the patient's clinical status, the patient is then weaned of his or her medications.
RESULTS
Savcenko and associates (2002) of our group have recently updated our 10-year experience with VATS thymectomy. A total of 47 procedures have been performed, of which 36 patients were able to be contacted and followed up with clear delineation of their clinical status. In the total series, there has been no mortality and no significant morbidity. In one patient (2%), conversion to a limited lateral thoracotomy was necessary in order to contain control of bleeding from a thymic vein. With limited thoracotomy
P.2642
conversion, containment of bleeding was adequate and no transfusion was necessary. The mean length of stay in all patients was 1.64 days, with a median length of stay of 1 day and a range of 0 to 8 days. Recently in selected patients, our group performed the procedure on an outpatient basis. The mean length of follow-up is now 53 months, with the longest patient being followed for 126 months.
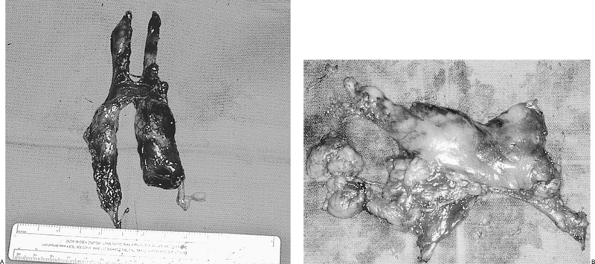 |
Fig. 178-5. Two thymus specimens after removal. A. Specimens from a younger patient with a well-defined gland. B. Specimen is from an older patient with extensive mediastinal fatty tissue. |
Table 178-2. Preoperative MGFA* Clinical Classification | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Our group has recently reclassified all our patients according to the new Myasthenia Gravis Foundation of America (MGFA) clinical classification as a part of the report by Savcenko and colleagues (2002), as presented in Table 178-2. All of the postoperative patients also have been reclassified on the basis of the new MGFA postintervention status results classification (Table 178-3) and further defined the MGFA postintervention status in relation to the preoperative MGFA clinical class (Table 178-4).
OTHER VIDEO-ASSISTED EXPERIENCE
Yim and the group in Hong Kong have published their experience in three reports: one as part of a multicenter experience with me and my colleagues (1996) and as single center reports in 1995 and 1999. They initially described the right-sided approach, and their experience is substantially the same as our own. Mineo and colleagues (2000) have championed a left-sided approach. They reported their results in 31 patients. There were no deaths or major complications, and the mean hospital stay was 5.2 days. At a mean follow-up of 39 months and 100% complete, the remission and improvement rates were 36% and 96%, respectively.
Table 178-3. MGFA* Postintervention Status Results | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Mineo's group has published two other reports on video-assisted thymectomy. The first in 1996 described the technique and advantages of adjuvant pneumomediastinum. The other report (1998) described their results with completion thymectomy in patients with refractory myasthenia who had previously undergone a thymectomy by another approach. In eight patients who had undergone a transcervical (n = 6) or transsternal (n = 2) thymectomy, the patients underwent reoperation by left thoracoscopic approach at a mean of 129 months. Gross or microscopic tissue was found in all patients, and the procedure was performed without mortality, but with two patients developing myasthenic crisis. Six of the eight patients achieved symptomatic improvement. This was further updated by Pompeo and co-workers in 2000.
Two other reports described further technique modifications. Ng and collaborators (1998) described advantages in their experience with a lateral rather than supine patient positioning. Novellino and colleagues in 1994 described a technique for bilateral thoracoscopic approaches combined with transcervical open approach to complete dissection of the upper poles.
Table 178-4. MGFA* Postintervention Status in Relationship to Preoperative MGFA Clinical Class | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
P.2643
Ruckert and co-workers (2000) performed a prospective trial of 20 patients who were randomly allocated to a thoracoscopic thymectomy or median sternotomy. Immediate postoperative lung function was reduced by 35% after thoracoscopic thymectomy and 65% after median sternotomy. By the third postoperative day, recovery of pulmonary function was complete after thoracoscopic thymectomy, but was still only 55% of baseline after median sternotomy. Their conclusions were that less pronounced impairment and faster recovery of pulmonary function after thoracoscopic thymectomy clearly defined this as minimally invasive.
OTHER LESS INVASIVE APPROACHES
Other minimally invasive approaches that have been described include an infrasternal mediastinoscopic approach by Uchiyama and colleagues (2001). They recently reported their experience in 23 patients, in which the approach was successful in 21 patients. There were no deaths and only one phrenic nerve complication.
Takeo and co-workers (2001) recently published a case report of using a sternal elevating method to increase the visualization between the sternum and the heart to facilitate videoscopic approaches.
Thymectomy by a partial sternotomy has been described by Pego-Fernandes and associates (2002). They reported their experience in 478 patients over a 26-year period. The complete remission rate was 12.7%, with a significant improvement rate of 62.5% and mild improvement rate of 17.4%. There was no improvement in 7.4% of patients.
A mini-sternotomy approach by using a reversed-T upper sternotomy was described by Grandjean and colleagues (2000). This experience was limited to a case report.
Lastly, a case report of a single patient undergoing thoracoscopic thymectomy using robotic assistance was described by Ashton and co-workers (2003). The procedure was performed using the DaVinci surgical system through a bilateral thoracoscopic approach. Their conclusion was that the procedure could be performed safely and effectively.
CONCLUSIONS
Despite 10 years of experience with video-assisted approaches for performance of thymectomy and myasthenia gravis, experience is still relatively limited, both by the technical challenges performed by the procedure as well as by the lack of compelling clinical data to justify routine widespread clinical adoption. Voiced concerns regarding the ability to perform a complete and total thymectomy by this approach have been frequent. Comparison of the adequacy of surgical resection by the various techniques has been confounded by the relatively small size of most surgical series, the variability in the preoperative classification between different series, the undulating nature of the disease, the relatively short follow-up period in most series, and the lack of uniformity of clinical classification of both preoperative status and postintervention follow-up. Hopefully, implementation and universal adaptation of the recently published MGFA classification will allow meaningful comparison between various surgical techniques.
Table 178-5. Video-Assisted Thymectomy | |
|---|---|
|
Benefits to video-assisted and all minimally invasive approaches include performance of a less invasive surgical procedure as manifested by less pain, less pulmonary dysfunction, and less exacerbation of myasthenia compared with more invasive standard approaches (Table 178-5). The additional benefit of better cosmesis is a significant, but not critical, bonus. Mandatory to all approaches is that completeness of thymectomy is tantamount to the best clinical response. Unfortunately, experience with all minimally invasive approaches is still limited and there are enough variables impacting analysis of outcomes that it cannot be definitively stated that the outcomes are comparable with open approaches. As minimally invasive and video-assisted techniques continue to become more widely used and surgeon experience with more complex procedures is gained, broader experience from more centers can be expected. Reporting of results by all techniques using a standardized classification (MGFA) should allow a more meaningful comparison assessment among the different approaches.
REFERENCES
Ashton RC Jr, et al: Totally endoscopic robotic thymectomy for myasthenia gravis. Ann Thorac Surg 75:569, 2003.
Cooper JD, et al: An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 45:242, 1988.
Grandjean JG, Lucchi M, Mariani MA: Reversed-T upper mini-sternotomy for extended thymectomy in myasthenic patients. Ann Thorac Surg 70:1423, 2000.
Mack MJ, Scruggs G: Video assisted thoracic surgery for myasthenia gravis. Chest Surg Clin North Am 8:809, 1998.
Mack MJ, et al: Results of video-assisted thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg 112:1352, 1996.
Mineo TC, et al: Adjuvant pneumomediastinum in thoracoscopic thymectomy for myasthenia gravis. Ann Thorac Surg 62:1210, 1996.
P.2644
Mineo TC, et al: Video-assisted completion thymectomy in refractory myasthenia gravis. J Thorac Cardiovasc Surg 115:252, 1998.
Mineo TC, et al: Thoracoscopic thymectomy in autoimmune myasthenia: results of left-sided approach. Ann Thorac Surg 69:1537, 2000.
Ng JW, et al: Video-assisted thymectomy in patients with myasthenia gravis: lateral versus supine position [Letter]. Thorac Cardiovasc Surg 115:265, 1998.
Novellino L, et al: Extended thymectomy, without sternotomy, performed by cervicotomy and thoracoscopic technique for the treatment of myasthenia gravis. Int Surg 79:378, 1994.
Pego-Fernandes PM, et al: Thymectomy by partial sternotomy for the treatment of myasthenia gravis. Ann Thorac Surg 74:204, 2002.
Pompeo E, et al: Thoracoscopic completion thymectomy in refractory nonthymomatous myasthenia. Ann Thorac Surg 70:918, 2000.
Ruckert JC, Walter M, Muller JM: Pulmonary function after thoracoscopic thymectomy versus median sternotomy for myasthenia gravis. Ann Thorac Surg 70:1656, 2000.
Savcenko M, et al: Video-assisted thymectomy for myasthenia gravis: an update of a single institution experience. Eur J Cardiothorac Surg 22:978, 2002.
Takeo S, Sakada T, Yano T: Video-assisted extended thymectomy in patients with thymoma by lifting the sternum. Ann Thorac Surg 71:1721, 2001.
Uchiyama A, et al: Infrasternal mediastinoscopic thymectomy in myasthenia gravis: surgical results in 23 patients. Ann Thorac Surg 72:1902, 2001.
Yim AP, et al: Video-assisted thoracoscopic thymectomy for myasthenia gravis. Chest 108:1440, 1995.
Yim APC, et al: Video-assisted thoracoscopic thymectomy for myasthenia gravis. Semin Thorac Cardiovasc Surg 11:65, 1999.
EAN: 2147483647
Pages: 203