126 - Esophageal pH Studies in Esophageal Disease
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Esophagus > Section XXIII - Benign Esophageal Disease > Chapter 147 - Barrett's Esophagus
function show_scrollbar() {}
Chapter 147
Barrett's Esophagus
Thomas G. Paulson
Brian J. Reid
DEFINITION
Any review of Barrett's esophagus is complicated by the fact that the definition of the condition has changed multiple times over the past 30 years. Barrett's esophagus had been defined as an arbitrary length, usually 2 to 3 cm, of columnar epithelium lining in the esophagus without regard to histology, but this definition did not take into account that three types of columnar mucosa (fundic gland, cardiac gland and intestinal metaplasia) could be detected in the distal esophagus, as reported by Paull and co-authors (1976). The definition then changed to either a columnar lining of an arbitrary length, usually 3 cm, or intestinal metaplasia of any length. Studies in the late 1990s showed that the majority of cases with esophageal intestinal metaplasia had segment lengths of 3 cm or less, and the definition was changed again.
The present definition requires both endoscopic recognition of columnar lining and biopsy-established intestinal metaplasia in the esophagus. Sampliner and associates (1988) defined short-segment Barrett's esophagus as those cases with 3 cm or less of columnar lining and intestinal metaplasia, whereas long-segment Barrett's esophagus includes those with more than 3 cm of columnar lining and intestinal metaplasia. Since fundic gland and cardiac gland mucosa are not associated with an increased risk of esophageal adenocarcinoma, histologic verification of intestinal metaplasia is required for a diagnosis of Barrett's esophagus.
EPIDEMIOLOGY
Barrett's esophagus is the only known precursor to esophageal adenocarcinoma. Columnar epithelium in the distal esophagus was first described by the British surgeon Norman Barrett (1950), who attributed the condition to a congenital short esophagus. Three years later, Allison and Johnstone (1953) correctly concluded that the columnar lining was acquired as a complication of chronic gastroesophageal reflux disease (GERD). Barrett (1957), reviewing the evidence in an eloquent manuscript, concurred.
Esophageal adenocarcinoma arising in ectopic gastric mucosa was reported by Morson and Belcher in 1952. Naef and colleagues (1975) published the first large series linking GERD to Barrett's esophagus and esophageal adenocarcinoma. Prior to 1975, adenocarcinomas constituted less than 4% of esophageal cancers, as reported in studies by Turnbull and Goodner (1968) and Raphael (1966), Bosch (1979) and Cederqvist (1980) and their colleagues; however, as reported by Brown and Devesa (2002), by 1996 the incidence of esophageal adenocarcinomas among white men exceeded that of squamous cell carcinoma. Bollshweiller and coauthors (2001) found that the prevalence of esophageal adenocarcinoma varies from country to country, yet as indicated by a large numbers of studies, including those by Moller (1992), Tuyns (1992), Powell and Allum (1992) and Liabeuf and Faivre (1997), as well as by Hansson (1993), McKinney (1995), Thomas (1996), Hansen (1997) and Levi (1998) and their associates, its incidence is rising dramatically among white men in almost all Western, industrialized nations, with the highest rates in Scotland. Rates have also increased in the United Kingdom, Scandinavia, France, Switzerland, and New Zealand.
In 1991, Blot and colleagues reported that esophageal adenocarcinoma had the most rapidly increasing incidence of any cancer in the United States. The rising incidence was first detected in white men, who remain the most affected group. Evaluating Surveillance, Epidemiology, and End Results (SEER) data from 1974 to 1994, Devesa and collaborators (1998) found that the incidence of esophageal adenocarcinoma rose more than 350% in white men. During the same period, rates of squamous cell carcinoma dropped by 35% in the same population. Increases in incidence of esophageal adenocarcinoma were greater in older (>65) patients than in younger ones. Similar rates of increase in the United States are seen for women and African Americans, although as reported by Brown and Devesa (2002), the absolute incidence in these groups is much lower than that of
P.2218
white men. However, even within countries there are large regional variations in the incidence of esophageal adenocarcinoma. For example, Kubo and Corley (2002) found that since 1974 the incidence in white men increased 800% in Seattle, Washington, compared with 300% in Utah.
Relatively little information exists concerning time trends for the incidence of gastroesophageal reflux disease and Barrett's esophagus during the period in which esophageal adenocarcinoma increased so dramatically. A population-based study of GERD by Locke and co-workers (1997) reported that approximately 40% of the adult population has symptomatic reflux and 18% have weekly reflux, results similar to a hospital-based study by Nebel and coauthors (1976) 20 years earlier. However, recent analyses of discharge diagnoses from a U.S. Veterans Administration database by Brown and Devesa (2002) showed that the age-adjusted incidence rate of reflux disease per 1,000 person-years increased 275% and 250% in white and African American men, respectively, over the past three decades.
Defining the incidence of Barrett's esophagus during this period is confounded by increased use of endoscopy, changes in the definition of Barrett's esophagus, and by the fact that a significant fraction of patients with Barrett's esophagus experience no symptoms that would cause them to seek medical attention. Three studies have attempted to estimate the incidence of Barrett's esophagus per 1,000 endoscopies. One study from the United States by Conio and associates (2001) found that the incidence of Barrett's esophagus increased 28-fold from 1965 to 1997, while gastroscopic examinations increased 22-fold over the same time period. This study concluded that most of the increased incidence may have been due to detection. A British study by Caygill and colleagues (1999) reported an 8-fold increase in the number of Barrett's cases per 1,000 endoscopies from 1977 to 1996 and concluded that either the incidence of Barrett's esophagus or its detection rate was increasing. In contrast, a study from Scotland by Prach and collaborators (1997) reported a remarkable 50-fold increase in Barrett's esophagus during the latter half of the 1980s, but this may have been due to increased awareness and detection.
Few studies have examined the prevalence of Barrett's esophagus in different ethnicities. Spechler and co-workers (2002) found that African Americans and Asians appear to have a significantly lower prevalence of Barrett's, whereas the rates for Hispanics were found by Bersentes and coauthors (1998) to be similar to those of Caucasians. Hassall (1997) reported that Barrett's esophagus and esophageal adenocarcinoma are rare but not unknown in children.
ETIOLOGY
Gastroesophageal Reflux
As indicated by Chow (1995), Vaughan (1995) and Lagergren (1999a) and their associates, the strongest and most consistent risk factors identified for esophageal adenocarcinoma are symptomatic gastroesophageal reflux and being overweight. Lagergren (1999a) and Farrow (2000) and their colleagues reported that persons with heartburn and regurgitation experience a sevenfold increase in esophageal adenocarcinoma risk. Lagergren and collaborators (1999a) examined the association between GERD and esophageal adenocarcinoma in a large population-based study in Sweden. Those having reflux symptoms for 12 to 20 years were five times more likely to develop esophageal adenocarcinoma than those without reflux (Fig. 147-1). The frequency, severity, and duration of the reflux symptoms were associated with greater risk, with those having severe reflux for more than 20 years having a relative risk of 43.5 (95% confidence interval; 18.3 to 103.5).
The presence of heartburn, its frequency, and its duration have all been associated with Barrett's esophagus, as reported by Eloubeidi and Provenzale (2001) and by Lieberman (1997) and Gerson (2002) and their colleagues. Patients with Barrett's typically have greater 24-hour esophageal acid exposure than those having GERD without Barrett's, which has been reported by Neumann and Cooper (1994) and by Iascone (1983), Gillen (1987), Champion (1994), Singh (1994), Coenraad (1998) and Oberg (1999) and their co-workers. The magnitude of reflux, as determined by pH monitoring, was found by Fass and coauthors (2001) to correlate with increasing length of the Barrett's segment. Although chronic gastroesophageal reflux is the main risk factor for developing Barrett's esophagus, patients with Barrett's have been reported by Hirschowitz (1996) to have normal levels of acid secretion.
Orlando (1995) describes gastric refluxate as containing hydrochloric acid, pepsin, conjugated and deconjugated bile salts, and pancreatic enzymes such as trypsin and lipase. Although all can cause esophageal injury, gastric pH is the main determinate of which substances are noxious. A growing body of evidence implicates bile as having a role in the genesis of Barrett's esophagus, particularly studies by Muller-Lissner (1986), Vaezi and Richter (1996, 1997), and DeMeester (1997) and those by Iftikhar (1993), Champion (1994), Vaezi (1995), Caldwell (1995), Kauer (1995a, 1995b), Marshall (1997), Stein (1998) and Nehra (1999) and their associates. Champion (1994), Kauer (1995b), Caldwell (1995) and Stein (1998) and their colleagues all report that patients with Barrett's esophagus have higher levels of bile in their reflux than those with uncomplicated esophagitis or normal controls. In one large study, mean esophageal bile exposure increased progressively from GERD without mucosal injury (N = 19) to erosive esophagitis (N = 45) to Barrett's esophagus (N = 33), with the highest levels found in early esophageal adenocarcinoma (N = 14) (p < 0.01). These results are consistent with studies by Pera (1989, 1993), Clark (1994) and Miwa (1995) and their collaborators showing that bile reflux potentiates the development of esophageal cancer in animal models. Hopwood and co-workers (1981) found that reflux
P.2219
of bile acids at the concentrations observed in patients with Barrett's causes damage to the esophageal epithelium in the presence of acidic pH. Mixed acid and bile acid reflux was the dominant pattern of reflux in patients with severe mucosal injury.
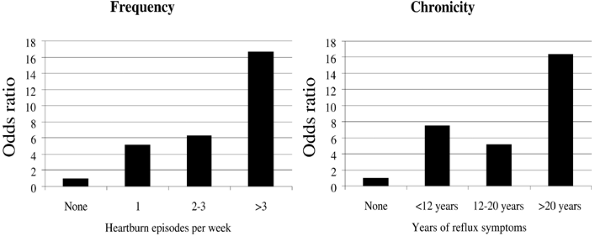 |
Fig. 147-1. Heartburn as a risk factor for esophageal adenocarcinoma. Odds ratios for developing esophageal adenocarcinoma in patients with symptoms of reflux, separated by frequency or duration of symptoms. Adapted from Lagergren J, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 340:825, 1999a. |
The junction between the esophagus and the stomach is delineated by the lower esophageal sphincter (LES), a region of smooth muscle at the distal end of the esophagus that produces a positive-pressure sphincter. The gastroesophageal flap valve, the diaphragmatic crura, the phrenicoesophageal ligament, the mucosal rosette, and the acute angle of His all contribute to anatomic barriers to reflux. Displacements of these structures, hiatal hernias, or reduction in muscle tone of the lower esophageal sphincter commonly occur in patients with GERD and Barrett's esophagus. Loughney (1998) and Fass (2001) and their coauthors report that patients with long-segment Barrett's have significantly lower LES pressure than short-segment patients, with both being significantly lower than normal controls. Further, Cameron (1999) found that hiatal hernias are typically larger in patients with long-segment Barrett's than those with short segments or those with GERD without Barrett's esophagus. Sontag and associates (1991) report that the mechanisms by which environmental exposures modify the normal anatomic barriers to reflux are poorly understood.
Obesity
Vaughan (1995), Brown (1995) and Chow (1998) and their colleagues have reported obesity as a major risk factor for esophageal adenocarcinoma, and Brown and Devesa (2002) find obesity increasing at an epidemic rate in Western societies over the same time period in which the incidence of esophageal adenocarcinoma has increased alarmingly. Studies by Vaughan (1995), Chow (1998) and Lagergren (1999b) and their collaborators have shown that persons who are overweight, as measured by increased body mass index (BMI) or waist-to-hip ratio, are at increased risk of esophageal adenocarcinoma, with a dose-dependent relation with increasing quartiles of BMI. The role of obesity in the genesis of Barrett's esophagus is unknown, but is possibly due to a combination of increased reflux due to mechanical pressure on the abdomen (particularly in men) and the effects of a diet high in fat and calories and low in fruits and vegetables, as reported by Brown (1995), Tzonou (1996), Zhang (1997) and Ward (1997) and their co-workers.
Other Risk Factors
Kabat (1993), Brown (1995), Tzonou (1996), Zhang (1997) and Mayne (2001) and their coauthors have identified cigarette smoking and a high-fat diet as other factors suspected of increasing the risk of esophageal adenocarcinoma. Studies by Gray (1992), Kabat (1993), Brown (1994), Vaughan (1995), Zhang (1996) and Gammon (1997) and their associates identified smoking as a potential risk factor for developing esophageal adenocarcinoma, although not as strong as for squamous cell carcinoma. Interestingly, smoking cessation does not appear to reduce the risk of esophageal adenocarcinoma until about 30 years
P.2220
later, suggesting smoking acts as an initiator in progression to esophageal adenocarcinoma. Smoking trends have mirrored the increases in esophageal adenocarcinoma, with a lag of about 30 years, consistent with any smoking being a risk for adenocarcinoma and continued smoking being a risk for squamous cell carcinoma, as reported by Kabat (1993), Brown (1994) and Gammon (1997) and their colleagues.
Dahms (1987) and Sartori (1991) and their collaborators have reported Barrett's developing after long-term treatment with chemotherapeutic agents; however, very few of the reported cases meet the current definition of Barrett's esophagus, and other studies by Herrera (1992) and Peters (1993) and their co-workers have failed to find an association between chemotherapy and development of Barrett's.
Aspirin and Nonsteroidal Antiinflammatory Drugs
The possible role of aspirin and nonsteroidal antiinflammatory drugs (NSAIDs) in reducing the risk of esophageal cancers has only recently received attention. Support for this line of study comes from evidence of cyclooxygenase-2 (COX-2) overexpression in Barrett's epithelium and esophageal adenocarcinoma in studies by Vainio and Morgan (1998) and by Eberhart (1994), Wilson (1998), Hwang (1998), McCarthy (1999), Fu (1999) and Zimmermann (1999) and their coauthors. Epidemiologic studies by Schreinemachers and Everson (1994) and Funkhouser and Sharp (1995), as well as by Thun (1993), Farrow (1998) and Langman (2000) and their associates, have found a protective effect of NSAIDs on the development of esophageal adenocarcinoma, with odds ratios from 0.1 to 0.6. A recent meta-analysis by Corley and colleagues (2003) of the reported studies found a combined odds ratio of 0.57.
Heredity
A heritable component predisposing to Barrett's esophagus has been postulated based on isolated cases of familial aggregation in studies by Gelfand (1983) as well as by Crabb (1985) and Jochem (1992) and their collaborators, although whether this represents a genetic predisposition to GERD or to developing Barrett's is unclear, as discussed by Cameron (1992) and Everhart and co-workers (1983). One recent case control study by Chak and coauthors (2002) has reported evidence for familial aggregation of Barrett's esophagus and esophageal adenocarcinoma, although other studies by Lagergren (2000), Dhillon (2001) and Romero (2002) and their associates have failed to identify a genetic component.
CLINICAL PRESENTATION
The distribution of Barrett's esophagus across the population is decidedly skewed, with men outnumbering women 4 to 1 in most series and whites outnumbering other ethnicities. As reported by Phillips and Wong (1991), patients typically present with symptoms of GERD, including heartburn and regurgitation. They may also present with odynophagia, dysphagia, noncardiac chest pain, hematemesis, melena, or occult gastrointestinal blood loss as well as less common extraesophageal symptoms such as hoarseness, asthma, and dental erosion, as reported by Bozymski (1985), Orlando (1995), Phillips and Wong (1991) and Sjogren and Johnson (1983). Patients with asthma have been found by Sontag and collaborators (1992) to have esophagitis or Barrett's esophagus or both more frequently than the population in general. Dysphagia in patients with Barrett's esophagus can result from stricture, a motility disorder, or esophageal adenocarcinoma.
Heartburn and regurgitation are common symptoms, occurring in approximately 40% of adult Americans, with 18% having weekly heartburn, making it impossible to predict which persons will have Barrett's esophagus on the basis of reflux alone. Winters (1987) and Hirota (1999) and their co-workers report that only 5% to 12% of patients who have symptomatic GERD will have Barrett's esophagus confirmed by endoscopy and biopsy, and Gerson and coauthors (2002) indicate that some patients with Barrett's esophagus may be asymptomatic. Increased frequency and duration of heartburn were associated with increased likelihood of Barrett's esophagus and esophageal adenocarcinoma by Lagergren (1999a) and Lieberman (1997) and their associates. However, patients with Barrett's esophagus can experience less pain with chronic reflux than those with esophagitis alone, and Eloubeidi and Provenzale (2001), as well as Johnson (1987) and Trimble (1995) and colleagues, have reported that the severity of reflux symptoms is negatively associated with Barrett's esophagus. As a consequence, patients with Barrett's esophagus may delay seeking medical attention, resulting in most patients having well-established disease at first diagnosis; Lagergren and colleagues (1999a) reported that 40% of patients who developed esophageal adenocarcinoma have no antecedent symptoms of reflux.
Dysphagia is an alarm symptom requiring expeditious evaluation for the possibility of an esophageal adenocarcinoma. Similarly, acute gastrointestinal bleeding, which may be due to an esophageal ulcer or severe erosive esophagitis, requires early endoscopy to establish the cause of the bleeding as well as to provide potential therapy if the bleeding persists. If no cause for chronic blood loss is found at colonoscopy, then the upper gastrointestinal tract should be evaluated endoscopically, including careful evaluation of the esophagus for Barrett's esophagus and esophagitis.
DIFFERENTIAL DIAGNOSIS AND DIAGNOSTIC STUDIES
The differential diagnosis of Barrett's esophagus depends on the stage of disease. One of us (BJR) and Thomas (1995)
P.2221
report that in the patient who presents with symptomatic GERD, the differential diagnosis consists of three major conditions: gastroesophageal reflux disease without Barrett's esophagus, Barrett's esophagus without cancer, and Barrett's esophagus with early esophageal adenocarcinoma.
In patients who present with dysphagia, the differential diagnosis includes benign peptic stricture with or without Barrett's esophagus, esophageal adenocarcinoma, esophageal squamous cell carcinoma, adenocarcinoma of the gastroesophageal junction, corrosive stricture, and motor disorders including achalasia and scleroderma. Patients with benign peptic strictures may have a long history of symptomatic GERD, although patients with esophageal adenocarcinoma and adenocarcinoma of the gastroesophageal junction may give similar histories.
Endoscopy and Biopsy
Endoscopic Landmarks and Biopsy Technique
Endoscopy with multiple, systematic biopsies is the standard for the diagnosis of Barrett's esophagus. Three endoscopic landmarks, which are measured relative to distance from the incisors, are required to fully characterize the patient with Barrett's esophagus: the ora serrata or squamocolumnar junction, the end of the tubular esophagus, and the diaphragmatic impression.
The ora serrata, which represents the junction between normal white squamous lining of the esophagus and salmon-pink Barrett's lining, is displaced proximally into the tubular esophagus and marks the proximal end of the Barrett's segment. The ora serrata may be jagged, with tongues of salmon-pink intestinal epithelium interdigitated with the white squamous epithelium (Fig. 147-2). Islands of pink intestinal epithelium may exist above the ora serrata; conversely, white islands of squamous epithelium may exist below it.
The salmon pink of Barrett's mucosa is indistinguishable from normal gastric mucosa. Therefore, identifying the distal end of the Barrett's segment requires assessment of the second landmark, the end of the tubular esophagus, which typically occurs approximately 1 cm proximal to flattening of the gastric folds. The end of the tubular esophagus also represents the proximal margin of any hiatal hernia. The diaphragmatic impression, which can be recognized as a concentric opening that varies with respiration, marks the distal margin of the hiatal hernia. Levine and one of us (BJR) (1991) suggest that four-quadrant biopsies should be obtained using the turn and suck method at 2-cm intervals throughout the columnar-lined esophagus from the distal end of the Barrett's segment to the ora serrata to confirm the presence of intestinal metaplasia with goblet cells. The combination of endoscopic evidence of columnar lining within the esophagus and intestinal metaplasia on biopsy is diagnostic of Barrett's esophagus.
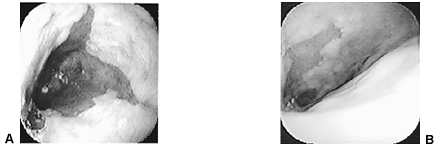 |
Fig. 147-2. Endoscopic images of Barrett's esophagus. A. Long-segment Barrett's esophagus (segment length equal to or greater than 3 cm). B. Short-segment Barrett's esophagus (segment length less than 3 cm). |
Severe erosive esophagitis may make recognition of the ora serrata difficult, as well as complicating the diagnosis of dysplasia due to active inflammation and injury of the mucosa. Treatment with a proton pump inhibitor can reduce inflammation, allowing easier identification of endoscopic landmarks, as well as more accurate classification of dysplasia.
A number of stains have been proposed for detection or evaluation of Barrett's mucosa, including toluidine blue, Lugol's iodine, indigo carmine, and methylene blue. However, Canto (1999) reports that their use has not been reproducibly shown to improve the ability to identify Barrett's epithelium or dysplasia, and they are not routinely used. These stains may be useful in identifying residual Barrett's epithelium remaining after ablation therapy.
Barrett's Segment Length
In a study by Spechler and collaborators (1994), 18% of patients undergoing upper endoscopy were found to have intestinal metaplasia in biopsies from, or proximal to, the gastroesophageal junction, most of whom did not have classic long-segment Barrett's esophagus. Cases of Barrett's esophagus can be divided into three categories: long segment (>3 cm) Barrett's esophagus, short segment ( 3 cm) Barrett's esophagus, and cardia intestinal metaplasia (in which the gastroesophageal junction appears normal endoscopically, but biopsies show intestinal metaplasia), as suggested by Hirota and co workers (1999). Endoscopic biopsies are recommended to evaluate all cases with endoscopically visible columnar mucosa in the esophagus, and surveillance is recommended for early detection of cancer in long and short segment Barrett's esophagus. However, surveillance is not recommended for cardia intestinal metaplasia, and screening biopsies are not recommended if the esophagogastric junction appears normal endoscopically.
The distinction between short and long segment Barrett's esophagus is a historical accident that is not based on clinical or biological data, and esophageal adenocarcinoma can develop in Barrett's segments of all sizes, as discussed by Schnell (1992) and Levine (1993) and their coauthors. Menke-Pluymers (1993) and Avidan (2002) and their associates
P.2222
suggest in retrospective case control studies that increasing Barrett's segment length may be associated with increased risk of cancer when segment length is viewed as a continuous variable rather than an arbitrary distinction at 3 cm. However, Rudolph and colleagues (2000) report that prospective cohort studies have not found segment length to be a significant predictor of subsequent progression to cancer, although there has been a non clinically significant trend in that direction. Although there have been anecdotal accounts of increases and decreases in Barrett's segment length over time, Cameron (1992) reports that the Barrett's segment is established relatively rapidly and does not change.
PATHOLOGY
The histopathologic definition of Barrett's esophagus has changed markedly over the past 30 years. Initially, three different types of epithelia were considered to be part of the spectrum of Barrett's esophagus. As described by Orlando (1995), Spechler and Goyal (1986), and by Paull and associates (1976), these epithelia, which were called by a variety of names, included gastric fundic gland mucosa, cardiac gland mucosa ( junctional epithelium ), and intestinal ( specialized ) metaplasia. However, gastric fundic and cardiac gland mucosa are histologically normal epithelia that can be found in the distal esophagus. Neither fundic gland nor cardiac gland mucosa has been shown to be associated with an increased risk of cancer, as reported by Haggitt (1978), Skinner (1983) and McArdle (1992) and their co-workers. In contrast, intestinal ( specialized ) metaplasia, containing goblet and other mucus-secreting cells, is abnormal and has a defined risk of progressing to cancer (Fig. 147-3).
At the present time, histopathologic classification of dysplasia is typically used for risk stratification in Barrett's esophagus, according to the scale suggested by Haggitt (1994) and by one of us (BJR) (1988) and Montgomery (2001) and respective coauthors:
Negative for dysplasia
Indefinite for dysplasia
Low-grade dysplasia
High-grade dysplasia
Cancer.
Dysplasia is defined as an unequivocal neoplastic epithelium confined within the basement membrane of the gland within which it arose. Dysplastic glands may retain their normal configuration, but more often have irregular or grossly distorted architecture. Cells with crowded, stratified, hyperchromatic nuclei line the glands. In some cases the large hyperchromatic nuclei contain large nucleoli and show a loss of nuclear polarity but lack crowding and stratification. In low-grade dysplasia (LGD), crypt architecture is preserved with minimal distortion. Nuclei may be stratified near the base of the crypts, but not near the luminal surface. Nuclei are large, crowded, and hyperchromatic, and mitotic features may be present in the upper portions of the crypt. Goblet and columnar cell mucus is usually diminished or absent. In high-grade dysplasia (HGD), crypt architecture is usually quite distorted, composed of branching and budding crypts, with intraglandular bridging of the epithelium to form a cribriform pattern of back to back glands, with a villiform configuration at the mucosal surface (Fig. 147-3B). The same nuclear abnormalities as found in LGD are seen, but stratification reaches the luminal surface, there is loss of nuclear polarity, and nuclei vary greatly in size, shape, and staining. Mucus is generally absent and abnormalities extend to the mucosal surface. Mosaic patterns of metaplasia, LGD, and HGD may be found throughout the Barrett's segment. According to Haggitt (1994), when individual malignant-appearing cells infiltrate
P.2223
the lamina propria, the diagnosis of invasive carcinoma (intramucosal carcinoma) is made (Fig. 147-3C).
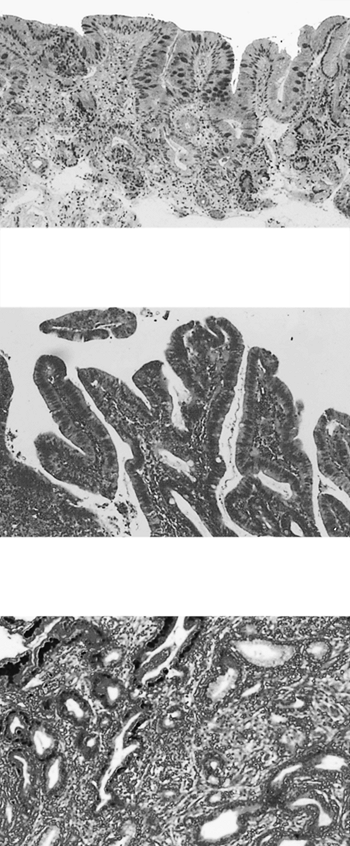 |
Fig. 147-3. Pathology of Barrett's esophagus and esophageal adenocarcinoma. A. Barrett's metaplasia without dysplasia. B. Barrett's esophagus with high-grade dysplasia. C. A well-differentiated adenocarcinoma arising in Barrett's esophagus, invading the submucosa. |
One of us (BJR) (1988) and Montgomery (2001) and respective associates have reported that the histopathologic assessment of dysplasia has several limitations, including significant observer variation in diagnosis even among expert pathologists. Alikhan and colleagues (1999) have reported that community pathologists agree with the diagnoses of specialty pathologists in only 30% to 35% of cases for all grades of dysplasia. Because of the observer variation in diagnosis, Wright (1997) and Sampliner (2002) recommend a second opinion from an experienced Barrett's pathologist for all suspected cases of high-grade dysplasia before intervention is considered.
Flow Cytometry
DNA content flow cytometry measures the amount of DNA per cell in biopsies from human tissue, allowing analysis of DNA ploidy (aneuploid and elevated 4N/tetraploid fractions) and cell cycle fractions. Flow cytometry has been evaluated in a number of cross-sectional studies in Barrett's esophagus as well as longitudinal investigations (see Biomarkers, later in this chapter) and can be used as an adjunct to histology in evaluating risk for progression to esophageal adenocarcinoma. Rabinovitch (1992) has suggested that guidelines for collection, preparation, data acquisition, and analysis of biopsies by flow cytometry should be carefully followed to prevent potential artifacts, including false aneuploid peaks and aggregation of nuclei that may produce falsely elevated 4N populations. When guidelines are followed, Bergers and collaborators (1996) have found that interlaboratory agreement with regard to detection of abnormal ploidies is very good, and one of us (BJR) (1988) and Montgomery (2001) and respective co-workers reported an interobserver agreement twice as high as the diagnosis of dysplasia. Uniformity in measurement of elevated 4N populations across different laboratories has not been assessed and will require additional cooperative validation.
Biopsies from the Barrett's segment can be processed to isolate nuclei that are then stained with a fluorescent dye, usually propidium iodide or 4 ,6 diamindino 2 phenylindole dihydrochloride (DAPI) that binds quantitatively to DNA; the stained nuclei are then interrogated by a laser, and fluorescence is measured. Rabinovitch (1992) demonstrated that a normal biopsy from Barrett's esophagus has a diploid peak (2N) that represents the cells in the G0/G1 interval of the cell cycle, a plateau between 2N and 4N that represents cells in DNA synthesis (S phase), and another smaller (normally <6% of cells) peak that represents cells in the G2/M phases of the cell cycle (4N) (Fig. 147-4A). Chromosomal errors that develop during neoplastic progression generate flow cytometric abnormalities, including
P.2224
aneuploid populations that represent clones of cells with abnormal (neither 2N nor 4N) DNA contents (see Fig. 147-4B) and tetraploid cell populations in which the 4N fraction is greater than 6% (Fig. 147-4C). Both of these abnormalities have been found by Teodori (1998), and by one of us (BJR) (2000b) and Rabinovitch (2001) and respective coauthors, to identify a subset of patients with a higher risk of progression to esophageal adenocarcinoma.
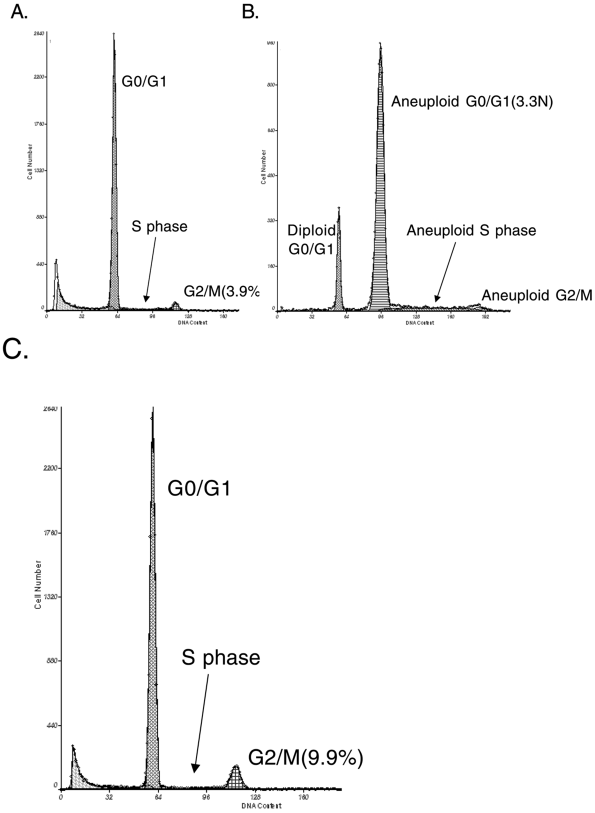 |
Fig. 147-4. Examples of flow cytometric analyses of biopsy samples from patients with Barrett's esophagus. A. DNA content flow histogram with a diploid cell population from a patient with Barrett's esophagus. B. An abnormal DNA content flow histogram with an aneuploid cell population from a patient with Barrett's esophagus. C. An abnormal DNA content flow histogram with an increased 4N cell population from a patient with Barrett's esophagus. |
Flow cytometric analysis can be subject to artifacts that arise due to suboptimal biopsy processing, such as false near-diploid aneuploids as a result of autolysis of fresh tissue left at room temperature or false-positive tetraploid populations due to aggregation (clumping) of nuclei, as reported by Alanen (1989) and one of us (BJR) (2000b) and respective associates. As suggested by Rabinovitch (1992), proper handling, storage, and processing of biopsies can prevent these artifacts, and critical examination during flow cytometric analysis can identify those samples in which artifacts have occurred, allowing reprocessing and reanalysis to be performed.
ENDOSCOPIC SCREENING FOR BARRETT'S ESOPHAGUS IN PATIENTS WITH GASTROESOPHAGEAL REFLUX DISEASE
The guidelines of the American College of Gastroenterology, reported by Sampliner (2002), state that patients with chronic GERD symptoms are those most likely to have Barrett's esophagus and should undergo upper endoscopy. Although Barrett's esophagus is the only known precursor to esophageal adenocarcinoma, Bytzer (1999) and Dulai (2002) and collaborators found that most esophageal adenocarcinomas arise in patients who were not known to have Barrett's prior to the cancer diagnosis. In a Danish study, Bytzer and co-workers (1999) found that only 1.3% of 524 patients who developed esophageal adenocarcinoma had been previously diagnosed with Barrett's. Dulai and coauthors (2002) reported that only about 5% of patients with esophageal adenocarcinoma in the United States had a prior diagnosis of Barrett's esophagus. Given the median ages of diagnosis of Barrett's esophagus (40 years) and of development
P.2225
of esophageal adenocarcinoma (64 years), Cameron (1993) estimated that 20 to 30 years are required for the development of cancer. Thus, there is ample time to detect patients at risk if sufficiently sensitive diagnostics can be employed. This provides the rationale for recommending screening of patients with GERD to detect those with Barrett's esophagus, especially considering that frequency and duration of reflux symptoms are suggested to be associated with Barrett's esophagus and esophageal adenocarcinoma, as discussed by Eloubeidi and Provenzale (2001) as well as by Lieberman (1997) and Lagergren (1999a) and their associates. Sampliner (2002) points out that the specifics of the recommendation concerning the timing of the endoscopy by patient age and duration of symptoms have not yet been defined.
However, recommendations for routine screening of GERD patients to detect those with Barrett's esophagus are controversial. The rationale against routine screening, discussed by Shaheen and collaborators (2002), is based on the observation that 90% to 95% of patients with GERD do not have Barrett's, the low absolute incidence of esophageal adenocarcinoma, and the chance that the number of endoscopic complications resulting from a national screening program might rival the benefit of early detection given the small numbers of cancers. Conio (2001) and Eckardt (2001) and their co-workers have reported that the lifespan of patients with Barrett's esophagus is comparable with that of the general population, whereas others have reported that it is shortened. However, van der Burgh (1996), Shaheen (2000), Conio (2001) and Eckardt (2001) and their coauthors indicate that more than 90% of Barrett's patients die of other causes, which has also been used to challenge a national policy of routine screening. Finally, Lagergren (1999a) and Gerson (2002) and their associates suggest there may be a substantial reservoir of asymptomatic individuals with Barrett's esophagus who are at risk for progression to esophageal adenocarcinoma but would not be detected if screening were limited to those with GERD.
Computer modeling studies by Soni (2000) and Inadomi (2003) and their colleagues have concluded that screening GERD patients for Barrett's esophagus may be cost-effective; however, these are based on assumptions that may be difficult to achieve in practice. At the present time, there is no national policy on screening. Shaheen and collaborators (2002) conclude that physicians should make the decision on a case-by-case basis, with appropriate counseling of the patient and informed consent, weighing the risks and benefits of screening and surveillance if Barrett's esophagus is found versus empiric therapy for GERD.
Endoscopic Biopsy Surveillance of Barrett's Esophagus
Sampliner and co-workers (2002) recommend endoscopic biopsy surveillance for patients with Barrett's esophagus. Although the goal of surveillance is to detect cancer early while there is still a favorable prognosis, only a few studies have addressed the effectiveness of surveillance in improving survival for patients with esophageal adenocarcinoma. The bulk of the available evidence supports surveillance. Small studies, such as those by Collard (2002) and by Peters (1994), Wright (1996), Streitz (1998), and van Sandick (1998) and their coauthors, consistently report improved survival for those patients enrolled in surveillance who do progress to esophageal adenocarcinoma relative to patients who are not in surveillance. Combining those studies that used similar criteria for tumor staging indicates that those patients who develop esophageal adenocarcinoma while in a surveillance program have significantly earlier-stage tumors than those who develop cancer outside of surveillance (Fig. 147-5). Support for surveillance was recently increased by a population-based study in California by Corley and associates (2002) that reported markedly improved survival for Barrett's patients who were enrolled in surveillance and subsequently progressed to cancer compared with Barrett's patients whose cancers were detected by symptoms rather than surveillance. In this study, patients whose cancers were detected by surveillance had a 5-year survival of 73.3%, compared with 0% for cancers detected as a result of symptoms. Farrow and Vaughan (1996) report that available data indicate that the survival advantage for patients whose tumors are detected in situpersists for up to 15 years after diagnosis (Table 147-1).
Although surveillance is recommended for patients with Barrett's esophagus, there are variations in the actual practices of endoscopists and pathologists. Falk and colleagues (2000) investigated the endoscopic surveillance practice patterns in the United States and found considerable variation in surveillance intervals, biopsy technique (random vs. four-quadrant), and recommendations for esophagectomy. In another study, Ofman and collaborators (2001) also found substantial room for improvement in the quality of care during surveillance of Barrett's esophagus in the community. Compared with standards established at specialty centers and accepted in the literature, community practice had only about 60% to 70% adherence to standards for identification of endoscopic landmarks, interpretations of pathology, and endoscopist pathologist communication, as well as only 40% adherence to recommended biopsy methods. Although these studies did not evaluate the outcome of surveillance, they are of concern, especially with regard to a recent report from Macdonald and co-workers (2000) of the complete failure of endoscopic surveillance to detect curable cancers in a large surveillance cohort in England. In this study, which did not use the recommended four-quadrant, 2-cm biopsy protocol described in the next section, only one of five cancers (20%) was detected as a result of surveillance.
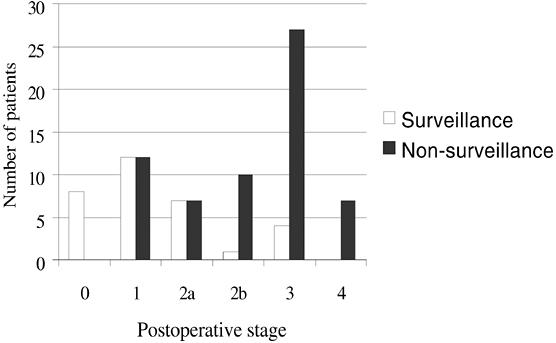 |
Fig. 147-5. Tumor stage at diagnosis in surveyed and nonsurveyed patients. Distribution of tumor stage at postoperative diagnosis in patients enrolled in surveillance programs versus those not enrolled in surveillance who present with esophageal adenocarcinoma. Adapted from Corley DA, et al: Surveillance and survival in Barrett's adenocarcinomas: a population-based study. Gastroenterology 122:633, 2002; Streitz JM Jr, Andrews CW Jr, Ellis FH Jr: Endoscopic surveillance of Barrett's esophagus. Does it help? J Thorac Cardiovasc Surg 105:383, 1993; and van Sandick JW, et al: Impact of endoscopic biopsy surveillance of Barrett's oesophagus on pathological stage and clinical outcome of Barrett's carcinoma. Gut 43:216, 1998. |
P.2226
Surveillance Technique
Levine and one of us (BJR) (1991) recommend that patients who have a columnar-lined esophagus should have biopsies taken in four quadrants every 2 cm as well as targeted biopsies of any visible endoscopic lesions using a therapeutic endoscope, jumbo biopsy forceps, and the turn and suck biopsy technique. The American College of Gastroenterology, as reported by Sampliner (2002), recommends surveillance intervals based on dysplasia grade. If the biopsies show intestinal metaplasia without high-grade dysplasia, then the patient should be entered into periodic surveillance with endoscopic biopsies taken in four quadrants at 2-cm intervals throughout the columnar lining. The present recommendations of the American College of Gastroenterology for patients without dysplasia on two consecutive endoscopies are to lengthen surveillance to 3-year intervals. Patients who have low-grade dysplasia are recommended
P.2227
to have a repeat endoscopy and then be entered into annual surveillance until no dysplasia is detected.
Table 147-1. Early Detection of Esophageal Adenocarcinoma: Survival of Surveyed and Nonsurveyed Cases | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
If the initial biopsies show high-grade dysplasia, then the diagnosis should be confirmed by a second opinion from an experienced Barrett's pathologist. An early, repeat endoscopy, generally within a month, should be performed with intensive biopsies in four quadrants at 1-cm intervals searching for a possible coexisting cancer. Suspected high-grade dysplasia should be confirmed by a second opinion from an experienced Barrett's pathologist. Heitmiller and coauthors (1996) recommend confirmation of the diagnoses of high-grade dysplasia by two experienced Barrett's pathologists before considering intervention. Buttar and associates (2001a) suggest that if there is an endoscopic lesion such as a nodule or ulcer, endoscopic ultrasound-guided endoscopic mucosal resection may help stage the lesion and guide therapy. If the evaluation shows only high-grade dysplasia, then three courses of action are possible: careful surveillance, with intervention reserved for cancer; endoscopic ablation therapy; and esophagectomy. These are discussed in Treatment.
Consensus regarding diagnosis was developed based on studies conducted at specialty centers using experienced gastrointestinal pathologists, but there are limitations to the degree in which the results can be extrapolated to the general clinical setting. There is marked observer variation in the diagnosis of dysplasia even among expert pathologists. The best agreement is achieved by comparing the combined diagnoses of negative, indefinite, and low-grade dysplasia with the combined diagnoses of high-grade dysplasia and esophageal cancer. However, even with this division into two categories, agreement between different pathologists (interobserver agreement) is lower than the agreement achieved by the same experienced pathologist on two occasions (intraobserver agreement). Community pathologists agree with specialty pathologists in only about 30% to 35% of cases, which is an obvious problem in extrapolating surveillance intervals from specialty studies to the general clinical setting. It is recommended that an experienced Barrett's pathologist review all cases with suspected high-grade dysplasia prior to intervention.
Only high-grade dysplasia appears to confer a significantly increased risk of progression. For example, one of us (BJR) and colleagues (2000b) found that there was no significant difference in rates of progression of negative, indefinite, and low-grade dysplasia to cancer with up to 14 years of prospective follow-up.
Both low- and high-grade dysplasia appear to regress in some individuals. For example, Weston (2001), Schnell (2001) and Sharma (2002) and their collaborators all reported that only about 3% of patients with low-grade dysplasia progress to cancer, whereas approximately 65% regress to no dysplasia.
All of these observations indicate the need for more objective, reproducible, and predictive markers to assess the risk of progressing to esophageal adenocarcinoma. A number of different genetic and cytometric markers, including molecular assessment of p53 status and flow cytometry, are presently being assessed in prospective studies as predictors of the risk of progressing from Barrett's to cancer. Recently, one of us (BJR) and co-workers (2003) proposed alternative surveillance strategies for centers that have access to reliable and reproducible flow cytometry, as subsequently described.
RADIOLOGY
Although endoscopic biopsy evaluation is the standard for evaluating Barrett's esophagus, some patients may have suggestive findings detected on a barium esophagogram ordered for other reasons. Agha (1986) noted that radiologic findings, although suggestive of potential Barrett's esophagus, are not diagnostic by themselves. A finding of mucosal irregularity, thickening, or nodularity during barium contrast esophagogram may indicate either Barrett's esophagus or reflux esophagitis. Robbins and Vincent (1980), Atkinson and Robertson (1988), and M. S. Levine (1983) and Vincent (1984) and their associates believe that the presence of stricture may suggest Barrett's esophagus since a peptic stricture can be found in patients with Barrett's, typically at the ora serrata. Finally, Som and Wolf (1956), Robbins and Vincent (1980), and Vincent and colleagues (1984) report that some patients with Barrett's present with normal esophagograms. Regardless of radiologic findings, diagnosis of Barrett's esophagus requires detection of intestinal metaplasia in biopsies from endoscopically visible columnar mucosa in the esophagus.
CLINICAL COURSE AND COMPLICATIONS
Van der Burgh (1996), Eckardt (2001) and Conio (2001) and their collaborators report that most patients with Barrett's esophagus live out their lives without complications from their condition. Nevertheless, patients with Barrett's esophagus are at risk for benign and malignant complications of chronic gastroesophageal reflux disease.
Benign Complications
Patients with Barrett's esophagus may develop a number of acute or chronic benign complications of chronic gastroesophageal reflux, including stricture, gastrointestinal bleeding, aspiration, and asthma. Barrett's esophagus has been reported by Spechler (1992) in up to 40% of patients with peptic strictures and in 10% to 15% of patients with erosive esophagitis. Hassall (1993) and Orlando (1995) both found that benign acid peptic strictures tend to present at the ora serrata, accompanied by inflammation and ulceration in the surrounding tissue. Benign strictures are usually associated with solid dysphagia and may be associated with
P.2228
a decrease in heartburn symptoms, since the stricture blocks passage of food to the stomach as well as reflux back into the esophagus.
Esophageal ulcers can occur either within the Barrett's segment or in squamous esophagitis proximally. Their symptoms, including pain and odynophagia, may be difficult to differentiate from those of chronic reflux, and they may be discovered incidentally during endoscopy. Orlando (1995) reports that acute gastrointestinal bleeding in patients with Barrett's esophagus is rare and typically occurs in the presence of an esophageal ulcer. However, Barrett's ulcers, erosive esophagitis, or both may be associated with chronic gastrointestinal blood loss manifested by heme-occult positivity or iron-deficiency anemia. Therefore, it is important to consider a possible upper gastrointestinal source for chronic gastrointestinal bleeding in patients in whom colonoscopy is negative, especially if they have upper gastrointestinal symptoms. In such cases, upper endoscopy should be considered, with careful evaluation for possible Barrett's esophagus.
Esophageal Adenocarcinoma
The most feared complication of Barrett's esophagus is esophageal adenocarcinoma. Haggitt (1994), as well as Cameron (1985) and Hameeteman (1989) and their co-workers, found that patients with Barrett's have a 30- to 50-fold increased risk for developing esophageal adenocarcinoma relative to the general population. The exact magnitude of the risk to the individual patient with Barrett's esophagus has not been well established, and Shaheen and coauthors (2000) suggest that the risk may have been magnified by selective publication of small studies with extreme results. Recent studies from the United States by Drewitz (1997), O'Connor (1999), Conio (2001) and Spechler (2001) and their associates have estimated the annual rate of progression from Barrett's esophagus to esophageal adenocarcinoma to be on the order of 0.035% to 0.07%. However, Kubo and Corley (2002) found that the incidence of esophageal adenocarcinoma shows marked regional variation within and outside the United States, and it is unclear whether or not this represents different rates of progression from Barrett's to cancer versus differences in other stages of progression. For example, the United Kingdom has an increased incidence of esophageal adenocarcinoma relative to the United States as reported by Bollschweiler and colleagues (2001), but Jankowski and collaborators (2002) observed that it also has an estimated annual rate of progression from Barrett's esophagus to esophageal adenocarcinoma that appears to be twice as high as that reported in the United States.
Natural History of High-Grade Dysplasia in Barrett's Esophagus
Among dysplasia grades, high-grade dysplasia has the greatest risk of esophageal adenocarcinoma. Wright (1997) and Collard (2002) have estimated the prevalence of coexisting cancer in patients referred to surgery for an endoscopic biopsy diagnosis of high-grade dysplasia to be about 40%. Wright (1997) indicates that many of these studies have been criticized because they did not use rigorous biopsy protocols and because they sometimes included patients with obvious signs of cancer at endoscopy. However, coexisting cancers remain a problem even when systematic biopsy protocols are used. Studies by Cameron and Carpenter (1997) and by Peters (1994) and Falk (1999) and their co-workers using four-quadrant, 2-cm biopsies have reported 11% to 55% unsuspected coexisting cancers in esophagectomy specimens.
Three prospective studies have estimated the risk of high-grade dysplasia progressing to cancer during follow-up. In one study from a tertiary center by one of us (BJR) and coauthors (2000b), the 5-year cumulative incidence of cancer was 59%, whereas a second study from a Veterans Administration (VA) hospital by Schnell and associates (2001) reported only a 9% 5-year cumulative incidence of cancer. The reasons for the differences in the two studies are probably multifactorial, including different pathologists, exclusion of four cancers in the first year as coexisting in the VA study, and a large referral population in the tertiary care center that may have selective referral of high-risk cases. One other prospective cohort study of 15 patients with unifocal high-grade dysplasia by Weston and colleagues (2000) reported that 27% progressed to cancer. Recent data from a randomized trial of photodynamic therapy by Overholt and collaborators (2003), available in abstract form only at the time of writing this chapter, reported that 28% of patients with high-grade dysplasia randomized to proton pump inhibitor therapy progressed to cancer in 2 years. Buttar and co-workers (2001b) have reported retrospective longitudinal data in which focal high-grade dysplasia, defined by five or fewer crypts with high grade, had only a 14% 3-year cumulative incidence of cancer, whereas more diffuse high-grade dysplasia had a 56% 3-year cumulative incidence of cancer. Although these data suggest that localized high-grade dysplasia may have a lower risk of progression, the patients underwent multiple interventions designed to prevent cancer, including endoscopic mucosal resection, photodynamic therapy, and, in some cases, esophagectomy, that may have compromised interpretation of the results.
Weston and coauthors (2000) reported that 47% of patients with unifocal high-grade dysplasia regressed to less than high grade without progression to cancer or redeveloping high-grade dysplasia for as long as 84 months. Regression of high-grade dysplasia was also observed in 38.6% of patients randomized to omeprazole 20 mg twice daily in the photodynamic therapy trial by Overholt and associates (2003) that was previously noted. Because of the possibility of regression, it is now recommended that patients with suspected high-grade dysplasia have an early repeat endoscopy with review of biopsies by an experienced
P.2229
Barrett's pathologist before intervention is undertaken.
Natural History of Negative, Indefinite, and Low-Grade Dysplasia in Barrett's Esophagus
Although most guidelines recommend more frequent surveillance intervals for patients with low-grade dysplasia, there is relatively little evidence to support this practice. In studies by Weston (2000), Schnell (2001) and Sharma (2002) and their colleagues, the incidence of cancer in patients with low-grade dysplasia is only 2% to 3%, whereas the chance of regression to no dysplasia is approximately 65%. In a prospective study reported by one of us (BJR) and collaborators (2000b) with up to 14 years of follow-up, there were no significant differences among negative, indefinite, and low-grade dysplasia with regard to progression to cancer. One computer modeling study by Ofman and co-workers (2000) reported that transient diagnoses of dysplasia were a major factor escalating the cost of care for patients with Barrett's esophagus.
Biomarkers
Because of the problems with dysplasia, many investigators have evaluated biomarkers for possible use in risk stratification of Barrett's esophagus. This literature is confusing for clinicians, largely because many molecular biologists have overstated the clinical importance of discovery research. For example, more than 60 biomarkers have been proposed for Barrett's esophagus, but most of these biomarkers have not advanced beyond the discovery phase. At the present time, one of us (BJR) and coauthors (2003) report that only p16 status, p53 status, and ploidy of Barrett's biopsies have been evaluated in large-scale prospective studies, and flow cytometric assessment of ploidy is nearest to routine clinical application.
Natural History of DNA Content Flow Cytometric Abnormalities (Tetraploidy, Aneuploidy)
Two prospective studies by Teodori (1998) and Rabinovitch (2001) and their co-workers have shown that flow cytometry, when properly performed, detects a subset of patients who are at intermediate risk of progression to cancer, with lower risk than patients with high-grade dysplasia but increased risk relative to the average Barrett's patient. Patients with flow cytometric abnormalities may benefit from annual surveillance, as is practiced in some centers, but reports by one of us (BJR) and associates (2000b, 2003) suggest that the presence of flow cytometric abnormalities is not an indication for esophagectomy or ablation because most of these patients follow a benign course.
THERAPY
Barrett's Esophagus without High-Grade Dysplasia or Cancer
Sampliner (2002) reports that the goals of therapy in patients with Barrett's esophagus without high-grade dysplasia or cancer are to control symptoms and maintain a healed mucosa. Medical and surgical interventions are targeted at reducing or eliminating gastroesophageal reflux. Whether or not complete abolition of gastroesophageal reflux can reduce the risk of cancer in Barrett's esophagus is an open issue, and there are no randomized trials of antireflux therapy that address this question.
Medical Therapy
Medical therapy is the more common form of treatment and consists primarily of proton pump inhibitors (PPIs) to inhibit production of gastric acid secretion. Triadafilopoulos (2000) and Feldman and co-workers (1993) report that PPIs are more effective than H2 receptor blockers in controlling symptoms and healing erosive esophagitis. Triadafilopoulos (2000) and Hameeteman and Tytgat (1986) also report that PPIs can be remarkably effective, relieving symptoms in up to 95% of patients and healing erosive esophagitis and Barrett's ulcers. Studies by Champion (1994), Marshall (1998) and Menges (2001) and their coauthors also have shown that PPIs decrease duodenogastroesophageal ( bile ) reflux, probably by decreasing the volume of secretions in the stomach. Because patients with Barrett's esophagus usually have more severe reflux than other GERD patients, most require PPIs rather than H2 receptor blockers for suppression of symptoms and healing of esophageal lesions. Discontinuing medical treatment in patients with Barrett's esophagus almost always results in reemergence of the reflux symptoms, suggesting that most patients with severe GERD and Barrett's esophagus will require medication indefinitely.
Studies by Ouatu-Lascar (1999) and Sampliner (2002) and their associates have shown that some Barrett's patients whose symptoms are relieved by PPI therapy may continue to have asymptomatic reflux. In the study by Ouatu-Lascar and colleagues (1999), residual, asymptomatic reflux was associated with increased proliferation as assessed by proliferating cell nuclear antigen staining (S phase of the cell cycle). Umansky and collaborators (2001) also reported that PPI therapy was associated with decreases in proliferation as measured by a number of cell cycle markers, including p16, cyclin D1, and cyclin E. The clinical relevance of these results is presently unclear for several reasons. Prospective studies by Rabinovitch (2001) and one of us (BJR) (2003) and respective co-workers find no evidence that hyperproliferation is an independent predictor of progression to cancer in Barrett's esophagus, and therefore normalization of proliferation cannot be assumed to modulate the risk of cancer. In addition, Sampliner and coauthors (2002) report that ablation
P.2230
can reverse Barrett's epithelium to neosquamous epithelium even in the presence of residual reflux, whereas some patients fail reversal even when acid exposure is normalized as assessed by 24-hour pH monitoring.
Studies by Sampliner (1994), and by Sampliner (1988, 1990), Gore (1993), Haag (1999) and Sharma (1999) and their associates, have evaluated the effect of medical therapy on regression of Barrett's epithelium. The general consensus reached by Neumann (1995) and Haag (1999) and their colleagues was that neither medical therapy with H2 receptor blockers nor proton pump inhibitor therapy causes complete regression of Barrett's epithelium in relatively short-term studies. However, Sampliner (1994), Gore (1993) and Neumann (1995) and their collaborators found that PPI therapy is associated with the appearance of neosquamous islands in the Barrett's segment. Sharma (1998) and Biddlestone (1998) and their co-workers indicated that these neosquamous islands should be biopsied during endoscopic surveillance because they can conceal residual buried Barrett's epithelium in 38% of cases. As reported by Sampliner and Fass (1993) and by one of us (BJR) and coauthors (2000a), residual and concealed Barrett's epithelium can progress to high-grade dysplasia and cancer.
Surgical Therapy
The details of antireflux surgery are covered elsewhere in this volume; this chapter covers the relationship of control of reflux to Barrett's epithelium and the risk of progression to cancer. Spechler and associates (2001) indicate that surgical interventions have the advantage of repairing the cause of the reflux, rather than just treating the symptoms, thereby potentially eliminating the need for indefinite medical therapy, although some patients continue to receive antireflux medications after surgery. Esophageal adenocarcinomas arising after apparently successful antireflux surgery have been observed, as they have after medical therapy with proton pump inhibitors, as reported by Naef (1975), Haggitt (1978), Williamson (1990), McDonald (1996), Hakansson (1997), and one of us (BJR) (2000a) and respective colleagues.
A population-based study in Sweden by Ye and collaborators (2001) addressed the relationship of antireflux surgery to development of esophageal and gastric cardia adenocarcinomas. In this study, a cohort of 35,274 men and 31,691 women with discharge diagnoses of gastroesophageal reflux were compared with a second cohort of 6,406 men and 4,671 women who underwent antireflux surgery. Cancers developing within the first year were excluded. The standardized incidence ratios (SIRs) among the reflux cohort of men for developing esophageal and gastric cardia adenocarcinoma were 6.3 and 2.4, respectively, supporting the association between GERD and esophageal adenocarcinoma that has been documented in other studies, as previously described. The risk for esophageal adenocarcinoma increased with follow-up time in the reflux cohort. Among men who had undergone antireflux surgery, risks were also elevated for esophageal adenocarcinoma (SIR = 14.1) and for gastric cardia adenocarcinoma (SIR = 5.3). In contrast to the reflux cohort, the risk of esophageal adenocarcinoma remained relatively stable after surgery, although the small number of cases precluded any definitive conclusions. The authors concluded that GERD is a risk factor for esophageal adenocarcinoma as well as gastric cardia carcinoma, although to a lesser degree. The risk of developing cancer in this population remained elevated after antireflux surgery. Because this was not a randomized trial, there are multiple possible confounders in the study, including the possibilities that patients referred for surgery may have had more severe disease, more Barrett's esophagus, or other risk factors for cancer. Because of the nature of the study, the adequacy of the antireflux surgery also could not be addressed, further confounding interpretation.
Oberg and co-workers (1999), in an observational, nonrandomized study, described progressive detection of intestinal metaplasia in short segments of columnar-lined esophagus of patients who were treated medically but not in those whose reflux was treated surgically. The results are intriguing but difficult to interpret because the medically treated group was substantially older than those treated surgically (median age difference of 12 years). Wetscher and coauthors (2001) reported that 12 of 83 GERD patients treated medically developed Barrett's esophagus, compared with none of 42 patients treated surgically. However, in contrast to the Oberg study, this study used only a single baseline endoscopy with four-quadrant biopsies, and the rapid appearance of intestinal metaplasia within 24 months is most consistent with sampling error.
In the period between the description of Barrett's esophagus by Barrett in 1950 and Naef and associates' classic series in 1975, a series of case reports and case series by Goldman (1960), Mossberg (1966) and Endo (1974) convincingly documented proximal migration of columnar epithelium in the setting of chronic gastroesophageal reflux. However, most subsequent literature and clinical experience, as reported by Cameron (1992) and later by Cameron and Arora (2002), has suggested that the Barrett's segment length goes to fixation early and then does not change in follow-up. At the present time, there is no compelling evidence that either medical or surgical antireflux therapy prevents the development of cancer in persons who already have Barrett's esophagus.
Barrett's Esophagus with High-Grade Dysplasia with and without Early Esophageal Adenocarcinoma
Three options are available for the patient who has high-grade dysplasia without esophageal adenocarcinoma: careful surveillance, with intervention reserved for those who progress to cancer; endoscopic ablation; and esophagectomy.
P.2231
None guarantees a perfect outcome, but in experienced, high-volume centers, each appears to have a high success rate, although long-term data are currently incomplete for ablation therapies. There are two therapeutic options for patients who have high-grade dysplasia with early esophageal adenocarcinoma documented by endoscopic biopsies: esophagectomy and endoscopic ablation.
Survival after esophageal adenocarcinoma is dependent upon the stage at which the cancer is diagnosed. Farrow and Vaughan (1996) examined the survival of 2,986 patients with esophageal adenocarcinoma registered in the SEER database from 1973 through 1991. Survival was found to be dependent upon stage at diagnosis, with 5-year survival for patients with carcinoma in situ being 10-fold greater than those with locally invasive or metastatic disease. However, a diagnosis of in situ disease occurred in only 1% of the cases. Because survival of patients with in situ disease persisted for more than 15 years, improved identification of high-risk individuals is warranted.
Surveillance
Careful surveillance may be considered in some patients who have high-grade dysplasia without biopsy-proven esophageal adenocarcinoma, but only with full recognition that nearly 40% of such patients may have an undetected, coexisting cancer, as will be discussed. However, observations by Weston (2000) and Overholt (2003) and their colleagues that high-grade dysplasia can regress in nearly 40% of cases suggest that some patients may benefit from more conservative management than either esophagectomy or endoscopic ablation. One of us (BJR) (2000b) and Schnell (2001) and respective co-workers have reported large, long-term research studies of surveillance of high-grade dysplasia. Both use multiple, frequent endoscopies (at 0, 3, 6, and 9 months in one; at 0, 1, 3, 6, and 9 months in the other) to search for coexisting cancers in the first year. One of us (BJR) and collaborators (2000b) used a four-quadrant biopsy protocol every centimeter in the esophagus. Both centers use a single, experienced Barrett's pathologist and detailed pathology evaluations that record mucosal type, degree of inflammation, and grade of dysplasia for every biopsy. Both studies also employ a joint clinical pathology management meeting at which team decisions are made concerning recommendations for surveillance intervals or treatment.
The risks of surveillance of high-grade dysplasia should be considered relative to the risks of other courses of action, including ablation and esophagectomy. The risks of continued surveillance include patient anxiety, endoscopic complications, and development of an advanced, incurable cancer. Any patient who enters into surveillance of high-grade dysplasia should have extensive counseling concerning these risks as well as the possibilities of esophageal resection and ablation. The patient should meet with a surgeon to discuss resection and be offered the opportunity to discuss ablation with an expert in the field. Endoscopic complications of intensive surveillance occur in about 1% of endoscopies. The most common is chest or epigastric pain during or after endoscopy. Levine and coauthors (2000) reported that rarer complications include bleeding associated with simultaneous stricture dilatation, atrial dysrhythmias, and respiratory arrest.
The most concerning risk is the possibility of developing an advanced, incurable cancer during surveillance. There is some evidence presented by Weston (2000), one of us (BJR) (2000b) and Schnell (2001) and respective associates that this may vary according to the surveillance volume of high-grade dysplasia at an institution. In the center with the most experience, advanced cancers were reported in 3% of cases, whereas 25% of cancers detected by surveillance were advanced in the lowest-volume center. Surveillance of high-grade dysplasia should only be undertaken in experienced high-volume institutions that use a team approach with the management safeguards just described. Surveillance of high-grade dysplasia has not been proven to consistently detect early, curable cancers outside of a research study. The results of practice pattern surveys and quality-of-care studies performed by Falk (2000) and Ofman (2001) and their colleagues indicate that there is substantial room for improvement in adherence to recommended endoscopic and pathologic standards of care for patients with Barrett's esophagus. Therefore, surveillance of high-grade dysplasia should only be undertaken if the gastroenterologist is willing to assume responsibility that endoscopic and pathologic care will meet the standards developed in research studies, which are not generally attained in community surveillance.
Esophagectomy
Treatment options for patients with advanced esophageal adenocarcinoma are discussed elsewhere in this volume, as are different surgical options for esophagectomy. This section focuses on patients in whom high-grade dysplasia with or without an early esophageal adenocarcinoma has been diagnosed during endoscopic screening or surveillance for Barrett's esophagus. Several studies by Collard (2002), Farrow and Vaughan (1996), and by Peters (1994), Streitz (1998), van Sandick (1998) and Corley (2002) and their collaborators indicate that such patients typically have earlier-stage disease than those detected when they become symptomatic and that they have improved survival with esophageal resection (see Table 147-1).
Esophagectomy in a high-volume institution can be performed for either high-grade dysplasia or high-grade dysplasia with biopsy-proven early esophageal adenocarcinoma. One of the strongest arguments for esophagectomy in patients with high-grade dysplasia is the chance of a coexisting cancer. In a meta-analysis of 243 patients who underwent esophagectomy for high-grade dysplasia between 1983 and 2001, Collard (2002) reported that 94 (38%) were
P.2232
found to have cancer in the surgical specimen. Wright (1997) found that many of these cases may be justifiably criticized because of inadequate endoscopic biopsy evaluation. However, it is also clear that a single four-quadrant, 2-cm biopsy protocol does not reliably exclude the possibility of an undetected, coexisting cancer, as reported by Cameron and Carpenter (1997), DeMeester and DeMeester (1999), and by Peters (1994), Falk (1999) and Nigro (1999) and their co-workers. Esophagectomy with complete resection of Barrett's epithelium by an experienced esophageal surgeon in a high-volume center is the only option that can be considered curative and eliminates the need for further endoscopic biopsy surveillance.
The disadvantage of esophagectomy is that it is a serious operation with a significant morbidity and mortality. Studies from high-volume specialty centers with experienced esophageal surgeons have reported relatively low operative mortalities. Heitmiller and coauthors (1996) reported a 1.7% esophagectomy mortality for 60 patients with high-grade dysplasia in Barrett's esophagus. There was an overall complication rate of 29% in this series, but major complications fell from 10% in the period encompassing 1982 to 1994 to 3% in the period 1994 to 2001. A recent randomized comparison of extended transthoracic resection versus limited transhiatal resection by Hulscher and associates (2002) also reported operative mortalities in the 2% to 5% range. Morbidities included pulmonary (pneumonia and atelectasis) and cardiac complications, anastomotic leakage, vocal cord paralysis, chylous leakage, and wound infection.
Multiple studies over the past 5 years indicate that the mortality of esophagectomy is significantly greater in low-volume institutions than in high-volume centers (Table 147-2). Begg and colleagues (1998), in a review of SEER statistics, reported that the mortality of esophagectomy in low-volume centers (defined as performing fewer than six procedures annually) was 17.3%, compared with 3.4% in high-volume centers with six or more resections annually (p < 0.001). Similarly, Patti and collaborators (1998), reviewing data from the California Office of Statewide Health Planning and Development, reported that hospitals that performed fewer than 30 esophagectomies in the 5-year period 1990 to 1994 had a 16% mortality rate, compared with 4.8% in those with 30 or more resections (p < 0.0001). More than 80% of esophagectomies in this study were performed in low-volume institutions. Dimick and co-workers (2001) evaluated hospital volume, mortality, length of stay, and hospital costs in Maryland from 1984 to 1999. The results showed that mortality, length of stay, and costs were all decreased in high-volume institutions. Similarly, a recent study of the national Medicare claims database by Birkmeyer and coauthors (2002) reported that the mortality of esophagectomy decreased as hospital volume increased.
Table 147-2. Effect of Hospital Volume on Operative Mortality for Esophagectomy | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Given the consistency of these data across multiple population-based studies, it seems it is in the best interest of the patient that esophagectomy be performed only in high-volume institutions with expertise in the operative and perioperative management of esophageal cancer. Clearly, the esophagectomy volume of a hospital is a factor that must be considered in deciding on the appropriate course of action for a patient in whom endoscopic biopsies reveal high-grade dysplasia without endoscopic or biopsy evidence of cancer.
Endoscopic Ablation for High-Grade Dysplasia
Photodynamic therapy (PDT) is the ablation method that has the greatest reported experience with high-grade dysplasia in Barrett's esophagus and may be considered for nonsurgical candidates. When short-term downstaging of high-grade dysplasia is used as the metric, PDT has been reported by Wang (1999) to be successful in 88% of cases. However, longer-term follow-up reports indicate a more complicated situation. Selvasekar and associates (2001) recently reviewed all four published series of PDT for high-grade dysplasia in 114 patients with Barrett's esophagus. Only 45% of patients were believed to have complete regression of Barrett's esophagus to neosquamous epithelium; 55% had residual Barrett's esophagus, including 9% with residual dysplasia. The authors concluded that continued endoscopic surveillance was essential for patients treated by ablation. An additional concern are reports in most ablation studies of Barrett's metaplasia remaining under neosquamous epithelium, raising the possibility of the development of a concealed cancer undetectable by endoscopic examination, as discussed by Wolfsen (2002) Overholt (1999), and one of us (BJR) (2000a) and respective colleagues. Complications of PDT reported by Wang and Nijhawan (2000), Wolfsen and Ng (2002), and Overholt and collaborators (1999)
P.2233
include nausea, chest pain, strictures, atrial fibrillation, pleural effusions, congestive heart failure, perforation, and phototoxicity.
Reports by Wang (1999) and by Overholt and co-workers (1999) point out patients who had high-grade dysplasia downstaged as a result of PDT, but who subsequently redeveloped high-grade dysplasia or progressed to esophageal adenocarcinoma. Krishnadath and coauthors (2000) evaluated three such cases, showing that PDT did not completely ablate some Barrett's clones with p16, p53, and ploidy abnormalities that subsequently progressed to high-grade dysplasia during follow-up of 16 to 37 months. Recently, Krishnadath and associates (2001) reported that patients whose high-grade dysplasia contains p53 mutations may have a relative resistance to PDT. This study was also of concern because it reported the appearance of new p53 mutations in two cases of neosquamous epithelium after PDT.
Long-term outcomes are not yet available of PDT for large numbers of patients with high-grade dysplasia in Barrett's esophagus. Two-year follow-up results of a randomized trial of PDT versus omeprazole for high-grade dysplasia have been reported by Overholt and collaborators (2003) in abstract form at the time this chapter was written. The results show that PDT plus omeprazole was more effective at eliminating high-grade dysplasia than omeprazole alone. As well, there was a statistically significant reduction in the rate of progression to cancer in the PDT arm. However, 13% of patients treated with PDT plus omeprazole still progressed to esophageal adenocarcinoma within 2 years, compared with 28% in the omeprazole-alone arm.
Recently, Attwood and co-workers (2003) have reported a prospective series of 29 patients with high-grade dysplasia treated by argon-beam plasma coagulation and followed for a mean of 37 months (range 7 to 78 months). High-grade dysplasia was ablated or downstaged in 25 patients (86%). However, 4 patients progressed to cancer (14%). Complications included one perforation, which was followed by resection and death from respiratory complications. Estimated 5-year survival was 82%.
Endoscopic Ablation Therapy for Early Esophageal Adenocarcinoma
At the present time, endoscopic ablation therapy should probably only be considered for the patient who is judged not to be a candidate for esophagectomy after being evaluated by an experienced esophageal surgeon in a high-volume center. Two ablation techniques might be considered in this setting: endoscopic mucosal resection (EMR) and photodynamic therapy.
Buttar and coauthors (2001a) report endoscopic ultrasound-guided EMR might be considered for local therapy of a cancer arising in an endoscopically visible lesion. Pacifico and Wang (2002) report that EMR can provide more accurate staging than endoscopic ultrasound and can determine whether the cancer has invaded more deeply than can be treated with ablation therapy. The literature on EMR is deficient in long-term follow-up studies because the technique has only recently been used in patients with Barrett's esophagus. Although initial complete remissions have been reported in more than 95% of patients, a relatively high frequency of metachronous cancers can be expected because synchronous cancers have been reported in endoscopic and surgical series and because the residual Barrett's esophagus may have extensive high-grade, cytometric, or molecular changes that place it at risk for progression to cancer. Ell and associates (2000) report that EMR has also been used with some success in the treatment of early esophageal adenocarcinomas and high-grade dysplasia, although follow-up data for this treatment are limited. If EMR is undertaken, May and colleagues (2002) suggest that continued careful endoscopic surveillance is required, because 21 of 70 patients treated by EMR in the largest series developed recurrent or metachronous cancers within 3 years, even though the initial estimated complete response was 98%.
Photodynamic therapy might also be considered in the patient who is not an optimal surgical candidate, but relatively little long-term follow-up data are available. Sibille and co-workers (1995) have reported 5-year survival data. In their series, the complete response rate at 6 months was 87%, but the 5-year survival and 5-year disease-specific survivals were 25% and 74%, respectively. Overholt and coauthors (1999) reported on 100 patients who were followed for a mean of 19 months; 13 patients had esophageal adenocarcinoma, of which 10 were judged to be ablated. Two patients developed metastatic cancer. In another series using 5-aminolevulinic acid, Gossner and associates (1998) reported that 17 (77%) of 22 patients were successfully ablated. In a more recent series using 5-aminolevulinic acid reported by May and colleagues (2002), there was an initial 100% complete response rate (32 of 32) to PDT, but 7 (22%) developed recurrent or metachronous cancer within 3 years. McCaughan and collaborators (1996) reported that the only significant variable affecting survival after PDT was clinical stage at time of treatment. Tan and co-workers (1999) have reported failure of 5-aminolevulinic acid PDT in tumors more than 1 cm in size or more than 2 mm in depth. Corti and coauthors (2000) found that radiation therapy may improve the overall remission rate in those patients who do not respond to PDT alone. Recently, Wolfsen and colleagues (2002) reported apparently complete ablation of 13 of 14 esophageal adenocarcinomas using porfimer sodium PDT. In the remaining case, the cancer was concealed beneath neosquamous epithelium and invaded the submucosa.
A retrospective review from the Mayo Clinic, reported by Pacifico and associates (2003), compared patients with early esophageal adenocarcinoma treated by EMR and PDT (N = 24) with those treated by esophagectomy (N = 64) between 1996 and 2001. Eighty-three percent of patients in the EMR/PDT group (20 of 24) remained free of cancer over a follow-up interval of 12 2 months, compared with
P.2234
100% of patients treated surgically who were followed for 19 3 months. There were no procedure-related deaths in the EMR/PDT group, compared with one death (1.5%) in the surgery group.
In summary, esophagectomy remains the only treatment that can be described as curative for early esophageal adenocarcinoma and the only procedure eliminating the need for continued endoscopic biopsy surveillance. Although initial complete response rates for ablation therapies are high, published series show treatment failures in approximately 13% to 30% of cases over relatively short follow-up intervals. Ginsberg (2003) cautions that patients considering ablation therapy for high-grade dysplasia and early esophageal adenocarcinoma should understand that this approach remains investigational and that they will need continued careful surveillance, perhaps for the rest of their lives. Although ablation may be considered for elderly patients with comorbid diseases that make them poor surgical candidates, the available data do not warrant widespread general application of endoluminal approaches for younger and fitter patients.
REFERENCES
Agha FP: Radiologic diagnosis of Barrett's esophagus: critical analysis of 65 cases. Gastrointest Radiol 11:123, 1986.
Alanen KA, Joensuu H, Klemi PJ: Autolysis is a potential source of false aneuploid peaks in flow cytometric DNA histograms. Cytometry 10: 417, 1989.
Alikhan M, et al: Variable pathologic interpretation of columnar lined esophagus by general pathologists in community practice. Gastrointest Endosc 50:23, 1999.
Allison PR, Johnstone AS: The oesophagus lined with gastric mucus membrane. Thorax 8:87, 1953.
Atkinson M, Robertson CS: Benign oesophageal stricture in Barrett's columnar epithelialised oesophagus and its responsiveness to conservative management. Gut 29:1721, 1988.
Attwood SE, et al: Argon beam plasma coagulation as therapy for high-grade dysplasia in Barrett's esophagus. Clin Gastroenterol Hepatol 1:258, 2003.
Avidan B, et al: Hiatal hernia size, Barrett's length, and severity of acid reflux are all risk factors for esophageal adenocarcinoma. Am J Gastroenterol 97:1930, 2002.
Barrett NR: Chronic peptic ulcer of the oesophagus and oesophagitis. Br J Surg 38:175, 1950.
Barrett NR: The lower esophagus lined by columnar epithelium. Surgery 41:881, 1957.
Begg CB, et al: Impact of hospital volume on operative mortality for major cancer surgery. JAMA 280:1747, 1998.
Bergers E, et al: Interlaboratory reproducibility of semiautomated cell cycle analysis of flow cytometry DNA-histograms obtained from fresh material of 1,295 breast cancer cases [see comments]. Hum Pathol 27:553, 1996.
Bersentes K, et al: Prevalence of Barrett's esophagus in Hispanics is similar to Caucasians. Dig Dis Sci 43:1038, 1998.
Biddlestone LR, et al: The histopathology of treated Barrett's esophagus: squamous reepithelialization after acid suppression and laser and photodynamic therapy. Am J Surg Pathol 22:239, 1998.
Birkmeyer JD, et al: Hospital volume and surgical mortality in the United States. N Engl J Med 346:1128, 2002.
Blot WJ, et al: Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 265:1287, 1991.
Bollschweiler E, et al: Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer 92:549, 2001.
Bosch A, Frias Z, Caldwell WL: Adenocarcinoma of the esophagus. Cancer 43:1557, 1979.
Bozymski EM: Barrett's esophagus: endoscopic characteristics. In Spechler SJ, Goyal RK (eds): Barrett's Esophagus: Pathophysiology, Diagnosis and Management. New York: Elsevier Science, 1985, p. 113.
Brown LM, Devesa S: Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 11:235, 2002.
Brown LM, et al: Adenocarcinoma of the esophagus and esophagogastric junction in white men in the United States: alcohol, tobacco, and socioeconomic factors. Cancer Causes Control 5:333, 1994.
Brown LM, et al: Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst 87:104, 1995.
Buttar NS, et al: Combined endoscopic mucosal resection and photodynamic therapy for esophageal neoplasia within Barrett's esophagus. Gastrointest Endosc 54:682, 2001a.
Buttar NS, et al: Extent of high-grade dysplasia in Barrett's esophagus correlates with risk of adenocarcinoma. Gastroenterology 120:1630, 2001b.
Bytzer P, et al: Adenocarcinoma of the esophagus and Barrett's esophagus: a population-based study. Am J Gastroenterol 94:86, 1999.
Caldwell MT, et al: Ambulatory oesophageal bile reflux monitoring in Barrett's oesophagus. Br J Surg 82:657, 1995.
Cameron AJ: Barrett's esophagus and adenocarcinoma: from the family to the gene. Gastroenterology 102:1421, 1992.
Cameron AJ: Epidemiologic studies and the development of Barrett's esophagus. Endoscopy 25:635, 1993.
Cameron AJ: Barrett's esophagus: prevalence and size of hiatal hernia. Am J Gastroenterol 94:2054, 1999.
Cameron AJ, Arora AS: Barrett's esophagus and reflux esophagitis: is there a missing link? Am J Gastroenterol 97:273, 2002.
Cameron AJ, Carpenter HA: Barrett's esophagus, high-grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol 92: 586, 1997.
Cameron AJ, et al: The incidence of adenocarcinoma in columnar-lined (Barrett's) esophagus. N Engl J Med 313:857, 1985.
Canto MI: Vital staining and Barrett's esophagus. Gastrointest Endosc 49:S12, 1999.
Caygill CP, et al: A single centre's 20 years' experience of columnar-lined (Barrett's) oesophagus diagnosis. Eur J Gastroenterol Hepatol 11:1355, 1999.
Cederqvist C, et al: Adenocarcinoma of the oesophagus. Acta Chir Scand 146:411, 1980.
Chak A, et al: Familial aggregation of Barrett's oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut 51:323, 2002.
Champion G, et al: Duodenogastroesophageal reflux: relationship to pH and importance in Barrett's esophagus. Gastroenterology 107:747, 1994.
Chow WH, et al: The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA 274:474, 1995.
Chow WH, et al: Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst 90:150, 1998.
Clark GW, et al: Effect of gastroduodenal juice and dietary fat on the development of Barrett's esophagus and esophageal neoplasia: an experimental rat model. Ann Surg Oncol 1:252, 1994.
Coenraad M, et al: Is Barrett's esophagus characterized by more pronounced acid reflux than severe esophagitis? Am J Gastroenterol 93: 1068, 1998.
Collard JM: High-grade dysplasia in Barrett's esophagus. The case for esophagectomy. Chest Surg Clin N Am 12:77, 2002.
Conio M, et al: Secular trends in the epidemiology and outcome of Barrett's oesophagus in Olmsted County, Minnesota. Gut 48:304, 2001.
Corley DA, et al: Surveillance and survival in Barrett's adenocarcinomas: a population-based study. Gastroenterology 122:633, 2002.
Corley DA, et al: Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 124:47, 2003.
Corti L, et al: Outcome of patients receiving photodynamic therapy for early esophageal cancer. Int J Radiat Oncol Biol Phys 47:419, 2000.
Crabb DW, et al: Familial gastroesophageal reflux and development of Barrett's esophagus. Ann Intern Med 103:52, 1985.
Dahms BB, et al: Barrett's esophagus in three children after antileukemia chemotherapy. Cancer 60:2896, 1987.
P.2235
DeMeester SR: Management of Barrett's esophagus free of dysplasia. Semin Thorac Cardiovasc Surg 9:279, 1997.
DeMeester SR, DeMeester TR: The diagnosis and management of Barrett's esophagus. Adv Surg 33:29, 1999.
Devesa SS, et al: Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 83:2049, 1998.
Dhillon PK, et al: Family history of cancer and risk of esophageal and gastric cancers in the United States. Int J Cancer 93:148, 2001.
Dimick JB, et al: Hospital volume is related to clinical and economic outcomes of esophageal resection in Maryland. Ann Thorac Surg 72:334, 2001.
Drewitz DJ, et al: The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol 92:212, 1997.
Dulai GS, et al: Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 122:26, 2002.
Eberhart CE, et al: Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107:1183, 1994.
Eckardt VF, et al: Life expectancy and cancer risk in patients with Barrett's esophagus: a prospective controlled investigation. Am J Med 111:33, 2001.
Ell C, et al: Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology 118:670, 2000.
Eloubeidi MA, Provenzale D: Clinical and demographic predictors of Barrett's esophagus among patients with gastroesophageal reflux disease: a multivariable analysis in veterans. J Clin Gastroenterol 33:306, 2001.
Endo M: A case of Barrett epithelization followed up for five years. Endoscopy 6:48, 1974.
Everhart CW Jr, et al: Barrett's esophagus: inherited epithelium or inherited reflux? J Clin Gastroenterol 5:357, 1983.
Falk GW, et al: Jumbo biopsy forceps protocol still misses unsuspected cancer in Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 49:170, 1999.
Falk GW, et al: Practice patterns for surveillance of Barrett's esophagus in the United States. Gastrointest Endosc 52:197, 2000.
Farrow DC, Vaughan TL: Determinants of survival following the diagnosis of esophageal adenocarcinoma (United States). Cancer Causes Control 7:322, 1996.
Farrow DC, et al: Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev 7:97, 1998.
Farrow DC, et al: Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control 11:231, 2000.
Fass R, et al: Correlation of oesophageal acid exposure with Barrett's oesophagus length. Gut 48:310, 2001.
Feldman M, et al: Treatment of reflux esophagitis resistant to H2-receptor antagonists with lansoprazole, a new H+/K(+)-ATPase inhibitor: a controlled, double-blind study. Lansoprazole Study Group. Am J Gastroenterol 88:1212, 1993.
Fu S, et al: Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology 116:1319, 1999.
Funkhouser EM, Sharp GB: Aspirin and reduced risk of esophageal carcinoma. Cancer 76:1116, 1995.
Gammon MD, et al: Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst 89: 1277, 1997.
Gelfand MD: Barrett esophagus in sexagenarian identical twins. J Clin Gastroenterol 5:251, 1983.
Gerson LB, et al: Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology 123:461, 2002.
Gillen P, et al: Barrett's oesophagus: pH profile. Br J Surg 74:774, 1987.
Ginsberg GG: Endoluminal therapy for Barrett's with high-grade dysplasia and early esophageal adenocarcinoma. Clin Gastroenterol Hepatol 1:241, 2003.
Goldman M: Barrett syndrome. Case Reports 39:104, 1960.
Gore S, et al: Regression of columnar lined (Barrett's) oesophagus with continuous omeprazole therapy. Aliment Pharmacol Ther 7:623, 1993.
Gossner L, et al: Photodynamic ablation of high-grade dysplasia and early cancer in Barrett's esophagus by means of 5-aminolevulinic acid. Gastroenterology 114:448, 1998.
Gray JR, et al: Cigarette and alcohol use in patients with adenocarcinoma of the gastric cardia or lower esophagus. Cancer 69:2227, 1992.
Haag S, et al: Regression of Barrett's esophagus: the role of acid suppression, surgery, and ablative methods. Gastrointest Endosc 50:229, 1999.
Haggitt RC: Barrett's esophagus, dysplasia, and adenocarcinoma. Hum Pathol 25:982, 1994.
Haggitt RC, et al: Adenocarcinoma complicating columnar epithelium-lined (Barrett's) esophagus. Am J Clin Pathol 70:1, 1978.
Hakansson HO, et al: Development of adenocarcinoma in Barrett's oesophagus after successful antireflux surgery. Eur J Surg 163:469, 1997.
Hameeteman W, Tytgat GN: Healing of chronic Barrett ulcers with omeprazole. Am J Gastroenterol 81:764, 1986.
Hameeteman W, et al: Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology 96:1249, 1989.
Hansen S, et al: Esophageal and gastric carcinoma in Norway 1958 1992: incidence time trend variability according to morphological subtypes and organ subsites. Int J Cancer 71:340, 1997.
Hansson LE, et al: Increasing incidence of both major histological types of esophageal carcinomas among men in Sweden. Int J Cancer 54:402, 1993.
Hassall E: Barrett's esophagus: new definitions and approaches in children. J Pediatr Gastroenterol Nutr 16:345, 1993.
Hassall E: Columnar-lined esophagus in children. Gastroenterol Clin North Am 26:533, 1997.
Heitmiller RF, et al: Barrett's esophagus with high-grade dysplasia. An indication for prophylactic esophagectomy. Ann Surg 224:66, 1996.
Herrera JL, et al: Barrett's esophagus: lack of association with adjuvant chemotherapy for localized breast carcinoma. Gastrointest Endosc 38: 551, 1992.
Hirota WK, et al: Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology 116:277, 1999.
Hirschowitz BI: Gastric acid and pepsin secretion in patients with Barrett's esophagus and appropriate controls. Dig Dis Sci 41:1384, 1996.
Hopwood D, et al: Effects of bile acids and hydrogen ion on the fine structure of oesophageal epithelium. Gut 22:306, 1981.
Hulscher JB, et al: Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 347:1662, 2002.
Hwang D, et al: Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst 90:455, 1998.
Iascone C, et al: Barrett's esophagus. Functional assessment, proposed pathogenesis, and surgical therapy. Arch Surg 118:543, 1983.
Iftikhar SY, et al: Bile reflux in columnar-lined Barrett's oesophagus. Ann R Coll Surg Engl 75:411, 1993.
Inadomi JM, et al: Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 138:176, 2003.
Jankowski JA, et al: Esophageal adenocarcinoma arising from Barrett's metaplasia has regional variations in the West. Gastroenterology 10: 1053, 2002.
Jochem VJ, et al: Familial Barrett's esophagus associated with adenocarcinoma. Gastroenterology 102:1400, 1992.
Johnson DA, et al: Esophageal acid sensitivity in Barrett's esophagus. J Clin Gastroenterol 9:23, 1987.
Kabat GC, et al: Tobacco, alcohol intake, and diet in relation to adenocarcinoma of the esophagus and gastric cardia. Cancer Causes Control 4: 123, 1993.
Kauer WK, et al: Does duodenal juice reflux into the esophagus of patients with complicated GERD? Evaluation of a fiberoptic sensor for bilirubin. Am J Surg 169:98, 1995a.
Kauer WK, et al: Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized. Ann Surg 222:525, 1995b.
Krishnadath KK, et al: Persistent genetic abnormalities in Barrett's esophagus after photodynamic therapy. Gastroenterology 119:624, 2000.
Krishnadath KK, et al: p53 mutations in Barrett's esophagus predict poor response to photodynamic therapy. Gastroenterology 120:A-413, 2001.
Kubo A, Corley DA: Marked regional variation in adenocarcinomas of the esophagus and the gastric cardia in the United States. Cancer 95:2096, 2002.
Lagergren J, et al: Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 340:825, 1999a.
Lagergren J, et al: Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 130:883, 1999b.
P.2236
Lagergren J, et al: Heredity and risk of cancer of the esophagus and gastric cardia. Cancer Epidemiol Biomarkers Prev 9:757, 2000.
Langman MJ, et al: Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ 320:1642, 2000.
Levi F, et al: Esophageal and gastric carcinoma in Vaud, Switzerland, 1976 1994. Int J Cancer 75:160, 1998.
Levine DS, Reid BJ: Endoscopic biopsy technique for acquiring larger mucosal samples. Gastrointest Endosc 37:332, 1991.
Levine DS, et al: An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology 105:40, 1993.
Levine DS, et al: Safety of a systematic endoscopic biopsy protocol in patients with Barrett's esophagus. Am J Gastroenterol 95:1152, 2000.
Levine MS, et al: Barrett esophagus: reticular pattern of the mucosa. Radiology 147:663, 1983.
Liabeuf A, Faivre J: Time trends in oesophageal cancer incidence in Cote d'Or (France), 1976 93. Eur J Cancer Prev 6:24, 1997.
Lieberman DA, et al: Risk factors for Barrett's esophagus in community-based practice. GORGE consortium. Gastroenterology Outcomes Research Group in Endoscopy. Am J Gastroenterol 92:1293, 1997.
Locke GR 3rd, et al: Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 112:1448, 1997.
Loughney T, et al: Esophageal manometry and ambulatory 24-hour pH monitoring in patients with short and long segment Barrett's esophagus. Am J Gastroenterol 93:916, 1998.
Macdonald CE, Wicks AC, Playford OJ: Final results from 10 year cohort of patients undergoing surveillance for Barrett's oesophagus: observational study. BMJ 321:1252, 2000.
Marshall RE, Anggiansah A, Owen WJ: Bile in the oesophagus: clinical relevance and ambulatory detection. Br J Surg 84:21, 1997.
Marshall RE, et al: Effect of omeprazole 20 mg twice daily on duodenogastric and gastro-oesophageal bile reflux in Barrett's oesophagus. Gut 43:603, 1998.
May A, et al: Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 14:1085, 2002.
Mayne ST, et al: Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev 10:1055, 2001.
McArdle JE, et al: Distribution of dysplasias and early invasive carcinoma in Barrett's esophagus [see comments]. Hum Pathol 23:479, 1992.
McCarthy CJ, et al: Cyclooxygenase-2 expression in gastric antral mucosa before and after eradication of Helicobacter pyloriinfection. Am J Gastroenterol 94:1218, 1999.
McCaughan JS Jr, et al: Photodynamic therapy for esophageal malignancy: a prospective twelve-year study. Ann Thorac Surg 62:1005, 1996.
McDonald ML, et al: Barrett's esophagus: does an antireflux procedure reduce the need for endoscopic surveillance? J Thorac Cardiovasc Surg 111:1135, 1996.
McKinney A, et al: Oesophageal and gastric cancer in Scotland 1960 1990. Br J Cancer 71:411, 1995.
Menges M, et al: Increased acid and bile reflux in Barrett's esophagus compared to reflux esophagitis, and effect of proton pump inhibitor therapy. Am J Gastroenterol 96:331, 2001.
Menke-Pluymers MB, et al: Risk factors for the development of an adenocarcinoma in columnar-lined (Barrett) esophagus. The Rotterdam Esophageal Tumor Study Group. Cancer 72:1155, 1993.
Miwa K, et al: Duodenogastric reflux and foregut carcinogenesis. Cancer 75:1426, 1995.
Moller H: Incidence of cancer of oesophagus, cardia and stomach in Denmark. Eur J Cancer Prev 1:159, 1992.
Montgomery E, et al: Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol 32:368, 2001.
Morson BC, Belcher JR: Adenocarcinoma of the oesophagus and ectopic gastric mucosa. Br J Cancer 6:127, 1952.
Mossberg SM: The columnar-lined esophagus (Barrett syndrome) an acquired condition? Gastroenterology 50:671, 1966.
Muller-Lissner SA: Bile reflux is increased in cigarette smokers. Gastroenterology 90:1205, 1986.
Naef AP, Savary M, Ozzello L: Columnar-lined lower esophagus: an acquired lesion with malignant predisposition. Report on 140 cases of Barrett's esophagus with 12 adenocarcinomas. J Thorac Cardiovasc Surg 70:826, 1975.
Nebel OT, et al: Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis 21:953, 1976.
Nehra D, et al: Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut 44:598, 1999.
Neumann CS, Cooper BT: 24 hour ambulatory oesophageal pH monitoring in uncomplicated Barrett's oesophagus. Gut 35:1352, 1994.
Neumann CS, Iqbal TH, Cooper BT: Long term continuous omeprazole treatment of patients with Barrett's oesophagus. Aliment Pharmacol Ther 9:451, 1995.
Nigro JJ, et al: Occult esophageal adenocarcinoma: extent of disease and implications for effective therapy. Ann Surg 230:433, 1999.
Oberg S, et al: The extent of Barrett's esophagus depends on the status of the lower esophageal sphincter and the degree of esophageal acid exposure. J Thorac Cardiovasc Surg 117:572, 1999.
O'Connor JB, Falk GW, Richter JE: The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol 94:2037, 1999.
Ofman JJ, et al: The economic impact of the diagnosis of dysplasia in Barrett's esophagus. Am J Gastroenterol 95:2946, 2000.
Ofman JJ, et al: The quality of care in Barrett's esophagus: endoscopist and pathologist practices. Am J Gastroenterol 96:876, 2001.
Orlando RC: Reflux esophagitis. In Yamada T (ed): Textbook of Gastroenterology. 2nd Ed. Philadelphia: JB Lippincott Company, 1995, p. 1214.
Ouatu-Lascar R, Fitzgerald RC, Triadadilopoulos G: Differentiation and proliferation in Barrett's esophagus and the effects of acid suppression. Gastroenterology 117:327, 1999.
Overholt BF, Panjehpour M, Haydek JM: Photodynamic therapy for Barrett's esophagus: follow-up in 100 patients. Gastrointest Endosc 49:1, 1999.
Overholt BF, et al: International, multicenter, partially blinded, randomised study of the efficacy of photodynamic therapy (PDT) using porfimer sodium (POR) for the ablation of high-grade dysplasia (HGD) in Barrett's esophagus (BE): results of 24-month follow-up. Gastroenterology 124:A-20, 2003.
Pacifico RJ, Wang KK: Nonsurgical management of Barrett's esophagus with high-grade dysplasia. Surg Oncol Clin N Am 11:321, 2002.
Pacifico RJ, et al: Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett's esophagus. Clin Gastroenterol Hepatol 1:252, 2003.
Patti MG, et al: A hospital's annual rate of esophagectomy influences the operative mortality rate [see comments]. J Gastrointest Surg 2:186, 1998.
Paull A, et al: The histologic spectrum of Barrett's esophagus. N Engl J Med 295:476, 1976.
Pera M, et al: Influence of esophagojejunostomy on the induction of adenocarcinoma of the distal esophagus in Sprague-Dawley rats by subcutaneous injection of 2,6-dimethylnitrosomorpholine. Cancer Res 49: 6803, 1989.
Pera M, et al: Influence of pancreatic and biliary reflux on the development of esophageal carcinoma. Ann Thorac Surg 55:1386, 1993.
Peters FT, et al: Is chemotherapy associated with development of Barrett's esophagus? Dig Dis Sci 38:923, 1993.
Peters JH, et al: Outcome of adenocarcinoma arising in Barrett's esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 108:813, 1994.
Phillips RW, Wong RK: Barrett's esophagus. Natural history, incidence, etiology, and complications. Gastroenterol Clin N Am 20:791, 1991.
Powell J, Allum WH: Oesophageal cancer in Britain. BMJ 304:1380, 1992.
Prach AT, et al: Increasing incidence of Barrett's oesophagus: education, enthusiasm, or epidemiology? Lancet 350:933, 1997.
Rabinovitch PS: Practical considerations for DNA content and cell cycle analysis. In Bauer KD, Duque RE, Shankey TV (eds): Clinical Flow Cytometry: Principles and Applications. Baltimore: Williams and Wilkins, 1992, p. 117.
Rabinovitch PS, et al: Predictors of progression in Barrett's esophagus III: baseline flow cytometric variables. Am J Gastroenterol 96:3071, 2001.
Raphael HA, et al: Primary adenocarcinoma of the esophagus: 18-year review and review of literature. Ann Surg 164:785, 1966.
P.2237
Reid BJ, Thomas Jr CR: Esophageal neoplasms. In Yamada T, et al. (eds): Textbook of Gastroenterology. Philadelphia: JB Lippincott, 1995, p. 1256.
Reid BJ, et al: Observer variation in the diagnosis of dysplasia in Barrett's esophagus. Hum Pathol 19:166, 1988.
Reid BJ, et al: Optimizing endoscopic biopsy detection of early cancers in Barrett's high-grade dysplasia. Am J Gastroenterol 95:3089, 2000a.
Reid BJ, et al: Predictors of progression to cancer in Barrett's esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol 95:1669, 2000b.
Reid B, et al: Biomarkers in Barrett's esophagus: a guideline for clinicians. Gastrointest Clin N Am 2003 (in press).
Robbins AH, Vincent ME: Columnar-lined esophagus (Barrett's esophagus) updated. Contemp Diagn Radiol 3:1, 1980.
Romero Y, et al: Barrett's esophagus: prevalence in symptomatic relatives. Am J Gastroenterol 97:1127, 2002.
Rudolph RE, Vaughn TL, Storer B: The effect of segment length on the risk of neoplastic progression in patients with Barrett's esophagus. Ann Intern Med 133:748, 2000.
Sampliner RE: Effect of up to 3 years of high-dose lansoprazole on Barrett's esophagus. Am J Gastroenterol 89:1844, 1994.
Sampliner RE: Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett's esophagus. Am J Gastroenterol 97:1888, 2002.
Sampliner RE, Fass R: Partial regression of Barrett's esophagus an inadequate endpoint. Am J Gastroenterol 88:2092, 1993.
Sampliner RE, et al: Squamous mucosa overlying columnar epithelium in Barrett's esophagus in the absence of anti-reflux surgery. Am J Gastroenterol 83:510, 1988.
Sampliner RE, et al: Lack of impact of therapy on extent of Barrett's esophagus in 67 patients. Dig Dis Sci 35:93, 1990.
Sampliner RE, et al: Impact of esophageal acid exposure on the endoscopic reversal of Barrett's esophagus. Am J Gastroenterol 97:270, 2002.
Sartori S, et al: Barrett esophagus after chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF): an iatrogenic injury? Ann Intern Med 114:210, 1991.
Schnell TG, Sontag SJ, Cheijfec G: Adenocarcinomas arising in tongues or short segments of Barrett's esophagus. Dig Dis Sci 37:137, 1992.
Schnell TG, et al: Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology 120:1607, 2001.
Schreinemachers DM, Everson RB: Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 5:138, 1994.
Selvasekar C, et al: Photodynamic therapy and the alimentary tract. Aliment Pharmacol Ther 15:899, 2001.
Shaheen NJ, Provenzale D, Sandler RS: Upper endoscopy as a screening and surveillance tool in esophageal adenocarcinoma: a review of the evidence. Am J Gastroenterol 97:1319, 2002.
Shaheen NJ, et al: Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology 119:333, 2000.
Sharma P, et al: Squamous islands in Barrett's esophagus: what lies underneath? Am J Gastroenterol 93:332, 1998.
Sharma P, et al: Durability of new squamous epithelium after endoscopic reversal of Barrett's esophagus. Gastrointest Endosc 50:159, 1999.
Sharma P, et al: Natural history of low grade dysplasia an infrequent finding which usually regresses preliminary results from the Barrett's Esophagus Study. Gastroenterology 122:A20, 2002.
Sibille A, et al: Long-term survival after photodynamic therapy for esophageal cancer. Gastroenterology 108:337, 1995.
Singh P, Taylor RH, Colin-Jones DG: Esophageal motor dysfunction and acid exposure in reflux esophagitis are more severe if Barrett's metaplasia is present. Am J Gastroenterol 89:349, 1994.
Sjogren RW Jr, Johnson LF: Barrett's esophagus: a review. Am J Med 74:313, 1983.
Skinner DB, et al: Barrett's esophagus. Comparison of benign and malignant cases. Ann Surg 198:554, 1983.
Som ML, Wolf BS: Peptic ulcer of the esophagus and esophagitis in gastric-lined esophagus. JAMA 162:641, 1956.
Soni A, Sampliner RE, Sonnenberg A: Screening for high-grade dysplasia in gastroesophageal reflux disease: is it cost-effective? Am J Gastroenterol 95:2086, 2000.
Sontag SJ, et al: The importance of hiatal hernia in reflux esophagitis compared with lower esophageal sphincter pressure or smoking. J Clin Gastroenterol 13:628, 1991.
Sontag SJ, et al: Prevalence of oesophagitis in asthmatics. Gut 33:872, 1992.
Spechler SJ: Epidemiology and natural history of gastro-oesophageal reflux disease. Digestion 51(suppl 1):24, 1992.
Spechler SJ, Goyal RK: Barrett's esophagus. N Engl J Med 315:362, 1986.
Spechler SJ, et al: Prevalence of metaplasia at the gastro-oesophageal junction. Lancet 344:1533, 1994.
Spechler SJ, et al: Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA 285:2331, 2001.
Spechler SJ, et al: Racial differences in the frequency of symptoms and complications of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 16:1795, 2002.
Stein HJ, et al: Bile reflux in benign and malignant Barrett's esophagus: effect of medical acid suppression and Nissen fundoplication. J Gastrointest Surg 2:333, 1998.
Streitz JM Jr, Andrews CW Jr, Ellis FH Jr: Endoscopic surveillance of Barrett's esophagus. Does it help? J Thorac Cardiovasc Surg 105:383, 1993.
Streitz JM Jr, et al: Endoscopic surveillance of Barrett's esophagus: a cost-effectiveness comparison with mammographic surveillance for breast cancer. Am J Gastroenterol 93:911, 1998.
Tan WC, et al: Photodynamic therapy using 5-aminolaevulinic acid for oesophageal adenocarcinoma associated with Barrett's metaplasia. J Photochem Photobiol B 53:75, 1999.
Teodori L, et al: DNA/protein flow cytometry as a predictive marker of malignancy in dysplasia-free Barrett's esophagus: thirteen-year follow-up study on a cohort of patients. Cytometry 34:257, 1998.
Thomas RJ, et al: Incidence trends in oesophageal and proximal gastric carcinoma in Victoria. Aust N Z J Surg 66:271, 1996.
Thun MJ, et al: Aspirin use and risk of fatal cancer. Cancer Res 53:1322, 1993.
Triadafilopoulos G: Proton pump inhibitors for Barrett's oesophagus. Gut 46:144, 2000.
Trimble KC, et al: Lowered oesophageal sensory thresholds in patients with symptomatic but not excess gastro-oesophageal reflux: evidence for a spectrum of visceral sensitivity in GORD. Gut 37:7, 1995.
Turnbull AD, Goodner JT: Primary adenocarcinoma of the esophagus. Cancer 22:915, 1968.
Tuyns AJ: Oesophageal cancer in France and Switzerland: recent time trends. Eur J Cancer Prev 1:275, 1992.
Tzonou A, et al: Diet and risk of esophageal cancer by histologic type in a low-risk population. Int J Cancer 68:300, 1996.
Umansky M, et al: Proton pump inhibitors reduce cell cycle abnormalities in Barrett's esophagus. Oncogene 20:7987, 2001.
Vaezi MF, Richter JE: Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology 111:1192, 1996.
Vaezi MF, Richter JE: Bile reflux in columnar-lined esophagus. Gastroenterol Clin North Am 26:565, 1997.
Vaezi MF, Singh S, Richter JE: Role of acid and duodenogastric reflux in esophageal mucosal injury: a review of animal and human studies. Gastroenterology 108:1897, 1995.
Vainio H, Morgan G: Cyclo-oxygenase 2 and breast cancer prevention. Non-steroidal anti-inflammatory agents are worth testing in breast cancer. BMJ 317:828, 1998.
van der Burgh A, et al: Oesophageal cancer is an uncommon cause of death in patients with Barrett's oesophagus. Gut 39:5, 1996.
van Sandick JW, et al: Impact of endoscopic biopsy surveillance of Barrett's oesophagus on pathological stage and clinical outcome of Barrett's carcinoma. Gut 43:216, 1998.
Vaughan TL, et al: Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 4:85, 1995.
Vincent ME, et al: The reticular pattern as a radiographic sign of the Barrett esophagus: an assessment. Radiology 153:333, 1984.
Wang KK: Current status of photodynamic therapy of Barrett's esophagus. Gastrointest Endosc 49:S20, 1999.
Wang KK, Nijhawan PK: Complications of photodynamic therapy in gastrointestinal disease. Gastrointest Endosc Clin N Am 10:487, 2000.
Ward MH, et al: Risk of adenocarcinoma of the stomach and esophagus with meat cooking method and doneness preference. Int J Cancer 71:14, 1997.
Weston AP, et al: Long-term follow-up of Barrett's high-grade dysplasia. Am J Gastroenterol 95:1888, 2000.
P.2238
Weston AP, et al: p53 protein overexpression in low grade dysplasia (LGD) in Barrett's esophagus: immunohistochemical marker predictive of progression. Am J Gastroenterol 96:1355, 2001.
Wetscher GJ, et al: Efficacy of medical therapy and antireflux surgery to prevent Barrett's metaplasia in patients with gastroesophageal reflux disease. Ann Surg 234:627, 2001.
Williamson WA, et al: Effect of antireflux operation on Barrett's mucosa. Ann Thorac Surg 49:537, 1990.
Wilson KT, et al: Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res 58:2929, 1998.
Winters C Jr, et al: Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 92:118, 1987.
Wolfsen HC, Ng CS: Cutaneous consequences of photodynamic therapy. Cutis 69:140, 2002.
Wolfsen HC, et al: Photodynamic therapy for dysplastic Barrett esophagus and early esophageal adenocarcinoma. Mayo Clin Proc 77:1176, 2002.
Wright TA: High-grade dysplasia in Barrett's oesophagus. Br J Surg 84:760, 1997.
Wright TA, et al: Cost effectiveness of detecting Barrett's cancer. Gut 39:574, 1996.
Ye W, et al: Risk of adenocarcinomas of the esophagus and gastric cardia in patients with gastroesophageal reflux diseases and after antireflux surgery. Gastroenterology 121:1286, 2001.
Zhang ZF, et al: Adenocarcinomas of the esophagus and gastric cardia: medical conditions, tobacco, alcohol, and socioeconomic factors. Cancer Epidemiol Biomarkers Prev 5:761, 1996.
Zhang ZF, et al: Adenocarcinomas of the esophagus and gastric cardia: the role of diet. Nutr Cancer 27:298, 1997.
Zimmermann KC, et al: Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res 59:198, 1999.
READING REFERENCE
Sampliner RE: Practice guidelines on the diagnosis, surveillance, and therapy of Barrett's esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 93:1028, 1998.
EAN: 2147483647
Pages: 203
- The Second Wave ERP Market: An Australian Viewpoint
- Enterprise Application Integration: New Solutions for a Solved Problem or a Challenging Research Field?
- The Effects of an Enterprise Resource Planning System (ERP) Implementation on Job Characteristics – A Study using the Hackman and Oldham Job Characteristics Model
- A Hybrid Clustering Technique to Improve Patient Data Quality
- Relevance and Micro-Relevance for the Professional as Determinants of IT-Diffusion and IT-Use in Healthcare