115 - Novel Strategies for Lung Cancer Immunotherapy
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Esophagus > Section XXI - Operative Procedures in the Management of Esophageal Disease > Chapter 132 - Transhiatal Esophagectomy Without Thoracotomy
function show_scrollbar() {}
Chapter 132
Transhiatal Esophagectomy Without Thoracotomy
Mark B. Orringer
Historically, Denk (1913) performed the first reported blunt transmediastinal esophageal resection without thoracotomy using a vein stripper in cadavers. The British surgeon Turner (1933) reported the first successful transhiatal blunt esophagectomy for carcinoma and used an antethoracic skin tube to reestablish alimentary continuity at a second stage. With the advent of endotracheal anesthesia, however, esophageal resection under direct vision became possible, and transhiatal esophagectomy (THE) without thoracotomy became a seldom-used procedure reserved primarily for the patient undergoing a laryngopharyngectomy for carcinoma and restoration of alimentary continuity with the stomach, as described by Ong and Lee (1960) and LeQuesne and Ranger (1966). With the advent of endotracheal intubation, safe transthoracic operations became a reality, and during this time, reports by Rehn (1898), Llobet (1900), and Torek (1915) of resection of the thoracic esophagus began to appear.
The earliest reports of successful transthoracic esophagectomy and intrathoracic esophagogastric anastomosis for carcinoma were by Ohsawa (1933), Marshall (1938), Adams and Phemister (1938), and Churchill and Sweet (1942). Garlock (1946) and Carter (1947) popularized the combined left thoracoabdominal incision for carcinoma of the lower one third of the esophagus, as had been used by Ohsawa in 1933. Ivor Lewis (1946) popularized the right combined thoracic and abdominal approach for esophageal resection and reconstruction, citing the advantages over a left-sided approach of improved access to the upper two thirds of the esophagus, exposure of the entire course of the esophagus after division of the azygos vein, and protection of the opposite pleural cavity provided by the descending thoracic aorta. Ong (1971) favored a right thoracotomy for resection of midthird esophageal tumor, but advocated a cervical esophagogastric anastomosis. McKeown (1972, 1976) also preferred a cervical esophagogastric anastomosis and described his use of three incisions (i.e., laparotomy, right thoracotomy, and cervical) for resection of esophageal carcinoma and esophageal reconstruction with stomach. But some variation of a thoracoabdominal esophageal resection and reconstruction with an intrathoracic esophagogastric anastomosis was the most popular approach into the 1980s.
With the exception of a few reported series of transthoracic esophageal resection and reconstruction for carcinoma performed with a hospital mortality lower than 5% by Akiyama and associates (1981), Mitchell (1987), and Mathisen and colleagues (1988b), this operation remained a formidable one in the hands of surgeons worldwide, with mortality rates ranging from 15% to 40% in series reported through the 1970s and early 1980s by Ellis (1983) and Posthlethwait (1983), and averaging 33%, as noted in the reviews by Giuli and Gignoux (1980) and Earlam and Cunha-Melo (1980). Even in more recent reports, such as that of Muller and associates (1990), published between 1980 and 1990, the mean mortality rate for resection of esophageal carcinoma, although nearly halved compared with the previous decade, was still 13%. Pulmonary complications caused by splinting and atelectasis associated with a combined thoracic and abdominal operation in a debilitated patient with esophageal obstruction, and mediastinitis and sepsis after an intrathoracic esophageal anastomotic leak, remain the leading causes of death after esophageal resection in virtually every reported series.
Against this backdrop of more than 40 years of experience with transthoracic esophageal resection, I and Sloan (1978) reawakened interest in the technique of THE without thoracotomy as an alternative approach with potentially less risk and morbidity than the more traditional transthoracic resection. By avoiding a thoracotomy, it was argued that this approach lessens the physiologic impact of a combined thoracic and abdominal operation. Furthermore, mediastinitis from an anastomotic leak is virtually eliminated, since the cervical esophageal anastomosis results in a transient, easily drained salivary fistula if a leak occurs. The technique of THE was criticized by some as being an unsafe operation that violates basic surgical principles of adequate
P.2005
exposure and hemostasis and denies the patient with carcinoma a complete mediastinal lymph node dissection. After 1978, a number of published reports described several surgeons' early experience with THE and discussed the indications and relative morbidity and mortality of transhiatal versus transthoracic esophagectomy. Many of these THE series represent the researchers' early results with this procedure, and they are not reflective of the true morbidity and mortality of the operation performed after gaining greater experience and facility with it. Katariya and colleagues (1994) reviewed 1,353 patients undergoing THE who were reported in the surgical literature between 1981 and 1992. Sixteen of the papers referenced (69.5%) were series of 50 patients or less and as such were not a true reflection of the type of results that can be achieved by more experienced surgical teams. In the past decade, there have been at least nine different reports on THE and 37 series comparing transthoracic esophagectomy and THE. Table 132-1 lists the series totaling more than 6,300 patients.
In a recently published meta-analysis of transthoracic versus transhiatal resection for carcinoma of the esophagus by Hulscher and associates (2001), perioperative blood loss was significantly higher after transthoracic resection (mean 1,001 mL vs. 728 mL), and transthoracic resection carried a higher risk for pulmonary complications, chylothorax, and wound infection. The overall mean anastomotic leak rate was greater with transhiatal resection (13.6% vs. 7.2%), as was the rate of vocal cord paralysis (9.5% vs. 3.5%). The length of postoperative intensive care after transthoracic resection was significantly longer (11.2 days vs. 9.1 days), and the hospital stay significantly prolonged (21 days vs. 17.8 days). The overall hospital mortality rate was significantly higher after transthoracic resections (9.2% vs. 5.7%). There was no significant difference in survival at 3 or 5 years postoperatively between those undergoing transthoracic and transhiatal esophagectomies. The largest reported THE series was published by me and my colleagues in 1999 and described our 20-year retrospective experience with this operation in 1,085 patients undergoing esophageal resection for diseases of the intrathoracic esophagus, 285 (26%) with benign disease and 800 (74%) with carcinoma; the total hospital mortality rate for the procedure was 3.8%, 4.2% in those undergoing THE for carcinoma. This chapter reviews our technique of THE without thoracotomy and the results we have obtained with this operation.
GENERAL CONSIDERATIONS OF TRANSHIATAL ESOPHAGECTOMY
THE has been possible in 97% of our patients requiring an esophagectomy for either benign or malignant disease. Without question, our comfort level with this procedure is far greater than 10 to 15 years ago and in part explains our general use of THE in virtually all of our patients requiring an esophagectomy. In patients with esophageal carcinoma, the operation is contraindicated if bronchoscopic evidence exists of tracheobronchial invasion, not contiguity, by the tumor. Histologic documentation of distant metastatic
P.2006
disease (i.e., biopsy-proven liver or supraclavicular or distant lymph node metastasis) associated with an intrathoracic esophageal carcinoma should be a contraindication to esophagectomy, because these patients with stage IV carcinomas have an average survival of approximately 6 months, and the risks of esophagectomy generally outweigh the short-term benefits in this population. As documented by Quint and associates (1985), computed tomographic scanning of the chest and upper abdomen in the preoperative assessment of these patients is helpful in defining the local extent of the tumor and the presence of distant metastatic disease, but this diagnostic modality is not a reliable indicator of resectability. Computed tomographic (CT) can demonstration of contiguity of an esophageal tumor with adjacent organs does not prove that invasion is present and that the tumor is unresectable. In our experience, the same applies to endoscopic ultrasonography, which we have not used on a regular basis in our patient population to predict resectability. In patients with benign esophageal disease, particularly those who have had previous esophageal operations, and especially an esophagomyotomy that may produce adhesions between the esophageal submucosa and adjacent aorta, the surgeon must be prepared to convert to a transthoracic esophagectomy if on palpation of the esophagus through the diaphragmatic hiatus periesophageal adhesions prevent a safe transhiatal resection. At present, the single most important contraindication to THE is the surgeon's assessment at the time of transhiatal evaluation of the esophagus that resection using this technique is unsafe. Newer modalities of staging esophageal carcinoma, such as endoscopic ultrasonography, as reported by Hiele (1997), Rice (1998), and Reed (1999) and their colleagues, and positron emission tomographic scanning, as noted by Luketich (1997) and Block (1997) and their co-workers, may allow improved selection of patients for esophagectomy.
Table 132-1. Reports on Transhiatal Esophagectomies Since 1990 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Anesthetic Management
Intraarterial blood pressure is monitored with a radial artery catheter so that prolonged hypotension resulting from cardiac displacement by the surgeon's hand inserted into the posterior mediastinum during the transhiatal dissection can be avoided. Two large-bore intravenous catheters, generally placed in peripheral arm veins, should be available for rapid volume replacement if required, but the arms are placed at the patient's sides during the operation so that the surgeon has unimpeded access to the neck, chest, and abdomen. If central venous pressure is to be monitored intraoperatively, and this is not routine, a right neck vein should be used to keep the left neck free for the operation. An unshortened standard endotracheal tube is used rather than a larger, more bulky double-lumen tube. If a posterior membranous tracheal tear occurs during the transhiatal dissection, the tube can be advanced into the left main-stem bronchus and one-lung anesthesia maintained while the tracheal injury is dealt with. Because transient hypotension caused by cardiac displacement can occur during a transhiatal dissection, inhalation agents are decreased and inspired oxygen concentration increased during this phase of the operation. THE requires a close working relationship between the anesthetist and the surgeon.
Operative Technique
The patient is positioned supine, the head turned toward the right, and the neck extended by placing a small folded sheet beneath the scapulae. The skin is prepared and draped from the mandible to the pubis and anterior to both midaxillary lines. The arms are padded and placed at the patient's sides. A table-mounted, self-retaining (upper-hand) retractor is used to facilitate the abdominal operation. Two suction lines, one at the head of the table and one from below, are used routinely. THE is executed in three phases: the abdominal, mediastinal, and cervical phases.
Abdominal Phase
The supraumbilical midline incision extends from the xiphoid to the umbilicus (Fig. 132-1). The triangular ligament of the liver is divided and the left lobe of the liver retracted to the right. The stomach is inspected to be certain that scarring or shortening from prior disease, or involvement of a significant portion of the upper stomach by tumor, does not preclude its use for a cervical esophagogastric anastomosis. The right gastroepiploic artery is identified early and protected throughout the operation.
Separation of the greater omentum from the stomach is begun high along the greater curvature at an avascular site where the right gastroepiploic artery terminates as it enters the stomach or anastomoses with small branches of the left gastroepiploic artery. The lesser sac behind the greater omentum is then entered at this point, and working progressively upward along the greater curvature of the stomach, the left gastroepiploic and short gastric vessels are divided and ligated sequentially, avoiding both injury to the gastric wall, which may result in later necrosis, and damage to the adjacent spleen. After the high greater curvature of the stomach has been mobilized away from the omentum and spleen, the peritoneum overlying the esophageal hiatus is incised and the esophagogastric junction encircled with a rubber drain. The filmy gastrohepatic omentum is incised beginning at the midpoint of the lesser curvature of the stomach and proceeding superiorly along the lesser curvature toward the hiatus. The left gastric vein is isolated, ligated, and divided. The left gastric artery is isolated, ligated, and divided at its origin from the celiac axis, sweeping all adjacent lymph nodes that can be removed with the specimen toward the stomach. The right gastric artery is protected throughout the gastric mobilization. Next, the greater omentum along the lower half of the greater curvature of the stomach is separated from the right gastroepiploic artery at least 1.5 to 2.0 cm inferior to the vessel to avoid inadvertent injury to it. A generous Kocher's maneuver is performed to ensure maximum mobility of the stomach. A pyloromyotomy extending from 1.5 cm on the stomach through the pylorus and onto the duodenum for 0.5 to 1.0 cm is performed using the cutting current of a needle-tipped electrocautery and a fine-tipped mosquito clamp for dissection of the gastric and duodenal muscle away from the underlying submucosa. If the pyloric or duodenal mucosa is violated, the tear is repaired with several 5 0 Prolene
P.2007
(Ethicon, Inc., Somerville, NJ, U.S.A.) sutures and reinforced with adjacent omentum. Two metallic hemostatic clips are placed adjacent to the pylorus to facilitate its identification on subsequent radiographic examinations. A 14F rubber jejunostomy tube is inserted and secured with a Witzel's maneuver. This tube is not brought out through the anterior abdominal wall until the thoracic phase of the operation is completed.
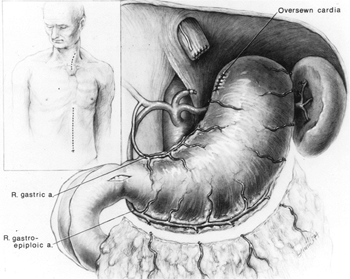 |
Fig. 132-1. Cervical and supraumbilical midline abdominal incisions used for transhiatal esophagectomy. When the stomach is used to replace the esophagus, the left gastric and left gastroepiploic vessels are divided. The right gastric and right gastroepiploic arteries supply the mobilized stomach. A pyloromyotomy and Kocher's maneuver are routinely performed. From Orringer MB, Sloan H: Substernal gastric bypass of the excluded esophagus for palliation of esophageal carcinoma. J Thorac Cardiovasc Surg 70:836, 1975. With permission. |
With downward traction on the rubber drain encircling the esophagogastric junction, mobilization of the distal 5 to 10 cm of esophagus from the mediastinum is initiated through the diaphragmatic hiatus (Fig. 132-2). Dissecting bluntly along the prevertebral fascia posterior to the esophagus and anteriorly behind the pericardium, mobility of the esophagus within the posterior mediastinum is assessed through the diaphragmatic hiatus, and if no contraindication exists to proceeding with the THE, attention is now turned to the neck.
Cervical Phase
A 5- to 6-cm incision paralleling the anterior border of the left sternocleidomastoid muscle and beginning just below the cricoid cartilage is used. The platysma and omohyoid fascial layers are divided, the sternocleidomastoid muscle and carotid sheath and its contents retracted laterally, and the larynx and trachea medially. No metal retractor should be placed against the tracheoesophageal groove to avoid injury to the recurrent laryngeal nerve. Ligation of the middle thyroid vein and inferior thyroid artery is performed when necessary.
The dissection is carried posteriorly to the prevertebral fascia medial to the carotid sheath. The prevertebral space behind the cervical esophagus is developed by blunt finger dissection, constantly keeping against the esophagus. The tracheoesophageal groove is developed by dissecting posterior to it and against the cervical esophagus to avoid injury to the recurrent laryngeal nerve. The cervical esophagus is encircled with a rubber drain and is retracted superiorly as blunt dissection of the upper thoracic esophagus from the superior mediastinum is performed, again keeping the volar aspects of the fingers against the esophagus (see Fig. 132-2). A 10-cm length of esophagus is mobilized in this fashion down to the level of the carina.
Transhiatal Dissection
THE is performed in an orderly sequential fashion. If the tumor-containing portion of the esophagus feels mobile and separable from adjacent structures as assessed by grasping the mass through the diaphragmatic hiatus and rocking it, THE is likely possible. The dissection begins posterior to the esophagus. One hand inserted through the diaphragmatic hiatus posterior to the esophagus is advanced superiorly, while the other inserted through the cervical incision is advanced downward along the prevertebral fascia. Traction is maintained on both rubber drains encircling either end of the esophagus to facilitate the dissection. A half sponge on a stick is advanced along the prevertebral fascia from the cervical incision until it meets the hand inserted from below
P.2008
through the diaphragmatic hiatus (Fig. 132-3). Once the posterior mediastinal tunnel is thus established, a 28F Argyle Saratoga sump catheter is inserted through the cervical wound and into the posterior mediastinum and is connected to suction at the upper end of the table. This suction catheter maintains a dry field during the subsequent posterior mediastinal dissection and facilitates identification and division of the lateral esophageal attachments under direct vision through the diaphragmatic hiatus. Surgeons whose glove size is larger than 7 or 7.5 may at times induce unacceptable hypotension as the hand inserted through the diaphragmatic hiatus displaces the heart. Particular effort should be made to keep the hand flattened posteriorly during the mediastinal dissection, as elevation of the hand anteriorly typically induces hypotension.
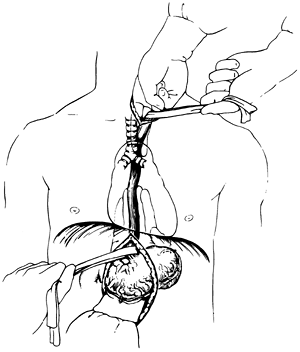 |
Fig. 132-2. Transhiatal esophagectomy is initiated as a midline dissection with the volar aspects of the fingers against the esophagus. Traction on rubber drains encircling either end of the esophagus facilitate the dissection. From Orringer MB: Surgical options for esophageal resection and reconstruction with stomach. In Baue AE, et al (eds): Glenn's Thoracic and Cardiovascular Surgery. 5th Ed. Norwalk, CT: Appleton&Lange, 1991, p. 787. With permission. |
The anterior esophageal dissection is performed as a mirror image of the posterior mobilization, again placing traction on the rubber drains encircling either end of the esophagus and dissecting with the volar aspect of the fingers against the anterior esophagus (Fig. 132-4). As the esophagus is mobilized away from the pericardium and carina, care must be taken to avoid injury to the posterior membranous trachea. With the assistance of narrow retractors placed into the diaphragmatic hiatus, mobile paraesophageal and subcarinal lymph nodes may be dissected with the specimen under direct vision. Long (13-inch) right-angle clamps inserted through the hiatus are used to dissect, mobilize, and clamp the lateral esophageal attachments. These are divided and ligated sequentially. As experience is gained with this procedure, more of the esophageal mobilization is done under direct vision through the hiatus, ligating the esophageal attachments. Fingers introduced through the cervical incision dissect downward against the anterior wall of the esophagus, sweeping it away from the trachea and avoiding injury to the posterior membranous trachea (Fig. 132-5). A carefully controlled half sponge on a stick facilitates this anterior dissection. Once the anterior and posterior esophageal dissections have been completed,
P.2009
the lateral esophageal attachments must now be divided. Traction is placed on the rubber drain encircling the cervical esophagus to deliver it out of the superior mediastinum. The lateral esophageal attachments are progressively dissected away from the esophagus until 8 to 10 cm of upper esophagus have been circumferentially mobilized. The esophagus is then permitted to retract back into the superior mediastinum. The right hand is inserted through the diaphragmatic hiatus anterior to the esophagus, which is retracted downward by the drain encircling its lower end. The hand is advanced upward into the superior mediastinum until the transition from the circumferentially mobilized upper esophagus to the adjacent segment with its intact lateral attachments can be identified (Fig. 132-6). The esophagus is trapped against the prevertebral fascia between the index and middle fingers, and with a downward raking motion of the hand, the remaining lateral periesophageal attachments are avulsed (Fig. 132-7). During this lateral dissection, vagal fibers passing from the hilum of the lungs onto the esophagus may be hooked by the index finger, identified through the diaphragmatic hiatus, grasped with a long right-angle clamp, divided, and ligated. At times, dense subcarinal or subaortic tissue requires fracture by firm pressure between the index finger and thumb.
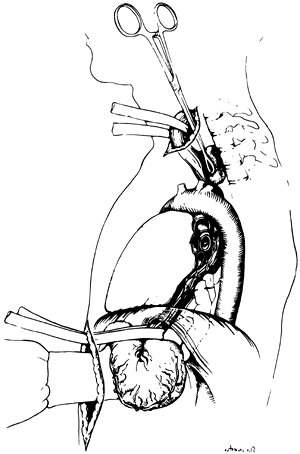 |
Fig. 132-3. A half sponge on a stick inserted through the cervical incision is advanced until it meets the hand inserted through the diaphragmatic hiatus posterior to the esophagus. From Orringer MB: Surgical options for esophageal resection and reconstruction with stomach. In Baue AE, et al (eds): Glenn's Thoracic and Cardiovascular Surgery. 5th Ed. Norwalk, CT: Appleton&Lange, 1991, p. 787. With permission. |
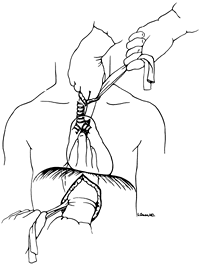 |
Fig. 132-4. The anterior transhiatal mobilization is a mirror image of the posterior dissection, again performed with the volar aspects of the fingers against the esophagus and using traction applied to drains around either end of the esophagus. Care must be taken to avoid injury to the posterior membranous trachea. From Orringer MB: Surgical options for esophageal resection and reconstruction with stomach. In Baue AE, et al (eds): Glenn's Thoracic and Cardiovascular Surgery. 5th Ed. Norwalk, CT: Appleton&Lange, 1991, p. 787. With permission. |
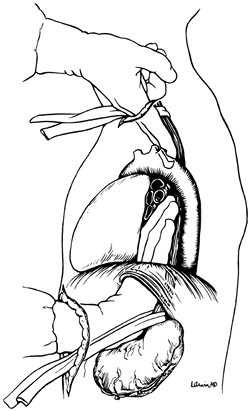 |
Fig. 132-5. In the process of dissecting the anterior esophagus, the hand should be pressed posteriorly against the esophagus to minimize cardiac displacement. From Orringer MB: Surgical options for esophageal resection and reconstruction with stomach. In Baue AE, et al (eds): Glenn's Thoracic and Cardiovascular Surgery. 5th Ed. Norwalk, CT: Appleton&Lange, 1991, p. 787. With permission. |
Once the entire intrathoracic esophagus has been mobilized, several inches of esophagus are gently pulled into the cervical wound, and the esophagus is divided obliquely in the neck with the GIA surgical stapler (Fig. 132-8), intentionally leaving redundancy in the length of remaining cervical esophagus that will facilitate later construction of the cervical esophagogastric anastomosis. The stomach is then delivered out of the abdomen and the attached esophagus
P.2010
drawn downward out of the posterior mediastinum through the diaphragmatic hiatus. Immediately after delivering the esophagus from the posterior mediastinum, narrow deep Deaver retractors are placed into the diaphragmatic hiatus, the posterior mediastinum is inspected for bleeding from the esophageal bed, and the mediastinal pleura is inspected to determine if entry into either pleural cavity has occurred and a chest tube is required. A large abdominal gauze pack is inserted through the diaphragmatic hiatus into the posterior mediastinum and left in place to tamponade any minor bleeding as the stomach is prepared for its relocation into the chest. Similarly, narrow-gauge packs are gently inserted through the cervical incision into the upper mediastinum while continually protecting the recurrent laryngeal nerve from direct injury. If entry into either pleural cavity has occurred, a chest tube is inserted as needed in the respective anterior axillary line.
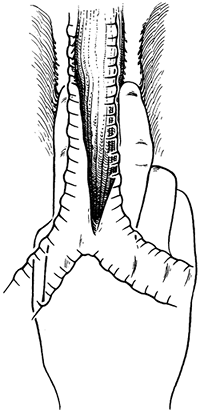 |
Fig. 132-6. After circumferential mobilization of the upper thoracic esophagus has been achieved through the cervical incision, the right hand is inserted through the diaphragmatic hiatus and advanced upward into the superior mediastinum until the undivided lateral esophageal attachments can be felt. From Orringer MB: Transhiatal esophagectomy. In Dudley H, Pories WJ, Carter D (eds): Rob and Smith's Operative Surgery. 4th Ed. London: Butterworth-Heinemann, 1983a, p. 192. With permission. |
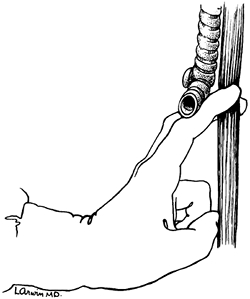 |
Fig. 132-7. Avulsion of filmy lateral esophageal attachments is achieved by a downward raking motion of the index and middle fingers, which have trapped the esophagus against the spine. Tougher vagal fibers that course along the esophagus may be delivered downward until visible through the hiatus and then divided under direct vision. From Orringer MB: Transhiatal blunt esophagectomy without thoracotomy. In Cohn LH (ed): Modern Technics in Surgery Cardiothoracic Surgery. New York: Futura, 1983b, p. 62. With permission. |
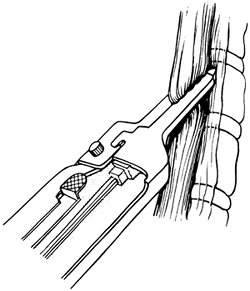 |
Fig. 132-8. The cervical esophagus is divided obliquely with the GIA stapler. From Orringer MB, Sloan H: Esophageal replacement after blunt esophagectomy. In Nyhus LM, Baker RJ (eds): Mastery of Surgery. Boston: Little, Brown, 1984, p. 426. With permission. |
The mobilized stomach and attached esophagus are placed on the anterior chest, and the gastric fundus is grasped and retracted superiorly. For tumors of the distal esophagus and cardia, an area along the lesser curvature at the level of the second vascular arcade from the cardia is cleared of fat and blood vessels between clamps and ties. The gastric fundus is retracted superiorly, as the lesser curvature of the stomach is progressively divided with sequential applications of the 5-cm GIA stapler approximately 4 to 6 cm distal to palpable tumor (Fig. 132-9). After each application of the stapler, the stomach is progressively stretched cephalad, thereby straightening the natural bend of the
P.2011
lesser curvature to the right and maximizing the subsequent upward reach of the stomach. For tumors of the upper and middle esophagus and in cases of benign disease requiring esophagectomy, as little stomach as possible is sacrificed to preserve gastric submucosal collateral circulation to the fundus. After dividing the proximal stomach, the esophagus is removed from the field, and the gastric staple suture line is oversewn with a running 4-0 polypropylene Lembert stitch. Traction on the stomach should be maintained as the suture line is oversewn to avoid purse-stringing the lesser curvature and subsequent interference with the upward reach of the stomach.
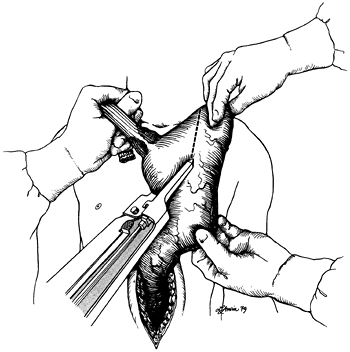 |
Fig. 132-9. After completion of the transhiatal mobilization of the esophagus, division of the cervical esophagus, and mobilization of the stomach with the attached esophagus out of the abdomen and onto the anterior chest, traction is applied to the gastric fundus at the point that reaches most superior to the neck. For tumors of the distal esophagus and esophagogastric junction, the GIA surgical stapler is applied to the stomach 4 to 6 cm distal to the esophagogastric junction. For upper and middle third esophageal tumors and for benign disease requiring an esophagectomy, as little stomach as possible is resected to maximize gastric submucosal collateral flow to the fundus. From Orringer MB, Sloan H: Esophageal replacement after blunt esophagectomy. In Nyhus LM, Baker RJ (eds): Mastery of Surgery. Boston: Little, Brown, 1984, p. 426. With permission. |
The abdominal pack that was placed into the mediastinum through the diaphragmatic hiatus and those in the superior mediastinum through the cervical incision are now removed, and the entire hand and forearm are inserted through the posterior mediastinum until three fingers emerge from the neck wound to ensure an adequate posterior mediastinal tunnel to accommodate the stomach. The gastric fundus is then grasped gently by the fingertips of one hand, which guide the stomach through the diaphragmatic hiatus and upward anterior to the spine, beneath the aortic arch, and into the superior mediastinum (Fig. 132-10). A Babcock clamp inserted into the superior mediastinum through the cervical incision is used to deliver the gastric fundus into the neck wound until the stomach can be grasped with the fingers and gently pulled upward. Great care is taken to avoid traumatizing the stomach in this process. The Babcock clamp is not completely closed, and the stomach is delivered into the neck more by pushing from below than traction from above. A healthy pink noncontused gastric fundus tip in the neck wound is the goal of this repositioning of the stomach. Suturing of the stomach to rubber traction drains, as originally advocated by me, suction apparatuses, and bowel bags are avoided to minimize gastric trauma. Typically 4 to 5 cm of stomach is delivered into the neck wound. It is temporarily held in place by inserting a narrow moistened gauze pack into the thoracic inlet posterior to the stomach to prevent the stomach from slipping back into the posterior mediastinum. A moistened gauze is placed temporarily over the neck wound and the color of the stomach intermittently assessed as the abdomen is closed. The stomach is carefully palpated to be certain that it has not become twisted within the posterior mediastinum
P.2012
during its positioning in the chest. In the rare situation in which mediastinal fibrosis from prior inflammation or irradiation results in posterior mediastinal narrowing that does not accommodate the stomach, it may be necessary to develop a retrosternal tunnel, resect the sternoclavicular joint, and place the stomach retrosternally.
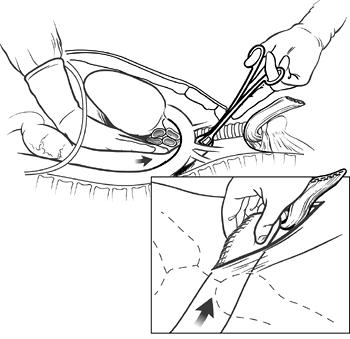 |
Fig. 132-10. The mobilized stomach is gently manipulated by one hand through the diaphragmatic hiatus upward beneath the aortic arch into the superior mediastinum until the tip of the gastric fundus can be grasped with a Babcock clamp inserted through the cervical incision. The clamp is applied gently, not completely racheting the handle, and is used to deliver the gastric fundus into the neck wound until it can be grasped with the fingertips (inset). Then, 4 to 6 cm of the stomach are brought into the neck wound more from pushing below in the chest than from pulling from above in the neck. From Orringer MB, Marshall B, Iannettoni MD: Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 119:277, 2000. With permission. |
To avoid contamination of the abdominal wound by oral bacteria, the cervical anastomosis is not begun until the abdominal portion of the operation is completed. The diaphragmatic hiatus is inspected and narrowed with No. 1 silk crural sutures as needed so that it loosely accommodates three fingers of the surgeon's hand alongside the stomach. The anterior gastric wall is sutured at several points to the edge of the diaphragmatic hiatus with interrupted No. 3-0 silk sutures to prevent subsequent intrathoracic herniation of abdominal viscera alongside the intrathoracic stomach as described by Reich (1996) and Heitmiller (1997) and their colleagues. The feeding jejunostomy tube is brought out through a stab wound in the left upper quadrant of the abdomen, and the jejunum is fixed against the anterior abdominal wall with several interrupted sutures. The jejunostomy tube is sutured to the skin. The abdominal incision is closed without routine use of abdominal or mediastinal drains.
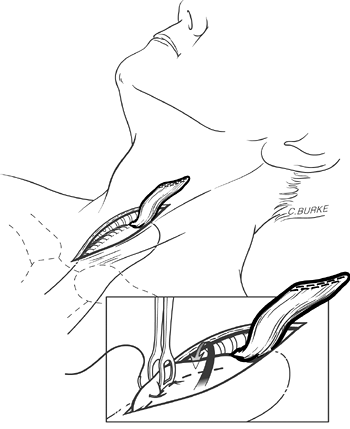 |
Fig. 132-11. When the stomach has been mobilized properly, 4 to 5 cm rests comfortably above the level of the clavicles along the cervical prevertebral fascia, well behind the end of the divided esophagus, which is retracted superiorly with an Allis clamp. The oversewn gastric staple line along the lesser curvature side of the stomach is toward the patient's right. A Babcock clamp (inset) grasps the anterior wall of the stomach low in the neck wound where it emerges from the posterior mediastinum at the thoracic inlet, and the gastric staple line (dotted line) is rotated more medially. The stomach is elevated several more centimeters into the wound, and a seromuscular 3-0 cardiovascular silk traction suture is placed distal to the clamp. From Orringer MB, Marshall B, Iannettoni MD: Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 119:277, 2000. With permission. |
The cervical esophagogastric anastomosis is then performed using the 3-cm long, 3.5-mm EndoGIA stapler, as reported by myself and associates (2000). A side-to-side anastomosis is constructed with the stapler inserted directly through the cervical incision (Figs. 132-11 through 132-17). After the stapler has been fired and removed from the field, a 16F nasogastric tube is inserted across the anastomosis and into the intrathoracic stomach for postoperative gastric decompression. When the anastomosis is completed, metallic hemostatic clips are placed on either side of the anastomosis for future radiographic localization of the anastomosis. At the conclusion of the operation, the cervical anastomosis is located approximately 20 cm from the upper incisors or 4 to 5 cm distal to the upper esophageal sphincter (Fig. 132-18), and the pyloromyotomy site rests 2 to 3 cm below the diaphragmatic hiatus. The cervical wound is closed loosely with absorbable suture over a small rubber drain. A portable
P.2013
chest radiograph is obtained in the operating room to ensure that no unrecognized pneumothorax or hemothorax exists, that the endotracheal tube is in proper position, and that the tip of the nasogastric tube is in good position above the level of the diaphragmatic hiatus. With meticulous attention to preoperative pulmonary physiotherapy, including complete abstinence from cigarette smoking for at least 2 weeks and regular use of an incentive inspirometer, and postoperative epidural anesthesia to improve respiratory dynamics, neither postoperative mechanical ventilation nor intensive care nursing is required routinely.
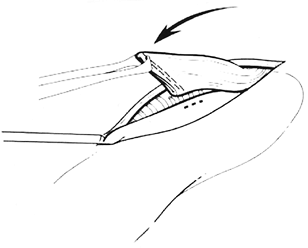 |
Fig. 132-12. The traction suture elevates the anterior gastric wall into the field and is fixed to the drapes with a hemostat. A point on the anterior gastric wall is selected for a 1.5-cm vertical gastrotomy (dotted line), which is performed with the cutting current of a needletip electrocautery device. The gastrotomy must be located far enough inferior to the tip of the gastric fundus to allow subsequent full insertion of the 3-cm long staple cartridge. Placement of the gastrotomy also must take into consideration the remaining length of cervical esophagus and should be performed with the realization that when the traction suture on the stomach is eventually removed, the stomach will partially retract downward into the thoracic inlet. Therefore, some redundancy in the length of the cervical esophagus should be allowed as the anastomosis is constructed. Modified from Orringer MB, Marshall B, Iannettoni MD: Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 119:277, 2000. With permission. |
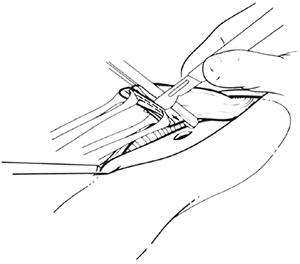 |
Fig. 132-13. An atraumatic vascular forceps serves as a guide for amputation of the cervical esophageal staple suture line, which is sent to the pathology department as the proximal esophageal margin. The original oblique placement of the stapler used to divide the cervical esophagus was purposeful, because the anterior tip of the esophagus should be longer than the posterior corner in construction of the anastomosis. Modified from Orringer MB, Marshall B, Iannettoni MD: Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 119:227, 2000. With permission. |
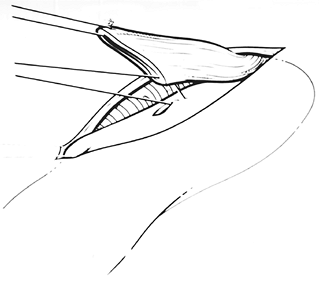 |
Fig. 132-14. Two full-thickness anastomotic stay sutures (4-0 polyglycolic acid) are placed, one from the anterior tip of the cut cervical esophagus, and one at the midpoint of the upper edge of the transverse gastrotomy and the posterior corner of the esophagus. Modified from Orringer MB, Marshall B, Iannettoni MD: Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 119: 277, 2000. With permission. |
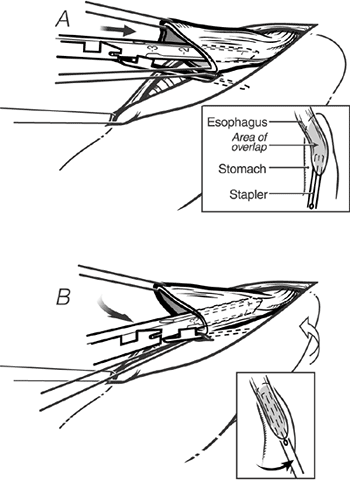 |
Fig. 132-15. A. With downward traction on the anastomotic stay sutures, the 3-cm long EndoGIA staple cartridge is inserted, the thinner anvil portion into the stomach, and the thicker staple-bearing portion into the esophagus. B. The staple cartridge is advanced into the esophagus and stomach. To achieve alignment of the posterior wall of the cervical esophagus and the anterior wall of the stomach, as the stapler is inserted and advanced into the esophagus and stomach, it is rotated so that it is pointing toward the patient's right ear (insets). From Orringer MB, Marshall B, Iannettoni MD: Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 119:277, 2000. With permission. |
Transhiatal Esophagectomy for Carcinoma of the Esophagogastric Junction
The technique of THE and proximal partial gastrectomy is applicable in most patients with carcinoma limited to the cardia and proximal stomach. It is generally possible to resect
P.2014
the proximal stomach in such a way that a 4- to 6-cm gastric margin still exists beyond the tumor (Fig. 132-19). So long as the entire greater curvature of the gastric fundus can be preserved, the point that reaches most cephalad to the neck is available for construction of the cervical anastomosis. Carcinomas of the cardia have a greater tendency to metastasize superiorly in the submucosal lymphatics of the esophagus rather than distally into the stomach, and recurrent carcinoma within the gastric remnant is uncommon and seldom enough to produce recurrent dysphagia before the patient succumbs to metastatic disease. For all practical purposes, the traditional proximal hemigastrectomy, which has long been performed for carcinoma of the cardia, provides no better a cancer operation and wastes valuable stomach that can be used for esophageal replacement and a cervical esophagogastric anastomosis. It further commits the surgeon to an intrathoracic esophagogastric anastomosis with its potential attendant complications.
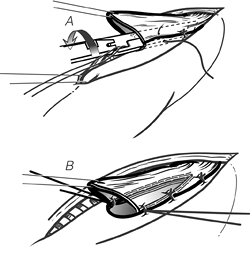 |
Fig. 132-16. A. The stapler is closed, thereby approximating the jaws, but before firing it, two suspension sutures between the anterior stomach and the adjacent esophagus are placed on either side. B. When the knife assembly of the stapler is advanced, the common wall between the esophagus and stomach is cut, and a 3-cm long side-to-side anastomosis created. Corner sutures are then placed at either side of the gastrotomy. From Orringer MB, Marshall B, Iannettoni MD: Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 119:277, 2000. With permission. |
When performing a THE for an esophagogastric junction tumor, the upper esophagus should not be divided until the surgeon is relatively certain that it is possible to preserve the entire greater curvature of the stomach in performance of the proximal gastrectomy. If the THE is performed first, and then the esophagogastric junction tumor is found to involve so much of the stomach that a proximal hemigastrectomy is required, gastric length is inadequate to reach to the neck, and the patient is left with a cervical esophagostomy and feeding tube, an unacceptable result of an operation being performed to relieve dysphagia.
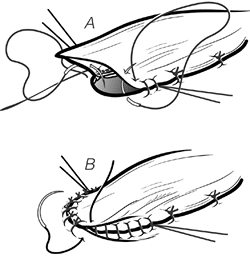 |
Fig. 132-17. The gastrotomy and remaining open esophagus are approximated in two layers: a running inner layer of 4 0 monofilament absorbable suture (A) and an outer interrupted layer (B), which incorporates the anterior wall of the upper esophagus. From Orringer MB, Marshall B, Iannettoni MD: Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 119:277, 2000. With permission. |
Results of Transhiatal Esophagectomy
Katariya and associates (1994) undertook a comprehensive collective review of the complications of THE in 1,353 patients culled from 23 papers published between 1981 and 1992. All but 12 of the operations were for cancer. Eighteen (1.3%) were converted to open thoracotomies for control of hemorrhage. Tracheal injuries occurred in nine (0.67%). The most common postoperative complications were thoracic or pulmonary, which the researchers grouped together as including pneumothoraces, pleural effusions, pneumonias, empyemas, and respiratory failure, and which occurred in nearly 50% of the patients. Such an inclusive complication category is of relatively little significance. The need for a chest tube because of entry into a pleural cavity is much less of a complication than pneumonia, empyema, or respiratory failure that prolongs hospitalization. Additional complications included anastomotic leak (15.1%), clinically detected recurrent laryngeal nerve injury (11.3%), cardiac arrhythmias, myocardial infarction or tamponade (11.9%), incidental splenectomy (2.6%), and chylothorax (0.7%). The
P.2015
30-day mortality rate for the 1,353 patients was 7.1%. As indicated previously, many of these reports represented the investigators' initial experience with THE.
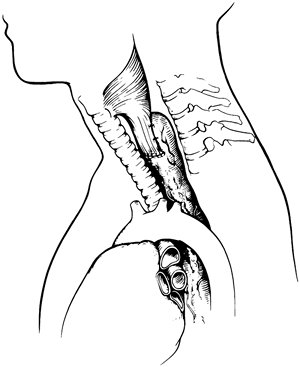 |
Fig. 132-18. Final position of the intrathoracic stomach in the neck after transhiatal esophagectomy and cervical esophagogastrostomy. The two sutures on either side of the anastomosis placed between the back of the cervical esophagus and the adjacent stomach limit tension on the anastomosis. These anterior suspension sutures are safer and preferable to tacking sutures between the stomach and the prevertebral fascia, which carry the risk of needle inoculation of the spine. From Iannettoni MD, Whyte RI, Orringer MB: Catastrophic complications of the cervical esophagogastric anastomosis. J Thorac Cardiovasc Surg 110:1493, 1995. With permission. |
Between 1976 and 1998, 1,085 patients with diseases of the intrathoracic esophagus underwent THE without thoracotomy at our institution, as recorded by myself and colleagues (1999). Two hundred eighty-five (26%) had benign disease necessitating esophageal replacement, and 800 (74%) had carcinoma (Table 132-2). The patients with benign disease ranged in age from 14 to 89 years (average 51 years), whereas the ages of those with carcinoma ranged from 28 to 92 years (average 64 years). Two hundred twenty-six (28%) of the patients with carcinoma were 70 years of age or older. Among the patients with carcinoma, 555 (69%) had adenocarcinomas (5 upper third, 53 middle third, and 497 lower third or cardia), and 225 (28%) had squamous cell carcinomas (28 upper, 121 middle, and 76 lower third). Twelve patients had adenosquamous carcinoma, 2 anaplastic, 2 poorly differentiated, 2 small cell, 1 undifferentiated, and 11 signet ring cell carcinoma. Barrett's mucosa was found in association with 287 (36%) of these carcinomas, including 275 adenocarcinomas, 7 squamous cell carcinomas, and 5 adenosquamous carcinomas.
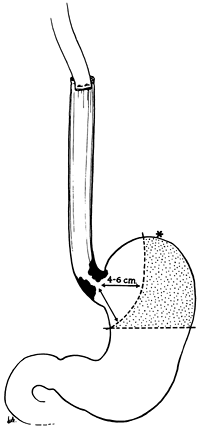 |
Fig. 132-19. Transhiatal esophagectomy for carcinoma of the cardia. Transection of the stomach along the lesser curvature 4 to 6 cm distal to palpable tumor preserves the point (asterisk) that reaches most cephalad and stomach that was formerly removed with a standard hemigastrectomy (stippled area). From Orringer MB, Sloan H: Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 76:643, 1978. With permission. |
Mediastinal inflammation from prior operations, perforations, or radiation therapy has not been a contraindication to THE. Two hundred thirty-four (29%) of the cancer patients had undergone radiation therapy before THE. One hundred forty-six (52%) of the patients with benign disease had undergone one or more prior esophageal operations or periesophageal operations, including antireflux repairs (85 patients), esophagomyotomy (60 patients), vagotomy (15 patients), esophagogastrostomy (4 patients), colonic interposition (4 patients), repair of perforation (3 patients), esophageal exclusion (3 patients), esophagoplasty (2 patients),
P.2016
laryngopharyngectomy (2 patients), proximal gastrectomy (3 patients), and sclerotherapy and resection of leiomyoma (1 patient each). Four patients with acute caustic injuries were treated with emergent THE, cervical esophagostomy, and feeding jejunostomy followed by delayed esophageal reconstruction 2 to 8 weeks later. One patient with a malfunctioning antiperistaltic retrosternal colonic bypass of a caustic esophageal stenosis underwent removal of the retrosternal colon, THE, and cervical esophagogastric anastomosis.
Table 132-2. Indications for Transhiatal Esophagectomy (1,085 Patients) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Esophageal resection and reconstruction were performed at the same operation in all but six patients, five with benign disease and one with carcinoma (Table 132-3). The stomach was used as the esophageal substitute in 782 (96%) of these patients who underwent immediate esophageal replacement. Six of the 25 patients with acute or chronic caustic injuries required either partial or total gastric resections. Colon was used to replace the esophagus in these latter six patients and in patients with carcinoma who had undergone prior gastric resection for peptic ulcer disease that precluded use of the stomach as an esophageal substitute. The posterior mediastinal route was used for the esophageal substitute in all but 20 patients, in whom either residual posterior mediastinal tumor or fibrosis and narrowing prevented adequate positioning of the stomach for a tension-free cervical anastomosis. The retrosternal route was used in these latter 20 patients. Removal of accessible subcarinal, periesophageal, and celiac axis lymph nodes was performed routinely in patients with carcinoma, but no attempt was made to perform an en bloc wide resection of the esophagus with its contiguous lymph node bearing tissues. Postsurgical tumor, node, metastasis (TNM) staging of these carcinomas demonstrated that 46% were either transmurally invasive into periesophageal adventitia or metastatic beyond regional lymph nodes (stage III or IV tumors) (Table 132-4).
Table 132-3. Esophageal Reconstruction after Transhiatal Esophagectomy (1,085 Patients) | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||
Three intraoperative deaths occurred from mediastinal hemorrhage that occurred during mobilization of the esophagus in one upper and two distal third carcinomas. Six patients experienced inordinate intraoperative bleeding, two intraabdominal associated with portal hypertension from cirrhosis, three from a torn azygos vein during mobilization of a midthird carcinoma, and one from splenic injury. Excluding these six patients with inordinate blood loss, measured intraoperative blood loss averaged 689 mL (Table 132-5). Entry into one or both pleural cavities was identified intraoperatively and treated with a chest tube(s) in 831 (77%) of the patients. Four intraoperative membranous tracheal lacerations occurred. Three involved the high membranous trachea and were repaired through a partial upper sternal split. One involved the membranous carina and required a right thoracotomy for repair. Thirty-four patients (3%) required a splenectomy because of intraoperative injury.
Five patients experienced mediastinal bleeding postoperatively that required thoracotomy for control within 24 hours of the procedure. Three had carcinoma and two had a
P.2017
megaesophagus of achalasia and were found to have arterial bleeding in the esophageal bed. Left recurrent laryngeal nerve injury causing hoarseness occurred in 74 patients (7%). This hoarseness was transient in 50 of these patients and resolved spontaneously within 2 to 12 weeks. Twenty-four patients (<1%) had true vocal cord paralysis, and seven of these required subsequent Teflon vocal cord injections. This complication can be virtually eliminated by avoiding placement of any retractor against the tracheoesophageal groove during the operation. Chylothorax occurred in 18 patients (<1%), 12 with carcinoma, and 6 with benign disease. This complication is managed best by aggressive transthoracic ligation of the injured thoracic duct within 7 to 10 days of the operation, as I and my associates (1988) recommended. The overall anastomotic leak rate after cervical esophagogastric anastomosis was 13%, and the leaks were more common in patients requiring retrosternal placement of the stomach as well as those with a history of prior operations at the gastroesophageal junction, which may have contributed to relative devascularization of the fundus during mobilization of the stomach for esophageal substitution. All but 9 of the 146 anastomotic leaks in this series were successfully treated by opening the cervical wound at the bedside and local packing until healing occurred. Necrosis of the upper stomach at the thoracic inlet necessitated take-down of the stomach from the chest and a cervical esophagostomy in nine patients.
Table 132-4. Postsurgical Tumor, Node, Metastasis (TNM) Staging of 800 Intrathoracic Esophageal Carcinomas | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 132-5. Measured Intraoperative Blood Loss with Transhiatal Esophagectomy | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
Among the 1,085 patients undergoing THE, 34 deaths occurred among 800 patients with carcinoma (4.2%) and 7 deaths among 285 patients with benign disease (2.4%), for an overall hospital mortality rate of 3.8% (41 deaths). There were three intraoperative deaths caused by hemorrhage. Overall, 49% of these patients were discharged within 10 days of the operation, 28% within 2 weeks, and 21% within 3 weeks. Six patients in this entire series died of respiratory insufficiency occurring early after THE. Of the 258 patients with benign esophageal disease treated by visceral esophageal substitution with stomach, the average follow-up is 44 months (range 1 213 months). Cervical esophagogastric anastomotic dilation is used liberally for any complaint of cervical dysphagia occurring during postoperative follow-up. With this liberal use of dilation therapy, of the 258 patients with benign disease undergoing esophageal replacement with stomach, only 56 (22%) have never required a postoperative esophageal dilation. At the time of their latest follow-up evaluation, however, 163 (63%) of these patients with benign disease are eating a regular unrestricted diet and have no dysphagia; 41 (16%) have occasional mild dysphagia that requires no treatment; 43 (17%) require an occasional esophageal dilation but swallow well between treatments; and 10 patients (4%) have severe dysphagia requiring regular dilations. One hundred fifty-seven (61%) have no regurgitation of gastric contents. Seventy-eight patients (30%) have occasional regurgitation if they eat a large meal and then assume a recumbent position or lie prone. They sleep comfortably in the horizontal position, however, on only one or two pillows, and do not regard their reflux as a significant problem. Nineteen patients (7%) have more regular and troublesome nocturnal regurgitation and therefore sleep with the head of their bed elevated. Two
P.2018
(<1%) of the patients have experienced pulmonary complications caused by aspiration. Postoperative dumping (postprandial nausea, cramping, diaphoresis, or diarrhea) has been mild, requiring no treatment in 28%, moderate and controlled with occasional medication in 10%, or severe or poorly controlled in 2%. Fifty-nine percent have experienced no dumping symptoms. The ability to eat comfortably after a cervical esophagogastric anastomosis for benign disease has generally been gratifying.
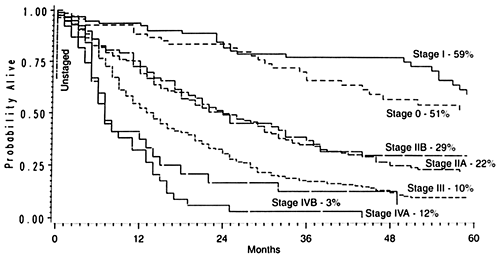 |
Fig. 132-20. Stage-dependent Kaplan-Meier actuarial survival curves in patients undergoing transhiatal esophagectomy for carcinoma of the intrathoracic esophagus and cardia. From Orringer MB, Marshall B, Iannettoni MD: Transhiatal esophagectomy clinical experience and refinements. Ann Surg 230:392, 1999. With permission. |
Of the 800 patients undergoing THE for carcinoma, 764 left the hospital alive. No follow-up was available on 31 patients (4%). Patients were followed for up to 195 months (mean 27 months). The overall 2-year Kaplan-Meier actuarial survival rate was 47%; the 5-year survival rate was 23%. Those with lower third or cardia carcinomas had a 5-year survival rate of 26% compared with 13% for those with middle third cancers, and 24% for those with upper third tumors. One unique subset of 217 patients received preoperative chemoradiation therapy prior to THE. Among these, 49 (23%) were complete responders (T0 N0 tumors on final pathology). The 2-year actuarial survival rate in this group was 86%; the 5-year rate, 48%. As expected, the stage of the resected tumor was an important determinant of survival after THE, those with stage 0 and I tumors living considerably longer than those with more advanced disease (Fig. 132-20, Table 132-6). The survival rate after THE for patients with adenocarcinoma was better than that after THE for squamous cell carcinoma. There was an overall statistically significant (p <0.01) survival advantage for adenocarcinoma; and this advantage approached statistical significance (p = 0.06) at 5 years, where the survival rate for those with adenocarcinoma was 24% and the survival rate for those with squamous cell carcinoma was 17% (Fig. 132-21).
Table 132-6. Kaplan-Meier Survival after Transhiatal Esophagectomy by Tumor Stage | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
The technique described of the stapled side-to-side cervical esophagogastric anastomosis has evolved over more than two decades after multiple attempts to reduce the anastomotic leak rate and other complications of this anastomosis. Although nearly 98% of cervical esophagogastric anastomotic leaks are managed relatively simply by open drainage, packing the neck wound, and jejunostomy tube feedings for nutritional support until healing is complete, some catastrophic complications associated with the cervical esophagogastric anastomosis have been reported by Iannettoni and associates (1995). Among these are gastric tip necrosis caused by ischemia of the upper 3 to 5 cm of the mobilized stomach above the thoracic inlet, and epidural abscess caused by bacterial seeding of the intervertebral disc from a suspension stitch between the stomach and cervical prevertebral fascia. Care must be taken in mobilizing the stomach to absolutely minimize trauma to the gastric tip, which will be anastomosed to the cervical esophagus. Traction sutures used to pull the stomach through the posterior mediastinum and into the neck wound should be avoided, and suspension sutures between the stomach and cervical prevertebral fascia are similarly now no longer used.
Despite interrupted, running, single- or double-layered manual anastomotic techniques, the postoperative anastomotic leak rate varies between 10% and 15%. Although the acute complications of a cervical esophagogastric anastomotic leak are clearly less morbid than those associated with an intrathoracic leak, the long-term sequelae are
P.2019
hardly inconsequential. Nearly 50% of cervical esophagogastric anastomotic leaks heal with a subsequent anastomotic stricture, and a need for life-long anastomotic dilatations represents a poor result of an esophageal resection and reconstruction intended to provide comfortable swallowing. I and my associates (2000) reported that the side-to-side stapled cervical esophagogastric anastomosis was used in more than 100 patients with a 2.7% leak rate, less need for postoperative anastomotic dilations, more comfortable swallowing, and greater patient satisfaction.
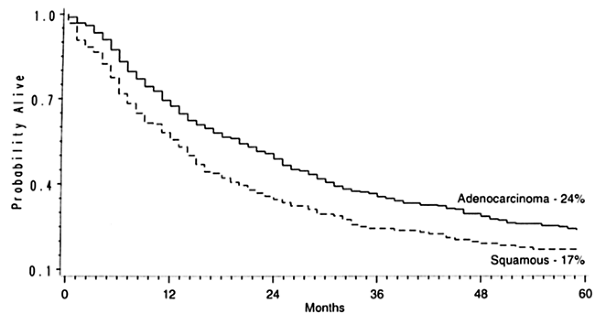 |
Fig. 132-21. Histology-dependent Kaplan-Meier survival curves in patients undergoing transhiatal esophagectomy for carcinoma of the intrathoracic esophagus and cardia. From Orringer MB, Marshall B, Iannettoni MD: Transhiatal esophagectomy clinical experience and refinements. Ann Surg 230:392, 1999. With permission. |
Most recently, minimally invasive video-assisted surgical techniques have been reported by Sadanaga and colleagues (1994), Swanstrom and Hansen (1997) and Luketich and associates (2000) as adjuncts to transhiatal dissection of the esophagus.
TRANSSTERNAL APPROACH TO THE CERVICOTHORACIC ESOPHAGUS
Anterior exposure of the low cervical and upper intrathoracic esophagus to the level of the carina is facilitated by performance of a partial upper median sternotomy (Fig. 132-22), an approach originally described by Waddell and Scannell (1957) and more recently used by myself (1984). This approach has utility in patients undergoing THE for middle or upper third esophageal malignancies that have shown no preoperative bronchoscopic evidence of tracheobronchial invasion. If transcervical and transhiatal mobilization of the esophagus, approaching the tumor from either end, suggests that there may be adherence of the tumor to the trachea, a partial anterior sternotomy allows direct visualization and sharp dissection of the esophagus from the posterior membranous trachea. After mobilizing the tumor away from the airway, the THE can be performed as described. This technique is also useful in elderly patients with cervical osteoarthritis that prevents them from extending the neck, or in obese patients with a short bull neck habitus that makes routine exposure of the cervical esophagus difficult. When positioning a patient for a THE, the cricoid cartilage is the anatomic landmark for the upper esophageal sphincter, and the length between the cricoid cartilage and the sternal notch represents the length of cervical esophagus that is available for the operation through a cervical incision. If the habitus of the patient prevents extension of the neck, and on palpation the cricoid cartilage is in close approximation to the suprasternal notch, there is an insufficient length of cervical esophagus, and extension of the cervical incision down onto the anterior chest and a partial upper sternotomy allow mobilization of the upper esophagus for the transhiatal resection and subsequent esophagogastric anastomosis. Among the 1,085 patients undergoing THE, a partial upper sternal split was required in 100 (9%), 37 with benign disease, and 63 with carcinoma, to gain access to the cervicothoracic esophagus that was hampered either by a bull-neck habitus, inability to extend the neck because of arthritis, or adherence of the tumor to the upper trachea.
Resection of the Cervicothoracic Esophagus
Resection of the cervicothoracic esophagus is most often required for malignancies of laryngotracheal, esophageal, or thyroid origin. The surgical treatment of these tumors may be complicated by the need to resect the larynx, either because of direct invasion by the tumor or because the proximal extent of the tumor necessitates division of the
P.2020
pharynx and reestablishment of alimentary continuity, which precludes salvage of the larynx. Similarly, severe caustic or radiation stenoses involving the esophageal introitus may leave no alternative but to divide the proximal pharynx where there is an adequate lumen and sacrifice the larynx if the ability to swallow is to be restored. Resection of the cervicothoracic esophagus may pose other challenges for the surgeon in addition to the need for a laryngopharyngectomy. At times, to achieve an adequate distal tracheal margin, the high retrosternal trachea must be divided, and it may be impossible to elevate the remaining trachea sufficiently out of the superior mediastinum and over the sternum for construction of a standard tracheostomy in the suprasternal notch. Furthermore, when a stomal recurrence after laryngectomy for carcinoma involves the cervicothoracic esophagus but is otherwise localized, resection of the proximal trachea and tracheostome with adjacent anterior cervical skin involved by tumor creates the added problem of coverage of the great vessels of the neck and superior mediastinum as well as establishment of a satisfactory airway. There has been considerable enthusiasm for the use of free jejunal grafts to replace the cervicothoracic esophagus, and experience with this technique has been reported by Jurkiewicz (1984), Kasai and Nishihira (1986), Sasaki and associates (1980), Wang and associates (1986), Coleman and associates (1987), Paletta and Jurkiewicz (1996), and Carlson and associates (1992). More recently, radial forearm free flaps for pharyngoesophageal reconstruction have been advocated by Colin and Haughey (1998). Advocates of total esophagectomy and esophageal replacement with either stomach or colon in these situations argue that a free jejunal graft, with its need for microvascular and multiple intestinal anastomoses, only further complicates an already technically demanding operation and may fail to achieve as adequate a distal esophageal margin.
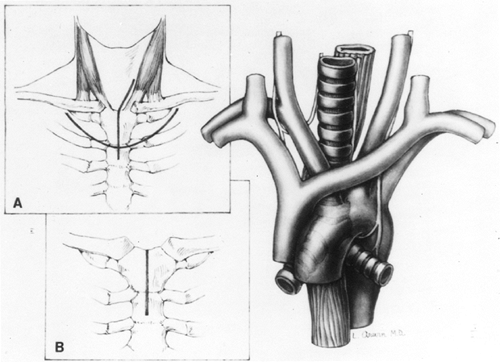 |
Fig. 132-22. Exposure of the upper mediastinum through a partial upper median sternotomy (main drawing). A. The usual left cervical skin incision used for transhiatal esophagectomy extends onto the anterior chest wall in the midline for a partial sternal split. Occasionally, a curved anterior thoracic skin incision with elevation of a skin flap can be used to avoid a scar in the low anterior neck. B. The sternotomy incision extending from the suprasternal notch through the manubrium. A small rib spreader inserted between the divided sternal edges provides the needed exposure to the cervicothoracic esophagus. From Orringer MB: Partial median sternotomy: anterior approach to the upper thoracic esophagus. J Thorac Cardiovasc Surg 87: 124, 1984. With permission. |
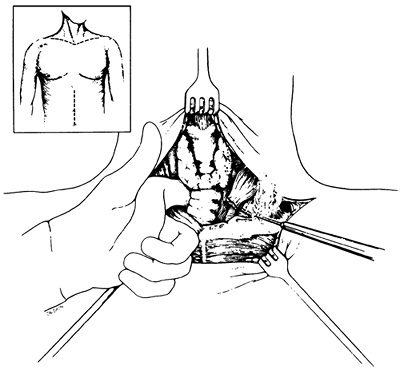 |
Fig. 132-23. Extended supraclavicular collar and supraumbilical upper midline abdominal incisions used for laryngopharyngoesophagectomy and pharyngogastric anastomosis for treatment of cervicothoracic esophageal carcinoma. Inset. Documentation that the tumor is free of the carotid sheaths and prevertebral fascia is obtained, and if the tumor requires resection of the retrosternal trachea, the sternocleidomastoid muscles are detached from their sternal and clavicular attachments. From Orringer MB: Anterior mediastinal tracheostomy with and without cervical exenteration. Ann Thorac Surg 54:628, 1992. With permission. |
At times, particularly in an elderly patient in whom loss of the larynx and the ability to communicate with speech may constitute more of a social disability, a localized cervical esophageal cancer may be resected by dividing the proximal esophagus virtually at the level of the cricopharyngeus sphincter within only 2 to 3 cm of the tumor. THE combined with a gastric pull-up and anastomosis to the residual upper sphincter may achieve adequate palliation. In patients with cervicothoracic esophageal malignancies, resection is facilitated by a partial median sternotomy that allows access to the upper thoracic esophagus as described by myself (1984) (see Fig. 132-22). When concomitant laryngopharyngectomy is required as well as division of the high retrosternal trachea, resection is greatly facilitated by removing the anterior thoracic breast plate, which includes the medial clavicles, upper manubrium of the sternum, and adjacent medial first, and occasionally second, ribs. This approach provides wide exposure of the superior mediastinum and its contents (Figs. 132-23 and 132-24) and division of the retrosternal trachea for construction of an anterior mediastinal tracheostomy. It was initially used by Sisson and associates (1962) and Grillo (1966) for patients undergoing anterior mediastinal tracheostomy for laryngotracheal pathology alone. However, it is also applicable in the aforementioned patients requiring a cervical exenteration (i.e., laryngopharyngoesophagectomy). Grillo and Mathisen (1990) have reported their series of 18 patients undergoing cervical exenteration, including 14 who required mediastinal tracheostomy. They emphasized concern about the possibility of innominate artery erosion postoperatively, a complication that has been associated with the need to bring the divided mediastinal trachea up and over the innominate artery to reach the anterior upper thoracic skin. They advocated prophylactic division of the innominate artery after preoperative aortic arch angiography and intraoperative encephalographic monitoring after clamping the vessel for 10 minutes before dividing it. They divided the artery in 7 of 14 patients undergoing anterior mediastinal tracheostomy. Mathisen and colleagues (1988a) have subsequently reported use of omental pedicles interposed between the mediastinal great vessels, trachea, and skin to minimize subsequent erosion of the great vessels. For patients undergoing anterior mediastinal tracheostomy and concomitant cervical exenteration for laryngeal, thyroid, or cervicothoracic esophageal malignancies, transposition of the divided trachea beneath and to the right of the innominate artery was emphasized as an important technical maneuver to avoid later innominate artery erosion in patients in whom the slightest concern exists regarding tension between
P.2021
the trachea and innominate artery (Fig. 132-25). This technique, as I reported (1992), was used in 10 of 34 patients undergoing cervical exenteration and concomitant anterior mediastinal tracheostomy at our institution. Reestablishment of alimentary continuity with a pharyngogastric anastomosis was used in almost every case. The operative mortality rate among these 34 patients was 18%, and the pharyngogastric anastomotic leak rate 29%, an incidence related both to the high reach required of the stomach as well as prior radiation therapy in many of these patients. In patients with tracheal stomal recurrence after prior laryngectomy or a malignant pharyngocutaneous fistula above an established tracheostome, a wide en bloc resection of anterior cervical skin adjacent to the proximal trachea and
P.2022
pharynx must be excised (Fig. 132-26). After removal of the 3- to 5-cm margin around the stoma and involved skin, a thoracoacromial flap of skin and subcutaneous tissue is elevated off the pectoralis muscle and rotated medially to resurface the skin over the lower neck and superior mediastinum. Iatrogenic hypoparathyroidism and hypothyroidism related to resection of the thyroid gland and adjacent parathyroid glands in the process of the cervical exenteration is common and occurred in nine patients. Anterior mediastinal tracheostomy combined with cervical exenteration, THE, and one-stage pharyngogastric anastomosis is a formidable technical undertaking. It may be difficult at times to justify such a procedure when long-term survival of patients with cervicothoracic esophageal and tracheal malignancies is seldom achieved. Nevertheless, this approach does provide efficient palliation and, occasionally, cure in these patients, and excellent results with acceptable morbidity can be achieved with careful patient selection, meticulous operative technique, and intensive postoperative care.
 |
Fig. 132-24. Resection of the anterior breast plate in preparation for resection of cervicothoracic esophageal carcinoma and construction of an anterior mediastinal tracheostomy. A. The breast plate includes the medial clavicles, adjacent first ribs, and upper sternum, and occasionally the medial second ribs as well (dotted lines). B. The trachea is divided obliquely leaving the posterior membranous portion, which must reach furthest anteriorly to the skin (asterisk) as long as possible. From Orringer MB: Anterior mediastinal tracheostomy with and without cervical exenteration. Ann Thorac Surg 54:628, 1992. With permission. |
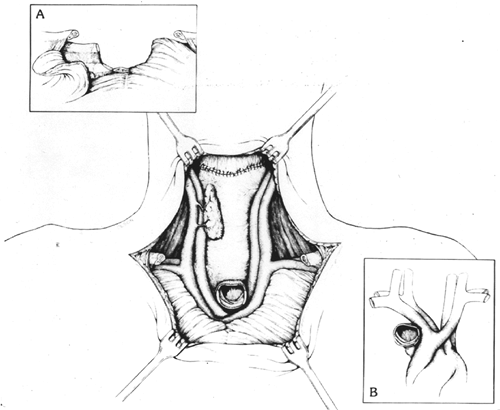 |
Fig. 132-25. Completed pharyngogastric anastomosis after cervical exenteration. One thyroid lobe and adjacent parathyroid tissue are preserved whenever possible (main illustration). A. The reflected pectoralis major muscle is used to cover the edges of the divided bony chest wall. B. Transposition of the tracheal stump beneath and to the right of the innominate artery is used whenever the least bit of pressure is on the innominate artery by the overlying trachea. This eliminates tension on the vessel when the trachea is sutured to the skin and the potential for subsequent tracheoinnominate artery erosion. From Orringer MB: Anterior mediastinal tracheostomy with and without cervical exenteration. Ann Thorac Surg 54:628, 1992. With permission. |
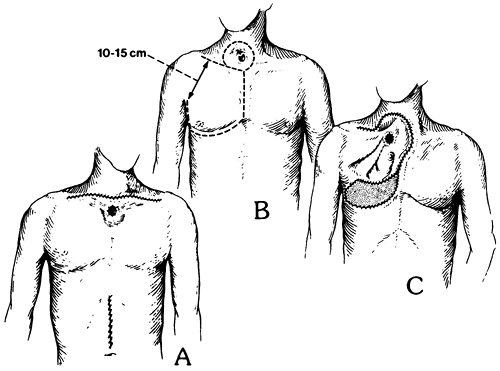 |
Fig. 132-26. A. Extended collar incision, anterior mediastinal tracheostomy, and upper midline abdominal incision after cervical exenteration, transhiatal esophagectomy, and pharyngogastric anastomosis. The tracheostome is often incorporated into the cervical incision as it is closed, rather than creating a separate stoma. B. When a tracheal stomal recurrence necessitates resection of the surrounding anterior cervical skin as well as the adjacent esophagus, a thoracoacromial nipple flap (dotted line) based on the thoracoacromial artery and preserving at least a 10- to 15-cm wide base is elevated and used for later reconstruction. C. After completion of the laryngopharyngoesophagectomy and the pharyngogastric anastomosis, the thoracoacromial flap is rotated and used to resurface the skin of the anterior neck and superior mediastinum. The mediastinal tracheostomy as well as a split-thickness skin graft used to resurface the anterior thorax are shown. From Orringer MB: Anterior mediastinal tracheostomy with and without cervical exenteration. Ann Thorac Surg 54:628, 1992. With permission. |
Lam and associates (1981) from Hong Kong reported one of the world's largest series of pharyngogastric anastomosis after laryngopharyngoesophagectomy in 157 patients, 67 with carcinoma of the hypopharynx, 7 with carcinoma of the cervical esophagus, and 83 with carcinoma of the larynx involving the hypopharynx. The hospital mortality rate was 31%, and the anastomotic leak rate was 23%, further attesting to the substantial magnitude of these operations. Only two innominate artery erosions occurred in this series, and these researchers did not use an anterior mediastinal tracheostomy in their patients.
Although a pharyngogastric anastomosis is technically feasible in most North American white patients, because of the maximum cephalad reach required of the stomach, pharyngogastric anastomotic disruption remains a significant postoperative problem in these patients. Furthermore, from a functional standpoint, the long-term results of a pharyngogastric anastomosis are not nearly as good as with a standard cervical esophagogastric anastomosis. In the latter, the upper esophageal sphincter is preserved and subsequent regurgitation from the intrathoracic stomach is a relatively minor problem. After a pharyngogastric anastomosis, however, with the upper esophageal sphincter resected, postoperative regurgitation during coughing, bending, or reclining is a major problem.
REFERENCES
Adams WE, Phemister DB: Carcinoma of the lower thoracic esophagus: report of a successful resection and esophagogastrostomy. J Thorac Surg 7:621, 1938.
Akiyama H, et al: Principles of surgical treatment for carcinoma of the esophagus: analysis of lymph node involvement. Ann Surg 194:438, 1981.
Averbach A, et al: Results of curative therapy for esophageal cancer in a community training hospital. Int Surg 87:31, 2002.
Beik AI, Jaffray B, Anderson JR: Transhiatal oesophagectomy: a comparison of alternative techniques in 68 patients. J R Coll Surg Edinb 25:25, 1996.
Berdejo L: Transhiatal versus transthoracic esophagectomy for clinical stage I esophageal carcinoma. Hepatogastroenterology 42:789, 1995.
Block MI, et al: Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg 64:770, 1997.
Bolton JS, Ochsner JL, Abdoh A: Surgical management of esophageal cancer: a decade of change. Ann Surg 219:475, 1994.
Bonavina L: Early oesophageal cancer: results of a European multicentre survey. Br J Surg 82:98, 1995.
Bousamra M 2nd, Haasler GB, Parviz M: A decade of experience with transthoracic and transhiatal esophagectomy. Am J Surg 183:162, 2002.
Boyle, MJ, et al: Transhiatal versus transthoracic esophagectomy: complication and survival rates. Am Surg 65:1137, 1999.
Carlson GW, Schusterman MA, Guillamondegui OM: Total reconstruction of the hypopharynx and cervical esophagus: a 20 year experience. Ann Plast Surg 29:408, 1992.
Carter BN: The combined thoraco-abdominal approach with particular reference to its employment in splenectomy. Surg Gynecol Obstet 84:1019, 1947.
Chu KM, et al: A prospective randomized comparison of transhiatal and transthoracic resection for lower-third esophageal carcinoma. Am J Surg 174:320, 1997.
Churchill ED, Sweet RH: Transthoracic resection of tumors of the stomach and esophagus. Ann Surg 115:897, 1942.
Coleman JJ III, et al: Ten years experience with the free jejunal autograft. Am J Surg 154:394, 1987.
Colin WB, Haughey BH: Pharyngoesophageal reconstruction, the method of Bruce H. Haughey. In Gates G (ed): Current Therapy in Otolaryngology Head and Neck Surgery. 6th Ed. St. Louis: Mosby-Year Book, 1998, pp. 285 288.
de Boer AG, et al: Transhiatal vs. extended transthoracic resection in oesophageal carcinoma: patients' utilities and treatment preferences. Br J Cancer 86:851, 2002.
P.2023
Denk W: Zur Radikaloperation des Oesophaguskarfzentralbl. Chirurgie 40:1065, 1913.
Doty JR, et al: Postesophagectomy morbidity, mortality, and length of hospital stay after preoperative chemoradiation therapy. Ann Thorac Surg 74:227, 2002.
Dudhat SB, Shinde SR: Transhiatal esophagectomy for squamous cell carcinoma of the esophagus. Dis Esophagus 11:226, 1998.
Earlam R, Cunha-Melo JR: Oesophageal squamous cell carcinoma. I. A critical review of surgery. Br J Surg 67:381, 1980.
Ellis FH Jr: Carcinoma of the esophagus. CA Cancer J Clin 33:264, 1983.
Garlock JH: Combined abdominothoracic approach for carcinoma of cardia and lower esophagus. Surg Gynecol Obstet 83:737, 1946.
Gertsch P, et al: Long-term results of transhiatal esophagectomy for esophageal carcinoma. A multivariate analysis of prognostic factors. Cancer 72:2312, 1993.
Gillinov AM, Heitmiller RF: Strategies to reduce pulmonary complications after transhiatal esophagectomy. Dis Esophagus 11:43, 1998.
Giuli R, Gignoux M: Treatment of carcinoma of the esophagus. Retrospective study of 2400 patients. Ann Surg 192:44, 1980.
Gluch L, et al: Comparison of outcomes following transhiatal or Ivor Lewis esophageactomy for esophageal carcinoma. World J Surg 23:271, 1999.
Goldminc M, et al: Oesophagectomy by transhiatal approach or thoracotomy: a prospective randomized trial. Br J Surg 80:367, 1993.
Grillo HC: Terminal or mural tracheostomy in the anterior mediastinum. J Thorac Cardiovasc Surg 51:422, 1966.
Grillo HC, Mathisen DJ: Cervical exenteration. Ann Thorac Surg 49:401, 1990.
Gupta NM: Oesophagectomy without thoracotomy: first 250 patients. Eur J Surg 162:455, 1996.
Hagen JA, Peters JH, DeMeester TR: Superiority of extended en bloc esophagectomy for carcinoma of the lower esophagus and cardia. J Thorac Cardiovasc Surg 106:850, 1993.
Heitmiller RF, Gillinov AM, Jones B: Transhiatal herniation of colon after esophagectomy and gastric pull-up. Ann Thorac Surg 63:534, 1997.
Hiele M, et al: Relation between endoscopic ultrasound findings and outcome of patients with tumors of the esophagus or esophagogastric junction. Gastrointest Endosc 45:381, 1997.
Horstmann O, et al: Transhiatal oesophagectomy compared with transthoracic resection and systematic lymphadenectomy for the treatment of oesophageal cancer. Eur J Surg 161:557, 1995.
Hulscher JB, et al: Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 347:1662, 2002.
Hulscher JBF, et al: Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 72:306, 2001.
Iannettoni MD, Whyte RI, Orringer MB: Catastrophic complications of the cervical esophagogastric anastomosis. J Thorac Cardiovasc Surg 110:1493, 1995.
Jacobi CA, et al: Surgical therapy of esophageal carcinoma: the influence of surgical approach and esophageal resection on cardiopulmonary function. Eur J Cardiothorac Surg 11:32, 1997.
Junginger T, Dutkowski P: Selective approach to the treatment of oesophageal cancer. Br J Surg 83:1473, 1996.
Jurkiewicz MJ: Reconstructive surgery of the cervical esophagus. J Thorac Cardiovasc Surg 88:893, 1984.
Kasai M, Nishihira T: Reconstruction using pedicled jejunal segments after resection for carcinoma of the cervical esophagus. Surg Gynecol Obstet 163:145, 1986.
Katariya K, et al: Complications of transhiatal esophagectomy. J Surg Oncol 57:157, 1994.
Lam KH, et al: Pharyngogastric anastomosis following pharyngolaryngoesophagectomy. Analysis of 157 cases. World J Surg 5:509, 1981.
LeQuesne LP, Ranger D: Pharyngogastrectomy with immediate pharyngogastric anastomosis. Br J Surg 53:105, 1966.
Lewis I: The surgical treatment of carcinoma of the esophagus with special reference to a new operation for growths of the middle third. Br J Surg 34:18, 1946.
Llobet AF. L'operation de Nassilov: la premi re intervention Buenos Aires. (Nassilous operation: first in Buenos Aires.) Rev Chir Paris 21: 647, 1900. (Cited by Saint JH: Arch Surg 19:53, 1929.)
Luketich JD, et al: Role of positron emission tomography in staging esophageal cancer. Ann Thorac Surg 64:765, 1997.
Luketich JD, et al: Minimally invasive esophagectomy. Ann Thorac Surg 70:906, 2000.
Marshall SF: Carcinoma of the esophagus. Surg Clin North Am 18:643, 1938.
Mathisen DJ, et al: The omentum in the management of complicated cardiothoracic problems. J Thorac Cardiovasc Surg 95:677, 1988a.
Mathisen DJ, et al: Transthoracic esophagectomy: a safe approach to carcinoma of the esophagus. Ann Thorac Surg 45:137, 1988b.
McKeown KC: Trends in oesophageal resection for carcinoma, with special reference to total oesophagectomy. Ann R Coll Surg Engl 51:213, 1972.
McKeown KC: Total three-stage oesophagectomy for cancer of the esophagus. Br J Surg 63:259, 1976.
Millikan KW, et al: A 15-year review of esophagectomy for carcinoma of the esophagus and cardia. Arch Surg 130:617, 1995.
Mitchell RL: Abdominal and right thoracotomy approach as standard procedure for esophagogastrectomy with low morbidity. J Thorac Cardiovasc Surg 93:205, 1987.
Muller JM, et al: Surgical therapy of oesophageal carcinoma. Br J Surg 77:845, 1990.
Naunheim KS, et al: Esophagectomy in the septuagenarian. Ann Thorac Surg 56:880, 1993.
Ohsawa T: The surgery of the esophagus. Arch Jpn Chir 10:605, 1933.
Ong GB: Resection and reconstruction of the esophagus. Curr Probl Surg 8:1, 1971.
Ong GB, Lee TC: Pharyngogastric anastomosis after oesophagopharyngectomy for carcinoma of the hypopharynx and cervical oesophagus. Br J Surg 48:193, 1960.
Orringer MB: Transhiatal esophagectomy. In Dudley H, Pories WJ, Carter D (eds): Rob and Smith's Operative Surgery. 4th Ed. London: Butterworth-Heinemann, 1983a, p. 192.
Orringer MB: Transhiatal blunt esophagectomy without thoracotomy. In Cohn LH (ed): Modern Technics in Surgery Cardiothoracic Surgery. New York: Futura, 1983b, p. 62.
Orringer MB: Partial median sternotomy: anterior approach to the upper thoracic esophagus. J Thorac Cardiovasc Surg 87:124, 1984.
Orringer MB: Surgical options for esophageal resection and reconstruction with stomach. In Baue AE, et al (eds): Glenn's Thoracic and Cardiovascular Surgery. 5th Ed. Norwalk, CT: Appleton&Lange, 1991, p. 787.
Orringer MB: Anterior mediastinal tracheostomy with and without cervical exenteration. Ann Thorac Surg 54:628, 1992.
Orringer MB, Bluett M, Deeb GM: Aggressive treatment of chylothorax complicating transhiatal esophagectomy without thoracotomy. Surgery 104:720, 1988.
Orringer MB, Marshall B, Iannettoni MD: Transhiatal esophagectomy: clinical experience and refinements. Ann Surg 230:392, 1999.
Orringer MB, Marshall B, Iannettoni MD: Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 119:277, 2000.
Orringer MB, Marshall B, Iannettoni MD: Transhiatal esophagectomy for treatment of benign and malignant esophageal disease. World J Surg 25:196, 2001.
Orringer MB, Sloan H: Substernal gastric bypass of the excluded esophagus for palliation of esophageal carcinoma. J Thorac Cardiovasc Surg 70:836, 1975.
Orringer MB, Sloan H: Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 76:643, 1978.
Orringer MB, Sloan H: Esophageal replacement after blunt esophagectomy. In Nyhus LM, Baker RJ (eds): Mastery of Surgery. Boston: Little, Brown, 1984, p. 426.
Pac M, et al: Transhiatal versus transthoracic esophagectomy for esophageal cancer. J Thorac Cardiovasc Surg 106:205, 1993.
Paletta CE, Jurkiewicz MJ: Esophageal replacement: microvascular jejunal transplantation. In Baue AE, et al (eds): Glenn's Thoracic and Cardiovascular Surgery. 6th Ed. Norwalk, CT: Appleton&Lange, 1996, pp. 931 938.
Pommier RF, et al: Relationships between operative approaches and outcomes in esophageal cancer. Am J Surg 175:422, 1998.
Posthlethwait RW: Complications and deaths after operations for esophageal carcinoma. J Thorac Cardiovasc Surg 85:827, 1983.
Putnam JB Jr, et al: Comparison of three techniques of esophagectomy within a residency training program. Ann Thorac Surg 57:319, 1994.
Quint LE, et al: Esophageal carcinoma: CT findings. Radiology 155:171, 1985.
Rao YG, et al: Transhiatal esophagectomy for benign and malignant conditions. Am J Surg 184:136, 2002.
P.2024
Reed CE, et al: Esophageal cancer staging: improved accuracy by endoscopic ultrasound of celiac lymph nodes. Ann Thorac Surg 67:319, 1999.
Rehn L: Operationen an dem Brustabschnitt der Speiserohre. (Operations on the esophagus in the region of the chest.) Verh Deutsch Ges Chir 27:448, 1898 (Cited by Saint JH: Arch Surg 19:53, 1929).
Reich H, Lo AY, Harvey JC: Diaphragmatic herniation following transhiatal esophagectomy. Scand J Thorac Cardiovasc Surg 30:101, 1996.
Rentz J, et al: Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg 125:1114, 2003.
Rice TW, et al: Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg 65:787, 1998.
Sadanaga N, et al: Laparoscopy-assisted surgery: a new technique for transhiatal esophageal dissection. Am J Surg 168:355, 1994.
Sasaki TM, et al: Free jejunal graft reconstruction after extensive head and neck surgery. Am J Surg 139:650, 1980.
Sisson GA, Straehley CJ Jr, Johnson NE: Mediastinal dissection for recurrent cancer after laryngectomy. Laryngoscope 72:1064, 1962.
Stark SP, et al: Transhiatal versus transthoracic esophagectomy for adenocarcinoma of the distal esophagus and cardia. Am J Surg 172:478, 1996.
Svanes K, et al: Morbidity, ability to swallow, and survival after oesophagectomy for cancer of the oesophagus and cardia. Eur J Surg 161:669, 1995.
Swanstrom LL, Hansen P: Laparoscopic total esophagectomy. Arch Surg 132:943, 1997.
Thomas P, et al: Changing patterns and surgical results in adenocarcinoma of the oesophagus. Br J Surg 84:119, 1997.
Tilanus HW, et al: Esophagectomy with or without thoracotomy. Is there any difference? J Thorac Cardiovasc Surg 105:898, 1993.
Torek F: The operative treatment of carcinoma of the oesophagus. Ann Surg 61:385, 1915.
Torres AJ, et al: Two-field radical lymphadenectomy in the treatment of esophageal carcinoma. Dis Esophagus 12:137, 1999.
Turner GG: Excision of the thoracic oesophagus for carcinoma with construction of extrathoracic gullet. Lancet 2:315, 1933.
Uravic M, et al: Transhiatal esophagectomy for carcinoma of the esophagus our ten years experience. Zentralbl Chir 127:956, 2002.
van Sandick JW, et al: Transhiatal esophagus resection without thoracotomy for carcinoma: complications, hospital mortality and prognosis in 115 patients. Ned Tijdschr Geneeskd 144:2061, 2000.
Vigneswaran WT, et al: Transhiatal esophagectomy for carcinoma of the esophagus. Ann Thorac Surg 56:838, 1993.
Waddell WR, Scannell JG: Anterior approach to carcinoma of the superior mediastinal and cervical segments of the esophagus. J Thorac Surg 33:663, 1957.
Wang ID Sun YE, Chen Y: Free jejunal grafts for reconstruction of pharynx and cervical esophagus. Ann Otol Rhinol Laryngol 95:348, 1986.
EAN: 2147483647
Pages: 203