113 - Novel Systemic Therapy for Advanced Non-Small-Cell Lung Cancer
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Esophagus > Section XXI - Operative Procedures in the Management of Esophageal Disease > Chapter 130 - Modified Heller Esophagomyotomy
Chapter 130
Modified Heller Esophagomyotomy
John M. Streitz Jr.
Esophageal achalasia is a benign condition of unknown etiology characterized by aperistalsis of the esophageal body and a high-amplitude lower esophageal sphincter (LES) that fails to relax completely during swallowing. These features result in a functional esophageal obstruction at the LES and the typical symptoms of dysphagia and regurgitation of undigested food. Although medical treatment aimed at smooth muscle relaxation occasionally improves symptoms in early cases, as noted by Coccia and colleagues (1991), effective and long-lasting relief of symptoms requires the mechanical disabling of the LES, either by forceful endoscopic dilation or surgical esophagomyotomy.
The two most important controversies today concerning the treatment of achalasia are whether esophagomyotomy or forceful dilation should be the initial form of therapy, and whether a limited myotomy alone or a complete myotomy with an associated antireflux procedure represents the better surgical treatment. There is considerable clinical evidence to support all three forms of therapy (dilation, limited myotomy, and myotomy with antireflux procedure), as well as considerable controversy. Each can be considered an acceptable choice as primary treatment of achalasia. With recent advances in laparoscopic surgery, a trend toward laparoscopic myotomy has supplanted the transthoracic approach in many centers. Nonetheless, the transthoracic, limited esophagomyotomy without an antireflux procedure, as described by Ellis (1993) and colleagues (1967), remains an effective treatment for achalasia in most cases, especially when laparoscopy is not feasible due to scarring from prior abdominal surgery, or when other considerations require a thoracic approach, such as resection of an epiphrenic diverticulum or the need to extend the myotomy proximally in cases of vigorous achalasia. It is a safe, simple procedure with a high long-term success rate and a low incidence of postoperative gastroesophageal reflux (GER), and should be in the repertoire of all thoracic surgeons.
HISTORICAL ASPECTS
Achalasia has been recognized for more than three centuries. The only treatment available until early in the last century was bougienage. In 1674, Willis is reported by Ellis and Olsen (1969) to have provided his patient with a whalebone to the end of which he attached a button of sponge with which the patient performed periodic esophageal dilations, apparently with success for many years. This early success with bougienage was probably unusual. Nevertheless, dilation remained the only therapy for this disorder until the beginning of the 20th century, when forceful dilation with expanding dilators, both mechanical and inflatable, was introduced by Von Mikulicz (1903) and Russel (1898).
Operations to widen or bypass the narrowed distal esophagus were developed early in the 20th century, including retrograde dilation of the cardia through a gastrotomy and esophageal resection. Barrett and Franklin (1949) recorded that cardioplasty and esophagogastrostomy enjoyed particular favor initially because of early good results. They noted, however, that later results showed that severe reflux symptoms and esophagitis were almost inevitable sequelae of these two procedures.
In 1914, Heller published the results of his first esophagomyotomy for cardiospasm using paired anterior and posterior incisions, which included the distal 8 cm of the esophagus but ended at the esophagogastric junction. As noted by Payne (1989), the success of this and subsequent cases gradually permitted myotomy to supplant the more radical bypass and sphincter-destroying procedures. Heller's operation, which Zaaijer (1923) modified mainly by the omission of the posterior myotomy, is the operation of choice for the surgical treatment of esophageal achalasia today.
PREOPERATIVE EVALUATION
As with any esophageal operation, the successful surgical management of achalasia depends on an accurate diagnosis.
P.1976
Advanced cases of achalasia with long-standing symptoms and esophageal dilation on radiographic examination present little diagnostic challenge (Fig. 130-1). Early cases, however, may be difficult to distinguish from peptic stricture, scleroderma, and malignant obstruction on clinical and radiographic grounds alone. In addition, achalasia must be distinguished from its vigorous form and from hypertensive LES and functional swallowing disorders (see Chapters 125 and 141). Therefore, esophageal manometry and endoscopy are essential preoperative diagnostic tests. The typical manometric findings consist of simultaneous, low-amplitude contractions in the body of the esophagus, and an LES usually of higher than normal amplitude, which fails to relax completely during swallowing. Although weight loss is a common feature of the disease, most people today seek attention before they have suffered severe nutritional derangement, and therefore preoperative nutritional support is rarely necessary.
 |
Fig. 130-1. Contrast radiograph shows the typical features of achalasia: a dilated, aperistaltic esophageal body and a tapered distal narrowing, or bird's beak appearance, at the gastroesophageal junction. |
CHOICE OF THERAPY
The choice of forceful dilation or esophagomyotomy as initial therapy for patients with achalasia is controversial. Proponents of dilation support its use because it is effective in two thirds of cases, is easily and safely performed, and is less invasive and less expensive than myotomy, as emphasized by Richter (1991). O'Connor and colleagues (2002) have demonstrated a cost-effectiveness advantage for initial forceful dilation. They point out that although surgical myotomy is more effective, its high initial expense makes it less cost effective than endoscopic therapy. In a decision-analysis model comparing surgical and endoscopic therapy, Urbach and colleagues (2001) showed that long-term results were slightly better with surgical myotomy than with endoscopic dilation, but that the difference was small enough that patient preference and local expertise should be taken into account when choosing a treatment strategy.
Supporters of myotomy as initial therapy argue that it is effective in 90% of cases, and that improvement in most cases is long lasting, whereas dilation must be repeated in many patients because of recurrent symptoms, and dilation carries a 1% to 5% risk for esophageal perforation and a mortality rate of 0.5%, compared with a 0.2% mortality rate for myotomy, as recorded by Ellis (1976) and Okike and colleagues (1979).
Only a few comparisons of the two techniques performed at the same institution have been published, the results of which are listed in Table 130-1. The study by Csendes and colleagues (1981) remains the only prospective randomized evaluation, and Okike and colleagues (1979) summarized the extensive experience at the Mayo Clinic with both forms of therapy. Both studies reinforce the finding of collective reviews, such as that of Ferguson (1991), that myotomy provides better long-term relief of symptoms than forceful dilation. An unsuccessful forceful dilation has not adversely affected the results obtained with subsequent open esophagomyotomy, however, and for that reason, some physicians have routinely advised forceful dilation as the initial form of therapy. With the emergence of laparoscopic esophagomyotomy, reports of higher mucosal perforation rates and poorer results of surgery following unsuccessful forceful dilation have appeared, such as those by Morino (1997), Bell (1997), and Peillon (2001) and their colleagues. [These findings are in contrast to the report by Dolan and co-workers (2002), who showed no difference in outcome for patients who underwent surgery after pneumatic dilation.] Prior botulinum toxin (Botox, Allergan, Inc., Irvine, CA, U.S.A.) injection also appears to increase the surgical
P.1977
perforation rate, due to inflammation at the site of injection, making dissection of the mucosa away from the muscle layer difficult, as noted by Sharp and associates (2002). In light of these findings, many surgeons, such as Suarez and co-workers (2002), now favor a primary laparoscopic esophagomyotomy as initial therapy rather than forceful dilation or Botox injection.
Table 130-1. Results of Forceful Dilation versus Esophagomyotomy | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||
In certain cases the proper choice of initial therapy is clear. Patients with severe medical illness, who are not suitable surgical candidates, should undergo initial endoscopic therapy. Surgery is clearly indicated for those patients with associated conditions requiring operation, such as epiphrenic diverticulum and paraesophageal hernia, and for patients with vigorous achalasia who require proximal extension of the esophagomyotomy for relief of pain as well as dysphagia. Patients with preoperative reflux symptoms, shown by pH testing to have GER, can be expected to have worsening of their reflux following weakening of the LES by forceful dilation, and these patients are better treated with myotomy and partial fundoplication.
TECHNIQUE OF OPERATION
The transthoracic, modified Heller esophagomyotomy is performed through a left posterolateral thoracotomy, entering the chest through the seventh intercostal space or the bed of the unresected eighth rib. The rib may be divided posteriorly to improve exposure, although this is usually unnecessary. The use of a double-lumen endotracheal tube allows deflation of the left lung, after which the mediastinal pleura is opened longitudinally, anterior to the descending aorta. The distal esophagus is mobilized, encircled with a Penrose drain, and elevated out of the mediastinum (Fig. 130-2). Gentle traction on the esophagus elevates the gastroesophageal junction into view through the hiatus without any division of the phrenoesophageal attachments. Incision of the diaphragm or enlargement of the hiatus is unnecessary.
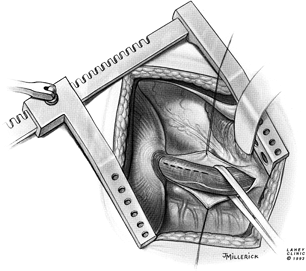 |
Fig. 130-2. Exposure of the esophagus for esophagomyotomy. Thoracotomy is through the seventh interspace or the bed of the eighth rib. The mediastinal pleura has been opened and the esophagus is encircled. Proposed site of myotomy is indicated. |
With the esophagus stretched by traction from the left thumb over the supporting index finger beneath, a scalpel is used to begin the esophagomyotomy (Fig. 130-3). The incision is started on the left anterolateral surface of the esophagus several centimeters above the gastroesophageal junction, avoiding the left vagus nerve. Pressure exerted on the muscle layers from the index finger below causes the muscle edges to separate as they are divided, revealing the smooth submucosal layer beneath the divided fibers of the circular muscle layer.
As the myotomy is extended caudally, the thick-walled muscle of the esophageal body, hypertrophied from chronic distal obstruction, gives way to the thinner muscle layers in the region of the physiologic LES. The myotomy is carried through the LES zone, ending a short distance onto the stomach, usually about 0.5 cm. Jara and colleagues (1979) showed that extension of the myotomy more than 2 cm onto the stomach results in reflux in 100% of cases. Ellis and associates (1967) stated that limiting the myotomy to less than 1 cm onto the stomach reduces the LES pressure enough to relieve dysphagia but leaves a short intraabdominal high-pressure zone that prevents reflux. Precise identification of the gastroesophageal junction is difficult using external landmarks; however, the gastric submucosa has a distinct appearance that allows relatively precise division of the distal muscular layers of the esophagus. As the myotomy is carried
P.1978
caudally, the smooth and pale esophageal submucosa abruptly meets the distinct transverse veins of the more vascular gastric submucosa (Fig. 130-4). The myotomy is extended 3 to 5 mm beyond this point. The myotomy is extended cephalad 5 to 7 cm above the gastroesophageal junction through the hypertrophied esophageal muscle, far enough proximally to ensure complete division of the LES. This extension can be performed easily using a scissors to dissect between the submucosa and muscular layers (Fig. 130-5). The muscle is then dissected off of the submucosa over one half of the esophageal circumference to separate the cut muscle edges 180 degrees in order to prevent their reapproximation and the recurrence of symptoms. The esophageal mucosa bulges out between the separated muscle edges, confirming that all circular muscle fibers have been divided. Inadvertent incision of the mucosa is unusual, but repair of a perforation is easily performed using a running or interrupted absorbable suture. A partial fundoplication as a buttress over the injured area may be performed, but it is not usually necessary.
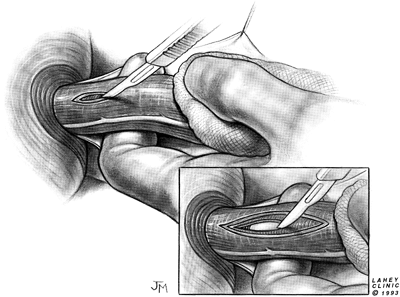 |
Fig. 130-3. Esophagomyotomy is begun with a scalpel. The esophagus is stretched gently over the supporting index finger and the muscle edges separate as they are divided. |
 |
Fig. 130-4. Distal extent of esophagomyotomy. Incision of the muscle layers ends 3 to 5 mm beyond the transition to gastric submucosa, which is marked by the presence of prominent transverse veins. |
When tension on the esophagus is released after completion of the myotomy, the gastroesophageal junction returns to its normal subdiaphragmatic position (Fig. 130-6). The mediastinal pleura is closed with a running suture, and the chest is closed routinely. A single posterior chest tube is left for pleural drainage. This is usually removed on the first postoperative day.
POSTOPERATIVE MANAGEMENT
Mortality is unusual with this operation, averaging less than 0.2% in a collective review by Andreollo and Earlam (1987) of over 5,000 procedures. Recovery from surgery usually is uncomplicated. The pleural tube is removed on the first postoperative morning, and a liquid diet is begun, with solid food added as tolerated. Most patients are ready to leave the hospital within 3 to 4 days.
POSTOPERATIVE COMPLICATIONS
The most frequent complications are those seen with any thoracic operation: atelectasis, wound infection, pneumonia, or phlebitis. Early postoperative complications specifically related to myotomy are uncommon. Empyema may occur, which in almost all cases is due to esophageal leakage
P.1979
from unrecognized mucosal injury at the time of myotomy. Paraesophageal hiatus hernia is an unusual complication of esophagomyotomy, accounting for less than 1% of such hernias, as noted by myself and Ellis (1990). When it does occur, Fletcher (1978), among others, advises that repair of the hernia should be undertaken promptly because of the possibility of incarceration and strangulation, a risk similar to that seen with primary paraesophageal hernia. Risk for herniation can be minimized by gentle retraction on the esophagus and avoidance of any disruption of the phrenoesophageal attachments. If the latter occurs, postoperative GER may result.
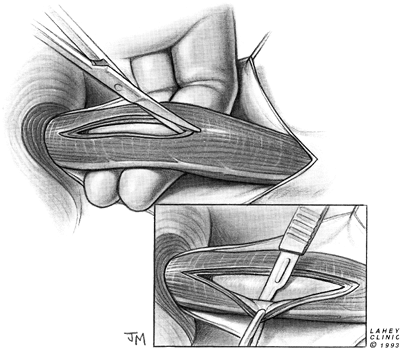 |
Fig. 130-5. Myotomy is extended cephalad, and the muscle is dissected off of the esophageal submucosa. |
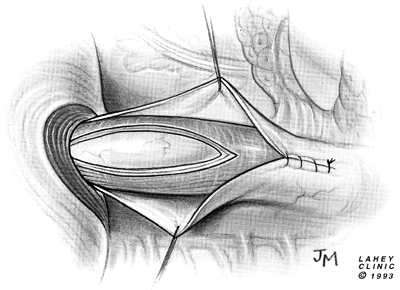 |
Fig. 130-6. The completed esophagomyotomy. Note return of the gastroesophageal junction to its normal subdiaphragmatic position. |
ASSOCIATED CONDITIONS
Gastroesophageal Reflux
Some surgeons add an antireflux procedure to esophagomyotomy. A small percentage of patients with achalasia have heartburn-like symptoms, and in some of them, as reported by Jamieson (1991) and Shoenut and associates (1995), ambulatory pH monitoring confirms that the symptoms are attributable to GER. This situation may arise in untreated patients and, more commonly, as recorded by Smart and colleagues (1987), in those who have previously undergone weakening of the LES by forceful dilation or by esophagomyotomy. This small group of patients with typical acid reflux symptoms, confirmed by pH monitoring, should undergo both a complete myotomy, extending 2 cm onto the stomach, and an antireflux repair, since GER can be expected to worsen after division of the already incompetent LES. It is in this group of patients that a laparoscopic myotomy with partial fundoplication shows clear advantage over other approaches.
A distinction should be made between patients demonstrating repeated episodes of acid reflux on pH testing and those whose study shows evidence of a persistent low pH from poor clearance of a single acid exposure or from acidic food residue in the esophagus. The latter instance does not necessitate the addition of an antireflux procedure because acid clearance in the distal esophagus can be expected to improve after myotomy.
The antireflux procedure chosen should be a partial rather than total fundoplication to minimize the risk for residual postoperative dysphagia. Although several types of partial fundoplications have been used, including the Hill posterior
P.1980
gastropexy and the Dor fundoplication, as reported by Rosato (1991), Desa (1990), Veiga-Fernandes (1981), and Crookes (1989) and their colleagues, the modified Belsey (1966) Mark IV repair has been performed most often. The usual operative technique is modified by the omission of the middle suture in the region of the myotomy (Figs. 130-7 to 130 9).
Diaphragmatic Hernia
Sliding hiatal hernia in association with achalasia, once thought rare by Binder and associates (1965), among others, has been reported by Okike (1979) and Scott (1985) and their colleagues to be present preoperatively in 8% to 26% of surgically treated cases of achalasia. Most surgeons, including Black and co-workers (1976), believe that hernia repair in such cases is an important factor in preventing postoperative GER. Although myotomy alone does not commonly result in GER, the addition of a hiatal hernia to myotomy in the dog results in incompetence of the residual antireflux mechanism. Also, indirect clinical evidence supports the concept that hiatal attachments and a normally located gastroesophageal junction are important antireflux mechanisms following myotomy. Andreollo and Earlam (1987) recorded that the use of a laparotomy approach without the addition of an antireflux repair is twice as likely to lead to GER as one performed via thoracotomy, presumably because of the disruption of the phrenoesophageal attachments necessary to gain access to the intrathoracic esophagus.
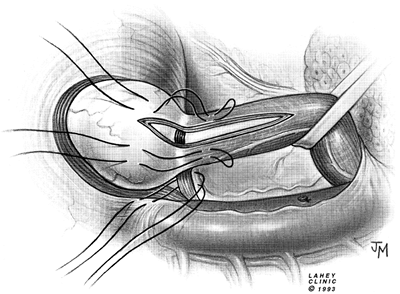 |
Fig. 130-7. Modified Belsey antireflux repair. Myotomy is extended 2 cm onto the stomach. Two mattress sutures lateral to the myotomy are placed 2 cm above and below gastroesophageal junction. |
Small hernias are easily repaired by restoring the gastroesophageal junction to its intraabdominal location by passing two mattress sutures through the esophageal wall
P.1981
on each side of the myotomy, then through the hiatus, and through the diaphragm, effectively fixing its location. Larger hernias may require a more complete anatomic repair of the Allison type with closure of the enlarged hiatus posteriorly.
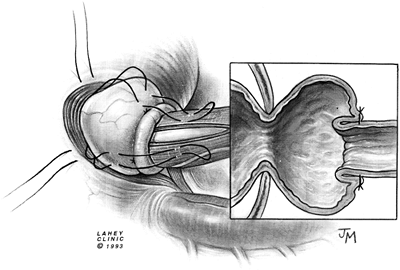 |
Fig. 130-8. Second layer of mattress sutures is placed. |
Primary paraesophageal hiatus hernia is unusual in association with achalasia. Anatomic repair of the hernia and limited esophagomyotomy can yield excellent results (Fig. 130-10), but if extensive dissection at the hiatus is necessary, it may be prudent to include an antireflux procedure.
Epiphrenic Diverticulum
Most epiphrenic diverticula arise in the presence of an esophageal motility disorder, predominantly achalasia, as noted by myself and colleagues (1992). The surgical treatment of achalasia in these patients is the same as for those without a diverticulum. Following excision of the diverticulum and a two-layer closure of the defect on one side, the esophagus is rotated 180 degrees for performance of esophagomyotomy in the usual way on the opposite side (Figs. 130-11 and 130-12).
Surgery Following Endoscopic Perforation
Forceful dilation of the LES results in esophageal perforation in as many as 5% of cases. Although conservative therapy may be used in contained tears, a transmural perforation requires prompt repair. Several surgical approaches to this problem have been advocated. Some surgeons, such as Slater and Sicular (1982), recommend a two-layer closure of the mucosal and muscular rents, with rotation of the mobilized esophagus and performance of an esophagomyotomy on the contralateral side, similar to the technique of diverticulectomy and esophagomyotomy in patients with achalasia (see Fig. 130-11). Others argue that the muscular tear caused by forceful dilation is a sufficient therapeutic myotomy, and prefer mucosal closure alone with some form of buttressing over the repair. Hunt and associates (2000) advocate a laparotomy approach with mucosal closure and a fundoplication as a buttress, either anterior or posterior, depending on the location of the perforation. Because most perforations occur posterolaterally, they have found that laparoscopic repair is usually not possible. Urbani and Mathisen (2000) prefer to approach such perforations through a left posterolateral thoracotomy, performing a mucosal closure buttressed by a pedicled intercostal muscle flap, which is sutured to the edges of the torn muscularis. Results with all techniques are excellent provided prompt and meticulous surgery is performed.
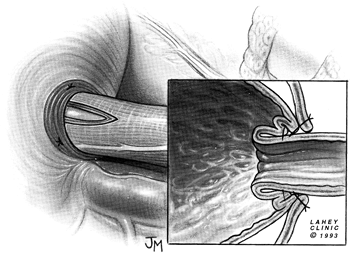 |
Fig. 130-9. Mattress sutures are tied, creating a 180-degree fundoplication around the intraabdominal portion of the esophagomyotomy. |
RESULTS
During the past four decades, consistently good results with the modified Heller esophagomyotomy have been reported from many centers around the world. Results from some representative reports are summarized in Table 130-2. (Similar short-term results have been reported for laparoscopic esophagomyotomy and partial fundoplication, which are shown in Table 130-3.)
Improvement is obtained in 90% or more of patients undergoing surgery for the first time. Results are somewhat less satisfactory for patients undergoing reoperation, with only about three fourths reporting improvement, as recorded by Ellis and colleagues (1984). Although operation is successful in relieving symptoms despite the stage of the
P.1982
disease, the results are more likely to be excellent than good or fair when the degree of esophageal dilatation is mild.
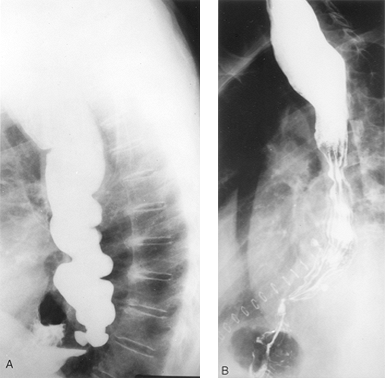 |
Fig. 130-10. Paraesophageal hernia and achalasia. A. Preoperative contrast radiograph shows a paraesophageal hernia in a patient with vigorous achalasia. B. Same patient after esophagomyotomy and Allison repair of paraesophageal hernia. |
Reports by Black (1976) and Jara (1979) and their associates, as well as by Ellis and Cole (1965), of myotomy alone included an 18% to 48% incidence of postoperative GER. The reason for this occurrence is not clear, but it presumably is attributed to overextension of the myotomy onto the stomach and disruption of hiatal anatomy. These reports have led some surgeons to advocate the addition of an antireflux procedure to myotomy in every case. In fact, however, the reported incidence of clinically important GER following limited esophagomyotomy is low in most series. Results of several large series concerning limited myotomy without antireflux repair, especially the work of Ellis and colleagues (1984), attest to both the efficacy of this procedure and its low incidence of postoperative GER (see Table 130-2). In addition, the results of postoperative pH monitoring from two series reported by Shoenut (1990) and Thomson (1987) and their associates confirm the clinical impression that abnormal GER after limited esophagomyotomy is unusual. In fact, similar rates of GER are reported both with and without antireflux repair. In two large collective reviews, by Andreollo and Earlam (1987) and Ferguson (1991), the average incidence of GER following transthoracic esophagomyotomy
P.1983
with added antireflux repair ranged from 7.3% to 10.7%, compared with 7.7% to 9.7% following myotomy alone. In addition, Pai and colleagues (1984) compared their experience performing esophagomyotomy both with and without an antireflux procedure and found a lower incidence of GER in patients undergoing limited esophagomyotomy alone.
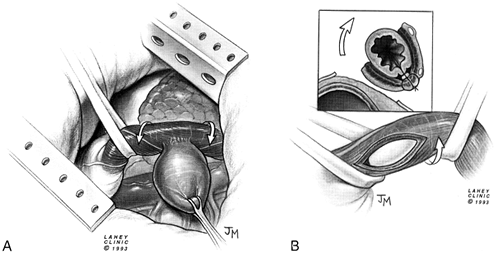 |
Fig. 130-11. Epiphrenic diverticulum and achalasia. A. Exposure of a diverticulum for excision by rotation of the esophagus. B. Esophagomyotomy is performed on the side of the esophagus opposite the diverticulectomy. |
I and my associates (1996) have shown with postoperative pH monitoring that the incidence of symptomatic GER following transthoracic, limited esophagomyotomy is low. Of 14 patients studied, 13 (93%) had a normal number of acid reflux episodes, and 10 (71%) had normal esophageal acid contact time (pH <4.0 of <4.5%). One patient had symptomatic acid reflux and three others demonstrated increased acid reflux without symptoms or esophagitis. Similar rates of asymptomatic acid reflux demonstrated by pH monitoring have been reported by Graham (1997) and Patti (1999) and their colleagues following laparoscopic myotomy with fundoplication. The significance of asymptomatic GER is unknown, but could conceivably lead to clinical symptoms or signs of esophageal injury with time. Ellis (1993) notes, however, that poor results following a transthoracic limited myotomy were due to GER in only 5% of patients of 179 patients studied. For those patients followed from 10 to 20 years postoperatively, this rate of GER remained low, with only a 4% incidence of reflux esophagitis.
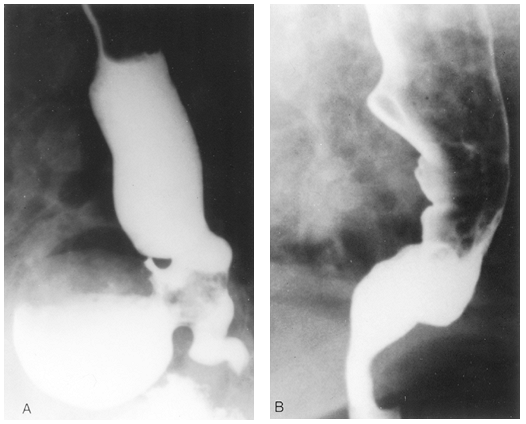 |
Fig. 130-12. Preoperative (A) and postoperative (B) contrast radiographs shows the appearance of achalasia and associated epiphrenic diverticulum and the appearance after diverticulectomy and limited esophagomyotomy. |
Table 130-2. Results of Primary Esophagomyotomy without Antireflux Procedure | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
If the surgeon chooses to perform an associated antireflux procedure, it should be a partial rather than a total fundoplication to minimize the risk for postoperative dysphagia. A 360-degree wrap of the distal esophagus in the absence of peristalsis may lead to an obstructive distal high-pressure zone. Duranceau (1982) and Topart (1992) and their colleagues demonstrated this fact with careful long-term follow-up of a group of patients with achalasia
P.1984
treated by esophagomyotomy and a total fundoplication. Although their early results showed good symptomatic improvement, longer follow-up revealed a high failure rate due to obstructive symptoms, with 5 of 17 patients ultimately requiring reoperation. Similar results were demonstrated by Stipa and associates (1990) in a comparison of the Belsey and Nissen operations in patients undergoing esophagomyotomy. Those who had undergone a Nissen fundoplication were five times as likely to report failure of the procedure because of residual obstructive symptoms, as were those who had undergone the Belsey repair. According to Leonardi and co-workers (1977), a partial fundoplication restores a distal high-pressure zone of lower amplitude than that resulting from total fundoplication, and therefore it is less likely to cause obstructive symptoms.
Table 130-3. Results of Laparoscopic Esophagomyotomy and Partial Fundoplication | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
To ensure complete division of the obstructing LES zone, some surgeons, including Rosato and colleagues (1991), Belsey (1966), and Skinner (1984), advocate an extension of the myotomy well onto the stomach followed by the necessary reconstruction of an antireflux mechanism. This extension is performed out of concern that precise titration of the myotomy, making it neither too short nor too long, can be difficult to perform repeatedly and reliably, and may lead to a high rate of failure because of an incomplete myotomy or GER. Although excellent results have been reported with this more extensive approach by surgeons such as Bonavina (1992), Little (1988), and Csendes (1988) and their colleagues, it seems to complicate an otherwise simple procedure unnecessarily and carries the risks inherent in antireflux surgery. These same arguments are used in the current debate over whether video-assisted approaches to esophagomyotomy require the addition of an antireflux procedure.
VIDEO-ASSISTED APPROACHES
Recent advances in thoracoscopic and laparoscopic surgery have led to their use in the treatment of achalasia. Both a limited thoracoscopic esophagomyotomy and a complete laparoscopic myotomy with partial fundoplication, either anterior (Dor) or posterior (Toupet), have demonstrated early success, as reported by a number of researchers, including Maher (1997), as well as Pellegrini (1993), Hunter (1997), and Rosati (1998) and their colleagues. The rate of mucosal perforation during video-assisted myotomy, ranging from 11% to 25% as reported by Raiser (1996), Hunter (1997), Vogt (1997), Rosati (1998), Sharp (2002), and Ackroyd (2001) and their associates, attests to the technical demands of these approaches and their mandatory learning curve.
Thoracoscopic esophagomyotomy without an antireflux procedure showed initial promise, but proved to have a high rate of incomplete myotomy and residual dysphagia, 27% as reported by Patti and associates (1999). Later follow-up of this same group of patients showed a 60% rate of postoperative GER. For these reasons, Patti (1999), Sharp (2002), and Bloomston (2000) and their colleagues, among others, have abandoned the thoracoscopic approach in favor of a laparoscopic esophagomyotomy. The experience of Nguyen and colleagues (1998) with the thoracoscopic Belsey Mark IV antireflux procedure emphasizes the technical difficulty of thoracoscopic surgery of the distal esophagus and cardia. They found significantly poorer results using thoracoscopy compared with the standard open operation, which they now consider the preferable approach. Notwithstanding some reports of successful thoracoscopic esophagomyotomy, such as the one by Maher and associates (2001), a consensus seems to exist that if a transthoracic approach is used, an open operation provides a better outcome than thoracoscopic surgery. It is important to bear in mind that the principal goal of surgery for achalasia is long-term relief of symptoms, not a small incision or a short hospital stay, and surgeons should choose their procedure accordingly.
Laparoscopic esophagomyotomy in recent years has become the procedure of choice in numerous centers for the surgical treatment of achalasia. No agreement exists regarding the need for, or type of, an antireflux procedure to be used in association with esophagomyotomy. This dispute is an extension of the same one that surrounded open operations. Laparoscopic results comparable with open operations have been reported by numerous investigators, both with and without an associated antireflux procedure.
Laparoscopic esophagomyotomy with a partial fundoplication, either anterior (Dor) or posterior (Toupet), has been shown by Dempsey (1999), Heniford (2001), and Ackroyd (2001) and their colleagues, among others, to provide results equivalent to open myotomy, with around 90% of patients displaying a good or excellent result (see Table 130-3). [The report by Heniford and associates (2001) provides a detailed description of their operative technique.] Both Donahue (2002) and Patti (1999) and their colleagues, who prefer an anterior fundoplication, report a 10% to 11% rate of postoperative dysphagia requiring endoscopic dilation, with variable results. The cause was usually a fibrotic stenosis at the myotomy site.
Laparoscopic myotomy without an antireflux procedure has been performed with good results by Boulez (1997), Sharp (2002), and Kjellin (1999) and their associates, and others, with an incidence of symptomatic GER comparable to those procedures employing a fundoplication.
Advances in computer-assisted, robotic surgery may provide increased precision for laparoscopic esophagomyotomy. Melvin and colleagues (2001) have reported success with a robotic laparoscopic myotomy and Toupet fundoplication. More experience and longer follow-up with this technique will be required to assess its clinical value and cost effectiveness.
Ellis and associates (1992) report that long-term follow-up of patients undergoing a transthoracic limited esophagomyotomy without an antireflux procedure demonstrates no significant deterioration in the operative success rate over 10 to 20 years of postoperative follow-up, with 89% remaining improved, and failure rates for GER and incomplete myotomy of
P.1985
5% and 2%, respectively. This high degree of success and low complication rate attest to the efficacy of the operation and remain the standards against which the long-term results of newer approaches should be measured.
REFERENCES
Ackroyd R, et al: Laparoscopic cardiomyotomy and anterior partial fundoplication for achalasia. Surg Endosc 15:683, 2001.
Andreollo NA, Earlam RJ: Heller's myotomy for achalasia: is an added antireflux procedure necessary? Br J Surg 74:765, 1987.
Barrett NR, Franklin RH: Concerning the unfavourable late results of certain operations performed in the treatment of cardiospasm. Br J Surg 37:194, 1949.
Bell RC: Laparoscopic closure of esophageal perforation following pneumatic dilatation for achalasia. Report of two cases. Surg Endosc 11:476, 1997.
Belsey R: Functional disease of the esophagus. J Thorac Cardiovasc Surg 52:164, 1966.
Binder HJ, et al: Rarity of hiatus hernia in achalasia. N Engl J Med 272:680, 1965.
Black J, Vorbach AN, Collis JL: Results of Heller's operation for achalasia of the oesophagus. The importance of hiatal repair. Br J Surg 63:949, 1976.
Bloomston M, et al: Videoscopic Heller myotomy for achalasia results beyond short-term follow-up. J Surg Res 92:150, 2000.
Bonavina L, et al: Primary treatment of esophageal achalasia. Long-term results of myotomy and Dor fundoplication. Arch Surg 127:222, 1992.
Boulez J, et at: Oesocardiomyotomie de Heller sans anti-reflux par voie laparoscopique. Ann Chir 51:232, 1997.
Castrini G, Pappalardo G, Mobarhan S: New approach to esophagocardiomyotomy report of forty cases. J Thorac Cardiovasc Surg 84:575, 1982.
Coccia G, et al: Prospective clinical and manometric study comparing pneumatic dilatation and sublingual nifedipine in the treatment of oesophageal achalasia. Gut 32:604, 1991.
Crookes PF, Wilkinson AJ, Johnston GW: Heller's myotomy with partial fundoplication. Br J Surg 76:99, 1989.
Csendes A, et al: A prospective randomized study comparing forceful dilatation and esophagomyotomy in patients with achalasia of the esophagus. Gastroenterology 80:789, 1981.
Csendes A, et al: Late subjective and objective evaluation of the results of esophagomyotomy in 100 patients with achalasia of the esophagus. Surgery 104:469, 1988.
Dempsey DT, et al: Comparison of outcomes following open and laparoscopic esophagomyotomy for achalasia. Surg Endosc 13:747, 1999.
Desa LA, Spencer J, McPherson S: Surgery for achalasia cardiae: the Dor operation. Ann R Coll Surg Engl 72:128, 1990.
Dolan K, et al: Does pneumatic dilatation affect the outcome of laparoscopic cardiomyotomy? Surg Endosc 16:84, 2002.
Donahue PE, et al: Achalasia of the esophagus: treatment controversies and the method of choice. Ann Surg 203:505, 1986.
Donahue PE, et al: Floppy Dor fundoplication after esophagocardiomyotomy for achalasia. Surgery 132:716, 2002.
Duranceau A, LaFontaine ER, Vallieres B: Effects of total fundoplication on function of the esophagus after myotomy for achalasia. Am J Surg 143:22,1982.
Effler DB, et al: Primary surgical treatment for esophageal achalasia. Surg Gynecol Obstet 132:1057, 1971.
Ellis F, Cole FL: Reflux after cardiomyotomy. Gut 6:80, 1965.
Ellis FH Jr: Management of oesophageal achalasia. Clin Gastroenterol 5:89, 1976.
Ellis FH Jr: Oesophagomyotomy for achalasia: a 22 year experience. Br J Surg 80:882, 1993.
Ellis FH Jr, Crozier RE, Watkins E Jr: Operation for esophageal achalasia. Results of esophagomyotomy without an antireflux operation. J Thorac Cardiovasc Surg 88:344, 1984.
Ellis FH Jr, Olsen AM: The development of surgery for esophageal achalasia. In Dunphy JE (ed): Achalasia of the Esophagus. Philadelphia: WB Saunders, 1969.
Ellis FH Jr, et al: Esophagomyotomy for esophageal achalasia: experimental, clinical, and manometric aspects. Ann Surg 166:640, 1967.
Ellis FH Jr, et al: Ten to 20-year clinical results after short esophagomyotomy without an antireflux procedure (modified Heller operation) for esophageal achalasia. Eur J Cardiothorac Surg 6:86, 1992.
Ferguson MK: Achalasia: current evaluation and therapy. Ann Thorac Surg 52:336, 1991.
Finley RJ, et al: Laparoscopic Heller myotomy improves esophageal emptying and the symptoms of achalasia. Arch Surg 136:892, 2001.
Fletcher PR: Acute hiatal hernia with oesophageal perforation following Heller's operation. Br J Surg 65:486, 1978.
Goulbourne IA, Walbaum PR: Long-term results of Heller's operation for achalasia. J R Coll Surg Edinb 30:101, 1985.
Graham AJ, et al: Laparoscopic esophageal myotomy and anterior fundoplication for the treatment of achalasia. Ann Thorac Surg 64:785, 1997.
Heller E: Extramuk se cardiaplastik beim chronischen cardiospasmus mit dilatation des oesophagus. Mitteilungen Aus Den Grenzgebieten der Medizin und Chirurgie 27:141, 1914.
Heniford BT, et al: Laparoscopic anterior esophageal myotomy and Toupet fundoplication for achalasia. Am Surg 67:1059, 2001.
Hunt DR, et al: Management of esophageal perforation after pneumatic dilation for achalasia. J Gastrointest Surg 4:411, 2000.
Hunter JG, et al: Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg 225:655, 1997.
Jamieson GG: Gastroesophageal reflux following myotomy for achalasia. Hepatogastroenterology 38:506, 1991.
Jara FM, et al: Long-term results of esophagomyotomy for achalasia of esophagus. Arch Surg 114:935, 1979.
Kjellin AP, et al: Laparoscopic myotomy without fundoplication in patients with achalasia. Eur J Surg 165:1162, 1999.
Leonardi HK, et al: An experimental study of the effectiveness of various antireflux operations. Ann Thorac Surg 24:215, 1977.
Little AG, et al: Surgical treatment of achalasia: Results with esophagomyotomy and Belsey repair. Ann Thorac Surg 45:489, 1988.
Luketich JD, et al: Outcomes after minimally invasive esophagomyotomy. Ann Thorac Surg 72:1909, 2001
Maher JW: Thoracoscopic esophagomyotomy for achalasia: maximum gain, minimal pain. Surgery 122:836, 1997.
Maher JW, Conklin J, Heitshusen DS: Thoracoscopic esophagomyotomy for achalasia: preoperative patterns of acid reflux and long-term follow-up. Surgery 130:570, 2001.
Mayberry JF, Smart HL, Atkinson M: Audit of surgical and pneumatic/hydrostatic treatment of achalasia in a defined population. J R Soc Med 79:708, 1986.
Melvin WS et al: Computer-assisted robotic Heller myotomy: initial case report. J Laparoendosc Adv Surg Tech A 11:251, 2001.
Morino M, et al: Preoperative pneumatic dilation represents a risk factor for laparoscopic Heller myotomy. Surg Endosc 11:359, 1997.
Nguyen NT, et al: Preliminary results of thoracoscopic Belsey Mark IV antireflux procedure. Surgical Laparosc Endosc 8:185, 1998.
O'Connor JB, et al: The cost-effectiveness of treatment strategies for achalasia. Dig Dis Sci 47:1516, 2002.
Okike N, et al: Esophagomyotomy versus forceful dilation for achalasia of the esophagus: results in 899 patients. Ann Thorac Surg 28:119, 1979.
Pai GP, et al: Two decades of experience with modified Heller's myotomy for achalasia. Ann Thorac Surg 38:201, 1984.
Patti MG, et al: Minimally invasive surgery for achalasia: an 8-year experience with 168 patients. Ann Surg 230:587, 1999.
Payne WS: Heller's contribution to the surgical treatment of achalasia of the esophagus. 1914. Ann Thorac Surg 48:876, 1989
Peillon C, et al: Achalasia: the case for primary laparoscopic treatment. Surg Laparosc Endosc Percutan Tech 11:71, 2001
Pellegrini CA, et al: Thoracoscopic esophageal myotomy in the treatment of achalasia. Ann Thorac Surg 56:680, 1993.
Richter JE: Achalasia: whether the knife or balloon? Not such a difficult question. Am J Gastroenterol 86:810, 1991.
Rosati R, et al: Evaluating results of laparoscopic surgery for esophageal achalasia. Surg Endosc 12:270, 1998.
Rosato EF, et al: Transabdominal esophagomyotomy and partial fundoplication for treatment of achalasia. Surg Gynecol Obstet 173:137, 1991.
Russel JC: Diagnosis and treatment of spasmodic stricture of the oesophagus. BMJ 1:1450, 1898.
Sauer L, Pellegrini CA, Way LW: The treatment of achalasia. A current perspective. Arch Surg 124:929, 1989.
Scott HW Jr, et al: Surgical management of esophageal achalasia. South Med J 78:1309, 1985.
P.1986
Sharp KW, et al. 100 consecutive minimally invasive Heller myotomies: lessons learned. Ann Surg 235:631, 2002.
Shoenut JP, et al: Esophageal reflux before and after isolated myotomy for achalasia. Surgery 108:876, 1990.
Shoenut JP, et al: Reflux in untreated achalasia patients. J Clin Gastroenterol 20:6, 1995.
Skinner DB: Myotomy and achalasia. Ann Thorac Surg 37:183, 1984.
Slater G, Sicular AA: Esophageal perforations after forceful dilatation in achalasia. Ann Surg 195:186, 1982.
Smart HL, et al: Twenty-four hour oesophageal acidity in achalasia before and after pneumatic dilatation. Gut 28:883, 1987.
Stipa S, et al: Heller-Belsey and Heller-Nissen operations for achalasia of the esophagus. Surg Gynecol Obstet 170:212, 1990.
Streitz JM Jr, Ellis FH Jr: Iatrogenic paraesophageal hiatus hernia. Ann Thorac Surg 50:446, 1990.
Streitz JM Jr, Glick ME, Ellis FH Jr: Selective use of myotomy for treatment of epiphrenic diverticula. Manometric and clinical analysis. Arch Surg 127:585, 1992.
Streitz JM Jr, et al: Objective assessment of gastroesophageal reflux after short esophagomyotomy for achalasia with the use of manometry and pH monitoring. T Thorac Cardiovascular Surg 111:107, 1996.
Suarez J, et al: Laparoscopic myotomy vs. Endoscopic dilation in the treatment of achalasia. Surg Endosc 16:75, 2002.
Thomson D, et al: Reflux patterns following limited myotomy without fundoplication for achalasia. Ann Thorac Surg 43:550, 1987.
Topart P, et al: Long-term effect of total fundoplication on the myotomized esophagus. Ann Thorac Surg 54:1046, 1992.
Urbach DR, et al: A decision analysis of the optimal initial approach to achalasia: laparoscopic Heller myotomy with partial fundoplication, thoracoscopic Heller myotomy, pneumatic dilatation, or botulinum toxin injection. J Gastrointest Surg 5:192, 2001.
Urbani M, Mathisen DJ: Repair of esophageal perforation after treatment for achalasia. Ann Thorac Surg 69:1609, 2000.
Veiga-Fernandes F, Pinheiro MF, Didia-Guerreiro: Cardiomyotomy associated with antireflux surgery in the treatment of achalasia. World J Surg 5:697, 1981.
Vogt D, et al. Successful treatment of esophageal achalasia with laparoscopic Heller myotomy and Toupet fundoplication. Am J Surg 174:709, 1997.
Von Mikulicz J: Small contributions to the surgery of the intestinal tract. Boston Med Surg J 148:608, 1903.
Zaaijer JH: Cardiospasm in the aged. Ann Surg 77:615, 1923.
Zaninotto G, et al. Treatment of esophageal achalasia with laparoscopic Heller myotomy and Dor partial anterior fundoplication: prospective evaluation of 100 consecutive patients. J Gastrointest Surg 4:282, 2000.
READING REFERENCE
Tomlinson P, Grant AF: A review of 74 patients with oesophageal achalasia: The results of Heller's cardiomyotomy, with and without Nissen fundoplication. Aust NZ J Surg 51:48, 1981.
EAN: 2147483647
Pages: 203