6 - Infectious Diseases
Editors: Schrier, Robert W.
Title: Internal Medicine Casebook, The: Real Patients, Real Answers, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > Chapter 6 - Infectious Diseases
function show_scrollbar() {}
Chapter 6
Infectious Diseases
Robert T. Schooley
Urinary Tract Infection
What host factors lead to the development of urinary tract infections (UTIs), and how are these factors different for men and women?
What organisms commonly cause lower UTIs?
What are the signs and symptoms of lower UTI, and how do these differ from those of pyelonephritis?
Discussion
What host factors lead to the development of UTIs, and how are these factors different for men and women?
Improper hygiene, sexual activity, incontinence, urinary tract instrumentation, contraceptive diaphragms with or without spermicides, diabetes mellitus, a genetic predisposition, and dehydration are all factors that can increase the
P.221
likelihood of a UTI. Most UTIs are caused by endogenous flora originating from the gastrointestinal tract. These organisms have been shown to colonize the vaginal introitus and periurethral area before UTI occurs. Women who wipe their perineal area from the posterior to anterior direction after defecation, rather than vice versa, or those who are incontinent of stool, may be subject to more frequent colonization of the short female urethra with Enterobacteriaceae. The longer urethra in men makes access to the bladder more difficult for enteric flora; however, this flora may be introduced to the normally sterile bladder area as the result of Foley catheterization or cystoscopy. One of the natural defenses against cystitis after urethral colonization is the mechanical flushing of the urinary bladder, which takes place during urination. Obviously, anyone who is dehydrated cannot benefit frequently from this natural defense mechanism. Sexual activity can predispose women to acquiring UTI. In addition, as the result of a poorly understood mechanism, women who use a diaphragm for contraception, especially with spermicides, seem to be more susceptible to urethral colonization and UTI. It has been proposed that this predisposition might be due, at least in part, to a shift in vaginal microbial flora caused by the activity of spermicides. Diabetes mellitus may predispose to UTI through a variety of mechanisms, including the defective chemotaxis of leukocytes, phagocytic defects, and enhanced growth conditions for bacteria. Genetically determined factors, such as the type and number of receptors on uroepithelial cells to which bacteria may attach, also appear to heighten susceptibility to UTIs.What organisms commonly cause lower UTIs?
Escherichia coli causes most (up to 80%) of the community-acquired uncomplicated UTIs, with Klebsiella, Enterobacter, and Proteus organisms more likely to cause complicated or hospital-acquired UTIs. These are all gram-negative organisms that usually originate from the patient's own gastrointestinal flora. There are, however, several gram-positive organisms that occur as urinary pathogens. Staphylococcus saprophyticus, a coagulase-negative Staphylococcus organism, causes 20% or more of the UTIs in women 16 to 35 years of age. Streptococcus faecalis causes 2% to 3% of the UTIs in otherwise healthy young women. When Staphylococcus aureus is found in the urine, a bacteremic infection of the kidney should be suspected.
Chlamydia, Ureaplasma, Mycoplasma, and Neisseria gonorrhoeae are sexually transmitted pathogens that usually cause vaginal or cervical infections; however, they may be implicated in cases of acute urethral syndrome in which Gram's-stained urine samples exhibit pyuria without bacteriuria.
Pseudomonas and Serratia are more commonly nosocomial gram-negative pathogens that are not usually seen in community-acquired, uncomplicated UTIs.
What are the signs and symptoms of lower UTI, and how do these differ from those of pyelonephritis?
The term lower UTI actually encompasses cystitis and urethritis, as well as prostatitis. Symptoms classically include urinary frequency, urgency, dysuria,
P.222
and suprapubic discomfort. Signs may include fever, cloudy or foul-smelling urine, and hematuria. Because upper UTIs (i.e., pyelonephritis, acute lobar nephritis, and a perinephric abscess) often start as cystitis, the same signs and symptoms may exist; however, the fever is usually more severe, and may be accompanied by shaking chills. An upper UTI is often accompanied by costovertebral angle tenderness on the involved side. Elderly people and those with diabetes may exhibit fewer signs and symptoms than otherwise normal hosts.
Case
A 19-year-old, sexually active woman presents to the emergency room complaining of a 2-day history of urinary frequency, burning, and urgency. She denies vaginal discharge or itching, fever, chills, nausea, vomiting, back pain, abdominal pain, or hematuria. She has no history of UTI or a sexually transmitted disease. She recently began using a diaphragm for birth control, and reports that her last menstrual period occurred 3 weeks ago. She has only one sexual partner, who denies penile discharge or burning on urination. On physical examination, she is noted to be afebrile with a normal blood pressure and pulse. There is no costovertebral angle tenderness. Her abdomen is soft and there is mild suprapubic tenderness in response to palpation. A urinalysis reveals 1+ protein, 2+ leukocytes, and 1+ blood. The urine pH is 5.6. Gram's staining of an unspun urine specimen reveals abundant polymorphonuclear leukocytes and moderate gram-negative rods. A clean-catch urine specimen is sent to the microbiology laboratory for culture.
The emergency room physician diagnoses an uncomplicated UTI and prescribes trimethoprim-sulfamethoxazole (TMP-SMX), one double-strength tablet twice a day for 3 days.
What other therapeutic options would have been appropriate in this patient?
What can this woman do to help prevent recurrent UTIs?
Should this woman's sexual partner be evaluated for UTI?
Was the Gram's staining an important diagnostic test, and in what way did the findings alter the management of this case?
What is the value of knowing the urine pH in this setting?
What other diagnostic or laboratory tests should have been performed?
What would be an appropriate analgesic for a patient with UTI who is experiencing severe urethral discomfort?
What side effects of therapy should this woman know about?
What possible consequences could arise if this woman does not comply with therapy?
Case Discussion
What other therapeutic options would have been appropriate in this patient?
TMP-SMX remains the drug of choice for the empirically based treatment of uncomplicated UTIs. For sulfa-allergic patients, ampicillin, amoxicillin, a first-generation cephalosporin, or a quinolone is the appropriate alternative. Therapy may then be modified on the basis of the urine culture results and the sensitivities of the infecting organism. Enterococci are not susceptible to either TMP-SMX or
P.223
cephalosporins, which points out the utility of performing urine Gram's staining when deciding on antibiotic therapy. The prevalence of ampicillin-resistant E. coli may be as high as 30% in some communities, and this needs to be considered when selecting an appropriate antibiotic. S. saprophyticus responds to ampicillin, TMP-SMX, and the quinolones. In the past, treatment of lower UTIs for 5 to 7 days was recommended. Short course therapy with agents that achieve high and sustained urinary concentrations (single dose with one or two double-strength TMP/SMX or 3 g of amoxicillin) will usually suffice for uncomplicated infections. Failures of short course therapy are indications that complicating factors requiring more extensive evaluation might be present. In general, single-dose therapy is contraindicated in patients with known anatomic or functional abnormalities, or with immunocompromising diseases such as diabetes mellitus. After single-dose therapy urine cultures should be performed 1 to 2 weeks later, to document the cure. In the event of treatment failure, a longer course of the appropriate antibiotic should be administered and an evaluation of potentially complicating factors should be undertaken.Regardless of the pathogen and the choice of antibiotics, aggressive oral hydration is a reasonable recommendation in the management of an uncomplicated UTI. Although there is no evidence that hydration improves the results of appropriate antimicrobial therapy, it does dilute the bacteria and removes infected urine by frequent bladder emptying.
What can this woman do to help prevent recurrent UTIs?
Some women find that switching to another method of birth control considerably reduces the frequency of recurrent bacterial UTIs. Thorough cleansing of the perineal area before sexual relations may decrease the incidence of postcoital UTI in those prone to UTI; however, most patients find this to be an impractical and not completely effective preventive measure.
Choosing another method of birth control may not be necessary for most women if they remember to drink a large glass of water before intercourse and void after intercourse; however, studies have shown that diaphragm usage is an independent risk factor for UTI. Regular antibiotic prophylaxis should be reserved for those patients with a history of multiple recurrent UTIs, or complicated UTI or upper tract infections, or for immunocompromised hosts. The disadvantages of ongoing prophylaxis include the development of drug-related side effects and colonization with multidrug-resistant organisms.
Should this woman's sexual partner be evaluated for UTI?
No. Although lower UTIs in women are associated with sexual activity, this is not a sexually transmitted disease. The infecting organisms are usually endogenous flora. Healthy men without predisposing factors such as urinary tract instrumentation or diabetes mellitus rarely get lower UTIs. Bacterial prostatitis does not put his sexual partner at risk for cystitis.
Was the Gram's staining an important diagnostic test, and in what way did the findings alter the management of this case?
When bacteriuria is found in Gram's-stained, uncentrifuged urine, this is a very specific finding for the diagnosis of UTI. The finding of microscopic bacteriuria corresponds to urine culture colony counts of 105/mL in more than 90% of such
P.224
specimens. Distinguishing between gram-positive and gram-negative infections can be quite useful in making therapeutic decisions.What is the value of knowing the urine pH in this setting?
Alkaline urine may be caused by infection with Proteus species, which produce urease. The presence of nonalkaline urine in this patient makes infection with a urea-splitting organism unlikely.
What other diagnostic or laboratory tests should have been performed?
The physical examination and diagnostic studies performed in an emergency room setting should be directed toward elucidating the nature of the patient's chief complaint and history. A pelvic examination would be appropriate if the patient had reported symptoms of increased vaginal discharge, dyspareunia, or exposure to a known sexually transmitted disease in the partner. The indications for performing cultures for sexually transmitted pathogens are similar to those for a pelvic examination. Chlamydia, Ureaplasma, N. gonorrhoeae, or Mycoplasma infection should have been considered in this patient if no organisms were seen on the Gram's-stained urine specimens, or if subsequent routine bacterial cultures grew no organisms.
Intravenous pyelography and a renal ultrasound examination should be reserved for when a complicated UTI or upper UTI such as pyelonephritis is suspected. A pregnancy test should be performed in any woman of childbearing age before prescribing an antibiotic that may be contraindicated in pregnancy.
What would be an appropriate analgesic for a patient with UTI who is experiencing severe urethral discomfort?
Phenazopyridine hydrochloride is a urinary tract analgesic agent that exerts a topical analgesic effect on the mucosa of the urinary tract through an unknown mechanism of action. The side effects are minimal, and include the urine acquiring a red or orange color that may stain fabric. It is usually not necessary to prescribe more than a 2-day supply to patients with uncomplicated UTIs who are receiving appropriate antibiotic therapy. Opioid analgesics are relatively contraindicated in UTI because they may cause acute urinary retention.
What side effects of therapy should this woman know about?
Vaginal candidiasis commonly develops after antimicrobial therapy because antibiotics eliminate much of the normal vaginal flora and create an ideal environment for the overgrowth of Candida albicans. Hypersensitivity reactions may occur with any antibiotic; however, TMP-SMX may rarely also be associated with interstitial nephritis, aseptic meningitis, Stevens-Johnson syndrome, or erythema multiforme. A careful history to rule out known drug allergy is important.
What possible consequences could arise if this woman does not comply with therapy?
The consequences of noncompliance with therapy include continuing symptoms, the induction of antibiotic-resistant strains of microorganisms, and, most important, ascending infection leading to acute pyelonephritis or even a perinephric abscess.
Suggested Readings
Bent S, Saint S. The optimal use of diagnostic testing in women with acute uncomplicated cystitis. Am J Med 2002;113(Suppl 1A):20S.
P.225
Dunagan WC, Ridner ML. Manual of medical therapeutics, 26th ed. Boston: Little, Brown and Company, 1995:257.
Fihn SD, Latham RH, Roberts P, et al. Association between diaphragm use and urinary tract infection. JAMA 1985;254:240.
Hooten TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infections in young women. N Engl J Med 1996;335:468.
Latham RH, Running K, Stamm WE. Urinary tract infection in young adult women caused by Staphylococcus saprophyticus. JAMA 1983;250:3063.
Leibovici L, Alpert G, Laor L, et al. Urinary tract infections and sexual activity in young women. Arch Intern Med 1987;147:345.
Norrby SR. Short term treatment of uncomplicated lower urinary tract infections in women. Rev Infect Dis 1990;12:458.
Rubin RH, Fang LST, Jones SR, et al. Single-dose amoxicillin therapy for urinary tract infection. JAMA 1980;244:561.
Sobel JD, Kaye D. Urinary tract infections. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases, 6th ed. New York: Elsevier Science, 2005:875.
Stamey TA. Pathogenesis and treatment of urinary tract infections. Baltimore: Williams & Wilkins, 1980.
The Acquired Immunodeficiency Syndrome
What are the principles of antiretroviral chemotherapy?
When should antiretroviral chemotherapy be started?
What are the most important human immunodeficiency virus HIV-1 associated opportunistic infections, and the treatments used in the HIV-1 infected individuals who live in developed countries?
Discussion
What are the principles of antiretroviral chemotherapy?
HIV-1 associated morbidity and mortality is the direct result of immunosuppression mediated by viral replication. The goal of antiretroviral chemotherapy is to drive plasma HIV-1 levels to below the limits of detection with the most sensitive available assay. This approach affords two major benefits: (a) Successful suppression of viral replication arrests destruction of the immune response and allows for immune reconstitution. This, in turn, results in a dramatic decline in HIV-1 associated morbidity and mortality. (b) The emergence of drug resistance can be eliminated or greatly reduced by driving viral replication rates to extremely low levels.
Suppression of plasma HIV-1 RNA to levels of 20 copies/mL is currently best achieved through the use of a combination regimen containing at least three agents. The inclusion of multiple agents is required both for potency and for interposing a significant genetic barrier to the virus with respect to the emergence of resistance. HIV-1 replication occurs at the rate of approximately 10 billion viral particles per day in each infected person. With the
P.226
replicative infidelity of HIV-1's reverse transcription mechanism, this high level of replication rapidly results in the creation of a diverse quasispecies of virus. Therefore, it is likely that at the institution of therapy, viral variants exist that are resistant to each currently available agent. The use of multiple agents with nonoverlapping resistance mechanisms requires the virus to make multiple genetic changes in each virion in order to persist in the presence of all agents in the regimen.At present, reduction of HIV-1 RNA to 20 copies/mL is best achieved with the selection of two nucleoside analogs usually administered as fixed dose combinations (and an anchor drug efavirenz or a ritonavir-boosted protease inhibitor). Although there is no single regimen that is appropriate for all patients, the nucleoside combination of tenofovir and emtricitabine with efavirenz has become the most frequent initial combination regimen. The recent introduction of a single tablet containing these two nucleosides and efavirenz has provided the first once-daily single pill antiretroviral regimen. Other fixed-dose nucleoside combinations, including either zidovudine and lamivudine or abacavir and lamivudine, may also be used in combination with efavirenz.
Although efavirenz is potent and well tolerated in most patients, it cannot be used in 15% to 20% of patients. Efavirenz is associated with central nervous system (CNS) side effects that require up to 10% of patients to seek another drug. Efavirenz is also teratogenic and should not be used in sexually active women of childbearing age who are not using effective birth control methods. Because transmission of drug-resistant viruses is increasingly frequent, it is best to check drug susceptibility before initiating antiretroviral drugs. Transmitted drug resistance to efavirenz is found in approximately 10% of patients initiating therapy for the first time in certain locations in the United States and Europe. In patients for whom efavirenz is not an optimal choice, a ritonavir-boosted protease inhibitor (usually r/lopinavir or r/atazanavir) is generally the best initial choice. Appropriate management of antiretroviral chemotherapy is both an art and a science that is best accomplished by physicians with substantial experience in management of patients with HIV-1 infection.
When should antiretroviral chemotherapy be started?
There is no single answer that is appropriate for every patient. Ongoing viral replication is always damaging to the immune response of the host. On the other hand, current antiretroviral regimens may be associated with side effects, and require significant discipline to achieve the level of viral suppression associated with durable success. As CD4 cell counts decline, patients are at greater risk for HIV-1 associated opportunistic infections. Rising plasma HIV-1 RNA levels are associated with more rapid immunologic and clinical disease progression. Adequate suppression of HIV-1 is best achieved in patients with high CD4 cell counts and low plasma HIV-1 RNA levels. Therefore, all things being equal, it could be argued that early institution of therapy is associated with the best chance of long-term success. The desire to start therapy early must be balanced by a consideration of long-term toxicities and the commitment of the patient to strict adherence of the regimen chosen. In general, the urgency to
P.227
start therapy increases as CD4 cell counts fall and the plasma HIV-1 RNA levels rise. Most experts recommend treatment for any patient with HIV-1 related symptoms and that the therapy be started for asymptomatic HIV-1 infected persons as their CD4 cell count passes into the 250 to 350 cell/mL range.What are the most important HIV-1 associated opportunistic infections, and the treatments used in the HIV-1 infected individuals who live in developed countries?
In general, the risk of various HIV-1 related infections increases with disease progression and declining CD4 cell counts. Two notable exceptions to this rule, however, are pneumococcal pneumonia and tuberculosis. All HIV-1 infected patients are at increased risk for acquiring pneumococcal pneumonia and sepsis. Whether the administration of pneumococcal vaccine can prevent or lessen the severity of pneumococcal disease in these patients has not been proved, but the current practice is to administer pneumococcal vaccine to all HIV-1 infected patients whose CD4 lymphocyte counts exceed 500/mm3. The response to vaccination in patients with counts of less than 500/mm3 is likely to be enhanced if they are on effective antiretroviral therapy at the time of vaccination.
Tuberculosis is one of the few HIV-1 related infections that is transmissible to immunocompetent persons. HIV-infected persons are at increased risk for acquiring tuberculosis regardless of the stage of their HIV-1 infection. Because patients with HIV-1 infection have reduced cellular immunity, a threshold of 5 cm is considered to be a positive tuberculin skin test. As the CD4 cell counts fall, patients may become anergic and the tuberculin skin test further loses it sensitivity.
Oral candidiasis (thrush) most frequently occurs when the CD4 lymphocyte count falls below 300/mm3. Thrush can usually be treated with topical antifungal agents (nystatin swish and swallow, or clotrimazole troches), but more severe cases, especially when esophageal lesions are present, may require systemic antifungal agents such as fluconazole.
Early in the acquired immunodeficiency syndrome (AIDS) epidemic, Pneumocystic jiroveci (formerly carinii) pneumonia was the most common AIDS-defining illness. With the advent of effective prophylactic regimens, this illness has become much less frequent. Pneumocystis pneumonia is usually treated with TMP-SMX. Alternatively, intravenous pentamidine, oral trimethoprim/ dapsone, or oral atovaquone can be used in sulfa-allergic patients.
HIV-1 infected persons with less than 200 CD4 lymphocytes/mm3 are at risk for several types of CNS infections. One of the most common causes of intracranial masses in HIV-1 infected patients, Toxoplasma gondii, is treated with pyrimethamine and sulfadiazine. Cryptococcus neoformans, Histoplasma capsulatum, and Coccidioides immitis can cause CNS disease or disseminated disease in HIV-1 infected patients; infection with these pathogens is usually treated with amphotericin B.
Patients with less than 50 CD4 lymphocytes/mm3 are at risk for suffering disseminated infection with Mycobacterium avium [mycobacterium avium complex (MAC)] or ocular or systemic cytomegalovirus infections. Disseminated
P.228
MAC is commonly manifested clinically by the appearance of systemic symptoms (fever, weight loss, night sweats, and anemia). Treatment with a combination of two or three active agents is required for MAC infection. Cytomegalovirus retinitis presents with painless loss of vision and may be accompanied by systemic evidence of infection, manifest by fever, weight loss, or gastrointestinal symptoms. Treatment with ganciclovir (or valganciclovir), foscarnet, or cidofovir is usually effective.
Case
A 32-year-old woman is found to be HIV-1 seropositive at the time of a life insurance physical examination. The patient has had no prior serious medical illnesses although she has experienced increased vaginal itching during the last year. Her physical examination is normal except for vaginal thrush. Her social history reveals that she has been married to the same man for the last 3 years. He is also healthy. Upon detailed questioning, he admitted to having experimented with sex with men on several occasions while traveling to San Francisco 8 years earlier. He is subsequently found to be HIV-1 seropositive with a CD4 cell count of 860 cells/mm3 and a plasma HIV-1 RNA level of 13,000 copies/mL.
The laboratory evaluation reveals that she has a positive enzyme-linked immunosorbent assay (ELISA) for antibodies to HIV-1. HIV-1 seropositivity was confirmed by a Western blot assay. Her purified protein derivative (PPD) is negative, as is her serology for T. gondii. Her rapid plasma reagin (RPR) is negative. Her hematocrit is 43. Her white blood cell count is 5,200/mm3. Her CD4 cell count is 340 cells/mm3. Her plasma HIV-1 RNA level is 143,000 copies/mL.
What would you recommend to her with respect to antiretroviral chemotherapy?
How would you alter your recommendations if you learned she is pregnant at the time of presentation? If she were pregnant, is it likely that her child would be infected?
What would you recommend to her husband with respect to antiretroviral chemotherapy?
Case Discussion
What would you recommend to her with respect to antiretroviral chemotherapy?
You should recommend to her that she initiate antiretroviral therapy. Although her CD4 cell count is in a range that would prompt some practitioners to recommend deferring therapy, the presence of vaginal thrush is a clinical indicator of HIV-1 disease and places her at greater risk for an opportunistic infection than a woman with the same CD4 cell count and no symptoms. Therapy should not be initiated until a viral susceptibility test result has been obtained. In her case, it reveals that her virus is resistant to efavirenz, likely reflecting the acquisition of the drug-resistant virus from her husband. Because of this, you should recommend a regimen that uses a boosted protease inhibitor as the anchor drug such as tenofovir, emtricitabine, and r/lopinavir.
How would you alter your recommendations if you learned she is pregnant at the time of presentation? If she were pregnant, is it likely that her child would be infected?
The general approach to antiretroviral chemotherapy in pregnancy should be the same as it is in a nonpregnant woman. Effective management of an infected
P.229
woman requires close collaboration among an internist or infectious disease specialist with experience using antiretroviral therapy, an obstetrician with experience dealing with HIV-1 infected mothers, and a pediatrician with HIV-1 expertise. The dual goals of therapy in this setting are to suppress viral replication to benefit the mother and to decrease the risk of transmission of HIV-1 to her baby. With her CD4 cell count and plasma HIV-1 RNA level, she is at risk for disease progression over the next several years and, as mentioned in the preceding text, most experts would recommend antiretroviral chemotherapy to her once she is through her first trimester of pregnancy. Although there is no evidence that tenofovir places fetuses at risk, there is more experience with zidovudine and lamivudine in pregnancy; so it would be preferable to use these agents instead of tenofovir and emtricitabine. Because her virus is not susceptible to efavirenz, it would not be used. Nonetheless, even if her virus were susceptible to the drug, it should not be used in pregnancy because of concerns about teratogenicity. Although d4T and ddI are used less frequently these days, they should be avoided whenever possible in pregnant women because of lactic acidosis, hepatic steatosis, and pancreatitis. Nevirapine has been associated with severe hepatitis, especially in women with CD4 cell count more than 250/mm3 and should be avoided in this patient for that reason. Therefore, she would best be treated with a protease inhibitor as the anchor drug in her regimen. Although nelfinavir is often used in pregnancy, concerns about its potency dampen the enthusiasm for it even in this setting. R/lopinavir is a very reasonable choice, although there are data that suggest increased metabolism of lopinavir during pregnancy. Therefore, drug levels should be followed-up.Before the advent of antiretroviral chemotherapy, the likelihood of transmitting HIV-1 from mother to child was in the range of 25%. Zidovudine monotherapy administered during the third trimester of pregnancy, coupled with intravenous zidovudine during delivery and 6 weeks of zidovudine for the infant, reduced the risk of perinatal transmission to 8%. More potent contemporary antiretroviral chemotherapeutic regimens have reduced this risk to below 1%. The goal of therapy in the mother should be to reduce plasma HIV-1 RNA levels to less than 20 copies/mL by delivery. The baby should also receive antiretroviral chemotherapy as part of the perinatal transmission prevention strategy. The neonate should not be breastfed, regardless of the mother's plasma HIV-1 level, in view of the risk of transmission of HIV-1 by this route.
What would you recommend to her husband with respect to antiretroviral chemotherapy?
Her husband has been infected for more than 5 years and has maintained a low plasma HIV-1 RNA level and a near normal CD4 cell count. Although he is technically not a long-term nonprogressor because he has not been documented to be infected for more than 10 years, his plasma HIV-1 RNA level and CD4 cell count predict that his disease progression risk is very low. Most experts would not recommend antiretroviral chemotherapy to him at this point. Although antiretroviral chemotherapy is not indicated, he should undergo a full initial evaluation for HIV-1 including a PPD, an RPR, and T. gondii and cytomegalovirus serology and should be followed-up at 3- to 6-month intervals for evidence of a rising plasma HIV-1
P.230
RNA level and/or a falling CD4 cell count. He should also be vaccinated against pneumococcal disease. It would also be prudent to test his virus for resistance to antiretroviral drugs to guide selection of his regimen when he requires therapy. Even if his virus is found to be susceptible to efavirenz, it should not be relied upon in his case because it is presumed that his wife acquired her resistant virus from him and it is known that drug-resistant virus may be overgrown by wild type virus in the plasma. In these situations, the lifelong persistence of drug-resistant minor species variants leads to treatment failure if these agents are used.
Suggested Readings
Barnes PF, Bloch AP, Davidson PT, et al. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med 1991;324:1644.
Carpenter CJ, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV Infection in 1998. JAMA 1998;280:78 86.
Connor EM, Sperling RS, Gelber R, et al. Pediatric ACTG Protocol 076 Study Group. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med 1994;331:1173 1180.
Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-na ve patients: a 3-year randomized trial. JAMA 2004;292:191.
Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. AIDS Treatment Guidelines Panel of the Department of Health and Human Services, Web site (http://AIDSinfo.nih.gov), 2006.
Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society USA Panel. JAMA, 2006;296:827 843.
Ho DD, Neumann AU, Perelson AS. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 1995;373:123 126.
Kitahata MM, Koepsell TD, Deyo RA, et al. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients' survival. N Engl J Med 1996;334(11):701 706.
Masur H, Ognibene FP, Yarchoan R, et al. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann Intern Med 1989;111:223.
Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med 1998;338:853 860.
Cellulitis
What factors predispose to the development of cellulitis?
What are the signs and symptoms of cellulitis?
What organisms most frequently cause cellulitis?
P.231
Discussion
What factors predispose to the development of cellulitis?
Although any person can acquire cellulitis, there are several factors that heighten the risk of this infection. Any compromise of skin integrity can introduce organisms into the skin and subcutaneous tissues. Therefore, surgical procedures, trauma, the placement of intravenous catheters, burns, and bite wounds are all factors that predispose to the development of cellulitis. The risk for development of cellulitis is also increased in hosts whose sensation is impaired, such as diabetic patients with peripheral neuropathy whose ability to perceive and react appropriately to trauma is diminished.
Impaired arterial circulation also predisposes to the development of cellulitis. Host immune mechanisms, such as polymorphonuclear leukocytes and complement, are delivered through the circulation. Therefore, if the host circulation is impaired, normal immune mechanisms, which might easily eradicate an organism, cannot be mounted. This is why cellulitis is more frequent in patients with impaired arterial circulation, such as those with diabetes and smokers with peripheral vascular disease.
Patients whose venous and lymphatic drainage is compromised are also less able to clear bacteria from their bodies, and are consequently predisposed to cellulitis. Patients with chronic edema of the lower extremities are particularly vulnerable to cellulitis, which may spread very rapidly. A distinctive form of cellulitis has been found in patients whose saphenous veins have been removed for coronary artery bypass grafting. These patients, who most likely have both venous insufficiency and impaired lymphatic drainage, have been found to acquire cellulitis at the site of the saphenous venectomy. Frequently, the portal of entry for the infection is associated with tinea pedis. Besides the treatment of cellulitis, the tinea pedis should be treated with a topical antifungal agent.
Immunocompromised patients, such as those undergoing chemotherapy or transplantation procedures, are also vulnerable to cellulitis. The infection in these patients may be more difficult to diagnose because the characteristic symptoms and signs may be more subtle owing to the antiinflammatory properties of the immunosuppression.
What are the signs and symptoms of cellulitis?
The classic appearance exhibited by cellulitis is a hot, swollen, red, and tender skin lesion. The patient may be febrile, and regional lymphadenopathy is common. Acute lymphangitis, indicated by red streaks coursing up the patient's limb from the site of the cellulitis, signifies the spread of infection along subcutaneous lymphatic channels. Not all cases of cellulitis are associated with lymphangitis, but it may be the harbinger of serious systemic illness with bacteremia.
What organisms most frequently cause cellulitis?
The most common causes of cellulitis in general are group A streptococci and S. aureus. These gram-positive cocci are normal constituents of the
P.232
human skin flora and are easily introduced into wounds by trauma. Other streptococci may also occasionally cause cellulitis. Although one could rely on -lactamase resistant penicillins or cephalosporins in the past to treat most cases of community-acquired S. aureus infection, there has been a dramatic increase in methicillin-resistant S. aureus infection among patients with community-acquired infection in many parts of the United States. In these areas, presumptive therapy with vancomycin or linezolid, pending identification and susceptibility testing, is required. Practitioners must be aware of local conditions in making empiric antimicrobial choices.Less common pathogens may also be introduced into a wound by trauma. For example, soil-contaminated wounds may become infected with fungi or Clostridium species. Animal bite wounds may become infected with bacteria from the animal's mouth. Erysipeloid, caused by Erysipelothrix rhusiopathiae, is a cellulitis that affects people who handle salt-water fish, shellfish, poultry, meat, or animal hides. Various Vibrio species may cause cellulitis in people with wounds exposed to salt water or raw seafood.
Less frequently, cellulitis may be acquired through bacteremia. Rare cases of pneumococcal cellulitis have been reported. Immunocompromised patients may also acquire cellulitis by means of a bacteremia caused by organisms, such as C. neoformans or E. coli, that are not usual causes of cellulitis in healthy hosts.
Case
A 27-year-old man presents to the emergency room complaining of pain in his right hand. He was well until the previous day, when he sustained a deep scratch at the base of his right thumb while playing with his cat. He washed the wound and bandaged it tightly to stop the bleeding. Overnight, however, his palm began to swell, turned red, and became increasingly painful.
His blood pressure is 120/70 mm Hg, heart rate is 90 beats per minute, respiratory rate is 12 per minute, and temperature is 38.5 C (101.3 F). Physical examination findings are notable for a laceration on the right thenar eminence that is 2 cm long and 0.5 cm deep. The wound is partially crusted over with blood, with a small amount of serosanguineous discharge. The surrounding tissue is erythematous, hot, and exquisitely tender. There are two red streaks ascending the lower half of his anterior forearm. He has a tender, mobile, 1-cm lymph node in the right axilla. There is full range of motion without discomfort in any of the digits or the wrist of his right upper extremity. Neurologic examination of the hand reveals normal findings, and Allen's test result is normal.
The following laboratory data are found: white blood cell count, 15,000/mm3, with a differential count of 75% polymorphonuclear leukocytes, 5% band forms, 17% lymphocytes, 2% monocytes, and 1% eosinophils. His serum chemistry values are normal. A radiographic study of the hand reveals no evidence of a foreign body or subcutaneous emphysema. Gram's staining of the serosanguineous discharge from the wound reveals large numbers of small gram-negative rods and a few gram-positive cocci in chains. Samples of the discharge and blood are sent for culture.
The patient was born and raised in the United States. He has been in good health before this illness and has no history of hospitalizations. He recalls having had a tetanus
P.233
booster shot 7 years ago. He has no history of allergic reactions to medications. His 7-year-old cat was also born and raised in the United States, has received all appropriate vaccinations, and is apparently healthy.
What infectious agents should be considered as possible causes of this patient's cellulitis?
What would be the most appropriate antibiotic treatment for this patient?
In addition to antibiotics, what other measures should be taken to treat this cellulitis?
Case Discussion
What infectious agents should be considered as possible causes of this patient's cellulitis?
Group A streptococci and S. aureus must always be considered as potential causes of cellulitis because they are the most common etiologic agents. In the event of animal bites or scratches, the oral flora of the animal may be an important source of infection as well. Pasteurella multocida is found in the oropharynx of 50% to 70% of healthy cats and 12% to 60% of healthy dogs. This gram-negative rod is frequently implicated in infections resulting from cat bites or scratches, and is found less often in wounds inflicted by dogs. Other important animal oral flora to consider in patients with bites and scratch wounds include aerobic and anaerobic streptococcal organisms, as well as gram-negative anaerobes such as Bacteroides species and Fusobacterium. Organisms found in soil, such as Clostridia species, may also be transmitted by scratches or bites.
The rapid tempo of this patient's illness, with the development of an exquisitely painful cellulitis within 24 hours of a cat scratch, is characteristic of P. multocida infection, although such a rapid course may also be seen in the setting of streptococcal infections. It would be unusual, however, for a staphylococcal infection to progress this rapidly. Moreover, the discharge from a staphylococcal infection would more likely be purulent than serosanguineous. The finding of many gram-negative rods on the Gram's-stained specimen of the wound discharge also suggests a P. multocida infection, or a gram-negative anaerobic infection. However, a few gram-positive cocci in chains were also found, making streptococcal infection a part of the differential diagnosis.
What would be the most appropriate antibiotic treatment for this patient?
This patient has a serious hand infection, along with an impending systemic illness. Anyone with such a serious hand infection should be hospitalized and receive intravenous antibiotics to prevent advancing infection, as well as to avert the potentially devastating consequences of suboptimal therapy. Penicillin is the drug of choice for P. multocida infections, and would also be effective for the management of both streptococcal and anaerobic infections. Therefore, intravenous penicillin would be the best antibiotic in this case. For patients who are allergic to penicillin, tetracycline is the best alternative drug for the treatment of P. multocida infections. The patient should also be seen in consultation with a hand surgeon to be certain that surgical intervention for drainage or decompression is not required.
In addition to antibiotics, what other measures should be taken to treat this cellulitis?
Overestimating the efficacy of antibiotics, and underestimating the critical roles played by debridement, drainage, wound elevation, and immobilization, are probably the most frequent mistakes made in the treatment of cellulitis. Drainage of a closed-space infection and removal of necrotic tissue are essential for curing any infection. Even when the appropriate antibiotics are administered, an infection can worsen if abscesses or necrotic tissue are not drained or removed. The reason for this is that abscesses and necrotic tissue are not well vascularized, making them inaccessible to both the antibiotics and the host immune mechanisms, such as polymorphonuclear leukocytes and complement, which are normally conveyed through the bloodstream. Therefore, in these inaccessible regions bacteria can freely multiply and, in some instances, such infection can result in sepsis and death despite an appropriate antibiotic regimen.
Abscesses tend to develop in the setting of P. multocida infection. In addition, the hand contains several physiologic spaces, such as the thenar eminence, that can serve as pockets of infection. Therefore, a P. multocida cellulitis of the hand may require surgical debridement and drainage. Incision of a hand wound should not be performed by a novice, because there is a great potential for damaging internal structures or creating wounds that would result in serious contractures. A hand surgeon should be consulted for this purpose.
The objective of elevation and immobilization in the treatment of cellulitis is to diminish the edema, which impedes the blood flow to an infected region. Elevation of the affected limb above the level of the heart is necessary to achieve optimal results. In the event of a lower extremity cellulitis, merely placing the affected limb on a chair while seated is not adequate because the abdominal contents still exert pressure on the lymphatic vessels in this position, thereby perpetuating the edema. In addition to the measures just described, this patient should receive a tetanus booster shot. Any patient with a bite or deep scratch wound who has not had a tetanus booster shot within the preceding 5 years should receive one.
P.234
Suggested Readings
Centers for Disease Control. Diphtheria, tetanus, and pertussis: guidelines for vaccine prophylaxis and other preventive measures. Ann Intern Med 1985;103:896.
Elliot DL, Tolle SW, Goldberg L, et al. Pet-associated illness. N Engl J Med 1985;313:985.
Francis DP, Holmes MA, Brandon G. Pasteurella multocida: infections after domestic animal bites and scratches. JAMA 1975;233:42.
Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 2005;352:1436 1444.
Goldstein EJC. Bites. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases, 6th ed. New York: Elsevier Science, 2005:3552.
Miller LG, Perdreau-Remington F, Reig G, et al. Fourteen patients with necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 2005;352:1445.
Stevens DL, Herr D, Lamperis H, et al. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2002;34:1481.
P.235
Swartz MN, Pasternak MS. Cellulitis and subcutaneous tissue infections. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practices of infectious diseases, 6th ed. New York: Elsevier Science, 2005:1172.
A Late Complication of Tuberculosis
What are the goals of the modern drug treatment of active pulmonary tuberculosis?
What factors are likely to promote relapse?
What factors are likely to foster the acquisition of drug-resistant disease?
Discussion
What are the goals of the modern drug treatment of active pulmonary tuberculosis?
Fundamental to the modern drug treatment of tuberculosis is the use of multiple-drug regimens. There are two goals to this approach.
The first object of multiple-drug treatment is to prevent the emergence of resistant organisms. The findings from early studies on the use of streptomycin dramatically demonstrated the futility of monotherapy, in that patients with severe disease showed an initial gratifying response to treatment, but after some weeks their condition began to deteriorate. Their sputum smears became positive for organisms once again, and drug-resistant disease developed. It is believed that monotherapy selects for, rather than induces the mutation of, resistant organisms. Therefore, the larger the population of organisms, the higher the likelihood that resistant organisms are present. Therefore, in the setting of an asymptomatic primary infection that involves few organisms, monotherapy (usually consisting of isoniazid) can be used safely as prophylaxis. In patients with active disease (especially cavitating pulmonary disease in which the burden of infection is immense), the probability of resistant organisms is high. Mutations leading to drug resistance are unlinked, however, so the use of two drugs (e.g., isoniazid plus rifampin) effectively prevents the emergence of secondary drug resistance (i.e., drug resistance acquired during treatment).
The second goal of therapy is to shorten the duration of treatment. To achieve a lasting cure in a high proportion of cases, regimens that comprise only rifampin plus isoniazid must be continued for 9 months. However, this can be reduced to 6 months by the addition of pyrazinamide for the first 2 months. Pyrazinamide is a powerful sterilizing drug that may exert its effect by acting on special subpopulations of organisms, such as those in a more acid environment. It has been shown that there is no additional benefit in continuing this expensive drug beyond the first 2 months.
A factor to be considered when planning multidrug treatment is that initial drug resistance exists when the disease is caused by organisms that are resistant to at least one drug before any treatment is given. When this is suspected on epidemiologic grounds, an additional drug (usually streptomycin
P.236
or ethambutol) is added to the regimen during the first 2 months, while the results of drug susceptibility studies are awaited. This approach reduces the risk of only one effective drug being given.What factors are likely to promote relapse?
Relapse (i.e., the endogenous reactivation of previously treated tuberculosis) is most likely to occur during the first year after the end of treatment and in those patients who initially had more extensive disease. Patients who discontinue their treatment early are most likely to have a relapse. Therefore, ensuring compliance with treatment is central to preventing relapse.
What factors are likely to foster the acquisition of drug-resistant disease?
For the reasons already outlined, drug-resistant organisms emerge when a patient effectively receives only monotherapy. This may occur for a variety of reasons, and the following illustrates how it can happen. A patient on rifampin plus isoniazid may sell his powerful red rifampin capsules to his friends (or the witch doctor) for use in the treatment of gonorrhea and then take only the isoniazid himself, resulting in isoniazid monotherapy! This man acquires isoniazid resistance, cough recurs, and ethambutol is added by a kindly physician, which effectively now constitutes ethambutol monotherapy. Soon, resistance to ethambutol emerges and the man's health continues to decline. Perhaps he will start taking his rifampin, which means he is receiving rifampin monotherapy.
Such patients first need to know that they must either take both medications and get better, or take neither and get worse, but at least, in this latter instance, the disease remains drug susceptible. It is always a mistake to add one drug at a time to a failing regimen. Instead, at least two new drugs should be added to protect against the emergence of resistance to each other. Fully supervised therapy prevents scenarios such as these from happening, but, unfortunately, at present it is not feasible on a global scale.
Case
A 73-year-old man is admitted because of a 3-month history of intermittent hemoptysis. Approximately once a week he has been coughing up small amounts of blood-streaked sputum, but, on the day before admission, he started to cough frequently and produced approximately half a cupful of red and clotted blood over a 24-hour period. The patient had emigrated to America in the 1940s after having been interned in a labor camp in Europe during World War II. A medical examination at the time of his liberation revealed he had tuberculosis. He was then admitted to a sanatorium, where he stayed for 18 months, with treatment consisting of artificial pneumothorax. In the 1950s, he had a relapse and was treated for 18 months with isoniazid, paraaminosalicylic acid, and streptomycin. He continued to smoke a pack of cigarettes per day until an attack of pneumonia 5 years before, which caused him to stop smoking. For the past year, he has been increasingly disabled by exertional dyspnea, such that he is now unable to climb a flight of stairs without stopping. He has also had recurrent exacerbations of breathlessness with productive cough, but no previous hemoptysis. He has recently noted increasing ankle edema. He has no history of weight loss or fever.
P.237
On examination, he is found to be thin and anxious, afebrile, and normotensive, with a regular pulse of 110 beats per minute. He is slightly tachypneic and has both central and peripheral cyanosis. The jugular venous pulse is visible approximately 3 cm above the clavicle when he is at an angle of 45 degrees. He has a discrete, firm, nontender lymph node that is enlarged to approximately 2 cm in the right supraclavicular fossa. The trachea is deviated to the right, and the right upper chest is noted to be indrawn below the clavicle; it is dull to percussion and bronchial breath sounds are heard. The apex of the heart is not palpable and auscultation of the heart reveals a loud pulmonary second sound. He has bilateral ankle edema.
The chest radiographic study on admission depicts bilateral, severe fibrotic lung disease, which is most marked in the upper lobes, with elevation of the hila; the abnormalities are more pronounced on the right. The horizontal fissure is elevated on the right and projects upward. Deviation of the trachea to the right is confirmed. There are thin-walled cavities bilaterally, and a cavity on the right is found to contain an opacity that is outlined by a crescent-shaped rim of air. A review of his laboratory records reveals that serum precipitins for aspergillus were found in his blood on a test sent from the outpatient department earlier in the month.
What is the most likely diagnosis in this patient?
If massive hemoptysis supervenes, how should this be managed?
What is the most useful investigation to confirm the diagnosis?
Should the patient receive intravenous amphotericin B?
What additional late complication of tuberculosis does this patient exhibit, and how can this be relieved?
Case Discussion
What is the most likely diagnosis in this patient?
Diagnoses that should be considered in this patient include aspergilloma, reactivation of the tuberculosis, carcinoma of the bronchus, and bronchiectasis. However, this patient exhibits the classic clinical picture of aspergilloma. Aspergillus colonizes and grows saprophytically in cavities created by preexisting lung disease (typically those caused by tuberculosis, although occasionally other diseases such as sarcoidosis, bronchiectasis, or pulmonary fibrosis can cause the formation of cavities hospitable to such infection). A fungus ball develops in the preexisting cavity, which is lined with bronchial epithelium or granulation tissue. Chest radiographic studies, tomograms, or computed tomographic (CT) scans can show the rounded opacity within the cavity, together with a crescent-shaped rim of air between the cavity and its wall. The ball may lie free within the cavity (in which case it can be seen to change position on decubitus chest radiographs) or it may be attached by granulation tissue. Often the patient is asymptomatic, but hemoptysis is the most important complication of aspergilloma. Serum precipitins to aspergillus are further supportive evidence for this diagnosis because these are found in 90% to 95% of cases of aspergilloma.
In this patient, reactivation of the tuberculosis is less likely than aspergilloma because, despite a 3-month history, the patient has not lost weight or had a fever. In
P.238
addition, the cavities seen on the chest radiographs have thin walls, suggesting the presence of inactive disease. However, radiologically, it is impossible to distinguish with certainty between active and inactive tuberculosis.Carcinoma of the bronchus might also be expected to cause weight loss, and it is an important consideration in patients with a history of tuberculosis because they are at higher risk than the general population because of the carcinoma (usually adenocarcinoma or alveolar cell carcinoma) that can form in scarred tissue, as an aftermath of infection. This patient is also at risk for bronchial carcinoma because of his long history of smoking.
Tuberculosis commonly leads to bronchiectasis, which would undoubtedly coexist in a patient like this who has severe destructive lung disease. However, this patient's episode is dominated by hemoptysis, rather than by the expectoration of copious, purulent sputum, which would be more suggestive of an exacerbation of bronchiectasis.
If massive hemoptysis supervenes, how should this be managed?
The major risk from aspergilloma is life-threatening hemoptysis. The prognosis for pulmonary aspergilloma is negatively influenced by a number of factors including the documentation of increasing size or numbers of the aspergillomas on chest radiographs, severe underlying lung disease, increasing Aspergillus-specific immunoglobulin G (IgG) antibodies, immunosuppression such as corticosteroids or HIV infection, and the presence of sarcoidosis. Surgical removal is the preferred treatment in the setting of life-threatening hemoptysis due to aspergilloma, although in this patient (as in many with aspergilloma), severe underlying lung disease indicates the likelihood of a poor outcome after pulmonary surgery. Embolization of the bronchial artery has been used successfully to control severe hemoptysis due to tuberculosis, among other causes, but has not been successful in the management of severe hemoptysis caused by aspergilloma, probably because of the large collateral circulation involved.
While arrangements are made for definitive management, a patient with severe hemoptysis should be positioned on the side of the suspected source (in this case, the right side) to minimize flooding of the unaffected lung with blood. Sedation of the patient is likely to be required. An intravenous line should be established and blood crossmatched and administered when needed.
What is the most useful investigation to confirm the diagnosis?
No investigation (other than pathologic analysis of the surgical specimen) is specific in confirming the diagnosis of aspergilloma, but Aspergillus precipitins are present in a high proportion of cases of aspergilloma and can serve to confirm the diagnosis in a patient with the characteristic clinical and radiologic presentation, such as that described here. Sputum culture for Aspergillus organisms is less helpful because it may yield no organisms if the cavity does not communicate with the bronchus. Skin tests with Aspergillus antigens are also less reliable.
Microscopic examination of the sputum for acid-alcohol fast bacilli should be done in a case such as this to exclude coexisting active tuberculosis, although, both clinically and radiologically, this is a less likely diagnosis. A reasonable precaution
P.239
would be to place the patient in respiratory isolation until negative smear results have been obtained.Should the patient receive intravenous amphotericin B?
There have been no prospective studies comparing the outcome in patients with aspergilloma treated with intravenous amphotericin B versus the outcome in untreated patients. The findings from retrospective studies suggest, however, that this treatment confers no beneficial effect, and this is not surprising, given that the fungal ball is isolated from the bloodstream. Asymptomatic patients may simply be monitored and resolution may occur spontaneously. Prophylactic surgical removal may be considered in patients who are fit for the procedure because of the potential of aspergilloma to cause fatal hemoptysis, and because surgery may effect a lasting cure. However, the poor exercise tolerance and the clinical findings indicating respiratory failure in this case suggest that the patient would be unlikely to tolerate the procedure. This could be confirmed by formal pulmonary function tests. Poor prognostic indicators would be an arterial blood gas analysis showing an elevated partial pressure of carbon dioxide (PCO2) and a forced expiratory volume of less than 1 L per second.
What additional late complication of tuberculosis does this patient exhibit, and how can this be relieved?
The patient has central cyanosis, indicating that he has hypoxia at rest. This is an additional late complication of pulmonary tuberculosis, probably exacerbated in his case by chronic obstructive pulmonary disease due to smoking. The hypoxia has resulted in pulmonary vasoconstriction and, hence, in pulmonary hypertension (indicated by the loud pulmonary second sound heard on auscultation of the heart). This, in turn, has resulted in right ventricular failure (indicated by the raised jugular venous pressure and edema), or cor pulmonale. Continuous administration of oxygen is indicated for relief of this syndrome.
Suggested Readings
Akbari JG, Varma PK, Neema PK, et al. Clinical profile and surgical outcome for pulmonary aspergilloma: a single center experience. Ann Thoracic Surg 2005;80:1067.
Glimp RA, Bayer AS. Pulmonary aspergilloma: diagnostic and therapeutic considerations. Arch Intern Med 1983;143:303.
Greene R. The radiological spectrum of pulmonary aspergillosis. Med Mycol 2005;43(Suppl 1):S147.
Kauffman C. Quandary about treatment of aspergillomas persists. Lancet 1996;347:1640.
Kim YT, Kang MC, Sung SW, et al. Good long-term outcomes after surgical treatment of simple and complex pulmonary aspergilloma. Ann Thorac Surg 2005;79:294.
Mitchison DA. Basic mechanisms of chemotherapy. Chest 1979;76(6 Suppl):771.
Shapiro MJ, Albelda SM, Mayock RL, et al. Severe hemoptysis associated with pulmonary aspergilloma: percutaneous intracavitary treatment. Chest 1988;94:1225.
Stevens DA, Virginia L, Kan VL, et al. Practice guidelines for diseases caused by aspergillus. Clin Infect Dis 2000;30:696.
P.240
Sepsis
Is there a clinical distinction between bacteremia and sepsis?
What is the distinction between chills and rigors?
What factors are associated with a poor prognosis in the setting of gram-negative sepsis?
Discussion
Is there a clinical distinction between bacteremia and sepsis?
It is important to differentiate among bacteremia, sepsis, and septic shock. Bacteremia is defined as the presence of viable bacteria in the blood, as demonstrated by a positive blood culture. Bacteremias may be further classified as transient, sustained, or intermittent, depending on the length of time blood cultures are positive. Transient bacteremias are common and last for only several minutes. When multiple blood cultures are positive over the course of several hours to several days, this indicates a sustained bacteremia. Intermittent bacteremias are those in which the blood cultures are intermittently positive. Sepsis is a clinical term that refers to a physiologic state that is associated with severe infection. In septic shock, there is hypotension (systolic blood pressure <90 mm Hg or a one-third reduction from the prior systolic blood pressure) and evidence of end-organ damage secondary to reduced blood flow.
What is the distinction between chills and rigors?
It is very important to know the difference between chills and rigors. Rigor (a true shaking chill) is very often associated with bacteremia. The patient may experience teeth chattering and body tremors that usually last for 15 to 30 minutes. A chill is more appropriately described as a chilly sensation, not a clinical presentation. Rigors may be seen in the setting of viral infections as well as bacteremias.
What factors are associated with a poor prognosis in the setting of gram-negative sepsis?
Despite advances in supportive therapy, the mortality rate associated with gram-negative septic shock approaches 40%. Factors that contribute to this poor prognosis are increased age, poor nutritional status, steroid use, cirrhosis, diabetes, congestive heart failure, and granulocytopenia. Outcome is also adversely affected by volume depletion, inappropriate antibiotic use, and delay in therapy.
Case
A 74-year-old white man with Alzheimer's disease is brought to the emergency room by ambulance after a 1-day history of fever and mental status changes. On arrival in the emergency room, his blood pressure is found to be 100/60 mm Hg, heart rate is 100 beats per minute, temperature is 38.5 C (101.3 F), and respiratory rate is 24 per minute. The patient is unable to give any history; however, his wife states that he had been in his usual health until the evening before admission, when he began to complain of generalized abdominal pain and had become more confused than usual.
P.241
Physical examination reveals an agitated elderly man who is in no acute distress. His oral mucosa is dry and the lung examination reveals decreased breath sounds at the bases bilaterally. Cardiac examination reveals sinus tachycardia. Abdominal examination reveals normal bowel sounds and a palpable mass in the lower abdomen extending from 2 cm below the umbilicus down to the pelvis. Rectal examination reveals an enlarged, firm prostate, and the stool is heme negative. His extremities are cool and clammy and there is decreased skin turgor.
Admission laboratory results are as follows: white blood cell count, 16,000/mm3 with a differential count of 85% polymorphonuclear leukocytes, 10% band forms, and 5% lymphocytes; hematocrit, 47%; creatinine, 2.3 mg/dL; blood urea nitrogen, 40 mg/dL; sodium, 141 mEq/L; potassium, 4.5 mEq/L; chloride, 107 mEq/L; and carbon dioxide, 17 mEq/L. Arterial blood gas measurement performed on room air reveals a pH of 7.29, a PO2 of 68 mm Hg, and a PCO2 of 30 mm Hg. His chest radiographic findings are unremarkable. The patient is asked for a urine specimen but is able to void only 5 mL of cloudy, dark yellow urine, which is sent to the laboratory for urinalysis and culture.
A Foley catheter was subsequently placed and 500 mL of foul-smelling urine was obtained. A Gram's stain revealed numerous polymorphonuclear cells and gram-negative rods. On repeat examination, his abdomen was found to be soft and the mass had disappeared.
What system is the likely source of infection in this patient, and how could infection at this site explain his other signs and symptoms?
What group of organisms is most likely associated with the sepsis syndrome in this patient, and how does this group differ from the other major groups of bacteria and fungi?
How does endotoxin affect macrophages, and what chemical signals are produced by macrophages to contribute to the sepsis syndrome?
Of what should the initial management of a patient with the sepsis syndrome consist?
Case Discussion
What system is the likely source of infection in this patient, and how could infection at this site explain his other signs and symptoms?
The most likely diagnosis that fits with this patient's constellation of symptoms is urosepsis. As brought to light by the physical examination, the patient has the signs of septic shock impaired tissue perfusion, hypotension, and lactic acidosis in association with positive blood cultures. Tachycardia, tachypnea, and oliguria are also usually seen in the setting of genitourinary, gastrointestinal, biliary, and gynecologic infections, and therefore are not specific to urosepsis. Abnormalities in mental status may also be a feature of the initial presentation, even without infection in the CNS. In elderly patients, the symptoms of mental obtundation may be subtle and consist only of withdrawal or agitation, and they may constitute the sole indication of severe infection. The chest radiographic study in this patient was negative with no evidence of an infiltrate, making pneumonia unlikely. However, the clinician must always keep in mind that with hydration an infiltrate may blossom, so the patient's
P.242
respiratory status should be monitored closely. Because the mental status changes may be the only early manifestation of sepsis in the elderly, more often than not a lumbar puncture yields normal fluid. This patient's physical examination findings were also remarkable for an abdominal mass, and indeed he had complained of diffuse abdominal pain for at least 1 day before admission. Certainly, elderly patients may have appendicitis, diverticulitis with an abscess, or a colon carcinoma with subsequent bacteremia, and all these conditions must be included in the differential diagnosis. He was noted to have an enlarged prostate and also had difficulty voiding.What group of organisms is most likely associated with the sepsis syndrome in this patient, and how does this group differ from the other major groups of bacteria and fungi?
The organisms most frequently isolated in the blood of patients with the sepsis syndrome are gram-negative bacilli. Shock occurs less frequently in the setting of bacteremia due to gram-positive organisms. This difference may stem from variations in the host response to different bacterial cell wall constituents. The lipopolysaccharide portion of the cell wall of gram-negative bacilli (called endotoxin) elicits a vigorous inflammatory response when injected intravenously into animals. The inflammatory response to lipoteichoic acid, a cell wall constituent of gram-positive organisms, is much less pronounced. This patient had not been hospitalized, nor had he undergone any kind of instrumentation. Therefore, the most likely organism to cause a UTI with subsequent bacteremia and sepsis syndrome in this patient is E. coli. Other potential gram-negative organisms that may precipitate septic shock include Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterobacter aerogenes, and Serratia marcescens. The primary portal of entry is the genitourinary tract, but the gastrointestinal tract, respiratory tract, and skin are also important sources of bacteremia. Enterococcus must also be considered as a potential cause of this patient's illness because it can frequently cause prostatitis. Other gram-positive organisms, such as coagulase-positive and coagulase-negative Staphylococcus species, can certainly cause bacteremia and sepsis syndrome; however, this occurs most commonly in hospitalized patients who have had some type of intravascular device installed. Gram's stains are particularly useful in establishing presumptive diagnoses in UTIs, as it was in this case.
How does endotoxin affect macrophages, and what chemical signals are produced by macrophages to contribute to the sepsis syndrome?
Several bacterial factors are powerful mediators of sepsis, and one of the most potent is endotoxin. As stated, endotoxin is the lipopolysaccharide component of the cell wall in gram-negative bacteria. It appears that when cell injury occurs with the activation of immune defenses or the initiation of antimicrobial therapy, bacterial cell lysis takes place and the titer of detectable endotoxin in the patient's blood rises dramatically. Endotoxin binds to the CD14 molecule on the surface of macrophages that activate one of the members of the Toll-like receptor family (TLR-4). TLR-4 triggering activates a cascade of inflammatory cytokines including tumor necrosis factor (TNF- ) and a number of additional downstream mediators including interleukin (IL)-1, IL-2, IL-6, and platelet-activating factor. After the release of TNF- , IL-1, and platelet-activating factor, arachidonic acid is metabolized to form leukotrienes,
P.243
thromboxane A2, and prostaglandin E2. IL-1 and IL-6 activate T cells to produce interferon- , IL-2, IL-4, and granulocyte macrophage colony-stimulating factor. The coagulation cascade and complement system are also activated (Fig. 6-1). Clinically, this phenomenon results in a low central venous or pulmonary capillary wedge pressure, as well as a marked decrease in total systemic vascular resistance. In addition, there is a compensatory increase in cardiac output in an attempt to maintain arterial perfusion. The end result of this process is an increase in cardiac output, a marked fall in peripheral vascular resistance, and hypotension. If uncontrolled, progressive lactic acidosis ensues, ultimately leading to death.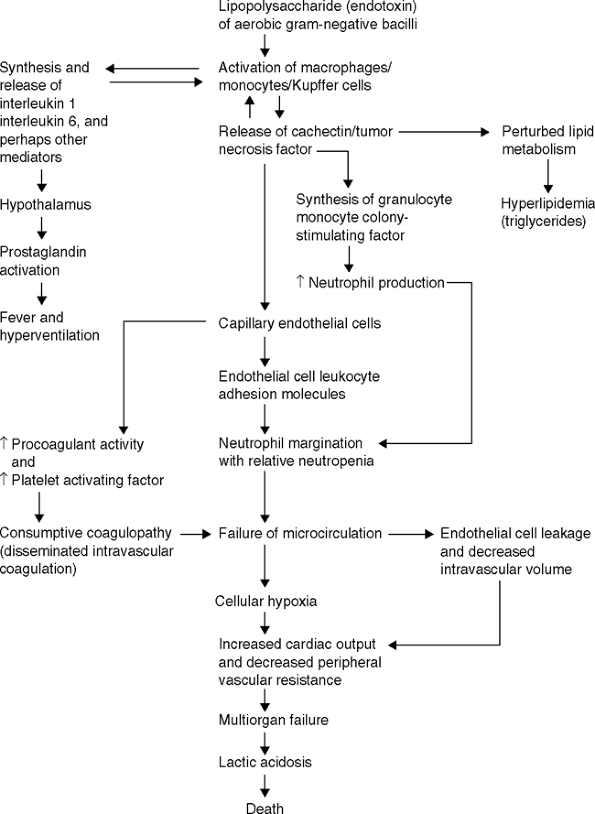
Figure 6-1 Pathogenesis of the microvascular injury and death due to endotoxin shock. (From
Karchmer AW, Barza M, Drew WL, et al. Infectious disease medicine (MKSAP IX). Philadelphia: American College of Physicians, 1991
.)Of what should the initial management of a patient with the sepsis syndrome consist?
Although antibiotic therapy is the mainstay of treatment of sepsis caused by gram-negative organisms, the amelioration of underlying conditions, elimination of predisposing factors, drainage of abscesses, removal of infected foreign bodies, and adequate supportive care are also of paramount importance for curing the infection. It is critical to remember that the immediate therapeutic intervention should be directed at increasing cardiac output and oxygen delivery to prevent or minimize hypoperfusion and reduce tissue hypoxia. An optimal intravascular volume must be restored and maintained. Fluid requirements are very unpredictable because of capillary leak, and, at the very least, central venous pressures should be monitored so that these requirements can be appropriately met. Respiratory status and acid base disturbances can be observed with serial arterial blood gas measurements, which are also helpful in determining a patient's prognosis. The chance for survival is reduced in patients who are acidemic. The next step is to obtain appropriate cultures and administer appropriate bactericidal agents. The antibiotic regimen should be chosen on the basis of the presumed source of the bacteria and the susceptibility pattern of organisms from that source. Antimicrobial therapy should be adjusted on the basis of microbiologic data as they become available. Additional therapies that are under investigation are based on the emerging knowledge of the pathophysiologic sequence of bacteremic shock. The conduct and interpretation of many of these studies have been complicated by a lack of precision of entry criteria that make the extrapolation of study results to clinical practice, difficult.
Suggested Readings
Abraham E, Laterre PF, Garg R, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med 2005;353:1332.
Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA 2003;290:238.
Cross AS, Opal SM. A new paradigm for the treatment of sepsis: is it time to consider combination therapy? Ann Intern Med 2003;138:502.
Munford RE. Sepsis, severe sepsis and septic shock. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practices of infectious diseases, 6th ed. New York: Elsevier Science, 2005:906.
Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med 2003;9:517.
P.244
P.245
Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA 1997;278:234.
Takeda K, Kaisho T, Akira S. Toll like receptor. Annu Rev Immunol 2003;21:335.
Endocarditis
What types of organisms can cause infective endocarditis?
What are some important factors that increase the risk for the development of endocarditis?
What are the two clinical types of bacterial endocarditis and their clinical characteristics?
What conditions call for surgical intervention?
Are there any differences in the characteristics of endocarditis between the intravenous drug abuse population and non drug abusers?
Discussion
What types of organisms can cause infective endocarditis?
There are many organisms that can infect the heart and cause endocarditis. The most common ones are traditional bacteria, but other organisms including fungi, Rickettsia, and Chlamydia may invade myocardial tissues and produce disease.
What are some important factors that increase the risk for the development of endocarditis?
The two major classes of risk factors contributing to the development of endocarditis include structural damage to cardiac tissue in contact with blood and conditions associated with bacteremia. Underlying heart diseases such as rheumatic valvular damage, a bicuspid aortic valve, patent ductus arteriosus, and small ventricular septal defects cause damaged tissue or abnormal blood flow, conditions under which bacteria can adhere to the surface and cause infection. Also implicated for the same reasons are prosthetic valves. Intravenous drug abusers are at risk for endocarditis because their valves are being constantly bombarded with impurities such as talc, which causes scarring of the valves, and also because they mix their drug of choice with contaminated water. Nosocomial infections may result from the placement of intravenous catheters or pacemaker wires, or from wound infections or genitourinary manipulation. Elderly patients are also at increased risk for endocarditis.
What are the two clinical types of bacterial endocarditis and their clinical characteristics?
Although there is overlap, the two clinical types of bacterial endocarditis are acute and subacute. Acute bacterial endocarditis is most commonly associated with intravenous drug abuse, intravenous catheter infection, and prosthetic valve infections. These infections may be rapidly fatal if left untreated, and surgical repair or replacement of the damaged valve may be necessary. Subacute
P.246
endocarditis develops most often in the setting of structural heart disease (e.g., mitral valve prolapse), a history of rheumatic heart disease, or prosthetic valves. It also affects elderly patients, or it may occur in the setting of no known valvular disease. Its onset tends to be more indolent. Symptoms such as weakness, fatigue, night sweats, and weight loss may have existed for weeks to months before diagnosis. Its onset may be related to antecedent events such as dental work, although no definite predisposing event is apparent in most cases. Because some patients may have multiple risk factors and exhibit a variable clinical picture, their disease cannot be easily classified. It is always important to keep in mind the maxim that if you don't think about endocarditis, you won't diagnose it!What conditions call for surgical intervention?
Surgical intervention should be considered if (a) there is more than one embolic event; (b) bacteremia persists despite 2 to 3 weeks of adequate antibiotic therapy; (c) there is progressive or refractory congestive heart failure; (d) there is significant valvular dysfunction resulting in moderate to severe congestive heart failure as demonstrated by echocardiography or other laboratory techniques; (e) local suppurative complications arise, as reflected by the appearance of new, persistent electrocardiographic conduction disturbances, echocardiographic evidence of a paravalvular abscess or fistula, purulent pericarditis, or persistent unexplained fever despite appropriate antibiotic therapy; (f) there is fungal endocarditis; or (g) there is appropriately treated prosthetic valve endocarditis due to drug-resistant organisms that recur despite appropriate antimicrobial therapy.
Are there any differences in the characteristics of endocarditis between the intravenous drug abuse population and nonaddict population?
There are several characteristics of endocarditis relatively unique to intravenous drug abusers, although these are only generalizations. A history of documented prior heart disease is unusual, and the incidence of tricuspid valve involvement is approximately 50% in this population, which is much higher than that in the nonaddict population. In addition, a murmur is frequently undetectable and there is isolated tricuspid valve involvement, unlike the murmurs of aortic or mitral valve insufficiency seen most commonly in the nonaddict population.
Case
A 27-year-old white man presents to the emergency room with a chief complaint of fevers, shaking chills, cough, and headache of 2 days' duration. He denies nausea, vomiting, diarrhea, or dysuria. History reveals that the patient smokes one pack of cigarettes per day, drinks a six-pack of beer per day, and has recently started skin-popping cocaine. He has had no previous hospitalizations nor has he undergone any surgical procedures.
Physical examination reveals a temperature of 39.0 C (102.2 F), blood pressure of 120/80 mm Hg, pulse of 114 beats per minute, and respiratory rate of 18 per minute. His conjunctivae are normal. His oral mucosa is moist and his dentition is good. Lung examination reveals some coarse rhonchi bilaterally. Cardiac examination reveals a grade 2/6
P.247
systolic murmur that is heard best at the left sternal border but does not radiate. Abdominal and extremity findings are unremarkable. Neurologic examination reveals nonfocal findings, although the patient does complain of a global headache. There is no meningismus.
Laboratory values are as follows: white blood cell count, 18,000/mm3 (85% polymorphonuclear cells, 10% bands, and 5% lymphocytes); hematocrit, 38%; and platelets, 170,000/mm3. A chest radiographic study reveals bilateral nodular infiltrates. The patient is admitted to the medical service for further evaluation and treatment.
What type of endocarditis does this patient likely have?
What is the likely cause of his pulmonary infiltrates?
What are the most common offending pathogens in this setting?
What would you prescribe as an initial antibiotic regimen?
Case Discussion
What type of endocarditis does this patient likely have?
This patient's clinical presentation illustrates a case of acute endocarditis. Tricuspid valve (right-sided) endocarditis is most likely because it is commonly associated with intravenous drug abuse, although the mitral and aortic valves could also be involved.
What is the likely cause of his pulmonary infiltrates?
The cause of this patient's pulmonary infiltrates is septic emboli that have traveled to the lung. In both acute and subacute bacterial endocarditis, signs and symptoms of embolic phenomena may appear. These episodes of vascular occlusion cause pain in the chest (pulmonary or coronary), abdomen (mesenteric or splenic), or the extremities. Bone pain (particularly vertebral and sacroiliac) is also common because of the hematogenous spread of infection to these sites. Other embolic phenomena that may occur include hematuria (emboli to the kidneys), blindness resulting from retinal artery occlusion, and acute neurologic symptoms (stroke, meningitis, seizures, and headache). Certainly, cardiac involvement such as congestive heart failure may occur in this setting as the result of progressive valvular insufficiency or myocarditis; however, this would be evidenced by the finding of Kerley's B lines or fluffy pulmonary infiltrates on chest radiographic studies.
What are the most common offending pathogens in this setting?
The organism that would most likely be the source of this patient's infection is S. aureus. This organism accounts for approximately 20% of the cases of endocarditis in the general population, and for 55% of the cases associated with intravenous drug abuse. It should therefore be suspected as the etiologic agent in infections associated with a history of intravenous drug abuse, as well as in the context of acute embolic phenomena and acute bacterial endocarditis. Coagulase-negative staphylococci are common in the setting of prosthetic valve endocarditis, but not in the setting of nonprosthetic valve associated infection. Streptococci account for approximately 70% of all cases of native valvular bacterial endocarditis in the nonaddict population, and infection due to the various species is broken down as follows: 40% due to viridans streptococci; 10% due to enterococci (group E
P.248
streptococci); and 20% due to other nonhemolytic, microaerophilic, anaerobic, or nonenterococcal group D streptococci. Approximately 10% of the cases are caused by other fastidious organisms, such as fungi and gram-negative bacilli.What would you prescribe as an initial antibiotic regimen?
The initial treatment of suspected acute bacterial endocarditis should be directed toward S. aureus because it is the most common organism in patients with acute bacterial endocarditis. The clinician should always draw three to four blood specimens for culture before initiating antibiotic therapy. After this is done, vancomycin (1 g intravenously every 12 hours) plus gentamicin (1 mg/kg intravenously every 8 hours) are appropriate as an initial combination until the culture results are known. This combination covers both S. aureus (methicillin sensitive and resistant) and enterococci infections, and, with few exceptions, any other likely bacteria. In the past, initial therapy might have consisted of nafcillin and gentamicin but the increasing incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections makes vancomycin a more prudent empiric choice, especially in communities in which MRSA is frequent. The antimicrobial regimen should be adjusted on the basis of results from the clinical microbiology laboratory, including blood cultures and susceptibility tests. Although there are a variety of recommendations in the literature, it is generally agreed that a prolonged administration of relatively high doses of bactericidal agents is indicated. With the exception of infection caused by highly resistant organisms, it is usually fairly easy to obtain a good symptomatic response (e.g., decline in fever and decreased myalgias) and sterilization of blood cultures within a few days of the start of therapy. A bacteriologic cure with sterilization of the lesions is much more difficult, however, because although valvular lesions are bathed in blood, the valves themselves are relatively avascular. Bacteria in vegetations are surrounded by fibrin. This, in combination with the high flow rates in the cardiac chambers, makes it difficult for phagocytic cells to adhere to the site of infection. Therefore, prolonged treatment with high doses of bactericidal antibiotics is essential for cure. There is invitro and invivo evidence that low-dose gentamicin in combination with semisynthetic penicillin effects more rapid killing of staphylococci and sterilization of valves than does penicillin alone. This suggests that the addition of gentamicin (1 mg/kg every 8 hours for 3 to 5 days) is a reasonable regimen (if the patient has no contraindications to aminoglycoside use, such as renal failure) in an attempt to clear the bacteremia rapidly and minimize damage to the heart valves. There are, however, no data from randomized, blinded studies showing that this approach has an impact on the ultimate clinical outcome. The use of combination therapy has also permitted shorter course therapy of right-sided S. aureus infective endocarditis in intravenous drug users.
Suggested Readings
Brandriss MW, Lambert JS. Cardiac infections. In: Reese RE, Betts RF, eds. A practical approach to infectious diseases, 3rd ed. Boston: Little, Brown and Company, 1991:278.
Chambers HF, Korzeniowski OM, Sande MA, et al. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine (Baltimore) 1983;62:170.
P.249
Chambers HF, Miller RT, Newman MD. Right-sided Staphylococcus aureus endocarditis in intravenous drug abusers: two-week combination therapy. Ann Intern Med 1988;109:619.
DiNuble M. Abbreviated therapy for right sided Staphylococcus aureus endocarditis in injecting drug users. Ann Intern Med 1994;121:873.
DiSalvo G, Habib G, Pergola V, et al. Echocardiography predicts embolic events in infective endocarditis. J Am Coll Cardiol 1991;37:1069.
Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 2005;352:1436 1444.
Miller LG, Perdreau-Remington F, Reig G, et al. Fourteen patients with necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 2005;352:1445.
Moon MR, Stinson EB, Miller DC. Surgical treatment of infective endocarditis. Prog Cardiovasc Dis 1997;40:239.
Sullam PM, Drake TA, Sande MA. Pathogenesis of endocarditis. Am J Med 1985;78:110.
Fever and Abdominal Pain
What is the single best test to evaluate the febrile patient with abdominal pain?
What are the most important pathogens in the bowel flora?
Besides obstruction, ischemia, and injury involving the gut (and its outpouchings), what are the other causes of peritonitis?
What are several examples of extraperitoneal diseases that can present with abdominal pain as a prominent symptom?
Discussion
What is the single best test to evaluate the febrile patient with abdominal pain?
The febrile patient with abdominal pain can be a daunting prospect. The differential diagnosis in this setting ranges from benign, self-limited infections such as viral enteritis to severe, life-threatening infections such as peritonitis resulting from an ischemic bowel. However, despite the availability of a tremendous variety of imaging procedures and tests of bodily fluids, the single best approach to diagnosis in a patient with fever and abdominal pain remains a careful history and physical examination. Sometimes the information yielded is sufficient to make a diagnosis. More often tests are necessary, but a careful clinical evaluation narrows the list of questions that need to be answered by tests. Fishing with a long series of tests without well-considered clinical questions occasionally hooks the true culprit, but more often nets a catch of red herrings.
What are the most important pathogens in the bowel flora?
A common concern in the febrile patient with abdominal pain is the possible contamination of the peritoneal space with pathogens from the bowel. Although a great variety of organisms live in the gut, the number of important pathogens is, fortunately, small. The Enterobacteriaceae are perhaps the best
P.250
known such pathogens. Anaerobes are the dominant organisms in the colon and, of this class Bacteroides species are the important pathogens. Finally, streptococci, especially enterococci, can be prominent pathogens (they are also important because of their resistance to a number of commonly used antibiotics, such as cephalosporins).Besides obstruction, ischemia, and injury involving the gut (and its outpouchings), what are the other causes of peritonitis?
Preexisting ascitic fluid, especially that due to hepatic cirrhosis, can become infected and cause peritonitis. Salpingitis and endometritis can lead to peritonitis through direct extension of the infection out of the open abdominal ostium of the tube. Primary peritonitis is an unusual form of bacterial peritonitis that has no clear predisposing factors; it most often affects children. Finally, there are noninfectious causes of peritonitis, including bleeding into the peritoneum, which can cause pain and low-grade fevers, plus the rare familial Mediterranean fever.
What are several examples of extraperitoneal diseases that can present with abdominal pain as a prominent symptom?
Lower lobe pneumonia can be a source of considerable abdominal pain and tenderness. Neuritic pain resulting from a variety of causes (infectious causes include herpes zoster, Lyme disease, and tabes dorsalis) can produce severe abdominal pain, which is convincing enough at times to prompt performance of an exploratory laparotomy.
Case
A 24-year-old woman comes to the emergency room because of a 4-day history of abdominal pain, which she describes as a sharp, progressively severe pain in the right lower chest and upper abdomen that is exacerbated by taking a deep breath, walking, or sitting erect. She feels nauseated, but has not vomited. At home she has had fevers as high as 38 C (100.4 F), but no rigors. She has had no previous similar episodes and has never undergone abdominal surgery. She denies cough or dyspnea, fatty food intolerance, jaundice or dark urine, dysuria, or urinary frequency. She has never been pregnant; her last menstrual period began 1 week ago and is now ending. Her past medical history is unremarkable; her only medication is an oral contraceptive. She drinks socially on weekends, but does not use tobacco. Her family history is notable in that her mother had a cholecystectomy at 34 years.
On physical examination, she is found to be a mildly obese young woman who is in moderate distress and lying curled up on her right side. Her temperature is 37.8 C (100.04 F), blood pressure is 96/60 mm Hg, and pulse is 110 beats per minute. Examination of her head and neck yield unremarkable findings; specifically, there is no scleral icterus or cervical adenopathy. Her chest is clear to auscultation and percussion, although she is unable to take a deep breath because of the pain in her right lower chest. She has hypoactive bowel sounds and exhibits substantial tenderness in the right upper quadrant associated with a positive Murphy's sign (an inability to take a deep breath during deep palpation of the right upper quadrant). The edge of her liver is not palpable
P.251
and the span, by percussion, is normal. No masses or tenderness are found elsewhere in the abdomen, and the spleen is not palpable. Rectal examination reveals no tenderness and the stool is guaiac negative. Her skin and extremities appear normal.
She has a white blood cell count of 10,500/mm3 with 85% segmented neutrophils and 7% band forms, a hematocrit of 39%, and a platelet count of 216,000/mm3. Serum electrolyte and creatinine values are normal. The aspartate aminotransferase (AST) level is elevated at 56 U/mL (normal, <30 IU/mL), but her serum bilirubin, alkaline phosphatase, and amylase levels are normal. Urinalysis is notable for 20 to 50 white blood cells, 20 to 50 red blood cells, many bacteria, and many epithelial cells per high-power field. She is thought to have acute cholecystitis, but an ultrasound scan of the liver, biliary ducts, and pancreas is negative.
What are the various ways for you to proceed at this point?
What is the most likely diagnosis based on the findings from your further investigations?
What pathogens can cause salpingitis with perihepatitis?
What noninvasive tests are helpful for confirming a specific cause of salpingitis with perihepatitis?
Case Discussion
What are the various ways for you to proceed at this point?
To elucidate the nature of this patient's disorder, you decide to proceed with the following: radionuclide biliary (lidofenin) scanning, a chest radiographic study, repeat urinalysis and culture, serologic tests for hepatitis A, B, and C, serum -human chorionic gonadotropin (hCG) measurement, and sexual history and pelvic examination.
Additional case details: Because it is 6:00 p.m., the nuclear medicine facilities are not available, and therefore it is not possible to have a radionuclide biliary scan performed. The chest radiographic study is normal. Repeat urinalysis on a catheterized specimen yields normal findings, but the culture results are pending, as are the results of serologic tests for hepatitis A, B, and C. The serum -hCG level shows that she is not pregnant. Sexual history and pelvic examination reveal she is sexually active with a new partner in the last month. Because she uses an oral contraceptive, her partner does not use condoms. She has had genital warts and yeast infections in the past, but has no known history of other sexually transmitted diseases. She does not use intravenous drugs, has never received a blood transfusion, and has had no occupational exposure to blood. On pelvic examination, her external genitalia are found to be normal. There is a small amount of dark blood from the cervical os, and mild tenderness with cervical motion and palpation of the right adnexa. The size of the uterus is normal and there are no adnexal masses.
What is the most likely diagnosis based on the findings from your further investigations?
All these tests investigate important causes of acute abdominal pain and fever. Interpretation of the results allows a fairly confident diagnosis to be made. The
P.252
initial concern was acute cholecystitis, but the normal ultrasound findings make this diagnosis unlikely, although they do not completely rule it out because a single stone may be lodged in the distal common duct and be missed on ultrasound scanning. Arguing against cholecystitis are her age and lack of previous pregnancies. Another possibility is right lower lobe pneumonia. In most cases of pneumonia, respiratory symptoms are the chief complaint, but lower lobe pneumonia, by irritating the parietal pleura overlying the diaphragm, can assume the characteristics of an abdominal presentation. In this case, the normal chest radiographic findings and lack of pulmonary symptoms make this diagnosis very unlikely.Patients with pyelonephritis can experience pain anteriorly (in the upper and mid-abdomen) as well as the classic costovertebral angle tenderness in the back. However, the lack of lower urinary tract symptoms (dysuria, urinary frequency, and suprapubic pain) in this patient is not conclusive evidence against this diagnosis because these symptoms are frequently mild or even absent in patients with upper UTIs. The initial urinalysis revealed many white blood cells, a finding that at first blush seems to confirm the diagnosis of pyelonephritis. However, there are many epithelial cells as well, which makes it impossible to tell whether the white blood cells came from the urinary or the reproductive tract. A catheterized urine specimen answers this question, and the subsequent normal urinalysis findings almost rule out the possibility of pyelonephritis. Rarely, if there is infection causing an obstruction of the ureter, the urinalysis results can be normal. Such patients are usually severely ill, however, rendering this diagnosis very unlikely in this case.
Although acute viral hepatitis can cause pronounced right upper quadrant pain and tenderness, it is a very unlikely diagnosis in this patient. At the onset of symptoms, the transaminase levels in the setting of viral hepatitis are markedly elevated usually exceeding 10 times normal. The minor elevation in the AST level in this patient would be very atypical of acute viral hepatitis.
The possibility of a ruptured ectopic pregnancy should always be considered in a young woman with abdominal pain and vaginal bleeding, but the serum -hCG measurement rules out this possibility.
Acute salpingitis (infection of the fallopian tubes or pelvic inflammatory disease) can be manifested by right upper quadrant pain. This symptom is thought to arise as a result of secretions from the infected tube leaking into the peritoneum and traveling up the right pericolic gutter to the right upper quadrant. This can produce infection of the hepatic capsule, termed perihepatitis (or Fitz-Hugh Curtis syndrome). Surprisingly, the symptoms of perihepatitis are frequently much more prominent than those stemming from the original focus of infection in the tube. Therefore, these patients are admitted frequently and sometimes taken to surgery for treatment of a presumed cholecystitis. There are no pathognomonic laboratory or imaging findings that can confirm this diagnosis; this requires laparoscopy. However, acute salpingitis should be seriously considered in this patient a sexually active woman with right upper quadrant pain, fevers, and no signs of cholecystitis. With the additional factor that she has a new sexual partner coupled with the finding of right adnexal tenderness, salpingitis with perihepatitis becomes the most likely diagnosis.
What pathogens can cause salpingitis with perihepatitis?
Neisseria gonorrhea is the classic cause of this syndrome. In Fitz-Hugh's original description, Gram's staining of the fluid from the hepatic capsule showed gram-negative diplococci. Since then, as in the case of acute urethritis, it has become clear that Chlamydia trachomatis is a common cause of acute salpingitis with perihepatitis.
What noninvasive tests are helpful for confirming a specific cause of salpingitis with perihepatitis?
Gram's staining of a cervical smear, although relatively insensitive (50%) for detecting gonococci, is specific enough (95%) to be used as the basis for presumptive therapy if results are positive. A cervical culture for gonorrhea allows the detection of smear-negative cases. It is possible to culture Chlamydia, but this is a relatively expensive procedure and the means of doing so are not available in many clinics and small hospitals. However, a number of antigen detection systems (using, for example, an ELISA) have been developed and marketed. These have an acceptable sensitivity and specificity, with results available in 24 hours or less.
P.253
Suggested Readings
Fitz-Hugh T. Acute gonococcic peritonitis of the right upper quadrant in women. JAMA 1934;102:2094.
Katzman DK, Friedman IM, McDonald CA, et al. Chlamydia trachomatis Fitz-Hugh Curtis syndrome without salpingitis in female adolescents. Am J Dis Child 1988;142:996.
Muller-Schoop JW, Wang SP, Munzinger J, et al. Chlamydia trachomatis as possible cause of peritonitis and perihepatitis in young women. BMJ 1978;1:1022.
Sholes D, Stergachis A, Heidrich FE, et al. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med 1996;334:1362.
Soper DE, Brockwell NJ, Dalton HP, et al. Observations concerning the microbial etiology of acute salpingitis. Am J Obstet Gynecol 1994;170:1008.
Wood JJ, Bolton JP, Cannon SR, et al. Biliary-type pain as a manifestation of genital tract infection: the Curtis Fitz-Hugh syndrome. Br J Surg 1982;69:251.
Central Nervous System Infection
What principles are important in selecting an antimicrobial regimen to treat a CNS infection?
How do cerebrospinal fluid (CSF) findings such as the protein and glucose levels, the white blood cell count, and differential help determine the differential diagnosis of a CNS infection?
What are the most common pathogens causing bacterial meningitis, and how does the prevalence of the bacterial pathogens that cause meningitis vary, depending on the age of the host?
P.254
Discussion
What principles are important in selecting an antimicrobial regimen to treat a CNS infection?
The blood brain barrier functions to help prevent the entry of circulating pathogens into the CNS. Unfortunately, however, it also has the effect of decreasing antibiotic penetration into the CSF. Cephalosporins, penicillins, chloramphenicol, and TMP-SMX are the commonly used antibiotics that demonstrate good CSF penetration. In contrast, the aminoglycosides have extremely poor CSF penetration, and an infection requiring aminoglycoside therapy must usually be managed with the intrathecal administration of these antibiotics. Other drugs, such as vancomycin, exhibit intermediate CSF penetration, and their efficacy depends on the presence of inflamed meninges to permit a therapeutic level of antibiotic to be reached.
Another important principle is to choose an empiric antibiotic regimen that covers the most likely pathogens. This choice therefore depends on the epidemiologic background of the patient and on his or her recent exposure history. After the pathogen has been identified and the drug susceptibility determined, therapy can be more specifically tailored. Finally, as with any new drug regimen, the patient's history of drug allergy should be carefully reviewed.
How do CSF findings such as the protein and glucose levels, the white blood cell count, and differential help determine the differential diagnosis of a CNS infection?
In adults, the normal range of the CSF glucose level is 45 to 80 mg/dL. A glucose level of less than 30 mg/dL suggests bacterial, fungal, or tuberculous meningitis. An elevated level may be seen in the setting of diabetes mellitus. A CSF protein level greater than 150 mg/dL suggests bacterial meningitis, and an extremely high protein level (>350 mg/dL) suggests a complete block of the spinal canal, as seen in certain cases of epidural abscesses or tumors. The normal range for the lumbar CSF protein level in adults is 9 to 58 mg/dL. A white blood cell count greater than 1,200/mm3 suggests bacterial meningitis. However, a lesser count does not necessarily imply viral infection because bacterial meningitis is also frequently associated with this finding. Neutrophil predominance (>50%) also suggests bacterial meningitis, although there is considerable overlap with other types of meningitis in this regard. Lymphocyte predominance may be seen in the context of tuberculous, viral, fungal, partially treated bacterial, or aseptic meningitis.
What are the most common pathogens causing bacterial meningitis, and how does the prevalence of the bacterial pathogens that cause meningitis vary, depending on the age of the host?
The four most common pathogens causing meningitis for all age-groups are Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis, and E. coli. However, there is considerable variation in the prevalence of these various pathogens among the different age-groups. The highest attack rate of bacterial meningitis occurs in the very young and very old. Lower attack rates are seen in young to middle-aged adults. In the United States, infants up to
P.255
1 month of age most commonly acquire group B streptococcus, E. coli, and Listeria meningitis; children 1 month to 5 years of age predominantly acquire H. influenzae meningitis (up to 70% of the cases). Forty percent of the patients 5 to 29 years of age acquire N. meningitidis infection, and S. pneumoniae is the most common meningitis pathogen in patients 29 years of age and older. Elderly patients are more vulnerable to Listeria monocytogenes, gram-negative bacilli, and pneumococcus.
Case
A 79-year-old man who is a resident of a nursing home is brought to the emergency room by the nursing home staff. He had been in his usual state of health until that morning, when headache, fever, and chills developed. He slept through breakfast, after which his caretakers found him to be lethargic, and this prompted them to bring him to the hospital. On initial examination he is found to be stuporous. His temperature is 102.6 F (39 C), and he has prominent nuchal rigidity. Funduscopic examination reveals no evidence of papilledema. A grade 2/6 systolic ejection murmur is found on cardiac examination. Pulmonary auscultation reveals the presence of bibasilar fine crackles. An indwelling Foley catheter is in place, which the nursing home staff explains he has required for the past 18 months because of urinary incontinence. A past medical history is notable for two episodes of UTIs, both occurring after the insertion of the Foley catheter, and mild chronic interstitial lung disease.
A CT scan of the head reveals no evidence of increased intracranial pressure, no shift or mass effect, and no intracranial bleeding, but atrophic changes consistent with age. A lumbar puncture is performed, blood and urine cultures are obtained, and appropriate therapy is begun.
What is the most likely pathogenesis of this man's meningitis?
What aspects of the emergency room management should have been different in this case?
What empiric intravenous antibiotic therapy would be most appropriate to treat the bacterial meningitis in a patient of this age, and how long should he be treated?
What physical findings could point to an anatomic source of bacterial meningitis?
What CSF findings would be expected if this patient has bacterial meningitis?
If the Gram's staining of the CSF and the cultures had yielded no organisms in this patient, what should you suspect?
If this patient had experienced the gradual onset of fevers, headache, and nuchal rigidity, what other possible diagnoses might you have entertained?
Should the patient be treated with dexamethasone?
Case Discussion
What is the most likely pathogenesis of this man's meningitis?
Several possible scenarios could explain the presence of bacteria in normally sterile CSF, despite an intact blood brain barrier. These include any of a number of
P.256
processes leading to the development of bacteremia and meningeal seeding. One of the more common sources of infection is nasopharyngeal colonization by bacteria, which is then followed by sinusitis, local invasion, bacteremia, and meningeal seeding. Another pathogenic mechanism is trauma (such as an occult skull fracture), leading to a breach in the blood brain barrier and the entry of skin flora.The most likely scenario in this patient is that a plugged Foley catheter led to the reflux of bladder contents into the ureters up to the kidneys, with consequent seeding of the bloodstream by urinary pathogens. Bacteremia can then lead to meningitis, especially in the immunocompromised or elderly host. Although the mechanism of bacterial transport across a presumably intact blood brain barrier is largely unknown, the findings from some studies have suggested that a high concentration of bacteria in the bloodstream, and the presence of bacterial virulence factors such as antiphagocytic polysaccharide capsules, the S-fimbriae of E. coli, or other components of bacterial cell walls, may facilitate this process.
What aspects of the emergency room management should have been different in this case?
The head CT scan was unnecessary because the funduscopic and nonfocal neurologic findings were sufficient to rule out a significant intracranial mass effect. Otherwise, the management of this patient was correct, and it illustrated a number of important concepts. It is important to attempt to identify the pathogen in a patient with meningitis before the initiation of antibiotic therapy. Lumbar puncture should be delayed or deferred only in the following two instances. First, lumbar puncture should not be performed if a minor delay in therapy could be hazardous, as in patients in bacterial shock or those who face a high risk of bacterial shock because of the rapid onset of purpura or because of low blood pressure. It also should not be performed when there is a possible danger of uncal herniation, as in the event of rapidly developing coma, focal neurologic signs, convulsions, or papilledema. Otherwise, appropriate management consists of quickly excluding papilledema, focal neurologic signs, shock, and purpura, followed by prompt lumbar puncture and the subsequent administration of appropriate antibiotics.
The respiratory isolation of patients with suspected meningitis is appropriate only when there is a strong suspicion of N. meningitidis, Mycobacterium tuberculosis, or H. influenzae type b (in pediatric patients). The close contacts of patients with N. meningitidis (such as the person performing an intubation) should receive prophylactic rifampin treatment (10 mg/kg orally twice a day for 2 days, to a maximum of 600 mg twice a day).
What empiric intravenous antibiotic therapy would be most appropriate to treat the bacterial meningitis in a patient of this age, and how long should he be treated?
The approaches to age-specific empiric antibiotic therapy for bacterial meningitis are based on knowledge of the most common pathogens that affect each group, as already outlined. For infants younger than 1 month, a combination of ampicillin and gentamicin or ampicillin and cefotaxime is a reasonable empiric antibiotic choice. The combination of vancomycin plus a third-generation cephalosporin is appropriate for infants 1 to 24 months of age. For children aged 2 years or older and adults younger than 50, vancomycin and a third-generation cephalosporin are
P.257
appropriate. Ampicillin should be added to this regimen for those older than 50 years to ensure coverage for L. monocytogenes. Once the pathogen and its susceptibility pattern are known, therapy can be individualized. The duration of antibiotic therapy for bacterial meningitis is still largely based on tradition: 10 to 14 days for acute bacterial meningitis caused by any of the three major meningeal pathogens, and approximately 3 weeks for gram-negative bacillary meningitis. The dosages of the antibiotics for the treatment of meningitis are usually higher than those used for other infections to ensure adequate bactericidal activity in the CSF. For example, intravenous penicillin G should be given every 4 hours to a total daily dose of 20 to 24 million units, and 2 g of ceftriaxone should be given every 12 hours. The aminoglycosides, which do not adequately traverse the blood brain barrier, must be administered intrathecally as well as intravenously when indicated, as in the event of meningitis due to a highly resistant gram-negative organism.What physical findings could point to an anatomic source of bacterial meningitis?
Important physical findings that are clues to an anatomic source of bacterial meningitis include otitis media, sinusitis, skull fracture, or other evidence of cranial trauma such as CSF leaking from the external auditory meatus, neurosurgical scars, or the presence of a ventriculostomy shunt.
Most cases of meningitis result from the attachment of bacteria to epithelial cells of the nasopharyngeal and oropharyngeal mucosa, followed by transgression of the mucosal barrier. These events are also associated with the development of otitis media and sinusitis. Any anatomic breach of the blood brain barrier, either through trauma or neurosurgery, can introduce bacteria into the CNS. Meningitis develops in up to 30% of patients who have a ventriculoatrial or ventriculoperitoneal shunt. Other pertinent physical findings in a patient with meningitis include a dermal sinus or mastoiditis.
What CSF findings would be expected if this patient has bacterial meningitis?
The clinician would expect to see the following constellation of findings: a low glucose level, a high protein content, and an elevated white blood cell count, with a neutrophil predominance (see earlier discussion).
If the Gram's staining of the CSF and the cultures had yielded no organisms in this patient, what should you suspect?
The lack of organisms on Gram's-stained CSF specimens does not rule out bacterial meningitis; however, this test should be performed on a centrifuged sediment of CSF. Negative findings are encountered in 10% to 20% of patients with bacterial meningitis whose CSF cultures are positive for organisms. In cases of partially treated bacterial meningitis, Gram's staining of the CSF more often yields negative findings. An acid-fast smear of spun CSF is only rarely positive in cases of tuberculous meningitis.
If this patient had experienced the gradual onset of fevers, headache, and nuchal rigidity, what other possible diagnoses might you have entertained?
Fungal meningitis, a brain abscess, tuberculous meningitis, and carcinomatous meningitis all tend to be rather insidious in onset and assume a more chronic course than that seen with acute bacterial meningitis. The onset of tuberculous meningitis may be occasionally rapid in an immunocompromised host, but this would usually
P.258
occur only in the course of miliary tuberculosis or with the rupture of a subependymal tubercle. More commonly, however, patients with tuberculous meningitis have symptoms for more than 2 weeks. One of the hallmarks of tuberculous meningitis is the development of ocular palsies, seen in 30% to 70% of the cases.An epidemiologic history is useful in diagnosing chronic meningitis. Prior exposure to tuberculosis, a history of skin test positivity, or recent travel to or residence in areas endemic to Histoplasma or Coccidioides is important information to elicit. Carcinomatous meningitis occurs usually in the setting of a known underlying malignancy.
Should the patient be treated with dexamethasone?
The adjunctive use of dexamethasone in the treatment of bacterial meningitis has been the subject of clinical investigation for many years. As in the case of antibiotic selection, current recommendations about the use of dexamethasone are age dependent. Clinical trials best support the use of dexamethasone in children with H. influenzae type b meningitis and in adults with known or suspected pneumococcal meningitis. The use of dexamethasone in infants and children with pneumococcal meningitis remains controversial. Concerns have been raised that the use of dexamethasone in children and adults with pneumococcal meningitis due to resistant organisms might be detrimental because a reduction in meningeal inflammation caused by the dexamethasone therapy might compromise penetration of vancomycin across the meninges. Although these concerns have been raised, clinical data are insufficient in either direction to make a clinically validated recommendation.
Suggested Readings
de Gans J, van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med 2002;347:1549 1556.
Quagliarello V, Scheld WM. Bacterial meningitis: pathogenesis, pathophysiology and progress. N Engl J Med 1992;327:864.
Scheld WM, Whitley RJ, Durak DT. Cerebrospinal fluid in central nervous system infections. In: Gillin BG, Weingarten K, Gamache PW, et al. eds. Infections of the central nervous system. New York: Raven Press, 1991:861.
Schoenbaum SC, Gardner P, Shillito J. Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations and therapy. J Infect Dis 1975;131:543.
Tunkel AR. Bacterial meningitis. Philadelphia: Lippincott Williams & Wilkins, 2001.
Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004;39:1267.
Tunkel AR, Wispelway B, Scheid WM. Bacterial meningitis: recent advances in pathophysiology and treatment. Ann Intern Med 1990;112:610.
Fever of Unknown Origin
What are the definitions of fever and fever of unknown origin?
What is the pathogenesis of fever?
What are the general categories of disease that can cause fever, and which general categories are the most commonly encountered?
Which laboratory tests should be routinely performed in a patient with fever of unknown origin?
In most large series, what percentage of patients with fever of unknown origin have been found to evade diagnosis?
P.259
Discussion
What are the definitions of fever and fever of unknown origin?
Fever is defined as an elevation of the body temperature. The normal temperature may vary from person to person, and ranges from 97.0 F to 99.2 F (36.1 C to 37.5 C) in healthy people. The temperature can also demonstrate a diurnal variation, in that it tends to be somewhat lower early in the morning. It is important to document a fever over the course of an entire day, and, to do this, patients should be instructed to keep a log of their temperature at home.
An elevated temperature can be the hallmark of infection; however, a patient with a serious infection can be hypothermic or even have a normal temperature, especially if he or she is elderly or immunosuppressed. Not all fevers are caused by infections.
Petersdorf and Beeson originally defined fever of unknown origin as fever that exceeds 38.3 C (100.94 F) on several occasions, lasts at least 3 weeks, and defies diagnosis after at least 1 week of routine study in the hospital. The 1-week routine study is thought to eliminate most short-lived fevers (e.g., viral illness, postoperative fever, and factitious fever). It has been suggested that this last criterion (hospital admission) should be modified to 1 week of intelligent and intensive investigation, which for most patients could be done on an outpatient basis. This definition does not apply to immunocompromised patients, however.
What is the pathogenesis of fever?
The body temperature is closely regulated within a certain normal range, and fever occurs when the core body temperature exceeds this range. There exists a balance between net heat production and heat loss. Heat is produced through body metabolism and muscle activity; heat is lost by means of dissipation through the skin and the lungs.
A central regulator of body temperature is the preoptic nucleus of the anterior hypothalamus. The hypothalamus controls body temperature by stimulating the autonomic nervous system to produce peripheral vasodilation and sweating. The hypothalamus can also cause heat to be conserved by bringing about cutaneous vasoconstriction. Shivering can also increase heat production.
In the setting of infection or other inflammatory states, mononuclear phagocytes produce cytokines such as IL-1 and TNF that are capable of raising the set point of the hypothalamus. This initiates the complex mechanisms that produce pyrexia. IL-1 appears to stimulate the hypothalamus through a prostaglandin mechanism, which explains why prostaglandin inhibitors such as aspirin are effective antipyretic agents.
What are the general categories of disease that can cause fever, and which general categories are the most commonly encountered?
Fevers that defy all attempts at diagnosis pose a challenge to the clinician. Because many causes of fever of unknown etiology are obscure on the initial evaluation of a patient, it is helpful to categorize the diagnostic possibilities into groups according to the likelihood of causing the fever.
There are numerous disease states associated with fever, but infections warrant special attention. Most infectious causes are obvious to the evaluating clinician once a careful history and physical examination coupled with routine diagnostic tests are completed. Certain systemic infectious diseases that are particularly associated with fever of unknown origin include tuberculosis (particularly the extrapulmonary form) and bacterial endocarditis. A complete list of infectious causes of fever of unknown origin is beyond the scope of this text; however, pyogenic bacterial, fungal, mycobacterial, viral, rickettsial, parasitic, and spirochetal infections have all been associated with prolonged fever. Localized causes of fever of unknown etiology include intraabdominal, perinephric, prostatic, and tooth abscesses, hepatobiliary infections, and pelvic infections. These sources of infection can be occult and need to be considered in a patient with a perplexing fever.
Other general categories of fever include malignancy and collagen-vascular disorders. Less common miscellaneous disorders include sarcoidosis, inflammatory bowel disorders, pulmonary embolli, thyroiditis, a retroperitoneal hematoma, granulomatous hepatitis, allergic reactions (drug fevers), and inherited diseases (familial Mediterranean fever). Factitious and fabricated fevers have also been described, but these constitute a diagnosis of exclusion. Finally, a significant minority of fevers with an undetermined cause are idiopathic.
Which laboratory tests should be routinely performed in a patient with fever of unknown origin?
Almost nowhere in the practice of medicine are an in-depth history and complete physical examination as essential as in the evaluation of a patient with fever of unknown origin, and, as Petersdorf observed in 1969, it is important to remember that at the end of the needle, the x-ray tube, and even the scalpel, is a sick patient who deserves the most thoughtful diagnostic approach of which we are capable.
The appropriate evaluation of each patient with fever of unknown origin needs to be individualized. Attention should be paid to the patient's exposure history, travel history, occupation, animal exposure, hobbies, and medications. The examination should be thorough and particular attention should focus on the lymphoid organs, skin, heart, eye grounds, and conjunctivae in a search for evidence of occult disease, such as bacterial endocarditis, malignancy, and vasculitis.
Blood cultures should be done routinely, as well as a complete blood count with a differential. Chest radiographic studies should also be obtained to rule out infection or malignancy. Various other diagnostic studies, including radiologic examinations and blood tests, should also be performed as dictated
P.261
by the nature of the clinical presentation. Certain serologic tests (such as a Lyme antibody test) may be indicated in the appropriate epidemiologic setting. Clearly, a random search for answers is not appropriate.In most large series, what percentage of patients with fever of unknown origin have been found to evade diagnosis?
In the few large trials that have examined this question, 5% to 25% of patients with fever of unknown origin have been found to elude a specific diagnosis. Table 6-1 summarizes the observations from three of the major series of patients with fever of unknown origin.
P.260
Case
A 61-year-old white man is seen because of a fever. He was well until 2 months before, when he noted the onset of fatigue, fever, chills, and weight loss. Temperatures as high as 40 C (104 F) have occurred in a cyclic manner (every 2 to 3 days), but resolve with acetaminophen. He denies headaches, arthralgias, visual disturbances, abdominal pain, and diarrhea. His medical history is remarkable for asthma, environmental allergies for which he is undergoing immunotherapy, and a hiatal hernia. His family history is unremarkable. The patient does not consume alcohol or smoke cigarettes. He is a retired fireman and has not traveled or had exposure to ill contacts. He has no pets or other animal exposures. There are none of the usually recognized risk factors for HIV infection. He is taking no medications.
On physical examination, the patient is found to be a tired-appearing, elderly man. His blood pressure is 146/85 mm Hg; pulse, 106 beats per minute; respirations, 20 per minute; and temperature, 38.3 C (100.94 F). The head, eyes, ears, nose, and throat examination is remarkable for the finding of dry mucous membranes; his oropharynx is clear and the tympanic membranes are normal. There is no lymphadenopathy except for a small, 1.5 2-cm, nontender lymph node in the right inguinal area. The heart sounds are unremarkable except for a regular tachycardia. The lungs are clear to auscultation and percussion. Abdominal examination reveals normal bowel sounds, and no hepatosplenomegaly or masses are palpated. Prostate and rectal findings are normal and a test for occult blood is negative. His skin appears jaundiced. The neurologic findings are normal.
Table 6-1 Summary of Study Findings in Patients with Fever of Unknown Origina | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
P.262
A chest radiograph is normal. A CT scan of the abdomen reveals enlarged portacaval lymph nodes. The serum electrolyte values are normal, and the following laboratory data are reported: white blood cell count, 4,000/ L; hemoglobin, 11.4 g/dL; and platelet count, 134,000/mm3. The differential count reveals 33% segmented neutrophils, 6% band forms, 18% lymphocytes, 4% reactive lymphocytes, 20% mononuclear cells, and 15% eosinophils. The albumin content is 3.1 mg/dL; total bilirubin, 2.8 mg/dL; alanine aminotransferase, 31 IU/L; AST, 35 IU/L; alkaline phosphatase, 242 IU/L; and lactate dehydrogenase, 567 IU/L. All blood cultures are negative. The erythrocyte sedimentation rate is 50 mm per hour. A PPD of Mycobacterium tuberculosis skin test is negative, as is the serum antinuclear antibody test.
A bone marrow biopsy specimen shows mild chronic inflammation and extensive granulomatosis. The granulomatous foci are composed of eosinophils, small lymphocytes with irregular nuclei, and histiocytes. Routine bacterial, acid-fast bacilli, and fungal cultures and stains are negative. A test for urinary Histoplasma antigen is negative. A needle biopsy specimen of the liver reveals sinusoidal dilatation, triaditis, bile stasis, and focal periportal fibrosis with granulomas and dilatation of the portal venous channels.
The patient is begun empirically on a regimen of isoniazid, ethambutol, and rifampin for a presumptive diagnosis of extrapulmonary tuberculosis, but there is little attendant improvement in his clinical status.
What is the most likely diagnosis in this patient?
What diagnostic test should be performed next?
What disorders could be causing the granulomas and fever in this patient?
Case Discussion
What is the most likely diagnosis in this patient?
The most likely cause of this patient's illness is lymphoma. M. tuberculosis is one of the most common organisms to be cultured from patients with fever of unknown origin and, therefore, it is important to rule it out, especially considering that the number of cases of M. tuberculosis infection have been increasing in the United States since the mid-1980s. However, the diagnosis may be delayed because it can take cultures 4 to 6 weeks to become positive, although smears of sputum or other appropriate clinical specimens may be positive when stained with acid-fast stain. Occasionally, the PPD skin test is negative, especially in patients with disseminated disease. This emphasizes the importance of using control skin tests in addition to the PPD test. This patient reported no exposure to tuberculosis, and his condition did not improve on antituberculous medications, making this disease less likely.
Certain bacterial infections are prone to disseminate and infect the reticuloendothelial system, including Brucella and Listeria species. Although Brucella infections can be associated with lymphadenopathy and fever, this patient had had no contact with large animals or occupational exposures that would place him at risk for brucellosis. In addition, assuming the laboratory is alerted to this possibility, bone marrow cultures can be positive in a large percentage of patients with Brucella.
P.263
Collagen-vascular disorders and vasculitis are other causes of fever of unknown origin. Among them are diseases such as polymyalgia rheumatica, systemic lupus erythematosus, mixed connective tissue disorders, and juvenile rheumatoid arthritis. The lack of appropriate symptoms in this patient, and a negative antinuclear antibody test, make this category of disease less likely.
Giant cell arteritis deserves special mention because 15% of patients with this disease can present with fever. Often, the sedimentation rate in these patients exceeds 100 mm per hour. The lack of visual disturbances, temporal artery tenderness, or jaw claudication does not completely rule out this diagnosis, and occasionally a temporal artery biopsy is indicated to elucidate the situation. In one large series, giant cell arteritis was found to be the most common cause of fever of unknown origin in patients older than 50 years.
Numerous malignancies have been associated with fever. Neoplasms of the reticuloendothelial system are the most common class of tumors causing fever. Fever in a patient of this age who exhibits both weight loss and adenopathy suggests a malignancy. A cyclic pattern of fevers, such as that demonstrated by this patient, suggests but does not clinch a diagnosis of Hodgkin's disease.
Patients with lymphoma can present with recurrent fever that remains obscure. Other malignancies associated with fever include non Hodgkin's lymphoma, renal cell carcinoma, and atrial myxomas.
What diagnostic test should be performed next?
The physician should always proceed in a logical and stepwise manner in the evaluation of a patient such as this one. The workup should start with a detailed history and physical examination, followed by directed laboratory evaluations and not a random searching for an answer. This patient underwent a very extensive workup, including routine blood tests, radiologic evaluations, and cultures that did not yield a diagnosis. The next most logical step would be to perform an excisional lymph node biopsy. It is important to try to obtain the entire lymph node, for the purposes of both histologic examination and the performance of special stains and cultures. Occasionally, fine-needle aspiration of a lymph node can be a rapid and reliable method for diagnosis, but the amount of material obtained may not be adequate for complete histologic confirmation of lymphoma. If no peripheral lymph nodes are amenable to biopsy, a laparotomy with sampling of intraabdominal nodes may be needed but this should not be undertaken until noninvasive radiographic studies have been utilized to evaluate the intrathoracic and intraabdominal cavities for abnormalities that could focus the surgical diagnostic intervention.
However, if a CT scan or ultrasound study detects an intraabdominal abnormality that cannot be cultured or sampled for biopsy percutaneously, laparotomy may be essential to obtain adequate material for histologic studies and culture.
In terms of infectious diseases, certain serologic tests can be invaluable in the evaluation of a patient with a fever of undetermined etiology. Rising antibody titers can be diagnostic for certain infectious diseases, but often acute and convalescent titers need to be determined as a pair to confirm the existence of an acute infection. There are specific serologic tests for many infectious diseases, including those caused
P.264
by Brucella, Francisella tularensis, and Coxiella burnetii, but results would unlikely be positive in this setting unless there is an exposure history for these organisms.Infection with HIV must be sought, especially if the accepted epidemiologic risk factors exist (e.g., homosexual exposures, intravenous drug abuse, and blood product transfusion before the widespread screening for HIV).
What disorders could be causing the granulomas and fever in this patient?
Granulomas in the liver and bone marrow are nonspecific findings. Because these organs are rich in reticuloendothelial cells, they can respond to antigens and form granulomas. Granulomas are known to be associated with a number of febrile diseases such as infections. Among the infectious causes of granuloma are tuberculosis, fungal infections (e.g., histoplasmosis), brucellosis, Q fever, tularemia, schistosomiasis, syphilis, and Whipple's disease.
Among the noninfectious causes of granuloma, sarcoidosis is the most common. Hepatic granulomas can also be found in the setting of connective tissue diseases, hypersensitivity reactions, primary liver diseases, and malignancy. Of the malignancies, hepatic granulomas can be seen with lymphomas. Finally, in nearly a third of the patients with hepatic granulomas, the cause cannot be ascertained, and these cases are deemed idiopathic.
Suggested Readings
Arnow JP, Flaherty JP. Fever of unknown origin. Lancet 1997;350:575.
Corey L, Boeckh M. Persistent fever in patients with neutropenia. N Engl J Med 2002;346:222.
Jacoby GA, Swartz MN. Fever of undetermined origin. N Engl J Med 1973;289:1407.
Larson EB, Feathersone HJ, Petersdorf RG. Fever of undetermined origin: diagnosis and follow-up of 105 cases, 1970 1980. Medicine (Baltimore) 1982;61:269.
Petersdorf RG. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore) 1961;40:1.
Petersdorf RG. Fever of unknown origin: an old friend revisited. Arch Intern Med 1992;152:21.
Pneumonia
What symptoms and physical, laboratory, and radiographic findings are commonly observed in patients with community-acquired pneumonia?
What are the common causes of community-acquired pneumonia?
What is the role of the spleen in combating bacterial infections?
Discussion
What symptoms and physical, laboratory, and radiographic findings are commonly observed in patients with community-acquired pneumonia?
The clinical findings in patients with community-acquired pneumonia are diverse, but can often be helpful in formulating a differential diagnosis. In
P.265
the setting of bacterial pneumonia, the symptoms are often acute at onset. Frequently, there are shaking rigors, high fever, and cough productive of purulent sputum. On physical examination, the patient may appear ill, and signs of lobar consolidation are often found on chest examination. A complete blood count may reveal a brisk leukocytosis with a left shift, and a chest radiographic study may show segmental or lobar infiltrates.Atypical pneumonia (e.g., due to viruses, or Rickettsia, Chlamydia, or Mycoplasma organisms) may also be acute at onset, but the cough is usually dry and nonproductive and rigors are absent. Chest examination may reveal fine diffuse rales, or findings may be normal. Skin examination may reveal a rash. A complete blood count may show a mild leukocytosis, or results may be normal. A chest radiographic study typically shows the presence of diffuse infiltrates throughout both lungs.
Pneumonia due to anaerobic organisms (e.g., aspiration pneumonia) is usually insidious at onset and the fever may be low grade. The cough may be productive of foul-smelling sputum. The patient's dentition may be poor and he or she may have foul-smelling breath. Chest examination may reveal consolidation in the lower lung fields. A mild leukocytosis and lower lobe infiltrates (particularly in the right lower lobe) may be seen on chest radiographic films.
Pulmonary tuberculosis is also insidious at onset. The fever may be accompanied by drenching night sweats, and cough is usually productive. Chest auscultation may reveal signs of upper lobe or apical consolidation. The complete blood count is often normal and chest radiographic studies may show upper lobe infiltrates, often with cavitation. Calcified hilar lymph nodes, which are a residual effect from the primary tuberculous infection, are often observed.
What are the common causes of community-acquired pneumonia?
The differential diagnosis of community-acquired pneumonia is broad, but can be narrowed considerably by the findings obtained from a careful history and physical examination, sputum Gram's staining, and chest radiographic evaluation. Bacterial pneumonia can be caused by S. pneumoniae, H. influenzae, S. aureus, Branhamella catarrhalis, and Legionella pneumophila. Uncommon causes of bacterial pneumonia include Yersinia pestis (plague), F. tularensis (tularemia), and Bacillus anthracis (anthrax). Atypical pneumonias are commonly due to Mycoplasma species or respiratory viruses. Less common causes of atypical pneumonia include Chlamydia species (psittacosis), C. burnetii (Q fever), H. capsulatum, C. immitis, and M. tuberculosis. Anaerobic or cavitary pneumonia is most commonly caused by oral anaerobes or by M. tuberculosis. Less common causes include Mycobacterium kansasii, H. capsulatum, C. immitis, and Blastomyces dermatitidis.
The initial assessment of patients with pneumonia should include a careful occupational and social history to determine whether there has been exposure to water-cooling facilities (L. pneumophila), wild animals (tularemia or plague), birds (psittacosis), or farm animals (anthrax or Q fever), and whether there has been a recent loss of consciousness (aspiration pneumonia) or exposure to people with tuberculosis. Likewise, the travel history is also important
P.266
in narrowing the differential diagnosis. Recent travel to the southwestern deserts of the United States would suggest coccidioidomycosis; exposure to bird droppings or bat guano in the Midwest would suggest histoplasmosis.What is the role of the spleen in combating bacterial infections?
The spleen is part of the reticuloendothelial system and is important in clearing certain bacterial pathogens from the bloodstream. Asplenic people are susceptible to sepsis stemming from encapsulated bacteria (pneumococci, H. influenzae, and N. meningitidis). They should therefore be vaccinated against these infections, preferably before splenectomy if done electively, because the spleen is also important in the development of an antibody response to these vaccines.
Case
A 64-year-old woman from Topeka, Kansas, presents with an 8-hour history of fever, rigors, and a cough productive of blood-tinged sputum. She has been in good health all of her life except for abdominal trauma that necessitated a splenectomy 30 years ago. As you are examining her, she experiences shaking rigors and her fever is found to be 39 C (102.6 F); she also complains of a pleuritic pain over the right posterior chest. Physical examination reveals an ill-appearing woman with a persistent cough productive of purulent sputum. There is dullness to percussion, egophony, and moist rales in the right posterior chest. Her white blood cell count is 15,000/mm3 and a chest radiographic study shows a dense consolidation in the right lower lobe with air bronchograms. Gram's staining of a sputum sample reveals numerous neutrophils and abundant intracellular gram-positive diplococci.
What is the most likely diagnosis in this patient?
What does the differential diagnosis of pneumonia consist of in this patient?
On the basis of the sputum findings, what is the most likely cause of this patient's condition?
What would be the most appropriate treatment for this patient?
Case Discussion
What is the most likely diagnosis in this patient?
The rapid onset of symptoms and purulent sputum are findings most suggestive of acute bacterial pneumonia. This diagnosis is further indicated by the lobar consolidation depicted on the chest radiographic study. Although a pulmonary embolism can cause the sudden onset of pleuritic chest pain, hemoptysis, and fever, it would be unusual for rigors and purulent sputum to occur in this setting. Tuberculosis would usually assume a more subacute presentation. Bronchogenic carcinoma can present with bronchial obstruction and a postobstructive pneumonia, although a hilar or perihilar mass would likely be found on chest radiographic studies. This patient's presentation would be unusual for atypical pneumonias, such as those caused by viruses or Mycoplasma or Chlamydia species. The presence of lancet
P.267
shaped diplococci in the sputum Gram's stain and the positive urinary test result for pneumococcal antigen further support the diagnosis.What does the differential diagnosis of pneumonia consist of in this patient?
This patient's signs and symptoms are most consistent with those of acute community-acquired bacterial pneumonia. The most common cause of community-acquired bacterial pneumonia is S. pneumoniae (pneumococcus). Other potential causes include H. influenzae, anaerobes (aspiration pneumonia), and L. pneumophila (usually spread by contaminated aerosols generated by air-conditioning systems, humidifiers, and bath showers). The diagnosis of bacterial pneumonia can usually be easily and rapidly made through the examination of a Gram's-stained specimen of expectorated sputum. This simple and inexpensive test would also be a key to determining the most appropriate therapy for this patient. The lack of dominant bacteria on the Gram's-stained sputum sample suggests the possibility of less common causes of acute lobar pneumonia such as L. pneumophila, tuberculosis, or fungi (coccidioidomycosis or histoplasmosis). The diagnosis of pneumonia due to L. pneumophila is made usually on the basis of sputum culture findings or on those yielded by a direct fluorescent antibody stain of the sputum. Likewise, if pulmonary tuberculosis is suspected, sputum acid-fast staining should be performed. A tuberculin skin test can screen for previous exposure to tuberculosis, but is of little value in the evaluation of active pulmonary infection. Fungal pneumonias should be considered if the patient has been exposed to bird or bat feces, has been involved in spelunking (histoplasmosis), or has traveled to Sonoran desert areas in the southwestern United States (coccidioidomycosis).
On the basis of the sputum findings, what is the most likely cause of this patient's condition?
The presentation and Gram's stain findings are indicative of pneumonia due to S. pneumoniae (pneumococcus), the most common cause of bacterial pneumonia in adults. The elderly, debilitated, and immunosuppressed are especially prone to pneumococcal pneumonia. The splenectomy in this patient also predisposes her to sepsis caused by encapsulated bacteria such as S. pneumoniae, H. influenzae, and N. meningitidis.
What would be the most appropriate treatment for this patient?
There are two aspects to decision making in this patient: whether she can be treated as an outpatient and what antibiotics she should receive. A very well validated tool termed the Pneumonia Severity Index (PSI) has been developed that provides excellent guidance about whether patients should best be treated in an inpatient or an outpatient setting. The PSI combines demographic features of the patient such as age and sex, clinical features of the patient, and underlying conditions to provide a score that provides excellent guidance about whether a patient can safely be managed as an outpatient (Fig. 6-2). Once the most appropriate setting for initial treatment has been determined, a decision about initial antibiotics must be made. In the past, for a patient such as this one, it would have been acceptable to initiate therapy with penicillin. Over the last several years, the prevalence of strains of S. pneumoniae that are resistant to penicillin has risen sharply in the United States.
P.268
Although there is controversy about whether most patients with pneumococcal pneumonia with moderately resistant organisms can be treated with penicillin or amoxicillin, this patient is acutely ill and is further compromised by having had a splenectomy. Therefore, it would be imprudent to empirically use penicillin or amoxicillin in the absence of susceptibility testing data. This patient would best be treated with a combination of azithromycin or clarithromycin plus a -lactam (cefotaxime, ceftriaxone, ampicillin-sulbactam, or ertapenem).
Figure 6-2 Pneumonia Severity Index. (From
Halm EA, Teirstein AS. Management of community-acquired pneumonia. N Engl J Med 2002;347:2039
.)Corticosteroids have no role in the treatment of uncomplicated pneumococcal pneumonia. A pneumococcal vaccine is administered to prevent pneumococcal infection in high-risk patients (e.g., asplenic people or those with a chronic pulmonary
P.269
disease or underlying immunodeficiency), but has no role in the management of acute pneumococcal pneumonia. Pneumococcal vaccination is also recommended for all people older than 65 years, and some physicians advocate vaccination for all people older than 55 years. Because of the splenectomy, this patient should receive pneumococcal vaccination as soon as she has recovered from the acute illness.
Suggested Readings
Bisno AL, Freeman JC. The syndrome of asplenia, pneumococcal sepsis, and disseminated intravascular coagulation. Ann Intern Med 1970;72:389.
Broome CV, Breiman RF. Pneumococcal vaccine: past, present and future. N Engl J Med 1991;325:1506.
Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997;336:243.
Gutierrez F, Rodriguez JC, Ayelo A, et al. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin Infect Dis 2003;36:286.
Halm EA, Teirstein AS. Management of community-acquired pneumonia. N Engl J Med 2002;347:2039.
Jacoby GA, Archer GL. New mechanisms of bacterial resistance to antimicrobial agents. N Engl J Med 1991;324:601.
Karlowski JA, Thornsberry C, Jones ME, et al. Factors associated with relative rates of antimicrobial resistance among Streptococcus pneumoniae in the United States: results of the TRUST surveillance program (1998 2002). Clin Infect Dis 2003;36:963.
Mandell LA. Relationship of penicillin resistance to mortality in pneumococcal pneumonia. Curr Infect Dis Rep 2001;3:9.
Mandell LA, Bartlett JG, Dowell SF, et al. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis 2003;37:1405.
Ward J. Antibiotic-resistant Streptococcus pneumoniae: clinical and epidemiologic aspects. Rev Infect Dis 1981;3:254.
EAN: 2147483647
Pages: 14