33 - Bone Marrow
Editors: Mills, Stacey E.
Title: Histology for Pathologists, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > X - Female Genital System > 41 - Uterus and Fallopian Tubes
function show_scrollbar() {}
41
Uterus and Fallopian Tubes
Michael R. Hendrickson
Kristen A. Atkins
Richard L. Kempson
Introduction
The fallopian tubes and uterus in many ways constitute a natural anatomic and functional unit. They both derive embryologically from the m llerian duct. Taken together, they provide the locations for the fusion of the descending egg and the ascending spermatozoon, the implantation of the resulting blastocyst, the incubation of the developing gestation, and they ultimately provide the mechanism for the delivery of the conceptus at term. They have a common anatomic organization and share common responses to a changing steroidal milieu. Looking beyond normal structure and function to pathology, the fallopian tube and the uterus, together with the ovarian surface epithelium, comprise what has been termed the extended m llerian system (1,2), which gives rise to a common set of neoplasms and nonneoplastic metaplastic epithelial changes.
P.1012
This chapter emphasizes those aspects of normal histology and immunohistochemistry relevant to the diagnostic pathologist and focuses on those features of the normal uterus and fallopian tubes that, because of their striking appearance or unfamiliarity, raise the issue of pathologic alterations.
Accounts of conventional light microscopic appearance of the fallopian tube and uterus have not changed substantially over the past several decades. This is in sharp contrast to the impressive gains in our knowledge of the biochemical and physiologic details of the normal function of these organs. Parallel advances have been made in microsurgery and radiologic imaging techniques. Increasing use of in vitro fertilization and embryo transfer technology has exploited these advances, and that in turn has prompted a return to many ancient questions: Why do women menstruate? What exactly does the endometrium do? Does it have an endocrine function? What are the functions of the many endometrial secretion products? Which of these endometrial contributions are essential to initiating and successfully sustaining a gestation? There has been an explosion of knowledge concerning the hormonal control of the reproductive system, fueled in large part by efforts to induce ovulation with pharmacologic agents. This has led in recent decades to a much more extensive knowledge of the neuroendocrine regulation of the menstrual cycle, the detailed anatomy and endocrinology of ovarian folliculogenesis, ovulation and corpus luteum function, the mechanism of action and genetics of steroid receptors, and the mechanisms responsible for normal menstrual bleeding. Paradoxically, most of this information is currently not of direct relevance to diagnostic pathologists. The practical orientation of this work notwithstanding, to ignore this knowledge would impart a distinctly dated character to this chapter. Therefore, we include a rough outline of some of this information and direct the interested reader to sources with a more detailed treatment of these issues.
This chapter first discusses the embryology and gross anatomy of the uterus and the fallopian tubes and then turns to the normal histology of the cervix, endometrium, myometrium, fallopian tube, and broad ligament.
Embryology
The uterus and fallopian tubes have a complex developmental history (3,4,5,6,7,8,9,10,11). For poorly understood reasons, precursors of both male and female internal genitalia are laid down early in each embryo in a manner analogous to the initial development of the bipotential gonad. This is known as the indifferent stage of genital development. Upon completion of this indifferent stage, definitive female differentiation is accompanied by regression of the male anlage, whereas male differentiation is accompanied by regression of the female anlage. Topographically, both of these systems are intimately related to the developing urinary tract, and, not surprisingly, anomalous development of the internal genitalia is often accompanied by anomalies of the urinary tract. Fetal sexual differentiation is completed during the first half of gestation; the last half is marked primarily by growth of the newly established genitalia. Relevant milestones have been summarized by Ramsey (12) (Figure 41.1).
The Indifferent Stage
By the 6th week of fetal life the urogenital sinus and the mesonephric (wolffian) ducts are well established. At this time the paired m llerian (paramesonephric) ducts begin their development. These structures are formed by an invagination of the celomic epithelium adjacent to that investing each developing ovary. The m llerian ducts are intimately related to the mesonephric ducts, and their normal formation appears in fact to be dependent on the presence of the mesonephros.
As the m llerian ducts grow caudally, they approach the midline where the distal portions fuse. Shortly after this fusion, the apposed medial duct walls disappear, bringing the two lumina into continuity to form a single cavity. Further downward growth of the fused m llerian structures (now termed the uterovaginal primordium) brings them into contact with the urogenital sinus. At this stage both the mesonephric ducts and the m llerian ducts are present in the fetus.
Female Differentiation
The differentiation of the indifferent internal genitalia into male or female structures depends on whether the fetus possesses ovaries or testes. In the male fetus, the Leydig cells and the Sertoli cells in the developing testes secrete testosterone and a nonsteroidal m llerian inhibiting substance respectively; the latter, antim llerian hormone (ATM) is a member of the transforming growth factor- family of glycoprotein differentiation factors (13). The net effect of this secretory activity is to ensure the persistence, differentiation, and growth of the mesonephric ducts to form the male genital system and the regression of the m llerian system. In the absence of a secreting testis (e.g., in a normal female fetus with ovaries or in a fetus with nonfunctioning gonads) the m llerian structures persist, whereas the mesonephric ducts regress. The nonfused portions of the m llerian ducts form the fallopian tubes; the fused segments develop into the uterus and probably the upper third of the vagina. Incomplete fusion of the caudal portion of the m llerian ducts results in a spectrum of uterovaginal abnormalities (14).
P.1013
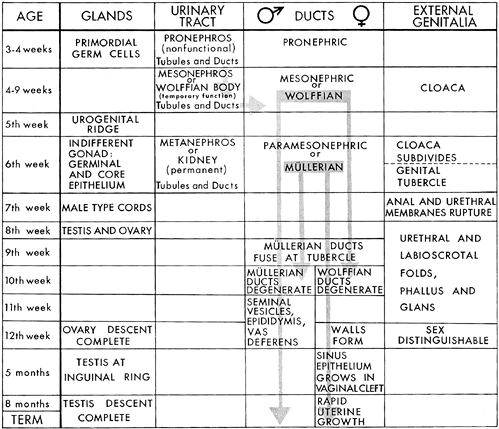 |
Figure 41.1 Chart showing interrelations and time sequence of events in the development of genitourinary system. Reprinted with permission from: Ramsey E. Embryology and developmental defects of the female reproductive tract. In: Danforth DN, Scott JR, eds. Obstetrics and Gynecology. 5th ed. Philadelphia: JB Lippincott; 1986:106 119. |
By the 21st week, the uterus and vagina are well formed. In contrast to the adult cervix, the cervix of the prenatal uterus is disproportionately large and makes up two thirds of the length of the organ. The second half of gestation is marked by uterine growth; from the 28th week to birth, a period of approximately 10 weeks, the fetal uterus doubles in size. However, the earlier cervicocorpus disproportion is maintained into childhood.
The events described above are driven, at least in part, by the expression of secreted ligands of the wingless (WNT) gene family and transcriptional regulators of the homeobox (HOX) gene family (13,15).
Gross Anatomy
Premenarchal Uterus and Fallopian Tubes
Neonatal Period
At birth the uterus averages about 4 cm in length, and its bulk and shape are dominated by its disproportionately large cervix (the cervicofundal ratio is approximately 3 5:1) (Figure. 41.2). The impact of the maternal hormonal environment is reflected in the markedly thickened rugal vaginal mucosa typically present at birth and, to a certain extent, in the histologic appearance of the endometrium, which is most often proliferative or weakly secretory. Maternal estrogen also results in cervical squamous cell maturation with glycogen storage. These mucosal changes regress shortly after birth (16,17,18).
Infancy
Uterine growth continues into the second year of life, at which time it reaches a plateau that persists until the premenarchal growth spurt at about 9 years of age. Until approximately age 13, the cervix continues to account for greater than half of the uterine length.
Adult Uterus and Fallopian Tubes
General Relations and Attachments
The uterus is located anterior to the rectum and posterior to the bladder (Figure. 41.3). It is covered anteriorly and posteriorly by a reflection of pelvic peritoneum that continues laterally to form the anterior and posterior leaves of the broad
P.1014
ligament. The posterior peritoneal reflection forms the uterine wall of the pouch of Douglas and covers a longer segment of the uterine isthmus than does the anterior peritoneal reflection. The tentlike broad ligaments house the major uterine vessels and the efferent lymphatic trunks; they also contain the fallopian tubes at their apices. Each ovary is attached to the ipsilateral uterine cornu by the utero-ovarian ligament, which is situated posterolateral and inferior to the uterine attachment of the fallopian tubes. The round ligaments arise anterolateral and inferior to the attachment of the fallopian tubes and pass anteriorly to insert into the canal of Nuck. These anatomic relations are of obvious importance
P.1015
to the surgeon but also of value to the pathologist because they often enable proper orientation of the hysterectomy specimen. The anterior surface of the uterus is distinguished by its longer bare region (i.e., lacking peritoneum) and the anteriorly directed stump of the round ligament. The posterior surface is more extensively covered by peritoneum, and the utero-ovarian ligament is attached to the posterior cornual aspect of the uterus. The uterus is anchored to its surroundings by a number of connective tissue bands; notable among them are the cardinal, uterosacral, and pubocervical ligaments (11,18,19,20).
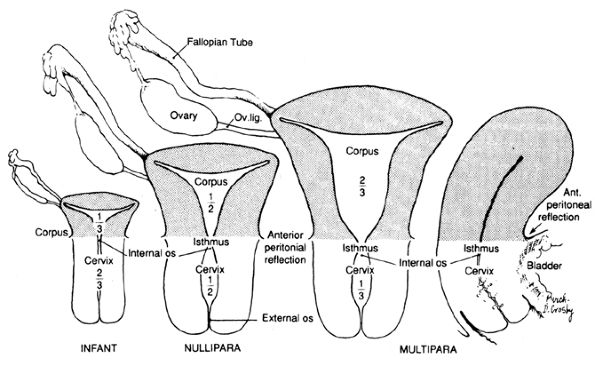 |
Figure 41.2 Drawings illustrating comparative sizes of prepubertal, mature nonparous, and parous uteri. The relative proportions of uterine corpus and cervix are seen to change with age and parity. Frontal and sagittal sections are presented. (After Ranice W. Crosby.) Reprinted with permission from: Ramsey E. Development of the human uterus and relevance to the adult condition. In: Chard T, Grudzinskas JG, eds. The Uterus. New York: Cambridge University Press; 1994:41 53. |
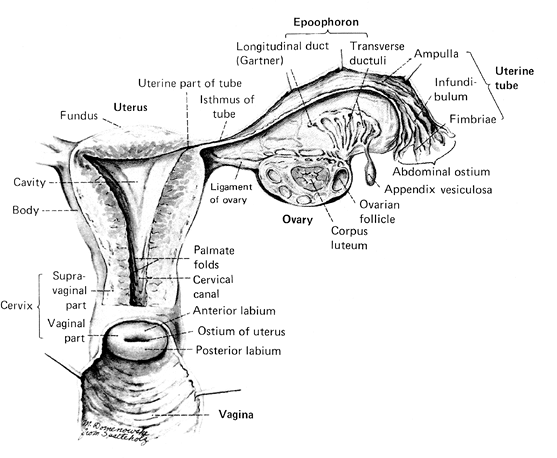 |
Figure 41.3 The normal internal female genitalia. Reprinted with permission from: Crafts R, Krieger H. Gross anatomy of the female reproductive tract, pituitary, and hypothalamus. In: Danforth D, Scott J, eds. Obstetrics and Gynecology. Philadelphia: JB Lippincott; 1986:64. |
Gross Anatomic Features of the Uterus
The adult nulliparous uterus is a hollow, pear-shaped muscular organ weighing 40 to 80 g and measuring approximately 7 to 8 cm along its long axis, 5.0 cm at its broadest extent (cornu to cornu), and 2.5 cm in anteroposterior dimension. These measurements vary considerably as a function of age, phase of the menstrual cycle, and parity. In general, high parity and youth are positively correlated with increasing uterine size (21). The adult uterus consists of an expanded body, the corpus, and a smaller cervix. That portion of the corpus cephalad to a line connecting the origin of the two fallopian tubes is called the fundus. The cornua are the two lateral regions of the fundus associated with the intramural portion of the fallopian tubes. The remainder of the corpus tapers from the fundus into the isthmus or the lower uterine segment, which shares histologic features with both of the uterine segments that it bridges: the uterine corpus and the endocervix. The existence of an anatomically and functionally significant lower uterine segment has been disputed by some authorities (22). The uterine cavity has the approximate configuration of the uterus, but its internal dimensions are much smaller, reflecting the substantial thickness of the uterine wall. The cavity is triangular, and the apices of this potential space are continuous with the lumina of the fallopian tubes at the two cornua and with the endocervical canal at the internal os. The length of the cavity is approximately 6.0 cm. Again, these measurements vary considerably with the age and parity of the individual (23). The cervix is roughly cylindrical and normally measures approximately 3 to 4 cm in length (24). It is pierced through its center by the endocervical canal. Traditionally, the endocervical canal has been described as having an external os that opens onto the exocervix and an internal os that separates the endocervical canal from the endometrial cavity. Although the former is a reasonable anatomic landmark, the latter is not because grossly, the transition from endometrial cavity to endocervix is gradual, without abrupt anatomic demarcation between endocervix and endometrium. This is histologically mirrored by the gradual transition of the mucosa in this region from endocervical type to endometrial type. The mucosal surface of the endocervical canal is deeply clefted to form the plicae palmatae. The lateral connective tissue attachments of the uterus are referred to as the parametria; they contain vessels, nerves, lymphatics, and lymph nodes.
The normal myometrium consists of two strata: an outer longitudinal muscle layer covering the fundus and an inner circular submucosal muscle layer extending to surround the internal os and the tubal ostia. There is an interposed thick middle layer, richly populated by vessels and composed of randomly interdigitating fibers (25). The magnetic resonance imaging (MRI) correlate of these layers is the outer zone and the submucosal low intensity halo junctional zone (26,27). Functionally, the junctional zone appears to be more involved with menstruation while the outer zone assumes a prominent role in gestation and parturition.
Parenthetically, Toth has described two lateral subserosally situated longitudinal bands of distinctive muscle fibers, the fasciculus cervicoangularis (28,29). On occasion, epithelium that is immunohistochemically and histologically similar to cervical mesonephric remnants is present within this bundle, suggesting that these structures represent the vestiges of the wolffian (mesonephric) duct which is more commonly encountered in the cervical stroma ( mesonephric rests ) and lateral vagina (Gardner's duct and derivative cysts).
Substantial deviations from the nulliparous adult uterus naturally occur throughout adult life. The uterus undergoes small-amplitude changes in size during the menstrual cycle, attaining its greatest volume during the secretory phase (27). During pregnancy, of course, the uterus enlarges much more dramatically to accommodate the growing conceptus. This growth is due largely to myocyte hypertrophy and hyperplasia, an increase in uterine vasculature, and in extracellular matrix; the net weight increases 10-fold during pregnancy. After delivery, uterine size rapidly decreases, and over the ensuing weeks a striking resorption of connective tissue occurs that is associated with a decrease in the size of individual myocytes (30). However, the uterus generally does not return completely to its nulliparous size and weight. Prior pregnancy (parity) can be deduced from several gross features. The multiparous nongravid uterus tends to weigh more in consequence of its thicker and more prominently layered muscular walls; this increase in weight is proportional to the patient's parity (21). The vasculature of the multiparous uterus tends to be more prominent. The most suggestive changes of previous pregnancy, however, are seen in the cervix. The nulliparous circular small external os is transformed after pregnancy into a slit that forms prominent anterior and posterior lips. In addition, healed cervical lacerations may be pronounced, and enough endocervical tissue may reside on the exocervix to give it a red granular appearance near the os. With the waning of ovarian hormone synthesis during the menopausal years, the uterus involutes and atrophies. This is reflected by a decrease in its weight and its dimensions. On occasion the endocervical
P.1016
canal is almost completely obliterated. Exogenous estrogens administered during this period sometimes maintain uterine weight artificially despite the loss of ovarian hormonal support.
Gross Anatomic Features of the Fallopian Tubes
The fallopian tubes are hollow, epithelium-lined muscular structures 11 to 12 cm in length that run through the apex of the broad ligament to span the uterine cornu medially and the ovary laterally. Each tube is divided into four anatomic segments. The intramural segment begins at the funnel-like uppermost recess of the uterine cornu and ends where the tube emerges from the uterine wall. The course of this 8-mm, pinpoint lumened segment varies from straight to highly convoluted (31). Beyond the uterine wall the proximal tube continues for 2 to 3 cm as the isthmus, a thick-walled, narrow-calibered segment that merges into a comparatively thin-walled expanded area, the ampulla. The distal tube ends in the trumpet-shaped infundibulum whose mouth opens into the peritoneal cavity and is fringed by approximately 25 fimbria. One of these, the ovarian fimbrium, attaches to the ovary. At the time of ovulation the infundibulum forms a cap over the ovarian surface to create the ovarian bursa. The tubal mucosa and the underlying endosalpingeal stroma are thrown up into longitudinal, branching folds (the plicae) whose branches increase in complexity from the isthmus to the infundibulum. The plicae terminate in the fimbria. At the time of ovulation the fimbria sweep over the surface of the ovary to facilitate egg capture (13,32,33,34,35).
Uterine and Tubal Vasculature
The major arterial supply of the uterus derives from the right and left uterine arteries, which arise from the corresponding hypogastric (internal iliac) arteries. The uterine artery divides into ascending and descending branches laterally at the level of the uterine isthmus. The ascending uterine artery anastomoses freely with the ovarian artery (a branch of the aorta) in the mesosalpinx, whereas the descending branch anastomoses with the vaginal arterial supply. Both the ascending and descending uterine arteries give rise to a complex network of circumferentially arranged subserosal arteries: the arcuate arteries. These, in turn, give rise to a series of radial arteries that penetrate the myometrium. Each of these radial vessels branches, in the inner third of the myometrium, into straight arteries (supplying the basalis) and spiral arteries that become the spiral arteries of the endometrium (36,37,38).
A striking characteristic of the adult intramyometrial uterine arteries is their marked tortuosity. This, no doubt, has to do with the variation in uterine size during reproductive life. In the postmenopausal years, striking degenerative changes may be seen in the uterine arteries, including intimal proliferation, fibrosis, and medial calcification. The severity of these changes is typically out of proportion to degenerative changes in nonuterine arteries. The venous drainage of the uterus parallels its arterial supply.
Uterine and Tubal Lymphatics
Lymphatics are present in both the cervix and the corpus. In the endometrium these vessels are intimately associated with the glands of the functionalis. The myometrium and cervical stroma contain a complex labyrinth of lymphatics that course toward the subserosal plexus. The channels forming the latter ramify over the entire surface of the uterus, and the confluence of these channels forms the major efferent lymphatic trunks of the uterus. The chief interest in lymphatic drainage for the pathologist is as a guide to the dissemination of carcinoma. The major lymph node groups draining cervical and endometrial carcinoma are indicated in Figures 41.4 and 41.5.
In both the mucosal and muscular layers, lymphatic anastomoses exist between the cervical and corpus systems, and on occasion cervical carcinomas may take advantage of this route to spread to the corpus. Whether the converse is true is unclear. Moreover, whether or not corpus carcinoma, once having invaded the cervix, then behaves like cervical carcinoma in terms of its lymphatic metastatic distribution is also unclear, even though this is a common clinical
P.1017
assumption. Indeed, involvement of cervical draining nodes by endometrial carcinoma does not necessarily imply cervical involvement. For further detail, the reader is referred to specialty works and textbooks of gynecologic oncology (39,40,41).
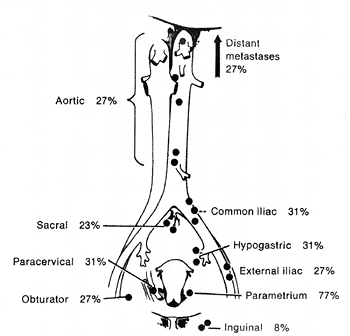 |
Figure 41.4 Percentage of involvement of draining lymph nodes in untreated patients with cervical cancer. Reprinted with permission from: Henriksen E. The lymphatic spread of carcinoma of the cervix and of the body of the uterus: a study of 420 necropsies. Am J Obstet Gynecol 1949;58:924 942. |
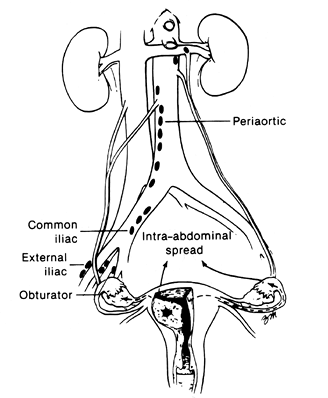 |
Figure 41.5 Paths of spread available to carcinoma of the endometrium. Not only may carcinoma cells metastasize to the pelvic and periaortic lymph nodes, but they also may spread through the fallopian tube to the peritoneum or they may invade through the myometrium into the broad ligament and ovary. Reprinted with permission from: DiSaia P, Creasman W, eds. Clinical Gynecologic Oncology. 3rd ed. St. Louis: CV Mosby; 1989:84 93. |
Tubal lymphatics accompany the ovarian vessels and drain into nodes near the right and left renal veins and the presacral and common iliac nodes. Lymphatic spread of tubal malignancy may reach extrapelvic sites early in its dissemination (42,43).
Uterine Cervix
The uterine cervix (or neck ) is the elongate fibromuscular portion of the uterus that measures 2.5 to 3.0 cm. A part of this structure protrudes into the upper part of the vagina (vaginal part, portio vaginalis), whereas the remainder lies above the vaginal vault (supravaginal portion, portio supravaginalis). The outer surface of the vaginal portion of the cervix is known variously as the ectocervix or exocervix. It is covered, at least in part, by stratified squamous epithelium that is continuous with, and histologically identical to, the mucosa of the vaginal fornices. That portion of the cervix, in relation to the endocervical canal, is known as the anatomic endocervix. The endocervical canal, lined for the most part by mucin-secreting epithelium that blends at one end with the squamous epithelium of the exocervix and with the epithelium of the lower uterine segment at its other end, brings the vagina into communication with the endometrial cavity. The anatomic opening of the endocervical canal onto the exocervix is known as the external os. In parous women, this most often takes on a slitlike configuration that serves to divide the exocervix into anterior and posterior lips (18,44). This particular geometry is thought to be important in uterine function during gestation (45). The upper limit of the endocervical canal is known as the internal os. This is not a distinct orifice; rather, there is a gradual, funnel-shaped widening of the endocervical canal and a transition from endocervical epithelium into the endometrial epithelium of the lower uterine segment. The junction of the endocervical glandular mucosa with the squamous epithelium of the exocervix is known as the squamocolumnar junction. This junction does not always lie at the external os; in fact, the squamocolumnar junction typically is located on the exocervix, where it can easily be inspected with the culposcope. This is further discussed in the section devoted to the transformation zone.
The uterine cervix obviously plays an important role in the anatomic support of the internal genitalia and plays an active role in labor and delivery, but arguably its primary role is the production of cervical mucous. Cervical mucous acts as a functional gate that prevents vaginal microorganisms from gaining access to the upper genital tract and (except for a small midcycle window before ovulation) denies sperm access to the uterus and fallopian tubes. At midcycle the chemical composition of the cervical mucous changes and its viscosity decreases. This has the effect of allowing the passage of sperm into the upper genital tract. These changes are the basis of the Spinnbarkeit and fern tests. In addition, the cervical mucous plays an important role in removing seminal plasma constituents (preventing sperm phagocytosis) and in providing a suitable environment for sperm storage, capacitation, and migration (46,47).
The following discussion first focuses on the epithelium of the exocervix, the endocervix, and the transformation zone and then turns to the stroma of the cervix and the changes that occur in the cervix during pregnancy.
Epithelium of the Exocervix
The squamous epithelium covering the exocervix is normally noncornified, and it grows, matures, and accumulates glycogen in its upper layers in response to circulating estrogens, most notably, estradiol (Figure 41.6). Because low blood levels of estrogen are the rule during childhood and the postmenopausal years, the squamous cells of the cervix do not proliferate or mature, and glycogen is not stored in the upper layers of the epithelium during these
P.1018
periods unless estrogen is made available as a result of therapy or functioning ovarian tumors (18). In the immediate postnatal period, the squamous epithelium of the newborn cervix is fully mature due to maternal estrogen, but the epithelium quickly becomes atrophic and glycogen disappears as estrogen levels decrease.
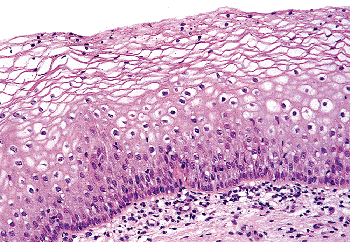 |
Figure 41.6 Mature squamous epithelium of the exocervix demonstrating a normal maturation sequence from basal cells to superficial cells. The cleared cytoplasm indicating glycogen storage should not be confused with koilocytosis. |
The estrogenically stimulated cervical squamous epithelium of the sexually mature woman can be divided into three layers: the basal/parabasal cell layer, the midzone layer (or stratum spongiosum), and the superficial layer (Figure 41.6). The basal cell layer is composed of cells with scant cytoplasm and oval to cuboidal nuclei with dense chromatin. These cells are usually mitotically inactive and do not mark immunohistochemically with proliferation markers; for example, Ki-67 and proliferating cell nuclear antigen (PCNA) (48). The cells immediately above the basal layer comprise the lower portion of the midzone layer and are known as parabasal cells, a term often used in cytologic circles. The parabasal cells are somewhat larger than the basal cells due to their increased cytoplasm, and the nuclei have slightly less dense chromatin. In contrast to the basal layer, mitotic figures are usually present but are not abnormal or particularly numerous in the normal epithelium. This layer also displays proliferation markers (48). The midzone layer is composed of cells with even more abundant cytoplasm and somewhat smaller vesicular nuclei. These are known as intermediate cells. Glycogen accumulates in most intermediate cells, and this imparts a finely granular or clear appearance to the cytoplasm. The superficial cells contain small, rounded, regular pyknotic nuclei, and their cytoplasm is abundant and clear as a result of even greater glycogen accumulation. Keratinization occurs in both the superficial and intermediate cells and renders them flat and platelike when they are spread on a slide. The cytoplasmic clearing characteristic of normal intermediate and superficial cells is often perinuclear. Because perinuclear clearing is also a feature of cells (koilocytes) infected by human papillomavirus (HPV), there is a potential for misinterpreting normal epithelial cells containing glycogen as abnormal. However, koilocytes not only feature perinuclear clearing of the cytoplasm, but their nuclei are larger, and these nuclei possess a more undulating nuclear membrane than do the nuclei found in intermediate and superficial cells (giving rise to the appearance of koilocytes sometimes described as raisinoid or pruneoid ). Moreover, the nuclear chromatin of koilocytes has a ropy texture in contrast to the homogenous appearance of normal cells. The cervical squamous mucosa undergoes cyclic changes during the menstrual cycle similar to the estrogen progesterone-induced changes in the vaginal mucosa, although the cells composing the latter are a more liable index of hormonal status. During the luteal phase and pregnancy, when progesterone levels are high, there is a predominance of intermediate cells.
The exocervical epithelium in postmenopausal women (not receiving a supplement of estrogen therapy) is composed mainly of basal and parabasal cells that feature scant cytoplasm and little or no cytoplasmic glycogen (Figure 41.7). The cells may have the same degree of nucleus-to-cytoplasm ratio shift toward the nucleus as do the cells composing cervical intraepithelial neoplasia (CIN). Consequently, atrophic epithelium is a part of the differential diagnosis of CIN, and care should be taken when a diagnosis of CIN is contemplated in a postmenopausal woman. However, the basal and parabasal cells in atrophic epithelia do not demonstrate the nuclear abnormalities and high mitotic index usually seen in the cells constituting the neoplastic epithelium in high grade CIN (high grade squamous intraepithelial lesion SIL).
Endocrine cells have been identified in the squamous epithelium of the exocervix by immunohistochemical techniques; their function is unknown, but they are thought to give rise to the rare cervical carcinoid tumors (49,50,51,52,53,54,55,56). Langerhans cells also are present in the ectocervical epithelium, as well as in the transformation zone (57,58,59). They
P.1019
are involved in antigen presentation to T-lymphocytes. Melanin-containing cells have been reported in the cervical epithelium and provide a plausible cell of origin for the uncommon cervical melanoma and blue nevus (60).
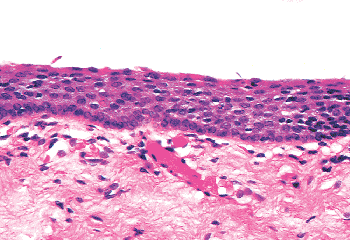 |
Figure 41.7 Postmenopausal atrophy of the cervical squamous epithelium. The immature cells can resemble the cells in high-grade squamous intraepithelial lesion SIL (cervical intraepithelial neoplasia CIN). |
Epithelium of the Endocervix
The anatomic endocervix extends from the external os to the internal os, but endocervical glandular epithelium is not exclusively limited to this anatomic area, particularly during the reproductive years. Rather, endocervical epithelium occupies significant regions of the anatomic exocervix during childhood and after the menarche. The shift of the endocervical epithelium out of the canal onto the exocervix is discussed in more detail below in the section devoted to the transformation zone.
The endocervix is lined by a single layer of mucin-secreting epithelium composed of cells with small, often basilar, nuclei above which is mucin-filled cytoplasm that imparts a picket fence appearance (Figure 41.8). Goblet cells are sometimes encountered (Figure 41.9). The nuclei are generally small, elongate, and have rather dense chromatin. They tend to overlap one another. When the endocervical epithelium has been damaged and is regenerating, the nuclei may become larger and more rounded, but mitotic figures are difficult to find in nonneoplastic endocervical cells (61). If one encounters endocervical epithelium containing easily found mitotic figures, consideration should be given to the possibility of a pathologic process such as well-differentiated carcinoma or carcinoma in situ, particularly if the nuclei are enlarged and nucleoli are prominent. Nucleoli are usually not prominent in resting endocervical cells, but they may become so during regeneration, pregnancy, and neoplastic transformation. Mitotic figures may be found in the constituent glandular cells of cervical endometriosis.
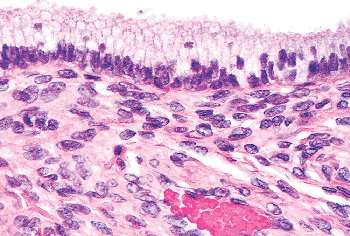 |
Figure 41.8 Normal endocervical mucosa with most nuclei in the characteristic basilar location. Enlargement of these nuclei and loss of apical mucin are features that should cause a closer inspection of the endocervical glands to ensure that neoplastic transformation is not present. |
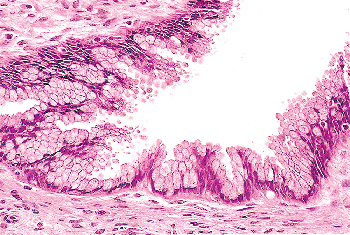 |
Figure 41.9 Goblet cells in the endocervix. Not infrequently, the nuclei of mucin-containing cells are displaced to the base of the cell and compressed by cytoplasmic mucin to produce a goblet cell. The presence of goblet cells and neuroendocrine cells in the normal endocervical mucosa tends to destabilize the conventional distinction in ovarian pathology between m llerian (i.e., cervical) mucinous and intestinal mucinous differentiation. |
Other types of cells may be identified in the endocervical epithelium. Ciliated cells are almost always present and can be a useful marker of a benign process when the appearance of the endocervical glandular epithelium raises concerns about well-differentiated adenocarcinoma (62). When ciliated cells are numerous, the term ciliary (or tubal) metaplasia is often used (Figures 41.10A and 41.10B) (63,64,65,66,67,68,69). Ciliated cells themselves can develop enlarged dense nuclei and thus come to resemble neoplastic cells (Figure 41.10C). As a result, care should be taken to look for cilia before diagnosing in situ neoplastic transformation of the endocervix. Immunohistochemistry may be of aid in this distinction; Marques et al. found a combination of vimentin and carcinoembryonic antigen (CEA) helpful: adenocarcinoma in situ tended to be CEA positive and vimentin negative while the opposite was true for tubal metaplasia (70).
Subcolumnar reserve cells that have the potential to differentiate into ciliated and mucous secretory cells have been reported to populate the endocervix, even though there is evidence that the differentiated mucous cells are capable of division without the intercession of reserve cells (61,62). It is easy to confuse the lymphocytes that have populated the glandular epithelium with epithelial reserve cells (71).
Endocrine cells also are present within the endocervical epithelium. Their normal function is unclear, but it is generally held that they give rise to the endocrine neoplasms such as carcinoids and neuroendocrine carcinomas that occasionally are encountered in the cervix (49,54).
The endocervical epithelium not only lines the surface of the endocervical canal, it also dips, to a variable degree, into the underlying stroma to form elongate clefts (Figure 41.11A).
P.1020
In histologic sections, these clefts typically are cut transversely, imparting the false impression that true endocervical glands are present within the stroma. However, true glands have different epithelia lining their ductal and secretory portions. In contrast, the endocervical mucosa has a more or less uniform appearance whether it lines the surface or the deep-lying glands. Further evidence that these are not true glands was provided in an study conducted over 40 years ago by Fluhmann (72,73). He demonstrated, by means of serial sections and three-dimensional reconstructions, that what appeared to be endocervical glands within the stroma are actually complex protrusions of the endocervical lining that form clefts into the underlying stroma. When the endocervical epithelium lining the stromal clefts proliferates, side channels grow out from the clefts, giving rise to a histologic pattern that even more closely suggests acini of glands (Figure 41.11B). Fluhmann labeled these side channels tunnel clusters ; we also refer to them by a more euphonious designation: Fluhmann's lumens. When secretion inspissates in tunnel clusters, either because of obstruction or because of the viscosity of the secretions, it appears as bright eosinophilic
P.1021
material, an eye-catching pattern resembling the thyroid (see Figure 41.16). Having now discharged our obligation to anatomic accuracy, we shall continue to use the terms endocervical gland(s) and cleft(s) interchangeably.
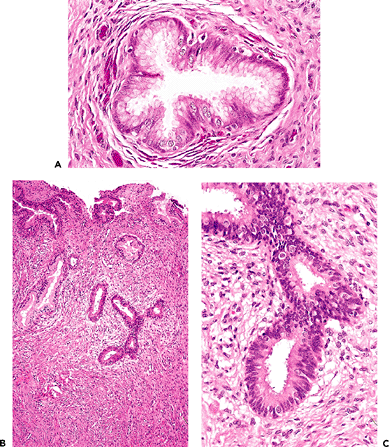 |
Figure 41.10 A. Ciliated cells in the endocervix. The normal cervical mucinous epithelium consists of an admixture of mucin-containing cells and a smaller population of ciliated cells. The population of ciliated cells undergoes cyclic variation with the menstrual cycle. B. Cervical tubal metaplasia. When ciliated cells are prominent they may simulate endocervical glandular dysplasia or carcinoma in situ. At low magnification the glands feature a prominence of nuclei, a feature shared with glandular dysplasia. C. Cervical tubal metaplasia. Higher magnification shows prominent cilia, the hallmark of ciliated cell metaplasia. |
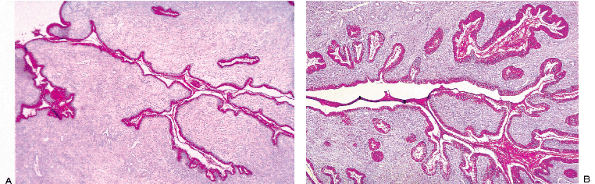 |
Figure 41.11 A. Tangential section of the endocervix stained with PAS to show how the gland clefts extend into the stroma and branch to form channels. B. When the endocervical mucosa undergoes hyperplasia and increases its surface area, as in pregnancy, the branches of the clefts proliferate and form even more collaterals ( tunnel clusters ). |
The depth to which benign endocervical glands can extend in the cervical stroma varies from cervix to cervix. They can be found as deep as 1 cm but usually are found at a depth of less than 5 mm (68,74,75). This anatomic variation becomes important when considering a diagnosis of minimal deviation adenocarcinoma (76,77). In this form of adenocarcinoma, the cytologic features differ only minimally from normal endocervical epithelium and the diagnosis depends to a large extent on the identification of abnormally shaped glands at an inappropriate depth within the cervical stroma. The trick here is to compare the depth of the glands in question with noncontroversially benign glands in the immediate neighborhood. Additionally useful in establishing a diagnosis of malignancy is a search for glands around nerves or vessels, an irregular lobster claw glandular configuration, and a granulation tissue stromal host response. We have not personally encountered a clinically malignant endocervical glandular proliferation that featured ciliated cells and, in our opinion, this finding argues strongly against a diagnosis of adenocarcinoma.
Endocervical cells show only minimal morphologic changes during the menstrual cycle, and even this amounts only to a shifting of the basally situated nuclei to a midcell position at the height of the proliferative phase. These minor cytologic changes are in contrast to the dramatic biochemical changes that occur within the endocervical cells during the menstrual cycle (46). Throughout the proliferative phase of the cycle, the endocervical cells secrete mucus of lower viscosity than at other times of the cycle. This is thought to aid penetration of the cervical canal by spermatozoa (46). When progesterone levels attain their zenith during the luteal phase, the endocervical glandular secretion becomes thick and scant. It is at this stage that the secretion may become inspissated and more visible in histologic sections. During pregnancy the number of tunnel clusters increases, and when this phenomena is extreme the term cervical glandular hyperplasia is often used (78). Pregnancy also causes the secretions of the endocervical cells to thicken and form a mucous plug that blocks the endocervical canal (47,79).
Epithelium of the Transformation Zone
The endocervical mucosa shifts over the various divisions of the anatomic cervix throughout life (18,44,80). At birth, the endocervical mucosa resides on the exocervix in two thirds of infants but it quickly moves back into the anatomic endocervical canal where in most girls it remains until near the menarche. After the onset of puberty, the endocervical mucosa again moves out onto the exocervix, usually more prominently on the anterior portion than on the posterior part (Figure 41.12A). The mechanism whereby endocervical mucosa changes location is apparently a mechanical one caused by swelling of the stroma of the cervical tissue in response to hormonal stimulation. As the lips of the cervix swell, they roll anteriorly and posteriorly, pulling the endocervical mucosa out of the canal onto the exocervix. The exposed endocervical tissue is often referred to as ectropion. Because the exposed endocervical mucosa appears red and ulcerated to the naked eye, it also has been interpreted as an erosion. There is, in fact, no erosion of the mucosa, rather the process is one of physiologic ectopy. After menarchial ectropion occurs, the endocervical tissue is gradually replaced by squamous epithelium throughout the reproductive years. The area where the glandular tissue is being replaced by squamous epithelium is known as the transformation zone. The junction between the two types of epithelium is labeled the squamocolumnar junction (44,81).
P.1022
Two squamocolumnar junctions are usually recognized (Figure 41.12B). The original squamocolumnar junction is the point where the native (original) exocervical squamous epithelium joins the endocervical glandular epithelium and is out on the exocervix during the reproductive years (80). This junction is usually sharply defined and it is anatomically fixed. After squamous metaplasia has replaced endocervical tissue, the original squamocolumnar junction is the fusion point between the new squamous epithelium laid down in the transformation zone and the native squamous epithelium (Figure 41.13). The functional squamocolumnar junction is the point of active replacement of columnar endocervical epithelium by squamous cells. This junction is often irregular and patchy, and it changes its contours and its locations during reproductive life. The functional squamocolumnar junction is usually implied when the term squamocolumnar junction is used without a modifier and the area between the two squamocolumnar junctions is the transformation zone. During pregnancy, particularly the first pregnancy, even more endocervical tissue moves out onto the exocervix, enlarging the area of ectopic endocervical epithelium. This phenomenon also can occur during progestogen therapy.
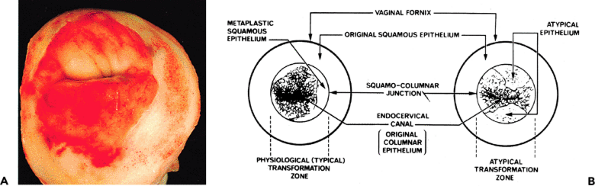 |
Figure 41.12 A. Multiparous cervix during the reproductive years. Note the slitlike configuration of the external os and the erythematous endocervical tissue out on the anatomic exocervix. This endocervical tissue undergoes conversion to squamous epithelium throughout the reproductive years. The squamocolumnar junction is visible as a sharp line between the white squamous epithelium and the erythematous glandular tissue. B. Diagram of the cervix demonstrating the transformation zone. On the left is a typical transformation zone in which metaplastic squamous epithelium is replacing endocervical columnar epithelium. Squamocolumnar junction refers to the original squamocolumnar junction. On the right is a nontypical transformation zone in which the metaplastic process is composed of dysplastic squamous cells and hence the process is cervical intraepithelial neoplasia. Reprinted with permission from: Fox H. Haines and Taylor Obstetrical and Gynaecological Pathology. 3rd ed. Philadelphia: WB Saunders; 1987. |
Because endocervical glandular epithelium is present on the exocervix, the transformation zone can be visualized with the aid of a colposcope. This is fortunate because neoplastic change begins most commonly in the transformation zone, and neoplastic transformation is accompanied by structural alterations that can be recognized using the colposcope. The combination of papilloma virus detection, cytologic preparations, colposcopic examination, biopsy, and local destruction of intraepithelial abnormalities in the transformation zone under colposcopic visualization is a powerful tool for the early detection and successful treatment of in situ neoplastic processes involving the cervix.
In the latter years of reproductive life the functional squamocolumnar junction reaches the area near the anatomic external os and, reversing its menarchal journey, begins to move up the anatomic endocervical canal. By the perimenopausal years, the squamocolumnar junction is usually concealed within the endocervical canal above the external os.
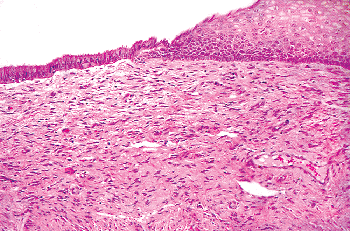 |
Figure 41.13 Squamocolumnar junction with a distinct transition from mature squamous epithelium on the right to endocervical glandular tissue on the left. Such a sharp change can be seen at the original squamocolumnar junction as well as the junction formed by squamous epithelium with endocervical tissue in the transformation zone when squamous epithelium is mature. |
P.1023
Two mechanisms are thought to be operative in transforming endocervical mucinous epithelium to squamous epithelium: squamous epithelialization and squamous metaplasia (3). The first involves the direct ingrowth of mature native squamous epithelium from the exocervix. This process is usually labeled squamous epithelialization. During squamous epithelialization, mature squamous cells come to lie beneath the endocervical glandular cells. They push the endocervical cells off the basement membrane, and gradually the columnar cells degenerate and are sloughed. Squamous epithelialization initially spares the openings of the underlying endocervical glands, and at this stage the openings to the glands have the appearance of pores when examined with the colposcope. Eventually, the ingrowth of squamous epithelium involves the orifices of the glandular clefts and then it can extend for varying distances down into the cleft spaces (Figures 41.14 and 41.15). When this process involves the orifice, it may plug the opening and if the mucinous epithelium below continues to secrete, a mucin-filled cyst (Nabothian cyst) or tunnel clusters filled with eosinophilic secretion result (Figure 41.16). If squamous epithelialization involves the cleft and its ramifying tunnels, squamous epithelium will be surrounded by endocervical stroma. Consequently, histologic sections taken in an area of squamous epithelialization may show Nabothian cysts, mucification of tunnel clusters, and/or islands of benign squamous epithelium in the stroma beneath the surface epithelium.
When the endocervical clefts undergo squamous epithelialization, care must be taken not to confuse the deep-lying benign squamous cells with invasive carcinoma. Although the cells in squamous epithelialization may have enlarged nuclei and prominent nucleoli, they do not demonstrate the anaplasia, the pleomorphism, the chromatin abnormalities, or the abnormal mitotic figures characteristic of invasive carcinoma. Moreover, the benign cells conform to the rounded configuration of the preexisting cleft and do not infiltrate the stroma irregularly. Typically there is no granulation tissue host response to squamous epithelialization, although chronic inflammation may be present. If squamous epithelialization involves tunnel clusters, small groups of squamous cells come to lie deep within the cervical stroma, imparting an architectural pattern that even more resembles infiltrating squamous cell carcinoma. Squamous epithelialization seems to be stimulated by chronic inflammation and local trauma, including cauterization or laser surgery.
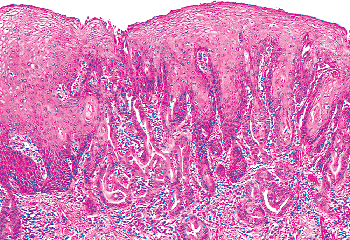 |
Figure 41.14 Squamous epithelialization of the endocervix. Note the mature squamous epithelium extending into endocervical gland clefts (PAS positive glandular structures surrounded by stroma). This process can mimic invasive carcinoma. |
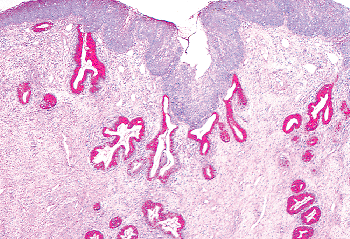 |
Figure 41.15 Conversion to squamous epithelium in the cervix may occur more rapidly on the surface than in the clefts, causing squamous epithelium to overlie endocervical gland clefts. When the newly laid down squamous epithelium blocks the orifices of the clefts, nabothian cysts or mucification of tunnel clusters, as seen in Figure 41.16, may result. |
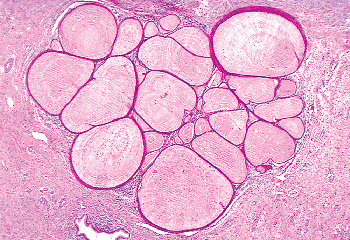 |
Figure 41.16 When the newly formed squamous epithelium in the transformation zone covers the endocervical gland cleft orifices and secretion continues, the tunnel clusters fill up with secretion that may become inspissated ( mucification ). |
P.1024
The second mechanism thought to contribute to the conversion of endocervical mucinous epithelium to squamous epithelium entails first the proliferation of endocervical reserve cells, and then the differentiation of these cells into squamous cells rather than mucin-producing cells (82). This process, known as squamous metaplasia or prosoplasia, can be distinguished from squamous epithelialization because, unlike the cells in squamous epithelialization, the reserve cells initially do not have squamous characteristics; rather, they appear as cuboidal cells with round nuclei growing beneath the mucinous epithelium (Figure 41.17). In fact, these cuboidal cells are identical in appearance to the basal or parabasal cells of the squamous epithelium. After the reserve cells proliferate and stratify, they differentiate into squamous cells that initially have only slightly increased amounts of cytoplasm (immature squamous metaplasia). Later the cells may fully mature to glycogen-containing squamous cells indistinguishable from the superficial cells of the exocervix (see Figure 41.13). Confusingly, squamous metaplasia is commonly used as a generic term for both metaplasia and squamous epithelialization.
Immature squamous metaplastic cells without fully developed squamous characteristics or glycogen accumulation can come to occupy most or all of the thickness of an epithelium (83,84) (Figure 41.18). Because fully mature squamous cells are not present toward the surface and because the cytoplasm of the immature cells is relatively scant and its nuclei often are elongate, immature squamous metaplasia can bear a close resemblance to high grade intraepithelial neoplasia (dysplasia carcinoma in situ). However, the nuclei in immature squamous metaplasia are uniform, chromatin abnormalities are minimal at most, and nuclear contours are usually smooth. Although mitotic figures may be present, in immature squamous metaplasia abnormal forms are not found. The possibility of immature squamous metaplasia should be considered in each case where a diagnosis of intraepithelial neoplasia is contemplated.
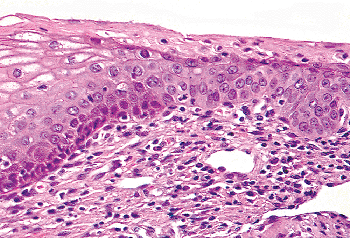 |
Figure 41.17 Functional squamocolumnar junction with metaplastic epithelium on the right. Note that in this example maturation has proceeded to the parabasal cell stage with abrupt keratinization rather than the normal maturation sequence to superficial cells as seen on the left. |
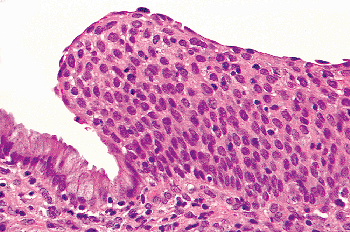 |
Figure 41.18 Immature squamous metaplasia on the right. The constituent cells do not demonstrate evidence of maturation and are similar to cells normally present in the basal layer of squamous epithelium. This type of metaplasia can be confused with high-grade squamous intraepithelial neoplasia. |
Squamous metaplasia is usually patchy, giving rise to the characteristic irregularity of the functional squamocolumnar junction (Figures 41.19A, B). As squamous metaplasia proceeds, the islands of squamous cells form bridges to other centers of metaplasia, ultimately producing a solid area of squamous epithelium.
Whatever the mechanism either squamous epithelialization or squamous metaplasia squamous replacement of mucinous epithelium on the exocervix is a normal process that must be distinguished from in situ and invasive neoplasms. Features that are often found in neoplasia but not in metaplasia or epithelialization are moderate to marked pleomorphism, lack of maturation sequence (this may be present in immature squamous metaplasia), irregular nuclear outlines, and abnormal mitotic figures. Nucleoli are usually inconspicuous in cervical intraepithelial neoplasia but are often prominent in metaplasia, and epithelialization (a notable exception is immature squamous metaplasia), and reactive changes in response to cervicitis.
Cervical Stroma
In contrast to the wall of the uterine corpus, which is predominately muscular, the stroma of the exocervix is mainly fibrous tissue admixed with elastin through which run infrequent strands of smooth muscle (85,86,87,88). A large number of vessels course through the stroma. A rich capillary network interfaces with the epithelium at the stromal epithelial junction. This interface is irregular and features
P.1025
fingers of connective tissue containing vessels overlain by a squamous cell mucosa of variable thickness. Much of the endocervical stroma is also fibroelastic tissue, but at the upper end of the endocervix the superficial fibrous stroma blends imperceptibly into the endometrial stroma of the lower uterine segment. Consequently, the superficial stroma of the upper endocervix and the stroma of the lower uterine segment has a hybrid endometrial cervical appearance. This can cause localization problems when it is important to determine whether a neoplastic process in a curettage specimen involves the endometrium or the endocervix, or both. We think the presence of unequivocal endometrial stroma, as determined by high cellularity (closely packed nuclei), should be present before interpreting tissue as originating from the endometrium on the basis of the stroma alone. Of course, if one type of normal glands is present, whether endocervical or endometrial, these glands can suggest the origin of the tissue, but both types of glands or even hybrid glands may be present in the transition area between the endocervix and the lower uterine segment. The endocervix contains a greater number of smooth muscle fibers in its deeper stroma than does the exocervix, and in the lower uterine segment these blend into the myometrium.
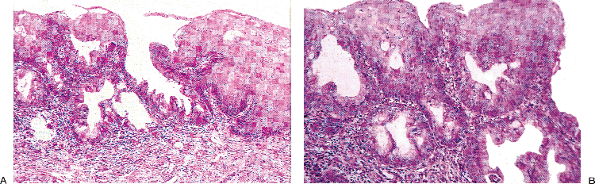 |
Figure 41.19 A. Islands of metaplastic squamous epithelium in the transformation zone at the functional squamocolumnar junction. These islands will eventually coalesce. B. Higher power photomicrograph of the area of squamous metaplasia demonstrated in A. |
The cervix bridges the sterile environment of the uterine cavity and the microbiological jungle of the lower genital tract. It is not surprising that this important immunologic role (both humoral and cellular) would be marked by a conspicuous lymphoid presence (44). Thus, large numbers of T-lymphocytes normally populate the endocervical stroma (89). B-lineage lymphoid cells, manifest as either plasma cells or germinal centers, are also commonly encountered.
In addition, dendritic cells are numerous in the cervix; a subset of these are Langerhans cells (immature dendritic cells that express MHC class II antigens and the CD4 receptor on their surface) that are involved in internalizing antigen and presenting it to T-lymphocytes in the regional lymph nodes (58,90,91,92).
The relevance of these observations to the surgical pathologist is chiefly to discourage the overuse of chronic cervicitis ; the presence of lymphoid tissue in the cervix is as normal as its presence in the small intestine.
In our opinion, a diagnosis of chronic cervicitis should be withheld unless the lymphoid infiltrate is very heavy and/or lymphoid nodules are numerous. Particularly important for the diagnosis of chronic cervicitis are large numbers of plasma cells. Scattered plasma cells are normal in the cervix. Acute cervicitis is not uncommon, but true inflammatory erosion or microabscesses are rare in the cervix.
Lymphocytes also may migrate into the endocervical epithelium, and in this location they may assume the appearance of cleared cells. Such cells have been misconstrued as reserve cells in the past (71).
Remnants of the wolffian duct commonly known as mesonephric rests can be found in the endocervical stroma of the lateral portions of the cervix in about a third of women (93) (Figure 41.20A, B). Usually these are deep in the stroma, but occasionally they are found near the surface and they can even blend with the endocervical gland clefts. Mesonephric rests are tubular structures lined by a single row of cuboidal cells with a central, round, cytologically bland nucleus. Typically the tubules form lumens that contain hyalin-like, eosinophilic secretions. Architecturally, there is usually a central elongate duct surrounded by smaller tubules. The combination of deep stromal location, the hyalin-like secretions, and the cuboidal cells usually serves to make identification of mesonephric rests straightforward. Even though tunnel clusters may ramify from a central cleft and contain eosinophilic secretion, they are lined by endocervical mucin-producing cells. The importance of this vestigial structure lies in its mimicry of
P.1026
well-differentiated adenocarcinoma. Mitotic figures are usually absent in mesonephric rests and the chromatin of the cells is bland. Moreover, mesonephric rests do not exhibit the raggedly infiltrative growth of carcinoma even though they are located deep in the stroma. Rarely, atypical hyperplastic and neoplastic processes may involve mesonephric remnants (78,93,94). Mesonephric proliferations, benign and malignant, often express CD10 (95,96).
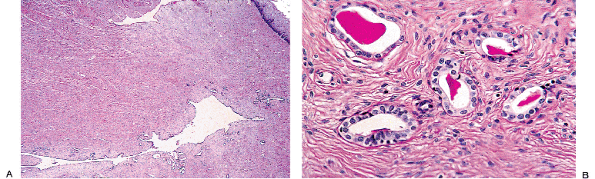 |
Figure 41.20 A. Mesonephric remnants in the cervix. A long cleftlike space deep in the stroma surrounded by tubules is the characteristic architectural finding. B. Ducts lined by bland cuboidal cells containing lumenal PAS-positive eosinophilic secretion are key features of mesonephric remnants. The blandness of the constituent cells and the organization around a central cleft are the most helpful features in distinguishing this from well-differentiated adenocarcinoma. |
Multinucleated giant cells rarely are found in the normal superficial endocervical stroma. These cells have enlarged and sometimes bizarre-shaped nuclei with smudged chromatin similar to those seen in fibroepithelial stromal polyps (97). They should not be mistaken for a neoplasm (98,99,100).
Cervix During Pregnancy
During pregnancy the endocervical epithelium proliferates so that its mucus-secreting surface increases. This proliferation leads to both the formation of polypoid protrusions of endocervical epithelium into the endocervical canal and an increased number of tunnel clusters budding off preexisting clefts within the cervical stroma. The overall impression is one of an increase in the amount of endocervical tissue and, consequently, this normal process is often termed endocervical glandular hyperplasia, or when numerous small glands are packed together, microglandular hyperplasia. Identical changes can be produced by artificial progestogens. The endocervical mucus during pregnancy is thick and functions as a plug to seal off the endometrial cavity from the vagina (22,101). Arias-Stella reaction may be seen in the endocervical glandular cells (102). As in the endometrium, the large cells with prominent nucleoli characteristic of the Arias-Stella reaction can raise concern about clear cell carcinoma, but the absence of mitotic figures and the gestational setting should quickly eliminate this possibility.
The stroma of the cervix undergoes a complex series of biochemical and biomechanical changes during pregnancy and parturition that taken together are known as cervical ripening (103). The initial change seems to be extensive destruction of collagen fibers by various collagenases accompanied by the accumulation of gel-like acid mucopolysaccharides. This process causes the cervix to soften, a process that reaches its zenith immediately before parturition. As a result, the cervix is easily effaced by the presenting part of the emerging infant. Thus, the usually cylindrical cervix is transformed into a thin saccular structure. The increased fluid in the cervical stroma during pregnancy causes the cervical lips to roll further out into the vagina, everting more of the endocervical mucosa beyond the external os. Squamous epithelialization and metaplasia rapidly ensue, and at the time of delivery there is often considerable immature squamous epithelium in the transformation zone. As noted previously, the cells in immature squamous metaplasia can closely resemble those found in intraepithelial neoplasia, so caution should be exercised when examining cervical specimens taken from pregnant women.
The cervical stromal cells, particularly those near the surface of the endocervical canal, may undergo decidual change during pregnancy (Figure 41.21A, B). Cervical decidual reaction is typically patchy, and at low power this focal replacement of the cervical stroma by aggregates of epithelioid cells can resemble invasive large cell nonkeratinizing carcinoma. Awareness of this physiologic process during pregnancy and close attention to the cytologic features of the suspect cells should avoid misdiagnosis (104).
Normal findings in the cervix that have relevance to histopathologic differential diagnosis are presented in Table 41.1.
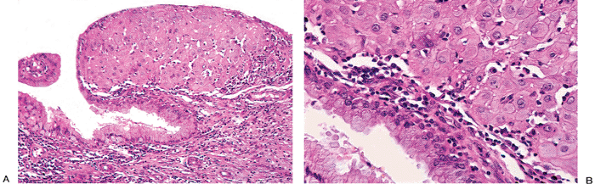 |
Figure 41.21 A. and B. Decidual reaction in the cervix. The sheetlike arrangement of the cells can mimic squamous cell carcinoma, but the nuclei are bland (see Figure 41.18). |
P.1027
Endometrium
Tissue Sampling and Associated Problems
A variety of endometrial tissue sampling techniques are available to the clinician. These techniques differ with respect to their indications, their limitations, and their associated complications (105,106,107,108,109,110,111,112,113). Endometrial curettage (cervical dilation and endometrial curettage D and C) entails the removal of most of the uterine mucosa by scraping with a sharp curette. Under ideal circumstances, the excision is complete or nearly complete. Endometrial biopsy (EMB) involves the removal of a more limited sample of tissue than does the complete curettage and is performed with a smaller curette. Single strips of endometrium are usually taken from both the anterior and the posterior fundal surfaces. Even though the sample is limited, the accuracy of diagnosis approximates that of the D and C. The chief advantage of this technique is that it does not require cervical dilation (and hence does not require anesthesia). EMB thus combines convenience and low cost, with little sacrifice in diagnostic accuracy. The major limitation of EMB lies in its inherent potential to miss focal lesions, such as polyps and localized carcinomas. Accordingly, when carcinoma is suspected clinically, a negative biopsy must be followed by a complete D and C, because only this technique ensures the absence of carcinoma. Hysteroscopy in combination with endometrial sampling is thought by some to increase the detection rate of uterine abnormalities (114); others disagree (115). If EMB is performed as part of an infertility workup (but see below), tissue should be obtained well into the presumed secretory phase; that is, 2 to 3 days before the time of the next menstrual period as estimated by clinical and laboratory findings. Although principle biopsy in the late luteal phase might destroy an early gestation, in practice this seems not to be the case (116).
Three artifacts of sectioning and tissue preparation should be mentioned at this point. A frequent finding in endometrial curettings is the telescoped gland, which is characterized by an inside-out gland within the lumen of a gland with a normal configuration. This artifact is seen when an intussuscepted or telescoped gland (produced by the traumatic removal of the tissue) is cross-sectioned, and it occurs most frequently in straight glands. Another artifact is the result of sectioning and involves the tangential cutting of a gland to produce a pseudo-gland-within-gland pattern. Confusion with adenocarcinoma can be avoided by attention to cytologic detail, comparison with surrounding glands, knowledge that the gland-within-gland pattern in carcinoma is usually extensive, and awareness of this topologic problem. A third artifact involves tangential sectioning of the endometrial surface to produce pseudocystic and pseudobudded glands. Poor fixation can sometimes result in the retraction of endometrial glands from their surrounding stromal envelope. Moreover, cytoplasmic vacuolization may be a result of autolysis and can simulate early secretory vacuolated epithelium.
Histology of the Normal Endometrium
The normal endometrium has a multiplicity of constantly changing normal patterns that depend on the nature and intensity of ovarian hormonal stimulation. The purpose of this section is to analyze the morphology of the normal nongravid endometrium in some detail from three points of view. First, we discuss regional variations, then the individual components of the endometrium; finally, using this background, we describe the temporal variations in the histology of the endometrium that occur throughout life.
P.1028
Table 41.1 Normal Findings in the Cervix that have Relevance to Histopathologic Differential Diagnosis (see text for Additional Differential Diagnostic Clues) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
P.1029
Regional Variations
The uterine lining can be divided into two regions on the basis of its morphology: the mucosa of the lower uterine segment and the mucosa of the corpus proper. The mucosa of the lower uterine segment (isthmus) is in general thinner than the fundal mucosa. The glands and stroma tend to be only sluggishly responsive to hormonal stimulation, and in consequence this portion of the endometrium most often lags behind the rest of the endometrium in its development. The morphologic transition from endocervical mucosa to lower uterine segment mucosa is gradual, and in fact the hybrid endocervical endometrial appearance of both the glands and the stroma of the lower uterine segment serves to identify this zone in endometrial curettings (Figures 41.22).
The major portion of the uterine lining, the corpus mucosa proper, is normally fully responsive to hormonal stimulation. Two layers can be readily identified within the endometrium throughout this region: the lowermost is labeled the basalis and the overlying one the functionalis. The basalis is that zone of weakly proliferative glands and associated dense-spindled stroma immediately adjacent to the myometrium (Figure 41.23A, B). Characteristically, the junction of the basalis and myometrium is irregular, and smooth muscle and endometrial stroma interdigitate and blend together at this point (Figure 41.24). When florid, this irregularity may give the false impression that endometrial tissue is pathologically isolated within the myometrium. This deception is particularly important when evaluating the presence or absence of superficial myometrial invasion
P.1030
in patients with endometrial adenocarcinoma. Of less importance is the confusion it creates in the diagnosis of adenomyosis. The basalis, despite its unimpressive, inactive, and undifferentiated appearance, plays a crucial role in the endometrial economy because it constitutes the reserve cell layer of the endometrium. After the bulk of the overlying functionalis is shed during menstruation, or after the functionalis is removed by curettage, the basalis and the residual deep functionalis are responsible for regenerating the endometrium. The remaining surface epithelium of the lower uterine segment also participates in this regeneration (117).
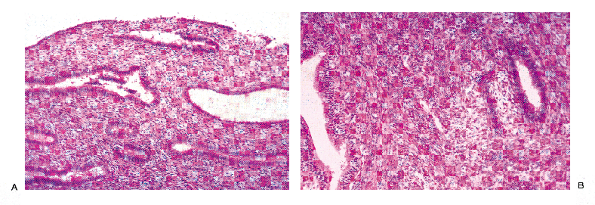 |
Figure 41.22 The lower uterine segment contains stroma and glands that are either hybrid between those seen in the endocervix and those in the fundus or a mixture of endometrial and endocervical glands and stroma. A. The stroma appears fibrous but more cellular than that typically found in the endocervix. B. An endometrial gland and an endocervical gland are found next to each other in this area of the lower uterine cervix. |
The appearance of the basalis is relatively constant throughout the menstrual cycle. Specifically, the glands usually appear weakly proliferative; that is, they possess pseudostratified elongate nuclei, rare mitotic figures, and dense, intensely basophilic chromatin. Most importantly, they lack secretory change (see Figure 41.23), and the stroma is spindled and nondecidualized. A notable exception to this generalization is the basalis during the latter half of pregnancy, which usually exhibits secretory glandular changes and stromal decidualization. The importance of recognizing the basalis of the endometrium lies in not mistaking it for the functionalis in a curettage specimen. This confusion would result in an erroneous impression that this weakly proliferative appearance represented the fully developed state of the functionalis.
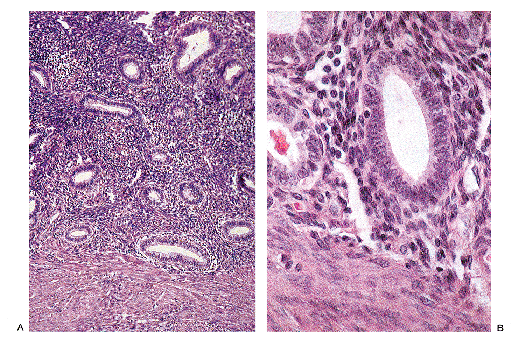 |
Figure 41.23 A. and B. The basalis of the endometrium is demonstrated. Throughout the menstrual cycle the basalis maintains a weakly proliferative appearance. As a result, dating of endometrium should be performed on fragments containing surface epithelium. |
It is the functionalis that exhibits the protean changes so characteristic of the normal endometrium. This layer has been traditionally divided into two strata the compactum and the spongiosum based on the morphologic
P.1031
appearances of each during the late secretory phase of the menstrual cycle and during pregnancy. Unless otherwise specified, the term endometrium refers to the functionalis in the subsequent discussion.
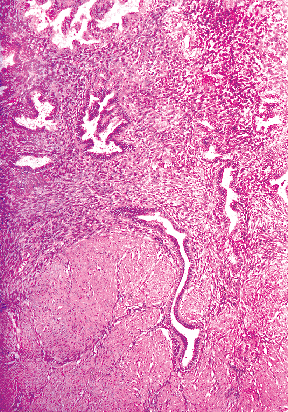 |
Figure 41.24 This is an example of an irregular endometrial myometrial junction. This phenomenon is important to think about when determining whether or not adenocarcinoma is superficially invading the myometrium. |
Individual Components of the Endometrium
The normal endometrium consists of both epithelial (surface and glandular) and mesenchymal (stromal and vascular) elements, which during reproductive years first synchronously proliferate, then differentiate, and finally disintegrate at roughly monthly intervals.
Epithelial Elements
The endometrial glandular and surface epithelia are both composed of four morphologically distinct cells, two of which are functional variants of the same cell.
Proliferative and Basalis-Type Cells.
The proliferative cells of the functionalis and the basalis-type cells are morphologically similar. These cells both have high nucleus-to-cytoplasm ratios and elongate sausage-shaped nuclei with dense chromatin and inconspicuous nucleoli. The cytoplasm is scant and generally basophilic to amphophilic (see Figure 41.33). Mitotic figures are common in the cells that compose the glands of the functionalis during the proliferative phase. When proliferative cells are the predominant cell type composing the epithelium (as in the proliferative endometrium), the nuclei appear pseudostratified.
Secretory Cells.
The characteristic cytoplasmic differentiation of the endometrial epithelial cell is nonmucinous secretion. Shortly after ovulation, secretory products accumulate in a subnuclear location in the proliferative cells; these products gradually shift to a supranuclear position and are ultimately discharged into the glandular lumens. This sequence of changes results in two easily recognizable secretory cell types: vacuolated and nonvacuolated secretory cells (see Figures 41.35B and 41.36C). Although vacuolated cells may have a nucleus similar to those seen in proliferative phase cells, the nonvacuolated secretory cells possess nuclei that are distinct from those seen in the undifferentiated proliferative phase cells. In contrast to the dense, intensely basophilic elongate nuclei of the proliferative cells, the nuclei of the nonvacuolated secretory cells are rounded and vesicular, they have uniformly dispersed chromatin, and occasionally nucleoli become prominent. The nonvacuolated secretory cells have uniform, moderately dense eosinophilic cytoplasm and often a frayed luminal border (see Figure 41.36C).
Another type of secretory cell is encountered, one that closely resembles the secretory cell of the uterine (fallopian) tube. This cell has an elongate nucleus with coarse chromatin, a moderate amount of densely eosinophilic cytoplasm, and a rounded luminal bleb similar to those found in apocrine glands. These cells are common in the surface epithelium and occasionally may line an entire endometrial gland. Some of these cells may in fact represent exhausted ciliated cells.
Ciliated Cells.
The ciliated cells of the endometrium have received little emphasis in the past. However, they are consistently present in endometrial specimens and presumably represent one line of differentiation open to the basalis-type cell. These cells are more prominent near the uterine isthmus and during the proliferative phase (118,119).
Ciliated cells have distinctive round, smoothly contoured vesicular nuclei containing finely stippled chromatin (Figure 41.25). Although the nuclear features remain relatively unchanged throughout cell development, the configuration and location of ciliated cells vary as a function of the stage of ciliogenesis. The earliest identifiable ciliated cells are situated adjacent to the basal lamina of the gland and are roughly pyramidal in shape. They possess distinctively clear cytoplasm with central round nuclei. A rounded cytoplasmic zone containing eosinophilic fibrillary material can be identified with routine stains. This zone corresponds to the intracytoplasmic cilia seen with the electron microscope. When the growing ciliated cells reach the luminal surface, the cilia are exposed to the glandular lumen. Initially the
P.1032
luminal surface of the ciliated cell is concave, but as the cell continues its development, this surface becomes convex, and ultimately the cilia may pinch off as a merocrine secretion. During this stage the cell has a characteristic fusiform-to-pear shape. Ciliated cells can come to predominate the cellular population of glands, and when they do the term ciliary metaplasia has been used.
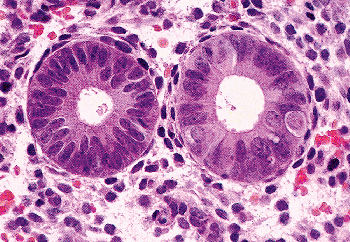 |
Figure 41.25 Proliferative phase glands with ciliated cells in the gland on the right. The round cell with clear cytoplasm at the 3-o'clock position has the characteristic appearance of ciliated cells before they have extruded their cilia into the glandular lumen. The other ciliated cells have a pyramidal shape. |
The Gland as a Whole.
The normal endometrial gland is lined by the aforementioned cells arranged in a nonstratified cuboidal-to-columnar epithelium, which during the proliferative phase deceptively appears to be stratified (i.e., it is pseudostratified). During the early proliferative phase, the glands are straight and have narrow lumens (Figure 41.26). Beginning in the midproliferative period and lasting throughout the rest of the cycle, the glands exhibit increasing degrees of coiling, but not branching. This culminates in the serrated saw-toothed appearance of the glands in the late secretory and menstrual endometrium. The surface epithelium is composed predominantly of apocrine-like secretory cells and ciliated cells, and has a relatively constant appearance throughout the cycle.
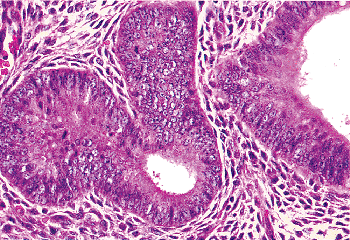 |
Figure 41.26 Proliferative phase glands and stroma. Note the elongate rather than rounded shape of the stromal cell nuclei. However, it is not uncommon for stromal cell nuclei to be elongate. |
Mesenchymal Elements
Cellular Elements
Endometrial Stroma
The endometrial stromal cell is the predominant cellular component of the stroma, and its appearance varies greatly with the stage of the menstrual cycle. During the early proliferative phase these cells have scant indistinct cytoplasm and dense oval-to-fusiform nuclei (Figure 41.27). As the menstrual cycle proceeds, the stromal cells become more elongate and acquire more cytoplasm. During the late proliferative phase and well into the secretory phase, electron microscopy shows increasing amounts of rough endoplasmic reticulum and both intra- and extracytoplasmic collagen. Toward the end of the secretory phase, the perivascular stromal cells become rounded, acquire more cytoplasm, and develop vesicular nuclei with occasionally prominent nucleoli. Cytoplasmic borders become more fully developed and gradually the entire endometrial stroma is transformed into sheets of cells, polygonal cells with sharp and distinct cytoplasmic borders, abundant cytoplasm, and centrally placed vesicular nuclei (Figure 41.28A, B).
This unique m llerian stromal transformation is called decidualization when fully developed (e.g., during pregnancy) and predecidualization when partially developed (e.g., during the late secretory phase of the menstrual cycle) (120). Ultrastructurally, the abundant cytoplasm of the decidual cell is populated by dilated rough endoplasmic
P.1033
reticulum, Golgi apparatus, and distinctly small mitochondria. Decidual cells form basal lamina and have complex intercellular interdigitations and tight junctions. The prominent intercellular borders are due to the accumulation of pericellular matrix (121).
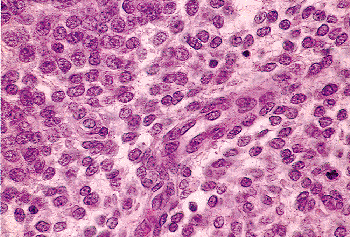 |
Figure 41.27 The proliferative phase endometrial stromal cells have scant, hard-to-discern cytoplasm and usually round nuclei (see Figure 41.26). Thin walled tubular blood vessels populate the endometrial stroma. |
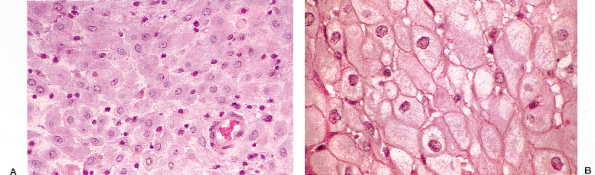 |
Figure 41.28 A. and B. These photomicrographs demonstrate decidual reaction during pregnancy. The cells have abundant cytoplasm and sharp cell margins. |
Thus, the decidua is the specialized endometrium of pregnancy and plays an active role in implantation and in mediating the relationship between the fetoplacental unit and the mother. The decidua secretes a host of products (prolactin, relaxin, renin, insulin-like growth factors (IGFs) and insulin-like growth factor binding proteins (IGFBPs) involved in the paracrine and autocrine regulation of the feto-maternal interface (13,122). In short, the endometrium of pregnancy functions as an endocrine organ. In addition, decidual cells appear to be capable of phagocytosis and are thought to play a role dismantling the collagen scaffolding at the implantation site (106).
Hematolymphoid Cells
A second prominent cellular constituent, particularly in the late secretory phase and during pregnancy, is what historically has been referred to as the stromal granulocyte but is now known to be a uterine natural killer (uNK) cell (123). These are rounded cells with bilobed nuclei, and have pale cytoplasm that contains eosinophilic granules. Their immunoprofile differs from that of blood natural killer (NK) cells. Their number appears to be positively correlated with the degree of predicidualization or decidualization in the surrounding endometrium; indeed, the number of such cells was used by Noyes as a dating criterion. This close association has suggested to some workers that uNKs play a role in the control of trophoblast invasion and spiral artery remodelling in human pregnancy and suggest that uNK cells in the late secretory phase and in early decidua may be important in initiating and maintaining decidualization. Alternatively, the death of uNK cells might be an early event in the onset of endometrial breakdown at menstruation (124,125,126).
In addition to this unique uNK cell, the endometrium contains other leukocyte subsets whose precise composition is menstrual cycle-dependent. These subsets include neutrophils and eosinophils (both rare until the premenstrual phase); macrophages; mast cells, and T-lymphocyte populations (present throughout the cycle but increasing perimenstrually) (13,127,128). Lymphoid follicles are commonly seen in the basalis of normal endometria (Figure 41.29).
Plasma cells are routinely seen in postpartum endometrial samplings and as an associated feature in a variety of pathological settings (endometrial polyps, endometrial carcinoma, etc.) It has traditionally been held that, outside these settings, plasma cells are normally not present in the endometrium and when identified, suggest (usually subclinical) endometritis. Certainly this is plausible when large numbers are present, although recent studies have raised questions about the clinical relevance of scattered plasma cells (129,130). In fact, small numbers of
P.1034
B-lymphocytes and plasma cells can be detected in endometrial samples using flow cytometry (127).
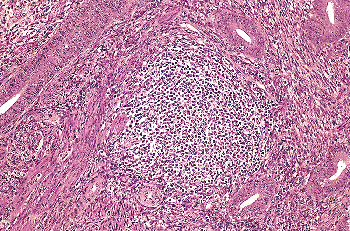 |
Figure 41.29 Lymphoid follicle. Scattered lymphoid follicles may be encountered in clinically normal women with otherwise unremarkable endometria. When present in large numbers and when associated with plasma cells, this finding is pathological. |
Occasionally, cells with bean-shaped nuclei and abundant vacuolated lipid-containing cytoplasm are present in the stroma of endometria stimulated by estrogen. These are termed stromal foam cells and probably represent modifications of endometrial stromal cells (Figure 41.30). Similar appearing foam cells are seen as a component of an inflammatory infiltrate, particularly one produced by foreign material (e.g., keratin).
Reticulin Framework
The endometrial stromal cells elaborate a reticulin framework that becomes progressively denser as the endometrium develops during the menstrual cycle, so that by the late secretory phase each stromal cell is enmeshed in reticulin. This framework undergoes dissolution during menstruation. The stromal intercellular space is also rich in high-molecular-weight mucopolysaccharides during the midproliferative and late secretory phase.
Vascular Elements
The radial arteries of the endometrium derive from the myometrial arcuate system. As the radial arteries course toward the uterine cavity they give off basal branches and then continue as endometrial spiral arteries. The basal arteries are unresponsive to steroid hormones, whereas the spiral arteries respond to varying hormone levels both by proliferation and, during the luteal phase of the menstrual cycle, by intermittent contraction (131,132).
Angiogenesis, the formation of new vessels, is central to the menstrual cycle and to implantation and subsequent gestation. As concerns the menstrual cycle: there must be repair of the vessels ruptured during the menstrual phase, then rapid growth of vessels during the proliferative phase, further development of spiral arteries during the secretory phase and, to come full circle, the dismantling of the vasculature that leads into the menstrual phase (13). All these stages are closely monitored by activators and inhibitors (133,134,135,136).
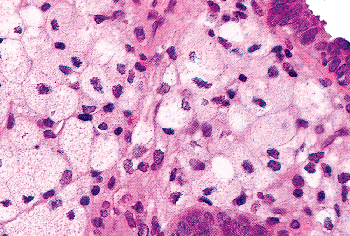 |
Figure 41.30 Stromal foam cells. Most likely these are modified endometrial stromal cells. In an inflammatory setting, stromal foam cells are probably macrophages. |
During implantation and gestation, angiogenesis is again pivotal in negotiating the hookup of the feto-placental unit to the maternal circulation. This entails an extensive remodelling of the placental bed spiral arteries to form the utero-placental vessels (137,138).
Ultrastructural Features and Immunohistochemistry
Both transmission and scanning electron microscopic study of the endometrium has produced an immense body of literature (109,139,140). Despite its scientific interest and the high aesthetic quality of many of the electron micrographs, little of this literature is currently of diagnostic relevance to the surgical pathologist. Mention should be made of two distinctive ultrastructural features found in the early secretory glandular cell: the giant mitochondrion complex (141) and the nucleolar channel system (142). These two features seem to be the earliest postovulatory morphologic change in the endometrial glandular cell.
Normal endometrial and endocervical epithelium have some differences in their immunohistochemical profile. Endometrial glands are positive for low and high molecular weight cytokeratins, vimentin, and occasionally CEA while endocervical epithelium expresses CEA but is negative for vimentin and low-molecular-weight cytokeratin (96). The endometrial stroma and mesonephric remnants are typically strongly positive for CD10 while the endometrial and endocervical glands are not. Occasionally normal smooth muscle cells express CD10 and about 40% of leiomyomas contain CD10 positive cells usually focally. Both the endometrial stroma and smooth muscle express vimentin, muscle specific actin, smooth muscle actin, and Bcl-2. Smooth muscle cells typically express desmin and caldesmon, whereas endometrial stromal cells do not. The combination of desmin (or caldesmon) and CD10 can be helpful in distinguishing smooth muscle tumors from endometrial stromal sarcoma.
The endometrium produces many secreted proteins that serve local cell signaling functions important for the developing endometrium and embryo. There is a large and growing body of literature concerning these products of endometrium, a topic beyond the scope of this chapter. However, there have been a number of excellent recent reviews of this subject (13,107,143,144,145,146).
Temporal Variations
Unlike morphologically unchanging epithelia, such as vaginal or gastrointestinal mucosa, that have an essentially constant appearance throughout the cell's lifetime, the
P.1035
endometrium undergoes dramatic temporal morphologic changes. The changes are cyclic and particularly striking during the reproductive years. These changes can be conveniently considered under six headings: newborn, premenarchal, perimenarchal, reproductive, perimenopausal, and postmenopausal years (107,147,148).
Newborn
The genitalia of the newborn girl respond to the high levels of circulating maternal and placental steroids by a transient burst of precocious development. The endometrium may be well developed and have either a proliferative or, less commonly, a secretory appearance. Within 2 weeks these changes have regressed, and the long hormonal quiescence of the premenarchal years begins. This quiescent period is characterized by a thin endometrium populated by inactive glands set within a spindled inactive stroma. Rarely, estrogen-secreting lesions of the ovary (follicular cysts, sex cord gonadal stromal tumors) may cause abnormal endometrial growth, with resulting abnormal bleeding as part of the syndrome of precocious pseudopuberty. The endometrium under these circumstances is proliferative or hyperplastic. That this phenomenon occurs suggests that the inactive appearance of the normal endometrium during this period results from an absence of hormonal stimulation rather than from transient end-organ unresponsiveness (10).
Menarche
The onset of uterine bleeding (menarche) is one of the many changes that signal the maturation of the reproductive system. In the United States, this usually occurs between 12 and 15 years of age. Characteristically, the perimenarchal period is marked by greater variability in the length of individual cycles than is seen in the reproductive years and by the occurrence of anovulatory cycles (148). Disordered proliferative endometria are commonly encountered in this setting (see section Disordered Proliferative Endometrium ).
Reproductive Years
The reproductive years are characterized by regularly occurring, roughly monthly, cycles, the end of which is signaled by menstrual bleeding (149,150). The dominant ovarian steroid secreted during the first half of the menstrual cycle is estradiol (E2), which induces endometrial proliferation. The second half of the cycle (beginning after ovulation) is hormonally dominated by both progesterone and estradiol, and this combination of hormones induces endometrial glandular secretion and stromal predecidualization. With the withdrawal of corpus luteum steroidal support, the endometrium is shed, setting the stage for the next cycle. These regularly recurring cycles may be interrupted by pregnancies, but after the termination of pregnancy, cycling is soon restored.
The biochemistry of the steroid molecules and their receptors that are responsible for the remarkable morphologic changes of the menstrual cycle have been the subject of intense study (151,152,153). Steroid molecules are hydrophobic and easily diffuse through cell membranes and freely enter all cells. The endometrium, vaginal mucosa, and other steroid-sensitive tissues are target organs by virtue of the presence of high-affinity, high-specificity, low-capacity saturable receptors for E2 and progesterone. These nucleus-based receptors are absent in nonresponsive cells. In addition to being highly responsive to circulating hormones, the endometrium also synthesizes substances such as glycoproteins that affect the hypothalamic-pituitary-ovary axis and the endometrium itself (see above). A detailed description of hormone regulation is beyond the scope of this chapter, but several excellent reviews on the topic have been published and can be found in the general references listed at the end of the chapter. In broad terms, the steroid molecule combines with the appropriate receptor, and the steroid receptor complex becomes associated with a nonhistone nucleoprotein. The net effect of this linkage is to alter both qualitatively and quantitatively DNA-dependent RNA transcription. The consequence is an altered profile of protein biosynthesis. Furthermore, the unique response of a particular target cell type depends on what specific growth and differentiation program is initiated by the steroidal signal. The endometrium is a major target organ for this continual barrage of steroidal information. It responds by undergoing the dramatic morphologic alterations that constitute the normal menstrual cycle as well as producing proteins important for hypothalamic feedback, modulation of placental hormone secretion, regulation of macrophages, and regeneration after menses.
Morphology of the Normal Menstrual Cycle Endometrium
The first day of the menstrual cycle has conventionally been identified as the first day of menstrual flow. Menses usually lasts for less than 5 days and is followed by the endometrial proliferative phase, the length of which exhibits great variation (9 20 days), but on average it lasts for 10 days. After ovulation, the coordinated and highly predictable series of stromal and glandular changes characteristic of the secretory (luteal) phase takes place. The traditional view is that the length of this phase is constant (14 days), and it is this alleged constancy that provides the basis for endometrial dating. Due to the sensitivity and specificity of serum hormonal studies, it is now uncommon for the surgical pathologist to be called upon to date the endometrium for infertility purposes. However, given the prevalence of polycystic ovarian disease, dysfunctional uterine bleeding, and iatrogenic hormonal use and because some gynecologists want endometrial dating it remains important for the surgical pathologist
P.1036
to recognize when ovulation has occurred and the normal responses of the endometrium to various hormonal stimuli. The following discussion is a concise description of the normal menstrual cycle with more detailed information in the accompanying figures. (Figures 41.31 and 41.32, Table 41.2) (106,107,117,154,155,156).
Proliferative Endometrial Phase (Ovarian Follicular Phase)
The proliferative phase spans the time from the previous menstrual period to ovulation. The endometrium responds to rising estrogen levels by synchronous proliferation of glands, stroma, and vessels. During the first third of the proliferative phase (early proliferative), the rate of growth of all three of these elements is coordinated, and as a consequence both vessels and glands are noncoiled. After a few days the growth of both glands and vessels outstrips that of the stroma; as a result, these tubular structures become coiled (mid- and late proliferative) (Figure 41.33).
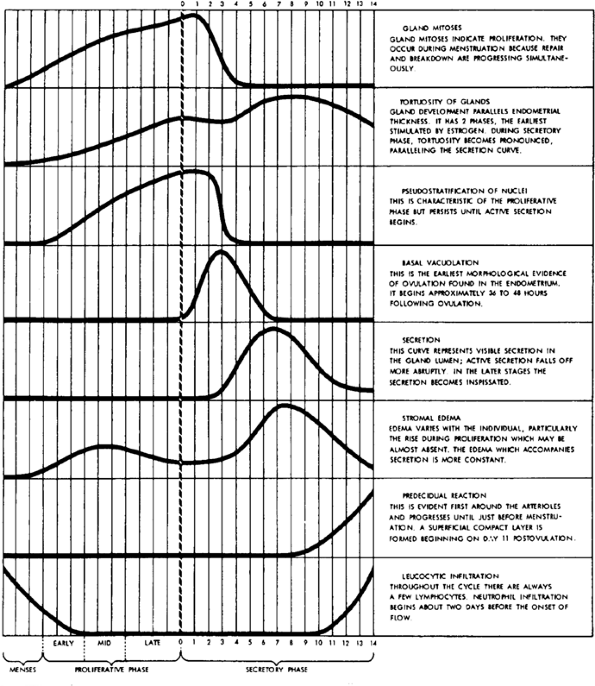 |
Figure 41.31 Approximate qualitative changes in eight morphologic criteria found to be most useful in dating human endometrium. Reprinted with permission from: Noyes R. Normal phases of the endometrium. In: Hertig A, Norris H, Abell M, eds. The Uterus. Baltimore: Williams & Wilkins; 1973:110 135. |
The glands are lined by mitotically active pseudostratified columnar cells with high nuclear to cytoplasmic ratios and dense chromatin (Figure 41.34). Mitotic figures are almost always easy to find. These cells are present throughout the proliferative phase and even into early secretion. After about 10 to 11 days, irregular subnuclear vacuoles begin to appear. During the last 2 days mitotic activity decreases, glandular coiling becomes more prominent, and vacuoles are easily found.
The interval period is the 48 hours between ovulation and the presence of uniformly vacuolated cells indicative of postovulatory day 2. Mitotic figures are present during this period, and the cells retain their proliferative nuclear features.
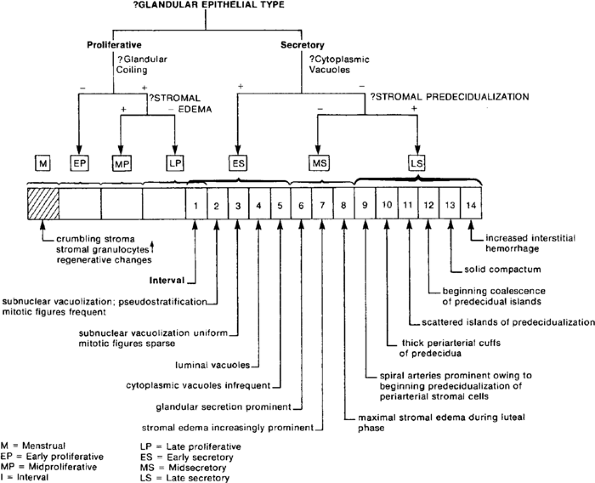 |
Figure 41.32 Decision tree for endometrial dating. Reprinted with permission from: Hendrickson M, Kempson R. Surgical Pathology of the Uterine Corpus. Philadelphia: WB Saunders; 1980. |
P.1037
Endometrial Secretory Phase (Ovarian Luteal Phase)
Ovulation is mediated by the luteinizing hormone (LH) surge, a synchronous burst of LH and follicle-stimulating hormone secretion that peaks on the 14th day of the ideal 28-day cycle. Ovulation occurs roughly 10 to 12 hours after this peak. The length of time from the last menstrual period to the day of ovulation for an individual woman is the length of her follicular phase. This surge of pituitary hormones initiates a complex series of events that results in the expulsion of the oocyte from the developed tertiary follicle and the transformation of the follicle into a corpus luteum. As a consequence of this transformation, the biosynthetic profile of the follicle changes, and both estradiol and large quantities of progesterone, are secreted. The biosynthetic lifetime of the corpus luteum defines the ovarian luteal phase that corresponds to endometrial secretory development. Both ovarian and endometrial luteal phases last 14 days on average, but this can be highly variable (see section Relevance of Endometrial Dating to Diagnostic Surgical Pathologists ).
The endometrium, which has proliferated and has been primed by estrogen, responds to the simultaneous stimulation of estrogen and progesterone by differentiating in a distinctive fashion. The morphologic changes can be divided into four periods: interval, early secretory, midsecretory, and late secretory (see Figure 41.32). The first 24 to 36 hours of the secretory phase are morphologically silent because the endometrium, for the most part, retains its late proliferative appearance, although scattered nonuniform subnuclear vacuoles may appear. This morphologically indeterminate endometrium is termed interval. The first unequivocal light microscopic indication that ovulation has occurred is the presence of uniform subnuclear vacuoles involving more than 50% of the endometrial glands. Over the next few days these vacuoles shift from a subnuclear to a supranuclear location. By the fifth postovulatory day, most of the secretion has been discharged into the gland lumen. The morphologic hallmark then of the early secretory phase (postovulatory days 2 to 5) is the vacuolated gland (Figure 41.35A, B).
The midsecretory phase lasts from postovulatory days (PODs) 5 to 9 and is characterized by nonvacuolated, prominently coiled secretory glands set within a spindled edematous stroma. Luminal secretion is most prominent during this period, and the overall appearance is one of
P.1038
glandular crowding (157). The secretory cells usually have round somewhat vesicular nuclei. This serves to separate them from the nuclei found in early secretory phase cells (Figure 41.36A, B, C). The distinctive feature of the late secretory endometrium (PODs 10 14) is stromal predecidualization. This diagnostic stromal change is heralded by an increased prominence of the spiral arteries. By the 10th postovulatory day, cuffs of predecidual cells are present around these arteries, initially involving the part of the vessel adjacent to the surface of the endometrium (Figure 41.37A, B). Subsequently, islands of predecidual cells appear in the superficial compactum. By POD 13 these islands become confluent. The extent of predecidualization is roughly paralleled by the degree of stromal infiltration by stromal granulocytes, although some investigators have suggested that the intensity of this infiltration is more closely correlated with the time of onset of menses (158). The appearance of the glands during the late secretory phase is not significantly different from their appearance during the midsecretory phase. They are lined by nonvacuolated secretory cells with round vesicular nuclei, and during the later days of this period, the glands typically have the saw-toothed appearance sometimes referred to as secretory exhaustion. As the late secretory phase progresses, increasing numbers of secretory cells exhibit apoptosis and apoptotic bodies accumulate within stromal macrophages.
Table 41.2 Decision Tree for Endometrial Dating | |
|---|---|
|
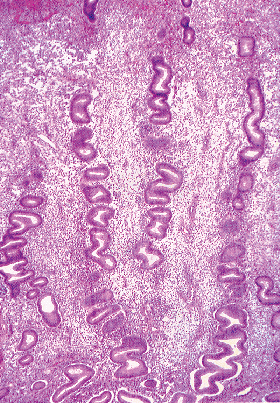 |
Figure 41.33 Midproliferative endometrium. Note the early coiling and synchronously developed glands. |
P.1039
Menstruation
Menstrual Phase (cycle days 1 4).
The abrupt withdrawal of both estrogen and progesterone accompanying the demise of the corpus luteum initiates menstrual bleeding. The molecular events initiating and guiding the controlled bleeding of menstruation are bewilderingly complex in contrast to the relatively straighforward light microscopic picture (159). The endometrium on the first day of menstrual bleeding (cycle day 1) is thin and compact. It is composed of the basalis and relative to the fully developed secretory endometrium a substantially shrunken, dense functionalis. The basalis maintains the relatively constant histologic appearance it has throughout the endometrial cycle. The shrinkage of the functionalis is largely due to deflation consequent on the withdrawal of interstitial fluid. The constituent glands and stroma of the functionalis fragment and crumble. Fibrin thrombi appears in vessels and within the
P.1040
P.1041
stroma. As the stroma disintegrates, the endometrial glands are randomly arranged and may artificially crowd together (Figure 41.38). The degenerative atypia of these glands, the necrotic background, and the close approximation of glands may suggest a diagnosis of malignancy. The general strategy of not making a diagnosis of malignancy when well-preserved glands and stroma are absent, should prevent such a mistake (Figure 41.39).
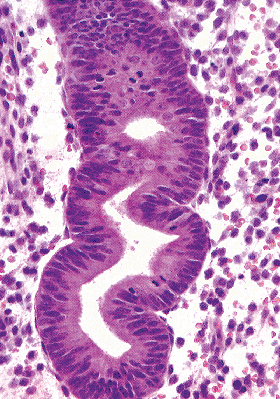 |
Figure 41.34 High-power view of a proliferative phase gland. The constituent cells are pseudostratified, and the characteristically elongate glandular nuclei have dense chromatin. |
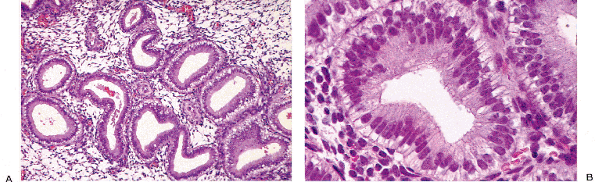 |
Figure 41.35 A. and B. Early secretory endometrium with subnuclear vacuoles. The nuclei retain the dense chromatin of the proliferative phase. Uniformly present cytoplasmic vacuoles are the most useful marker of the first third of the secretory phase. |
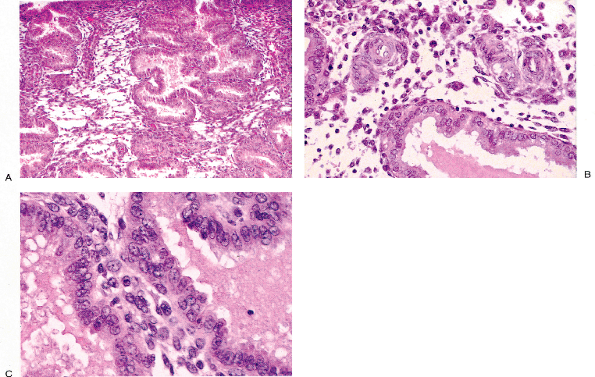 |
Figure 41.36 Midsecretory endometrium. The hallmarks of this period can be seen in these three photomicrographs. At low magnification (A), extreme glandular coiling and stromal edema are apparent. At a somewhat higher magnification (B), the coiled spiral arteries are seen within an edematous stroma. Perivascular predecidual reaction has not occurred. At yet higher magnification (C), the characteristically round vesicular nuclei of the midsecretory endometrium are apparent (contrast with proliferative phase nuclei in Figure 41.26). |
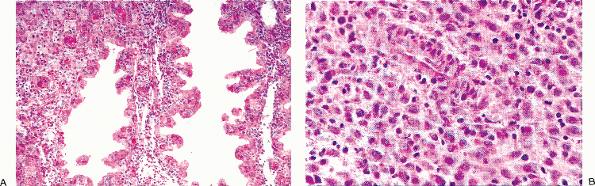 |
Figure 41.37 Late secretory endometrium. At low magnification (A), the serrated appearance of the gland reflects their coiled state. The stroma cells have undergone predecidual reaction (B). Predecidual reaction begins around the spiral arteries, and this reaction serves to distinguish midsecretory endometria from late secretory endometria. |
 |
Figure 41.38 Stromal breakdown. Disintegrating endometrium may simulate endometrial malignancy. A. Sheets of disintegrating stromal cells may simulate an endometrial stromal neoplasm. B. Higher magnification shows individual cell necrosis and inflammation, findings that should raise the possibility of disintegrating nonneoplastic endometrium. C. Confirmation of this possibility is made by finding inflamed epithelium associated with degenerating stromal cells. In this case, characteristic epithelium-covered spherules are produced. |
There is rapid onset of endometrial repair during this time. Numerous studies have demonstrated the outgrowth of epithelium from the stumps of the disrupted endometrial glands and ingrowth from the intact areas of the cornual and isthmic endometrium. This process begins during the 2nd or 3rd day and is completed by the 4th or 5th day. This early repair phase is thought by some to be estrogen independent as is the initial regrowth of the vasculature (160). In normal women, almost 50% of menstrual blood loss occurs on the first day (106,161,162).
Endometrial Morphology During the Luteal Phase of the Cycle of Conception
If implantation of a blastocyst occurs, it will be during the midsecretory phase (PODs 6 8), and this event is associated with a resurgence of glandular secretion and a persistence of stromal edema. After this time, biopsy findings will
P.1042
feature prominent glandular secretion (which in a nongravid cycle would have subsided), stromal edema, and stromal predecidualization (Figure 41.40) (163,164).
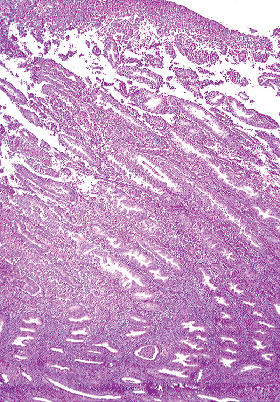 |
Figure 41.39 A low-power view of a menstrual endometrium showing the unresponsive basalis in the lower part of the photomicrograph and the functionalis disintegrating near the surface. |
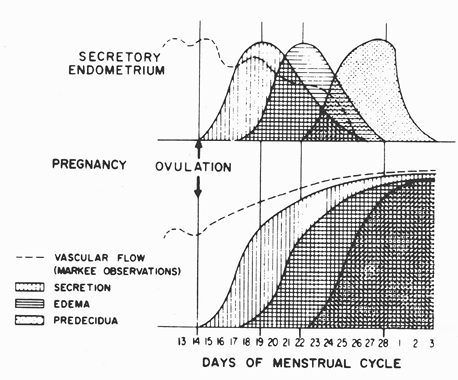 |
Figure 41.40 The transition from secretory to gestational endometrium is shown. The simultaneous occurrence of intense secretion, edema, and predecidual reaction and maintained vascular flow characterizes the early endometrial gestational responses. Reprinted with permission from: Arias-Stella J. Gestational endometrium. In: Hertig A, Norris H, Abell M, eds. The Uterus. Baltimore: Williams & Wilkins; 1973:185 212. |
The developing predecidua is gradually converted to decidua after POD 14 of the luteal phase of conception. This transformation is complete by the end of the first month of gestation. The fully developed decidual reaction is distinctive. Almost all of the endometrial stroma is converted into pavementlike sheets of epithelioid cells with prominent cytoplasmic margins and central vesicular nuclei (see Figure 41.28). In the superficial portion of the functionalis, the glands are compressed and their lining becomes flattened and endothelium-like. This compact sheetlike zone, the zona compactum, overlies saw-toothed scalloped glands, the zona spongiosum, which continue to exhibit secretory features. Many of the glands in the spongiosum are lined by cells with enlarged nuclei that often have atypical nuclear features approaching those of the Arias-Stella reaction (Figure 41.41). Scattered stromal granulocytes (uterine NK cells) are present. Decidual cells may exhibit substantial nuclear pleomorphism and cytologic atypia. This is particularly prominent in the region of the implantation site. In addition, this region is also infiltrated by intermediate trophoblastic cells, which normally have a bizarre cytologic appearance. These cells are positive for both keratin and hPL. The infiltration of decidua and the underlying myometrium by trophoblasts in the past has been termed syncytial metritis, not a particularly felicitous label because the process has little to do with inflammation (Figure 41.42). Accordingly, the current term for this process is implantation site reaction and is diagnostic of intrauterine pregnancy (165). Both placental site reaction and decidual atypia may incorrectly suggest malignancy, but confusion with adenocarcinoma is avoided by noting the secretory setting of these findings as well as the clinical history.
Although a fully developed decidual reaction is a constant feature of intrauterine pregnancy, it is by no means a diagnostic finding. An identical decidual reaction may be seen in extrauterine (ectopic) pregnancy; also, it can be produced by progestational agents or can be associated with persistence of the corpus luteum unassociated with pregnancy (e.g., corpus luteum cyst). For this reason, intrauterine implantation should only be diagnosed in the presence
P.1043
of chorionic villi or the presence of intermediate trophoblasts associated with enlarged vessels replaced by hyalin, or with fragments of fibrinoid matrix (165).
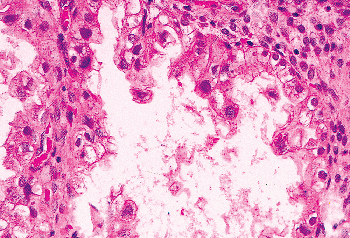 |
Figure 41.41 Gestational endometrium. This gland is lined by cells with enlarged dense nuclei characteristic of the Arias-Stella reaction. |
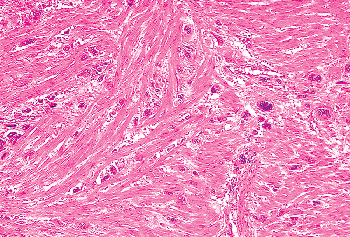 |
Figure 41.42 Myometrium beneath an implantation site containing infiltrating trophoblasts. This must not be misinterpreted as evidence of gestational trophoblastic disease. |
Distressingly, on rare occasions, the finding of trophoblastic tissue in a curetting does not exclude an ectopic gestation and suggests that when clinical suspicion is high, the presence of either chorionic villi or an implantation site should not preclude further workup of a possible ectopic pregnancy (166).
Previous intrauterine pregnancy is strongly suggested in the postpartum endometrium by the presence of cuffs of hyalinized decidua around sclerotic ectatic spiral arteries. The decidual cells composing this cuff often have hyperchromatic smudged and degenerate nuclei, and the vessels are often thrombosed. These findings have been referred to as subinvolution of the placental site and can be responsible for postpartum hemorrhage (167). This presumably occurs because these severely altered vessels are incapable of contraction. With time, the foci of hyalinization shrink and the nuclei may disappear, leaving a small pink scar resembling an ovarian corpus atreticum. These distinctive foci have been referred to as pregnancy plaques, and they may persist in the basalis for many years. Similar lesions featuring intermediate trophoblasts have been termed placental site nodule and placental site plaque (167). Occasionally, a placental site nodule (PSN) or exaggerated placental site can be confused for a gestational trophoblastic tumor such as placental site trophoblastic tumor (PSTT) or epithelioid trophoblastic tumor (ETT). While conventional H&E morphology is still very useful in distinguishing placental site nodules and plaques, immunohistochemical profiles can also be helpful. Studies over the last decade have shown that there are a variety of intermediate trophoblasts involved in implantation and establishing vascular connections to the maternal blood flow. These trophoblasts seem to have distinct immunohistochemical properties, some of which are shared with the cells of gestational trophoblastic tumors (GTTs). An exaggerated placental site is characterized by infiltration of individual cells with preservation of normal structures and mitoses essentially absent. Plancetal site trophoblastic tumors are characterized by mitotically active confluent masses of cells that disrupt normal architectures. All trophoblasts express pancytokeratins, so while this may be helpful in highlighting the trophoblasts it does little to predict the aggressive potential of the proliferation. Kurman et al. have established algorithms for evaluating placental site nodules and trophoblastic tumors, using a combination of human placental lactogen (hPL), p63 and Ki67. Briefly, the intermediate trophoblasts of an exaggerated placental site is hPL positive, p63 negative, and has a Ki67 activity of less than 1%. A PTT will have the same immunohistochemical profile except the Ki67 is much greater. The intermediate trophoblasts in a placental site nodule are hPL negative, p63 positive and have a Ki67 activity of less than 10%. Epitheloid trophoblastic tumor can sometimes be confused with a placental site nodule but has a Ki67 activity of greater than 10%. Occasionally, the diagnostic dilemma is between placental site nodule and squamous cell carcinoma; in this scenario, inhibin and cytokeratin 18 can be useful as PSNs are postitive for both of these antibodies while squamous cell carcinomas are negative (168,169,170).
Hyperprogestational states (particularly pregnancy) are sometimes associated with the distinctive glandular change to which Arias-Stella first drew attention in 1954 and that now bears his name (171,172). Most commonly this change is encountered in endometrial glands, but on occasion it may be present in foci of endometriosis or adenomyosis, in endocervical glands, in uterine (fallopian) tube epithelium, or in the glands within polyps. The Arias-Stella (AS) phenomenon characteristically involves a focus of tightly packed glands whose extreme coiling and collapse throw the lining epithelium into prominent papillary folds. This epithelium is composed of cells exhibiting marked nuclear pleomorphism and hyperchromatism. The nuclei typically have a smudged appearance. The cell cytoplasm may be strikingly hypervacuolated and cleared (clear cells) or densely eosinophilic (dark cells) (see Figure 41.41). One or the other cell type may predominate from area to area. Occasionally the eosinophilic cells may line the glands in a hobnail fashion. Mitotic figures only rarely are present. Elsewhere the endometrium usually exhibits the other changes one would anticipate with progestational stimulation, such as secretory glands and a stromal decidual reaction.
Less striking degrees of nuclear hyperchromasia, nuclear pleomorphism, and cytoplasmic vacuolization are, in our experience, constant features of the secretory glands of every pregnancy. The Arias-Stella reaction appears to represent the extreme end of the morphologic spectrum of the glandular epithelial changes seen in all pregnancies.
An endometrial Arias-Stella change may be present in a variety of clinical settings, including normal intrauterine
P.1044
pregnancy, extrauterine pregnancy, gestational trophoblastic disease, and persistent corpus luteum. In a retrospective review of patients coded as having Arias-Stella reaction, 16% had an extrauterine gestation. It also may be produced by the administration of ovulation-inducing drugs or progestational agents (171).
Because of the marked nuclear atypia of the epithelium lining the closely packed glands, the Arias-Stella phenomenon can be confused with adenocarcinoma, particularly with the architectural and cytologic features of clear cell carcinoma. This difficulty largely can be avoided by remembering that the Arias-Stella phenomenon occurs in a secretory setting; that is, more conventional secretory glands and decidualized or predecidualized stroma are usually found elsewhere in the specimen and the patient is premenopausal. Moreover, glandular mitotic figures tend not to be a prominent feature, although they may occasionally be encountered (173). Clear cell carcinoma (like any carcinoma) is fundamentally a proliferative process, and the constituent cells not only possess malignant features but also exhibit mitotic activity. Most importantly, clear cell carcinoma of the endometrium develops almost exclusively in postmenopausal women. Immunochemistry has been useful in separating the two; p53 and Ki-67 staining are absent in Arias-Stella (AS) and are present in clear cell carcinoma (174).
Occasionally in a gestational setting glandular nuclei may exhibit marked nuclear clearing reminiscent of herpetic infections (Figure 41.43) (175).
Endometrial Vasculature
The transition from the endometrium of early gestation to that of fully developed pregnancy is marked by the accelerated development of the endometrial vasculature, resulting in increased thickness of the spiral arteries, as reflected in their mean cross-sectional diameter (176,177).
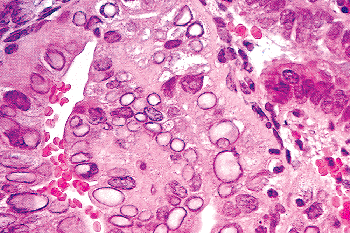 |
Figure 41.43 Nuclear clearing of the glandular cells in the endometrium may be seen in a gestational setting. This change should not be misconstrued as evidence of herpes virus infection. |
Perimenopausal and Postmenopausal Years
With the waning of hormonal function in the fifth decade of life, a woman enters the perimenopause, during which time uterine bleeding characteristically again becomes erratic, and the length of time between bleeding episodes lengthens. Thus, both the perimenopause and the perimenarche may be marked by erratic ovarian function and consequent dysfunctional uterine bleeding (178).
The end of ovarian follicular development and ovulation results in cessation of the menstrual periods and the menopause. Thereafter, the uterus enters a second inactive period, and the endometrial glandular epithelium, as in the premenarchal years, is typically atrophic. However, the glandular architecture and thickness of the endometrium may vary considerably. Several different patterns may be seen in the peri- and postmenopausal endometrium, which are described in the following sections.
Atrophic Endometrium
Atrophic epithelium is nonstratified and composed of a single layer of flattened to cuboidal cells. Mitotic figures are not present, the nucleus-to-cytoplasm ratio is high, and there is usually no specific cytoplasmic differentiation, although cilia may be present occasionally. The defining and only constant feature of atrophic endometria is the atrophic epithelial lining of its constituent glands, which may have any configuration, including cystic dilatation and glandular crowding. The stroma is spindled and is neither predecidualized nor decidualized. The nuclei may be densely pyknotic (as in postmenopausal endometria or endometrial polyps) or plump (as in endometria associated with oral contraceptives). Stromal disintegration may be present (Figure 41.44) (179,180,181).
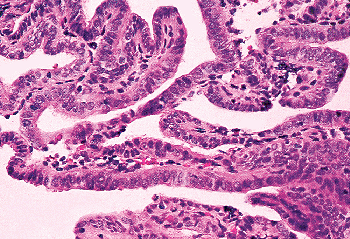 |
Figure 41.44 Endometrial atrophy as seen in an endometrial sampling. On occasion, atrophic surface epithelium may be removed in coiled masses and simulate hyperplasia because the glands are closely approximated. Reprinted with permission from: Hendrickson M, Kempson R. The uterine corpus. In: Sternberg S, ed. Diagnostic Surgical Pathology. New York: Raven; 1994:2091 2193. |
P.1045
Atrophic endometria can occur in a variety of clinical settings. It is the normal state during the premenarchal and later postmenopausal years. During the reproductive years, atrophic patterns may be seen in association with premature ovarian failure or, more commonly, in patients taking oral contraceptives.
Weakly Proliferative Endometrium
Weakly proliferative epithelium is nonstratified, although some degree of nuclear pseudostratification may be present, and its constituent cells are thin (pencil shape). This epithelium differs from the normal proliferative epithelium in the paucity of mitotic figures, the greater density of the nuclear chromatin, and, in the usual case, the overall lack of organization of the glands. The glands may be of any configuration, but the glands-to-stroma ratio is almost always near unity or there can be a slight stromal predominance. The stroma is spindled; stromal cell nuclei may be densely pyknotic or plump.
The morphologic variability of the weakly proliferative endometrium parallels that of the atrophic endometrium. The difference between them is solely based on the appearance of the epithelial cells: weakly proliferative rather than flattened or cuboidal. The clinical settings in which these two endometria occur overlap considerably. It is conceptually convenient to regard the weakly proliferative endometrium as lying on a continuum between normal and atrophic endometrium; the weakly proliferative patterns may then be conceived of as representing a transition between normal proliferation and atrophy. Weakly proliferative endometria are most often encountered in patients in the peri- or postmenopausal years; these endometria appear to be poorly supported by low levels of endogenous or exogenous estrogen. This pattern is normal in the hormonally hyporesponsive lower uterine segment of the normally cycling premenopausal women.
During the perimenopausal period some women are prone to chronic stromal breakdown due to the inconsistent hormonal milieu and anovulatory cycles. This can result in surface metaplasias such as eosinophilic or papillary metaplasia. The former is characterized by enlarged cells with abundant pink cytoplasm and conspicuous necleoli. The latter often form small papillary aggregates which layer the surface epithelium but can be detached and free-floating in an endometrial sampling. When atypia is present in these cells, this benign process can be mistaken for serous papillary carcinoma or its precursor, endometrial intraepithelial neoplasia. In this setting Ki67 and p53 can be useful adjuvant tests as eosinophillic metaplasia has a low Ki67 index and is usually only weakly for p53 while EIC is often strongly positive for both. While this panel will not give a definitive answer, it can prove helpful in confirming the histologic impression (182).
Disordered Proliferative Endometrium
We regard disordered proliferation as the morphologic bridge spanning normaly proliferating endometrium and endometrial hyperplasia. It is the endometrial pattern encountered in women experiencing sporadic anovulatory cycles and thus is most commonly encountered in the perimenopausal and perimenarchal years. It is also commonly seen in women receiving estrogen therapy. Disordered proliferation differs from normal proliferation by virtue of a loss of synchrony of glandular development so that some glands are tubular, whereas others are cystically dilated or have complex shapes. Budding may be present so that there may be a shift in the glands-to-stroma ratio in favor of the glands, but this shift is never more than 2:1. When glandular predominance is so marked that there is a shift in the glands-to-stroma ratio of 3:1 in favor of the glands, a diagnosis of hyperplasia is warranted (Figure 41.45).
We think that disordered proliferation (in the sense above) represents the response of a normal endometrium to sporadic, unopposed estrogen stimulation. There is no evidence that this pattern is a marker for an increased risk for the subsequent development of endometrial carcinoma. Because the term hyperplasia connotes to many clinicians such an increased risk, we part company with those pathologists who include this pattern in the hyperplasia group.
Histologically, the variously shaped glands are lined by normal proliferative cells with elongate dense nuclei that are most often pseudostratified. Mitotic figures are usually present and may be numerous. Stromal cells are spindled with plump nuclei.
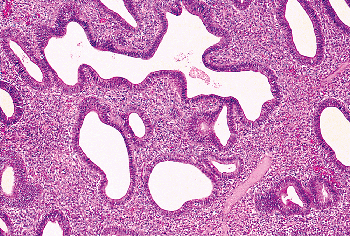 |
Figure 41.45 Disordered proliferation is the result of anovulatory cycles and is normal during the perimenopausal years. It is often found in the endometrium of women receiving estrogen therapy. Disordered proliferation is characterized by nonsynchronous growth of the glands including budding, but the glands-to-stroma ratio is unity or with a slight glandular predominance. This pattern of endometrial growth serves as a bridge between normal proliferation and hyperplasia. |
P.1046
Relevance of Endometrial Dating to Diagnostic Surgical Pathologists
The endometrial biopsy (EB) no longer plays the major role it used to play in the evaluation of the infertility patient for three reasons. First, historically, the EB's primary function was to serve as a bioassay for assessing a patient's endocrinological state; this role has been largely replaced by a variety of sophisticated and user-friendly direct methods of measuring circulating steroid and gonadotropin levels. Second, the EB's other function was to detect organic uterine disease. This role has been rendered irrelevant by the general availability of a large number of sophisticated imaging techniques. Finally, over recent years, many carefully controlled studies have established that, as a screening test for distinguishing fertile from infertile couples, the endometrial biopsy has poor test characteristics. That is, setting aside organic causes of infertility, the endometrial biopsy, functioning as a bioassay, is unreliable in separating fertile from infertile women. This is due largely to the substantial morphologic heterogeneity of the normally functioning endometrium (183,184,185,186,187,188).
The latest edition of a widely used infertility text asserts: Taken together, the body of available evidence supports the conclusion that traditional endometrial histologic dating is not a valid diagnostic tool. Consequently, endometrial dating cannot be used to guide the clinical management of women with reproductive failure and should no longer be regarded as an important element of their evaluation (189).
That said, the EB is still employed by some infertility specialists. In a recent survey of infertility physicians, 12% routinely ordered this test as part of the workup of the infertile couple, chiefly for its bioassay function (187,190).
Its deficiencies notwithstanding, the EB, when it is ordered for fertility evaluation, can provide much useful information. Its chief function is to determine whether ovulation has occurred and documenting anovulatory cycles points toward an ovarian factor in the patient's infertility. Again, organic reproductive tract disease may be detected on the endometrial biopsy; for example, endometrial poylps, leiomyomas, endometritis, hyperplasia, or carcinoma.
What modern studies do suggest is that the obsessive fine-tuning of the postovulatory morphologic date provides no useful clinical information. Historically, this fine-tuning of the postovulatory morphologic date was designed to detect luteal phase deficiency (LPD). Briefly, LPD can be thought of as a subtle form of ovulatory dysfunction manifested by a relatively short luteal phase. This results in low cumulative serum levels of progesterone in the luteal phase of the cycle and a consequent underdevelopment of secretory endometrium. LPD is a relatively uncommon syndrome, that (like congestive heart failure) has many etiologies. LPD is thought by many experts to be responsible for some cases of infertility and to be remediable by using a variety of techniques, chiefly ovulation induction (13,189). Historically, LPD was defined in terms of a greater than 3-day disparity between the woman's chronologic date of ovulation and her endometrial morphologic date when this disparity occured more than sporadically. The diagnosis of this condition is now largely based on an assessment of the integrated luteal phase progesterone and not on a histological assessment of the endometrium (188,191,192).
What follows is a stripped down version of endometrial dating for those who are asked to provide a fine-tuned morphologic date (i.e., more than proliferative, early-, mid- or late-secretory); most endometrial biopsies do not require this.
The precise details of the morphologic patterns corresponding to standard cycle dates are presented in Table 41.2 and Figures 41.31 and 41.32.
Some general points are important to keep in mind when using these aids:
1) Morphologic dates and chronological dates
In evaluating the endometrium, it is important to carefully distinguish between the morphologic postovulatory date assigned to a morphologically normal endometrium and the chronologic postovulatory date (POD) (106,147,193,194). The morphologic date is a summary characterization of the histologic development of the endometrium based on an assessment of glandular and stromal features. The morphologic findings may be summarized either in terms of postovulatory days, cycle days, or phases. For example, the morphologic pattern associated with a particular standard postovulatory day is assigned the number of that day; for example, POD 12 refers to the pattern seen on POD 12 of the standard cycle. Equivalently, this morphologic information can be conveyed using cycle day or phase (e.g., late secretory).
2) Endometrial dating should be reported as a range of 2 days
Research in the last two decades has shown the fallacy in using a standard 28 day cycle for all women. It is now known that both the follicular and leuteal phases vary among women (195). Adding to this biological heterogeneity is the poor interobserver reproducibility of the conventional Noyes' dating.
Noyes and Haman reported that two observers agreed on the same morphologic date 29% of the time, were within one day of each other in 63% of the cases, and were within 2 days of one another in 82% of cases (196). These results have been duplicated in more recent studies (154,197,198,199). As a consequence, the results of endometrial dating should be reported as a range of 2 days to reflect this variability.
3) Determining whether the patient has ovulated
Deciding whether a woman has ovulated by examining an endometrial biopsy (EMB) taken in what is presumed to be her late luteal phase is usually a trivial matter; the
P.1047
presence of a late secretory endometrium means ovulation. Deciding whether ovulation has occurred in the early luteal phase is more problematic. The characteristic light microscopic postovulatory secretory changes in the endometrium lag behind actual ovulation by at least 1 day. A biopsy on POD 2 usually reveals subnuclear vacuoles in the vast majority of glandular cells in the superficial functionalis but these may be spotty (interval pattern). Such vacuolization may be seen in late proliferative endometria as well as in other types of nonsecretory endometria and is, for this reason, not diagnostic of ovulation. Because of the ambiguous morphologic picture in the early postovulatory period, endometrial biopsies are usually obtained well into the presumed luteal phase of the cycle, preferably on PODs 11 to 13. We require uniform subnuclear vacuolization in 50% of the glands before diagnosing the earliest morphologic evidence of ovulation; this usually is present by POD 3.
4) Some practical details
Accurate endometrial dating requires attention to a number of details (193). Dating features should be sought only in fragments of endometrial functionalis, which are lined by surface epithelium; fragments of basalis and lower uterine segment should be ignored. The assigned date should be of the most developed area and should be based on features near the surface epithelium (the egg's eye view of Noyes) (193).
The preconditions for assigning a morphologic date to an endometrium are described as follows. Assigning a date to an EMB is obviously not possible in the absence of an adequate specimen, in a noncycling endometrium (e.g., nonsecretory pattern other than proliferative), in a patient being treated with medication that alters the morphology of the endometrium, or in an endometrium that is inflamed or that houses an intrauterine device. Thus, a precondition for applying the dating criteria is that one is dealing with a roughly normal endometrial pattern. Scanning power examination should establish the presence of pattern uniformity (apart from the expected variation due to sectioning randomly oriented fragments and normal out of phase curettage inhabitants such as fragments of the lower uterine segment, cervical epithelium, and stratum basalis), the absence of significant budding and branching of glands, and the absence of necrosis and inflammation. Examination at higher power should exclude the presence of significant epithelial nuclear atypia and the absence of significant numbers of stromal plasma cells.
A checklist for the contents of the pathology report of endometrial biopsies is provided in Table 41.3.
Endometrial Myometrial Junction
The endometrial myometrial junction is normally irregular, a phenomenon that must be kept in mind when assessing the presence or absence of myoinvasion by endometrial adenocarcinoma and in diagnosing superficial adenomyosis (see Figure 41.24).
Table 41.3 The Contents of the Pathology Report for an Infertility Endometrial Biopsy | |
|---|---|
|
Apoptosis and the Endometrium
The role of programmed cell death or apoptosis in the remodeling of the endometrium was mentioned earlier in this chapter. Apoptosis and its hormonal control in both human and animal endometrium is an area of active investigation by a number of groups (200,201,202,203).
Withdrawal of progesterone in the rabbit endometrium correlates with the development of apoptosis (204,205,206,207).
Bcl-2 is a protooncogene initially described in the (14,18) translocation in follicular lymphoma. It has been shown to prolong cell survival by preventing apoptosis. Gompel et al. studied Bcl-2 expression immunohistochemically in the normal endometrium (208). They found that Bcl-2 predominated in glandular cells and reached a maximum at the end of the follicular phase but disappeared at the onset of secretory activity. Maia et al. found a precipitous drop in Bcl-2 after ovulation, further supporting an increase in Bcl-2 in response to estrogen and a decrease in response to progesterone (209). In addition, this group found an elevated p53 expression in the proliferative phase but a drop in the late luteal phase. These results strongly suggest hormone-dependent regulation of Bcl-2 expression and cell cycling.
The role of tumor necrosis factor alpha (TNF- ) in the induction of apoptosis was explored by Tabibzadeh et al. (210). They found that the TNF receptor as well as Fas protein were expressed in endometrial epithelium throughout the entire menstrual cycle and were most prominent in the
P.1048
basalis. They concluded that endometrial epithelium, by expressing receptors for TNF- and Fas protein, can respond to ligands that regulate apoptosis.
Normal findings in the endometrium that have relevance to histopathologic differential diagnosis are shown in Table 41.4.
Myometrium
The bulk of the myometrium comprises smooth muscle cells, but an important contribution is made by extracellular components such as collagen and elastin. The smooth muscle within the corpus is more concentrated relative to collagen and elastin than the muscle in either the cervix or the lower uterine segment. This distribution of muscle is consistent with the passive role of the cervix during parturition; the uterine contents propelled by fundal contractions are thought to passively dilate a cervix previously softened by the action of collagenase. The uterine smooth muscle cells are spindled, with blunt-ended fusiform nuclei. Their cytoplasmic volume depends on whether the patient is pregnant (211,212,213,214). Scattered normal mitotic figures may be encountered, particularly during the secretory phase of the endometrial cycle (215). Characteristic ultrastructural features of smooth muscle include (a) numerous dense, 60- to 80-A myofilaments without cross striations, which almost fill the cytoplasm; (b) small round dense bodies along the trajectory of the filaments; (c) dense plaques arranged along the inner aspect of the plasma membrane; and (d) plasma membrane related vesicles that may play a role in ionized calcium movement across the plasma membrane during contraction. In addition, there is the usual complement of cytoplasmic organelles, including smooth and rough endoplasmic reticulum, mitochondria, and a Golgi apparatus. Typically these organelles arrange themselves around the nucleus, which often has an irregular shape. These ultrastructural features reflect the dual function of the uterine myocyte: muscular contraction and collagen and elastin synthesis. The ultrastructural appearance varies with the levels of circulating steroidal hormones. In particular, estrogen appears to sharply increase myocyte protein synthesis. This correlates morphologically in an increased volume of rough endoplasmic reticulum and increased numbers of cytoplasmic contractile elements. The biochemistry and electrophysiology of the myometrium have been extensively reviewed (214,216,217). The histologic and ultrastructural appearance of smooth muscle cells differ substantially from those of the endometrial stromal cell. These differences are set out in Table 41.5. However, it should be noted that cells with a hybrid smooth muscle stromal phenotype occur normally at the endometrial myometrial junction and that this phenotypic ambiguity is sometimes expressed by spindle cell neoplasms of the uterine corpus (218). Some uterine smooth muscle cells have been shown to express some classes of keratins (219,220,221,222). The immunohistochemical profile of myometrial cells is presented in Table 41.5.
Pregnancy-Related Changes
To accommodate the growing fetus and to prepare for its role in fetal expulsion, the uterus undergoes a 10-fold increase in size and weight during pregnancy both by hypertrophy and, to a much lesser extent, by hyperplasia. Normal mitotic figures are often increased and may be present in large numbers. Uterine growth during pregnancy appears to be largely promoted by estradiol, whereas progesterone probably functions to inhibit uterine contractions during gestation. The light microscopic appearance of the hypertrophied uterine smooth muscle cells of pregnancy is distinctive. They are enlarged and have abundant, rather glassy cytoplasm and vesicular elongate nuclei with occasionally prominent nucleoli. Changes occur in the ultrastructural appearance of the smooth muscle cells as well. In addition to an increase in size and the number of myofilaments, there is a striking increase in the number of gap junctions (223,224). These establish the contact between cells required for the coordinated uterine contractions that expel the term infant (225). These myometrial changes are closely coordinated with the dramatic structural changes of cervical ripening required for cervical effacement (see section Uterine Cervix ). In the postpartum period, the uterus undergoes an extraordinary 85% reduction in weight within 3 weeks of delivery (25). This weight loss is primarily due to a reduction in individual cell volume rather than a reduction in cell number. In addition, a large amount of collagen is degraded over this brief period. Complete return of the uterus to the nulliparous weight does not occur if gestation has proceeded beyond the second trimester.
The Fallopian Tube
Histology of the Fallopian Tube
The fallopian tube is lined by a nonstratified epithelium that is separated from the endosalpingeal stroma by a basement membrane. Each of the tubal segments is lined by a mixture of three basic cell types: ciliated cells, secretory cells, and intercalated (peg) cells (Figures 41.46A, B and 41.47). In recent years it has become apparent that the peg cell is, in reality, a stage in the cyclic variation during the menstrual cycle of the secretory cell (226). The relative number of these cells differs in each of the anatomic
P.1049
P.1050
P.1051
regions of the tube. In addition, many investigators believe that the numbers of the three types of cells within each of these anatomic regions undergo regular variations throughout the menstrual cycle (227,228,229,230,231,232). Ciliated cells are most prominent at the ovarian (distal) end of the tube particularly in the fimbrial mucosa and predominate during midcycle; their numbers diminish progressively to achieve a nadir at the time of menstruation (Figure 41.47). In a gestational cycle the number of cilia continue to decrease. Ciliary movement rather than muscular contractions is chiefly responsible for the movement of the egg toward the site of fertilization: the ampulloisthmic junction.
Table 41.4 Normal Findings in the Endometrium that have Relevance to Histopathologic Differential Diagnosis (see text for Additional Differential Diagnostic Clues) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Secretory cells are most prominent toward the uterine end of the tube and undergo cyclic changes in cell height and appearance, reflecting their elaboration, accumulation, and discharge of oviduct secretions as the menstrual cycle proceeds. Most often they have ovoid, somewhat dense nuclei, and they may contain an apical vacuole (Figure 41.48A, B). The oviduct fluid secreted by these cells serves many important functions and has been the subject of a review (233).
Intercalated cells have been thought to represent either effete secretory cells or some type of reserve cells. They have a thin dense nucleus and little cytoplasm. Endocrine cells have been noted in the fallopian tube; their function is as mysterious here as it is in the uterus (234). The basal cells reported in the early literature have been shown to be lymphocytes, which may represent a tubal component of a mucosa-associated lymphoid system (235,236,237,238,239). Scattered lymphocytes and occasional lymphoid follicles should be considered to be within normal limits and constitute part of the mucosa-associated lymphoid system (240).
Ciliogenesis is promoted by estradiol and deciliation by progesterone. Prolonged exposure to progestogens (whether endogenously as in pregnancy or exogenously) or withdrawal of estrogen (as in the postmenopausal years) leads to epithelial atrophy. Postmenopausal estrogen administration leads to regrowth of cilia.
Mitotic figures are rarely seen in the fallopian tube epithelium, so no cyclic regeneration occurs as in the endometrium. Both the transmission and scanning electron microscopic appearance of the normal tubal mucosa have been extensively documented over the past two decades.
Of interest to the diagnostic pathologist are the abnormalities of ciliogenesis found in patients with Kartagener's syndrome (241,242).
P.1052
Table 41.5 Comparison of Differentiated Features for Endometrial Stromal Cells and Smooth Muscle Cells | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
P.1053
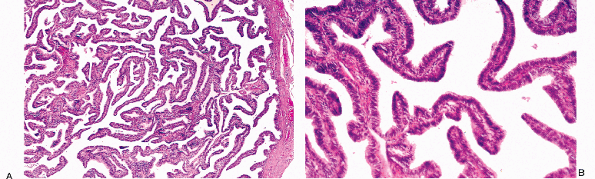 |
Figure 41.46 A. and B. Ampulla of the fallopian tube. Note the long slender plicae or folds resting on the muscularis. |
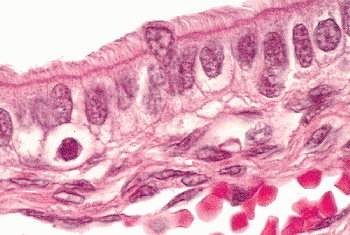 |
Figure 41.47 This high-power photomicrograph of tubal epithelium shows numerous ciliated cells with a compressed secretory cell nucleus above the level of the ciliated cells. The cell with clear cytoplasm is probably a lymphocyte. |
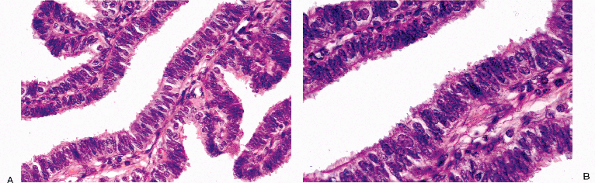 |
Figure 41.48 A. and B. In this area the tubal epithelial cells are crowded, a pattern that is common in the fallopian tube when ciliated cells are not numerous. |
P.1054
With the advent of genetic testing for BRCA-1 and BRCA-2 gene mutations, prophylactic salpingo-oophorectomies are being performed. Although there are no inherent morphological changes in the epithelium of a fallopian tube from a woman who harbors a BRCA-1 or BRCA-2 gene mutation, she is at an increased risk of having an in situ or invasive carcinoma. Therefore, in this setting the entire fallopian tube should be submitted for histological evaluation and scrutinized for occult malignancy (243,244).
Myosalpinx
The myosalpinx is composed of an inner circular layer and an outer longitudinal layer. The isthmus near the uterotubal junction also possesses an inner longitudinal layer. The presence of the muscular layer can be particularly helpful to the pathologist when trying to distinguish a tubo-ovarian complex from an ovarian serous neoplasm.
Physiology
The details of the physiology of the tube are beyond the scope of this chapter; interested readers are referred to published reviews (229,245). Unresolved mysteries include how the sperm moves so rapidly from the vagina to the ampulla (in some cases within 5 minutes) and how the tube manages to orchestrate fertilization by ensuring that spermatozoa moving toward the ovary and an egg moving away from the ovary meet in the appropriate part of the fallopian tube.
Fallopian Tube in Pregnancy
The fallopian tube has already played its part when the fertilized ovum implants in the endometrium. It is now inactive throughout the gestational period. A muted version of endometrial decidual change often occurs in the endosalpingeal stroma during the latter part of pregnancy, while the epithelium of the fallopian tube undergoes atrophy (246) (Figure 41.49). The fallopian tube is the most common site of ectopic gestation. A review of physiologic factors in its development has been presented (247). Morphologic changes in the fallopian tube can be produced by birth control pills and of course tubal ligation (248,249,250).
Normal findings in the fallopian tubes that have relevance to histopathologic diagnosis can be found in Table 41.6.
Paraovarian and Paratubal Structures
The broad ligament and environs are populated by a variety of tubular and cystic structures with the propensity to form clinically or surgically noticeable cysts (38,251). These are indicated in Figure 41.50. Many are lined by m llerian-type epithelium. Walthard rests are universal findings over the serosal surface of the fallopian tubes. They are lined by transitional-type epithelium. A more or less constant finding in sections that include the peritubal soft tissue are the tortuous remnants of the mesonephric ducts. These are lined by cuboidal epithelium and possess a fibromyovascular cuff.
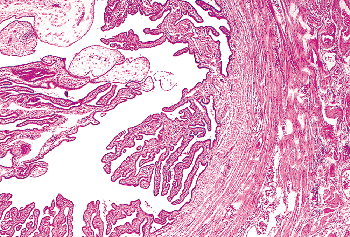 |
Figure 41.49 Fallopian tube containing decidual cells (upper left). The stromal cells of the plicae frequently undergo decidual change during pregnancy. |
Supplemental Readings
A chronicle of the changing concepts of the uterus through the ages is provided by Ramsey (10), and a fascinating discussion of the role of preconceptions in filtering the facts of uterine anatomy is provided by Laqueur (252). There has been interesting speculation on the evolutionary purposes of menstruation (253,254).
Several textbooks of gynecological pathology provide coverage of normal uterine and fallopian tube anatomy and the differential diagnostic issues raised by departures from typical appearances.
Fox H, Wells M, eds. Haines and Taylor Obstetrical and Gynaecological Pathology. 5th ed. Edinburgh, Scotland: Churchill Livingstone; 2003.
Robboy S, Anderson M, Russell P. Pathology of the Female Reproductive Tract. New York: Churchill Livingstone; 2001.
Kurman RJ, ed. Blaustein's Pathology of the Female Genital Tract. 5th ed. New York: Springer-Verlag; 2002.
Several extensive treatises on reproductive endocrinology have been published or revised in recent years:
Strauss JF III, Barbieri RL. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 5th ed. Philadelphia: Elsevier Saunders; 2004.
Speroff L, Fritz MA, eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
P.1055
Table 41.6 Normal Findings in the Uterine (Fallopian) Tube and Uterine Serosa that have Relevance to Histopathologic Differential Diagnosis (see text for Additional Differential Diagnostic Clues) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
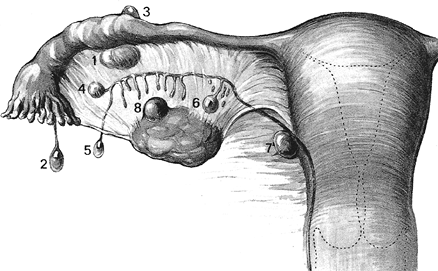 |
Figure 41.50 Topography of various cysts encountered in the female internal genitalia. 1, Paraovarian cyst of paramesonephric origin (type I); 2, hydatid cyst of Morgagni (paramesonephric origin); 3, subserosal m llerian cyst (paramesonephric origin); 4, paraovarian cyst of mesonephric origin (type II); 5, Kobelt's cyst (appendix vesiculosa); 6, cyst of the paroophoron; 7, duct cyst; 8, cyst of the rete ovarii. Reprinted with permission from: Janovski N, Paramanandhan T. Ovarian Tumors, Tumors and Tumor-like Conditions of the Ovaries, Fallopian Tubes and Ligaments of the Uterus. Philadelphia: WB Saunders; 1973:191 194. |
P.1056
Adashi EY, Rock JA, Rosenwaks Z, eds. Reproductive Endocrinology, Surgery, and Technology. Philadelphia: Lippincott-Raven; 1996.
Ferin M, Jewelewicz R, Warren M. The Menstrual Cycle: Physiology, Reproductive Disorders, and Infertility. New York: Oxford University Press; 1993.
Two monographs devoted exclusively to the biology of the uterus are listed as follows:
Chard T, Grudzinskas JG, eds. The Uterus. New York: Cambridge University Press; 1994.
Wynn RM, Jollie WP, eds. Biology of the Uterus. 2nd ed. New York: Plenum Medical Book Company; 1989.
More general treatments of the territory surveyed in this chapter with more emphasis on basic science issues and less emphasis on practical surgical pathology diagnostics are listed as follows.
Uterine Cervix
Wright TC, Ferenczy A. Anatomy and histology of the cervix. In: Kurman RJ, ed. Blaustein's Pathology of the Female Genital Tract. 5th ed. New York: Springer-Verlag; 2002:207-224.
Singer A, Chow C. Anatomy of the cervix and physiological changes in cervical epithelium. In: Fox H, Wells M, eds. Haines and Taylor Obstetrical and Gynaecological Pathology. 5th ed. Vol 1. New York: Churchill Livingstone; 2003:247 272.
Uterine Endometrium and Myometrium
Buckley CH. Normal endometrium and non-proliferative conditions of the endometrium. In: Fox H, Wells M, eds. Haines and Taylor Obstetrical and Gynaecological Pathology. 5th ed. New York: Churchill Livingstone; 2003:391 441.
Giudice LC, Ferenczy A. The endometrial cycle: morphological and biochemical events. In: Adashi EY, Rock JA, Rosenwaks Z, eds. Reproductive Endocrinology, Surgery, and Technology. Vol 1. Philadelphia: Lippincott-Raven; 1996:271 300.
Mutter GL, Ferenczy A. Anatomy and histology of the uterine corpus. In: Kurman RJ, ed. Blaustein's Pathology of the Female Genital Tract. 5th ed. New York: Springer-Verlag; 2002:383 420.
Warren M, Li T, Klentzeris D. Cell biology of the endometrium: histology, cell types and menstrual changes. In: Chard T, Grudzinskas J, eds. The Uterus. New York: Cambridge University Press; 1994:94 125.
Normal Gestational Findings
Shih I-M, Mazur MT, Kurman RJ. Gestational trophoblastic disease and related lesions. In: Kurman RJ, ed. Blaustein's Pathology of the Female Genital Tract. New York: Springer-Verlag; 2002:1193 1250.
Fallopian Tube
Brenner RM, Slayden OD. The fallopian tube cycle. In: Adashi EY, Rock JA, Rosenwaks Z, eds. Reproductive Endocrinology, Surgery, and Technology. Vol 1. Philadelphia: Lippincott-Raven; 1996:326 339.
Honor L. Pathology of the fallopian tube and broad ligament. In: Fox H, Wells M, eds. Haines and Taylor Obstetrical and Gynaecological Pathology. 5th ed. New York: Churchill Livingstone; 2003:585 634.
Wheeler JE. Diseases of the fallopian tube. In: Kurman RJ, ed. Blaustein's Pathology of the Female Genital Tract. 5th ed. New York: Springer-Verlag; 2002:617 648.
References
1. Lauchlan S. Metaplasias and neoplasias of M llerian epithelium. Histopathology 1984;8:543 557.
2. Lauchlan SC. The secondary mullerian system revisited. Int J Gynecol Pathol 1994;13:73 79.
3. Moore K, Persaud TVN. The Developing Human: Clinically Oriented Embryology. 7th ed. Philadelphia: WB Saunders; 2003:207 221.
4. Acien P. Embryological observations on the female genital tract. Hum Reprod 1992;7:437 445.
5. O'Rahilly R. Prenatal human development. In: Wynn RM, Jolli WP, eds. Biology of the Uterus. 2nd ed. New York: Plenum Medical Book Company; 1989:35 56.
6. Jost A, Vigier B, Prepin J, Perchellet JP. Studies on sex differentiation in mammals. Recent Prog Horm Res 1973;29:1 41.
7. Gondos B. Development of the reproductive organs. Ann Clin Lab Sci 1985;15:363 373.
8. Szamborski J, Laskowska H. Some observations on the developmental histology of the human foetal uterus. Biol Neonat 1968;13:298 314.
9. Gray CA, Bartol FF, Tarleton BJ, et al. Developmental biology of uterine glands. Biol Reprod 2001;65:1311 1323.
10. Ramsey E. Development of the human uterus and relevance to the adult condition. In: Chard T, Grudzinskas JG, eds. The Uterus. New York: Cambridge University Press; 1994:41 53.
11. McLean JM. Embryology and anatomy of the female genital tract. In: Fox H, Wells M, eds. Haines and Taylor Obstetrical and Gynaecological Pathology. Edinburgh, Scotland: Churchill Livingstone; 2003:1 40.
12. Ramsey E. Embryology and developmental defects of the female reproductive tract. In: Danforth DN, Scott JR, eds. Obstetrics and Gynecology. 5th ed. Philadelphia: JB Lippincott; 1986:106 119.
13. Strauss JF III, Lessey BA. The structure, function, and evaluation of the female reproductive tract. In: Strauss JF III, Barbieri RL, eds. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 5th ed. Philadelphia: Elsevier Saunders; 2004:255 306.
14. Patton G, Kistner R. Atlas of Infertility Surgery. Boston: Little, Brown and Company; 1984.
15. Tulac S, Nayak NR, Kao LC, et al. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab 2003;88:3860 3866.
16. Haber HP, Mayer EI. Ultrasound evaluation of uterine and ovarian size from birth to puberty. Pediatr Radiol 1994;24:11 13.
17. Nussbaum A, Sanders R, Jones M. Neonatal uterine morphology as seen on real-time US. Radiology 1986;160:641 643.
18. Singer A, Chow C. Anatomy of the cervix and physiological changes in cervical epithelium. In: Fox H, Wells M, eds. Haines and Taylor Obstetrical and Gynaecological Pathology. Edinburgh, Scotland: Churchill Livingstone; 2003:247 272.
19. Eddy CA, Pauerstein CJ. Anatomy and physiology of the fallopian tube. Clin Obstet Gynecol 1980;23:1177 1193.
P.1057
20. Williams PL, Warwick R, Dyson M, Bannister LH. Gray's Anatomy of the Human Body. 37th ed. New York: Churchill Livingstone; 1989.
21. Langlois PL. The size of the normal uterus. J Reprod Med 1970;4:220 228.
22. Calder AA. The cervix during pregnancy. In: Chard T, Grudzinskas JG, eds. The Uterus. New York: Cambridge University Press; 1994:288 307.
23. Kurz K, Tadesse E, Haspels A. In vivo measurements of uterine cavities in 795 women of fertile age. Contraception 1984;29:495 510.
24. Zemlyn S. The length of the uterine cervix and its significance. J Clin Ultrasound 1981;9:267 269.
25. Finn C, Porter D. The Uterus. Acton, MA: Publishing Sciences Group; 1975.
26. Togashi K, Nakai A, Sugimura K. Anatomy and physiology of the female pelvis: MR imaging revisited. J Magn Reson Imaging 2001;13:842 849.
27. Hoad CL, Raine-Fenning NJ, Fulford J, Campbell BK, Johnson IR, Gowland PA. Uterine tissue development in healthy women during the normal menstrual cycle and investigations with magnetic resonance imaging. Am J Obstet Gynecol 2005;192:648 654.
28. Toth A. Studies on the muscular structure of the human uterus. II. Fasciculi cervicoangulares: vestigial or functional remnant of the mesonephric duct? Obstet Gynecol 1977;49:190 196.
29. Toth S, Toth A. Undescribed muscle bundle of the human uterus: fasciculus cervicoangularis. Am J Obstet Gynecol 1974;118:979 984.
30. Huszar G, Naftolin F. The myometrium and uterine cervix in normal and preterm labor. N Engl J Med 1984;311:571 581.
31. Merchant RN, Prabhu SR, Chougale A. Uterotubal junction morphology and clinical aspects. Int J Fertil 1983;28:199 205.
32. Vizza E, Correr S, Muglia U, Marchiolli F, Motta PM. The three-dimensional organization of the smooth musculature in the ampulla of the human fallopian tube: a new morpho-functional model. Hum Reprod 1995;10:2400 2405.
33. Croxatto HB. Physiology of gamete and embryo transport through the fallopian tube. Reprod Biomed Online 2002;4:160 169.
34. Talbot P, Geiske C, Knoll M. Oocyte pickup by the mammalian oviduct. Mol Biol Cell 1999;10:5 8.
35. Gordts S, Campo R, Rombauts L, Brosens I. Endoscopic visualization of the process of fimbrial ovum retrieval in the human. Hum Reprod 1998;13:1425 1428.
36. Greiss FC Jr, Rose JC. Vascular physiology of the nonpregnant uterus. In: Wynn R, Jollie W, eds. Biology of the Uterus. 2nd ed. New York: Plenum Medical Book Company; 1989:69 88.
37. Ramsey E. Vascular anatomy. In: Wynn R, Jollie W, eds. Biology of the Uterus. 2nd ed. New York: Plenum Medical Book Company; 1989:57 68.
38. Wydrzynski M. Anatomical principles of microsurgery of the tubal arteries. Anat Clin 1985;7:233 236.
39. DiSaia PJ, Creasman WT. Clinical Gynecologic Oncology. 5th ed. St. Louis: Mosby; 1997:viii, 657.
40. Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer 1993;71(4 suppl):1702 1709.
41. Plentl A, Friedman E. Lymphatic System of the Female Genitalia: The Morphologic Basis of Oncologic Diagnosis and Therapy. Philadelphia: WB Saunders; 1971.
42. Klein M, Rosen A, Lahousen M, et al. Lymphogenous metastasis in the primary carcinoma of the fallopian tube. Gynecol Oncol 1994;55(pt 1):336 338.
43. Klein M, Rosen AC, Lahousen M, Graf AH, Rainer A. Lymphadenectomy in primary carcinoma of the fallopian tube. Cancer Lett 1999;147:63 66.
44. Wright TC, Ferenczy A. Anatomy and histology of the cervix. In: Kurman RJ, ed. Blaustein's Pathology of the Female Genital Tract. 5th ed. New York: Springer-Verlag, 2002:207 224.
45. Aspden RM. The importance of a slit-like lumen cross-section for the mechanical function of the cervix. Br J Obstet Gynaecol 1987;94:915 916.
46. Gorodeski G. The cervical cycle. In: Adashi E, Rock J, Rosenwaks Z, eds. Reproductive Endocrinology, Surgery, and Technology. Philadelphia: Lippincott-Raven; 1996:301 324.
47. Gipson IK. Mucins of the human endocervix. Front Biosci 2001;6:D1245 D1255.
48. Konishi I, Fujii S, Nonogaki H, Nanbu Y, Iwai T, Mori T. Immunohistochemical analysis of estrogen receptors, progesterone receptors, Ki-67 antigen, and human papillomavirus DNA in normal and neoplastic epithelium of the uterine cervix. Cancer 1991;68:1340 1350.
49. Albores-Saavedra J, Gersell D, Gilks CB, et al. Terminology of endocrine tumors of the uterine cervix: results of a workshop sponsored by the College of American Pathologists and the National Cancer Institute. Arch Pathol Lab Med 1997;121:34 39.
50. Fetissof F, Arbeille B, Boivin F, Sam-Giao M, Henrion C, Lansac J. Endocrine cells in ectocervical epithelium. An immunohistochemical and ultrastructural analysis. Virchows Arch A Pathol Anat Histopathol 1987;411:293 298.
51. Fetissof F, Berger G, Dubois MP, et al. Endocrine cells in the female genital tract. Histopathology 1985;9:133 145.
52. Fetissof F, Dubois MP, Heitz PU, Lansac J, Arbeille-Brassart B, Jobard P. Endocrine cells in the female genital tract. Int J Gynecol Pathol 1986;5:75 87.
53. Fetissof F, Heitzman A, Machet MC, Lansac J. Unusual endocervical lesions with endocrine cells. Pathol Res Pract 1993;189:928 939.
54. Fetissof F, Serres G, Arbeille B, de Muret A, Sam-Giao M, Lansac J. Argyrophilic cells and ectocervical epithelium. Int J Gynecol Pathol 1991;10:177 190.
55. Scully R, Aguirre P, DeLellis R. Argyrophilia, serotonin, and peptide hormones in the female genital tract and its tumors. Int J Gynecol Pathol 1984;3:51 70.
56. Chan JK, Tsui WM, Tung SY, Ching RC. Endocrine cell hyperplasia of the uterine cervix. A precursor of neuroendocrine carcinoma of the cervix? Am J Clin Pathol 1989;92:825 830.
57. Hussain LA, Kelly CG, Fellowes R, et al. Expression and gene transcript of Fc receptors for IgG, HLA class II antigens and Langerhans cells in human cervico-vaginal epithelium. Clin Exp Immunol 1992;90:530 538.
58. Miller CJ, McChesney M, Moore PF. Langerhans cells, macrophages and lymphocyte subsets in the cervix and vagina of rhesus macaques. Lab Invest 1992;67:628 634.
59. Morelli AE, di Paola G, Fainboim L. Density and distribution of Langerhans cells in the human uterine cervix. Arch Gynecol Obstet 1992;252:65 71.
60. Osamura RY, Watanabe K, Oh M. Melanin-containing cells in the uterine cervix: histochemical and electron-microscopic studies of two cases. Am J Clin Pathol 1980;74:239 242.
61. Hiersche HD, Nagl W. Regeneration of secretory epithelium in the human endocervix. Arch Gynecol 1980;229:83 90.
62. Gould PR, Barter RA, Papadimitriou JM. An ultrastructural, cytochemical, and autoradiographic study of the mucous membrane of the human cervical canal with reference to subcolumnar basal cells. Am J Pathol 1979;95:1 16.
63. Ismail SM. Cone biopsy causes cervical endometriosis and tubo-endometrioid metaplasia. Histopathology 1991;18:107 114.
64. Jonasson JG, Wang HH, Antonioli DA, Ducatman BS. Tubal metaplasia of the uterine cervix: a prevalence study in patients with gynecologic pathologic findings. Int J Gynecol Pathol 1992;11:89 95.
65. Novotny DB, Maygarden SJ, Johnson DE, Frable WJ. Tubal metaplasia. A frequent potential pitfall in the cytologic diagnosis of endocervical glandular dysplasia on cervical smears. Acta Cytol 1992;36:1 10.
66. Pacey F, Ayer B, Greenberg M. The cytologic diagnosis of adenocarcinoma in situ of the cervix uteri and related lesions. III. Pitfalls in diagnosis. Acta Cytol 1988;32:325 330.
67. Suh KS, Silverberg SG. Tubal metaplasia of the uterine cervix. Int J Gynecol Pathol 1990;9:122 128.
68. Young RH, Clement PB. Pseudoneoplastic glandular lesions of the uterine cervix. Semin Diagn Pathol 1991;8:234 249.
69. Oliva E, Clement PB, Young RH. Tubal and tubo-endometrioid metaplasia of the uterine cervix. Unemphasized features that may cause problems in differential diagnosis: a report of 25 cases. Am J Clin Pathol 1995;103:618 623.
P.1058
70. Marques T, Andrade LA, Vassallo J. Endocervical tubal metaplasia and adenocarcinoma in situ: role of immunohistochemistry for carcinoembryonic antigen and vimentin in differential diagnosis. Histopathology 1996;28:549 550.
71. Peters WM. Nature of basal and reserve cells in oviductal and cervical epithelium in man. J Clin Pathol 1986;39:306 312.
72. Fluhmann C. The Cervix Uteri and Its Diseases. Philadelphia: WB Saunders; 1961.
73. Fluhmann CF. The nature and development of the so-called glands of the cervix uteri. Am J Obstet Gynecol 1957;74:753 768.
74. Clement PB, Young RH. Deep nabothian cysts of the uterine cervix. A possible source of confusion with minimal-deviation adenocarcinoma (adenoma malignum). Int J Gynecol Pathol 1989;8:340 348.
75. Teshima S, Shimosato Y, Kishi K, Kasamatsu T, Ohmi K, Uei Y. Early stage adenocarcinoma of the uterine cervix. Histopathologic analysis with consideration of histogenesis. Cancer 1985;56:167 172.
76. Bertrand M, Lickrish GM, Colgan TJ. The anatomic distribution of cervical adenocarcinoma in situ: implications for treatment. Am J Obstet Gynecol 1987;157:21 25.
77. Gilks CB, Reid PE, Clement PB, Owen DA. Histochemical changes in cervical mucus-secreting epithelium during the normal menstrual cycle. Fertil Steril 1989;51:286 291.
78. Nucci MR. Symposium part III: tumor-like glandular lesions of the uterine cervix. Int J Gynecol Pathol 2002;21:347 359.
79. Lagow E, DeSouza MM, Carson DD. Mammalian reproductive tract mucins. Hum Reprod Update 1999;5:280 292.
80. McDonnell JM, Emens JM, Jordan JA. The congenital cervicovaginal transformation zone in sexually active young women. Br J Obstet Gynaecol 1984;91:580 584.
81. Burch DJ, Spowart KJ, Jesinger DK, Randall S, Smith SK. A dose-ranging study of the use of cyclical dydrogesterone with continuous 17 beta oestradiol. Br J Obstet Gynaecol 1995;102:243 248.
82. Forsberg JG. Cervicovaginal epithelium: its origin and development. Am J Obstet Gynecol 1973;115:1025 1043.
83. Crum CP, Egawa K, Fu YS, et al. Atypical immature metaplasia (AIM). A subset of human papilloma virus infection of the cervix. Cancer 1983;51:2214 2219.
84. Duggan MA. Cytologic and histologic diagnosis and significance of controversial squamous lesions of the uterine cervix. Mod Pathol 2000;13:252 260.
85. Aspden RM. Collagen organisation in the cervix and its relation to mechanical function. Coll Relat Res 1988;8:103 112.
86. Kiwi R, Neuman MR, Merkatz IR, Selim MA, Lysikiewicz A. Determination of the elastic properties of the cervix. Obstet Gynecol 1988;71:568 574.
87. Leppert PC, Cerreta JM, Mandl I. Orientation of elastic fibers in the human cervix. Am J Obstet Gynecol 1986;155:219 224.
88. Leppert PC, Yu SY. Three-dimensional structures of uterine elastic fibers: scanning electron microscopic studies. Connect Tissue Res 1991;27:15 31.
89. Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology 1999;96:272 277.
90. Edwards JN, Morris HB. Langerhans' cells and lymphocyte subsets in the female genital tract. Br J Obstet Gynaecol 1985;92:974 982.
91. Hughes RG, Norval M, Howie SE. Expression of major histocompatibility class II antigens by Langerhans' cells in cervical intraepithelial neoplasia. J Clin Pathol 1988;41:253 259.
92. Roncalli M, Sideri M, Gie P, Servida E. Immunophenotypic analysis of the transformation zone of human cervix. Lab Invest 1988;58:141 149.
93. Ferry JA, Scully RE. Mesonephric remnants, hyperplasia, and neoplasia in the uterine cervix. A study of 49 cases. Am J Surg Pathol 1990;14:1100 1111.
94. Seidman JD, Tavassoli FA. Mesonephric hyperplasia of the uterine cervix: a clinicopathologic study of 51 cases. Int J Gynecol Pathol 1995;14:293 299.
95. Oliva E. CD10 expression in the female genital tract: does it have useful diagnostic applications? Adv Anat Pathol 2004;11:310 315.
96. McCluggage WG, Oliva E, Herrington CS, McBride H, Young RH. CD10 and calretinin staining of endocervical glandular lesions, endocervical stroma and endometrioid adenocarcinomas of the uterine corpus: CD10 positivity is characteristic of, but not specific for, mesonephric lesions and is not specific for endometrial stroma. Histopathology 2003;43:144 150.
97. Nucci MR, Young RH, Fletcher CD. Cellular pseudosarcomatous fibroepithelial stromal polyps of the lower female genital tract: an underrecognized lesion often misdiagnosed as sarcoma. Am J Surg Pathol 2000;24:231 240.
98. Abdul-Karim FW, Cohen RE. Atypical stromal cells of lower female genital tract. Histopathology 1990;17:249 253.
99. Clement PB. Multinucleated stromal giant cells of the uterine cervix. Arch Pathol Lab Med 1985;109:200 202.
100. Metze K, Andrade LA. Atypical stromal giant cells of cervix uteri evidence of Schwann cell origin. Pathol Res Pract 1991;187:1031 1038.
101. Ledger WL, Anderson AB. The influence of steroid hormones on the uterine cervix during pregnancy. J Steroid Biochem 1987;27:1029 1034.
102. Nucci MR, Young RH. Arias-Stella reaction of the endocervix: a report of 18 cases with emphasis on its varied histology and differential diagnosis. Am J Surg Pathol 2004;28:608 612.
103. Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol 1995;38:267 279.
104. Pisharodi LR, Jovanoska S. Spectrum of cytologic changes in pregnancy. A review of 100 abnormal cervicovaginal smears, with emphasis on diagnostic pitfalls. Acta Cytol 1995;39:905 908.
105. Manganiello PD, Burrows LJ, Dain BJ, Gonzalez J. Vabra aspirator and pipelle endometrial suction curette. A comparison. J Reprod Med 1998;43:889 892.
106. Mutter GL, Ferenczy A. Anatomy and histology of the uterine corpus. In: Kurman RJ, ed. Blaustein's Pathology of the Female Genital Tract. 5th ed. New York: Springer-Verlag; 2002:383 420.
107. Giudice LC, Ferenczy A. The endometrial cycle: morphologic and biochemical. In: Adashi EY, Rock JA, Rosenwaks Z, eds. Reproductive Endocrinology, Surgery, and Technology. Philadelphia: Lippincott-Raven; 1996:271 300.
108. Buckley CH. Normal endometrium and non-proliferative conditions of the endometrium. In: Fox H, Wells M, eds. Haines and Taylor Obstetrical and Gynaecological Pathology. 5th ed. London: Churchill Livingstone; 2003:391 442.
109. Warren M, Li T, Klentzeris D. Cell biology of the endometrium: histology, cell types and menstrual changes. In: Chard T, Grudzinskas JG, eds. The Uterus. New York: Cambridge University Press; 1994:94 124.
110. Cooper JM, Erickson ML. Endometrial sampling techniques in the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am 2000;27:235 244.
111. Chambers JT, Chambers SK. Endometrial sampling: When? Where? Why? With what? Clin Obstet Gynecol 1992;35:28 39.
112. Mihm LM, Quick VA, Brumfield JA, Connors AF Jr, Finnerty JJ. The accuracy of endometrial biopsy and saline sonohysterography in the determination of the cause of abnormal uterine bleeding. Am J Obstet Gynecol 2002;186:858 860.
113. Revel A, Shushan A. Investigation of the infertile couple: hysteroscopy with endometrial biopsy is the gold standard investigation for abnormal uterine bleeding. Hum Reprod 2002;17:1947 1949.
114. Tahir MM, Bigrigg MA, Browning JJ, Brookes ST, Smith PA. A randomised controlled trial comparing transvaginal ultrasound, outpatient hysteroscopy and endometrial biopsy with inpatient hysteroscopy and curettage. Br J Obstet Gynaecol 1999;106:1259 1264.
115. Ben-Yehuda OM, Kim YB, Leuchter RS. Does hysteroscopy improve upon the sensitivity of dilatation and curettage in the diagnosis of endometrial hyperplasia or carcinoma? Gynecol Oncol 1998;68:4 7.
116. Hill GA, Herbert CM III, Parker RA, Wentz AC. Comparison of late luteal phase endometrial biopsies using the Novak curette or PIPELLE endometrial suction curette. Obstet Gynecol 1989;73(pt 1):443 445.
117. Ferenczy A, Bergeron C. Histology of the human endometrium: from birth to senescence. Ann N Y Acad Sci 1991;622:6 27.
P.1059
118. Denholm R, More IA. Atypical cilia of the human endometrial epithelium. J Anat 1980;131(pt 2):309 315.
119. Comer MT, Andrew AC, Leese HJ, Trejdosiewicz LK, Southgate J. Application of a marker of ciliated epithelial cells to gynaecological pathology. J Clin Pathol 1999;52:355 357.
120. Kearns M, Lala P. Life history of decidual cells: a review. Am J Reprod Immunol 1983;3:78 82.
121. Iwahashi M, Muragaki Y, Ooshima A, Yamoto M, Nakano R. Alterations in distribution and composition of the extracellular matrix during decidualization of the human endometrium. J Reprod Fertil 1996;108:147 155.
122. Speroff L, Fritz MA. The uterus: embryology, histology, and endocrinology of the uterus and menstruation. Anatomical abnormalities and leiomyomas. In: Speroff L, Fritz MA, eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004:113 144.
123. Whitelaw PF, Croy BA. Granulated lymphocytes of pregnancy. Placenta 1996;17:533 543.
124. Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol 2005;42:511 521.
125. King A. Uterine leukocytes and decidualization. Hum Reprod Update 2000;6:28 36.
126. Kayisli UA, Guzeloglu-Kayisli O, Arici A. Endocrine-immune interactions in human endometrium. Ann N Y Acad Sci 2004;1034:50 63.
127. Givan AL, White HD, Stern JE, et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol 1997;38:350 359.
128. Tabibzadeh S. Proliferative activity of lymphoid cells in human endometrium throughout the menstrual cycle. J Clin Endocrinol Metab 1990;70:437 443.
129. Kiviat N, Wolner-Hanssen P, Eschenbach D, et al. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol 1990;14:167 175.
130. Achilles SL, Amortegui AJ, Wiesenfeld HC. Endometrial plasma cells: do they indicate subclinical pelvic inflammatory disease? Sex Transm Dis 2005;32:185 188.
131. Ramsey E. Vascular anatomy. In: Wynn RM, ed. Biology of the Uterus. New York: Plenum Press; 1977:59 76.
132. Ramsey E. Anatomy of the human uterus. In: Chard T, Grudzinskas JG, eds. The Uterus. New York: Cambridge University Press; 1994:18 40.
133. Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction 2001;121:181 186.
134. Taylor RN, Lebovic DI, Hornung D, Mueller MD. Endocrine and paracrine regulation of endometrial angiogenesis. Ann N Y Acad Sci 2001;943:109 121.
135. Rees MC, Bicknell R. Angiogenesis in the endometrium. Angiogenesis 1998;2:29 35.
136. Albrecht ED, Pepe GJ. Steroid hormone regulation of angiogenesis in the primate endometrium. Front Biosci 2003;8:D416 D429.
137. Anin SA, Vince G, Quenby S. Trophoblast invasion. Hum Fertil (Camb) 2004;7:169 174.
138. Lyall F. Priming and remodelling of human placental bed spiral arteries during pregnancy a review. Placenta 2005;26(suppl A):S31 S36.
139. Spornitz UM. The functional morphology of the human endometrium and decidua. Adv Anat Embryol Cell Biol 1992;124:1 99.
140. Cornillie FJ, Lauweryns JM, Brosens IA. Normal human endometrium. An ultrastructural survey. Gynecol Obstet Invest 1985;20:113 129.
141. Dockery P, Rogers AW. The effects of steroids on the fine structure of the endometrium. Baillieres Clin Obstet Gynaecol 1989;3:227 248.
142. Dockery P, Pritchard K, Warren MA, Li TC, Cooke ID. Changes in nuclear morphology in the human endometrial glandular epithelium in women with unexplained infertility. Hum Reprod 1996;11:2251 2256.
143. Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science 2002;296:2185 2188.
144. Tazuke SI, Giudice LC. Growth factors and cytokines in endometrium, embryonic development, and maternal: embryonic interactions. Semin Reprod Endocrinol 1996;14:231 245.
145. Mylonas I, Jeschke U, Wiest I, et al. Inhibin/activin subunits alpha, beta-A and beta-B are differentially expressed in normal human endometrium throughout the menstrual cycle. Histochem Cell Biol 2004;122:461 471.
146. Stavreus-Evers A, Koraen L, Scott JE, Zhang P, Westlund P. Distribution of cyclooxygenase-1, cyclooxygenase-2, and cytosolic phospholipase A2 in the luteal phase human endometrium and ovary. Fertil Steril 2005;83:156 162.
147. Speroff L, Fritz MA, eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
148. Treloar A, Boynton R, Behn B, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil 1967;12(pt 2):77 126.
149. Hall J. Neuroendocrine control of the menstrual cycle. In: Strauss J, Barbieri R, eds. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Philadelphia: Elsevier Saunders; 2004:195 212.
150. Hodgen GD. Neuroendocrinology of the normal menstrual cycle. J Reprod Med 1989;34(1 suppl):68 75.
151. Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev 2005;26:465 478.
152. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Cell communication. In: Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th ed. New York: Garland Science; 2002:831 906.
153. Rhen T, Cidlowski JA. Steroid hormone action. In: Strauss J, Barbieri R, eds. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 5th ed. Philadelphia: Elsevier Saunders; 2004:155 174.
154. Li T, Dockery P, Rogers A, Cooke I. How precise is histologic dating of endometrium using the standard dating criteria? Fertil Steril 1989;51:759 763.
155. Strauss JI, Gurpide E. The endometrium: regulation and dysfunction. In: Yen S, Jaffe R, eds. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. Philadelphia: WB Saunders; 1991:309 356.
156. Wynn RM. The human endometrium: cyclic and gestational changes. In: Wynn R, Jollie W, eds. Biology of the Uterus. 2nd ed. New York: Plenum Medical Book Company; 1989:289 332.
157. Milwidsky A, Palti Z, Gutman A. Glycogen metabolism of the human endometrium. J Clin Endocrinol Metab 1980;51:765 770.
158. Daly D, Tohan N, Doney T, Maslar I, Riddick D. The significance of lymphocytic-leukocytic infiltrates in interpreting late luteal phase endometrial biopsies. Fertil Steril 1982;37:786 791.
159. Critchley HO, Kelly RW, Brenner RM, Baird DT. The endocrinology of menstruation a role for the immune system. Clin Endocrinol (Oxf) 2001;55:701 710.
160. Rogers PA, Lederman F, Taylor N. Endometrial microvascular growth in normal and dysfunctional states. Hum Reprod Update 1998;4:503 508.
161. Salamonsen LA. Tissue injury and repair in the female human reproductive tract. Reproduction 2003;125:301 311.
162. Ludwig H, Spornitz UM. Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Ann N Y Acad Sci 1991;622:28 46.
163. Hertig A. Gestational hyperplasia of endometrium: a morphologic correlation of ova, endometrium, and corpora lutea during early pregnancy. Lab Invest 1964;13:1153 1191.
164. Parr M, Parr E. The implantation reaction. In: Wynn R, Jollie W, eds. Biology of the Uterus. 2nd ed. New York: Plenum Medical Book Company; 1989:233 278.
165. O'Connor D, Kurman R. Intermediate trophoblast in uterine curettings in the diagnosis of ectopic pregnancy. Obstet Gynecol 1988;72:665 670.
P.1060
166. Gruber K, Gelven PL, Austin RM. Chorionic villi or trophoblastic tissue in uterine samples of four women with ectopic pregnancies. Int J Gynecol Pathol 1997;16:28 32.
167. Young RH, Kurman RJ, Scully RE. Placental site nodules and plaques. A clinicopathologic analysis of 20 cases. Am J Surg Pathol 1990;14:1001 1009.
168. Shih IM, Seidman JD, Kurman RJ. Placental site nodule and characterization of distinctive types of intermediate trophoblast. Hum Pathol 1999;30:687 694.
169. Shih IM, Kurman RJ. Ki-67 labeling index in the differential diagnosis of exaggerated placental site, placental site trophoblastic tumor, and choriocarcinoma: a double immunohistochemical staining technique using Ki-67 and Mel-CAM antibodies. Hum Pathol 1998;29:27 33.
170. Shih IM, Kurman RJ. p63 expression is useful in the distinction of epithelioid trophoblastic and placental site trophoblastic tumors by profiling trophoblastic subpopulations. Am J Surg Pathol 2004;28:1177 1183.
171. Arias-Stella J. The Arias-Stella reaction: facts and fancies four decades after. Adv Anat Pathol 2002;9:12 23.
172. Huettner PC, Gersell DJ. Arias-Stella reaction in nonpregnant women: a clinicopathologic study of nine cases. Int J Gynecol Pathol 1994;13:241 247.
173. Arias-Stella J Jr, Arias-Velasquez A, Arias-Stella J. Normal and abnormal mitoses in the atypical endometrial change associated with chorionic tissue effect [corrected]. Am J Surg Pathol 1994;18:694 701.
174. Vang R, Barner R, Wheeler DT, Strauss BL. Immunohistochemical staining for Ki-67 and p53 helps distinguish endometrial Arias-Stella reaction from high-grade carcinoma, including clear cell carcinoma. Int J Gynecol Pathol 2004;23:223 233.
175. Mazur M, Hendrickson M, Kempson R. Optically clear nuclei. An alteration of endometrial epithelium in the presence of trophoblast. Am J Surg Pathol 1983;7:415 423.
176. Lichtig C, Deutch M, Brandes J. Vascular changes of endometrium in early pregnancy. Am J Clin Pathol 1984;81:702 707.
177. Hustin J, Wells M. Pathology of the pregnant uterus. In: Fox H, Wells M, eds. Haines and Taylor Obstetrical and Gynaecological Pathology. 5th ed. Edinburgh, Scotland: Churchill Livingstone; 2003:1327 1357.
178. Taffe JR, Dennerstein L. Menstrual patterns leading to the final menstrual period. Menopause 2002;9:32 40.
179. Archer DF, McIntyre-Seltman K, Wilborn WW Jr, et al. Endometrial morphology in asymptomatic postmenopausal women. Am J Obstet Gynecol 1991;165:371 322.
180. Choo YC, Mak KC, Hsu C, Wong TS, Ma HK. Postmenopausal uterine bleeding of nonorganic cause. Obstet Gynecol 1985;66:225 228.
181. Moodley M, Roberts C. Clinical pathway for the evaluation of postmenopausal bleeding with an emphasis on endometrial cancer detection. J Obstet Gynaecol 2004;24:736 741.
182. Quddus MR, Sung CJ, Zheng W, Lauchlan SC. p53 immunoreactivity in endometrial metaplasia with dysfunctional uterine bleeding. Histopathology 1999;35:44 49.
183. Haney AF. Endometrial biopsy: a test whose time has come and gone. Fertil Steril 2004;82:1295 1296, 1301 1302.
184. Coutifaris C, Myers ER, Guzick DS, et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril 2004;82:1264 1272.
185. Murray MJ, Meyer WR, Zaino RJ, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril 2004;81:1333 1343.
186. Myers ER, Silva S, Barnhart K, et al. Interobserver and intraobserver variability in the histological dating of the endometrium in fertile and infertile women. Fertil Steril 2004;82:1278 1282.
187. Glatstein IZ, Harlow BL, Hornstein MD. Practice patterns among reproductive endocrinologists: further aspects of the infertility evaluation. Fertil Steril 1998;70:263 269.
188. Balasch J. Investigation of the infertile couple: investigation of the infertile couple in the era of assisted reproductive technology: a time for reappraisal. Hum Reprod 2000;15:2251 2257.
189. Speroff L, Fritz MA. Female infertility. In: Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004:1013 1069.
190. Glatstein IZ, Harlow BL, Hornstein MD. Practice patterns among reproductive endocrinologists: further aspects of the infertility evaluation. Fertil Steril 1998;70:263 269.
191. Balasch J, Fabregues F, Creus M, Vanrell J. The usefulness of endometrial biopsy for luteal phase evaluation in infertility. Hum Reprod 1992;7:973 977.
192. Peters AJ, Lloyd RP, Coulam CB. Prevalence of out-of-phase endometrial biopsy specimens. Am J Obstet Gynecol 1992;166(pt 1):1738 1746.
193. Noyes R. Normal phases of the endometrium. In: Hertig A, Norris H, Abell M, eds. The Uterus. Baltimore: Williams & Wilkins; 1973:110 135.
194. Noyes R, Hertig A, Rock J. Dating the endometrial biopsy. Fertil Steril 1950;1:3 25.
195. McNeely MJ, Soules MR. The diagnosis of luteal phase deficiency: a critical review [see comments]. Fertil Steril 1988;50:1 15.
196. Noyes R, Haman J. Accuracy of endometrial dating: correlation of endometrial dating with basal body temperature and menses. Fertil Steril 1953;4:504 517.
197. Li T, Rogers A, Lenton E, Dockery P, Cooke I. A comparison between two methods of chronological dating of human endometrial biopsies during the luteal phase, and their correlation with histologic dating. Fertil Steril 1987;48:928 932.
198. Gibson M, Badger GJ, Byrn F, Lee K, Korson R, Trainer T. Error in histologic dating of secretory endometrium: variance component analysis. Fertil Steril 1991;56:242 247.
199. Scott RT, Snyder RR, Strickland DM, et al. The effect of interobserver variation in dating endometrial histology on the diagnosis of luteal phase defects. Fertil Steril 1988;50:888 892.
200. Otsuki Y. Apoptosis in human endometrium: apoptotic detection methods and signaling. Med Electron Microsc 2001;34:166 173.
201. Kokawa K, Shikone T, Nakano R. Apoptosis in the human uterine endometrium during the menstrual cycle. J Clin Endocrinol Metab 1996;81:4144 4147.
202. Konno R, Igarashi T, Okamoto S, et al. Apoptosis of human endometrium mediated by perforin and granzyme B of NK cells and cytotoxic T lymphocytes. Tohoku J Exp Med 1999;187:149 155.
203. Sivridis E, Giatromanolaki A. New insights into the normal menstrual cycle-regulatory molecules. Histol Histopathol 2004;19:511 516.
204. Rotello RJ, Lieberman RC, Purchio AF, Gerschenson LE. Coordinated regulation of apoptosis and cell proliferation by transforming growth factor beta 1 in cultured uterine epithelial cells. Proc Natl Acad Sci U S A 1991;88:3412 3415.
205. Gerschenson LE, Rotello RJ. Apoptosis: a different type of cell death. FASEB J 1992;6:2450 2455.
206. Rotello RJ, Hocker MB, Gerschenson LE. Biochemical evidence for programmed cell death in rabbit uterine epithelium. Am J Pathol 1989;134:491 495.
207. Rotello RJ, Lieberman RC, Lepoff RB, Gerschenson LE. Characterization of uterine epithelium apoptotic cell death kinetics and regulation by progesterone and RU 486. Am J Pathol 1992;140:449 456.
208. Gompel A, Sabourin JC, Martin A, et al. Bcl-2 expression in normal endometrium during the menstrual cycle. Am J Pathol 1994;144:1195 1202.
209. Maia H Jr, Maltez A, Studart E, Athayde C, Coutinho EM. Ki-67, Bcl-2 and p53 expression in endometrial polyps and in the normal endometrium during the menstrual cycle. BJOG 2004;111:1242 1247.
210. Tabibzadeh S, Zupi E, Babaknia A, Liu R, Marconi D, Romanini C. Site and menstrual cycle-dependent expression of proteins of the tumour necrosis factor (TNF) receptor family, and BCL-2 oncoprotein and phase-specific production of TNF alpha in human endometrium. Hum Reprod 1995;10:277 286.
211. Cole W, Garfield R. Ultrastructure of the myometrium. In: Wynn R, Jollie W, eds. Biology of the Uterus. 2nd ed. New York: Plenum Medical Book Company; 1989:455 504.
P.1061
212. Garfield R, Yallampalli C. Structure and function of uterine muscle. In: Chard T, Grudzinskas JG, eds. The Uterus. New York: Cambridge University Press; 1994:54 93.
213. Huszar G, Walsh M. Biochemistry of the myometrium and cervix. In: Wynn R, Jollie W, eds. Biology of the Uterus. 2nd ed. New York: Plenum Medical Book Company; 1989:355 402.
214. Kao C. Electrophysiological properties of uterine smooth muscle. In: Wynn R, Jollie W, eds. Biology of the Uterus. 2nd ed. New York: Plenum Medical Book Company; 1989:403 454.
215. Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol 1989;160:637 641.
216. Hamoir G. Biochemistry of the myometrium. In: Wynn R, ed. Biology of the Uterus. New York: Plenum Press; 1977:377 421.
217. Marshall J. The physiology of the myometrium. In: Hertig A, Norris H, Abell M, eds. The Uterus. Baltimore: Williams & Wilkins; 1973:89 109.
218. Oliva E, Clement PB, Young RH, Scully RE. Mixed endometrial stromal and smooth muscle tumors of the uterus: a clinicopathologic study of 15 cases. Am J Surg Pathol 1998;22:997 1005.
219. Azumi N, Ben-Ezra J, Battifora H. Immunophenotypic diagnosis of leiomyosarcomas and rhabdomyosarcomas with monoclonal antibodies to muscle-specific actin and desmin in formalin-fixed tissue. Mod Pathol 1988;1:469 474.
220. Brown D, Theaker J, Banks P, Gatter K, Mason D. Cytokeratin expression in smooth muscle and smooth muscle tumours. Histopathology 1987;11:477 486.
221. Langloss J, Kurman R, Bratthauer G. Expression of keratin by normal and neoplastic smooth muscle cells of the human uterus. 1990.
222. Norton AJ, Thomas JA, Isaacson PG. Cytokeratin-specific monoclonal antibodies are reactive with tumours of smooth muscle derivation. An immunocytochemical and biochemical study using antibodies to intermediate filament cytoskeletal proteins. Histopathology 1987;11:487 499.
223. Clement PB, Young RH, Scully RE. Nontrophoblastic pathology of the female genital tract and peritoneum associated with pregnancy. Semin Diagn Pathol 1989;6:372 406.
224. Silverberg S, Kurman R. Tumors of the uterine corpus and gestational trophoblastic disease. 3rd series, fascicle 3. In: Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1992:191 206.
225. Garfield RE, Hayashi RH. Appearance of gap junctions in the myometrium of women during labor. Am J Obstet Gynecol 1981; 140:254 260.
226. Brenner R, Slayden O. The fallopian tube cycle. In: Adashi E, Rock J, Rosenwaks Z, eds. Reproductive Endocrinology: Surgery and Technology. Vol 1. Philadelphia: Lippincott-Raven; 1996:325 339.
227. Bonilla-Musoles F, Ferrer-Barriendos J, Pellicer A. Cyclical changes in the epithelium of the fallopian tube. Studies with scanner electron microscopy (SEM). Clin Exp Obstet Gynecol 1983;10:79 86.
228. Donnez J, Casanas-Roux F, Caprasse J, Ferin J, Thomas K. Cyclic changes in ciliation, cell height, and mitotic activity in human tubal epithelium during reproductive life. Fertil Steril 1985;43:554 559.
229. Jansen RP. Endocrine response in the fallopian tube. Endocr Rev 1984;5:525 551.
230. Lindenbaum ES, Peretz BA, Beach D. Menstrual-cycle-dependent and -independent features of the human fallopian tube fimbrial epithelium: an ultrastructural and cytochemical study. Gynecol Obstet Invest 1983;16:76 85.
231. Verhage HG, Bareither ML, Jaffe RC, Akbar M. Cyclic changes in ciliation, secretion and cell height of the oviductal epithelium in women. Am J Anat 1979;156:505 521.
232. Menezo Y, Guerin P. The mammalian oviduct: biochemistry and physiology. Eur J Obstet Gynecol Reprod Biol 1997;73:99 104.
233. Leese HJ. The formation and function of oviduct fluid. J Reprod Fertil 1988;82:843 856.
234. Sivridis E, Buckley C, Fox H. Argyrophil cells in normal, hyperplastic, and neoplastic endometrium. J Clin Pathol 1984;37:378 381.
235. Constant O, Cooke J, Parsons CA. Reformatted computed tomography of the female pelvis: normal anatomy. Br J Obstet Gynaecol 1989;96:1047 1053.
236. de Castro A, Yebra C, Aznar F, et al. Measurement of the endometrial cavity length using Wing Sound I. Adv Contracept 1987;3:133 137.
237. Hricak H. MRI of the female pelvis: a review. AJR Am J Roentgenol 1986;146:1115 1122.
238. Morris H, Emms M, Visser T, Timme A. Lymphoid tissue of the normal fallopian tube a form of mucosal-associated lymphoid tissue (MALT). Int J Gynecol Pathol 1986;5:11 22.
239. Boehme M, Donat H. Identification of lymphocyte subsets in the human fallopian tube. Am J Reprod Immunol 1992;28:81 84.
240. Kutteh WH, Blackwell RE, Gore H, Kutteh CC, Carr BR, Mestecky J. Secretory immune system of the female reproductive tract. II. Local immune system in normal and infected fallopian tube. Fertil Steril 1990;54:51 55.
241. Lurie M, Tur-Kaspa I, Weill S, Katz I, Rabinovici J, Goldenberg S. Ciliary ultrastructure of respiratory and fallopian tube epithelium in a sterile woman with Kartagener's syndrome. A quantitative estimation. Chest 1989;95:578 581.
242. Halbert SA, Patton DL, Zarutskie PW, Soules MR. Function and structure of cilia in the fallopian tube of an infertile woman with Kartagener's syndrome. Hum Reprod 1997;12:55 58.
243. Agoff SN, Mendelin JE, Grieco VS, Garcia RL. Unexpected gynecologic neoplasms in patients with proven or suspected BRCA-1 or -2 mutations: implications for gross examination, cytology, and clinical follow-up. Am J Surg Pathol 2002;26:171 178.
244. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 2002;346:1609 1615.
245. Lindblom B, Wilhelmsson L, Wikland M, Hamberger L, Wiqvist N. Prostaglandins and oviductal function. Acta Obstet Gynecol Scand Suppl 1983;113:43 46.
246. Green LK, Kott ML. Histopathologic findings in ectopic tubal pregnancy. Int J Gynecol Pathol 1989;8:255 262.
247. Pulkkinen MO, Talo A. Tubal physiologic consideration in ectopic pregnancy. Clin Obstet Gynecol 1987;30:164 172.
248. Donnez J, Casanas-Roux F, Ferin J. Macroscopic and microscopic studies of fallopian tube after laparoscopic sterilization. Contraception 1979;20:497 509.
249. Donnez J, Casanas-Roux F, Ferin J, Thomas K. Tubal polyps, epithelial inclusions, and endometriosis after tubal sterilization. Fertil Steril 1984;41:564 568.
250. Mills SE, Fechner RE. Stromal and epithelial changes in the fallopian tube following hormonal therapy. Hum Pathol 1980;11(5 suppl):583 585.
251. Blackwell PM, Fraser IS. Superficial lymphatics in the functional zone of normal human endometrium. Microvasc Res 1981;21:142 152.
252. Laqueur T. Making Sex: Body and Gender from the Greeks to Freud. Cambridge, MA: Harvard University Press; 1990.
253. Profet M. Menstruation as a defense against pathogens transported by sperm. Q Rev Biol 1993;68:335 386.
254. Strassmann BI. The evolution of endometrial cycles and menstruation. Q Rev Biol 1996;71:181 220.
255. Crafts R, Krieger H. Gross anatomy of the female reproductive tract, pituitary, and hypothalamus. In: Danforth D, Scott J, eds. Obstetrics and Gynecology. Philadelphia: JB Lippincott; 1986:64.
256. Henriksen E. The lymphatic spread of carcinoma of the cervix and of the body of the uterus; a study of 420 necropsies. Am J Obstet Gynecol 1949;58:924 942.
257. DiSaia P, Creasman W, eds. Clinical Gynecologic Oncology. 3rd ed. St. Louis: CV Mosby; 1989:84 93.
258. Hendrickson M, Kempson R. Surgical Pathology of the Uterine Corpus. Philadelphia: WB Saunders; 1980.
259. Arias-Stella J. Gestational endometrium. In: Hertig A, Norris H, Abell M, eds. The Uterus. Baltimore: Williams & Wilkins; 1973:185 212.
260. Hendrickson M, Kempson R. The uterine corpus. In: Sternberg S, ed. Diagnostic Surgical Pathology. New York: Raven; 1994:2091 2193.
261. Janovski N, Paramanandhan T. Ovarian Tumors, Tumors and Tumor-like Conditions of the Ovaries, Fallopian Tubes and Ligaments of the Uterus. Philadelphia: WB Saunders; 1973:191 194.
262. Anderson MC, Hartley RB. Cervical crypt involvement by intraepithelial neoplasia. Obstet Gynecol 1980;55:546 550.
P.1062
263. Clement PB, Young RH, Scully RE. Stromal endometriosis of the uterine cervix. A variant of endometriosis that may simulate a sarcoma. Am J Surg Pathol 1990;14:449 455.
264. Clement PB. Pathology of endometriosis. Pathol Annu 1990;25(pt 1):245 295.
265. Baker PM, Clement PB, Bell DA, Young RH. Superficial endometriosis of the uterine cervix: a report of 20 cases of a process that may be confused with endocervical glandular dysplasia or adenocarcinoma in situ. Int J Gynecol Pathol 1999;18:198 205.
266. Young RH, Scully RE. Atypical forms of microglandular hyperplasia of the cervix simulating carcinoma. A report of five cases and review of the literature. Am J Surg Pathol 1989;13:50 56.
267. Leslie KO, Silverberg SG. Microglandular hyperplasia of the cervix: unusual clinical and pathological presentations and their differential diagnosis. Prog Surg Pathol 1984;5:95 114.
268. Hendrickson MR, Longacre TA, Kempson RL. The uterine corpus. In: Mills SE, ed. Diagnostic Surgical Pathology. 4th ed. New York: Raven Press; 2004:2435 2542.
269. Russack V, Lammers R. Xanthogranulomatous endometritis. Report of six cases and a proposed mechanism of development. Arch Pathol Lab Med 1990;114:929 932.
270. Johannisson E, Parker R, Landgren B, Diczfalusy E. Morphometric analysis of the human endometrium in relation to peripheral hormone levels. Fertil Steril 1982;38:564 571.
271. Jones R, Mammel J, Shepard M, Fisher R. Recovery of chlamydia trachomatis from the endometrium of women at risk for chlamydial infection. Am J Obstet Gynecol 1986;155:35 39.
272. Poropatich C, Rojas M, Silverberg S. Polymorphonuclear leukocytes in the endometrium during the normal menstrual cycle. Int J Gynecol Pathol 1987;6:230 234.
273. Winkler B, Reumann W, Mitao M, Gallo L, Richart R, Crum C. Chlamydial endometritis. A histological and immunohistochemical analysis. Am J Surg Pathol 1984;8:771 778.
274. Farhood AI, Abrams J. Immunohistochemistry of endometrial stromal sarcoma (ESS). Hum Pathol 1991;22:224 230.
275. Binder SW, Nieberg RK, Cheng L, al-Jitawi S. Histologic and immunohistochemical analysis of nine endometrial stromal tumors: an unexpected high frequency of keratin protein positivity. Int J Gynecol Pathol 1991;10:191 197.
276. Dabbs DJ. Diagnostic Immunohistochemistry. New York: Churchill Livingstone; 2002.
277. Devaney K, Tavassoli FA. Immunohistochemistry as a diagnostic aid in the interpretation of unusual mesenchymal tumors of the uterus. Mod Pathol 1991;4:225 231.
278. Farhood AI, Abrams J. Immunohistochemistry of endometrial stromal sarcoma. Hum Pathol 1991;22:224 230.
279. Franquemont DW, Frierson HF Jr, Mills SE. An immunohistochemical study of normal endometrial stroma and endometrial stromal neoplasms. Evidence for smooth muscle differentiation. Am J Surg Pathol 1991;15:861 870.
280. Brown DC, Theaker JM, Banks PM, Gatter K, Mason D. Cytokeratin expression in smooth muscle and smooth muscle tumours. Histopathology 1987;11:477 486.
281. Gown AM, Boyd HC, Chang Y, Ferguson M, Reichler B, Tippens D. Smooth muscle cells can express cytokeratins of simple epithelium. Immunocytochemical and biochemical studies in vitro and in vivo. Am J Pathol 1988;132:223 232.
282. Ramaekers FC, Pruszczynski M, Smedts F. Cytokeratins in smooth muscle cells and smooth muscle tumours. Histopathology 1988;12:558 561.
283. Rizeq MN, van de Rijn M, Hendrickson MR, Rouse RV. A comparative immunohistochemical study of uterine smooth muscle neoplasms with emphasis on the epithelioid variant. Hum Pathol 1994;25:671 677.
284. Balaton AJ, Vuong PN, Vaury P, Baviera EE. Plexiform tumorlet of the uterus: immunohistological evidence for a smooth muscle origin. Histopathology 1986;10:749 754.
285. Sullinger J, Scully RE. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic and immunohistochemical study [abstract]. Mod Pathol 1989;2:93A.
286. Lillemoe I, Perrone T, Norris H, Dehner L. Myogenous phenotype of epithelial-like areas in endometrial stromal sarcomas. Arch Pathol Lab Med 1991;115:215 219.
287. McCluggage WG, Shah V, Walsh MY, Toner PG. Uterine tumour resembling ovarian sex cord tumour: evidence for smooth muscle differentiation. Histopathology 1993;23:83 85.
288. Fetissof F, Berger G, Dubois MP, Philippe A, Lansac J, Jobard P. Female genital tract and Peutz-Jeghers syndrome: an immunohistochemical study. Int J Gynecol Pathol 1985;4:219 229.
289. Saffos RO, Rhatigan RM, Scully RE. Metaplastic papillary tumor of the fallopian tube a distinctive lesion of pregnancy. Am J Clin Pathol 1980;74:232 236.