20 - Urinary Calculi
Authors: Macfarlane, Michael T.
Title: Urology, 4th Edition
Copyright 2006 Lippincott Williams & Wilkins
> Table of Contents > Part Two - Selected Topics > Chapter 20 - Urinary Calculi
Chapter 20
Urinary Calculi
Stone Formation
The genesis of urinary stones requires both crystal formation and aggregation. Crystal formation occurs when concentrations of the stone components reach supersaturation within the urine at a specific temperature and pH. Intermittent supersaturation occurs frequently, for example, after meals or during periods of dehydration. These crystals must then aggregate to form stones and be retained within the kidney. Normal urine contains inhibitors of crystal formation and aggregation. Citrate and magnesium are inhibitors of crystal formation. Tamm-Horsfall protein and nephrocalcin are potent inhibitors of crystal aggregation.
Types and Causes of Stones
Calcium Stones (75%)
Calcium oxalate, as either a monohydrate or dihydrate (less dense), is a major component of most urinary stones. Calcium phosphate (apatite) is the second most common component of stones and is usually found in association with calcium oxalate. Both are highly insoluble salts in urine. Factors that are significant in calcium stone formation are discussed later. Hypercalciuria is the direct antecedent of most calcium stones.
![]() Causes of Hypercalciuria
Causes of Hypercalciuria
Increased intestinal absorption the mechanism of excessive intestinal calcium absorption that occurs in patients with idiopathic hypercalciuria is unknown; however, it is believed to be the most common etiology, accounting for approximately 50% of cases. Serum calcium is usually normal in the idiopathic group. Excessive vitamin D intake will also produce increased intestinal absorption resulting in hypercalcemia and hypercalciuria.
Decreased renal reabsorption the second major cause of idiopathic hypercalciuria is thought to be a renal leak mechanism. The loss of calcium in the urine leads to increased parathyroid hormone (PTH), which in turn causes elevated 1,25-vitamin D3 and increased intestinal absorption to maintain normal serum levels of calcium.
Increased bone resorption the most common etiology of increased bone resorption is hyperparathyroidism; however, it accounts for less than 5% of stone patients. Parathyroid hyperplasia or adenomas secrete excessive amounts of PTH. PTH causes (a) increased calcium reabsorption in the proximal convoluted tubule, (b) increased 1,25-vitamin D3, and (c) increased bone demineralization and calcium release from bone. Patients will have both elevated PTH and serum calcium levels. Other less frequent causes of resorptive hypercalciuria include chronic immobilization, metastatic cancer to bone, multiple myeloma, and vitamin D intoxication.
P.124
![]() Differentiation of Hypercalciuric States
Differentiation of Hypercalciuric States
| Type of Hypercalciuria | Serum Ca2+ | Urine Ca2+ | Serum PTH |
|---|---|---|---|
| Absorptive | Normal | Normal | Normal or increased |
| Renal | Normal | Increased | Increased |
| Idiopathic | Normal | Increased | Normal |
| Resorptive | Increased | Increased | Increased or normal |
![]() Hyperoxaluria
Hyperoxaluria
Oxalate is a major component of most calcium stones and has an effect on crystallization that is ten times greater than that of calcium. Eighty percent of urinary oxalate comes from endogenous production in the liver (40% from ascorbic acid, 40% from glycine) and 20% from dietary sources. Foods high in oxalate are tea, coffee, beer, rhubarb, cocoa, spinach, and other green leafy vegetables. The primary site of oxalate absorption is the distal bowel (colon). Patients with small bowel resection (or bypass) or inflammatory bowel disease can have increased oxalate absorption.
![]() Hyperuricosuria
Hyperuricosuria
Uric acid promotes calcium oxalate crystal formation. Hyperuricosuria with normal urine pH (>5.5) is frequently associated with calcium stones.
P.125
![]() Hypocitraturia
Hypocitraturia
Citrate in the urine has an inhibitory effect on stone formation by binding with calcium in the urine and thereby decreasing calcium oxalate and calcium phosphate crystal formation. A low urinary citrate level is associated with calcium nephrolithiasis in 20% to 60% of patients with stone formation. Conditions that can result in hypocitraturia are renal tubular acidosis (RTA), strenuous exercise, enteric hyperoxaluria, and diets high in animal protein.
![]() Type I (Distal) Renal Tubular Acidosis
Type I (Distal) Renal Tubular Acidosis
Type I RTA is caused by an inability of the distal nephron to establish and maintain a hydrogen ion gradient between the tubular fluid and the blood (see Chapter 28). It causes primarily calcium phosphate stone formation in up to 70% of adults with the disorder because of hypocitraturia and hypercalciuria. The diagnosis of Type I RTA is made by finding hypokalemia, hyperchloremia, metabolic acidosis, and a urine pH of 5.5 or higher. Giving potassium citrate corrects the hypocitraturia, hypokalemia, and metabolic acidosis.
Infection Stones (Struvite) (15%)
Magnesium ammonium phosphate (MgNH4PO4-6H2O) or triple phosphate stones occur in the setting of persistently high urinary pH caused by urea-splitting bacteria, resulting in high ammonia production. Alkaline pH higher than 7.2 markedly reduces the solubility of magnesium ammonium phosphate in urine, resulting in its precipitation. The major urea-splitting organisms include Proteus species, Pseudomonas, and Klebsiella. Neurogenic bladder and foreign bodies in the urinary tract (e.g., catheters and sutures even chromic catgut) have a high association with formation of struvite calculi. Struvite stones have been shown to contain numerous infective bacteria within their structure where antibiotics cannot penetrate; therefore, they must be removed if infection is to be cured. Prophylaxis against recurring magnesium ammonium phosphate stones requires maintenance of sterile urine (long-term suppressive antibiotics), high urine volumes, and decreased urinary phosphate levels (limit dietary phosphate ingestion and intestinal absorption by administering phosphate-binding aluminum hydroxide gels). Struvite calculi account for most staghorn stones.
P.126
Uric Acid Stones (5% 10%)
Uric acid is a product of purine metabolism and is excreted in the urine. Stones form in the setting of low urine volume, low pH (acid urine), and high levels of urinary uric acid (hyperuricosuria). They are the only radiolucent urinary calculi. Uric acid stone formers can be categorized into two major groups: those with high blood levels of uric acid and those with normal levels.
![]() Hyperuricosuria without Hyperuricemia
Hyperuricosuria without Hyperuricemia
Causes of isolated uric acid stones include a consistently abnormal low pH (e.g., patients with chronic diarrheal states or those taking acidifying medications), excessive water loss (especially from the gastrointestinal tract), uricosuric drugs (e.g., salicylates and thiazides), and high-protein diets. Management of these patients consists of increasing urinary volume to 2 L/day (or 1 L urine output for every 300 mg uric acid in a 24-hour urine collection), limiting dietary proteins to less than 90 g/day, and alkalinizing urine (to a level between pH 6.5 and 7.0).
![]() Hyperuricosuria with Hyperuricemia
Hyperuricosuria with Hyperuricemia
Elevated serum uric acid levels can be caused by gout, myeloproliferative disorders (acute leukemia), or neoplastic disease and the Lesch-Nyhan syndrome (an inborn error of metabolism owing to a deficiency of the enzyme hypoxanthine-guanine phosphoribosyltransferase). These patients will usually require treatment with allopurinol 300 mg/day, in addition to the previous measures.
Cystine Stones (1%)
Cystinuria is the result of an inherited autosomal recessive defect in the renal tubular reabsorption of four amino acids: cystine, ornithine, lysine, and arginine (mnemonic COLA). Normal urinary cystine levels are less than 100 mg per 24 hours; however, homozygous cystinurics excrete in excess of 600 mg/day. Ask about a family history of stone disease or recurrent stones and look for the characteristic hexagonal cystine crystals or a positive cyanide nitroprusside test in the urine. Stones may also occur in heterozygous cystinurics that excrete less than 300 mg/day. Cystine stones occur in acid urine. Management of cystinuria consists of increasing urinary volume to 3 to 4 L/day (or >1 L urine output for every 300 mg cystine in a 24-hour urine collection) and urinary alkalinization to pH greater than 7.0. In resistant cases, D-penicillamine (250 mg q6h) or -mercaptopropionylglycine
P.127
(MPG) (250 mg q6h) can be used to enhance cystine solubility in urine. Cystine stones can occasionally form staghorns.
Management of Urolithiasis
Management of urolithiasis has undergone a major revolution since the development of extracorporeal shock wave lithotripsy (ESWL) and advances in endoscopic equipment. The combination of ESWL and endourologic technology has almost eliminated major open stone surgery. Approximately 50% of symptomatic stones will require surgical intervention, and 50% can be expected to pass spontaneously. Stones of less than 5 mm in diameter should generally be given 4 to 8 weeks to pass on their own if definitive indications for removal do not exist. Stones of greater than 10 mm are unlikely to pass, and interventional therapy should be planned.
Most Common Sites for Hang-Up of Ureteral Stones
Ureteropelvic junction
Pelvic brim where ureter crosses iliac vessels
Just outside bladder at base of broad ligament in females or where vas deferens crosses ureter in males
Ureterovesical junction in intramural ureter
Indications for Early Intervention
High-grade urinary obstruction
Persistent infection despite antibiotics
Uncontrollable pain
Impairment of renal function
Stone Dissolution
Unfortunately, most stones (i.e., calcium stones) cannot be dissolved. Uric acid or cystine stones can frequently be dissolved by alkalinization of the urine. If there is significant ureteral obstruction, a stent should be placed. Oral alkalinization (sodium bicarbonate or potassium citrate) may require several weeks. Titrate urine pH with Nitrazine paper. Direct irrigation of the stone via percutaneous or retrograde catheters with an alkaline solution is occasionally successful. Hyperuricosuria can be managed by a low-purine diet or allopurinol if urine uric acid levels exceed 1,200 mg/day. A diet low in methionine can help lower urinary cystine levels. Dissolution therapy should be given
P.128
1 month before deeming it a failure. Failure to dissolve the stones will require surgical intervention.
![]() Dissolution of Uric Acid Stones
Dissolution of Uric Acid Stones
Increase urine volume to at least 2 L/day.
Titrate urine pH to 6.5 to 7.0 with potassium citrate [10 20 mEq orally (PO) twice a day (bid) to four times a day (qid)].
Adopt low-purine diet (decrease red meats).
Use allopurinol 300 mg/day if patient is unresponsive to low-purine diet or if urine uric acid is greater than 1,200 mg/day.
![]() Dissolution of Cystine Stones
Dissolution of Cystine Stones
Alkalinize urine to pH greater than 7.0 with potassium citrate.
Increase fluid intake to greater than 4 L/day.
Restrict foods high in methionine such as meat and dairy products.
Administer D-penicillamine or -MPG if unresponsive.
Surgical Management
![]() Treatment Modalities
Treatment Modalities
Extracorporeal shock wave lithotripsy. ESWL uses shock waves produced by electrical spark gap or electromagnetic or piezo-electric energy sources. The shock wave is then transmitted through a water medium to the patient. The stone is positioned at the second focal point of the shock wave by a three-dimensional fluoroscopic scanning system or by ultrasound imaging. The similar impedance of the human body and water allows efficient energy transfer to the stone, resulting in fragmentation (note that cystine stones respond poorly to ESWL).
Endourology. Stone extraction can often be performed by manually grasping the stone with special forceps or by trapping the stone within one of several instruments that look like a wire basket and then removing the stone. Stones too large to be directly removed endoscopically must first be fragmented into smaller pieces using laser, electrohydraulic, or ultrasound techniques. Manually crushing the stone is also an option under certain circumstances. Stones can be approached endoscopically in a retrograde fashion through the urethra and ureter or in an anterograde fashion with a percutaneous nephrostomy.
Open surgery. Open surgery for stones such as a pyelolithotomy, nephrolithotomy, or ureterolithotomy is rarely necessary if access to ESWL and endourologic technology is available.
P.129
![]() Treatment by Stone Location
Treatment by Stone Location
Kidney. ESWL and/or endourologic stone fragmentation via a nephrostomy tract or a retrograde approach is the treatment of choice. Open surgical removal is occasionally necessary for complicated cases or large staghorn calculi.
Ureter. Upper ureteral stones above the iliac crest can generally be treated with primary ESWL. Lower ureteral stones can be repositioned endoscopically to above the iliac crest and then treated with ESWL. Primary ureteroscopic extraction with or without laser lithotripsy is effective for lower ureteral stones and some upper ureteral stones.
Bladder and urethra. Vesical calculi can generally be removed cystoscopically with laser or electrohydraulic lithotripsy. Urethral stones can usually be grasped with forceps and removed or pushed back into the bladder with or without the use of laser lithotripsy. Open cystolithotomy is occasionally necessary.
Workup of Stone-Forming Patients
Initial evaluation and acute management of the stone patient are covered in the section on management of urolithiasis and in Chapter 6. A brief guide follows to help approach stone-forming patients after acute therapy has been rendered and the stone removed. Diagnosing the underlying etiology of a patient's stone disease is essential if future stone recurrences are to be avoided. Approximately 15% of patients will have a recurrence within 1 year and nearly 50% within 5 years. Patients with their first stone will generally be given a limited screen looking for significant metabolic risk factors. Patients with recurrent stones or with abnormalities on their first stone screen should undergo a more extensive workup.
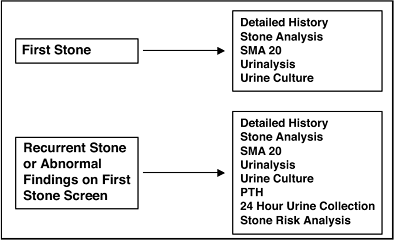 |
No Caption Available |
P.130
History
A detailed history is essential. Question about infections, medications (including herbal supplements), diet (including vitamins), bowel disease or surgery, gout, renal disease, bone or parathyroid disease, and any family history of stone disease. Particular attention should be paid to fluid intake and any dietary indiscretions. A dietary diary for 1 week is helpful.
Metabolic Workup
![]() Stone Analysis
Stone Analysis
Results of a stone chemical analysis are invaluable in guiding management. Every effort should be made to retrieve the stone.
![]() Blood Tests
Blood Tests
SMA-20 will include electrolytes, serum calcium, uric acid, and phosphorus. A PTH level should be obtained.
![]() Urine Assessment
Urine Assessment
Urinalysis (include pH and check for crystals) and urine culture should be obtained.
A cyanide nitroprusside test of the urine can be used to screen for suspected cystinuria.
A 24-hour urine collection on a random diet should be performed for calcium, phosphorus, magnesium, oxalate, uric acid, citrate, total volume, and creatinine and then repeated on a restricted diet (avoid meat and restrict sodium, oxalate, and calcium).
Stone Prevention
Prevention of recurrent stones is an important aspect of the overall care of the stone-forming patient. High fluid intake with the goal of more than 2-L urine output per day is the single most important modification that can be recommended for all stone-forming patients. All patients should also be encouraged to eliminate any dietary excesses (especially protein and phosphates), and moderate sodium restriction is beneficial for patients with hypercalciuria. Potassium citrate has become increasingly important in the management of all stone-forming patients except infection stones.
P.131
Potassium citrate alkalinizes the urine for patients with uric acid or cystine stones and supplements urinary citrate levels and its inhibitory effect on calcium stone formation. Patients with hyperuricemia should be put on a low-protein diet (<90 g/day) and allopurinol if urine uric acid levels exceed 1,200 mg/day or serum uric acid levels are abnormal.
Urinary alkalinization is usually accomplished by giving oral potassium citrate and titrating the dose to the appropriate urine pH monitored with Nitrazine paper. The suggested dosages of potassium citrate are only starting points and should be adjusted as needed to accomplish the goal.
Calcium Stone Forming Patients
The mainstay in preventing recurrent calcium oxalate or calcium phosphate stones is to increase fluid intake and supplement with potassium citrate. The addition of a thiazide diuretic is indicated for high urinary calcium levels and allopurinol for high urinary uric acid levels (>1,200 mg/day). Hyperuricosuria has been shown to promote calcium oxalate stone formation. Moderate sodium restriction is also beneficial to patients with hypercalciuria, especially if they are taking a thiazide diuretic.
Dietary calcium restriction, once a mainstay of preventing calcium nephrolithiasis, has come into question. Recent studies have reported that a higher intake of dietary calcium was strongly associated with a decreased risk of kidney stones. Dietary calcium restriction is thus inappropriate. However, dietary calcium supplementation has not been shown to be protective against calcium stones.
![]() Normocalciuria (and Normocalcemia)
Normocalciuria (and Normocalcemia)
Increase fluid (>2 L/day) and prescribe potassium citrate (Urocit-K 20 mEq PO bid).
![]() Hypercalciuria (and Normocalcemia)
Hypercalciuria (and Normocalcemia)
Increase fluid (>3 L/day) and prescribe potassium citrate (Urocit-K 20 mEq PO bid) and thiazide diuretic (HCTZ 25 mg PO bid).
![]() Hypercalcemia
Hypercalcemia
The patient should be worked up for hyperparathyroidism.
P.132
![]() Hyperuricosuria
Hyperuricosuria
Increase fluid (>3 L/day) and prescribe potassium citrate (Urocit-K 20 mEq PO bid) and allopurinol [Zyloprim 300 mg PO daily (qd)] if urine uric acid levels exceed 1,200 mg/day.
![]() Hyperuricemia
Hyperuricemia
Increase fluid (>3 L/day) and prescribe potassium citrate (Urocit-K 20 mEq PO bid) and allopurinol (Zyloprim 300 mg PO qd).
![]() Hyperoxaluria
Hyperoxaluria
Patients with increased oxalate absorption in the gut are treated as for other findings and dietary oxalate restriction. In addition, calcium carbonate (1 2 g with each meal) or cholestyramine will inhibit oxalate absorption.
![]() Type I (Distal) Renal Tubular Acidosis
Type I (Distal) Renal Tubular Acidosis
Type I RTA causes primarily calcium phosphate stone formation because of hypocitraturia and hypercalciuria. Giving potassium citrate corrects both hypocitraturia and metabolic acidosis.
Struvite Stone Formers
Prevention of recurrent magnesium ammonium phosphate stones requires complete surgical removal of any stone, alleviation of any obstruction, and maintenance of sterile urine. Occasionally acetohydroxamic acid (250 mg PO q8h), a reversible inhibitor of urease, may be used to help prevent crystallization of struvite.
Uric Acid Stone Formers
Patients with hyperuricemia should be managed with increased fluid (>3 L/day), potassium citrate (Urocit-K 20 mEq PO bid), and allopurinol (Zyloprim 300 mg PO qd). Patients with hyperuricosuria and normal serum uric acid levels should be treated with allopurinol if urinary uric acid levels exceed 1,200 mg/day. All patients who form uric acid stones should have their urine alkalinized to pH 6.5 to 7.0. It should not be raised above 7.0. Titrate urine pH with Nitrazine paper. Allopurinol inhibits xanthine oxidase and decreases the production of uric acid. It is dosed at either 300 mg PO qd or 100 mg PO tid.
P.133
Cystine Stone Formers
Restrict foods high in methionine such as meat and dairy products. Fluid intake should be increased (>3 L/day) and the urine should be alkalinized with potassium citrate (Urocit-K 20 mEq PO bid). A urine pH of higher than 7.0 is the goal. If alkalinization fails, then D-penicillamine and -MPG are used to complex with cystine and form soluble compounds.
EAN: 2147483647
Pages: 44
- ERP Systems Impact on Organizations
- The Second Wave ERP Market: An Australian Viewpoint
- Enterprise Application Integration: New Solutions for a Solved Problem or a Challenging Research Field?
- Distributed Data Warehouse for Geo-spatial Services
- Development of Interactive Web Sites to Enhance Police/Community Relations