11. Apoptosis is a property of many or all cells
27.10 Reorganization of the cell at mitosis |
| Key terms defined in this section |
| Centrioles are small hollow cylinders consisting of microtubules that become located near the poles during mitosis. They reside within the centrosomes. Procentriole is an immature centriole, formed in the vicinity of a mature centriole. |
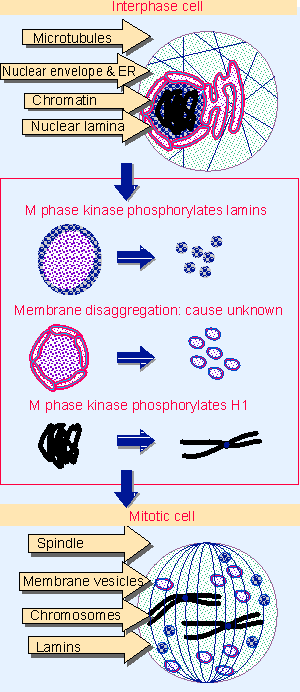 |
Figure 27.32 Major changes in the cell at mitosis involve the chromosomes, nuclear envelope, nuclear lamina, and microtubules. |
The culmination of the cell cycle is the act of division, when the chromosomes segregate into two diploid sets and the other components of the cell are partitioned between the two daughter cells. The change in cell structure is dramatic, as summarized in Figure 27.32. The division between nucleus and cytoplasm is abolished, and the cytoskeleton is entirely reorganized. The relevant events include:
- Condensation of chromatin to give recognizable chromosomes.
- Dissolution of the nuclear lamina and breakdown of the nuclear envelope. The lamina dissociates into individual lamin subunits, and the nuclear envelope, endoplasmic reticulum, and Golgi apparatus break down into small membrane vesicles. (Nuclear dissolution is typical of animal cells but does not occur, for example, in the yeasts, S. cerevisiae and S. pombe, where mitosis involves nuclear division.)
- Dissociation and reconstruction of microtubules into a spindle. Microtubules dissociate into tubulin dimers, which reassemble into microtubules extended from the mitotic microtubule organizing centers.
- Reorganization of actin filaments to replace the usual network by the contractile ring that pinches the daughter cells apart at cytokinesis.
All of these changes are reversible; following the separation of daughter cells, the actin filaments resume their normal form, the microtubular spindle is dissolved, the nuclear envelope reforms, and chromosomes take a more dispersed structure in the form of interphase chromatin. Modification of appropriate substrate proteins (which could be either the structural subunits themselves or proteins associated with them) provides a plausible means to control the passage of mitosis. The question then becomes how the mitotic changes and their reversal depend upon the activation and inactivation of M phase kinase (for review see McIntosh and Koonce, 1989; King et al., 1994).
The best characterized substrate for M phase kinase is histone H1. As noted previously, we do not know what role the phosphorylation of H1 plays at either G1/S or M phase transitions. It is a reasonable assumption, however, that M phase kinase acts directly on chromatin by phosphorylating H1 and other target proteins, and that this is the cause of chromosomal condensation.
Nuclear integrity is abandoned when the underlying lamina dissociates into its constituent lamins, and the nuclear envelope breaks down into vesicles. Lamins are phosphorylated during mitosis, and the presence of phosphate groups on only two serine residues per lamin is sufficient to cause dissociation of the lamina. Mutations that change these serines into alanines prevent the lamina from dissociating at mitosis. So the reversible phosphorylation of these two serine residues induces a structural change in the individual lamin subunits that controls their ability to associate into the lamina.
This phosphorylation event appears to be the direct responsibility of the M phase kinase, which can cause the nuclear lamina to dissociate in vitro. The phosphorylation and dephosphorylation of lamins and lamin-associated proteins may be sufficient to account for the dissolution and reformation of the nuclear envelope. The reorganization of the endoplasmic reticulum and Golgi is not well defined (Peter et al., 1990; Foisner and Gerace, 1993).
The reorganization of microtubules into the spindle has been extensively described, but cannot yet be connected to the action of M phase kinase. Microtubules extend from organizing centers that are found in the centrosomes and at the kinetochores. Microtubules themselves consist of dimers of α-tubulin and β-tubulin. Within the centrosome there is a related protein, γ-tubulin, which is part of a complex that may provide the actual nucleating source. The direct interaction of this complex with the αβ tubulin dimers of microtubules remains to be characterized (for review see Murray and Szostak, 1985; Mitchison, 1988).
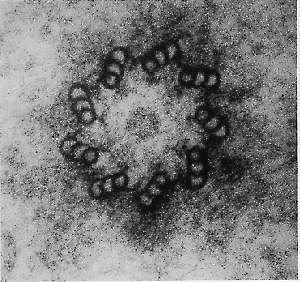 |
Figure 27.33 The centriole consists of nine microtubule triplets, apparent in cross-section as the wall of a hollow cylinder. Photograph kindly provided by A. Ross. |
The structure of centrosomes is not well defined, but in animal cells a centrosome contains a pair of centrioles, surrounded by a dense amorphous region. The centriole is a small hollow cylinder whose wall consists of a series of triplet fused microtubules. A centriole is shown in cross-section in Figure 27.33.
The function of the centriole in mitosis is not clear. Originally it was thought that it might provide the structure to which microtubules are anchored at the pole, but the fibers seem instead to terminate in the amorphous region around the centrioles. It is possible that the centriole is concerned with orienting the spindle; it may also have a role in establishing directionality for cell movement. However, there are cell types in which centrosomes do not appear to contain centrioles.
Centrioles have their own cycle of duplication. When born at mitosis, a cell inherits two centrioles. During interphase they reproduce, so that at the start of mitosis there are four centrioles, two at each pole. Probably only the parental centriole is functional.
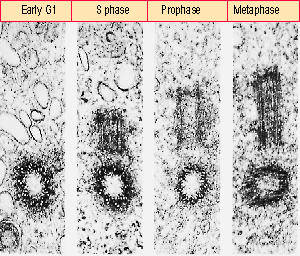 |
Figure 27.34 A centriole reproduces by forming a procentriole on a perpendicular axis; the procentriole is subsequently extended into a mature centriole. Photograph kindly provided by J. B. Rattner and S. G. Phillips. |
The centriole cycle is illustrated in Figure 27.34. Soon after mitosis, a procentriole is elaborated perpendicular to the parental centriole. It has the same structure as the mature parental centriole, but is only about half its length. Later during interphase, it is extended to full length. It plays no role in the next mitosis, but becomes a parental centriole when it is distributed to one of the daughter cells. The orientation of the parental centriole at the mitotic pole is responsible for establishing the direction of the spindle.
How are centrioles reproduced? The precise elaboration of the procentriole adjacent to the parental centriole suggests that some sort of template function is involved. The parental centriole cannot itself be seen to reproduce or divide, but it could provide some nucleating structure onto which tubulin dimers assemble to extend the procentriole. Could a centriole be assembled in the absence of a pre-existing centriole?
| Reviews | |
| King, R. W., Jackson, P. K., and Kirschner, M. W. (1994). Mitosis in transition. Cell 79, 563-571. | |
| McIntosh, J. R. and Koonce, M. P. (1989). Mitosis. Science 246, 622-628. | |
| Mitchison, T. J. (1988). Microtubule dynamics and kinetochore function in mitosis. Ann. Rev. Cell Biol. 4, 527-549. | |
| Murray, A. W. and Szostak, J. W. (1985). Chromosome segregation in mitosis and meiosis. Ann. Rev. Cell Biol. 1, 289-315. | |
| Research | |
| Foisner, R. and Gerace, L. (1993). Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell 73, 1267-1279. | |
| Peter, M. et al. (1990). In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 61, 591-602. | |